Abstract
Aim:
To assess the use of extracorporeal cardiopulmonary resuscitation (ECPR), compared with manual or mechanical cardiopulmonary resuscitation (CPR), for out-of-hospital cardiac arrest (OHCA) and in-hospital cardiac arrest (IHCA) in adults and children.
Methods:
The PRISMA guidelines were followed. We searched Medline, Embase, and Evidence-Based Medicine Reviews for randomized clinical trials and observational studies published before May 22, 2018. The population included adult and pediatric patients with OHCA and IHCA of any origin. Two investigators reviewed studies for relevance, extracted data, and assessed risk of bias using the ROBINS-I tool. Outcomes included short-term and long-term survival and favorable neurological outcome.
Results:
We included 25 observational studies, of which 15 studies were in adult OHCA, 7 studies were in adult IHCA, and 3 studies were in pediatric IHCA. There were no studies in pediatric OHCA. No randomized trials were included. Results from individual studies were largely inconsistent, although several studies in adult and pediatric IHCA were in favor of ECPR. The risk of bias for individual studies was overall assessed to be critical, with confounding being the primary source of bias. The overall quality of evidence was assessed to be very low. Heterogeneity across studies precluded any meaningful meta-analyses.
Conclusions:
There is inconclusive evidence to either support or refute the use of ECPR for OHCA and IHCA in adults and children. The quality of evidence across studies is very low.
Keywords: ECPR, Extracorporeal cardiopulmonary resuscitation, Cardiac arrest
Introduction
Extracorporeal cardiopulmonary resuscitation (ECPR) is an advanced rescue therapy, where an extracorporeal circuit is employed, to support circulation in patients with cardiac arrest refractory to conventional CPR [1]. ECPR maintains vital organ perfusion while potential reversible causes of the cardiac arrest can be identified and treated.
ECPR is recognized by the American Heart Association (AHA) [2,3] and the European Resuscitation Council (ERC) [4,5] as a therapy which can be considered in select cardiac arrest patients, when rapid expert deployment is possible. However, the benefits of applying ECPR are not clear and optimal patient selection and timing of the therapy are not well-understood [6]. Furthermore, the ethical considerations related to using and studying ECPR are complex [7]. Given the recent increase in the availability and usage of ECPR for cardiac arrest [8–10], there is a need for a review of the evidence to guide the international consensus on ECPR in cardiac arrest.
The objective of this systematic review was to inform the update of the International Liaison Committee on Resuscitation (ILCOR) treatment recommendations by assessing the use of ECPR, compared to manual or mechanical cardiopulmonary resuscitation (CPR), for OHCA and IHCA of all causes in adults and children.
Methods
Protocol and registration
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. The PRISMA checklist is provided in the Supplementary Contents. The protocol and amendments were prospectively submitted to the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42018085404). The protocol is provided in the Supplementary Contents. The review was commissioned by ILCOR.
Eligibility criteria
We used the PICO (Population, Intervention, Comparison, Outcome) format to frame the study question: Among adults (≥18 years) and children (< 18 years) with cardiac arrest in any setting (out-of-hospital or in-hospital) (P), does the use of ECPR, including extracorporeal membrane oxygenation or cardiopulmonary bypass, during cardiac arrest (I), compared to manual CPR and/or mechanical CPR (C), change survival at hospital discharge, long-term survival, neurological outcome at discharge, and/or long-term neurological outcome (O).
Outcomes with similar time frames (i.e. short-term [hospital discharge, 28-days, 30-days, and 1-month] and long-term [3-months, 6-months, and 1-year]) were combined into single categories. Long-term survival reported as hazard ratios (i.e. survival analysis), irrespective of length of follow-up, was also considered. Return of spontaneous circulation (ROSC) was not included as an outcome since it is difficult to meaningfully define in this patient population.
Randomized trials, non-randomized controlled trials, and observational studies (cohort studies and case-control studies) with a control group (i.e. patients not receiving ECPR) were included. Animal studies, ecological studies, case series, case reports, reviews, abstracts, editorials, comments, and letters to the editor were not included. There were no limitations on publication period or study language. The population included patients with IHCA or OHCA of any origin, without age restriction. Studies with ≤5 patients receiving ECPR or studies that did not report timing of ECPR (i.e. not clear whether ECPR was used during or after cardiac arrest) were excluded.
Studies exclusively assessing the use of extracorporeal life support for cardiac and/or respiratory failure after sustained ROSC were not included. Studies reporting the use of extracorporeal circulation for accidental hypothermia, pulmonary embolism, overdoses, or other conditions were included if cardiac arrest was documented. Studies assessing cost-effectiveness of ECPR were considered for a descriptive summary.
Information sources and search strategy
We searched the following electronic bibliographic databases on December 19, 2017: Medline, Embase, and Evidence-Based Medicine Reviews (which includes the Cochrane Library). The search was repeated on May 22, 2018 to capture any articles published during the review process. We used a combination of various search terms for cardiac arrest and extracorporeal circulation. The bibliographies of included articles were reviewed for potential additional articles. To identify ongoing trials, we searched the International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/) (which includes entries in ClinicalTrials.gov) on March 13, 2018. The search strategies for each database and the Clinical Trials Registry Platform are provided in eTables 1–2 in the Supplementary Contents.
Study selection
Two reviewers, using pre-defined screening criteria, independently screened all titles and abstracts retrieved from the systematic review. The reviewers were blinded to authors and journal titles during the screening stage. Any disagreement regarding inclusion or exclusion were resolved via discussion between the reviewers and with a third reviewer as needed. The Kappa-value for inter-observer variance was calculated. In case of only weak or moderate agreement between reviewers (i.e. a Kappa<0.80 [12]) a third reviewer reviewed all excluded titles and abstracts to ensure optimized sensitivity. Two reviewers then reviewed the full text-reports of all potentially relevant publications passing the first level of screening. Any disagreement regarding eligibility was resolved via discussion.
Data collection and data items
Two reviewers using a pre-defined standardized data extraction form extracted data as pertinent to the PICO (see “Eligibility criteria”). Missing statistical parameters (i.e. odds ratios) of importance and variance measures (i.e. confidence intervals) were calculated if data permitted. Any discrepancies in the extracted data were identified and resolved with discussion and consensus.
