Abstract
The mammalian retina is the most unique tissue among those that display robust circadian/diurnal oscillations. The retina is not only a light sensing tissue that relays light information to the brain, it has its own circadian “system” independent from any influence from other circadian oscillators. While all retinal cells and retinal pigment epithelium (RPE) possess circadian oscillators, these oscillators integrate by means of neural synapses, electrical coupling (gap junctions), and released neurochemicals (such as dopamine, melatonin, adenosine, and ATP), so the whole retina functions as an integrated circadian system. Dysregulation of retinal clocks not only causes retinal or ocular diseases, it also impacts the circadian rhythm of the whole body, since the light information transmitted from the retina entrains the brain clock that governs the body circadian rhythms. In this review, how circadian oscillations in various retinal cells are integrated, how retinal diseases affect daily rhythms, and how modern electronic displays might affect human health are discussed.
Keywords: retina, photoreceptor, circadian rhythm
Introduction
The vertebrate retina is the most unique tissue among those that display robust circadian/diurnal oscillations. The retina is not only a light sensing tissue that relays light information to the brain, it has its own circadian “system” that prepares the retina to anticipate the upcoming dawn or dusk. In lower vertebrates and invertebrates, there are multiple light sensing tissues or organs in addition to the retina, such as the pineal gland, regions in the deep brain, skin, and even the entire body. In higher mammalian species, these tissues or organs lose the ability to detect light except the retina, even though they still have their circadian oscillator machinery intact.
The neural retina is not a homogenous tissue. It is packed with different types of neurons and glia cells and is highly organized in stratified layers (Dowling, 1970). All of the retinal cells are known to have circadian oscillators, so the circadian signals from retinal cells have to integrate to allow the retina to function as an independent circadian system. The major retinal neurons and glial cells are the photoreceptors, bipolar cells, ganglion cells, horizontal cells, amacrine cells, and the Müller glia (Dowling, 1970; Dowling & Werblin, 1971). The electroretinogram (ERG) is often used to record retinal light responses under various illuminating conditions with the a-wave reflecting the photoreceptor responses of the outer retina while the b-wave reflects the inner retinal responses. The ERG a-wave, b-wave, or both display circadian rhythms in a species-dependent manner (Dearry & Barlow, 1987; Fowlkes et al., 1987; Hawlina et al., 1992; Hankins et al., 1998; Manglapus et al., 1998; Manglapus et al., 1999; McGoogan & Cassone, 1999; Hankins et al., 2001; Miranda-Anaya et al., 2002; Ren & Li, 2004; Cameron et al., 2008; Cameron & Lucas, 2009; Danilenko et al., 2009; Danilenko et al., 2011; Sengupta et al., 2011; Jackson et al., 2012), indicating that the retinal light sensitivities and responses are governed by the internal circadian system (Barlow, 2001). Thus, retinas do not merely respond to ambient light passively, the circadian system in the retina actively regulates the overall light responses and prepares the retina to adapt to the upcoming dawn or dust (Green & Besharse, 2004). The canonical circadian core genes (such as Per 1–3, Bmal1, Clock, Cry 1–2, RevErb, and Ror) and their molecular mechanisms are detected in the retina [for a review of the molecular mechanism of circadian oscillation, please see Bell-Pedersen et al., 2005; Storch et al., 2007; Tosini et al., 2008; McMahon et al., 2014; Besharse & McMahon, 2016]. While we will not address the specific molecular mechanism of circadian oscillation in this review, we will address the circadian oscillations in various retinal neurons and how they integrate.
I. Circadian oscillation in the photoreceptors
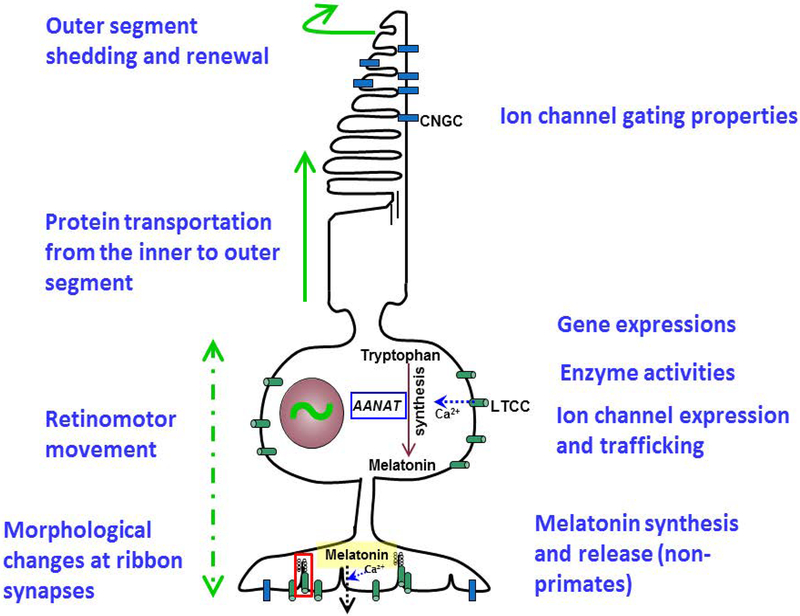
Among retinal cells, the circadian oscillation in photoreceptors is studied the most (Young, 1978; LaVail, 1980; Burnside et al., 1982; Fisher et al., 1983; Cahill & Besharse, 1993; Pierce et al., 1993; Stenkamp et al., 1994; Cahill & Besharse, 1995; Green & Besharse, 2004; Menger et al., 2005; Hasegawa & Cahill, 1998; 1999b; a; Manglapus et al., 1999; Valenciano et al., 1999; Ko et al., 2001; 2003; Hasegawa & Cahill, 2004; Ko et al., 2004a; Menger et al., 2005; Chaurasia et al., 2006; Ko et al., 2006; Sakamoto et al., 2006; Ko et al., 2007; Ivanova et al., 2008; Ribelayga et al., 2008; Jackson et al., 2009; Ko et al., 2009b; Haque et al., 2010; Ko et al., 2013; Li et al., 2013; Jin et al., 2015; Zhang et al., 2015; Baba et al., 2018; Pierce & Besharse, 1985; 1988; Pierce et al., 1993; Manglapus et al., 1999; Tosini et al., 2007). Rod and cone photoreceptors are the first neurons to detect light and relay this information to the bipolar cells and then to ganglion cells for vision. Photoreceptors in lower vertebrates have autonomous circadian oscillators, and they function independently in the absence of other retinal inputs (Hasegawa & Cahill, 1998; Ko et al., 2001; Hasegawa & Cahill, 2004). While the core circadian clock genes and proteins are all detected in mammalian photoreceptors (Tosini et al., 2007; Schneider et al., 2010; Sandu et al., 2011; Liu et al., 2012; Dkhissi-Benyahya et al., 2013), whether the mammalian photoreceptors are as autonomous as the ones in lower vertebrates remains controversial. As the photoreceptors strongly rely on lighting conditions to drive their circadian oscillation shown in rodents (Sandu et al., 2011; Besharse & McMahon, 2016), the isolated rat photoreceptors display circadian oscillations of clock genes in cultures, which demonstrates that photoreceptor clocks can function independently without inputs from other retinal cells (Tosini et al., 2007). The photoreceptor oscillators lead to morphological, physiological, biochemical, and molecular changes that ultimately regulate photoreceptor function and physiology in a circadian fashion (Figure 1; Green & Besharse, 2004; Tosini et al., 2008; Ko et al., 2009a; Ko et al., 2010; Besharse & McMahon, 2016). In vertebrate rod photoreceptors, outer segment shedding and renewal is a continuous process, but the rate of which is under circadian control (Young, 1978; LaVail, 1980; Reme et al., 1986; Dearry & Barlow, 1987; Grace et al., 1999). In some lower vertebrates such as teleosts, amphibian, and fish, the inner segments of rod and cone photoreceptors undergo sustained contraction and elongation, known as retinomotor movement, in response to changes in ambient illumination as well as the circadian cycles (Burnside & Ackland, 1984; Pierce & Besharse, 1985; 1988; Stenkamp et al., 1994; Burnside, 2001; Menger et al., 2005). While cones remain in a contracted state during the day and elongated at night or after dark adaptation, rods contract at night (Menger et al., 2005). The molecular machinery regulating photoreceptor elongation requires microtubule shuttling and assembly by dynein-1 (Lewis et al., 2018). It is reasonable to postulate that the rate of dynein-1 movement might be governed by the photoreceptor oscillators, with rods and cones anti-phase to each other.
Figure 1. There is circadian regulation in the vertebrate photoreceptor.
CNGC: cGMP-gated cation channel; LTCC: L-type voltage-gated calcium channel; AANAT: Aralkylamine N-acetyltransferase, also known as arylalkylamine N-acetyltransferase or serotonin N-acetyltransferase, the key enzyme of melatonin synthesis.
Photoreceptors form specialized ribbon synapses with bipolar cells and horizontal cells. The numbers and ultrastructure of synaptic ribbons in both photoreceptors and bipolar cells undergo changes depending on the time of day and light intensities (Vollrath et al., 1989; Vollrath & Spiwoks-Becker, 1996; Adly et al., 1999; Balkema et al., 2001; Spiwoks-Becker et al., 2004; Hull et al., 2006). In addition to the circadian oscillator genes, several photoreceptor-specific genes such as opsin and rhodopsin (Korenbrot & Fernald, 1989; Pierce et al., 1993; Green & Besharse, 1996; Bernard et al., 1997; von Schantz et al., 1999; Yu et al., 2007), ion channels (Ko et al., 2001; Ko et al., 2004a; Ko et al., 2007; Ko et al., 2010), and enzyme activities (Besharse & Iuvone, 1983; Ko et al., 2001; 2004b; Ko et al., 2009b; Ko et al., 2009c; Huang et al., 2012; Huang et al., 2013; Ko et al., 2013; Huang et al., 2015) are also under circadian control that ultimately contribute to the circadian regulation of photoreceptor physiology and function. Photoreceptors are more susceptible to light damage during the subjective night (Organisciak et al., 2000; Vaughan et al., 2002), which likely reflects circadian regulation of the expression and distribution of retinal crystallins, the chaperone proteins trafficking in and out of the photoreceptor outer segments that is protective to photoreceptors (Organisciak et al., 2011).
The cGMP-gated cation channel (CNGC) is the last step of the phototransduction process that takes place in the outer segments in response to light-induced hyperpolarization of photoreceptors. Light induces a G-protein mediated phototransduction cascade that results in a fall in local intracellular cGMP and leads to the closure of CNGCs, reduced cation influx, and membrane hyperpolarization. In the absence of photon stimulation, the intracellular cGMP concentration is relatively high, which causes the opening of CNGCs and influx of cations (Na+ and Ca2+) to depolarize photoreceptors in the dark. Thus, CNGCs carry the photoreceptor “dark current” and serve essential roles in the light-dependent changes in photoreceptor membrane potential and subsequent neural processing (Cobbs et al., 1985; Pugh Jr. & Lamb, 2000; Kramer & Molokanova, 2001; Lamb & Pugh, 2004). Even though the opening or closure of CNGCs is dependent on light stimulation, the affinity of CNGCs to cGMP is under circadian regulation in avian cone photoreceptors (Ko et al., 2001; Chae et al., 2007). The apparent affinity of CNGCs to cGMP is higher at the subjective night than during the subjective day suggesting a substantially larger dark current during the subjective night than subjective day. The complex cell-signaling involving adenylyl cyclase, cAMP, Ras-MAP kinase (MAPK), calcium/calmodulin-dependent kinase II (CaMKII), and tyrosine kinase leads to the circadian rhythmicity of tyrosine phosphorylation on the 85-kDa auxiliary subunit of cone CNGCs and provides one of the final steps in the circadian regulation of CNGCs (Ko et al., 2001; 2003; 2004b; Chae et al., 2007). Because the CNGCs in photoreceptors are essential components of the visual phototransduction cascade, they may also play a role in the light entrainment of photoreceptor circadian oscillators. Since the gating of CNGCs is under circadian control in cone photoreceptors, these channels represent an example of an entity that is both an input to and an output from the circadian oscillator (Ko et al., 2001). Roenneberg and Merrow (Roenneberg & Merrow, 1999) have presented models of circadian oscillator systems in which pathways that lead to entrainment of the core oscillators (i.e. the circadian inputs) can themselves be regulated by the oscillators (i.e. they are also components of circadian outputs). One feature of these models is that they contain additional feedback loops that can markedly enhance the stability of the overall oscillator system. Therefore, the circadian regulation of CNGCs in retinal photoreceptors represents an adaptation to enhance the stability of retinal circadian oscillators.
L-type calcium channels (LTCCs) mediate a voltage-dependent and depolarization-induced calcium influx and regulate diverse biological processes such as contraction, secretion, neurotransmission, differentiation, and gene expression in many different cell types (Barnes & Kelly, 2002; Benitah et al., 2002; Catterall et al., 2005; Thorneloe & Nelson, 2005; Dolphin, 2006; Catterall & Few, 2008; Dolphin, 2009; Joiner & Lee, 2015). There are three major LTCCs expressed in the vertebrate retina: Cav1.2, Cav1.3, and Cav1.4 (Ahlijanian et al., 1990; Strom et al., 1998; Firth et al., 2001; Morgans, 2001; Barnes & Kelly, 2002; Xu et al., 2002; Haeseleer et al., 2004; Morgans et al., 2005; Cristofanilli et al., 2007; Ko et al., 2007; Wu et al., 2007; Kersten et al., 2010; Mizuno et al., 2010; Xing et al., 2012; Zou et al., 2012; Knoflach et al., 2013; Liu et al., 2013; Lee et al., 2015; Haeseleer et al., 2016; Shi et al., 2017a). In photoreceptors, Cav1.4 is exclusively expressed at the synaptic terminals and responsible for the tonic release of glutamate in the dark as a result of depolarization-evoked activation of LTCCs (Morgans, 2001; Haeseleer et al., 2004; Knoflach et al., 2013; Haeseleer et al., 2016). Cav1.3 is also detected at the synaptic terminal (Firth et al., 2001; Shi et al., 2017a), but it might not be as essential for mediating neurotransmitter release as Cav1.4 (Shi et al., 2017a; Shi et al., 2017b). Both Cav1.2 and Cav1.3 are largely expressed in the inner segments and cell bodies, in which calcium influx into this part of the photoreceptors is involved in calcium-dependent gene expression, metabolism, and homeostasis (Miller et al., 1994; Korenbrot, 1995; Wilkinson & Barnes, 1996; Firth et al., 2001; Barnes & Kelly, 2002; Krizaj & Copenhagen, 2002; Xu et al., 2002; Ko et al., 2007; Ko et al., 2009a; Krizaj, 2012; Joiner & Lee, 2015).
