Abstract
The increasing prevalence of obesity is alarming because it is a risk factor for cardiovascular and metabolic diseases (such as type 2 diabetes). The occurrence of these comorbidities in obese patients can arise from white adipose tissue (WAT) dysfunctions, which affect metabolism, insulin sensitivity and promote local and systemic inflammation. In mammals, WAT depots at different anatomical locations (subcutaneous, preperitoneal and visceral) are highly heterogeneous in their morpho-phenotypic profiles and contribute differently to homeostasis and obesity development, depending on their ability to trigger and modulate WAT inflammation. This heterogeneity is likely due to the differential behavior of cells from each depot. Numerous studies suggest that adipose-derived stem/stromal cells (ASC; referred to as adipose progenitor cells, in vivo) with depot-specific gene expression profiles and adipogenic and immunomodulatory potentials are keys for the establishment of the morpho-functional heterogeneity between WAT depots, as well as for the development of depot-specific responses to metabolic challenges. In this review, we discuss depot-specific ASC properties and how they can contribute to the pathophysiology of obesity and metabolic disorders, to provide guidance for researchers and clinicians in the development of ASC-based therapeutic approaches.
Keywords: White adipose tissue, Metabolic diseases, Obesity, Adipose-derived stromal/stem cells, Adipose depot, Inflammation
Core tip: White adipose tissue (WAT) depots at different anatomical locations are highly heterogeneous in morphology and phenotype, and contribute differently to the development of obesity and metabolic disorders. Here, we discuss the role of adipose-derived stem/stromal cells (ASC) in the development of obesity and metabolic disorders, by reviewing the data suggesting that depot-specific ASC/adipose progenitor cells help to develop the specific responses of each WAT depot to metabolic challenges. In particular, we address the importance of ASC-dependent immunomodulation in the inflammatory response associated with obesity, providing guidance for future research on the use ASC-based therapeutic approaches.
INTRODUCTION
The World Health Organization defines obesity as abnormal or excessive fat accumulation that represents a risk to health. Obesity can develop due to an imbalance between energy intake and expenditure by the organism, and it is strongly related to environmental factors, such as high caloric food consumption and sedentary lifestyle. In addition, the intestinal microbiota, stress levels, endocrine and genetic profiles can also contribute to increase an individual’s susceptibility to obesity[1,2]. The increasing prevalence of obesity is alarming because it is a risk factor for several diseases, including hypertension, ischemic cardiovascular disease, dyslipidemia, insulin-resistance, diabetes, metabolic syndrome[3-7] and also cancer[8-11].
The primary site for energy storage in humans is white adipose tissue (WAT)[12]. The discovery that metabolic diseases such as obesity and type-2 diabetes arise from WAT dysfunctions has revealed immune and endocrine non-classical functions of WAT, which strongly impact on metabolism, insulin sensitivity and promote local and systemic inflammation[13-15]. Mammalian WAT depots found in distinct anatomical locations are highly heterogeneous in their morpho-phenotypic profiles[16,17]. The differential accumulation of fat in specific anatomical depots (rather than the total body fat mass) is the crucial factor that determines the clinical outcomes of obesity and other metabolic diseases. Depot-specific adipose-derived stem/stromal cells (ASC) could be pivotal to determine the different pathophysiological roles of each depot, by modulating the depot’s gene expression profile and its adipogenic and immunomodulatory potentials. Therefore, a deep understanding of the contribution of depot-specific ASC to the differential properties and pathogenicity of WAT depots can be crucial for developing new therapeutic approaches against metabolic disorders.
In this review, we discuss the current knowledge on depot-dependent ASC properties and how they can contribute to the pathophysiology of obesity and metabolic disorders. The discussion here aims to provide guidance for researchers and clinicians in the future use ASC in therapeutic strategies against obesity and related pathologies.
WHITE ADIPOSE TISSUE DEPOTS: A MATTER OF ANATOMICAL LOCATION OR INHERENT PROPERTIES?
In most mammals species, fat storage occurs mainly in WAT, inside specialized cells called adipocytes[18], which accumulate triglyceride molecules (consisting of glycerol and fatty acid chains). Adipocytes can dramatically alter their size according to changes in metabolic demand. After a meal, insulin stimulates WAT to store energy in the form of neutral lipids, mainly triacylglycerol, in a process known as lipogenesis. Conversely, adipocytes provide free fat acids to be metabolized by the organism through lipolysis, in periods of fasting[19].
In humans, WAT is distributed in two main depots – the subcutaneous and the visceral WAT - with distinct structure, cell content, gene expression and secretion profiles, as well as responsiveness to neuro-endocrine stimuli. The subcutaneous WAT is distributed along the body surface, forming the hypodermis, with distinct depots in the abdominal, femoral, gluteal, facial and cranial regions. On the other hand, visceral WAT surrounds the organs of the abdominal cavity, and is also found in smaller amounts around the heart (epicardial visceral WAT), stomach (epigastric visceral WAT) and blood vessels (perivascular visceral WAT)[16,17,20].
Evidence links obesity and metabolic dysfunction to the total body fat mass, particularly in the abdominal region[5]. In the abdominal WAT, subcutaneous WAT is subdivided by the Scarpa’s fascia into superficial and deep depots[21,22], while visceral WAT is subdivided into omental (surrounding the surface of the intestines), mesenteric (deeply within the intestines) and retroperitoneal (near the kidneys, at the back) fat depots[16,23]. In the 1950s, Vague[24] showed that the anatomical fat distribution could have important metabolic implications, with certain distributions favoring diabetes and atherosclerosis. Krotkiewsk et al[25] showed that subjects with a higher waist-to-hip ratio had increased blood pressure, low carbohydrate tolerance and high insulin plasma levels. By connecting clinical, epidemiological and physiological evidence with WAT measurements, different research groups concluded that visceral fat accumulation (central obesity) is more strongly associated with higher metabolic and cardiovascular risk, while subcutaneous fat accumulation in the thighs and hips (peripheral obesity) is associated with a lower risk of these diseases [26-30].
However, it remained unclear whether the differential impact on systemic metabolism was due to the anatomical location of the WAT depot, to intrinsic properties of the cells in each depot, or both. WAT depot transplantation in mice shed light on the influence of depot anatomical location on systemic metabolism. Both lean and obese mice had increased glucose tolerance, insulin sensitivity and reduced body weight after receiving a transplant of subcutaneous WAT from lean mice into the visceral cavity[31-34]. The metabolic improvement exerted by subcutaneous WAT transplanted into a different anatomical location suggested that subcutaneous and visceral WAT depots are intrinsically different.
The studies mentioned above triggered the search for intrinsic biological differences between depots that could explain the link between depot heterogeneity and metabolic complications, both in lean and obese rodents and humans. Indeed, gene expression analysis revealed significant differences in hundreds of genes between distinct adipose tissue depots[35-37]. Moreover, visceral WAT has a higher triglyceride turnover compared to subcutaneous WAT, probably due to a higher sensitivity to the lipolytic function of catecholamines and a lower sensitivity to the antilipolytic effects of insulin[38-40].
Thus far, the vast majority of studies on the heterogeneity of abdominal WAT depots focused on the comparison between subcutaneous and visceral WAT. However, abdominal WAT comprises not only these two types of depots but also the preperitoneal (also known as endoabdominal or extraperitoneal) WAT, located between the transverse fascia and the parietal peritoneum[41]. Interestingly, the preperitoneal WAT has the highest size variation during weight loss by dieting, compared with subcutaneous and visceral WAT[42]. Like subcutaneous and visceral WAT, preperitoneal WAT can also be identified in non-obese and obese subjects by computer-tomography and ultrasonography[43-46].
Suzuki et al[43] suggested that the abdominal wall fat index (AFI) determined by ultrasonography could be a novel indicator of visceral fat deposition. This study showed that the AFI - which represents the ratio between the preperitoneal WAT maximum thickness (Pmax) and the subcutaneous WAT minimum thickness (Smin) - positively correlates with the visceral to subcutaneous WAT ratio (V/S). These data indicate that the thickness of the preperitoneal WAT depot is positively associated with the visceral depot mass. Moreover, the AFI correlated positively with the plasma levels of triglycerides and with the basal insulin levels in obese individuals, but was inversely correlated with high density lipoprotein levels[43]. Whether preperitoneal and visceral WAT depots have similar properties or even similar impact on metabolic dysfunctions remains controversial. While some studies showed that the preperitoneal WAT maximum thickness or the AFI are associated with cardiovascular risk factors[47,48], others indicated that visceral WAT thickness showed a better association with cardiovascular risk factors compared with subcutaneous and preperitoneal WAT thickness[49,50]. Similarly to visceral WAT, the preperitoneal WAT is covered by the peritoneum; however, visceral (but not preperitoneal) WAT contains portal vein circulation[51,52]. A functional comparison of the preperitoneal WAT depot with subcutaneous and visceral WAT, both in lean and in metabolically disrupted patients, is necessary to clarify the impact of each WAT depot on metabolic and cardiovascular disease risks.
Therefore, the relationship between different WAT depots and systemic homeostasis and the development of metabolic diseases is mainly dependent on the intrinsic properties rather than the anatomical location of each depot. The metabolic and genetic differences observed between abdominal whole WAT depots could be related to the behavior of the cells that dwell in each depot.
