Abstract
Background
Postoperative nausea and vomiting (PONV) is a common complication following general anaesthesia. It may be associated with patient dissatisfaction, increased costs of treatment, and unintended admission to hospital.
Supplemental intravenous crystalloid administration in the perioperative period may be a simple intervention to prevent PONV.
Objectives
To assess whether supplemental intravenous crystalloid administration prevents PONV in patients undergoing surgical procedures under general anaesthesia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 7), MEDLINE (1946 to August 2018), Embase (1947 to August 2018), and the Cumulative Index of Nursing and Allied Health Literature (CINAHL; 1971 to August 2018). We searched clinical trials registers for ongoing or unpublished completed studies (August 2018), handsearched conference proceedings of anaesthesiology societies, as published in three major journals (British Journal of Anaesthesia, European Journal of Anaesthesiology, and Anesthesiology; August 2018), and conducted backward and forward citation searching of relevant articles.
Selection criteria
We included randomized controlled trials of participants older than six months undergoing surgical procedures under general anaesthesia and given supplemental perioperative intravenous crystalloids, defined as a volume larger than that received by a comparator group, to prevent PONV.
Data collection and analysis
We used the standard methodological procedures described by Cochrane.
Main results
We included 41 studies (4224 participants). Participants underwent ambulatory or short length of stay surgical procedures, and were predominantly American Society of Anesthesiology (ASA) class I or II. There is one study awaiting classification and three ongoing studies. All studies took place in surgical centres, and were conducted in geographically diverse settings. Risk of bias was generally unclear across all domains.
Supplemental intravenous crystalloid administration probably reduces the cumulative risk of postoperative nausea (PON) (risk ratio (RR) 0.62, 95% confidence interval (CI) 0.51 to 0.75; 18 studies; 1766 participants; moderate‐certainty evidence). When the postoperative period was divided into early (first six hours postoperatively) and late (at the time point closest to or including 24 hours postoperatively) time points, the intervention reduced the risk of early PON (RR 0.67, 95% CI 0.58 to 0.78; 20 studies; 2310 participants; moderate‐certainty evidence) and late PON (RR 0.47, 95% CI 0.32 to 0.69; 17 studies; 1682 participants; moderate‐certainty evidence).
Supplemental intravenous crystalloid administration probably reduces the risk of postoperative vomiting (POV) (RR 0.50, 95% CI 0.40 to 0.63; 20 studies; 1970 participants; moderate‐certainty evidence). The intervention specifically reduced both early POV (RR 0.56, 95% CI 0.41 to 0.76; 19 studies; 1998 participants; moderate‐certainty evidence) and late POV (RR 0.48, 95% CI 0.29 to 0.79; 15 studies; 1403 participants; moderate‐certainty evidence).
Supplemental intravenous crystalloid administration probably reduces the need for pharmacologic treatment of PONV (RR 0.62, 95% CI 0.51 to 0.76; 23 studies; 2416 participants; moderate‐certainty evidence).
The effect of supplemental intravenous crystalloid administration on the risk of unplanned postoperative admission to hospital is unclear (RR 1.05, 95% CI 0.77 to 1.43; 3 studies; 235 participants; low‐certainty evidence).
No studies reported serious adverse events that may occur following supplemental perioperative intravenous crystalloid administration (i.e. admission to high‐dependency unit, postoperative cardiac or respiratory complication, or death).
Authors' conclusions
There is moderate‐certainty evidence that supplemental perioperative intravenous crystalloid administration reduces PON and POV, in ASA class I to II patients receiving general anaesthesia for ambulatory or short length of stay surgical procedures. The intervention probably also reduces the risk of pharmacologic treatment for PONV. The effect of the intervention on the risk of unintended postoperative admission to hospital is unclear. The risk of serious adverse events resulting from supplemental perioperative intravenous crystalloid administration is unknown as no studies reported this outcome. The one study awaiting classification may alter the conclusions of the review once assessed.
Plain language summary
Extra intravenous fluid given during surgery to prevent nausea and vomiting
Review question
This review looks at whether giving extra intravenous fluid to people during general anaesthesia prevents nausea and vomiting after their surgery is done.
Background
Nausea and vomiting is a common complication after having general anaesthetic for surgery. About 30% of people suffer from nausea and vomiting after surgery, even after receiving medication intended to prevent it.
During surgery, a patient receives salt‐containing fluid through an intravenous drip and the amount of fluid given may affect how they feel afterwards. Some complications, like nausea and vomiting, may be reduced after getting extra intravenous fluid during surgery. Some complications, like shortness of breath, may be worse with extra fluid.
Search date
The search was up‐to‐date as of August 2018.
Study characteristics
We looked at studies where people had general anaesthesia for surgery, and received larger or smaller amounts of intravenous fluid, and were later checked to see if they developed nausea and vomiting after their surgeries were done. We found 41 studies, with 4224 participants analysed in our review.
Key results
Our review suggests that giving people extra intravenous fluid during surgery under general anaesthesia probably decreases the risk of having either nausea or vomiting after surgery, and probably reduces the need for medication to treat nausea.
It is unclear how giving extra intravenous fluid affects the risk of unexpectedly needing hospital admission after minor surgery. No studies looked at whether extra intravenous fluid makes other complications worse.
Certainty of the evidence
There are two reasons why the conclusions of this review may not be exactly correct. First, many of the studies were not designed perfectly. Second, the studies did not agree on exactly how helpful the extra intravenous fluids were for preventing nausea and vomiting. Most studies did find it at least somewhat helpful.
Summary of findings
Summary of findings for the main comparison. Supplemental IV crystalloid compared to control IV crystalloid volume for postoperative nausea and vomiting.
| Supplemental IV crystalloid compared to comparator IV crystalloid volume for preventing postoperative nausea and vomiting. | |||||
| Patient or population: participants aged 6 months or older undergoing surgical procedures under general anaesthesia Setting: surgical centres in North America, South America, Europe, Africa, and Asia Intervention: perioperative administration of IV crystalloid volume larger than that received by the comparator group Comparator: perioperative administration of an IV crystalloid volume smaller than that received by the intervention group | |||||
| Outcome | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Risk with comparator IV crystalloid | Risk with supplemental IV crystalloid | ||||
| Risk of PON, defined as the presence of subjective nausea, reported dichotomously or based on a study‐defined dichotomous threshold on a continuous scale such as a VAS | Cumulative events, explicitly reported for the entire study period | RR 0.62 (0.51 to 0.75) | 1766 (18 RCTs) | ⊕⊕⊕⊝ moderate1 | |
| 482 per 1000 | 183 fewer per 1000 (120 to 236 fewer) |
||||
| Early events, occurring in the first 6 hours postoperatively | RR 0.67 (0.58 to 0.78) | 2310 (20 RCTs) |
⊕⊕⊕⊝ moderate1 | ||
| 307 per 1000 | 101 fewer per 1000 (68 to 129 fewer) | ||||
| Late events, occurring at the time point closest to or including 24 hours postoperatively | RR 0.47 (0.32 to 0.69) |
1682 (17 RCTs) |
⊕⊕⊕⊝ moderate1 | ||
| 187 per 1000 | 99 fewer per 1000 (58 to 127 fewer) |
||||
| Risk of POV, reported dichotomously by any discrete episodes of vomiting | Cumulative events, explicitly reported for the entire study period | RR 0.50 (0.40 to 0.63) | 1970 (20 RCTs) | ⊕⊕⊕⊝ moderate1 | |
| 295 per 1000 | 147 fewer per 1000 (109 to 177 fewer) |
||||
| Early events, occurring in the first 6 hours postoperatively | RR 0.56 (0.41 to 0.76) | 1998 (19 RCTs) |
⊕⊕⊕⊝ moderate1 | ||
| 106 per 1000 | 47 fewer per 1000 (25 to 63 fewer) |
||||
| Late events, occurring at the time point closest to or including 24 hours postoperatively | RR 0.48 (0.29 to 0.79) |
1403 (15 RCTs) |
⊕⊕⊕⊝ moderate1 | ||
| 68 per 1000 | 35 fewer per 1000 (14 to 48 fewer) |
||||
| Risk of requiring pharmacologic treatment for PONV, reported dichotomously as the use of any medication intended to treat nausea or vomiting during the postoperative period | Cumulative events, explicitly reported for the entire study period | RR 0.62 (0.51 to 0.76) | 2416 (23 RCTs) | ⊕⊕⊕⊝ moderate1 | |
| 284 per 1000 | 108 fewer per 1000 (68 to 139 fewer) |
||||
| Risk of unintended postoperative admission to hospital, reported dichotomously as admission to an inpatient unit of a participant after an intended ambulatory surgical procedure | Cumulative events, explicitly reported for the entire study period | RR 1.05 (0.77 to 1.43) | 235 (3 RCTs) | ⊕⊕⊝⊝ low2 | |
| 288 per 1000 | 14 more per 1000 (66 fewer to 124 more) |
||||
| Risk of suffering a serious adverse event, reported dichotomously as the occurrence of any of: admission to high‐dependency unit, postoperative cardiac or respiratory complication, or death | Cumulative events, explicitly reported for the entire study period | ‐ | This outcome was not reported for included trials | ‐ | |
| ‐ | ‐ | ||||
| * For all outcomes, the assumed and corresponding risks (and their 95% CI) are based on the proportion of events in the comparator and intervention groups, respectively. CI: confidence interval; PON: postoperative nausea;POV: postoperative vomiting; RR: risk ratio; VAS: visual analogue scale | |||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate‐certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low‐certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low‐certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1Downgraded one level due to risk of publication bias, following inspection of funnel plot.
2Downgraded two levels due to imprecision and inconsistency.
Background
Description of the condition
Postoperative nausea and vomiting (PONV) is a common and dreaded complication following anaesthesia. In the absence of risk factors, the baseline risk of PONV is 10%. The presence of female gender, history of motion sickness or PONV, non‐smoking status, and the use of postoperative opioids increase PONV risk to as high as 79% (Apfel 1999). Even with the administration of prophylactic antiemetic medications, the risk of PONV can still be approximately 30% (Habib 2006; Watcha 1992). Complications after surgery lead to patient dissatisfaction and it has been shown that patients rank PONV a highly undesirable complication, wishing to avoid it even more than postoperative pain (Eberhart 2002; Macario 1999; Myles 2000). PONV is so distressing that patients are willing to pay out of pocket to prevent its occurrence (Gan 2001).
Although not usually life‐threatening, PONV may lead to complications commonly associated with vomiting, including dehydration, electrolyte imbalance, and aspiration of gastric contents. In some surgical cases, PONV has also led to: wound complications, oesophageal rupture, subcutaneous emphysema, pneumomediastinum, and bilateral pneumothoraces (Atallah 2004; Bremner 1993; Schumann 1999; Temes 1999; Thompson 1978). PONV is among the most frequently observed complications in the postanaesthesia care unit (PACU), and its presence correlates strongly with delayed discharge from the PACU, unanticipated admission following ambulatory surgery, and increased costs (Gold 1989; Parra‐Sanchez 2012; Wetchler 1992). Therefore, PONV leads not only to patient dissatisfaction, but also increased costs related to length of hospital stay.
PONV hinders patient mobilization and delays resumption of oral intake of food, fluids, and medications. Therefore its prevention is typically included in early recovery after surgery programmes (Mortensen 2014). Preventing PONV positively impacts patient satisfaction, surgical outcomes, and resource utilization.
There are numerous prophylactic treatments for PONV. For instance, ondansetron 4 mg intravenously (0.1 mg/kg in children) is a commonly used pharmacologic antiemetic. Dexamethasone 4 mg to 10 mg (0.1 mg/kg in children) has also been demonstrated to have antiemetic properties, and is safe to use in a surgical population (De Oliviera 2013; Polderman 2018). Other medications shown to prevent PONV in meta‐analysis include tropisetron, dolasetron, cyclizine, granisetron, and droperidol. Droperidol is rarely used due to its association with QT prolongation, and related US Food and Drug Administration (FDA) black box warning (Guy 1991; McCormick 2002). Pharmacologic interventions are often used in combination (Apfel 2004), and multimodal prophylaxis is recommended in patients predicted to be at high risk of PONV (Gan 2014). An upcoming Cochrane Review will examine the use of pharmacologic prophylaxis for PONV (Weibel 2017).
Anaesthetic technique also influences the risk of PONV. The use of volatile anaesthetics increases the risk of PONV, while the use of regional anaesthesia, or total intravenous anaesthesia with propofol, is comparatively protective against nausea and vomiting (Borgeat 2003; Scuderi 2000). Administration of intravenous dextrose‐containing solutions may also prevent PONV (Dabubondoc 2013)
There are non‐pharmacologic approaches to PONV prevention as well. Acupuncture, specifically acustimulation of the P6 acupoint, reduces PONV by 30% when used in combination with ondansetron 4 mg intravenous in comparison to ondansetron alone (Lee 2015). Additionally, inhalation of isopropyl alcohol vapours has been demonstrated to reduce requirements for rescue anti‐emetic medications, when compared to saline placebo (Hines 2018).
Description of the intervention
This Cochrane systematic review examined the effect of supplemental perioperative intravenous crystalloid administration on PONV.
Intravenous crystalloids are widely administered before, during, and after procedures requiring general anaesthesia. They are inexpensive and have relatively few adverse effects. A prior systematic review has suggested that supplemental intravenous crystalloids may be effective in preventing PONV (Apfel 2012). These authors noted that intravenous administration of 15 mL to 30 mL/kg may generally be regarded as a substantial supplemental volume, whereas 0 mL to 3 mL/kg might be considered "restrictive". However, studies of supplemental perioperative intravenous crystalloids were noted to vary widely on the specific volumes administered.
How the intervention might work
Investigation of the effect of perioperative intravenous crystalloid administration on PONV was initially motivated by the results of observational studies suggesting that perioperative volume status influenced postoperative complication rates (Dawson 1980; Fahy 1969; Shires 1961). This work showed that PONV was among the most prevalent events after surgery and motivated subsequent inquiry into the relationship between perioperative volume resuscitation and PONV (Keane 1986; Spencer 1988; Yogendran 1995).
Multiple reviews have explained the complex physiology of nausea and vomiting (Blackburn 2015; Borison 1953; Palazzo 1984). Briefly, the vomiting centre, located in the lateral reticular formation of the medulla, co‐ordinates efferent activity to the respiratory, gastrointestinal, and abdominal musculature to produce vomiting. This centre receives afferent stimuli from a variety of sites: the pharynx, gastrointestinal tract chemo‐ and stretch receptors, the brain (including vestibular information from cranial nerve VIII), aortic baroreceptors, and the chemoreceptor trigger zone. The chemoreceptor trigger zone is a neural centre physiologically outside of the blood‐brain barrier, which provides afferent information to the vomiting centre in response to noxious stimuli in the blood.
Patients typically present for surgery with a fluid deficit secondary to fasting, bleeding, bowel preparation, and other causes of dehydration. Preoperative orthostatic hypotension is associated with PONV, and preoperative volume expansion reduces intraoperative gut hypoperfusion. It has been proposed that brainstem, vestibular, and intestinal hypoperfusion, with concomitant ischaemia, may mediate nausea and vomiting (Gan 1997; Pusch 2002a; Pusch 2002b). Supplemental intravenous crystalloids could serve to mitigate this effect; however, no proven explanation for the putative role of volume status in this model exists.
A prior Cochrane Review found that buffered intravenous solutions were not superior to non‐buffered intravenous solutions for preventing postoperative vomiting (Bampoe 2017).
Why it is important to do this review
Despite evidence‐based, multimodal prophylactic regimens, PONV remains a prevalent clinical problem (Gan 2007). The use of pharmacologic agents alone reduces the risk of PONV but increases the risk of side effects (Alkaissi 2004). Intravenous crystalloids are an attractive treatment modality because they are relatively inexpensive and have few side effects. Many different intravenous fluid interventions have been tested in a wide variety of surgical and anaesthetic contexts. Not surprisingly, results have been conflicting. Previous reviewers have suggested the presence of reporting bias in the early literature (Apfel 2012). Moreover, there is a lack of consensus regarding the volume of supplemental intravenous crystalloid required for PONV prophylaxis, or the ideal timing of its administration.
We conducted this systematic review to consolidate knowledge from the existing literature, so that perioperative clinicians may be provided a comprehensive assessment of the influence of intravenous crystalloid supplementation on the risk of PONV.
Objectives
To assess whether supplemental intravenous crystalloid administration reduces PONV in patients undergoing surgical procedures under general anaesthesia.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) that evaluated the effect of supplemental perioperative intravenous crystalloid administration for the prevention of PONV.
We did not exclude any study based on language of publication or publication status.
Types of participants
We included participants older than six months, undergoing any type of surgical procedure performed under general anaesthesia. For subgroup and sensitivity analyses, we defined children as six months to 17 years, and adults as 18 years or older.
Types of interventions
We included studies that examined supplemental perioperative intravenous crystalloid administration. Given the lack of agreement in the literature on specific volumes administered, we defined the intervention as an intravenous crystalloid volume larger than that received by a comparator group. The comparator is defined as an intravenous crystalloid volume smaller than that received by an intervention group, and we also included studies in which the comparator received no supplemental perioperative intravenous crystalloid. We included studies regardless of the timing of administration, including preoperative, intraoperative, postoperative, or a combination of these. Timing of administration was classified by the point at which administration was initiated.
We also included studies that administered dextrose‐containing crystalloids, but since intravenous dextrose may independently reduce PONV (Dabubondoc 2013), we conducted sensitivity analyses to ensure that the inclusion of these studies did not influence our overall meta‐analyses.
We excluded non‐intravenous routes of crystalloid administration (i.e. oral).
We excluded studies that compared only supplemental intravenous colloids to a comparator. However, we included studies including both colloids and crystalloids, as long as they had an intervention group receiving only supplemental crystalloid, in a volume greater than that received by a comparator group that also received only crystalloid.
Types of outcome measures
The following outcomes were subject to meta‐analysis.
Primary outcomes
Risk of PON, defined as the presence of subjective nausea, reported dichotomously or based on a study‐defined dichotomous threshold on a continuous scale such as a visual analogue scale (VAS).
Risk of POV, reported dichotomously by any discrete episodes of vomiting.
Secondary outcomes
Risk of requiring pharmacologic treatment for PONV, reported dichotomously as the use of any medication intended to treat nausea or vomiting during the postoperative period.
Risk of unintended postoperative admission to hospital, reported dichotomously as admission of a participant to an inpatient unit after an intended ambulatory surgical procedure.
Risk of suffering a serious adverse event, reported dichotomously as the occurrence of any of: admission to high‐dependency unit, postoperative cardiac or respiratory complication, or death.
The risk of PONV was reported in a minority of studies. PONV was also inconsistently defined across studies, casting doubt on the meaningfulness of analysing this nebulous outcome. As such, we opted to focus on analysis of PON and POV, which study investigators defined in a more consistent manner.
For the risk of PON, when continuous data were reported (e.g. using a visual analogue scale), we analysed these separately from dichotomous data in order to better characterize the magnitude of effect.
We measured POV dichotomously, based on the presence or absence of vomiting during the postoperative period. Some studies presented retching, the production of emetogenic movements without the expulsion of gastric contents, on its own or with vomiting. We combined retching and vomiting data when it would clearly not cause a unit of analysis error.
Although our analyses focused on the risk of these outcomes over the cumulative study period, when the data were available we also analysed the risk of these outcomes occurring at different postoperative time points postoperatively (i.e. early, late). In accordance with prior reviews on this topic, the early postoperative period was defined as the highest incidence of PON or POV within six hours after surgery, while the late postoperative period was defined as the time period reporting PON or POV nearest to 24 hours after surgery (Apfel 2012).
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2011). We did not apply restrictions to language or publication status.
We searched the following databases for relevant trials (August 2018).
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 7).
MEDLINE (1946 to August 2018).
Embase (1947 to August 2018).
Cumulative Index of Nursing and Allied Health (CINAHL; 1971 to August 2018).
We developed the initial search strategy using MEDLINE, identifying relevant index terms and the keywords to cover the concepts of the perioperative period, nausea and vomiting, intravenous administration, and crystalloid fluids. This search strategy was then adapted to the other electronic databases. All search strategies can be found in Appendix 1.
We scanned the following trials registries for ongoing and unpublished trials (August 2018).
The World Health Organization International Clinical Trials Registry Platform (WHO ICTRP).
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We scanned the reference lists and citations of included trials and any relevant systematic reviews identified for further references to additional trials. When necessary we attempted to contact trial authors for additional information.
We screened conference proceedings of anaesthesiology societies, published in three major anaesthesiology journals, from the two preceding years: British Journal of Anaesthesia, European Journal of Anaesthesiology, and Anesthesiology. This search was completed on 4 August 2018.
Data collection and analysis
Selection of studies
We merged search results using Covidence, and removed duplicated records. Two review authors (KJ, MW) read titles and abstracts and removed obviously irrelevant reports. We resolved discrepancies in the title and abstract screening by discussion with two other review authors (RG, SB). Two authors (KJ, MW) retrieved the full text of the potentially relevant reports. Four authors (KJ, MW, RG, SB) examined the full‐text reports to determine which met the eligibility criteria, and made final decisions on study inclusion. We recorded the number of studies retrieved at each stage and reported this information using a PRISMA flow diagram (Moher 2009), where we reported brief details of closely related papers excluded from this review (Figure 1).
1.
