Abstract
Purpose:
Recent trends including the use of proton therapy and administration of reduced-doses of cyclophosphamide have been adapted in head and neck rhabdomyosarcoma (HN-RMS) to reduce late morbidity. Our primary goal was to analyze local control and survival outcomes after photon versus proton irradiation in pediatric patients with HN-RMS, with the secondary goal of analyzing the effect of cyclophosphamide dose on disease outcomes.
Methods and Materials:
This single-institution cohort study was comprised of 76 pediatric HN-RMS patients treated with definitive chemoradiation from 2000 to 2018. Fifty-one patients (67%) received intensity-modulated photon radiation therapy (IMRT) and 25 (33%) proton therapy.
Results:
Local failure (LF) at 2 years was 12.5% for parameningeal RMS and 0% for orbital RMS and other head and neck sites (p=0.24). Patients treated with protons were more likely to have received reduced-dose cyclophosphamide (p<0.0001). The 2-year LF was 7.9% in the IMRT cohort versus 14.6% in the proton cohort (p=0.07), with no difference in survival outcomes. Cumulative cyclophosphamide dose was significantly associated with 2-year LF: 0% for cumulative dose of >20g/m2 versus 15.3% for ≤20g/m2 (p=0.04). Among parameningeal RMS patients (n=59), both cumulative cyclophosphamide dose and dose-intensity were associated with local failure (p=0.01). There was a trend toward worse event-free survival for parameningeal RMS patients who received reduced-dose-intensity cyclophosphamide (59.2% versus 70.6%, p=0.11).
Conclusions:
Both dose-intensity and cumulative cyclophosphamide dose seem to play an important role in achieving local control for HN-RMS patients treated with either protons or photons. Longer follow-up is needed to further assess disease outcomes with proton therapy.
SUMMARY
Efforts to reduce late morbidity in head and neck rhabdomyosarcoma include proton therapy and reduced-doses of cyclophosphamide. We show that both the dose-intensity and cumulative cyclophosphamide dose seem to play a significant role in achieving local control for patients with head and neck rhabdomyosarcoma treated with definitive irradiation using either photons or protons.
INTRODUCTION
Head and neck rhabdomyosarcoma (HN-RMS) accounts for approximately 35% of all rhabdomyosarcomas and is a uniquely difficult disease to treat given the young patient age and critical anatomy of the head and neck. HN-RMS is often subdivided into three groups based on anatomical and prognostic considerations, including parameningeal, (15% of all RMS), orbital (10%), and other head and neck sites (10%).1 Parameningeal tumors are defined as those arising from the nasopharynx, nasal cavity, paranasal sinuses, middle ear, mastoid, infratemporal fossa, and pterygopalatine space, and are typically associated with a more guarded prognosis compared to orbital and other head and neck tumors.2 Most patients with HN-RMS present with unresectable disease secondary to anatomical and functional considerations. As such, these patients are treated with definitive chemoradiation, with the goal of maintaining form and function.3 Local relapse is the dominant form of failure for HN-RMS and results in a high mortality rate as well as devastating physical and functional consequences in the children that survive.
In 1999, our institution began using intensity-modulated radiation therapy (IMRT) to treat all patients with HN-RMS, with the aim of 1) improving local control via improved target coverage and 2) reducing late morbidity via a reduction in dose to surrounding normal tissue. 4 Proton therapy holds further promise in sparing normal tissue and subsequently decreasing late morbidity. Data from a phase II study at Massachusetts General Hospital and MD Anderson (including 44 patients with HN-RMS) showed that disease outcomes after proton RT were similar to those observed after photon RT on historical trials.5 For patients with embryonal parameningeal RMS, local control at 3 years was 59%.6 However, long-term disease outcomes for proton therapy for RMS are limited, and there has never been a direct comparison of proton versus photon therapy for RMS establishing their equivalence.
