Abstract
This phase Ib study randomized patients with stable sickle cell disease (SCD) aged 18–65 years to twice‐daily PF‐04447943 (a phosphodiesterase 9A inhibitor; 5 or 25 mg) or placebo, with/without hydroxyurea coadministration, for up to 29 days. Blood samples were collected at baseline and various posttreatment time points for assessments of PF‐04447943 pharmacokinetics (PKs)/pharmacodynamics (PDs). Change from baseline in potential SCD‐related biomarkers was evaluated. Of 30 patients, 15 received hydroxyurea and 28 completed the study. PF‐04447943, with/without hydroxyurea, was generally well tolerated, with no treatment‐related serious adverse events. Plasma PF‐04447943 exposure was dose proportional. Twice‐daily PF‐04447943 25 mg significantly reduced the number and size of circulating monocyte‐platelet and neutrophil‐platelet aggregates and levels of circulating soluble E‐selectin at day 29 vs. baseline (adjusted P < 0.15). PF‐04447943 demonstrated PK/PD effects suggestive of inhibiting pathways that may contribute to vaso‐occlusion. This study also provides guidance regarding biomarkers for future SCD studies.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑In patients with sickle cell disease (SCD), enhanced adhesiveness of sickle‐shaped RBC red blood cells to the endothelium, adhesive interactions among sickled RBCs, leukocytes, and the endothelium contribute to vaso‐occlusion, which is responsible for numerous complications in SCD.
what question did this study address?
☑This study assessed safety, tolerability, pharmacokinetics, and pharmacodynamics of PF‐04447943, a selective phosphodiesterase 9A inhibitor, with and without coadministration of hydroxyurea in patients with stable SCD and evaluated possible biomarkers for use in future SCD studies.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑PF‐04447943 was well tolerated. The number and size of circulating monocyte‐platelet and neutrophil‐platelet aggregates and the level of circulating soluble E‐selectin were significantly reduced from baseline in patients who received PF‐04447943 25 mg twice daily, suggesting that PF‐04447943 has beneficial effects that may help reduce parameters that contribute to vaso‐occlusion in patients with SCD.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑This study provides guidance regarding biomarkers (leukocyte‐platelet aggregates and soluble E‐selectin) for use in future SCD studies.
A single amino acid mutation in the gene encoding the β‐globin subunit of hemoglobin results in sickle cell disease (SCD), which is characterized by production and polymerization of sickle hemoglobin (HbS) and sickle‐shaped red blood cells (RBCs).1, 2 In the United States, SCD affects ~100,000 individuals.3 Although reliable global estimates for SCD are unavailable, the estimated incidence of sickle cell anemia is 306,000 newborns per year.4 In SCD, enhanced adhesiveness of sickle‐shaped RBCs to the endothelium and adhesive interactions between sickled RBCs and leukocytes (neutrophils, monocytes, and lymphocytes) with the endothelium5, 6, 7, 8 contribute to vaso‐occlusion.9, 10 Vaso‐occlusion is responsible for numerous complications and morbidities in SCD, the phenotypic hallmark being vaso‐occlusive crisis (VOC), a painful, recurring condition associated with hospitalization,11 acceleration of organ damage,12 and premature death.13 .
Cellular adhesion contributes to vaso‐occlusion in SCD, and these cellular adhesive properties may be modulated by intracellular levels of cyclic guanosine monophosphate (cGMP).2, 14 Hydroxyurea, indicated for treatment of SCD, increases the expression of fetal hemoglobin (HbF), which inhibits HbS polymerization. In addition, hydroxyurea may act as a nitric oxide donor to stimulate the production of cGMP.2, 14, 15 Intracellular levels of cGMP are modulated by the enzyme phosphodiesterase 9A (PDE9A), a very‐high–affinity cGMP‐specific phosphodiesterase.2 PDE9A RNA are expressed in the brain and in neutrophils and reticulocytes isolated from individuals with SCD.2, 16 PDE9 plays a role in modulating intracellular levels of cGMP, and a working model is that the degradation of cGMP by PDE9A in neutrophils decreases cGMP levels in SCD, thereby contributing to vascular dysfunction and increasing cell adhesion in leukocytes. PDE9 inhibition increases cGMP levels, leading to reductions in the formation of heterotypic blood cell aggregates and in the inflammatory response.2
PF‐04447943 (Pfizer Inc, Collegeville, PA) is a potent, selective inhibitor of PDE9A,16, 17 with a 50% maximal inhibitory concentration for recombinant human PDE9A of 12 nM and > 78‐fold selectivity for PDE9A over other PDE enzymes.16 Studies in preclinical models demonstrated accumulation of cGMP in mouse brain tissue and dose‐dependent increases in cerebral spinal fluid in the rat after systemic administration of PF‐04447943.18 PF‐04447943 was also shown to achieve improvements in synaptic plasticity and cognitive performance in several rodent cognition models, including working, episodic, and spatial memory, auditory gating, and attention.17, 19 In two SCD models, tumor necrosis factor alpha–treated normal wild‐type mice and the Townes model of SCD,20 PF‐04447943, in combination with hydroxyurea as prophylaxis, improved cellular and plasma pharmacodynamic (PD) markers, including cellular aggregates and soluble adhesion plasma markers.21, 22 Previous clinical studies assessed the pharmacokinetics (PKs)/PDs of PF‐04447943 in healthy human volunteers23, 24 and demonstrated its beneficial effects on cognition in preclinical models17, 19 and in patients with Alzheimer disease, in whom PF‐04447943 was shown to be safe and well tolerated.25
The objectives of this study were to evaluate the safety, tolerability, PKa, and PD of PF‐04447943, with or without coadministration of hydroxyurea, in patients with stable SCD and to evaluate possible biomarkers for use in future SCD studies.
Methods
Patients
Eligible adults aged 18–65 years with a confirmed diagnosis of stable SCD (HbSS or HbS‐β0 thalassemia), defined as no significant complications for ≥ 1 month prior to baseline visit (e.g., VOC, acute chest syndrome, or complication requiring hospitalization) and no blood transfusions for ≥ 2 months. Patients receiving hydroxyurea were required to be on a stable dose for ≥ 8 weeks before participation, with intent to remain on the same dose throughout the trial. Patients not receiving hydroxyurea could not begin hydroxyurea during the study. All patients of childbearing potential were required to use highly effective contraception during the study and for 30 days after the last trial medication dose. Women were considered to be of nonchildbearing potential if they were postmenopausal, had undergone a hysterectomy and/or bilateral oophorectomy, or had medically confirmed ovarian failure.
