Key Points
Question
Which individual facial grading item contributes most to the estimation of facial palsy–related quality of life?
Findings
In this cohort study of 920 patients with facial palsy, there were 529 with absent synkinesis (flaccid group) and 391 with synkinesis (nonflaccid group). Oral commissure movement with smile was the eFACE item that contributed most in the estimation of facial palsy–related quality of life in groups with facial palsy (relative weight, 0.108 for the flaccid group and 0.025 for the nonflaccid group).
Meaning
The present study demonstrates that the function of individual facial regions is not equally important for the estimation of facial palsy–related quality of life; the ability to smile is of greatest importance in patients with flaccid and nonflaccid facial palsy.
This cohort study examines the influence of individual facial grading items on the statistical estimation of facial palsy–related quality of life among patients with flaccid and nonflaccid facial palsy.
Abstract
Importance
Study of the association of regional facial dysfunction with quality of life will lead to a better understanding of quality of life in facial palsy.
Objective
To determine the association of regional facial dysfunction with facial palsy–related quality of life.
Design, Setting, and Participants
This retrospective cohort analysis included patients with flaccid and nonflaccid (synkinetic) facial palsy treated at a tertiary care facial nerve center; the flaccid facial palsy group included 529 patients, and the nonflaccid facial palsy group included 391 patients. Data were included from all patients with facial palsy who had an eFACE score and Facial Clinimetric Evaluation (FaCE) scale total score acquired at the same time from February 1, 2014, through October 31, 2017. Linear regression analysis was performed to calculate the amount of variance in quality of life explained by the severity of facial palsy (eFACE). A relative weight analysis was performed for the contribution of each individual eFACE item in estimating quality of life.
Main Outcomes and Measures
Facial palsy severity was measured using all 15 individual eFACE items (rated on a scale of 0 to 200, where 0 represents complete flaccidity, 100 represents a balanced aesthetic appearance, and 200 represents the worst imaginable hypertonia of a patient with synkinesis, with a transformation used for values from 101 to 200), and facial palsy–related quality of life was measured using the FaCE scale total score (range, 0 [worst] to 100 [best]).
Results
Data of 920 individual patients (59.5% female; mean [SD] age, 48.6 [16.6] years) were available. The eFACE composite score accounted for 21.2% of the quality-of-life variance in the flaccid group and 13.9% in the nonflaccid group. With the use of all 15 individual eFACE items, these proportions increased to 29.7% and 16.8%, respectively. In both groups, oral commissure movement with smile was found to be the most important contributing item (relative weight, 0.108 [95% CI, 0.075-0.148] for the flaccid group and 0.025 [95% CI, 0.005-0.052] for the nonflaccid group). Items related to the function of periocular muscles were found to be of low importance.
Conclusions and Relevance
The present study suggests that the function of individual facial regions is not equally important for estimating facial palsy–related quality of life. The ability to smile is of greatest importance among patients with flaccid and nonflaccid facial palsy. The true importance of periocular function in the estimation of quality of life should be studied further in future research.
Level of Evidence
NA.
Introduction
Facial palsy may range in severity from near-normal facial function to crippling facial disfigurement. Facial palsy severity has been recognized to influence patient-perceived quality of life to varying degrees, with some studies describing an association1,2,3 and others describing no such association.4,5 Measurement scales and study populations differ greatly between studies, and most studies that did not find an association between facial palsy severity and quality of life used the House-Brackmann Scale.6 Studies that have found an association between severity of facial palsy and quality of life have found the strength of the association to be weak1,2,3 and often used the composite score of the Sunnybrook Facial Grading System,7 thereby calculating the mean scores for disability of different facial zones. Most well-established facial grading systems apply a regional assessment of facial function. For example, the recently developed eFACE facial grading system assesses 15 items, including 4 static, 7 dynamic, and 4 synkinesis items, enabling generation of a composite score and static, dynamic, and synkinesis subscores.8 Although the composite score is an effective approach for presenting a gross impression of facial function, valuable information about regional facial function is lost in composite scores. Individual regional facial grading items may each have a different influence on perceived quality of life that is not represented in composite scores. The aim of this study was to examine the influence of individual facial grading items on the statistical estimation of facial palsy–related quality of life by means of relative weight analysis and thereby achieve a better understanding of the association of regional facial dysfunction with quality of life.
