Summary
Genetic cardiovascular (CV) diseases encompass major groups of Mendelian (>90%) and non-Mendelian, (matrilinear) familial (F) disorders including cardiomyopathies (CMP), aneurysmal diseases, ventricular and supraventricular arrhythmogenic disorders, and pulmonary hypertension (PH). In F-cardiomyopathies (F-CMP), a pathologic mutation provides specific vs. phenotype-based descriptive diagnosis, early, pre-clinical diagnosis in families. Subsets of dilated cardiomyopathies (DCM) (e.g. cardiolaminopathies) include genotype in indications for primary prevention of sudden cardiac death (SCD). Pathologic mutations are one of the major diagnostic criteria for arrhythmogenic CMP. For F-aneurysms, syndromic and non-syndromic, genetics plays a diagnostic role. Syndromes are recognized on phenotypes, while non-syndromic aneurysms are diagnosed on imaging and genetic testing. Risk of events varies, according to the genetic cause. In F-Arrhythmogenic disorders, genetics offers diagnostic, therapeutic, and prognostic benefits, in particular Long QT Syndrome (LQTS), Short QT Syndrome (SQTS), Brugada Syndrome (BS), and Catecholaminergic Polymorfic Ventricular Tachycardia (CPVT); medications may vary according to genotypes. F-atrial arrhythmias, typically atrial fibrillation (AF), are still poorly investigated. F-pulmonary hypertension may have genetic basis (Groups I and V, last classification). Early diagnosis is mandatory given the new available drugs that influence the natural history of the disease. In the future, clinical family screening, genetic counselling, and testing should become systematic; treatments are going to be driven by genetic cause.
Genetic cardiovascular diseases
The majority of genetic CV diseases are single gene disorders characterized by either syndromic presentation or exclusive involvement of the heart (i.e. CMP) or vessels (i.e. heritable aortopathies). Their overall burden is difficult to establish: some diseases are common [i.e. hypertrophic cardiomyopathy (HCM)], while others are extremely rare [restrictive cardiomyopathy (RCM), arterial tortuosity syndrome (ATS), etc.). Most of them demonstrate clinical and genetic heterogeneity. The inheritance is autosomal dominant (AD), autosomal recessive (AR), or X-linked (XL) either dominant (rare) or recessive (more common).1 Most of these diseases are transmitted in AD mode (up to 80% of cases): CMPs,2 aneurysmal diseases, both syndromic [e.g. Marfan syndrome (MFS)] and non-syndromic,3 primary pulmonary hypertension (PPH);4 AF.5 Autosomal recessive CV diseases are less common, but include rare CMPs such as atrial dilated cardiomyopathy (ADCM) caused by homozygous mutations in NPPA gene;6 sick sinus syndrome (SSS) caused by mutations in SCN5A gene;7,8 ATS,9 lysosomal storage diseases involving heart and vessels:10 parental consanguinity or geographically isolated populations should be explored. X-linked diseases, frequently demonstrate recessive inheritance (XLR): heterozygous females are phenotypically healthy or manifest minor/mild phenotypes. Examples include XLR defects of Dystrophin or Emerin11,12 or Danon Disease and Anderson Fabry disease, which cannot be simply defined as XLR because females may manifest a later onset and milder phenotype than males.
Work-up in genetic cardiovascular diseases: common principles
The fundament of clinical genetics in CV diseases is the deep phenotyping in probands and relatives. The clinical evaluation is preceded by genetic counselling with pedigree generation. Genetic visit of the proband aims at exploring the phenotypic traits related with the suspected disease/syndrome. For systemic/multi-organ diseases/syndromes, multidisciplinary evaluation may add important clues and specifications on each observed trait. Imaging and biomarkers systematically support the phenotype characterization. Clinical family screening [clinical visit, electrocardiogram (ECG), and two dimension Trans Toracic Echocardiography (2D-TTE)] is performed irrespective of genetic testing: it provides immediate diagnoses in relatives [e.g. bicuspid aortic valve (BAV), aneurysmal aortopathy, early CMP, congenital heart disease (CHD), PH]. Once the clinical diagnosis is formulated in the proband, genetic testing may analyse either a single disease gene (e.g. AD MFS or XL Danon Disease) or multi-gene panels in genetically heterogeneous diseases [e.g. HCM or familial-thoracic aortic aneurysm/thoracic aortic aneurysm and dissection (F-TAA/TAAD)]. When a potential disease-causing mutation is identified in the proband, cascade genetic testing is offered to relatives. The parallel achievement of phenotype and genetic data gives the essential data for segregation studies in families. This implies that mutations that are sufficient, by themselves, to cause the observed phenotype, should be present in all affected family members and absent in non-affected ones. The age-related penetrance of the disease has to be considered in segregation studies. For this reason, long-term monitoring of relatives can be uniquely useful.
