Abstract
Upright hyperventilation occurs in ~25% of our patients with postural tachycardia syndrome (POTS). Poikilocapnic hyperventilation alone causes tachycardia. Here, we examined changes in respiration and hemodynamics comprising cardiac output (CO), systemic vascular resistance (SVR), and blood pressure (BP) measured during head-up tilt (HUT) in three groups: patients with POTS and hyperventilation (POTS-HV), patients with panic disorder who hyperventilate (Panic), and healthy controls performing voluntary upright hyperpnea (Voluntary-HV). Though all were comparably tachycardic during hyperventilation, POTS-HV manifested hyperpnea, decreased CO, increased SVR, and increased BP during HUT; Panic patients showed both hyperpnea and tachypnea, increased CO, and increased SVR as BP increased during HUT; and Voluntary-HV were hyperpneic by design and had increased CO, decreased SVR, and decreased BP during upright hyperventilation. Mechanisms of hyperventilation and hemodynamic changes differed among POTS-HV, Panic, and Voluntary-HV subjects. We hypothesize that the hyperventilation in POTS is caused by a mechanism involving peripheral chemoreflex sensitization by intermittent ischemic hypoxia.
NEW & NOTEWORTHY Hyperventilation is common in postural tachycardia syndrome (POTS) and has distinctive cardiovascular characteristics when compared with hyperventilation in panic disorder or with voluntary hyperventilation. Hyperventilation in POTS is hyperpnea only, distinct from panic in which tachypnea also occurs. Cardiac output is decreased in POTS, whereas peripheral resistance and blood pressure (BP) are increased. This is distinct from voluntary hyperventilation where cardiac output is increased and resistance and BP are decreased and from panic where they are all increased.
Keywords: hyperventilation, postural tachycardia, systemic vascular resistance
INTRODUCTION
Standing upright can evoke symptoms, such as lightheadedness, headache, nausea, fatigue, cognitive deficits, and exercise intolerance, relieved by recumbency (39, 42). This defines orthostatic intolerance (OI). Signs such as marked changes in heart rate (HR), blood pressure (BP), or respiration may also be present. Postural tachycardia syndrome (POTS) is a common form of chronic OI in which excess upright tachycardia is associated with OI symptoms in the absence of hypotension (20, 26, 40). Postural hyperventilation has been reported in the “hyperventilation syndrome” (28), which has been regarded as a psychologically induced event (14). For example, hyperventilation is present in many but not all patients with panic disorder, and postural stress may trigger onset of panic, dyspnea, and hyperventilation (17). Hyperventilation is excessive ventilation, or ventilation out of proportion to metabolic demand, such that falls and a respiratory alkalosis ensues.
Dyspnea (shortness of breath), hyperventilation, and hypocapnia [reduced carbon dioxide (CO2) in the blood] in the absence of cardiopulmonary disease have been reported in association with POTS and typically take the form of postural hypocapnic hyperpnea (hyperpnea is excessive depth of breathing) (9, 34, 43, 47). We observed hypocapnic hyperpnea in POTS in response to a rapid initial orthostatic decrease in cardiac output (CO) and cerebral blood flow (CBF) (9) during poikilocapnic (uncontrolled CO2) experiments. Poikilocapnic hyperventilation produces sinus tachycardia (29), and hypocapnia reduces CBF, resulting in symptoms and maladaptive effects, including impaired neuronal activation (22). We recently demonstrated that postural hypocapnic hyperpnea can cause POTS, rather than resulting from POTS (9). Data suggests that ischemia of the carotid body (“stagnant hypoxia”) (9) is the candidate mechanism for hyperventilation and results primarily from low CO and secondarily from sympathetic vasoconstriction of the artery to the carotid body (9, 11).
We hypothesize that postural hyperventilation causing POTS is identifiably different from voluntary hyperpneic hyperventilation and from hyperventilation in panic disorder. Differences comprise distinctive changes in BP, CO, and systemic vascular resistanc, whereas excessive tachycardia is present in all.
