Abstract
The organic anion transporting polypeptides (OATPs) are a superfamily of drug transporters involved in the uptake and disposition of a wide array of structurally divergent endogenous and exogenous substrates, including steroid hormones, bile acids, and commonly used drugs, such as anti-infectives, antihypertensives, and cholesterol lowering agents. In the past decade, OATPs, primarily OATP1A2, OATP1B1, and OATP1B3, have emerged as potential mediators of chemotherapy disposition, including drugs such as methotrexate, doxorubicin, paclitaxel, docetaxel, irinotecan and its important metabolite 7-ethyl-10-hydroxycamptothecin, and certain tyrosine kinase inhibitors. Furthermore, OATP family members are polymorphic and numerous studies have shown OATP variants to have differential uptake, disposition, and/or pharmacokinetics of numerous drug substrates with important implications for interindividual differences in efficacy and toxicity. Additionally, certain OATPs have been found to be overexpressed in a variety of human solid tumors, including breast, liver, colon, pancreatic, and ovarian cancers, suggesting potential roles for OATPs in tumor development and progression and as novel targets for cancer therapy. This review focuses on the emerging roles for selected OATPs in cancer pharmacology, including preclinical and clinical studies suggesting roles in chemotherapy disposition, the pharmacogenetics of OATPs in cancer therapy, and OATP overexpression in various tumor tissues with implications for OATPs as therapeutic targets.
Introduction
The solute carrier superfamily encompasses many transporters that play important roles in the uptake and distribution of both endogenous compounds and xenobiotics (Hagenbuch and Stieger, 2013). Among these are the organic anion transporting polypeptides (OATPs), classified in the SLCO family, which transport a large number of structurally diverse amphipathic substrates including steroid hormones (which are important for proliferation of hormone-dependent cancers) (De Bruyn et al., 2011), bile acids, statins, antihypertensives, antibiotics, antifungals, and chemotherapeutic agents (Hagenbuch and Stieger, 2013). Since the discovery of the first OATP in 1994 (Jacquemin et al., 1994), over 300 OATP family members have been identified in over 40 species, including 11 human transporters and many transporters in rats and mice (Hagenbuch and Stieger, 2013).
While many human OATPs have direct rodent orthologs, this is not necessarily the case for OATP1A2, OATP1B1, and OATP1B3, in which the rodent transporters have high sequence homology to the human transporters but are not direct orthologs. There are four known members of the Oatp1a family in mice (Oatp1a1, Oatp1a4, Oatp1a5, and Oatp1a6), in contrast to one human member (OATP1A2), but only one mouse Oatp1b transporter (Oatp1b2) compared with two human transporters (OATP1B1 and OATP1B3). Based on localization patterns of these transporters, it is thought that murine Oatp1b2 is the closest ortholog to human OATP1B1 and OATP1B3, but Oatp1a1 and Oatp1a4 may also fulfill similar functions. For OATP1A2, the closest murine isoform Oatp1a4 exhibits 72% amino acid sequence homology; however, additional isoforms Oatp1a1, Oatp1a5, and Oatp1a6 may also have similar roles based on tissue localization. While Oatp1b2 null mice are often used to mimic the loss of OATP1B1 and OATP1B3 function, this is potentially problematic because the Oatp1a transporters may be able to partially recover substrate transport when Oatp1b2 is absent. For this reason, the utilization of Oatp1a/Oatp1b null mice to prevent compensation from Oatp1a transporters when studying the impact of the loss of Oatp1b function on substrate transport may be a significant factor to consider (Iusuf et al., 2012). Additionally, several humanized mouse strains have been created using Oatp1a/Oatp1b null mice with expression of human OATP1A2, OATP1B1, or OATP1B3 in the liver parenchymal cells. These humanized mice have allowed for better study of the effect of each human transporter individually. The limitations for the use of these mouse models were summarized in a recent review and should be considered when interpreting data from mouse model studies given the lack of direct rodent orthologs for important OATP transporters (Durmus et al., 2016). To date, no mouse model has been published for OATP2B1.
The genes encoding several human OATPs (in particular, SLCO1B1, SLCO1B3, and SLCO1A2) are located on chromosome 12, while genes for other OATPs are spread throughout the rest of the genome. All members of the OATP family contain 12 putative transmembrane domains with conserved cysteine residues in an extracellular loop between transmembrane domains 9 and 10, as well as signature sequences containing N-glycosylation sites in other extracellular loops (Hagenbuch and Meier, 2004; Konig, 2011). The OATPs are organized into families (in which members have at least 40% sequence homology; denoted by a number) and subfamilies (in which members have at least 60% sequence homology; denoted by a letter), with individual members of each subfamily identified numerically. The OATP designation is used for human transporters, while Oatp is used for nonhuman transporters (Hagenbuch and Meier, 2004).
Because of their wide range of transported substrates, OATPs have important roles both in the kinetics of drug disposition and in drug-drug interactions when two or more xenobiotics that are a substrate for the same OATP are given concurrently (Kindla et al., 2009; Shitara, 2011; König et al., 2013). Additionally, several drugs are known to inhibit the function of one or more OATP, notably inhibition of OATP1B1 and OATP1B3 by macrolide antibiotics and rifampicin, inhibition of OATP1B1 by cyclosporine (Clarke and Cherrington, 2012; König et al., 2013), and inhibition of all OATPs by rifamycin (Hagenbuch and Meier, 2004). Intestinal OATP2B1 is inhibited by baicalin, cefixime, and fruit juices (most notably apple juice; also orange and grapefruit juices) (Dresser et al., 2002; Clarke and Cherrington, 2012; Shirasaka et al., 2013; Fujita et al., 2016), while OATP1A2 is inhibited by grapefruit juice (specifically its component naringin) (Glaeser et al., 2007; Clarke and Cherrington, 2012; Rebello et al., 2012). Testosterone and progesterone (and steroid hormones closely related to them) inhibit OATP2B1-mediated transport but are not substrates for OATP2B1 (Grube et al., 2006a). These inhibitors may be clinically useful as a therapeutic strategy to increase oral bioavailability and increase systemic exposure for drugs with high first-pass metabolism by the liver, given the important role of OATPs in taking up substrates into hepatocytes for further processing (Hagenbuch and Stieger, 2013). The International Transporter Consortium has published guidelines regarding recommended testing to determine if new drugs are inhibitors of OATPs (Giacomini et al., 2010). Difficulty can arise when studying the effect of OATP inhibitors since some of these compounds also inhibit cytochrome P450 (P450) enzymes and other transporters (Koenen et al., 2011). Additionally, data for localization and transport of compounds by OATPs can vary based on the cell type and method of detection used, which likely contributes to some conflicting results in the literature that are summarized in this review.
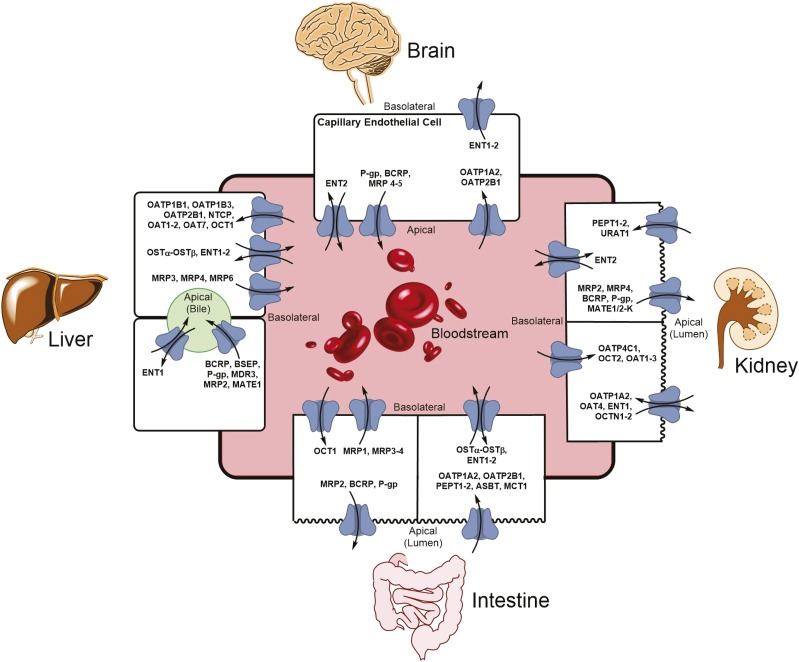
Transport by OATPs is sodium independent, pH dependent, and electroneutral, and OATP substrates tend to be highly albumin bound in plasma circulation (Hagenbuch and Meier, 2004). The exact mechanism of transport by OATPs is not fully known and may vary by substrate for an individual transporter (Hagenbuch and Stieger, 2013). Closely related OATPs may have different substrate profiles, as is the case for OATP1B1 and OATP1B3, which each have unique substrates. However, they also have shared substrate transport capacity for classes of drugs such as statins, angiotensin receptor antagonists, and human immunodeficiency virus protease inhibitors. OATPs have been found in nearly every human tissue, commonly (but not always) located in the basolateral membrane of polarized cells (Konig, 2011). Certain OATPs, such as OATP1B1, OATP1B3, OATP1A2, and OATP2B1, are primarily expressed in tissues important to drug disposition, including liver, kidney, and intestine, and at the blood-brain barrier (Fig. 1). The regulation of OATP expression is complex, with both transcriptional and post-transcriptional components (Hagenbuch and Stieger, 2013). This review will focus on four widely studied OATPs and their roles in cancer pharmacology: OATP1A2, OATP1B1, OATP1B3, and OATP2B1. Table 1 provides an overview of each of these key OATPs including major sites of expression in normal tissues and prototypical substrates.
Fig. 1.
Organ-specific expression of drug transporters. The expression of transporters at the apical and basolateral membranes of various tissues plays a crucial role in the transport of both endogenous and exogenous substrates across membranes. In the liver and kidney, transporters mediate movement of substrates into hepatocytes or the proximal tubule cells, respectively, where the substrates may undergo biotransformation or be excreted unmodified into the bile or urine via other transporters. In the intestine, transporters are involved in the selective movement of substrates into and out of enterocytes, which affects the oral bioavailability of drugs. In the capillary endothelial cells of the blood-brain barrier, transporters control which substrates can cross from the bloodstream into brain cells, thus limiting the ability of certain substrates to penetrate brain tissue. For the liver, the bloodstream compartment refers to the portal venous circulation. ASBT, apical sodium-dependent bile acid transporter; BCRP, breast cancer resistance protein; ENT, equilibrative nucleoside transporter; MATE, multidrug and toxic compound extrusion; MCT, monocarboxylate transporter; MRP, multidrug resistance-associated protein; NTCP, sodium/taurocholate cotransporting polypeptide; OAT, organic anion transporter; OST, organic solute and steroid transporter; PEPT, peptide transporter; P-gp, P-glycoprotein.
TABLE 1.
Overview of selected SLCO transporters
The table was adapted from Ho and Kim (2010) and Konig (2011).
| Gene Symbol | Protein | Normal Tissue Distribution | Cellular Localization | Example Substrates |
|---|---|---|---|---|
| SLCO1A2 (formerly SLC21A3) | OATP1A2 (formerly OATP-A, OATP1) | Brain (blood-brain barrier), kidney, liver, intestine, eye, placenta, prostate, testis | BL (ciliary body epithelia) | Chemotherapy: atrasentan, chemotherapy-bile acid conjugates (i.e., Bamet-R2, Bamet-UD2, chlorambucil taurocholate), docetaxel, imatinib, methotrexate, microcystin-LR |
| AP (kidney, hepatocytes, intestine) | Other: xenobiotics: BSP, deltorphin, fexofenadine, gadolinium dye for MRI, ouabain, rosuvastatin, levofloxacin, DPDPE, rocuronium, saquinavir; endogenous: bile salts, T4, T3, PGE2, E2G, E3S, DHEAS | |||
| SLCO1B1 (formerly SLC21A6) | OATP1B1 (formerly LST-1, OATP-C, OATP2) | Liver, placenta | BL (hepatocytes) | Chemotherapy: daunorubicin, mitoxantrone, atrasentan, chemotherapy-bile acid conjugates (i.e., Bamet-R2, Bamet-UD2), cytarabine, etoposide, flavopiridol, gimatecan, hydroxyurea, methotrexate, microcystin-LR, carboplatin, cisplatin, oxaliplatin, SN-38 (irinotecan active metabolite), sorafenib-β-D-glucuronide, docetaxel, paclitaxel |
| Other: xenobiotics: olmesartan, valsartan, atrasentan, temocaprilat, pitavastatin, pravastatin, cerivastatin, atorvastatin, rosuvastatin, rifampin, caspofungin, enalapril, BSP, phalloidin, troglitazone, bosentan, benzylpenicillin, nafcillin, some cephalosporins; endogenous: bilirubin, bilirubin-glucuronides, GCA, leukotriene C4, DHEAS, thromboxane B2, T4, T3, PGE2, TCA, E2G, E3S | ||||
| SLCO1B3 (formerly SLC21A8) | OATP1B3 (formerly LST-2, OATP8) | Liver, placenta | BL (hepatocytes) | Chemotherapy: atrasentan, hydroxyurea, methotrexate, microcystin-LR, carboplatin, cisplatin, oxaliplatin, SN-38, docetaxel, paclitaxel, imatinib, sorafenib- β-D-glucuronide |
| Other: xenobiotics: fexofenadine, deltorphin, ouabain, digoxin, rosuvastatin, valsartan, pitavastatin, BSP, rifampin, repaglinide, telmisartan, olmesartan, enalapril, DPDPE; endogenous: bilirubin conjugates, DHEAS, T3, T4, E2G, E3S, GCA, TCA, CCK-8 | ||||
| SLCO2B1 (formerly SLC21A9) | OATP2B1 (formerly OATP-B) | Widely expressed: liver, brain (blood-brain barrier), eye, small intestine, heart, kidney, lung, ovary, pancreas, placenta, skeletal muscle, spleen, testis | BL (hepatocytes, ciliary body epithelium); AP (intestine) | Chemotherapy: erlotinib, SN-38Other: xenobiotics: benzylpenicillin, bosentan, BSP, ezetimibe, fexofenadine, glibenclamide, glyburide, montelukast, statins, sulfasalazine, telmisartan; endogenous: E3S, DHEAS, TCA |
AP, apical; Bamet-R2, cis-diamminechloro-cholylglycinate-platinum(II); Bamet-UD2, cis-diammine-bisursodeoxycholate-platinum(II); BL, basolateral; BSP, bromosulfophthalein; CCK-8, cholecystokinin octapeptide; DPDPE, [D-penicillamine2,5] encephalin; E2G, estradiol-17β-glucuronide; GCA, glycocholate; MRI, magnetic resonance imaging; PGE2, prostaglandin E2; T3, triiodothyronine; T4, thyroxine; TCA; taurocholate.
OATP1A2 is found predominantly in the brain at the blood-brain barrier (Gao et al., 2000; Tamai et al., 2000; Kusuhara and Sugiyama, 2005), testis (Tamai et al., 2000), renal proximal tubule cells (Kullak-Ublick et al., 1995), liver cholangiocytes but not hepatocytes (Lee et al., 2005), prostate (Tamai et al., 2000), and ciliary body epithelium (Gao et al., 2005). There are conflicting data regarding intestinal expression of OATP1A2, but it is generally not considered to be highly expressed in this tissue (Tamai et al., 2000; Glaeser et al., 2007; Hilgendorf et al., 2007; Meier et al., 2007; Tamai, 2012; Gröer et al., 2013; Drozdzik et al., 2014). OATP1B1 and OATP1B3 are found almost exclusively at the basolateral membrane of hepatocytes under normal conditions (Tamai et al., 2000; Cui et al., 2003; Konig, 2011; Iusuf et al., 2012). However, one study found low OATP1B3 mRNA expression in the kidney (Hilgendorf et al., 2007), and there are conflicting data indicating expression in the intestine with one study finding OATP1B1 and OATP1B3 mRNA expression in the small intestine (Glaeser et al., 2007) and another finding neither protein nor mRNA expression of these transporters along the entire intestinal tract (Drozdzik et al., 2014).
