Abstract
Inositol 1,4,5-trisphosphate receptors (IP3Rs), by releasing Ca2+ from the endoplasmic reticulum (ER) of animal cells, allow Ca2+ to be redistributed from the ER to the cytosol or other organelles, and they initiate store-operated Ca2+ entry (SOCE). For all three IP3R subtypes, binding of IP3 primes them to bind Ca2+, which then triggers channel opening. We are now close to understanding the structural basis of IP3R activation. Ca2+-induced Ca2+ release regulated by IP3 allows IP3Rs to regeneratively propagate Ca2+ signals. The smallest of these regenerative events is a Ca2+ puff, which arises from the nearly simultaneous opening of a small cluster of IP3Rs. Ca2+ puffs are the basic building blocks for all IP3-evoked Ca2+ signals, but only some IP3 clusters, namely those parked alongside the ER–plasma membrane junctions where SOCE occurs, are licensed to respond. The location of these licensed IP3Rs may allow them to selectively regulate SOCE.
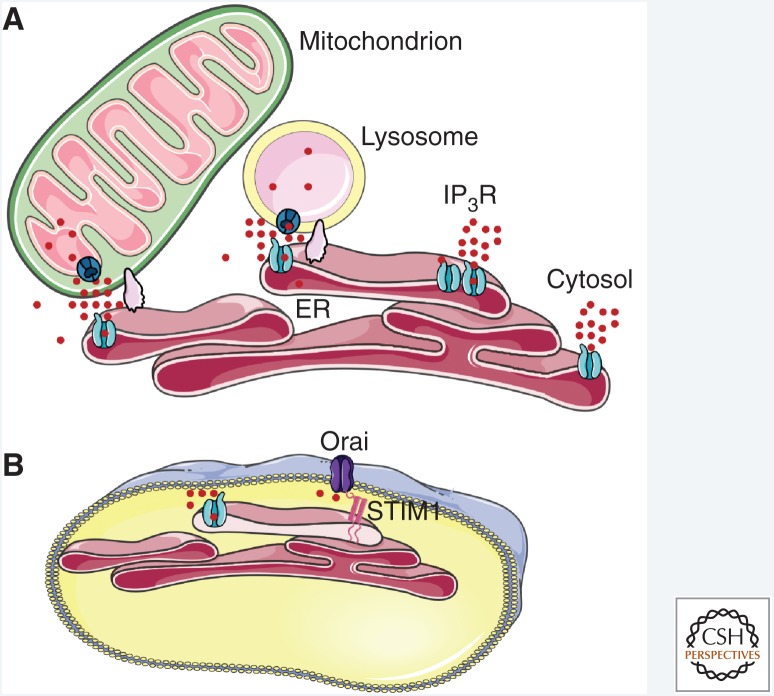
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are expressed in most animal cells, including single-celled protozoa (Prole and Taylor 2011). They mediate release of Ca2+ from intracellular stores, primarily the endoplasmic reticulum (ER) (Berridge 1993) and Golgi apparatus (Pizzo et al. 2011; Wong et al. 2013; Rodriguez-Prados et al. 2015). IP3Rs are also expressed in the nuclear envelope and nucleoplasmic reticulum (Echevarría et al. 2003), where they may selectively generate nuclear Ca2+ signals, although cytosolic Ca2+ signals also invade the nucleoplasm (Bading 2013). IP3R-mediated Ca2+ fluxes across ER membranes increase the cytosolic Ca2+ concentration ([Ca2+]c), and when these signals occur close to other organelles, mitochondria (Csordas et al. 2018) or lysosomes (Lopez Sanjurjo et al. 2013; Garrity et al. 2016; Atakpa et al. 2018), for example, they allow their low-affinity uptake systems to resequester the Ca2+. The accompanying decrease in ER luminal Ca2+ concentration is also important because it activates stromal interaction molecule 1 (STIM1), which then accumulates at ER–plasma membrane (PM) junctions. Within these narrow junctions, STIM1 in the ER membrane interacts directly with Orai1, which is a hexameric Ca2+ channel in the PM (Hou et al. 2012; Yen and Lewis 2018), causing it to open (Prakriya and Lewis 2015). The resulting store-operated Ca2+ entry (SOCE) is almost universally associated with IP3-evoked Ca2+ release. Hence, in response to the many extracellular stimuli that evoke IP3 formation, IP3Rs allow Ca2+ to be rapidly redistributed from the ER to the cytosol or other organelles and, by controlling the Ca2+ content of the ER, IP3Rs control Ca2+ flowing into the cell through SOCE (Fig. 1).
Figure 1.
IP3 receptors deliver Ca2+ to the cytosol and organelles. (A) By releasing Ca2+ from the endoplasmic reticulum (ER), IP3Rs can deliver Ca2+ to the cytosol, to other IP3Rs to ignite regenerative signals, or to the close appositions (membrane contact sites, supported by scaffold proteins) between the ER and other organelles. The latter include mitochondria and lysosomes, which can then accumulate Ca2+ via their low-affinity uptake systems from the high local Ca2+ concentration provided by IP3Rs. (B) Loss of Ca2+ from the ER also activates STIM1, which then binds to Orai at ER–plasma membrane (PM) junctions to initiate store-operated Ca2+ entry (SOCE).
The cytosolic Ca2+ signals evoked by IP3Rs or SOCE regulate diverse physiological responses, including exocrine secretion (Futatsugi et al. 2005), gluconeogenesis (Wang et al. 2012), embryological development (Kume et al. 1997; Uchida et al. 2010), transcription (Kar et al. 2012), nerve growth (Takei et al. 1998), and migration (Wei et al. 2009). The ability of IP3Rs to deliver Ca2+ to the mitochondrial uniporter (MCU) allows regulation of oxidative phosphorylation (Cardenas et al. 2010, 2016) and apoptosis (La Rovere et al. 2016), and Ca2+ delivery to lysosomes may allow them to accumulate Ca2+, which regulates their activities (Xu and Ren 2015). Dysregulation of IP3Rs is implicated in human diseases including Huntington's disease, Alzheimer's disease, amyotrophic lateral sclerosis, ataxias, autism, and cancer (Berridge 2016). Despite the importance of IP3Rs in both normal physiology and disease, the only known antagonists of IP3Rs (heparin, caffeine, and Xestospongin) lack specificity (Saleem et al. 2014). There is a pressing need for selective, membrane-permeant IP3R antagonists.
Ca2+-binding sites, which include those on the proteins that decode Ca2+ signals, are so abundant in cytosol that only some 1% of Ca2+ entering the cytosol remains free, although that buffering capacity does vary widely between cell types (Schwaller 2012). A cytosolic Ca2+ ion therefore spends the most time held by a buffer with which it moves more slowly than when free, and when it dissociates it is likely to diffuse freely for only a brief interval before it is recaptured by another buffer. Schwaller (2012) has suggested an apt analogy with Velcro to describe this behavior, which ensures that cytosolic Ca2+ diffuses slowly (Allbritton et al. 1992). This is an important feature that allows Ca2+ to linger at open channels and underpins its ability to serve as a local messenger. Hitherto, it has been assumed that IP3 diffuses freely in cytosol, based largely on measurements from cytoplasmic extracts of Xenopus oocytes in a rightly influential paper (Allbritton et al. 1992). Hence, the widely promulgated assumption has been that Ca2+ is a local messenger, while IP3 is a global messenger. However, IP3Rs in Xenopus ooctyes are concentrated in a narrow rim beneath the PM, whereas they are distributed throughout the cytoplasm of more typical cells (Thillaiappan et al. 2017). The cytoplasmic density of IP3Rs considered alongside their affinity for IP3 and the necessity for an IP3R to bind four molecules of IP3 before it can open (Alzayady et al. 2016) suggest that IP3Rs may, and prior to their activation, appreciably buffer IP3 (Taylor and Konieczny 2016). Estimates of IP3 diffusion in SH-SY5Y neuroblastoma cells, derived from measuring the extent to which IP3 focally released from a caged precursor spreads to initiate local Ca2+ signals, have elegantly confirmed that diffusion of IP3 in cells (diffusion coefficient, D ∼ 10 µm2/sec) is ∼30-fold slower than expected (Dickinson et al. 2016) and comparable to Ca2+ diffusion (D = 13–65 µm2/sec) (Allbritton et al. 1992). This suggests that both intracellular messengers, IP3 and Ca2+, can act locally within the confines of a typical cell (Dickinson et al. 2016). The activities of many cells are coordinated by Ca2+ waves that spread between cells (Leybaert and Sanderson 2012). Diffusion of IP3 through intercellular gap junctions is one means by which such Ca2+ waves are thought to propagate, but that idea was influenced by the assumption that IP3 diffusion is unhindered (Leybaert 2016). The discovery that IP3 diffuses slowly may require reappraisal of current thinking on how intercellular Ca2+ waves propagate and it invites speculation that there may be “highways” between cells wherein IP3 buffering is reduced to facilitate faster intercellular diffusion.
In a contribution to the first edition of this collection, we reviewed the history of IP3Rs (Taylor and Tovey 2012), noting that it was entwined with that of ryanodine receptors (RyRs), the close cousins of IP3Rs. The cross fertilization between studies of these two major families of intracellular Ca2+ release channels, with their many structural and functional similarities (Seo et al. 2012; des Georges et al. 2016), continues to provide important insight. That interplay will again be apparent in this review. We focus on recent progress toward understanding the structural basis of IP3R activation, evidence that IP3Rs are regulated by many additional proteins, and the organization of IP3Rs within ER membranes and the implications of that for SOCE. Other reviews provide readers with broader overviews (Foskett et al. 2007), historical perspectives (Berridge 2005; Rossi and Taylor 2019), and more focused considerations of IP3Rs and disease (Berridge 2016; Hisatsune and Mikoshiba 2017; Egorova and Bezprozvanny 2018), their regulation by proteolysis (Wang and Yule 2018) and other signals (Prole and Taylor 2016; Taylor 2017), the evolution of IP3Rs (Alzayady et al. 2015), and relationships between SOCE and IP3Rs (Taylor and Machaca 2019; Thillaiappan et al. 2019). We begin with a short overview of IP3Rs.