METHODS
Subjects.
The POTS group was comprised of 16 subjects aged 13–26 yr old (mean age 18.1 ± 1.2 yr; 14 women, 2 men) with POTS as defined by standard criteria (40) and who additionally complained of shortness of breath when upright. All had normal pulmonary function tests and no discernible cardiopulmonary or systemic illness. We have regarded these dyspneic patients as a distinct subgroup of POTS denoted “postural hyperpnea” because tidal volume (TV) increases with orthostatic stress but respiratory rate (RR) does not (9). We recently described a distinct subset of patients with POTS who exhibit hyperventilation elicited by the imposition of an orthostatic challenge. All of these had normal pulmonary function tests and no discernible cardiopulmonary or systemic illness. They exhibited postural hyperventilation in the form of hypocapnic hyperpnea causing reduced CBF velocity (CBFv), consequent lightheadedness, and increased BP. This POTS-HV comprised ~25% of our enrolled patients.
All patients were recruited from our Center for Hypotension, which is an outpatient referral center for patients with POTS, OI, and other disorders related to autonomic nervous system dysfunction. All patients with POTS had day-to-day symptoms of OI for 6 mo or more, and symptom relief was provided by recumbency and exhibited characteristic features such as lightheadedness, diaphoresis, warmth, nausea, hyperventilation, pallor, and a postdrome of fatigue and headache following imposition of an orthostatic challenge, along with age-dependent changes in upright HR. Signs and symptoms of OI and an excessive increase in HR without hypotension were evident within 10 min of head-up tilt (HUT) (26, 31, 37). If age was <19 yr, excessive tachycardia was defined as an increase in HR of at least 40 beats/min or a HR >120 beats/min in the absence of postural hypotension (41) during the 10 min upright tilt table test. If age was >19 yr, excessive tachycardia was defined by an orthostatic HR increment exceeding 30 beats/min (40). Other medical problems that could explain these signs or symptoms had been ruled out. We excluded potential enrollees with a history of respiratory disease, sleep apnea, obesity, or anxiety disorders. Patients with diagnosed panic disorder were specifically excluded from this group.
The panic disorder group was comprised of 7 subjects aged 15–22 yr old (mean age 16.4 ± 2.5 yr; 5 women, 2 men). Patients were diagnosed with recurrent panic attacks by a psychiatrist, fulfilling criteria contained in DSM-V-TR Axis I Disorders (1). These patients with panic disorder were referred for complaints of upright tachycardia, shortness of breath, or symptoms of hyperventilation, such as lightheadedness, numbness, and tingling of the arms and around the mouth, during panic attacks triggered by orthostasis.
There were two groups of healthy volunteers: one that performed voluntary hyperpnea during HUT (designated Voluntary-HV) and a second group of true controls that breathed normally, against which all other groups (POTS-HV, Panic, and Voluntary HV) were compared. Healthy control subjects were nonsmokers with no known medical conditions, taking no medications, with a normal physical exam and electrocardiogram. Healthy control subjects were included only if free of systemic illness and of OI of any type. We studied 7 Voluntary-HV subjects, aged 17–25 yr (mean age 19.1 ± 2.6 yr; 5 women, 2 men) and 10 true control subjects, aged 18–25 yr (mean age 18.8 ± 2.2 yr; 8 women, 2 men).
Patients and control subjects were required to refrain from all medications, including anxiolytic medications, for at least 2 wk before the study, with the exception of contraceptive or thyroid medications. Since all enrollees with POTS were either on no medication or able to tolerate being off of medication for at least 2 wk before testing, and all were able to complete the entire day of testing, they could be described as being “moderately” affected by their condition. Enrollees were required to stop ingestion of xanthine-, caffeine-, or alcohol-containing substances 72 h before study. A light breakfast was permitted on testing day if eaten 2 or more hours before testing.
