Key Points
Question
What are the associations between aspirin dose and duration of use and the risk of developing hepatocellular carcinoma?
Findings
In this population-based study of 2 nationwide, prospective cohorts of 87 507 men and 45 864 women, self-reported regular use of standard dose (325 mg) aspirin tablets at least 2 or more times per week was associated with a significantly 49% reduced risk of developing hepatocellular carcinoma. The observed benefit of aspirin was both dose and duration dependent, appearing with aspirin use for 5 years or more, at a dose of 1.5 or more standard tablets per week.
Meaning
Use of at least 1.5 standard aspirin tablets per week may represent a feasible strategy for primary prevention against hepatocellular carcinoma; however, potential benefits must be carefully balanced with hazards.
Abstract
Importance
Prospective data on the risk of hepatocellular carcinoma (HCC) according to dose and duration of aspirin therapy are limited.
Objective
To examine the potential benefits of aspirin use for primary HCC prevention at a range of doses and durations of use within 2 prospective, nationwide populations.
Design, Setting, and Participants
Pooled analysis of 2 prospective US cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. Data were accessed from November 1, 2017, through March 7, 2018. A total of 133 371 health care professionals who reported data on aspirin use, frequency, dosage, and duration of use biennially since 1980 in women and 1986 in men were included. Individuals with a cancer diagnosis at baseline (except nonmelanoma skin cancer) were excluded.
Main Outcomes and Measures
Cox proportional hazards regression models were used to calculate multivariable adjusted hazard ratios (HRs) and 95% CIs for HCC.
Results
Of the 133 371 participants, 87 507 were women and 45 864 were men; in 1996, the median time of follow-up, the mean (SD) age was 62 (8) years for women and 64 (8) years for men. Over more than 26 years of follow-up encompassing 4 232 188 person-years, 108 incident HCC cases (65 women, 43 men) were documented. Compared with nonregular use, regular aspirin use (≥2 standard-dose [325-mg] tablets per week) was associated with reduced HCC risk (adjusted HR, 0.51; 95% CI, 0.34-0.77). This benefit appeared to be dose related: compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 standard-dose tablets per week, 0.51 (95% CI, 0.30-0.86) for more than 1.5 to 5 tablets per week, and 0.49 (95% CI, 0.28-0.96) for more than 5 tablets per week (P for trend = .006). Significantly lower HCC risk was observed with increasing duration (P for trend = .03); this decrease was apparent with use of 1.5 or more standard-dose aspirin tablets per week for 5 or more years (adjusted HR, 0.41; 95% CI, 0.21-0.77). In contrast, use of nonaspirin nonsteroidal anti-inflammatory drugs was not significantly associated with HCC risk (adjusted HR, 1.09; 95% CI, 0.78-1.51).
Conclusions and Relevance
This study suggests that regular, long-term aspirin use is associated with a dose-dependent reduction in HCC risk, which is apparent after 5 or more years of use. Similar associations were not found with nonaspirin NSAIDs. Further research appears to be needed to clarify whether aspirin use represents a feasible strategy for primary prevention against HCC.
This pooled analysis of the Nurses’ Health Study and the Health Professionals Follow-up Study examines the association between long-term use of aspirin and the development of hepatocellular carcinoma in adult men and women.
Introduction
Hepatocellular carcinoma (HCC) represents the second leading cause of cancer death worldwide.1,2 In the United States, the incidence of HCC has risen in the past 40 years3 to an estimated 6.7 cases per 100 000 person-years in 2012.4 At the same time, rates of HCC mortality are accelerating more rapidly than those for any other cancer.5 Most patients with HCC receive the diagnosis at a late stage, with limited treatment options and median survival of less than 1 year.1,3 With such a poor prognosis, there is a need to develop effective preventive strategies to reduce the incidence and mortality of HCC.
A body of experimental6,7,8 and epidemiologic9,10 evidence suggests that aspirin may prevent incident HCC via inhibition of the proinflammatory cyclooxygenase-2 (COX-2) enzyme.6,7,8 However, to date, the optimal aspirin dosage and duration of use for chemoprevention remain undefined. Prospective evidence specifically addressing aspirin dose and duration is limited to 2 previous cohort studies that assessed aspirin use at only 1 point in time.9,10 As individual patterns of medication use may change over time, prospectively updated medication data are important for accurately estimating risk, particularly in diseases with prolonged latency.11,12 Moreover, it remains controversial whether nonaspirin nonsteroidal anti-inflammatory drugs (NSAIDs), which share several mechanisms of action with aspirin, exert similar anti-HCC effects. Some prior studies reported a null association,9,10 while others found inverse associations.13,14 Given the paucity of data and the accelerating incidence of HCC, understanding the potential chemopreventive benefits of aspirin represents an important unmet need.11
To address this issue, we examined the dose- and duration-dependent associations between aspirin use and HCC risk in 2 prospective, nationwide cohorts: the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS). These 2 populations permit a comprehensive examination of long-term aspirin use at a wide range of doses and primary HCC prevention.
