Abstract
Background
Unilateral cerebral palsy (CP) is a condition that affects muscle control and function on one side of the body. Children with unilateral CP experience difficulties using their hands together secondary to disturbances that occur in the developing fetal or infant brain. Often, the more affected limb is disregarded. Constraint‐induced movement therapy (CIMT) aims to increase use of the more affected upper limb and improve bimanual performance. CIMT is based on two principles: restraining the use of the less affected limb (for example, using a splint, mitt or sling) and intensive therapeutic practice of the more affected limb.
Objectives
To evaluate the effect of constraint‐induced movement therapy (CIMT) in the treatment of the more affected upper limb in children with unilateral CP.
Search methods
In March 2018 we searched CENTRAL, MEDLINE, Embase, CINAHL, PEDro, OTseeker, five other databases and three trials registers. We also ran citation searches, checked reference lists, contacted experts, handsearched key journals and searched using Google Scholar.
Selection criteria
Randomised controlled trials (RCTs), cluster‐RCTs or clinically controlled trials implemented with children with unilateral CP, aged between 0 and 19 years, where CIMT was compared with a different form of CIMT, or a low dose, high‐dose or dose‐matched alternative form of upper‐limb intervention such as bimanual intervention. Primarily, outcomes were bimanual performance, unimanual capacity and manual ability. Secondary outcomes included measures of self‐care, body function, participation and quality of life.
Data collection and analysis
Two review authors independently screened titles and abstracts to eliminate ineligible studies. Five review authors were paired to extract data and assess risk of bias in each included study. GRADE assessments were undertaken by two review authors.
Main results
We included 36 trials (1264 participants), published between 2004 and 2018. Sample sizes ranged from 11 to 105 (mean 35). Mean age was 5.96 years (standard deviation (SD) 1.82), range three months to 19.8 years; 53% male and 47% participants had left hemiplegia. Fifty‐seven outcome measures were used across studies. Average length of CIMT programs was four weeks (range one to 10 weeks). Frequency of sessions ranged from twice weekly to seven days per week. Duration of intervention sessions ranged from 0.5 to eight hours per day. The mean total number of hours of CIMT provided was 137 hours (range 20 to 504 hours). The most common constraint devices were a mitt/glove or a sling (11 studies each).
We judged the risk of bias as moderate to high across the studies.
Key results: Primary outcomes at primary endpoint (immediately after intervention)
CIMT versus low‐dose comparison (e.g. occupational therapy)
We found low‐quality evidence that CIMT was more effective than a low‐dose comparison for improving bimanual performance (mean difference (MD) 5.44 Assisting Hand Assessment (AHA) units, 95% confidence interval (CI) 2.37 to 8.51).
CIMT was more effective than a low‐dose comparison for improving unimanual capacity (Quality of upper extremity skills test (QUEST) ‐ Dissociated movement MD 5.95, 95% CI 2.02 to 9.87; Grasps; MD 7.57, 95% CI 2.10 to 13.05; Weight bearing MD 5.92, 95% CI 2.21 to 9.6; Protective extension MD 12.54, 95% CI 8.60 to 16.47). Three studies reported adverse events, including frustration, constraint refusal and reversible skin irritations from casting.
CIMT versus high‐dose comparison (e.g. individualised occupational therapy, bimanual therapy)
When compared with a high‐dose comparison, CIMT was not more effective for improving bimanual performance (MD −0.39 AHA Units, 95% CI −3.14 to 2.36). There was no evidence that CIMT was more effective than a high‐dose comparison for improving unimanual capacity in a single study using QUEST (Dissociated movement MD 0.49, 95% CI −10.71 to 11.69; Grasp MD −0.20, 95% CI −11.84 to 11.44). Two studies reported that some children experienced frustration participating in CIMT.
CIMT versus dose‐matched comparison (e.g. Hand Arm Bimanual Intensive Therapy, bimanual therapy, occupational therapy)
There was no evidence of differences in bimanual performance between groups receiving CIMT or a dose‐matched comparison (MD 0.80 AHA units, 95% CI −0.78 to 2.38).
There was no evidence that CIMT was more effective than a dose‐matched comparison for improving unimanual capacity (Box and Blocks Test MD 1.11, 95% CI −0.06 to 2.28; Melbourne Assessment MD 1.48, 95% CI −0.49 to 3.44; QUEST Dissociated movement MD 6.51, 95% CI −0.74 to 13.76; Grasp, MD 6.63, 95% CI −2.38 to 15.65; Weightbearing MD −2.31, 95% CI −8.02 to 3.40) except for the Protective extension domain (MD 6.86, 95% CI 0.14 to 13.58).
There was no evidence of differences in manual ability between groups receiving CIMT or a dose‐matched comparison (ABILHAND‐Kids MD 0.74, 95% CI 0.31 to 1.18). From 15 studies, two children did not tolerate CIMT and three experienced difficulty.
Authors' conclusions
The quality of evidence for all conclusions was low to very low. For children with unilateral CP, there was some evidence that CIMT resulted in improved bimanual performance and unimanual capacity when compared to a low‐dose comparison, but not when compared to a high‐dose or dose‐matched comparison. Based on the evidence available, CIMT appears to be safe for children with CP.
Plain language summary
Constraint‐induced movement therapy in the treatment of the upper limb in children with unilateral cerebral palsy
Review question
Does constraint‐induced movement therapy (CIMT) improve arm and hand use in children with unilateral cerebral palsy (CP)?
What is the aim of this review?
To find out if CIMT helps children with unilateral (hemiplegic) CP to use their hands more effectively.
Key messages
CIMT may work better than another upper‐limb therapy carried out at low intensity (low dose) for improving children’s ability to use both hands together. CIMT appears no more effective than another upper‐limb therapy carried out at a high dose or equal dose. CIMT appears to be safe. More well‐designed research is needed for strong conclusions to be made.
What was studied in the review?
Children with unilateral CP have difficulty using two hands together. Most daily activities need co‐ordinated use of two hands together, so clinicians use CIMT to help children with unilateral CP improve upper‐limb ability. There is no one type of CIMT, although it always involves a constraint (e.g. mitt, sling, cast) on the less affected arm, accompanied by intensive therapy with the more affected arm.
What are the main results of the review?
Thirty‐six studies were found. Children were involved in CIMT from 20 to 504 hours. CIMT studies were divided into three categories.
CIMT compared with a low‐dose comparison group (children had 0 to 25 hours of comparison therapy; and the amount of therapy was much lower than the amount of CIMT)
CIMT may improve bimanual ability (that is, using both hands together; low‐quality evidence) and unilateral capacity (that is, one‐handed ability using the more affected hand; very low‐quality evidence) more than low dose. Three studies reported that a small number of children experienced frustration or refused to wear the constraint, or had reversible skin irritations from casting.
CIMT compared with a high‐dose comparison group (children had more than 25 hours of bimanual therapy or another form of intensive therapy and the amount was less than CIMT)
CIMT appeared no more effective than a high‐dose comparison therapy on bimanual ability (low‐quality evidence) or unimanual capacity (very low‐quality evidence). Two studies reported that some children experienced frustration from participating in CIMT.
CIMT compared with a dose‐matched comparison group (children received the same amount of bimanual therapy as the CIMT group).
CIMT appeared no more effective than dose‐matched therapy on bimanual ability, unimanual capacity (low‐quality evidence) or manual ability (very low‐quality evidence). From 15 studies, two children did not tolerate CIMT and three had difficulty getting used to CIMT.
How up to date is this review?
The review includes studies published up to March 2018.
Summary of findings
Background
Description of the condition
Cerebral palsy (CP) is an umbrella term, which describes “a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non‐progressive disturbances that occurred in the developing fetal or infant brain” (Rosenbaum 2009, p 9). The definition also specifies that the motor disorders that characterise CP often co‐exist with epilepsy; musculoskeletal, behaviour and communication problems; and difficulties with sensation, perception and cognition. CP is considered the most common cause of physical disability in childhood. In many developed countries, CP is estimated to be present in 1.9 to 2.1 children per 1000 live births (ACPR 2016).
Unilateral CP, also called hemiplegic CP, is common; 39% of children with CP in Australia have this form (ACPR 2016). Upper‐limb dysfunction can range from mildly to profoundly impaired depending on the timing, site, extent and nature of the brain lesion (Holmefur 2013; Holmström 2010). Reduced ability to use the more‐affected upper limb in daily activities is associated with musculoskeletal deformity, disorders of posture and movement, and impaired sensory and cognitive function (Arner 2008; Bodimeade 2013; Brown 1987; Eliasson 1995; Klingels 2012; Steenbergen 2006). The potential impact of impaired upper‐limb function on restrictions to participation in daily life has resulted in extensive clinical and research endeavours, by occupational therapists and others, to devise and evaluate interventions to improve upper‐limb function in this specific group of children (Beckung 2002; Fauconnier 2009; Ziviani 2008).
Upper‐limb interventions employed in recent years to improve unilateral capacity, bimanual performance and task performance in children with unilateral CP include intra‐muscular Botulinum toxin‐A injections (Hoare 2010; Hoare 2013), casting (Autti‐Rämö 2006), orthoses and Lycra splinting (Elliott 2011; Imms 2016a; Jackman 2014), surgery (Van Heest 2015), strengthening programs (Rameckers 2015), virtual reality (Snider 2010; Weiss 2014), home programs (Novak 2009), goal‐directed training (Löwing 2010), action observation therapy (Kirkpatrick 2016; Sgandurra 2013), robotics (Gilliaux 2015), electrical stimulation (Xu 2015; Yıldızgören 2014), repetitive transcranial magnetic stimulation (TMS) (Gillick 2014; Kirton 2016a (CIMT + r TMS)), sensory cueing (Dong 2017), mirror therapy (Bruchez 2016), gaming (Chiu 2014) and Cognitive Orientation to daily Occupational Performance (Cameron 2017). Along with bimanual therapy (Facchin 2011; Gelkop 2015; Gordon 2007; Green 2013; Hoare 2013; Sakzewski 2011), constraint‐induced movement therapy (CIMT) is one of two interventions that were developed specifically for children with unilateral CP.
Description of the intervention
The two key components that define CIMT are restraint of the less affected upper limb, with the addition of intensive, structured, upper‐limb therapy (Eliasson 2014a). The definition and implementation of these two components is diverse across clinical and research environments. The types of restraints used in studies to date include splints, slings, mitts/gloves and casts. These have been applied from one hour per day to 24 hours a day, over a period of two weeks to two months or more. Intervention has been delivered individually or in groups, in the home, clinic, during inpatient programs, or novel environments such as embedded in circus‐ or pirate‐themed camps. The nature of intensive upper‐limb therapy for the more affected arm and hand has also varied greatly. Some studies reported the approach to therapy in detail, but for most, the descriptions are brief (Sakzewski 2016). Many studies used eclectic approaches or approaches that are difficult to classify according to named frameworks. Several used descriptors such as 'play' and 'involvement in functional activity', whilst some were clear that the intervention involved shaping and repetition. A few studies used goal‐oriented therapy based on motor learning principles and some added bimanual therapy. Several studies did not include an intensive upper‐limb therapy alongside constraint, rather they maintained the child’s low‐intensity pre‐study therapy.
The absence of clarity around a specific definition of CIMT was addressed by an expert panel, which met to scope the state of knowledge about CIMT and to make recommendations for future clinical and research directions (Eliasson 2014a). The panel proposed four main classifications of CIMT.
Signature CIMT (sCIMT), which is derived from the original model developed by Taub 2004, for adults with hemiparesis following stroke. It is defined as restraint of the unaffected upper limb for 90% of the waking day for at least two weeks, while engaging the child in intensive upper‐limb therapy for three or more hours per day.
Modified CIMT (mCIMT), which comprises variation to the signature model, specifically the type of restraint, nature of intensive therapy, and the hours per day and duration in weeks of the program.
Hybrid CIMT (hCIMT), which is the result of efforts by clinicians and researchers to combine CIMT and bimanual forms of intervention into intervention packages. Defined as hCIMT by Eliasson 2014a, it is based on the premise that CIMT, as a unilateral intervention, may result in improved unilateral upper‐limb ability, but practice of bimanual functional activities is necessary to transfer these improvements into daily life.
Forced use therapy, which involves use of restraint of the less affected upper limb, without including an intensive, upper‐limb intervention.
We used these definitions in this review to classify the types of CIMT across studies (See Characteristics of included studies).
How the intervention might work
CIMT used with children with unilateral CP aims to address two different but linked mechanisms to improve unilateral capacity and bimanual performance: developmental disregard and use‐dependent cortical re‐organisation (Taub 2007).
The term developmental disregard is used to describe behaviours of children with unilateral CP who have learned to suppress use of, and therefore to disregard, their more affected upper limb (DeLuca 2003). From an early age many children with unilateral CP discover it is more efficient and effective to complete tasks using the less affected hand, even if there is only mild impairment in the more affected limb (Kuhtz‐Buschbeck 2000; Krumlinde‐Sundholm 1998). Families and clinicians, particularly occupational therapists, often note a discrepancy between actual use of the limb in daily activities and the capacity for upper‐limb use observed in a clinic situation (Sutcliffe 2009; Zielinski 2014a; Zielinski 2014b). Therapists, therefore, create the opportunity, experience and environment that optimises a child's ability to use their more affected limb. This experience aims to reverse the behavioural aspect of suppression of use of the affected limb and use appropriate rewards to motivate a child to master increasingly challenging upper‐limb movements and tasks. The intensive but targeted upper‐limb practice in which children engage during CIMT, and which is facilitated by restraint of the less affected hand, is intended to overcome developmental disregard by counter‐conditioning or reducing the suppression of motor activity (Morris 2001).
Increased and more effective use of the more affected limb during CIMT aims to induce expansion of the contralateral cortical area controlling movement of the more affected limb (Friel 2014). This activity‐dependent, cortical re‐organisation may serve as the neural basis for permanent increase in use of the affected limb in daily activities following treatment. Several studies provide evidence that potential exists for such activity‐dependent neuroplasticity in children with unilateral CP following CIMT (Cope 2010; Juenger 2007; Manning 2015; Sutcliffe 2007; Sutcliffe 2009).
Why it is important to do this review
Four recent systematic reviews concluded that CIMT was more effective for improving upper‐limb function than low intensity or standard care interventions and equally effective as an alternative, upper‐limb intervention delivered at a similar dose (Dong 2013; Chen 2014; Sakzewski 2014; Chiu 2016). This latter evidence is important as it allows families choice of effective interventions to suit individual child and family preferences, needs and resources. Chen 2014 provided additional insights – reporting that effect sizes were larger immediately after intervention than at later endpoints, and that home‐ and clinic‐based interventions resulted in larger effects than camp‐based intervention. Chen 2014 also reported that type of restraint, amount of daily use, and duration of therapy did not impact outcome.
Despite the increasing clarity around the effectiveness of CIMT, more work is required to understand the minimum dose that is effective, allowing children and families to make choices that minimise burden and costs of intervention. The advent of hybrid interventions is relatively recent and a greater understanding of whether there are additive effects of combining unilateral and bimanual interventions is required. Finally, more high‐quality randomosed controlled trials (RCTs) are using outcome measures that are validated for use with children with unilateral CP. This will allow for meta‐analyses, which will result in trustworthy conclusions regarding the effectiveness of CIMT, allow determination of clinically important outcomes and clarification of duration of effect over time. This Cochrane Review of the most up‐to‐date literature addresses contemporary issues in this field of research. This is important to inform families of children with CP, service providers, clinicians and researchers of the state‐of ‐the‐art in relation to clinical applications of CIMT and directions for future research.
Objectives
To evaluate the effect of constraint‐induced movement therapy (CIMT) in the treatment of the more affected upper limb in children with unilateral cerebral palsy (CP).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trial (RCTs), cluster‐RCTs or clinically controlled trials. See Differences between protocol and review.
Types of participants
Participants diagnosed with unilateral CP, aged between birth and 19 years. We only included studies involving a subset of children with unilateral CP if separate data were available for these children.
Types of interventions
In the original 2007 review (Hoare 2007a; Hoare 2007b), we used definitions of constraint‐induced movement therapy (CIMT) described by Taub 2002 [pers comm]. For this update, we used the definitions outlined in a more recent expert consensus paper: signature CiMT (sCIMT); modified CIMT (mCIMT); hybrid CMIT (hCIMT); and forced use therapy (Eliasson 2014a). In this report, we use 'CIMT' as an umbrella term to encompass all specific types of CIMT (Eliasson 2014a).
We included studies that evaluated sCIMT, mCIMT, hCIMT or forced use therapy compared to usual care, conventional therapy, bimanual therapy, variations of sCIMT, mCIMT, hCIMT or forced‐use therapy; alternative, upper‐limb interventions; or no treatment. We also included studies where CIMT was combined with a concurrent intervention provided CIMT could be isolated as defining the intervention group from the comparison group, and that any co‐intervention was implemented in each group in an identical manner. For example, an eligible comparison would be CIMT plus Botulinum toxin‐A injections versus bimanual therapy plus Botulinum toxin‐A injections, while an ineligible comparison would be CIMT plus bimanual therapy compared with CIMT. We excluded studies where CIMT was combined with lower‐limb intervention.
Dosage of CIMT was defined as total hours of intervention calculated with the following formula.
Total hours of CIMT intervention = therapist‐led intervention + parent‐led intervention + other intervention (e.g. usual care) + forced use (Table 5).
1. Dosage of CIMT.
| Study | Therapist‐led (hours) | Parent‐led (hours) | Other (hours) | Total therapy (hours) | Forced use (hours) | Total therapy + forced used (hours) |
| Aarts 2010 | 72 | 26.4 | ‐ | 98.4 | ‐ | 98.4 |
| Abd El‐Kafy 2014 | 80 | 40 | ‐ | 120 | ‐ | 120 |
| Abootalebi 2010 | 105 | Not reported | 6.75 | 111.75 | 140 | 252 |
| Al‐Oraibi 2011 | 96 | Not reported | ‐ | 96 | ‐ | 96 |
| Charles 2006 | 60 | 10 | ‐ | 70 | ‐ | 70 |
| Chen 2014 | 112 | ‐ | ‐ | 112 | ‐ | 112 |
| Choudhary 2013 | 20 | 56 | 8.4 | 84.4 | ‐ | 84.4 |
| Christmas 2018 | ‐ | 42 | ‐ | 42 | 462 | 504 |
| de Brito Brandão 2010 | 30 | ‐ | 2.25 | 32.25 | 107.75 | 140 |
| DeLuca 2012 | 126 | ‐ | ‐ | 126 | 90 | 216 |
| Deppe 2013 | 80 | ‐ | ‐ | 80 | ‐ | 80 |
| Dong 2017 | 15 | 60 | ‐ | 75 | ‐ | 75 |
| Eliasson 2011 | ‐ | 112 | ‐ | 112 | ‐ | 112 |
| Eliasson 2018 | ‐ | 36 | ‐ | 36 | ‐ | 36 |
| Eugster‐Buesch 2012 | ‐ | 84 | 2 | 86 | ‐ | 86 |
| Facchin 2011 | 90 | 120 | ‐ | 210 | ‐ | 210 |
| Gelkop 2015 | 96 | ‐ | ‐ | 96 | ‐ | 96 |
| Gharib 2010 | 126 | Not reported | 13.5 | 139.5 | ‐ | 139.5 |
| Gordon 2011 | 90 | 15 | ‐ | 105 | ‐ | 105 |
| Hoare 2013 | 16 | 152 | ‐ | 168 | ‐ | 168 |
| Hosseini 2010 | 60 | Not reported | ‐ | 60 | ‐ | 60 |
| Kirton 2016a (CIMT + r TMS) | 80 | 10 | ‐ | 90 | ‐ | 90 |
| Rostami 2012a | 15 | 10 | ‐ | 25 | 143 | 168 |
| Rostami 2012b | 18 | Not reported | 4 | 22 | 118 | 140 |
| Sabour 2012 | 60 | ‐ | 4.5 | 64.5 | ‐ | 64.5 |
| Sakzewski 2011 | 60 | ‐ | ‐ | 60 | ‐ | 60 |
| Sakzewski 2015a | 60 | ‐ | ‐ | 60 | ‐ | 60 |
| Sakzewski 2015b | 30 | ‐ | ‐ | 30 | ‐ | 30 |
| Smania 2009 | 10 | Not reported | ‐ | 10 | 270 | 280 |
| Sung 2005 | 6 | Not reported | ‐ | 6 | 498 | 504 |
| Taub 2004 | 126 | ‐ | ‐ | 126 | 126 | 252 |
| Taub 2011 | 90 | ‐ | ‐ | 90 | 90 | 180 |
| Wallen 2011 | 16 | 112 | ‐ | 128 | ‐ | 128 |
| Xu 2012 | 30 | 10 | ‐ | 40 | ‐ | 40 |
| Yu 2012 | 20 | Not reported | ‐ | 20 | ‐ | 20 |
| Zafer 2016 | 2 | 24 | ‐ | 26 | 22 | 48 |
We calculated the dosage of forced use in models of CIMT where constraint devices were worn outside of therapist‐ or parent‐led intervention hours, such as when children wore a cast for 24 hours a day and were participating in therapy for SIX hours per day. For studies where constraint was worn for 90% of waking hours or 24 hours per day, we estimated that time involved in forced use was equivalent to 12 hours per day. In the example given above, hours of therapy per day = six hours (therapist‐ or parent‐led) + (12 hours forced use ‐ six hours therapist‐ or parent‐led) = 12 hours.
To achieve the objectives of our review related to intensity of comparison intervention, we categorised comparison interventions according to total dosage calculated as follows.
Total hours of comparison intervention = therapist‐led intervention + parent‐led intervention + other intervention (e.g. usual care) (Table 6).
2. Dosage of comparison interventions.
| Study | Therapist‐led (hours) | Parent‐led (hours) | Total therapy (hours) | Forced use (hours) | Total therapy + forced use (hours) |
| Low dose | |||||
| Abootalebi 2010 | 6.75 | Not reported | 6.75 | ‐ | 6.75 |
| Al‐Oraibi 2011 | 16 | Not reported | 16 | ‐ | 16 |
| Charles 2006 | 0 | 0 | 0 | ‐ | 0 |
| Choudhary 2013 | 9.33 | Time not specified | 9.33 | ‐ | 9.33 |
| de Brito Brandão 2010 | 2.25 | 0 | 2.25 | ‐ | 2.25 |
| Dong 2017 | 7.5 | 0 | 7.5 | ‐ | 7.5 |
| Eliasson 2011 | Not reported | Not reported | Not reported | ‐ | 0 |
| Eliasson 2018 | 0 | Time not specified | Not reported | ‐ | Not reported |
| Eugster‐Buesch 2012 | Not reported | Not reported | Not reported | ‐ | 0 |
| Facchin 2011 | 15 | Not reported | 15 | ‐ | 15 |
| Gharib 2010 | 13.5 | Not reported | 13.5 | ‐ | 13.5 |
| Hosseini 2010 | Not reported | Not reported | Not reported | ‐ | 0 |
| Rostami 2012b | 4 | Not reported | 4 | ‐ | 4 |
| Sabour 2012 | 4.5 | Not reported | 4.5 | ‐ | 4.5 |
| Taub 2004 | 6.6 | Not reported | 6.6 | ‐ | 6.6 |
| Taub 2011 | 6.75 | Not reported | 6.75 | ‐ | 6.75 |
| Yu 2012 | 10 | Not reported | 10 | ‐ | 10 |
| High dose | |||||
| Chen 2014 | 30 | 0 | 30 | ‐ | 30 |
| Hoare 2013 | 14 | 16.2 | 30.2 | ‐ | 30.2 |
| Sakzewski 2015a | 9 | 36 | 45 | ‐ | 45 |
| Wallen 2011 | 8 | 36.8 | 44.8 | ‐ | 44.8 |
| Dose‐matched | |||||
| Aarts 2010 | 12 | 89.6 | 101.6 | ‐ | 101.6 |
| Abd El‐Kafy 2014 | 120 | Time not specified | 120 | ‐ | 120 |
| Deppe 2013 | 80 | 0 | 80 | ‐ | 80 |
| Dong 2017 | 15 | 60 | 75 | ‐ | 75 |
| Facchin 2011 | 90 | 120 | 210 | ‐ | 210 |
| Gelkop 2015 | 96 | Not reported | 96 | ‐ | 96 |
| Gordon 2011 | 90 | 15 | 105 | ‐ | 105 |
| Kirton 2016a (CIMT + r TMS) | 80 | 10 | 90 | ‐ | 90 |
| Rostami 2012b | 22 | Not reported | 22 | ‐ | 22 |
| Sakzewski 2011 | 60 | 0 | 60 | ‐ | 60 |
| Sakzewski 2015b | 30 | Not reported | 30 | ‐ | 30 |
| Smania 2009 | 10 | Time not specified | 10 | ‐ | 10 |
| Sung 2005 | 6 | Not reported | 6 | ‐ | 6 |
| Xu 2012 | 30 | 10 | 40 | ‐ | 40 |
| Zafer 2016 | 2 | 22 | 26 | ‐ | 26 |
| Different form of CIMT | |||||
| Christmas 2018 | 0 | 42 | 42 | 0 | 42 |
| DeLuca 2012 | 63 | 0 | 63 | 149 | 212 |
| Rostami 2012a | 15 | 10 | 168 | 143 | 311 |
The following categories were included.
Low dose: total hours of intervention = range 0 to 25 hours and a substantial difference from experimental‐group dosage with forced‐use dosage excluded.
High dose: total hours of intervention > 25 hours but less than experimental‐group dosage with forced‐use dosage excluded.
Dose‐matched: experimental and comparison groups received equal dosages of therapist‐ + parent‐led + other interventions. Time spent in forced use was excluded from the CIMT dosage for this comparison.
Other form of CIMT: when CIMT was compared head‐to‐head with another form of CIMT such as delivered at a different dose or in a different environment.
Types of outcome measures
In the original review (Hoare 2007a; Hoare 2007b), we broadly grouped outcome measures according to the domains of the International Classification of Functioning, Disability and Health (ICF) (WHO 2001). For this review update, we categorised measures into primary or secondary outcomes, to better reflect the expected effect of CIMT (Eliasson 2014a). The goal of CIMT is to improve unilateral upper‐limb ability to transfer into improved bimanual functional performance (self‐care, manual ability, individual performance). The primary outcomes, therefore, focused on both bimanual and unimanual function. Secondary measures included those that CIMT may effect but are not the primary target of intervention.
We considered outcome measures ineligible for inclusion if they: 1) did not possess adequate reported validity or reliability (or both) for children with CP; 2) were standardised assessments that were invalidated because the administration or scoring was adapted; or 3) both. Ineligible measures and the reasons for ineligibility are listed in Table 7.
3. Ineligible outcome measures reported in included studies, and reasons for being ineligible.
| Measure | Study reported in | Reason for exclusion |
| 9‐Hole Peg Test |
Choudhary 2013 Xu 2012 |
No evidence of validity or reliability as an outcome measure in cerebral palsy |
| Accelerometry |
Dong 2017 Gordon 2011 |
No evidence of validity or reliability as an outcome measure in cerebral palsy |
| Active range of motion |
Dong 2017 Hosseini 2010 Taub 2011 Xu 2012 |
No evidence of validity or reliability as an outcome measure in cerebral palsy |
| Bimanual Function composite and Unimanual Function composite | Hosseini 2010 | No evidence of validity or reliability as an outcome measure in cerebral palsy |
| Bruininks‐Oseretsky Test of Motor Proficiency |
Charles 2006 Chen 2014 Dong 2017 Hosseini 2010 Rostami 2012a Rostami 2012b |
Used the modified form with no evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Caregiver Functional Use Scale |
Charles 2006 Dong 2017 Hosseini 2010 |
No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Child Arm Use Test | Taub 2004 | No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Children’s Hand‐use Experience Questionnaire (CHEQ): number of items completed independently, % of items child completed independently where affected hand was used as a support or with grip | Sakzewski 2015a | Amer 2016 recommends that these scales are not used due to misfitting items. |
| Developmental Activities Screening Inventory | Taub 2004 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Emerging Behavior Scale | Taub 2004 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Erhardt Developmental Prehension Assessment | Sung 2005 | No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Function test | Smania 2009 | No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Functional Magnetic Resonance Imaging | Sakzewski 2011 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Globe Rating Scale | Xu 2012 | No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Inventory of New Motor Behaviors | Taub 2011 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Isometric shoulder torque | Abd El‐Kafy 2014 (using Biodex) | No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Jebsen Taylor Test of Hand Function |
Charles 2006 de Brito Brandão 2010 Dong 2017 Gordon 2011 Hosseini 2010 Sabour 2012 Sakzewski 2011 Sakzewski 2015a Sakzewski 2015b |
Used the modified form with no evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Neuromapper (H reflex) | Abootalebi 2010 | No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Parent interview/investigator‐developed questionnaire |
Al‐Oraibi 2011 Eliasson 2018 Eugster‐Buesch 2012 Wallen 2011 |
No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Passive range of motion | Taub 2011 | Used a modified form with no evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Peabody Developmental Motor Scales | Original version: Abootalebi 2010 | No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Version 2: Xu 2012 (used in a non‐standardised manner, i.e. with children older than standardisation sample) | ||
| Pediatric Motor Activity Log | Original version: Taub 2004, DeLuca 2012; Hoare 2013 | At least three versions of this test exist (Wallen 2013). Original version: One study Lin 2012 provides insufficient evidence for reliability and validity for this version used. PMAL – Revised (Wallen version) Wallen 2009b. No data have been collected for testing psychometric robustness using this version of the PMAL‐R, so insufficient evidence exists to support its use. PMAL – Revised (Uswatte version) Uswatte 2012. Evidence exists for reliability of this version so it is eligible for inclusion. |
| Wallen version: Wallen 2011 | ||
| Unspecified version: Rostami 2012a, Rostami 2012b, Chen 2014 | ||
| Pinch strength | Kirton 2016a (CIMT + r TMS) | No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| QUEST total score |
Abd El‐Kafy 2014 Choudhary 2013 Christmas 2018 Facchin 2011 Gharib 2010 Taub 2004 Zafer 2016 |
Total score is reported to have poor construct validity, see Thorley 2012 |
| QUEST: Dissociated Movement and Grasp domains (adapted) | DeLuca 2012 | Used the adapted version |
| Reaching kinematics/3D kinematics |
Chen 2014 Gordon 2011 |
No evidence of validity or reliability as an outcome measure in children with cerebral palsy |
| Shriners Hospital for Children Upper Extremity Evaluation |
DeLuca 2012 Kirton 2016a (CIMT + r TMS) |
Data reported for a single group |
| Social life ability scale for Chinese infant‐junior school students | Xu 2012 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Surface EMG | Xu 2012 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Stereognosis | Sakzewski 2011 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Toddler Arm Use Test | Taub 2004 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Transcranial Magnetic Stimulation | Sakzewski 2011 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Upper Extremity Function test | Xu 2012 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
| Use test | Smania 2009 | No evidence for validity or reliability as an outcome measure in children with cerebral palsy |
EMG: Electromyography PMAL: Pediatric Motor Activity Log. QUEST: Quality of Upper Extremity Skills Test
We deemed the following measures eligible for inclusion.
Primary outcomes
Bimanual
Kids‐Assisting Hand Assessment (Kids‐AHA; Holmefur 2007; Holmefur 2009; Holmefur 2016; Krumlinde‐Sundholm 2003; Krumlinde‐Sundholm 2007; Krumlinde‐Sundholm 2012)
Hand Assessment for Infants (HAI) ‐ both hands score (Krumlinde‐Sundholm 2017)
Unimanual
Melbourne Assessment of Unilateral Upper Limb Function or Melbourne Assessment 2 (Melbourne Assessment 2; Randall 2008; Randall 2012)
Box and Blocks Test (Jongbloed‐Pereboom 2013)
Quality of Upper Extremity Skills Test (QUEST) ‐ Dissociated movement domain (Thorley 2012)
QUEST ‐ Grasp domain (Thorley 2012)
QUEST ‐ Weight‐bearing domain (Thorley 2012)
QUEST ‐ Protective extension domain (Thorley 2012)
Shriner’s Hospital Upper Extremity Evaluation (SHUEE; Davids 2006)
Pediatric Motor Activity Log (PMAL) ‐ Revised (Uswatte 2012b)
Hand Assessment for Infants (HAI) ‐ Unimanual score (Krumlinde‐Sundholm 2017)
Manual ability
ABILHAND‐Kids (Arnould 2004; Bleyenheuft 2017)
Children’s Hand‐use Experience Questionnaire (CHEQ) ‐ Effectiveness of grasp, Time to do task and Bothered scales only (Amer 2016; Sköld 2011)
Birmingham Bimanual Questionnaire (Christmas 2018)
Adverse events
We recorded adverse events for each included study (See Table 8).
4. Adverse events.
| Study | Adverse events |
| CIMT versus low‐dose comparison | |
| Abootalebi 2010 | The study did not mention the presence or absence of adverse events. |
| Al‐Oraibi 2011 | The study did not mention the presence or absence of adverse events. |
| Charles 2006 | One child who was unable to tolerate CIMT was removed from study. |
| Choudhary 2013 | The study did not mention the presence or absence of adverse events. The study reported that the children tolerated the treatment well. |
| de Brito Brandão 2010 | The study did not mention the presence or absence of adverse events. |
| Dong 2017 | The study reported that “No major adverse events were reported” (p 4), however, “There were two dropouts from the CIMT group in the first week of treatment, because the children did not tolerate the intervention and complained about inconvenience during physical activities at school” (p 4). |
| Eliasson 2011 | One child was unable to tolerate CIMT. |
| Eliasson 2018 | The study reported that there were no adverse events. |
| Eugster‐Buesch 2012 | The study reported there were no adverse events to the affected hand. Parent questionnaire reported the CIMT program caused frustration in completing activities (2/11), splint refusal was observed (6/11) and completing the programme was exhausting (6/11) |
| Facchin 2011 | The published trial protocol (Facchin 2009) specified that the unaffected limb would be monitored using Quality of Upper Extremity Skills Test (QUEST) and Besta Scales. The study reported no significant or sustained, long‐term adverse effects for the unaffected limb. Facchin 2009 also specified that behaviour change would be assessed using the Child Behaviour Checklist and family stress would be monitored using Parenting Stress Index but no data were provided for either of these outcomes at any time point. |
| Gharib 2010 | The study did not mention the presence or absence of adverse events. |
| Hosseini 2010 | The study did not mention the presence or absence of adverse events. |
| Rostami 2012b | The study did not mention the presence or absence of adverse events. |
| Sabour 2012 | The study did not mention the presence or absence of adverse events. |
| Taub 2004 | The study reported that all children adapted to CIMT within 1 to 2 days. The study also reported that 2 children in CIMT group with a history of behaviour management problems experienced behaviour control difficulties, and that 3 (DeLuca 2006) or 4 (Taub 2004) children in CIMT group experienced minor and reversible skin irritations from casting. All children maintained full range of movement and functional movement skills in the arm that had a cast applied. |
| Taub 2011 | The study reported that the children generally coped well with the cast, with few complaints after the first day. |
| Yu 2012 | The study did not mention the presence or absence of adverse events. |
| CIMT versus high‐dose comparison | |
| Chen 2014 | The study reported that there were no adverse events bu that “some children experienced frustration in the early stages of constraint‐induced therapy”. |
| Hoare 2013 | The study reported there were no adverse events resulting from CIMT. |
| Sakzewski 2015a | The study reported that 1 child in the hybrid‐CIMT group had a seizure, which was unrelated to the intervention. |
| Wallen 2011 | The study reported minor adverse events in 5 children in the CIMT group due to a lack of acceptance of the CIMT mitt and frustration/refusal to co‐operate. The study reported there were no adverse events due to the comparison intervention. |
| CIMT versus dose‐matched comparison | |
| Aarts 2010 | The study reported that there were no adverse events. |
| Abd El‐Kafy 2014 | The study did not mention the presence or absence of adverse events. |
| Deppe 2013 | The study did not mention the presence or absence of adverse events. |
| Dong 2017 | The study reported that “No major adverse events were reported” (p 4), but that “There were two dropouts from the CIMT group in the first week of treatment, because the children did not tolerate the intervention and complained about inconvenience during physical activities at school” (p 4). |
| Facchin 2011 | The published trial protocol (Facchin 2009) specified that the unaffected limb would be monitored using QUEST and Besta Scales. They reported no significant or sustained, long‐term adverse effects for the unaffected limb. Facchin 2009 also specified that behaviour change would be assessed using the Child Behaviour Checklist and family stress would be monitored using Parenting Stress Index, however, no data were provided for either of these outcomes at any time point. |
| Gelkop 2015 | The study reported that there were no adverse events. |
| Gordon 2011 | The study reported that there were no adverse events. |
| Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS) | The study authors reported that all participants completed all stages with no dropouts or adverse events. Also, although headache was reported in 11% of repetitive Transcranial Magnetic Stimulation (rTMS) group, it was mild and self‐limiting. Additional side effects (tingling, nausea) were reported in < 3% of sessions. |
| Rostami 2012b | The study did not mention the presence or absence of adverse events. |
| Sakzewski 2011 | The study reported that there were no “major” adverse events. |
| Sakzewski 2015b | The study did not mention the presence or absence of adverse events. |
| Smania 2009 | The study authors stated that one child was excluded following commencement of Modified CIMT (mCIMT) due to behaviour difficulties manifesting on commencement of mCIMT. The parents of 3 children reported resistance to wearing the mitten for the first few days. |
| Sung 2005 | The study reported no decline in hand function of the immobilized unaffected arm after 6 weeks in the forced‐use therapy group, or any cases of joint stiffness or skin problems. |
| Xu 2012 | The study reported that there were no adverse events. |
| Zafer 2016 | The study did not mention the presence or absence of adverse events. |
| CIMT versus different form CIMT | |
| Christmas 2018 | The study reported that there were no serious adverse events. 12 non‐serious adverse events related to the intervention were identified for the prolonged restraint group: 2 children had minor bruising because of a fall and 10 had small areas of skin abrasions. |
| DeLuca 2012 | The study reported that there were no adverse events. |
| Rostami 2012a | The study did not mention the presence or absence of adverse events. |
CIMT: constraint‐induced movement therapy.
Secondary outcomes
Individualised measures of performance
Canadian Occupational Performance Measure (COPM; Carswell 2004; Cusick 2006; Cusick 2007)
Goal Attainment Scaling (GAS; Cusick 2006)
Self‐care
Pediatric Evaluation of Disability Inventory (PEDI) ‐ Self‐Care Functional Skills domain (Feldman 1990; James 2014)
PEDI ‐ Self‐Care Caregiver Assistance domain (Feldman 1990; James 2014)
Functional Independence Measure for Children (WeeFIM; James 2014)
Body function
Grip strength (for example, Jamar Dynamometer) (Klingels 2010)
Modified Ashworth Scale ‐ Elbow (Clopton 2005; Klingels 2010)
Modified Ashworth Scale ‐ Wrist (Klingels 2010)
Two‐point discrimination (Klingels 2010)
Passive Range of Motion (PROM; Glazier 1997; Klingels 2010)
Modified Tardieu Scale (MTS; Gracies 2010; Mackey 2004)
Participation
Children’s Assessment of Participation and Enjoyment (CAPE; Sakzewski 2007)
Assessment of Life Habits (LIFE‐H; Noreau 2007)
Quality of life
Cerebral Palsy Quality of Life Questionnaire for Children (CP QOL) ‐Child/self report (Davis 2013)
CP QOL ‐ Child/Caregiver report (Davis 2013)
KIDSCREEN‐52 (The Kid Screen Group Europe)
Pediatric Quality of Life Inventory (PEDSQOLTM) 4.0 ‐ Generic Core Scale (Varni 2008)
PEDSQOLTM 3.0 ‐ Cerebral Palsy Module (Varni 2006)
PEDSQOLTM ‐ Infant Scale (Varni 2011)
Parenting and family measures
Parenting Sense of Competence Scale (Gilmore 2009)
Other
Pediatric Arm Function Test (PAFT; Uswatte 2012a)
School Function Assessment (SFA; Sakzewski 2007)
Besta Scale (Rosa‐Rizzotto 2014)
Video Observations Aarts and Aarts (VOAA‐DD; Aarts 2007; Aarts 2009; Houwink 2013)
Alberta Infant Motor Scales (AIMS; Piper 1992)
Timing of outcome assessment
An additional objective for this review update was to examine the maintenance of effects of CIMT following intervention.
The primary endpoint was immediately following CIMT.
Due to variation in the timing of endpoints following CIMT, we categorised the secondary endpoints as follows.
Two weeks to four months following CIMT
Five to six months following CIMT
Seven to 12 months following CIMT
Main outcomes for 'Summary of findings' table
We selected the follow‐up period immediately postintervention as the time point for the 'Summary of findings' tables, as we considered this to be a time of peak effect for CIMT. Considering the available data and validity/reliability of outcome measures, two review authors (BH, MW) selected the following outcomes for inclusion through consensus.
Bimanual, measured by the Kids‐AHA (Holmefur 2007; Holmefur 2009; Holmefur 2016; Krumlinde‐Sundholm 2003; Krumlinde‐Sundholm 2007; Krumlinde‐Sundholm 2012)
Unimanual, measured by the Melbourne Assessment 2 (Randall 2008; Randall 2012) and the QUEST, Grasps domain (Thorley 2012)
Manual ability, measured by the ABILHAND‐Kids (Arnould 2004; Bleyenheuft 2017)
Self‐care, measured by the PEDI, Self‐Care Functional Skills domain (Feldman 1990; James 2014)
Individualised measures of performance, measured by the COPM (Carswell 2004; Cusick 2006; Cusick 2007).
Adverse events, as reported by trial authors
Search methods for identification of studies
We ran searches up to 2006 for the previous versions of this review (Hoare 2007a; Hoare 2007b). For this update, we revised the search strategy and searched some additional databases (Differences between protocol and review). We limited the updated searches to the period 2006 onwards.
Electronic searches
We searched the databases and trials registers listed below in September 2016 and March 2018. No language restrictions were applied to the search strategy. Search strategies used for this review update are reported in Appendix 1.
Central Register of Controlled Trials (CENTRAL; 2018, Issue 2), in the Cochrane Library (searched 26 March 2018).
MEDLINE Ovid (1946 to March week 3 2018).
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (searched 22 March 2018).
MEDLINE Epub Ahead of Print Ovid (searched 22 March 2018).
Embase Ovid (1974 to 21 March 2018).
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 22 March 2018).
PsycInfo Ovid (1967 to March week 2 2018).
Science Citation Index ‐ Extended Web of Science (1970 to 22 March 2018).
PEDro (www.pedro.org.au; searched 23 March 2018).
OTseeker (www.otseeker.com; searched 23 March 2018).
Cochrane Database of Systematic Reviews (CDSR; 2018, Issue 3), part of the Cochrane Library (searched 26 March 2018).
ClinicalTrial.gov (clinicaltrials.gov; searched 23 March 2018).
WHO International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en; searched 23 March 2018).
Australian New Zealand Clinical Trials Registry (ANZCTR; www.anzctr.org.au; searched 23 March 2018).
Searching other resources
We undertook the following, additional searches.
Conversations with colleagues and key authors in this field.
Searches of reference lists of relevant articles, systematic reviews and conference abstracts.
Forward and backward citation searches of included studies using Google Scholar (scholar.google.com.au).
-
Handsearching of the following key journals from 2007 to 2018:
Developmental Medicine and Child Neurology;
Physical and Occupational Therapy in Pediatrics;
Archives of Physical Medicine and Rehabilitation;
Journal of Child Neurology;
Journal of Rehabilitation Medicine;
Pediatric Physical Therapy;
American Journal of Occupational Therapy;
NeuroRehabilitation; and
Clinical Rehabilitation.
Google Scholar (scholar.google.com.au), using the search terms 'constraint therapy' and 'cerebral palsy'.
Data collection and analysis
Selection of studies
We managed all references generated by the search strategy using EndNote (EndNote). We eliminated duplicates. Two review authors (BH and MW) independently conducted an initial screening of titles and abstracts to exclude references that clearly did not meet the inclusion criteria (Criteria for considering studies for this review). Next, we obtained full‐text papers for those that provided insufficient information in the abstract to judge eligibility, and those that met the inclusion criteria. We linked multiple publications on the same study. Two review authors (BH and MW) independently evaluated the retrieved papers for relevance. We recorded the process in a PRISMA flow chart (Moher 2009); see Figure 1. We did not disagree on the inclusion/exclusion status of any abstract or article, therefore a third review author was not required. We applied no restrictions to language, date or status of publication. We sought assistance with translation, when necessary, from the Cochrane Developmental, Psychosocial and Learning Problems editorial team.
1.
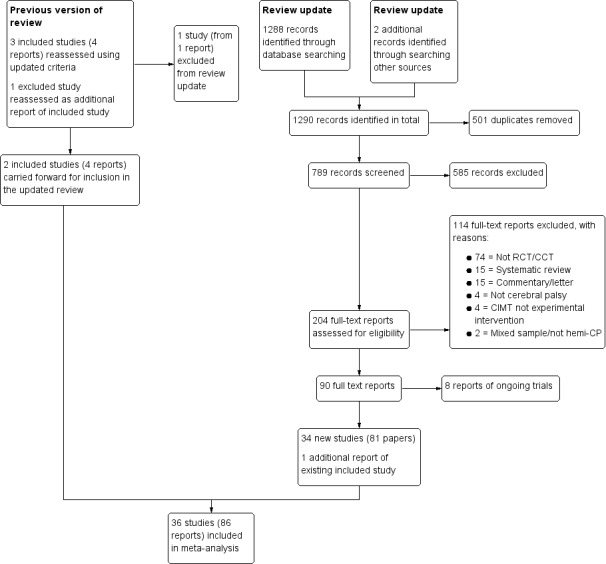
Study flow diagram
Data extraction and management
We tailored and updated the data extraction form to the requirements of this review. We piloted the form prior to commencing the original 2007 review (Hoare 2007a; Hoare 2007b). Five review authors (BH, MW, MJ, MT, CI) were paired, allocated included trials and independently extracted data from the included trials. We assembled and compared multiple publications of the same study to ensure completeness and to identify possible contradictions. If we identified contradictions, we sought additional information from the study authors. We extracted details on the study population, study environment, intervention, study methodology and outcomes of each study, to enable quality appraisal, evaluation of external validity and data analysis. Each pair of review authors resolved disagreements by discussion. We sought additional information from the study authors, if required. For cluster‐randomised trials, we extracted the number of clusters in the trial, the average size of clusters, the unit of randomisation, and the statistical methods used to analyse the trial. We also recorded estimates of the intra‐cluster correlation (ICC) coefficient for each outcome when they were reported.
Assessment of risk of bias in included studies
The pairs of review authors independently assessed the risk of bias of each trial, according to the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), and set out in Appendix 2, across the following domains: sequence generation; allocation concealment; blinding; incomplete outcome data; selective reporting; and other sources of bias. This assessment consisted of two parts: (1) a succinct description of the evidence used in making assignation of study quality for each domain, which included verbatim quotes from the paper or correspondence with the trial author(s), or a comment from the review author about procedures used to avoid bias, or both; and (2) an assessment of risk of bias (resulting in assignment of a judgement of ‘low’, ‘high’ or ‘unclear' risk of bias) for each of the domains. We contacted the trial authors for additional information if the publication did not provide adequate information to enable informed ratings. Discrepancies within the pairs were resolved by discussion. A third review author was consulted to resolve disagreement, if required. In the event that the review authors had undertaken the studies included in the review, independent review authors, who were not associated with these studies, extracted the data, assessed the risk of bias and populated the 'Risk of bias' tables.
Measures of treatment effect
Continuous data
We followed the Cochrane Handbook for Systematic Reviews of Interventions preferred method for handling continuous variables (Deeks 2011) and methods used in the original review (Hoare 2007a; Hoare 2007b). For primary outcomes, we assessed mean change scores and the standard deviation (SD) of the mean difference (MD), as opposed to comparing means and SD at specific time points. This approach considers differences in baseline performance, which is an issue for research involving small sample sizes and heterogeneous populations such as children with CP. We contacted the authors of included studies to obtain additional data to enable use of mean change scores for analysis, if required. When mean change scores and the SD of the MD were not available, we used the mean and SD at each time point (Deeks 2011). We used the MD and relevant 95% confidence intervals (CIs) when trials used the same rating scale or test to pool results across studies for an outcome. We used the standardised mean difference (SMD) and relevant 95% CI to pool trials that used different rating scales or tests.
Dichotomous data
No study included dichotomous data. We outline methods for handling dichotomous data in future updates of the review in the Differences between protocol and review section and Table 9.
5. Unused methods.
| Method | Approach |
| Measures of treatment effect |
Dichotomous data As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), we will report the odds ratio (OR) with a 95% confidence interval in future updates of this review, as most studies with a dichotomous outcome report the OR. |
| Unit of analysis issues |
Studies with multiple treatment groups If a trial includes three or more groups, we will consider the nature of the intervention and control arms, and, where appropriate, combine the data from two treatment arms that are similar and have the same control group, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c; Higgins 2011c). |
| Assessment of reporting biases | In future updates of this review, we will draw funnel plots from the outcome data and explore and discuss possible reasons for any visual asymmetry of the funnel plot (e.g. chance, publication bias or true heterogeneity) (Egger 1997). |
| Subgroup analysis | As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), in future updates of this review, we will explore the following characteristics using subgroup analyses when there are 10 or more studies for inclusion in a meta‐analysis.
|
| Sensitivity analysis | Where heterogeneity is substantial (I2 > 50%), we will explore the possible causes of heterogeneity in a sensitivity analysis, in which we will omit individual studies one at a time, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). |
CIMT: constraint‐induced movement therapy. hCMIT: hybrid CMIT. mCIMT modified CMIT. sCMIT: signature CIMT
Unit of analysis issues
Cross‐over trials
CIMT aims to have a lasting effect and we anticipated that effects would have carry‐over beyond a wash‐out period into the cross‐over period (Charles 2006). Therefore, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), we included data from the first intervention period only for RCTs using a cross‐over design (Eliasson 2011; Smania 2009; Taub 2004).
Cluster‐randomised trials
For cluster‐randomised trials that were randomised using clusters, we extracted the number of clusters in the trial, the average size of clusters, and the unit of randomisation. Where possible, we documented the statistical methods used to analyse the trial. We examined the methods for adjustments for clustering or other covariates. Where study authors had adjusted results for clustering, we extracted means, SD, and the number of participants in each treatment group, and included these data in the meta‐analyses. Where study authors had not adjusted results for clustering, we followed the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Studies with multiple treatment groups
For multi‐arm trials we either selected one pair of interventions that most closely matched our inclusion criteria and excluded the others, or we grouped the data so the only difference between the groups was CIMT.
Dealing with missing data
We attempted to contact the trial investigators of included studies when there was incomplete reporting of data or additional data were required (e.g. requesting change data). We reported our correspondences, and outcomes, in the Characteristics of included studies tables. When authors of included studies were unable to provide additional data, we included all of the data that were available in the review. Where data such as SD were not available, we used the CI and group size to calculate a SD using the calculator and methods according to Higgins 2011c. We assessed the risk of bias arising from incomplete outcome data as part of the overall 'Risk of bias' assessment (Assessment of risk of bias in included studies).
Assessment of heterogeneity
We pooled study data in a meta‐analysis for outcomes with data from at least two homogenous studies (studies that investigated the effects of CIMT on similar populations and reported similar outcomes). We explored heterogeneity initially through visual exploration of the forest plots and considered the I2 statistic, which describes the percentage of variability in the effect estimates due to heterogeneity (Higgins 2002). In addition, we considered the Tau2 statistic for each meta‐analysis, and compared the magnitude of heterogeneity with the distribution values for general physical health and adverse event and pain and quality of life/functioning – nonpharmacologic (median = 0.050, 95% CI 0.00 to 4.00). We considered heterogeneity in the meta‐analysis to be substantial when the Tau2 value was greater than 0.05 (Rhodes 2015).
Assessment of reporting biases
We considered the possible influence of publication and small study biases on review findings. In the current review, if we suspected or found direct evidence for selective outcome reporting, we contacted study authors for additional information.
Data synthesis
Comparisons of interest were CIMT versus low dose, high dose and dose‐matched, and CIMT other forms of CIMT. We did not pool data from these four comparisons together in a single meta‐analysis. We believe that the effect sizes for each of these comparisons are likely to vary considerably and that it is not theoretically justifiable to include interventions with vastly different treatment dosages in one comparison group. In the original 2007 review (Hoare 2007a; Hoare 2007b), we planned to calculate pooled effects using a fixed‐effect model across trials, using the same outcome in similar populations. However, due to the limited number of included trials, no pooled analyses were possible. For this update, we used a random‐effects model for each meta‐analysis, as we could not assume the effects being estimated in the different studies were identical due to the nature of CIMT provided (e.g. difference in treatment dosage, restraint type etc.) (DerSimonian 1986). We considered separate meta‐analyses for the timing of follow‐up, including immediately postintervention (zero to two weeks), two weeks to four months, five to six months, and seven to 12 months following CIMT. For several outcomes we were not able to pool data in a meta‐analysis because data were only available from a single study or change from baseline data were not available. For these studies, we presented data (mean with SD, or mean difference (MD) with 95% CI) from the CIMT and comparison groups in tables, for a narrative description of the results.
Two review authors (BH, MW) used the GRADE approach to assess the quality of the body of evidence for each outcome in each comparison (Guyatt 2008). We reported our GRADE ratings for all outcomes for comparisons of CIMT versus low dose, CIMT versus high dose and CIMT versus dose‐matched, and a comparison of different forms of CIMT in the Effects of interventions section. We also presented GRADE ratings for outcomes where there were sufficient data to conduct meta‐analyses for comparisons in 'Summary of findings' tables, which we constructed using GRADEpro (GradePro GDT 2015; Schünemann 2013). Consistent with criteria applied by (Ryan 2017), and to ensure consistency of GRADE judgements, we applied the criteria below for all key comparisons.
Limitations of studies: downgrade once if less than 75% of included studies are at low risk of bias across all 'Risk of bias’ domains.
Inconsistency: downgrade once if heterogeneity is statistically significant (P < 0.10) and I2 > 40%, or if data were from a single study only.
Indirectness: downgrade once if more than 50% of the participants are outside the target group.
Imprecision: downgrade once if fewer than 400 participants for continuous data and fewer than 300 events for dichotomous data (Guyatt 2011).
Publication bias: downgrade where there is direct evidence of publication bias.
We summarised the adverse events in Table 8.
Subgroup analysis and investigation of heterogeneity
We were unable to conduct any subgroup analyses due to the small number of studies in each comparison. These have been archived in Table 9 for use in future updates of this review, should data permit.
Sensitivity analysis
We assessed the influence of our analysis model by re‐analysing data using a fixed‐effect model instead of a random‐effects model for all outcomes included in a pooled analyses, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions section (Sterne 2011).
Results
Description of studies
Results of the search
For the previous version of this review (Hoare 2007a; Hoare 2007b), we screened 214 references and identified three included studies. The database searches for this update found 1288 records; we found two additional records by searching Google Scholar. After removing obvious duplicates, we screened the titles and abstracts of 789 records. Of these, we excluded 585 irrelevant records and obtained 204 full‐text reports for further scrutiny. Two review authors (BH, MW) independently examined the full‐text versions and agreed to include 34 new studies (from 81 reports) of sCIMT, mCIMT, hybrid therapy or forced use, plus one additional report of a study already included, making a total of 36 included studies from 86 reports. We also identified eight ongoing studies (Ongoing studies).
Four studies were published in Persian with English abstracts (Abootalebi 2010; Gharib 2010; Hosseini 2010; Sabour 2012). We later identified an English manuscript for Hosseini 2010. The remaining three studies were assessed and data extracted by two independent Persian speaking health professionals (Associate Professor Mehdi Rassafiani and Dr Fakher Rahim).
See Figure 1 for the study selection process.
Included studies
Three randomised or controlled clinical trials of CIMT, with a total of 70 participants, were included in the original review (Eliasson 2005; Sung 2005; Taub 2004). We retained two of these studies (Sung 2005; Taub 2004). We excluded the trial by Eliasson 2005 from this update as no randomisation was used and we did not consider the methods to meet the requirements for a controlled clinical trial as defined in Box 6.3.a of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). This review therefore includes 36 original and independent studies (Aarts 2010; Abd El‐Kafy 2014; Abootalebi 2010; Al‐Oraibi 2011; Charles 2006; Chen 2014; Choudhary 2013; Christmas 2018; de Brito Brandão 2010; DeLuca 2012; Deppe 2013; Dong 2017; Eliasson 2011; Eliasson 2018; Eugster‐Buesch 2012; Facchin 2011; Gelkop 2015; Gharib 2010; Gordon 2011; Hoare 2013; Hosseini 2010; Kirton 2016a (CIMT + r TMS); Rostami 2012a; Rostami 2012b; Sabour 2012; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Smania 2009; Sung 2005; Taub 2004; Taub 2011; Wallen 2011; Xu 2012; Yu 2012; Zafer 2016). The 36 trials included a total of 1264 participants and took place between 2004 and 2018. Details for each study are provided in Characteristics of included studies tables.
Design
Of the 36 included studies, 35 were randomised controlled trials (RCTs) and one was a cluster‐RCT (Facchin 2011). The study by Facchin 2011 included 105 participants across 21 rehabilitation sites where each participating clinical centre was randomised to one of three interventions (e.g. centre A was randomised to deliver mCIMT; centre D was randomised to deliver Bimanual Intensive Rehabilitation programme and so on). In this way, all children enrolled in a particular clinical centre participated in the intervention randomly assigned to that centre. The study authors report that no significant differences among inter‐ and intra‐cluster variabilities were observed in children enrolled in the trial. We therefore included the data in meta‐analyses.
Most trials compared two groups, that is, CIMT versus a comparison intervention. Three trials included a three‐group design (Dong 2017; Facchin 2011; Xu 2012) and two trials included a four‐group design (Kirton 2016a (CIMT + r TMS); Rostami 2012b).
One trial (Xu 2012) included three groups comparing mCIMT+Functional Electrical Stimulation (FES), mCIMT alone and occupational therapy (OT) alone. As the mCIMT+FES group combined two distinct interventions we did not consider this group to be sufficiently similar to the mCIMT alone group to be combined to create a single pair‐wise comparison. Therefore, we excluded this group from comparison and selected the groups that most closely matched our inclusion criteria (mCIMT alone and OT alone).
Facchin 2011 included three groups comparing mCIMT with a high‐dose, bimanual, intensive rehabilitation group and a low‐dose, traditional rehabilitation group. These groups were all deemed to meet our inclusion criteria and were analysed in separate analyses. Therefore, combining data from the two comparison groups was not required.
Rostami 2012b included a four‐group design including mCIMT+Virtual Reality (VR), VR alone, mCIMT alone and a low‐dose comparison. The nature of these interventions allowed CIMT to be isolated from co‐interventions across three comparisons. This included mCIMT(+VR) versus dose‐matched VR, mCIMT versus dose‐matched VR and mCIMT versus low‐dose usual care. No data were available for analysis however.
The study by Kirton 2016a (CIMT + r TMS) included a four‐group design comparing CIMT+ repetitive Transcranial Magnetic Stimulation (rTMS), intensive motor learning therapy + rTMS, CIMT+sham rTMS and intensive motor learning therapy+sham rTMS. The nature of these groups allowed CIMT to be isolated from co‐interventions across two comparisons: CIMT(+rTMS) versus dose‐matched intensive motor learning therapy (+rTMS) and CIMT(+ sham rTMS) versus dose‐matched motor learning (+ sham rTMS). To allow analysis of data from these two comparisons we set up two study IDs for this study. Kirton 2016a (CIMT + r TMS) examines the comparison of CIMT( + rTMS) versus dose‐matched intensive motor learning therapy (+ rTMS) and Kirton 2016b (CIMT + sham TMS) examines the comparison CIMT(+ sham) versus dose‐matched intensive motor learning therapy (+ sham).
The type of CIMT provided in the studies included the following.
Signature CIMT used in two studies (Kirton 2016a (CIMT + r TMS); Taub 2004).
Modified CIMT used in 24 studies (Abd El‐Kafy 2014; Al‐Oraibi 2011; Chen 2014; Choudhary 2013; Christmas 2018; Dong 2017; Eliasson 2011; Eliasson 2018; Eugster‐Buesch 2012; Facchin 2011; Gelkop 2015; Gordon 2011; Hoare 2013; Hosseini 2010; Rostami 2012a; Rostami 2012b; Sakzewski 2011; Sakzewski 2015b; Smania 2009; Sung 2005; Wallen 2011; Xu 2012; Yu 2012; Zafer 2016).
Hybrid CIMT used in 10 studies (Aarts 2010; Abootalebi 2010; Charles 2006; de Brito Brandão 2010; DeLuca 2012; Deppe 2013; Gharib 2010; Sabour 2012; Sakzewski 2015a; Taub 2011).
We identified no studies of forced‐use therapy alone. However, in 11 studies, children used constraints to limit less affected upper‐limb function for periods of time in addition to the times they were engaged in structured therapy (Abootalebi 2010; Christmas 2018; de Brito Brandão 2010; DeLuca 2012; Rostami 2012a; Rostami 2012b; Smania 2009; Sung 2005; Taub 2004; Taub 2011; Zafer 2016).
We classified the comparison groups as follows.
Low‐dose comparison used in 17 studies (Abootalebi 2010; Al‐Oraibi 2011; Charles 2006; Choudhary 2013; de Brito Brandão 2010; Dong 2017; Eliasson 2011; Eliasson 2018; Eugster‐Buesch 2012; Facchin 2011; Gharib 2010; Hosseini 2010; Rostami 2012b; Sabour 2012; Taub 2004; Taub 2011; Yu 2012).
High‐dose comparison used in five studies (Chen 2014; DeLuca 2012; Hoare 2013; Wallen 2011; Sakzewski 2015a).
Dose‐matched comparison used in 17 studies (Aarts 2010; Abd El‐Kafy 2014; Deppe 2013; Dong 2017; Facchin 2011; Gelkop 2015; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Rostami 2012a; Rostami 2012b; Sakzewski 2011; Sakzewski 2015b; Smania 2009; Sung 2005; Xu 2012; Zafer 2016).
Different form of CIMT used in three studies (Christmas 2018; DeLuca 2012; Rostami 2012a).
Of the 36 included trials, we were able to undertake 40 comparisons. Multiple comparisons were possible for three studies (Dong 2017; Facchin 2011; Rostami 2012b), due to multi‐group designs. The trial by Kirton 2016a (CIMT + r TMS) allowed two independent comparisons in the same comparison group (i.e. CIMT versus dose‐matched) (Kirton 2016a (CIMT + r TMS) and Kirton 2016b (CIMT + sham TMS)). We set up two study IDs to allow analysis of data from both comparisons: Kirton 2016a (CIMT + r TMS) examines the comparison of CIMT(+ rTMS) versus dose‐matched intensive motor learning therapy (+ rTMS), and Kirton 2016b (CIMT + sham TMS) examines the comparison CIMT(+ sham) versus dose‐matched intensive motor learning therapy (+ sham).
We undertook the following comparisons.
CIMT versus low dose (17 comparisons: Abootalebi 2010; Al‐Oraibi 2011; Charles 2006; Choudhary 2013; de Brito Brandão 2010; Dong 2017; Eliasson 2011; Eliasson 2018; Eugster‐Buesch 2012; Facchin 2011; Gharib 2010; Hosseini 2010; Rostami 2012b; Sabour 2012; Taub 2004; Taub 2011; Yu 2012).
CIMT versus high dose (four comparisons: Chen 2014; Hoare 2013; Sakzewski 2015a; Wallen 2011).
CIMT versus dose‐matched (16 comparisons (15 studies): Aarts 2010; Abd El‐Kafy 2014; Deppe 2013; Dong 2017; Facchin 2011; Gelkop 2015; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Rostami 2012b; Sakzewski 2011; Sakzewski 2015b; Smania 2009; Sung 2005; Xu 2012; Zafer 2016).
CIMT versus different form of CIMT (three comparisons: Christmas 2018; DeLuca 2012; Rostami 2012a).
Sample sizes
There was considerable variation in sample size between studies. The 36 included studies randomised 1264 participants with unilateral cerebral palsy (CP), with sample sizes ranging from 11 participants in Smania 2009 to 105 participants in Facchin 2011 (mean = 35; median = 31). Ten (28%) studies included sample sizes of fewer than 20 participants.
Participant characteristics
Across the 36 included studies, participant characteristics were inconsistently reported using data for either the whole sample or following dropout. Of the 1195 participants for whom data were reported, 633 (53%) were boys and 562 were girls. Eight studies did not report side of hemiplegia. For the remaining 28 trials, 471 participants (47%) had left hemiplegia and 529 right hemiplegia. One study did not report the age of participants (Sabour 2012). Of the remaining 35 studies, the mean age of participants was 5.96 years (SD 1.82), range three months to 19.8 years.
Twelve studies, including a total of 415 participants, classified children using the Manual Ability Classsification System (MACS) Eliasson 2006. Of the 425 children, 119 (28.6%) were classified at MACS I, 245 (59.1%) at MACS II, 49 (11.8%) at MACS III and 2 (0.05%) at MACS IV. Eight studies including a total of 383 participants classified children using the Gross Motor Function Classification System (GMFCS) Palisano 2008; 250 (65.3%) were classified at GMFCS I, 132 (34.5%) at GMFCS II and 1 at GMFCS III.
The most common criteria for inclusion of participants were active range of motion at the wrist/fingers in the more affected upper limb and adequate intellectual ability. Sixteen studies specified that participants required the ability to extend the wrist at least 20° and the fingers at least 10° from full flexion at the metacarpophalangeal joints (Abd El‐Kafy 2014; Abootalebi 2010; Charles 2006; Chen 2014; Deppe 2013; Dong 2017; Gelkop 2015; Gordon 2011; Hosseini 2010; Rostami 2012a; Rostami 2012b; Sabour 2012; Wallen 2011; Xu 2012; Yu 2012; Zafer 2016). A further six studies included only those children who could grasp or release with the more affected hand (Eugster‐Buesch 2012; Gelkop 2015; Gharib 2010; Sakzewski 2015b; Smania 2009; Hoare 2013). The study by Eliasson 2011 specifically included participants with any severity level of decreased hand function. In 16 studies, children needed to be able to follow simple or one‐stage commands (Abd El‐Kafy 2014; Abootalebi 2010; Choudhary 2013; de Brito Brandão 2010; DeLuca 2012; Dong 2017; Eliasson 2011; Eugster‐Buesch 2012; Gharib 2010; Hoare 2013; Rostami 2012a; Sakzewski 2011; Smania 2009; Wallen 2011; Xu 2012; Yu 2012). Two studies required participants to have normal intellectual function (Al‐Oraibi 2011; Gelkop 2015), and four studies specified children required an intellectual quotient (IQ) of > 70, measured using standardised assessment tools (Charles 2006; Gordon 2011; Hosseini 2010; Sabour 2012).
Twenty studies excluded participants if they had upper‐limb Botulinum toxin‐A injections in the six months prior to commencing CIMT (Abd El‐Kafy 2014; Abootalebi 2010; Charles 2006; Chen 2014; Choudhary 2013; de Brito Brandão 2010; DeLuca 2012; Deppe 2013; Dong 2017; Facchin 2011; Gelkop 2015; Gordon 2011; Hoare 2013; Rostami 2012a; Rostami 2012b; Sabour 2012; Sakzewski 2011; Sakzewski 2015b; Taub 2011; Xu 2012). Seventeen studies also excluded children who had recent or prior upper‐limb surgery (Abd El‐Kafy 2014; Abootalebi 2010; Charles 2006; Choudhary 2013; Deppe 2013; Eliasson 2011; Gharib 2010; Gordon 2011; Hoare 2013; Hosseini 2010; Rostami 2012a; Rostami 2012b; Sabour 2012; Sakzewski 2015a; Sakzewski 2015b; Sung 2005; Xu 2012). Studies also excluded participants due to current or uncontrolled seizures (14 studies), visual impairment (14 studies), muscle contractures or modified Ashworth Scale scores of > 3 (11 studies), or hearing impairment (four studies). Four studies did not report exclusion criteria (Al‐Oraibi 2011; Eugster‐Buesch 2012; Taub 2004; Xu 2012).
Location of studies
Studies were conducted across 19 countries. Five studies were conducted in Australia (Hoare 2013; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Wallen 2011) and five in the USA (Charles 2006; DeLuca 2012; Gordon 2011; Taub 2004; Taub 2011). Other countries with multiple studies included Iran (four studies: Abootalebi 2010; Gharib 2010; Hosseini 2010; Sabour 2012), Italy (two studies: Facchin 2011; Smania 2009), China (two studies: Dong 2017; Xu 2012), Korea (two studies: Sung 2005; Yu 2012), and Sweden (two studies: Eliasson 2011; Eliasson 2018). Single studies were completed in the Netherlands (Aarts 2010), Germany (Deppe 2013), Switzerland (Eugster‐Buesch 2012), Brazil (de Brito Brandão 2010), Canada (Kirton 2016a (CIMT + r TMS)), Jordan (Al‐Oraibi 2011), Egypt (Abd El‐Kafy 2014), Israel (Gelkop 2015), Taiwan (Chen 2014), India (Choudhary 2013) and Pakistan (Zafer 2016).
CIMT mode of delivery
Dosage of CIMT
See summary of CIMT dosage in Table 5.
When the total amount of CIMT was calculated (therapist‐led intervention + parent‐led intervention + other intervention (e.g. usual care) + forced use), the mean number of hours provided across included studies was 129 hours (range 20 hours (Yu 2012) to 504 hours (Christmas 2018; Sung 2005). When the forced use component was removed, the average total dosage was 79 hours (range six hours (Sung 2005) to 210 hours (Facchin 2011).
The average length of CIMT programs was five weeks, ranging from one week (Sakzewski 2015b) to 12 weeks (Eliasson 2018). The duration of daily intervention sessions ranged from 0.5 hours (Eliasson 2018; Sung 2005) to eight hours per day (Kirton 2016a (CIMT + r TMS)). Frequency of therapist‐ and/or parent‐led intervention sessions ranged from twice weekly (Smania 2009; Sung 2005) to seven days per week (Abootalebi 2010; Chen 2014; DeLuca 2012; Eliasson 2011; Eugster‐Buesch 2012; Gharib 2010; Hoare 2013; Wallen 2011).
All studies provided information on the amount of therapist‐led intervention provided. On average, 56 hours of CIMT was provided by therapists during a CIMT program (range 0 to 126 hours). In three studies, implementation of CIMT was parent‐led (Eliasson 2011; Eliasson 2018; Eugster‐Buesch 2012).
Nine studies did not provide information about if, or how much, parent‐led intervention was provided in the CIMT protocol (Abootalebi 2010; Al‐Oraibi 2011; Gharib 2010; Hosseini 2010; Rostami 2012b; Smania 2009; Sung 2005; Taub 2011; Yu 2012). Ten studies did not include parent‐led intervention sessions. Where reported, there was an average dosage of 34 hours of parent‐led intervention, ranging from 10 (Charles 2006; Kirton 2016a (CIMT + r TMS); Rostami 2012a; Xu 2012) to 152 hours (Hoare 2013).
In seven studies, usual care continued during the CIMT intervention period (Abootalebi 2010; Choudhary 2013; de Brito Brandão 2010; Eugster‐Buesch 2012; Gharib 2010; Rostami 2012b; Sabour 2012). Mean total dosage of other interventions across these studies was six hours, ranging from two hours (Eugster‐Buesch 2012) to 14 hours (Gelkop 2015).
CIMT protocols in 11 studies included forced use defined as use of a constraint outside of therapist‐ or parent‐led intervention (Abootalebi 2010; Christmas 2018; de Brito Brandão 2010; DeLuca 2012; Rostami 2012a; Rostami 2012b; Smania 2009; Sung 2005; Taub 2004; Taub 2011; Zafer 2016). The average total dose of forced use was 161 hours, ranging from 22 hours (Zafer 2016) to 498 hours (Sung 2005).
Type of constraint
A range of methods were used to constrain use of the less affected upper limb. The most common included a mitt/glove (Al‐Oraibi 2011; Chen 2014; Eliasson 2011; Eliasson 2018; Gelkop 2015; Hoare 2013; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Smania 2009; Wallen 2011), or a sling (Aarts 2010; Abd El‐Kafy 2014; Abootalebi 2010; Charles 2006; Choudhary 2013; de Brito Brandão 2010; Gordon 2011; Sabour 2012; Yu 2012; Zafer 2016). Each method was used in 11 studies. Seven studies used a splint (Dong 2017; Facchin 2011; Gharib 2010; Hosseini 2010; Rostami 2012a; Rostami 2012b; Xu 2012), seven used a cast (Christmas 2018; DeLuca 2012; Eugster‐Buesch 2012; Kirton 2016a (CIMT + r TMS); Sung 2005; Taub 2004; Taub 2011), and the remaining study used a bandage to fix the child's arm to their trunk (Deppe 2013).
Therapy provider
The delivery of CIMT was undertaken by a diverse range of therapists, parents, teachers or other interventionists. Most commonly, CIMT was delivered by a combination of therapists and parents (17 studies ‐ Aarts 2010; Abd El‐Kafy 2014; Abootalebi 2010; Al‐Oraibi 2011; Chen 2014; Choudhary 2013; Eliasson 2011; Eliasson 2018; Facchin 2011; Gharib 2010; Hoare 2013; Kirton 2016a (CIMT + r TMS); Taub 2004; Taub 2011; Wallen 2011; Xu 2012; Zafer 2016), followed by delivery by therapists alone (11 studies ‐ de Brito Brandão 2010; DeLuca 2012; Deppe 2013; Dong 2017; Gelkop 2015; Rostami 2012a; Rostami 2012b; Sabour 2012; Smania 2009; Sung 2005; Yu 2012), parents alone (one study ‐ Eugster‐Buesch 2012), therapist and interventionists (physiotherapists, students and volunteers, three studies ‐ Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b), or parents and unspecified interventionists ("trained interventionists", graduate and undergraduate students, teachers; three studies ‐ Charles 2006; Christmas 2018Gordon 2011).
Therapy location
Most often CIMT was delivered in clinical treatment centres (nine studies) (Aarts 2010; Chen 2014; Choudhary 2013; de Brito Brandão 2010; Deppe 2013; Rostami 2012b; Sabour 2012; Sung 2005; Yu 2012), or a combination of clinical treatment centres and home (eight studies) (Abd El‐Kafy 2014; Abootalebi 2010; Al‐Oraibi 2011; Facchin 2011; Gharib 2010; Hoare 2013; Wallen 2011; Xu 2012). Other treatment environments included home‐based (Eliasson 2018; Eugster‐Buesch 2012; Rostami 2012a; Taub 2004; Zafer 2016), home and community settings (Christmas 2018; DeLuca 2012; Taub 2011), home and pre‐school (Eliasson 2011), school (Dong 2017; Gelkop 2015), theme camps (Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b), and camps and home (Kirton 2016a (CIMT + r TMS)).
CIMT was most commonly delivered to children individually (21 studies) (Abd El‐Kafy 2014; Abootalebi 2010; Al‐Oraibi 2011; Christmas 2018; de Brito Brandão 2010; DeLuca 2012; Deppe 2013; Dong 2017; Eliasson 2011; Eliasson 2018; Eugster‐Buesch 2012; Facchin 2011; Gharib 2010; Hoare 2013; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sung 2005; Taub 2004; Taub 2011; Wallen 2011; Zafer 2016). Eleven studies implemented CIMT in group‐based models (Aarts 2010; Charles 2006; Chen 2014; Choudhary 2013; Gordon 2011; Sabour 2012; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Xu 2012; Yu 2012). Two studies combined both delivery methods (Gelkop 2015; Kirton 2016a (CIMT + r TMS)).
Twenty‐two studies reported the provision of home programs for implementation of CIMT. Ten studies reported no home program being provided (de Brito Brandão 2010; DeLuca 2012; Deppe 2013; Dong 2017; Sabour 2012; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Smania 2009; Sung 2005), and four studies did not specify whether a home program was provided (Gelkop 2015; Hosseini 2010; Rostami 2012b; Yu 2012).
Models of practice
Equal numbers of studies reported using shaping (11 studies) (Aarts 2010; Abd El‐Kafy 2014; Charles 2006; Chen 2014; Choudhary 2013; de Brito Brandão 2010; DeLuca 2012; Deppe 2013; Kirton 2016a (CIMT + r TMS); Taub 2004; Taub 2011) or motor learning theory (12 studies) (Eliasson 2011; Eliasson 2018; Facchin 2011; Gelkop 2015; Gordon 2011; Hoare 2013; Sabour 2012; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Smania 2009; Wallen 2011) to guide the implementation of CIMT. Other models of practice were described as fine/gross motor activities (seven studies) (Christmas 2018; Dong 2017; Rostami 2012a; Rostami 2012b; Sung 2005; Xu 2012) and motor training (Al‐Oraibi 2011). The model of practice was not described in four studies (Abootalebi 2010; Eugster‐Buesch 2012; Gharib 2010; Yu 2012).
Fidelity
Six studies provided a detailed description of the intervention model and implementation methods in published study protocols (Eliasson 2018; Facchin 2011; Hoare 2013; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b). Kirton 2016a (CIMT + r TMS) provided supplementary information detailing the intervention using the Template for Intervention Description and Replication (TIDieR) checklist and guide (Hoffmann 2014). We did not attempt to obtain unpublished intervention protocols from other studies. Only a single study (DeLuca 2012) reported methods to evaluate treatment fidelity. This involved the following: "The therapists in the study videotaped their intervention activities 3 times each week (for a total of 12 sessions) to evaluate treatment fidelity. They also maintained systematic daily treatment logs that included the specific skills and activities practiced, frequency of administration, any behavioral or logistical challenges encountered, and daily progress observed. The experienced clinical research staff at University of Alabama monitored fidelity by reviewing and analysing the videotapes and intervention logs using a fidelity checklist developed for the study" (Case‐Smith 2012, p 18/19).
Comparison interventions
Low‐dose comparison groups
Seventeen studies employed a low‐dose comparison intervention (Abootalebi 2010; Al‐Oraibi 2011; Charles 2006; Choudhary 2013; de Brito Brandão 2010; Dong 2017; Eliasson 2011; Eliasson 2018; Eugster‐Buesch 2012; Facchin 2011; Gharib 2010; Hosseini 2010; Rostami 2012b; Sabour 2012; Taub 2004; Taub 2011; Yu 2012). In most of these studies, insufficient information was provided about the specific nature of the intervention. Thirteen of these studies described the comparison intervention as occupational therapy, usual care or conventional/traditional therapy (Abootalebi 2010; Choudhary 2013; de Brito Brandão 2010; Dong 2017; Eliasson 2011; Eugster‐Buesch 2012; Facchin 2011; Gharib 2010; Rostami 2012b; Sabour 2012; Taub 2004; Taub 2011; Yu 2012); nine of which specified that intervention was delivered by occupational therapists (suggesting upper‐limb intervention was included). The remainder of the interventions were delivered by physiotherapists (n = 1) or did not specify the intervention providers. Other comparison interventions were described as neuro‐developmental therapy (NDT) (two studies: Al‐Oraibi 2011; Hosseini 2010) and infant massage (one study: Eliasson 2011). Most studies provided very few details of the nature of low‐dose comparison interventions. Insufficient information was given by Hosseini 2010 to name the low‐dose intervention.
The average total dose for the 13 studies which reported dosage information was 7.9 hours (range 0 to 16 hours). None of these studies, however, reported information about the dose of home program included in the intervention. Four studies did not specify intervention dosage (Eliasson 2011; Eliasson 2018; Eugster‐Buesch 2012; Hosseini 2010), and one specified that no comparison intervention was provided (Charles 2006). For 12 of the studies which provided information on intervention frequency, low‐dose interventions were carried out over two to 10 weeks with therapists from zero to seven days per week in sessions of 20 to 60 minutes per day. Three studies specified that no home program was included (Charles 2006; de Brito Brandão 2010; Dong 2017), two included a home program but gave no information on dose (Choudhary 2013; Eliasson 2018) and the remaining 12 studies did not mention the inclusion of a home program.
