Abstract
Separation of a homogeneous mixture of different components to reach an ordered out-of-equilibrium state in solution has attracted continuous attention. While this can be achieved using external chemical fuels or photo energy, an alternative energy source is heat. Here we realize a temperature-controlled cycle of transitions between ordered and disordered states based on a mixture of two kinds of building blocks that self-assemble into cubic structures (nanocubes). An almost statistical mixture of nanocubes (disordered state) is thermodynamically most stable at lower temperature (25 °C), while homoleptic assemblies composed of single components are preferentially produced at higher temperature (100 °C) followed by rapid cooling. The scrambling of the building blocks between the nanocubes takes place through the exchange of free building blocks dissociated from the nanocubes. Based on this mechanism, it is possible to accelerate, retard, and perfectly block the scrambling by the guest molecules encapsulated in the nanocubes.
In this paper, the authors study the temperature-controlled dynamic behavior of a system of nanocubes self-assembled from two different building blocks. Non-intuitively, the disordered, equilibrium state (a mixture of heteroleptic cubes) and the ordered, out-of-equilibrium state (a mixture of homoleptic cubes) are cycled by heating and subsequent rapid cooling.
Introduction
Two kinds of different gases put into a box spontaneously mix together to reach a homogeneous fluid in equilibrium. Such a disordered state is thermodynamically favored and entropy is the quantity that determines which direction a reaction spontaneously proceeds to. Thus, the separation of each component from a homogeneous mixture (towards ordered state) needs external energy or help of Maxwell’s demon if we want to realize the separation without any energy except for information1–4. The homogeneity of a mixture of gases in equilibrium derives from their negligibly weak interactions. Contrary to mixing of gases, molecular self-assembly of multiple components can spontaneously form homoleptic self-assemblies composed of single components through self-sorting under thermodynamic control with the aid of intermolecular interactions between well-designed molecular building blocks that discriminate between oneself and others5–19. Furthermore, in the case where a statistical mixture of components in molecular assemblies is thermodynamically preferred, recent progress on adaptive chemistry has realized the transition of such disordered states into an ordered out-of-equilibrium state by external photo or chemical energy20–34.
