Abstract
Background:
Childhood maltreatment is a major risk factor for psychopathology. However, some maltreated individuals appear remarkably resilient to the psychiatric effects while manifesting the same array of brain abnormalities as maltreated individuals with psychopathology. Hence, a critical aim is to identify compensatory brain alterations that enable resilient individuals to maintain mental well-being despite alterations in stress-susceptible regions.
Method:
Network models were constructed from diffusion tensor imaging and tractography in physically healthy unmedicated 18–25-year-olds (n=342, n=192 maltreated) to develop network based explanatory models.
Results:
First, we determined that susceptible and resilient individuals had the same alterations in global fiber stream network architecture using two different definitions of resilience (i.e., 1 – no lifetime history of Axis I or II disorders, 2 – no clinically significant symptoms of anxiety, depression, anger-hostility or somatization). Second, we confirmed an a priori hypothesis that right amygdala nodal efficiency was lower in asymptomatic resilient than in susceptible participants or controls. Third, we identified 8 other nodes with reduced nodal efficiency in resilient individuals and showed that nodal efficiency moderated the relationship between maltreatment and psychopathology. Fourth, we found that models based on global network architecture and nodal efficiency could delineate group membership (control, susceptible, resilient) with 75%, 82% and 80% cross-validated accuracy.
Conclusions:
Together these findings suggest that sparse fiber networks with increased small-worldness following maltreatment render individuals vulnerable to psychopathology if abnormalities occur in specific nodes, but that decreased ability of certain node to propagate information throughout the network mitigates the effects and leads to resilience.
Keywords: abuse and neglect, childhood adversity, brain network architecture, early life stress, depression, anxiety
Introduction
Maltreatment and household dysfunction is a major risk factor for psychopathology associated with 33%, 54%, 64% and 67% of the population attributable risk for anxiety disorders (1), depression (2), addiction (3) and suicide attempts (4), respectively. Abuse and neglect are also recognized as major risk factors for personality and psychotic disorders (5–22) and to hasten onset and exacerbate the course of bipolar disorder (23–32). A critical unanswered question is why some maltreated individuals develop psychopathology while others remain resilient.
We have hypothesized that maltreatment increases risk by altering trajectories of brain development. The most consistent findings are smaller midsagittal area (33–37) or decreased fractional anisotropy (38, 39) of the corpus callosum and lower hippocampal volume in adults (40–51). Maltreatment is also associated with attenuated development of the anterior cingulate (52–55), orbitofrontal (55–57) and dorsolateral prefrontal cortex (54, 58), and with enhanced amygdala response to threat (59, 60) and alterations in functional connectivity (61–64).
Maltreatment also appears to effect brain network architecture (65). We recently reported that maltreated individuals had diffusion tensor imaging (DTI) networks with reduced degree, strength and global efficiency but increased pathlength and small-worldness (66). Puetz et al (67) reported similar alteration in maltreated children.
We originally presumed that psychiatric and neurobiological susceptibility would align and that brain abnormalities would be detectable in maltreated individuals with psychopathology but not in those without psychopathology. However, that turns out not to be the case. Indeed, studies have reported a compelling set of structural and functional brain differences in maltreated individuals with no history of psychopathology (39, 52, 60, 66, 68–80). Thus, the relationship between maltreatment, brain changes and psychopathology remain unclear.
Two major possibilities arise. First, these alterations may have no relevance to psychopathology, which would call into question numerous circuit-based models. Alternatively, there may be specific neurobiological alterations that enable resilient subjects to effectively compensate for abnormalities in stress-susceptible structures. Hence, we sought to determine if psychiatrically susceptible and resilient subjects had the same overall differences in network architecture and to identify specific differences that may enable resilient individuals to maintain mental well-being.
We theorized that susceptibility and resilience could be explained by integrating the multiple ‘hit’ concept (81, 82) with network architecture. Brain network architecture needs to balance the opposing demands of integration and segregation in order to combine the presence of functionally specialized and segregated modules with a robust number of connecting links (83). This tradeoff can be seen in network properties such as small-worldness, which reflect the ratio of local clustering coefficients to overall pathlength. We found that maltreated individuals had slightly sparser networks with increased small-worldness resulting from intact local modular architecture but lower connectivity between modules (66). The weaker degree of integration between clusters in maltreated individuals renders their networks more vulnerable and puts them at increased risk for psychopathology by making it harder to effectively compensate for ‘second hit’ abnormalities that might occur within a cluster, community or node. Until both processes occur these individuals may appear resilient. True resilience occurs in maltreated individuals with both global network and specific nodal abnormalities but who are effectively compensated, which may result from partially isolating and limiting the impact of problematic nodes. Hence, we tested the hypothesis that susceptible and resilient individuals have comparable abnormalities in global network architecture but that resilient individuals have a lower degree of right amygdala centrality, as right amygdala hyperreactivity to threatening stimuli is the most consistent functional abnormality in maltreated individuals (59, 60). Additionally, we conducted exploratory analyses to identify other nodes with abnormal centrality in resilient individuals.
Methods and Materials
Subject Recruitment
The Partners Healthcare institutional review board approved this study and all subjects provided written informed consent. Recruitment followed previously reported methods (51, 65, 66) – (see supplement). The sample was enriched to increase the number of participants exposed to three or more types of maltreatment. Subjects were selected based on exposure not psychiatric history since selecting subjects for any specific disorder or for none could bias the results by including the most affected or resilient subjects.
Subject Assessments
Assessment, evaluations and Structured Clinical Interviews for DSM-IV Axis I and II psychiatric disorders (84) were conducted by mental health professionals blind to the neuroimaging results. The Maltreatment and Abuse Chronology of Exposure (MACE) scale was used to evaluate type and timing of maltreatment during childhood (85). (See S2 for additional information).
Current symptom of depression, anxiety, somatization and anger-hostility were assessed using Kellner’s Symptom Questionnaire (86), which has good psychometric properties and reliably reveals strong associations between self-reported exposure and psychopathology (39, 85, 87–89). Parental education and perceived financial sufficiency (90) were collected as these are important risk factor for maltreatment and may also have effects on trajectories of brain development.
Criteria for susceptibility or resilience
Resilience refers to the dynamic process leading to some aspect of positive adaptation (e.g., emotional well-being) in the face of significant adversity (91). We defined resilience in two ways. In both instances we designated subjects’ reporting exposure to two or more types of maltreatment as ‘at risk’, as this degree of exposure increased odds of lifetime history of MDD by 4.67-fold (95% CI 2.68–8.39, p < 10−8). One classification was made based on presence or absence of history of major Axis I or II DSM-IV disorders. The second classification was made based on presence or absence of clinically significant symptoms on Kellner’s Symptom Questionnaire. Hence, we used both lifetime categorical and current state dimensional definitions.
MRI Data Acquisition
MRI scans were acquired using 3T Siemens TIM Trio (Erlangen, Germany) using previously reported methods (66) (see S3). Briefly, multiple diffusion-weighted images were acquired in 72 directions. Scan parameters were: b=1000 sec/mm2; echo time (TE)/repetition time (TR)=81 msec/6sec; matrix=128×128 on 240mmx240mm field of view (FOV); slices 3.5mm without gap.
MRI Analysis and Network Construction
DTI scans were analyzed and unweighted networks constructed using previously published methods (66) (see S4). Scans were parcellated into 90 regions using the AAL template (92) and number of fiber streams interconnecting each of these nodal regions were calculated. Fiber streams connecting nodes were defined as edges, which were classified as 0 (no interconnecting fiber streams) or 1. Graph theory was used to calculate global network parameters including: global efficiency, degree, transitivity, vulnerability and small-worldness. These network parameters have been described in detail in previous papers (66, 93–99) (R package: igraph, brainGraph).
Statistical Procedures
Statistical Assessment of Group Differences in Global Network Architecture.
An omnibus MANOVA test was used to determine if there were significant multivariate group differences in network architecture. Individual ANCOVA analyses were performed to delineate which specific global network measures differed between the groups. Tukey post-hoc tests were used to determine significance of the two group contrasts within each of the significant main effects. Covariates included age, gender, parental education and financial sufficiency.
