Abstract
Background:
Central nervous system (CNS) metastases represent a significant source of morbidity and mortality for patients with ALK-positive non-small cell lung cancer (NSCLC). Alectinib has demonstrated robust CNS activity in both crizotinib-naïve and crizotinib-resistant settings. However, the CNS efficacy of alectinib has not been established in patients with untreated symptomatic, large CNS metastases.
Methods:
In this retrospective study, patients were eligible if they had advanced ALK-positive NSCLC with large (defined as ≥1 cm) or symptomatic CNS metastases and received alectinib. Medical records and radiographic imaging were reviewed to determine treatment outcomes. CNS efficacy was assessed per the modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Results:
Of 19 patients, 15 (79%) had measurable CNS disease at baseline and were evaluable for response. CNS objective response rate (CORR) in these patients was 73.3% (95% CI, 44.9% to 92.2%), CNS disease control rate (CDCR) was 100.0% (95% CI, 78.2% to 100.0%), and median CNS duration of response (CDOR) was 19.3 months (95% CI, 14.3 months to not evaluable). In 18 evaluable patients with measurable and/or nonmeasurable baseline CNS disease, CORR was 72.2% (95% CI, 46.5% to 90.3%), CDCR was 100.0% (95% CI, 81.5% to 100.0%), and median CDOR was 17.1 months (95% CI, 14.3 to not evaluable). All eight patients with symptoms attributable to CNS metastases had clinical improvement upon starting alectinib. Six patients (32%) eventually required salvage brain radiotherapy.
Conclusions:
Alectinib demonstrated meaningful CNS efficacy in ALK-positive NSCLC patients with untreated, symptomatic or large brain metastases.
Keywords: alectinib, ALK, non-small cell lung cancer, central nervous system, brain metastases
INTRODUCTION
Clinical outcomes for patients with advanced anaplastic lymphoma kinase (ALK) fusion-positive non-small cell lung cancer (NSCLC) have improved considerably with the development of ALK-targeted tyrosine kinase inhibitors (TKIs). However, central nervous system (CNS) metastases remain a substantial cause of morbidity and mortality. Up to 30–40% of patients with advanced ALK-positive NSCLC present with brain metastases at initial diagnosis.1–3 Across multiple trials of second-generation ALK-TKIs, roughly 60% of crizotinib-resistant patients have CNS metastases.4–8 As patients live longer with sequential TKI therapies, their cumulative risk of acquiring CNS disease increases further—to over 70% at 5 years after the initial diagnosis— 3 highlighting the need to establish optimal CNS treatment strategies.
Alectinib is a second-generation ALK-TKI with activity against the most common crizotinib-resistant mutations. Preclinical data support the ability of alectinib, which is not a P-glycoprotein substrate, to penetrate the blood-brain barrier. 9 In clinic, alectinib has demonstrated potent activity in patients with advanced ALK-positive NSCLC.1, 5–7 In a pooled analysis of two phase II studies, alectinib demonstrated robust CNS activity, with a CNS objective response rate (CORR) of 64%, CNS disease control rate (CDCR) of 90%, and median CNS duration of response (CDOR) of 10.8 months among crizotinib-resistant patients with baseline measurable CNS disease.10 Similarly robust intracranial activity was reported in the randomized phase III ALEX trial, which demonstrated superior efficacy of alectinib versus crizotinib as first-line therapy in advanced ALK-positive NSCLC.1
While the CNS activity of alectinib is firmly established, it is notable that all clinical trials of alectinib excluded patients with symptomatic or unstable CNS metastases. As a result, patients with relatively large brain metastases or leptomeningeal disease (LMD), both of which are more likely to be associated with symptoms compared to small parenchymal lesions, may not have met eligibility for clinical trials. Thus, the effectiveness of alectinib in patients with symptomatic and/or large CNS lesions is unknown. At present, standard therapy for symptomatic or large brain lesions remains surgical resection, stereotactic radiosurgery (SRS), whole brain radiotherapy (WBRT), or some combination thereof, which may delay the initiation of systemic therapy and cause short- and long-term neurotoxicities including radionecrosis and cognitive impairment.11–14
Herein, we report the CNS efficacy of alectinib in ALK-positive NSCLC, focusing on patients with baseline CNS metastases that were symptomatic or ≥1 cm in size.