Among all hepatic transporters, OATP1B1 and OATP1B3 were found to have the highest and second highest, respectively, protein abundance in healthy Caucasian subjects (Burt et al., 2016); in another study OATP1B1 was among the most abundant transporters in terms of liver protein levels, while OATP1B3 protein levels were moderate compared with other transporters (Hilgendorf et al., 2007). OATP2B1 is ubiquitously expressed in fetal and adult tissues and notably is found at the apical membrane of enterocytes (Kobayashi et al., 2003; Sai et al., 2006; Meier et al., 2007; Iusuf et al., 2012; Tamai, 2012), the basolateral membrane of hepatocytes (Kullak-Ublick et al., 2001), the apical membrane of renal proximal tubule cells, and the blood-brain barrier (Iusuf et al., 2012), as well as in the heart (Grube et al., 2006b), pancreas, lung, ovary, testis, spleen (Tamai et al., 2000), ciliary body epithelium (Gao et al., 2005), and placenta (St-Pierre et al., 2002).
The Role of OATP Transporters in Chemotherapy Disposition
It is widely known that OATPs represent an important family of transporters with important roles in drug uptake and distribution. A growing number of chemotherapeutic agents have been shown to be transported by OATP1A2, OATP1B1, OATP1B3, and OATP2B1, primarily in preclinical studies utilizing in vitro cell-based systems or in vivo animal models (Thakkar et al., 2015; Durmus et al., 2016). Emerging data suggest potential roles for these OATPs in relation to the clinical pharmacology of specific chemotherapeutic agents. In addition, chemotherapy agents may affect the transport of other substances by OATPs and contribute to drug-drug interactions. There are little published data regarding chemotherapeutic agent–specific transporter-mediated drug-drug interactions. Marada et al. (2015) used human embryonic kidney (HEK) cells stably transfected with human OATP1B1 and OATP1B3 to study the interaction of 26 different chemotherapy drugs with these transporters by assessing the change in transport of prototypic substrates—estrone-3-sulfate (E3S) for OATP1B1 and cholecystokinin octapeptide for OATP1B3—before and after addition of the chemotherapy agent. For OATP1B1, transport of E3S was significantly increased by irinotecan and decreased by paclitaxel and vinblastine; for OATP1B3, transport of cholecystokinin octapeptide was significantly decreased by chlorambucil, paclitaxel, vinblastine, vincristine, mitoxantrone, and etoposide (Marada et al., 2015). This study suggests that the transport of OATP substrates may be affected by coadministration of chemotherapeutic agents with potential implications for clinically significant drug-drug interactions; this is an area that needs further study to assess if clinically relevant concentrations of these agents affect OATP substrate pharmacokinetics. A preclinical mouse model for such studies has been developed using methotrexate as an OATP substrate (Durmus et al., 2015).
Methotrexate.
Methotrexate is an antimetabolite chemotherapy agent whose primary mechanism of action is via inhibition of dihydrofolate reductase, a key enzyme in the folate pathway, leading to termination of DNA synthesis. The methotrexate disposition pathway is complex, with many transporters and enzymes involved (Mikkelsen et al., 2011). While the main path for excretion of methotrexate is renally mediated, it also undergoes hepatic uptake and enterohepatic recirculation, which contribute to its overall disposition (Walling, 2006). In recent years, variants in genes encoding the hepatic transporters OATP1B1 and OATP1B3 have been found to have a significant effect on methotrexate clearance in several large genome-wide association studies, with OATP1B1 variants having a larger effect than variation in any other gene (Treviño et al., 2009; Ramsey et al., 2012, 2013).
Methotrexate is transported in vitro by OATP1B1, OATP1B3 (Abe et al., 2001; van de Steeg et al., 2010; Durmus et al., 2015), and OATP1A2 (Badagnani et al., 2006; van de Steeg et al., 2010). Oatp1a/1b deficient mice have significantly higher plasma methotrexate levels and reduced liver methotrexate levels compared with wild type (van de Steeg et al., 2011), with these differences able to be partially or completely rescued by transgenic expression of human OATP1B1, OATP1B3, or OATP1A2 (van de Steeg et al., 2009, 2012). OATP1A2 transport of methotrexate is noted to be both saturable and pH dependent, with 7-fold higher transport in the presence of an acidic extracellular environment (Badagnani et al., 2006). Transport of methotrexate by OATB1B1 and OATP1B3 is inhibited by rifampicin (Durmus et al., 2015).
Taxanes.
The taxane chemotherapy agents are mitotic inhibitors that work by disrupting microtubule function. Docetaxel pharmacokinetics are widely variable (Baker et al., 2006), which has clinical implications since reduced clearance is associated with increased risk of dose-limiting toxicities (Bruno et al., 1998, 2003) and clearance may also impact survival (Bruno et al., 2003). There are several differences in the pharmacokinetics of docetaxel and paclitaxel, but both are metabolized by CYP3A4 and other P450 enzymes and are transported by ABCB1, ABCG2, ABCC1, and ABCC2 in addition to OATPs (Baker et al., 2006; Oshiro et al., 2009).
Docetaxel is predominantly dependent on hepatic disposition in humans; ∼75% is excreted in bile but only ∼5% in urine (Cortes and Pazdur, 1995). Docetaxel is transported in vitro by OATP1B3 (Smith et al., 2005; Oshiro et al., 2009; de Graan et al., 2012; Obaidat et al., 2012; Nieuweboer et al., 2014; Yamada et al., 2014; Iusuf et al., 2015; Lee et al., 2015). Significantly decreased docetaxel transport into prostate cancer cells was seen in patient-derived xenografts found to have downregulation of OATP1B3 (de Morrée et al., 2016). There are conflicting data for OATP1B1 with some studies showing no transport (Smith et al., 2005; Baker et al., 2009; Yamada et al., 2014) and other more recent studies suggesting that OATP1B1-mediated docetaxel transport occurs (de Graan et al., 2012; Nieuweboer et al., 2014; Lee et al., 2015). Docetaxel is transported by mouse Oatp1b2 (de Graan et al., 2012; Lee et al., 2015). Slco1b2 knockout mice show significantly higher plasma levels and decreased liver-to-plasma ratios of docetaxel (Iusuf et al., 2015; Lee et al., 2015). Oatb1b2-deficient mice have markedly increased docetaxel area under the plasma time-concentration curve (AUC) (de Graan et al., 2012) and Oatp1a/1b knockout mice have higher plasma AUC and reduced liver-to-plasma AUC ratios compared with wild type, with intestinal absorption of docetaxel not affected (Iusuf et al., 2015). Collectively, these data suggest OATP1B transporters are involved in hepatic docetaxel uptake and clearance.
Paclitaxel is transported significantly by OATP1B3 (Smith et al., 2005; Oshiro et al., 2009; Svoboda et al., 2011; Park et al., 2016). There are conflicting data for OATP1B1-mediated transport of paclitaxel, with some negative data (Smith et al., 2005) and other data showing paclitaxel transport by OATP1B1 in vitro and the ability of OATP1B1 or OATP1B3 transfected into cells to improve cytotoxicity of paclitaxel (Svoboda et al., 2011). Paclitaxel is also transported by mouse Oatp1a/1b (van de Steeg et al., 2010), with Slco1a/1b null mice having higher AUC and lower liver uptake for paclitaxel compared with wild type, and transport was noted to be saturable at the highest doses of paclitaxel (van de Steeg et al., 2011). Human hepatoma HepG2 cells with significantly reduced OATP1B3 expression show paclitaxel resistance (Takano et al., 2009). The clinically used drug vehicle Cremophor inhibits OATP1B3 transport of paclitaxel (Smith et al., 2005), which has the potential to cause drug-drug interactions.
Irinotecan/7-Ethyl-10-Hydroxycamptothecin.
Irinotecan is a camptothecin derivative that exerts an antitumor effect by inhibition of topoisomerase I, resulting in double-stranded DNA breaks and cell death. It is spontaneously converted to an active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), by hepatic carboxylesterase and butyrylcholinesterase (de Man et al., 2018). SN-38 is believed to play a role in both the efficacy and toxicity associated with irinotecan-containing regimens, especially diarrhea (Kehrer et al., 2001; Fujita et al., 2016). SN-38 is further metabolized to an inactive form, SN-38-glucuronide, by uridine diphosphate glucuronosyltransferases, most prominently by the 1A1 isoform (UGT1A1); this glucuronide form is transported to the intestinal lumen via bile (Lokiec et al., 1995; de Man et al., 2018). Other transporters and enzymes, besides OATPs, involved in irinotecan disposition include ABCB1, ABCC1-2, ABCG2, CYP3A4, and CYP3A5. Irinotecan is primarily eliminated in the bile (66%), but irinotecan and its metabolites can also be found in the urine (de Man et al., 2018).
The parent drug irinotecan does not seem to be a substrate for OATP1B1 (Nozawa et al., 2005a; Oostendorp et al., 2009; Iusuf et al., 2014) or OATP1B3 (Iusuf et al., 2014), but these transporters are important for disposition of the active metabolite SN-38 (Yamaguchi et al., 2008; Oostendorp et al., 2009; Obaidat et al., 2012; Fujita et al., 2014). OATP1B1 does not transport SN-38 glucuronide (Nozawa et al., 2005a). OATP2B1 transports SN-38 in Xenopus oocytes (Fujita et al., 2016), but not in HEK293 cells (Fujita et al., 2014). Oatp1a/1b null mice have markedly increased plasma exposure of irinotecan and SN-38 and significantly decreased liver-to-plasma ratios of these compounds compared with wild type. When the human transporters OATP1B1 or OATP1B3 were introduced into these mice, SN-38 plasma levels reverted to the same levels as in the wild-type mice; however, irinotecan plasma levels were not affected, suggesting that SN-38, but not irinotecan, is a substrate for OATP1B1 and OATP1B3 in vivo (Iusuf et al., 2014).
Gastrointestinal toxicity (such as diarrhea) is the most common dose-limiting side effect of irinotecan and is due to SN-38 accumulation in intestinal tissues (Fujita et al., 2016). SN-38 is transported by OATP2B1 (Fujita et al., 2016) which is expressed in the intestines (Tamai, 2012). OATP2B1-mediated SN-38 transport is inhibited by apple juice (in a dose-dependent manner) and cefixime, among other compounds, with mice given apple juice concurrently with SN-38 having less gastrointestinal toxicity than control mice (Fujita et al., 2016). Additionally, the concurrent use of cyclosporine (a known potent OATP inhibitor) to reduce biliary excretion of irinotecan metabolites (through various transporters) has been shown to improve the toxicity profile of irinotecan in patients with metastatic colon cancer (Chester et al., 2003); the concept of utilizing an OATP inhibitor to alter the pharmacokinetic parameters of chemotherapeutic agents is an area that warrants additional investigation.
Tyrosine Kinase Inhibitors.
Tyrosine kinase inhibitors (TKIs) are chemotherapy agents that disrupt cell signaling via inhibition of tyrosine kinases. There are numerous TKIs in routine clinical use that have slightly different pharmacokinetic properties, but all are transported into and out of cells by various members of the solute carrier and ATP-binding cassette (ABC) families and are primarily metabolized by various P450 enzymes (Neul et al., 2016; Gong et al., 2017). Transport of TKIs by OATPs has been the subject of many recent studies (Hu et al., 2008, 2009, 2014; Yamakawa et al., 2011; Obaidat et al., 2012; Zimmerman et al., 2013; Vasilyeva et al., 2015; Bins et al., 2017; Bauer et al., 2018). In general, elimination of TKIs is primarily hepatic and mediated by P450 enzymes (O’Brien and Moghaddam, 2017), with renal clearance playing a small role for some agents (van Erp et al., 2009).
Erlotinib is transported by OATP2B1 in vitro (Bauer et al., 2018). Sorafenib transport by OATP1B1 was negative in some studies (Hu et al., 2009; Bins et al., 2017), but another study showed that OATP1B1- and OATP1B3-transfected cells transported sorafenib significantly higher than vector control in vitro. However, the in vivo mouse data showed no difference in sorafenib pharmacokinetics between wild-type and Oatp1b2-deficienct mice, and no change in sorafenib transport in humanized OATP1B1 or OATP1B3 mice compared with Oatp1a/b null mice (Zimmerman et al., 2013). Sorafenib is not transported by OATP1A2 or OATP1B3 (Hu et al., 2009). Sorafenib has an important metabolite, sorafenib-β-D-glucuronide, which is transported into hepatocytes by OATP1B1 (Bins et al., 2017) and mouse Oatp1a/1b (Vasilyeva et al., 2015). In contrast to the sorafenib data in the Zimmerman et al. (2013) study, sorafenib-β-D-glucuronide transport was affected in Oatp1b2 deficient or Oatp1a/1b null mice with these mice having significantly higher sorafenib-β-D-glucuronide plasma AUC compared with wild type, and this difference in AUC partially corrected to wild type in mice transgenic for human OATP1B1 or OATP1B3. The Zimmerman et al. (2013) data overall suggest that in vivo OATP1B1 and OATP1B3 are not important for sorafenib transport but are important for transport of its glucuronide metabolite. Imatinib is transported by OATP1A2 and OATP1B3 (Hu et al., 2008; Yamakawa et al., 2011; Obaidat et al., 2012). However, while imatinib transport by OATP1A2 is seen in vitro, imatinib absorption in cancer patients was not found to be associated with function-altering OATP1A2 variants or with coadministration of rosuvastatin (a known OATP1A2 inhibitor), suggesting that an alternate transport pathway may also be important in vivo (Eechoute et al., 2011).
TKIs also have implications in drug-drug interactions involving OATPs. Sorafenib and other TKIs (axitinib, nilotinib, pazopanib, and lapatinib) are inhibitors of OATP1B1 in vitro; however, in vivo data are conflicting and it is likely that transport through other mechanisms becomes important when OATP1B1 is inhibited in these cases (Polli et al., 2008; Hu et al., 2014). Coadministration of sorafenib changes the pharmacokinetics of docetaxel, a known OATP substrate, which may have clinical implications since sorafenib is being incorporated into protocols for many different solid tumors due to its favorable side effect profile and efficacy against these tumors (Awada et al., 2012).
Anthracyclines.
The anthracyclines, including doxorubicin, daunorubicin, and mitoxantrone, have several mechanisms that contribute to cytotoxicity: disruption of DNA and RNA synthesis via intercalation, inhibition of topoisomerase II, generation of free oxygen radicals, and interference with histone function. Approximately 50% of doxorubicin’s disposition is mediated by biliary elimination, suggesting hepatic uptake and clearance play important roles in its disposition (Danesi et al., 2002). Multiple transporters are involved in doxorubicin disposition, including uptake by SLC22A16 and efflux by ABCC1, ABCG2, and ABCB1. It also undergoes metabolism via several pathways (Thorn et al., 2011).