Here we present a simple example of a temperature-controlled reversible transition between ordered and disordered states consisting of a mixture of two kinds of molecular building blocks that self-assemble into hexameric cubic structures, i.e. nanocubes, with different thermal stabilities. Because of a structural similarity between the two components (A and B for example), scrambling of the building blocks between the nanocubes (A6 and B6) spontaneously takes place at 25 °C to reach an almost statistical mixture of nanocubes composed of two kinds of building blocks, AnB6–n (n = 0–6), a disordered state. Then, a metastable ordered state (a mixture of homoleptic nanocubes composed of a single component, A6 and B6) is spontaneously recovered by heating at 100 °C and subsequent rapid cooling. The reversible transition between the two states is realized by the change in the energy landscape of the system consisting of two kinds of building blocks in response to the temperature. It is also found that the scrambling is accelerated, retarded, or blocked by guest molecules encapsulated in the nanocubes, which enables us to lock and unlock a metastable state by the encapsulation and release of hydrophobic molecules in the nanocubes.
Results
Scrambling between nanocubes
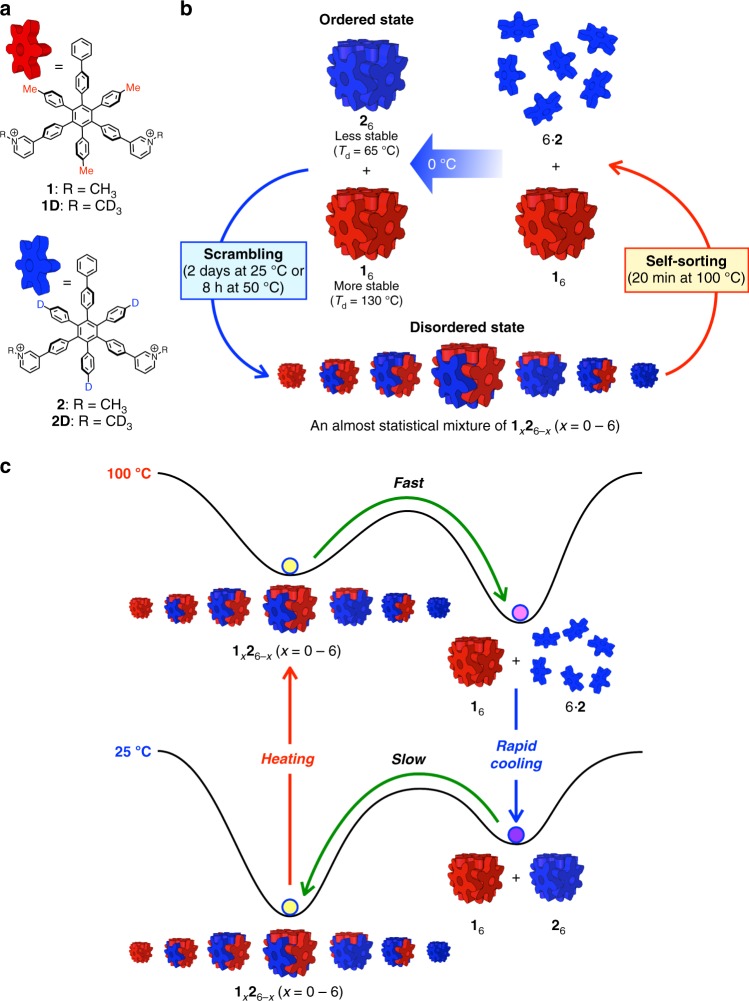
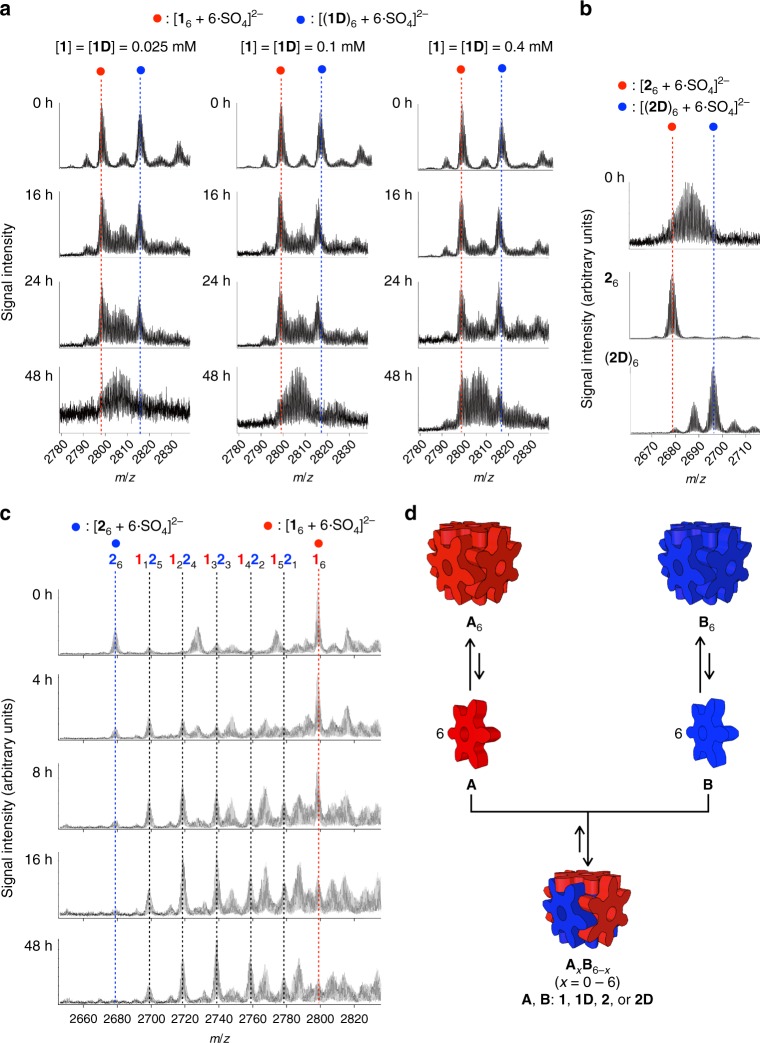
Gear-shaped amphiphiles (GSA: 1·Cl2 and 2·Cl2), which are C2v-symmetric hexaphenylbenzene (HPB) derivatives possessing three kinds of substituents on the periphery of HPB, self-assemble into a cube-shaped structure (nanocubes, 16 and 26) in water driven by the hydrophobic effect and van der Waals (vdW) and cation–π interactions between GSA molecules (Fig. 1a)35–37. Though the structures of 1 and 2 are very similar, 16 is thermally more stable than 26 because vdW interactions around p-tolyl methyl groups of 16 significantly enhance its thermal stability (disassembly temperature (Td) of 16 is 130 °C, while that of 26 is 65 °C). To investigate the relation between the thermal and kinetic stabilities of the nanocubes, scrambling of GSAs between nanocubes assembled from non-deuterated GSAs (16 or 26) and from partially deuterated GSAs ((1D)6 or (2D)6) was monitored by electrospray ionization-time-of flight (ESI-TOF) mass spectrometry. When aqueous solutions of 16 and (1D)6 were mixed at room temperature ([1] = [1D] = 0.025 mM), the scrambling was clearly monitored by change in the distribution of mass signals for [1x(1D)6–x(SO4)6]2– (x = 0–6), which captured two electrons during the ionization (an aqueous H2SO4 solution was added just before the mass measurement in order to detect the nanocubes as negative ion species), and an almost statistical signal pattern was observed in 2 days (Fig. 2a). In contrast, the scrambling for a mixture of thermally less stable nanocubes, 26 and (2D)6, finished just after the mixing of the two solutions (Fig. 2b), indicating that the rate of the scrambling is related to the thermal stability of the nanocubes. It was also found that the rate of the scrambling is dependent on the concentration; the scrambling took place faster at lower concentration of the nanocubes (Fig. 2a). This result indicates that the scrambling does not occur by the collision of the nanocubes but through the exchange of monomer GSAs dissociated from the nanocubes (Fig. 2d) even though the signals for free GSAs were not observed by 1H NMR spectroscopy.
Fig. 1.