Key exclusion criteria included: major surgery within 3 months or serious infection within 1 month of baseline visit; history of cerebrovascular accident, seizure disorder, clinically significant orthostatic blood pressure changes or symptoms, malignancy (except basal cell or squamous cell cancer treated and resolved for ≥ 5 years); history or evidence of cardiac disease; diagnosis of acute hepatitis; family history of long QT syndrome and/or electrocardiogram (ECG) abnormalities, Fridericia's corrected QT > 450 millisecond or QRS interval > 120 millisecond by 12‐lead ECG at screening; use of concomitant medications that prolong the QT/QTc interval or lower seizure threshold; systemic therapy with CYP3A inhibitors (within 7 days or 5 half‐lives), or inducers (within 28 days) or PDE5 inhibitors (within 7 days) prior to first dose of trial medication; creatinine clearance < 60 mL/min, hemoglobin < 6 g/dL, alanine aminotransferase/serum glutamic pyruvic transaminase and aspartate aminotransferase/serum glutamic oxaloacetic transaminase > 2 × upper limit of normal; any condition affecting drug absorption; positive urine screening for illicit drug use; regular alcohol consumption of > 14 (women) or 21 (men) drinks per week within 6 months of screening; and for patients taking hydroxyurea, no concomitant use of live vaccines, didanosine, stavudine, or interferon was allowed.
Study design
This multiple‐dose, investigator‐blinded and patient‐blinded, sponsor‐unblinded, randomized, placebo‐controlled, phase Ib study was conducted at 18 centers in Belgium, France, Italy, The Netherlands, the United Kingdom, and the United States between December 2014 and September 2016. The study protocol, amendments, and informed consent documentation were approved by the institutional review boards and/or independent ethics committees at each participating investigational site. The study was conducted in compliance with the ethical principles of the Declaration of Helsinki and with all International Council for Harmonisation Good Clinical Practice guidelines; all patients provided written informed consent prior to study participation. This study is registered with clinicaltrials.gov (NCT02114203).
After a 28‐day screening period, patients were randomized in a 3:1 active‐placebo ratio to PF‐04447943 5 mg, PF‐04447943 25 mg, or placebo through an interactive response system. Study medication was taken orally twice daily with water, with or without food, ~12 hours apart for 29 (+1) days. Patients stayed in the clinical research unit from baseline to day 2; after discharge, they were treated on an outpatient basis, with visits on days 7 (± 2), 14 (± 2), 21 (± 2), and 29 (±1). All patients were seen for a follow‐up visit 30 (± 2) days after the day 29 visit.
Patients received PF‐04447943 25 mg or placebo twice daily (cohort 1). The dose level for cohort 2 was established based on a review of emerging safety and PK data from the first eight patients in cohort 1 at day 7, along with day 29 biomarker data (Figure 1). Following this review, the PF‐04447943 dose of 5 mg twice daily was chosen for investigation in cohort 2 based on PK/PD modeling of available in vitro and in vivo data. Exposure‐response modeling of in vitro PDE9 inhibition and cGMP data were used to inform dose selection. The 5‐mg and 25‐mg doses were chosen because these doses were expected to provide information on different parts of the exposure‐response relationship (i.e., 5‐mg dose on the linear region of the curve and 25‐mg dose on the plateau region of the curve). Hydroxyurea dose adjustments were at the investigator's discretion and may have been implemented to maintain an absolute neutrophil count of at least ~2.0 × 109/L and an absolute reticulocyte count of at least ~100 × 109/L.
Figure 1.
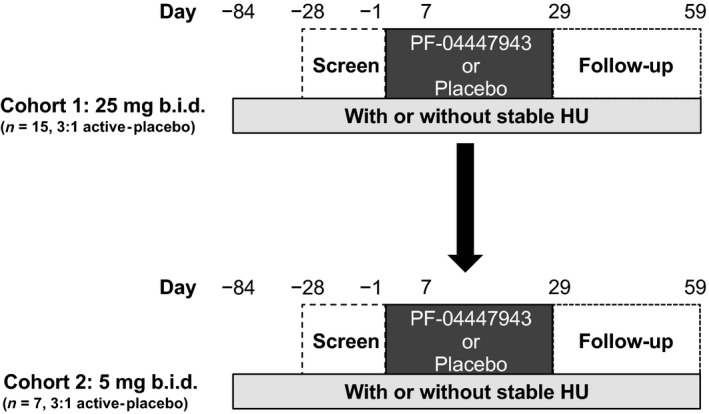
Schematic of the study design. Cohort 1 received 25 mg PF‐04447943 or placebo twice a day for 29 days (n = 15,a 3:1 active‐placebo ratio). Cohort 2 received 5 mg PF‐04447943 or placebo twice a day for 29 days (n = 7, 3:1 active‐placebo ratio). CI, confidence interval; HU, hydroxyurea; ICAM, intercellular adhesion molecule; RBC, red blood cell; VCAM, vascular adhesion molecule. aOne patient discontinued after randomization (n=16) but before receiving study drug.
Safety assessments
Adverse events (AEs), serious adverse events (SAEs), laboratory parameters, physical examinations, and ECGs were reviewed on an ongoing basis. All AEs were coded using the Medical Dictionary for Regulatory Activities version 19.0. All monitors were Pfizer, Inc. employees or employees of the contract research organization paid by Pfizer to monitor the study.
PK assessments
Blood samples (3 mL) for PK analysis of PF‐04447943 were collected before dosing on the day 1, 2, 7, and 29 visits, at 0.5, 1, 2, 4, 8, and 12 hours after dosing on day 1, and at 1 and 2 hours after dosing on day 7. Samples taken prior to dosing required at least a 4‐hour fast. Samples were collected into tubes containing dipotassium ethylenediaminetetraacetic acid and centrifuged at ~1,700 g for 10 minutes at 4°C, and plasma (≥ 1.5 mL) was collected and stored at –20°C until analysis. Plasma samples were analyzed at Wuxi AppTec (Shanghai, China) with a validated, sensitive, and specific high‐performance liquid chromatography tandem mass spectrometric method. The lower limit of quantification (LLOQ) was 1.00 ng/mL. Plasma samples with PF‐04447943 concentrations below the LLOQ were reported as < 1.00 ng/mL.
PF‐04447943 PK parameters were derived from plasma concentration‐time profiles using noncompartmental analysis. Samples below the LLOQ were set to 0, and actual collection times were used for PK analyses. Parameters analyzed were area under the concentration‐time curve from time 0–12 hours postdose (AUC0‐12; determined by the linear/log trapezoidal method); dose‐normalized AUC0‐12 (AUC0‐12 [dn], determined by AUC0‐12/dose); peak plasma concentration (Cmax); dose‐normalized Cmax (Cmax [dn] determined by Cmax/dose); and time to Cmax (Tmax).
