Significance
Mitochondria are the compartments in animal cells that produce the most energy and are often targets of bacterial toxins during infection. In response, hosts employ an adaptive transcriptional response known as the mitochondrial unfolded protein response (UPRmt) to maintain mitochondrial function and eliminate the toxic bacteria. Here, we demonstrate that the pathogen Pseudomonas aeruginosa exploits a negative regulatory mechanism built into the UPRmt to prevent activation of the antibacterial response. Impressively, if the negative regulator ZIP-3 is inhibited, worms are resistant to infection as they are able to effectively activate the UPRmt. The pathogen potentially evolved means to impair the UPRmt because a virulence determinant that ordinarily maintains biofilm metabolism perturbs mitochondrial function eliciting the antibacterial response.
Keywords: mitochondrial UPR, ZIP-3, ATFS-1, UPRmt, immunity
Abstract
Mitochondria generate most cellular energy and are targeted by multiple pathogens during infection. In turn, metazoans employ surveillance mechanisms such as the mitochondrial unfolded protein response (UPRmt) to detect and respond to mitochondrial dysfunction as an indicator of infection. The UPRmt is an adaptive transcriptional program regulated by the transcription factor ATFS-1, which induces genes that promote mitochondrial recovery and innate immunity. The bacterial pathogen Pseudomonas aeruginosa produces toxins that disrupt oxidative phosphorylation (OXPHOS), resulting in UPRmt activation. Here, we demonstrate that Pseudomonas aeruginosa exploits an intrinsic negative regulatory mechanism mediated by the Caenorhabditis elegans bZIP protein ZIP-3 to repress UPRmt activation. Strikingly, worms lacking zip-3 were impervious to Pseudomonas aeruginosa-mediated UPRmt repression and resistant to infection. Pathogen-secreted phenazines perturbed mitochondrial function and were the primary cause of UPRmt activation, consistent with these molecules being electron shuttles and virulence determinants. Surprisingly, Pseudomonas aeruginosa unable to produce phenazines and thus elicit UPRmt activation were hypertoxic in zip-3–deletion worms. These data emphasize the significance of virulence-mediated UPRmt repression and the potency of the UPRmt as an antibacterial response.
Metazoans differentiate pathogenic and commensal bacteria in part by monitoring the integrity of essential intracellular activities. For example, the opportunistic pathogen Pseudomonas aeruginosa secretes multiple toxins that perturb host protein synthesis and mitochondrial function (1–3). Interestingly, disruption of either process, independent of bacterial infection, elicits immune response activation (4–7). P. aeruginosa secretes multiple toxins capable of perturbing oxidative phosphorylation (OXPHOS), including cyanide, siderophores, and phenazines, which impair OXPHOS complex IV, host iron acquisition, and electron transport, respectively (2, 3, 8, 9).
One mechanism by which cells respond to mitochondrial dysfunction is by activating the mitochondrial unfolded protein response (UPRmt), which is regulated by the bZIP protein ATFS-1, a unique transcription factor that harbors both a mitochondrial targeting (MTS) and nuclear localization (NLS) sequence. ATFS-1 is efficiently imported into healthy mitochondria via its MTS and degraded in the mitochondrial matrix. However, mitochondrial perturbations such as perturbed proteostasis and OXPHOS impairment reduce mitochondrial protein import, which in turn causes the accumulation of ATFS-1 in the cytosol. ATFS-1 can then traffic to the nucleus via its NLS. In the nucleus, ATFS-1 induces a transcriptional program that includes a mitochondrial recovery program, as well as an antibacterial response involving peptides and secreted lysozymes that defend against bacterial pathogens (10, 11). The presence of both the MTS and a NLS allows ATFS-1 to respond to mitochondrial import deficiency as a surrogate for OXPHOS function and also serves as a mechanism to detect and defend against pathogens that perturb mitochondrial function (12).