Methods
Patients and Study Overview
We selected a retrospective cohort of all patients with facial palsy treated at Massachusetts Eye and Ear Infirmary, Boston, from February 1, 2014, through October 31, 2017. Criteria for inclusion in this study were the presence of an eFACE assessment and a Facial Clinimetric Evaluation (FaCE) scale score obtained at the same time. Patients were excluded if clinical notes were missing, the FaCE scale was incomplete, or they had bilateral facial palsy because the eFACE system is primarily intended for unilateral facial palsy. This study was approved by the institutional review board of the Massachusetts Eye and Ear Infirmary, and all patients provided written informed consent.
Since its development in early 2014, the eFACE facial grading system has been our standard measure of clinician-graded facial function. The eFACE consists of 4 static, 7 dynamic, and 4 synkinesis items.8 Ratings of each item are used to calculate static, dynamic, and synkinesis subscores and an eFACE total score (range, 0 [worst] to 100 [best]). Nine eFACE items are rated on a scale of 0 (worst) to 100 (best). Four items regarding static characteristics and 2 items regarding dynamic characteristics relate to the nasolabial fold (NLF) and are rated on a scale of 0 to 200, where 0 represents complete flaccidity, 100 represents a balanced aesthetic appearance, and 200 represents the worst imaginable hypertonia of a patient with synkinesis. When calculating the eFACE total score or subscores, a transformation is used for values from 101 to 200. These hyperkinetic values are transformed to their absolute distance from 200 (200 − item score) to be comparable to the flaccid values. The same transformation into standardized eFACE scores was used for all analyses.
We routinely use the patient-reported FaCE scale1 as a measure of facial palsy–related quality of life at our institution. The FaCE scale consists of 15 items with 5-point Likert scale–type answers that are transformed to a FaCE total score (range, 0 [worst] to 100 [best]).
The eFACE and FaCE scale assessments are routinely performed during the initial consultation and thereafter, by indication. If more than 1 pair of FaCE and eFACE scores was present, the assessments of the senior author (T.A.H.) were used in this study for consistency. In addition, we searched medical records for general patient characteristics.
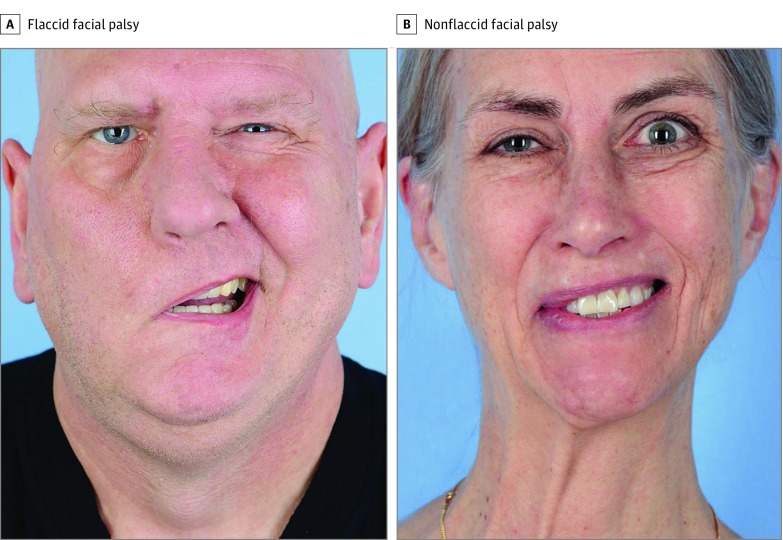
We divided the cohort between a group in whom synkinesis was absent (flaccid group) and a group in whom synkinesis was present (nonflaccid group), as determined by the eFACE synkinesis score, for all analyses. Patients with a synkinesis score of 100 were included in the flaccid group, and patients with a synkinesis score ranging from 0 to 99 were included in the nonflaccid group. Representative photographs of patients from each group are presented in the Figure.