Risk of bias in individual studies
Two investigators independently assessed risk of bias for the included studies. Risk of bias was assessed by the ROBINS-I tool [13] for observational studies. In the ROBINS-I tool, risk of bias is assessed within specified domains, including (1) bias due to confounding, (2) bias in selection of participants into the study, (3) bias in classification of interventions, (4) bias due to deviations from intended interventions (5) bias due to missing data, (6) bias in measurement of outcomes, (7) bias in selection of the reported result, and (8) overall bias [13]. Bias assessments were tabulated with explanations when studies were downgraded. Since assessments are inherently subjective and there are no strict and objective criteria to judge bias within the ROBINS-I tool [13], disagreements were resolved via discussion between the two investigators. Bias was assessed per study rather than per outcome, since there were no meaningful differences in bias across outcomes.
Data synthesis and confidence in cumulative evidence
Studies were assessed for clinical (i.e. participants, interventions, and outcomes), methodological (i.e. study design or risk of bias), and statistical heterogeneity [14]. Separate meta-analyses were planned for adult IHCA, adult OHCA, pediatric IHCA, and pediatric OHCA as described in the protocol.
The quality of the overall evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology ranging from very low quality of evidence to high quality of evidence [15]. Detailed assessment of overall risk of bias, inconsistency, indirectness, imprecision and potential other issues such as publication bias were tabulated.
Review Manager (The Cochrane Collaboration, 2014) was used to generate forest plots.
Results
Study selection
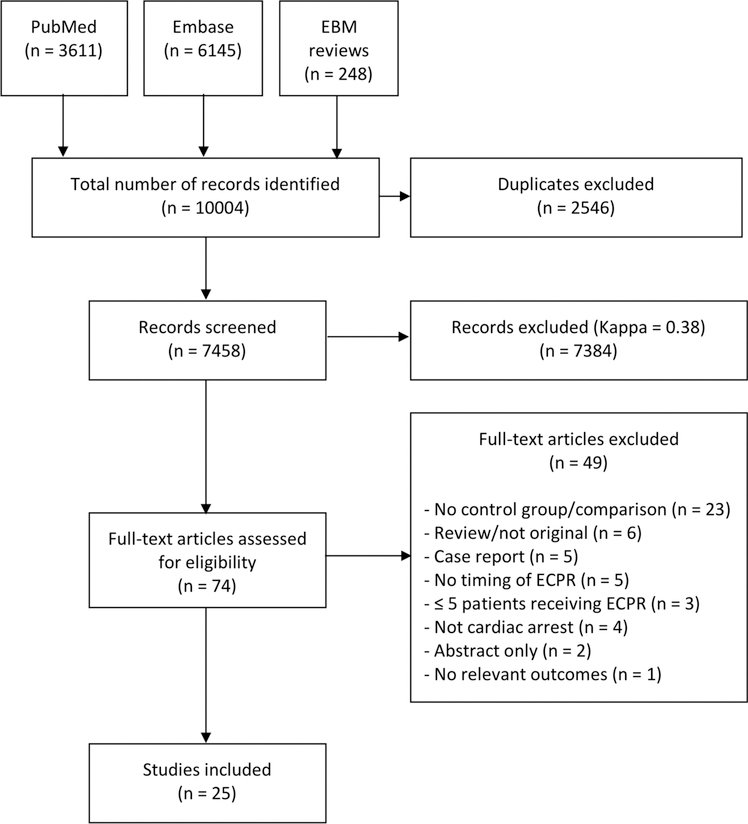
The search strategy identified 7458 records of which 74 records were eligible for full-text review. The Kappa for identifying records during the initial screening of the first search was 0.38 prompting review by a third reviewer. A PRISMA diagram of the study selection process is presented in Fig. 1. No randomized clinical trials were identified. Twenty-five observational studies met all of the inclusion criteria and none of the exclusion criteria. [16–40] Fifteen studies were in adult OHCA [16–30], 7 studies were in adult IHCA [31–37], and 3 studies were in pediatric IHCA [38,17–40]. We identified no studies in pediatric OHCA. An overview of each included study is provided in Tables 1–3 and details are provided in the Supplementary Contents. We identified 5 ongoing clinical trials in adult OHCA on the International Clinical Trials Registry Platform. An overview of each trial is provided in Table 4. We did not identify any studies assessing the cost-effectiveness of ECPR in cardiac arrest.
Fig. 1.
PRISMA diagram.
Out of 7458 screened records, 74 articles were assessed for eligibility, and 25 studies were included.
Table 1.
