Abstract
Opioids serve a vital role in the current analgesic array of treatment options. They are useful in acute instances involving severe pain associated with trauma, surgery, and terminal diseases such as cancer. In the past three decades, multiple receptor isoforms and conformations have been reported throughout literature. Most of these studies conducted systemic analyses of opioid receptor function, often generalizing findings from receptor systems in central nervous tissue or exogenously expressing immortalized cell lines as common mechanisms throughout physiology. However, a culmination of innovative experimental data indicates that opioid receptor systems are differentially modulated depending on their anatomic expression profile. Importantly, opioid receptors expressed in the peripheral nervous system undergo regulation uncommon to similar receptors expressed in central nervous system tissues. This distinctive characteristic begs one to question whether peripheral opioid receptors maintain anatomically unique roles, and whether they may serve an analgesic advantage in providing pain relief without promoting addiction.
Introduction
Opioid receptor systems belong to one of three isoform families: μ, δ, or κ (Lord et al., 1977). Each isoform is composed of a seven-transmembrane–spanning G protein–coupled receptor (GPCR) that signals through Gαi/o subunits to inhibit adenylyl cyclase activity, reducing the production of cAMP and subsequent protein kinase A (PKA) activity. Activation of the accompanying Gβγ subunits results in activation of inward rectifying K+ channels (Christie et al., 1987) and inhibition of voltage-gated Ca+2 channels (Gross and Macdonald, 1987), thereby hyperpolarizing neurons in which opioid receptors are activated. In several cell models, opioid receptor activation stimulates Gβ displacement from Gαi/o subunits and subsequent extracellular signal-regulated kinase/mitogen-activated protein kinase phosphorylation (Belcheva et al., 2003). However, it is well accepted that all three isoforms produce similar effects across physiology, resulting in outcome measurements appropriately reproducible between research groups and models.
Opioid receptors were originally and widely considered susceptible to canonical desensitization mechanisms, in which agonist activation of the GPCR stimulates β-arrestin–dependent internalization (Bohn et al., 1999, 2000; Gainetdinov et al., 2004). Within this model, opioid receptor activation triggers G protein receptor kinase 2/3 (GRK 2/3) to phosphorylate residues on the intracellular face of opioid receptors to uncouple the receptor from G proteins and attract β-arrestin association with the receptor (Zhang et al., 1998). Clathrin-mediated internalization then occurs, directing vesicles to degradation or recycling to the plasma membrane. This cycle represents a common desensitization mechanism for many GPCRs, including μ, δ, and κ. However, recent work has discovered anatomic differences in opioid receptor desensitization, as well as responsiveness.
Central Opioid Receptor Regulation
Opioid receptor function in vivo was initially characterized utilizing central nervous system (CNS) tissue, including brain and spinal cord. These studies, including work from the laboratories of Macdonald Christie, John Traynor, Mark Von Zastrow, and Chris Evans, among many others, found receptor behavior similar to studies in immortalized cell lines. All three isoforms of opioid receptors are constitutively sensitive to agonist activation, and undergo canonical, clathrin-mediated GPCR desensitization. Opioid receptor regulation in central nervous tissue involves dynamic protein kinase A phosphorylation events that dictate receptor function (Bernstein and Welch, 1998; Chakrabarti et al., 1998), G protein receptor kinase events that mediate receptor desensitization (Hasbi et al., 1998; Chakrabarti et al., 2001), β-arrestin association that can dictate downstream signaling function (Appleyard et al., 1999; Bohn et al., 1999, 2000; Zhang et al., 1999), and modulation by regulators of G protein signaling (Huang et al., 2015; Dripps et al., 2017). Importantly, these regulatory processes occur similarly throughout the CNS as well as in exogenously overexpressing immortalized cell lines. However, new and innovative studies have discovered unique differences in the regulation of opioid receptors in peripheral nervous tissue that could bear pharmacological utility.