Methods
Participants
The NHS enrolled 121 700 US female registered nurses, aged 30 to 55 years, in 1976; the HPFS enrolled 51 529 male health professionals, aged 40 to 75 years, in 1986.15,16 Participants returned questionnaires at enrollment and biennially thereafter, providing data on lifestyle, medical history, and disease outcomes; dietary intake was assessed every 4 years using a validated semiquantitative food frequency questionnaire.17,18 In both cohorts, follow-up rates consistently exceed 90%.19 Data were accessed from November 1, 2017, through March 7, 2018. For the current study, we included all individuals who completed the medication portion of the questionnaire when aspirin use was first assessed (1980 NHS; 1986 HPFS).20,21 Consistent with prior publications, we excluded individuals with missing aspirin information or anyone with a cancer diagnosis at baseline, except nonmelanoma skin cancer, as these tumors generally have little clinical effect, and thus are not expected to be associated with recall bias or lifestyle changes.20,22 No participants had a missing date of HCC diagnosis. The NHS and HPFS were approved by the institutional review boards of the Harvard T. H. Chan School of Public Health and Partners Healthcare, Boston, Massachusetts. These were voluntary questionnaires returned to study staff by the NHS/HPFS participants.
Assessment of Aspirin Use and Covariates
Beginning in 1980 in NHS, biennial questionnaires asked participants whether they took aspirin most weeks, number of tablets taken per week, and years of aspirin use. Beginning in 1986 in HPFS, participants were asked whether they used aspirin 2 or more times per week, and the number of tablets taken per week (beginning in 1992). In both cohorts, participants were specifically asked about standard-dose (325-mg) aspirin tablets.20,21 Between 1994 and 1998, participants were asked to convert intake of 4 low-dose (81-mg) aspirin tablets to 1 standard-dose aspirin tablet. Since 2000, participants report regular use of low-dose aspirin separately from standard-dose aspirin. Consistent with prior analyses,20,21 regular aspirin use was defined as aspirin use 2 or more times per week; nonregular use included consumption of aspirin fewer than 2 times per week or no use. Reasons for aspirin use included headache, musculoskeletal pain, and primary cardiovascular disease prevention (NHS),21 and cardiovascular disease risk reduction, pain, and headache (HPFS).20 Consistent with prior publications,20,22 covariates were selected a priori as potential confounders (eMethods in the Supplement).
Ascertainment of HCC
Hepatocellular carcinoma diagnoses were obtained from biennial questionnaires and the National Death Index.23 Researchers obtained permission from participants or next of kin to acquire medical records, pathology reports, imaging results, and death certificates, and 2 blinded physicians abstracted data confirming each HCC diagnosis, along with anatomic features underlying cirrhosis (diagnosed histopathologically or through imaging), and presence of viral hepatitis.
Statistical Analysis
Follow-up time accrued from the date of baseline questionnaire return to the date of HCC diagnosis, all-cause mortality, or end of follow-up (January 31, 2012 [HPFS] or June 1, 2012 [NHS]), whichever came first. We used Cox proportional hazards regression models conditioned on age (years), questionnaire cycle, and sex (ie, cohort) (in pooled analyses) to estimate multivariable-adjusted hazard ratios (HRs) and 95% CIs of the association between regular aspirin use and HCC risk. All relevant covariates were updated biennially and included as time-varying covariates. Consistent with prior analyses,22,24,25 we used cumulative mean values for dietary factors, physical activity, and alcohol use to minimize variance and better reflect long-term patterns. The proportionality assumption was not violated.
For dose analyses, we calculated cumulative mean aspirin intake from all questionnaires before each 2-year interval.20,21 Duration of medication use (years) was estimated by summing all previous intervals of regular use before each interval.20,21 Linear trend was assessed using the median of each category as a continuous variable. Consistent with previous analyses,20 we examined time since discontinuation of aspirin use among former aspirin users. We evaluated whether the effects of aspirin vary among strata defined by putative HCC risk factors and tested the significance of interactions using the log likelihood ratio test. We also separately examined the association between acetaminophen and nonaspirin NSAIDs and HCC risk.
As a sensitivity analysis, we assessed the latency of aspirin use and HCC risk using a lag of 4 years. Next, we excluded any individual with HCC associated with chronic viral hepatitis B or C (n = 21). Finally, we excluded any individual with HCC and confirmed cirrhosis (n = 41) or unknown cirrhosis status (n = 20). All statistical analyses were performed using SAS, version 9.4 (SAS Institute), with a 2-sided significance P value <.05.
Results
Among 133 371 participants (87 507 women; 45 864 men), we documented 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC) over more than 26 years of follow-up, encompassing 4 232 188 person-years. At the midpoint of follow-up (1996), the mean (SD) age was 62 (8) years for women and 64 (8) years for men. Regular aspirin users were more likely to be older, former smokers, and to regularly use statins and multivitamins, compared with nonregular users (eTable 1 in the Supplement).