| Study | Country | Years of inclusion | Inclusion criteria | Exclusion criteria | Patients analyzed (n) |
|---|---|---|---|---|---|
| Agostinucci et al. [16] | France | 2005–2010 | Use of load-distributing band | Not Reported | 285 |
| Cesana et al. [17] | Italy | 2011–2015 | Age 18–75 years, witnessed, ischemic etiology, absence of comorbidities precluding ICU admission | Not Reported | 148 |
| Choi et al. [18] | Korea | 2011–2015 | Non-traumatic, age ≤75, witnessed, bystander CPR or no-flow time ≤5 min, prehospital low-flow time ≤30 min and >10 min of conventional CPR at ED, absence of severe comorbidities | DNR, poor performance status or terminal illness, trauma, intracranial hemorrhage, acute aortic dissection, ROSC within 10 min of ED arrival | 60 |
| Hase et al. [19] | Japan | 1999–2003 | Cardiac etiology | Not Reported | 100 |
| Kim et al. [20] | Korea | 2006–2013 | Age >18 years, non-traumatic | Not Reported | 104 |
| Lee et al. [21] | Korea | 2009–2014 | Not Reported | Not Reported | 955 |
| Maekawa et al. [22] | Japan | 2000–2004 | Cardiac etiology, age >16 years, witnessed, CPR duration >20 min | DNR, dead prior to hospital arrival | 48 |
| Poppe et al. [23] | Austria | 2003–2014 | Age >18 years, ongoing CPR | Not Reported | 96 |
| Sakamoto et al. [24] | Japan | 2008–2011 | Shockable rhythm, cardiac arrest on arrival, 45 min from cardiac arrest onset to hospital arrival, no ROSC within 15 min after hospital arrival | Age <20 or >75 years, poor level of activities of daily living, non-cardiac etiology, body temperature <30 C, no informed consent | 454 |
| Schober et al. [25] | Austria | 2002–2012 | Cardiac origin, CPR duration >30 min | Clinical indication for ECPR | 239 |
| Siao et al. [26] | Taiwan | 2011–2013 | Age 18–75 years, ventricular fibrillation, no-flow time < 5 min, refractory cardiac arrest | Head trauma or active bleeding, severe sepsis, initial non-shockable rhythm, terminal malignancy, history of neurological deficits | 60 |
| Tanno et al. [27] | Japan | 2000–2004 | Age >16 years, cardiac etiology | Not Reported | 398 |
| Venturini et al. [28] | USA | 2011–2016 | CPR in cardiac catheterization laboratory, mechanical chest compressions | Not Reported | 31 |
| Yannopoulos et al. [29] | USA | 2015–2016 | Age 18–75 years, cardiac etiology, shockable rhythm, 3 direct current shocks, amiodarone, eligible mechanical CPR, time to CCL < 30 min | Nursing home resident, DNR, known terminal illness, significant bleeding | 188 |
| Yannopoulos et al. [30] | USA | 2015–2016 | Age 18–75 years, cardiac etiology, shockable rhythm, 3 direct current shocks, amiodarone, eligible mechanical CPR, transfer time from scene to CCL < 30 min | Nursing home resident, DNR, known terminal illness, significant bleeding | 232 |
ECPR refers to extracorporeal cardiopulmonary resuscitation, CPR refers to cardiopulmonary resuscitation, ED refers to emergency department, ICU refers to intensive care unit, DNR refers to do-not-resuscitate, ROSC refers to return of spontaneous circulation; CCL refers to cardiac catheterization laboratory.
All studies compared ECPR vs. no ECPR whereas Sakamoto compared emergency departments with ECPR vs. emergency departments with no ECPR.
There was some overlap between the studies by Hase, Maekawa and Tanno, and between Yannopolous (2016 + 2017).
Table 3.
Characteristics of studies in pediatric in-hospital cardiac arresta.
| Study | Country | Years of inclusion | Inclusion criteria | Exclusion criteria | Patients analyzed (n) |
|---|---|---|---|---|---|
| Lasa et al. [38] | USA | 2000 – 2011 | Age < 18 years, CPR duration ≥10 min | Hospitals with no ECPR cases, events in the delivery room or rehabilitation facility or same-day surgery center, obstetric and traumatic events | 3,756 |
| Odegard et al. [39] | USA | 2004 – 2009 | Cardiac arrest during cardiac catheterization | Not Reported | 70 |
| Ortmann et al. [40] | USA | 2000 – 2008 | Age <18 years, cardiac admission | Not Reported | Medical: 574 Surgical: 640 |
ECPR refers to extracorporeal cardiopulmonary resuscitation, CPR refers to cardiopulmonary resuscitation.
There was some overlap between the studies by Lasa and Ortmann.
Table 4.
Overview of ongoing randomized clinical trials registered online.
| Title | IDa | Country | Estimated Completion Date | Objective | Patients (n) |
|---|---|---|---|---|---|
| Hyperinvasive Approach in Cardiac Arrest | NCT01511666 | Czech Republic | May 2018 | Determine the advantage of prehospital intra-arrest hypothermia, mechanical CPR, ECLS, and early invasive assessment vs. standard of care. | 170 |
| Emergency Cardiopulmonary Bypass for Cardiac Arrest | NCT01605409 | Austria | May 2018 | Determine the feasibility of ECPB installed in an ED vs. standard of care | 40 |
| ECPR for Refractory Out-Of-Hospital Cardiac Arrest | NCT03065647 | USA | December 2018 | Determine the feasibility of expedited transport to an ED capable of initiating ECPR vs. standard of care | 30 |
| A Comparative Study Between a Pre-hospital and an In-hospital Circulatory Support Strategy in Refractory Cardiac Arrest | NCT02527031 | France | March 2019 | Determine the advantage of pre-hospital ECMO vs. in-hospital ECMO | 210 |
| Early Initiation of Extracorporeal Life Support in Refractory OHCA | NCT03101787 | Netherlands | May 2019 | Determine the effect of ECPR in ED vs. standard of care | 110 |
OHCA refers to out-of-hospital cardiac arrest, ROSC refers to return of spontaneous circulation, ECPB refers to emergency cardiopulmonary bypass, ECPR refers to extracorporeal cardiopulmonary resuscitation, ECMO refers to extracorporeal membrane oxygenation, CCPR refers to conventional cardiopulmonary resuscitation, ED refers to emergency department, CPR refers to cardiopulmonary resuscitation, ECLS refers to extracorporeal life support.
All studies were registered at clinicaltrials.gov.