Major genetic cardiovascular diseases
Familial cardiomyopathies
The clinical diagnosis of CMP in probands does not need, by itself, genetic testing. The phenotype-based diagnosis of HCM, DCM, RCM, or arrhythmogenic cardiomyopathy (ACM) (or genetic mimics vs. acquired phenocopies) is feasible on the basis of clinical data and imaging. The recent introduction of the non-dilated hypokinetic cardiomyopathy (NDHC) by the ESC13 and of the HCM < 15 mm in relatives of probands, expands the possibility of diagnosing DCM and HCM in patients that would not fulfil past WHO criteria. Familial CMP is diagnosed when two or more members of the same family are affected: the family screening defines the familial vs. non-familial (sporadic) disease (Figure 1). Genetic CMP can be diagnosed in unique affected family member when the disease-causing mutation is identified (de novo mutation, small families, and adoption). The clinical management and diagnoses of CMP such as dilated cardiolaminopathies14 or ACM (in Task Force-2010 criteria, a pathologic mutation in disease gene is a major diagnostic criterion) are influenced by the genetic cause. The novel diagnoses of NDHC13 and HCM < 15 mm16 are supported by the identification of the genetic causes, especially in relatives of affected probands. The feasibility of genetic testing deserves a few considerations: with NGS techniques, the time for testing is shorter but costs remain high (needs for confirmation with a different method, complex bioinformatics analysis, and high number of genes to be tested). Therefore, although costs and time are abated with respect to the past, resources needed to run these programs are still consistent. The sustainability therefore is an open issue, given the little institutional investments done in the setting of clinical and molecular genetics in the public care.
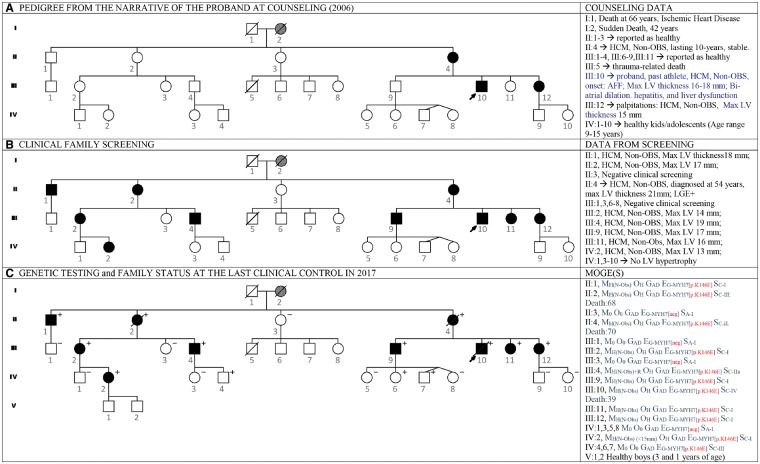
Figure 1.
The figure shows the pedigree of a family with autosomal dominant hypertrophic cardiomyopathy, the diagnostic impact of clinical family screening, and the phenotype–genotype description. The proband, III: 10, was diagnosed with hypertrophic cardiomyopathy at the age of 30 years, when he developed heart failure with atrial fibrillo-flutter (AFF). His medical history was characterized by recurrence of the atrial arrhythmias, ablations, and further recurrence of atrial fibrillo-flutter. He further suffered pneumonia with sepsis, pleuritis, and pericardial effusion, liver failure and progressed to end-stage heart failure. (A) The panel shows the first pedigree of the family, before performing clinical family screening and genetic testing. According to the narrative of the proband and to the health status of his relatives the only affected family members were II: 4, and III: 12. His grand-mother was probably affected (I: 2). (B) The panel shows the pedigree after completion of family screening: seven additional members were affected and asymptomatic, all unaware of their disease status. (C) The panel shows the pedigree after the completion of genetic testing: there are three healthy carriers in the third generation. Individual IV: 2 decided to have pregnancy and was supported and monitored during both pregnancy and delivery; her children are healthy and have not been tested yet. In the right column, phenotype and cause of the cardiomyopathies are precisely described using MOGE(S) nosology.14 In the right column, the summary of clinical data shows that genetically negative family members are not affected, supporting the segregation of the genotype with the phenotype up to the third generation. Future clinical monitoring of the family will demonstrate whether the healthy carriers of the fourth generation will develop the disease, further confirming the segregation of the phenotype with the genotype in the family. This is especially important in this family because the mutation identified in MYH7 is not reported.
