Abstract
Background and study aim
Standard endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) procedures involve use of no-suction or suction aspiration techniques. A new aspiration method, the stylet slow-pull technique, involves slow withdrawal of the needle stylet to create minimum negative pressure. The aim of this study was to compare the sensitivity of EUS-FNA using stylet slow-pull or suction techniques for malignant solid pancreatic lesions using a standard 22-gauge needle.
Patients and methods
Consecutive patients presenting for EUS-FNA of pancreatic mass lesions were randomized to the stylet slow-pull or suction techniques using a 22- gauge needle. Both techniques were standardized for each pass until an adequate specimen was obtained, as determined by rapid on-site cytology examination. Patients were crossed over to the alternative technique after four nondiagnostic passes.
Results
Of 147 patients screened, 121 (mean age 64±13.8 years) met inclusion criteria and were randomized to the stylet slow-pull technique (n = 61) or the suction technique (n = 60). Technical success rates were 96.7% and 98.3% in the slow-pull and suction groups, respectively (P>0.99). The sensitivity for malignancy of EUS-FNA was 82% in the slow-pull group and 69% in the suction group (P =0.10). The first-pass diagnostic rate (42.6% vs. 38.3%; P =0.71), acquisition of core tissue (60.6% vs. 46.7%; P =0.14), and the median (range) number of passes to diagnosis (2 [1 – 3] vs. 1 [1–2]; P =0.71) were similar in the slow-pull and suction groups, respectively.
Conclusions
The stylet slow-pull and suction techniques both offered high and comparable diagnostic sensitivity with a mean of 2 passes required for diagnosis of solid pancreatic lesions. The endosonographer may choose either technique during FNA.
Introduction
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was first introduced in the early 1990s [1], Since then, this technique has widely disseminated and has become the current standard of care for establishing tissue diagnosis in patients with a suspected neoplasm that is accessible via EUS-FNA [2].
The current standard of practice for EUS-FNA of solid pancreatic lesions utilizes either a 22- or 25-gauge FNA needle, with reported diagnostic sensitivities ranging from 70% to 94% on the basis of cytopathologic diagnosis [3–5], Several clinical trials have evaluated various methodologic variations that may improve diagnostic yield, including different needle sizes [6], the use of a stylet [5], rapid on-site evaluation [7,8], and the application of suction [9], However, the ideal technique for EUS-FNA has not yet been established, Furthermore, the lack of a core specimen for histologic diagnosis creates challenges when attempting to obtain a diagnosis in diseases such as lymphoma, neuroendocrine tumors, or autoimmune pancreatitis, whereby immunohistochemical staining or intact histologic architecture is important.
Emerging data suggest that needle aspiration techniques have a direct effect on the yield of EUS-FNA, Conventionally, when performing EUS-FNA, negative pressure is applied using suction with a 10- or 20-mL syringe, However, the use of suction may increase blood contamination [10], To avoid this, other techniques have been used, including absence of suction or a new method called the “stylet slow-pull” technique, in which the stylet is slowly and continuously withdrawn as the needle moves to-and-fro within the target lesion, creating minimal negative pressure.
A prospective, single-arm study showed that the slow-pull technique, in conjunction with a ProCore needle (EchoTip ProCore; Cook Medical, Bloomington, Indiana, USA), had a high diagnostic yield and histologic core tissue acquisition for diagnosis of various abdominal masses without the presence of an onsite cytopathologist [11], A recent retrospective study showed that the slow-pull technique, in conjunction with a standard 25-gauge FNA needle, was associated with improved diagnostic yield in pancreatic masses [12].
There are no published prospective, randomized trials comparing the stylet slow-pull and standard suction techniques for EUS-FNA using standard needles, The primary aim of this study was to compare the sensitivities of the stylet slow-pull technique and standard suction technique for EUS-FNA of malignant solid pancreatic mass lesions using a standard FNA needle, The secondary aims were the first pass diagnostic rate, yield of core tissue, diagnostic accuracy, and adverse event rate.
Patients and methods
All consecutive patients between the ages of 18 and 90 years presenting for EUS-FNA of solid pancreatic mass lesions detected on abdominal imaging (computed tomography scan, magnetic resonance imaging or ultrasound) at two tertiary academic centers (Johns Hopkins University, Maryland, and Cleveland Clinic, Ohio, USA) from September 2013 to December.2014 were screened for inclusion, The study was approved by the Institutional Review Boards for Human Research at both institutions, and complied with Health Insurance Portability and Accountability ACT regulations, The trial was registered at Clin-icalTrials,gov (NCT01936467), All patients provided informed consent for participation in the study.
