Abstract
Copper homeostasis in pathogenic bacteria is critical for cuproprotein assembly and virulence. However, in vivo biochemical analyses of these processes are challenging, which has prevented defining and quantifying the homeostatic interplay between Cu+-sensing transcriptional regulators, chaperones, and sequestering molecules. The cytoplasm of Pseudomonas aeruginosa contains a Cu+-sensing transcriptional regulator, CueR, and two homologous metal chaperones, CopZ1 and CopZ2, forming a unique system for studying Cu+ homeostasis. We found here that both chaperones exchange Cu+, albeit at a slow rate, reaching equilibrium after 3 h, a time much longer than P. aeruginosa duplication time. Therefore, they appeared as two separate cellular Cu+ pools. Although both chaperones transferred Cu+ to CueR in vitro, experiments in vivo indicated that CopZ1 metallates CueR, eliciting the translation of Cu+ efflux transporters involved in metal tolerance. Although this observation was consistent with the relative Cu+ affinities of the three proteins (CopZ1 < CueR < CopZ2), in vitro and in silico analyses also indicated a stronger interaction between CopZ1 and CueR that was independent of Cu+. In contrast, CopZ2 function was defined by its distinctly high abundance during Cu2+ stress. Under resting conditions, CopZ2 remained largely in its apo form. Metal stress quickly induced CopZ2 expression, and its holo form predominated, reaching levels commensurate with the cytoplasmic Cu+ levels. In summary, these results show that CopZ1 acts as chaperone delivering Cu+ to the CueR sensor, whereas CopZ2 functions as a fast-response Cu+-sequestering storage protein. We propose that equivalent proteins likely play similar roles in most bacterial systems.
Keywords: copper, Pseudomonas, homeostasis, metal ion-protein interaction, metalloprotein, metal sensor, metallochaperone, stress response, transcriptional regulation
Introduction
Copper is a micronutrient required as a redox cofactor by multiple enzymes (e.g. Cu-superoxide dismutases, cytochrome oxidases, etc.) (1–4). Nevertheless, free Cu+/2+ is toxic as it disrupts Fe-S centers and generates free radicals (5, 6). Copper antibacterial properties and the role in innate immunity are the direct consequence of this cellular toxicity (7, 8). These deleterious cellular effects have enabled the identification of molecules conferring tolerance to Cu+/2+ in bacterial systems (1, 9, 10). These include cytoplasmic metal sensing transcriptional regulators, chaperone proteins, and transmembrane efflux systems. In vitro biochemical studies have shown high affinity Cu+ bind to these proteins leading to the virtual absence of unbound metal with Cu+ movement via ligand exchange among interacting proteins (1, 9, 11, 12). These observations have not, however, produced an integrated description of the molecular interplay leading to cellular Cu+/2+ homeostasis. This is, there is a conceptual gap between the phenotypical observations (effect of Cu+/2+ on cell growth rate) and the biochemical characterization of isolated molecules. Consider for instance, the limited information on how Cu+ reaches compartmentally restricted target cupro-proteins or that no plasma membrane transporters enabling Cu+/2+ influx have been characterized, except for CcoA that provides copper for cytochrome c oxidase assembly (13). Further complicating the analysis, Cu+ distribution/sensing molecules are not ubiquitous, as different bacterial species have solved Cu+/2+ tolerance using alternative strategies (1, 7, 9, 14).
Assuming that copper homeostasis is enabled by an integrated molecular system distributing Cu+ to various targets, we characterized this molecular network in Pseudomonas aeruginosa under nondeleterious extracellular Cu2+ stress. This is, the system was studied under steady state conditions where Cu+ influx is equal to efflux, cells have the capacity to sequester cytoplasmic Cu+ excess, and there is no change on cellular growth rate (15). Genome-wide transcriptomic analysis revealed the presence of cytoplasmic (CueR) and periplasmic (CopS/R) Cu+ sensing regulators, their corresponding regulons, multiple metal chaperones, and specific Cu+ efflux and influx systems bridging the membranes separating cellular compartments (15). This was later complemented by mathematical simulation of fluxes and compartmental pools describing the experimental metal uptake kinetics (16). These computational models support novel homeostatic elements revealed by the architecture of the CueR and CopS/R regulons; for instance, the participation of the CusCBA system mobilizing cytoplasmic Cu+, or the significant role that periplasmic proteins might play in the response to external Cu2+. However, whereas these approaches gave an initial picture of the homeostatic network, they provided little information on the intra-compartmental metal distribution. How is Cu+ exchanged among soluble proteins in each compartment? Which molecules chaperone Cu+ and interact with the alternative transporters? How is the measured excess Cu+ stored under steady state conditions?
Considering the fate of Cu+ in P. aeruginosa cytoplasm, the interplay of CueR and the two identified cytoplasmic metal chaperones appears key for the metal distribution. CueR is a member of the MerR family (17, 18). It forms homodimers with three domains: a N-terminal DNA-binding domain, a central dimerization helix, and a C-terminal region where two cysteines are responsible for metal binding (12, 17). CueR homologs bind Cu+ with 10−19–10−21 m affinities (12, 19) and both forms of the regulator, CueRapo and CueRholo, bind the cognate promoter regions (20, 21). The high binding affinity of the sensor has led to assume that there is no “free” cytoplasmic Cu+ (12). Quite relevant, it is unknown how CueR acquires the metal. Although CopZ-like Cu+ chaperones appear to deliver the metal to other transcriptional regulators such as CopY (22–24) and CsoR (25). Interestingly, P. aeruginosa genome encodes for two cytoplasmic Cu+ chaperones, CopZ1 and CopZ2 (15). Both are under control of CueR, binding Cu+ with 1:1 stoichiometry and high affinity, CopZ1 4 × 10−15 m and CopZ2 8 × 10−17 m (15). This multiplicity of copper chaperones has been also observed in Streptomyces lividans that has four CopZ-like Cu+ chaperones (25). Even though their Cu+ affinities have been determined (CopZ-1317 10−17 m and CopZ-3079 10−18 m), their possible function is obscure as they both appear to interact similarly with CsoR. A bioinformatic approach has also shown the presence of various CopZ homologous proteins in some Rhizobiales species and a correlation with multiple Cu+-ATPases encoded in the genomes was observed (26).
In this study, we report the distinct roles of P. aeruginosa Cu-chaperones, CopZ1 and CopZ2. In vitro and in vivo studies show that CopZ1 delivers metal to CueR via protein-protein interactions; whereas the more abundant CopZ2 serves as the Cu+ storage pool providing a fast-homeostatic response to high Cu+ level conditions. These properties provide a mechanistical model to explain the various equilibria among cytoplasmic Cu+-binding proteins and a novel strategy for Cu+ tolerance via metal binding to a cytoplasmic protein.
Results
CopZ1 and CopZ2 have distinct roles in P. aeruginosa
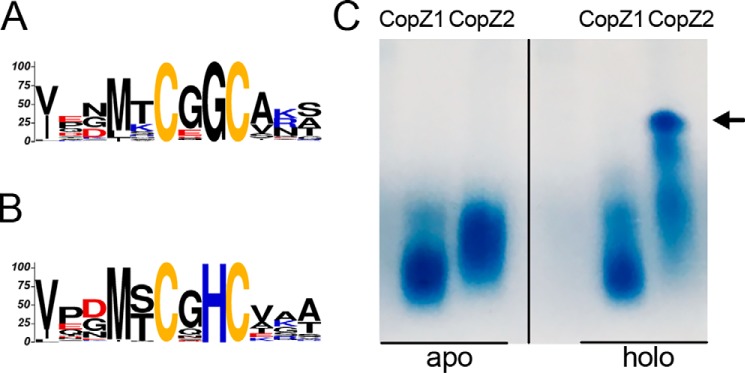
The structural similarities between CopZ1 and CopZ2, sharing 37% sequence identity and the invariant CXXC Cu-binding motif, might suggest an analogous functional role. Analysis of 896 homologous sequences shows that these can be divided in two subgroups of chaperones. The CopZ1-like subgroup (544 sequences), including all eukaryotic chaperones, shows a conserved CXGC sequence in the metal-binding region (Fig. 1A). Instead, CopZ2-like proteins (352 sequences) are only present in prokaryotes and the Cu+-binding loop has an invariant His (MXCXHC) (Fig. 1B). This His is likely responsible for the higher Cu+ affinity observed in P. aeruginosa CopZ2 (15), as it has been shown that its removal decreases S. lividans CopZs affinity for Cu+ (25). Moreover, the His is probably determinant of their different behavior in the presence of Cu+ where CopZ2 (but not CopZ1) forms Cu+-mediated multimeric structures even with equimolar Cu+ as observed in native PAGE gels (Fig. 1C). It is known that in various conditions, chaperones form protein clusters binding multiple Cu+ ions (27–31). However, it is not our goal here to analyze these structures, but rather point out the different structural and Cu+-binding properties that might lead to the alternative roles shown below.
Figure 1.
Structural differences of CopZ1 and CopZ2. Conserved Cu+ binding motifs of (A) CopZ1-like and (B) CopZ2-like proteins. C, native PAGE gel of purified CopZ1 and CopZ2 in the absence (left) and presence of equimolar amounts of Cu+ (right). The vertical dividing line in panel C indicates where the image has been spliced; all signals were from an identical original image and have not been altered. Arrow indicates multimeric structures of CopZ2.
