Key Points
Question
Is there a benefit of continuing bevacizumab treatment beyond disease progression in patients with non–small cell lung cancer (NSCLC)?
Findings
In this randomized clinical trial of 485 patients with advanced, nonsquamous NSCLC, the primary end point was not met; median overall survival was not significantly different between groups. No new safety signals were identified with bevacizumab treatment beyond disease progression.
Meaning
Continued bevacizumab treatment beyond disease progression did not demonstrate survival benefit.
This randomized clinical trial assesses the efficacy and safety of continuous bevacizumab treatment plus standard of care vs standard of care alone beyond first progression in patients with non–small cell lung cancer.
Abstract
Importance
Bevacizumab treatment beyond progression has been investigated in breast and metastatic colorectal cancers. Avastin in All Lines Lung (AvaALL) is the first randomized phase 3 study of bevacizumab across multiple lines of treatment beyond progression in non–small cell lung cancer (NSCLC).
Objective
To assess the efficacy and safety of continuous bevacizumab treatment beyond first progression in NSCLC.
Design, Setting, and Participants
AvaALL was a randomized, open-label, phase 3b trial, conducted from 2011 to 2015 in 123 centers worldwide. Patients with nonsquamous NSCLC previously treated with first-line bevacizumab plus platinum-doublet chemotherapy and at least 2 cycles of bevacizumab maintenance were randomized (1:1) at first progression to receive bevacizumab plus standard of care (SOC) or SOC alone.
Interventions
Patients received bevacizumab (7.5 or 15 mg/kg intravenously every 21 days) and/or investigator’s choice of SOC. For subsequent lines, patients treated with bevacizumab received SOC with or without bevacizumab; the SOC arm received SOC only.
Main Outcomes and Measures
The primary outcome was overall survival (OS). Secondary outcomes included progression-free survival from first to second (PFS2) and third progression (PFS3), time to second (TTP2) and third progression (TTP3), and safety.
Results
Between June 2011 and January 2015, 485 patients (median age, 63.0 years [range, 26-84 years]; 293 [60.4%] male) were randomized. Median OS was not significantly longer with bevacizumab plus SOC vs SOC alone: 11.9 (90% CI, 10.2-13.7) vs 10.2 (90% CI, 8.6-11.9) months (hazard ratio [HR], 0.84; 90% CI, 0.71-1.00; P = .104). Median PFS2 was numerically longer with bevacizumab plus SOC vs SOC alone: 5.5 (90% CI, 4.2-5.7) vs 4.0 (90% CI, 3.4-4.3) months (HR, 0.83; 90% CI, 0.70-0.98; P = .06). Median PFS3 appeared longer with bevacizumab plus SOC vs SOC alone: 4.0 (90% CI, 2.9-4.5) vs 2.6 (90% CI, 2.3-2.9) months (HR, 0.63; 90% CI, 0.49-0.83), as did TTP2 and TTP3. Grade 3/4 adverse events were more frequent with bevacizumab plus SOC (186 [76.5%]) vs SOC alone (140 [60.3%]). No new safety signals were observed.
Conclusions and Relevance
The primary end point was not met; however, OS was underpowered according to initial statistical assumptions. Continued therapy beyond first progression led to improved PFS3 (but not PFS2), TTP2, and TTP3. Although a result with P = .06 for PFS2 would conventionally be considered significant at a specified 2-sided α of .10, in the absence of adjustments for multiplicity, this result could be a chance finding. No new safety signals were identified with bevacizumab treatment beyond progression.