Biomarker assessments
A panel of biomarkers preselected as those relevant to SCD included: a panel of soluble adhesion molecules (E‐selectin, P‐selectin, intracellular adhesion molecule, and vascular adhesion molecule (Myriad/RBM; Austin, TX), leukocyte‐platelet aggregates (neutrophil‐platelet and monocyte‐platelet aggregates; Center for Platelet Research Studies (CPRS); Boston, MA); MAC‐1 integrin expression on monocytes and neutrophils (CPRS); microparticles (endothelial‐, RBC‐, and platelet‐derived microparticles; CPRS), and coagulation markers (tissue factor; thrombin‐antithrombin complexes; prothrombin fragments F1 + 2; D‐dimer; Pfizer Clinical Pathology, Groton, CT). Biomarker samples were collected at baseline and prior to dosing on days 1, 2, 7, 14, 21, and 29. There was no a priori ranking of the biomarkers. Based on studies in SCD mice, we hypothesized that cellular aggregates and soluble plasma adhesion biomarkers may be impacted.21,22 Microparticles were not examined in the SCD mouse models.
HbF (%) levels were determined at baseline and prior to dosing on day 29 (Covance Laboratories, Indianapolis, IN). Low HbF levels were defined as < 10% and high baseline HbF levels were ≥ 10%, based on median of the sample distribution.
Neutrophil‐platelet and monocyte‐platelet aggregate number and size were measured as previously described.26, 27 Within 30 minutes of sample collection, 0.3 mL of citrate‐anticoagulated blood was added to 1.2 mL FacsLysing solution (Becton Dickinson, Canaan, CT), mixed gently, then refrigerated until analysis. Samples were centrifuged (5 minutes, 500 g), and the cells were resuspended in 300 μL 10 mM 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid, 0.15 m NaCl, 0.5% bovine serum albumin, and pH 7.4. Samples were stained with a mixture of R Phycoerythrin‐Cyanin 5.1 (PC5)‐conjugated antihuman CD14 monoclonal antibody (directed against the lipopolysaccharide receptor, a monocyte identifier; Beckman Coulter, Carlsbad, CA) and phycoerythrin‐conjugated CD42a monoclonal antibody (directed against glycoprotein IX, a platelet identifier; Pharmingen, San Jose, CA) or immunoglobulin G1kappa (isotype control; Pharmingen). Sample analysis was performed in a FACSCalibur flow cytometer with CellQuest software (Becton Dickinson).26, 27 CD11b on the surface of monocytes and neutrophils in whole unfixed blood was stained with the above mixture of antibodies with the addition of Alexa488‐CD11b (clone ICRF44; Biolegend, San Diego, CA). Monocytes were identified by forward and side light scatter and CD14 expression. The presence of CD42a on monocytes indicated formation of heterotypic aggregates with activated platelets. Results are reported as the number (%) of monocyte‐platelet or neutrophil‐platelet aggregates and the size (mean fluorescence intensity of CD42a) of the aggregates.
Platelet‐rich plasma prepared from citrate‐anticoagulated blood was used to measure RBC‐, endothelial‐, and platelet‐derived microparticles. Samples were stained with fluorescein isothiocyanate–conjugated CD61 (clone Y2/51; Dako/Fisher Scientific, GPIIIa, for identification of platelet‐derived particles), PE‐CD235a (EBioscience/Fisher Scientific, for identification of RBC‐derived microparticles), and separately with fluorescein isothiocyanate‐CD61 and PE‐CD146 (BD Pharmingen, for identification of endothelial‐derived microparticles). Background staining was determined using fluorescently labeled isotype‐matched normal mouse immunoglobulin G.
Statistical analyses
A maximum sample size of 8−16 patients per cohort was determined to satisfy the need to minimize exposure to PF‐04447943 and the need to have sufficient numbers of patients to enable useful conclusions to be drawn. A simulation study showed that under reasonable assumptions regarding biomarker variability and correlations between biomarkers, a total sample size of 32 would result in a minimum of 80% power for detecting a difference (one‐sided P < 0.15) between at least one of two active treatment levels and placebo for at least 1 of 15 biomarkers when using the Holm‐Bonferroni stepdown method, if the true effect size was 50% change from baseline.
The PK concentration analysis population comprised all enrolled patients treated with PF‐04447943 who had ≥ 1 plasma concentration reading, and the PK parameter analysis population comprised all enrolled patients treated with PF‐04447943 who had ≥ 1 of the PK parameters of interest. PK parameters were summarized descriptively by dose. The safety analysis set comprised all patients who received ≥ 1 dose of study medication. AEs, laboratory data, vital signs, and ECGs were summarized descriptively by dose. Biomarker analyses included all subjects who received ≥ 1 dose of study drug (active or placebo) and had baseline evaluation and ≥ 1 observed measure after baseline.
All biomarker values were log‐transformed for the analysis. Changes from baseline in log‐transformed biomarkers were evaluated with repeated‐measures analysis of covariance models for each dose cohort. Models containing terms for time point (each day/combination) and log‐transformed baseline biomarker were fit for each biomarker. Additional models for change at day 29, including log‐transformed baseline biomarker, dose plus background hydroxyurea therapy (yes/no) or baseline HbF (low/high), and corresponding interaction terms for dose by background hydroxyurea therapy or dose by baseline HbF were used to evaluate the biomarker changes in patients with and without hydroxyurea and in patients with high and low baseline HbF. Changes from baseline for each time point are presented as point estimates, 95% confidence intervals, and P values. The Holm‐Bonferroni stepdown procedure was applied to determine which biomarkers showed a significant change from baseline (one‐sided adjusted P < 0.15). All analyses were performed using R 3.3.228 and SAS 9.4 (SAS Institute, Cary, NC).
Results
Patients
Of 30 patients randomized (men, n = 12; women, n = 18), 29 received twice‐daily PF‐04447943 (25 mg, n = 15; 5 mg, n = 7) or placebo (n = 7), and 28 completed the study. One patient discontinued after randomization but before receiving study drug owing to difficulty in collecting blood samples for PK analysis, and one patient receiving PF‐04447943 in cohort 1 withdrew from the study because of an SAE. Of the 30 patients randomized, 15 were taking hydroxyurea. The planned size of cohort 1 was expanded to 16 patients prior to moving to cohort 2 so that sufficient biomarker data could be obtained to enable statistically meaningful conclusions to be drawn. Cohort 2 was assigned a dose of 5 mg twice daily to allow for better characterization of dose‐response relationship of the biomarker effects. The demographic and baseline clinical characteristics of all randomized patients are summarized in Table 1.