STROMAL-VASCULAR FRACTION AND THE INHERENT PROPERTIES OF WAT DEPOTS
WAT is composed of two main cell fractions: mature unilocular adipocytes and stromal-vascular cells, known as the stromal-vascular fraction (SVF). After enzymatic digestion of the adipose tissue and centrifugation, the adipocytes float to the surface, while SVF cells sediment to the pellet[53].
Adipocytes have the fundamental role of accumulating triacylglycerols during periods of caloric excess, and then breaking this reservoir into free fatty acids when energy consumption is required. Mature adipocytes are equipped with enzymes and regulatory proteins to perform lipolysis and lipogenesis, which are orchestrated by hormones, cytokines and other factors involved in energy metabolism[16].
The adipose SVF is highly heterogeneous, and can be sub-divided into hematopoietic and stromal compartments[53]. The hematopoietic compartment comprises cells that express CD45, including lymphocytes (Natural Killer, helper and regulatory T cells, and B cells)[54], eosinophils[55], neutrophils[56], hematopoietic progenitors[57], mast cells[58] and macrophages. Notably, the presence of macrophages has been repeatedly reported in human and murine adipose tissue[59-61]. The percentage of macrophages varies according to the presence of pathophysiological conditions, such as obesity, which is characterized by monocytic/macrophagic infiltration into adipose tissue[62,63].
The stromal compartment of the adipose SVF is composed of mesenchymal and endothelial cells associated with blood vessels. Zimmerlin et al[64] distinguished the following four cell subpopulations in the stromal SVF compartment, using a combination of in situ immunolabeling and cell sorting: (1) Pericytes/mesenchymal stem cells (MSC; CD146+/CD34-/CD31-); (2) Adipocyte progenitors/Pre-adipocytes (CD146-/CD34+/CD31-); (3) Endothelial progenitor cells (CD31+/CD34+); and (4) Mature endothelial cells (CD31+/CD34-). All cells in the stromal compartment are negative for the pan-hematopoietic marker CD45. In the adipose tissue, MSC give rise to endothelial progenitors and pre-adipocytes, which differentiate into endothelial cells and adipocytes, respectively. Therefore, adipose MSC can maintain or increase adipocyte numbers, thereby modulating the adipose tissue lipid store capacity, as well as its ability for homeostasis or regeneration through adipogenesis[65].
SVF culture generates a population of adherent cells characterized by the expression of mesenchymal markers including CD44, CD73, CD90 and CD105, but negative for CD45 and CD31[66,67]. These cells can differentiate in vitro into mature cells of mesodermal lineages, such as adipocytes, osteoblasts and chondrocytes[66-69]. These combined phenotypic features and differentiation properties are diagnostic of ASC[70]. These cells can also lead to angiogenesis, by differentiating directly into endothelial cells[71], by interacting with endothelial cells to induce vascular formation[72], or by secreting angiogenic factors such as VEGF, HGF, FGF and PDGF[73-75]. The angiogenic potential of ASC has important therapeutic implications. ASC secrete different types of chemical mediators, including cytokines and growth factors, which have paracrine activities that stimulate local cell survival and proliferation, angiogenesis, differentiation of local stem cells, and reduce apoptosis[75-77]. Moreover, ASC can suppress mixed lymphocyte reaction[78] and their low immunogenicity could enable their safe use in allogeneic transplants, as part of cell-based regenerative therapies[79]. Therefore, the differentiation capacity of ASC and their trophic effects directly contribute to adipose tissue homeostasis, cell renewal, tissue repair and tissue immunogenic balance[80].
Lafontan et al[81] postulated that metabolic and genetic differences observed between abdominal whole WAT depots could be related to the unique properties of the cells that dwell in each of these depots. Besides, these unique cell properties could also account for the different responses of each depot to metabolic challenges[81,82]. Proteomic analysis of adipocytes and SVF cells isolated from subcutaneous and visceral WAT from lean subjects showed that the SVF could have a higher contribution to the functional differences observed between these depots[83].
The in vivo counterparts of the cultured ASC still remain to be defined and studies sometimes refer to these cells as mesenchymal stem/stromal cells. Throughout this review the term “ASC” only will be used for the adherent cells derived from the SVF with the diagnostic features mentioned previously, which we use as criteria for ASC identification[70]. In contrast, when describing resident adipose cells with progenitor potential in vivo, the term “adipose progenitors” will be used instead. Given the ability of ASC/adipose progenitors to govern adipose tissue development and homeostasis, some studies have suggested that depot-specific ASC with unique cell-autonomous properties could be responsible for the morpho-functional heterogeneity of WAT depots[84-86].
RELATIONSHIP BETWEEN OBESITY-INDUCED INFLAMMATION AND ADIPOGENESIS
WAT inflammation in obesity
The ability of adipocytes to increase in size (adipocyte hypertrophy) during lipogenesis was believed to be the only mechanism by which adult WAT expands upon insulin stimulation. However, it is now widely accepted that an increase in adipocytes number - or adipose tissue hyperplasia - also contributes to WAT mass gain through the recruitment and differentiation of adipose progenitors, in a process known as adipogenesis[2]. Therefore, the ability of WAT to expand during life in response to metabolic needs depends not only on adipocytes, but also on the adipogenic potential of adipose progenitors. Other factors such as vasculature and extracellular matrix remodeling also contribute to the plasticity of adipose tissue and influence adipocyte hypertrophy and adipogenesis from stem cells[87].
During the development of obesity, WAT expands to an extent that leads to chronic tissue inflammation[62], which is associated with an increased risk of type-2 diabetes and cardiovascular disease[88]. The first functional connection between obesity and inflammation was the observation that obese WAT secretes large amounts of the proinflammatory cytokine tumor necrosis factor (TNF)-α, and that this cytokine had a direct role in obesity-induced insulin resistance[89,90]. As well as increased levels of proinflammatory cytokines, obese WAT also exhibits low level of anti-inflammatory mediators[89,91]. The discovery that obesity is characterized by macrophage accumulation in adipose tissue added a new dimension to our understanding of how obesity propagates inflammation, as macrophage recruitment is an important factor in promoting insulin resistance[62,63]. A clue to the origin of these recruited macrophages came from the observation that, in CD45.2 mice transplanted with bone marrow cells from CD45.1 mice, 85% of the adipose tissue macrophage (F4/80+) cell population had the CD45.1 marker. Therefore, during obesity development, the expanding WAT secretes chemoattractants (such as the mouse chemoattractant protein-1, MCP-1, and the macrophage inflammatory protein-1α, MIP-1α) that recruit monocytes from the bone marrow to adipose tissue[62,63].
In obesity, the infiltrating macrophages adopt a proinflammatory (“M1”) phenotype, becoming a source of proinflammatory cytokines such as IL-1β and TNF-α[63], which trigger local and systemic insulin resistance[62]. These infiltrating macrophages differ from adipose tissue resident (“M2”) macrophages, which exhibit anti-inflammatory characteristics[92,93]. In mice, high-fat diets turn the secretion pattern of M2 macrophages into M1, by the reduction of IL-10 and arginase levels, and the increase in TNF-α and iNOS levels[94]. Diet-induced obesity increases the expression of the M1 marker CD11c in WAT, while decreasing CD206 expression, which is typical of M2 macrophages[95].
The poorly-defined mechanisms that initiate inflammation and connect the inflammatory scenario of obese WAT to other diseases are the subject of intense investigation, in a research area known as “metabolic inflammation”[96]. Metabolically altered adipose tissue cells may interact with immune cells to initiate the inflammatory process. Interactions between immune and metabolic cells occurs in other metabolic tissues and organs (liver, muscle and pancreas) in obese individuals, suggesting that metabolic inflammation could be a systemic feature of obesity[97].
Immune-metabolic interactions occur in obesity between adipocytes or SVF cells and macrophages. Indeed, adipocyte hypertrophy is a potential trigger for macrophage accumulation in WAT[98]. In association with the large increase in protein synthesis, hypertrophied adipocytes display mitochondrial and endoplasmic reticulum stress, which could lead to the activation of inflammatory signaling pathways[99-101]. In line with this hypothesis, hypertrophied adipocytes in obese individuals change their intrinsic secretion profile towards a proinflammatory phenotype (characterized by high TNF-α and low adiponectin levels)[19,102,103]. TNF-α could stimulate pre-adipocytes and endothelial cells to secrete MCP-1, attracting monocytes from the bone marrow[62,63]. In addition, pro-inflammatory cytokines and fatty acids secreted by hypertrophic adipocytes can lead recruited macrophages towards an M1 proinflammatory phenotype[104]. Moreover, groups of hypoxic and hypertrophic adipocytes undergo necrosis, and are cleared by macrophage phagocytosis. Indeed, macrophages form crown-like structures around necrotic adipocytes in obese WAT, in a typical chronic inflammatory response[95,105].