Familial arrhythmogenic diseases
Inherited atrial diseases
Inherited atrial diseases include rare ADCM, autosomal recessive, or dominant SSS, and a certain proportion of lone AF. Atrial dilated cardiomyopathy is a rare AR disease characterized by ‘clinical onset in adulthood, bi-atrial dilatation up to giant size, early supraventricular arrhythmias with progressive loss of atrial electric activity to atrial standstill, thrombo-embolic complications, stable, normal left ventricular function, and New York Heart Association functional class during the long-term course of the disease, and severely decreased levels of atrial natriuretic peptide’.6 Sick sinus syndrome is clinically characterized by sinus bradycardia, sinus arrest, chronotropic incompetence, and susceptibility to atrial arrhythmias.17 Sick sinus syndrome can be sporadic, such as the common SSS observed in old patients,18 or familial, typically manifesting in young patients. Familial SSS is either AD or AR.19,20 The genetic diagnosis (SCN5A gene in SSS1 and HCN4 in SSS2)8,19,20 contributes to identify at-risk family members, to schedule monitoring for prevention of arrhythmias, and to support decisions for ablation of atrial arrhythmias or pacemaker (PM) implantation. Atrial fibrillation can be sporadic or familial;21 about 30% of patients have a positive family history and individuals with at least one parent with AF have a 85% relative risk of developing AF.22 Numerous disease genes have been identified to date.23 The role of clinical genetics is to exclude other diseases that can explain the arrhythmia. In fact, some of the disease genes may also cause other heritable atrial disease.6 Genes such as SCN5A that cause LQTS, SQTS, or BS may also cause AF, and long-term follow-up of mutation carriers is necessary to exclude other arrhythmogenic phenotypes or explore why the same mutations may manifest different arrhythmogenic phenotypes.
Inherited channelopathies and life-threatening ventricular arrhythmias
Cardio-channelopathies encompass a large genetically heterogeneous group of electrical abnormalities that can be associated with unexpected sudden death.1 The major groups of channelopathies associated with risk of sudden death include diseases that affect the QT interval, BS, and CPVT. Long QT Syndrome encompasses genetically heterogeneous diseases, AD in the majority of cases, and sharing prolonged QT interval on resting 12-lead surface ECG in the absence of structural heart disease. Inheritance is AR in the rare Jervell and Lange-Nielsen syndrome. Patients with LQTS may be asymptomatic during their entire life or manifest syncope, aborted cardiac arrest or SCD. The latter can be the first manifestation of the disease. In 90% of cases, mutations are identified in KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3) genes.24 The remaining 10% of mutations occurs in genes encoding other ion channels or proteins interacting with ion channels. The diagnosis of LQTS is based on consensus criteria25 and supported by a score system that sums points assigned to ECG findings (baseline and after exercise stress), evidence of torsade de pointes, history of syncope, congenital deafness, family history, and members with LQTS, unexplained SCD below age 30 among immediate family members.26 Genotype is not included in the score. Short QT Syndrome is an inherited arrhythmogenic disease associated with increased risk of AF, ventricular tachycardia (VT)/ventricular fibrillation (VF), and SCD.27 Current estimates indicate a prevalence of 0.02% to 0.1% in the adult population and 0.05% in children/adolescents.28 The diagnosis is done when QT interval is ≤330 ms in the absence of tachycardia or bradycardia. Gain-of-function mutations in three genes encoding potassium channels, KCNQ1, KCNH2, and KCNJ2, have been associated with the SQTS.25 Brugada Syndrome is a genetically heterogeneous, AD arrhythmogenic disease characterized by ST elevation with negative T wave in the right precordial leads in the absence of structural cardiac abnormalities.29 The disease burden ranges from 5 to 20 individuals in 10 000. Although mean age of patients manifesting life-threatening arrhythmias is around 40, arrhythmic events can occur at any age; arrhythmias typically occur while sleeping or at rest or after heavy meals or during episodes of fever.29 Pathogenic mutations are identified in about 30% of patients, with SCN5A gene accounting for the majority of cases and other genes accounting for about 5% of genotyped cases.30 Overlap phenotypes (long QT, short QT, coved-type ST-segment elevation in the right precordial leads) are possible in affected members of the same families (e.g. SCN5A). A recent ‘oligogenetic inheritance’ hypothesis postulates that mutations in more than one disease gene is needed to induce a clinical phenotype.31 Ajmaline provocation test supports clinical diagnosis especially in patients with equivocal baseline ECG pattern as unique evidence of possible disease;25 other conditions potentially manifesting a Brugada-like ECG patterns (ischaemic heart disease, hyperkalaemia, hypercalcaemia, ACM, myocarditis, mechanical compression of the right ventricle outflow tract, or pulmonary embolism) should be excluded. In symptomatic patients, risk stratification includes history of VT/VF, syncope, and spontaneous coved-type ST-segment elevation. In asymptomatic patients