Key Points
Question
Does the cricoid pressure prevent pulmonary aspiration in patients undergoing rapid sequence induction of anesthesia?
Findings
In this randomized, noninferiority double-blind trial involving 3472 patients, the results failed to demonstrate the noninferiority of a sham procedure in preventing pulmonary aspiration compared with the cricoid pressure. Mortality, pneumonia, and length of stay did not differ significantly between groups, and differences in intubation time and laryngoscopic exposure suggest more difficulties in the Sellick group.
Meaning
This large randomized trial failed to demonstrate the noninferiority of a sham procedure to prevent pulmonary aspiration during rapid sequence induction of anesthesia.
Abstract
Importance
The use of cricoid pressure (Sellick maneuver) during rapid sequence induction (RSI) of anesthesia remains controversial in the absence of a large randomized trial.
Objective
To test the hypothesis that the incidence of pulmonary aspiration is not increased when cricoid pressure is not performed.
Design, Setting, and Participants
Randomized, double-blind, noninferiority trial conducted in 10 academic centers. Patients undergoing anesthesia with RSI were enrolled from February 2014 until February 2017 and followed up for 28 days or until hospital discharge (last follow-up, February 8, 2017).
Interventions
Patients were assigned to a cricoid pressure (Sellick group) or a sham procedure group.
Main Outcomes and Measures
Primary end point was the incidence of pulmonary aspiration (at the glottis level during laryngoscopy or by tracheal aspiration after intubation). It was hypothesized that the sham procedure would not be inferior to the cricoid pressure. The secondary end points were related to pulmonary aspiration, difficult tracheal intubation, and traumatic complications owing to the tracheal intubation or cricoid pressure.
Results
Of 3472 patients randomized, mean (SD) age was 51 (19) years and 1777 (51%) were men. The primary end point, pulmonary aspiration, occurred in 10 patients (0.6%) in the Sellick group and in 9 patients (0.5%) in the sham group. The upper limit of the 1-sided 95% CI of relative risk was 2.00, exceeding 1.50, failing to demonstrate noninferiority (P = .14). The risk difference was −0.06% (2-sided 95% CI, −0.57 to 0.42) in the intent-to-treat population and −0.06% (2-sided 95% CI, −0.56 to 0.43) in the per protocol population. Secondary end points were not significantly different among the 2 groups (pneumonia, length of stay, and mortality), although the comparison of the Cormack and Lehane grade (Grades 3 and 4, 10% vs 5%; P <.001) and the longer intubation time (Intubation time >30 seconds, 47% vs 40%; P <.001) suggest an increased difficulty of tracheal intubation in the Sellick group.
Conclusions and Relevance
This large randomized clinical trial performed in patients undergoing anesthesia with RSI failed to demonstrate the noninferiority of the sham procedure in preventing pulmonary aspiration. Further studies are required in pregnant women and outside the operating room.
Trial Registration
ClinicalTrials.gov Identifier: NCT02080754
This randomized clinical trial investigates whether the incidence of pulmonary aspiration is increased when cricoid pressure is not performed.
Introduction
Induction of anesthesia induces a loss of protective upper airway reflexes and may be associated with pulmonary aspiration.1 The incidence of anesthesia-induced pulmonary aspiration is very low (0.03%) in elective surgery2 when preoperative fasting rules have been complied and in the absence of risk factors for regurgitation of gastric contents such as pregnancy or morbid obesity.3,4 However, in emergency conditions, noncompliance with preoperative fasting rules and delayed gastric emptying markedly increase the risk of pulmonary aspiration.5 In this context, a rapid sequence induction (RSI) of anesthesia is recommended to minimize the risk of aspiration, combining the use of a short-acting hypnotic and a muscle relaxant, mainly succinylcholine, associated with the application of a manual pressure to the cricoid cartilage (known as Sellick maneuver). The goal of the cricoid pressure is to compress the esophagus between the cricoid cartilage and the fifth cervical vertebra. The cricoid pressure was described more than 45 years ago6 and is widely recommended, although its efficacy has been poorly documented.7 Several studies have challenged its efficacy because occlusion of the esophagus is often uncomplete,8 and it could even facilitate the opening of the lower esophageal sphincter.9 Moreover, the cricoid pressure may increase airway obstruction, compromising mask ventilation,10 and/or alter the glottis vision increasing the rate of difficult tracheal intubation,7 although this last point was not confirmed in a randomized study.11 Lastly, although all anesthesiologists have been trained to perform the cricoid pressure, it is thought to be difficult to perform appropriately,7 and some rare but severe traumatic injuries (esophageal or cricoid cartilage rupture) have been reported.12,13 This multicenter, noninferiority randomized clinical trial was conducted to test the hypothesis that the incidence of pulmonary aspiration is not increased when cricoid pressure is not performed during a RSI of anesthesia.