In a further attempt to spare late morbidity in children with RMS, there has been a reduction in cyclophosphamide dose-intensity and cumulative dose over time from the Intergroup Rhabdomyosarcoma Study IV (IRS-IV) in the 1990’s to the current Children’s Oncology Group (COG) studies. Specifically, the cyclophosphamide dose was reduced for low-risk patients on IRS V (“D” COG series), and then for intermediate and high-risk patients in the ARST series.7-11 Specifically, earlier generation studies administered 12-14 cyclophosphamide doses, each at 2.2 g/m2, whereas later generation studies reduced the dosage to 1.2 g/m2 and the total number of doses to 7-14. It is unknown whether this dose de-escalation has significantly affected local control over time, but it is plausible that systemic therapy may influence local control for RMS as has been shown in Ewing sarcoma,12 head and neck cancer,13 lung cancer,14 rectal cancer,15 and cervical cancer,16 among others.
It is imperative to ensure that efforts focused on reducing late morbidity such as proton therapy and cyclophosphamide dose reduction do not compromise disease outcomes. As such, our primary goal was to evaluate local control and survival outcomes after photon (IMRT) versus proton irradiation in pediatric patients with HN-RMS, with the secondary goal of investigating the effect of cyclophosphamide dose on disease outcomes in a well-characterized, single institution cohort.
METHODS AND MATERIALS
This is a cohort study of all pediatric patients with HN-RMS treated with chemoradiation at Memorial Sloan Kettering Cancer Center (MSK) from January 2000 to June 2018. Of the 90 patients with HN-RMS treated during this timeframe, we included 76 patients ≤25 years of age in our analysis. Tumor location was parameningeal in 59 patients (78%), orbital in 9 (12%), and other head and neck sites in 8 (10%). A waiver of authorization was received from the MSK Institutional Review Board to perform the analyses in this study.
Treatment
Radiation therapy
All patients received radiation therapy (RT) as part of local control. Seventy-one patients (93%) received definitive radiation at a median time of 13 weeks from initiation of chemotherapy. Five patients (7%) underwent surgical resection followed by adjuvant RT for local control. In total, 51 patients (67%) received IMRT and 25 (33%) received proton therapy. The decision for IMRT versus proton therapy was largely one of timing; all patients treated from 1/2000 to 6/2013 received IMRT, while all but 4 patients treated from 7/2013-6/2018 received proton therapy. These 4 patients did not receive proton therapy due to logistical and/or health barriers preventing travel to the proton center. The radiation doses were similar between the two groups and were given as per standard COG guidelines, with 36 Gy used for postoperative margin-negative patients; 41.4 Gy for postoperative margin-positive patients; and 50.4 Gy for definitive treatment of gross disease (with the exception of orbital primaries in whom 2 patients received 45 Gy as per ARST0331). A relative biologic effectiveness (RBE) of 1.1 was used to calculate the proton dose in GyRBE units.17
Chemotherapy
Thirty-seven patients (48%) received chemotherapy on or according to COG protocols including D9803 (n=5), D9602 (n=3), ARST0331 (n=10), ARST0531 (n=12), ARST0431 (n=3), ARST08P1 (n=2), and ARST1431 (n=1). Twenty-four patients (32%) were treated on an MSK phase II pilot protocol IRB 03-099 consisting of irinotecan, carboplatin, cyclophosphamide, doxorubicin, vincristine, ifosfamide and etoposide.18 Eight patients (11%) were treated as per a National Cancer Institute pilot study of dose-dense, sequentially administered vincristine, doxorubicin, and cyclophosphamide with etoposide and ifosfamide. Five patients (7%) were treated as per European protocols. The remaining patients (n=3) received a combination of standard chemotherapy agents off protocol. The dose-intensity of cyclophosphamide and the cumulative dose of all alkylating agents (expressed in cyclophosphamide equivalents, subsequently referred to as cumulative cyclophosphamide dose) were collected for each patient. For the purpose of analysis, reduced dose-intensity of cyclophosphamide was defined as <2g/m2 per cycle (typically given as 1.2g/m2), while standard dose-intensity was considered anything above 2 g/m2 per cycle.
