Abstract
An unprecedented reaction of thiourea derivatives with 6β-bromoandrostenedione has been discovered for the formation of aminothiazolo-androstenones via a simple, safer, cascade protocol that enables the syntheses of novel molecules by using readily available reagents. The reaction mechanism of product formation has been rationalized by density functional theory calculations. This benign methodology accentuates a domino protocol deploying a renewable solvent, ethanol, while generating novel compounds that display potent growth inhibitory effects in in vitro studies for several cancer cell lines at submicromolar concentrations.
Graphical Abstract
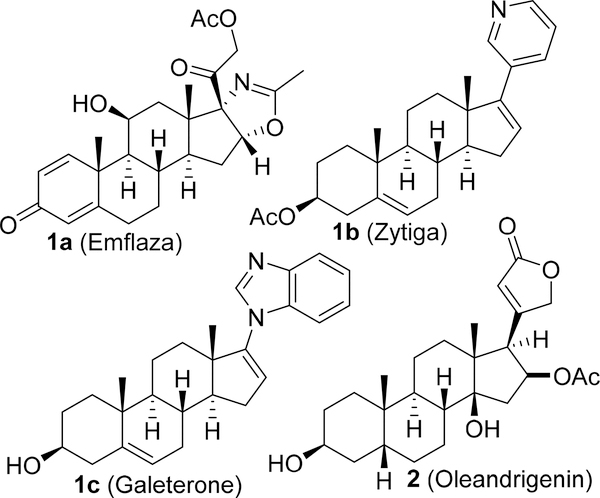
Steroidal hormones are involved in a number of biological signaling processes1,2 with a large number being of natural products isolated from various plants and microorganisms. These molecules are known for their wide-ranging biological activities,3 and therefore, not surprisingly, assorted synthetic derivatives have been reported in the pursuit of drugs, drug candidates, and other useful entities such as herbicides.4–12 Steroidal derivatives comprise one of the broadest spectra of the therapeutic class of compounds and are being used extensively in modern medicine to treat different anomalies, including cancer.10,13 Both natural and synthetic steroidal derivatives are known for their therapeutic properties such as agonists of cell-surface G-protein coupled bile acid receptor 1 (GP-BAR1),8 neuroactive,9 anticancer,14 anti-Alzheimer,15 and several other medicinal properties.16 Thiazole derivatives are another class of pharmacologically important compounds with several approved drugs in this category including dasatinib and ritonavir.17,18 Heterocyclic rings comprise several steroidal-based drugs including recently approved Emflaza (deflazacort) to treat Duchenne muscular dystrophy (DMD) and Zytiga (abiraterone acetate) to treat metastatic castration-resistant prostate cancer.19–23 Heterocycle-attached androstanes, galeterone22 and oleandrigenin,23 are examples of a drug in clinical trials and a natural product, respectively (Figure 1).
Figure 1.
Representative examples of heterocycle containing steroidal drugs (1a, 1b, and 1c) and a natural product (2).