High‐dose comparison groups
Four studies employed a high‐dose comparison intervention (Chen 2014; Hoare 2013; Sakzewski 2015a; Wallen 2011). These interventions were intensive, individualised occupational therapy (Sakzewski 2015a; Wallen 2011), bimanual occupational therapy (Hoare 2013), or intensive traditional rehabilitation delivered by physiotherapists (Chen 2014).
The average total dose, including therapist delivered and home program hours for the four high‐dose comparison interventions was 37.5 hours (range 30 to 45 hours). These interventions were carried out with therapists over four to eight weeks, one to two days per week, in sessions of 45 minutes per day to four hours per day resulting in total, therapist delivered doses of eight hours to 30 hours. Three of the studies included a home program and specified total doses ranging from 16.2 to 36.8 hours (Hoare 2013; Sakzewski 2015a; Wallen 2011).
Dose‐matched comparison groups
Fifteen studies employed a high‐dose comparison intervention. The majority of these were described as either Hand Arm Bimanual Intensive Training (HABIT) (Gelkop 2015; Gordon 2011) or bimanual interventions (Deppe 2013; Facchin 2011; Sakzewski 2011; Sakzewski 2015b; Zafer 2016), or conventional care delivered by occupational therapists and/or physiotherapists (Aarts 2010; Abd El‐Kafy 2014; Smania 2009; Sung 2005; Xu 2012). One study each used “intensive motor therapy” (Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS)), virtual reality (Rostami 2012b) or “Remind to Move” (a wrist‐worn sensory cueing device to alert children to do customised movement tasks with the affected upper extremity) (Dong 2017).
The average total dose, including therapist delivered and home program hours for the 15 dose‐matched interventions was 71.4 hours (range six to 210 hours). This is lower than the dose we report for the dose‐matched CIMT interventions (129 hours) as the forced use component integral to several of the CIMT studies (for example, those using casting for 24 hours per day as a means of constraint) was factored into the average dose. Dose‐matched comparison interventions were carried out by therapists over one to 10 weeks, from one day per fortnight to six days per week, in sessions of 30 minutes per day to eight hours per day resulting in total doses of therapist guided intervention of two hours to 120 hours. Seven of nine studies which specified using a home program as part of the intervention reported total doses of home programs ranging from 10 to 120 hours.
Different form of CIMT comparison groups
Three studies employed a different form of CIMT as the comparison intervention. DeLuca 2012 used a high‐dose hCIMT intervention delivered three hours per day instead of six hours per day ‐ the form was otherwise identical. Rostami 2012a compared clinic‐based CIMT with home‐based CIMT delivered by an occupational therapist. More recently, Christmas 2018 compared prolonged constraint using a custom‐made semi‐rigid cast with intermittent hand holding.
The average total dose, including therapist‐delivered, forced use (restraint worn most of the waking day) and home program hours across the three studies which used a different form of CIMT as a comparison intervention was 91 hours (range 42 to 168 hours). In two of the studies, interventions were carried out with therapists, over two to three weeks, from five to seven days per week, in sessions of 90 minutes per day to three hours per day resulting in total doses of 15 to 63 therapist‐delivered hours. One study specified that no home program was included (DeLuca 2012) and the other study reported 101 hours of home program (Rostami 2012a). In the third study (Christmas 2018), hand holding was used as a form of restraint by families in usual settings for 42 hours, one hour per day, over three blocks of two weeks during in a 10‐week period.
Outcomes
We have summarised the included outcomes in Table 10. Excluded outcomes and reasons for exclusion are provided in Table 7.
6. Included outcomes.
| Outcomes | Measure | Study reported in |
| Primary outcomes | Bimanual | |
| Hand Assessment for Infants (HAI) ‐ Both hands score | Eliasson 2018 | |
| Kids‐Assisting Hand Assessment (Kids‐AHA) | Aarts 2010; Al‐Oraibi 2011; Christmas 2018; DeLuca 2012; Deppe 2013; Eliasson 2011; Eliasson 2018; Gelkop 2015; Gordon 2011; Hoare 2013; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Wallen 2011 | |
| Unimanual | ||
| Melbourne Assessment (Melbourne Assessment of Unilateral Upper Limb Function or Melbourne Assessment 2) | Aarts 2010; Deppe 2013; Eugster‐Buesch 2012; Sakzewski 2011; Sakzewski 2015a: Sakzewski 2015b | |
| Hand Assessment for Infants (HAI) ‐ Unimanual score | Krumlinde‐Sundholm 2017 | |
| Box and Blocks Test | Sakzewski 2015a; Sung 2005; Yu 2012 | |
| Quality of Upper Extremity Skills Test (QUEST) (Dissociated movement domain) | Abd El‐Kafy 2014; Choudhary 2013; Christmas 2018; Facchin 2011; Gelkop 2015; Gordon 2011; Hoare 2013; Taub 2004 | |
| Quality of Upper Extremity Skills Test (QUEST) (Grasps domain) | Abd El‐Kafy 2014; Choudhary 2013; Christmas 2018; Facchin 2011; Gelkop 2015; Gordon 2011; Hoare 2013 | |
| Quality of Upper Extremity Skills Test (QUEST) (Weighbearing domain) | Abd El‐Kafy 2014; Choudhary 2013; Christmas 2018; Facchin 2011; Gelkop 2015 | |
| Quality of Upper Extremity Skills Test (QUEST) (Protective extension domain) | Abd El‐Kafy 2014; Choudhary 2013; Christmas 2018; Facchin 2011; Gelkop 2015 | |
| Shriner’s Hospital Upper Extremity Evaluation (SHUEE) | DeLuca 2012 (Data not available for either treatment group) | |
| Pediatric Motor Activity Log ‐ Revised; Uswatte 2012b version (version for which there is evidence for validity and reliability) | Taub 2011 | |
| Manual ability | ||
| ABILHAND‐Kids | Aarts 2010 | |
| Birmingham Bimanual Questionnaire | Christmas 2018 | |
| Children’s Hand‐use Experience Questionnaire (CHEQ) | Sakzewski 2015a (Effectiveness of grasp, Time to do task and Bothered scales only) | |
| Secondary outcomes | Individualised measures of performance | |
| Canadian Occupational Performance Measure (COPM) | Aarts 2010; Gordon 2011; Hoare 2013; Sakzewski 2011; Sakzewski 2015a: Sakzewski 2015b; Wallen 2011 | |
| Goal Attainment Scaling (GAS) | Aarts 2010; Gordon 2011; Hoare 2013; Wallen 2011 | |
| Self‐care | ||
| Pediatric Evaluation of Disability Inventory (PEDI ‐ Self‐Care Functional Skills domain) | de Brito Brandão 2010; Deppe 2013; Hoare 2013; Gordon 2011 | |
| Pediatric Evaluation of Disability Inventory (PEDI ‐ Self‐Care Caregiver Assistance domain) | de Brito Brandão 2010; Hoare 2013; Gordon 2011 | |
| Pediatric Evaluation of Disability Inventory ‐ Computer Adaptive Test (PEDI‐CAT) | Boyd 2017 (ongoing study) | |
| Functional Independence Measure for Children (WeeFIM) | Chen 2014; Sung 2005; Yu 2012 | |
| Body function | ||
| Grip strength (for example, Jamar Dynamometer) | Charles 2006; Sakzewski 2011; Xu 2012; Yu 2012 | |
| Modified Ashworth Scale (MAS): Elbow | Abootalebi 2010; Charles 2006; Hoare 2013; Taub 2011; Wallen 2011 | |
| MAS: Wrist | Abootalebi 2010; Charles 2006; Hoare 2013; Taub 2011; Wallen 2011; Xu 2012 | |
| 2‐point discrimination | Charles 2006; Sakzewski 2011 | |
| Passive Range of Motion (PROM) | Hoare 2013; Taub 2011; Hosseini 2010 | |
| Modified Tardieu Scale (MTS) | Hoare 2013; Sakzewski 2011; Wallen 2011 | |
| Participation | ||
| Children’s Assessment of Participation and Enjoyment (CAPE) | Sakzewski 2011 | |
| Assessment of Life Habits (LIFE‐H) |
Sakzewski 2011 Sakzewski 2015a |
|
| Quality of Life | ||
| Cerebral Palsy Quality of Life Questionnaire for Children (CP QOL‐Child/self‐report) | Sakzewski 2011 | |
| Cerebral Palsy Quality of Life Questionnaire for Children ‐ Caregiver Report (CP QOL‐Child/caregiver report) | Chen 2014; Sakzewski 2015a | |
| KIDSCREEN‐52 | Sakzewski 2011 | |
| Pediatric Quality of Life Inventory (PedsQL) ‐ various versions | Christmas 2018; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS) | |
| Parental and family measures | ||
| Parenting Sense of Competence Scale (PSCS) | Eliasson 2018 | |
| Other | ||
| Alberta Infant Motor Scales (AIMS) | Eliasson 2018 | |
| Besta Scale | Facchin 2011 | |
| Pediatric Arm Function Test (PAFT) | Abd El‐Kafy 2014; Taub 2011 | |
| School Function Assessment (SFA) | Sakzewski 2011 | |
| Video Observations Aarts and Aarts (VOAA‐DD) | Aarts 2010 | |
A total of 57 outcome measures were used across all included trials. Thirty (52%) of these measures were only used in a single trial. The mean number of outcomes used in each trial was four (range one to 14). The most commonly used measure was the Assisting Hand Assessment (AHA), which was used in 15 trials (Aarts 2010; Al‐Oraibi 2011; Christmas 2018; DeLuca 2012; Deppe 2013; Eliasson 2011; Eliasson 2018; Gelkop 2015; Gordon 2011; Hoare 2013; Kirton 2016a (CIMT + r TMS); Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Wallen 2011). We did not include data from five studies in any of the analyses for a combination of reasons: none of the included outcome measures possessed adequate reported validity or reliability (or both) for children with CP; standardised assessments were invalidated because the administration or scoring was adapted; and/or the data were not reported or made available (Abd El‐Kafy 2014; Hosseini 2010; Rostami 2012a; Rostami 2012b; Smania 2009).
Funding sources
Five studies failed to report on funding (Abd El‐Kafy 2014; Choudhary 2013; Gelkop 2015; Smania 2009; Yu 2012); two studies reported receiving no funding (Deppe 2013; Zafer 2016); for three studies we did not have a translation available to assess funding (Abootalebi 2010; Gharib 2010; Sabour 2012); 13 studies reported being funded by research councils (de Brito Brandão 2010 ; Charles 2006; Chen 2014; Christmas 2018; Eliasson 2011; Eliasson 2018; Gordon 2011; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Taub 2004; Taub 2011; Wallen 2011); eleven studies reported being funded by the host institution (Al‐Oraibi 2011; de Brito Brandão 2010; DeLuca 2012; Dong 2017; Facchin 2011; Hoare 2013; Hosseini 2010; Rostami 2012a; Rostami 2012b; Sung 2005; Xu 2012); two studies were funded by charitable foundations (Aarts 2010; Eugster‐Buesch 2012); and two studies reported multiple sources of funding (Eliasson 2018; Kirton 2016a (CIMT + r TMS)).
Excluded studies
We excluded an additional 114 studies in this update, making a total of 136 excluded studies in this review. See Characteristics of excluded studies tables. We excluded studies because they: were not randomised or controlled trials (92 studies); were systematic or narrative reviews (16 studies); were commentaries or letters (16 studies); did not include participants with CP (five studies); the samples were not diagnosed with unilateral CP or had mixed diagnoses (two studies); or did not evaluate CIMT (four studies). We elaborate on the reasons for exclusion for several studies here.
Eliasson 2005 was included in the previous version of this review (Hoare 2007a; Hoare 2007b); however this study did not use a randomisation method for group allocation so was excluded in this update.
Gordon 2008 was excluded as it used quasi randomisation.
Lin 2011 was excluded as children with hemiplegic or quadriplegic CP were included and data were not reported separately.
Gillick 2010; Gillick 2014 and Gillick 2018 were excluded as CIMT could not be isolated as defining the intervention group from the comparison group. These studies examined the effects of repetitive transcranial magnetic stimulation (rTMS).
Klingels 2013 was excluded as it aimed to measure the additional effects of an intensive therapy program, not the effects of CIMT.
Vaghela Vishwas 2014 was considered for inclusion, however it could not be determined if the study used a randomised‐controlled design, and attempts to contact the authors for clarification were unsuccessful.
Ongoing studies
We identified eight ongoing studies; these are described in the Characteristics of ongoing studies tables. No data from these studies have been included in the review.
One ongoing study, which we categorised as CIMT compared with a low‐dose intervention, included infants randomised to a waiting‐list control group compared with 28 days of CIMT combined with sensory kit and reach training (Chorna 2015).
We categories four studies as dose‐matched. The study by Boyd 2017 and colleagues randomised infants aged three to nine months to infant‐friendly, parent‐delivered CIMT or a dose‐matched bimanual intervention. The Chamudot 2016 study randomised infants to home‐based intervention, with or without constraint. NCT02918890 is comparing 90 hours of CIMT with 90 hours Hand Arm Bimanual Intensive Training (HABIT). NCT02346825 is also recruiting infants and has three intervention groups: 1) intensive plus cast (continuous constraint); 2) intensive plus splint (part‐time constraint); and 3) intensive and no constraint with the following comparisons: intensive plus splint (part‐time constraint) versus intensive and no constraint (dose‐matched category); intensive plus cast (continuous constraint) versus intensive and no constraint (high‐dose comparison); and intensive plus cast (continuous constraint) versus intensive plus splint (part‐time constraint) (‘Other form of CIMT’).
We categorised two studies as ‘Other form of CIMT’: NCT02875054 is comparing children participating in CIMT wearing a cast for either 24 hours or three hours per day, while NCT02840643 is comparing outcomes for children when equivalent doses of hCIMT are delivered in different orders (90 hours CIMT followed by 90 hours intensive bimanual hand therapy and vice versa).
The clinical trials registry for NCT02808195 provides insufficient information to categorise the study. This study is comparing upper‐limb training using CIMT versus a Kinect upper‐limb motor rehabilitation system.
All but two of these studies are using an assessment from the Assisting Hand Assessment (AHA) family and will contribute to the uniformity of data once included in this review. Additionally, the inclusion of studies of infants will extend the understanding of effects of CIMT to this younger age group.
Risk of bias in included studies
For details see Figure 2 and Figure 3.
2.
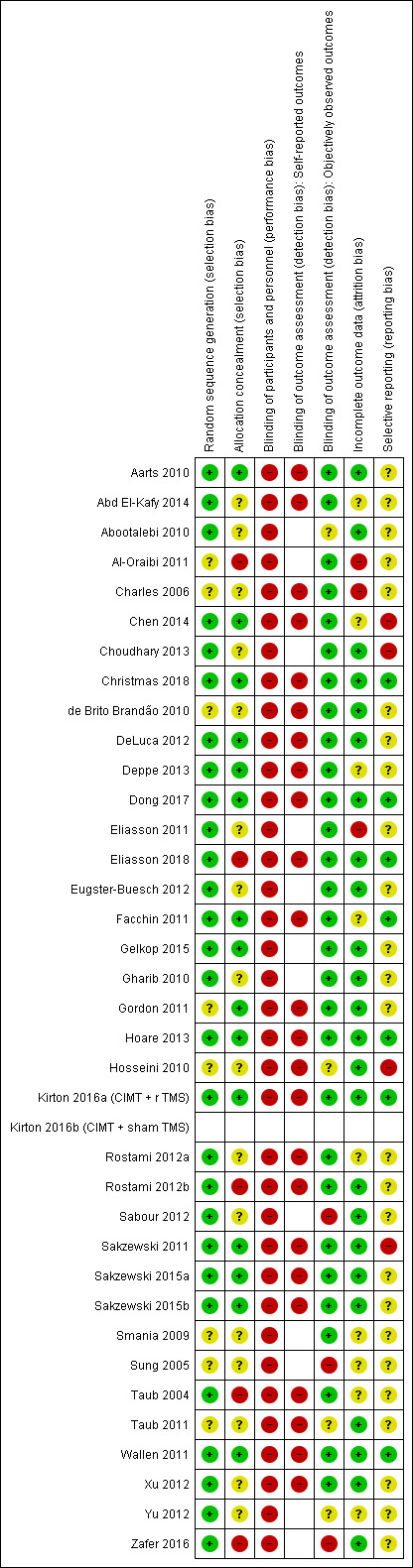
'Risk of bias' summary: Review authors' judgements about each 'Risk of bias' item for each included study. Note: Not all studies used self‐reported outcome measures, so a 'Risk of bias' rating could not be ascribed. This explains the absence of ratings for some of the studies. No ratings are entered for Kirton 2016b (CIMT + sham TMS), as it is the same study as Kirton 2016a (CIMT + r TMS), immediately above it in the 'Risk of bias' summary.
3.
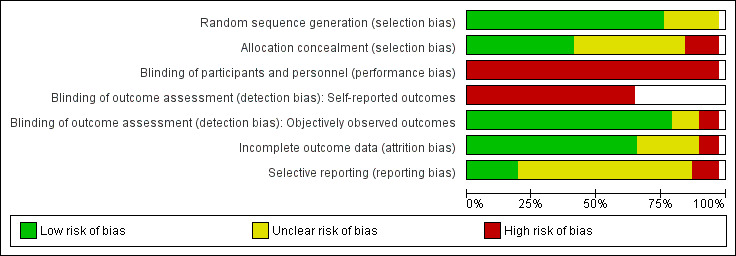
'Risk of bias' graph: Review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies. Note: Not all studies used self‐reported outcome measures, so a 'Risk of bias' rating could not be ascribed. This explains the absence of data in the corresponding domain in this graph. The total risk is < 100% because data were entered only once for Kirton 2016a (CIMT + r TMS) and Kirton 2016b (CIMT + sham TMS).
Allocation
Random sequence generation
We rated 28 studies (78%) that met the criteria for adequate random sequence generation at low risk of bias. Information for eight studies was unclear or we did not find sufficient information to permit judgement (Al‐Oraibi 2011; Charles 2006; de Brito Brandão 2010; Gordon 2011; Hosseini 2010; Smania 2009; Sung 2005; Taub 2011).
Allocation concealment
We rated 15 studies (41%) that used adequate methods to conceal the allocation sequence at low risk of bias. We rated 15 that did not provide enough information regarding the allocation concealment procedures at unclear risk of bias (Abd El‐Kafy 2014; Abootalebi 2010; Charles 2006; Choudhary 2013; de Brito Brandão 2010; Eliasson 2011; Eugster‐Buesch 2012; Gharib 2010; Hosseini 2010; Sabour 2012; Smania 2009; Sung 2005; Taub 2011; Xu 2012; Yu 2012) and a further five studies, which did not use adequate procedures for allocation concealment, at high risk of bias (Al‐Oraibi 2011; Eliasson 2018; Rostami 2012b; Taub 2004; Zafer 2016).
Blinding
Due to the overt nature of CIMT it was not possible to blind study participants, families and personnel from knowledge of which intervention a participant received. We therefore judged all 36 trials at high risk of performance bias.
Twenty‐four studies used self‐ or parent‐reported outcome measures (Aarts 2010; Abd El‐Kafy 2014; Charles 2006; Chen 2014; Christmas 2018; de Brito Brandão 2010; DeLuca 2012; Deppe 2013; Dong 2017; Eliasson 2018; Facchin 2011; Gordon 2011; Hoare 2013; Hosseini 2010; Kirton 2016a (CIMT + r TMS); Rostami 2012a; Rostami 2012b; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Taub 2004; Taub 2011; Wallen 2011; Xu 2012). Due to the overt nature of CIMT it was not possible to blind participants and proxies (parents) for these measures and we judged all 24 trials to be at high risk of performance bias.
All studies included observational‐based tests. Twenty nine studies (81%) reported blinding of outcome assessors. We rated these studies at low risk of bias. We judged four studies that did not provide sufficient information to permit judgement at unclear risk of bias (Abootalebi 2010; Hosseini 2010; Taub 2011; Yu 2012), and three studies that reported that the outcome assessors were not blinded at high risk of bias (Sabour 2012; Sung 2005; Zafer 2016).
Incomplete outcome data
We rated nine trials, which did not provide sufficient information about missing data, at unclear risk of bias (Abd El‐Kafy 2014; Chen 2014; Deppe 2013; Facchin 2011; Rostami 2012a; Smania 2009; Sung 2005; Taub 2004; Yu 2012). Additionally, three of these studies did not report information on dropouts or missing data (Rostami 2012a; Sung 2005; Taub 2004).
We rated three studies at high risk of bias due to high attrition rates (Al‐Oraibi 2011; Charles 2006; Eliasson 2011). The studies by Eliasson 2011 and Al‐Oraibi 2011 report attrition rates exceeding 20% and 30%, respectively, which were unbalanced across groups and contributed to high risk of bias ratings for this domain. A large proportion of the sample (33%) was not included in analysis in the study by Charles 2006. The attrition rates were unbalanced across groups and it is possible the attrition rates would affect outcomes.
Twenty‐four studies provided adequate information about missing data and all had rates less than 20% (Aarts 2010; Abootalebi 2010; Choudhary 2013; Christmas 2018; de Brito Brandão 2010; DeLuca 2012; Dong 2017; Eliasson 2018; Eugster‐Buesch 2012; Gelkop 2015; Gharib 2010; Gordon 2011; Hoare 2013; Hosseini 2010; Kirton 2016a (CIMT + r TMS); Rostami 2012b; Sabour 2012; Sakzewski 2011; Sakzewski 2015a; Sakzewski 2015b; Taub 2011; Wallen 2011; Xu 2012; Zafer 2016). We rated these studies at low risk of attrition bias. We also rated Gharib 2010, who reported a high rate of attrition (19.2%), which was balanced across groups and below the threshold of 20%, at low risk of bias.
Selective reporting
Five studies had published study protocols (Eliasson 2018; Facchin 2011; Hoare 2013; Sakzewski 2011; Sakzewski 2015a). Eleven studies had trial registrations (Charles 2006; Chen 2014; Christmas 2018; Dong 2017; Eliasson 2018; Gordon 2011; Hoare 2013; Kirton 2016a (CIMT + r TMS); Sakzewski 2011; Sakzewski 2015a; Wallen 2011). These allowed judgement of the completeness of reporting of the studies' pre‐specified outcomes. Three of these studies did not report data that were specified in the trial registration or protocol (Chen 2014; Choudhary 2013; Sakzewski 2011); we rated these studies at high risk of reporting bias. We also rated Hosseini 2010 at high risk of reporting bias as they did not report some of the outcomes specified in the manuscript.
We considered trials in which it was not possible to find any registry record or publicly available report as having insufficient information to permit judgement for this criteria. This included 25 (70%) studies (Aarts 2010; Abd El‐Kafy 2014; Abootalebi 2010; Al‐Oraibi 2011; Charles 2006; de Brito Brandão 2010; DeLuca 2012; Deppe 2013; Eliasson 2011; Eugster‐Buesch 2012; Gelkop 2015; Gharib 2010; Gordon 2011; Rostami 2012a; Rostami 2012b; Sabour 2012; Sakzewski 2015a; Sakzewski 2015b; Smania 2009; Sung 2005; Taub 2004; Taub 2011; Xu 2012; Yu 2012; Zafer 2016).
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Constraint induced movement therapy (CIMT) compared to low‐dose comparison for children with unilateral cerebral palsy.
| Constraint induced movement therapy compared to low‐dose comparison for children with unilateral cerebral palsy | ||||||
| Patient or population: children with unilateral cerebral palsy Setting: mixed (home, clinic, laboratory, pre‐school) Intervention: constraint induced movement therapy Comparison: low‐dose comparison | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with low‐dose comparison | Risk with constraint induced movement therapy | |||||
| Bimanual performance Assessed with: Kids‐Assisting Hand Assessment Scale from: 0 to 100 Follow‐up: immediately postintervention | The mean bimanual performance in the control groups ranged from 0.57 to 1.0 AHA units | The mean bimanual performance in the intervention groups was 5.44 AHA units higher (2.37 higher to 8.51 higher) |
‐ | 39 (2 RCTs)c | ⊕⊕⊝⊝ Lowa,b | Higher score indicates improved bimanual performance. |
| Unimanual capacity Assessed with: Melbourne Assessment Scale from: 0 to 100 Follow‐up: immediately postintervention | The mean unimanual capacity in the control group was−0.05 points | The mean unimanual capacity in the intervention group was 1.98 points higher (1.55 lower to 5.51 higher) | ‐ | 23 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | Higher score indicates improved unimanual capacity. |
| Unimanual capacity Assessed with: Quality of Upper Extremity Skills Test ‐ Grasps Scale from: 0 to 100 Follow‐up: immediately postintervention | The mean unimanual capacity in the control groups ranged from 0.9 to 2.5 points | The mean unimanual capacity in the intervention groups was 7.57 points higher (2.10 higher to 13.05 higher) | ‐ | 103 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,e | Higher score indicates improved unimanual capacity. |
| Manual ability ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies measured manual ability. |
| Self‐care ‐ not measured | See comment | See comment | ‐ | ‐ | See comment | No studies measured self‐care. |
| Individualised measures of performance ‐ not measured | See comment | See comment | ‐ | ‐ | See comment | No studies measured individualised performance. |
| Adverse events | The presence or absence of adverse events were not mentioned in 8/16 studies. | ‐ | 454 (16 RCTs) | ‐ | ||
| 3 studies reported 4 children were unable to tolerate constraint induced movement therapy | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias (all studies are at high risk of bias because it is not possible to blind personnel or participants to group allocation). bDowngraded one level due to small sample size (number of participants < 400). cDowngraded one level due to inconsistency (heterogeneity statistically significant: P < 0.10, I2 > 40%). dDowngraded one level because results are from a single study. eTrial by Choudhary 2013 was registered in Clinical Trials Registry of India. Register stated one of the outcomes was: “To assess parent's perception of improvement in upper extremity function after four weeks of therapy and eight week follow‐up, using parent questionnaire.” No parent perception data were reported. We did not downgrade the body of evidence for unimanual capacity based on this finding.
Summary of findings 2. Constraint induced movement therapy (CIMT) compared to high‐dose comparison for children with unilateral cerebral palsy.
| Constraint induced movement therapy compared to high‐dose comparison for children with unilateral cerebral palsy | ||||||
| Patient or population: children with unilateral cerebral palsy Setting: mixed (home, clinic, camp) Intervention: constraint induced movement therapy Comparison: high‐dose comparison | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with high‐dose comparison | Risk with constraint induced movement therapy | |||||
| Bimanual performance Assessed with: Assisting Hand Assessment‐Kids Scale from: 0 to 100 Follow‐up: immediately postintervention | The mean bimanual performance in the control groups ranged from0.8 to 7 AHA units | The mean bimanual performance in the intervention groups was 0.39 AHA units lower (3.14 lower to 2.36 higher) | ‐ | 126 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | Higher score indicates improved bimanual performance. |
| Unimanual capacity Assessed with: Melbourne Assessement Scale from: 0 to 100 Follow‐up: immediately postintervention | The mean unimanual capacity in the control group was 1.2 points | The mean unimanual capacity in the intervention group was 2 points lower (5.36 lower to 1.36 higher) | ‐ | 43 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | Higher score indicates improved unimanual capacity. |
| Unimanual capacity Assessed with: Quality of Upper Extremity Skills Test ‐ Grasp Scale from: 0 to 100 Follow‐up: immediately postintervention | The mean unimanual capacity in the control group was 3.31 points | The mean unimanual capacity in the intervention group was 0.2 points lower (11.84 lower to 11.44 higher) | ‐ | 34 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | Higher score indicates improved unimanual capacity. |
| Manual ability ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies measured manual ability. |
| Self‐care Assessed with: Pediatric Evaluation of Disability Inventory ‐ Self‐Care Functional Skills Domain Scale from: 0 to 73 Follow‐up: immediately postintervention | The mean self‐care in the control group was8.04 points | The mean self‐care in the intervention group was 1.52 points higher (3.1 lower to 6.14 higher) | ‐ | 34 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | Higher score indicates improved self‐care. |
| Individualised measures of performance Assessed with: Canadian Occupational Performance Measure ‐ Performance Scale from: 0 to 10 Follow‐up: immediately postintervention | The mean individualised measure of performance in the control groups ranged from 3.07 to 3.4 points | The mean individualised measure of performance in the intervention groups was 0.02 points lower (0.72 lower to 0.69 higher) | ‐ | 126 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | Higher score indicates improved parent‐rated occupational performance. |
| Adverse events | 3/4 studies reported no significant adverse events resulting from CIMT. | ‐ | 186 (4 RCTs) | ‐ | ||
| The remaining study reported 1 child receiving Hybrid CIMT had a seizure unrelated to intervention. Minor adverse events included frustrations and lack of acceptance of CIMT mitt (n = 6 children). | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due risk of bias (all studies are at high risk of bias because it is not possible to blind personnel or participants to group allocation). bDowngraded one level due to small sample size (number of participants < 400). cThe study protocol by Sakzewski 2015a was published and the study was retrospectively registered with ANZCTR. Secondary outcomes listed in the published protocol were not reported in the publication of study results including: Assessment of Life Habits (LIFE‐H) and Cerebral Palsy Quality of Life Questionnaire (self‐ and parent‐report). We did not downgrade the body of evidence for bimanual performance based on this finding. dDowngraded one level because results are from a single study.
Summary of findings 3. Constraint induced movement therapy (CIMT) compared to dose‐matched comparison for children with unilateral cerebral palsy.
| Constraint induced movement therapy compared to dose‐matched comparison for children with unilateral cerebral palsy | ||||||
| Patient or population: children with unilateral cerebral palsy Setting: mixed (home, clinic, pre‐school, laboratory, camp) Intervention: constraint induced movement therapy Comparison: dose‐matched comparison | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with dose‐matched comparison | Risk with constraint induced movement therapy | |||||
| Bimanual performance Assessed with: Assisting Hand Assessment ‐ Kids Scale from: 0 to 100 Follow‐up: immediately postintervention | The mean bimanual performance in the control groups ranged from 1.2 to 9.5 AHA units | The mean bimanual performance in the intervention groups was 0.8 AHA units higher (0.78 lower to 2.38 higher) | ‐ | 229 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | Higher score indicates improved bimanual performance. |
| Unimanual capacity Assessed with: Melbourne Assessment Scale from: 0 to 100 Follow‐up: immediately postintervention | The mean unimanual capacity in the control groups ranged from −0.8 to 7.1 points | The mean unimanual capacity in the intervention groups was 1.48 points higher (0.49 lower to 3.44 higher) | ‐ | 203 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | Higher score indicates improved unimanual capacity. |
| Unimanual capacity Assessed with: Quality of Upper Extremity Skills Test ‐ Grasp Scale from: 0 to 100 Follow‐up: immediately postintervention | The mean unimanual capacity in the control groups ranged from 3.7 to 10.8 points | The unimanual capacity in the intervention group was 6.63 points higher (2.38 lower to 15.65 higher) | ‐ | 124 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,d | Higher score indicates improved unimanual capacity. |
| Manual ability Assessed with: ABILHAND‐Kids Scale from: −10 to 10 Follow‐up: immediately postintervention | The mean manual ability in the control groups ranged from −0.08 to 0.22 logits | The mean manual ability in the interventions group was 0.52 logits higher (0.41 lower to 1.46 higher) | ‐ | 95 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,d | Higher score indicates improved manual ability. |
| Self‐care Assessed with: Pediatric Evaluation of Disability Inventory ‐ Self‐Care Functional Skills domain Scale from: 0 to 73 Follow‐up: immediately postintervention | The mean self‐care in the control groups ranged from 1.4 to 3.4 points | The mean self‐care in the intervention groups was 1.09 points lower (2.42 lower to 0.24 higher) | ‐ | 45 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Higher score indicates improved self‐care. |
| Individualised measures of performance Assessed with: Canadian Occupational Performance Measure ‐ Performance Scale from: 0 to 10 Follow‐up: immediately postintervention | The mean individualised measures of performance in the control groups ranged from 1.2 to 3.4 points | The mean individualised measures of performance in the intervention groups was0.08 points higher (1.29 lower to 1.46 higher) | ‐ | 191 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | Higher score indicates improved occupational performance. |
| Adverse events | 10/15 studies reported the presence or absence of adverse events. Of these, 7 studies reported no adverse events. Facchin 2011 specifically monitored changes on the less affected limb and found no detrimental effect following CIMT. Three studies reported minor adverse events including inability to tolerate CIMT (Dong 2017) and behavioural difficulties and resistance to wearing the mitt (Smania 2009). Kirton 2016a (CIMT + r TMS) reported 11% of children who received rTMS in conjunction with CIMT experienced headaches and < 3% reported tingling and nausea. | ‐ | 569 (15 RCTs) | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias (all trials are at high risk of bias because it is not possible to blind personnel or participants to group allocation). bDowngraded one level due to small sample size (number of participants < 400). cProtocol available for Sakzewski 2011. Neurovascular changes (functional Magnetic resonance imaging, functional connectivity), and brain (re)organisation (Transcranial Magnetic Stimulation) listed in protocol but not reported or addressed in the publications. We did not downgrade the body of evidence based on this finding. dDowngraded one level due to inconsistency (heterogeneity statistically significant: P < 0.10, I2 > 40%).
Summary of findings 4. Constraint induced movement therapy (CIMT) compared to different forms CIMT for children with unilateral cerebral palsy.
| Constraint induced movement therapy compared to different forms CIMT for children with unilateral cerebral palsy | ||||||
| Patient or population: children with unilateral cerebral palsy Setting: mixed (home, clinic) Intervention: Constraint induced movement therapy Comparison: different forms CIMT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with different forms constraint induced movement therapy | Risk with constraint induced movement therapy | |||||
| Bimanual performance Assessed with: Assisting Hand Assessment ‐ Kids Scale from: −10.26 to 8.72 Follow‐up: immediately postintervention | The mean bimanual performance in the control group was 0.84 AHA logits | The mean bimanual performance in the intervention group was 2.19 AHA logits higher (1.15 lower to 5.53 higher) | ‐ | 60 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c | Different scale units (logit scale and AHA unit scale) and different reporting (time point and change from baseline) precluded meta‐analysis. Higher score indicates improved bimanual performance |
|
Bimanual performance
assessed with: Assisting Hand Assessment ‐ Kids
Scale from: 0 to 100 Follow‐up: immediately postintervention |
The mean bimanual performance in the control group was 5.3 AHA units | The mean bimanual performance in the intervention group was3.70 AHA units higher (1.27 lower to 8.67 higher) | ‐ | |||
| Unimanual capacity ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies measured unimanual capacity using the Melbourne Assessment 2 |
|
Unimanual capacity
Assessed with: Quality of Upper Extremity Skills Test ‐ Grasp Scale from: 0 to 100 Follow‐up: immediately postintervention |
The mean unimanual capacity in the control group was −0.5 points | The mean unimanual capacity in the intervention group was 3.70 points higher (1.91 lower to 8.71 higher) | 60 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b,c | Higher score indicates improved bimanual performance | |
| Manual Ability ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies measured manual ability using the ABILHAND‐Kids |
| Self‐care ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies measured self‐care using the Pediatric Evaluation of Disability Inventory |
| Individualised measures of performance ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies measured individual performance using the Canadian Occupational Performance Measure |
| Adverse events | 2 studies reported no adverse events | ‐ | 94 (3 RCTs) | ‐ | ||
| 1 study did not report the presence or absence of adverse events | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias (all trials are at high risk of bias because it is not possible to blind personnel or participants to group allocation). bDowngraded one level because results are from a single study. cDowngraded one level due to small sample size (number of participants < 400).
In the following sections, and for each comparison category describing the effects of intervention, we first present findings for primary outcomes from pooled results for each available outcome at each time point, and then describe findings from single studies where no data were available to pool from other studies. This information is then presented for secondary outcomes. Note, we do not mention outcome measures and time points if there were no available data for these variables.
A summary of the quality of evidence is provided for outcomes included in the 'Summary of findings' tables both below and in the 'Summary of findings' tables.
1. CIMT versus a low‐dose comparison
Seventeen studies contributed to this comparison
Pooled results
Primary outcomes
Bimanual
We found evidence that CIMT is more effective than a low‐dose comparison for bimanual performance assessed with the KidsAHA (scale from 0 to 100) immediately postintervention (mean difference (MD) 5.44 AHA units, 95% confidence interval (CI) 2.37 to 8.51; 2 studies, 39 participants; zero heterogeneity: I2 = 0%, Tau2 = 0.00; Al‐Oraibi 2011; Eliasson 2011). See Analysis 1.1.
1.1. Analysis.
Comparison 1 CIMT versus a low‐dose comparison, Outcome 1 Assisting Hand Assessment.
Unimanual
We found evidence from three domains of the QUEST (dissociated movement, grasp, and protective extension ‐ each domain scores range from 0 to100) that CIMT is more effective than a low‐dose comparison both immediately postintervention, and at the two‐week to four‐month follow‐up period:
dissociated movement: immediately postintervention = MD 5.95, 95% CI 2.02 to 9.87; 3 studies, 121 participants; moderate to high heterogeneity: I2 = 43% (moderate), Tau2 = 4.94 (high); Choudhary 2013; Facchin 2011; Taub 2004; two‐week to four‐month follow‐up period = MD 5.80, 95% CI 2.29 to 9.31; 1 study, 31 participants; Choudhary 2013. See Analysis 1.2
grasp: immediately postintervention = MD 7.57, 95% CI 2.10 to 13.05; 2 studies, 103 participants; high heterogeneity: I2 = 66%, Tau2 = 10.35; Choudhary 2013; Facchin 2011; two‐week to four‐month follow‐up period = MD 6.50, 95% CI 2.03 to 10.97; 1 study, 31 participants; Choudhary 2013. Analysis 1.3
protective extension: immediately postintervention = MD 12.54, 95% CI 8.60 to 16.47; 2 studies, 103 participants; zero heterogeneity: I2 = 0%, Tau2 = 0.00; Choudhary 2013; Facchin 2011; two‐week to four‐month follow‐up period = MD 11.10, 95% CI 6.22 to 15.98; 1 study, 31 participants; Choudhary 2013. See Analysis 1.4.
1.2. Analysis.
Comparison 1 CIMT versus a low‐dose comparison, Outcome 2 Quality of Upper Extremity Skills Test (QUEST) ‐ Dissociated Movement.
1.3. Analysis.
Comparison 1 CIMT versus a low‐dose comparison, Outcome 3 QUEST ‐ Grasps.
1.4. Analysis.
Comparison 1 CIMT versus a low‐dose comparison, Outcome 4 QUEST ‐ Protective Extension.
In the remaining domain of the QUEST scale (weightbearing) CIMT appears to be more effective than a low‐dose comparison immediately postintervention (MD 5.92 points, 95% CI 2.21 to 9.63; 2 studies, 103 participants; zero heterogeneity: I2 = 0%, Tau2 = 0.00; Choudhary 2013; Facchin 2011), but not at the two‐week to four‐month follow‐up period (MD 4.50 points, 95% CI −1.55 to 10.55; 1 study, 31 participants; Choudhary 2013). See Analysis 1.5.
1.5. Analysis.
Comparison 1 CIMT versus a low‐dose comparison, Outcome 5 QUEST ‐ Weightbearing.
Manual ability
No study measure this outcome.
Adverse events
We were unable to conduct a meta‐analysis for this outcome.
Secondary outcomes
Individualised measures of performance
No study measured this outcome.
Self‐care
We were unable to conduct a meta‐analysis for this outcome.
Body function
In a meta‐analysis of two studies with 68 participants (Charles 2006; Dong 2017), we found no differences between the groups in grip strength at immediately postintervention (standardised mean difference (SMD) −0.14, 95% CI −0.61 to 0.34; heterogeneity: I2 = 0%, Tau2 = 0.00) and at the two‐week to four‐month follow‐up period (SMD −0.12, 95% CI −0.59 to 0.36; zero heterogeneity: I2 = 0%, Tau2 = 0.00). See Analysis 1.6.
1.6. Analysis.
Comparison 1 CIMT versus a low‐dose comparison, Outcome 6 Grip Strength.
We found no differences between the groups in passive resistance to stretch at the:
elbow assessed with the Modified Ashworth scale Elbow domain (scored from 0 to 4) immediately postintervention (MD 0.00, 95% CI −0.42 to 0.42; 2 studies, 33 participants; heterogeneity: I2 = 0%, Tau2 = 0.00); Abootalebi 2010; Charles 2006) and at the five‐ to six‐month follow‐up period (MD 0.32, 95% CI −0.43 to 1.07; 1 study, 22 participants); see Analysis 1.7; and
wrist assessed with the Modified Ashworth scale (scored from 0 to 4) immediately postintervention (MD 0.71, 95% CI −0.07 to 1.49; 2 studies, 34 participants; I2= 48%; Abootalebi 2010; Charles 2006) and at the two‐week to four‐month follow‐up period (MD 0.55, 95% CI −0.41 to 1.51; 1 study, 22 participants); see Analysis 1.8.
1.7. Analysis.
Comparison 1 CIMT versus a low‐dose comparison, Outcome 7 Modified Ashworth Scale (MAS) ‐ Elbow.
1.8. Analysis.
Comparison 1 CIMT versus a low‐dose comparison, Outcome 8 MAS ‐ Wrist.
Participation
No study measured this outcome.
Quality of life
No study measured this outcome.
Parenting and family measures
We were unable to conduct a meta‐analysis for this outcome.
Other
We were unable to conduct a meta‐analysis for any other outcome.
Single‐study results
Primary outcomes
Bimanual
Eliasson 2018 (27 participants) found no differences between the CIMT and low‐dose comparison groups in bimanual performance assessed with the AHA at the 18‐month follow‐up period (MD 17.16 AHA units, 95% CI −2.59 to 36.91). See Analysis 1.9.1
1.9. Analysis.
Comparison 1 CIMT versus a low‐dose comparison, Outcome 9 Data table.
| Data table | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period |
CIMT (mean) |
SD | N |
Low dose (mean) |
SD | N | Mean difference (95% CI) |
| Assisting Hand Assessment [AHA units] | ||||||||
| Eliasson 2018 | 18‐month follow‐up | 51.83 | 21.91 | 18 | 34.67 | 25.95 | 9 | 17.16 (−2.59 to 36.91) |
| Eliasson 2018 | ||||||||
| Hand Assessment for Infants ‐ Bimanual | ||||||||
| Eliasson 2018 | Baseline to immediately following intervention (change) | 10.09 | 8.21 | 18 | 4.82 | 10.17 | 13 | 5.27 (−1.43 to 11.97) |
| Eliasson 2018 | ||||||||
| Hand Assessment for Infants ‐ Unimanual | ||||||||
| Eliasson 2018 | Baseline to immediately following intervention (change) | 2.83 | 3.65 | 18 | 0.31 | −5.0 | 13 | 2.52 (−0.68 to 5.72) |
| Eliasson 2018 | ||||||||
| Melbourne Assessment | ||||||||
| Eugster‐Buesch 2012 | Baseline to immediately following intervention (change) | 1.93 | 4.86 | 12 | −0.05 | 3.74 | 11 | 1.98 (−1.55 to 5.51) |
| Eugster‐Buesch 2012 | Baseline to 2 weeks to 4 months post‐treatment (change) | 1.96 | 4.88 | 12 | 1.84 | 5.23 | 11 | 0.12 (−4.02 to 4.26) |
| QUEST ‐ Grasps | ||||||||
| Gharib 2010 | Baseline | 70.96 | 8.96 | 11 | 71.06 | 9.33 | 10 | |
| Gharib 2010 | Immediately following intervention (time point data) | 80.58 | 10.43 | 11 | 71.10 | 9.19 | 10 | 9.48 (1.09 to 17.87) |
| QUEST ‐ Dissociated Movement | ||||||||
| Gharib 2010 | Baseline | 77.09 | 10.09 | 11 | 75.04 | 11.99 | 10 | |
| Gharib 2010 | Immediately following intervention (time point data) | 83.77 | 6.13 | 11 | 77.68 | 10.90 | 10 | 6.09 (−1.58 to 13.76) |
| QUEST ‐ Weightbearing | ||||||||
| Gharib 2010 | Baseline | 82.43 | 15.54 | 11 | 76.72 | 8.02 | 10 | |
| Gharib 2010 | Immediately following intervention (time point data) | 86.85 | 14.13 | 11 | 78.24 | 7.29 | 10 | 8.61 (−0.88 to 18.10) |
| QUEST ‐ Protective extension | ||||||||
| Gharib 2010 | Baseline | 82.07 | 18.32 | 11 | 76.56 | 11.33 | 10 | |
| Gharib 2010 | Immediately following intervention (time point data) | 85.53 | 19.02 | 11 | 79.98 | 10.98 | 10 | 5.55 (−7.59 to 18.69) |
| Box and Blocks | ||||||||
| Yu 2012 | Baseline | 15.7 | 3.5 | 10 | 11.8 | 4.0 | 10 | |
| Yu 2012 | Immediately following intervention (time point) | 18.6 | 3.7 | 10 | 12.4 | 4.0 | 10 | 6.20 (2.82 to 9.58) |
| Pediatric Motor Activity Log ‐ Revised | ||||||||
| Taub 2011 | Baseline to immediately following intervention (change) | 2.2 | 0.5 | 10 | 0.1 | 0.3 | 10 | 2.10 (1.74 to 2.46) |
| Taub 2011 | ||||||||
| Pediatric Evaluation of Disability Inventory (PEDI): Self‐care ‐ Functional Skills domain | ||||||||
| de Brito Brandão 2010 | Baseline to immediately following intervention (change) | 6.68 | 6.16 | 8 | 1.04 | 3.02 | 7 | 5.64 (0.82 to 10.46) |
| de Brito Brandão 2010 | Baseline to 2 weeks to 4 months post‐treatment (change) | 9.57 | 3.99 | 8 | 2.7 | 2.41 | 7 | 6.87 (3.58 to 10.16) |
| PEDI: Self‐care ‐ Caregiver Assistance domain | ||||||||
| de Brito Brandão 2010 | Baseline to immediately following intervention (change) | 7.9 | 7.78 | 8 | −0.9 | 4.64 | 7 | 8.80 (2.41 to 15.19) |
| de Brito Brandão 2010 | Baseline to 2 weeks to 4 months post‐treatment (change) | 7.5 | 9.67 | 8 | 1.41 | 2.86 | 7 | 6.09 (−0.94 to 13.12) |
| Functional Independence Measure for Children (WeeFIM) ‐ Total Score | ||||||||
| Yu 2012 | Baseline | 71.5 | 11.2 | 10 | 70.3 | 11.6 | 10 | |
| Yu 2012 | Immediately following intervention (time point) | 74.9 | 10.4 | 10 | 71.9 | 11.4 | 10 | 3.00 (−6.56 to 12.56) |
| MAS ‐ Shoulder | ||||||||
| Abootalebi 2010 | Baseline to immediately following intervention (change) | 0.33 | 0.52 | 6 | 1.33 | 0.82 | 6 | −1.00 (−1.78 to −0.22) |
| Abootalebi 2010 | ||||||||
| Sabour 2012 | Baseline | 0.58 | 0.51 | 12 | 0.88 | 0.68 | 13 | |
| Sabour 2012 | Immediately following intervention (time point) | 0.50 | 0.52 | 12 | 1.15 | 0.62 | 13 | −0.65 (−1.10 to −0.20) |
| MAS ‐ Elbow | ||||||||
| Sabour 2012 | Baseline | 1.45 | 0.33 | 12 | 1.46 | 0.85 | 13 | |
| Sabour 2012 | Immediately following intervention (time point) | 1.54 | 0.33 | 12 | 1.53 | 0.74 | 13 | 0.01 (−0.43 to 0.45) |
| MAS ‐ Wrist | ||||||||
| Sabour 2012 | Baseline | 1.45 | 0.46 | 12 | 1.26 | 0.56 | 13 | |
| Sabour 2012 | Immediately following intervention (time point) | 1.33 | 0.38 | 12 | 1.11 | 0.58 | 13 | 0.22 (−0.16 to 0.60) |
| Grip strength | ||||||||
| Yu 2012 | Baseline | 9.0 | 3.3 | 10 | 10.3 | 3.3 | 10 | |
| Yu 2012 | Immediately following intervention (time point) | 10.5 | 3.6 | 10 | 10.5 | 3.3 | 10 | 0.00 (−0.88 to 0.88) |
| 2 point discrimination | ||||||||
| Charles 2006 | Baseline to immediately following intervention (change) | 0.91 | 1.64 | 11 | 1.29 | 2.05 | 11 | −0.38 (−1.93 to 1.17) |
| Charles 2006 | Baseline to 5 to 6 months postintervention (change) | 0.55 | 4.10 | 11 | −1.14 | 2.73 | 11 | 1.69 (−1.22 to 4.60) |
| Parenting Sense of Competence Scale (PSOC) ‐ Mother | ||||||||
| Eliasson 2018 | Baseline to immediately following intervention (change) | −1.31 | 7.18 | 16 | 0.00 | 5.72 | 13 | −1.31 (−6.01 to 3.39) |
| Eliasson 2018 | ||||||||
| PSOC ‐ Father | ||||||||
| Eliasson 2018 | Baseline to immediately following intervention (change) | 3.25 | −17.0 | 16 | −5.08 | 8.95 | 12 | 8.33 (−1.42 to 18.08) |
| Eliasson 2018 | ||||||||
| Besta Scale ‐ Global score | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | 0.23 | 0.39 | 39 | 0.06 | 0.35 | 33 | 0.17 (−0.00 to 0.34) |
| Facchin 2011 | ||||||||
| Besta Scale ‐ Grasp (affected side) | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | 0.30 | 0.57 | 39 | 0.06 | 0.45 | 33 | 0.24 (0.00 to 0.48) |
| Facchin 2011 | ||||||||
| Besta Scale ‐ Bimanual use | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | 0.24 | 0.56 | 39 | 0.16 | 0.39 | 33 | 0.08 (−0.14 to 0.30) |
| Facchin 2011 | ||||||||
| Besta Scale ‐ Activities of Daily Living (ADL) (2 to 6 years) | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | 0.22 | 0.47 | 28 | 0.05 | 0.50 | 24 | 0.17 (−0.10 to 0.44) |
| Facchin 2011 | ||||||||
| Besta Scale ‐ ADL (7 to 8 years) | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | −0.19 | 0.27 | 11 | 0.17 | 0.19 | 9 | −0.36 (−0.56 to −0.16) |
| Facchin 2011 | ||||||||
Eliasson 2018 (31 participants) also found no differences between the CIMT and low‐dose comparison groups in bimanual performance assessed with the HAI at immediately postintervention (P = 0.14). Our calculations using change‐from‐baseline data were consistent (MD 5.27 HAI units, 95% CI −1.43 to 11.97). See Analysis 1.9.2.
Unimanual
There was no clear difference between CIMT and low‐dose comparison groups (baby massage) for the more affected upper limb assessed using change from baseline to immediately postintervention on the HAI ‐ Unimanual assessment scale (MD 2.52 HAI units, 95% CI −0.68 to 5.72, 31 participants; Eliasson 2018). See Analysis 1.9.3.
Eugster‐Buesch 2012 found no difference between the CIMT and low‐dose comparison groups in unimanual capacity assessed with the Melbourne Assessment (scores range from 0 to 122) at any time point (post‐test: P = 0.30; two weeks: P = 0.19; three months: P = 0.96). Our calculations using change‐from‐baseline data were consistent (immediately postintervention: MD 1.98, 95% CI −1.55 to 5.51, 23 participants; two‐week to four‐month follow‐up period: MD 0.12, −4.02 to 4.26, 23 participants). See Analysis 1.9.4.
Gharib 2010 (21 participants) showed that CIMT is more effective than a low‐dose comparison for unimanual capacity assessed with the QUEST at immediately postintervention on the Grasp domain (scale from 0 to 100) (MD 9.48, 95% CI 1.09 to 17.87) but not on the Dissociated movement (MD 6.09, 95% CI −1.58 to 13.76), Weightbearing (MD 8.61, 95% CI −0.88 to 18.10) or Protective extension domains (MD 5.55, 95% CI −7.59 to 18.69). See Analysis 1.9.5 to Analysis 1.9.8).
Yu 2012 (20 participants) showed that CIMT is more effective than a low‐dose comparison for unimanual capacity assessed with the Box and Blocks Test (scored as the number of blocks grasped and moved to another spot) at immediately postintervention (P < 0.05; effect size and exact P value not reported). Using the postintervention means and SD provided for each group, our calculations were consistent: MD 6.20, 95% CI 2.82 to 9.58; Analysis 1.9.9).
Change‐from‐baseline data from Taub 2011 (20 participants) showed that CIMT is more effective than a low‐dose comparison for unimanual capacity assessed with the Pediatric Motor Activity Log‐Revised (PMAL‐R) at immediately postintervention. See Analysis 1.9.10.
Adverse events
See Table 8. Eight studies (Abootalebi 2010, Al‐Oraibi 2011, de Brito Brandão 2010, Gharib 2010, Hosseini 2010, Rostami 2012b, Sabour 2012, Yu 2012) did not mention the presence or absence of adverse events. Four children across three studies (Charles 2006, Dong 2017, Eliasson 2011) were unable to tolerate a constraint‐based intervention. In the study by Eugster‐Buesch 2012, parents were asked specifically about difficulties experienced with CIMT: 2/11 reported that children experienced frustration; 6/11 reported (unspecified) splint refusal; and 6/11 found completing the program exhausting. Two studies reported that children tolerated CIMT well (Choudhary 2013; Taub 2011), and three further studies specified that there were no major adverse events (Dong 2017; Eliasson 2018; Facchin 2011). Two studies monitored less affected hand use and, although noting no loss of movement or function from CIMT, reported minor and reversible skin irritations from casting (Eugster‐Buesch 2012; Facchin 2011).
Secondary outcomes
Self‐care
Change‐from‐baseline data from de Brito Brandão 2010 (15 participants) showed that CIMT is more effective than a low‐dose comparison for self‐care assessed with the Functional Skills domain (Scale from: 0 to 73) (MD 5.64, 95% CI 0.82 to 10.46) and Caregiver Assistance domains (MD 8.80, 95% CI 2.41 to 15.19) of the PEDI‐Self‐Care at immediately postintervention, and only the Functional Skills domain of the PEDI‐Self‐Care at the two‐week to four‐month follow‐up period (MD 6.87, 95%CI 3.58 to 10.16). See Analysis 1.9.11 and 1.9.12.
Yu 2012 (20 participants) showed that CIMT is more effective than a low‐dose comparison for self‐care assessed with the Functional Independence measure for children (WeeFIM scale from 18 to 126) ) at immediately postintervention (P < 0.05; effect size and exact P value not reported). Using postintervention means and SD provided for each group, we found no evidence of a difference between the groups: MD 3.00, 95% CI −6.56 to 12.56). See Analysis 1.9.13
Body function
Change‐from‐baseline data from Abootalebi 2010 (MD −1.00, 95% CI −1.78 to −0.22; 12 participants) and time‐point data from Sabour 2012 (MD −0.65, 95% CI −1.10 to −0.20; 25 participants) showed that CIMT was more effective than a low‐dose comparison at reducing muscle stiffness at the shoulder, as assessed with the MAS scale (scores from 0 to 4), at immediately postintervention. Using postintervention means and SD from Sabour 2012 (25 participants), we found no differences between the groups regarding elbow (MD 0.01, 95% CI −0.43 to 0.45) or wrist stiffness (MD 0.22, 95% CI −0.16 to 0.60). See Analysis 1.9.14 to Analysis 1.9.16.
Time‐point data from Yu 2012 (20 participants) showed no difference between the CIMT and low‐dose comparison groups for grip strength assessed using a hand dynamometer at immediately postintervention (MD 0.00kg, 95% CI −0.88 to 0.88). Similarly, change‐from‐baseline data from Charles 2006 (22 participants) found no evidence of a difference between the CIMT and low‐dose comparison groups for body function assessed by the two‐point discrimination test (patient perception of at immediately postintervention (MD −0.38 mm, 95% CI −1.93 to 1.17) or at the six‐month follow‐up period (MD −1.69 mm, 95% CI −1.22 to 4.60). See Analysis 1.9.17 and Analysis 1.9.18.
Parenting and family measures
Change‐from‐baseline data from Eliasson 2018 showed no differences between the CIMT and low‐dose comparison groups in parental competence assessed with the Parenting Sense of Competence Scale (PSCS; scale from 0 to 96; Eliasson 2018) immediately postintervention in mothers (MD −1.31 points, 95% −6.01 to 3.39; 29 mothers) or fathers (MD 8.33 points, 95% −1.42 to 18.08; 28 fathers). See Analysis 1.9.19 and 1.9.20.
Other
Change from baseline data from showed no differences between the CIMT and low‐dose comparison groups in any domain of the Besta scale (Affected limb function ‐ domains global, grasp, bimanual use, and activities for daily living See Analysis 1.9.21 to Analysis 1.9.24.
Sensitivity analysis
To assess the influence of our analysis model on the results, we repeated the pooled analyses for Bimanual capacity (AHA), Unilateral capacity (QUEST), and body function (Grip strength and MAS) using a fixed‐effect model instead of a random‐effects model. This had no impact on any outcome (analyses not shown), except MAS scores (scores from 0 to 4) at the wrist at immediately postintervention, where we found evidence that a low‐dose comparison was more effective than CIMT for reducing wrist muscle stiffness (MD 0.66, 95% CI 0.12 to 1.21; 2 studies; P = 0.16, I2 = 48%; Abootalebi 2010; Charles 2006; analysis not shown).
Quality of evidence for primary outcomes
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that CIMT is more effective than a low‐dose comparison for improving bimanual performance ‐ assessed using the Kids‐Assisting Hand Assessment (AHA) in children with CP at immediately postintervention. There is very low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for inconsistency, once for imprecision, once for publication bias) that CIMT is more effective than a low‐dose comparison for improving unimanual capacity measured using the QUEST ‐ Grasp domain at immediately postintervention and at the two‐week to‐ four‐month follow‐up period. There is very low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for inconsistency, once for imprecision) that CIMT is not more effective than a low‐dose comparison for improving unimanual capacity measured using the Melbourne Assessment. See Table 1.
2. CIMT versus high‐dose comparison
Pooled results
Primary outcomes
Bimanual
In a meta‐analysis of three studies (Hoare 2013; Sakzewski 2015a; Wallen 2011), we found no differences between the CIMT and high‐dose comparison groups in bimanual performance assessed with the Kids‐AHA (scale form 0 to 100) at immediately postintervention (MD −0.39 AHA units, 95% CI −3.14 to 2.36; 126 participants; moderate to high heterogeneity: I2 = 31% (moderate); Tau2 = 1.90 (high)), or at the two‐week to four‐month follow‐up period (MD −0.91 AHA units, 95% CI −5.06 to 3.23; 127 participants; high heterogeneity: I2 = 57%; Tau2 = 7.66). See Analysis 2.1.
2.1. Analysis.
Comparison 2 CIMT versus a high‐dose comparison, Outcome 1 Assisting Hand Assessment.
Unimanual
We were unable to conduct a meta‐analysis for this outcome.
Manual ability
No study measured this outcome.
Adverse events
We were unable to conduct a meta‐analysis for this outcome.
Secondary outcomes
Individualised measures of performance
In two separate meta‐analyses involving three studies (Hoare 2013; Sakzewski 2015a; Wallen 2011), we found no differences between the CIMT and high‐dose comparison groups for:
occupational performance ‐ assessed using the Canadian occupational performance measure (COPM) immediately postintervention (MD −0.02, 95% CI −0.72 to 0.69; 126 participants; low heterogeneity: I2 = 13%, Tau2 = 0.05; Analysis 2.2.1), and at the two‐week to four‐month follow‐up period (MD −0.22, 95% CI −0.87 to 0.43; 127 participants; zero heterogeneity: I2 = 0%, Tau2 = 0.00; Analysis 2.2.2); and
satisfaction with performance ‐ assessed using the COPM satisfaction scale at immediately postintervention (MD −0.33, 95% CI −1.22 to 0.55; 126 participants; low heterogeneity: I2 = 23%, Tau2 = 0.14; Analysis 2.3.1), and at the two‐week to four‐month follow‐up period (MD −0.21, 95% CI −1.24 to 0.82; 127 participants; moderate heterogeneity: I2 = 52%, Tau2 = 0.43; Analysis 2.3.2).
2.2. Analysis.
Comparison 2 CIMT versus a high‐dose comparison, Outcome 2 Canadian Occupational Performance Measure (COPM) ‐ Performance.
2.3. Analysis.
Comparison 2 CIMT versus a high‐dose comparison, Outcome 3 COPM ‐ Satisfaction.
There were no differences in the percentage of goals achieved at the 'expected', 'greater than expected' or 'much greater than expected' levels between groups receiving CIMT or a high‐dose comparison at immediately postintervention or at the two‐week to four‐month follow‐up period (Hoare 2013; Wallen 2011). See Table 11.
7. Outcomes from Goal Attainment Scaling.
| Study | Assessment period | CIMT | n | Comparison | n | Mean difference (95% CI) |
| CIMT versus high‐dose comparison | ||||||
| Hoare 2013 | Percentage of goals achieved at 'expected', 'greater than expected' or 'much greater than expected' level at immediately postintervention | 65% | 17 | 65% | 17 | NA |
| Percentage of goals achieved at 'expected', 'greater than expected' or 'much greater than expected' level at 2 weeks to 4 months postintervention | 67% | 17 | 61% | 17 | NA | |
| Wallen 2011 | Percentage of goals achieved at 'expected', 'greater than expected' or 'much greater than expected' level at immediately postintervention | 75% | 25 | 73% | 25 | NA |
| Percentage of goals achieved at 'expected', 'greater than expected' or 'much greater than expected' level at 2 weeks to 4 months postintervention | 84% | 25 | 81% | 25 | NA | |
| CIMT versus dose‐matched comparison | ||||||
| Aarts 2010 | Percentage of children that showed an increase of 2 points or more, compared with baseline, at immediately postintervention | 82% | 28 | 23% | 22 | NA |
| Percentage of children that showed an increase of 2 points or more, compared with baseline, at 2 weeks to 4 months postintervention | 86% | 28 | 36% | 22 | NA | |
| Gordon 2011 | Mean t score (SD) at immediately postintervention (time‐point data) | 51.0 (SD 7.47) |
21 | 59.1 (SD 7.69) |
21 | −8.10 (−12.69 to −3.51) |
| Mean t score (SD) at 2 weeks to 4 months postintervention (time‐point data) | 54.5 (SD 6.59) | 21 | 61.3 (SD 7.03) |
21 | −6.80 (−10.92 to −2.68) | |
| Mean t score (SD) at 6 months postintervention (time‐point data) | 59.0 (SD 7.03) | 21 | 63.8 (SD 7.25) |
21 | −4.80 (−9.12 to −0.48) | |
CI: confidence interval. CIMT: constraint‐induced movement therapy. SD: standard deviation.
Self‐care
We were unable to conduct a meta‐analysis for this outcome.
Body function
We were unable to conduct a meta‐analysis for this outcome as we could not pool the data using SMD. The study by Wallen 2011 reported R1 values while the study by Hoare 2013 calculated the R2 minus R1 differential. A higher R1 value suggests lower spasticity whereas a higher R2 − R1 differential suggests higher spasticity.
Participation
No study measured this outcome.
Quality of life
We were unable to conduct a meta‐analysis for this outcome.
Parenting and family measures
No study measured this outcome.
Other
No study measured any other outcomes.
Single‐study results
Primary outcome
Unimanual
Change‐from‐baseline data from Hoare 2013 (34 participants) showed no differences between the CIMT and high‐dose comparison groups in unimanual capacity assessed with the following two domains of the QUEST (scale 0 to 100 for both domains) at immediately postintervention and at the two‐week to four‐month follow‐up period:
dissociated movement (immediately postintervention: MD 0.49, 95% CI −10.71 to 11.69; two‐week to four‐month follow‐up period: MD −6.21, 95% CI −15.77 to 3.35; See Analysis 2.4.1); and
grasp (MD −0.20, 95% CI −11.84 to 11.44; two‐week to four‐month follow‐up period: MD 7.96, 95% CI −1.59 to 17.51; See Analysis 2.4.2).
2.4. Analysis.
Comparison 2 CIMT versus a high‐dose comparison, Outcome 4 Data table.
| Data table | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | CIMT(mean) | SD | N | High dose (mean) | SD | N | Mean difference[95% CI] |
| Quality of Upper Extremity Skills Test (QUEST) ‐ Dissociated Movement | ||||||||
| Hoare 2013 | Baseline to immediately following intervention (change) | 3.6 | 19.05 | 17 | 3.11 | 13.87 | 17 | 0.49 [‐10.71, 11.69] |
| Hoare 2013 | Baseline to 2 weeks to 4 months post‐intervention (change) | ‐2.43 | 17.51 | 17 | 3.78 | 9.88 | 17 | ‐6.21 [‐15.77, 3.35] |
| Hoare 2013 | ||||||||
| QUEST ‐ Grasps | ||||||||
| Hoare 2013 | Baseline to immediately following intervention (change) | 3.11 | 19.81 | 17 | 3.31 | 14.39 | 17 | ‐0.20 [‐11.84, 11.44] |
| Hoare 2013 | Baseline to 2 weeks to 4 months post‐intervention (change) | 8.58 | 8.84 | 17 | 0.62 | 18.05 | 17 | 7.96 [‐1.59, 17.51] |
| Hoare 2013 | ||||||||
| Melbourne Assessment | ||||||||
| Sakzewski 2015a | Baseline to immediately following intervention (change) | ‐1.1 | 5.5 | 24 | 1.2 | 5.2 | 18 | ‐2.30 [‐5.56, 0.96] |
| Sakzewski 2015a | Baseline to 2 weeks to 4 months post‐intervention (change) | ‐1.0 | 5.0 | 24 | 1.0 | 6.0 | 19 | ‐2.00 [‐5.36, 1.36] |
| Sakzewski 2015a | ||||||||
| Pediatric Evaluation of Disability Inventory (PEDI): Self‐care ‐ Functional Skills domain | ||||||||
| Hoare 2013 | Baseline to immediately following intervention (change) | 9.56 | 7.95 | 17 | 8.04 | 5.59 | 17 | 1.52 [‐3.10, 6.14] |
| Hoare 2013 | Baseline to 2 weeks to 4 months post‐intervention (change) | 10.35 | 7.0 | 17 | 12.19 | 8.28 | 17 | ‐1.84 [‐6.99, 3.31] |
| Hoare 2013 | ||||||||
| PEDI: Self‐care ‐ Caregiver Assistance domain | ||||||||
| Hoare 2013 | Baseline to immediately following intervention (change) | 9.43 | 10.8 | 17 | 9.09 | 9.27 | 17 | 0.34 [‐6.43, 7.11] |
| Hoare 2013 | Baseline to 2 weeks to 4 months post‐intervention (change) | 9.91 | 16.19 | 17 | 12.59 | 12.95 | 17 | ‐2.68 [‐12.54, 7.18] |
| Hoare 2013 | ||||||||
| Functional Independence Measure for Children (WeeFIM) | ||||||||
| Chen 2014 | Baseline to immediately following intervention (change) | 3.04 | 0.98 | 23 | 2.32 | 0.48 | 22 | 0.72 [0.27, 1.17] |
| Chen 2014 | Baseline to 2 weeks to 4 months post‐intervention (change) | 5.22 | 1.44 | 23 | 4.36 | 0.73 | 22 | 0.86 [0.20, 1.52] |
| Chen 2014 | Baseline to 5 to 6 months post‐intervention (change) | 7.26 | 2.03 | 23 | 6.0 | 1.11 | 22 | 1.26 [0.31, 2.21] |
| Modified Ashworth Scale (MAS) ‐ Elbow flexors | ||||||||
| Wallen 2011 | Baseline to immediately following intervention (change) | ‐0.16 | 0.85 | 25 | ‐0.06 | 0.91 | 25 | ‐0.10 [‐0.59, 0.39] |
| Wallen 2011 | Baseline to 2 weeks to 4 months post‐intervention (change) | ‐0.18 | 0.93 | 25 | ‐0.08 | 0.83 | 25 | ‐0.10 [‐0.59, 0.39] |
| Wallen 2011 | ||||||||
| MAS ‐ Wrist flexors | ||||||||
| Wallen 2011 | Baseline to immediately following intervention (change) | ‐0.2 | 0.62 | 25 | ‐0.04 | 0.75 | 25 | ‐0.16 [‐0.54, 0.22] |
| Wallen 2011 | Baseline to 2 weeks to 4 months post‐intervention (change) | 0.0 | 0.74 | 25 | 0.04 | 0.69 | 25 | ‐0.04 [‐0.44, 0.36] |
| Wallen 2011 | ||||||||
| Modified Tardieu Scale (MTS) ‐ Elbow flexors | ||||||||
| Hoare 2013 | Baseline to immediately following intervention (change) (R2 ‐ R1) |
‐15.9 | 35.16 | 17 | ‐21.77 | 36.87 | 17 | 5.87 [‐18.35, 30.09] |
| Hoare 2013 | Baseline to 2 weeks to 4 months post‐intervention (change) (R2 ‐ R1) |
‐10.29 | 46.15 | 17 | ‐3.53 | 48.31 | 17 | ‐6.76 [‐38.52, 25.00] |
| Hoare 2013 | ||||||||
| Wallen 2011 | Baseline to immediately following intervention (change) (R1 only) | 4.6 | 31.0 | 25 | ‐1.36 | 41.91 | 25 | 3.28 [‐19.68, 26.24] |
| Wallen 2011 | Baseline to 2 weeks to 4 months post‐intervention (change) (R1 only) | ‐0.52 | 37.15 | 25 | 1.32 | 49.71 | 25 | ‐1.84 [‐26.17, 22.49] |
| Wallen 2011 | ||||||||
| MTS ‐ Wrist flexors | ||||||||
| Hoare 2013 | Baseline to immediately following intervention (change) (R2 ‐ R1) |
‐2.94 | 9.85 | 17 | ‐12.65 | 23.92 | 17 | 9.71 [‐2.59, 22.01] |
| Hoare 2013 | Baseline to 2 weeks to 4 months post‐post‐intervention (change) (R2 ‐ R1) |
‐4.12 | 11.76 | 17 | ‐12.65 | 22.58 | 17 | 8.53 [‐3.57, 20.63] |
| Hoare 2013 | ||||||||
| Wallen 2011 | Baseline to immediately following intervention (change) (R1 only) | 10.36 | 23.64 | 25 | 0.32 | 29.31 | 25 | 10.04 [‐4.72, 24.80] |
| Wallen 2011 | Baseline to 2 weeks to 4 months post‐intervention (change) (R1 only) | 3.08 | 32.32 | 25 | ‐6.96 | 30.30 | 25 | 10.04 [‐7.33, 27.41] |
| Wallen 2011 | ||||||||
| Cerebral Palsy Quality of Life (CP QOL) (Proxy) ‐ Social Wellbeing and Acceptance | ||||||||
| Chen 2014 | Baseline to immediately following intervention (change) | 9.4 | 1.9 | 11 | 6.3 | 5.4 | 11 | 3.10 [‐0.28, 6.48] |
| Chen 2014 | Baseline to 2 weeks to 4 months post‐intervention (change) | 14.5 | 3.1 | 11 | 10.1 | 5.2 | 11 | 4.40 [0.82, 7.98] |
| Chen 2014 | ||||||||
| CP QOL (Proxy) ‐ Function | ||||||||
| Chen 2014 | Baseline to immediately following intervention (change) | 10.0 | 3.9 | 11 | 8.6 | 5.5 | 11 | 1.40 [‐2.58, 5.38] |
| Chen 2014 | Baseline to 2 weeks to 4 months post‐intervention (change) | 13.8 | 6.4 | 11 | 11.6 | 5.8 | 11 | 2.20 [‐2.90, 7.30] |
| Chen 2014 | ||||||||
| CP QOL (Proxy) ‐ Participation and Physical Health | ||||||||
| Chen 2014 | Baseline to immediately following intervention (change) | 8.3 | 5.4 | 11 | 8.7 | 4.9 | 11 | ‐0.40 [‐4.71, 3.91] |
| Chen 2014 | Baseline to 2 weeks to 4 months post‐intervention (change) | 11.7 | 8.0 | 11 | 12.1 | 5.3 | 11 | ‐0.40 [‐6.07, 5.27] |
| Chen 2014 | ||||||||
| CP QOL (Proxy) ‐ Emotional Wellbeing and Self‐esteem | ||||||||
| Chen 2014 | Baseline to immediately following intervention (change) | 10.2 | 3.8 | 11 | 8.5 | 5.5 | 11 | 1.70 [‐2.25, 5.65] |
| Chen 2014 | Baseline to 2 weeks to 4 months post‐intervention (change) | 14.8 | 4.5 | 11 | 12.5 | 5.0 | 11 | 2.30 [‐1.68, 6.28] |
| Chen 2014 | ||||||||
| CP QOL (Proxy) ‐ Pain and Impact of Disability (lower score = better) | ||||||||
| Chen 2014 | Baseline to immediately following intervention (change) | 11.9 | 3.1 | 11 | 10.2 | 6.4 | 11 | 1.70 [‐2.50, 5.90] |
| Chen 2014 | Baseline to 2 weeks to 4 months post‐intervention (change) | 18.6 | 10.1 | 11 | 14.3 | 6.0 | 11 | 4.30 [‐2.64, 11.24] |
| Chen 2014 | ||||||||
| CP QOL (Proxy) ‐ Access | ||||||||
| Chen 2014 | Baseline to immediately following intervention (change) | 9.5 | 2.9 | 11 | 8.9 | 5.3 | 11 | 0.60 [‐2.97, 4.17] |
| Chen 2014 | Baseline to 2 weeks to 4 months post‐intervention (change) | 14.5 | 6.4 | 11 | 11.6 | 7.0 | 11 | 2.90 [‐2.71, 8.51] |
| Chen 2014 | ||||||||
| CP QOL (Proxy) ‐ Family Health | ||||||||
| Chen 2014 | Baseline to immediately following intervention (change) | 10.8 | 4.5 | 11 | 9.9 | 5.0 | 11 | 0.90 [‐3.08, 4.88] |
| Chen 2014 | Baseline to 2 weeks to 4 months post‐intervention (change) | 14.5 | 2.1 | 11 | 12.8 | 1.7 | 11 | 1.70 [0.10, 3.30] |
| Chen 2014 | ||||||||
Sakzewski 2015a found no differences between the CIMT and high‐dose comparison groups in unimanual capacity assessed by change scores from baseline to immediately postintervention on the Melbourne Assessment scale (MD −2.30, 95% CI −5.56 to 0.96; 42 participants) and at the two‐week to four‐month follow‐up period ((MD −2.00, 95% CI −5.36 to 1.36; 43 participants). See Analysis 2.4.3.
Adverse events
See Table 8. Four studies reported on adverse events (Chen 2014; Hoare 2013; Sakzewski 2015a; Wallen 2011). Of these, two studies reported that some children experienced some frustration from participating in CIMT (Chen 2014; Wallen 2011), and two reported no adverse events related to CIMT (Hoare 2013; Sakzewski 2015a).
Single‐study results
Self‐care
Change‐from‐baseline data from Hoare 2013 (34 participants) showed no differences between the CIMT and high‐dose comparison groups on the PEDI Self‐care ‐ Functional skills (scale from 0 to 73) or Caregiver assistance domains at immediately postintervention (Functional skills: MD 1.52, 95% CI −3.10 to 6.14; Caregiver Assistance: MD 0.34, 95% CI −6.43 to 7.11) and at the two‐week to four‐month follow‐up period (Functional skills: MD −1.84, 95% CI −6.99 to 3.31; Caregiver assistance: MD −2.68, 95% CI −12.54 to 7.18). See Analysis 2.4.4 and Analysis 2.4.5.
Change‐from‐baseline from Chen 2014 (45 participants) showed evidence that CIMT was more effective than a high‐dose comparison on the WeeFIM (scale from 18 to 126) at immediately postintervention (MD 0.72 , 95% CI 0.27 to 1.17), at the two‐week to four‐month follow‐up period (MD 0.86, 95% CI 0.20 to 1.52) and the six‐month follow‐up period (MD 1.26, 95% CI 0.31 to 2.21). See Analysis 2.4.6.
Body function
Change‐from‐baseline data from Wallen 2011 (50 participants) showed no differences between the CIMT and high‐dose comparison groups in passive resistance to stretch assessed with the MAS at immediately postintervention or at the two‐week to four‐month follow‐up period for the elbow (immediately postintervention: MD −0.10, 95% CI −0.59 to 0.39; two‐week to four‐month follow‐up: MD −0.10, 95% CI −0.59 to 0.39; see Analysis 2.4.7) or the wrist (immediately postintervention: MD −0.16, 95% CI −0.54 to 0.22; two‐week to four‐month follow‐up period: MD −0.04, 95% CI −0.44 to 0.36; Analysis 2.4.8).
Using change‐from‐baseline means and SDs provided for each group by Wallen 2011 (50 participants), our calculations found no difference in R1 values between the CIMT and high‐dose comparison groups at immediately postintervention or at the two‐week to four‐month follow‐up period for elbow flexors measured using the Modified Tardieu scale (immediately postintervention: MD 3.28, 95%CI −19.68 to 26.24; two‐week to four‐month follow‐up: MD 1.84, 95% CI −26.17 to 22.49; see Analysis 2.4.9) or wrist flexors (immediately postintervention: MD 10.04, 95%CI − 4.72 to 24.8; two‐week to four‐month follow‐up: MD 10.04, 95% CI −7.33 to 27.41; see Analysis 2.4.10. This outcome was consistent for the results reported by Hoare 2013 (34 participants) using R2 minus R1 differential data.
Quality of life
Change‐from‐baseline data from Chen 2014 (22 participants) demonstrated no difference between the CIMT and high‐dose comparison groups at immediately postintervention on outcomes from all seven domains of the Cerebral Palsy Quality of Life (CP QOL) parent proxy version. At the two‐week to four‐month follow‐up, there was evidence that CIMT was more effective than a high‐dose comparison for the CP QOL parent proxy version Social well‐being and acceptance, and Family health domains, but there was no evidence of a difference in the remaining five domains (function, participation and physical health, emotional well‐being and self‐esteem, pain and impact of disability, access, and family health) See Analysis 2.4.11 to Analysis 2.4.17.
Sensitivity analysis
We repeated the pooled analyses for bimanual (assessed using AHA) and individualised measures of performance (assessed using Canadian Occupational Performance Measure; COPM) using a fixed‐effect model instead of a random‐effects model. Using a fixed‐effect model, we found no evidence to suggest that CIMT is more effective than a high‐dose comparison at immediately postintervention (MD 5.44 AHA units, 95% CI 2.37 to 8.51; 3 studies; P < 0.001; Hoare 2013; Sakzewski 2015a; Wallen 2011; analysis not shown), with no change in heterogeneity (I2 = 0%). At the two‐week to four‐month follow‐up period, a fixed‐effect model resulted in a change in the effect size, in favour of the high‐dose comparison, however the outcome was not significant (MD −1.47 AHA units, 95% CI −4.03 to 1.09; 3 studies; P = 0.10; I2 = 57%; Hoare 2013; Sakzewski 2015a; Wallen 2011; analysis not shown). A fixed‐effect model had no impact on occupational performance and satisfaction with performance assessed by the COPM at immediately postintervention and at the two‐week to four‐month follow‐up (analyses not shown).