Peripheral Opioid Receptor Priming
In 2011, members of the Tesmer group found that GRK2/3 inhibition in locus coeruleus neurons only partially blocks agonist-induced μ desensitization, indicating that other desensitization mechanisms may participate, or that GRK2/3 may play another role (Thal et al., 2011). Although this was one of the first reports identifying anatomic distinctions in opioid receptor desensitization, it was almost a decade earlier in which Stein and Schafer discovered anatomic differentiation in opioid receptor agonist responsiveness. Zollner et al. (2003) identified μ responsiveness in peripheral sensory neurons isolated from the dorsal root ganglia (DRG) as significantly greater following painful inflammation of the innervated tissue. These results were later confirmed by Clarke and colleagues across all three isoforms in both in vivo and in vitro models (Patwardhan et al., 2005; Berg et al., 2007, 2011). In these studies, the application of an inflammatory mediator, bradykinin (BK), stimulated increased responsiveness of opioid receptor signaling in peripheral nervous tissues. Importantly, basal opioid receptor responsiveness was considerably low, relative to other studies conducted with central nervous tissues (Whistler and von Zastrow, 1998; Arttamangkul et al., 2008; Dang et al., 2009). This disparity intimated that a type of braking mechanism was unique to peripheral nervous tissues expressing opioid receptors and naively repressing receptor responsiveness. However, the molecular players of this mechanism remained undiscovered, prompting additional studies to highlight potential targets to increase peripheral opioid efficacy.
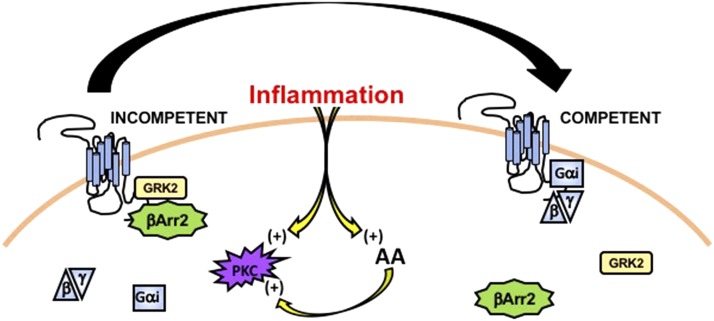
The unique physiology and phenotype of peripheral nervous system tissues, including dorsal root and trigeminal ganglia neurons, serve as an important starting point for examining potential players in the mechanism that supports naive desensitization of opioid receptors. Peripheral sensory neurons have a bipolar phenotype, with afferent innervations that can extend over a meter in length from the cell body. Due to this length, many effects seen on opioid receptors in the periphery are likely due to temporally discriminative changes in protein–protein interactions and receptor coupling, in contrast to more time-extensive modifications in receptor translation and insertion (Jung et al., 2012). Therefore, investigations centered around post-translational mechanisms that would either stimulate or inhibit protein associations within short time frames appropriately represent previously published in vivo findings. In 2015, work by the Clarke group identified that δ functional competence in peripheral trigeminal sensory neurons was positively regulated by arachidonic acid (AA) and BK pretreatment (Sullivan et al., 2015). Additional work revealed various sensitivities to cyclooxygenase (COX) or lipoxygenase (LOX) inhibition, suggesting that COX and LOX metabolites of AA also stimulate opioid receptor functional competence. Importantly, BK and AA both activate protein kinase C (PKC) (O’Flaherty and Nishihira, 1987; Burgess et al., 1989), as do COX and LOX metabolites (Shearman et al., 1989; Liu et al., 1991; Abayasekara et al., 1993; Castrillo et al., 2003), suggesting that PKC may play an important role in reversing naive opioid receptor desensitization in peripheral afferent sensory neurons (Fig. 1).
Fig. 1.
Illustration of inflammation stimulating a conversion of the opioid receptor system from a state of incompetence to that of competence for activation on a peripheral nerve ending. βArr2, β-arrestin 2.