Aspirin Use and HCC Risk
The age-standardized HCC incidence rate was significantly lower among regular aspirin users (2.1 cases per 100 000 person-years) compared with nonregular users (5.2 cases per 100 000 person-years, P < .001). Regular aspirin use was associated with significantly lower HCC risk compared with nonregular use (multivariable HR, 0.51; 95% CI, 0.34-0.77) (Table 1), with similar estimates in women and men. The results were unchanged after further adjustment for regular use of nonaspirin NSAIDs (ie, ≥2 tablets per week; model 3). The results were similar after further accounting for cumulative mean coffee consumption and adherence to a healthy diet (Alternate Healthy Eating Index 201024; multivariable HR, 0.49; 95% CI, 0.30-0.79). In stratified models, the inverse associations between regular aspirin use and HCC risk were consistent across all prespecified groups (all P > .05 for interaction) (eTable 2 in the Supplement).
Table 1. Regular Aspirin Use and Risk of Hepatocellular Carcinoma Among 133 371 Women and Men in the NHS (1980-2012) and HPFS (1986-2012) Cohorts.
| Characteristic | Aspirin Use | |
|---|---|---|
| Nonregular | Regulara | |
| Women | ||
| Cases per person-years | 44/1 900 916 | 21/1 229 095 |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.53 (0.32-0.90) |
| Model 2 (95% CI)b | 1 [Reference] | 0.49 (0.29-0.84) |
| Model 3 (95% CI)c | 1 [Reference] | 0.49 (0.28-0.83) |
| Men | ||
| Cases per person-years | 27/593 041 | 16/509 136 |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.55 (0.29-1.02) |
| Model 2 (95%CI)b | 1 [Reference] | 0.54 (0.30-1.01) |
| Model 3 (95% CI)c | 1 [Reference] | 0.54 (0.30-1.02) |
| Pooled | ||
| Cases per person-years | 71/2 493 957 | 37/1 738 231 |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.54 (0.36-0.80) |
| Model 2 (95% CI)b | 1 [Reference] | 0.51 (0.34-0.77) |
| Model 3 (95% CI)c | 1 [Reference] | 0.51 (0.34-0.77) |
Abbreviations: HPFS, Health Professionals Follow-up Study; HR, hazard ratio; NHS, Nurses’ Health Study.
Regular aspirin use was defined as consumption of 2 or more standard-dose (325-mg) aspirin tablets or more per week (vs nonregular use) and modeled as a time-varying covariate.
Model 2 was conditioned on age (continuous years) and year of questionnaire return, and adjusted for sex (ie, cohort), race/ethnicity (white vs nonwhite), body mass index (continuous measure), alcohol intake (0-4.9, 5.0-14.9, ≥15.0 g/d), smoking status (current vs prior vs never), physical activity (<3.0, 3.0-8.9, ≥9.0 metabolic equivalent task hours per week), diabetes (yes vs no), hypertension (yes vs no), dyslipidemia (yes vs no), regular multivitamin use (≥2 multivitamin tablets per week vs no), regular use of oral antidiabetic medications (yes vs no) and regular use of statins (yes vs no). All relevant covariates were updated over time.
Model 3 includes model 2 covariates plus regular use of nonaspirin nonsteroidal anti-inflammatory drugs (≥2 nonaspirin nonsteroidal anti-inflammatory drug tablets per week vs none), assessed as a time-varying covariate.
Aspirin Dose and Duration of Use
A dose-dependent reduction in HCC risk was observed with increasing cumulative mean aspirin dose, without departures from linearity (P for trend = .006) (Table 2). The apparent benefit of aspirin emerged with use of 1.5 to fewer than 5 standard-dose aspirin tablets per week. Compared with nonusers, the multivariable HRs (model 3) in aspirin users were 0.87 (95% CI, 0.51-1.48) for 0.5 to 1.5 standard-dose tablets per week, 0.51 (95% CI, 0.30-0.86) for 1.5 to fewer than 5 tablets per week, and 0.49 (95% CI, 0.28-0.96) for 5 or more tablets per week. After further adjustment for aspirin duration (model 4), the association between aspirin dose and HCC risk remained statistically significant (P for trend = .005).
Table 2. Cumulative Mean Aspirin Dose and Risk of Hepatocellular Carcinoma in 13 371 Women (1980-2012) and Men (1986-2012) in the Pooled NHS and HPFS Cohorts.
| Characteristic | No. of Weekly Aspirin Tablets (Cumulative Mean) | P Value for Trenda | |||
|---|---|---|---|---|---|
| <0.5 | 0.5 to <1.5 | 1.5 to <5 | ≥5 | ||
| Cases per person-years | 46/1 425 726 | 23/867 338 | 24/1 207 350 | 15/731 776 | |
| Model 1, HR (95% CI)b | 1 [Reference] | 0.83 (0.49-1.40) | 0.50 (0.30-0.84) | 0.55 (0.30-1.01) | .008 |
| Model 2, HR (95% CI)c | 1 [Reference] | 0.86 (0.50-1.46) | 0.48 (0.28-0.82) | 0.48 (0.26-0.89) | .003 |
| Model 3, HR (95% CI)d | 1 [Reference] | 0.87 (0.51-1.48) | 0.51 (0.30-0.86) | 0.49 (0.28-0.96) | .006 |
| Model 4, HR (95% CI)e | 1 [Reference] | 1.20 (0.65-2.20) | 0.56 (0.32-0.99) | 0.37 (0.17-0.81) | .005 |
Abbreviations: HR, hazard ratio; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study.