Quality of evidence
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that CIMT is not more effective than a high‐dose comparison for improving bimanual performance in children with CP at immediately postintervention. There is very low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for inconsistency, once for imprecision) that CIMT is not more effective than a high‐dose comparison for improving unimanual capacity on the Melbourne Assessment and the QUEST ‐ Grasp domain. There is very low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for inconsistency, once for imprecision) that CIMT is not more effective than a high‐dose comparison for improving self‐care skills on the PEDI Self‐care ‐ Functional skills domain. There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that CIMT is not more effective than a high‐dose comparison for improving parent‐rated occupational performance assessed with COPM at immediately postintervention or at the two‐to‐four‐month postintervention period. See Table 2.
3. CIMT versus dose‐matched comparison
Pooled results
Primary outcomes
Bimanual
See Analysis 3.1. We found no differences between CIMT and dose‐matched comparison groups in bimanual performance assessed with the AHA (scale from 0 to 100) at:
3.1. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 1 Assisting Hand Assessment.
immediately postintervention (MD 0.80 AHA units, 95% CI −0.78 to 2.38; 6 studies (7 comparisons), 229 participants; low heterogeneity: I2 = 21%, Tau2 = 0.92; Aarts 2010; Gelkop 2015; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b);
two‐week to four‐month follow‐up period (MD 1.81 AHA units, 95% CI −0.10 to 3.73; 4 studies (5 comparisons), 149 participants; moderate to high heterogeneity: I2 = 22% (moderate), Tau2 = 1.06 (high); Aarts 2010; Gelkop 2015; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS))
five‐ to six‐month follow‐up period (MD −0.04 AHA units, 95% CI −1.56 to 1.49; 5 studies 4 studies (5 comparisons), 163 participants; low heterogeneity: I2 = 2%, Tau2 = 0.07; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b); or
the seven‐ to 12‐month follow‐up period (MD 0.70 AHA units, 95% CI −2.53 to 3.93; 1 study, 57 participants; Sakzewski 2011).
Unimanual
See Analysis 3.2.We found differences between CIMT and dose‐matched comparison groups in unimanual speed and dexterity assessed with the Box and Blocks test (scored as number of blocks transferred from one box to another within 60 seconds) at immediately postintervention (MD 1.11, 95% CI −0.06 to 2.28; 2 studies, 72 participants; low heterogeneity: I2 = 0%, Tau2 = 0.00; Sakzewski 2015a; Sung 2005), and at the two‐week to four‐month follow‐up (MD −0.10, 95% CI −3.66 to 3.46; 1 study, 41 participants; Sakzewski 2015a).
3.2. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 2 Box and Blocks Test.
See Analysis 3.3. We found no differences between CIMT and dose‐matched comparison groups in unimanual capacity assessed with the Melbourne Assessment (scale from 0 to 100) at:
3.3. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 3 Melbourne Assessment.
immediately postintervention (MD 1.48, 95% CI ‐0.49 to 3.44; 5 studies (6 comparisons), 203 participants; moderate to high heterogeneity: I2 = 33% (moderate), Tau2 = 1.95 (high); Aarts 2010; Deppe 2013; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b);
two‐week to four‐month follow‐up period (MD 1.36, 95% CI −1.28 to 4.00; 2 studies (3 comparisons), 95 participants; low heterogeneity: I2 = 0%, Tau2 = 0.00; Aarts 2010; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS)); and
the seven‐ to 12‐month follow‐up period (MD −1.00, 95% CI −4.39 to 2.39; 1 study, 57 participants; Sakzewski 2011).
However, we did find evidence that CIMT was more effective at improving unimanual uppe‐ limb function assessed with the Melbourne Assessment (scale from 0‐100) than a dose‐matched comparison at the five‐ to six‐month follow‐up period (MD 3.18, 95% CI 0.85 to 5.50; 3 studies (4 comparisons), 120 participants; low heterogeneity: I2 = 4%, Tau2 = 0.29; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b).
See Analysis 3.4. We found no differences between CIMT and dose‐matched comparison groups in unimanual capacity assessed with the Dissociated movement domain of the QUEST (scale from 0 to 100) at:
3.4. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 4 Quality of Upper Extremity Skills Test (QUEST) ‐ Dissociated Movement.
immediately postintervention (MD 6.51, 95% CI −0.74 to 13.76; 3 studies, 124 participants; Facchin 2011; Gelkop 2015; Gordon 2011);
two‐week to four‐month follow‐up period (MD 3.74, 95% CI −0.29 to 7.77; 2 studies, 52 participants; Gelkop 2015; Gordon 2011); and
five‐ to six‐month follow‐up period (MD 0.70, 95% CI −3.87 to 5.27; 1 study, 42 participants; Gordon 2011).
See Analysis 3.5. We found no differences between CIMT and dose‐matched comparison groups in unimanual capacity assessed with the Grasp domain of the QUEST (scale from 0 to 100) at:
3.5. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 5 QUEST ‐ Grasp.
immediately postintervention (MD 6.63, 95% CI −2.38 to 15.65; 3 studies, 124 participants; Facchin 2011; Gelkop 2015; Gordon 2011);
two‐week to four‐month follow‐up period (MD 1.18, 95% CI −5.12 to 7.49; 2 studies, 52 participants; Gelkop 2015; Gordon 2011); or
five‐ to six‐month follow‐up period (MD 1.70, 95% CI −6.32 to 9.72; 1 study, 42 participants; Gordon 2011).
Heterogenity was high for both domains of the QUEST at immediately postintervention (Dissociated movement: I2 = 86%. Tau2 = 34.44; Graps: I2 = 84%, 13%, Tau2 = 53.17).
We found no differences between CIMT and dose‐matched comparison groups in unimanual capacity assessed with the Weightbearing domain on the QUEST (scale from 0 to 100) at immediately postintervention (MD −2.31, 95% CI −8.02 to 3.40; 2 studies, 82 participants; zero heterogeneity: I2 = 0%, Tau2 = 0.00; Facchin 2011; Gelkop 2015), or at the two‐week to four‐month follow‐up period (MD 8.10, 95% CI −21.90 to 38.10; 1 study, 10 participants; Gelkop 2015). See Analysis 3.6.
3.6. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 6 QUEST ‐ Weightbearing.
We found evidence that CIMT was more effective than a dose‐matched comparison at improving unimanual capacity assessed with the Protective extension domain of the QUEST (scale from 0 to 100) at immediately postintervention (MD 6.86, 95% CI 0.14 to 13.58; 2 studies, 82 participants; low heterogeneity: 0%, Tau2 = 0.00; Facchin 2011; Gelkop 2015), but not at the two‐week to four‐month follow‐up period (MD 4.80, 95% CI −10.08 to 19.68; 1 study, 10 participants; Gelkop 2015). See Analysis 3.7.
3.7. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 7 QUEST ‐ Protective Extension.
Manual ability
A meta‐analysis of 2 studies (3 comparisons) involving 95 participants (Aarts 2010; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS)) found no differences between CIMT and dose‐matched comparison groups in manual ability assessed with the ABILHAND‐Kids (scale from ‐10 to 10) at immediately postintervention (MD 0.52, 95% CI −0.41 to 1.46; high heterogeneity: I2 = 74%, Tau2 = 0.47), or at the two‐week to four‐month follow‐up period (MD 0.06, 95% CI −0.51 to 0.62; low heterogeneity: I2 = 21%, Tau2 = 0.07). However, there was evidence that CIMT was more effective than a dose‐matched comparison at the five‐ to six‐month follow‐up period (MD 0.74, 95% CI 0.31 to 1.18; zero heterogeneity: I2 = 0%, Tau2 = 0.00; See Analysis 3.8.
3.8. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 8 Abilhand‐Kids.
Adverse events
We were unable to conduct a meta‐analysis for this outcome.
Secondary outcomes
Individualised measures of performance
See Analysis 3.9. We found no differences between CIMT and dose‐matched comparison groups for occupational performance assessed with the COPM (scale from 0 to 10) at:
3.9. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 9 Canadian Occupational Performance Measure (COPM) ‐ Performance.
immediately postintervention (MD 0.08, 95% CI −1.29 to 1.46; 5 studies (6 comparisons), 191 participants; high heterogeneity: I2 = 89%,Tau2 = 2.54; Aarts 2010; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b);
two‐week to four‐month follow‐up period (MD 0.55, 95% CI −1.45 to 2.55; 2 studies (3 comparisons), 95 participants; high heterogeneity: I2 = 88%, Tau2 = 2.71; Aarts 2010; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS));
five‐ to six‐month follow‐up period (MD −0.30, 95% CI −1.01 to 0.41; 3 studies (4 comparisons), 110 participants; zero heterogeneity: I2 = 0%, Tau2 = 0.00; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b); or
seven‐ to 12‐month follow‐up period (MD 0.10, 95% CI −0.83 to 1.03; 1 study, 57 participants; Sakzewski 2011).
See Analysis 3.10. We found no differences between CIMT and dose‐matched comparison groups in satisfaction with performance assessed with the COPM (scale from 0 to 10) at:
3.10. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 10 COPM ‐ Satisfaction.
immediately postintervention (MD 0.47, 95% CI −0.99 to 1.92; 5 studies (6 comparisons), 191 participants; high heterogeneity: I2 = 84%, Tau2 = 2.54; Aarts 2010; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b);
two‐week to four‐month follow‐up period (MD 1.10, 95% CI −0.24 to 2.43; 2 studies (3 comparisons), 95 participants; high heterogeneity: I2 = 60%, Tau2 = 0.84; Aarts 2010; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS));
five‐ to six‐month follow‐up period (MD 0.17, 95% CI −0.63 to 0.98; 3 studies (4 comparisons), 121 participants; zero heterogeneity: I2 = 0%, Tau2 = 0.00; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b); or
seven‐ to 12‐month follow‐up period (MD 0.90, 95% CI −0.31 to 2.11; 1 study, 57 participants; Sakzewski 2011).
Heterogeneity was high for both occupational performance and satisfaction with performance, especially at immediately postintervention, due to a much larger effect size in the Aarts 2010 study.
Self‐care
We found no differences between CIMT and dose‐matched comparison groups in self‐care ability assessed with PEDI Self‐care Functional skills (scale from 0‐73) domain at immediately postintervention (MD −1.09, 95% CI −2.42 to 0.24; 2 studies, 45 participants; low heterogeneity: I2 = 8%, Tau2 = 0.12; Deppe 2013; Gordon 2011; Analysis 3.11).
3.11. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 11 Pediatric Evaluation of Disability Inventory: Self‐care ‐ Functional Skills domain.
Body function
We found no differences between CIMT and dose‐matched comparison groups in grip strength of the impaired hand at:
immediately postintervention (SMD 0.16, 95% CI −0.13 to 0.46; 4 studies (5 comparisons), 194 participants; low heterogeneity: I2 = 7%, Tau2 = 0.01; Dong 2017; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Xu 2012);
the two‐week to four‐month follow‐up period (SMD 0.32, 95% CI −0.02 to 0.66; 3 studies (4 comparisons), 137 participants; zero heterogeneity: I2 = 0%, Tau2 = 0.00; Dong 2017; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Xu 2012);
the five‐ to six‐month follow‐up period (SMD 0.20, 95% CI −0.14 to 0.54; 3 studies (4 comparisons), 144 participants; low heterogeneity: I2 = 6%, Tau2 = 0.01; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Xu 2012); or
the seven‐ to 12‐month follow‐up period (SMD −0.02, 95% CI −0.61 to 0.57; 1 study, 44 participants; Sakzewski 2011)..
See Analysis 3.12.
3.12. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 12 Grip Strength (impaired hand).
Participation
We were unable to conduct a meta‐analysis for this outcome.
Quality of life
We found evidence from one study (45 participants) with two comparisons, Kirton 2016a (CIMT + r TMS) and Kirton 2016b (CIMT + sham TMS), that a dose‐matched comparison was more effective than CIMT for quality of life assessed with the Speech and Communication domain of the child‐reported Pediatric Quality of Life Inventory (PedsQoLTM) 3 Cerebral Palsy (CP) (scores on all dimensions from 0 to 100) Module at the five‐ to six‐month follow‐up period only (MD −13.50, 95% CI −24.94 to −2.06). This was not sustained at the seven‐ to 12‐month follow‐up period (MD −7.19, 95% CI −32.97 to 18.59). We found no differences between CIMT and dose‐matched comparison groups for all other domains of the child‐reported PedsQoLTM 3 CP Module. See Analysis 3.13 to Analysis 3.19.
3.13. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 13 Pediatric Quality of Life Inventory (PedsQLTM) 3.0 Cerebral Palsy (CP) Module (3.0) – Child Daily Activities.
3.19. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 19 PedsQLTM 3.0 CP Module – Child Speech and Communication.
We found evidence from one study (45 participants) with two comparisons, Kirton 2016a (CIMT + r TMS) and Kirton 2016b (CIMT + sham TMS), that CIMT was more effective than a dose‐matched comparison for quality of life, assessed with both domains of the parent proxy version of the PedsQoLTM 3 CP Module scale, at immediately postintervention: Move and Balance (MD 13.82, 95% CI 5.78 to 21.87) and Fatigue (MD 11.02, 95% CI 0.81 to 21.23). This was not sustained for either domain at the five‐ to six‐month follow‐up period or at the seven‐ to 12‐month follow‐up period. With the exception of Eating activities at 12 months postintervention (MD 9.78, 95% CI 2.01 to 17.56; 1 study (2 comparisons), 45 participants; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS)), we found no differences between the CIMT and dose‐matched comparison groups for quality of life assessed with any other domain of the parent proxy version of the PedsQoLTM 3 CP Module. See Analysis 3.20 to Analysis 3.26 .
Parenting and family measures
No study measured this outcome.
Other
We were unable to conduct a meta‐analysis any other outcomes.
Single‐study results
Primary outcomes
Bimanual
We analysed change‐from‐baseline data on the AHA from Deppe 2013 (29 participants) as a single study because scaled scores rather than AHA units were available. There was no difference between CIMT and dose‐matched comparison groups in bimanual ability assessed with the AHA at immediately postintervention (MD 1.00, 95% CI −2.63 to 4.63; Analysis 3.27.1).
3.27. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 27 Data table.
| Data table | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | CIMT(mean) | SD | N | Dose‐matched (mean) | SD | N | Mean difference [95% CI] |
| Assisting Hand Assessment (Scaled score) | ||||||||
| Deppe 2013 | Baseline to immediately following intervention (change) NB: Scaled score not AHA units. Excluded from metaanalysis |
5.8 | 5.1 | 16 | 4.8 | 3.8 | 13 | 1.00 [‐2.63, 4.63] |
| Deppe 2013 | ||||||||
| Deppe 2013 | ||||||||
| QUEST ‐ Dissociated Movement | ||||||||
| Zafer 2016 | Baseline | 52.41 | 8.14 | 10 | 50.43 | 7.37 | 10 | |
| Zafer 2016 | Immediately following intervention (time point data) | 85.91 | 3.12 | 10 | 82.71 | 2.47 | 10 | 3.20 [0.73, 5.67] |
| Zafer 2016 | ||||||||
| QUEST ‐ Grasp | ||||||||
| Zafer 2016 | Baseline | 53.13 | 7.20 | 10 | 52.10 | 5.87 | 10 | |
| Zafer 2016 | Immediately following intervention (time point data) | 87.90 | 3.13 | 10 | 83.00 | 3.21 | 10 | 4.90 [2.12, 7.68] |
| Zafer 2016 | ||||||||
| QUEST ‐ Weightbearing | ||||||||
| Zafer 2016 | Baseline | 72.97 | 6.96 | 10 | 70.42 | 6.87 | 10 | |
| Zafer 2016 | Immediately following intervention (time point data) | 81.86 | 7.78 | 10 | 75.36 | 6.91 | 10 | 6.50 [0.05, 12.95] |
| Zafer 2016 | ||||||||
| QUEST ‐ Protective Extension | ||||||||
| Zafer 2016 | Baseline | 73.69 | 6.18 | 10 | 72.15 | 6.07 | 10 | |
| Zafer 2016 | Immediately following intervention (time point data) | 80.80 | 3.25 | 10 | 78.80 | 2.24 | 10 | 2.00 [‐0.45, 4.45] |
| Zafer 2016 | ||||||||
| Pediatric Evaluation of Disability Inventory: Self‐care ‐ Caregiver Assistance domain | ||||||||
| Gordon 2011 | Baseline to immediately following intervention (change) | 0.25 | 0.46 | 8 | 1.25 | 1.39 | 8 | ‐1.00 [‐2.01, 0.01] |
| Gordon 2011 | ||||||||
| Gordon 2011 | ||||||||
| Functional Independence Measure for Children (WeeFIM) | ||||||||
| Sung 2005 | Baseline to immediately following intervention (change) | 1.94 | 1.7 | 18 | 1.15 | 2.2 | 13 | 0.79 [‐0.64, 2.22] |
| Sung 2005 | ||||||||
| Sung 2005 | ||||||||
| Modified Ashworth Scale (Wrist) | ||||||||
| Xu 2012 | Baseline to immediately following intervention (change) | ‐0.05 | 0.15 | 22 | ‐0.13 | 0.22 | 23 | 0.08 [‐0.03, 0.19] |
| Xu 2012 | Baseline to 2 weeks to 4 months post‐intervention (change) | ‐0.07 | 0.18 | 22 | ‐0.17 | 0.32 | 23 | 0.10 [‐0.05, 0.25] |
| Xu 2012 | Baseline to 5 to 6 months post‐intervention (change) | ‐0.02 | 0.19 | 22 | ‐0.24 | 0.37 | 23 | 0.22 [0.05, 0.39] |
| 2‐point discrimination | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐0.1 | 1.8 | 27 | 0.5 | 2.4 | 23 | ‐0.60 [‐1.79, 0.59] |
| Sakzewski 2011 | Baseline to 2 weeks to 4 months post‐intervention (change) | ‐0.1 | 2.8 | 26 | 0.2 | 2.8 | 22 | ‐0.30 [‐1.89, 1.29] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 0.1 | 3.8 | 22 | ‐0.2 | 2.5 | 18 | 0.30 [‐1.66, 2.26] |
| Assessment of Life Habits (LIFE‐H) ‐ Total Score | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐0.2 | 1.4 | 28 | 0.5 | 1.7 | 29 | ‐0.70 [‐1.51, 0.11] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 0.0 | 1.2 | 27 | 0.6 | 1.7 | 25 | ‐0.60 [‐1.41, 0.21] |
| Sakzewski 2011 | ||||||||
| LIFE‐H ‐ Recreation | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐0.2 | 2.7 | 29 | 0.3 | 3.0 | 28 | ‐0.50 [‐1.98, 0.98] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 0.4 | 3.4 | 28 | 1.7 | 3.0 | 24 | ‐1.30 [‐3.04, 0.44] |
| Sakzewski 2011 | ||||||||
| LIFE‐H ‐ Nutrition | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 0.1 | 1.6 | 29 | 0.1 | 1.6 | 29 | 0.00 [‐0.82, 0.82] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 0.2 | 0.1 | 1.9 | 0.3 | 1.4 | 25 | ‐0.10 [‐0.65, 0.45] |
| Sakzewski 2011 | ||||||||
| LIFE‐H ‐ Personal Care | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 0.5 | 0.9 | 29 | 0.6 | 1.4 | 28 | ‐0.10 [‐0.71, 0.51] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 0.8 | 1.1 | 28 | 0.8 | 1.3 | 24 | 0.00 [‐0.66, 0.66] |
| Sakzewski 2011 | ||||||||
| LIFE‐H ‐ Education | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐0.2 | 2.4 | 29 | 0.5 | 2.7 | 28 | ‐0.70 [‐2.03, 0.63] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | ‐0.5 | 2.4 | 27 | 0.5 | 2.8 | 24 | ‐1.00 [‐2.44, 0.44] |
| Sakzewski 2011 | ||||||||
| Children’s Assessment of Participation and Enjoyment (CAPE) ‐ Diversity | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐0.5 | 6.9 | 32 | ‐1.3 | 8.4 | 31 | 0.80 [‐3.00, 4.60] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | ‐1.0 | 7.3 | 32 | Missing | Missing | ||
| Sakzewski 2011 | ||||||||
| Children’s Assessment of Participation and Enjoyment (CAPE) ‐ Intensity | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 0.1 | 0.5 | 32 | ‐0.0 | 0.4 | 31 | 0.10 [‐0.12, 0.32] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 0.0 | 0.4 | 29 | ‐0.0 | 0.5 | 29 | 0.00 [‐0.23, 0.23] |
| Sakzewski 2011 | ||||||||
| Cerebral Palsy Quality of Life (child report) ‐ Social well‐being and acceptance | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 3.8 | 11.2 | 18 | ‐0.2 | 10.9 | 17 | 4.00 [‐3.32, 11.32] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 4.7 | 15 | 16 | ‐1.8 | 9.9 | 14 | 6.50 [‐2.50, 15.50] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 5.3 | 16.3 | 17 | 3.3 | 13.8 | 15 | 2.00 [‐8.43, 12.43] |
| Cerebral Palsy Quality of Life (child report) ‐ Function | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 8.1 | 10.3 | 18 | 3.8 | 7.2 | 17 | 4.30 [‐1.56, 10.16] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 8.7 | 16.4 | 16 | 1.9 | 9.5 | 14 | 6.80 [‐2.65, 16.25] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 8.7 | 15.5 | 17 | 8.3 | 12.3 | 15 | 0.40 [‐9.25, 10.05] |
| Cerebral Palsy Quality of Life (child report) ‐ Emotional well‐being and self‐esteem | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 4.0 | 9.4 | 18 | ‐1.8 | 11.2 | 17 | 5.80 [‐1.07, 12.67] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 3.2 | 13.6 | 16 | 0.9 | 13.2 | 14 | 2.30 [‐7.30, 11.90] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1.1 | 14.4 | 17 | 4.3 | 13 | 15 | ‐3.20 [‐12.69, 6.29] |
| Cerebral Palsy Quality of Life (child report) ‐ Participation and physical health | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 4.5 | 12.4 | 18 | 3.7 | 9.4 | 17 | 0.80 [‐6.47, 8.07] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 8.4 | 13.9 | 16 | 6.3 | 14.3 | 14 | 4.71 [‐2.55, 11.98] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 6.5 | 16.1 | 17 | 12.4 | 18.9 | 15 | ‐5.90 [‐18.15, 6.35] |
| Cerebral Palsy Quality of Life (child report) ‐ Pain and impact of disability (lower score = better) | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐5.4 | 17.7 | 18 | ‐10.6 | 23.1 | 17 | 5.20 [‐8.49, 18.89] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | ‐6.8 | 26.1 | 16 | ‐7.1 | 18.7 | 14 | 0.30 [‐15.81, 16.41] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | ‐6.3 | 23.6 | 17 | ‐11.5 | 19 | 15 | 5.20 [‐9.58, 19.98] |
| Cerebral Palsy Quality of Life (Proxy) ‐ Social well‐being and acceptance | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 6.1 | 11 | 29 | 2.4 | 6.9 | 31 | 3.70 [‐0.98, 8.38] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 4.7 | 10.2 | 27 | 3.9 | 11.1 | 27 | 0.80 [‐4.89, 6.49] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 4.2 | 12.7 | 28 | 2.2 | 8.8 | 27 | 2.00 [‐3.76, 7.76] |
| Cerebral Palsy Quality of Life (Proxy) ‐ Function | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 5.6 | 10.6 | 29 | 7.8 | 9.7 | 31 | ‐2.20 [‐7.35, 2.95] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 4.6 | 10.6 | 27 | 7.9 | 10.2 | 27 | ‐3.30 [‐8.85, 2.25] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 2.2 | 11.3 | 28 | 8.1 | 9.9 | 27 | ‐5.90 [‐11.51, ‐0.29] |
| Cerebral Palsy Quality of Life (Proxy) ‐ Participation and physical health | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 5.3 | 12.2 | 29 | 7.8 | 9.1 | 31 | ‐2.50 [‐7.98, 2.98] |
| Sakzewski 2011 | Baseline to 5 to 6 months post intervention (change) | 2.4 | 12 | 27 | 9.5 | 13.6 | 27 | ‐7.10 [‐13.94, ‐0.26] |
| Sakzewski 2011 | Baseline to 7 to 12 months post intervention (change) | 3.6 | 12.7 | 28 | 9 | 12.5 | 27 | ‐5.40 [‐12.06, 1.26] |
| Cerebral Palsy Quality of Life (Proxy) ‐ Emotional well‐being and self‐esteem | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 3.8 | 9.8 | 29 | 5.4 | 9.9 | 31 | ‐1.60 [‐6.59, 3.39] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 2.3 | 13.3 | 27 | 4.3 | 11.9 | 27 | ‐2.00 [‐8.73, 4.73] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1 | 11.6 | 28 | 4.1 | 11.9 | 27 | ‐3.10 [‐9.31, 3.11] |
| Cerebral Palsy Quality of Life (Proxy) ‐ Pain and impact of disability (lower score = better) | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 2.8 | 13.6 | 29 | ‐2.2 | 14.4 | 31 | 5.00 [‐2.08, 12.08] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | ‐0.2 | 13.9 | 27 | ‐1.1 | 19.6 | 27 | 0.90 [‐8.16, 9.96] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 0.3 | 14.9 | 28 | ‐3.7 | 17.2 | 27 | 4.00 [‐4.52, 12.52] |
| Cerebral Palsy Quality of Life (Proxy) ‐ Access | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 2.9 | 18 | 29 | 3.2 | 15.8 | 31 | ‐0.30 [‐8.89, 8.29] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 2.9 | 14.6 | 27 | 2.4 | 21.1 | 27 | 0.50 [‐9.18, 10.18] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 2.7 | 15.8 | 28 | 3.7 | 17.6 | 27 | ‐1.00 [‐9.85, 7.85] |
| Cerebral Palsy Quality of Life (Proxy) ‐ Family health | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 2.8 | 13 | 29 | 4.4 | 11.3 | 31 | ‐1.60 [‐7.78, 4.58] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 2.7 | 6.5 | 27 | 7.8 | 13.3 | 27 | ‐5.10 [‐10.68, 0.48] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1.2 | 13.6 | 28 | 9 | 14.3 | 27 | ‐7.80 [‐15.18, ‐0.42] |
| KIDSCREEN ‐ Physical Wellbeing | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 5.3 | 10.4 | 21 | 0.2 | 7.1 | 18 | 5.10 [‐0.43, 10.63] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 4.7 | 12.7 | 19 | 1.3 | 7.5 | 17 | 3.40 [‐3.33, 10.13] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 3.7 | 9.2 | 19 | 0.4 | 6.5 | 16 | 3.30 [‐1.92, 8.52] |
| KIDSCREEN ‐ Psychological Wellbeing | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 4.5 | 7.5 | 21 | ‐2.6 | 7.9 | 18 | 7.10 [2.24, 11.96] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 5.2 | 9.4 | 19 | 0.1 | 10.5 | 17 | 5.10 [‐1.44, 11.64] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 3.8 | 8.6 | 19 | ‐0.5 | 6.3 | 16 | 4.30 [‐0.65, 9.25] |
| KIDSCREEN ‐ Mood and Emotions | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 7.7 | 9.1 | 20 | 3.4 | 10.6 | 17 | 4.30 [‐2.13, 10.73] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 4.1 | 7.1 | 18 | ‐0.0 | 8.1 | 17 | 4.10 [‐0.96, 9.16] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 2.6 | 7.4 | 19 | 2.9 | 9.1 | 16 | ‐0.30 [‐5.86, 5.26] |
| KIDSCREEN ‐ Self‐perception | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 4.7 | 8.3 | 20 | ‐3.2 | 8.4 | 18 | 1.38 [‐5.05, 7.81] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 5.1 | 12.3 | 18 | ‐2.7 | 8.7 | 17 | 4.10 [‐0.96, 9.16] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1.5 | 10.9 | 19 | ‐1.2 | 12.5 | 16 | ‐0.30 [‐5.86, 5.26] |
| KIDSCREEN ‐ Autonomy | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 3.5 | 10.3 | 20 | 2.4 | 9 | 18 | 1.10 [‐5.04, 7.24] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 3.6 | 8.9 | 18 | 0.4 | 7.6 | 17 | 3.20 [‐2.27, 8.67] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 4.2 | 8 | 19 | 1.4 | 10.2 | 16 | 2.80 [‐3.36, 8.96] |
| KIDSCREEN ‐ Parent Relations | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 2.1 | 6.5 | 21 | 0.1 | 6.2 | 18 | 2.00 [‐1.99, 5.99] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 2.5 | 9.5 | 19 | 1.6 | 7.7 | 16 | 0.90 [‐4.80, 6.60] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1.7 | 9.3 | 19 | 2.4 | 7.9 | 16 | ‐0.70 [‐6.40, 5.00] |
| KIDSCREEN ‐ Financial Resources | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 0.8 | 6.5 | 20 | 2.1 | 10.1 | 17 | ‐1.30 [‐6.88, 4.28] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 4 | 7.7 | 18 | 2.1 | 9.1 | 16 | 1.90 [‐3.80, 7.60] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 2.5 | 8.7 | 19 | 3 | 9.7 | 15 | ‐0.50 [‐6.78, 5.78] |
| KIDSCREEN ‐ Social Supports + Peers | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 1.6 | 13.2 | 20 | 0.6 | 7.1 | 18 | 1.00 [‐5.65, 7.65] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 5.5 | 15.7 | 18 | 1 | 12.2 | 17 | 4.50 [‐4.79, 13.79] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 3.8 | 10.8 | 19 | 1.8 | 9.8 | 16 | 2.00 [‐4.83, 8.83] |
| KIDSCREEN ‐ School Environment | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 3.5 | 12.6 | 21 | ‐1 | 9.3 | 17 | 4.50 [‐2.47, 11.47] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 3.3 | 12.7 | 19 | ‐0.5 | 9.5 | 16 | 3.80 [‐3.57, 11.17] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 0.6 | 8.0 | 19 | 0.6 | 11.2 | 16 | 0.00 [‐6.56, 6.56] |
| KIDSCREEN ‐ Social Acceptance | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐0.2 | 7.9 | 21 | ‐1 | 8.1 | 17 | 0.80 [‐4.32, 5.92] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | ‐2.7 | 8.8 | 19 | ‐1.4 | 13.4 | 16 | ‐1.30 [‐8.97, 6.37] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 2.9 | 7.4 | 19 | 2.6 | 12.1 | 15 | 0.30 [‐6.67, 7.27] |
| KIDSCREEN (Parent Proxy) ‐ Physical Wellbeing | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 1.4 | 12.5 | 31 | 0.5 | 7.7 | 31 | 0.90 [‐4.27, 6.07] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 2.9 | 11.9 | 29 | 1.4 | 8.8 | 28 | 1.50 [‐3.92, 6.92] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 3.0 | 12.6 | 30 | 0.3 | 8 | 25 | 2.70 [‐2.79, 8.19] |
| KIDSCREEN (Parent Proxy) ‐ Psychological Wellbeing | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 2.6 | 11.3 | 31 | 1.0 | 6.3 | 31 | 1.60 [‐2.95, 6.15] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 1.9 | 9.3 | 29 | 0.5 | 9.1 | 28 | 1.40 [‐3.38, 6.18] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1.5 | 10.6 | 30 | ‐0.5 | 7.1 | 25 | 2.00 [‐2.70, 6.70] |
| KIDSCREEN (Parent Proxy) ‐ Mood and Emotions | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 1.5 | 7.5 | 30 | ‐1.1 | 6.2 | 31 | 2.60 [‐0.86, 6.06] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 0.7 | 7.3 | 28 | ‐0.1 | 6.6 | 28 | 0.80 [‐2.85, 4.45] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1.1 | 8.5 | 29 | 1 | 7.6 | 25 | 0.10 [‐4.19, 4.39] |
| KIDSCREEN (Parent Proxy) ‐ Self‐perception | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 2.5 | 10.6 | 31 | 0.7 | 7.6 | 30 | 1.80 [‐2.82, 6.42] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 2.7 | 9.7 | 29 | ‐1.1 | 8.9 | 28 | 3.80 [‐1.03, 8.63] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 4.5 | 9.8 | 30 | 1.2 | 8.9 | 25 | 3.30 [‐1.65, 8.25] |
| KIDSCREEN (Parent Proxy) ‐ Autonomy | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐0.5 | 8.9 | 31 | 1.1 | 8.1 | 31 | ‐1.60 [‐5.84, 2.64] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 1.8 | 8.6 | 28 | 0.6 | 11 | 28 | 1.20 [‐3.97, 6.37] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1.8 | 7.6 | 30 | 0.6 | 6.8 | 25 | 1.20 [‐2.61, 5.01] |
| KIDSCREEN (Parent Proxy) ‐ Parent Relations | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 3.6 | 9.6 | 31 | ‐0.2 | 6.3 | 31 | 3.80 [‐0.24, 7.84] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 2.4 | 8.5 | 28 | 0.4 | 5.9 | 28 | 2.00 [‐1.83, 5.83] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 3.2 | 10 | 30 | 1 | 6.5 | 25 | 2.20 [‐2.19, 6.59] |
| KIDSCREEN (Parent Proxy) ‐ Financial Resources | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 2.2 | 8.5 | 26 | ‐2.3 | 7.2 | 29 | 4.50 [0.31, 8.69] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | 3.3 | 7.7 | 24 | 2.6 | 10.3 | 27 | 0.70 [‐4.26, 5.66] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 3.3 | 8.3 | 26 | 0.9 | 8.8 | 25 | 2.40 [‐2.30, 7.10] |
| KIDSCREEN (Parent Proxy) ‐ Social Supports + Peers | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐2.4 | 10.8 | 27 | 1.8 | 6.9 | 30 | ‐4.20 [‐8.96, 0.56] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | ‐0.6 | 10.3 | 25 | 1 | 9.4 | 26 | ‐1.60 [‐7.02, 3.82] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1.6 | 8/9 | 26 | 2.6 | 8.9 | 25 | ‐1.00 [‐5.65, 3.65] |
| KIDSCREEN (Parent Proxy) ‐ School Environment | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | 2.1 | 5.3 | 29 | 0.6 | 7 | 30 | 1.50 [‐1.66, 4.66] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | ‐0.1 | 8.2 | 28 | 1.5 | 9.8 | 27 | ‐1.60 [‐6.38, 3.18] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 0.7 | 8.3 | 29 | 0.6 | 8.6 | 24 | 0.10 [‐4.48, 4.68] |
| KIDSCREEN (Parent Proxy) ‐ Social Acceptance | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐0.2 | 11.3 | 29 | 3.1 | 9.6 | 29 | ‐3.30 [‐8.70, 2.10] |
| Sakzewski 2011 | Baseline to 5 to 6 months post‐intervention (change) | ‐3.4 | 10.7 | 27 | 2.3 | 9.6 | 26 | ‐5.70 [‐11.17, ‐0.23] |
| Sakzewski 2011 | Baseline to 7 to 12 months post‐intervention (change) | 1 | 12 | 28 | 4.4 | 12.5 | 23 | ‐3.40 [‐10.17, 3.37] |
| Video Observation Aarts & Aarts: Determine Developmental Disregard (VOAA‐DDD) ‐ Performance | ||||||||
| Aarts 2010 | Baseline to immediately following intervention (change) | 9.1 | 17.0 | 28 | ‐0.7 | 16 | 22 | 9.80 [0.62, 18.98] |
| Aarts 2010 | Baseline to 2 weeks to 4 months post‐intervention (change) | 10.9 | 14.2 | 28 | 4.0 | 13.7 | 22 | 6.90 [‐0.87, 14.67] |
| Aarts 2010 | ||||||||
| VOAA:DDD ‐ Capacity | ||||||||
| Aarts 2010 | Baseline to immediately following intervention (change) | 14.9 | 19.7 | 28 | 1.9 | 13.6 | 22 | 13.00 [3.75, 22.25] |
| Aarts 2010 | Baseline to 2 weeks to 4 months post‐intervention (change) | 10.4 | 21.0 | 28 | ‐2.3 | 12.3 | 22 | 12.70 [3.38, 22.02] |
| Aarts 2010 | ||||||||
| VOAA‐DDD ‐ Developmental Disregard | ||||||||
| Aarts 2010 | Baseline to immediately following intervention (change) | ‐6.6 | 14.8 | 28 | ‐0.5 | 17.4 | 22 | ‐6.10 [‐15.21, 3.01] |
| Aarts 2010 | Baseline to 2 weeks to 4 months post‐intervention (change) | ‐1.7 | 13.2 | 28 | ‐4.0 | 18.3 | 22 | 2.30 [‐6.78, 11.38] |
| Aarts 2010 | ||||||||
| School Function Assessment | ||||||||
| Sakzewski 2011 | Baseline to immediately following intervention (change) | ‐0.3 | 2.9 | 21 | 1 | 2.1 | 19 | ‐1.30 [‐2.86, 0.26] |
| Sakzewski 2011 | Baseline to 6 months (change) | ‐0.3 | 2.2 | 19 | 1.2 | 2.6 | 14 | ‐1.50 [‐3.18, 0.18] |
| Sakzewski 2011 | ||||||||
| Besta Scale ‐ Global score | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | 0.23 | 0.39 | 39 | 0.23 | 0.29 | 33 | 0.00 [‐0.16, 0.16] |
| Facchin 2011 | ||||||||
| Facchin 2011 | ||||||||
| Besta Scale ‐ Grasp (affected side) | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | 0.30 | 0.57 | 39 | 0.09 | 0.38 | 33 | 0.21 [‐0.01, 0.43] |
| Facchin 2011 | ||||||||
| Facchin 2011 | ||||||||
| Besta Scale ‐ Bimanual use | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | 0.24 | 0.56 | 39 | 0.28 | 0.44 | 33 | ‐0.04 [‐0.27, 0.19] |
| Facchin 2011 | ||||||||
| Facchin 2011 | ||||||||
| Besta Scale ‐ Activities of Daily Living (ADL) (2 to 6 years) | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | 0.22 | 0.47 | 28 | 0.25 | 0.33 | 28 | ‐0.03 [‐0.24, 0.18] |
| Facchin 2011 | ||||||||
| Facchin 2011 | ||||||||
| Besta Scale ‐ ADL use (7 to 8 years) | ||||||||
| Facchin 2011 | Baseline to immediately following intervention (change) | ‐0.19 | 0.27 | 11 | 0.00 | 0.0 | 5 | Not estimable |
| Facchin 2011 | ||||||||
| Facchin 2011 | ||||||||
Unimanual
Time‐point data on the QUEST from Zafer 2016 (20 participants) showed no evidence that CIMT was more effective than a dose‐matched comparison at immediately postintervention on the Dissociated movement scale from 0 to 100) (MD 3.20, 95% CI 0.73 to 5.67; Analysis 3.27.2), Grasp (MD 4.90, 95% CI 2.12 to 7.86; Analysis 3.27.3) and Weightbearing domains (MD 6.50, 95% CI 0.05 to 12.95; Analysis 3.27.4), or the Protective Extension domain of the QUEST ‐ scale (MD 2.00, 95% CI 0.45 to 4.45;(Analysis 3.27.5).
Adverse events
See Table 8. Six studies (134 participants) reported that no adverse events were experienced in the CIMT group (Aarts 2010; Gelkop 2015; Gordon 2011; Kirton 2016a (CIMT + r TMS); Sakzewski 2011; Xu 2012). One of these studies, which combined CIMT with rTMS reported headache and additional side effects of rTMS experienced by at least 11% of participants (Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS)).
Four studies mentioned adverse effects. In two studies, two children did not tolerate CIMT and were unable to complete the intervention (Dong 2017), and three others experienced difficulty getting used to CIMT at the outset of intervention (Smania 2009). Two studies that monitored the function of the less affected hand reported no adverse events (Facchin 2011; Sung 2005).
Five studies did not mention the presence or absence of adverse events (Abd El‐Kafy 2014; Deppe 2013; Rostami 2012b; Sakzewski 2015b; Zafer 2016).
Single‐study results
Individualised measures of performance
Two studies reported conflicting results for goal attainment scale (GAS), which could not be pooled in a meta‐analysis (Aarts 2010; Gordon 2011). Aarts 2010 found that participants who received CIMT achieved a substantially higher percentage of goals at the 'expected', 'greater than expected' or 'much greater than expected' level than the dose‐matched comparison at immediately postintervention (CIMT: 82%, dose‐matched comparison: 23%) and at the two‐week to four‐month follow‐up period (CIMT: 82%, dose‐matched comparison: 36%). Using the postintervention mean T scores and SD provided for each group by Gordon 2011, our calculations found evidence that a dose‐matched comparison was more effective than CIMT for goal attainment at immediately postintervention (MD −8.10, 95% CI −12.69 to −3.51, 42 participants), at the two‐week to four‐month follow‐up period (MD −6.80, 95% CI −10.92 to −2.68) and at the five‐ to six‐month follow‐up period (MD −4.80, 95% CI −9.12 to −0.48). See Table 11.
Self‐care
Change‐from‐baseline data showed no differences between the CIMT and dose‐matched comparison groups in the amount of caregiver assistance required for self‐care, assessed with the PEDI Self‐care Caregiver domain assistance (Scale from 0 to 100) (MD −1.00, 95% CI −2.01 to −0.01; 1 study, 16 participants; Gordon 2011; Analysis 3.27.6) or the WeeFIM total score (from 18 to 126) (MD −0.79, 95% CI −0.64 to 2.22; 1 study, 31 participants; Sung 2005; Analysis 3.27.7), at immediately postintervention.
Body function
Change‐from‐baseline data from Xu 2012 (45 participants) showed no differences between the CIMT and dose‐matched comparison groups for wrist flexors assessed with the MAS (scored from 0 to 4) at immediately postintervention (MD −0.08, 95% CI −0.03 to 0.19), and at the two‐week to four‐month follow‐up period (MD 0.10, 95% CI −0.05 to 0.25), but did show evidence that CIMT was more effective than a dose‐matched comparison at the five‐ to six‐month follow‐up period (MD 0.22, 95% CI 0.05 to 0.39). See Analysis 3.27.8.
Change‐from‐baseline data from Sakzewski 2011 (63 participants) showed no differences between the CIMT and dose‐matched comparison groups for tactile discrimination, assessed with the two‐point discrimination test (The smallest distance (mm) between two points that still results in the perception of two distinct stimuli is recorded as the patient's two‐point threshold), at immediately postintervention (MD −0.60 mm, 95% CI −1.79 to 0.59, 50 participants), at the two‐week to four‐month follow‐up period (MD −0.30 mm, 95% CI −1.89 to 1.29, 48 participants), and at the five‐ to six‐month follow‐up period (MD 0.30 mm, 95% CI −1.66 to 2.26, 40 participants). See Analysis 3.27.9.
Participation
Change‐from‐baseline data from Sakzewski 2011 (63 participants)showed no differences between the CIMT and dose‐matched comparison groups for participation assessed with the Assessment of Life Habits (LIFE‐H) (scores from 0 to 9) (total score or four domains) at immediately postintervention or at the five‐ to six‐month follow‐up period. See Analysis 3.27.10 to Analysis 3.27.14.
Change‐from‐baseline to immediately postintervention data from Sakzewski 2011 (63 participants) showed similar results for participation assessed with both the Diversity (scores from 0 to 55) and Intensity (scores from 1 to 7) domains of the CAPE. See Analysis 3.27.15 and Analysis 3.27.16.
Quality of life
Change‐from‐baseline data from Sakzewski 2011 (63 participants) showed no differences between the CIMT and dose‐matched comparison groups in quality of life assessed with the five domains of the child‐report version of the CPQOL at immediately postintervention, at the five‐ to six‐month follow‐up period and the seven‐ to 12‐month follow‐up period. See Analysis 3.27.17 to Analysis 3.27.21.
Change‐from‐baseline data from Sakzewski 2011 showed that a dose‐matched comparison was more effective than CIMT for quality of life assessed by the Function and Family health domains on the parent proxy version of the CPQOL (scale from 0 to 100) at the seven‐ to 12‐month follow‐up period, and the Partcipation and Physical health domains at the five‐ to six‐month follow‐up though this was not sustained at the seven‐ to 12‐month follow‐up period See Analysis 3.27.22 to See Analysis 3.27.28.
Change‐from‐baseline data from Sakzewski 2011 on all nine domains of the child‐report version of KIDSCREEN, showed no differences between the CIMT and dose‐matched comparison groups at all time points, the exception being the Psychological Well‐being domain, where there was evidence that CIMT was more effective than a dose‐matched comparison at immediately postintervention. This was not sustained at the five‐ to six‐month or seven‐ to 12 month follow‐up periods. See Analysis 3.27.29 to Analysis 3.27.38.
Change‐from‐baseline data from Sakzewski 2011, on the parent proxy version of KIDSCREEN, showed that CIMT was more effective than a dose‐matched comparison for quality of life assessed the Financial Resources domain at immediately postintervention and the Social Acceptance domain at the 7‐ to 12‐month follow‐up period, but not for the remaining eight other domains at all other time points. See Analysis 3.27.39 to Analysis 3.27.48.
Other
Change‐from‐baseline data from Aarts 2010 (50 participants) showed no evidence that CIMT was more effective than a dose‐matched comparison at immediately postintervention for developmental disregard assessed by the Performance and Capacity domains of the Video Observations Aarts and Aarts ‐Determine Developmental Disregard (VOAA‐DDD). This was sustained at the two‐week to four‐month follow‐up period for the Capacity domain but not the Performance domain. However, there were no differences between the CIMT and dose‐matched comparison groups for the VOAA‐DDD Developmental disregard domain. See Analysis 3.27.49 to Analysis 3.27.51.
Change‐from‐baseline data from Sakzewski 2011 (30 participants) showed no differences between the CIMT and dose‐matched comparison groups for performance of functional tasks at school assessed by the School Function Assessment. See Analysis 3.27.
Change‐from‐baseline data from Facchin 2011 showed no differences between the CIMT and dose‐matched comparison groups for hand function assessed by the Besta scale global score or for any other domain, at immediately postintervention. See Analysis 3.27.53 to Analysis 3.27.57.
Sensitivity analysis
To assess the influence of our analysis model on the results, we repeated the pooled analyses for Bimanual (AHA),Manual ability (ABILHAND‐Kids), Unimanual dexterity (Box and Blocks Test, Melbourne Assessment, QUEST), Self‐care (PEDI), Individualised measures of performance (COPM), Body function (Grip strength) and Quality of life (CPQOL) using a fixed‐effect model instead of a random‐effects model. This had no impact for the outcomes Unimanual dexterity (assessed with the Box and Blocks Test, QUEST ‐ Weightbearing, QUEST ‐ Protective extension, PEDI Self‐care domain) and body function (functional skills and Grip strength).
Using a fixed‐effect model, we again found no evidence that CIMT is more effective than a dose‐matched comparison for improving bimanual performance at immediately postintervention, at the five‐ to six‐month follow‐up period, or at the seven‐ to 12‐month follow‐up period, with no change in heterogeneity (analysis not shown). However, using a fixed‐effect model resulted in a change in the effect size at the two‐week to four‐month follow‐up, in favour of CIMT with no change in heterogeneity (MD 1.60 AHA units, 95% CI 0.00 to 3.19; P = 0.27; 4 studies (5 comparisons); low heterogeneity: I2 = 21%; Aarts 2010; Gelkop 2015; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); analysis not shown).
Using a fixed‐effect model also provided evidence that CIMT was more effective than a dose‐matched comparison for a range of other outcomes at immediately postintervention, including the following.
Bimanual, assessed with ABILHAND‐Kids (MD 0.73 AHA units, 95% CI 0.34 to 1.11; 2 studies (3 comparisons); P = 0.02; high heterogeneity: I2 = 74%; Aarts 2010; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); analysis not shown).
Unimanual, assessed with the Melbourne Assessment (scored from 0 to 122) (MD 1.63, 95% CI 0.11 to 3.14; 5 studies (6 comparisons); P = 0.19; moderate heterogeneity: I2 = 33%; Aarts 2010; Deppe 2013; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b; analysis not shown), the QUEST ‐ Dissociated Movement domain (MD 4.40, 95% CI 1.92 to 6.88; 3 studies; P = 0.001; high heterogeneity: I2 = 86%; Facchin 2011; Gelkop 2015; Gordon 2011; analysis not shown), and the QUEST ‐ Grasp domain (MD 5.97, 95% CI 2.49 to 9.46; 3 studies; P = 0.002; high heterogeneity: I2 = 84%; Facchin 2011; Gelkop 2015; Gordon 2011; analysis not shown).
Individualised measures of performance, assessed with COPM Performance (scored from 0 to 10) (MD 0.74, 95% CI 0.31 to 1.16; 5 studies (6 comparisons); P < 0.001; high heterogeneity: I2 = 89%; Aarts 2010; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b; analysis not shown), and COPM Satisfaction (MD 1.03, 95% CI 0.52 to 1.54; 5 studies (6 comparisons); P < 0.001; high heterogeneity: I2 = 84%; Aarts 2010; Gordon 2011; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); Sakzewski 2011; Sakzewski 2015b; analysis not shown).
These outcomes were only persistent at the two‐week to four‐month follow‐up period for occupational performance measured with the COPM (MD 1.39, 95% CI 0.81 to 1.97; 2 studies (3 comparisons), 95 participants; P < 0.001; high heterogeneity: I2 = 88%; Aarts 2010; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); analysis not shown) and occupational satisfaction also measured with the COPM (MD 1.53, 95% CI 0.84 to 2.21; 2 studies (3 comparisons), number of participants?; P = 0.08; high heterogeneity: I2 = 84%; Aarts 2010; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); analysis not shown).
Applying a fixed‐effect model for quality of life outcomes measured using the PedsQLTM 3 CP Module ‐ scale from 0 to 100 used in multiple comparison groups included in the study by Kirton (45 participants) provided evidence that CIMT was more effective than a dose‐matched comparison at immediately postintervention for domains of the parent/proxy report version, including the following.
School Activities (MD 11.51, 95% CI 1.92 to 21.10; 1 study (2 comparisons); P = 0.15; moderate heterogeneity: I2 = 52%; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); analysis not shown).
Pain and Hurt (MD 14.99, 95% CI 4.87 to 25.12; 1 study (2 comparisons); P = 0.004; high heterogeneity: I2 = 76%; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); analysis not shown).
Eating Activities (MD 10.35, 95% CI 2.67 to 18.03; 1 study (2 comparisons),; P = 0.04; high heterogeneity: I2 = 76%; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); analysis not shown).
This was persistent at the five‐ to six‐month follow‐up period for the Pain and Hurt domain only (MD 9.86, 95% CI 0.59 to 19.12; 1 study (2 comparisons); P = 0.008, I2 = 86%; Kirton 2016a (CIMT + r TMS); Kirton 2016b (CIMT + sham TMS); analysis not shown).
Quality of evidence
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that CIMT is not more effective than a dose‐matched comparison for improving bimanual performance (assessed with the AHA), unimanual capacity (assessed with the Melbourne Assessment) or self‐care (assessed with the PEDI Self‐care Functional skills domain) in children with CP at immediately postintervention. There is very low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for inconsistency, once for imprecision) that CIMT is not more effective than a dose‐matched comparison for improving unimanual capacity (assessed with the QUEST ‐ Grasp domain), manual ability (assessed with ABILHAND‐Kids) or parent‐reported occupational performance (assessed with the COPM) at immediately postintervention. See Table 3.
4. CIMT versus different forms of CIMT
Pooled results
Primary outcomes
Bimanual
We were unable to conduct a meta‐analysis, as the two studies that reported data on this outcome, Christmas 2018 and DeLuca 2012, reported different AHA units.
Unimanual
No study measured this outcome.
Manual ability
No study measured this outcome.
Adverse events
We were unable to conduct a meta‐analysis for this outcome.
Secondary outcomes
Individualised
No study measure this outcome.
Self‐care
No study measure this outcome.
Body function
No study measure this outcome.
Participation
No study measure this outcome.
Quality of life
Change‐from‐baseline data from Christmas 2018, (43 participants) showed no differences between prolonged CIMT versus manual CIMT for quality of life, assessed by the five domains of the child‐report versions of the PedsQLTM ‐ CP Module and the PedsQLTM ‐ Generic Core Scale (total score and five domain scores), at immediately postintervention and at the five‐ to six‐month follow‐up period. See Analysis 4.1 to Analysis 4.11.
4.1. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 1 Pediatric Quality of Life Inventory ( PedsQLTM) 3.0 Cerebral Palsy (CP) Module – Parent Daily Activities.
| Pediatric Quality of Life Inventory ( PedsQLTM) 3.0 Cerebral Palsy (CP) Module – Parent Daily Activities | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 9.8 | 22.8 | 20 | 8.6 | 28 | 23 | 1.20 [‐13.99, 16.39] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | 5.5 | 16.7 | 22 | 7.6 | 26.6 | 23 | ‐2.10 [‐15.02, 10.82] |
4.11. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 11 PedsQLTM 4.0 Generic Core Scale ‐ Nursery Functioning.
| PedsQLTM 4.0 Generic Core Scale ‐ Nursery Functioning | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 11.3 | 19 | 15 | ‐2.9 | 24 | 17 | 14.20 [‐0.72, 29.12] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐8.6 | 24 | 16 | ‐1.6 | 21.1 | 16 | ‐7.00 [‐22.66, 8.66] |
Change‐from‐baseline data from Christmas 2018 also showed no differences between prolonged CIMT versus manual CIMT for quality of life, assessed by the Psychosocial Functioning, Social Functioning, Physical Functioning and Physical Summary domains of the PedsQLTM Infant Scale, at immediately postintervention or at the five‐ to six‐month follow‐up period. However, Christmas 2018 did find evidence of a difference between prolonged CIMT versus a manual CIMT for quality of life on the Emotional Functioning, Cognitive Functioning, Psychological Functioning and the Summary domains of the PedsQLTM Infant Scale, at the five‐ to six‐month follow‐up period. Evidence of a difference at immediately postintervention was only present for the Cognitive Functioning domain. See analysis Analysis 4.12 to Analysis 4.19.
4.12. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 12 PedsQLTM Infant Scale ‐ Summary.
| PedsQLTM Infant Scale ‐ Summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 0.17 | 42 | 4 | ‐10.0 | 12 | 7 | 10.17 [‐31.94, 52.28] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐2.5 | 1.6 | 4 | ‐9.4 | 3.2 | 5 | 6.90 [3.69, 10.11] |
4.19. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 19 PedsQLTM Infant Scale ‐ Cognitive Functioning.
| PedsQLTM Infant Scale ‐ Cognitive Functioning | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 6.5 | 7.9 | 4 | ‐15.5 | 18.6 | 7 | 22.00 [6.20, 37.80] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | 4.6 | 5.7 | 4 | ‐10.6 | 13.7 | 5 | 15.20 [1.96, 28.44] |
Parenting and family measures
No study measure this outcome.
Other
No study measure any other outcomes.
Single‐study results
Primary outcome
Bimanual
Time‐point data from DeLuca 2012 (18 participants) showed no difference between six hours of CIMT versus three hours of CIMT for bimanual performance assessed with the Kids‐AHA at immediately postintervention (MD 2.19 logit scores, 95% CI −1.15 to 5.53 . See Analysis 4.20.
4.20. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 20 Assisting Hand Assessment (logits, time‐point data).
| Assisting Hand Assessment (logits, time‐point data) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | CIMT (6 hours) | SD | N | CIMT (3 hours) | SD | N | Mean difference[95% CI] |
| DeLuca 2012 | Post‐intervention (time point data) | 3.03 | 3.9 | 9 | 0.84 | 3.3 | 9 | 2.19 [‐1.15, 5.53] |
Similarly, time‐point data from Christmas 2018 (60 participants) showed no difference between prolonged CIMT versus manual CIMT for bimanual performance assessed with the AHA (Version 4.4) (Logit‐based scale form 0 to 100) at immediately postintervention (MD 3.70 AHA units, 95% CI −1.27 to 8.67 . See Analysis 4.21.
4.21. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 21 Assisting Hand Assessment (AHA units, change‐from‐baseline data).
| Assisting Hand Assessment (AHA units, change‐from‐baseline data) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 9.0 | 8.8 | 29 | 5.3 | 10.8 | 31 | 3.70 [‐1.27, 8.67] |
Unimanual
Change‐from‐baseline data from Christmas 2018 (60 participants) showed no difference between prolonged CIMT versus manual CIMT for unimanual capacity assessed with the following domains of the QUEST (scale 0% to 100%) at immediately postintervention: Dissociated movement (MD −0.60, 95% CI −5.11 to 3.91), Grasp (MD 3.40, 95% CI −1.91 to 8.71) and Weightbearing domains (MD 2.20, 95% CI −5.20 to 9.60). See Analysis 4.22 to Analysis 4.25.
4.2. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 2 PedsQLTM 3.0 CP Module – Parent Move & Balance.
| PedsQLTM 3.0 CP Module – Parent Move & Balance | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 8.0 | 23.0 | 22 | ‐2.1 | 27.0 | 22 | 10.10 [‐4.72, 24.92] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐2.0 | 20.6 | 22 | ‐6.9 | 22.3 | 23 | 4.90 [‐7.64, 17.44] |
4.25. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 25 QUEST ‐ Dissociated Movement.
| QUEST ‐ Dissociated Movement | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 1.6 | 8.3 | 29 | 2.2 | 9.5 | 31 | ‐0.60 [‐5.11, 3.91] |
Manual ability
Christmas 2018 (50 participants) showed a difference between prolonged CIMT versus manual CIMT for manual ability assessed with the Birmingham Bimanual Questionnaire (scored as a percentage) at immediately postintervention (P = 0.019). This was not sustained at five to six months postintervention (P = 0.87). Our calculations using change‐from‐baseline data were consistent, with an MD of 16.90 (95% CI 3.31 to 30.49; 50 participants) at immediately postintervention that was not sustained at five to six months postintervention (MD of 1.10, 95% −12.33 to 14.53; 48 participants). See analysis Analysis 4.26.
4.26. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 26 The Birmingham Bimanual Questionnaire.
| The Birmingham Bimanual Questionnaire | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 20.9 | 25.29 | 23 | 4.0 | 23.4 | 27 | 16.90 [3.31, 30.49] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | 3.1 | 25.1 | 21 | 2.0 | 21.4 | 27 | 1.10 [‐12.33, 14.53] |
Adverse events
See Table 8. Two of the three studies that contributed to this comparison reported on adverse events (Christmas 2018; DeLuca 2012; Rostami 2012a). One study reported no adverse events (DeLuca 2012). Christmas 2018 reported no serious adverse events and 12 non‐serious adverse events related to interventions for the prolonged restraint group: two children had minor bruising because of a fall and 10 had small areas of skin abrasions. The remaining study did not mention the presence or absence of adverse events (Rostami 2012a).
Sensitivity analysis
We were unable to conduct a sensitivity analysis for this comparison as no data were available.
Quality of evidence
There is very low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for inconsistency, once for imprecision) that six hours of CIMT is not more effective than three hours of CIMT for improving bimanual performance assessed with the Kids‐AHA. There is also very low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for inconsistency, once for imprecision) that prolonged constraint is not more effective than manual constraint for improving bimanual performance assessed with the Kids‐AHA. See Table 4.
Discussion
This update of our original systematic review includes 36 rrandomised controlled triald (RCTs) evaluating the effectiveness of constraint‐induced movement therapy (CIMT) in children with unilateral cerebral palsy (CP). Enormous diversity among the studies included: a broad range of constraint devices, models and dosage of therapy; outcome measures; settings; and comparison interventions. To improve homogeneity, we grouped analyses according to relative dosage of comparison intervention (low, high, matched), or comparison of different forms of CIMT. Our primary outcomes were bimanual performance, unimanual capacity, manual ability and adverse events. Secondary outcomes included individualised measures of performance, self‐care, body function, participation and quality of life outcomes.
Summary of main results
Primary outcomes ‐ bimanual performance, unimanual capacity, manual ability and adverse events
Outcomes from this review of 36 RCTs, using 57 outcome measures, provided weak evidence that CIMT was more effective than a low‐dose comparison for improving bimanual performance and unimanual capacity in children with unilateral CP, aged three months to 19 years of age. There was also weak evidence that CIMT was not more effective than a high‐dose or dose‐matched comparison group for improving bimanual performance and unimanual capacity. Very low‐quality evidence suggested no difference in manual ability between CIMT and dose‐matched interventions. Manual ability was not measured in CIMT compared with low‐dose or high‐dose comparison interventions. From our understanding, CIMT is likely to be the most frequently evaluated therapy intervention for children with CP using high‐level, RCTs methods. However, the overall strength of evidence remains weak due to small sample sizes, inability to blind children and therapists to the intervention, and the number of different outcome measures used across included studies with many showing no evidence of validity or reliability for use with children with CP.
The outcomes of this review did not support the suggestion that CIMT, as a unilateral intervention, yields more improvement in the unimanual capacity of the more affected arm compared with bimanual therapy (Dong 2013), when compared with high‐dose or dose‐matched interventions, most of which were bimanual interventions. This also applied to the suggestion that more improvement in bimanual performance was observed following bimanual therapy when compared with CIMT (Dong 2013). Aside from the very small study by Gelkop 2015 (six children in CIMT group) that demonstrated a very large, and likely imprecise effect size (11.7 Assisting Hand Assessment (AHA) units), the amount of change on the AHA immediately following CIMT ranged from −2.0 AHA units to 6.43 AHA units, and at the two‐week to four‐month follow‐up period from 0.60 AHA units to 7.1 AHA units. The data demonstrated that CIMT high‐dose and dose‐matched therapy can improve bimanual performance and unimanual capacity with equivalent effect.
Overall, the evidence for safety is incomplete because only 20 out of 36 studies reported collecting data on adverse events; it is unclear whether or not this reflects an absence of adverse events or a failure to report them. Studies reported a small number of participants as being unable to tolerate CIMT due to frustration and lack of acceptance of the restraint device, especially in the first few days of a CIMT program. Only nine children from approximately 472 participants receiving CIMT in the 20 studies that addressed adverse events were unable to continue CIMT. There was no evidence of a decline in hand function or increased joint stiffness in the less affected hand as a result of constraint (Facchin 2011; Sung 2005). CIMT appeared to be a safe intervention for children with unilateral CP.
Secondary outcomes ‐ individualised measures of performance, self‐care, body function, participation and quality of life outcomes
The broad range of secondary outcomes precluded meta‐analysis for most outcomes. Guidance on the effectiveness of intervention for secondary outcomes, therefore, is based largely on the results of single studies.
There were no data relating to individualised measures of performance ( Goal Attainment Scaling (GAS), Canadian Occupational Performance Measure (COPM)) in the CIMT versus a low‐dose intervention comparison. Overall, we found no evidence of a difference between CIMT and a high‐dose or dose‐matched comparison for improving individualised measures of performance (GAS, COPM). The exception was the conflicting results on the GAS from the single studies by Aarts 2010 and Gordon 2011. Aarts 2010 found evidence that CIMT was more effective than a dose‐matched comparison group (usual care), while Gordon 2011 found evidence that a dose‐matched comparison group (Hand Arm Bimanual Intensive Training (HABIT)) was more effective than CIMT. These conflicting results are perhaps related to the nature of the CIMT and comparison interventions in each study. Aarts 2010 used the hybrid CMIT (hCIMT) model embedded in a pirate‐theme setting, where CIMT was provided for six weeks followed by twoweeks of bimanual intervention, which targeted family goals. Goals were established using the COPM and an individualised home program established to practice the goal activities. The usual care comparison intervention did not specify whether it included bimanual training or practice of goal tasks. In contrast, the CIMT protocol used by Gordon 2011 provided two weeks of CIMT alone using a sling for six hours per day. The authors stated that the "CIMT group was unable to practice bimanual goals and, instead, practiced unimanual movement components comprising the goal" (p 3) for up to 30 minutes a day. The bimanual group, however, practiced their goals: so it is unsurprising that this group achieved higher scores on the GAS.
For self‐care outcomes (Evaluation of Disability Inventory (PEDI), Functional Independence Measure for Children (WeeFIM)), we were able to conduct a pooled analysis for the PEDI Functional Skills domain only (Deppe 2013; Gordon 2011), for CIMT compared with dose‐matched interventions. We found no evidence of a difference between CIMT and dose‐matched comparison interventions. Data from single studies provided evidence that CIMT was more effective than a low‐dose comparison on the WeeFIM and both domains of the PEDI (de Brito Brandão 2010; Yu 2012). There were conflicting results when CIMT was compared with a high‐dose intervention. Data from the single study by Hoare 2013 found no evidence of a difference between groups for both domains of the PEDI, while outcomes on the WeeFIM in the study by Chen 2014, found evidence that CIMT was more effective than a high‐dose comparison.
For outcomes related to body function, we were able to conduct a pooled analysis for grip strength and the Modified Ashworth Scale (MAS) (wrist, elbow and shoulder) only, for CIMT compared with low‐dose interventions. We identified no evidence of differences. Results of two single studies reinforced these findings (Abootalebi 2010; Sabour 2012), except for shoulder muscle stiffness measured using the MAS. We found no evidence of differences between CIMT and high‐dose interventions in two single studies for MAS at the wrist and elbow or modified Tardieu scale (wrist and elbow) (Hoare 2013; Wallen 2011). A single study evaluated MAS at the elbow for the CIMT versus dose‐matched comparison and provided no evidence of differences (Xu 2012). Overall, there is no evidence of differences in effects on body function outcomes between CIMT and low‐dose, high‐dose and dose‐matched interventions.
The limited number of studies including measures of participation and quality of life precluded the pooling of data for meta‐analysis across studies in any of the comparison group categories. However, due to multiple intervention groups for the study by Kirton 2016 (Kirton 2016a (CIMT + r TMS) and Kirton 2016b (CIMT + sham TMS)), we were able to pool data from the PedsQL for CIMT compared to a dose‐matched intervention. We found no evidence of a difference between CIMT and a dose‐matched comparison for any of the seven domains of the child‐report version, except for the Speech and Communication domain for which we found evidence that the comparison intervention was more effective than CIMT at the five‐to‐six‐month follow‐up period. For the parent‐proxy version of the PedsQL, we found evidence that CIMT was more effective than a dose‐matched comparison for the Movement and Balance and Fatigue domains at immediately postintervention only (out of seven domains).
Two studies found no evidence of a difference between CIMT and a high‐dose comparison (Chen 2014) or dose‐matched comparison (Sakzewski 2011) on any domain of the child‐report version of the Cerebral Palsy ‐ Quality of Life (CP‐QOL) immediately postintervention, or between CIMT and a dose‐matched comparison at any longer term follow‐up period (Sakzewski 2011). Chen 2014 provided evidence that CIMT was more effective than a high‐dose comparison for the CP‐QOL Social Well‐being and Acceptance and Family Health domains at the two‐week to four‐month follow‐up period. Sakzewski 2011 obtained the same outcome on the child‐report version of the KIDSCREEN, except for the Psychological Well‐being domain where there was evidence that CIMT was more effective at immediately postintervention only.
Sakzewski 2011 also used the parent proxy versions of the CPQOL and KIDSCREEN. They found evidence that a dose‐matched comparison was more effective than CIMT for the Function domain and Family Health domains of the CPQOL at the seven‐ to 12‐month follow‐up period and for the Partcipation and Physical Health domains at the five‐ to six‐month follow‐up period. Using the KIDSCREEN, they also found evidence that CIMT was more effective than a dose‐matched comparison for the Financial Resources domain at immediately postintervention and the Social Acceptance domain at the seven‐ to ‐12‐month follow‐up period.
In summary, no study comparing CIMT with a low‐dose intervention measured quality of life, and results for the other comparisons were inconsistent; effects were observed for just a few of the many domains on quality of life measures at varying endpoints.
A single study assessed participation using two measures (Assessment of Life Habits (LIFE‐H), Children’s Assessment of Participation and Enjoyment (CAPE)) for the CIMT versus dose‐matched comparison (Sakzewski 2011). It identified no evidence of differences between groups on any domains.
Overall completeness and applicability of evidence
Although many of the RCTs included in this review evaluated the use of CIMT in children with unilateral CP, we downgraded the quality and strength of evidence due to small sample sizes, inability to blind children and therapists to intervention, and heterogeneity in dosage of CIMT and comparison interventions and outcomes measured.
Many gaps in the evidence base remain (Eliasson 2014a). Outcomes from this review largely related to the short‐term effects of CIMT in children with CP. It is likely that the longer‐term effect of CIMT will be difficult to establish. Longer‐term outcomes from a single block of CIMT are susceptible to influence from other treatments and ongoing child development (Eliasson 2014a). More importantly, intensive blocks of upper‐limb intervention, such as CIMT, are not considered a one‐off intervention. Children experience periods of rapid development and improvement in skills, so the cumulative effect of multiple blocks of CIMT or bimanual therapy (or both) requires further investigation.The few studies ‐ excluded from this review ‐ that specifically investigated the effect of repeated CIMT, found that children maintained improvements from the first programme of CIMT and made further gains after a second, with one year in between blocks (Charles 2006). DeLuca 2015 reported similar outcomes from up to three blocks of CIMT with intervals of between four to 40 months. These findings support a model of block‐based, upper‐limb intervention for children with unilateral CP with defined breaks in between. However, further studies investigating the repeated effects of CIMT are required (Eliasson 2014a), and pragmatic research methods, such as comparative effectiveness studies, should be supported (Damiano 2014; Hoare 2014).
Across studies, there was variable and often incomplete reporting of dosage of CIMT. It has been acknowledged that calculation for dosage is complex due to the number of factors to consider (Eliasson 2014a). This can include the time the constraint device is worn per day, the duration, frequency and length of therapy provided with a therapist or at home with a parent, or both. We propose future studies use consistent and standardised guidelines for calculation of total dosage of CIMT as used in this review (i.e. Total hours of CIMT intervention = therapist‐led intervention + parent‐led intervention + other intervention (e.g. usual care) + forced use during waking hours). Careful monitoring and reporting of the actual dosage of CIMT using log books or other technology will assist in understanding the implications of actual dosage of CIMT, as this often does not reflect the dosage reported in a study protocol (Eliasson 2005; Hoare 2013). A major limitation in reporting of studies of CIMT, also identified by Sakzewski 2016, relates to the specific content and dosage of comparison interventions. Studies' reporting of content and dose was frequently inadequate for interpretation of results and replication of interventions. The findings from this review highlight the need for future trials of CIMT to use the Template for Intervention Description and Replication (TIDieR) checklist and guidelines for reporting interventions (Hoffmann 2014). Achieving consistency and a high standard of reporting of interventions will allow for adequate identification of the influence of intervention characteristics, such as dosage and content of interventions, and other important contextual aspects of intervention protocols.
The small number of studies included in each comparison did not allow for subgroup analyses for child characteristics such as age, and intervention characteristics such as the dosage or model of CIMT. Most studies included in this review also did not report individual data following CIMT. While the main effects and between‐group differences demonstrated positive improvements, it was evident from the large standard deviations (SDs) and wide confidence intervals (CIs) within studies that not all children with unilateral CP improved following CIMT. Hoare 2014 identified that, in the few trials where individual responses have been reported, 18% to 65% of children did not demonstrate change on the AHA greater than the smallest detectable difference of five AHA units. We encourage future studies to report supplementary data for individual participants to enable an understanding of the child characteristics that influence response to CIMT and identify children who are likely to respond positively to CIMT. Such supplementary data could include baseline and follow‐up data from clinical outcomes alongside individual characteristics such as age, MACS (Eliasson 2006) or mini‐MACS levels (Eliasson 2017), cognitive function and brain lesion characteristics (if available).
We explored heterogeneity by following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). This included visual exploration of the forest plots and consideration of the I2 statistic for each meta‐analysis (Higgins 2002). Simulation studies (Kontopantelis 2012), however, have shown that estimates of heterogeneity variance (I2 values) are inaccurate when the number of studies in a meta‐analysis is small, which is the case for this review. We acknowledge that not calculating and presenting the 95% CI for heterogeneity variance, as it requires conducting meta‐analysis outside RevMan 5 (Review Manager 2014), is a limitation of this review and readers should consider limitation of this when interpreting the results.
In summary, the applicability of the evidence from this review relates to children with CP from three months to 19 years of age (mean age of 5.96 years). Children from the included studies were a representative sample of children with unilateral CP (Eliasson 2006), with most classified at MACS levels I and II (88%). Knowledge of the effects of CIMT for children younger than 12 months of age is growing and currently the focus of several ongoing studies (Boyd 2017; NCT02346825).
Quality of the evidence
Using the GRADE criteria (Guyatt 2008), we rated the quality of evidence for all comparisons as low, due to high risk of bias (including lack of blinding due to impossibility of blinding participants) and imprecision (small number of participants). We judged 28 studies (78%) to have low risk of bias for random sequence generation. However, we judged the methods for allocation concealment as being low risk in only 15 trials (41%). We rated 16 studies as having unclear risk of bias due to insufficient information to permit judgement. We rated five studies as at high risk of bias. Ratings of risk of bias should be considered when assessing the quality of evidence as intervention effect estimates may be exaggerated in studies with a high risk of bias. As an example, intervention effects may be inflated by 15% in trials with unclear or inadequate allocation concealment (Savović 2012).
One of the strongest features of the quality of evidence in this review is the blinding of outcome assessors for objectively‐observed outcomes across 29 included studies. Only two studies did not use blinded assessors for objectively‐observed outcomes and four studies did not report blinding. Adding further weight to the strength of evidence for studies, we chose to include only those outcome measures with known validity and reliability in children with CP. We also considered standardised assessments that were administered or scored in an adapted manner invalid. We rated all trials as being at high risk of bias for the category of blinding of participants. Failure to blind of participants and therapy providers (personnel) is unavoidable when examining the effect of CIMT but nevertheless introduces risk of performance bias. Twenty‐four trials used self‐ or parent‐reported outcomes so we created an additional item for rating risk of bias: blinding of outcome assessment: self‐reported outcomes. We judged all 24 trials that used self‐reported outcomes as being at high risk of bias given that lack of, or unclear, double‐blinding is associated with a 22% (on average) exaggeration of intervention effects (Savović 2012).
We rated 24 studies (67%) at low risk of bias for incomplete outcome data because of low levels of missing data and reported intention‐to‐treat analyses. We judged the risk of attrition bias as unclear in nine studies. We considered three studies to be at high risk of bias due to high attrition rates or unbalanced attrition rates across groups, as it is possible that attrition rates may affect outcomes. While the extent and direction of bias is unpredictable, excluding participants from the analysis can result in biased estimates of treatment effects (Nüesch 2009). We rated 25 studies (69%) at unclear risk of reporting bias as they were either not registered or not preceded by a published trial protocol. Eleven trials had accessible trial registrations and only five had a published trial protocol. However, this is improving with time ‐ the five protocols were published since 2011.
Sample sizes were small ‐ the number of participants included in the pooled analyses ranged from 34 to 229 participants. Fifty per cent of the included trials had a sample size ≤ 30 participants. Ten trials (28%) had a sample size fewer than 20 participants. The small sample sizes across the three categories of comparison interventions precluded or limited the ability to pool data, which was further eroded by inconsistent use of outcomes measures. Where we were able to pool data for meta‐analysis, the relatively small number of participants included in the analysis led to large within‐study variations and may have resulted in analyses that lacked statistical power. With the exception of the Gelkop 2015 study, there is no strong indication of bias due to sample size on visual inspection of forest plots ‐ bias would be evident if a disproportionate number of smaller studies reported positive findings than negative findings. Our analyses did not identify any differences in treatment effect between CIMT and a dose‐matched comparison (Analysis 3.4; Analysis 3.5), which included data from Gelkop 2015. Although inclusion of data from Gelkop 2015 did not influence the overall outcome, the size of treatment effects for these outcomes may have been inflated.
Potential biases in the review process
A common source of bias in systematic reviews is the failure to identify all relevant studies. We attempted to minimise this bias by performing thorough database searches, including searching for studies in all languages, searching reference lists of included studies and relevant systematic reviews, and corresponding with the authors of the included studies. We are confident that this review includes all of the published and unpublished evidence that meets our inclusion criteria (Criteria for considering studies for this review).
Five review authors were paired, allocated included trials, independently extracted data and assessed risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The process of two review authors independently reviewing abstracts and extracting data (with a third review author moderating disagreements) minimised bias. Some of the review authors were also authors on included trials; however, they did not review their own papers to limit the potential for bias. We actively attempted to contact study authors to clarify points that were unclear or absent and obtain any missing, inconsistent or incomplete data. Not all study authors responded and some were unwilling or unable to provide the requested information. As a result, the study methodology of some trials remained unclear and there were gaps in the data available for analyses. Two review authors, using the GRADE approach, collaborated to reach consensus on the quality of the body of evidence for each outcome in each comparison group category (Guyatt 2008).
Agreements and disagreements with other studies or reviews
Since 2005, there have been 14 systematic or narrative reviews of CIMT in children with CP (Andersen 2013; Brady 2009; Charles 2005; Chen 2014a; Chiu 2016, Dong 2013; Eliasson 2014a; Huang 2009; Klepper 2017; Nascimento 2009; Oh 2014; Sakzewski 2009; Sakzewski 2014; Tervahauta 2017). Two broader systematic reviews of interventions for children with CP have also included studies of CIMT (Novak 2013; Tinderholt Myrhaug 2014). The review by Oh 2014 was not published in English. Reviews published prior to 2010 all provided preliminary support for the use of CIMT in children with CP but reinforced the need for further research before advocating for CIMT to be used as part of standard clinical practice (Charles 2005; Brady 2009; Huang 2009; Nascimento 2009; Sakzewski 2009). Since 2010, 30 additional RCTs have been published and subsequent systematic reviews concluded that CIMT was more effective for improving upper‐limb function than low‐intensity or standard care interventions, and equally effective as an alternative, upper‐limb intervention delivered at a similar dose. Although the outcomes of our review are consistent with these findings, our use of GRADE principles to rate more objectively the quality of evidence and strength of recommendations has resulted in more tempered conclusions about the effects of CIMT. Below we present other methodological differences that should also be highlighted.
Our findings are inconsistent with that of the systematic review by Dong 2013, regarding differential outcomes of bimanual compared with CIMT. Dong 2013 concluded that "CIMT yields more improvements in the unimanual capacity of the impaired arm compared with bimanual therapy". Further, the authors state "a potential benefit of bimanual therapy is that participants may see more improvement in both bimanual performance and self‐determined overall life goals" (Dong 2013, p 133). In comparison, our systematic review found no evidence of a difference in this outcome. There are several reasons for the discrepancy in findings between the two systematic reviews. The Dong 2013 review reported including seven RCTs of children with unilateral CP, aged two to 16 years old. Of these seven studies, one, Hung 2011 (in Gordon 2011), was a subset of children from a larger study (Gordon 2011), which was also included in Dong's review, therefore duplicating findings. In addition, three of the included studies reported different outcomes from the same study by Sakzewski 2011. Consequently, only four unique studies were actually included, where our review analysed the results of significantly more studies. Dong 2013 rated the methodological quality of included trials using the PEDro scale (Maher 2003), but a narrative review of individual study findings guided conclusions rather than consideration of the quantitative data available. The report of differential outcomes for bimanual versus CIMT is not supported by the evidence. We are concerned that the incorrect conclusions in the Dong 2013 review have been reported therefore perpetuating the erroneous findings.
