Abstract
The ability of cells to respond to mechanical forces is critical for numerous biological processes. Emerging evidence indicates that external mechanical forces trigger changes in nuclear envelope structure and composition, chromatin organization, and gene expression. However, it remains unclear if these processes originate in the nucleus or are downstream of cytoplasmic signals. This review discusses recent findings supporting a direct role of the nucleus in cellular mechanosensing and highlights novel tools to study nuclear mechanotransduction.
Introduction
Cells are constantly being exposed to mechanical forces, such as shear forces on endothelial cells1, compressive forces on chondrocytes2, and tensile forces in myocytes3. The cells’ ability to sense and respond to these mechanical cues are critical for numerous biological processes, including embryogenesis4, 5, development4, 5, and tissue homeostasis6, 7. While it has long been recognized that mechanical forces can influence cell morphology and behavior8, 9, the understanding of the molecular pathways involved in mechanosensing, and how disruption of these pathways can give rise to various diseases, is still evolving10–13. Stretch activated ion-channels, adhesions complexes, cell-cell-junctions, and cytoskeletal components have all been identified as mechanosensitive elements that can activate cellular signaling pathways such Rho-family GTPases or the mitogen-activated protein kinase–extracellular signal-regulated kinase (MAPK–ERK), induce nuclear translocation of the transcriptional regulators YAP/TAZ and MKL1, and ultimately result in expression of mechanoresponsive genes (see 14–18 for review). Over the last two decades the question whether the nucleus itself can sense mechanical stimuli has received increasing attention19, 20. Such ‘nuclear mechanotransduction’ could provide a more rapid and direct method to transduce forces into cellular events21, 22 and act in concert with or independent of cytoplasmic mechanotransduction pathways. In this scenario, forces applied to the nucleus via the cytoskeleton may modulate the effect of cytoplasmic signals, or even be sufficient to directly trigger changes in gene expression. Such multifaceted mechanotransduction may enable cells to distinguish between small forces only affecting the cell surface, and larger forces resulting in large-scale cell and nuclear deformations. Spurred in part by advances in biophysical, biochemical, and imaging assays, multiple mechanisms have been proposed to explain how forces acting on the nucleus could influence chromatin organization, transcription, and other cellular processes19, 22–24. However, distinguishing between nuclear events that are downstream of cytoplasmic mechanosensitive signaling pathways, and those that reflect true nuclear mechanotransduction events, remains challenging.
One aspect that is universally accepted now is that extracellular and cytoplasmic forces are transmitted across the nuclear envelope to the nuclear interior, where they can cause deformation of chromatin and nuclear bodies20, 25–27. Intriguingly, a recent study demonstrated that force application to the nucleus can induce chromatin stretching and expression of a reporter transgene28. These findings provide some of the most direct evidence to date for the nucleus as a mechanoresponsive organelle. Below we discuss current findings that support nuclear mechanotransduction, explain potential molecular mechanisms, and highlight emerging technologies to study nuclear mechanotransduction.
The nucleus and the nuclear lamina
The nucleus is the largest and stiffest organelle in the cell29, 30. It can be broadly separated into the nuclear interior, which houses chromatin, nuclear bodies and other intranuclear elements, and the surrounding nuclear envelope. The nuclear envelope is comprised of the outer and inner nuclear membranes (ONM and INM, respectively), which contain a large number of membrane-bound proteins31, 32, as well as nuclear pore complexes (NPCs) that control entry of large molecules into the nuclear interior33. Underneath the INM lies the nuclear lamina, a filamentous protein network comprised of A-type and B-type lamins, and lamin binding proteins34, 35. In mammalian somatic cells, the major A-type lamin isoforms are lamin A and C, encoded by the LMNA gene. One major motivation to study the role of the nucleus in mechanotransduction came from the identification of LMNA mutations as the genetic cause for various forms of muscular dystrophy and cardiomyopathy36–38. Diseases caused by lamin mutations (commonly referred to as laminopathies) remain both intriguing and perplexing. Although A-type lamins are nearly ubiquitously expressed, many of the LMNA mutations predominantly affect mechanically active tissue, i.e., skeletal muscle, cardiac muscle, and tendons. These tissue-specific disease phenotypes suggest that defects in the nucleus can impair the ability of cells to respond appropriately to mechanical forces. It is now well recognized that the nuclear lamina governs numerous biological functions, both biophysical and biochemical, including determining nuclear size and stiffness39–43, regulating translocation and activity of transcription factors44–47, interacting with chromatin and regulating its epigenetic state48, 49, and controlling cell polarization and migration50–52. Consequently, cells lacking lamin A/C or expressing disease-causing mutations display severe defects in nuclear stability53–55, cytoskeletal dynamics47, 51, and nucleo-cytoskeletal force transmission55, 56. Furthermore, lamin A/C-deficient and mutant cells fail to adequately activate mechanoresponsive genes when subjected to mechanical stimulation43, 57, 58, suggesting an important role of the nucleus, and lamin A/C in particular, in cellular mechanotransduction. However, it remains incompletely understood to what extent lamins directly respond to mechanical stress in vivo, and if changes in lamin levels and organization are downstream of other mechanotransduction pathways26, 59–61. The importance of the nuclear lamina in fundamental biological processes is highlighted by the early death of mice that lack functional lamin A/C. These mice are born without any overt defects, but develop severe muscular dystrophy and dilated cardiomyopathy and die at 2–8 weeks of age62, 63. Uncovering how lamins mediate nuclear processes and mechanosensitive gene expression will not only enhance our understanding of mechanotransduction per se, but may also provide insights into the pathophysiology of laminopathies, with the potential to inform therapeutic approaches for these currently incurable diseases.
Force transmission to the nucleus
Work by the Ingber group in the 1990s provided some of the first evidence that forces can be transmitted from the cell surface to the nucleus via the cytoskeleton20. It is now recognized that these forces are transmitted across the nuclear envelope through the Linker of Nucleoskeleton and Cytoskeleton (LINC) complex64, 65. The LINC complex is comprised of nesprin proteins that reside within the ONM and contain a C-terminal KASH (Klarsicht, ANC-1, Syne Homology) domain, which interacts with SUN (Sad1 Unc-84) domain proteins located on the INM. The SUN proteins in turn bind to the nuclear lamina, nuclear pores, and chromatin (Fig. 1A)66. On the cytoplasmic side, nesprins can interact with each other and with all major cytoskeletal filaments. The composition of the LINC complex and LINC complex associated proteins vary with cell type. Furthermore, both nesprin-1 and −2 contain alternative start and stop sites that produce a number of isoforms, including the so-called “giant” variants, which contain an N-terminal actin-binding domain67. Nesprin-1 and −2 can bind to actin filaments67 and the microtubule associated motor proteins kinesin68 and dynein69; nesprin-3 binds to plectin70, which connects to intermediate filaments; nesprin-4 interacts with kinesin-171 (Fig. 1A). Additional KASH domain proteins and LINC complex associated proteins have recently been characterized and are often cell-type specific. We refer the readers to excellent recent reviews on the LINC complex for further details34, 66, 67, 72.
Figure 1.