Recent studies have evaluated the role of OATPs in doxorubicin disposition (Durmus et al., 2014; Lee et al., 2017). Doxorubicin uptake and transport by OATPs in vitro vary according to the model system that is used. Doxorubicin is transported by OATP1A2-expressing HEK293 (Durmus et al., 2014), HeLa, and Madin-Darby canine kidney II cells (Lee et al., 2017), while OATP1B1- and OATP1B3-mediated doxorubicin transport is seen in Madin-Darby canine kidney II cells (Lee et al., 2017) but not in HeLa (Lee et al., 2017) or HEK293 cells (Durmus et al., 2014). Results of in vivo studies in rodents have shown that doxorubicin uptake and disposition are significantly decreased in Oatp1a/1b null mice compared with wild type (Durmus et al., 2014; Lee et al., 2017) and that transgenic expression of human OATPs either completely (for OATP1A2) or partially (for OATP1B1 and OATP1B3) reverts doxorubicin pharmacokinetics to wild-type values (Durmus et al., 2014). These data together suggest that OATP1A2, OATP1B1, and OATP1B3 are all involved in doxorubicin transport. The related agents daunorubicin and mitoxantrone are transported by OATP1B1 in vitro, and deficiency of mouse Oatp1b2 results in significantly increased plasma AUC of both drugs (Drenberg et al., 2016).
Platins.
The platinum chemotherapy agents work by crosslinking DNA and therefore disrupting DNA synthesis and repair. These compounds are transported by SLC31A1, ABCC2, ABCG2, and MDR1 (P-glycoprotein) (Marsh et al., 2009; Dasari and Tchounwou, 2014), and importantly by organic cation transporters (OCTs), OCT1, OCT2, and OCT3, encoded by SLC22 genes (Wagner et al., 2016). A number of enzymes are involved in converting platinum-based chemotherapy drugs to inactive metabolites, such as myeloperoxidase and superoxide dismutase (Marsh et al., 2009). The elimination of platinum agents is almost exclusively through the kidneys (Burger et al., 2011).
Cisplatin, carboplatin, and oxaliplatin are transported in vitro by OATP1B1 and OATP1B3, and cytotoxicity of these agents in human tumor cells was found to be enhanced with increasing OATP1B3 mRNA expression (Lancaster et al., 2013). Oatp1b2 knockout mice have significantly reduced liver-to-plasma ratios of cisplatin compared with wild type (Lancaster et al., 2013). Bile acid-cisplatin derivatives have been developed as liver-trophic drugs that could become concentrated in bile and help overcome resistance to platinum agents (Criado et al., 1997, 2000). Two of these, Bamet-R2 (cis-diamminechloro-cholylglycinate-platinum(II)) and Bamet-UD2 (cis-diammine-bisursodeoxycholate-platinum(II)), are transported by OATP1B1 and to a lesser extent by OATP1A2 (Briz et al., 2002).
Summary: OATPs in Chemotherapy Disposition.
While a growing number of chemotherapeutic agents have been shown to be OATP substrates in vitro, it remains yet to be determined if OATPs are important to the clinical pharmacology of all these purported anticancer agents. However, a series of follow-up or complementary studies in animal models using Oatp knockout mice or transgenic human OATP mice have suggested OATP transporters are important to the clinical pharmacology of agents such as methotrexate, taxanes, and anthracyclines. Additional studies to evaluate the roles of OATP transporters in chemotherapy disposition are warranted due to the narrow therapeutic indices associated with them in cancer therapeutics. A more comprehensive evaluation and understanding of the clinical pharmacology of these drugs would yield important knowledge to better optimize chemotherapy dosing and combination therapies that may improve therapeutic efficacy while mitigating serious drug-mediated adverse effects.
Pharmacogenetics of OATP Transporters
The SLCO genes are polymorphic, with common (minor allele frequency >5%) variants identified in OATP1B1, OATP1B3, and OATP1A2. Some of these variants affect the expression, localization, and/or function of the transporter and can significantly alter the disposition of xenobiotics and endogenous substrates (Konig, 2011). Table 2 contains information on functional consequences of selected polymorphisms in SLCO1A2, SLCO1B1, SLCO1B3, and SLCO2B1, and this topic was also reviewed in detail recently (Zhou et al., 2017).
TABLE 2.
Pharmacogenomics of OATPs: selected variants and functional consequences
In vitro and in vivo functional studies as well as pharmacokinetic-pharmacogenetic correlative studies were included in the literature review for this table.
| Gene | Nucleotide Change | Amino Acid Change | rs Number | Functional Consequencea | Reference |
|---|---|---|---|---|---|
| SLCO1A2 | c.−1167G>A | Intronic | rs4148977 | Decreased imatinib clearance | Yamakawa et al. (2011) |
| c.−1094G>A | Intronic | rs4148978 | Decreased imatinib clearance | ||
| c.−423G>A | Intronic | rs3764043 | Increased imatinib clearance | ||
| c.38T>C | Ile13Thr | rs10841795 | Increased transport of multiple substrates including methotrexate | Badagnani et al. (2006), Gong and Kim (2013) | |
| c.404A>T | Asn135Ile | rs45502302 | Decreased transport | Badagnani et al. (2006), Gong and Kim (2013) | |
| c.502C>T | Arg168Cys | rs11568564 | Decreased transport of multiple substrates including methotrexate | ||
| c.516A>C | Glu172Asp | rs11568563 | Decreased transport of multiple substrates including methotrexate | ||
| c.559G>A | Ala187Thr | rs750165758 | Decreased transport | Lee et al. (2005) | |
| c.833delA | Asn278Met | rs11568555 | Decreased transport of multiple substrates including methotrexate | Badagnani et al. (2006) | |
| SLCO1B1 | g.21130388G>A | Intronic | rs4149015 | Decreased transport of multiple substrates including SN-38 | Niemi et al. (2004), Han et al. (2008) |
| c.217T>C (*2) | Phe73Leu | rs56101265 | Decreased transport of multiple substrates including docetaxel | Tirona et al. (2001), Gong and Kim (2013), Lee et al. (2015) | |
| c.245T>C | Val82Ala | rs56061388 | Decreased transport | Tirona et al. (2001), Gong and Kim (2013) | |
| c.388A>G (*1b) | Asn130Asp | rs2306283 | Decreased transport of some substrates including cytarabine; increased or unchanged transport for other substrates | Kameyama et al. (2005), Han et al. (2008), Gong and Kim (2013), Drenberg et al. (2016) | |
| c.467A>G | Glu156Gly | rs72559745 | Decreased transport | Tirona et al. (2001), Gong and Kim (2013) | |
| c.521T>C (*5) | Val174Ala | rs4149056 | Decreased transport of multiple substrates including SN-38, docetaxel, cytarabine, etoposide, mitoxantrone, and daunorubicin; decreased clearance of methotrexate | Tirona et al. (2001), Kameyama et al. (2005), Nozawa et al. (2005a), Han et al. (2008), Gong and Kim (2013), Ramsey et al. (2013), Lee et al. (2015), Drenberg et al. (2016) | |
| c.578T>G | Leu193Arg | rs72559746 | Decreased to abolished transport of some substrates | Michalski et al. (2002), Gong and Kim (2013) | |
| c.1007C>G | Pro336Arg | rs72559747 | Decreased for some substrates, unchanged for others | Nishizato et al. (2003), Gong and Kim (2013) | |
| c.1058T>C | Ile353Thr | rs55901008 | Decreased transport | Tirona et al. (2001), Gong and Kim (2013) | |
| c.1294A>G | Asn432Asp | rs56387224 | Decreased transport | ||
| c.1463G>C | Gly488Ala | rs59502379 | Decreased transport of multiple substrates including docetaxel | Tirona et al. (2001), Gong and Kim (2013), Lee et al. (2015) | |
| c.1964A>G | Asp655Gly | rs56199088 | Decreased transport | Tirona et al. (2001), Gong and Kim (2013), Lee et al. (2015) | |
| c.2000A>G | Glu667Gly | rs55737008 | Decreased transport | Tirona et al. (2001), Gong and Kim (2013) | |
| c.1865+248G>A | Intronic | rs4149081 | Decreased methotrexate clearance | Treviño et al. (2009), Ramsey et al. (2013) | |
| c.1865+4846T>C | Intronic | rs11045879 | Decreased methotrexate clearance | ||
| SLCO1B3 | c.1599-5676A>G | Intronic | rs11045585 | Decreased or unchanged docetaxel clearance | Chew et al. (2011), de Graan et al. (2012) |
| c.439A>G | Thr147Ala | rs57585902 | Decreased or unchanged docetaxel transport | Baker et al. (2009), Chew et al. (2011), Lee et al. (2015) | |
| c.699G>A | Met233Ile | rs7311358 | Decreased transport of multiple substrates including paclitaxel | Schwarz et al. (2011), Gong and Kim (2013), Park et al. (2016) | |
| c.1559A>C | His520Pro | rs559692629 | Decreased transport of multiple substrates; decreased or unchanged docetaxel transport | Baker et al. (2009), Chew et al. (2011), Schwarz et al. (2011), Lee et al. (2015) | |
| c.1564G>T | Gly522Cys | rs72559743 | Decreased transport | Letschert et al. (2004), Gong and Kim (2013) | |
| c.1679T>C | Val560Ala | rs12299012 | Decreased transport of multiple substrates; decreased or unchanged docetaxel transport | Baker et al. (2009), Chew et al. (2011), Schwarz et al. (2011), Gong and Kim (2013), Lee et al. (2015) | |
| SLCO2B1 | c.601G>A | Val201Met | rs35199625 | Decreased transport | Ho et al. (2006) |
| c.1175C>T | Thr392Ile | rs1621378 | Decreased transport | Nozawa et al. (2002), Gong and Kim (2013) | |
| c.1457C>T | Ser486Phe | rs2306168 | Decreased transport | Nozawa et al. (2002), Gong and Kim (2013) | |
| c.1526G>A | Arg509His | rs140407559 | Decreased transport | (Nozawa et al. (2002) |
Increased and decreased refer to transport function of the variant transporter compared with the corresponding wild-type transporter. For some variants, effect on transport function varies by substrate; in these cases, the predominant effect on transporter function is listed.
OATP1A2.
Multiple function-altering variants in SLCO1A2 have been reported (Lee et al., 2005; Zhou et al., 2015). Several SLCO1A2 variants show altered transport of methotrexate, including the hyperfunctional c.38T>C variant, hypofunctional c.516A>C, and c.502C>T variants, and nonfunctional c.833delA variant (Badagnani et al., 2006). The c.516A>C variant has been reported as hypofunctional for other substrates as well, and some SLCO1A2 variants have substrate-specific alteration of transport (Lee et al., 2005). Imatinib clearance is significantly affected by several single nucleotide polymorphisms (SNPs) in SLCO1A2, with patients carrying at least one copy of each of the intronic variants c.−1167G>A and c.−1094G>A having significantly reduced drug clearance compared with wild type, and patients who do not have the intronic variant c.−423G>A (homozygous wild type) have increased clearance (Yamakawa et al., 2011). The clinical significance of altered drug transport function associated with SLCO1A2 variants has not been determined to date. Further study of SLCO1A2 variation as it relates to chemotherapy disposition will be helpful in the future given this transporter’s role at the blood-brain barrier and the importance of developing chemotherapeutic agents that penetrate the brain tissue for certain diagnoses, most notably malignant brain tumors.
OATP1B1.
SLCO1B1 variants have been widely studied with regard to impact on the pharmacokinetics of many drugs (Niemi et al., 2011; Shitara, 2011), and notably in the incidence of adverse drug reactions in patients treated with statins (Link et al., 2008; Jiang et al., 2016). The SLCO1B1 c.521T>C variant, which has a minor allele frequency of 12%–18% in European and East Asian populations with lower frequency (1.9%) in sub-Saharan African populations (Pasanen et al., 2008), has been the most extensively studied of the SLCO1B1 variants and is associated with significantly impaired hepatic uptake, and thus corresponding increased plasma levels, of many substrates (Tirona et al., 2001; Nishizato et al., 2003; Mwinyi et al., 2004; Niemi et al., 2004, 2005a,b; Kameyama et al., 2005; Nozawa et al., 2005a; Katz et al., 2006; Ho et al., 2007; Zhang et al., 2007; Xiang et al., 2009). The c.521T>C variant has been identified as a significant risk factor for statin-induced myopathy (Link et al., 2008) and other statin-induced adverse drug reactions (Jiang et al., 2016). A case-control analysis of patients in a large randomized trial study on the effectiveness of additional reductions in cholesterol and homocysteine found an odds ratio for myopathy of 4.5 per copy of the variant C allele, and homozygous variant (CC) patients had an odds ratio of 16.9 for development of myopathy compared with TT patients (Link et al., 2008). Another commonly studied SLCO1B1 variant is the c.388A>G variant (Tirona et al., 2001), which has no apparent relationship with statin adverse drug reactions (Jiang et al., 2016).
Several SLCO1B1 haplotypes with differential drug transport compared with wild type have been identified. SLCO1B1*1a is the reference allele, with the *1b haplotype including the c.388A>G variant, the *5 haplotype having the c.521T>C variant, and the *15 haplotype containing both the c.388A>G and c.521T>C variants (Tirona et al., 2001). There are conflicting data regarding the function of the *1b haplotype, with transport of some substrates being no different compared with transport by the wild-type protein (Tirona et al., 2001; Michalski et al., 2002; Nozawa et al., 2002; Iwai et al., 2004; Lee et al., 2015), while in other studies it appears to be hyperfunctional for some substrates (Michalski et al., 2002; Mwinyi et al., 2004; Ieiri et al., 2009; Nies et al., 2013; Crona et al., 2016; Drenberg et al., 2016) and hypofunctional for others (Michalski et al., 2002; Drenberg et al., 2016). Some data suggest that the c.388A>G variant causes increased expression of OATP1B1 at the plasma membrane (Nozawa et al., 2002; Nies et al., 2013). The *5 and *15 haplotypes are hypofunctional, including significantly reduced in vitro transport of cytarabine, daunorubicin, etoposide, mitoxantrone (Drenberg et al., 2016), and docetaxel (de Graan et al., 2012; Lee et al., 2015). However, in a clinical study, no effect on docetaxel clearance was found for patients carrying the SLCO1B1 variants g.21130388G>A (promoter variant), c.388A>G, or c.521T>C compared with wild type (de Graan et al., 2012).
The effect of other SLCO1B1 variants on chemotherapy disposition has been studied. Several studies have examined the relationship between SLCO1B1 genotype and the high interpatient variability seen in methotrexate clearance (Treviño et al., 2009; Lopez-Lopez et al., 2011; Ramsey et al., 2012, 2013). In 115 pediatric leukemia patients, those who were homozygous for the intronic SLCO1B1 variant c.1865+248G>A or c.1865+4846T>C had significantly higher plasma methotrexate levels following high-dose methotrexate therapy compared with patients with the wild-type or heterozygous variant genotypes, although the number of patients homozygous for these variants was low. High methotrexate plasma levels at 72 hours after the start of infusion were significantly correlated with development of global toxicity, vomiting, and renal toxicity, suggesting genetic variants that affect methotrexate plasma levels may also increase rates of methotrexate-associated toxicities (Lopez-Lopez et al., 2011).