The systematic review and meta‐analysis by Chen 2014a included 27 RCTs. Consistent with Dong 2013, there were errors of duplication of findings: the findings from one study that were reported in more than one publication, as if they were multiple studies, including Hung 2011 (a subset of children from Gordon 2011), Hsin 2012 (a subset of children from Chen 2014), Fedrizzi 2012 (follow‐up data from Facchin 2011) and Geerdink 2013 (additional data from Aarts 2010). Chen 2014a included each of these papers as separate studies. That said, however, the outcomes from the Chen 2014a review were consistent with our findings: CIMT provided a medium, beneficial effect when compared with conventional therapy; a large effect when compared with a lower‐dose comparison group; and a small effect when compared with a dose‐equivalent group. However, there were differences that are important to highlight. Chen 2014a pooled outcomes from multiple studies with highly variable dosages of experimental and comparison interventions, that were assessed using a range of unrelated measures (many with no psychometric data for use in children with CP) in a meta‐analysis, and calculated the effect size using the standardised mean difference (SMD). This approach requires careful consideration when interpreting the results. The study by Rostami 2012b demonstrated a significantly larger effect size when compared with other studies. When closely examined, outcomes from this study included the Pediatric Motor Activity Log (PMAL) (version used was unknown) and the Bruninks‐Oseretsky Test of Motor Proficiency, subtest 8. We excluded both of these outcomes from our review update as there is no evidence of validity or reliability as an outcome measure in children with CP (see Table 7). The authors of the Chen 2014a study note that the presence of a dose‐equivalent comparison group had significant associations with study effect size. This is unsurprising given how the effect size (Cohen’s d) is calculated: by subtracting the mean of the control/comparison group from the mean of the intervention group and dividing by the SD of the control group (or a pooled SD from both groups); the larger the difference between groups and the smaller the variability between groups, the larger the effect size. Therefore, CIMT compared with a control group receiving no treatment/usual care will result in a much larger Cohen’s d estimate compared with a control group receiving a treatment of equal intensity (e.g. HABIT). Due to the heterogeneity between studies, we caution against comparing effect sizes between studies of CIMT with substantially different dosages of comparison intervention and outcome measures. The outcomes of our review support this, where, on average, the effect estimate for outcomes from the AHA were approximately 10 times greater when CIMT was compared with a low‐dose comparison (usual care) than with a high‐dose or dose‐matched comparison. This provides support for our decision not to pool data from the four comparisons together in a single meta‐analysis. We propose that it is not theoretically justifiable to include interventions with vastly different treatment dosages in one comparison group. Readers should carefully consider the type of control/comparison intervention and the variability in response to treatment, particularly when estimates are calculated using Cohen’s d.
Using a systematic review with meta‐analysis design, Chiu 2016 aimed to address a number of questions relating to the effect of CIMT in children with unilateral CP. In keeping with our review, Chiu 2014 found evidence that CIMT was more effective than no/sham intervention, but no evidence that CIMT was better than the same dose of upper‐limb therapy without restraint. The Chiu 2014 review also highlights the problem of not clearly identifying unique studies, as the authors had incorrectly classified several included reports as single studies, and thus duplicated data from the same cohort of children (Geerdink 2013 same cohort as Aarts 2010; De Brito Brandao 2012 same cohort as Gordon 2011; and DeLuca 2006 same cohort as Taub 2004), or subset of cohort of children (Hsin 2012 subset of cohort from Chen 2014). There are also important methodological differences between the Chiu 2014 review and this review. The authors of Chiu 2014 classified the comparison groups in Wallen 2011 and Hisn 2012 (in Chen 2014) as dose‐matched comparisons, whereas we classified these trials as high‐dose comparisons. Like Chen 2014a, the authors of Chiu 2014 pooled data, using SMD, from measures that we argue are conceptually incompatible and should not be pooled. These included the AHA, the Jebsen Taylor Test of Hand Function (JTTHF), 9 hole peg test (9HPT) and the QUEST. These outcomes measure different constructs: bimanual performance, unilateral speed and dexterity and quality of movement. Adopting a random‐effects model and using the SMD does not account for pooling measures with substantially different and clinically implausible constructs. The pooled effect favoured CIMT for improving activity and participation outcomes, whereas visual inspection of the forest plots and the I2 value indicated substantial heterogeneity (I2 value of 65% and 84% for each model (Figure 2 and Figure 4 in Chiu 2014)). The authors did not address the possible reasons for the significantly larger effects in the Rostami 2012b and Taub 2011 studies, nor did they repeat the data analyses with these studies excluded. Additionally, the Chiu 2016 review included studies that used measures with no demonstrated reliability or validity for children with CP, potentially inflating the resulting treatment effects. For example, Rostami 2012b used the Bruninks‐Oseretsky Test of Motor Proficiency and the PMAL (version used unknown), and Taub 2011 used the Inventory of New Motor Activities and Programs Instrument. We excluded these outcomes from our review as having no evidence of validity or reliability as an outcome measure in children with CP (Table 7). Another important difference to highlight relates to the classification of outcome measures. Chiu 2016 classified the AHA, PMAL and Caregiver Functional Use Survey as participation measures. However, consistent with the International Classification of Functioning, Disability and Health (ICF) (WHO 2001) and categorisation in literature (Hoare 2011), these outcomes are not measures of participation but activity level.
The systematic review by Klepper 2017 aimed to compare mCIMT and bimanual therapy of equal intensity in children with unilateral CP. It included five studies from eight papers (Facchin 2011; Gelkop 2015; Gordon 2011; Hoare 2013; Sakzewski 2011). The authors planned to use the SMD to calculate effect sizes for each outcome (upper‐limb function, individualised goals, self‐care, and caregiver assistance), but were unable to undertake a meta‐analysis due to heterogeneity and the small number of included studies. Of the 43 comparisons, they reported two as statistically significant. These were both studies that included a subset of children from a larger study (Gordon 2011). Consistent with outcomes from our review, the data did not demonstrate a superior effect for CIMT compared with bimanual therapy for improving a range of unimanual and bimanual outcomes. The authors made a weak, non‐specific recommendation for either CIMT or bimanual therapy to improve a child’s performance of daily functional activities on the basis of results of the COPM and the PEDI. They used GRADE to guide their interpretation of the overall quality of the evidence for each outcome and the strength of the recommendations (Guyatt 2008), which they judged to be moderate. They made a strong recommendation for either CIMT or bimanual therapy to improve quality of unimanual capacity and bimanual performance. Using GRADE, our overall recommendations were judged as weak due to the limitations in included studies.
The findings from the most recent review by Tervahauta 2017 included six studies from nine papers (Charles 2006; Facchin 2011; Gelkop 2015; Sakzewski 2011; Sakzewski 2015a; Zafer 2016), and echoed our own results. In that review, Tervahauta 2017 rated methodological quality using the American Academy of Cerebral Palsy and Developmental Medicine criteria, and excluded hybrid CIMT (hCMIT) interventions. They aimed to evaluate the effect of CIMT compared with bimanual therapy and to identify if a particular model of CIMT was superior. They calculated effect sizes using SMD at immediately postintervention. Unlike previous systematic reviews with meta‐analyses, the authors chose not to combine data in a meta‐analysis due to the considerable clinical and methodological heterogeneity across studies. They found no evidence of a superior effect for any model of CIMT and no effect for treatment specificity (that is, there was no evidence that CIMT was more effective than bimanual intervention in improving unimanual function, and no evidence that bimanual intensive training was more effective than CIMT in improving bimanual function). Overall, the outcomes of this review were guided by robust methodology and in agreement with the outcomes of the currently reported review.
Authors' conclusions
Implications for practice.
This review found weak evidence that, compared with an intervention carried out at low intensity, constraint‐induced movement therapy (CIMT) is more effective at improving bimanual performance and unimanual capacity in children with unilateral cerebral palsy (CP). CIMT appears no more effective, however, than another upper‐limb therapy that is carried out intensively (i.e. the intensive, high‐dose and dose‐matched comparison interventions). The 17 low‐dose comparison interventions were generally not described in sufficient detail to provide a clear indication of the nature of the intervention, although interventionists in nine studies were occupational therapists, implying that an upper‐limb intervention was included. In contrast, the majority of high‐dose and dose‐matched comparison interventions were intensive, bimanual interventions that were therapist‐led and more clearly defined. Consequently, the outcomes of this review provide support for the implementation of well‐defined, time‐limited, goal‐directed blocks of CIMT or bimanual therapy at an intensity greater than low‐dose comparison interventions (i.e. the intensive, high‐dose and dose‐matched interventions). The challenge now for clinicians is to implement these outcomes into clinical practice and to identify potential barriers and enablers for implementation in their local context (Sakzewski 2014b). Generally speaking, CIMT did not appear to impact body structure and function outcomes, such as grip strength, muscle stiffness and spasticity, and had no consistent effect on quality of life. Although there was minimal research on participation outcomes, it is hypothesised that CIMT and bimanual interventions may not have a direct effect on children's participation (Imms 2016a).
Although we were unable to examine the impact of different modes of delivery of CIMT, such as signature CIMT (sCIMT), modified CMIT (mCIMT) or hybrid CMIT (hCIMT), our review shows that CIMT can be implemented in a range of modes and settings, that constraint can be achieved using various devices, and the accompanying intervention can be delivered by interventionists other than therapists, including parents and students. Our review indicates that the specific mode of CIMT intervention is a lesser issue than implementation of an intensive, carefully‐targeted and well‐supported programme. However, maintenance of treatment fidelity is essential. Clinicians should ensure that the two key ingredients across all models of CIMT are maintained: 1) restraint of the well‐functioning upper limb (irrespective of device/type); and 2) intensive, structured training (irrespective of type) (Eliasson 2014a). The mean number of hours of CIMT provided across studies was 129 hours (range = 20 hours in Yu 2012 to 504 hours in Sung 2005). We did not identify any study that concluded that short‐term constraint methods, such as occasional hand holding, were effective. No study provided CIMT for a period longer than 10 weeks. Clincians, therefore, should view CIMT as a relatively short‐term intervention that is provided for a defined period, and carefully evaluate outcomes before and after implementation using valid and reliable measures.
The high‐dose and dose‐matched comparison interventions, predominantly intensive occupational therapy and bimanual interventions, offer evidence‐based options for families. As with CIMT, these interventions should be implemented with adherence to intervention fidelity, including the nature of intervention and dose.
It is important for clinicians to educate children and families about the outcomes of this review. CIMT appears to be a safe intervention for children with unilateral CP. Families should feel confident that, on average, active engagement in a well‐defined, intensive program of CIMT or bimanual therapy can lead to improvements in bimanual performance and unimanual capacity. Discussions with families will include the magnitude of the benefit, the uncertainty of long‐term benefits of the blocks of intervention, and the need to continually monitor children's upper‐limb function and occupational performance to identify appropriate timing of further episodes of intensive, upper‐limb interventions or implementation of alternative means of achieving child‐ and family‐centred goals. It should be emphasised that not all children respond to CIMT (Hoare 2015). The challenge remains for researchers to identify the most appropriate of these interventions to implement with individual children. This review was not able to identify the characteristics of children who could be advised to participate in one or the other of CIMT or bimanual interventions. In the meantime, clinicians should consider the specific goals for individual children and families and choose the most developmentally appropriate, family‐centred, and convenient of these approaches (Hoare 2017). Factors, in addition to child and family characteristics and preferences, which may impact of intervention selection include therapist expertise, costs of implementing the intervention, funding and service delivery models, and resource availability.
Implications for research.
The current evidence for CIMT in children with unilateral CP mostly comprises small studies at high or unclear risk of bias, and that use a wide range of outcome measures. Larger, more rigorous and more adequately reported randomised controlled trials (RCTs) in the future should aim to develop sequentially knowledge of the effect of CIMT in children with unilateral CP. Future research is required to address three high‐priority areas for future CIMT research identified by expert consensus (Eliasson 2014a). These are: 1) the effect of age on the treatment effect; 2) the effect of repeated CIMT; and 3) the minimum dosage of CIMT required to impact outcomes. Our findings also indicate that there are no further advantages to be gained by conducting studies of CIMT compared with low‐dose interventions. Efforts to tease out optimal dosage, age effects and other critical questions must focus on intensive delivery of both CIMT and comparison interventions. We also recommend that future studies of CIMT in children with CP undertake cost‐benefit analyses, to determine the impact and cost‐effectiveness of the diverse models of upper‐limb intervention, to assist with future knowledge translation.
Inadequate reporting of both CIMT and comparison interventions was common and substantial in this review. We recommend that future trials use the Template for Intervention Description and Replication (TIDieR) checklist and guideline for reporting interventions (Hoffmann 2014). The TIDieR checklist guides study authors to provide details on the rationale for the interventions included in the study, materials required, procedures followed, and to specify who provided the intervention, modes of delivery, where, when and how much intervention was provided, any intervention tailoring available, modifications made to the intervention during study implementation, and planned and actual intervention adherence or fidelity. This information can be provided as supplementary information or included in a published trial protocol. A full description of all comparison interventions is required.
We located 86 published papers or abstracts (range 1 to 10 per study) reporting findings from the 36 studies included in this review. During the literature search and data extraction process, it was frequently difficult and time consuming to establish whether a publication resulted from an existing study and was a duplicate; reported a separate set of outcomes or contained a subgroup of participants; or was a unique study by the same group of authors. In the Agreements and disagreements with other studies or reviews section of this review, we have identified that other researchers have not recognised multiple publications from the same study and have inadvertently synthesised findings from duplicate publications of the same study. To avoid confusion, future studies of CIMT should consider publishing a single manuscript for reporting study results. At the very least, each publication emanating from the same study using the same cohort or subgroups of children should explicitly refer to previous publications and clearly articulate the relationship of the publication to the study as a whole. Trial registration is frequently mandatory and publication of a study protocol is becoming more common ‐ both will assist systematic reviewers and others synthesising evidence to identify and accurately analyse and interpret findings.
We recommend that all authors of trials investigating CIMT in children with CP follow the CONSORT guidelines for reporting RCTs (Schulz 2010) and the extended HARMS guidelines for reporting adverse events (Ioannidis 2004). Although relatively few adverse events potentially related to the CIMT were reported, only 50% of included studies reported monitoring this outcome and the absence of adverse events cannot be confirmed. Implementation of a standardised method of recording and reporting adverse events would ensure more consistent and deliberate reporting.
The choice of outcome measure should be carefully matched to the expected effect of the intervention in all research evaluating health‐related interventions. We strongly recommend future studies use reliable outcome measures that have been validated for children with CP and their families and are matched to the aims of CIMT ‐ improving unimanual capacity and bimanual performance. Understanding its impact on individualised goals related to self‐care and ability to complete other everyday activities is also relevant to consider. Fifty‐seven outcome measures were used by the 36 included studies included in this review and over half of these were used in a single study. This severely limits the ability to pool data for meta‐analysis, slowing the development of further knowledge in this area of research. Uniform follow‐up periods after completion of CIMT could also be adopted to enable more accurate meta‐analysis of studies. Studies should adhere to the standardised procedures for the administration and scoring of outcome measures. When these procedures are modified, the validity and reliability of the outcome is not maintained and the integrity of the measure is threatened (Eliasson 2014a). Unless studies are investigating the cumulative and longitudinal effects of multiple blocks of CIMT or bimanual therapy (or both), outcome measures should also reflect the potential impact of a short‐term intensive block of upper‐limb activity level intervention. Although CIMT has demonstrated domain‐specific changes in quality of life in some studies included in this review, a single block of CIMT does not aim to change multi‐dimensional constructs such as quality of life (Gilson 2014) or participation (Adair 2015; Imms 2016b). Selection of outcome measures in future studies of CIMT should reflect this and efforts should also be made to minimise the potential for assessment burden for children and their families. Research has also repeatedly demonstrated there is no evidence that CIMT either improves, or leads to deterioration in, body function and structure outcomes. Studies could justifiably avoid adding to assessment burden and research waste by refraining from further measurement of these types of variables.
What's new
| Date | Event | Description |
|---|---|---|
| 13 May 2019 | Amended | Title added to Plain Language Summary. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 12 December 2018 | New search has been performed | Full update of review |
| 12 December 2018 | New citation required but conclusions have not changed | Thirty‐four new studies included in review. |
| 18 March 2018 | New search has been performed | Review updated following a new search in March 2018. |
| 13 November 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the Cochrane Developmental, Psychosocial, and Learning Problems editorial team for their guidance and support.
We would sincerely like to thank the following people for their support in undertaking this review:
Kathryn Duncan, Senior Library Coodinator, Library Academic and Research Services, Australian Catholic University, Melbourne, Australia;
Associate Professor Mehdi Rassafiani, Department of Occupational Therapy, University of Social Welfare and Rehabilitation Sciences, Tehrān, Iran;
Dr Fakher Rahim, Ahvaz Jondishapour University of Medical Sciences, Ahvaz, Iran;
Dr Jason Wasiak, The University of Melbourne, Melbourne, Australia;
Dr Kirsty Stewart, Occupational therapist, The Children's Hospital at Westmead, Sydney, Australia;
Miranda Cumpston, former Head of Learning & Support, Cochrane Central Executive, Melbourne Australia; and
All authors who kindly provided data ‐ especially Dr Leanne Sakzewski.
Appendices
Appendix 1. Search Strategies 2006 onwards
Central Register of Controlled Trials (CENTRAL), in the Cochrane Library
CENTRAL strategy used 2006 to 2016
(Title, Abstract, Keywords) = "cerebral palsy" OR hemipleg* AND (Title, Abstract, Keywords) = CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice" OR restrain*
CENTRAL strategy 2016 onwards
#1 MeSH descriptor: [Cerebral Palsy] this term only #2 MeSH descriptor: [Hemiplegia] this term only #3 "cerebral pals*":ti,ab,kw #4 Hemipleg*:ti,ab,kw #5 {or #1‐#4} #6 (CIMT or mCIMT or "CI therap*" or forced or "massed practice" or restrain*):ti,ab,kw #7 constrain* #8 #6 or #7 #9 #5 and #8 with Publication Year from 2016 to 2018, in Trials
MEDLINE Ovid
1 cerebral palsy/ 2 cerebral pals$.tw. 3 hemiplegia/ 4 hemipleg$.tw. 5 (unilateral adj3 spastic$).tw. 6 unilateral CP.tw. 7 or/1‐6 8 Restraint, Physical/ 9 (constrain$ adj10 (movement$ or therap$)).tw. 10 CIMT.tw. 11 mCIMT.tw. 12 CI therap$.tw. 13 forced.tw. 14 massed practice.tw. 15 or/8‐14 16 randomized controlled trial.pt. 17 controlled clinical trial.pt. 18 randomi#ed.ab. 19 placebo$.ab. 20 drug therapy.fs. 21 randomly.ab. 22 trial.ab. 23 groups.ab. 24 or/16‐23 25 exp animals/ not humans.sh. 26 24 not 25 27 7 and 15 and 26
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid
1 cerebral pals$.tw. 2 hemipleg$.tw. 3 (unilateral adj3 spastic$).tw. 4 unilateral CP.tw. 5 or/1‐4 6 (constrain$ adj10 (movement$ or therap$)).tw. 7 CIMT.tw. 8 mCIMT.tw. 9 CI therap$.tw. 10 forced.tw. 11 massed practice.tw. 12 or/6‐11 13 5 and 12 14 (random$ or trial$ or control$ or group$ or placebo$ or blind$ or prospectiv$ or longitudinal$ or meta‐analys$ or systematic review$).tw. 15 13 and 14
MEDLINE Epub Ahead of Print Ovid
1 cerebral pals$.tw. 2 hemipleg$.tw. 3 (unilateral adj3 spastic$).tw. 4 unilateral CP.tw. 5 or/1‐4 6 (constrain$ adj10 (movement$ or therap$)).tw. 7 CIMT.tw. 8 mCIMT.tw. 9 CI therap$.tw. 10 forced.tw. 11 massed practice.tw. 12 or/6‐11 13 5 and 12 14 (random$ or trial$ or control$ or group$ or placebo$ or blind$ or prospectiv$ or longitudinal$ or meta‐analys$ or systematic review$).tw. 15 13 and 14
Embase Ovid
1 cerebral palsy/ 2 cerebral pals$.tw. 3 hemiplegia/ 4 hemipleg$.tw. 5 (unilateral adj3 spastic$).tw. 6 unilateral CP.tw. 7 or/1‐6 8 constraint induced therapy/ 9 movement therapy/ 10 (constrain$ adj10 (movement$ or therap$)).tw. 11 CIMT.tw. 12 mCIMT.tw. 13 CI therap$.tw. 14 forced.tw. 15 massed practice.tw. 16 or/8‐15 17 7 and 16 18 exp Clinical trial/ 19 Randomized controlled trial/ 20 Randomization/ 21 Single blind procedure/ 22 Double blind procedure/ 23 triple blind procedure/ 24 Crossover procedure/ 25 Placebo/ 26 Randomi#ed.tw. 27 RCT.tw. 28 (random$ adj3 (allocat$ or assign$)).tw. 29 randomly.ab. 30 groups.ab. 31 trial.ab. 32 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 33 Placebo$.tw. 34 Prospective study/ 35 (crossover or cross‐over).tw. 36 prospective.tw. 37 or/18‐36 38 17 and 37 39 remove duplicates from 38
CINAHL EBSCOhost
S31 S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 S30 TI (evaluat* study or evaluat* research) or AB (evaluate* study or evaluat* research) or TI (effectiv* study or effectiv* research) or AB (effectiv* study or effectiv* research) OR TI (prospectiv* study or prospectiv* research) or AB(prospectiv* study or prospectiv* research) or TI (follow‐up study or follow‐up research) or AB (follow‐up study or follow‐up research) S29 placebo* S28 crossover* or "cross over*" S27 (MH "Crossover Design") S26 (tripl* N3 mask*) or (tripl* N3 blind*) S25 (trebl* N3 mask*) or (trebl* N3 blind*) S24 (doubl* N3 mask*) or (doubl* N3 blind*) S23 (singl* N3 mask*) or (singl* N3 blind*) S22 (clinic* N3 trial*) or (control* N3 trial*) S21 (random* N3 allocat* ) or (random* N3 assign*) S20 randomis* or randomiz* S19 (MH "Meta Analysis") S18 (MH "Clinical Trials+") S17 MH random assignment S16 S7 AND S15 S15 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 S14 massed practice S13 forced S12 CI therap* S11 mCIMT S10 CIMT S9 (constrain* N10 (movement* or therap*)) S8 (MH "Constraint‐Induced Therapy") S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 S6 unilateral CP S5 (unilateral N3 spastic*) S4 hemipleg* S3 (MH "Hemiplegia") S2 cerebral pals* S1 (MH "Cerebral Palsy")
PsycInfo EBSCO
S1 DE "Cerebral Palsy" OR DE "Hemiplegia" S2 TI ("cerebral pals*" OR hemipleg* OR "unilateral CP" ) OR AB ( "cerebral pals*" OR hemipleg* OR "unilateral CP") S3 TI unilateral N3 spastic* OR AB unilateral N3 spastic* S4 S1 OR S2 OR S3 S5 DE "Physical Restraint" S6 TI ( CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice" ) OR AB ( CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice" ) S7 TI ( (movement* OR therap*) N10 constrain* ) OR AB ( (movement* OR therap*) N10 constrain* ) S8 S5 OR S6 OR S7 S9 (MR "clinical trial") OR (MR "treatment outcome") S10 TI ( randomi#ed OR placebo* OR randomly OR trial OR groups ) OR AB ( randomi#ed OR placebo* OR randomly OR trial OR groups ) S11 S9 OR S10 S12 (PO "animal") NOT (PO "human") S13 S11 NOT S12 S14 S4 AND S8 AND S13
Science Citation Index ‐ Extended Web of Science
Strategy used 2006 to 2016
TOPIC: (("cerebral palsy" OR hemipleg*)) AND TOPIC: (CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice") Indexes=SCI‐EXPANDED
Strategy used 2016 onwards
# 8#6 AND #3 Indexes=SCI‐EXPANDED Timespan=2016‐2018 # 7#6 AND #3 Indexes=SCI‐EXPANDED Timespan=All years #6 #5 OR #4 Indexes=SCI‐EXPANDED Timespan=All years #5 TS=(constrain* Near/3 (movement* or therap*)) Indexes=SCI‐EXPANDED Timespan=All years #4 TS=(CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice") Indexes=SCI‐EXPANDED Timespan=All years #3 #2 OR #1 Indexes=SCI‐EXPANDED Timespan=All years #2 TS=(hemipleg*) Indexes=SCI‐EXPANDED Timespan=All years #1 TS= ("cerebral palsy") Indexes=SCI‐EXPANDED Timespan=All years
PEDro (www.pedro.org.au)
Note: Search terms cannot be combined in PEDro, so we undertook two separate searches.
Search 1
(Abstract/Title) =“cerebral palsy”
AND
(Method) = clinical trial
Search 2
(Abstract/Title) =hemiplegia
AND
(Method) = clinical trial
OTseeker (www.otseeker.com)
Search Any Field
("cerebral palsy"OR hemipleg*) AND (CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice" OR restrain*)
Cochrane Database of Systematic Reviews (CDSR), part of the Cochrane Library
#1 MeSH descriptor: [Cerebral Palsy] this term only #2 MeSH descriptor: [Hemiplegia] this term only #3 "cerebral pals*":ti,ab,kw #4 Hemipleg*:ti,ab,kw #5 {or #1‐#4} #6 (CIMT or mCIMT or "CI therap*" or forced or "massed practice" or restrain*):ti,ab,kw #7 constrain* #8 #6 or #7 #9 #5 and #8 in Cochrane Reviews (Reviews and Protocols)
ClinicalTrials.gov (https://clinicaltrials.gov)
Search field: Other terms
("cerebral palsy" OR hemipleg*) AND (CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice" OR restrain*)
WHO ICTRP (http://apps.who.int/trialsearch/AdvSearch.aspx)
(Condition) = ("cerebral palsy" OR hemipleg*)
AND
(Intervention) = (CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice" OR restrain*)
ANZCTR (www.anzctr.org.au)
Note: Interface only allows 100 characters in the search, therefore we undertook two searches.
Search 1
(Search terms) = "cerebral palsy" AND (CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice" OR restrain*)
Search 2
hemipleg* AND (CIMT OR mCIMT OR "CI therap*" OR forced OR "massed practice" OR restrain*)
Appendix 2. Criteria for assigning 'Risk of bias' judgements
We used the domains from the Cochrane Handbook for Systematic Reviews of Interventions to appraise the quality and risk of bias of included articles (Higgins 2011a). The domains below outline the criteria used and any interpretations required that were specific to this topic.
Sequence generation (selection bias)
We described methods used to generate the allocation sequence using quotes from the reference, when possible. We assigned comments such as ‘probably done’ or ‘probably not done’ to supplement any ambiguous quotes. We then assigned each study to one of the following categories.
Low risk of bias: adequate method used for randomisation (e.g. computer generated, table of random numbers)
High risk of bias: inadequate method of randomisation used (e.g. case file number, date of birth, alternate numbers)
Unclear risk of bias: uncertainty about whether an appropriate method of randomisation was used
Allocation concealment (selection bias)
We assigned each included trial to one of the following categories.
Low risk of bias: adequate concealment of allocation (e.g. pre‐numbered or coded identical containers administered serially to participants)
High risk of bias: allocation not adequately concealed (e.g. alternate assignment)
Unclear risk of bias: uncertainty existed about whether allocation was adequately concealed (e.g. study authors did not describe allocation methods)
Blinding
We addressed three potential areas of blinding.
Blinding of participants and personnel (performance bias)
Due to the overt nature of CIMT intervention, blinding of participants and intervention providers to intervention is not possible. Therefore, we rated all included studies at high risk of bias on this domain.
Blinding of outcome assessment: self‐reported outcomes (detection bias)
As blinding of participants and their families to intervention is not possible, self‐reported outcomes could not be considered to be completely blinded to intervention group. Consequently, we rated all self‐reported outcome assessments at high risk of bias.
Blinding of outcome assessment: objectively‐observed outcomes (detection bias)
Blinding of outcome assessor(s) and data analyst(s) from knowledge of intervention allocation is possible. We evaluated and graded the method used to ensure blinding as follows.
Low risk of bias: blinding was likely effective
High risk of bias: detection bias due to knowledge of the allocated interventions by outcome assessors
Unclear risk of bias: blinding not described in sufficient detail
Incomplete outcome data (attrition bias)
We extracted the numbers of, and reason(s) for, attrition or exclusion of participants, and whether attrition was balanced between groups and analysed appropriately (e.g. intention‐to‐treat (ITT) analysis), and graded the risk of bias as follows.
Low risk of bias: < 20% missing data; handling of incomplete outcome data was complete and unlikely to have produced bias
High risk of bias: ≥ 20% missing data; attrition bias due to amount, nature or handling of incomplete outcome data
Unclear risk of bias: insufficient reporting of attrition/exclusions to permit judgment of ‘Low risk of bias’ or ‘High risk of bias’ (e.g. number randomised not stated, no reasons for missing data provided)
Selective reporting bias (reporting bias)
Selective reporting bias may be evident in several ways (Higgins 2011c). For example: a trial protocol was available and some of the proposed outcome measures were not included in the published trial manuscript; the methods section of the published study identified an outcome measure that was not subsequently reported; and the results of subscales of a full measurement scale or a subset of events were selectively reported. We assigned each included study to one of the following quality criteria.
Low risk of bias: studies reported all prespecified outcomes
High risk of bias: any of the above‐mentioned selective reporting was evident in the study
Unclear risk if bias: it is uncertain whether selective reporting bias was avoided
Other sources of bias
Other sources of bias may include baseline imbalance, early stopping and cointervention. We described the nature of the bias and it graded as follows.
Low risk of bias: no other bias detected
High risk of bias: bias due to problems not covered elsewhere in the table
Unclear risk of bias: there may be a risk of bias, but there is either insufficient information to assess whether an important risk of bias exists, or insufficient rationale or evidence that an identified problem will introduce bias
Data and analyses
Comparison 1. CIMT versus a low‐dose comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Assisting Hand Assessment | 2 | 39 | Mean Difference (IV, Random, 95% CI) | 5.44 [2.37, 8.51] |
| 2 Quality of Upper Extremity Skills Test (QUEST) ‐ Dissociated Movement | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Immediately postintervention | 3 | 121 | Mean Difference (IV, Random, 95% CI) | 5.95 [2.02, 9.87] |
| 2.2 2‐week to 4‐month follow‐up | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 5.8 [2.29, 9.31] |
| 3 QUEST ‐ Grasps | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Immediately postintervention | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 7.57 [2.10, 13.05] |
| 3.2 2‐week to 4‐month follow‐up | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 6.5 [2.03, 10.97] |
| 4 QUEST ‐ Protective Extension | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Immediately postintervention | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 12.54 [8.60, 16.47] |
| 4.2 2‐week to 4‐month follow‐up | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 11.10 [6.22, 15.98] |
| 5 QUEST ‐ Weightbearing | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Immediately postintervention | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 5.92 [2.21, 9.63] |
| 5.2 2‐week to 4‐month follow‐up | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 4.5 [‐1.55, 10.55] |
| 6 Grip Strength | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Immediately postintervention | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.61, 0.34] |
| 6.2 2‐week to 4‐month follow‐up | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.59, 0.36] |
| 7 Modified Ashworth Scale (MAS) ‐ Elbow | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Immediately postintervention | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.42, 0.42] |
| 7.2 5‐ to 6‐month follow‐up | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.43, 1.07] |
| 8 MAS ‐ Wrist | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Immediately postintervention | 2 | 34 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐0.07, 1.49] |
| 8.2 5‐ to 6‐month follow‐up | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.41, 1.51] |
| 9 Data table | Other data | No numeric data | ||
| 9.1 Assisting Hand Assessment [AHA units] | Other data | No numeric data | ||
| 9.2 Hand Assessment for Infants ‐ Bimanual | Other data | No numeric data | ||
| 9.3 Hand Assessment for Infants ‐ Unimanual | Other data | No numeric data | ||
| 9.4 Melbourne Assessment | Other data | No numeric data | ||
| 9.5 QUEST ‐ Grasps | Other data | No numeric data | ||
| 9.6 QUEST ‐ Dissociated Movement | Other data | No numeric data | ||
| 9.7 QUEST ‐ Weightbearing | Other data | No numeric data | ||
| 9.8 QUEST ‐ Protective extension | Other data | No numeric data | ||
| 9.9 Box and Blocks | Other data | No numeric data | ||
| 9.10 Pediatric Motor Activity Log ‐ Revised | Other data | No numeric data | ||
| 9.11 Pediatric Evaluation of Disability Inventory (PEDI): Self‐care ‐ Functional Skills domain | Other data | No numeric data | ||
| 9.12 PEDI: Self‐care ‐ Caregiver Assistance domain | Other data | No numeric data | ||
| 9.13 Functional Independence Measure for Children (WeeFIM) ‐ Total Score | Other data | No numeric data | ||
| 9.14 MAS ‐ Shoulder | Other data | No numeric data | ||
| 9.15 MAS ‐ Elbow | Other data | No numeric data | ||
| 9.16 MAS ‐ Wrist | Other data | No numeric data | ||
| 9.17 Grip strength | Other data | No numeric data | ||
| 9.18 2 point discrimination | Other data | No numeric data | ||
| 9.19 Parenting Sense of Competence Scale (PSOC) ‐ Mother | Other data | No numeric data | ||
| 9.20 PSOC ‐ Father | Other data | No numeric data | ||
| 9.21 Besta Scale ‐ Global score | Other data | No numeric data | ||
| 9.22 Besta Scale ‐ Grasp (affected side) | Other data | No numeric data | ||
| 9.23 Besta Scale ‐ Bimanual use | Other data | No numeric data | ||
| 9.24 Besta Scale ‐ Activities of Daily Living (ADL) (2 to 6 years) | Other data | No numeric data | ||
| 9.25 Besta Scale ‐ ADL (7 to 8 years) | Other data | No numeric data |
Comparison 2. CIMT versus a high‐dose comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Assisting Hand Assessment | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Immediately postintervention | 3 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐3.14, 2.36] |
| 1.2 2‐week to 4‐month follow‐up | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐5.06, 3.23] |
| 2 Canadian Occupational Performance Measure (COPM) ‐ Performance | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Immediately postintervention | 3 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.72, 0.69] |
| 2.2 2‐week to 4‐month follow‐up | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.87, 0.43] |
| 3 COPM ‐ Satisfaction | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Immediately postintervention | 3 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐1.22, 0.55] |
| 3.2 2‐week to 4‐month follow‐up | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐1.24, 0.82] |
| 4 Data table | Other data | No numeric data | ||
| 4.1 Quality of Upper Extremity Skills Test (QUEST) ‐ Dissociated Movement | Other data | No numeric data | ||
| 4.2 QUEST ‐ Grasps | Other data | No numeric data | ||
| 4.3 Melbourne Assessment | Other data | No numeric data | ||
| 4.4 Pediatric Evaluation of Disability Inventory (PEDI): Self‐care ‐ Functional Skills domain | Other data | No numeric data | ||
| 4.5 PEDI: Self‐care ‐ Caregiver Assistance domain | Other data | No numeric data | ||
| 4.6 Functional Independence Measure for Children (WeeFIM) | Other data | No numeric data | ||
| 4.7 Modified Ashworth Scale (MAS) ‐ Elbow flexors | Other data | No numeric data | ||
| 4.8 MAS ‐ Wrist flexors | Other data | No numeric data | ||
| 4.9 Modified Tardieu Scale (MTS) ‐ Elbow flexors | Other data | No numeric data | ||
| 4.10 MTS ‐ Wrist flexors | Other data | No numeric data | ||
| 4.11 Cerebral Palsy Quality of Life (CP QOL) (Proxy) ‐ Social Wellbeing and Acceptance | Other data | No numeric data | ||
| 4.12 CP QOL (Proxy) ‐ Function | Other data | No numeric data | ||
| 4.13 CP QOL (Proxy) ‐ Participation and Physical Health | Other data | No numeric data | ||
| 4.14 CP QOL (Proxy) ‐ Emotional Wellbeing and Self‐esteem | Other data | No numeric data | ||
| 4.15 CP QOL (Proxy) ‐ Pain and Impact of Disability (lower score = better) | Other data | No numeric data | ||
| 4.16 CP QOL (Proxy) ‐ Access | Other data | No numeric data | ||
| 4.17 CP QOL (Proxy) ‐ Family Health | Other data | No numeric data |
Comparison 3. CIMT versus a dose‐matched comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Assisting Hand Assessment | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Immediately postintervention | 7 | 229 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.78, 2.38] |
| 1.2 2‐week to 4‐month follow‐up | 5 | 149 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐0.10, 3.73] |
| 1.3 5‐ to 6‐month follow‐up | 5 | 163 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐1.56, 1.49] |
| 1.4 7‐ to 12‐month follow‐up | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐2.53, 3.93] |
| 2 Box and Blocks Test | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Immediately postintervention | 2 | 72 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐0.06, 2.28] |
| 2.2 2‐week to 4‐month follow‐up | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐3.66, 3.46] |
| 3 Melbourne Assessment | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Immediately postintervention | 6 | 203 | Mean Difference (IV, Random, 95% CI) | 1.48 [‐0.49, 3.44] |
| 3.2 2‐week to 4‐month follow‐up | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐1.28, 4.00] |
| 3.3 5‐ to 6‐month follow‐up | 4 | 120 | Mean Difference (IV, Random, 95% CI) | 3.18 [0.85, 5.50] |
| 3.4 7‐ to 12‐month follow‐up | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐4.39, 2.39] |
| 4 Quality of Upper Extremity Skills Test (QUEST) ‐ Dissociated Movement | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Immediately postintervention | 3 | 124 | Mean Difference (IV, Random, 95% CI) | 6.51 [‐0.74, 13.76] |
| 4.2 2‐week to 4‐month follow‐up | 2 | 52 | Mean Difference (IV, Random, 95% CI) | 3.74 [‐0.29, 7.77] |
| 4.3 5‐ to 6‐month follow‐up | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐3.87, 5.27] |
| 5 QUEST ‐ Grasp | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Immediately postintervention | 3 | 124 | Mean Difference (IV, Random, 95% CI) | 6.63 [‐2.38, 15.65] |
| 5.2 2‐week to 4‐month follow‐up | 2 | 52 | Mean Difference (IV, Random, 95% CI) | 1.18 [‐5.12, 7.49] |
| 5.3 5‐ to 6‐month follow‐up | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐6.32, 9.72] |
| 6 QUEST ‐ Weightbearing | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Immediately postintervention | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐2.31 [‐8.02, 3.40] |
| 6.2 2‐week to 4‐month follow‐up | 1 | 10 | Mean Difference (IV, Random, 95% CI) | 8.10 [‐21.90, 38.10] |
| 7 QUEST ‐ Protective Extension | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Immediately postintervention | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 6.86 [0.14, 13.58] |
| 7.2 2‐week to 4‐month follow‐up | 1 | 10 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐10.08, 19.68] |
| 8 Abilhand‐Kids | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Immediately postintervention | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.41, 1.46] |
| 8.2 2‐week to 4‐month follow‐up | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.51, 0.62] |
| 8.3 5‐ to 6‐month follow‐up | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.74 [0.31, 1.18] |
| 9 Canadian Occupational Performance Measure (COPM) ‐ Performance | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Immediately postintervention | 6 | 191 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐1.29, 1.46] |
| 9.2 2‐week to 4‐month follow‐up | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐1.45, 2.55] |
| 9.3 5‐ to 6‐month follow‐up | 4 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.01, 0.41] |
| 9.4 7‐ to 12‐month follow‐up | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.83, 1.03] |
| 10 COPM ‐ Satisfaction | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Immediately postintervention | 6 | 191 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.99, 1.92] |
| 10.2 2‐week to 4‐month follow‐up | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐0.24, 2.43] |
| 10.3 5‐ to 6‐month follow‐up | 4 | 121 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.63, 0.98] |
| 10.4 7‐ to 12‐month follow‐up | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.31, 2.11] |
| 11 Pediatric Evaluation of Disability Inventory: Self‐care ‐ Functional Skills domain | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐2.42, 0.24] |
| 12 Grip Strength (impaired hand) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Immediately postintervention | 5 | 194 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.13, 0.46] |
| 12.2 2‐week to 4‐month follow‐up | 4 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.02, 0.66] |
| 12.3 5‐ to 6‐month follow‐up | 4 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.14, 0.54] |
| 12.4 7‐ to 12‐month follow‐up | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.61, 0.57] |
| 13 Pediatric Quality of Life Inventory (PedsQLTM) 3.0 Cerebral Palsy (CP) Module (3.0) – Child Daily Activities | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐10.32, 8.91] |
| 13.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐3.75 [‐12.33, 4.82] |
| 13.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐15.53, 12.81] |
| 14 PedsQLTM 3.0 CP Module – Child School Activities | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 2.25 [‐11.70, 16.19] |
| 14.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐14.89, 15.20] |
| 14.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐14.98, 15.69] |
| 15 PedsQLTM 3.0 CP Module – Child Move & Balance | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 3.55 [‐5.63, 12.73] |
| 15.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 4.13 [‐4.91, 13.17] |
| 15.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐13.02, 11.11] |
| 16 PedsQLTM 3.0 CP Module – Child Pain and Hurt | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 7.42 [‐6.58, 21.42] |
| 16.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 2.89 [‐11.77, 17.54] |
| 16.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 3.91 [‐7.85, 15.67] |
| 17 PedsQLTM 3.0 CP Module – Child Fatigue | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 2.77 [‐16.35, 21.89] |
| 17.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 8.34 [‐3.39, 20.07] |
| 17.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 5.39 [‐7.54, 18.33] |
| 18 PedsQLTM 3.0 CP Module – Child Eating Activities | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐6.01 [‐15.81, 3.79] |
| 18.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐3.81 [‐20.02, 12.40] |
| 18.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐2.38 [‐12.88, 8.12] |
| 19 PedsQLTM 3.0 CP Module – Child Speech and Communication | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐12.60 [‐37.82, 12.62] |
| 19.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐13.50 [‐24.94, ‐2.06] |
| 19.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐7.19 [‐32.97, 18.59] |
| 20 PedsQLTM 3.0 CP Module – Parent Daily Activities | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 3.51 [‐3.07, 10.08] |
| 20.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 1.23 [‐6.51, 8.96] |
| 20.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 4.26 [‐4.08, 12.59] |
| 21 PedsQLTM 3.0 CP Module – Parent School Activities | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 12.98 [‐1.64, 27.60] |
| 21.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 13.38 [‐8.95, 35.71] |
| 21.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 8.74 [‐3.32, 20.80] |
| 22 PedsQLTM 3.0 CP Module – Parent Move & Balance | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 13.82 [5.78, 21.87] |
| 22.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 8.10 [‐0.79, 17.00] |
| 22.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 3.88 [‐13.22, 20.98] |
| 23 PedsQLTM 3.0 CP Module – Parent Pain and Hurt | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 9.41 [‐15.49, 34.31] |
| 23.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 13.89 [‐12.35, 40.13] |
| 23.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 3.04 [‐6.42, 12.51] |
| 24 PedsQLTM 3.0 CP Module – Parent Fatigue | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 24.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 11.02 [0.81, 21.23] |
| 24.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 7.37 [‐2.72, 17.47] |
| 24.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 10.72 [‐2.78, 24.21] |
| 25 PedsQLTM 3.0 CP Module – Parent Eating Activities | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 25.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 11.44 [‐4.50, 27.38] |
| 25.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 3.15 [‐4.03, 10.32] |
| 25.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 9.78 [2.01, 17.56] |
| 26 PedsQLTM 3.0 CP Module – Parent Speech and Communication | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 26.1 Immediately postintervention | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐30.49, 30.12] |
| 26.2 5‐ to 6‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐22.39, 19.06] |
| 26.3 7‐ to 12‐month follow‐up | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐11.07, 8.35] |
| 27 Data table | Other data | No numeric data | ||
| 27.1 Assisting Hand Assessment (Scaled score) | Other data | No numeric data | ||
| 27.2 QUEST ‐ Dissociated Movement | Other data | No numeric data | ||
| 27.3 QUEST ‐ Grasp | Other data | No numeric data | ||
| 27.4 QUEST ‐ Weightbearing | Other data | No numeric data | ||
| 27.5 QUEST ‐ Protective Extension | Other data | No numeric data | ||
| 27.6 Pediatric Evaluation of Disability Inventory: Self‐care ‐ Caregiver Assistance domain | Other data | No numeric data | ||
| 27.7 Functional Independence Measure for Children (WeeFIM) | Other data | No numeric data | ||
| 27.8 Modified Ashworth Scale (Wrist) | Other data | No numeric data | ||
| 27.9 2‐point discrimination | Other data | No numeric data | ||
| 27.10 Assessment of Life Habits (LIFE‐H) ‐ Total Score | Other data | No numeric data | ||
| 27.11 LIFE‐H ‐ Recreation | Other data | No numeric data | ||
| 27.12 LIFE‐H ‐ Nutrition | Other data | No numeric data | ||
| 27.13 LIFE‐H ‐ Personal Care | Other data | No numeric data | ||
| 27.14 LIFE‐H ‐ Education | Other data | No numeric data | ||
| 27.15 Children’s Assessment of Participation and Enjoyment (CAPE) ‐ Diversity | Other data | No numeric data | ||
| 27.16 Children’s Assessment of Participation and Enjoyment (CAPE) ‐ Intensity | Other data | No numeric data | ||
| 27.17 Cerebral Palsy Quality of Life (child report) ‐ Social well‐being and acceptance | Other data | No numeric data | ||
| 27.18 Cerebral Palsy Quality of Life (child report) ‐ Function | Other data | No numeric data | ||
| 27.19 Cerebral Palsy Quality of Life (child report) ‐ Emotional well‐being and self‐esteem | Other data | No numeric data | ||
| 27.20 Cerebral Palsy Quality of Life (child report) ‐ Participation and physical health | Other data | No numeric data | ||
| 27.21 Cerebral Palsy Quality of Life (child report) ‐ Pain and impact of disability (lower score = better) | Other data | No numeric data | ||
| 27.22 Cerebral Palsy Quality of Life (Proxy) ‐ Social well‐being and acceptance | Other data | No numeric data | ||
| 27.23 Cerebral Palsy Quality of Life (Proxy) ‐ Function | Other data | No numeric data | ||
| 27.24 Cerebral Palsy Quality of Life (Proxy) ‐ Participation and physical health | Other data | No numeric data | ||
| 27.25 Cerebral Palsy Quality of Life (Proxy) ‐ Emotional well‐being and self‐esteem | Other data | No numeric data | ||
| 27.26 Cerebral Palsy Quality of Life (Proxy) ‐ Pain and impact of disability (lower score = better) | Other data | No numeric data | ||
| 27.27 Cerebral Palsy Quality of Life (Proxy) ‐ Access | Other data | No numeric data | ||
| 27.28 Cerebral Palsy Quality of Life (Proxy) ‐ Family health | Other data | No numeric data | ||
| 27.29 KIDSCREEN ‐ Physical Wellbeing | Other data | No numeric data | ||
| 27.30 KIDSCREEN ‐ Psychological Wellbeing | Other data | No numeric data | ||
| 27.31 KIDSCREEN ‐ Mood and Emotions | Other data | No numeric data | ||
| 27.32 KIDSCREEN ‐ Self‐perception | Other data | No numeric data | ||
| 27.33 KIDSCREEN ‐ Autonomy | Other data | No numeric data | ||
| 27.34 KIDSCREEN ‐ Parent Relations | Other data | No numeric data | ||
| 27.35 KIDSCREEN ‐ Financial Resources | Other data | No numeric data | ||
| 27.36 KIDSCREEN ‐ Social Supports + Peers | Other data | No numeric data | ||
| 27.37 KIDSCREEN ‐ School Environment | Other data | No numeric data | ||
| 27.38 KIDSCREEN ‐ Social Acceptance | Other data | No numeric data | ||
| 27.39 KIDSCREEN (Parent Proxy) ‐ Physical Wellbeing | Other data | No numeric data | ||
| 27.40 KIDSCREEN (Parent Proxy) ‐ Psychological Wellbeing | Other data | No numeric data | ||
| 27.41 KIDSCREEN (Parent Proxy) ‐ Mood and Emotions | Other data | No numeric data | ||
| 27.42 KIDSCREEN (Parent Proxy) ‐ Self‐perception | Other data | No numeric data | ||
| 27.43 KIDSCREEN (Parent Proxy) ‐ Autonomy | Other data | No numeric data | ||
| 27.44 KIDSCREEN (Parent Proxy) ‐ Parent Relations | Other data | No numeric data | ||
| 27.45 KIDSCREEN (Parent Proxy) ‐ Financial Resources | Other data | No numeric data | ||
| 27.46 KIDSCREEN (Parent Proxy) ‐ Social Supports + Peers | Other data | No numeric data | ||
| 27.47 KIDSCREEN (Parent Proxy) ‐ School Environment | Other data | No numeric data | ||
| 27.48 KIDSCREEN (Parent Proxy) ‐ Social Acceptance | Other data | No numeric data | ||
| 27.49 Video Observation Aarts & Aarts: Determine Developmental Disregard (VOAA‐DDD) ‐ Performance | Other data | No numeric data | ||
| 27.50 VOAA:DDD ‐ Capacity | Other data | No numeric data | ||
| 27.51 VOAA‐DDD ‐ Developmental Disregard | Other data | No numeric data | ||
| 27.52 School Function Assessment | Other data | No numeric data | ||
| 27.53 Besta Scale ‐ Global score | Other data | No numeric data | ||
| 27.54 Besta Scale ‐ Grasp (affected side) | Other data | No numeric data | ||
| 27.55 Besta Scale ‐ Bimanual use | Other data | No numeric data | ||
| 27.56 Besta Scale ‐ Activities of Daily Living (ADL) (2 to 6 years) | Other data | No numeric data | ||
| 27.57 Besta Scale ‐ ADL use (7 to 8 years) | Other data | No numeric data |
3.14. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 14 PedsQLTM 3.0 CP Module – Child School Activities.
3.15. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 15 PedsQLTM 3.0 CP Module – Child Move & Balance.
3.16. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 16 PedsQLTM 3.0 CP Module – Child Pain and Hurt.
3.17. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 17 PedsQLTM 3.0 CP Module – Child Fatigue.
3.18. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 18 PedsQLTM 3.0 CP Module – Child Eating Activities.
3.20. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 20 PedsQLTM 3.0 CP Module – Parent Daily Activities.
3.21. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 21 PedsQLTM 3.0 CP Module – Parent School Activities.
3.22. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 22 PedsQLTM 3.0 CP Module – Parent Move & Balance.
3.23. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 23 PedsQLTM 3.0 CP Module – Parent Pain and Hurt.
3.24. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 24 PedsQLTM 3.0 CP Module – Parent Fatigue.
3.25. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 25 PedsQLTM 3.0 CP Module – Parent Eating Activities.
3.26. Analysis.
Comparison 3 CIMT versus a dose‐matched comparison, Outcome 26 PedsQLTM 3.0 CP Module – Parent Speech and Communication.
Comparison 4. CIMT versus different form of CIMT.
4.3. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 3 PedsQLTM 3.0 CP Module – Parent Pain and Hurt.
| PedsQLTM 3.0 CP Module – Parent Pain and Hurt | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 0.6 | 19.2 | 20 | 6.0 | 15.5 | 23 | ‐5.40 [‐15.93, 5.13] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐5.7 | 23.9 | 22 | 2.2 | 21.9 | 23 | ‐7.90 [‐21.31, 5.51] |
4.4. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 4 PedsQLTM 3.0 CP Module – Parent Fatigue.
| PedsQLTM 3.0 CP Module – Parent Fatigue | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | ‐8.6 | 25.4 | 21 | ‐1.0 | 20.9 | 23 | ‐7.60 [‐21.42, 6.22] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐6.8 | 19.7 | 22 | ‐7.6 | 21.6 | 23 | 0.80 [‐11.27, 12.87] |
4.5. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 5 PedsQLTM 3.0 CP Module – Parent Eating Activities.
| PedsQLTM 3.0 CP Module – Parent Eating Activities | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 2.8 | 20.7 | 22 | 5.7 | 20.3 | 23 | ‐2.90 [‐14.89, 9.09] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | 0.6 | 22.2 | 22 | ‐6.7 | 20.6 | 23 | 7.30 [‐5.23, 19.83] |
4.6. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 6 PedsQLTM 4.0 Generic Core Scale ‐ Total Score.
| PedsQLTM 4.0 Generic Core Scale ‐ Total Score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 3.9 | 11.3 | 15 | 1.2 | 15.3 | 17 | 2.70 [‐6.55, 11.95] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐6.0 | 17.3 | 16 | ‐4.5 | 13 | 16 | ‐1.50 [‐12.10, 9.10] |
4.7. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 7 PedsQLTM 4.0 Generic Core Scale ‐ Psychosocial Summary.
| PedsQLTM 4.0 Generic Core Scale ‐ Psychosocial Summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 2.9 | 14.2 | 15 | ‐1.9 | 18 | 17 | 4.80 [‐6.37, 15.97] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐6.0 | 19.1 | 16 | ‐5.0 | 15 | 16 | ‐1.00 [‐12.90, 10.90] |
4.8. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 8 PedsQLTM 4.0 Generic Core Scale ‐ Physical Summary.
| PedsQLTM 4.0 Generic Core Scale ‐ Physical Summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 4.4 | 14.6 | 22 | 9.5 | 21.9 | 23 | ‐5.10 [‐15.93, 5.73] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐6.5 | 19.2 | 21 | ‐6.2 | 20.2 | 24 | ‐0.30 [‐11.82, 11.22] |
4.9. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 9 PedsQLTM 4.0 Generic Core Scale ‐ Emotional Summary.
| PedsQLTM 4.0 Generic Core Scale ‐ Emotional Summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 1.9 | 18.7 | 22 | 5.4 | 17.8 | 23 | ‐3.50 [‐14.18, 7.18] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | 1.9 | 20.6 | 21 | 0.2 | 13.5 | 24 | 1.70 [‐8.63, 12.03] |
4.10. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 10 PedsQLTM 4.0 Generic Core Scale ‐ Social Functioning.
| PedsQLTM 4.0 Generic Core Scale ‐ Social Functioning | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | ‐10.5 | 18.0 | 22 | ‐6.0 | 25.4 | 23 | ‐4.50 [‐17.32, 8.32] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐19.8 | 24.5 | 21 | ‐18.2 | 21.5 | 24 | ‐1.60 [‐15.16, 11.96] |
4.13. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 13 PedsQLTM Infant Scale ‐ Psychosocial Summary.
| PedsQLTM Infant Scale ‐ Psychosocial Summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 1.1 | 3.8 | 4 | ‐10.5 | 16 | 7 | 11.60 [‐0.82, 24.02] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | 1.5 | 2.9 | 4 | ‐8.2 | 3.5 | 5 | 9.70 [5.52, 13.88] |
4.14. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 14 PedsQLTM Infant Scale ‐ Physical Summary.
| PedsQLTM Infant Scale ‐ Physical Summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 3.4 | 8.2 | 4 | ‐5.0 | 9.0 | 7 | 8.40 [‐2.04, 18.84] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐3.8 | 7.5 | 4 | ‐6.6 | 8.1 | 5 | 2.80 [‐7.42, 13.02] |
4.15. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 15 PedsQLTM Infant Scale ‐ Physical Functioning.
| PedsQLTM Infant Scale ‐ Physical Functioning | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 3.1 | 16.2 | 4 | ‐9.1 | 18.0 | 7 | 12.20 [‐8.53, 32.93] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐8.9 | 21.2 | 4 | ‐11.4 | 20.1 | 5 | 2.50 [‐24.74, 29.74] |
4.16. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 16 PedsQLTM Infant Scale ‐ Physical Symptoms.
| PedsQLTM Infant Scale ‐ Physical Symptoms | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | ‐5.5 | 11.4 | 4 | ‐10.0 | 6.0 | 7 | 4.50 [‐7.52, 16.52] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐8.0 | 9.9 | 4 | ‐11 | 7.7 | 5 | 3.00 [‐8.82, 14.82] |
4.17. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 17 PedsQLTM Infant Scale ‐ Emotional Functioning.
| PedsQLTM Infant Scale ‐ Emotional Functioning | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | ‐1.9 | 7.8 | 4 | ‐12.3 | 18.8 | 7 | 10.40 [‐5.49, 26.29] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | 2.6 | 5.7 | 4 | ‐13.1 | 7.0 | 5 | 15.70 [7.40, 24.00] |
4.18. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 18 PedsQLTM Infant Scale ‐ Social Functioning.
| PedsQLTM Infant Scale ‐ Social Functioning | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | ‐1.3 | 6.3 | 4 | 3.6 | 12.8 | 7 | ‐4.90 [‐16.21, 6.41] |
| Christmas 2018 | Baseline to 5 to 6 months post‐intervention (change) | ‐2.5 | 5.0 | 4 | ‐1.0 | 2.2 | 5 | ‐1.50 [‐6.77, 3.77] |
4.22. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 22 Quality of Upper Extremity Skills Test (QUEST) ‐ Grasps.
| Quality of Upper Extremity Skills Test (QUEST) ‐ Grasps | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 2.9 | 10.3 | 29 | ‐0.5 | 10.7 | 31 | 3.40 [‐1.91, 8.71] |
4.23. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 23 QUEST ‐ Weightbearing.
| QUEST ‐ Weightbearing | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | 2.1 | 18.3 | 29 | ‐0.1 | 9.15 | 31 | 2.20 [‐5.20, 9.60] |
4.24. Analysis.
Comparison 4 CIMT versus different form of CIMT, Outcome 24 QUEST ‐ Protective extension.
| QUEST ‐ Protective extension | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Assessment period | mCIMT (prolonged constraint) | SD | N | mCIMT (manual constraint) | SD | N | Mean difference[95% CI] |
| Christmas 2018 | Immediately following intervention (change) | ‐0.43 | NR | 29 | ‐2.0 | 19.9 | 31 | Not calculated |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aarts 2010.
| Methods |
Design: single‐centre, single‐blind, randomised controlled trial Comparison groups reported by study authors: mCIMT followed by bimanual training (mCIMT‐BiT) vs usual care (occupational therapist (OT) /Physical therapist (PT)) Country: the Netherlands Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis:CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) Cerebral palsy with unilateral or severely asymmetric, bilateral spastic movement impairment (b) Aged 2.5 to 8 years (c) Manual Ability Classification System (MACS) Levels I, II, or III Exclusion criteria (a) Intellectual disability such that simple tasks could not be understood or executed (i.e. developmental age less than 2 years) (b) Inability to combine the study protocol with the regular school programme (c) Inability to walk independently without a walking aid Participants: 52 children with unilateral spastic CP were randomised Randomisation method: within 48 hours after inclusion, each participant was randomised to the intervention or comparison group by throwing a dice Dropouts: n = 2 from comparison group withdrew immediately after randomisation due to family circumstances Number of participants who received intended treatment: n = 50 Number of participants who were analysed: total sample: n = 50; mean age = 4.9 years SD 1.5 years (calculated by review authors); 28 males, 22 females; 28 left hemiplegia; MACS I n = 16, MACS II n = 22, MACS III n = 12; GMFCS I = 48, II = 2 Intervention group: n = 28; mean age = 4.8 years SD 1.3 years; 14 males, 14 females; 14 left hemiplegia, 14 right hemiplegia; MACS I n = 9, MACS II n = 12, MACS III n = 7; GMFCS I =27, II =1 Comparison group: n = 22; mean age = 5.1 years SD 1.7 years; 14 males, 8 females; 14 left hemiplegia, 8 right hemiplegia; MACS I n = 7, MACS II n = 10, MACS III n = 5; GMFCS I = 21, II = 1 |
|
| Interventions |
Intervention group (mCIMT‐BiT) Treatment dosage Length: six weeks of mCIMT followed by 2 weeks of bimanual training using a Pirate theme (8 weeks total) Duration: 3‐hour sessions Frequency: 3 afternoons per week for 8 weeks (9 hours per week) Total dose of therapy time: 72 hours Description Type of restraint device: sling Hours per day restraint worn: 54 hours (average per day ˜ 1 hour 17 minutes) Treatment environment: primary rehabilitation centre Individual or group: group and individual Therapy provider: occupational therapists, physiotherapists, therapy assistant Models of practice: shaping and repetitive task practice. During the last 2 weeks, the emphasis was on task specific exercises in goal‐directed bimanual play and self‐care activities without restraint. These two weeks were used to train individual goals that were set by the parents using Goal Attainment Scaling. Home programme: quote: parents "were asked to stimulate their child to use the affected arm and hand at home as much as possible and to register the duration of specific periods of stimulation on the child’s daily record form" (p.511). 3.3 hours additional stimulation at home was achieved per week (total therapy plus stimulation time = 12.3 ± 1.9 hours per week) Comparison group (dose‐matched usual care) Treatment dosage Length: 8 weeks Duration: therapist delivered: 0.5‐ to 1‐hour sessions Frequency: twice per week Total dose of therapy time: 2 hours (1.5 hours/week) Note: therapy was planned as 72 hours dose‐matched ‐ 1.5 hours per week with therapist plus 7.5 hours per week with parents and teachers. Planned dose = 9 hours per week. Actual dose = 12.7 ± 2.1 hours per week) Treatment environment: participating rehabilitation centres Individual or group: individual Therapy provider: occupational therapists, physiotherapists Models of practice: quote: The "child was engaged in exercises to stretch the affected arm, to improve weight‐bearing capacity, and to use the affected arm and hand as a good assist" p.511). Home programme: quote: "Parents and teachers were instructed to stimulate the children at least 7.5 hours a week to use the affected arm as an assist in daily activities. Parents and teachers received oral and written instructions about activities they were expected to train at home or at (pre)school. Parents and teachers were asked to register the duration of specific periods of stimulation on the child’s daily record form" (p.511) |
|
| Outcomes |
Assessment time points: baseline. Week 9 (immediately following intervention). Week 17 (8 weeks after completion of intervention) (2 weeks to 4 months postintervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: authors provided change data for AHA units and ABILHAND‐Kids logits Fundings sources: Johanna Children Fund (JKF; grant number 2007/0199‐110) Study author declaration: "The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised by throwing a dice with equal probabilities" |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were randomised by throwing a dice with equal probabilities" Comment: dice rolling assumes concealed allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including ABILHAND‐Kids, COPM, GAS was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quotes: Assessments were done by "..therapist...blinded for group allocation". AHA and Melbourne Assessments scored "..blinded for group allocation and test session." Independent statistician was "..blinded for group allocation" Comments: assessors, scorers and statistician blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two children withdrew from usual care group due to family circumstances" Comment: specified that no participants were lost to follow‐up or changed group allocation thereafter. Total follow‐up rate was 96%. Rates and reasons for attrition were not unequally distributed and were unlikely to affect outcomes Quote: “…loss to follow‐up of 2 participants in the UC group immediately after randomization prevented a true intention‐to‐treat analysis” Comment: it appears participants were analysed in the group to which they were randomised and that missing data were not imputed for analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement. Video Observations Aarts and Aarts (VOAA‐DDD) was not reported in primary paper |
Abd El‐Kafy 2014.
| Methods |
Design: single‐centre randomised controlled trial Comparison groups reported by study authors: CIMT vs conventional non‐structured therapy programme Country: Egypt Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) Diagnosis of congenital unilateral CP confirmed by MRI (b) Aged 4 to 8 years (c) MAS 1‐2 in the upper limb (d) MACS Levels II‐IV (e) Ability to extend the wrist a least 20° and fingers 10° from full flexion (f) Cognitively competent and able to follow instructions (g) No serious or recurring medical conditions (h) Not receiving other interventions to improve upper‐limb function Exclusion criteria (a) Visual problems that would prevent child from performing the intervention (b) Balance problems that would put child at risk of falling when wearing a restraint (c) Uncontrolled seizures (d) Botulinum toxin‐A injections to upper limb in last 6 months, or plan to receive it during study period (e) Other muscle tone control medications within three months of pre‐treatment testing (f) Fixed contractures or stiffness in affected upper limb that would limit activity engagement (g) Previous CIMT or forced use therapy (h) Orthopaedic or neurological surgery in upper limb Participants: 30 children with unilateral CP Randomisation method: allocated randomly on computerised basis using SPSS Dropouts: n = 3; intervention n = 1 (inability to continue intervention), comparison n = 2 (n = 1 died, n = 1 travel difficulties) Number of participants who received intended treatment: n = 27 (90%), intervention n = 14, comparison n = 13 Number of participants who were analysed: total sample: n=27; mean age = 6.1 years SD 1.5 years (calculated by review authors); 12 males, 15 females; 3 left hemiplegia, 24 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n=14; mean age = 6.0 years SD 1.7 years; 7 males, 7 females; 2 left hemiplegia, 12 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n=13; mean age = 6.2 years SD 1.3 years; 5 males, 8 females; 1 left hemiplegia, 12 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (CIMT) Treatment dosage Length: 4 weeks Duration: 6 hours per day Frequency: 5 days per week for 4 weeks Total dose of therapy time: 120 hours (planned: 80 in clinic, 40 at home) Description Type of restraint device: sling strapped to the child’s trunk at the distal end and sewn shut Hours per day restraint worn: 6 hours per week day Treatment environment: home and clinic Individual or group: not specified – assume individual Therapy provider: two therapists (OTs and/or PTs) and parents Models of practice: shaping, repetitive practice Home programme: list of treatment activities, including arm reaching, weight‐bearing and strengthening, manipulative, arm‐hand and postural reactions exercises, and upper‐limb self‐care activities. Restraint was worn for home programme. Comparison group (dose‐matched, conventional unstructured therapy programme) Treatment dosage Length: 4 weeks Duration: 6 hours per day Frequency: 5 days per week Total dose of therapy time: 120 hours (planned: 80 in clinic, 40 at home) Treatment environment: clinic and home Individual or group: not specified – assume individual Therapy provider: two therapists (OTs and/or PTs) and parents Models of practice: 'conventional’ therapy Home programme: list of treatment activities, including arm reaching, weight‐bearing and strengthening, manipulative, arm‐hand and postural reactions exercises, and upper‐limb self‐care activities i.e., same as treatment group but without wearing the restraint. |
|
| Outcomes |
Pre‐treatment: immediately following intervention, 3 months following end of treatment (2 weeks to 4 months postintervention) Primary outcome measure
Pediatric Arm Functional Test (PAFT) (Uswatte 2012) (% score; range 0 to 100) Quality of Upper Extremity Skills Test (QUEST) ‐ Total score (% score; range 0 to 100). Reason for exclusion: Total score is reported to have poor construct validity (Thorley 2012). Isometric shoulder torque. Reason for exclusion: No evidence of validity or reliability in CP |
|
| Notes | No data from this study have been included in this review as the data reported in the manuscript were not able to be included in meta‐analysis. Reported as “mean rank” with unclear analytical procedure Additional information sought from authors: authors have not responded to attempts to contact them requesting change data for eligible outcomes including QUEST and PAFT Fundings sources: nil mentioned Study author declaration: the authors report no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The recruited children were allocated randomly on a computerized base using SPSS (version 16) into two equal groups of 15 children each" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided and therefore unable to make a judgement of either low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including PAFT was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "The evaluators (physical therapists and occupational therapist) who performed all assessments throughout the study did not take part in the intervention program. They also had not been informed regarding which group each evaluated child belonged to (blind assessors)" Comment: blinding of outcome assessment assessed to be low risk of bias for PAFT, QUEST, isokinetic muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: one child from CIMT group was not included in analysis as the child discontinued intervention due to frustration, attrition therefore due to intervention. Two children from the comparison group were not included in analysis (one died, one had long distance between home and clinic). Rate of follow‐up is high (90%) and attrition is unlikely to affect outcomes. It is unclear whether the reason for attrition in the CIMT is likely to affect outcomes. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit a judgement of low or high risk |
Abootalebi 2010.
| Methods |
Design: randomised controlled trial Comparison groups reported by study authors: CIMT plus usual care vs usual care alone Country: Iran Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) At least 20° of extension in the affected wrist joint and at least 10° of extension in the joints of the fingers (b) Able to do grabbing and be able to understand simple instructions Exclusion criteria (a) Aged 4 to 6 years of age (b) Severe mental difficulties such as mental retardation and vision problems that prevent or interfere with the test (c) History of orthopaedic surgery of the upper limb (d) Presence of fixed contractures (e) Botulinum toxin injections in upper‐limb muscle structure during the last six months (or during the study) (f) Exacerbation of difficulties with balance while wearing a sling (g) Severe behavioural difficulties such as hyperactivity, lack of focus, aggression (h) Using high‐doses of anticonvulsants Participants: 12 children aged 4 to 6 years with unilateral CP Randomisation method: Quote: “Persons in the study were randomly divided into treatment and control groups. In this sampling, the name of the children were put in a pot, then name of each child withdrawn from the pot and included in the treatment or control group in turn”. Dropouts: n = 1 from comparison group (due to difficulty continuing the treatment) Number of participants who received intended treatment: intervention n = 6, comparison n = 6 Number of participants who were analysed: total sample: n = 12; mean age = 59.91 months SD 9.15 months, range = 48 to 72 months; 5 males, 7 females; 7 left hemiplegia, 5 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n = 6; mean age = 61.17 months SD 8.87 months; 2 males, 4 females; 2 left hemiplegia, 4 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n = 6; mean age = 58.17 months SD 9.24 month; 3 males, 3 females; 3 left hemiplegia, 3 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (Hybrid) Treatment dosage Length: 21 consecutive days Duration: CIMT: 5 hours per day. OT: 45 minutes 3 times per week Frequency: CIMT: 21 consecutive days, OT: 3 times per week for 3 weeks Total dose of therapy time: face‐to‐face time with therapist: 105 (CIMT) + 6.75 (OT) hours = 112.75 hours. Description Type of restraint device:sling Hours per day restraint worn: 90% of waking hours Treatment environment: clinic and home Individual or group: individual Therapy provider: occupational therapist Models of practice: both intervention and comparison group received regular (current) occupational therapy (three session per week for 45 minutes each session). In addition, the CIMT group wore a sling for 90% of waking hours for 21 consecutive days and received 5 hours per day of therapy Home programme: parents were instructed to keep their children busy with activities that help them to use their affected hand Comparison group (low dose) Treatment dosage Length: 3 weeks Duration: 45 minutes Frequency: 3 sessions per week Total dose of therapy time: 6.75 hours Description Treatment environment: clinic Individual or group: individual Therapy provider: occupational therapist Models of practice: not reported Home programme: not reported |
|
| Outcomes |
Assessment time points: baseline, 3 weeks (immediately following intervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: authors provided change data for MAS Fundings sources: translation not available. Study author declaration: translation not available. Note: published in Persian ‐ data extraction and risk of bias were kindly completed by Associate Professor Mehdi Rassafiani, Department of Occupational Therapy, University of Social Welfare and Rehabilitation Sciences, Tehrān, Iran and Dr Fakher Rahim, Ahvaz Jondishapour University of Medical Sciences, Ahvaz, Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Persons in the study were randomly divided into treatment and control group. In this sampling, the name of the children were put in a pot, then name of each child withdrawn from the pot and included in the treatment or control group in turn” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Children’s name put in a pot and were randomly divided into experimental and control groups” Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Unclear risk | Comment: not reported. Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “One child in control group was not able to complete the study due to the family problem and therefore was removed from the data analysis” Comment: follow‐up rate was high, rates and reasons for attrition unlikely to be due to treatment or to affect outcomes. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Al‐Oraibi 2011.
| Methods |
Design: single‐centre randomised controlled trial Comparison groups reported by study authors: CIMT vs neurodevelopmental therapy (NDT) Country: Jordan Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Unilateral CP (b) Normal intellectual ability (c) Co‐operative family Exclusion criteria
Participants: 20 children with unilateral CP Randomisation method: not clearly stated Dropouts: n = 6; intervention n = 3, comparison n = 3 (per group reasons not given, n = 2 due to technical problems, n = 4 due to family situation) Number of participants who received intended treatment: n = 14 (70%), intervention n = 7, comparison n = 7 Number of participants who were analysed: total sample: n = 14; mean age = 56 months SD 23.8 months (calculated by review authors), range = 22 months to 105 months; 10 males, 4 females; 7 left hemiplegia, 7 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n = 7; mean age = 47 months SD 19 months, range = 22 months to 71 months, ; 4 males, 3 females; 2 left hemiplegia, 5 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n = 7; mean age = 65 months SD 26 months, range = 25 months to 105 months, mean age = 65 months SD 26 months; 6 males, 1 female; 5 left hemiplegia, 2 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 8 weeks Duration: 2 hours per day Frequency: 6 days per week Total dose of therapy time: 96 hours: mean achieved was 56.6 hours (SD 25.7 hours) of expected 96 hours Description Type of restraint device: custom‐made glove that prevented grasp Hours per day restraint worn: 2 hours per day, 6 days per week Treatment environment: clinic and home Individual or group: individual Therapy provider: OTs and parents Models of practice: motor training Home programme: fine motor activities that were demonstrated in therapy sessions Comparison group (low dose/NDT): Treatment dosage Length: 8 weeks Duration: 1‐hour sessions Frequency: 2 sessions per week Total therapy time: 16 hours Treatment environment: clinic once per week, home once per week Individual or group: individual Therapy provider: physiotherapists who had undertaken basic NDT course and had at least 4 years experience Models of practice: original NDT method Home programme: not stated |
|
| Outcomes |
Assessment time points: baseline, postintervention (immediately following intervention) Primary Outcome Measures
|
|
| Notes | Mean change data were calculated by review authors from data provided by study authors Fundings sources: Department of Habilitation Services for Children and Youth, Research Unit, Stockholm and Karolinska Hospital, Stockholm; Swedish International Development Cooperation, Swedish Research Links Programme Study author declaration: the authors report no declaration of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After the children were recruited, the randomisation was performed using Manual Ability Classification System (MACS) level and age as factors for stratification. The randomisation procedure was performed by the first author and a study coordinator” Comment: insufficient information given about the sequence generation process to permit judgement of risk of bias. |
| Allocation concealment (selection bias) | High risk | Quote: “The randomisation procedure was performed by the first author and a study coordinator”. “One of them was responsible for conducting the pre‐ and post‐intervention assessments and coordinating the programme” Comment: the randomisation was completed by one of the investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “The video recordings in the present study were analysed by an individual who was unaware of the aim of the project” Comment: blinding likely as aim of project was to compare groups |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Data for 6 (of 20) children were missing (30%). In two cases due to technical problems and four children dropped out of the project. The main reason for dropouts was problems related to the family situation which made it impossible to fulfil their commitment and arrange transportation for the weekly visit to the centre" Comment: a large proportion of sample was not included in analysis (30%). Insufficient information is available to determine whether the rates and reasons for attrition were balanced across groups or were likely to affect outcome. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement of risk of bias |
Charles 2006.
| Methods |
Design: single‐centre, single‐blind, randomised controlled, cross‐over trial Comparison groups reported by study authors: CIMT vs delayed intervention control group (children received no treatment) Country: USA Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Ability to extend the wrist at least 20° and the fingers at least 10° from full flexion at the metacarpophalangeal joints (b) 50% difference between the involved and non‐involved hand on the Jebsen–Taylor Test of Hand Function (c) Scored within 1 SD of the mean on the Kaufman Brief Intelligence Test (d) Willingness to agree to intervention and testing procedures and travel to Columbia University for participation Exclusion criteria (a) Health problems not associated with CP (b) Seizures (c) Visual problems that would interfere with carrying out the intervention or testing (d) Severely increased muscle tone (modified Ashworth score greater than 3) (e) Orthopaedic surgery on the involved upper limb (f) Dorsal rhizotomy (g) Botulinum toxin therapy in the upper‐limb musculature during the past 6 months or wishing to receive it within the period of study (h) Intrathecal baclofen (i) Balance problems while wearing the sling Participants: 33 children with unilateral CP Randomisation method: randomisation was performed in groups of four children (i.e. rolling admission) with the intention to achieve an equal number in both the treatment and control groups; dropouts were replaced immediately. Dropouts: n = 4; intervention n = 3 (n = 2 withdrew before receiving intervention, n = 1 removed from intervention because interventionists felt child was unable to tolerate procedure), comparison n = 1 (participant declined to participate). Lost to follow‐up: intervention n = 5, comparison n = 2 Number of participants who received intended treatment: intervention n = 16, comparison n = 13 Number of participants who were analysed: total sample: n = 22; mean age = 6 years 8 months SD 1 year 4 months, range = 4 to 8 years; 14 males, 8 females; 12 left hemiplegia, 10 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n = 11; mean age = 6 years 9 months SD 2 years 2 months; 5 males, 6 females; 8 left hemiplegia, 3 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n = 11; mean age = 6 years 8 months SD 2 years 1 month; 9 males, 2 females; 4 left hemiplegia, 7 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (Hybrid CIMT) Treatment dosage Length: 2 weeks Duration: 6 hours per day Frequency: 10 of 12 days Total dose of therapy time: face‐to‐face time with therapist = 60 hours Description Type of restraint device: Quote: "Children in the treatment group wore a sling on the non‐involved upper limb for the entire time during an intervention session (6 hours) and the sling was removed at the end of each session. The sling was strapped to the child’s trunk and the distal end was sewn shut to prevent use of the non‐involved hand" (p.636‐637) Hours per day restraint worn: 6 hours: Quote: "time out of the sling during the 6‐hour period was allowed for designated activities (e.g. toileting) and could not exceed 30 minutes per day" (p.637) Treatment environment: University clinical laboratory Individual or group: groups of 2 to 4 children Therapy providers: “trained interventionists” Models of practice: Quote: "During each 6‐hour session each child received individualised instruction from a trained interventionist involving specific practice of designated target movements. Children were engaged in play and functional activities that provided two types of structured practice (shaping and repetitive task practice) using the involved upper limb, especially the hand" (p.637) Home programme: Quote: "At the end of each day, each child in the treatment group went home with an exercise programme that involved practice with the involved limb (without any restraint) for 1 hour, which was extended to 2 hours per day for 6 months after the intervention. Parents kept activity logs to monitor compliance" (p.637 Comparison Group (low dose): children in this group received no treatment during the study period |
|
| Outcomes |
Assessment time points: baseline; 1 week postintervention (immediately following intervention); 1 month postintervention; 6 months postintervention (5 to 6 months postintervention) Primary outcome measure
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: authors provided change data for MAS, grip strength and 2‐point discrimination Question: Further description of the randomisation and allocation concealment procedures Reply: Quote: "In regard to the randomization and allocation concealment procedures: randomization and allocation was the responsibility solely of the project manager. Once randomization/allocation was completed, each study participant was given a code (by the project manager) for de‐identification and evaluation purposes. Thus the evaluators were blinded to allocation" Fundings sources: NIH grant HD 40961 from the National Center for Medical Rehabilitation Research (National Institute of Child Health and Human Development). Study author declaration: no declaration provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including CFUS was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "The same evaluator, blind as to group assignment, performed all testing of a specific child" (p. 638) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 19 children were allocated to the CIMT group, 11 were analysed (Reasons: 2 withdrew before receiving treatment; 1 could not tolerate it; 5 lost to follow‐up). 14 children were allocated to control, 11 were analysed (Reasons: 1 withdrew before receiving treatment; 2 lost to follow‐up). A large proportion of the sample was not included in analysis (33%). The attrition rates were unbalanced across groups and it is possible the attrition rates would affect outcomes. An as‐treated analysis was completed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Chen 2014.