IP3 RECEPTORS ARE REGULATED BY IP3 AND Ca2+
Vertebrate genomes encode subunits for three closely related IP3R subunits (IP3R1–3), which assemble into homo- and heterotetrameric channels. The subunits are enormous (∼2700 residues), such that IP3Rs and RyRs (which are even larger, 4 × ∼5000 residues/RyR) are the largest known ion channels. The IP3R subtypes differ in their patterns of expression between tissues (Taylor et al. 1999) and perhaps in their subcellular distributions (Vervloessem et al. 2015), they have different affinities for IP3 (IP3R2 > IP3R1 > IP3R3) (Iwai et al. 2007), they differ in their associations with other proteins and in their modulation by additional signals (Prole and Taylor 2016), they appear to differ in their capacity to sustain oscillatory Ca2+ signals (Miyakawa et al. 1999; Wang and Yule 2018), and the functional consequences of perturbing IP3Rs differ for the different subtypes (Hisatsune and Mikoshiba 2017). Despite the differences, the core functional properties of all IP3Rs are similar and so too are their structures, consistent with the sequence conservation (∼70%) between subtypes (Fan et al. 2015, 2018; Paknejad and Hite 2018). All IP3Rs form large-conductance cation channels with relatively weak selectivity for Ca2+ over K+ (PCa/PK ∼ 7) (Foskett et al. 2007). The large conductance, which allows a single IP3R to conduct ∼105 Ca2+/sec or 1000 Ca2+ ions for each 10-msec opening (Vais et al. 2010), is important because it permits small numbers of IP3Rs to rapidly deliver large local Ca2+ signals to the cytosol. The second feature common to all IP3Rs, although historically it has spawned some controversy, is their biphasic regulation by [Ca2+]c. The activity of all IP3Rs is enhanced by modest increases in [Ca2+]c and inhibited by more substantial increases (Iino 1990; Bezprozvanny et al. 1991; Foskett et al. 2007). Whether IP3Rs are also regulated directly by Ca2+ within the ER lumen remains a contentious and unresolved issue (Irvine 1990; Nunn and Taylor 1992; Vais et al. 2012).
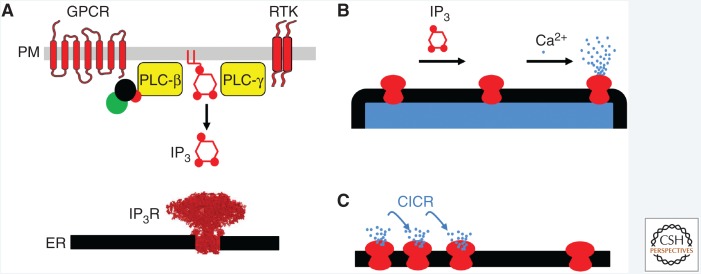
Activation of IP3Rs requires binding of both IP3 to all four of its subunits (Alzayady et al. 2016) and Ca2+ binding (Finch et al. 1991; Marchant and Taylor 1997). The simplest scheme envisages two Ca2+-binding sites associated with the IP3R (Marshall and Taylor 1994). Biophysical analyses tentatively suggest that the stimulatory Ca2+-binding site may be closer to the pore than the inhibitory site (Vais et al. 2012). Different schemes have been proposed to explain the interaction between IP3 and Ca2+, with IP3 proposed to regulate only the inhibitory Ca2+-binding site (reducing its affinity for Ca2+) (Mak et al. 1998; Vais et al. 2012) or both the inhibitory (reducing its Ca2+ affinity) and stimulatory (increasing its Ca2+ affinity) sites (Marchant and Taylor 1997; Adkins and Taylor 1999). Whatever the detailed mechanism, the outcome is that IP3 primes IP3Rs to respond to stimulation by Ca2+, by either divorcing the stimulatory and inhibitory effects, or by directly promoting Ca2+ binding to the stimulatory site (Fig. 2A,B). This interplay has important implications because it allows IP3Rs, in the presence of IP3, to propagate Ca2+ signals regeneratively by Ca2+-induced Ca2+ release (CICR) (Fig. 2C). We return to this feature later, but first we consider progress toward understanding the structural basis of how IP3 and Ca2+ binding together lead to opening of a large-conductance channel through which Ca2+ can leave the ER.
Figure 2.
IP3 receptors are stimulated by IP3 and Ca2+. (A) Many receptors, including G-protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs), can stimulate phospholipase C (PLC), leading to production of IP3, which then binds to IP3Rs in the endoplasmic reticulum (ER). (B) IP3 binding to IP3R primes them to bind Ca2+, which then stimulates the channel to open, allowing Ca2+ to flow out of the ER. (C) This dual regulation of IP3Rs by IP3 and Ca2+ allows them to mediate regenerative signals propagated by Ca2+-induced Ca2+ release (CICR). PM, Plasma membrane.
HOW TO OPEN AN IP3 RECEPTOR
How does IP3 binding to a site, the IP3-binding core (IBC), located ∼7 nm from the constriction within the closed channel, lead to channel opening? Progress toward answering this question has come from high-resolution crystal structures of the amino-terminal region of the IP3R, which includes the IBC (Bosanac et al. 2002, 2005; Lin et al. 2011; Seo et al. 2012) and of the entire cytosolic region (Hamada et al. 2017). These analyses capture structures of only one subunit of the tetrameric IP3R. Cryoelectron microscopy (cryo-EM) structures of IP3R1 (Fan et al. 2015, 2018) and of IP3R3 with and without IP3 and Ca2+ (Paknejad and Hite 2018) capture different states of the complete protein. Structural analyses of RyR fragments (Amador et al. 2009; Tung et al. 2010; Kimlicka et al. 2013; Liu et al. 2015) and of complete structures of RyR1 and RyR2 in various states (Efremov et al. 2015; Yan et al. 2015; Zalk et al. 2015; des Georges et al. 2016; Peng et al. 2016) also provide insight into the workings of IP3Rs.
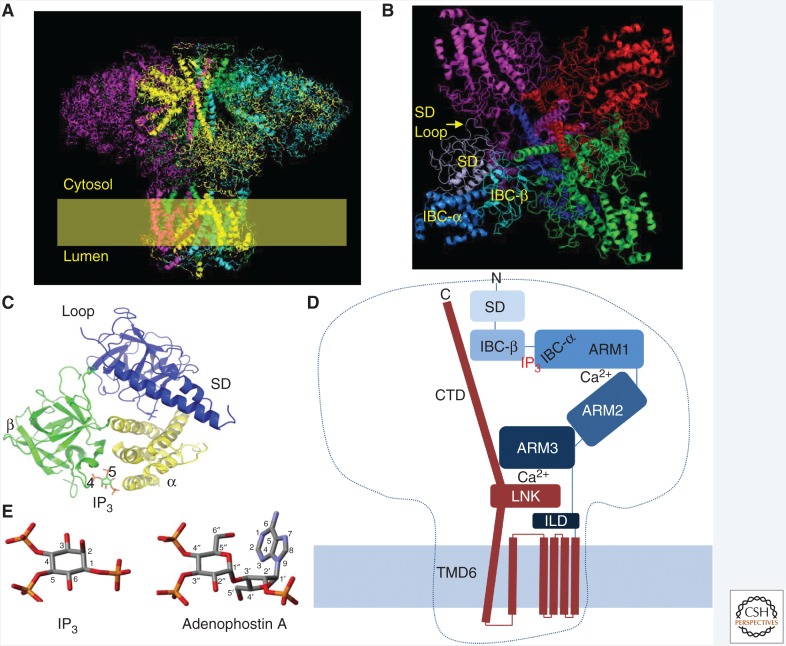
The structure of the IP3R resembles a square mushroom, most of which (∼90%) is in the cytosol (Fig. 3A; Fan et al. 2015; Paknejad and Hite 2018). Most of the stalk is embedded in the ER membrane and the cap, with a diameter of ∼25 nm, extends ∼13 nm into the cytosol. The large size of IP3Rs is relevant, not only for the opportunities it provides for cryo-EM analysis and the technical challenges it presents to crystallographers and molecular biologists, but also for its cellular functions. IP3Rs may, for example, be too large to fit within the narrow ER–PM junctions where SOCE occurs (Thillaiappan et al. 2017), but their large size allows IP3Rs to accrete accessory proteins (see below) and it may allow them to more effectively deliver Ca2+ to the surface of juxtaposed organelles, like mitochondria or lysosomes (Fig. 1A).
Figure 3.
IP3 receptor structure. (A) Cryoelectron microscopy (cryo-EM) of IP3R1 shows its tetrameric mushroom-like structure. (From Fan et al. 2015; adapted, with permission, from Springer Nature © 2015.) Subunits are color-coded. A similar structure has been reported for IP3R3 (Paknejad and Hite 2018). (B) View from the cytosol. (C) The amino-terminal region of each IP3R subunit comprises the suppressor domain (SD) with the “hot spot” loop through which it contacts an adjacent subunit; and the IP3-binding core (IBC), with its α and β domains. The essential 4- and 5-phosphates of IP3 interact predominantly with residues on the inner surface of the β and α domains, respectively, to trigger partial closure of clam-like IBC. (D) Schematic representation showing a single IP3R1 subunit, highlighting the IBC, where IP3 binds, two Ca2+-binding sites at interfaces between ARM1 and ARM2 domains, and between the LNK and ARM3 domains (Paknejad and Hite 2018). The α-helical rod (carboxy-terminal domain [CTD]) extending from LNK to the cap of the mushroom was resolved in structures from one laboratory (Fan et al. 2015, 2018), but not in the structures determined by another laboratory (Paknejad and Hite 2018). It is clear that conformational changes initiated by IP3 binding must pass through a critical nexus formed by the LNK (from the pore region) and intervening lateral domains (ILDs) (from the cytosolic domain). (E) Structures of IP3 and adenophostin A, showing how the latter has structures equivalent to the essential 4- and 5-phosphates of IP3.