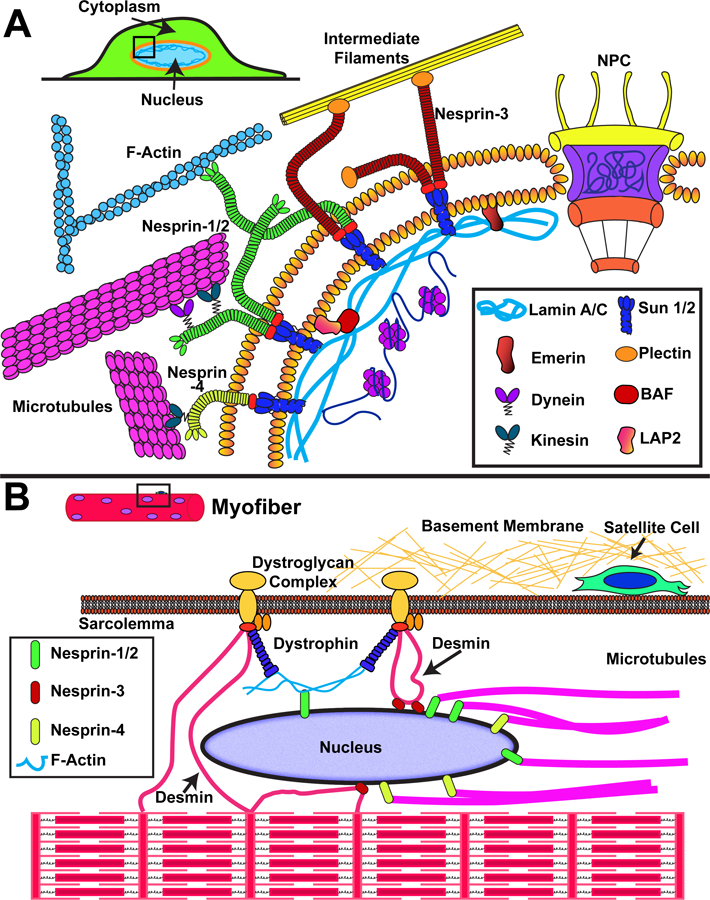
Schematic overview of nuclear envelope proteins involved in force transmission to the nucleus. (A) Force transmission to the nucleus involves interaction of cytoskeletal elements (actin filaments, intermediate filaments, microtubules) with nesprin proteins on the ONM, which transmit force through SUN domain proteins on the INM to the nuclear lamina and interior. (B) Organization of the cytoskeletal network within muscle cells, including the highly ordered actin-myosin structures to form contractile sarcomeres and myofibrils. Nuclei are positioned at the periphery of the cell, where they interact with the muscle-specific proteins dystrophin (through actin filaments) and desmin. Additional proteins such as LINC complex proteins and lamins may be involved in anchoring the myonuclei and place and transmitting forces between the nucleus and cytoskeleton.
Whereas external forces can be applied to the nucleus independent of the LINC complex, for example, during compression of the nucleus73, or cell migration through confined environments74, cells in many cases require an intact LINC complex to effectively transmit forces between the cytoskeleton and the nucleus. Consequently, depletion or expression of dominant-negative nesprin and SUN proteins severely impairs nucleo-cytoskeletal force transmission27 and mechanosensitive gene expression28, 75. Nonetheless, it remains to be tested whether the impaired mechanotransduction is due to the role of LINC complex components in intracellular force transmission, or whether these proteins contribute through other functions, such as serving as signaling scaffolds or regulating other aspects of nuclear organization, including chromatin mobility and nuclear envelope tethering49, 76. Force-induced nuclear deformation further require an intact and adequately tensed cytoskeletal network77, 78 to transmit forces from the cell surface to the nucleus22. If the actin cytoskeleton is disrupted through pharmacological or genetic approaches, force transmission to the nucleus is impaired78, 79, which is accompanied by changes in chromatin dynamics77. Notably, mechanically-induced changes in the nucleus, cytoskeleton, and extracellular matrix appear to be interrelated. For example, the mechanical properties of the extracellular matrix affect both cytoskeletal organization80 and the expression of lamin A/C61, 81, resulting in cells finely tuned with their physical environment.
The intricate relationship between the cytoskeletal network, nuclear mechanics, and the mechanical environment is particularly important in skeletal and cardiac muscle cells. These contractile cells have a highly organized cytoskeleton, including a specialized perinuclear network that anchors the nucleus in place (Fig. 1B). Desmin is a muscle-specific cytoplasmic intermediate filament that interacts with the nuclear envelope through plectin 182. This interaction is important for myofiber health83, and functional loss of plectin releases tension on the nucleus and results in altered expression of mechanoresponsive genes82. LINC complex proteins have similarly important functions in muscle cells. The LINC complex is required for myonuclear movement84–87, including the effective spacing of nuclei along the myofiber length. Loss of LINC complex function causes muscular dystrophies88–90, suggesting that adequately connecting the nucleus to the cytoskeleton is crucial for skeletal muscle health and maintenance. This idea is further supported by the finding that LMNA mutations that cause muscular dystrophy and dilated cardiomyopathy result in impaired nucleo-cytoskeletal coupling55, 91, 92 and loss of structural function, whereas LMNA mutations associated with lipodystrophy have little or no effect on nuclear mechanics and nucleo-cytoskeletal force transmission55, 91.
Although striated muscle are the tissues impacted most by disruption in nuclear mechanics and nucleo-cytoskeletal coupling, many other cell types are also affected by impaired nucleo-cytoskeletal force transmission93, 94. For example, T-cell activation requires proper lamin A/C and LINC complex function to regulate T-cell receptor clustering and F-actin formation93. In fibroblasts and endothelial cells, depletion of lamin A/C or disruption of the LINC complex reduces migration capabilities94–96. Similarly, the LINC complex is important in outer hair cells for hearing97, proper function of the ciliary rootlets in photoreceptors and ependymal cells98, hair follicle structure99, and radial neuronal migration during neurogenesis100. These findings demonstrate the broad importance of nucleo-cytoskeletal force transmission on cellular function.
Potential mechanisms for nuclear mechanotransduction
The negative effects of lamin mutations and LINC complex disruption are well documented, but the underlying molecular mechanisms remain incompletely understood. External forces are transmitted across the cytoskeleton to the nucleus, where they result in substantial deformation101–103. These forces and deformations could modulate transcriptional activity and chromatin organization through a number of mechanisms.
One potential mechanism to transduce forces acting onto the nucleus into altered transcriptional activity is by modulating the physical organization of chromatin. The spatial location of the DNA with the nucleus exists in a non-random organization. This “4D nucleome” (meaning the 3D chromatin architecture and its change over time) is important for transcriptional regulation and cellular functions104–107. Heterochromatic DNA, which is tightly wrapped around histones and largely inaccessible for the transcriptional machinery, is often localized to the nuclear periphery49. This peripheral localization promotes gene silencing, while repositioning of genes towards the nuclear interior generally facilitates gene activation108, although additional regulations apply. Thus, force-induced changes in gene positioning relative to the nuclear periphery could alter the transcriptional activity of specific genes and contribute to nuclear mechanotransduction. Supporting this idea, altering cytoskeletal organization and tension by culturing cells on micropatterned substrates alters nuclear shape and chromosome distribution, accompanied by changes in gene expression103, 109. It remains unclear to what extent these changes are the direct result of altered cytoskeletal forces acting on the nucleus versus upstream signaling pathways that may be sensitive to cytoskeletal organization. Extrinsic force application to cells can also induce repositioning of nuclear bodies and the associated chromatin110–112, which could affect additional nuclear processes. Lastly, whereas changes in chromatin organization may lay downstream of forces acting on the nucleus, the epigenetic state of chromatin also contributes to the mechanical properties of the nucleus: chromatin decondensation increases nuclear deformability, and chromatin condensation decreases nuclear deformability110, 113–116, both of which may occur independently of changes in lamin levels117. Thus, changes in nuclear organization, even when downstream of other pathways, can have a direct effect on nuclear deformation and may thus modulate other nuclear mechanotransduction processes.
