Key Points
Question
Is nanoparticle albumin-bound (nab)–paclitaxel plus gemcitabine safe and effective for treating adult patients with advanced cholangiocarcinoma?
Findings
In this single-arm, phase 2 clinical trial, intravenous treatment with nab-paclitaxel, 125 mg/m2, and gemcitabine, 1000 mg/m2, on days 1, 8, and 15 of every 28 days was well tolerated. The primary trial end point of 6-month progression-free survival rate of 61% failed to reject the null hypothesis of 55%, although the efficacy of this regimen may be similar to the standard chemotherapy regimens in cholangiocarcinoma.
Meaning
This regimen has a tolerable safety profile and may be considered an acceptable alternative option for treating patients with advanced or metastatic cholangiocarcinoma.
This single-arm, 2-stage, phase 2 clinical trial assesses the safety and efficacy of intravenous gemcitabine plus nanoparticle albumin-bound–paclitaxel for treatment of adults with advanced cholangiocarcinoma.
Abstract
Importance
Gemcitabine with platinum has limited efficacy for treatment of advanced cholangiocarcinoma, necessitating an evaluation of alternative drug combinations. Recent evidence suggests that paclitaxel may potentiate gemcitabine activity.
Objective
To evaluate whether gemcitabine plus nanoparticle albumin-bound (nab)–paclitaxel is safe and effective for treatment of advanced cholangiocarcinoma.
Design, Setting, and Participants
This single-arm, 2-stage, phase 2 clinical trial was conducted at 23 community and academic centers across the United States and Europe. Patients aged 18 years or older enrolled between September 2014 and March 2016 had confirmed advanced or metastatic cholangiocarcinoma without prior systemic therapy, and had an Eastern Cooperative Oncology Group Performance Status score of 0 to 1 and a Child-Pugh score less than 8. Previous surgery, radiation, or liver-directed therapies were permitted.
Interventions
Patients received intravenous nab-paclitaxel, 125 mg/m2, followed by gemcitabine, 1000 mg/m2, on days 1, 8, and 15 of each 28-day treatment cycle until disease progression or unacceptable toxic effects.
Main Outcomes and Measures
The primary outcome was improvement in 6-month progression-free survival (PFS) rate (null and alternative hypotheses of 55% and 70%, respectively) in the evaluable population. Secondary outcomes included median overall survival (OS), PFS, time to progression, best overall response rate, disease control rate, safety and toxicity, and association of change in carbohydrate antigen 19-9 with survival.
Results
Seventy-four patients with a median age of 62 (range, 36-87) years, including 44 women (60%), were enrolled. Patients received a median of 6 (range, 1-18) treatment cycles, and the median follow-up was 10.2 (range, 0.6-27.3) months. The observed 6-month PFS rate of 61% (95% CI, 48%-73%) did not favor the alternative hypothesis. Median PFS was 7.7 (95% CI, 5.4-13.1) months, median OS was 12.4 (95% CI, 9.2-15.9) months, and median time to progression was 7.7 (95% CI, 6.1-13.1) months. The confirmed best overall response rate and disease control rate were 30% and 66%, respectively. Hazard ratios for an association between a change in serum carbohydrate antigen 19-9 and median PFS as well as median OS were 2.02 (95% CI, 0.86-4.75) (P = .10) and 1.54 (95% CI, 0.64-3.71) (P = .34), respectively. The most common treatment-related hematologic and nonhematologic adverse events at grade 3 or higher were neutropenia (43%) and fatigue (14%), respectively.
Conclusions and Relevance
Although the trial did not meet its primary efficacy end point, the results indicate that a nab-paclitaxel plus gemcitabine regimen was well tolerated and may be an alternative option to current therapeutic approaches for advanced cholangiocarcinoma.