PKC is an important kinase and signaling molecule in neurons, including peripheral sensory neurons. As a post-translational modifier, it reacts to changes in lipid composition, intracellular Ca+2, and numerous upstream regulators to target ion channels, GPCRs, and other proteins to significantly change activities and downstream functions. Indeed, Brackley et al. (2016) discovered that PKC activation by BK was necessary to reverse functional incompetence of δ in peripheral DRG neurons. Results from this work demonstrated that application of the inflammatory mediator BK stimulated PKC to phosphorylate Raf kinase inhibitory protein. Phosphorylation of this scaffolding protein stimulates dimerization and sequestration of GRK2, which was found to be naively associated with δ in sensory neurons (Fig. 1). Importantly, expression of the kinase-dead mutant GRK2-K220R (Freedman et al., 1995) in sensory neurons failed to increase δ competence, suggesting that kinase activity of GRK2 was not the limiting factor for δ functional incompetence, but rather steric blockade of signaling molecules that would associate with the receptor. In this model, GRK2 occupation of G protein binding sites would prevent functional activation of pathways downstream from δ, thereby rendering the receptor system incompetent. Additional work corroborates the importance of basal participation by GPCR desensitization machinery as β-arrestin plays a role similar to GRK2 in μ and δ functional incompetence in trigeminal sensory neurons (Sullivan et al., 2016). Importantly, recent findings agree in that opioid receptors expressed in peripheral sensory neurons exist in a functionally desensitized state, unlike in neurons from central nervous tissues. Furthermore, inflammatory activation of PKC primes the opioid receptors for full responsiveness to agonist stimulation, providing a mechanism for this unique level of anatomically distinct receptor regulation.
GRK Biochemistry
Transient GRK isoform association with the opioid receptor has been detailed previously in multiple cell models. Homologous desensitization of opioid receptors is most often initiated by kinase phosphorylation of amino acids along the C terminus of the receptor. For μ, GRK2/3 phosphorylation predominantly occurs following high-efficacy opioid agonist administration, whereas GRK5 serves to phosphorylate the receptor following morphine (low-desensitizing agonist) (Schulz et al., 2004; Doll et al., 2012). Importantly, PKC has also demonstrated a role in receptor phosphorylation, although this typically occurs following activation of a coexpressing Gαq-coupled GPCR (Bailey et al., 2004; Doll et al., 2011; Feng et al., 2011). GRK2 is also responsible for postagonist phosphorylation and internalization of δ (Guo et al., 2000; Kouhen et al., 2000) and κ (Appleyard et al., 1997; McLaughlin et al., 2003) in both immortalized and CNS cell models. Brackley et al. (2017) found that PKA phosphorylation of GRK2 drives plasma membrane targeting and constitutive association with δ in peripheral DRG neurons. GRK2 phosphorylation in this neuronal model was mediated by A-kinase anchoring protein (AKAP) 79/150, thereby utilizing a scaffolding protein necessary for post-translational upregulation of several pain-sensing transient receptor potential channels, including transient receptor potential family V1 (Jeske et al., 2008, 2009; Schnizler et al., 2008; Zhang et al., 2008) and transient receptor potential family A1 (Brackley et al., 2017). Importantly, the expression of an AKAP mutant with a deleted PKA binding domain resulted in a functionally competent δ in sensory neurons, indicating an important scaffolding role for AKAP in the naive and inhibitory association of GRK2 with δ in peripheral nervous tissue.