Patients were excluded at the time of EUS if the lesion was determined to be a cyst, as FNA would only yield cystic fluid, Other exclusion criteria included uncorrectable coagulopathy (international normalized ratio >1,5) or thrombocytopenia (platelet <50000), pregnant women (women of childbearing age underwent urine pregnancy testing), or refusal to participate in the study.
All procedures were performed using linear array echoendo-scopes (Olympus UCT140 - Olympus America, Center Valley, Pennsylvania, USA; or Pentax EG-387OUTK-Hoya, Tokyo, Japan), Patients were placed in the left lateral decubitus position under conscious or deep sedation with monitored anesthesia care, Patients who met the inclusion criteria were randomized to the stylet slow-pull technique or standard suction technique using a 22-gauge needle (Expect; Boston Scientific, Marlborough, Massachusetts, USA).
Procedure technique
After completion of the endosonographic examination, the pancreatic mass was evaluated with color Doppler to avoid intervening vessels. The needle was sharpened by withdrawing the stylet approximately 2 mm, and then advanced into the lesion. In patients randomized to the stylet slow-pull technique, 15 to-and-fro movements within the lesion were performed, with simultaneous minimal negative pressure provided by pulling the needle stylet slowly and continuously to half the length of the stylet ( Fig. 1). In patients randomized to the suction technique, 15 to-and-fro movements within the lesion were performed with the use of a 10-mL suction syringe (
Fig. 1). In patients randomized to the suction technique, 15 to-and-fro movements within the lesion were performed with the use of a 10-mL suction syringe ( Fig. 2). Both stylet slow-pull and suction FNA techniques were performed within 25 – 30 seconds. The fanning technique was performed at the discretion of the endoscopist.
Fig. 2). Both stylet slow-pull and suction FNA techniques were performed within 25 – 30 seconds. The fanning technique was performed at the discretion of the endoscopist.
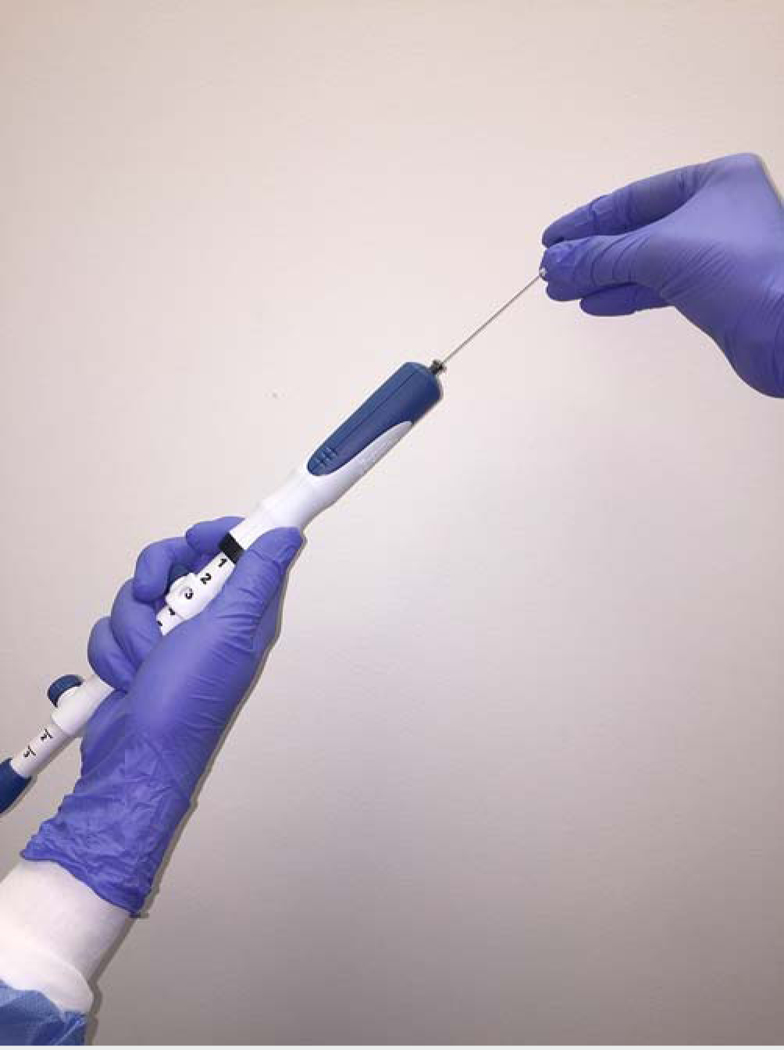
 Fig. 1
Fig. 1
Stylet slow-pull technique: the endoscopist performs to- and-fro movements within the lesion while the assistant pulls the needle stylet slowly and continuously to half the length of the stylet.
