Abstract
We evaluated the relation of fruit and vegetable consumption, including specific fruits and vegetables, with incident breast cancer characterized by menopausal status, hormone receptor status, and molecular subtypes. Fruit and vegetable consumption, cumulatively averaged across repeated, validated questionnaires, was examined in relation to risk of invasive breast cancer among 182,145 women initially aged 27–59y in the Nurses’ Health Study (NHS, 1980–2012) and NHSII (1991–2013). Cox proportional hazards regression, adjusted for known risk factors, was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), and assessed tumors by hormone receptor status and molecular subtypes. We prospectively documented 10,911 invasive breast cancer cases. Greater intake of total fruits and vegetables, especially cruciferous and yellow/orange vegetables, was associated with significantly lower breast cancer risk (>5.5 versus ≤2.5 servings/day HR=0.89, 95%CI=0.83–0.96; Ptrend=0.006). Intake of total vegetables was especially associated with lower risk of estrogen receptor negative tumors (HR per 2 additional servings/day as a continuous variable=0.84, 95%CI=0.77–0.93; Pheterogeneity=0.02). Among molecular subtypes, higher intake of total fruits and vegetables (HR per 2 additional servings/day as a continuous variable) was most strongly associated with lower risk of human epidermal growth factor receptor 2 (HER2)-enriched (HR=0.79, 95%CI=0.67–0.93), basal-like (HR=0.84, 95%CI=0.72–0.97), and luminal A (HR=0.94, 95%CI=0.89–0.99), but not with luminal B tumors (Pheterogeneity=0.03). In conclusion, our findings support that higher intake of fruits and vegetables, and specifically cruciferous and yellow/orange vegetables, may reduce the risk of breast cancer, especially those that are more likely to be aggressive tumors.
Introduction
Worldwide, breast cancer is the most common cancer in women and the second leading cause of cancer death, with variation in incidence around the world.1 Breast cancer is a heterogeneous disease representing multiple tumor types with specific pathological features and biological behaviors, different responses to therapeutics, and variable survival.2 Fruits and vegetables are rich in potentially anti-carcinogenic nutrients including fiber, vitamins C and E, carotenoids, and other bioactive substances,3–5 and higher intakes have been hypothesized to reduce cancer risk. Despite inconsistencies across individual studies of breast cancer risk,6–20 inverse associations with intake of vegetables, but not fruits, were observed in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.19 In contrast, higher intake of fruits was associated with lower risk of breast cancer in a meta-analysis including 15 prospective cohort studies.21 Evidence of associations by estrogen receptor (ER) status and molecular subtypes of breast cancer is sparse, with inconsistent results.10, 12,15,17,19, 20, 22 In a large pooled analysis of 20 studies20 as well as the EPIC cohort,19 total vegetable consumption was particularly associated with ER-negative, but not ER-positive tumors. In our prior analysis in the Nurses’ Health Study (NHS), healthier dietary patterns were suggestively associated with a lower risk of human epidermal growth factor receptor 2 (HER2)-enriched breast cancer, and this appeared to be due to high consumption of fruit.22 Given that fruits and vegetables vary widely in nutrients, examination of specific fruit and vegetable subgroups is also important; however, little is known about the relationship with breast cancer.11, 15, 17, 20, 23 In some studies, inverse associations have been observed with ER-negative tumors, including apples/pears, peaches/nectarines, strawberries, and lettuce in the pooled study,20 and berries and peaches/nectarines in the NHS.23 In a previous publication among younger women in the NHSII, we observed a suggested but non-significant inverse association between pre-menopausal total fruit consumption and incident breast cancer.24 Although prior assessments were suggestive, they were limited in power, particularly for specific fruits and vegetables, as well as aggressive subtypes of breast cancer.
Pooling data from the NHS and NHSII cohorts allowed us to evaluate the relation of fruit and vegetable consumption, including specific fruits and vegetables, with incident breast cancer in a large group of women with up to nine assessments of diet and a large number of breast cancer cases characterized by menopausal status, hormone receptor status, and molecular subtypes.
SUBJECTS AND METHODS
Study Population
As ongoing prospective cohort studies of US female registered nurses, the NHS started in 1976 with 121,700 women aged 30–55 years, and the NHSII began in 1989 with 116,429 women aged 25–42 years. Participants first completed semi-quantitative food frequency questionnaires (SFFQ) beginning in 1980 (NHS, n=98,047) or 1991 (NHSII, n=97,813). Participants were excluded for implausible total energy intake (<600 or >3500 kcal/day), cancer diagnosis (except non-melanoma skin cancer) before the baseline questionnaire, or having left blank >10 items (NHS) or >70 items (NHSII) on the baseline SFFQ, or left blank all fruit and/or vegetable items, leaving 88,301 women in the NHS and 93,844 women in the NHSII for analysis. The cumulative follow-up rates exceed 95% in both cohorts. This study was approved by the Institutional Review Board at Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health. Consent was implied by the return of completed questionnaires.
Dietary Assessment
In the NHS, participants completed a 61-item SFFQ in 1980, followed by SFFQs expanded to 116–130 items in 1984, 1986, and every four years thereafter. In the NHSII, in 1991 and every four years thereafter, dietary intake was measured with a ~130-item SFFQ (questionnaires available at http://www.nurseshealthstudy.org/participants/questionnaires). Questions included the frequency of consumption for a specified serving of each food item in nine categories from “never or less than once/month” through “6 or more times/day.” The validity of the SFFQ has been documented by comparison with more detailed methods25–27 and biomarkers of intake.26 Intake of fruits and vegetables in these cohorts has been associated with lower risk of other diseases, including diabetes28 and coronary heart disease.29
In the 1980 SFFQ, we calculated total fruit consumption by summing the consumption of five individual fruits including apples/pears, oranges, peaches/plums/apricots, bananas, and other fruits. Total vegetable consumption was calculated by summing the consumption of ten individual vegetable items including green beans, broccoli, cabbage/cauliflower/Brussels sprouts, carrots, corn, spinach/other greens, peas/lima beans, winter squash, sweet potatoes, and tomatoes/tomato juice. Ten individual fruits were asked consistently since 1984 in the NHS and 1991 in the NHSII: grapes/raisins, peaches/plums/apricots, prunes, bananas, cantaloupe/melon, apples/pears, oranges, grapefruits, strawberries, and blueberries (Appendix Table 1). We calculated total fruit intake (not including juices) by summing the intake amounts of all individual fruits that were asked consistently or sporadically during follow-up. Individual vegetables have also been reported in the Appendix Table 1. We consistently asked about most of them in SFFQs, except mushroom, onion, beet, alfalfa sprouts, and sauerkraut, which we inquired about sporadically. Individual items were summed to create total vegetables (not including potatoes) (Appendix Table 1). Total fruit juice intake was calculated by summing the intake levels of juices reported in SFFQs. We did not inquire about type or brand of fruit juices in the SFFQs. Five subgroups of vegetables included green leafy vegetables, yellow/orange vegetables, tomatoes, cruciferous vegetables, and other vegetables. Fruits and vegetables were also grouped by the content of vitamin C (≥40 mg/100g), α-carotene (≥3000 mcg/100g), β-carotene (≥3000 mcg/100g), and lutein (≥10 mg/100g).29–32
Identification of Breast Cancer Cases and Molecular Subtypes of Breast Cancer
Cases of breast cancer were identified on biennial follow-up questionnaires; the National Death Index was searched for nonresponders. Participants (or next of kin) were asked for permission to obtain relevant hospital records and pathology reports. Because accuracy was high for self-reporting (99%), breast cancer diagnoses (n=883) without medical records were included in the analysis. We collected breast cancer tissue for approximately 70% of cases and constructed tumor microarrays (TMA) to assess tumor characteristics by immunohistochemistry; details are described elsewhere.22, 33, 34 Immunohistochemical staining, with results read manually by a study pathologist, was performed for ER, progesterone receptor (PR), HER2, cytokeratin 5/6 (CK5/6), and epidermal growth factor receptor (EGFR). ER, PR, and HER2 status for cases without TMAs was extracted from medical records. Molecular subtypes were defined according to ER, PR, HER2, CK5/6, and EGFR status in combination with histologic grade: Luminal A (ER-positive and/or PR-positive, and HER2-negative with grade 1 or 2); luminal B (ER-positive, and/or PR-positive, and HER2-positive; or ER-positive, and/or PR-positive, and HER2-negative with grade 3); HER2-enriched (ER-negative, PR-negative, and HER2-positive); basal-like (ER-negative, PR-negative, HER2-negative, and CK5/6-positive and/or EGFR-positive); unclassified tumors lacked expression for all five markers.