Results
P. aeruginosa Perturbs Mitochondrial Function and Impairs UPRmt Activation.
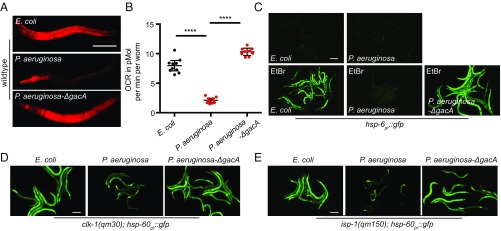
Using the UPRmt transcriptional reporter strains hsp-6pr::gfp and hsp-60pr::gfp, in which GFP expression is regulated by mitochondrial chaperone promoters, we sought to better understand the relationship between P. aeruginosa exposure, mitochondrial dysfunction, and UPRmt activation. Exposure of Caenorhabditis elegans to P. aeruginosa has been shown to result in modest activation of hsp-6pr::gfp (12, 13). Consistent with the pathogen causing mitochondrial dysfunction, mitochondrial membrane potential was depleted within 3 h of exposure to P. aeruginosa, and oxygen consumption was also impaired (Fig. 1 A and B and SI Appendix, Fig. S1A).
Fig. 1.
P. aeruginosa perturbs mitochondrial function and represses the UPRmt. (A) Images of TMRE-stained wild-type worms following 15 h of exposure to E. coli, P. aeruginosa, or P. aeruginosa-∆gacA. (Scale bar, 0.1 mm.) (B) Oxygen consumption rates (OCRs) of wild-type worms following 12 h of exposure to E. coli, P. aeruginosa, or P. aeruginosa-∆gacA. n = 10; error bars indicate mean ± SD; ****P < 0.03 (Student’s t test). (C) hsp-6pr::gfp worms on E. coli, P. aeruginosa, or P. aeruginosa-∆gacA exposed to a control or 30 μg/mL ethidium bromide. Images were obtained 27 h after exposure. (Scale bar, 0.1 mm.) (D) clk-1(qm30);hsp-60pr::gfp worms exposed to E. coli, P. aeruginosa, or P. aeruginosa-∆gacA for 48 h. (Scale bar, 0.1 mm.) (E) isp-1(qm150);hsp-60pr::gfp worms exposed to E. coli, P. aeruginosa, or P. aeruginosa-∆gacA for 48 h. (Scale bar, 0.1 mm.)
Diverse forms of mitochondrial stress are known to cause UPRmt activation. For example, when worms were raised on the nonpathogenic food source Escherichia coli (OP50), impairment of mitochondrial genome replication due to ethidium bromide (EtBr) exposure resulted in hsp-6pr::gfp activation (Fig. 1C) (4). Surprisingly, when worms were exposed to both P. aeruginosa and EtBr, hsp-6pr::gfp was impaired (Fig. 1C). Similarly, P. aeruginosa infection impaired UPRmt activation caused by the clk-1(qm30) or isp-1(qm150) mutant alleles, which impair ubiquinone biosynthesis and OXPHOS, respectively (14) (Fig. 1 D and E and SI Appendix, Fig. S1B). Interestingly, UPRmt repression required the P. aeruginosa two-component regulator GacA (Fig. 1 C–E), which is required for the expression of most virulence genes in P. aeruginosa (15–17). Together, these data suggest that P. aeruginosa evolved a mechanism to impair the UPRmt during infection.
ZIP-3 Negatively Regulates UPRmt Activation.
We next sought to identify the mechanism(s) by which the UPRmt is inhibited during P. aeruginosa exposure by focusing on host factors. We previously found that ATFS-1 regulates expression of the gene encoding the bZIP transcription factor ZIP-3 by binding the zip-3 promoter (18) and mediating the induction of zip-3 mRNA transcription during mitochondrial dysfunction caused by spg-7(RNAi), clk-1 mutation, and paraquat treatment (SI Appendix, Fig. S1 C–E) (4). zip-3 was also transcriptionally induced in a strain expressing constitutively active ATFS-1, which harbors a mutation that impairs the MTS and promotes its nuclear accumulation (11, 19) (SI Appendix, Fig. S1F). Importantly, the bZIP domain of ZIP-3 was one of four C. elegans bZIP domains previously found to dimerize with the bZIP domain of ATFS-1 in vitro (20), suggesting heterodimer formation between ATFS-1 and ZIP-3 may affect ATFS-1 function.