Circadian regulation of LTCCs has been observed in gold fish retinal bipolar cells (Hull et al., 2006), chick cone photoreceptors (Ko et al., 2007; Ko et al., 2009b; Shi et al., 2009; Huang et al., 2012; Huang et al., 2013; Ko et al., 2013; Huang et al., 2015), and other non-retinal neurons (Kim et al., 2005; Nahm et al., 2005). In both retinal cases, the average maximal current amplitudes of LTCCs are significantly larger at midnight than at midday, while the activation and channel gating kinetics do not change throughout the course of a day. In chick retinas, the main factor contributing to the circadian regulation of LTCC current amplitudes is the expression of functional L-VGCCα1 subunits, with mRNA levels and protein expression of Cav1.3 being rhythmic (Ko et al., 2007). The Ras-MAPK-CaMKII signaling pathway that regulates the circadian rhythms of CNGCs also serves as part of the circadian output to regulate LTCCs. However, the circadian regulation of LTCCs and CNGCs are different. While the expression of Cav1.3 varies throughout the day with varying maximal amplitudes, the CNGC currents remain constant, but its gating properties exhibit changes throughout the day (Ko et al., 2001; Ko et al., 2007). In addition to Ras-MAPK-CaMKII signaling, phosphatidylinositol 3 kinase (PI3K)-protein kinase B (AKT) signaling (Ko et al., 2009b), calcineurin (Huang et al., 2012), mechanistic/mammalian target of rapamycin complex 1 (mTORC1, (Huang et al., 2013), and AMP-activated protein kinase (AMPK, (Huang et al., 2015) are all involved in the circadian regulation of LTCCs. Furthermore, microRNA-26a regulates LTCCs at the post-transcriptional level (Shi et al., 2009). In photoreceptors, not only is the activation (phosphorylation) of the complex signaling network rhythmic, these networks further regulate the circadian rhythms of LTCCs and CNGCs and thus the physiology of photoreceptors. Several molecules in the signaling network including AKT, mTORC1, and AMPK that regulate LTCCs are also involved in cellular metabolism. The overall production of ATP displays a diurnal rhythm in avian retinas (Huang et al., 2015). Thus, it is reasonable to assume that at the cellular level, metabolism and energy production in photoreceptors is under circadian regulation.
While cone photoreceptors are responsible for daytime and color vision and rod photoreceptors are for nighttime and dim-light vision (Dowling, 1987), there is a circadian regulation in the interaction between rods and cones (Wang & Mangel, 1996; Manglapus et al., 1998; Manglapus et al., 1999; Ribelayga et al., 2002; Ribelayga et al., 2008; Ribelayga & Mangel, 2010). The ERG recordings using monochromatic light sources indicate a day and night difference in the dynamics of rod- or cone-dominance (Manglapus et al., 1998; Manglapus et al., 1999). There is a day-night change in spectral sensitivity as the cones dominate during the day while the rods dominate during the night, even when animals are kept in constant darkness for several days. The process is mostly governed by the photoreceptor circadian clocks. As cone photoreceptors specifically form synapses with cone bipolar cells and rod photoreceptors synapse onto rod bipolar cells, this rod-cone shift takes place at the photoreceptor-to-bipolar cell synapses (Manglapus et al., 1998). Furthermore, the rod-cone shifts are modulated by dopamine, a neurotransmitter released from the amacrine cells in the inner retina (Manglapus et al., 1999). The modulatory action of dopamine in the retinal circadian system will be discussed in the following section.
Even though the synapse between a photoreceptor and a bipolar cell is rod/cone specific, rod and cone photoreceptors in the vertebrate retina are anatomically connected or coupled by gap junctions (Raviola & Gilula, 1973; Yang & Wu, 1989; Krizaj et al., 1998; Bloomfield & Dacheux, 2001; Hornstein et al., 2005) meaning rod inputs can enter the cone circuitry (Ribelayga et al., 2008). The rod-cone coupling is remarkably strong at night but weak during the day in goldfish, rabbit, and mice (Ribelayga et al., 2008; Ribelayga & Mangel, 2010). This rhythmic rod-cone coupling is mediated by the circadian regulation of the phosphorylation of connexin 36 (Cx36), the gap junction protein between rods and cones, in which Cx36 phosphorylation is higher at night than during the day (Zhang et al., 2015). The rod-cone coupling is also subjected to modulation by dopamine (Witkovsky et al., 1988; Wang & Mangel, 1996; Krizaj et al., 1998; Ribelayga et al., 2002; 2004; Jin et al., 2015), since both rods and cones express dopaminergic receptors, while horizontal cells do not (Witkovsky et al., 1988; Cohen et al., 1992; Yazulla & Lin, 1995; Krizaj et al., 1998; Ko et al., 2003).
II. Circadian oscillators in the inner retinal cells
The core clock genes are all detected in the inner retinal neurons (bipolar, horizontal, amacrine, and ganglion cells) especially in the dopaminergic (DA) amacrine cells (Witkovsky et al., 2003; Gabriel et al., 2004; Ruan et al., 2006; Liu et al., 2012). Based on morphological characteristics and neurotransmitters released, there are 30–40 types of amacrine cells in the inner retina (Remington, 2012). While the core clock genes oscillate in amacrine cells, whether these gene transcripts cycle rhythmically in other inner cells (bipolar neurons, horizontal neurons, and ganglion neurons) are not clear. Imaging of a bioluminescent reporter of Per2Luc shows the circadian cycling of the core circadian gene Per2 across the whole retina (Ruan et al., 2008), as well as in isolated retinal layers, including the photoreceptor layer, inner retinal layer, and the ganglion cell layer (Jaeger et al., 2015). Interestingly, in mixed dissociated retinal cell cultures excised from the PERIOD2 (PER2)::LUC (Per2Luc) knock-in mice, the Per2Luc bioluminescent oscillation maintains similar amplitudes without dampening, while the Per2Luc oscillations show dampening after a few cycles in the individual retinal layers (Jaeger et al., 2015), which might imply that the overall circadian rhythm in the retina requires the integration from all cellular oscillators. Müller glial cells are proposed to play a role in integrating the whole retinal circadian system, since all the core clock genes oscillate in these glial cells, and the circadian rhythmicity of these glial cells is able to sustain in isolation from other cell types (Ruan et al., 2006; Xu et al., 2016). A single Müller cell extends the entire thickness of the retina and makes contacts with multiple retinal neurons. Furthermore, there are gap junctions (or tight junctions) between Müller cells, as well as between Müller cells and photoreceptors (Dowling, 1987; Rich et al., 1995). Light or dark information coming from the photoreceptors could have been relayed to these glial cells through the junctions. As Müller cells are critical in regulation of ion buffering and neurotransmitter recycling (Dowling, 1987), it is reasonable to postulate that Müller glial cells are able to synchronize the circadian rhythm of the whole retina. However, within the retina, not all signals are synchronized in the same circadian phase. For example, the dopamine released from the DA amacrine cells peaks during the day time, while melatonin diffused from the photoreceptors and the pineal cells peaks at night (Besharse & Iuvone, 1983; Robertson & Takahashi, 1988; Zatz, 1989; Iuvone, 1990; Benloucif & Dubocovich, 1996; Nikaido & Takahashi, 1996; Adachi et al., 1998; Tosini, 2000; Tosini & Dirden, 2000; Iuvone et al., 2002; Miranda-Anaya et al., 2002; Besharse et al., 2004; Fukuhara et al., 2004; Iuvone et al., 2005; Peters & Cassone, 2005; Baba et al., 2009; Tosini et al., 2012; Bernard et al., 1997; Ebihara et al., 1997). These neurochemical signals serve as synchronizing signals to integrate various circadian oscillators in the retina.
III. Circadian integration in the retina
a. The retinal dopamine-melatonin anti-phase relationship.
The two major neurotransmitters that serve as “day” and “night” signals in the retina are dopamine and melatonin, respectively (Figure 2; Dubocovich, 1983; Adachi et al., 1998), and the retinal dopamine is always in an opposite circadian/diurnal phase to retinal melatonin (Besharse & Iuvone, 1983; Dubocovich, 1983; Adachi et al., 1998). Dopamine has long been known to regulate retinal physiology (Dowling, 1990; 1991). While dopamine is synthesized and release from the DA amacrine cells (Iuvone et al., 1978; Dubocovich et al., 1985; Dowling, 1990), dopamine receptors are distributed throughout the whole retina (Krizaj et al., 1998; Witkovsky, 2004; Jackson et al., 2009; Keeley & Reese, 2010; Jackson et al., 2012). The synthesis and release of dopamine is light-driven as well as circadian regulated in birds and amphibians, but in mammalian retinas that are melatonin-deficient, their retinal dopamine content only displays a diurnal rhythm (Iuvone et al., 1978; Nir et al., 2000a; b; Valenciano et al., 2000; Zhang et al., 2018). While previous studies showed that light stimulated DA amacrine cells by activating rods, cones, and the melanopsin-expressing intrinsically photosensitive retina ganglion cells (ipRGCs; please see a later section for details on ipRGCs; Zhang et al., 2007; Zhang et al., 2008; Newkirk et al., 2013; Vuong et al., 2015), a more comprehensive examination shows that it is the cone photoreceptors that drive the light signals through multiple neural circuits, including both ON and OFF bipolar cells, as well as a cone-driven retrograde signaling pathway from ipRGCs, to modulate the dopamine release from DA amacrine cells (Qiao et al., 2016).
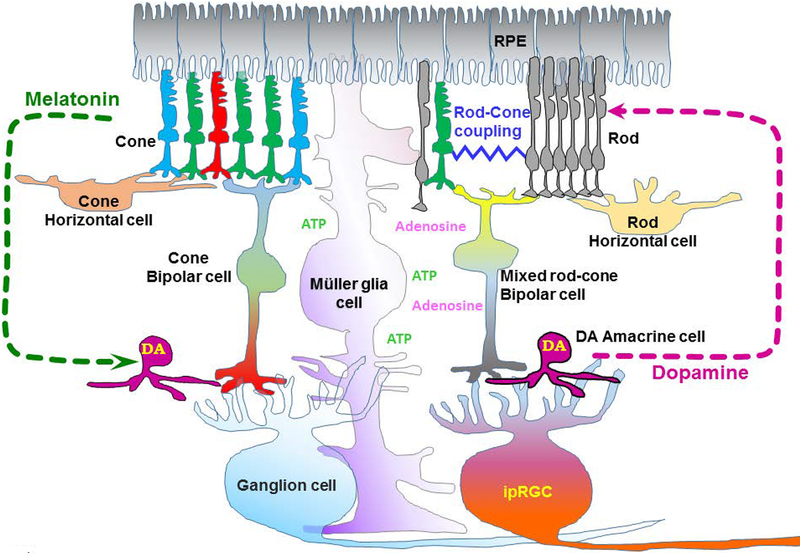
Figure 2. A schematic diagram of the circadian system in the retina.
All retinal cells and retinal pigment epithelium (RPE) have circadian oscillators. Photoreceptors release melatonin at night that inhibits dopaminergic amacrine cells (DA) from releasing dopamine. During the day, dopamine released from the amacrine cells inhibits the melatonin synthesis and release from the photoreceptors. As the gap junction couplings between rods and cones are stronger at night, the intrinsic clock-driven signals, as well as dopamine, uncouple these electric synapses between rods and cones. The intrinsic photosensitive retinal ganglion cell (ipRGC) receives inputs from photoreceptors through bipolar cells, as well as receiving dopamine from AII amacrine cells. The ipRGCs directly relay the light signal to the SCN for photoentrainment.
As the light stimulates dopamine synthesis (Iuvone et al., 1978), dopamine is able to modulate retina daytime vision by enhancing the contrast sensitivity and spatial resolution of light-adapted retinas (Jackson et al., 2012). In retina-specific dopamine deficient mice, their light-adapted ERG responses, contrast sensitivity, acuity, and the retinal circadian rhythms are dampened, but their dark-adapted ERG responses appear normal (Jackson et al., 2012). Thus, dopamine is critical for regulating retinal light adaption and daytime vision. In addition, dopamine is able to act like the light/daytime signal (Manglapus et al., 1999) to entrain (Hasegawa & Cahill, 1999b) and phase-shift the retinal clocks in amphibians (Green et al., 1995; Steenhard & Besharse, 2000), and exogenous dopamine inhibits melatonin production from the photoreceptors at night (Hasegawa & Cahill, 1999b; a; Zawilska & Iuvone, 1992; Nguyen-Legros et al., 1996; Tosini & Dirden, 2000). Dopamine shifts the rod-cone dominance (Manglapus et al., 1999) as well as the rod-cone coupling toward the “daytime” phase in birds, fish, and mammals (Witkovsky et al., 1988; Wang & Mangel, 1996; Krizaj et al., 1998; Ribelayga et al., 2002; 2004; Jin et al., 2015). Interestingly, while dopamine is able to phase-shift and modulate photoreceptor properties as mentioned above, cone photoreceptors are involved in the photic regulation of retinal DA amacrine cells (Qiao et al., 2016).