While the M1 profile is pro-inflammatory, the potentiation of M2 pathways in macrophages appears to reduce metabolic inflammation (or “metainflammation”), improving insulin sensitivity[103]. The M2 phenotype of resident adipose-tissue macrophages is maintained by the paracrine action of lymphocytes and eosinophils; however, in obesity, the recruitment of these cells to WAT is suppressed[106,107]. Tolerogenic CD4+ T-regulatory cells (Tregs) are also downregulated in WAT during obesity, which could lead to metainflammation[108,109]. Aside from Tregs, other leukocytes, including NK, NKT and mast cells, have a yet poorly-defined role in metainflammation[110-112]. Further studies on the temporal and spatial immune-metabolic interactions between leukocytes and WAT cells should shed light on the mechanisms underlying inflammation in obesity, to identify potential targets for clinical intervention.
Complex molecular signaling pathways may link metabolic challenges (e.g., excessive fat storage) with inflammation in obesity[113], including pathways involving the NLRP3 inflammasome, a cytoplasmic protein complex that promotes the conversion of pro-cytokines into active cytokines, which are then secreted[114]. NLRP3 inflammasome activity can be modulated by several metabolites, including fatty acids, and the activation of this complex can interfere with insulin signaling[115,116]. Inflammasome activity can be triggered by endogenous or exogenous stress signals (e.g., cytokines, free fatty acids, glucose, reactive oxygen species, ATP), which function as “pathogen-associated molecular patterns” that interact with pattern recognition receptors, especially toll-like receptors (TLRs), in WAT cells. The interaction of stress signals with TLR4, for example, activates the nuclear factor-κB pathway, which increases NLRP3 expression[116-118].
Adipose progenitors could be key regulators of macrophage recruitment and activation in WAT[84]. Indeed, human ASC express active TLRs, including TLR4, whose activation results in the secretion of the pro-inflammatory cytokines IL-6 and IL-8[119]. Moreover, adipose progenitors express molecules that favor immune differentiation, such as osteopontin, which was identified as one of the factors involved in macrophage accumulation during diet-induced obesity[120]. In line with this notion, we showed that human ASC secrete MCP-1 in vitro[121], and that mouse ASC populations enriched in pre-adipocytes (CD34+ ASC) could be responsible for most of the MCP-1 secretion in mice[122]. In addition, we observed that ASC can support in vitro hematopoiesis, with a tendency to generate macrophages from hematopoietic progenitors[67]. Moreover, while adipocytes are the main source of hormones that regulate energy metabolism (such as adiponectin and leptin), inflammatory cytokines are mostly secreted by cells from the SVF[123]. Therefore, adipose progenitors can be key players in the regulation of the metabolic inflammation established during obesity, acting as a key source of secreted immune-mediators in adipose tissue, both in normal and in pathological conditions[124].
Although macrophage infiltration in obese adipose tissue potentiates inflammation and favors the development of comorbidities, the pro-inflammatory cytokines secreted by infiltrating macrophages with an M1-phenotype could also decrease WAT mass by stimulating adipocyte lipolysis and inhibiting adipogenesis[98]. In fact, classically activated M1 macrophages impair insulin signaling and adipogenesis in adipocytes, by both direct and paracrine signals[94]. The immune and metabolic interactions that occur within WAT may have evolved as a mechanism to regain homeostasis, in order to prevent the obesity-associated mobility impairment that makes animals more vulnerable to predators[125].
The mechanisms regulating adipogenesis and inflammatory responses from stromal cells have been the subject of several studies, using various in vivo and in vitro model systems[126-129]. These studies have shown that the TNF-receptor superfamily molecule CD40 is expressed during adipogenic differentiation and interacts with surrounding immune cells, modulating adipocyte inflammatory responses and insulin resistance[127,128]. Additionally, a study by Tous et al[129] identified sphingosine kinase-1 as a potential therapeutic target to attenuate chronic inflammation in obesity and related metabolic diseases, as this molecule regulates the pro-inflammatory response in adipose progenitors.
Impact of inflammation induction on ASC functionality
ASC functionality is directly affected by obesity-induced inflammation[121,130]. Some studies have reported an inverse correlation between the body-mass index (BMI, a commonly used obesity indicator) and ASC differentiation capacity[130-132]. In agreement with these data, our studies and those of others demonstrated that ASC from obese subjects have decreased ability to differentiate into adipocytes in vitro, when compared with those from lean subjects, as assessed by intracellular lipid accumulation and/or the expression of adipogenic genes[121,130-133]. Isakson et al[134] suggested that the inflammatory state in adipose tissue may be responsible for the impaired adipocyte differentiation observed in obesity. Indeed, inflammatory cytokines are anti-adipogenic[135], and it is possible that ASC from obese patients carry a “memory” of differentiation inhibition from the inflammatory environment in vivo, and which manifests itself as impaired adipogenesis in vitro. Pro-inflammatory macrophages secrete factors that impair human adipogenesis from ASC in vitro[136,137], and there is a negative correlation between the adipogenic capacity of obese ASC and the up-regulation of inflammatory genes[130,138]. In contrast, some studies reported that ASC from obese donors showed higher expression of adipogenic genes, suggesting that obese ASC are more potent in adipogenesis[138,139]. A recent study showed that ASC from obese pigs (given a high-fat diet) exhibited increased adipogenic potential relative to those from lean pigs, at the onset of obesity[140]. The discrepancies between studies on the impact of inflammation on the adipogenic potential of ASC could be due to differences in the methods used to evaluate adipogenesis, or to the use of donors with different adiposity grades, or at different stages of obesity development.
The pro-angiogenic potential of ASC is also altered in obesity. ASC from morbidly obese individuals have higher mRNA and protein expression of the anti-angiogenic factor TSP-1 than ASC from lean individuals[130]. In addition, “lean” ASC (i.e., those differentiated from adipose tissue of lean individuals) had increased capacity to form tube-like networks while “obese” ASC (derived from obese individuals) were not responsive to angiogenic stimuli[141], showing a reduced capacity to form capillary-like structures[142]. Moreover, extracellular vesicles from obese ASC exhibited lower levels of angiogenic-related factors and, consequently, reduced angiogenic potential compared with those derived from lean ASC[143].
The ASC differentiation capacity is also disrupted in patients with type-2 diabetes mellitus. Global gene expression profiling revealed that ASC from type-2 diabetes donors have low levels of adipogenic genes compared with those from non-diabetic donors[144], indicating a decreased potential for adipogenic differentiation in diabetes. Additionally, ASC from diabetic rats were less effective at forming microvessels in vivo than those from non-diabetic animals[145].
Obesity also alters the immunomodulatory properties of ASC, and their ability to secrete chemical mediators. ASC isolated from patients with different adiposity grades exhibit different secretion patterns[146,147]. In particular, we demonstrated that ASC from morbidly obese patients secrete more proinflammatory cytokines, such as IL-6 and IL-8[121], which is in agreement with data from other groups showing that obese ASC display up-regulation of inflammatory genes (including IL-6, IL-8, IL-10 and MCP-1) compared with lean ASC[138,148]. In addition to the increased expression of inflammatory markers, obese ASC had increased migration and phagocytosis capacity compared with lean ASC. Besides, ASC from obese individuals show reduced capacity to activate the M2 macrophage phenotype and to suppress lymphocyte proliferation[149]. Therefore, the immunomodulatory properties of ASC are altered in obesity, which may be related to the role of adipose progenitors as key regulators of the immune response during obesity development. As well as in obesity, alterations in immunomodulatory properties are observed in patients with type-2 diabetes mellitus[149], and global gene expression profiling revealed that genes involved in inflammation are upregulated in ASC from type-2 diabetes patients[144]. Recently, Liu and colleagues[150] showed that ASC derived from mice with type-2 diabetes are less effective at restricting CD4+T lymphocyte proliferation and pro-inflammatory “polarization” (during pro-inflammatory immune phenotype acquisition) than ASC from lean mice.
Collectively, these data show that obesity and other immune metabolic pathologies disrupt ASC/adipose progenitor functionality, favoring a pro-inflammatory response. This response, in turn, impairs ASC adipogenic capacity, which may reduce the ability of adipose progenitors to generate new adipocytes in WAT depots, ultimately leading to ectopic fat storage. Overall, evidence from a large number of studies indicate that ASC/adipose progenitors are key regulators of the immune response in obesity and other metabolic disorders, highlighting the potential of ASC use in cell-based regenerative therapies.
REGIONAL DIFFERENCES IN ASC FUNCTIONALITY IN OBESITY AND THEIR EFFECT ON FAT EXPANSION AND DISTRIBUTION PATTERNS
ASC behavior in WAT depots in obesity
Numerous studies evaluated the behavior of ASC derived from different WAT depots (in both rodents and humans; Table 1), to test the hypothesis that the properties of depot-specific ASC could account for some of the differences in morphology, function and response to metabolic challenges observed between WAT depots.
Table 1.