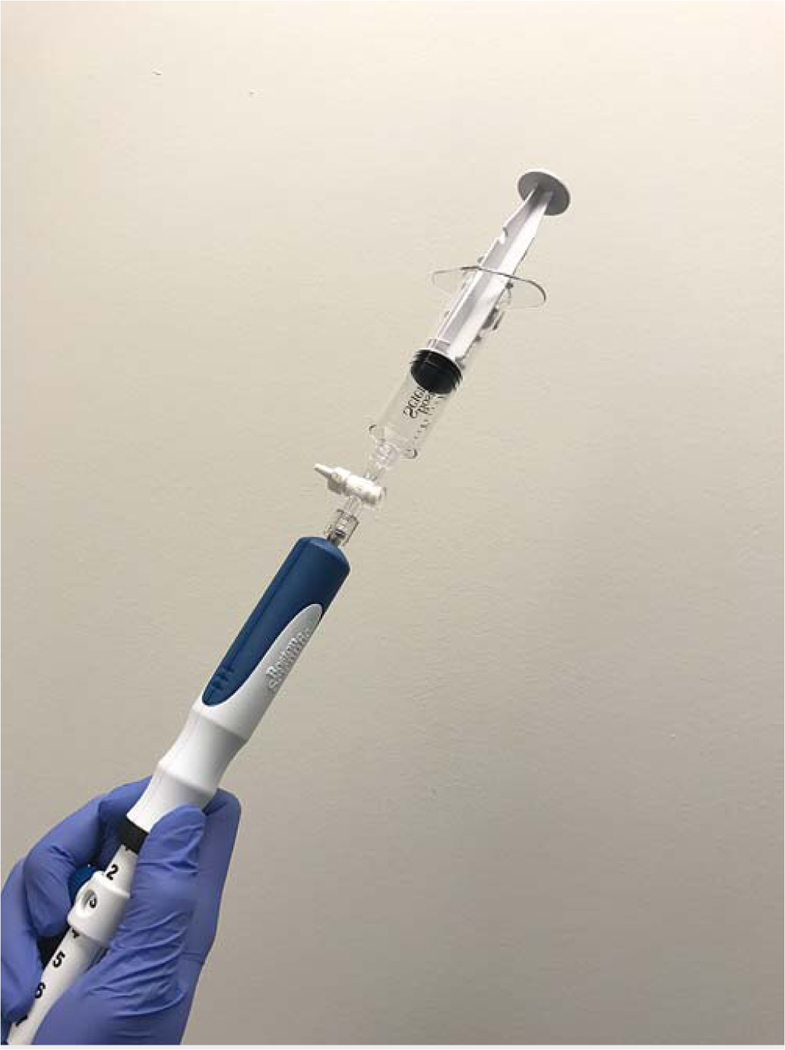
 Fig. 2
Fig. 2
Suction technique: the endoscopist performs to-and-fro movements within the lesion with the use of 10mL suction syringe.
After the specimen was procured, it was assessed by rapid on-site cytology examination (ROSE). The cytopathology technician processing the slides and the cytopathology physician were blinded to the aspiration technique. FNA passes were terminated once ROSE showed adequate cytologic material. If adequate material was not obtained after four passes, patients were crossed over to the other arm, and FNA was performed until the on-site cytotechnician confirmed adequate cytologic material up to a maximum of six additional passes. The purpose of the crossover was to ensure sufficient tissue was obtained for a diagnosis.
All patients were observed for immediate complications in the recovery room for 1–2 hours before discharge. All patients were followed up with telephone calls 24–72 hours postprocedure, and any adverse events were documented.
Preparation of cytologic specimen
After FNA, the obtained material was expressed onto glass slides by reinsertion of the stylet. The material was assessed macroscopically by the in-room cytotechnician. The lengths of all macroscopically visible core specimens (whitish pieces of tissue expelled from the needle) were measured carefully with a ruler. The core specimen was removed from the glass slide, placed into preservative solution and submitted for cell-block preparation. The remainder of the specimen was smeared.
Definitions
Malignant lesions were defined as those that were adenocarcinoma, neuroendocrine tumors or metastases to the pancreas.