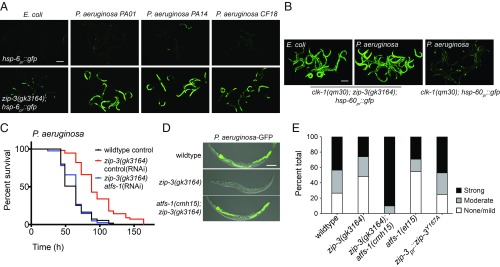
Intriguingly, when raised on E. coli, zip-3(gk3164) loss-of-function mutants displayed modest UPRmt activation (Fig. 2A and SI Appendix, Fig. S2A), suggesting that ZIP-3 is either required for mitochondrial function or functions as a negative regulator of activated ATFS-1. Impressively, the UPRmt was strongly activated in zip-3(gk3164) worms raised on several pathogenic P. aeruginosa strains within 12 h (Fig. 2A and SI Appendix, Fig. S2B), unlike in wild-type worms (21). Moreover, while UPRmt activation in clk-1(qm30) worms was repressed during P. aeruginosa infection (Fig. 1D and SI Appendix, Fig. S1B), clk-1(qm30);zip-3(gk3164) worms displayed robust activation of the UPRmt following 48 h P. aeruginosa exposure (Fig. 2B). These data demonstrate that ZIP-3 is required for UPRmt inhibition during P. aeruginosa infection.
Fig. 2.
The bZIP protein ZIP-3 is a negative regulator of ATFS-1 and the UPRmt. (A) hsp-6pr::gfp or zip-3(gk3164);hsp-6pr::gfp worms on E. coli or pathogenic P. aeruginosa strains PA01, PA14, and CF1821. (Scale bar, 0.1 mm.) (B) clk-1(qm30);zip-3(gk3164);hsp-60pr::gfp and clk-1(qm30);hsp-60pr::gfp worms on E. coli or P. aeruginosa. (Scale bar, 0.1 mm.) (C) Survival of wild-type and zip-3(gk3164) worms on control or atfs-1(RNAi) exposed to P. aeruginosa. Statistics are in SI Appendix, Table S5. (D) Representative photomicrographs of wild-type, zip-3(gk3164), and zip-3(gk3164);atfs-1(cmh15) worms raised on E. coli and exposed to P. aeruginosa-GFP for 48 h. Images are overlays of differential interference contrast (DIC) and GFP. (Scale bar, 0.1 mm.) (E) Quantification of intestinal colonization of wild-type, zip-3(gk3164), zip-3(gk3164);atfs-1(cmh15), atfs-1(et15), and zip-3pr::zip-3Y167A worms. White, gray, and black bars denote no or mild infection, moderate infection, and strong infection, respectively. Thirty worms were analyzed per treatment.
We next sought to determine the physiological impact of UPRmt inhibition by P. aeruginosa during infection. Impressively, zip-3(gk3164) worms survived significantly longer than wild-type worms during P. aeruginosa exposure (Fig. 2C), in a manner dependent on atfs-1 (Fig. 2C). Furthermore, the prolonged survival of zip-3(gk3164) worms on P. aeruginosa was comparable to the prolonged survival conferred by the constitutively active allele atfs-1(et15) (SI Appendix, Fig. S2C). As with the atfs-1(et15) strain (12), the intestinal colonization of P. aeruginosa was reduced in zip-3(gk3164) relative to wild-type worms, which also required atfs-1 (Fig. 2 D and E). Combined, these data indicate that zip-3–deletion worms are resistant to P. aeruginosa in a manner dependent on UPRmt activation, suggesting that zip-3 negatively regulates atfs-1.
ZIP-3 Stability Is Regulated by the Ubiquitin Ligase WWP-1 and Proteasomal Degradation.