Previous studies in peripheral sensory neurons primarily examined initial opioid receptor responsiveness, and not desensitized responses, so little is known concerning the potential for anatomic distinction in receptor desensitization. Given that GRK2 constitutively associates with δ, is it possible that receptor desensitization follows a non-GRK2 mechanism? Phosphorylation at T394 of μ has been reported to be critical to acute D-Ala2, N-MePhe4, Gly-ol-enkephalin desensitization and not morphine but has no effect on chronic desensitization to either (Li et al., 2013). This is also the case for Chinese hamster ovary cells (Pak et al., 1997) and animals (Wang et al., 2016), suggesting similar desensitization mechanisms for μ in peripheral and central nervous tissues. Furthermore, phosphorylation of T394 μ appears to be GRK2-dependent (Zhang et al., 1998), suggesting that the kinase potentially governs both preagonist and postagonist functional competence of peripheral opioid receptors. Less information is available for δ and κ in peripheral sensory neurons; however, given their similarities in mechanisms of agonist-dependent desensitization, it can be presumed that all three isoforms follow similar patterns of desensitization, with similar dependencies on GRKs. Importantly, can the anatomic distinctions of opioid receptors between peripheral and central nervous tissues be targeted pharmacologically to increase receptor responsiveness in the periphery and reduce systemic side effects?
On the intracellular side, blocking constitutive GRK2 association with an opioid receptor would induce functional receptor competence and the possibility for analgesia. Tesmer et al. (2010), Thal et al. (2012), Homan and Tesmer (2015), and Bouley et al. (2017) have conducted a number of studies on the efficacies of various GRK inhibitors on GPCR function and physiology. However, as Brackley et al. (2016) found, GRK2 kinase activity was not a rate-limiting step in maintaining δ in a desensitized state. Rather, GRK2 provides steric hindrance to G protein activation by δ. Given the large number of proteins and enzymes that constitute the GRK interactome, it becomes difficult to speculate whether other intracellular pathways would be affected, thereby modifying tissue physiology beyond an opioid receptor. However, current and unpublished studies stretch the treatment possibilities for GRK inhibitors to unconsidered realms, providing hope for multiple diseases.
Opioid Receptor Translocation
Opioid receptor function is dependent on subcellular localization (Stoeber et al., 2018). Similar to many CNS models, δ function in DRG neurons is dependent upon plasma membrane expression (Scherrer et al., 2006). However, several studies have discovered mechanisms unique to primary afferent neurons that regulate opioid receptor function based on receptor translocation. For example, subcellular localization of δ to the plasma membrane was increased in DRG neurons following ipsilateral exposure to complete Freund’s adjuvant (Gendron et al., 2006). BK, an inflammatory mediator increased in vivo following complete Freund’s adjuvant administration, was reported to increase the number of DRG neurons positively expressing δ on its cell surface (Pettinger et al., 2013). Additionally, nerve growth factor activation of tropomysin receptor kinase A in DRG increases μ agonist efficacy by increasing receptor translocation to peripheral nerve terminals (Mousa et al., 2007). Pradhan et al. (2013) reported in 2013 that inflammation increases N-type Ca+2 channel contribution to δ-mediated analgesia in DRG neurons in a β-arrestin two-dependent manner. This intimates that β-arrestin 2 affects subcellular localization of the δ receptor isoform and its functional conjugation to Gβγ-mediated inhibition of voltage-gated calcium channel, a primary mechanism that supports hyperpolarization of primary afferent neurons. Interestingly, research from the same group found that δ receptor responses were increased in DRG neurons with β-arrestin 1 knocked out (Mittal et al., 2013). However, this mechanism was tied to Rho-associated protein kinase and LIM kinase-dependent translocation mechanisms to the plasma membrane, such that exposure to δ-specific agonists resulted in increased δ receptor expression on the plasma membrane.
Multiple sources indicate varying amounts of δ/μ coexpression in primary afferent neurons, including values as low as 5% total (Scherrer et al., 2009) to approximately 30% (Bardoni et al., 2014). Despite their functional similarities/differences, coexpression of the two isoforms appears to regulate function in the periphery. Reduced δ expression concomitantly reduces cell surface expression and function of μ in DRG neurons (Walwyn et al., 2009). Unique to this pairing, others have demonstrated functional heterodimerization of μ and δ isoforms in peripheral afferents (Wang et al., 2010), even demonstrating dynamic changes in δ, μ, and κ isoform expression in DRG afferents following inflammation (Ji et al., 1995). Together, these reports indicate a unique interplay of cross-functionalization between opioid receptor isoforms that maintain unique characteristics in the peripheral nervous system.