Within the ER membrane, there are probably 24 transmembrane domains (TMDs), six contributed by residues toward the carboxy-terminal end of each IP3R subunit, although a recent report suggests the possible presence of two additional TMDs between TMD1 and TMD2 in IP3Rs (Fan et al. 2018; Paknejad and Hite 2018) and perhaps also in RyR (des Georges et al. 2016). The structure of the IP3R transmembrane region is similar in RyRs and, to a lesser extent, in voltage-gated cation channels, most of which have six TMDs per subunit. The ion-conducting path is lined by the four tilted TMD6 helices, which twist around each other. At the luminal end, there is a short (∼1 nm) “selectivity filter” within which conserved backbone carbonyls may form a cation-binding site, allowing hydrated cations to pass in single file. The selectivity filter, its supporting pore-loop helix and a flexible luminal loop are all formed by residues linking TMD5 to TMD6. Near the cytosolic end of TMD6, a narrow hydrophobic constriction blocks movement of ions in the closed channel. Minimally, the hydrophobic side chains of these residues (Phe2586 and Ile2590 in IP3R1) must move for the pore to open, but there may also be changes around the pore helix to displace a positively charged residue (His2541) that might otherwise impede cation movements (Fan et al. 2015). Opening of RyR1 is associated with splaying and bowing of TMD6, such that the occluding hydrophobic side chain is displaced (des Georges et al. 2016). A similar mechanism may open the IP3R pore (Fan et al. 2018). TMD6 extends well beyond the ER membrane (∼1.5 nm) and then terminates in a short α-helical bundle (the linker, LNK) that includes a Zn2+-finger motif and aligns parallel with the ER membrane (Fan et al. 2015; Paknejad and Hite 2018). The functional significance of the Zn2+- finger is unknown. Hence, structures formed by the TMD5-6 loop guard the luminal entrance to the pore, while the cytosolic exit is formed by the extended TMD6. Each of these regions is enriched in negatively charged residues that probably contribute to the cation selectivity of the IP3R.
IP3 binding to the clam-like IBC initiates IP3R activation (Fig. 3B,C). The IBC is located toward the amino terminal of the primary sequence of each subunit, and comprises two domains (α and β), with the pocket between them providing the positively charged residues that interact with IP3 (Bosanac et al. 2002; Seo et al. 2012; Paknejad and Hite 2018). All four subunits must bind IP3 before the channel can open (Alzayady et al. 2016). It is less clear whether all stimulatory Ca2+-binding sites must be occupied for channel opening (Vais et al. 2012). The amino-terminal region forms a triangular structure at the top of the mushroom, with the suppressor domain (SD) (residues 1–223; also known as β-trefoil domain 1 [BTF1]) and IBC-β (BTF2) lining the cytosolic exit at the top of the IP3R. The subunits interact through a loop within the SD that contacts IBC-β of a neighboring subunit (Fig. 3B,C; Seo et al. 2012; Fan et al. 2015; Hamada et al. 2017; Paknejad and Hite 2018). Mutations within this “hot spot” loop disrupt gating of IP3R (Yamazaki et al. 2010) and RyR (Amador et al. 2009). IBC-α sits behind the β-trefoil structures at the tip of a series of largely α-helical domains (ARM 1–3) that extend in a boomerang-like shape to meet the LNK domain (Fig. 3A,D).
The two critical phosphate groups of IP3 (P-4 and P-5) interact predominantly with basic residues (Arg and Lys) lining opposing sides of the IBC clam: P-4 with IBC-β and P-5 with IBC-α (Fig. 3C; Bosanac et al. 2002). These interactions allow IP3 to partially close the clam-like IBC (Lin et al. 2011; Seo et al. 2012; Paknejad and Hite 2018), and they elegantly rationalize the long-established conclusion that all known agonists of IP3Rs have structures equivalent to the 4- and 5-phosphates of IP3. The structures also demonstrate how endogenous dephosphorylation of IP3 to 1,4-IP2 effectively terminates Ca2+ signaling. The importance of the clam closure is reinforced by results with adenophostin A analogs. Adenophostin A is a fungal product with 10-fold greater affinity for IP3Rs than IP3, and with structures, its 3″- and 4″-phosphates, equivalent to the 5- and 4-phosphates of IP3, respectively (Fig. 3E; Rossi et al. 2010; but see Fan et al. 2018). Whereas loss of the 5-phosphate group from IP3 abolishes activity, loss of the equivalent phosphate from adenophostin A (3″-phosphate) leaves some residual activity because the adenine moiety of adenophostin A can interact with IBC-α and so presumably mediate clam closure in the absence of the usual phosphate group (Sureshan et al. 2009). Closure of the clam-like IBC by IP3 binding is linked to IP3R gating through the SD, but the exact sequence of conformational changes is unresolved. It may be that movement of IBC-α relative to a firmly anchored IBC-β/SD reorients the ARM domains (Paknejad and Hite 2018), or it may be that tethering of IBC-α to the SD causes reorientation of the SD and disruption of intersubunit interactions (Li et al. 2009; Seo et al. 2012; des Georges et al. 2016). It is, however, clear that IP3-evoked conformational changes must pass through the contacts between ARM3 and LNK domains to reach the occluded pore (Fig. 3D).
ARM3 terminates in the intervening lateral domain (ILD), which sits between the cytosolic structures and the TMDs. ILD runs largely parallel to the ER membrane, and comprises two β strands (which lie immediately beneath ARM3) followed by a helix-turn-helix motif that links to TMD1 (Fig. 3D). The LNK domain (an extension of TMD6) is positioned between the β- and α-helical components of the ILD. Hence, interleaved structures formed by extensions of ARM3 (ILD) and TMD6 (LNK) form a critical nexus between the cytosolic region and the pore of the IP3R. Mutations within ILD disrupt IP3R function (Hamada et al. 2017), and the LNK domain contributes a conserved residue to a Ca2+-binding site at the base of the ARM3 domain (Fig. 3D; Paknejad and Hite 2018). This Ca2+-binding site, formed by residues at the interface of the cytosolic (base of ARM3) and pore (LNK domain) regions, is absolutely conserved in RyRs and IP3Rs (des Georges et al. 2016). We note that in both RyR (Glu4032 in RyR1) (Du and MacLennan 1998) and IP3R1 (Glu2100) (Miyakawa et al. 2001), a conserved glutamate was proposed to contribute to the stimulatory Ca2+-binding site. Indeed, and somewhat perplexingly, mutation of this residue affected both stimulation and inhibition of IP3Rs by Ca2+ (Miyakawa et al. 2001). It is now clear from structural analyses that these conserved glutamates do not coordinate Ca2+ in either RyR (des Georges et al. 2016) or IP3R (Paknejad and Hite 2018). It is equally clear that the EF-hand domain of RyR, which projects from a structure equivalent to ARM3 of the IP3R, is absent from IP3Rs (Fan et al. 2015), and nor does the EF-hand provide the essential Ca2+ regulation of RyRs (Guo et al. 2016). Hence, the conserved Ca2+-binding site at the interface between the cytosolic (ARM3) and channel domains (LNK) suggests an appealing, but untested, link between Ca2+ and gating of the IP3R, namely that IP3 binding stabilizes this Ca2+-binding site and Ca2+ binding to it then leads to opening of the pore. This proposal also aligns with the suggestion that the stimulatory Ca2+-binding site may be close to the pore (Vais et al. 2012). The high-resolution structure of IP3R3 recently identified another Ca2+-binding site, which is also formed by residues provided by different domains across an interface between them (ARM1 and ARM2) (Paknejad and Hite 2018). It is not yet clear how (or whether) either of these Ca2+-binding sites relates to stimulation and inhibition of IP3Rs by cytosolic Ca2+. It is, however, intriguing that both sites are formed by residues contributed by different domains, consistent with IP3-evoked rigid-body domain movements influencing whether Ca2+ binds to the sites.
Recent progress has brought us close to seeing how IP3 binding to the IBC causes pore residues some 7 nm away to move and allow Ca2+ to pass from the ER lumen to the cytosol (Fan et al. 2018; Paknejad and Hite 2018). IP3 initiates IP3R activation by causing closure of the IBC. That conformational change must then pass onward through a critical nexus formed between the cytoplasmic and pore domains at the ILD–LNK complex. Since IP3 primes IP3Rs to bind Ca2+, which then triggers channel opening (Fig. 2B; Adkins and Taylor 1999), we speculate that an intervening step between IP3 binding to the IBC and pore opening involves rearrangement of Ca2+-binding sites at the ARM1–ARM2 interface or at the LNK–ARM3 nexus. The conformational changes evoked by Ca2+ binding must then pass through the ILD–LNK complex to cause movement of a hydrophobic residue in TMD6 and allow opening of the pore (Fig. 3D).
IP3 RECEPTORS AS SIGNALING HUBS
IP3 and Ca2+ are the essential regulators of IP3R gating (Fig. 2), but many intracellular signals, including ATP (Wagner and Yule 2012), cAMP (Taylor 2017), H+ (Worley et al. 1987), NADH (Kaplin et al. 1996), and the redox state (Joseph 2010; Joseph et al. 2018), can modulate this regulation. IP3Rs can also be regulated by covalent modifications, including phosphorylation, controlled by more than a dozen protein kinases and phosphatases, ubiquitination (Wojcikiewicz 2018), transglutaminase-mediated cross-linking of Gln-Lys residues (Hamada et al. 2014), and perhaps nitrosylation (Pan et al. 2008). Proteolysis of IP3Rs by caspase-3 (Hirota et al. 1999) or calpains (Magnusson et al. 1993) may contribute to their degradation. But after limited proteolysis, IP3R fragments remain associated as a functional channel. Intriguingly, native and cleaved IP3Rs respond differently, suggesting that proteolysis can provide more subtle regulation than merely down-regulating IP3Rs (Alzayady et al. 2013; Wang et al. 2017).