Adult out-of-hospital cardiac arrest
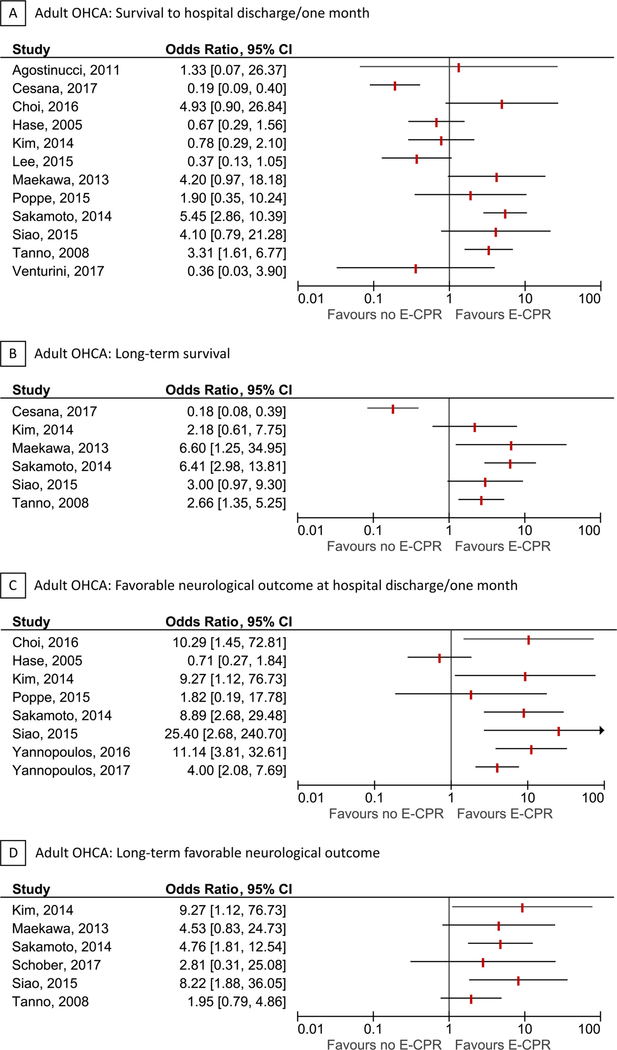
Fifteen of the included studies were in adult OHCA [16–30]. Eight studies were performed in Asia [18–22,24,26,27], 4 studies in Europe [16,17,23,25], and 3 studies in North America [28,17–30]. Three studies included both OHCA and IHCA patients [17,21,28]. The cohort and/or time-frame was overlapping for some studies [19,22,27,29,30]. Years of patient inclusion ranged from 1999 to 2015. The majority of studies defined the exposure as “ECPR use”, whereas one study [24] defined the exposure as “ECPR availability” and two studies [29,30] defined exposure as a “ECPR strategy”. The median age of exposed patients ranged from 46 to 59 years. Twelve studies reported survival to hospital discharge, 6 studies reported long-term survival, 8 studies reported favorable neurological outcome at hospital discharge, and 6 studies reported long-term favorable neurological outcomes. All studies defined favorable neurological outcome as a Cerebral Performance Category score of 1–2. Forests plots of each outcome are presented in Fig. 2. Additional details for each individual study are provided in Table 1 and the Supplementary Contents.
Fig. 2.
Forest plots for adult out-of-hospital cardiac arrest.
Forest plots for survival to hospital discharge/one month (A), long-term survival (B), favorable neurological outcome at hospital discharge/one month (C), and long-term favorable neurological outcome (D) in adult out-of-hospital cardiac arrest. The vertical red lines indicate odds ratios. Horizontal lines indicate 95% confidence intervals of the estimate. The studies are ordered by alphabetical order within each outcome. The forest plots for long-term outcomes are representative of all included patients, independent of survival to hospital discharge. The studies by Cesana et al. Lee et al. and Venturini et al. included both out-of-hospital cardiac arrest and in-hospital cardiac arrest patients. There was some overlap between the studies by Hase, Maekawa and Tanno, and between Yannopolous (2016 + 2017).
OHCA refers to out-of-hospital cardiac arrest.
Adult in-hospital cardiac arrest
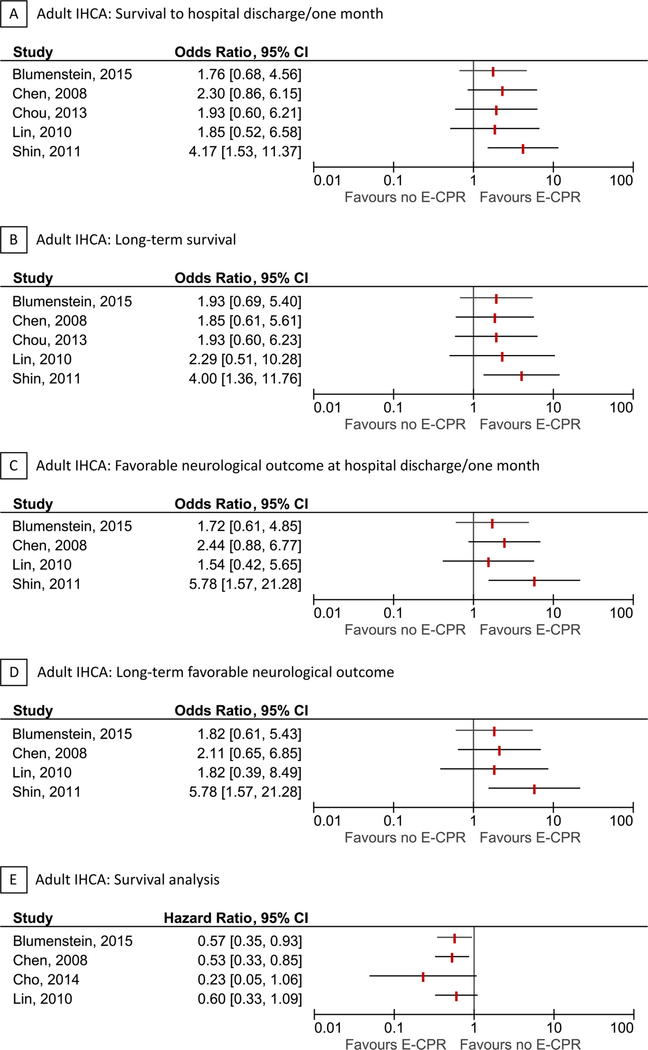
Seven of the included studies were in adult IHCA [31–37]. Six studies were performed in Asia [32,32–37] and one study was performed in Europe [31]. The cohort and/or time-frame was overlapping for some studies [32,33,35,32–37]. Years of patient inclusion ranged from 2001 to 2013. The majority of studies defined the exposure as “ECPR use”, whereas two studies [36,37] defined the exposure as “ECPR attempt”. The median age of exposed patients ranged from 57 to 72 years. Six studies reported survival to hospital discharge, 6 studies reported long-term survival, 5 studies reported favorable neurological outcome at hospital discharge, and 5 studies reported long-term favorable neurological outcome. Four studies reported survival analyses with length of follow-up ranging from 1 to 3 years. All studies defined favorable neurological outcome as a Cerebral Performance Category score of 1–2. Forests plots of each outcome are presented in Fig. 3. Additional details for each individual study are provided in Table 2 and the Supplementary Contents.
Fig. 3.
Forest plots for adult in-hospital cardiac arrest.