Peripheral Opioid Targets
Spahn et al. (2017) designed a μ-specific agonist uniquely selective for receptor association in inflamed tissues only. Utilizing computer modeling of μ at low pH values, thereby attempting to recreate inflammatory conditions for agonist association, they designed a new agonist that only binds to μ when the pH is low and in a state of inflammatory flux. Importantly, this compound was capable of stimulating Gαi signaling when applied following inflammatory injury without affecting central autonomic centers, thereby avoiding depressed respiration, sedation, or addiction. This aspect was used by Jamshidi et al. (2015) while reporting on the functional selectivity of the peripherally restrictive κ opioid agonist U50488, which avoids CNS modulation of mood, and is especially important in reference to recent work on κ as a peripheral pain target (Snyder et al., 2018). Taken together, recent findings are important for understanding the anatomic complexity and differential possibilities of peripheral opioid receptors when designing safer and newer opioids for the treatment of pain.
Conclusions and Future Directions
A considerable amount of peripheral opioid receptor research has focused on the δ and μ receptor isoforms. However, previous work on the κ receptor system identifies that specific κ agonists may hold analgesic property isoforms in peripheral nervous tissue (Su et al., 1998; Labuz et al., 2006; Berg et al., 2011; Cunha et al., 2012), despite little biochemical evidence supporting whether κ opioid receptors are regulated similarly to δ and μ. Given recent breakthroughs associated with receptor dimerizm and allosteric agonists, multiple research groups are consistently identifying new mechanisms unique to peripheral opioid receptor systems that can be manipulated to provide accurate analgesia without central side effects. This remains an important goal in patient care in the generational combat against the worldwide opioid crisis.
Acknowledgments
We thank the countless researchers whose research and theories are represented in this review, as well as those whom we were unable to include due to limitations on available space.
Abbreviations
- AA
arachidonic acid
- AKAP
A-kinase anchoring protein
- BK
bradykinin
- CNS
central nervous system
- COX
cyclooxygenase
- DRG
dorsal root ganglia
- GPCR
G protein–coupled receptor
- GRK
G protein receptor kinase
- LOX
lipoxygenase
- PKA
protein kinase A
- PKC
protein kinase C
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Jeske.
Footnotes
N.A.J. was supported by National Institutes of Health [Grants DA042220 and NS082746].
References
- Abayasekara DR, Jones PM, Persaud SJ, Michael AE, Flint AP. (1993) Prostaglandin F2 alpha activates protein kinase C in human ovarian cells. Mol Cell Endocrinol 91:51–57. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Celver J, Pineda V, Kovoor A, Wayman GA, Chavkin C. (1999) Agonist-dependent desensitization of the kappa opioid receptor by G protein receptor kinase and beta-arrestin. J Biol Chem 274:23802–23807. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Patterson TA, Jin W, Chavkin C. (1997) Agonist-induced phosphorylation of the kappa-opioid receptor. J Neurochem 69:2405–2412. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. (2008) Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol 74:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Kelly E, Henderson G. (2004) Protein kinase C activation enhances morphine-induced rapid desensitization of mu-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol 66:1592–1598. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Tawfik VL, Wang D, François A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, et al. (2014) Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron 81:1443. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Tan Y, Heaton VM, Clark AL, Coscia CJ. (2003) Mu opioid transactivation and down-regulation of the epidermal growth factor receptor in astrocytes: implications for mitogen-activated protein kinase signaling. Mol Pharmacol 64:1391–1401. [DOI] [PubMed] [Google Scholar]
- Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. (2007) Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther 321:839–847. [DOI] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Sanchez TA, Silva M, Patwardhan AM, Milam SB, Hargreaves KM, Clarke WP. (2011) Regulation of κ-opioid receptor signaling in peripheral sensory neurons in vitro and in vivo. J Pharmacol Exp Ther 338:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein MA, Welch SP. (1998) mu-Opioid receptor down-regulation and cAMP-dependent protein kinase phosphorylation in a mouse model of chronic morphine tolerance. Brain Res Mol Brain Res 55:237–242. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498. [DOI] [PubMed] [Google Scholar]
- Bouley R, Waldschmidt HV, Cato MC, Cannavo A, Song J, Cheung JY, Yao XQ, Koch WJ, Larsen SD, Tesmer JJG. (2017) Structural determinants influencing the potency and selectivity of indazole-paroxetine hybrid G protein-coupled receptor kinase 2 inhibitors. Mol Pharmacol 92:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackley AD, Gomez R, Akopian AN, Henry MA, Jeske NA. (2016) GRK2 constitutively governs peripheral delta opioid receptor activity. Cell Rep 16:2686–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackley AD, Gomez R, Guerrero KA, Akopian AN, Glucksman MJ, Du J, Carlton SM, Jeske NA. (2017) A-kinase anchoring protein 79/150 scaffolds transient receptor potential A 1 phosphorylation and sensitization by metabotropic glutamate receptor activation. Sci Rep 7:1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess GM, Mullaney I, McNeill M, Dunn PM, Rang HP. (1989) Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J Neurosci 9:3314–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo A, Través PG, Martín-Sanz P, Parkinson S, Parker PJ, Boscá L. (2003) Potentiation of protein kinase C zeta activity by 15-deoxy-delta(12,14)-prostaglandin J(2) induces an imbalance between mitogen-activated protein kinases and NF-kappa B that promotes apoptosis in macrophages. Mol Cell Biol 23:1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Law PY, Loh HH. (1998) Distinct differences between morphine- and [D-Ala2,N-MePhe4,Gly-ol5]-enkephalin-mu-opioid receptor complexes demonstrated by cyclic AMP-dependent protein kinase phosphorylation. J Neurochem 71:231–239. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Oppermann M, Gintzler AR. (2001) Chronic morphine induces the concomitant phosphorylation and altered association of multiple signaling proteins: a novel mechanism for modulating cell signaling. Proc Natl Acad Sci USA 98:4209–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. (1987) Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol 32:633–638. [PubMed] [Google Scholar]
- Cunha TM, Souza GR, Domingues AC, Carreira EU, Lotufo CM, Funez MI, Verri WA, Jr, Cunha FQ, Ferreira SH. (2012) Stimulation of peripheral kappa opioid receptors inhibits inflammatory hyperalgesia via activation of the PI3Kγ/AKT/nNOS/NO signaling pathway. Mol Pain 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Napier IA, Christie MJ. (2009) Two distinct mechanisms mediate acute mu-opioid receptor desensitization in native neurons. J Neurosci 29:3322–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Pöll F, Koch T, Höllt V, Schulz S. (2011) Agonist-selective patterns of µ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol 164:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C, Pöll F, Peuker K, Loktev A, Glück L, Schulz S. (2012) Deciphering µ-opioid receptor phosphorylation and dephosphorylation in HEK293 cells. Br J Pharmacol 167:1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dripps IJ, Wang Q, Neubig RR, Rice KC, Traynor JR, Jutkiewicz EM. (2017) The role of regulator of G protein signaling 4 in delta-opioid receptor-mediated behaviors. Psychopharmacology (Berl) 234:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Li Z, Wang JB. (2011) Protein kinase C-mediated phosphorylation of the μ-opioid receptor and its effects on receptor signaling. Mol Pharmacol 79:768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman NJ, Liggett SB, Drachman DE, Pei G, Caron MG, Lefkowitz RJ. (1995) Phosphorylation and desensitization of the human beta 1-adrenergic receptor: involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. J Biol Chem 270:17953–17961. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27:107–144. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O’Donnell D, Vincent JP, Stroh T, Beaudet A. (2006) Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci 26:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RA, Macdonald RL. (1987) Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc Natl Acad Sci USA 84:5469–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wu Y, Zhang W, Zhao J, Devi LA, Pei G, Ma L. (2000) Identification of G protein-coupled receptor kinase 2 phosphorylation sites responsible for agonist-stimulated delta-opioid receptor phosphorylation. Mol Pharmacol 58:1050–1056. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Polastron J, Allouche S, Stanasila L, Massotte D, Jauzac P. (1998) Desensitization of the delta-opioid receptor correlates with its phosphorylation in SK-N-BE cells: involvement of a G protein-coupled receptor kinase. J Neurochem 70:2129–2138. [DOI] [PubMed] [Google Scholar]