The Institutional Review Board of New York Medical College reviewed and approved this protocol. Each subject received a detailed description of all protocols and was given an opportunity to have their questions answered. Signed informed consent was obtained from all participants or their parents.
Instrumentation.
All subjects were instrumented in a similar fashion by the same operator. Height and weight were measured, and body mass index was calculated. During instrumentation, all subjects lay supine on an electronic motorized tilt table (Colin Medical Instruments Corp., San Antonio, TX) with a footboard. An electrocardiograph measured HR from the beat-to-beat cardiac electrical interval. Beat-to-beat BP was monitored by a volume clamp method using finger arterial plethysmography (Finometer; FMS, Amsterdam, the Netherlands) on the right middle or index finger and corrected for tilt angle using ModelFlow software. Finometer data were calibrated to brachial artery pressure and intermittently checked against oscillometric BP measurements.
The Finometer uses the Modelflow algorithm to estimate beat-to-beat CO by pulse-wave analysis (15). Before experiments began, Modelflow CO was calibrated against an Innocor inert gas rebreathing CO measurement (Innovision, Denmark) while supine. We computed systemic vascular resistance (SVR) by dividing the time average arterial pressure [mean arterial pressure (MAP)] by the Modelflow CO averaged over each cardiac cycle. The first value of SVR was also calculated by measuring a manual BP, calculating the estimated MAP from the formula MAP = (systolic BP + 2diastolic BP)/3, and dividing that result by the measured (supine) CO. Thereafter, continuous SVR was calculated from Modelflow data. To estimate TV, respiratory plethysmography (Respitrace, NIMS Scientific, Miami Beach, FL) calibrated by measurements made using a pneumotachogram (Hans Rudolph, Shawnee KS) and nasal cannula capnography combined with a pulse oximeter (Smith Medical PM, Waukesha, WI) measured respiration, end tidal CO2 (ETCO2), and oxygen saturation. Transcranial Doppler (TCD) (Neurovision; Multigon, Yonkers, NY) measured CBFv of the left middle cerebral artery (MCA) using a 2-MHz probe fixed to the subject’s head by a custom-made headband. Signals were acquired at 200 samples/s, multiplexed, and A/D-converted using custom software. These measurements are routine during our tilt table studies.
Protocol.
Subjects arrived at 9:30 AM. Following instrumentation, subjects remained awake and supine for 30 min to acclimate. Baseline data was comprised of continuously measured HR, BP, RR, TV, expiratory minute volume (V̇e), ETCO2, CO, SVR, and CBFv. For purposes of comparison, we used averaged data for the 10 min immediately preceding tilt to determine baseline supine values. Mean ETCO2 over the last 10 min of the rest period was defined as baseline CO2 for each subject throughout the protocol.
After supine data collections were complete, all subjects were tilted upright over 8 s to 70° for 10 min or as tolerated. Voluntary hyperventilation in healthy volunteers began 2 min after initiation of upright tilt by having them take maximum breaths at their supine RR for two minutes. This emulates the hyperpneic breathing typically seen in most patients with POTS. Healthy volunteers had ETCO2 values that ranged between 38 and 43 Torr supine, which decreased with upright tilt but was never less than 33 Torr. We, therefore, defined hyperventilation by an ETCO2 consistently <30 Torr during upright tilt. Continuously measured HR, BP, ETCO2, respiratory data, CO, SVR, and CBFv data were recorded for offline analysis.
Normalized respiratory impedance data was used to calculate RR and estimate TV and V̇e. Data were detrended to remove artifact, and TV was obtained as peak-to-trough volume per breath. V̇e was determined by averaging TV over 1 min and multiplying by the RR.
Data analysis.
All data were continuously sampled at 200 Hz, converted with an analog-to-digital converter (DI-720 DataQ Ind, Milwaukee, WI) connected to a personal computer, and analyzed offline.