The expression of dopamine receptors in the retina is also under circadian control (Nir et al., 2002). In the mouse retina, the D1, D2, D4, and D5 dopamine receptors mediate dopamine’s bioactivities (Jackson et al., 2009). However, not all dopamine receptors contribute to the circadian regulation of the retina. Through binding to the D2/D4 receptors, administering dopamine during the subjective night shifts CNGCs from its high affinity to cGMP to a lower daytime-like affinity in avian cone photoreceptors, while inhibition of D1 receptors does not have any effects on cone photoreceptors (Ko et al., 2003). In mice, deletion of D5 receptors have no impact on retinal function, but knock out of D4 or D1 receptors have distinct visual deficits (Jackson et al., 2012). Even though dopamine acting through D1 receptors is necessary to maintain retinal spatial resolution in mice, it is the D4 receptors that mediate the circadian regulation of light-adapted ERG (Jackson et al., 2012). Dopamine shifts the rod-cone coupling toward the “daytime” phase through D2-like receptors in fish and mice (Ribelayga & Mangel, 2003; 2007; Ribelayga et al., 2008). Furthermore, it is through D2-like receptors that dopamine inhibits melatonin synthesis in the photoreceptors (Nguyen-Legros et al., 1996). Hence, it is reasonable to conclude that through D2/D4 receptors, dopamine serves as a daytime signal to modulate the retinal circadian rhythm.
As mentioned earlier, both dopamine and melatonin are important circadian signals in the retina that have reciprocal and antagonistic relationships with each other (Iuvone, 1986; Nguyen-Legros et al., 1996; Ebihara et al., 1997; Adachi et al., 1998; Tosini & Dirden, 2000). Melatonin is synthesized and secreted from the retina photoreceptors in lower vertebrates (such as avian, amphibian, reptile, fish) and most mammalian species, and all retinal cells express melatonin receptors (Dubocovich, 1985; 1991; Tosini et al., 2008; Tosini et al., 2012; Acuna-Castroviejo et al., 2014). While it is still debatable whether the retina is able to synthesize and secrete melatonin in humans and higher primates (Reiter et al., 2010; Hardeland et al., 2012; Acuna-Castroviejo et al., 2014; Bonmati-Carrion et al., 2014), it is clear that the retinal melatonin level is higher at night than during the day, in part through the melatonin released from the pineal gland at night reaching the retina through blood circulation (Acuna-Castroviejo et al., 2014; Bonmati-Carrion et al., 2014; Blasiak et al., 2016). The transcription of arylalkylamine N-acetyltransferase (AANAT), the key melatonin synthesis enzyme, is under circadian control, so at night, the content of retinal melatonin is higher than the day time (Besharse & Iuvone, 1983; Iuvone & Besharse, 1983; Dubocovich, 1988; 1991; Bernard et al., 1999; Iuvone et al., 2005; Tosini et al., 2008; Haque et al., 2010; Tosini et al., 2012). In the carp retina, melatonin serves as an autocrine or paracrine signal and modulates neurotransmission from cones and cone-driven bipolar cells (Huang et al., 2005). Administration of exogenous melatonin in Xenopus and carps increases the amplitude of dark-adapted scotopic ERG (Wiechmann et al., 2003; Ping et al., 2008). Administration of exogenous melatonin during the daytime reduces the ERG b-wave amplitude in diurnal birds (Lu et al., 1995) but increases the ERG a- and b-wave amplitudes in nocturnal mice (Baba et al., 2009). In humans, oral intake of melatonin during the day decreases the amplitude of the cone ERG (Gagne et al., 2009), and the amplitude of the cone and mixed rod-cone responses are negatively correlated with the endogenous melatonin concentration in the saliva (Rufiange et al., 2002). Constant administration of melatonin abolishes the ERG circadian rhythms in chickens (McGoogan & Cassone, 1999). Interestingly, although melatonin is known to regulate the disk shedding of photoreceptor outer segments in Xenopus and rats (Besharse & Dunis, 1983; White & Fisher, 1989), when comparing the circadian rhythm of disk shedding between melatonin-proficient (C3H+/+) and melatonin-deficient (C57/BL6) mice, the disk shedding is rhythmic in both mouse strains, and exogenous melatonin does not affect the circadian rhythmicities, which questions the contribution of melatonin in regulating the rhythmic disk shedding in mice (Grace et al., 1999). Thus, how disk shedding in mammalian species is regulated requires further investigation (Tosini et al., 2012).
The synthesis of melatonin is not only governed by the photoreceptor circadian clock, light and dopamine are also able to inhibit the production and secretion of melatonin from photoreceptors as mentioned previously. As dopamine through D2/D4 receptors inhibits the synthesis and release of melatonin, melatonin appears to regulate the circadian rhythm of dopamine in the retina. At night, melatonin inhibits the dopamine production from amacrine cells (Dubocovich, 1983; Adachi et al., 1998). In fish, the rhythmic dopamine release in the retina depends on the activation of melatonin receptors (Ribelayga et al., 2002). In mice, melatonin also regulates the circadian rhythm of retinal dopamine. In a comparison between melatonin-proficient (C3H+/+) and melatonin-deficient (C57BL6) mice, C3H+/+ mice display strong circadian oscillations of retinal dopamine content and metabolism, while C57BL6 mice do not (Doyle et al., 2002). However, if the melatonin-deficient (C57BL6) mice are given daily injections of melatonin, these mice exhibit a robust circadian rhythm of retinal dopamine content even if the mice are kept in constant darkness (Doyle et al., 2002). Therefore, in the retina, not only do dopamine and melatonin reciprocally regulate each other, dopamine serves as a “day signal” while melatonin serves as a “night signal” to orchestrate the circadian oscillators existing in various retinal cell types enabling the whole retina to function and adjust across 12 magnitudes of ambient light changes harmoniously throughout the course of a day (Green & Besharse, 2004; Besharse & McMahon, 2016).
b. Adenosine triphosphate (ATP): the circadian regulation of retinal metabolism and the role of ATP in inter-retinal communication.
In addition to melatonin and dopamine that serve as neural signals in the retina to regulate the overall retinal circadian rhythm, ATP has been proposed as an inter-retinal signal to synchronize the retinal clocks, and the metabolic state in the retina is under circadian regulation (Dmitriev & Mangel, 2000; 2001; 2004; Huang et al., 2015). As shown in the fish and rabbit retina, the retinal pH values oscillate in a circadian fashion (Dmitriev & Mangel, 2000; 2001), and overall pH changes in the retina reflects changes in its metabolic state (Dmitriev & Mangel, 2004). In the avian retina, the overall ATP level displays a diurnal rhythm that is nearly antiphase to the circadian oscillation of AMP-activated protein kinase (AMPK) activation (Huang et al., 2015). AMP-activated protein kinase (AMPK) is a cellular energy sensor (Hardie, 2007a). When the intracellular AMP to ATP ratio rises, AMPK is activated (phosphorylated) and promotes catabolic pathways while inhibiting anabolic ones so that more ATP is generated (Hardie, 2007a; b; Towler & Hardie, 2007). Among all retinal neurons, the photoreceptors consume the most energy (Wong-Riley, 2010), and their energy consumption is highly compartmentalized (Linton et al., 2010; Wei et al., 2012). There is heavy energy expenditure in the outer segments of photoreceptors where phototransduction and protein transport for outer segment renewal are taking place in response to various light intensities (Korenbrot, 1995; Koutalos & Yau, 1996). In the dark, most of the energy consumption is in the inner segments and synaptic terminals to maintain the dark currents and neurotransmitter release (Wong-Riley, 2010). The retinal photoreceptors have a higher metabolic activity in the dark, which means that on one hand, ATP is hydrolyzed in an accelerated rate to support tonic neurotransmitter release. On the other hand, mitochondria will have to produce more ATP to sustain photoreceptor activities. As a result, the mitochondrial enzymes that are responsible for ATP production should be more active in darkness, which is supported by Huang et al. (2004) where the mitochondrial enzymes cytochrome C oxidase III and adenosine triphosphatase-6 are down-regulated by higher light intensity (Huang et al., 2004). The overall ATP production might be lower in the presence of bright light. Therefore, the retinal energy expenditure and production can be light intensity-dependent as a reflection of acute light/dark adaptation, as well as circadian clock-regulated.
In addition to serving as a cellular energy source, ATP is also a neurotransmitter in the retina, and ATP receptors are present in all retinal neurons (Ho et al., 2014). ATP and other purines (ADP, AMP, adenosine, adenine, and hypoxanthine) are tonically released in the retina particularly in the dark (Perez et al., 1986), and such release is increased by neuronal activity (Neal & Cunningham, 1994). However, Müller glia cells are also able to generate, release, and accumulate extracellular ATP (Loiola & Ventura, 2011). Interestingly, in cultured cortical glia cells, extracellular ATP accumulation displays circadian rhythms (Womac et al., 2009; Burkeen et al., 2011). Hence, extracellular ATP could serve as a neurotransmitter to communicate among multiple circadian oscillators that exist in different retinal cell types (Ruan et al., 2006; Liu et al., 2012). In addition, the extracellular ATP can further be converted into adenosine, which could contribute to the circadian rhythm of retinal adenosine with a higher concentration at night than during the day (Ribelayga & Mangel, 2005). Retinal adenosine is known to regulate circadian rhythms of photoreceptor coupling, as well as retinal light/dark adaptation (Ribelayga & Mangel, 2005; 2007; Ribelayga et al., 2008; Li et al., 2013). Therefore, the circadian rhythm of retinal ATP might not only reflect the circadian control of energy status, but it might implicate that ATP and its metabolite adenosine could serve as neuromodulators to coordinate the various circadian oscillators in the retina to integrate the overall retinal circadian rhythm in the whole retina. As evidence, there is a circadian regulation of retinal light responses as measured by electroretinogram (ERG) in humans and animals (Dearry & Barlow, 1987; Fowlkes et al., 1987; Hawlina et al., 1992; Hankins et al., 1998; Manglapus et al., 1998; Manglapus et al., 1999; McGoogan & Cassone, 1999; Hankins et al., 2001; Miranda-Anaya et al., 2002; Ren & Li, 2004; Cameron et al., 2008; Cameron & Lucas, 2009). As heterogeneous as the retina is, through multiple mechanisms, including the rod-cone coupling, Muller glial cells, inter-retinal synaptic network, dopamine / melatonin inverse regulation, and ATP / adenosine, the retina as a whole has its own circadian system that allows it to anticipate the upcoming light changes at dawn and dusk and adapt to daily ambient illumination.
IV. The relationship between retinal photoreceptors and the retinal pigment epithelium (RPE)
As stated above, the outer segments (OS) of photoreceptors are constantly shedding and renewing, the rate of which is under circadian control. The outermost tips of photoreceptor OS are surrounded by RPE cells. RPE cells phagocytose the shed tips of the OS, degrade the shed disk membrane, and further recycle molecules involved in phototransduction back to the photoreceptors (Mazzoni et al., 2014). If phagocytosis is defective and the OS debris accumulates in the interphotoreceptor space, it causes photoreceptor degeneration (Dowling & Sidman, 1962; Bok & Hall, 1971; Mullen & LaVail, 1976; Anderson et al., 1983; Sheedlo et al., 1989) as seen in inherited retinal degeneration diseases such as retinitis pigmentosa and inherited maculopathies (LaVail & Mullen, 1976; Mullen & LaVail, 1976; Bertolotti et al., 2014), and in Royal College of Surgeons Rats (Caldwell & McLaughlin, 1985). While the rod OS tip clearance occurs at dawn or with light stimulation (LaVail, 1976b; a; Besharse et al., 1977; Fisher et al., 1983; LaVail, 1980; Reme et al., 1986; Ruggiero et al., 2012; Laurent et al., 2017), the timing of cone OS clearance varies by species, depending on whether the animal’s retina is rod-dominant (Young, 1977; O’Day & Young, 1978; Fisher et al., 1983) or cone-dominant (Anderson et al., 1978; Young, 1978; Immel & Fisher, 1985; Bobu et al., 2006; Bobu & Hicks, 2009). Even though photoreceptor oscillators appear to control the OS shedding and renewal, the rate of phagocytosis of shed OS requires circadian control from the RPE, as RPE cells also have intact circadian oscillators (Baba et al., 2010; Ruggiero et al., 2012; Baba et al., 2017; Laurent et al., 2017), and cultured RPE isolated from the Per2Luc mouse displays circadian oscillations in luciferase activities (Baba et al., 2017). Thus, the rhythmic oscillations of the autophagy process in RPE cells are controlled by RPE cells themselves. In this regard, while photoreceptors and RPE cells have independent circadian oscillators, the shed disks from photoreceptor OS that are to be engulfed and degraded in RPE cells require synchronization from both. Even though administration of melatonin induces disk shedding in Xenopus retinas (Besharse & Dunis, 1983) and increases phagocytosis in rat RPE cells (White & Fisher, 1989), the rhythmic disk shedding is still present in melatonin-deficient mice (Grace et al., 1999). Since dopamine is able to phase-shift the circadian rhythm of RPE via the D2 receptors expressed in the RPE (Baba et al., 2017), dopamine is likely to be the neurochemical signal that synchronizes photoreceptors and the RPE.
Like photoreceptors, the protein expression of Cav1.3 is under circadian control in the RPE (Muller et al., 2014; Reichhart & Strauss, 2014; Genewsky et al., 2015). Interestingly, in Cav1.3 knockout mice, the RPE lacks peak phagocytic activity following morning photoreceptor shedding and retained a higher number of phagosomes at a later time of the day. The big potassium (BK) channel is a voltage-dependent and calcium-modulated large conductance potassium channel and is also expressed abundantly in the RPE, but its mRNA expression is not under circadian control (Muller et al., 2014). In BK knockout mice, the RPE retains normal phagocytic capability, but its rhythmic phagocytosis is phase-shifted out of synchronization from the photoreceptor disk shedding. Thus, both Cav1.3 and BK channels might be important to regulate the circadian timing in RPE phagocytosis in synchronization with photoreceptors for the disk shedding.
V. Melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs): their role in photoentrainment
The primary visual pathway starts with the rods and cones transmitting light information to bipolar cells, ganglion cells, and onto the lateral geniculate nucleus in the thalamus for image processing (Dowling, 1987). About 2–5% of retinal ganglion cells are able to detect light directly, the intrinsically photosensitive retinal ganglion cells (ipRGCs; Provencio et al., 1998; Berson et al., 2002; Hattar et al., 2002; Hattar et al., 2003; Lucas et al., 2003; Chaurasia et al., 2005; Hannibal et al., 2014). After leaving the eye, the axons of ipRGCs directly project to the suprachiasmatic nucleus (SCN), the central clock, through the retinohypothalamic tract (Hendrickson et al., 1972; Moore & Lenn, 1972; Hattar et al., 2002; Hattar et al., 2003; Lucas et al., 2003). This is the non-visual pathway mediating circadian inputs of light information into the SCN for photoentrainment. There are major electrical properties of ipRGCs that are different from the traditional photoreceptors (rods and cones). When light reaches the photoreceptor OS, through a G protein-coupled phosphodiesterase-mediated phototransduction process, it causes a decrease of intracellular cGMP, closure of CNGCs, and hyperpolarization of photoreceptors, which further decreases the release of glutamate from photoreceptors to other inner neurons (Edwards, 1995; Zagotta & Siegelbaum, 1996; Kaupp & Seifert, 2002). When light reaches the ipRGCs, it initiates an intracellular phosphoinositide signaling cascade leading to transient receptor potential (TRP) channels opening and cell depolarization, as such, ipRGCs fire action potentials and release glutamate onto SCN neurons (Berson et al., 2002; Panda et al., 2005; Warren et al., 2006; Hartwick et al., 2007; Sekaran et al., 2007). The traditional photoreceptors use rhodopsin and opsins as their chromophores (Yau & Baylor, 1989; Edwards, 1995), but the ipRGCs use melanopsin, a chromophore first identified from the skin of amphibians (Provencio et al., 1998; Hattar et al., 2002; Hattar et al., 2003; Lucas et al., 2003).
There are at least 5 subtypes of ipRGCs (M1-M5), and they have different morphological characteristics, retinal distribution, and possibly different functions (Schmidt et al., 2011b). Even though these ipRGCs directly send light information to the SCN, they still receive inputs from rods and cones, and their light responses and mRNA levels are modulated by these inputs (Berson et al., 2002; Sakamoto et al., 2004; Lucas et al., 2012). In addition, these ipRGCs have complex relationships with other retinal neurons. Dopamine receptors are expressed in retinal ganglion cells (McMahon et al., 2014), and dopaminergic amacrine neurons directly interact with ipRGC dendrites (Vugler et al., 2007). As such, not only does dopamine regulate melanopsin expression in ipRGCs (Sakamoto et al., 2005), it directly modulates the light responses of ipRGCs through D1 receptors (Van Hook et al., 2012). Furthermore, ipRGCs might send retrograde signals that modulate the retinal light responses. The intraretinal axon collaterals of ipRGCs project to dopaminergic amacrine cells and influence retinal light adaptation, since the deletion of M1 ipRGCs attenuates retinal light adaptation (Prigge et al., 2016). During development, ablation of ipRGCs causes mislocalization of a subset of cone photoreceptors in dark-reared animals (Tufford et al., 2018).
Even though rods, cones, and ipRGCs all provide light information for circadian entrainment, ipRGCs play a more significant role in overall photoentrainment and circadian regulation of the whole body. Patients with glaucoma, in which their RGCs (including ipRGCs) are degenerated, often suffer from circadian dysregulation related syndromes, such as sleep disorders (Gracitelli et al., 2015; Guo et al., 2017) and autonomic nervous system dysfunction (Kashiwagi et al., 2000) that are caused in part by a deficiency of circadian entrainment (Guido et al., 2010). Other ocular diseases related to retinal degeneration (such as macular degeneration, diabetic retinopathy, or inherited blindness) that dampens or prevents light inputs to the SCN may also impact the overall circadian rhythm of patients. Patients with Smith-Magenis Syndrome, a genetic disorder, have abnormal daily rhythms with sleep disturbances (daytime sleepiness, nocturnal awakenings, difficulty falling asleep at night), and they have high melatonin levels during the day but low levels at night. These patients apparently have a deficit in melanopsin positive ipRGC photoreception (Barboni et al., 2018), which reinforces the importance of ipRGCs in photoentrainment.
As mentioned earlier, light suppresses melatonin release from the pineal gland and the retina. This is because the pineal gland is under the control of the SCN through the autonomic system, and the SCN directly receives light inputs from ipRGCs (for reviews see (Macchi & Bruce, 2004; Guido et al., 2010; Hardeland, 2012). Among the wavelengths of visible light, the 460 nm blue light suppresses melatonin twice as much as the 555 nm green light and shifts the circadian rhythms by twice the duration (3.5 hr versus 1.5 hr; Gooley et al., 2010). Evidently, some blind people are not able to see images due to degenerated photoreceptors, but they still have conscious light perception with intense light stimulus at 481 nm, which is the peak spectral sensitivity of melanopsin (Zaidi et al., 2007). This discovery led to intensive research on the influence of blue lights and health (Tosini et al., 2016). Most modern electronic displays (including cell phones, computers, tablets, and televisions) emit blue lights through their screens, and energy efficient lighting such as the light-emitting diode (LED) and curlicue compact fluorescent lightbulbs emit more blue lights than the traditional incandescent lightbulbs, which has raised concerns on possible adverse effects of human health (Tosini et al., 2016). People who wear “blue-light goggles” that allow more blue light into the eyes while concurrently looking at computer monitors have significantly decreased nighttime melatonin levels compared to subjects only exposed to monitor light at night (Figueiro et al., 2011). Attenuation of blue light by wearing short wavelength-blocking glasses prior to bedtime significantly increases the night time melatonin level and improves the sleep quality and duration (Ostrin et al., 2017).
While ipRGCs project to the SCN, they also project to other brain areas (Schmidt et al., 2011a; Schmidt et al., 2011b), including the limbic system (amygdala, habenula, and dorsal thalamus), which is important in mood regulation and cognitive function (Lazzerini Ospri et al., 2017). The amygdala plays an important role in learned fear (fear-conditioning), but light enhances mice fear-conditioning responses, in which rods, cones, and ipRGCs are required (Warthen et al., 2011). Keeping adult diurnal rodents in a short photoperiod (such as light:darkness at 5:19 hr) for a period of time causes depression-like behavior (Ashkenazy-Frolinger et al., 2010; Leach et al., 2013). When animals are entrained to 12:12 light-dark cycles with dim light during the light-phase, they also display depressive behavior similar to animals in a short photoperiod (Deats et al., 2015; Ikeno et al., 2016). If diurnal rodents are entrained to light-dark cycles but with dim light at night, they have impaired cognition and depression without circadian alterations (Fonken et al., 2012). Thus, because ipRGCs receive light information from rods and cones and project to the limbic system, different light settings (such as dim lights or short photoperiods) might affect mood and cognitive function (Lazzerini Ospri et al., 2017).
VI. The circadian clocks and ocular diseases
As the circadian clocks in the retina regulate retinal metabolism, signaling, gene expression, and light sensitivity, altered clock function is likely to affect retinal health. Dysregulation of the core clock genes has been regarded as a key factor in the pathogenesis of several ocular diseases. The Bmal1 knockout mice display premature aging symptoms, and their ocular abnormalities include corneal neovascularization, keratinization, and progressive inflammation (Yang et al., 2016), while Clock-deficient mice have cataracts (Kondratov et al., 2006). In addition, photoreceptor viability during aging is significantly reduced in Bmal1 knockouts, as they lose 20–30% of their photoreceptors at 8–9 months old compared to the wild type mice of the same age (Baba et al., 2018). Per1/Per2 knockout mice have scattered retinal deformations as well as reduced cone opsin expression (Ait-Hmyed et al., 2013). These data suggest that either disruption of circadian rhythms in the eye or the retina would cause these ocular diseases, or the core circadian genes (Bmal1, Clock, Per1, Per2) might have other functions such as supporting the proper development and health of the retina. Disruption of retinal dopamine rhythms causes myopia in chickens (Stone et al., 1989; Stone et al., 1990; McMahon et al., 2014), while lens-induced myopia changes the expression of circadian clock genes and melatonin receptors in the retina (Stone et al., 2011). Thus, the circadian rhythm in the retina is important for refractive error development (Stone et al., 2013; Chakraborty et al., 2018). As mentioned previously, photoreceptors are more sensitive to light damage during the subjective night (Organisciak et al., 2000; Vaughan et al., 2002). In rodent models of type 1 or type 2 diabetes, the expression of several circadian genes is down regulated in the retina (Kuriyama et al., 2004; Busik et al., 2009; Wang et al., 2014; Lahouaoui et al., 2016), but there is no such change in the SCN. It is possible that changes in retinal circadian genes correlate to the diabetic disease progression. However, it also implies that dysregulation of the retinal circadian clocks in diabetes might exacerbate or even contribute to diabetic retinopathy.
While the dysregulation of the retinal clock genes might lead to various ocular diseases mentioned above, certain ocular diseases that manifest death of RGCs (such as glaucoma) or decreased light input to the retina (such as in aging eyes or eyes with cataracts) affect the whole body circadian timing. Glaucoma is a widespread ocular disease and a major cause of blindness (Quigley & Broman, 2006). Dysregulation of intraocular pressure (IOP) due to aging, hypertension, or the inability of diurnal oscillations of IOP contributes to the pathogenesis of glaucoma (Quigley, 1993; Friedman et al., 2004; Boland & Quigley, 2007; Choi & Kook, 2015). Patients with glaucoma often have disturbed sleep problems and dampened diurnal rhythms (Zizi et al., 2002; Jean-Louis et al., 2008; Lanzani et al., 2012). In a rat model of glaucoma with ~50–70% of RGCs degenerated, these glaucomatous rats require higher light intensities for entrainment, take more days to re-adjust to a shifted light-dark cycle, and show significantly higher variability in locomotor activity onsets compared to normal rats (Drouyer et al., 2008). Age-related eye changes such as crystalline lens yellowing, cataract development, or miosis progression (decreased pupillary area) cause a progressive loss of light transmission into the retina, which leads to a decrease of light inputs to the SCN for circadian photoreception (Turner et al., 2010). Deficiency in light reception eventually causes a significant decrease in systemic melatonin even to undetectable levels, along with decreased amplitudes in daily oscillations of several hormones (Monk et al., 1997; Oren et al., 1997; Van Someren et al., 2002; Danilenko et al., 2009). Thus, weakened SCN control of the body circadian rhythms due to decreased light inputs from the retina, aging, or both may increase the risk of all-cause morbidity (Hastings et al., 2003; Turner et al., 2010). Cataract surgery not only recovers vision, it further improves circadian photoreception and reduces the adverse effect of chronodisruption in these patients (Turner & Mainster, 2008; Turner et al., 2010). Therefore, the deficiency in light reception into the retina due to ocular diseases or aging contributes to dysregulation of the whole body circadian rhythm that has a profound adverse impact on health.
VII. Summary
The retina has a fascinating circadian system in that all retinal cells have their own oscillators, and yet all of these circadian oscillators are integrated for the rhythmic regulation of retinal physiology and function. Thus, the retina does not just passively respond to ambient light, its circadian system actively prepares the retina to anticipate the changes in light intensity that come with dawn and dust. Dysregulation of the retinal circadian system has adverse impacts on photoreceptors, RPE, RGCs, and possibly the lens and the cornea (McMahon et al., 2014), which implies that the retinal circadian system further affects other ocular tissues beyond the neural retina. In disease states such as diabetes, downregulation of the clock genes in the retina might further exacerbate the development of diabetic retinopathy. Since the retina transmits light information to the SCN that further controls the circadian rhythm of the entire body, degenerated retinas dampen the light input to the SCN which further affects the body’s daily rhythms. As we live in a modern society with advanced lighting for both day and night, excessive illumination not only has adverse impacts on the retina, light pollution may have a strong impact on human health that has been underestimated (Contin et al., 2016). While advancements in technology shifts usage from the old incandescent lightbulbs to more energy efficient LED lighting and with many electronics emitting blue lights, the adverse effects of blue lights on human daily rhythms and health have been a research focus in recent years. As more research on the use of LEDs on health will be needed, maybe with the continued evolution of scientific technology, future illumination devices will be more environment and human health friendly.
Acknowledgements
Research in the author’s laboratory was previously funded by NIH and currently supported by the Department of Veterinary Integrative Biosciences and College of Veterinary Medicine and Biomedical Sciences, Texas A&M University.
Abbreviations list:
- AANAT
arylalkylamine N-acetyltransferase
- AKT
protein kinase B
- AMPK
AMP-activated protein kinase
- BK
big conductance potassium channel
- Bmal1
Brain and muscle Arnt-like protein-1
- Ca2+
calcium
- CaMKII
Calcium/calmodulin-dependent kinase II
- CNGC
cGMP-gated cation channel
- Na+
sodium
- Cry
Cryptochrome
- Cx36
connexin 36
- DA
dopaminergic
- ERG
electroretinogram
- ipRGC
intrinsically photosensitive retinal ganglion cell
- K+
potassium
- LED
light-emitting diode
- LTCC
L-type calcium channel
- Luc
lucerferase
- MAPK
mitogen-activated protein kinase
- mTORC1
mechanistic/mammalian target of rapamycin complex 1
- OS
outer segment of the photoreceptor
- Per
Period
- PI3K
phosphatidylinositol 3 kinase
- Ror
retinoic acid receptor-related orphan receptors
- RPE
retinal pigment epithelium
- SCN
suprachiasmatic nucleus
- TRP
transient receptor potential
Footnotes
Conflicts of Interest: The author declares no competing financial interests.
