The introduction of pneumococcal conjugate vaccines (PCVs) into childhood vaccination programs has reduced carriage of vaccine serotypes and pneumococcal disease. The 10-valent PCV was introduced in Iceland in 2011.
KEYWORDS: Iceland, Streptococcus pneumoniae, adults, epidemiology, lower respiratory tract, molecular epidemiology, pneumococcus, pneumonia, vaccination
ABSTRACT
The introduction of pneumococcal conjugate vaccines (PCVs) into childhood vaccination programs has reduced carriage of vaccine serotypes and pneumococcal disease. The 10-valent PCV was introduced in Iceland in 2011. The aim of this study was to determine PCV impact on the prevalence of serotypes, genetic lineages, and antimicrobial-resistant pneumococci isolated from the lower respiratory tract (LRT) of adults. Pneumococci isolated between 2009 and 2017 at the Landspitali University Hospital were included (n = 797). The hospital serves almost three-quarters of the Icelandic population. Isolates were serotyped and tested for antimicrobial susceptibility, and the genome of every other isolate collected between 2009 and 2014 was sequenced (n = 275). Serotypes and multilocus sequence types (STs) were extracted from the genome data. Three study periods were defined, 2009 to 2011 (PreVac), 2012 to 2014 (PostVac-I), and 2015 to 2017 (PostVac-II). The total number of isolates and vaccine-type (VT) pneumococci decreased from PreVac to PostVac-II (n = 314 versus n = 230 [p = 0.002] and n = 170 versus n = 33 [p < 0.001], respectively), but non-vaccine-type (NVT) pneumococci increased among adults 18 to 64 years old (n = 56 versus n = 114 [p = 0.008]). Serotype 19F decreased in the PostVac-II period; these isolates were all multidrug resistant (MDR) and were members of the Taiwan19F-14 PMEN lineage. Serotype 6A decreased among adults ≥65 years old in the PostVac-II period (p = 0.037), while serotype 6C increased (p = 0.021) and most serotype 6C isolates were MDR. Nonencapsulated Streptococcus pneumoniae (NESp) isolates increased among adults 18 to 64 years old in the PostVac-II period, and the majority were MDR (p = 0.028). An overall reduction in the number of LRT samples and pneumococcus-positive cultures and significant changes in the serotype distribution became evident within 4 years, thereby demonstrating a significant herd effect.
INTRODUCTION
Pneumococcus is an important human pathogen that causes significant morbidity and mortality worldwide (1). It is one of the most important human pathogens in community-acquired and nosocomial pneumonia, causing an estimated two-thirds of all cases of bacterial pneumonia and resulting in hospitalization and death among older adults (2–6). One of its main virulence factors is a polysaccharide capsule (serotype), and nearly 100 different serotypes have been identified to date (7, 8).
Pneumococcal conjugate vaccines (PCVs) have been included in the infant vaccination program of more than 100 countries (9), which has resulted in a significant reduction in disease and circulating vaccine serotypes within those countries. Protection of the unvaccinated adult population through herd immunity is generally observed later than vaccine-induced immunity among the pediatric population (10–12). Importantly, serotype replacement and the circulation of antimicrobial-resistant pneumococcal lineages expressing non-vaccine serotypes can also occur (13–16).
In April 2011, the 10-valent PCV (PHiD-CV, Synflorix; GSK) was introduced into the national infant immunization program in a 2 + 1 vaccine schedule, without catch-up vaccination. The vaccine directly targets serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F, but the potential cross-protection against vaccine-related serotypes 6A and 19A seems to vary among studies (17–21). Pneumococcal vaccines had not previously been a part of the routine infant immunization program in Iceland. In this study, we analyzed the impact of PHiD-CV implementation among pneumococci recovered from the lower respiratory tract (LRT) of adult patients. The distribution of pneumococcal serotypes and genetic lineages along with changes in antimicrobial resistance rates were assessed before and after vaccine implementation.
MATERIALS AND METHODS
Study population and bacterial isolates.
All pneumococci isolated from lower respiratory tract (LRT) samples taken from adults ≥18 years of age with suspected pneumonia and submitted to the Department of Clinical Microbiology at Landspitali University Hospital in Iceland between 2009 and 2017 were included in the study. When two or more pneumococcal isolates of the same phenotype (i.e., the same serotype and antimicrobial susceptibility pattern) were identified from the same patient within 30 days, they were considered to be from the same infection, and only the first isolate was included in the subsequent analyses.
The Department of Clinical Microbiology serves as the reference laboratory for the whole country and is the primary microbiology laboratory for the greater Reykjavík capital area. The study was divided into three different periods for the analyses, 3 years prior to vaccination (2009 to 2011, PreVac), 1 to 3 years postvaccination (2012 to 2014, PostVac-I), and 4 to 6 years postvaccination (2015 to 2017, PostVac-II).
The primary catchment area for the Landspitali University Hospital was considered to be within a 100-km driving distance from the hospital, and the population demographic information for this referral region was obtained from Statistics Iceland (www.statice.is). The population sizes of the referral region for adults ≥18 years of age (which includes over 70% of Icelandic adults ≥18 years old) were 170,042 (PreVac), 177,490 (PostVac-I), and 186,724 (PostVac-II). The prevalences of the pneumococcal isolates were calculated using the population size of the referral region as the denominator. The population sizes stratified by age group (18 to 64 and ≥65 years old) are shown in Table S1 in the supplemental material.
Serotyping.
Serotypes were determined for all available isolates with ImmuLex pool antisera (State Serum Institute, Copenhagen, Denmark) and/or by a multiplex PCR (mPCR) method, which included 78 sets of serogroup/serotype-specific primer pairs. Serotypes of serogroup 6 were identified as previously described (22). Nonencapsulated Streptococcus pneumoniae (NESp) isolates (i.e., those that were negative for cpsA and positive for lytA) were tested for the cpsB gene, which is essential for encapsulation (23), using a previously published PCR method (24).
DNA extraction, whole-genome sequencing, and phylogenetic analysis.
DNA extraction and whole-genome sequencing (WGS) were performed as previously described on every other pneumococcal isolate from the years 2009 to 2014 (22). Multilocus sequence types (STs) and clonal complexes (CCs) were defined in the standard manner. Genome annotation, gene clustering, and sequence alignments were performed using Prokka and Roary. FastTree and ClonalFrameML were used to reconstruct the phylogenetic tree as described in our previous paper (22). Note that a core genome threshold of 99.6% was calculated for this data set.
Antibiotic susceptibility testing.
All isolates were tested for antimicrobial susceptibility to chloramphenicol, erythromycin, tetracycline, trimethoprim-sulfamethoxazole, and clindamycin using disk diffusion tests. Oxacillin disks (1 µg) were used to screen for penicillin resistance. E-tests were used to measure the MIC (bioMérieux, France) of penicillin and ceftriaxone on oxacillin-resistant isolates. Multidrug resistance (MDR) was defined as nonsusceptibility to three or more classes of antimicrobials (regardless of penicillin susceptibility). Susceptibility testing was performed according to the methods and criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (25).
Statistical analyses.
