This cohort study evaluates the association between image-based place-pitch mapping using flat-panel computed tomography with speech and pitch perception in individuals with cochlear implants.
Key Points
Question
Is an individualized, image-guided approach to place-pitch cochlear implant programming associated with improved pitch-scaling performance without undermining speech perception accuracy?
Findings
In this interventional cohort study of 17 cochlear implant users, significant improvement in pitch-scaling performance with an image-guided approach to postimplantation programming was observed. The greatest association occurred with major pitch reversals (notes spaced 1.65 semitones or greater) in the low- and high-frequency ranges.
Meaning
An image-based approach toward cochlear implant mapping may improve pitch perception outcomes by reducing place-pitch mismatch.
Abstract
Importance
Cochlear implant users generally display poor pitch perception. Flat-panel computed tomography (FPCT) has recently emerged as a modality capable of localizing individual electrode contacts within the cochlea in vivo. Significant place-pitch mismatch between the clinical implant processing settings given to patients and the theoretical maps based on FPCT imaging has previously been noted.
Objective
To assess whether place-pitch mismatch is associated with poor cochlear implant–mediated pitch perception through evaluation of an individualized, image-guided approach toward cochlear implant programming on speech and music perception among cochlear implant users.
Design, Setting, and Participants
A prospective cohort study of 17 cochlear implant users with MED-EL electrode arrays was performed at a tertiary referral center. The study was conducted from June 2016 to July 2017.
Interventions
Theoretical place-pitch maps using FPCT secondary reconstructions and 3-dimensional curved planar re-formation software were developed. The clinical map settings (eg, strategy, rate, volume, frequency band range) were modified to keep factors constant between the 2 maps and minimize confounding. The acclimation period to the maps was 30 minutes.
Main Outcomes and Measures
Participants performed speech perception tasks (eg, consonant-nucleus-consonant, Bamford-Kowal-Bench Speech-in-Noise, vowel identification) and a pitch-scaling task while using the image-guided place-pitch map (intervention) and the modified clinical map (control). Performance scores between the 2 interventions were measured.
Results
Of the 17 participants, 10 (58.8%) were women; mean (SD) was 59 (11.3) years. A significant median increase in pitch scaling accuracy was noted when using the experimental map compared with the control map (4 more correct answers; 95% CI, 0-8). Specifically, the number of pitch-scaling reversals for notes spaced at 1.65 semitones or greater decreased when an image-based approach to cochlear implant programming was used vs the modified clinical map (4 mistakes; 95% CI, 0.5-7). Although there was no observable median improvement in speech perception during use of an image-based map, the acute changes in frequency allocation and electrode channel deactivations used with the image-guided maps did not worsen consonant-nucleus-consonant (−1% correct phonemes, 95% CI, −2.5% to 6%) and Bamford-Kowal-Bench Speech-in-Noise (0.5-dB difference; 95% CI, −0.75 to 2.25 dB) median performance results relative to the clinical maps used by the patients.
Conclusions and Relevance
An image-based approach toward ochlear implant mapping may improve pitch perception outcomes by reducing place-pitch mismatch. Studies using a longer acclimation period with chronic stimulation over months may help assess the full range of the benefits associated with personalized image-guided cochlear implant mapping.
Introduction
Deficits in pitch perception experienced by cochlear implant (CI) users have been well described,1,2 including the fact that CIs are frequently out of tune. One of the key variables that affects CI-mediated pitch processing is electrode placement relative to the tonotopic frequency gradient of the spiral ganglion neurons. Cochlear implant electrode placement is affected by both anatomic and surgical factors. Despite inevitable variations in final electrode placement from patient to patient, we have adopted a one-size-fits-all approach toward cochlear implant place-pitch mapping, meaning that cochlear implants are activated with more or less the same default place-pitch map (for a given device and company).
In 1960, Greenwood3 experimentally derived a mathematical function that describes the between the spatial location of the hair cells along the organ of Corti and the corresponding frequency. Later, Stakhovskaya and colleagues4,5 conducted anatomic studies on cadaver temporal bones to determine the relationship between the organ of Corti and the spiral ganglion cells. By combining these 2 functions, we can identify a relationship between electrode position and spiral ganglion frequency, which we will refer to hereafter as the modified Greenwood function. This function characterizes the relationship between the distance along the basilar membrane and the corresponding characteristic frequency of the spiral ganglion neurons at that distance.
