Abstract
Emerging evidence has shown that both prostatic basal and luminal cells are able to initiate oncogenic transformation. However, despite the diversity of tumor initiating cells, most prostate cancer cells express the androgen receptor (AR) and depend on androgens for their growth and expansion, implicating an essential role of androgen signaling in prostate tumorigenesis. Prostatic basal cells express p63 and are able to differentiate into luminal, neuroendocrine, and basal cells. Here, we directly assessed the essential role of androgen signaling in prostatic p63-expressing cell initiated oncogenic transformation and tumor formation. Using novel and relevant mouse models, we demonstrated that, with stabilized β-catenin expression, prostatic p63-expressing cells possess the ability to initiate oncogenic transformation and, in the presence of androgens, they further transdifferentiate into luminal-like tumor cells and develop adenocarcinomas. Castration prior to activating stabilized β-catenin sensitizes p63-expressing cells and increases their sensitivity to androgens, resulting in aggressive and fast growing tumor phenotypes. These findings are consistent with what have been observed in human prostate cancers, demonstrating an essential role for androgen signaling in prostate cancer initiation and progression. This study also provides fresh insight into developing new therapeutic strategies for better treating prostate cancer patients.
Keywords: β-catenin, p63, prostate cancer, the androgen receptor, Wnt Signaling
INTRODUCTION
Prostate cancer is the most common non-cutaneous malignancy in men, contributing to 27,500 deaths annually in the US 1. Androgen signaling, mediated through the androgen receptor (AR) and its ligands, testosterone and 5α-dihydrotestosterone (DHT) is essential for prostate cancer initiation and progression 2–4. Three cell populations in prostatic epithelium have been well defined, which include luminal, basal, and neuroendocrine cells. Both prostatic luminal and basal cells can be efficient targets for initiating oncogenic transformation 5–8. Early transplantation experiments showed that prostatic basal cells but not luminal cells were able to efficiently induce prostate tumor initiation 9, 10. However, recent studies using genetically engineered mouse models indicated that prostatic luminal cells are more sensitive to be targeted for prostate cancer initiation in comparison to basal cells 5–7. Intriguingly, despite the diversity of tumor-initiating cells in the prostate, almost all tumor cells developed from the above mouse models express the AR and depend on androgens for their growth and expansion 5–7. This is very similar to what has been observed in human primary prostate cancer, suggesting a decisive role of the androgen-signaling pathway in prostate cancer initiation and development.
Emerging evidence has shown an important role of the Wnt signaling pathways in prostate development and tumorigenesis 11, 12. Wnt responsive cells appear to possess stem/progenitor cell properties in the mouse prostate 13. Abnormal expression of Wnt ligands, receptors, and effectors has been identified in prostate tumor and surrounding cells, suggesting paracrine regulatory mechanisms in prostate tumorigenesis 14, 15. Castration can elevate Wnt signaling and promote cell survival in the mouse prostate 16. The interaction between Wnt and androgen signaling pathways have been observed in prostate cancer cells 17–19. Co-expression of the AR and stabilized β-catenin in mouse prostatic luminal cells has been shown to accelerate tumor development and enhance tumor invasion and progression 20.
Prostatic basal cells express Ck5, Ck14, and p63, and contain prostatic stem/progenitor cell properties 21. Activation of oncogenic signaling in mouse Ck5 or Ck14 expressing cells induces transformation, and initiates tumor formation 5–7. The recent lineage tracing study identified several subpopulations of prostatic basal cells according to their different expression patterns of p63, Ck5 and Ck14 proteins 22. It appears that the majority of prostatic basal cells express p63, and those basal cells that express p63 but not Ck5 and Ck14 are more potent to differentiate into other prostatic epithelial lineages than other types of basal cells 22. Since p63 is a homologue of the p53 tumor suppressor 23, 24, it is unclear whether prostatic p63-expressing cells have the potential for initiating oncogenic transformation. In this study, we address these important questions using a diverse group of novel and relevant mouse models. We observed that prostatic p63 expressing cells possess the ability to initiate oncogenic transformation and, in the presence of androgens, these cells are able to further transdifferentiate into luminal-like tumor cells and develop to adenocarcinomas. In addition, we also found that castration can sensitize prostatic p63-expressing cells to androgens. With stabilized β-catenin expression, these androgen hypersensitive cells can develop into aggressive and fast growing tumors. These results illustrate a novel molecular mechanism by which androgen signaling in prostatic basal cells initiates tumor development and progression, providing fresh insight into our current knowledge to develop new therapeutic strategies for the treatment of advanced prostate cancer.
RESULTS
Conditional expression of stabilized β-catenin in prostatic p63-expressing cells at embryonic stages induces cell proliferation and abnormal cell foci formation.
Although it has been shown that prostatic p63 expressing cells possess stem/progenitor cell properties, their cellular potential to be targeted for oncogenic transformation still remains unclear. The expression of p63 was observed exclusively in cells localized in urogenital sinus epithelium (UGE) starting at or before embryonic day 13.5, E13.5 (Supplemental Fig. 1A1–3’) 25, 26. To directly assess the ability of embryonic p63-expressing cells in initiating oncogenic transformation, we generated RosamTmG/+:p63CreERT2/+ and RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ mice (Fig. 1A), in which the expression of mGFP, alone or with a stabilized form of β-catenin can be simultaneously induced in p63-expressing cells through tamoxifen (TM) induction 27. To evaluate the cellular identity of p63-expressing cells, we administered TM at E13.5 to the above mice and then analyzed them at E18.5 (Fig. 1B). Using triple IF approaches, we detected the co-expression of p63 and mGFP with E-cadherin, CK8, Ck5, AR, or Ki67 in UGE areas of RosamTmG/+:p63CreERT2/+ embryos (Supplemental Figure 1C-G). Our results demonstrates that prostatic p63-expressing cells are of epithelial origin and multipotent and possess proliferative characteristics. Intriguingly, we observed scattered cell clusters in the UGS areas of RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ embryos (arrows, Fig. 1C1-E1). The expression of stabilized β-catenin was detected in the cytoplasm and nucleus of cells within those cell foci, which directly links the expression of stabilized β-catenin to the formation of cell foci. Co-expression of stabilized β-catenin with mGFP, p63, and E-cadherin also appeared in the cell clusters (Arrowheads Fig. 1C4-E4), demonstrating the origin of those proliferative cell clusters deriving from p63-expressing epithelial cells. We also observed co-staining of stabilized β-catenin with Ck8 but no or very weak Ck5 in the cell clusters (arrowheads, Fig. 1F1-F2, and Supplemental Fig. 2A1–4 and 2B1–4). Using triple IF assays, we further confirmed the co-expression of mGFP and stabilized β-catenin with p63 and Ck8 but not Ck5 in those cell foci (arrowheads, Fig. 1G5-I5). These data suggest that p63+Ck8+ and Ck5- cells may be a more proliferative cell population within the β-catenin-induced cell population. We also observed that co-expression of AR or Ki67 with stabilized β-catenin in the above cell clusters (arrowheads, Fig. 1F3–4, Supplemental Fig. 2C1–4 and 2D1–4). The expression of Ck8 and AR but not Ck5 within these cell foci suggest that stabilized β-catenin can induce abnormal cell proliferation and luminal-like cell cluster formation in embryonic UGS tissues.
Figure 1. Conditional expression of stabilized β-catenin in p63-expressing cells at embryonic stage induces atypical cluster formation.