A likelihood ratio test (26) was used to test the null hypothesis of equality when comparing the rate (r1) of a certain serotype, CC, or ST in a given age group PreVac to the rate (r2) of the same serotype, CC, or ST in the same age group in PostVac-II (for serotypes) and PostVac-I (for CC/ST). For the CCs and STs, PreVac was compared to PostVac-I (2012 to 2014), as there was no genome sequencing done on pneumococcal isolates after 2014. The two-sided Fisher’s exact test was used to calculate the p values for antimicrobial resistance by using R version 3.3.2. The level of significance for all tests was ≤0.05. Simpson’s diversity index was calculated to assess the change in ST diversity after vaccine implementation (27).
Ethics.
This study was approved by The National Bioethics Committee (VSNb2013010015/03.07) and the appropriate authorities at Landspitali University Hospital in Iceland.
RESULTS
Demographics.
The laboratory received 17,762 samples during the study period (Fig. 1). This yielded 814 pneumococcal isolates, of which 17 isolates were not stored or were nonviable, leaving 797 isolates for further analyses. About 10% of the LRT samples originated from adults residing outside of the primary catchment area. The total number of pneumococcal isolates decreased significantly from 314 (184.7/100,000 adults) PreVac to 230 (123.2/100,000 adults) PostVac-II (p = 0.002; Table 1). The median age of the patients with confirmed pneumonia was 75.2 years. More than half (430/797, 54.0%) of all isolates obtained were from patients ≥65 years of age, and in this age group, the total number of isolates decreased from 191 (719.8/100,000 adults aged ≥65 years) in the PreVac period to 102 (314.7/100,000 adults aged ≥65 years) in the PostVac-II period (p < 0.001; Table 2).
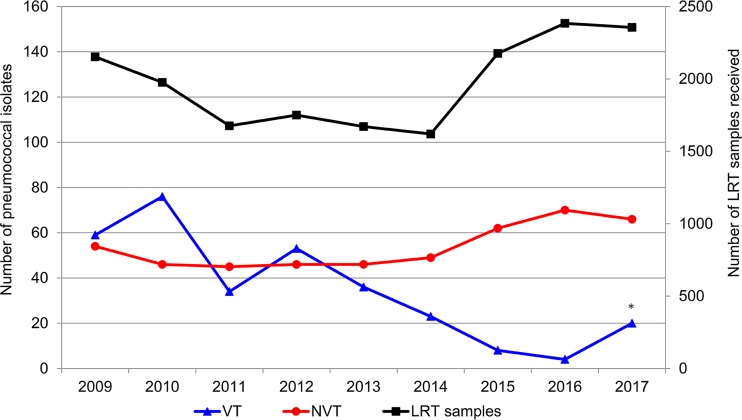
FIG 1.
Annual number of isolates belonging to serotypes targeted by PHiD-CV (vaccine-type [VT]), serotypes not targeted by PHiD-CV (non-vaccine-type [NVT]), and lower respiratory tract (LRT) samples received by the laboratory. *, Seven isolates of serotype 19F were detected in the same patient in 2017.
TABLE 1.
Serotype distribution for each study year, 2009 to 2017, in adults ≥18 years of age
| Serotype | No. of isolates per yr |
PreVac isolates (2009–2011) |
PostVac-I isolates (2012–2014) |
PreVac vs PostVac-I p value | PostVac-II isolates (2015–2017) |
PreVac vs PostVac-II p value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | No. | No. per 100,000 | No. | No. per 100,000 | No. | No. per 100,000 | |||
| 3 | 11 | 9 | 5 | 9 | 3 | 8 | 6 | 7 | 7 | 25 | 14.7 | 20 | 11.3 | 0.558 | 20 | 10.7 | 0.485 |
| 4 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.6 | 0 | 0 | Nca | 1 | 0.5 | 0.965 |
| 6A | 10 | 4 | 4 | 5 | 4 | 0 | 1 | 1 | 1 | 18 | 10.6 | 9 | 5.1 | 0.221 | 3 | 1.6 | 0.016 |
| 6B | 9 | 10 | 2 | 11 | 7 | 3 | 3 | 3 | 1 | 21 | 12.3 | 21 | 11.8 | 0.927 | 7 | 3.7 | 0.052 |
| 6C | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 7 | 5 | 1 | 0.6 | 2 | 1.1 | 0.719 | 14 | 7.5 | 0.021 |
| 7F | 0 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 1.2 | 1 | 0.6 | 0.683 | 2 | 1.1 | 0.951 |
| 8 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 | 0 | 0 | Nc | 0 | 0 | Nc |
| 9V | 3 | 4 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 9 | 5.3 | 2 | 1.1 | 0.136 | 0 | 0 | Nc |
| 9A | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1.2 | 2 | 1.1 | 0.977 | 0 | 0 | Nc |
| 9N | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 1 | 0.6 | 2 | 1.1 | 0.136 | 4 | 2.1 | Nc |
| 10 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 | 0 | 0 | Nc | 0 | 0 | Nc |
| 11A | 5 | 6 | 3 | 2 | 6 | 3 | 7 | 4 | 9 | 14 | 8.2 | 11 | 6.2 | 0.641 | 20 | 10.7 | 0.617 |
| 14 | 8 | 7 | 0 | 3 | 3 | 1 | 0 | 0 | 1 | 15 | 8.8 | 7 | 3.9 | 0.229 | 1 | 0.5 | 0.008 |
| 15A | 1 | 1 | 0 | 1 | 3 | 1 | 2 | 2 | 4 | 2 | 1.2 | 5 | 2.8 | 0.470 | 8 | 4.3 | 0.230 |
| 15B/C | 3 | 0 | 5 | 3 | 5 | 3 | 4 | 6 | 1 | 8 | 4.7 | 11 | 6.2 | 0.694 | 11 | 5.9 | 0.749 |
| 16F | 2 | 3 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 5 | 2.9 | 2 | 1.1 | 0.426 | 1 | 0.5 | 0.231 |
| 17F | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.6 | 1 | 0.6 | 0.984 | 0 | 0 | Nc |
| 18B | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 | 0 | 0 | Nc | 0 | 0 | Nc |
| 18C | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1.2 | 0 | 0 | Nc | 0 | 0 | Nc |
| 19F | 30 | 45 | 24 | 29 | 23 | 15 | 1 | 1 | 18b | 99 | 58.2 | 67 | 37.7 | 0.068 | 20b | 10.7 | <0.001 |