- Homan KT, Tesmer JJ. (2015) Molecular basis for small molecule inhibition of G protein-coupled receptor kinases. ACS Chem Biol 10:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, Kato HE, Livingston KE, Thorsen TS, Kling RC, et al. (2015) Structural insights into µ-opioid receptor activation. Nature 524:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi RJ, Jacobs BA, Sullivan LC, Chavera TA, Saylor RM, Prisinzano TE, Clarke WP, Berg KA. (2015) Functional selectivity of kappa opioid receptor agonists in peripheral sensory neurons. J Pharmacol Exp Ther 355:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. (2008) A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 138:604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. (2009) A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain 146:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hökfelt T. (1995) Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci 15:8156–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Yoon BC, Holt CE. (2012) Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci 13:308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhen OM, Wang G, Solberg J, Erickson LJ, Law PY, Loh HH. (2000) Hierarchical phosphorylation of delta-opioid receptor regulates agonist-induced receptor desensitization and internalization. J Biol Chem 275:36659–36664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuz D, Berger S, Mousa SA, Zöllner C, Rittner HL, Shaqura MA, Segovia-Silvestre T, Przewlocka B, Stein C, Machelska H. (2006) Peripheral antinociceptive effects of exogenous and immune cell-derived endomorphins in prolonged inflammatory pain. J Neurosci 26:4350–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ma F, Gu Y, Huang LY. (2013) Analgesic tolerance of opioid agonists in mutant mu-opioid receptors expressed in sensory neurons following intrathecal plasmid gene delivery. Mol Pain 9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Timar J, Howlett J, Diglio CA, Honn KV. (1991) Lipoxygenase metabolites of arachidonic and linoleic acids modulate the adhesion of tumor cells to endothelium via regulation of protein kinase C. Cell Regul 2:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. (1977) Endogenous opioid peptides: multiple agonists and receptors. Nature 267:495–499. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Xu M, Mackie K, Chavkin C. (2003) Phosphorylation of a carboxyl-terminal serine within the kappa-opioid receptor produces desensitization and internalization. J Biol Chem 278:34631–34640. [DOI] [PubMed] [Google Scholar]
- Mittal N, Roberts K, Pal K, Bentolila LA, Fultz E, Minasyan A, Cahill C, Pradhan A, Conner D, DeFea K, et al. (2013) Select G-protein-coupled receptors modulate agonist-induced signaling via a ROCK, LIMK, and β-arrestin 1 pathway. Cell Rep 5:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA, Cheppudira BP, Shaqura M, Fischer O, Hofmann J, Hellweg R, Schäfer M. (2007) Nerve growth factor governs the enhanced ability of opioids to suppress inflammatory pain. Brain 130:502–513. [DOI] [PubMed] [Google Scholar]
- O’Flaherty JT, Nishihira J. (1987) Arachidonate metabolites, platelet-activating factor, and the mobilization of protein kinase C in human polymorphonuclear neutrophils. J Immunol 138:1889–1895. [PubMed] [Google Scholar]