Trial Registration
ClinicalTrials.gov identifier: NCT02181634
Introduction
The incidence of cholangiocarcinoma (CCA), classified as intrahepatic and extrahepatic (perihilar and distal), is increasing globally, including in the United States.1 Advanced CCAs are aggressive cancers with a median survival in patients of less than 12 months.2 The options for systemic therapy are limited, with a 5-year overall survival (OS) rate of approximately 5% despite treatment.3 The standard first-line systemic therapy of gemcitabine and a platinum analogue for advanced unresectable and metastatic CCA has limited efficacy, necessitating an evaluation of alternative drug combinations.2,4
Paclitaxel can inhibit the gemcitabine-metabolizing enzyme cytidine deaminase to increase the intratumoral concentration of the active metabolites of gemcitabine.5 Standard paclitaxel has considerable toxicity compared with the nanoparticle albumin-bound (nab) colloidal formulation, nab-paclitaxel (nabP), which has less vehicle-related hypersensitivity reactions, neurotoxicity, and neutropenia.6,7,8 On the basis of preclinical evidence of the potential synergism and clinical efficacy of nabP and gemcitabine in treating breast and pancreatic cancer,9,10,11 we conducted a phase 2, single-arm trial to assess the safety and efficacy of nabP and gemcitabine therapy for patients with advanced or metastatic CCA.
Methods
Study Design
This was a phase 2, single-arm, 2-stage clinical trial (protocol No. PrE0204) with a planned enrollment of 70 patients to obtain 67 evaluable patients (trial protocol in Supplement 1). In stage 1, 35 evaluable patients were planned for, and if at least 21 patients were alive and progression free at 6 months, stage 2 would commence by enrolling an additional 32 evaluable patients. Stage 2 enrollment was permitted before the completion of the 6-month follow-up in stage 1 in the absence of significant grade 3 or higher toxic effects. The study protocol was approved by the ethics committee or institutional review board at each site. The study was conducted in accordance with the Declaration of Helsinki12 and with the Good Clinical Practice Guidelines of the International Conference on Harmonization. Participating study sites and the PrECOG, LLC (Philadelphia, Pennsylvania) data safety monitoring board reviewed the safety data. All patients provided written informed consent before enrollment.
Outcomes
The primary outcome of the present study was the clinical efficacy of nabP plus gemcitabine therapy as determined by assessing improvement in the 6-month progression-free survival (PFS) rate. Secondary outcomes included evaluation of the median OS, PFS, and time to progression (TTP) rates and the best overall response rate (ORR) and disease control rate (DCR); the safety and toxicity profile of the treatment regimen; and the association of the maximum change in serum carbohydrate antigen 19-9 (CA19-9) level with survival. Exploratory outcomes, including enumeration of circulating tumor cells13 and immunohistochemical evaluation of cytidine deaminase, human equilibrative nucleoside transporter 1,13 and secreted protein acidic and rich in cysteine14 protein expression levels, are pending and will be reported in a future article.
Patient Eligibility
Key patient eligibility requirements included being 18 years of age or older; pathologic confirmation of CCA, advanced or metastatic stage; no prior systemic therapy, disease radiographically measurable per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; an Eastern Cooperative Oncology Group Performance Status score of 0 to 1; and a Child-Pugh score less than 8. Previous surgery, radiation, or liver-directed therapies were permitted.
Investigational Treatment
On days 1, 8, and 15 of each 28-day treatment cycle, patients received intravenous nabP, 125 mg/m2, followed by intravenous gemcitabine, 1000 mg/m2, each for 30 minutes. Treatment was permitted until disease progression or development of an unacceptable toxic effect.
Assessments and Study End Points
To evaluate treatment response, computed tomography or magnetic resonance imaging was performed at baseline and every 8 weeks, and response assessment was defined per RECIST, version 1.1.15,16 Serial serum CA19-9 level measurements were performed at baseline and every 8 weeks thereafter.
The PFS was calculated from the date of the first study treatment to either the date of documented disease progression or death from any cause, whichever occurred first. The OS was defined as the time from enrollment until death or censored at last patient contact. The TTP was calculated from date of first study treatment to date of disease progression. Among patients with a baseline serum CA19-9 level of 40 U/mL or higher, the proportion of patients with a 50% or greater decrease from baseline was measured.17
All toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. The trial monitoring included 2 interim safety evaluations before the completion of accrual.