In view of the importance of heterocycle-bearing steroidal derivatives, a large number of methodologies and synthetic schemes have been described,24–30 often involving multistep synthesis.6–9,31 Recently, Stanley et al. have reported the synthesis of heteroarylated steroidal diene by using bismuth triflate as a catalyst.32
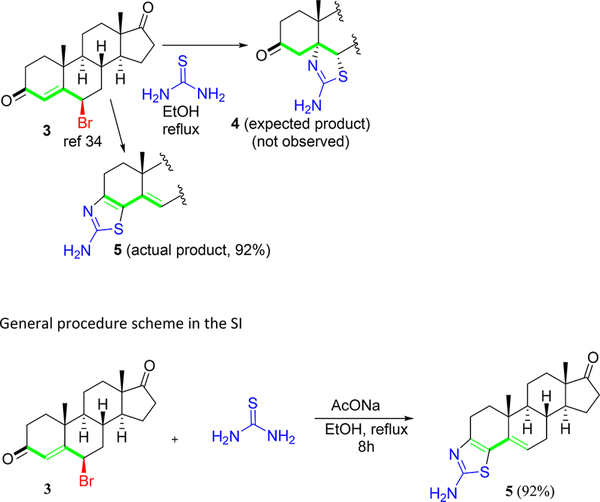
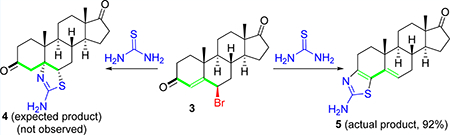
In our pursuit of synthesizing bioactive heterocycles,33–36 we envisaged the synthesis of thiazolino-androstanedione derivatives via our recently developed methodology that entails a 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) mediated domino reaction of γ-bromo enones (3) with thioamides and thioureas to form thiazoline products (4) under refluxing conditions.37 Surprisingly, hitherto unknown 3,4-thiazolo-androstenone product (5) was formed instead of the expected 5,6-thiazolino-androstenedione derivative (4) (Scheme 1). The reaction was conducted in various solvents (see Table 1, Supporting Information (SI)), and to our delight, the reaction occurred in a renewable and recyclable solvent, ethanol (EtOH), without compromising the yield and purity and precluded anhydrous reaction conditions or inert atmosphere. Product 5 was formed in 92% yield on a gram scale synthesis, and the pure material was isolated simply by filtration followed by washing with ethanol and water.
Scheme 1.
Synthesis of Thiazolo-androstenone Derivatives (5)
Table 1.
NCI Data for Selected Cell Lines for Two Compoundsa
| GI50 |
|||
|---|---|---|---|
| cancer panel | cell line | 17 | 23 |
| leukemia | CCRF-CEM | 1.90 | 2.45 |
| K-562 | 1.86 | 2.86 | |
| MOLT-4 | 1.68 | 2.49 | |
| RPMI-8226 | 0.86 | 2.43 | |
| SR | 0.85 | 2.32 | |
| NSCLC | HOP-62 | 2.07 | 1.42 |
| NCI-H522 | 1.63 | 1.65 | |
| colon cancer | HCC-2998 | 3.55 | 1.90 |
| HCT-116 | 1.59 | 1.49 | |
| HCT-15 | 1.58 | 2.68 | |
| HT29 | 1.49 | 2.55 | |
| CNS cancer | SF-295 | 1.19 | 1.81 |
| SF-539 | 1.34 | 1.62 | |
| SNB-75 | 1.52 | 2.19 | |
| U251 | 1.59 | 1.69 | |
| melanoma | LOX IMVI | 1.67 | 1.64 |
| MALME-3M | 1.94 | 1.99 | |
| M14 | 2.36 | 1.85 | |
| SK-MEL-2 | 1.86 | 2.19 | |
| SK-MEL-28 | 1.52 | 2.31 | |
| SK-MEL-5 | 2.02 | 1.86 | |
| UACC-62 | 1.54 | 1.72 | |
| ovarian cancer | IGROV1 | 1.89 | 2.11 |
| OVCAR-3 | 1.72 | 2.18 | |
| renal cancer | 786–0 | 1.43 | 1.62 |
| ACHN | 1.78 | 2.08 | |
| CAKI-1 | 1.99 | 2.83 | |
| RXF 393 | 1.45 | 1.74 | |
| TK-10 | 1.97 | 2.46 | |
| UO-31 | 1.67 | 1.34 | |
| prostate cancer | PC-3 | 1.88 | 1.86 |
| DU-145 | 1.93 | 2.04 | |
| breast cancer | MCF7 | 1.37 | 2.31 |
| BT-549 | 1.54 | 1.49 | |
| MDA-MB-468 | 2.01 | 1.93 | |
GI50 = concentration of a compound that causes 50% growth inhibition.39
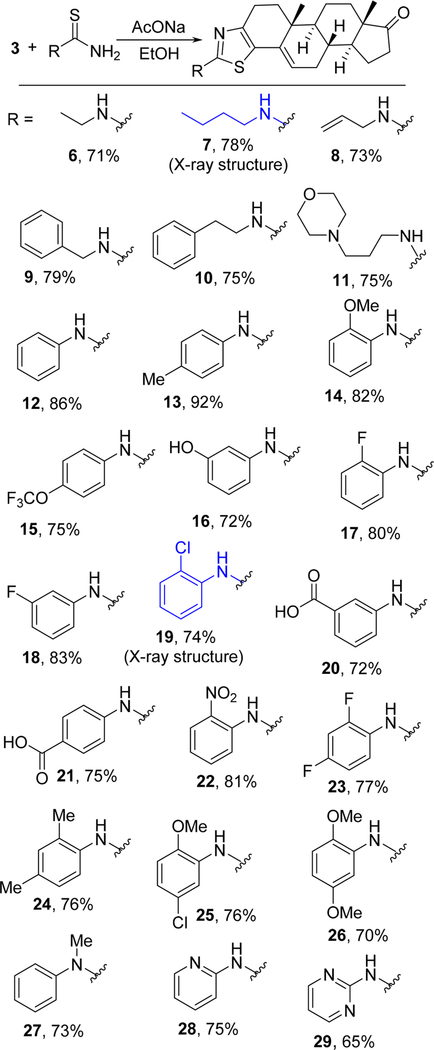