| Methods |
Design: single‐centre, randomised controlled trial Comparison groups reported by study authors: home‐based CIMT vs traditional rehabilitation Country: Taiwan Other: trial registered at CinicalTrials.gov (NCT01076257) Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs high dose |
|
| Participants |
Inclusion criteria (a) Congenital unilateral spastic CP (b) Considerable nonuse of the more affected upper limb (amount of use score on the Pediatric Motor Activity Log < 2.5) (c) Active extension of the wrist and metacarpophalangeal joint ≥10° (d) No excessive muscle tone before starting treatment (Modified Ashworth Scale ≤2 for any joint on the upper limb) Exclusion criteria (a) Severe cognitive, visual, or auditory disorder (b) Severe concurrent illness or disease not typically associated with CP (c) Active medical conditions such as pneumonia (d) Any major surgery or nerve blockage (such as botulinum toxin‐A or phenol injection) within 6 months before interventions (e) Poor cooperation during assessments Participants: 48 (abstract states 45) children with unilateral spastic CP were randomised Randomisation method: children were first stratified by age (6 to 8 years; 9 to 12 years) and then allocated using unlabelled sealed envelopes containing numbers generated by a statistician external to the study Dropouts: n = 3; intervention n = 1 (excluded from analysis as unable to complete the reach‐to‐grasp kinematic analysis), comparison n = 2 (n = 1 lost to follow‐up due to tight family schedule and lack of transportation, n = 1 excluded from analysis as unable to complete reach to grasp) Number of participants who received intended treatment: n = 48 Number of participants who were analysed: total sample: n = 45; mean age = 8.7 years SD 1.92 years (calculated by authors), range = 6 to 12 years; 21 males, 24 females; 22 left hemiplegia, 23 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n=23; mean age = 8.7 years SD 1.9 years; 11 males, 12 females; 11 left hemiplegia, 12 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n=22; mean age = 8.7 year, SD 2.0 years; 5 males, 6 females; 11 left hemiplegia, 11 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 4 weeks Duration: 3.5 to 4 hours (individualised treatment with physiotherapist and home programme) Frequency: 7 days per week Total dose of therapy time: 98 to 112 hours Description Type of restraint device: elastic bandage and restraint mitten that limited wrist and individual finger movement Hours per day restraint worn: mean = 3.5 hours per day (obtained from Chen 2013) Treatment environment: home Individual or group: individual Therapy provider: physiotherapist Models of practice: focused on functional training of the more affected upper limb by applying principles of shaping and repetitive task practice Home programme: was included in home‐based intervention protocol Comparison group (high dose) Treatment dosage Length: 4 weeks Duration: 3.5 to 4 hours (individualised treatment with physiotherapist and home programme) Frequency: twice per week Total dose of therapy time: 28 to 32 hours Treatment environment: home Individual or group: individual Therapy provider: physiotherapist Models of practice: functional unilateral or bilateral upper‐limb training using the principles of activity oriented approaches, neurodevelopmental therapy techniques, and motor learning and control Home programme: was included in home‐based intervention protocol |
|
| Outcomes |
Assessment time points: baseline (data reported in Chen 2013; 2014, Hsin 2012); 4 weeks (immediately following intervention‐ data reported in Chen 2013; 2014, Hsin 2012); 3 months (Chen 2014) (2 weeks to 4 months postintervention); 6 months (Chen 2014) (5 to 6 months postintervention) Primary outcome measures
Secondary outcome measure
|
|
| Notes | Information and data from Chen (2014) extracted and used in this review Additional information sought from authors: authors provided change data for the WeeFIM. CPQOL data requested but authors unable to provide Fundings sources: National Science Council, Taiwan (NSC 98‐2314‐B‐182‐006‐MY3) Study author declaration: the authors declare that there is no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Stratified allocation was used to randomly assign children to constraint‐induced therapy or traditional rehabilitation. Children were first stratified by age. Children aged 6–8 years were randomized using two sets of sealed envelopes, and children aged 9–12 years using another two sets of sealed envelopes. The method of randomization was the same for children in both age strata. Each two sets of envelopes included 30 unlabelled envelopes containing a number ranging from 1 to 30, and 30 sealed envelopes, labelled from 1 to 30, with group allocation (constraint‐induced therapy or traditional rehabilitation)” |
| Allocation concealment (selection bias) | Low risk | Quote: "A table of random numbers was generated by a statistician outside the department, and 15 randomly selected numbers in the range from 1 to 30 were assigned to the constraint induced therapy group and the remaining 15 numbers to the traditional rehabilitation group. After inclusion, the child drew an unmarked envelope to get a number and was allocated by its matching marked envelope sequence generated from a random numbers table by a statistician external to research department" (Chen 2013) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including CP‐QOL and PMAL was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "A certified occupational therapist blinded to the group allocation was trained to properly administer the outcome measures" (Chen 2013) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “1 child in the TR group was unable to complete the follow‐up because of a tight family schedule and lack of transportation to the site of the posttest assessments. One child in each of the home‐based CIT and TR groups was excluded from the analysis because their motor ability was insufficient to complete the standardized study procedure of the reach‐to‐grasp kinematic analysis. Consequently, a total of 45 children, 23 in the home‐based CIT group and 22 in the TR group, completed the intervention, posttest, and follow‐up measures" (Chen 2014) Comment: follow‐up rate was high (94%). Rates and reasons for attrition were balanced across groups and are unlikely to affect outcomes. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | High risk | Comment: trial registered at CinicalTrials.gov (NCT01076257). PMAL and PDMS‐2 Visual Motor Integration scale not reported at 3 or 6 months. CP‐QOL data were not reported for whole sample at any time point |
Choudhary 2013.
| Methods |
Design: single‐centre, single‐blind, parallel groups, randomised controlled trial Comparison groups reported by study authors: mCIMT vs usual care Country: India Other:trial was registered in Clinical Trials Registry of India Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Aged 3 to 8 years (b) Unilateral CP (c) Minimum difference of 10 points between upper limbs on QUEST (d) Able to understand simple one‐step commands (e) Able to sit without support (f) Able to see 1 inch object from 1 metre Exclusion criteria (a) Uncontrolled epilepsy (seizure frequency of more than 1 episode/month during past 3 months) (b) MAS ≥3 at shoulder, elbow or wrist (c) Recent orthopaedic surgery or casting in preceding 6 months (d) Splint on the affected upper limb (e) Botulinum toxin or phenol in upper limb during past 6 months or plan to receive it during study period (f) Taking tone‐modifying agents such as baclofen, tizanidine, benzodiazepines or dantrolene Participants: 31 children with unilateral CP Randomisation method: a computer‐generated random number table was used Dropouts: n = 0 (at primary outcome measure point) Number of participants who received intended treatment: n = 31 (100%); intervention n = 16, comparison n = 15 Number of participants who were analysed: total sample: n = 31; mean age = 60.53 months SD 17.67 months (calculated by review authors); 18 males, 13 females; 18 left hemiplegia, 13 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n = 16; mean age = 58.5 months SD 17.7 months; 8 males, 8 females; 9 left hemiplegia, 7 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n = 15; mean age = 62.7 months SD 18.0 months; 10 males, 5 females; 4 left hemiplegia, 11 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 4 weeks Duration: 2 hours per session Frequency: 10 sessions over 4 weeks Total dose of therapy time: 20 hours Description Type of restraint device: arm sling Hours per day restraint worn: worn while intervention was given i.e. 2 hours per day for 10 days. An additional home programme was completed with sling for 1 hour per day on intervention days and for 2 hours per day on days with no intervention Treatment environment: clinic and home Individual or group: groups of 4 children Therapy provider: trained occupational therapist and first investigator (discipline unknown). Parents carried out conventional therapy home programme after training by blinded OT. Models of practice: shaping, specific task practice Home programme: “Exercise plan" that involved practice with involved upper limb with restraint of non‐affected upper limb for 1 hour per day, or 2 hours per day on non‐intervention days. Additionally, 20 minutes per day of "conventional" OT home programme was completed and included stretching, strengthening, bimanual hand activities and ADLs Comparison group (low dose) Treatment dosage Length: 4 weeks Duration: 20 minutes Frequency: daily Total dose of therapy time: not reported but calculated as 9.4 hours Treatment environment: home Individual or group: individual Therapy provider: parent Models of practice: not specified Home programme: "Conventional" OT home programme of stretching, strengthening, bimanual hand activities and ADL |
|
| Outcomes |
Assessment time points: baseline; 4 weeks from baseline (immediately after intervention);12 weeks from baseline (8 weeks after stopping intervention) (2 weeks to 4 months postintervention) Primary outcome measure
Secondary outcome measures
|
|
| Notes | Median and range data converted to mean and SD using Wan 2014 method Fundings sources: nil mentioned Study author declaration: no declaration provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer generated random number table was used. Two groups were generated using block randomisation method, using a block size of six" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “Allocation to the groups was concealed from the outcome assessor”. " Evaluation was done by a separate physical therapist masked to the group assignment” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “One patient in mCIMT group received five sessions of supervised intervention but did not return for the scheduled visit thereafter”. “The primary analysis was intention to treat. For missing values of outcome measures we carried forward the last observations” Comment: rates of attrition were low, balanced across groups and are unlikely to affect outcomes |
| Selective reporting (reporting bias) | High risk | Comment: Trial was registered in Clinical Trials Registry of India. Register stated one of the outcomes was: quote “To assess parent's perception of improvement in upper extremity function after four weeks of therapy and eight week follow up, using parent questionnaire.” No parent perception data were reported |
Christmas 2018.
| Methods |
Design: parallel‐group, randomised controlled trial Comparison groups reported by study authors: caregiver‐directed prolonged CIMT vs caregiver‐directed intermittent manual CIMT Country: UK Other (Protocol or registration number): ISCTN Registry (58484608) Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs different form of CIMT |
|
| Participants |
Inclusion Criteria (a) Hemiplegic cerebral palsy irrespective of cognitive impairment (b) Aged 18 months to 4 years Exclusion Criteria (a) Contra‐indication to the intervention such as a skin condition that prohibited the use of a persistent immobilisation device (b) Episode of prolonged constraint‐induced movement therapy lasting two weeks or more in the previous six months Participants: 62 children with hemiplegic cerebral palsy were randomised Randomisation method: following baseline assessment, the site therapist telephoned the independent Primary Care Clinical Research and Trials Unit at the University of Birmingham for randomisation ensuring concealed allocation. A balanced blocked randomisation schedule stratified by centre was used Dropouts: n = 2; intervention n = 1 (withdrew prior to intervention), comparison n = 1 (family moved from the area) Number of participants who received intended treatment: CI intervention group: n= 29/30; control group: n= 31/32 Number of participants who were analysed: total sample: n = 60; mean age = 2 years 6 months SD 1 year 0 months (calculated by authors), range not reported; 32 males, 30 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported. Intervention group: n = 30; mean age = 2 years 8 months SD 1 year 2 months, range not reported; 19 males, 11 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported. Comparison group: n = 32; mean age = 29 years months SD 1 year 0 months; 13 males, 19 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention Group (mCIMT – prolonged constraint) Treatment dosage Length: 6 weeks consisting of 3 blocks of 2 weeks completed over a 10 week period (2 week break between blocks) Duration: 1 hour Frequency: 7 days per week Total dose of therapy time: 504 hours; face‐to‐face time with therapist = 0 hours Description Type of restraint device: custom‐made semi‐rigid cast (3M soft cast) or wrist splint extending from the metacarpal heads to above the wrist, crepe bandage enclosing the fingers and thumb Hours per day restraint worn: 24 hours Treatment environment: usual settings – home and pre‐school Individual or group: individual Therapy providers: parents or pre‐school workers Models of practice: the interventions aimed to promote mass practice of the affected upper limb to improve grasp, release, reaching, in‐hand manipulation and use as an assisting hand during bimanual activity. The practice was embedded in the context of functional tasks or usual child‐friendly play for a total of 1 hour, which could be divided to fit with the child’s usual routine. To encourage participation, the activity aimed to be enjoyable with substantial verbal encouragement and praise. If the therapist found there were no toys available, a small number of suitable toys were provided Home programme: all provided at home or other usual settings Comparison Group (mCIMT different form ‐ manual constraint) Treatment dosage Length: 6 weeks consisting of 3 blocks of 2 weeks completed over a 10‐week period (2‐week break between blocks) Duration: 1 hour Frequency: 7 days per week Total dose of therapy time: 43 hours; face‐to‐face time with therapist = 0 hours Description Type of restraint device: holding was intermittent and hand‐over‐hand, never forceful Hours per day restraint worn: holding restraint conducted little and often by caregiver during therapy (1 hour per day) Treatment environment: usual settings – home and pre‐school Individual or group: individual Therapy providers: parents or pre‐school workers Models of practice: the interventions aimed to promote mass practice of the affected upper limb to improve grasp, release, reaching, in‐hand manipulation and use as an assisting hand during bimanual activity. The practice was embedded in the context of functional tasks or usual child‐friendly play for a total of 1 hour, which could be divided to fit with the child’s usual routine. To encourage participation, the activity aimed to be enjoyable with substantial verbal encouragement and praise. If the therapist found there were no toys available, a small number of suitable toys were provided Home programme: all provided at home or other usual settings |
|
| Outcomes |
Assessment time points:baseline: 10 weeks (immediately following intervention); 24 weeks after start of intervention (only mailed questionnaires were completed at 24 weeks) Primary outcome measure (include units and scale range)
Secondary outcome measures
|
|
| Notes |
Adverse events No serious adverse events. 12 non‐serious adverse events related to interventions were identified for the prolonged restraint group: 2 children had minor bruising because of a fall and 10 had small areas of skin abrasions Funding sources: P.M.C. was funded by the West Midlands Strategic Health Authority as part of a Clinical Academic Doctorate Fellowship. C.S. was supported by a NIHR Senior Investigator’s award and C.C. receives funding from the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care for West Midlands Programme (CLAHRC‐WM). The Nancie Finnie Cerebral Palsy Charity provided funding for the project. This article presents independent research partly funded by the National Institute for Health Research (NIHR) Study author declarations:the author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“Following informed consent and the baseline assessment, the site therapist telephoned the Primary Care Clinical Research and Trials Unit at the University of Birmingham for randomisation. The unit was independent of the research team ensuring concealed allocation. A balanced blocked randomisation schedule stratified by centre (nQuery Advisor 7.0, Statistical Solutions, USA) generated by a statistician was used” Comment: randomisation schedule generated by statistician in an independent unit |
| Allocation concealment (selection bias) | Low risk | Quote: “Following informed consent and the baseline assessment, the site therapist telephoned the Primary Care Clinical Research and Trials Unit at the University of Birmingham for randomization. The unit was independent of the research team ensuring concealed allocation. A balanced blocked randomization schedule stratified by centre (nQuery Advisor 7.0, Statistical Solutions, USA) generated by a statistician was used”. Comment: participants allocated by independent unit ensuring allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding of self‐reported outcomes was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “AHA, the primary outcome, was completed by the primary investigator “blinded to patient allocations” Quote: “Safeguards were put in place to maintain blinding of the assessor because families and therapists could not be blinded to group allocation. These included reminder to parents not to discuss group allocation in front of the trial assessor, research notes kept in a locked filing cabinet, adverse events reported to the trial coordinator rather than the principal investigator, data analysis commencing after the trial database was locked, reminder on the trial assessor’s mobile phone and email not to disclose group allocation. Inadvertent un‐blinding was recorded on the trial database” Quote: “The assessor was aware of group allocation for only 8% (5/62) of the participants” Comment: small proportion of group allocation was revealed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “At primary endpoint, 10 weeks, data were unable to be collected for 1 participant for each group due to drop out prior to intervention (n=1) and family moved from area (n=1)” Quote: “..no missing data for the Assisting Hand Assessment” For secondary outcome measures: Quote: “The QUEST was 89% (55/62) complete at baseline and 91% (55/60) at 10 weeks. The Pediatric Quality of Life Inventory in combination with the Cerebral Palsy module was returned for 96% (49/51) at baseline and 94% (48/51) at the 10‐ and 24‐week assessments. The Pediatric Quality of Life Inventory infant scale was 100% (11/11) complete at all time points. The Birmingham Bimanual Questionnaire response was 81% (50/62) at baseline, 97% (60/62) at 10 weeks and 95% (59/62) at 24 weeks. There was a 94% (58/62) response rate for the diaries and 87% (54/62) for the parent questionnaires.” Comment: no missing data for the primary outcome measure at baseline or follow‐up. Low rate of attrition and low rates of missing data for secondary outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes specified in ISCTN Registry (58484608) were reported in the publication |
de Brito Brandão 2010.
| Methods |
Design: single‐centre randomised controlled trial Comparison groups reported by study autho rs: bimanual plus CIMT vs usual care Country: Brazil Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Spastic unilateral CP (b) Able to comprehend verbal commands and execute activities proposed during intervention Exclusion criteria (a) Associated pathologies or movement disorders (b) Surgery or botulinum toxin‐A injections 6 months prior to study beginning Participants: 16 participants with spastic unilateral CP were randomised Randomisation method: participants were randomly allocated by draw of sealed envelopes. Dropouts: n = 1 from comparison group due to “family problems” Number of participants who received intended treatment: n = 15 Number of participants who were analysed: total sample: n = 15; mean age = 5.9 years SD 1.2 years (calculated by review authors from Table 5), range = 4 years to 8 years 8 months; 8 males, 7 females; numbers for side of hemiplegia not reported; MACS I n = 4, MACS II n = 7, MACS III n = 4; GMFCS I n = 10, GMFCS II n = 5. Intervention group: n=8; mean age = 6.1 years SD 1.4 years (calculated by review authors from Table 5), range = 4 years, 6 months to 7 years 4 months; 4 males, 4 females; numbers for side of hemiplegia not reported; MACS I n=2, MACS II n=4, MACS III n=2; GMFCS I n=6, GMFCS II n=2. Comparison group: n=7; mean age = 5.7 years SD 1.1 years (calculated by review authors from Table 5), range = 4 years to 7 years 4 months; 4 males, 3 females; numbers for side of hemiplegia not reported; MACS I n=2, MACS II n=3, MACS III n=2; GMFCS I n=4, GMFCS II n=3. |
|
| Interventions |
Intervention group (Hybrid CIMT) Treatment dosage Length: 2 weeks of CIMT followed by 1 week of bimanual training (3 weeks total) Duration: 3 hours daily for 2 weeks of CIMT, followed by 3 x 45‐minute daily sessions of bimanual training for 1 week Frequency: daily (weekdays) Total dose of therapy time: 32 hours 15 minutes Description Type of restraint device: resting splint over wrist and fingers and a sling Hours per day restraint worn: planned = 10; actual = not reported Treatment environment: clinic Individual or group: individual Therapy provider: occupational therapist Models of practice: shaping, positive feedback and rewards Home programme: no Comparison group (low dose) Treatment dosage Length: 3 weeks Duration: 1 x 45‐minute session per week Frequency: weekly Total dose of therapy time: 2 hours 15 minutes Description Treatment environment: not reported Individual or group: individual Therapy provider: occupational therapist Models of practice: sessions were functionally orientated and included training of bimanual activities and sensory stimulation Home programme: no |
|
| Outcomes |
Assessment time points: baseline (1 week prior to intervention); 1 week after intervention (5 weeks from baseline) (immediately following intervention); 1 month after intervention (8 weeks from baseline) (2 weeks to 4 months postintervention) Primary outcome measure
Outcome measures
|
|
| Notes |
Additional information sought from authors: authors provided change data for MAS, grip strength and 2‐point discrimination Fundings sources: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and by Fundação de Apoioà Pesquisa do Estado de Minas Gerais (FAPEMIG), Brazil Study author declaration: no declaration provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:“ Participants were randomly allocated into intervention and control groups by draw of sealed envelopes”. Comment: insufficient information provided to determine random component in sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “ Participants were randomly allocated into intervention and control groups by draw of sealed envelopes” Comment: insufficient information provided to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including PEDI Self‐care ‐ Functional skills and Caregiver assistance domains was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "All assessments ... by an examiner blinded as to the children's groups (intervention or control)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The child who discontinued treatment was not included in the statistical analyses" Comment: one child from the usual care group dropped out due to family problems. Rates of attrition were low, balanced across groups and are unlikely to affect outcomes |
| Selective reporting (reporting bias) | Unclear risk | Comment: No study protocol located. Insufficient information to permit judgement |
DeLuca 2012.
| Methods |
Design: multi‐centre, single‐blinded, randomised controlled trial Comparison groups reported by study authors: ACQUIREc (CIMT plus bimanual activity; 6 hours per day) vs ACQUIREc (3 hours per day) Country: USA Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs different form of CIMT |
|
| Participants |
Inclusion criteria (a) Aged 3 to 6 years (b) Capable of following simple instructions and communicating effectively Exclusion criteria (a) Use of botulinum toxin‐A injections within the past 6 months (b) Previous participation in a formal CIMT programme (c) Presence of major uncontrolled seizures or comorbid medical conditions (d) Presence of visual impairment Participants: 18 children with unilateral CP Randomisation method: after screening and enrolment, children were randomised by the Data Coordinating and Analysis Center. Dropouts: n = 0 at postintervention assessment, n = 3; intervention n = 2, comparison n = 1 ‐ at 6‐month assessment Number of participants who received intended treatment: n = 18 Number of participants who were analysed: total sample: n = 18; mean age = 48.06 months SD = 11.64 months; 10 males, 8 females; 11 left hemiplegia, 7 right hemiplegia; MACS I n = 0, MACS II n = 15, MACS III n = 2, MACS IV n = 1; GMFCS not reported Intervention group: hybrid CIMT 6 hours per day: n = 9; mean age not reported; 5 males, 4 females; 4 left hemiplegia, 5 right hemiplegia; MACS I n = 0, MACS II n = 7, MACS III n = 1, MACS IV n = 1; GMFCS not reported Comparison group: hybrid CIMT 3 hours per day: n = 9; mean age not reported; 5 males 4 females; 7 left hemiplegia, 2 right hemiplegia; MACS I n = 0, MACS II n = 8, MACS III n = 1, MACS IV n = 0; GMFCS not reported |
|
| Interventions |
Intervention group (Hybrid CIMT) Treatment dosage Length: 26 days Duration: 6 hours per day Frequency: 18 days of CIMT followed by 3 days of bimanual (i.e. 21 days) over a 26‐day (3‐week) period Total dose of therapy time: 126 hours Description Type of restraint device: continuous cast, univalved for removal once per week, cast extended axilla to finger tips Hours per day restraint worn: 24 hours per day for 18 days Treatment environment: naturalistic settings: home, community, home‐like residence, park Individual or group: individual Therapy provider: occupational therapists Models of practice: "The therapist works individually within a structured treatment format, guided by principles of learning theory. When a child demonstrates a new skill or movement, the therapist provides reinforcement (primarily verbal praise, smiles, and supportive gestures) and then “shapes” movement by increasing demands for more precision, strength, fluency, and/or automaticity – a technique labelled “successive approximations.” Young children also receive intrinsic reinforcement for their efforts (e.g. solving puzzle, activating toy, completing self‐help task). Therapists ask parents and children to identify favourite activities, reinforcers, and personal goals for upper‐limb skills to determine the content and conduct of sessions. A central feature of ACQUIREc therapy is the “MR3 cycle,” an acronym for the 4 successive features of movement, reinforcement, repetition, and refinement which is an ongoing cyclical pattern that progresses in small increments as the child’s skills increase." (DeLuca 2012 p.136) Home programme: nil Comparison group (high dose, different form of hybrid CIMT – 3 hours) Treatment dosage Length: 26 days Duration: 3 hours per day Frequency: 18 days of CIMT followed by 3 days of bimanual (i.e. 21 days) over a 26‐day (‐ week) period Total dose of therapy time: 63 hours Description Type of restraint device: continuous cast, univalved for removal once per week, cast extended axilla to finger tips Hours per day restraint worn: 24 hours per day for 18 days Treatment environment: naturalistic settings: home, community, home‐like residence, park Individual or group: individual Therapy provider: occupational therapists Models of practice: "The therapist works individually within a structured treatment format, guided by principles of learning theory. When a child demonstrates a new skill or movement, the therapist provides reinforcement (primarily verbal praise, smiles, and supportive gestures) and then “shapes” movement by increasing demands for more precision, strength, fluency, and/or automaticity – a technique labelled “successive approximations.” Young children also receive intrinsic reinforcement for their efforts (e.g., solving puzzle, activating toy, completing self‐help task). Therapists ask parents and children to identify favourite activities, reinforcers, and personal goals for upper‐limb skills to determine the content and conduct of sessions. A central feature of ACQUIREc therapy is the “MR3 cycle,” an acronym for the 4 successive features of movement, reinforcement, repetition, and refinement which is an ongoing cyclical pattern that progresses in small increments as the child’s skills increase." (DeLuca 2012 p.136) Home programme: nil |
|
| Outcomes |
Assessment time points: baseline; within 1‐week postintervention (immediately following intervention); 1 month postintervention (2 weeks to 4 months postintervention); 6 months postintervention (5 to 6 months postintervention) Primary outcome measure
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: authors responded to request for information (AHA unit, QUEST change data), but appropriate data were not made available for meta‐analysis. Question: In your manuscript you report “We used only the Dissociated Movement and Grasp/Release sections of the QUEST; the revised protocol used 27 of 36 items”. Can you confirm if the “27 of the 36 items” refers to removing 9 of the items from the Dissociated movement and Grasp sections of the test? Reply: "Yes we did do items from the Dissociated Movement and Grasp section of the QUEST, but did not include the posture questions of the grasp section and did not duplicate the Grasp questions within the Dissociated Movement section" Fundings sources: Whitney S. Fox and Daniel S. Goldberg; Occupational Therapy Department, School of Health Professions; Biomedical Research Support Fund at the University of Alabama at Birmingham and the Georgetown University Center on Health and Education Study author declaration: authors declared no financial relationships related to this article and no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were randomized by the Data Coordinating and Analysis Center" using a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Quote: "Children were randomized by the Data Coordinating and Analysis Center". Comment: appears to be independently completed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including PMAL was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "...therapists who were not associated with treatment and were blinded to children’s treatment group administered a battery of standardized assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all children were followed up at 1‐week and 1‐month endpoints. Three of 18 children were lost to follow‐up at 6 months. Rates of attrition were balanced across groups and are unlikely be due to treatment or affect outcomes |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information given to permit judgement of low risk or high risk |
Deppe 2013.
| Methods |
Design: single‐centre, single‐blind, randomised controlled trial Comparison groups reported by study authors: Kid‐CIMT plus bimanual vs bimanual Country: Germany Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) Unilateral spastic CP or acquired non‐progressive central hemiplegia with other aetiologies (stroke, traumatic brain injury, non‐traumatic intracranial haemorrhage) (b) Age 3 years to 12 years (c) Active movement of wrist and MCP joints with extension from full flexion of at least 20° Exclusion criteria (a) Inability to stand and walk independently (b) Upper‐limb treatment with botulinum toxin within 6 months (c) Upper‐limb orthopaedic surgery within 1 year (d) Uncontrolled epilepsy (e) Insufficient cognitive ability to understand tasks and instructions (f) Severe behaviour problems Participants: 33 children with unilateral CP were recruited (in addition to 14 children with other unilateral diagnoses) Randomisation method: computer‐generated list of random numbers in concealed envelopes Dropouts: n = 5; intervention n = 2 (n = 1 family reasons, n = 1 behaviour problems), comparison n = 3 (n = 1 family reasons, n = 1 behaviour problems, n = 1 interfering disease); unclear which diagnostic group, although appears that 4/5 had CP Number of participants who received intended treatment: n = 29 Number of participants who were analysed: total sample: n = 29; mean age = 6 years 4 months SD 2 years 3 months (calculated by review authors); 13 males, 16 females; 8 left hemiplegia 21 right hemiplegia; MACS I n = 9, MACS II n = 18, MACS III n = 2; GMFCS not reported Intervention group: n= 1 6; mean age = 5 years 11 months SD 1 year 6 months; 6 left hemiplegia, 10 right hemiplegia; MACS I n = 5, MACS II n = 11, MACS III n = 0; GMFCS not reported Comparison group: n = 13; mean age = 6 years 10 months SD 2 years 6 months; 2 left hemiplegia, 11 right hemiplegia; MACS I n = 4, MACS II n = 7, MACS III n = 2; GMFCS not reported |
|
| Interventions |
Intervention group (Hybrid CIMT) Treatment dosage Length: 4 weeks Duration: 4 x 60‐minute sessions per day Frequency: 5 days per week Total dose of therapy time: 80 hours (60 hours CIMT plus 20 hours bimanual) Description Type of restraint device: arm (including shoulder, elbow, hand and fingers) fixed to the trunk using elastic bandages Hours per day restraint worn: 4 hours plus during one meal per day Treatment environment: clinic Individual or group: individual Therapy provider: experienced physiotherapists, occupational therapists, sport and music therapists, and educationalists Models of practice: shaping, with a focus on sensation, mobilisation and activity Home programme: no Comparison group (dose‐matched) Treatment dosage Length: 4 weeks Duration: 4 x 60‐minute sessions per day Frequency: 5 days per week Total dose of therapy time: 80 hours Treatment environment: clinic Individual or group: individual Therapist provider: experienced physiotherapists, occupational therapists, sport and music therapists, and educationalists Models of practice: shaping with a focus on sensation, mobilisation and activity Home programme: no |
|
| Outcomes |
Assessment time points: baseline; post treatment (within 1 week postintervention): Assisting Hand Assessment and Melbourne Assessment (immediately following intervention); two weeks after completion of intervention: Pediatric Evaluation of Disability Inventory (self‐care domain) (2 weeks to 4 months postintervention) Primary outcome measures
Secondary outcome measure
|
|
| Notes | Study included participants with other forms of hemiplegia (e.g. ABI), however data are provided for CP specific subgroup Additional information sought from authors: authors responded to request for information (AHA unit 0‐100 data), but appropriate data were not made available for meta‐analysis. AHA data from this study is therefore reported in Analysis 3.27 Fundings sources: this research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors Study author declaration: No declaration provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible children were selected at random for kid‐CIMT or intensive bimanual training by employing a computer‐generated list of randomized numbers in concealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated list of numbers in concealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including PEDI was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "Assessments were performed independently by experienced therapists that did not participate in the treatment and were blinded for group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 29 of 33 children with CP were analysed. Although it was reported that two children dropped out from kids‐CIMT group and 3 from IBT, it is unclear which group the non‐CP child was allocated. Although it is possible the dropouts were equal, it is also possible they were not. Insufficient information was given to determine whether the rates and reasons were balanced across groups. An as‐treated analysis was completed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Dong 2017.
| Methods |
Design: randomised controlled trial Comparison groups reported by study authors: CIMT versus Remind to Move (RTM) vs conventional rehabilitation Country: Hong Kong Other: trial registered at ClinicalTrials.gov (NCT02645331) Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis
|
|
| Participants |
Inclusion criteria (a) Aged 5 to 16 years of age (b) Ability to follow instructions (c) Ability to grasp and release light objects, with at least 20° of extension of the wrist and 10° of the metacarpophalangeal joints of the fingers (from full flexion of the affected hand) (d) Manual Ability Classification System (MACS) Levels I to III Exclusion criteria (a) Severe intellectual disability as defined by the Hong Kong Wechsler Intelligence Scale for Children (b) Visual or auditory disorder (c) Subject to seizures or having health problems not associated with CP (d) Predominant spasticity or contracture more than 3 on the Modified Ashworth Scale in wrist and finger flexors, forearm pronators, and/or thumb adductors (e) Botulinum neurotoxin injections and/or surgical interventions in the 6‐month period before the study Participants: 73 children with unilateral CP Randomisation method: a computer‐generated list of random numbers and concealed envelopes; this was done by an assistant not involved in the study. Dropouts: n = 3; intervention n = 2 (“children did not tolerate intervention and complain inconvenience after constraint”), comparison n = 1 ("parent refused conventional rehabilitation”) Number of participants who received intended treatment: n = 71; CIMT n=22, RMT n=25, low dose n=24 Number of participants who were analysed: Total sample: n=73; mean age = 11 years 10 months SD 3 years 1 months, range = 6 years 1 month to 16 years 7 months; 44 males, 29 females; 37 left hemiplegia, 36 right hemiplegia; MACS I n = 20, MACS II n=38, MACS III n=15; GMFCS I n=37, GMFCS II n=36. Intervention group (CIMT): n=24; mean age = 11 years 1 month SD 2 years 7 months; 15 males, 9 females; 12 left hemiplegia, 12 right hemiplegia; MACS I n=7, MACS II n=13, MACS III n=4; GMFCS I n = 12, GMFCS II n = 12 RTM group: n = 25; mean age = 12 years 1 months SD 3 years 3 month; 15 males, 10 females; 11 left hemiplegia, 14 right hemiplegia; MACS I n=5, MACS II n = 15, MACS III n = 5; GMFCS I n = 14, GMFCS II n=11 Low dose group: n = 24; mean age = 12 years 2 months SD 3 years 2 months; 14 males, 9 females; 14 left hemiplegia, 9 right hemiplegia; MACS I n = 8, MACS II n=10, MACS III n = 6; GMFCS I n = 11, GMFCS II n = 13 |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 3 weeks Duration: 5 hours per day Frequency: 5 days per week Total dose of therapy time: 75 hours (15 hours structured with therapist, 60 unstructured with teacher/parent). Face‐to‐face time with therapist = 15 hours Description Type of restraint device: custom‐made, volar, resting hand splint Hours per day restraint worn: 5 hours per day Treatment environment: school Individual or group: individual Therapy provider: occupational therapist Models of practice: Structured: “This training included both fine‐motor and gross motor activities, general movements for range of motion and voluntary repetition of desired movements, and age appropriate self‐care and play activities” (p.2). Unstructured: “Continued typical school routine and performed the predetermined upper‐limb movements independently, although supervised by the teachers or parents” (p.3) Home programme: nil Comparison group 1 (dose‐matched, Remind to Move) Treatment dosage Length: 3 weeks Duration: a sensory cueing wrist watch was worn 5 hours per day Frequency: 5 days per week Total dose of therapy time: 75 hours (15 hours structured with therapist, 60 unstructured with teacher/parent). Face‐to‐face time with therapist = 15 hours Description Children wore a sensory cueing wrist watch device (PolyU Technology & Consultancy Co. Ltd, Hong Kong; US patent US‐2010‐0160834‐A1) on the more‐affected arm. It emitted a vibration cue at 15‐minute intervals which reminded them to make predetermined movements Treatment environment: school Individual or group:individual Therapy provider: occupational therapist Models of practice: the shaping practice was similar in both RTM intervention and CIMT. "During the individual shaping practice session, children worked one‐to‐one with the occupational therapist, to guide the components of movement and the sequence of tasks; the children were asked to use their more affected hand to assist the functional hand to complete the bimanual shaping tasks, or to perform the structured unimanual practice with the affected hand freely. During the unstructured training session, the children were encouraged to complete customized movement tasks independently once they felt the vibration cues from the wristwatch. The teachers and parents avoided providing any verbal cues to get the children to use their affected hand" (p.3) Home programme: nil Comparison group 2 (low dose) Treatment dosage Length: 3 weeks Duration: 1 hour per day Frequency: 2 to 3 days per week Total dose of therapy time: maximum = 9 hours Description Treatment environment: school Individual or group: not reported Therapy provider: not reported Models of practice: hand splinting, muscle strengthening and stretching, neurodevelopmental facilitation techniques Home programme: nil |
|
| Outcomes |
Assessment time points: baseline; Immediately following intervention; 1‐month postintervention (2 weeks to 4 months postintervention); 3 months postintervention (2 weeks to 4 months postintervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: authors provided mean change and the standard deviation of mean change data for: Grip strength Fundings sources: VAD and KF were partially supported by the General Research Fund 2012/13, University Grants Committee, Hong Kong SAR (5608/12M). The funding source had no role in conduct of the study or writing of the report. Study author declaration: KF has a US patent of the sensory cueing wristwatch (US‐2010‐0160834‐A1). Y‐FC, SSWT, and LMSW have stated that they had no interests which might be perceived as posing a conflict or bias. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A sample of 73 eligible participants, stratified according to the levels of MACS, was randomly allocated to three groups (to receive either RTM, CIMT, or conventional rehabilitation), using a computer‐generated list of random numbers and concealed envelopes; this was done by a teaching assistant not involved in the study” |
| Allocation concealment (selection bias) | Low risk | Quote: “….using a computer‐generated list of random numbers and concealed envelopes; this was done by a teaching assistant not involved in the study” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including CFUS was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “The assessments, except the parental questionnaire, were performed by an experienced occupational therapist in paediatrics, who was trained to use the assessments by the investigators, and was blinded to the group allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “There were two dropouts from the CIMT group in the first week of treatment, because the children did not tolerate the intervention and complained about inconvenience during physical activities at school. One participant dropped out from the conventional rehabilitation group because his parents rejected the randomized group allocation.” “Seventy‐three participants were randomized, with a dropout rate at 4.1%, which is less than the predicted rate of 10% in the power calculation” Comment: the majority of the sample was therefore included (96%) and attrition was reasonably balanced across groups. Reasons for attrition were related to the intervention but unlikely to affect outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: trial registered at ClinicalTrials.gov: NCT02645331. All outcomes reported in trial registration are reported in the publication |
Eliasson 2011.
| Methods |
Design: single research centre with children and therapists recruited from various therapy centres. Assessor‐blinded, randomised controlled cross‐over trial with washout period Comparison groups reported by study authors: Eco‐CIMT vs ordinary paediatric rehabilitation Country: Sweden Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Unilateral CP (b) Aged 18 months to 5 years (c) Any severity level of decreased hand function (d) Ability to cooperate in the testing procedure (e) Parents willing to commit to the eight‐week intervention procedure Exclusion criteria (a) Visual or behavioural problems that would interfere with treatment or testing procedures (b) Botulinum toxin injection in the last six months (c) Included in another intensive training programme (d) Undergone surgery (e) Unstable medical situation during the study period Participants: 33 children with unilateral CP were randomised Randomisation method: participants were stratified according to age and level of hand function (mild, moderate, severe), recruited consecutively, randomised by computer‐generated list of random numbers (after consent). No other information given Dropouts: n=8/33; n = 6 dropped out while in the Eco‐CIMT arm of the trial (n = 1 did not tolerate Eco‐CIMT, n = 2 did not attend evaluation appointment, n = 3 completed less than 25 hours of the expected 112 hours of intervention [25 hours of training was cut‐off point for inclusion in the study]), n = 2 dropped out while in the low dose arm of the trial (n = 2 did not fulfil control criteria due to changed medical condition) Number of participants who received intended treatment: see above Number of participants who were analysed: total sample: n = 25; mean age = 28.8 months SD=11.2 months; 18 males, 7 females; side of hemiplegia not reported; MACS not reported but most of sample < 4 years; GMFCS not reported Intervention group: n = 12; mean age = 26.1 months 95% CI = 20 to 32 months; 9 males, 3 females; side of hemiplegia not reported; MACS not reported but most of sample < 4 years; GMFCS not reported Comparison group: n = 13; mean age = 31.2 months 95% CI = 24 to 39 months; 9 males, 4 females; side of hemiplegia not reported; MACS not reported but most of sample < 4 years; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 8 weeks Duration:2 hours Frequency: daily Total dose of therapy time: planned = 112 hours, actual time approximately 65.5 hours Description Type of restraint device: fabric mitt with stiff volar insert Hours per day restraint worn: 2 hours Treatment environment: home and preschool Individual or group:individual Therapy provider: parents or preschool teacher with once per week supervision from child’s usual therapists Models of practice: not clearly specified – connected with Dynamic Systems Theory, motor learning, Bronfernbrenner's ecological model of child development, based on each child’s Assisting Hand Assessment Home programme: Eco‐CIMT delivered as a home programme Comparison group (low dose) Treatment dosage Length: 8 weeks Duration: not specified, described as physio twice per month, OT once per month Frequency: not specified, described as physio twice per month, OT once per month Total dose of therapy time: not reported Description Treatment environment: not reported Individual or group: individual Therapy provider: occupational therapists and physiotherapists Models of practice: not reported: therapists were asked to maintain the child’s ordinary treatments during the usual care period Home programme: not reported |
|
| Outcomes |
Assessment time points: baseline; 8 weeks (immediately after intervention) Primary outcome measure
Secondary outcome measures
|
|
| Notes | Standards deviations were calculated by review authors from raw data provided by authors. Only data from the first intervention period were analysed. Data from 6 months (pre cross‐over intervention), 8 months (after cross‐over intervention) excluded from analysis Fundings sources: Swedish Research Council, Stockholm City Council, and the FOU Committee Study author declaration: no declarations given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The children were recruited consecutively. The randomization was produced by using a computer generated list of random number after the consent form was filled in" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement of low or high risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "The AHA video recordings were coded and scored by a blinded assessor who did not know any of the children, the time of assessment or their group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 18 children were allocated to the CIMT group, 12 were analysed. (Reasons: 1 did not tolerate intervention; 2 did not attend follow‐up; 1 did not achieve target of 25 hours of intervention; 1 did not complete control (cross‐over) period). 15 children were allocated to the comparison group, 13 were analysed (Reasons: 2 did not achieve target of 25 hours of intervention during CIMT (cross‐over) intervention). A large proportion of the sample was not included in analysis (24%). The rates of loss to follow‐up were unbalanced across groups and likely to affect outcomes. An as‐treated analysis was completed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Eliasson 2018.
| Methods |
Design: single‐centre, assessor‐blinded, randomised controlled trial Comparison groups reported by study authors: baby CIMT vs baby massage Country: Sweden Other (Protocol or registration number): NCT01864811 Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion Criteria (a) Between 3 and 8 months of corrected age and a ≥15% difference between the two hands assessed by the HAI (b) Considered at high risk of developing unilateral CP, that is, had a known neonatal event that affected the brain, and/or clinical signs that had been identified by a child neurologist or physiotherapist using assessments such as the Alberta Infant Motor Scale (AIMS) or Hammersmith Infant Neurological Examination (HINE) Exclusion Criteria (a) Severe visual impairment. (b) Seizures that could not be controlled by antiepileptic drugs (c) Families who were not able to communicate in either English or Swedish Participants: 37 infants were enrolled and 19 assigned in randomised fashion to receive baby‐CIMT and 18 to baby‐massage. Six infants did not fulfil the diagnostic criteria at 12 months of age and were therefore excluded. The children excluded had bilateral CP (n = 2) or exhibited no sign of CP (n = 4) at 12 months of age Randomisation method: randomisation was stratified by age (3–4, 5–6, and 7–8 months, corrected for prematurity) and neonatal event (neonatal arterial stroke at a gestational age ≥ week 37, preterm birth at < week 37, and unknown/other) and performed after the first assessment when the consent form was completed. A list of random numbers associated with these stratification factors was generated before initiation of the intervention and was known only to the first author, who assigned the families to the different interventions Dropouts: none Number of participants who received intended treatment: CI intervention group: n = 18; Control group: n = 13 Number of participants who were analysed: total sample: n = 31; mean age 5.6 month, SD 1.7; 16 males, 15 females; 15 left hemiplegia, 16 right hemiplegia Intervention group: n = 18; mean age = 6 months SD 1.7 weeks, range = not reported; 8 males, 10 females; 7 left hemiplegia, 11 right hemiplegia. Comparison group: n = 13; mean age = 5 months SD 1.6 weeks, range = not reported; 8 males, 5 females; 8 left hemiplegia, 5 right hemiplegia |
|
| Interventions |
Intervention Group (mCIMT) Treatment dosage Length:18 weeks (6 weeks on, 6 weeks off, 6 weeks on) Duration: 30 minutes Frequency: 6 days per week Total dose of therapy time: planned = 36 hours, actual time 35 hours SD 10 hours Description Type of restraint device: mitten or something similar Hours per day restraint worn: 30 minutes Treatment environment: home Individual or group: individual Therapy providers: therapists and parents Models of practice: dynamic systems theory, motor learning theory and Bronfenbrenner and Morris’s ecological model of child development. The training included several components in which grasping action and toy exploration was the main focus. The choice of toys depended on the infant’s individual ability to perform motor actions in combination with their cognitive ability. The specific focus for each week were specified depending on the infant’s ability and progress Home programme: baby‐CIMT was delivered in the home environment Comparison Group (Baby massage) Treatment dosage Length: 18 weeks (6 weeks on, 6 weeks off, 6 weeks on) Duration: 5 – 30 minutes Frequency: 6 days per week Total therapy time: planned = 72 occasions, actual occasions 52 SD 26 occasions Treatment environment: home Individual or group: individual Therapy provider: parents Models of practice: baby massage. Parents were taught to massage each body part in sequence using slow and gentle strokes, smooth circular movements, and gentle squeezing depending on the body part. Home programme: baby massage was delivered in the home environment |
|
| Outcomes |
Assessment time points: baseline; 6 weeks (immediately after first 6 weeks of baby CIMT); 12 weeks (immediately after 6‐week break); 18 weeks (immediately after second 6 weeks of baby CIMT); 12 months of age; 18 months of age Primary outcome measure
Secondary outcome measures
Measures used at baseline to describe sample
Measure used to compare groups at 18‐month follow‐up
Outcome measure excluded in this Cochrane review
|
|
| Notes |
Additional information sought from authors: authors provided mean age for the whole sample along with mean change and the standard deviation of mean change data for HAI and PSCS immediately following intervention (18 weeks). Group data (mean, SD) for the AHA at 2 years were also provided. Question: The 2014 protocol states the AIMS was to be undertaken at baseline and 12 months. The 12 month data was not reported in the 2018 publication. Can you clarify if data for the AIMS was collected at 12 months? Reply: Regarding AIMS, I have the data at about 12 month but that is sometimes close to end of intervention, sometimes after a long time period if the babies, depending on the inclusion age 3‐8 month. Therefore, we decided not to report 12 month of age data. Adverse events: there were no adverse events Funding sources: the project was financially supported by the Swedish Research Council (grant nos. 521‐211‐2655 and 521‐2011‐456), Promobilia (grant no. 11006), Stiftelsen Frimurare‐Barnhuset in Stockholm, and Foundation Olle Engkvist Byggmästare as well as by grants to LS from the Stockholm City Council, to LE from the Health Care Sciences Postgraduate School and to LKS from the Strategic Research Programme in Care Sciences at Karolinska Institutet. The authors have no conflicts of interest to declare Study author declarations: the authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors, Lena Sjöstrand, Linda Ek and Kristina Tedroff, declare that they have no competing interests. Lena Krumlinde‐Sundholm and Ann‐Christin Eliasson are stockholders in Handfast AB a company for educational purpose. LKS is working as AHA teacher |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Eligible children were randomised to the interventions. Randomisation was stratified by age (3–4, 5–6, and 7–8 months, corrected or prematurity) and neonatal event (neonatal arterial stroke at a gestational age ≥week 37, preterm birth at<week 37, and unknown/other) and performed after the first assessment when the consent form was completed. A list of random numbers associated with these stratification factors was generated before initiation of the intervention and was known only to the first author (ACE), who assigned the families to the different interventions” |
| Allocation concealment (selection bias) | High risk | Quote:“A list of random numbers associated with these stratification factors was generated before initiation of the intervention and was known only to the first author (ACE), who assigned the families to the different interventions”. Comment: investigator enrolling participants could possibly foresee assignments and thus introduce selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including the Parenting Sense of Competence (PSCS) not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “Only the assessors of the video recordings of HAI and Assisting Hand Assessment (AHA) (LE) and the brain scans (FL) were blinded to group allocation” (p. 193, Eliasson 2018) Quote: “The occupational therapist responsible for data collection (i.e., administration and filming of HAI and AHA) will not be blinded to group allocation” (p. 5, Eliasson 2014) Comment: the therapist collecting data was not blinded however, the assessors of the video recordings were blinded and not likely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The 37 infants eligible to participate were all enrolled and 19 assigned in randomised fashion to receive baby‐CIMT and 18 to baby‐massage…all families fulfilled the interventions and there were no dropouts. However, six infants did not fulfil the diagnostic criteria at 12 months of age and were therefore excluded, since the baby‐CIMT is currently considered appropriate for infants with unilateral CP. The children excluded had bilateral CP (n= 2) or exhibited no sign of CP (n= 4) at 12 months of age. The final group of 31 infants were further analysed” |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available. All outcomes except the AIMS were used and reported. The AIMS was proposed as an outcome measure at baseline and 12 months in the protocol but not reported in the paper. The authors report that due to the variable age at recruitment i.e. between 3 and 8 months for this study a consistent follow‐up period for the AIMS this was not possible. |
Eugster‐Buesch 2012.
| Methods |
Design: multi‐centre, single‐blind, randomised controlled trial Comparison groups reported by study authors: forced use vs usual care Country: Switzerland Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) 6 to16 years (b) Unilateral CP (c) GMFCS Levels I or II (d) Able to lift impaired arm against gravity and grasp a lightweight item such as pen from a desk (e) Secure balance while standing and sitting (f) Able to understand and follow therapists’ instruction Exclusion criteria
Participants: 23 children with unilateral CP Randomisation method: not clearly stated Dropouts: n = 0 prior to primary end point; 14 lost to follow‐up at 12 months Number of participants who received intended treatment: n = 23 (100%); intervention n = 12, comparison n = 11 Number of participants who were analysed: total sample: n = 23; mean age = 10 years 8 months SD 7 years 9 months, range = 6 years 0 months to 16 year 11 months; 12 males, 11 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported Intervention group: n = 12; mean age = 9.8 years SD 3.5 years, range = 6 years 0 months to 15 years 6 months; 5 males, 7 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported Comparison group: n = 11; mean age = 11.7 years SD = 3.7 years, range = 6 years 1 month to 16 years 11 months; 7 males, 4 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 2 weeks Duration: 2 hours per day of age appropriate ADL and play, 4 hours per day without formal therapy or training; 1 hour per week of usual therapy. Frequency: daily Total dose of therapy time: 84 hours of constraint use which included 2 hours of ADL activities Description Type of restraint device: removable Softcast with Velcro fastenings (forearm to fingertips) Hours per day restraint worn: planned: 6 hours per day, actual: 72% of participants (n = 8) reported having always (45%) or often (27%) reached the 6 hours/day target for duration of cast wear. The rest achieved the target sometimes (n = 2) or rarely (n = 1). Treatment environment: home and clinic (participants had 1 session of therapy each week) Individual or group: individual Therapy provider: parents Models of practice: none prescribed Home programme: all the therapy was completed at home Comparison group (low dose) No information on the comparison group in this study were reported |
|
| Outcomes | Assessment time points: baseline: 2 weeks prior to intervention; Pretest: immediately prior to intervention; Post‐test 1: Immediately following intervention; Post‐test 2: 2 weeks after intervention (2 weeks to 4 months postintervention); Post‐test 3: 3 months after intervention (2 weeks to 4 months postintervention); Post‐test 4: 12 months after intervention (7 to 12 months postintervention) Primary outcome measure
Investigator developed questionnaire. Reason for exclusion: No evidence of validity or reliability in CP |
|
| Notes | 12‐month data not reported by study authors. Additional information sought from authors Question: Following review of your study we would like to seek clarification on how many children were recruited to the study, how many children were randomised to each group and the number of dropouts at each assessment for each group. Reply: CONSORT diagram sent. Summary as follows: Assessed for eligibility: n = 27; Excluded n = 4 Allocated to group: CIMT (n=12); comparison (n=11) Received allocated intervention: CIMT (n = 12 ); comparison (n = 11) Lost to follow‐up (postintervention): CIMT (n = 0 ); comparison ( n =1) Lost to follow‐up (2 weeks postintervention): CIMT (n = 0 ); comparison (n = 0) Lost to follow‐up (3 months postintervention): CIMT (n = 1 ); comparison (n = 1) Lost to follow‐up (12 months postintervention): CIMT (n = 5 ); comparison (n = 9) Fundings sources: Stiftung Cerebral, Switzerland. Study author declaration: the authors report no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned either into control group (C) or intervention group (I) by the study coordination center using sealed envelopes” Comment: Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “The raters were blinded to group allocation of a child and were not involved in the recruiting process or in the therapy sessions” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: additional data from authors confirmed that 1 child from the comparison group was not assessed immediately after intervention (due to illness), one child from each group was not assessed at 3 months (reasons unknown), and that only 14 children, mostly from treatment group were assessed at 12 months. There is, therefore, a low risk of bias up to the 3 months follow‐up with minimal and balanced drop out; and high risk of bias at 12 months with high and unbalanced drop out |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Facchin 2011.
| Methods |
Design: multi‐centre, prospective, cluster‐randomised controlled trial involving 21 rehabilitation sites Type of cluster: intervention type (CIMT, bimanual or traditional treatment) Cluster size: 21 sites Number of clusters in each arm: 7 (mCIMT), 7 (Bimanual Intensive Rehabilitation programme), 7 (Traditional treatment) Adjusted for clustering: no Comparison groups reported by study authors: CIMT vs bimanual intensive rehabilitation (IRP) vs traditional treatment Country: Italy Other: a trial protocol was published Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis
|
|
| Participants |
Inclusion criteria (a) Aged 2 to 8 years (b) Unilateral CP Exclusion criteria (a) Previous constraint therapy (b) Injections of antispasticity drugs (e.g. botulinum toxin) in previous 6 months Participants: 105 children with unilateral CP (power calculation estimated 111 children were required, 113 were recruited). Randomisation method: cluster randomisation: Quote: “Each clinical center was randomized to a treatment option (i.e., mCIMT was randomly assigned to center A; IRP to center D; and ST to center F). In this way, all children enrolled in center A underwent the treatment selected for that center” Dropouts: n =113; randomised n = 105, completed intervention n = 105, analysed n = 104. Although a sample size calculation estimated that 111 were required, 37 in each group, 113 children were recruited as follows:
Number of participants who received intended treatment: intervention n = 39, IRP n = 33, low dose n = 33 Number of participants who were analysed: total sample: n = 105; mean age = 3.96 years SD 2.02 years; 53 males, 52 females; 49 left hemiplegia, 56 right hemiplegia; MACS not reported; GMFCS not reported Intervention group (mCIMT): n = 39; mean age = 4.36 years SD 2.11 years; 19 males, 20 females; 15 left hemiplegia, 24 right hemiplegia; MACS not reported; GMFCS not reported Comparison group 1 (IRP): n = 33; mean age = 3.27 SD 1.77 years; 17 males, 16 females; 18 left hemiplegia, 15 right hemiplegia; MACS not reported; GMFCS not reported. Comparison group 2 (low dose): n = 33; mean age = 4.18 years SD 2.04 years; 17 males, 16 females; 16 left hemiplegia, 17 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 10 weeks Duration: 3 hours per day Frequency: 7 days per week Total dose of therapy time: 210 hours. Face‐to‐face time with therapist = 210 hours Description Type of restraint device: “Comfortable fabric glove with a built‐in volar stiff plastic splint ... with the thumb kept in a fixed position tight against the index finger” (Facchin 2009, p.221). Photo in protocol Facchin 2009 shows gutter splint for forearm/wrist and fingers Hours per day restraint worn: 3 hours Treatment environment: clinic and home Individual or group: individual Therapy provider: for half of clinic sessions (1.5 hours) physiotherapists provided therapy and remaining half (1.5 hours) parents were supervised to conduct therapy Models of practice: unimanual activities according to motor learning approach during play sessions and ADLs Home programme: 3 hours constraint for the 4 non‐clinic days per week Comparison group 1 (dose‐matched) Treatment dosage Length: 10 weeks Duration: 3 hours per day Frequency: 7 days per week Total dose of therapy time: 210 hours. Face‐to‐face time with therapist = 210 hours Description Treatment environment: clinic and home Individual or group: iIndividual Therapy provider: for half of clinic sessions (1.5 hours) physiotherapists provided therapy and remaining half (1.5 hours) parents were supervised to conduct therapy Models of practice: bimanual activities according to motor learning approach during play sessions and ADLs Home programme: 3 hours intervention for the 4 non‐clinic days per week Comparison group 2 (low dose) Treatment dosage Length: 10 weeks Duration: pre‐school and school aged children: 40 to 60 minutes per week. Infants: 1 hour per week Frequency: pre‐school and school aged children: once per week. Infants: twice a week Total dose of therapy time: pre‐school and school aged children: 10 hours. Infants: 20 hours Description Treatment environment: not reported Individual or group: not reported Therapy provider: pre‐school and school aged children: physiotherapists. Infants: occupational therapists Models of practice: pre‐school and school aged children: not reported. Infants: neurodevelopmental therapy Home programme: not reported |
|
| Outcomes |
Assessment time points: baseline (0 weeks); 10 weeks (immediately following intervention); 3 months postintervention (2 weeks to 4 months postintervention); 6‐month postintervention (5 to 6 months postintervention); 12 months postintervention (7 to 12 months postintervention) Primary outcome
Measures used to monitor adverse events
Measures identified as covariates
Treatment satisfaction and compliance perceived by parents |
|
| Notes |
Additional information sought from authors Authors provided mean change and the standard deviation of mean change data for QUEST and Besta Scale for immediately following intervention. Question 1: Were the sites randomised to treatment before participants were recruited? Reply 1: We confirm that the sites were randomised to treatment before participants were recruited. Each site was randomized to a treatment approach (Intensive Bimanual, Intensive CIMT or Standard treatment) and subsequently patients were recruited Question 2: Did researchers/staff at each site know the intervention assigned to their site whilst they were recruiting participants? Reply 2: Once the site was randomized, the researchers/staff at each site were aware of which treatment they were recruiting for, since during the recruitment phase they were in charge of explaining the type of treatment they would have eventually administered to children and families (this phase was required for Ethical Committee approval of the project) Question 3: Did participants know the intervention to which they would be allocated when they consented to participate? Reply 3: For the same reason, when participants consented to participate, they were aware of which treatment their consensus was for Fundings sources: Veneto Region Government, Regional Epidemiological Observatory for Sick Children. Pierfranco e Luisa Mariani Foundation. Monitoring and Innovation on Health Technology and Organization (MIHTO), University of Padua spin‐off Study author declaration: no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each clinical center (n=21) was randomized to a treatment option (i.e., mCIMT was randomly assigned to center A; IRP, to center D; and ST, to center F). In this way, all children enrolled in center A underwent the treatment selected for that center (mCIMT in the example)" |
| Allocation concealment (selection bias) | Low risk | Quote: “To each cluster (the clinical center), a treatment group was randomly assigned and the cluster developed only that treatment" (p. 163, Fedrizzi, 2013) Comment: Sites were randomised to treatment before participants were recruited |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including Parenting Stress Index, Besta scale for parents and Child Behavior Checklist was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote:“Two supervisors of outcome measures examined the videotapes of all evaluations of patients from each treatment group, and they were blinded to the treatment allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “A sample of 111 participants has been recruited….Thirty seven cases were enrolled in seven centers for mCIMT, 37 cases in seven centers for IRP, and 37 cases in seven centers for ST.” (p. 541 Facchin, 2011) Quote: “105 patients were recruited and assigned to the treatment groups: mCIMT (n = 39), bimanual IRP (n = 33), and ST (n = 33)…..One of the centers of the mCIMT group asked to add two extra patients to the experimental group because of organizational reasons (more than one patient reaching the center at the same time in the final recruitment phase) so that the expected amount of 37 was exceeded (p. 544 Facchin, 2011). “ST and IRP groups had a 10% dropout rate before the trial started because of minor reasons” (p. 544 Facchin, 2011) Quote: “One patient recruited in the IRP group withdrew from the study because the family moved and did not undergo the posttreatment assessment” (p. 544 Facchin, 2011) Comment: inconsistent reporting of numbers of participants and drop outs. Insufficient information to permit judgment |
| Selective reporting (reporting bias) | Low risk | Comment: Study protocol available. All outcomes were used and reported, or deviations from the protocol were adequately explained. Secondary outcomes were utilised as covariates in the analysis |
Gelkop 2015.
| Methods |
Design: single‐centre, single‐blind, randomised controlled trial Comparison groups reported by study authors: CIMT vs HABIT Country: Israel Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison type reported by study authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) Aged 18 months to 7 years (b) Congenital hemiplegia (c) Ability to extend wrist 20° (d) Ability to release objects from the hand (e) Age‐appropriate cognitive ability as identified by child’s placement in age‐appropriate classes and based on evaluations by school psychologists Exclusion criteria (a) Received an intensive therapeutic intervention involving the upper extremities or botulinum toxin therapy in the upper limb within the past 6 months (b) Any intended new treatment within the study period Participants: 12 children with congenital hemiplegia Randomisation method: two groups were created by matching children according to age, cognitive level (class level), and initial hand function as determined by AHA and QUEST scores. The two groups as a whole were then randomised using concealed allocation to receive either CIMT or HABIT. Additional information from authors: "Children were randomized offsite, using a random number generator, by an individual with no knowledge of or participation in the study" Dropouts: nil Number of participants who received intended treatment: n = 12 Number of participants who were analysed: total sample: n = 12; mean age = 4.29 years SD 1.65 years (calculated by review authors); 2 males, 10 females; 6 left hemiplegia, 6 right hemiplegia; MACS I n=2, MACS II n = 4, MACS III n = 3; GMFCS not reported Intervention group: n =6; mean age = 4.25 years SD 1.58 years; 1 male, 5 females; 3 left hemiplegia, 3 right hemiplegia; MACS I n = 1, MACS II n = 1, MACS III n = 2; GMFCS not reported Comparison group: n = 6; mean age = 4.33 years SD 1.86 years; 1 male 5 females; 3 left hemiplegia, 3 right hemiplegia; MACS I n = 1, MACS II n=3, MACS III n = 1; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 8 weeks Duration: 2 hours per day Frequency: 6 days per week for 8 weeks (12 hours per week) Total dose of therapy time: 96 hours Description Type of restraint device: custom made mitt Hours per day restraint worn: 2 hours Treatment environment: child’s regular preschool or kindergarten Individual or group: group (1 hour per day) and individual (1 hour per day) Therapy provider: occupational therapists and occupational therapy assistants Models of practice: intensive, progressive task practice based on a motor learning approach. "Task difficulty was graded by changing the task constraints, which required progressive skill in hand use and increasingly providing tasks involving greater difficulty.... Strategies for grading activities and changing the constraints of the tasks were discussed in group meetings. Children participated in whole‐ and part‐task practice" (p.29). "Interventionists tracked compliance by recording daily task performance using a daily log in which they indicated the activity performed and time spent on each activity....Activities included activities of daily living (e.g., cleaning, eating) and playing with an assortment of child‐friendly games performed indoors and outdoors" (p.30) Home programme: not reported Comparison group (ose‐matched) Treatment dosage Length: 8 weeks Duration: 2 hours per day Frequency: 6 days per week for 8 weeks (12 hours per week) Total dose of therapy time: 96 hours Description Treatment environment: child’s regular preschool or kindergarten Individual or group: group (1 hour per day) and individual (1 hour per day) Therapy provider: occupational therapists and occupational therapy assistants Models of practice: children "engaged in age‐appropriate fine and gross motor bimanual activities.... Activities were chosen based on the ability of the child’s paretic hand.... Task demands were graded and the children encouraged to be active in identifying movements to complete an action (i.e., problem solving). Interventionists avoided using verbal requests to use the paretic hand as much as possible, and instead modified the environment by providing tasks that required the use of both hands to elicit desired movements" (p.30) Home programme: not reported |
|
| Outcomes |
Assessment time points: baseline 1 (0 weeks); baseline 2 (9 weeks). Data for baseline period 2 (immediately prior to intervention) were used for meta‐analysis in this review; 17 weeks (immediately following intervention). Week 26 (8 weeks after completion of intervention) (2 weeks to 4 months postintervention) Primary outcome measures:
|
|
| Notes | Standard deviation data were calculated from 95% CI data reported in the paper for immediately post‐intervention data Additional information sought from authors Authors provided mean change and the standard deviation of mean change data for: AHA and QUEST Question 1: “Two participants (one from each group) were unable to complete the assessment due to lack of cooperation”. Can you clarify if these two children were unable to be assessed at all time points Reply 1: These children were not assessed at all time points for the QUEST (i.e. all subtests) Question 2: Further description of the randomisation and allocation concealment procedures Reply 2: Children were randomized offsite, using a random number generator, by an individual with no knowledge of or participation in the study. Question 3: Can you clarify if the treatment provided was 5 or 6 days per week. (Note: Clarification sought because authors report CIMT consisted of 2 hours per day treatment sessions, 6 days a week for 8 weeks (total dosage 96 hours) (p. 29). Total dose in text (p. 30) indicated total dose of 80 hours (consistent with 5 days per week of therapy) Reply 3: Treatment was provided 6 days per week Fundings sources: no funding reported Study author declaration: the authors report no conflict of interest. The authors alone were responsible for the content and writing of this article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two groups were created by matching children according to age, cognitive level (class level), and initial hand function as determined by AHA and QUEST scores. The two groups as a whole were then randomized using concealed allocation to receive either CIMT or HABIT" Further information obtained from the authors: "Children were randomized offsite, using a random number generator, by an individual with no knowledge of or participation in the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "The two groups as a whole were then randomized using concealed allocation to receive either CIMT or HABIT" Further information obtained from the authors: "Children were randomized offsite, using a random number generator, by an individual with no knowledge of or participation in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "All children were assessed by physical therapists blinded to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intent to treat principles were used for the analysis" Comment: missing data were imputed using appropriate methods. One child in each group was unable to complete the QUEST, therefore, missing data were balanced across groups and unlikely to be related to outcome |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Gharib 2010.
| Methods |
Design: single‐blind randomised clinical trial Comparison groups reported by study authors: CIMT plus usual care vs usual care Country: Iran Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Unilateral spastic CP (b) Age between 18 and 72 months (c) Spasticity in affected elbow flexor < 3 on Modified Ashworth Scale (d) Ability of overall mass grasp (e) Ability to follow instructions Exclusion criteria (a) Previously received CIMT (b) Achieved 100/100 for QUEST total score for non‐affected upper limb (c) Ability to understand and carry out verbal and physical commands (d) Attention disorders, vision and typical audiology problems (e) Orthopaedic neurological problems in the upper extremities (f) Uncontrolled seizures (g) Feeling pain following the use of splints (h) Parental reports of autism and behavior problems (i) Not participating in therapy sessions over three consecutive sessions Participants: 21 children with unilateral CP Randomisation method: “... after first assessment, children were randomly divided into two groups of intervention and control using a lottery pot” Dropouts: n = 26 were randomised; intervention n=14, comparison n = 12. Three children in intervention group and 2 children in comparison group dropped out “due to non‐compliance”. No further reasons were provided Number of participants who received intended treatment: intervention n =11, comparison n=10 Number of participants who were analysed: total sample: n=21; mean age = 47.29 month, SD 18.35 months; 9 males, 12 females; 5 left hemiplegia, 16 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n = 11; mean age = 46.5 months SD 17.5 months; 4 males, 7 females; 3 left hemiplegia, 8 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n = 10; mean age = 48.1 months SD 19.2 months; 5 males, 5 females; 2 left hemiplegia, 8 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (Hybrid CIMT) Treatment dosage Length: 6 weeks Duration: usual care OT: 45 minutes 3 times per week; CIMT: 3 hours per day Frequency: daily. Total dose of therapy time: CIMT (126 hours) + usual care OT (13.5 hours) = 139.5 hours. Face‐to‐face time with therapist = 13.5 hours Description Type of restraint device: splint Hours per day restraint worn: 3 hours Treatment environment: home (CIMT) and Clinic (OT) Individual or group: individual Therapy provider: parent (CIMT) and occupational therapist (OT) Models of practice: at the beginning of each session parents provided oral and written reports to the therapists on how tasks were performed in the CIMT home program Home programme: to ensure consistent use of the restraint and implementation of CIMT at home, therapists phone parents weekly to discuss the programme and follow‐up progress Comparison group (low dose Treatment dosage Length: 6 weeks Duration: 45 minutes Frequency: 3 sessions a week for 6 weeks (2.25 hours per week) Total dose of therapy time: 13.5 hours Description: Treatment environment: clinic Individual or group: individual Therapy provider: occupational therapist Models of practice: not described Home programme: not reported |
|
| Outcomes |
Assessment time points: baseline; 6 weeks (immediately postintervention) Primary outcome measure
Secondary outcome measures
|
|
| Notes |
Note: published in Persian ‐ data extraction and risk of bias were kindly completed by Associate Professor Mehdi Rassafiani, Department of Occupational Therapy, University of Social Welfare and Rehabilitation Sciences, Tehrān, Iran and Dr Fakher Rahim, Ahvaz Jondishapour University of Medical Sciences, Ahvaz, Iran Associate Professor Mehdi contacted the authors for further information about the nature of intervention provided. Details are follows: Both groups Both intervention and control group received 45 minutes regular occupational therapy (OT) three times a week for the 6 week study period Hybrid CIMT group The intervention group had CIMT as well. Parents were given a splint to be used by their children at home and were trained to do activities for three hours per day while wearing the splint. The parents were trained and checked for CIMT after each session of regular OT. At the beginning of each session of regular OT, parents gave a written and oral report of how activities have been done. Also, to ensure use of the splint and doing exercises at home, telephone follow‐up was conducted during the week. Therefore, all the instruction and training to the parent were done in the clinic and CIMT were done at home by parent. Therapist provided oral instruction and demonstrated how to do the activities at home and there was not any written instruction to be used at home by parents Fundings sources: translation not available Study author declaration: translation not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “... after first assessment, children were randomly divided into two groups of intervention and control using a lottery pot” |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “The evaluation was done at the beginning and end of 6 weeks in both groups by a Master of Occupational Therapy Student who was blinded to groups” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Three children in intervention group and two in control group were not able to complete the study” ‐ “due to non‐compliance” Comment: high rate of attrition (19.2%), which is balanced across groups. Rate < 20% therefore judged as low risk |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Gordon 2011.
| Methods |
Design: single‐centre, randomised controlled trial Comparison groups reported by study authors: CIMT vs HABIT Country: USA Other: trial registered on ClinicalTrials.gov (NCT00305006) Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) Ability to extend wrist >20° and fingers at the metacarpophalangeal joints >10° from full flexion (b) Ability to lift the more affected arm 15 cm above a table surface and grasp light objects (c) >50% difference in Jebsen‐Taylor Test of Hand Function (JTTHF) scores between the two hands (d) Mainstream school (e) Kaufman Brief Intelligence test score > 70 (f) Ability to follow instructions during screening and to complete the testing Exclusion criteria (a) Health problems unassociated with CP (b) Current untreated seizures (c) Visual problems interfering with treatment/testing (d) Severe muscle tone (Modified Ashworth >3.5) (e) Orthopaedic surgery of affected hand within 1 year (f) Botulinum toxin therapy in upper limb within 6 months, or planned during treatment period (g) Balance problems precluding wearing a sling Participants: 44 participants with unilateral CP Randomisation method: offsite, concealed randomisation. Method not specified. Dropouts: n = 2; intervention (n = 1 family changed mind regarding participation), comparison n = 1 (failure to complete pre‐test) Number of participants who received intended treatment: n=42 Number of participants who were analysed: total: n = 42; mean age = 6 years 4 months SD 2 years 0 months, range = 3.5 to 10 years; 20 male, 22 female; 18 left hemiplegia, 24 right hemiplegia; MACS I n = 5, MACS II n = 35, MACS III n = 2; GMFCS not reported Intervention group: n = 21; mean age = 6 years 3 months SD 2 years 2 months; 9 males, 12 females; 6 left hemiplegia, 15 right hemiplegia; MACS I n = 2, MACS II n=18, MACS III n = 1; GMFCS not reported Comparison group: n = 21; mean age = 6 years 4 months SD 1 year 11 months; 11 males, 10 females; 12 left hemiplegia, 9 right hemiplegia; MACS I n=3, MACS II n = 17, MACS III n = 1; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 3 weeks (15 consecutive weekdays) Duration: 6 hours per day Frequency: daily (weekdays) Total dose of therapy time: planned = 90 hours, actual = not reported Description Type of restraint device: sling (closed ended) strapped to body Hours per day restraint worn: planned = 90 hours, actual = not reported Treatment environment: clinic: day‐camp model Individual or group: group: 2 to 5 participants per group; involved group work as well as 1:1 time. Ratio of therapists to participants was 1:1 Therapy provider: occupational therapists and physiotherapists were primary interventionists (always present within the group), assisted by graduate students from Kinesiology, Neuroscience, Speech Pathology and Psychology as well as undergraduate students Models of practice: enjoyable, intensive task practice based on motor learning approaches. Targeted movements and temporal and spatial coordination were practiced in whole or part within the context of completing tasks. "Participants performed unilateral fine‐motor and manipulative gross motor activities that elicited general movements of interest and included a range of age‐appropriate, unimanual functional and play activities. The interventionist provided assistance where appropriate" (p.3) Home programme: families were asked to encourage 1‐hour daily practice at home (without constraint) of unimanual tasks during intervention and for the 6 months following intervention Comparison group (dose‐matched) Treatment dosage Length: 3 weeks (15 consecutive weekdays) Duration: hours per day Frequency: daily (weekdays) Total dose of therapy time: planned = 90 hours, actual = not reported Description Treatment environment: clinic: day‐camp model Individual or group: group: 2 to 5 participants per group; involved group work as well as 1:1 time. Ratio of therapists to participants was 1:1 Therapy provider: occupational therapists and physiotherapists were primary interventionists (always present within the group), assisted by graduate students from Kinesiology, Neuroscience, Speech Pathology and Psychology as well as undergraduate students Models of practice: enjoyable intensive task practice based on motor learning approaches. Targeted movements and temporal and spatial coordination were practiced in whole or part within the context of competing tasks. Activities were selected to increase "in complexity from a nondominant passive assist (e.g. stabilising paper while drawing) to active manipulator (e.g., reorienting paper while cutting) using increasingly complex bimanual coordination and participants’ interests. Task demands were graded, and participants were engaged in active problem solving. Interventionists avoided verbal prodding to use the paretic hand and instead constrained the environment by providing tasks necessitating the use of both hands to elicit desired movements. Part practice included both bilateral symmetrical (e.g., reaching toward object[s] with both hands) and asymmetrical (e.g., pulling apart objects) movements" (p.4) Home programme: families were asked to encourage 1 hour daily bimanual practice at home (without constraint) during intervention and for the 6 months following intervention |
|
| Outcomes |
Assessment time points: baseline. Within 2 days of treatment ending (immediately following intervention); 1 month after treatment (2 weeks to 4 months postintervention); 6 months after treatment (5 to 6 month postintervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: PEDI and QUEST data requested and received from authors for subset reported immediately following intervention in De Brito Brandao (2012) Fundings sources: Thrasher Research Fund; CVS Landmark Cares; the Brazilian government agencies National Counsel of Technological and Scientific Development (CNPq) and Foundation for Research Support of Minas Gerais (FAPEMIG). Study author declaration: the author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants (4‐10 in each camp) were randomized offsite using concealed allocation stratified by age and JTTHF screening score” (p.4) Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants (4‐10 in each camp) were randomized offsite using concealed allocation” (p.4) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including COPM, GAS, PEDI was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote “....by a physical therapist blinded to group allocation (verified following testing)” (p. 3) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One participant dropped out after randomization (unaware of group allocation), and another was excluded after the intervention for inability to comply with testing procedures" (p. 5) Comment: Missing data is low, balanced across intervention groups and unlikely to affect outcomes. Analysis was by intention to treat. The method for handling missing data was not specified. |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial registered on Clinical Trials.gov (NCT00305006). Does not specify outcome measures. Insufficient information to permit judgement |
Hoare 2013.
| Methods |
Design: single‐centre, assessor‐blinded, prospective, randomised controlled, trial Comparison groups reported by study authors: mCIMT vs bimanual occupational therapy (both groups also had botulinum toxin‐A injections) Country: Australia Other: trial registered at Australian Clinical Trials Register (ACTRN12605000002684) Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs high dose |
|
| Participants |
Inclusion criteria (a) Diagnosis of congenital spastic unilateral CP (b) Aged 18 months to 6 years (c) Active movement of the affected upper limb such that the child was able to reach forward to an elevated position and able to grasp a cube from a table top and release it in a large container (d) Able to attend to tasks and follow simple one stage commands (e) Moderate levels of muscle tone (i.e. 1‐2 on Modified Ashworth Scale) (f) Moderate levels of spasticity (i.e. 1‐2 on Modified Tardieu Scale) (g) No fixed contracture in target group of muscles to be injected with BoNT‐A (h) Appropriate for upper limb BoNT‐A as assessed by a rehabilitation physician Exclusion criteria (a) BoNT‐A injections in the upper limb in the past 12 months (b) Prior upper‐limb surgery Participants: 35 children with congenital unilateral CP were randomised Randomisation method: following consent, children were block‐randomised into pairs matched by age (± 6 months) using a computer‐generated set of random numbers, creating an allocation sequence that was contained in individual opaque envelopes for use by the chief investigator. As children were recruited, the next envelope in the sequence was opened and the child assigned to the stated group. All randomisation, sequence generation and preparation of group allocation materials were performed by a third party who had no direct contact with the clinical aspects of the trial Dropouts: n = 1 dropped out following randomisation but prior to baseline assessment or receiving botulinum toxin injections due to family stressors unrelated to the trial Number of participants who received intended treatment: n = 34 Number of participants who were analysed: total sample: n=34; mean age = 35.80 months SD 15.75 months; 20 males, 14 females; 16 left hemiplegia, 18 right hemiplegia; sample too young for MACS; GMFCS not reported Intervention group: n = 17; mean age = 36.06 months SD 15.61 months; 11 males, 6 females; 11 left hemiplegia, 6 right hemiplegia; sample too young for MACS; GMFCS not reported Comparison group: n = 17; mean age = 35.55 months SD 16.39 months; 9 males, 8 females; 5 left hemiplegia, 12 right hemiplegia; sample too young for MACS; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT): NOTE: Both groups received botulinum toxin‐A injections, so the defining difference between the groups was mCIMT or bimanual occupational therapy Treatment dosage Length: 8 weeks Duration: 3 hours Frequency: daily Total dose of therapy time: planned: with therapist =16 hours, including home programme = 168 hours; actual = 98.54 hours (95% CI = 81.98 to 115.1) Description Type of restraint device: neoprene mitt Hours per day restraint worn: 3 hours Treatment environment: 2 x 45 to 60 minute sessions per week in a clinic with an occupational therapist plus home programme carried out by family aiming to achieve 3 hours per day (minimum of 30 minutes per occasion and including time spent at clinic) Individual or group: individual Therapy provider: principal investigator (occupational therapist) and family Models of practice: based on motor learning theory, learning was facilitated by practicing skills and opportunity for massed practice. "Unimanual tasks were selected to facilitate repetitive practice of movements and skills of the impaired limb (e.g. grasp, release, holding and transporting)" (p.3) Home programme: same as clinic‐based intervention. Mitt worn for minimum 30 minute sessions and unimanual "tasks were selected to facilitate repetitive practice of movements and skills of the affected limb (e.g. grasp, release, holding and transporting)" (p.3) Comparison group (high dose) NOTE: Both groups received botulinum toxin‐A injections, so the defining difference between the groups was mCIMT or bimanual occupational therapy Treatment dosage Length: 8 weeks Duration: 2 x 45 to 60 minute sessions per week with an occupational therapist. A home programme was encouraged but no time requirements were provided. Frequency: 2 x 45 to 60 minute sessions per week with an occupational therapist. Home programme encouraged but no time requirements were provided Total dose of therapy time: planned: minimum 12 to 16 hours, actual therapy time = 31.63 hours (95% CI = 15.39 to 47.86) which was significantly lower than mCIMT group P < 0.001) Description Treatment environment: 2 x 45‐60 minute sessions per week in a clinic with an occupational therapist and home programme carried out by family Individual or group: individual Therapy provider: principal investigator (occupational therapist) and family Models of practice: therapy: "targeted the development of specific hand skills and motor planning abilities using repetitive practice of bimanual activities. Knowledge of the Assisting Hand Assessment item difficulty hierarchy" for bimanual skills "served as a guide for selecting specific activities, but children were not trained to complete the assessment tasks. Treatment incorporated components of motor learning and cognitive‐based motor intervention" (p.3) Home programme: families were "encouraged to undertake a home programme, but no time requirements were specified. This was based on current clinical practice and designed to reflect differences in treatment intensity between [bimanual occupational therapy and mCIMT] protocols (p.3) |
|
| Outcomes |
Assessment time points: baseline – following randomisation and pre‐botulinum toxin‐A injections; 1 month following botulinum toxin‐A injections (prior to starting intervention); 3 months (immediately after intervention); 6 months post‐botulinum toxin‐A injections (2 weeks to 4 months postintervention) Primary outcome measure
Secondary outcome measures
|
|
| Notes | All children received botulinum toxin‐A injections to the affected upper limb: Botox®, under general anaesthetic, maximum dose of 15U/kg (up to 400U), dilution 100U/1mL Additional information sought from authors: authors provided mean change and the standard deviation of mean change data for AHA, QUEST, PEDI, COPM, PROM, MTS Fundings sources: La Trobe University, Southern Health, and Allergan Australia Pty Ltd. Study author declaration: Allergan Australia provided partial support by providing the BoNT‐A (Botox) used in the study, by payment of research assistants for blinded administration and scoring of assessments, and video‐editing services. The authors have no pecuniary interest in Allergan. BH is an occupational therapist and has received sponsorship from Allergan Australia to attend and teach at conferences and meetings but has no personal financial interest in Botox or any related product. CI is co‐investigator of an RCT investigating the effect of repeat injections of BoNT‐A and occupational therapy in the upper limbs of children with unilateral CP that has received support from Allergan Australia. In 2008, CI received a grant from Allergan Australia to present results of this trial at the American Academy of Cerebral Palsy and Developmental Medicine in Atlanta, but has no personal financial interest in Botox or any related product. HBR has received sponsorship from Allergan Australia to attend and teach at conferences and meetings but has no personal financial interest in Botox or any related product. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following consent and prior to baseline assessment, children were randomized into pairs matched by age (SD 6mo)....The set of random numbers was used to create an allocation sequence that was contained in individual opaque envelopes for use by the chief investigator. As children were recruited, the next envelope in the sequence was opened and the child assigned to the stated group" |
| Allocation concealment (selection bias) | Low risk | Quote: "All randomisation, sequence generation, and preparation of group allocation materials were performed by a third party who had no direct contact with the clinical aspects of the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including COPM, GAS, PEDI, PMAL was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: Outcomes were administered "by a senior occupational therapist blinded to group assignment”. The primary outcome AHA and the QUEST were “scored by assessors blinded to group allocation and order of assessment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: full data sets were obtained except for 1 participant from the bimanual occupational therapy group who dropped out (due to family stressors unrelated to group allocation) following randomisation but prior to baseline assessment. This missing data is unlikely to be related to true outcome. Analysis was by intention to treat |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available. All outcomes were used and reported |
Hosseini 2010.