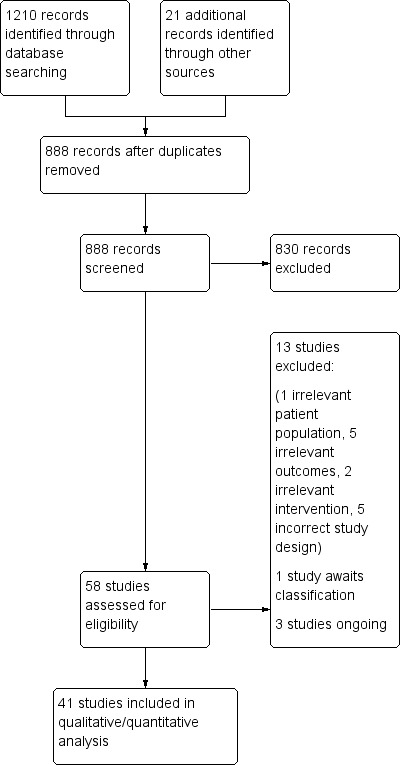
Study flow diagram.
Data extraction and management
Two review authors (KJ, MW) independently read the included studies and extracted the following data using a Cochrane template data extraction form (Appendix 2).
Participants: total number of participants randomized to each group.
Interventions: details of intervention and comparison (including type of intravenous crystalloid, crystalloid volumes used, and timing of intravenous crystalloid administration).
Outcomes: study outcomes as measured and reported by study authors (to include types of assessment measures, and time of measurement).
Outcome data: results of outcome measures.
We resolved discrepancies by discussion with two other review authors (RG, SB). After agreement, one study author (KJ) entered study data and information for evaluation of the risk of bias into Review Manager 5 (Review Manager 2014). We attempted to contact study authors to obtain additional information when required.
Assessment of risk of bias in included studies
Two review authors (KJ, MW), independently assessed the retained studies with the Cochrane 'Risk of bias' tool (Higgins 2011; Review Manager 2014). We resolved disagreements by discussion with the assistance of a third review author (RG). As is standard, we considered the following methodological criteria:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incompleteness of outcome data (attrition bias);
selective outcome reporting (reporting bias);
other sources of bias.
We considered randomization adequate when generated by a computer or random number table algorithm. We considered concealment adequate if the study prevented participant recruiters, investigators, and participants from knowing the allocation of each subsequent study participant (e.g. central randomization by a third party, or the use of sequential, opaque, sealed envelopes). We considered blinding adequate if measures were clearly described that would reasonable prevent participants and personnel from being aware of group allocation (e.g. intervention completed while patient was anaesthetized, using a concealed intravenous crystalloid container and pump operated by a third party not otherwise responsible for patient care). We considered outcome data adequate if all dropouts or withdrawals were accounted for, or if the number of dropouts was small (< 20%) and similar for both interventions. We considered trials as having a low risk of reporting bias if each measurement stated in the methods section was included in the results. We considered non‐intention‐to‐treat as selective reporting.
Measures of treatment effect
Using Review Manager 5 (Review Manager 2014), we presented the results for dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs) and for continuous data as mean differences (MDs) with 95% CIs. We planned to adapt data presented with different scales as standardized mean differences (SMDs) and 95% CIs. For SMDs, we considered 0.2 a small effect, 0.5 a moderate effect and 0.8 a large effect.
Unit of analysis issues
For studies containing greater than two groups, we merged the data from the similar groups when they were equivalent according to the criteria of our protocol (Jewer 2016). When this was not feasible, we entered the data separately and divided the comparator equally.
If we had identified cluster‐randomized trials, we would have meta‐analysed standard errors and effect estimates using the generic inverse‐variance method in Review Manager 5 (Review Manager 2014)
Dealing with missing data
When data were missing, we attempted to contact the corresponding author. We did not treat medians and means as equivalent. When possible, we calculated missing statistics from other quoted statistics. When participant dropout was encountered, we used an intention‐to‐treat analysis. We explored the effect of missing data using 'best‐case' and 'worst‐case' scenario sensitivity analyses.
Assessment of heterogeneity
We considered clinical heterogeneity during evaluation of the manuscripts, prior to pooling the results. We quantified statistical heterogeneity by calculating the I2 statistic, judging the amount of heterogeneity as low (I2 < 40%), moderate (I2 = 40% to 75%), or high (I2 > 75%) (Guyatt 2011; Higgins 2003).
Assessment of reporting biases
Publication bias is introduced when medical journals are more likely to report studies favouring one treatment than they are to report studies favouring another treatment. In intervention studies, manuscripts may be more likely to be published if they demonstrate efficacy of an intervention over a placebo or control arm. As greater than 10 studies contributed to each of our primary outcomes, we used visual funnel plot analysis in our assessment of reporting bias (Duval 2000). We planned to use the classical fail‐safe number had this not been the case (< 10 studies).
For each outcome, we constructed a funnel plot in Review Manager 5 (Review Manager 2014), with the standard error or precisions (1/standard error) on the y‐axis and the logarithm of the odds ratio on the x‐axis. When no publication bias or small study effects were present, the graph had the shape of an inverted funnel. A vertical line at the logarithm of the effect size found (log odds ratio) would divide the studies such that they are evenly distributed on each side of the line. This provided an estimation of the putative publication bias‐free effect size.
Data synthesis
We analysed data with Review Manager 5 using random‐effects models for all comparisons, given the anticipated moderate to high amount of heterogeneity across studies (Higgins 2003; Review Manager 2014). Random‐effects models give the same results as fixed‐effect models in the absence of statistical heterogeneity. When there is statistical heterogeneity, random‐effects models widen the confidence interval, thus decreasing the chance of finding an effect when there is none. They may increase the weight of smaller studies.
Subgroup analysis and investigation of heterogeneity
In the event of moderate (I2 = 40% to 75%) or high (I2 > 75%) statistical heterogeneity, we started with visual inspection of the forest plots, then proceeded with the following a priori subgroup analyses:
volume of supplemental intravenous crystalloid administered (control: intervention volume ratio of less than 1:3 or greater than 1:3);
timing of supplemental intravenous crystalloid administration (preoperative, intraoperative, or postoperative);
age (6 months to 17 years, 18 years or older).
For outcomes that have a moderate or high level of heterogeneity after subgroup analyses, the results of the subgroup analyses are only presented in a narrative manner.
For outcomes involving multiple studies with paediatric participants, the subgroup results for paediatric participants are also specifically reported, to elucidate this important source of clinical heterogeneity, and to provide specific guidance for clinicians working with this specific patient population.
Sensitivity analysis
We performed sensitivity analyses for outcomes involving studies that used dextrose‐containing fluids, as this is an intervention that independently reduces the risk of PONV (Dabubondoc 2013). The volume of supplemental intravenous crystalloid administered varied in each study, therefore we conducted sensitivity analyses to determine the effect of including studies that infused larger absolute volumes of supplemental intravenous crystalloid to their respective comparator groups (i.e. 10 mL/kg or more). We also sought to assess the influence of studies at relatively higher risk of bias. For each outcome involving studies with one or fewer domains at high or unclear risk of bias, we performed a sensitivity analysis using only those studies with low risk of bias.
'Summary of findings' table and GRADE
The GRADE approach appraises the certainty of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The certainty of a body of evidence takes into consideration within‐study risk of bias (methodological quality), the directness of the evidence, the heterogeneity of the data, the precision of effect estimates, and the risk of publication bias. We used the principles of the GRADE system (Guyatt 2008; Santesso 2016), to provide an overall assessment of the certainty of the body of evidence associated with each outcome.
We used GRADEpro software to create Table 1 (GRADEpro GDT).
Results
Description of studies
See: Included studies; Excluded studies; Studies awaiting classification; and Ongoing studies.
Results of the search
We identified 1210 records from database searches, plus 21 records from forward and backward citation searches, grey literature searches, and clinical trials registry searches. After excluding duplicates, we scrutinized the titles and abstracts of 888 records. From these, we assessed 58 full reports for eligibility, of which we excluded 13. Details of excluded studies are in the table Characteristics of excluded studies.
Included studies
We included 41 studies in the review (Ali 2003; Amireh 2009; Ashok 2017; Behdad 2011; Bennett 1999; Bhukal 2012; Chaudhary 2008; Chauhan 2013; Chohedri 2006; Cook 1990; Dagher 2009; Egeli 2004; Elgueta 2013; Elhakim 1998; Goodarzi 2006; Gwak 2007; Hashish 2007; Heidari 2012; Heshmati 2004; Holte 2004; Ismail 2017; Keane 1986; Lambert 2009; Lee 2009; Magner 2004; Maharaj 2005; McCaul 2003; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Ooi 1992; Paganelli 2008; Sharma 2010; Shin 2007; Singh 2013; Soleimani 2018; Spencer 1988; Yilmaz 2014; Yogendran 1995; Yoon 2008).
Study selection is detailed in Figure 1.
One of the included studies was a completed, unpublished RCT with data available on ClinicalTrials.gov (Yilmaz 2014). Five studies were published in a non‐English language: two in Farsi (Behdad 2011; Najafianaraki 2010), two in Korean (Shin 2007, Yoon 2008), and one in Portuguese (Paganelli 2008). These studies were translated for interpretation and included in the meta‐analysis.
Three of the included studies did not report data in sufficient detail to use in any analysis (Bennett 1999; Egeli 2004; Singh 2013). We were unable to obtain further details from the authors.
For further details, see Characteristics of included studies.
Participants
The 41 RCTs included in the review reported data from 4224 participants.
Thirty‐four studies exclusively enrolled adult participants. One study enrolled predominantly adults but also had participants as young as 12 years old (Shin 2007). Six studies enrolled paediatric participants, encompassing various age ranges: three to seven years (Ashok 2017), four to 18 years (Egeli 2004), one to 12 years (Elgueta 2013; Goodarzi 2006), six to 12 years (Heshmati 2004), and two to 15 years (Yilmaz 2014).
Thirty‐two studies included participants classified as ASA I or II (Ali 2003; Amireh 2009; Ashok 2017; Bennett 1999; Bhukal 2012; Chaudhary 2008; Chauhan 2013; Chohedri 2006; Cook 1990; Elgueta 2013; Elhakim 1998; Goodarzi 2006; Gwak 2007; Hashish 2007; Heidari 2012; Holte 2004; Ismail 2017; Keane 1986; Lambert 2009; Lee 2009; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Ooi 1992; Sharma 2010; Shin 2007; Soleimani 2018; Spencer 1988; Yilmaz 2014; Yoon 2008). Three studies included participants classified as ASA I to III (Maharaj 2005; Paganelli 2008; Yogendran 1995), and two studies included participants classified as ASA I only (Behdad 2011; Heshmati 2004; Magner 2004). Four studies did not report the ASA classification of their participants (Dagher 2009; Egeli 2004; McCaul 2003; Singh 2013).
One study specifically described selecting for participants at high risk for PONV (Bhukal 2012). The other studies were inconsistent in their reporting of baseline risk factors for PONV.
All studies enrolled participants undergoing surgery with general anaesthesia. One study also included participants receiving deep sedation (Bennett 1999).
There were a variety of elective surgeries performed in these studies, performed on an ambulatory basis or with a short length of stay (i.e. one day). Among studies that solely focused on one type of surgical procedure, seven studies focused on laparoscopic gynaecologic surgery (Chauhan 2013; Hashish 2007; Lambert 2009; Magner 2004; Maharaj 2005; McCaul 2003; Monti 1999), six studies focused on laparoscopic cholecystectomy (Amireh 2009; Holte 2004; Ismail 2017; Lee 2009; Paganelli 2008; Sharma 2010), six studies focused on otorhinolaryngologic procedures (Behdad 2011; Dagher 2009; Egeli 2004; Elgueta 2013; Heshmati 2004; Yilmaz 2014), two studies focused on unspecified laparoscopic surgeries (Cook 1990; Murshed 2012), two studies focused on therapeutic abortion (Elhakim 1998; Ooi 1992), one study focused on strabismus repair (Goodarzi 2006), one study focused on open cholecystectomy (Chaudhary 2008), one study focused on dental extractions (Bennett 1999), one study focused on orthopaedic surgery (Heidari 2012), one study focused on cervical cerclage (Najafianaraki 2010), and one study focused on breast cancer surgery (Soleimani 2018). Twelve studies involved a heterogeneous mix of surgical procedures, typically of an abdominal or gynaecologic nature (Ali 2003; Ashok 2017; Bhukal 2012; Chohedri 2006; Gwak 2007; Keane 1986; Onyando 2014; Shin 2007; Singh 2013; Spencer 1988; Yogendran 1995; Yoon 2008).
Settings
All studies took place in surgical centres. Twenty‐one studies took place in Asia, eight in Europe, six in North America, four in Africa, and two in South America. Six studies were completed in each of India and Iran. Five studies were completed in each of the UK and the USA. The remaining studies originated from other countries (i.e. Bangladesh, Brazil, Canada, Chile, Denmark, Egypt, Ireland, Jordan, Kenya, Lebanon, South Korea, and Turkey).
Interventions
Twenty‐nine of the 41 included studies used Ringer's lactate as their intervention supplemental crystalloid (Ali 2003; Amireh 2009; Ashok 2017; Behdad 2011; Chaudhary 2008; Chauhan 2013; Cook 1990; Dagher 2009; Elgueta 2013; Elhakim 1998; Goodarzi 2006; Gwak 2007; Hashish 2007; Heidari 2012; Heshmati 2004; Holte 2004; Ismail 2017; Lambert 2009; Lee 2009; Magner 2004; Maharaj 2005; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Sharma 2010; Singh 2013; Spencer 1988; Yoon 2008). Five studies used normal saline (Bennett 1999; Bhukal 2012; Chohedri 2006; Paganelli 2008; Yilmaz 2014). One study used both Ringer's lactate and normal saline (Soleimani 2018). One study used an acetate‐containing balanced crystalloid solution called Plasmalyte (Yogendran 1995).
One study used 5% dextrose in saline (Ooi 1992), one study used 5% dextrose in Ringer's lactate (Egeli 2004), two studies used a combination of 5% dextrose in water and Ringer's lactate (Keane 1986; McCaul 2003). One study had a study arm using Ringer's lactate and a study arm using 5% dextrose in Ringer's lactate (Cook 1990). Finally, one study had a study arm using Ringer's lactate and a study arm using 5% dextrose in water (Shin 2007); however, the latter arm was not included in analyses as 5% dextrose in water is not a crystalloid solution. Three studies used more than one type of intravenous crystalloid solution (Cook 1990; McCaul 2003; Soleimani 2018).
Supplemental crystalloid administration started before induction of anaesthesia (preoperatively) in 24 studies (Ali 2003; Amireh 2009; Bennett 1999; Chaudhary 2008; Chauhan 2013; Chohedri 2006; Cook 1990; Dagher 2009; Hashish 2007; Heidari 2012; Holte 2004; Lambert 2009; Lee 2009; Magner 2004; Maharaj 2005; Monti 1999; Murshed 2012; Onyando 2014; Ooi 1992; Sharma 2010; Shin 2007; Singh 2013; Yilmaz 2014; Yogendran 1995), after induction of anaesthesia (intraoperatively) in 15 studies (Ashok 2017; Behdad 2011; Bhukal 2012; Elgueta 2013; Elhakim 1998; Goodarzi 2006; Gwak 2007; Heshmati 2004; Ismail 2017; Keane 1986; McCaul 2003; Najafianaraki 2010; Paganelli 2008; Spencer 1988; Yoon 2008), and after emergence from anaesthesia (postoperatively) in one study (Egeli 2004). One study started supplemental crystalloid administration preoperatively for one study arm, and intraoperatively in another study arm (Soleimani 2018).
Generally, intervention groups were administered a volume of intravenous supplemental crystalloid of at least 10 mL/kg. There were a minority of studies where the comparator groups were administered a volume of intravenous supplemental crystalloid comparable to this volume (Ashok 2017; Chauhan 2013; Dagher 2009; Goodarzi 2006; Hashish 2007; Holte 2004; Ismail 2017; Magner 2004; Paganelli 2008; Sharma 2010; Yilmaz 2014).
Details of the intervention are presented in Table 2.
1. Crystalloid volumes used in included studies.
| Study | Timing | Comparator group | Intervention group(s) |
| Ali 2003 | Preoperative | RL 2 mL/kg | RL 15 mL/kg |
| Amireh 2009 | Preoperative | No crystalloid bolus | RL 10 mL/kg |
| Ashok 2017 | Intraoperative | RL 10 mL/kg | RL 30 mL/kg |
| Behdad 2011 | Intraoperative | RL 4 mL/kg | RL 10 mL/kg |
| Intraoperative | RL 20 mL/kg | ||
| Bennett 1999 | Preoperative | NS 1 to 2 mL/kg | NS 15 mL/kg |
| Bhukal 2012 | Intraoperative | NS 4 mL/kg | NS 10 mL/kg |
| Chaudhary 2008 | Preoperative | RL 2 mL/kg | RL 12 mL/kg |
| Chauhan 2013 | Preoperative | RL 10 mL/kg | RL 30 mL/kg |
| Chohedri 2006 | Preoperative | NS 2 mL/kg | NS 20 mL/kg |
| Cook 1990 | Preoperative | No crystalloid bolus | RL 20 mL/kg |
| Preoperative | RL 20 mL/kg with 1 g/kg dextrose | ||
| Dagher 2009 | Preoperative | RL 10 mL/kg | RL 30 mL/kg |
| Egeli 2004 | Postoperative | No crystalloid bolus | D5RL 60 to 120 mL/hour |
| Elgueta 2013 | Intraoperative | RL 10 mL/kg/hour | RL 30 mL/kg/hour |
| Elhakim 1998 | Intraoperative | No crystalloid bolus | RL 1000 mL |
| Goodarzi 2006 | Intraoperative | RL 10 mL/kg/hour | RL 30 mL/kg/hour |
| Gwak 2007 | Intraoperative | RL 6 mL/kg/hour | RL 18 mL/kg/hour |
| Hashish 2007 | Preoperative | RL 10 mL/kg | RL 30 mL/kg |
| Heidari 2012 | Preoperative | No crystalloid bolus | RL 10 mL/kg |
| Heshmati 2004 | Intraoperative | No crystalloid bolus | RL 4 mL/kg/hour |
| Holte 2004 | Preoperative | RL 15 mL/kg | RL 40 mL/kg |
| Ismail 2017 | Intraoperative | RL 10 mL/kg | RL 30 mL/kg |
| Keane 1986 | Mixed | No crystalloid bolus | Intraoperative RL 1000 mL then postoperative 1000 mL D5W |
| Lambert 2009 | Preoperative | No crystalloid bolus | RL 900 to 1000 mL |
| Lee 2009 | Preoperative | RL 5 mL/kg/hour | RL 30 mL/kg/hour |
| Magner 2004 | Preoperative | RL 10 mL/kg | RL 30 mL/kg |
| Maharaj 2005 | Preoperative | RL 2 mL/kg per hour fasted | RL 3 mL/kg per house fasted |
| McCaul 2003 | Intraoperative | No crystalloid bolus | RL 1.5 mL/kg per hour fasted |
| Intraoperative | D5RL 1.5 mL/kg per hour fasted | ||
| Monti 1999 | Preoperative | No crystalloid bolus | RL 1000 mL |
| Murshed 2012 | Preoperative | RL 1.5 mL/kg per hour fasted | RL 15 mL/kg |
| Najafianaraki 2010 | Preoperative | RL 2 mL/kg per hour fasted | RL 2 mL/kg per hour fasted then RL 10 mL/kg |
| Onyando 2014 | Preoperative | No crystalloid bolus | RL "maintenance" rate per hour fasted (maximum 1000 mL) |
| Ooi 1992 | Preoperative | No crystalloid bolus | 20 mL/kg 4% dextrose/0.18% saline solution |
| Paganelli 2008 | Intraoperative | NS 10 mL/kg/hour | NS 1000 mL bolus then 10 mL/kg/hour |
| Sharma 2010 | Preoperative | RL 10 mL/kg | RL 20 mL/kg |
| Preoperative | RL 30 mL/kg | ||
| Shin 2007 | Preoperative | RL 2 mL/kg | RL 20 mL/kg |
| Singh 2013 | Preoperative | No crystalloid bolus | RL 30 mL/kg |
| Soleimani 2018 | Preoperative | NS 1.5 mL/kg/hour | NS 1.5 mL/kg/hour then RL 5 mL/kg |
| Intraoperative | NS 1.5 mL/kg/hour then RL 5 mL/kg | ||
| Spencer 1988 | Intraoperative | No crystalloid bolus | RL 1000 mL |
| Yilmaz 2014 | Intraoperative | NS 10 mL/kg/hour | NS 20 mL/kg/hour |
| Yogendran 1995 | Preoperative | Plasmalyte 2 mL/kg | Plasmalyte 20 mL/kg |
| Yoon 2008 | Intraoperative | RL 2 mL/kg | RL 18 mL/kg |
D5RL: dextrose 5% in Ringer's Lactate; D5W: dextrose 5% in water, NS: normal saline, RL: Ringer's Lactate
Comparators
In all studies, participants in the comparator group received a smaller volume of perioperative crystalloid than did participants in the intervention group, or they received no perioperative crystalloid.
In 26 studies, both the comparator group and the intervention group received the same type of intravenous crystalloid (Ali 2003; Ashok 2017; Behdad 2011; Bennett 1999; Bhukal 2012; Chaudhary 2008; Chauhan 2013; Chohedri 2006; Dagher 2009; Elgueta 2013; Goodarzi 2006; Gwak 2007; Hashish 2007; Holte 2004; Ismail 2017; Lee 2009; Magner 2004; Maharaj 2005; Murshed 2012; Najafianaraki 2010; Paganelli 2008; Sharma 2010; Shin 2007; Yilmaz 2014; Yogendran 1995; Yoon 2008). In 14 studies, the comparator group did not receive any perioperative intravenous crystalloid bolus (Amireh 2009; Cook 1990; Egeli 2004; Elhakim 1998; Heidari 2012; Heshmati 2004; Keane 1986; Lambert 2009; McCaul 2003; Monti 1999; Onyando 2014; Ooi 1992; Singh 2013; Spencer 1988).
