Abstract
BACKGROUND
Advanced pancreatic cancer (aPC) has a poor prognosis with limited survival benefit from current standard treatment. Viscum album extracts (VAE) are used by many cancer patients, showing immune-stimulating effects, improved quality of life, and a survival benefit in patients with aPC.
CASE SUMMARY
A 59-year-old architect developed epigastric pain. A cystic lesion of the pancreas of 45-mm diameter was detected. In a follow-up magnetic resonance imaging, about one year later, multiple lesions were seen in the corpus and the tail of the pancreas; CA-19-9 was elevated to 58.5 U/mL. A distal pancreatectomy with splenectomy was performed, and a tumor of 7 cm × 5 cm × 3.5 cm was excised. Histologic investigation showed an intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma with invasion of the lymph vessels, perineural invasion, and positive nodes (2/27); surgical margins showed tumor cells, and the tumor was classified as pT3 N1 M0 R1. The patient was treated with radiation of the tumor bed and capecitabine/oxaliplatin followed by gemcitabine and FOLFIRINOX. Seven months after surgery, a liver metastasis was detected and treatment with FOLFIRINOX was started. Four months after detection of the metastasis, the patient opted for additional treatment with VAE. Another month later, the metastasis was treated with radiofrequency ablation (RFA). Eight months later, the hepatic lesion recurred and was again treated with RFA. The continuous VAE treatment was increased in dose, and the patient stayed recurrence-free for the next 39 mo in good health and working full-time (as of the time this case report was written).
CONCLUSION
We present the case of a patient with aPC with R1-resection with development of liver metastasis during the course of treatment who showed an overall survival of 63 mo and a relapse-free survival of 39 mo under increasing VAE therapy. The possible synergistic effect on tumor control of RFA treatment and immune-stimulatory effects of VAE should be further investigated.
Keywords: Pancreatic cancer, Integrative medicine, Viscum album extract, Radiofrequency ablation, Case report
Core tip: We herewith present a case report of a patient with advanced pancreatic cancer with a survival time of 63 mo despite 2 times of relapse und continuous treatment with Viscum album extracts. The patient preserved a good quality of live, regained his weight after weight loss during chemotherapy and is currently working full-time in his job.
INTRODUCTION
Pancreatic cancer (PC) is a common malignancy (incidence 12.6/100.000 in the United States[1]), with a dismal prognosis (5-year survival of 2%[2]), especially in cases of locally advanced disease (median survival of 8-12 mo[3]). Intraductal papillary mucinous neoplasm (IPMN)-associated invasive adenocarcinoma of the pancreas is a histologic subtype of PC that has to be kept distinct from mucinous cystic neoplasms of the pancreas[4]; IPMN-associated invasive adenocarcinoma shows a better prognosis due to earlier onset of clinical symptoms compared to pancreatic ductal adenocarcinoma (PDA). However, prognosis of IPMN-associated and standard PDA are equally poor when compared by stage[5]. In all PC patients in which R0-resection seems feasible, surgery is recommended. R0-resected patients show a strong survival benefit compared to R1-resected patients[6]. Type of chemotherapy regimen - as primary or adjuvant treatment - is chosen according to tumor stage and performance status[7]. Radiofrequency ablation (RFA) is increasingly used in advanced PC (aPC); the procedure is safe, but comparative randomized trials on effectiveness are lacking[8-10].
Viscum album (European mistletoe, Viscum album L.) is a hemiparasitic shrub, growing on different host trees (oak, apple, pine and others). Aqueous Viscum album extracts (VAE) show a variety of antineoplastic properties including cytotoxic effects, apoptosis induction, immune stimulation, down-regulation of cancer genes (e.g., transforming growth factor β and matrix-metalloproteinases), reduction of cell migration, and reduction of tumor angiogenesis[11-14]. Active compounds of VAE are especially mistletoe lectins and viscotoxins, but also oligo- and polysaccharides, flavonoids, and triterpene acids[14]. Injectable forms of VAE preparations (typically for subcutaneous use, or - as off-label use - for intralesional or intravenous application) are commercially available, and are used as supportive cancer therapy [15]. VAE show a common range of local reactions such as erythema at the injection site and general reactions like fever and flu-like symptoms. Rarely, pseudo allergic reactions can occur. VAE are safe, even when used in higher dosages [16].
CASE PRESENTATION
Chief complaints
A 59-year-old architect developed epigastric pain. He had a body mass index (BMI) of 30 (body weight of 92 kg, height of 175 cm) and was a nonsmoker.
