Key Points
Question
Are patients with breast cancer treated with autologous fat transfer at an increased risk of cancer relapse compared with those who receive conventional breast reconstruction?
Findings
In this cohort study examining 300 affected breasts reconstructed with autologous fat transfer and 300 matched control patients, no significant difference in the rate of locoregional recurrence was observed after 5-year follow-up.
Meaning
Exposure to autologous fat transfer was not found to be associated with increased rates of cancer relapse, thereby confirming the short-term findings of previous studies that clinical evidence does not suggest the presence of such risks.
Abstract
Importance
Autologous fat transfer (AFT or fat grafting) has become an invaluable tool for the correction of disfiguring deformities after breast cancer surgery. However, clinical and animal studies have shown conflicting results regarding its oncologic safety.
Objective
To determine whether exposure to AFT vs conventional breast reconstruction is associated with increased rates of cancer relapse in patients with breast cancer.
Design, Setting, and Participants
This matched cohort study involved retrospective medical record review to identify all patients in a local patient database receiving AFT between 2006 and 2014. Each AFT case was matched with a nonexposed control patient with similar baseline characteristics. The mean (SD) follow-up of patients receiving AFT was 9.3 (4.9) years including 5.0 (1.7) years following AFT. Control patients were followed up for a mean (SD) of 8.6 (1.8) years from the primary surgery. Patients were identified through the local patient database of the Tergooi Hospital in Hilversum, the Netherlands. A total of 287 patients with breast cancer (300 affected breasts) who received AFT for breast reconstruction after cancer were included in the intervention group. Each AFT case was matched with a respective control patient based on age, type of oncologic surgery, tumor invasiveness, and disease stage. In addition, individual AFT-control pairs were selected to have the same locoregional recurrence–free interval at baseline. Data were analyzed between 2016 and 2017.
Exposures
Reconstruction with AFT vs conventional breast reconstruction or none.
Main Outcomes and Measures
Primary end points were the cumulative incidences of oncologic events in AFT and control patients and their respective hazard ratios.
Results
Of the 587 total patients, all were women and the mean age was 48.1 years for the patients undergoing AFT and 49.4 years for the control patients. Eight locoregional recurrences were observed in the treatment group (287 patients) and 11 among the control group (300 patients), leading to an unadjusted hazard ratio of 0.63 (95% CI, 0.25-1.60; P = .33). No increased locoregional recurrence rates were seen in relevant subgroups based on the type of oncological surgery, tumor invasiveness, or pathological stage. In addition, no increased risks with AFT were detected with respect to distant recurrences or breast cancer–specific mortality.
Conclusions and Relevance
No significant differences in the locoregional recurrence rates between the AFT and control groups were observed after 5 years of follow-up. These findings confirm the results of previous studies; therefore, clinical evidence suggesting that AFT is associated with increased risk for cancer relapse is still lacking.
This matched cohort study investigates whether exposure to autologous fat transfer vs conventional breast reconstruction is associated with increased rates of cancer relapse in patients with breast cancer.
Introduction
Breast cancer is the most common malignancy in women, with a global burden that surpasses all other cancers.1 Fortunately, through improved early detection and treatment, its mortality is gradually decreasing. In addition, advancements in neoadjuvant tumor-shrinking drug regimens have made it technically feasible to preserve the breast in most patients, thereby reducing morbidity and improving quality of life. Although oncoplastic and breast reconstructive techniques can successfully restore the overall breast shape and volume after breast-conserving therapy (BCT) as well as mastectomy, they are often unable to eliminate remaining smaller deformities that are often equally disfiguring and stigmatizing for the patient. Since its popularization by Coleman2 in the late 1990s, autologous fat transfer (AFT or fat grafting), has been increasingly used for this purpose.