Funding sources
Six studies disclosed a funding source. Of these, five studies cited an academic source of funding, such as a hospital or university department (Ashok 2017; Chauhan 2013; Holte 2004; Maharaj 2005; Yilmaz 2014), while one study disclosed funding from an industry source (Spencer 1988, Baxter Health Care). Two studies stated that they had no funding (Bhukal 2012; Soleimani 2018). For the remaining studies, the source of funding was unclear.
Excluded studies
We excluded 13 studies for not meeting the inclusion criteria (Abraham‐Nording 2012; Alnema 2011; Apfel 2012; Brandstrup 2003; Cuthbertson 2011; Dabubondoc 2013; Gaiser 2002; Heidari 2011; Holte 2007a; Holte 2007b; Lei 2017; Mintz 2004; Yavuz 2014). Five were not focused on PONV (Abraham‐Nording 2012; Brandstrup 2003; Cuthbertson 2011; Holte 2007a; Holte 2007b), five were not randomized controlled trials (Alnema 2011; Apfel 2012; Lei 2017; Mintz 2004; Yavuz 2014), one studied participants undergoing neuraxial anaesthetic (Gaiser 2002), and two did not have an intervention group receiving intravenous crystalloids (Dabubondoc 2013; Heidari 2011).
See Characteristics of excluded studies.
Studies awaiting classification
We identified one study in a clinical trial register that was terminated; we will await publication of study results before assessing eligibility (Laws 2003).
For further details, see Characteristics of studies awaiting classification
Ongoing studies
We identified three ongoing studies (NCT03141645; NCT03142464; NCT03485443).
One study aims to investigate preoperative intravenous fluid administration in participants 18 years or older, undergoing laparoscopic cholecystectomy (NCT03141645). They will compare participants receiving preoperative intravenous fluid administration against two groups: one that receives intraoperative ondansetron, and one that receives neither preoperative intravenous fluid nor intraoperative ondansetron. The primary outcome is PONV within the first postoperative 24 hours. The study hypothesis is that participants receiving preoperative intravenous fluid administration and patients receiving intraoperative ondansetron will have a similar reduction in risk of PONV, compared with the comparator group receiving neither.
One study aims to examine postoperative intravenous fluid administration in participants 18 years or older, undergoing laparoscopic cholecystectomy (NCT03142464). The study compares a restrictive fluid administration strategy against their usual practice of postoperative fluid administration. The primary outcome is renal function, reflected by serum creatinine, while nausea rated on a visual analogue scale (VAS) is a secondary outcome measure.
One study aims to evaluate the effect of intraoperative hydration on postoperative vomiting in paediatric patients undergoing otorhinolaryngological surgery (NCT03485443). They will compare participants receiving an intervention of normal saline at a rate of 30 mL/kg/hour during the intraoperative period with a comparison group receiving normal saline at a rate of 10 mL/kg/hour during the intraoperative period. The primary outcomes assessed will be postoperative nausea and vomiting in the PACU. Rescue antiemetic administration will also be documented, as will intensity of postoperative pain.
For further details, see Characteristics of ongoing studies.
Risk of bias in included studies
We assessed the risk of bias in the included studies in terms of allocation sequence generation, blinding, incomplete reporting of outcome data, and selective reporting. Risk of bias was generally low to moderate across all included studies, but eight studies were at high risk of bias (Bennett 1999; Egeli 2004; Keane 1986; Lambert 2009; McCaul 2003; Monti 1999; Soleimani 2018; Yogendran 1995). Three studies were at low risk of bias across all domains (Amireh 2009; Holte 2004; Ismail 2017), while nine studies had one domain at unclear risk of bias but were otherwise at low risk of bias (Ali 2003; Ashok 2017; Bhukal 2012; Chauhan 2013; Elgueta 2013; Gwak 2007; Magner 2004; Maharaj 2005; Murshed 2012). Outcomes that included data from studies at higher risk were subject to further sensitivity analyses to assess the influence of these studies on results.
For details, see Figure 2, Figure 3, and Characteristics of included studies
2.
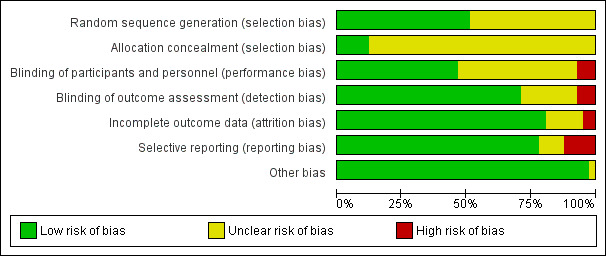
'Risk of bias' graph.
3.
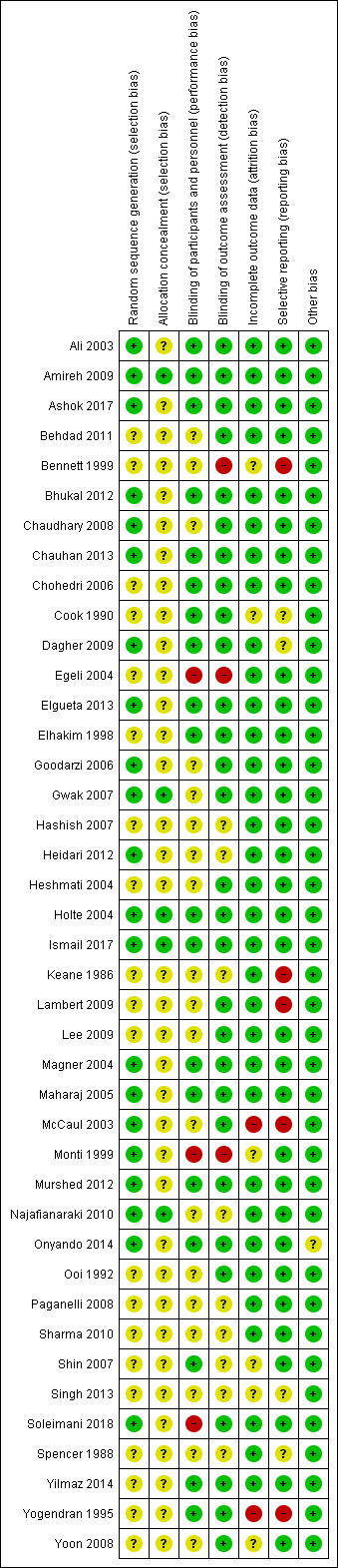
'Risk of bias' summary.
Allocation
All studies were RCTs. Twenty‐one studies provided adequate information to determine that the randomization process was prospective and unpredictable (Ali 2003; Amireh 2009; Ashok 2017; Bhukal 2012; Chaudhary 2008; Chauhan 2013; Dagher 2009; Elgueta 2013; Goodarzi 2006; Gwak 2007; Heidari 2012; Holte 2004; Ismail 2017; Magner 2004; Maharaj 2005; McCaul 2003; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Soleimani 2018). In the remaining studies, the randomization process was not adequately described to exclude randomization bias.
Allocation concealment was adequate if study personnel were unaware of the allocation of each subsequent study participant (i.e. using sequentially numbered, opaque, sealed envelopes or centralized third‐party allocation). Five studies sufficiently described effective methods to conceal group allocations from study personnel (Amireh 2009; Gwak 2007; Holte 2004; Ismail 2017; Najafianaraki 2010). The remaining studies provided insufficient details on their allocation process.
Blinding
Participants and personnel were adequately blinded in 19 studies (Ali 2003; Amireh 2009; Ashok 2017; Bhukal 2012; Chauhan 2013; Chohedri 2006; Cook 1990; Dagher 2009; Elgueta 2013; Elhakim 1998; Holte 2004; Ismail 2017; Magner 2004; Maharaj 2005; Murshed 2012; Onyando 2014; Shin 2007; Yilmaz 2014; Yogendran 1995). In one study supplemental intravenous crystalloid was administered over 24 hours postoperatively, so blinding of participants and care staff would have been very difficult (Egeli 2004). In one study, outcome assessors were blinded, but patients and anaesthesia personnel were not described as blinded during the preoperative and intraoperative periods, respectively (Soleimani 2018). In one study, blinding was not mentioned at all (Monti 1999). In the remaining studies, blinding of either participants or personnel was not adequately described to rule out a lack of blinding.
As all outcomes were evaluated in the postoperative period, adequate blinding of the outcome assessor was theoretically possible in all studies where fluid administration occurred preoperatively or intraoperatively. Twenty‐nine studies stated that the outcome assessor was blinded to study group (Ali 2003; Amireh 2009; Ashok 2017; Behdad 2011; Bhukal 2012; Chaudhary 2008; Chauhan 2013; Chohedri 2006; Cook 1990; Dagher 2009; Elgueta 2013; Elhakim 1998; Goodarzi 2006; Gwak 2007; Heshmati 2004; Holte 2004; Ismail 2017; Lambert 2009; Lee 2009; Magner 2004; Maharaj 2005; McCaul 2003; Murshed 2012; Onyando 2014; Ooi 1992; Soleimani 2018; Yilmaz 2014; Yogendran 1995; Yoon 2008). In one study, fluid administration took place postoperatively and outcome assessors were not blinded (Egeli 2004). In one study, participants received the intervention preoperatively while awake and later completed a self‐administered questionnaire (Bennett 1999). In one study, blinding was not mentioned at all (Monti 1999). Outcome assessor blinding was not adequately described in the remaining studies, therefore they had an unclear risk of bias.
Incomplete outcome data
We judged six studies to have unclear risk of attrition bias: for not reporting participant counts (Singh 2013); for not providing reasons for exclusions (Cook 1990); for inadequately reporting results to facilitate evaluation of attrition (Monti 1999); for having > 20% attrition with balanced withdrawals amongst study groups (Shin 2007); for having > 10% attrition with balanced withdrawals (Bennett 1999); and for < 10% attrition with balanced withdrawals (Yoon 2008). Two studies had high risk of attrition bias, where approximately 10% of participants were excluded or lost to follow‐up without indication of their allocation (McCaul 2003; Yogendran 1995). The remaining studies reported no participant losses during the trial, or only a small number of participants that was unlikely to substantially affect study results.
Selective reporting
Five studies were at high risk of reporting bias: four for not completing an intention‐to‐treat analysis for excluded participants (Bennett 1999; Lambert 2009; McCaul 2003; Yogendran 1995), and one for failing to report results of a planned outcome, vomiting (Keane 1986). Two studies had an unclear risk of bias, as they did not complete an intention‐to‐treat analysis but were only missing two participants each (Cook 1990; Dagher 2009). One paper failed to report several planned outcomes, but these were not pertinent to this review, so the paper was placed at unclear risk of reporting bias (Spencer 1988). One study did not report their data in adequate detail to rule out reporting bias, and was at unclear risk (Singh 2013). The remaining papers were at low risk of reporting bias.
Other potential sources of bias
In one study, a statistically significant difference in operative time existed between the intervention and the comparator despite prospective randomization and allocation concealment, but it was unclear whether this would bias other study results (Onyando 2014).
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
1. Risk of PON, defined as the presence of subjective nausea, reported dichotomously or based on a study‐defined dichotomous threshold on a continuous scale such as a VAS
Studies reporting risk of PON
Thirty‐two studies (3268 participants) assessed PON (Ali 2003; Amireh 2009; Behdad 2011; Bennett 1999; Chaudhary 2008; Chauhan 2013; Chohedri 2006; Cook 1990; Dagher 2009; Elhakim 1998; Gwak 2007; Hashish 2007; Heshmati 2004; Ismail 2017; Keane 1986; Lambert 2009; Magner 2004; Maharaj 2005; McCaul 2003; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Ooi 1992; Paganelli 2008; Sharma 2010; Shin 2007; Singh 2013; Soleimani 2018; Spencer 1988; Yogendran 1995; Yoon 2008). Three of these studies reported data in insufficient detail to be used in our meta‐analysis of PON, and we were unable to obtain further details from the authors (Bennett 1999; Bhukal 2012; Singh 2013).
Most studies reported PON data dichotomously (Ali 2003; Amireh 2009; Behdad 2011; Chauhan 2013; Chohedri 2006; Cook 1990; Dagher 2009; Gwak 2007; Hashish 2007; Heshmati 2004; Ismail 2017; Keane 1986; Lambert 2009; Magner 2004; Maharaj 2005; McCaul 2003; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Ooi 1992; Paganelli 2008; Sharma 2010; Shin 2007; Spencer 1988; Yogendran 1995; Yoon 2008). In studies presenting continuous data, several grading scales were used: five‐point Likert scale (Bennett 1999; Bhukal 2012), 10 cm or 100 mm VAS (Elhakim 1998; Sharma 2010), 0 to 10 verbal grading scale (Maharaj 2005), and ordinal grading scale (Magner 2004). In some studies, investigators assessed nausea on a continuous scale and converted this measurement to a dichotomous value using a threshold level, for instance 1 on a 0 to 10 verbal scale or 50 mm on a 100 mm VAS (Ali 2003; Amireh 2009; Chaudhary 2008; Gwak 2007; Maharaj 2005; Onyando 2014). One study reported both dichotomous and continuous data for PON (Maharaj 2005).
Risk of PON, when cumulative events were explicitly reported for the entire study period
Eighteen studies (1766 participants) reported dichotomous data for risk of PON during the cumulative study period (Ali 2003; Amireh 2009; Behdad 2011; Chauhan 2013; Dagher 2009; Gwak 2007; Heshmati 2004; Ismail 2017; Lambert 2009; Magner 2004; Maharaj 2005; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Ooi 1992; Sharma 2010; Yoon 2008). Supplemental intravenous crystalloid decreased risk of PON during the cumulative study period (risk ratio (RR) 0.62, 95% confidence interval (CI) 0.51 to 0.75; Analysis 1.1; Figure 4). This outcome had moderate statistical heterogeneity (I2 = 57%) that could not be reduced by subgroup analyses for: the relative amount of crystalloid administered, timing of crystalloid administration, or age (i.e. paediatric participants). We rated the certainty of this evidence using GRADE as moderate, having been downgraded due to risk of publication bias, as indicated by inspection of a funnel plot generated from included study data.
1.1. Analysis.
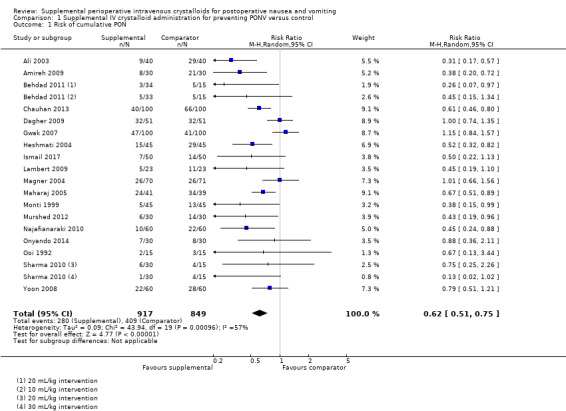
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 1 Risk of cumulative PON.
4.
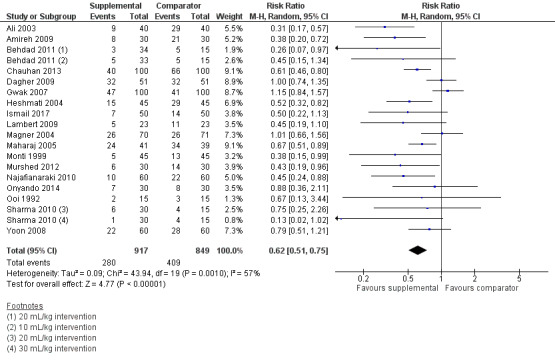
Forest plot of comparison: 1 Supplemental IV crystalloid administration for preventing PONV versus control, outcome: 1.5 Risk of overall PON (when cumulative nausea events were explicitly reported for the entire study period), as measured by the presence of subjective nausea, reported dichotomously or based on a study‐defined dichotomous threshold on a continuous scale such as a VAS.
One study (30 participants) in this analysis used a dextrose‐containing solution in the intervention group (Ooi 1992), and a sensitivity analysis found that inclusion of this study did not substantially affect the RR or statistical heterogeneity. The inclusion of studies where comparator group participants received at least 10 mL/kg of supplemental intravenous crystalloid did not substantially affect the RR. We performed a sensitivity analysis involving only studies at low risk of bias (Ali 2003; Amireh 2009; Chauhan 2013; Gwak 2007; Ismail 2017; Maharaj 2005; Murshed 2012), and this did not substantially affect the RR.
Risk of PON during specific time points (i.e. early and late postoperative period)
Twenty studies (2310 participants) reported dichotomous data on risk of PON in the early postoperative period (Ali 2003; Amireh 2009; Chauhan 2013; Chohedri 2006; Cook 1990; Dagher 2009; Gwak 2007; Hashish 2007; Ismail 2017; Keane 1986; Magner 2004; Maharaj 2005; McCaul 2003; Murshed 2012; Onyando 2014; Paganelli 2008; Shin 2007; Spencer 1988; Yogendran 1995; Yoon 2008); of these, three studies (410 participants) used a dextrose‐containing solution (Cook 1990; Keane 1986; McCaul 2003). Seventeen studies (1682 participants) reported dichotomous data on risk of PON in the late postoperative period (Ali 2003; Amireh 2009; Cook 1990; Dagher 2009; Gwak 2007; Hashish 2007; Ismail 2017; Magner 2004; Maharaj 2005; McCaul 2003; Murshed 2012; Onyando 2014; Paganelli 2008; Shin 2007; Spencer 1988; Yogendran 1995; Yoon 2008); of these, two studies (98 participants) used a dextrose‐containing solution (Cook 1990; McCaul 2003).
Supplemental intravenous crystalloids decreased the risk of early PON (RR 0.67, 95% CI 0.58 to 0.78; Analysis 1.2). Heterogeneity was low (I2 = 9%). Supplemental intravenous crystalloids decreased the risk of late PON (RR 0.47, 95% CI 0.32 to 0.69; Analysis 1.3; Figure 5). Heterogeneity was low (I2 = 38%). We rated the certainty of evidence using GRADE as moderate for both early and late time points, having been downgraded due to risk of publication bias, as indicated by inspection of a funnel plot generated from included study data.
1.2. Analysis.
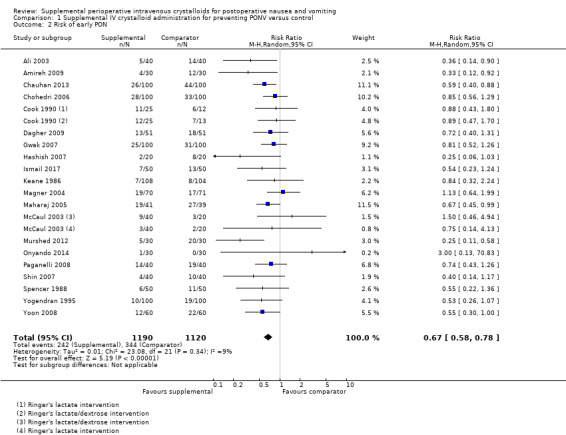
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 2 Risk of early PON.
1.3. Analysis.
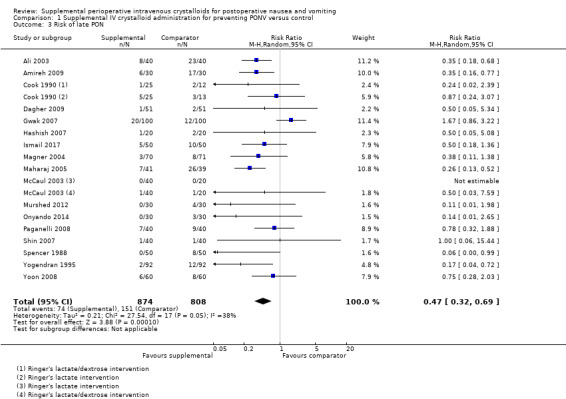
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 3 Risk of late PON.
5.
Forest plot of comparison: 1 Supplemental IV crystalloid administration for preventing PONV versus control, outcome: 1.9 Risk of pharmacologic treatment for PONV.
A sensitivity analysis found that inclusion of dextrose‐containing solutions did not substantially affect the RR or statistical heterogeneity for risk of early PON. For risk of late PON, removing dextrose‐containing solutions increased statistical heterogeneity (I2 = 47%), but did not substantially affect the RR. The inclusion of studies where comparator group participants received at least 10 mL/kg of supplemental intravenous crystalloid did not substantially affect the RR for risk of early PON or late PON. We performed sensitivity analyses involving only studies at low risk of bias for early PON (Ali 2003; Amireh 2009; Chauhan 2013; Elgueta 2013; Gwak 2007; Ismail 2017; Magner 2004; Maharaj 2005; Murshed 2012) and late PON (Ali 2003; Amireh 2009; Elgueta 2013; Gwak 2007; Ismail 2017; Magner 2004; Maharaj 2005; Murshed 2012), but the RR was not substantially affected in either case.
Risk of PON, when reported using continuous data
Five studies (415 participants) reported continuous data for early PON (Chaudhary 2008; Elhakim 1998; Maharaj 2005; Sharma 2010; Soleimani 2018). One study reported both dichotomous and continuous data for early and late PON, and was accordingly included in analyses of PON as a dichotomous outcome, as well as analyses of PON as a continuous outcome (Maharaj 2005).