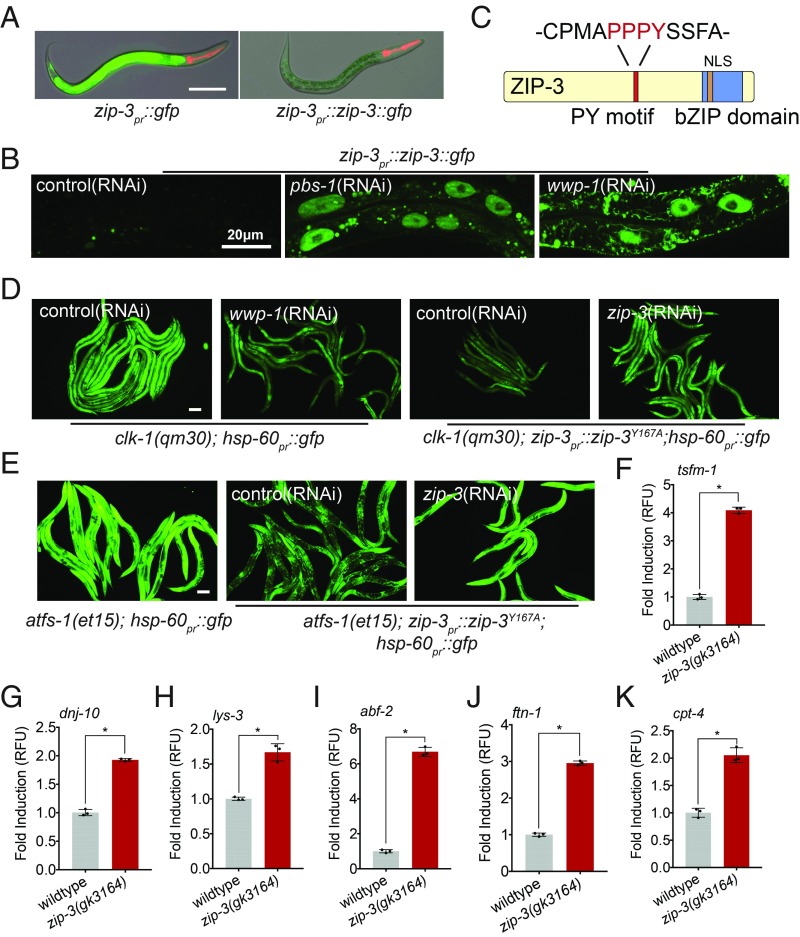
To better understand the mechanism by which ZIP-3 is regulated and impacts the UPRmt, transgenic strains were generated in which GFP or a ZIP-3::GFP fusion protein was expressed via the zip-3 promoter. GFP was expressed at high levels, indicating that the zip-3 promoter was active (Fig. 3A). However, ZIP-3::GFP was difficult to detect, suggesting that the fusion protein has a relatively short half-life (Fig. 3A). Impressively, inhibition of a core proteasomal subunit via pbs-1(RNAi) caused ZIP-3::GFP accumulation within intestinal nuclei (Fig. 3B), indicating that in addition to transcriptional regulation, ZIP-3 is also regulated by protein stability.
Fig. 3.
ZIP-3 is regulated by proteasomal degradation and represses atfs-1–dependent transcription. (A) Representative photomicrographs of zip-3pr::gfp and zip-3pr::zip-3::gfp worms raised on E. coli. Images are overlays of differential interference contrast (DIC) and GFP. (Scale bar, 0.1 mm.) (B) Representative intestinal images of zip-3pr::zip-3::gfp in worms on control, pbs-1(RNAi), or wwp-1(RNAi). (Scale bar, 0.02 mm.) (C) ZIP-3 protein. (D) clk-1(qm30);hsp-60pr::gfp worms on control or wwp-1(RNAi) and clk-1(qm30);zip-3Y167A;hsp-60pr::gfp on control or zip-3(RNAi). (Scale bar, 0.1 mm.) Quantification is given in SI Appendix, Fig. S3C. (E) atfs-1(et15);hsp-60pr::gfp or atfs-1(et15);zip-3Y167A;hsp-60pr::gfp on control or zip-3(RNAi). (Scale bar, 0.1 mm.) Quantification is given in SI Appendix, Fig. S3D. (F–K) tsfm-1, dnj-10, lys-3, abf-2, ftn-1, and cpt-4 transcripts as determined by qRT-PCR in wild-type or zip-3(gk3164) worms on P. aeruginosa (n = 3, ±SD); *P < 0.05 (Student’s t test). RFU, relative fluorescence unit.
We next generated a strain expressing GFP::ZIP-3 from the native locus via CRISPR-Cas9. Importantly, GFP::ZIP-3 prevented UPRmt activation during exposure to P. aeruginosa, unlike zip-3 deletion, indicating that the fusion protein was functional (SI Appendix, Fig. S3A). To identify potential ubiquitin ligases that regulate GFP::ZIP-3 degradation, we initially examined previous transcriptional profiling data of those mRNAs induced during mitochondrial dysfunction (4). Of the seven ubiquitin ligases examined (SI Appendix, Table S6), only wwp-1(RNAi) caused an accumulation of GFP::ZIP-3 within intestinal nuclei (Fig. 3B). WWP-1 is a well-conserved HECT domain ubiquitin ligase with over 20 known substrates in mammals (22). WW domain ubiquitin ligases bind specifically to PY motifs (-PPxY-) within their substrates. Interestingly, ZIP-3 harbors a single PY motif (-PPPY-) (Fig. 3C), suggesting that WWP-1 interacts directly with ZIP-3 to ubiquitinate and target it for degradation (23, 24). To examine the role of the PY motif, the -PPxY- motif in GFP::ZIP-3 was altered to either -PPxA- or -PPxF- via CRISPR. Furthermore, like wwp-1(RNAi), either amino acid substitution caused accumulation of GFP::ZIP-3 in the intestinal nuclei (SI Appendix, Fig. S3B). Combined, these data indicate that ZIP-3 is recognized and ubiquitinated by WWP-1 and degraded by proteasomes.