MATERIALS AND METHODS
Patients and Data Collection
Patients were diagnosed with advanced ALK - positive NSCLC between 2008–2017 and treated with alectinib at Massachusetts General Hospital. Patients could have received prior ALK-TKI(s) or chemotherapy. Baseline untreated, active CNS metastases that were ≥1 cm and/or symptomatic were required. The 1 cm cut-off was selected as clinicians may use this as a threshold to determine whether to proceed with CNS-RT.12 Prior CNS-RT was allowed if the above criteria were met, with unequivocal tumor growth post-radiation. Data were extracted on clinicopathologic features under an institutional review board-approved protocol. The data cut-off date was January 20, 2018.
Assessments
Tumor responses were determined using the modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.15 Up to five intracranial (≥5 mm) and up to five extracranial target lesions were included. CNS disease assessment could be done only by magnetic resonance imaging (MRI). Disease assessment scans were obtained at baseline, between weeks 6–8, then every 2–4 months until treatment discontinuation (unless indicated otherwise), and retrospectively reviewed by radiologists. Response confirmation occurred at least 4 weeks after the initial response.
Statistical Analyses
CDCR was defined as the percentage of patients who had a best CNS response of complete response (CR), partial response (PR), or stable disease (SD). CORR and CDCR were estimated with a 95% confidence interval (CI) based on the exact binomial distribution. CDOR was defined as the time from the first CNS response until CNS progression. Time to CNS progression was calculated from the alectinib start date until CNS progression, and the cumulative incidence of CNS progression was estimated with the end of alectinib as a competing risk. Overall time to progression (TTP) was calculated from the alectinib start date until the earliest date of CNS or extracranial progression. Patients continuing on alectinib without progression at data cut-off were censored on the last follow-up date on alectinib for CDOR, time to CNS progression, TTP and duration of alectinib. TTP, CDOR, and duration of treatment were estimated using the Kaplan-Meier method with 95% CIs calculated using the log-log transformation. Analysis was performed using SAS 9.4 (SAS Institute Inc, NC).
RESULTS
Patient Characteristics
Nineteen patients were eligible for this study (Table 1, Supplementary Table 1). Two patients (11%) received alectinib as first-line therapy. Seventeen (89%) had received prior ALK-TKIs: 10 (53%), crizotinib; 5 (26%), crizotinib and ceritinib; 1 (5%), crizotinib and brigatinib; 1 (5%), crizotinib, ceritinib, and brigatinib (Supplementary Table 2). Fifteen patients switched from the preceding TKI because of disease progression (4 extracranial, 10 CNS only, 1 both CNS and extracranial), and two due to intolerable toxicities. Eight (42%) had received prior CNS-RT (Table 1). However, all had active untreated CNS metastases at the time of starting alectinib. The median duration between prior RT and alectinib start was 16.5 months (range, 6.0–31.5 months).
Table 1.
Baseline characteristics of patients enrolled in the study.
| Baseline characteristic | N=19, n (%) |
|---|---|
| Age, median in years (range) | 42 (19–69) |
| Sex | |
| Male | 8(42) |
| Female | 11(58) |
| Race | |
| White | 13(68) |
| Asian | 4(21) |
| Other | 2(11) |
| Smoking history | |
| Never | 14(74) |
| Light (≤ 10 pack-years) | 4(21) |
| Heavy (>10 pack-years) | 1(5) |
| Histology | |
| Adenocarcinoma | 19(100) |
| Stage at diagnosis# | |
| III | 7(37) |
| IV | 12(63) |
| Alectinib line of therapy | |
| First | 2(11) |
| Second | 8(42) |
| Third | 5(26) |
| Fourth or greater | 4(21) |
| Symptomatic CNS metastases | |
| No | 11(58) |
| Yes | 8(42) |
| Size of largest brain metastasis | |
| < 1 cm | 3(16) |
| 1 cm to < 2 cm | 10(53) |
| ≥ 2 cm | 6(32) |
| Leptomeningeal disease | |
| No | 15(79) |
| Yes | 4(21) |
| Radiation therapy | |
| No prior brain radiation | 11(58) |
| Prior brain radiation | 8(42) |
| Type of brain RT | |
| Prior WBRT | 4(21) |
| Prior brain SRS | 3(16) |
| Prior WBRT and SRS | 1 (5) |
| RT to alectinib interval | |
| Within 4 weeks | 0 |
| > 4 weeks to ≤6 months | 1(5) |
| > 6 months | 7(37) |
Staging per the American Joint Committee on Cancer 7th edition.