Statistical Assessment of Group Differences in Right Amygdala Centrality.
For these analyses we compared groups based on current symptoms as this resulted in groupings of similar size that were closely matched for maltreatment history. MANOVA was used to test the hypothesis that the asymptomatic group differed from symptomatic and unexposed controls. We focused on three a priori measures of nodal centrality: 1) nodal efficiency (Neff), which measures the ability of a node to propagate information with other nodes in the network; 2) degree centrality, which is the number of connecting nodes; and 3) closeness centrality, which is the average distance from a node to all other nodes in the network. Together, these provide a good understanding of the connectivity and influence of a particular node on the network. Age and sex were included as covariates.
Exploratory identification of additional nodes that differ between susceptible and resilient participants.
The aim was to identify other nodes that differ significantly between susceptible and resilient participants, which is complicated by the number of potential nodal comparisons. To limit this number, we first chose a priori to use Neff as the single measure of centrality. Then we used random forest regression with conditional inference trees (RFR-CIT) and elastic nets to identify a sparse set of nodes that might best predict this group difference (see S5).
Classifying susceptible, resilient and unexposed controls based on network and nodal parameters.
RFR-CIT was used in multiclass prediction model (see S6) to assess how well 5 measures of global network architecture plus Neff values could predict the correct classification of the 310 symptomatic, asymptomatic and healthy control participants using 10-fold cross-validation. This process was also repeated restricting the number of nodes to only those identified as significant in the prior analysis.
Results
The sample consisted of N=342 participants (132M/210F) 21.7 ± 2.5 years of age. Key demographic features are indicated in Supplemental Table S1. Fifty-six percent of the participants had moderate-to-high-exposure to maltreatment (mean 4.1 ± 2.1 types) and the remainder were no-to-low exposure controls (0.4 ± 0.5 types).
Resilient versus Susceptible by Lifetime Diagnosis
Clinical and demographic characteristics.
Based on these criteria there were N=143 (52M/91F) susceptible, N=49 (18M/31F) resilient and N=95 (40M/55F) unexposed controls. There were no differences between groups in gender (Χ2= 0.86, df = 2, p = .65) but a modest difference in age (Table 1). Table 1 indicates number of types and severity of maltreatment for the different groups. Multivariate analyses indicate marked overall group differences in exposure (Pillai-Barlett Trace (PBT)= 0.750, F22,540 = 14.71 p < 10−16). Susceptible subjects reported greater exposure to all types of maltreatment than controls while resilient subjects reported greater exposure to most types. Susceptible subjects reported greater overall exposure than resilient subjects (PBT = 0.217, F11,176 = 4.432, p < 10−5) and greater exposure to sexual abuse, witnessing violence to siblings, parental verbal abuse and emotional neglect.
Table 1.
Groups based on lifetime history of psychopathology meeting Axis I or II DSM-IV criteria
| Control (1) | Resilient (2) | Susceptible (3) | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | F | p | |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| 22.1±2.46 | 21.7±2.46 | 21.2±2.46 | p > .60 | p < .02 | p > .30 | 4.4 | p < .02 | |
| Parental Education (years) | ||||||||
| 16.6±3.15 | 14.7±3.15 | 14.8±3.15 | p < .01 | p < 10−4 | p > .90 | 10.6 | p < 10−4 | |
| Financial sufficiency during childhood | ||||||||
| 3.36±0.90 | 2.94±0.90 | 2.76±0.90 | p < .10 | p < 10−5 | p > .40 | 13.1 | p < 10−5 | |
| Number of Types of Maltreatment | ||||||||
| 0.58±1.62 | 2.96±1.55 | 4.36±1.59 | p < 10−11 | p < 10−12 | p < 10−5 | 147.2 | p < 10−43 | |
| Sexual Abuse | ||||||||
| 0.05±1.78 | 0.59±1.71 | 1.87±1.75 | p > 0.4 | p < 10−10 | p < 10−4 | 30.8 | p < 10−12 | |
| Parental Verbal Abuse | ||||||||
| 1.92±2.75 | 5.82±2.63 | 7.22±2.69 | p < 10−11 | p < 10−12 | p < .02 | 101.6 | p < 10−33 | |
| Parental non-verbal emotional abuse | ||||||||
| 2.02±2.04 | 4.33±1.95 | 4.98±1.99 | p < 10−7 | p < 10−12 | p > .20 | 58.3 | p < 10−21 | |
| Parental physical abuse | ||||||||
| 2.14±2.44 | 4.03±2.33 | 4.86±2.39 | p < .001 | p < 10−11 | p > .10 | 34.0 | p < 10−13 | |
| Witnessing interparental violence | ||||||||
| 0.67±2.26 | 1.34±2.16 | 1.79±2.21 | p > .30 | p < .003 | p > .40 | 6.70 | p < .01 | |
| Witnessing violence towards siblings | ||||||||
| 0.19±1.62 | 0.739±1.55 | 1.49±1.58 | p > .20 | p<10−6 | p < .02 | 17.9 | p < 10−7 | |
| Peer emotional bullying | ||||||||
| 3.0±2.96 | 6.6±2.84 | 7.47±2.9 | p < 10−8 | p < 10−12 | p > .20 | 63.6 | p < 10−22 | |
| Peer physical bullying | ||||||||
| 0.51±2.44 | 2.57±2.34 | 2.49±2.39 | p < 10−4 | p < 10−6 | p > .90 | 20.3 | p < 10−8 | |
| Emotional neglect | ||||||||
| 0.87±2.02 | 1.88±1.93 | 3.09±1.97 | p < .05 | p < 10−11 | p <.002 | 33.7 | p < 10−13 | |
| Physical neglect | ||||||||
| 0.44±1.82 | 0.933±1.74 | 1.62±1.78 | p > .40 | p < 10−4 | p <.07 | 11.7 | p < 10−4 | |
Exposure to maltreatment was controlled for age, sex, parental education and financial sufficiency.
Overall, 60%, 54%, 41%, 24%, 15% and 4% of susceptible participants had histories of major depression, one or more anxiety disorders, one or more personality disorders, ADHD, eating disorders, or bipolar disorder, respectively. By definition none of the resilient subjects or controls had a history of psychopathology.
Network Architecture.
There was a significant multivariate group effect on overall network architecture (PBT = 0.088, F10,552 = 2.545, p = .005), which was not influenced by inclusion of mean FA values (see S7). There were significant network differences between susceptible subjects and controls (PBT =0.069, F5,227 = 3.353, p = .006) and between resilient subjects and controls (PBT =0.092, F5,133 = 2.688, p = 0.02) but not between susceptible and resilient (PBT =0.023, F5,182 = 0.856, p = .51).
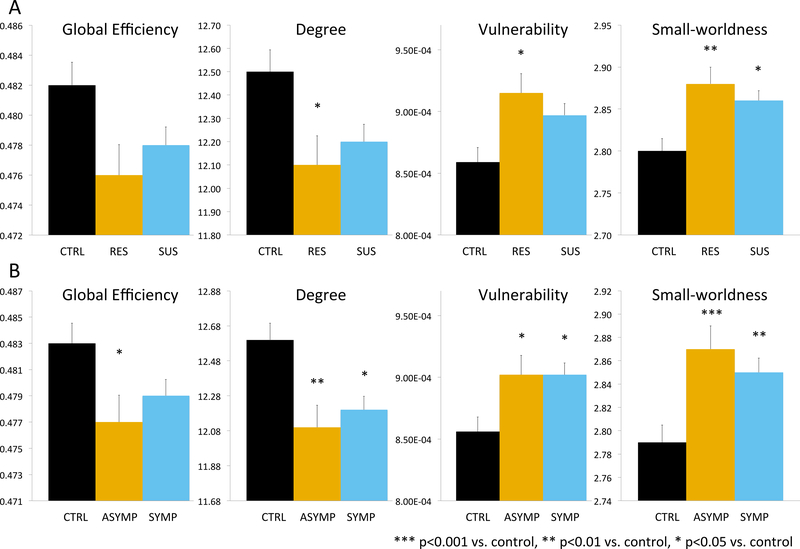
As seen in Figure 1A there were significant group differences in univariate measures of degree, vulnerability and small-worldness, with most significant comparisons between unexposed controls and resilient participants despite their lower degree of exposure.