Sensitivity was defined as the true positive rate whereby the test was the final cytologic diagnosis (smears and cell block). The gold standard was defined as follows: for resectable cases, surgical histology was considered the gold standard. For unre-sectable or benign cases, final cytologic diagnosis (with compatible clinical outcome) at 6-month follow-up was considered the gold standard. Negative cytology was confirmed with clinical data and/or imaging at 6-month follow-up. Specificity was defined as the proportion of patients correctly identified on cytology as having no malignancy to all patients without malignancy, as confirmed in comparison with the gold standard.
Outcome measures
The primary outcome was sensitivity of EUS-FNA using either the stylet slow-pull technique or the suction technique for malignant pancreatic solid lesions. Secondary outcomes were firstpass diagnostic rate, yield of core tissue, diagnostic accuracy, and adverse events.
Randomization
Computer-generated randomization assignments were obtained before study enrollment using the block randomization method by a statistician. These were then placed in sequentially numbered sealed opaque envelopes and opened by the research fellow or endoscopist during the procedure when patients met criteria for study inclusion. Patients were randomized equally to the two techniques (1:1 allocation).
Statistical analysis
It was assumed that the sensitivity of the “standard suction” technique would be 74% based on past studies using this technique [3–5]. We hypothesized that the stylet slow-pull technique would produce a clinically significant increase of 20% in sensitivity. Based on these assumptions, the target total sample size was 121 accounting for a 10% dropout rate. This was done with α = 5% based on a two-sided test and power (1 -β) = 80%.
Baseline characteristics of the patient population, pancreatic mass lesions, technical details, and procedure outcomes were summarized as means (SD) or medians (with interquartile range [IQR] and range) for continuous data, and as frequencies and proportions for categorical data. For the comparison of categorical data, the chi-squared test was used. Fisher’s exact test was used when the assumptions for chi-squared test were not met. The two-sample t test or the Wilcoxon rank-sum test was used for the comparison of continuous data (two independent samples). Statistical significance was determined as P< 0.05 (two-tailed test). Data sets were compiled using Microsoft Excel, and all statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, New York, USA).
Results
A total of 147 patients with pancreatic mass lesions were screened for inclusion between September 2013 and December 2014. Of these, 26 were excluded from the study because a predominantly cystic lesion was found (n = 9), the lack of an appreciable mass on EUS (n = 14), unavailable on-site pathology (n = 2), and respiratory distress precluding completion of the examination (n = 1). The remaining 121 patients (Johns Hopkins, n = 100, Cleveland Clinic, n = 21; mean age 64± 13.8 years; 57.9% male) were randomized to undergo EUS-FNA with the slow-pull (n = 61) or suction (n = 60) techniques ( Fig. 3).
Fig. 3).
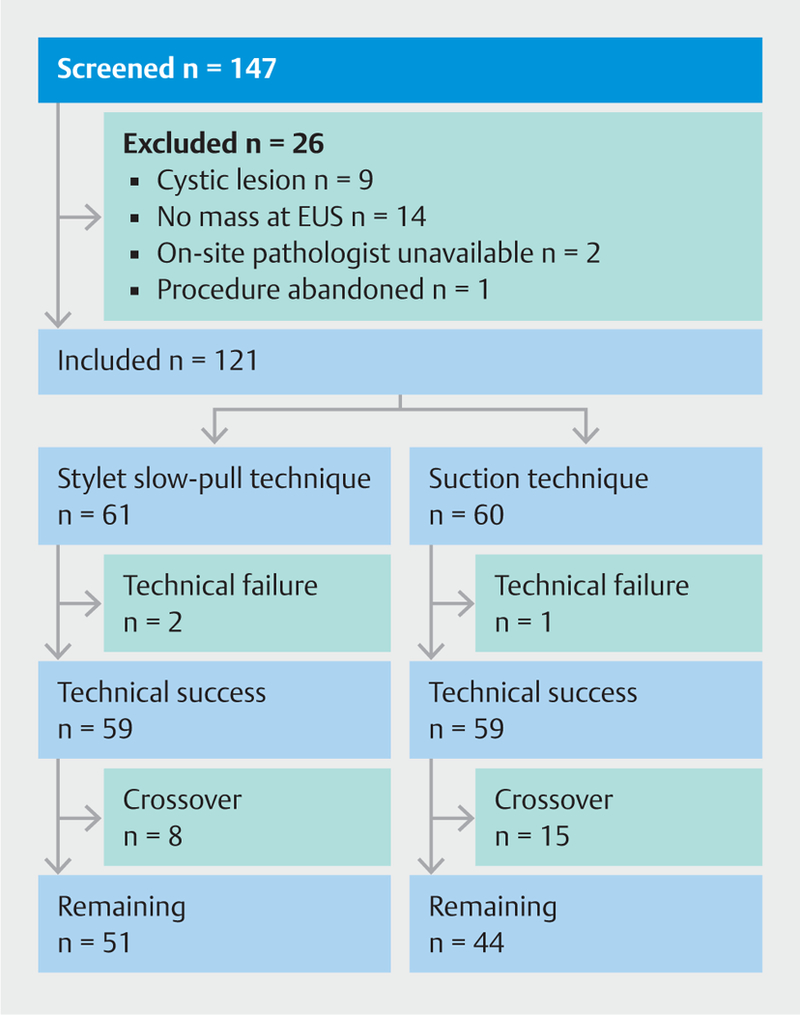
 Fig.3
Fig.3
Patient flow through the study. EUS, endoscopic ultrasound.