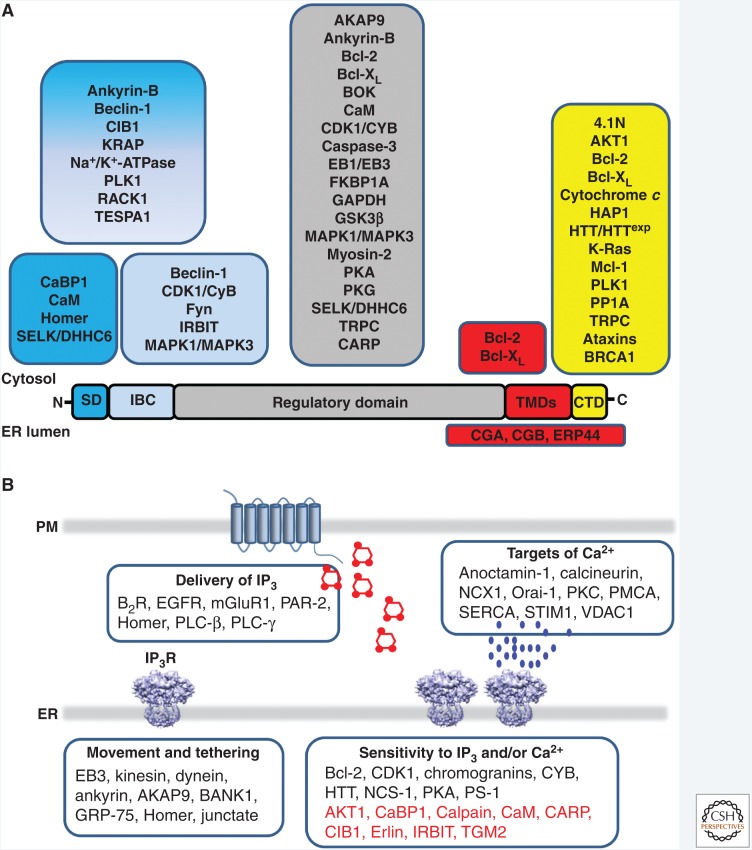
More impressive still is the huge array of proteins that associate with IP3Rs (Fig. 4A; Prole and Taylor 2016). These proteins, which may associate with cytosolic or luminal parts of the IP3R, can regulate the distribution of IP3Rs (Geyer et al. 2015), their affiliation with signaling pathways that deliver IP3 (Tu et al. 1998) or cAMP (Tovey et al. 2008) to IP3Rs, the sensitivity of Ca2+ release to IP3 and Ca2+, and they may allow IP3Rs to deliver Ca2+ to specific decoding proteins (Fig. 4B; Szabadkai et al. 2006). Many of these modulatory influences are likely to be context specific, determined, for example, by IP3R subtype, cell type, and perhaps contingent on interactions between modulators (Ivanova et al. 2014; Prole and Taylor 2016). IP3R-binding protein released with IP3 (IRBIT), for example, is a protein that competes with IP3 for binding to the IBC, but it does so only after IRBIT phosphorylation (Ando et al. 2014).
Figure 4.
IP3 receptors interact with many accessory proteins. (A) Proteins that interact with IP3Rs shown according to the regions of the IP3R with which they interact. (From Prole and Taylor 2016; adapted under the terms of the Creative Commons Attribution License [CC BY, 2016].) AKAP9, A-kinase anchoring protein 9; AKT1, RAC-α serine/threonine protein kinase; BANK1, B-cell scaffold protein with ankyrin repeats; Bcl-2, B-cell lymphoma 2; B2R, bradykinin B2 receptor; BRCA1, breast and ovarian cancer susceptibility gene 1; CaBP1, Ca2+-binding protein 1; CaM, calmodulin; CARP, carbonic anhydrase-related protein; CDK1, cyclin-dependent kinase 1; CIB1, Ca2+- and integrin-binding protein 1; CYB, cyclin-B1; EB3, end-binding protein 3; EGFR, epidermal growth factor receptor; GRP-75, glucose-regulated protein 75; HTT, huntingtin; IRBIT, IP3-binding protein released with IP3; mGluR1, metabotropic glutamate receptor 1; NCS-1, neuronal Ca2+-sensor 1; PAR-2, protease-activated receptor 2; PKA, protein kinase A; PLC-β, phospholipase Cβ; PLC-γ, phospholipase Cγ, PS-1/PS-2, presenilin 1/2; NCX1, Na+/Ca2+ exchanger 1; PKC, protein kinase C; PMCA, plasma membrane Ca2+-ATPase; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; STIM1, stromal interaction molecule 1; TGM2, transglutaminase-2; VDAC1, voltage-dependent anion channel 1. Proteins shown in red inhibit the activity of IP3Rs. Original sources for additional interactions shown here are ataxins (Chen et al. 2008; Liu et al. 2009), CARP (Hirota et al. 2003), and BRCA1 (Hedgepeth et al. 2015). (B) Examples of proteins shown according to whether they facilitate delivery of IP3 to IP3Rs, intracellular distribution of IP3Rs, IP3R activation or delivery of Ca2+ to specific targets.
Modulatory proteins also provide links between IP3Rs and human diseases, additional to those arising from loss or mutation of IP3Rs (Berridge 2016; Casey et al. 2017; Hisatsune and Mikoshiba 2017; Terry et al. 2018). The mutant forms of Huntingtin associated with Huntington's disease, mutant ataxins associated with spinocerebellar ataxias, and mutant presenilins associated with inherited forms of Alzheimer's disease, for example, have each been reported to enhance IP3-evoked Ca2+ signals (Chen et al. 2008; Cheung et al. 2008, 2010; Liu et al. 2009; Egorova and Bezprozvanny 2018).
IP3R-evoked Ca2+ signals may also be targets for cancer therapeutics (Vervloessem et al. 2018). Transfer of Ca2+ from the ER to mitochondria via IP3Rs stimulates mitochondrial ATP production, but excessive Ca2+ transfer triggers apoptosis (Cardenas et al. 2010). ER-mitochondria Ca2+ transfer can thereby promote cell survival or death, according to the magnitude of the transfer. It has been suggested that tumor cells are “addicted” to ER-mitochondria Ca2+ transfer and so particularly susceptible to its inhibition because they lack the robust, protective autophagy response of normal cells (Cardenas et al. 2016). Here, inhibition of Ca2+ transfer to mitochondria might provide an opportunity to selectively kill cancer cells by necrosis (Cardenas et al. 2016). Conversely, exaggerating the transfer of Ca2+ from ER to mitochondria can trigger apoptosis. The tumor suppressors, Bap1 (Bononi et al. 2013) and PTEN (Kuchay et al. 2017), achieve this by protecting IP3Rs from proteosomal degradation, allowing sustained Ca2+ transfer to mitochondria and enhanced sensitivity to stimuli that promote apoptosis. The proapoptotic protein, Bok, achieves the same effect by protecting IP3Rs from cleavage by caspase (Schulman et al. 2013). Other anti-apoptotic members of the Bcl-2 family of proteins (e.g., Bcl-2, Bcl-XL) are also proposed to influence ER-mitochondrial Ca2+ transfer, and thereby apoptosis, but by regulating the activity, rather than the expression, of IP3Rs (discussed in Vervloessem et al. 2018). These interactions are now attracting interest as potential therapeutic targets in cancer.
Most accessory proteins that affect IP3-evoked Ca2+ release appear to do so indirectly by influencing IP3 or Ca2+ binding or the interplay between them. However, a few proteins, including Gβγ, which may mimic IP3 (Zeng et al. 2003), and the Ca2+-binding proteins, CIB1(Ca2+- and integrin-binding protein) (White et al. 2006) and CaBP1 (Yang et al. 2002), have been claimed to reversibly gate IP3Rs directly, bypassing the need for IP3. However, the suggestion that CaBP1 stimulates IP3Rs has been challenged by three different groups (Haynes et al. 2004; Nadif Kasri et al. 2004; Li et al. 2013). There is a need to establish whether there are additional physiological means, other than through IP3 and Ca2+, to stimulate IP3R activation.
We need also to consider whether the scaffolding of signaling proteins by IP3Rs serves only to funnel information toward an IP3-evoked Ca2+ signal, or might these scaffolds fulfil additional, and possibly unrelated, roles. There is, for example, evidence that IP3Rs, independent of their ability to release Ca2+ from the ER, can modulate SOCE (Chakraborty et al. 2016) and, since the mechanisms are not yet clear (Thillaiappan et al. 2019), they may arise through scaffolding of proteins by IP3Rs.
Space limitations forbid comprehensive discussion of IP3Rs and their accessory proteins (additional examples can be found in Prole and Taylor 2016). Instead, we show some of the proteins that intercede at different levels, from facilitating delivery of IP3 to IP3Rs to guiding presentation of their Ca2+ signals to specific targets (Fig. 4B). A clear theme is that assembly of signaling proteins around IP3Rs provides many opportunities for local integration and processing of information, before it is returned to the cell as a Ca2+ signal.
LICENSING IP3 RECEPTORS TO RESPOND
High-resolution optical microscopy with fluorescent Ca2+ indicators has revealed the subcellular organization of the Ca2+ signals evoked by IP3. Since these recordings have succeeded in observing the openings of single IP3Rs in situ, they have been aptly named “optical patch-clamp” recording (Parker and Smith 2010). The results of these analyses show that low concentrations of IP3 evoke short-lived openings of single IP3Rs (“Ca2+ blips”). Greater concentrations of IP3 evoke “Ca2+ puffs,” which typically last ∼100 msec and report the coordinated opening of a few IP3Rs within a small cluster. These are thought to arise when Ca2+ released by one IP3R rapidly ignites the activity of its IP3-bound neighbors through CICR (Fig. 2C; Smith and Parker 2009). Ca2+ puffs may be the building blocks of all IP3-evoked Ca2+ signals because all three IP3R subtypes can generate Ca2+ puffs with broadly similar properties (Mataragka and Taylor 2018). As stimulus intensities increase further, Ca2+ diffusing from one Ca2+ puff can recruit the activity of a more distant site, generating a regenerative Ca2+ wave that spreads across the cell (Rooney et al. 1990; Bootman et al. 1997; Marchant et al. 1999). Further increases in stimulus intensity increase the frequency of the Ca2+ waves. These are manifest as Ca2+ spikes or oscillations at the whole-cell level, as first reported by Peter Cobbold (Woods et al. 1986). Hence, both the spatial and temporal organization of cytosolic Ca2+ signals changes with stimulus intensity, and this has important functional consequences (Thurley et al. 2014; Samanta and Parekh 2017). It is immediately apparent that both the coregulation of IP3Rs by Ca2+ and IP3, and the geographical relationships between IP3Rs, are important determinants of how far Ca2+ signals progress through this hierarchy of Ca2+ release events (Fig. 2).