Statistical Analysis
In this 2-stage design, the first-stage analysis required that 21 of the first 35 evaluable patients be alive and progression free at 6 months to continue accrual. At completion, the trial required more than 43 of 67 evaluable patients to be alive and progression free at 6 months to conclude that the 6-month PFS rate was at least 70% (vs a null hypothesis of 55%) based on historical data from the Advanced Biliary Cancer (ABC)–02 and BINGO (Gemcitabine and Oxaliplatin With or Without Cetuximab in Advanced Biliary-Tract Cancer) trials.2,4 This design had a 20% chance of falsely identifying the therapy as statistically nonsignificant if the true treatment success rate was 70% and a 5% chance of falsely concluding the therapy as significant if the true success rate was 55%. There was greater than 0.66 probability that the study would terminate at the first stage if the null hypothesis was true.
Distributions of PFS, TTP, and OS were estimated using the Kaplan-Meier approach (with pointwise confidence intervals for time-to-event outcomes), and univariate testing was performed using the log-rank test. Descriptive statistics were used for all clinical demographic data. The ORR and the DCR were summarized by number and percentage, with associations evaluated via the Fisher exact test. The preplanned determination of the association between change in serum CA19-9 level and survival was evaluated using a Cox proportional hazards regression model and the log-rank test. Safety data summaries described the incidence of adverse events (including severity and association with drug or treatment) and grade 3 or 4 toxic effects. For the final analysis, the data cutoff date was December 15, 2017, and 19 patients who were alive were censored for the survival analysis. Statistical analyses were completed using SAS (SAS Institute Inc), version 9.3. A 2-sided P < .05 was considered statistically significant.
Results
Patients
Seventy-four patients were enrolled at 23 community and academic centers across the United States and Europe between September 2014 and March 2016. The baseline demographic and disease characteristics of the patients are summarized in Table 1. The median age of the participants was 62 (range, 36-87) years and included 44 women (60%) and 68 white individuals (92%). Sixty-one patients (82%) had intrahepatic CCA. The patients received a median of 6 (range, 1-18) treatment cycles, and the median follow-up was 10.2 (range, 0.6-27.3) months.
Table 1. Patient Baseline Demographic and Disease Characteristics (Safety Population).
| Characteristic | Result |
|---|---|
| Total No. of patients progression free | 74 |
| Age, median (range), y | 62 (36-87) |
| Female, No. (%) | 44 (60) |
| Race, No. (%) | |
| White | 68 (92) |
| African American | 3 (4) |
| Asian | 1 (1) |
| Other | 2 (3) |
| Ethnicity, No. (%) | |
| Hispanic | 1 (1) |
| Not Hispanic | 72 (97) |
| Not reported | 1 (1) |
| Time since initial diagnosis, days | |
| Mean (SD) | 114.2 (409.5) |
| Median (range) | 22 (4-3146) |
| ECOG performance status, No. (%) | |
| 0 | 33 (45) |
| 1 | 41 (55) |
| Tumor location, No. (%) | |
| Intrahepatic | 61 (82) |
| Extrahepatic, perihilar | 4 (5) |
| Extrahepatic, distal | 9 (12) |
| AJCC stage in 69 patients, No. (%) | |
| II | 11 (16) |
| III | 3 (4) |
| IV | 55 (80) |
| CA19-9, median (range), U/mL | 158 (1-380 670) |
Abbreviations: AJCC, American Joint Committee on Cancer; CA19-9, carbohydrate antigen 19-9; ECOG, Eastern Cooperative Oncology Group.