History of present illness
Nothing to declare.
History of past illness
The patient had a history of bronchial asthma and suffered from gastroesophageal reflux disease, which was treated with esomeprazole.
Personal and family history
His father had died from metastatic colon cancer.
Laboratory examinations
CA-19-9 was elevated to 58.5 U/mL.
Imaging examinations
A cystic lesion of the pancreas of 45-mm diameter was seen in ultrasound and confirmed by computed tomography (CT) scan. In a follow-up magnetic resonance imaging (MRI), about one year after initial symptoms, multiple lesions were seen in the corpus and the tail of the pancreas (Figure 1A and B). A splenopancreatectomy was performed, and a tumor of 7 cm × 5 cm × 3.5 cm of the pancreatic tail was excised. Histologic investigation showed a poorly demarcated IPMN-associated hypermuzinous invasive adenocarcinoma with invasion of the lymph vessels, perineural invasion, and positive nodes (2/27); the tumor was 1 mm in the ventral resection margin, and the dorsal margin showed tumor parts (Figure 2). The tumor was classified as pT3 N1 M0 R1.
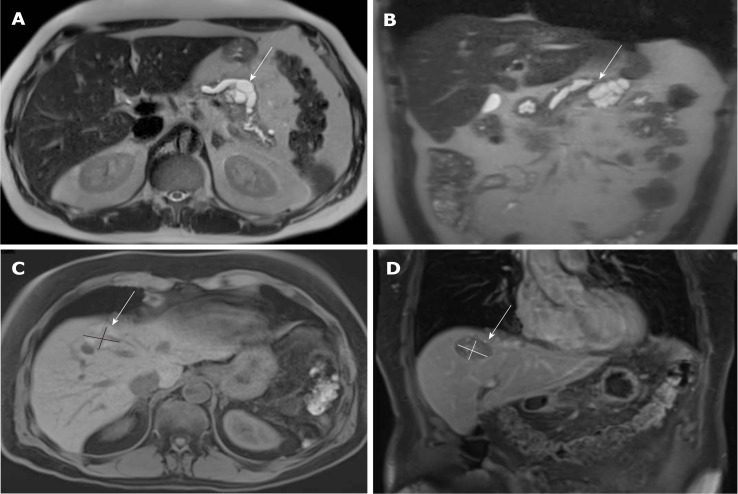
Figure 1.
Magnetic resonance imaging images of the tumor lesions. A and B: primary tumor lesion 2 mo before the initial diagnosis: cystic lesion at the location between body and tail of the pancreas with a size of 43 mm × 38 mm × 30 mm; dilatation of the pancreatic duct of 13 mm (A: T2 axial, B: T2 coronal); C and D: Relapsed hepatic lesion in segment VIII in month 20 after initial diagnosis (C: T1 axial, D: T1 coronal).
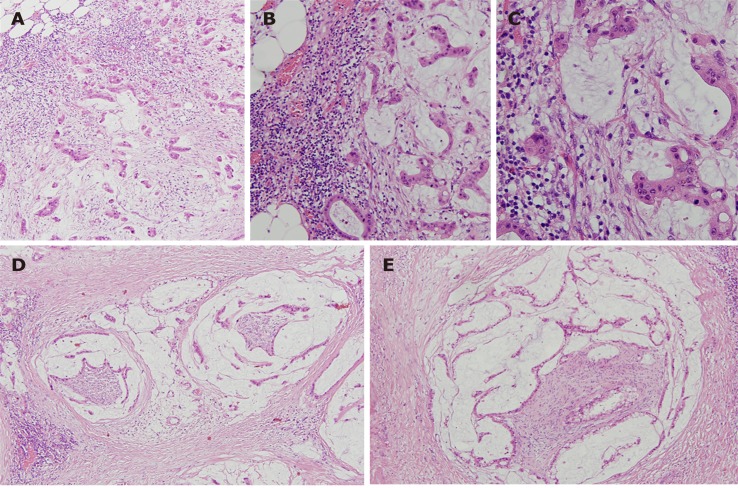
Figure 2.
Patient’s tumor, haematoxylin and eosin stain. A: × 100; B: × 200; C :( × 400) invasive mucinous adenocarcinoma; D and E: (× 100) perineural invasion.
FINAL DIAGNOSIS
A patient with aPC.
TREATMENT
The patient was treated with radiation of the tumor bed (59.4 Gy) and capecitabine and oxaliplatin followed by gemcitabine and 8 cycles of FOLFIRINOX (folinic acid 390 mg, fluorouracil 4700 mg, irinotecan 340 mg, oxaliplatin 150 mg).