Methods
Study Design
The IRIS (Sellick Interest in Rapid Sequence Induction) study was a multicenter, randomized, double-blind, noninferiority trial aiming to assess the cricoid pressure during RSI in adults by comparing the incidence of pulmonary aspiration whether this maneuver is applied or feigned. The protocol was registered in ClinicalTrials.gov and is available in Supplement 1. Recruitment began in February 2014 and ended (including follow-up) in February 2017. The study was approved by the Comité de Protection des Personnes Ile-de-France VI, Paris, France. Written informed consent was obtained from the patient or a close relative/surrogate in case of emergency conditions. Should such a person be absent, the patient was randomized according to the specifications of emergency consent authorized by the ethical committee and the patient was asked to give his/her consent for the continuation of the trial when his/her condition allowed. The reporting of this study followed the Consolidated Standards of Reporting Trials statement extended to noninferiority trials.14
Setting and Participants
Patients undergoing any type of surgery under general anesthesia requiring an RSI were eligible for enrollment. The inclusion criteria were patients 18 years and older with a full stomach (<6 hours fasting) or the presence of at least 1 risk factor for pulmonary aspiration (emergency conditions, body mass index >30 [calculated as weight in kilograms divided by height in meters squared], previous gastric surgery [sleeve, bypass, or gastrectomy], ileus, early [<48 hours] postpartum, diabetic gastroparesia, gastroesophageal reflux, hiatus hernia, preoperative nausea/vomiting, and pain).15,16 The exclusion criteria were refusal of patient to participate, younger than 18 years, pregnancy, inclusion in another randomized trial, lack of national health care insurance, contraindication for the use of the cricoid pressure or succinylcholine, pneumonia or pulmonary contusion, upper respiratory tract abnormalities, consciousness disorders, and patients requiring an alternative technique to laryngoscopy.
We assessed the body mass index, Mallampati score,17 mouth opening, and thyromental distance, enabling calculation of the risk of difficult tracheal intubation (post hoc).18 The anesthesia and intubation procedures were standardized, following French guidelines.19 When the patient had a nasogastric tube before anesthesia, the decision to remove it was left to the attending anesthesiologist as well as the decision to administer antacid before anesthesia. After preoxygenation (either until an expired oxygen fraction >90% had been obtained or following 4 forced vital capacity inspirations in emergency cases), anesthesia was induced using a rapid active hypnotic (propofol or thiopental or etomidate or ketamine) and succinylcholine (1 mg/kg), which provides excellent intubation conditions.20 The choice of the hypnotic was left to the anesthesiologists. The use of rocuronium was not authorized. Tracheal intubation was performed in the sniffing position and using MacIntosh laryngoscope with a metallic blade because a plastic blade increases the rate of difficult tracheal intubation.21 We measured the time to intubation (delay between insertion of the laryngoscope and inflation of the tracheal tube cuff). Correct positioning of the tracheal tube was confirmed by monitoring of end-tidal carbon dioxide. The decision to administer opioids was left to the anesthesiologist.
Intervention
Patients were randomly allocated in a 1:1 ratio to 1 of the following 2 groups: Sellick group and sham group. In the Sellick group, an expected pressure equivalent to 30 N was applied with the first 3 fingers on the cricoid cartilage.22 All operators had been trained to perform this maneuver, which is applied as a routine practice. However, a special training session was performed before the beginning of the study in each center using a mannequin and the syringe model.23 This model enables reproduction of the recommended pressure using an obtruded 50-mL syringe and reducing its volume from 40 mL to 33 mL, this training being repeated each month. Only trained individuals were authorized to perform the cricoid pressure. In the sham group, the investigator did not apply any pressure. To ensure appropriate blinding of the rest of the team, an opaque cover was applied in both groups masking if the investigator applied pressure. To maintain appropriate blinding in case of difficult tracheal intubation, the unique unblinded investigator who applied the cricoid pressure could not replace the blind investigator who performed tracheal intubation. Among junior operators, only those with more than 1 year of training (2 years for nurse) were authorized to perform tracheal intubation.
The randomization list was computer generated, balanced by blocks of undisclosed size (n = 6), and stratified on the center. Allocation concealment was achieved using a centralized, secure, interactive, web-response system accessible from each study center (Randoweb).24
Study Outcomes
The primary end point was the incidence of pulmonary aspiration as detected either at visually the glottis level during laryngoscopy or by tracheal aspiration just after tracheal intubation. The secondary end points were related to pulmonary aspiration (frequency of suspected aspiration pneumonia within 24 hours requiring chest radiography and aspiration pneumonia when patients had both pulmonary aspiration and new infiltrates at the chest radiography; chest radiography was repeated within 24 hours if initially normal), difficult tracheal intubation (Cormack and Lehane grade, reflecting intubation conditions,25 at intubation and if cricoid pressure was interrupted,22 frequency of difficult and impossible intubation, the need of mask ventilation, oxygen desaturation [<92%], and cricoid pressure interruption) and traumatic complications owing to the tracheal intubation or cricoid pressure (esophageal rupture and cricoid cartilage fracture). Pneumonia was considered as severe when at least 1 of the following items was present: decrease in oxygen saturation greater than 10% compared with the value before anesthesia; ratio of partial pressure arterial oxygen to fraction of inspired oxygen less than 300; requirement of mechanical ventilation (invasive or not); and prolonged hospital stay. Difficult tracheal intubation was defined as that requiring more than 2 attempts and/or any alternative technique (except gum elastic bougie).18,26 We recorded mortality at day 28 or at hospital discharge, admission to intensive care unit (ICU), lengths of hospital and ICU stays, and any adverse events declared, even if not associated with the intervention. Adverse events included the following categorical end points: pulmonary aspiration, aspiration pneumonia, difficult and impossible tracheal intubation, traumatic complications, postoperative reintubation, admission into ICU, and mortality; serious adverse events included the following end points: severe aspiration pneumonia, impossible tracheal intubation, postoperative reintubation, admission into ICU, and mortality.