Trial Registration
clinicaltrialsregister.eu Identifier: 2010-022645-14; ClinicalTrials.gov identifier: NCT01351415
Introduction
Retrospective analyses suggested a survival benefit of bevacizumab continuation after induction therapy in advanced non–small cell lung cancer (NSCLC).1,2 The randomized, phase 2 West Japan Oncology Group (WJOG) 5910L trial in advanced nonsquamous NSCLC demonstrated a modest progression-free survival (PFS) benefit and a nonsignificant finding of improved overall survival (OS) with bevacizumab beyond progression.3 The Avastin Registry: Investigation of Effectiveness and Safety (ARIES) observational analysis suggested that cumulative bevacizumab use after progression prolonged OS in patients with metastatic colorectal cancer.4 These data were confirmed in a phase 3, open-label trial (ML18147).5 The phase 3 TANIA breast cancer study also showed improved PFS with continued bevacizumab plus chemotherapy vs chemotherapy alone.6
The open-label, randomized phase 3b Avastin in All Lines Lung (AvaALL) study assessed the efficacy and safety of bevacizumab beyond first progression in advanced NSCLC following bevacizumab maintenance therapy.
Methods
Study Design
AvaALL investigated standard-of-care (SOC) chemotherapy with or without bevacizumab beyond first progression in patients with advanced, nonsquamous NSCLC. The study was undertaken in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines; written informed consent was obtained from all patients. The study was approved by local institutional review boards at each study site (protocol available in Supplement 1).
At first progression, patients were randomized 1:1 to receive second-line investigator’s choice of SOC with or without bevacizumab (7.5 or 15 mg/kg every 21 days). At second and third progression, patients receiving bevacizumab received SOC with or without bevacizumab; the SOC arm received SOC only. Beyond third progression, bevacizumab was continued at the investigator’s discretion, in the absence of unacceptable toxic effects or consent withdrawal. The same dose of bevacizumab (investigator’s choice) was continued throughout all lines of treatment.
Objectives
The primary objective was OS beyond first progression of continuous bevacizumab therapy vs SOC. Secondary objectives included PFS from randomization at first progression to second (PFS2) and third progression (PFS3), time to progression (TTP) from randomization at first progression to second (TTP2) and third progression (TTP3) (eFigure 1 in Supplement 2), and safety.
Patients
Inclusion criteria were as follows: nonsquamous NSCLC progressing following first-line bevacizumab (4-6 cycles) plus platinum-doublet chemotherapy, and at least 2 cycles of bevacizumab maintenance monotherapy prior to first progression; more than 2 consecutive cycles of bevacizumab between end of first-line and first day of second-line treatment; at least 1 measurable lesion (Response Evaluation Criteria In Solid Tumors, version 1.1); and Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2. Patients with asymptomatic treated brain metastases were eligible if treatment was completed at least 28 days before randomization.
Exclusion criteria were as follows: mixed non–small cell and small cell tumors or mixed adenosquamous carcinomas with predominant squamous component; epidermal growth factor receptor mutation-positive disease; grade at least 2 hemoptysis 3 months or less before randomization; tumor invading a major blood vessel on imaging; radiotherapy 28 days or less before randomization.
Statistical Analyses
Approximately 416 OS events were required to achieve 80% power for the stratified log-rank test at a 1-sided 5% significance level (overall 10% 2-sided, P < .10) for the final OS analysis (500 randomized patients); this would detect a difference between median OS of 10 months (SOC) vs 12.8 months (bevacizumab) (corresponding hazard ratio [HR], 0.78). Allowing for a 2% dropout rate, 250 patients per arm were planned.