Baseline patient and lesion characteristics were similar in both groups ( Table 1). Similar numbers of prior nondiagnostic EUS-FNA had been performed in each group (slow-pull n = 8, suction n = 7; P>0.90) at outside institutions. The majority of masses were located within the head of the pancreas (slow-pull 68.9% and suction 51.7%; P = 0.06). There was no difference in mean size of the mass (3.2± 1.6 vs. 2.9±1.2cm; P = 0.23), vascular involvement (86.9% vs. 93.3%; P = 0.37), cystic component (16.4% vs. 20.0%; P = 0.64) or presence of biliary stent (18.0% vs. 13.3%; P = 0.46) in the slow-pull and suction groups, respectively (
Table 1). Similar numbers of prior nondiagnostic EUS-FNA had been performed in each group (slow-pull n = 8, suction n = 7; P>0.90) at outside institutions. The majority of masses were located within the head of the pancreas (slow-pull 68.9% and suction 51.7%; P = 0.06). There was no difference in mean size of the mass (3.2± 1.6 vs. 2.9±1.2cm; P = 0.23), vascular involvement (86.9% vs. 93.3%; P = 0.37), cystic component (16.4% vs. 20.0%; P = 0.64) or presence of biliary stent (18.0% vs. 13.3%; P = 0.46) in the slow-pull and suction groups, respectively ( Table 2). ROSE was provided by a cyto-technician alone in 100 patients, and by both cytotechnician and pathologist in 21 cases.
Table 2). ROSE was provided by a cyto-technician alone in 100 patients, and by both cytotechnician and pathologist in 21 cases.
 Table 1
Table 1
Patient baseline characteristics.
| Stylet slow-pull technique (n = 61) | Standard suction technique (n = 60) | P value | |
|---|---|---|---|
| Patient age, mean (SD), years | 64.2 (13.8) | 64.1 (13.8) | 0.96 |
| Male, n (%) | 38 (62.3) | 32 (53.3) | 0.36 |
| Prior history, n (%) | |||
| ▪ Chronic pancreatitis | 2(3.3) | 3 (5.0) | 0.68 |
| ▪ Chemotherapy | 1 (1.6) | 3 (5.0) | 0.36 |
| ▪ Radiation therapy | 3(4.9) | 3 (5.0) | >0.99 |
| Prior nondiagnostic FNA, n (%) | 8(13.1) | 7(11.7) | >0.90 |
| Antiplatelet therapy, n (%) | 14(23.0) | 6 (10.0) | 0.09 |
FNA, fine-needle aspiration.
 Table 2
Table 2
Masscharacteristics.
| Stylet slow-pull technique (n = 61) | Standard suction technique (n = 60) | P value | |
|---|---|---|---|
| Mass location, n (%) | |||
| ▪ Uncinate/Head | 42 (68.9) | 31 (51.7) | 0.06 |
| ▪ Neck | 1 (1.6) | 6 (10.0) | 0.06 |
| ▪ Body | 13(21.3) | 14(23.3) | 0.83 |
| ▪ Tail | 5(8.2) | 9(15.0) | 0.27 |
| Mass size, mean (SD) | 3.2 (1.6) | 2.8 (1.2) | 0.23 |
| ▪ Median | 2.9 | 2.80 | |
| ▪ IQR | 2.0 – 4.2 | 2.2 – 3.6 | |
| Cystic component, n (%) | 10(16.4) | 12 (20.0) | 0.64 |
| Vascular involvement, arterial/venous, n (%) | 53 (86.9) | 56 (93.3) | 0.37 |
| EUS features of chronic pancreatitis, n (%) | 7(11.5) | 11 (18.3) | 0.32 |
| Biliary stent present, n (%) | 11 (18.0) | 8(13.3) | 0.46 |
| ▪ Metal | 5(8.2) | 1 (1.7) | 0.21 |
| ▪ Plastic | 6(9.8) | 7(11.7) | 0.78 |
IQR, interquartile range; EUS, endoscopic ultrasound.