Calculated using cumulative mean aspirin dose (continuous), among regular aspirin users.
Model 1 was conditioned on age (continuous years), year of questionnaire return, and sex (ie, cohort).
Model 2 includes model 1 covariates plus race/ethnicity (white vs nonwhite), body mass index (continuous measure), alcohol intake (0-4.9, 5.0-14.9, ≥15.0 g/d), smoking status (current vs prior vs never), physical activity (<3.0, 3.0-8.9, ≥9 metabolic equivalent task–hours per week), diabetes (yes vs no), hypertension (yes vs no), dyslipidemia (yes vs no), regular multivitamin use (≥2 multivitamin tablets per week vs no), regular use of oral antidiabetic medications (yes vs no), and regular use of statins (yes vs no). All relevant covariates were updated over time.
Model 3 includes model 2 regular use of nonaspirin nonsteroidal anti-inflammatory drugs (≥2 nonaspirin nonsteroidal anti-inflammatory drug tablets per week vs no), assessed as a time-varying covariate.
Model 4 includes model 3 covariates plus duration of regular aspirin use (continuous, years).
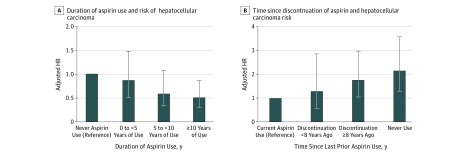
Risk estimates for HCC decreased significantly with increasing duration of regular aspirin use (P for trend = .03 among aspirin users) (Figure, A; eTable 3 in the Supplement). Compared with nonregular use, less than 5 years of aspirin use was not associated with significantly reduced HCC risk. However, the multivariable HR for HCC was 0.62 (95% CI, 0.34-1.13) for 5 to less than 10 years of use, and 0.55 (95% CI, 0.32-0.93) for 10 or more years of use. Furthermore, when former aspirin users were compared with current aspirin users (Figure, B; eTable 4 in the Supplement), increasing years since last prior use of aspirin was associated with significantly increased HCC risk (HR for discontinuation ≥8 years ago compared with current aspirin use, 1.77; 95% CI, 1.06-2.97; P for linear trend = .006), and the benefit of aspirin was no longer evident beyond 8 years since last aspirin use. A joint analysis of dose and duration (eTable 5 in the Supplement) suggested that the apparent benefit of aspirin was evident with regular use of 1.5 or more standard-dose tablets per week, for more than 5 years.
Figure. Hepatocellular Carcinoma Risk in Relation to Duration of Aspirin Use and Time Since Aspirin Discontinuation.
A, Duration of aspirin use and hepatocellular carcinoma risk. Categories of aspirin use duration were compared with individuals reporting never-use of aspirin (reference). The P value for trend was calculated using continuous duration of use (months) among aspirin users, compared with the lowest reported duration of use. P for trend = .03. B, Time since discontinuation of aspirin and hepatocellular carcinoma risk. Current aspirin use (reference group) was defined as consumption of 2 or more standard-dose (325-mg) aspirin tablets per week on the most recent questionnaire. Among prior aspirin users, time since discontinuation of regular use was defined as nonregular use on the most recent questionnaire but regular aspirin use less than 8 or 8 or more years in the past. P for trend was calculated using continuous elapsed time in months since last regular aspirin use among prior aspirin users. The multivariable-adjusted model was conditioned on age (continuous years), year of questionnaire return and sex (ie, cohort), and was further adjusted for race/ethnicity (white vs nonwhite), body mass index (continuous measure), alcohol intake (0-4.9, 5.0-14.9, ≥15.0 g/d), smoking status (current vs prior vs never), physical activity (<3.0, 3.0-8.9, ≥9 metabolic equivalent task–hours per week), diabetes (yes vs no), hypertension (yes vs no), dyslipidemia (yes vs no), regular multivitamin use (≥2 multivitamin tablets per week vs no), regular use of oral antidiabetic medications (yes vs no), and regular use of statins (yes vs no). All relevant covariates were updated over time. P for trend = .006. HR indicates hazard ratio; error bars, 95% CI.
Nonaspirin NSAIDs and Acetaminophen Use
Compared with nonregular use, no HCC risk reduction was observed with regular nonaspirin NSAID use (≥2 times per week) (eTable 6 in the Supplement), nor was an association found between increasing duration of NSAID use and HCC risk (HR for ≥10 years of use compared with nonuse, 1.06; 95% CI, 0.74-1.50; P for linear trend = .20 among users). Similarly, no association was found between regular acetaminophen use and HCC risk (eTable 6 in the Supplement) or between increasing duration of acetaminophen use and HCC risk (HR for ≥10 years of use compared with nonuse, 0.88; 95% CI, 0.54-1.45; P for linear trend = .42 among users).