| 19A | 5 | 3 | 2 | 3 | 4 | 6 | 4 | 5 | 3 | 10 | 5.9 | 13 | 7.3 | 0.730 | 12 | 6.4 | 0.891 |
| 20 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 | 0 | 0 | Nc | 0 | 0 | Nc |
| 21 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 4 | 1 | 0.6 | 2 | 1.1 | 0.719 | 6 | 3.2 | 0.217 |
| 22F | 4 | 3 | 5 | 5 | 1 | 3 | 5 | 4 | 1 | 12 | 7.1 | 9 | 5.1 | 0.619 | 10 | 5.4 | 0.670 |
| 23F | 8 | 11 | 7 | 7 | 4 | 4 | 2 | 0 | 0 | 26 | 15.3 | 15 | 8.5 | 0.219 | 2 | 1.1 | 0.001 |
| 23A | 3 | 0 | 0 | 1 | 1 | 2 | 5 | 2 | 6 | 3 | 1.8 | 4 | 2.3 | 0.832 | 13 | 7.0 | 0.111 |
| 23B | 0 | 1 | 3 | 0 | 2 | 3 | 5 | 4 | 1 | 4 | 2.4 | 5 | 2.8 | 0.859 | 10 | 5.4 | 0.337 |
| 24F | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | Nc | 2 | 1.1 | Nc |
| 31 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 | 0 | 0 | Nc | 0 | 0 | Nc |
| 33F | 2 | 1 | 1 | 3 | 0 | 0 | 1 | 1 | 0 | 4 | 2.4 | 3 | 1.7 | 0.774 | 2 | 1.1 | 0.536 |
| 34 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.6 | 0 | 0 | Nc | 1 | 0.5 | 0.965 |
| 35F | 0 | 1 | 1 | 3 | 3 | 4 | 1 | 3 | 3 | 2 | 1.2 | 10 | 5.6 | 0.123 | 7 | 3.7 | 0.298 |
| 35B | 2 | 1 | 3 | 4 | 1 | 2 | 3 | 7 | 4 | 6 | 3.5 | 7 | 3.9 | 0.895 | 14 | 7.5 | 0.289 |
| Not determinedc | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 4 | 1 | 0.6 | 3 | 1.7 | 0.518 | 4 | 2.1 | 0.396 |
| NESpd | 2 | 5 | 6 | 4 | 7 | 8 | 11 | 13 | 11 | 13 | 7.6 | 19 | 10.7 | 0.534 | 35 | 18.7 | 0.054 |
| Total | 113 | 122 | 79 | 99 | 82 | 72 | 70 | 74 | 86 | 314 | 184.7 | 253 | 142.5 | 0.043 | 230 | 123.2 | 0.002 |
| VTe | 59 | 77 | 34 | 53 | 37 | 23 | 9 | 4 | 20 | 170 | 100.0 | 113 | 63.7 | 0.013 | 33 | 17.7 | <0.001 |
| NVTf | 54 | 45 | 45 | 46 | 45 | 49 | 61 | 70 | 66 | 144 | 84.7 | 140 | 78.9 | 0.693 | 197 | 105.5 | 0.184 |
| All LRT samples | 2,153 | 1,976 | 1,676 | 1,750 | 1,671 | 1,620 | 2,176 | 2,384 | 2,356 | 5,805 | 3,413.9 | 5,041 | 2,840.2 | <0.001 | 6,916 | 3,703.9 | 0.003 |
| Percentage of LRT samples positive for pneumococci | 5.2 | 6.2 | 4.7 | 5.7 | 4.9 | 4.4 | 3.2 | 3.1 | 3.7 | 5.4 | 5.0 | 3.3 | |||||
Nc, not calculated.
Seven isolates of serotype 19F were detected in the same patient in 2017.
Serotypes other than those included in the multiplex PCR panel of the study.
NESp, nonencapsulated S. pneumoniae.
Serotypes detected in the study that are targeted by PHiD-CV (4, 6B, 7F, 9V, 14, 18C, 19F, and 23F).
Serotypes detected in the study that are not targeted by PHiD-CV.
TABLE 2.
Serotype distribution within each age group during the PreVac (2009 to 2011), PostVac-I (2012 to 2014), and PostVac-II (2015 to 2017) periods in LRT samples
| Serotype | 18–64 yrs |
≥65 yrs |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PreVac isolates (2009–2011) |
PostVac-I isolates (2012–2014) |
PreVac vs PostVac-I p value | PostVac-II isolates (2015–2017) |
PreVac vs PostVac-II p value | PreVac isolates (2009–2011) |
PostVac-I isolates (2012–2014) |
PreVac vs PostVac-I p value | PostVac-II isolates (2015–2017) |
PreVac vs PostVac-II p value | |||||||
| No. | No. per 100,000 | No. | No. per 100,000 | No. | No. per 100,000 | No. | No. per 100,000 | No. | No. per 100,000 | No. | No. per 100,000 | |||||
| 3 | 9 | 6.3 | 9 | 6.1 | 0.963 | 11 | 7.1 | 0.851 | 16 | 60.3 | 11 | 37.7 | 0.425 | 9 | 27.8 | 0.209 |
| 4 | 1 | 0.7 | 0 | 0 | Nca | 1 | 0.6 | 0.973 | 0 | 0 | 0 | 0 | Nc | 0 | 0 | Nc |
| 6A | 6 | 4.2 | 2 | 1.3 | 0.325 | 1 | 0.6 | 0.170 | 12 | 45.2 | 7 | 24.0 | 0.371 | 2 | 6.2 | 0.037 |
| 6B | 3 | 2.1 | 13 | 8.8 | 0.095 | 5 | 3.2 | 0.688 | 18 | 67.8 | 8 | 27.4 | 0.143 | 2 | 6.2 | 0.005 |
| 6C | 0 | 0 | 2 | 1.3 | Nc | 7 | 4.5 | Nc | 1 | 3.8 | 0 | 0 | Nc | 7 | 21.6 | 0.191 |
| 7F | 1 | 0.7 | 1 | 0.7 | 0.988 | 1 | 0.6 | 0.973 | 1 | 3.8 | 0 | 0 | Nc | 1 | 3.1 | 0.926 |
| 8 | 0 | 0 | 0 | 0 | Nc | 0 | 0 | Nc | 1 | 3.8 | 0 | 0 | Nc | 0 | 0 | Nc |
| 9V | 2 | 1.4 | 2 | 1.3 | 0.983 | 0 | 0 | Nc | 7 | 26.4 | 0 | 0 | Nc | 0 | 0 | Nc |
| 9A | 0 | 0 | 0 | 0 | Nc | 0 | 0 | Nc | 2 | 7.5 | 2 | 6.9 | 0.950 | 0 | 0 | Nc |
| 9N | 0 | 0 | 1 | 0.7 | Nc | 3 | 1.9 | Nc | 1 | 3.8 | 1 | 3.4 | 0.965 | 1 | 3.1 | 0.926 |
| 11A | 7 | 4.9 | 6 | 4.0 | 0.824 | 11 | 7.1 | 0.601 | 7 | 26.4 | 5 | 17.1 | 0.665 | 9 | 27.8 | 0.946 |
| 14 | 7 | 4.9 | 3 | 2.0 | 0.379 | 0 | 0 | Nc | 8 | 30.1 | 4 | 13.7 | 0.381 | 1 | 3.1 | 0.068 |
| 15A | 0 | 0 | 4 | 2.7 | Nc | 6 | 3.9 | Nc | 2 | 7.5 | 1 | 3.4 | 0.662 | 2 | 6.2 | 0.895 |
| 15B/C | 4 | 2.8 | 3 | 2.0 | 0.781 | 9 | 5.8 | 0.400 | 4 | 15.1 | 8 | 27.4 | 0.508 | 2 | 6.2 | 0.482 |