| Methods |
Design: single‐blind, randomised controlled trial Comparison groups reported by study authors: CIMT vs conventional therapy Country: Iran Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Ability to extend wrist joint more than 20° and fingers in metacarpophalangeal joints at least 10° from full flexion (b) More than 50% difference between involved and non‐involved hands in Jebson Taylor Test of Hand Function (c) Ability to raise involved hand from surface of table more than 15 centimetres (d) Score of at least 70 on Color Raven Test of IQ (e) Willingness to participate in the research Exclusion criteria (a) Health difficulties not related to CP (b) Treatment‐resistant seizures (c) Visual problems that would interfere with carrying out the test (d) Modified Ashworth Score average score greater than 3.5 in upper limbs (e) Orthopaedic surgery on involved hand (f) Rhizotomy in the last year (g) Botulinum toxin treatment in muscles of upper limbs in the last six months or during the study (h) Use of intrathecal baclofen in the six months before intervention or during the study (i) Balance problems while wearing splint Participants: 28 children with unilateral CP were recruited and allocated equally to groups Randomisation method: “Participants have been selected based on stratified random sampling method. In this method, after providing sampling framework, persons based on inclusion and exclusion criteria have been classified in 4 levels, then samples has been selected randomly in two groups.” (p.51). No additional information was reported Dropouts: intervention n = 2, comparison n = 1 (reasons were beginning of school season and length of sessions every day; reasons per group were not given) Number of participants who received intended treatment: intervention n = 12, comparison n = 13 Number of participants who were analysed: total sample: n = 25; mean age = 7 years 5 months SD 5 years 4 months, range = not reported; 13 males, 12 females; 15 left hemiplegia, 10 right hemiplegia; MACS: not reported; GMFCS: not reported Intervention group: n = 12; mean age = 7 years 10 months SD 7 years 6 months; 6 males, 6 females; 8 left hemiplegia, 4 right hemiplegia; MACS: not reported; GMFCS: not reported Comparison group: n = 13; mean age = 7 years 10 months SD 1 years 5 months; 7 males, 6 females; 7 left hemiplegia, 6 right hemiplegia; MACS: not reported; GMFCS: not reported |
|
| Interventions |
Intervention Group (mCIMT) Treatment dosage Length: 10 days Duration: 6 hours per day Frequency: 10 days Total dose of therapy time: face‐to‐face time with therapist = 60 hours Description Type of restraint device: splint Hours per day restraint worn: not reported Treatment environment: not reported Individual or group: not reported Therapy providers: not reported Models of practice: not reported Home programme: not reported Comparison Group (low dose) Treatment dosage Length: unclear Duration: unclear Frequency: unclear Total dose of therapy time: unclear Description Treatment environment: not reported Individual or group: not reported Therapy provider: not reported Models of practice: NDT Home programme: not reported |
|
| Outcomes |
Assessment time points: baseline; 2 weeks (Immediately postintervention) Primary outcome measures
No information on scoring/measurement units or direction and magnitude of scales were provided Hand‐grip strength using handheld goniometer Passive range of motion – muscle groups not specified Modified Ashworth Scale – muscle groups not specified Two‐point discrimination Bruininks‐Oseretsky Test of Motor Proficiency – subscales used were: Manual Dexterity with non‐involved and involved hands separately, Bilateral Coordination, Upper‐Limb Coordination. Reason for exclusion: No established reliability or validity in CP Jebsen Taylor Hand Function Test. Reason for exclusion: No established reliability or validity in CP Active range of motion – muscle groups not specified. Reason for exclusion: No established reliability or validity in CP Caregiver Functional Use Survey. Reason for exclusion: No established reliability or validity in CP Unimanual function composite (for involved and uninvolved hands separately) – composite scores from Manual Dexterity and Jebsen Taylor Hand Function Test. Reason for exclusion: No established reliability or validity in CP Bimanual Function composite – composite score from Bilateral Coordination, Upper‐Limb Coordination and Caregiver. Reason for exclusion: No established reliability or validity in CPFunctional Use Survey. Reason for exclusion: No established reliability or validity in CP |
|
| Notes |
Additional information sought from authors: letter emailed to corresponding author at: soortigi.ot@gmail.com on 22/7/2016 and reminder on 21/8/2016. No response from authors. No data available for inclusion in the review Fundings sources: Pediatric Neurorehabilitation Center of University of Social Welfare and Rehabilitation Sciences Study author declaration: no declaration given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants have been selected based on stratified random sampling method. In this method, after providing sampling framework, persons based on inclusion and exclusion criteria have been classified in 4 levels, then samples has been selected randomly in two groups”. Page 51 Comment:the authors do not report the nature of the strata nor any further details of the methods used to generate the allocation sequence. Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Participants have been selected based on stratified random sampling method. In this method, after providing sampling framework, persons based on inclusion and exclusion criteria have been classified in 4 levels, then samples has been selected randomly in two groups.” Page 51 Quote: “Finally, randomly the participants were placed in constraint induced movement therapy and conventional therapy groups”. Comment: not described. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “This research has been performed with single blinded, randomized, control trial” page 51 Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including CFUS was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Unclear risk | Quote: “This research has been performed with single blinded, randomized, control trial…” page 51 Comment: not described. Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “…due to beginning school season and being too long session in every day, 3 children failed (2 children in CIMT and 1 in conventional group).” Page 51 Comment: three of 28 children dropped out of intervention and therefore were not included in the analysis (89% completed). Reason for missing outcome data are unlikely to be related to true outcome and numbers were balanced across groups Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | High risk | Comment: no study protocol located. Results for some of the measures specified in the paper were not reported |
Kirton 2016a (CIMT + r TMS).
| Methods |
Design: single‐centre, assessor‐blinded, factorial design, randomised controlled clinical trial Comparison groups reported by study authors: CIMT plus rTMS vs intensive motor learning therapy plus rTMS vs CIMT plus sham rTMSvs intensive motor therapy plus sham rTMS Country: Canada Other: Trial registered at Clinicaltrials.gov (NCT01189058) Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis
|
|
| Participants |
Inclusion criteria (a) Symptomatic hemiparesis (including perceived functional limitations by child and parent) (b) MRI‐confirmed unilateral perinatal ischaemic stroke (c) Age 6 to 19 years (d) Term birth (> 35 weeks) (e) Written informed consent/assent Exclusion criteria (a) Additional neurologic abnormality (b) Multifocal stroke (c) Severe hemiparesis (Manual Ability Classification System V or <20°finger/wrist extension) or predominant dystonia (d) Developmental delay precluding compliance (e) Unstable epilepsy (f) TMS contraindication (g) CIMT within 6 months, upper‐limb surgery, or botulinum toxin within 12 months. Presence of stroke‐side motor evoked potentials was not required Participants: 45 children with perinatal stroke and hemiparesis Randomisation method: "The statistician used random size blocks for group balance while accommodating camp sizes of 4 to 8 participants. Randomization was not stratified because of the sample size, unknown prognostic factors, and grouping by age to optimize psychosocial benefits" (p.2) Dropouts: all participants completed all interventions and outcomes. Two participants randomized to rTMS crossed over and were reassigned to sham because of high resting motor thresholds (> 90%) precluding rTMS Number of participants who received intended treatment: n = 11 CIMT (plus rTMS), n = 10 intensive motor learning therapy (plus rTMS), n = 11 CIMT (plus sham), n = 12 intensive motor learning therapy (plus sham) Number of participants who were analysed: total sample: n = 45; mean age = 11.58 years SD 3.84 years, range = 6.23 to 19.79 years; 28 males, 17 females; 25 left hemiplegia, 20 right hemiplegia; MACS I n = 17, MACS II n = 28, GMFCS not reported Intervention group 1 (CIMT plus rTMS): n = 12; mean age = 13 years 3 months SD 3 years 8 months; 10 males, 2 females; 8 left hemiplegia, 4 right hemiplegia; MACS I n = 6, MACS II n = 6; GMFCS not reported Comparison group 1: (Intensive motor learning therapy plus rTMS) n = 10; mean age = 12 years 2 months SD 4 years 2 months; 5 males, 5 females; 4 left hemiplegia, 6 right hemiplegia; MACS I n = 2, MACS II n = 8, GMFCS not reported Intervention group 2 (CIMT plus sham): n = 11; mean age = 10 years 7 months SD 3 years 8 months; 6 males, 5 females; 8 left hemiplegia, 3 right hemiplegia; MACS I n = 5, MACS II n = 6; GMFCS not reported Comparison group 2: (Intensive motor learning therapy plus sham) n = 12; mean age = 10 years 4 months SD 3 years 6 months; 7 males, 5 females; 5 left hemiplegia, 7 right hemiplegia; MACS I n = 4, MACS II n = 8; GMFCS not reported |
|
| Interventions |
Intervention group (sCIMT) Treatment dosage Length: 2 weeks Duration: 8 hours per day Frequency: 10 consecutive weekdays Total dose of therapy time: 80 hours plus home programme during the 2‐week intervention period (1 hour per day = 10 hours) plus maintenance home programme (15 minutes/day for 22 weeks = 27.5 hours) Description Type of restraint device: custom‐fit, bivalved, removable cast from below the elbow to the distal interphalangeal joint Hours per day restraint worn: 90% waking hours x 12 days (Monday to ‐Friday + middle weekend). Target was 100% of the daily camp (8 hours per day). Treatment environment: clinic (goal‐directed, peer‐supported motor learning camp) and home Individual or group: individual (2 hours per day) and group (1 staff to 3 children ratio; 5.5 hours per day) Therapy provider: occupational therapists, child life therapists, volunteers and allied health professionals Models of practice: principles included: i) unimanual tasks which best elicit the target movements,ii) repetitive practice, iii) shaping (incremental increases in task difficulty) and iv) positive feedback. Individual sessions: "Target movements were selected based on the child’s goals and current functioning of the affected hand/arm. Unimanual therapeutic activities that incorporated the target movement and considered the subject’s interests were chosen for the 1:1 therapy. All tasks were presented in a manner that ensured initial partial success and kept the subject engaged and motivated in therapy activities. Tasks were altered after the subject was able to achieve approximately 80% success of the trial. Tasks were gradually increased in difficulty by adjusting one variable within the task (temporal, spatial, accuracy or resistance). Each activity was presented multiple times in a row for a minimum of ten minutes per session. Modeling (if needed), cueing, and positive feedback was provided with each activity trial." (TiDieR guidelines in supplementary information, p.3) Group sessions: Provided "by a multidisciplinary team of allied health care professionals (>7 years experience working with children with CP) including child life therapists, occupational therapists and occupational therapy assistants. Activities were developed with diverse contributors from the ACH Therapeutic Arts programme including art, music, and horticultural therapists (not included in provider ratio). OTs supporting group programming ensured consistency with the principles above and adaptations of activities to suit individual subject abilities and goals. Volunteers also assisted completion of group activities. When possible, these included past program participants and youth with perinatal stroke and hemiparesis. Subject/provider was typically 1:1, but at times less if the subjects had relatively good functioning of the affected limb and no behavioural concerns" (TiDieR guidelines in supplementary information, p.4) Home programme: "During the 2‐week intervention, participants were prescribed 60 min/evening of goal‐directed upper‐limb activities. Following completion of the 2 week programme, participants received a structured bimanual home programme (15 min/d) based on evolution of their goals with a “transfer package” to promote integration into daily activities. Therapists met with families at 2 and 4 months and were available by phone to adjust therapy as needed" (p.2) Comparison group (dose‐matched) Treatment dosage Length: 2 weeks Duration: 8 hours per day Frequency: 10 consecutive weekdays Total dose of therapy time: 80 hours plus home programme during the 2‐week intervention period (1 hour per day = 10 hours) plus maintenance home programme (15 minutes/day for 22 weeks = 27.5 hours) Description Treatment environment: clinic (goal‐directed, peer‐supported motor learning camp) and home Individual or group: individual (2 hours per day) and group (1 staff to 3 children ratio; 5.5 hours per day) Therapy provider: occupational therapists and allied health professionals Models of practice: "Interventions were individualized to the specific goals of each child. Tasks were graded and selected according to relative function with increasing complexity and geared to age‐appropriate activities of daily living. Assistive technologies including virtual reality and video games were used. Group activities incorporating upper‐limb training were delivered by occupational therapists and allied health professionals. Activities were sports, horticultural and music therapy, creative gaming (e.g., “Rock Band”), and therapeutic arts. During breaks (0.5 hours per day), an upper‐limb activity of the child’s choice was encouraged, and activities of daily living were focused on during lunch/snack times (2 hours per day)" (p.2) Home programme: "During the 2‐week intervention, participants were prescribed 60 min/evening of goal‐directed upper‐limb activities. Following completion of the 2 week programme, participants received a structured bimanual home programme (15 min/d) based on evolution of their goals with a “transfer package” to promote integration into daily activities. Therapists met with families at 2 and 4 months and were available by phone to adjust therapy as needed" (p.2) |
|
| Outcomes |
Assessment time points: baseline; 1 week (immediately following intervention); 2 month (2 weeks to 4 months postintervention); 6 months (5 to 6 months postintervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: authors provided mean change and the standard deviation of mean change data for: AHA, PedsQOL, Abilhand‐Kids, grip strength, COPM and the Melbourne Assessment Question 1: Describe how you assured allocation concealment during randomisation Reply 1: Statistician maintained running database of all consented subjects by study number and performed each randomisation once the block size was determined (for the next camp) Question 2: We note from the trial register that the Shriners Hospital Upper Extremity Evaluation (SHUEE), pinch strength and rPMAL, TMAL were planned as outcome measures but these have not been reported in your publication. Can you confirm if these were used? Reply 2: These outcomes were originally intended but multiple issues were encountered during the trial that prevented accurate data. The SHUEE and PMAL were administered incorrectly on early subjects, the pinch meter was found to have calibration errors, etc all of which prevented comparable data across the entire population at trial completion Additional information: Box and blocks was erroneously omitted from the measures protocol in the 1st 2 camps and therefore not collected. Grip strength was collected as a safety outcome and is reported Fundings sources: Heart and Stroke Foundation of Canada, Alberta. Children’s Hospital Foundation. The funder had no role in study design, data collection, analysis or interpretation, or writing of the report. The corresponding author had full access to the data and final responsibility for the decision to submit. Study author declaration: the authors report no disclosures relevant to the manuscript |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“Participants were randomized as a group before each camp (1:1) to rTMS/sham and 1:1 to CIMT/none” (p. 2) |
| Allocation concealment (selection bias) | Low risk | Quote from email: “Statistician maintained running database of all consented subjects by study number and performed each randomization once the block size was determined (for the next camp)” Comment: Additional information obtained from authors confirm allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including ABILHAND‐Kids, PedsQL and COPM was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “Structured application was performed by the same certified occupational therapist blinded to patient characteristics and treatment allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All participants completed all interventions and outcomes. Two participants randomized to rTMS crossed over and were reassigned to sham because of high resting motor thresholds (>90%) precluding rTMS. None of the following intention‐to‐treat analysis findings or conclusions were altered by the secondary per‐protocol analysis" |
| Selective reporting (reporting bias) | Low risk | Comment: Data from measures listed as secondary outcomes in the publication were not reported. These included grip strength and the box and blocks test. Trial registered at Clinicaltrials.gov/NCT01189058 listed the Shriners Hospital Upper Extremity Evaluation (SHUEE), pinch strength, rPMAL, Tween Motor Activity Log (tMAL) listed in protocol but not reported or addressed in the publication Response from authors: “These outcomes were originally intended but multiple issues were encountered during the trial that prevented accurate data – the SHUEE and PMAL were administered incorrectly on early subjects, the pinch meter was found to have calibration errors, etc all of which prevented comparable data across the entire population at trial completion” |
Kirton 2016b (CIMT + sham TMS).
| Methods | To allow analysis of data from these two comparisons we set up two study IDs for this study. Kirton 2016a (CIMT + r TMS) examines the comparison of CIMT(+rTMS) versus dose‐matched motor learning (+rTMS) while Kirton 2016b (CIMT + sham TMS) examines the comparison CIMT(+sham) versus dose‐matched motor learning (+sham). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Rostami 2012a.
| Methods |
Design: single‐blind, randomised controlled trial Comparison groups reported by study authors: home‐based mCIMT vs clinic‐based mCIMT Country: Iran Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs different of form CIMT |
|
| Participants |
Inclusion criteria (a) Spastic unilateral CP (b) At least 20° wrist and 10° fingers extension from full flexion (c) Greater movement deficits of one upper limb (score less than 2.5 on the amount of use scale of the Pediatrics Motor Activity Log) (d) Muscle tone less than 3 on Modified Ashworth Scale (e) Comprehend and execute simple verbal commands Exclusion criteria (a) Health problems not associated with CP (b) Seizures (c) Untreated visual problems that would interfere with performing intervention or testing (d) Orthopaedic surgery on the more‐involved upper limb (e) Botulinum toxin therapy in the upper limb during the past 6 months or within the period of study (g) Balance problems while wearing the splint Participants: 14 children with spastic unilateral CP were randomised Randomisation method: "Performed with SPSS software, so that numbers 1 to 14 according to different children were enrolled to the software; and by random sampling subtest, seven children elected and assigned to the home group and others to the clinic group" (P.2) Dropouts: not reported Number of participants who received intended treatment: intervention n=7, comparison n=7 Number of participants who were analysed: total sample: n = 14; mean age = 4 years 1 month SD 6 years 2 months, range = 4 years 1 month to 8 years 4 months; 9 males, 5 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported Intervention group: n = 7; mean age = 6 years 3 months SD not reported; gender not reported; side of hemiplegia not reported; MACS not reported; GMFCS not reported Comparison group: n = 7; mean age = 6 years 3 months SD not reported; gender not reported; side of hemiplegia not reported; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention Group (mCIMT) Treatment dosage Length: 2 weeks Duration: 1.5 hours Frequency: 5 x per week for 10 sessions Total dose of therapy time: 15 hours. Face‐to‐face time with therapist: 15 hours Description Type of restraint device: thermoplastic splint Hours per day restraint worn: "Most of waking hours and removed it just for bathing, sleeping and short resting periods during the day" Treatment environment: home Individual or group: individual Therapy provider: occupational therapist Models of practice: daily activities such as reaching, grasping and manipulating objects or toys, fine motor skills, dressing and undressing, eating, grooming, according to the children’s age and capabilities. Used child’s own toys in familiar daily routines Home programme: 1 hour per day Comparison Group (Different form of mCIMT) Treatment dosage Length: 2 weeks Duration: 1.5 hours Frequency: 5 times per week for 10 sessions Total dose of therapy time: 15 hours. Face‐to‐face time with therapist: 15 hours Description Type of restraint device: thermoplastic splint Hours per day restraint worn: "Most of waking hours and removed it just for bathing, sleeping and short resting periods during the day" Treatment environment: clinic Individual or group: individual Therapy provider: occupational therapist Models of practice: daily activities such as reaching, grasping and manipulating objects or toys, fine motor skills, dressing and undressing, eating, grooming, according to the children’s age and capabilities. Used toys and other tools from the clinic Home programme: 1 hour per day |
|
| Outcomes |
Assessment time points: baseline 1 (8 days before intervention); baseline 2 (day before start of treatment); 2 weeks (Immediately following intervention); 3 months (2 weeks to 4 months postintervention) Primary outcome measures
Outcome measures
|
|
| Notes |
Additional information sought from authors: none sought as all measures had no evidence of validity or reliability in CP Fundings sources: Ahvaz Jundishapur University of Medical Sciences (grant no. U‐89071). Study author declaration: none of the authors have any financial or other interests relating to this manuscript |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed with SPSS software, so that numbers 1 to 14 according to different children were enrolled to the software; and by random sampling subtest, seven children elected and assigned to the home group and others to the clinic group” |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including PMAL was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "Bruininks–Oseretsky Test scores were measured by an evaluator blinded to intervention groups” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: study did not specify whether there were dropouts. Rate of attrition and relationship to outcomes is unable to be assessed. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Rostami 2012b.
| Methods |
Design: randomised controlled trial Comparison groups reported by study authors: CIMT plus virtual reality vs virtual reality vs CIMT vs usual care Country: Iran Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors (not used in meta‐analysis as no data available)
|
|
| Participants |
Inclusion criteria (a) Spastic unilateral CP (b) At least 20° of wrist extension and 10° active finger extension from full flexion (c) More movement deficits in one upper limb (less than 2.5 on the Amount of Use scale on the PMAL) (d) Muscle tone less than 3 on the Modified Ashworth Scale (e) Age range between 6 to 12 years (f) Normal or corrected to normal vision and hearing Exclusion criteria (a) Health problems not associated with CP (b) Seizures (c) Hemispatial neglect (d) Orthopaedic surgery on the involved upper limb (e) Botulinum toxin therapy for the affected upper limb within past 6 months (f) Balance problems Participants: 32 children with spastic unilateral CP were randomised Randomisation method: once baseline evaluations were completed, children were matched based on age and randomly assigned to one of 4 study groups using a computer generated random number list. Randomisation process was performed by one of the researchers blinded to the intervention types Dropouts: n = 0 Number of participants who received intended treatment: CIMT n = 8, VR plus CIMT n = 8, VR n = 8, control n = 8 Number of participants who were analysed: total sample: n = 32; mean age = 8 years 1 month SD not reported, range = 6 years 2 months to 11 years 8 months; 14 males; 18 females; 18 left hemiplegia; 14 right hemiplegia; MACS not reported; GMFCS not reported CIMT group: n = 8; mean age = 8 years 4 months SD not reported; 4 males; 4 females; 6 left hemiplegia, 2 right hemiplegia; MACS not reported; GMFCS not reported CIMT plus VR group: n = 8; mean age = 8 years 2 months SD not reported; 4 males, 4 females; 5 left hemiplegia, 3 right hemiplegia; MACS not reported; GMFCS not reported VR group: n = 8; mean age = 7 years 8 months SD not reported; 3 males, 5 females; 3 left hemiplegia, 5 right hemiplegia; MACS not reported; GMFCS not reported Low‐dose comparison group: n = 8; mean age = 8 years 0 months SD not reported; 3 males, 5 females; 4 left hemiplegia, 4 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT plus VR) Treatment dosage Length: 4 weeks Duration: 1.5 hours Frequency: 3 days per week Total dose of therapy time: 22 hours (18 hours virtual therapy + continued routine therapy (2 x 0.5 ‐hour sessions per week = 4 hours total)) Description Type of restraint device: Volar resting splint extending from fingertips to the proximal forearm Hours per day restraint worn: 5 hours Treatment environment: clinic Individual or group: individual Therapy provider: therapist – no further detail provided Models of practice: children selected their favourite games, while therapist choose the appropriate handles and suitable aspects of games including required range of motion, strength, speed, accuracy, and difficulty, according to the children’s abilities Home programme: not reported Intervention Group (mCIMT) Treatment dosage Length: 4 weeks Duration: 1.5 hours Frequency: 3 days per week Total dose of therapy time: 22 hours (18 hours CIMT + continued routine therapy (2 x 0.5 hour sessions per week = 4 hours total)) Description Type of restraint device: Volar resting splint extending from fingertips to the proximal forearm Hours per day restraint worn: 5 hours Treatment environment: clinic Individual or group: individual Therapy provider: therapist – no further detail provided Models of practice: intervention included daily activities such as reaching, grasping, manipulating objects or toys, dressing and undressing, eating, and grooming, according to the child’s age and abilities. Frequent and immediate visual and auditory feedback about the success of the action was presented to children by the system to encourage both participation and attention and to increase the child’s knowledge of their performance either during practice or at the end of practice Home programme: not reported Comparison group (VR Group) Treatment dosage Length: 4 weeks Duration: 1.5 hours Frequency: 3 times per week Total dose of therapy time: 22 hours (18 hours VR + continued routine therapy (2 x 0.5 hour sessions per week = 4 hours total)) Description Treatment environment: clinic Individual or group: iIndividual Therapy provider: therapist – no further detail provided Models of practice: children selected their favourite games, while therapist choose the appropriate handles and suitable aspects of games including required range of motion, strength, speed, accuracy, and difficulty, according to the children’s abilities Home programme: not reported Comparison group (low dose) Treatment dosage Length: 4 weeks Duration: 0.5 hours Frequency: 2 times per week Total dose of therapy time: 4 hours Description Treatment environment: not reported Individual or group:individual Therapy provider: therapist Models of practice: neurodevelopmental facilitation techniques, range of motion exercises, and stretching Home programme: not reported |
|
| Outcomes |
Assessment time points: baseline 1 week prior to intervention; baseline 1 day prior to intervention; postintervention (immediately following intervention); 3 months (2 weeks to 4 months postintervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Fundings sources: Ahvaz Jundishapur University of Medical Sciences (grant no. U‐89071) Study author declaration: no declaration given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Once baseline evaluations were completed, children were matched based on age and randomly assigned to one of 4 study groups (VR, modified CIMT, combined VR and modified CIMT, or control) using a computer generated random number list” |
| Allocation concealment (selection bias) | High risk | Quote: “Randomisation process was performed by one of the researchers blinded to the intervention types” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: binding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: binding for self‐reported outcomes including PMAL was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “An assessor blinded to group assignment administered the BOTMP” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the CONSORT diagram indicated that all children received intervention and completed follow‐up assessment. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Sabour 2012.
| Methods |
Design: multi‐centre randomised controlled trial Comparison groups reported by study authors: CIMT plus bimanual training (Hybrid mCIMT) plus OT vs OT alone Country: Iran Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Aged 5 to 10 years (b) Unilateral CP (c) Ability to extend wrist 20° (d) Ability to extend fingers 10° (e) > 75 IQ score according to Raven Exclusion criteria (a) Botulinum toxin therapy in the upper limb within the past 6 months (b) History of orthopaedic surgery in the affected arm (c) Hearing and visual disabilities (d) Balance and protective reaction impairment Participants: 25 children with unilateral CP Randomisation method: “... 25 chosen children were randomly divided into intervention and control groups according to the random table” Dropouts: none Number of participants who received intended treatment: intervention n = 12, comparison n = 13 Number of participants who were analysed: total sample: n = 25; mean age = not reported; 11 males, 14 females; 15 left hemiplegia, 10 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n = 12; mean age = 93.6 months SD 14.2 months; 4 males, 8 females; 8 left hemiplegia, 4 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n = 13; mean age = 85.4 months SD 17.2 months; 7 males, 6 females; 7 left hemiplegia, 6 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention Group (Hybrid CIMT) Treatment dosage Length: 2 weeks (weekdays) Duration: 6 hours per day (hybrid) 45 minutes, 3 times per week (OT) Frequency: daily (weekdays) (hybrid); 3 times per week (OT) Total dose of therapy time: 60 + 4.5 hours = 64.5 hours. Face‐to‐face time with therapist = 64.5 hours Description Type of restraint device: sling Hours per day restraint worn: 3 hours Treatment environment: clinic Individual or group: group (n = 4) Therapy provider: occupational therapist Models of practice: “Children in intervention group received regular occupational therapy (minutes, 3 times per week), and a combination of CIMT and bimanual training as follow. At first, sound upper limb was restrained (by sling) for three hours and the children practiced structured activities based on movement learning principles with affected upper limb. Then the sling was removed and the children practiced bimanual activities for three more hours. This process was continued for 10 days in two consecutive weeks” Home programme: none Comparison Group (low dose) Treatment dosage Length: 2 weeks Duration: 45 minutes Frequency: 3 times per week Total dose of therapy time: 4.5 hours Description Treatment environment: clinic Individual or group: individual Therapy provider: occupational therapist Models of practice: not described Home programme: not described |
|
| Outcomes |
Assessment time points: baseline; 2 weeks (Immediately postintervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Note: Published in Persian ‐ data extraction and risk of bias were kindly completed by Associate Professor Mehdi Rassafiani, Department of Occupational Therapy, University of Social Welfare and Rehabilitation Sciences, Tehrān, Iran and Dr Fakher Rahim, Ahvaz Jondishapour University of Medical Sciences, Ahvaz, Iran Additional information sought from authors: data for the Modified Ashworth Scale (shoulder, elbow and wrist flexors) was requested from authors. Data files were reported by authors to be lost therefore not available for inclusion in the review Fundings sources: translation not available Study author declaration: translation not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Children were randomly divided into experimental and control groups according to the random table” |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | High risk | Comment: outcome assessors were not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no drop outs in this trial. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Sakzewski 2011.
| Methods |
Design: multi‐centre, assessor‐blinded, matched pairs, randomised comparison trial Comparison groups reported by study authors: mCIMT vs bimanual training – both interventions were delivered in an intensive circus‐themed day camp Country: Australia Other: trial registered at Australian Clinical Trials Register (ACTRN12609000912280) Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) Congenital hemiplegia (b) Aged 5 to 16 years (c) Ability to follow instructions (determined during screening assessment and in consultation with caregivers) (d) Predominant spasticity with Modified Ashworth Scale (MAS) grades of between 1 and ≤3 for wrist flexors, forearm pronators and/or thumb adductors interfering with upper‐limb function Exclusion criteria (a) Predominant dystonia and/or muscle contracture (MAS >3) (b) Previous upper‐limb orthopaedic surgery (c) Serial casting or botulinum toxin‐A injections in the upper limb in the 6 months before commencement of intervention Participants: 63 children with congenital spastic hemiplegia were randomised Randomisation method: children were matched (for age, sex, side of hemiplegia and upper‐limb function according to Melbourne Assessment) and the pairs randomly assigned to groups using a computer list of random numbers and concealed envelope opened by non‐study personnel Dropouts:Intervention: all completed intervention; 3 weeks: no dropouts; 26 weeks: 3/32 (n = 1 unable to be contacted, n = 2 failed to attend assessment); 52 weeks: 2/32 (n = 2 failed to attend) Comparison: 30/32 completed intervention (n = 1 injured prior to baseline, n = 1 refused to return); 3 weeks: 2/32 (same participants as those who did not complete intervention); 26 weeks: 3/32 (n = 1 broke arm, n = 2 same participants as those who did not complete intervention); 52 weeks: 5/32 (n = 1 upper‐limb surgery, n = 2 unable to be contacted, n = 2 same participants as those who did not complete intervention) Number of participants who received intended treatment: Intervention:all completed intervention, comparison n = 30/32 Number of participants who were analysed: data were available for children who were randomised and participated in baseline assessment. Total sample: n = 63; mean age = 10 years 2 months SD 2.7 years, range 5 to 16years: 33 males, 30 females; 27 left hemiplegia, 36 right hemiplegia; MACS I n = 16, MACS II n = 46, MACS III n = 1; GMFCS I n = 16, GMFCS II n = 47 Intervention group: n = 32; mean age = 10 years 1 month (95% CI = 9.1 to 11.0 years); 17 males, 15 females; 16 left hemiplegia, 16 right hemiplegia; MACS I n = 8, MACS II n = 23, MACS III n = 1; GMFCS I n = 8, GMFCS II n = 24 Comparison group: n = 31; mean age = 10 years 2 months (95% CI = 9.2 to 11.1 years); 16 males, 15 females; 11 left hemiplegia, 20 right hemiplegia; MACS I n = 8, MACS II n = 23, MACS III n = 0; GMFCS I n = 8, GMFCS II n = 23 |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 10 days during 2 weeks Duration: 6 hours per day Frequency: 10 days during 2 weeks Total dose of therapy time: 60 hours Description Type of restraint device: tailor‐made glove with volar plastic insert to prevent grasp Hours per day restraint worn: 6 hours (removed for toileting, aerial circus activities, and the low ropes course because of safety). Actual hours worn 58% of children received the allocated 60 hours of intervention, no additional data given regarding mean or range of amount of actual intervention Treatment environment: circus theme in community sporting facilities Individual or group: groups of 9‐13 children with 1 therapist to 2 children Therapy provider: occupational therapists and physiotherapists with student and other volunteers Models of practice: interventions used a goal‐directed, activity‐based framework, employing principles of motor learning, including specific task practice, fostering problem solving (individual and within the group framework), and modifying task and environmental constraints to support goal attainment. One to two individual goals were addressed when the glove was removed for no longer than 15 minutes per day Home programme: nil Comparison group (dose‐matched Treatment dosage Length: 10 days during 2 weeks Duration: 6 hours per day Frequency: 10 days during 2 weeks Total dose of therapy time: 60 hours Description Treatment environment: circus theme in community sporting facilities Individual or group: groups of 9‐13 children with 1 therapist to 2 children Therapy provider: occupational therapists and physiotherapists with student and other volunteers Models of practice: interventions used a goal‐directed, activity‐based framework, employing principles of motor learning, including specific task practice, fostering problem solving (individual and within the group framework), and modifying task and environmental constraints to support goal attainment Home programme: nil |
|
| Outcomes |
Assessment time points: baseline; 3 weeks (immediately following intervention); 26 weeks (5 to 6 months postintervention);52 weeks (7 to 12 months postintervention) Primary outcome measures
Secondary outcome measures
Measures used at baseline to describe sample
|
|
| Notes |
Additional information sought from authors: data published was presented as Estimated Mean Difference (EMD). Authors contacted and provided mean change and the standard deviation of mean change data for all included outcomes Question: We understand from previous correspondence that outcomes including passive range of motion, Modified Ashworth Scale and Modified Tardieu Scale were used to assess eligibility rather than used as outcomes in this study. Can you confirm this is accurate? Reply: Yes Fundings sources: National Health and Research Council of Australian (NHMRC) funding the INCITE project (468300), NHMRC Dora Lush Post Graduate Scholarship (LS; 384488), and NHMRC Career Development Grant (RB; 473840). Study author declaration: no commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Once matched, children were randomized to pairs using a computer generated list of random numbers” |
| Allocation concealment (selection bias) | Low risk | Quote: Children were randomised using “concealed envelopes opened by non‐study personnel” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including COPM, Life‐H, CAPE, CPQOL, KIDSCREEN and SFA was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “The primary outcome measures were videotaped and scored in random order by trained occupational therapists masked to group allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the CONSORT diagram detailed flow of participants and adequacy of data. The maximum attrition was 10% at 26 weeks follow‐up, was balanced across groups and unlikely to be related to true outcome. Analysis was by intention to treat. Methods for handing missing data was not reported |
| Selective reporting (reporting bias) | High risk | Comment: Australian Clinical Trials Register (ACTRN12609000912280). Protocol available. Neurovascular changes (functional MRI, functional connectivity), and brain (re)organisation (TMS) listed in protocol but not reported or addressed in the publication |
Sakzewski 2015a.
| Methods |
Design: multi‐centre, assessor‐blinded, matched pairs, pragmatic randomised comparison trial Comparison groups reported by study authors: hybrid CIMT (mCIMT plus bimanual training) vs individualised standard care Country: Australia Other: trial registered at Australian Clinical Trials Register (ACTRN12613000181707) Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs high dose |
|
| Participants |
Inclusion criteria: (a) Unilateral C (b) Aged 5 to 16 years (c) Ability to follow instructions (d) Predominant spasticity ‐ Modified Ashworth Scale greater than 1 and no more than 3 for wrist flexors, forearm pronators, and/or thumb adductors interfering with upper‐limb function Exclusion criteria (a) Predominant dystonia (b) Muscle contracture (Modified Ashworth Scale grade >3) (c) Previous orthopaedic surgery on an upper limb Participants: 53 children with unilateral CP Randomisation method: children were matched in pairs according to age (12 months), sex, and level on the Manual Ability Classification System. They were then randomised within the matched pairs using a computer‐generated list of random numbers placed in concealed envelopes opened by non‐study personnel Dropouts: n = 9: intervention n= 3/28 (11%) (n = 1 behaviour difficulties, n = 1 family circumstances, n = 1 baseline assessment incorrectly administered), comparison n = 6/25 (24%) (n = 1 ineligible, n = 4 family circumstances, n = 1 withdrew as unwell) Number of participants who received intended treatment: intervention n = 25/28, comparison n = 19/25 Number of participants who were analysed: n = 44/53 (83%): intervention n = 25/28, comparison n = 19/25 Participant characteristics: total sample: n = 53; mean age =7 years 7 months SD 2 years 4 months; 37 males, 16 females; 26 left hemiplegia, 27 right hemiplegia; MACS I n = 26, MACS II n = 26; GMFCS I n=38, GMFCS I n=15 Intervention group: n = 28; mean age = 8.0 years SD 2.5 years; 19 males, 9 females; 13 left hemiplegia, 15 right hemiplegia; MACS I n = 12, MACS II n = 16; GMFCS I n = 19, GMFCS II n = 9 Comparison group: n = 25; mean age = 7.6 years SD 2.0 years, 18 males, 7 females; 13 left hemiplegia, 12 right hemiplegia; MACS I n = 15, MACS II n = 10; GMFCS I n = 19, GMFCS I n = 6 |
|
| Interventions |
Intervention group (Hybrid CIMT) Treatment dosage Length: 2 weeks (10 days) for Hybrid CIMT – 1 week of CIMT followed by 1 week of bimanual, all in a circus themed day camp Duration: 6 hours per day Frequency: 5 days per week for 10 days Total dose of therapy time: 60 hours Description Type of restraint device: individually tailored glove Hours per day restraint worn: 6 hours per day for 5 days in Week 1 Treatment environment: day‐camp format in a circus‐themed community facility Individual or group: groups of 10‐15 children Therapy provider: five occupational therapists and one physiotherapist, supported by volunteer therapists and therapy students with a therapist to child ratio of 1:2 Models of practice: collaborative goal setting with the child and family occurred during baseline assessment to determine therapy priorities. The child participated in 45 hours of direct targeted (activity‐based goal‐directed upper‐limb therapy using principles of motor learning, goal‐directed training, and fine and gross manipulation) and 10 hours of indirect therapy in more general gross motor activities (e.g. circus activities such as tumbling), gross unstructured upper‐limb activities (e.g. parachute and ball games), and group debriefing. In the modified CIMT week, the unimpaired arm was constrained and therapeutic activities were performed predominantly with the impaired hand. During circus aerial activities, the gloves were removed and fingers of the unimpaired hand taped to simulate the glove. The glove was only removed for toileting and aerial circus activities. During the second week, a bimanual approach focused on activities requiring coordinated use of both hands using repetitive task practice of bimanual activities. Results from the baseline Assisting Hand assessment (AHA) and understanding of the item hierarchy informed specific treatment activities and strategies Home programme: nil Comparison group (high dose) Length: 12 weeks Duration: 1.5 hours per week direct intervention for 6 weeks plus 30 minutes per day of home programme for these 6 weeks and subsequent 6 weeks Frequency: 6 days per week Total dose of therapy time: 45 hours Treatment environment: clinic and home Individual or group: individual Therapy provider: 16 hospital‐ and community‐based paediatric occupational therapists who were experienced in providing therapy to children with unilateral CP. Families completed home programme Models of practice: direct therapy sessions included 1 hour of therapy provided by a paediatric occupational therapist directly with the child, and 0.5 hours for home programme development and demonstration. Collaborative goal setting with the child and family occurred during baseline assessment to determine therapy priorities. Therapy consisted of targeted and structured, activity‐based, goal‐directed upper‐limb therapy using principles of motor learning and addressed parent/child‐identified functional goals. A manual is available from the authors Home programme: as described above |
|
| Outcomes |
Assessment time points: baseline; 13 weeks (immediately following intervention); 26 weeks (post baseline) (2 weeks to 4 months postintervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: authors provided mean change and the standard deviation of mean change data for: AHA, Melbourne Assessment, COPM, Box and Blocks. Question 1: We note from the 2013 protocol that the CPQOL and LIFE‐H were reported as outcomes used in this study however results from these outcomes were not reported in your 2015 paper. Are you able to share this data for inclusion in our review? Reply 1: There was an error in the photocopying of these assessment forms at 2 time points, therefore the data were incomplete and assessments unable to be scored. Analyses could not be completed Question 2: Request for DMQ data Reply 2: Data was obtained at baseline only Question 3: Could you clarify if the Pediatric Volitional Questionnaire was added following publication of your study protocol? Reply 3: In the studies where we used the DMQ and PVQ, data was only collected at a single time point. These measures were not included as outcome measures but rather as discriminative and descriptive measures. For the PVQ, data was obtained during the intervention period Fundings sources: LM was supported by a National Health and Medical Research Council (NHMRC) Scholarship (1039832) and a University of Queensland Research scholarship. RNB was supported by a Career Development Fellowship from the NHMRC of Australia (1037220). LS was supported by an NHMRC TRIP fellowship (1036183). This project was supported by funding from a NHMRC grant (COMBiT project grant: 1003887). Study author declaration: the authors stated they had no interests which might be perceived as posing a conflict or bias. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Children were matched in pairs according to age (12 months), sex, and level on the Manual Ability Classification System. They were then randomized within the matched pairs using a computer‐generated list of random numbers placed in concealed envelopes opened by non‐study personnel" |
| Allocation concealment (selection bias) | Low risk | Quote: Children were "randomized within the matched pairs using a computer‐generated list of random numbers placed in concealed envelopes opened by non‐study personnel" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "The primary outcome measures were videotaped and scored in random order by trained occupational therapists masked to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Six children withdrew from standard care and three from hybrid‐CIMT" Comment: this represents attrition rates of 24% and 11% respectively. Analysis was by intention to treat. Methods for handling missing data were not specified Further information obtained from authors: "Generalised linear modelling accounts for missing data i.e. will not list wise delete therefore all available data from each timepoint was included in analysis" |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol was published and the study was retrospectively registered with ANZCTR. Secondary outcomes listed in the published protocol were not reported in the publication of study results including: LIFE‐H and CP‐QOL (self‐ and parent‐report). Authors report this was due to data collection errors. The published protocol specified that postintervention assessment (13 weeks) was the primary endpoint whereas the trials registry entry, nominated both endpoints as primary endpoints and the publication of study results specified that 26 week assessment was the primary endpoint |
Sakzewski 2015b.
| Methods |
Design: randomised controlled trial Comparison groups reported by study authors: mCIMT vs bimanual therapy Country: Australia Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) Unilateral CP (b) Aged 5 to 16 years (c) Predominantly spasticity which interfered with upper‐limb function (d) Minimal ability to grasp with the impaired upper limb Exclusion criteria (a) Previous surgery to the upper limb (b) Upper‐limb intramuscular botulinum toxin‐A injections in the previous 6 months Participants: 18 children with CP were recruited Randomisation method: children were matched in pairs according to age (12 month age bands), gender and side of hemiplegia. Once matched, children were randomised within the pairs using a computer‐generated list of random numbers and concealed envelopes opened by non‐study personnel Dropouts: n = 3: intervention (n = 1 family circumstances, n = 2 failed to attend 26 weeks assessments), comparison n = 0. Last observation was carried forward for all missing data so data sets for 18 children were analysed Number of participants who received intended treatment: intervention n = 8, comparison n = 9 Number of participants who were analysed: total sample: n = 18; mean age = 8 years 6 months SD = 1 year 6 months; range not given; 9 males, 9 females; 7 left hemiplegia, 11 right hemiplegia; MACS I n = 4, MACS II n = 14, MACS III n = 0; GMFCS I n = 12, GMFCS II n = 6 Intervention group: n = 9; mean age = 8.7 years SD 1.5 years months; 5 males, 4 females; 3 left hemiplegia, 6 right hemiplegia; MACS I n = 3, MACS II n = 6; GMFCS I n = 6, GMFCS II n = 3 Comparison group: n = 9; mean age = 8.9 years SD 1.5 years; 4 males, 5 females; 4 left hemiplegia, 5 right hemiplegia; MACS I n = 1, MACS II n = 8; GMFCS I n = 6, GMFCS II n = 3 |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 5 days Duration: 6 hours per day Frequency: daily for 5 days Total dose of therapy time: face‐to‐face time with therapist = 30 hours Description Type of restraint device: customised glove with solid thermoplastic volar insert to prevent grasp. Hours per day restraint worn: 6 hours. Removed only for toileting and aerial circus activities (fingers were taped to restrict manipulation) Treatment environment: circus‐themed day camp in a community facility Individual or group: groups of 10 to 15 children Therapy provider: 5 occupational therapists, one physiotherapist, volunteer therapists and therapy students, ratio of 1 therapist for 2 children Models of practice: activity‐based goal directed upper‐limb therapy using the principles of motor learning. Collaborative goal setting with child and family determined intervention priorities. Children worked collaboratively in pairs, therapy and circus activities were completed predominantly with the impaired hand Home programme: nil mentioned Comparison group (dose ‐matched Treatment dosage: Length: 5 days Duration: 6 hours per day Frequency: daily for 5 days Total dose of therapy time: face‐to‐face time with therapist = 30 hours Description Treatment environment: circus‐themed day camp in a community facility Individual or group: groups of 10 to 15 children Therapy provider: 5 occupational therapists, one physiotherapist, volunteer therapists and therapy students, ratio of 1 therapist for 2 children Models of practice: activity‐based goal directed upper‐limb therapy using the principles of motor learning. Collaborative goal setting with child and family determined intervention priorities. activities focused on the co‐ordinated use of both hands using repetitive task practice of bimanual activities. Home programme: nil mentioned |
|
| Outcomes |
Assessment time points: baseline; immediately postintervention; 26 weeks (5 to 6 months postintervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: authors provided mean change and the standard deviation of mean change data for: AHA, Melbourne Assessment and COPM Question: Due to the design of this study (comparison of groups from two separate studies), this paper does not meet the strict inclusion criteria for our review. However, we note from this manuscript that you have completed an unpublished RCT. We would like to include data from this study and therefore would like to request further information. Do you have a manuscript for this specific RCT? Would you be willing to share data for inclusion in our review Reply: The data presented in this paper for the low dose group is the RCT you are referring to. There is not a separate paper for that study, it is embedded in this dosing paper. The methodology is exactly the same as INCITE only lower dose Fundings sources: National Health and Medical Research Council (NHMRC) project Grant (368500), NHMRC TRIP fellowship (LS 1036183), NHMRC Career Development Fellowship (RB 1037220). Study author declaration: the authors have no conflict of interests to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Children were matched in pairs according to age (12 month age bands), gender and side of hemiplegia. Once matched, children were randomized within the pairs using a computer generated list of random numbers and concealed envelopes opened by non‐study personnel” |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was using “a computer generated list of random numbers and concealed envelopes opened by non‐study personnel" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including COPM was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “The primary outcome measures were videotaped and scored in random order by trained occupational therapists masked to group allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: one child in the mCIMT group dropped out due to family circumstances and 2 children in the mCIMT group did not attend the 26‐week assessment. With no dropouts and full data sets for the bimanual therapy group, this represents unbalanced outcomes data. Note: all missing data were carried forward so data for the full sample were analysed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information is available to determine presence or absence of selective outcome reporting |
Smania 2009.
| Methods |
Design: randomised controlled trial with cross‐over. Following randomisation, children participated in 5 weeks of intervention, completed an assessment and there was a 4‐week suspension of therapeutic intervention (washout period). This was followed by another assessment, 5 weeks of intervention (cross‐over), an assessment and a final assessment 4 weeks after completion of the second arm of intervention Comparison groups reported by study authors: mCIMT vs conventional physiotherapy Country: Italy Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion Criteria (a) Aged 1 to 10 years (b) Mild to moderate paresis enabling reach and grasp of a pellet (c) Active participation in the proposed activities (d) Good physical health (e) Parent consent for participation Exclusion Criteria (a) Presence of severe behaviour disturbances (b) Severe developmental or intellectual retardation (score < 60 on the Brunet‐Lezine developmental quotient test, Terman‐Merril intelligence quotient test or the WISC‐R) Participants: 11 children with unilateral CP were randomised Randomisation method: not described Dropouts: intervention n = 1 (reported to experience a “sudden manifestation of a severe aggressive behavior. This aggressive behavior, consistent with nervousness and refusal to participate in the treatment sessions manifested soon after the beginning of the mCIT program”) Number of participants who received intended treatment: intervention n = 6, comparison n = 5 Number of participants who were analysed: total sample: n = 10; mean age = 3 years 4 months SD 1 year 11 months, range = 1 to 9 years; 7 males, 3 females; 6 left hemiplegia, 4 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: not reported Comparison group: not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 5 weeks Duration: 1 hour per session Frequency: 2 x weekly Total dose of therapy time: 10 hours Description Type of restraint device: cotton mitt Hours per day restraint worn: 8 hours Treatment environment: not reported Individual or group: not reported Therapy provider: physiotherapist Models of practice: based on principles of motor learning, which emphasised self‐generated actions repeated in playful and motivational settings, with appropriate level of successful learning Home programme: parents asked to “stimulate child to use arm at home”. No frequency and intensity not specified or reported Comparison group (dose‐matched): Treatment dosage Length: 5 weeks Duration: 1 hour per session Frequency: 2 x weekly Total dose of therapy time: 10 hours Description Treatment environment: not specified Individual or group: not reported Therapy provider: physiotherapist Models of practice: based on principles of motor learning, which emphasised self‐generated actions repeated in playful and motivational settings, with appropriate level of successful learning Home programme: “Therapist gave indications on home exercises” |
|
| Outcomes |
Assessment time points: Baseline 1 (2 days prior to treatment) Baseline 2 (1 day prior to treatment) (mean of two testing sessions used for analysis) Postintervention: 5 weeks (1 day immediately after treatment and 2 days immediately after treatment) (mean of two testing sessions used for analysis) Final follow‐up assessment: 4 weeks from end of intervention (2 weeks to 4 months postintervention) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: none sought as all measures had no evidence of validity or reliability in CP Fundings sources: no funding declared Study author declaration: no declaration given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: A “restricted randomization cross‐over design” was used p.494 Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: “Testing sessions were videotaped…..The examiner was blinded with regards to the aim of the study and the treatment the patients received" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “One patient was excluded from the study because of the sudden manifestation of a severe aggressive behavior”. Comment: one child (out of 11) was unable to continue CIMT and had no follow‐up data. The missing data are likely to be related to the CIMT intervention and true outcome, but constituted a small proportion of the sample size. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Sung 2005.
| Methods |
Design: single‐centre, single‐blind, randomised controlled trial. Comparison groups reported by study authors: forced use therapy vs conventional OT Country: Korea Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) Unilateral CP (b) Good health (c) 8 years old or younger (d) Able to walk independently Exclusion criteria (a) Severe paralysis of the upper limb (b) Cognitive dysfunction that rendered them unable to cooperate during testing (c) Insecure ambulators Participants: 31 children with unilateral CP Randomisation method: not reported Dropouts: not reported Number of participants who received intended treatment: not reported Number of participants who were analysed: total sample: n=31; mean age = 37.39 months SD 19.33 months (calculated by review authors); 15 males, 16 females; 10 left hemiplegia, 21 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n = 18; mean age = 33.2 months SD 8.1 months; 10 males, 8 females; 3 left hemiplegia, 15 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n = 13; mean age = 43.2 months SD 27.9 months; 5 males, 8 females; 7 left hemiplegia, 6 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 6 weeks Duration: 30 minutes Frequency: 2 days per week for 6 weeks (1 hour per week) Total dose of therapy time: 6 hours Description Type of restraint device: short arm Scotchcast, below elbow to fingertips Hours per day restraint worn: 24 hours per day for 6 weeks Treatment environment: outpatient rehabilitation centre Individual or group: individual Therapy provider: occupational therapists Models of practice: stretching exercises for 5 to 10 minutes. Therapeutic goal setting. Tasks such as reaching, grasping, holding, manipulating an object, bearing weight on the arm, and making hand gestures were divided into small component skills, which were worked on individually and later chained together to complete a target activity. Incorporated activities of daily living including eating, grooming, dressing, and using the toilet, into the therapy sessions Home programme: parents were encouraged children to use the affected hand during daily routine activities Comparison group (dose‐matched) Length: 6 weeks Duration: 30 minutes Frequency: 2 days per week for 6 weeks (1 hour per week) Total dose of therapy time: 6 hours Treatment environment: outpatient rehabilitation centre Individual or group: individual Therapy provider: occupational therapists Models of practice: stretching exercises for 5 to 10 minutes. Therapeutic goal setting. Tasks such as reaching, grasping, holding, manipulating an object, bearing weight on the arm, and making hand gestures were divided into small component skills, which were worked on individually and later chained together to complete a target activity. Incorporated activities of daily living including eating, grooming, dressing, and using the toilet, into the therapy sessions Home programme: not reported |
|
| Outcomes |
Assessment time points: baseline; 6 weeks after completion of intervention (2 weeks to 4 months postintervention) Primary Outcome Measure(s)
Outcome Measures
|
|
| Notes |
Fundings sources: Asan Institute for Life Science for the Promotion of Science for Young Scientists (grant no. 2003‐181). Study author declaration: no commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Thirty‐one patients with hemiplegic CP....were recruited and randomly assigned to the FUT group (n=18) or the control group (n=13)" Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | High risk | Comment: no blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study did not report whether or not there had been drop outs. Completion of an intention‐to‐treat analysis was not specified. Insufficient information to permit judgement of risk of bias associated with attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Taub 2004.
| Methods |
Design: single‐centre, single‐blind, randomised controlled trial with cross‐over Comparison groups reported by study authors: CIMT vs usual care Country: USA Other: no protocol or trial registration identified Groups defined by Cochrane authors:
Comparison defined by Cochrane authors and used in meta‐analysis:CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Diagnosis of CP resulting in hemiparesis or substantially greater deficit in movement of 1 upper limb in comparison to the other (b) Good health (c) ≤ 8 years old (d) For children <18 months of age ‐ an etiology of stroke confirmed by magnetic resonance imaging findings Exclusion criteria
Participants: n = 18; intervention n = 9, comparison n = 9 Randomisation method: “Achieved by assigning patients according to the group designation indicated on a folded piece of paper, taped closed, and drawn from a jar set up before the beginning of subject enrolment” Dropouts: not reported Number of participants who received intended treatment: n = 18 Number of participants who were analysed: total sample: n=18; mean age = 41.2 months SD 27.9 months (calculated by review authors), range 7 to 96 months; 13 males, 5 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported Intervention group: n = 9; mean age = 39 months SD 28.1 months (calculated by review authors); 7 males, 2 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported Comparison group: n = 9; mean age = 43.4 months SD 29.2 months (calculated by review authors); 6 males, 3 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (sCIMT) Treatment dosage Length: Three weeks Duration: 6 hours per day Frequency: 7 days per week for 3 weeks (42 hours per week) Total dose of therapy time: 126 hours Description Type of restraint device: Long‐arm bivalved cast (upper arm to fingertips) Hours per day restraint worn: 24 hours Treatment environment: home Individual or group: individual Therapy provider: occupational therapist or physiotherapist. Same 3 therapists as usual care Models of practice: shaping. Interesting and useful activities were presented to the child in ways that provided immediate, frequent, and repetitive rewards (primarily verbal praise, smiles, and supportive gestures, with some food and toys) for the child’s efforts and increasingly functional use of the more‐impaired limb. Tasks such as reaching, grasping, holding, manipulating an object, bearing weight on the arm, and making hand gestures were divided into their small component skills, which were worked on individually and later chained together to comprise a target activity. When the child demonstrated a new movement skill, the therapist proceeded to shape this by increasing the demands for more precision, strength, fluency, automaticity, and/or functional versatility. Also incorporated everyday tasks (e.g., dressing/undressing, eating, bathing, and grooming) in the therapy sessions. Shaping tasks were selected by considering 1) the family and child’s goals, 2) the intrinsic motivating properties of an activity, 3) promotion of independence by acquisition of age‐appropriate self‐help skills, and 4) the movements that therapists believed had the greatest potential for improvement. Parents were encouraged to join in therapy‐related activities and encourage their child to use newly acquired skills when the therapist was not present. When a child showed signs of fatigue, frustration, or reduced interest, the therapist adapted the activities but did not cease the therapy Home programme: none. CIMT was implemented with therapist in the home environment Comparison group (low dose) Length: 3 weeks Duration: not reported Frequency: not reported Total dose of therapy time: mean of 2.2 hours per week Treatment environment: not reported Individual or group: not reported Therapy provider: occupational therapists and/or physiotherapists Models of practice: not reported Home programme: not reported |
|
| Outcomes |
Assessment time points: baseline; immediately following intervention; 3 weeks after end of intervention (2 weeks to 4 months postintervention) For the CIMT group only, additional time points were at 3 and 6 months Primary outcome measure
Outcome measures
|
|
| Notes | Mean change data for individual QUEST domains available in De Luca (2002) Fundings sources: Alabama Health Services Foundation, the Civitan International Research Center, the National Institute of Child Health and Human Development of the National Institutes of Health, the Administration on Developmental Disabilities, and the Maternal and Child Health Bureau. Study author declaration: no declarations given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Achieved by assigning patients according to the group designation indicated on a folded piece of paper, taped closed, and drawn from a jar set up before the beginning of subject enrolment” Taub 2004 |
| Allocation concealment (selection bias) | High risk | Comment: not described. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including PMAL, DASI‐II was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "Videotapes of these sessions (TAUT) were scored independently by 2 experienced pediatric occupational therapists (interrater reliability .98) who were blind to the treatment group and pre‐ or posttreatment status of the children" (p.306 Taub, 2004) Quote: "Both therapists were unaware of the treatment period or group status of the children involved" (p. 934 DeLuca, 2006) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study did not report whether or not there had been drop outs. Completion of an intention‐to‐treat analysis was not specified. Insufficient information to permit judgement of risk of bias associated with attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Taub 2011.
| Methods |
Design: single‐centre randomised controlled trial with cross‐over Comparison groups reported by study authors: CIMT vs usual care Country: USA Other: no protocol or trial registration identified Groups defined by Cochrane authors:
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Stroke in prenatal, perinatal or very early antenatal period confirmed by MRI (b) Congenital hemiparesis (c) Aged 2 to 6 years (d) No serious or recurring medical complications (e) Living within 40 miles of clinic or willing to temporarily locate to the area Exclusion criteria (a) Score of > 2.5 on the Pediatric Motor Activity Log for the more affected limb (b) Uncontrolled seizures (c) Botulinum toxin‐A injection in the upper limb or other spasticity medications within 3 months of intervention (d) Fixed contractures in the upper limb (4 or more on the Ashworth scale) (e) Previous CIMT or forced use therapy Participants: 22 children with congenital hemiparesis Randomisation method: children were assigned randomly in blocks of 4 ‐ no further details provided Dropouts: n = 2: comparison n = 2 (dropped out prior to intervention, n = 1 seizures, n = 1 indefinite hospitalisation) Number of participants who received intended treatment: n = 20 (91%): intervention n = 10, comparison n = 10 Number of participants who were analysed: total sample: n = 20; mean age = 3.65 years SD 1.42 years (calculated by review authors); 4 males, 16 females; 6 left hemiplegia, 14 right hemiplegia; MACS not reported; GMFCS not reported Intervention group: n = 10; mean age = 4 years SD 1.2 years; 2 males, 8 females; 2 left hemiplegia, 8 right hemiplegia; MACS not reported; GMFCS not reported Comparison group: n = 10; mean age = 3.3 years SD 1.6 years; 2 males, 8 females; 4 left hemiplegia, 6 right hemiplegia; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (hybrid CIMT) Treatment dosage Length: 3 weeks (15 consecutive weekdays) ‐ 13 days of CIMT and 2 days of bimanual activities Duration: 6 hours per day Frequency: each week day Total dose of therapy time: 90 hours Description Type of restraint device: long arm cast including hand and fingers to above elbow (univalved for skin check only) Hours per day restraint worn: 24 hours Treatment environment: home and community Individual or group: individual Therapy provider: a therapist (profession unspecified) Models of practice: shaping ‐ the more affected arm was trained intensively by a behavioral procedure termed ‘‘shaping ‐ where the child is required to improve performance, usually in small steps, at each iteration of a movement to obtain a reward (enthusiastic praise, encouraging exclamations, and other signs of approval by the therapist)" At the beginning of the fourteenth day of treatment, the cast was removed and the child received training in using the more affected arm in bilateral activities for the final 2 days of treatment Throughout, a ‘‘transfer package,’’ was used to induce transfer of therapeutic gains from the treatment period to usual life activities Home programme: written list of training tasks given to caregiver to complete over weekends. Caregivers were trained in the shaping of movements.Home programme provided post treatment to encourage continuation of training – weekly phone calls from therapist carried out for first month post treatment Comparison group (low dose) Length: 3 weeks (although can presume they continued for the 6‐month control period) Duration: 1‐2 hours Frequency: 1‐2 sessions per week Total dose of therapy time: not reported Description Treatment environment: not specified Individual or group: not specified Therapy provider: occupational therapist or physiotherapist Models of practice: not stated Home programme: not stated |
|
| Outcomes | Assessment time points: baseline. PMAL collected daily during treatment. Immediately following intervention (following 15 weekdays of treatment); 4 weeks post baseline; 6 months post baseline (2 weeks to 4 months postintervention) Primary outcome measure
Outcome measures
|
|
| Notes |
Additional information sought from authors: authors contacted but declined to provide MAS data and PAFT data (to enable pooling with a second study) Fundings sources: National Institutes of Health (5R13NS040925‐09), the National Institutes of Health Office of Rare Diseases Research, the Child NeurologySociety, and the Children’s Hemiplegia and Stroke Association, Grant HD040692 from the National Center for Medical Rehabilitation Research of NICHD. Study author declaration: the authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children were assigned randomly in blocks of 4" Comment: insufficient information given to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including PMAL was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Unclear risk | Comment: not described. Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two children assigned to the control group dropped out before treatment began, 1 because of a seizure and 1 because of an indefinite hospitalization" Comment: amount of, and reasons for, missing data is not likely to affect outcomes. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Wallen 2011.
| Methods |
Design: multi‐centre, assessor‐blinded, pragmatic randomised controlled trial Comparison groups reported by study authors: mCIMT vs intensive occupational therapy Country: Australia Other: trial registered at Australian Clinical Trials Register (ACTRN12607000446460) Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs high dose |
|
| Participants |
Inclusion criteria (a) Spastic unilateral CP (b) Aged 18 months to 8 years (c) Able to achieve at least 10° wrist extension and/or finger extension (d) Functional PROM (120° shoulder flexion and abduction; 30 to 120° elbow movement; neutral wrist and finger extension; minimum 45° supination) (e) Capable of co‐operating for assessment and therapy (f) Access to weekly occupational therapy (g) Parents committed to the study Exclusion criteria (a) Children who had engaged in a new or altered intervention for their upper limb in the 4 months preceding randomisation Participants: 50 participants with spastic unilateral CP Randomisation method: allocation sequence, consisting of randomly permuted blocks of 2 or 4, was generated by the independent National Health and Medical Research Council Clinical Trials Centre prior to commencement of recruitment Dropouts: n = 0 Number of participants who received intended treatment: n = 49; comparison n = 1 (withdrew halfway through treatment due to fractured more affected arm – this child was analysed according to principles of intention‐to‐treat) Number of participants who were analysed: total sample: n = 50; mean age = 48.6 months SD 21 months; 27 males, 23 females; 27 left hemiplegia, 23 right hemiplegia; MACS I n = 2, MACS II n = 3, MACS III n = 8, MACS IV n = 1; GMFCS I n = 33, GMFCS II n = 15, GMFCS III n = 1 Intervention group: n = 25; mean age = 28.8 months SD 21.9 months; 17 males, 12 females; 16 left hemiplegia, 9 right hemiplegia; MACS I n = 1, MACS II n = 20, MACS III n = 2, MACS IV n = 0; GMFCS I n = 22, GMFCS II n = 3, GMFCS III n = 0 Comparison group: n = 25; mean age = 48.8 months SD 20.5 months; 10 males, 15 females; 11 left hemiplegia, 14 right hemiplegia; MACS I n = 1, MACS II n = 17, MACS III n= 6, MACS IV n = 1; GMFCS I n = 11, GMFCS II n = 12, GMFCS III n = 1 |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 8 weeks Duration: planned = 2 hours per day (minimum of 30‐minute sessions). Actual = mean 1.3 hours per day (SD 0.6), range 0.4 to 2.3 hours. NOTE: 1 x weekly OT session was included in these data Frequency: daily Total dose of therapy time: mean = 72.8 hours (based on 1.3 hours, 7 days per week for 8 weeks) Description Type of restraint device: fabric mitt with thermoplastic volar insert Hours per day restraint worn: planned = 2 hours. Actual = 1.3 hours Treatment environment: clinic (1 hour weekly), home (daily) Individual or group: individual Therapy provider: parents provided home programme, occupational therapists provided 1 hour per week Models of practice: therapy, completed while the mitt was worn, was based on motor learning principles and involved self‐generated voluntary repetitions of specific movements of the affected upper limb, which were incorporated into play activities. The particular movements were those required to complete activities of daily living selected by parents as priorities for intervention, but which were lacking in the child’s upper‐limb movement repertoire Home programme: yes – based on Novak 2009 principles Comparison group (high dose) Length: 8 weeks Duration: suggested = 20 minutes per day (0.33 hour). Actual = mean 0.8 hours (SD 0.6), range 0.3 to 2.6 Frequency: daily Total dose of therapy time: 44.8 hours (mean 0.8 hours intervention x 56 days) Description Treatment environment: home and clinic (weekly) Individual group: individual Therapy provider: parents provided home programme, occupational therapist once per week Models of practice: intensive OT aimed to achieve parents’ goals, and included techniques aimed at minimising impairment (e.g. stretching, casting, splinting) and enhancing activities (e.g. motor training, environmental modification, and practice of specific goal activities) Home programme: completed based on Novak et al. principles (Novak 2009)p |
|
| Outcomes |
Assessment time points: baseline: 10 weeks from randomisation (immediately following intervention); 6 months from randomisation (2 to 4 months postintervention). Primary outcome measure
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: author provided mean change and standard deviation of the mean change for: AHA Units, MAS (for elbow and wrist flexors and pronators) and COPM Additional information provided by authors
Fundings sources: Margaret Wallen was supported by an Allergan Doctoral Scholarship from the Cerebral Palsy Alliance Research Foundation. Rob Herbert was supported by a fellowship from the Australian National Health and Medical Research Council. Study author declaration: no declarations given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation sequence, consisting of randomly permuted blocks of 2 or 4, was generated by the independent National Health and Medical Research Council Clinical Trials Centre before commencement of recruitment" |
| Allocation concealment (selection bias) | Low risk | Quote: "..the investigator telephoned an independent off‐site research assistant to be informed of the participant’s allocation. This process ensured that the allocation sequence remained concealed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes including PMAL, COPM, GAS was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "Data assessors video‐recorded the AHA, which was later scored blinded to allocation and timing of assessment by an otherwise uninvolved accredited rater" Comment: assessor blinding at 10 weeks was 86%, at 6 months 80% (100% postintervention) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All participants were included in the analysis and were analysed in the group to which they were randomized (i.e. in accordance with the principles of intention to treat). "Missing data were imputed by carrying forward the last value" Comment: one child in the intensive OT group did not complete intervention due to a broken arm. Amount of, and reasons for, attrition is unlikely to affect outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: trial registered at Autralia New Zealand Clinical Trials Register (ANZCTR: 2607000446460). All outcomes reported |
Xu 2012.
| Methods |
Design: single‐centre, assessor‐blinded, randomised controlled trial Comparison groups reported by study authors: CIMT vs dose‐matched OT vs CIMT plus FES Country: China Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) 2 – 14 year old children with unilateral CP (b) Ability to extend the wrist ≥ 20° and the metacarpophalangeal joint 10° from full flexion (c) 20% to 80% difference between the involved and non‐involved hand on the globe rating scale scores (unknown scale) (d) Informed consent from parents (e) Ability to comply with study instructions Exclusion criteria (a) Any health problems that are not associated with CP (b) Contractures that limit functional arm and hand use (c) Uncontrolled seizures (d) Botulinum toxin injection in the upper limb during the last 6 months or scheduled to receive it within the period of study (e) Orthopaedic surgery on the involved upper limb (f) Visual and balance problems that would prevent them from carrying out the intervention or assessment (g) Prior exposure to constraint therapy (h) Interventions such as baclofen, dantrium and artane, etc Participants: 75 children with unilateral CP Randomisation method: participants were allocated by a random number produced by computerized method of minimisation. The stratification included the age (≤4 years and > 4 years) and globe rating scale scores (≤ 5 and > 5) Dropouts: CIMT n = 2/24 discontinued treatment and were not followed up. OT n=3/26 were not followed up, 1 = discontinued treatment, 2= lost to follow‐up. CIMT plus FES n = 2/25 were not followed up, 1 = discontinued treatment, 1= lost to follow‐up Number of participants who received intended treatment: 71 of 75 received intended intervention, a total of 68 of 75 were followed up Number of participants who were analysed: total sample: n = 68; mean age = 55 months SD 33 months, range 24 to 149 months; 25 males, 43 females; 30 left hemiplegia, 38 right hemiplegia; MACS not reported; GMFCS I n = 60; GMFCS II n = 8 Intervention group (CIMT): n = 22; mean age = 54.6 months SD 36.6 months; 25 males, females; 12 left hemiplegia, 10 right hemiplegia; MACS not reported; GMFCS I n = 19, II n = 3 Comparison group 1 (OT): n = 23; mean age = 54.7 months SD 30.8 months; 11 males, 12 females; 8 left hemiplegia 15 right hemiplegia; MACS not reported; GMFCS I n = 21, II n = 2 Comparison group 2 (CIMT plus FES): n = 23; mean age = 56.8 months SD 34 months; 7 males, 16 females; 10 left hemiplegia, 13 right hemiplegia; MACS not reported; GMFCS I n = 20; II n = 3 |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 2 weeks then home programme only for 6 months Duration: 3 hours per day at centre for 2 weeks plus 1 hour per day at home during initial 2 weeks, then 2 hours per day at home for 6 months Frequency: 10 days over the 2 weeks Total dose of therapy time: planned = 30 hours (plus 10 at home) during 2‐week period plus home programme following the 2 weeks. Actual hours not reported Description Type of restraint device: below elbow resting splint Hours per day restraint worn: 4 hours Treatment environment: children’s hospital and home Individual or group: groups of 2‐4 children Therapy provider: occupational therapists and families Models of practice: individualised instruction from occupational therapists involving the specific practice of designated target movements using play and functional activities that provided the structured and intensive practice using the involved hand (e.g. hand exercise, dancing, ball, card, manipulating and board games, puzzles, bowling, painting, eating and putting away games). When the target movement was performed successfully, task difficulty was increased by changing either temporal or spatial/ accuracy task constraints. Children were provided positive reinforcement with verbal praise and toys throughout a task for performance of target movements Home programme: 1 hour per day at home during initial 2 weeks, then 2 hours per day at home for 6 months. Daily activity logs and fortnightly phone calls from therapists to assist with adherence to daily home programme Comparison group (dose‐matched) Treatment dosage Length: 2 weeks then home programme only for 6 months Duration: 3 hours per day at centre. 1 hour per day at home during initial 2 weeks, then 2 hours per day at home for 6 months Frequency: 10 days over the 2 weeks Total dose of therapy: planned = 30 hours (plus 10 at home) during 2 week period plus home programme following the 2 weeks. Actual hours not reported Description Treatment environment: children’s hospital and home Individual or group: not reported Therapy provider: occupational therapists and families Models of practice: individually tailored advice and treatment (based on NDT, motor learning, stretching, strength and co‐ordination training, and task specific training), and provision of orthoses according to individual goals and clinical reasoning, aimed at reducing spasticity, improving hand function and ADL Home programme: 1 hour per day at home during initial 2 weeks, then 2 hours per day at home for 6 months. Daily activity logs and fortnightly phone calls from therapists to assist with adherence to daily home programme Comparison group 1 (mCIMT plus FES) CIMT component Length: 2 weeks then home programme only for 6 months Duration: 3 hours per day at centre for 2 weeks plus 1 hour per day at home during initial 2 weeks, then 2 hours per day at home for 6 months Frequency: 10 days over the 2 weeks Total dose of therapy: planned = 30 hours (plus 10 at home) during 2‐week period plus home programme following the 2 weeks. Actual hours not reported Description Type of restraint device: below elbow resting splint Hours per day restraint worn: 4 hours Treatment environment: children’s hospital and home Individual or group: groups of 2‐4 children Therapy provider: occupational therapists and families Models of practice: individualised instruction from occupational therapists involving the specific practice of designated target movements using play and functional activities that provided the structured and intensive practice using the involved hand (e.g. hand exercise, dancing, ball, card, manipulating and board games, puzzles, bowling, painting, eating and putting away games). When the target movement was performed successfully, task difficulty was increased by changing either temporal or spatial/ accuracy task constraints. Children were provided positive reinforcement with verbal praise and toys throughout a task for performance of target movements Home programme: 1 hour per day at home during initial 2 weeks, then 2 hours per day at home for 6 months. Daily activity logs and fortnightly phone calls from therapists to assist with adherence to daily home programme FES component Length: 2 weeks concurrently with CIMT programme Duration: 20 minutes Frequency: 10 days over the 2 weeks Total dose of therapy time: planned = 200 minutes. Actual hours not reported Description Treatment environment: centre Individual or group: individual Therapy provider: not reported Models of practice: FES was applied for 20 minutes 5 times a week for 2 weeks on extensor carpi radialis and extensor digitorum of the affected upper limb using MyoTrac Infiniti dual channel neuromuscular electrical stimulation unit (Quebec, Canada) and reusable carbonised‐rubber electrodes. The active electrode was placed on the motor point and the inactive electrode was placed distally over the same muscle group. The active electrode was cut to size, so that only the same muscle group would be stimulated. Frequency = 50 Hz, pulse rate = 30 pulses per second with 300 μs of amplitude, and the amplitude was maximum 100 mA. ON time = 12 seconds with 1 second of rise and decay and an OFF time of 12 seconds. Amplitude was increased slowly to the child’s tolerance without causing discomfort Home programme: no FES was completed at home |
|
| Outcomes |
Assessment time points: baseline; 2 weeks (immediately postintervention); 3 months postintervention (2 weeks to 4 months postintervention); 6 months postintervention (5 to 6 months postintervention) Primary outcome measure
Secondary outcome measures
|
|
| Notes |
Fundings sources: Grant HD 2009J1‐C531 from Bureau of Science and Technology of Guangzhou Municipality, Guangzhou, China. Study author declaration: no declaration given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...subjects were allocated in an unbiased manner by a random number produced by computerized method of minimization" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: blinding for self‐reported outcomes was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Low risk | Quote: "Outcome measurements were assessed by three independent evaluators who were not aware of the treatment group of each patient" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Evidence: low rates of missing data (2/25; 2/24, 3/26) Comment: reasons for missing data given and distributed evenly across groups. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement of high or low risk |
Yu 2012.
| Methods |
Design: single‐centre, randomised controlled trial Comparison groups reported by study authors: mCIMT vs traditional therapy Country: Korea Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs low dose |
|
| Participants |
Inclusion criteria (a) Unilateral CP (b) Not undertaken CIMT in previous 2 years (c) Voluntary movement not limited when non‐affected side is restrained (d) No difficulties in performing PROM exercised and some active ROM (voluntary wrist extension and voluntary finger extension of 10° degrees or more) on affected side (e) No cognitive deficits (able to understand the instructions of therapists) Exclusion criteria
Participants: 24 participants with unilateral CP were randomised Randomisation method: table of random sampling numbers used, allocation concealment unclear Dropouts: n = 4 dropouts. Reasons for dropouts were not reported Number of participants who received intended treatment: unclear if dropouts received treatment Number of participants who were analysed: total sample: n = 20; mean age = 9.4 years SD 0.34 years (calculated by review authors); 13 males, 7 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported Intervention group: n = 10; mean age = 9.4 years SD 0.3 years; 7 males, 3 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported Comparison group: n = 10; mean age = 9.4 years SD 0.4 years; 6 males; 4 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 10 weeks Duration: 1 hour per session Frequency: 2 sessions per week Total dose of therapy time: 20 hours Description Type of restraint device: sling and splint made of a light material. Arm position during restraint was 90° elbow flexion, 20° wrist extension, and 20° finger joint flexion Hours per day restraint worn: planned = unclear. Actual = unclear. Treatment environment: clinic Individual or group: group (size not specified) Therapy provider: experienced physical therapists Models of practice: unclear Home programme: unclear Comparison group (low dose) Length: 10 weeks Duration: 30 minute sessions Frequency: 2 sessions per week Total dose of therapy time: 10 hours Description Treatment environment: clinic (assumed, not specified) Individual or group: group (size not reported) Therapy provider: experienced physical therapists Models of practice: no details of intervention given Home programme: unclear |
|
| Outcomes |
Assessment time points: baseline; 10 weeks (immediately post‐intervention) Primary outcome measures
|
|
| Notes |
Additional information sought from authors: authors contacted via email (otsalt@nate.com) on 09/05/2015 and 03/07/2015 for change from baseline data but no response received Fundings sources: nil funding reported Study author declaration : no declaration given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomized using a table of random sampling numbers” |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | Unclear risk | Quote: “single‐blind analysis” Comment: does not specify who is blinded. Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: four dropouts, but the reasons or group were not specified. Completion of an intention‐to‐treat analysis was not specified. Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
Zafer 2016.