After identification of the formed product, thiazolo-androstenone (5), under the optimized conditions, we carried out the reaction of substituted thiourea derivatives under the same reaction conditions; the expected products were formed in good to excellent yield. This reaction is very general for a wide range of substituted thiourea derivatives. Reaction of alkyl-substituted thioureas with the electrophile (3) afforded the products 6–10 in very good yields (71–79%). Morpholine, a hydrophilic substituent, attached to alkyl thiourea reacted smoothly to give the corresponding product (11) in 75% yield. Similarly, arylthiourea derivatives also reacted with the electrophile and delivered products without affecting the average yield and purity; N-phenyl thiourea provided (12) in 86% yield. Electron-donating groups on the aryl ring of thiourea provided the desired compounds without compromising the yield and purity; namely, toluenyl product (13) in 92% yield as well as methoxy-, trifluoromethoxy-, and hydroxyphenyl-substituted derivatives (14, 15, and 16) are formed efficiently. In identifying the scope of the methodology, substrates with electronwithdrawing groups on the phenyl ring were also reacted with electrophile (3), and the expected products were produced such as fluoro- and chloro-substituted entities (17, 18, and 19) in 80, 83, and 74% yields, respectively. The single-crystal structure of compound 19 (CCDC 1858408) is available in the Supporting Information. Carboxylic acid substituted products (20 and 21) were formed in an average yield of ~73%.
This general methodology also tolerated strong electron-withdrawing substituents; the nitro group on the phenyl ring afforded 22 in 81% yield, while disubstituted products 23–26 were also formed efficiently (70–77%). N,N-Disubstituted thiourea did not hamper the reaction either, and the expected product 27 was formed in 73% yield along with pyridine and pyrimidine products 28 and 29 (Scheme 2).
Scheme 2.
Reaction of Various Thiourea Derivatives with 6β-Bromoandrostenedione (3)
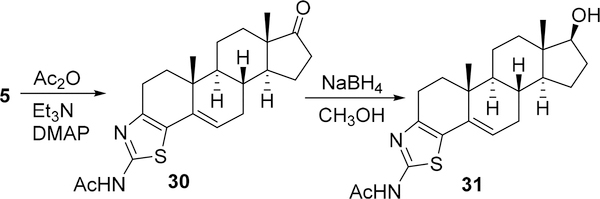
To test the scope of the methodology to generate a library of new molecules as potential therapeutic agents, one of the compounds (5) was synthesized on multigram scale and further derivatized by simple transformations. Reaction of the amino-thiazolo derivative 5 with acetic anhydride formed acetamido product 30, which on NaBH4 reduction afforded the hydroxy product 31 (Scheme 3). The average yields of these reactions are 93%, and the products were simply obtained by filtering and washing the solid with methanol and water. In our preliminary in vitro anticancer studies, hydroxy compounds have shown several times better activity than the parent ketone compounds (data not shown).