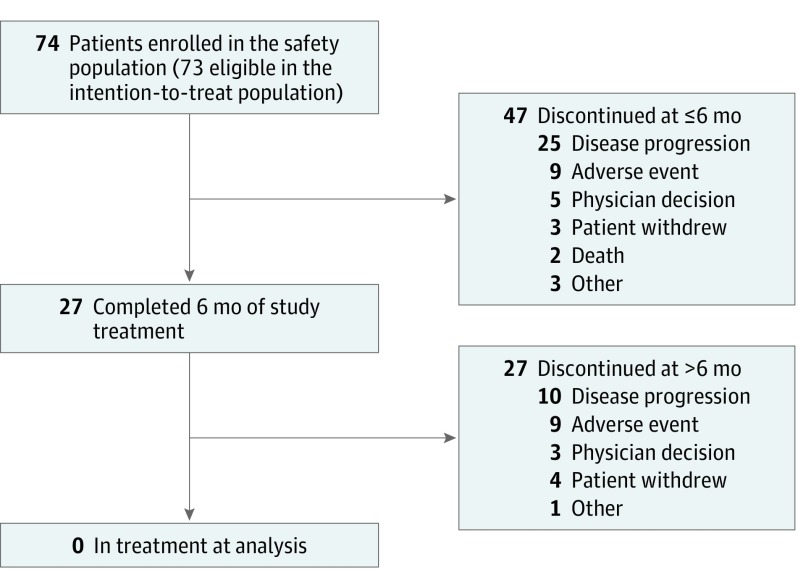
Patient disposition is summarized in Figure 1. Of the 73 patients in the intention-to-treat population (who received ≥1 dose of study treatment and were eligible for efficacy assessments), 47 (64%) discontinued treatment within 6 months of study initiation (25 [34%] discontinued due to progression), and 27 (36%) discontinued after 6 months (10 [14%] discontinued due to progression). In the stage 1 analysis, 19 of 35 patients were alive and progression free at 6 months, providing an observed 6-month PFS of 54%, which did not favor the alternative hypothesis. The trial was not halted, however, because the patient accrual for stage 2 had already been completed.
Figure 1. Patient Disposition.
Efficacy Results
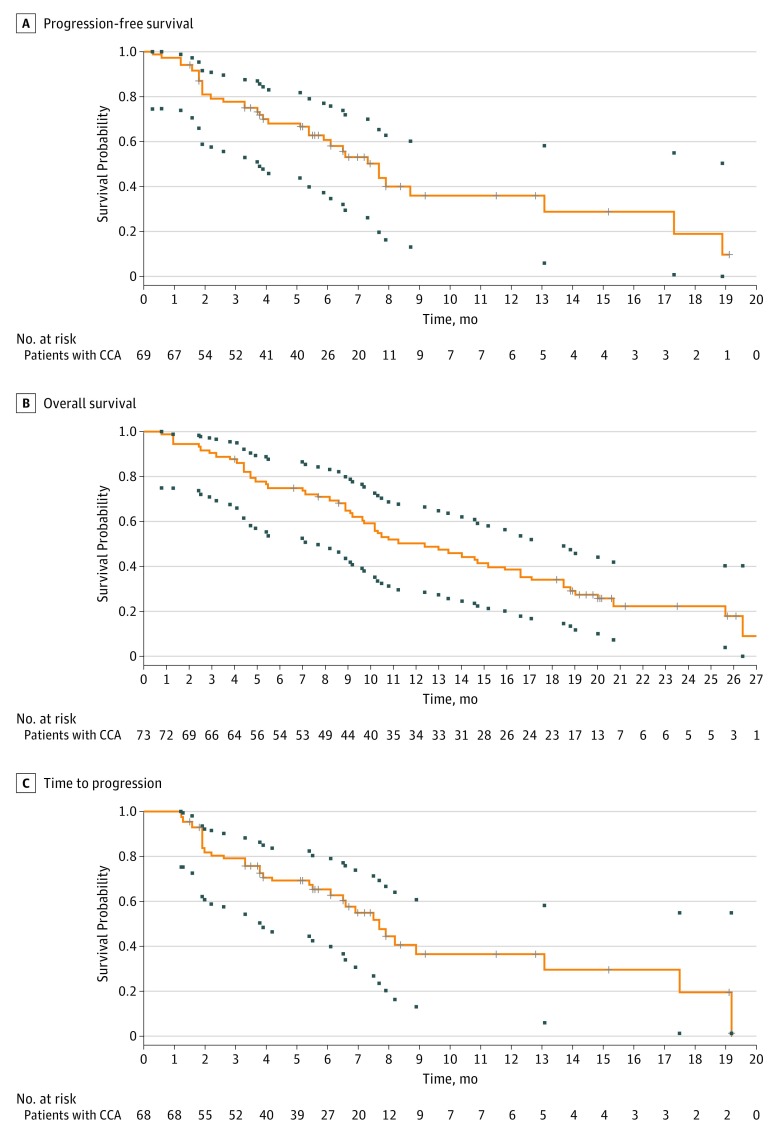
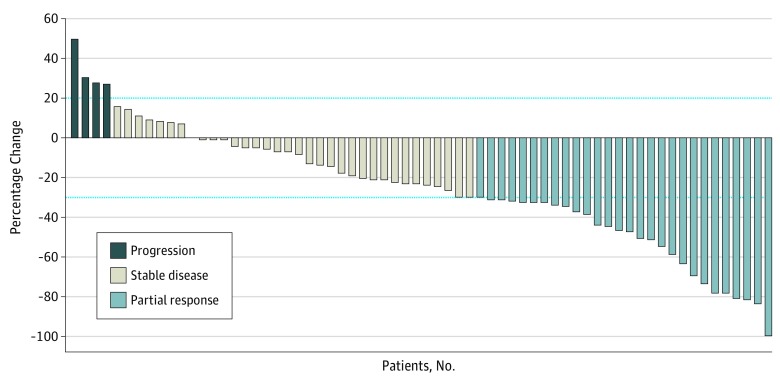
For the primary objective, the observed PFS rate at 6 months was 61% (95% CI, 48%-73%), which did not achieve the alternative hypothesis of 70%. It should be noted that the primary end point may be considered more descriptive because stage I analysis failed to reject the null hypothesis. The median PFS was 7.7 (95% CI, 5.4-13.1) months (Figure 2A), the median OS was 12.4 (95% CI, 9.2-15.9) months (Figure 2B), and the median TTP was 7.7 (95% CI, 6.1-13.1) months (Figure 2C). The confirmed best ORR and DCR were 30% and 66%, respectively (Figure 3). The hazard ratio for an association between the change in serum CA19-9 level and the median PFS as well as median OS were 2.02 (95% CI, 0.86-4.75) (P = .10) and 1.54 (95% CI, 0.64-3.71) (P = .34), respectively (eFigure in Supplement 2).
Figure 2. Kaplan-Meier Analyses of Survival Outcomes in the Intention-to-Treat Population.
The boxes represent pointwise confidence intervals for time-to-event outcomes. Tick marks represent censored patients. Equal precision confidence intervals (small boxes) extend to the last event times. CCA indicates cholangiocarcinoma.
Figure 3. Waterfall Plot of Best Response per Response Evaluation Criteria in Solid Tumors, Version 1.1.
Best overall response rate is 30% and disease control rate is 66%. The dashed line above the x-axis represents 20% increase in sum of target lesions from nadir, and the dashed line below the x-axis represents a 30% decrease in sum of target lesions from baseline.
Safety
The study protocol permitted dose modifications but no change to the administration schedule for treatment-related adverse events. A total of 72 patients (97%) experienced a treatment-related adverse event (eTable in Supplement 2), the most common of which were fatigue (52 patients [70%]), neutropenia (50 patients [68%]), and peripheral neuropathy (41 patients [55%]). Overall, 61 patients (82%) experienced a grade 3 or higher treatment-related adverse event (Table 2). The most common treatment-related hematologic and nonhematologic adverse events at grade 3 or higher were neutropenia (32 patients [43%]) and fatigue (10 patients [14%]), respectively.