In addition to changes in gene or chromosome positioning, mechanical forces may directly alter chromatin organization and transcription. In vitro experiments indicate that 5 pN of force is sufficient to decondense single chromatin fibers118. Recent work from the Wang and Belmont labs demonstrated that applying forces to the cell surface results in instantaneous stretching of chromatin inside the nucleus, associated with rapid induction of transcription of a transgene located within that chromatin region28. Notably, the level of transcription correlated with the frequency and magnitude of the applied forces, and disruption of the LINC complex abolished the force-mediated transcription response28. The finding that force-induced transcription occurred extremely rapidly (<30 seconds) suggests that the stretching of chromatin alters the accessibility of the transcriptional machinery to the gene or its activity, rather than altering the epigenetic state of the locus. Though highly intriguing, such directly mediated modulation in gene expression has yet to be demonstrated for endogenous genes. Furthermore, it remains to be seen whether this mechanism of modulating gene transcription only applies to genes that are already “primed” for transcription, or if it could also activate silenced genes, such as those in heterochromatic regions. Intriguingly, prolonged force application induces an increase in heterochromatin and transcriptional repression4, which could serve as a negative feedback mechanism. Lastly, it is unclear how force-induced chromatin stretching would be able to confer specificity, as it is likely that multiple genomic loci would be subjected to a similar level of mechanical force, and direct association between mechanoresponsive genes and LINC complex components have not been demonstrated to date.
Force-induced molecular crowding could present another potential nuclear mechanotransduction mechanism. Nuclear deformation could also alter nuclear processes by local crowding and exclusion of soluble factors in areas where chromatin has been compacted. For example, exclusion of DNA damage repair factors delay repair of DNA breaks119, 120. Similar exclusion of transcriptional regulators or chromatin remodelers could alter transcriptional activity.
Recent studies revealed that mechanical stress can induce conformational and post-translational changes (e.g. phosphorylation) in nuclear envelope proteins (Fig. 2)26, 60, 61, 121. Force application on the nucleus results in apical-to-basal differences in the conformation of lamin A/C, as evidenced by the masking of certain C- and N-terminal epitopes under tension60. Exposing isolated nuclei to shear stress exposes a cryptic cysteine residue (Cys552) in the Ig-domain of lamin A/C, which is normally inaccessible during periods of low mechanical stress61. It remains to be seen whether this residue can become exposed under physiological forces in intact cells, as the N-terminal portion of the Ig-domain appears largely inaccessible during periods of high mechanical stress in vivo60. Recent findings further indicate that reduced cytoskeletal tension, for example, when cells are cultured on soft substrates, results in increased lamin A/C phosphorylation, which is associated with increased solubility and degradation61, 121. In contrast, increased cytoskeletal tension results in decreased lamin A/C phosphorylation and higher lamin A/C levels121. Similarly, force application to isolated nuclei through the LINC complex causes phosphorylation of the INM protein emerin26, which binds to lamin AC. It is unclear whether these phosphorylation events are triggered by increased residue accessibility after force-induced conformational changes, or if force application is modulating the activity of nuclear kinases such as Src122. Regardless of the specific mechanism, mutations of the relevant Tyr74 and Tyr95 sites in emerin results in decreased stress fiber formation and decreased expression of SRF-dependent genes26. In response to prolonged force application, emerin may also serve to reinforce the actin network at the ONM and facilitate chromatin remodeling4. Although additional work is needed to elucidate the specific pathways involved, including whether emerin and lamin are downstream of other mechanosensitive signaling events and which biochemical signals are activated by their phosphorylation, these findings demonstrate the relevance of nuclear envelope proteins in modulating transcriptional activity and nuclear and cytoskeletal organization.
Figure 2.
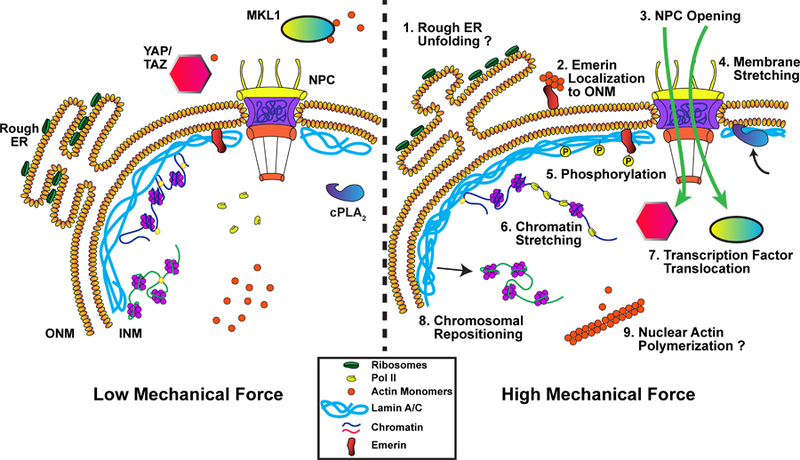
Proposed mechanisms for how the cell nucleus could respond directly to mechanical forces. (1) Stretching of the nuclear membrane could alter the conformation of the rough ER, exposing more ribosomes to the cytoplasm. (2) Force application promotes translocation of emerin from the INM to the ONM, modulating chromatin organization and facilitating actin polymerization at the ONM. (3) Increased membrane tension could open nuclear pore complexes (NPC) and modulate NPC permeability. (4) Stretching of the nuclear membrane recruits cPLA2 to the INM. (5) Force transmission to the nucleus results in post-translational modification and altered dynamics of lamin A/C and INM proteins such as emerin (see also (2)), which can modulate the mechanical properties of the nucleus and induce downstream signaling. (6) External forces can induce chromatin stretching, altering polymerase and transcription factor accessibility and activity. (7) Nuclear pore opening and sequestration at the nuclear envelope can modulate localization and activity of transcription factors. (8) Forces acting on the nucleus may reposition chromatin domains, altering their transcriptional activity. (9) Mechanically induced polymerization of nuclear actin can modulate export and activity of the transcriptional regulator MKL1, and affect other nuclear processes that require monomeric actin.
Force-induced stretching of the nuclear membranes could present an additional mechanism for nuclear mechanotransduction. Hypotonic swelling of the nuclear membranes results in the translocation of nucleoplasmic phospholipases A2 (cPLA2) to the INM, which is inhibited when the nucleus is stabilized by either F-actin or lamin A/C123. This translocation directly activates cPLA2 and 5-LOX123, which are required for the production of the chemotactic eicosanoids that attract leukocytes to sites of injury in vivo 123. Since the underlying nuclear lamina is substantially stiffer than the nuclear membranes, it mechanically shields the nuclear membranes from large forces. At the same time, the nuclear lamina can tolerate substantially larger area strains than lipid membranes115, 116. Thus, nuclear envelope composition and organization could dramatically modulate the stretch response of the nuclear membrane. Furthermore, since the nuclear membranes are continuous with the endoplasmic reticulum (ER), stretching of the nuclear membrane is expected to increase the membrane tension in the adjacent rough ER124. It will be interesting to determine whether increased membrane tension on the nucleus can alter the organization of the rough ER, and possibly the distribution of ER membrane-bound proteins125. For example, polysomes are enriched in ER sheets rather than ER tubules126, thus reducing membrane curvature could increase their exposure to the cytosol (Fig. 2).