A temperature-controlled cycle of transitions between ordered and disordered states in a mixture of nanocubes (16 and 26). a The chemical structures of GSAs used in this research. b A temperature-controlled cycle of the conversion between the ordered and disordered states. An almost statistical mixture of the nanocubes (disordered state) was produced at 25 or 50 °C, while a mixture of two kinds of nanocubes composed of single GSAs was preferentially produced by heating at 100 °C followed by rapid cooling at 0 °C. There are positional isomers for 1x26−x (x = 2–4). c A schematic representation of change in the energy landscape of a mixture of two kinds of GSAs, 1 and 2, in response to the temperature. An almost statistical mixture of nanocubes 1x26–x (x = 0–6) is thermodynamically most stable at 25 °C, while a mixture of 16 and monomer 2 is thermodynamically most stable at 100 °C because decomposition temperatures of the nanocubes composed of more GSAs 2 are lower than 100 °C
Fig. 2.
Monitoring of scrambling of GSAs between the nanocubes by ESI-TOF mass spectrometry and scrambling mechanism. a The scrambling of GSAs between 16 and (1D)6 was monitored in different concentrations. Right after the mixing of aqueous solutions of 16 and (1D)6, strong signals for the homoleptic nanocubes (indicated by solid blue and red circles) were observed. These signals became weak with time and new signals assigned to heteroleptic nanocubes appeared in between the two signals. The scrambling takes place faster in lower concentration of the nanocubes. b The scrambling of GSAs between 26 and (2D)6. The scrambling was completed right after the mixing of 26 and (2D)6, which is much faster than the scrambling between 16 and (1D)6. c The scrambling of GSAs between 16 and 26 was monitored after mixing of aqueous solutions of 16 and 26. The conversion of the less stable nanocube (26) into heteroleptic nanocubes, 1x26−x (x = 1–5), is faster than that of 16. d The scrambling of GSAs between the nanocubes A6 and B6 takes place through the exchange of monomer GSAs dissociated from the nanocubes. After the formation of heteroleptic nanocubes AxB6−x (x = 1–5), the scrambling of AxB6−x (x = 1–5) with the less stable homoleptic nanocube takes place faster than with the more stable one
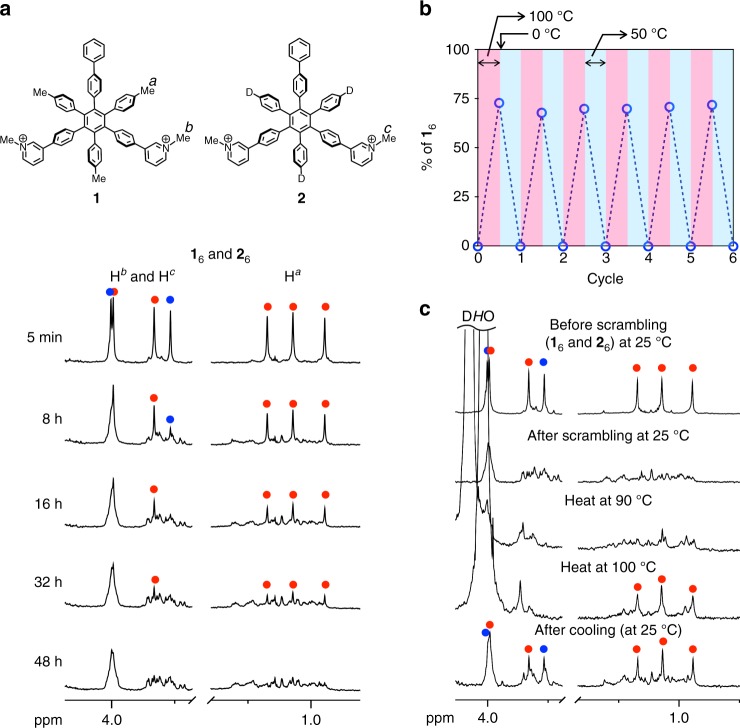
Next, the scrambling between the two different nanocubes (16 and 26) was monitored by ESI-TOF mass spectrometry (Fig. 2c). When 16 and 26 ([1] = [2] = 0.4 mM) were mixed at 25 °C, the signal for 26 decreased faster than that for 16 and finally reached an almost statistical compositional mixture of the nanocubes in 2 days. The scrambling in the same concentration of the nanocubes ([1] = [2] = 0.4 mM) was monitored by 1H NMR spectroscopy (Fig. 3a and Supplementary Fig. 3). The 1H NMR spectra of a 1:1 mixture of 16 and 26 at 25 °C changed for 2 days, which is consistent with the time taken for the convergence of mass signals of a mixture of 16 and 26. As observed by the mass measurements, the 1H NMR signals for 26 decreased faster than those for 16, indicating that the heteroleptic nanocubes, 1x26−x (x = 1–5), tend to preferentially exchange GSAs with 26 because the scrambling between less stable nanocubes takes place faster, which supports the idea that the scrambling takes place through the exchange of monomer GSAs dissociated from the nanocubes. The scrambling was accelerated by heating and reached convergence in 8 h at 50 °C.
Fig. 3.