Functional aspects of human adipose-derived stem/stromal cells derived from different adipose depots
| ASC depot origin | Species | Metabolic status of subjects | Gender | Sample number (n) | Functional aspects of ASC | Publications |
| SC and VC | Human | Non-obese | Male and female | 18 | Proliferation: SC > VC | Baglioni et al[85], 2012 |
| Adipogenic potential: SC > VC | ||||||
| Adiponectin secretion by ASC-derived adipocytes: SC > VC | ||||||
| Lipolysis susceptibility of ASC-derived adipocytes: VC > SC | ||||||
| SC, PP, VC | Human | Morbidly obese | Female | 12 | Adipogenic potential: PP > SC > VC | Silva et al[86], 2017 |
| IL-6, IL-8, MCP-1, G-CSF secretion: VC > SC = PP | ||||||
| PAI-1 secretion: SC=PP > VC | ||||||
| Adiponectin secretion by ASC-derived adipocytes: PP > SC = VC | ||||||
| SC e VC | Human | Obese | Male and female | 29 | Proliferation: SC > VC | van Harmelen et al[131], 2004 |
| Adipogenic potential: SC = VC | ||||||
| SC and VC | Human | Non-obese | Female | 5 | Surface markers (CD31-, CD34-, CD45-, CD73+, CD90+, CD105+): SC = VC | Kim et al[151], 2016 |
| Proliferation: SC > VC ; | ||||||
| Adipogenic potential: SC > VC ; | ||||||
| Genetic pattern: SC ≠ VC | ||||||
| Lipid biosynthesis and metabolism genes expression: VC > SC | ||||||
| DNA-dependent transcription: SC > VC | ||||||
| SC and VC | Mice and human | Non-obese and obese | Male and female | 198 (human) | Genome-wide expression profiles (including embrionic development and pattern specification genes): SC ≠ VC | Gesta et al[152], 2006 |
| SC, VC | Human | Lean and obese | Male and female | 12 | Proliferation: SC > VC | Tchkonia et al[153], 2007 |
| Adipogenic potential: SC > VC mesenteric > VC omentum | ||||||
| Induced-apoptosis susceptibility VC > SC | ||||||
| Genome-wide expression profiles (including early development genes): SC ≠ VC | ||||||
| SC and VC | Human | Obese | Female | 8 | MCP-1, IL-6, IL-8, CCL-5 secretion: VC > SC | Zhu et al[154], 2015 |
| SC and VC | Human | Non-obese | Male and female | 15 | MCP-1, eotaxin, IL-1ra, IL-6, GM-CSF, VEGF secretion: VC > SC | Perrini et al[155], 2013 |
| SC and PP | Human | Non-obese and obese | Male | 8 | Proliferation: SC > PP | Fernández et al[156], 2010 |
| Adipogenic potential: PP > SC | ||||||
| SC and VC | Human | Lean and obese | Female | 14 | Adipogenic potential: SC > VC | Hauner et al[159], 1988 |
| SC and VC | Human | Not stated | Not stated | Not stated | Adipogenic potential: SC > VC | Adams et al[160], 1997 |
| SC and VC | Human | Non-obese and obese | Male and female | 12 | Adipogenic potential: SC > VC | Niesler et al[161], 1998 |
| Susceptibility to induced apoptosis: VC > SC | ||||||
| SC and VC | Human | Non-obese and obese | Male and female | Not stated | Adipogenic potential: SC > VC | Digby et al[162], 2000 |
| SC, VC | Human | Obese | Male and female | 16 | Adipogenic potential: SC > VC mesenteric > VC omentum | Tchkonia et al[163], 2002 |
| SC, VC | Human | Obese | Male and female | 18 | Adipogenic potential: SC > VC omentum | Tchkonia et al[164], 2005 |
| Resistance to induced apoptosis: SC > VC omentum | ||||||
| Proliferation: SC = VC mesenteric > VC omentum | ||||||
| SC, VC | Human | Overweight and obese | Male and female | 31 | Adipogenic potential: SC > VC | Tchkonia et al[165], 2006 |
| Resistance to induced apoptosis: SC > VC mesenteric > VC omentum | ||||||
| SC and VC | Human | Not stated | Not stated | 21 | Proliferation: SC = VC | Toyoda et al[166]2009 |
| Adipogenic and osteogenic potential: SC > VC | ||||||
| SC and VC | Mice | Non-obese and obese | Not stated | Not stated | Proliferation: SC > VC | Macotela et al[167], 2012 |
| Adipogenic potential: SC > VC | ||||||
| SC and VC | Mice | Not stated | Male | Not stated | Adipogenic potential: SC > VC | Meissburger et al[168], 2016 |
| SV and VC | Mice | High-fat diet | Male and female | Not stated | Proliferation in response to high-fat diet: SC > VC | Joe et al[169], 2009 |
| Adipogenic potential: SC > VC | ||||||
| SC and VC | Human | Non-obese and obese | Male and female | 18 | Adipogenic potential: SC = VC | Shahparaki et al[170], 2002 |
| SC and VC | Human | Non-obese | Male and female | 13 | ASC-derived adipocytes C/EBP, AP-2 and adiponection expression: SC > VC | Perrini et al[171], 2008 |
| Adiponectin secretion of ASC-derived adipocytes: VC > SC | ||||||
| Stimulated glucose uptake ASC-derived adipocytes: VC > SC | ||||||
| SC e VC | Mice | Lean | Male | Not stated | MMP14 expression: SC = VC | Tokunaga et al[172], 2014 |
| MMP8 and MMP13: VC > SC |
ASC: Adipose-derived stem/stromal cells; G-CSF: Granulocyte colony-stimulating factor; GM-CSF: Granulocyte-Macrophage colony-stimulating factor; IL: Interleukine; MCP-1: Monocyte chemoattractant protein-1; MMP: Matrix metaloproteinase; PP: Preperitoneal; SC: Subcutaneous; VEGF: Vascular endothelial growth factor; VC: Visceral.
Baglioni et al[85] reported that, for both lean and overweight subjects, ASC derived from subcutaneous WAT depots have higher growth rate and adipogenic potential than those derived from visceral WAT depots. In addition, adipocytes derived from subcutaneous ASC have greater capacity to secrete adiponectin and are less susceptible to lipolysis than adipocytes derived from visceral ASC. Therefore, functional differences between subcutaneous and visceral WAT depots could originate from differences in depot-specific stem cells. Moreover, microarray analysis revealed that the genes differentially expressed between subcutaneous and visceral ASC are implicated in energy and lipid metabolism; importantly, genes involved in cholesterol biosynthesis and triacylglycerol metabolism were upregulated in visceral ASC[151]. Genome-wide expression profiles of ASC derived from subcutaneous and visceral depots are highly distinct, in particular for the expression of genes responsible for early development, which gave rise to the idea that adipose depots exist as individual mini-organs[152,153].
Numerous studies have compared depot-specific ASC from lean and obese subjects, to investigate if the differences between depot-specific ASC may account for the differential responses of adipose depots during the development of metabolic dysfunctions. We have recently demonstrated that ASC from the visceral depot secreted the highest levels of IL-6 and IL-8 compared with ASC derived from subcutaneous and preperitoneal WAT depots[86]. Other studies also reported increased secretion of pro-inflammatory, pro-angiogenic and pro-migratory molecules (IL-6[154,155], IL-8[154], CCL-5[154], MCP-1[86,154,155], G-CSF[86], GM-CSF[155], eotaxin[155], IL-1ra[155] and VEGF[155]) by ASC from the visceral depot, when compared with those derived from the subcutaneous depot. Therefore, visceral ASC appear to secrete more pro-inflammatory cytokines than subcutaneous ASC, both in obese and in non-obese states, which is in line with the stronger pro-inflammatory pattern adopted by visceral WAT in response to metabolic challenges.
Fernández et al[156] were the first to report the isolation of ASC from the preperitoneal WAT depot. These authors observed that preperitoneal ASC have a higher adipogenic potential than those derived from the subcutaneous WAT depot. Comparing ASC from abdominal subcutaneous, preperitoneal and visceral WAT depots of morbidly obese women, we demonstrated that preperitoneal ASC have the highest ability to differentiate to the adipogenic lineage in vitro. In addition, we observed that ASC derived from the visceral depot had the lowest adipogenic potential[86], which could be explained by the strongly pro-inflammatory milieu established in this depot during obesity. For example, IL-6 production in visceral WAT is 3 fold higher than in the subcutaneous depot[146,157]. Moreover, the macrophage accumulation observed in WAT depots during obesity development[62,63] is particularly high in the visceral compared with the subcutaneous WAT depot[158]. We have recently demonstrated that, compared with subcutaneous SVF cells, visceral SVF populations have higher numbers of CD14+CD206- cells, a phenotype associated with M1 macrophages[86].
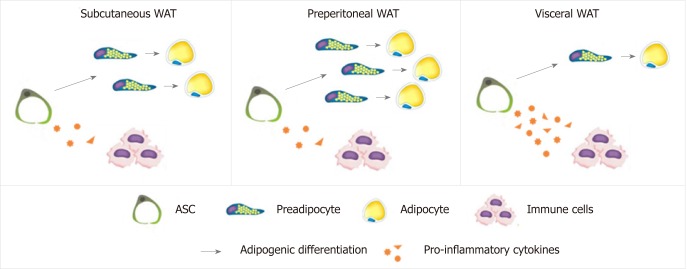
Although some studies showed that visceral ASC have higher adipogenic potential than those from subcutaneous depots, others studies reported the opposite, both in humans[85,86,151,153,159-166] and in mice[152,167-169], with no differences reported in two studies in humans[131,170]. Thus, there is currently no clear consensus regarding the differences in adipogenic potential between depot-specific ASC populations. Differences in donor adiposity grades and sex, in ASC isolation and adipogenic induction protocols, as well as in methods of adipogenic evaluation could account for this discrepancy, and highlight the importance of technical standardization in this area[173]. Nevertheless, as most studies suggest that there are differences in the adipogenic capacity of ASC derived from distinct WAT depots, together with differences regarding their pro-inflammatory potential (Figure 1), it is likely that ASC/adipogenic precursors contribute to establish distinct fat distribution and expansion patterns between depots, and the balance between hypertrophy and hyperplasia during obesity development.