An extreme form of nuclear mechanotransduction is force-induced nuclear membrane rupture. Compressive forces on the nucleus generated by actomyosin contractility can increase intranuclear pressure and result in nuclear membrane blebbing and transient loss of nuclear envelope integrity (i.e., nuclear envelope rupture)79, 127–130. Although these phenomena were first observed in cells deficient for lamin A/C, cells carrying lamin A/C mutations91, cells with lower levels of B-type lamins,79 and cancer cells with a compromised nuclear lamina54, it is now apparent that all cells regularly exhibit transient nuclear envelope rupture. Defects in the nuclear lamina, increased actomyosin contractility, and external confinement can dramatically increase the incidence of nuclear envelope rupture from a few percent to the majority of cells79, 131. Cells typically restore nuclear envelope integrity and remain viable, but loss of nuclear envelope integrity results in uncontrolled exchange of cytoplasmic and nuclear proteins91, 128, mislocalization of organelles54, and DNA damage128, 129. The effect of nuclear envelope rupture on cell signaling, chromatin organization, gene expression, and long-term outcome remain incompletely understood and are topics of active investigation. Transcriptome analysis of nuclear rupture induced by severe cell compression revealed activation of DNA damage response pathways, metabolism, and nucleolar RNA production132. Recent findings additionally point to an important function of cGAS, a cytoplasmic DNA binding protein first recognized for its activation of the STING pathways when encountering viral DNA in the cytoplasm133. The latest findings indicate that cGAS can also be activated when exposed to genomic DNA after nuclear envelope breakdown of micronuclei134–137.
Increased nuclear membrane tension could also potentiate cytoplasmic signaling pathways by altering the permeability of NPCs (Fig. 2). Current models generated from the atomic structures of NPC components suggest that the NPC can undergo conformational changes that constrict or dilate the NPC in response to mechanical force138–140. Force-mediated alterations to NPC conformations could arise from increase in nuclear membrane tension or force transmission through LINC complex proteins and nuclear lamins. Both Sun1 and lamin A/C interact with NUP153141, 142, a protein that comprises a portion of the NPC basket143. In support of this mechanism, recent work by the Roca-Cusachs group found that direct force application to the nucleus is sufficient to promote nuclear entry of YAP, a mechanosensitive transcription factor73. The increase in nuclear YAP localization occurs through increased nuclear import of YAP, mediated by an increase in the permeability of the NPC for larger proteins, and the partial unfolding of YAP to further promote transit through the NPC73. Besides an increase in NPC permeability, other nuclear envelope proteins may modulate the import/export of mechanosensitive transcription factors such as YAP/TAZ and MKL1 47, 57, 144 through additional mechanisms (Fig. 2). Lamin A/C has also been shown to sequester transcription factors, such as retinoblastoma protein145, 146 and c-Fos44, at the nuclear periphery and thereby control their activity within the nucleus. Through these mechanisms, the nuclear lamina may further modulate gene expression and cell behavior.
Whereas short-term force application has been shown to rapidly induce transcription28, 43, long-term force application (12 h) can result in a global increase in heterochromatin and transcriptional repression4, suggesting that there may be a different response to force application depending on the duration of stimulation. Future studies will also need to consider differences in the response across cell type, as certain cell types may have an increased susceptibility to chromatin stretching resulting from differences in lamin A/C expression61. Lastly, while it appears that chromatin stretching can rapidly increase gene activation and Pol II recruitment (Fig. 2), prolonged mechanical stimulation likely activates mechanoresponsive feedback mechanisms that further influence gene expression, nuclear organization, and nucleo-cytoskeletal force transmission. Intriguingly, mechanical force application to isolated nuclei via nesprins results in lamin A/C recruitment and emerin phosphorylation, causing nuclear stiffening26. Thus, biochemical signaling pathways activated by mechanoresponsive genes could result in similar feedback loops which alter the responsiveness of the cell to further mechanical forces.
Technologies to study nuclear mechanotransduction
One major challenge in the field of nuclear mechanotransduction is uncoupling changes in nuclear structure, organization, and transcription that are directly due to force application to the nucleus from those that are secondary to changes in cytoplasmic mechanosensitive signaling pathways. Address this challenge requires (1) improvements in the temporal resolution of nuclear events to distinguish between immediate and downstream consequences; (2) enhanced detection of force-induced changes in chromatin organization and local transcription; (3) direct measurements of intranuclear and perinuclear forces; and (4) experimental approaches that can physically separate nuclear and cytoplasmic mechanotransduction contributions.
One method to study the force-induced relocation of genes within the nucleus or the local stretching and unfolding of chromatin loops within a single chromosomal region is to insert arrays of LacO sequences into specific genomic loci, and then fluorescently label these site with GFP-LacI (Fig. 3)28. This reporter system allows for assessing how effective chromatin stretching, measured by an increased distance between adjacent GFP-LacI loci, corresponds to changes in gene expression of the reporter gene, which can be quantified by fluorescence in situ hybridization (FISH) against the RNA transcript. Recent developments in labeling specific genomic regions of endogenous genes using CRISPR/Cas9 and related systems could help overcome the challenge of having to insert large LacO arrays or using bacterial artificial chromosome (BAC) reporters, and may even enable multi-color imaging by using dCas9 constructs from different bacterial species, each tagged with a different fluorophore (Fig. 3)147, 148. Measuring changes in the 4D nucleome could be further aided by the use of super-resolution microscopy, which allows resolving features down to 20–100 nm in intact cells149 (Table 1). In addition to optical microscopy based approaches, changes to chromosomal arrangement can be studied using sequence-based technology, such as Hi-C, which is based on the chromosome conformation capture (3C)-based methodology150 (Fig. 3). Hi–C can detect chromatin interactions across the entire genome, both within and between chromosomes, by covalently crosslinking protein/DNA complexes in their in situ configuration followed by deep sequencing. Whereas Hi-C is traditionally performed on large cell numbers (~106 cells), approaches are currently in development to extent this technique to smaller cell numbers and even single cells151. Changes in the accessibility of DNA regions may provide additional information on force-induced changes in chromatin organization, which could modulate transcriptional activity. One exciting approach is Assay for Transposase-Accessible Chromatin sequencing (ATAC-seq), which identifies accessible chromatin regions based on the insertion of a hyperactive transposase and subsequent genome fragmentation and sequencing152. Applying Hi-C and ATAC-seq analyses to cells in high- and low-force environments, or to cells before and after nuclear force application should provide detailed information on how external forces alters the spatial interactome of chromatin, which could be further coupled with RNA-seq analysis to determine if chromatin changes corresponds to a change in gene transcription.
Figure 3.