Table 2. Treatment-Related AEs at Grade 3 or Higher.
| Event | Patients, No. (%) (N = 74) |
|---|---|
| All hematologic AEs | |
| Neutropenia | 32 (43) |
| Thrombocytopenia | 12 (16) |
| Anemia | 11 (15) |
| Leukopenia | 7 (10) |
| Hemolytic uremic syndrome | 3 (4) |
| Febrile neutropenia | 2 (3) |
| Nonhematologic AEs in ≥5% of patients | |
| Fatigue | 10 (14) |
| Elevated alkaline phosphatase level | 7 (10) |
| Peripheral neuropathy | 6 (8) |
| Diarrhea | 5 (7) |
| Elevated alanine aminotransferase level | 4 (5) |
| Hyponatremia | 4 (5) |
Abbreviation: AE, adverse event.
Discussion
This single-arm, phase 2 multicenter trial evaluated the efficacy of the combination of nabP and gemcitabine treatment in patients with advanced or metastatic CCA. The PFS rate at 6 months was observed to be 61% in the intention-to-treat population and did not favor the alternative hypothesis. Nevertheless, the primary end point in this trial, along with the secondary efficacy end points of median PFS of 7.7 months and median OS of 12.4 months, was similar to that in the phase 3 ABC-02 trial (median PFS of 8.0 months and median OS of 11.7 months).2 These data are also similar to those observed in the phase 2 gemcitabine plus oxaliplatin trial and gemcitabine plus capecitabine trials (median PFS of 6.1 and 6.2 months, and median OS of 12.4 and 12.7 months, respectively).4,18 The present trial enrolled only patients with CCA, whereas the other mentioned trials also enrolled patients with gallbladder and ampullary cancers, which may have impacted the results. The observed best ORR of 30% in the present study is higher than that reported for patients with CCA in the gemcitabine plus oxaliplatin (20%) and gemcitabine plus cisplatin (19%) arms of the BINGO and ABC-02 trials, respectively.2,4 The median follow-up time in the present trial was 10.2 months, with 19 patients alive at the time of data cutoff, and further follow-up may affect the overall survival estimate.
The combination treatment of nabP plus gemcitabine showed an acceptable safety profile among patients with CCA, and no new unexpected toxicities were observed in the present study. The most common treatment-related grade 3 or higher adverse events included neutropenia, thrombocytopenia, fatigue, and anemia and are consistent with those reported in the phase 3 Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT), which evaluated gemcitabine plus nabP for treatment of pancreatic adenocarcinoma.10 On the basis of the adverse event profile, nabP plus gemcitabine treatment may be considered for patients who are not otherwise considered candidates for cisplatin-based therapy, specifically those with renal dysfunction.
A recent phase 2, single-arm trial with liposomal paclitaxel plus gemcitabine treatment of 39 patients with unresectable or metastatic biliary cancers conducted in the Republic of Korea19 reported a comparable median PFS and OS of 5.9 months and 11.9 months, respectively. The ORR was also comparable at 26%. It is unlikely that a randomized phase 2 or 3 clinical trial will be conducted with a noninferior statistical design. The addition of taxanes to gemcitabine, however, appears to be effective for treatment of CCA and is now being evaluated in combination with gemcitabine and cisplatin with encouraging preliminary data.20
Limitations
There are inherent limitations to the present trial, including the lack of a concurrent control arm and the small patient population. However, this multicenter study was conducted in both academic and community centers, which increases the generalizability of the data. The rate of enrollment was more robust than anticipated for this rare cancer, enabling completion of accrual even before the stage 1 data could be analyzed.
Conclusions
In summary, treatment with nabP plus gemcitabine for patients with advanced CCA was found to have an acceptable safety profile. Although the trial did not meet its primary efficacy end point, the PFS and OS were comparable to those of the gemcitabine plus cisplatin and gemcitabine plus oxaliplatin regimens in the ABC-02 and BINGO trials, respectively.2,4 As such, we conclude that combination nabP and gemcitabine therapy is well tolerated and may be considered as an alternative regimen to current therapeutic approaches in advanced CCA.