Historically, clinical application of the modified Greenwood function has been limited by the metallic artifact and temporal bone attenuation that occurs with postimplantation imaging. In the past decade, flat-panel computed tomographic (FPCT) imaging has emerged as a technology capable of high-spatial resolution volumetric and dynamic imaging. Flat-panel computed tomographic imaging combined with secondary reconstructions in the cochlear axis allows for clear visualization of electrode channels in vivo that has previously been unattainable.6 A previous study demonstrated the feasibility of using high-resolution secondary reconstructions of FPCT data sets for postoperative cochlear implant imaging.7 Theoretical place-pitch maps were calculated based on the FPCT findings and compared with the participant’s clinical place-pitch map. The results of the study revealed significant place-pitch mismatch between the clinical maps and the FPCT-based maps, with deviations up to 1.5 octaves.
These shifts may affect pitch perception by causing a discrepancy between the frequency that an individual electrode channel should be associated with and the frequency that has been assigned to it. Correcting the place-pitch mismatch may lead to clinical benefits with respect to pitch perception. In the present study, we evaluated the association between individualized, image-guided place-pitch mapping and speech and pitch perception in cochlear implant users. We hypothesized that image-guided maps may lead to improved pitch perception in cochlear implant users without a corresponding decrease in speech perception abilities.
Methods
Study Design and Subjects
We conducted a prospective cohort study among 17 cochlear implant users. The inclusion criteria for participant eligibility consisted of adults (aged ≥18 years) who had undergone full electrode insertion of a MED-EL CI. The exclusion criteria consisted of cochlear implant users with medical conditions that may prevent participation in this study (eg, vision problems, cognitive disorders), image-guided maps with less than 7 activated electrodes, and experimentally derived frequency shifts greater than 7 half-tone steps in either direction. This study was approved by an institutional review board committee of the University of California, San Francisco. Written informed consent was obtained from all study participants, and financial compensation was provided.
A 2-sided significance level with α = .05 and a power (1 β) of 80 required a sample size of 16 individuals exposed to 2 arms (OpenEpi, version 3). Of the 17 people who were originally enrolled in the study, 1 cochlear implant user was ineligible to continue owing to an inability to meet inclusion criteria (minimum of 7 activated electrodes and full electrode insertion). Two cochlear implant users were unable to remain in the study because of unforeseen personal emergencies. Nonetheless, we adopted an intention-to-treat approach toward our analyses. Our participants’ demographics consisted of 7 men and 10 women, with a mean (SD) age of 59 (11.3) years (range, 48-79 years). There were 10 unilateral cochlear implant users and 7 bilateral cochlear implant users. The eTable in the Supplement provides more information regarding the demographic details.
Overall, the study included 2 visits. During the first visit, participants underwent FPCT image acquisition of their CIs. Participants returned for their second visit to undergo auditory perception testing. We provided a 30-minute acclimation period to both control maps and experimental maps for participants before testing them on speech and music perception tasks. The auditory environment during the acclimation period was conversation between the study coordinator (N.T.J.) and the research participant. The order of testing (control map vs experimental map first) was randomized across all participants.
Imaging and Reconstruction
Participants had FPCT imaging performed on a flat-panel angiography system with commercially available software (DynaCT; Siemens). We obtained FPCT temporal bone images and created high-resolution secondary reconstructions using previously described and optimized parameters (Figure 1).6 DICOM data processing was performed using open-source medical image viewing software (OsiriX; Pixmeo). Our measurement methods have been described in prior studies.7,8,9 For each cochlear implant array, we measured the electrode channel’s position as a percentage of distance along the cochlear duct length rather than the distance along the electrode array. Our measurements did not account for scalar excursions.
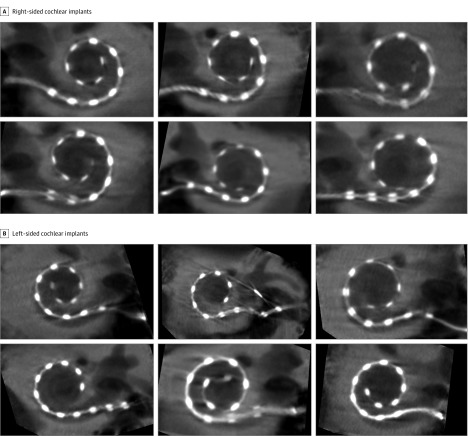
Figure 1. Assessment of Cochlear Implants In Vivo Using High-Resolution Secondary Reconstructions of Flat-Panel Computed Tomographic Imaging.
A, Six right-sided cochlear implants are depicted. B, Six left-sided cochlear implants are depicted. The following measures were used in this study: voxel size, 0.02 mm; 512 × 512 section matrix; Hounsfield units kernel types; sharp image characteristics; 0.5-mm distance; and 0.1-mm image thickness.