| 16F | 0 | 0 | 0 | 0 | Nc | 1 | 0.6 | Nc | 5 | 18.8 | 2 | 6.9 | 0.401 | 0 | 0 | Nc |
| 17F | 1 | 0.7 | 1 | 0.7 | 0.988 | 0 | 0 | Nc | 0 | 0 | 0 | 0 | Nc | 0 | 0 | Nc |
| 18B | 0 | 0 | 0 | 0 | Nc | 0 | 0 | Nc | 1 | 3.8 | 0 | 0 | Nc | 0 | 0 | Nc |
| 18C | 1 | 0.7 | 0 | 0 | Nc | 0 | 0 | Nc | 1 | 3.8 | 0 | 0 | Nc | 0 | 0 | Nc |
| 19F | 39 | 27.2 | 22 | 14.8 | 0.126 | 7 | 4.5 | 0.001 | 60 | 226.1 | 45 | 154.3 | 0.199 | 13b | 40.1 | <0.001 |
| 19A | 5 | 3.5 | 6 | 4.0 | 0.973 | 7 | 4.5 | 0.765 | 5 | 18.8 | 7 | 24.0 | 0.784 | 5 | 15.4 | 0.835 |
| 20 | 0 | 0 | 0 | 0 | Nc | 0 | 0 | Nc | 1 | 3.8 | 0 | 0 | Nc | 0 | 0 | Nc |
| 21 | 0 | 0 | 2 | 1.3 | Nc | 4 | 2.6 | Nc | 1 | 3.8 | 0 | 0 | Nc | 2 | 6.2 | 0.786 |
| 22F | 8 | 5.6 | 7 | 4.7 | 0.525 | 5 | 3.2 | 0.524 | 4 | 15.1 | 2 | 6.9 | 0.536 | 5 | 15.4 | 0.982 |
| 23F | 14 | 9.8 | 6 | 4.0 | 0.264 | 0 | 0 | Nc | 12 | 45.2 | 9 | 30.9 | 0.566 | 2 | 6.2 | 0.037 |
| 23A | 2 | 1.4 | 3 | 2.0 | 0.983 | 6 | 3.9 | 0.375 | 1 | 3.8 | 1 | 3.4 | 0.965 | 7 | 21.6 | 0.191 |
| 23B | 1 | 0.7 | 3 | 2.0 | 0.715 | 4 | 2.6 | 0.388 | 3 | 11.3 | 2 | 6.9 | 0.715 | 6 | 18.5 | 0.638 |
| 24F | 0 | 0 | 0 | 0 | Nc | 1 | 0.6 | Nc | 0 | 0 | 0 | 0 | Nc | 1 | 3.1 | Nc |
| 31 | 1 | 0.7 | 0 | 0 | Nc | 0 | 0 | Nc | 0 | 0 | 0 | 0 | Nc | 0 | 0 | Nc |
| 33F | 0 | 0 | 3 | 2.0 | Nc | 2 | 1.3 | Nc | 4 | 15.1 | 0 | 0 | Nc | 0 | 0 | Nc |
| 34 | 0 | 0 | 0 | 0 | Nc | 1 | 0.6 | Nc | 1 | 3.8 | 0 | 0 | Nc | 0 | 0 | Nc |
| 35F | 1 | 2.1 | 5 | 3.4 | 0.272 | 2 | 1.3 | 0.731 | 1 | 3.8 | 5 | 17.1 | 0.293 | 5 | 15.4 | 0.332 |
| 35B | 3 | 2.1 | 2 | 1.3 | 0.749 | 7 | 4.5 | 0.441 | 3 | 11.3 | 5 | 17.1 | 0.703 | 7 | 21.6 | 0.522 |
| Not determinedc | 1 | 2.1 | 3 | 2.0 | 0.514 | 1 | 0.6 | 0.973 | 1 | 3.8 | 0 | 0 | NC | 3 | 9.0 | 0.585 |
| NESpd | 6 | 4.2 | 7 | 4.7 | 0.886 | 25 | 16.2 | 0.028 | 7 | 26.4 | 12 | 41.1 | 0.532 | 10 | 30.9 | 0.833 |
| Total | 123 | 85.7 | 116 | 78.2 | 0.641 | 128 | 82.9 | 0.864 | 191 | 719.8 | 137 | 469.8 | 0.011 | 102 | 314.7 | <0.001 |
| VTe | 67 | 46.7 | 47 | 31.7 | 0.176 | 14 | 9.1 | <0.001 | 107 | 403.2 | 65 | 222.9 | 0.022 | 19 | 61.7 | <0.001 |
| NVTf | 56 | 39.0 | 69 | 46.5 | 0.519 | 114 | 73.9 | 0.008 | 84 | 316.6 | 72 | 246.9 | 0.201 | 83 | 253.0 | 0.367 |
Nc, not calculated.
Seven isolates of serotype 19F were detected in the same patient in 2017.
Serotypes other than those included in the multiplex PCR panel of the study.
NESp, nonencapsulated S. pneumoniae.
Serotypes detected in the study that are targeted by PHiD-CV (4, 6B, 7F, 9V, 14, 18C, 19F, and 23F).
Serotypes detected in the study that are not targeted by PHiD-CV.
Serotyping.
Among all 797 pneumococcal isolates, 789 (99.0%) were successfully serotyped, but 8 isolates were of serotypes other than those included in the mPCR scheme and were not characterized further. Overall, 31 serotypes were detected, 28 in the PreVac period and 24 each in the PostVac-I and PostVac-II periods. Overall, isolates of serotypes 4, 6B, 7F, 9V, 14, 18C, 19F, and 23F, which are targeted by PHiD-CV (vaccine type [VT]), decreased significantly between the PreVac and PostVac-II periods (p < 0.001; Table 1). VT serotypes 1 and 5 were not detected in the study. The prevalence of isolates with serotypes not targeted by PHiD-CV (non-vaccine type [NVT]) did not change significantly (p = 0.184; Table 1). VTs were most prevalent in 2010 (77/122; 63.1%) and least prevalent in 2016 (4/74; 5.4%) (Table 1 and Fig. 1).
Overall, among vaccine-related serotypes 6A and 19A, the frequency of serotype 19A did not change during the study period (PreVac, n = 10; PostVac-I, n = 13; and PostVac-II, n = 12), but serotype 6A decreased significantly from the PreVac to PostVac-II period (n = 18 versus n = 3, respectively; p = 0.016; Table 1). Notably, serotype 6C increased significantly from PreVac to PostVac-II (n = 1 versus n = 14, respectively; p = 0.021; Table 1).
Analyses by age group revealed that while the prevalence of VT pneumococci decreased significantly in both age groups, the prevalence of NVT and NESp isolates increased significantly in adults aged 18 to 64 years (p = 0.008 and 0.028, respectively). There was a nonsignificant decrease in the incidence of serotype 3 in the ≥65-year age group (from 60.3/100,000 adults in the PreVac period to 27.8/100,000 adults in the PostVac-II period). There was no significant change in the overall prevalence of NVT or NESp isolates in the ≥65-year age group (Table 2).
CC/MLST.