Forest plots for survival to hospital discharge/ one month (A), long-term survival (B), favorable neurological outcome at hospital discharge/one month (C), long-term favorable neurological outcome (D), and survival analysis (E) in adult in-hospital cardiac arrest. The vertical red lines indicate odds ratios or hazard ratios. Horizontal lines indicate 95% confidence intervals of the estimate. For the survival analysis (hazard ratios from Cox proportional hazard models) with time-to-death as the outcome, estimates below 1 are in favor of ECPR. The studies are ordered by alphabetical order within each outcome. The forest plots for long-term outcomes are representative of all included patients, independent of survival to hospital discharge. There was some overlap between the studies by Chen and Lin, and between Cho and Shin (2011 + 2013).
IHCA refers to in-hospital cardiac arrest.
Table 2.
| Study | Country | Years of inclusion | Inclusion criteria | Exclusion criteria | Patients analyzed (n) |
|---|---|---|---|---|---|
| Blumenstein et al. [31] | Germany | 2009 – 2013 | Cardiovascular admission, witnessed | Not Reported | 353 |
| Chen et al. [32] | Taiwan | 2004 – 2006 | Age 18–75 years, CPR duration > 10 min, cardiac etiology, witnessed | Previous irreversible brain damage, terminal malignancy, DNR | 92 |
| Cho et al. [33] | Korea | 2001 – 2013 | Pulmonary embolism | Non-survivors of CPR | 20 |
| Chou et al. [34] | Taiwan | 2006 – 2010 | Age >18 years, acute myocardial infarction, CPR > 10 min | Terminal malignancy, previously irreversible brain damage, DNR, ROSC within 10 min | 66 |
| Lin et al. [35] | Taiwan | 2004 – 2006 | Age 18–75 years, cardiac etiology, CPR duration >10 min, ROSC | Not Reported | 54 |
| Shin et al. [36,37] | Korea | 2003 – 2009 | Age 18–80 years, CPR duration > 10 min, witnessed | Previous neurologic damage, intracranial hemorrhage, terminal malignancy, traumatic origin with bleeding, septic origin, organ failure despite maximal therapy, DNR | 120 |
ECPR refers to extracorporeal cardiopulmonary resuscitation, CPR refers to cardiopulmonary resuscitation, DNR refers to do-not-resuscitate, ROSC refers to return of spontaneous circulation.
All studies compared ECPR vs. no ECPR whereas Shin et al. compared ECPR attempt vs. no ECPR attempt.
There was some overlap between the studies by Chen and Lin, and between Cho and Shin (2011 + 2013).
The studies by Shin (2011 + 2013) included the same patient population, but reported different outcomes.
Pediatric in-hospital cardiac arrest
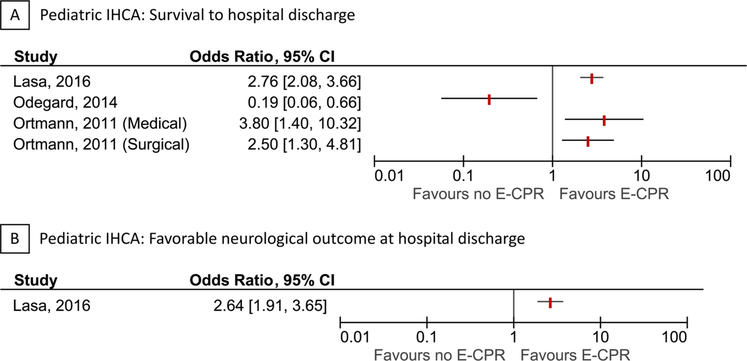
Three of the included studies were in pediatric IHCA [38–40]. All studies were performed in North America, of which two studies [38,40] were from the Get With The Guidelines® registry. Years of patient inclusion ranged from 2000 to 2011. All studies defined the exposure as “ECPR use”. All studies reported survival to hospital discharge, whereas only one study reported favorable neurological outcome at hospital discharge. Favorable neurological outcome was defined as a Pediatric Cerebral Performance Category score of 1–3. Forests plots of each outcome are presented in Fig. 4. Additional details for each individual study are provided in Table 3 and the Supplementary Contents.
Fig. 4.
Forest plots for pediatric in-hospital cardiac arrest.
Forest plots for survival to hospital discharge (A) and favorable neurological outcome at hospital discharge (B) in pediatric in-hospital cardiac arrest. The vertical red lines indicate odds ratios. Horizontal lines indicate 95% confidence intervals of the estimate. The studies are ordered by alphabetical order within each outcome. The 95% confidence interval reported by Ortmann et al. (medical-group) was non-symmetric and therefore re-estimated. There was some overlap between the studies by Lasa et al. and Ortmann et al
IHCA refers to in-hospital cardiac arrest.
Risk of bias for individual studies
The risk of bias within individual studies was judged overall as critical for all studies, with confounding being the primary source. Risk of selection bias was judged to be low for the majority of studies. Few studies were at moderate risk of bias for missing data. The majority of studies did not report any missing data and were therefore classified as low risk of bias, but the risk of bias could also be considered “unknown”. All studies were at moderate risk for selective reporting since none provided a pre-registered protocol. The remaining ROBINS-I domains were all judged to be at low risk of bias. A detailed list of risk of bias assessments is provided in eTable 3 in the Supplementary Contents.
Quality of evidence across studies
The overall quality of evidence across all studies were judged to be of very low quality. GRADE summary tables and additional details are provided in eTable 4–6 in the Supplementary Contents.
Meta-analyses, meta-regression, and publication bias
The critical risk of bias and heterogeneity between studies did not allow for any meaningful meta-analyses. We were not able to conduct meta-regression or test for publication bias because too few studies were identified.
Discussion
In this systematic review, we identified studies comparing the use of ECPR to manual or mechanical CPR for OHCA and IHCA in adult and pediatric patients. We identified 25 observational studies, of which 15 studies were in adult OHCA, 7 studies were in adult IHCA, and 3 studies were in pediatric IHCA. No randomized clinical trials were identified, though several are ongoing as noted on the International Clinical Trials Registry Platform. Results from studies in OHCA were inconsistent. Studies in adult and pediatric IHCA were generally in favor of ECPR, although the risk of bias for individual studies was overall assessed to be critical. The quality of evidence was very low across all outcomes.