Assessment of other variables
Data on potential breast cancer risk factors were obtained from the biennial questionnaires, including age, weight, history of benign breast disease, family history of breast cancer, smoking, ages at menarche, menopause, and first birth, parity, menopausal status, postmenopausal hormone use, oral contraceptive use, alcohol consumption, and physical activity, updated with the most recent information, if available. Body mass index (BMI) at age 18 and height were obtained from the baseline questionnaire. Weight change since age 18 was calculated at each questionnaire cycle.
Statistical Analysis
Data from the NHS and NHSII were pooled. Participants contributed person-years from the date of return of the baseline SFFQ (NHS, 1980; NHSII, 1991) to the date of any cancer diagnosis except non-melanoma skin cancer, death, or end of follow-up (NHS, June 1, 2012; NHSII, June 1, 2013), whichever occurred first. To minimize within-person variation and reduce measurement error in exposures, we calculated the cumulative average of dietary intake by averaging repeated measures through follow-up. We categorized total and subgroups of fruits and vegetables in five groups based on the frequency of intake. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) in the pooled data, using the lowest category of intake as the reference. Models stratified by age in months, calendar year of the current questionnaire cycle, and cohort. Multivariable models included the covariates described above and energy intake (kcal/day). We replaced missing covariate data with carried-forward method for continuous variables and missing indicator method for categorical variables. Linear trend was examined by modeling the median value for fruit/vegetable categories as a continuous variable. We also evaluated the association between each two servings per week of individual fruit, vegetable, and fruit juice consumption. For this analysis, because some of the fruit, vegetable, and fruit juice items (including grapes/raisins, blueberries, strawberries, grapefruits, prunes, cantaloupe/melon, apple juice, other juice, lettuce, cauliflower, kale/mustard greens/chard, Brussels sprouts, eggplant/zucchini, celery, and mixed vegetables) were not asked in 1980 SFFQ, the follow-up started from 1984. In secondary analyses, we additionally adjusted for dietary fiber, animal fat, and a modified alternate healthy eating index (AHEI) score that excluded fruits and vegetables.
To assess potential differences in the relation between intakes of fruits and vegetables, and incident breast cancer by BMI at age 18, family history of breast cancer, history of benign breast disease, and smoking status, cross-product terms were added to the multivariable model and evaluated with a likelihood ratio test. To examine differential associations of fruit and vegetable consumption with breast cancer risk by hormone receptor status and molecular subtypes, we used proportional hazards regression models with a data duplication method for competing risks.35 To take advantage of repeated diet assessments in these cohorts and evaluate the latency between fruit and vegetable consumption and breast cancer incidence, analyses were performed using varying lag times. For example, in the NHS for the 0–4 year latency interval, fruit and vegetable consumption in 1980 was related to breast cancer risk between the 1980 and 1984 follow-up period; consumption in 1984 was related to risk between 1984 and 1986; consumption in 1986 was related to risk between 1986 and 1990, and so on. For the 4–8 year latency interval, fruit and vegetable consumption in 1980 was related to breast cancer risk between 1984 and 1986; consumption in 1984 was related to risk between 1986 and 1990, and so on.36 To evaluate the difference between HRs for fruit or vegetable items, we evaluated the P-value for heterogeneity with the Q statistic.37,38 To examine whether the associations with breast cancer risk were heterogeneous among individual fruits, two fully-adjusted models were fitted: one with total fruit consumption and the other with total fruit consumption plus consumption of individual fruits excluding apples (which had the most similar association as the total fruit consumption) to avoid over-fitting. The same analyses were done for total and individual vegetables, excluding carrots (which had the most similar association as the total vegetable consumption). The likelihood ratio test tested whether the model including individual fruits or vegetables had better fit than total fruit or total vegetable consumption only. We also performed a multivariable stepwise Cox proportional hazards analysis to select the independent fruit and vegetable items. All P-values were two-sided. SAS version 9.3 (SAS Institute, Inc., Cary NC) was used for all analyses.
Results
Total fruit and vegetable Consumption and Dietary and Lifestyle Factors
Over 4,309,000 person-years of follow up (mean follow-up time=23.7 years), 10,911 invasive cases of breast cancer were documented. In both cohorts, higher total fruit and vegetable consumption was associated with lower prevalence of smoking, lower animal fat consumption, higher level of physical activity, higher fiber consumption, and earlier age at menarche (Table 1). In the NHS, participants with higher intakes of fruits and vegetables consumed less alcohol, whereas in the NHSII this association was opposite. In the NHSII, higher consumption of total fruits and vegetables was associated with lower prevalence of oral contraceptive use.
Table 1.
Age and age-standardized characteristics according to consumption of total fruits and vegetables among women enrolled in the Nurses’ Health Study (n=88,301) in 1980 and Nurses’ Health Study II (n=93,844) in 1991
| Nurses’ Health Study | Nurses’ Health Study II | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤2.5 servings/day | >2.5 to 3.5 servings/day | >3.5 to 4.5 servings/day | >4.5 to 5.5 servings/day | >5.5 servings/day | ≤2.5 servings/day | >2.5 to 3.5 servings/day | >3.5 to 4.5 servings/day | >4.5 to 5.5 servings/day | >5.5 servings/day | |
| Number | 32,287 | 21,429 | 15,096 | 8,868 | 10,621 | 27,479 | 20,989 | 17,355 | 11,647 | 16,374 |
|
| ||||||||||
| Mean | ||||||||||
| Total fruit intake, servings/day | 0.6 | 1.2 | 1.7 | 2.2 | 3.4 | 0.4 | 0.8 | 1.1 | 1.4 | 2.1 |
| Total vegetable intake, servings/day | 1.1 | 1.8 | 2.3 | 2.7 | 3.8 | 1.3 | 2.2 | 2.8 | 3.5 | 5.2 |
| Fruit juice, servings/day | 0.9 | 1.0 | 1.1 | 1.1 | 1.2 | 0.6 | 0.9 | 1.0 | 1.1 | 1.3 |
| Age, years | 45.1 | 46.2 | 46.8 | 47.5 | 47.9 | 35.7 | 36.1 | 36.2 | 36.4 | 36.6 |
| BMI at age 18, kg/m2 | 21.2 | 21.3 | 21.4 | 21.5 | 21.6 | 21.1 | 21.2 | 21.2 | 21.4 | 21.6 |
| Current BMI, kg/m2 | 24.3 | 24.4 | 24.5 | 24.6 | 24.6 | 24.6 | 24.6 | 24.5 | 24.7 | 24.8 |
| Physical activity, MET-hrs/wk | 11.4 | 13.5 | 15.4 | 16.8 | 18.7 | 15.5 | 18.7 | 21.2 | 24.2 | 29.8 |
|
| ||||||||||
| Alcohol consumption, g/day | 7.2 | 6.3 | 5.8 | 5.8 | 5.4 | 2.9 | 3.1 | 3.2 | 3.2 | 3.3 |
| Total fiber intake, g/day | 8.9 | 12.1 | 14.2 | 16.2 | 20.5 | 14.4 | 17.2 | 18.9 | 20.5 | 24.0 |
| Animal fat intake, % energy | 30.8 | 29.6 | 28.7 | 27.7 | 25.4 | 18.7 | 17.9 | 17.3 | 16.8 | 15.5 |
| Total energy intake, kcal | 1397 | 1545 | 1642 | 1745 | 1910 | 1490 | 1708 | 1849 | 1980 | 2200 |
|
| ||||||||||
| % | ||||||||||
|
| ||||||||||
| Current smokers | 36 | 28 | 25 | 22 | 21 | 16 | 12 | 11 | 10 | 10 |
| Ever used oral contraceptives | 48 | 49 | 48 | 48 | 47 | 86 | 85 | 85 | 84 | 82 |
| Family history of breast cancer in mother or sisters | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| History of benign breast disease | 24 | 25 | 25 | 25 | 25 | 9 | 10 | 10 | 10 | 10 |
| Nulliparous | 6 | 6 | 6 | 6 | 7 | 30 | 26 | 24 | 25 | 27 |
| Age at menarche <12 years | 21 | 22 | 24 | 24 | 24 | 23 | 23 | 24 | 26 | 28 |
Fruit and vegetable consumption and breast cancer incidence
Results from the age-adjusted models were generally similar to multivariable models, so only multivariable results are presented. Higher consumption of total fruits and vegetables was associated with lower breast cancer incidence (>5.5 vs. ≤2.5 servings/day; HR=0.89, 95%CI=0.83–0.96; Ptrend=0.006) (Table 2). Total fruits and total vegetables, separately, were both associated inversely with lower breast cancer incidence (>2.5 servings/day vs. ≤4 servings/week of fruits; HR=0.91, 95%CI=0.84–0.99; Ptrend=0.08; >4.5 vs. ≤1.5 servings/day of vegetables; HR=0.91, 95%CI=0.84–1.00; Ptrend=0.03). Fruit juice consumption was not associated with breast cancer risk.