Supplemental intravenous crystalloids decreased the severity of early PON on a 100 mm VAS (mean difference (MD) ‐16.38, 95% CI ‐21.81 to ‐10.96; Analysis 1.4). Statistical heterogeneity was moderate (I2 = 47%), but there were insufficient studies to conduct planned subgroup analyses.
1.4. Analysis.
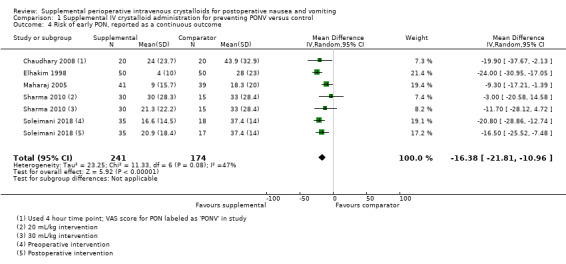
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 4 Risk of early PON, reported as a continuous outcome.
Five studies (415 participants) reported continuous data assessing late PON (Chaudhary 2008; Elhakim 1998; Maharaj 2005; Sharma 2010; Soleimani 2018). On a 100 mm VAS, supplemental intravenous crystalloids decreased the severity of nausea (MD ‐9.62, 95% CI ‐14.91 to ‐4.32; Analysis 1.5). Statistical heterogeneity was high (I2 = 71%), but there were insufficient studies to conduct planned subgroup analyses.
1.5. Analysis.
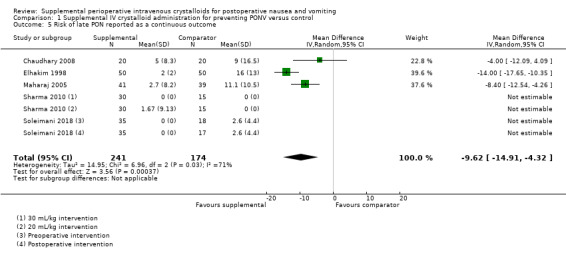
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 5 Risk of late PON reported as a continuous outcome.
There were insufficient studies to conduct sensitivity analyses for dextrose‐containing solutions, for comparator group volume infused, or for risk of bias.
2. Risk of POV, reported dichotomously by any discrete episodes of vomiting
Studies reporting risk of POV
Thirty‐one studies (3105 participants) evaluated POV (Ali 2003; Amireh 2009; Ashok 2017; Behdad 2011; Bhukal 2012; Chaudhary 2008; Chauhan 2013; Chohedri 2006; Cook 1990; Dagher 2009; Elgueta 2013; Elhakim 1998; Gwak 2007; Hashish 2007; Heidari 2012; Heshmati 2004; Ismail 2017; Magner 2004; Maharaj 2005; McCaul 2003; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Paganelli 2008; Sharma 2010; Shin 2007; Singh 2013; Spencer 1988; Yilmaz 2014; Yoon 2008); however, one study did not report sufficiently detailed data to be included our analyses for risk of POV (Singh 2013).
Four studies (500 participants) reported POV in paediatric participants, aged 6 months to 18 years (Ashok 2017; Elgueta 2013; Heshmati 2004; Yilmaz 2014).
Risk of POV, when cumulative events were explicitly reported for the entire study period
Twenty studies (1970 participants) provided overall data for POV across all time points (Ali 2003; Amireh 2009; Ashok 2017; Behdad 2011; Bhukal 2012; Chaudhary 2008; Dagher 2009; Elgueta 2013; Gwak 2007; Heidari 2012; Heshmati 2004; Ismail 2017; Magner 2004; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Sharma 2010; Yilmaz 2014; Yoon 2008); of these, four studies (500 participants) included paediatric participants (Ashok 2017; Elgueta 2013; Heshmati 2004; Yilmaz 2014). Supplemental intravenous crystalloids decreased the cumulative risk of POV over the entire study period (RR 0.50, 95% CI 0.40 to 0.63; Analysis 1.6; Figure 6). Heterogeneity was low (I2 = 31%). We rated the certainty of this evidence using GRADE as moderate, having been downgraded due to risk of publication bias, as indicated by inspection of a funnel plot generated from included study data.
1.6. Analysis.
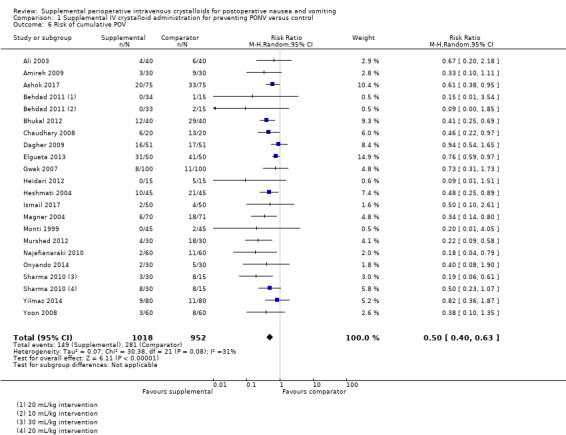
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 6 Risk of cumulative POV.
6.
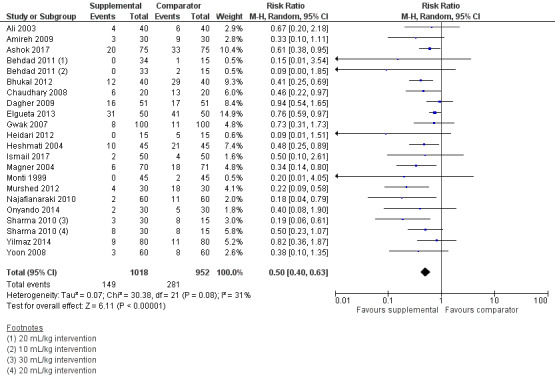
Forest plot of comparison: 1 Supplemental IV crystalloid administration for preventing PONV versus control, outcome: 1.6 Risk of cumulative POV.
For paediatric participants, supplemental intravenous crystalloid administration also reduced the cumulative risk of POV over the entire study period, but to a lesser degree than for adults (RR 0.69, 95% CI 0.57 to 0.85; I2 = 0%).
There were insufficient studies to conduct sensitivity analyses for dextrose‐containing solutions. The inclusion of studies where comparator group participants received at least 10 mL/kg of supplemental intravenous crystalloid did not substantially affect the RR. A sensitivity analysis involving only studies at low risk of bias did not substantially affect the RR (Ali 2003; Amireh 2009; Ashok 2017; Bhukal 2012; Elgueta 2013; Gwak 2007; Ismail 2017; Magner 2004; Murshed 2012).
Risk of POV during specific time points (i.e. early and late postoperative period)
We analysed 19 studies (1998 participants) for early POV (Ali 2003; Amireh 2009; Chauhan 2013; Chohedri 2006; Cook 1990; Dagher 2009; Elhakim 1998; Gwak 2007; Hashish 2007; Ismail 2017; Magner 2004; Maharaj 2005; McCaul 2003; Murshed 2012; Onyando 2014; Paganelli 2008; Shin 2007; Spencer 1988; Yoon 2008); of these, two studies (98 participants) used dextrose‐containing solutions (Cook 1990; McCaul 2003). Fifteen studies (1403 participants) assessed late POV (Ali 2003; Amireh 2009; Cook 1990; Dagher 2009; Elhakim 1998; Gwak 2007; Hashish 2007; Ismail 2017; Magner 2004; McCaul 2003; Murshed 2012; Onyando 2014; Paganelli 2008; Shin 2007; Yoon 2008); of these, two studies (98 participants) used dextrose‐containing solutions (Cook 1990; McCaul 2003).
Supplemental intravenous crystalloids decreased early POV (RR 0.56, 95% CI 0.41 to 0.76; Analysis 1.7). Statistical heterogeneity was low (I2 = 0%). Supplemental intravenous crystalloids also decreased postoperative vomiting late POV (RR 0.48, 95% CI 0.29 to 0.79; Analysis 1.8). Statistical heterogeneity was low (I2 = 0%). We rated the certainty of this evidence using GRADE as moderate for both time points, having been downgraded due to risk of publication bias, as indicated by inspection of a funnel plot generated by included study data.
1.7. Analysis.
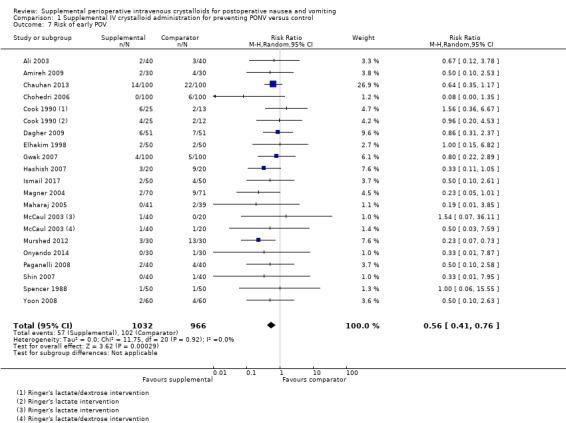
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 7 Risk of early POV.
1.8. Analysis.
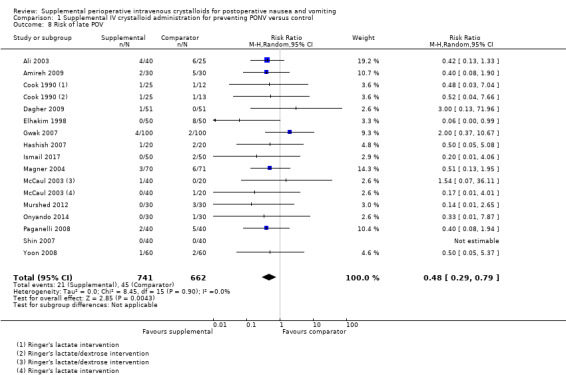
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 8 Risk of late POV.
A sensitivity analysis found that inclusion of dextrose‐containing solutions did not substantially affect the RR or statistical heterogeneity for risk of either early or late POV. The inclusion of studies where comparator group participants received at least 10 mL/kg of supplemental intravenous crystalloid did not substantially affect the RR for risk of early POV or late POV. A sensitivity analysis of studies at low risk of bias did not substantially affect the RR for risk of early POV (Ali 2003; Amireh 2009; Chauhan 2013; Gwak 2007; Ismail 2017; Magner 2004; Maharaj 2005; Murshed 2012) or for late POV (Ali 2003; Amireh 2009; Gwak 2007; Ismail 2017; Magner 2004; Murshed 2012).
Secondary outcomes
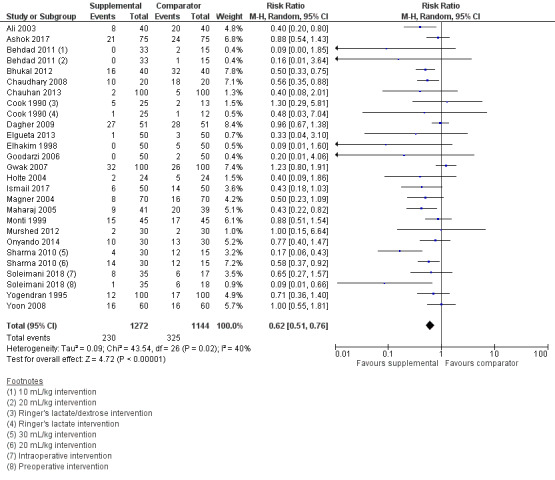
1. Risk of requiring pharmacologic treatment for PONV
Twenty‐three studies (2416 participants) measured the use of postoperative antiemetic medications (Ali 2003; Ashok 2017; Behdad 2011; Bhukal 2012; Chaudhary 2008; Chauhan 2013; Cook 1990; Dagher 2009; Elgueta 2013; Elhakim 1998; Goodarzi 2006; Gwak 2007; Holte 2004; Ismail 2017; Magner 2004; Maharaj 2005; Monti 1999; Murshed 2012; Onyando 2014; Sharma 2010; Soleimani 2018; Yogendran 1995; Yoon 2008); of these, one study (38 participants) used a dextrose‐containing solution (Cook 1990), and three studies (350 participants) examined pharmacologic treatment of PONV in paediatric participants, ranging from 1 to 12 years old (Ashok 2017; Elgueta 2013; Goodarzi 2006). One study measured the use of postoperative antiemetic medications but did not provide sufficient data to analyse this outcome (Singh 2013).
Supplemental intravenous crystalloids decreased the risk of requiring pharmacologic treatment of PONV (RR 0.62, 95% CI 0.51 to 0.76; Analysis 1.9; Figure 5). We rated the certainty of this evidence using GRADE as moderate, having been downgraded due to risk of publication bias, as indicated by inspection of a funnel plot generated from included study data.
1.9. Analysis.
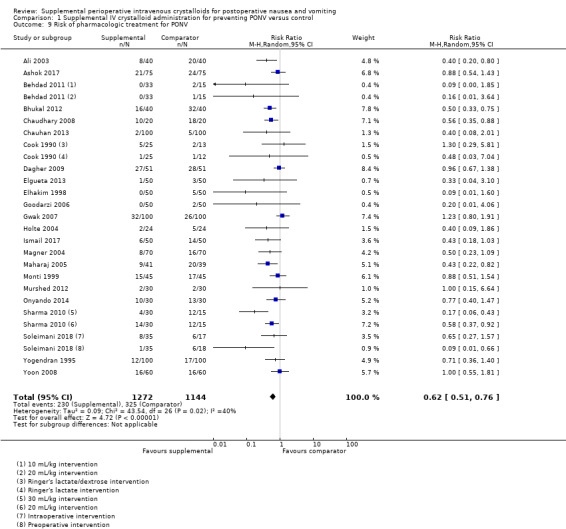
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 9 Risk of pharmacologic treatment for PONV.
This outcome had moderate statistical heterogeneity (I2 = 40%). The RR was not affected by any of our planned subgroup analyses: timing of fluid administration, relative volume of supplemental intravenous crystalloid administered, or age. A sensitivity analysis found that inclusion of dextrose‐containing solutions did not substantially affect the RR or statistical heterogeneity.
For paediatric participants, supplemental intravenous crystalloid administration did not appear to reduce the risk of requiring pharmacologic treatment of PONV (RR 0.81, 95% CI 0.50 to 1.30; I2 = 0%).
The inclusion of studies where comparator group participants received at least 10 mL/kg of supplemental intravenous crystalloid did not substantially affect the RR. A sensitivity analysis of studies at low risk of bias did not substantially affect the RR (Ali 2003; Ashok 2017; Bhukal 2012; Chauhan 2013; Elgueta 2013; Gwak 2007; Holte 2004; Ismail 2017; Magner 2004; Maharaj 2005; Murshed 2012).
2. Risk of unintended postoperative admission to hospital
Three studies (235 participants) quantified the rate of unplanned admission to hospital after ambulatory surgery (Ali 2003; Cook 1990; Maharaj 2005); of these, one study (38 participants) used a dextrose‐containing solution
Supplemental intravenous crystalloid administration did not affect this outcome (RR 1.05, 95% CI 0.77 to 1.43; Analysis 1.10). Heterogeneity was low (I² = 0%). We rated the certainty of this evidence using GRADE as low, having been downgraded due to imprecision and inconsistency of the results of included studies.
1.10. Analysis.
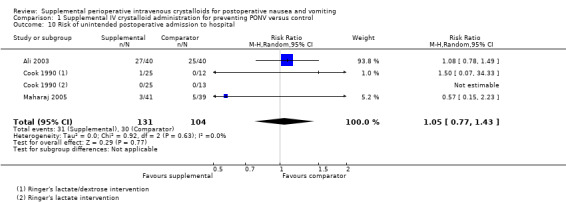
Comparison 1 Supplemental IV crystalloid administration for preventing PONV versus control, Outcome 10 Risk of unintended postoperative admission to hospital.
There were insufficient studies to carry out planned sensitivity analyses for dextrose‐containing solutions, comparator group volume infused, or for studies at low risk of bias.
3. Risk of suffering a serious adverse event (any of: admission to high‐dependency unit, postoperative cardiac or respiratory complication, or death)
We found no information about this outcome in the included studies.
Discussion
Summary of main results
We included 41 trials with a total of 4224 participants in this meta‐analysis. Combination of the results of these studies showed that supplemental perioperative intravenous crystalloid administration probably reduces the risk of PON in the overall postoperative period (RR 0.62, 95% CI 0.51 to 0.75; moderate‐certainty evidence), and specifically during the early (RR 0.67, 95% CI 0.58 to 0.78; moderate‐certainty evidence) and late (RR 0.47, 95% CI 0.32 to 0.69; moderate‐certainty evidence) time points. Supplemental perioperative intravenous crystalloid administration probably reduces the risk of POV in the overall postoperative period (RR 0.50, 95% CI 0.40 to 0.63; moderate‐certainty evidence), as well as during early (RR 0.56, 95% CI 0.41 to 0.76; moderate‐certainty evidence) and late (RR 0.48, 95% CI 0.29 to 0.79; moderate‐certainty evidence) time points. The certainty of the evidence for all PON and POV outcomes, as assessed using GRADE, is rated as moderate. Supplemental perioperative intravenous crystalloid administration probably reduces the risk for treatment with antiemetic rescue medication (RR 0.62, 95% CI 0.51 to 0.76; moderate‐certainty evidence). The effect of the intervention on the risk of unintended postoperative hospital admission after ambulatory surgery is unclear (RR 1.05, 95% CI 0.77 to 1.43; 3 studies; 235 participants; low‐certainty evidence). No studies reported serious adverse events with this intervention (i.e. admission to high‐dependency unit, postoperative cardiac or respiratory complication, or death).
Overall completeness and applicability of evidence
The majority of trials enrolled only ASA I to II patients, for ambulatory or short length of stay procedures (i.e. one day) (Ali 2003; Amireh 2009; Ashok 2017; Behdad 2011; Bennett 1999; Bhukal 2012; Chaudhary 2008; Chauhan 2013; Chohedri 2006; Cook 1990; Elgueta 2013; Elhakim 1998; Goodarzi 2006; Gwak 2007; Hashish 2007; Heidari 2012; Holte 2004; Ismail 2017; Keane 1986; Lambert 2009; Lee 2009; Monti 1999; Murshed 2012; Najafianaraki 2010; Onyando 2014; Ooi 1992; Sharma 2010; Shin 2007; Soleimani 2018; Spencer 1988; Yilmaz 2014; Yoon 2008). Otherwise, there was significant diversity amongst the included studies. Participants' baseline risk of PONV likely varied between studies, but this information was insufficiently reported to specifically analyse. Participants underwent a wide range of surgical procedures. Anaesthetic technique was varied, including induction and maintenance agents, use of muscle relaxants and reversal agents, intraoperative opioid administration, and pharmacologic PONV prophylaxis. Trials took place in a number of countries across the developed, emerging, and developing world. Although this variation likely introduced heterogeneity into the results, it also suggests that conclusions are generalizable to a sizeable scope of ambulatory surgical populations.
We found that PONV was very inconsistently defined across studies, so we had to focus on the related and more precisely defined outcomes of PON and POV. Most trials included in this review reported on one of our primary outcomes (i.e. risk of PON, risk of POV, or risk of PONV). There were few studies reporting continuous data for risk of PON; although far fewer studies and patients were pooled, we were able to assess the effect of supplemental intravenous crystalloid administration on PON severity. Nonetheless, this presents an area for further research.
Very few studies examined potential harms that patients may experience from vigorous volume administration. For instance, no studies examined the risk of serious adverse events (i.e. admission to high‐dependency unit, postoperative cardiac or respiratory complication, or death), and no studies examined PACU length of stay. This is clearly a deficiency in the existing literature.
Due to differences in the way that studies defined the volume of supplemental intravenous crystalloid that was administered to patients, it was not possible to compare absolute volume administered across studies. Where applicable, we conducted subgroup analyses of supplemental intravenous crystalloid volume administered relative to comparator and intervention groups, and this was not found to be influential. Moreover, we conducted sensitivity analyses omitting studies where comparator groups received a volume of intravenous supplemental crystalloid comparable to most studies' intervention groups (10 mL/kg or more), and this appeared to have negligible influence on the effect of the intervention. However, more work may be required to elucidate an optimal dosing for this intervention.
Despite these limitations, there is sufficient data to suggest that supplemental intravenous crystalloid administration may be helpful to reduce the risk of PONV. The varied settings do provide a degree of generalizability, albeit in an ambulatory setting with generally healthy patients. These results may not be easily generalized to more comorbid patients, or more extensive surgical cases where hospital length of stay is expected to exceed one or two days.
Quality of the evidence
The vast majority of studies reported a consistent direction of effect, with overlap of confidence intervals, and pooled participant numbers exceeded optimal effect size calculations, so we rated down no primary outcome for imprecision. Assessment of population, interventions, and outcomes of all included studies discovered no risk of indirectness. We completed a thorough grey literature search.
However, for both risk of PON and risk of POV, inspection of funnel plots strongly suggested the risk of publication bias so we downgraded the evidence strength of these outcomes to moderate.
For the outcome of risk of requiring pharmacologic treatment of PONV, there were similar concerns, as well as inclusion of relatively more studies at risk of bias, accounting for > 10% of participants in the analysis (Monti 1999; Soleimani 2018; Yogendran 1995; 395 participants). Subsequently, we decided to further downgrade this outcome to low.
For the outcome of unintended postoperative admission to hospital, there was a concern about imprecision, as indicated by wide confidence intervals indicating appreciable benefit and harm, as well as small sample sizes, in a limited number of analysed studies. There was inconsistency in effect. On account of these concerns, we downgraded this outcome to low.