risk stratification includes male gender and fragmented QRS, which is a marker of conduction abnormality and a predictor of prognosis.32 Catecholaminergic Polymorfic Ventricular Tachycardia is a rare disease (1:10 000) that typically presents with syncope or cardiac arrest triggered by exercise or emotion in children/adolescents.33 Mortality is high in unrecognized and untreated patients. The baseline ECG is normal, but exercise induces the polymorphic ventricular arrhythmias; a few patients may demonstrate bidirectional VT.34 The disease is genetically heterogeneous: CPVT1 (60–70%) is AD and caused by mutations in the gene RYR2 gene. CPVT2 is AR and is associated with mutations in the cardiac Calsequestrin gene (CASQ2) both involved in myocyte calcium homeostasis.35 Less common genes include KCNJ2 (CPVT3), Calmodulin (CALM1), and Triadin (TRDN).36 Risk factors for arrhythmias are young age, male gender, history of cardiac arrest, arrhythmias while taking beta-blockers, and mutation in the c-terminus of the RYR2 gene.33,36
Familial aneurysmal diseases: thoracic aorta (F-TAA)
Heritable aneurysmal diseases include more than 40 genetically different disorders.37 Thoracic aorta is most commonly affected. Thoracic aortic aneurysm may occur in syndromes or be the unique (or main) manifestation of the disease. Syndromic diseases such as MFS (Table 1), vascular Ehlers-Danlos syndrome (EDS-IV), and Loeys-Dietz syndromes (LDS) are characterized by phenotypic traits that usually allow clinical diagnosis.3 Recent identification of novel disease genes for LDS and overlapping phenotypes assigns to genetic testing a diagnostic role.37 When TAA/TAAD occurs as isolated trait genetic testing plays an essential diagnostic role (Figure 2). Non-syndromic F-TAA/TAAD are diagnosed on imaging data. They can be grouped according to the molecular pathway/structure in which disease genes are involved3,38 and include defects in the structural and functional proteins of vascular smooth muscle cells, extracellular matrix (ECM), or transforming growth factor (TGF-β) signalling pathway.38 The former include ACTA2 (smooth muscle alpha-actin), MYH11 (myosin heavy chain 11), MYLK (myosin light chain kinase), and PRKG1 (cyclic guanosine monophosphate-dependent protein kinase). Extracellular matrix genes include Fibrillin 1 and 2, COL3A1, MFAP5 that encodes the ECM component MAGP-2 and MAT2A that encodes methionine adenosyltransferase II alpha (MAT IIa). Transforming growth factor-β pathway includes TGFBR1 (LDS type 1), TGFBR2 (LDS type 2), SMAD3 (LDS type 3), TGFB2 (LDS type 4), and TGFB3 (LDS type 5). Bicuspid aortic valve is common in the general population (1–2%); in 25% of cases, it is associated with dilation of the ascending aorta.39 Bicuspid aortic valve-associated aortopathy should be distinguished from that recurring in CHD (such as hypoplastic left heart syndrome, coarctation of aorta, and septal defects) and in syndromes (Andersen-Tawil, DiGeorge, Noonan, LEOPARD, and Turner).39,40 Non-syndromic F-BAV has been associated with mutations in NOTCH139 and in GATA540 and in isolated FTAAD-TGFBR1, FTAAD-TGFBR2, FTAAD-SMAD3, FTAAD-TGFB2, and FTAAD-ACTA2.3 The major clinical risk in genetic TAA, syndromic and non-syndromic, is dissection and rupture of the aorta; non-aortic arteries can be involved in EDS-IV, ATS, and LDS. The identification of the disease-causing mutation in each proband provides the tool for early, preclinical diagnosis, tailored monitoring, and preventive surgery. Surgical indications are guided by the imaging characteristics of the aneurysm (size in particular), rate of progression, family history of rupture, coexistence of risk factors (hypertension), or electively planned (e.g. in fertile women who plan pregnancy). Although guidelines provide general indications, each patient should be cared for on individual basis.
Table 1.
Marfan syndrome—diagnostic criteria (J Med Genet 2010;47:476–85)
| (A) Diagnosis of Marfan syndrome | ||
| In the absence of family history | Aortic root dilatation Z score ≥ 2 AND ectopia lentis | |
| Aortic root dilatation Z score ≥ 2 AND FBN1 | ||
| Aortic root dilatation Z score ≥ 2 AND systemic score ≥ 7a | ||
| Ectopia lentis AND FBN1 with known aortic root dilatation | ||
| In the presence of family history | Aortic root dilatation Z score ≥ 2 AND ectopia lentis | |
| Aortic root dilatation Z score ≥ 2 AND FBN1 | ||
| Aortic root dilatation Z score ≥ 2 AND systemic score ≥ 7a | ||
| Ectopia lentis AND FBN1 with known aortic root dilatation | ||
| (B) Systemic score | Value | |
| Skeletal | ||
| Reduced upper segment/lower segment and increased arm span/height | 1 | |
| Wrist AND thumb sign | 3 | |
| Wrist OR thumb sign | 1 | |
| Pectus carinatum deformity | 2 | |
| Pectus excavatum or chest asymmetry | 1 | |
| Hindfoot deformity | 2 | |
| Plain flat foot | 1 | |
| Spontaneous pneumothorax | 2 | |
| Dural ectasia | 2 | |
| Protrusio acetabuli | 2 | |
| Scoliosis or thoracolumbar kyphosis | 1 | |
| Reduced elbow extension | 1 | |
| Facies | ||
| Three of five facial features | 1 | |
| Dolichocephaly | ||
Downward slanting palpebral fissures |
||
Enophthalmos |
||
| Retrognathia | ||
Malar hypoplasia |
||
| Skin | ||
| Skin striae | 1 | |
| Eye | ||
| Severe myopia (>3 diopters) | 1 | |
| Heart | ||
| Mitral valve prolapse | 1 | |
A score of ≥ 7 is considered a positive systemic score.