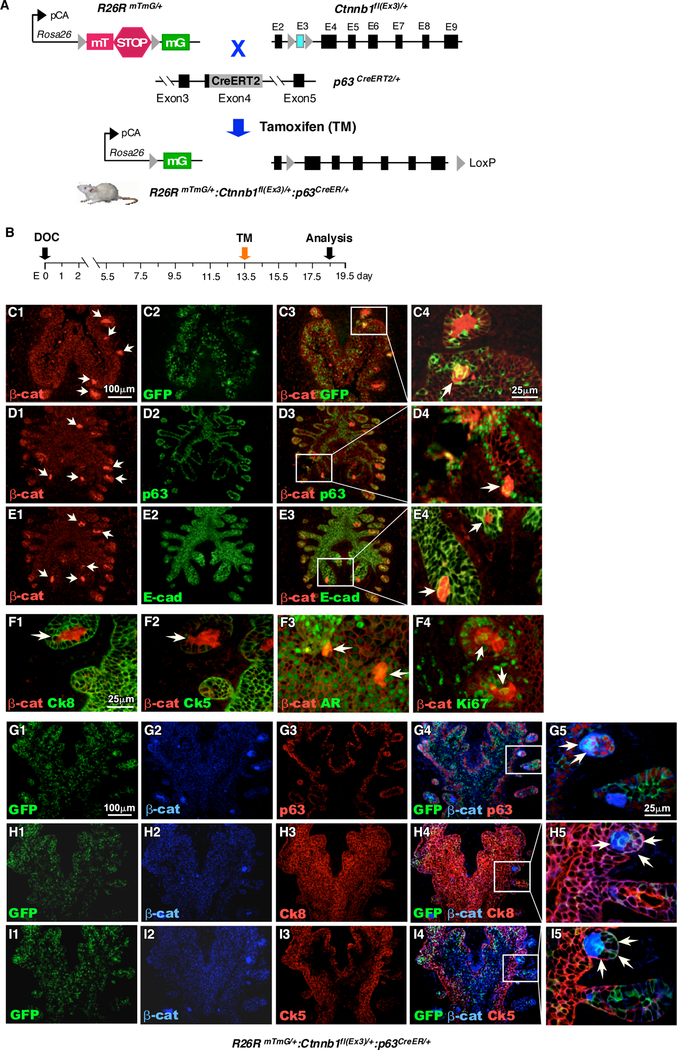
A. Schematic illustration of transgenic alleles and tamoxifen-inducible recombination events present in RosamTmG/+, Ctnnb1fl(Ex3)/+, and p63CreERT2/+ mice. The expression of either membrane-targeted tdTomato (mT) or membrane-targeted EGFP (mG) is regulated by the pCA promoter, consisting of a chicken β-actin core promoter with a CMV enhancer, through Cre/LoxP mediated recombination. B. Schematic representation depicting experimental timeline for labeling and analyzing UGS tissues during embryonic stage. Pregnant females received a single dose of tamoxifen on E13.5 and UGS tissues were isolated at E18.5. C-E. Immunofluorescent staining of UGS tissues from RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ embryos using antibodies against β-catenin (red), (C) GFP, (D) p63, (E) E-cad (green). F. Immunofluorescent staining of UGS tissues from RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ embryos using antibodies against β-catenin (red) and (F1) Ck8, (F2) Ck5, (F3) AR or (F4) Ki67 (green) G-I. Triple immunofluorescent staining of GFP (green), beta-catenin (blue) with (G) p63, (H) Ck8 or (I) Ck5 (red). Arrows (C-I) denote proliferative intraepithelial cell clusters expressing stabilized β-catenin (n=3).
Expression of stabilized β-catenin induces cell proliferation and initiates oncogenic transformation in prostatic prepubescent p63 expressing cells.
Next, we assessed the potential of prostatic prepubescent p63 expressing cells in oncogenic transformation. We injected TM in RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ mice at postnatal day 14, P14 (Fig. 2A). The expression of mGFP appeared in prostate epithelium across different lobes at P21 (Fig. 2B-B’). Histological analyses reveal intraepithelial cluster lesions in four prostatic lobes (Fig. 2C1–3), which are mainly located in the basal side of prostate glands and display nuclear hyperchromasia (Fig. 2C1’−3’). IHC analyses showed that the atypical cells within those cell clusters express GFP, p63, and stabilized β-catenin in adjacent tissue sections (pink arrows, Fig. 2D1–3’). The co-expression of mGFP and β-catenin, or mGFP and p63 was further detected in the atypical cell clusters on the adjacent tissue sections using co-IF approaches (arrowheads, Supplemental Fig. 3A1–3A2”). Interestingly, co-expression of AR or Ki67 with stabilized β-catenin was also observed in some atypical cells (Supplemental Fig. 3B1–3B2”), suggesting a regulatory role of androgen-signaling in prepubescent p63-expressing cell mediated oncogenic transformation. Co-IF analyses further showed that these prepubescent atypical cells were immunoreactive to Ck5, slightly to Ck14, but not to Ck8 antibody (Arrowheads, Fig. 2E1–3’), demonstrating the basal cell origin of these atypical cell clusters.
Figure 2. Conditional expression of stabilized β-catenin in prostatic prepubescent p63 expressing cells induces cell proliferation and oncogenic transformation.
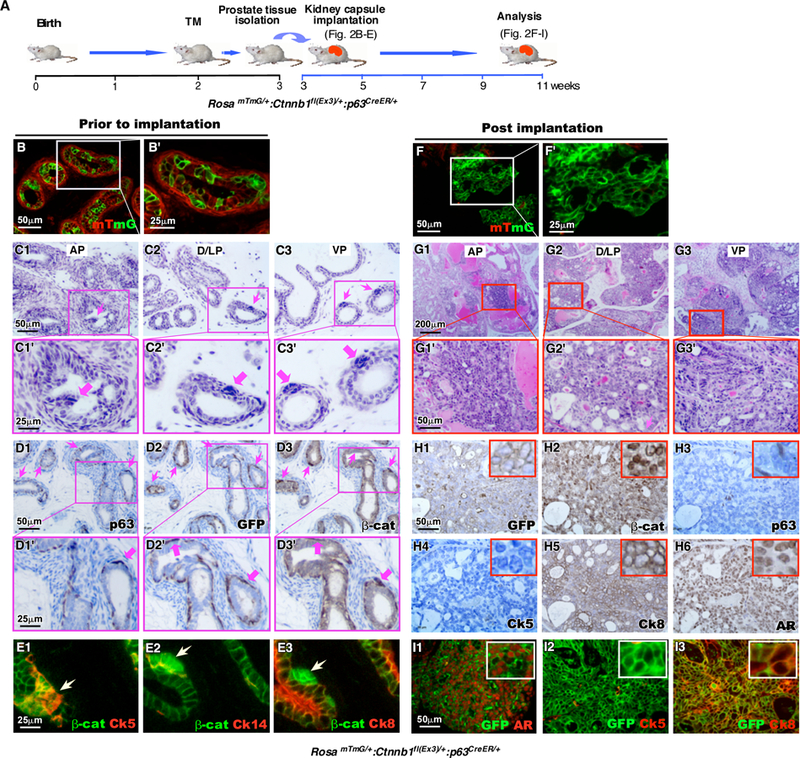
A. Schematic representation of experimental design. RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ mice (n=6) received a single tamoxifen injection on P14. Prostate tissues were isolated and dissected into individual lobes one week later (P21). Half of each lobe was implanted under the kidney capsule of SCID mice, while the other half was preserved for histological and immunofluorescent analyses. Kidney grafts. B and F. mTmG fluorescence of (B-B’) P21 prostate tissues prior to implantation under the kidney capsule and (F-F’) graft tissues eight weeks after implantation in the kidney. C. H&E stained sections of different prostatic lobes, (C1-C1’) anterior prostate (AP), (C2-C2’) dorsolateral prostate (D/LP), and (C3-C3’) ventral prostate (VP) from RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ mice at P21. Immunohistochemical staining of sequential sections of the same tissues with (D1-D1’) p63, (D2-D2’) GFP or (D3-D3’) β-catenin antibodies (brown). Sections are counterstained with hematoxylin (blue). E. Immunofluorescent staining of stabilized β-catenin (green) with (E1) Ck5, (E2) Ck14 or (E3) Ck8 (red). G. H&E stained sections of graft tissues derived from different prostatic lobes. H. IHC staining of sequential sections of graft tissues with (H1) GFP, (H2) β-catenin, (H3) p63, (H4) Ck5, (H5) Ck8, or (H6) AR antibodies (brown). Sections are counterstained with hematoxylin (blue). I. Immunofluorescent images of graft tissues generated from RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ prostates stained with antibodies against GFP (green) and (I1) AR, (I2) Ck5, or (I3) Ck8 (red).