Between 2009 and 2014, 567 pneumococcal isolates were recovered and 275 (48.5%) of these isolates were sequenced, among which 41 different CCs (31 CCs PreVac and 32 CCs PostVac-I) and 73 STs (56 STs PreVac and 47 STs PostVac-I) were detected. There was no difference in the ST diversity between the two periods (Simpson’s diversity indexes of the STs were 0.97 PreVac and 0.96 PostVac-I).
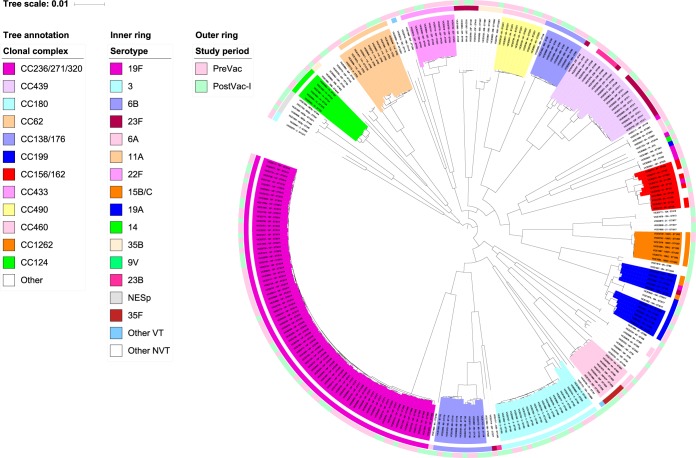
A phylogenetic tree was created with concatenated sequences of 1,130 full-length coding loci found in 99.6% of the pneumococcal genomes. The tree was annotated with CC designations and serotypes (Fig. 2).
FIG 2.
Midrooted phylogenetic tree, created from 1,221 full-length coding loci found in 99.6% of 275 genomes from LRT samples, annotated with CC designations. Serotypes (inner circle) are presented using the same colors as the appropriate CC where possible. Study periods (outer circle) are also presented.
The most prevalent CC in both study periods was CC236/271/32019F, which comprised 31.2% (49/157 [28.8/100,000 adults]) of the PreVac pneumococci, but this CC decreased to 24.6% (29/118 [16.3/100,000 adults]) in the PostVac-I period (p = 0.010) (Fig. 2; see also Table S3 in the supplemental material). Minimal and insignificant changes in the prevalence of other CCs were observed, although the prevalence of CC43322F decreased in the PostVac-I period (p = 0.042) (Fig. 2; Table S3).
Antimicrobial resistance.
Approximately one-third of pneumococci were resistant to penicillin and/or erythromycin in all three study periods, and there was no significant change in those resistance rates over time (Table 3; see also Table S2 in the supplemental material). MDR pneumococci decreased from 34.4% in the PreVac period to 23.9% in the PostVac-II period (p = 0.010; Table 4). Prior to PHiD-CV implementation, 87.9% (102/116) of isolates that were penicillin nonsusceptible were also MDR, but this was reduced to 72.9% (51/70; p = 0.016) in the PostVac-II period (Table 5). This reduction was related mainly to the decrease in MDR serotype 19F pneumococci.
TABLE 3.
PNSP serotypes during the PreVac (2009 to 2011) and PostVac-II (2015 to 2017) periods
| PNSP serotype | PreVac isolates |
PostVac-II isolates |
p value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 6A | 0 | 0 | 1 | 1.4 | 0.458 |
| 6B | 8 | 6.9 | 5 | 7.1 | 1.000 |
| 6C | 0 | 0 | 5 | 7.1 | 0.007 |
| 9V | 4 | 3.4 | 0 | 0 | Nca |
| 9A | 1 | 0.9 | 0 | 0 | 1.000 |
| 11A | 0 | 0 | 1 | 1.4 | 0.376 |
| 14 | 3 | 2.6 | 1 | 1.4 | 1.000 |
| 15A | 0 | 0 | 5 | 7.1 | 0.007 |
| 15B/C | 0 | 0 | 1 | 1.4 | 0.376 |
| 19F | 91 | 78.4 | 20b | 28.6 | <0.001 |
| 19A | 2 | 1.7 | 0 | 0 | Nc |
| 22F | 0 | 0 | 2 | 2.9 | 0.209 |
| 23F | 1 | 0.9 | 0 | 0 | 1.000 |
| 23A | 0 | 0 | 3 | 4.3 | 0.094 |
| 23B | 0 | 0 | 3 | 4.3 | 0.094 |
| 35B | 0 | 0 | 6 | 8.6 | 0.003 |
| Not determinedc | 1 | 0.9 | 0 | 0 | 1.000 |
| NESpd | 5 | 4.3 | 17 | 24.3 | <0.001 |
| Total PNSP | 116 | 100 | 70 | 100 | 0.121 |
| Total pneumococcal isolates | 314 | 36.9 | 230 | 30.4 | 0.002 |
Nc, not calculated.
Seven isolates of serotype 19F were detected in the same patient in 2017.
Serotypes other than those included in the multiplex PCR panel of the study.
NESp, nonencapsulated S. pneumoniae.
TABLE 4.
MDR pneumococcal serotypes during the PreVac (2009 to 2011) and PostVac-II (2015 to 2017) periods
| MDR serotype | PreVac isolates |
PostVac-II isolates |
p value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 6A | 1 | 0.9 | 0 | 0 | 1.000 |
| 6B | 9 | 8.3 | 5 | 9.1 | 1.000 |
| 6C | 1 | 0.9 | 9 | 16.4 | <0.001 |
| 14 | 2 | 1.9 | 1 | 1.8 | 1.000 |
| 15A | 1 | 0.9 | 5 | 9.1 | 0.017 |
| 15B/C | 0 | 0 | 1 | 1.8 | 0.337 |
| 19F | 87 | 80.6 | 20a | 36.4 | <0.001 |
| 23F | 1 | 0.9 | 0 | 0 | 1.000 |
| 23A | 0 | 0 | 1 | 1.8 | 0.337 |
| 35B | 0 | 0 | 1 | 1.8 | 0.337 |
| Not determinedb | 1 | 0.9 | 0 | 0 | 1.000 |
| NESpc | 5 | 4.6 | 12 | 21.8 | <0.001 |
| Total MDR serotypes | 108 | 100 | 55 | 100 | 0.010 |
| Total pneumococcal isolates | 314 | 34.4 | 230 | 23.9 | 0.002 |
Seven isolates of serotype 19F were detected in the same patient in 2017.
Serotypes other than those included in the multiplex PCR panel of the study.
NESp, nonencapsulated S. pneumoniae.
TABLE 5.
PNSP serotypes that were also MDR PreVaC (2009 to 2011) and PostVac-II (2015 to 2017)
| PNSP/MDR serotype | PreVac isolates |
PostVac-II isolates |
p value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 6B | 8 | 6.9 | 5 | 7.1 | 1.000 |
| 6C | 0 | 0 | 5 | 7.1 | 0.007 |
| 14 | 2 | 1.7 | 1 | 1.4 | 1.000 |
| 15A | 0 | 0 | 5 | 7.1 | 0.007 |
| 15B/C | 0 | 0 | 1 | 1.4 | 0.376 |
| 19F | 87 | 75.0 | 20a | 28.6 | <0.001 |
| 23A | 0 | 0 | 1 | 1.4 | 0.376 |
| 35B | 0 | 0 | 1 | 1.4 | 0.376 |
| Not determinedb | 1 | 0.9 | 0 | 0 | 1.000 |
| NESpc | 4 | 3.4 | 12 | 17.1 | 0.002 |
| Total PNSP/MDR | 102 | 87.9 | 51 | 72.9 | 0.016 |
| Total PNSP | 116 | 100 | 70 | 100 | 0.121 |
Seven isolates of serotype 19F were detected in the same patient in 2017.