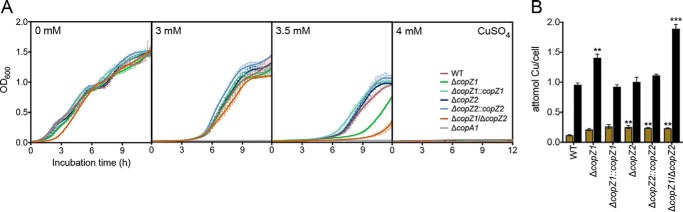
Searching for biological evidences of their distinct functions, we examined the contributions of P. aeruginosa CopZ1 and CopZ2 to the overall cellular tolerance to Cu+ stress. Strains carrying deletion mutants in the coding genes (ΔcopZ1, ΔcopZ2, and ΔcopZ1/ΔcopZ2) were grown in LB medium containing various Cu2+ concentrations (Fig. 2A). The P. aeruginosa ΔcopA1 mutant strain lacking the Cu+-ATPase responsible for cytoplasmic Cu+ efflux was included as a control (32). In the absence of added metal, the growth rate of all strains was similar to that of the wildtype (WT) cells. No significant differences were detected in the growth rate of ΔcopZ1 and ΔcopZ2 in the presence of low 0–3 mm Cu2+. However, the ΔcopZ1 mutant was more susceptible to metal toxicity at higher, 3.5 mm Cu2+ levels. A slightly more pronounced phenotype was observed in the double mutant ΔcopZ1/ΔcopZ2, whereas the susceptibility to Cu2+ was abrogated in the ΔcopZ1::copZ1-complemented strain. This altered tolerance to Cu2+ in ΔcopZ1 was similar to that observed for deletion mutants of homologous genes in Bacillus subtilis, Agrobacterium tumefaciens, and Enterococcus hirae (22, 33–35); albeit, mutation of CopZ in Listeria monocytogenes and Pseudomonas fluorescens did not lead to a diminished metal tolerance (36, 37). The distinct growth phenotypes of ΔcopZ1 and ΔcopZ2 under Cu2+ stress were supported by determinations of whole cell Cu levels (Fig. 2B). Treatment of cells for 10 min with 0.5 mm CuSO4 was chosen to examine effects on the overall Cu homeostasis. We have shown that these conditions raise cellular Cu but do not affect cellular growth rates (15, 16). Fig. 2B shows that all mutant stains presented small increments in basal Cu levels. More relevant, both ΔcopZ1 and ΔcopZ1/ΔcopZ2 mutant strains showed significantly higher levels of cellular Cu upon exposure to CuSO4. On the other hand, the ΔcopZ2 mutation did not affect the cell copper levels paralleling its lack of growth phenotype. These differences in Cu2+ tolerance and cellular metal levels observed in ΔcopZ1 and ΔcopZ2 strains point out distinct functional roles for the corresponding proteins.
Figure 2.
Contribution of CopZ1 and CopZ2 to P. aeruginosa Cu+ tolerance and metal content. A, growth rate of WT, ΔcopZ1, ΔcopZ2, ΔcopZ1/ΔcopZ2, ΔcopA1, as well as copZ1 and copZ2 complemented strains in the presence of 0–4 mm CuSO4. B, intracellular Cu levels before (ochre) and after (black) 10 min exposure to 0.5 mm CuSO4. Data are the mean ± S.E. of three independent experiments. Significant differences from values in the WT strain as determined by unpaired two-tailed Student's t test are: **, p < 0.01, ***, p < 0.001.
Cu+ exchange among CopZ1 and CopZ2
Cu+ transfer between homologous CopZ proteins and the structurally similar N-terminal metal-binding domains present in Cu+-ATPases is well characterized (38–40). This hinted at a possible Cu+ exchange among CopZ1 and CopZ2; although, the large difference in their Cu+ affinities might limit metal transfer from CopZ2 to CopZ1 (CopZ2 KD = 8 × 10−17 m; CopZ1 KD = 4 × 10−15 m (15)). To explore the CopZs/Cu+ exchange, we performed bidirectional in vitro Cu+ transfer assays. This is a CopZ was loaded with equimolar amounts of Cu+, incubated in the presence of its apo homolog, separated with affinity Ni-NTA2 resin, and Cu+ associated with each CopZ measured. All assays were performed in the presence of reducing 10 mm ascorbic acid. Fig. 3A shows that CopZ1holo transferred bound Cu+, although not all, to CopZ2apo. Alternatively, as expected, a much limited transfer was apparent from CopZ2holo to CopZ1apo (Fig. 3B). In both cases, control experiments performed in the absence of acceptor protein show that <5% of Cu+ was dissociated from the holo donor after a 10-min reaction (Fig. 3, A and B). The Cu+-exchange was not due to dissociation from donor and subsequent binding to acceptor. Although these experiments show a predominant transfer of Cu+ from CopZ1 to CopZ2, it was apparent that equilibrium was not reached under the experimental conditions. The ratio of the CopZ1/CopZ2 Cu+ binding KD yields a Keq = 42; yet, if the levels of the CopZ1apo/holo and CopZ2apo/holo at the end of the 10-min exchange were considered, a reaction quotient smaller than that expected from the Keq was observed (Fig. 3C). Alternatively, longer Cu+ exchange experiments showed that after a 3-h incubation, the reaction quotient for the exchange reaction reached a value of 53, quite close to the calculated Keq. These data clearly show the anticipated Cu+ exchange and that the predicted equilibrium is eventually reached. This indicates that at equilibrium the CopZ2holo predominates over CopZ1holo. Perhaps more important, the data reveals a quite slow Cu+ exchange. Considering the P. aeruginosa duplication time of 25–35 min in LB media (41), it is apparent that the CopZ1/CopZ2 metal exchange would not reach equilibrium and that both chaperones would function as relatively independent Cu+ pools.
Figure 3.
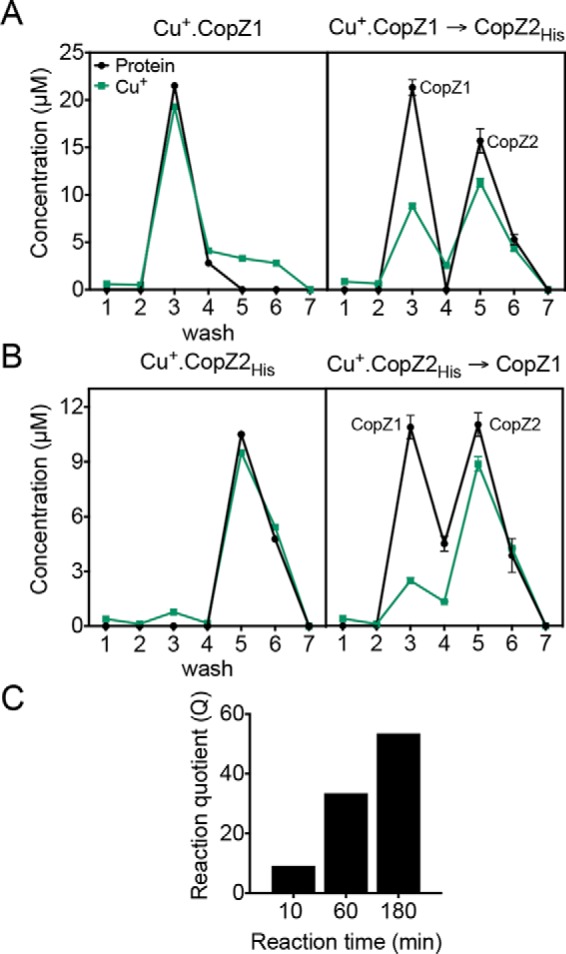
Cu+ exchange among CopZ1 and CopZ2. A and B, protein (black) and Cu+ (green) in the washes and eluates from Ni-NTA resin (wash 1–4, elution 5–7). A, Cu+ transfer from CopZ1holo in the absence (left) and presence of CopZ2apo (right); and B, Cu+ transfer from CopZ2holo in the absence (left) and presence of CopZ1apo (right). Data are the mean ± S.E. of three independent experiments. C, Cu+ transfer reaction from CopZ1holo to CopZ2apo after various incubation times expressed as a reaction quotient Q = ([CopZ1apo] × [CopZ2holo] × [CopZ1holo]−1 × [CopZ2apo]−1) for CopZ1holo + CopZ2apo ⇋ CopZ1apo + CopZ2holo.
CueR receives Cu+ from both CopZ1 and CopZ2 in vitro
Cu+ transfer from CopZ-like chaperones to CueR has been assumed but not experimentally demonstrated; although chaperones supply the metal to other transcriptional regulators (22–25). This hypothesis was tested by measuring Cu+ transfer from the holo forms of CopZ1 and CopZ2 to CueRapo. P. aeruginosa CueR was heterologously expressed and affinity purified. A limited fraction (10–12%) of the resulting protein contained bound Cu+ that could not be removed by extensive treatment with various chelators. The purified protein showed a high affinity for Cu+ (KD = 2.5 ± 1.0 × 10−16 m) (Fig. S1). Noticeably, this constant is lower than that reported for Escherichia coli CueR (10−21 m) or Salmonella enterica CueR (3 × 10−19 m) and closer to that observed for other Cu+ sensors like Streptococcus pneumoniae CopY (10−17 m) or Mycobacterium tuberculosis (10−18 m) (12, 19, 42, 43). However, when compared in the context of CopZ1.Cu+ and CopZ2.Cu+ KD values, the halfway CueR KD hinted to possible different interactions with each chaperone.
Fig. 4A shows that both CopZ1holo and CopZ2holo deliver Cu+ to CueRapo. However, CopZ1 appeared to transfer larger amounts of Cu+ to CueR, with a larger fraction of CopZ2 remaining in the holo form after the assay (CopZ1holo 27 ± 1%; CopZ2 63 ± 1%; p < 0.03). As in the CopZ1/CopZ2 exchange experiments (Fig. 3), no free Cu+ was detected in eluates from columns loaded with CopZ1holo or CopZ2holo in the absence of CueR (not shown). It has been shown that both CueRapo and CueRholo bind with similar dissociation constants to the promoter region of genes under its control (44). Exploring the effect of DNA binding on the CueR-CopZs interactions, the Cu+ transfer from CopZ1holo or CopZ2holo to CueRapo bound to the promoter region of copZ2 (PcopZ2) (Table S1) was also examined. The interaction of CueRapo-PcopZ2 was confirmed using an electrophoretic mobility shift assay (Fig. S2). Again, both chaperones delivered Cu+ to the sensor and the transfer was not significantly different from the experiments in the absence of DNA (Fig. 4B). These results point out that the activation of CueR occurs via direct Cu+ transfer from the chaperones. Moreover, the data agree with the relative affinities of the three molecules for Cu+ (CopZ2 > CueR > CopZ1).
Figure 4.