Table 2.
Hazard ratios and 95% confidence intervals of fruit and vegetable consumption in relation to incident invasive breast cancer (n=10,911 cases) in Nurses’ Health Study and Nurses’ Health Study II.
| Consumption Levels | Ptrend | Per 2 servings/day | |||||
|---|---|---|---|---|---|---|---|
| Total fruit and vegetable intake | |||||||
|
| |||||||
| Amount of intake | ≤2.5 servings/day | >2.5 to 3.5 servings/day | >3.5 to 4.5 servings/day | >4.5 to 5.5 servings/day | >5.5 servings/day | ||
| No. of cases/person-years in NHS | 1,292/457,076 | 1,683/542,469 | 1,768/525,605 | 1,324/376,169 | 1,544/453,806 | ||
| No. of cases/person-years in NHSII | 722/450,298 | 761/452,651 | 693/405,921 | 516/281,609 | 608/363,466 | ||
| Model 1 | 1 | 0.97 (0.92–1.03) | 0.99 (0.93–1.05) | 1.00 (0.94–1.07) | 0.94 (0.88–1.00) | 0.11 | 0.98 (0.95–1.00) |
| Model 2 | 1 | 0.94 (0.88–1.00) | 0.94 (0.88–1.00) | 0.95 (0.89–1.02) | 0.89 (0.83–0.96) | 0.006 | 0.96 (0.93–0.98) |
|
| |||||||
| Total fruit intake | |||||||
|
| |||||||
| Amount of intake | ≤4 servings/week | >4 to 6 servings/week | >6 servings/week to 1.5 servings/day | >1.5 to 2.5 servings/day | >2.5 servings/day | ||
| No. of cases/person-years in NHS | 1,023/365,517 | 963/298,599 | 2,311/718,399 | 2,400/682,790 | 914/289,822 | ||
| No. of cases/person-years in NHSII | 802/497,615 | 583/337,772 | 1,061/612,398 | 677/391,529 | 177/114,632 | ||
| Model 1 | 1 | 1.02 (0.96–1.10) | 0.98 (0.92–1.04) | 1.01 (0.95–1.07) | 0.91 (0.85–0.99) | 0.05 | 0.94 (0.90–1.00) |
| Model 2 | 1 | 1.00 (0.94–1.07) | 0.95 (0.90–1.01) | 0.99 (0.93–1.05) | 0.91 (0.84–0.99) | 0.08 | 0.94 (0.89–1.00) |
|
| |||||||
| Total vegetable intake | |||||||
|
| |||||||
| Amount of intake | ≤1.5 servings/day | >1.5 to 2.5 servings/day | >2.5 to 3.5 servings/day | >3.5 to 4.5 servings/day | >4.5 servings/day | ||
| No. of cases/person-years in NHS | 1,155/421,021 | 2,547/815,680 | 2,282/651,837 | 1,034/299,308 | 593/167,280 | ||
| No. of cases/person-years in NHSII | 483/308,753 | 1,034/610,319 | 898/518,716 | 487/287,107 | 398/229,049 | ||
| Model 1 | 1 | 0.98 (0.92–1.04) | 1.01 (0.95–1.07) | 0.96 (0.89–1.03) | 0.98 (0.90–1.06) | 0.54 | 0.99 (0.95–1.02) |
| Model 2 | 1 | 0.93 (0.88–0.99) | 0.94 (0.88–1.00) | 0.89 (0.82–0.96) | 0.91 (0.84–1.00) | 0.03 | 0.95 (0.91–0.99) |
|
| |||||||
| Fruit juice Intake | |||||||
|
| |||||||
| Amount of intake | ≤1 serving/week | >1 to 4 servings/week | >4 to 6 servings/week | >6 servings/week to 1.5 servings/day | >1.5 servings/day | ||
| No. of cases/person-years in NHS | 800/278,959 | 1,497/462,795 | 1,140/356,775 | 2,237/720,158 | 1,908/520,020 | ||
| No. of cases/person-years in NHSII | 464/301,403 | 994/586,374 | 468/271,764 | 704/401,579 | 670/392,752 | ||
| Model 1 | 1 | 1.02 (0.95–1.09) | 1.05 (0.97–1.13) | 1.03 (0.96–1.10) | 1.09 (1.01–1.16) | 0.01 | 1.07 (1.01–1.13) |
| Model 2 | 1 | 0.99 (0.92–1.06) | 1.02 (0.94–1.10) | 0.99 (0.93–1.06) | 1.03 (0.96–1.10) | 0.31 | 1.03 (0.97–1.09) |
Model 1 stratified by cohort, calendar year, and age in months.
Model 2 stratified by cohort, calendar year, and age in months and adjusted for family history of breast cancer (yes, no), history of benign breast disease (yes, no), height (<1.60, 1.60 to <1.65, 1.65 to <1.70, 1.70 to <1.75, and ≥1.75 meters), BMI at age 18 years (<18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25.0 to <30, ≥30.0 kg/m2), weight change since age 18 (continuous), smoking (never, past, current 1 to 14/day, current 15 to 24/day, current ≥25/day), physical activity (quintiles of MET-hr per week, missing), oral contraceptive use (never, < 2 years, 2 to <5 years, 5 to <10 years, ≥10 years), alcohol intake (g/day, quintiles), total energy intake (kcal/day, quintiles), age at menarche (<12, 12, 13, 14, >14 years), parity and age at first birth (nulliparous, parity ≤2 and age at first birth <25 years, parity≤2 and age at first birth 25 to <30 years, parity ≤2 and age at first birth ≥30 years, parity 3 to 4 and age at first birth <25 years, parity 3 to 4 and age at first birth 25 to <30 years, parity 3 to 4 and age at first birth ≥30 years, parity ≥5 and age at first birth <25 years, parity ≥5 and age at first birth ≥25 years), and menopausal status, age at menopause, and postmenopausal hormone use (premenopausal, postmenopausal and age at menopause<50 years and never postmenopausal hormone use, postmenopausal and age at menopause<50 years and past postmenopausal hormone use, postmenopausal and age at menopause<50 years and current postmenopausal hormone use, postmenopausal and age at menopause≥50 years and never postmenopausal hormone use, postmenopausal and age at menopause≥50 years and past postmenopausal hormone use, postmenopausal and age at menopause≥50 years and current postmenopausal hormone use, missing).
Among subgroups of vegetables, green leafy vegetables (>1 servings/day vs. ≤2 servings/week; HR=0.93, 95%CI=0.87–0.99; Ptrend=0.03), yellow/orange vegetables (>5 vs. ≤2 servings/week; HR=0.91, 95%CI=0.84–0.99; Ptrend=0.004), and cruciferous vegetables (>5 vs. ≤2 servings/week; HR=0.90, 95%CI=0.84–0.96; Ptrend=0.0002) were associated with lower breast cancer risk (Table 3). For yellow/orange and cruciferous vegetables combined, >4 vs. ≤2 servings/week of each was associated with a 17% lower risk of breast cancer (HR=0.83, 95%CI=0.76–0.91). With mutual adjustment for subgroups, the association with cruciferous vegetable consumption was not materially changed (>5 vs. ≤2 servings/week; HR=0.92, 95%CI=0.85–0.98; Ptrend=0.008), however, the association for yellow/orange vegetables was attenuated (>5 vs. ≤2 servings/week; HR=0.94, 95%CI=0.86–1.03; Ptrend=0.14). When examining subgroups of fruits and vegetables defined by micronutrient content, those rich in vitamin C (>1 servings/day vs. ≤2 servings/week; HR=0.89, 95%CI=0.82–0.95; Ptrend=0.004), α-carotene (≥3 servings/week vs. <2 servings/month; HR=0.91, 95%CI=0.84–0.99; Ptrend=0.02), and β-carotene (>1 servings/day vs. ≤2 servings/week; HR=0.87, 95%CI=0.80–0.94; Ptrend=0.0004) were each inversely associated with breast cancer risk.
Table 3.