There were some common pitfalls affecting the risk of bias in this literature. For the majority of included studies, there was insufficient description of measures to ensure random sequence generation and allocation concealment. Similarly, the nature of the intervention and its timing made it possible in many instances that blinding of participants and personnel could be compromised. However, we performed sensitivity analyses of studies at low risk of bias where possible, and it was reassuring that the inclusion of studies at relatively higher risk of bias did not appear to affect our estimate of risk in any outcome.
Potential biases in the review process
In general, we followed the protocols and procedures outlined by Cochrane in order to minimize any procedural bias in this review. In completing the meta‐analysis, we elected in several instances to deviate from the published protocol (Jewer 2016), which may have biased the review. For a complete list of protocol deviations and their justification, see Differences between protocol and review. Deviations that may have significantly biased the review are described below.
As included studies completed outcome assessments at a wide range of times, it was necessary to define time points for pooling of data. This was not identified a priori. We opted to define our time points in accordance with existing definitions of early and late PONV, as described in a previous meta‐analysis (Apfel 2012).
Intervention arms in several included studies prompted post‐hoc decisions on eligibility. Specifically, six papers used dextrose‐containing crystalloids (Cook 1990; Egeli 2004; Keane 1986; McCaul 2003; Ooi 1992; Shin 2007). Previous research has reported that intravenous dextrose may reduce PONV (Dabu‐Bondoc 2013). We decided to include these studies in pooled results and complete a sensitivity analysis where applicable, which suggested a negligible impact on risk reduction across all affected outcomes.
Different studies assessed and reported PON as a dichotomous or a continuous outcome. Since the majority of studies used dichotomous data, these results likely have greater precision, and are accordingly emphasized in this review. To provide an estimate of the clinical reduction in nausea, we decided to report continuous data separately. We did not contact authors for the data required to convert them to dichotomous outcomes.
Clinical heterogeneity was anticipated, therefore we planned to complete several subgroup analyses when there was also statistical heterogeneity (i.e. I2 > 40%). Specifically, we examined the relative volume of supplemental intravenous crystalloid administered between intervention and comparator groups, the timing of administration, and participant age. The subgroups definitions were chosen to best reflect the spectrum of populations and interventions present in analysed data.
The protocol intended to report length of stay in PACU as a secondary outcome. This outcome was not reported in the included studies. This was replaced with an alternative secondary outcome, unintended postoperative admission to hospital after ambulatory surgery, which was reported by three studies (Ali 2003; Cook 1990; Maharaj 2005). We chose this outcome to similarly address the potential system cost of PONV.
Finally, despite our comprehensive search for studies, one unclassified study remains, which may be a source of potential bias.
Agreements and disagreements with other studies or reviews
Prior to our meta‐analysis, the most comprehensive review of supplemental intravenous crystalloid administration for preventing PONV included 15 randomized controlled trials (Apfel 2012). The results of that review demonstrated statistically significant decreases in early, late, and cumulative PON, cumulative POV, late and cumulative PONV, and postoperative antiemetic administration. Pooled effect sizes for early and late POV and early PONV suggested a risk reduction, but 95% confidence intervals could not rule out a type I error.
Our meta‐analysis furthers the work completed in that review. By identifying new publications and completing a thorough grey literature search up to August 2018, we have included 26 additional studies, more than doubling the number of participants. This allowed for a more highly powered analysis, which explains the increased precision of our results when compared to the previous meta‐analysis. Improved power also likely explains why some outcomes, specifically early and late POV, were found to have significant risk reductions, when this was not the case in the prior analysis (Apfel 2012).
Authors' conclusions
Implications for practice.
This meta‐analysis demonstrates that supplemental perioperative intravenous crystalloid administration is probably effective in preventing postoperative nausea and vomiting (PONV) in American Society of Anesthesiology (ASA) class I to II patients, who receive general anaesthesia, for ambulatory or short length of stay (i.e. one‐day) surgical procedures. Evidence suggests that the intervention probably reduces the cumulative risk for PON and POV in the postoperative period, as well as during early and late time points specifically. Supplemental intravenous crystalloid administration may reduce the risk of requiring pharmacologic treatment for PONV. The effects of the intervention on the risk of unplanned postoperative admission to hospital after ambulatory surgery are unclear. The risk of serious adverse events resulting from vigorous perioperative intravenous crystalloid administration are unknown, as no identified studies reported this outcome.
The one study awaiting classification may alter the conclusions of the review once assessed.
Implications for research.
Current evidence on the use of supplemental intravenous crystalloid administration for preventing PONV is limited by several choices in the assessment of outcomes. Notably, time points for evaluation are inconsistently reported; a uniform choice of early and late time points would make comparison and pooling of results more straightforward. Presenting cumulative data for these time points, for example in the first six postoperative hours and thereafter, would facilitate future meta‐analysis.
Further reporting of continuous data for nausea severity (e.g. visual analogue scale, VAS) would allow for better assessment of the clinical impact of prophylactic interventions.
Assessment of duration of post anaesthesia care unit (PACU) stay, unintended hospital admission, and perhaps post‐discharge hospital admission would also provide valuable information about the value of this intervention.
Future studies could also be strengthened by including outcomes evaluating the potential harm of volume administration, such as cardiorespiratory complications, anastomotic dehiscence, and electrolyte abnormalities. Given that these are occur relatively infrequently, surrogate outcomes could also be considered, such as perioperative weight gain, which has been associated with serious adverse events (Brandstrup 2003).
Comparative studies of pharmacologic and non‐pharmacologic antiemetic therapies would better allow clinicians to determine the relative utility of interventions such as prophylactic intravenous crystalloid administration. It would also allow for the completion of cost benefit analyses to determine the most efficient means of PONV prophylaxis.
Only six of the 41 studies included in this review examined paediatric participants (Ashok 2017; Egeli 2004; Elgueta 2013; Goodarzi 2006; Heshmati 2004; Yilmaz 2014), and one of these did not report data in sufficient detail for analysis (Egeli 2004). We were only able to pool data for two outcomes (i.e. cumulative risk of POV, and risk of pharmacologic treatment of PONV). It is clear that an increased focus on paediatric research is needed to quantify the utility of this intervention in this population, while a more consistently defined age range for paediatric patients would allow for more direct comparison between studies.
What's new
| Date | Event | Description |
|---|---|---|
| 2 April 2019 | Amended | Typos corrected |
Acknowledgements
We would like to thank Michael Bennett (content editor), Vibeke E Horstmann (statistical editor), Leopold Eberhart, Daniel Molano Franco, Bita Mesgarpour (peer reviewers), Marie Kakhu (consumer referee), and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review.
We acknowledge Jill Hayden, for her advice in the development of this review, and Julie Scott for her contribution to the review protocol (Jewer 2016). In the completion of the review, her role was assumed by Michael Wong.
We offer many thanks to Danial Sayyad for translating our Persian studies.
Finally, we recognize Jane Cracknell (Managing Editor, Cochrane Anesthesia) for her patient guidance and correction of various drafts of this review.
Appendices
Appendix 1. PONV and IV crystalloid therapy search strategies
MEDLINE (1946 to August 2018)
| 1 | Rehydration Solutions/ |
| 2 | Isotonic Solutions/ |
| 3 | fluid therapy/ |
| 4 | crystalloid.mp. |
| 5 | fluid*.tw. |
| 6 | hydrat*.tw. |
| 7 | rehydrat*.tw. |
| 8 | isotonic solution*.tw. |
| 9 | ((ringer* or isotonic or salt) adj2 solution*).tw. |
| 10 | salt solution*.tw. |
| 11 | or/1‐10 |
| 12 | exp Administration, Intravenous/ |
| 13 | iv.tw. |
| 14 | "i v".tw. |
| 15 | intravenous*.tw. |
| 16 | or/12‐15 |
| 17 | ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. |
| 18 | "Postoperative Nausea and Vomiting"/ |
| 19 | postoperative complications/ |
| 20 | ponv.tw. |
| 21 | (exp postoperative care/ or postoperative care.tw. or recovery.tw. or exp "perioperative period"/) and (nause* or vomit* or emesis or emeses or emet* or queasiness or queasy).tw. |
| 22 | (("post operative" or postoperati* or perioperative or "peri‐operative" or surger* or surgical* or postsurg* or intraoperative or anesthe* or anaesthe* or postanesthe* or postanaesthe*) and (nause* or vomit* or emesis or emeses or queasiness or queasy)).tw. |
| 23 | or/18‐22 |
| 24 | 11 and 16 and 17 and 23 |
Embase (1947 to August 2018)
| No. | Search Terms |
| #28 | ('intravenous drug administration'/exp OR intravenous*:ab,ti OR iv:ab,ti OR 'i v':ab,ti) AND (('rehydration'/de OR 'fluid resuscitation'/de OR 'crystalloid'/exp OR 'isotonic solution'/exp) OR crystalloid OR (fluid*:ab,ti OR hydrat*:ab,ti OR rehydrat*:ab,ti) OR ((ringer* OR isotonic OR salt) NEAR/2 solution*):ab,ti) AND (('postoperative nausea and vomiting'/exp OR 'postoperative complication'/de) OR ponv:ab,ti OR ('post operative':ab,ti OR postoperati*:ab,ti OR perioperative:ab,ti OR 'peri‐operative':ab,ti OR surger*:ab,ti OR surgical*:ab,ti OR postsurg*:ab,ti OR intraoperative:ab,ti OR anesthe*:ab,ti OR anaesthe*:ab,ti OR postanesthe*:ab,ti OR postanaesthe*:ab,ti AND (nause*:ab,ti OR vomit*:ab,ti OR emesis:ab,ti OR emeses:ab,ti OR queasiness:ab,ti OR queasy:ab,ti)) OR (('postoperative period'/exp OR 'perioperative period'/exp OR 'anaesthetic recovery'/exp) AND (nause*:ab,ti OR vomit*:ab,ti OR emesis:ab,ti OR emeses:ab,ti OR queasiness:ab,ti OR queasy:ab,ti))) AND (('clinical trial'/exp OR 'randomised controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp) OR random*:ab OR 'randomised controlled trial*':ab,ti OR 'randomised controlled trial*':ab,ti OR rct:ab,ti OR 'single blind*':ab,ti OR 'double blind*':ab,ti OR ((treble OR triple) NEAR/1 blind*):ab,ti OR placebo:ab,ti) |
| #27 | ('clinical trial'/exp OR 'randomised controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp) OR random*:ab OR 'randomised controlled trial*':ab,ti OR 'randomised controlled trial*':ab,ti OR rct:ab,ti OR 'single blind*':ab,ti OR 'double blind*':ab,ti OR ((treble OR triple) NEAR/1 blind*):ab,ti OR placebo:ab,ti |
| #26 | placebo:ab,ti |
| #25 | ((treble OR triple) NEAR/1 blind*):ab,ti |
| #24 | 'double blind*':ab,ti |
| #23 | 'single blind*':ab,ti |
| #22 | rct:ab,ti |
| #21 | 'randomised controlled trial*':ab,ti |
| #20 | 'randomised controlled trial*':ab,ti |
| #19 | random*:ab |
| #18 | 'clinical trial'/exp OR 'randomised controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp |
| #17 | ('postoperative nausea and vomiting'/exp OR 'postoperative complication'/de) OR ponv:ab,ti OR ('post operative':ab,ti OR postoperati*:ab,ti OR perioperative:ab,ti OR 'peri‐operative':ab,ti OR surger*:ab,ti OR surgical*:ab,ti OR postsurg*:ab,ti OR intraoperative:ab,ti OR anesthe*:ab,ti OR anaesthe*:ab,ti OR postanesthe*:ab,ti OR postanaesthe*:ab,ti AND (nause*:ab,ti OR vomit*:ab,ti OR emesis:ab,ti OR emeses:ab,ti OR queasiness:ab,ti OR queasy:ab,ti)) OR (('postoperative period'/exp OR 'perioperative period'/exp OR 'anaesthetic recovery'/exp) AND (nause*:ab,ti OR vomit*:ab,ti OR emesis:ab,ti OR emeses:ab,ti OR queasiness:ab,ti OR queasy:ab,ti)) |
| #16 | ('postoperative period'/exp OR 'perioperative period'/exp OR 'anaesthetic recovery'/exp) AND (nause*:ab,ti OR vomit*:ab,ti OR emesis:ab,ti OR emeses:ab,ti OR queasiness:ab,ti OR queasy:ab,ti) |
| #15 | nause*:ab,ti OR vomit*:ab,ti OR emesis:ab,ti OR emeses:ab,ti OR queasiness:ab,ti OR queasy:ab,ti |
| #14 | 'postoperative period'/exp OR 'perioperative period'/exp OR 'anaesthetic recovery'/exp |
| #13 | 'post operative':ab,ti OR postoperati*:ab,ti OR perioperative:ab,ti OR 'peri‐operative':ab,ti OR surger*:ab,ti OR surgical*:ab,ti OR postsurg*:ab,ti OR intraoperative:ab,ti OR anesthe*:ab,ti OR anaesthe*:ab,ti OR postanesthe*:ab,ti OR postanaesthe*:ab,ti AND (nause*:ab,ti OR vomit*:ab,ti OR emesis:ab,ti OR emeses:ab,ti OR queasiness:ab,ti OR queasy:ab,ti) |
| #12 | ponv:ab,ti |
| #11 | 'postoperative nausea and vomiting'/exp OR 'postoperative complication'/de |
| #10 | ('rehydration'/de OR 'fluid resuscitation'/de OR 'crystalloid'/exp OR 'isotonic solution'/exp) OR crystalloid OR (fluid*:ab,ti OR hydrat*:ab,ti OR rehydrat*:ab,ti) OR ((ringer* OR isotonic OR salt) NEAR/2 solution*):ab,ti |
| #9 | 'intravenous drug administration'/exp OR intravenous*:ab,ti OR iv:ab,ti OR 'i v':ab,ti |
| #8 | 'i v':ab,ti |
| #7 | iv:ab,ti |
| #6 | intravenous*:ab,ti |
| #5 | 'intravenous drug administration'/exp |
| #4 | ((ringer* OR isotonic OR salt) NEAR/2 solution*):ab,ti |
| #3 | fluid*:ab,ti OR hydrat*:ab,ti OR rehydrat*:ab,ti |
| #2 | crystalloid |
| #1 | 'rehydration'/de OR 'fluid resuscitation'/de OR 'crystalloid'/exp OR 'isotonic solution'/exp |
Cochrane (CENTRAL; 2018, Issue 7)
| Search ID | Search Hits |
| #1 | intravenous* or iv or i.v. |
| #2 | (fluid* or hydrat* or rehydrat* or crystalloid*):ab,ti,kw |
| #3 | ((ringer* or isotonic or salt) near/2 solution*):ab,ti,kw |
| #4 | #2 or #3 |
| #5 | (("post operative" or postoperati* or perioperative or "peri‐operative" or surger* or surgical* or postsurg* or intraoperative or anesthe* or anaesthe* or postanesthe* or postanaesthe*) and (nause* or vomit* or emesis or emeses or emet* or queasiness or queasy)):ti,ab,kw |
| #6 | ponv |
| #7 | #6 or #5 |
| #8 | #7 and #4 and #1 |
CINAHL (1971 to August 2018)
| Search ID# | Search Terms |
| S26 | S11 AND S22 AND S23 AND S24 AND S25 |
| S25 | S12 OR S13 OR S14 OR S15 |
| S24 | S16 OR S17 |
| S23 | S18 OR S19 |
| S22 | S20 OR S21 |
| S21 | (MH "Vomiting") OR (MH "Nausea") OR (MH "Nausea and Vomiting+") |
| S20 | TI ( nause* or vomit* or emesis or emeses or emet* or queasiness or queasy ) OR AB ( nause* or vomit* or emesis or emeses or emet* or queasiness or queasy ) |
| S19 | TI ( ("post operative" or postoperati* or perioperative or "peri‐operative" or surger* or surgical* or postsurg* or intraoperative or anesthe* or anaesthe* or postanesthe* or postanaesthe*) ) OR AB ( "post operative" or postoperati* or perioperative or "peri‐operative" or surger* or surgical* or postsurg* or intraoperative or anesthe* or anaesthe* or postanesthe* or postanaesthe* ) |
| S18 | (MH "Postoperative Complications") OR (MH "Postoperative Period") OR (MH "Postoperative Care") OR (MH "Intraoperative Care") OR (MH "Perioperative Care+") |
| S17 | intravenous* or iv or "i v" |
| S16 | (MH "Administration, Intravenous+") OR (MH "Injections, Intravenous") OR (MH "Intravenous Therapy+") |
| S15 | TI ( fluid* OR crystalloid* OR hydrat* OR rehydrat* ) OR AB ( fluid* OR crystalloid* OR hydrat* OR rehydrat* ) |
| S14 | ((ringer* OR isotonic OR salt) N2 solution*) |
| S13 | (MH "Fluid Resuscitation") OR (MH "Fluid Therapy") |
| S12 | (MH "Crystalloid Solutions+") OR (MH "Isotonic Solutions") OR (MH "Rehydration Solutions") |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 |
| S10 | (MH "Placebos") |
| S9 | (MH "Random Assignment") |
| S8 | TI trial |
| S7 | PT Clinical trial |
| S6 | (MH "Clinical Trials+") |
| S5 | TI randomi?ed N1 control* |
| S4 | AB randomised controlled trials |
| S3 | AB placebo* |
| S2 | AB random* |
| S1 | (singl* OR doubl* OR trebl* OR tripl*) N1 (blind* OR mask*) |
Appendix 2. Data extraction form
| Review title or ID | Supplemental perioperative IV crystalloids for PONV |
| Study ID (e.g. Smith 2001) | |
| Report ID | |
| Report ID of other reports of this study including errata or retractions | |
| Notes | |
General Information
| Date form completed (dd/mm/yyyy) | |
| Name/ID of person extracting data | |
| Reference citation | |
| Study author contact details | |
| Publication type (e.g. full report, abstract, letter) | |
| Notes: | |
Study eligibility
| Study Characteristics | Eligibility criteria (Insert inclusion criteria for each characteristic as defined in the Protocol) |
Eligibility criteria met? | Location in text or source (pg & ¶/fig/table/other) | |||
| Yes | No | Unclear | ||||
| Type of study | Randomized controlled trial | |||||
| Quasi‐randomized controlled trial | ||||||
| Participants | ||||||
| Types of intervention | ||||||
| Types of comparison | ||||||
| Types of outcome measures | ||||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
Characteristics of included studies
Methods
| Descriptions as stated in report/paper | Location in text or source(pg & ¶/fig/table/other) | ||
| Aim of study(e.g. efficacy, equivalence, pragmatic) | |||
| Design(e.g. parallel, cross‐over, non‐RCT) | |||
| Unit of allocation(by individuals, cluster/ groups or body parts) | |||
| Start date | |||
| End date | |||
| Duration of participation(from recruitment to last follow‐up) | |||
| Ethical approval needed/obtained for study | Yes No Unclear | ||
| Notes: | |||
Participants
| Description Include comparative information for each intervention or comparison group if available |
Location in text or source (pg & ¶/fig/table/other) | ||
| Population description (from which study participants are drawn) | |||
| Setting (including location and social context) | |||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method of recruitment of participants (e.g. phone, mail, clinic patients) | |||
| Informed consent obtained | Yes No Unclear | ||
| Total no. randomized (or total pop. at start of study for non‐RCTs) | |||
| Clusters (if applicable, no., type, no. people per cluster) | |||
| Baseline imbalances | |||
| Withdrawals and exclusions (if not provided below by outcome) | |||
| Age | |||
| Sex | |||
| Race/ethnicity | |||
| Severity of illness | |||
| Co‐morbidities | |||
| Other relevant sociodemographics | |||
| Subgroups measured | |||
| Subgroups reported | |||
| Notes: | |||
Intervention groups
Copy and paste table for each intervention and comparison group
Intervention Group 1
| Description as stated in report/paper | Location in text or source (pg & ¶/fig/table/other) | |
| Group name | ||
| No. randomized to group (specify whether no. people or clusters) | ||
| Theoretical basis (include key references) | ||
| Description (include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing (e.g. frequency, duration of each episode) | ||
| Delivery (e.g. mechanism, medium, intensity, fidelity) | ||
| Providers (e.g. no., profession, training, ethnicity etc. if relevant) | ||
| Co‐interventions | ||
| Economic information (i.e. intervention cost, changes in other costs as result of intervention) | ||
| Resource requirements (e.g. staff numbers, cold chain, equipment) | ||
| Integrity of delivery | ||
| Compliance | ||
| Notes: | ||
Outcomes
Copy and paste table for each outcome.