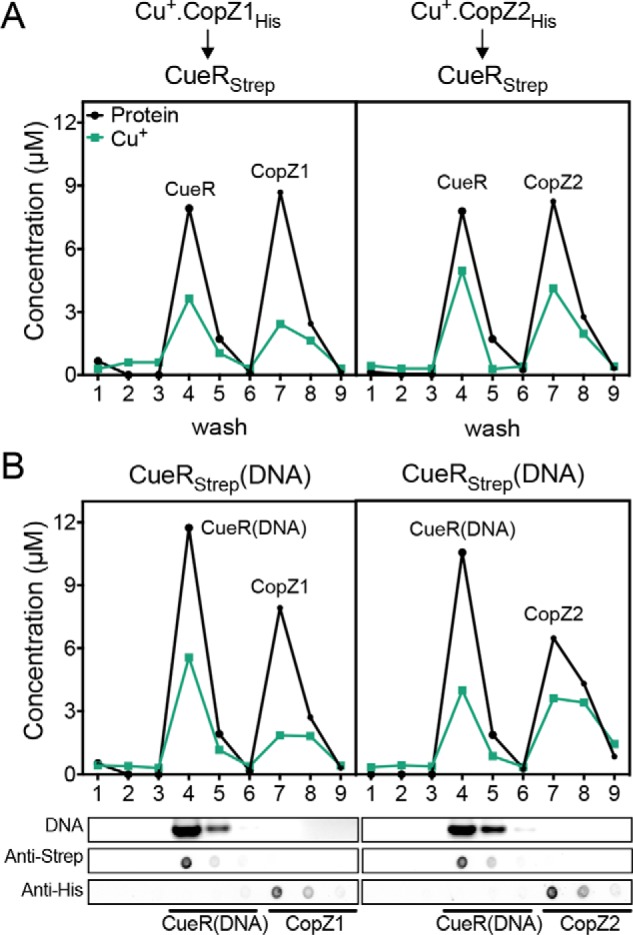
Cu+ transfer from CopZsholo to CueRapo. Experiments were performed in the absence (A) and presence (B) of PcopZ2. Data are from a representative Cu+ transfer experiment from CopZ1holo (left) and CopZ2holo (right). Protein (black) and Cu+ (green) contents are shown in washes and eluates from Ni-NTA resin (wash 1–6, elution 7–9). Lower panels show fractions analyzed for DNA content (top panel), Strep-tagged CueR (second panel), and His-tagged CopZs (bottom panel).
Stronger interaction of CueR with CopZ1
It has been shown that movement of Cu+ within a Cu+ homeostatic network occurs via highly specific protein-protein interactions ensuring the absence of free Cu+ (1, 22–25, 45). Exploring whether the metal transfer from chaperones to CueR is governed by structural aspects and metal affinities, we investigated the interaction of chaperones with CueR in the absence of metal. Co-purification of the proteins at increasing CueR:CopZs ratios showed a stronger interaction of CopZ1 with the sensor (Fig. 5), in agreement with the larger Cu+ transfer from CopZ1 to CueR (Fig. 4). This evidence was further supported by in silico docking experiments. These required modeling the P. aeruginosa molecules using structures of homologous proteins as templates. The available E. coli CueRapo structure lacks the metal-binding Cys in the C-terminal end of the protein and was not suitable for these studies (46). Consequently, CueRholo was selected as docking receptor, whereas apo forms of CopZs were used as ligands. E. coli CueRholo was used to model P. aeruginosa CueR; whereas S. typhimurium GolBapo and the Thermus thermophilus CopZapo served as templates of CopZ1 and CopZ2, respectively. It could be argued that the relevant dockings to consider were that of the holo forms of CopZs with the CueRapo. However, the similarities of the holo and apo CopZs structures suggested that the calculated interactions would be quite similar for both forms (r.m.s. deviations between GolBapo (4Y2K) and GolBholo (4Y2I) is 0.094 Å (47)). Furthermore, considering that only the protein-protein interactions would be analyzed, the metal ion was likely to play a minor role.
Figure 5.
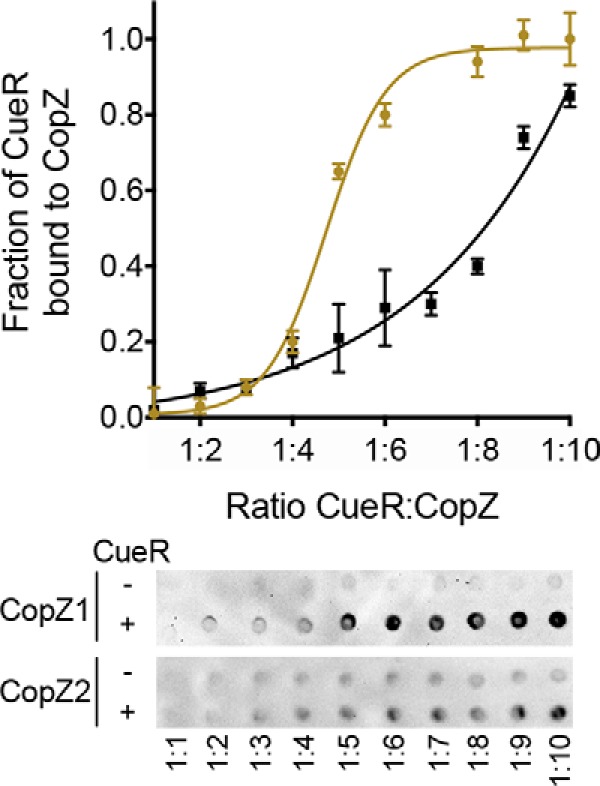
In vitro interaction between CopZs and CueR. Co-purification of isolated proteins by Strep-Tactin®XT batch affinity chromatography using 10 μm Strep-tagged CueRapo dimer and varying concentrations of His-tagged CopZsapo (10–100 μm). His-tagged proteins co-purified with CueR were immunostained to calculate their relative abundance as a measure of the fraction of CueR bound to CopZs (CopZ1, ochre, and CopZ2, black). Curves were fitted to a sigmoidal equation giving a K½ value of 1:4.73 ± 0.13 for CopZ1 and ≥1:9 for CopZ2 (units are CueR:CopZ ratios). Data are the mean of two replicates of an immunostaining representative from three independent experiments.
Docking simulations were restricted to a distance range of <10 Å between metal-binding Cys in the chaperones and CueR. The best docking solutions were selected by applying a maximum 2 Å r.m.s. deviation cut off, clustered, and ranked according to the HADDOCK score (Table S2) (48). This initial analysis clearly showed that the CueRholo-CopZ1apo pair conformers presented lower bonding (electrostatic and desolvation) energies when compared with the CueRholo-CopZ2apo. Although these results indicated a more stable CueR-CopZ1 interaction, docking energies cannot be directly linked to binding free energies (48). Toward obtaining a more conclusive evaluation, the interacting residues at the protein interfaces (10 Å cut off) in each cluster were selected, their r.m.s. deviations were calculated and plotted against the HADDOCK score. Fig. 6A shows that in general CueRholo-CopZ1apo clusters of docking solutions had much lower i-l − r.m.s. deviations than the CueRholo-CopZ2apo clusters. This is, the proteins were closer, and less disperse, in the CueRholo-CopZ1apo interactions. Moreover, if only those clusters statistically significant are considered (Clusters 1 and 10 Table S2, Fig. 6A, red dots), the strength of the CueRholo-CopZ1apo interaction compared with CueRholo-CopZ2apo become more evident.
Figure 6.
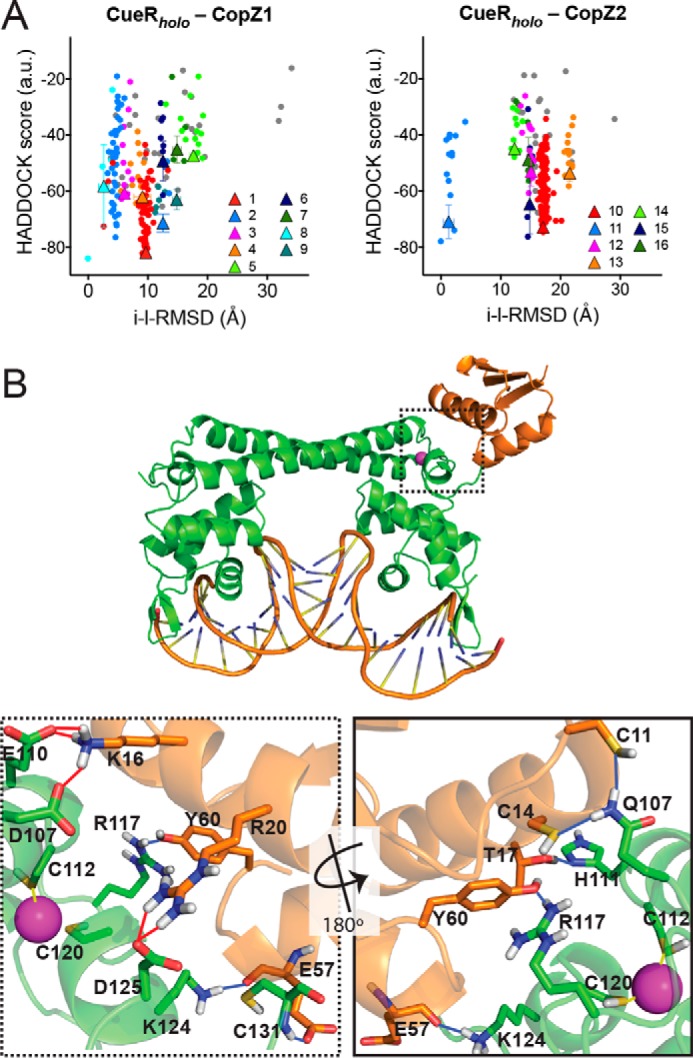
In silico CopZs interaction with CueR. A, intermolecular docking scores as a function of the interface-ligand r.m.s. deviations for residues in the intermolecular contact area (10 Å cutoff). Individual clusters numbered as shown in Table S2 are indicated in various colors. Conformations that did not fit in any cluster are shown as gray dots. The clusters averages are indicated as triangles with error bars. B, CueRholo-CopZ1apo interaction model. The conformer with lower HADDOCK score from cluster 1 is shown. CueR (green), CopZ1 (orange), DNA, Cu+ (cyan), binding residues (sticks), H-bonds (blue lines), and salt bridges (red lines) are represented. Dotted frame shows a detail of the interaction site and the continues frame a 180° rotated view (bottom panels).