Baseline data was recorded for 10 min before upright tilt. The first minute of tilt following a prolonged supine rest often includes a period of hemodynamic instability known as “initial orthostatic hypotension” (IOH) (52). IOH is caused by the rapid translocation of blood from the upper body to the lower body and the normal lag in the onset of compensatory adrenergic vasoconstriction. For these reasons, the first minute of tilt is often avoided for purposes of analysis. However, our prior data showed that this time period may be critical to initiating hypocapnic hyperpnea (9). Therefore, we retained early physiological measurements following the initiation of upright tilt and graphically displayed BP, CO, CBV, HR, and SVR during this early “initial” tilt epoch. Thereafter, data were tabulated at 1 min after tilt and again when respirations had become stable in patients with POTS and in patients with panic disorder. In patients with POTS-HV, respirations became stable upon tilt and remained stable up to ~4 min. In Voluntary HV, respirations became stable by 2–3 min and became stable in the panic group by 1–2 min. Measurements were made in healthy volunteer hyperventilators (Voluntary HV) during the last 20 s of voluntary hyperventilation when respirations were stable. Data was time-averaged during these time periods.
Data presentation and statistics.
There were four groups in all: hyperventilating POTS (POTS-HV), panic disorder (Panic), voluntary hyperventilators (Voluntary HV), and healthy control volunteers who underwent tilt table testing without voluntary hyperventilation. The data from healthy, nonhyperventilating volunteers were used to evaluate differences in supine baseline hemodynamic and cardiopulmonary data compared with POTS-HV and Panic subjects. It was additionally used as a visual, but not a statistical, comparison of the response to orthostasis. All tabular results are reported as mean ± SE, and statistical differences between values shown in the tables were calculated using t-test with significance set at P ≤ 0.05, comparing Healthy with POTS-HV and with Panic subjects.
Graphic data are depicted as mean ± SE. Data were obtained from original time series averaged over 15-s intervals centered at the time markers. Data were collected and analyzed by the same investigator throughout.
We used repeated-measures ANOVA (rmANOVA) to compare study groups on outcomes over time intervals. To address our hypotheses, we were interested in how the study groups differed in outcome measures over time during the tilt. As such, we focused on the “group × time interaction” in the rmANOVA. We assumed a covariance structure of compound symmetry. Reported P values reflect the interaction term using the Greenhouse-Geisser correction. To control for multiple comparisons, pairwise comparisons among groups, following a significant omnibus rmANOVA test, were conducted using Scheffe’s test. Statistical significance was set at P ≤ 0.05. Results were calculated by using GraphPad Prism version 4.0.
Sample size estimation.
For sample size estimation, we assumed a repeated measures design with measurements at baseline and four subsequent time points within each study participant. Basing our calculations on HR and assuming a within-subject correlation of HR measures of 0.50 on repeated assessment, a sample size of 7 individuals per group with 5 assessments would provide 80% power to detect a standardized effect size of 0.85 for the between-group comparison and more than 80% power to detect a group-by-time interaction effect size of 1.0 or larger. This sample size incorporates a Greenhouse-Geisser correction to the F-test.
RESULTS
Height was 162 ± 4 cm in POTS-HV, 168 ± 4 cm in Voluntary HV, and 166 ± 3 cm in the Panic group, whereas weights were 58 ± 2 kg, 63 ± 2 km, and 61 ± 4 kg, respectively. Height was 169 ± 3 cm, whereas weight was 67 ± 2 kg for control subjects. There were no differences in these parameters either within or between groups.
Supine baseline data.
Baseline supine data for BP, HR, CO, SVR, CBFv, V̇e, and ETCO2 are shown in Table 1. There was no significant difference in CO, SVR, V̇e, MAP, systolic BP, diastolic BP, pulse pressure, ETCO2, or mean CBFv between POTS-HV, Panic, and Voluntary HV. HR was significantly increased in POTS-HV but not in Panic.
Table 1.