Hazard ratios and 95% confidence intervals of subgroups of fruit and vegetable consumption in relation to incident invasive breast cancer (n=10,911 cases) in Nurses’ Health Study and Nurses’ Health Study II.
| Consumption Levels | Ptrend | |||||
|---|---|---|---|---|---|---|
| Green leafy vegetable intake | ||||||
| Amount of intake | ≤2 servings/week | >2 to 4 servings/week | >4 to 6 servings/week | >6 servings/week to 1 serving/day | >1 servings/day | |
| No. of cases/person-years in NHS | 1,500/567,610 | 2,163/659,719 | 1,848/534,085 | 723/213,002 | 1,367/372,934 | |
| No. of cases/person-years in NHSII | 604/403,641 | 870/538,425 | 757/415,130 | 284/177,224 | 785/419,358 | |
| Model 1 | 1 | 1.02 (0.96–1.08) | 1.02 (0.96–1.09) | 1.02 (0.94–1.10) | 1.04 (0.98–1.11) | 0.28 |
| Model 2 | 1 | 0.95 (0.90–1.01) | 0.93 (0.87–0.99) | 0.92 (0.85–0.99) | 0.93 (0.87–0.99) | 0.03 |
|
| ||||||
| Yellow/orange vegetable intake | ||||||
|
| ||||||
| Amount of intake | ≤2 servings/week | >2 to 3 servings/week | >3 to 4 servings/week | >4 to 5 servings/week | >5 servings/week | |
| No. of cases/person-years in NHS | 4,108/1,305,886 | 1,657/470,261 | 931/293,064 | 457/129,100 | 458/156,630 | |
| No. of cases/person-years in NHSII | 1,953/1,188,668 | 631/352,322 | 361/191,770 | 154/104,970 | 201/116,123 | |
| Model 1 | 1 | 1.00 (0.95–1.05) | 0.96 (0.91–1.02) | 0.94 (0.87–1.02) | 0.90 (0.83–0.98) | 0.007 |
| Model 2 | 1 | 0.98 (0.93–1.03) | 0.95 (0.89–1.01) | 0.93 (0.85–1.01) | 0.91 (0.84–0.99) | 0.004 |
|
| ||||||
| Tomato intake | ||||||
|
| ||||||
| Amount of intake | ≤2 servings/week | >2 to 4 servings/week | >4 to 6 servings/week | >6 servings/week to 1 serving/day | >1 servings/day | |
| No. of cases/person-years in NHS | 1,675/595,564 | 3,132/950,188 | 1,815/518,863 | 498/157,262 | 479/128,381 | |
| No. of cases/person-years in NHSII | 750/511,096 | 1,197/658,061 | 794/456,451 | 202/115,244 | 357/212,870 | |
| Model 1 | 1 | 1.05 (1.00–1.11) | 1.07 (1.01–1.13) | 1.05 (0.96–1.14) | 1.11 (1.02–1.20) | 0.009 |
| Model 2 | 1 | 1.02 (0.97–1.07) | 1.03 (0.97–1.09) | 1.02 (0.93–1.11) | 1.06 (0.98–1.15) | 0.21 |
|
| ||||||
| Cruciferous vegetable intake | ||||||
|
| ||||||
| Amount of intake | ≤2 servings/week | >2 to 3 servings/week | >3 to 4 servings/week | >4 to 5 servings/week | >5 servings/week | |
| No. of cases/person-years in NHS | 3,030/1,024,004 | 1,729/476,923 | 1,190/364,159 | 705/192,392 | 955/297,017 | |
| No. of cases/person-years in NHSII | 1,458/880,950 | 681/385,353 | 466/262,993 | 281/161,688 | 414/262,876 | |
| Model 1 | 1 | 1.01 (0.96–1.06) | 0.95 (0.89–1.00) | 0.97 (0.90–1.04) | 0.91 (0.85–0.96) | 0.0007 |
| Model 2 | 1 | 0.97 (0.92–1.02) | 0.92 (0.87–0.98) | 0.94 (0.87–1.01) | 0.90 (0.84–0.96) | 0.0002 |
|
| ||||||
| Other vegetable intake | ||||||
| Amount of intake | ≤2 servings/week | >2 to 4 servings/week | >4 to 6 servings/week | >6 servings/week to 1 serving/day | >1 servings/day | |
| No. of cases/person-years in NHS | 554/222,662 | 2,183/694,458 | 2,286/681,612 | 912/264,363 | 1,676/491,969 | |
| No. of cases/person-years in NHSII | 251/154,205 | 821/490,953 | 905/504,966 | 320/194,177 | 1,003/609,606 | |
| Model 1 | 1 | 1.03 (0.96–1.12) | 1.03 (0.95–1.11) | 1.04 (0.95–1.13) | 0.99 (0.91–1.07) | 0.17 |
| Model 2 | 1 | 0.99 (0.92–1.07) | 0.98 (0.90–1.06) | 0.99 (0.90–1.08) | 0.94 (0.86–1.02) | 0.06 |
|
| ||||||
| Fruits and vegetables high in vitamin C | ||||||
|
| ||||||
| Amount of intake | ≤2 servings/week | >2 to 4 servings/week | >4 to 6 servings/week | >6 servings/week to 1 serving/day | >1 servings/day | |
| No. of cases/person-years in NHS | 821/322,755 | 1,787/566,451 | 1,836/548,713 | 825/229,135 | 2,342/688,031 | |
| No. of cases/person-years in NHSII | 444/271,768 | 954/577,239 | 823/468,741 | 286/164,045 | 793/472,149 | |
| Model 1 | 1 | 0.99 (0.92–1.06) | 0.97 (0.91–1.04) | 1.02 (0.94–1.10) | 0.94 (0.88–1.00) | 0.04 |
| Model 2 | 1 | 0.94 (0.87–1.00) | 0.91 (0.85–0.97) | 0.95 (0.87–1.03) | 0.89 (0.82–0.95) | 0.004 |
|
| ||||||
| Fruits and vegetables high in α-carotene | ||||||
|
| ||||||
| Amount of intake | <2 servings/month | 2 to <4 servings/month | 1 to <2 servings/week | 2 to <3 servings/week | ≥3 servings/week | |
| No. of cases/person-years in NHS | 665/191,717 | 2,070/649,455 | 2,586/802,189 | 1,123/316,909 | 1,162/391,617 | |
| No. of cases/person-years in NHSII | 451/284,886 | 889/529,046 | 1,033/599,456 | 471/266,650 | 456/273,655 | |
| Model 1 | 1 | 0.95 (0.89–1.02) | 0.94 (0.88–1.00) | 0.92 (0.85–0.99) | 0.91 (0.84–0.98) | 0.01 |
| Model 2 | 1 | 0.95 (0.88–1.01) | 0.92 (0.86–0.99) | 0.89 (0.82–0.96) | 0.91 (0.84–0.99) | 0.02 |
|
| ||||||
| Fruits and vegetables high in β-carotene | ||||||
|
| ||||||
| Amount of intake | ≤2 servings/week | >2 to 4 servings/week | >4 to 6 servings/week | >6 servings/week to 1 serving/day | >1 servings/day | |
| No. of cases/person-years in NHS | 736/301,972 | 1,381/455,777 | 1,697/508,135 | 851/241,899 | 2,946/847,277 | |
| No. of cases/person-years in NHSII | 249/173,435 | 612/379,126 | 696/414,291 | 324/186,699 | 1,419/800,339 | |
| Model 1 | 1 | 1.01 (0.93–1.09) | 1.00 (0.93–1.08) | 1.04 (0.95–1.13) | 1.00 (0.93–1.07) | 0.66 |
| Model 2 | 1 | 0.94 (0.86–1.01) | 0.90 (0.83–0.98) | 0.92 (0.84–1.01) | 0.87 (0.80–0.94) | 0.0004 |
|
| ||||||
| Fruits and vegetables high in lutein | ||||||
|
| ||||||
| Amount of intake | ≤1 serving/month | >1 to 3 servings/month | >3 to 4 servings/month | >1 to 3 servings/week | >3 servings/week | |
| No. of cases/person-years in NHS | 797/254,275 | 1,944/581,492 | 1,410/457,320 | 2,820/820,986 | 630/233,258 | |
| No. of cases/person-years in NHSII | 861/565,092 | 1,084/622,253 | 492/299,831 | 723/389,612 | 140/76,717 | |
| Model 1 | 1 | 1.04 (0.98–1.11) | 1.03 (0.97–1.11) | 1.04 (0.98–1.10) | 0.98 (0.89–1.07) | 0.39 |
| Model 2 | 1 | 1.01 (0.95–1.07) | 1.00 (0.93–1.07) | 0.97 (0.92–1.04) | 0.94 (0.86–1.03) | 0.07 |
Model 1 stratified by cohort, calendar year, and age in months.