Scheme 3.
Derivatization of Heterocycle-Fused Steroidal Molecules
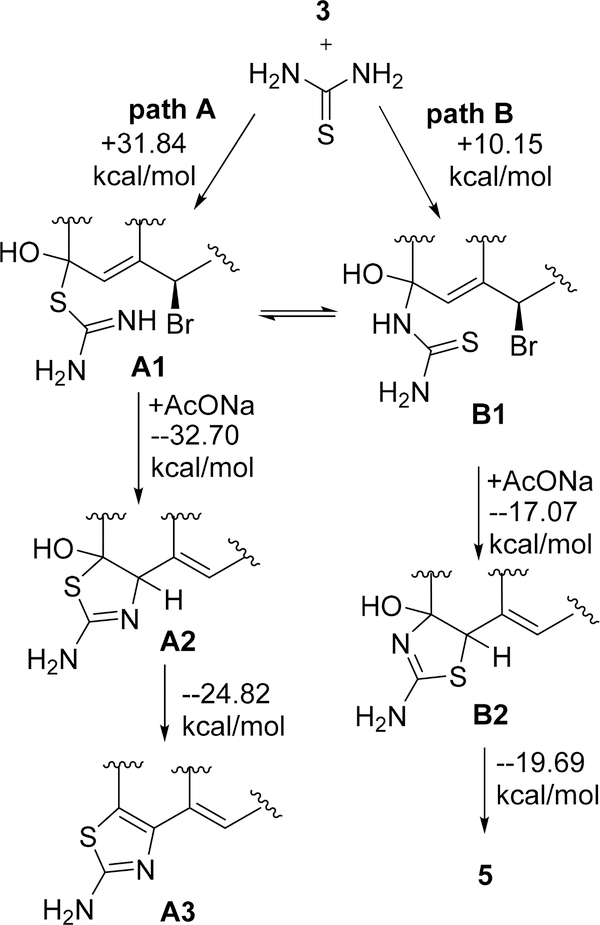
There are four possible pathways for thiourea to undergo reaction with the electrophile, 6β-bromoandrostenedione, to create three different products (Scheme 4 and the SI).
Scheme 4.
Plausible Mechanism for the Formation of Product (5) Using M06-2X/6-311++G(d,p) + PCM (Solvent = EtOH)
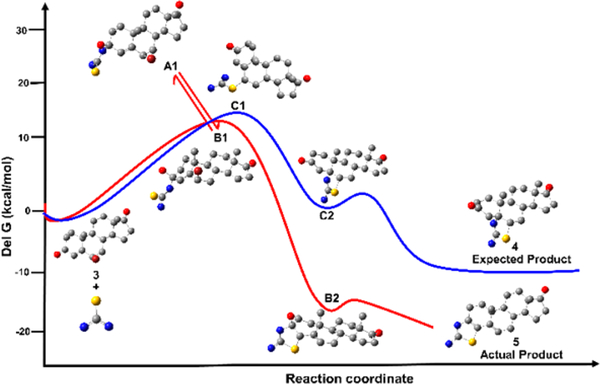
We computed the feasibility of these four pathways by using a hybrid-density functional method (M06–2X)/6–311++G(d,p) + PCM = EtOH as implemented in the Gaussian 09 suite of programs.34 The expected product 4, based on our previous report,37 is the least favorable path, and the SN2′ reaction of thiourea with β-bromoandrostenedione to generate thiazolo-androstenone is also not favorable (SI). Nucleophilic addition of thiourea to carbonyl of β-bromoandrostenedione (3) can form two possible intermediates, hemithioacetal (path A) or hemiaminal (path B). Gibb’s free energy for the formation of hemithioacetal (A1, + 31.84 kcal/mol) and hemiaminal (B1, + 10.15 kcal/mol) is endergonic, which is achievable by refluxing the reaction mixture. We believe the formation of hemithioacetal (A1) and hemiaminal (B1) is reversible under the reaction conditions. Intramolecular SN2′ reaction of these intermediates leads to the formation of thiazoline derivatives (A2 and B2). This intramolecular reaction of hemithioacetal is more favorable than that of hemiaminal (−32.70 kcal/mol vs −17.07 kcal/mol). The final step, elimination of water, is also more favorable for the hemithioacetal derivative than that of the hemiaminal (−24.82 kcal/mol vs −19.69 kcal/mol) to produce the final products A3 and 5, respectively (Scheme 4). Among the three steps for the formation of possible products, the first step is reversible and endergonic while the last two steps are irreversible and exergonic. Actual product 5 is formed because of the less activation energy for the first step, as a result of the nucleophilic addition to form hemiaminal B1. The energy profile diagram is shown in Figure 2.