OUTCOME AND FOLLOW-UP
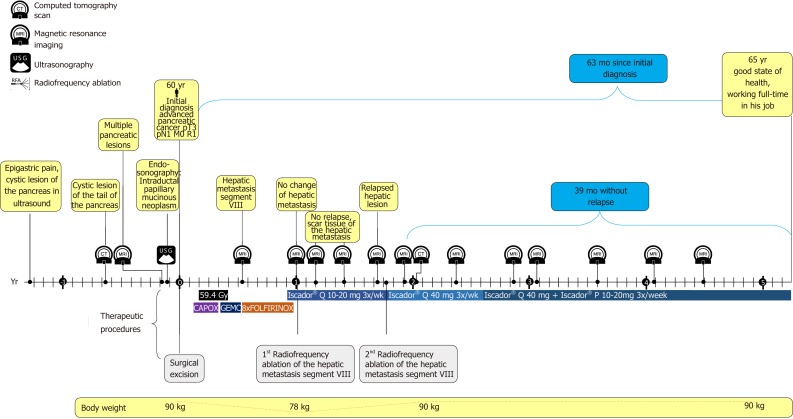
About 7 mo after surgery, a liver metastasis 2 cm in diameter in segment VIII was detected, and therefore treatment with FOLFIRINOX was started. Four months after detection of the metastasis the patient opted for additional continuous treatment with VAE (Iscador Qu 10 mg, 3 injections per week). Another month later, the metastasis was still unchanged despite chemotherapy and therefore treated with RFA. Eight months later, the hepatic lesion recurred within the scar tissue of the first metastasis, showing a lesion about 2 cm in diameter (Figure 1C and D). It was again treated by RFA and by a dose increase of VAE (Figure 3). The lesion regressed again and the patient stayed recurrence-free for the next 39 mo (as of the writing of this case report; for details of the disease and treatment course, see Figure 3).
Figure 3.
Timeline of the patient with advanced pancreatic cancer. Gy: Radiotherapy in Gray; CAPOX: Capecitabine + oxaliplatin; GEMC: Gemcitabine; FOLIFIRINOX: Leucovorin + fluorouracil + irinotecan + oxaliplatin.
DISCUSSION
We present a case of a 59-year-old patient with an aPC who underwent resection but showed positive margins (R1), with two relapses (liver metastases) after radiochemotherapy and RFA of metastases. The patient continuously received an adjunct treatment with VAE in increasing doses beginning shortly after detection of the liver metastasis and continuing until present. He showed prolonged survival and good health 63 mo after initial diagnosis. The patient had an advanced primary tumor, perineural invasion, lymph vascular invasion, node involvement, and positive resection margins - all of which are independent negative prognostic factors[17]. In this patient, surgical excision was possible - a circumstance associated with an improved prognosis. However, median survival of PC patients who have undergone surgical excision, regardless of stage or other prognostic factors, is 19 mo (interquartile range, 10-42 mo)[18].
VAE was repeatedly investigated in PC and showed cytotoxic effects in cell lines and animal studies (PA-TU-8902, PAXF 736, PAXF 546)[19-21]. Tröger et al[22] found a survival benefit for aPC patients solely treated with VAE in a randomized controlled trial on overall survival. Similar results were seen by Axtner et al[23] in an integrative oncology registry. In a phase 1 study of VAE and Gemcitabine in patients with advanced solid tumors (including PC) it could be shown, that VAE did not affect the pharmacokinetics of Gemcitabine[24]. VAE in PC is currently further investigated in clinical trials (NCT02948309).
RFA acts through direct thermal cell damage but shows additional immune stimulatory properties as tumor antigens and a variety of cytokines are released from the treated tumor tissue; increased numbers of tumor-specific T cells have been detected in RFA-treated patients[10]. Combination therapy of RFA with different immune-stimulatory agents showed promising results[25,26]. The combination of VAE with radiation - which, like RFA, leads to cell damage and to a release of antigens - proved to be more effective than radiation alone in animal studies[27,28]. VAE shows several immune-stimulating properties like proliferation induction and increase of antigen presentation of dendritic cells, proliferation of CD4+ T cells, increase of natural killer cell-mediated cytotoxicity, and release of cytokines[14].
CONCLUSION
We presume that VAE acted synergistically with RFA, leading to tumor control. Reports of combined treatment with RFA and VAE should be collected to determine whether further investigations in this area are worthwhile.