Since activating stabilized β-catenin causes severe skin lesions and mortality in 4 to 6-week-old mice 28, we implanted prostate tissues isolated from the above RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ mice under the renal capsule of NOD/SCID mice to further assess the fate of the clustered p63-expressing cells, (Fig. 2A). The grafts were collected 8 weeks post the implantation and appeared much larger in comparison to the sizes prior to the implantation. Most of cells within grafted tissues appeared mGFP positive (Fig. 2F and F’), suggesting their origin of p63-expressing cells. Based on guidelines recommended by The Mouse Models of Human Cancers Consortium Prostate Pathology Committee in 2013 29, we observed typical pathological changes resembling mouse high-grade PINs and multi-focal intracystic adenocarcinomas in the grafted tissues derived from four different prostatic lobes (Fig. 2G1–3’). Specifically, atypical intraductal proliferation of glandular cells with cribriform and partly solid patterns appears pronounced in the lesions. Both atypical and tumor cells show marked intraductal expansion with loss of cell polarity in the lumens. Some of the cells display nuclear atypia and hyperchromasia, with prominent nuclear atypia and occasional mitotic activities. As shown above, development of HGPIN and intracystic adenocarcinomas from a scatter of atypical proliferative cell clusters (Fig. 2C1–3) provides direct evidence that prostatic p63-expressing cells are able to initiate oncogenic transformation and promote tumor formation. Most atypical and tumor cells in the above lesions showed positive staining with mGFP, β-catenin, Ck8, or AR antibody (Fig. 2H1, 2, 5, and 6). However, only very few p63 or Ck5 positive cells appeared in grafted samples (Fig. 2H3–4). Co-expression of mGFP and stabilized β-catenin appears in most cells within the grafted tissues (Supplemental Fig. 3D1–3) while only a few scattered cells co-express mGFP with p63 (Supplemental Fig. 3E1–3, Supplemental Table 2) or Ck5 (Fig. 2I2, and Supplemental Fig. 3H1–3). However, co-expression of both AR and Ck8, prostatic luminal cell markers, was observed in most atypical and tumor cells (Fig. 2I1 and 2I3, and Supplemental Fig. 3F1–3 and 3I1–3). Increased mGFP+Ki67+ cells also appeared in the grafted tissues (Supplemental Fig. 3G1–3). These data indicate that prepubescent p63-expressing cells with activating stabilized β-catenin expression are able to be transformed oncogenically, transdifferentiated, and form PIN and tumor lesions. Selective expansion of Ck8 and AR positive cells in the grafted tissues further suggests an essential role of androgen signaling for the transdifferentiation and growth of transformed p63-expressing cells.
Androgen deprivation sensitizes p63-expressing cells and enhances stabilized β-catenin induced prostate tumorigenesis.
We next further assessed the effect of androgens on prostatic p63-expressing cell initiated oncogenic transformation. A group of RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ mice (n=4) were administered TM at ages of 8 weeks and analyzed at ages of 16 weeks (Experiment 1, Fig. 3A). We observed only intraepithelial cluster lesions derived from the basal layer in all of four prostatic lobes (Fig. 3B1–3’) but did not detect any other pathological abnormalities. Most atypical cells within clusters appear mGFP positive, suggesting their origin of p63 expressing cells (arrowheads, Fig. 3C1–3). Co-IF analyses showed that the majority of cells within clusters co-express stabilized β-catenin with p63, AR, or Ck8, but only few with Ck5 (Fig. 3D1-D4“, Supplemental Table 3). In contrast, another group of RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ mice (n=6) were first castrated, then administered TM and supplemented androgens, and analyzed at age of 16 weeks (Experiment 2, Fig. 3A). Intriguingly, histologic analyses revealed pathologic lesions resembling mouse high-grade PIN and intracystic adenocarcinoma in all of four different prostatic lobes, featured typical intraductal proliferation of clusters and tufts with polygonal shapes and hyperchromatic nuclei, protruding into lumens (Fig. 3E1’−3’). Based on the guidelines recommended by The Mouse Models of Human Cancers Consortium Prostate Pathology Committee, these mice exhibited more aggressive lesions than the ones presented in Experiment 1 29 (Fig. 3E1–3’ versus 3B1–3’). Positive staining of GFP appeared in those proliferative cell clusters, indicating they originate from p63-expressing cells (Fig. 3F1–3). All of the atypical or tumor cells expressed stabilized β-catenin (Fig. 3G1). Co-IF analyses showed that the majority of atypical cells co-express stabilized β-catenin with Ck8 or AR (Fig. 3G3”-G4”, Supplemental Table 3). In contrast, only few cells showed co-expression of stabilized β-catenin with p63 or Ck5 (Fig. 3G1”-G2”, Supplemental Table 3). These results demonstrate that castration can sensitize p63-expressing cells and enhance their abilities for proliferation and forming aggressive and fast growing tumor phenotypes upon activating stabilized β-catenin expression.
Figure 3. Androgen deprivation sensitizes p63-expressing cells and enhances prostate tumorigenesis induced by stabilized β-catenin upon replacement of androgen.

A. Schematic depicting experimental timeline for castration, TM injection, androgen replacement and analysis. Experiment 1: RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ mice were injected with tamoxifen at age of eight weeks and prostate tissues were isolated at age of sixteen weeks (n=4). Experiment 2: RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ mice were castrated at age of eight weeks, injected with tamoxifen at age of twelve weeks followed by androgen replacement at age of 13.5 weeks. Prostate tissues were isolated and dissected into individual lobes at age of 17.5 weeks (n=6). B. H&E stained sections of different prostatic lobes, (B1) AP, (B2) D/LP and (B3) VP, isolated from mice described in Experiment 1. C. Fluorescent images of mT (red) and mG (green) expression in the different prostate lobes, (C1) AP, (C2) D/LP and (C3) VP of mice described in Experiment 1. D. Immunofluorescent staining of prostate tissues from RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ using antibodies against β-catenin (green) and (D1-D1”) p63, (D2-D2”) Ck5, (D3-D3”) Ck8 or (D4-D4”) AR (red). E. H&E stained sections of different prostatic lobes, (E1) AP, (E2) D/LP and (E3) VP, isolated from RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ described in Experiment 2. F. Fluorescent images of mT (red) and mG (green) expression in the different prostate lobes, (F1) AP, (F2) D/LP and (F3) VP of mice described in Experiment 2. G. Immunofluorescent staining of prostate tissues from RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ using antibodies against β-catenin (green) and (G1-G1”) p63, (G2-G2”) Ck5, (G3-G3”) Ck8 or (G4-G4”) AR (red). Arrows (B-G) denote proliferative intraepithelial cell clusters expressing stabilized β-catenin.