Figure 2.
Probable potential energy surface of formation of actual and expected products calculated using M06–2X/6–311++G(d,p) + PCM.
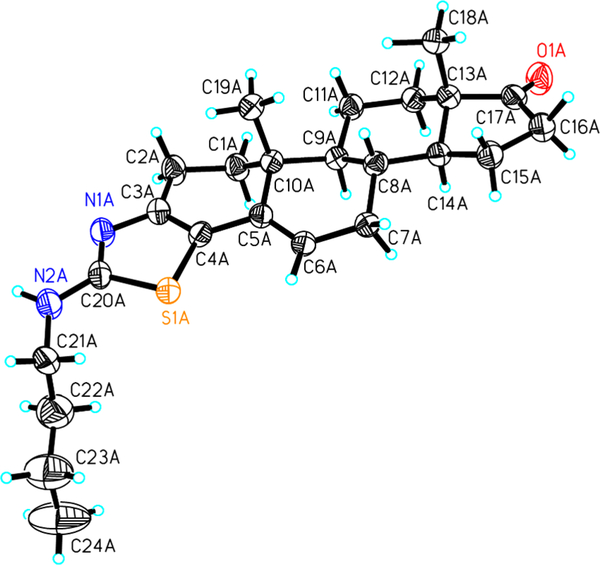
Structures with absolute stereochemistry have been confirmed by single-crystal diffraction. The ORTEP diagrams (7 and 19) show the regiospecific reaction of this methodology in which N and S of thiazole are attached to C-3 and C-4, respectively (Figure 3 and the SI).
Figure 3.
ORTEP diagram of compounds 7 (CCDC 1858409).
We have evaluated some of the aforementioned compounds by screening them in NCI’s 60 cancer cell lines,38 and several entities have shown promising activity against several cancer cell lines at submicromolar concentrations; in vitro testing results for compounds 17 and 23 against NCI-60 cancer cell lines are shown in the SI.
These molecules have shown potent activity against several cancer cell lines including the growth inhibition of leukemia cell lines: RPMI-8226 and SR with 50% growth inhibition (GI50) values at submicromolar concentration; two of the nonsmall cell lung cancer (NSCLC) cell lines were inhibited at low μM concentration. Compound 17 inhibited four of six central nervous system (CNS) cell lines with GI50 values of <2 μM concentration including four cell lines of the colon cancer; 17 inhibited the growth of glioblastoma (SF-295) and gliosarcoma (SF-539) cell lines with GI50 values of 1.19 and 1.34 μM, respectively. Five melanoma cell lines and six renal cancer cell lines were inhibited at low micromolar concentration with GI50 values <2 μM. These molecules (17 and 23) have also shown promising activity against ovarian cancer, prostate cancer, and breast cancer cell lines (Table 1).