Abbreviations: CNS, central nervous system; RT, radiation therapy; WBRT, whole brain radiotherapy; SRS, stereotactic radiosurgery
Eight patients (42%) had baseline symptoms attributable to the CNS disease, the most common being headache (n=4). CNS disease-related symptoms in the remaining four of 8 patients included: focal seizures (n=2), ataxia and incontinence (n=1), and lower extremity numbness/tingling and weakness (n=1) (Supplementary Table 1). Five (26%) required the initiation of steroids pre-alectinib. The median size of the largest brain metastasis was 1.3 cm (range, <1 mm to 4.9 cm). Five patients (26%) had edema surrounding brain metastases, and four (21%) had LMD. All patients started alectinib at 600 mg twice daily. Four required dose reductions (450 mg twice daily, n=3; 300 mg twice daily, n=1) because of toxicities.
Intracranial Efficacy
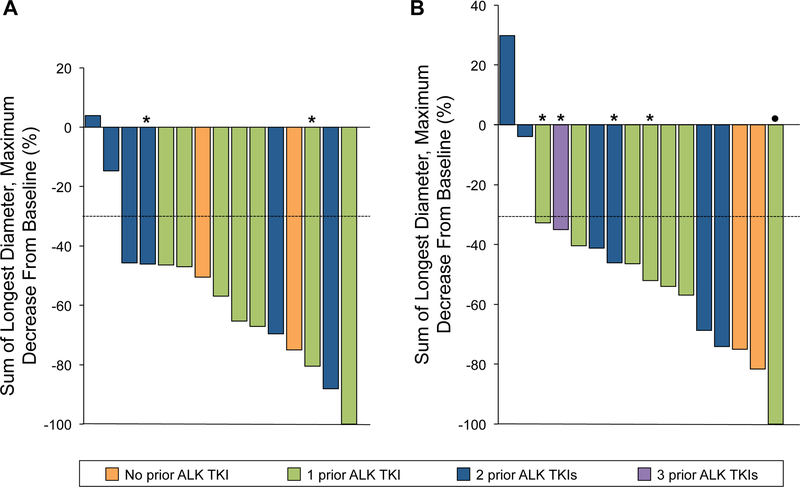
Sixteen patients (84%) had measurable CNS disease at baseline, of whom 15 were evaluable for response. In this group, 11 had CNS responses with one CR (6.7%) and 10 PRs (66.7%) observed, resulting in a CORR of 73.3% (95% CI, 44.9% to 92.2%) (Figure 1A). The median time to CNS response was 1.7 months (range, 1.5 to 12.6 months). The CDCR was 100.0% (95% CI, 78.2% to 100.0%), and median CDOR was 19.3 months (95% CI, 14.3 months to not evaluable) after 64% of events (Table 2).
Figure 1.
Tumor responses to alectinib. (A) Maximal percent change in target intracranial lesions from baseline in patients with measurable CNS disease. (B) Maximal percent change in target lesions from baseline in patients with measurable overall disease. Patients unevaluable for response or with nonmeasurable disease are not shown. Dotted horizontal line shows the 30% threshold for partial response (PR). Asterisk indicates stable disease. Dot indicates PR.
Table 2.