The essence of AFT involves injecting a patient’s own liposuctioned fat into a soft tissue deformity, hence the popular term lipofilling. Its minimal invasiveness and autologous nature make it an extremely attractive procedure in breast reconstruction. Over the years, studies have reported encouraging clinical results, reflected by high patient and surgeon satisfaction.3 In addition to restoring volume deformities, AFT has also been demonstrated to improve scar appearance,4,5 alleviate pain,6,7 and even induce tissue regeneration after radiotherapy.8,9 These extra effects are thought to be mediated by the activation of mesenchymal stem cells found in adipose tissue, referred to as adipose-derived stem cells.10 Adipose-derived stem cells are believed to play a crucial role in the survival of adipocytes after fat transfer by stimulating angiogenesis and tissue regeneration through a number of cytokines and growth factors.11
Despite its clinical benefits and favorable regenerative properties, the application of AFT in patients with breast cancer has been restricted by 2 main factors: the fear that it can interfere with breast cancer imaging and that intentionally placing regenerative cells in a previous tumor bed could increase the risk of locoregional recurrence (LRR). While studies have already demonstrated that macrocalcifications resulting from fat necrosis after AFT do not seem to hinder the detection of breast cancer,12,13,14 the question regarding the risk of recurrence remains a topic of much debate. Over the past decade, fundamental research has shown that adipose-derived stem cells can stimulate cancer growth and proliferation in nude mice experiments.15,16,17 Although it is questionable whether the interaction between human fat tissue and cancer cells injected in immunodeficient mice can accurately reflect the clinical setting, this uncertainty has inevitably led to a decrease in the application of AFT in patients with breast cancer, particularly in patients with BCT.18
Investigating the potential oncologic risks with AFT in clinical trials has been a great challenge because AFT lacks a comparable alternative to use in a control group. Because this renders setting up randomized clinical trials impractical and even unethical, studies published in the last decade consisted chiefly of retrospective case series and cohorts, which have been unable to provide definite answers.
A major breakthrough came with the publication of what is, to our knowledge, the first matched cohort on the subject by Petit et al19 from the European Institute of Oncology in Milan, Italy. By matching their AFT group with control individuals based on all relevant baseline characteristics, this study aimed to minimize the influence of confounders to quantify the true association of AFT with cancer relapse. It inspired several subsequent studies to adopt this study design in the pursuit of more reliable clinical data on the topic.20,21,22,23,24 Unfortunately, most of these studies have been limited by a relatively short follow-up in the context of evaluating oncologic outcomes, typically 2 to 3 years after exposure to AFT. The objective of this study is to evaluate the oncologic risks of AFT in patients with breast cancer after significantly longer follow-up than reported in the literature and provide crucial evidence on this highly relevant question.
Methods
Patients
Patients were identified through a retrospective medical record review of the local patient database of Tergooi Hospital in Hilversum, the Netherlands. This study was reviewed by the local ethics committee, but official approval and patient consent were not required because it did not fall under the Medical Research Involving Human Subjects Act. All female patients with histopathologically confirmed breast cancer diagnosis who underwent AFT for the correction of contour deformities of the breast between January 2006 and August 2014 were included. Exclusion criteria consisted of the absence of primary breast cancer (eg, in prophylactic mastectomy), history of locoregional recurrence, and missing histopathological or oncological data. Patients with breast cancer treated in roughly the same period were used as control patients.
Treatment
All AFT procedures were performed by the senior author (A.T.). After tumescent infiltration, fat tissue was usually harvested from the abdomen or upper legs using a closed low-pressure suction system (0.5 atm) and a 3-mm multiple-hole cannula. Centrifugation was occasionally used to remove excess blood or oil from ruptured adipocytes. The purified fat was subsequently transferred to 10-mL syringes and reinjected percutaneously into the breast deformity with a 2-mm blunt cannula in multiple passes and tissue planes to allow for maximum scatter of the fat droplets. A forked cannula was used to perforate scar adhesions and fibrosis whenever necessary.
Outcomes
The primary end point of this study was the cumulative incidence of LRR, while distant recurrence events and breast cancer–specific mortality were secondary end points. All patient records were reviewed thoroughly to obtain relevant demographic, surgical, and oncologic data. Documentation from the routine checkups by the local oncologic team (surgeon and oncologist), radiological imaging, and histopathological findings were used to identify oncologic events. In addition, the Dutch Nationwide Network and Registry of Histo- and Cytopathology was used as an extra screening tool to ensure no oncologic events were missed, especially in patients receiving their routine oncologic checkups elsewhere. To ensure optimal quality of data reporting, the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology statement were followed.25
Statistical Analysis
Using 1:1 nearest-neighbor propensity score matching, each patient undergoing AFT was individually paired with a respective control patient based on age, type of oncologic surgery, tumor invasiveness, and disease stage. To account for the latency period between the oncologic surgery and exposure to AFT in the intervention group, each control patient was also matched to have an LRR-free interval corresponding to the time to AFT (Figure 1). Differences in baseline characteristics were assessed using the Fisher exact test or χ2 test for the categorical variables and independent-samples t test or the Mann-Whitney U test for continuous variables. In the case of missing data, logistic and stochastic regression imputation were performed dichotomous and categorical variables, respectively. The primary end points were assessed in the form of cumulative incidence curves by the Kaplan-Meier method with corresponding hazard ratios (HR). Differences were tested using a 2-sided log-rank test. Analyses of LRR events were performed per tumor (breast), while distant recurrences and mortality were investigated per patient. Additional sensitivity analyses included evaluating the LRR event rates per patient to reduce the risk of confounding in patients with bilateral disease. Finally, multivariable Cox proportional hazards models were fitted to adjust for potential confounding variables. All statistical analyses were performed using the R Studio, version 1.0.136 (R Programming),26 and statistical significance was set at P less than .05 (2-sided).