A large clinical study (Treviño et al., 2009) and a follow-up study including additional patients (Ramsey et al., 2013), which utilized a genome-wide association study approach, found that SNPs in SLCO1B1 were significantly associated with methotrexate clearance. The SLCO1B1 variants c.1865+248G>A and c.1865+4846T>C were in linkage disequilibrium with each other and with c.521T>C, all of which were associated with decreased methotrexate clearance (Treviño et al., 2009). Interestingly, those carrying wild-type alleles at these positions had increased incidence of gastrointestinal toxicity (per National Cancer Institute Cancer Therapy Evaluation Program toxicity grading) compared with those with variant alleles, presumably due to enhanced clearance and resultant lower plasma exposure with corresponding higher gastrointestinal tract methotrexate exposure via elimination pathways. In the follow-up study, the SNP most significantly associated with methotrexate clearance was c.521T>C, in which homozygous variant (CC) patients had methotrexate clearance that was on average 13% lower than homozygous wild-type (TT) patients. This genotype explained 2% of the variation in methotrexate clearance; a higher proportion explained than that for age and sex combined. In this study, three other significant SNPs in SLCO1B1 were in significant linkage disequilibrium with c.521T>C (Ramsey et al., 2013). Another study utilizing the same patient population indicated that, after controlling for c.521T>C, 15 additional SNPs in SLCO1B1, all nonsynonymous and some rare (minor allele frequency <5%), were significantly associated with methotrexate clearance, with these SNPs either causing increased or decreased clearance compared with wild type. These data showed that even rare damaging SNPs can have a significant impact on methotrexate clearance (Ramsey et al., 2012).
A separate study replicated the finding that c.1865+248G>A and c.1865+4846T>C are associated with methotrexate disposition; in this study, these variants were associated with higher average plasma methotrexate levels, including levels above the upper limit of the desired range (Li et al., 2015). A recent study in adult patients with hematologic malignancies receiving high-dose methotrexate suggests patients with the SLCO1B1 variant c.388A>G or c.521T>C exhibit differential urinary endogenous metabolomic profiles that may interact with renal organic anion transporters to modulate risk for methotrexate-induced toxicities, illustrating the link between SLCO1B1 SNPs and methotrexate clearance and/or toxicity may be multifactorial and more complex than previously thought (Martinez et al., 2018).
Regarding irinotecan and SN-38 disposition, Xenopus oocytes expressing the SLCO1B1*15 allele showed markedly reduced transport for SN-38, while the *5 allele conferred a more modest reduction in SN-38 transport (Nozawa et al., 2005a). Patients with the *15 haplotype had 3-fold reduction in irinotecan clearance and significantly higher AUC for both irinotecan and SN-38 compared with *1a/*1a (Xiang et al., 2006). In patients with nonsmall cell lung cancer treated with irinotecan and cisplatin, the SLCO1B1 variants c.521T>C and g.21130388G>A were found to affect the pharmacokinetics of SN-38. Patients carrying at least one copy of the c.521T>C variant allele or those homozygous for the g.21130388G>A promoter variant had a significantly higher AUC of the important irinotecan metabolite SN-38 compared with wild-type patients. Additionally, SN-38 clearance was significantly lower in the g.21130388AA patients and there was a trend toward lower clearance in patients with at least one copy of the c.521T>C variant. None of the variants in this study were associated with tumor response or pharmacokinetics of the parent drug irinotecan (Han et al., 2008). However, in another study, the SLCO1B1*5 haplotype was associated with significantly increased irinotecan AUC, but neither the SLCO1B1*1b nor the SLCO1B1*5 haplotype was associated with SN-38 pharmacokinetics (Innocenti et al., 2009). In a study by Crona et al. (2016), the c.521T>C variant was associated with increased irinotecan AUC in a discovery cohort of patients; however, this finding did not replicate in a validation cohort, and this variant was not associated with SN-38 pharmacokinetics. Thus, the relationship between these SLCO1B1 variants and the pharmacokinetics of irinotecan and SN-38 is not consistently defined and warrants further study in larger populations of patients to determine whether SLCO1B1 variants influence irinotecan and/or SN-38 pharmacokinetics.
However, SLCO1B1 variants have been linked to irinotecan-induced toxicities. The SLCO1B1 c.521T>C variant was associated with increased risk of irinotecan-induced grade 3/4 neutropenia in a cohort of adult Japanese patients with various cancers who were treated either with irinotecan monotherapy or irinotecan plus cisplatin (Sai et al., 2010). A patient with the SLCO1B1*15/15 genotype as well as another at-risk genotype was reported to have excessive accumulation of SN-38 in the plasma circulation, which led to life-threatening diarrhea and neutropenia (Takane et al., 2009). Patients with at least one copy of the c.521T>C variant allele who were treated with irinotecan had higher rates of grade 4 neutropenia compared with wild type, and patients homozygous for the c.388A>G variant, had higher rates of grade 3 diarrhea following irinotecan therapy (Han et al., 2008). In advanced cancer patients treated with single-agent irinotecan, the SLCO1B1*1b haplotype was associated with a lower absolute neutrophil count nadir when comparing *1b/1b patients to *1a/1a or *1a/1b patients (Innocenti et al., 2009); conversely, the c.388A>G variant was associated with a protective effect against neutropenia following irinotecan therapy in another study (Crona et al., 2016). With regard to outcomes, patients with the c.521T>C variant had increased SN-38 exposure compared with wild type, and those with the SLCO1B1 c.388GG genotype had significantly longer progression-free survival compared with the c.388AA genotype (Teft et al., 2015). Collectively, these genetic association studies demonstrate that SLCO1B1 variation may play an important role in the interindividual variability in chemotherapy disposition and response for several OATP1B1 chemotherapeutic substrates.
OATP1B3.
The functional consequences of various SLCO1B3 variants, especially c.334T>G and c.699G>A, have been studied, for which there are substrate-specific alterations in transport compared with wild type (Letschert et al., 2004; Schwarz et al., 2011). OATP1B3 transports testosterone in vitro, and cells expressing OATP1B3 containing either the c.334T>G or c.699G>A variant transported testosterone at a similar rate compared with wild type, while the presence of both variants concurrently resulted in significant impairment of testosterone transport (Hamada et al., 2008). This has potential implications for the growth of hormone-dependent tumors such as prostate cancer.
Several SLCO1B3 variants have been found to affect docetaxel transport compared with wild type, including c.699G>A, c.1559A>C, c.1679T>C, and the haplotype containing c.334T>G and c.699G>A in vitro (Lee et al., 2015); however, results from clinical studies on the effect of SLCO1B3 variants on docetaxel elimination are mixed. In a study on the effect of the SLCO1B3 haplotype on docetaxel pharmacokinetics in cancer patients, the variants c.359+76G>A (intronic), c.699G>A, c.1599-5676A>G (intronic), and c.*347_*348insA were used to construct haplotypes that had a significant impact on docetaxel clearance and AUC. These haplotypes explained 29% and 22% of the variability in docetaxel clearance and AUC, respectively; specifically, one haplotype resulted in a 30% decrease in clearance and 40% increase in AUC, while another haplotype increased clearance by 50% compared with the reference haplotype (Chew et al., 2012). However, in a study on patients treated with docetaxel, no relationship was found between the c.699G>A, c.1559A>C, c.1679T>C, c.334T>G, c.439A>G, or c.767G>C variant and docetaxel pharmacokinetics (Baker et al., 2009). Patients with nasopharyngeal carcinoma who were homozygous for the intronic SLCO1B3 variant c.1599-5676A>G had significantly higher AUC and lower clearance of docetaxel in one study, while multiple other SLCO1B3 variants (including c.334T>G, c.1559A>C, c.1679T>C, c.699G>A, and c.1599-5676A>G) had no effect on docetaxel pharmacokinetics, although the number of patients with these variants in this study was low (Chew et al., 2011). Another study similarly found no effect of the variants c.334T>G, c.699G>A, and c.1599-5676A>G on docetaxel clearance (de Graan et al., 2012).
Taxane-induced transport and toxicities have been studied with respect to SLCO1B3 variation. For paclitaxel, significantly reduced transport was found for oocytes expressing the c.699A>G variant, while no effect on paclitaxel transport was found for the c.334A>G variant (Park et al., 2016). A clinical study found no relationship between paclitaxel-associated neurotoxicity and the SLCO1B3 variants c.334T>G and c.699G>A in 118 cancer patients after adjusting for age and treatment schedule (Leskelä et al., 2011). The c.1599-5676A>G variant in SLCO1B3 has been associated with grade 3/4 docetaxel-induced leukopenia and neutropenia in some studies (Kiyotani et al., 2008; Yamada et al., 2014), but had no effect on these toxicities or other hematologic abnormalities in another study (Choi et al., 2015). Thus, the consequences of SLCO1B3 variation to taxane-induced differential transport or risk for drug-mediated toxicity have not as yet been clearly defined.
OATP Expression in Tumor Tissues and Their Potential as Therapeutic Targets
The differential expression of OATPs in cancerous versus normal tissues has been studied as a way to develop novel diagnostic or therapeutic strategies for these tumors (Okabe et al., 2008). OATPs that are highly expressed in cancerous tissues with little to no expression in corresponding normal tissues may be useful as diagnostic markers and/or therapeutic targets, since malignant tissues that have upregulation of OATPs transporting chemotherapeutic substrates may be more sensitive to the cytotoxic effects of these agents. This makes screening for OATP expression prior to starting therapy an attractive way to increase the chance of response to a chemotherapy regimen (Buxhofer-Ausch et al., 2013). Table 3 contains information on the overexpression of OATP1A2, OATP1B1, OATP1B3, and OATP2B1 in various cancer tissues, which has recently been reviewed in more detail (Thakkar et al., 2015).
TABLE 3.
Differential OATP protein expression in cancer tissues
See the text for references for this table.
| Protein | Malignant Tissue Distributiona |
|---|---|
| OATP1A2 | Increased expression: breast, pancreas, bone, some lung cancer cell lines |
| Decreased expression: colon, some lung cell lines | |
| OATP1B1 | Increased expression: breast, pancreas, prostate, ovary, lung, colon |
| Decreased expression: liver | |
| OATP1B3 | Increased expression: colon, pancreas, lung, breast, prostate, ovary, lung, stomach, gallbladder |
| Decreased expression: liver | |
| OATP2B1 | Increased expression: lung (conflicting evidence), breast, colon, bone |
| Decreased expression: none reported |
Increased and decreased refer to expression of the transporter in malignant tissue compared with normal tissue of the same type.
While OATP1B3 is predominantly expressed in liver under normal conditions, it has been detected in various malignant tissues including tumors of the cervix, intestines, liver, lung, pancreas, and genitourinary tract (Lancaster et al., 2013). A cancer-specific form of OATP1B3, also referred to as cancer-type OATP1B3, has been identified in which an alternative splice site is used, resulting in a protein that is structurally different from that found in normal liver; the cancer-specific OATP1B3 transcript has been detected in malignant tissues from the colon (Nagai et al., 2012; Han et al., 2013; Thakkar et al., 2013; Sun et al., 2014), pancreas (Han et al., 2013; Thakkar et al., 2013), and lung (Nagai et al., 2012; Sun et al., 2014). Some commonly used detection methods for wild-type OATP1B3 do not detect this cancer-specific OATP1B3; therefore, studies that do not show expression of OATP1B3 in cancerous tissues must be interpreted with caution if they did not search for the cancer-specific variant (Evangeli et al., 2017). The transport function of the cancer-specific OATP1B3 variant for at least some substrates is significantly decreased or abolished (Thakkar et al., 2013; Sun et al., 2014), although transport of other substrates is similar to that of the wild-type transporter (Imai et al., 2013).
Additionally, OATP1A2, OATP1B1, OATP1B3, and OATP2B1 transport many endogenous steroid hormones and hormone conjugates including estradiol, E3S, and dehydroepiandrosterone sulfate (DHEAS) ( Kullak-Ublick et al., 1998, 2001; König et al., 2000; Cui et al., 2001; Tamai et al., 2001; Pizzagalli et al., 2003; Nozawa et al., 2004a,b; Grube et al., 2006a; Miyagawa et al., 2009; Maeda et al., 2010; De Bruyn et al., 2011; Yang et al., 2011; Buxhofer-Ausch et al., 2013). Steroid hormones and their conjugates are important for the proliferation of tumor cells in hormone-dependent malignancies (Nozawa et al., 2004b, 2005b; Hamada et al., 2008; Hong and Chen, 2011). However, there is generally redundant transport of these hormones by one or more OATPs in addition to other transporters; for example, in one study OATP1B3 transported E3S efficiently but only accounted for 6% of total E3S transport in breast cancer cells (Maeda et al., 2010). The expression of OATPs in hormone-dependent cancers has been studied and linked to the level of differentiation in the tumor specimens, which may be used as a marker of disease stage (Pressler et al., 2011).
Breast Tumors.
OATP1A2, OATP1B1, OATP1B3, and OATP2B1 have all been found in breast cancer cells at levels similar to or higher than those found in normal breast tissue (Pizzagalli et al., 2003; Al Sarakbi et al., 2006; Bleasby et al., 2006; Miki et al., 2006; Muto et al., 2007; Meyer zu Schwabedissen et al., 2008; Kindla et al., 2011; Banerjee et al., 2012; Stute et al., 2012; Buxhofer-Ausch et al., 2013). Treatment with chemotherapy changes the expression levels of these transporters in a cell line–dependent manner (Stute et al., 2012). Hormone-dependent breast tumor cells have been shown to have increased transport of E3S compared with hormone-independent tumors, possibly due to increased OATP expression in hormone-dependent tumors (Banerjee et al., 2012−2014).
Estrogens are important for development and growth of hormone-dependent breast cancers, with certain estrogen derivatives being taken up into tumor cells to be converted to their active forms in situ (Hong and Chen, 2011). Estrogen concentrations in tumors have been found to be significantly higher than levels in the plasma circulation, suggesting an active process resulting in accumulation of intratumoral estrogens (Pasqualini et al., 1996). E3S is an important source of estrogen for breast cancer cells and is found at particularly high levels in postmenopausal breast cancer patients (Pasqualini et al., 1996). E3S is converted to estradiol in a series of enzymatic reactions, and estradiol drives cell proliferation (Nozawa et al., 2004b, 2005b; Banerjee et al., 2013; Matsumoto et al., 2015). E3S transport into breast cancer cells is apparently by an active carrier-mediated process given its uptake kinetics (Nozawa et al., 2004b). Due to their transport of steroid hormones, OATPs have been considered important to proliferation in breast cancer cells (Banerjee et al., 2012).
OATP-mediated transport of hormone conjugates may be useful as a therapeutic target (Banerjee et al., 2012). A study examining the plasma levels of estrone, estrone conjugates, and androstenedione in postmenopausal women with resected early stage estrogen receptor positive breast cancer (prior to aromatase inhibitor therapy) found that several genetic variants in SLCO1B1, including c.521T>C and c.463C>A, were associated with higher levels of these hormones compared with wild type. This is clinically relevant because estrone conjugates are converted to estrone by the enzyme steroid sulfatase, and steroid sulfatase inhibitors are available for clinical use (Dudenkov et al., 2017). Additionally, expression of OATPs in tumor specimens may be associated with disease prognosis. Overexpression of OATPs has also been associated with higher tumor grade (Al Sarakbi et al., 2006; Matsumoto et al., 2015). However, increased OATP expression may also make breast tumors more susceptible to chemotherapeutic agents that are transported by that OATP, as was suggested in a study where OATP1B3 expression was associated with improved prognosis in estrogen receptor positive patients only, possibly due to increased uptake of tamoxifen in these patients (Muto et al., 2007). Recently, it has been shown that OATP1A2 expression may predict pathologic response to neoadjuvant chemotherapy in triple-negative breast cancer patients as well (Hashimoto et al., 2014).
Prostate and Other Genitourinary Tumors.