Overall survival was defined as time from randomization at first progression to date of death. Progression-free survival was defined as time from randomization until progression or death, and TTP as time from randomization until objective tumor progression; neither were adjusted for multiple testing. Progression-free survival, TTP, and OS were calculated using Kaplan-Meier methodology and between-treatment differences tested by stratified log-rank test (10% significance level). Hazard ratios and 90% confidence intervals were estimated on a stratified Cox model. Adverse events (AEs) were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Results
Patients
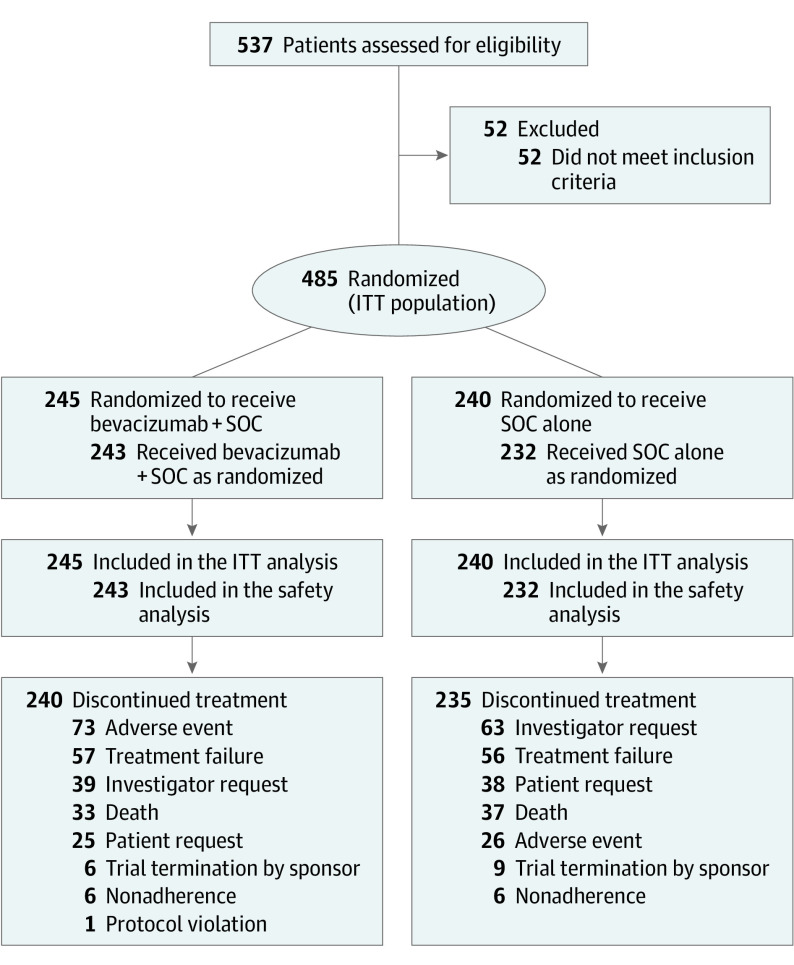
Between June 2011 and January 2015, 485 patients were randomized (bevacizumab, n = 245; SOC, n = 240), of whom 475 received treatment (bevacizumab, n = 243; SOC, n = 232) (Figure 1). Baseline characteristics were balanced (eTable 1 in Supplement 2).
Figure 1. Patient Disposition.
ITT indicates intent to treat; SOC, standard of care.
Efficacy
The final cutoff date was June 24, 2016. The primary analysis was conducted 60 months after first patient enrollment (prespecified in the protocol); the database closed with 387 OS events. Median OS (Figure 2) was numerically longer with bevacizumab plus SOC vs SOC but was not statistically significant (11.9 [90% CI, 10.2-13.7] vs 10.2 [90% CI, 8.6-11.9] months; stratified HR, 0.84; 90% CI, 0.71-1.00; P = .104). Subgroup analyses showed similar results (eFigure 2 in Supplement 2), except in never smokers or patients older than 75 years.
Figure 2. Kaplan-Meier Plot of Overall Survival (OS) in the Intent-to-Treat Population.