Model 2 stratified by cohort, calendar year, and age in months and adjusted for family history of breast cancer (yes, no), history of benign breast disease (yes, no), height (<1.60, 1.60 to <1.65, 1.65 to <1.70, 1.70 to <1.75, and ≥1.75 meters), BMI at age 18 years (<18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25.0 to <30, ≥30.0 kg/m2), weight change since age 18 (continuous), smoking (never, past, current 1 to 14/day, current 15 to 24/day, current ≥25/day), physical activity (quintiles of MET-hr per week, missing), oral contraceptive use (never, < 2 years, 2 to <5 years, 5 to <10 years, ≥10 years), alcohol intake (g/day, quintiles), total energy intake (kcal/day, quintiles), age at menarche (<12, 12, 13, 14, >14 years), parity and age at first birth (nulliparous, parity ≤2 and age at first birth <25 years, parity≤2 and age at first birth 25 to <30 years, parity ≤2 and age at first birth ≥30 years, parity 3 to 4 and age at first birth <25 years, parity 3 to 4 and age at first birth 25 to <30 years, parity 3 to 4 and age at first birth ≥30 years, parity ≥5 and age at first birth <25 years, parity ≥5 and age at first birth ≥25 years), and menopausal status, age at menopause, and postmenopausal hormone use (premenopausal, postmenopausal and age at menopause<50 years and never postmenopausal hormone use, postmenopausal and age at menopause<50 years and past postmenopausal hormone use, postmenopausal and age at menopause<50 years and current postmenopausal hormone use, postmenopausal and age at menopause≥50 years and never postmenopausal hormone use, postmenopausal and age at menopause≥50 years and past postmenopausal hormone use, postmenopausal and age at menopause≥50 years and current postmenopausal hormone use, missing).
When examining individual fruits and vegetables, the associations appeared stronger per 2 servings/week of winter squash (HR=0.90, 95%CI=0.83–0.98), broccoli (HR=0.96, 95%CI=0.92–0.99), cabbage (HR=0.93, 95%CI=0.89–0.99), and cauliflower (HR=0.92, 95%CI=0.87–0.98), (PHeterogeneity=0.63 for individual fruits; PHeterogeneity=0.009 for individual vegetables) (Appendix Figure S1). The goodness of fit was not significantly improved by adding individual fruit consumption (except apples) to the model with total fruit consumption or adding individual vegetable consumption (except carrots) to the model with total vegetable consumption. Using stepwise selection analyses with individual fruits and vegetables (including apples/pears, oranges, broccoli, cabbage, cauliflower, carrots, winter squash, lettuce, eggplant/zucchini), carrots remained significant (p<0.05).
Fruit and vegetable consumption and breast cancer subtypes
In analyses by tumor hormone receptor status, higher consumption of fruits and vegetables was more strongly associated with ER-negative than ER-positive tumors (Table 4) (for example, per 2 servings/day of total fruit and vegetable intake, ER-negative, HR=0.88, 95%CI=0.83–0.94; Pheterogeneity=0.02). Similar results were observed for ER-negative/PR-negative compared with ER-positive/PR-negative or ER-positive/PR-positive. Higher consumption of green leafy, yellow/orange, tomato and other vegetables, as well as fruits and vegetables rich in vitamin C, α-carotene,β-carotene and lutein was each associated with lower risk of ER-negative cancer. Higher consumption of cruciferous vegetables was associated with lower risk of both ER-positive and ER-negative breast cancer. In contrast, higher consumption of fruit juice was associated with higher risk of ER-negative breast cancer (data not shown).
Table 4.
Consumption of fruits and vegetables in relation to risk of overall breast cancer by estrogen and progesterone receptor status and molecular subtypes among women in the Nurses’ Health Study and Nurses’ Health Study II
| Breast cancer subtype | No. of cases | Total fruit and vegetable intake (per 2 servings/day) | Total fruit intake (per 2 servings/day) | Total vegetable intake (per 2 servings/day) |
|---|---|---|---|---|
|
| ||||
| HR (95%CI) | HR (95%CI) | HR (95%CI) | ||
|
| ||||
| Estrogen receptor positive | 7,464 | 0.96 (0.93–0.99) | 0.94 (0.88–1.01) | 0.96 (0.91–1.00) |
| Estrogen receptor negative | 1,794 | 0.88 (0.83–0.94) | 0.91 (0.79–1.04) | 0.84 (0.77–0.93) |
| P for Heterogeneity | 0.02 | 0.63 | 0.02 | |
|
| ||||
| Estrogen and progesterone receptor positive | 6,128 | 0.96 (0.93–1.00) | 0.96 (0.89–1.04) | 0.95 (0.90–1.00) |
| Estrogen receptor positive and progesterone receptor negative | 1,214 | 0.95 (0.88–1.03) | 0.87 (0.74–1.03) | 0.99 (0.89–1.11) |
| Estrogen and progesterone receptor negative | 1,573 | 0.88 (0.82–0.94) | 0.88 (0.76–1.02) | 0.85 (0.77–0.94) |
| P for Heterogeneity | 0.07 | 0.39 | 0.08 | |
|
| ||||
| Luminal A | 2,459 | 0.94 (0.89–0.99) | 0.90 (0.80–1.02) | 0.93 (0.86–1.01) |
| Luminal B | 1,080 | 0.98 (0.90–1.06) | 0.99 (0.84–1.18) | 0.94 (0.84–1.06) |
| HER2-enriched | 262 | 0.79 (0.67–0.93) | 0.58 (0.40–0.82) | 0.78 (0.62–1.00) |
| Basal-like | 331 | 0.84 (0.72–0.97) | 0.89 (0.66–1.21) | 0.83 (0.67–1.03) |
| Unclassified | 99 | 1.16 (0.89–1.49) | 1.36 (0.80–2.32) | 1.17 (0.80–1.72) |
| P for Heterogeneity | 0.03 | 0.04 | 0.36 | |
Stratified by cohort, calendar year, and age in months and adjusted for family history of breast cancer (yes, no), history of benign breast disease (yes, no), height (<1.60, 1.60 to <1.65, 1.65 to <1.70, 1.70 to <1.75, and ≥1.75 meters), BMI at age 18 years (<18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25.0 to <30, ≥30.0 kg/m2), weight change since age 18 (continuous), smoking (never, past, current 1 to 14/day, current 15 to 24/day, current ≥25/day), physical activity (quintiles of MET-hr per week, missing), oral contraceptive use (never, < 2 years, 2 to <5 years, 5 to <10 years, ≥10 years), alcohol intake (g/day, quintiles), total energy intake (kcal/day, quintiles), age at menarche (<12, 12, 13, 14, >14 years), parity and age at first birth (nulliparous, parity ≤2 and age at first birth <25 years, parity≤2 and age at first birth 25 to <30 years, parity ≤2 and age at first birth ≥30 years, parity 3 to 4 and age at first birth <25 years, parity 3 to 4 and age at first birth 25 to <30 years, parity 3 to 4 and age at first birth ≥30 years, parity ≥5 and age at first birth <25 years, parity ≥5 and age at first birth ≥25 years), and menopausal status, age at menopause, and postmenopausal hormone use (premenopausal, postmenopausal and age at menopause<50 years and never postmenopausal hormone use, postmenopausal and age at menopause<50 years and past postmenopausal hormone use, postmenopausal and age at menopause<50 years and current postmenopausal hormone use, postmenopausal and age at menopause≥50 years and never postmenopausal hormone use, postmenopausal and age at menopause≥50 years and past postmenopausal hormone use, postmenopausal and age at menopause≥50 years and current postmenopausal hormone use, missing).
When examining molecular subtypes of breast cancer, we found that each 2 servings/day of total fruit and vegetable consumption were most strongly associated with lower risk of HER2-enriched (HR=0.79, 95%CI=0.67–0.93), basal-like (HR=0.84, 95%CI=0.72–0.97), and luminal A (HR=0.94, 95%CI=0.89–0.99), compared with luminal B (HR=0.98, 95%CI=0.90–1.06) tumors (Pheterogeneity=0.03) (Table 4). Among subgroups, high intake of yellow/orange vegetables, tomato, fruits and vegetables rich in vitamin C, and fruits and vegetables rich in α-carotene was each associated with lower risk of HER2-enriched cancer (data not shown). High intake of cruciferous vegetables was associated with lower risk of luminal A (for each serving/day; HR=0.82, 95%CI=0.69–0.98) and basal-like tumors (for each serving/day; HR=0.58, 95%CI=0.36–0.93).