- Pak Y, O’Dowd BF, George SR. (1997) Agonist-induced desensitization of the mu opioid receptor is determined by threonine 394 preceded by acidic amino acids in the COOH-terminal tail. J Biol Chem 272:24961–24965. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. (2005) Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci 25:8825–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinger L, Gigout S, Linley JE, Gamper N. (2013) Bradykinin controls pool size of sensory neurons expressing functional δ-opioid receptors. J Neurosci 33:10762–10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A, Smith M, McGuire B, Evans C, Walwyn W. (2013) Chronic inflammatory injury results in increased coupling of delta opioid receptors to voltage-gated Ca2+ channels. Mol Pain 9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Tóth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gavériaux-Ruff C, et al. (2006) Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci USA 103:9691–9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. (2008) Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 28:4904–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Höllt V. (2004) Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J 23:3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman MS, Naor Z, Sekiguchi K, Kishimoto A, Nishizuka Y. (1989) Selective activation of the gamma-subspecies of protein kinase C from bovine cerebellum by arachidonic acid and its lipoxygenase metabolites. FEBS Lett 243:177–182. [DOI] [PubMed] [Google Scholar]
- Snyder LM, Chiang MC, Loeza-Alcocer E, Omori Y, Hachisuka J, Sheahan TD, Gale JR, Adelman PC, Sypek EI, Fulton SA, et al. (2018) Kappa opioid receptor distribution and function in primary afferents. Neuron 99:1274–1288.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, Durmaz V, Sabri P, Reidelbach M, Machelska H, et al. (2017) A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 355:966–969. [DOI] [PubMed] [Google Scholar]
- Stoeber M, Jullié D, Lobingier BT, Laeremans T, Steyaert J, Schiller PW, Manglik A, von Zastrow M. (2018) A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 98:963–976.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Wachtel RE, Gebhart GF. (1998) Inhibition of calcium currents in rat colon sensory neurons by K- but not mu- or delta-opioids. J Neurophysiol 80:3112–3119. [DOI] [PubMed] [Google Scholar]
- Sullivan LC, Berg KA, Clarke WP. (2015) Dual regulation of δ-opioid receptor function by arachidonic acid metabolites in rat peripheral sensory neurons. J Pharmacol Exp Ther 353:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LC, Chavera TS, Jamshidi RJ, Berg KA, Clarke WP. (2016) Constitutive desensitization of opioid receptors in peripheral sensory neurons. J Pharmacol Exp Ther 359:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer JJ, Tesmer VM, Lodowski DT, Steinhagen H, Huber J. (2010) Structure of human G protein-coupled receptor kinase 2 in complex with the kinase inhibitor balanol. J Med Chem 53:1867–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, et al. (2012) Paroxetine is a direct inhibitor of G protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol 7:1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DM, Yeow RY, Schoenau C, Huber J, Tesmer JJ. (2011) Molecular mechanism of selectivity among G protein-coupled receptor kinase 2 inhibitors. Mol Pharmacol 80:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, John S, Maga M, Evans CJ, Hales TG. (2009) Delta receptors are required for full inhibitory coupling of mu-receptors to voltage-dependent Ca(2+) channels in dorsal root ganglion neurons. Mol Pharmacol 76:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, et al. (2010) Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci USA 107:13117–13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Barbier E, Chiu YT, He Y, Zhan J, Bi GH, Zhang HY, Feng B, Liu-Chen LY, Wang JB, et al. (2016) T394A mutation at the μ opioid receptor blocks opioid tolerance and increases vulnerability to heroin self-administration in mice. J Neurosci 36:10392–10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. (1998) Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci USA 95:9914–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA 95:7157–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Law PY, Barak LS, Caron MG. (1999) Agonist-specific regulation of delta-opioid receptor trafficking by G protein-coupled receptor kinase and beta-arrestin. J Recept Signal Transduct Res 19:301–313. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li L, McNaughton PA. (2008) Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59:450–461. [DOI] [PubMed] [Google Scholar]
- Zollner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schafer M. (2003) Painful inflammation-induced increase in mu-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol Pharmacol 64:202–210. [DOI] [PubMed] [Google Scholar]