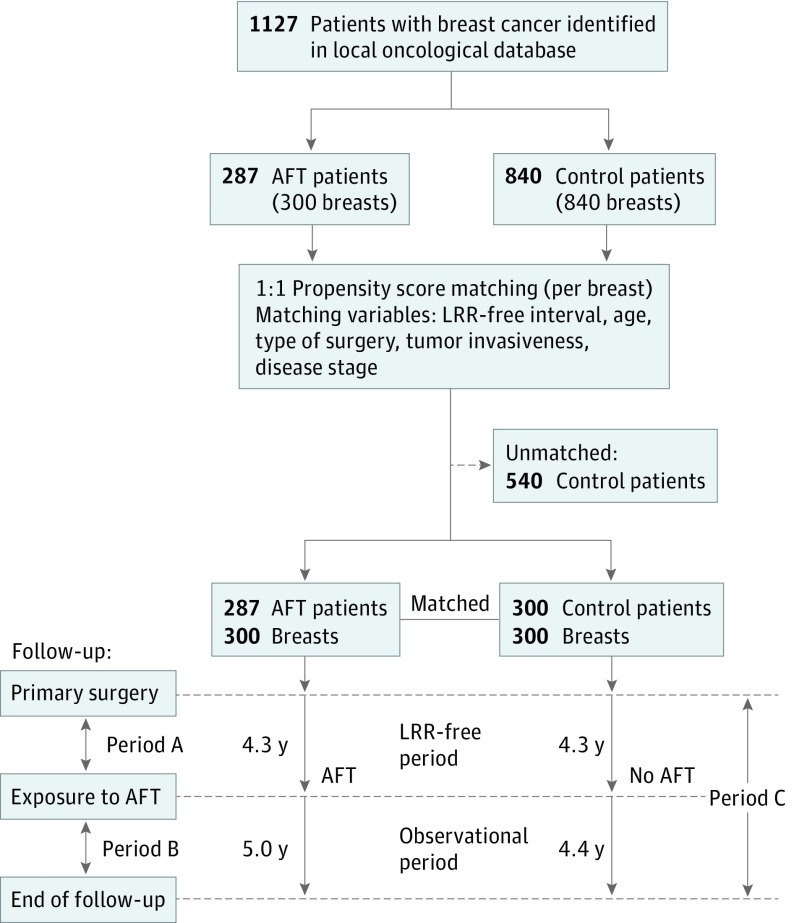
Figure 1. Matched Cohort Study Design: Each Autologous Fat Transfer (AFT) Case Matched With a Control Case Not Treated With AFT Based on Relevant Baseline Characteristics.
The follow-up was composed of 3 distinct periods, corresponding to the time from oncologic surgery to AFT (period A), AFT to the end of the follow-up (period B), and the total oncologic follow-up (period C). Oncologic data were collected for the length of the observation period. LRR indicates locoregional recurrence.
Results
Patients
A total of 287 patients with breast cancer who underwent breast reconstruction with AFT met the inclusion criteria. Thirteen of them were diagnosed as having a second primary tumor in the contralateral breast during the oncologic follow-up and underwent an additional reconstruction with AFT. The resulting 300 breasts affected by breast cancer that were subsequently reconstructed with AFT (AFT cases) were matched to 300 control patients from the local oncologic database who did not receive AFT (Figure 1). The baseline characteristics of the patients are presented in Table 1. The AFT and control groups did not differ significantly with regard to age, type of oncologic surgery, tumor invasiveness, histological grade, receptor expression, or disease stage. Similarly, no significant differences were detected in the administered (neo)adjuvant treatments, except for the number of patients receiving hormonal therapy (119 [40%] and 151 [50%] in the AFT and control groups, respectively; P = .01) (Table 1).
Table 1. Baseline Characteristics.