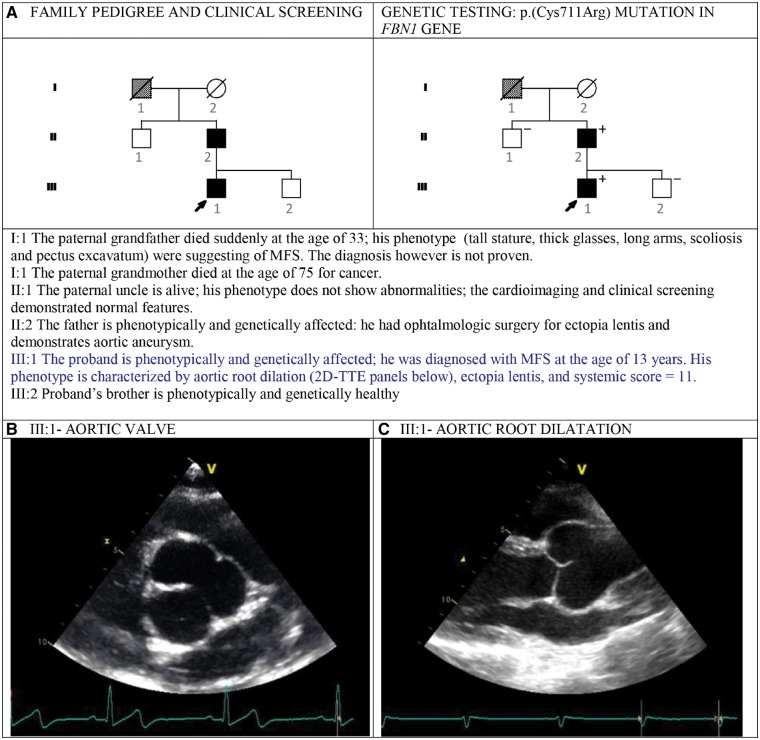
Figure 2.
The figure shows the pedigree of this small family with autosomal dominant Marfan syndrome (A) and the 2D-TTE view (B, C) of the aortic root of family member III: 1 demonstrating aortic root aneurysm.
Familial pulmonary hypertension
Pulmonary arterial hypertension is a rare (1–2 per 100 000 and 1 per 1 000 000 people), life-threatening disease. Pulmonary hypertension is defined as an increase in mean pulmonary arterial pressure (PAPm) ≥25 mmHg at rest as assessed by right heart catheterization.41 Current clinical classification is based on the principles of similarities in pathobiology, clinical features, and therapeutic options.4 More than 40 different diseases are divided into five groups.4 Groups 1 and 5 include forms of pulmonary hypertension of genetic origin (Table 2). For these latter, a clinically oriented approach is feasible for familial AD PPH in which mutations of bone morphogenetic protein receptor 2 (BMPR2) recur in up to 80% of patients and families. Less common disease genes, such as SMAD9, play a role in the same BMP pathway as downstream modulators of the BMP signalling pathway and cause a phenotypically similar, AD disease. Other disease genes such as ALK-1 and Endoglin (ENG) account for a minority of familial PPH. Defects in other rare disease genes such as CAVEOLIN 3 (CAV3) or genes coding ion channels such as KCNH3, result in a similar phenotype, but via different mechanisms. Pulmonary veno-occlusive hypertension is allelic at the PPH1 locus (BMPR2), while the recessive form is caused by mutations in EIF2AK4.42 Finally, Group 5 PH includes a variety of acquired and heritable diseases such as the AD lymphangioleiomyomatosis43 and lysosomal storage diseases such as glycogen storage disease types 1 and 344,45 and Gaucher disease type 1.46,47 Clinical genetics with counselling and pedigree construction, search for known risk factors and annotation of novel potential risk factors, family investigation, and genetic testing are currently being translated in the clinical setting. Clinical attention to PH is encouraged by the availability of new medical treatments (endothelin receptor antagonists, phosphodiesterase type-5 inhibitors, soluble guanylate cyclase stimulators, and prostanoids) that continue to be trialled in patients with idiopathic, heritable, drug-induced, and connective tissue disease-associated pulmonary hypertension.
Table 2.