Serotypes other than those included in the multiplex PCR panel of the study.
NESp, nonencapsulated S. pneumoniae.
Serotype 19F isolates were the most prevalent penicillin-nonsusceptible pneumococci (PNSP) and MDR pneumococci in all three study periods. A total of 78.4% of PNSP and 80.6% of MDR pneumococci recovered in the PreVac period were serotype 19F, but this decreased to 28.6% and 36.4%, respectively, in the PostVac-II period (p < 0.001 for both; Tables 3 and 4). Nearly all (91.8%) of the sequenced PNSP serotype 19F isolates were members of the internationally distributed MDR lineage CC236/271/32019F (Taiwan19F-14; Table 3 and Fig. 2; see also Table S3 in the supplemental material).
There were three serotypes, 6C, 15A, and 35B, that were not associated with penicillin resistance in the PreVac period, but each serotype described 7 to 9% of the PNSP by the PostVac-II period (Table 3). Similarly, there were changes among the MDR pneumococci of all three of these serotypes; in particular, there were significant increases in MDR serotype 6C and 15A pneumococci (16.4% [n = 9] and 9.1% [n = 5] PostVac-II, respectively) (Tables 3 to 5). Among all PNSP isolates, NESp isolates increased from 4.3% to 24.3% from the PreVac period to the PostVac-II period (p < 0.001), of which 17.1% were also MDR (p = 0.002; Tables 3 to 5). The overall number of isolates of serotypes 6C, 15A, and 35B and that of the NESp isolates was relatively low, and only every other isolate from 2009 to 2014 was chosen for genome sequencing. Therefore, only a few of each of these pneumococci were selected for genome sequencing, and it is difficult to draw any major conclusions about the genetic lineages associated with these PNSP or MDR pneumococci, except to say that the characterized STs corresponded to widely distributed genetic lineages such as ST344NT and ST3156B (Fig. 2; Table S3).
DISCUSSION
The results of this study demonstrated an indirect (herd) effect of PHiD-CV among adults by decreasing the overall number of LRT samples and the proportion of those samples that were positive for pneumococci and by causing a reduction in the proportion of pneumococci that were of vaccine serotypes. The vaccine-induced herd effect leading to a reduction in the incidence of pneumococcal serotypes in unvaccinated children and adults has been widely studied for invasive pneumococcal disease (IPD) (10, 28–31), but few studies have documented a herd effect on vaccine serotypes in adults with pneumonia (11, 32).
High vaccine coverage (>70 to 80%) leads to extensive herd protection in a population (33), which becomes evident in the adult population within a few years following vaccine implementation (10, 12). At the beginning of the PostVac-II period, over 97% of Icelandic children <5 years of age were fully vaccinated (34). We assessed the differences in the prevalences of pneumococcal serotypes between two postimplementation periods by comparing the PreVac period (2009 to 2011) to both the PostVac-I (2012 to 2014) and PostVac-II (2015 to 2017) periods. The relatively rapid establishment of herd protection and the decline of VTs in adults (29, 30, 33) could partly be explained by the high vaccine uptake in Iceland, a country where vaccines are generally well accepted (35). However, serotype replacement of NVTs has been observed where PCVs have been implemented (36–38), and this was also the case in our study, although serotype replacement was significant only in adults aged 18 to 64 years.
The penicillin-nonsusceptible and multidrug-resistant serotype 19F isolates were all members of the globally distributed CC236/271/32019F lineage. Serotype 19F was the most prevalent PNSP/MDR serotype in all study periods, although it decreased significantly in the PostVac-II period. The prevalence of PNSP/MDR serotype 19F was unusually high in 2017 compared to that in the two previous years, but this was partly because one 77-year-old immunodeficient patient contributed 7 of the 18 isolates detected that year. However, fluctuations in the prevalence of serotype 19F are also known to occur following PCV introduction, and this will need to be monitored in Iceland going forward (11, 38). Adult infections are frequently a reflection of nasopharyngeal carriage among young children (31), but interestingly, serotype 19F was not detected in 2017 among healthy Icelandic children or in Icelandic children with acute otitis media (22). Therefore, it is possible that older children and/or older adults can serve as a reservoir for serotype 19F, and this maintains serotype 19F in the unvaccinated population after vaccine introduction. Serotypes 1 and 5 were not detected in this study. These serotypes are rare in Iceland. Serotype 1 was last detected in 2012, serotype 5 was last detected in 1996, and both serotypes were recovered from patients with IPD (S. J. Quirk, G. Haraldsson, M. Á. Hjálmarssdóttir, H. Erlendsdóttir, and K. G. Kristinsson, unpublished data).
Among vaccine-related serotypes, the prevalence of serotype 6A decreased significantly in the second PostVac period even though it is not a direct target of the vaccine, and other countries that have implemented PHiD-CV have also described similar results (18, 20). Furthermore, in our previous study, the prevalence of serotype 6A decreased in Icelandic children 1 to <4 years of age with acute otitis media (22). The possible cross-protection against serotype 19A through the serotype 19F conjugate has been widely debated (17–21); however, the Icelandic adult population did not appear to benefit from the childhood vaccinations against serotype 19A, since the incidence of serotype 19A did not change between the study periods. Before vaccine implementation in Iceland, serotype 19A was more commonly found in IPD and nasopharyngeal carriage than in non-IPD (acute otitis media and pneumonia), and serotype 19F was predominant in non-IPD (22, 39).
Serotypes 6C and 15A have been reported as upcoming PNSP and MDR serotypes following PCV introduction (13, 40, 41), but among adults in Iceland, these serotypes were detected only in low numbers in the PostVac periods of the study. Our group has also detected MDR isolates of serotype 6C that were members of CC3156B/6C and ST3866C (a double locus variant of PMEN Poland6B-20) among children (22, 39), but it remains to be seen whether CC3156C will replace CC236/271/32019F as a major MDR lineage in Iceland.
Notably, the prevalence of NESp isolates increased significantly, and they were the most frequently detected pneumococci in the PostVac-II period in adults aged 18 to 64 years. The opposite was seen in the United States, where NESp isolates decreased in adults between the ages of 50 and 64 years, with a parallel increase among adults ≥65 years of age (32).
Increased vaccine pressure caused by PCVs might open an environmental niche that NESp is adept to employ. This could explain the increased prevalence of NESp isolates in the PostVac-II period, or the increase might simply be a natural trend that reflects longer-term variation (42, 43). Our data support the need for continued surveillance of pneumococci in Iceland. It should be noted that some serotypes were found only in low numbers; therefore, the statistical power for detecting a difference in the frequency between the PreVac and PostVac periods among these serotypes was low.