Prostate cancer specimens have been found to have increased protein and/or mRNA expression of OATP1B1 (Buxhofer-Ausch et al., 2013) and OATP1B3 (Hamada et al., 2008; Pressler et al., 2011; Buxhofer-Ausch et al., 2013; Lancaster et al., 2013) compared with normal prostate or benign prostatic hyperplasia specimens. Increased OATP1B3 expression has been correlated with increased tumor grade (Pressler et al., 2011). OATP1B1 and OATP1B3 expression is increased in metastatic lesions from prostate cancer that has not responded to androgen deprivation therapy compared with untreated prostate cancer (Wright et al., 2011).
Androgen-depleting therapies are the current mainstay of treatment of prostate cancer; however, over time, many patients become resistant to this therapy and progress to castration-resistant prostate cancer, which can be fatal (Cho et al., 2014). Upregulation of some OATPs is also believed to allow prostate cancer cells to continue to grow by more effectively scavenging low levels of circulating androgens and other steroids such as E3S during androgen-depleting therapies; therefore, therapies to reduce OATP-mediated steroid hormone transport could represent a treatment strategy for resistant prostate cancer (Cho et al., 2014). The adrenal steroid hormone DHEAS, which is not affected by androgen-depleting therapies, has also been considered as a possible pharmacological target given its role in cell proliferation under androgen-depleted conditions, which can cause tumor resistance to antiandrogen therapies (Evaul et al., 2010; Arakawa et al., 2012). DHEAS is transported by OATP1A2, OATP1B1, OATP1B3, and OATP2B1 (Kullak-Ublick et al., 1998, 2001; Cui et al., 2001; Pizzagalli et al., 2003; Grube et al., 2006a; Yang et al., 2011). Under conditions of androgen depletion, the expression of OATP1A2 significantly increases, and in OATP1A2 knockdown cells DHEAS does not stimulate cell growth, suggesting that OATP1A2 is important for DHEAS-mediated growth of prostate cancer cells (Arakawa et al., 2012). In contrast, two docetaxel-resistant patient-derived xenografts were found to have marked decrease in SLCO1B3 expression, which reduced the uptake of docetaxel into these cells (de Morrée et al., 2016). Thus, up- and downregulation of OATP transporters in prostate cancer cells may have varying effects depending on whether a cytotoxic agent or tumor growth–promoting endogenous substrate is being transported.
Due to their role in steroid transport, OATPs may impact prostate cancer prognosis. In one study, carriers of the reference SNP (rs) 12422149 in SLCO1B1 or the rs4149117 in SLCO1B3 had a higher rate of prostate cancer–specific mortality compared with wild type (Wright et al., 2011). In men with prostate cancer, those with at least one copy of the SLCO1B3 c.334T>G variant have been shown to have longer time to response to androgen deprivation therapy (Sharifi et al., 2008) and a trend toward shorter time to progression to castration-resistant prostate cancer (Fujimoto et al., 2013) compared with wild type, likely due to increased transport of testosterone into the tumor by the T allele (Sharifi et al., 2008). In another study, patients with the SLCO1B3 c.334GG/699AA haplotype had longer median survival (8.5 vs. 6.4 years) and improved survival to 10 years (42% vs. 23%) compared with patients with TT/AA or TG/GA haplotypes (Hamada et al., 2008). This evidence suggests that SLCO1B3 variation may aid in prognostication for prostate cancer.
The SLCO2B1 variant c.935G>A has been shown to affect DHEAS uptake, with the variant transporter having less DHEAS transport compared with wild type; accordingly, a cell growth assay showed that the wild-type transporter was associated with increased cell proliferation (Yang et al., 2011). Patients with prostate cancer with at least one copy of the variant allele at rs12422149 in SLCO2B1 have longer time to progression on antiandrogen therapy, suggesting an improved prognosis conferred by the variant genotype, likely due to reduced transport of DHEAS into the tumor by the variant allele (Yang et al., 2011; Fujimoto et al., 2013). Two other SLCO2B1 variants (rs1077858 and rs1789693) also affect time to progression in prostate cancer, with the variant alleles being associated with longer time to progression. Patients with more than one risk genotype in SLCO2B1, or with an at-risk genotype in both SLCO1B3 (which did not reach significance on their own) and SLCO2B1, had progressively worse outcomes as the number of at-risk genotypes increased (Yang et al., 2011).
Hepatic Tumors.
OATP1B1 and OATP1B3, while highly expressed in normal liver tissues, are generally downregulated in hepatic tumors to varying degrees in different studies (Kinoshita and Miyata, 2002; Cui et al., 2003; Vavricka et al., 2004; Zollner et al., 2005; Libra et al., 2006; Pressler et al., 2011; Wlcek et al., 2011; Vasuri et al., 2011; Buxhofer-Ausch et al., 2013). In one study, 45% of hepatocellular carcinoma specimens lacked staining for both OATP1B1 and OATP1B3 (Vasuri et al., 2011). In addition, the pattern of OATP1B3 staining is different in malignant cells (cytoplasmic) versus normal tissue (membranous) (Libra et al., 2006), and progressive loss of OATP1B3 staining is seen as tumor grade increases, suggesting that the lowest OATP1B3 expression is associated with the most aggressive tumors (Lockhart et al., 2008). Neither OATP1B1 nor OATP1B3 expression was found in lymph node metastases of hepatocellular carcinoma or in liver metastases from adenocarcinoma of the colon, rectum, or pancreas, and normal liver tissue surrounding these metastatic lesions maintained normal OATP1B1 and OATP1B3 expression (Cui et al., 2003). OATP2B1 mRNA levels were found to be lower in malignant versus normal cells in some studies (Pressler et al., 2011; Wlcek et al., 2011), but in another study OATP2B1 mRNA expression was similar or higher in malignant versus normal hepatic cells (Libra et al., 2006). Other OATPs are also overexpressed in malignant versus normal hepatic tissue (Wlcek et al., 2011).
The conjugation of drugs to bile acids has been studied as a way to selectively deliver these drugs, including chemotherapy agents, to the liver (Kramer et al., 1992). There is evidence that uptake of bile acid derivatives into human hepatic cancer cells is OATP mediated (Kullak-Ublick et al., 1996, 1997). An imaging agent that is a bile acid derivative is transported by OATP1B3, which is significantly underexpressed in liver tumors compared with normal liver; therefore, the use of this agent could allow for improved visualization of liver tumors (Libra et al., 2006). Decreased protein expression of these OATP transporters in malignant tissues may be important given the role of these transporters in the uptake of chemotherapy agents, with reduced expression leading to decreased tumoral uptake of these drugs.
Colorectal and Other Gastrointestinal Tumors.
OATP1A2 mRNA has been detected in colon adenocarcinoma specimens (Tamai et al., 2000), but was found to be expressed at lower levels in colon neoplasia specimens compared with normal colon, which has implications for the use of chemotherapy agents transported by OATP1A2 in the management of gastrointestinal cancers (Ballestero et al., 2006). OATP1B1 and OATP1B3 mRNA are highly expressed in various cancers of the gastrointestinal tract including the colon, despite not being found in normal colon tissue (Abe et al., 2001; Ballestero et al., 2006; Buxhofer-Ausch et al., 2013), with higher OATP1B1 expression associated with higher tumor grade in colon cancer specimens (Pressler et al., 2011). OATP1B3 has been detected in gastric and gallbladder cancers (Abe et al., 2001; Lancaster et al., 2013). In one study, out of 30 colorectal cancer specimens studied, 27 were positive for OATP1B3 by immunohistochemistry, with the remaining three specimens expressing a mutant form of OATP1B3 that caused the antibody binding for the immunohistochemical staining to fail; the OATP1B3 staining in these samples was primarily cytoplasmic, in contrast to the membranous pattern found in normal liver (Evangeli et al., 2017). Another study similarly found that while none of the normal colon specimens had OATP1B3 mRNA present, four out of the seven colon cancer specimens studied had detectable OATP1B3 mRNA that was almost exclusively a cancer-specific isoform different from that found in normal liver (Nagai et al., 2012).
Overexpression of OATP1B3 mRNA in a colorectal cancer cell line was associated with reduced drug-induced apoptosis of these cells and reduced transcriptional activity of the tumor suppressor p53; these functions are apparently directly related to OATP1B3-mediated transport, since inclusion of a point mutation that abolishes OATP1B3 transport also removed the antiapoptosis and reduced p53 features of these cells (Lee et al., 2008). OATP2B1 mRNA has been detected in colon adenocarcinoma samples (Tamai et al., 2000; Bleasby et al., 2006; Kleberg et al., 2012) with higher expression in neoplastic colon specimens compared with normal colon (Kleberg et al., 2012).
Pancreatic Tumors.
OATP1B3 is expressed in pancreatic cancer samples at higher levels than in normal pancreas (Abe et al., 2001; Kounnis et al., 2011, 2015; Buxhofer-Ausch et al., 2013; Hays et al., 2013; Lancaster et al., 2013). OATP1A2 (Kounnis et al., 2011) and OATP1B1 (Kounnis et al., 2011, 2015) are also highly expressed in pancreatic tumors. OATP1B3 expression was found to be highest in the nonmalignant conditions of pancreatic hyperplasia and pancreatitis, and was also high in stage 1 pancreatic adenocarcinoma, with lower levels of expression seen in stage 2 or 3 adenocarcinoma, suggesting that OATP1B3 expression could be useful as a marker to detect premalignant lesions or early stage pancreatic cancer (Hays et al., 2013).
Lung Tumors.
OATP1A2 (Brenner et al., 2015) and OATP2B1 (Bleasby et al., 2006; Brenner et al., 2015) mRNA are expressed at low levels in normal lung epithelium. OATP1A2 mRNA was found at similar (Monks et al., 2007) or lower (Brenner et al., 2015) levels in malignant lung tissue compared with normal tissue, while OATP2B1 mRNA was found to be upregulated in malignant lung tissue compared with normal in one study (Brenner et al., 2015) and downregulated in malignant tissue in another study (Monks et al., 2007). Minor OATP2B1 mRNA expression in lung tumor specimens was detected in another study, although quantification compared with normal tissue was not done (Bleasby et al., 2006). OAPT1B1 and OATP1B3 have also been shown to be expressed in some lung tumor specimens (Buxhofer-Ausch et al., 2013), and SLCO1B3 mRNA was found in malignant lung cells (Lancaster et al., 2013). No mRNA expression of OATP1B3 was found in normal lung tissues, while the cancer-specific OATP1B3 was found in two out of five lung cancer specimens studied (Nagai et al., 2012).
Conclusions
Over the past two decades, OATPs have increasingly become recognized as important mediators of drug uptake and disposition. More recently, it has become apparent that OATPs have potentially important emerging roles in cancer pharmacology. Transport of many chemotherapy agents is mediated by OATPs, which may have significant implications for chemotherapy disposition and drug-drug interactions. OATPs are polymorphic with numerous function-altering variants that can alter the disposition, efficacy, and toxicity profiles of chemotherapy agents; this is especially notable for drugs that traditionally have narrow therapeutic indices and has important implications for personalized and precision medicine initiatives. OATPs are widely and differentially expressed in several normal and neoplastic tissues and transport many substrates that are of importance in cancer therapy and tumor progression. Transport of endogenous steroid hormones is associated with cell proliferation in some hormone-dependent cancers, and overexpression of OATPs in these tumors may lead to more aggressive disease. Differential expression of OATPs in neoplastic tissues may provide novel therapeutic strategies to preferentially deliver chemotherapy to tumor tissues with OATP overexpression to enhance tumor killing while minimizing damage to normal tissues.
Acknowledgments
We thank Michael L. Schulte for assistance with figure preparation.
Abbreviations
- ABC
ATP-binding cassette
- AUC
area under the plasma time-concentration curve
- Bamet-R2
cis-diamminechloro-cholylglycinate-platinum(II)
- Bamet-UD2
cis-diammine-bisursodeoxycholate-platinum(II)
- DHEAS
dehydroepiandrosterone sulfate
- E3S
estrone-3-sulfate
- HEK
human embryonic kidney
- OATP
organic anion transporting polypeptide
- OCT
organic cation transporter
- P450
cytochrome P450
- rs
reference single nucleotide polymorphism
- SN-38
7-ethyl-10-hydroxycamptothecin
- SNP
single nucleotide polymorphism
- TKI
tyrosine kinase inhibitor
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Schulte, Ho.
Footnotes
This work was supported by the National Institutes of Health (NIH) National Institute of General Medical Sciences [Awards T32 GM007569 (to R.R.S.) and R01 GM099924 (to R.H.H.)], NIH National Cancer Institute [Award K12 CA090625 (to R.R.S.)], Alex’s Lemonade Stand Foundation [Young Investigator Award 17-00375 (to R.R.S.)], Rally Foundation [Fellowship Award (to R.R.S.)], and Hyundai Hope on Wheels [Scholar Award (to R.H.H.)].