| Variable | No. (%) | P Value | |
|---|---|---|---|
| AFT | Controls | ||
| No. of cases | 300 | 300 | NA |
| Age, mean (SD), y | 48.1 (9.0) | 49.4 (8.4) | .26 |
| Oncologic surgery | |||
| BCT | 139 (46) | 150 (50) | .41 |
| MST | 161 (54) | 150 (50) | |
| Tumor invasiveness | |||
| In situ | 39 (13) | 40 (13) | >.99 |
| Invasive | 261 (87) | 260 (87) | |
| Grade (B&R) | |||
| 1 | 39 (15) | 28 (9.3) | .05 |
| 2 | 99 (38) | 144 (48) | |
| 3 | 114 (38) | 105 (35) | |
| Unknown | 27 (9.0) | 23 (7.7) | |
| Stage | |||
| 0 | 39 (13) | 40 (14) | .95 |
| I | 99 (33) | 102 (33) | |
| II | 114 (38) | 107 (36) | |
| III | 48 (16) | 51 (17) | |
| IV | 0 (0) | 0 (0) | |
| ER status | |||
| Positive | 206 (69) | 211 (70) | .74 |
| Negative | 42 (14) | 44 (15) | |
| Unknown | 52 (17) | 45 (15) | |
| PR status | |||
| Positive | 171 (57) | 168 (56) | .35 |
| Negative | 74 (25) | 87 (29) | |
| Unknown | 57 (18) | 45 (15) | |
| Her-2-neu | |||
| Overexpression | 45 (15) | 48 (16) | .92 |
| No overexpression | 183 (59) | 183 (61) | |
| Unknown | 72 (24) | 69 (23) | |
| TNBC2 | |||
| Yes | 24 (8.0) | 26 (8.7) | .80 |
| No | 220 (73) | 224 (75) | |
| Unknown | 56 (19) | 50 (17) | |
| Chemotherapy | |||
| Yes | 164 (45) | 162 (46) | .94 |
| No | 136 (55) | 138 (54) | |
| Radiotherapy | |||
| Yes | 182 (60) | 180 (60) | .93 |
| No | 118 (40) | 120 (40) | |
| Hormonal therapy | |||
| Yes | 119 (40) | 151 (50) | .01 |
| No | 181 (60) | 149 (50) | |
Abbreviations: AFT, autologous fat transfer; BCT, breast-conserving therapy; B&R, malignancy grade according to the Bloom and Richardson grading system; ER, estrogen receptor expression; MST, mastectomy; NA, not applicable; PR, progesterone receptor expression; TNBC, triple-negative breast cancer.
Treatment
Autologous fat transfer was performed as the only reconstructive modality in 112 (37.3%) of the breast reconstructions, which involved primarily sequelae of BCT and subsequent radiotherapy. In patients treated with mastectomy, AFT was indicated for remaining soft tissue deformities after reconstruction with implants in 83 breasts (29.0%), autologous flaps in 71 breasts (23.6%), and less frequently after hybrid reconstructions (34 breasts [11.3%]). On average, 1.8 AFT procedures (range, 1-9) were required to achieve the desired result, but in 152 cases (50.7%), a single treatment was sufficient.
Follow-up
The mean (SD) length of the oncologic follow-up (period C) in the intervention group was 9.3 (4.9) years, including an observational period after exposure to AFT of 5.0 (1.7) years (period B). Control patients had a slightly shorter oncologic follow-up of 8.6 (1.8) years, with a corresponding observational period of 4.4 (2.1) years (Figure 1).
Oncologic Events
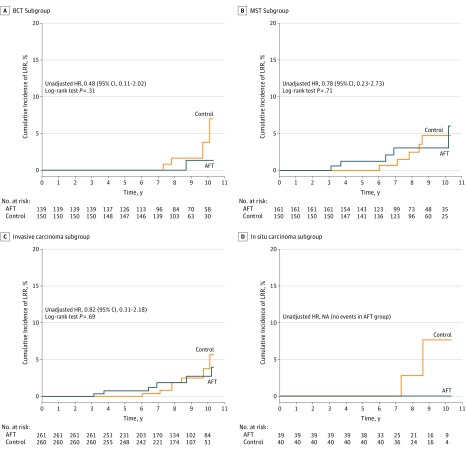
A total of 8 LRR events were observed in the AFT group and 11 in the control group, resulting in comparable cumulative incidence curves (unadjusted HR, 0.63; 95% CI, 0.25-1.60; P = .33) (Figure 2 and Table 2). No significant differences were observed in relevant subgroups (Figure 3; eFigure 1 in the Supplement). Sensitivity analysis was performed for the LRR events per patient (as opposed as per tumor/breast) but did not result in significant changes in the HRs (HR, 0.64; 95% CI, 0.25-1.62; P = .34). An additional sensitivity analysis involved only the subgroup undergoing AFT within 5 years after the primary surgery (ie, period A, ≤5 years), which also showed no increased risk of LRR (HR, 0.63; 95% CI, 0.22-1.78; P = .38).
Figure 2. Incidence Rate of Locoregional Recurrence (LRR) for the Whole Cohort.
AFT indicates autologous fat transfer; HR, hazard ratio.