Fig. 6B shows a molecular model of the CueRholo-CopZ1apo interaction where a bonding network composed mainly by salt bridges and hydrogen bonds is observed. Again, comparison of these bonding interfaces shows the stronger interaction in the case of the CueRholo-CopZ1apo pair. Ten H-bonds and 7 salt bridges were observed at the CueRholo-CopZ1apo interface, whereas CueRholo-CopZ2apo presented 7 H-bonds and 2 salt bridges (Table S3). In both cases, a common binding site in CueR (Glu110, Arg117, Asp119, Lys124, and Cys131) appeared to be involved in intermolecular hydrogen bonds and hydrophobic contacts.
In summary, in vivo and in silico analyses support the hypothesis that CopZ1 is the chaperone that interacts with and delivers the metal to CueR. Moreover, those indicate that the protein-protein recognition, independent of the Cu+ transfer event, has a contributing effect to the targeting and distribution of Cu+ within the cell.
CopZ1 mediates the transcriptional regulation of CopA1 expression
The described experiments suggest that CopZ1 might have a main role as Cu+ donor to CueR. Testing this idea, the dependence of CueR-mediated transcription on CopZ1 and CopZ2 was studied in vivo. The expression of the copA1 gene was used as reporter of CueR activity. CopA1 is the P-type ATPase responsible for cytoplasmic Cu+ efflux (32). The P. aeruginosa copZ1, copZ2, and copA1 genes are all under control of CueR (15). As a first step in these experiments, their expression kinetic upon exposure to external 0.5 mm Cu2+ was determined. Fig. 7A shows that the transcriptional response to external Cu2+ takes place within 5 min of exposure. Importantly, an earlier increase in copZ1 and copZ2 transcript levels was observed followed by higher expression of copA1. Transcript levels decayed after 5 min exposure consistent with the system reaching steady state (15). It was also notable that expression levels of copZ1 are substantially lower than those of copZ2 (∼8 times at 5 min). Based on these results, the expression of copA1 in P. aeruginosa strains was measured after 2 min of exposure to Cu2+ (Fig. 7B). As previously shown, deletion of CueR completely abolished the expression of copA1 (15). Most importantly, copA1 expression was diminished in ΔcopZ1, whereas the ΔcopZ2 strain showed a slightly increased expression of the transporter. Cells lacking both copZ1/copZ2 displayed an impaired phenotype similar to that of ΔcopZ1. Validating these results, complementation of the mutant strains resulted in restoration of the copA1 expression. These results clearly support the role of CopZ1 delivering Cu+ to CueR to elicit the cellular response to increased Cu+ levels and argue against CopZ2 transferring Cu+ to CueR in vivo. Moreover, these observations explain the critical role of CopZ1, but not CopZ2, conferring tolerance to high (3.5 mm) external Cu2+ levels (Fig. 2). The higher expression of CopA1 in the ΔcopZ2 strain is in agreement with the role of CopZ2 shown in the following experiments.
Figure 7.
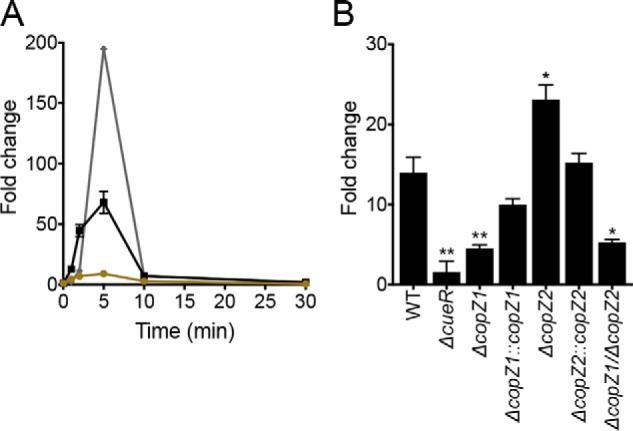
Transcriptional levels of copZ1, copZ2, and copA1 upon Cu2+ stress. A, kinetics of expression at RNA level of copZ1 (ochre circles), copZ2 (black squares), and copA1 (gray diamonds) upon 0.5 mm CuSO4 treatment. B, copA1 expression in ΔcueR, ΔcopZ1, ΔcopZ2, ΔcopZ1/ΔcopZ2 mutants and complemented strains after 2 min of 0.5 mm CuSO4 treatment. Data are the mean ± S.E. of three independent experiments. Significant differences from the WT as determined by unpaired two-tailed Student's t test are: *, p < 0.05; **, p < 0.01.
CopZ2 is more abundant than CopZ1 in vivo
The described results show that CopZ1 supplies Cu+ for CueR activation. Then, what role does CopZ2 play in the response to Cu+ stress? The large transcriptional up-regulation of copZ2 (Fig. 7A) indicates that CopZ2 abundance might rise significantly in the presence of high intracellular Cu+. The in vivo levels of CopZ1 and CopZ2 were measured in mutant strains complemented with the corresponding gene carrying a 3′ His tag coding sequence under control of its own promoter (500 bp upstream sequence). Distinct from other bacteria, P. aeruginosa copZ genes are located in single-gene operons, preceded by a CueR operator sequence (Fig. S3). Complemented strains were challenged with 0.5 mm Cu2+ and the produced His-tagged CopZs were detected by immunostaining (Fig. 8). Resulting signals were calibrated with a standard curve of purified His-tagged protein. Fig. 8A shows that CopZ2 was significantly more abundant than CopZ1 during the response to Cu+ stress. In fact, CopZ2 levels increased comparably to the intracellular Cu+ concentration (15). CopZ2 appeared as an early response to Cu+, reaching maximum protein levels 5–10 min after the initial exposure to Cu2+ (Fig. 8B). Importantly, chaperone levels remained elevated under steady state conditions again as the intracellular Cu+ content does (15). In contrast to CopZ2, CopZ1 showed a much more attenuated response to metal stress (Fig. 8, A and B). CopZ1 increased 2–3 times, whereas CopZ2 levels rose ∼10 times (Fig. 8B). In bacteria, there is a strict cellular Cu quota, about 104 atoms per cell (12), half or less are expected to be in the cytoplasm (16). These numbers correlate well with the amount of CopZ2 encountered in the cytoplasm during steady state, 40 fmol of CopZ2/μg of total protein. This represents a copy number of ∼9,000 CopZ2 molecules/cell. Although this is a rough estimation, it is apparent that CopZ2 provides enough Cu+ storage in the cytoplasm, at least within certain ranges, during Cu+/2+ stress.
Figure 8.
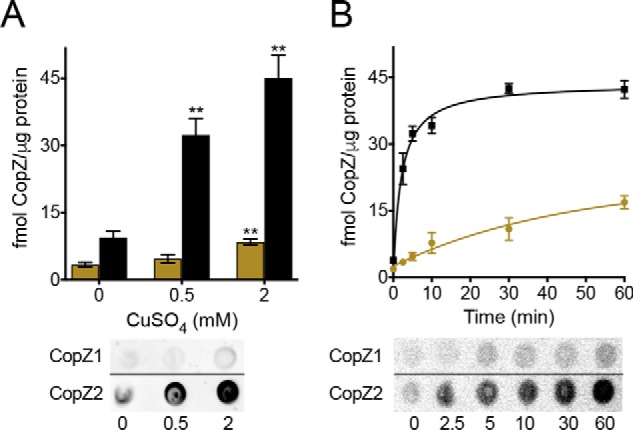
In vivo abundance of CopZ1 (ochre) and CopZ2 (black) proteins. ΔcopZ1 and ΔcopZ2 were complemented with the corresponding His-tagged gene under control of the native promoter. His-tagged proteins were immunostained and their abundance calculated using a standard curve of pure His-tagged protein. The horizontal dividing lines indicate where the images have been spliced; signals were from an identical original image and have not been altered. CopZs levels were determined after exposure to (A) different CuSO4 concentrations during 10 min or (B) 0.5 mm CuSO4 during different times. Data are the mean ± S.E. of three independent experiments. Significant differences from values in the absence of CuSO4 as determined by unpaired two-tailed Student's t test were: **, p < 0.01.
CopZ2 is fully metallated upon Cu stress
The significant increase of CopZ2 upon Cu2+ exposure suggests that this might act as a Cu+ buffer protein. Moreover, the small but sizable CopZ2 pool present at low Cu+ levels might serve as fast response preceding the synthesis of Cu+ efflux transporters. These ideas were examined by measuring the apo/holo ratio of each chaperone before and after 10 min stress with 0.5 mm Cu2+. In these experiments, again, mutant strains complemented with genes carrying His tag coding sequences and under the control of their own promoters were used. After incubation in the presence of Cu2+, cells were washed, homogenized in the presence of 10 mm maleimide, and the levels of alkylated (apo form) and non-alkylated (holo form) of the chaperones quantified by MS. Both chaperones contain Cys only in the Cu+-binding motifs (Fig. 1, A and B), residues that are protected from maleimide alkylation when Cu+ is coordinated (Fig. S4). The relative increases of CopZ1 and CopZ2 levels upon Cu+ stress determined by MS were comparable with those measured using immunostaining (Fig. S5).
Fig. 9 shows the apo/holo ratios of CopZ2 in cells growing in LB media with no added Cu2+ and for CopZ1 and CopZ2 upon Cu+2 exposure. Being a lower abundance chaperone, in the absence of external Cu2+, peptides containing the metal-binding motif of CopZ1 were under the method detection limit and the relative levels of CopZ1holo and CopZ1apo could not be quantified. This was not the case in the presence of Cu2+, when CopZ1 was found fully metallated (Fig. 9A). Distinct was the case of CopZ2, which was observed partially metallated (44%) in the absence of Cu2+ but largely metallated (∼98%) upon metal stress (Fig. 9B). Then, it is apparent that CopZ2 functions as a cytoplasmic Cu+ storage system of P. aeruginosa.
Figure 9.