The goal of ECPR is to support patients with cardiac arrest by providing time for recovery, diagnostics, and/or treatment of potentially reversible causes. The use of ECPR is complex and requires local expertise, specialized equipment, rigorous patient selection, and careful timing [2,3,6]. The location of cardiac arrest is of particular relevance in this context, since patients who experience OHCA are significantly different from patients who experience IHCA [41–44]. Patients with IHCA tend to have shorter low-flow time and are more likely to have rapid access to a dedicated ECPR response team. While the use of in-hospital extracorporeal life support has increased over the past decade [8–10], ECPR is not readily available for pre-hospital use and patients who experience OHCA are reliant on rapid transportation to ECPR capable hospitals [45].
The included studies were all assessed to have a critical risk of confounding potentially limiting internal validity. First, the final decision to perform ECPR is generally made on a case-by-case basis, which may limit the comparability between those receiving ECPR following a period of CPR and those with no ECPR. The factors driving the decision to use ECPR are based on clinical assessments of the underlying disease, the assumption that conventional CPR will not be effective, and boundaries set by deployment protocols. These factors may be related to outcomes and could therefore bias the results. Second, many studies only reported unadjusted results [16–19,23–25,27–30,34,39] or did not adjust adequately for important confounders. For instance, very few studies accounted for pre-cardiac arrest performance status or activities of daily living [22] and none of the studies adjusted for intra-cardiac arrest variables (e.g., end-tidal CO2, lactate, pH, potassium). In addition, studies accounting for past-medical history [21,31,32,35–38], used crude measurements (e.g., renal disease vs. no renal disease, cardiac disease vs. no cardiac disease), which increases the risk of residual confounding. Third, most studies adjusted for “CPR duration” [20–22,26,31–33,35–38,40]. This is problematic, since “CPR duration” could be a mediator on the causal pathway between ECPR and outcomes [46] and because “CPR duration” is defined differently for patients receiving ECPR (time to ECPR, which was rarely well-defined) and no ECPR (time to ROSC or death). Adjusting for “CPR duration” using traditional methods is therefore likely to introduce biased results, although the direction of this bias can be difficult to predict [47]. Some studies also adjusted for treatments after the cardiac arrest (e.g., targeted temperature management) [20,22,26,36,37], which may bias the results, since these variables cannot be direct confounders of the relationship between ECPR and outcomes [47]. These limitations illustrate the need for rigorous randomized clinical trials or alternative study designs minimizing bias to clarify the role of ECPR in cardiac arrest.
The vast majority of the included studies were single-center studies [17–22,25–37,39], with varying inclusion criteria and settings. Some studies in adult OHCA restricted their inclusion criteria to patients with a witnessed cardiac arrest, very short no-flow times, and/or required a certain duration of conventional CPR prior to ECPR [17,18,22,25,26]. Three studies assessed the availability and/or use of ECPR in the cardiac catheterization laboratory [28–30]. The results of these studies are not easily applicable to other settings. Studies in adult and pediatric IHCA were less diverse, although one adult study restricted inclusion to patients with cardiac arrest caused by acute pulmonary embolism [33]. ECPR technology [1] and costs [48] may also have varied across studies and time. The high-degree of heterogeneity between studies limited our ability to perform meta-analyses and reduced the generalizability of the included studies.
While we report on the use of ECPR in relation to outcomes, we did not evaluate patient selection, indication, and prognostication related to ECPR. A recent position paper by Abrams et al. has highlighted some of these issues, proposing that ECPR may be initiated by rapid-response teams within 15 min of conventional CPR in patients without severe comorbidities [6], although there is little evidence to support such a recommendation. Systematic reviews in IHCA [49] and OHCA [50] recently assessed prognostic factors of favorable outcome in adult patients receiving ECPR. Both reviews found initial shockable rhythms, short low-flow time, and low lactate values at admission to be associated with better outcomes. In the context of resource utilization, we did not identify any cost-effectiveness studies for ECPR specific to cardiac arrest. One study reported hospital costs without performing a cost-effectiveness analysis [51] and two studies conducted cost-effectiveness analyses for ECPR primarily including non-cardiac arrest patients [52,53]. Understanding the clinical benefits of ECPR relative to the resource utilization is particularly important given the recent increased use of ECPR.
Conclusions
There is inconclusive evidence to either support or refute the use of ECPR for OHCA and IHCA in adults and children. The quality of evidence across studies is very low. Future investigations should be cautious of issues related to internal validity. Randomized clinical trials are needed to better inform clinical practice.
Supplementary Material
Acknowledgements
The authors would like to thank Teruko Kishibe, St. Michael’s Hospital, Toronto, ON, Canada, for preparing and conducting the systematic searches.
Conflicts of interest
None of the authors have any conflicts of interest to report. Dr. Andersen was compensated by the American Heart Association on behalf of ILCOR for his work related to this systematic review. Dr. Deakin is the ILCOR domain lead for “Defibrillation”, a member of the ERC and Treasure of the Resuscitation Council (UK). Dr. Guerguerian is the vicechair of the Pediatric Task Force for Get With The Guidelines. Dr. Donnino is supported by grant K24HL127101 and R01HL136705 from the National Heart, Lung, and Blood Institute.
Footnotes
International Liaison Committee On Resuscitation Task Force Investigators
Besides the authors Michael W. Donnino, Charles Deakin, Lars W. Andersen, and Jerry Nolan, members of the International Liaison Committee on Resuscitation Advanced Life Support Task Force include: Jasmeet Soar, Clifton Callaway, Bernd Boettiger, Tonia Nicholson, Edison Paiva, Michael Parr, Tzong-Luen Wang, Brian O’Neil, Peter Morley, Katherine Berg, Michelle Welsford, Ian Drennan, Joshua Reynolds, Robert Neumar, and Claudio Sandroni.
Besides the author Anne-Marie Guerguerian, members of the International Liaison Committee on Resuscitation Pediatric Task Force include: Ian Maconochie, Richard Aickin, Allan de Caen, Dianne Atkins, Peter Meaney, Kee-Chong Ng, Gabrielle Nuthall, Amelia Reis, Naoki Shimizu, Vinay Nadkarni, Robert Bingham, Janice Tijssen, Yong-Kwang Gene Ong, Thomaz Bittencourt Couto, Steve Schexnayder, Patrick Van de Voorde, Mary Fran Hazinski, Eric Layonas, Shinichiro Ohshimo, and Barney Scholefield.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resuscitation.2018.07.029.