Statistical analysis
Local failure (LF) was defined as relapse at the primary tumor site occurring as a first event (with or without simultaneous distant failure). Radiation treatment plans and imaging at the time of relapse were reviewed to determine the location of the failure in relationship to the radiation treatment fields. LF was correlated with modality of radiation (IMRT versus protons), tumor size, tumor site, histology, nodal status, timing of RT, and cyclophosphamide dosing (both dose-intensity and cumulative dose). Among the subset of patients with parameningeal disease and embryonal histology with available imaging, a volumetric response analysis was performed by comparing tumor volume on MRI prior to induction chemotherapy with tumor volume on MRI prior to RT. Ellipsoid tumor volumes were calculated using the 3-dimensional measurements collected and relative percent volume reduction was subsequently calculated as previously defined.6,19 Event-free survival (EFS) was calculated as the time from diagnosis to disease progression, including local and/or distant relapse. Overall survival (OS) was calculated as the time from diagnosis to death from any cause. Patients without an event were censored at the time of last follow-up. A competing-risks analysis was used to assess the cumulative incidence of LF, while the Kaplan-Meier method was used to assess EFS and OS. Cumulative incidence curves among subgroups were compared utilizing Gray’s method, while survival curves were compared with the Mantel log-rank test. P ≤0.05 was considered significant. Multivariate analysis was not able to be performed given the small number of events.
RESULTS
The median follow-up of surviving patients was 10 years (range, 1-17) in the IMRT cohort versus 2 years (range, 0.5-5) in the proton cohort. Patients treated with protons were similar in age to those treated with IMRT, with a median age of 7 years (range, 3-22) in the proton cohort versus 8 years (range, 2-25) in the IMRT cohort. There were no significant differences in gender, race, tumor site, tumor size, stage and group between the two cohorts (Table 1). Patients treated with protons were more likely to have embryonal histology (96% versus 71%, p=0.01). Patients treated with protons were also more likely to be treated with reduced-dose intensity cyclophosphamide (88% vs 32%, p<0.0001). The median dose-intensity and cumulative dose of cyclophosphamide for patients treated with proton therapy were 1.2g/m2/cycle and 13.1g/m2, respectively, versus 3.2g/m2/cycle and 17.5g/m2 in the IMRT cohort.
Table 1.
Baseline patient and treatment characteristics
| IMRT cohort N=51 |
Proton cohort N=25 |
P Value | |
|---|---|---|---|
| Age at Radiation (Mean) | 10.0 | 7.8 | 0.15 |
| Gender | |||
| Male | 19 (37) | 13 (52) | 0.22 |
| Female | 32 (63) | 12 (48) | |
| Race | |||
| White | 41 (80) | 20 (80) | 0.95 |
| Black | 6 (12) | 3 (12) | |
| Asian | 3 (6) | 1 (4) | |
| Other | 1 (2) | 1 (4) | |
| Histology | |||
| Embryonal | 36 (71) | 24 (96) | 0.01 |
| Alveolar | 15 (29) | 1 (4) | |
| Tumor location | |||
| Parameningeal | 42 (82) | 17 (68) | 0.16 |
| Orbital | 6 (12) | 3 (12) | |
| Other head and neck | 3 (6) | 5 (20) | |
| Tumor size | |||
| <5cm | 27 (53) | 14 (56) | 0.80 |
| ≥5cm | 24 (47) | 11 (44) | |
| Nodal status | |||
| N0 | 25 (49) | 18 (72) | 0.06 |
| N1 | 26 (51) | 7 (28) | |
| Stage | |||
| 1 | 9 (18) | 8 (32) | 0.48 |
| 2 | 9 (18) | 5 (20) | |
| 3 | 23 (45) | 9 (36) | |
| 4 | 10 (20) | 3 (12) | |
| Group | |||
| 2 | 3 (6) | 2 (8) | 0.61 |
| 3 | 38 (75) | 20 (80) | |
| 4 | 10 (20) | 3 (12) | |
| Cyclophosphamide dose per cycle | |||
| Reduced | 16 (32) | 21 (88) | <0.0001 |
| Standard | 34 (68) | 3 (12) | |
| Cumulative alkylating agent dose | |||
| ≤20g/m2 | 28 (57) | 16 (70) | 0.31 |
| >20g/m2 | 21 (43) | 7 (30) |
Abbreviations: IMRT = intensity-modulated radiation therapy
Local Failure
Among the entire cohort, 10 patients experienced a local failure at a median time of 1.0 years from RT, for a 2-year LF of 9.9%. There were no local failures among patients with orbital and other and head neck site tumors. The 2-year LF for patients with parameningeal RMS was 12.5%. LF did not differ by tumor size (<5cm versus ≥5cm, p=0.69), nodal status (p=0.68), or timing from chemotherapy to start of RT (≤4 weeks versus >4 weeks, p=0.80). There was a trend toward increased local failure in patients with embryonal RMS, although this was not statistically significant (2-year LF 10.6% versus 6.8, p=0.33).