Outcome 1
| Description as stated in report/paper | Location in text or source (pg & ¶/fig/table/other) | ||
| Outcome name | |||
| Time points measured (specify whether from start or end of intervention) | |||
| Time points reported | |||
| Outcome definition (with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
| Unit of measurement (if relevant) | |||
| Scales: upper and lower limits (indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
| Assumed risk estimate (e.g. baseline or population risk noted in Background) | |||
| Power (e.g. power & sample size calculation, level of power achieved) | |||
| Notes: | |||
Other
| Study funding sources (including role of funders) | ||
| Possible conflicts of interest (for study authors) | ||
| Notes: | ||
Data and analyses
Comparison 1. Supplemental IV crystalloid administration for preventing PONV versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of cumulative PON | 18 | 1766 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.51, 0.75] |
| 2 Risk of early PON | 20 | 2310 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.58, 0.78] |
| 3 Risk of late PON | 17 | 1682 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.32, 0.69] |
| 4 Risk of early PON, reported as a continuous outcome | 5 | 415 | Mean Difference (IV, Random, 95% CI) | ‐16.38 [‐21.81, ‐10.96] |
| 5 Risk of late PON reported as a continuous outcome | 5 | 415 | Mean Difference (IV, Random, 95% CI) | ‐9.62 [‐14.91, ‐4.32] |
| 6 Risk of cumulative POV | 20 | 1970 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.40, 0.63] |
| 7 Risk of early POV | 19 | 1998 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.41, 0.76] |
| 8 Risk of late POV | 15 | 1403 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.29, 0.79] |
| 9 Risk of pharmacologic treatment for PONV | 23 | 2416 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.51, 0.76] |
| 10 Risk of unintended postoperative admission to hospital | 3 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.77, 1.43] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ali 2003.
| Methods |
Design: double‐blind, prospective randomized controlled trial Country: USA Multisite: no International: no Treatment timing: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): laparoscopic or gynaecological surgery Randomization unit: participants Analysis unit: individual |
|
| Participants |
Screened participants were excluded if they:
Randomized to:
No withdrawals were stated. Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
Primary outcomes:
Outcomes were reported in the following time periods: 0 to 1 hour, 1 to 24 hours, and 0 to 24 hours postoperatively Secondary outcomes included:
|
|
| Notes | Trial registration: not found Funder: none stated A priori sample size estimation: stated on page 782 Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was based on computer‐generated codes that were maintained in sequentially numbered, opaque envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Randomization was based on computer‐generated codes that were maintained in sequentially numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The anaesthesia provider, the postoperative study investigator, and the PACU nurses were blinded to allocation of the groups, as were the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The anaesthesia provider, the postoperative study investigator and the PACU nurses were blinded to allocation of the groups, as were the participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Amireh 2009.
| Methods |
Design: double‐blind, prospective randomized controlled trial Country: Jordan Multisite: no International: no Treatment timing: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): laparoscopic cholecystectomy Randomization unit: participants Analysis unit: individual |
|
| Participants |
Screened participants were excluded if:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
Primary outcomes:
These outcomes were reported in the following time periods: 0 to 1 hour, 1 to 24 hours, and 0 to 24 hours postoperatively Secondary outcomes included:
|
|
| Notes | Trial registration: not found Funder: none stated A priori sample size estimation: not stated Conducted: August 2003 to May 2004 Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were prospectively and randomly divided into 2 groups. |
| Allocation concealment (selection bias) | Low risk | Randomization was performed by the nurse in the preoperative holding area who picked 1 of a prearranged and sealed 60 similar envelopes.= |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participant, the anaesthesia provider, the postoperative study investigator, and the nurses in recovery area and on the wards were unaware of the participants's group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The participant, the anaesthesia provider, the postoperative study investigator, and the nurses in recovery area and on the wards were unaware of the participant's group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Ashok 2017.
| Methods |
Design: double‐blind, prospective randomized controlled trial Country: India Multisite: no International: no Treatment timing: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): lower abdominal and penile surgeries of less than 60 minutes duration Randomization unit: participants Analysis unit: individual |
|
| Participants |
Screened participants were excluded if:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: Clinical Trial Registry of India REF/2015/06/009178 Funder: Department of Anesthesiology and Intensive Care, Post Graduate Institute of Medical Education and Research, Chandigarh, India. A priori sample size estimation: stated on page 3 Conducted: dates not stated Declared conflicts of interest: authors report no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Computer‐generated randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants, parents or guardians, surgeons, PACU nurses, and the investigator performing the postoperative assessment were blinded to the group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The participants, parents or guardians, surgeons, PACU nurses, and the investigator performing the postoperative assessment were blinded to the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants lost to follow‐up (2 from the intervention arm, 3 from the control) are accounted for with reasons for exclusion provided. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Behdad 2011.
| Methods |
Design: double‐blind, prospective randomized controlled trial Country: Iran Multisite: no International: no Treatment timing: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): tympanomastoidectomy Randomization unit: participants Analysis unit: individual |
|
| Participants |
Screened participants were excluded:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
These outcomes were all measured in the recovery room until discharge from the recovery room. |
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: stated Conducted: summer 2009 Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper states participants were (quote) "randomly allocated" but does not explain further. |
| Allocation concealment (selection bias) | Unclear risk | Paper states participants were (quote) "randomly allocated" but does not explain further. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is stated that the study is (quote) "double‐blind", but it is not clear that the surgical care team was blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study personnel measuring outcomes were not aware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Bennett 1999.
| Methods |
Design: blind, prospective randomized controlled trial Country: USA Multisite: no International: no Treatment timing: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): extraction of at least 2 impacted third molars Randomization unit: participants Analysis unit: individual |
|
| Participants |
Screened participants were excluded:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
Outcomes were reported for these outcomes at these time intervals. |
|
| Notes | Trial registration: not found Funder: none stated A priori sample size estimation: not completed Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper states (quote) "patients were randomly allocated" but no further information on methodology provided. |
| Allocation concealment (selection bias) | Unclear risk | Paper states (quote) "patients were randomly allocated" but no further information on methodology provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is stated that the trial is (quote) "double‐blind" but no further information on fluid administration is provided. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants complete questionnaires, and would be aware of whether or not they received the (preoperative) fluid intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13 participants are excluded, but they appear to be evenly distributed between the intervention and control groups. |
| Selective reporting (reporting bias) | High risk | All outcomes are reported, but an intention‐to‐treat analysis is not completed. |
| Other bias | Low risk | There were no other sources of bias. |
Bhukal 2012.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: India Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): elective non‐laparoscopic surgeries under general anaesthesia Randomization unit: participants Analysis unit: individual | |
| Participants |
Screened participants were excluded:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated Intraoperative bolus of either 10 mL/kg or 4 mL/kg of normal saline solution |
|
| Outcomes |
These outcomes were reported as median scores for an immediate postoperative assessment, and at 2, 6, and 24 hours postoperatively |
|
| Notes | Trial registration: not found Funder: 'nil' A priori sample size estimation: not completed Conducted: dates not stated Declared conflicts of interest: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was performed using a Tippet random chart. |
| Allocation concealment (selection bias) | Unclear risk | Knowledge of Tippet chart by investigators not stated explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study (quote) "double blinded"; anaesthesiologist's role not clear but no obvious influence. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperative questioning was conducted by an anaesthesiologist blinded to the intraoperative fluid management. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Chaudhary 2008.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: India Multisite: no International: no Treatment duration: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): elective open cholecystectomy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
Primary outcomes:
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was performed using a computer‐generated random table. |
| Allocation concealment (selection bias) | Unclear risk | Random allocation was performed using a computer‐generated random table. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Fluid administered prior to anaesthesia, concealment not explained, though all participants received some amount of IV fluid. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The observer collecting postoperative data was blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported. |
| Other bias | Low risk | There were no other sources of bias. |
Chauhan 2013.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: India Multisite: no International: no Treatment duration: perioperative period Follow‐up: 4 hours postoperatively Operative procedure(s): elective gynaecological laparoscopy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not found Funder: "Pubmed articles, National Medical Library (AIIMS)" listed as 'source of support' A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using sealed envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Randomization was performed using sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention IV fluid is not administered by a member of the care team. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The text states that (quote) "the investigator" administers the fluid preoperatively and interviews the patient postoperatively, but the paper states the investigator was blind to the patient's allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Chohedri 2006.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Iran Multisite: no International: no Treatment duration: preoperative period Follow‐up: until discharge from the ambulatory surgical unit Operative procedure(s): general, orthopaedic, and gynaecologic surgeries Randomization unit: participants Analysis unit: individual | |
| Participants |
Screened participants were excluded if they had a history of:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper states participants were (quote) "randomly allocated" but does not explain further. |
| Allocation concealment (selection bias) | Unclear risk | Paper states participants were (quote) "randomly allocated" but does not explain further. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The attending anaesthesiologist and recovery room nurses were blind to the participants' allocation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The anaesthesiologist assessing the adverse outcomes was blind to the participants' allocation group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Cook 1990.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: England Multisite: no International: no Treatment duration: preoperative period Follow‐up: 3 days postoperatively Operative procedure(s): ambulatory laparoscopy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Two participants were apparently randomized but not included in the results. No reason for exclusion was given. Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper states (quote) "each patient was allocated at random" but no further information on methodology provided. |
| Allocation concealment (selection bias) | Unclear risk | Paper states (quote) "each patient was allocated at random" but no further information on methodology provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Infusion bags were hidden to prevent unblinding, but it was not specified how this applied to the (quote) "no preoperative fluid" group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were made by an author blinded to treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two patients were not assessed. Material impact questionable as this represents a small fraction of the participants but no explanation was given. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported. An intention‐to‐treat analysis was not completed. |
| Other bias | Low risk | There were no other sources of bias. |
Dagher 2009.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Lebanon Multisite: no International: no Treatment duration: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): thyroidectomy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: stated on page 188 Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was performed using a computer‐generated number table. |
| Allocation concealment (selection bias) | Unclear risk | Random allocation was performed using a computer‐generated number table. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The anaesthesiologist taking care of the participant may have been aware of the group assignment, but the participant and PACU nurses were not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collection was completed by a blinded observer. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants excluded for protocol violations, but no apparent material impact. |
| Selective reporting (reporting bias) | Unclear risk | Intention‐to‐treat analysis was not completed for 2 excluded participants. All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Egeli 2004.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Turkey Multisite: no International: no Treatment duration: postoperative period Follow‐up: 1 week postoperatively Operative procedure(s): adenotonsillectomy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on the randomization protocol was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information on the randomization protocol was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and their parents were aware of group allocation as the intervention was postoperative IV fluid administration. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The (unblinded) parent completed the questionnaire and used the McGrath Face Scale to assess pain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Elgueta 2013.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Chile Multisite: no International: no Treatment duration: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): elective otorhinolaryngological surgery Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: NCT01575600 Funder: departmental funding A priori sample size estimation: stated on page 608 Conducted: July 2010 to March 2012 Declared conflicts of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was performed using a computer‐generated number table. |
| Allocation concealment (selection bias) | Unclear risk | Random allocation was performed using a computer‐generated number table. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, their parents, and medical staff were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators performing the postoperative assessments were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants were lost to follow‐up, 3 from the control group and 4 from the intervention group. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. An intention‐to‐treat analysis was performed. |
| Other bias | Low risk | The authors stated they had no conflict of interest to declare and had departmental funding only. |
Elhakim 1998.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Egypt Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 48 hours postoperatively Operative procedure(s): ambulatory termination of pregnancy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: none stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on the randomization protocol was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information on the randomization protocol was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | IV fluid containers were covered by a large paper bag. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded anaesthetist performed the study assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were noted. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Goodarzi 2006.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: USA Multisite: no International: no Treatment duration: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): ambulatory strabismus surgery under general anaesthesia Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was carried out using a computer program. |
| Allocation concealment (selection bias) | Unclear risk | Randomization was carried out using a computer program. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | PACU nurses were blinded to the allocation group, but blinding of the anaesthesiologist caring for participants was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collection was completed by a single investigator blinded to the technique of fluid therapy. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Gwak 2007.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: South Korea Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): laparotomy, laparoscopic abdominal or gynaecological surgery Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Groups with the same intraoperative fluid management but different inspired concentrations of oxygen were combined for meta‐analysis. Trial registration: not stated Funder: not stated A priori sample size estimation: stated on page S33 Conducted: dates not stated Declared conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was conducted by computer‐generated codes that were maintained in sequentially numbered envelopes. |
| Allocation concealment (selection bias) | Low risk | Randomization was conducted by computer‐generated codes that were maintained in sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | PACU nurses were blinded to the allocation group, but blinding of the anaesthesiologist caring for participants was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The postoperative study investigator was blinded to the group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were noted. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Hashish 2007.
| Methods | Design: prospective randomized controlled trial Country: Egypt Multisite: no International: no Treatment duration: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): diagnostic gynaecological laparoscopy for infertility Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated
|
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding of participants and personnel provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were noted. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Heidari 2012.
| Methods | Design: prospective randomized controlled trial Country: Iran Multisite: no International: no Treatment duration: preoperative period Follow‐up: unclear, "in the recovery room and surgical ward" Operative procedure(s): elective orthopaedic procedures Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation software was used to produce a simple randomized list of 2 equal groups. |
| Allocation concealment (selection bias) | Unclear risk | Random allocation software was used to produce a simple randomized list of 2 equal groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not stated. Fluid intervention occurs when participant is awake with no stated sham treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding status of outcome assessor not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were noted. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Heshmati 2004.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Iran Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): tonsillectomy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the allocation process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of care staff, including the intraoperative anaesthesiologist, was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses responsible for outcome assessment were blinded to allocation group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were noted. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Holte 2004.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Denmark Multisite: no International: no Treatment duration: preoperative period Follow‐up: 3 days postoperatively Operative procedure(s): laparoscopic cholecystectomy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: "The study was supported by grants from the University of Copenhagen and the Danish Research Council (no. 22‐01‐0160)" A priori sample size estimation: stated on page 894 Conducted: 23 October 2001 to 20 August 2002 Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was completed using serially numbered, sealed, and opaque envelopes based on an externally generated computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Randomization was completed using serially numbered, sealed, and opaque envelopes based on an externally generated computer‐generated list of random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Infusion bags were hidden in opaque sacks, ensuring blinding of the patient and care staff. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators obtaining patient data were not present during fluid infusion. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Ismail 2017.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Egypt Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): laparoscopic cholecystectomy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: both groups received dexamethasone 5 mg IV at the beginning of surgery |
|
| Outcomes |
|
|
| Notes | Trial registration: NCT02726308 Funder: not stated A priori sample size estimation: stated Conducted: May 2015 to December 2015 Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was based on computer‐generated codes maintained in sequentially numbered, opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Randomization was based on computer‐generated codes maintained in sequentially numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and members of the surgical team were blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses collecting data in PACU were blind to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Keane 1986.
| Methods | Design: prospective randomized controlled trial Country: Ireland Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 6 hours postoperatively Operative procedure(s): breast biopsy, varicose vein ligation, dilatation and curettage, and inguinal hernia repair Randomization unit: participants Analysis unit: individual | |
| Participants |
No exclusion criteria were provided. Randomized to:
All patients completed the first questionnaire (6 hours postoperatively), which evaluated PONV. 36 (17%) and 28 (24%) of eligible patients did not complete the second and third questionnaires, respectively. These questionnaires did not evaluate PONV. Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The blinding process of personnel was not described. Participants were aware of their allocation status but not the purpose of the IV fluid. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors completing questionnaires was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83% of participants responded to the second questionnaire. 76% of participants responded to the 3rd questionnaire. Neither of these questionnaires examined PONV outcomes. |
| Selective reporting (reporting bias) | High risk | Vomiting data were not reported. |
| Other bias | Low risk | There were no other sources of bias. |
Lambert 2009.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: USA Multisite: no International: no Treatment duration: perioperative period Follow‐up: discharge from postanaesthetic care unit to hospital or same‐day surgical care unit to home Operative procedure(s): laparoscopic gynaecological surgery Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: stated on page 112 Conducted: not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the allocation process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear whether participants and anaesthesiologists were blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses collecting data in PACU were blind to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for 6 participants were removed from the final sample due to protocol violations; both groups were equally affected. |
| Selective reporting (reporting bias) | High risk | An intention‐to‐treat analysis was not completed. |
| Other bias | Low risk | There were no other sources of bias. |
Lee 2009.
| Methods | Design: blinded, prospective randomized controlled trial Country: South Korea Multisite: no International: no Treatment duration: preoperative/intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): laparoscopic cholecystectomy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and the perioperative team was not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessor was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Magner 2004.
| Methods | Design: double‐blind, prospective, randomized controlled trial. Country: UK Multisite: no International: no Treatment duration: preoperative period Follow‐up: 48 hours postoperatively Operative procedure(s): gynaecological laparoscopy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated. |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: stated on page 382 Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was completed using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Randomization was completed using a computer‐generated random number sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Fluid was administered in the preoperative area; the participant and perioperative team were not aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was completed by a blind investigator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were noted. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Maharaj 2005.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Ireland Multisite: no International: no Treatment duration: preoperative period Follow‐up: 72 hours postoperatively Operative procedure(s): diagnostic gynaecological laparoscopy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Excluded after randomization:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: "funded from departmental resources" A priori sample size estimation: stated on page 676 Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes were used to determine group allocation. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were used to determine group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, anaesthesiologists, and PACU nurses were blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigator completing patient assessments in PACU was blind to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were noted. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
McCaul 2003.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: UK Multisite: no International: no Treatment duration: intraoperative period Follow‐up: morning of the first postoperative day Operative procedure(s): elective gynaecological laparoscopy Randomization unit: participants Analysis unit: individual | |
| Participants |
Participants were excluded after randomization:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not found Funder: none stated A priori sample size estimation: stated on page 441 Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unmarked envelopes were used to determine group allocation. |
| Allocation concealment (selection bias) | Unclear risk | Unmarked envelopes were used to determine group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were anaesthetized at the time of fluid administration. Blinding of personnel, including the attending anaesthesiologist, was not explained. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were assessed by a blinded interviewer using a standardized questionnaire in the PACU. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 participants were excluded after randomization. No explanation was given for their exclusion. The groups to which each excluded patient was assigned was not identified. |
| Selective reporting (reporting bias) | High risk | An intention‐to‐treat analysis was not completed. |
| Other bias | Low risk | There were no other sources of bias. |
Monti 1999.
| Methods | Design: prospective randomized controlled trial Country: USA Multisite: no International: no Treatment duration: preoperative period Follow‐up: until discharge from the ambulatory care unit Operative procedure(s): gynaecological laparoscopy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using a random distribution table. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned using a random distribution table. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of withdrawals was not stated and denominators were not provided for any reported results. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Murshed 2012.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Bangladesh Multisite: no International: no Treatment duration: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): elective laparoscopic surgery Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The blind envelope method was used to randomize participants to groups. |
| Allocation concealment (selection bias) | Unclear risk | The blind envelope method was used to randomize participants to groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participant, anaesthesia provider, and PACU nurses were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study investigator was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Najafianaraki 2010.
| Methods | Design: double‐blind randomized controlled trial Country: Iran Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): Shirodkar's operation (cervical cerclage) Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was completed using a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Randomization was completed using a table of random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not stated that participants or surgical personnel were blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is stated that ward staff were blinded to group allocation, but no outcome assessor is explicitly identified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Onyando 2014.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Kenya Multisite: no International: no Treatment duration: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): gynaecological surgery Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: stated on page 13 Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was completed using a computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Randomization was completed using a computer‐generated table of random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants, the anaesthetist, PACU nurses, and post‐surgical ward nurses were unaware of the group assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperative care nurses documented outcomes and were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Unclear risk | The duration of anaesthesia was significantly shorter for the experimental group. |
Ooi 1992.
| Methods | Design: single‐blind, prospective randomized controlled trial Country: UK Multisite: no International: no Treatment duration: perioperative period Follow‐up: not specified Operative procedure(s): therapeutic abortion Randomization unit: participants Analysis unit: individual | |
| Participants |
No exclusion criteria were provided. Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: stated on page 576 Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not stated that participants or personnel were blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All postoperative testing was completed by a blinded observer. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was excluded. This was explained in the text. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Paganelli 2008.
| Methods | Design: blind, prospective randomized controlled trial Country: Brazil Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): laparoscopic cholecystectomy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not found Funder: none stated A priori sample size estimation: stated on page 19 Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as being "blind" but the blinding status of perioperative personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study is described as being "blind" but the blinding status of the outcome assessor was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Sharma 2010.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: India Multisite: no International: no Treatment duration: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): laparoscopic cholecystectomy Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated; post hoc calculation provided on page 385 Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as being "blind" but the blinding status of the perioperative personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study is described as being "blind" but the blinding status of the outcome assessor was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
Shin 2007.
| Methods | Design: blind, prospective randomized controlled trial Country: South Korea Multisite: no International: no Treatment duration: preoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): ambulatory surgeries Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, but the randomization process was not described. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned, but the allocation process was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study fluid was hidden in an opaque bag to maintain blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The blinding of the outcome assessor is not explained. However, mechanisms to maintain blinding of the perioperative team were described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 77.5% of participants completed the study. The loss to follow‐up was approximately equal between low and high‐volume interventions. The reasons for attrition were explained. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. An intention‐to‐treat analysis was completed. |
| Other bias | Low risk | There were no other sources of bias. |
Singh 2013.
| Methods | Design: randomized controlled trial Country: India Multisite: no International: no Treatment duration: preoperative period Follow‐up: (quote) "in the post anesthesia care unit" Operative procedure(s): ambulatory surgeries Randomization unit: participants Analysis unit: individual | |
| Participants |
Randomized to:
|
|
| Interventions |
|
|
| Outcomes |
Participants were monitored in the postanaesthesia care unit for:
Vomiting was recorded as number of episodes. Antiemetic treatment with ondansetron (4 mg) and dexamethasone (8 mg) was measured, for nausea scores more than 5 out of a maximum of 10, or a vomiting episode. |
|
| Notes |
Trial registration: not stated
Funder: not stated
A priori sample size estimation: not stated
Conducted: dates not stated
Declared conflicts of interest: not stated Only available as an abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, but the randomization process was not described. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the allocation process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not described in this study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not described in this study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess, due to very limited presentation of data. Only P values and relative risk reported. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess, due to very limited presentation of data. Only P values and relative risk reported. |
| Other bias | Low risk | No other sources of bias were stated. |
Soleimani 2018.