To further assess the androgen dependency of the above castration sensitized cells, we implanted prostate tissues isolated from RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ mice (Experiment 2, Fig. 3A) under the renal capsule of either intact or castrated NOD/SCID mice (Figure 4A). The grafted tissues were analyzed at 12 weeks after transplantation. We observed regressed prostate glands with scattered residues of PIN lesions in implanted tissues isolated from castrated mice (Fig. 4B1–3’), demonstrating the requirement of androgens for the above cells. Analyses of cells within the above regressed glands showed weak staining of β-catenin, Ck8, and AR (Fig. 4C3, 4, and 6), and no staining for mGFP, p63, and Ck5 (Fig. 4C1, 2, and 5). In contrast, the grafted tissues from four different lobes of intact mice showed fast growing and aggressive tumor lesions that feature multiple sheets of invasive carcinoma with marked nuclear atypia, pleomorphism, and frequent mitotic activities (Fig. 4D1–3’). These tumor cells showed positive staining of mGFP, β-catenin, Ck8, and AR, but no or very weak staining of p63 or Ck5 on adjacent sections (Fig. 4E1–6). Co-IF analyses showed that most tumor cells co-expressed stabilized β-catenin with Ck8 and AR (Supplemental Fig. 4C-C” and 4D-D”), but not p63 and Ck5 (Supplemental Fig. 4A-A” and 4B-B”). These data confirm an essential role of androgens in promoting the growth of castration-sensitized p63-expressing cells. Since most tumor cells were AR and Ck8 positive, it further indicates the importance of androgens in transdifferentiating and expanding prostatic luminal-type of tumor cells from those castration-sensitized p63-expressing cells.
Figure 4. Tumorigenesis caused by stabilization of stabilized β-catenin in sensitized p63-expressing cells is androgen dependent.
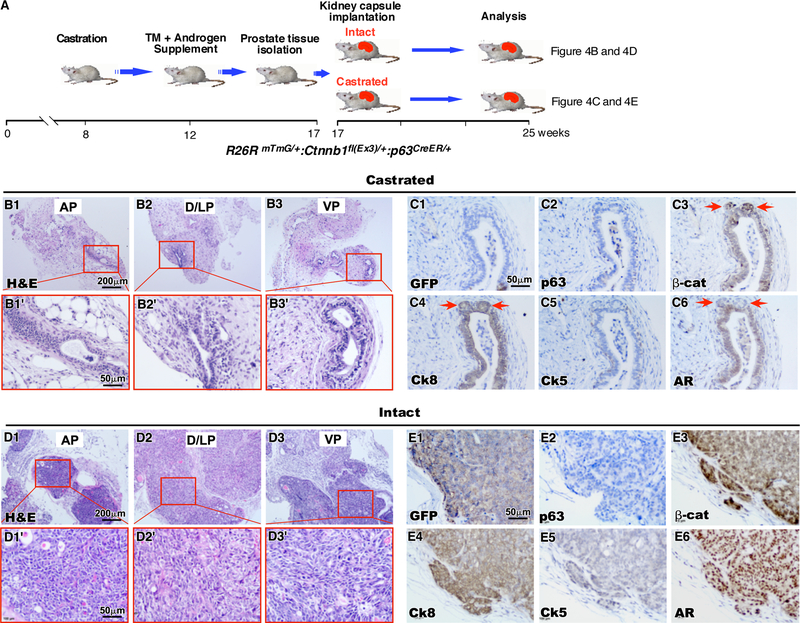
A. Schematic depicting experimental timeline for kidney capsule transplantation in both castrated and intact SCID mice. RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ were castrated at eight weeks of age. Four weeks later, the mice were injected with a single dose of tamoxifen and were supplemented with androgen. At 16 weeks of age, these mice prostate tissues were isolated and implanted under the kidney capsule of either intact or castrated SCID mice. Tissue grafts were dissected twelve weeks later (n=4). B. H&E stained sections of graft tissues derived from different prostatic lobes, (B1) AP, (B2) D/LP and (B3) VP, in castrated SCID mice. C. IHC staining of sequential sections of graft tissues harvested from castrated SCID mice with (C1) GFP, (C2) p63, (C3) β-catenin, (C4) Ck8, (C5) Ck5 or (C6) AR (brown). Sections are counterstained with hematoxylin (blue). D. H&E stained sections of graft tissues derived from different prostatic lobes, (D1) AP, (D2) D/LP and (D3) VP, harvested from intact SICD mice. E. IHC staining of sequential sections of graft tissues from intact SCID mice with (E1) GFP, (E2) p63, (E3) β-catenin, (E4) Ck8, (E5) Ck5 or (E6) AR (brown). Sections are counterstained with hematoxylin (blue).
Determining the effect of abnormal activation of β-catenin in inducing prostatic oncogenesis in tissue recombinants.
We then performed the tissue recombination assays to directly assess the oncogenic effect of p63-expressing cells upon stabilization of β-catenin. Both RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ and RosamTmG/+:p63CreERT2/+ mice were castrated at age of 8 weeks, injected with TM at 12 weeks, and sacrificed at 20 weeks (Fig. 5A). Single cell suspensions prepared from prostate tissues of the above mice were transplanted with embryonic UGSM cells isolated from wild type mice under the renal capsule of either castrated or intact NOD/SCID mice. Mice were sacrificed and grafts were analyzed twelve weeks post implantation. In intact mice, grafts derived from prostate tissues of RosamTmG/+:p63CreERT2/+ mice appeared small and whitish, but, in contrast, ones from RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ mice were large, yellowish, and containing blood vessels on the surface. Histologically, tubular epithelial structures with prostatic-like ducts appeared in samples of graft tissues from RosamTmG/+:p63CreERT2/+ controls (Fig. 5B1–2). Pathological lesions resembling typical high-grade PIN and multi-focal intracystic adenocarcinomas revealed in graft samples implanted with prostatic epithelial cells of RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ mice (arrows, Fig. 5C1–2). Most tumor cells showed positive staining with GFP, β-catenin, Ck8, E-cadherin, and AR antibodies (Fig. 5D1, 2, 5–7), only few cells stained with p63 and Ck5 antibodies (Fig. 5D3–4, Supplemental Table 5). There was no staining with synaptophysin (Fig. 5D8). Co-IF analyses showed co-expression of GFP with β-catenin, Ck8, and AR in the majority of tumor cells, confirming prostatic luminal cell properties (Fig. 5E1–4. H1–4, and I1–4). However, only a few of cells showed co-staining of GFP with p63 or CK5 (Fig. 5F1–4 and G1–4). In addition, a significant portion of tumor cells showed positive staining of Ki67 (Fig. 5J1–4). In contrast, grafts derived from prostate tissues of RosamTmG/+:Ctnnb1(ex3)fl/+:p63CreERT2/+ or RosamTmG/+;p63CreERT2/+ appeared small and underdeveloped in castrated mice. Histologically, grafted tissues from RosamTmG/+:Ctnnb1(ex3)fl/+;p63CreERT2/+ mice failed to develop normal epithelium and only showed tissues mainly composed of prostatic stromal cells (Supplemental Fig. 5). These data suggest that the growth of prostate epithelium is solely dependent on androgens. Taken together, the above data further demonstrate the transformative ability of castration-sensitized p63-expressing cells in developing HGPIN and prostatic tumors and the requirement of androgens in castration-sensitized p63-expressing cell initiated tumor formation.