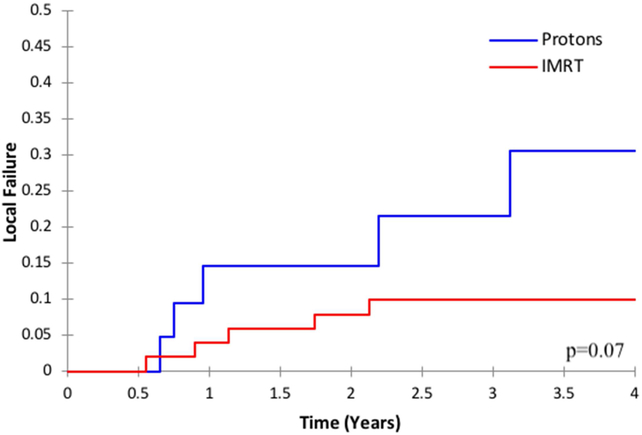
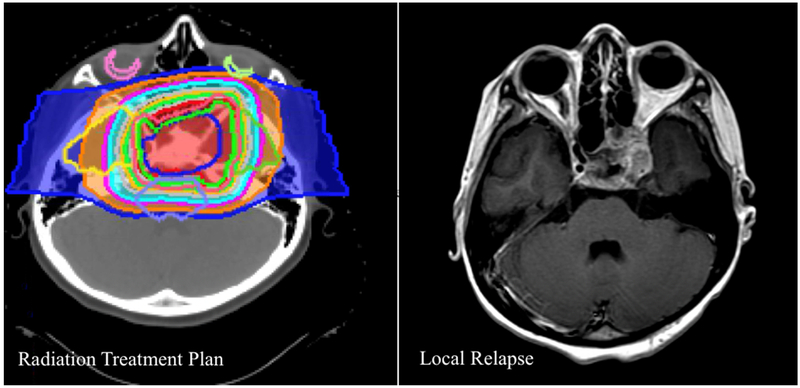
Patients treated with IMRT had a 2-year LF rate of 7.9% compared to 14.6% in patients treated with protons (p=0.07, Figure 1). All proton and IMRT local relapses were within the radiation field in the high-dose region; there were no marginal failures. See Figure 2 for an example of an in-field local failure after proton therapy.
Figure 1.
Local failure by radiation modality (protons versus intensity-modulated radiation, therapy, IMRT)
Figure 2.