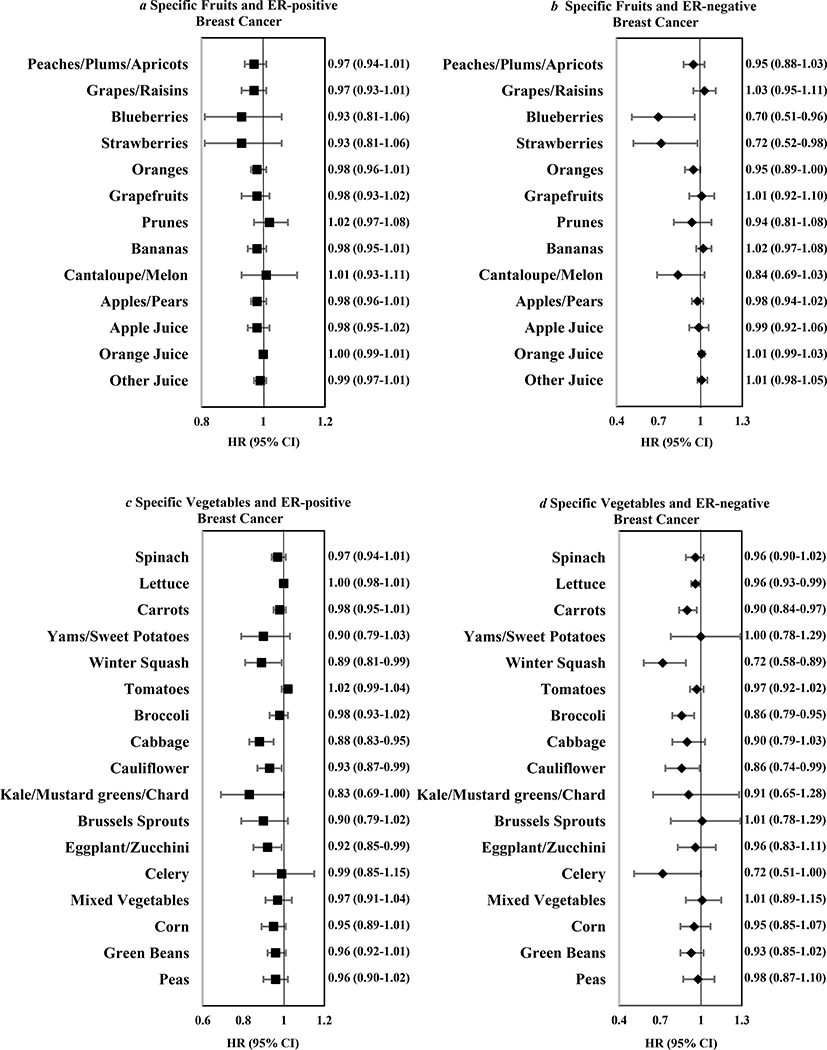
Examining individual fruits and vegetables with ER-negative breast cancer, higher intakes of blueberries, strawberries, lettuce, carrots, winter squash, broccoli, and cauliflower were associated with reduced risk (Figure 1). Using stepwise selection (including blueberries, strawberries, oranges, lettuce, carrots, winter squash, broccoli, cauliflower, celery), carrots and winter squash remained significant (p<0.05).
Figure 1.
Multivariable hazard ratios and 95% confidence intervals for every 2 servings/week of specific fruits (a and b) and vegetables (c and d) in relation to ER positive cancers (7,464 cases) and ER negative cancers (1,794 cases) among 182,145 women in the Nurses’ Health Studies
Subgroup Analyses
In separate evaluations of pre- and postmenopausal breast cancer, the associations between total fruit and vegetable consumption and subgroups of fruit and vegetable consumption produced similar HRs, although among premenopausal women, some of the associations were not significant due to the smaller sample size (Appendix Tables S2, S3). The associations between total fruit or total vegetable consumption and breast cancer incidence did not differ by BMI at age 18, smoking status, history of benign breast disease, and family history of breast cancer (p-interaction>0.05).
To evaluate the importance of timing of fruit and vegetable consumption in relation to breast cancer risk, we conducted analyses using only baseline dietary data (NHS, 1980; NHSII, 1991) without updating, as well as latency analyses assessing intake 0–4, 4–8, 8–12, 12–16, and 16–20 years prior to diagnosis. With baseline intake, total fruit and vegetable consumption was significantly associated with lower breast cancer risk (>5.5 vs. ≤2.5 servings/day; HR=0.91, 95%CI=0.85–0.98; Ptrend=0.009), as was total vegetable consumption (>4.5 vs. ≤1.5 servings/day; HR=0.92, 95%CI=0.84–1.00; Ptrend=0.01). However, baseline total fruit consumption was not associated with risk (>2.5 servings/day vs. ≤4 servings/week; HR=0.95, 95%CI=0.89–1.02; Ptrend=0.17). In the time-lagged analyses, total fruit and vegetable consumption was associated with significantly decreased breast cancer risk 8–12 years after exposure, but not for shorter latency periods (Table 5). Total fruit consumption was more strongly associated with breast cancer risk for longer time lags, 12–16 years after exposure. Total vegetable consumption was associated with lower risk for 8–12 and 12–16 years after exposure. However, lower risk of ER-negative or ER-negative/PR-negative cancer was observed with high intake of total fruits and vegetables 4–8 years before breast cancer diagnosis (Appendix Table S4).
Table 5.
Time lagged analyses on breast cancer according to fruit and vegetable consumption in pooled data from Nurses’ Health Study and Nurses’ Health Study II.
| No. of cases | Consumption Levels | Ptrend | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total fruit and vegetable intake | |||||||
| ≤2.5 servings/day | >2.5 to 3.5 servings/day | >3.5 to 4.5 servings/day | >4.5 to 5.5 servings/day | >5.5 servings/day | |||
| 0–4 year lag | 9,362 | 1 | 0.95 (0.89–1.01) | 0.98 (0.92–1.05) | 0.94 (0.87–1.01) | 0.94 (0.88–1.01) | 0.12 |
| 4–8 year lag | 8,584 | 1 | 0.98 (0.91–1.05) | 0.91 (0.85–0.98) | 0.97 (0.90–1.05) | 0.99 (0.92–1.06) | 0.94 |
| 8–12 year lag | 7,529 | 1 | 0.90 (0.84–0.97) | 0.89 (0.83–0.96) | 0.92 (0.85–1.00) | 0.90 (0.83–0.97) | 0.05 |
| 12–16 year lag | 6,083 | 1 | 0.95 (0.88–1.03) | 0.94 (0.86–1.02) | 0.93 (0.85–1.02) | 0.89 (0.82–0.97) | 0.01 |
| 16–20 year lag | 4,631 | 1 | 0.97 (0.89–1.06) | 0.94 (0.86–1.03) | 0.94 (0.85–1.04) | 0.89 (0.80–0.98) | 0.02 |
|
| |||||||
| Total fruit intake | |||||||
|
| |||||||
| ≤4 servings/week | >4 to 6 servings/week | >6 servings/week to 1.5 servings/day | >1.5 to 2.5 servings/day | >2.5 servings/day | |||
| 0–4 year lag | 9,362 | 1 | 0.99 (0.91–1.06) | 0.98 (0.92–1.04) | 0.97 (0.91–1.03) | 0.93 (0.86–1.01) | 0.08 |
| 4–8 year lag | 8,584 | 1 | 0.99 (0.92–1.07) | 0.98 (0.92–1.05) | 1.01 (0.94–1.08) | 0.97 (0.89–1.05) | 0.67 |
| 8–12 year lag | 7,529 | 1 | 0.95 (0.87–1.03) | 0.92 (0.86–0.99) | 0.97 (0.90–1.04) | 0.96 (0.88–1.04) | 0.70 |
| 12–16 year lag | 6,083 | 1 | 0.99 (0.90–1.08) | 0.92 (0.85–0.99) | 0.93 (0.86–1.01) | 0.91 (0.83–1.00) | 0.05 |
| 16–20 year lag | 4,631 | 1 | 1.03 (0.93–1.13) | 0.92 (0.84–1.00) | 0.93 (0.85–1.02) | 0.91 (0.82–1.02) | 0.05 |
|
| |||||||
| Total vegetable intake | |||||||
|
| |||||||
| ≤1.5 servings/day | >1.5 to 2.5 servings/day | >2.5 to 3.5 servings/day | >3.5 to 4.5 servings/day | >4.5 servings/day | |||
| 0–4 year lag | 9,362 | 1 | 0.95 (0.89–1.01) | 0.94 (0.88–1.01) | 0.94 (0.87–1.01) | 0.94 (0.87–1.02) | 0.23 |
| 4–8 year lag | 8,584 | 1 | 0.95 (0.89–1.02) | 0.89 (0.83–0.96) | 0.95 (0.88–1.03) | 0.95 (0.87–1.03) | 0.36 |
| 8–12 year lag | 7,529 | 1 | 0.90 (0.84–0.96) | 0.87 (0.81–0.94) | 0.91 (0.83–0.99) | 0.89 (0.82–0.98) | 0.09 |
| 12–16 year lag | 6,083 | 1 | 0.93 (0.86–1.00) | 0.92 (0.84–0.99) | 0.91 (0.83–1.00) | 0.88 (0.80–0.97) | 0.03 |
| 16–20 year lag | 4,631 | 1 | 0.97 (0.89–1.06) | 0.98 (0.89–1.07) | 0.93 (0.83–1.04) | 0.90 (0.80–1.02) | 0.08 |
Stratified by cohort, calendar year, and age in months and adjusted for family history of breast cancer (yes, no), history of benign breast disease (yes, no), height (<1.60, 1.60 to <1.65, 1.65 to <1.70, 1.70 to <1.75, and ≥1.75 meters), BMI at age 18 years (<18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25.0 to <30, ≥30.0 kg/m2), weight change since age 18 (continuous), smoking (never, past, current 1 to 14/day, current 15 to 24/day, current ≥25/day), physical activity (quintiles of MET-hr per week, missing), oral contraceptive use (never, < 2 years, 2 to <5 years, 5 to <10 years, ≥10 years), alcohol intake (g/day, quintiles), total energy intake (kcal/day, quintiles), age at menarche (<12, 12, 13, 14, >14 years), parity and age at first birth (nulliparous, parity ≤2 and age at first birth <25 years, parity≤2 and age at first birth 25 to <30 years, parity ≤2 and age at first birth ≥30 years, parity 3 to 4 and age at first birth <25 years, parity 3 to 4 and age at first birth 25 to <30 years, parity 3 to 4 and age at first birth ≥30 years, parity ≥5 and age at first birth <25 years, parity ≥5 and age at first birth ≥25 years), and menopausal status, age at menopause, and postmenopausal hormone use (premenopausal, postmenopausal and age at menopause<50 years and never postmenopausal hormone use, postmenopausal and age at menopause<50 years and past postmenopausal hormone use, postmenopausal and age at menopause<50 years and current postmenopausal hormone use, postmenopausal and age at menopause≥50 years and never postmenopausal hormone use, postmenopausal and age at menopause≥50 years and past postmenopausal hormone use, postmenopausal and age at menopause≥50 years and current postmenopausal hormone use, missing).