Considerable evidence suggests that most IP3Rs are mobile within ER membranes (Ferreri-Jacobia et al. 2005; Fukatsu et al. 2010; Pantazaka and Taylor 2011; Smith et al. 2014; Thillaiappan et al. 2017) and that IP3 and/or Ca2+ can regulate clustering of IP3Rs (Wilson et al. 1998; Iwai et al. 2005; Tateishi et al. 2005; Chalmers et al. 2006; Tojyo et al. 2008; Rahman and Taylor 2009; Pantazaka and Taylor 2011). There is also evidence that clustering may be required for cells to generate effective Ca2+ signals (Geyer et al. 2015). There is, however, a conundrum because, whereas most IP3Rs are mobile, the Ca2+ puffs evoked by IP3 repeatedly initiate at the same fixed sites within a cell (Thomas et al. 1998; Smith and Parker 2009; Smith et al. 2009b; Keebler and Taylor 2017; Mataragka and Taylor 2018). Why should a small fraction of the IP3Rs in a cell assume complete responsibility for generating Ca2+ puffs?
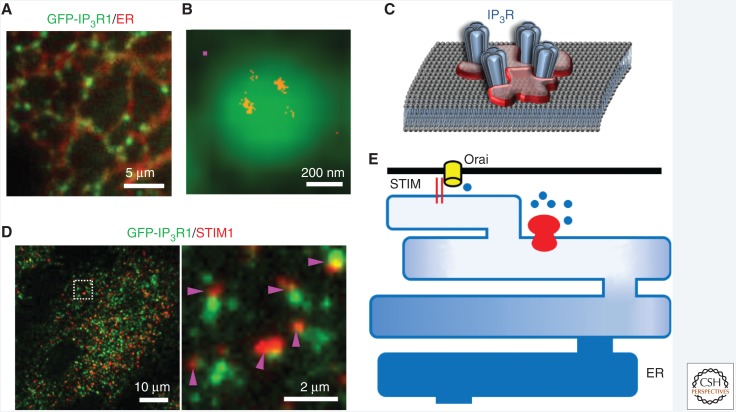
We recently addressed this problem using HeLa cells in which gene editing was used to tag endogenous IP3R1 with EGFP so that we could simultaneously observe the distribution of IP3Rs and the Ca2+ signals they evoke (Thillaiappan et al. 2017). The results show that most IP3Rs are assembled into small clusters or puncta, and while the number of IP3Rs within each cluster varies considerably, there is an average of ∼8 IP3Rs per cluster. Super-resolution imaging reveals that although IP3Rs remain within their puncta, they are often too far apart (>100 nm) for the IP3Rs to be held together by direct interactions between them (Fig. 5A,B). Instead, we suggest that a scaffold, which may be formed by proteins or lipids, corrals IP3Rs into puncta (Fig. 5C). The scaffold has yet to be identified. Most IP3R puncta, which are present in ER throughout the cell, are mobile (>70%). Most IP3Rs move by diffusion in ER membranes, but a small faction is moved directionally by microtubule motors. Others have shown (Smith et al. 2009a; Ellefsen and Parker 2018), and we have confirmed (Keebler and Taylor 2017; Thillaiappan et al. 2017), that although IP3Rs are present throughout the cell, almost all Ca2+ puffs arise from IP3Rs within ER that lies close to the PM. This occurs whether IP3 is delivered to the cell through endogenous receptor-activated signaling pathways, which might locally deliver IP3 immediately beneath the PM, or by flash-photolysis of caged-IP3, which uniformly delivers IP3 throughout the cell. Hence, the propensity of near-PM IP3Rs to respond does not arise from selective delivery of IP3. Indeed, exactly the same Ca2+ release sites respond after photolysis of caged-IP3 or stimulation of endogenous signaling pathways (Keebler and Taylor 2017; Lock et al. 2017). Furthermore, it is only the immobile IP3R puncta adjacent to the PM that initiate Ca2+ puffs (Thillaiappan et al. 2017). It is, therefore, only a small fraction of the several thousand IP3Rs expressed in a cell that are competent, or “licensed,” to respond. It will be important to identify the factor that licenses this small cohort of IP3Rs to respond to IP3.
Figure 5.
Immobile IP3 receptor clusters initiate Ca2+signals. (A) HeLa cells with endogenous IP3R1 tagged with EGFP showing IP3R puncta (green) within endoplasmic reticulum (ER) membranes (red). (B) Diffraction-limited image of a punctum recorded using total internal reflection fluorescence (TIRF) microscopy, and the superimposed super-resolution (STORM) image showing IP3Rs (red) within the punctum. The magenta square indicates the approximate size of a single tetrameric IP3R. (C) IP3Rs appear to be diffusively distributed within a punctum, suggesting the need for a scaffold to corral IP3Rs. (D) TIRF images of a HeLa cell in which the ER has been depleted of Ca2+ by treatment with thapsigargin, showing the distribution of STIM1 (red) and IP3R (green). The enlarged image shows that stromal interaction molecule (STIM) puncta form alongside the ER where the immobile IP3Rs that are licensed to respond are parked. (Data results for A–D are from Thillaiappan et al. 2017.) (E) Licensed IP3Rs parked alongside the ER–PM junctions where store-operated Ca2+ entry (SOCE) occurs may allow substantial loss of Ca2+ from that part of the ER without causing appreciable Ca2+ loss from the remaining ER. The ER–PM junction with its associated licensed IP3Rs may constitute the basic functional unit for SOCE.
We noted in a preceding section that IP3R activation and SOCE are an almost universal partnership (Fig. 1B). It is, therefore, intriguing that the ER–PM junctions where STIM1 accumulates after loss of Ca2+ from the ER sit alongside the sites where licensed IP3Rs reside (Fig. 5D; Thillaiappan et al. 2017). This too may have functional consequences by allowing active IP3Rs to locally and substantially deplete ER specifically located alongside ER–PM junctions. This provides a possible answer to another problem, namely, that Ca2+ within the ER lumen has many roles beyond Ca2+ signaling—it is required for protein folding, for example—yet STIM1 is activated only after substantial loss of Ca2+ from the ER (Brandman et al. 2007; Luik et al. 2008; Bird et al. 2009). How does the ER regulate SOCE without compromising its other functions? We suggest that licensed IP3Rs held alongside ER–PM junctions may be the basic functional units of SOCE, allowing IP3 to cause a substantial loss of Ca2+ from the ER that regulates SOCE without trespassing into the remaining ER (Fig. 5E; Thillaiappan et al. 2017, 2019; Taylor and Machaca 2019).
CONCLUDING REMARKS
IP3Rs allow Ca2+ to be redistributed, in response to receptor activation, from the ER lumen to the cytosol or other organelles, and they ultimately control SOCE. Coregulation of IP3Rs by IP3 and Ca2+ endows IP3Rs with a capacity to mediate regenerative Ca2+ signals, which give rise to a hierarchy of Ca2+-release events as stimulus intensities increase. Recent progress with x-ray crystallography and cryo-EM have brought us closer to understanding how IP3 binding to a site located some 7 nm from the pore, triggers Ca2+ binding and thence opening of the pore. High-resolution measurements of Ca2+ signals in living cells have revealed that only a small cohort of immobile IP3R clusters, parked alongside the ER–PM junctions where SOCE occurs, are licensed to respond. These IP3Rs both mediate Ca2+ puffs and they may selectively regulate SOCE.
ACKNOWLEDGMENTS
Work from the authors’ laboratory is supported by the Biotechnology and Biological Sciences Research Council UK (Grant No. BB/P005330/1 to C.W.T.) and the Wellcome Trust (Grant No. 101844 to C.W.T.).