Figure 1.
Adipogenic and pro-inflammatory potentials of adipose-derived stem/stromal cells derived from different abdominal adipose tissue depots. Adipose-derived stem/stromal cells (ASC) from different abdominal adipose tissues have different adipogenic and immunomodulatory properties. Pre-peritoneal ASC have the highest capacity to generate new adipocytes by adipogenesis and low pro-inflammatory profile. ASC from visceral abdominal depot have the highest capacity to secrete pro-inflammatory cytokines such as interleukine (IL)-1ra, IL-6 and IL-8 together with the lowest adipogenic potential. ASC: Adipose-derived stem/stromal cells; WAT: White adipose tissue.
Fat distribution and expansion capacity of different WAT depots
As mentioned earlier, WAT can expand through increases in the size of adipocytes (hypertrophy), as well as by increases in the number of adipocytes (hyperplasia, through adipogenesis). Obese individuals where the visceral WAT is expanded preferentially have a greater risk of developing other metabolic and cardiovascular diseases than those who have more subcutaneous WAT expansion[174-176], which has a protective role against the metabolic complications of obesity induced by high-fat diets[177,178].
Hypertrophic adipocytes are associated with adipose tissue dysfunction and inflammation[179-181], while adipocyte hyperplasia is associated with improved insulin sensitivity and other metabolic parameters[182], indicating that the balance between hypertrophy and hyperplasia during WAT expansion can determine the effect of adipose tissue expansion on metabolic disease development. A comparison of WAT depots suggested that hyperplasia contributes to subcutaneous WAT expansion more than to the expansion of visceral WAT, after a high-fat diet[169]. Given the association of hypertrophic adipocytes with adipose tissue dysfunction, the preferential expansion of visceral WAT by hypertrophy rather than hyperplasia could represent the mechanism underlying the link between visceral WAT expansion and obesity. On the other hand, the preferential expansion of subcutaneous WAT in humans by hyperplasia may explain why subcutaneous WAT expansion is considered comparatively “healthier” than visceral WAT expansion.
However, lineage-tracing experiments in transgenic male mice have challenged this view, by detecting an increase in the formation of new adipocytes in the epididymal visceral WAT, with no measurable adipocyte formation in the subcutaneous WAT, in mice given a high-fat diet[183,184]. Later studies demonstrated that in vivo hyperplasia in WAT varies according to the specific depot and the sex, being influenced by sex hormones[185]. While males have higher potential for expansion by hyperplasia in the visceral WAT only, females exhibit WAT hyperplasia in both visceral and subcutaneous depots after a high-fat diet[185]. This may also occur in humans, since obesity development in men is associated predominantly with visceral WAT expansion, while obesity development in women involves subcutaneous WAT expansion[22,186]. Adding further complexity to this issue, Tchoukalova et al[187] reported that overfeeding in humans induces different mechanisms of WAT expansion in upper- and lower-body subcutaneous WAT depots: while upper-body abdominal subcutaneous WAT predominantly expands by adipocyte hypertrophy, lower-body subcutaneous WAT preferentially expands by adipocyte hyperplasia. Moreover, differences in preadipocyte replication or apoptosis could explain the differential patterns of expansion between upper- and lower-body subcutaneous WAT depots.
The numerous in vitro and in vivo studies described in this review suggests that the different physiopathological properties of distinct WAT depots could be attributed to the intrinsic properties - including gene expression, adipogenic and angiogenic potentials, and inflammatory behavior - of adipose progenitors cells within each adipose compartment. However, Jeffery et al[185] recently challenged this hypothesis by demonstrating, in a series of elegant transplantation experiments in transgenic mice, that donor adipose progenitor cells behave as resident progenitors after transplantation. As previously described, levels of hyperplasia were only detected in visceral WAT of male mice fed with a high-fat diet, but not in subcutaneous WAT, indicating that subcutaneous adipose progenitors did not enter adipogenesis[184]. Importantly, when subcutaneous adipose progenitors were injected into the visceral WAT depot, they proliferated in response to the high-fat diet, but neither subcutaneous nor visceral adipose progenitors proliferated when transplanted into the subcutaneous WAT depot[185]. These exciting data may suggest that adipose progenitors from distinct WAT depots, despite having different developmental origins[188,189], are functionally plastic and capable of responding to high-fat diets according to cell-extrinsic factors of the depot microenvironment. Therefore, these new data suggest that, irrespective of their origin, adipose progenitors behave according to the WAT depot in which they dwell. Although it is clear that ASC from distinct fat depots contribute differently to obesity, further studies are now necessary to clarify the contribution of cell-extrinsic and/or intrinsic factors in obesity development.
CONCLUSION
Adipose progenitors play an important role in obesogenic WAT growth and the regulation of adipogenesis by these cells may be used in novel therapeutic strategies against obesity and related diseases. There is no doubt that ASC from different WAT depots have distinct properties, which are not totally autonomous, as the distinct microenvironments of each WAT depot influence the function of adipose progenitor in WAT expansion. Moreover, distinct in vivo niches of adipose progenitors may account for the differential susceptibilities of adipose depot to the development of metabolic dysfunction. Future studies on adipose progenitor niches, considering the depot-specific microenvironment and the influence of sex influence on adipose progenitor activation, should elucidate the regulatory signals that govern adipose progenitor function. Ultimately, these studies may allow adipose progenitors to be targeted in therapeutic approaches to prevent obesity development or to allow obese individuals to reach a healthier metabolic status.
ACKNOWLEDGEMENTS
The authors thank the National Council for Scientific and Technological Development (CNPq), the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) and the Coordination of High Education Personnel Improvement (CAPES) for financial support.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: September 29, 2018
First decision: October 19, 2018
Article in press: February 28, 2019
Specialty type: Cell and tissue engineering
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cardile V, Tanabe S S-Editor: Wang JL L-Editor: A E-Editor: Song H
Contributor Information
Karina Ribeiro Silva, Laboratory of Tissue Bioengineering, Directory of Metrology Applied to Life Sciences, National Institute of Metrology, Quality and Technology, Duque de Caxias, RJ 25250-020, Brazil; Post-Graduation Program of Biotechnology, National Institute of Metrology, Quality and Technology, Duque de Caxias, RJ 25250-020, Brazil.
Leandra Santos Baptista, Laboratory of Tissue Bioengineering, Directory of Metrology Applied to Life Sciences, National Institute of Metrology, Quality and Technology, Duque de Caxias, RJ 25250-020, Brazil; Post-Graduation Program of Biotechnology, National Institute of Metrology, Quality and Technology, Duque de Caxias, RJ 25250-020, Brazil; Multidisciplinary Center for Biological Research (Numpex-Bio), Federal University of Rio de Janeiro Campus Duque de Caxias, Duque de Caxias, RJ 25245-390, Brazil. leandra.baptista@gmail.com.