The association for total fruit and vegetable intake and breast cancer was attenuated after adjustment for fiber consumption, as a constituent of fruits and vegetables, (>5.5 vs. ≤2.5 servings/day; HR=0.92, 95%CI=0.83–1.01; Ptrend=0.21). However, the associations remained significant for cruciferous vegetables, and fruits and vegetables rich in β-carotene after additional adjustment for fiber consumption (Appendix Table S5). Additional adjustment for animal fat or AHEI did not change the results (Appendix Table S5). We observed similar finding for total fruit and vegetable intake where energy intake was included in the models as continuous variables (>5.5 vs. ≤2.5 servings/day; HR for total fruits and vegetables=0.90, 95%CI: 0.84–0.96; Ptrend=0.01). In Appendix Table S6, the results were presented for NHS and NHSII separately. We did not observe significant heterogeneity between the two cohorts and total fruit and vegetable consumption was associated with lower risk of breast cancer in the NHS and NHSII.
Discussion
In this large analysis, pooling repeated measures from two large prospective cohorts, higher total fruit and vegetable consumption during adulthood was associated with a modest, statistically significant lower invasive breast cancer incidence, with the strongest associations for ER-negative, HER2-enriched, and basal-like tumors. The inverse associations appeared to be strongest for consumption eight or more years before diagnosis. Higher intakes of yellow/orange vegetables, cruciferous vegetables, green leafy vegetables as well as fruits and vegetables rich in vitamin C, α-carotene, and β-carotene were associated with lower breast cancer incidence. The associations differed significantly among individual vegetables: higher intakes of winter squash, broccoli, cabbage, and cauliflower were significantly associated with lower incidence of breast cancer.
Fruit and vegetable consumption has been hypothesized to reduce risk of breast cancer, but many studies have not observed associations. In a pooled analysis of 20 prospective cohort studies with 34,526 cases of breast cancer, including the NHS and NHSII,20 consumption of total fruits and vegetables was not associated with overall risk of breast cancer, but vegetable consumption was inversely associated with risk of ER-negative/PR-negative tumors (highest vs. lowest quintile; HR=0.84, 95%CI=0.75–0.93; Ptrend=0.001). Total vegetable consumption was associated with lower risk of overall breast cancer as well as ER-negative/PR-negative tumors in a recent EPIC study (10,197 breast cancer cases).19 A limitation of most of the studies was that diet was assessed with a single questionnaire at baseline, and in many studies, the follow-up was relatively short. Our latency analyses suggested weak associations with 0–8 year lags. Our results indicate that fruit and vegetable intake may be important eight or more years before diagnosis, which is consistent with an effect acting in the early stages of carcinogenesis. Our findings also strongly support a greater benefit of both total fruit and vegetable and total vegetable consumption for reduction of ER-negative breast cancer, which may be due to the dominant role of hormonal exposures in the etiology of ER-positive tumors. Consistent with our earlier NHS report included 792 ER-negative cases,23 higher intake of strawberries and blueberries was associated with substantially lower risk of ER-negative tumors; as were observed for strawberries in the pooled analysis.20 However, these foods were not included in many other studies.
In addition to analyses by ER/PR status, we observed significant heterogeneity in the association between fruit and vegetable consumption by tumor molecular subtypes, with stronger associations with HER2-enriched and basal-like tumors, more aggressive forms of breast cancer with few identified preventive factors.39 While these associations may reflect the ER-negative component of these subtypes, future studies are warranted to replicate the stronger associations we observed with HER2-enriched tumors to understand the role of HER2 in the underlying mechanisms. To our knowledge, the current study is the first to evaluate the risk of breast cancer by molecular subtype in relation to fruit and vegetable consumption.
With widely varying nutrient and phytochemical content of fruits and vegetables, we observed, as expected, heterogeneity in breast cancer association by individual items. Particularly notable was the inverse association with cruciferous vegetables including cauliflower, cabbage, and broccoli. Although associations with Brussels sprouts and kale/mustard greens/chard were not significant, the 95% CIs were wide given relatively low consumption and they were not assessed in early questionnaires. Cruciferous vegetables are hypothesized to prevent cancer given that they are rich sources of bioactive compounds including isothiocyanates and indoles that suppress mutagenic and carcinogenic activity in laboratory models.40 In the large pooled analysis, cruciferous vegetable consumption was not significantly associated with lower breast cancer risk,41 but an inverse association was observed in the Black Women’s Health Study.15
Consistent with our prior finding of reduced breast cancer risk with higher fiber intake,5 and the biologically plausible role of fiber in fruits and vegetables, the inverse association with total fruit and vegetable intake was attenuated with additional adjustment for fiber. However, the associations with cruciferous vegetables and β-carotene-rich fruits and vegetables were not attenuated with adjustment for fiber, suggesting other constituents of fruits and vegetables such as micronutrients that may also be important. For example, carotenoids have been hypothesized to reduce cancer risk through antioxidant or antiproliferative activity.42 This is consistent with prior studies where both dietary intake and circulating levels of carotenoids have been inversely associated with breast cancer, especially ER-negative tumors.4, 43, 44 Our results also support a role of vitamin C, which also may act as an antioxidant in reducing breast cancer risk.
Our use of pooled data from two, large, well-established cohorts with long-term follow-up, many repeated diet assessments, and a large number of cases, allowed the detection of modest reductions in risk, examination of specific fruits and vegetables, and assessment of breast cancer risk by hormone receptor status, molecular subtype, and menopausal status. While residual confounding cannot be excluded, the incorporation of updated, detailed data on lifestyle factors and other potential confounders had minimal effects on associations. Although the majority of participants were white educated females, the underlying biologic mechanisms were not likely to differ substantially by race.45,46 Type I error is possible given that we made multiple comparisons. However, the central finding of an inverse association with fruits and vegetables, particularly for ER-negative cases, was our primary hypothesis, and additional analyses supported these results.
In conclusion, our findings from two, large prospective cohorts support the hypothesis that total fruit and vegetable consumption are associated with lower breast cancer incidence, particularly the more aggressive tumors including ER-negative, HER2-enriched, and basal-like. Yellow/orange and cruciferous vegetables appear to be particularly beneficial. Notably, these associations for cruciferous vegetables are independent of fiber intake. Finally, fruit and vegetable intake may be important in reducing tumor initiation given the importance of intake 8 or more years before diagnosis. Increased intake of fruits and vegetables has numerous health benefits, including the potential of reducing the breast cancer risk. Our findings support current guidelines for cancer prevention47 that recommend a diet high in fruits and vegetables, and suggest the importance of translational studies to understand the underlying mechanisms with cancer incidence.
Supplementary Material
Impact.
Based on these results, high intake of fruits and vegetables may reduce the risk of breast cancer, especially aggressive tumors.
Acknowledgments
FUNDING: The study was supported by the National Institutes of Health Grants (R01 CA050385, UM1 CA176726, UM1 CA186107, P01 CA87969) and a grant from The Breast Cancer Research Foundation.