Figure 1.
Group differences in global network architecture based on lifetime psychiatric history (A) and current clinically significant symptoms of depression, anxiety, anger-hostility or somatization.. CTRL – individuals with no history of maltreatment nor Axis-I or II DSM-IV disorders. RES – individuals with history of maltreatment but no history of psychopathology. SUS – individuals with history of maltreatment and psychopathology. ASYMP - history of maltreatment but no clinically significant symptoms. SYMP - history of maltreatment and current clinically significant symptoms.
Resilient versus Susceptible by Current Symptoms
Clinical and demographic characteristics.
To avoid confusion, we refer to the susceptible maltreated subjects with clinically significant symptoms as ‘symptomatic’ and resilient maltreated subjects without as ‘asymptomatic’. There were N=118 (52M/66F) controls without symptoms, N=86 (36M/50F) asymptomatic maltreated participants and N=106 (34M/72F) symptomatic maltreated participants. Table 2 indicates current symptoms and exposure history. There were no differences between groups in gender (Χ2= 3.68, df = 2, p = 0.159) but a modest difference in age (Table 2).
Table 2.
Groups based on current symptoms
| Control (1) | Asymptomatic (2) | Symptomatic (3) | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | F | p | |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| 22.0 ± 2.45 | 21.7±2.45 | 20.9±2.45 | p < .60 | p < 10−2 | p < .08 | 5.67 | p < 10−2 | |
| Parental Education (years) | ||||||||
| 16.6 ± 3.12 | 14.8±3.12 | 14.8±3.12 | p < 10−3 | p < 10−4 | p > .90 | 12.18 | p < 10−5 | |
| Financial sufficiency during childhood | ||||||||
| 3.38 ± 0.853 | 3.02±0.853 | 2.62±0.853 | p < .02 | p < 10−9 | p < .01 | 21.77 | p < 10−8 | |
| Number of Types of Maltreatment | ||||||||
| 0.337 ± 1.61 | 3.61±1.6 | 4.5±1.62 | p < 10−11 | p < 10−11 | p < .01 | 204.2 | p < 10−54 | |
| Depression | ||||||||
| 3.23 ± 3.75 | 4.28±3.72 | 11.8±3.78 | p < .20 | p < 10−11 | p < 10−11 | 147.8 | p < 10−42 | |
| Anxiety | ||||||||
| 4.31 ± 3.43 | 5.55±3.41 | 12.7±3.46 | p > .10 | p < 10−11 | p < 10−11 | 168.8 | p < 10−46 | |
| Somatization | ||||||||
| 3.77 ± 3.84 | 4.97±3.81 | 10.9±3.86 | p > .10 | p < 10−11 | p < 10−11 | 96.1 | p < 10−30 | |
| Anger-hostility | ||||||||
| 3.57 ± 3.49 | 4.37±3.46 | 10.2±3.51 | p > .40 | p < 10−11 | p < 10−11 | 102.3 | p < 10−32 | |
| Sexual Abuse | ||||||||
| 0.1 ± 1.69 | 1.21±1.68 | 1.78±1.7 | p < 10−4 | p < 10−10 | p > .10 | 28.0 | p < 10−10 | |
| Parental Verbal Abuse | ||||||||
| 1.43 ± 2.65 | 6.49±2.63 | 7.42±2.66 | p < 10−11 | p < 10−11 | p > .10 | 162.6 | p < 10−46 | |
| Parental non-verbal emotional abuse | ||||||||
| 1.66 ± 2.00 | 4.57±1.98 | 5.25±2.01 | p < 10−11 | p < 10−11 | p > .10 | 99.8 | p < 10−32 | |
| Parental physical abuse | ||||||||
| 1.92 ± 2.43 | 4.74±2.41 | 4.9±2.44 | p < 10−11 | p < 10−11 | p > .90 | 51.9 | p < 10−18 | |
| Witnessing interparental violence | ||||||||
| 0.232 ± 2.20 | 1.64±2.18 | 2±2.21 | p < 10−4 | p < 10−6 | p > .70 | 19.8 | p < 10−7 | |
| Witnessing violence towards siblings | ||||||||
| 0.0293 ± 1.55 | 1.14±1.54 | 1.59±1.56 | p < 10−5 | p < 10−10 | p > .20 | 29.3 | p < 10−11 | |
| Peer emotional bullying | ||||||||
| 3.33±2.84 | 6.66±2.82 | 7.59±2.86 | p < 10−11 | p < 10−11 | p > .10 | 68.2 | p < 10−23 | |
| Peer physical bullying | ||||||||
| 0.539±2.22 | 2.39±2.21 | 2.57±2.24 | p < 10−7 | p < 10−8 | p > .90 | 28.0 | p < 10−10 | |
| Emotional neglect | ||||||||
| 0.697±1.98 | 2.45±1.96 | 3.18±1.99 | p < 10−7 | p < 10−11 | p > .10 | 45.6 | p < 10−16 | |
| Physical neglect | ||||||||
| 0.191±1.77 | 1.37±1.76 | 1.7±1.78 | p < 10−4 | p < 10−7 | p> .60 | 21.9 | p < 10−8 |
Exposure to maltreatment was controlled for age, sex, parental education and financial sufficiency.
There were marked group differences in degree of exposure to maltreatment (PBT = 0.716, F22,584 = 14.801 p < 10−16). Both symptomatic and asymptomatic groups reported greater exposure to all types of maltreatment than controls. There was only a minor multivariate difference in exposure to maltreatment in symptomatic versus asymptomatic subjects (PBT = 0.113, F11,176 = 2.045, p = 0.03) with no significant univariate differences in exposure to any of the 10 types of maltreatment. On the other hand, there were marked differences between symptomatic and asymptomatic subjects in symptom scores, but no differences between asymptomatic participants and controls on any of the symptom scales (Table 2). Asymptomatic participants also did not differ from controls in lifetime prevalence of Axis I or II disorders associated with increased aggression.
Network Architecture
There was a significant multivariate group effect on network architecture (PBT = 0.083, F10,596 = 2.594, p = .004). There were significant differences between symptomatic subjects and controls (PBT = 0.061, F5,212 = 2.762, p < 0.02) and between asymptomatic participants and controls (PBT = 0.093, F5,192 = 3.955, p < 0.002) but not between symptomatic and asymptomatic (PBT =0.023, F5,182 = 0.856, p > .5). As seen in Figure 1B there were group differences in univariate measures of global efficiency, degree, vulnerability and small-worldness.
Differences in Amygdala Centrality Between Symptomatic and Asymptomatic Maltreated Participants
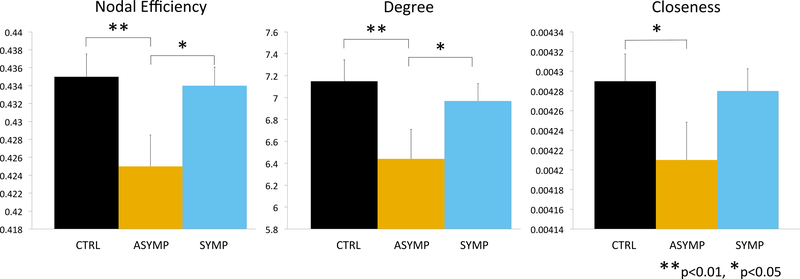
Multivariate connectivity measures were significantly lower in asymptomatic than symptomatic participants on the right side (PBT = 0.045, F3,186 = 2.94, p = .035). Univariate comparisons indicated that these groups differed in measures of Neff (F1,188 = 5.52, p < .02) and degree centrality (F1,188 = 3.94, p < .05) but not closeness (F1,188 = 3.03, p = .083) (Figure 2). Asymptomatic subjects also showed multivariate differences from controls in right amygdala connectivity (PBT = 0.051, F3,200 = 3.57, p = .015), whereas susceptible subjects did not differ from controls (PBT = 0.003, F3,220 = 0.20, p = .90). Asymptomatic participants had significantly lower Neff (F1,200 = 7.84, p < .006), degree centrality (F1,200 = 7.83, p < .006) and closeness (F1,200 = 4.66, p < 0.04) than controls. There was no significant main or interactive effect of sex on right amygdala Neff.