Although it is plausible that concurrent NSAID and aspirin use might have affected our findings, analyses mutually adjusting for the other agent did not significantly affect the estimates of hazard for each dose category of aspirin (HR for ≥5 cumulative mean weekly aspirin tablets vs nonregular use, 0.49; 95% CI, 0.28-0.96; P for dose trend = .006; Table 2), or each duration category of aspirin (HR for ≥10 years of use vs never-use, 0.55; 95% CI, 0.32-0.93; P for duration trend = .03; eTable 3 in the Supplement). Similarly, analyses mutually adjusting for regular aspirin use did not significantly affect the estimates of hazard for each duration category of nonaspirin NSAIDs (HR for ≥10 years of use compared with nonuse, 1.09; 95% CI, 0.76-1.54; P for linear trend = .19 among users). Furthermore, our results were unchanged after restricting the cohort to regular users of 1 medication (but not both). Among nonregular NSAID users, the multivariable HR with regular aspirin use was 0.48 (95% CI, 0.31-0.75); conversely, among nonregular aspirin users, the multivariable HR with regular NSAID use was 0.98 (95% CI, 0.87-1.10).
Sensitivity Analyses
Sensitivity analyses showed that, first, our results were similar after excluding any case of HCC diagnosed within 4 years of follow-up (n = 7; multivariable HR, 0.53; 95% CI, 0.35-0.81). Second, we excluded any case of HCC associated with underlying viral hepatitis B or hepatitis C (n = 18) and observed similar results (multivariable HR, 0.60; 95% CI, 0.39-0.93). Third, we excluded ever-users of statins, and our results again were unchanged (multivariable HR, 0.49; 95% CI, 0.31-0.78). Fourth, we examined the association between regular aspirin use and risk of noncirrhotic HCC (n = 47) and found that regular aspirin use was associated with a significant reduction in noncirrhotic HCC risk (multivariable HR, 0.53; 95% CI, 0.83-0.87). Finally, we compared the associations of aspirin by HCC subtype (ie, cirrhotic vs noncirrhotic HCC) and found that the associations were similar (HR for cirrhotic HCC, 0.49; 95% CI, 0.29-0.84; HR for noncirrhotic HCC, 0.53; 95% CI, 0.83-0.87; P for heterogeneity = .53).
Discussion
In 2 nationwide, prospective cohorts of US men and women, long-term, regular aspirin use (≥2 times per week) was associated with a significantly reduced risk of developing HCC. The apparent benefit of aspirin was both dose and duration dependent, appearing after 5 or more years of use, with 1.5 or more standard-dose aspirin tablets per week. Similar benefit was observed in men and women and in participants with and without underlying cirrhosis. In contrast, no significant HCC risk reduction was observed with regular use of nonaspirin NSAIDs.
Our results extend previous data suggesting that aspirin may protect against incident HCC.9,10 However, prospective evidence specifically addressing both aspirin dose and duration derive from a single published study in which current use of less than 163 mg of aspirin daily was associated with a significantly reduced HCC risk (HR, 0.39; 95% CI, 0.17-0.91), but neither a dose-response relationship nor a duration-response relationship was observed.10 While broadly consistent with our results, that analysis lacked updated dosage or duration data and could not account for additional, potentially relevant medication or dietary confounders.11 In contrast, with prospectively updated aspirin data over a long length of follow-up, our study provides more compelling evidence of a potential dose- and duration-dependent association between aspirin use and HCC risk. Our findings suggest that significant HCC risk reduction might be achieved with long-term use of dose equivalents as low as 70 mg/d or a daily 81-mg aspirin tablet.
Several lines of evidence support the presence of a dose-dependent relationship between aspirin and HCC risk. The proinflammatory COX-2 enzyme is overexpressed in inflammation-associated cancers,26,27,28,29 including HCC,6,7,8 and experimental data indicate that higher cumulative doses of aspirin are required to fully inhibit COX-2.30 COX-2 overexpression activates proinflammatory signaling cascades relevant to hepatocarcinogenesis, including protein kinase 3, mammalian target of rapamycin, and nuclear factor κ B pathways, which promote cellular proliferation and angiogenesis.6 Aspirin inhibits nuclear factor κ B31 and protein kinase 332 signaling, with effects that are maximized at higher doses. Finally, in HCC tumors, COX-2 overexpression is associated with reduced disease-free survival,33 while selective COX-2 inhibition reduces liver cancer cell proliferation.7
Our findings suggest that the anti-HCC effects of aspirin may require consistent use for 5 or more years, and this finding is supported by prior data.10 We observed that the benefits of aspirin progressively diminish after discontinuation and are no longer evident after more than 8 years since last use. These findings support the hypothesis that sustained aspirin use confers progressive HCC protection and are consistent with our understanding of the prolonged latency underlying hepatocarcinogenesis. Although comparable prospective data in HCC are lacking, findings from analogous studies of aspirin use for colorectal cancer prevention show that a minimum of 6 years of regular use is necessary for significant chemoprevention.34
In the absence of viral hepatitis or significant alcohol intake, the pathogenesis of HCC is likely mediated through the progression of nonalcoholic fatty liver disease (NAFLD). Accumulating evidence suggests that nearly 40% of NAFLD-associated HCCs may arise in noncirrhotic livers.22 To our knowledge, this is the first prospective US study to show chemopreventive benefits of aspirin against both noncirrhotic and cirrhotic HCC, albeit with limited numbers of cases. Although it remains unknown whether aspirin reduces NAFLD fibrosis, 2 cross-sectional studies found that aspirin use is associated with a reduced prevalence of NAFLD35 and lower serum indices of fibrosis severity.36 Collectively, these findings highlight the importance of continued efforts to understand aspirin’s hepatoprotective effects, particularly in light of the growing at-risk NAFLD population.