Importantly, ZIP-3 stabilization caused by either wwp-1(RNAi) or ZIP-3PPxA was sufficient to repress clk-1(qm30)–induced UPRmt activation (Fig. 3D and quantified in SI Appendix, Fig. S3C), consistent with ZIP-3 being a negative regulator of ATFS-1. We next examined the impact of ZIP-3 stabilization on UPRmt activation caused by the constitutively active allele atfs-1(et15). ATFS-1et15 harbors an impaired MTS, which causes nuclear accumulation of ATFS-1 and constitutive activation of the UPRmt independent of mitochondrial stress (19). Impressively, ZIP-3PPxA reduced UPRmt activation in atfs-1(et15) worms (Fig. 3E and quantified in SI Appendix, Fig. S3D), indicating that ZIP-3 inhibits the activated form of ATFS-1, rather than perturbing mitochondrial function, consistent with ZIP-3 harboring an NLS and being localized in the nucleus (Fig. 3B and SI Appendix, Fig. S3B).
ZIP-3 Limits a Subset of ATFS-1–Dependent Transcripts That Confer Resistance to P. aeruginosa.
Because the resistance of zip-3–deletion worms to P. aeruginosa required atfs-1, we identified the atfs-1–regulated transcripts increased in zip-3(gk3164) worms exposed to P. aeruginosa (25), as they potentially comprise genes that confer pathogen resistance. Following 18 h of P. aeruginosa exposure, 1,082 mRNAs were induced in zip-3(gk3164) worms relative to wild-type worms (SI Appendix, Fig. S4A and Table S1). Of those, the induction of 108 mRNAs required atfs-1 during mitochondrial dysfunction caused by spg-7(RNAi) (26) (SI Appendix, Fig. S4 B and C and Tables S2 and S3), consistent with ZIP-3 inhibiting activated ATFS-1. These mRNAs include components involved in mitochondrial recovery (Fig. 3 F and G), innate immunity (Fig. 3 H and I), iron acquisition (Fig. 3J), and fat metabolism (Fig. 3K). Of note, many of the mRNAs altered in zip-3(gk3164) worms exposed to P. aeruginosa were not affected by atfs-1 deletion during mitochondrial stress (SI Appendix, Fig. S4C), indicating ZIP-3 has roles independent of ATFS-1 and the UPRmt. Furthermore, zip-3(gk3164);atfs-1(cmh15) worms developed slower than either the zip-3(gk3164) or atfs-1(cmh15) mutants alone, also consistent with atfs-1–independent roles for zip-3 (SI Appendix, Fig. S5).
C. elegans Responds to P. aeruginosa–Produced Phenazines to Activate the UPRmt.
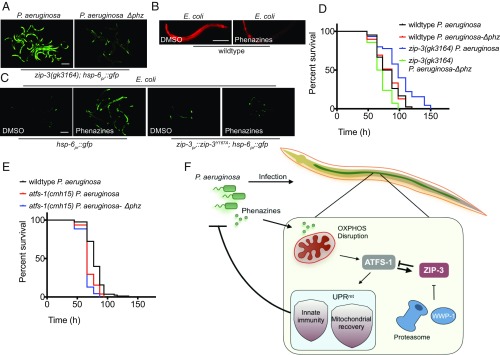
Next, we took advantage of the robust UPRmt activation that occurred in zip-3(gk3164) worms exposed to P. aeruginosa to identify the pathogen-produced molecules that perturb mitochondrial function and activate the UPRmt. Interestingly, P. aeruginosa unable to produce phenazines (P. aeruginosa-∆phz) (27, 28) did not activate the UPRmt in worms lacking zip-3 (Fig. 4A). Moreover, phenazine treatment was sufficient to perturb mitochondrial function (Fig. 4B) and activate the UPRmt (Fig. 4C) in worms raised on nonpathogenic E. coli, consistent with phenazines being redox-active compounds that can perturb OXPHOS (3, 29). Importantly, worms expressing degradation-resistant ZIP-3Y167A were unable to activate the UPRmt upon phenazine exposure (Fig. 4C), consistent with ZIP-3 repressing UPRmt activation during P. aeruginosa exposure. Of note, P. aeruginosa strains unable to produce cyanide or multiple siderophores still caused UPRmt activation, suggesting that in this assay, the most potent mitochondrial toxins are phenazines (SI Appendix, Fig. S6).