References
- [1].Conrad SA, Broman LM, Taccone FS, Lorusso R, Malfertheiner MV, Pappalardo F, et al. The extracorporeal life support organization Maastricht treaty for nomenclature in extracorporeal life support. A position paper of the extracorporeal life support organization. Am J Respir Crit Care Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132(18 Suppl. 2):S444–64. [DOI] [PubMed] [Google Scholar]
- [3].de Caen AR, Berg MD, Chameides L, Gooden CK, Hickey RW, Scott HF, et al. Part 12: pediatric advanced life support: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132(18 Suppl. 2):S526–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Soar J, Nolan JP, Böttiger BW, Perkins GD, Lott C, Carli P, et al. European Resuscitation Council Guidelines for resuscitation 2015: section 3. Adult advanced life support. Resuscitation 2015;95:100–47. [DOI] [PubMed] [Google Scholar]
- [5].Maconochie IK, Bingham R, Eich C, López-Herce J, Rodríguez-Núñez A, Rajka T, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 6. Paediatric life support. Resuscitation 2015;95:223–48. [DOI] [PubMed] [Google Scholar]
- [6].Abrams D, Garan AR, Abdelbary A, Bacchetta M, Bartlett RH, Beck J, et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med 2018. [DOI] [PubMed] [Google Scholar]
- [7].Riggs KR, Becker LB, Sugarman J. Ethics in the use of extracorporeal cardiopulmonary resuscitation in adults. Resuscitation 2015;91:73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Richardson AS, Schmidt M, Bailey M, Pellegrino VA, Rycus PT, Pilcher DV. ECMO Cardio-Pulmonary Resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation 2017;112:34–40. [DOI] [PubMed] [Google Scholar]
- [9].McCarthy FH, McDermott KM, Kini V, Gutsche JT, Wald JW, Xie D, et al. Trends in U.S. Extracorporeal membrane oxygenation use and outcomes: 2002–2012. Semin Thorac Cardiovasc Surg 2015;27(2):81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Paden ML, Rycus PT, Thiagarajan RR, Registry E. Update and outcomes in extracorporeal life support. Semin Perinatol 2014;38(2):65–70. [DOI] [PubMed] [Google Scholar]
- [11].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22(3):276–82. [PMC free article] [PubMed] [Google Scholar]
- [13].Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. 2011. Version 5.1.0. [Updated March 2011].
- [15].Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Agostinucci JM, Ruscev M, Galinski M, Gravelo S, Petrovic T, Carmeaux C, et al. Out-of-hospital use of an automated chest compression device: facilitating access to extracorporeal life support or non-heart-beating organ procurement. Am J Emerg Med 2011;29(9):1169–72. [DOI] [PubMed] [Google Scholar]
- [17].Cesana F, Avalli L, Garatti L, Coppo A, Righetti S, Calchera I, et al. Effects of extracorporeal cardiopulmonary resuscitation on neurological and cardiac outcome after ischaemic refractory cardiac arrest. Eur Heart J Acute Cardiovasc Care 2017. p. [DOI] [PubMed] [Google Scholar]
- [18].Choi DH, Kim YJ, Ryoo SM, Sohn CH, Ahn S, Seo DW, et al. Extracorporeal cardiopulmonary resuscitation among patients with out-of-hospital cardiac arrest. Clin Exp Emerg Med 2016;3(3):132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hase M, Tsuchihashi K, Fujii N, Nishizato K, Kokubu N, Nara S, et al. Early defibrillation and circulatory support can provide better long-term outcomes through favorable neurological recovery in patients with out-of-hospital cardiac arrest of cardiac origin. Circ J 2005;69(11):1302–7. [DOI] [PubMed] [Google Scholar]
- [20].Kim SJ, Jung JS, Park JH, Park JS, Hong YS, Lee SW. An optimal transition time to extracorporeal cardiopulmonary resuscitation for predicting good neurological outcome in patients with out-of-hospital cardiac arrest: a propensity-matched study. Crit Care 2014;18(5):535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee SH, Jung JS, Lee KH, Kim HJ, Son HS, Sun K. Comparison of extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: is extracorporeal cardiopulmonary resuscitation beneficial? Korean J Thorac Cardiovasc Surg 2015;48(5):318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Maekawa K, Tanno K, Hase M, Mori K, Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med 2013;41(5):1186–96. [DOI] [PubMed] [Google Scholar]
- [23].Poppe M, Weiser C, Holzer M, Sulzgruber P, Datler P, Keferbock M, et al. The incidence of “load&go” out-of-hospital cardiac arrest candidates for emergency department utilization of emergency extracorporeal life support: a one-year review. Resuscitation 2015;91:131–6. [DOI] [PubMed] [Google Scholar]
- [24].Sakamoto T, Morimura N, Nagao K, Asai Y, Yokota H, Nara S, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation 2014;85(6):762–8. [DOI] [PubMed] [Google Scholar]
- [25].Schober A, Sterz F, Herkner H, Wallmueller C, Weiser C, Hubner P, et al. Emergency extracorporeal life support and ongoing resuscitation: a retrospective comparison for refractory out-of-hospital cardiac arrest. Emerg Med J 2017;34(5):277–81. [DOI] [PubMed] [Google Scholar]
- [26].Siao FY, Chiu CC, Chiu CW, Chen YC, Chen YL, Hsieh YK, et al. Managing cardiac arrest with refractory ventricular fibrillation in the emergency department: conventional cardiopulmonary resuscitation versus extracorporeal cardiopulmonary resuscitation. Resuscitation 2015;92:70–6. [DOI] [PubMed] [Google Scholar]
- [27].Tanno K, Itoh Y, Takeyama Y, Nara S, Mori K, Asai Y. Utstein style study of cardiopulmonary bypass after cardiac arrest. Am J Emerg Med 2008;26(6):649–54. [DOI] [PubMed] [Google Scholar]
- [28].Venturini JM, Retzer E, Estrada JR, Friant J, Beiser D, Edelson D, et al. Mechanical chest compressions improve rate of return of spontaneous circulation and allow for initiation of percutaneous circulatory support during cardiac arrest in the cardiac catheterization laboratory. Resuscitation 2017;115:56–60. [DOI] [PubMed] [Google Scholar]