ACKNOWLEDGEMENTS
We are thankful to Dr. Helmut Kiene (IFAEMM) for revision of the manuscript.
Footnotes
Informed consent statement: Informed consent was received from the patient for the publication of the report and accompanying images. The patient read the submission version of the report and confirmed its content.
Conflict-of-interest statement: The authors have declared no conflicts of interest.
Peer-review started: November 1, 2018
First decision: December 20, 2018
Article in press: March 2, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
CARE Checklist (2016) statement: This case report was prepared following the CARE Guideline.
P-Reviewer: Ramia JM, Sandhu DS, Sugimoto M, Yang F S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
Contributor Information
Paul G Werthmann, Department of Methodology, Institute for Applied Epistemology and Medical Methodology (IFAEMM), Freiburg 79111, Germany; Center for Complementary Medicine, Institute for Environmental Health Sciences and Hospital Infection Control, Medical Center - University of Freiburg, Freiburg 79106, Germany. paul.werthmann@ifaemm.de.
Robert Kempenich, Department of Oncology, General Practitioner with Specialization in Oncology, Strasbourg F-67000, France.
Gerlinde Lang-Avérous, Department of Pathology, Hôpital de Hautepierre, University Hospital of Strasbourg, Strasbourg F-67000, France.
Gunver S Kienle, Department of Methodology, Institute for Applied Epistemology and Medical Methodology (IFAEMM), Freiburg 79111, Germany; Center for Complementary Medicine, Institute for Environmental Health Sciences and Hospital Infection Control, Medical Center - University of Freiburg, Freiburg 79106, Germany.
References
- 1.National Cancer Institute. SEER Cancer Stat Facts: Pancreas Cancer [Internet]. 2018 [cited July 16, 2018] Available from: URL: https://seer.cancer.gov/statfacts/html/pancreas.html. [Google Scholar]
- 2.Tabernero J, Chiorean EG, Infante JR, Hingorani SR, Ganju V, Weekes C, Scheithauer W, Ramanathan RK, Goldstein D, Penenberg DN, Romano A, Ferrara S, Von Hoff DD. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20:143–150. doi: 10.1634/theoncologist.2014-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crippa S, Salvia R, Warshaw AL, Domínguez I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY, Mino-Kenudson M, Capelli P, Pederzoli P, Castillo CF. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571–579. doi: 10.1097/SLA.0b013e31811f4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poultsides GA, Reddy S, Cameron JL, Hruban RH, Pawlik TM, Ahuja N, Jain A, Edil BH, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghaneh P, Kleeff J, Halloran CM, Raraty M, Jackson R, Melling J, Jones O, Palmer DH, Cox TF, Smith CJ, O'Reilly DA, Izbicki JR, Scarfe AG, Valle JW, McDonald AC, Carter R, Tebbutt NC, Goldstein D, Padbury R, Shannon J, Dervenis C, Glimelius B, Deakin M, Anthoney A, Lerch MM, Mayerle J, Oláh A, Rawcliffe CL, Campbell F, Strobel O, Büchler MW, Neoptolemos JP European Study Group for Pancreatic Cancer. The Impact of Positive Resection Margins on Survival and Recurrence Following Resection and Adjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269:520–529. doi: 10.1097/SLA.0000000000002557. [DOI] [PubMed] [Google Scholar]
- 7.Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, Arnold D ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 8.Hua YQ, Wang P, Zhu XY, Shen YH, Wang K, Shi WD, Lin JH, Meng ZQ, Chen Z, Chen H. Radiofrequency ablation for hepatic oligometastatic pancreatic cancer: An analysis of safety and efficacy. Pancreatology. 2017;17:967–973. doi: 10.1016/j.pan.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 9.Pandya GJ, Shelat VG. Radiofrequency ablation of pancreatic ductal adenocarcinoma: The past, the present and the future. World J Gastrointest Oncol. 2015;7:6–11. doi: 10.4251/wjgo.v7.i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 11.Beztsinna N, de Matos MBC, Walther J, Heyder C, Hildebrandt E, Leneweit G, Mastrobattista E, Kok RJ. Quantitative analysis of receptor-mediated uptake and pro-apoptotic activity of mistletoe lectin-1 by high content imaging. Sci Rep. 2018;8:2768. doi: 10.1038/s41598-018-20915-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podlech O, Harter PN, Mittelbronn M, Pöschel S, Naumann U. Fermented mistletoe extract as a multimodal antitumoral agent in gliomas. Evid Based Complement Alternat Med. 2012;2012:501796. doi: 10.1155/2012/501796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elluru SR, Duong Van Huyen JP, Delignat S, Prost F, Heudes D, Kazatchkine MD, Friboulet A, Kaveri SV. Antiangiogenic properties of viscum album extracts are associated with endothelial cytotoxicity. Anticancer Res. 2009;29:2945–2950. [PubMed] [Google Scholar]