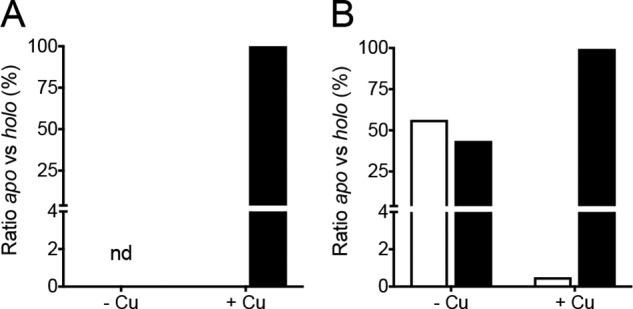
Apo/holo ratios of CopZs by alkylation-MS analysis. ΔcopZ complemented strains were cultured in the absence and presence of 0.5 mm CuSO4 during 10 min. A, CopZ1, and B, CopZ2, Cys were alkylated with maleimide and enriched protein preparations analyzed by MS/MS. The apo (white) and holo (black) ratios were quantified by summing the fragment intensity to obtain the molar fraction of modified versus nonmodified Cys in Cu-binding site-containing peptides. Data are the average of two independent experiments.
Exploring alternative cytoplasmic Cu+ sequestering mechanisms, other than CopZ2, we observed that no changes in GSH levels are detected in the WT cells challenged with 0.5 mm Cu2+, nor in the ΔcopZ2 or ΔcopZ1/ΔcopZ2 mutant strains (Fig. S6). Albeit, these mutants showed higher GSH basal levels. This agrees with our previous observations that Cu+ stress does not promote changes in the expression of GSH biosynthesis genes, PA2140 (metallothionein-like) or the csp3 gene (15). However, if CopZ2 plays a central role in the cytoplasmic Cu+ sequestration, how do bacteria overcome the absence of copZ2 or why does the ΔcopZ2 strain show a normal Cu+ tolerance (Fig. 2A)? As shown in Fig. 7B, there is an elevated expression of copA1 in the ΔcopZ2 strain. We hypothesize that this leads to a more efficient extrusion of Cu+ from the cytoplasm to compensate for a loss of chaperoning function. Then, the high affinity CopZ2 is likely to participate in the stabilization of Cu+ pools in the cytoplasm of P. aeruginosa.
Discussion
Bacterial Cu+/2+ homeostasis is linked to a number of physiological processes through the metallation of key cuproenzymes including multicopper oxidases, cytochrome c oxidases, superoxide dismutase, nitrous-oxide reductase, among others. Mechanisms of copper influx and efflux are, of course, instrumental to determine the cellular Cu+/2+ quota. However, a basic analysis makes clear that Cu+/2+ homeostasis is highly dependent on the metal cytoplasmic fate. In this compartment, the metal sensor is metallated and chaperones distribute metal to efflux transporters and cuproproteins. Moreover, various cytoplasmic molecules (GSH, Csp3, and metallothionein) have been proposed to have a putative role in metal sequestration. Here, we report how the singular roles and interplay of two P. aeruginosa Cu+ chaperones, CopZ1 and CopZ2, enable the control of Cu+ homeostasis in the cytoplasmic compartment (Fig. 10). Although metal exchange between the two chaperones is kinetically restricted (Fig. 3), CopZ1 metallates the sensor CueR (Fig. 4) and has a direct influence on the transcriptional control of the CueR regulon (Fig. 7), whereas CopZ2 sequester Cu+, acting in the fast response to Cu+ stress (Figs. 8 and 9).
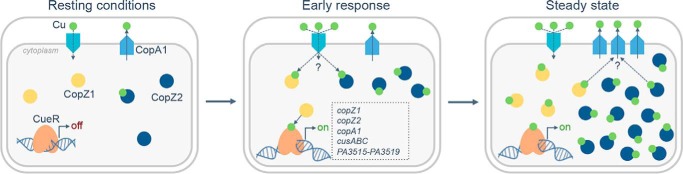
Figure 10.
Model of Cu+ homeostasis via CopZ1, CopZ2, and CueR interplay in the cytoplasm of P. aeruginosa. Three different landscapes are represented. Resting conditions (left) in the absence of Cu-stress, where CopZ1 (yellow) and CopZ2 (blue) appear to be at similar levels and CopZ2 is only partially metallated. Early response (central panel) takes place within 1–3 min of external Cu2+ exposure. Once Cu enters into the cytoplasm, CopZ1 metallates the sensor CueR (orange) leading to transcriptional activation of the CueR regulon genes (dotted box), whereas CopZ2 acts as an early Cu+ storage system. The CopZ2 pool increases immediately and become fully metallated. As steady state is reached (right panel), intracellular Cu+ and CopZ2 levels remain constant as a result of equal Cu+ influx and efflux rates. CopZ1 and CopZ2 constitute independent Cu+ pools, working coordinately to maintain Cu+ homeostasis in the cytoplasm of P. aeruginosa.
Cu+ exchange among P. aeruginosa chaperones is kinetically restricted
Analysis of CopZ1 and CopZ2 structures, their distinct behavior binding metals and forming dimers (Fig. 1), and particularly the alternative phenotypes of mutant strains (Fig. 2), indicate that these proteins might have different cellular functions, and consequently constitute independent Cu+ pools. It has long been established that Cu+ chaperones exchange metal with structurally similar domains present in P1B1-type ATPases (38–40). Then, Cu+ exchange among CopZ1 and CopZ2 could be expected such as they would reach a thermodynamic equilibrium dictated by their KD for the metal. Results from CopZ1/CopZ2 Cu+ exchange experiments showed that whereas the metal exchange occurs, equilibrium is slowly reached after a few hours (Fig. 3). P. aeruginosa doubling time in rich media is 25–35 min. Then, it is probable that the CopZ1/CopZ2 Cu+ exchange operates far from equilibrium and the chaperones constitute two functionally separated metal pools.
CueR receives Cu+ from CopZ1
At the center of Cu+ homeostasis are the periplasmic and cytoplasmic metal sensors. CueR, a typical member of the MerR family, is the cytoplasmic Cu+-activated transcriptional regulator in P. aeruginosa (15, 49). PaCueR has a KD 1–3 orders of magnitude larger than those reported for homologous proteins (12, 19). However, CueR still satisfices the paradigm that there is no free Cu+ in the cell cytoplasm as one free Cu+ per cell would yield a 10−8 m concentration, formally 8 orders of magnitude higher than the KD of 2.5 × 10−16 m. Conversely, this KD fits the observed interplay of CueR with CopZ1, which has a higher KD (lower metal affinity), and CopZ2, which has a lower KD.
Several lines of evidence show that CueR obtains Cu+ from CopZ1. We observed that in vitro CopZ1holo transfers Cu+ to the CueRapo. Moreover, CopZ1holo is more efficient than CopZ2holo metallating CueRapo (Fig. 4). In principle, this observation could be just due to the relative KD values of sensor and chaperones; this is the selectivity that would be driven only by the relative Cu+-binding constants. In fact, metallation from a chaperone with lower affinity for Cu+ than the sensor appears to be the case in S. pneumoniae for CupA(site 2)/CopY (42, 50). However, in vitro and in silico experiments showed that, largely independent of the metal ion, the transcription factor interacts more favorably with CopZ1 than CopZ2 (Figs. 5 and 6). Then, it is apparent that the protein-protein interaction should be thermodynamically shallow for CopZ1-CueR. These physicochemical principles driving the molecular behavior explain the in vivo control of copA1 transcription by CopZ1 but not by CopZ2 (Fig. 7). This is particularly relevant under conditions of Cu+ stress when the pool of CopZ2 is roughly 1 order of magnitude higher than that of CopZ1 (Fig. 8).
CopZ2 serves as a Cu+ sequestering pool
The participation of Cu+ sequestering molecules in mechanisms of metal tolerance has been postulated (51). Among these, GSH, metallothionein (PA2140), and Cps3 (PA2107) are present in P. aeruginosa. However, none of these genes or enzymes related to GSH biosynthesis are induced during the exposure of P. aeruginosa to Cu2+, even though under these conditions cells reach four times higher Cu2+ levels compared with resting conditions (15). Moreover, GSH levels do not increase during exposure to 0.5 mm Cu2+ (Fig. S6), nor that the Δcsp3 mutant strains are sensitive to high Cu2+ levels (not shown). Alternatively, upon P. aeruginosa exposure to 0.5 mm Cu2+ we observed a high induction of copZ2 that resulted in a large increase in CopZ2 proteins pools (Figs. 7 and 8). The manner in which the system integrates the cytoplasmic copper homeostatic network is interesting (Fig. 10). Under resting conditions both CopZ1 and CopZ2 appear to be at similar levels and CopZ2 is only partially metallated (Fig. 10, left panel). Upon Cu2+ stress, CopZ1 metallates the sensor CueR leading to transcriptional activation of the CueR regulon genes, including copZ1, copZ2, and copA1 (Fig. 10, central panel). The CopZ2 pool increases immediately and CopZ1 remains quite close to basal levels, whereas both chaperones, however, are fully metallated (Fig. 10, right panel). Rough estimations suggest that the levels of CopZ2 might suffice to sequester all the Cu+ excess under the tested experimental conditions. In addition, the fast increase in CopZ2 together with the slow rate of metal exchange support the idea that both chaperones constitute independent Cu+ pools. Although this idea is relevant when considering cytoplasmic Cu+ homeostasis, it is likely to have important implications for Cu+ distribution to cuproproteins and membrane transporters.
In summary, this study provides a novel model for copper homeostasis in bacteria. In P. aeruginosa the fate of cytoplasmic Cu+ is determined by the interplay between the metallosensor CueR and two CopZ chaperones (Fig. 10). CopZ1 acts as chaperone delivering Cu+ to the CueR sensor, whereas CopZ2 functions as a fast response Cu+ sequestering system.
Experimental procedures
Bacterial strains
Bacterial strains, plasmids, and oligonucleotides used in this study are listed in Table S1. P. aeruginosa PAO1 served as WT strain. P. aeruginosa strains were grown at 37 °C in LB medium supplemented with 25 μg/ml of irgasan (WT and mutant strains) or 30 μg/ml of gentamicin (complemented strains). E. coli strains were grown at 37 °C in LB medium supplemented with 30 μg/ml of kanamycin, 100 μg/ml of ampicillin, or 10 μg/ml of gentamicin, depending on the plasmids selection.