Example of an in-field local failure in the high-dose region after proton therapy for a suprasellar rhabdomyosarcoma
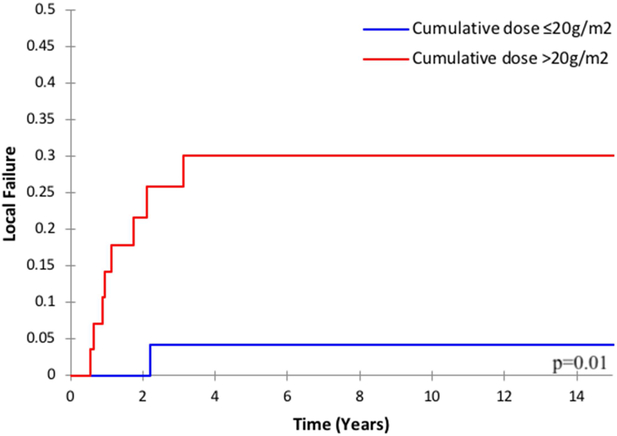
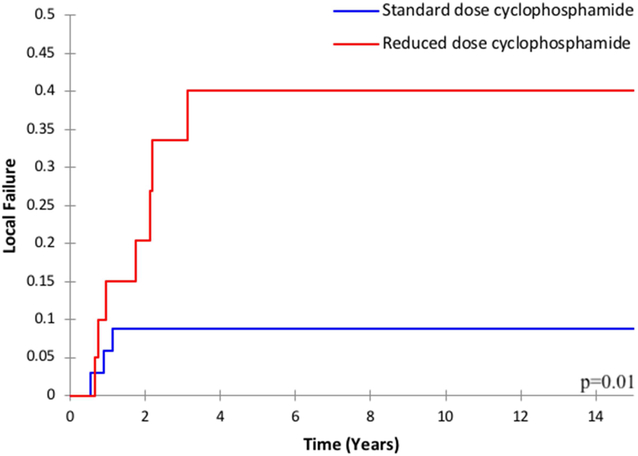
Among the entire cohort, the cumulative dose of cyclophosphamide was associated with LF: the 2-year LF was 15.3% for patients who received a cumulative dose of ≤20g/m2 versus 0% for >20g/m2 (p=0.04). There was also a trend toward increased local failure among patients who received reduced dose-intensity of cyclophosphamide (2-year LF 12.7% versus 8.1%, p=0.08). Among the subgroup of patients with parameningeal RMS (n=59), both the cumulative dose and dose-intensity were associated with local failure. The 2-year LF was 21.6% for cumulative dose ≤20g/m2 versus 0% for >20g/m2 (p=0.01, Figure 3a); and the 2-year LF was 20.4% for patients who received reduced dose-intensity cyclophosphamide versus 8.8% for standard dose (p=0.01, Figure 3b).
Figure 3.
Local failure among patients with parameningeal rhabdomyosarcoma by a) cumulative cyclophosphamide dose and b) cyclophosphamide dose-intensity
To further evaluate the effect of cyclophosphamide dose independent of the use of radiation modality / protons, we limited our analysis to include only those treated with IMRT. Among patients treated with IMRT, cumulative cyclophosphamide dose was again significantly associated with local failure (2-year LF 14.5% for ≤20g/m2 versus 0% for >20g/m2, p=0.04), although dose-intensity was not (p=0.64).
Volumetric Response Analysis
Among the subgroup of patients with parameningeal RMS and embryonal histology who received RT at week 12+/−4 and had available imaging (n=26), median tumor size pre-chemotherapy was 35.4 cm3 (range, 1.1-159.1 cm3), with a median relative percent volume reduction after induction chemotherapy of 74.9% (range, 9.4-100%). The initial tumor volume was larger for patients who developed a local failure than patients with local control, although this was not statistically significant (median 50.6 cm3 versus 23.2 cm3, p=0.32). In addition, patients who developed a local failure had a lower relative percent volume reduction after induction chemotherapy compared to patients who experienced local control (median 28.9% versus 82.2%, p=0.01). Patients who received reduced dose-intensity cyclophosphamide also had a lower relative percent volume reduction after induction chemotherapy compared to patients who received standard dose (median 54.4% versus 83.6%, p=0.05). However, cumulative cyclophosphamide dose was not associated with relative percent volume reduction after induction chemotherapy (relative reduction 80.1% for cumulative dose >20g/m2 versus 72.2% for ≤20g/m2, p=0.85).
Event-free and Overall Survival
Fourteen of 76 patients developed a distant failure as a first event for a 2-year cumulative incidence of distant failure of 18.7%. Among all patients, the 2-year EFS and OS were 74.6% and 84.1%, respectively. There were no differences in EFS or OS between the IMRT and proton cohorts (p=0.25 and p=0.58). There was also no difference in EFS or OS by cumulative cyclophosphamide dose (p=0.62 and p=0.65) or dose-intensity (p=0.64 and p=0.25). For patients with parameningeal RMS, there was a trend toward worse EFS in patients who received reduced dose-intensity cyclophosphamide (2-year EFS 59.2% versus 70.6%, p=0.11) and reduced cumulative dose (2-year EFS 60.2% versus 80.0%, p=0.21).