In the setting of chronic liver disease, any potential benefit from aspirin must be weighed against bleeding risk. In a recent study of Korean patients with chronic hepatitis B, aspirin monotherapy was not associated with significantly increased bleeding risk (HR, 1.11; 95% CI, 0.48-2.54); however, aspirin dose was not assessed.37 Within these cohorts, a dose-dependent association between regular aspirin use and bleeding risk was previously identified,38 which underscores the need for future research in populations with well-phenotyped liver disease. Nonetheless, our findings support the potential incorporation of low-dose aspirin into personalized HCC primary prevention strategies that carefully balance benefits with hazards.
Consistent with 2 prior studies,9,10 but not others,13,14 we did not observe significant HCC risk reduction with nonaspirin NSAIDs. This result might be explained by established differences in the actions of aspirin and nonaspirin NSAIDs on COX isoforms. Although aspirin irreversibly inhibits COX isoenzymes, NSAIDs do so reversibly,39 producing transient effects lasting for only a portion of the dosing interval,40 which may minimize anti-HCC effects. In addition, nonaspirin NSAIDs disrupt the intestinal barrier, increasing delivery of proinflammatory cytokines to the liver that promote a hepatic inflammatory response, whereas such effects have not been observed with aspirin.41 Finally, aspirin has numerous unique COX-independent anti-inflammatory actions. For example, aspirin blocks platelet thromboxane and thus inhibits sphingosine-1-phosphate, a bioactive lipid that promotes cellular proliferation and angiogenesis.42 Pharmacologic inhibitors of sphingosine kinase show promise experimentally for preventing HCC43 and may enhance the HCC chemotherapeutic response.44
Strengths and Limitations
Strengths of this study include a large, well-characterized population with prospectively updated aspirin data over more than 26 years of follow-up, permitting detailed assessments of the potential benefits of aspirin at a range of doses over time. We confirmed all HCC cases, without reliance on administrative codes that may not identify noncirrhotic HCC or accurately distinguish HCC from other liver cancers. Furthermore, to our knowledge, this represents the first prospective US cohort study to couple prospectively updated aspirin data with important lifestyle, dietary, and medication covariates relevant to aspirin use and HCC risk, thereby minimizing misclassification and offering more precise estimations of HCC risk.
We acknowledge several limitations. First, as an observational study, our results are not as definitive as those of a randomized clinical trial. Until recently, HCC primary prevention trials have not been feasible; however, the emergence of personalized methods for HCC risk prediction may soon offer a means to efficiently identify high-risk subpopulations in whom the benefits of aspirin might be apparent with shorter durations of use.45 Second, aspirin use was self-selected, long-term data on tolerability were lacking, and we cannot exclude the possibility of residual confounding. However, aspirin was primarily used for analgesia,46 and acetaminophen, an analgesic used for similar purposes but with a distinct mechanism of action, was not associated with HCC risk. Third, participants in these cohorts are predominantly white, and several sensitivity analyses were limited by small numbers of cases, underscoring the need for research in larger, well-phenotyped, and diverse populations. Nevertheless, our age-standardized incidence of HCC is comparable to the broader US white population.4 Moreover, estimates were unchanged after excluding cases of HCC arising within 4 years of follow-up.
Fourth, aspirin use was assessed by questionnaire, which could lead to recall bias; however, the prospective design of this study minimizes this risk, because all aspirin exposure data were collected before the diagnosis of cancer. Fifth, data regarding individual statin types or dosages were not available; however, our findings were unchanged after adjusting for statin use and excluding statin users. Finally, despite careful multivariable adjustment and ascertainment of cirrhotic HCC, these cohorts lack more-detailed clinical information regarding liver disease, including NAFLD fibrosis or cirrhosis. As patients with advanced liver disease may be advised against aspirin use, this lack of use raises the possibility of confounding by indication. However, the apparent benefits of aspirin also extended to noncirrhotic HCC, without significant heterogeneity in patterns of use. Moreover, in analyses restricted to former aspirin users, the findings were similar.