Image-Guided Map: Experimental
We used the modified Greenwood function3,4 to calculate the theoretical characteristic frequency for each electrode channel. These ideal center frequencies were used to determine the lower and upper frequency boundaries for each channel in a clinical system software with a MAX programming interface for programming for MED-EL (MAESTRO System Software 6.0) CIs. Extracochlear electrodes, channels that required frequency shifts of greater than 7 semitones, and theoretical frequencies that exceed the frequency boundaries of 70 to 8500 Hz were deactivated in the experimental maps. We used a fixed bandwidth of 70 to 8500 Hz when setting frequency allocations; all place-pitch maps were custom programmed to this defined frequency range.
Modified Clinical Map: Control
For the control map, we used the patient’s current, most frequently used clinical map. Certain settings were held constant among all modified clinical and experimental maps, such as strategy high-definition continuous interleaved sampling, default volume (100% [range, 0%-100%]), stimulation rate, frequency band range (70-8500 Hz), logarithmic frequency bands, maplaw logarithmic compression (1000 [range, 0-8000]), and sensitivity settings (75% [range, 0%-100%]). The purpose of standardizing these settings was to minimize potential confounding that may arise from variations unrelated to center frequencies.
Testing Environment
Regardless of the hearing status of the contralateral ear, all participants were asked to remove any assistive hearing device and occlude the contralateral ear with an earplug to avoid any confounding that could arise from residual hearing during testing. Bilateral cochlear implant users were tested using their better ear. Each participant completed the speech and pitch tasks in a soundproof acoustic chamber. Loudspeakers (SS-MB150H; Sony) were placed directly in front of the seated participant’s face, and the user interface was displayed on a touch-screen monitor located inside the sound booth. All acoustic stimuli were sampled at 44.1 kHz and 16-bit resolution. The stimuli were presented via a mixer (MultiMix 6 USB; Alesis), a stereo power amplifier (PCA2; Pyle), and a single calibrated loudspeaker, located approximately 0.6 meter from the participant at an average level of 65-dB sound pressure level.
Sound Perception Tasks
All individuals participated in the consonant-nucleus-consonant (CNC) test,10 the Bamford-Kowal-Bench Speech-in-Noise (BKB-SIN) test,11 the Iowa vowel recognition test,12 and a pitch scaling task developed in our laboratory. Regarding the CNC test, each participant completed 2 lists of 50 words (150 phonemes per list [score range, 0%-100%]) per map, and we determined the mean of the phoneme scores. For the BKB-SIN test, cochlear implant users completed 1 paired list (2 lists of 10 sentences) for practice and 2 paired lists per map (score range, 23.5 dB [lowest) to −1.5 dB [best]). The mean signal-to-noise ratio (SNR) for 50% correct score from the 2 paired lists was determined. The vowel recognition task consists of an 8-alternative identification paradigm, including 6 monophthongs and 2 diphthongs. Each vowel was preceded by /h/ and followed by /d/. Tokens for these closed-set tests were digitized from the natural productions of a male speaker. Each participant completed a total of 80 trials (10 trials per token) per map.
Pitch perception and interval associations were assessed using a pitch scaling test that involved both an ordinal component (pitch ranking) and a parametric component (assessing relative pitch distance between tones) (Figure 2). Participants ranked a total of 71 auditory tokens from lowest to highest pitch with a 100-point scale (0, lowest pitch in a set of tokens; 100, highest pitch in a set of tokens). These tokens spanned a frequency spectrum of 70 to 8500 Hz on a logarithmic scale. The first set was made up of 11 tokens spanning 70 to 8500 Hz. The remaining 10 sets focused on smaller pitch intervals and consisted of 6 tokens 1.65 semitones apart (ie, total of 71 tokens).
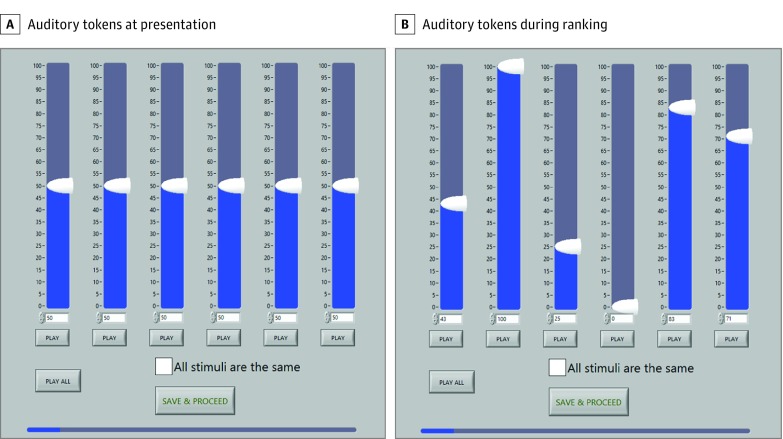
Figure 2. User Interface for the Pitch-Scaling Task.