The technical success rates were 96.7% and 98.3% in slow-pull and suction groups, respectively (P>0.99). Of the three technical failures, one patient had an uncinate lesion and the 22-gauge needle could not be advanced outside the echoendo-scope. Therefore, a 25-gauge needle was used to successfully perform three passes. The remaining two patients had a firm mass in the pancreatic body and tail, which could not be successfully punctured with the 22-gauge needle. Subsequent successful passes were made with a 25-gauge needle. Fellow involvement was also similar in both groups (37.7% vs. 41.7%; P = 0.66) ( Table 3).
Table 3).
 Table 3
Table 3
Procedural details
| Stylet slow-pull technique (n = 61) | Standard suction technique (n = 60) | P value | |
|---|---|---|---|
| Technical success, n (%) | 59 (96.7) | 59 (98.3) | >0.99 |
| Crossover, n (%) | 8(13.1) | 15 (25.0) | 0.10 |
| Fanning, n (%) | 24(39.3) | 26 (43.3) | 0.71 |
| First-pass diagnostic, n (%) | 26(42.6) | 23 (38.3) | 0.71 |
| No. passes to diagnosis, mean (SD) | 2.0(1.3) | 1.9 (1.8) | 0.71 |
| ▪ Median | 2 | 1 | |
| ▪ IQR | 1 – 3 | 1 – 2 | |
| First-pass core, n (%) | 24(39.3) | 15 (25.0) | 0.12 |
| Core obtained, n (%) | 37 (60.6) | 28 (46.7) | 0.14 |
| Length of core, mean (SD), mm | 2.9(2.2) | 3.0 (3.0) | 0.82 |
| ▪ Median | 2.5 | 2.0 | |
| ▪ IQR | 0.6 – 4.5 | 1.0 – 5.0 | |
| ROSE, n (%) | |||
| ▪ Cytotechnician | 53 (86.9) | 47 (78.3) | 0.23 |
| ▪ Cytopathologist | 8(14) | 13(21.7) | 0.23 |
| Fellow involved, n (%) | 23 (37.7) | 25 (41.7) | 0.66 |
IQR, interquartile range; ROSE, rapid on-site evaluation
The primary outcome of sensitivity of EUS-FNA was 82% in the slow-pull group and 69% in the suction group (P = 0.10). The first pass diagnostic rate (42.6% vs. 38.3 %; P = 0.71), acquisition of core tissue (60.6% vs. 46.7 %; P = 0.14), median (range) number of passes to diagnosis (2 [1 –3] vs. 1 [1 –2]; P = 0.71), and diagnostic accuracy (80% vs. 70%; P =0.14) were similar in slow-pull and suction groups, respectively. There was no difference in the crossover rate (13.1 % vs. 25.0%; P =0.10) or use of fanning technique (39.3% vs. 43.3%; P = 0.71) in the slow-pull and suction groups, respectively. There was also no difference in the diagnostic yield between those who had fanning and those who did not (P = 0.53). The overall sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the slow-pull and suction techniques were 82 %, 67 %, 96%, 29% and 69%, 80%, 97%, 19%, respectively ( Table 4,
Table 4,  Table 5).
Table 5).
 Table 4
Table 4
Stylet slow-pull technique group.
| Diagnosis following stylet slow-pull tech nique |
Gold standard diagnosis | |
|---|---|---|
| Positive for malignancy |
Negative for malignancy |
|
| Positive for malignancy | 45 | 2 |
| Negative for malignancy | 10 | 4 |
| Diagnostic accuracy, % (95%CI) | ||
| ▪ Sensitivity | 82 (68–90) | |
| ▪ Specificity | 67 (24–94) | |
| ▪ NPV | 29 (19 – 57) | |
| ▪ PPV | 96 (84 – 99) | |
NPV, negative predictive value; PPV, positive predictive value
 Table 5
Table 5
Standard suction technique group.
| Diagnosis following standard suction technique |
Gold standard diagnosis | |
|---|---|---|
| Positive for malignancy |
Negative for malignancy |
|
| Positive for malignancy | 38 | 1 |
| Negative for malignancy | 17 | 4 |
| Diagnostic accuracy, % (95%CI) | ||
| ▪ Sensitivity | 69 (55– 80) | |
| ▪ Specificity | 80 (29–98) | |
| ▪ NPV | 19 (6– 42) | |
| ▪ PPV | 97 (84 – 99) | |
NPV, negative predictive value; PPV, positive predictive value
A total of 35 patients underwent surgical resection. There was no difference in the final diagnosis between the two groups ( Table 6). There were no procedure-related adverse events in either group.