Conclusions
This study demonstrates that long-term aspirin use appears to be associated with a reduced risk of HCC in a dose- and duration-dependent manner. Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer.34 Research to uncover the mechanisms by which aspirin inhibits hepatocarcinogenesis may facilitate the development of HCC primary prevention strategies.
eMethods. Ascertainment of Covariates in the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) and Construction of Multivariable Models
eTable 1. Age-Standardized Characteristics of Study Participants from NHS and HPFS Cohorts in 1996 (n = 133 371)
eTable 2. Stratified Analyses: Regular Aspirin Use and Risk of Hepatocellular Carcinoma in the Pooled Study Population (n = 133 371)
eTable 3. Duration of Aspirin Use and Risk of Hepatocellular Carcinoma in Women (1980-2012) and Men (1986-2012) in the Pooled NHS and HPFS cohorts (n = 133 371)
eTable 4. Time Since Discontinuation of Aspirin and Risk of Incident Hepatocellular Carcinoma (HCC) in the Pooled NHS and HPFS Cohorts (n = 133 371)
eTable 5. Joint Analysis of Aspirin Dose and Duration of Use and Risk of Incident HCC in the Pooled NHS and HPFS Population (n = 133 371)
eTable 6. Regular Use of Non-Aspirin Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) or Acetaminophen and Risk of Hepatocellular Carcinoma (HCC) in the Pooled NHS and HPFS Cohorts (n = 133 371)
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10-29. doi: 10.3322/caac.20138 [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi: 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 4.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology. 2017;152(4):812-820.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312-1337. doi: 10.1002/cncr.29936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Cai W, Chu ESH, et al. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene. 2017;36(31):4415-4426. doi: 10.1038/onc.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern MA, Schubert D, Sahi D, et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36(4, pt 1):885-894. doi: 10.1053/jhep.2002.36125 [DOI] [PubMed] [Google Scholar]
- 8.Foderà D, D’Alessandro N, Cusimano A, et al. Induction of apoptosis and inhibition of cell growth in human hepatocellular carcinoma cells by COX-2 inhibitors. Ann N Y Acad Sci. 2004;1028:440-449. doi: 10.1196/annals.1322.052 [DOI] [PubMed] [Google Scholar]
- 9.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808-1814. doi: 10.1093/jnci/djs452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrick JL, Sahasrabuddhe VV, Chan AT, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Cancer Prev Res (Phila). 2015;8(12):1156-1162. doi: 10.1158/1940-6207.CAPR-15-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11(1):45-54. doi: 10.1038/nrgastro.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy IG, Pim CP. An aspirin a day: the allure (and distraction) of chemoprevention. J Natl Cancer Inst. 2012;104(23):1782-1784. doi: 10.1093/jnci/djs462 [DOI] [PubMed] [Google Scholar]
- 13.Pang Q, Jin H, Qu K, et al. The effects of nonsteroidal anti-inflammatory drugs in the incident and recurrent risk of hepatocellular carcinoma: a meta-analysis. Onco Targets Ther. 2017;10:4645-4656. doi: 10.2147/OTT.S143154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang IC, Chang J, Kim K, Park SM. Aspirin use and risk of hepatocellular carcinoma in a national cohort study of Korean Adults. Sci Rep. 2018;8(1):4968. doi: 10.1038/s41598-018-23343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894-900. doi: 10.1093/oxfordjournals.aje.a114319 [DOI] [PubMed] [Google Scholar]
- 16.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81-86. doi: 10.1097/00001648-199601000-00014 [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51-65. doi: 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 18.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858-867. doi: 10.1093/ije/18.4.858 [DOI] [PubMed] [Google Scholar]
- 19.Rimm EB, Stampfer MJ, Colditz GA, Giovannucci E, Willett WC. Effectiveness of various mailing strategies among nonrespondents in a prospective cohort study. Am J Epidemiol. 1990;131(6):1068-1071. doi: 10.1093/oxfordjournals.aje.a115598 [DOI] [PubMed] [Google Scholar]
- 20.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134(1):21-28. doi: 10.1053/j.gastro.2007.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan AT, Manson JE, Feskanich D, Stampfer MJ, Colditz GA, Fuchs CS. Long-term aspirin use and mortality in women. Arch Intern Med. 2007;167(6):562-572. doi: 10.1001/archinte.167.6.562 [DOI] [PubMed] [Google Scholar]
- 22.Simon TG, King LY, Chong DQ, et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: Results from two prospective cohort studies. Hepatology. 2018;67(5):1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016-1019. doi: 10.1093/oxfordjournals.aje.a117191 [DOI] [PubMed] [Google Scholar]
- 24.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009-1018. doi: 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Willett WC, Rimm EB, Stampfer MJ, Giovannucci EL. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. BMJ. 2015;351:h4238. doi: 10.1136/bmj.h4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131-2142. doi: 10.1056/NEJMoa067208 [DOI] [PubMed] [Google Scholar]