Figure 5. Stabilization of β-catenin in sensitized p63-expressing cells resulted in oncogenesis in tissue recombinants.
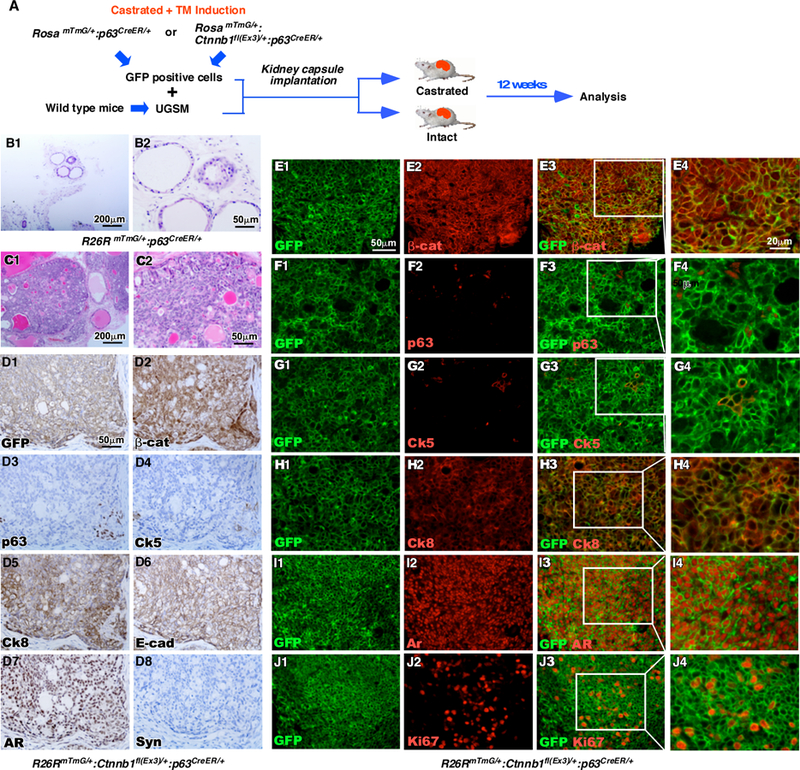
A. Schematic depicting preparation of recombinants and timeline for kidney capsule transplantation. RosamTmG/+ p63CreERT2/+ or RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ mice were castrated at the age of eight weeks, injected with TM at the age of twelve weeks, and sacrificed eight weeks later. Prostate tissues were isolated and cells were sorted for GFP expression using FACS. GFP-positive cells were implanted under the kidney capsule of intact SCID mice along with wild-type urogenital sinus mesenchyme tissue. Tissue grafts were isolated twelve weeks later (n=3). B-C. H&E stained sections of tissue graft recombinants made of UGSM and GFP-positive epithelial cells from the prostates of either (B) RosamTmG/+ p63CreERT2/+ or (C) RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ mice. D. IHC staining of sequential sections of tissues grafts from RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ mice using antibodies against (D1) GFP, (D2) β-catenin, (D3) p63, (D4) Ck5, (D5) Ck8, (D6) E-cad, (D7) AR or (D8) Synaptophysin (brown). Sections are counterstained with hematoxylin (blue). E-J. Immunofluorescent staining of tissue recombinants generated from RosamTmG/+ Ctnnb1fl(Ex3)/+ p63CreERT2/+ mice using antibodies against GFP (green) and (E) β-catenin, (F) p63, (G) Ck5, (H) Ck8, (I) AR, or (J) Ki67 (red).
Androgen signaling plays a promotional role in p63-expressing cell mediated oncogenic transformation and tumor formation.
Androgen signaling is mainly regulated through the AR 2, 3. To directly evaluate the role of the AR in p63-expressing cells, we generated Ctnnb1(ex3)fl/+;R26hARL/+:p63CreERT2/+ mice, in which both the human AR and stabilized β-catenin transgenes are co-expressed in p63-expressing cells (Fig. 6A) 30. As designed in Figure 6B, we performed a series of experiments to directly test the effect of AR on stabilized β-catenin mediated oncogenic transformation in prostatic p63-expressing cell using Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ mice and Ctnnb1(ex3)fl/+:p63CreERT2/+ controls. Typical proliferative cell clusters and PIN lesions appeared in four prostatic lobes of Ctnnb1(ex3)fl/+or Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ mice (Fig. 6C1–3, and 6E1–3), respectively. Pathological analyses revealed much worse and more aggressive lesions in the prostate of Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ compound mice than of Ctnnb1(ex3)fl/+;p63CreERT2/+ controls based on the guidelines recommended by The Mouse Models of Human Cancers Consortium Prostate Pathology Committee 29. Specifically, the lesions feature nuclear atypia and hyperchromasia and protrude or fill in lumens (Fig. 6E1–3). Positive staining of β-catenin and Ck8 appeared in prostate tissues of the both genotype mice but the expression of human AR transgene was only observed in tissues of Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ compound mice (Fig. 6C4–6 and 6E4–6). To evaluate the fate of the above atypical cells, we transplanted the above prostate tissues isolated from both genotype mice under the renal capsules in the intact NOD/SCID mice (Fig. 6B). At 6 weeks after the transplantation, we analyzed the above mice and observed the formation of prostatic invasive adenocarcinomas in the grafts of prostatic tissues from Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ mice (Fig. 6F1–3). In contrast, only PIN lesions were observed in the samples derived from the prostates of Ctnnb1(ex3)fl/+:p63CreERT2/+ mice (Fig. 6D1–3). Again, only grafted tissues from Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ mice showed transgenic AR expression (Fig. 6F5) and an overall increase in androgen receptor expression (Supplemental Figure 6E). Using co-IF approaches, we assessed the co-expression of Ki67 and stabilized β-catenin in the above tissues samples (Supplemental Fig. 6A-A” and 6B-B”). Ki67 immunostaining was quantified by counting a total of 1000 β-catenin expressing cells from five fields in each sample. Representative data from three individual experiments were shown that the epithelial proliferative index increased from 50 in grafted samples of Ctnnb1(ex3)fl/+:p63CreERT2/+ mice to 150 in ones of Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ mice (Supplemental Fig. 6C), suggesting a promoting role of human AR transgene expression in the growth of prostatic β-catenin expressing cells. To investigate the molecular basis underlying transgenic AR and stabilized β-catenin expression in promoting prostate tumor cell growth, we performed real-time quantitative RT-PCR (qPCR) to assess the expression of Wnt/β-catenin downstream targets in RNA samples isolated from grafted tissues of both Ctnnb1(ex3)fl/+;p63CreERT2/+ and Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ mice. We observed a significant increase in expression of Lef1, c-Myc, Axin2, CD44, and Bex1 in tissues of Ctnnb1(ex3)fl/+;R26hARL/+:p63CreERT2/+ mice as compared to Ctnnb1(ex3)fl/+:p63CreERT2/+ mice (Supplemental Fig. 6D). Taken together, these results suggest that the expression of human AR transgene can enhance stabilized β-catenin mediated tumor growth and progression, which may be through increasing the expression of β-catenin downstream target genes.