Scrambling and induced-sorting of GSAs between the nanocubes (16 and 26). The scrambling and induced-sorting were monitored by 1H NMR spectroscopy (500 MHz, D2O, p-tolyl methyl and N-methyl group regions, [1] = [2] = 0.4 mM). a The monitoring of the scrambling of GSAs between 16 and 26 at 298 K. Red and blue solid circles indicate the signals for 16 and 26, respectively. b A plot of the existence ratio of 16 in a 1:1 mixture of 1 and 2 during six cycles of the transitions between the ordered and disordered states by changing temperature. c 1H NMR monitoring of the transition in the first cycle. Red and blue solid circles indicate the signals for 16 and 26, respectively. The temperatures at which the measurements were carried out are indicated in the spectra
Interconversion between ordered and disordered states
Considering the fact that 16 (Td = 130 °C) is thermally more stable than 26 (Td = 65 °C) due to the vdW interactions around p-tolyl methyl groups in 1, nanocubes composed of more 2 would be less stable than those composed of more 1. Hence, at higher temperature than Td of 26, the nanocubes composed of more 2 should be disassembled to lead to a mixture of monomer 2 and the nanocubes composed of more 1. Then rapid cooling of the mixture of the nanocubes (mainly 16) and free 2 thus obtained leads to a mixture mainly containing homoleptic nanocubes (16 and 26) through faster self-assembly of monomer 2 into 26 than the scrambling (Fig. 1b, c). As expected, heating of a mixture of 16 and 26 at 100 °C led to the homoleptic 16 in 83% yield (Fig. 3c and Supplementary Fig. 5). Subsequent rapid cooling at 0 °C gave homoleptic nanocubes (ordered state) in 70.7 ± 0.7% yield (Fig. 3c and Supplementary Fig. 6). At 50 °C, this mixture was gradually converted into an almost statistical mixture of nanocubes 1x26–x (x = 0–6) (disordered state), which is thermodynamically most stable at 50 °C. The interconversion between the ordered and disordered states is repeatable simply by changing the temperature of the solution without any degradation because the GSAs are completely stable even at 100 °C (Fig. 3b).
Guest effect on scrambling
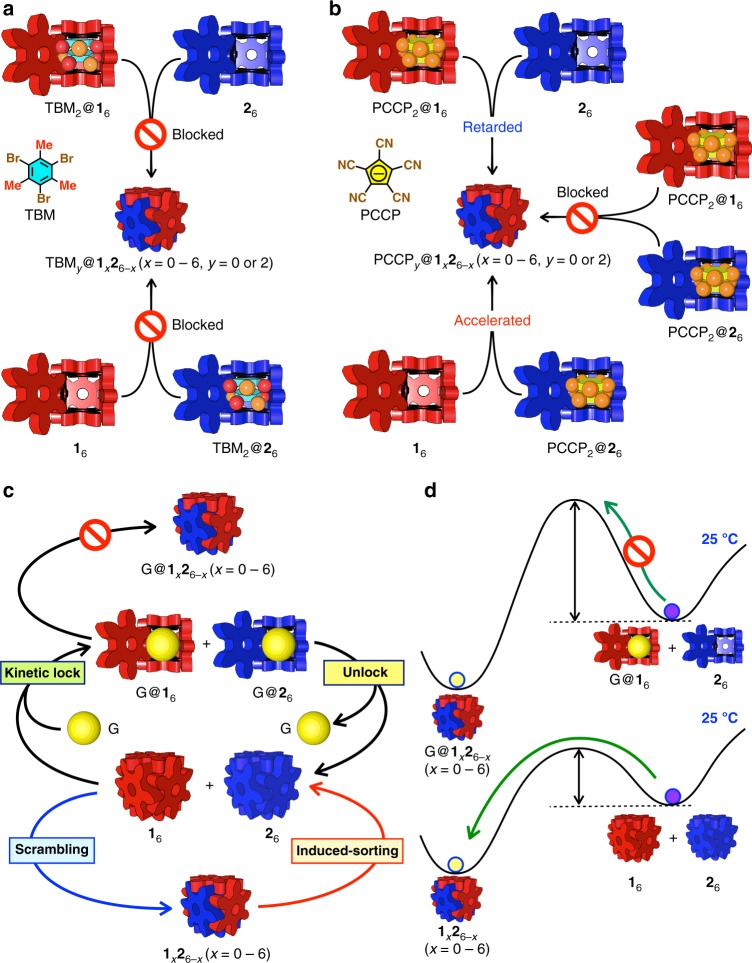
As the nanocubes can encapsulate neutral and anionic species in their about-1-nm-sized hydrophobic inner space to lead to thermally more stable nanocubes37, the guest species would prevent the dissociation of the nanocubes into the monomers, thereby retarding the scrambling. When aqueous solutions of 16 encapsulating two molecules of 1,3,5-tribromomesitylenes (TBM), TBM2@16 (Td > 150 °C), and of 26 (Td = 65 °C) were mixed, nothing happened for 6 months (Supplementary Fig. 7); the scrambling of GSAs and the distribution of TBM molecules between the nanocubes were perfectly blocked (Fig. 4a). The same result was found when aqueous solutions of TBM2@26 (Td = 135 °C) and 16 (Td = 130 °C) were mixed (Supplementary Fig. 8). These results indicate that the encapsulated hydrophobic guest molecules stabilize the nanocubes to change the energy landscape, resulting in the suppression of the dissociation of free GSAs (Fig. 4d). Interestingly, when insoluble TBM existed in an aqueous solution of TBM2@26 and 16, the scrambling took place slowly. (It took 26 days to reach equilibration, which is longer than the time for the scrambling between 16 and 26, 2 days.) (Supplementary Fig. 9). Insoluble TBM molecules were gradually encapsulated in 16, during which the structure of the 16 nanocube would partially be broken or free GSAs would be dissociated. Hence, these (partially) dissociated GSAs of 1 should promote the scrambling. On the other hand, no scrambling took place when insoluble TBM was present in a solution of TBM2@16 and 26, though gradual encapsulation of TBM in 26 was observed (Supplementary Fig. 10). In this case, though the encapsulation of TBM into 26 caused the dissociation of monomer GSA 2, the dissociation of monomer GSA 1 was strongly prevented by the encapsulation of TBM into 16 (Td > 150 °C), so the scrambling did not take place. These results indicate that whether the scrambling takes place or not is determined by the existence of monomer GSAs (1 and 2) and that in the case where the thermal stabilities of the two nanocubes are significantly different the rate-determining step of the scrambling is the dissociation of monomer GSAs from a thermally more stable nanocube.