Figure 2.
Group differences in centrality of right amygdala based on history of maltreatment and current clinically significant symptoms of depression, anxiety, anger-hostility or somatization.
Exploratory Differences in Centrality Between Symptomatic and Asymptomatic Maltreated Participants
Elastic net provided a simple 5-node model that included right amygdala followed by left frontal inferior pars triangularis, right middle cingulum, right paracentral lobule and left supplemental motor area (Table S2). RFR-CIT provided a 9-node model that independently included all the nodes identified in the elastic net plus left and right olfactory cortex and left and right postcentral gyrus.
As seen in Table 3 the same basic pattern held with lower Neff values for resilient subjects than for susceptible or controls for all identified nodes, though not all of these differences were statistically significant. Controls and susceptible participants did not differ on any of these Neff measures. There were no main or interactive effects of sex on Neff in any of these regions.
Table 3.
Group differences in nodal efficiency of nodes identified by random forest regression.
| Control (1) | Resilient (2) | Susceptible (3) | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | F | p | |
|---|---|---|---|---|---|---|---|---|
| Olfactory (R): MNI(x, y, z)=(10,16,−11) | ||||||||
| 0.491±0.046 | 0.472±0.046 | 0.483±0.046 | p < .01 | p > .10 | p > .10 | 4.64 | p < .02 | |
| Amygdala (R): MNI(x, y, z)=(27,1,−18) | ||||||||
| 0.434±0.025 | 0.425±0.025 | 0.435±0.025 | p < .02 | p > .80 | p < .01 | 4.33 | p < .02 | |
| Frontal Inferior Triangularis (L): MNI=(−46,30,14) | ||||||||
| 0.443±0.030 | 0.434±0.030 | 0.445±0.030 | p < .02 | p > .60 | p < .01 | 3.64 | p < .03 | |
| Postcentral Gyrus (L): MNI(x, y, z)=(−42,−23,49) | ||||||||
| 0.505±0.028 | 0.495±0.028 | 0.501±0.028 | p < .02 | p > .20 | p > .10 | 3.41 | p < .04 | |
| Olfactory (L): MNI(x, y, z)=(−8,15,−11) | ||||||||
| 0.482±0.047 | 0.467±0.047 | 0.479±0.047 | p < .03 | p > .50 | p < .08 | 2.95 | p < .06 | |
| Midcingulate Area (R): MNI(x, y, z)=(8,−9,40) | ||||||||
| 0.513±0.032 | 0.504±0.032 | 0.514±0.032 | p < .06 | p > .80 | p < .02 | 2.74 | p < .07 | |
| Paracentral Lobule (R) : MNI(x, y, z)=(7,−32,68) | ||||||||
| 0.424±0.017 | 0.421±0.017 | 0.426±0.017 | p > .10 | p > .30 | p < .04 | 2.34 | p < .10 | |
| Supplementary Motor Area (L): MNI(x, y, z)=(−5,5,61) | ||||||||
| 0.428±0.030 | 0.423±0.030 | 0.432±0.030 | p > .20 | p > .30 | p < .04 | 2.28 | p < .10 | |
| Postcentral Gyrus (R): MNI(x, y, z)=(41,−25,53) | ||||||||
| 0.499±0.025 | 0.495±0.025 | 0.501±0.025 | p > .30 | p > .40 | p > .10 | 1.41 | p > .20 |
Means and standard deviations adjusted to remove variance attributable to age and gender.
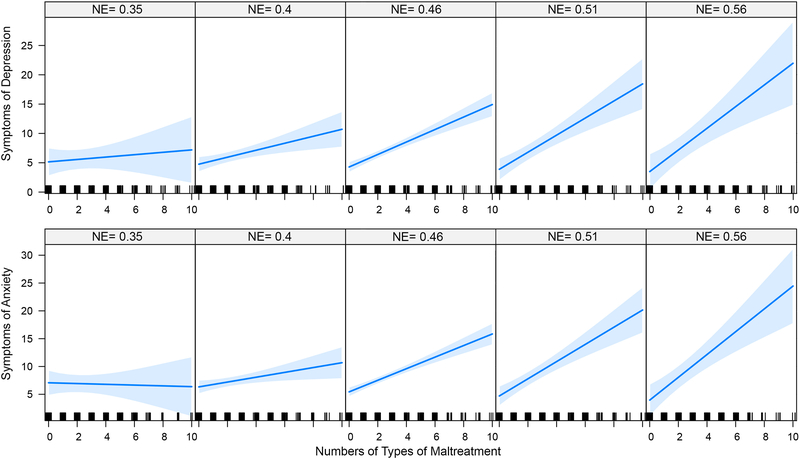
A key consideration is whether reduced Neff moderates the relationship between maltreatment and psychopathology. Hence, interactive effects of Neff for the 9 identified nodes on the association between multiplicity of maltreatment and symptom of anxiety or depression were assessed using ANCOVA. As illustrated in Figure 3 there were significant interactive effects such that a strong linear relationship between MACE multiplicity scores and depression or anxiety emerged in individuals with high but not low Neff in left frontal inferior pars triangularis. Figure 4 illustrates group differences in primary and secondary network interconnections of the pars triangularis. Similarly, there was a significant interactive effect between Neff in left olfactory area and anxiety ratings (p < .04) and left middle cingulum and depression (p = .05). These results were not corrected for multiple comparisons due to the low power of tests for moderation with continuous variables (100). Statistical associations between measures of Neff and Kellner symptom scores are discussed in S8.
Figure 3.
Moderating influence of nodal efficiency of left inferior triangularis on relationship between multiplicity of exposure to maltreatment and current symptoms of depression (F1,317 = 4.45, p < 0.04 and anxiety (F1,317 = 8.30, p = 0.004) on Kellner’s Symptom Questionnaire. The plots derive from the ANCOVA model and show the slope of the relationship between MACE score and symptom ratings with nodal efficiency fixed at values ranging from lowest to highest at equispaced intervals.
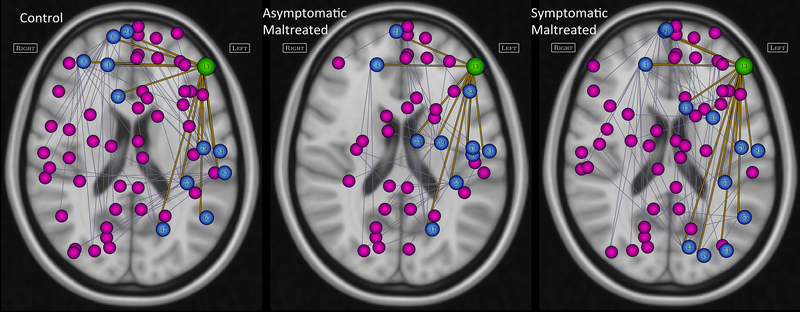
Figure 4.
Differences in primary and secondary nodal interconnections of the left inferior frontal gyrus pars triangularis in controls, asymptomatic and symptomatic maltreated groups. Pars triangularis is illustrated as this node emerged as the most significant moderator of the relationship between exposure to maltreatment (number of different types) and current symptoms of depression and anxiety, Green circle identifies the location of the pars triangularis, blue circles indicate primary interconnections, magenta circles indicate secondary interconnections, Region name corresponding to nodal numbers can be found in the automated anatomical labeling (AAL) system (92).