Figure 6. Androgen signaling plays a promotional role in oncogenic transformation induced by stabilized β-catenin in p63-expressing cells.
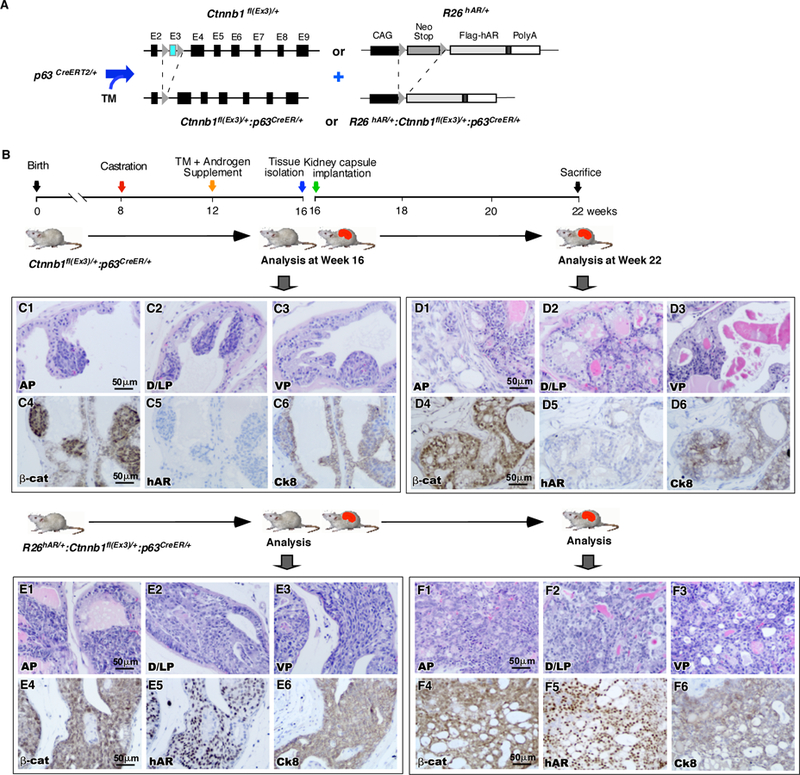
A. Illustration of transgenic alleles present in R26hARL/+Ctnnb1fl(Ex3)/+p63CreERT2/+ mice. Expression of the R26hARL/+transgene is driven by a modified CAG promoter and is activated by Cre-mediated removal of a Lox-Stop-Lox neomycin cassette. B. Schematic of experimental timeline. R26hARL/+Ctnnb1fl(Ex3)/+p63CreERT2/+ and Ctnnb1fl(Ex3)/+p63CreERT2/+ mice were castrated at eight weeks of age and injected with tamoxifen at age of 12 weeks. Then the mice were supplemented with androgen pellets. At the age of 16 weeks, mice were sacrificed, the prostates were harvested and implanted under the kidney capsule of SCID mice. Tissue grafts were isolated from SCID mice six weeks later (n=3). C. Histological analyses of prostate tissues isolated from Ctnnb1fl(Ex3)/+p63CreERT2/+ mice prior to kidney grafting. H&E stained sections of the (C1) AP, (C2) D/LP and (C3) VP. IHC staining of sequential section of prostatic tissues with antibodies for (C4) β-catenin, (C5) h-AR or (C6) Ck8 (brown). D. Histological analyses of prostate tissues isolated from R26hARL/+Ctnnb1fl(Ex3)/+p63CreERT2 mice prior to kidney grafting. H&E stained sections of the (D1) AP, (D2) D/LP or (D3) VP. IHC staining of sequential sections with (D4) β-catenin, (D5) h-AR or (D6) Ck8 antibodies (brown). E. Histological analyses of tissue grafts derived from Ctnnb1fl(Ex3)/+p63CreERT2 mice. H&E stained sections of graft tissues derived from the (E1) AP, (E2) D/LP or (E3) VP. IHC staining of sequential sections with (E4) β-catenin, (E5) h-AR or (E6) Ck8 antibodies (brown). F. Histological analyses of tissue grafts derived from R26hARL/+Ctnnb1fl(Ex3)/+p63CreERT2 mice. H&E stained sections of grafts derived from the (F1) AP, (F2) D/LP or (F3) VP. IHC staining of sequential sections with (F4) β-catenin, (F5) h-AR or (F6) Ck8 antibodies (brown).
DISCUSSION
Emerging evidence has shown that both prostatic luminal and basal epithelial cells can be targeted for prostate cancer initiation 5–7. Both mouse Ck5 and Ck14 expressing cells are able to induce oncogenic transformation and initiate tumor formation 5–7. However, most prostate cancers developed in those mouse models contain prostatic luminal cell properties and are androgen dependent 5–7. Here, we used a variety of relevant mouse models and in vivo systems to directly address the significance of androgen signaling in oncogenic transformation and tumor development initiated from prostatic p63-expressing cells. We demonstrate that activating Wnt oncogenic signaling by expressing stabilized β-catenin in prostatic p63-expressing cells is able to induce cell proliferation and the formation of atypical cell clusters in different prostatic lobes at embryonic, prepubescent, and adult stages. Intriguingly, despite the androgen insensitive nature of prostatic p63-expressing cells, androgens are still essential for these cells to grow and develop to androgen-dependent, luminal cell type prostate tumors. These findings are consistent with what have been observed in human prostate cancers, in which the majority of tumor cells are androgen-sensitive and possess luminal cell properties, providing new insight into the molecular mechanisms for prostate cancer initiation and progression.
Lineage tracing studies showed that prostatic p63-expressing cells are multipotent and able to develop to both basal and luminal cells 31, 32. Interestingly, the expression of p63 or Ck8, the basal or luminal cell markers, respectively, appears mutually exclusive during the course of prostate development and maturation in the adult prostate 32, 33. In this study, we observed that although the expression of stabilized β-catenin in prostatic p63-expressing cells induced oncogenic transformation, these cells needed to further transdifferentiate into prostatic luminal cells and then grow and expand in the presence of androgens. Using both IHC and co-IF approaches, we confirmed that most tumor cells express both Ck8 and AR, the cellular markers for prostatic luminal cells. In this study, we also observed that there was no or very limited growth of p63-expressing atypical cell clusters in castrated SCID host in comparison to intact counterparts. In addition, we also observed a significant regression in re-grafted prostate tumor samples isolated from intact hosts into castrated hosts. These above results demonstrate an indispensable role of androgens in prostatic basal cell initiated prostate cancer, which are also consistent with the similar observation in the conditional deletion of Pten tumor suppressor in prostatic Ck14 positive cells 6. Our data also implicate that the androgen-enriched microenvironment in the prostate selectively promotes the transdifferentiation of prostatic p63-expressing cells to androgen sensitive cells with prostatic luminal cell properties. The above observations are also similar to what happens in human prostate cancer, in which the majority of primary cancer cells possesses luminal cell properties and are ligand-dependent although they can further develop to androgen-independent statues after androgen deprivation therapy (ADT). Interestingly, we did not observe the direct expansion of transformed p63-expressing cells in the prostate of Ctnnb1(ex3)fl/+:p63CreERT2/+ mice. Instead, our data depicts expansion of Ck8+ and AR+ luminal-like cells arising from the p63-expressing basal cells. Although the molecular mechanisms underlying the inability of oncogenic transformed basal cells in developing into AR negative and androgen independent tumors in the mouse prostate are unclear currently, it may imply a repressive role of androgens in the growth of non-luminal prostatic cells in the prostate of the above mice. Therefore, further investigation using the above mouse models will provide fresh insight into developing more effective treatments for prostate cancer and prevent CRPC development.