Statistical Analysis
Because of the wide range of pulmonary aspiration rate reported, we chose the estimate reported by Martin et al5: 2.8%. The population included patients requiring tracheal intubation in emergency conditions in an academic center performed by an anesthesiology team but not in an operating room5 and was thought to be closed to that expected in our study. The sham procedure was considered noninferior to the cricoid pressure if the incidence of pulmonary aspiration was not more than 50% higher (relative risk of 1.5). A difference of less than 50% was considered clinically negligible because aspiration is a rare event that may occur despite the use of the cricoid pressure and also because the pressure itself is associated with adverse effects.7 The sample size was calculated at 1717 patients per study arm, for a total of 3434 (α= 0.05; β = 0.20) (NQuery Advisor 7.0, Statistical Solutions Ltd).27 Taking into account an incidence of consent withdrawal of 1.5%, our total target sample size comprised 3500 patients.
Characteristics of the intention-to-treat (ITT) population were expressed as number (percentage) for qualitative variables, and mean (SD) or median (interquartile range [IQR]) for quantitative variables, depending on their distribution. The analysis of the primary end point was performed based primarily on the per protocol (PP) population and repeated on the ITT population. The PP population was defined as patients without protocol violation. Noninferiority was assessed on the upper limit of the 1-sided 95% CI of the relative risk of aspiration (sham group/Sellick group). Because of the low event rate, a bias-corrected and accelerated bootstrap CI was computed using 10 000 resamples.28 If the upper limit of the CI was lower than 1.5, the noninferiority hypothesis would be accepted. The 2-sided (precision) 95% CI was also reported. We also calculated the risk difference (post hoc) and its 2-sided 95% CI, as recommended.14 The secondary end points were compared with a superiority hypothesis on the ITT population and using available data. Qualitative variables were compared using the Pearson χ2 test, Fisher exact test, or Cochrane-Armitage test for trend, and continuous variables were compared using the Wilcoxon rank sum test. No interim analysis was planned. All superiority tests were 2-sided, and P values of less than .05 were considered significant. Statistical analysis was performed using R software, version 3.4.1 (R Programming).
Results
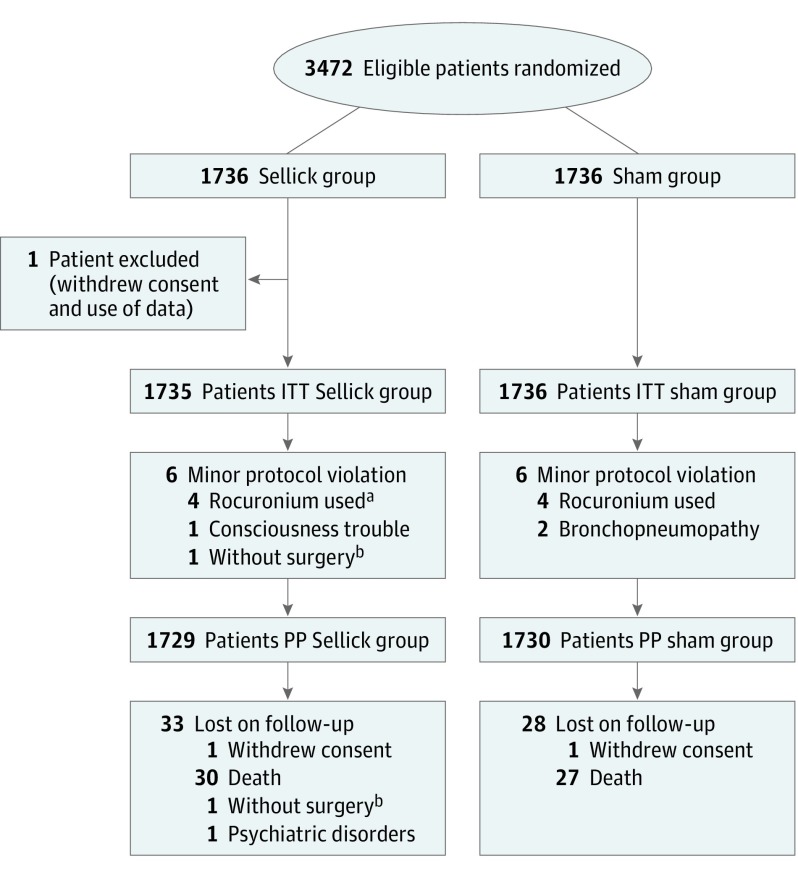
Twelve centers were invited to participate and 2 declined. The 10 participating centers recruited 3472 patients (Figure 1; eTable 1 in Supplement 2). One patient was excluded because he withdrew consent and data use. No major protocol violation occurred, and minor violations were observed in 12 (0.3%), including use of rocuronium instead of succinylcholine (n = 8), preexisting signs of pneumonia (n = 2), preexisting disturbed consciousness (n = 1), and lack of surgery (n = 1). Two patients withdrew consent. Therefore, 1735 patients in the Sellick group and 1736 in the sham group were analyzed on an ITT basis; 1729 patients in the Sellick group and 1730 in the sham group were analyzed on a PP analysis.