References:
- Acuna-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, Rosales-Corral S, Tan DX & Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci, 71, 2997–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi A, Nogi T & Ebihara S (1998) Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res, 792, 361–369. [DOI] [PubMed] [Google Scholar]
- Adly MA, Spiwoks-Becker I & Vollrath L (1999) Ultrastructural changes of photoreceptor synaptic ribbons in relation to time of day and illumination. Invest Ophthalmol Vis Sci, 40, 2165–2172. [PubMed] [Google Scholar]
- Ahlijanian MK, Westenbroek RE & Catterall WA (1990) Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron, 4, 819–832. [DOI] [PubMed] [Google Scholar]
- Ait-Hmyed O, Felder-Schmittbuhl MP, Garcia-Garrido M, Beck S, Seide C, Sothilingam V, Tanimoto N, Seeliger M, Bennis M & Hicks D (2013) Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci, 37, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK & Steinberg RH (1978) Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci, 17, 117–133. [PubMed] [Google Scholar]
- Anderson DH, Stern WH, Fisher SK, Erickson PA & Borgula GA (1983) Retinal detachment in the cat: the pigment epithelial-photoreceptor interface. Invest Ophthalmol Vis Sci, 24, 906–926. [PubMed] [Google Scholar]
- Ashkenazy-Frolinger T, Kronfeld-Schor N, Juetten J & Einat H (2010) It is darkness and not light: Depression-like behaviors of diurnal unstriped Nile grass rats maintained under a short photoperiod schedule. J Neurosci Methods, 186, 165–170. [DOI] [PubMed] [Google Scholar]
- Baba K, DeBruyne JP & Tosini G (2017) Dopamine 2 Receptor Activation Entrains Circadian Clocks in Mouse Retinal Pigment Epithelium. Sci Rep, 7, 5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Iuvone PM & Tosini G (2009) Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A, 106, 15043–15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Ribelayga CP, Michael Iuvone P & Tosini G (2018) The Retinal Circadian Clock and Photoreceptor Viability. Adv Exp Med Biol, 1074, 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Sengupta A, Tosini M, Contreras-Alcantara S & Tosini G (2010) Circadian regulation of the PERIOD 2::LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis, 16, 2605–2611. [PMC free article] [PubMed] [Google Scholar]
- Balkema GW, Cusick K & Nguyen TH (2001) Diurnal variation in synaptic ribbon length and visual threshold. Vis Neurosci, 18, 789–797. [DOI] [PubMed] [Google Scholar]
- Barboni MTS, Bueno C, Nagy BV, Maia PL, Vidal KSM, Alves RC, Reiter RJ, do Amaral FG, Cipolla-Neto J & Ventura DF (2018) Melanopsin System Dysfunction in Smith-Magenis Syndrome Patients. Invest Ophthalmol Vis Sci, 59, 362–369. [DOI] [PubMed] [Google Scholar]
- Barlow R (2001) Circadian and efferent modulation of visual sensitivity. Prog Brain Res, 131, 487–503. [DOI] [PubMed] [Google Scholar]
- Barnes S & Kelly ME (2002) Calcium channels at the photoreceptor synapse. Adv Exp Med Biol, 514, 465–476. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL & Zoran MJ (2005) Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet, 6, 544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitah JP, Gomez AM, Fauconnier J, Kerfant BG, Perrier E, Vassort G & Richard S (2002) Voltage-gated Ca2+ currents in the human pathophysiologic heart: a review. Basic Res Cardiol, 97 Suppl 1, I11–18. [DOI] [PubMed] [Google Scholar]
- Benloucif S & Dubocovich ML (1996) Melatonin and light induce phase shifts of circadian activity rhythms in the C3H/HeN mouse. J Biol Rhythms, 11, 113–125. [DOI] [PubMed] [Google Scholar]
- Bernard M, Guerlotte J, Greve P, Grechez-Cassiau A, Iuvone MP, Zatz M, Chong NW, Klein DC & Voisin P (1999) Melatonin synthesis pathway: circadian regulation of the genes encoding the key enzymes in the chicken pineal gland and retina. Reproduction, nutrition, development, 39, 325–334. [DOI] [PubMed] [Google Scholar]
- Bernard M, Iuvone PM, Cassone VM, Roseboom PH, Coon SL & Klein DC (1997) Avian melatonin synthesis: photic and circadian regulation of serotonin N-acetyltransferase mRNA in the chicken pineal gland and retina. J Neurochem, 68, 213–224. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA & Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science, 295, 1070–1073. [DOI] [PubMed] [Google Scholar]
- Bertolotti E, Neri A, Camparini M, Macaluso C & Marigo V (2014) Stem cells as source for retinal pigment epithelium transplantation. Prog Retin Eye Res, 42, 130–144. [DOI] [PubMed] [Google Scholar]
- Besharse JC & Dunis DA (1983) Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science, 219, 1341–1343. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Hollyfield JG & Rayborn ME (1977) Photoreceptor outer segments: accelerated membrane renewal in rods after exposure to light. Science, 196, 536–538. [DOI] [PubMed] [Google Scholar]
- Besharse JC & Iuvone PM (1983) Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature, 305, 133–135. [DOI] [PubMed] [Google Scholar]
- Besharse JC & McMahon DG (2016) The Retina and Other Light-sensitive Ocular Clocks. J Biol Rhythms, 31, 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Zhuang M, Freeman K & Fogerty J (2004) Regulation of photoreceptor Per1 and Per2 by light, dopamine and a circadian clock. Eur J Neurosci, 20, 167–174. [DOI] [PubMed] [Google Scholar]
- Blasiak J, Reiter RJ & Kaarniranta K (2016) Melatonin in Retinal Physiology and Pathology: The Case of Age-Related Macular Degeneration. Oxid Med Cell Longev, 2016, 6819736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA & Dacheux RF (2001) Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res, 20, 351–384. [DOI] [PubMed] [Google Scholar]
- Bobu C, Craft CM, Masson-Pevet M & Hicks D (2006) Photoreceptor organization and rhythmic phagocytosis in the nile rat Arvicanthis ansorgei: a novel diurnal rodent model for the study of cone pathophysiology. Invest Ophthalmol Vis Sci, 47, 3109–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobu C & Hicks D (2009) Regulation of retinal photoreceptor phagocytosis in a diurnal mammal by circadian clocks and ambient lighting. Invest Ophthalmol Vis Sci, 50, 3495–3502. [DOI] [PubMed] [Google Scholar]
- Bok D & Hall MO (1971) The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. The Journal of cell biology, 49, 664–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland MV & Quigley HA (2007) Risk factors and open-angle glaucoma: classification and application. J Glaucoma, 16, 406–418. [DOI] [PubMed] [Google Scholar]
- Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, Reiter R, Hardeland R, Rol MA & Madrid JA (2014) Protecting the melatonin rhythm through circadian healthy light exposure. International journal of molecular sciences, 15, 23448–23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkeen JF, Womac AD, Earnest DJ & Zoran MJ (2011) Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J Neurosci, 31, 8432–8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside B (2001) Light and circadian regulation of retinomotor movement. Prog Brain Res, 131, 477–485. [DOI] [PubMed] [Google Scholar]
- Burnside B & Ackland N (1984) Effects of circadian rhythm and cAMP on retinomotor movements in the green sunfish, Lepomis cyanellus. Invest Ophthalmol Vis Sci, 25, 539–545. [PubMed] [Google Scholar]
- Burnside B, Evans M, Fletcher RT & Chader GJ (1982) Induction of dark-adaptive retinomotor movement (cell elongation) in teleost retinal cones by cyclic adenosine 3’,’5-monophosphate. J Gen Physiol, 79, 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Li Calzi S, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME & Grant MB (2009) Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. The Journal of experimental medicine, 206, 2897–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM & Besharse JC (1993) Circadian clock functions localized in xenopus retinal photoreceptors. Neuron, 10, 573–577. [DOI] [PubMed] [Google Scholar]
- Cahill GM & Besharse JC (1995) Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog. Retinal Eye Res, 14, 267–291. [Google Scholar]
- Caldwell RB & McLaughlin BJ (1985) Freeze-fracture study of filipin binding in photoreceptor outer segments and pigment epithelium of dystrophic and normal retinas. J Comp Neurol, 236, 523–537. [DOI] [PubMed] [Google Scholar]
- Cameron MA, Barnard AR & Lucas RJ (2008) The electroretinogram as a method for studying circadian rhythms in the mammalian retina. J Genet, 87, 459–466. [DOI] [PubMed] [Google Scholar]
- Cameron MA & Lucas RJ (2009) Influence of the rod photoresponse on light adaptation and circadian rhythmicity in the cone ERG. Mol Vis, 15, 2209–2216. [PMC free article] [PubMed] [Google Scholar]
- Catterall WA & Few AP (2008) Calcium channel regulation and presynaptic plasticity. Neuron, 59, 882–901. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP & Striessnig J (2005) International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev, 57, 411–425. [DOI] [PubMed] [Google Scholar]
- Chae KS, Ko GY & Dryer SE (2007) Tyrosine phosphorylation of cGMP-gated ion channels is under circadian control in chick retina photoreceptors. Invest Ophthalmol Vis Sci, 48, 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Ostrin LA, Nickla DL, Iuvone PM, Pardue MT & Stone RA (2018) Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol Opt, 38, 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Haque R, Pozdeyev N, Jackson CR & Iuvone PM (2006) Temporal coupling of cyclic AMP and Ca/calmodulin-stimulated adenylyl cyclase to the circadian clock in chick retinal photoreceptor cells. J Neurochem, 99, 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, Natesan A, Zatz M, Tosini G, Liu C, Korf HW, Iuvone PM & Provencio I (2005) Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem, 92, 158–170. [DOI] [PubMed] [Google Scholar]
- Choi J & Kook MS (2015) Systemic and Ocular Hemodynamic Risk Factors in Glaucoma. Biomed Res Int, 2015, 141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs WH, Barkdoll AE 3rd & Pugh EN Jr. (1985) Cyclic GMP increases photocurrent and light sensitivity of retinal cones. Nature, 317, 64–66. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S & O’Malley KL (1992) Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci U S A, 89, 12093–12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contin MA, Benedetto MM, Quinteros-Quintana ML & Guido ME (2016) Light pollution: the possible consequences of excessive illumination on retina. Eye (Lond), 30, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Mizuno F & Akopian A (2007) Disruption of actin cytoskeleton causes internalization of Ca(v)1.3 (alpha 1D) L-type calcium channels in salamander retinal neurons. Mol Vis, 13, 1496–1507. [PubMed] [Google Scholar]
- Danilenko KV, Plisov IL, Cooper HM, Wirz-Justice A & Hebert M (2011) Human cone light sensitivity and melatonin rhythms following 24-hour continuous illumination. Chronobiol Int, 28, 407–414. [DOI] [PubMed] [Google Scholar]
- Danilenko KV, Plisov IL, Wirz-Justice A & Hebert M (2009) Human retinal light sensitivity and melatonin rhythms following four days in near darkness. Chronobiol Int, 26, 93–107. [DOI] [PubMed] [Google Scholar]
- Dearry A & Barlow RB Jr. (1987) Circadian rhythms in the green sunfish retina. J Gen Physiol, 89, 745–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deats SP, Adidharma W & Yan L (2015) Hypothalamic dopaminergic neurons in an animal model of seasonal affective disorder. Neurosci Lett, 602, 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Coutanson C, Knoblauch K, Lahouaoui H, Leviel V, Rey C, Bennis M & Cooper HM (2013) The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci, 70, 3435–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV & Mangel SC (2000) A circadian clock regulates the pH of the fish retina. J Physiol, 522 Pt 1, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV & Mangel SC (2001) Circadian clock regulation of pH in the rabbit retina. J Neurosci, 21, 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV & Mangel SC (2004) Retinal pH reflects retinal energy metabolism in the day and night. J Neurophysiol, 91, 2404–2412. [DOI] [PubMed] [Google Scholar]
- Dolphin AC (2006) A short history of voltage-gated calcium channels. Br J Pharmacol, 147 Suppl 1, S56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC (2009) Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol, 19, 237–244. [DOI] [PubMed] [Google Scholar]
- Dowling JE (1970) Organization of vertebrate retinas. Invest Ophthalmol, 9, 655–680. [PubMed] [Google Scholar]
- Dowling JE (1987) The retina, an approachable part of the brain. Belknap-Harvard, Cambridge, MA. [Google Scholar]
- Dowling JE (1990) Functional and pharmacological organization of the retina: dopamine, interplexiform cells, and neuromodulation. Res Publ Assoc Res Nerv Ment Dis, 67, 1–18. [PubMed] [Google Scholar]
- Dowling JE (1991) Retinal neuromodulation: the role of dopamine. Vis Neurosci, 7, 87–97. [DOI] [PubMed] [Google Scholar]
- Dowling JE & Sidman RL (1962) Inherited retinal dystrophy in the rat. The Journal of cell biology, 14, 73–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE & Werblin FS (1971) Synaptic organization of the vertebrate retina. Vision Res, Suppl 3, 1–15. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W & Menaker M (2002) Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci, 19, 593–601. [DOI] [PubMed] [Google Scholar]
- Drouyer E, Dkhissi-Benyahya O, Chiquet C, WoldeMussie E, Ruiz G, Wheeler LA, Denis P & Cooper HM (2008) Glaucoma alters the circadian timing system. PLoS One, 3, e3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML (1983) Melatonin is a potent modulator of dopamine release in the retina. Nature, 306, 782–784. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML (1985) Characterization of a retinal melatonin receptor. J Pharmacol Exp Ther, 234, 395–401. [PubMed] [Google Scholar]
- Dubocovich ML (1988) Pharmacology and function of melatonin receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 2, 2765–2773. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML (1991) Melatonin receptors in the central nervous system. Adv Exp Med Biol, 294, 255–265. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Lucas RC & Takahashi JS (1985) Light-dependent regulation of dopamine receptors in mammalian retina. Brain Res, 335, 321–325. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Adachi A, Hasegawa M, Nogi T, Yoshimura T & Hirunagi K (1997) In vivo microdialysis studies of pineal and ocular melatonin rhythms in birds. Biological signals, 6, 233–240. [DOI] [PubMed] [Google Scholar]
- Edwards SC (1995) Involvement of cGMP and calcium in the photoresponse in vertebrate photoreceptor cells. J Fla Med Assoc, 82, 485–488. [PubMed] [Google Scholar]
- Figueiro MG, Wood B, Plitnick B & Rea MS (2011) The impact of light from computer monitors on melatonin levels in college students. Neuro endocrinology letters, 32, 158–163. [PubMed] [Google Scholar]
- Firth SI, Morgan IG, Boelen MK & Morgans CW (2001) Localization of voltage-sensitive L-type calcium channels in the chicken retina. Clin Experiment Ophthalmol, 29, 183–187. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Pfeffer BA & Anderson DH (1983) Both rod and cone disc shedding are related to light onset in the cat. Invest Ophthalmol Vis Sci, 24, 844–856. [PubMed] [Google Scholar]
- Fonken LK, Kitsmiller E, Smale L & Nelson RJ (2012) Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms, 27, 319–327. [DOI] [PubMed] [Google Scholar]