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Adkins CE, Taylor CW. 1999. Lateral inhibition of inositol 1,4,5-trisphosphate receptors by cytosolic Ca2+. Curr Biol 9: 1115–1118. 10.1016/S0960-9822(99)80481-3 [DOI] [PubMed] [Google Scholar]
- Allbritton NL, Meyer T, Stryer L. 1992. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258: 1812–1815. 10.1126/science.1465619 [DOI] [PubMed] [Google Scholar]
- Alzayady KJ, Chandrasekhar R, Yule DI. 2013. Fragmented inositol 1,4,5-trisphosphate receptors retain tetrameric architecture and form functional Ca2+ release channels. J Biol Chem 288: 11122–11134. 10.1074/jbc.M113.453241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzayady KJ, Sebe-Pedros A, Chandrasekhar R, Wang L, Ruiz-Trillo I, Yule DI. 2015. Tracing the evolutionary history of inositol, 1, 4, 5-trisphosphate receptor: Insights from analyses of Capsaspora owczarzaki Ca2+ release channel orthologs. Mol Biol Evol 32: 2236–2253. 10.1093/molbev/msv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzayady KJ, Wang L, Chandrasekhar R, Wagner LE II, Van Petegem F, Yule DI. 2016. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci Signal 9: ra35 10.1126/scisignal.aad6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador FJ, Liu S, Ishiyama N, Plevin MJ, Wilson A, Maclennan DH, Ikura M. 2009. Crystal structure of type I ryanodine receptor amino-terminal β-trefoil domain reveals a disease-associated mutation “hot spot” loop. Proc Natl Acad Sci 106: 11040–11044. 10.1073/pnas.0905186106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H, Kawaai K, Mikoshiba K. 2014. IRBIT: A regulator of ion channels and ion transporters. Biochim Biophys Acta 1843: 2195–2204. 10.1016/j.bbamcr.2014.01.031 [DOI] [PubMed] [Google Scholar]
- Atakpa P, Thillaiappan NB, Mataragka S, Prole DL, Taylor CW. 2018. IP3 receptors preferentially associate with ER-lysosome contact sites and selectively deliver Ca2+ to lysosomes. Cell Rep 25: 3180–3193. 10.1016/j. celrep.2018.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H. 2013. Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci 14: 593–608. 10.1038/nrn3531 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 1993. Inositol trisphosphate and calcium signalling. Nature 361: 315–325. 10.1038/361315a0 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2005. Unlocking the secrets of cell signaling. Annu Rev Physiol 67: 1–21. 10.1146/annurev.physiol.67.040103.152647 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2016. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96: 1261–1296. 10.1152/physrev.00006.2016 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754. 10.1038/351751a0 [DOI] [PubMed] [Google Scholar]
- Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RR, Putney JW Jr. 2009. STIM1 is a calcium sensor specialized for digital signaling. Curr Biol 19: 1724–1729. 10.1016/j.cub.2009.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP, Pinton P. 2013. Identification of PTEN at the ER and MAMs and its regulation of Ca2+ signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ 20: 1631–1643. 10.1038/cdd.2013.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ, Lipp P. 1997. Cooking with calcium: The recipes for composing global signals from elementary events. Cell 91: 367–373. 10.1016/S0092-8674(00)80420-1 [DOI] [PubMed] [Google Scholar]
- Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, et al. 2002. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature 420: 696–700. 10.1038/nature01268 [DOI] [PubMed] [Google Scholar]
- Bosanac I, Yamazaki H, Matsu-ura T, Michikawa T, Mikoshiba K, Ikura M. 2005. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol Cell 17: 193–203. 10.1016/j.molcel.2004.11.047 [DOI] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. 2007. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131: 1327–1339. 10.1016/j.cell.2007.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, et al. 2010. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142: 270–283. 10.1016/j.cell.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, Muller M, McNeal A, Lovy A, Jana F, Bustos G, Urra F, Smith N, Molgo J, Diehl JA, et al. 2016. Selective vulnerability of cancer cells by inhibition of Ca2+ transfer from endoplasmic reticulum to mitochondria. Cell Rep 14: 2313–2324. 10.1016/j.celrep.2016.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JP, Hirouchi T, Hisatsune C, Lynch B, Murphy R, Dunne AM, Miyamoto A, Ennis S, van der Spek N, O'Hici B, et al. 2017. A novel gain-of-function mutation in the ITPR1 suppressor domain causes spinocerebellar ataxia with altered Ca2+ signal patterns. J Neurol 264: 1444–1453. 10.1007/s00415-017-8545-5 [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Deb BK, Chorna T, Konieczny V, Taylor CW, Hasan G. 2016. Mutant IP3 receptors attenuate store-operated Ca2+ entry by destabilizing STIM-Orai interactions in Drosophila neurons. J Cell Sci 129: 3903–3910. 10.1242/jcs.191585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers M, Schell MJ, Thorn P. 2006. Agonist-evoked inositol trisphosphate receptor (IP3R) clustering is not dependent on changes in the structure of the endoplasmic reticulum. Biochem J 394: 57–66. 10.1042/BJ20051130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Tang TS, Tu H, Nelson O, Pook M, Hammer R, Nukina N, Bezprozvanny I. 2008. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci 28: 12713–12724. 10.1523/jneurosci.3909-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. 2008. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58: 871–883. 10.1016/j.neuron.2008.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. 2010. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal 3: ra22 10.1126/scisignal.2000818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Weaver D, Hajnoczky G. 2018. Endoplasmic reticular-mitochondrial contactology: Structure and signaling functions. Trends Cell Biol 28: 523–540. 10.1016/j.tcb.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Georges A, Clarke OB, Zalk R, Yuan Q, Condon KJ, Grassucci RA, Hendrickson WA, Marks AR, Frank J. 2016. Structural basis for gating and activation of RyR1. Cell 167: 145–157.e17. 10.1016/j.cell.2016.08.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson GD, Ellefsen KL, Dawson SP, Pearson JE, Parker I. 2016. Hindered cytoplasmic diffusion of inositol trisphosphate restricts its cellular range of action. Sci Signal 9: ra108 10.1126/scisignal.aag1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du GG, Maclennan DH. 1998. Functional consequences of mutations of conserved, polar amino acids in transmembrane sequences of the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J Biol Chem 273: 31867–31872. 10.1074/jbc.273.48.31867 [DOI] [PubMed] [Google Scholar]
- Echevarría W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. 2003. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol 5: 440–446. 10.1038/ncb980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremov RG, Leitner A, Aebersold R, Raunser S. 2015. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 517: 39–43. 10.1038/nature13916 [DOI] [PubMed] [Google Scholar]
- Egorova PA, Bezprozvanny I. 2018. Inositol 1,4,5-trisphosphate receptors and neurodegenerative disorders. FEBS J 285: 3547–3565. 10.1111/febs.14366 [DOI] [PubMed] [Google Scholar]
- Ellefsen KL, Parker I. 2018. Dynamic Ca2+ imaging with a simplified lattice light-sheet microscope: A sideways view of subcellular Ca2+ puffs. Cell Calcium 71: 34–44. 10.1016/j.ceca.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Baker ML, Wang Z, Baker MR, Sinyagovskiy PA, Chiu W, Ludtke SJ, Serysheva II. 2015. Gating machinery of InsP3R channels revealed by electron cryomicroscopy. Nature 527: 336–341. 10.1038/nature15249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Baker MR, Wang Z, Seryshev AB, Ludtke SJ, Baker ML, Serysheva II. 2018. Cryo-EM reveals ligand induced allostery underlying InsP3R channel gating. Cell Res 28: 1158–1170. 10.1038/s41422-018-0108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri-Jacobia M, Mak DOD, Foskett JK. 2005. Translational mobility of the type 3 inositol 1,4,5-trisphosphate receptor Ca2+ release channel in endoplasmic reticulum membrane. J Biol Chem 280: 3824–3831. 10.1074/jbc.M409462200 [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. 1991. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science 252: 443–446. 10.1126/science.2017683 [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658. 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukatsu K, Bannai H, Inoue T, Mikoshiba K. 2010. Lateral diffusion of inositol 1,4,5-trisphosphate receptor type 1 in Purkinje cells is regulated by calcium and actin filaments. J Neurochem 114: 1720–1733. 10.1111/j.1471-4159.2010.06885.x [DOI] [PubMed] [Google Scholar]
- Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, et al. 2005. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309: 2232–2234. 10.1126/science.1114110 [DOI] [PubMed] [Google Scholar]
- Garrity AG, Wang W, Collier CM, Levey SA, Gao Q, Xu H. 2016. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. eLife 5: e15887 10.7554/eLife.15887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M, Huang F, Sun Y, Vogel SM, Malik AB, Taylor CW, Komarova YA. 2015. Microtubule-associated protein EB3 regulates IP3 receptor clustering and Ca2+ signaling in endothelial cells. Cell Rep 12: 79–89. 10.1016/j.celrep.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Sun B, Xiao Z, Liu Y, Wang Y, Zhang L, Wang R, Chen SR. 2016. The EF-hand Ca2+ binding domain is not required for cytosolic Ca2+ activation of the cardiac ryanodine receptor. J Biol Chem 291: 2150–2160. 10.1074/jbc.M115.693325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K, Terauchi A, Nakamura K, Higo T, Nukina N, Matsumoto N, Hisatsune C, Nakamura T, Mikoshiba K. 2014. Aberrant calcium signaling by transglutaminase-mediated posttranslational modification of inositol 1,4,5-trisphosphate receptors. Proc Natl Acad Sci 111: E3966–E3975. 10.1073/pnas.1409730111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K, Miyatake H, Terauchi A, Mikoshiba K. 2017. IP3-mediated gating mechanism of the IP3 receptor revealed by mutagenesis and X-ray crystallography. Proc Natl Acad Sci 114: 4661–4666. 10.1073/pnas.1701420114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LP, Tepikin AV, Burgoyne RD. 2004. Calcium-binding protein 1 is an inhibitor of agonist-evoked, inositol 1,4,5-trisphosphate-mediated calcium signaling. J Biol Chem 279: 547–555. 10.1074/jbc.M309617200 [DOI] [PubMed] [Google Scholar]
- Hedgepeth SC, Garcia MI, Wagner LE II, Rodriguez AM, Chintapalli SV, Snyder RR, Hankins GD, Henderson BR, Brodie KM, Yule DI, et al. 2015. The BRCA1 tumor suppressor binds to inositol 1,4,5-trisphosphate receptors to stimulate apoptotic calcium release. J Biol Chem 290: 7304–7313. 10.1074/jbc.M114.611186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota J, Furuichi T, Mikoshiba K. 1999. Inositol 1,4,5-trisphosphate receptor type 1 is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner. J Biol Chem 274: 34433–34437. 10.1074/jbc.274.48.34433 [DOI] [PubMed] [Google Scholar]