Table 1.
Demographics and baseline clinical characteristics: all randomized patients a
| Characteristic | PF‐04447943 5 mg (n = 7) | PF‐04447943 25 mg (n = 16) | Placebo (n = 7) |
|---|---|---|---|
| Sex, n | |||
| Male | 4 | 6 | 2 |
| Female | 3 | 10 | 5 |
| Age, y | |||
| Mean (SD) | 37.9 (10.6) | 36.3 (11.0) | 39.4 (14.0) |
| Range | 24–52 | 23–57 | 25–62 |
| Race, n | |||
| White | 1 | 0 | 0 |
| Black | 6 | 15 | 7 |
| Other | 0 | 1 | 0 |
| Hydroxyurea use, n | |||
| Yes | 6 | 6 | 3 |
| No | 1 | 10 | 4 |
| Weight, kg | |||
| Mean (SD) | 64.0 (10.5) | 66.9 (8.7) | 69.5 (10.8) |
| Range | 48.0–76.0 | 54.1–84.3 | 52.3–82.1 |
| BMI, kg/m2 | |||
| Mean (SD) | 22.6 (2.4) | 23.5 (3.2) | 24.0 (4.6) |
| Range | 18.8–25.5 | 18.1–30.0 | 18.4–30.8 |
BMI, body mass index.
One patient discontinued after randomization but before receiving study drug. All other patients received twice‐daily dosing.
Safety
No deaths occurred during the study. SAEs were reported by three patients, two in the PF‐04447943 5‐mg twice‐daily group (three events of sickle cell pain crisis in one patient, of which two occurred during the nontreatment follow‐up period on days 56 and 83; biliary colic in one patient, which developed during the nontreatment follow‐up on day 44) and one in the PF‐04447943 25 mg twice‐daily group (sickle cell pain crisis, pneumonia) who withdrew from the study on day 8. None of the SAEs were considered related to the study drug by the investigators. No patients had dose reductions or temporary discontinuations attributable to AEs. The most frequently reported treatment‐related AEs (≥ two patients in any cohort) were headache (5 mg twice daily, 0%; 25 mg twice daily, 27%; placebo, 14%), dizziness (5 mg twice daily, 0%; 25 mg twice daily, 13%; placebo, 0%) and fatigue (5 mg twice daily, 0%; 25 mg twice daily, 27%; placebo, 29%). In total, four patients experienced sickle cell pain crisis, one (14%) in the 5 mg twice‐daily group, two (13%) in the 25 mg twice‐daily group, and one (14%) in the placebo group.
PKs
PK parameters and median plasma concentration‐time curves were derived following single oral doses of PF‐04447943 at 5 and 25 mg on day 1 (Table 2 and Figure S1). Based on geometric mean postdose (AUC0‐12) and observed Cmax values, plasma PF‐04447943 exposure seemed to be dose proportional. Moderate interpatient variability based on the geometric percent coefficient of variation was observed (Table 2). The Cmax values on day 7 were similar to those on day 1.
Table 2.
Summary of pharmacokinetic parameters following administration of single oral doses of PF‐04447943 5 mg and 25 mg a
| Parameter | PF‐04447943 5 mg (n = 7) | PF‐04447943 25 mg (n = 15) |
|---|---|---|
| Day 1 | ||
| AUC0−12, ng·h/mL | 242 (35) | 1170 (29) |
| AUC0−12 (dn), ng·h/mL/mg | 48 (35) | 47 (29) |
| Cmax, ng/mL | 45.8 (39) | 248.2 (31) |
| Cmax (dn), ng/mL/mg | 9.2 (39) | 9.9 (31) |
| Tmax, hb | 1.9 (1.0–4.0) | 1.0 (0.5–4.1) |
| Day 7 | ||
| Cmax, ng/mL | 59.5 (33.1) | 284.0 (28.6) |
AUC0−12, area under the concentration‐time curve from time 0−12 hours postdose; Cmax, maximum concentration; dn, dose normalized to 1 mg; Tmax, time to Cmax.
aValues are geometric mean (geometric percent coefficient of variation) unless otherwise noted; bValues are median (range).
Biomarkers
An exploratory objective of this study was to evaluate biomarkers that may be informative in demonstrating the pharmacologic effect of PF‐04447943 in SCD. In both PF‐04447943 groups, no consistent changes from baseline were observed prior to day 29 (data not shown). Overall percent changes were observed in cellular aggregates and soluble E‐selectin at day 29 in the 25 mg twice‐daily group (Figure 2 ). PF‐04447943 treatment did not significantly alter HbF or Hb levels, MAC‐1 expression on monocytes or neutrophils, circulating microparticles, or coagulation parameters at day 29 (Figure 2 ), as well as a panel of clinical laboratory data (results not shown). At day 29, treatment with PF‐04447943 25 mg twice daily significantly reduced the number and size of circulating monocyte‐platelet aggregates from baseline by 52% and 36%, respectively (adjusted P = 0.025 and P = 0.140, respectively) and the number and size of neutrophil‐platelet aggregates by 46% and 34%, respectively (adjusted P = 0.095 and P = 0.018, respectively) (Figures 2 and 3 a,b). In the 5 mg twice‐daily group, the levels of circulating soluble E‐selectin were reduced by 11% (adjusted P = 0.064; Figures 2 and 3 c). A trend was observed in the decrease in the levels of soluble P‐selectin but lacked statistical significance (Figures 2 and 3 c). In the 5‐mg twice‐daily group, trends were similar to those in the 25 mg twice‐daily group but most lacked statistical significance, with the exception of neutrophil‐platelet aggregate size (−43%; adjusted P = 0.020; Figure 3 b). No significant changes from baseline in any biomarker were observed at day 29 in the placebo group (Figure 3 ).
Figure 2.