Multiclass Predictive Classification
A key consideration is how well can network information predict class membership. Using RFR-CIT for multiclass classification indicated that measures of global network architecture (i.e., global efficiency, small-worldness, degree, transitivity and vulnerability) and Neff of 90 nodes were able to correctly categorize controls, asymptomatic and symptomatic participants in the sample with 97.8% accuracy (p < 10−15) and kappa = 0.966. As seen in Table 4 all groups were identified in the sample with better than 97% balanced accuracy. Cross-validated predictive accuracy was also good with the model identifying the out of bag sample with overall accuracy of 71.6% (p < 0.02) and kappa 0.557. Balanced accuracy for each class ranged from 79% to 89%.
Table 4.
Multiclass classification within actual sample and within cross-validated out-of-bag samples.
| Global Measures plus 90 nodes | |||||
| Within Sample | Class: cntrls | Class: resilient | Class: susceptible | Overall Measures | |
| Sensitivity | 0.947 ± 0.009 | 0.996 ± 0.006 | 1.000 ± 0.000 | Accuracy | 0.978 ± 0.004 |
| Specificity | 0.998 ± 0.003 | 0.978 ± 0.004 | 0.993 ± 0.004 | P-value | < 10−15 |
| Balanced Accuracy | 0.973 ± 0.005 | 0.987 ± 0.003 | 0.996 ± 0.002 | Kappa | 0.966 ± 0.006 |
| Cross-Validated (OOB) | |||||
| Sensitivity | 0.601 ± 0.018 | 0.978 ± 0.016 | 0.884 ± 0.031 | Accuracy | 0.716 ± 0.019 |
| Specificity | 0.985 ± 0.009 | 0.803 ± 0.008 | 0.861 ± 0.018 | P-value | 0.018 ± 0.062 |
| Balanced Accuracy | 0.793 ± 0.011 | 0.891 ± 0.009 | 0.873 ± 0.02 | Kappa | 0.557 ± 0.03 |
| Global Measures plus 9 nodes | |||||
| Within Sample | Class: cntrls | Class: resilient | Class: susceptible | Overall Measures | |
| Sensitivity | 0.876 ± 0.012 | 0.935 ± 0.012 | 0.848 ± 0.012 | Accuracy | 0.878 ± 0.007 |
| Specificity | 0.94 ± 0.007 | 0.909 ± 0.007 | 0.976 ± 0.005 | P-value | < 10−15 |
| Balanced Accuracy | 0.908 ± 0.007 | 0.922 ± 0.007 | 0.912 ± 0.007 | Kappa | 0.814 ± 0.011 |
| Cross-Validated (OOB) | |||||
| Sensitivity | 0.623 ± 0.012 | 0.793 ± 0.041 | 0.749 ± 0.018 | Accuracy | 0.69 ± 0.01 |
| Specificity | 0.871 ± 0.011 | 0.801 ± 0.004 | 0.884 ± 0.01 | P-value | < 10−7 |
| Balanced Accuracy | 0.747 ± 0.009 | 0.797 ± 0.022 | 0.817 ± 0.011 | Kappa | 0.521 ± 0.015 |
Restricting the model to 5 global and 9 nodal measures yielded within sample accuracy of 87.8% and kappa = 0.814. Cross-validated performance was also highly significant with balanced accuracy for each class ranging from 74.7 to 81.7% versus chance accuracy of 33%. As this approach may lead to overfitting (101) (albeit slight given the sample size) we confirmed the result by dividing the data into balanced training (67% of subjects) and test sets. Selecting the 5 global measures and most important 9 nodes that discriminated asymptomatic from symptomatic in the training set and creating a predictive model on the training set fit the test set with 73%, 79% and 82% balanced accuracy. See S9 for additional information.
Discussion
Susceptible and resilient individuals differed markedly from controls but not from each other in global network architecture. Hence, maltreatment appears to affect network architecture regardless of psychiatric outcome. Symptomatic and asymptomatic participants however differed from each other in Neff of the right amygdala and in eight other nodes. Measures of global network architecture and Neff classified subjects as symptomatic, asymptomatic or non-maltreated with good predictive accuracy. Hence, this appears to be a promising approach to understanding neurobiological differences between susceptible and resilient maltreated individuals.
According to this model both susceptible and resilient individuals have sparse global networks, but resilient individuals differ from susceptible and controls in the Neff of a small subset of nodes. We propose that one or more of these nodes may function abnormally in susceptible individuals whose sparse network may be unable to compensate for these abnormalities. Resilient participants, on the other hand, may be able to effectively compensate, as these nodes have a diminished ability to propagate information throughout their network.
Identified nodes have interesting associations with psychopathology. The anterior portion of the middle cingulate (aka dorsal anterior cingulate (dACC)) is the component of the cingulate cortex selectively activated by pain (102) and by stimuli that elicit fear (103) and may be vulnerable to chronic pain and stress syndromes (103). The supplementary motor area (SMA) is strongly interconnected with the anterior midcingulate (104) and plays a critical role in planning and sequencing of movements through its connection to motor cortex. This node, which also contains pre-SMA, interconnects with caudate and putamen (105) and appears to be involved in aspects of depression such as psychomotor retardation (106). Antidepressant response to ketamine is specifically associated with increased blood flow in SMA and dACC (107). The olfactory cortex in the AAL template corresponds most closely to subcallosal gyrus in the Harvard-Oxford Maximum Probability Atlas, and contains portions of Brodmann Area 25, which is strongly implicated in the neurobiology of refractory depression (108). Thickness of the pars triangularis correlates with severity ratings in patients with panic disorder (109). Further, the pars triangularis contains a portion of Broca’s area and could potentially play a role in the self-castigating ‘voices’ that maltreated individuals often perceive. Reducing Neff of this region may enable maltreated individuals to more effectively compensate by helping to quiet this internal voice. The paracentral lobule is associated with control of urination and defecation, which can be compromised by stress or anxiety. Higher levels of trait anxiety have been associated with stronger interconnections between amygdala and paracentral lobule and between amygdala and dACC (110).
We do not know if reduced Neff was a compensatory adaptation that enables some individuals to regain mental well-being following exposure to maltreatment or if low Neff was a preexisting protective factor. Longitudinal studies have identified different trajectories of recovery in trauma exposed individuals. Bonanno et al (111) identified two resilience patterns – minimal impact and emergent resilience. Low Neff in specific nodes prior to acute exposure may predispose individuals to show a minimal impact resilience pattern. In contrast, adaptive changes in Neff over time may be more characteristic of individuals following an emergent resilience pattern with gradual abatement of symptoms following exposure.
Interestingly, Kozisek et al (112) postulated that antidepressants work by increasing brain derived neurotrophic factor to enhance neural plasticity allowing for therapeutic alterations in network connections. Our findings suggest that diminishing Neff in these specific nodes may be a potentially beneficial neuroplastic change and it would be important to ascertain if this occurs following successful treatment.
A few prior studies have identified neurobiological differences between susceptible and resilient maltreated participants. De Bellis et al (113) and Morey et al (114) compared maltreated children with and without PTSD and found that those with PTSD had decreased right ventromedial prefrontal, posterior cerebral and cerebellar gray matter volumes. In contrast, those without PTSD had larger left amygdala and right hippocampal volumes. Herringa et al (115) found that relatively resilient maltreated youths (fewer internalizing symptoms) had greater prefrontal-amygdala interconnectivity to negative images than more susceptible maltreated youths or controls. This suggest that reducing amygdaloid influence on the global network or enhancing top-down regulation of the amygdala may each lead to symptom attenuation. These findings are consistent with our observation that susceptible and resilient individuals differ in a small number of regions or pathways that may facilitate compensation.
Our theoretical model posits that psychopathology emerges from the “two-hit” combination of vulnerable network architecture and abnormal nodal function. A key question is which hit comes first? We have previously reported that right amygdala volume was most significantly affected by exposure to maltreatment at 10–11 years of age (116). We also reported that fiber tracts that differed most strongly between maltreated individuals and controls were primarily association and cortico-limbic pathways (66) that continue to actively myelinate during adolescence and early adulthood and may be particularly vulnerable at that time (117, 118). Hence, it may be the case that nodal abnormalities precede a developmental change in global network architecture that occurs during adolescence or early adulthood and may help explain the marked increase in prevalence of certain psychiatric disorders that occurs during this time (119).