- Hirota J, Ando H, Hamada K, Mikoshiba K. 2003. Carbonic anhydrase-related protein is a novel binding protein for inositol 1,4,5-trisphosphate receptor type 1. Biochem J 372: 435–441. 10.1042/bj20030110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune C, Mikoshiba K. 2017. IP3 receptor mutations and brain diseases in human and rodents. J Neurochem 141: 790–807. 10.1111/jnc.13991 [DOI] [PubMed] [Google Scholar]
- Hou X, Pedi L, Diver MM, Long SB. 2012. Crystal structure of the calcium release-activated calcium channel Orai. Science 338: 1308–1313. 10.1126/science.1228757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. 1990. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122. 10.1085/jgp.95.6.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine RF. 1990. “Quantal” Ca2+ release and the control of Ca2+ entry by inositol phosphates—A possible mechanism. FEBS Lett 263: 5–9. 10.1016/0014-5793(90)80692-C [DOI] [PubMed] [Google Scholar]
- Ivanova H, Vervliet T, Missiaen L, Parys JB, De Smedt H, Bultynck G. 2014. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim Biophys Acta 1843: 2164–2183. 10.1016/j.bbamcr.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Iwai M, Tateishi Y, Hattori M, Mizutani A, Nakamura T, Futatsugi A, Inoue T, Furuichi T, Michikawa T, Mikoshiba K. 2005. Molecular cloning of mouse type 2 and type 3 inositol 1,4,5-trisphosphate receptors and identification of a novel type 2 receptor splice variant. J Biol Chem 280: 10305–10317. 10.1074/jbc.M413824200 [DOI] [PubMed] [Google Scholar]
- Iwai M, Michikawa T, Bosanac I, Ikura M, Mikoshiba K. 2007. Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J Biol Chem 282: 12755–12764. 10.1074/jbc.M609833200 [DOI] [PubMed] [Google Scholar]
- Joseph SK. 2010. Role of thiols in the structure and function of inositol trisphosphate receptors. Curr Top Membr 66: 299–322. 10.1016/S1063-5823(10)66013-9 [DOI] [PubMed] [Google Scholar]
- Joseph SK, Young M, Alzayady K, Yule DI, Ali M, Booth DM, Hajnóczky G. 2018. Redox regulation of type-I inositol trisphosphate receptors in intact mammalian cells. J Biol Chem 293: 17464–17476. 10.1074/jbc.RA118.005624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplin AI, Snyder SH, Linden DJ. 1996. Reduced nicotinamide adenine dinucleotide-selective stimulation of inositol 1,4,5-trisphosphate receptors mediates hypoxic mobilization of calcium. J Neurosci 16: 2002–2011. 10.1523/jneurosci.16-06-02002.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Nelson C, Parekh AB. 2012. CRAC channels drive digital activation and provide analog control and synergy to Ca2+-dependent gene regulation. Curr Biol 22: 242–247. 10.1016/j.cub.2011.12.025 [DOI] [PubMed] [Google Scholar]
- Keebler MV, Taylor CW. 2017. Endogenous signalling pathways and caged-IP3 evoke Ca2+ puffs at the same abundant immobile intracellular sites. J Cell Sci 130: 3728–3739. 10.1242/jcs.208520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimlicka L, Lau K, Tung CC, Van Petegem F. 2013. Disease mutations in the ryanodine receptor N-terminal region couple to a mobile intersubunit interface. Nat Commun 4: 1506 10.1038/ncomms2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchay S, Giorgi C, Simoneschi D, Pagan J, Missiroli S, Saraf A, Florens L, Washburn MP, Collazo-Lorduy A, Castillo-Martin M, et al. 2017. PTEN counteracts FBXL2 to promote IP3R3- and Ca2+-mediated apoptosis limiting tumour growth. Nature 546: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S, Muto A, Inoue T, Suga K, Okano H, Mikoshiba K. 1997. Role of inositol 1,4,5-trisphosphate receptor in ventral signaling in Xenopus embryos. Science 278: 1940–1943. 10.1126/science.278.5345.1940 [DOI] [PubMed] [Google Scholar]
- La Rovere RM, Roest G, Bultynck G, Parys JB. 2016. Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 60: 74–87. 10.1016/j.ceca.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Leybaert L. 2016. IP3, still on the move but now in the slow lane. Sci Signal 9: fs17 10.1126/scisignal.aal1929 [DOI] [PubMed] [Google Scholar]
- Leybaert L, Sanderson MJ. 2012. Intercellular Ca2+ waves: mechanisms and function. Physiol Rev 92: 1359–1392. 10.1152/physrev.00029.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chan J, Haeseleer F, Mikoshiba K, Palczewski K, Ikura M, Ames JB. 2009. Structural insights into Ca2+-dependent regulation of inositol 1,4,5-trisphosphate receptors by CaBP1. J Biol Chem 284: 2472–2481. 10.1074/jbc.M806513200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Enomoto M, Rossi AM, Seo MD, Rahman T, Stathopulos PB, Taylor CW, Ikura M, Ames JB. 2013. CaBP1, a neuronal Ca2+ sensor protein, inhibits inositol trisphosphate receptors by clamping inter-subunit interactions. Proc Natl Acad Sci 110: 8507–8512. 10.1073/pnas.1220847110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Baek K, Lu Z. 2011. Apo and InsP3-bound crystal structures of the ligand-binding domain of an InsP3 receptor. Nat Struct Mol Biol 18: 1172–1174. 10.1038/nsmb.2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, Pulst SM, Bezprozvanny I. 2009. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci 29: 9148–9162. 10.1523/jneurosci.0660-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sun B, Xiao Z, Wang R, Guo W, Zhang JZ, Mi T, Wang Y, Jones PP, Van Petegem F, et al. 2015. Roles of the NH2-terminal domains of cardiac ryanodine receptor in Ca2+ release activation and termination. J Biol Chem 290: 7736–7746. 10.1074/jbc.M114.618827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JT, Smith IF, Parker I. 2017. Comparison of Ca2+ puffs evoked by extracellular agonists and photoreleased IP3. Cell Calcium 63: 43–47. 10.1016/j.ceca.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Sanjurjo CI, Tovey SC, Prole DL, Taylor CW. 2013. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J Cell Sci 126: 289–300. 10.1242/jcs.116103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. 2008. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454: 538–542. 10.1038/nature07065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson A, Haug LS, Walaas I, Ostvold AC. 1993. Calcium-induced degradation of the inositol (1,4,5)-trisphosphate receptor/Ca2+ channel. FEBS Lett 323: 229–232. 10.1016/0014-5793(93)81345-Z [DOI] [PubMed] [Google Scholar]
- Mak DOD, McBride S, Foskett JK. 1998. Inositol 1,4,5-tris-phosphate activation of inositol tris-phosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci 95: 15821–15825. 10.1073/pnas.95.26.15821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant JS, Taylor CW. 1997. Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr Biol 7: 510–518. 10.1016/S0960-9822(06)00222-3 [DOI] [PubMed] [Google Scholar]
- Marchant J, Callamaras N, Parker I. 1999. Initiation of IP3-mediated Ca2+ waves in Xenopus oocytes. EMBO J 18: 5285–5299. 10.1093/emboj/18.19.5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall ICB, Taylor CW. 1994. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem J 301: 591–598. 10.1042/bj3010591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataragka S, Taylor CW. 2018. All three IP3 receptor subtypes generate Ca2+ puffs, the universal building blocks of IP3-evoked Ca2+ signals. J Cell Sci 131: jcs220848 10.1242/jcs.220848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. 1999. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J 18: 1303–1308. 10.1093/emboj/18.5.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Mizushima A, Hirose K, Yamazawa T, Bezprozvanny I, Kurosaki T, Iino M. 2001. Ca2+-sensor region of IP3 receptor controls intracellular Ca2+ signaling. EMBO J 20: 1674–1680. 10.1093/emboj/20.7.1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadif Kasri N, Holmes AM, Bultynck G, Parys JB, Bootman MD, Rietdorf K, Missiaen L, McDonald F, De Smedt H, Conway SJ, et al. 2004. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J 23: 312–321. 10.1038/sj.emboj.7600037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn DL, Taylor CW. 1992. Luminal Ca2+ increases the sensitivity of Ca2+ stores to inositol 1,4,5-trisphosphate. Mol Pharmacol 41: 115–119. [PubMed] [Google Scholar]
- Paknejad N, Hite RK. 2018. Structural basis for the regulation of inositol trisphosphate receptors by Ca2+ and IP3. Nat Struct Mol Biol 25: 660–668. 10.1038/s41594-018-0089-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Zhang X, Song K, Wu X, Xu J. 2008. Exogenous nitric oxide-induced release of calcium from intracellular IP3 receptor-sensitive stores via S-nitrosylation in respiratory burst-dependent neutrophils. Biochem Biophys Res Commun 377: 1320–1325. 10.1016/j.bbrc.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Pantazaka E, Taylor CW. 2011. Differential distribution, clustering, and lateral diffusion of subtypes of the inositol 1,4,5-trisphosphate receptor. J Biol Chem 286: 23378–23387. 10.1074/jbc.M111.236372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I, Smith IF. 2010. Recording single-channel activity of inositol trisphosphate receptors in intact cells with a microscope, not a patch clamp. J Gen Physiol 136: 119–127. 10.1085/jgp.200910390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Shen H, Wu J, Guo W, Pan X, Wang R, Chen SR, Yan N. 2016. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 354: aah5324 10.1126/science.aah5324 [DOI] [PubMed] [Google Scholar]
- Pizzo P, Lissandron V, Capitanio P, Pozzan T. 2011. Ca2+ signalling in the Golgi apparatus. Cell Calcium 50: 184–192. 10.1016/j.ceca.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. 2015. Store-operated calcium channels. Physiol Rev 95: 1383–1436. 10.1152/physrev.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prole DL, Taylor CW. 2011. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS ONE 6: e26218 10.1371/journal.pone.0026218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prole DL, Taylor CW. 2016. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J Physiol 594: 2849–2866. 10.1113/JP271139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T, Taylor CW. 2009. Dynamic regulation of IP3 receptor clustering and activity by IP3. Channels 3: 226–232. 10.4161/chan.3.4.9247 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Prados M, Rojo-Ruiz J, Aulestia FJ, Garcia-Sancho J, Alonso MT. 2015. A new low-Ca2+ affinity GAP indicator to monitor high Ca2+ in organelles by luminescence. Cell Calcium 58: 558–564. 10.1016/j.ceca.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Rooney TA, Sass EJ, Thomas AP. 1990. Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J Biol Chem 265: 10792–10796. [PubMed] [Google Scholar]
- Rossi AM, Taylor CW. 2019. IP3 receptors: Lessons from analyses ex cellula. J Cell Sci 132 10.1242/jcs.222463 [DOI] [PubMed] [Google Scholar]