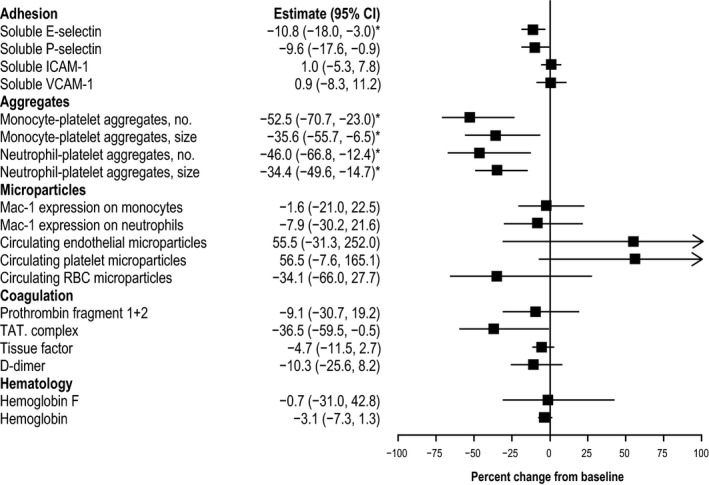
Percent change from baseline at day 29 in biomarkers with PF‐04447943 25 mg twice daily. The percent changes from baseline to day 29 in adhesion, aggregates, microparticle, coagulation, and hematology biomarkers are depicted in the forest plot. Estimates and 95% confidence intervals are based on repeated measures model. Statistically significant (P < 0.15 after multiplicity correction using Holm‐Bonferroni stepdown procedure) changes from baseline are indicated by an asterisk. CI, confidence interval; ICAM, intercellular adhesion molecule; MPA, monocyte‐platelet aggregates; NPA, neutrophil‐platelet aggregate; RBC, red blood cell; TAT, thrombin‐antithrombin; VCAM, vascular adhesion molecule.
Figure 3.
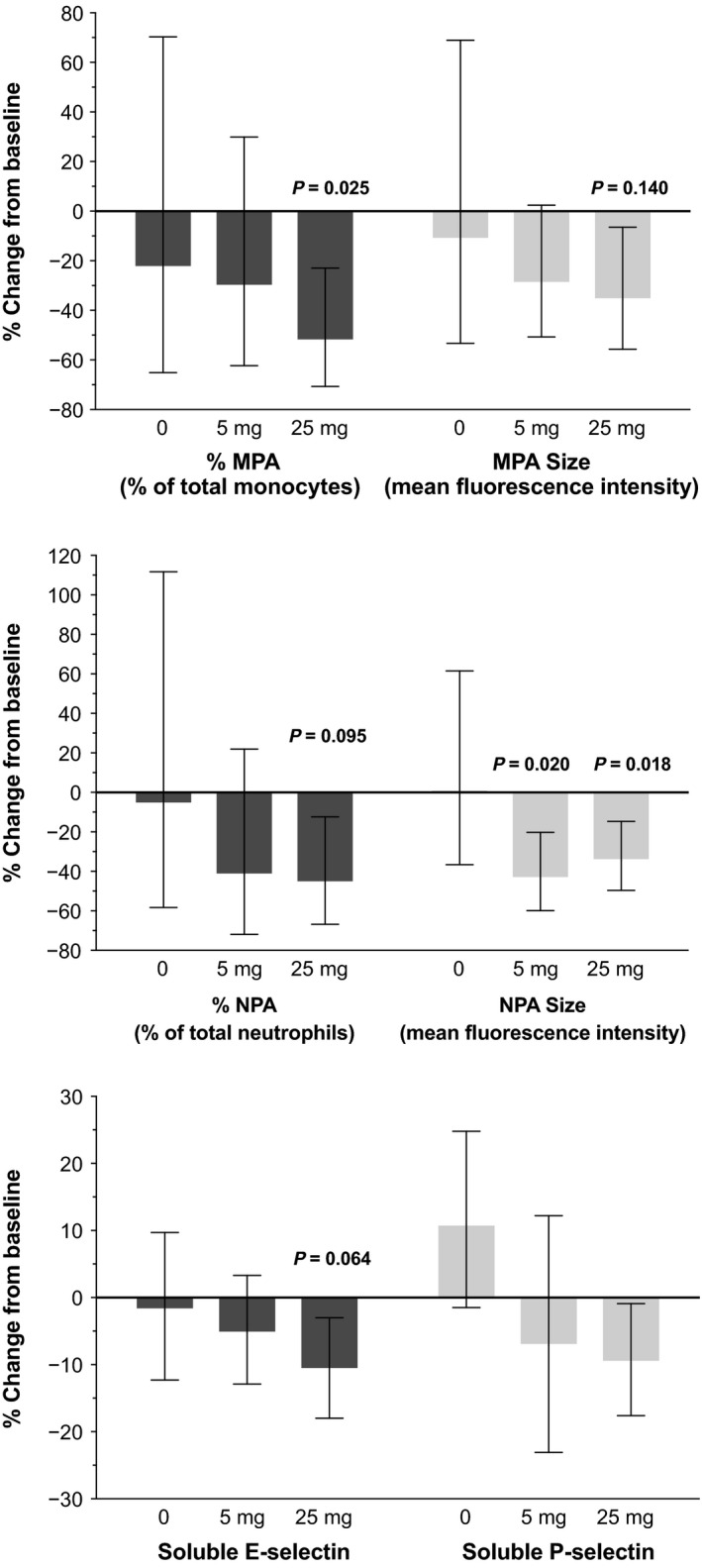
Percent change from baseline at day 29 in cellular aggregates and soluble P‐selectin and E‐selectin levels. (a) Change in monocyte‐platelet aggregates, number, and size. (b) Change in neutrophil‐platelet aggregates, number, and size. (c) Change in plasma‐soluble E‐selectin and P‐selectin. Bars and whiskers represent estimates and 95% confidence intervals (CIs), based on repeated measures model. The P values of statistically significant (P < 0.15 after multiplicity correction using Holm‐Bonferroni stepdown procedure) changes from baseline are reported above corresponding estimates. MPA, monocyte‐platelet aggregates; NPA, neutrophil‐platelet aggregate.
Baseline HbF levels were determined at study initiation. As expected, patients who were on stable hydroxyurea had significantly elevated baseline HbF levels compared with patients not on hydroxyurea (Figure 4 a). No significant changes were observed at day 29 in HbF level in the 5‐mg or 25‐mg twice‐daily groups (Figure 2 ). Additional analyses found no significant differences in biomarker changes at day 29 in patients with and without hydroxyurea cotherapy and in patients with low and high baseline HbF levels (Figure 4 b and S2 ).
Figure 4.