Table 2. Multivariable Cox Regression Analysis.
| Outcome | Events, No. | Unadjusted HR | Adjusted HR | |||
|---|---|---|---|---|---|---|
| AFT | Controls | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| LRR | 8 | 11 | 0.63 (0.25-1.60) | .33 | NA | NA |
| DR | 22 | 21 | 0.94 (0.52-1.72) | .85 | 0.98 (0.54-1.79)a | .95 |
| Overall mortality | 8 | 33 | 0.20 (0.09-0.44) | <.001 | 0.20 (0.09-0.44)b | <.001 |
| Mortality (breast cancer related) | 7 | 16 | 0.37 (0.15-0.91) | .02 | 0.38 (0.15-0.92)c | .03 |
Abbreviations: AFT, autologous fat transfer; DR, distant recurrence; HR, hazard ratio; LRR, locoregional recurrence; NA, not adjusted because none of the baseline variables were identified as confounders (change in HR for AFT vs control of greater than 5% after addition of variable to the model).
Adjusted for grade and hormonal therapy.
Adjusted for grade, hormonal therapy, and tumor stage.
Adjusted for hormonal therapy and tumor stage.
Figure 3. Incidence Rate of Locoregional Recurrence in the Subgroups With Breast-Conserving Therapy (BCT), Mastectomy (MST), Invasive, and In Situ Carcinomas.
AFT indicates autologous fat transfer; HR, hazard ratio; LRR, locoregional recurrence; NA, not applicable.
The number of distant recurrence events were similar and without significant differences between the AFT and control groups (HR, 0.94, 95% CI, 0.52-1.72; P = .85) (Table 2; eFigure 2 in the Supplement). Lastly, a much higher mortality was observed in control patients (n = 33) compared with patients undergoing AFT (n = 8) with an HR of 0.20 (95% CI, 0.09-0.44; P < .001), as shown in eFigure 3 in the Supplement. The difference was also significant when analysis was restricted to only the breast cancer–specific mortality (HR, 0.37; 95% CI, 0.15-0.91; P = .02) (eFigure 4 in the Supplement). After adjusting for potential confounding variables (eTable in the Supplement), the multivariable Cox regression model did not result in notable changes in the HRs of LRR, distant recurrence, and mortality between the AFT and control groups (Table 2).
Discussion
The oncologic safety of AFT in patients with breast cancer has been a highly controversial topic in the past decade. The conflicting evidence from the molecular and clinical arenas has engendered divergent and even polarized opinions among plastic surgeons as well as oncologists on whether the clinical benefits of AFT outweigh its potential risks. Despite the large number of publications on this topic, studies have been unable to provide convincing evidence, largely owing to their limited methodologic quality and the lack of suitable control groups. A number of research groups have dealt with this problem by evaluating oncologic events in patients undergoing AFT and nonexposed control patients from the same institution.27,28,29,30,31,32,33,34 Although this study design can provide a fair indication of the recurrence rates in that particular clinic, potential confounding by other baseline variables limits its capability in deducing the specific oncologic risks associated with AFT. The pathophysiology of cancer relapse is multifactorial and depends on many other factors, such as the age at presentation, morphological tumor characteristics, type of surgery, and (neo)adjuvant therapy regimens.35 Therefore, the absolute risks of AFT can only be determined if studies correct sufficiently for these confounders. The publication of what is, to our knowledge, the first matched cohort (the group from the European Institute of Oncology by Petit et al19 in 2012) set the new benchmark for this study design that can provide the considerably higher level of evidence without the need for randomization. Since then, 5 such studies, with a total of 897 patients undergoing AFT, have been published.20,21,22,23,24 Unfortunately, an important limitation remains the relatively short follow-up (mean, 3.1 years) and the scarcity of oncologic data for patients with BCT, who have the highest theoretical risk of developing a local relapse after AFT.