- 14.Singh BN, Saha C, Galun D, Upreti DK, Bayry J, Kaveri SV. European Viscum album: a potent phytotherapeutic agent with multifarious phytochemicals, pharmacological properties and clinical evidence. RSC Adv. 2016 [Google Scholar]
- 15.Kienle GS, Kiene H. Complementary cancer therapy: a systematic review of prospective clinical trials on anthroposophic mistletoe extracts. Eur J Med Res. 2007;12:103–119. [PubMed] [Google Scholar]
- 16.Kienle GS, Grugel R, Kiene H. Safety of higher dosages of Viscum album L. in animals and humans--systematic review of immune changes and safety parameters. BMC Complement Altern Med. 2011;11:72. doi: 10.1186/1472-6882-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JW, Bhandari M, Astill DS, Wilson TG, Kow L, Brooke-Smith M, Toouli J, Padbury RT. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford) 2010;12:101–108. doi: 10.1111/j.1477-2574.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J, Ahuja N, Makary MA, Cameron JL, Eckhauser FE, Choti MA, Hruban RH, Pawlik TM, Wolfgang CL. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford) 2014;16:83–90. doi: 10.1111/hpb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissenstein U, Kunz M, Urech K, Baumgartner S. Interaction of standardized mistletoe (Viscum album) extracts with chemotherapeutic drugs regarding cytostatic and cytotoxic effects in vitro. BMC Complement Altern Med. 2014;14:6. doi: 10.1186/1472-6882-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burger AM, Mengs U, Schüler JB, Fiebig HH. Antiproliferative activity of an aqueous mistletoe extract in human tumor cell lines and xenografts in vitro. Arzneimittelforschung. 2001;51:748–757. doi: 10.1055/s-0031-1300110. [DOI] [PubMed] [Google Scholar]
- 21.Rostock M, Huber R, Greiner T, Fritz P, Scheer R, Schueler J, Fiebig HH. Anticancer activity of a lectin-rich mistletoe extract injected intratumorally into human pancreatic cancer xenografts. Anticancer Res. 2005;25:1969–1975. [PubMed] [Google Scholar]
- 22.Tröger W, Galun D, Reif M, Schumann A, Stanković N, Milićević M. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: a randomised clinical trial on overall survival. Eur J Cancer. 2013;49:3788–3797. doi: 10.1016/j.ejca.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 23.Axtner J, Steele M, Kröz M, Spahn G, Matthes H, Schad F. Health services research of integrative oncology in palliative care of patients with advanced pancreatic cancer. BMC Cancer. 2016;16:579. doi: 10.1186/s12885-016-2594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansky PJ, Wallerstedt DB, Sannes TS, Stagl J, Johnson LL, Blackman MR, Grem JL, Swain SM, Monahan BP. NCCAM/NCI Phase 1 Study of Mistletoe Extract and Gemcitabine in Patients with Advanced Solid Tumors. Evid Based Complement Alternat Med. 2013;2013:964592. doi: 10.1155/2013/964592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gameiro SR, Higgins JP, Dreher MR, Woods DL, Reddy G, Wood BJ, Guha C, Hodge JW. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS One. 2013;8:e70417. doi: 10.1371/journal.pone.0070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Staveley-O'Carroll KF, Kimchi ET. Potential of Radiofrequency Ablation in Combination with Immunotherapy in the Treatment of Hepatocellular Carcinoma. J Clin Trials. 2016;6 doi: 10.4172/2167-0870.1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurin M, Zarković N, Hrzenjak M, Ilić Z. Antitumorous and immunomodulatory effects of the Viscum album L. preparation Isorel. Oncology. 1993;50:393–398. doi: 10.1159/000227217. [DOI] [PubMed] [Google Scholar]
- 28.von Bodungen U, Ruess K, Reif M, Biegel U. Kombinierte Anwendung von Strahlentherapie und adjuvanter Therapie mit einem Mistelextrakt (Viscum album L.) zur Behandlung des oralen malignen Melanoms beim Hund: Eine retrospektive Studie. Complement Med Res. 2017;24:358–363. doi: 10.1159/000485743. [DOI] [PubMed] [Google Scholar]