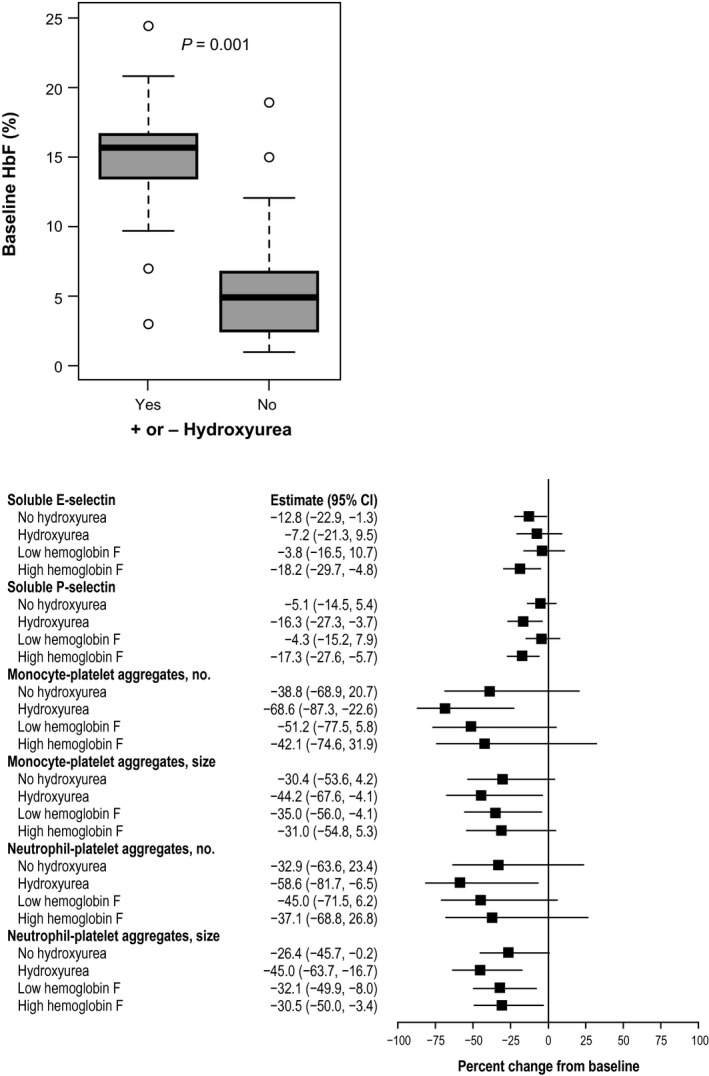
Analysis of biomarkers by hydroxyurea (HU) use and fetal hemoglobin (HbF) levels. (a) Box and whiskers plot of HbF baseline levels (%) in patients (n = 27) with and without HU treatment. Boxes present area between 25th and 75th percentile values. Whiskers extend 1.5 of interquartile range from the box. Line within each box is the median value. The circles represent individual observations that could be considered outliers. (b) The percent changes from baseline at day 29 for biomarkers in subgroups of patients (PF‐04447943 25 mg twice daily), with vs. without HU cotreatment, and low vs. high baseline HbF levels. Low HbF levels were defined as < 10%, and high baseline HbF levels were 10% or higher, based on median of the sample distribution. Forest plot presents estimates and 95% confidence intervals, based on repeated measures model.
Discussion
This study represents the first evaluation of PF‐04447943, a PDE9A inhibitor, in patients with SCD. At the doses assessed (5 and 25 mg twice daily), PF‐04447943 displayed dose‐proportional PK and was well tolerated throughout the 29‐day treatment period. Although all SAEs reported occurred in patients who received PF‐04447943, all were expected AEs in patients with SCD, and none were considered related to PF‐04447943 in the judgment of the investigators. Safety, tolerability, and PK parameters were in agreement with previous clinical studies of PF‐04447943 in healthy volunteers and patients with Alzheimer disease.25, 29
As for treatment‐related changes in biomarkers, the number and size of circulating monocyte‐platelet and neutrophil‐platelet aggregates and the level of circulating soluble E‐selectin were significantly reduced from baseline in patients who received PF‐04447943 25 mg twice daily, suggesting that PF‐04447943 has beneficial effects that may help reduce parameters that contribute to vaso‐occlusion in SCD. Owing to a high degree of interpatient variability and possibly relatively small sample size, no apparent synergistic effect was observed when PF‐04447943 was given with hydroxyurea for the biomarkers analyzed.
There are no existing validated biomarkers for SCD. However, our clinical findings are consistent with changes observed preclinically with a combination of PF‐04447943 and hydroxyurea in two models of SCD.21, 22 In these studies, the dynamics of cellular interactions were visualized using intravital microscopy. In the first model, C57BL/6J wild‐type mice challenged with tumor necrosis factor alpha, which induces an acute inflammatory response in the cremaster microcirculation characterized by endothelial neutrophil adhesion and the formation of multicellular aggregates, co‐administration of hydroxyurea and PF‐04447943 prior to the challenge reduced endothelial neutrophil adhesion and formation of neutrophil‐platelet aggregates and increased the number and velocity of rolling neutrophils compared with results in saline‐treated mice. In addition, levels of plasma soluble E‐selectin and vascular adhesion molecule‐1 were reduced with the combination of PF‐04447943 and hydroxyurea. In a second model, using Townes SCD mice, which experienced an acute inflammatory response and vaso‐occlusion upon cremaster muscle exposure, pretreatment with the combination of PF‐04447943 and hydroxyurea also decreased neutrophil adhesion and increased neutrophil rolling.21 A response was also observed in the number of neutrophil‐platelet aggregates; administration of PF‐04447943 alone resulted in a decrease in neutrophil‐platelet aggregates with a more pronounced effect observed with the coadministration of hydroxyurea. In a prophylactic setting with repeat dosing, Townes SCD mice treated with PF‐04447943 in combination with hydroxyurea twice daily for 4 weeks achieved an increase in neutrophil rolling, a reduction in neutrophil adhesion, a 73% reduction in the formation of neutrophil‐platelet aggregates, and an 11% decrease in plasma‐soluble E‐selectin compared with vehicle‐treated mice.22
Taking together these previously published murine results and the current human results, we propose a working model for SCD in which treatment with PF‐04447943 with or without hydroxyurea reduces the steady‐state concentrations of monocyte‐platelet and neutrophil‐platelet aggregates as well as the chronic, moderate activation state of endothelial cells.