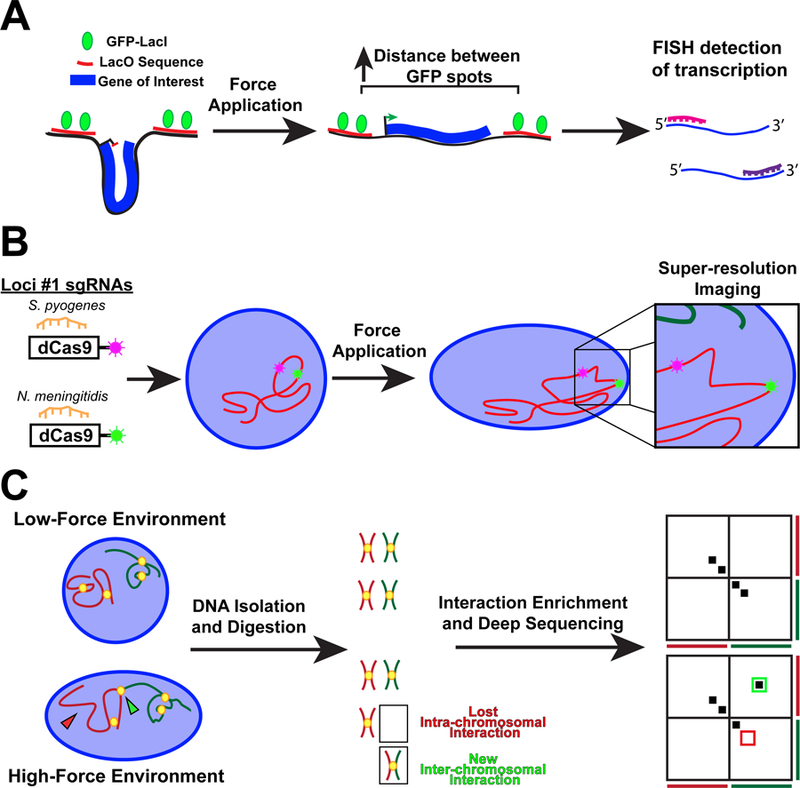
Technologies to study the effect of force transmission to the nucleus on genome organization and gene regulation. (A) Schematic of a reporter transgene to measure chromatin stretching. The transgene is flanked by two fluorescently labeled regions of DNA. An increase in the distance between the fluorescent spots indicates effective chromatin stretching. Changes to the level of transcript of the transgene can be assessed by RNA fluorescence in situ hybridization, allowing to correlate force-induced chromatin stretch with changes in transgene expression. (B) Specific endogenous DNA loci can be fluorescently labeled using CRISPR-dCas9 from different species. Changes to the positioning and spacing between adjacent loci following force application can be determined with high resolution by fluorescence microscopy. (C) Hi-C maps genome-wide chromatin interactions using deep sequencing, with changes to the interaction profile being displayed using heatmaps. Interactions appear as hot spots off the diagonal.
Table 1.
Examples of super-resolution microscopy and their application to study nuclear processes and structures.
| Type of microscopy | Mechanism of action | Application to imaging nuclear structures |
|---|---|---|
| Stimulated emission depletion (STED) | Enhances resolution by depleting fluorescence in specific regions of the sample while leaving a center focal spot active to emit fluorescence. This is achieved by generating a “doughnut” around the focal spot using a second depletion laser beam. | • γ-H2AX foci colocalizing with Ku foci160 • Mobility of proteins being imported into the nucleus161 |
| Spatially modulated illumination (SMI) | Spatially modulated illumination (SMI) microscopy achieves higher spatial resolution by modulating the illuminating light along the optical axis, after which the sample is moved through a standing wave field at precise axial steps. This technique provides improved z-axis resolution for each of the fluorophores162 | • Chromatin compaction of specific loci163 • Live cell measurements of a tet-operator repeat insert in U2OS cells164 |
| Structured illumination microscopy (SIM) | Similar to SMI in that it generates a spatially modulated illumination pattern; however this occurs along the object plane (x,y) rather than the optical (z) plane165. Multiple images are acquired and then computationally combined to generate an image with twice the resolution as traditional widefield microscopy165. | • RecA bundle formation and localization166 • NPCs colocalization with channels in the lamin network and peripheral heterochromatin167 |
| Photo-activated localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM) | Identify precise locations of individual fluorophores by using photoswitchable fluorophores to achieve optical isolation of the signal168. | • Volume of chromatin in different epigenetic states169 • H2B localization in interphase cells170 |
Molecular tension sensors can provide insights into the forces applied across specific cellular structures. Biophysical measurements on intact cells and isolated nuclei indicate that ~1–10 nN are required to induce substantial nuclear deformation116, 153, 154. The recent development of a nesprin tension biosensor has enabled the first measurements of forces transmitted across the LINC complex25, 155. Using an artificial nesprin-2giant construct containing a FRET-based tension module, Conway and colleagues demonstrated that force transmission changed with both myosin activity and cell elongation, and that the basal and apical sections of the nucleus are exposed to different forces25. Potential limitations of the current version of the tension sensor include a low signal-to-noise ratio, the insertion site of the FRET tension module, and its force range limit of ~6 pN156, 157, motivating further work in this area.
Lastly, one way to circumvent the confounding cytoplasmic signaling events that arise from applying force at the cell surface is to study isolated nuclei, or to use micromanipulation to apply force in close proximity to the nucleus158, 159. Using magnetic beads bound to the cytoplasmic domain of nesprins allows studying the role of the LINC complex in nuclear mechanotransduction and targeting specific nesprin isoforms26. One limitation of using isolated nuclei is that the isolation procedure may perturb nuclear structure, as well as the chemical composition of the nuclear interior (e.g., ion concentrations, ATP-levels, molecular crowding), which could affect nuclear mechanics and other nuclear processes42. Furthermore, working with isolated nuclei limits experiments to studying factors that originate within the nucleus, and excludes studying the import of cytoplasmic factors. Disrupting the LINC complex in intact cells allows exchange of biochemical molecules and can help identify events that require force transmission to the nucleus and nuclear deformation159. However, external force application may still induce nuclear deformation through LINC-complex independent mechanisms.
Future Perspective
The field of mechanobiology has substantially evolved and advanced in the past two decades, greatly enhancing our knowledge of how mechanical cues govern cell behavior. It is now well recognized that nuclear envelope proteins play a crucial role in the cellular response to mechanical stimuli, and that forces are transmitted from the cell surface and cytoskeleton to the nuclear interior. Increasing findings suggest that the nucleus can act as a cellular mechanosensor. Nonetheless, many questions remain, including to what extent the nucleus itself responds to mechanical forces, where such nuclear mechanotransduction processes occurs, and if these nuclear processes complement or act in parallel or downstream of cytoplasmic signaling pathways. To untangle further the profound interplay between the nucleus, cytoskeleton, and cell surface will take an integrative approach that employs biophysical assays, genetic manipulation, high-throughput genomic and proteomics, and live-cell imaging with high spatial and temporal resolution. Furthermore, experimental approaches must be employed that attempt to uncouple nuclear changes due to indirect mechanisms (i.e., cytoplasmic signal that modulate chromatin organization and transcription) from force-induced, nucleus-intrinsic events, for example, by utilizing models in which nuclear force transmission is disrupted while other cytoplasmic mechanosensitive pathways remain intact. Unraveling the force-sensitive molecular regulatory networks controlled by the nucleus and the nuclear lamina will not only increase our understanding of cellular mechanotransduction, but may also spur the development of novel therapeutic approaches to treat the currently incurable diseases that arise from impaired nuclear mechanics and mechanotransduction.