Fig. 4.
P. aeruginosa-secreted phenazines allow worms to detect the pathogen and initiate a protective antibacterial response. (A) zip-3(gk3164);hsp-6pr::gfp worms on wild-type P. aeruginosa or P. aeruginosa-∆phz. (Scale bar, 0.1 mm.) (B) Representative images of TMRE-stained wild-type worms on E. coli treated with DMSO or phenazines. (Scale bar, 0.1 mm.) (C) hsp-6pr::gfp or zip-3Y167A;hsp-6pr::gfp worms raised on E. coli treated with DMSO or phenazines. (Scale bar, 0.1 mm.) (D) Survival of wild-type and zip-3(gk3164) worms exposed to P. aeruginosa or P. aeruginosa-∆phz. Statistics are given in SI Appendix, Table S5. (E) Survival of wild-type and atfs-1(cmh15) worms exposed to P. aeruginosa or P. aeruginosa-∆phz. Statistics are given in SI Appendix, Table S5. (F) Schematics of the interactions between P. aeruginosa, mitochondrial perturbation, and UPRmt repression via ZIP-3.
Last, we sought to gain insight into the relationship between virulence-mediated UPRmt repression (Fig. 1) and the P. aeruginosa-produced phenazines that perturb mitochondrial function and activate the UPRmt. One possibility is that UPRmt repression increases phenazine potency by impairing a mitochondrial stress response. However, wild-type worm survival was similar upon exposure to wild type or P. aeruginosa-∆phz (Fig. 4D), suggesting the associated mitochondrial perturbation (Figs. 1A and 4B) did not decrease survival. Alternatively, UPRmt repression may prevent the activation of an antimicrobial response initiated in response to phenazine-dependent mitochondrial perturbation (Fig. 4C).
To further examine these models, wild-type and zip-3–deletion worms were exposed to wild type or P. aeruginosa-∆phz. Surprisingly, zip-3(gk3164) worms were not resistant to P. aeruginosa-∆phz as they were to wild-type P. aeruginosa. In fact, the pathogenic strain unable to produce phenazines was considerably more toxic to worms lacking zip-3 (Fig. 4D). We next examined the impact of P. aeruginosa-∆phz in atfs-1(cmh15) worms that cannot activate the UPRmt. Consistent with the UPRmt regulating an antibacterial response, atfs-1(cmh15) worms were more sensitive to P. aeruginosa than wild-type worms (Fig. 4E). However, survival of atfs-1(cmh15) worms was similar when exposed to P. aeruginosa or P. aeruginosa-∆phz (Fig. 4E). These findings suggest that the mitochondrial dysfunction caused by phenazines allows the host to initiate an antimicrobial response to prolong survival. However, in the absence of phenazines, the host is unable to engage the UPRmt, resulting in decreased survival.
Discussion
The interactions between host cells and P. aeruginosa that facilitate pathogen detection and host response remain unclear. Our findings indicate that C. elegans detects P. aeruginosa through phenazine-mediated disruption of OXPHOS and responds by initiating the UPRmt via the transcription factor ATFS-1. However, the P. aeruginosa virulence response engages a host negative regulatory mechanism. ZIP-3 impairs active ATFS-1 and the associated mitochondrial-protective and antibacterial response, limiting a pathway that impairs intestinal colonization and prolongs host survival.
Studies in multiple organisms have suggested that phenazines are virulence factors that impair electron transport and mitochondrial function (30, 31). However, phenazines serve multiple functions for Pseudomonas species independent of infection. Perhaps most intriguing, phenazines are required to maintain redox balance in Pseudomonas biofilms where the internal bacteria are hypoxic (32, 33). Perhaps similarly, the P. aeruginosa lawn used in the C. elegans slow-killing assay is hypoxic as well (34). In both scenarios, phenazines serve as electron shuttles to maintain redox balance throughout the biofilm by facilitating the transfer of electrons to available oxygen. As electron shuttles, phenazines can also impair the eukaryotic electron transport chain.