Data suggest a role for selectins in VOC, and targeting leukocyte selectin adhesion may be therapeutic in SCD.9, 10, 30 Preclinical and clinical data from studies of rivipansel (previously known as GMI‐1070), a pan selectin antagonist with E‐selectin selectivity, provide supporting evidence that changes in these biomarkers may lead to beneficial clinical changes in these patients, albeit from data obtained in an acute hospital setting for VOC. Mechanistically, rivipansel targets the direct interaction of vascular endothelial cells with leukocytes, whereas modulation of cGMP is upstream. In the Berkeley SCD mouse model using intravital microscopy, treatment with rivipansel administered after a challenge increased the leukocyte rolling fraction and the velocity of rolling leukocytes, reduced leukocyte adhesion, inhibited RBC–white blood cell interactions, and improved blood flow and survival of the SCD mouse following the challenge.30 In a phase I study, intravenous administration of rivipansel in patients with clinically stable SCD resulted in decreases in markers of leukocyte activation, platelet activation, vascular inflammation (including soluble E‐selectin and intracellular adhesion molecule‐1), monocyte activation, and coagulation system activation.31 In a randomized, placebo‐controlled phase II study of hospitalized patients with SCD and VOC, rivipansel showed trends toward improved time to VOC resolution, length of stay, and parenteral opioid requirement.32
Several limitations of the current study deserve mention. Although, as expected, HbF levels were higher at baseline in patients receiving hydroxyurea, the biomarker trends in patients receiving PF‐04447943, a PDE9 inhibitor, were similar for both groups, suggesting that the PDE9‐mediated effects are separate from those mediated by HbF. However, the small sample size did not allow for definitive conclusions to be made regarding safety or for meaningful comparisons between patients receiving and not receiving hydroxyurea with respect to additivity or synergy. The duration of the study was not long enough to capture changes in HbF. Although a PK/PD relationship for PF‐04447943–mediated elevations in CSF cGMP was established in the normal healthy‐volunteer study,24 the assessment of plasma cGMP levels found no significant changes with PF‐04447943 treatment in the current study (data not shown). Plasma cGMP does not specifically reflect PDE9 activity due to the activity of other PDEs in plasma, and a cellular assay for cGMP based on levels in neutrophils was not feasible owing to large blood volume requirements. Future studies of a longer duration are needed to determine whether the observed biomarker changes lead to clinically meaningful reductions in the frequency, duration, and severity of VOC.
In conclusion, multiple doses of PF‐04447943, with and without hydroxyurea, administered to patients with stable SCD were generally well tolerated and showed PD effects suggestive of a protective effect against vaso‐occlusion. The plasma exposure of PF‐04447943 seemed to be dose proportional over the dose range tested. The time‐dependent and dose‐dependent reductions in E‐selectin and in the size and number of neutrophil‐platelet and monocyte‐platelet aggregates observed with PF‐04447943 25 mg twice daily bridge previous preclinical and clinical data and provide guidance for dosing in future clinical studies of PF‐04447943, and perhaps other drugs, in patients with SCD.
Clinicaltrials.gov Identifier
Funding
This study was sponsored by Pfizer.
Conflict of Interest
R.J.C., D.B., D.R., D.D.P., and N.C. are employees of Pfizer Inc and may own stock/options in the company. B.T. was an employee of Pfizer, Inc at the time of this study. J.H. is a consultant for Bluebird Bio, GBT, and Pfizer, and a member of the Speakers’ Bureau for Addmedica, Novartis, and Terumo BCT. A.D.M. has served as a consultant for Instrumentation Laboratory; receives research funding from Baxalta, Eisai, GLSynthesis, Ionis, Ironwood, Pfizer, and Sysmex; and is a member of advisory committees for AstraZeneca and Janssen. A.L.F. has received research funding from Baxalta, Eisai, GLSynthesis, Ionis, Ironwood, Pfizer, and Sysmex.
Author Contributions
All authors wrote the manuscript. R.J.C., D.B., D.D.P., B.T., and N.C. designed the research. J.H. performed the research. D.B., D.R., D.D.P., A.D.M., and A.L.F. analyzed the data.
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer, Inc. will provide access to individual deidentified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or the European Union or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supporting information
Figure S1. Analysis of biomarkers by fetal hemoglobin (HbF) levels and hydroxyurea use.
Figure S2. Analysis of biomarkers by fetal hemoglobin (HbF) levels and hydroxyurea use. Changes from baseline at day 29 for biomarkers in subgroups of patients (PF‑04447943 25 mg twice daily) with low vs. high baseline HbF levels (A), and with hydroxyurea (HU) co‐treatment vs. without HU (B). Low HbF levels were defined as <10% and high baseline HbF levels were 10% or higher, based on median of the sample distribution. Forest plot presents estimates and 95% confidence intervals, based on repeated measures model.
Acknowledgments
This study was sponsored by Pfizer. Medical writing and editorial support were provided by Bina J. Patel, PharmD, of Peloton Advantage (Parsippany, NJ) and funded by Pfizer, Inc. No author received an honorarium related to the development of this manuscript.