- 27.Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol. 2005;26(5):1393-1399. [PubMed] [Google Scholar]
- 28.Lim HY, Joo HJ, Choi JH, et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6(2):519-525. [PubMed] [Google Scholar]
- 29.Yip-Schneider MT, Barnard DS, Billings SD, et al. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21(2):139-146. doi: 10.1093/carcin/21.2.139 [DOI] [PubMed] [Google Scholar]
- 30.Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373-2383. doi: 10.1056/NEJMra052717 [DOI] [PubMed] [Google Scholar]
- 31.Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998;95(2):681-686. doi: 10.1073/pnas.95.2.681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38(3):756-768. doi: 10.1053/jhep.2003.50380 [DOI] [PubMed] [Google Scholar]
- 33.Chen G, Li X, Yang J, et al. Prognostic significance of cyclooxygenase-2 expression in patients with hepatocellular carcinoma: a meta-analysis. Arch Med Sci. 2016;12(5):1110-1117. doi: 10.5114/aoms.2016.61916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2(6):762-769. doi: 10.1001/jamaoncol.2015.6396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen H, Shahzad G, Jawairia M, Bostick RM, Mustacchia P. Association between aspirin use and the prevalence of nonalcoholic fatty liver disease: a cross-sectional study from the Third National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2014;40(9):1066-1073. doi: 10.1111/apt.12944 [DOI] [PubMed] [Google Scholar]
- 36.Jiang ZG, Feldbrügge L, Tapper EB, et al. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther. 2016;43(6):734-743. doi: 10.1111/apt.13515 [DOI] [PubMed] [Google Scholar]
- 37.Lee M, Chung GE, Lee JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66(5):1556-1569. doi: 10.1002/hep.29318 [DOI] [PubMed] [Google Scholar]
- 38.Huang ES, Strate LL, Ho WW, Lee SS, Chan AT. Long-term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011;124(5):426-433. doi: 10.1016/j.amjmed.2010.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knights KM, Mangoni AA, Miners JO. Defining the COX inhibitor selectivity of NSAIDs: implications for understanding toxicity. Expert Rev Clin Pharmacol. 2010;3(6):769-776. doi: 10.1586/ecp.10.120 [DOI] [PubMed] [Google Scholar]
- 40.Loll PJ, Picot D, Ekabo O, Garavito RM. Synthesis and use of iodinated nonsteroidal antiinflammatory drug analogs as crystallographic probes of the prostaglandin H2 synthase cyclooxygenase active site. Biochemistry. 1996;35(23):7330-7340. doi: 10.1021/bi952776w [DOI] [PubMed] [Google Scholar]
- 41.Tugendreich S, Pearson CI, Sagartz J, Jarnagin K, Kolaja K. NSAID-induced acute phase response is due to increased intestinal permeability and characterized by early and consistent alterations in hepatic gene expression. Toxicol Pathol. 2006;34(2):168-179. doi: 10.1080/01926230600611752 [DOI] [PubMed] [Google Scholar]
- 42.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funaki M, Kitabayashi J, Shimakami T, et al. Peretinoin, an acyclic retinoid, inhibits hepatocarcinogenesis by suppressing sphingosine kinase 1 expression in vitro and in vivo. Sci Rep. 2017;7(1):16978. doi: 10.1038/s41598-017-17285-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grbčić P, Tomljanović I, Klobučar M, Kraljević Pavelić S, Lučin K, Sedić M. Dual sphingosine kinase inhibitor SKI-II enhances sensitivity to 5-fluorouracil in hepatocellular carcinoma cells via suppression of osteopontin and FAK/IGF-1R signalling. Biochem Biophys Res Commun. 2017;487(4):782-788. doi: 10.1016/j.bbrc.2017.04.100 [DOI] [PubMed] [Google Scholar]
- 45.Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526-549. doi: 10.1016/j.jhep.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manson JE, Stampfer MJ, Colditz GA, et al. A prospective study of aspirin use and primary prevention of cardiovascular disease in women. JAMA. 1991;266(4):521-527. doi: 10.1001/jama.1991.03470040085027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Ascertainment of Covariates in the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) and Construction of Multivariable Models
eTable 1. Age-Standardized Characteristics of Study Participants from NHS and HPFS Cohorts in 1996 (n = 133 371)
eTable 2. Stratified Analyses: Regular Aspirin Use and Risk of Hepatocellular Carcinoma in the Pooled Study Population (n = 133 371)
eTable 3. Duration of Aspirin Use and Risk of Hepatocellular Carcinoma in Women (1980-2012) and Men (1986-2012) in the Pooled NHS and HPFS cohorts (n = 133 371)
eTable 4. Time Since Discontinuation of Aspirin and Risk of Incident Hepatocellular Carcinoma (HCC) in the Pooled NHS and HPFS Cohorts (n = 133 371)
eTable 5. Joint Analysis of Aspirin Dose and Duration of Use and Risk of Incident HCC in the Pooled NHS and HPFS Population (n = 133 371)
eTable 6. Regular Use of Non-Aspirin Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) or Acetaminophen and Risk of Hepatocellular Carcinoma (HCC) in the Pooled NHS and HPFS Cohorts (n = 133 371)