| Methods |
Design: randomised controlled trial Comparison groups reported by study authors: CIMT vs bimanual therapy Country: Pakistan Other: no protocol or trial registration identified Groups defined by Cochrane authors
Comparison defined by Cochrane authors and used in meta‐analysis: CIMT vs dose‐matched |
|
| Participants |
Inclusion criteria (a) Spastic unilateral CP (b) Aged 1.5 to 12 years (c) 10° wrist extension and 10° finger extension (d) A range of 40 to 60 on the grasp and dissociated movement domains of the QUEST Exclusion criteria
Participants: 20 children with spastic unilateral CP were randomised Randomisation method: “Randomly divided”. No further information given Dropouts: n = 2: one from each group ‐ group not specified (n = 1 inability to attend due to exams, n = 1 unable to attend follow‐up assessment) Number of participants who received intended treatment: intervention n = 9, comparison n = 9 Number of participants who were analysed: total sample: n = 18; mean age = 8 years 10 months SD 3 years 1 month, range = 1 year 6 months to 12 years; 15 males, 3 females; side of hemiplegia not reported; MACS not reported; GMFCS not reported Intervention group: not reported Comparison group: not reported |
|
| Interventions |
Intervention group (mCIMT) Treatment dosage Length: 2 weeks Duration: 2 hours of therapy per day plus additional 4 hours per day of constraint Frequency: 6 days per week Total dose of therapy time: 26 hours. Therapists spent 2 hours with child and family at the start of intervention and thereafter contacted the family once. Parents were then responsible for the treatment programme Description Type of restraint device: “Mitt that was constraining the hand and the elbow was constrained by sling strapped to the trunk” Hours per day restraint worn: 6 hours Treatment environment: home Individual or group: individual Therapy provider: parents Models of practice: personalised activities which comprised unimanual daily activities to practice reach, grasp, manipulation, release and weight bearing. Home programme: as above Comparison group (dose‐matched) Treatment dosage Length: 2 weeks Duration: 2 hours of therapy per day Frequency: 6 days per week. Total dose of therapy time: 26 hours. Therapists spent 2 hours with child and family at the start of intervention and thereafter contacted the family once. Parents were then responsible for the treatment programme Description Treatment environment: home Individual or group: individual Therapy provider: parents Models of practice: personalised activities which comprised bimanual daily activities to practice reach, grasp, manipulation, release and weight bearing. Home programme: as above |
|
| Outcomes |
Assessment time points: baseline; Immediately postintervention (2 weeks) Primary outcome measures
Secondary outcome measures
|
|
| Notes |
Additional information sought from authors: additional details regarding randomisation procedures, allocation concealment, blinding of data assessors, age range, amount of therapist contact with child and family, and reasons for drop outs were obtained from the authors. The authors were unable to provide data requested for side of hemiplegia, participant characteristics for each intervention group separately and change data for the QUEST Question 1: Further information on methods used to randomise children to each group Reply 1: Coin was tossed for every other participant to be placed in one group and immediate next participant was consequently placed in the other group Question 2: Further information on methods use for allocation concealment Reply 2: Allocation of patients to the groups was done by data assessor herself so there was no allocation concealment Question 3: Were data assessors blinded to group allocation? Reply 3: No Question 4: The protocol reports input from a therapist at the start of the programme, can further information please be provided about the intensity, that is, the number of hours and how frequently the therapist were in contact with families Reply 4: At the start of the programme 2 hours were given to the patient by the therapist and in a period of 2 weeks therapist contacted the family once Question 5: One child from each group was dropped due to lack of follow‐up. Can you please provide detail about the reasons for these dropouts Reply 5: The reason for the dropout of one child was clash between his exams and treatment period the other child dropped out for his parents could not manage to bring out time for follow‐up Fundings sources: no funding support received Study author declaration: no disclosures to make |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were “randomly divided into two groups” Additional information from authors: “Coin was tossed for every other participant to be placed in one group and immediate next participant was consequently placed in the other group” Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | High risk | Additional information from authors: “Allocation of patients to the groups was done by data assessor herself so there was no allocation concealment” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Additional information from authors: "Participants and personnel were unable to be blinded" |
| Blinding of outcome assessment (detection bias) Objectively observed outcomes | High risk | Additional information from authors: "Outcome assessors were not blinded to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “One child from each group was dropped due to lack of follow up” Additional information from authors: “The reason for the dropout of one child was clash between his exams and treatment period the other child dropped out for his parents could not manage to bring out time for follow‐up” Comment: reasons for missing outcome data unlikely to be related to true outcome. Completion of an intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol located. Insufficient information to permit judgement |
ADLs: activities of daily living AHA: Assisting Hand Assessment BoNT‐A: Botox C: control CAPE: Children’s Assessment of Participation and Enjoyment CFUS:Caregiver Functional Use Survey CIMT: Constraint‐induced movement therapy COPM: Canadian Occupational Performance Measure CP: cerebral palsy EMG: electromyography Eco‐CIMT: Ecological‐Constraint‐induced movement therapy FES: Functional Electrical Stimulation FUT: Forced use therapy GAS: Goal Attainment Scaling GMFCS: Gross Motor Function Classification System HAI: Hand assessment for infants I: intervention lb: pounds MACS: Manual Ability Classification System MAS: Modified Ashworth Scale mCIMT: Modified CIMT mCIMT‐BiT: Modified CIMT followed by bimanual training MRI: magnetic resonance imaging MTS: Modified Tardieu Scale OT: occupational therapist PMAL: Pediatric Motor Activity Log PMAL‐R: Pediatric Motor Activity Log revised PROM: Passive Range of Motion PT: physical therapist ROM: range of motion RTM: Remind to Move rTMS: repetitive Transcranial Magnetic Stimulation SD: standard deviation TMS: Transcranial Magnetic Stimulation SPSS: Statistics software package VR: virtual reality WeeFIM: Functional Independence Measure for Children
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersen 2013 | Not a randomised controlled trial. Discussion paper. |
| Ardakani 2010 | Not a randomised controlled trial. |
| Aschner 2012 | Not a randomised controlled trial. |
| Ballaz 2012 | Not a randomised controlled trial. |
| Basu 2012a | Not a randomised controlled trial. |
| Bonnier 2006 | Not a randomised controlled trial. |
| Boyd 2001 | Not a randomised controlled trial. Systematic review. |
| Brady 2009 | Not a randomised controlled trial. Systematic review. |
| Brandão 2009 | Not a randomised controlled trial. |
| Brekke 2004 | Not a randomised controlled trial. Conference proceedings abstract. |
| Buesch 2010 | Does not include children with CP. Not a randomised controlled trial. |
| Cao 2015 | Not a randomised controlled trial. |
| Charles 2001 | Not a randomised controlled trial. |
| Charles 2005 | Not a randomised controlled trial. Systematic review. |
| Chen 2014a | Not a randomised controlled trial. Systematic review. |
| Chevignard 2008 | Does not include children with CP. Not in English. |
| Cheyne 2013 | Not a randomised controlled trial. |
| Chiu 2016 | Not a randomised controlled trial. Systematic review. |
| Christman 2015 | Not a randomised controlled trial. |
| Cimolin 2012 | Does not include children with CP. Not in English. |
| Cohen‐Holzer 2010 | Not a randomised controlled trial. Conference proceedings abstract. |
| Cohen‐Holzer 2011 | Not a randomised controlled trial. |
| Cohen‐Holzer 2016 | Not a randomised controlled trial. |
| Coker 2009 | Not a randomised controlled trial. |
| Coker 2010 | Not a randomised controlled trial. |
| Cope 2008 | Not a randomised controlled trial. |
| Cope 2010 | Not a randomised controlled trial. |
| Crocker 1997 | Not a randomised controlled trial. |
| DeLuca 2003 | Not a randomised controlled trial. |
| DeLuca 2015 | Not a randomised controlled trial. |
| Dickerson 2007 | Not a randomised controlled trial. |
| Dong 2013 | Not a randomised controlled trial. Systematic review. |
| Echols 2000 | Not a randomised controlled trial. |
| Eliasson 2003 | Not a randomised controlled trial. |
| Eliasson 2005 | Randomisation was not used. |
| Eliasson 2009 | Not a randomised controlled trial. |
| Eliasson 2014a | Not a randomised controlled trial. Expert consensus. |
| Eliasson 2015 | Not a randomised controlled trial. |
| Fergus 2008 | Not a randomised controlled trial. |
| Fetters 2004 | Not a randomised controlled trial. Critical appraisal. |
| Ganapathy Sankar 2015 | Not a randomised controlled trial. |
| Geerdink 2015 | Not a randomised controlled trial. |
| Gillick 2010 | Not a randomised controlled trial. |
| Gillick 2014 | Evaluation of the effect of Primed Repetitive Transcranial Magnetic Stimulation, not CIMT. |
| Gillick 2015 | Evaluation of the effect of Primed Repetitive Transcranial Magnetic Stimulation, not CIMT. |
| Gillick 2018 | Evaluation of the effect of Transcranial Magnetic Stimulation, not CIMT. |
| Glover 2002 | Not a randomised controlled trial. |
| Gordon 2001 | Not a randomised control trial. Abstract. |
| Gordon 2005 | Not a randomised controlled trial. Discussion paper. |
| Gordon 2006 | Not a randomised controlled trial. |
| Gordon 2007 | Not a randomised controlled trial. |
| Gordon 2008 | Not a randomised controlled trial. Does not include children with CP. |
| Gordon 2010 | Not a randomised controlled trial. Discussion paper. |
| Gordon 2011a | Not a randomised controlled trial. Discussion paper. |
| Gordon 2011b | Not a randomised controlled trial. Commentary. |
| Hackman 2000 | Not a randomised controlled trial. Conference proceedings abstract. |
| Hart 2005 | Not a randomised controlled trial. Editorial. |
| Haynes 2012 | Not a randomised controlled trial. |
| Hoare 2008 | Not a randomised controlled trial. Commentary. |
| Hoare 2014 | Not a randomised controlled trial. Commentary |
| Hoare 2015 | Not a randomised controlled trial. Commentary |
| Huang 2009 | Not a randomised controlled trial. Systematic review. |
| Huang 2010 | Not a randomised controlled trial. Discussion paper. |
| Islam 2014 | Not a randomised controlled trial. |
| Juenger 2007 | Not a randomised controlled trial. |
| Juenger 2013 | Not a randomised controlled trial. |
| Karman 2003 | Not a randomised controlled trial. |
| Kim 2015a | Not a randomised controlled trial. Commentary |
| Klepper 2017 | Not a randomised controlled trial. Systematic review. |
| Klingels 2013 | Evaluation of the effect of intensive therapy program, not CIMT. |
| Kong 2013 | Not a randomised controlled trial. |
| Kuhnke 2008 | Not a randomised controlled trial. |
| Kwon 2014 | Not a randomised controlled trial. |
| Lavinder 2007 | Not a randomised controlled trial. |
| Lee 2010 | Not a randomised controlled trial. Conference proceedings abstract. |
| Leon‐Santos 2008 | Not a randomised controlled trial. Systematic review |
| Lin 2011 | Mixed sample. Includes children with hemiplegia and quadriplegia |
| Lowes 2014a | Not a randomised controlled trial. |
| Lowes 2014b | Not a randomised controlled trial. Pilot study |
| Maitre 2011 | Not a randomised controlled trial. Conference proceedings abstract. |
| Manning 2014 | Not a randomised controlled trial. |
| Manning 2015 | Not a randomised controlled trial. |
| Manning 2016 | Not a randomised controlled trial. |
| Martin 2008 | Not a randomised controlled trial. |
| Mcconnell 2014 | Not a randomised controlled trial. |
| Motta 2010 | Not a randomised controlled trial. |
| Nascimento 2009 | Not a randomised controlled trial. Systematic review. |
| Naylor 2005 | Case‐series design |
| NCT02957708 | Not a randomised controlled trial. Unpublished. |
| Newman 2008 | Not a randomised controlled trial. |
| Nordstrand 2013 | Not a randomised controlled trial. |
| Novak 2013 | Not a randomised controlled trial. Systematic review |
| Nwaobi 1987 | Not a randomised controlled trial. Not CIMT protocol. |
| Oh 2014 | Not a randomised controlled trial. Systematic review. Not published in English. |
| Pardeep 2010 | Not a randomised controlled trial. |
| Park 2009 | Not a randomised controlled trial. |
| Pidcock 2009 | Not a randomised controlled trial. Discussion paper. |
| Pierce 2002 | Not a randomised controlled trial. |
| Psychouli 2010 | Not a randomised controlled trial. |
| Psychouli 2016 | Not a randomised controlled trial. |
| Ramachandran 2011 | Not a randomised controlled trial. |
| Ramey 2012 | Not a randomised controlled trial. Letter to the editor. |
| Reidy 2012 | Not a randomised controlled trial. |
| Reidy 2018 | Not a randomised controlled trial. |
| Rickards 2014 | Not a randomised controlled trial. |
| Ries 2006 | Not a randomised controlled trial. |
| Roberts 2015 | Not a randomised controlled trial. |
| Rocca 2013 | Not a randomised controlled trial. |
| Sakzewski 2009 | Not a randomised controlled trial. Systematic review and meta‐analysis. |
| Sakzewski 2012 | Not a randomised controlled trial. Critical appraisal. |
| Sakzewski 2014 | Not a randomised controlled trial. Systematic review and meta‐analysis. |
| Schrank 2013 | Not a randomised controlled trial. |
| Seema 2015 | Not a randomised controlled trial. |
| Shetty 2014 | Not a randomised controlled trial. |
| Staudt 2014 | Not a randomised controlled trial. Commentary. |
| Stearns 2009 | Not a randomised controlled trial. |
| Sterling 2013 | Not a randomised controlled trial. |
| Sterr 2002 | Does not include children with CP. Paediatric and adult population. Not a CIMT protocol |
| Sutcliffe 2007 | Not a randomised controlled trial. |
| Sutcliffe 2009 | Not a randomised controlled trial. |
| Taub 2007 | Not a randomised controlled trial. Discussion paper. |
| Tervahauta 2017 | Not a randomised controlled trial. Systematic review |
| Thakkar 2014 | Not a randomised controlled trial. |
| Thompson 2015 | Not a randomised controlled trial. |
| Tinderholt Myrhaug 2014 | Not a randomised controlled trial. Systematic review |
| Tucker 2015 | Conference proceedings abstract |
| Vaghela Vishwas 2014 | Unable to determine if sample was randomly allocated to groups. Authors contacted but no response received. |
| Wallen 2004 | Not a randomised controlled trial. Discussion paper. Critical appraisal. |
| Wallen 2008 | Not a randomised controlled trial. |
| Walther 2009 | Not a randomised controlled trial. |
| Wang 2013 | Not a randomised controlled trial. Systematic review |
| Willis 2002 | Does not include children with CP. |
| Wu 2013 | Not a randomised controlled trial. |
| Yasukawa 1990 | Not a randomised controlled trial. |
| Yu 2012b | Not a randomised controlled trial. Commentary. |
| Zipp 2012 | Not a randomised controlled trial. |
CIMT: constraint‐induced movement therapy; CP: cerebral palsy
Characteristics of ongoing studies [ordered by study ID]
Boyd 2017.
| Trial name or title | REACH: Multisite randomised trial of Rehabilitation very EArly in Congenital Hemiplegia |
| Methods |
Aim: to compare the efficacy of infant‐friendly mCIMT (Baby mCIMT) to infant‐friendly bimanual therapy (Baby BIM) on upper limb, cognitive and neuroplasticity outcomes Design: single‐blind, randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Infant‐friendly modified constraint‐induced movement therapy (mCIMT) Involves wearing a material glove/sock on the unimpaired hand to encourage use of the impaired hand in play‐based activity with the impaired arm/hand. mCIMT will be provided in the home by parents/caregivers to the infant by using the glove/sock and engaging the infant in specific play‐based activities. mCIMT will commence between 3‐9 months corrected age, and be provided for 20 minutes per day (can be done in 2 by 10‐minute blocks) for 5 days per week up to 6 months of age. Between 6 and 9 months of age, therapy will be provided for 30 minutes per day (can be done in 3 by 10 minute blocks) for 5 days per week. Between 9 and 12 months of age, therapy will be provided for 40 minutes per day (can be done in 2 by 20‐minute blocks) for 5 days per week. Between 12 and 15 months of age, therapy will be provided for 40 minutes per day (can be done in 2 by 20‐minute blocks) for 5 days per week. Parents will be supported by an experienced occupational therapist/physiotherapist who will do regular monthly home visits and fortnightly Skype calls for 6 months until the infant is 12‐15 months of age to ensure that therapy is child and family friendly. Parents will document the intervention in a training log, and therapists will video record home‐visit therapy sessions. Infant‐friendly bimanual therapy (BIM) Comprises play‐based activity designed to utilise equal activity of both the impaired and unimpaired upper limbs. BIM will be provided in the home by parents/caregivers to the infant by engaging the infant in age appropriate bimanual play activities. BIM will commence between 3‐9 months corrected age, and be provided for 20 minutes per day (can be done in 2 by 10‐minute blocks) for 5 days per week up to 6 months of age. Between 6 and 9 months of age, therapy will be provided for 30 minutes per day (can be done in 3 by 10‐minute blocks) for 5 days per week. Between 9 and 12 months of age, therapy will be provided for 40 minutes per day (can be done in 2 by 20‐minute blocks) for 5 days per week. Between 12 and 15 months of age, therapy will be provided for 40 minutes per day (can be done in 2 by 20‐minute blocks) for 5 days per week. Parents will be supported by an experienced occupational therapist/physiotherapist who will do regular monthly home visits and fortnightly Skype calls for 6 months until the infant is 12‐15 months of age to ensure that therapy is child and family friendly. Parents will document the intervention in a training log, and therapists will video record home visit therapy sessions. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 15/03/2016 |
| Contact information | Prof Roslyn Boyd Queensland Cerebral Palsy and Rehabilitation Research Centre School of Medicine, University of Queensland Centre for Children's Health Research 62 Graham Street South Brisbane, Queensland 4101 Australia Phone +61 7 3069 7372 Email r.boyd@uq.edu.au |
| Notes | Funding: Australian National Health and Medical Research Council (NHMRC) Project Grant for REACH 1059332; NHMRC Early Career Fellowship no.1090828 (LS); NHMRC Research Fellowship (RB) 1105038. This study is funded by the Australian National Health and Medical Research Council (NHMRC) for a project grant no 1078877. The NHMRC has provided people support for the following team members: a Research Fellowship (RB, 1105038), Early Career Fellowship (LS, No1090828, KW, No 631712). |
Chamudot 2016.
| Trial name or title | Constraint induced movement therapy (CIMT) in babies home program |
| Methods |
Aim: to test the efficacy of a mCIMT treatment in babies diagnosed with hemiplegia, treated in a home program, as compared to a control group of babies receiving a parallel home program but with no CIMT Design: randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Constraint induced movement therapy (mCIMT) Two‐month home program that includes restricting the non hemiplegic hand an hour a day during play Active play Two‐month home program that includes active use of hemiplegic hand during play one hour a day |
| Outcomes |
Primary outcome Assisting Hand Assessment (Time Frame: after two months of treatment) |
| Starting date | May 2011 |
| Contact information | Prof. Gross‐ Tsur Varda, Shaare Zedek Medical Center |
| Notes |
Chorna 2015.
| Trial name or title | Early childhood constraint therapy in cerebral palsy |
| Methods |
Aim: (related to review topic) demonstrate that CIMT improves the sensory and motor function of an affected upper extremity in young children with asymmetric CP . Design: randomised controlled trial with waiting list‐control |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Constraint therapy The CIMT intervention includes 3 components: (1) use of a removable soft constraint for 6 hours per day, with a non‐invasive wear monitor (2) home‐use of a sensory kit (15 minutes per day) and; (3) and a reach training tool (10 minutes per day). Comparison group is a waiting‐list control. Intervention lasts 28 days. |
| Outcomes |
Primary outcomes
|
| Starting date | October 1, 2015 |
| Contact information | Olena Chorna, Nationwide Children's Hospital, MM, CCRP 614‐355‐6721 olena.chorna@nationwidechildrens.org |
| Notes |
NCT02346825.
| Trial name or title | The baby CHAMP study (Children With Hemiparesis Arm and Movement Project) (The Baby CHAMP) |
| Methods |
Aim 1: to test the efficacy of 3 different constraint conditions used as part of administering a standardised form of therapy known as ACQUIRE. The 3 constraint conditions are: i) continuous constraint, ii) part‐time constraint, and iii) no constraint Aim 2: to monitor stress levels and safety risks related to use of constraint in the 3 conditions identified above (Aim 1) Design: randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intensive plus cast Children in this group will have 3 hours of daily therapy each weekday for 4 weeks while wearing a full‐arm cast on their stronger arm and hand. Parents will be required to do 45 minutes of daily therapy for which they will be trained Intensive plus splint Children in this group will have 3 hours of daily therapy each weekday for 4 weeks while wearing a part‐time splint on their stronger arm and hand. Parents will be required to do 45 minutes of daily therapy for which they will be trained Intensive no constraint Children in this group will have 3 hours of daily therapy each weekday for 4 weeks but will not wear a constraint. Parents will be required to do 45 minutes of daily therapy for which they will be trained |
| Outcomes |
Primary outcomes
|
| Starting date | January 2014 |
| Contact information | Stephanie C DeLuca, 540‐526‐2098 stephdeluca@vt.edu Laura Bateman, 540‐526‐2033 laurapb2@vt.edu |
| Notes |
NCT02808195.
| Trial name or title | Comparative effectiveness of a Kinect‐based unilateral arm training system vs constraint‐induced therapy for children with cerebral palsy |
| Methods |
Aims: to evaluate the effectiveness of Kinect‐based upper limb motor rehabilitation system (ULMTS) program on motor performance and functional outcomes Design: randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Constraint‐induced therapy Training of the more affected arm and restraint of the less affected arm Kinect‐based constraint‐induced therapy Training of the more affected arm and restraint of the less affected arm by kinect‐game |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | August 2016 |
| Contact information | Hao‐Ling Chen, National Taiwan University Hospital, 886‐2‐3366‐8162, hlchen@ntu.edu.tw |
| Notes |
NCT02840643.
| Trial name or title | Combined constraint therapy and bimanual therapy for children with unilateral brain injury |
| Methods |
Aim: to examine efficacy of combined unimanual and bimanual intensive therapy in children with unilateral brain injury Design: randomised controlled trial with cross‐over |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Bimanual hand therapy Children will receive 90 hours (6 hours/day, 5 days/week, 3 weeks) of intensive bimanual hand therapy, which involves actively using both hands in play‐based activities, games, arts and crafts, and activities of daily living. The different arms of the study will receive blocks of CIMT and bimanual therapy, in different orders Constraint therapy Children will receive 90 hours (6 hours/day, 5 days/week, 3 weeks) of intensive CIMT, which involves actively using the impaired hand in play‐based activities, games, arts and crafts, and activities of daily living. The different arms of the study will receive blocks of CIMT and bimanual therapy, in different orders |
| Outcomes |
Primary Assisting Hand Assessment (Time Frame: Day 1 of Intervention and day 180 of intervention) Secondary
|
| Starting date | July 2011 |
| Contact information | Contact: Kelly Au, OTR/L, Blythedale Children's Hospital, 914‐831‐2459, kellya@blythedale.org |
| Notes | Sponsor: Blythedale Children's Hospital |
NCT02875054.
| Trial name or title | Camp High 5: Evaluation of the effect on upper limb function |
| Methods |
Aim: to evaluate unimanual and bimanual upper‐limb function as well as compare outcomes of varied cast wear in children with hemiplegic CP following a hybrid camp model of mCIMT and hand‐arm bimanual intensive training (HABIT) Design: randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Continued casting Participants with 24‐hour cast wear (continued casting) for the entire duration of the constraint portion of camp (2 initial weeks) Intermittent casting Participants who wear a univalve cast for 3 hours of constraint camp with home exercise program of 2 hours cast wear on the weekends (intermittent casting).Interventions |
| Outcomes |
Primary outcomes
|
| Starting date | June 2016 |
| Contact information | Renat Sukhov, MD, New York University Medical School |
| Notes |
NCT02918890.
| Trial name or title | Intensive unimanual (CIMT) and bimanual training (HABIT) in children with hemiplegia |
| Methods |
Aim: improve the use of the affected hand and quality of overall movement in a fun, social setting Design: randomised controlled trial |
| Participants |
Inclusion Criteria
Exclusion Criteria
|
| Interventions |
Constraint‐induced movement therapy (CIMT) 90 hours Hand‐arm bimanual intensive therapy (HABIT) 90 hours |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | September 2014 |
| Contact information | Claudio Ferre 212‐678‐3332 cpresearch@tc.columbia.edu |
| Notes |
AHA: Assisting Hand Assessment CIMT: Constraint‐induced movement therapy CP: cerebral palsy GMFCS: Gross Motor Function Classification System MACS: Manual Ability Classification System mCIMT: modified CIMT MRI: magnetic resonance imaging PMAL: Pediatric Motor Activity Log PMAL‐R: Pediatric Motor Activity Log revised SD: standard deviation
Differences between protocol and review
The protocol for this review was published in 2002 (Hoare 2002), and the first review in 2007 (Hoare 2007a; Hoare 2007b). Due to changes in methodology for conducting systematic reviews and a substantial increase in the number of included studies, there are several differences between the 2002 protocol, the original 2007 review and this update. These are described below.
-
Review group management
The protocol and 2007 review were managed by the Movement Disorders Reveiw Group. Management has been transferred to the Cochrane Developmental, Psychosocial and Learning Problems Review Group.
-
Authorship
Dr Jason Wasiak stepped down from the authorship team and Dr Margaret Wallen, Ms Megan Thorley and Ms Michelle Jackman joined the authorship team.
-
Due to the large number of CIMT RCTs, we have excluded quasi‐RCTs in this update.
-
In the original 2007 review (Hoare 2007a; Hoare 2007b), we used definitions of CIMT, as described by Taub 2002 [pers comm]. For this update, we used definitions outlined in a more recent expert consensus paper (Eliasson 2014a), which include sCIMT, mCIMT, hCIMT or forced‐use therapy. We use 'CIMT' as an umbrella term to encompass all specific types of CIMT (Eliasson 2014a).
We excluded studies where CIMT was combined with a concurrent intervention and CIMT could not be isolated as defining the intervention group from a comparison group, and studies where CIMT was combined with lower limb intervention.
To achieve the objectives of our review related to intensity of comparison intervention, for this update, we categorised groups according to the total dosage of treatment. Total dose was calculated using the following calculation: therapist‐led intervention + parent‐led intervention + other intervention (e.g. usual care) = total hours of intervention. This included low‐dose, high‐dose and dose‐matched comparisons.
-
In the original 2007 review (Hoare 2007a; Hoare 2007b), we broadly grouped outcome measures according to domains of the International Classification of Functioning, Disability and Health (ICF) (WHO 2001). For this review update, we categorised measures into primary or secondary outcomes, to better reflect the expected effect of CIMT (Eliasson 2014a). The ultimate goal of CIMT is to improve functional performance (self‐care, manual ability, individual performance) that typically requires the use of both hands together (bimanual), so the primary outcomes in this review focused on both bimanual and unimanual function. Secondary measures now include those that CIMT may effect but are not the primary target of intervention.
We also updated the eligibility criteria for outcome measures. We did not include measures if they: 1) did not possess adequate reported validity or reliability for children with CP (or both); 2) were standardised assessments that were invalidated because the administration or scoring was adapted; or 3) both. We listed ineligible measures and the reasons for ineligibility in Table 7.
In the original review (Hoare 2007a; Hoare 2007b), we had adverse events as a secondary outcome. For this update, we moved adverse events to the list of primary outcomes, in line with MECIR standards.
-
Search methods for identification of studies
We updated and amended the search strategy used for the original review (Hoare 2007a; Hoare 2007b, following advice from Cochrane Developmental, Psychosocial and Learning Problems. We searched the following additional databases: MEDLINE In‐Process & Other Non‐Indexed Citations, MEDLINE Epub Ahead of Print, Science Citation Index, PEDro, OTseeker, ClinicalTrial.gov, WHO International Clinical Trials Registry Platform and Australian New Zealand Clinical Trials Registry. We also handsearched the following journals from 2007 onwards: Developmental Medicine and Child Neurology, Physical and Occupational Therapy in Pediatrics, Archives of Physical Medicine and Rehabilitation, Journal of Child Neurology, Journal of Rehabilitation Medicine, Pediatric Physical Therapy, American Journal of Occupational Therapy, NeuroRehabilitation, Clinical Rehabilitation.
-
Data extraction and management
We developed and tailored an updated data extraction form to meet the requirements of the review and new studies.
Due to the large number of studies in this update, five review authors (BH, MW, MJ, MT, CI) independently extracted data from the included trials.
-
Assessment of risk of bias in included studies
In the protocol (Hoare 2002) and 2007 review (Hoare 2007a; Hoare 2007b), tworeview authors independently assessed study quality using an adaptation of the method outlined in Schulz 1995. This is no longer consistent with Cochrane Review methods. For this update, five review authors (including BH, MW, MJ, MT, CI) were paired, allocated included trials and undertook independent assessment of methodological quality of each trial across six domains, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
-
Dichotomous data. We intended to present the relative risk (or risk ratio) with a 95% confidence interval (CI), and calculate the number needed to treat for an additional beneficial outcome as an absolute measure of treatment effect (Hoare 2002). For this review update, we decided to report the odds ratio (OR) with a 95% CI, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), as most studies with a dichotomous outcome report the OR. However, no included outcome measures in the review update reported dichotomous data.
-
Cross‐over trials. The 2002 protocol did not address the issue of cross‐over trials (Hoare 2002). We did not consider cross‐over designs to be a suitable method for children with CP as CIMT is likely to have a lasting effect, which will carry over into the cross‐over period (Charles 2006). For this review update, we only included data from the first intervention period for RCTs using a cross‐over design, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Studies with multiple treatment groups. The 2002 protocol did not address the issue of multiple treatment groups in a single trial (Hoare 2002), and the 2007 review did not include studies with three or more groups (Hoare 2007a; Hoare 2007b). For this update, when a trial included three or more groups, we planned to consider the nature of the intervention and control arms, and where appropriate, combine the data from two treatment arms that were similar and had the same control group, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, section 16.5.4, section 7.7.3.8 and Table 7.7a (Higgins 2011c). For this review update, we deemed no treatment arms in studies with multiple treatment groups to be similar (Dong 2017; Facchin 2011; Kirton 2016a (CIMT + r TMS); Xu 2012; Rostami 2012b). Therefore, combining data from the two treatment arms was not appropriate.
-
We did not address the issue of assessment of heterogeneity in the 2002 protocol (Hoare 2002), but did for this review update because it is important to consider to what extent the results of studies are consistent. We considered the Tau2 statistic for each meta‐analysis, and compared the magnitude of heterogeneity with the distribution values for general physical health and adverse event and pain and quality of life/functioning – nonpharmacologic (median = 0.05, 95% CI 0.00 to 4.00). We considered heterogeneity in the meta‐analysis to be substantial when the Tau2 value was greater than 0.05 (Rhodes 2015).
-
Assessment of reporting biases
There were insufficient studies with similar outcomes included in this review to investigate publication bias and other small‐study effects using statistical methods or funnel plots. See Table 9.
-
In the original 2007 review (Hoare 2007a; Hoare 2007b), we planned to calculate pooled effects using a fixed‐effect model across trials using the same outcome in similar populations (Hoare 2002). Due to the limited number of included studies in 2007, no pooled analysis were possible. For this update, we used a random‐effects model, as we could not assume the effects being estimated in the different studies were identical due to the nature of CIMT provided (e.g. difference in treatment dosage, restraint type etc.). Also, where pooling of data was not possible within a meta‐analysis (i.e. outcome data only available from a single study), we presented data from the treatment and comparison groups (mean, SD) and mean difference (MD) (95% CI) in tables, to facilitate a narrative description of the results.
For this update, we assessed the overall quality of the evidence associated with the result of each meta‐analysis using the GRADE approach (GradePro GDT 2015). We summarised the effect estimates and GRADE ratings for our primary outcomes in a 'Summary of findings' table. The GRADE approach was not developed when the protocol for this review was developed (Hoare 2002), and was not a requirement during development of the original review in 2007 (Hoare 2007a; Hoare 2007b).
-
Subgroup analysis and investigation of heterogeneity
The 2002 protocol did not address the issue of subgroup analysis (Hoare 2002). For this review update, we planned to conduct a number of subgroup analyses to establish whether there is a different effect of CIMT on child or intervention characteristics (Table 9), but could not due to the small number of studies in each comparison.
-
At the time of the original 2007 review (Hoare 2007a; Hoare 2007b), we had no plans to conduct a sensitivity analysis due to the limited of trials. For this review update, we assessed the influence of our analysis model by re‐analysing data using a fixed‐effect model instead of a random‐effects model for all outcomes included in a pooled analyses (Sterne 2011), to examine how the results of the meta‐analysis change under these different analysis models. We had also planned to explore the possible causes of heterogeneity, where this was substantial (I2 > 50%), in a sensitivity analysis, but did not.
Contributions of authors
Brian Hoare: guarantor, conceived the review, designed the review, co‐ordinated the review, designed the search strategy, developed the inclusion/exclusion criteria, searched the literature, screened retrieved papers against inclusion criteria, organised retrieval of papers, selected studies, appraised quality of papers, extracted data from papers, sent additional requests for data to authors of included trials, entered data into Review Manager 5 (Review Manager 2014), analysed the data, interpreted the data, managed the data, and contributed to writing the review.
Margaret Wallen (co‐primary author): gurantor, designed the review, development of inclusion/exclusion criteria, screening search results, screening retrieved papers against inclusion criteria, study selection, appraising quality of papers, extracting data from papers, additional data requests for included trials, data analysis, interpretation of data, data management for the review, and contributed to writing the review.
Megan Thorley: extracted data from papers, and appraised the quality of papers.
Michelle Jackman: extracted data from papers, and appraised the quality of papers.
Leeanne Carey: contributed to writing the review.
Christine Imms: extracted data from papers, appraised the quality of papers, interpreted the data, and contributed to writing the review.
Sources of support
Internal sources
-
Cerebral Palsy Alliance, Australia.
BH received a Career Development Grant from the Cerebral Palsy Alliance (CDG9116) in 2017. Part of these funds were used to support the preparation of this update.
External sources
None, Other.
Declarations of interest
Brian Hoare* is employed by Monash Health. In 2014, he received an honorarium from Allergan Australia for travel to Sri Lanka as part of a multi‐disciplinary team to teach and train local physicians and therapists in the management of the upper limb in children with CP. The honoraria covered flights and accommodation for the trip, which were paid for directly by Allergan. This update does not review products manufactured by Allergan and Brian Hoare has no personal financial interest in Allergan, Botox®, or any related product.
*Brian Hoare, Christine Imms and Leeanne Carey are authors on the included study Hoare 2013, and were not involved in assessing the eligibility of this study for inclusion, extracting data from this study for purposes of this review, assessing the risk of bias in this study, or grading the quality of the evidence from this study.
Margaret Wallen is an author on the included study Wallen 2011 and was not involved in assessing the eligibility of this study for inclusion, extracting data from this study, assessing the risk of bias in this study, or grading the quality of the evidence from this study.
Megan Thorley ‐ none known.
Michelle Jackman ‐ none known.
Leeanne Carey* ‐ none known.
Christine Imms* is employed by the Australian Catholic University (ACU). ACU has provided support to CI for travel to conferences in which presentations were made about research in CP. CI received a philanthropic travel grant and other support from her university employer for unrelated studies.
Shared 1st authorship
Edited (no change to conclusions)
References
References to studies included in this review
Aarts 2010 {published and unpublished data}
- Aarts PB, Burg J, Oostendorp RA. Forced use applied to the affected upper limb of children with cerebral palsy. Developmental Medicine and Child Neurology 2005;47(s103):3. [DOI: 10.1111/j.1469-8749.2005.tb12573.x] [DOI] [Google Scholar]
- Aarts PB, Hartingsveldt M, Anderson PG, Tillaar I, Burg J, Geurts AC. The pirate group intervention protocol: description and a case report of a modified constraint‐induced movement therapy combined with bimanual training for young children with unilateral spastic cerebral palsy. Occupational Therapy International 2012;19(2):76‐87. [DOI: 10.1002/oti.321; PUBMED: 21751275 ] [DOI] [PubMed] [Google Scholar]
- Aarts PB, Jongerius PH, Geerdink YA, Limbeek J, Geurts AC. Effectiveness of modified constraint‐induced movement therapy in children with unilateral spastic cerebral palsy: a randomized controlled trial. Neurorehabilitation and Neural Repair 2010;24(6):509‐18. [DOI: 10.1177/1545968309359767; PUBMED: 20424191 ] [DOI] [PubMed] [Google Scholar]
- Aarts PB, Jongerius PH, Geerdink YA, Limbeek J, Geurts AC. Modified constraint‐induced movement therapy combined with bimanual braining (mCIMT‐BiT) in children with unilateral spastic cerebral palsy: how are improvements in arm‐hand use established?. Research in Developmental Disabilities 2010;32(1):271‐9. [DOI: 10.1016/j.ridd.2010.10.008.; PUBMED: 21051191 ] [DOI] [PubMed] [Google Scholar]
- Crajé C, Aarts PB, Nijhuis‐van der Sanden M, Steenbergen B. Action planning in typically and atypically developing children (unilateral cerebral palsy). Research in Developmental Disabilities 2010;31(5):1039‐46. [DOI: 10.1016/j.ridd.2010.04.007; PUBMED: 20451346] [DOI] [PubMed] [Google Scholar]
- Geerdink Y, Aarts PB, Geurts AC. Motor learning curve and long‐term effectiveness of modified constraint‐induced movement therapy in children with unilateral cerebral palsy: a randomized controlled trial. Research in Developmental Disabilities 2013;34(3):923‐31. [DOI: 10.1016/j.ridd.2012.11.011; PUBMED: 23291509 ] [DOI] [PubMed] [Google Scholar]
Abd El‐Kafy 2014 {published data only}
- Abd El‐Kafy EM, Elshemy SA, Alghamdi MS. Effect of constraint‐induced therapy on upper limb functions: a randomized control trial. Scandinavian Journal of Occupational Therapy 2014;21(1):11‐23. [DOI: 10.3109/11038128.2013.837505; PUBMED: 24325594] [DOI] [PubMed] [Google Scholar]
Abootalebi 2010 {published and unpublished data}
- Abootalebi S, Khoshnevisan A, Kohan AH, Pishyareh E, Rahgozar M. The effects of "constraint‐induced movement therapy" on fine motor skills in children with hemiplegic cerebral palsy. Tehran University Medical Journal 2010;68(2):128‐36. [EMBASE: 2010402289; tumj.tums.ac.ir/article‐1‐375‐en.html] [Google Scholar]
Al‐Oraibi 2011 {published and unpublished data}
- Al‐Oraibi S, Eliasson AC. Implementation of constraint‐induced movement therapy for young children with unilateral cerebral palsy in Jordan: a home‐based model. Disability and Rehabilitation 2011;33(21‐22):2006‐12. [DOI: 10.3109/09638288.2011.555594; PUBMED: 21332299 ] [DOI] [PubMed] [Google Scholar]
Charles 2006 {published and unpublished data}
- Charles JR, Gordon AM. A repeated course of constraint‐induced movement therapy results in further improvement. Developmental Medicine and Child Neurology 2007;49(10):770‐3. [DOI: 10.1111/j.1469-8749.2007.00770.x; NCT00305006; PUBMED: 17880647] [DOI] [PubMed] [Google Scholar]
- Charles JR, Wolf SL, Schneider JA, Gordon AM. Efficacy of a child‐friendly form of constraint‐induced movement therapy in hemiplegic cerebral palsy: a randomized control trial. Developmental Medicine and Child Neurology 2006;48(8):635‐42. [DOI: 10.1017/S0012162206001356; NCT00305006; PUBMED: 16836774] [DOI] [PubMed] [Google Scholar]
Chen 2014 {published and unpublished data}
- Chen CL, Kang LJ, Hong WH, Chen FC, Chen HC, Wu CY. Effect of therapist‐based constraint‐induced therapy at home on motor control, motor performance and daily function in children with cerebral palsy: a randomized controlled study. Clinical Rehabilitation 2013;27(3):236‐45. [DOI: 10.1177/0269215512455652; NCT01076257; PUBMED: 22952304] [DOI] [PubMed] [Google Scholar]
- Chen CL, Lin KC, Kang LJ, Wu CY, Chen HC, Hsieh YW. Potential predictors of functional outcomes after home‐based constraint‐induced therapy for children with cerebral palsy. American Journal of Occupational Therapy 2014;68(2):159‐66. [DOI: 10.5014/ajot.2014.009860; NCT01076257; PUBMED: 24581402] [DOI] [PubMed] [Google Scholar]
- Chen HC, Chen CL, Kang LJ, Wu CY, Chen FC, Hong WH. Improvement of upper extremity motor control and function after home‐based constraint‐induced therapy in children with unilateral cerebral palsy: immediate and long‐term effects. Archives of Physical Medicine and Rehabilitation 2014;95(8):1423‐32. [CENTRAL: CN‐00998721; DOI: 10.1016/j.apmr.2014.03.025; EMBASE: 2014508144; NCT01076257; PUBMED: 24742939] [DOI] [PubMed] [Google Scholar]
- Chen HC, Kang LJ, Chen CL, Lin KC, Chen FC, Wu KP. Younger children with cerebral palsy respond better than older ones to therapist‐based constraint induced therapy at home on functional outcomes and motor control. Physical & Occupational Therapy in Pediatrics 2016;36(2):171‐85. [DOI: 10.3109/01942638.2015.1101042; NCT01076257; PUBMED: 26643052] [DOI] [PubMed] [Google Scholar]
- Hsin YJ, Chen FC, Lin KC, Kang LJ, Chen CL, Chen CY. Efficacy of constraint‐induced therapy on functional performance and health‐related quality of life for children with cerebral palsy: a randomized controlled trial. Journal of Child Neurology 2012;27(8):992‐9. [DOI: 10.1177/0883073811431011; NCT01076257; PUBMED: 22241704] [DOI] [PubMed] [Google Scholar]
Choudhary 2013 {published data only}
- Choudhary A, Gulati S, Kabra M, Singh UP, Sankhyan N, Pandey RM, et al. Efficacy of modified constraint induced movement therapy in improving upper limb function in children with hemiplegic cerebral palsy: a randomized controlled trial. Brain & Development 2013;35(9):870‐6. [DOI: 10.1016/j.braindev.2012.11.001; CTRI/2008/091/000231; PUBMED: 23238223] [DOI] [PubMed] [Google Scholar]
Christmas 2018 {published and unpublished data}
- Christmas PM. A Randomised Controlled Trial and Systematic Review Comparing Two Methods of Constraint Induced Movement Therapy to Improve Upper Limb Function in Pre‐School Children with Hemiplegic Cerebral Palsy [PhD thesis]. Birmingham (UK): University of Birmingham, 2015. [etheses.bham.ac.uk/6571/4/Christmas16PhD.pdf] [Google Scholar]
- Christmas PM, Sackley C, Feltham MG, Cummins C. A randomized controlled trial to compare two methods of constraint‐induced movement therapy to improve functional ability in the affected upper limb in pre‐school children with hemiplegic cerebral palsy: CATCH TRIAL. Clinical Rehabilitation 2018;32(7):909‐18. [DOI: 10.1177/0269215518763; PUBMED: 29552921] [DOI] [PubMed] [Google Scholar]
de Brito Brandão 2010 {published and unpublished data}
- Brandão MD, Gordon A, Mancini MC. The effects of constraint‐induced movement therapy (CIMT) and hand‐arm bimanual intensive training (HABIT) on the daily functioning of children with cerebral palsy. Developmental Medicine & Child Neurology 2011;53(Suppl 5):30. [DOI: 10.1111/j.1469-8749.2011.04112.x] [DOI] [Google Scholar]
- Mancini MC, Brandão MB, Dupin A, Drummond AF, Chagas PSC, Assis MG. How do children and caregivers perceive their experience of undergoing the CIMT protocol?. Scandinavian Journal of Occupational Therapy 2013;20(5):343‐8. [DOI: 10.3109/11038128.2013.799227; PUBMED: 23713691 ] [DOI] [PubMed] [Google Scholar]
- Brito Brandão M, Mancini MC, Vaz DV, Pereira de Melo AP, Fonseca ST. Adapted version of constraint‐induced movement therapy promotes functioning in children with cerebral palsy: a randomized controlled trial. Clinical Rehabilitation 2010;24(7):639‐47. [DOI: 10.1177/0269215510367974; PUBMED: 20530645] [DOI] [PubMed] [Google Scholar]
DeLuca 2012 {published data only}
- Case‐Smith J, DeLuca SC, Stevenson R, Ramey S. A multi‐center randomized controlled trial of pediatric constraint‐induced movement therapy: 6‐month follow‐up. Developmental Medicine & Child Neurology 2011;53(Suppl 5):28‐9. [DOI: 10.1111/j.1469-8749.2011.04112.x; E4; NCT00991692] [DOI] [Google Scholar]
- Case‐Smith J, DeLuca SC, Stevenson R, Ramey SL. Multicenter randomized controlled trial of pediatric constraint‐induced movement therapy: 6‐month follow‐up. American Journal of Occupational Therapy 2012;66(1):15‐23. [DOI: 10.5014/ajot.2012.002386; NCT00991692; PUBMED: 22389937] [DOI] [PubMed] [Google Scholar]
- DeLuca S, Case‐Smith J, Echolls K, Lowenhaupt S, Lowes L, Ramey SL, et al. A multicenter clinical trial of pediatric constraint‐Induced therapy in children with CP: what is the dose?. Developmental Medicine & Child Neurology 2010;52(Suppl 5):25‐6. [DOI: 10.1111/j.1469-8749.2010.03755.x; NCT00991692] [DOI] [Google Scholar]
- DeLuca SC, Case‐Smith J, Stevenson R, Ramey SL. Constraint‐induced movement therapy (CIMT) for young children with cerebral palsy: effects of therapeutic dosage. Journal of Pediatric Rehabilitation Medicine 2012;5(2):133‐42. [DOI: 10.3233/PRM-2012-0206; NCT00991692; PUBMED: 22699104] [DOI] [PubMed] [Google Scholar]
Deppe 2013 {published data only}
- Deppe W, Thuemmler K, Fleischer J, Berger C, Meyer S, Wiedemann B. Modified constraint‐induced movement therapy versus intensive bimanual training for children with hemiplegia ‐ a randomized controlled trial. Clinical Rehabilitation 2013;27(10):909‐20. [CENTRAL: CN‐00996843; DOI: 10.1177/0269215513483764; PUBMED: 23818409 ] [DOI] [PubMed] [Google Scholar]
- Deppe W, Thümmler K, Fleischer J, Berger C, Pelz S. Constraint‐induced movement therapy compared with bimanual therapy in hemiparetic children ‐ what is (more) effective?. European Journal of Paediatric Neurology 2010;14(6):547. [DOI: 10.1016/j.ejpn.2010.09.001] [DOI] [Google Scholar]
- Deppe W, Thümmler K, Fleischer J, Berger C, Pelz S. Constraint‐induced movement therapy versus equally intensive bimanual training for children with central hemiparesis: a comparative study. Developmental Medicine & Child Neurology 2010;52(Suppl 5):27‐8. [DOI: 10.1111/j.1469-8749.2010.03755.x; EMBASE: 70324831] [DOI] [Google Scholar]
Dong 2017 {published and unpublished data}
- Dong VA, Fong KN, Chen YF, Tseng SS, Wong LM. 'Remind‐to‐move' treatment versus constraint‐induced movement therapy for children with hemiplegic cerebral palsy: a randomized controlled trial. Developmental Medicine & Child Neurology 2017;59(2):160‐7. [DOI: 10.1111/dmcn.13216; PUBMED: 27503605] [DOI] [PubMed] [Google Scholar]
- Fong KN, Jim ES, Dong VA, Cheung HK. 'Remind to move': a pilot study on the effects of sensory cueing treatment on hemiplegic upper limb functions in children with unilateral cerebral palsy. Clinical Rehabilitation 2013;27(1):82‐9. [DOI: 10.1177/0269215512448199; PUBMED: 22801471 ] [DOI] [PubMed] [Google Scholar]
Eliasson 2011 {published and unpublished data}
- Eliasson A‐C, Shaw K, Berg E, Krumlinde‐Sundholm L. An ecological approach of constraint induced movement therapy for 2‐3‐year‐old children: a randomized control trial. Research in Developmental Disabilities 2011;32(6):2820‐8. [DOI: 10.1016/j.ridd.2011.05.024; PUBMED: 21700416] [DOI] [PubMed] [Google Scholar]
Eliasson 2018 {published data only}
- Eliasson A‐C, Nordstrand L, Ek L, Lennartsson F, Sjöstrand L, Tedroff K, et al. The effectiveness of Baby‐CIMT in infants younger than 12 months with clinical signs of unilateral‐cerebral palsy: an explorative study with randomized design. Research in Developmental Disabilities 2018;72:191‐201. [DOI: 10.1016/j.ridd.2017.11.006; PUBMED: 29175749] [DOI] [PubMed] [Google Scholar]
- Eliasson A‐C, Sjöstrand L, Ek L, Krumlinde‐Sundholm L, Tedroff K. Efficacy of baby‐CIMT: study protocol for a randomised controlled trial on infants below age 12 months, with clinical signs of unilateral CP. BMC Pediatrics 2014;14:141. [DOI: 10.1186/1471-2431-14-141; PMC4062504; PUBMED: 24903062] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrand L, Holmefur M, Kits A, Eliasson A‐C. Improvements in bimanual hand function after baby‐CIMT in two‐year old children with unilateral cerebral palsy: a retrospective study. Research in Developmental Disabilities 2015;41‐42:86‐93. [DOI: 10.1016/j.ridd.2015.05.003; PUBMED: 26100242] [DOI] [PubMed] [Google Scholar]
Eugster‐Buesch 2012 {published data only}
- Eugster‐Buesch F, Bruin ED, Boltshauser E, Steinlin M, Küenzle C, Müller E, et al. Forced‐use therapy for children with cerebral palsy in the community setting: a single‐blinded randomized controlled pilot trial. Journal of Pediatric Rehabilitation Medicine 2012;5(2):65‐74. [DOI: 10.3233/PRM-2012-0198; PUBMED: 22699097] [DOI] [PubMed] [Google Scholar]
Facchin 2011 {published and unpublished data}
- Facchin P, Rosa‐Rizzotto M, Turconi AC, Pagliano E, Fazzi E, Stortini M, et al. Multisite trial on efficacy of constraint‐induced movement therapy in children with hemiplegia: study design and methodology. American Journal of Physical Medicine & Rehabilitation 2009;88(3):216‐30. [DOI: 10.1097/PHM.0b013e3181951382; PUBMED: 19847131] [DOI] [PubMed] [Google Scholar]
- Facchin P, Rosa‐Rizzotto M, Visonà Dalla Pozza L, Turconi AC, Pagliano E, Signorini S, et al. Multisite trial comparing the efficacy of constraint‐induced movement therapy with that of bimanual intensive training in children with hemiplegic cerebral palsy: postintervention results. American Journal of Physical Medicine & Rehabilitation 2011;90(7):539‐53. [DOI: 10.1097/PHM.0b013e3182247076; PUBMED: 21765273] [DOI] [PubMed] [Google Scholar]
- Fedrizzi E, Rosa‐Rizzotto M, Turconi AC, Pagliano E, Fazzi E, Pozza LV, et al. Unimanual and bimanual intensive training in children with hemiplegic cerebral palsy and persistence in time of hand function improvement: 6‐month follow‐up results of a multisite clinical trial. Journal of Child Neurology 2013;28(2):161‐75. [CENTRAL: CN‐00969460; DOI: 10.1177/0883073812443004; PUBMED: 22580904] [DOI] [PubMed] [Google Scholar]
- Rosa‐Rizzotto M, Visonà Dalla Pozza L, Turconi AC, Facchin P, Fedrizzi E. Constraint‐induced movement therapy vs bimanual intensive training in hemiplegic cerebral palsy: preliminary results. European Journal of Paediatric Neurology 2010;14(6):547‐8. [Google Scholar]
- Rosa‐Rizzotto M, Visonà Dalla Pozza L, Turconi AC, Tornetta L, Andreucci E, Zambonin F, et al. GIPCI Study Group. The perception of involved professionals towards research feasibility and usefulness: lessons from the multi‐site trial on efficacy of constraint induced movement therapy in children with hemiplegia. European Journal of Physical and Rehabilitation Medicine 2010;46(3):369‐76. [PUBMED: 20927003] [PubMed] [Google Scholar]
- Turconi AC, Diella E. The constraint therapy in the hemiplegic child: from research to clinical practice [La constraint therapy nel bambino emiplegico: dalla ricerca alla pratica clinica]. MR Giornale Italiano di Medicina Riabilitativa 2011;25(2/3):105‐9. [www.minervamedica.it/it/riviste/medicina‐riabilitativa/articolo.php?cod=R47Y2011N02A0105] [Google Scholar]
Gelkop 2015 {published and unpublished data}
- Gelkop N, Burshtein DG, Lahav A, Brezner A, Al‐Oraibi S, Ferre CL, et al. Efficacy of constraint‐induced movement therapy and bimanual training in children with hemiplegic cerebral palsy in an educational setting. Physical & Occupational Therapy in Pediatrics 2015;35(1):24‐39. [DOI: 10.3109/01942638.2014.925027; PUBMED: 24983295] [DOI] [PubMed] [Google Scholar]
Gharib 2010 {published data only}
- Gharib M, Hosseyni A, Fahimmi N, Salehi M. Effect of modified constraint induced movement therapy on quality of upper extremity skills in children with hemiplegic cerebral palsy. Journal of Gorgan University of Medical Sciences 2010;12(3):29‐36. [goums.ac.ir/journal/article‐1‐776‐en.pdf] [Google Scholar]
Gordon 2011 {published and unpublished data}
- Gordon AM, Hung YC, Brandao M, Ferre CL, Kuo HC, Friel K, et al. Bimanual training and constraint‐induced movement therapy in children with hemiplegic cerebral palsy: a randomized trial. Neurorehabilitation and Neural Repair 2011;25(8):692‐702. [DOI: 10.1177/1545968311402508; PUBMED: 21700924] [DOI] [PubMed] [Google Scholar]
- Hung YC, Casertano L, Hillman A, Gordon AM. The effect of intensive bimanual training on coordination of the hands in children with congenital hemiplegia. Research in Developmental Disabilities 2011;32(6):2724‐31. [DOI: 10.1016/j.ridd.2011.05.038; PUBMED: 21715141] [DOI] [PubMed] [Google Scholar]
- Brito Brandão M, Gordon AM, Mancini MC. Functional impact of constraint therapy and bimanual training in children with cerebral palsy: a randomized controlled trial. American Journal of Occupational Therapy 2012;66(6):672‐81. [DOI: 10.5014/ajot.2012.004622; PUBMED: 23106987] [DOI] [PubMed] [Google Scholar]
- Brito Brandão M, Gordon AM, Mancini MC. The effects of constraint‐induced movement therapy (CIMT) and hand‐arm bimanual intensive training (HABIT) on the daily functioning of children with cerebral palsy. Developmental Medicine & Child Neurology 2011;53(Suppl 5):30. [DOI: 10.1111/j.1469-8749.2011.04112.x] [DOI] [Google Scholar]
Hoare 2013 {published and unpublished data}
- Hoare B, Imms C, Villanueva E, Rawicki HB, Matyas T, Carey L. Intensive therapy following upper limb botulinum toxin A injection in young children with unilateral cerebral palsy: a randomized trial. Developmental Medicine & Child Neurology 2013;55(3):238‐47. [DOI: 10.1111/dmcn.12054; EMBASE: 23236956; ACTRN12605000002684; PUBMED: 23236956] [DOI] [PubMed] [Google Scholar]
- Hoare BJ, Imms C, Rawicki HB, Carey L. Modified constraint‐induced movement therapy or bimanual occupational therapy following injection of botulinum toxin‐A to improve bimanual performance in young children with hemiplegic cerebral palsy: a randomised controlled trial methods paper. BMC Neurology 2010;10:58. [DOI: 10.1186/1471-2377-10-58; ACTRN12605000002684; PMC2909943; PUBMED: 20602795] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosseini 2010 {published data only}
- Hossein SM, Sourtiji H, Taghizadeh A. Effect of child friendly constraint induced movement therapy on unimanual and bimanual function in hemiplegia. Iranian Rehabilitation Journal 2010;8(12):50‐4. [irj.uswr.ac.ir/article‐1‐193‐en.pdf] [Google Scholar]
- Sourtiji H, Hosseini SM, Mohamadian F. Effectiveness of ICF‐based modified constraint induced movement therapy on hand functions in children with hemiplegic cerebral palsy. Journal of Research in Rehabilitation Sciences 2011;7(Suppl 5):613‐20. [www.sid.ir/En/Journal/ViewPaper.aspx?ID=256550] [Google Scholar]
Kirton 2016a (CIMT + r TMS) {published and unpublished data}
- Kirton A, Andersen J, Herrero M, Carsolio L, Nettel‐Aguirre A, Keess J, et al. Brain stimulation and constraint for perinatal stroke hemiparesis: the PLASTIC CHAMPS trial. European Journal of Paediatric Neurology 2015;19(Suppl 1):S10. [DOI: 10.1016/S1090-3798(15)30030-1; NCT01189058; OP29 – 2815] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirton A, Andersen J, Herrero M, Nettel‐Aguirre A, Carsolio L, Damji O, et al. Brain stimulation and constraint for perinatal stroke hemiparesis: the PLASTIC CHAMPS trial. Neurology 2016;86(18):1659‐67. [DOI: 10.1212/WNL.0000000000002646; 27029628; NCT01189058; PMC4854585] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirton 2016b (CIMT + sham TMS) {published and unpublished data}
- Kirton A, Andersen J, Herrero M, Nettel‐Aguirre A, Carsolio L, Damji O, et al. Brain stimulation and constraint for perinatal stroke hemiparesis: the PLASTIC CHAMPS trial. Neurology 2016;86(18):1659‐67. [DOI: 10.1212/WNL.0000000000002646; NCT01189058; PMC4854585 ; PUBMED: 27029628] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rostami 2012a {published and unpublished data}
- Rostami HR, Malamiri RA. Effect of treatment environment on modified constraint‐induced movement therapy results in children with spastic hemiplegic cerebral palsy: a randomized controlled trial. Disability and Rehabilitation 2012;34(1):40‐4. [DOI: 10.3109/09638288.2011.585214; PUBMED: 21851293] [DOI] [PubMed] [Google Scholar]
Rostami 2012b {published and unpublished data}
- Rostami HR, Arastoo AA, Nejad SJ, Mahany MK, Malamiri, RA, Goharpey S. Effects of modified constraint‐induced movement therapy in virtual environment on upper‐limb function in children with spastic hemiparetic cerebral palsy: a randomised controlled trial. NeuroRehabilitation 2012;31(4):357–65. [DOI: 10.3233/NRE-2012-00804; PUBMED: 23232158] [DOI] [PubMed] [Google Scholar]
- Rostami HR, Arastoo AA, Nejad SJ, Malamiri RA, Mahany MK, Goharpey S. Efficacy of combined virtual reality with constraint‐induced movement therapy on upper limb function of children with hemiparetic cerebral palsy. Journal of Research in Rehabilitation Sciences 2011;7(4):499‐508. [www.sid.ir/En/Journal/ViewPaper.aspx?ID=255960] [Google Scholar]
Sabour 2012 {published data only}
- Sabour HE, Rassafiani M, Hosseini SA, Akbarfahimi N, Karimloo M. The effect of combination of constraint induced movement therapy with bimanual intensive therapy on upper limb function of children with hemiplegic cerebral palsy. Journal of Research in Rehabilitation Sciences 2012;8(Suppl 8):1312‐8. [sid.ir/En/Journal/ViewPaper.aspx?ID=350278] [Google Scholar]
Sakzewski 2011 {published and unpublished data}
- Boyd R, Sakzewski L, Ziviani J, Abbott DF, Badawy R, Gilmore R, et al. INCITE: a randomised trial comparing constraint induced movement therapy and bimanual training in children with congenital hemiplegia. BMC Neurology 2010;10:4. [DOI: 10.1186/1471-2377-10-4; ACTRN12609000912280; PMC2832893 ; PUBMED: 20064275] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R, Ziviani J, Sakzewski L, Shields N, Boyd RN. A balancing act: children's experience of modified constraint‐induced movement therapy. Developmental Neurorehabilitation 2010;13(2):88‐94. [DOI: 10.3109/17518420903386161; ACTRN12609000912280; PUBMED: 20222769] [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Carlon S, Shields N, Ziviani J, Boyd R. Impact of intensive upper limb rehabilitation on quality of life in a randomised trial for children with congenital hemiplegia. Developmental Medicine & Child Neurology 2012;54(Suppl 5):28‐9. [DOI: 10.1111/j.1469-8749.2012.04289.x; ACTRN12609000912280; F2.G3] [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Carlon S, Shields N, Ziviani J, Ware RS, Boyd RN. Impact of intensive upper limb rehabilitation on quality of life: a randomized trial in children with unilateral cerebral palsy. Developmental Medicine & Child Neurology 2012;54(5):415‐23. [DOI: 10.1111/j.1469-8749.2012.04272.x; ACTRN12609000912280; PUBMED: 22429002] [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Ziviani J, Abbott DF, Macdonell RA, Jackson GD, Boyd RN. Equivalent retention of gains at 1 year after training with constraint‐induced or bimanual therapy in children with unilateral cerebral palsy. Neurorehabilitation and Neural Repair 2011;25(7):664‐71. [DOI: 10.1177/1545968311400093; ACTRN12609000912280; PUBMED: 21427273] [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Ziviani J, Abbott DF, Macdonell RA, Jackson GD, Boyd RN. Participation outcomes in a randomized trial of 2 models of upper‐limb rehabilitation for children with congenital hemiplegia. Archives of Physical Medicine and Rehabilitation 2011;92(4):531‐9. [DOI: 10.1016/j.apmr.2010.11.022; ACTRN12609000912280; PUBMED: 21440700] [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Ziviani J, Abbott DF, Macdonell RA, Jackson GD, Boyd RN. Randomized trial of constraint‐induced movement therapy and bimanual training on activity outcomes for children with congenital hemiplegia. Developmental Medicine & Child Neurology 2011;53(4):313‐20. [DOI: 10.1111/j.1469-8749.2010.03859.x; ACTRN12609000912280; PUBMED: 21401585] [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Ziviani J, Boyd R. The relationship between unimanual capacity and bimanual performance in children with congenital hemiplegia. Developmental Medicine & Child Neurology 2010;52(9):811‐6. [DOI: 10.1111/j.1469-8749.2009.03588.x; ACTRN12609000912280; PUBMED: 20132142] [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Ziviani J, Boyd RN. A randomized trial of novel upper limb rehabilitation in children with congenital hemiplegia. Developmental Medicine & Child Neurology 2010;52(Suppl 2):8‐9. [DOI: 10.1111/j.1469-8749.2009.03595.x; ACTRN12609000912280; PhD.3] [DOI] [Google Scholar]
- Sakzewski L, Ziviani J, Boyd RN. Best responders after intensive upper‐limb training for children with unilateral cerebral palsy. Archives of Physical Medicine and Rehabilitation 2011;92(4):578‐84. [DOI: 10.1016/j.apmr.2010.12.003; ACTRN12609000912280; PUBMED: 21440702 ] [DOI] [PubMed] [Google Scholar]
Sakzewski 2015a {published and unpublished data}
- Boyd RN, Ziviani J, Sakzewski L, Miller L, Bowden J, Cunnington R, et al. COMBIT: protocol of a randomised comparison trial of COMbined modified constraint induced movement therapy and bimanual intensive training with distributed model of standard upper limb rehabilitation in children with congenital hemiplegia. BMC Neurology 2013;13:68. [DOI: 10.1186/1471-2377-13-68; ACTRN12613000181707; PMC3750247; PUBMED: 23809257] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L, Ziviani J, Ware RS, Boyd RN. Does context matter? Mastery motivation and therapy engagement of children with cerebral palsy. Physical & Occupational Therapy in Pediatrics 2016;36(2):155‐70. [DOI: 10.3109/01942638.2015.1076556; ACTRN12613000181707; PUBMED: 26565438] [DOI] [PubMed] [Google Scholar]
- Miller L, Ziviani J, Ware RS, Boyd RN. Mastery motivation as a predictor of occupational performance following upper limb intervention for school‐aged children with congenital hemiplegia. Developmental Medicine & Child Neurology 2014;56(10):976‐83. [DOI: 10.1111/dmcn.12471; ACTRN12613000181707; PUBMED: 24766637] [DOI] [PubMed] [Google Scholar]
- Miller L, Ziviani J, Ware RS, Boyd RN. Mastery motivation in children with congenital hemiplegia: individual and environmental associations. Developmental Medicine & Child Neurology 2014;56(3):267‐74. [DOI: 10.1111/dmcn.12356; PUBMED: 24341437] [DOI] [PubMed] [Google Scholar]
- Miller L, Ziviani J, Ware RS, Boyd RN. Mastery motivation: a way of understanding therapy outcomes for children with unilateral cerebral palsy. Disability and Rehabilitation 2015;37(16):1439‐45. [DOI: 10.3109/09638288.2014.964375; ACTRN12613000181707; PUBMED: 25259559 ] [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Miller L, Ziviani J, Abbott DF, Rose S, Macdonell RA, et al. Randomized comparison trial of density and context of upper limb intensive group versus individualized occupational therapy for children with unilateral cerebral palsy. Developmental Medicine & Child Neurology 2015;57(6):539‐47. [DOI: 10.1111/dmcn.12702; PUBMED: 25627092] [DOI] [PubMed] [Google Scholar]
Sakzewski 2015b {published and unpublished data}
- Sakzewski L, Provan K, Ziviani J, Boyd RN. Comparison of dosage of intensive upper limb therapy for children with unilateral cerebral palsy: how big should the therapy pill be?. Research in Developmental Disabilities 2015;37:9‐16. [DOI: 10.1016/j.ridd.2014.10.050; PUBMED: 25460215] [DOI] [PubMed] [Google Scholar]
Smania 2009 {published data only}
- Smania N, Aglioti SM, Cosentino A, Camin M, Gandolfi M, Tinazzi M, et al. A modified constraint‐induced movement therapy (CIT) program improves paretic arm use and function in children with cerebral palsy. European Journal of Physical and Rehabilitation Medicine 2009;45(4):493‐500. [NCT00473447; PUBMED: 20032907] [PubMed] [Google Scholar]
Sung 2005 {published data only}
- Sung IY, Ryu JS, Pyun SB, Yoo SD, Song WH, Park MJ. Efficacy of forced‐use therapy in hemiplegic cerebral palsy. Archives of Physical Medicine and Rehabilitation 2005;86(11):2195‐8. [DOI: 10.1016/j.apmr.2005.05.007; PUBMED: 16271570] [DOI] [PubMed] [Google Scholar]
Taub 2004 {published and unpublished data}
- DeLuca S. Intensive Movement Therapy with Casting for Children with Hemiparetic Cerebral Palsy: A Randomised Controlled Trial [Dissertation]. Birmingham (US): University of Alabama at Birmingham, 2002. [NCT00061139] [Google Scholar]
- DeLuca SC, Echols K, Law CR, Ramey SL. Intensive pediatric constraint‐induced therapy for children with cerebral palsy: randomized, controlled, crossover trial. Journal of Child Neurology 2006;21(11):931‐8. [DOI: 10.1177/08830738060210110401; NCT00061139; PUBMED: 17092457] [DOI] [PubMed] [Google Scholar]
- Taub E, Ramey SL, DeLuca S, Echols K. Efficacy of constraint‐induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics 2004;113(2):305‐12. [NCT00061139; PUBMED: 14754942] [DOI] [PubMed] [Google Scholar]
Taub 2011 {published data only (unpublished sought but not used)}
- Taub E, Griffin A, Uswatte G, Gammons K, Nick J, Law CR. Treatment of congenital hemiparesis with pediatric constraint‐induced movement therapy. Journal of Child Neurology 2011;26(9):1163‐73. [DOI: 10.1177/0883073811408423; PMC3674837; PUBMED: 21771948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallen 2011 {published and unpublished data}
- Wallen M, Ziviani J, Naylor O, Evans R, Novak I, Herbert RD. Modified constraint‐induced therapy for children with hemiplegic cerebral palsy: a randomized trial. Developmental Medicine & Child Neurology 2011;53(12):1091‐9. [DOI: 10.1111/j.1469-8749.2011.04086.x; ACTRN12607000446460; PUBMED: 21923854] [DOI] [PubMed] [Google Scholar]
Xu 2012 {published data only}
- Xu K, He L, Mai J, Yan X, Chen Y. Muscle recruitment and coordination following constraint‐induced movement therapy with electrical stimulation on children with hemiplegic cerebral palsy: a randomized controlled trial. PLOS One 2015;10(10):e0138608. [DOI: 10.1371/journal.pone.0138608; ChiCTR‐TRC‐13004041; PMC4599892; PUBMED: 26452230] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Wang L, Mai J, He L. Efficacy of constraint‐induced movement therapy and electrical stimulation on hand function of children with hemiplegic cerebral palsy: a controlled clinical trial. Disability and Rehabilitation 2012;34(4):337‐46. [DOI: 10.3109/09638288.2011.607213; ChiCTR‐TRC‐13004041; PUBMED: 21961441] [DOI] [PubMed] [Google Scholar]
Yu 2012 {published data only (unpublished sought but not used)}
- Yu J, Kang H, Jung J. Effects of modified constraint‐induced movement therapy on hand dexterity, grip strength and activities of daily living of children with cerebral palsy: a randomized control trial. Journal of Physical Therapy Science 2012;24(10):1029‐31. [DOI: 10.1589/jpts.24.1029] [DOI] [Google Scholar]
Zafer 2016 {published and unpublished data}
- Zafer H, Amjad I, Malik AN, Shaukat E. Effectiveness of constraint induced movement therapy as compared to bimanual therapy in upper motor function outcome in child with hemiplegic cerebral palsy. Pakistan Journal of Medical Sciences 2016;32(1):181‐4. [DOI: 10.12669/pjms.321.8491; PMC4795864; PUBMED: 27022371] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Andersen 2013 {published data only}
- Andersen JC, Majnemer A, O'Grady K, Gordon AM. Intensive upper extremity training for children with hemiplegia: from science to practice. Seminars in Pediatric Neurology 2013;20(2):100‐5. [DOI: 10.1016/j.spen.2013.06.001; PUBMED: 23948684 ] [DOI] [PubMed] [Google Scholar]
Ardakani 2010 {published data only}
- Ardakani MJN, Reza Olyaei G, Abdolvahab M, Bagheri H, Jalili M, Zadeh SF. The effects and maintenance of constraint‐induced therapy on spasticity and function of upper extremity in hemiplegic cerebral palsy children 6 to 12 years old. Journal of Modern Rehabilitation 2010;4(3‐4):41‐7. [mrj.tums.ac.ir/article‐1‐93‐en.html] [Google Scholar]
Aschner 2012 {published data only}
- Aschner A. Impact of Constraint‐Induced Movement Therapy on Brain Functioning in Children with Cerebral Palsy [Thesis]. Nashville (TN): Vanderbilt University, 2012. [discoverarchive.vanderbilt.edu/handle/1803/5060] [Google Scholar]
Ballaz 2012 {published data only}
- Ballaz L, Huffenus A‐F, Lamarre C, Koclas L, Lemay M. Effect of forced use therapy on posture in children with hemiplegic cerebral palsy: a pilot study. Journal of Rehabilitation Medicine 2012;44(3):268‐71. [DOI: 10.2340/16501977-0920; PUBMED: 22278090] [DOI] [PubMed] [Google Scholar]
Basu 2012a {published data only}
- Basu A, Eyre JA. A plea for consideration of the less affected hand in therapeutic approaches to hemiplegia. Developmental Medicine & Child Neurology 2012;54(4):380‐2. [DOI: 10.1111/j.1469-8749.2012.04242.x; PUBMED: 22348374] [DOI] [PubMed] [Google Scholar]
Bonnier 2006 {published data only}
- Bonnier B, Eliasson A‐C, Krumlinde‐Sundholm L. Effects of constraint‐induced movement therapy in adolescents with hemiplegic cerebral palsy: a day camp model. Scandanavian Journal of Occupational Therapy 2006;13(1):13‐22. [PUBMED: 16615411] [DOI] [PubMed] [Google Scholar]
Boyd 2001 {published data only}
- Boyd RN, Morris ME, Graham HK. Management of upper limb dysfunction in children with cerebral palsy: a systematic review. European Journal of Neurology 2001;8(Suppl. 5):150‐66. [DOI: 10.1046/j.1468-1331.2001.00048.x; PUBMED: 11851744] [DOI] [PubMed] [Google Scholar]
Brady 2009 {published data only}
- Brady K, Garcia T. Constraint‐induced movement therapy (CIMT): pediatric applications. Developmental Disabilities Research Reviews 2009;15(2):102‐11. [DOI: 10.1002/ddrr.59; 19489088] [DOI] [PubMed] [Google Scholar]
Brandão 2009 {published data only}
- Brandão MB, Mancini MC, Vaz DV, Bueno AM, Furtado SR, Coelho ZA. Effects of constraint‐induced movement therapy in children with hemiplegia: a single case experimental study. Revista Brasileira de Fisioterapia 2009;13(6):527‐34. [DOI: 10.1590/S1413-35552009005000064] [DOI] [Google Scholar]
Brekke 2004 {published data only}
- Brekke EL, Ehler LA, Furze JA, Goulet C. Constraint induced therapy for a child with cerebral palsy: a case study. Pediatric Physical Therapy 2004;16(1):71. [DOI: 10.1097/01.PEP.0000115223.79541.5C] [DOI] [Google Scholar]
Buesch 2010 {published data only}
- Buesch FE, Schlaepfer B, Bruin ED, Wohlrab G, Ammann‐Reiffer C, Meyer‐Heim A. Constraint‐induced movement therapy for children with obstetric brachial plexus palsy: two single‐case series. International Journal of Rehabilitation Research 2010;33(2):187‐92. [DOI: 10.1097/MRR.0b013e3283310d6e; PUBMED: 19738482] [DOI] [PubMed] [Google Scholar]
Cao 2015 {published data only}
- Cao J, Khan B, Hervey N, Tian F, Delgado MR, Clegg NJ, et al. Evaluation of cortical plasticity in children with cerebral palsy undergoing constraint‐induced movement therapy based on functional near‐infrared spectroscopy. Journal of Biomedical Optics 2015;20(4):046009. [DOI: 10.1117/1.JBO.20.4.046009; PMC4479242; PUBMED: 25900145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Charles 2001 {published data only}
- Charles J, Lavinder G, Gordon AM. Effects of constraint‐induced therapy on hand function in children with hemiplegic cerebral palsy. Pediatric Physical Therapy 2001;13(2):68‐76. [PUBMED: 17053660] [PubMed] [Google Scholar]
Charles 2005 {published data only}
- Charles J, Gordon AM. A critical review of constraint‐induced movement therapy and forced use in children with hemiplegia. Neural Plasticity 2005;12(2‐3):245‐61. [DOI: 10.1155/NP.2005.245; PMC2565448; PUBMED: 16097492] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2014a {published data only}
- Chen Y‐P, Pope S, Tyler D, Warren GL. Effectiveness of constraint‐induced movement therapy on upper‐extremity function in children with cerebral palsy: a systematic review and meta‐analysis of randomized controlled trials. Clinical Rehabilitation 2014;28(10):939‐53. [DOI: 10.1177/0269215514544982; PUBMED: 25125440] [DOI] [PubMed] [Google Scholar]
Chevignard 2008 {published data only}
- Chevignard M, Azzi V, Abada G, Lemesle C, Bur S, Toure H, et al. The effectiveness of constraint‐induced movement therapy for children with hemiplegia following acquired brain injury [Intérêt de la thérapie par contrainte induite chez l’enfant hémiplégique après lésion cérébrale acquise]. Annales de Réadaptation et de Médecine Physique 2008;51(4):238–47. [DOI: 10.1016/j.annrmp.2008.01.013; PUBMED: 18395284 ] [DOI] [PubMed] [Google Scholar]
Cheyne 2013 {published data only}
- Cheyne DO, Fehlings D. Can neuroimaging help identify effective strategies for constraint therapy in congenital hemiparesis?. Developmental Medicine & Child Neurology 2013;55(10):882‐3. [DOI: 10.1111/dmcn.12238; PUBMED: 23937171 ] [DOI] [PubMed] [Google Scholar]
Chiu 2016 {published data only}
- Chiu H‐C, Ada L. Constraint‐induced movement therapy improves upper limb activity and participation in hemiplegic cerebral palsy: a systematic review. Journal of Physiotherapy 2016;62(3):130‐7. [DOI: 10.1016/j.jphys.2016.05.013; PUBMED: 27323932 ] [DOI] [PubMed] [Google Scholar]
Christman 2015 {published data only}
- Christman E, McAllister K, Claar K, Kaufman S, Page SJ. Occupational therapists' opinions of two pediatric constraint‐induced movement therapy protocols. American Journal of Occupational Therapy 2015;69(6):6906180020. [DOI: 10.5014/ajot.2015.019042; PUBMED: 26565095 ] [DOI] [PubMed] [Google Scholar]
Cimolin 2012 {published data only}
- Cimolin V, Beretta E, Piccinini L, Turconi AC, Locatelli F, Galli M, et al. Constraint‐induced movement therapy for children with hemiplegia after traumatic brain injury: a quantitative study. Journal of Head Trauma Rehabilitation 2012;27(3):177‐87. [DOI: 10.1097/HTR.0b013e3182172276; PUBMED: 21522025 ] [DOI] [PubMed] [Google Scholar]
Cohen‐Holzer 2010 {published data only}
- Cohen‐Holzer M, Reinstein R, Rotem H, Katz‐Leurer M. The effect of combined training‐constraint and bimanual use on hand function of children with cerebral palsy hemiparesis. Developmental Medicine & Child Neurology 2010;52(Suppl 5):28. [DOI: 10.1111/j.1469-8749.2010.03755.x; E6] [DOI] [Google Scholar]
Cohen‐Holzer 2011 {published data only}
- Cohen‐Holzer M, Katz‐Leurer M, Reinstein R, Rotem H, Meyer S. The effect of combining daily restraint with bimanual intensive therapy in children with hemiparetic cerebral palsy: a self‐control study. NeuroRehabilitation 2011;29(1):29‐36. [DOI: 10.3233/NRE-2011-0674; PUBMED: 21876293] [DOI] [PubMed] [Google Scholar]
Cohen‐Holzer 2016 {published data only}
- Cohen‐Holzer M, Sorek G, Schless S, Kerem J, Katz‐Leurer M. The influence of a constraint and bimanual training program using a variety of modalities, on upper extremity functions and gait parameters among children with hemiparetic cerebral palsy: a case series. Physical & Occupational Therapy in Pediatrics 2016;36(1):17‐27. [DOI: 10.3109/01942638.2014.990549; PUBMED: 25521486] [DOI] [PubMed] [Google Scholar]
Coker 2009 {published data only}
- Coker P, Lebkicher C, Harris L, Snape J. The effects of constraint‐induced movement therapy for a child less than one year of age. NeuroRehabilitation 2009;24(3):199‐208. [DOI: 10.3233/NRE-2009-0469; PUBMED: 19458426] [DOI] [PubMed] [Google Scholar]
Coker 2010 {published data only}
- Coker P, Karakostas T, Dodds C, Hsiang S. Gait characteristics of children with hemiplegic cerebral palsy before and after modified constraint‐induced movement therapy. Disability and Rehabilitation 2010;32(5):402‐8. [DOI: 10.3109/09638280903171592; PUBMED: 20095954 ] [DOI] [PubMed] [Google Scholar]
Cope 2008 {published data only}
- Cope SM, Forst HC, Bibis D, Liu X‐C. Modified constraint‐induced movement therapy for a 12‐month‐old child with hemiplegia: a case report. American Journal of Occupational Therapy 2008;62(4):430‐7. [DOI: 10.5014/ajot.62.4.430; PUBMED: 18712005] [DOI] [PubMed] [Google Scholar]
Cope 2010 {published data only}
- Cope SM, Liu X‐C, Verber MD, Cayo C, Rao S, Tassone JC. Upper limb function and brain reorganization after constraint‐induced movement therapy in children with hemiplegia. Developmental Neurorehabilitation 2010;13(1):19‐30. [DOI: 10.3109/17518420903236247; PUBMED: 20067342] [DOI] [PubMed] [Google Scholar]
Crocker 1997 {published data only}
- Crocker MD, MacKay‐Lyons M, McDonnell E. Forced use of the upper extremity in cerebral palsy: a single case study design. American Journal of Occupational Therapy 1997;51(10):824‐33. [DOI: 10.5014/ajot.51.10.824; PUBMED: 9394143] [DOI] [PubMed] [Google Scholar]
DeLuca 2003 {published data only}
- DeLuca SC, Echols K, Ramey SL, Taub E. Pediatric constraint‐induced movement therapy for a young child with cerebral palsy: two episodes care. Physical Therapy 2003;83(11):1003‐13. [PUBMED: 14577827] [PubMed] [Google Scholar]
DeLuca 2015 {published data only}
- DeLuca SC, Ramey SL, Trucks MR, Wallace DA. Multiple treatments of pediatric constraint‐induced movement therapy (pCIMT): a clinical cohort study. American Journal of Occupational Therapy 2015;69(6):6906180010. [DOI: 10.5014/ajot.2015.019323; PMC4643376; PUBMED: 26565094] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickerson 2007 {published data only}
- Dickerson AE, Brown LE. Pediatric constraint‐induced movement therapy in a young child with minimal active arm movement. American Journal of Occupational Therapy 2007;61(5):563‐73. [PUBMED: 17944294] [DOI] [PubMed] [Google Scholar]
Dong 2013 {published data only}
- Dong VA‐Q, Tung IH‐H, Siu HW‐Y, Fong KN‐K. Studies comparing the efficacy of constraint‐induced movement therapy and bimanual training in children with unilateral cerebral palsy: a systematic review. Developmental Neurorehabilitation 2013;16(2):133‐43. [DOI: 10.3109/17518423.2012.702136; PUBMED: 22946588] [DOI] [PubMed] [Google Scholar]
Echols 2000 {published data only}
- Echols K, DeLuca SC, Taub E, Ramey S. Constraint‐induced movement therapy in young children: a protocol and outcomes compared to traditional measures. Pediatric Physical Therapy 2000;12(4):210. [12] [Google Scholar]
Eliasson 2003 {published data only}
- Eliasson A‐C, Bonnier B, Krumlinde‐Sundholm L. Clinical experience of constraint induced movement therapy in small children with hemiplegic cerebral palsy ‐‐ a day camp model. Developmental Medicine & Child Neurology 2003;45(5):357‐9. [PUBMED: 12729152] [DOI] [PubMed] [Google Scholar]
Eliasson 2005 {published and unpublished data}
- Eliasson A‐C, Krumlinde‐Sundholm L, Shaw K, Wang C. Effects of constraint‐induced movement therapy in young children with hemiplegic cerebral palsy: an adapted model. Developmental Medicine & Child Neurology 2005;47(4):266‐75. [PUBMED: 15832550] [DOI] [PubMed] [Google Scholar]
Eliasson 2009 {published data only}
- Eliasson A‐C, Shaw K, Pontén E, Boyd RN, Krumlinde‐Sundholm L. Feasibility of a day‐camp model of modified constraint‐induced movement therapy with and without botulinum toxin A injection for children with hemiplegia. Physical & Occupational Therapy in Pediatrics 2009;29(3):311‐33. [PUBMED: 19842858] [DOI] [PubMed] [Google Scholar]
Eliasson 2014a {published data only}
- Eliasson A‐C, Krumlinde‐Sundholm L, Gordon AM, Feys H, Klingels K, Aarts PB, et al. Guidelines for future research in constraint‐induced movement therapy for children with unilateral cerebral palsy: an expert consensus. Developmental Medicine & Child Neurology 2014;56(2):125‐37. [DOI: 10.1111/dmcn.12273; PUBMED: 24266735] [DOI] [PubMed] [Google Scholar]
Eliasson 2015 {published data only}
- Eliasson A‐C. What can be learned from reporting no‐treatment effect of distribution of upper limb training?. Developmental Medicine & Child Neurology 2015;57(6):498. [DOI: 10.1111/dmcn.12706] [DOI] [PubMed] [Google Scholar]
Fergus 2008 {published data only}
- Fergus A, Buckler J, Farrell J, Isley M, McFarland M, Riley B. Constraint‐induced movement therapy for a child with hemiparesis: a case report. Pediatric Physical Therapy 2008;20(3):271‐83. [DOI: 10.1097/PEP.0b013e318181e569; PUBMED: 18703966] [DOI] [PubMed] [Google Scholar]
Fetters 2004 {published data only}
- Fetters L, Figueiedo EM, Keane‐Miller D, McSweeney DJ, Tsao C‐C. Critically appraised topics 67. Is constraint‐induced therapy an effective intervention for the treatment of upper extremity dysfunction in children with spastic hemiplegic cerebral palsy (CP)?. Pediatric Physical Therapy 2004;16(1):77‐9. [DOI: 10.1097/01.PEP.0000116783.25230.8C] [DOI] [PubMed] [Google Scholar]
Ganapathy Sankar 2015 {published data only}
- Ganapathy Sankar U. Constraint induced movement therapy (CIMT) for children with hemiplegic cerebral palsy to improve upper extremity function: pilot study. International Journal of Science and Research 2015;4(5):2524‐7. [Paper ID: SUB154817; www.ijsr.net/archive/v4i5/SUB154817.pdf] [Google Scholar]
Geerdink 2015 {published data only}
- Geerdink YA, Aarts P, Burg J, Steenbergen B, Geurts A. Intensive upper limb intervention with self‐management training is feasible and promising for older children and adolescents with unilateral cerebral palsy. Research in Developmental Disabilities 2015;43‐44:97‐105. [DOI: 10.1016/j.ridd.2015.06.013; PUBMED: 26164301] [DOI] [PubMed] [Google Scholar]
Gillick 2010 {published data only}
- Gillick BT, Koppes A. Gross motor outcomes in children with hemiparesis involved in a modified constraint‐induced therapy program. Journal of Pediatric Rehabilitation Medicine 2010;3(3):171‐5. [DOI: 10.3233/PRM-2010-0126; PUBMED: 21791848] [DOI] [PubMed] [Google Scholar]
Gillick 2014 {published data only}
- Gillick B, Krach LE, Feyma T, Rich TL, Moberg K, Thomas W, et al. Primed low‐frequency repetitive transcranial magnetic stimulation and constraint‐induced movement therapy in pediatric hemiparesis: a randomized controlled trial. Developmental Medicine & Child Neurology 2014;56(1):44‐52. [DOI: 10.1111/dmcn.12243; NCT01104064; PMC3864983; PUBMED: 23962321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillick 2015 {published data only}
- Gillick BT, Krach LE, Feyma T, Rich TL, Moberg K, Menk J, et al. Safety of primed repetitive transcranial magnetic stimulation and modified constraint‐induced movement therapy in a randomized controlled trial in pediatric hemiparesis. Archives of Physical Medicine and Rehabilitation 2015;96(Suppl 4):S104‐13. [DOI: 10.1016/j.apmr.2014.09.012; NCT01104064; PMC4380609; PUBMED: 25283350] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillick 2018 {published data only}
- Gillick B, Rich T, Nemanich S, Chen C‐Y, Menk J, Mueller B, et al. Transcranial direct current stimulation and constraint‐induced therapy in cerebral palsy: a randomized, blinded, sham‐controlled clinical trial. European Journal of Paediatric Neurology 2018;22(3):358‐68. [DOI: 10.1016/j.ejpn.2018.02.001; NCT 02250092; PMC5899638; PUBMED: 29456128] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glover 2002 {published data only}
- Glover JE, Matter CA, Yoell C, Speed S. The effectiveness of constraint induced movement therapy in two young children with hemiplegia. Pediatric Rehabilitation 2002;5(3):125‐31. [DOI: 10.1080/1363849021000039326; PUBMED: 12581474] [DOI] [PubMed] [Google Scholar]
Gordon 2001 {published data only}
- Gordon AM. Constraint‐induced therapy in children with hemiplegic cerebral palsy. Developmental Medicine and Child Neurology 2001;43(Suppl 87):6‐7. [DOI: 10.1111/j.1469-8749.2001.tb04119.x] [DOI] [PubMed] [Google Scholar]
Gordon 2005 {published data only}
- Gordon AM, Charles J, Wolf SL. Methods of constraint induced movement therapy for children with hemiplegic cerebral palsy: development of a child‐friendly intervention for improving upper‐extremity function. Archives of Physical Medicine and Rehabilitation 2005;86(4):837‐44. [DOI: 10.1016/j.apmr.2004.10.008; PUBMED: 15827942] [DOI] [PubMed] [Google Scholar]
Gordon 2006 {published data only}
- Gordon AM, Charles J, Wolf SL. Efficacy of constraint‐induced movement therapy on involved upper‐extremity use in children with hemiplegic cerebral palsy is not age‐dependent. Pediatrics 2006;117(3):e363‐73. [DOI: 10.1542/peds.2005-1009; PUBMED: 16510616] [DOI] [PubMed] [Google Scholar]
Gordon 2007 {published data only}
- Gordon A, Connelly A, Neville B, Vargha‐Khadem F, Jessop N, Murphy T, et al. Modified constraint‐induced movement therapy after childhood stroke. Developmental Medicine & Child Neurology 2007;49(1):23‐7. [DOI: 10.1111/j.1469-8749.2007.0072a.x; PUBMED: 17209972] [DOI] [PubMed] [Google Scholar]
Gordon 2008 {published data only}
- Gordon AM, Chinnan A, Gill S, Petra E, Hung YC, Charles J. Both constraint‐induced movement therapy and bimanual training lead to improved performance of upper extremity function in children with hemiplegia. Developmental Medicine & Child Neurology 2008;50(12):957‐8. [PUBMED: 19160464] [DOI] [PubMed] [Google Scholar]
Gordon 2010 {published data only}
- Gordon AM, Okita SY. Augmenting pediatric constraint‐induced movement therapy and bimanual training with video gaming technology. Technology and Disability 2010;22(4):179‐91. [DOI: 10.3233/TAD-2010-0302] [DOI] [Google Scholar]
Gordon 2011a {published data only}
- Gordon AM. To constrain or not to constrain, and other stories of intensive upper extremity training for children with unilateral cerebral palsy. Developmental Medicine & Child Neurology 2011;53 Suppl 4:56‐61. [DOI: 10.1111/j.1469-8749.2011.04066.x; PUBMED: 21950396] [DOI] [PubMed] [Google Scholar]
Gordon 2011b {published data only}
- Gordon AM. Is it time to remove the restraints?. Developmental Medicine & Child Neurology 2011;53(4):292‐3. [DOI: 10.1111/j.1469-8749.2010.03906.x; PUBMED: 21401580] [DOI] [PubMed] [Google Scholar]
Hackman 2000 {published data only}
- Hackman FC, Kauffman KD. Home implementation of constraint induced movement therapy in a child with hemiplegia: a comparison of impact on child and family life to gross and fine motor functional gains. Pediatric Physical Therapy 2000;12(4):214. [21] [Google Scholar]
Hart 2005 {published data only}
- Hart H. Can constraint therapy be developmentally appropriate and child friendly?. Developmental Medicine & Child Neurology 2005;47(6):363. [DOI: 10.1017/S0012162205000708; onlinelibrary.wiley.com/doi/pdf/10.1111/j.1469‐8749.2005.tb01152.x] [DOI] [PubMed] [Google Scholar]
Haynes 2012 {published data only}
- Haynes MP, Phillips D. Modified constraint induced movement therapy enhanced by a neuro‐development treatment‐based therapeutic handling protocol: two case studies. Journal of Pediatric Rehabilitation Medicine 2012;5(2):117‐24. [DOI: 10.3233/PRM-2012-0203; PUBMED: 22699102] [DOI] [PubMed] [Google Scholar]
Hoare 2008 {published data only}
- Hoare B. Unravelling the cerebral palsy upper limb. Developmental Medicine & Child Neurology 2008;50(12):887. [DOI: 10.1111/j.1469-8749.2008.03130.x] [DOI] [PubMed] [Google Scholar]
Hoare 2014 {published data only}
- Hoare BJ. Putting some excitement into constraint‐induced movement therapy. Developmental Medicine & Child Neurology 2014;56(1):5‐6. [DOI: 10.1111/dmcn.12272; PUBMED: 23980669] [DOI] [PubMed] [Google Scholar]
Hoare 2015 {published data only}
- Hoare BJ. Constraint therapy, the panacea for unilateral cerebral palsy?. Developmental Medicine & Child Neurology 2015;57(1):12‐3. [DOI: 10.1111/dmcn.12596; PUBMED: 25315321] [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang H‐H, Fetters L, Hale J, McBride A. Bound for success: a systematic review of constraint‐induced movement therapy in children with cerebral palsy supports improved arm and hand use. Physical Therapy 2009;89(11):1126‐41. [DOI: 10.2522/ptj.20080111; PUBMED: 19729391] [DOI] [PubMed] [Google Scholar]
Huang 2010 {published data only}
- Huang W‐C, Chen Y‐J, Chien C‐L, Kashima H, Lin K‐C. Constraint‐induced movement therapy as a paradigm of translational research in neurorehabilitation: reviews and prospects. American Journal of Translational Research 2010;3(1):48‐60. [PMC2981425; PUBMED: 21139805] [PMC free article] [PubMed] [Google Scholar]
Islam 2014 {published data only}
- Islam M, Nordstrand L, Holmström L, Kits A, Forssberg H, Eliasson A‐C. Is outcome of constraint‐induced movement therapy in unilateral cerebral palsy dependent on corticomotor projection pattern and brain lesion characteristics?. Developmental Medicine & Child Neurology 2014;56(3):252‐8. [DOI: 10.1111/dmcn.12353; PUBMED: 24341408] [DOI] [PubMed] [Google Scholar]
Juenger 2007 {published data only}
- Juenger H, Linder‐Lucht M, Walther M, Berweck S, Mall V, Staudt M. Cortical neuromodulation by constraint‐induced movement therapy in congenital hemiparesis: an FMRI study. Neuropediatrics 2007;38(3):130‐6. [DOI: 10.1055/s-2007-985904; PUBMED: 17985262] [DOI] [PubMed] [Google Scholar]
Juenger 2013 {published data only}
- Juenger H, Kuhnke N, Braun C, Ummenhofer F, Wilke M, Walther M, et al. Two types of exercise‐induced neuroplasticity in congenital hemiparesis: a transcranial magnetic stimulation, functional MRI, and magnetoencephalography study. Developmental Medicine & Child Neurology 2013;55(10):941‐51. [DOI: 10.1111/dmcn.12209; PUBMED: 23937719] [DOI] [PubMed] [Google Scholar]
Karman 2003 {published data only}
- Karman N, Maryles J, Baker RW, Simpser E, Berger‐Gross P. Constraint‐induced movement therapy for hemiplegic children with acquired brain injuries. Journal of Head Trauma Rehabilitation 2003;18(3):259‐67. [PUBMED: 12802168] [DOI] [PubMed] [Google Scholar]
Kim 2015a {published data only}
- Kim G, Blitz J. Commentary on “Constraint‐induced movement therapy in children aged 5 to 9 years with cerebral palsy: a day camp model". Pediatric Physical Therapy 2015;27(1):81. [DOI: 10.1097/PEP.0000000000000113; PUBMED: 25521269] [DOI] [PubMed] [Google Scholar]
Klepper 2017 {published data only}
- Klepper SE, Clayton Krasinski D, Gilb MC, Khalil N. Comparing unimanual and bimanual training in upper extremity function in children with unilateral cerebral palsy. Pediatric Physical Therapy 2017;29(4):288‐306. [DOI: 10.1097/PEP.0000000000000438; PUBMED: 28953170] [DOI] [PubMed] [Google Scholar]
Klingels 2013 {published and unpublished data}
- Klingels K, Feys H, Molenaers G, Verbeke G, Daele S, Hoskens J, et al. Randomized trial of modified constraint‐induced movement therapy with and without an intensive therapy program in children with unilateral cerebral palsy. Neurorehabilitation and Neural Repair 2013;27(9):799‐807. [DOI: 10.1177/1545968313496322; PUBMED: 23901061] [DOI] [PubMed] [Google Scholar]
Kong 2013 {published data only}
- Kong E‐J, Chun K‐A, Jeong J‐H, Cho I‐H. Brain SPECT analysis after constraint‐induced movement therapy in young children with hemiplegic cerebral palsy: case report. Nuclear Medicine and Molecular Imaging 2013;47(2):119‐24. [DOI: 10.1007/s13139-013-0200-1; PMC4041966; PUBMED: 24900092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuhnke 2008 {published data only}
- Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint‐induced movement therapy?. Developmental Medicine & Child Neurology 2008;50(12):898‐903. [DOI: 10.1111/j.1469-8749.2008.03119.x; PUBMED: 18811703] [DOI] [PubMed] [Google Scholar]
Kwon 2014 {published data only}
- Kwon J‐Y, Chang WH, Chang HJ, Yi S‐H, Kim M‐Y, Kim E‐H, et al. Changes in diffusion tensor tractographic findings associated with constraint‐induced movement therapy in young children with cerebral palsy. Clinical Neurophysiology 2014;125(12):2397‐403. [DOI: 10.1016/j.clinph.2014.02.025; PUBMED: 24746686] [DOI] [PubMed] [Google Scholar]
Lavinder 2007 {published data only}
- Lavinder G. Constraint‐Induced Therapy in the Upper Extremity for Children with Hemiplegic Cerebral Palsy [PhD thesis]. New York (NY): Teachers College, Columbia University, 2007. [Google Scholar]
Lee 2010 {published data only}
- Lee Z. Effects of constraint‐induced movement therapy on cortical reorganization in patients with hemiplegic cerebral palsy. Developmental Medicine & Child Neurology 2010;52(Suppl 4):37‐8. [DOI: 10.1111/j.1469-8749.2010.03682.x; PP 8.13] [DOI] [Google Scholar]
Leon‐Santos 2008 {published data only}
- León‐Santos MR, Romero‐Torres MD, Conjero‐Casares JA. Efficacy of constraint‐induced movement therapy in children with cerebral palsy [Eficacia de la terapia de movimiento inducido por restricción en niños con parálisis cerebral]. Rehabilitación 2008;42(4):199‐204. [DOI: 10.1016/S0048-7120(08)74587-0; medes.com/publication/42602] [DOI] [Google Scholar]
Lin 2011 {published data only}
- Lin K‐C, Wang T‐N, Wu C‐Y, Chen C‐l, Chang K‐C, Lin Y‐C, et al. Effects of home‐based constraint‐induced therapy versus dose‐matched control intervention on functional outcomes and caregiver well‐being in children with cerebral palsy. Research in Developmental Disabilities 2011;32(5):1483‐91. [DOI: 10.1016/j.ridd.2011.01.023; PUBMED: 21429706] [DOI] [PubMed] [Google Scholar]
Lowes 2014a {published data only}
- Lowes LP, Mayhan M, Orr T, Batterson N, Tonneman JA, Meyer A, et al. Pilot study of the efficacy of constraint‐induced movement therapy for infants and toddlers with cerebral palsy. Physical & Occupational Therapy in Pediatrics 2014;34(1):4‐21. [DOI: 10.3109/01942638.2013.810186; PMC4162395; PUBMED: 23848499] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowes 2014b {published data only}
- Lowes LP, Lo WD, Alfano LN, Case‐Smith J. Authors' response to evidence to practice commentary. Physical & Occupational Therapy in Pediatrics 2014;34(1):25‐9. [DOI: 10.3109/01942638.2014.880260; PUBMED: 24460081] [DOI] [PubMed] [Google Scholar]
Maitre 2011 {published data only}
- Maitre N, Henderson G, Gogliotti S, Simmons A, Key A. Constraint‐induced movement therapy improves neural processing efficiency and neurobehavioral function in children with hemiparetic cerebral palsy. Developmental Medicine & Child Neurology 2011;53(Suppl 5):27. [DOI: 10.1111/j.1469-8749.2011.04112.x; E2] [DOI] [Google Scholar]
Manning 2014 {published data only}
- Manning KY. Resting State Functional Magnetic Resonance and Diffusion Tensor Imaging of Hemiplegic Cerebral Palsy Patients Treated with Constraint‐Induced Movement Therapy: Predictors and Clinically Correlated Evidence of Neuroplasticity [Masters thesis]. London (ON): The University of Western Ontario, 2014. [Google Scholar]
Manning 2015 {published data only}
- Manning KY, Fehlings D, Mesterman R, Gorter JW, Switzer L, Campbell C, et al. Resting state and diffusion neuroimaging predictors of clinical improvements following constraint‐induced movement therapy in children with hemiplegic cerebral palsy. Journal of Child Neurology 2015;30(11):1507‐14. [DOI: 10.1177/0883073815572686] [DOI] [PubMed] [Google Scholar]
Manning 2016 {published data only}
- Manning KY, Menon RS, Gorter JW, Mesterman R, Campbell C, Switzer L, et al. Neuroplastic sensorimotor resting state network reorganisation in children with hemiplegic cerebral palsy treated with constraint induced movement therapy. Journal of Child Neurology 2016;31(2):220‐6. [DOI: 10.1177/0883073815588995; PUBMED: 26078420] [DOI] [PubMed] [Google Scholar]
Martin 2008 {published data only}
- Martin A, Burtner PA, Poole J, Philips J. Case report: ICF‐level changes in a preschooler after constraint‐induced movement therapy. American Journal of Occupational Therapy 2008;62(3):282‐8. [PUBMED: 18557004] [DOI] [PubMed] [Google Scholar]
Mcconnell 2014 {published data only}
- McConnell K, Johnston L, Kerr C. Efficacy and acceptability of reduced intensity constraint‐induced movement therapy for children aged 9‐11 years with hemiplegic cerebral palsy: a pilot study. Physical & Occupational Therapy in Pediatrics 2014;34(3):245‐59. [DOI: 10.3109/01942638.2013.866611; PUBMED: 24341455] [DOI] [PubMed] [Google Scholar]
Motta 2010 {published data only}
- Motta F, Antonello CE, Stignani C. Forced‐use, without therapy, in children with hemiplegia: preliminary study of a new approach for the upper limb. Journal of Pediatric Orthopedics 2010;30(6):582‐7. [DOI: 10.1097/BPO.0b013e3181e88ee4; PUBMED: 20733424] [DOI] [PubMed] [Google Scholar]
Nascimento 2009 {published data only}
- Nascimento LR, Glória AE, Habib ES. Effects of constraint‐induced movement therapy as a rehabilitation strategy for the affected upper limb of children with hemiparesis: systematic review of the literature [Efeitos da terapia de movimento induzido por restrição como estratégia de reabilitação do membro superior acometido de crianças hemiparéticas: revisão sistemática da literatura]. Revista Brasieria de Fisioterapia 2009;13(2):97‐102. [DOI: 10.1590/S1413-35552009005000022; pdfs.semanticscholar.org/a5c1/7666ffe113011efa630f58629ea7da92a344.pdf] [DOI] [Google Scholar]
Naylor 2005 {published data only}
- Naylor CE, Bower E. Modified constraint‐induced movement therapy for young children with hemiplegic cerebral palsy: a pilot study. Developmental Medicine & Child Neurology 2005;47(6):365–9. [DOI: 10.1111/j.1469-8749.2005.tb01155.x; PUBMED: 15934484] [DOI] [PubMed] [Google Scholar]
NCT02957708 {unpublished data only}
- NCT02957708. Constraint‐induced movement therapy and self‐regulation for children with cerebral palsy [Effectiveness of modified constraint‐induced movement therapy and self‐regulation learning for children with hemiplegic cerebral palsy]. clinicaltrials.gov/ct2/show/NCT02957708 (first received 22 September 2016). [NCT02957708]
Newman 2008 {published data only}
- Newman CJ, Vuilleumier L, Vuilleumier A, Jaton R, Holenweg‐Gross C. Induced‐stress motor therapy: development of a new orthosis [Therapie motrice par contrainte induite: developpement d'une nouvelle orthose]. ergotherapeute.ch/media/documents/Newman%20et%20al.,%20version%20finale,%20PP%202008.pdf (accessed 23 January 2012).