- [29].Yannopoulos D, Bartos JA, Martin C, Raveendran G, Missov E, Conterato M, et al. Minnesota resuscitation consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-Hospital refractory ventricular fibrillation. J Am Heart Assoc 2016;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yannopoulos D, Bartos JA, Raveendran G, Conterato M, Frascone RJ, Trembley A, et al. Coronary artery disease in patients with out-of-Hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol 2017;70(9):1109–17. [DOI] [PubMed] [Google Scholar]
- [31].Blumenstein J, Leick J, Liebetrau C, Kempfert J, Gaede L, Gross S, et al. Extracorporeal life support in cardiovascular patients with observed refractory in-hospital cardiac arrest is associated with favourable short and long-term outcomes: a propensity-matched analysis. Eur Heart J Acute Cardiovasc Care 2016;5(7):13–22. [DOI] [PubMed] [Google Scholar]
- [32].Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008;372(9638):554–61. [DOI] [PubMed] [Google Scholar]
- [33].Cho YH, Kim WS, Sung K, Jeong DS, Lee YT, Park PW, et al. Management of cardiac arrest caused by acute massive pulmonary thromboembolism: importance of percutaneous cardiopulmonary support. ASAIO J 2014;60(3):280–3. [DOI] [PubMed] [Google Scholar]
- [34].Chou TH, Fang CC, Yen ZS, Lee CC, Chen YS, Ko WJ, et al. An observational study of extracorporeal CPR for in-hospital cardiac arrest secondary to myocardial infarction. Emerg Med J 2014;31(6):441–7. [DOI] [PubMed] [Google Scholar]
- [35].Lin JW, Wang MJ, Yu HY, Wang CH, Chang WT, Jerng JS, et al. Comparing the survival between extracorporeal rescue and conventional resuscitation in adult in-hospital cardiac arrests: propensity analysis of three-year data. Resuscitation 2010;81(7):796–803. [DOI] [PubMed] [Google Scholar]
- [36].Shin TG, Choi JH, Jo IJ, Sim MS, Song HG, Jeong YK, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med 2011;39(1):1–7. [DOI] [PubMed] [Google Scholar]
- [37].Shin TG, Jo IJ, Sim MS, Song YB, Yang JH, Hahn JY, et al. Two-year survival and neurological outcome of in-hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int J Cardiol 2013;168(4):3424–30. [DOI] [PubMed] [Google Scholar]
- [38].Lasa JJ, Rogers RS, Localio R, Shults J, Raymond T, Gaies M, et al. Extracorporeal Cardiopulmonary Resuscitation (E-CPR) during pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge: a report from the American Heart Association’s Get With The Guidelines-Resuscitation (GWTG-R) registry. Circulation 2016;133(2):165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Odegard KC, Bergersen L, Thiagarajan R, Clark L, Shukla A, Wypij D, et al. The frequency of cardiac arrests in patients with congenital heart disease undergoing cardiac catheterization. Anesth Analg 2014;118(1):175–82. [DOI] [PubMed] [Google Scholar]
- [40].Ortmann L, Prodhan P, Gossett J, Schexnayder S, Berg R, Nadkarni V, et al. Outcomes after in-hospital cardiac arrest in children with cardiac disease: a report from Get With the Guidelines–Resuscitation. Circulation 2011;124(21):2329–37. [DOI] [PubMed] [Google Scholar]
- [41].Moler FW, Meert K, Donaldson AE, Nadkarni V, Brilli RJ, Dalton HJ, et al. Inhospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med 2009;37(7):2259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fredriksson M, Aune S, Bång A, Thorén AB, Lindqvist J, Karlsson T, et al. Cardiac arrest outside and inside hospital in a community: mechanisms behind the differences in outcome and outcome in relation to time of arrest. Am Heart J 2010;159(5):749–56. [DOI] [PubMed] [Google Scholar]
- [43].Engsig M, Søholm H, Folke F, Gadegaard PJ, Wiis JT, Molin R, et al. Similar long-term survival of consecutive in-hospital and out-of-hospital cardiac arrest patients treated with targeted temperature management. Clin Epidemiol 2016;8:761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moskowitz A, Holmberg MJ, Donnino MW, Berg KM. In-hospital cardiac arrest: are we overlooking a key distinction? Curr Opin Crit Care 2018;24(3):151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Singer B, Reynolds JC, Lockey DJ, O’Brien B. Pre-hospital extra-corporeal cardiopulmonary resuscitation. Scand J Trauma Resusc Emerg Med 2018;26(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Andersen LW, Grossestreuer AV, Donnino MW. “Resuscitation time bias”-a unique challenge for observational cardiac arrest research. Resuscitation 2018;125:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20(4):488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Harvey MJ, Gaies MG, Prosser LA. U.S. and International In-Hospital Costs of Extracorporeal Membrane Oxygenation: a Systematic Review. Appl Health Econ Health Policy 2015;13(4):341–57. [DOI] [PubMed] [Google Scholar]
- [49].D’Arrigo S, Cacciola S, Dennis M, Jung C, Kagawa E, Antonelli M, et al. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Resuscitation 2017;121:62–70. [DOI] [PubMed] [Google Scholar]
- [50].Debaty G, Babaz V, Durand M, Gaide-Chevronnay L, Fournel E, Blancher M, et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation 2017;112:1–10. [DOI] [PubMed] [Google Scholar]
- [51].Yuan-Hsi T, Meng-Yu W, Feng-Chun T, Hai-Jing C, Pyng Jing L. Costs associated with extracorporeal life support used in adults: a single-center study. Acta Cardiol Sin 2011;27:221–8. [Google Scholar]
- [52].St-Onge M, Fan E, Mégarbane B, Hancock-Howard R, Coyte PC. Venoarterial extracorporeal membrane oxygenation for patients in shock or cardiac arrest secondary to cardiotoxicant poisoning: a cost-effectiveness analysis. J Crit Care 2015;30(2). p. 437. [DOI] [PubMed] [Google Scholar]
- [53].Mahle WT, Forbess JM, Kirshbom PM, Cuadrado AR, Simsic JM, Kanter KR. Costutility analysis of salvage cardiac extracorporeal membrane oxygenation in children. J Thorac Cardiovasc Surg 2005;129(5):1084–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.