References
- Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, Adachi H, Fujiwara K, Okabe M, Suzuki T, et al. (2001) LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 120:1689–1699. [DOI] [PubMed] [Google Scholar]
- Al Sarakbi W, Mokbel R, Salhab M, Jiang WG, Reed MJ, Mokbel K. (2006) The role of STS and OATP-B mRNA expression in predicting the clinical outcome in human breast cancer. Anticancer Res 26:4985–4990. [PubMed] [Google Scholar]
- Arakawa H, Nakanishi T, Yanagihara C, Nishimoto T, Wakayama T, Mizokami A, Namiki M, Kawai K, Tamai I. (2012) Enhanced expression of organic anion transporting polypeptides (OATPs) in androgen receptor-positive prostate cancer cells: possible role of OATP1A2 in adaptive cell growth under androgen-depleted conditions. Biochem Pharmacol 84:1070–1077. [DOI] [PubMed] [Google Scholar]
- Awada A, Hendlisz A, Christensen O, Lathia CD, Bartholomeus S, Lebrun F, de Valeriola D, Brendel E, Radtke M, Delaunoit T, et al. (2012) Phase I trial to investigate the safety, pharmacokinetics and efficacy of sorafenib combined with docetaxel in patients with advanced refractory solid tumours. Eur J Cancer 48:465–474. [DOI] [PubMed] [Google Scholar]
- Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, Kawamoto M, Johns SJ, Ferrin TE, Carlson EJ, et al. (2006) Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther 318:521–529. [DOI] [PubMed] [Google Scholar]
- Baker SD, Sparreboom A, Verweij J. (2006) Clinical pharmacokinetics of docetaxel: recent developments. Clin Pharmacokinet 45:235–252. [DOI] [PubMed] [Google Scholar]
- Baker SD, Verweij J, Cusatis GA, van Schaik RH, Marsh S, Orwick SJ, Franke RM, Hu S, Schuetz EG, Lamba V, et al. (2009) Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther 85:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestero MR, Monte MJ, Briz O, Jimenez F, Gonzalez-San Martin F, Marin JJ. (2006) Expression of transporters potentially involved in the targeting of cytostatic bile acid derivatives to colon cancer and polyps. Biochem Pharmacol 72:729–738. [DOI] [PubMed] [Google Scholar]
- Banerjee N, Allen C, Bendayan R. (2012) Differential role of organic anion-transporting polypeptides in estrone-3-sulphate uptake by breast epithelial cells and breast cancer cells. J Pharmacol Exp Ther 342:510–519. [DOI] [PubMed] [Google Scholar]
- Banerjee N, Fonge H, Mikhail A, Reilly RM, Bendayan R, Allen C. (2013) Estrone-3-sulphate, a potential novel ligand for targeting breast cancers. PLoS One 8:e64069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee N, Miller N, Allen C, Bendayan R. (2014) Expression of membrane transporters and metabolic enzymes involved in estrone-3-sulphate disposition in human breast tumour tissues. Breast Cancer Res Treat 145:647–661. [DOI] [PubMed] [Google Scholar]
- Bauer M, Matsuda A, Wulkersdorfer B, Philippe C, Traxl A, Ozvegy-Laczka C, Stanek J, Nics L, Klebermass EM, Poschner S, et al. (2018) Influence of OATPs on hepatic disposition of erlotinib measured with positron emission tomography. Clin Pharmacol Ther 104:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bins S, van Doorn L, Phelps MA, Gibson AA, Hu S, Li L, Vasilyeva A, Du G, Hamberg P, Eskens F, et al. (2017) Influence of OATP1B1 function on the disposition of sorafenib-β-D-glucuronide. Clin Transl Sci 10:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasby K, Castle JC, Roberts CJ, Cheng C, Bailey WJ, Sina JF, Kulkarni AV, Hafey MJ, Evers R, Johnson JM, et al. (2006) Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica 36:963–988. [DOI] [PubMed] [Google Scholar]
- Brenner S, Klameth L, Riha J, Schölm M, Hamilton G, Bajna E, Ausch C, Reiner A, Jäger W, Thalhammer T, et al. (2015) Specific expression of OATPs in primary small cell lung cancer (SCLC) cells as novel biomarkers for diagnosis and therapy. Cancer Lett 356:517–524. [DOI] [PubMed] [Google Scholar]
- Briz O, Serrano MA, Rebollo N, Hagenbuch B, Meier PJ, Koepsell H, Marin JJ. (2002) Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chloro-cholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. Mol Pharmacol 61:853–860. [DOI] [PubMed] [Google Scholar]
- Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, et al. (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16:187–196. [DOI] [PubMed] [Google Scholar]
- Bruno R, Olivares R, Berille J, Chaikin P, Vivier N, Hammershaimb L, Rhodes GR, Rigas JR. (2003) α-1-Acid glycoprotein as an independent predictor for treatment effects and a prognostic factor of survival in patients with non-small cell lung cancer treated with docetaxel. Clin Cancer Res 9:1077–1082. [PubMed] [Google Scholar]
- Burger H, Loos WJ, Eechoute K, Verweij J, Mathijssen RH, Wiemer EA. (2011) Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist Updat 14:22–34. [DOI] [PubMed] [Google Scholar]
- Burt HJ, Riedmaier AE, Harwood MD, Crewe HK, Gill KL, Neuhoff S. (2016) Abundance of hepatic transporters in Caucasians: a meta-analysis. Drug Metab Dispos 44:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxhofer-Ausch V, Secky L, Wlcek K, Svoboda M, Kounnis V, Briasoulis E, Tzakos AG, Jaeger W, Thalhammer T. (2013) Tumor-specific expression of organic anion-transporting polypeptides: transporters as novel targets for cancer therapy. J Drug Deliv 2013:863539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JD, Joel SP, Cheeseman SL, Hall GD, Braun MS, Perry J, Davis T, Button CJ, Seymour MT. (2003) Phase I and pharmacokinetic study of intravenous irinotecan plus oral ciclosporin in patients with fuorouracil-refractory metastatic colon cancer. J Clin Oncol 21:1125–1132. [DOI] [PubMed] [Google Scholar]
- Chew SC, Sandanaraj E, Singh O, Chen X, Tan EH, Lim WT, Lee EJ, Chowbay B. (2012) Influence of SLCO1B3 haplotype-tag SNPs on docetaxel disposition in Chinese nasopharyngeal cancer patients. Br J Clin Pharmacol 73:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SC, Singh O, Chen X, Ramasamy RD, Kulkarni T, Lee EJ, Tan EH, Lim WT, Chowbay B. (2011) The effects of CYP3A4, CYP3A5, ABCB1, ABCC2, ABCG2 and SLCO1B3 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of docetaxel in nasopharyngeal carcinoma patients. Cancer Chemother Pharmacol 67:1471–1478. [DOI] [PubMed] [Google Scholar]
- Cho E, Montgomery RB, Mostaghel EA. (2014) Minireview: SLCO and ABC transporters: a role for steroid transport in prostate cancer progression. Endocrinology 155:4124–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JR, Kim JO, Kang DR, Shin JY, Zhang XH, Oh JE, Park JY, Kim KA, Kang JH. (2015) Genetic variations of drug transporters can influence on drug response in patients treated with docetaxel chemotherapy. Cancer Res Treat 47:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Cherrington NJ. (2012) Genetics or environment in drug transport: the case of organic anion transporting polypeptides and adverse drug reactions. Expert Opin Drug Metab Toxicol 8:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Pazdur R. (1995) Docetaxel. J Clin Oncol 13:2643–2655. [DOI] [PubMed] [Google Scholar]
- Criado JJ, Domínguez MF, Medarde M, Fernández ER, Macías RIR, Marín JJG. (2000) Structural characterization, kinetic studies, and in vitro biological activity of new cis-diamminebis-cholylglycinate(O,O′) Pt(II) and cis-diamminebis-ursodeoxycholate(O,O′) Pt(II) complexes. Bioconjug Chem 11:167–174. [DOI] [PubMed] [Google Scholar]
- Criado JJ, Herrera MC, Palomero MF, Medarde M, Rodriguez E, Marin JJG. (1997) Synthesis and characterization of a new bile acid and platinum(II) complex with cytostatic activity. J Lipid Res 38:1022–1032. [PubMed] [Google Scholar]
- Crona DJ, Ramirez J, Qiao W, de Graan AJ, Ratain MJ, van Schaik RH, Mathijssen RH, Rosner GL, Innocenti F. (2016) Clinical validity of new genetic biomarkers of irinotecan neutropenia: an independent replication study. Pharmacogenomics J 16:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, König J, Keppler D. (2001) Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol Pharmacol 60:934–943. [DOI] [PubMed] [Google Scholar]
- Cui Y, König J, Nies AT, Pfannschmidt M, Hergt M, Franke WW, Alt W, Moll R, Keppler D. (2003) Detection of the human organic anion transporters SLC21A6 (OATP2) and SLC21A8 (OATP8) in liver and hepatocellular carcinoma. Lab Invest 83:527–538. [DOI] [PubMed] [Google Scholar]
- Danesi R, Fogli S, Gennari A, Conte P, Del Tacca M. (2002) Pharmacokinetic-pharmacodynamic relationships of the anthracycline anticancer drugs. Clin Pharmacokinet 41:431–444. [DOI] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB. (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn T, Ye ZW, Peeters A, Sahi J, Baes M, Augustijns PF, Annaert PP. (2011) Determination of OATP-, NTCP- and OCT-mediated substrate uptake activities in individual and pooled batches of cryopreserved human hepatocytes. Eur J Pharm Sci 43:297–307. [DOI] [PubMed] [Google Scholar]
- de Graan AJ, Lancaster CS, Obaidat A, Hagenbuch B, Elens L, Friberg LE, de Bruijn P, Hu S, Gibson AA, Bruun GH, et al. (2012) Influence of polymorphic OATP1B-type carriers on the disposition of docetaxel. Clin Cancer Res 18:4433–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. (2018) Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet 57:1229–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morrée ES, Böttcher R, van Soest RJ, Aghai A, de Ridder CM, Gibson AA, Mathijssen RH, Burger H, Wiemer EA, Sparreboom A, et al. (2016) Loss of SLCO1B3 drives taxane resistance in prostate cancer. Br J Cancer 115:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenberg CD, Paugh SW, Pounds SB, Shi L, Orwick SJ, Li L, Hu S, Gibson AA, Ribeiro RC, Rubnitz JE, et al. (2016) Inherited variation in OATP1B1 is associated with treatment outcome in acute myeloid leukemia. Clin Pharmacol Ther 99:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. (2002) Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther 71:11–20. [DOI] [PubMed] [Google Scholar]
- Drozdzik M, Gröer C, Penski J, Lapczuk J, Ostrowski M, Lai Y, Prasad B, Unadkat JD, Siegmund W, Oswald S. (2014) Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine. Mol Pharm 11:3547–3555. [DOI] [PubMed] [Google Scholar]
- Dudenkov TM, Ingle JN, Buzdar AU, Robson ME, Kubo M, Ibrahim-Zada I, Batzler A, Jenkins GD, Pietrzak TL, Carlson EE, et al. (2017) SLCO1B1 polymorphisms and plasma estrone conjugates in postmenopausal women with ER+ breast cancer: genome-wide association studies of the estrone pathway. Breast Cancer Res Treat 164:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmus S, Lozano-Mena G, van Esch A, Wagenaar E, van Tellingen O, Schinkel AH. (2015) Preclinical mouse models to study human OATP1B1- and OATP1B3-mediated drug-drug interactions in vivo. Mol Pharm 12:4259–4269. [DOI] [PubMed] [Google Scholar]
- Durmus S, Naik J, Buil L, Wagenaar E, van Tellingen O, Schinkel AH. (2014) In vivo disposition of doxorubicin is affected by mouse Oatp1a/1b and human OATP1A/1B transporters. Int J Cancer 135:1700–1710. [DOI] [PubMed] [Google Scholar]
- Durmus S, van Hoppe S, Schinkel AH. (2016) The impact of organic anion-transporting polypeptides (OATPs) on disposition and toxicity of antitumor drugs: insights from knockout and humanized mice. Drug Resist Updat 27:72–88. [DOI] [PubMed] [Google Scholar]
- Eechoute K, Franke RM, Loos WJ, Scherkenbach LA, Boere I, Verweij J, Gurney H, Kim RB, Tirona RG, Mathijssen RH, et al. (2011) Environmental and genetic factors affecting transport of imatinib by OATP1A2. Clin Pharmacol Ther 89:816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangeli L, Ioannis S, Valentinos K, Antigony M, Elli I, Eleftheria H, Vasiliki G, Evangelos B. (2017) SLCO1B3 screening in colorectal cancer patients using high-resolution melting analysis method and immunohistochemistry. Tumour Biol 39:1010428317691176. [DOI] [PubMed] [Google Scholar]
- Evaul K, Li R, Papari-Zareei M, Auchus RJ, Sharifi N. (2010) 3β-hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology 151:3514–3520. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Kubo T, Inatomi H, Bui HT, Shiota M, Sho T, Matsumoto T. (2013) Polymorphisms of the androgen transporting gene SLCO2B1 may influence the castration resistance of prostate cancer and the racial differences in response to androgen deprivation. Prostate Cancer Prostatic Dis 16:336–340. [DOI] [PubMed] [Google Scholar]
- Fujita D, Saito Y, Nakanishi T, Tamai I. (2016) Organic anion transporting polypeptide (OATP)2B1 contributes to gastrointestinal toxicity of anticancer drug SN-38, active metabolite of irinotecan hydrochloride. Drug Metab Dispos 44:1–7. [DOI] [PubMed] [Google Scholar]
- Fujita K, Sugiura T, Okumura H, Umeda S, Nakamichi N, Watanabe Y, Suzuki H, Sunakawa Y, Shimada K, Kawara K, et al. (2014) Direct inhibition and down-regulation by uremic plasma components of hepatic uptake transporter for SN-38, an active metabolite of irinotecan, in humans. Pharm Res 31:204–215. [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. (2000) Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther 294:73–79. [PubMed] [Google Scholar]
- Gao B, Huber RD, Wenzel A, Vavricka SR, Ismair MG, Remé C, Meier PJ. (2005) Localization of organic anion transporting polypeptides in the rat and human ciliary body epithelium. Exp Eye Res 80:61–72. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. International Transporter Consortium (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, et al. (2007) Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther 81:362–370. [DOI] [PubMed] [Google Scholar]
- Gong IY, Kim RB. (2013) Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab Pharmacokinet 28:4–18. [DOI] [PubMed] [Google Scholar]
- Gong L, Giacomini MM, Giacomini C, Maitland ML, Altman RB, Klein TE. (2017) PharmGKB summary: sorafenib pathways. Pharmacogenet Genomics 27:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröer C, Brück S, Lai Y, Paulick A, Busemann A, Heidecke CD, Siegmund W, Oswald S. (2013) LC-MS/MS-based quantification of clinically relevant intestinal uptake and efflux transporter proteins. J Pharm Biomed Anal 85:253–261. [DOI] [PubMed] [Google Scholar]
- Grube M, Köck K, Karner S, Reuther S, Ritter CA, Jedlitschky G, Kroemer HK. (2006a) Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol 70:1735–1741. [DOI] [PubMed] [Google Scholar]
- Grube M, Köck K, Oswald S, Draber K, Meissner K, Eckel L, Böhm M, Felix SB, Vogelgesang S, Jedlitschky G, et al. (2006b) Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther 80:607–620. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2004) Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Stieger B. (2013) The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med 34:396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada A, Sissung T, Price DK, Danesi R, Chau CH, Sharifi N, Venzon D, Maeda K, Nagao K, Sparreboom A, et al. (2008) Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in Caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res 14:3312–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE, Kim HT, Lee JS. (2008) Influence of the organic anion-transporting polypeptide 1B1 (OATP1B1) polymorphisms on irinotecan-pharmacokinetics and clinical outcome of patients with advanced non-small cell lung cancer. Lung Cancer 59:69–75. [DOI] [PubMed] [Google Scholar]
- Han S, Kim K, Thakkar N, Kim D, Lee W. (2013) Role of hypoxia inducible factor-1α in the regulation of the cancer-specific variant of organic anion transporting polypeptide 1B3 (OATP1B3), in colon and pancreatic cancer. Biochem Pharmacol 86:816–823. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Tatsumi S, Takeda R, Naka A, Ogane N, Kameda Y, Kawachi K, Shimizu S, Sakai M, Kamoshida S. (2014) Expression of organic anion-transporting polypeptide 1A2 and organic cation transporter 6 as a predictor of pathologic response to neoadjuvant chemotherapy in triple negative breast cancer. Breast Cancer Res Treat 145:101–111. [DOI] [PubMed] [Google Scholar]