This study addresses both these issues. It represents one of the largest matched cohort studies, with almost half of its study population consisting of patients with BCT (n = 139). In addition, this is the first study to reach 5-year follow-up after exposure to AFT. Each patient undergoing AFT was matched with a respective control patient based on age, type of oncologic surgery, tumor invasiveness, and disease stage. This produced groups that were very comparable and did not display significant differences in most of the remaining baseline characteristics, thereby limiting the influence of confounders. As with other matched cohort studies, each control patient was paired to have an LRR-free interval matching period A in the respective patient undergoing AFT. This step is crucial to avoid comparing patients at different stages of their oncologic follow-up because it has been demonstrated that cancer recurrence rates are highest in the first few years after surgery.36
Survival analysis showed a slightly lower LRR rate in AFT cases compared with controls, which was not statistically significant (Figure 2). Also, no notable differences were observed between subgroups based on the type of surgery (Figure 3A and B), invasiveness (Figure 3C and D), disease stage (eFigure 1A-D in the Supplement), and the presence of triple-negative breast cancer (eFigure 1E and F in the Supplement). To exclude the possibility that the per breast analysis could have introduced bias by treating patients with bilateral breast as 2 distinct cases, a sensitivity analysis was performed based on the 287 unilateral AFT cases and their respective controls, but the result was not significant. To evaluate whether the inclusion of patients with long LRR-free interval could result in an underestimation of the overall LRR rate, an additional sensitivity analysis involved only patients with latency period (period A) shorter than 5 years. Likewise, this factor did not appear to have significant influence on the results. Dividing the total LRR events by the number of patient-years after exposure (period B) resulted in incidence rates of 0.53% and 0.83% per year in the AFT and control groups, respectively. This rate was slightly lower than those reported by other matched cohort studies (0.7%-1.9% per year).19,20,21,22,23,24 This could be possibly explained by the longer observational period in this cohort because the gradual decrease in the risk of cancer relapse with time could ultimately translate into a lower incidence rate with the increasing length of oncologic follow-up. Moreover, it is comforting that no alarming LRR rates were observed in patients with BCT because the application of AFT is often avoided in this group owing to fears of spreading tumor cells and possibly activating dormant cancer cells. These findings are in line with published studies.19,21,24 On the other hand, a contrasting observation regarding the in situ carcinomas was made in the studies by Petit et al,19,23 where unlike in this study, a significantly higher rate of LRR was seen in patients undergoing AFT. With the limited oncologic data for this particular subgroup, this would be an interesting subject to be investigated by larger studies in the future.
No notable findings were seen with the rate of distant recurrences because these did not differ significantly between the groups. On the other hand, an astonishing 4-fold higher overall mortality was seen in the control group. This was also true for the breast cancer–specific mortality, which was more than twice as high in control patients. Both of these could not be adequately explained by the influence of confounders (Table 2). Such striking differences raise the question of whether a certain preselection has produced a group of patients undergoing AFT with a more favorable prognosis than the control patients despite their similar baseline characteristics. Interestingly enough, several other matched cohorts have also reported 2 to 3 times higher mortality in their matched control groups,19,21,24 which suggests either a positive effect of AFT or breast reconstruction itself on the prognosis of patients with breast cancer or confounding by other factors not investigated by the studies so far. This should be an interesting subject to be evaluated by future research.
Limitations
The retrospective design is a limitation with this study; however, this is a well-recognized issue in the area of AFT because setting up prospective or randomized trials is highly challenging owing to practical and ethical concerns. Therefore, propensity score matching was implemented to control for variables that were known or presumed confounders for cancer relapse and thus select control patients with similar baseline characteristics. Although a 1:2 matching would have been superior in reducing the risk of selection bias in the control patients, it was not possible owing to the limited size of the local database. In addition, matching was also limited to 4 key variables in addition to the latency period (period A). Although matching minimized differences between groups with regard to most baseline parameters, it is possible that other confounding variables may have been overlooked, which could explain the prominent differences in the mortality rates. Although this study did not demonstrate significant differences in the recurrence rates between AFT and control patients, it did not possess sufficient statistical power to eliminate the possibility that an association between AFT and cancer relapse may still be present.
Conclusions
There is an urgent need to investigate whether AFT could potentially compromise the oncologic safety in patients with breast cancer, before a false sense of security promotes wide adoption in clinical practice. The findings of this matched cohort study show no significant differences in the LRR between patients undergoing AFT and control patients after 5-year oncologic follow-up. In line with reported rates from other published matched cohorts, there is no clinical evidence so far to suggest that AFT leads to increased rates of cancer relapse in patients with breast cancer. To provide definite evidence on the matter, oncologic data from several matched cohorts could be pooled, ideally in the form of individual patient data meta-analysis, to increase their statistical power and allow more reliable assessment of the specific risks within subgroups.
eTable. Univariable Cox-regression analysis of oncological events
eFigure 1. Incidence Rate of Locoregional Recurrence in the Subgroups With Stage 0-4 and Triple-Negative and Non–Triple-Negative Breast Cancer Cases
eFigure 2. Incidence Rate of Distant Recurrence in All Patients
eFigure 3. Incidence Rate of the Overall Mortality in All Patients
eFigure 4. Incidence Rate of Breast Cancer–Specific Mortality in All Patients
References
- 1.Breast cancer fact sheets 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed January 18, 2018.