- Fowlkes DH, Karwoski CJ & Proenza LM (1987) Circadian modulation of the electroretinogram (a‐ and b‐waves) in the diurnal lizard Anolis carolinensis. Biological Rhythm Research, 18, 147–168. [Google Scholar]
- Friedman DS, Wilson MR, Liebmann JM, Fechtner RD & Weinreb RN (2004) An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. American journal of ophthalmology, 138, S19–31. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GC, Storm DR, Iuvone PM & Tosini G (2004) Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neurosci, 24, 1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R, Lesauter J, Banvolgyi T, Petrovics G, Silver R & Witkovsky P (2004) AII amacrine neurons of the rat retina show diurnal and circadian rhythms of parvalbumin immunoreactivity. Cell Tissue Res, 315, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne AM, Danilenko KV, Rosolen SG & Hebert M (2009) Impact of oral melatonin on the electroretinogram cone response. J Circadian Rhythms, 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genewsky A, Jost I, Busch C, Huber C, Stindl J, Skerka C, Zipfel PF, Rohrer B & Strauss O (2015) Activation of endogenously expressed ion channels by active complement in the retinal pigment epithelium. Pflugers Arch, 467, 2179–2191. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA & Lockley SW (2010) Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med, 2, 31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace MS, Chiba A & Menaker M (1999) Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis Neurosci, 16, 909–918. [DOI] [PubMed] [Google Scholar]
- Gracitelli CP, Duque-Chica GL, Roizenblatt M, Moura AL, Nagy BV, Ragot de Melo G, Borba PD, Teixeira SH, Tufik S, Ventura DF & Paranhos A Jr. (2015) Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology, 122, 1139–1148. [DOI] [PubMed] [Google Scholar]
- Green CB & Besharse JC (1996) Identification of a novel vertebrate circadian clock-regulated gene encoding the protein nocturnin. Proc Natl Acad Sci U S A, 93, 14884–14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB & Besharse JC (2004) Retinal circadian clocks and control of retinal physiology. J Biol Rhythms, 19, 91–102. [DOI] [PubMed] [Google Scholar]
- Green CB, Cahill GM & Besharse JC (1995) Regulation of tryptophan hydroxylase expression by a retinal circadian oscillator in vitro. Brain Res, 677, 283–290. [DOI] [PubMed] [Google Scholar]
- Guido ME, Garbarino-Pico E, Contin MA, Valdez DJ, Nieto PS, Verra DM, Acosta-Rodriguez VA, de Zavalia N & Rosenstein RE (2010) Inner retinal circadian clocks and non-visual photoreceptors: novel players in the circadian system. Prog Neurobiol, 92, 484–504. [DOI] [PubMed] [Google Scholar]
- Guo ZZ, Jiang SM, Zeng LP, Tang L, Li N, Xu ZP & Wei X (2017) ipRGCs: possible causation accounts for the higher prevalence of sleep disorders in glaucoma patients. Int J Ophthalmol, 10, 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F & Palczewski K (2004) Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nature neuroscience, 7, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Williams B & Lee A (2016) Characterization of C-terminal Splice Variants of Cav1.4 Ca2+ Channels in Human Retina. J Biol Chem, 291, 15663–15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins MW, Jones RJ & Ruddock KH (1998) Diurnal variation in the b-wave implicit time of the human electroretinogram. Vis Neurosci, 15, 55–67. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Jones SR, Jenkins A & Morland AB (2001) Diurnal daylight phase affects the temporal properties of both the b-wave and d-wave of the human electroretinogram. Brain Res, 889, 339–343. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Kankipati L, Strang CE, Peterson BB, Dacey D & Gamlin PD (2014) Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J Comp Neurol, 522, 2231–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Ali FG, Biscoglia R, Abey J, Weller J, Klein D & Iuvone PM (2010) CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase mRNA in chicken cone photoreceptors. J Neurochem, 113, 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R (2012) Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. TheScientificWorldJournal, 2012, 640389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, Madrid JA, Tan DX & Reiter RJ (2012) Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. Journal of pineal research, 52, 139–166. [DOI] [PubMed] [Google Scholar]
- Hardie DG (2007a) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol, 8, 774–785. [DOI] [PubMed] [Google Scholar]
- Hardie DG (2007b) Biochemistry. Balancing cellular energy. Science, 315, 1671–1672. [DOI] [PubMed] [Google Scholar]
- Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ & Pickard GE (2007) Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci, 27, 13468–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M & Cahill GM (1998) Cyclic AMP resets the circadian clock in cultured Xenopus retinal photoreceptor layers. J Neurochem, 70, 1523–1531. [DOI] [PubMed] [Google Scholar]
- Hasegawa M & Cahill GM (1999a) Modulation of rhythmic melatonin synthesis in Xenopus retinal photoreceptors by cyclic AMP. Brain Res, 824, 161–167. [DOI] [PubMed] [Google Scholar]
- Hasegawa M & Cahill GM (1999b) A role for cyclic AMP in entrainment of the circadian oscillator in Xenopus retinal photoreceptors by dopamine but not by light. J Neurochem, 72, 1812–1820. [DOI] [PubMed] [Google Scholar]
- Hasegawa M & Cahill GM (2004) Regulation of the circadian oscillator in Xenopus retinal photoreceptors by protein kinases sensitive to the stress-activated protein kinase inhibitor, SB 203580. J Biol Chem, 279, 22738–22746. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB & Maywood ES (2003) A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci, 4, 649–661. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM & Yau KW (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science, 295, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG & Yau KW (2003) Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature, 424, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawlina M, Jenkins HG & Ikeda H (1992) Diurnal variations in the electroretinographic c-wave and retinal melatonin content in rats with inherited retinal dystrophy. Doc Ophthalmol, 79, 141–150. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Wagoner N & Cowan WM (1972) An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z Zellforsch Mikrosk Anat, 135, 1–26. [DOI] [PubMed] [Google Scholar]
- Ho T, Vessey KA & Fletcher EL (2014) Immunolocalization of the P2X4 receptor on neurons and glia in the mammalian retina. Neuroscience, 277, 55–71. [DOI] [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Li PH & Schnapf JL (2005) Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J Neurosci, 25, 11201–11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Ko ML & Ko GY (2013) A new functional role for mechanistic/mammalian target of rapamycin complex 1 (mTORC1) in the circadian regulation of L-type voltage-gated calcium channels in avian cone photoreceptors. PLoS One, 8, e73315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Ko ML, Vernikovskaya DI & Ko GY (2012) Calcineurin serves in the circadian output pathway to regulate the daily rhythm of L-type voltage-gated calcium channels in the retina. Journal of cellular biochemistry, 113, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Shi L, Lin CH, Kim AJ, Ko ML & Ko GY (2015) A New Role for AMP-Activated Protein Kinase (AMPK) in the Circadian Regulation of L-Type Voltage-Gated Calcium Channels (L-VGCCs) in Late-Stage Embryonic Retinal Photoreceptors. J Neurochem, 135, 727–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Lee SC & Yang XL (2005) Modulation by melatonin of glutamatergic synaptic transmission in the carp retina. J Physiol, 569, 857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Li F, Alvarez RA, Ash JD & Anderson RE (2004) Downregulation of ATP synthase subunit-6, cytochrome c oxidase-III, and NADH dehydrogenase-3 by bright cyclic light in the rat retina. Invest Ophthalmol Vis Sci, 45, 2489–2496. [DOI] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S & von Gersdorff H (2006) Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J Neurophysiol, 96, 2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno T, Deats SP, Soler J, Lonstein JS & Yan L (2016) Decreased daytime illumination leads to anxiety-like behaviors and HPA axis dysregulation in the diurnal grass rat (Arvicanthis niloticus). Behav Brain Res, 300, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immel JH & Fisher SK (1985) Cone photoreceptor shedding in the tree shrew (Tupaia belangerii). Cell Tissue Res, 239, 667–675. [DOI] [PubMed] [Google Scholar]
- Iuvone PM (1986) Evidence for a D2 dopamine receptor in frog retina that decreases cyclic AMP accumulation and serotonin N-acetyltransferase activity. Life sciences, 38, 331–342. [DOI] [PubMed] [Google Scholar]
- Iuvone PM (1990) Development of melatonin synthesis in chicken retina: regulation of serotonin N-acetyltransferase activity by light, circadian oscillators, and cyclic AMP. J Neurochem, 54, 1562–1568. [DOI] [PubMed] [Google Scholar]
- Iuvone PM & Besharse JC (1983) Regulation of indoleamine N-acetyltransferase activity in the retina: effects of light and dark, protein synthesis inhibitors and cyclic nucleotide analogs. Brain Res, 273, 111–119. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Brown AD, Haque R, Weller J, Zawilska JB, Chaurasia SS, Ma M & Klein DC (2002) Retinal melatonin production: role of proteasomal proteolysis in circadian and photic control of arylalkylamine N-acetyltransferase. Invest Ophthalmol Vis Sci, 43, 564–572. [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK & Neff NH (1978) Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science, 202, 901–902. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC & Chaurasia SS (2005) Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res, 24, 433–456. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Alonso-Gomez AL & Iuvone PM (2008) Dopamine D4 receptors regulate intracellular calcium concentration in cultured chicken cone photoreceptor cells: relationship to dopamine receptor-mediated inhibition of cAMP formation. Brain Res, 1207, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Chaurasia SS, Zhou H, Haque R, Storm DR & Iuvone PM (2009) Essential roles of dopamine D4 receptors and the type 1 adenylyl cyclase in photic control of cyclic AMP in photoreceptor cells. J Neurochem, 109, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM & McMahon DG (2012) Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci, 32, 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger C, Sandu C, Malan A, Mellac K, Hicks D & Felder-Schmittbuhl MP (2015) Circadian organization of the rodent retina involves strongly coupled, layer-specific oscillators. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 29, 1493–1504. [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, Zizi F, Lazzaro DR & Wolintz AH (2008) Circadian rhythm dysfunction in glaucoma: A hypothesis. J Circadian Rhythms, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin NG, Chuang AZ, Masson PJ & Ribelayga CP (2015) Rod electrical coupling is controlled by a circadian clock and dopamine in mouse retina. J Physiol, 593, 1597–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner ML & Lee A (2015) Voltage-Gated Cav1 Channels in Disorders of Vision and Hearing. Current molecular pharmacology, 8, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K, Tsumura T, Ishii H, Ijiri H, Tamura K & Tsukahara S (2000) Circadian rhythm of autonomic nervous function in patients with normal-tension glaucoma compared with normal subjects using ambulatory electrocardiography. J Glaucoma, 9, 239–246. [DOI] [PubMed] [Google Scholar]
- Kaupp UB & Seifert R (2002) Cyclic nucleotide-gated ion channels. Physiol Rev, 82, 769–824. [DOI] [PubMed] [Google Scholar]
- Keeley PW & Reese BE (2010) Morphology of dopaminergic amacrine cells in the mouse retina: independence from homotypic interactions. J Comp Neurol, 518, 1220–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten FF, van Wijk E, van Reeuwijk J, van der Zwaag B, Marker T, Peters TA, Katsanis N, Wolfrum U, Keunen JE, Roepman R & Kremer H (2010) Association of whirlin with Cav1.3 (alpha1D) channels in photoreceptors, defining a novel member of the usher protein network. Invest Ophthalmol Vis Sci, 51, 2338–2346. [DOI] [PubMed] [Google Scholar]
- Kim DY, Choi HJ, Kim JS, Kim YS, Jeong DU, Shin HC, Kim MJ, Han HC, Hong SK & Kim YI (2005) Voltage-gated calcium channels play crucial roles in the glutamate-induced phase shifts of the rat suprachiasmatic circadian clock. Eur J Neurosci, 21, 1215–1222. [DOI] [PubMed] [Google Scholar]
- Knoflach D, Kerov V, Sartori SB, Obermair GJ, Schmuckermair C, Liu X, Sothilingam V, Garrido MG, Baker SA, Glosmann M, Schicker K, Seeliger M, Lee A & Koschak A (2013) Cav1.4 IT mouse as model for vision impairment in human congenital stationary night blindness type 2. Channels (Austin), 7, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY-P, Jian K, Shi L & Ko ML (2010) Circadian regulation of ion channels in photoreceptors In Dartt DA (ed) Encyclopedia of the Eye. Elsevier Ltd., Oxford, pp. 296–301. [Google Scholar]
- Ko GY, Ko M & Dryer SE (2004a) Circadian and cAMP-dependent modulation of retinal cone cGMP-gated channels does not require protein synthesis or calcium influx through L-type channels. Brain Res, 1021, 277–280. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML & Dryer SE (2001) Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron, 29, 255–266. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML & Dryer SE (2003) Circadian phase-dependent modulation of cGMP-gated channels of cone photoreceptors by dopamine and D2 agonist. J Neurosci, 23, 3145–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Ko ML & Dryer SE (2004b) Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J Neurosci, 24, 1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Liu Y, Lyon RF, Roper M, Dryer SE & Ko ML (2006) Protein expression and somatostatin modulation of calcium channels are under circadian control in chick retina photoreceptors. Invest Ophthalmol Vis Sci, 47, ARVO E-abstract: 5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Shi L & Ko ML (2009a) Circadian regulation of ion channels and their functions. J Neurochem, 110, 1150–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Jian K, Shi L & Ko GY (2009b) Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. J Neurochem, 108, 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Liu Y, Dryer SE & Ko GY (2007) The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J Neurochem, 103, 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Shi L, Huang CC, Grushin K, Park SY & Ko GY (2013) Circadian phase-dependent effect of nitric oxide on L-type voltage-gated calcium channels in avian cone photoreceptors. J Neurochem, 127, 314–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Shi L & Ko GY (2009c) Circadian controls outweigh acute illumination effects on the activity of extracellular signal-regulated kinase (ERK) in the retina. Neurosci Lett, 451, 74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV & Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev, 20, 1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot JI (1995) Ca2+ flux in retinal rod and cone outer segments: differences in Ca2+ selectivity of the cGMP-gated ion channels and Ca2+ clearance rates. Cell Calcium, 18, 285–300. [DOI] [PubMed] [Google Scholar]