We have discovered an efficient domino protocol to synthesize novel thiazolo-androstenone derivatives by using readily available starting materials under mild reaction conditions in benign and recyclable solvent. A large number of novel and therapeutically useful molecules are thus readily accessible via this general pathway, and interestingly, these thiazolo-androstenone derivatives could be further derivatized to generate a large library of active compounds. Further derivatization, associated anticancer studies, and mode of action of this class of compounds are currently underway and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to the INBRE for a pilot grant (Grant No. 224658). This publication was made possible by the Research Technology Core of the Arkansas INBRE Program, supported by a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429-16, from the National Institutes of Health to record the mass spectrometry data. We are thankful to Dr. Victor, Director/Senior Scientist X-ray Crystallography Laboratory University of Kansas (NSF-MRI grant CHE-0923449), for recording the crystal structures of compounds.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.8b02587.
1H and 13C NMR spectra, DFT calculation data, and X-ray diffraction data (PDF)
Accession Codes
CCDC 1858408–1858409 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
REFERENCES
- (1).Cheskis BJ Regulation of cell signalling cascades by steroid hormones. J. Cell. Biochem 2004, 93, 20–27. [DOI] [PubMed] [Google Scholar]
- (2).Wilkenfeld SR; Lin C; Frigo DE Communication between genomic and non-genomic signaling events coordinate steroid hormone actions. Steroids 2018, 133, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dai J; Yoshida WY; Kelly M; Williams P Pregnane-10,2-carbolactones from a Hawaiian Marine Sponge in the Genus Myrmekioderma. J. Nat. Prod 2016, 79, 1464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Liu J; Zhang D; Sun X; Ding T; Lei B; Zhang C Structure-activity relationship of brassinosteroids and their agricultural practical usages. Steroids 2017, 124, 1–17. [DOI] [PubMed] [Google Scholar]
- (5).Calle JM; Pérez AJ; Simonet AM; Guerra JO; Macías FA Steroidal Saponins from Furcraea hexapetala Leaves and Their Phytotoxic Activity. J. Nat. Prod 2016, 79, 2903–2911. [DOI] [PubMed] [Google Scholar]
- (6).Kudova E; Chodounska H; Slavikova B; Budesinsky M; Nekardova M; Vyklicky V; Krausova B; Svehla P; Vyklicky L A New Class of Potent N-Methyl-D-Aspartate Receptor Inhibitors: Sulfated Neuroactive Steroids with Lipophilic D-Ring Modifications. J. Med. Chem 2015, 58, 5950–66. [DOI] [PubMed] [Google Scholar]
- (7).Festa C; Renga B; D’Amore C; Sepe V; Finamore C; De Marino S; Carino A; Cipriani S; Monti MC; Zampella A; Fiorucci S Exploitation of cholane scaffold for the discovery of potent and selective farnesoid X receptor (FXR) and G-protein coupled bile acid receptor 1 (GP-BAR1) ligands. J. Med. Chem 2014, 57, 8477–95. [DOI] [PubMed] [Google Scholar]
- (8).Sepe V; Renga B; Festa C; D’Amore C; Masullo D; Cipriani S; Di Leva FS; Monti MC; Novellino E; Limongelli V; Zampella A; Fiorucci S Modification on ursodeoxycholic acid (UDCA) scaffold. discovery of bile acid derivatives as selective agonists of cell-surface G-protein coupled bile acid receptor 1 (GP-BAR1). J. Med. Chem 2014, 57, 7687–701. [DOI] [PubMed] [Google Scholar]