Following the financial crisis in Iceland in 2008, physicians were advised to reduce test samples at the Landspitali University Hospital, and as a result, fewer LRT samples were received from 2010 to 2014. In the following years, when the effect of the crisis subsided, the number of LRT samples gradually increased again, but at the same time, the number of pneumococcal isolates continued to decrease. This decrease was particularly evident among patients aged ≥65 years, and PCV introduction into childhood immunization programs has been shown to be very beneficial for older adults (11, 33).
Most of the LRT samples in this study were sputum samples; thus, some pneumococcal isolates may represent colonization, although colonization among adults is rare (44), and sputum samples should have been collected only from patients with suspected pneumonia and not healthy adults. Therefore, using the number of positive pneumococcal isolates as a proxy for pneumococcal pneumonia could be considered a weakness. In general, the financial crisis mentioned above was the only known factor influencing sampling. Vaccine uptake area, guidelines, the health care system, and microbiological methods remained the same during the study period.
The herd effect became evident in our study 3 to 4 years after PHiD-CV implementation and was associated with significant changes in both the serotype distribution and the number of pneumococcal isolates cultured from the lower respiratory tract samples of adults. Pneumococcus was the most frequent pathogen recovered from adults with pneumonia in Iceland prior to vaccination (45); hence, the protection of older adults through pneumococcal vaccination and herd immunity is of the utmost importance.
Supplementary Material
ACKNOWLEDGMENTS
This was an investigator-initiated study that was funded by GlaxoSmithKline Biologicals S.A. Further grants that supported this study were received from the Landspitali University Hospital Research Fund, The Eimskip University Fund, the Wellcome Trust (research fellowship 083511/Z/07/Z and grant 04992/Z/14/Z to A.B.B.), and the John Fell Fund (grant 123/734 to A.B.B.). Work at the Wellcome Sanger Institute was supported by Wellcome core funding grant number 206194.
GlaxoSmithKline Biologicals S.A. was provided the opportunity to review a draft version of this manuscript, but the authors are solely responsible for final content and interpretation. The authors received no financial support or other form of compensation related to the development of the manuscript.
We thank the staff at the Department of Clinical Microbiology for collecting the pneumococcal isolates from the LRT samples. We also thank other members of the VIce study group.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01766-18.
REFERENCES
- 1.World Health Organization. 2018. Pneumococcal disease. World Health Organization, Geneva, Switzerland: http://www.who.int/ith/diseases/pneumococcal/en/. [Google Scholar]
- 2.Fung HB, Monteagudo-Chu MO. 2010. Community-acquired pneumonia in the elderly. Am J Geriatr Pharmacother 8:47–62. doi: 10.1016/j.amjopharm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Torres A, Blasi F, Peetermans WE, Viegi G, Welte T. 2014. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis 33:1065–1079. doi: 10.1007/s10096-014-2067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson DA, Musher DM, Jacobson JW, Verhoef J. 1993. A brief history of the pneumococcus in biomedical research: a panoply of scientific discovery. Clin Infect Dis 17:913–924. doi: 10.1093/clinids/17.5.913. [DOI] [PubMed] [Google Scholar]
- 5.Song JY, Nahm MH, Moseley MA. 2013. Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance. J Korean Med Sci 28:4–15. doi: 10.3346/jkms.2013.28.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjarnason A, Lindh M, Westin J, Andersson LM, Baldursson O, Kristinsson KG, Gottfredsson M. 2017. Utility of oropharyngeal real-time PCR for S. pneumoniae and H. influenzae for diagnosis of pneumonia in adults. Eur J Clin Microbiol Infect Dis 36:529–536. doi: 10.1007/s10096-016-2829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geno KA, Saad JS, Nahm MH. 2017. Discovery of novel pneumococcal serotype 35D, a natural WciG-deficient variant of serotype 35B. J Clin Microbiol 55:1416–1425. doi: 10.1128/jcm.00054-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reijtman V, Fossati S, Hernandez C, Sommerfleck P, Bernaldez P, Litterio M, Berberian G, Regueira M, Lopardo H. 2013. Serotype distribution of pneumococci isolated from pediatric patients with acute otitis media and invasive infections, and potential coverage of pneumococcal conjugated vaccines. Rev Argent Microbiol 45:27–33. [PubMed] [Google Scholar]
- 9.World Health Organization. 2016. Immunization coverage. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs378/en/. [Google Scholar]
- 10.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigo C, Bewick T, Sheppard C, Greenwood S, McKeever TM, Trotter CL, Slack M, George R, Lim WS. 2015. Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. Eur Respir J 45:1632–1641. doi: 10.1183/09031936.00183614. [DOI] [PubMed] [Google Scholar]
- 12.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. 2011. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 11:760–768. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 13.van der Linden M, Perniciaro S, Imöhl M. 2015. Increase of serotypes 15A and 23B in IPD in Germany in the PCV13 vaccination era. BMC Infect Dis 15:207. doi: 10.1186/s12879-015-0941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchiya M, Urushibara N, Aung MS, Morimoto S, Ito M, Kudo K, Sumi A, Kobayashi N. 2016. Emerging non-PCV13 serotypes of noninvasive Streptococcus pneumoniae with macrolide resistance genes in northern Japan. New Microbes New Infect 9:66–72. doi: 10.1016/j.nmni.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano S, Fujisawa T, Ito Y, Chang B, Suga S, Noguchi T, Yamamoto M, Matsumura Y, Nagao M, Takakura S, Ohnishi M, Ihara T, Ichiyama S. 2016. Serotypes, antimicrobial susceptibility, and molecular epidemiology of invasive and non-invasive Streptococcus pneumoniae isolates in paediatric patients after the introduction of 13-valent conjugate vaccine in a nationwide surveillance study conducted in Japan in 2012–2014. Vaccine 34:67–76. doi: 10.1016/j.vaccine.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Duvvuri VR, Deng X, Teatero S, Memari N, Athey T, Fittipaldi N, Gubbay JB. 2016. Population structure and drug resistance patterns of emerging non-PCV-13 Streptococcus pneumoniae serotypes 22F, 15A, and 8 isolated from adults in Ontario, Canada. Infect Genet Evol 42:1–8. doi: 10.1016/j.meegid.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Hammitt LL, Akech DO, Morpeth SC, Karani A, Kihuha N, Nyongesa S, Bwanaali T, Mumbo E, Kamau T, Sharif SK, Scott JA. 2014. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health 2:e397–e405. doi: 10.1016/S2214-109X(14)70224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jokinen J, Rinta-Kokko H, Siira L, Palmu AA, Virtanen MJ, Nohynek H, Virolainen-Julkunen A, Toropainen M, Nuorti JP. 2015. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children—a population-based study. PLoS One 10:e0120290. doi: 10.1371/journal.pone.0120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knol MJ, Wagenvoort GH, Sanders EA, Elberse K, Vlaminckx BJ, de Melker HE, van der Ende A. 2015. Invasive pneumococcal disease 3 years after introduction of 10-valent pneumococcal conjugate vaccine, the Netherlands. Emerg Infect Dis 21:2040–2044. doi: 10.3201/eid2111.140780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naucler P, Galanis I, Morfeldt E, Darenberg J, Ortqvist A, Henriques-Normark B. 2017. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis 65:1780–1789. doi: 10.1093/cid/cix685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domingues CM, Verani JR, Montenegro Renoiner EI, de Cunto Brandileone MC, Flannery B, de Oliveira LH, Santos JB, de Moraes JC. 2014. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med 2:464–471. doi: 10.1016/S2213-2600(14)70060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quirk SJ, Haraldsson G, Erlendsdottir H, Hjalmarsdottir MA, van Tonder AJ, Hrafnkelsson B, Sigurdsson S, Bentley SD, Haraldsson A, Brueggemann AB, Kristinsson KG. 2018. Effect of vaccination on pneumococci isolated from the nasopharynx of healthy children and the middle ear of children with otitis media in Iceland. J Clin Microbiol 56:e01046-18. doi: 10.1128/JCM.01046-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morona JK, Paton JC, Miller DC, Morona R. 2000. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol Microbiol 35:1431–1442. [DOI] [PubMed] [Google Scholar]
- 24.Kurola P, Erkkila L, Kaijalainen T, Palmu AA, Hausdorff WP, Poolman J, Jokinen J, Kilpi TM, Leinonen M, Saukkoriipi A. 2010. Presence of capsular locus genes in immunochemically identified encapsulated and unencapsulated Streptococcus pneumoniae sputum isolates obtained from elderly patients with acute lower respiratory tract infection. J Med Microbiol 59:1140–1145. doi: 10.1099/jmm.0.016956-0. [DOI] [PubMed] [Google Scholar]
- 25.European Committee on Antimicrobial Susceptibility Testing. 2012. Breakpoint tables for interpretation of MICs and zone diameters.