- Korenbrot JI & Fernald RD (1989) Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature, 337, 454–457. [DOI] [PubMed] [Google Scholar]
- Koutalos Y & Yau KW (1996) Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci, 19, 73–81. [DOI] [PubMed] [Google Scholar]
- Kramer RH & Molokanova E (2001) Modulation of cyclic-nucleotide-gated channels and regulation of vertebrate phototransduction. J Exp Biol, 204, 2921–2931. [DOI] [PubMed] [Google Scholar]
- Krizaj D (2012) Calcium stores in vertebrate photoreceptors. Adv Exp Med Biol, 740, 873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D & Copenhagen DR (2002) Calcium regulation in photoreceptors. Frontiers in bioscience : a journal and virtual library, 7, d2023–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Gabriel R, Owen WG & Witkovsky P (1998) Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. J Comp Neurol, 398, 529–538. [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K, Sasahara K, Kudo T & Shibata S (2004) Daily injection of insulin attenuated impairment of liver circadian clock oscillation in the streptozotocin-treated diabetic mouse. FEBS Lett, 572, 206–210. [DOI] [PubMed] [Google Scholar]
- Lahouaoui H, Coutanson C, Cooper HM, Bennis M & Dkhissi-Benyahya O (2016) Diabetic retinopathy alters light-induced clock gene expression and dopamine levels in the mouse retina. Mol Vis, 22, 959–969. [PMC free article] [PubMed] [Google Scholar]
- Lamb TD & Pugh EN Jr. (2004) Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res, 23, 307–380. [DOI] [PubMed] [Google Scholar]
- Lanzani MF, de Zavalia N, Fontana H, Sarmiento MI, Golombek D & Rosenstein RE (2012) Alterations of locomotor activity rhythm and sleep parameters in patients with advanced glaucoma. Chronobiol Int, 29, 911–919. [DOI] [PubMed] [Google Scholar]
- Laurent V, Sengupta A, Sanchez-Bretano A, Hicks D & Tosini G (2017) Melatonin signaling affects the timing in the daily rhythm of phagocytic activity by the retinal pigment epithelium. Exp Eye Res, 165, 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM (1976a) Rod outer segment disc shedding in relation to cyclic lighting. Exp Eye Res, 23, 277–280. [DOI] [PubMed] [Google Scholar]
- LaVail MM (1976b) Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science, 194, 1071–1074. [DOI] [PubMed] [Google Scholar]
- LaVail MM (1980) Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci, 19, 407–411. [PubMed] [Google Scholar]
- LaVail MM & Mullen RJ (1976) Role of the pigment epithelium in inherited retinal degeneration analyzed with experimental mouse chimeras. Exp Eye Res, 23, 227–245. [DOI] [PubMed] [Google Scholar]
- Lazzerini Ospri L, Prusky G & Hattar S (2017) Mood, the Circadian System, and Melanopsin Retinal Ganglion Cells. Annu Rev Neurosci, 40, 539–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach G, Adidharma W & Yan L (2013) Depression-like responses induced by daytime light deficiency in the diurnal grass rat (Arvicanthis niloticus). PLoS One, 8, e57115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Wang S, Williams B, Hagen J, Scheetz TE & Haeseleer F (2015) Characterization of Cav1.4 complexes (alpha11.4, beta2, and alpha2delta4) in HEK293T cells and in the retina. J Biol Chem, 290, 1505–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TR, Zareba M, Link BA & Besharse JC (2018) Cone myoid elongation involves unidirectional microtubule movement mediated by dynein-1. Mol Biol Cell, 29, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP & O’Brien J (2013) Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. J Neurosci, 33, 3135–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton JD, Holzhausen LC, Babai N, Song H, Miyagishima KJ, Stearns GW, Lindsay K, Wei J, Chertov AO, Peters TA, Caffe R, Pluk H, Seeliger MW, Tanimoto N, Fong K, Bolton L, Kuok DL, Sweet IR, Bartoletti TM, Radu RA, Travis GH, Zagotta WN, Townes-Anderson E, Parker E, Van der Zee CE, Sampath AP, Sokolov M, Thoreson WB & Hurley JB (2010) Flow of energy in the outer retina in darkness and in light. Proc Natl Acad Sci U S A, 107, 8599–8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kerov V, Haeseleer F, Majumder A, Artemyev N, Baker SA & Lee A (2013) Dysregulation of Ca(v)1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels (Austin), 7, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z & Ribelayga CP (2012) Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS One, 7, e50602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiola EC & Ventura AL (2011) Release of ATP from avian Muller glia cells in culture. Neurochemistry international, 58, 414–422. [DOI] [PubMed] [Google Scholar]
- Lu J, Zoran MJ & Cassone VM (1995) Daily and circadian variation in the electroretinogram of the domestic fowl: effects of melatonin. J Comp Physiol A, 177, 299–306. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG & Yau KW (2003) Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science, 299, 245–247. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Lall GS, Allen AE & Brown TM (2012) How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Prog Brain Res, 199, 1–18. [DOI] [PubMed] [Google Scholar]
- Macchi MM & Bruce JN (2004) Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol, 25, 177–195. [DOI] [PubMed] [Google Scholar]
- Manglapus MK, Iuvone PM, Underwood H, Pierce ME & Barlow RB (1999) Dopamine mediates circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci, 19, 4132–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglapus MK, Uchiyama H, Buelow NF & Barlow RB (1998) Circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci, 18, 4775–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni F, Safa H & Finnemann SC (2014) Understanding photoreceptor outer segment phagocytosis: use and utility of RPE cells in culture. Exp Eye Res, 126, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoogan JM & Cassone VM (1999) Circadian regulation of chick electroretinogram: effects of pinealectomy and exogenous melatonin. Am J Physiol, 277, R1418–1427. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Iuvone PM & Tosini G (2014) Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res, 39, 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger GJ, Koke JR & Cahill GM (2005) Diurnal and circadian retinomotor movements in zebrafish. Vis Neurosci, 22, 203–209. [DOI] [PubMed] [Google Scholar]
- Miller JL, Picones A & Korenbrot JI (1994) Differences in transduction between rod and cone photoreceptors: an exploration of the role of calcium homeostasis. Curr Opin Neurobiol, 4, 488–495. [DOI] [PubMed] [Google Scholar]
- Miranda-Anaya M, Bartell PA & Menaker M (2002) Circadian rhythm of iguana electroretinogram: the role of dopamine and melatonin. J Biol Rhythms, 17, 526–538. [DOI] [PubMed] [Google Scholar]
- Mizuno F, Barabas P, Krizaj D & Akopian A (2010) Glutamate-induced internalization of Ca(v)1.3 L-type Ca(2+) channels protects retinal neurons against excitotoxicity. J Physiol, 588, 953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Billy BD, Kennedy KS & Kupfer DJ (1997) The effects on human sleep and circadian rhythms of 17 days of continuous bedrest in the absence of daylight. Sleep, 20, 858–864. [DOI] [PubMed] [Google Scholar]
- Moore RY & Lenn NJ (1972) A retinohypothalamic projection in the rat. J Comp Neurol, 146, 1–14. [DOI] [PubMed] [Google Scholar]
- Morgans CW (2001) Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci, 42, 2414–2418. [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L & Taylor WR (2005) Photoreceptor calcium channels: insight from night blindness. Vis Neurosci, 22, 561–568. [DOI] [PubMed] [Google Scholar]
- Mullen RJ & LaVail MM (1976) Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science, 192, 799–801. [DOI] [PubMed] [Google Scholar]
- Muller C, Mas Gomez N, Ruth P & Strauss O (2014) CaV1.3 L-type channels, maxiK Ca(2+)-dependent K(+) channels and bestrophin-1 regulate rhythmic photoreceptor outer segment phagocytosis by retinal pigment epithelial cells. Cell Signal, 26, 968–978. [DOI] [PubMed] [Google Scholar]
- Nahm SS, Farnell YZ, Griffith W & Earnest DJ (2005) Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J Neurosci, 25, 9304–9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M & Cunningham J (1994) Modulation by endogenous ATP of the light-evoked release of ACh from retinal cholinergic neurones. Br J Pharmacol, 113, 1085–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk GS, Hoon M, Wong RO & Detwiler PB (2013) Inhibitory inputs tune the light response properties of dopaminergic amacrine cells in mouse retina. J Neurophysiol, 110, 536–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A & Trouvin JH (1996) Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J Neurochem, 67, 2514–2520. [DOI] [PubMed] [Google Scholar]
- Nikaido SS & Takahashi JS (1996) Calcium modulates circadian variation in cAMP-stimulated melatonin in chick pineal cells. Brain Res, 716, 1–10. [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R & Iuvone PM (2000a) Diurnal metabolism of dopamine in dystrophic retinas of homozygous and heterozygous retinal degeneration slow (rds) mice. Brain Res, 884, 13–22. [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R & Iuvone PM (2000b) Diurnal metabolism of dopamine in the mouse retina. Brain Res, 870, 118–125. [DOI] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M & Iuvone PM (2002) Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci, 22, 2063–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Day WT & Young RW (1978) Rhythmic daily shedding of outer-segment membranes by visual cells in the goldfish. The Journal of cell biology, 76, 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren DA, Giesen HA & Wehr TA (1997) Restoration of detectable melatonin after entrainment to a 24-hour schedule in a ‘free-running’ man. Psychoneuroendocrinology, 22, 39–52. [DOI] [PubMed] [Google Scholar]
- Organisciak D, Darrow R, Barsalou L, Rapp C, McDonald B & Wong P (2011) Light induced and circadian effects on retinal photoreceptor cell crystallins. Photochemistry and photobiology, 87, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Kutty RK & Wiggert B (2000) Circadian-dependent retinal light damage in rats. Invest Ophthalmol Vis Sci, 41, 3694–3701. [PubMed] [Google Scholar]
- Ostrin LA, Abbott KS & Queener HM (2017) Attenuation of short wavelengths alters sleep and the ipRGC pupil response. Ophthalmic Physiol Opt, 37, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB & Jegla T (2005) Illumination of the melanopsin signaling pathway. Science, 307, 600–604. [DOI] [PubMed] [Google Scholar]
- Perez MT, Ehinger BE, Lindstrom K & Fredholm BB (1986) Release of endogenous and radioactive purines from the rabbit retina. Brain Res, 398, 106–112. [DOI] [PubMed] [Google Scholar]
- Peters JL & Cassone VM (2005) Melatonin regulates circadian electroretinogram rhythms in a dose- and time-dependent fashion. Journal of pineal research, 38, 209–215. [DOI] [PubMed] [Google Scholar]
- Pierce ME & Besharse JC (1985) Circadian regulation of retinomotor movements. I. Interaction of melatonin and dopamine in the control of cone length. J Gen Physiol, 86, 671–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ME & Besharse JC (1988) Circadian regulation of retinomotor movements: II. The role of GABA in the regulation of cone position. J Comp Neurol, 270, 279–287. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML & Takahashi JS (1993) Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron, 10, 579–584. [DOI] [PubMed] [Google Scholar]
- Ping Y, Huang H, Zhang XJ & Yang XL (2008) Melatonin potentiates rod signals to ON type bipolar cells in fish retina. J Physiol, 586, 2683–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge CL, Yeh PT, Liou NF, Lee CC, You SF, Liu LL, McNeill DS, Chew KS, Hattar S, Chen SK & Zhang DQ (2016) M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina. J Neurosci, 36, 7184–7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP & Rollag MD (1998) Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A, 95, 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh EN Jr. & Lamb TD (2000) Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation In Stravenga DG, Degrip WJ, Pugh EN Jr. (eds) Molecular Mechanisms in Visual Transduction Elsevier Science Amsterdam, The Netherlands: pp. 183–255. [Google Scholar]
- Qiao SN, Zhang Z, Ribelayga CP, Zhong YM & Zhang DQ (2016) Multiple cone pathways are involved in photic regulation of retinal dopamine. Sci Rep, 6, 28916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA (1993) Open-angle glaucoma. The New England journal of medicine, 328, 1097–1106. [DOI] [PubMed] [Google Scholar]
- Quigley HA & Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol, 90, 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E & Gilula NB (1973) Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci U S A, 70, 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart N & Strauss O (2014) Ion channels and transporters of the retinal pigment epithelium. Exp Eye Res, 126, 27–37. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX & Fuentes-Broto L (2010) Melatonin: a multitasking molecule. Prog Brain Res, 181, 127–151. [DOI] [PubMed] [Google Scholar]
- Reme C, Wirz-Justice A, Rhyner A & Hofmann S (1986) Circadian rhythm in the light response of rat retinal disk-shedding and autophagy. Brain Res, 369, 356–360. [DOI] [PubMed] [Google Scholar]
- Remington LA (2012) Chapter 4: Retina Clinical Anatomy and Physiology of the Visual System Butterworth-Heinemann, Elsevier Inc., Oxyford, UK. [Google Scholar]
- Ren JQ & Li L (2004) A circadian clock regulates the process of ERG b- and d-wave dominance transition in dark-adapted zebrafish. Vision Res, 44, 2147–2152. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Cao Y & Mangel SC (2008) The circadian clock in the retina controls rod-cone coupling. Neuron, 59, 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C & Mangel SC (2003) Absence of circadian clock regulation of horizontal cell gap junctional coupling reveals two dopamine systems in the goldfish retina. J Comp Neurol, 467, 243–253. [DOI] [PubMed] [Google Scholar]
- Ribelayga C & Mangel SC (2005) A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci, 25, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C & Mangel SC (2007) Tracer coupling between fish rod horizontal cells: modulation by light and dopamine but not the retinal circadian clock. Vis Neurosci, 24, 333–344. [DOI] [PubMed] [Google Scholar]
- Ribelayga C & Mangel SC (2010) Identification of a circadian clock-controlled neural pathway in the rabbit retina. PLoS One, 5, e11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y & Mangel SC (2002) Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells. J Physiol, 544, 801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y & Mangel SC (2004) A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J Physiol, 554, 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich KA, Figueroa SL, Zhan Y & Blanks JC (1995) Effects of Muller cell disruption on mouse photoreceptor cell development. Exp Eye Res, 61, 235–248. [DOI] [PubMed] [Google Scholar]