CNS efficacy of alectinib in patients with symptomatic or large CNS metastases at baseline.
| Response | Measurable baseline CNS disease (N=15)* | Measurable and/or nonmeasurable baseline CNS disease (N=18)* |
|---|---|---|
| CNS response rate | ||
| Responders, No. | 11 | 13 |
| CORR, % (95% CI) | 73.3 (44.9 to 92.2) | 72.2 (46.5 to 90.3) |
| Best overall response, No. (%) | ||
| Complete response | 1 (6.7) | 3 (16.7) |
| Partial response | 10 (66.7) | 10 (55.6) |
| Stable disease | 4 (26.7) | 5 (27.8) |
| Progressive disease | 0 | 0 |
| CNS disease control rate (DCR) | ||
| No. | 15 | 18 |
| CDCR, % (95% CI) | 100.0 (78.2 to 100.0) | 100.0 (81.5 to 100.0) |
| Median CDOR, months (95% CI) | 19. 3 (14.3 to not evaluable) | 17.1 (14.3 to not evaluable) |
| Patients included in analysis, No. (%) | 11 (100.0) | 13 (100.0) |
| Patients with event, No. (%) | 7 (63.6) | 8 (61.5) |
| Cumulative incidence rate for CNS PD, % (95% CI) | ||
| Patients included in analysis, No. | 16 | 19 |
| 6 months | 12.5 (1.9–33.7) | 15.8 (3.7–35.6) |
| 12 months | 12.5 (1.9–33.7) | 21.1 (6.2–41.7) |
| 18 months | 39.4 (15.1–63.2) | 45.3 (20.6–61.2) |
| 24 months | 66.9 (34.3–86.0) | 70.5 (39.6–87.7) |
One patient with baseline measurable CNS disease could not be evaluated for CNS disease response due to missing scans.
Abbreviations: CNS, central nervous system; CORR, CNS objective response rate; CDOR, CNS duration of response; PD, progressive disease; 95% CI, 95% confidence interval.
In 18 evaluable patients with measurable and/or nonmeasurable CNS disease at baseline, the CORR was 72.2% (95% CI, 46.5% to 90.3%) with 3 CRs (16.7%) and 10 PRs (55.6%), and the median time to response was 1.8 months (range, 1.5 to 12.6 months). The CDCR was 100.0% (95% CI, 81.5% to 100.0%). After 62% of events, the median CDOR was 17.1 months (95% CI, 14.3 to not evaluable) (Table 2).
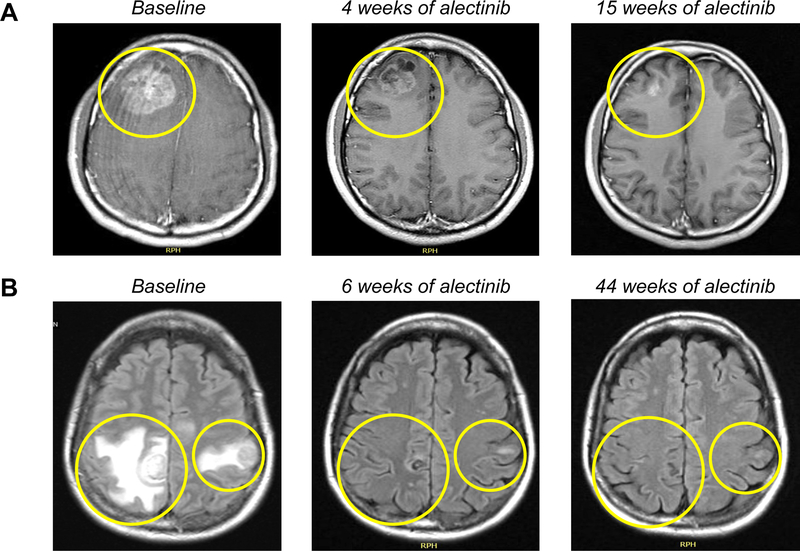
All eight patients (100%) with symptoms attributable to CNS metastases experienced clinical improvement after starting alectinib. This included four patients with symptomatic LMD (patients 2, 17, 19, and 20 in Supplementary Table 1), of whom two had CRs, one had PR, and one had SD as the best CNS response. All five patients who required steroids prior to alectinib were able to taper off steroids once they began alectinib, without recrudescence of neurologic symptoms. Figure 2 demonstrates representative intracranial responses to alectinib in two patients with symptomatic brain metastases.
Figure 2.