As detailed in this study, we observed a much more aggressive prostate tumor phenotype in Ctnnb1(ex3)fl/+:p63CreERT2/+ mice that were castrated prior to TM administration in comparison to their counterparts that received TM directly (Fig. 3). These data suggest that castration can sensitize p63-expressing cells and enhance their abilities in oncogenic transformation, and androgen dependent cell growth and expansion. Our observations also provide a new insight into the mechanism underlying prostate cancer progression and castration resistant prostate cancer (CRPC) development. Although ADT is an effective treatment for prostate cancer, it eventually fails in nearly all cases of prostate cancer, and consequently patients develop CRPC, which is far more aggressive than the primary disease. Many mechanisms underlying prostate cancer progression and CRPC development have been elucidated. Specifically, it has been suggested that ADT may sensitize prostate cancer cells making them hypersensitive to androgens. These hypersensitive tumor cells can then grow and expand using alternative sources of androgens to develop and progress to more aggressive tumor phenotypes. Intra-tumoral synthesis of androgens has been detected in many patients with advanced diseases, providing direct evidence supporting the above hypothesis for disease progression 34. Therefore, better and combined therapeutic strategies should be considered to overcome the shortcomings of the current ADT in order to eliminate and reduce the androgen-hypersensitive tumor cells.
Multiple lines of evidence indicate abnormally activating Wnt/β-catenin signaling pathways can promote cell proliferation and induce oncogenic transformation 35. Expression of stabilized β-catenin in prostatic luminal cells results in hyperplasia and prostatic intraepithelial neoplasia (PIN) 36, 37. In addition to its oncogenic effect, stabilized β-catenin can also change cellular differentiation fate, which further results in either preventing prostate maturation or inducing transdifferentiation into squamous epithelium in the mouse prostate 36–38. As detailed in this study, we showed that the expression of stabilized β-catenin in prostatic p63-expressing cells induce the formation of proliferative cell cluster, hyperplasia, and PIN formation at different stages of prostate development and maturation. In the presence of androgens, these transformed p63-expressing atypical cells further transdifferentiate to prostatic luminal cells and progress to lesions of high grade PIN and prostatic adenocarcinoma. These observations imply an essential role for androgen signaling in enhancing prostate tumor initiation and progression. It has been shown that an enrichment of AR and Wnt signaling pathways occurs in early-onset prostate cancer, but not in elderly-onset prostate cancer 39, highlighting a clinical significance for a collaborative role of androgen and Wnt signaling pathways. To further validate the critical role of the AR in prostate tumorigenesis, we generated Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ mice in this study. We observed more aggressive PIN and adenocarcinoma lesions in Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+compound mice than in Ctnnb1(ex3)fl/+:p63CreERT2/+ counterparts. A significant increase in the number of Ki67 positive cells was observed in prostate tumor tissues of Ctnnb1(ex3)fl/+:R26hARL/+:p63CreERT2/+ in comparison to ones of Ctnnb1(ex3)fl/+:p63CreERT2/+. We searched for the molecular targets that are responsible for PIN and prostatic adenocarcinoma development in the above mouse models, and observed higher expression of Lef1, c-Myc, Axin2, Cd44, and Bex1 in the prostatic tissues of Ctnnb1(ex3)fl/+;R26hARL/+:p63CreERT2/+ compared to those from Ctnnb1(ex3)fl/+:p63CreERT2/+ mice. A protein-protein interaction between AR and β-catenin has been identified in prostate cancer cells 40–42. As we observed in the above experiments, the AR can enhance β-catenin-mediated transcription to result in prostate cancer aggressiveness and progression through the interaction. Further investigating the interaction between androgen and Wnt signaling pathways during course of prostate cancer initiation and progression may provide fresh insight in designing more effective therapeutic strategies for the treatment of advance prostate cancer patients.
MATERIALS AND METHODS
Mouse mating and genotyping.
P63CreERT2 was generated as described previously 22. RosamTmG (Jackson Laboratories; stock 7676) mice 43 were provided by Dr. Liqun Luo. Ctnnb1(ex3)fl mice were obtained from Dr. Makoto M. Taketo 27. R26hARL/+ mice were generated as described previously 30. All animal experiments performed in this study were approved by the Institutional Animal Care and Use Committee at Beckman Research Institute/City of Hope.
RosamTmG/mTmG and p63CreERT2/+ mice were crossed to generate RosamTmG/+ p63CreERT2/+ mice, which were then used to generate both RosamTmG/+ p63CreERT2/+ and RosamTmG/+ Ctnnb1(Ex3)fl/+ p63CreERT2/+ mice. We then bred p63CreERT2/+ and Ctnnb1(Ex3)fl/fl mice to generate Ctnnb1(Ex3)fl/+ p63CreERT2/+ mice. We further crossed p63CreERT2/+ Ctnnb1(Ex3)fl/fl and R26hARL/+ mice to generate both Ctnnb1(Ex3)fl/+ p63CreERT/+2 and R26hARL/+ Ctnnb1(Ex3)fl/+ p63CreERT/+2 mice. Genomic DNA samples isolated from mouse tail tips or embryo yolk sacs were used for genotyping with appropriate primers (see Supplemental Table 6) as described previously 44, 45.
Mouse procedures.
Castration of adult male mice was performed as described previously 46. For androgen supplement, testosterone pellets (12.5 mg, Innovative Research of America) were placed in mice subcutaneously. For tamoxifen induction, adult mice received a single intraperitoneal injection of 80μg/g body weight tamoxifen (Sigma), prepubescent mice (injected at P14) received a total dose of 1mg of tamoxifen, and pregnant mothers received a single intraperitoneal injection totaling 80μg/g body weight.
In vivo prostate regeneration assay.
Prostatic tissues were collected from different ages of mice and dissected into AP, DLP, and VP. Half of each prostatic lobe was implanted under the renal capsule of SCID mice and the rest of the tissues were prepared for histological analyses. The SCID mice were sacrificed and the graft tissues were collected and used for regrafting and histological analyses. For regeneration assays, either prostatic tissues or recombinants were prepared and implanted under the renal capsule of SCID mice. For tissue recombination assays, mouse urogenital sinus mesenchyme (UGSM) cells collected and cultured as reported previously 47 were combined with approximately 1×105 of mGFP positive prostate epithelial cells collected from cell sorting, and then implanted under the renal capsule of 8-week-old castrated or intact SCID mice as described previously 13, 20.
Histology and immunostaining.
Serial sections were prepared from prostatic tissues for hematoxylin-eosin (H&E) staining or immunohistochemistry (IHC) 13, 20. For immunofluorescence (IF) staining and detection of mTmG signals, mouse tissues were fixed in 4% PFA at 4°C overnight, cryoprotected in 30% sucrose at 4°C overnight, and embedded in OCT (Tissue-Tek). Five micron sections were obtained for mTmG and IF analyses 13. Both primary and second antibodies used in this study were listed in Supplemental Table 8.