- (9).Qian M; Krishnan K; Kudova E; Li P; Manion BD; Taylor A; Elias G; Akk G; Evers AS; Zorumski CF; Mennerick S; Covey DF Neurosteroid analogues. 18. Structure-activity studies of ent-steroid potentiators of gamma-aminobutyric acid type A receptors and comparison of their activities with those of alphaxalone and allopregnanolone. J. Med. Chem 2014, 57, 171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bansal R; Acharya PC Man-made cytotoxic steroids: exemplary agents for cancer therapy. Chem. Rev 2014, 114, 6986–7005. [DOI] [PubMed] [Google Scholar]
- (11).Le Bideau F; Dagorne S Synthesis of transition-metal steroid derivatives. Chem. Rev 2013, 113, 7793–850. [DOI] [PubMed] [Google Scholar]
- (12).El-Desoky E-SI; Reyad M; Afsah EM; Dawidar AA Synthesis and chemical reactions of the steroidal hormone 17alpha-methyltestosterone. Steroids 2016, 105, 68–95. [DOI] [PubMed] [Google Scholar]
- (13).Moreno Y Banuls L; Urban E; Gelbcke M; Dufrasne F; Kopp B; Kiss R; Zehl M Structure-activity relationship analysis of bufadienolide-induced in vitro growth inhibitory effects on mouse and human cancer cells. J. Nat. Prod 2013, 76, 1078–84. [DOI] [PubMed] [Google Scholar]
- (14).Ning X; Yang Y; Deng H; Zhang Q; Huang Y; Su Z; Fu Y; Xiang Q; Zhang S Development of 17β-hydroxysteroid dehydrogenase type 3 as a target in hormone-dependent prostate cancer therapy. Steroids 2017, 121, 10–16. [DOI] [PubMed] [Google Scholar]
- (15).Ji ZH; Xu ZQ; Zhao H; Yu XY Neuroprotective effect and mechanism of daucosterol palmitate in ameliorating learning and memory impairment in a rat model of Alzheimer’s disease. Steroids 2017, 119, 31–35. [DOI] [PubMed] [Google Scholar]
- (16).Larik FA; Saeed A; Shahzad D; Faisal M; El-Seedi H; Mehfooz H; Channar PA Synthetic approaches towards the multi target drug spironolactone and its potent analogues/derivatives. Steroids 2017, 118, 76–92. [DOI] [PubMed] [Google Scholar]
- (17).Rouf A; Tanyeli C Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem 2015, 97, 911–927. [DOI] [PubMed] [Google Scholar]
- (18).Ayati A; Emami S; Asadipour A; Shafiee A; Foroumadi A Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem 2015, 97, 699–718. [DOI] [PubMed] [Google Scholar]
- (19).FDA. FDA approves drug to treat Duchenne muscular dystrophy. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm540945.htm.
- (20).Madhra MK; Sriram HM; Inamdar M; Sharma MK; Prasad M; Joseph S Improved Procedure for Preparation of Abiraterone Acetate. Org. Process Res. Dev 2014, 18, 555–558. [Google Scholar]
- (21).Szychowski J; Truchon J-F; Bennani YL Natural Products in Medicine: Transformational Outcome of Synthetic Chemistry. J. Med. Chem 2014, 57, 9292–9308. [DOI] [PubMed] [Google Scholar]
- (22).Antonarakis ES; Bastos DA Galeterone for the treatment of advanced prostate cancer: the evidence to date. Drug Des., Dev. Ther 2016, 10, 2289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Fuke C; Arao T In Oleander toxins; Springer GmbH, 2005; pp 519–526. [Google Scholar]
- (24).Vitellozzi L; McAllister GD; Genski T; Taylor RJK Organometallic Routes to Novel Steroids Containing Heterocyclic C-17 Side-Chains. Synthesis 2016, 48, 48–56. [Google Scholar]
- (25).Zhang BL; Song LX; Li YF; Li YL; Guo YZ; Zhang E; Liu HM Synthesis and biological evaluation of dehydroepiandroster-one-fused thiazole, imidazo[2,1-b]thiazole, pyridine steroidal analogues. Steroids 2014, 80, 92–101. [DOI] [PubMed] [Google Scholar]