Representative intracranial responses to alectinib in patients. (A) Axial post-contrast T1-weighted MRI images of response in a patient (patient 1, Supplementary Table 1) who previously received crizotinib and ceritinib. Brain imaging pursued for headaches revealed new metastatic lesions (largest, 4.9 cm with edema and midline shift). The patient declined neurosurgical intervention. He began steroids and alectinib 600 mg twice daily, resulting in complete resolution of headaches and a marked radiographic response (70% intracranial and 67% extracranial tumor reduction as the best response). Steroids were discontinued within three weeks without recurrent headaches. He received alectinib for over two years with CDOR of 17 months. (B) Axial FLAIR MRI images of response to alectinib in a previously untreated patient (patient 6, Supplementary Table 1) with symptomatic large brain metastases. This patient started steroids and first-line alectinib with clinical and radiographic response (75% intracranial tumor reduction, and extracranial CR). Steroids were completely tapered within two months of alectinib. At data cutoff, she remains on alectinib beyond 14 months with an ongoing response.
Overall Efficacy
Seventeen of 19 patients were evaluable for overall (intracranial and extracranial) tumor response. The confirmed overall ORR was 62.5% (95% CI, 38.6% to 81.5%) in the patients with measurable disease, with 10 of 16 patients having PRs (Figure 1B). The overall DCR was 93.8% (15/16; 95% CI, 69.8% to 99.8%). Additionally, one patient had nonmeasurable disease at baseline, with non-CR/non-PD as best response.
Fifteen patients (79%) ultimately had disease progression (10 CNS only; 3 extracranial only; 2 both CNS and extracranial) on alectinib. The median time to CNS progression was 18.6 months (95% CI, 15.9 months to not evaluable) (Supplementary Figure 1A). The 6-month and 12-month intracranial progression rates were 15.8% (95% CI, 3.7% to 35.6%) and 21.1% (95% CI, 6.2% to 41.7%), respectively. The median overall TTP was 16.2 months (95% CI, 5.2 to 20.7 months) (Supplementary Figure 1B).
Subsequent Therapies
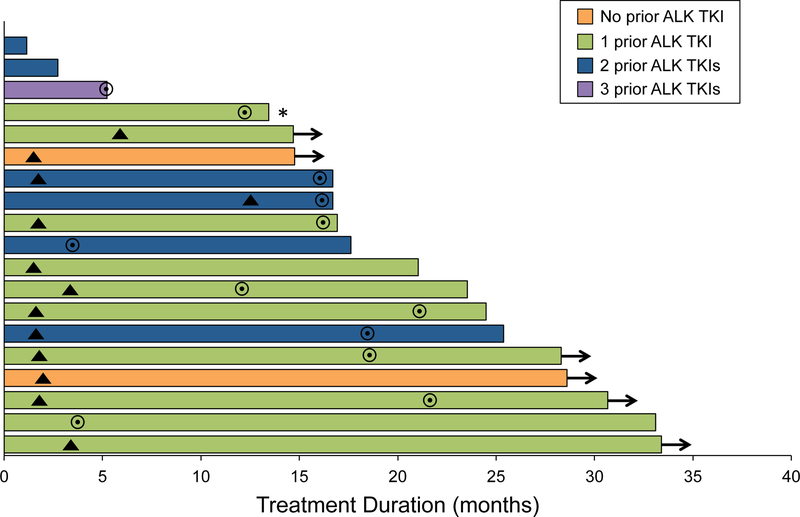
The median duration of alectinib was 21.0 months (95% CI, 16.7 to 33.1 months) (Figure 3). Six patients (32%) required salvage brain SRS (5 while on alectinib, 1 following alectinib discontinuation). The median interval between the start of alectinib and post-alectinib SRS was 15.8 months (range, 3.9 to 34.2 months).
Figure 3.
Duration of treatment. Arrows indicate patients continuing on alectinib at data cutoff. Triangles indicate the time points at which a CNS response was first observed. Circles represent the time points of CNS disease progression. Asterisk indicates a patient whose disease was unevaluable for CNS response.
Among 15 patients who had disease progression on alectinib, two (13%) required SRS only for CNS disease and were continuing on alectinib at data cutoff. The remaining patients received the following subsequent therapies: dose intensification of alectinib (6/15, 40%), lorlatinib (4/15, 27%), brigatinib (2/15, 13%), and nivolumab (1/15, 7%). Among the six patients who received higher dosing of alectinib (900 mg twice daily, n=5; 750 mg twice daily, n=1),16 five had CNS-only and one had both intracranial and extracranial progression on the standard dosing. This strategy allowed all six patients to stay on alectinib for a median duration of 7.6 additional months (2.3 to 9.9+ months), with three continuing on the higher dose of alectinib at data cutoff. None of the patients experienced a grade 3 or higher treatment-related adverse event on the higher dosing of alectinib (according to the Common Terminology Criteria for Adverse Events version 4.03), and none required a dose reduction.