| Methods | Design: double‐blind randomized controlled trial Country: Iran Multisite: no International: no Treatment duration: preoperative and intraoperative periods Follow‐up: 24 hours postoperatively Operative procedure(s): ambulatory surgeries Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: IRCT201602116481N8 Funder: none declared A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was completed using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Randomization was completed using a table of random numbers, but it is not clear how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and anaesthesia personnel was not described, and the intervention occurred both preoperatively, at a time when patients could be aware of group allocation, and intraoperatively, when anaesthesia providers could be aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The person completing the assessments did not know the goals of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No other sources of bias were stated. |
Spencer 1988.
| Methods | Design: prospective randomized controlled trial Country: UK Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 72 hours Operative procedure(s): gynaecological surgery Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria were not stated Randomized to:
Main characteristics of participants:
|
|
| Interventions |
|
|
| Outcomes |
Secondary outcomes included at 6 hours:
At 72 hours:
|
|
| Notes | Trial registration: not stated Funder: Baxter Health Care, a manufacturer of IV fluids A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the allocation process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not described in this study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not described in this study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were noted. |
| Selective reporting (reporting bias) | Unclear risk | Urinary retention and thirst were not reported, but these outcomes are not relevant to this review. |
| Other bias | Low risk | There were no other sources of bias. |
Yilmaz 2014.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Turkey Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): tonsillectomy or adenotonsillectomy, or both Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: NCT02177201 Funder: sponsored by Adnan Menderes University A priori sample size estimation: not stated Conducted: August 2013 to August 2014 Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the randomization process was provided. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but no information on the allocation process was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, care providers, and investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants, 3 from each arm, were excluded after beginning the study due to "meeting exclusion criteria". |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. Data were presented as an intention‐to‐treat analysis. |
| Other bias | Low risk | There were no other sources of bias. |
Yogendran 1995.
| Methods | Design: double‐blind, prospective randomized controlled trial Country: Canada Multisite: no International: no Treatment duration: preoperative period Follow‐up: until discharge from ambulatory surgery department Operative procedure(s): ambulatory gynaecological, orthopaedic, and general surgical operations Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes | Trial registration: not stated Funder: not stated A priori sample size estimation: not stated Conducted: dates not stated Declared conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but the randomization process is not described. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but the randomization process is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The attending anaesthesiologist and PACU nurses were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigator who collected data postoperatively was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 92% of participants are reached for postoperative interview, and their allocation status is not described in the text. |
| Selective reporting (reporting bias) | High risk | There was no intention‐to‐treat analysis. |
| Other bias | Low risk | There were no other sources of bias. |
Yoon 2008.
| Methods | Design: blinded, prospective randomized controlled trial Country: South Korea Multisite: no International: no Treatment duration: intraoperative period Follow‐up: 24 hours postoperatively Operative procedure(s): gynaecologic surgery Randomization unit: participants Analysis unit: individual | |
| Participants |
Exclusion criteria included:
Randomized to:
Main characteristics of participants:
|
|
| Interventions |
Co‐interventions: none stated |
|
| Outcomes |
|
|
| Notes |
Trial registration: not stated Funder: not stated A priori sample size estimation: stated on page 167 Conducted: dates not stated Declared conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but the randomization process was not explained. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly allocated, but the allocation process was not explained. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel were not explained. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessor did not know the patient's group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Nine randomized participants were lost to follow‐up; an intention‐to‐treat analysis was not completed. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were no other sources of bias. |
ASA: American Society of Anesthesiologists Physical Status Classification; BMI: body mass index; kg: kilogram; IM: intramuscular; IV: intravenous; PACU: post anaesthesia care unit; PONV: postoperative nausea and vomiting; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham‐Nording 2012 | This study does not evaluate postoperative nausea and vomiting. |
| Alnema 2011 | This publication is not a randomized controlled trial. |
| Apfel 2012 | This publication is not a randomized controlled trial. |
| Brandstrup 2003 | This study does not evaluate postoperative nausea and vomiting |
| Cuthbertson 2011 | This study does not evaluate postoperative nausea and vomiting. |
| Dabubondoc 2013 | This study does not use supplemental IV fluids as an intervention. |
| Gaiser 2002 | This study does not include participants having a surgical procedure performed under general anaesthesia or monitored anaesthetic care. |
| Heidari 2011 | This study does not use supplemental IV crystalloids as an intervention. |
| Holte 2007a | This study does not evaluate postoperative nausea and vomiting. |
| Holte 2007b | This study does not evaluate postoperative nausea and vomiting. |
| Lei 2017 | This publication is not a randomized controlled trial. |
| Mintz 2004 | This publication is not a randomized controlled trial. |
| Yavuz 2014 | This publication is not a randomized controlled trial. |
IV: intravenous
Characteristics of studies awaiting assessment [ordered by study ID]
Laws 2003.
| Methods | Design: randomized controlled trial Country: UK Multisite: no Treatment duration: intraoperative period Follow‐up: until 3 days post surgery Operative procedure(s): tonsillectomy Randomization unit: participants Analysis unit: individual |
| Participants | Children undergoing tonsillectomy |
| Interventions | 20 mL 4%/saline 0.18% intravenous fluid bolus versus no intravenous fluid bolus |
| Outcomes | The incidence and severity of vomiting, pain and activity disturbance will be monitored and recorded for 3 days post surgery. |
| Notes | Trial ended in 2003 due to "Objectives no longer viable." Data not yet published. |
Characteristics of ongoing studies [ordered by study ID]
NCT03141645.
| Trial name or title | Comparison of IV fluid loading and ondansetron in reduction of PONV after LC |
| Methods | Prospective randomized controlled trial |
| Participants | Estimated 153 participants; laparoscopic cholecystectomy |
| Interventions | Preoperative bolus of 10 mL/kg Ringer's lactate or no preoperative bolus A second intervention group will receive ondansetron IV but no additional IV crystalloid |
| Outcomes | Postoperative nausea and vomiting up to 24 hours |
| Starting date | June 2017 |
| Contact information | minkcheerful@hotmail.com |
| Notes | Data collection pending |
NCT03142464.
| Trial name or title | Intravenous fluids after laparoscopic cholecystectomy |
| Methods | Prospective randomized controlled trial |
| Participants | 100 participants aged 18 years and older; laparoscopic cholecystectomy |
| Interventions | Postoperative infusion of 5% glucose with normal saline or Ringer's lactate (at the discretion of the surgeon) or no postoperative fluid |
| Outcomes | Postoperative nausea and vomiting up to 24 hours Serum creatinine and thirst will also be reported |
| Starting date | July 2015 |
| Contact information | — |
| Notes | Study completed, no data reported |
NCT03485443.
| Trial name or title | Effects of fluid infusion on postoperative vomiting in paediatric patients undergoing otorhinolaryngological surgery |
| Methods | Prospective randomized controlled trial |
| Participants | 160 participants aged 2 to 14 years old; otorhinolaryngological surgery |
| Interventions | Intraoperative infusion of normal saline at a rate of 10 mL/kg/hour or 30 mL/kg/hour |
| Outcomes | Postoperative nausea and vomiting events Administration of rescue antiemetics and intensity of pain will also be reported |
| Starting date | April 2018 |
| Contact information | dr_snnylmz@hotmail.com |
| Notes | — |
IV: intravenous; LC: laparoscopic cholecystectomy; PONV: postoperative nausea and vomiting
Differences between protocol and review
We made the following changes to the published protocol (Jewer 2016):
We updated Description of the condition to provide a more comprehensive review of PONV.
We updated Description of the intervention, because the definition of supplemental intravenous crystalloid in this section did not match our definition used in Types of interventions. In Description of the intervention, we originally defined supplemental intravenous crystalloid as > 2 mL/kg. In the interest of clarity and consistency we opted to define it as a volume larger than that received by the comparator group. We also included a statement that we included studies in which the comparator received no intravenous fluids, because it may not otherwise be apparent to readers that this would be the case, even though it is in accordance with our definition of the intervention.
We removed a reference in Description of the intervention, because we had cited a withdrawn Cochrane Review of pharmacologic prophylaxis for PONV. This has now been superceded by a new Cochrane Review in progress (Weibel 2017).
We simplified the Objectives section. The wording of the primary objective has been slightly revised for clarity. The secondary objective was removed to reflect that the review's primary focus is the volume of supplemental intravenous crystalloid, because timing of crystalloid administration was only intended to be assessed for its potential confounding effect.
We updated Types of participants to reflect that no study specifically examined adults older than 65 years. The age ranges for adult and paediatric patient classification were slightly changed to reflect how the majority of studies classified these demographic groups (i.e. adults were 18 and older).
We updated Types of interventions. The protocol stated that we would include studies regardless of the timing of administration, including preoperative, intraoperative, postoperative, or a combination of these. However, it was unclear how timing would be classified if administration spanned more than one of these periods. We now specify that it is based on the time point at which fluid administration was initiated.
We revised the Types of outcome measures; Primary outcomes; and Secondary outcomes sections. We discovered that the primary outcome PONV was very inconsistently defined in the literature, so this was removed in favour of emphasizing the other primary outcomes of PON and POV. Furthermore, the included studies reported outcomes at different time points, and it was necessary to define time points for the purpose of meta‐analysis (i.e. cumulative, early, late). Our definitions were based on those used in a previous meta‐analysis on supplemental intravenous crystalloid administration for PONV (Apfel 2012). The secondary outcome PACU length of stay, which was intended to reflect the systems cost of PONV, was not reported in any included studies, therefore it was removed. In its place, we added the secondary outcome of unintended postoperative admission to hospital, which was reported in our included studies. To ensure compliance with Cochrane Review standards, we revised the wording of all outcomes to more explicitly define how and when they were measured.
We used Covidence, not Refworks, to merge search results.
We reorganized Assessment of risk of bias in included studies in the interest of clarity but did not change the content of this section.
We initially planned to carry out data analysis with fixed‐effect models, but we opted to use random‐effects models because we anticipated a moderate to high amount of heterogeneity across studies.
We lowered the threshold for moderate heterogeneity in Subgroup analysis and investigation of heterogeneity to use a more stringent threshold (I2 > 40%) than that planned in the protocol (I2 > 50%). This is in line with the GRADE guideline on inconsistency (Guyatt 2011).
A second revision to the section Subgroup analysis and investigation of heterogeneity involved the subgroup analysis examining the relative volume of supplemental crystalloids administered to the intervention group. Our protocol stated that we would examine the ratio of fluids given to the intervention and control groups as < 1:1.5, between 1:1.5 and 1:3, and > 1:3. However, no studies had an intervention group receiving a volume less than 1.5 times that of the comparator, therefore the subgroup analysis was sub‐optimal. We instead performed a subgroup analysis for a ratio of intervention compared to control volume of < 1:3 and > 1:3.
A third revision to the section Subgroup analysis and investigation of heterogeneity involved participant age. Because no study exclusively assessed populations older than 65 years, we eliminated the protocol subgroup > 65 years. Our original age subgroup of 18 to 65 years is now inclusive of all adults greater than 18 years of age.
In the protocol, it was anticipated that issues requiring sensitivity analyses would not become apparent until the meta‐analysis was underway. The Sensitivity analysis section has been updated to reflect our examination of studies using dextrose‐containing solutions, studies administering higher volumes of intravenous supplemental crystalloid to their comparator groups, as well as those at risk of bias.
Protocol author J Scott ended her involvement with the review before data collection, and M Wong was subsequently recruited. R Parker was added as an author for her contribution to the literature search and relevant sections of the review.
Contributions of authors
James K Jewer (KJ), Michael Wong (MW), Robin Parker (RP), Sally J Bird (SB), Ashraf S Habib (AH), Ronald B George (RG)
Conceiving the review: Julie Scott
Co‐ordinating the review: KJ and MW
Undertaking manual searches: RP, KJ, MW
Screening search results: KJ and MW
Organizing retrieval of papers: RP, KJ and MW
Screening retrieved papers against inclusion criteria: KJ, MW, SB, AH, RP
Appraising quality of papers: KJ, MW, SB, AH, RP
Abstracting data from papers: KJ, MW
Writing to authors of papers for additional information: KJ
Providing additional data about papers: KJ
Obtaining and screening data on unpublished studies: KJ, MW
Data management for the review: KJ
Entering data into Review Manager 5 (Review Manager 2014): KJ
RevMan statistical data: KJ
Interpretation of data: KJ, MW, AH, RG
Statistical inferences: KJ, MW, RG
Writing the review: KJ, MW, RG, SB, RP, AH
Securing funding for the review: N/A
Guarantor for the review (one author): RG
Person responsible for reading and checking review before submission: KJ
Declarations of interest
James K Jewer: no conflict of interest to disclose.
Michael J Wong: no conflict of interest to disclose.
Robin Parker: no conflict of interest to disclose.
Sally J Bird: no conflict of interest to disclose.
Ashraf S Habib received research support from Acacia Pharma (2015 to 2016) and was an advisory board member for Mylan (2014) and Baxter (2015). He has a grant pending from Trevena Inc (2016 to 2017). None of those entities produce the intervention of interest except for Baxter, which manufactures intravenous fluids. Acacia and Baxter are pharmaceutical companies that manufacture antiemetics for the management of PONV, but antiemetic medications are not the intervention of interest in this review. The advisory board relationship with Baxter was not related to intravenous fluids.
Ronald B George: no conflict of interest to disclose.
Edited (no change to conclusions)
References
References to studies included in this review
Ali 2003 {published data only}
- Ali SZ, Taguchi A, Holtmann B, Kurz A. Effect of supplemental pre‐operative fluid on postoperative nausea and vomiting. Anaesthesia 2003;58(8):780‐4. [PUBMED: 12859471] [DOI] [PubMed] [Google Scholar]
Amireh 2009 {published data only}
- Amireh AM, Al‐Ghoul YA, Jabir IA. Pre‐operative intravenous fluid supplements: a simple and inexpensive method to reduce post‐operative nausea and vomiting among patients underwent laparoscopic cholecystectomy. Journal of the Royal Medical Services 2009;16(3):31‐5. [Google Scholar]
Ashok 2017 {published data only}
- Ashok V, Bala I, Bharti N, Jain D, Samujh R. Effects of intraoperative liberal fluid therapy on postoperative nausea and vomiting in children ‐ a randomized controlled trial. Pediatric Anesthesia 2017;27(8):810‐5. [PUBMED: 28585750 ] [DOI] [PubMed] [Google Scholar]
Behdad 2011 {published data only}
- Behdad S, Ayatollahi V, Khoushbin M, Hajiesmaeli MR, Baradaranfar MH, Abbasi HR. Comparing the efficacy of intraoperative administration of three doses of Ringer solution on PONV prevention in patients undergoing tympanomastoidectomy surgery. Iranian Journal ofAnesthesiology and Critical Care 2011;23(4):11‐6. [Google Scholar]
Bennett 1999 {published data only}
- Bennett J, McDonald T, Lieblich S, Piecuch J. Perioperative rehydration in ambulatory anesthesia for dentoalveolar surgery. Oral Surgery Oral Medicine Oral Pathology 1999;88(3):279‐84. [PUBMED: 10503854] [DOI] [PubMed] [Google Scholar]
Bhukal 2012 {published data only}
- Bhukal I, Srinivas N, Solanki SL, Yaddanapudi LN, Jain A. A randomized study to compare the efficacy of two intravenous fluid regimens of normal saline on the incidence of postoperative nausea and vomiting. Anesthesia: Essays and Researches 2012;6(1):21‐4. [PUBMED: 25885496 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaudhary 2008 {published data only}
- Chaudhary S, Sethi AK, Motiani P, Adatia C. Pre‐operative intravenous fluid therapy with crystalloids or colloids on post‐operative nausea and vomiting. Indian Journal of Medical Research 2008;127(6):577‐81. [PUBMED: 18765877] [PubMed] [Google Scholar]
Chauhan 2013 {published data only}
- Chauhan G, Madan D, Gupta K, Kashyap C, Maan P, Nayar P. Effect of intraoperative intravenous crystalloid infusion on post‐operative nausea and vomiting after diagnostic gynaecological laparoscopy: comparison of 30 mL/kg and 10 mL/kg and to report the effect of the menstrual cycle on the incidence of post‐operative nausea and vomiting. Anesthesia: Essays and Researches 2013;7(1):100‐4. [PUBMED: 25885729] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chohedri 2006 {published data only}
- Chohedri AH, Matin M, Khosravi A. The impact of operative fluids on the prevention of postoperative anesthetic complications in ambulatory surgery. Middle East Journal of Anesthesiology 2006;18(6):1147‐56. [PUBMED: 17263269] [PubMed] [Google Scholar]
Cook 1990 {published data only}
- Cook R, Anderson S, Riseborough M, Blogg CE. Intravenous fluid load and recovery: a double‐blind comparison in gynaecological patients who had day‐case laparoscopy. Anaesthesia 1990;45(10):826‐30. [PUBMED: 2146903] [DOI] [PubMed] [Google Scholar]
Dagher 2009 {published data only}
- Dagher CF, Abboud B, Richa F, Abouzeid H, El‐Khoury C, Doumit C, et al. Effect of intravenous crystalloid infusion on postoperative nausea and vomiting after thyroidectomy: a prospective, randomized, controlled study. European Journal of Anaesthesiology 2009;26(3):188‐91. [PUBMED: 19237980] [DOI] [PubMed] [Google Scholar]
Egeli 2004 {published data only}
- Egeli E, Harputluoglu U, Ozturk O, Oghan F, Kocak S. Can post‐adenotonsillectomy morbidity be reduced by intravenous 24 h hydration in pediatric patients following adenotonsillectomy?. International Journal of Pediatric Otorhinolaryngology 2004;68(8):1047‐51. [PUBMED: 15236891 ] [DOI] [PubMed] [Google Scholar]
Elgueta 2013 {published data only}
- Elgueta MF, Echevarria GC, Fuente N, Cabrera F, Valderrama A, Cabezón R, et al. Effect of intravenous fluid therapy on postoperative vomiting in children undergoing tonsillectomy. British Journal of Anaesthesia 2013;110(4):607‐14. [PUBMED: 23257991 ] [DOI] [PubMed] [Google Scholar]
Elhakim 1998 {published data only}
- Elhakim M, El‐Sebiae S, Kaschef N, Essawi GH. Intravenous fluid and postoperative nausea and vomiting after day‐case termination of pregnancy. Acta Anaesthesiologica Scandinavica 1998;42(2):216‐9. [PUBMED: 9509206] [DOI] [PubMed] [Google Scholar]
Goodarzi 2006 {published data only}
- Goodarzi M, Matar MM, Shafa M, Townsend JE, Gonzalez I. A prospective randomized blinded study of the effect of intravenous fluid therapy on postoperative nausea and vomiting in children undergoing strabismus surgery. Pediatric Anesthesia 2006;16(1):49‐53. [PUBMED: 16409529 ] [DOI] [PubMed] [Google Scholar]
Gwak 2007 {published data only}
- Gwak MS, Choi SJ, Yoon JS, Lee SM, Hahm TS, Gil JY, et al. The effect of high FiO2 plus liberal intraoperative fluid on the early PONV and pain in patients undergoing intra‐abdominal surgery. Korean Journal of Anesthesiology 2007;52(6):S32‐6. [DOI: 10.4097/kjae.2007.52.6.S32] [DOI] [Google Scholar]
Hashish 2007 {published data only}
- Hashish, SA, Yossef IA, Ibrahim IT. Crystalloid infusion versus ephedrine injection in prevention of nausea and vomiting after gynecological laparoscopy. El‐Minia Medical Bulletin 2007;2:153‐9. [Google Scholar]
Heidari 2012 {published data only}
- Heidari SM, Saghaei M, Shafiee Z. Effect of preoperative volume loading on the intraoperative variability of blood pressure and postoperative nausea and vomiting. Medicinski Arhiv 2012;66(2):94‐6. [PUBMED: 22486138] [DOI] [PubMed] [Google Scholar]
Heshmati 2004 {published data only}
- Heshmati F, Afshar AH, Mahoori A, Zeinali MB, Abbasivash R. High intravenous fluid therapy prevents post‐tonsillectomy nausea and vomiting. Iranian Journal of Medical Sciences 2004;29(2):72‐4. [Google Scholar]
Holte 2004 {published data only (unpublished sought but not used)}
- Holte K, Klarskov B, Christensen DS, Lund C, Nielsen KG, Bie P, et al. Liberal versus restrictive fluid administration to improve recovery after laparoscopic cholecystectomy [2004]. Annals of Surgery 2004;240(5):892‐9. [PUBMED: 15492573] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismail 2017 {published data only}
- Ismail EA, Bakri MH, Abd‐Elshafy SK. Dexamethasone alone versus in combination with intra‐operative super‐hydration for postoperative nausea and vomiting prophylaxis in female patients undergoing laparoscopic cholecystectomy: a randomized clinical trial. Korean Journal of Anesthesiology 2017;70(5):535‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keane 1986 {published data only}
- Keane PW, Murray PF. Intravenous fluids in minor surgery. Their effect on recovery from anaesthesia. Anaesthesia 1986;41(6):635‐7. [PUBMED: 3728935] [DOI] [PubMed] [Google Scholar]
Lambert 2009 {published data only}
- Lambert KG, Wakim JH, Lambert NE. Preoperative fluid bolus and reduction of postoperative nausea and vomiting in patients undergoing laparoscopic gynecologic surgery. American Association of Nurse Anesthetists Journal 2009;77(2):110‐4. [PUBMED: 19388505] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee YC, Kim JM, Lee DY. Administration of large fluid volumes decreases the incidence of postoperative nausea and vomiting as effectively as ondansetron [복강경하 담낭 절제술 시 대용량의 수액 투여는 Ondansetron의 효과와동일하게 수술 후 오심 및 구토를 감소시킨다]. Korean Journal of Anesthesiology 2009;56(4):403‐7. [DOI: 10.4097/kjae.2009.56.4.403] [DOI] [PubMed] [Google Scholar]
Magner 2004 {published data only}
- Magner JJ, McCaul C, Carton E, Gardiner J, Buggy D. Effect of intraoperative intravenous crystalloid infusion on postoperative nausea and vomiting after gynaecological laparoscopy: comparison of 30 and 10 mL/kg. British Journal of Anaesthesia 2004;93(3):381‐5. [PUBMED: 15220164 ] [DOI] [PubMed] [Google Scholar]
Maharaj 2005 {published data only (unpublished sought but not used)}
- Maharaj CH, Kallam SR, Malik A, Hassett P, Grady D, Laffey JG. Preoperative intravenous fluid therapy decreases postoperative nausea and pain in high risk patients. Anesthesia and Analgesia 2005;100(3):675‐82. [PUBMED: 15728051 ] [DOI] [PubMed] [Google Scholar]
McCaul 2003 {published data only}
- McCaul C, Moran C, O'Cronin D, Naughton F, Geary M, Carton E, et al. Intravenous fluid loading with or without supplementary dextrose dose not prevent nausea, vomiting, and pain after laparoscopy. Canadian Journal of Anesthesia 2003;50(5):440‐4. [PUBMED: 12734150] [DOI] [PubMed] [Google Scholar]
Monti 1999 {published data only}
- Monti S, Pokorny M. Preop fluid bolus reduces risk of post op nausea and vomiting: a pilot study. Internet Journal of Advanced Nursing Practice 1999;4(2):1‐5. [Google Scholar]
Murshed 2012 {published data only}
- Murshed H, Islam MZ, Wahab MA, Al‐Azad MA. Effect of preoperative bolus supplemental fluid on postoperative nausea and vomiting ‐ a prospective study. Journal of Armed Forces Medical College Bangladesh 2012;8(2):16‐21. [DOI: 10.3329/jafmc.v8i2.16342] [DOI] [Google Scholar]
Najafianaraki 2010 {published data only}
- Najafianaraki A. The effect of fluid therapy on postoperative nausea and vomiting (PONV) after Shirodkar's operation. Iranian South Medical Journal 2010;13(1):52‐8. [Google Scholar]
Onyando 2014 {published data only}
- Onyando AA. The effect of preoperative volume loading on the incidence of postoperative nausea and vomiting at Kenyatta National Hospital. University of Nairobi 2014. [http://hdl.handle.net/11295/75354]
Ooi 1992 {published data only}
- Ooi LG, Goldhill DR, Griffiths A, Smith C. IV fluids and minor gynaecological surgery: effect on recovery from anesthesia. British Journal of Anaesthesia 1992;68(6):576‐9. [PUBMED: 1610630] [DOI] [PubMed] [Google Scholar]
Paganelli 2008 {published data only}
- Paganelli AL. Influence of perioperative volume replacement on postoperative nausea and vomiting [Influência da reposiçāo volêmica perioperatória sobre náuseas e vômitos no pósoperatório]. Universidade Federal de Santa Catarina 2008.