Figure 1. Flow of Participants Through the Study.
ITT indicates intention to treat; PP, per protocol.
aIncluding 1 patient with allergy to succinylcholine.
bThis refers to the same patient.
The baseline characteristics of the 2 groups were well balanced (Table 1). Patients underwent mainly abdominal surgery (n = 2116; 61%), endoscopy (n = 484; 14%), orthopedic surgery (n = 462; 13%), head and neck surgery (n = 123; 4%), and cardiothoracic surgery (n = 68; 2%) (198 missing values).
Table 1. Main Baseline Characteristicsa.
| Variable | No. (%) | ||
|---|---|---|---|
| Sellick Group (n = 1735) | Sham Group (n = 1736) | Total (N = 3471) | |
| Age, mean (SD), y | 51 (20) | 51 (19) | 51 (19) |
| Men | 893 (51) | 884 (51) | 1777 (51) |
| BMI, mean (SD) | 26.4 (6.8) | 26.7 (7.2) | 26.6 (7.0) |
| Regurgitation risk factors | |||
| Emergency condition | 1150 (66) | 1137 (65) | 2287 (66) |
| Nonfasting | 169 (10) | 169 (10) | 338 (10) |
| Gastroesophageal reflux | 351 (20) | 328 (19) | 679 (20) |
| Hiatal hernia | 110 (6) | 116 (7) | 226 (7) |
| Diabetic gastroparesis | 63 (4) | 85 (5) | 148 (4) |
| Ileus | 559 (32) | 540 (31) | 1099 (32) |
| Nausea and/or vomiting | 399 (23) | 391 (23) | 790 (23) |
| Obesity (BMI >30) | 212 (12) | 261 (15) | 473 (14) |
| Pain | 399 (23) | 372 (21) | 771 (22) |
| Postpartum (<48 h) | 3 (0.2) | 0 | 3 (0.1) |
| Previous gastric surgeryb | 87 (5) | 88 (5) | 175 (5) |
| Others | 158 (9) | 141 (8) | 299 (9) |
| Factors, No. (IQR) | 2 (1-3) | 2 (1-3) | 2 (1-3) |
| Difficult intubation risk factors | |||
| Mouth opening, mean (SD), mm | 46 (10) | 47 (10) | 47 (10) |
| Missing values, No. | 40 | 40 | 80 |
| Thyromental distance, mean (SD), mm | 81 (18) | 81 (18) | 81 (18) |
| Missing values, No. | 44 | 46 | 90 |
| Mallampati score | |||
| 1 | 778 (45) | 786 (46) | 1564 (46) |
| 2 | 749 (44) | 698 (41) | 1447 (42) |
| 3 | 172 (10) | 211 (12) | 383 (11) |
| 4 | 15 (0.9) | 16 (0.9) | 31 (0.9) |
| Missing values, No. | 21 | 25 | 46 |
| Receding mandible | 90 (5) | 79 (5) | 169 (5) |
| Missing values, No. | 19 | 19 | 38 |
| Difficult intubation risk | |||
| Low risk | 1082 (65) | 1091 (66) | 2173 (63) |
| Intermediate risk | 571 (34) | 563 (34) | 1134 (34) |
| High risk | 16 (1) | 8 (0.5) | 24 (0.7) |
| Missing values, No. | 66 | 74 | 140 |
| Nasogastric tube aspiration before | 256 (15) | 246 (14) | 502 (14) |
| Nasogastric tube in place | 218 (13) | 221 (13) | 439 (13) |
| Missing values, No. | 3 | 4 | 7 |
| Antacid medication | 206 (12) | 203 (12) | 409 (12) |
| Missing values, No. | 4 | 5 | 9 |
| Anesthetic induction | |||
| Propofol | 1546 (89) | 1560 (90) | 3106 (90) |
| Ketamine | 161 (9) | 165 (9) | 326 (9) |
| Etomidate | 154 (9) | 139 (8) | 293 (8) |
| Thiopental | 32 (2) | 42 (2) | 74 (2) |
| Combined administration | 158 (9) | 166 (10) | 324 (9) |
| Missing values, No. | 1 | 0 | 1 |
| Opioid administration | 497 (29) | 544 (31) | 1041 (30) |
| Missing values, No. | 1 | 1 | 1 |
| Dose of succinylcholine, mg (SD) | 81 (20) | 81 (22) | 81 (21) |
| Missing values, No. | 4 | 5 | 9 |
| Nondepolarizing muscular relaxant | 972 (56) | 998 (58) | 1970 (57) |
| Missing values, No. | 0 | 5 | 5 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range.
No significant difference between groups.
This includes sleeve, bypass, and gastrectomy.