Construction of P. aeruginosa mutant and complemented strains
ΔcopZ1 mutant strain was a gift from Dr. S. Lory (Harvard Medical School) (49). ΔcopZ2 and ΔcopZ1/ΔcopZ2 double mutant strains were constructed using the two-step allelic exchange method (52). Briefly, 500-bp regions flanking the copZ2 gene were amplified by PCR. A mutant allele resulted from splicing by overlapping PCR. A mutation replacing Cu-binding Cys for Ala residues, followed by a stop codon and a PvuII site, were introduced to produce a nonfunctional copZ2 mutant allele. This was cloned into the allelic exchange vector pDONRPEX18Gm, transformed into the donor E. coli S17.1, and transferred into the WT or ΔcopZ1 strains by conjugation. Single crossover mutants were selected in 10 μg/ml of gentamicin LB plates, and unmarked double crossover mutants were isolated by counter-selection in 15% sucrose, no salt LB plates. Double crossover bacterial strains were selected in 25 μg/ml of irgasan, LB agar, and screened by restriction digest of PCR products. Deletions were confirmed by sequencing.
Mutant strains were complemented with the corresponding gene carrying a 3′ His tag coding sequence under control of the native promoter using the mini-Tn7 insertion system (53). The genes and their 500-bp upstream promoter regions were amplified by PCR. The 3′ primer included a His tag coding sequence. Amplicons were cloned into a pUC18-mini-Tn7-Gm vector. Resulting plasmids and helper plasmid pTNS2 were co-transformed into the corresponding ΔcopZ1 or ΔcopZ2 strains, followed by selection in 30 μg/ml of gentamicin, on LB plates. Complemented strains were verified by PCR.
Cu2+ sensitivity assay
Overnight cultures were diluted in 25 μg/ml of irgasan, LB medium, adjusted to 0.05 OD600, and supplemented with the indicated CuSO4 concentration. Cell growth (OD600) was monitored every 10 min for 10 h using an Epoch 2 Microplate Spectrophotometer (BioTek) at 37 °C with continuous shaking. Cu uptake was measured in whole cells using atomic absorption spectroscopy as described (15).
Protein expression and purification
CopZ1 and CopZ2 containing a His6 tag joined by a TEV-cleavage site were expressed in E. coli BL21(DE3)pLysS cells and purified as described (15). When required, purified protein was subjected to TEV protease cleavage overnight and reapplied to the Ni-NTA column to obtain untagged proteins. Flow-through fractions were collected and buffer exchanged in 3-kDa Centricons to 25 mm HEPES (pH 8), 100 mm sucrose, 150 mm NaCl, and 1 mm DTT.
The cueR (PA4778) gene was amplified from genomic DNA using a 3′-end primer that introduced a Strep-tag coding sequence and a stop codon, cloned into a pBAD-topo vector (Invitrogen) and expressed in E. coli BL21(DE3) cells. CueR was affinity purified using Strep-Tactin®XT Superflow® columns (IBA). Isolated CueR contained 0.08–0.12 eq of Cu bound. Attempts to remove the residual Cu with combinations of metal chelators (tetrathiomolybdate, KCN, BCS, and EDTA) were not successful.
Purified proteins were stored in 20% glycerol, 25 mm HEPES (pH 8), 100 mm sucrose, 150 mm NaCl, and 1 mm DTT at −80 °C. Protein concentrations were determined in accordance to Bradford (54), thiol levels were measured using Ellman's method (55), and bound Cu+ was determined by atomic absorption spectroscopy (15). In all cases, protein purity was ≥90% as estimated by SDS-PAGE followed by Coomassie Brilliant Blue staining. CopZs oligomerization was analyzed by Blue Native PAGE electrophoresis (56).
Cu+ binding and transfer
Cu+-loaded proteins were prepared by slow addition of CuSO4 in the presence of 10 mm ascorbic acid to reduced proteins (3 h preincubation in 5 mm tris(2-carboxyethyl)phosphine, 4 °C). Unbound Cu+ was removed by passage through Sephadex PD-10 columns (CueR) or by washing after binding to Ni-NTA resin (CopZ1 and CopZ2). Cu+ transfer reactions were performed using apo/holo partners with different tags. 10 nmol of His-tagged CopZsholo were incubated 30 min with Ni-NTA resin. Unbound protein and free Cu+ were washed with 20 mm imidazole, buffer H (25 mm HEPES (pH 8), 150 mm NaCl, 10 mm ascorbic acid). 10 nmol of TEV-protease–cleaved CopZsapo, or 5 nmol of the Strep-tag CueRapo dimer in the presence or absence of 5 nmol of PcopZ2 (dsDNA copZ2 promoter region) were incubated 10 min at room temperature with resin-bound CopZsholo. Untagged proteins were collected in the washes with 20 mm imidazole, buffer H. His-tagged proteins were eluted with 300 mm imidazole, buffer H. Eluted proteins were verified by SDS-PAGE/Western blots immunostained with primary rabbit His tag antibody, pAb, and goat anti-rabbit IgG antibody-horseradish peroxidase (GeneScript), or Strep-Tactin®-horseradish peroxidase conjugate (IBA). Eluted DNA was observed by agarose gel electrophoresis.
In vitro interaction of CueR with CopZ1 and CopZ2
Interactions between regulator and chaperones were studied by assessing the co-purification of isolated proteins by batch affinity chromatography. 10 μm Strep-tagged CueRapo dimer was incubated 10 min at room temperature with 10–100 μm His-tagged CopZsapo in buffer containing 25 mm Tris-HCl (pH 8.0), 150 mm NaCl, 50 mm sucrose, 5 mm DTT, 0.2 mm BCS. Samples were incubated 10 min with 50 μl of Strep-Tactin®XT Superflow® resin (IBA) and centrifuged at 14,000 rpm for 1 min to collect unbound proteins in the supernatant. Resins were washed twice with buffer, 25 mm Tris-HCl (pH 8.0), 150 mm NaCl, and bound proteins were eluted with buffer supplemented with 50 mm biotin. Controls were performed by individually subjecting each CopZ to the same protocol, lacking the interacting partner. Bound proteins were loaded onto nitrocellulose blotting membrane and His-tagged proteins were immunostained as described above. Stained dots were recorded using ChemiDoc XRS+ Imager (Bio-Rad), and quantified using the Gilles Carpentier-Dot Blot Analyzer for ImageJ (57).
Gene expression analysis
Cells (mid-exponential phase) were incubated in 0.5 mm CuSO4 antibiotic-free LB medium. 0.5-ml aliquots were taken at the indicated times, stabilized with RNA protect Bacteria Reagent (Qiagen), and RNA was isolated with RNeasy Mini Kit (Qiagen). RNA was treated with DNase I, purified by phenol/chloroform extraction and ethanol precipitated. 1 μg of RNA was used for cDNA synthesis using the ProtoScript® II kit (New England BioLabs). Quantitative PCR were carried out with FastStart Essential DNA Green Master (Roche Applied Science) in a 10-μl final volume, using 0.25 μm of each primer. The efficiency of primer sets was evaluated by quantitative PCR in serial dilutions of WT cDNA. Results were normalized to 30S ribosomal protein S12 (PA4268) (32). The PfaffI method was used to compare samples (58).
CopZ1 and CopZ2 expression kinetics
CopZ1 and CopZ2 complemented strains (mid-exponential phase) were incubated in 0.5 mm CuSO4 antibiotic-free LB medium and 2-ml aliquots were taken at the indicated times. Cell pellets were resuspended in buffer, 25 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.3% SDS, and sonicated twice on ice. Supernatants were adjusted to 4 μg/μl of total protein, and 40 μg of protein samples were loaded onto nitrocellulose blotting membrane. As standard, a curve of 5–100 ng of His-tagged pure protein was also included in the blot. His-tagged proteins were detected as described above. Stained dots were quantified as described above.
In vivo CopZ1 and CopZ2 apo/holo equilibrium determinations
Protein alkylation and sample enrichment
CopZ1- and CopZ2-complemented strains (mid-exponential phase) were incubated in 0.5 mm CuSO4 antibiotic-free LB medium, 10 min at 37 °C. Cells were harvested by centrifugation (11,000 rpm, 3 min, 4 °C), pellets were resuspended in 25 mm Tris-HCl (pH 7.4), 150 mm NaCl, 10 mm maleimide and sonicated three times for 30 s in a dry ice/ethanol bath. Alkylation reaction was stopped after a 10-min incubation on ice by adding 20 mm l-cysteine. Cellular debris were removed by centrifugation at 16,000 rpm for 10 min at 4 °C, and supernatants were loaded into Ni-NTA columns. The collected His-tagged CopZs-enriched fractions were concentrated in 3-kDa Centricons.
In-solution digestion and LC-MS/MS analyses
Mass spectroscopy analysis was performed by the University of Massachusetts Mass Spectrometry Facility. CopZs-enriched fractions were lyophilized, treated with in 0.1% Protease Max (Promega), reduced with 2.25 mm DTT, alkylated with 5 mm iodoacetamide, and digested with 0.4 μg of trypsin (Promega). Peptides were cleaned using C18 zip-tips (OMIX), eluted in 80% acetonitrile, 1% formic acid, lyophilized, and re-suspended in 5% acetonitrile, 0.1% TFA. Peptides were analyzed by LC-MS/MS using a Waters NanoAcquity UPLC coupled to a Thermo Scientific Q Exactive Hybrid Quadrupole-Orbitrap mass spectrometer. Samples were loaded onto 100-μm inner diameter fused-silica pre-column packed with Magic C18AQ (2 cm × 5 μm (200 Å) (Bruker-Michrom) at a flow rate of 4.0 μl/min for 4 min with 5% acetonitrile, 0.1% formic acid. Peptides were eluted at 300 nl/min from 75 μm × 25 cm Magic C18AQ 3 μm (100 Å) particles with a linear gradient from 5 to 35% of mobile phase B (acetonitrile, 0.1% formic acid) in mobile phase A (0.1% formic acid), over 60 min. Ions were introduced by positive electrospray ionization into the Q Exactiva. Full MS scans from 300–1750 m/z were acquired followed by 10 MS/MS scans acquired under HCD fragmentation at a resolution of 17,500 (m/z 200).