A, Participants were presented with sets of 6 or 11 acoustic tokens of random order spanning a defined frequency spectrum on a logarithmic scale. B, Participants were instructed to rank these acoustic tokens from lowest to highest pitch on a 100-point scale, where 0 was defined as the lowest pitch and 100 was defined as the highest pitch in the set. The play all button allows the participant to play the acoustic tokens in the order that they ranked the tones. If the listener could not distinguish between any of the tones, they were instructed to select the all stimuli are the same button.
Statistical Analysis
Extreme outliers were assessed by inspection of a box plot for values greater than 3 box lengths from the edge of the box. We used Shapiro-Wilk tests (P > .05) of normality and normal Q-Q plots to determine whether there was normal distribution. For study analyses, we used 2-way repeated-measures analysis of variance and post hoc analyses with a Bonferroni adjustment. When the data were skewed (ie, not normally distributed), we used the Wilcoxon signed rank test to assess whether the differences in speech and pitch performance between an image-guided place-pitch map and a modified control map were significant.
The precision of the results and the range within which the true magnitude of the outcome could be expected were determined using 95% CIs. All statistical analyses were performed using SPSS Statistics, version 23 (IBM Corp).
Results
Consonant-Nucleus-Consonant Test
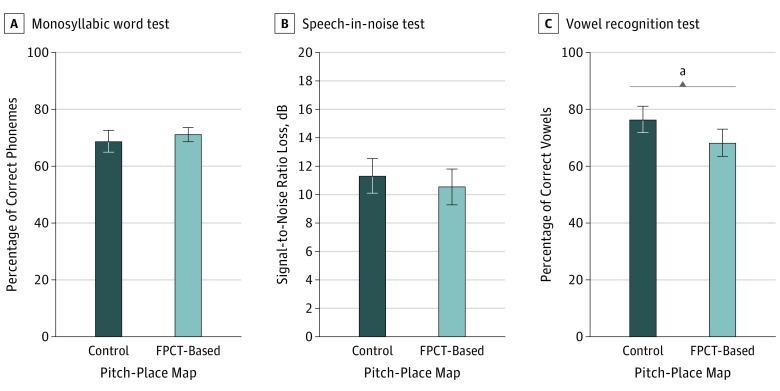
Sixteen cochlear implant users underwent speech perception testing to understand the association between an imaging-based pitch-place map and their clinically derived pitch-place map by CNC performance scores. The experimental pitch-place map elicited an improvement in CNC scores in 8 cochlear implant users compared with the modified clinical pitch-place map, whereas 2 cochlear implant users demonstrated no improvement and 6 cochlear implant users did not perform as well. A Wilcoxon signed rank test determined that there was not a significant median increase (−1.0% correct phonemes, 95% CI, −2.5% to 6.0%) in correct phonemes when participants used imaging-based pitch-place maps (72.0% correct phonemes) compared with the modified clinical maps (73.0% correct phonemes) (Figure 3A).
Figure 3. Speech Perception Performances While Using the Modified Clinical Map vs the Image-Based Experimental Map.
There was no significant difference between the consonant-nucleus-consonant test (150 phonemes per list) (A) and Bamford-Kowal-Bench Speech-in-Noise test (B) (−1.0% correct phonemes; 95% CI, −2.5% to 6.0%). On average, cochlear implant users did better on the Iowa vowel recognition test (80 trials) (C) when using their modified clinical maps compared with their image-based experimental maps (12% increase in correct vowel recognition; 95% CI, 1%-17%). FPCT indicates flat-panel computed tomography. Error bars indicate 95% CIs.
aStatistical significance with P < .05.
BKB-SIN Test
The experimental pitch-place map elicited an improvement in BKB-SIN scores in 7 cochlear implant users compared with the modified clinical pitch-place map, whereas 9 cochlear implant users did not perform as well on the BKB-SIN test. Although there was a positive change, a Wilcoxon signed rank test determined that it was not a significant median increase (0.5-dB difference; 95% CI, −0.75 dB to 2.25 dB) in BKB-SIN scores (SNR-50, 9.5 dB) compared with the modified clinical map (SNR-50, 11.5 dB) (Figure 3B).