- 26.Berger C. 2001. Statistical inference, 2nd ed Duxbury Press, N. Scituate, MA. [Google Scholar]
- 27.Simpson EH. 1949. Measurement of diversity. Nature 163:688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- 28.Kendall BA, Dascomb KK, Mehta RR, Stockmann C, Mason EO, Ampofo K, Pavia AT, Byington CL. 2016. Early Streptococcus pneumoniae serotype changes in Utah adults after the introduction of PCV13 in children. Vaccine 34:474–478. doi: 10.1016/j.vaccine.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Regev-Yochay G, Paran Y, Bishara J, Oren I, Chowers M, Tziba Y, Istomin V, Weinberger M, Miron D, Temper V, Rahav G, Dagan R. 2015. Early impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneumococcal disease: a nationwide surveillance study. Vaccine 33:1135–1142. doi: 10.1016/j.vaccine.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L. 2012. Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis 12:207. doi: 10.1186/1471-2334-12-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grau I, Ardanuy C, Cubero M, Benitez MA, Linares J, Pallares R. 2016. Declining mortality from adult pneumococcal infections linked to children's vaccination. J Infect 72:439–449. doi: 10.1016/j.jinf.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Mendes RE, Hollingsworth RC, Costello A, Jones RN, Isturiz RE, Hewlett D Jr, Farrell DJ. 2015. Noninvasive Streptococcus pneumoniae serotypes recovered from hospitalized adult patients in the United States in 2009 to 2012. Antimicrob Agents Chemother 59:5595–5601. doi: 10.1128/AAC.00182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsaban G, Ben-Shimol S. 2017. Indirect (herd) protection, following pneumococcal conjugated vaccines introduction: a systematic review of the literature. Vaccine 35:2882–2891. doi: 10.1016/j.vaccine.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Eythorsson E, Hrafnkelsson B, Erlendsdottir H, Gudmundsson SA, Kristinsson KG, Haraldsson A. 2018. Decreased acute otitis media with treatment failure after introduction of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine. Pediatr Infect Dis J 37:361–366. [DOI] [PubMed] [Google Scholar]
- 35.Óskarsson Ý, Guðnason Þ, Jónsdóttir GA, Kristinsson KG, Briem H, Haraldsson Á. 2015. Public opinion on childhood immunisations in Iceland. Vaccine 33:7211–7216. doi: 10.1016/j.vaccine.2015.10.125. [DOI] [PubMed] [Google Scholar]
- 36.Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, Thomas AR, Harrison LH, Bennett NM, Farley MM, Facklam RR, Jorgensen JH, Besser J, Zell ER, Schuchat A, Whitney CG. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 37.Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet 378:1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hays C, Vermee Q, Agathine A, Dupuis A, Varon E, Poyart C, Ploy MC, Raymond J. 2017. Demonstration of the herd effect in adults after the implementation of pneumococcal vaccination with PCV13 in children. Eur J Clin Microbiol Infect Dis 36:831–838. doi: 10.1007/s10096-016-2868-5. [DOI] [PubMed] [Google Scholar]
- 39.Hjálmarsdóttir MÁ, Quirk SJ, Haraldsson G, Erlendsdóttir H, Haraldsson Á, Kristinsson KG. 2017. Comparison of serotype prevalence of pneumococci isolated from middle ear, lower respiratory tract and invasive disease prior to vaccination in Iceland. PLoS One 12:e0169210. doi: 10.1371/journal.pone.0169210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neves FPG, Cardoso NT, Snyder RE, Marlow MA, Cardoso CAA, Teixeira LM, Riley LW. 2017. Pneumococcal carriage among children after four years of routine 10-valent pneumococcal conjugate vaccine use in Brazil: the emergence of multidrug resistant serotype 6C. Vaccine 35:2794–2800. doi: 10.1016/j.vaccine.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Setchanova L, Murdjeva M, Stancheva I, Alexandrova A, Sredkova M, Stoicheva T, Yoneva M, Kurchatova A, Mitov I. 2017. Serotype changes and antimicrobial nonsusceptibility rates of invasive and non-invasive Streptococcus pneumoniae isolates after implementation of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Bulgaria. Braz J Infect Dis 21:433–440. doi: 10.1016/j.bjid.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller LE, Robinson DA, McDaniel LS. 2016. Nonencapsulated Streptococcus pneumoniae: emergence and pathogenesis. mBio 7:e01792. doi: 10.1128/mBio.01792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langereis JD, de Jonge MI. 2017. Non-encapsulated Streptococcus pneumoniae, vaccination as a measure to interfere with horizontal gene transfer. Virulence 8:637–639. doi: 10.1080/21505594.2017.1309492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamaluba M, Kandasamy R, Ndimah S, Morton R, Caccamo M, Robinson H, Kelly S, Field A, Norman L, Plested E, Thompson BA, Zafar A, Kerridge SA, Lazarus R, John T, Holmes J, Fenlon SN, Gould KA, Waight P, Hinds J, Crook D, Snape MD, Pollard AJ. 2015. A cross-sectional observational study of pneumococcal carriage in children, their parents, and older adults following the introduction of the 7-valent pneumococcal conjugate vaccine. Medicine (Baltimore) 94:e335. doi: 10.1097/MD.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjarnason A, Asgeirsson H, Baldursson O, Kristinsson KG, Gottfredsson M. 2015. Mortality in healthcare-associated pneumonia in a low resistance setting: a prospective observational study. Infect Dis (Lond) 47:130–136. doi: 10.3109/00365548.2014.980842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.