- 2.Coleman SR. Structural fat grafting. Aesthet Surg J. 1998;18(5):386-388, 388. doi: 10.1016/S1090-820X(98)70098-6 [DOI] [PubMed] [Google Scholar]
- 3.Krastev TK, Alshaikh GAH, Hommes J, Piatkowski A, van der Hulst RRWJ. Efficacy of autologous fat transfer for the correction of contour deformities in the breast: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2018;S1748-6815(18)30175-X. [DOI] [PubMed] [Google Scholar]
- 4.Klinger M, Caviggioli F, Klinger FM, et al. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24(5):1610-1615. doi: 10.1097/SCS.0b013e3182a24548 [DOI] [PubMed] [Google Scholar]
- 5.Jaspers ME, Brouwer KM, van Trier AJ, Groot ML, Middelkoop E, van Zuijlen PP. Effectiveness of autologous fat grafting in adherent scars: results obtained by a comprehensive scar evaluation protocol. Plast Reconstr Surg. 2017;139(1):212-219. doi: 10.1097/PRS.0000000000002891 [DOI] [PubMed] [Google Scholar]
- 6.Juhl AA, Karlsson P, Damsgaard TE. Fat grafting for alleviating persistent pain after breast cancer treatment: a randomized controlled trial. J Plast Reconstr Aesthet Surg. 2016;69(9):1192-1202. doi: 10.1016/j.bjps.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Maione L, Vinci V, Caviggioli F, et al. Autologous fat graft in postmastectomy pain syndrome following breast conservative surgery and radiotherapy. Aesthetic Plast Surg. 2014;38(3):528-532. doi: 10.1007/s00266-014-0311-9 [DOI] [PubMed] [Google Scholar]
- 8.Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119(5):1409-1422. doi: 10.1097/01.prs.0000256047.47909.71 [DOI] [PubMed] [Google Scholar]
- 9.Panettiere P, Marchetti L, Accorsi D. The serial free fat transfer in irradiated prosthetic breast reconstructions. Aesthetic Plast Surg. 2009;33(5):695-700. doi: 10.1007/s00266-009-9366-4 [DOI] [PubMed] [Google Scholar]
- 10.Brown SA, Levi B, Lequeux C, Wong VW, Mojallal A, Longaker MT. Basic science review on adipose tissue for clinicians. Plast Reconstr Surg. 2010;126(6):1936-1946. doi: 10.1097/PRS.0b013e3181f44790 [DOI] [PubMed] [Google Scholar]
- 11.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292-1298. doi: 10.1161/01.CIR.0000121425.42966.F1 [DOI] [PubMed] [Google Scholar]
- 12.Carvajal J, Patiño JH. Mammographic findings after breast augmentation with autologous fat injection. Aesthet Surg J. 2008;28(2):153-162. doi: 10.1016/j.asj.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 13.Costantini M, Cipriani A, Belli P, et al. Radiological findings in mammary autologous fat injections: a multi-technique evaluation. Clin Radiol. 2013;68(1):27-33. doi: 10.1016/j.crad.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 14.Rubin JP, Coon D, Zuley M, et al. Mammographic changes after fat transfer to the breast compared with changes after breast reduction: a blinded study. Plast Reconstr Surg. 2012;129(5):1029-1038. doi: 10.1097/PRS.0b013e31824a2a8e [DOI] [PubMed] [Google Scholar]
- 15.Bertolini F, Petit JY, Kolonin MG. Stem cells from adipose tissue and breast cancer: hype, risks and hope. Br J Cancer. 2015;112(3):419-423. doi: 10.1038/bjc.2014.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eterno V, Zambelli A, Pavesi L, et al. Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget. 2014;5(3):613-633. doi: 10.18632/oncotarget.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17(1-2):93-106. doi: 10.1089/ten.tea.2010.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kling RE, Mehrara BJ, Pusic AL, et al. Trends in autologous fat grafting to the breast: a national survey of the American Society of Plastic Surgeons. Plast Reconstr Surg. 2013;132(1):35-46. doi: 10.1097/PRS.0b013e318290fad1 [DOI] [PubMed] [Google Scholar]
- 19.Petit JY, Botteri E, Lohsiriwat V, et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol. 2012;23(3):582-588. doi: 10.1093/annonc/mdr158 [DOI] [PubMed] [Google Scholar]
- 20.Fertsch S, Hagouan M, Munder B, et al. Increased risk of recurrence associated with certain risk factors in breast cancer patients after DIEP-flap reconstruction and lipofilling-a matched cohort study with 200 patients. Gland Surg. 2017;6(4):315-323. doi: 10.21037/gs.2017.03.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale KL, Rakha EA, Ball G, Tan VK, McCulley SJ, Macmillan RD. A case-controlled study of the oncologic safety of fat grafting. Plast Reconstr Surg. 2015;135(5):1263-1275. doi: 10.1097/PRS.0000000000001151 [DOI] [PubMed] [Google Scholar]
- 22.Petit JY, Maisonneuve P, Rotmensz N, Bertolini F, Rietjens M. Fat grafting after invasive breast cancer: a matched case-control study. Plast Reconstr Surg. 2017;139(6):1292-1296. doi: 10.1097/PRS.0000000000003339 [DOI] [PubMed] [Google Scholar]
- 23.Petit JY, Rietjens M, Botteri E, et al. Evaluation of fat grafting safety in patients with intraepithelial neoplasia: a matched-cohort study. Ann Oncol. 2013;24(6):1479-1484. doi: 10.1093/annonc/mds660 [DOI] [PubMed] [Google Scholar]
- 24.Silva-Vergara C, Fontdevila J, Weshahy O, Yuste M, Descarrega J, Grande L. Breast cancer recurrence is not increased with lipofilling reconstruction: a case-controlled study. Ann Plast Surg. 2017;79(3):243-248. doi: 10.1097/SAP.0000000000001106 [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RStudio Team RStudio: Integrated Development for R. http://www.rstudio.com/. Published 2015. Accessed September 1, 2017.