The study was supported by the National Institutes of Health Grants (R01 CA050385, UM1 CA176726, UM1 CA186107, P01 CA87969) and a grant from The Breast Cancer Research Foundation. The study sponsors were not involved in the study design and collection, analysis and interpretation of data, or the writing of the article or the decision to submit it for publication. The authors were independent from study sponsors. We would like to thank the participants and staff of the NHS and NHS II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Contributors: The authors’ responsibility was as follows: MSF, WYC, BR, RMT, WCW, and AHE: designed the research; MSF: analysis and wrote the manuscript; and MSF and AHE: had primary responsibility for the final content of the manuscript; and all authors: provided critical input in the writing of the manuscript and read and approved the final manuscript. The authors assume full responsibility for analyses and interpretation of these data.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Polyak K. Breast cancer: origin and evaluation. J Clin Invest. 2007;117(11):3155–63. doi: 10.1172/JCI33295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 4.Eliassen AH, Hendrickson SJ, Brinton LA, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. 2012;104:1905–16. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farvid MS, Eliassen AH, Cho E, et al. Dietary fiber intake in young adults and breast cancer risk. Pediatrics. 2016;137:e20151226. doi: 10.1542/peds.2015-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata A, Paganini-Hill A, Ross RK, Henderson BE. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamins supplements and cancer incidence among the elderly: a prospective study. Br J Cancer. 1992;66:673–9. doi: 10.1038/bjc.1992.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohan TE, Howe GR, Friedenreich CM, et al. Dietary fiber, vitamins A, C, and E, and risk of breast cancer: a cohort study. Cancer Causes Control. 1993;4:29–37. doi: 10.1007/BF00051711. [DOI] [PubMed] [Google Scholar]
- 8.Verhoeven DT, Assen N, Goldbohm RA, et al. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br J Cancer. 1997;75:149–55. doi: 10.1038/bjc.1997.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547–56. doi: 10.1093/jnci/91.6.547. [DOI] [PubMed] [Google Scholar]
- 10.Olsen A, Tjønneland A, Thomsen BL, et al. Fruits and vegetables intake differentially affects estrogen receptor negative and positive breast cancer incidence rates. J Nutr. 2003;133:2342–7. doi: 10.1093/jn/133.7.2342. [DOI] [PubMed] [Google Scholar]
- 11.van Gils CH, Peeters PH, Bueno-de-Mesquita HB, et al. Consumption of vegetables and fruits and risk of breast cancer. JAMA. 2005;293:183–93. doi: 10.1001/jama.293.2.183. [DOI] [PubMed] [Google Scholar]
- 12.Sonestedt E, Borgquist S, Ericson U, et al. Plant foods and oestrogen receptor alpha- and beta-defined breast cancer: observations from the Malmo Diet and Cancer cohort. Carcinogenesis. 2008;29:2203–9. doi: 10.1093/carcin/bgn196. [DOI] [PubMed] [Google Scholar]
- 13.George SM, Park Y, Leitzmann MF, et al. Fruit and vegetable intake and risk of cancer: a prospective cohort study. Am J Clin Nutr. 2009;89:347–53. doi: 10.3945/ajcn.2008.26722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler LM, Wu AH, Wang R, et al. A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am J Clin Nutr. 2010;91:1013–9. doi: 10.3945/ajcn.2009.28572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boggs DA, Palmer JR, Wise LA, et al. Fruit and vegetable intake in relation to risk of breast cancer in the Black Women’s Health Study. Am J Epidemiol. 2010;172:1268–79. doi: 10.1093/aje/kwq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Löf M, Sandin S, Lagiou P, et al. Fruit and vegetable intake and risk of cancer in the Swedish women’s lifestyle and health cohort. Cancer Causes Control. 2011;22:283–9. doi: 10.1007/s10552-010-9696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki R, Iwasaki M, Hara A, et al. Fruit and vegetable intake and breast cancer risk defined by estrogen and progesterone receptor status: the Japan Public Health Center-based Prospective Study. Cancer Causes Control. 2013;24:2117–28. doi: 10.1007/s10552-013-0289-7. [DOI] [PubMed] [Google Scholar]
- 18.Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) Am J Clin Nutr. 2014;100:394S–398S. doi: 10.3945/ajcn.113.071357. [DOI] [PubMed] [Google Scholar]
- 19.Emaus MJ, Peeters PH, Bakker MF, et al. Vegetable and fruit consumption and the risk of hormone receptor-defined breast cancer in the EPIC cohort. Am J Clin Nutr. 2016;103:168–77. doi: 10.3945/ajcn.114.101436. [DOI] [PubMed] [Google Scholar]
- 20.Jung S, Spiegelman D, Baglietto L, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. 2013;105:219–36. doi: 10.1093/jnci/djs635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aune D, Chan DS, Vieira AR, et al. Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012;134:479–93. doi: 10.1007/s10549-012-2118-1. [DOI] [PubMed] [Google Scholar]
- 22.Hirko KA, Willett WC, Hankinson SE, et al. Healthy dietary patterns and risk of breast cancer by molecular subtype. Breast Cancer Res Treat. 2016;155:579–88. doi: 10.1007/s10549-016-3706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung TT, Chiuve SE, Willett WC, et al. Intake of specific fruits and vegetables in relation to risk of estrogen receptor-negative breast cancer among postmenopausal women. Breast Cancer Res Treat. 2013;138:925–30. doi: 10.1007/s10549-013-2484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farvid MS, Chen WY, Michels KB, et al. Fruit and vegetable consumption in adolescence and early adulthood and breast cancer risk: population based study. BMJ. 2016;353:i2343. doi: 10.1136/bmj.i2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–67. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Lenart E. Willett WC. Nutritional Epidemiology. Oxford University Press, USA; 2013. Reproducibility and validity of food frequency questionnaires; pp. 96–141. [Google Scholar]
- 27.Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187:1051–1063. doi: 10.1093/aje/kwx328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muraki I, Imamura F, Manson JE, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–14. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 30.Steinmetz KA, Potter JD, Folsom AR. Vegetables, fruit, and lung cancer in the Iowa Women’s Health Study. Cancer Res. 1993;53:536–43. [PubMed] [Google Scholar]
- 31.Nutrient Database for Standard Reference, Release 14. Department of Agriculture ARS; 2001. [Google Scholar]
- 32.Holland GWA, Unwin ID, Buss DH, et al. The Composition of Foods. Cambridge UK: The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food; 1991. [Google Scholar]
- 33.Tamimi RM, Baer HJ, Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins LC, Marotti JD, Baer HJ, Tamimi RM. Comparison of estrogen receptor results from pathology reports with results from central laboratory testing. J Natl Cancer Inst. 2008;100:218–21. doi: 10.1093/jnci/djm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–32. [PubMed] [Google Scholar]
- 36.Willett WC. Willett WC. Nutritional Epidemiology. Oxford University Press, USA; 2013. Issues in analysis and presentation of dietary data; pp. 305–33. [Google Scholar]
- 37.DerSimonin R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 39.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9(Suppl 2):S73–81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuentes F, Paredes-Gonzalez X, Kong AT. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3′-diindolylmethane: Anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr Pharmacol Rep. 2015;1(3):179–196. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith-Warner SA, Spiegelman D, Yaun SS, et al. Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA. 2001;285:769–76. doi: 10.1001/jama.285.6.769. [DOI] [PubMed] [Google Scholar]
- 42.Bertram JS. Dietary carotenoids, connexins and cancer: what is the connection? Biochem Soc Trans. 2004;32:985–9. doi: 10.1042/BST0320985. [DOI] [PubMed] [Google Scholar]
- 43.Aune D, Chan DS, Vieira AR, et al. Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2012;96:356–73. doi: 10.3945/ajcn.112.034165. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Spiegelman D, Baglietto L, et al. Carotenoid intakes and risk of breast cancer defined by estrogen receptor and progesterone receptor status: a pooled analysis of 18 prospective cohort studies. Am J Clin Nutr. 2012;95:713–25. doi: 10.3945/ajcn.111.014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chlebowski RT, Anderson GL, Aragaki AK, Prentice R. Breast cancer and menopausal hormone therapy by race/ethnicity and body mass index. J Natl Cancer Inst. 2015;108 doi: 10.1093/jnci/djv327. pii: djv327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gram IT, Park SY, Kolonel LN, et al. Smoking and risk of breast cancer in a racially/ethnically diverse population of mainly women who do not drink alcohol: The MEC Study. Am J Epidemiol. 2015;182:917–25. doi: 10.1093/aje/kwv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.https://www.cancer.org/healthy/eat-healthy-get-active/acs-guidelines-nutrition-physical-activity-cancer-prevention/guidelines.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.