Figure. Representative Photographs of Patients With Right-Sided Facial Palsy.
Statistical Analysis
Descriptive statistics are given using numbers and frequencies, medians and interquartile ranges, and means and SDs when appropriate. We performed linear regression analyses to study the size of the association between the eFACE and FaCE scale total scores for both groups. The same analyses were also performed for the association of individual eFACE items with the FaCE scale total score. In linear regression, the model correlation coefficient (R2) represents the proportion of variance in the output variable (FaCE scale total score) that can be explained by the input variable (the eFACE total score or the 15 individual eFACE item subscores).
When determining the relative weight (defined as the proportionate contribution to the correlation coefficient) of individual eFACE items, multicollinearity may occur. Multicollinearity is a phenomenon whereby an estimator variable can be derived from another estimator variable, which might influence the regression coefficients of the estimator variables in a multiple regression model. The technique of relative weight analysis described by Johnson9 overcomes this problem and was used. In this technique, noncorrelating estimator variables are derived from the original correlating variables and are used to calculate the proportionate contribution of each individual original input variable.9 We determined the significance of relative weights based on the 95% CI.10 Relative weight estimation was performed using R software (version 3.4; R Foundation for Statistical Computing), and all other analyses were performed using SPSS software (version 25.0; IBM Corporation).
Results
The eFACE scores of 1304 unique patients were acquired during the study period. For 975 patients, a FaCE scale score was available from the same time. Fifty-five patients were excluded owing to missing data regarding clinical notes and facial photographs because some FaCE scale questions were not filled out or because they had bilateral facial palsy, leaving data from 920 patients (70.6%) available for this study (373 men [40.5%] and 547 women [59.5%]; mean [SD] age, 48.6 [16.6] years). The flaccid group included 529 patients; the nonflaccid group, 391. In the flaccid group, 282 patients (53.3%) were women, and the mean (SD) age was 49.5 (17.4) years. In the nonflaccid group, 265 patients (67.8%) were women, and the mean (SD) age was 47.3 (15.5) years. Median (interquartile range) eFACE total scores, 15 individual eFACE subscores, and FaCE scale total scores for both groups are presented in Table 1.
Table 1. Spread of Individual eFACE Items in the Flaccid and Nonflaccid Groups.
| Itema | Facial Palsy Group, Median (IQR) Score | |
|---|---|---|
| Flaccid (n = 529) | Nonflaccid (n = 391) | |
| eFACE total score | 51 (36-61) | 72 (65-83) |
| eFACE individual items | ||
| Resting browb | 85 (69-100) | 90 (80-100) |
| Resting PFWb | 80 (61-91) | 87 (75-98) |
| NLF depth at restb | 77 (51-91) | 87 (78-100) |
| OC at restb | 80 (57-94) | 90 (82-100) |
| Brow elevation | 4 (0-62) | 39 (8-89) |
| Gentle eye closure | 81 (58-97) | 100 (91-100) |
| Full eye closure | 94 (75-100) | 100 (100-100) |
| NLF depth with smileb | 38 (0-87) | 88 (75-100) |
| OC movement with smile | 7 (0-61) | 32 (10-76) |
| NLF orientation with smileb | 37 (0-82) | 78 (56-90) |
| Lower lip movement with /ee/ pronunciation | 54 (28-86) | 67 (38-89) |
| Ocular synkinesis | 100 (100-100) | 67 (30-93) |
| Midfacial synkinesis | 100 (100-100) | 78 (56-92) |
| Mentalis dimpling | 100 (100-100) | 74 (49-93) |
| Platysmal synkinesis | 100 (100-100) | 79 (52-94) |
| FaCE scale total score | 45 (31-59) | 67 (58-81) |
Abbreviations: FaCE, Facial Clinimetric Evaluation; IQR, interquartile range; NLF, nasolabial fold; OC, oral commissure, PFW, palpebral fissure width.