Supine baseline hemodynamic and cardiopulmonary data
| Healthy Volunteer | POTS Hyperventilation | Panic Disorder | |
|---|---|---|---|
| Heart rate, beats/min | 66 ± 3 | 82 ± 5* | 74 ± 5 |
| Systolic BP, mmHg | 115 ± 3 | 118 ± 2 | 114 ± 3 |
| Diastolic BP, mmHg | 60 ± 2 | 62 ± 2 | 68 ± 3 |
| MAP, mmHg | 79 ± 4 | 77 ± 4 | 81 ± 4 |
| Pulse pressure, mmHg | 54 ± 3 | 55 ± 4 | 47 ± 3 |
| Respiratory rate, breaths/min | 14 ± 1 | 15 ± 1 | 14 ± 1 |
| V̇e, l/min | 6.8 ± 0.7 | 7.4 ± 0.7 | 6.7 ± 0.7 |
| Tidal volume, liters | 0.49 ± 0.03 | 0.50 ± 0.08 | 0.51 ± 0.05 |
| ETCO2, Torr | 41.4 ± 0.6 | 38.2 ± 0.8 | 41.9 ± 0.5 |
| Cardiac output, l/min | 5.7 ± 0.4 | 5.4 ± 0.2 | 5.8 ± 0.4 |
| TPR, mmHg·l−1·min−1 | 16.3 ± 1.5 | 15.6 ± 1.5 | 15.1 ± 1.0 |
| Mean CBFv, cm/s | 72 ± 3 | 73 ± 6 | 75 ± 3 |
Values are means ± SE. BP, blood pressure; CBFv, cerebral blood flow velocity; ETCO2, end tidal carbon dioxide; MAP, mean arterial pressure; POTS, postural tachycardia syndrome; TPR, total peripheral resistance.
P < 0.05 difference from healthy volunteer control subject.
Upright tilt data.
It was uncommon for a patient with panic disorder to endure upright positioning for more than a few minutes of tilt, although one patient was able to endure 11 min. Postural hyperventilation in POTS was characterized by hyperpnea without tachypnea, as previously reported (9, 45).
Respiratory responses and CBFv.
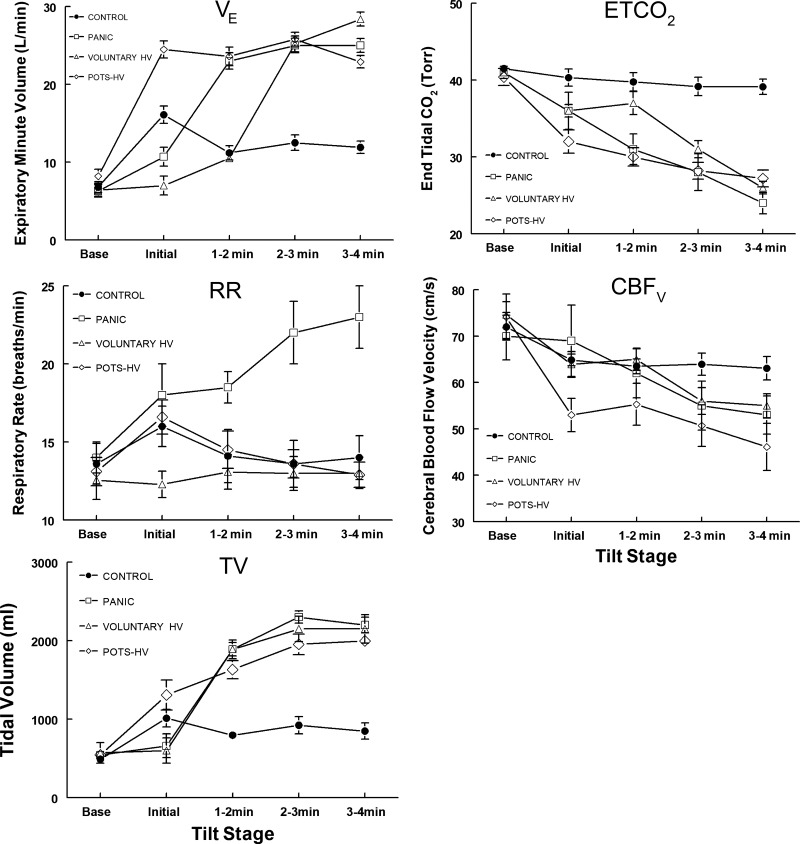
Figure 1 shows that TV immediately increased in POTS-HV with tilt and accounted for much of the V̇e increase (P < .001) because there was no increase in RR in this group. Rather, there was a small, albeit nonsignificant, decrease in RR with time following the initial tilt-induced increase. This contrasted with Panic in which TV increased only after the initial period and was associated with a significant increase in RR. By design, RR was fixed for the Voluntary HV group. Their TV increased during the period of voluntary hyperpnea to levels similar to those observed in POTS-HV. ETCO2 decreased similarly for patients with POTS-HV, Panic, and Voluntary HV enrollees, whereas CBFv decreased in parallel for all of these groups, as expected.