Sharma 2010 {published data only}
- Sharma CS, Gupta V, Dixi MB, Sadhu S, Joshi N. Effect of perioperative intravenous crystalloid infusion on postoperative nausea and vomiting after laparoscopic cholecystectomy. Journal of Anaesthesiology Clinical Pharmacology 2010;26(3):383‐6. [Google Scholar]
Shin 2007 {published data only}
- Shin YH, Ahn HJ, Choi SJ, Lee W, Lee BD. Comparison of dextrose water and Hartmann's solution in each low and high doses for adult ambulatory anesthesia: effect on recovery from anesthesia [외래 마취 환자에서 포도당 용액과 하트만 용액의 투여 용량이마취 회복에 미치는 영향]. Korean Journal of Anesthesiology 2007;52(1):55‐61. [DOI: 10.4097/kjae.2007.52.1.55] [DOI] [Google Scholar]
Singh 2013 {published data only}
- Singh M, Nagarakanti N. Role of preoperative hydration with balanced salt solution in decreasing the incidence of postoperative nausea and vomiting. Anesthesia and Analgesia 2013;116:S25. [Google Scholar]
Soleimani 2018 {published data only}
- Soleimani M, Mohammadi M, Teymourian H, Gholizadeh N, Khazaei Y, Safari F. The effect of fluid therapy in acute post‐operative complications of breast cancer; pain and post‐operative nausea and vomiting. International Journal of Cancer Management 2018;11(6):e67047. [Google Scholar]
Spencer 1988 {published data only}
- Spencer EM. Intravenous fluids in minor gynaecological surgery. Their effect on postoperative morbidity. Anaesthesia 1988;43(12):1050‐1. [PUBMED: 3232784] [DOI] [PubMed] [Google Scholar]
Yilmaz 2014 {unpublished data only}
- Yilmaz S. Effect of intraoperative crystalloid volume on postoperative vomiting (EICVPV). clinicaltrials.gov/ct2/show/NCT02177201 2014.
Yogendran 1995 {published data only}
- Yogendran S, Asokumar B, Cheng DC, Chung F. A prospective randomized double‐blinded study of the effect of intravenous fluid therapy on adverse outcomes on outpatient surgery. Anesthesia and Analgesia 1995;80(4):682‐6. [PUBMED: 7893018] [DOI] [PubMed] [Google Scholar]
Yoon 2008 {published data only}
- Yoon JS, Kim KM, Kim YH. The effects of amounts of intraoperative intravenous fluid administration on postoperative nausea and vomiting during gynecological surgery [부인과 수술 중 수액 투여량이 수술 후 오심 구토에 미치는 영향]. Korean Journal of Anesthesiology 2008;55(2):166‐70. [DOI: 10.4097/kjae.2008.55.2.166] [DOI] [Google Scholar]
References to studies excluded from this review
Abraham‐Nording 2012 {published data only}
- Abraham‐Nordling M, Hjern F, Pollack J, Prytz M, Borg T, Kressner U. Randomized clinical trial of fluid restriction in colorectal surgery. British Journal of Surgery 2012;99(2):186‐91. [PUBMED: 21948211] [DOI] [PubMed] [Google Scholar]
Alnema 2011 {published data only}
- Al‐Nema ZM, Mohammed SI, Ahmed FT. Effect of administration of crystalloid IV fluids preoperatively on postoperative nausea and vomiting. Iraqi Journal of Community Medicine 2011;24(4):339‐42. [Google Scholar]
Apfel 2012 {published data only}
- Apfel CC, Meyer A, Orhan‐Sungur M, Jalota L, Whelan RP, Jukar‐Rao S. Supplemental intravenous crystalloids for the prevention of postoperative nausea and vomiting: quantitative review. British Journal of Anaesthesia 2012;108(6):893‐902. [PUBMED: 22593126] [DOI] [PubMed] [Google Scholar]
Brandstrup 2003 {published data only}
- Brandstrup B, Tønnesen H, Beier‐Holgersen R, Hjortsø E, Ørding H, Lindorff‐Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor‐blinded multicenter trial. Annals of Surgery 2003;238(5):641‐8. [PUBMED: 14578723] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuthbertson 2011 {published data only}
- Cuthbertson BH, Campbell MK, Stott SA, Elders A, Hernández R, Boyers D, et al. A pragmatic multi‐centre randomised controlled trial of fluid loading in high‐risk surgical patients undergoing major elective surgery ‐ the FOCCUS study. Critical Care 2011;15(6):R296. [PUBMED: 22177541] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dabubondoc 2013 {published data only}
- Dabu‐Bondoc S, Vadivelu N, Shimono C, English A, Kosarussavadi B, Dai F, et al. Intravenous dextrose administration reduces postoperative antiemetic rescue treatment requirements and postanesthesia care unit length of stay. Anesthesia and Analgesia 2013;117(3):591‐6. [PUBMED: 22253268] [DOI] [PubMed] [Google Scholar]
Gaiser 2002 {published data only}
- Gaiser RR, Dong Y, Cheek TG, Gutsche BB. Does increased intravenous hydration decrease the incidence of nausea/vomiting following Cesarean section?. Anesthesiology 2002;96(Suppl 1):P83. [Google Scholar]
Heidari 2011 {published data only}
- Heidari SM, Saryazdi H, Shafa A, Arefpour R. Comparison of the effect of preoperative administration of Ringer's solution, normal saline and hypertonic saline 5% on postoperative nausea and vomiting: a randomized, double blinded clinical study. Pakistan Journal of Medical Science 2011;27(4):771‐4. [Google Scholar]
Holte 2007a {published data only}
- Holte K, Kristensen BB, Valentiner L, Foss NB, Husted H, Kehlet H. Liberal versus restrictive fluid management in knee arthroplasty: a randomized, double‐blind study. Anesthesia and Analgesia 2007;105(2):465‐74. [PUBMED: 17646507] [DOI] [PubMed] [Google Scholar]
Holte 2007b {published data only}
- Holte K, Foss NB, Andersen J, Valentiner L, Lund C, Bie P, et al. Liberal or restrictive fluid administration in fast‐track colonic surgery: a randomized, double‐blind study. British Journal of Anaesthesia 2007;99(4):500‐8. [PUBMED: 17681972 ] [DOI] [PubMed] [Google Scholar]
Lei 2017 {published data only}
- Lei Y, Huang Q, Zhang S, Chen G, Pei F. Clinical research on perioperative restrictive fluid therapy combined with preoperative urination training in total hip arthroplasty. Chinese Journal of Reparative and Reconstructive Surgery 2017;31(11):1295‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mintz 2004 {published data only}
- Mintz Y, Weiss YG, Rivkind AI. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor‐blinded multicenter trial. Annals of Surgery 2004;240(2):386‐8. [PUBMED: 15273568] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yavuz 2014 {published data only}
- Yavuz MS, Kazanci D, Turan S, Aydinli B, Selçuk G, Özgök A, et al. Investigation of the effects of preoperative hydration on the postoperative nausea and vomiting. BioMed Research International 2014 Jan 20 [Epub ahead of print]. [DOI: 10.1155/2014/302747; PUBMED: 24563861 ] [DOI] [PMC free article] [PubMed]
References to studies awaiting assessment
Laws 2003 {published data only}
- Laws D. Morbidity associated with perioperative intravenous fluid in children undergoing tonsillectomy. http://www.isrctn.com/ISRCTN26015483 (first received 12 September 2003). [ISRCTN: https://doi.org/10.1186/ISRCTN26015483]
References to ongoing studies
NCT03141645 {published data only}
- NCT03141645. Comparison of IV fluid loading and ondansetron in reduction of PONV after LC. https://clinicaltrials.gov/ct2/show/NCT03141645 (first received 5 May 2017).
NCT03142464 {published data only}
- NCT03142464. Intravenous fluids after laparoscopic cholecystectomy. https://clinicaltrials.gov/ct2/show/NCT03142464 (first received 5 May 2017).
NCT03485443 {published data only}
- NCT03142464. Effects of fluid infusion on postoperative vomiting in pediatric patients undergoing otorhinolaryngological surgery (FLUIDVOMIT). https://clinicaltrials.gov/ct2/show/NCT03485443 (first received 28 September 2018).
Additional references
Alkaissi 2004
- Alkaissi A, Gunnarsson H, Johnsson V, Evertsson K, Ofenbartl L, Kalman S. Disturbing post‐operative symptoms are not reduced by prophylactic antiemetic treatment in patients at high risk of post‐operative nausea and vomiting. Acta Anaesthesiologica Scandinavica 2004;48(6):761‐71. [PUBMED: 15196110] [DOI] [PubMed] [Google Scholar]
Apfel 1999
- Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross‐validations between two centers. Anesthesiology 1999;91(3):693‐700. [PUBMED: 10485781] [DOI] [PubMed] [Google Scholar]
Apfel 2004
- Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. New England Journal of Medicine 2004;350(24):2441‐51. [PUBMED: 15190136] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atallah 2004
- Atallah FN, Riu BM, Nguyen LB, Seguin PO, Fourcade OA. Boerhaave's syndrome after postoperative vomiting. Anesthesia and Analgesia 2004;98(4):1164‐6. [PUBMED: 15041618] [DOI] [PubMed] [Google Scholar]
Bampoe 2017
- Bampoe S, Odor PM, Dushianthan A, Bennett‐Guerrero E, Cro S, Gan TJ, et al. Perioperative administration of buffered versus non‐buffered crystalloid intravenous fluid to improve outcomes following adult surgical procedures. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD004089.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blackburn 2015
- Blackburn J, Spencer R. Postoperative nausea and vomiting. Anaesthesia and Intensive Care Medicine 2015;16(9):452‐6. [Google Scholar]
Borgeat 2003
- Borgeat A, Ekatodramis G, Schenker CA. Postoperative nausea and vomiting in regional anesthesia: a review. Anesthesiology 2003;98(2):530‐47. [PUBMED: 12552215] [DOI] [PubMed] [Google Scholar]
Borison 1953
- Borison HL, Wang SC. Physiology and pharmacology of vomiting. Pharmacological Reviews 1953;5(2):193‐230. [PUBMED: 13064033] [PubMed] [Google Scholar]
Bremner 1993
- Bremner WG, Kumar CM. Delayed surgical emphysema, pneumomediastinum and bilateral pneumothoraces after postoperative vomiting. British Journal of Anaesthesia 1993;71(2):296‐7. [PUBMED: 8123411] [DOI] [PubMed] [Google Scholar]
Dabu‐Bondoc 2013
- Dabu‐Bondoc S, Vadivelu N, Shimono C, English A, Kosarussavadi B, Dai F, et al. Intravenous dextrose administration reduces postoperative antiemetic rescue treatment requirements and postanesthesia care unit length of stay. Anesthesia and Analgesia 2013;117(3):591‐6. [PUBMED: 22253268] [DOI] [PubMed] [Google Scholar]
Dawson 1980
- Dawson B, Reed WA. Anaesthesia for day‐care surgery: a symposium (III). Anaesthesia for adult surgical out‐patients. Canadian Anaesthetists' Society Journal 1980;27(4):409‐11. [PUBMED: 7407673] [DOI] [PubMed] [Google Scholar]
De Oliviera 2013
- Oliviera GS Jr, Castro‐Alves LJ, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta‐analysis of randomized controlled trials. Anesthesia & Analgesia 2013;116(1):58‐74. [DOI: 10.1213/ANE.0b013e31826f0a0a] [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455‐63. [PUBMED: 10877304] [DOI] [PubMed] [Google Scholar]
Eberhart 2002
- Eberhart LHJ, Morin AM, Wulf H, Geldner G. Patient preferences for immediate postoperative recovery. British Journal of Aneaesthesia 2002;89(5):760‐1. [PUBMED: 12393775] [PubMed] [Google Scholar]
Fahy 1969
- Fahy A, Marshall M. Postanaesthetic morbidity in out‐patients. British Journal of Anaesthesia 1969;41(5):433‐8. [PUBMED: 5782913] [DOI] [PubMed] [Google Scholar]
Gan 1997
- Gan TJ, Mythen MG, Glass PS. Intraoperative gut hypoperfusion may be a risk factor for postoperative nausea and vomiting. British Journal of Anaesthesia 1997;78(4):476. [PUBMED: 9135331] [DOI] [PubMed] [Google Scholar]
Gan 2001
- Gan T, Sloan F, Dear Gde L, El‐Moalem HE, Lubarsky DA. How much are patients willing to pay to avoid postoperative nausea and vomiting?. Anesthesia and Analgesia 2001;92(2):393‐400. [PUBMED: 11159239] [DOI] [PubMed] [Google Scholar]
Gan 2007
- Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for Ambulatory Anesthesia. Society for ambulatory anesthesia guidelines for the management of postoperative nausea and vomiting. Anesthesia and Analgesia 2007;105(6):1615‐28. [PUBMED: 18042859] [DOI] [PubMed] [Google Scholar]
Gan 2014
- Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer T, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesthesia and Analgesia 2014;118(1):85‐113. [PUBMED: 24356162] [DOI] [PubMed] [Google Scholar]
Gold 1989
- Gold BS, Kitz DS, Lecky JH, Neuhaus JM. Unanticipated admission to the hospital following ambulatory surgery. JAMA 1989;262(21):3008‐10. [PUBMED: 2810644] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 6 August 2017. Hamilton: McMaster University (developed by Evidence Prime), 2015.
Guy 1991
- Guy JM, André‐Fouet X, Porte J, Bertrand M, Lamaud M, Verneyre H. Torsades de pointes and prolongation of the duration of QT interval after injection of droperidol. Annales de Cardiologie et d'Angeiologie 1991;40(9):541‐5. [PUBMED: 1776799] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Habib 2006
- Habib AS, Chen YT, Taguchi A, Hu XH, Gan TJ. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: a retrospective database analysis. Current Medical Research and Opinion 2006;22(6):1093‐9. [PUBMED: 16846542 ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hines 2018
- Hines S, Steels E, Chang A, Gibbons K. Aromatherapy for treatment of postoperative nausea and vomiting. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD007598.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015
- Lee A, Chan SK, Fan LT. Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD003281.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Macario 1999
- Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesthesia and Analgesia 1999;89(3):652‐8. [PUBMED: 10475299] [DOI] [PubMed] [Google Scholar]
McCormick 2002
- McCormick CG. FDA Alert: Current FDA report on droperidol status and basis for 'Black Box' warning. ASA Newsletter 2002;66(4):19‐20. [Google Scholar]
Moher 2009
- Moher D, Liberata A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:b2535. [PUBMED: 19622551] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortensen 2014
- Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, et al. Consensus guidelines for enhanced recovery after gastrectomy: enhanced recovery after surgery (ERAS) Society recommendations. British Journal of Surgery 2014;101(10):1209‐29. [PUBMED: 25047143] [DOI] [PubMed] [Google Scholar]
Myles 2000
- Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. British Journal of Anaesthesia 2000;84(1):6‐10. [PUBMED: 10740539] [DOI] [PubMed] [Google Scholar]
Palazzo 1984
- Palazzo MG, Strunin L. Anaesthesia and emesis. I: Etiology. Canadian Anaesthetists' Society Journal 1984;31(2):178‐87. [PUBMED: 6367902] [DOI] [PubMed] [Google Scholar]
Parra‐Sanchez 2012
- Parra‐Sanchez I, Abdallah R, You J, Zu AZ, Grady M, Cummings K, et al. A time‐motion economic analysis of postoperative nausea and vomiting in ambulatory surgery. Canadian Journal of Anesthesia 2012;59(4):366‐75. [PUBMED: 22223185] [DOI] [PubMed] [Google Scholar]
Polderman 2018
- Polderman JAW, Farhang‐Razi V, Dieren S, Kranke P, DeVries JH, Hollmann MW, et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD011940.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pusch 2002a
- Pusch F, Berger A, Wildling E, Tiefenthaler W, Krafft P. The effects of systolic arterial blood pressure variations on postoperative nausea and vomiting. Anesthesia and Analgesia 2002;94(6):1652‐5. [PUBMED: 12032046] [DOI] [PubMed] [Google Scholar]
Pusch 2002b
- Pusch F, Berger A, Wildling E, Zimpfer M, Moser M, Sam C, et al. Preoperative orthostatic dysfunction is associated with an increased incidence of postoperative nausea and vomiting. Anesthesiology 2002;96(6):1381‐5. [PUBMED: 12170050] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Santesso 2016
- Santesso N, Carrasco‐Labra A, Langendam M, Brignardello‐Petersen R, Mustafa RA, Heus P, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016;74:28‐39. [PUBMED: 26796947] [DOI] [PubMed] [Google Scholar]
Schumann 1999
- Schumann R, Polaner DM. Massive subcutaneous emphysema and sudden airway compromise after postoperative vomiting. Anesthesia and Analgesia 1999;89(3):796‐7. [PUBMED: 10475327] [DOI] [PubMed] [Google Scholar]
Scuderi 2000
- Scuderi PE, James RL, Harris L, Mims GR. Multimodal antiemetic management prevents early postoperative vomiting after outpatient laparoscopy. Anesthesia and Analgesia 2000;91(6):1408‐14. [PUBMED: 11093990] [DOI] [PubMed] [Google Scholar]
Shires 1961
- Shires T, Williams J, Brown F. Acute change in extracellular fluids associated with major surgical procedures. Annals of Surgery 1961;154:803‐10. [PUBMED: 13912109] [DOI] [PMC free article] [PubMed] [Google Scholar]
Temes 1999
- Temes R, Feteiha M, Mapel D, Crowell R, Wernly J. Esophageal rupture after regional anesthesia: report of two cases. Journal of Clinical Gastroenterology 1999;28(4):360‐3. [PUBMED: 10372939] [DOI] [PubMed] [Google Scholar]
Thompson 1978
- Thompson DP, Ashley FL. Face‐lift complications: a study of 922 cases performed in a 6‐year period. Plastic and Reconstructive Surgery 1978;61(1):40‐9. [PUBMED: 619386] [DOI] [PubMed] [Google Scholar]
Watcha 1992
- Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 192;77(1):162‐84. [PUBMED: 1609990] [DOI] [PubMed] [Google Scholar]
Weibel 2017
- Weibel S, Jelting Y, Pace N, Rücker G, Raj D, Schaefer MS, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta‐analysis. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012859] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetchler 1992
- Wetchler BV. Postoperative nausea and vomiting in day‐case surgery. British Journal of Anaesthesia 1992;69(7 Suppl 1):33S‐9S. [PUBMED: 1486012] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jewer 2016
- Jewer JK, Bird SJ, Scott JL, Habib AS, George RB. Supplemental perioperative intravenous crystalloids for postoperative nausea and vomiting. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012212] [DOI] [PMC free article] [PubMed] [Google Scholar]