DISCUSSION
CNS metastases are a common, challenging problem for patients with advanced lung cancer. ALK-positive NSCLC in particular is associated with a high frequency of brain metastases at initial diagnosis1–3 and high cumulative lifetime risk of CNS disease.3 Although a number of next-generation, brain-penetrable ALK inhibitors such as alectinib have entered the clinic, clinical trials have generally excluded patients with symptomatic or unstable CNS disease.1, 5–7 Therefore, the role of CNS-active TKIs in this population is undefined, and evidence-based guidance is lacking on the real-world question of how to optimally manage patients with symptomatic and/or large CNS metastases. These patients may by default be directed to traditional treatments such as surgical resection or RT despite the availability of brain-penetrant TKIs, which can be a less toxic and highly efficacious alternative.
In this retrospective analysis of 19 patients with symptomatic or large (≥1 cm) CNS metastases, we observed that alectinib had robust intracranial efficacy, with CORR of 73.3% in evaluable patients with measurable baseline CNS disease. These responses were rapid and durable. All patients with baseline neurologic symptoms experienced clinical improvement upon starting alectinib. Notably, the majority (68%) did not require subsequent salvage CNS-RT. This may be attributable to durable CNS disease control achieved with alectinib, availability of other highly CNS-active agents such as lorlatinib,17 or insufficient long-term follow-up. Our findings are supported by previous preclinical data for alectinib and are highly consistent with the intracranial efficacy observed in prior trials.6, 7, 10 To the best of our knowledge, however, this is the first study to demonstrate CNS efficacy of alectinib specifically in patients with symptomatic or large CNS metastases.
These results suggest that starting with alectinib— rather than proceeding with local CNS therapy first—may be considered a feasible CNS trea tment approach even in patients with large, symptomatic CNS metastases in the appropriate clinical context. Alectinib could induce a meaningful response in these patients while allowing the prompt initiation of systemic therapy and postponing (or eliminating) the need for brain radiation, with potential sparing from the associated short- and long-term neurotoxicities.11–14 For these patients, careful multi-disciplinary evaluation at the initial presentation involving the surgical, radiation oncology, and medical oncology teams will be critical, with close clinical follow-up upon starting alectinib and early radiologic response assessment if warranted. Of note, this study did not compare outcomes in patients treated with alectinib first versus CNS-RT followed by alectinib. Thus, the optimal management sequence remains to be determined, and will need to be tailored based on the clinical context, considering factors such as patient stability, extracranial disease status, and ready access to alectinib or salvage RT.
In this era of next-generation TKIs which effectively cross the blood-brain barrier (e.g., alectinib,1, 6, 7 brigatinib,8 or lorlatinib17 in ALK-positive NSCLC; osimertinib in EGFR-mutant NSCLC18), the question of how to integrate brain radiation and TKIs with robust CNS activity applies broadly across oncogene-driven NSCLCs. Could patients start with TKI first and safely defer radiation, and if so, (1) with which TKIs, (2) in which NSCLC subsets, and (3) for patients with which CNS disease characteristics? In a recent retrospective analysis, the use of upfront EGFR-TKI was associated with shorter overall survival and intracranial progression-free survival compared to SRS followed by EGFR-TKI, or WBRT followed by EGFR-TKI, in TKI-naïveEGFR-mutant NSCLC patients with brain metastases.19 However, the majority of patients in this study received the first-generation TKI erlotinib, which has inferior CNS activity/penetration compared to next-generation TKIs such as osimertinib.18 Further studies comparing upfront CNS-active TKI with CNS-RT followed by TKI are warranted across different subsets of oncogene-driven lung cancer.