Fig. 1.
Respiratory data and cerebral blood flow velocity (CBFv) are shown for all subjects before [baseline (Base)] and following head-up tilt measured at the time epochs shown. The increase in expiratory minute volume (V̇e) resulted from an increase in tidal volume (TV) in all groups compared with control (P < 0.001). Respiratory rate (RR) only increased significantly (P < 0.001) in the Panic group. End tidal CO2 (ETCO2) decreased similarly and significantly for all groups (P < 0.001), whereas CBFv decreased in parallel, as expected. HV, hyperventilation; Panic, panic disorder; POTS, postural tachycardia syndrome.
Hemodynamic responses to tilt.
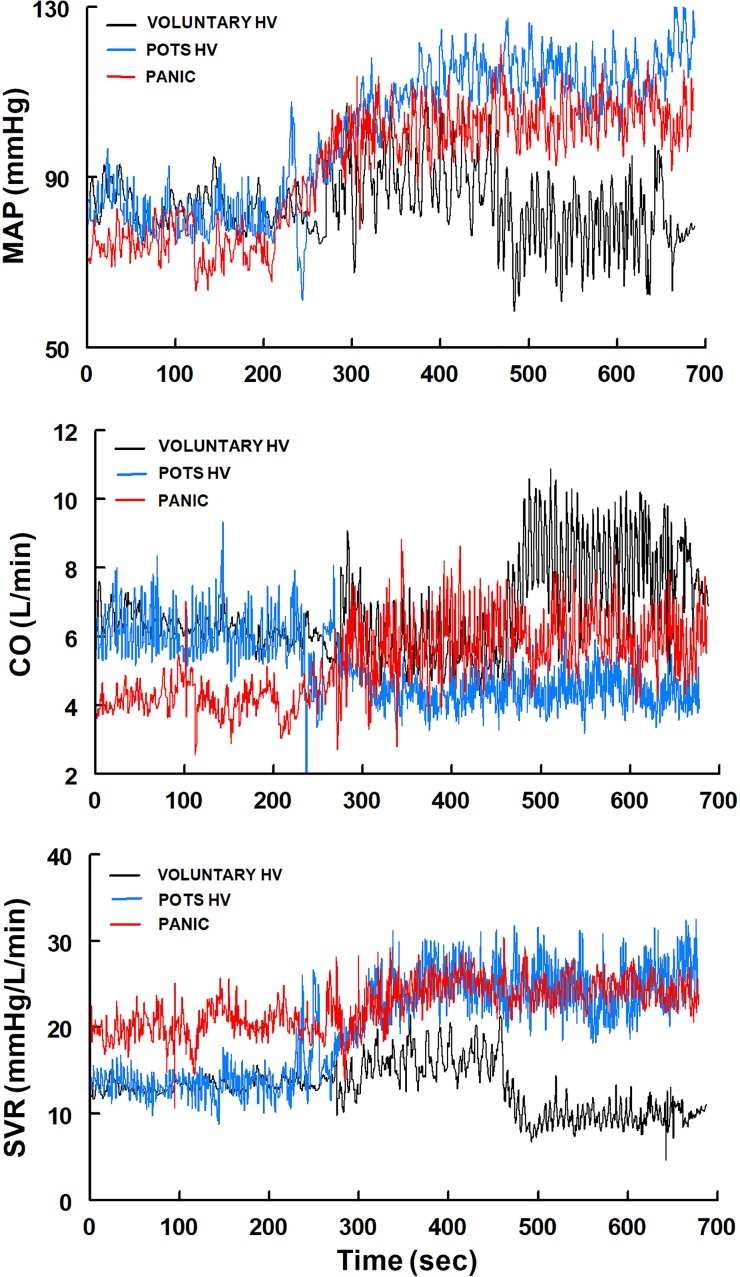
Figure 2 shows representative tracings of MAP, CO, and SVR for Voluntary HV, POTS-HV, and Panic. MAP increased in POTS-HV and Panic, whereas it decreased in Voluntary HV with the onset of upright hyperventilation. CO significantly decreased in POTS-HV but increased in Panic and Voluntary HV. SVR increased similarly in POTS-HV and Panic but decreased markedly in Voluntary HV.