Fig. 4.
The effect of guest molecules on the scrambling of GSAs between the nanocubes (16 and 26). a The encapsulation of hydrophobic guest molecules (TBM: 1,3,5-tribromomesitylene) in 16 or 26 blocked the scrambling. b Anionic guest molecule (PCCP: pentacyanocyclopentadienide) accelerated, retarded, and blocked the scrambling depending on the way of the encapsulation of PCCP in the nanocubes. c A cycle of the transition between the ordered and disordered states coupled with the kinetic lock and unlock of the ordered state by the encapsulation and release of guest molecules (G) in the nanocubes. G indicates two or three molecules of n-hexanes. Three molecules of n-hexanes are encapsulated in 16, while two in 26. The removal of G from the nanocubes was carried out by heating at 100 °C. d A schematic representation of change in the energy landscape of the scrambling of GSAs with hydrophobic guest molecules (G). A mixture of G@16 and 26 is kinetically stable at 25 °C (a) because the encapsulated hydrophobic guest molecules stabilize the nanocube, giving rise to high activation energy of the scrambling. The same effect works in the cases where the scrambling starts from a mixture of 16 and G@26 (a) and from a mixture of G@16 and G@26 (c)
Quite different results were observed when anionic species (NaPCCP: sodium pentacyanocyclopentadienide) was used as a guest molecule (Fig. 4b). When PCCP2@16 and 26 were mixed together, the scrambling was significantly retarded (65% of PCCP2@16 and 20% of 26 existed in the solution after 1 month) (Supplementary Fig. 11). Furthermore, the scrambling was perfectly blocked by the encapsulation of PCCP in both of the nanocubes (Supplementary Fig. 12). Surprisingly, the addition of 2 eq of PCCP in a 1:1 mixture of 16 and 26 accelerated the scrambling (the scrambling finished in 1 day, which is faster than that carried out in the absence of PCCP (2 days)) (Supplementary Fig. 13). Right after the addition of PCCP in a solution of 16 and 26, all PCCP molecules were selectively encapsulated in thermally less stable 26 under kinetic control. Then the exchange of PCCP between the nanocubes coincided with the scrambling of GSAs between the nanocubes. A simple mixing of 16 and PCCP2@26 also accelerated the scrambling (1 day) (Supplementary Fig. 14). The acceleration of the scrambling under this condition is ascribed to the dissociation of monomer 1 initiated by the transfer of PCCP encapsulated in 26 into 16 (PCCP2@26 + 16 PCCP2@16 + 26).
Because of the lower stability of the ordered state at 25 °C, spontaneous scrambling takes place to reach the disordered state. To control the timing of the scrambling, a guest-encapsulation-release system was coupled with the cycle of transitions (Fig. 4c and Supplementary Fig. 15). After the formation of a mixture of homoleptic nanocubes from the disordered state, a hydrophobic guest molecule (Hex: n-hexane) was added in the mixture to form the nanocubes that encapsulate the guest molecules, Hex3@16 and Hex2@26. The scrambling of the GSAs between the two nanocubes was blocked for 7 days, indicating that the metastable ordered state was kinetically locked. The removal of the guest molecules by heating at 100 °C is the trigger to unlock the ordered state to lead to a mixture of guest-free homoleptic nanocubes, 16 and 26, which then was converted into the disordered state again.
Discussion
In conclusion, a temperature-controlled cycle of transitions that is fabricated from two kinds of building blocks that self-assemble into homoleptic and heteroleptic cubic assemblies (nanocubes) with different thermal stability was realized. The scrambling experiments (intermolecular exchange) of the building blocks (GSAs) between the nanocubes indicate that the kinetic stability of the nanocube is closely related to the thermal stability of the nanocubes; a thermally more stable nanocube is kinetically more inert. The concentration effect on the rate of the scrambling and the results obtained from the scrambling experiments in the presence of guest molecules indicate that the scrambling takes place through the exchange of free GSAs dissociated from the nanocubes and that the rate of the scrambling is determined by the dissociation of free GSAs provided from a thermally more stable nanocube. The interconversion between the metastable ordered (a mixture of 16 and 26) and the thermodynamically most stable disordered states (an almost statistical mixture of 1x26–x (x = 0–6)) was realized simply by changing the temperature. The result that the induced-sorted state was preferred by heating at 100 °C and subsequent rapid cooling was realized because only the nanocubes composed of more 1 can survive at this temperature. The rate of the scrambling can be controlled by anionic guest species. The blocking of the scrambling by the encapsulation of guest molecules would enable to kinetically capture a certain metastable species that is selected from a dynamic combinatorial library of compositional and positional isomers of nanocubes consisting of several kinds of GSAs in response to external stimuli.