Unless otherwise indicated, transformed scores range from 0 (worst) to 100 (best).
Scores range from 0 to 200, where 0 represents complete flaccidity, 100 represents a balanced aesthetic appearance, and 200 represents the worst imaginable hypertonia of a patient with synkinesis.
Two linear regression analyses were performed with the eFACE total score as the input variable and FaCE scale total score as output variable. For the flaccid group, the model correlation was 0.212 (P < .001) (eFigure 1 in the Supplement). For the nonflaccid group, the model correlation was 0.139 (P < .001) (eFigure 2 in the Supplement). In a multiple regression model, with all 15 eFACE items as input variables instead of the eFACE total score and the FaCE scale total score as the output variables, the model R2 value increased with 0.085 to 0.297 (P < .001) for the flaccid group. In the nonflaccid group, the model R2 value increased with 0.029 to 0.168 (P < .001).
Results of the relative weight analysis showed that, for both groups, oral commissure movement with smile was the most important item in the estimation of the FaCE scale total score (relative weight, 0.108 [95% CI, 0.075-0.148] for the flaccid group and 0.025 [95% CI, 0.005-0.052] for the nonflaccid group). All 7 eFACE dynamic items (brow elevation [relative weight, 0.038; 95% CI, 0.021-0.062], NLF depth with smile [relative weight, 0.038; 95% CI, 0.023-0.057], NLF orientation with smile [relative weight, 0.037; 95% CI, 0.024-0.053], lower lip movement with /ee/ pronunciation [relative weight, 0.028; 95% CI, 0.011-0.054], gentle eye closure [relative weight, 0.010; 95% CI, 0.004-0.020], full eye closure [relative weight, 0.006; 95% CI, 0.001-0.012], and oral commissure with smile [relative weight, 0.108; 95% CI, 0.075-0.148]) and 2 static eFACE items (NLF depth at rest [relative weight, 0.015; 95% CI, 0.006-0.030] and oral commissure at rest [relative weight, 0.008; 95% CI, 0.002-0.018]) were significant in the estimation of the FaCE scale total score in the flaccid group. In the nonflaccid group, the eFACE items oral commissure with smile (relative weight, 0.025; 95% CI, 0.005-0.052), platysmal synkinesis (relative weight, 0.024; 95% CI, 0.002-0.055), and lower lip movement with /ee/ pronunciation (relative weight, 0.023; 95% CI, 0.003-0.056) were significant in the estimation of the FaCE scale total score (Table 2 and eFigure 3 in the Supplement).
Table 2. Relative Weights and 95% CIs in the Flaccid and Nonflaccid Groups.
| Item | Relative Weight (95% CI) | |
|---|---|---|
| Flaccid Group (n = 529) | Synkinesis Group (n = 391) | |
| eFACE static variables | ||
| Resting brow | 0.006 (−0.001 to 0.023) | 0.004 (−0.010 to 0.027) |
| Resting PFW | 0.004 (−0.001 to 0.013) | 0.006 (−0.009 to 0.026) |
| OC at rest | 0.008 (0.002 to 0.018)a | 0.002 (−0.022 to 0.006) |
| NLF depth at rest | 0.015 (0.006 to 0.030)a | 0.010 (−0.007 to 0.030) |
| eFACE dynamic variables | ||
| Brow elevation | 0.038 (0.021 to 0.062)a | 0.017 (−0.001 to 0.040) |
| Gentle eye closure | 0.010 (0.004 to 0.020)a | 0.002 (−0.022 to 0.006) |
| Full eye closure | 0.006 (0.001 to 0.012)a | 0.003 (−0.012 to 0.013) |
| NLF depth with smile | 0.038 (0.023 to 0.057)a | 0.008 (−0.008 to 0.032) |
| OC movement with smile | 0.108 (0.075 to 0.148)a | 0.025 (0.005 to 0.052)a |
| NLF orientation with smile | 0.037 (0.024 to 0.053)a | 0.014 (−0.002 to 0.038) |
| Lower lip movement with /ee/ | 0.028 (0.011 to 0.054)a | 0.023 (0.003 to 0.056)a |
| eFACE synkinesis variables | ||
| Ocular synkinesis | NA | 0.011 (−0.005 to 0.034) |
| Midface synkinesis | NA | 0.017 (−0.003 to 0.045) |
| Mentalis dimpling | NA | 0.003 (−0.021 to 0.007) |
| Platysmal synkinesis | NA | 0.024 (0.002 to 0.055)a |
Abbreviations: NA, not available; NLF, nasolabial fold, OC, oral commissure, PFW, palpebral fissure width.