- Robertson LM & Takahashi JS (1988) Circadian clock in cell culture: II. In vitro photic entrainment of melatonin oscillation from dissociated chick pineal cells. J Neurosci, 8, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T & Merrow M (1999) Circadian systems and metabolism. J Biol Rhythms, 14, 449–459. [DOI] [PubMed] [Google Scholar]
- Ruan GX, Allen GC, Yamazaki S & McMahon DG (2008) An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol, 6, e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Zhang DQ, Zhou T, Yamazaki S & McMahon DG (2006) Circadian organization of the mammalian retina. Proc Natl Acad Sci U S A, 103, 9703–9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufiange M, Dumont M & Lachapelle P (2002) Correlating retinal function with melatonin secretion in subjects with an early or late circadian phase. Invest Ophthalmol Vis Sci, 43, 2491–2499. [PubMed] [Google Scholar]
- Ruggiero L, Connor MP, Chen J, Langen R & Finnemann SC (2012) Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc Natl Acad Sci U S A, 109, 8145–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Iuvone PM & Tosini G (2006) Intraocular injection of kainic acid does not abolish the circadian rhythm of arylalkylamine N-acetyltransferase mRNA in rat photoreceptors. Mol Vis, 12, 117–124. [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM & Tosini G (2005) Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci, 22, 3129–3136. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C & Tosini G (2004) Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J Neurosci, 24, 9693–9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandu C, Hicks D & Felder-Schmittbuhl MP (2011) Rat photoreceptor circadian oscillator strongly relies on lighting conditions. Eur J Neurosci, 34, 507–516. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK & Hattar S (2011a) Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci, 34, 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S & Matynia A (2011b) Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci, 31, 16094–16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Tippmann S, Spiwoks-Becker I, Holthues H, Wolloscheck T, Spatkowski G, Engel L, Frederiksen U & Spessert R (2010) Unique clockwork in photoreceptor of rat. J Neurochem, 115, 585–594. [DOI] [PubMed] [Google Scholar]
- Sekaran S, Lall GS, Ralphs KL, Wolstenholme AJ, Lucas RJ, Foster RG & Hankins MW (2007) 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J Neurosci, 27, 3981–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Baba K, Mazzoni F, Pozdeyev NV, Strettoi E, Iuvone PM & Tosini G (2011) Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS One, 6, e24483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedlo HJ, Li L & Turner JE (1989) Photoreceptor cell rescue in the RCS rat by RPE transplantation: a therapeutic approach in a model of inherited retinal dystrophy. Prog Clin Biol Res, 314, 645–658. [PubMed] [Google Scholar]
- Shi L, Chang JY, Yu F, Ko ML & Ko GY (2017a) The Contribution of L-Type Cav1.3 Channels to Retinal Light Responses. Frontiers in molecular neuroscience, 10, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Ko ML & Ko GY (2009) Rhythmic expression of microRNA-26a regulates the L-type voltage-gated calcium channel alpha1C subunit in chicken cone photoreceptors. J Biol Chem, 284, 25791–25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Ko ML & Ko GY (2017b) Retinoschisin Facilitates the Function of L-Type Voltage-Gated Calcium Channels. Front Cell Neurosci, 11, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Glas M, Lasarzik I & Vollrath L (2004) Mouse photoreceptor synaptic ribbons lose and regain material in response to illumination changes. Eur J Neurosci, 19, 1559–1571. [DOI] [PubMed] [Google Scholar]
- Steenhard BM & Besharse JC (2000) Phase shifting the retinal circadian clock: xPer2 mRNA induction by light and dopamine. J Neurosci, 20, 8572–8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL, Iuvone PM & Adler R (1994) Photomechanical movements of cultured embryonic photoreceptors: regulation by exogenous neuromodulators and by a regulable source of endogenous dopamine. J Neurosci, 14, 3083–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Lin T, Iuvone PM & Laties AM (1990) Postnatal control of ocular growth: dopaminergic mechanisms. Ciba Found Symp, 155, 45–57; discussion 57–62. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM & Iuvone PM (1989) Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A, 86, 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, McGlinn AM, Baldwin DA, Tobias JW, Iuvone PM & Khurana TS (2011) Image defocus and altered retinal gene expression in chick: clues to the pathogenesis of ametropia. Invest Ophthalmol Vis Sci, 52, 5765–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Pardue MT, Iuvone PM & Khurana TS (2013) Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res, 114, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T & Weitz CJ (2007) Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell, 130, 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A & Meindl A (1998) An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet, 19, 260–263. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS & Nelson MT (2005) Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Canadian journal of physiology and pharmacology, 83, 215–242. [DOI] [PubMed] [Google Scholar]
- Tosini G (2000) Melatonin circadian rhythm in the retina of mammals. Chronobiol Int, 17, 599–612. [DOI] [PubMed] [Google Scholar]
- Tosini G, Baba K, Hwang CK & Iuvone PM (2012) Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res, 103, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M & Castanon-Cervantes O (2007) Localization of a circadian clock in mammalian photoreceptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 21, 3866–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G & Dirden JC (2000) Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neurosci Lett, 286, 119–122. [DOI] [PubMed] [Google Scholar]
- Tosini G, Ferguson I & Tsubota K (2016) Effects of blue light on the circadian system and eye physiology. Mol Vis, 22, 61–72. [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K & Iuvone PM (2008) The circadian clock system in the mammalian retina. BioEssays : news and reviews in molecular, cellular and developmental biology, 30, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler MC & Hardie DG (2007) AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res, 100, 328–341. [DOI] [PubMed] [Google Scholar]
- Tufford AR, Onyak JR, Sondereker KB, Lucas JA, Earley AM, Mattar P, Hattar S, Schmidt TM, Renna JM & Cayouette M (2018) Melanopsin Retinal Ganglion Cells Regulate Cone Photoreceptor Lamination in the Mouse Retina. Cell Rep, 23, 2416–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PL & Mainster MA (2008) Circadian photoreception: ageing and the eye’s important role in systemic health. Br J Ophthalmol, 92, 1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PL, Van Someren EJ & Mainster MA (2010) The role of environmental light in sleep and health: effects of ocular aging and cataract surgery. Sleep medicine reviews, 14, 269–280. [DOI] [PubMed] [Google Scholar]
- Valenciano AI, Alonso-Gomez AL & Iuvone PM (1999) Diurnal rhythms of tryptophan hydroxylase activity in Xenopus laevis retina: opposing phases in photoreceptors and inner retinal neurons. Neuroreport, 10, 2131–2135. [DOI] [PubMed] [Google Scholar]
- Valenciano AI, Alonso-Gomez AL & Iuvone PM (2000) Regulation of tryptophan hydroxylase activity in Xenopus laevis photoreceptor cells by cyclic AMP. J Neurochem, 74, 1961–1967. [DOI] [PubMed] [Google Scholar]
- Van Hook MJ, Wong KY & Berson DM (2012) Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur J Neurosci, 35, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Someren EJ, Riemersma RF & Swaab DF (2002) Functional plasticity of the circadian timing system in old age: light exposure. Prog Brain Res, 138, 205–231. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM & Organisciak DT (2002) Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochemistry and photobiology, 75, 547–553. [DOI] [PubMed] [Google Scholar]
- Vollrath L, Meyer A & Buschmann F (1989) Ribbon synapses of the mammalian retina contain two types of synaptic bodies--ribbons and spheres. J Neurocytol, 18, 115–120. [DOI] [PubMed] [Google Scholar]
- Vollrath L & Spiwoks-Becker I (1996) Plasticity of retinal ribbon synapses. Microsc Res Tech, 35, 472–487. [DOI] [PubMed] [Google Scholar]
- von Schantz M, Lucas RJ & Foster RG (1999) Circadian oscillation of photopigment transcript levels in the mouse retina. Brain Res Mol Brain Res, 72, 108–114. [DOI] [PubMed] [Google Scholar]
- Vugler AA, Redgrave P, Semo M, Lawrence J, Greenwood J & Coffey PJ (2007) Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Exp Neurol, 205, 26–35. [DOI] [PubMed] [Google Scholar]
- Vuong HE, Hardi CN, Barnes S & Brecha NC (2015) Parallel Inhibition of Dopamine Amacrine Cells and Intrinsically Photosensitive Retinal Ganglion Cells in a Non-Image-Forming Visual Circuit of the Mouse Retina. J Neurosci, 35, 15955–15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tikhonenko M, Bozack SN, Lydic TA, Yan L, Panchy NL, McSorley KM, Faber MS, Yan Y, Boulton ME, Grant MB & Busik JV (2014) Changes in the daily rhythm of lipid metabolism in the diabetic retina. PLoS One, 9, e95028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y & Mangel SC (1996) A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci U S A, 93, 4655–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL & Robinson DW (2006) The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci, 23, 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warthen DM, Wiltgen BJ & Provencio I (2011) Light enhances learned fear. Proc Natl Acad Sci U S A, 108, 13788–13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Schubert T, Paquet-Durand F, Tanimoto N, Chang L, Koeppen K, Ott T, Griesbeck O, Seeliger MW, Euler T & Wissinger B (2012) Light-driven calcium signals in mouse cone photoreceptors. J Neurosci, 32, 6981–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MP & Fisher LJ (1989) Effects of exogenous melatonin on circadian disc shedding in the albino rat retina. Vision Res, 29, 167–179. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Vrieze MJ, Dighe R & Hu Y (2003) Direct modulation of rod photoreceptor responsiveness through a Mel(1c) melatonin receptor in transgenic Xenopus laevis retina. Invest Ophthalmol Vis Sci, 44, 4522–4531. [DOI] [PubMed] [Google Scholar]
- Wilkinson MF & Barnes S (1996) The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J Gen Physiol, 107, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P (2004) Dopamine and retinal function. Doc Ophthalmol, 108, 17–40. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stone S & Besharse JC (1988) Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res, 449, 332–336. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, LeSauter J, Yan L, Johnson M, Zhang DQ, McMahon D & Silver R (2003) Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. J Neurosci, 23, 7670–7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womac AD, Burkeen JF, Neuendorff N, Earnest DJ & Zoran MJ (2009) Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci, 30, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT (2010) Energy metabolism of the visual system. Eye Brain, 2, 99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Marmorstein AD, Striessnig J & Peachey NS (2007) Voltage-dependent calcium channel CaV1.3 subunits regulate the light peak of the electroretinogram. J Neurophysiol, 97, 3731–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing W, Akopian A & Krizaj D (2012) Trafficking of presynaptic PMCA signaling complexes in mouse photoreceptors requires Cav1.4 alpha1 subunits. Adv Exp Med Biol, 723, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Zhao JW & Yang XL (2002) Expression of voltage-dependent calcium channel subunits in the rat retina. Neurosci Lett, 329, 297–300. [DOI] [PubMed] [Google Scholar]
- Xu L, Ruan G, Dai H, Liu AC, Penn J & McMahon DG (2016) Mammalian retinal Muller cells have circadian clock function. Mol Vis, 22, 275–283. [PMC free article] [PubMed] [Google Scholar]
- Yang G, Chen L, Grant GR, Paschos G, Song WL, Musiek ES, Lee V, McLoughlin SC, Grosser T, Cotsarelis G & FitzGerald GA (2016) Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med, 8, 324ra316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL & Wu SM (1989) Modulation of rod-cone coupling by light. Science, 244, 352–354. [DOI] [PubMed] [Google Scholar]
- Yau KW & Baylor DA (1989) Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci, 12, 289–327. [DOI] [PubMed] [Google Scholar]
- Yazulla S & Lin ZS (1995) Differential effects of dopamine depletion on the distribution of [3H]SCH 23390 and [3H]spiperone binding sites in the goldfish retina. Vision Res, 35, 2409–2414. [PubMed] [Google Scholar]
- Young RW (1977) The daily rhythm of shedding and degradation of cone outer segment membranes in the lizard retina. J Ultrastruct Res, 61, 172–185. [DOI] [PubMed] [Google Scholar]
- Young RW (1978) The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Invest Ophthalmol Vis Sci, 17, 105–116. [PubMed] [Google Scholar]
- Yu CJ, Gao Y, Li P & Li L (2007) Synchronizing multiphasic circadian rhythms of rhodopsin promoter expression in rod photoreceptor cells. J Exp Biol, 210, 676–684. [DOI] [PubMed] [Google Scholar]
- Zagotta WN & Siegelbaum SA (1996) Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci, 19, 235–263. [DOI] [PubMed] [Google Scholar]
- Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, Brainard GC, Gregory-Evans K, Rizzo JF 3rd, Czeisler CA, Foster RG, Moseley MJ & Lockley SW (2007) Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol, 17, 2122–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M (1989) Relationship between light, calcium influx and cAMP in the acute regulation of melatonin production by cultured chick pineal cells. Brain Res, 477, 14–18. [DOI] [PubMed] [Google Scholar]
- Zawilska JB & Iuvone PM (1992) Melatonin synthesis in chicken retina: effect of kainic acid-induced lesions on the diurnal rhythm and D2-dopamine receptor-mediated regulation of serotonin N-acetyltransferase activity. Neurosci Lett, 135, 71–74. [DOI] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE & McMahon DG (2008) Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A, 105, 14181–14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Zhou TR & McMahon DG (2007) Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. J Neurosci, 27, 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li H, Liu X, O’Brien J & Ribelayga CP (2015) Circadian clock control of connexin36 phosphorylation in retinal photoreceptors of the CBA/CaJ mouse strain. Vis Neurosci, 32, E009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Silveyra E, Jin N & Ribelayga CP (2018) A Congenic Line of the C57BL/6J Mouse Strain that is Proficient in Melatonin Synthesis. Journal of pineal research, e12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizi F, Jean-Louis G, Magai C, Greenidge KC, Wolintz AH & Heath-Phillip O (2002) Sleep complaints and visual impairment among older Americans: a community-based study. The journals of gerontology. Series A, Biological sciences and medical sciences, 57, M691–694. [DOI] [PubMed] [Google Scholar]
- Zou J, Lee A & Yang J (2012) The expression of whirlin and Cav1.3alpha(1) is mutually independent in photoreceptors. Vision Res, 75, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]