Trial Protocol
eTable. Treatment-Related Adverse Events (≥10% Frequency)
eFigure. Kaplan-Meier Curves for (A) Progression-Free Survival and (B) Overall Survival by Decline in CA19-9 (<50% vs ≥50%)
References
- 1.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13-21.e1. doi: 10.1016/j.cgh.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valle J, Wasan H, Palmer DH, et al. ; ABC-02 Trial Investigators . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 3.Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, Schulick RD. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg. 2007;11(11):1488-1496. doi: 10.1007/s11605-007-0282-0 [DOI] [PubMed] [Google Scholar]
- 4.Malka D, Cervera P, Foulon S, et al. ; BINGO investigators . Gemcitabine and Oxaliplatin With or Without Cetuximab in Advanced Biliary-Tract Cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15(8):819-828. doi: 10.1016/S1470-2045(14)70212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frese KK, Neesse A, Cook N, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2(3):260-269. doi: 10.1158/2159-8290.CD-11-0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ait-Oudhia S, Straubinger RM, Mager DE. Meta-analysis of nanoparticulate paclitaxel delivery system pharmacokinetics and model prediction of associated neutropenia. Pharm Res. 2012;29(10):2833-2844. doi: 10.1007/s11095-012-0775-8 [DOI] [PubMed] [Google Scholar]
- 7.Gianni L, Kearns CM, Giani A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol. 1995;13(1):180-190. doi: 10.1200/JCO.1995.13.1.180 [DOI] [PubMed] [Google Scholar]
- 8.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi: 10.1200/JCO.2005.04.937 [DOI] [PubMed] [Google Scholar]
- 9.Lau SC, Cheung WY. Evolving treatment landscape for early and advanced pancreatic cancer. World J Gastrointest Oncol. 2017;9(7):281-292. doi: 10.4251/wjgo.v9.i7.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548-4554. doi: 10.1200/JCO.2011.36.5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.28105 [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Murakami Y, Uemura K, et al. Human equilibrative nucleoside transporter 1 expression predicts survival of advanced cholangiocarcinoma patients treated with gemcitabine-based adjuvant chemotherapy after surgical resection. Ann Surg. 2012;256(2):288-296. doi: 10.1097/SLA.0b013e3182536a42 [DOI] [PubMed] [Google Scholar]
- 14.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25(3):319-325. doi: 10.1200/JCO.2006.07.8824 [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services Food and Drug Administration; Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. Guidance for industry: clinical trial endpoints for the approval of cancer drugs and biologics. https://www.fda.gov/downloads/Drugs/Guidances/ucm071590.pdf. Published May 2007. Accessed July 13, 2018.
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 17.Grunnet M, Christensen IJ, Lassen U, et al. Decline in CA19-9 during chemotherapy predicts survival in four independent cohorts of patients with inoperable bile duct cancer. Eur J Cancer. 2015;51(11):1381-1388. doi: 10.1016/j.ejca.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 18.Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110(6):1307-1312. doi: 10.1002/cncr.22902 [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Do YR, Song HS, et al. Multicenter phase II clinical trial of Genexol-PM® with gemcitabine in advanced biliary tract cancer. Anticancer Res. 2017;37(3):1467-1473. doi: 10.21873/anticanres.11471 [DOI] [PubMed] [Google Scholar]
- 20.Shroff RT, Borad MJ, Xiao L, et al. A phase II trial of gemcitabine (G), cisplatin (C), and nab-paclitaxel (N) in advanced biliary tract cancers (aBTCs) [ASCO abstract]. J Clin Oncol. 2017;35(15)(suppl):4018. doi: 10.1200/JCO.2017.35.15_suppl.4018 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Treatment-Related Adverse Events (≥10% Frequency)
eFigure. Kaplan-Meier Curves for (A) Progression-Free Survival and (B) Overall Survival by Decline in CA19-9 (<50% vs ≥50%)