Data analysis
Raw data files were processed with Proteome Discoverer (Thermo), and identified using Mascot Server (version 2.4) against the P. aeruginosa SwissProt FASTA file (manually updated to include the CopZ2 protein). Search parameters included variable modifications of carbamidomethyl cysteine (Cam-Cys) and maleimide cysteine (Mal-Cys). Tolerance for assignments was restricted to 10 ppm for precursors and 0.05 Da for fragments. Results were processed by Scaffold (Proteome Software, Inc.) utilizing the Trans-Proteomic Pipeline (Institute for Systems Biology) with threshold values set at 95% for peptides (0.1% false-discovery rate) and 99% for proteins (2 peptides minimum). The apo and holo ratios of the proteins were quantified by summing the fragment intensity to obtain the molar fraction of modified (Mal) versus nonmodified (Cam) cysteines in Cu-binding site-containing peptides.
Computational studies
Conserved residues in Cu-binding regions of CopZ-like chaperones
P. aeruginosa CopZs sequences were used as templates for BLAST searches of the nonredundant protein sequences database. Pseudomonas organisms were excluded. Sequences were sorted based on their Cu+-binding regions, CopZ1-like MxCxGC (543 sequences), or CopZ2-like MxCxHC (349 sequences). Both groups were aligned using the Muscle tool (Jalview) (59). Sequence logos were created using the WebLogo online tool (60).
Comparative modeling
Models of P. aeruginosa CueRholo, CopZ1apo, and CopZ2apo models were built using E. coli (Ag+)CueR (PDB ID 4WLW) (46); Salmonella typhimurium GolB (PDB ID 4Y2K) (47) for CopZ1, and the Thermus thermophilus CopZ (PDB ID 2ROE) (62) for CopZ2, as templates. Selected proteins share 48, 45, and 42% sequence identity with their P. aeruginosa homologues, respectively. Models were built using PRIME and optimized with Maestro protein preparation wizard (63). For protein-protein docking simulations, the Cu+ atom was kept in the metal-binding site of the CueR protein. The PROPKA program was employed to set the protonation states at pH 7.0 and structures were energy minimized using PRIME (64).
Protein-protein docking simulations
Simulations were done using the HADDOCK2.2 server (65). CueRholo was selected as receptor and apo chaperones were chosen as ligands. Chaperone Cu+-binding Cys were placed within 10 Å of CueR Cu+-binding residues (Cys112 and Cys120) as starting receptor-ligand positions (46). The CueR residues Glu110, His111, Gln113, Arg117, Asp119, Pro121, and Lys124 were set as active residues (allegedly directly involved in the interaction). For the chaperones CopZ1/CopZ2, residues Asn/Gly8, Thr10, Cys11, Gly/His13, Cys14, Lys/Arg16, Arg38, Ala/Glu58, Gly59, and Thr60 were selected as active residues. Passive residues were automatically defined around the active residues. Docking simulations were performed using the default parameters of the server easy interface. The top 200 docking solutions were clustered based on the fraction of common contacts, with a cutoff of 0.75 and clusters ranked according to the HADDOCK score for further analysis. Protein-protein interactions in the best conformations (according to the HADDOCK score) of each cluster were analyzed using PISA (66). The interactions of the best CueRholo-CopZ1 conformation were represented using PyMol (61).
Author contributions
L. N.-A. and D. R. data curation; L. N.-A. and D. R. formal analysis; L. N.-A. and D. R. investigation; L. N.-A. visualization; L. N.-A. methodology; L. N.-A. and D. R. writing-original draft; L. N.-A. and J. M. A. writing-review and editing; J. M. A. conceptualization; J. M. A. supervision; J. M. A. funding acquisition; J. M. A. project administration.
Supplementary Material
Acknowledgments
We thank the Center for Bioinformatics and Molecular Simulation, Universidad de Talca for the use of its computational resources. We thank Dr. Robert Dempski (Worcester Polytechnic Institute) and Dr. Daniel Raimunda (Instituto Investigación Médica Mercedes y Martín Ferreyra, Consejo Nacional de Investigaciones Científicas y Técnicas, UNC) for critical reading of the manuscript and helpful discussions.
This work was supported by National Institutes of Health Grant R01GM114949 (to J. M. A.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S6 and Tables S1–S3.
- Ni-NTA
- nickel-nitrilotriacetic acid
- r.m.s.
- root mean square
- BCS
- bathocuproinedisulfonic acid disodium salt
- TEV
- tobacco etch virus
- PDB
- Protein Data Bank.
References
- 1. Argüello J. M., Raimunda D., and Padilla-Benavides T. (2013) Mechanisms of copper homeostasis in bacteria. Front. Cell Infect. Microbiol. 3, 73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fraústo da Silva J. J. R., and Williams R. J. P. (2001) The Biological Chemistry of the Elements: the Inorganic Chemistry of Life, 2nd Ed., Oxford University Press, Oxford [Google Scholar]
- 3. Desideri A., and Falconi M. (2003) Prokaryotic Cu,Zn superoxidies dismutases. Biochem. Soc. Trans. 31, 1322–1325 10.1042/bst0311322 [DOI] [PubMed] [Google Scholar]
- 4. Brunori M., Giuffrè A., and Sarti P. (2005) Cytochrome c oxidase, ligands and electrons. J. Inorg. Biochem. 99, 324–336 10.1016/j.jinorgbio.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 5. Macomber L., and Imlay J. A. (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U.S.A. 106, 8344–8349 10.1073/pnas.0812808106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dupont C. L., Grass G., and Rensing C. (2011) Copper toxicity and the origin of bacterial resistance-new insights and applications. Metallomics 3, 1109–1118 10.1039/c1mt00107h [DOI] [PubMed] [Google Scholar]
- 7. Ladomersky E., and Petris M. J. (2015) Copper tolerance and virulence in bacteria. Metallomics 7, 957–964 10.1039/C4MT00327F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodgkinson V., and Petris M. J. (2012) Copper homeostasis at the host-pathogen interface. J. Biol. Chem. 287, 13549–13555 10.1074/jbc.R111.316406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osman D., and Cavet J. S. (2008) Copper homeostasis in bacteria. Adv. Appl. Microbiol. 65, 217–247 10.1016/S0065-2164(08)00608-4 [DOI] [PubMed] [Google Scholar]
- 10. Rensing C., and Grass G. (2003) Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27, 197–213 10.1016/S0168-6445(03)00049-4 [DOI] [PubMed] [Google Scholar]
- 11. Argüello J. M., Patel S. J., and Quintana J. (2016) Bacterial Cu+-ATPases: models for molecular structure-function studies. Metallomics 8, 906–914 10.1039/C6MT00089D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Changela A., Chen K., Xue Y., Holschen J., Outten C. E., O'Halloran T. V., and Mondragón A. (2003) Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 301, 1383–1387 10.1126/science.1085950 [DOI] [PubMed] [Google Scholar]
- 13. Ekici S., Yang H., Koch H. G., and Daldal F. (2012) Novel transporter required for biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. MBio 3, e00293–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hernández-Montes G., Argüello J. M., and Valderrama B. (2012) Evolution and diversity of periplasmic proteins involved in copper homeostasis in γ-proteobacteria. BMC Microbiol. 12, 249–263 10.1186/1471-2180-12-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quintana J., Novoa-Aponte L., and Argüello J. M. (2017) Copper homeostasis networks in the bacterium Pseudomonas aeruginosa. J. Biol. Chem. 292, 15691–15704 10.1074/jbc.M117.804492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parmar J. H., Quintana J., Ramírez D., Laubenbacher R., Argüello J. M., and Mendes P. (2018) An important role for periplasmic storage in Pseudomonas aeruginosa copper homeostasis revealed by a combined experimental and computational modeling study. Mol. Microbiol. 110, 357–369 10.1111/mmi.14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown N. L., Stoyanov J. V., Kidd S. P., and Hobman J. L. (2003) The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27, 145–163 10.1016/S0168-6445(03)00051-2 [DOI] [PubMed] [Google Scholar]
- 18. Ma Z., Jacobsen F. E., and Giedroc D. P. (2009) Coordination chemistry of bacterial metal transport and sensing. Chem. Rev. 109, 4644–4681 10.1021/cr900077w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Osman D., Piergentili C., Chen J., Chakrabarti B., Foster A. W., Lurie-Luke E., Huggins T. G., and Robinson N. J. (2015) Generating a metal-responsive transcriptional regulator to test what confers metal-sensing in cells. J. Biol. Chem. 290, 19806–198022 10.1074/jbc.M115.663427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen P., Keller A. M., Joshi C. P., Martell D. J., Andoy N. M., Benítez J. J., Chen T. Y., Santiago A. G., and Yang F. (2013) Single-molecule dynamics and mechanisms of metalloregulators and metallochaperones. Biochemistry 52, 7170–7183 10.1021/bi400597v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Outten F. W., Outten C. E., Hale J., and O'Halloran T. V. (2000) Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, CueR. J. Biol. Chem. 275, 31024–31029 10.1074/jbc.M006508200 [DOI] [PubMed] [Google Scholar]
- 22. Odermatt A., and Solioz M. (1995) Two trans-acting metalloregulatory proteins controlling expression of the copper-ATPases of Enterococcus hirae. J. Biol. Chem. 270, 4349–4354 10.1074/jbc.270.9.4349 [DOI] [PubMed] [Google Scholar]
- 23. Cobine P., Wickramasinghe W. A., Harrison M. D., Weber T., Solioz M., and Dameron C. T. (1999) The Enterococcus hirae copper chaperone CopZ delivers copper(I) to the CopY repressor. FEBS Lett. 445, 27–30 10.1016/S0014-5793(99)00091-5 [DOI] [PubMed] [Google Scholar]