Iowa Vowel Identification Test
Of the 16 cochlear implant users who underwent vowel testing, the experimental map elicited an improvement in vowel identification scores in 5 cochlear implant users compared with the control map, whereas 2 participants saw no improvement and 9 cochlear implant users did not perform as well on the vowel test. A Wilcoxon signed rank test determined that there was a significant median difference between the experimental and control maps. There was a median increase of 12% (95% CI, 1%-17%) in vowel recognition scores when the participant’s control maps were used compared with the experimental maps (Figure 3C).
Pitch-Scaling Test
We found that the experimental map elicited an improvement in pitch-scaling performance compared with control maps in 12 cochlear implant users, whereas 5 cochlear implant users performed better on the pitch-scaling task using the modified clinical maps. We used a Wilcoxon signed rank test and observed a significant median difference of 4 more correct answers (95% CI, 0-8) in the pitch-scaling task with the experimental map compared with the control map. These improvements occurred even though there were a greater number of deactivated electrode channels in the experimental map (n = 1.8) than in the control map (n = 0.4).
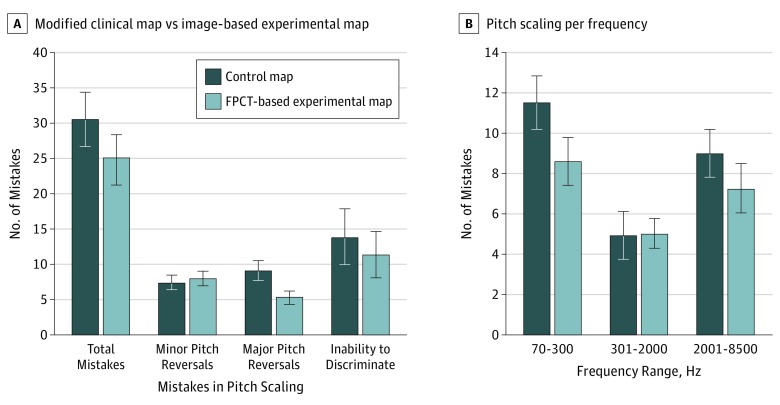
To understand the type of mistakes that occurred in these tasks, each error was subcategorized into 1 of the following groups: minor pitch reversal, major pitch reversal, and inability to perceive a difference between pitches. We defined a minor pitch reversal as a pitch reversal between 2 notes that are 1.65 semitones apart from one another. A major pitch reversal is a pitch reversal between 2 notes greater than 1.65 semitones apart from one another. When a participant ranked 2 pitches as equivalent, we characterized that event as an inability to discriminate. A Wilcoxon signed rank test determined that there was a significant increase in the number of major pitch reversals made with use of the control map compared with the experimental map (4 difference in mistakes; 95% CI, 0.5 to 7 mistakes). We did not observe a significant difference in the number of minor pitch reversals (a difference of 2 in mistakes; 95% CI, −2 to 4 mistakes) and lack of discriminability (0 difference in mistakes; 95% CI, −8 to 8 mistakes) between the 2 place-pitch maps (Figure 4A).
Figure 4. Mean Performance Data for the Pitch-Scaling Task Using the Modified Clinical Map vs the Image-Based Experimental Map.
A, Number of mistakes in the pitch-scaling task using the modified clinical map vs the image-based experimental map. The left column compares the total number of mistakes made during the pitch-scaling task between the control and experimental groups. These mistakes were broken down into subcategories: minor pitch reversals, major pitch reversals, and inability to discriminate between 2 pitches. There was a significant median difference of 4 more correct answers (95% CI, 0-8) in the pitch-scaling task with the experimental map compared with the control map. A Wilcoxon signed rank test determined that there was a significant increase in the number of major pitch reversals made with use of the control map compared with the experimental map (difference of 4 in number of mistakes; 95% CI, 0.5-7 mistakes). B, The association between the pitch-place map and frequency range and the number of pitch-scaling mistakes. Low-frequency range was defined as 70 to 300 Hz; midfrequency range, 301 to 2000 Hz; and high-frequency range, 2001 to 8500 Hz. A significant difference was noted in the total number of low-frequency (mean [SD] 5.100 [1.046]; 95% CI, 2.258-7.942) and high-frequency (mean [SD] 3.167 [0.804]; 95% CI, 0.981-5.352) range mistakes made in the pitch-scaling task between the control and the experimental maps. Error bars indicate 95% CIs. FPCT indicates flat-panel computed tomography.