Acute Toxicity
There was no difference in combined grade ≥3 acute toxicity between the proton and IMRT cohorts. Patients treated with protons were more likely to experience grade 3 dermatitis (20% versus 2%, p=0.006) and less likely to develop grade 3 mucositis (12% versus 20%, p=0.41).
DISCUSSION
Our primary objective was to analyze local control and other disease outcomes for HN-RMS after proton versus photon (IMRT) therapy. Although there was a trend toward worse local control in the proton arm on univariate analysis, this was non-significant. In addition, there were no differences in EFS or OS between the IMRT and proton groups. All proton local failures were in-field in the high dose region. As such, marginal misses with protons did not account for any of the local failures, similar to findings from MGH.6 Importantly, all 5 patients in our proton cohort who experienced a local relapse were treated with reduced-dose cyclophosphamide (1.2g/m2 per cycle) according to recent COG ARST protocols. Patients treated with protons were more likely to have received reduced-dose cyclophosphamide for two reasons. First, there has been a deescalation in cyclophosphamide dose over time on national COG protocols as discussed above, and the proton cohort was a more contemporary cohort of patients (treated since 2013) compared to the IMRT cohort (treated since 2000). Second, patients who received IMRT were treated at MSK’s main campus and were therefore more likely to be treated with a higher dose of alkylating agents on an institutional MSK protocol drug regimen; on the institutional MSK protocol IRB 03-099, patients receive 3.2g/m2 of cyclophosphamide per cycle in addition to 13g/m2 of ifosfamide per cycle.18
As patients in our cohort received a variety of cyclophosphamide dose regimens, a secondary objective was then to analyze the specific impact of cyclophosphamide dose on disease outcomes. Among the entire cohort, local failure was significantly worse in patients who received lower cumulative doses of cyclophosphamide. As patients with orbital and other head and neck site embryonal RMS were treated on low-risk protocols and did not experience any local failures in our cohort, we also analyzed the effect of cyclophosphamide dose specifically in patients with parameningeal RMS (comprising 78% of our cohort). For parameningeal RMS, a reduction in both dose-intensity and cumulative dose of cyclophosphamide significantly increased the rate of local failure. There was also a non-significant trend toward worse EFS in patients treated with a reduced dose-intensity of cyclophosphamide (2-year EFS 59.2% versus 70.6%, p=0.11). As we were unable to perform a multivariate analysis, we further analyzed the effect of cyclophosphamide dose in the subgroup of patients who received IMRT in order to eliminate radiation modality as a confounding factor. Among this group, cumulative cyclophosphamide dose again remained significantly associated with LF.
To compare our outcomes to the outcomes of patients with parameningeal RMS treated on the most recent IRSG/COG studies, the local failure was 16% on IRS-IV, 19% on D9803, and 27.6% on ARST0531 (Table 2).20-22 Importantly, the cyclophosphamide dose-intensity and cumulative dose on ARST0531 was significantly lower than that used on IRS-IV and D9803. The inferior local control on ARST0531 compared to historical cohorts is similar to the increase in local failure from 8.8% to 33.5% seen among patients in our cohort treated with reduced dose-intensity cyclophosphamide. Similarly, for patients with low-risk embryonal RMS (including orbital RMS) a reduction in cyclophosphamide dose from D9602 to ARST0331 was associated with worse local control.8,23 Additionally, for patients with intermediate-risk embryonal RMS, there was an improvement in failure-free survival on IRS IV compared to IRS III, largely attributed to the increased dose-intensity of the alkylating agent on IRS-IV compared to IRS-III (2.2g/m2 versus 1 g./m2).24 A single institution series from St. Jude Children’s Hospital also recently noted an increase in locoregional failures in parameningeal RMS after reduced-dose cyclophosphamide and delayed RT, although we did not find that local control was affected by the timing of RT.25
Table 2.