- (26).Martinez Botella G; Salituro FG; Harrison BL; Beresis RT; Bai Z; Shen K; Belfort GM; Loya CM; Ackley MA; Grossman SJ; Hoffmann E; Jia S; Wang J; Doherty JJ; Robichaud AJ Neuroactive Steroids. 1. Positive Allosteric Modulators of the (gamma-Aminobutyric Acid)A Receptor: Structure-Activity Relationships of Heterocyclic Substitution at C-21. J. Med. Chem 2015, 58, 3500–11. [DOI] [PubMed] [Google Scholar]
- (27).Li J; Huo H; Guo R; Liu B; Li L; Dan W; Xiao X; Zhang J; Shi B Facile and efficient access to Androsten-17-(1’,3′,4’)-pyrazoles and Androst-17beta-(1’,3′,4’)-pyrazoles via Vilsmeier reagents, and their antiproliferative activity evaluation in vitro. Eur. J. Med. Chem 2017, 130, 1–14. [DOI] [PubMed] [Google Scholar]
- (28).Metz TL; Lutovsky GA; Stanley LM An Acid-Catalyzed Addition and Dehydration Sequence for the Synthesis of Heteroarylated Steroidal Dienes. J. Org. Chem 2018, 83, 1643–1648. [DOI] [PubMed] [Google Scholar]
- (29).Ambrose AJ; Santos EA; Jimenez PC; Rocha DD; Wilke DV; Beuzer P; Axelrod J; Kanduluru AK; Fuchs PL; Cang H; Costa-Lotufo LV; Chapman E; Clair JJL Ritterostatin GN1N, a Cephalostatin–Ritterazine Bis-steroidal Pyrazine Hybrid, Selectively Targets GRP78. ChemBioChem 2017, 18, 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zolottsev VA; Tkachev YV; Latysheva AS; Kostin VA; Novikov RA; Timofeev VP; Morozevich GE; Kuzikov AV; Shumyantseva VV; Misharin AY Comparison of [17(20)E]-21-Norpregnene oxazolinyl and benzoxazolyl derivatives as inhibitors of CYP17A1 activity and prostate carcinoma cells growth. Steroids 2018, 129, 24–34. [DOI] [PubMed] [Google Scholar]
- (31).Michalak K; Morawiak M; Wicha J Synthetic Approach to the Core Structure of Oleandrin and Related Cardiac Glycosides with Highly Functionalized Ring D. Org. Lett 2016, 18, 6148–6151. [DOI] [PubMed] [Google Scholar]
- (32).Metz TL; Lutovsky GA; Stanley LM An Acid-Catalyzed Addition and Dehydration Sequence for the Synthesis of Heteroarylated Steroidal Dienes. J. Org. Chem 2018, 83, 1643. [DOI] [PubMed] [Google Scholar]
- (33).Alam MA; Alsharif Z; Alkhattabi H; Jones D; Delancey E; Gottsponer A; Yang T Hexafluoroisopropyl alcohol mediated synthesis of 2,3-dihydro-4H-pyrido[1,2-a]pyrimidin-4-ones. Sci. Rep 2016, 6, 36316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Alam MA, Cytotoxic agents, anticancer agents and the method of synthesizing the cytotoxic and anticancer agents. Provisional Patent filed, 2018.
- (35).Allison D; Delancey E; Ramey H; Williams C; Alsharif ZA; Al-khattabi H; Ontko A; Gilmore D; Alam MA Synthesis and antimicrobial studies of novel derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-Acinetobacter baumannii agents. Bioorg. Med. Chem. Lett 2017, 27, 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Alsharif Z; Ali MA; Alkhattabi H; Jones D; Delancey E; Ravikumar PC; Alam MA Hexafluoroisopropanol mediated benign synthesis of 2H-pyrido[1,2-a]pyrimidin-2-ones by using a domino protocol. New J. Chem 2017, 41, 14862–14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Alsharif ZA; Alam MA Modular synthesis of thiazoline and thiazole derivatives by using a cascade protocol. RSC Adv. 2017, 7, 32647–32651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).NCI. The NCI Development Therapeutics Program (DTP) https://dtp.cancer.gov/ (accessed Feb 17, 2016).
- (39).Holbeck SL; Collins JM; Doroshow JH Analysis of Food and Drug Administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol. Cancer Ther 2010, 9, 1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.