- Rossi AM, Riley AM, Potter BVL, Taylor CW. 2010. Adenophostins: High-affinity agonists of IP3 receptors. Curr Top Membr 66: 209–233. 10.1016/S1063-5823(10)66010-3 [DOI] [PubMed] [Google Scholar]
- Saleem H, Tovey SC, Molinski TF, Taylor CW. 2014. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br J Pharmacol 171: 3298–3312. 10.1111/bph.12685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta K, Parekh AB. 2017. Spatial Ca2+ profiling: Decrypting the universal cytosolic Ca2+ oscillation. J Physiol 595: 3053–3062. 10.1113/JP272860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman JJ, Wright FA, Kaufmann T, Wojcikiewicz RJ. 2013. The BCL-2 family member bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage. J Biol Chem 288: 25340–25349. 10.1074/jbc.M113.496570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller B. 2012. Cytosolic Ca2+ buffers. Cold Spring Harb Perspect Biol 2: a004051 10.1101/cshperspect.a004051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MD, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, et al. 2012. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature 483: 108–112. 10.1038/nature10751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Parker I. 2009. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc Natl Acad Sci 106: 6404–6409. 10.1073/pnas.0810799106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Wiltgen SM, Parker I. 2009a. Localization of puff sites adjacent to the plasma membrane: Functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium 45: 65–76. 10.1016/j.ceca.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Wiltgen SM, Shuai J, Parker I. 2009b. Ca2+ puffs originate from preestablished stable clusters of inositol trisphosphate receptors. Sci Signal 2: ra77 10.1126/scisignal.2000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Swaminathan D, Dickinson GD, Parker I. 2014. Single-molecule tracking of inositol trisphosphate receptors reveals different motilities and distributions. Biophys J 107: 834–845. 10.1016/j.bpj.2014.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureshan KM, Riley AM, Rossi AM, Tovey SC, Dedos SG, Taylor CW, Potter BVL. 2009. Activation of IP3 receptors by synthetic bisphosphate ligands. Chem Commun 1204–1206. 10.1039/b819328b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. 2006. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175: 901–911. 10.1083/jcb.200608073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Shin RM, Inoue T, Kato K, Mikoshiba K. 1998. Regulation of nerve growth mediated by inositol 1,4,5-trisphosphate receptors in growth cones. Science 282: 1705–1708. 10.1126/science.282.5394.1705 [DOI] [PubMed] [Google Scholar]
- Tateishi Y, Hattori M, Nakayama T, Iwai M, Bannai H, Nakamura T, Michikawa T, Inoue T, Mikoshiba K. 2005. Cluster formation of inositol 1,4,5-trisphosphate receptor requires its transition to open state. J Biol Chem 280: 6816–6822. 10.1074/jbc.M405469200 [DOI] [PubMed] [Google Scholar]
- Taylor CW. 2017. Regulation of IP3 receptors by cyclic AMP. Cell Calcium 63: 48–52. 10.1016/j.ceca.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Konieczny V. 2016. IP3 receptors: Take four IP3 to open. Sci Signal 9: pe1 10.1126/scisignal.aaf6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Machaca K. 2019. IP3 receptors and store-operated Ca2+ entry: A license to fill. Curr Opin Cell Biol 57: 1–7. 10.1016/j.ceb.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Taylor CW, Tovey SC. 2012. IP3 receptors: Toward understanding their activation. Cold Spring Harb Perspect Biol 2: a004010 10.1101/cshperspect.a004010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Genazzani AA, Morris SA. 1999. Expression of inositol trisphosphate receptors. Cell Calcium 26: 237–251. 10.1054/ceca.1999.0090 [DOI] [PubMed] [Google Scholar]
- Terry LE, Alzayady KJ, Furati E, Yule DI. 2018. Inositol 1,4,5-trisphosphate receptor mutations associated with human disease. Messenger 6: 29–44. [PMC free article] [PubMed] [Google Scholar]
- Thillaiappan NB, Chavda AP, Tovey SC, Prole DL, Taylor CW. 2017. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat Commun 8: 1505 10.1038/s41467-017-01644-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thillaiappan NB, Chakraborty P, Hasan G, Taylor CW. 2019. IP3 receptors and Ca2+ entry. Biochim Biophys Acta 10.1016/j.bbamcr.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Thomas D, Lipp P, Berridge MJ, Bootman MD. 1998. Hormone-evoked elementary Ca2+ signals are not stereotypic, but reflect activation of different size channel clusters and variable recruitment of channels within a cluster. J Biol Chem 273: 27130–27136. 10.1074/jbc.273.42.27130 [DOI] [PubMed] [Google Scholar]
- Thurley K, Tovey SC, Moenke G, Prince VL, Meena A, Thomas AP, Skupin A, Taylor CW, Falcke M. 2014. Reliable encoding of stimulus intensities within random sequences of intracellular Ca2+ spikes. Sci Signal 7: ra59 10.1126/scisignal.2005237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojyo Y, Morita T, Nezu A, Tanimura A. 2008. The clustering of inositol 1,4,5-trisphosphate (IP3) receptors is triggered by IP3 binding and facilitated by depletion of the Ca2+ store. J Pharm Sci 107: 138–150. 10.1254/jphs.08021FP [DOI] [PubMed] [Google Scholar]
- Tovey SC, Dedos SG, Taylor EJA, Church JE, Taylor CW. 2008. Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates direct sensitization of IP3 receptors by cAMP. J Cell Biol 183: 297–311. 10.1083/jcb.200803172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. 1998. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21: 717–726. 10.1016/S0896-6273(00)80589-9 [DOI] [PubMed] [Google Scholar]
- Tung CC, Lobo PA, Kimlicka L, Van Petegem F. 2010. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature 468: 585–588. 10.1038/nature09471 [DOI] [PubMed] [Google Scholar]
- Uchida K, Aramaki M, Nakazawa M, Yamagishi C, Makino S, Fukuda K, Nakamura T, Takahashi T, Mikoshiba K, Yamagishi H. 2010. Gene knock-outs of inositol 1,4,5-trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis. PLoS ONE 5: e12500 10.1371/journal.pone.0012500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H, Foskett JK, Mak DD. 2010. Unitary Ca2+ current through recombinant type 3 InsP3 receptor channels under physiological ionic conditions. J Gen Physiol 136: 687–700. 10.1085/jgp.201010513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H, Foskett JK, Ullah G, Pearson JE, Mak DOD. 2012. Permeant calcium ion feed-through regulation of single inositol 1,4,5-trisphosphate receptor channel gating. J Gen Physiol 140: 697–716. 10.1085/jgp.201210804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervloessem T, Yule DI, Bultynck G, Parys JB. 2015. The type 2 inositol 1,4,5-trisphosphate receptor, emerging functions for an intriguing Ca2+-release channel. Biochim Biophys Acta 1853: 1992–2005. 10.1016/j.bbamcr.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervloessem T, Kerkhofs M, La Rovere RM, Sneyers F, Parys JB, Bultynck G. 2018. Bcl-2 inhibitors as anti-cancer therapeutics: The impact of and on calcium signaling. Cell Calcium 70: 102–116. 10.1016/j.ceca.2017.05.014 [DOI] [PubMed] [Google Scholar]
- Wagner LE II, Yule DI. 2012. Differential regulation of the InsP3 receptor type-1 and -2 single channel properties by InsP3, Ca2+ and ATP. J Physiol 590: 3245–3259. 10.1113/jphysiol.2012.228320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yule DI. 2018. Differential regulation of ion channels function by proteolysis. Biochim Biophys Acta Mol Cell Res 1865: 1698–1706. 10.1016/j.bbamcr.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, Fischer WH, Chen J, Tabas I, Montminy M. 2012. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature 485: 128–132. 10.1038/nature10988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wagner LE II, Alzayady KJ, Yule DI. 2017. Region-specific proteolysis differentially regulates type 1 inositol 1,4,5-trisphosphate receptor activity. J Biol Chem 292: 11714–11726. 10.1074/jbc.M117.789917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. 2009. Calcium flickers steer cell migration. Nature 457: 901–905. 10.1038/nature07577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Yang J, Monteiro MJ, Foskett JK. 2006. CIB1, a ubiquitously expressed Ca2+-binding protein ligand of the InsP3 receptor Ca2+ release channel. J Biol Chem 281: 20825–20833. 10.1074/jbc.M602175200 [DOI] [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Smith AJ, Oliver JM, Oberdorf JA, Wojcikiewicz RJH. 1998. Calcium-dependent clustering of inositol 1,4,5-trisphosphate receptors. Mol Biol Cell 9: 1465–1478. 10.1091/mbc.9.6.1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz RJH. 2018. The making and breaking of inositol 1,4,5-trisphosphate receptor tetramers. Messenger 6: 45–49. 10.1166/msr.2018.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AK, Capitanio P, Lissandron V, Bortolozzi M, Pozzan T, Pizzo P. 2013. Heterogeneity of Ca2+ handling among and within Golgi compartments. J Mol Cell Biol 5: 266–276. 10.1093/jmcb/mjt024 [DOI] [PubMed] [Google Scholar]
- Woods NM, Cuthbertson KSR, Cobbold PH. 1986. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature 319: 600–602. 10.1038/319600a0 [DOI] [PubMed] [Google Scholar]
- Worley PF, Baraban JM, Supattapone S, Wilson VS, Snyder SH. 1987. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem 262: 12132–12136. [PubMed] [Google Scholar]
- Xu H, Ren D. 2015. Lysosomal physiology. Annu Rev Physiol 77: 57–80. 10.1146/annurev-physiol-021014-071649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Chan J, Ikura M, Michikawa T, Mikoshiba K. 2010. Tyr-167/Trp-168 in type1/3 inositol 1,4,5-trisphosphate receptor mediates functional coupling between ligand binding and channel opening. J Biol Chem 285: 36081–36091. 10.1074/jbc.M110.140129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Bai XC, Yan C, Wu J, Li Z, Xie T, Peng W, Yin CC, Li X, Scheres SH, et al. 2015. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517: 50–55. 10.1038/nature14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, McBride S, Mak DOD, Vardi N, Palczewski K, Haeseleer F, Foskett JK. 2002. Identification of a family of calcium sensors as protein ligands of inositol trisphosphate receptor Ca2+ release channels. Proc Natl Acad Sci 99: 7711–7716. 10.1073/pnas.102006299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M, Lewis RS. 2018. Physiological CRAC channel activation and pore properties require STIM1 binding to all six Orai1 subunits. J Gen Physiol 150: 1373–1385. 10.1085/jgp.201711985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, Hendrickson WA, Frank J, Marks AR. 2015. Structure of a mammalian ryanodine receptor. Nature 517: 44–49. 10.1038/nature13950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Mak DD, Li Q, Shin DM, Foskett JK, Muallem S. 2003. A new mode of Ca2+ signaling by G protein-coupled receptors: Gating of IP3 receptor Ca2+ release channels by Gβγ. Curr Biol 13: 872–876. 10.1016/S0960-9822(03)00330-0 [DOI] [PubMed] [Google Scholar]