Comparison of parameningeal local failure on recent COG/IRSG studies
| Study |
Cyclophosphamide dose per cycle (g/m2) |
Cumulative alkylating agent dose* |
Local failure for PM-RMS |
|---|---|---|---|
| IRS-IV14 | 2.2** | 26.4 | 16% |
| IRS-V (D9803)4 | 2.2 | 25.1-30.8 | 19% |
| ARST0531116 | 1.2 | 8.4-16.8 | 28% |
| MSK (current study) | |||
| Cohort A | |||
| Cohort B | 1.2 | 10 (median) | 33.5% |
| 2.2 | 24 (median) | 8.8% |
In cyclophosphamide equivalents
Or 9g/m2 of ifosfamide
It is unknown whether dose-intensity of cyclophosphamide or cumulative dose is the more significant factor affecting disease control. Similarly, the optimal dose-intensity of cyclophosphamide remains unknown. As the minority of the cyclophosphamide dose is given prior to the start of radiation in most studies, the dose-intensity of therapy prior to and during radiation is likely an important factor for local control. In fact, we found that volumetric response after induction chemotherapy correlated with both local control as well as dose-intensity of cyclophosphamide. The main concern with increased doses of cyclophosphamide include, in the short-term, cytopenias, and in the long-term, infertility, which is most evident in males treated at a young age.26 Dose intensification of cyclophosphamide beyond 2.2g/m2 to 3.6g/m2 as part of induction therapy for intermediate-risk RMS did not improve outcomes on a COG pilot.27 Of note, preclinical studies have demonstrated that cyclophosphamide has a sharp dose response curve. Thus, it is plausible that small changes in dose may significantly impact cell killing and disease control.28,29
The major limitations of our study are the retrospective design and the small number of events, which precludes the use of a multivariate analysis that could address the relative importance of the different factors associated with higher rates of recurrence. Patients were not randomized to receive protons versus IMRT or standard versus reduced-dose chemotherapy. Thus, there may be unexplained confounding factors affecting our results. However, the decision for the type of radiation used and the dose of chemotherapy given for a specific risk-group was based on timing and location of treatment, rather than clinical baseline characteristics that may influence disease control.
CONCLUSION
In summary, HN-RMS is a challenging site to treat given the critical anatomy of the head and neck region and the concern for late effects. Local failures in HN-RMS not only cause severe facial disfigurement and devastating functional consequences, but they also can be lifethreatening. Proton therapy and reduced-dose cyclophosphamide may both reduce late morbidity in this vulnerable patient population. Longer follow-up is needed in the proton cohort, although it appears that the dose of cyclophosphamide, rather than radiation modality, is likely the more important factor affecting local disease control. Efforts focused on further evaluating the optimal dose of cyclophosphamide or alkylating agents needed to balance disease control with toxicity are needed.
Acknowledgments
Funding: NIH grant P30 CA 008748
Footnotes
Conflict of interest: Dr. Wolden reports personal fees from YmAbs therapeutics, outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ries LA, Smith MA, Gurney JG, et al. : Cancer incidence and survival among children and adolescents: United States SEER program. Bethesda,MD: National Cancer Institute, SEER program, National Institutes of Health; 1999; Publication no. 99-4649. [Google Scholar]
- 2.Raney RB Jr., Tefft M, Newton WA, et al. : Improved prognosis with intensive treatment of children with cranial soft tissue sarcomas arising in nonorbital parameningeal sites. A report from the Intergroup Rhabdomyosarcoma Study. Cancer 59:147–55, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Casey DL, Wolden SL Rhabdomyosarcoma of the Head and Neck: A Multimodal Approach J Neurol Surg B Skull Base, 79 (2018), pp. 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolden SL, Wexler LH, Kraus DH, et al. Intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma Int J Radiat Oncol Biol Phys, 61 (2005), pp. 1432–1438 [DOI] [PubMed] [Google Scholar]