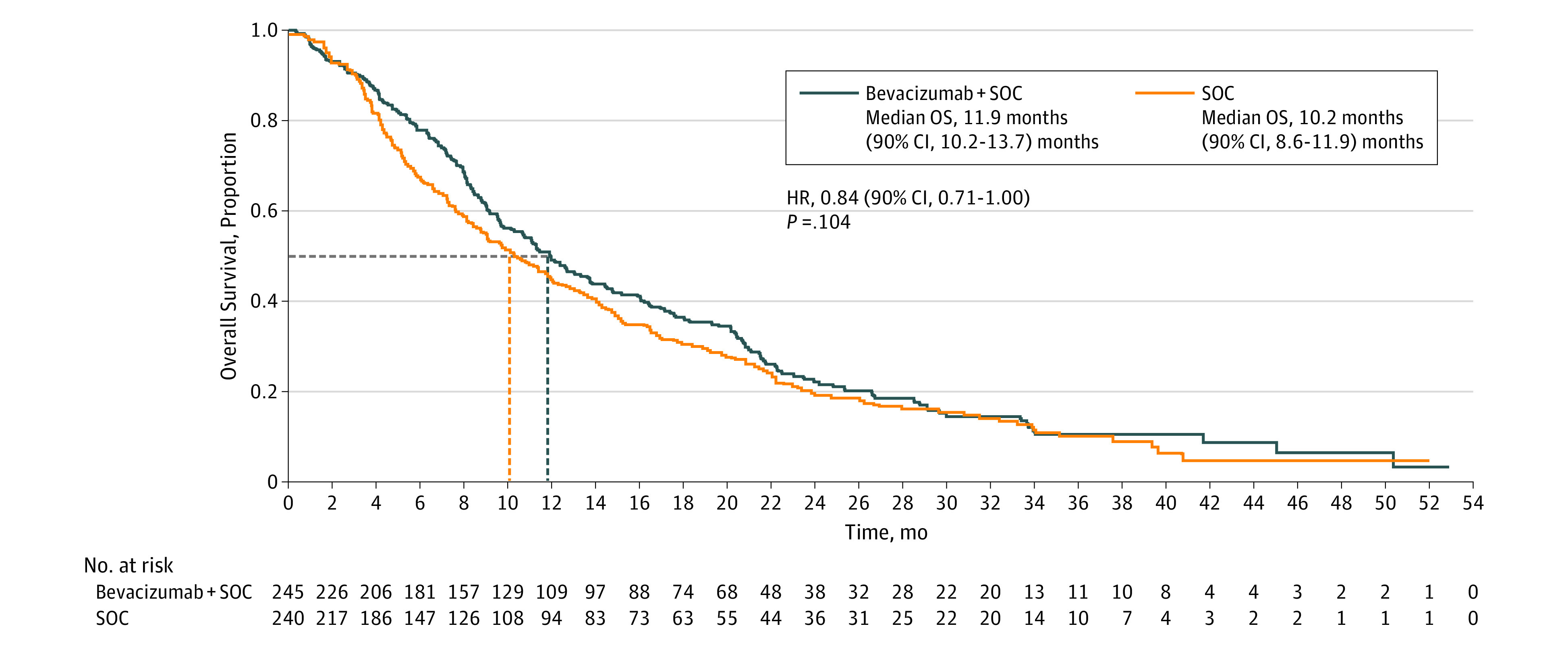
HR indicates hazard ratio; SOC, standard of care.
Median OS did not differ according to bevacizumab dose of 7.5 mg/kg plus SOC (11.4 vs 10.2 months SOC alone; stratified HR, 0.86; 90% CI, 0.69-1.07) or 15 mg/kg plus SOC (12.6 vs 10.2 months SOC alone; stratified HR, 0.84; 90% CI, 0.68-1.04). Median PFS2 was numerically longer with bevacizumab plus SOC vs SOC alone (5.5 [90% CI, 4.2-5.7] vs 4.0 [90% CI, 3.4-4.3] months; stratified HR, 0.83; 90% CI, 0.70-0.98; P = .06) (eFigure 3A in Supplement 2). Subgroup analysis showed similar findings (eFigure 3B in Supplement 2), except patients with ECOG PS 2 or never smokers. Median PFS3 was longer with bevacizumab vs SOC (4.0 [90% CI, 2.9-4.5] vs 2.6 [90% CI, 2.3-2.9] months; HR, 0.63; 90% CI, 0.49-0.83) (eFigure 4 in Supplement 2), as were TTP2 and TTP3 (eFigure 5 and eFigure 6 in Supplement 2).