- 24. Neubert M. J., Dahlmann E. A., Ambrose A., and Johnson M. D. (2017) Copper chaperone CupA and zinc control CopY regulation of the Pneumococcal cop operon. mSphere 2, e00372–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaplin A. K., Tan B. G., Vijgenboom E., and Worrall J. A. (2015) Copper trafficking in the CsoR regulon of Streptomyces lividans. Metallomics 7, 145–155 10.1039/C4MT00250D [DOI] [PubMed] [Google Scholar]
- 26. Cubillas C., Miranda-Sanchez F., Gonzalez-Sanchez A., Elizalde J. P., Vinuesa P., Brom S., and Garcia-de Los Santos A. (2017) A comprehensive phylogenetic analysis of copper transporting P1B ATPases from bacteria of the Rhizobiales order uncovers multiplicity, diversity and novel taxonomic subtypes. Microbiologyopen [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kihlken M. A., Leech A. P., and Le Brun N. E. (2002) Copper-mediated dimerization of CopZ, a predicted copper chaperone from Bacillus subtilis. Biochem. J. 368, 729–739 10.1042/bj20021036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Banci L., Bertini I., Del Conte R., Mangani S., and Meyer-Klaucke W. (2003) X-ray absorption and NMR spectroscopic studies of CopZ, a copper chaperone in Bacillus subtilis: the coordination properties of the copper ion. Biochemistry. 42, 2467–2474 10.1021/bi0205810 [DOI] [PubMed] [Google Scholar]
- 29. Urvoas A., Moutiez M., Estienne C., Couprie J., Mintz E., and Le Clainche L. (2004) Metal-binding stoichiometry and selectivity of the copper chaperone CopZ from Enterococcus hirae. Eur. J. Biochem. 271, 993–1003 10.1111/j.1432-1033.2004.04001.x [DOI] [PubMed] [Google Scholar]
- 30. Hearnshaw S., West C., Singleton C., Zhou L., Kihlken M. A., Strange R. W., Le Brun N. E., and Hemmings A. M. (2009) A tetranuclear Cu(I) cluster in the metallochaperone protein CopZ. Biochemistry 48, 9324–9326 10.1021/bi9011995 [DOI] [PubMed] [Google Scholar]
- 31. Kay K. L., Hamilton C. J., and Le Brun N. E. (2016) Mass spectrometry of B. subtilis CopZ: Cu(I)-binding and interactions with bacillithiol. Metallomics 8, 709–719 10.1039/C6MT00036C [DOI] [PubMed] [Google Scholar]
- 32. González-Guerrero M., Raimunda D., Cheng X., and Argüello J. M. (2010) Distinct functional roles of homologous Cu+ efflux ATPases in Pseudomonas aeruginosa. Mol. Mirobiol. 78, 1246–1258 10.1111/j.1365-2958.2010.07402.x [DOI] [PubMed] [Google Scholar]
- 33. Radford D. S., Kihlken M. A., Borrelly G. P., Harwood C. R., Le Brun N. E., and Cavet J. S. (2003) CopZ from Bacillus subtilis interacts in vivo with a copper exporting CPx-type ATPase CopA. FEMS Microbiol. Lett. 220, 105–112 10.1016/S0378-1097(03)00095-8 [DOI] [PubMed] [Google Scholar]
- 34. Gaballa A., and Helmann J. D. (2003) Bacillus subtilis CPx-type ATPases: characterization of Cd, Zn, Co and Cu efflux systems. Biometals 16, 497–505 10.1023/A:1023425321617 [DOI] [PubMed] [Google Scholar]
- 35. Nawapan S., Charoenlap N., Charoenwuttitam A., Saenkham P., Mongkolsuk S., and Vattanaviboon P. (2009) Functional and expression analyses of the cop operon, required for copper resistance in Agrobacterium tumefaciens. J. Bacteriol. 191, 5159–5168 10.1128/JB.00384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Corbett D., Schuler S., Glenn S., Andrew P. W., Cavet J. S., and Roberts I. S. (2011) The combined actions of the copper-responsive repressor CsoR and copper-metallochaperone CopZ modulate CopA-mediated copper efflux in the intracellular pathogen Listeria monocytogenes. Mol. Microbiol. 81, 457–472 10.1111/j.1365-2958.2011.07705.x [DOI] [PubMed] [Google Scholar]
- 37. Zhang X. X., and Rainey P. B. (2008) Regulation of copper homeostasis in Pseudomonas fluorescens SBW25. Environ. Microbiol. 10, 3284–3294 10.1111/j.1462-2920.2008.01720.x [DOI] [PubMed] [Google Scholar]
- 38. Lutsenko S. (2010) Human copper homeostasis: a network of interconnected pathways. Curr. Opin. Chem. Biol. 14, 211–217 10.1016/j.cbpa.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Banci L., Bertini I., McGreevy K. S., and Rosato A. (2010) Molecular recognition in copper trafficking. Nat. Prod. Rep. 27, 695–710 10.1039/b906678k [DOI] [PubMed] [Google Scholar]
- 40. Boal A. K., and Rosenzweig A. C. (2009) Structural biology of copper trafficking. Chem. Rev. 109, 4760–4779 10.1021/cr900104z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. LaBauve A. E., and Wargo M. J. (2012) Growth and laboratory maintenance of Pseudomonas aeruginosa. Curr. Protoc. Microbiol. 25, Chapter 6, Unit 6E.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glauninger H., Zhang Y., Higgins K. A., Jacobs A. D., Martin J. E., Fu Y., Coyne Rd H. J., Bruce K. E., Maroney M. J., Clemmer D. E., Capdevila D. A., and Giedroc D. P. (2018) Metal-dependent allosteric activation and inhibition on the same molecular scaffold: the copper sensor CopY from Streptococcus pneumoniae. Chem. Sci. 9, 105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma Z., Cowart D. M., Ward B. P., Arnold R. J., DiMarchi R. D., Zhang L., George G. N., Scott R. A., and Giedroc D. P. (2009) Unnatural amino acid substitution as a probe of the allosteric coupling pathway in a mycobacterial Cu(I) sensor. J. Am. Chem. Soc. 131, 18044–18045 10.1021/ja908372b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Joshi C. P., Panda D., Martell D. J., Andoy N. M., Chen T. Y., Gaballa A., Helmann J. D., and Chen P. (2012) Direct substitution and assisted dissociation pathways for turning off transcription by a MerR-family metalloregulator. Proc. Natl. Acad. Sci. U.S.A. 109, 15121–15126 10.1073/pnas.1208508109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Halloran T. V., and Culotta V. C. (2000) Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 275, 25057–25060 10.1074/jbc.R000006200 [DOI] [PubMed] [Google Scholar]
- 46. Philips S. J., Canalizo-Hernandez M., Yildirim I., Schatz G. C., Mondragón A., and O'Halloran T. V. (2015) Allosteric transcriptional regulation via changes in the overall topology of the core promoter. Science 349, 877–881 10.1126/science.aaa9809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei W., Sun Y., Zhu M., Liu X., Sun P., Wang F., Gui Q., Meng W., Cao Y., and Zhao J. (2015) Structural insights and the surprisingly low mechanical stability of the Au-S bond in the gold-specific protein GolB. J. Am. Chem. Soc. 137, 15358–15361 10.1021/jacs.5b09895 [DOI] [PubMed] [Google Scholar]
- 48. de Vries S. J., van Dijk M., and Bonvin A. M. (2010) The HADDOCK web server for data-driven biomolecular docking. Nat. Protoc. 5, 883–897 10.1038/nprot.2010.32 [DOI] [PubMed] [Google Scholar]
- 49. Thaden J. T., Lory S., and Gardner T. S. (2010) Quorum-sensing regulation of a copper toxicity system in Pseudomonas aeruginosa. J. Bacteriol. 192, 2557–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fu Y., Tsui H. C., Bruce K. E., Sham L. T., Higgins K. A., Lisher J. P., Kazmierczak K. M., Maroney M. J., Dann C. E. 3rd, Winkler M. E., and Giedroc D. P. (2013) A new structural paradigm in copper resistance in Streptococcus pneumoniae. Nat. Chem. Biol. 9, 177–183 10.1038/nchembio.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dennison C., David S., and Lee J. (2018) Bacterial copper storage proteins. J. Biol. Chem. 293, 4616–4627 10.1074/jbc.TM117.000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hmelo L. R., Borlee B. R., Almblad H., Love M. E., Randall T. E., Tseng B. S., Lin C., Irie Y., Storek K. M., Yang J. J., et al. (2015) Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nat. Protoc. 10, 1820 10.1038/nprot.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choi K. H., and Schweizer H. P. (2006) Mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat. Protoc. 1, 153–161 10.1038/nprot.2006.24 [DOI] [PubMed] [Google Scholar]
- 54. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 55. Ellman G. L. (1959) Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 56. Wittig I., Braun H.-P., and Schägger H. (2006) Blue native PAGE. Nat. Protoc. 1, 418–428 10.1038/nprot.2006.62 [DOI] [PubMed] [Google Scholar]
- 57. Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of Image analysis. Nat. Methods 9, 671–675 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Waterhouse A. M., Procter J. B., Martin D. M., Clamp M., and Barton G. J. (2009) Jalview version 2: a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Crooks G. E., Hon G., Chandonia J.-M., and Brenner S. E. (2004) WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 10.1101/gr.849004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Delano W. L. (2002) The PyMol Molecular Graphics System, Schrödinger, New York [Google Scholar]
- 62. Sakakibara D., Sasaki A., Ikeya T., Hamatsu J., Hanashima T., Mishima M., Yoshimasu M., Hayashi N., Mikawa T., Wälchli M., et al. (2009) Protein structure determination in living cells by in-cell NMR spectroscopy. Nature 458, 102–105 10.1038/nature07814 [DOI] [PubMed] [Google Scholar]
- 63. Schrödinger (2017) Maestro, Release 2017-3, Schrödinger, LLC, New York [Google Scholar]
- 64. Jacobson M. P., Pincus D. L., Rapp C. S., Day T. J., Honig B., Shaw D. E., and Friesner R. A. (2004) A hierarchical approach to all-atom protein loop prediction. Proteins 55, 351–367 10.1002/prot.10613 [DOI] [PubMed] [Google Scholar]
- 65. van Zundert G., Rodrigues J., Trellet M., Schmitz C., Kastritis P., Karaca E., Melquiond A., van Dijk M., De Vries S., and Bonvin A. (2016) The HADDOCK2.2 web server: user-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 428, 720–725 10.1016/j.jmb.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 66. Krissinel E., and Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 10.1016/j.jmb.2007.05.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.