- 27.Cohen O, Lam G, Karp N, Choi M. Determining the oncologic safety of autologous fat grafting as a reconstructive modality: an institutional review of breast cancer recurrence rates and surgical outcomes. Plast Reconstr Surg. 2017;140(3):382e-392e. doi: 10.1097/PRS.0000000000003576 [DOI] [PubMed] [Google Scholar]
- 28.Kim HY, Jung BK, Lew DH, Lee DW. Autologous fat graft in the reconstructed breast: fat absorption rate and safety based on sonographic identification. Arch Plast Surg. 2014;41(6):740-747. doi: 10.5999/aps.2014.41.6.740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kronowitz SJ, Mandujano CC, Liu J, et al. Lipofilling of the breast does not increase the risk of recurrence of breast cancer: a matched controlled study. Plast Reconstr Surg. 2016;137(2):385-393. doi: 10.1097/01.prs.0000475741.32563.50 [DOI] [PubMed] [Google Scholar]
- 30.Laporta R, Longo B, Sorotos M, Pagnoni M, Santanelli di Pompeo F. Breast reconstruction with delayed fat-graft-augmented DIEP flap in patients with insufficient donor-site volume. Aesthetic Plast Surg. 2015;39(3):339-349. doi: 10.1007/s00266-015-0475-y [DOI] [PubMed] [Google Scholar]
- 31.Masia J, Bordoni D, Pons G, Liuzza C, Castagnetti F, Falco G. Oncological safety of breast cancer patients undergoing free-flap reconstruction and lipofilling. Eur J Surg Oncol. 2015;41(5):612-616. doi: 10.1016/j.ejso.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 32.Mestak O, Hromadkova V, Fajfrova M, Molitor M, Mestak J. Evaluation of oncological safety of fat grafting after breast-conserving therapy: a prospective study. Ann Surg Oncol. 2016;23(3):776-781. doi: 10.1245/s10434-015-4908-2 [DOI] [PubMed] [Google Scholar]
- 33.Pinell-White XA, Etra J, Newell M, Tuscano D, Shin K, Losken A. Radiographic implications of fat grafting to the reconstructed breast. Breast J. 2015;21(5):520-525. doi: 10.1111/tbj.12450 [DOI] [PubMed] [Google Scholar]
- 34.Seth AK, Hirsch EM, Kim JY, Fine NA. Long-term outcomes following fat grafting in prosthetic breast reconstruction: a comparative analysis. Plast Reconstr Surg. 2012;130(5):984-990. doi: 10.1097/PRS.0b013e318267d34d [DOI] [PubMed] [Google Scholar]
- 35.Clemons M, Danson S, Hamilton T, Goss P. Locoregionally recurrent breast cancer: incidence, risk factors and survival. Cancer Treat Rev. 2001;27(2):67-82. doi: 10.1053/ctrv.2000.0204 [DOI] [PubMed] [Google Scholar]
- 36.Kwast AB, Groothuis-Oudshoorn KC, Grandjean I, et al. Histological type is not an independent prognostic factor for the risk pattern of breast cancer recurrences. Breast Cancer Res Treat. 2012;135(1):271-280. doi: 10.1007/s10549-012-2160-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Univariable Cox-regression analysis of oncological events
eFigure 1. Incidence Rate of Locoregional Recurrence in the Subgroups With Stage 0-4 and Triple-Negative and Non–Triple-Negative Breast Cancer Cases
eFigure 2. Incidence Rate of Distant Recurrence in All Patients
eFigure 3. Incidence Rate of the Overall Mortality in All Patients
eFigure 4. Incidence Rate of Breast Cancer–Specific Mortality in All Patients