Methods
General
1H and 13C NMR spectra were recorded using a Bruker AV-500 (500 MHz) spectrometer. High-resolution mass spectra (HRMS) were obtained using a Waters Xevo G2-S QTOF mass spectrometer. All reagents were obtained from commercial suppliers (TCI Co., Ltd., WAKO Pure Chemical Industries Ltd., KANTO Chemical Co., Inc., and Sigma-Aldrich Co.) and were used as received. GSAs 1·Cl2 and 2·Cl2 and compounds 3 and 4 were synthesized according to previously reported procedures35. See supplementary information for synthetic procedures, including Supplementary Figs. 16–19.
Host–guest complexation
The solutions of TBM2@16, TBM2@26 (TBM indicates 1,3,5-tribromomesitylene), PCCP2@16 (PCCP indicates pentacyanocyclopentadienide) and Hex3@16 (Hex indicates n-hexane) were prepared according to the literatures35, 37. Hex (1 μL) was added by a syringe to a D2O solution of 26 ([2] = 1.0 mM, 600 μL) in an NMR tube. The suspension was mixed by inverting the NMR tube four times and sonicated for 5 min to afford Hex2@26 (Supplementary Fig. 1). The formation of PCCP2@26 was confirmed by the titration experiments using the 1H NMR signals of the nanocube encapsulating PCCPs (Supplementary Fig. 2).
Monitoring of scrambling by ESI-TOF mass spectrometry
ESI-TOF mass spectra of the scrambling of the nanocubes in water were recorded on a SYNAPT G2-Si HDMS mass spectrometer (Waters, Massachusetts, Milford, USA) in negative ionization mode at 0.88 kV with a 0 V sampling cone voltage and source offset voltage, 4 V trap and 2 V transfer collision energy, and 1.5 mL/min trap gas flow. The scrambling experiment between 16 and 1D6 was presented as an example. Solutions of 16 and of 1D6 ([GSA] = 0.8 mM) in H2O were prepared separately. The solutions of 16 (150 μL) and of 1D6 (150 μL) were added to an Eppendorf microcentrifuge tube. Then, the concentration of GSAs was adjusted to be 0.4, 0.1, and 0.025 mM by addition of Milli-Q water. The scrambling of the GSAs was monitored by mass spectrometry. A portion of the mixture was taken. After addition of 1 mM H2SO4 aq., this solution was immediately injected into gold-coated glass capillaries made in house (~5 μL sample loaded per analysis) and the mass spectrum was recorded. The spectra were calibrated using 1 mg/mL CsI and analyzed using MassLynx software (Waters).
Monitoring of scrambling by 1H NMR spectroscopy
Solutions of 16 and of 26 ([GSA] = 2 mM) in D2O were prepared separately. A solution of TMACl (8 mM, TMACl indicates tetramethylammonium chloride) in D2O (15 μL), which was used as an internal standard, was added to an Eppendorf microcentrifuge tube. Then the solutions of 16 (120 μL) and of 26 (120 μL) in D2O and D2O (345 μL) were added to the Eppendorf microcentrifuge tube to adjust the concentration of GSAs to 0.4 mM or the solutions of 16 (150 μL) and of 26 (150 μL) in D2O and D2O (285 μL) were added to the Eppendorf microcentrifuge tube to adjust the concentration of GSAs to 0.5 mM. The scrambling of the GSAs was monitored at 25 °C by 1H NMR spectroscopy. The existence ratios of 16 and 26 were determined by their integrated values against that of the signal of TMA+. Three p-tolyl methyl signals observed at 0.5–2 ppm were used to determine the existence ratio of 16. As 26 lacks p-tolyl methyl groups, N-methyl signals at 3–4 ppm were used to determine the existence ratio of 26. In order to confirm the reproducibility of the measurements, the same experiments were carried out three times. Similar procedures were conducted for the scrambling of the nanocubes with guest molecules. See supplementary information for more details (Supplementary Figs. 3, 4 and 7–14).
Interconversion between ordered and disordered states in a mixture of nanocubes
An almost statistical mixture of the nanocubes 1x26–x (x = 0–6) was produced from a mixture of 16 and 26 ([1] = [2] = 0.5 mM) at 25 °C for 3 days or at 50 °C for 8 h. A mixture of 16 and 26 was preferentially produced by heating at 100 °C for 20 min followed by rapid cooling at 0 °C for 20 s. The induced-sorting of 16 and 26 was monitored by variable temperature 1H NMR measurements (Supplementary Figs. 5 and 6).