Indicates items contribute significantly based on the 95% CI.
Discussion
The aim of this study was to better understand the association of individual components of regional facial function with estimating quality of life in patients with facial palsy. We found that facial palsy–related quality of life is better estimated by regional facial function items than a composite score of facial function. In addition, quality of life can be estimated better in patients with flaccid compared with nonflaccid facial palsy.
Overall facial palsy severity is associated with facial palsy–related quality of life more closely in patients who do not have synkinesis (the flaccid group) compared with patients in whom synkinesis is present (nonflaccid group), with an explained variance of 21.2% and 13.9%, respectively. Estimating quality of life using all 15 eFACE items increased the model correlation to 29.7% in the flaccid group but only 16.8% in the nonflaccid group. This observation demonstrates that use of the regional facial function items instead of composite scores helps to better estimate facial palsy–related quality of life.
Although the proportion of variance explained may seem low (ie, 13.9%-29.7%), these levels are in line with findings in other studies of disease severity and disease-specific quality of life. Compared with other variables estimating disease-specific quality of life in facial palsy, our R2 values are relatively large. In a study investigating age, sex, and etiology and duration of palsy,3 the total proportion of variance was found to be 3.8% compared with 13.9% to 29.7% explained solely by facial palsy severity in the present study. Other factors influencing quality of life in facial palsy are yet to be unraveled.
The difference in explained variance between the flaccid and nonflaccid groups suggests that expert-rated severity of synkinesis correlates poorly with patient-reported severity of synkinesis. A future study of this association, with the use of the synkinesis-specific Synkinesis Assessment Questionnaire,11 for example, on an individual regional item level would provide additional insights. In addition, the stronger correlation between eFACE scores and FaCE scale total score in the flaccid group could partly be owing to the lack of specific synkinesis-related questions included in the FaCE scale.1 Development of a synkinesis-specific extension to the FaCE scale may improve understanding of quality of life in a population with nonflaccid facial palsy.
The single most important contributing eFACE item in the flaccid group was found to be oral commissure movement with smile. The third and fourth most important contributing factors were NLF depth with smile and NLF orientation with smile. Impairment in these items may be owing to lack of smile but also to midfacial flaccidity, which results in problems with speaking, eating, and drinking. Together, these 3 items account for two-thirds of the total R2 value, meaning that midfacial flaccidity is the single most important estimator of facial palsy–related quality of life. The relative importance of oral commissure movement with smile compared with oral commissure at rest also emphasizes the importance of a dynamic facial reconstruction for patients with flaccid facial palsy (Table 2 and eFigure 3 in the Supplement).
In the nonflaccid group, a more balanced contribution of each individual item to facial palsy–related quality of life was seen, although only 3 items contributed significantly. Although the most important contributor again was oral commissure movement with smile, the contribution of the items related to the NLF in the smile and midfacial synkinesis were much less important and not statistically significant. Patients with synkinesis usually have a good resting tone of the face and generally have fewer issues with speaking, eating, or drinking. Therefore, the inability to produce a balanced and sufficient smile likely represents the cause of quality-of-life impairment in this patient group.12 Only 1 synkinesis item (platysmal synkinesis) was found to have a significant effect on facial palsy–related quality of life. The relatively low importance of the other 3 synkinesis items suggests a poor correlation between expert-rated and patient-experienced severity of synkinesis.