Safety
Treatment exposure is reported in eTable 2 in Supplement 2. No new safety signals were identified. Adverse events of special interest (Table) and grade 3/4 AEs (eTable 3 in Supplement 2) were more frequent with bevacizumab plus SOC vs SOC alone (118 [48.6%] vs 63 [27.2%] and 186 [76.5%] vs 140 [60.3%], respectively). Sixteen bevacizumab-treated patients (6.6%) and 12 SOC-treated patients (5.2%) experienced grade 5 treatment-related AEs (eTable 4 and eTable 5 in Supplement 2).
Table. Adverse Events of Special Interest (AESIs) With Preferred Term Reported for at Least 1% of Patients Overall (Safety Population).
| System Organ Class and Preferred Term | No. (%) | |
|---|---|---|
| Bevacizumab Plus SOC (n = 243) | SOC Alone (n = 232) | |
| Any AESI | 118 (48.6) | 63 (27.2) |
| Arterial and venous thromboembolic events | 26 (10.7) | 21 (9.1) |
| Vascular disorders | 12 (4.9) | 14 (6.0) |
| Venous thrombosis | 3 (1.2) | 3 (1.3) |
| Deep vein thrombosis | 3 (1.2) | 2 (0.9) |
| Respiratory, thoracic, and mediastinal disorders | 12 (4.9) | 9 (3.9) |
| Pulmonary embolism | 11 (4.5) | 9 (3.9) |
| Hypertension | 55 (22.6) | 28 (12.1) |
| Vascular disorders | 54 (22.2) | 28 (12.1) |
| Hypertension | 53 (21.8) | 25 (10.8) |
| Proteinuria | 51 (21.0) | 24 (10.3) |
| Renal and urinary disorders | 51 (21.0) | 24 (10.3) |
| Proteinuria | 51 (21.0) | 23 (9.9) |
Abbreviation: SOC, standard of care.
Discussion
AvaALL is the first randomized phase 3 study to analyze bevacizumab across multiple lines of treatment beyond progression in NSCLC. The protocol-specified OS end point was not met. However, there was a nonsignificant finding of improved median OS in the bevacizumab vs the SOC arm. As a result of recruitment challenges, the analysis was performed after 60 months, at 387 OS events, and was therefore underpowered. Time to PFS2 was numerically longer with bevacizumab plus SOC vs SOC alone, although no formal testing was performed. These results align with WJOG 5910L, which was also underpowered as a result of enrollment issues.3 Both TTP2 and TTP3 were significantly longer with bevacizumab plus SOC vs SOC alone. However, no multiplicity adjustment was performed; therefore, these findings should be interpreted with caution.
Survival data in most subgroups were similar to the overall population, except for never smokers, patients older than 75 years, or those with ECOG PS 2. Similar results were reported for never smokers in WJOG 5910L.3 Never smokers have distinct molecular tumor profiles vs smokers,7,8 which may affect treatment response. Furthermore, patients older than 75 years and those with a higher ECOG PS may be more susceptible to AEs. Low patient numbers in these subgroups prevent definitive conclusions from being drawn. No unexpected AEs were reported, with more grade at least 3 AEs in the bevacizumab vs the SOC arm, consistent with previous studies.3,5
Since AvaALL began accrual, major changes have occurred to second-line SOC. Checkpoint inhibitors have been approved for locally advanced or metastatic NSCLC after disease progression on platinum-doublet chemotherapy.9 These have displaced pemetrexed and docetaxel from second line, while erlotinib is no longer approved in this setting.10,11 Recent trials have thus rendered the control arm of AvaALL outmoded.
Conclusions
A substantial benefit of bevacizumab therapy beyond progression in patients with NSCLC was not shown, but some improvements in efficacy were observed. No new safety signals were identified.