Frequency Range
We conducted a 2-way repeated-measures analysis of variance to evaluate whether there was an association between the place-pitch map (control, experimental) and/or frequency range (low, medium, high) and the number of pitch-scaling mistakes. Analysis of the studentized residuals showed that there was normality, as assessed by the Shapiro-Wilk test of normality and no outliers, as assessed by no studentized residuals greater than 3 SDs. The Mauchly test of sphericity indicated that the assumption of sphericity was met for the 2-way interaction, χ22 = 2.982. Data are mean (SE), unless otherwise stated. The main outcome of the place-pitch map was a significant difference in mistakes made between trials. The mean difference between the control and experimental maps was 1.533 (95% CI, 0.415 to 2.651) (Figure 4B). The main finding associated with frequency showed that there was a significant difference in mistakes made between frequency ranges. Post hoc analysis with a Bonferroni adjustment revealed a significantly greater number of mistakes made in pitch trials spanning the low-frequency range compared with the midfrequency range (5.100 [1.046]; (95% CI, 2.258 to 7.942). Similarly, there was a significantly greater number of mistakes made in pitch trials spanning the high-frequency range pitch compared with the midfrequency range (3.167 [0.804]; 95% CI, 0.981 to 5.352). There was a difference of mistakes (1.93 [0.987]; 95% CI, −0.748 to 4.615) between those made in pitch trials spanning the low-frequency and high-frequency ranges that was not significant.
Discussion
In this prospective study, we evaluated the association between image-guided place-pitch mapping and CI-mediated speech and pitch perception. The cochlear implant users’ performance improved on an ordinal and parametric pitch-scaling task when they used an image-guided place-pitch map rather than their clinical map settings. The number of mistakes made decreased with intervention despite a mean of 10 activated channels with the image-guided place-pitch map vs 12 activated channels with the modified control map.
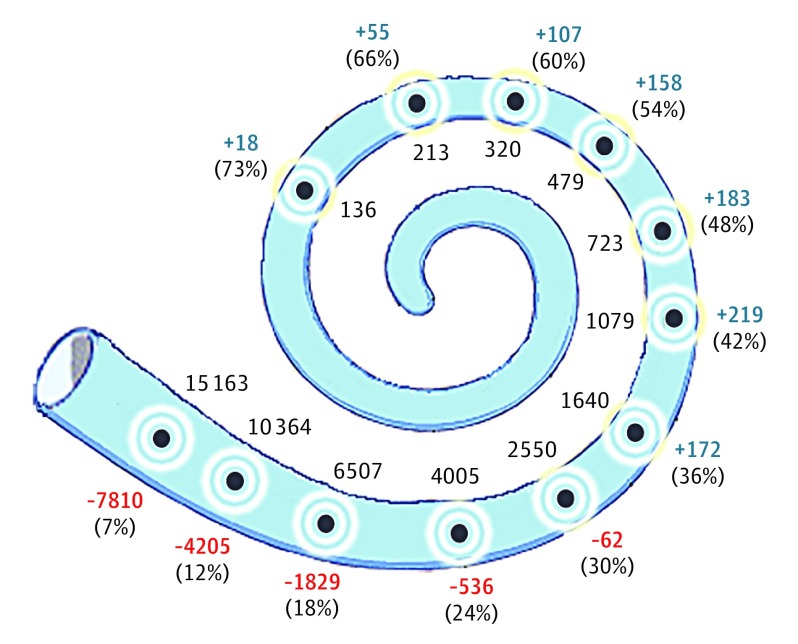
The most significant improvements were seen with pitch reversals between 2 notes greater than 1.65 semitones apart from one another, rather than smaller pitch intervals as we would have estimated based on earlier work from our research group. Our data also revealed that pitch-scaling mistakes were often made in the low- and high-frequency ranges. The observed benefits of the experimental map also had the greatest association with these frequency ranges, suggesting that the intervention corrected the pitch-place mismatch previously observed in the most apical and basal electrodes of the electrode array7 (Figure 5). Based on our findings, we cautiously suggest that an image-guided place-pitch map may provide a cochlear implant user with a more accurate representation of relative pitch intervals. We were concerned, however, that the image-guided approach would require electrode deactivations and large frequency shifts requiring greater acclimatization for cochlear implant users than we could provide in the present short-term study design. Speech perception was conserved and benefits in pitch performance were observed despite the short acclimation period and prior neuroplastic adaptations that had already occurred with long-term use of a clinical place-pitch map. We suspect that the significance of our pitch-scaling outcomes may be more robust with longer acclimation periods.
Figure 5. Place-Pitch Mismatch Among 17 Cochlear Implant Users.
Each gray dot depicts 1 of 12 electrode channels along the scala tympani. The black numbers along the medial edge of the cochlea report the mean theoretical center frequencies (hertz), using the modified Greenwood function4,5 and flat-panel computed tomographic imaging. Along the lateral aspect of the bony canal is the degree (hertz) to which the clinical map center frequency overshot (blue) or undershot (red) the image-guided map theoretical frequency. Within parenthesis is the aggregated percentage distance from the base of the organ of Corti for that particular electrode channel.