Strengths of this study include a large sample of carefully assessed and unmedicated participants imaged on the same scanner and who were recruited from the community. Another strength was the comprehensive assessment of maltreatment using the MACE, which assesses exposure to types of maltreatment not included in prior instruments.
A common limitation in resilience research is that resilient individuals are often exposed to lower levels of adversity than susceptible individuals (120) and this was a problem using criteria 1. Criteria 2 identified subjects who were resilient by criteria 1 but also included participants who experienced a diagnosable disorder but were now recovered. Interestingly, the key concern in having a resilient group with a lower degree of exposure than the susceptible group is that weaker effects in the resilient group may be secondary to their weaker degree of exposure. This was not a concern as the resilient group had abnormalities in global network architecture that were at least as strong as those seen in the susceptible group. Further, the asymptomatic group had much greater alterations in Neff in key nodes than symptomatic participants despite their slightly lower degree of exposure to adversity.
Our susceptible/symptomatic and resilient/asymptomatic groups were heterogeneous in lifetime diagnoses and participants within each group varied in exposure histories. More nuanced groups differences may be discernible in studies that focus on circumscribed categories such as trauma exposed children with and without PTSD (114).
Another limitation is reliance on retrospective self-report, which theoretically could be affected by memory impairment associated with psychopathology, and mood-congruent memory biases. Brewin et al (121), in a detailed review, found little evidence to support these criticisms. Retrospective reports of abuse are verifiable (122) and modern instruments for assessing maltreatment all show impressive test-retest reliability (e.g., Childhood Trauma Questionnaire r = 0.88 (123), MACE r = 0.91 (85)).
The finding that maltreated individuals without psychopathology have basically the same array of neuroimaging abnormalities as maltreated individuals with psychopathology has been perplexing (124). The present findings provide a potential answer to the puzzle by identifying additional abnormalities in resilient individuals that may enable them to effectively compensate. This is novel and important information as it suggests that effort to treat maltreated individuals with psychopathology do not have to focus on reversing maltreatment-related alterations but may instead foster specific nodal changes that bring their circuitry more into line with the circuitry of resilient individuals.
Supplementary Material
Acknowledgements.
This work was supported by National Institute of Mental Health Award MH091391, National Institute of Drug Abuse Award DA-017846 and National Institute of Child Health and Human Development Award HD-079484 to M.H.T. Portions of this work were introduced in an oral presentation at the Society of Biological Psychiatry annual meeting in 2017.
Footnotes
Disclosures. Dr. Teicher receives royalties from Pearson and consulting fees from Abide Therapeutics and Custom Learning Design. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. (2010): Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry: American Medical Association, pp 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF (2003): The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med, pp 268–277. [DOI] [PubMed] [Google Scholar]
- 3.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF (2003): Childhood Abuse, Neglect, and Household Dysfunction and the Risk of Illicit Drug Use: The Adverse Childhood Experiences Study. Pediatrics: American Academy of Pediatrics, pp 564–572. [DOI] [PubMed] [Google Scholar]
- 4.Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH (2001): Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA, pp 3089–3096. [DOI] [PubMed] [Google Scholar]
- 5.Afifi TO, Mather A, Boman J, Fleisher W, Enns MW, MacMillan H, et al. (2011): Childhood adversity and personality disorders: results from a nationally representative population-based study. Journal of psychiatric research, pp 814–822. [DOI] [PubMed] [Google Scholar]
- 6.Bierer LM, Yehuda R, Schmeidler J, Mitropoulou V, New AS, Silverman JM, et al. (2003): Abuse and neglect in childhood: relationship to personality disorder diagnoses. CNS Spectrums, pp 737–754. [DOI] [PubMed] [Google Scholar]
- 7.Cutajar MC, Mullen PE, Ogloff JRP, Thomas SD, Wells DL, Spataro J (2010): Psychopathology in a large cohort of sexually abused children followed up to 43 years. Child abuse & neglect, pp 813–822. [DOI] [PubMed] [Google Scholar]
- 8.Gratz KL, Latzman RD, Tull MT, Reynolds EK, Lejuez CW (2011): Exploring the Association Between Emotional Abuse and Childhood Borderline Personality Features: The Moderating Role of Personality Traits. Behavior Therapy, pp 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman JL, Perry JC, van der Kolk BA (1989): Childhood trauma in borderline personality disorder. The American journal of psychiatry: American Psychiatric Publishing, pp 490–495. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JG, Cohen P, Smailes EM, Skodol AE, Brown J, Oldham JM (2001): Childhood verbal abuse and risk for personality disorders during adolescence and early adulthood. Compr Psychiatry, pp 16–23. [DOI] [PubMed] [Google Scholar]
- 11.Lyons-Ruth K, Bureau J-F, Holmes B, Easterbrooks A, Brooks NH (2013): Borderline symptoms and suicidality/self-injury in late adolescence: prospectively observed relationship correlates in infancy and childhood. Psychiatry research, pp 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenzie MT (2013): Temperament and maltreatment in the emergence of borderline and antisocial personality pathology during early adolescence. J Can Acad Child Adolesc Psychiatry. [PMC free article] [PubMed] [Google Scholar]
- 13.Widom CS, Czaja SJ, Paris J (2009): A prospective investigation of borderline personality disorder in abused and neglected children followed up into adulthood. J Pers Disord, pp 433–446. [DOI] [PubMed] [Google Scholar]
- 14.Zanarini MC, Williams AA, Lewis RE, Reich RB, Vera SC, Marino MF, et al. (1997): Reported pathological childhood experiences associated with the development of borderline personality disorder. The American journal of psychiatry: American Psychiatric Publishing, pp 1101–1106. [DOI] [PubMed] [Google Scholar]
- 15.Alemany S, Arias B, Aguilera M, Villa H, Moya J, Ibáñez MI, et al. (2011): Childhood abuse, the BDNF-Val66Met polymorphism and adult psychotic-like experiences. Br J Psychiatry: The Royal College of Psychiatrists, pp 38–42. [DOI] [PubMed] [Google Scholar]
- 16.Arseneault L, Cannon M, Fisher HL, Polanczyk G, Moffitt TE, Caspi A (2011): Childhood trauma and children's emerging psychotic symptoms: A genetically sensitive longitudinal cohort study. The American journal of psychiatry: American Psychiatric Publishing Arlington, VA , pp 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bebbington P, Jonas S, Kuipers E, King M, Cooper C, Brugha T, et al. (2011): Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry: The Royal College of Psychiatrists, pp 29–37. [DOI] [PubMed] [Google Scholar]
- 18.Bendall S, Jackson HJ, Hulbert CA, McGorry PD (2008): Childhood trauma and psychotic disorders: a systematic, critical review of the evidence Schizophrenia bulletin: Oxford University Press, pp 568–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly M (2011): Childhood psychotic symptoms: link between non-consensual sex and later psychosis. Br J Psychiatry: The Royal College of Psychiatrists, pp 251–252- author reply 252. [DOI] [PubMed] [Google Scholar]
- 20.Fisher HL, Jones PB, Fearon P, Craig TK, Dazzan P, Morgan K, et al. (2010): The varying impact of type, timing and frequency of exposure to childhood adversity on its association with adult psychotic disorder Psychological medicine: Cambridge University Press, pp 1967–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaudiano BA, Zimmerman M (2010): The relationship between childhood trauma history and the psychotic subtype of major depression Acta psychiatrica Scandinavica: Blackwell Publishing Ltd, pp 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houston JE, Murphy J, Shevlin M, Adamson G (2011): Cannabis use and psychosis: re-visiting the role of childhood trauma Psychological medicine: Cambridge University Press, pp 2339–2348. [DOI] [PubMed] [Google Scholar]
- 23.Brown GR, McBride L, Bauer MS, Williford WO (2005): Impact of childhood abuse on the course of bipolar disorder: A replication study in U.S. veterans. Journal of affective disorders, pp 57–67. [DOI] [PubMed] [Google Scholar]
- 24.Daruy-Filho L, Brietzke E, Lafer B, Grassi Oliveira R (2011): Childhood maltreatment and clinical outcomes of bipolar disorder Acta psychiatrica Scandinavica: Blackwell Publishing Ltd, pp 427–434. [DOI] [PubMed] [Google Scholar]
- 25.Etain B, Mathieu F, Henry C, Raust A, Roy I, Germain A, et al. (2010): Preferential association between childhood emotional abuse and bipolar disorder. Journal of traumatic stress: Wiley Subscription Services, Inc., A Wiley Company, pp 376–383. [DOI] [PubMed] [Google Scholar]
- 26.Garno JL, Goldberg JF, Ramirez PM, Ritzler BA (2005): Impact of childhood abuse on the clinical course of bipolar disorder. The British Journal of Psychiatry: The Royal College of Psychiatrists, pp 121–125. [DOI] [PubMed] [Google Scholar]