Nordstrand 2013 {published data only}
- Nordstrand L, Eliasson A‐C. Six years after a modified constraint induced movement therapy (CIMT) program ‐‐ what happens when the children have become young adults?. Physical & Occupational Therapy in Pediatrics 2013;33(2):163‐9. [DOI: 10.3109/01942638.2013.757157; PUBMED: 23369068] [DOI] [PubMed] [Google Scholar]
Novak 2013 {published data only}
- Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Developmental Medicine & Child Neurology 2013;55(10):885‐910. [DOI: 10.1111/dmcn.12246; PUBMED: 23962350] [DOI] [PubMed] [Google Scholar]
Nwaobi 1987 {published data only}
- Nwaobi OM. Non‐dominant arm restraint and dominant arm function in a child with athetoid cerebral palsy: electromyographic and functional evaluation. Archives of Physical Medicine and Rehabilitation 1987;68(12):837‐9. [PUBMED: 3426382] [PubMed] [Google Scholar]
Oh 2014 {published data only}
- Oh Y‐J, Cho B‐H, Lee J‐S. Effects of constraint‐induced movement therapy for the upper‐extremity function in children with cerebral palsy suffering from hemiplegia: meta‐analysis. Journal of Korean Society of Occupational Therapy 2014;22(3):69‐83. [DOI: 10.14519/jksot.2014.22.3.06] [DOI] [Google Scholar]
Pardeep 2010 {unpublished data only}
- Pardeep M, Senthilkumar CB, Mamta S. Efficacy of modified constraint‐induced movement therapy in children with spastic hemiparetic cerebral palsy. Indian Journal of Physiotherapy and Occupational Therapy 2010;4(1):47‐53. [www.indianjournals.com/ijor.aspx?target=ijor:ijpot&volume=4&issue=1&article=013] [Google Scholar]
Park 2009 {published data only}
- Park ES, Rha D‐W, Lee JD, Yoo JK, Chang WH. The short‐term effects of combined modified constraint‐induced movement therapy and botulinum toxin injection for children with spastic hemiplegic cerebral palsy. Neuropediatrics 2009;40(6):269‐74. [DOI: 10.1055/s-0030-1252049; PUBMED: 20446220] [DOI] [PubMed] [Google Scholar]
Pidcock 2009 {published data only}
Pierce 2002 {published data only}
- Pierce SR, Daly K, Gallagher KG, Geshkoff AM, Schaumburg SW. Constraint‐induced therapy for a child with hemiplegic cerebral palsy: a case report. Archives of Physical Medicine and Rehabilitation 2002;83(10):1462‐3. [PUBMED: 12370887] [DOI] [PubMed] [Google Scholar]
Psychouli 2010 {published data only}
- Psychouli P, Burridge J, Kennedy C. Forced use as a home‐based intervention in children with congenital hemiplegic cerebral palsy: choosing the appropriate constraint. Disability and Rehabilitation: Assistive Technology 2010;5(1):25‐33. [DOI: 10.3109/17483100903121489; PUBMED: 19941438] [DOI] [PubMed] [Google Scholar]
Psychouli 2016 {published data only}
- Psychouli P, Kennedy CR. Modified constraint‐induced movement therapy as a home‐based intervention for children with cerebral palsy. Pediatric Physical Therapy 2016;28(2):154‐60. [DOI: 10.1097/PEP.0000000000000227; PUBMED: 26808960] [DOI] [PubMed] [Google Scholar]
Ramachandran 2011 {published data only}
- Ramachandran S, Thakur P. Upper extremity constraint‐induced movement therapy in infantile hemiplegia. Journal of Pediatric Neurosciences 2011;6(1):29‐31. [DOI: 10.4103/1817-1745.84403; PMC3173910; PUBMED: 21977084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramey 2012 {published data only}
- Ramey SL, DeLuca SC, Case‐Smith J, Stevenson R. Caution is warranted in interpreting data from a recent trial of modified constraint‐induced therapy. Developmental Medicine & Child Neurology 2012;54(5):477‐9; author reply 479‐81. [DOI: 10.1111/j.1469-8749.2012.04240.x; PUBMED: 22409421] [DOI] [PubMed] [Google Scholar]
Reidy 2012 {published data only}
- Reidy TG, Naber E, Viguers E, Allison K, Brady K, Carney J, et al. Outcomes of a clinic‐based pediatric constraint‐induced movement therapy program. Physical & Occupational Therapy in Pediatrics 2012;32(4):355‐67. [DOI: 10.3109/01942638.2012.694991; PUBMED: 22731797] [DOI] [PubMed] [Google Scholar]
Reidy 2018 {published data only}
- Reidy TG, Carney J, Schoenbrodt L, Pidcock FS. Reporting collateral effects of pediatric constraint induced movement therapy: parent observed speech and language changes. Journal of Interprofessional Education and Practice 2018;11:58‐63. [10.1016/j.xjep.2018.02.006] [Google Scholar]
Rickards 2014 {published data only}
- Rickards T, Sterling C, Taub E, Perkins‐Hu C, Gauthier L, Graham M, et al. Diffusion tensor imaging study of the response to constraint‐induced movement therapy of children with hemiparetic cerebral palsy and adults with chronic stroke. Archives of Physical Medicine and Rehabilitation 2014;95(3):506‐14.e1. [DOI: 10.1016/j.apmr.2013.08.245; PUBMED: 24055785] [DOI] [PubMed] [Google Scholar]
Ries 2006 {published data only}
- Ries JD, Leonard R. Is there evidence to support the use of constraint‐induced therapy to improve the quality or quantity of upper extremity function of a 2 ½‐year‐old girl with congenital hemiparesis? If so, what are the optimal parameters of this intervention?. Physical Therapy 2006;86(5):746‐52. [DOI: 10.1093/ptj/86.5.746] [DOI] [PubMed] [Google Scholar]
Roberts 2015 {published data only}
- Roberts H. Constraint Induced Movement Therapy with Armeo® Spring Pediatric Training for Children with Hemiplegic Cerebral Palsy: A Comparative Study [PhD thesis]. Denton (TX): Texas Woman’s University, 2015. [Google Scholar]
Rocca 2013 {published data only}
- Rocca MA, Turconi AC, Strazzer S, Absinta M, Valsasina P, Beretta E, et al. MRI predicts efficacy of constraint‐induced movement therapy in children with brain injury. Neurotherapeutics 2013;10(3):511‐9. [DOI: 10.1007/s13311-013-0189-2; PMC3701764; PUBMED: 23605556] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakzewski 2009 {published data only}
- Sakzewski L, Ziviani J, Boyd R. Systematic review and meta‐analysis of therapeutic management of upper‐limb dysfunction in children with congenital hemiplegia. Pediatrics 2009;123(6):e1111‐22. [DOI: 10.1542/peds.2008-3335; PUBMED: 19451190] [DOI] [PubMed] [Google Scholar]
Sakzewski 2012 {published data only}
- Sakzewski L. Bimanual therapy and constraint‐induced movement therapy are equally effective in improving hand function in children with congenital hemiplegia. Journal of Physiotherapy 2012;58(1):59. [DOI: 10.1016/S1836-9553(12)70075-9; PUBMED: 22341385] [DOI] [PubMed] [Google Scholar]
Sakzewski 2014 {published data only}
- Sakzewski L, Gordon A, Eliasson A‐C. The state of the evidence for intensive upper limb therapy approaches for children with unilateral cerebral palsy. Journal of Child Neurology 2014;29(8):1077‐90. [DOI: 10.1177/0883073814533150; PUBMED: 24820334] [DOI] [PubMed] [Google Scholar]
Schrank 2013 {published data only}
- Schrank JJ. Constraint‐induced movement therapy effects on gross motor function of a child with triplegic cerebral palsy. Pediatric Physical Therapy 2013;25(1):71‐8. [DOI: 10.1097/PEP.0b013e31827abaf4; PUBMED: 23288013] [DOI] [PubMed] [Google Scholar]
Seema 2015 {published data only}
- Seema SN, Kannabiran B. Effects of modified constrained induced movement therapy to improve the upper limb functional activities and gross manual dexterity on hemiparetic cerebral palsy children. International Journal of Neurorehabilitation 2015;2(3):169. [DOI: 10.4172/2376-0281.1000169] [DOI] [Google Scholar]
Shetty 2014 {published data only}
- Shetty R, Joshi A, Shibila J. The Magical Pouch Program: a case study of modified constraint induced movement therapy with bimanual training on a child with unilateral spastic cerebral palsy. Indian Journal of Occupational Therapy 2014;46(1):3‐9. [medind.nic.in/iba/t14/i1/ibat14i1p3.pdf] [Google Scholar]
Staudt 2014 {published data only}
- Staudt M, Berweck S. Is constraint‐induced movement therapy harmful in unilateral spastic cerebral palsy with ipsilateral cortico‐spinal projections?. Developmental Medicine & Child Neurology 2014;56(3):202‐3. [DOI: 10.1111/dmcn.12372; PUBMED: 24372093] [DOI] [PubMed] [Google Scholar]
Stearns 2009 {published data only}
- Stearns GE, Burtner PA, Keenan KM, Qualls C, Phillips J. Effects of constraint‐induced movement therapy on hand skills and muscle recruitment of children with spastic hemiplegic cerebral palsy. NeuroRehabilitation 2009;24(2):95‐108. [DOI: 10.3233/NRE-2009-0459; PUBMED: 19339749] [DOI] [PubMed] [Google Scholar]
Sterling 2013 {published data only}
- Sterling C, Taub E, Davis D, Rickards T, Gauthier LV, Griffin A, et al. Structural neuroplastic change after constraint‐induced movement therapy in children with cerebral palsy. Pediatrics 2013;131(5):e1664‐9. [DOI: 10.1542/peds.2012-2051; PUBMED: 23610209] [DOI] [PubMed] [Google Scholar]
Sterr 2002 {published data only}
- Sterr A, Freivogel S, Schmalohr D. Neurobehavioural aspects of recovery: assessment of the learned nonuse phenomenon in hemiparetic adolescents. Archives of Physical Medicine and Rehabilitation 2002;83(12):1726‐31. [DOI: 10.1053/apmr.2002.35660; PUBMED: 12474177] [DOI] [PubMed] [Google Scholar]
Sutcliffe 2007 {published data only}
- Sutcliffe TL, Gaetz WC, Logan WJ, Cheyne DO, Fehlings DL. Cortical reorganization after modified constraint‐induced movement therapy in pediatric hemiplegic cerebral palsy. Journal of Child Neurology 2007;22(11):1281‐7. [DOI: 10.1177/0883073807307084; PUBMED: 18006957] [DOI] [PubMed] [Google Scholar]
Sutcliffe 2009 {published data only}
- Sutcliffe TL, Logan WJ, Fehlings DL. Pediatric constraint‐induced movement therapy is associated with increased contralateral cortical activity on functional magnetic resonance imaging. Journal of Child Neurology 2009;24(10):1230‐5. [DOI: 10.1177/0883073809341268; PUBMED: 19805822] [DOI] [PubMed] [Google Scholar]
Taub 2007 {published data only}
- Taub E, Griffin A, Nick J, Gammons K, Uswatte G, Law CR. Pediatric CI therapy for stroke‐induced hemiparesis in young children. Developmental Neurorehabilitation 2007;10(1):3‐18. [PUBMED: 17608322] [DOI] [PubMed] [Google Scholar]
Tervahauta 2017 {published data only}
- Tervahauta MH, Girolami GL, Øberg GK. Efficacy of constraint‐induced movement therapy compared with bimanual intensive training in children with unilateral cerebral palsy: a systematic review. Clinical Rehabilitation 2017;31(11):1445‐56. [DOI: 10.1177/0269215517698834; PUBMED: 29050511] [DOI] [PubMed] [Google Scholar]
Thakkar 2014 {published data only}
- Thakkar P. Effect of modified constraint induced movement therapy on hand function of hemiplegic cerebral palsy. International Journal of Current Research and Review 2014;6(17):29‐36. [www.ijcrr.com/uploads/770_pdf.pdf] [Google Scholar]
Thompson 2015 {published data only}
- Thompson AM, Chow S, Vey C, Lloyd M. Constraint‐induced movement therapy in children aged 5 to 9 years with cerebral palsy: a day camp model. Pediatric Physical Therapy 2015;27(1):72‐80. [DOI: 10.1097/PEP.0000000000000111; PUBMED: 25521268] [DOI] [PubMed] [Google Scholar]
Tinderholt Myrhaug 2014 {published data only}
- Tinderholt Myrhaug H, Østensjø S, Larun L, Odgaard‐Jensen J, Jahnsen R. Intensive training of motor function and functional skills among young children with cerebral palsy: a systematic review and meta‐analysis. BMC Pediatrics 2014;14:292. [DOI: 10.1186/s12887-014-0292-5; CRD42013004023; PMC4265534; PUBMED: 25475608] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tucker 2015 {published data only}
- Tucker R, Westwater‐Wood S. Constraint induced movement therapy in hemiplegic cerebral palsy: are we ready for a guideline? A review of the literature. Physiotherapy 2015;101(Suppl 1):e1546. [DOI: 10.1016/j.physio.2015.03.1540; RR‐PO‐17‐18‐Sat] [DOI] [Google Scholar]
Vaghela Vishwas 2014 {published data only}
- Vaghela Vishwas G. To study the effects of Mcimt versus Cimt for young children with spastic hemiplegic cerebral palsy ‐ a comparative study. Indian Journal of Physiotherapy and Occupational Therapy ‐ An International Journal 2014;8(2):136‐41. [DOI: 10.5958/j.0973-5674.8.2.075] [DOI] [Google Scholar]
Wallen 2004 {published data only}
- Wallen M, Hoare B. There is weak evidence that forced‐use therapy provided for 1‐month without additional therapy improved the fine motor function of children with hemiparesis. Australian Occupational Therapy Journal 2004;51(2):110‐1. [DOI: 10.1111/j.1440-1630.2004.00435.x] [DOI] [Google Scholar]
Wallen 2008 {published data only}
- Wallen MA, Zivianni J, Herbert R, Evans R, Novak I. Modified constraint‐induced therapy for children with hemiplegic cerebral palsy: a feasibility study. Developmental Neurorehabilitation 2008;11(2):124‐33. [DOI: 10.1080/17518420701640897; PUBMED: 17943505] [DOI] [PubMed] [Google Scholar]
Walther 2009 {published data only}
- Walther M, Juenger H, Kuhnke N, Wilke M, Brodbeck V, Berweck S, et al. Motor cortex plasticity in ischemic perinatal stroke: a transcranial magnetic stimulation and functional MRI study. Pediatric Neurology 2009;41(3):171‐8. [DOI: 10.1016/j.pediatrneurol.2009.04.006; PUBMED: 19664531] [DOI] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang T‐N, Wu C‐Y, Chen C‐L, Shieh J‐Y, Lu L, Lin K‐C. Logistic regression analyses for predicting clinically important differences in motor capacity, motor performance, and functional independence after constraint‐induced therapy in children with cerebral palsy. Research in Developmental Disabilities 2013;34(3):1044‐51. [DOI: 10.1016/j.ridd.2012.11.012; PUBMED: 23291522] [DOI] [PubMed] [Google Scholar]
Willis 2002 {published data only}
- Willis JK, Morello A, Davie A, Rice JC, Bennett JT. Forced use treatment of childhood hemiparesis. Pediatrics 2002;110(1 Pt 1):94‐6. [PUBMED: 12093952] [DOI] [PubMed] [Google Scholar]
Wu 2013 {published data only}
- Wu WC, Hung JW, Tseng CY, Huang YC. Group constraint‐induced movement therapy for children with hemiplegic cerebral palsy: a pilot study. American Journal of Occupational Therapy 2013;67(2):201‐8. [DOI: 10.5014/ajot.2013.004374; PUBMED: 23433275] [DOI] [PubMed] [Google Scholar]
Yasukawa 1990 {published data only}
- Yasukawa A. Upper extremity casting: adjunct treatment for a child with hemiplegic cerebral palsy. American Journal of Occupational Therapy 1990;44(9):840‐6. [2221004] [DOI] [PubMed] [Google Scholar]
Yu 2012b {published data only}
- Yu S, Fetters L. Commentary on “Effects of constraint‐induced movement therapy on gait, balance, and functional locomotor mobility”. Pediatric Physical Therapy 2012;24(1):69. [DOI: 10.1097/PEP.0b013e31823e08d7; PUBMED: 22207473] [DOI] [PubMed] [Google Scholar]
Zipp 2012 {published data only}
- Zipp GP, Winning S. Effects of constraint‐induced movement therapy on gait, balance, and functional locomotor mobility. Pediatric Physical Therapy 2012;24(1):64‐8. [DOI: 10.1097/PEP.0b013e31823e0245; PUBMED: 22207472] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Boyd 2017 {published data only}
- Boyd RN, Ziviani J, Sakzewski L, Novak I, Badawi N, Pannek K, et al. REACH: study protocol of a randomised trial of rehabilitation very early in congenital hemiplegia. BMJ Open 2017;7(9):e017204‐19. [DOI: 10.1136/bmjopen-2017-017204; ACTRN12615000180516; PMC5623522; PUBMED: 28928195] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamudot 2016 {published data only}
- Chamudot R, Parush S, Gross‐Tsur V. Modified‐constraint induced movement therapy for infants with hemiplegia: a randomized trial. Developmental Medicine & Child Neurology 2016;58(Suppl 6):54‐5. [DOI: 10.1111/dmcn.13322; NCT01214902] [DOI] [Google Scholar]
Chorna 2015 {published data only}
- Chorna O, Heathcock J, Key A, Noritz G, Carey H, Hamm E, et al. Early childhood constraint therapy for sensory/motor impairment in cerebral palsy: a randomised clinical trial protocol. BMJ Open 2015;5(12):e010212. [DOI: 10.1136/bmjopen-2015-010212; NCT02567630; PMC4679990; PUBMED: 26644127] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02346825 {published data only}
- NCT02346825. The Baby CHAMP Study (Children With Hemiparesis Arm and Movement Project) (The Baby CHAMP) [Multisite RCT of 3 neurorehabilitation therapies for infants with asymmetrical cerebral palsy]. clinicaltrials.gov/ct2/show/NCT02346825 (first received 15 January 2015). [NCT02346825]
NCT02808195 {published data only}
- NCT02808195. Comparative effectiveness of a Kinect‐based unilateral arm training system vs constraint‐induced therapy for children with cerebral palsy. clinicaltrials.gov/ct2/show/NCT02808195 (first received 24 May 2016). [NCT02808195]
NCT02840643 {published data only}
- NCT02840643. Combined constraint therapy and bimanual therapy for children with unilateral brain injury. clinicaltrials.gov/ct2/show/NCT02840643 (first received 19 July 2016). [NCT00119132]
NCT02875054 {published data only}
- NCT02875054. Camp High 5: evaluation of the effect on upper limb function. clinicaltrials.gov/ct2/show/NCT02875054 (first received 12 August 2016). [NCT02875054]
NCT02918890 {published data only}
- NCT02918890. Intensive unimanual (CIMT) and bimanual training (HABIT) in children with hemiplegia. clinicaltrials.gov/ct2/show/NCT02918890 (first received 27 September 2016). [NCT02918890]
Additional references
Aarts 2007
- Aarts PB, Jongerius PH, Aarts MA, Hartingsveldt MJ, Anderson PG, Beumer A. A pilot study of the Video Observations Aarts and Aarts (VOAA): a new software program to measure motor behaviour in children with cerebral palsy. Occupational Therapy International 2007;14(2):113‐22. [DOI: 10.1002/oti.229; PUBMED: 17623383] [DOI] [PubMed] [Google Scholar]
Aarts 2009
- Aarts PB, Jongerius PH, Geerdink YA, Geurts AC. Validity and reliability of the VOAA‐DDD to assess spontaneous hand use with a video observation tool in children with spastic unilateral cerebral palsy. BMC Musculoskeletal Disorders 2009;10:145. [DOI: 10.1186/1471-2474-10-145; PMC2790438; PUBMED: 19939255] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACPR 2016
- Australian Cerebral Palsy Register. Report of the Australian Cerebral Palsy Register, Birth Years 1993‐2009. www.cpregister.com/pubs/pdf/ACPR‐Report_Web_2016.pdf (accessed 28 March 2016).
Adair 2015
- Adair B, Ullenhag A, Keen D, Granlund M, Imms C. The effect of interventions aimed at improving participation outcomes for children with disabilities: a systematic review. Developmental Medicine & Child Neurology 2015;57(12):1093‐104. [DOI: 10.1111/dmcn.12809; PUBMED: 26010935] [DOI] [PubMed] [Google Scholar]
Amer 2016
- Amer A, Eliasson AC, Peny‐Dahlstrand M, Hermansson L. Validity and test‐retest reliability of Children's Hand‐use Experience Questionnaire in children with unilateral cerebral palsy. Developmental Medicine & Child Neurology 2016;58(7):743‐9. [DOI: 10.1111/dmcn.12991; PUBMED: 26610725] [DOI] [PubMed] [Google Scholar]
Arner 2008
- Arner M, Eliasson AC, Nicklasson S, Sommerstein K, Hägglund G. Hand function in cerebral palsy. Report of 367 children in a population‐based longitudinal health care program. Journal of Hand Surgery 2008;33(8):1337‐47. [DOI: 10.1016/j.jhsa.2008.02.032; PUBMED: 18929198] [DOI] [PubMed] [Google Scholar]
Arnould 2004
- Arnould C, Penta M, Renders A, Thonnard JL. ABILHAND‐Kids: a measure of manual ability in children with cerebral palsy. Neurology 2004;63(6):1045‐52. [DOI: 10.1212/01.WNL.0000138423.77640.37; PUBMED: 15452296] [DOI] [PubMed] [Google Scholar]
Autti‐Rämö 2006
- Autti‐Rämö I, Suoranta J, Anttila H, Malmivaara A, Mäkelä M. Effectiveness of upper and lower limb casting and orthoses in children with cerebral palsy: an overview of review articles. American Journal of Physical Medicine and Rehabilitation 2006;85(1):89‐103. [DOI: 10.1097/01.phm.0000179442.59847.27; PUBMED: 16357554] [DOI] [PubMed] [Google Scholar]
Beckung 2002
- Beckung E, Hagberg G. Neuroimpairments, activity limitations, and participation restrictions in children with cerebral palsy. Developmental Medicine & Child Neurology 2002;44(5):309‐16. [DOI: 10.1111/j.1469-8749.2002.tb00816.x; PUBMED: 12033716] [DOI] [PubMed] [Google Scholar]
Bleyenheuft 2017
- Bleyenheuft Y, Gordon AM, Rameckers E, Thonnard JL, Arnould C. Measuring changes of manual ability with ABILHAND‐Kids following intensive training for children with unilateral cerebral palsy. Developmental Medicine & Child Neurology 2017;59(5):505‐11. [DOI: 10.1111/dmcn.13338; PUBMED: 27896811] [DOI] [PubMed] [Google Scholar]
Bodimeade 2013
- Bodimeade HL, Whittingham K, Lloyd O, Boyd RN. Executive function in children and adolescents with unilateral cerebral palsy. Developmental Medicine & Child Neurology 2013;55(10):926‐33. [DOI: 10.1111/dmcn.12195; PUBMED: 23809003] [DOI] [PubMed] [Google Scholar]
Brown 1987
- Brown J, Rensburg F, Walsh G, Lakie M, Wright GW. A neurological study of hand function of hemiplegic children. Developmental Medicine & Child Neurology 1987;29(3):287‐304. [DOI: 10.1111/j.1469-8749.1987.tb02482.x; PUBMED: 3596065] [DOI] [PubMed] [Google Scholar]
Bruchez 2016
- Bruchez R, Jequier Gygax M, Roches S, Fluss J, Jacquier D, Ballabeni P, et al. Mirror therapy in children with hemiparesis: a randomized observer‐blinded trial. Developmental Medicine & Child Neurology 2016;58(9):970‐8. [DOI: 10.1111/dmcn.13117; PUBMED: 27046296] [DOI] [PubMed] [Google Scholar]
Cameron 2017
- Cameron D, Craig T, Edwards B, Missiuna C, Schwellnus H, Polatajko HJ. Cognitive Orientation to daily Occupational Performance (CO‐OP): a new approach for children with cerebral palsy. Physical & Occupational Therapy in Pediatrics 2017;37(2):183‐98. [DOI: 10.1080/01942638.2016.1185500; PUBMED: 27282077] [DOI] [PubMed] [Google Scholar]
Carswell 2004
- Carswell A, McColl MA, Baptiste S, Law M, Polatajko HJ, Pollock N. The Canadian Occupational Performance Measure: a research and clinical literature review. Canadian Journal of Occupational Therapy 2004;71(4):210‐22. [DOI: 10.1177/000841740407100406; PUBMED: 15586853] [DOI] [PubMed] [Google Scholar]
Case‐Smith 2012
- Case‐Smith J, DeLuca SC, Stevenson R, Ramey SL. Multicenter randomized controlled trial of pediatric constraint‐induced movement therapy: 6‐month follow‐up. American Journal of Occupational Therapy 2012;66(1):15‐23. [DOI: 10.5014/ajot.2012.002386; NCT00991692; PUBMED: 22389937] [DOI] [PubMed] [Google Scholar]
Chiu 2014
- Chiu H‐C, Ada L, Lee H‐M. Upper limb training using Wii Sports Resort for children with hemiplegic cerebral palsy: a randomized, single‐blind trial. Clinical Rehabilitation 2014;28(10):1015‐24. [DOI: 10.1177/0269215514533709; PUBMED: 24849793] [DOI] [PubMed] [Google Scholar]
Clopton 2005
- Clopton N, Dutton J, Featherston T, Grigsby A, Mobley J, Melvin J. Interrater and intrarater reliability of the Modified Ashworth Scale in children with hypertonia. Pediatric Physical Therapy 2005;17(4):268‐74. [DOI: 10.1097/01.pep.0000186509.41238.1a; PUBMED: 16357682] [DOI] [PubMed] [Google Scholar]
Cusick 2006
- Cusick A, McIntyre S, Novak I, Lannin N, Lowe K. A comparison of goal attainment scaling and the Canadian Occupational Performance Measure for pediatric rehabilitation research. Pediatric Rehabilitation 2006;9(2):149‐57. [DOI: 10.1080/13638490500235581; PUBMED: 16449074] [DOI] [PubMed] [Google Scholar]
Cusick 2007
- Cusick A, Lannin NA, Lowe K. Adapting the Canadian Occupational Performance Measure for use in a paediatric clinical trial. Disability and Rehabilitation 2007;29(10):761‐6. [DOI: 10.1080/09638280600929201; PUBMED: 17457734] [DOI] [PubMed] [Google Scholar]
Damiano 2014
- Damiano DL. Meaningfulness of mean group results for determining the optimal motor rehabilitation program for an individual child with cerebral palsy. Developmental Medicine & Child Neurology 2014;56(12):1141‐6. [DOI: 10.1111/dmcn.12505; PMC4229436; PUBMED: 24919877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davids 2006
- Davids JR, Peace LC, Wagner LV, Gidewall MA, Blackhurst DW, Roberson WM. Validation of the Shriners Hospital for Children Upper Extremity Evaluation (SHUEE) for children with hemiplegic cerebral palsy. Journal of Bone and Joint Surgery 2006;88(2):326‐33. [DOI: 10.2106/JBJS.E.00298; PUBMED: 16452744] [DOI] [PubMed] [Google Scholar]
Davis 2013
- Davis E, Mackinnon A, Davern M, Boyd R, Bohanna I, Waters E, et al. Description and psychometric properties of the CP QOL‐Teen: a quality of life questionnaire for adolescents with cerebral palsy. Research in Developmental Disabilities 2013;34(1):344‐52. [DOI: 10.1016/j.ridd.2012.08.018; PUBMED: 22989577] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI: 10.1136/bmj.315.7109.629; PMC2127453; PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eliasson 1995
- Eliasson A‐C, Gordon AM, Forssberg H. Tactile control of isometric fingertip forces during grasping in children with cerebral palsy. Developmental Medicine & Child Neurology 1995;37(1):72‐84. [DOI: 10.1111/j.1469-8749.1995.tb11933.x; PUBMED: 7828788] [DOI] [PubMed] [Google Scholar]
Eliasson 2006
- Eliasson A‐C, Krumlinde‐Sundholm L, Rösblad B, Beckung E, Arner M, Ohrvall A‐M, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Developmental Medicine & Child Neurology 2006;48(7):549‐54. [DOI: 10.1017/S0012162206001162; PUBMED: 16780622] [DOI] [PubMed] [Google Scholar]
Eliasson 2017
- Eliasson A‐C, Ullenhag A, Wahlström U, Krumlinde‐Sundholm L. Mini‐MACS: development of the Manual Ability Classification System for children younger than 4 years of age with signs of cerebral palsy. Developmental Medicine & Child Neurology 2017;59(1):72‐8. [DOI: 10.1111/dmcn.13162; PUBMED: 27273427] [DOI] [PubMed] [Google Scholar]
Elliott 2011
- Elliott C, Reid S, Hamer P, Alderson J, Elliott B. Lycra® arm splints improve movement fluency in children with cerebral palsy. Gait & Posture 2011;33(2):214‐9. [DOI: 10.1016/j.gaitpost.2010.11.008; PUBMED: 21131201] [DOI] [PubMed] [Google Scholar]
EndNote [Computer program]
- Thomson Reuters. EndNote X7.4. New York (NY): Thomson Reuters, 2015.
Fauconnier 2009
- Fauconnier J, Dickinson HO, Beckung E, Marcelli M, McManus V, Michelsen SI, et al. Participation in life situations of 8‐12 year old children with cerebral palsy: cross sectional European study. BMJ 2009;338:b1458. [DOI: 10.1136/bmj.b1458; PMC2673343; PUBMED: 19395424] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldman 1990
- Feldman AB, Haley SM, Coryell J. Concurrent and construct validity of the Pediatric Evaluation of Disability Inventory. Physical Therapy 1990;70(10):602‐10. [PUBMED: 2217539] [DOI] [PubMed] [Google Scholar]
Friel 2014
- Friel KM, Williams PT, Serradj N, Chakrabarty S, Martin JH. Activity‐based therapies for repair of the corticospinal system injured during development. Frontiers in Neurology 2014;5:229. [DOI: 10.3389/fneur.2014.00229; PMC4241838; PUBMED: 25505443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilliaux 2015
- Gilliaux M, Renders A, Dispa D, Holvoet D, Sapin J, Dehez B, et al. Upper limb robot‐assisted therapy in cerebral palsy: a single‐blind randomized controlled trial. Neurorehabilitation and Neural Repair 2015;29(2):183‐92. [DOI: 10.1177/1545968314541172; PUBMED: 25015650] [DOI] [PubMed] [Google Scholar]
Gilmore 2009
- Gilmore L, Cuskelly M. Factor structure of the Parenting Sense of Competence scale using a normative sample. Child: Care, Health and Development 2009;35(1):48‐55. [DOI: 10.1111/j.1365-2214.2008.00867.x; PUBMED: 18991983] [DOI] [PubMed] [Google Scholar]
Gilson 2014
- Gilson K‐M, Davis E, Reddihough D, Graham K, Waters E. Quality of life in children with cerebral palsy: Implications for practice. Journal of Child Neurology 2014;29(8):1134‐40. [DOI: 10.1177/0883073814535502; PUBMED: 24870369] [DOI] [PubMed] [Google Scholar]
Glazier 1997
- Glazier JN, Fehlings DL, Steele CA. Test‐retest reliability of upper extremity goniometric measurements of passive range of motion and of sphygmomanometer measurements of grip strength in children with cerebral palsy and upper extremity spasticity. Developmental Medicine & Child Neurology 1997;39(Suppl 75):33‐4. [DOI: 10.1111/j.1469-8749.1997.tb07505.x; SP: 16] [DOI] [Google Scholar]
Gracies 2010
- Gracies JM, Burke K, Clegg NJ, Browne R, Rushing C, Fehlings D, et al. Reliability of the Tardieu Scale for assessing spasticity in children with cerebral palsy. Archives of Physical Medicine and Rehabilitation 2010;91(3):421‐8. [DOI: 10.1016/j.apmr.2009.11.017; PUBMED: 20298834] [DOI] [PubMed] [Google Scholar]
GradePro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 April 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Green 2013
- Green D, Schertz M, Gordon AM, Moore A, Schejter Margalit T, Farquharson Y, et al. A multi‐site study of functional outcomes following a themed approach to hand‐arm bimanual intensive therapy for children with hemiplegia. Developmental Medicine & Child Neurology 2013;55(6):527‐33. [DOI: 10.1111/dmcn.12113; PUBMED: 23458353] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD; PMC2335261; PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI: 10.1016/j.jclinepi.2011.01.012; PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI: 10.1002/sim.1186; PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoare 2010
- Hoare BJ, Wallen MA, Imms C, Villanueva E, Rawicki HB, Carey L. Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy (UPDATE). Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003469.pub4; PUBMED: 20091546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoare 2011
- Hoare B, Imms C, Randall M, Carey L. Linking cerebral palsy upper limb measures to the International Classification of Functioning, Disability and Health. Journal of Rehabilitation Medicine 2011;43(11):987‐96. [DOI: 10.2340/16501977-0886; PUBMED: 22031344] [DOI] [PubMed] [Google Scholar]
Hoare 2017
- Hoare B, Greaves S. Unimanual versus bimanual therapy in children with unilateral cerebral palsy: same, same, but different. Journal of Pediatric Rehabilitation Medicine 2017;10(1):47‐59. [DOI: 10.3233/PRM-170410; PUBMED: 28339410] [DOI] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI: 10.1136/bmj.g1687; PUBMED: 24609605] [DOI] [PubMed] [Google Scholar]
Holmefur 2007
- Holmefur M, Krumlinde‐Sundholm L, Eliasson A‐C. Interrater and intrarater reliability of the Assisting Hand Assessment. American Journal of Occupational Therapy 2007;61(1):79‐84. [DOI: 10.5014/ajot.61.1.79; PUBMED: 17302108] [DOI] [PubMed] [Google Scholar]
Holmefur 2009
- Holmefur M, Aarts P, Hoare B, Krumlinde‐Sundholm L. Test‐retest and alternate forms reliability of the Assisting Hand Assessment. Journal of Rehabilitation Medicine 2009;41(11):886‐91. [DOI: 10.2340/16501977-0448; PUBMED: 19841839] [DOI] [PubMed] [Google Scholar]
Holmefur 2013
- Holmefur M, Kits A, Bergström J, Krumlinde‐Sundholm L, Flodmark O, Forssberg H, et al. Neuroradiology can predict the development of hand function in children with unilateral cerebral palsy. Neurorehabilitation and Neural Repair 2013;27(1):72‐8. [DOI: 10.1177/1545968312446950; PUBMED: 22677505] [DOI] [PubMed] [Google Scholar]
Holmefur 2016
- Holmefur MM, Krumlinde‐Sundholm L. Psychometric properties of a revised version of the Assisting Hand Assessment (Kids‐AHA 5.0). Developmental Medicine & Child Neurology 2016;58(6):618‐24. [DOI: 10.1111/dmcn.12939; PUBMED: 26507383] [DOI] [PubMed] [Google Scholar]
Holmström 2010
- Holmström L, Vollmer B, Tedroff K, Islam M, Persson JK, Kits A, et al. Hand function in relation to brain lesions and corticomotor‐projection pattern in children with unilateral cerebral palsy. Developmental Medicine & Child Neurology 2010;52(2):145‐52. [DOI: 10.1111/j.1469-8749.2009.03496.x; PUBMED: 19807768] [DOI] [PubMed] [Google Scholar]
Houwink 2013
- Houwink A, Geerdink YA, Steenbergen B, Geurts AC, Aarts PB. Assessment of upper‐limb capacity, performance, and developmental disregard in children with cerebral palsy: validity and reliability of the revised Video‐Observation Aarts and Aarts module: Determine Developmental Disregard (VOAA‐DDD‐R). Developmental Medicine & Child Neurology 2013;55(1):76‐82. [DOI: 10.1111/j.1469-8749.2012.04442.x; PUBMED: 23095032] [DOI] [PubMed] [Google Scholar]
Imms 2016a
- Imms C, Wallen M, Elliott C, Hoare B, Randall M, Greaves S, et al. Minimising impairment: protocol for a multicentre randomised controlled trial of upper limb orthoses for children with cerebral palsy. BMC Paediatrics 2016;16:70. [DOI: 10.1186/s12887-016-0608-8; ANZ Clinical Trials Registry: U1111‐1164‐0572 ; PMC4882829; PUBMED: 27230616] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imms 2016b
- Imms C, Adair B, Keen D, Ullenhag A, Rosenbaum P, Granlund M. ‘Participation’: a systematic review of language, definitions, and constructs used in intervention research with children with disabilities. Developmental Medicine & Child Neurology 2016;58(1):29‐38. [DOI: 10.1111/dmcn.12932; PUBMED: 26411643] [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gøtzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [DOI: 10.5014/ajot.2013.006643; PUBMED: 15545678] [DOI] [PubMed] [Google Scholar]
Jackman 2014
- Jackman M, Novak I, Lannin N. Effectiveness of hand splints in children with cerebral palsy: a systematic review with meta‐analysis. Developmental Medicine & Child Neurology 2014;56(2):138‐47. [DOI: 10.1111/dmcn.12205; PUBMED: 23848480] [DOI] [PubMed] [Google Scholar]
James 2014
- James S, Ziviani J, Boyd R. A systematic review of activities of daily living measures for children and adolescents with cerebral palsy. Developmental Medicine & Child Neurology 2014;56(3):233‐44. [DOI: 10.1111/dmcn.12226; PUBMED: 23937056] [DOI] [PubMed] [Google Scholar]
Jongbloed‐Pereboom 2013
- Jongbloed‐Pereboom M, Nijhuis‐van der Sanden MW, Steenbergen B. Norm scores of the box and block test for children ages 3‐10 years. American Journal of Occupational Therapy 2013;67(3):312‐8. [DOI: 10.5014/ajot.2013.006643; PUBMED: 23597689] [DOI] [PubMed] [Google Scholar]
Kirkpatrick 2016
- Kirkpatrick E, Pearse J, James P, Basu A. Effect of parent‐delivered action observation therapy on upper limb function in unilateral cerebral palsy: a randomized controlled trial. Developmental Medicine & Child Neurology 2016;58(10):1049‐56. [DOI: 10.1111/dmcn.13109; PUBMED: 27038153] [DOI] [PubMed] [Google Scholar]
Klingels 2010
- Klingels K, Cock P, Molenaers G, Desloovere K, Huenaerts C, Jaspers E, et al. Upper limb motor and sensory impairments in children with hemiplegic cerebral palsy: can they be measured reliably?. Disability and Rehabilitation 2010;32(5):409‐16. [DOI: 10.3109/09638280903171469; PUBMED: 20095955] [DOI] [PubMed] [Google Scholar]
Klingels 2012
- Klingels K, Demeyere I, Jaspers E, Cock P, Molenaers G, Boyd R, et al. Upper limb impairments and their impact on activity measures in children with unilateral cerebral palsy. European Journal of Paediatric Neurology 2012;16(5):475‐84. [DOI: 10.1016/j.ejpn.2011.12.008; PUBMED: 22244966] [DOI] [PubMed] [Google Scholar]
Kontopantelis 2012
- Kontopantelis E, Reeves D. Performance of statistical methods for meta‐analysis when true study effects are non‐normally distributed: a simulation study. Statistical Methods in Medical Research 2012;21(4):409‐26. [DOI: 10.1177/0962280210392008; PUBMED: 21148194] [DOI] [PubMed] [Google Scholar]
Krumlinde‐Sundholm 1998
- Krumlinde‐Sundholm L, Eliasson A‐C, Forssberg H. Obstetric brachial plexus injuries: assessment protocol and functional outcome at age 5 years. Developmental Medicine & Child Neurology 1998;40(1):4‐11. [PUBMED: 9459211] [DOI] [PubMed] [Google Scholar]
Krumlinde‐Sundholm 2003
- Krumlinde‐Sundholm L, Eliasson A‐C. Development of the Assisting Hand Assessment: a Rasch‐built measure intended for children with unilateral upper limb impairment. Scandinavian Journal of Occupational Therapy 2003;10(1):16‐26. [DOI: 10.1080/11038120310004529; psycnet.apa.org/record/2018‐08451‐001] [DOI] [Google Scholar]
Krumlinde‐Sundholm 2007
- Krumlinde‐Sundholm L, Holmefur M, Kottorp A, Eliasson A‐C. The Assisting Hand Assessment: current evidence of validity, reliability, and responsiveness to change. Developmental Medicine & Child Neurology 2007;49(4):259‐64. [DOI: 10.1111/j.1469-8749.2007.00259.x; PUBMED: 17376135] [DOI] [PubMed] [Google Scholar]
Krumlinde‐Sundholm 2012
- Krumlinde‐Sundholm L. Reporting outcomes of the Assisting Hand Assessment: what scale should be used?. Developmental Medicine & Child Neurology 2012;54(9):807‐8. [DOI: 10.1111/j.1469-8749.2012.04361.x; PUBMED: 22803624] [DOI] [PubMed] [Google Scholar]
Krumlinde‐Sundholm 2017
- Krumlinde‐Sundholm L, Ek L, Sicola E, Sjöstrand L, Guzzetta A, Sgandurra G, et al. Development of the Hand Assessment for Infants: evidence of internal scale validity. Developmental Medicine & Child Neurology 2017;59(12):1276‐83. [DOI: 10.1111/dmcn.13585; PUBMED: 28984352] [DOI] [PubMed] [Google Scholar]
Kuhtz‐Buschbeck 2000
- Kuhtz‐Buschbeck JP, Krumlinde‐Sundholm L, Eliasson A‐C, Forssberg H. Quantitative assessment of mirror movements in children and adolescents with hemiplegic cerebral palsy. Developmental Medicine & Child Neurology 2000;42(11):728‐36. [DOI: 10.1111/j.1469-8749.2000.tb00034.x; PUBMED: 11104343] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lin 2012
- Lin K‐C, Chen H‐F, Chen C‐L, Wang T‐N, Wu C‐Y, Hsieh Y‐W, et al. Validity, responsiveness, minimal detectable change, and minimal clinically important change of the Pediatric Motor Activity Log in children with cerebral palsy. Research in Developmental Disabilities 2012;33(2):570‐7. [DOI: 10.1016/j.ridd.2011.10.003; PUBMED: 22119706] [DOI] [PubMed] [Google Scholar]
Löwing 2010
- Löwing K, Bexelius A, Carlberg EB. Goal‐directed functional therapy: a longitudinal study on gross motor function in children with cerebral palsy. Disability and Rehabilitation 2010;32(11):908‐16. [DOI: 10.3109/09638280903353422; PUBMED: 19852713] [DOI] [PubMed] [Google Scholar]
Mackey 2004
- Mackey AH, Walt SE, Lobb G, Stott SN. Intraobserver reliability of the modified Tardieu scale in the upper limb of children with hemiplegia. Developmental Medicine & Child Neurology 2004;46(4):267‐72. [DOI: 10.1111/j.1469-8749.2004.tb00481.x; PUBMED: 15077704] [DOI] [PubMed] [Google Scholar]
Maher 2003
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomised controlled trials. Physical Therapy 2003;83(8):713‐21. [PUBMED: 12882612] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2001
- Morris DM, Taub E. Constraint‐induced therapy approach to restoring function after neurological injury. Topics in Stroke Rehabilitation 2001;8(3):16‐30. [DOI: 10.1310/BLJX-M89N-PTPY-JDKW; PUBMED: 14523735] [DOI] [PubMed] [Google Scholar]
Noreau 2007
- Noreau L, Lepage C, Boissiere L, Picard R, Fougeyrollas P, Mathieu J, et al. Measuring participation in children with disabilities using the Assessment of Life Habits. Developmental Medicine & Child Neurology 2007;49(9):666‐71. [DOI: 10.1111/j.1469-8749.2007.00666.x; PUBMED: 17718822] [DOI] [PubMed] [Google Scholar]
Novak 2009
- Novak I, Cusick A, Lannin N. Occupational therapy home programs for cerebral palsy: double‐blind, randomized, controlled trial. Pediatrics 2009;124(4):e606‐14. [DOI: 10.1542/peds.2009-0288; PUBMED: 19770175] [DOI] [PubMed] [Google Scholar]
Nüesch 2009
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Bürgi E, Scherer M, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta‐epidemiological study. BMJ 2009;339:b3244. [DOI: 10.1136/bmj.b3244] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palisano 2008
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine & Child Neurology 2008;39(4):214–23. [DOI: 10.1111/j.1469-8749.1997.tb07414.x] [DOI] [PubMed] [Google Scholar]
Piper 1992
- Piper MC, Pinnell LE, Darrah J, Maguire T, Byrne PJ. Construction and validation of the Alberta Infant Motor Scale (AIMS). Canadian Journal of Public Health 1992;83(Suppl 2):S46‐50. [PUBMED: 1468050] [PubMed] [Google Scholar]
Rameckers 2015
- Rameckers EA, Janssen‐Potten YJ, Essers IM, Smeets RJ. Efficacy of upper limb strengthening in children with cerebral palsy: a critical review. Research in Developmental Disabilities 2015;36C:87‐101. [DOI: 10.1016/j.ridd.2014.09.024; PUBMED: 25462469] [DOI] [PubMed] [Google Scholar]
Randall 2008
- Randall M, Imms C, Carey L. Establishing validity of a modified Melbourne Assessment for children ages 2 to 4 years. American Journal of Occupational Therapy 2008;62(4):373‐83. [DOI: 10.5014/ajot.62.4.373; PUBMED: 18712000] [DOI] [PubMed] [Google Scholar]
Randall 2012
- Randall M, Imms C, Carey L. Further evidence of validity of the Modified Melbourne Assessment for neurologically impaired children aged 2 to 4 years. Developmental Medicine & Child Neurology 2012;54(5):424‐8. [DOI: 10.1111/j.1469-8749.2012.04252.x.; PUBMED: 22390189] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 2015
- Rhodes KM, Turner RM, Higgins JP. Predictive distributions were developed for the extent of heterogeneity in meta‐analyses of continuous outcome data. Journal of Clinical Epidemiology 2015;68(1):52‐60. [DOI: 10.1016/j.jclinepi.2014.08.012; PMC4270451; PUBMED: 25304503] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosa‐Rizzotto 2014
- Rosa‐Rizzotto M, Visona Dalla Pozza L, Corlatti A, Luparia A, Marchi A, Molteni F, et al. A new scale for the assessment of performance and capacity of hand function in children with hemiplegic cerebral palsy: reliability and validity studies. European Journal of Physical and Rehabiliation Medicine 2014;50(5):543‐56. [PUBMED: 24732444] [PubMed] [Google Scholar]
Rosenbaum 2009
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine & Child Neurology 2009;49(Suppl 109):8‐14. [DOI: 10.1111/j.1469-8749.2007.tb12610.x; PUBMED: 17370477] [DOI] [PubMed] [Google Scholar]
Ryan 2017
- Ryan JM, Cassidy EE, Noorduyn SG, O'Connell NE. Exercise interventions for cerebral palsy. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD011660.pub2; PUBMED: 28602046] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakzewski 2007
- Sakzewski L, Boyd R, Zivianni J. Clinimetric properties of participation measures for 5‐ to 13‐year old children with cerebral palsy: a systematic review. Developmental Medicine & Child Neurology 2007;49(3):232‐40. [DOI: 10.1111/j.1469-8749.2007.00232.x; PUBMED: 17355482] [DOI] [PubMed] [Google Scholar]
Sakzewski 2014b
- Sakzewski L, Ziviani J, Boyd RN. Delivering evidence‐based upper limb rehabilitation for children with cerebral palsy: barriers and enablers identified by three pediatric teams. Physical & Occupational Therapy in Pediatrics 2014;34(4):368‐83. [DOI: 10.3109/01942638.2013.861890; PUBMED: 24303800] [DOI] [PubMed] [Google Scholar]
Sakzewski 2016
- Sakzewski L, Reedman S, Hoffmann T. Do we really know what they were testing? Incomplete reporting of interventions in randomised trials of upper limb therapies in unilateral cerebral palsy. Research in Developmental Disabilities 2016;59:417‐27. [DOI: 10.1016/j.ridd.2016.09.018; PUBMED: 27736712] [DOI] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: Combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI: 10.3310/hta16350; PUBMED: 22989478] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI: 10.1001/jama.1995.03520290060030; PUBMED: 7823387] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; Vol. 340:c332. [DOI: 10.1136/bmj.c332; PMC2844940; PUBMED: 20332509] [DOI] [PMC free article] [PubMed]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sgandurra 2013
- Sgandurra G, Ferrari A, Cossu G, Guzzetta A, Fogassi L, Cioni G. Randomized trial of observation and execution of upper extremity actions versus action alone in children with unilateral cerebral palsy. Neurorehabilitation and Neural Repair 2013;27(9):808‐15. [DOI: 10.1177/1545968313497101; PUBMED: 23886886] [DOI] [PubMed] [Google Scholar]
Sköld 2011
- Sköld A, Hermansson LN, Krumlinde‐Sundholm L, Eliasson A‐C. Development and evidence of validity for the Children's Hand‐use Experience Questionnaire (CHEQ). Developmental Medicine & Child Neurology 2011;53(5):436‐42. [DOI: 10.1111/j.1469-8749.2010.03896.x; PUBMED: 21413973] [DOI] [PubMed] [Google Scholar]
Snider 2010
- Snider L, Majnemer A. Virtual reality: we are virtually there. Physical & Occupational Therapy in Pediatrics 2010;30(1):1‐3. [DOI: 10.3109/01942630903476131; PUBMED: 20170427] [DOI] [PubMed] [Google Scholar]
Steenbergen 2006
- Steenbergen B, Gordon AM. Activity limitation in hemiplegic cerebral palsy: evidence for disorders in motor planning. Developmental Medicine & Child Neurology 2006;48(9):780‐3. [DOI: 10.1017/S0012162206001666; PUBMED: 16904028] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting bias. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Taub 2002 [pers comm]
- Taub E. Re: Hello [personal communication]. Email to: B Hoare 14 November 2002.
The Kid Screen Group Europe
- The Kid Screen Group Europe. The KIDSCREEN Questionnaires. Quality of Life Questionnaires for Children and Adolescents. Handbook. Lengerich (DE): Pabst Science Publishers, 2006. [Google Scholar]
Thorley 2012
- Thorley M, Lannin N, Cusick A, Novak I, Boyd R. Construct validity of the Quality of Upper Extremity Skills Test for children with cerebral palsy. Developmental Medicine & Child Neurology 2012;54(11):1037‐43. [DOI: 10.1111/j.1469-8749.2012.04368.x; PUBMED: 22845645] [DOI] [PubMed] [Google Scholar]
Uswatte 2012a
- Uswatte G, Taub E, Griffin A, Rowe J, Vogtle L, Barman J. Pediatric Arm Function Test: reliability and validity for assessing more‐affected arm motor capacity in children with cerebral palsy. American Journal of Physical Medicine & Rehabilitation 2012;91(12):1060‐9. [DOI: 10.1097/PHM.0b013e318269ec76; PMC3501559; PUBMED: 23103486] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uswatte 2012b
- Uswatte G, Taub E, Griffin A, Vogtle L, Rowe J, Barman J. The Pediatric Motor Activity Log‐Revised: assessing real‐world arm use in children with cerebral palsy. Rehabilitation Psychology 2012;57(2):149‐58. [DOI: 10.1037/a0028516; PMC3375622; PUBMED: 22686553] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Heest 2015
- Heest AE, Bagley A, Molitor F, James MA. Tendon transfer surgery in upper‐extremity cerebral palsy is more effective than botulinum toxin injections or regular, ongoing therapy. Journal of Bone and Joint Surgery 2015;97(7):529‐36. [DOI: 10.2106/JBJS.M.01577; PUBMED: 25834076] [DOI] [PubMed] [Google Scholar]
Varni 2006
- Varni JW, Burwinkle TM, Berrin SJ, Sherman SA, Artavia K, Malcarne VL, et al. The PedsQL in pediatric cerebral palsy: reliability, validity, and sensitivity of the Generic Core Scales and Cerebral Palsy Module. Developmental Medicine & Child Neurology 2006;48(6):442‐9. [DOI: 10.1017/S001216220600096X; PUBMED: 16700934] [DOI] [PubMed] [Google Scholar]
Varni 2008
- Varni JW, Limbers CA. The PedsQL Multidimensional Fatigue Scale in young adults: feasibility, reliability and validity in a university student population. Quality of Life Research 2008;17(1):105‐14. [DOI: 10.1007/s11136-007-9282-5; PUBMED: 18027106] [DOI] [PubMed] [Google Scholar]
Varni 2011
- Varni JW, Limbers CA, Neighbors K, Schulz K, Lieu JE, Heffer RW, et al. The PedsQL™ Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Quality of Life Research 2011;20(1):45‐55. [DOI: 10.1007/s11136-010-9730-5; PUBMED: 20730626] [DOI] [PubMed] [Google Scholar]
Wallen 2009b
- Wallen M, Bundy A, Pont K, Ziviani J. Psychometric properties of the Pediatric Motor Activity Log used for children with cerebral palsy. Developmental Medicine & Child Neurology 2009;51(3):200‐8. [DOI: 10.1111/j.1469-8749.2008.03157.x; PUBMED: 19018839] [DOI] [PubMed] [Google Scholar]
Wallen 2013
- Wallen M, Ziviani J. Caution regarding the Pediatric Motor Activity Log to measure upper limb intervention outcomes for children with unilateral cerebral palsy. Developmental Medicine & Child Neurology 2013;55(6):497‐8. [DOI: 10.1111/dmcn.12057; PUBMED: 23336281] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI: 10.1186/1471-2288-14-135; PMC4383202; PUBMED: 25524443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2014
- Weiss PL, Tirosh E, Fehlings D. Role of virtual reality for cerebral palsy management. Journal of Child Neurology 2014;29(8):1119‐24. [DOI: 10.1177/0883073814533007; PUBMED: 24799367] [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. ICF: International Classification of Functioning, Disability and Health ‐ Short Version. www.who.int/iris/handle/10665/42417 (accessed 23 March 2006).
Xu 2015
- Xu K, He L, Mai J, Yan X, Chen Y. Muscle recruitment and coordination following constraint‐induced movement therapy with electrical stimulation on children with hemiplegic cerebral palsy: a randomized controlled trial. PLOS One 2015;10(10):e0138608. [DOI: 10.1371/journal.pone.0138608; PMC4599892; chictr.org: ChiCTR‐TRC‐13004041; PUBMED: 26452230] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yıldızgören 2014
- Yıldızgören MT, Nakipoğlu Yüzer GF, Ekiz T, Özgirgin N. Effects of neuromuscular electrical stimulation on the wrist and finger flexor spasticity and hand functions in cerebral palsy. Pediatric Neurology 2014;51(3):360‐4. [DOI: 10.1016/j.pediatrneurol.2014.05.009; PUBMED: 25011433] [DOI] [PubMed] [Google Scholar]
Zielinski 2014a
- Zielinski IM, Jongsma ML, Baas CM, Aarts PB, Steenbergen B. Unravelling developmental disregard in children with unilateral cerebral palsy by measuring event‐related potentials during a simple and complex task. BMC Neurology 2014;14:6. [DOI: 10.1186/1471-2377-14-6; PMC3893558; PUBMED: 24397355] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zielinski 2014b
- Zielinski IM, Steenbergen B, Baas CM, Aarts PB, Jongsma ML. Neglect‐like characteristics of developmental disregard in children with cerebral palsy revealed by event related potentials. BMC Neurology 2014;14:221. [DOI: 10.1186/s12883-014-0221-0; PMC4258290; PUBMED: 25433482] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziviani 2008
- Ziviani J, Wallen M. Participation: the ultimate challenge. In: Eliasson A‐C, Burtner PA editor(s). Improving Hand Function in Children with Cerebral Palsy: Theory, Evidence and Intervention. London (UK): Mac Keith Press, 2008:385‐95. [Google Scholar]
References to other published versions of this review
Hoare 2002
- Hoare BJ, Wasiak J. Constraint induced movement therapy in the treatment of the upper limb in children with spastic hemiplegic cerebral palsy. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD004149] [DOI] [PubMed] [Google Scholar]
Hoare 2007a
- Hoare B, Imms C, Carey L, Wasiak J. Constraint‐induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy: a Cochrane systematic review. Clinical Rehabilitation 2007;21(8):675‐85. [DOI: 10.1002/14651858.CD004149.pub2; PUBMED: 17846067] [DOI] [PubMed] [Google Scholar]
Hoare 2007b
- Hoare BJ, Wasiak J, Imms C, Carey L. Constraint‐induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004149.pub2; PUBMED: 17443542] [DOI] [PubMed] [Google Scholar]