Importantly, P. aeruginosa lacking phenazines remains pathogenic toward C. elegans and in the absence of ZIP-3 is considerably more toxic. We propose that the mitochondrial perturbation caused by phenazines is detected by ATFS-1 via mitochondrial surveillance, which in turn activates a response to promote mitochondrial function and eliminate the bacteria. However, P. aeruginosa exploits the host negative regulator ZIP-3 to repress UPRmt activation. Consistent with this model, UPRmt repression is dependent on the pathogenicity of P. aeruginosa (Fig. 1 D and E), while the phenazines that perturb mitochondrial function and activate the UPRmt are secreted independent of the virulence response (Fig. 4E) (3, 27, 29, 31).
We have shown that ZIP-3 is a labile negative regulator of ATFS-1 that is degraded by proteasomes in a manner dependent on the ubiquitin ligase WWP-1. ZIP-3 stabilization is sufficient to inhibit the UPRmt during mitochondrial dysfunction by impairing nuclear ATFS-1, likely by forming a heterodimer (20). However, it will be interesting to elucidate the inputs that determine ZIP-3 protein stability during mitochondrial stress. WWP-1 or ZIP-3 may receive additional inputs that either stimulate or impair ZIP-3 degradation. PY domain phosphorylation can influence interactions with WW domain ubiquitin ligases (35). Our data suggest that phosphorylation of the PY domain is required for ZIP-3 degradation as altering the PY domain from -PPPY- to -PPPF- within ZIP-3 prevented degradation (SI Appendix, Fig. S3B). However, the stimuli or potential tyrosine kinase or phosphatase remain to be identified. It will also be exciting to determine the function of ZIP-3–mediated negative regulation in the absence of pathogens as multiple consequences of prolonged UPRmt activation have been observed, including impaired development (19), loss of dopamine neurons (36), and the propagation of deleterious mitochondrial genomes (11, 37), indicating that UPRmt activation must be strictly regulated by both positive and negative regulators.
Materials and Methods
The full details of worm strains and bacteria strains are described in SI Appendix. The procedures for tetramethylrhodamine, ethyl ester (TMRE) staining, the oxygen consumption assay, qPCR, RNA sequencing combined with phenazine treatment, P. aeruginosa slow-killing assays, and P. aeruginosa intestinal accumulation assays are described in SI Appendix. Image and statistical analysis is also described in SI Appendix.
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetics Center for providing C. elegans strains [funded by the NIH Office of Research Infrastructure Programs (P40 OD010440)] and the University of Massachusetts Medical School Core Facility for deep sequencing. The work is supported by the Howard Hughes Medical Institute and National Institutes of Health Grants R01AG040061 and R01AG047182 (to C.M.H.) and R01AI130289 (to R.P.-W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession nos. GSE111325 and GSE113136).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817259116/-/DCSupplemental.