- 5.Ladra MM, Szymonifka JD, Mahajan A, et al. : Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol 32:3762–70, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladra MM, Mandeville HC, Niemierko A, et al. : Local failure in parameningeal rhabdomyosarcoma correlates with poor response to induction chemotherapy. Int J Radiat Oncol Biol Phys 92:358–67, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crist WM, Anderson JR, Meza JL, et al. : Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol 19:3091–102, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Walterhouse DO, Pappo AS, Meza JL, et al. : Reduction of cyclophosphamide dose for patients with subset 2 low-risk rhabdomyosarcoma is associated with an increased risk of recurrence: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Cancer 123:2368–2375, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raney RB, Walterhouse DO, Meza JL, et al. : Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol 29:1312–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arndt CA, Stoner JA, Hawkins DS, et al. : Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children's oncology group study D9803. J Clin Oncol 27:5182–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins DS, Chi YY, Anderson JR, et al. : Addition of Vincristine and Irinotecan to Vincristine, Dactinomycin, and Cyclophosphamide Does Not Improve Outcome for Intermediate-Risk Rhabdomyosarcoma: A Report From the Children's Oncology Group. J Clin Oncol 36:2770–2777, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grier HE, Krailo MD, Tarbell NJ, et al. : Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 348:694–701, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Pignon JP, le Maitre A, Maillard E, et al. : Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4–14, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Schaake-Koning C, van den Bogaert W, Dalesio O, et al. : Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med 326:524–30, 1992 [DOI] [PubMed] [Google Scholar]
- 15.De Caluwe L, Van Nieuwenhove Y, Ceelen WP: Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev:CD006041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eifel PJ, Winter K, Morris M, et al. : Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol 22:872–80, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Paganetti H, Niemierko A, Ancukiewicz M, et al. : Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 53:407–21, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Dharmarajan KV, Wexler LH, Wolden SL Concurrent radiation with irinotecan and carboplatin in intermediate- and high-risk rhabdomyosarcoma: A report on toxicity and efficacy from a prospective pilot phase II study Pediatr Blood Cancer, 60 (2013), pp. 242–247 [DOI] [PubMed] [Google Scholar]
- 19.Ferrari A, Miceli R, Meazza C, et al. : Comparison of the prognostic value of assessing tumor diameter versus tumor volume at diagnosis or in response to initial chemotherapy in rhabdomyosarcoma. J Clin Oncol 28:1322–8, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Donaldson SS, Meza J, Breneman JC, et al. : Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma--a report from the IRSG. Int J Radiat Oncol Biol Phys 51:718–28, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Wolden SL, Lyden ER, Arndt CA, et al. Local control for intermediate-risk rhabdomyosarcoma: Results from D9803 according to histology, group, site, and size: A report from the Children’s Oncology Group Int J Radiat Oncol Biol Phys, 93 (2015), pp. 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casey DL, Chi YY, Donaldson SS, et al. Increased Local Failure for Patients with Intermediate-Risk Rhabdomyosarcoma on ARST0531: A Report from the Children’s Oncology Group (2019) (under submission) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ermoian RP, Breneman J, Walterhouse DO, et al. : 45 Gy is not sufficient radiotherapy dose for Group III orbital embryonal rhabdomyosarcoma after less than complete response to 12 weeks of ARST0331 chemotherapy: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Pediatr Blood Cancer 64, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker KS, Anderson JR, Link MP, et al. : Benefit of intensified therapy for patients with local or regional embryonal rhabdomyosarcoma: results from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol 18:2427–34, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Lucas JT Jr., Pappo AS, Wu J, et al. : Excessive Treatment Failures in Patients With Parameningeal Rhabdomyosarcoma With Reduced-dose Cyclophosphamide and Delayed Radiotherapy. J Pediatr Hematol Oncol 40:387–390, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopp LM, Gupta P, Pelayo-Katsanis L, et al. : Late effects in adult survivors of pediatric cancer: a guide for the primary care physician. Am J Med 125:636–41, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Spunt SL, Smith LM, Ruymann FB, et al. : Cyclophosphamide dose intensification during induction therapy for intermediate-risk pediatric rhabdomyosarcoma is feasible but does not improve outcome: a report from the soft tissue sarcoma committee of the children's oncology group. Clin Cancer Res 10:6072–9, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Porrata LF, Adjei AA: The pharmacologic basis of high dose chemotherapy with haematopoietic stem cell support for solid tumours. Br J Cancer 85:484–9, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griswold DP Jr., Trader MW, Frei E 3rd, et al. : Response of drug-sensitive and - resistant L1210 leukemias to high-dose chemotherapy. Cancer Res 47:2323–7, 1987 [PubMed] [Google Scholar]