Of interest, lower lip movement with /ee/ pronunciation contributes significantly in both groups. Thus, asymmetry of the lower lip has a relatively high influence on quality of life in our sample. A low score on this item indicates weakness of the lower lip in both groups of patients because hypertonicity of the lower lip is very uncommon. Even patients with synkinesis are more likely to have persistent lower lip weakness after facial nerve insult and aberrant regeneration. Asymmetry of the lower lip can be effectively treated with botulinum toxin injections or depressor labii inferioris myectomy to the contralateral healthy lower lip.13,14,15 These interventions improve the esthetic appearance of the smile but do not restore movement. Several surgical techniques for dynamic restoration of the lower lip have been published,16 but only 1 nonrandomized study of 18 patients17 compared the effects of contralateral botulinum toxin injections with the effects of ipsilateral digastric muscle transfer. In that study,17 recipients of digastric muscle transfer were more likely to be dissatisfied with their outcome. Furthermore, pretreatment and posttreatment quality-of-life measurements are not available in the literature. Future prospective and randomized studies using validated preoperative and postoperative quality-of-life outcome measures are needed to understand the benefits of static vs dynamic treatment of lower lip asymmetry.
Items related to the function of the periocular muscles (gentle eye closure, full eye closure, and ocular synkinesis) were found to be of low importance in the flaccid and the nonflaccid groups. The effect of periocular function on quality of life has been investigated previously.18 However, the relative importance of individual regional items that make up the composite score has not. Clinically, we expected to find these items to be of greater importance because impairment in eye closure can cause great discomfort. We considered that this low weighting of periocular items may be owing to prior periocular surgery and so cross-referenced all treatment records and a consecutive sample of 50 medical records and found only a 3% to 6% rate of prior surgery. This finding leaves room for further investigation because we do not fully understand why these items are less important. Alternatively, the FaCE scale only incorporates 3 items related to ocular problems, all aimed at problems in patients with flaccid facial palsy.1 Thus, the use of the FaCE scale could have partly masked the true extent of the eye-related disability, especially in those with synkinesis.
In a recent study,19 the relative contribution of all eFACE items was compared with expert-rated disfigurement. Of the 5 significantly contributing items that were found, 3 were static (NLF depth at rest, oral commissure at rest, and palpebral fissure at rest). This finding may seem contrary to the finding of the present study that dynamic variables are of much greater importance in estimating quality of life. We hypothesize that dynamic variables are of greater importance in estimating quality of life because of the influence that dynamic asymmetries may have on interpersonal communication and the expression of emotion. A direct comparison is limited because of the difference between patient-reported quality of life vs expert-rated disfigurement constructs. Experts may be focused on different items of the eFACE, such as symmetry at rest, because of the clinical problems they know might arise from certain regional dysfunctions. In another study,20 layperson perception of global and regional facial palsy was studied. Of the regional facial palsies, zygomatic and/or buccal facial palsy generally scored worst on domains such as perceived normality, distress, intelligence, and trustworthiness as rated by a layperson. These negative impressions can cause problems in social interaction for patients, which is known to negatively influence quality of life.21 The importance of the midface variables may be related to this zone’s importance in interacting with other people.
Limitations
A limitation of our study is that we used clinician grading to determine severity of facial palsy. Although the eFACE instrument has been shown to be a reliable and valid method of assessing facial palsy severity,22,23,24 the use of scores by different clinicians can introduce an element of error. However, all eFACE assessments in our study sample were performed by clinicians who are experienced in the whole spectrum of facial palsy thereby limiting the degree of error. Data of 920 patients (70.6%) were included in this study, presenting a potential source of selection bias. The patients excluded did not differ from the included patients in characteristics such as sex and age. The FaCE scale total scores of the 55 patients excluded because of missing data were relatively high compared with the scores of the included patients. These high quality-of-life scores suggests that the present cohort might represent a slightly lower quality of life than other cohorts of patients with facial palsy because of the tertiary care facial nerve center setting. However, the excluded patients had relatively high eFACE scores as well, suggesting that the associations found can be generalized.