References
- 1.Mahajan-Miklos S, Tan MW, Rahme LG, Ausubel FM. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell. 1999;96:47–56. doi: 10.1016/s0092-8674(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 2.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Ray A, Rentas C, Caldwell GA, Caldwell KA. Phenazine derivatives cause proteotoxicity and stress in C. elegans. Neurosci Lett. 2015;584:23–27. doi: 10.1016/j.neulet.2014.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337:587–590. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwan DL, Kirienko NV, Ausubel FM. Host translational inhibition by Pseudomonas aeruginosa exotoxin A triggers an immune response in Caenorhabditis elegans. Cell Host Microbe. 2012;11:364–374. doi: 10.1016/j.chom.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melo JA, Ruvkun G. Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell. 2012;149:452–466. doi: 10.1016/j.cell.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunbar TL, Yan Z, Balla KM, Smelkinson MG, Troemel ER. C. elegans detects pathogen-induced translational inhibition to activate immune signaling. Cell Host Microbe. 2012;11:375–386. doi: 10.1016/j.chom.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher LA, Manoil C. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J Bacteriol. 2001;183:6207–6214. doi: 10.1128/JB.183.21.6207-6214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirienko NV, Ausubel FM, Ruvkun G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2015;112:1821–1826. doi: 10.1073/pnas.1424954112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker BM, Nargund AM, Sun T, Haynes CM. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet. 2012;8:e1002760. doi: 10.1371/journal.pgen.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin YF, et al. Maintenance and propagation of a deleterious mitochondrial genome by the mitochondrial unfolded protein response. Nature. 2016;533:416–419. doi: 10.1038/nature17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellegrino MW, et al. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature. 2014;516:414–417. doi: 10.1038/nature13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Samuel BS, Breen PC, Ruvkun G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature. 2014;508:406–410. doi: 10.1038/nature13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng J, Bussière F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 15.Heeb S, Haas D. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol Plant Microbe Interact. 2001;14:1351–1363. doi: 10.1094/MPMI.2001.14.12.1351. [DOI] [PubMed] [Google Scholar]
- 16.Brencic A, et al. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol Microbiol. 2009;73:434–445. doi: 10.1111/j.1365-2958.2009.06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Souza JT, Mazzola M, Raaijmakers JM. Conservation of the response regulator gene gacA in Pseudomonas species. Environ Microbiol. 2003;5:1328–1340. doi: 10.1111/j.1462-2920.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- 18.Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt) Mol Cell. 2015;58:123–133. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauthan M, Ranji P, Aguilera Pradenas N, Pitot C, Pilon M. The mitochondrial unfolded protein response activator ATFS-1 protects cells from inhibition of the mevalonate pathway. Proc Natl Acad Sci USA. 2013;110:5981–5986. doi: 10.1073/pnas.1218778110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinke AW, Baek J, Ashenberg O, Keating AE. Networks of bZIP protein-protein interactions diversified over a billion years of evolution. Science. 2013;340:730–734. doi: 10.1126/science.1233465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DG, et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006;7:R90. doi: 10.1186/gb-2006-7-10-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhi X, Chen C. WWP1: A versatile ubiquitin E3 ligase in signaling and diseases. Cell Mol Life Sci. 2012;69:1425–1434. doi: 10.1007/s00018-011-0871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey KF, Kumar S. Nedd4-like proteins: An emerging family of ubiquitin-protein ligases implicated in diverse cellular functions. Trends Cell Biol. 1999;9:166–169. doi: 10.1016/s0962-8924(99)01541-x. [DOI] [PubMed] [Google Scholar]
- 24.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 25.Deng P, Haynes CM. 2019 Differential expression analysis of wildtype and zip-3(gk3164) worms with next generation sequencing. Gene Expression Omnibus. Available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE111325. Deposited March 1, 2018.
- 26.Deng P, Haynes CM. 2019 Differential expression analysis of wildtype, atfs-1(tm4919) and zip-3(gk3164) worms with next generation sequencing. Gene Expression Omnibus. Available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE113136. Deposited April 13, 2018.
- 27.Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 28.Liberati NT, et al. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci USA. 2006;103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierson LS, III, Pierson EA. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol. 2010;86:1659–1670. doi: 10.1007/s00253-010-2509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cezairliyan B, et al. Identification of Pseudomonas aeruginosa phenazines that kill Caenorhabditis elegans. PLoS Pathog. 2013;9:e1003101. doi: 10.1371/journal.ppat.1003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recinos DA, et al. Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc Natl Acad Sci USA. 2012;109:19420–19425. doi: 10.1073/pnas.1213901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietrich LE, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321:1203–1206. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos I, Dietrich LE, Price-Whelan A, Newman DK. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res Microbiol. 2010;161:187–191. doi: 10.1016/j.resmic.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy KC, Andersen EC, Kruglyak L, Kim DH. A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science. 2009;323:382–384. doi: 10.1126/science.1166527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H, et al. Interactions of beta and gamma ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. J Biol Chem. 2002;277:13539–13547. doi: 10.1074/jbc.M111717200. [DOI] [PubMed] [Google Scholar]
- 36.Martinez BA, et al. Dysregulation of the mitochondrial unfolded protein response induces non-apoptotic dopaminergic neurodegeneration in C. elegans models of Parkinson’s disease. J Neurosci. 2017;37:11085–11100. doi: 10.1523/JNEUROSCI.1294-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gitschlag BL, et al. Homeostatic responses regulate selfish mitochondrial genome dynamics in C. elegans. Cell Metab. 2016;24:91–103. doi: 10.1016/j.cmet.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.