- 27.Hyun M, Friedman SD, Dunner DL (2000): Relationship of childhood physical and sexual abuse to adult bipolar disorder. Bipolar disorders, pp 131–135. [DOI] [PubMed] [Google Scholar]
- 28.Leverich GS, McElroy SL, Suppes T, Keck PE, Denicoff KD, Nolen WA, et al. (2002): Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry, pp 288–297. [DOI] [PubMed] [Google Scholar]
- 29.Lu W, Mueser KT, Rosenberg SD, Jankowski MK (2008): Correlates of adverse childhood experiences among adults with severe mood disorders. Psychiatr Serv: American Psychiatric Association, pp 1018–1026. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre RS, Soczynska JK, Mancini D, Lam C, Woldeyohannes HO, Moon S, et al. (2008): The relationship between childhood abuse and suicidality in adult bipolar disorder. Violence, pp 361–372. [DOI] [PubMed] [Google Scholar]
- 31.Post RM, Leverich GS, Xing G, Weiss RB (2001): Developmental vulnerabilities to the onset and course of bipolar disorder Development and psychopathology: Cambridge University Press, pp 581–598. [DOI] [PubMed] [Google Scholar]
- 32.Romero S, Birmaher B, Axelson D, Goldstein T, Goldstein BI, Gill MK, et al. (2009): Prevalence and correlates of physical and sexual abuse in children and adolescents with bipolar disorder. Journal of affective disorders, pp 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E (1997): Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Annals of the New York Academy of Sciences. 821:160–175. [DOI] [PubMed] [Google Scholar]
- 34.De Bellis MD, Keshavan MS, Clark DB, Casey BJ (1999): Developmental traumatology part II: brain development. Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- 35.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. (2002): Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological psychiatry. 52:1066–1078. [DOI] [PubMed] [Google Scholar]
- 36.Andersen SL, Tomoda A, Vincow ES, Valente E, Polcari A, Teicher MH (2008): Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci, pp 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL (2004): Childhood neglect is associated with reduced corpus callosum area. Biological psychiatry. 56:80–85. [DOI] [PubMed] [Google Scholar]
- 38.Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, et al. (2008): Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry research. 162:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teicher MH, Samson JA, Sheu YS, Polcari A, McGreenery CE (2010): Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. The American journal of psychiatry. 167:1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. (1997): Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry, pp 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM (2010): Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. Journal of psychiatric research. 44:799–807. [DOI] [PubMed] [Google Scholar]
- 42.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B (1997): Hippocampal volume in women victimized by childhood sexual abuse. Psychological medicine. 27:951–959. [DOI] [PubMed] [Google Scholar]
- 43.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD (2006): Hippocampal and amygdalar volumes in dissociative identity disorder. The American journal of psychiatry. 163:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. (2002): Childhood trauma associated with smaller hippocampal volume in women with major depression. The American journal of psychiatry. 159:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weniger G, Lange C, Sachsse U, Irle E (2009): Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. Journal of psychiatry & neuroscience : JPN. 34:383–388. [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen SL, Teicher MH (2008): Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci, pp 183–191. [DOI] [PubMed] [Google Scholar]
- 47.Brambilla P, Soloff PH, Sala M, Nicoletti MA, Keshavan MS, Soares JC (2004): Anatomical MRI study of borderline personality disorder patients. Psychiatry Res. 131:125–133. [DOI] [PubMed] [Google Scholar]
- 48.Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. (2000): Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry, pp 1115–1122. [DOI] [PubMed] [Google Scholar]
- 49.Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J (2003): Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry research. 122:193–198. [DOI] [PubMed] [Google Scholar]
- 50.Opel N, Redlich R, Zwanzger P, Grotegerd D, Arolt V, Heindel W, et al. (2014): Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, pp 2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teicher MH, Anderson CM, Polcari A (2012): Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA, pp E563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. (2006): Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry, pp 975–982. [DOI] [PubMed] [Google Scholar]
- 53.De Bellis MD, Keshavan MS, Spencer S, Hall J (2000): N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. The American journal of psychiatry. 157:1175–1177. [DOI] [PubMed] [Google Scholar]
- 54.Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, Teicher MH (2009): Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage. 47 Suppl 2:T66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, et al. (2003): Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological psychiatry. 53:879–889. [DOI] [PubMed] [Google Scholar]
- 56.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. (1999): Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. The American journal of psychiatry. 156:575–584. [DOI] [PubMed] [Google Scholar]
- 57.Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, et al. (2010): Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci: Society for Neuroscience, pp 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheu Y-S, Polcari A, Anderson CM, Teicher MH (2010): Harsh corporal punishment is associated with increased T2 relaxation time in dopamine-rich regions. NeuroImage, pp 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, et al. (2013): Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human brain mapping. 34:2899–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. (2012): Limbic Scars: Long-Term Consequences of Childhood Maltreatment Revealed by Functional and Structural Magnetic Resonance Imaging. Biol Psychiatry, pp 286–293. [DOI] [PubMed] [Google Scholar]
- 61.Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ (2014): Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress Anxiety, pp 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, et al. (2013): Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress Psychological medicine: Cambridge University Press, pp 507–518. [DOI] [PubMed] [Google Scholar]
- 63.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. (2013): Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA: National Acad Sciences, pp 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Dai Z, Peng H, Tan L, Ding Y, He Z, et al. (2014): Overlapping and segregated resting‐state functional connectivity in patients with major depressive disorder with and without childhood neglect. Human brain mapping, pp 1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teicher MH, Anderson CM, Ohashi K, Polcari A (2014): Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatry, pp 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohashi K, Anderson CM, Bolger EA, Khan A, McGreenery CE, Teicher MH (2017): Childhood maltreatment is associated with alteration in global network fiber-tract architecture independent of history of depression and anxiety. NeuroImage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puetz VB, Parker D, Kohn N, Dahmen B, Verma R, Konrad K (2017): Altered brain network integrity after childhood maltreatment: A structural connectomic DTI‐study. Human brain mapping, pp 855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paul R, Henry L, Grieve SM, Guilmette TJ, Niaura R, Bryant R, et al. (2008): The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatric disease and treatment. 4:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seckfort DL, Paul R, Grieve SM, Vandenberg B, Bryant RA, Williams LM, et al. (2008): Early Life Stress on Brain Structure and Function Across the Lifespan: A Preliminary Study. Brain imaging and behavior. 2:49. [Google Scholar]
- 70.Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, et al. (2011): Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of pediatrics & adolescent medicine. 165:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carballedo A, Lisiecka D, Fagan A, Saleh K, Ferguson Y, Connolly G, et al. (2012): Early life adversity is associated with brain changes in subjects at family risk for depression. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 13:569–578. [DOI] [PubMed] [Google Scholar]
- 72.Everaerd D, Gerritsen L, Rijpkema M, Frodl T, van Oostrom I, Franke B, et al. (2012): Sex modulates the interactive effect of the serotonin transporter gene polymorphism and childhood adversity on hippocampal volume. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 37:1848–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF (2012): Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. Journal of psychiatry & neuroscience : JPN. 37:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerritsen L, Tendolkar I, Franke B, Vasquez AA, Kooijman S, Buitelaar J, et al. (2012): BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Molecular psychiatry. 17:597–603. [DOI] [PubMed] [Google Scholar]