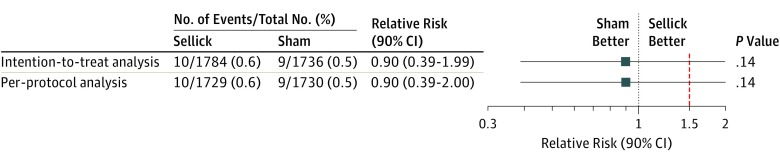
Concerning the primary end point, no missing value was observed. In the ITT population, pulmonary aspiration occurred in 10 patients (0.6%) in the Sellick group and in 9 patients (0.5%) in the sham group (relative risk, 0.90; 95% CI, 0.33-2.38) (Figure 2). In the PP analysis, pulmonary aspiration occurred in 10 patients (0.6%) in the Sellick group and in 9 patients (0.5%) in the sham group (relative risk, 0.90; 2-sided 95% CI, 0.33-2.38). In both the ITT and PP populations, the upper limit of the 1-sided 95% CI (1.99 and 2.00, respectively) exceeded the noninferiority margin of 1.5, thus noninferiority was not demonstrated (P = .14 in both analysis). The risk difference was −0.06% (2-sided 95% CI, −0.57 to 0.42) in the ITT population and −0.06% (2-sided 95% CI, −0.56 to 0.43) in the PP population. The same results were obtained in the subgroups of patients without nasogastric tube (n = 3032) and those requiring emergency surgery (n = 2286) (data not shown; post hoc analysis).
Figure 2. Comparison of the Incidence of Pulmonary Aspiration (Primary End Point) Between the Sellick Group and the Sham Group.
The points represent the estimates of relative risk (sham group/Sellick group), and the horizontal bars represent the associated 2-sided 90% CI. The upper limits are identical to those of the 1-sided 95% CI used in this study for establishing noninferiority. Clinical noninferiority of the sham procedure would be accepted if the upper limit of these intervals fell below the predefined noninferiority margin represented in red dotted line.
The incidence of difficult tracheal intubation was higher in the Sellick group but did not reach statistical significance, although the comparison of the Cormack and Lehane grade and the longer intubation time suggest an increased difficulty of tracheal intubation in the Sellick group (Table 2). The incidence of interruption of the cricoid pressure was more frequent in the Sellick group as well as the improvement in the Cormack and Lehane grade after its release (Table 2). All traumatic complications were related to tracheal intubation, and there was no significant difference between groups.
Table 2. Tracheal Intubation and Extubation.
| Variable | No. (%) | P Value | |
|---|---|---|---|
| Sellick Group (n = 1735) | Sham Group (n = 1736) | ||
| Tracheal intubation | |||
| Intubation time, median (IQR), s | 27 (19-40) | 23 (15-37) | <.001 |
| Intubation time >30 s | 792 (47) | 677 (40) | <.001 |
| Missing values, No. | 42 | 49 | NA |
| Operator | .33 | ||
| Senior anesthesiologist | 184 (11) | 162 (9) | |
| Junior anesthesiologist | 439 (25) | 465 (27) | |
| Senior nurse anesthetist | 990 (57) | 970 (56) | |
| Junior nurse anesthetists | 109 (6) | 125 (7) | |
| Missing values, No. | 13 | 14 | NA |
| Use of a bougie | 536 (21) | 546 (21) | .63 |
| Missing values, No. | 1 | 0 | NA |
| No. of attempts | |||
| 1 | 1585 (92) | 1589 (92) | .19 |
| 2 | 119 (7) | 126 (7) | |
| >2 | 28 (2) | 18 (1) | |
| Missing values, No. | 3 | 3 | NA |
| More than 1 operator | 70 (4) | 82 (5) | .32 |
| Missing values, No. | 5 | 4 | NA |
| Cormack and Lehane grade | |||
| 1 | 1285 (74) | 1381 (80) | <.001 |
| 2 | 270 (16) | 256 (15) | |
| 3 | 133 (8) | 76 (4) | |
| 4 | 42 (2) | 17 (1) | |
| Missing values, No. | 5 | 6 | NA |
| Interruption of the cricoid pressure | 246 (14) | 86 (5) | <.001 |
| Missing values, No. | 5 | 5 | NA |
| Improvement in Cormack and Lehane Grade after cricoid pressure interruption | 152 (62) | 28 (33) | <.001 |
| Missing values, No. | 1 | 1 | .55 |
| Vomiting | 6 (0.3) | 4 (0.2) | |
| Missing values, No. | 1 | 0 | |
| Difficult tracheal intubation | 72 (4) | 51 (3) | |
| More than 2 attempts | 51 | 39 | .05 |
| Other technique used | 31 | 21 | |
| Missing values, No. | 1 | 0 | |
| Impossible tracheal intubation | 0 | 1 | NA |
| Missing values, No. | 1 | 0 | >.99 |
| Mask ventilation required | 23 (1) | 20 (1) | NA |
| Missing values, No. | 2 | 0 | .64 |
| Oxygen desaturation (<92%) | 67 (4) | 66 (4) | NA |
| Missing values, No. | 4 | 0 | .92 |
| Tracheal extubation | |||
| Postoperative extubation | 1676 (97) | 1687 (97) | .42 |
| Missing values, No. | 3 | 1 | NA |
| Patients with NDMR and extubated | 930 (54) | 962 (55) | .29 |
| Missing values, No. | 1 | 0 | NA |
| Train-of-4 measurementa | 817 (88) | 835 (87) | .45 |
| Train-of 4, median (IQR), %a | 97 (91-100) | 97 (91-100) | .86 |
| Missing values, No. | 3 | 2 | NA |
| Reversal agent administereda | 515 (56) | 464 (56) | .78 |
| Missing values, No. | 3 | 1 | NA |
| Swallowing reflex present | 1659 (99) | 1667 (99) | .84 |
| Missing values, No. | 62 | 56 | NA |
| Postoperative dyspnea | 9 (0.5) | 11 (0.6) | .66 |
| Missing values, No. | 51 | 47 | NA |
| Postoperative oxygen desaturation (<92%) | 60 (4) | 62 (4) | .88 |
| Missing values, No. | 68 | 51 | NA |
| Postoperative NIV | 2 (0.1) | 5 (0.3) | .45 |
| Missing values, No. | 51 | 45 | NA |
| Postoperative tracheal reintubation | 2 (0.1) | 2 (0.1) | >.99 |
| Missing values, No. | 50 | 45 | NA |
| Traumatic complication | 17 (1) | 9 (0.5) | .11 |
| Missing values, No. | 3 | 0 | NA |
Abbreviations: IQR, interquartile range; NA, not applicable; NDMR, nondepolarizing muscular relaxant; NIV, noninvasive ventilation.