- Hays A, Apte U, Hagenbuch B. (2013) Organic anion transporting polypeptides expressed in pancreatic cancer may serve as potential diagnostic markers and therapeutic targets for early stage adenocarcinomas. Pharm Res 30:2260–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. (2007) Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos 35:1333–1340. [DOI] [PubMed] [Google Scholar]
- Ho RH, Choi L, Lee W, Mayo G, Schwarz UI, Tirona RG, Bailey DG, Stein CM, Kim RB. (2007) Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics 17:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RH, Kim RB. (2010) Uptake transporters, in Comprehensive Toxicology (McQueen CA. ed) pp 519–556, Elsevier, Oxford, UK. [Google Scholar]
- Ho RH, Leake BF, Kim RB, Wang Y. (2006) OATP2B1 allelic variants differentially transport rosuvastatin in vitro. Drug Metab Rev 38:240–241. [Google Scholar]
- Hong Y, Chen S. (2011) Aromatase, estrone sulfatase, and 17β-hydroxysteroid dehydrogenase: structure-function studies and inhibitor development. Mol Cell Endocrinol 340:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chen Z, Franke R, Orwick S, Zhao M, Rudek MA, Sparreboom A, Baker SD. (2009) Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin Cancer Res 15:6062–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA, Burger H, Baker SD, Sparreboom A. (2008) Interaction of imatinib with human organic ion carriers. Clin Cancer Res 14:3141–3148. [DOI] [PubMed] [Google Scholar]
- Hu S, Mathijssen RH, de Bruijn P, Baker SD, Sparreboom A. (2014) Inhibition of OATP1B1 by tyrosine kinase inhibitors: in vitro–in vivo correlations. Br J Cancer 110:894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieiri I, Higuchi S, Sugiyama Y. (2009) Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol 5:703–729. [DOI] [PubMed] [Google Scholar]
- Imai S, Kikuchi R, Tsuruya Y, Naoi S, Nishida S, Kusuhara H, Sugiyama Y. (2013) Epigenetic regulation of organic anion transporting polypeptide 1B3 in cancer cell lines. Pharm Res 30:2880–2890. [DOI] [PubMed] [Google Scholar]
- Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramírez J, Relling M, Chen P, Das S, Rosner GL, Ratain MJ. (2009) Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol 27:2604–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iusuf D, Hendrikx JJ, van Esch A, van de Steeg E, Wagenaar E, Rosing H, Beijnen JH, Schinkel AH. (2015) Human OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and clearance of docetaxel. Int J Cancer 136:225–233. [DOI] [PubMed] [Google Scholar]
- Iusuf D, Ludwig M, Elbatsh A, van Esch A, van de Steeg E, Wagenaar E, van der Valk M, Lin F, van Tellingen O, Schinkel AH. (2014) OATP1A/1B transporters affect irinotecan and SN-38 pharmacokinetics and carboxylesterase expression in knockout and humanized transgenic mice. Mol Cancer Ther 13:492–503. [DOI] [PubMed] [Google Scholar]
- Iusuf D, van de Steeg E, Schinkel AH. (2012) Functions of OATP1A and 1B transporters in vivo: insights from mouse models. Trends Pharmacol Sci 33:100–108. [DOI] [PubMed] [Google Scholar]
- Iwai M, Suzuki H, Ieiri I, Otsubo K, Sugiyama Y. (2004) Functional analysis of single nucleotide polymorphisms of hepatic organic anion transporter OATP1B1 (OATP-C). Pharmacogenetics 14:749–757. [DOI] [PubMed] [Google Scholar]
- Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. (1994) Expression cloning of a rat liver Na+-independent organic anion transporter. Proc Natl Acad Sci USA 91:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Tang Q, Feng J, Dai R, Wang Y, Yang Y, Tang X, Deng C, Zeng H, Zhao Y, et al. (2016) Association between SLCO1B1 -521T>C and -388A>G polymorphisms and risk of statin-induced adverse drug reactions: a meta-analysis. Springerplus 5:1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. (2005) Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics 15:513–522. [DOI] [PubMed] [Google Scholar]
- Katz DA, Carr R, Grimm DR, Xiong H, Holley-Shanks R, Mueller T, Leake B, Wang Q, Han L, Wang PG, et al. (2006) Organic anion transporting polypeptide 1B1 activity classified by SLCO1B1 genotype influences atrasentan pharmacokinetics. Clin Pharmacol Ther 79:186–196. [DOI] [PubMed] [Google Scholar]
- Kehrer DFS, Sparreboom A, Verweij J, de Bruijn P, Nierop CA, van de Schraaf J, Ruijgrok EJ, de Jonge MJA. (2001) Modulation of irinotecan-induced diarrhea by cotreatment with neomycin in cancer patients. Clin Cancer Res 7:1136–1141. [PubMed] [Google Scholar]
- Kindla J, Fromm MF, König J. (2009) In vitro evidence for the role of OATP and OCT uptake transporters in drug-drug interactions. Expert Opin Drug Metab Toxicol 5:489–500. [DOI] [PubMed] [Google Scholar]
- Kindla J, Rau TT, Jung R, Fasching PA, Strick R, Stoehr R, Hartmann A, Fromm MF, König J. (2011) Expression and localization of the uptake transporters OATP2B1, OATP3A1 and OATP5A1 in non-malignant and malignant breast tissue. Cancer Biol Ther 11:584–591. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Miyata M. (2002) Underexpression of mRNA in human hepatocellular carcinoma focusing on eight loci. Hepatology 36:433–438. [DOI] [PubMed] [Google Scholar]
- Kiyotani K, Mushiroda T, Kubo M, Zembutsu H, Sugiyama Y, Nakamura Y. (2008) Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci 99:967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleberg K, Jensen GM, Christensen DP, Lundh M, Grunnet LG, Knuhtsen S, Poulsen SS, Hansen MB, Bindslev N. (2012) Transporter function and cyclic AMP turnover in normal colonic mucosa from patients with and without colorectal neoplasia. BMC Gastroenterol 12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2003) Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther 306:703–708. [DOI] [PubMed] [Google Scholar]
- Koenen A, Kroemer HK, Grube M, Meyer zu Schwabedissen HE. (2011) Current understanding of hepatic and intestinal OATP-mediated drug-drug interactions. Expert Rev Clin Pharmacol 4:729–742. [DOI] [PubMed] [Google Scholar]
- Konig J. (2011) Uptake transporters of the human OATP family: molecular characteristics, substrates, their role in drug-drug interactions, and functional consequences of polymorphisms, in Drug Transporters (Fromm MF, Kim RB. eds) pp 1–28, Springer-Verlag, Berlin. [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000) Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 275:23161–23168. [DOI] [PubMed] [Google Scholar]
- König J, Müller F, Fromm MF. (2013) Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev 65:944–966. [DOI] [PubMed] [Google Scholar]
- Kounnis V, Chondrogiannis G, Mantzaris MD, Tzakos AG, Fokas D, Papanikolaou NA, Galani V, Sainis I, Briasoulis E. (2015) Microcystin LR shows cytotoxic activity against pancreatic cancer cells expressing the membrane OATP1B1 and OATP1B3 transporters. Anticancer Res 35:5857–5865. [PubMed] [Google Scholar]
- Kounnis V, Ioachim E, Svoboda M, Tzakos A, Sainis I, Thalhammer T, Steiner G, Briasoulis E. (2011) Expression of organic anion-transporting polypeptides 1B3, 1B1, and 1A2 in human pancreatic cancer reveals a new class of potential therapeutic targets. OncoTargets Ther 4:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W, Wess G, Schubert G, Bickel M, Girbig F, Gutjahr U, Kowalewski S, Baringhaus KH, Enhsen A, Glombik H, et al. (1992) Liver-specific drug targeting by coupling to bile acids. J Biol Chem 267:18598–18604. [PubMed] [Google Scholar]
- Kullak-Ublick GA, Beuers U, Paumgartner G. (1996) Molecular and functional characterization of bile acid transport in human hepatoblastoma HepG2 cells. Hepatology 23:1053–1060. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Fisch T, Oswald M, Hagenbuch B, Meier PJ, Beuers U, Paumgartner G. (1998) Dehydroepiandrosterone sulfate (DHEAS): identification of a carrier protein in human liver and brain. FEBS Lett 424:173–176. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Glasa J, Böker C, Oswald M, Grützner U, Hagenbuch B, Stieger B, Meier PJ, Beuers U, Kramer W, et al. (1997) Chlorambucil-taurocholate is transported by bile acid carriers expressed in human hepatocellular carcinomas. Gastroenterology 113:1295–1305. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, Meier PJ. (1995) Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology 109:1274–1282. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. (2001) Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 120:525–533. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. (2005) Active efflux across the blood-brain barrier: role of the solute carrier family. NeuroRx 2:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster CS, Sprowl JA, Walker AL, Hu S, Gibson AA, Sparreboom A. (2013) Modulation of OATP1B-type transporter function alters cellular uptake and disposition of platinum chemotherapeutics. Mol Cancer Ther 12:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Leake BF, Kim RB, Ho RH. (2017) Contribution of organic anion-transporting polypeptides 1A/1B to doxorubicin uptake and clearance. Mol Pharmacol 91:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Leake BF, Teft W, Tirona RG, Kim RB, Ho RH. (2015) Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance. Mol Cancer Ther 14:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Belkhiri A, Lockhart AC, Merchant N, Glaeser H, Harris EI, Washington MK, Brunt EM, Zaika A, Kim RB, et al. (2008) Overexpression of OATP1B3 confers apoptotic resistance in colon cancer. Cancer Res 68:10315–10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, Leake BF, Kim RB. (2005) Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem 280:9610–9617. [DOI] [PubMed] [Google Scholar]
- Leskelä S, Jara C, Leandro-García LJ, Martínez A, García-Donas J, Hernando S, Hurtado A, Vicario JC, Montero-Conde C, Landa I, et al. (2011) Polymorphisms in cytochromes P450 2C8 and 3A5 are associated with paclitaxel neurotoxicity. Pharmacogenomics J 11:121–129. [DOI] [PubMed] [Google Scholar]
- Letschert K, Keppler D, König J. (2004) Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8). Pharmacogenetics 14:441–452. [DOI] [PubMed] [Google Scholar]
- Li J, Wang XR, Zhai XW, Wang HS, Qian XW, Miao H, Zhu XH. (2015) Association of SLCO1B1 gene polymorphisms with toxicity response of high dose methotrexate chemotherapy in childhood acute lymphoblastic leukemia. Int J Clin Exp Med 8:6109–6113. [PMC free article] [PubMed] [Google Scholar]
- Libra A, Fernetti C, Lorusso V, Visigalli M, Anelli PL, Staud F, Tiribelli C, Pascolo L. (2006) Molecular determinants in the transport of a bile acid-derived diagnostic agent in tumoral and nontumoral cell lines of human liver. J Pharmacol Exp Ther 319:809–817. [DOI] [PubMed] [Google Scholar]
- Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R, et al. for the SEARCH Collaborative Group (2008) SLCO1B1 variants and statin-induced myopathy - a genomewide study. New Engl J Med 359:789–799. [DOI] [PubMed] [Google Scholar]
- Lockhart AC, Harris E, Lafleur BJ, Merchant NB, Washington MK, Resnick MB, Yeatman TJ, Lee W. (2008) Organic anion transporting polypeptide 1B3 (OATP1B3) is overexpressed in colorectal tumors and is a predictor of clinical outcome. Clin Exp Gastroenterol 1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokiec F, Canal P, Gay C, Chatelut E, Armand JP, Roché H, Bugat R, Gonçalvès E, Mathieu-Boué A. (1995) Pharmacokinetics of irinotecan and its metabolites in human blood, bile, and urine. Cancer Chemother Pharmacol 36:79–82. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, Piñan MA, Garcia-Miguel P, Navajas A, Garcia-Orad A. (2011) Polymorphisms of the SLCO1B1 gene predict methotrexate-related toxicity in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 57:612–619. [DOI] [PubMed] [Google Scholar]
- Maeda T, Irokawa M, Arakawa H, Kuraoka E, Nozawa T, Tateoka R, Itoh Y, Nakanishi T, Tamai I. (2010) Uptake transporter organic anion transporting polypeptide 1B3 contributes to the growth of estrogen-dependent breast cancer. J Steroid Biochem Mol Biol 122:180–185. [DOI] [PubMed] [Google Scholar]
- Marada VV, Flörl S, Kühne A, Burckhardt G, Hagos Y. (2015) Interaction of human organic anion transporter polypeptides 1B1 and 1B3 with antineoplastic compounds. Eur J Med Chem 92:723–731. [DOI] [PubMed] [Google Scholar]
- Marsh S, McLeod H, Dolan E, Shukla SJ, Rabik CA, Gong L, Hernandez-Boussard T, Lou XJ, Klein TE, Altman RB. (2009) Platinum pathway. Pharmacogenet Genomics 19:563–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Muhrez K, Woillard JB, Berthelot A, Gyan E, Choquet S, Andrès CR, Marquet P, Barin-Le Guellec C. (2018) Endogenous metabolites-mediated communication between OAT1/OAT3 and OATP1B1 may explain the association between SLCO1B1 SNPs and methotrexate toxicity. Clin Pharmacol Ther 104:687–698. [DOI] [PubMed] [Google Scholar]
- Matsumoto J, Ariyoshi N, Sakakibara M, Nakanishi T, Okubo Y, Shiina N, Fujisaki K, Nagashima T, Nakatani Y, Tamai I, et al. (2015) Organic anion transporting polypeptide 2B1 expression correlates with uptake of estrone-3-sulfate and cell proliferation in estrogen receptor-positive breast cancer cells. Drug Metab Pharmacokinet 30:133–141. [DOI] [PubMed] [Google Scholar]
- Meier Y, Eloranta JJ, Darimont J, Ismair MG, Hiller C, Fried M, Kullak-Ublick GA, Vavricka SR. (2007) Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos 35:590–594. [DOI] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. (2008) Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Res 68:9338–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski C, Cui Y, Nies AT, Nuessler AK, Neuhaus P, Zanger UM, Klein K, Eichelbaum M, Keppler D, Konig J. (2002) A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J Biol Chem 277:43058–43063. [DOI] [PubMed] [Google Scholar]
- Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, Ishida T, Ohuchi N, Blumberg B, Sasano H. (2006) Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res 66:535–542. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Thorn CF, Yang JJ, Ulrich CM, French D, Zaza G, Dunnenberger HM, Marsh S, McLeod HL, Giacomini K, et al. (2011) PharmGKB summary: methotrexate pathway. Pharmacogenet Genomics 21:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa M, Maeda K, Aoyama A, Sugiyama Y. (2009) The eighth and ninth transmembrane domains in organic anion transporting polypeptide 1B1 affect the transport kinetics of estrone-3-sulfate and estradiol-17β-D-glucuronide. J Pharmacol Exp Ther 329:551–557. [DOI] [PubMed] [Google Scholar]
- Monks NR, Liu S, Xu Y, Yu H, Bendelow AS, Moscow JA. (2007) Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1- and OATP1B3-expressing HeLa cells. Mol Cancer Ther 6:587–598. [DOI] [PubMed] [Google Scholar]
- Muto M, Onogawa T, Suzuki T, Ishida T, Rikiyama T, Katayose Y, Ohuchi N, Sasano H, Abe T, Unno M. (2007) Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer Sci 98:1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwinyi J, Johne A, Bauer S, Roots I, Gerloff T. (2004) Evidence for inverse effects of OATP-C (SLC21A6) *5 and *1b haplotypes on pravastatin kinetics. Clin Pharmacol Ther 75:415–421. [DOI] [PubMed] [Google Scholar]
- Nagai M, Furihata T, Matsumoto S, Ishii S, Motohashi S, Yoshino I, Ugajin M, Miyajima A, Matsumoto S, Chiba K. (2012) Identification of a new organic anion transporting polypeptide 1B3 mRNA isoform primarily expressed in human cancerous tissues and cells. Biochem Biophys Res Commun 418:818–823. [DOI] [PubMed] [Google Scholar]
- Neul C, Schaeffeler E, Sparreboom A, Laufer S, Schwab M, Nies AT. (2016) Impact of membrane drug transporters on resistance to small-molecule tyrosine kinase inhibitors. Trends Pharmacol Sci 37:904–932. [DOI] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M, Daly AK, Eichelbaum M, Kivistö KT, Neuvonen PJ. (2005a) Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther 77:468–478. [DOI] [PubMed] [Google Scholar]
- Niemi M, Kivistö KT, Hofmann U, Schwab M, Eichelbaum M, Fromm MF. (2005b) Fexofenadine pharmacokinetics are associated with a polymorphism of the SLCO1B1 gene (encoding OATP1B1). Br J Clin Pharmacol 59:602–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi M, Pasanen MK, Neuvonen PJ. (2011) Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev 63:157–181. [DOI] [PubMed] [Google Scholar]
- Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, Backman JT, Kerb R, Schwab M, Neuvonen PJ, et al. (2004) High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1). Pharmacogenetics 14:429–440. [DOI] [PubMed] [Google Scholar]
- Nies AT, Niemi M, Burk O, Winter S, Zanger UM, Stieger B, Schwab M, Schaeffeler E. (2013) Genetics is a major determinant of expression of the human hepatic uptake transporter OATP1B1, but not of OATP1B3 and OATP2B1. Genome Med 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuweboer AJ, Hu S, Gui C, Hagenbuch B, Ghobadi Moghaddam-Helmantel IM, Gibson AA, de Bruijn P, Mathijssen RH, Sparreboom A. (2014) Influence of drug formulation on OATP1B-mediated transport of paclitaxel. Cancer Res 74:3137–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, Takane H, Irie S, Kusuhara H, Urasaki Y, et al. (2003) Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther 73:554–565. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2004a) Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther 308:438–445. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. (2005a) Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos 33:434–439. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, Tsuji A, Yokoi T. (2002) Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther 302:804–813. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Suzuki M, Takahashi K, Yabuuchi H, Maeda T, Tsuji A, Tamai I. (2004b) Involvement of estrone-3-sulfate transporters in proliferation of hormone-dependent breast cancer cells. J Pharmacol Exp Ther 311:1032–1037. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Suzuki M, Yabuuchi H, Irokawa M, Tsuji A, Tamai I. (2005b) Suppression of cell proliferation by inhibition of estrone-3-sulfate transporter in estrogen-dependent breast cancer cells. Pharm Res 22:1634–1641. [DOI] [PubMed] [Google Scholar]
- Obaidat A, Roth M, Hagenbuch B. (2012) The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol 52:135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien Z, Moghaddam MF. (2017) A systematic analysis of physicochemical and ADME properties of all small molecule kinase inhibitors approved by US FDA from January 2001 to October 2015. Curr Med Chem 24:3159–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Szakács G, Reimers MA, Suzuki T, Hall MD, Abe T, Weinstein JN, Gottesman MM. (2008) Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol Cancer Ther 7:3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp RL, van de Steeg E, van der Kruijssen CM, Beijnen JH, Kenworthy KE, Schinkel AH, Schellens JH. (2009) Organic anion-transporting polypeptide 1B1 mediates transport of gimatecan and BNP1350 and can be inhibited by several classic ATP-binding cassette (ABC) B1 and/or ABCG2 inhibitors. Drug Metab Dispos 37:917–923. [DOI] [PubMed] [Google Scholar]
- Oshiro C, Marsh S, McLeod H, Carrillo MW, Klein T, Altman R. (2009) Taxane pathway. Pharmacogenet Genomics 19:979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Lim SM, Shin HJ, Cho A, Shin JG, Lee MG, Kim HR, Kim JH, Cho BC. (2016) Pharmacogenetic analysis of advanced non-small-cell lung cancer patients treated with first-line paclitaxel and carboplatin chemotherapy. Pharmacogenet Genomics 26:116–125. [DOI] [PubMed] [Google Scholar]
- Pasanen MK, Neuvonen PJ, Niemi M. (2008) Global analysis of genetic variation in SLCO1B1. Pharmacogenomics 9:19–33. [DOI] [PubMed] [Google Scholar]
- Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C. (1996) Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J Clin Endocrinol Metab 81:1460–1464. [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Varga Z, Huber RD, Folkers G, Meier PJ, St-Pierre MV. (2003) Identification of steroid sulfate transport processes in the human mammary gland. J Clin Endocrinol Metab 88:3902–3912. [DOI] [PubMed] [Google Scholar]
- Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, John-Williams LS, Koch KM, Serabjit-Singh CJ. (2008) The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos 36:695–701. [DOI] [PubMed] [Google Scholar]
- Pressler H, Sissung TM, Venzon D, Price DK, Figg WD. (2011) Expression of OATP family members in hormone-related cancers: potential markers of progression. PLoS One 6:e20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey LB, Bruun GH, Yang W, Treviño LR, Vattathil S, Scheet P, Cheng C, Rosner GL, Giacomini KM, Fan Y, et al. (2012) Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res 22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey LB, Panetta JC, Smith C, Yang W, Fan Y, Winick NJ, Martin PL, Cheng C, Devidas M, Pui CH, et al. (2013) Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood 121:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello S, Zhao S, Hariry S, Dahlke M, Alexander N, Vapurcuyan A, Hanna I, Jarugula V. (2012) Intestinal OATP1A2 inhibition as a potential mechanism for the effect of grapefruit juice on aliskiren pharmacokinetics in healthy subjects. Eur J Clin Pharmacol 68:697–708. [DOI] [PubMed] [Google Scholar]
- Sai K, Saito Y, Maekawa K, Kim SR, Kaniwa N, Nishimaki-Mogami T, Sawada J, Shirao K, Hamaguchi T, Yamamoto N, et al. (2010) Additive effects of drug transporter genetic polymorphisms on irinotecan pharmacokinetics/pharmacodynamics in Japanese cancer patients. Cancer Chemother Pharmacol 66:95–105. [DOI] [PubMed] [Google Scholar]
- Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, Artursson P, Tsuji A. (2006) Predominant contribution of organic anion transporting polypeptide OATP-B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal Caco-2 cells. Drug Metab Dispos 34:1423–1431. [DOI] [PubMed] [Google Scholar]
- Schwarz UI, Meyer zu Schwabedissen HE, Tirona RG, Suzuki A, Leake BF, Mokrab Y, Mizuguchi K, Ho RH, Kim RB. (2011) Identification of novel functional organic anion-transporting polypeptide 1B3 polymorphisms and assessment of substrate specificity. Pharmacogenet Genomics 21:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi N, Hamada A, Sissung T, Danesi R, Venzon D, Baum C, Gulley JL, Price DK, Dahut WL, Figg WD. (2008) A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int 102:617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka Y, Shichiri M, Murata Y, Mori T, Nakanishi T, Tamai I. (2013) Long-lasting inhibitory effect of apple and orange juices, but not grapefruit juice, on OATP2B1-mediated drug absorption. Drug Metab Dispos 41:615–621. [DOI] [PubMed] [Google Scholar]
- Shitara Y. (2011) Clinical importance of OATP1B1 and OATP1B3 in drug-drug interactions. Drug Metab Pharmacokinet 26:220–227. [DOI] [PubMed] [Google Scholar]
- Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. (2005) Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther 4:815–818. [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. (2002) Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab 87:1856–1863. [DOI] [PubMed] [Google Scholar]
- Stute P, Reichenbach A, Szuwart T, Kiesel L, Götte M. (2012) Impact of testosterone on the expression of organic anion transporting polypeptides (OATP-1A2, OATP-2B1, OATP-3A1) in malignant and non-malignant human breast cells in vitro. Maturitas 71:376–384. [DOI] [PubMed] [Google Scholar]
- Sun Y, Furihata T, Ishii S, Nagai M, Harada M, Shimozato O, Kamijo T, Motohashi S, Yoshino I, Kamiichi A, et al. (2014) Unique expression features of cancer-type organic anion transporting polypeptide 1B3 mRNA expression in human colon and lung cancers. Clin Transl Med 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda M, Wlcek K, Taferner B, Hering S, Stieger B, Tong D, Zeillinger R, Thalhammer T, Jäger W. (2011) Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: relevance for paclitaxel transport. Biomed Pharmacother 65:417–426. [DOI] [PubMed] [Google Scholar]
- Takane H, Kawamoto K, Sasaki T, Moriki K, Moriki K, Kitano H, Higuchi S, Otsubo K, Ieiri I. (2009) Life-threatening toxicities in a patient with UGT1A1*6/*28 and SLCO1B1*15/*15 genotypes after irinotecan-based chemotherapy. Cancer Chemother Pharmacol 63:1165–1169. [DOI] [PubMed] [Google Scholar]
- Takano M, Otani Y, Tanda M, Kawami M, Nagai J, Yumoto R. (2009) Paclitaxel-resistance conferred by altered expression of efflux and influx transporters for paclitaxel in the human hepatoma cell line, HepG2. Drug Metab Pharmacokinet 24:418–427. [DOI] [PubMed] [Google Scholar]
- Tamai I. (2012) Oral drug delivery utilizing intestinal OATP transporters. Adv Drug Deliv Rev 64:508–514. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. (2000) Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun 273:251–260. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nozawa T, Koshida M, Nezu J, Sai Y, Tsuji A. (2001) Functional characterization of human organic anion transporting polypeptide B (OATP-B) in comparison with liver-specific OATP-C. Pharm Res 18:1262–1269. [DOI] [PubMed] [Google Scholar]
- Teft WA, Welch S, Lenehan J, Parfitt J, Choi YH, Winquist E, Kim RB. (2015) OATP1B1 and tumour OATP1B3 modulate exposure, toxicity, and survival after irinotecan-based chemotherapy. Br J Cancer 112:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar N, Kim K, Jang ER, Han S, Kim K, Kim D, Merchant N, Lockhart AC, Lee W. (2013) A cancer-specific variant of the SLCO1B3 gene encodes a novel human organic anion transporting polypeptide 1B3 (OATP1B3) localized mainly in the cytoplasm of colon and pancreatic cancer cells. Mol Pharm 10:406–416. [DOI] [PubMed] [Google Scholar]
- Thakkar N, Lockhart AC, Lee W. (2015) Role of organic anion-transporting polypeptides (OATPs) in cancer therapy. AAPS J 17:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. (2011) Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 21:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Merino G, Kim RB. (2001) Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem 276:35669–35675. [DOI] [PubMed] [Google Scholar]
- Treviño LR, Shimasaki N, Yang W, Panetta JC, Cheng C, Pei D, Chan D, Sparreboom A, Giacomini KM, Pui CH, et al. (2009) Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol 27:5972–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Steeg E, Stránecký V, Hartmannová H, Nosková L, Hřebíček M, Wagenaar E, van Esch A, de Waart DR, Oude Elferink RP, Kenworthy KE, et al. (2012) Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest 122:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Steeg E, van der Kruijssen CM, Wagenaar E, Burggraaff JE, Mesman E, Kenworthy KE, Schinkel AH. (2009) Methotrexate pharmacokinetics in transgenic mice with liver-specific expression of human organic anion-transporting polypeptide 1B1 (SLCO1B1). Drug Metab Dispos 37:277–281. [DOI] [PubMed] [Google Scholar]
- van de Steeg E, van Esch A, Wagenaar E, van der Kruijssen CM, van Tellingen O, Kenworthy KE, Schinkel AH. (2011) High impact of Oatp1a/1b transporters on in vivo disposition of the hydrophobic anticancer drug paclitaxel. Clin Cancer Res 17:294–301. [DOI] [PubMed] [Google Scholar]
- van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, Kenworthy KE, Schinkel AH. (2010) Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest 120:2942–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp NP, Gelderblom H, Guchelaar HJ. (2009) Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 35:692–706. [DOI] [PubMed] [Google Scholar]
- Vasilyeva A, Durmus S, Li L, Wagenaar E, Hu S, Gibson AA, Panetta JC, Mani S, Sparreboom A, Baker SD, et al. (2015) Hepatocellular shuttling and recirculation of sorafenib-glucuronide is dependent on Abcc2, Abcc3, and Oatp1a/1b. Cancer Res 75:2729–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasuri F, Golfieri R, Fiorentino M, Capizzi E, Renzulli M, Pinna AD, Grigioni WF, D’Errico-Grigioni A. (2011) OATP 1B1/1B3 expression in hepatocellular carcinomas treated with orthotopic liver transplantation. Virchows Arch 459:141–146. [DOI] [PubMed] [Google Scholar]
- Vavricka SR, Jung D, Fried M, Grützner U, Meier PJ, Kullak-Ublick GA. (2004) The human organic anion transporting polypeptide 8 (SLCO1B3) gene is transcriptionally repressed by hepatocyte nuclear factor 3β in hepatocellular carcinoma. J Hepatol 40:212–218. [DOI] [PubMed] [Google Scholar]
- Wagner DJ, Hu T, Wang J. (2016) Polyspecific organic cation transporters and their impact on drug intracellular levels and pharmacodynamics. Pharmacol Res 111:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling J. (2006) From methotrexate to pemetrexed and beyond. A review of the pharmacodynamic and clinical properties of antifolates. Invest New Drugs 24:37–77. [DOI] [PubMed] [Google Scholar]
- Wlcek K, Svoboda M, Riha J, Zakaria S, Olszewski U, Dvorak Z, Sellner F, Ellinger I, Jäger W, Thalhammer T. (2011) The analysis of organic anion transporting polypeptide (OATP) mRNA and protein patterns in primary and metastatic liver cancer. Cancer Biol Ther 11:801–811. [DOI] [PubMed] [Google Scholar]
- Wright JL, Kwon EM, Ostrander EA, Montgomery RB, Lin DW, Vessella R, Stanford JL, Mostaghel EA. (2011) Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev 20:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Han Y, Neuvonen M, Pasanen MK, Kalliokoski A, Backman JT, Laitila J, Neuvonen PJ, Niemi M. (2009) Effect of SLCO1B1 polymorphism on the plasma concentrations of bile acids and bile acid synthesis marker in humans. Pharmacogenet Genomics 19:447–457. [DOI] [PubMed] [Google Scholar]
- Xiang X, Jada SR, Li HH, Fan L, Tham LS, Wong CI, Lee SC, Lim R, Zhou QY, Goh BC, et al. (2006) Pharmacogenetics of SLCO1B1 gene and the impact of *1b and *15 haplotypes on irinotecan disposition in Asian cancer patients. Pharmacogenet Genomics 16:683–691. [DOI] [PubMed] [Google Scholar]
- Yamada A, Maeda K, Kiyotani K, Mushiroda T, Nakamura Y, Sugiyama Y. (2014) Kinetic interpretation of the importance of OATP1B3 and MRP2 in docetaxel-induced hematopoietic toxicity. CPT Pharmacometrics Syst Pharmacol 3:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Kobayashi M, Okada M, Takeuchi T, Unno M, Abe T, Goto J, Hishinuma T, Mano N. (2008) Rapid screening of antineoplastic candidates for the human organic anion transporter OATP1B3 substrates using fluorescent probes. Cancer Lett 260:163–169. [DOI] [PubMed] [Google Scholar]
- Yamakawa Y, Hamada A, Shuto T, Yuki M, Uchida T, Kai H, Kawaguchi T, Saito H. (2011) Pharmacokinetic impact of SLCO1A2 polymorphisms on imatinib disposition in patients with chronic myeloid leukemia. Clin Pharmacol Ther 90:157–163. [DOI] [PubMed] [Google Scholar]
- Yang M, Xie W, Mostaghel E, Nakabayashi M, Werner L, Sun T, Pomerantz M, Freedman M, Ross R, Regan M, et al. (2011) SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 29:2565–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, He YJ, Gan Z, Fan L, Li Q, Wang A, Liu ZQ, Deng S, Huang YF, Xu LY, et al. (2007) OATP1B1 polymorphism is a major determinant of serum bilirubin level but not associated with rifampicin-mediated bilirubin elevation. Clin Exp Pharmacol Physiol 34:1240–1244. [DOI] [PubMed] [Google Scholar]
- Zhou F, Zhu L, Wang K, Murray M. (2017) Recent advance in the pharmacogenomics of human solute carrier transporters (SLCs) in drug disposition. Adv Drug Deliv Rev 116:21–36. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yuan J, Li Z, Wang Z, Cheng D, Du Y, Li W, Kan Q, Zhang W. (2015) Genetic polymorphisms and function of the organic anion-transporting polypeptide 1A2 and its clinical relevance in drug disposition. Pharmacology 95:201–208. [DOI] [PubMed] [Google Scholar]
- Zimmerman EI, Hu S, Roberts JL, Gibson AA, Orwick SJ, Li L, Sparreboom A, Baker SD. (2013) Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res 19:1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Fickert P, Silbert D, Fuchsbichler A, Zatloukal K, Denk H, Trauner M. (2005) Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver Int 25:367–379. [DOI] [PubMed] [Google Scholar]