Acknowledgements
We apologize to all authors whose work could not be cited due to space constraints. This work was supported by awards from the National Institutes of Health [R01 HL082792 and U54 CA210184], the Department of Defense Breast Cancer Research Program [Breakthrough Award BC150580], the National Science Foundation [CAREER Award CBET-1254846 and MCB-1715606], and a Fleming Postdoctoral Fellowship to TK.
Footnotes
Competing Financial Interests
The authors have no competing financial interests to declare.
References
- 1.Dewey CF Jr., Bussolari SR, Gimbrone MA Jr. & Davies PF The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103, 177–85 (1981). [DOI] [PubMed] [Google Scholar]
- 2.Freeman PM, Natarajan RN, Kimura JH & Andriacchi TP Chondrocyte cells respond mechanically to compressive loads. J Orthop Res 12, 311–20 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Magid A & Law DJ Myofibrils bear most of the resting tension in frog skeletal muscle. Science 230, 1280–2 (1985). [DOI] [PubMed] [Google Scholar]
- 4.Le HQ et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol 18, 864–75 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Lecuit T & Lenne PF Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol 8, 633–44 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Barnes JM, Przybyla L & Weaver VM Tissue mechanics regulate brain development, homeostasis and disease. J Cell Sci 130, 71–82 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews BD, Overby DR, Mannix R & Ingber DE Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci 119, 508–18 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Reidy MA & Bowyer DE Scanning electron microscopy of arteries. The morphology of aortic endothelium in haemodynamically stressed areas associated with branches. Atherosclerosis 26, 181–94 (1977). [DOI] [PubMed] [Google Scholar]
- 9.Tucker JB & Meats M Microtubules and control of insect egg shape. J Cell Biol 71, 207–17 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingber DE Mechanobiology and diseases of mechanotransduction. Ann Med 35, 564–77 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Jaalouk DE & Lammerding J Mechanotransduction gone awry. Nat Rev Mol Cell Biol 10, 63–73 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iskratsch T, Wolfenson H & Sheetz MP Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol 15, 825–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphrey JD, Dufresne ER & Schwartz MA Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15, 802–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muehlich S, Hermanns C, Meier MA, Kircher P & Gudermann T Unravelling a new mechanism linking actin polymerization and gene transcription. Nucleus 7, 121–5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panciera T, Azzolin L, Cordenonsi M & Piccolo S Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol 18, 758–770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross TD et al. Integrins in mechanotransduction. Curr Opin Cell Biol 25, 613–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z, Guo SS & Fassler R Integrin-mediated mechanotransduction. J Cell Biol 215, 445–456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy SE, Dubin AE & Patapoutian A Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 18, 771–783 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Fedorchak GR, Kaminski A & Lammerding J Cellular mechanosensing: getting to the nucleus of it all. Prog Biophys Mol Biol 115, 76–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maniotis AJ, Chen CS & Ingber DE Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A 94, 849–54 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambliss AB et al. The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction. Sci Rep 3, 1087 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Tytell JD & Ingber DE Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 10, 75–82 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Cho S, Irianto J & Discher DE Mechanosensing by the nucleus: From pathways to scaling relationships. J Cell Biol 216, 305–315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miroshnikova YA, Nava MM & Wickstrom SA Emerging roles of mechanical forces in chromatin regulation. J Cell Sci 130, 2243–2250 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Arsenovic PT et al. Nesprin-2G, a Component of the Nuclear LINC Complex, Is Subject to Myosin-Dependent Tension. Biophys J 110, 34–43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guilluy C et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol 16, 376–81 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lombardi ML et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem 286, 26743–53 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tajik A et al. Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater 15, 1287–1296 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caille N, Thoumine O, Tardy Y & Meister JJ Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech 35, 177–87 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Lammerding J Mechanics of the nucleus. Compr Physiol 1, 783–807 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dreger M, Bengtsson L, Schoneberg T, Otto H & Hucho F Nuclear envelope proteomics: novel integral membrane proteins of the inner nuclear membrane. Proc Natl Acad Sci U S A 98, 11943–8 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worman HJ & Schirmer EC Nuclear membrane diversity: underlying tissue-specific pathologies in disease? Curr Opin Cell Biol 34, 101–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jovanovic-Talisman T & Zilman A Protein Transport by the Nuclear Pore Complex: Simple Biophysics of a Complex Biomachine. Biophys J 113, 6–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Leeuw R, Gruenbaum Y & Medalia O Nuclear Lamins: Thin Filaments with Major Functions. Trends Cell Biol (2017). [DOI] [PubMed] [Google Scholar]
- 35.Ho CY & Lammerding J Lamins at a glance. J Cell Sci 125, 2087–93 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genschel J & Schmidt HH Mutations in the LMNA gene encoding lamin A/C. Hum Mutat 16, 451–9 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Bonne G et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 21, 285–8 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Fatkin D et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med 341, 1715–24 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Lammerding J et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem 281, 25768–80 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Zwerger M et al. Altering lamina assembly reveals lamina-dependent and -independent functions for A-type lamins. J Cell Sci 128, 3607–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broers JL et al. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet 13, 2567–80 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Dahl KN, Kahn SM, Wilson KL & Discher DE The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J Cell Sci 117, 4779–86 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Lammerding J et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 113, 370–8 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivorra C et al. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev 20, 307–20 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osmanagic-Myers S, Dechat T & Foisner R Lamins at the crossroads of mechanosignaling. Genes Dev 29, 225–37 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorner D et al. Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway. J Cell Biol 173, 83–93 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho CY, Jaalouk DE, Vartiainen MK & Lammerding J Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497, 507–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harr JC et al. Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J Cell Biol 208, 33–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solovei I et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152, 584–98 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Harada T et al. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J Cell Biol 204, 669–82 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JS et al. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys J 93, 2542–52 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davidson PM, Denais C, Bakshi MC & Lammerding J Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell Mol Bioeng 7, 293–306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robijns J et al. In silico synchronization reveals regulators of nuclear ruptures in lamin A/C deficient model cells. Sci Rep 6, 30325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vargas JD, Hatch EM, Anderson DJ & Hetzer MW Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus 3, 88–100 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zwerger M et al. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum Mol Genet 22, 2335–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Folker ES, Ostlund C, Luxton GW, Worman HJ & Gundersen GG Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc Natl Acad Sci U S A 108, 131–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertrand AT et al. Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J Cell Sci 127, 2873–84 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Cupesi M et al. Attenuated hypertrophic response to pressure overload in a lamin A/C haploinsufficiency mouse. J Mol Cell Cardiol 48, 1290–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buxboim A et al. Coordinated increase of nuclear tension and lamin-A with matrix stiffness out-competes Lamin-B Receptor which favors soft tissue phenotypes. Mol Biol Cell (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ihalainen TO et al. Differential basal-to-apical accessibility of lamin A/C epitopes in the nuclear lamina regulated by changes in cytoskeletal tension. Nat Mater 14, 1252–1261 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swift J et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sullivan T et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol 147, 913–20 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubben N et al. Post-natal myogenic and adipogenic developmental: defects and metabolic impairment upon loss of A-type lamins. Nucleus 2, 195–207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alam SG et al. The nucleus is an intracellular propagator of tensile forces in NIH 3T3 fibroblasts. J Cell Sci 128, 1901–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crisp M & Burke B The nuclear envelope as an integrator of nuclear and cytoplasmic architecture. FEBS Lett 582, 2023–32 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Chang W, Worman HJ & Gundersen GG Accessorizing and anchoring the LINC complex for multifunctionality. J Cell Biol 208, 11–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajgor D & Shanahan CM Nesprins: from the nuclear envelope and beyond. Expert Rev Mol Med 15, e5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson MH & Holzbaur EL Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 142, 218–28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fridolfsson HN, Ly N, Meyerzon M & Starr DA UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev Biol 338, 237–50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilhelmsen K et al. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol 171, 799–810 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roux KJ et al. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci U S A 106, 2194–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horn HF LINC complex proteins in development and disease. Curr Top Dev Biol 109, 287–321 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Elosegui-Artola A et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell (2017). [DOI] [PubMed] [Google Scholar]
- 74.Thiam HR et al. Perinuclear Arp2/3-driven actin polymerization enables nuclear deformation to facilitate cell migration through complex environments. Nat Commun 7, 10997 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banerjee I et al. Targeted ablation of nesprin 1 and nesprin 2 from murine myocardium results in cardiomyopathy, altered nuclear morphology and inhibition of the biomechanical gene response. PLoS Genet 10, e1004114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lottersberger F, Karssemeijer RA, Dimitrova N & de Lange T 53BP1 and the LINC Complex Promote Microtubule-Dependent DSB Mobility and DNA Repair. Cell 163, 880–93 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Makhija E, Jokhun DS & Shivashankar GV Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc Natl Acad Sci U S A 113, E32–40 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keeling MC, Flores LR, Dodhy AH, Murray ER & Gavara N Actomyosin and vimentin cytoskeletal networks regulate nuclear shape, mechanics and chromatin organization. Sci Rep 7, 5219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hatch EM & Hetzer MW Nuclear envelope rupture is induced by actin-based nucleus confinement. J Cell Biol 215, 27–36 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeung T et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60, 24–34 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Swift J & Discher DE The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J Cell Sci 127, 3005–15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Staszewska I, Fischer I & Wiche G Plectin isoform 1-dependent nuclear docking of desmin networks affects myonuclear architecture and expression of mechanotransducers. Hum Mol Genet 24, 7373–89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Konieczny P et al. Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J Cell Biol 181, 667–81 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stroud MJ et al. Nesprin 1alpha2 is essential for mouse postnatal viability and nuclear positioning in skeletal muscle. J Cell Biol 216, 1915–1924 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bone CR & Starr DA Nuclear migration events throughout development. J Cell Sci 129, 1951–61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collins MA et al. Emery-Dreifuss muscular dystrophy-linked genes and centronuclear myopathy-linked genes regulate myonuclear movement by distinct mechanisms. Mol Biol Cell 28, 2303–2317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roman W et al. Myofibril contraction and crosslinking drive nuclear movement to the periphery of skeletal muscle. Nat Cell Biol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Q et al. Nesprin-1 and −2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet 16, 2816–33 (2007). [DOI] [PubMed] [Google Scholar]
- 89.Puckelwartz MJ et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet 18, 607–20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stroud MJ, Banerjee I, Veevers J & Chen J Linker of nucleoskeleton and cytoskeleton complex proteins in cardiac structure, function, and disease. Circ Res 114, 538–48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Vos WH et al. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum Mol Genet 20, 4175–86 (2011). [DOI] [PubMed] [Google Scholar]
- 92.Zuela N, Zwerger M, Levin T, Medalia O & Gruenbaum Y Impaired mechanical response of an EDMD mutation leads to motility phenotypes that are repaired by loss of prenylation. J Cell Sci 129, 1781–91 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez-Granado JM et al. Nuclear envelope lamin-A couples actin dynamics with immunological synapse architecture and T cell activation. Sci Signal 7, ra37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khatau SB et al. The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration. Sci Rep 2, 488 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang W, Antoku S, Ostlund C, Worman HJ & Gundersen GG Linker of nucleoskeleton and cytoskeleton (LINC) complex-mediated actin-dependent nuclear positioning orients centrosomes in migrating myoblasts. Nucleus 6, 77–88 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.King SJ et al. Nesprin-1 and nesprin-2 regulate endothelial cell shape and migration. Cytoskeleton (Hoboken) 71, 423–34 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Horn HF et al. The LINC complex is essential for hearing. J Clin Invest 123, 740–50 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Potter C et al. Multiple Isoforms of Nesprin1 Are Integral Components of Ciliary Rootlets. Curr Biol 27, 2014–2022 e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stewart RM et al. Nuclear-cytoskeletal linkages facilitate cross talk between the nucleus and intercellular adhesions. J Cell Biol 209, 403–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X et al. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 64, 173–87 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khatau SB et al. A perinuclear actin cap regulates nuclear shape. Proc Natl Acad Sci U S A 106, 19017–22 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE & Mauck RL Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells. Biophys J 108, 2783–93 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramdas NM & Shivashankar GV Cytoskeletal control of nuclear morphology and chromatin organization. J Mol Biol 427, 695–706 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Geyer PK, Vitalini MW & Wallrath LL Nuclear organization: taking a position on gene expression. Curr Opin Cell Biol 23, 354–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen H et al. Functional organization of the human 4D Nucleome. Proc Natl Acad Sci U S A 112, 8002–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Misteli T Beyond the sequence: cellular organization of genome function. Cell 128, 787–800 (2007). [DOI] [PubMed] [Google Scholar]