- 75.Huang H, Gundapuneedi T, Rao U (2012): White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, pp 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, Paul RH (2013): Impact of early vs. late childhood early life stress on brain morphometrics. Brain imaging and behavior, 2012/12/19 ed, pp 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Philip NS, Sweet LH, Tyrka AR, Price LH, Bloom RF, Carpenter LL (2013): Decreased default network connectivity is associated with early life stress in medication-free healthy adults. European Neuropsychopharmacology, pp 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samplin E, Ikuta T, Malhotra AK, Szeszko PR, Derosse P (2013): Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. Journal of psychiatric research. 47:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. (2015): Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry, pp 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teicher MH, Anderson CM, Polcari A (2012): Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences of the United States of America. 109:E563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bale TL (2014): Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues Clin Neurosci: Les Laboratoires Servier, pp 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bayer TA, Falkai P, Maier W (1999): Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the "two hit hypothesis". Journal of psychiatric research: Elsevier pp 543–548. [DOI] [PubMed] [Google Scholar]
- 83.Sporns O, Honey CJ (2006): Small worlds inside big brains. PNAS: National Acad Sciences, pp 19219–19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.First MB, Spitzer RL, Gibbon M, Williams J (1997): Structured clinical interview for DSM-IV axis I disorders - clinician version (SCID-CV). Washington DC and London, England: American Psychiatry Association Press, Inc. [Google Scholar]
- 85.Teicher MH, Parigger A (2015): The ‘Maltreatment and Abuse Chronology of Exposure’ (MACE) scale for the retrospective assessment of abuse and neglect during development. PloS one: Public Library of Science, pp e0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kellner R (1987): A symptom questionnaire. The Journal of clinical psychiatry, pp 268–274. [PubMed] [Google Scholar]
- 87.Khan A, McCormack HC, Bolger EA, McGreenery CE, Vitaliano G, Polcari A, et al. (2015): Childhood Maltreatment, Depression, and Suicidal Ideation: Critical Importance of Parental and Peer Emotional Abuse during Developmental Sensitive Periods in Males and Females. Front Psychiatry, 3rd ed ed, pp 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teicher MH, Samson JA, Polcari A, McGreenery CE (2006): Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. The American journal of psychiatry. 163:993–1000. [DOI] [PubMed] [Google Scholar]
- 89.Teicher MH, Vitaliano GD (2011): Witnessing violence toward siblings: an understudied but potent form of early adversity. PloS one: Public Library of Science, pp e28852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH (2009): Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological psychiatry. 65:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luthar SS, Cicchetti D, Becker B (2000): The construct of resilience: a critical evaluation and guidelines for future work. Child development. 71:543–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15:273–289. [DOI] [PubMed] [Google Scholar]
- 93.Anderson A, Cohen MS (2013): Decreased small-world functional network connectivity and clustering across resting state networks in schizophrenia: an fMRI classification tutorial. Frontiers in human neuroscience: Frontiers, pp 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bassett DS, Bullmore E (2006): Small-World Brain Networks. Neuroscientist: SAGE Publications, pp 512–523. [DOI] [PubMed] [Google Scholar]
- 95.Bullmore E, Sporns O (2009): Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci: Nature Publishing Group, pp 186–198. [DOI] [PubMed] [Google Scholar]
- 96.Bullmore E, Barnes A, Bassett DS, Fornito A, Kitzbichler M, Meunier D, et al. (2009): Generic aspects of complexity in brain imaging data and other biological systems. NeuroImage, pp 1125–1134. [DOI] [PubMed] [Google Scholar]
- 97.Latora V, Marchiori M (2001): Efficient behavior of small-world networks. Phys Rev Lett, pp 198701. [DOI] [PubMed] [Google Scholar]
- 98.Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: uses and interpretations. NeuroImage, pp 1059–1069. [DOI] [PubMed] [Google Scholar]
- 99.Stam CJ, Reijneveld JC (2007): Graph theoretical analysis of complex networks in the brain. Nonlinear Biomedical Physics: BioMed Central Ltd, pp 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McClelland GH, Judd CM (1993): Statistical difficulties of detecting interactions and moderator effects. Psychol Bull. 114:376–390. [DOI] [PubMed] [Google Scholar]
- 101.Smialowski P, Frishman D, Kramer S (2010): Pitfalls of supervised feature selection. Bioinformatics. 26:440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lieberman MD, Eisenberger NI (2015): The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proc Natl Acad Sci U S A. 112:15250–15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vogt BA, Berger GR, Derbyshire SW (2003): Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 18:3134–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 4:215–222. [DOI] [PubMed] [Google Scholar]
- 105.Bozkurt B, Yagmurlu K, Middlebrooks EH, Karadag A, Ovalioglu TC, Jagadeesan B, et al. (2016): Microsurgical and Tractographic Anatomy of the Supplementary Motor Area Complex in Humans. World Neurosurg. 95:99–107. [DOI] [PubMed] [Google Scholar]
- 106.Bracht T, Federspiel A, Schnell S, Horn H, Hofle O, Wiest R, et al. (2012): Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS One. 7:e52238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. (2018): Persistent antidepressant effect of low-dose ketamine and activation in the supplementary motor area and anterior cingulate cortex in treatment-resistant depression: A randomized control study. J Affect Disord. 225:709–714. [DOI] [PubMed] [Google Scholar]
- 108.Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM (2011): The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 69:301–308. [DOI] [PubMed] [Google Scholar]
- 109.Kang EK, Lee KS, Lee SH (2017): Reduced Cortical Thickness in the Temporal Pole, Insula, and Pars Triangularis in Patients with Panic Disorder. Yonsei Med J. 58:1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greening SG, Mitchell DG (2015): A network of amygdala connections predict individual differences in trait anxiety. Hum Brain Mapp. 36:4819–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonanno GA, Diminich ED (2013): Annual Research Review: Positive adjustment to adversity--trajectories of minimal-impact resilience and emergent resilience. Journal of child psychology and psychiatry, and allied disciplines. 54:378–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kozisek ME, Middlemas D, Bylund DB (2008): Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacology & therapeutics. 117:30–51. [DOI] [PubMed] [Google Scholar]
- 113.De Bellis MD, Hooper SR, Chen SD, Provenzale JM, Boyd BD, Glessner CE, et al. (2015): Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Development and psychopathology. 27:1555–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morey RA, Haswell CC, Hooper SR, De Bellis MD (2016): Amygdala, Hippocampus, and Ventral Medial Prefrontal Cortex Volumes Differ in Maltreated Youth with and without Chronic Posttraumatic Stress Disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 41:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ (2016): Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 1:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH (2014): Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 97:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yakovlev PI, LeCours AR (1967): The myelogenetic cycles of regional maturation of the brain In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell. [Google Scholar]
- 118.Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, et al. (2014): The development of human amygdala functional connectivity at rest from 4 to 23 years: a crosssectional study. Neuroimage. 95:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB (2007): Age of onset of mental disorders: a review of recent literature. Current opinion in psychiatry. 20:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cicchetti D (1993): Developmental Psychopathology: Reactions, Reflections, Projections. Developmental Review, pp 471–502. [Google Scholar]
- 121.Brewin CR, Andrews B, Gotlib IH (1993): Psychopathology and early experience: a reappraisal of retrospective reports. Psychological bulletin. 113:82–98. [DOI] [PubMed] [Google Scholar]
- 122.Chu JA, Frey LM, Ganzel BL, Matthews JA (1999): Memories of childhood abuse: dissociation, amnesia, and corroboration. The American journal of psychiatry. 156:749–755. [DOI] [PubMed] [Google Scholar]
- 123.Bernstein DP, Fink L (1998): Childhood Trauma Questionnaire Manual. The Psychological Corporation. [Google Scholar]
- 124.Teicher MH, Samson JA, Anderson CM, Ohashi K (2016): The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci: Nature Research, pp 652–666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.