Genetic pulmonary hypertension
| PH WHO GROUP | TYPE | Inheritance | MIM | Gene | MIM | Protein |
|---|---|---|---|---|---|---|
| # phenotype | *Gene locus | |||||
| Primary pulmonary hypertension (PPH) | ||||||
| 1 | PPH1 | AD | BMPR2a | 600799 | Bone Morphogenetic protein receptor 2 | |
| 1 | PPH2 | AD | SMAD9 | 603295 | Mothers against decapentaplegic drosophila, homologue of, 9 | |
| 1 | PPH3 | AD | CAV1 | 601047b | Caveolin1 | |
| 1 | PPH4 | AD | KCNK3 | 603220 | Potassium channel, subfamily K, member 3 | |
| 1 | Dexfenfluramine-associated PH | AD | CYP1B1 | 601771 | Cytochrome P450; subfamily 1; polypeptide 1 | |
| Pulmonary veno-occlusive disease (PVOD) | ||||||
| 1 | PVOD1 | AD | BMPR2 | 600799 | Bone Morphogenetic protein receptor 2 | |
| 1 | PVOD2 | AR | EIF2AK4 | 609280 | Eukaryotic translation initiation factor-2, alpha kinase 4 | |
| Hereditary haemorrhagic telangiectasia (HHT) | ||||||
| 1 | HHT1 (Rendu-Osler-Weber) | AD | ENG | 131195 | Endoglein (CD105) | |
| 1 | HHT2 | AD | ACVRL1 | 601284 | Activin A receptor, type II-like 1 | |
| Lymphangioleiomyomatosis (LAM) | ||||||
| 5 | Tuberous sclerosis-1; LAM | AD | TSC1 | 605284 | Amartin | |
| 5 | Tuberous sclerosis-2 and LAM somatic mutations | AD | TSC2 | 191092 | Tuberin | |
| Lysosomal storage diseases | ||||||
| 5 | Glycogen Storage disease type 1 | AR | G6PC (1a) | 232200 | Glucose-6-phosphatase | |
| SLC37A4 (1b) | 232220 | Glucose-6-phosphate translocase | ||||
| 5 | Glycogen Storage disease types 3a and b | AR | AGL | 610860 | Glycogen debrancher enzyme | |
| 5 | Gaucher Disease type 1 (with or without splenectomy) | AR | GBA | 606463 | Acid beta-glucosidase | |
Including fenfluramine-/dexfenfluramine associated PH.
Disorders allelic at the same locus: ‘Lipodystrophy, congenital, generalized type 3’ and ‘Partial lipodystrophy, congenital cataracts and neurodegeneration syndrome’.
Current applications, limits, and future developments
Current applications
Genetic diagnosis is now part of the diagnostic work-up of familial CV diseases; it provides preclinical, early, and prenatal diagnosis and supports treatment decisions. Its translation is still limited for atrial arrhythmias and pulmonary hypertension, while it is substantially implementing in cardiomyopathies, ventricular arrhythmias and aneurysmal aortopathies. Limits include the small resources for genetics and cardiology, both genetic testing and clinical family screening, the still incomplete list of disease-causing genes and the complex interpretation of NGS data. A major limit for genetic arrhythmogenic diseases is the possible non-informative ECG at baseline in family screening and therefore segregation studies. The future, in general, is the expansion of clinical screening of relatives, the better segregation studies in families for both CMP and arrhythmogenic diseases and individual risk stratification for preventable catastrophic aortic dissection in families as well as the identification of pulmonary hypertension for early treatments with new medications.
Funding
Research for inherited cardiovascular diseases is supported by funds from EU project INHERITANCE (241924) and EURO-FDCM, by Italian Ministry of Health to the IRCCS Foundation Policlinico San Matteo of Pavia (GR-2009-1608713 and RF-PSM-2008-1145809), by charities for patients and families (MAGICA ONLUS and ASM Sardegna Onlus).
Conflict of interest: none declared.
References
- 1. Arbustini E, Favalli V, Narula N, Disabella E, Grasso M.. Genetic basis of cardiovascular disease In: Fuster V, Harrington RA, Narula J, Eapen ZJ, eds. Hurst's the Heart. McGraw Hill Education; 2017. p174–204. [Google Scholar]
- 2. Kimura A. Molecular genetics and pathogenesis of cardiomyopathy. J Hum Genet 2016;61:41–50. [DOI] [PubMed] [Google Scholar]
- 3. Bradley TJ, Bowdin SC, Morel CF, Pyeritz RE.. The expanding clinical spectrum of extracardiovascular and cardiovascular manifestations of heritable thoracic aortic aneurysm and dissection. Can J Cardiol 2016;32:86–99. [DOI] [PubMed] [Google Scholar]
- 4. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R.. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34–D41. [DOI] [PubMed] [Google Scholar]
- 5. Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, de Andrade M, Burnett JC, Olson TM.. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. New Engl J Med 2008;359:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Disertori M, Quintarelli S, Grasso M, et al. Autosomal recessive atrial dilated cardiomyopathy with standstill evolution associated with mutation of Natriuretic Peptide Precursor A. Circulation 2012;112:963520. [DOI] [PubMed] [Google Scholar]