This study had several additional limitations. It was a retrospective, single-institution analysis of a small number of patients, conducted at a tertiary academic center. Patients received alectinib as varying lines of therapy, although most had received prior ALK-TKIs such as crizotinib; the CORRs were consistent with those observed in previous phase II studies in crizotinib-refractory patients.6, 7 Alectinib is now the standard first-line therapy in advanced ALK-positive NSCLC.1 Future prospective studies evaluating outcomes in newly diagnosed patients (including those with symptomatic CNS metastases) treated with upfront alectinib may help establish the optimal CNS treatment strategy for these patients.
In summary, alectinib demonstrates robust, clinically meaningful CNS activity in ALK-positive NSCLC patients with symptomatic or large CNS metastases. Further studies comparing alectinib versus CNS-RT followed by alectinib are needed. Pending such studies, all patients presenting with symptomatic or large CNS metastases should be carefully evaluated by a multidisciplinary team. In appropriate patients, clinicians could potentially consider alectinib in lieu of RT as a viable initial CNS treatment option.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by a grant from the National Cancer Institute (R01CA164273, to A.T.S.), by Be a Piece of the Solution, and by the Targeting a Cure for Lung Cancer Research Fund at MGH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JJL has received honorarium from Chugai Pharma and Boehringer-Ingelheim. JFG has served as a compensated consultant or received honoraria from Bristol-Myers Squibb, Genentech/Roche, Ariad/Takeda, Loxo, Pfizer, Incyte, Novartis, Merck, Agios, Amgen, Regeneron, Oncorus, Jounce, Array, and Clovis Oncology. ATS has served as a compensated consultant or received honoraria from Pfizer, Novartis, Genentech/Roche, Ariad/Takeda, Ignyta, LOXO, Blueprint Medicines, KSQ Therapeutics, Daiichi Sankyo, EMD Serono, Taiho Pharmaceutical, TP Therapeutics, Foundation Medicine, Natera, and Guardant, and has received research funding from Pfizer, Novartis, and Roche/Genentech. The remaining authors have no financial interests to declare.
REFERENCES
- 1.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829–838. [DOI] [PubMed] [Google Scholar]
- 2.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917–929. [DOI] [PubMed] [Google Scholar]
- 3.Gainor JF, Tseng D, Yoda S, et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol 2017;2017. [DOI] [PMC free article] [PubMed]
- 4.Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119–1128. [DOI] [PubMed] [Google Scholar]
- 6.Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661–668. [DOI] [PubMed] [Google Scholar]
- 7.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490–2498. [DOI] [PubMed] [Google Scholar]
- 9.Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014;74:1023–1028. [DOI] [PubMed] [Google Scholar]
- 10.Gadgeel SM, Shaw AT, Govindan R, et al. Pooled Analysis of CNS Response to Alectinib in Two Studies of Pretreated Patients With ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:4079–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulahannan D, Khalifa J, Faivre-Finn C, et al. Emerging treatment paradigms for brain metastasis in non-small-cell lung cancer: an overview of the current landscape and challenges ahead. Ann Oncol 2017;28:2923–2931. [DOI] [PubMed] [Google Scholar]
- 12.Khandekar MJ, Piotrowska Z, Willers H, et al. Role of Epidermal Growth Factor Receptor (EGFR) Inhibitors and Radiation in the Management of Brain Metastases from EGFR Mutant Lung Cancers. Oncologist 2018. [DOI] [PMC free article] [PubMed]
- 13.Ou SH, Weitz M, Jalas JR, et al. Alectinib induced CNS radiation necrosis in an ALK+NSCLC patient with a remote (7 years) history of brain radiation. Lung Cancer 2016;96:15–18. [DOI] [PubMed] [Google Scholar]
- 14.Ou SH, Klempner SJ, Azada MC, et al. Radiation necrosis presenting as pseudoprogression (PsP) during alectinib treatment of previously radiated brain metastases in ALK-positive NSCLC: Implications for disease assessment and management. Lung Cancer 2015;88:355–359. [DOI] [PubMed] [Google Scholar]
- 15.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:1087–1095. [DOI] [PubMed] [Google Scholar]
- 16.Gainor JF, Chi AS, Logan J, et al. Alectinib Dose Escalation Reinduces Central Nervous System Responses in Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer Relapsing on Standard Dose Alectinib. J Thorac Oncol 2016;11:256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017. [DOI] [PMC free article] [PubMed]
- 18.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 19.Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naive Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 2017;35:1070–1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.