We also observed preservation of speech performance. Over the past couple of decades, CI-mediated speech perception has been optimized such that most cochlear implant users achieve excellent speech perception—a fact that raises the question of whether speech testing is the appropriate task to assess high-level cochlear implant performance. With this issue in mind, we found the speech perception outcomes in this study to be useful. Under our exclusion criteria, cochlear implant users could have up to 4 electrode channels deactivated13,14 and frequency shifts of up to 7 half-tone steps in either direction.15,16,17,18 Despite these liberal factors, CNC and BKB-SIN test performance did not worsen with the use of the image-guided map. However, vowel recognition scores slightly worsened with use of an image-guided map. We believe that cochlear implant users, when presented with a 0.4-second stimulus and a closed set of vowels to choose from, depended more heavily on acoustic familiarity provided by the long-term use of a place-pitch map than perceptual identification to complete this study task. However, further study is required to understand this observed difference (12%) in vowel recognition performance. It is possible that these vowel recognition outcomes might be obliterated under a longitudinal study design or longer acclimation period to the place-pitch map.
Limitations
There are several limitations to this study. Our image-guided frequency allocations were created using fixed-frequency bandwidths. By fixing the bandwidth, we ensured that the total bandwidth is not a confounding variable and that narrow filters (caused by desired frequencies close to 70 or 8500 Hz) were avoided. As a consequence of this approach, we accepted larger errors between the ideal MAESTRO center frequencies and the desired FPCT center frequencies for the most apical and basal electrodes and tolerated a greater number of electrode deactivations. In addition, we chose to use high-definition continuous interleaved sampling as the processing strategy for both the control and intervention maps because it offered a more direct comparison between image-guided and clinical maps already used. However, some of the participants were using the non–high-definition continuous interleaved sampling strategy in their daily lives as part of their processing strategy. Thus, using only high-definition continuous interleaved sampling for the reference condition with the default bandwidth may have provided a degree of novelty to the listener while eliminating the presence of fine structure on the lower channels (channels 1-4) as a potential confounding variable. Although the fine-structure–disabled channels would likely be altered in sound quality, the envelope-only channels should behave nearly identical to the users’ daily strategy.
Furthermore, we did not incorporate scalar excursions into our measurements. It is possible that the differences in distance due to scalar translocation skewed our frequency allocation calculations, and consequentially, produced less accurate place-pitch maps. We also used a short-term prospective study design. Although we observed an association between pitch perception and image-guided mapping even with such a short acclimation period, we recognize that longitudinal studies are necessary to determine whether these associations are persistent and transferrable.
Conclusions
We demonstrated an association between image-guided mapping and pitch perception in cochlear implant users. Pitch perception has been an elusive goal for many cochlear implant users.2,19 Despite the sensitive relationship between the location along the organ of Corti and the characteristic frequency, cochlear implant users are typically provided with a universal default place-pitch map on cochlear implant activation, which requires the users to adapt to the mismatch.20 However, the plasticity of the auditory system has limitations and, for many cochlear implant listeners, adaptation remains incomplete even after extended experience with a place-pitch map.21,22
If provided with a more accurate place-pitch map on device activation, cochlear implant users may not be required to overcome their expectations regarding perceptual sounds to as significant of a degree as they would with a default place-pitch map and, as a result, achieve maximum perceptual benefit at a much quicker rate. Further studies are under way to assess the benefits of a longer acclimation period with chronic stimulation over several months, which may help us to assess the full range of potential benefits of personalized image-guided cochlear implant mapping.