- 7. Walsh-Irwin C, Hannibal GB.. Sick sinus syndrome. AACN Adv Crit Care 2015;26:376–380. [DOI] [PubMed] [Google Scholar]
- 8. De Regibus V, Rordorf R, Giorgianni C, Canclini C, Vicentini A, Taravelli E, Petracci B, Savastano S, De Servi S, Arbustini E.. Autosomal recessive atrial disease presenting with sick sinus syndrome (SSS), right atrial fibrosis and biatrial dilatation: clinical impact of genetic diagnosis. Int J Cardiol 2016;208:67–69. [DOI] [PubMed] [Google Scholar]
- 9. Callewaert B, De Paepe A, Coucke P. Arterial tortuosity syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews2014. Seattle (WA): University of Washington, Seattle; 1993–2019. [PubMed]
- 10. Dasouki M, Jawdat O, Almadhoun O, Pasnoor M, McVey AL, Abuzinadah A, Herbelin L, Barohn RJ, Dimachkie MM.. Pompe disease: literature review and case series. Neurol Clin 2014;32:751.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diegoli M, Grasso M, Favalli V, Serio A, Gambarin FI, Klersy C, Pasotti M, Agozzino E, Scelsi L, Ferlini A, Febo O, Piccolo G, Tavazzi L, Narula J, Arbustini E.. Diagnostic work-up and risk stratification in X-linked dilated cardiomyopathies caused by dystrophin defects. J Am Coll Cardiol 2011;58:925–934. [DOI] [PubMed] [Google Scholar]
- 12. Koch AJ, Holaska JM, Emerin in health and disease. Semin Cell Dev Biol 2014;29:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, Duboc D, Gimeno J, de Groote P, Imazio M, Heymans S, Klingel K, Komajda M, Limongelli G, Linhart A, Mogensen J, Moon J, Pieper PG, Seferovic PM, Schueler S, Zamorano JL, Caforio ALP, Charron P.. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2016;37:1850–1858. [DOI] [PubMed] [Google Scholar]
- 14. Arbustini E, Narula N, Dec GW, Reddy KS, Greenberg B, Kushwaha S, Marwick T, Pinney S, Bellazzi R, Favalli V, Kramer C, Roberts R, Zoghbi WA, Bonow R, Tavazzi L, Fuster V, Narula J.. The MOGE (S) classification for a phenotype–genotype nomenclature of cardiomyopathy: endorsed by the World Heart Federation. J Am Coll Cardiol 2013;62:2046–2072. [DOI] [PubMed] [Google Scholar]
- 15. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 16. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H.. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Kardiologia Polska 2014;72:1054–1126. [DOI] [PubMed] [Google Scholar]
- 17. Short D. The syndrome of alternating bradycardia and tachycardia. Br Heart J 1954;16:208.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jensen PN, Gronroos NN, Chen LY, Folsom AR, deFilippi C, Heckbert SR, Alonso A.. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol 2014;64:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kodama T, Serio A, Disertori M, Bronzetti G, Diegoli M, Narula N, Grasso M, Mazzola S, Arbustini E.. Autosomal recessive paediatric sick sinus syndrome associated with novel compound mutations in SCN5A. Int J Cardiol 2013;167:3078–3080. [DOI] [PubMed] [Google Scholar]
- 20.Abe K, Machida T, Sumitomo N, Yamamoto H, Ohkubo K, Watanabe I, Makiyama T, Fukae S, Kohno M, Harrell DT, Ishikawa T, Tsuji Y, Nogami A, Watabe T, Oginosawa Y, Abe H, Maemura K, Motomura H, Makita N. Sodium channelopathy underlying familial sick sinus syndrome with early onset and predominantly male characteristics. Circ Arrhythm Electrophysiol 2014;7:511–517. [DOI] [PubMed] [Google Scholar]
- 21. Fox CS, Parise H, D'Agostino RB, Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ.. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA 2004;291:2851–2855. [DOI] [PubMed] [Google Scholar]
- 22. Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen W-K, Hammill SC, Packer DL, Olson TM.. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol 2003;41:2185–2192. [DOI] [PubMed] [Google Scholar]
- 23. Christophersen IE, Ellinor PT.. Genetics of atrial fibrillation: from families to genomes. J Hum Genet 2016;61:61–70. [DOI] [PubMed] [Google Scholar]
- 24. Nakano Y, Shimizu W.. Genetics of long-QT syndrome. J Hum Genet 2016;61:51–55. [DOI] [PubMed] [Google Scholar]
- 25. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang C-E, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C, Ackerman M, Belhassen B, Estes NAM, Fatkin D, Kalman J, Kaufman E, Kirchhof P, Schulze-Bahr E, Wolpert C, Vohra J, Refaat M, Etheridge SP, Campbell RM, Martin ET, Quek SC.. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013;15:1389–1406. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz PJ, Crotti L.. QTc behavior during exercise and genetic testing for the long-QT syndrome. Circulation 2011;124:2181–2184. [DOI] [PubMed] [Google Scholar]