Table 6). There were no procedure-related adverse events in either group.
 Table 6
Table 6
Final diagnosis.
| Final diagnosis, n (%) | Stylet slow-pull technique (n = 61) | Standard suction technique (n = 60) | P value |
|---|---|---|---|
| Pancreatic adenocarcinoma | 46(75.4) | 49(81.7) | 0.51 |
| Neuroendocrine tumor | 7(11.5) | 4(6.7) | 0.53 |
| Pancreatic metastasis | 2(3.3) | 2(3.3) | >0.99 |
| Total cases of malignancy | 55(90.2) | 55 (91.7) | 0.90 |
| Chronic inflammation/benign | 5(8.2) | 3 (5.0) | 0.72 |
| Other | 1 (1.6) | 2(3.3) | 0.62 |
For patients with negative FNA (n = 11), the overall follow-up was mean/median 9.7/8.6 months (range 0.4–28.9 months). Follow-up imaging was by computed tomography or magnetic resonance imaging at a mean/median follow-up of 5.9/5.1 months (range 0.3 – 17 months).
Discussion
The present prospective randomized trial demonstrated no difference in the sensitivity of EUS-FNA for diagnosing solid pancreatic mass lesions when performed using either the stylet slow-pull or suction techniques (82% vs. 69%; P = 0.10) using a standard 22-gauge FNA needle. There was also no difference in the first-pass diagnostic rate (42.6% vs. 38.3%)ormedian number of passes required (2 vs. 1) to achieve a diagnosis in the stylet slow-pull or suction groups, respectively. Although high PPV rates were noted in each group (96% and 97%), the NPV was low (29% and 19%), which is likely to be a reflection of the high prevalence of malignancy in our study population.
A recent retrospective study by Nakai et al. also compared the stylet slow-pull and suction techniques when performing EUS-FNA for pancreatic lesions [12]. Although a variety of standard needle types (no core or side-port needles; Expect - Boston Scientific; EchoTip - Cook Medical; or EZshot2 without side port - Olympus Medical Systems) and sizes (22 and 25 gauge) were used in the study, when subgroup analysis of procedures performed using a 22-gauge needle was performed, the authors also found no difference in sensitivity between the stylet slow-pull and suction techniques (79.2 % vs. 76%; P = 0.6).
In a bench-top study, Katanuma et al. elegantly demonstrated an exponential rise in suction force when 10mL negative pressure was applied to 25-, 22-, and 19-gauge needles, respectively [13]. No differences in the suction force generated using 10 mL were detected between standard and ProCore 22-gauge needles (EchoTip ProCore; Cook Medical). Remarkably, the suction force generated in a 22-gauge needle using the stylet slow-pull technique was very weak compared with the 10 mL syringe (1.4 vs. 23.7 mN). Nonetheless, our study and that of Nakai et al. have not shown a difference in diagnostic sensitivity between the stylet slow-pull and suction techniques. Thus, aside from suction force, other factors that influence the acquisition of cells from tissue are likely at play, and may be difficult to replicate in a bench-top study.
There was a higher rate of visible core specimen acquisition in the stylet slow-pull group in the current study (60.6% vs. 46.7 %; P =0.14), but the difference was not statistically significant. In a prospective single-arm study evaluating EUS-FNA of intra-abdominal solid mass lesions using a 22-gauge ProCore needle with the stylet slow-pull technique, the authors reported a visible core rate of 96%, which is much higher than the rates observed in our study within the slow-pull arm [11]. However, despite the higher visible core rate, the sensitivity for diagnosing pancreatic lesions was 91 %, which is similar to the results observed in our study. Paik et al. used 22-gauge ProCore needles (rather than standard FNA needles), which may be the reason for the higher core rate. Further studies comparing stylet slow-pull and suction techniques using ProCore needles are required to ascertain the benefit of the slow-pull technique in obtaining core tissue. Additionally, quantitative or qualitative methods to measure or describe the rate of blood contamination in the visible core specimens is needed to clarify the quality of core specimens that are being obtained with the present armamentarium of EUS needles.
One shortcoming of the stylet slow-pull technique is that reinsertion of the stylet is required for repeat passes, which prolongs the procedure time and can also be associated with a risk of inadvertent needle stick injury [14]. Furthermore, coordination with an assistant is required to perform the technique adequately.