Results are provided for patients with NMDR and extubated.
There was no significant difference concerning the extubation procedure (Table 2). Extubation was performed mostly in the operating room (n = 2610; 77%) and less frequently in the recovery room (n = 750; 23%). Most patients (n = 1703; 90%) who received a nondepolarizing muscular relaxant during surgery and were extubated postoperatively underwent either train-of-4 measurement to assess neuromuscular blockade and/or reversal of neuromuscular blockade.29 When considering other secondary end points, no significant difference was observed between the Sellick and the sham group, including the incidence of adverse events (Table 3; eTable 2 in the Supplement).
Table 3. Secondary End Points.
| Variable | No. (%) | Mean Difference, % (95% CI)a | P Value | |
|---|---|---|---|---|
| Sellick Group (n = 1735) | Sham Group (n = 1736) | |||
| Mortality | 30 (2) | 27 (2) | −0.2 (−1.0 to 0.7) | .69 |
| Suspected pneumonia within 24 h | 15 (0.9) | 10 (0.6) | −0.3 (−0.8 to 0.3) | .31 |
| Aspiration pneumonia | 4 (0.2) | 4 (0.2) | 0 (−0.3 to 0.3) | >.99 |
| Severe pneumonia | 2 (0.1) | 2 (0.1) | 0 (−0.2 to 0.2) | >.99 |
| Length of hospital stay, median (IQR), d | 3 (1-9) | 4 (1-9) | 0.3 (−0.2 to 0.8) | .89 |
| Length of hospital stay >28 d | 91 (5) | 93 (5) | 0.1 (−1.4 to 1.6) | .89 |
| Admission into ICU | 181 (10) | 201 (12) | 1.1 (−0.9 to 3.2) | .28 |
| Length of ICU stay, median (IQR), d | 5 (2-11) | 5 (2-10) | −0.4 (−1.9 to 1.1) | .58 |
| Adverse eventsb | 276 (16) | 256 (15) | −1.2 (−3.6 to 1.2) | .34 |
| Serious adverse eventsc | 200 (12) | 211 (12) | 0.6 (−1.5 to 2.8) | .57 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
Mean difference is calculated as Sellick group minus sham group.
Systematically included the following categorical end points: pulmonary aspiration, aspiration pneumonia, difficult and impossible tracheal intubation, traumatic complications, postoperative reintubation, admission into ICU, and mortality, which were present in 83% of patients with adverse events.
Systematically included the following categorical end points: severe aspiration pneumonia, impossible tracheal intubation, postoperative reintubation, admission into ICU, and mortality, which were present in 83% of patients with serious adverse events.
Discussion
This large, randomized double-blind trial in patients undergoing anesthesia with RSI failed to demonstrate the noninferiority (δ = 50%) of the sham procedure as compared with the cricoid pressure in preventing pulmonary aspiration. We also observed a low incidence of pulmonary aspiration (0.5%), and we did not observe any significant secondary end points except those suggesting a more difficult tracheal intubation in the Sellick group.
Although the cricoid pressure has been used in clinical practice for decades and is recommended by most countries during RSI, it remains controversial, including its effectiveness in preventing pulmonary aspiration as well its deleterious effects such as airway-related complications (interference with laryngeal exposure, difficult tracheal intubation, and mask ventilation)7 and rare traumatic complications.12,13 Some paradoxical effects, potentially favoring aspiration, have been reported such as opening of the lower esophageal sphincter.9 Moreover, application of an appropriate pressure to the cricoid cartilage may not be simple, and many studies have focused on its appropriate application and training, and 47% to 63% of operators may improperly apply cricoid pressure.7 The lack of a large randomized trial explains these controversies. In a systematic review, Algie et al30 identified only 1 small randomized trial (n = 40)31 and concluded that there is no relevant information available from randomized trials with respect to the application of cricoid pressure during RSI.