References
- 1.Conway B, Rene A. Obesity as a disease: no lightweight matter. Obes Rev. 2004;5:145–151. doi: 10.1111/j.1467-789X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 2.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 3.Björntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 4.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 5.Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 6.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body Mass Index, Abdominal Fatness, and Heart Failure Incidence and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Circulation. 2016;133:639–649. doi: 10.1161/CIRCULATIONAHA.115.016801. [DOI] [PubMed] [Google Scholar]
- 8.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 9.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 10.Font-Burgada J, Sun B, Karin M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016;23:48–62. doi: 10.1016/j.cmet.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Donohoe CL, Lysaght J, O'Sullivan J, Reynolds JV. Emerging Concepts Linking Obesity with the Hallmarks of Cancer. Trends Endocrinol Metab. 2017;28:46–62. doi: 10.1016/j.tem.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27:875–888. doi: 10.1038/sj.ijo.0802326. [DOI] [PubMed] [Google Scholar]
- 13.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 14.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 16.Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf) 2012;205:194–208. doi: 10.1111/j.1748-1716.2012.02409.x. [DOI] [PubMed] [Google Scholar]
- 17.Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. 2018;221:jeb162958. doi: 10.1242/jeb.162958. [DOI] [PubMed] [Google Scholar]
- 18.Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Blüher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 20.Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011;34 Suppl 2:S371–S379. doi: 10.2337/dc11-s250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 22.Lancerotto L, Stecco C, Macchi V, Porzionato A, Stecco A, De Caro R. Layers of the abdominal wall: anatomical investigation of subcutaneous tissue and superficial fascia. Surg Radiol Anat. 2011;33:835–842. doi: 10.1007/s00276-010-0772-8. [DOI] [PubMed] [Google Scholar]
- 23.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 25.Krotkiewski M, Björntorp P, Sjöström L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288:1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 28.von Eyben FE, Mouritsen E, Holm J, Montvilas P, Dimcevski G, Suciu G, Helleberg I, Kristensen L, von Eyben R. Intra-abdominal obesity and metabolic risk factors: a study of young adults. Int J Obes Relat Metab Disord. 2003;27:941–949. doi: 10.1038/sj.ijo.0802309. [DOI] [PubMed] [Google Scholar]
- 29.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 30.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 31.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hocking SL, Chisholm DJ, James DE. Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdominal compartment. Diabetologia. 2008;51:900–902. doi: 10.1007/s00125-008-0969-0. [DOI] [PubMed] [Google Scholar]
- 33.Foster MT, Softic S, Caldwell J, Kohli R, de Kloet AD, Seeley RJ. Subcutaneous Adipose Tissue Transplantation in Diet-Induced Obese Mice Attenuates Metabolic Dysregulation While Removal Exacerbates It. Physiol Rep. 2013;1:e00015. doi: 10.1002/phy2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hocking SL, Stewart RL, Brandon AE, Suryana E, Stuart E, Baldwin EM, Kolumam GA, Modrusan Z, Junutula JR, Gunton JE, Medynskyj M, Blaber SP, Karsten E, Herbert BR, James DE, Cooney GJ, Swarbrick MM. Subcutaneous fat transplantation alleviates diet-induced glucose intolerance and inflammation in mice. Diabetologia. 2015;58:1587–1600. doi: 10.1007/s00125-015-3583-y. [DOI] [PubMed] [Google Scholar]
- 35.Vidal H. Gene expression in visceral and subcutaneous adipose tissues. Ann Med. 2001;33:547–555. doi: 10.3109/07853890108995965. [DOI] [PubMed] [Google Scholar]
- 36.Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, Hudson TJ, Tchernof A. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 37.Linder K, Arner P, Flores-Morales A, Tollet-Egnell P, Norstedt G. Differentially expressed genes in visceral or subcutaneous adipose tissue of obese men and women. J Lipid Res. 2004;45:148–154. doi: 10.1194/jlr.M300256-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Bolinder J, Kager L, Ostman J, Arner P. Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis. Diabetes. 1983;32:117–123. doi: 10.2337/diab.32.2.117. [DOI] [PubMed] [Google Scholar]
- 39.Rebuffé-Scrive M, Andersson B, Olbe L, Björntorp P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism. 1989;38:453–458. doi: 10.1016/0026-0495(89)90198-4. [DOI] [PubMed] [Google Scholar]
- 40.Rebuffé-Scrive M, Anderson B, Olbe L, Björntorp P. Metabolism of adipose tissue in intraabdominal depots in severely obese men and women. Metabolism. 1990;39:1021–1025. doi: 10.1016/0026-0495(90)90160-e. [DOI] [PubMed] [Google Scholar]
- 41.Moore KL, Dalley AF. 4th ed. Rio de Janeiro: Guanabara Koogan; 2001. Anatomia orientada para a clínica; p. 1023. [Google Scholar]
- 42.Sabir N, Pakdemirli E, Sermez Y, Zencir M, Kazil S. Sonographic assessment of changes in thickness of different abdominal fat layers in response to diet in obese women. J Clin Ultrasound. 2003;31:26–30. doi: 10.1002/jcu.10129. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki R, Watanabe S, Hirai Y, Akiyama K, Nishide T, Matsushima Y, Murayama H, Ohshima H, Shinomiya M, Shirai K. Abdominal wall fat index, estimated by ultrasonography, for assessment of the ratio of visceral fat to subcutaneous fat in the abdomen. Am J Med. 1993;95:309–314. doi: 10.1016/0002-9343(93)90284-v. [DOI] [PubMed] [Google Scholar]
- 44.Tayama K, Inukai T, Shimomura Y. Preperitoneal fat deposition estimated by ultrasonography in patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1999;43:49–58. doi: 10.1016/s0168-8227(98)00118-1. [DOI] [PubMed] [Google Scholar]
- 45.Kawamoto R, Ohtsuka N, Nakamura S, Ninomiya D, Inoue A. Preperitoneal fat thickness by ultrasonography and obesity-related disorders. J Med Ultrason (2001) 2007;34:93–99. doi: 10.1007/s10396-007-0137-z. [DOI] [PubMed] [Google Scholar]
- 46.Guldiken S, Tuncbilek N, Okten OO, Arikan E, Tugrul A. Visceral fat thickness determined using ultrasonography is associated with anthropometric and clinical parameters of metabolic syndrome. Int J Clin Pract. 2006;60:1576–1581. doi: 10.1111/j.1742-1241.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 47.Nishina M, Kikuchi T, Yamazaki H, Kameda K, Hiura M, Uchiyama M. Relationship among systolic blood pressure, serum insulin and leptin, and visceral fat accumulation in obese children. Hypertens Res. 2003;26:281–288. doi: 10.1291/hypres.26.281. [DOI] [PubMed] [Google Scholar]
- 48.Kawamoto R, Kajiwara T, Oka Y, Takagi Y. Association between abdominal wall fat index and carotid atherosclerosis in women. J Atheroscler Thromb. 2002;9:213–218. doi: 10.5551/jat.9.213. [DOI] [PubMed] [Google Scholar]
- 49.Liu KH, Chan YL, Chan WB, Kong WL, Kong MO, Chan JC. Sonographic measurement of mesenteric fat thickness is a good correlate with cardiovascular risk factors: comparison with subcutaneous and preperitoneal fat thickness, magnetic resonance imaging and anthropometric indexes. Int J Obes Relat Metab Disord. 2003;27:1267–1273. doi: 10.1038/sj.ijo.0802398. [DOI] [PubMed] [Google Scholar]
- 50.Kim SK, Kim HJ, Hur KY, Choi SH, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr. 2004;79:593–599. doi: 10.1093/ajcn/79.4.593. [DOI] [PubMed] [Google Scholar]
- 51.Vague J, Vague P, Meignen JM. Amsterdam: Ex-cerpta Medica; 1985. Android and gynoid obesities, past and present. In: Vague J, Björntorp P, Guy-Grand B, editors. Metabolic complications of human obesities; p. 3. [Google Scholar]
- 52.Busetto L, Baggio MB, Zurlo F, Carraro R, Digito M, Enzi G. Assessment of abdominal fat distribution in obese patients: anthropometry versus computerized tomography. Int J Obes Relat Metab Disord. 1992;16:731–736. [PubMed] [Google Scholar]
- 53.Cousin B, Caspar-Bauguil S, Planat-Bénard V, Laharrague P, Pénicaud L, Casteilla L. [Adipose tissue: a subtle and complex cell system] J Soc Biol. 2006;200:51–57. doi: 10.1051/jbio:2006007. [DOI] [PubMed] [Google Scholar]
- 54.Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, André M, Casteilla L, Pénicaud L. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579:3487–3492. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 55.Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23:407–415. doi: 10.1016/j.tem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Cousin B, André M, Arnaud E, Pénicaud L, Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun. 2003;301:1016–1022. doi: 10.1016/s0006-291x(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 58.Anderson EK, Gutierrez DA, Hasty AH. Adipose tissue recruitment of leukocytes. Curr Opin Lipidol. 2010;21:172–177. doi: 10.1097/MOL.0b013e3283393867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cousin B, André M, Casteilla L, Pénicaud L. Altered macrophage-like functions of preadipocytes in inflammation and genetic obesity. J Cell Physiol. 2001;186:380–386. doi: 10.1002/1097-4652(2001)9999:9999<000::AID-JCP1038>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 60.Villena JA, Cousin B, Pénicaud L, Casteilla L. Adipose tissues display differential phagocytic and microbicidal activities depending on their localization. Int J Obes Relat Metab Disord. 2001;25:1275–1280. doi: 10.1038/sj.ijo.0801680. [DOI] [PubMed] [Google Scholar]
- 61.Curat CA, Miranville A, Sengenès C, Diehl M, Tonus C, Busse R, Bouloumié A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 62.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev. 2007;65:S7–12. doi: 10.1111/j.1753-4887.2007.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 66.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baptista LS, Pedrosa CGS, Silva KR, Otazú IB, Takyia CM, Dutra HS, Cláudio-da-silva C, Borojevic R, Rossi MID. Bone Marrow and Adipose Tissue-Derived Mesenchymal Stem Cells: How Close Are They? J Stem Cells. 2007;2:2. [Google Scholar]