The strengths of this study include its prospective, multicenter, and randomized design. The cytotechnician and the pathologist who made the final cytologic diagnosis were also blinded to the technique used to obtain specimens. However, the endo- sonographers could not be blinded to the procedure technique and this is a limitation of the study. Furthermore, although the cytotechnician was blinded to the technique used by the endo-sonographer, there is a small possibility that they may have witnessed the technique. Also, cellularity of the specimens was not quantified. Although the acquisition of core specimens was recorded, the ability to perform immunohistochemistry or assess tissue architecture was not quantified, as these characteristics were not the primary goal of this study and we had few nonpan- creatic adenocarcinoma lesions.
Another limitation of the study is the potential for a type 2 error. Our sample size calculation assumed a 20% gain in sensitivity with the stylet slow-pull technique. Hence, a smaller, yet clinically relevant difference in sensitivity may not have been detected by the sample size of the study. Furthermore, when taking into account the crossover rate, the dropout rate in our study is higher than the anticipated 10%. In the intention-to-treat analysis, the specificity and NPV were significantly higher in the suction group. Hence, it is possible that the study was underpowered to detect a difference between the stylet slow-pull and suction groups, and a larger sample size may have borne out a difference. Another limitation is that overall follow-up was limited to a mean of 6 months, and therefore false-negative results may have been undetected.
Our prospective randomized trial demonstrated that there is no difference in sensitivity or median number of passes to diagnosis when performing EUS-FNA of solid pancreatic lesions using either the stylet slow-pull or suction techniques when a standard 22-gauge needle is utilized. The suction technique may be advantageous as repeated stylet insertion and coordination with an assistant is not required. Hence, the standard suction method may indeed be the preferable technique for performing EUS-FNA of solid pancreatic mass lesions. Larger studies are needed to verify these results.
Footnotes
Competing interests
Boston Scientific provided a research grant for the study but had no involvement in study design, data collection or data analysis.
TRIAL REGISTRATION: Multi-center single-blinded randomized clinical trial NCT01936467 at clinicaltrials.gov
References
- [1].Vilmann P, Jacobsen GK, Henriksen FW et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc 1992; 38: 172–173 [DOI] [PubMed] [Google Scholar]
- [2].Dumonceau JM, Polkowski M, Larghi A et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2011; 43: 897–912 [DOI] [PubMed] [Google Scholar]
- [3].Itoi T, Sofuni A, Itokawa F et al. Current status of diagnostic endo-scopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc 2011; 23 : (Suppl. 01): 17–21 [DOI] [PubMed] [Google Scholar]
- [4].Bang JY, Hebert-Magee S, Trevino J et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc 2012; 76: 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wani S, Early D, Kunkel J et al. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: a prospective, single blind, randomized, controlled trial. Gastrointest Endosc 2012; 76: 328–335 [DOI] [PubMed] [Google Scholar]
- [6].Madhoun MF, Wani SB, Rastogi A et al. The diagnostic accuracy of 22- gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy 2013; 45: 86– 92 [DOI] [PubMed] [Google Scholar]
- [7].Hikichi T, Irisawa A, Bhutani MS et al. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol 2009; 44: 322–328 [DOI] [PubMed] [Google Scholar]
- [8].Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol 2011; 106: 1705–1710 [DOI] [PubMed] [Google Scholar]
- [9].Puri R, Vilmann P, Saftoiu A et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol 2009; 44: 499 – 504 [DOI] [PubMed] [Google Scholar]
- [10].Fabbri C, Polifemo AM, Luigiano C et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: a prospective comparative study with randomisation of needle sequence. Dig Liver Dis 2011; 43: 647 – 652 [DOI] [PubMed] [Google Scholar]
- [11].Paik WH, Park Y, Parkdo H et al. Prospective evaluation of new 22 gauge endoscopic ultrasound core needle using capillary sampling with stylet slow-pull technique for intra-abdominal solid masses.J Clin Gastroenterol 2015; 49: 199– 205 [DOI] [PubMed] [Google Scholar]
- [12].Nakai Y, Isayama H, Chang KJ et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci 2014; 59: 1578–1585 [DOI] [PubMed] [Google Scholar]
- [13].Katanuma A, Itoi T, Baron TH et al. Bench-top testing of suction forces generated through endoscopic ultrasound-guided aspiration needles. J Hepatobiliary Pancreat Sci 2015; 22: 379– 385 [DOI] [PubMed] [Google Scholar]
- [14].Rastogi A, Wani S, Gupta N et al. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc 2011; 74: 58 – 64 [DOI] [PubMed] [Google Scholar]