Kinetic lock and unlock of the induced-sorted state by the encapsulation and release of guest molecules
n-Hexane (Hex) (1 μL) was added via a syringe to a D2O solution of 16 and 26 ([1] = [2] = 0.5 mM) in an NMR tube. The suspension was mixed by shaking the NMR tube for 30 s to afford a D2O solution of Hex3@16 and Hex2@26. The encapsulated n-hexane was removed upon heating at 100 °C for 60 min and then a mixture of 16 and 26 was obtained by rapid cooling at 0 °C for 20 s (Supplementary Fig. 15).
Supplementary information
Acknowledgements
This research was supported by JSPS Grants-in-Aid for Scientific Research on Innovative Areas “Dynamical Ordering of Biomolecular Systems for Creation of Integrated Functions” (25102001 and 25102005), the Mitsubishi Foundation, Sekisui Integrated Research, and the Joint Research by Exploratory Research Center on Life and Living Systems (ExCELLS).
Author contributions
Y.-Y.Z., T.K., S.T., and S.H. conceived the project. S.H. prepared the manuscript and all the authors discussed the results and commented on the manuscript. Y.-Y.Z. synthesized all the GSAs and carried out NMR measurements. K.I. and S.U. carried out mass measurements. Y.H. and H.M. synthesized NaPCCP.
Data availability
The authors declare that all the other data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09495-1.
References
- 1.Yang R. Gas Separation by Adsorption Processes. Kent: Elsevier Science; 2014. [Google Scholar]
- 2.Bernardo P, Drioli E, Golemme G. Membrane gas separation: a review/state of the art. Ind. Eng. Chem. Res. 2009;48:4638–4663. doi: 10.1021/ie8019032. [DOI] [Google Scholar]
- 3.Leff H, Rex AF. Maxwell’s Demon: Entropy, Information, Computing. Bristol: Adam Hilger; 1990. [Google Scholar]
- 4.Leff, H. & Rex, A. F. Maxwell’s Demon 2: Entropy, Classical and Quantum Information, Computing (CRC Press, Boca Raton, 2002).
- 5.Liu M, Zhang L, Wang T. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015;115:7304–7397. doi: 10.1021/cr500671p. [DOI] [PubMed] [Google Scholar]
- 6.Jędrzejewska H, Szumna A. Making a right or left choice: chiral self-sorting as a tool for the formation of discrete complex structures. Chem. Rev. 2017;117:4863–4899. doi: 10.1021/acs.chemrev.6b00745. [DOI] [PubMed] [Google Scholar]
- 7.Pritchard VE, et al. Homochiral self-sorted and emissive IrIII metallo-cryptophanes. Chem. Eur. J. 2017;23:6290–6294. doi: 10.1002/chem.201701348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safont-Sempere MM, et al. Impact of molecular flexibility on binding strength and self-sorting of chiral π-surfaces. J. Am. Chem. Soc. 2011;133:9580–9591. doi: 10.1021/ja202696d. [DOI] [PubMed] [Google Scholar]
- 9.Makiguchi W, et al. Chirality- and sequence-selective successive self-sorting via specific homo- and complementary-duplex formations. Nat. Commun. 2015;6:7236. doi: 10.1038/ncomms8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safont-Sempere MM, Fernández G, Würthner F. Self-sorting phenomena in complex supramolecular systems. Chem. Rev. 2011;111:5784–5814. doi: 10.1021/cr100357h. [DOI] [PubMed] [Google Scholar]
- 11.Petryk M, Biniek K, Janiak A, Kwit M. Unexpected narcissistic self-sorting at molecular and supramolecular levels in racemic chiral calixsalens. CrystEngComm. 2016;18:4996–5003. doi: 10.1039/C6CE00256K. [DOI] [Google Scholar]
- 12.Sisco SW, Moore JS. Homochiral self-sorting of BINOL macrocycles. Chem. Sci. 2014;5:81–85. doi: 10.1039/C3SC52018H. [DOI] [Google Scholar]
- 13.Gidron O, et al. Homochiral [2]catenane and bis[2]catenane from alleno- acetylenic helicates—a highly selective narcissistic self-sorting process. J. Am. Chem. Soc. 2015;137:12502–12505. doi: 10.1021/jacs.5b08649. [DOI] [PubMed] [Google Scholar]
- 14.Prins LJ, De Jong F, Timmerman P, Reinhoudt DN. An enantiomerically pure hydrogen-bonded assembly. Nature. 2000;408:181–184. doi: 10.1038/35041530. [DOI] [PubMed] [Google Scholar]
- 15.Yan LL, et al. Stereocontrolled self-assembly and self-sorting of luminescent europium tetrahedral cages. J. Am. Chem. Soc. 2015;137:8550–8555. doi: 10.1021/jacs.5b03972. [DOI] [PubMed] [Google Scholar]