eTable. Demographic Information on Participants With Cochlear Implants
References
- 1.Limb CJ, Roy AT. Technological, biological, and acoustical constraints to music perception in cochlear implant users. Hear Res. 2014;308:13-26. doi: 10.1016/j.heares.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 2.Jiam NT, Caldwell MT, Limb CJ. What does music sound like for a cochlear implant user? Otol Neurotol. 2017;38(8):e240-e247. doi: 10.1097/MAO.0000000000001448 [DOI] [PubMed] [Google Scholar]
- 3.Greenwood DD. A cochlear frequency-position function for several species—29 years later. J Acoust Soc Am. 1990;87(6):2592-2605. doi: 10.1121/1.399052 [DOI] [PubMed] [Google Scholar]
- 4.Stakhovskaya O, Sridhar D, Bonham BH, Leake PA. Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol. 2007;8(2):220-233. doi: 10.1007/s10162-007-0076-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sridhar D, Stakhovskaya O, Leake PA. A frequency-position function for the human cochlear spiral ganglion. Audiol Neurootol. 2006;11(suppl 1):16-20. doi: 10.1159/000095609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearl MS, Roy A, Limb CJ. High-resolution secondary reconstructions with the use of flat panel CT in the clinical assessment of patients with cochlear implants. AJNR Am J Neuroradiol. 2014;35(6):1202-1208. doi: 10.3174/ajnr.A3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiam NT, Pearl MS, Carver C, Limb CJ. Flat-panel CT imaging for individualized pitch mapping in cochlear implant users. Otol Neurotol. 2016;37(6):672-679. doi: 10.1097/MAO.0000000000001060 [DOI] [PubMed] [Google Scholar]
- 8.Saeed SR, Selvadurai D, Beale T, et al. The use of cone-beam computed tomography to determine cochlear implant electrode position in human temporal bones. Otol Neurotol. 2014;35(8):1338-1344. doi: 10.1097/MAO.0000000000000295 [DOI] [PubMed] [Google Scholar]
- 9.Würfel W, Lanfermann H, Lenarz T, Majdani O. Cochlear length determination using cone beam computed tomography in a clinical setting. Hear Res. 2014;316:65-72. doi: 10.1016/j.heares.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 10.Lehiste I, Peterson GE. Linguistic considerations in the study of speech intelligibility. J Acoust Soc Am. 1959;31(3):280. doi: 10.1121/1.1907713 [DOI] [Google Scholar]
- 11.Bench J, Kowal A, Bamford J. The BKB (Bamford-Kowal-Bench) sentence lists for partially-hearing children. Br J Audiol. 1979;13(3):108-112. doi: 10.3109/03005367909078884 [DOI] [PubMed] [Google Scholar]
- 12.Tyler RS, Parkinson AJ, Woodworth GG, Lowder MW, Gantz BJ. Performance over time of adult patients using the Ineraid or nucleus cochlear implant. J Acoust Soc Am. 1997;102(1):508-522. [DOI] [PubMed] [Google Scholar]
- 13.Fu QJ, Shannon RV, Wang X. Effects of noise and spectral resolution on vowel and consonant recognition: acoustic and electric hearing. J Acoust Soc Am. 1998;104(6):3586-3596. doi: 10.1121/1.423941 [DOI] [PubMed] [Google Scholar]
- 14.Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110(2):1150-1163. doi: 10.1121/1.1381538 [DOI] [PubMed] [Google Scholar]
- 15.Tiffany WR, Bennett DN. Intelligibility of slow-played speech. J Speech Hear Res. 1961;4:248-258. [DOI] [PubMed] [Google Scholar]
- 16.Daniloff RG, Shriner TH, Zemlin WR. Intelligibility of vowels altered in duration and frequency. J Acoust Soc Am. 1968;44(3):700-707. doi: 10.1121/1.1911164 [DOI] [PubMed] [Google Scholar]
- 17.Nagafuchi M. Intelligibility of distorted speech sounds shifted in frequency and time in normal children. Audiology. 1976;15(4):326-337. doi: 10.3109/00206097609071792 [DOI] [PubMed] [Google Scholar]
- 18.Fu QJ, Shannon RV. Effects of electrode configuration and frequency allocation on vowel recognition with the Nucleus-22 cochlear implant. Ear Hear. 1999;20(4):332-344. doi: 10.1097/00003446-199908000-00006 [DOI] [PubMed] [Google Scholar]
- 19.Houtsma AJM. Pitch Perception In: Moore BCJ, ed. Hearing. Cambridge, MA: Academic Press; 1995:267-295. doi: 10.1016/B978-012505626-7/50010-8 [DOI] [Google Scholar]
- 20.Svirsky MA, Silveira A, Neuburger H, Teoh SW, Suárez H. Long-term auditory adaptation to a modified peripheral frequency map. Acta Otolaryngol. 2004;124(4):381-386. [PubMed] [Google Scholar]
- 21.Svirsky MA, Fitzgerald MB, Sagi E, Glassman EK. Bilateral cochlear implants with large asymmetries in electrode insertion depth: implications for the study of auditory plasticity. Acta Otolaryngol. 2015;135(4):354-363. doi: 10.3109/00016489.2014.1002052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagi E, Fu QJ, Galvin JJ III, Svirsky MA. A model of incomplete adaptation to a severely shifted frequency-to-electrode mapping by cochlear implant users. J Assoc Res Otolaryngol. 2010;11(1):69-78. doi: 10.1007/s10162-009-0187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Demographic Information on Participants With Cochlear Implants