Another limitation of our study is the constitution of the cohort studied. Our tertiary care center delivers care to the full spectrum of patients with facial palsy. This spectrum often includes patients who were not helped elsewhere or who referred themselves to our center. These patients may present with problems regarding their facial function when findings of the clinical examination of the face are nearly normal. We hypothesize that quality of life in this patient subgroup may be disproportionately low. We included these patients because we set out to study the full range of facial palsy and the effect of different regional impairments. For that reason, patients with minor problems in only 1 region should be included.
Conclusions
Our data demonstrate the importance and value of regional assessment of facial dysfunction in facial palsy, instead of the use of composite scores. The study of regional facial function items provides a better understanding of facial palsy–specific quality of life. The relative importance of regional facial function items can point a direction toward future clinical and research aims. The present study shows a discrepancy in the relative importance of regional facial function items between facial palsy–related quality of life and expert-rated disfigurement.
eFigure 1. Scatterplot of eFACE and FaCE Scale Total Scores for the Flaccid Group
eFigure 2. Scatterplot of eFACE and FaCE Scale Total Scores for the Nonflaccid Group
eFigure 3. Relative Weights of Individual eFACE Items to FaCE Scale Total Score
References
- 1.Kahn JB, Gliklich RE, Boyev KP, Stewart MG, Metson RB, McKenna MJ. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope. 2001;111(3):32-37. doi: 10.1097/00005537-200103000-00005 [DOI] [PubMed] [Google Scholar]
- 2.Ng JH, Ngo RY. The use of the Facial Clinimetric Evaluation Scale as a patient-based grading system in Bell’s palsy. Laryngoscope. 2013;123(5):1256-1260. doi: 10.1002/lary.23790 [DOI] [PubMed] [Google Scholar]
- 3.Kleiss IJ, Hohman MH, Susarla SM, Marres HA, Hadlock TA. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE Scale: a retrospective cohort study. Clin Otolaryngol. 2015;40(6):651-656. doi: 10.1111/coa.12434 [DOI] [PubMed] [Google Scholar]
- 4.Lassaletta L, Alfonso C, Del Rio L, Roda JM, Gavilan J. Impact of facial dysfunction on quality of life after vestibular schwannoma surgery. Ann Otol Rhinol Laryngol. 2006;115(9):694-698. doi: 10.1177/000348940611500908 [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Fung K, Lownie SP, Parnes LS. Assessing impairment and disability of facial paralysis in patients with vestibular schwannoma. Arch Otolaryngol Head Neck Surg. 2007;133(1):56-60. doi: 10.1001/archotol.133.1.56 [DOI] [PubMed] [Google Scholar]
- 6.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147. doi: 10.1177/019459988509300202 [DOI] [PubMed] [Google Scholar]
- 7.Ross BG, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. Otolaryngol Head Neck Surg. 1996;114(3):380-386. doi: 10.1016/S0194-5998(96)70206-1 [DOI] [PubMed] [Google Scholar]
- 8.Banks CA, Bhama PK, Park J, Hadlock CR, Hadlock TA. Clinician-graded electronic facial paralysis assessment: the eFACE. Plast Reconstr Surg. 2015;136(2):223e-230e. doi: 10.1097/PRS.0000000000001447 [DOI] [PubMed] [Google Scholar]
- 9.Johnson JW. A heuristic method for estimating the relative weight of predictor variables in multiple regression. Multivariate Behav Res. 2000;35(1):1-19. doi: 10.1207/S15327906MBR3501_1 [DOI] [PubMed] [Google Scholar]
- 10.Tonidandel S, Lebreton JM, Johnson JW. Determining the statistical significance of relative weights. Psychol Methods. 2009;14(4):387-399. doi: 10.1037/a0017735 [DOI] [PubMed] [Google Scholar]