Our primary end point was the occurrence of pulmonary aspiration either during laryngoscopy or tracheal aspiration. This end point is thought to directly assess the efficiency of the cricoid pressure and minimize lost on follow-up because it is recorded immediately after tracheal intubation. This end point is probably less sensitive than those using a biomarker32 but has the advantage of excluding aspirations that could occur intraoperatively or postoperatively and that cannot be prevented by the cricoid pressure. This study failed to demonstrate noninferiority because the incidence of aspiration was overestimated (2.8% expected vs 0.5% observed). We do not think that this was related to the inclusion of patients with a too-low risk of regurgitation because our study population reflects that in which RSI remains recommended, and most of our patients fulfilled at least 2 criteria or more for high risk of aspiration (Table 1). Several differences may explain this lower incidence of pulmonary aspiration in our study compared with that of Martin et al5 such as operating room vs nonoperating room setting (ie, more compliance with RSI recommendations), more frequent use of succinylcholine (99% vs 60%) and propofol (90% vs 18%), and less frequent cardiac arrests (0% vs 45%). When looking at other secondary end points (mortality, pneumonia, adverse effects, and length of stay) no indication was noted in favor of the cricoid pressure.
The cricoid pressure has been accused of leading to difficult tracheal intubation or even difficult mask ventilation.7,11 Our study confirmed that it adversely interferes with duration of intubation and laryngeal exposure but without significant increase in the incidence of difficult tracheal intubation (Table 2). This result is in agreement with that obtained in a randomized study.11 The main reason is that the cricoid pressure is usually interrupted when facing unexpected difficult tracheal intubation. Together with the lack of significant difference in traumatic complications, this result suggests that the interference of the cricoid pressure with airway control has previously been overestimated.
Our study has several strengths. To maximize the effectiveness of the cricoid pressure, we standardized anesthesia, tracheal intubation, and cricoid pressure procedures, which are considered essential in such a trial.7 We repeatedly trained the operators, using a simple simulation device,23 although all operators were already trained and frequently used the technique. We also carefully assessed residual neuromuscular blockade and swallowing reflex because postoperative aspiration may have influenced secondary end points (pneumonia, length of stay, and mortality). We did not standardize the use of gastric tubes, but excluding patients with a gastric tube did not change our results.
Limitations
Our study has several limitations. We excluded pregnant women and children, and thus, our results may not apply to obstetric and pediatric populations. This is important because pulmonary aspiration still remains a cause of maternal death.3 Further randomized trials are needed in these specific populations, and our study may help to obtained better ethical acceptance of such randomized studies.7 Our results may not apply to emergency conditions outside the operating room in which sedation and tracheal intubation conditions are worse, and pulmonary aspiration may have occurred before RSI.33 Our study population may have comprised patients with heterogeneous risk factors for pulmonary aspiration but there is no evidence-based indication for the exact weight of these factors.15 Very low levels of aspiration could have also been clinically unnoticed. Finally, our study took place in urban academic centers and might not be generalizable in other settings.
Conclusions
In what is, to our knowledge, the first large randomized trial performed in patients undergoing anesthesia with RSI, we failed to demonstrate the noninferiority (δ = 50%) of a sham procedure as compared with the cricoid pressure in preventing pulmonary aspiration. We did not observe any significant difference in pneumonia, length of stay, and mortality. Further randomized studies are required in pregnant women and in emergency conditions outside the operating room, both conditions not studied here.
Trial Protocol.