Fig. 2.
Representative tracings of mean arterial pressure (MAP), cardiac output (CO), and systemic vascular resistance (SVR) from a Voluntary HV, POTS-HV, and Panic subject. MAP increased in POTS-HV and Panic, decreased in Voluntary HV with the onset of upright hyperventilation. CO decreased in POTS-HV but increased in Panic and Voluntary HV. SVR increased similarly in POTS-HV and Panic but decreased markedly in Voluntary HV. HV, hyperventilation; Panic, panic disorder.
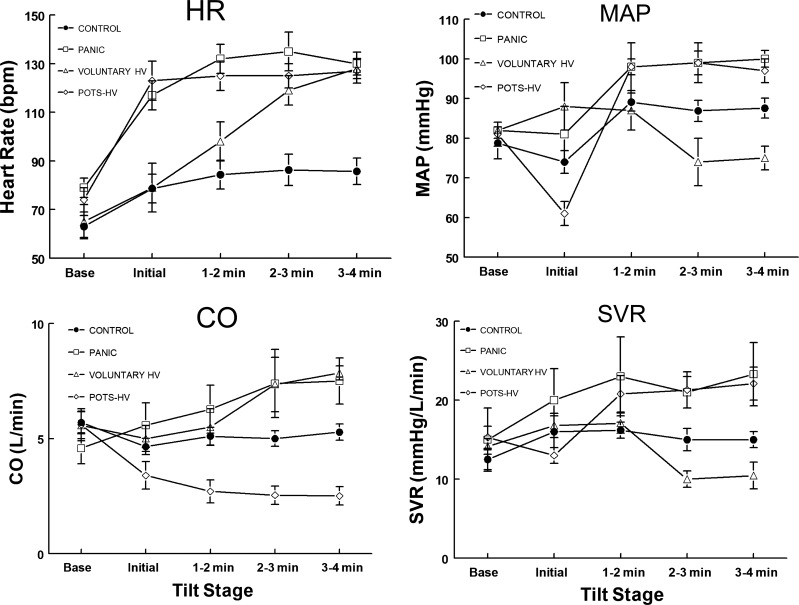
Representative findings were confirmed by data in Fig. 3, which shows the groups and normative control data. HR increased during periods of hyperventilation for all groups. MAP increased in POTS-HV and Panic, CO decreased in POTS-HV but increased in Panic and Voluntary HV, and SVR increased in POTS-HV and Panic but significantly decreased in Voluntary HV.
Fig. 3.