References
- 1. Piel, F.B. , Steinberg, M.H. & Rees, D.C. Sickle cell disease. N. Engl. J. Med. 376, 1561–1573 (2017). [DOI] [PubMed] [Google Scholar]
- 2. Almeida, C.B. et al Hydroxyurea and a cGMP‐amplifying agent have immediate benefits on acute vaso‐occlusive events in sickle cell disease mice. Blood 120, 2879–2888 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hassell, K.L. Population estimates of sickle cell disease in the U.S. Am. J. Prev. Med. 38, S512–S521 (2010). [DOI] [PubMed] [Google Scholar]
- 4. Piel, F.B. , Hay, S.I. , Gupta, S. , Weatherall, D.J. & Williams, T.N. Global burden of sickle cell anaemia in children under five, 2010‐2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 10, e1001484 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoover, R. , Rubin, R. , Wise, G. & Warren, R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood 54, 872–876 (1979). [PubMed] [Google Scholar]
- 6. Turhan, A. , Weiss, L.A. , Mohandas, N. , Coller, B.S. & Frenette, P.S. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc. Natl. Acad. Sci. USA 99, 3047–3051 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belcher, J.D. et al Critical role of endothelial cell activation in hypoxia‐induced vasoocclusion in transgenic sickle mice. Am. J. Physiol. Heart Circ. Physiol. 288, H2715–H2725 (2005). [DOI] [PubMed] [Google Scholar]
- 8. Zennadi, R. , Chien, A. , Xu, K. , Batchvarova, M. & Telen, M.J. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood 112, 3474–3483 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frenette, P.S. & Atweh, G.F. Sickle cell disease: old discoveries, new concepts, and future promise. J. Clin. Invest. 117, 850–858 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang, D. , Xu, C. , Manwani, D. & Frenette, P.S. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood 127, 801–809 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Powars, D.R. , Chan, L.S. , Hiti, A. , Ramicone, E. & Johnson, C. Outcome of sickle cell anemia: a 4‐decade observational study of 1056 patients. Medicine 84, 363–376 (2005). [DOI] [PubMed] [Google Scholar]
- 12. Platt, O.S. et al Pain in sickle cell disease. Rates and risk factors. N. Engl. J. Med. 325, 11–16 (1991). [DOI] [PubMed] [Google Scholar]
- 13. Darbari, D.S. et al Severe painful vaso‐occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS One 8, e79923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Canalli, A.A. , Franco‐Penteado, C.F. , Saad, S.T. , Conran, N. & Costa, F.F. Increased adhesive properties of neutrophils in sickle cell disease may be reversed by pharmacological nitric oxide donation. Haematologica 93, 605–609 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Cokic, V.P. et al Hydroxyurea induces fetal hemoglobin by the nitric oxide‐dependent activation of soluble guanylyl cyclase. J. Clin. Invest. 111, 231–239 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kleiman, R.J. et al Phosphodiesterase 9A regulates central cGMP and modulates responses to cholinergic and monoaminergic perturbation in vivo. J. Pharmacol. Exp. Ther. 341, 396–409 (2012). [DOI] [PubMed] [Google Scholar]
- 17. Hutson, P.H. et al The selective phosphodiesterase 9 (PDE9) inhibitor PF‐04447943 (6‐[(3S,4S)‐4‐methyl‐1‐(pyrimidin‐2‐ylmethyl)pyrrolidin‐3‐yl]‐1‐(tetrahydro‐2H‐py ran‐4‐yl)‐1,5‐dihydro‐4H‐pyrazolo[3,4‐d]pyrimidin‐4‐one) enhances synaptic plasticity and cognitive function in rodents. Neuropharmacology 61, 665–676 (2011). [DOI] [PubMed] [Google Scholar]
- 18. Verhoest, P.R. et al Design and discovery of 6‐[(3S,4S)‐4‐methyl‐1‐(pyrimidin‐2‐ylmethyl)pyrrolidin‐3‐yl]‐1‐(tetrahydro‐2H‐pyr an‐4‐yl)‐1,5‐dihydro‐4H‐pyrazolo[3,4‐d]pyrimidin‐4‐one (PF‐04447943), a selective brain penetrant PDE9A inhibitor for the treatment of cognitive disorders. J. Med. Chem. 55, 9045–9054 (2012). [DOI] [PubMed] [Google Scholar]
- 19. Vardigan, J.D. , Converso, A. , Hutson, P.H. & Uslaner, J.M. The selective phosphodiesterase 9 (PDE9) inhibitor PF‐04447943 attenuates a scopolamine‐induced deficit in a novel rodent attention task. J. Neurogenet. 25, 120–126 (2011). [DOI] [PubMed] [Google Scholar]
- 20. Wu, L.C. , Sun, C.W. , Ryan, T.M. , Pawlik, K.M. , Ren, J. & Townes, T.M. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood 108, 1183–1188 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jasuja, R. , Patel Hett, S. , Fruebis, J. & Pittman, D. PDE‐9 inhibition combined with hydroxyurea is beneficial in vaso‐occlusive crisis in mouse model of sickle cell disease [abstract]. Blood 124, 2694 (2014). [Google Scholar]
- 22. Jasuja, R. , Parks, E. , Murphy, J.E. & Pittman, D.D. Chronic administration of the PDE9 inhibitor PF‐04447943 reduces leukocyte‐platelet aggregates and markers of endothelial activation in a mouse model of sickle cell disease [abstract]. Blood 128, 1293 (2016). [Google Scholar]
- 23. Evans, R.M. et al Safety and pharmacokinetics of PF‐04447943, a PDEA inhibitor, in single and multiple dose phase 1 studies in healthy volunteers [abstract O3‐05‐04]. Alzheimers Dement. 6, S135 (2010). [Google Scholar]
- 24. Nicholas, T. et al PF‐04447943, a novel PDE9A inhibitor, increases CGMP levels in cerebrospinal fluid: translation from non‐clinical species to healthy human volunteers [abstract P2‐240]. Alzheimers Dement. 5, P330–P331 (2009). [Google Scholar]
- 25. Schwam, E.M. et al A multicenter, double‐blind, placebo‐controlled trial of the PDE9A inhibitor, PF‐04447943 Alzheimer's disease. Curr. Alzheimer Res. 11, 413–421 (2014). [DOI] [PubMed] [Google Scholar]
- 26. Michelson, A.D. , Barnard, M.R. , Krueger, L.A. , Frelinger, A.L. III & Furman, M.I. Evaluation of platelet function by flow cytometry. Methods 21, 259–270 (2000). [DOI] [PubMed] [Google Scholar]
- 27. Gerrits, A.J. , Frelinger, A.L. III & Michelson, A.D. Whole blood analysis of leukocyte‐platelet aggregates. Curr. Protoc. Cytom. 78, 6.15.1–6.15.10 (2016). [DOI] [PubMed] [Google Scholar]
- 28. R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing <https://www.r-project.org/>. (2016) Accessed: October 9, 2018.
- 29. Xie, R. , Tan, B. & Harnisch, L.O . Population pharmacokinetics of PF‐04447943 in health volunteers and adult patients with Alzheimer's disease or sickle cell disease [poster]. Presented at: Annual Meeting of the Population Approach Group in Europe; June 6–9, 2017; Budapest, Hungary.
- 30. Chang, J. , Patton, J.T. , Sarkar, A. , Ernst, B. , Magnani, J.L. & Frenette, P.S. GMI‐1070, a novel pan‐selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood 116, 1779–1786 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wun, T. et al Phase 1 study of the E‐selectin inhibitor GMI 1070 in patients with sickle cell anemia. PLoS One 9, e101301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Telen, M.J. et al Randomized phase 2 study of GMI‐1070 in SCD: reduction in time to resolution of vaso‐occlusive events and decreased opioid use. Blood 125, 2656–2664 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Analysis of biomarkers by fetal hemoglobin (HbF) levels and hydroxyurea use.
Figure S2. Analysis of biomarkers by fetal hemoglobin (HbF) levels and hydroxyurea use. Changes from baseline at day 29 for biomarkers in subgroups of patients (PF‑04447943 25 mg twice daily) with low vs. high baseline HbF levels (A), and with hydroxyurea (HU) co‐treatment vs. without HU (B). Low HbF levels were defined as <10% and high baseline HbF levels were 10% or higher, based on median of the sample distribution. Forest plot presents estimates and 95% confidence intervals, based on repeated measures model.