- 68.Hattori H, Sato M, Masuoka K, Ishihara M, Kikuchi T, Matsui T, Takase B, Ishizuka T, Kikuchi M, Fujikawa K, Ishihara M. Osteogenic potential of human adipose tissue-derived stromal cells as an alternative stem cell source. Cells Tissues Organs. 2004;178:2–12. doi: 10.1159/000081088. [DOI] [PubMed] [Google Scholar]
- 69.Guilak F, Awad HA, Fermor B, Leddy HA, Gimble JM. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41:389–399. [PubMed] [Google Scholar]
- 70.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 72.Merfeld-Clauss S, Gollahalli N, March KL, Traktuev DO. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A. 2010;16:2953–2966. doi: 10.1089/ten.tea.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vilahur G, Oñate B, Cubedo J, Béjar MT, Arderiu G, Peña E, Casaní L, Gutiérrez M, Capdevila A, Pons-Lladó G, Carreras F, Hidalgo A, Badimon L. Allogenic adipose-derived stem cell therapy overcomes ischemia-induced microvessel rarefaction in the myocardium: systems biology study. Stem Cell Res Ther. 2017;8:52. doi: 10.1186/s13287-017-0509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, Dilley RJ. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21:2189–2203. doi: 10.1089/scd.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 76.Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 77.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291:R880–R884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 78.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 79.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 80.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 81.Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 82.Lebovitz HE. The relationship of obesity to the metabolic syndrome. Int J Clin Pract Suppl. 2003:18–27. [PubMed] [Google Scholar]
- 83.Peinado JR, Jimenez-Gomez Y, Pulido MR, Ortega-Bellido M, Diaz-Lopez C, Padillo FJ, Lopez-Miranda J, Vazquez-Martínez R, Malagón MM. The stromal-vascular fraction of adipose tissue contributes to major differences between subcutaneous and visceral fat depots. Proteomics. 2010;10:3356–3366. doi: 10.1002/pmic.201000350. [DOI] [PubMed] [Google Scholar]
- 84.Cignarelli A, Perrini S, Ficarella R, Peschechera A, Nigro P, Giorgino F. Human adipose tissue stem cells: relevance in the pathophysiology of obesity and metabolic diseases and therapeutic applications. Expert Rev Mol Med. 2012;14:e19. doi: 10.1017/erm.2012.13. [DOI] [PubMed] [Google Scholar]
- 85.Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A, Borgogni E, Frontera S, Nesi G, Liotta F, Lucchese M, Perigli G, Francini F, Forti G, Serio M, Luconi M. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One. 2012;7:e36569. doi: 10.1371/journal.pone.0036569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silva KR, Côrtes I, Liechocki S, Carneiro JR, Souza AA, Borojevic R, Maya-Monteiro CM, Baptista LS. Characterization of stromal vascular fraction and adipose stem cells from subcutaneous, preperitoneal and visceral morbidly obese human adipose tissue depots. PLoS One. 2017;12:e0174115. doi: 10.1371/journal.pone.0174115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59:1075–1088. doi: 10.1007/s00125-016-3933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Freitas Lima LC, Braga VA, do Socorro de França Silva M, Cruz JC, Sousa Santos SH, de Oliveira Monteiro MM, Balarini CM. Adipokines, diabetes and atherosclerosis: an inflammatory association. Front Physiol. 2015;6:304. doi: 10.3389/fphys.2015.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 90.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 91.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 92.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 93.Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN, Bouloumié A. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 94.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 96.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 97.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–T59. doi: 10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 100.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 101.Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, Tamatani T, Yamagata K, Miyagawa J, Kitao Y, Hori O, Yamasaki Y, Ogawa S. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–663. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 102.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 103.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 104.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 105.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 106.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 108.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O'Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, Jobe BA, Roberts CT, Jr, Slifka MK, Marks DL. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, Andoh Y, Fujii S, Iwabuchi K, Onoé K, Tsutsui H. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 113.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne) 2013;4:52. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Finucane OM, Lyons CL, Murphy AM, Reynolds CM, Klinger R, Healy NP, Cooke AA, Coll RC, McAllan L, Nilaweera KN, O'Reilly ME, Tierney AC, Morine MJ, Alcala-Diaz JF, Lopez-Miranda J, O'Connor DP, O'Neill LA, McGillicuddy FC, Roche HM. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes. 2015;64:2116–2128. doi: 10.2337/db14-1098. [DOI] [PubMed] [Google Scholar]
- 116.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsutsui H, Imamura M, Fujimoto J, Nakanishi K. The TLR4/TRIF-Mediated Activation of NLRP3 Inflammasome Underlies Endotoxin-Induced Liver Injury in Mice. Gastroenterol Res Pract. 2010;2010:641865. doi: 10.1155/2010/641865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lombardo E, DelaRosa O, Mancheño-Corvo P, Menta R, Ramírez C, Büscher D. Toll-like receptor-mediated signaling in human adipose-derived stem cells: implications for immunogenicity and immunosuppressive potential. Tissue Eng Part A. 2009;15:1579–1589. doi: 10.1089/ten.tea.2008.0340. [DOI] [PubMed] [Google Scholar]
- 120.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschöp MH, Bruemmer D. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Silva KR, Liechocki S, Carneiro JR, Claudio-da-Silva C, Maya-Monteiro CM, Borojevic R, Baptista LS. Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women. Stem Cell Res Ther. 2015;6:72. doi: 10.1186/s13287-015-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou HR, Kim EK, Kim H, Claycombe KJ. Obesity-associated mouse adipose stem cell secretion of monocyte chemotactic protein-1. Am J Physiol Endocrinol Metab. 2007;293:E1153–E1158. doi: 10.1152/ajpendo.00186.2007. [DOI] [PubMed] [Google Scholar]
- 123.Siklova-Vitkova M, Klimcakova E, Polak J, Kovacova Z, Tencerova M, Rossmeislova L, Bajzova M, Langin D, Stich V. Adipose tissue secretion and expression of adipocyte-produced and stromavascular fraction-produced adipokines vary during multiple phases of weight-reducing dietary intervention in obese women. J Clin Endocrinol Metab. 2012;97:E1176–E1181. doi: 10.1210/jc.2011-2380. [DOI] [PubMed] [Google Scholar]
- 124.Itoh M, Suganami T, Hachiya R, Ogawa Y. Adipose tissue remodeling as homeostatic inflammation. Int J Inflam. 2011;2011:720926. doi: 10.4061/2011/720926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ye J, McGuinness OP. Inflammation during obesity is not all bad: evidence from animal and human studies. Am J Physiol Endocrinol Metab. 2013;304:E466–E477. doi: 10.1152/ajpendo.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ruiz-Ojeda FJ, Rupérez AI, Gomez-Llorente C, Gil A, Aguilera CM. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int J Mol Sci. 2016;17:E1040. doi: 10.3390/ijms17071040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou D, Samovski D, Okunade AL, Stahl PD, Abumrad NA, Su X. CD36 level and trafficking are determinants of lipolysis in adipocytes. FASEB J. 2012;26:4733–4742. doi: 10.1096/fj.12-206862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Poggi M, Jager J, Paulmyer-Lacroix O, Peiretti F, Gremeaux T, Verdier M, Grino M, Stepanian A, Msika S, Burcelin R, de Prost D, Tanti JF, Alessi MC. The inflammatory receptor CD40 is expressed on human adipocytes: contribution to crosstalk between lymphocytes and adipocytes. Diabetologia. 2009;52:1152–1163. doi: 10.1007/s00125-009-1267-1. [DOI] [PubMed] [Google Scholar]
- 129.Tous M, Ferrer-Lorente R, Badimon L. Selective inhibition of sphingosine kinase-1 protects adipose tissue against LPS-induced inflammatory response in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab. 2014;307:E437–E446. doi: 10.1152/ajpendo.00059.2014. [DOI] [PubMed] [Google Scholar]
- 130.Oñate B, Vilahur G, Ferrer-Lorente R, Ybarra J, Díez-Caballero A, Ballesta-López C, Moscatiello F, Herrero J, Badimon L. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 2012;26:4327–4336. doi: 10.1096/fj.12-207217. [DOI] [PubMed] [Google Scholar]
- 131.Van Harmelen V, Röhrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism. 2004;53:632–637. doi: 10.1016/j.metabol.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 132.Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50:151–157. doi: 10.1007/s00125-006-0496-9. [DOI] [PubMed] [Google Scholar]
- 133.Wu CL, Diekman BO, Jain D, Guilak F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: the effects of free fatty acids. Int J Obes (Lond) 2013;37:1079–1087. doi: 10.1038/ijo.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58:1550–1557. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang B, Berger J, Hu E, Szalkowski D, White-Carrington S, Spiegelman BM, Moller DE. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol Endocrinol. 1996;10:1457–1466. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- 136.Bilkovski R, Schulte DM, Oberhauser F, Mauer J, Hampel B, Gutschow C, Krone W, Laudes M. Adipose tissue macrophages inhibit adipogenesis of mesenchymal precursor cells via wnt-5a in humans. Int J Obes (Lond) 2011;35:1450–1454. doi: 10.1038/ijo.2011.6. [DOI] [PubMed] [Google Scholar]