Trial Protocol
eTable 1. Baseline Characteristics and Disease History of the ITT Population
eTable 2. Patients Exposed to Bevacizumab in Both Treatment Arms (Safety Population)
eTable 3. Grade 3 or 4 Adverse Events (Preferred Term Occurring in ≥10% of Patients with Grade 1 or 2 Events in Any Treatment Arm; Safety Population)
eTable 4. Treatment-Emergent Grade 5 Adverse Events by Body System and Preferred Term (Safety Population)
eTable 5. Cause of Death
eFigure 1. Timeline of Progressive Disease and Study Endpoints
eFigure 2. Forest Plot of OS by Subgroups
eFigure 3. PFS: (A) Kaplan-Meier Plot of PFS2 in the ITT Population; (B) Forest Plot of PFS2 by Subgroups
eFigure 4. PFS3 in the ITT Population
eFigure 5. TTP2 in the ITT Population
eFigure 6. TTP3 in the ITT Population
References
- 1.Kosty MP, Wozniak AJ, Jahanzeb M, et al. Effectiveness and safety of post-induction phase bevacizumab treatment for patients with non-small-cell lung cancer: results from the ARIES observational cohort study. Target Oncol. 2015;10(4):509-516. doi: 10.1007/s11523-014-0355-4 [DOI] [PubMed] [Google Scholar]
- 2.Nadler E, Yu E, Ravelo A, Sing A, Forsyth M, Gruschkus S. Bevacizumab treatment to progression after chemotherapy: outcomes from a US community practice network. Oncologist. 2011;16(4):486-496. doi: 10.1634/theoncologist.2010-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda M, Yamanaka T, Seto T, et al. Bevacizumab beyond disease progression after first-line treatment with bevacizumab plus chemotherapy in advanced nonsquamous non-small cell lung cancer (West Japan Oncology Group 5910L): an open-label, randomized, phase 2 trial. Cancer. 2016;122(7):1050-1059. doi: 10.1002/cncr.29893 [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Flick ED, Cohn AL, et al. Bevacizumab exposure beyond first disease progression in patients with metastatic colorectal cancer: analyses of the ARIES observational cohort study. Pharmacoepidemiol Drug Saf. 2014;23(7):726-734. doi: 10.1002/pds.3633 [DOI] [PubMed] [Google Scholar]
- 5.Bennouna J, Sastre J, Arnold D, et al. ; ML18147 Study Investigators . Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29-37. doi: 10.1016/S1470-2045(12)70477-1 [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G, Puglisi F, Cortes J, et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1269-1278. doi: 10.1016/S1470-2045(14)70439-5 [DOI] [PubMed] [Google Scholar]
- 7.Gibelin C, Couraud S. Somatic alterations in lung cancer: do environmental factors matter? Lung Cancer. 2016;100:45-52. doi: 10.1016/j.lungcan.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 8.Subramanian J, Govindan R. Molecular profile of lung cancer in never smokers. EJC Suppl. 2013;11(2):248-253. doi: 10.1016/j.ejcsup.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La-Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacotherapy. 2015;35(10):963-976. doi: 10.1002/phar.1643 [DOI] [PubMed] [Google Scholar]
- 10.Tarceva (erlotinib) [package insert]. Melville, NY: OSI Pharmaceuticals; 2004. https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/021743lbl.pdf. Accessed January 1, 2018.
- 11.Erlotinib summary of product characteristics. European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000618/WC500033994.pdf. Accessed January 1, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Characteristics and Disease History of the ITT Population
eTable 2. Patients Exposed to Bevacizumab in Both Treatment Arms (Safety Population)
eTable 3. Grade 3 or 4 Adverse Events (Preferred Term Occurring in ≥10% of Patients with Grade 1 or 2 Events in Any Treatment Arm; Safety Population)
eTable 4. Treatment-Emergent Grade 5 Adverse Events by Body System and Preferred Term (Safety Population)
eTable 5. Cause of Death
eFigure 1. Timeline of Progressive Disease and Study Endpoints
eFigure 2. Forest Plot of OS by Subgroups
eFigure 3. PFS: (A) Kaplan-Meier Plot of PFS2 in the ITT Population; (B) Forest Plot of PFS2 by Subgroups
eFigure 4. PFS3 in the ITT Population
eFigure 5. TTP2 in the ITT Population
eFigure 6. TTP3 in the ITT Population