- 107.Sexton T, Schober H, Fraser P & Gasser SM Gene regulation through nuclear organization. Nat Struct Mol Biol 14, 1049–55 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Zullo JM et al. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell 149, 1474–87 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Wang Y, Nagarajan M, Uhler C & Shivashankar GV Orientation and repositioning of chromosomes correlate with cell geometry-dependent gene expression. Mol Biol Cell 28, 1997–2009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spagnol ST & Dahl KN Spatially Resolved Quantification of Chromatin Condensation through Differential Local Rheology in Cell Nuclei Fluorescence Lifetime Imaging. PLoS One 11, e0146244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Booth-Gauthier EA, Alcoser TA, Yang G & Dahl KN Force-induced changes in subnuclear movement and rheology. Biophys J 103, 2423–31 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poh YC et al. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat Commun 3, 866 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spagnol ST, Armiger TJ & Dahl KN Mechanobiology of Chromatin and the Nuclear Interior. Cell Mol Bioeng 9, 268–276 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chalut KJ et al. Chromatin decondensation and nuclear softening accompany Nanog downregulation in embryonic stem cells. Biophys J 103, 2060–70 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dahl KN, Engler AJ, Pajerowski JD & Discher DE Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J 89, 2855–64 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stephens AD, Banigan EJ, Adam SA, Goldman RD & Marko JF Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol Biol Cell 28, 1984–1996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stephens AD et al. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Mol Biol Cell (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cui Y & Bustamante C Pulling a single chromatin fiber reveals the forces that maintain its higher-order structure. Proc Natl Acad Sci U S A 97, 127–32 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bennett RR et al. Elastic-Fluid Model for DNA Damage and Mutation from Nuclear Fluid Segregation Due to Cell Migration. Biophys J 112, 2271–2279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Irianto J et al. DNA Damage Follows Repair Factor Depletion and Portends Genome Variation in Cancer Cells after Pore Migration. Curr Biol 27, 210–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buxboim A et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol 24, 1909–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tifft KE, Bradbury KA & Wilson KL Tyrosine phosphorylation of nuclear-membrane protein emerin by Src, Abl and other kinases. J Cell Sci 122, 3780–90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Enyedi B, Jelcic M & Niethammer P The Cell Nucleus Serves as a Mechanotransducer of Tissue Damage-Induced Inflammation. Cell 165, 1160–70 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Enyedi B & Niethammer P Nuclear membrane stretch and its role in mechanotransduction. Nucleus 8, 156–161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heald R & Cohen-Fix O Morphology and function of membrane-bound organelles. Curr Opin Cell Biol 26, 79–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shibata Y et al. Mechanisms determining the morphology of the peripheral ER. Cell 143, 774–88 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Isermann P & Lammerding J Consequences of a tight squeeze: Nuclear envelope rupture and repair. Nucleus 8, 268–274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Denais CM et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raab M et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–62 (2016). [DOI] [PubMed] [Google Scholar]
- 130.Takaki T et al. Actomyosin drives cancer cell nuclear dysmorphia and threatens genome stability. Nat Commun 8, 16013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lammerding J & Wolf K Nuclear envelope rupture: Actin fibers are putting the squeeze on the nucleus. J Cell Biol 215, 5–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Le Berre M, Aubertin J & Piel M Fine control of nuclear confinement identifies a threshold deformation leading to lamina rupture and induction of specific genes. Integr Biol (Camb) 4, 1406–14 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Sun L, Wu J, Du F, Chen X & Chen ZJ Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gluck S et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 19, 1061–1070 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harding SM et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mackenzie KJ et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dou Z et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550, 402–406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chug H, Trakhanov S, Hulsmann BB, Pleiner T & Gorlich D Crystal structure of the metazoan Nup62*Nup58*Nup54 nucleoporin complex. Science 350, 106–10 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Stuwe T et al. Architecture of the fungal nuclear pore inner ring complex. Science 350, 56–64 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Solmaz SR, Blobel G & Melcak I Ring cycle for dilating and constricting the nuclear pore. Proc Natl Acad Sci U S A 110, 5858–63 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu Q et al. Functional association of Sun1 with nuclear pore complexes. J Cell Biol 178, 785–98 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jahed Z, Soheilypour M, Peyro M & Mofrad MR The LINC and NPC relationship - it’s complicated! J Cell Sci 129, 3219–29 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Fahrenkrog B & Aebi U The nuclear pore complex: nucleocytoplasmic transport and beyond. Nat Rev Mol Cell Biol 4, 757–66 (2003). [DOI] [PubMed] [Google Scholar]
- 144.Dupont S et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–83 (2011). [DOI] [PubMed] [Google Scholar]
- 145.Dorner D, Gotzmann J & Foisner R Nucleoplasmic lamins and their interaction partners, LAP2alpha, Rb, and BAF, in transcriptional regulation. FEBS J 274, 1362–73 (2007). [DOI] [PubMed] [Google Scholar]
- 146.Markiewicz E, Dechat T, Foisner R, Quinlan RA & Hutchison CJ Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell 13, 4401–13 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ma H et al. Multicolor CRISPR labeling of chromosomal loci in human cells. Proc Natl Acad Sci U S A 112, 3002–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shao S et al. Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res 44, e86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Donnert G et al. Macromolecular-scale resolution in biological fluorescence microscopy. Proc Natl Acad Sci U S A 103, 11440–5 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Belton JM et al. Hi-C: a comprehensive technique to capture the conformation of genomes. Methods 58, 268–76 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nagano T et al. Single-cell Hi-C reveals cell-to-cell variability in chromosome structure. Nature 502, 59–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Buenrostro JD, Wu B, Chang HY & Greenleaf WJ ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol 109, 21 29 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Neelam S et al. Direct force probe reveals the mechanics of nuclear homeostasis in the mammalian cell. Proc Natl Acad Sci U S A 112, 5720–5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krause M, Te Riet J & Wolf K Probing the compressibility of tumor cell nuclei by combined atomic force-confocal microscopy. Phys Biol 10, 065002 (2013). [DOI] [PubMed] [Google Scholar]
- 155.Arsenovic PT, Bathula K & Conway DE A Protocol for Using Forster Resonance Energy Transfer (FRET)-force Biosensors to Measure Mechanical Forces across the Nuclear LINC Complex. J Vis Exp (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grashoff C et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meng F, Suchyna TM, Lazakovitch E, Gronostajski RM & Sachs F Real Time FRET Based Detection of Mechanical Stress in Cytoskeletal and Extracellular Matrix Proteins. Cell Mol Bioeng 4, 148–159 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fedorchak G & Lammerding J Cell Microharpooning to Study Nucleo-Cytoskeletal Coupling. Methods Mol Biol 1411, 241–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lombardi ML, Zwerger M & Lammerding J Biophysical assays to probe the mechanical properties of the interphase cell nucleus: substrate strain application and microneedle manipulation. J Vis Exp (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Britton S, Coates J & Jackson SP A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J Cell Biol 202, 579–95 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bianchini P, Cardarelli F, Di Luca M, Diaspro A & Bizzarri R Nanoscale protein diffusion by STED-based pair correlation analysis. PLoS One 9, e99619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Failla AV, Spoeri U, Albrecht B, Kroll A & Cremer C Nanosizing of fluorescent objects by spatially modulated illumination microscopy. Appl Opt 41, 7275–83 (2002). [DOI] [PubMed] [Google Scholar]
- 163.Hildenbrand G et al. Nano-sizing of specific gene domains in intact human cell nuclei by spatially modulated illumination light microscopy. Biophys J 88, 4312–8 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reymann J et al. High-precision structural analysis of subnuclear complexes in fixed and live cells via spatially modulated illumination (SMI) microscopy. Chromosome Res 16, 367–82 (2008). [DOI] [PubMed] [Google Scholar]
- 165.Cremer C, Szczurek A, Schock F, Gourram A & Birk U Super-resolution microscopy approaches to nuclear nanostructure imaging. Methods 123, 11–32 (2017). [DOI] [PubMed] [Google Scholar]
- 166.Lesterlin C, Ball G, Schermelleh L & Sherratt DJ RecA bundles mediate homology pairing between distant sisters during DNA break repair. Nature 506, 249–53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schermelleh L et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320, 1332–6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Henriques R, Griffiths C, Hesper Rego E & Mhlanga MM PALM and STORM: unlocking live-cell super-resolution. Biopolymers 95, 322–31 (2011). [DOI] [PubMed] [Google Scholar]
- 169.Boettiger AN et al. Super-resolution imaging reveals distinct chromatin folding for different epigenetic states. Nature 529, 418–22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ricci MA, Manzo C, Garcia-Parajo MF, Lakadamyali M & Cosma MP Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. Cell 160, 1145–58 (2015). [DOI] [PubMed] [Google Scholar]