- 137.Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148:868–877. doi: 10.1210/en.2006-0687. [DOI] [PubMed] [Google Scholar]
- 138.Oñate B, Vilahur G, Camino-López S, Díez-Caballero A, Ballesta-López C, Ybarra J, Moscatiello F, Herrero J, Badimon L. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genomics. 2013;14:625. doi: 10.1186/1471-2164-14-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nuermaimaiti N, Liu J, Liang X, Jiao Y, Zhang D, Liu L, Meng X, Guan Y. Effect of lncRNA HOXA11-AS1 on adipocyte differentiation in human adipose-derived stem cells. Biochem Biophys Res Commun. 2018;495:1878–1884. doi: 10.1016/j.bbrc.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 140.Zhu XY, Ma S, Eirin A, Woollard JR, Hickson LJ, Sun D, Lerman A, Lerman LO. Functional Plasticity of Adipose-Derived Stromal Cells During Development of Obesity. Stem Cells Transl Med. 2016;5:893–900. doi: 10.5966/sctm.2015-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pérez LM, Bernal A, San Martín N, Gálvez BG. Obese-derived ASCs show impaired migration and angiogenesis properties. Arch Physiol Biochem. 2013;119:195–201. doi: 10.3109/13813455.2013.784339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pérez LM, Suárez J, Bernal A, de Lucas B, San Martin N, Gálvez BG. Obesity-driven alterations in adipose-derived stem cells are partially restored by weight loss. Obesity (Silver Spring) 2016;24:661–669. doi: 10.1002/oby.21405. [DOI] [PubMed] [Google Scholar]
- 143.Togliatto G, Dentelli P, Gili M, Gallo S, Deregibus C, Biglieri E, Iavello A, Santini E, Rossi C, Solini A, Camussi G, Brizzi MF. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int J Obes (Lond) 2016;40:102–111. doi: 10.1038/ijo.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Tienen FH, van der Kallen CJ, Lindsey PJ, Wanders RJ, van Greevenbroek MM, Smeets HJ. Preadipocytes of type 2 diabetes subjects display an intrinsic gene expression profile of decreased differentiation capacity. Int J Obes (Lond) 2011;35:1154–1164. doi: 10.1038/ijo.2010.275. [DOI] [PubMed] [Google Scholar]
- 145.Ferrer-Lorente R, Bejar MT, Tous M, Vilahur G, Badimon L. Systems biology approach to identify alterations in the stem cell reservoir of subcutaneous adipose tissue in a rat model of diabetes: effects on differentiation potential and function. Diabetologia. 2014;57:246–256. doi: 10.1007/s00125-013-3081-z. [DOI] [PubMed] [Google Scholar]
- 146.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 147.Zvonic S, Lefevre M, Kilroy G, Floyd ZE, DeLany JP, Kheterpal I, Gravois A, Dow R, White A, Wu X, Gimble JM. Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol Cell Proteomics. 2007;6:18–28. doi: 10.1074/mcp.M600217-MCP200. [DOI] [PubMed] [Google Scholar]
- 148.Pérez LM, de Lucas B, Lunyak VV, Gálvez BG. Adipose stem cells from obese patients show specific differences in the metabolic regulators vitamin D and Gas5. Mol Genet Metab Rep. 2017;12:51–56. doi: 10.1016/j.ymgmr.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Serena C, Keiran N, Ceperuelo-Mallafre V, Ejarque M, Fradera R, Roche K, Nuñez-Roa C, Vendrell J, Fernández-Veledo S. Obesity and Type 2 Diabetes Alters the Immune Properties of Human Adipose Derived Stem Cells. Stem Cells. 2016;34:2559–2573. doi: 10.1002/stem.2429. [DOI] [PubMed] [Google Scholar]
- 150.Liu MH, Li Y, Han L, Zhang YY, Wang D, Wang ZH, Zhou HM, Song M, Li YH, Tang MX, Zhang W, Zhong M. Adipose-derived stem cells were impaired in restricting CD4+T cell proliferation and polarization in type 2 diabetic ApoE-/- mouse. Mol Immunol. 2017;87:152–160. doi: 10.1016/j.molimm.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 151.Kim B, Lee B, Kim MK, Gong SP, Park NH, Chung HH, Kim HS, No JH, Park WY, Park AK, Lim JM, Song YS. Gene expression profiles of human subcutaneous and visceral adipose-derived stem cells. Cell Biochem Funct. 2016;34:563–571. doi: 10.1002/cbf.3228. [DOI] [PubMed] [Google Scholar]
- 152.Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 154.Zhu Y, Tchkonia T, Stout MB, Giorgadze N, Wang L, Li PW, Heppelmann CJ, Bouloumié A, Jensen MD, Bergen HR, 3rd, Kirkland JL. Inflammation and the depot-specific secretome of human preadipocytes. Obesity (Silver Spring) 2015;23:989–999. doi: 10.1002/oby.21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Perrini S, Ficarella R, Picardi E, Cignarelli A, Barbaro M, Nigro P, Peschechera A, Palumbo O, Carella M, De Fazio M, Natalicchio A, Laviola L, Pesole G, Giorgino F. Differences in gene expression and cytokine release profiles highlight the heterogeneity of distinct subsets of adipose tissue-derived stem cells in the subcutaneous and visceral adipose tissue in humans. PLoS One. 2013;8:e57892. doi: 10.1371/journal.pone.0057892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fernández M, Acuña MJ, Reyes M, Olivares D, Hirsch S, Bunout D, de la Maza MP. Proliferation and differentiation of human adipocyte precursor cells: differences between the preperitoneal and subcutaneous compartments. J Cell Biochem. 2010;111:659–664. doi: 10.1002/jcb.22753. [DOI] [PubMed] [Google Scholar]
- 157.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 158.Harman-Boehm I, Blüher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Klöting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–2247. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 159.Hauner H, Wabitsch M, Pfeiffer EF. Differentiation of adipocyte precursor cells from obese and nonobese adult women and from different adipose tissue sites. Horm Metab Res Suppl. 1988;19:35–39. [PubMed] [Google Scholar]
- 160.Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee VK, O'Rahilly S. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100:3149–3153. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Niesler CU, Siddle K, Prins JB. Human preadipocytes display a depot-specific susceptibility to apoptosis. Diabetes. 1998;47:1365–1368. doi: 10.2337/diab.47.8.1365. [DOI] [PubMed] [Google Scholar]
- 162.Digby JE, Crowley VE, Sewter CP, Whitehead JP, Prins JB, O'Rahilly S. Depot-related and thiazolidinedione-responsive expression of uncoupling protein 2 (UCP2) in human adipocytes. Int J Obes Relat Metab Disord. 2000;24:585–592. doi: 10.1038/sj.ijo.0801201. [DOI] [PubMed] [Google Scholar]
- 163.Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 164.Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M, Chinnappan D, Cartwright A, Jensen MD, Kirkland JL. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–E277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 165.Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 166.Toyoda M, Matsubara Y, Lin K, Sugimachi K, Furue M. Characterization and comparison of adipose tissue-derived cells from human subcutaneous and omental adipose tissues. Cell Biochem Funct. 2009;27:440–447. doi: 10.1002/cbf.1591. [DOI] [PubMed] [Google Scholar]
- 167.Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meissburger B, Perdikari A, Moest H, Müller S, Geiger M, Wolfrum C. Regulation of adipogenesis by paracrine factors from adipose stromal-vascular fraction - a link to fat depot-specific differences. Biochim Biophys Acta. 2016;1861:1121–1131. doi: 10.1016/j.bbalip.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 169.Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 170.Shahparaki A, Grunder L, Sorisky A. Comparison of human abdominal subcutaneous versus omental preadipocyte differentiation in primary culture. Metabolism. 2002;51:1211–1215. doi: 10.1053/meta.2002.34037. [DOI] [PubMed] [Google Scholar]
- 171.Perrini S, Laviola L, Cignarelli A, Melchiorre M, De Stefano F, Caccioppoli C, Natalicchio A, Orlando MR, Garruti G, De Fazio M, Catalano G, Memeo V, Giorgino R, Giorgino F. Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia. 2008;51:155–164. doi: 10.1007/s00125-007-0841-7. [DOI] [PubMed] [Google Scholar]
- 172.Tokunaga M, Inoue M, Jiang Y, Barnes RH 2nd, Buchner DA, Chun TH. Fat depot-specific gene signature and ECM remodeling of Sca1(high) adipose-derived stem cells. Matrix Biol. 2014;36:28–38. doi: 10.1016/j.matbio.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cleal L, Aldea T, Chau YY. Fifty shades of white: Understanding heterogeneity in white adipose stem cells. Adipocyte. 2017;6:205–216. doi: 10.1080/21623945.2017.1372871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Miyazaki Y, DeFronzo RA. Visceral fat dominant distribution in male type 2 diabetic patients is closely related to hepatic insulin resistance, irrespective of body type. Cardiovasc Diabetol. 2009;8:44. doi: 10.1186/1475-2840-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Livingston JN, Cuatrecasa P, Lockwood DH. Insulin insensitivity of large fat cells. Science. 1972;177:626–628. doi: 10.1126/science.177.4049.626. [DOI] [PubMed] [Google Scholar]
- 180.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 181.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 183.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17:376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Peña R, Church CD, Colman L, Berry R, Rodeheffer MS. The Adipose Tissue Microenvironment Regulates Depot-Specific Adipogenesis in Obesity. Cell Metab. 2016;24:142–150. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tchoukalova YD, Koutsari C, Votruba SB, Tchkonia T, Giorgadze N, Thomou T, Kirkland JL, Jensen MD. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring) 2010;18:1875–1880. doi: 10.1038/oby.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chau YY, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl A, Hastie N. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Krueger KC, Costa MJ, Du H, Feldman BJ. Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Reports. 2014;3:1147–1158. doi: 10.1016/j.stemcr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]