- 27. Khera S, Jacobson JT.. Short QT syndrome in current clinical practice. Cardiol Rev 2016;24:190–193. [DOI] [PubMed] [Google Scholar]
- 28. Guerrier K, Kwiatkowski D, Czosek RJ, Spar DS, Anderson JB, Knilans TK.. Short QT interval prevalence and clinical outcomes in a pediatric population. Circulation 2015;8:1460–1464. [DOI] [PubMed] [Google Scholar]
- 29. Adler A. Brugada syndrome: diagnosis, risk stratification, and management. Curr Opin Cardiol 2016;31:37–45. [DOI] [PubMed] [Google Scholar]
- 30. Watanabe H, Minamino T.. Genetics of Brugada syndrome. J Hum Genet 2016;61:57–60. [DOI] [PubMed] [Google Scholar]
- 31. Bezzina CR, Barc J, Mizusawa Y, Remme CA, Gourraud J-B, Simonet F, Verkerk AO, Schwartz PJ, Crotti L, Dagradi F, Guicheney P, Fressart V, Leenhardt A, Antzelevitch C, Bartkowiak S, Borggrefe M, Schimpf R, Schulze-Bahr E, Zumhagen S, Behr ER, Bastiaenen R, Tfelt-Hansen J, Olesen MS, Kääb S, Beckmann BM, Weeke P, Watanabe H, Endo N, Minamino T, Horie M, Ohno S, Hasegawa K, Makita N, Nogami A, Shimizu W, Aiba T, Froguel P, Balkau B, Lantieri O, Torchio M, Wiese C, Weber D, Wolswinkel R, Coronel R, Boukens BJ, Bézieau S, Charpentier E, Chatel S, Despres A, Gros F, Kyndt F, Lecointe S, Lindenbaum P, Portero V, Violleau J, Gessler M, Tan HL, Roden DM, Christoffels VM, Le Marec H, Wilde AA, Probst V, Schott J-J, Dina C, Redon R.. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, Ohe T, Zipes DP, Wu J.. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 2008;118:1697–1704. [DOI] [PubMed] [Google Scholar]
- 33. Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff J-M, Klug D, Hayashi M, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A.. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation 2009;119:2426–2434. [DOI] [PubMed] [Google Scholar]
- 34. Imberti JF, Underwood K, Mazzanti A, Priori SG.. Clinical challenges in catecholaminergic polymorphic ventricular tachycardia. Heart Lung Circ 2016;25:777–783. [DOI] [PubMed] [Google Scholar]
- 35. Nyegaard M, Overgaard MT, Søndergaard MT, Vranas M, Behr ER, Hildebrandt LL, Lund J, Hedley PL, Camm AJ, Wettrell G, Fosdal I, Christiansen M, Børglum AD.. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet 2012;91:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Werf C, Zwinderman AH, Wilde AA.. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace 2012;14:175–183. [DOI] [PubMed] [Google Scholar]
- 37. Arbustini E, Favalli V, Di Toro A, Giuliani L, Limongelli G.. Common presentation of rare diseases: aortic aneurysms and valves. Int J Cardiol 2018;257:358–365. [DOI] [PubMed] [Google Scholar]
- 38. Andelfinger G, Loeys B, Dietz H.. A decade of discovery in the genetic understanding of thoracic aortic disease. Can J Cardiol 2016;32:13–25. [DOI] [PubMed] [Google Scholar]
- 39. Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D.. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437:270–274. [DOI] [PubMed] [Google Scholar]
- 40. Bonachea EM, Chang S-W, Zender G, LaHaye S, Fitzgerald-Butt S, McBride KL, Garg V.. Rare GATA5 sequence variants identified in individuals with bicuspid aortic valve. Pediat Res 2014;76:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2015;37:67–119. [DOI] [PubMed] [Google Scholar]
- 42. Ma L, Bao R.. Pulmonary capillary hemangiomatosis: a focus on the EIF2AK4 mutation in onset and pathogenesis. Appl Clin Genet. 2015;8:181.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. von Ranke FM, Zanetti G, e Silva JLP, Neto CAA, Godoy MCB, Souza CA, Mançano AD, Souza AS, Escuissato DL, Hochhegger B, Marchiori E.. Tuberous sclerosis complex: state-of-the-art review with a focus on pulmonary involvement. Lung 2015;193:619–627. [DOI] [PubMed] [Google Scholar]
- 44. Alkhorayyef A, Ryerson L, Chan A, Phillipos E, Lacson A, Adatia I.. Pulmonary interstitial glycogenosis associated with pulmonary hypertension and hypertrophic cardiomyopathy. Pediat Cardiol 2013;34:462–466. [DOI] [PubMed] [Google Scholar]
- 45. Lee TM, Berman-Rosenzweig ES, Slonim AE, Chung WK.. Two cases of pulmonary hypertension associated with type III glycogen storage disease. JIMD Rep 2011;1:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Al-Naamani N, Roberts KE, Hill NS, Preston IR.. Imatinib as rescue therapy in a patient with pulmonary hypertension associated with Gaucher disease. Chest J 2014;146:e81–e83. [DOI] [PubMed] [Google Scholar]
- 47. de Boer GM, van Dussen L, van den Toorn LM, den Bakker MA, Hoek RAS, Hesselink DA, Hollak CEM, van Hal PTW.. Lung transplantation in Gaucher disease: a learning lesson in trying to avoid both scylla and charybdis. Chest J 2016;149:e1–e5. [DOI] [PubMed] [Google Scholar]