- 16.Ronson TK, Roberts DA, Black SP, Nitschke JR. Stacking interactions drive selective self-assembly and self-sorting of pyrene-based MII4L6 architectures. J. Am. Chem. Soc. 2015;137:14502–14512. doi: 10.1021/jacs.5b09920. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Q, et al. Self-sorting of heteroanions in the assembly of cross-shaped polyoxometalate clusters. J. Am. Chem. Soc. 2018;140:2595–2601. doi: 10.1021/jacs.7b11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, et al. Assembled molecular face-rotating polyhedra to transfer chirality from two to three dimensions. Nat. Commun. 2016;7:12469. doi: 10.1038/ncomms12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shigemitsu H, et al. An adaptive supramolecular hydrogel comprising self-sorting double nanofibre networks. Nat. Nanotech. 2018;13:165–172. doi: 10.1038/s41565-017-0026-6. [DOI] [PubMed] [Google Scholar]
- 20.Lehn JM. Perspectives in chemistry—steps towards complex matter. Angew. Chem. Int. Ed. 2013;52:2836–2850. doi: 10.1002/anie.201208397. [DOI] [PubMed] [Google Scholar]
- 21.Lehn JM. Perspectives in chemistry—aspects of adaptive chemistry and materials. Angew. Chem. Int. Ed. 2015;54:3276–3289. doi: 10.1002/anie.201409399. [DOI] [PubMed] [Google Scholar]
- 22.Herder M, Lehn JM. The photodynamic covalent bond: sensitized alkoxyamines as a tool to shift reaction networks out-of-equilibrium using light energy. J. Am. Chem. Soc. 2018;140:7647–7657. doi: 10.1021/jacs.8b03633. [DOI] [PubMed] [Google Scholar]
- 23.Boekhoven J, Hendriksen WE, Koper GJM, Eelkema R, van Esch JH. Transient assembly of active materials fueled by a chemical reaction. Science. 2015;349:1075–1079. doi: 10.1126/science.aac6103. [DOI] [PubMed] [Google Scholar]
- 24.Sorrenti A, Leira-Iglesias J, Markvoort AJ, de Greef TFA, Hermans TM. Non-equilibrium supramolecular polymerization. Chem. Soc. Rev. 2017;46:5476–5490. doi: 10.1039/C7CS00121E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osowska K, Miljanić OScaron. Oxidative kinetic self-sorting of a dynamic imine library. J. Am. Chem. Soc. 2011;133:724–727. doi: 10.1021/ja109754t. [DOI] [PubMed] [Google Scholar]
- 26.Vantomme G, Jiang S, Lehn JM. Adaptation in constitutional dynamic libraries and networks, switching between orthogonal metalloselection and photoselection processes. J. Am. Chem. Soc. 2014;136:9509–9518. doi: 10.1021/ja504813r. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, Yue L, Wang S, Lehn JM, Willner I. DNA-based multiconstituent dynamic networks: hierarchical adaptive control over the composition and cooperative catalytic functions of the systems. J. Am. Chem. Soc. 2018;140:12077–12089. doi: 10.1021/jacs.8b06546. [DOI] [PubMed] [Google Scholar]
- 28.Ragazzon G, Prins LJ. Energy consumption in chemical fuel-driven self-assembly. Nat. Nanotech. 2018;13:882–889. doi: 10.1038/s41565-018-0250-8. [DOI] [PubMed] [Google Scholar]
- 29.van Rossum SAP, Tena-Solsona M, van Esch JH, Eelkema R, Boekhoven J. Dissipative out-of-equilibrium assembly of man-made supramolecular materials. Chem. Soc. Rev. 2017;46:5519–5535. doi: 10.1039/C7CS00246G. [DOI] [PubMed] [Google Scholar]
- 30.Merindol R, Walther A. Materials learning from life: concepts for active, adaptive and autonomous molecular systems. Chem. Soc. Rev. 2017;46:5588–5619. doi: 10.1039/C6CS00738D. [DOI] [PubMed] [Google Scholar]
- 31.Zhao H, et al. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks. Nat. Nanotech. 2016;11:82–88. doi: 10.1038/nnano.2015.256. [DOI] [PubMed] [Google Scholar]
- 32.Kathan M, Hecht S. Photoswitchable molecules as key ingredients to drive systems away from the global thermodynamic minimum. Chem. Soc. Rev. 2017;46:5536–5550. doi: 10.1039/C7CS00112F. [DOI] [PubMed] [Google Scholar]
- 33.Hess H, Ross JL. Nonequilibrium assembly of microtubules: from molecules to autonomous chemical robots. Chem. Soc. Rev. 2017;46:5570–5587. doi: 10.1039/C7CS00030H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tena-Solsona M, Wanzke C, Riess B, Bausch AR, Boekhoven J. Self-selection of dissipative assemblies driven by primitive chemical reaction networks. Nat. Commun. 2018;9:2044. doi: 10.1038/s41467-018-04488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhan YY, et al. Hyperthermostable cube-shaped assembly in water. Commun. Chem. 2018;1:14. doi: 10.1038/s42004-018-0014-2. [DOI] [Google Scholar]
- 36.Zhan YY, Kojima T, Koide T, Tachikawa M, Hiraoka S. A balance between van der Waals and cation–π interactions that stabilizes hydrophobic assemblies. Chem. Eur. J. 2018;24:9130–9135. doi: 10.1002/chem.201801376. [DOI] [PubMed] [Google Scholar]
- 37.Zhan YY, et al. Induced-fit expansion and contraction of a self-assembled nanocube finely responding to neutral and anionic guests. Nat. Commun. 2018;9:4530. doi: 10.1038/s41467-018-06874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the other data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author upon request.