RNA Isolation and Reverse Transcription (RT)-Quantitative PCR (qPCR) Assays.
Mouse tissues were homogenized in RNA-Bee (TEL-TEST, Inc., Friendswood, TX) and total RNA was isolated as recommended by the manufacturer. Reverse transcription was carried out as described previously 20. For quantitative PCR, cDNA samples were mixed with SYBR GreenER qPCR Super Mix Universal (11762, Invitrogen) and appropriate primers (Supplemental Table 7) for amplifying specific gene expression in the MX 3005P thermocycler (Stratagene). Relative mRNA levels were calculated 20. Reactions were done in triplicate, and the values were normalized to GAPDH (glyceraldehyde3-phosphate dehydrogenase) expression levels.
Microscope image acquisition.
Images of H&E and IHC were acquired on an Axio Lab. A1 microscope using 10x and 40x Zeiss A-Plan objectives with a Canon EOS 1000D camera and using Axiovision software (Carl Zeiss). Images of immunofluorescence staining and mTmG signals and were acquired on an Nikon ECLIPSE E800 epi-fluorescence microscope using 10x and 40x Nikon Plan Fluor objectives with an QImaging RETIGA EXi camera and using QCapture software (QImaging). Statistical analyses were performed using 2-tailed Student’s t test or 2-way ANOVA.
Supplementary Material
ACKNOWLEDGEMENTS:
This work was supported by NIH grants R01CA070297 (ZS), R01CA151623 (ZS), R01CA166894 (ZS), R21CA190021 (ZS), R01DK104941 (ZS), R01CA193455 (JX), and R01CA112403 (JX).
Footnotes
CONFLICT OF INTEREST: The authors declared no conflict competing financial interest in relation to this study.
References:
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61: 212–236. [DOI] [PubMed] [Google Scholar]
- 2.Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology 2002; 60: 132–138; discussion 138–139. [DOI] [PubMed] [Google Scholar]
- 3.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol 2002; 20: 3001–3015. [DOI] [PubMed] [Google Scholar]
- 4.Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology 1988; 122: 552–562. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZA, Shen MM. Revisiting the concept of cancer stem cells in prostate cancer. Oncogene 2011; 30: 1261–1271. [DOI] [PubMed] [Google Scholar]
- 6.Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 2012; 21: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang ZA, Mitrofanova A, Bergren SK, Abate-Shen C, Cardiff RD, Califano A et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat Cell Biol 2013; 15: 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson DA, Witte ON. Stem cells in prostate cancer initiation and progression. J Clin Invest 2007; 117: 2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science 2010; 329: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawson DA, Zong Y, Memarzadeh S, Xin L, Huang J, Witte ON. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc Natl Acad Sci U S A 2010; 107: 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett 2006; 237: 22–32. [DOI] [PubMed] [Google Scholar]
- 12.Murillo-Garzon V, Kypta R. WNT signalling in prostate cancer. Nat Rev Urol 2017; 14: 683–696. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Johnson DT, Luong R, Yu EJ, Cunha GR, Nusse R et al. Wnt/beta-Catenin-Responsive Cells in Prostatic Development and Regeneration. Stem Cells 2015; 33: 3356–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med 2012; 18: 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol 2003; 201: 204–212. [DOI] [PubMed] [Google Scholar]
- 16.Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, Neilson EG et al. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res 2008; 68: 4709–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC. The androgen receptor can promote beta-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem 2002; 277: 17933–17943. [DOI] [PubMed] [Google Scholar]
- 18.Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res 2000; 60: 4709–4713. [PubMed] [Google Scholar]
- 19.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B et al. Linking beta-catenin to androgen-signaling pathway. J Biol Chem 2002; 277: 11336–11344. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Luong R, Johnson DT, Cunha GR, Rivina L, Gonzalgo ML et al. Androgen signaling is a confounding factor for beta-catenin-mediated prostate tumorigenesis. Oncogene 2016; 35: 702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D et al. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol 2002; 157: 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DK, Liu Y, Liao L, Wang F, Xu J. The prostate basal cell (BC) heterogeneity and the p63-positive BC differentiation spectrum in mice. Int J Biol Sci 2014; 10: 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 1998; 2: 305–316. [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Keyes WM, Papazoglu C, Zuber J, Li W, Lowe SW et al. TAp63 induces senescence and suppresses tumorigenesis in vivo. Nat Cell Biol 2009; 11: 1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol 2000; 157: 1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999; 398: 708–713. [DOI] [PubMed] [Google Scholar]
- 27.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J 1999; 18: 5931–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998; 95: 605–614. [DOI] [PubMed] [Google Scholar]
- 29.Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R et al. Animal models of human prostate cancer: the consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res 2013; 73: 2718–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu C, Luong R, Zhuo M, Johnson DT, McKenney JK, Cunha GR et al. Conditional expression of the androgen receptor induces oncogenic transformation of the mouse prostate. J Biol Chem 2011; 286: 33478–33488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 2009; 461: 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation 2001; 68: 270–279. [DOI] [PubMed] [Google Scholar]
- 33.Pignon J-C, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proceedings of the National Academy of Sciences 2013; 110: 8105–8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res 2008; 68: 6407–6415. [DOI] [PubMed] [Google Scholar]
- 35.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781–810. [DOI] [PubMed] [Google Scholar]
- 36.Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T et al. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene 2003; 22: 3875–3887. [DOI] [PubMed] [Google Scholar]
- 37.Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene 2011; 30: 1868–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruxvoort KJ, Charbonneau HM, Giambernardi TA, Goolsby JC, Qian CN, Zylstra CR et al. Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res 2007; 67: 2490–2496. [DOI] [PubMed] [Google Scholar]
- 39.Weischenfeldt J, Simon R, Feuerbach L, Schlangen K, Weichenhan D, Minner S et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell 2013; 23: 159–170. [DOI] [PubMed] [Google Scholar]
- 40.Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC. The androgen receptor can promote beta -catenin nuclear translocation independently of APC. J Biol Chem 2002. [DOI] [PubMed]
- 41.Truica CI, Hsiung G, Voeller HJ, Gelmann EP. beta-catenin mutation are not sufficient to activate Wnt signaling in prostate cells. AACR Annual Meeting Proceedings 2001; 42: 693. [Google Scholar]
- 42.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B et al. Linking beta-catenin to androgen signaling pathway. J Biol Chem 2002; 277: 11336–11344. [DOI] [PubMed] [Google Scholar]
- 43.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 2007; 45: 593–605. [DOI] [PubMed] [Google Scholar]
- 44.Beliakoff J, Lee J, Ueno H, Aiyer A, Weissman IL, Barsh GS et al. The PIAS-like protein Zimp10 is essential for embryonic viability and proper vascular development. Mol Cell Biol 2008; 28: 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng Y, Clark C, Luong R, Tu WH, Lee J, Johnson DT et al. The leucine zipper putative tumor suppressor 2 protein LZTS2 regulates kidney development. J Biol Chem 2011; 286: 40331–40342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugimura Y, Cunha GR, Donjacour AA. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod 1986; 34: 973–983. [DOI] [PubMed] [Google Scholar]
- 47.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci U S A 2003; 100 Suppl 1: 11896–11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