- 11.Mehta RP, WernickRobinson M, Hadlock TA. Validation of the Synkinesis Assessment Questionnaire. Laryngoscope. 2007;117(5):923-926. doi: 10.1097/MLG.0b013e3180412460 [DOI] [PubMed] [Google Scholar]
- 12.Coulson SE, O'dwyer NJ, Adams RD, Croxson GR. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol. 2004;25(6):1014-1019. doi: 10.1097/00129492-200411000-00026 [DOI] [PubMed] [Google Scholar]
- 13.Chen CK, Tang YB. Myectomy and botulinum toxin for paralysis of the marginal mandibular branch of the facial nerve: a series of 76 cases. Plast Reconstr Surg. 2007;120(7):1859-1864. doi: 10.1097/01.prs.0000287136.22709.77 [DOI] [PubMed] [Google Scholar]
- 14.Hussain G, Manktelow RT, Tomat LR. Depressor labii inferioris resection: an effective treatment for marginal mandibular nerve paralysis. Br J Plast Surg. 2004;57(6):502-510. doi: 10.1016/j.bjps.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Tulley P, Webb A, Chana JS, et al. Paralysis of the marginal mandibular branch of the facial nerve: treatment options. Br J Plast Surg. 2000;53(5):378-385. doi: 10.1054/bjps.2000.3318 [DOI] [PubMed] [Google Scholar]
- 16.Terzis JK, Kalantarian B. Microsurgical strategies in 74 patients for restoration of dynamic depressor muscle mechanism: a neglected target in facial reanimation. Plast Reconstr Surg. 2000;105(6):1917-1931. doi: 10.1097/00006534-200005000-00001 [DOI] [PubMed] [Google Scholar]
- 17.Butler DP, Leckenby JI, Miranda BH, Grobbelaar AO. Botulinum toxin therapy versus anterior belly of digastric transfer in the management of marginal mandibular branch of the facial nerve palsy: a patient satisfaction survey. Arch Plast Surg. 2015;42(6):735-740. doi: 10.5999/aps.2015.42.6.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henstrom DK, Lindsay RW, Cheney ML, Hadlock TA. Surgical treatment of the periocular complex and improvement of quality of life in patients with facial paralysis. Arch Facial Plast Surg. 2011;13(2):125-128. doi: 10.1001/archfacial.2011.9 [DOI] [PubMed] [Google Scholar]
- 19.Banks CA, Jowett N, Hadlock CR, Hadlock TA. Weighting of facial grading variables to disfigurement in facial palsy. JAMA Facial Plast Surg. 2016;18(4):292-298. doi: 10.1001/jamafacial.2016.0226 [DOI] [PubMed] [Google Scholar]
- 20.Li MK, Niles N, Gore S, Ebrahimi A, McGuinness J, Clark JR. Social perception of morbidity in facial nerve paralysis. Head Neck. 2016;38(8):1158-1163. doi: 10.1002/hed.24299 [DOI] [PubMed] [Google Scholar]
- 21.Macgregor FC. Facial disfigurement: problems and management of social interaction and implications for mental health. Aesthetic Plast Surg. 1990;14(4):249-257. doi: 10.1007/BF01578358 [DOI] [PubMed] [Google Scholar]
- 22.Banks CA, Jowett N, Hadlock TA. Test-retest reliability and agreement between in-person and video assessment of facial mimetic function using the eFACE facial grading system. JAMA Facial Plast Surg. 2017;19(3):206-211. doi: 10.1001/jamafacial.2016.1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong LSH, Eviston TJ, Low TH, Hasmat S, Coulson SE, Clark JR. Validation of the clinician-graded Electronic Facial Paralysis Assessment. Plast Reconstr Surg. 2017;140(1):159-167. doi: 10.1097/PRS.0000000000003447 [DOI] [PubMed] [Google Scholar]
- 24.Gaudin RA, Robinson M, Banks CA, Baiungo J, Jowett N, Hadlock TA. Emerging vs time-tested methods of facial grading among patients with facial paralysis. JAMA Facial Plast Surg. 2016;18(4):251-257. doi: 10.1001/jamafacial.2016.0025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Scatterplot of eFACE and FaCE Scale Total Scores for the Flaccid Group
eFigure 2. Scatterplot of eFACE and FaCE Scale Total Scores for the Nonflaccid Group
eFigure 3. Relative Weights of Individual eFACE Items to FaCE Scale Total Score