eTable 1. Recruitment in the 10 Academic Centers
eTable 2. Description of Adverse Events
References
- 1.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62. [DOI] [PubMed] [Google Scholar]
- 2.Olsson GL, Hallen B, Hambraeus-Jonzon K. Aspiration during anaesthesia: a computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986;30(1):84-92. [DOI] [PubMed] [Google Scholar]
- 3.Thomas TA, Cooper GM. Why Mothers Die 2000-2002: The Sixth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG; 2001:137-138. [Google Scholar]
- 4.Langeron O, Birenbaum A, Le Saché F, Raux M. Airway management in obese patient. Minerva Anestesiol. 2014;80(3):382-392. [PubMed] [Google Scholar]
- 5.Martin LD, Mhyre JM, Shanks AM, Tremper KK, Kheterpal S. 3,423 emergency tracheal intubations at a university hospital: airway outcomes and complications. Anesthesiology. 2011;114(1):42-48. [DOI] [PubMed] [Google Scholar]
- 6.Sellick BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2(7199):404-406. [DOI] [PubMed] [Google Scholar]
- 7.Salem MR, Khorasani A, Zeidan A, Crystal GJ. Cricoid pressure controversies: narrative review. Anesthesiology. 2017;126(4):738-752. [DOI] [PubMed] [Google Scholar]
- 8.Smith KJ, Dobranowski J, Yip G, Dauphin A, Choi PT. Cricoid pressure displaces the esophagus: an observational study using magnetic resonance imaging. Anesthesiology. 2003;99(1):60-64. [DOI] [PubMed] [Google Scholar]
- 9.Tournadre JP, Chassard D, Berrada KR, Boulétreau P. Cricoid cartilage pressure decreases lower esophageal sphincter tone. Anesthesiology. 1997;86(1):7-9. [DOI] [PubMed] [Google Scholar]
- 10.Shorten GD, Alfille PH, Gliklich RE. Airway obstruction following application of cricoid pressure. J Clin Anesth. 1991;3(5):403-405. [DOI] [PubMed] [Google Scholar]
- 11.Turgeon AF, Nicole PC, Trépanier CA, Marcoux S, Lessard MR. Cricoid pressure does not increase the rate of failed intubation by direct laryngoscopy in adults. Anesthesiology. 2005;102(2):315-319. [DOI] [PubMed] [Google Scholar]
- 12.Ralph SJ, Wareham CA. Rupture of the oesophagus during cricoid pressure. Anaesthesia. 1991;46(1):40-41. [DOI] [PubMed] [Google Scholar]
- 13.Heath KJ, Palmer M, Fletcher SJ. Fracture of the cricoid cartilage after Sellick’s manoeuvre. Br J Anaesth. 1996;76(6):877-878. [DOI] [PubMed] [Google Scholar]
- 14.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295(10):1152-1160. [DOI] [PubMed] [Google Scholar]
- 15.American Society of Anesthesiologists Committee Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114(3):495-511. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan V, Hashmi J, Singh R, Samra T, Aneja S. Comparative evaluation of gastric pH and volume in morbidly obese and lean patients undergoing elective surgery and effect of aspiration prophylaxis. J Clin Anesth. 2015;27(5):396-400. [DOI] [PubMed] [Google Scholar]
- 17.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429-434. [DOI] [PubMed] [Google Scholar]
- 18.Langeron O, Cuvillon P, Ibanez-Esteve C, Lenfant F, Riou B, Le Manach Y. Prediction of difficult tracheal intubation: time for a paradigm change. Anesthesiology. 2012;117(6):1223-1233. [DOI] [PubMed] [Google Scholar]
- 19.Molliex S, Berset JC, Billard V, et al. ; Société Français d’Anesthésie et de Réanimation . [Airway management in adult anesthesia except with the exception of difficult intubation. Recommendations of the jury. Short text--2000]. Ann Fr Anesth Reanim. 2003;22(8):745-749. [DOI] [PubMed] [Google Scholar]
- 20.Tran DT, Newton EK, Mount VA, Lee JS, Wells GA, Perry JJ. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2015;10(10):CD002788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amour J, Marmion F, Birenbaum A, et al. Comparison of plastic single-use and metal reusable laryngoscope blades for orotracheal intubation during rapid sequence induction of anesthesia. Anesthesiology. 2006;104(1):60-64. [DOI] [PubMed] [Google Scholar]
- 22.Herman NL, Carter B, Van Decar TK. Cricoid pressure: teaching the recommended level. Anesth Analg. 1996;83(4):859-863. [DOI] [PubMed] [Google Scholar]
- 23.Flucker CJ, Hart E, Weisz M, Griffiths R, Ruth M. The 50-millilitre syringe as an inexpensive training aid in the application of cricoid pressure. Eur J Anaesthesiol. 2000;17(7):443-447. [DOI] [PubMed] [Google Scholar]
- 24.Morice V. RandoWeb, an online randomization tool for clinical trials. Comput Methods Programs Biomed. 2012;107(2):308-314. [DOI] [PubMed] [Google Scholar]
- 25.Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39(11):1105-1111. [PubMed] [Google Scholar]
- 26.Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92(5):1229-1236. [DOI] [PubMed] [Google Scholar]
- 27.Blackwelder WC. “Proving the null hypothesis” in clinical trials. Control Clin Trials. 1982;3(4):345-353. [DOI] [PubMed] [Google Scholar]
- 28.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 29.Baillard C, Gehan G, Reboul-Marty J, Larmignat P, Samama CM, Cupa M. Residual curarization in the recovery room after vecuronium. Br J Anaesth. 2000;84(3):394-395. [DOI] [PubMed] [Google Scholar]
- 30.Algie CM, Mahar RK, Tan HB, Wilson G, Mahar PD, Wasiak J. Effectiveness and risks of cricoid pressure during rapid sequence induction for endotracheal intubation. Cochrane Database Syst Rev. 2015;18(11):CD011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills P, Poole T, Curran J. Cricoid pressure and the pressor response to tracheal intubation. Anaesthesia. 1988;43(9):788-791. [DOI] [PubMed] [Google Scholar]
- 32.Bohman JK, Kashyap R, Lee A, et al. A pilot randomized clinical trial assessing the effect of cricoid pressure on risk of aspiration. Clin Respir J. 2018;12(1):175-182. [DOI] [PubMed] [Google Scholar]
- 33.Higgs A, McGrath BA, Goddard C, et al. ; Difficult Airway Society; Intensive Care Society; Faculty of Intensive Care Medicine; Royal College of Anaesthetists . Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018;120(2):323-352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Recruitment in the 10 Academic Centers
eTable 2. Description of Adverse Events