Heart rate (HR), mean arterial pressure (MAP), cardiac output (CO), and systemic vascular resistance (SVR) from Voluntary HV, POTS-HV, Panic, and Control subjects. HR increased significantly (P < 0.001) during periods of hyperventilation for all groups. MAP increased in POTS-HV and Panic, whereas Voluntary HV was significantly reduced compared with all other groups (P < 0.025). CO decreased significantly (P < 0.005) in POTS-HV, in Panic (P < 0.025), and in Panic and Voluntary HV. SVR increased significantly in POTS-HV (P < 0.005) and Panic (P < 0.025) but decreased significantly (P < 0.01) in Voluntary HV. HV, hyperventilation; Panic, panic disorder; POTS, postural tachycardia syndrome.
DISCUSSION
Patterns of hyperventilation in POTS compared with panic.
Poikilocapnic hyperventilation is defined by an increase in V̇e, causing decreased blood CO2 and ETCO2. V̇e may be increased by an increase in RR alone (tachypnea), an increase in TV alone (hyperpnea), or a combination of both, depending on the nature of the stressor (50). In the absence of administered CO2, the respiratory response to pain or panic is a combination of hyperpnea and tachypnea, as observed here and described in the literature (6, 30, 53). In contrast, V̇e increased in POTS-HV by an increase in TV with either no change or a small decrease in RR, as shown previously (9). TV alone was increased during voluntary hyperventilation in healthy volunteers by design so as to mimic the isolated hyperpnea in POTS.
Three factors contribute to tachycardia during hyperventilation (29). In order of increasing potency, they are: 1) lung inflation reflexes, 2) effects because of ischemia of central medullary chemoreflexes and inspiratory neurons (7), and 3) direct actions of hypocapnia on the sinoatrial node (29). In healthy volunteers, with normal autonomic function, tachycardia is accompanied by a rise in CO and fall in SVR.
Our major findings contrast changes in CO, SVR, and BP during hyperventilation in patients with POTS-HV, panic disorder with hyperventilation, and in voluntary hyperpneic hyperventilation in healthy control subjects.
An outline of characteristic directional changes in BP, CO, and SVR is summarized in Table 2.
Table 2.
Characteristic directional hemodynamic changes during orthostatic hyperventilation
| Voluntary Hyperventilation | POTS Hyperventilation | Panic Disorder | |
|---|---|---|---|
| MAP, SBP, DBP | ↓ | ↑ | ↑ |
| Cardiac output | ↑ | ↓ | ↑ |
| SVR | ↓ | ↑ | ↑ |
DBP, diastolic blood pressure; MAP, mean arterial pressure; POTS, postural tachycardia syndrome; SBP, systolic blood pressure; SVR, systemic vascular resistance.
Hyperventilation in POTS.
All patients with POTS, regardless of hyperventilation, have thoracic hypovolemia and increased SVR, sufficient to prevent postural hypotension when upright, despite reduced preload and CO (13, 25, 44). Stroke volume and cardiac preload are reduced (27), whereas sympathoexcitation is increased (2). POTS-HV comprises ~25% of our POTS population. The relationship of hyperventilation causing upright tachycardia has been revived by reports from our laboratory (9, 43) and has been previously reported by others (34), following the definition of POTS (40). Hyperventilation during orthostasis in POTS appears to be driven by rapid and excessively decreased initial CO, BP, and CBF. Our data show that reduced CBF is immediately followed by hyperpnea and hypocapnia (9). A reduction in CBF implies a reduction in carotid artery and carotid body blood flows. Hypocapnia perpetuates the reduction in CBF (48). This orthostatic epoch of IOH (52) results in “stagnant hypoxia” of the carotid body (9, 19). The carotid body cannot distinguish stagnant hypoxia [also known as “ischemic hypoxia” (10)] from hypoxic hypoxia. Upright patients with POTS-HV have an atypical presentation of chronic intermittent hypoxia. Chronic intermittent hypoxia increases the sensitivity of the peripheral chemoreflex sensitivity to hypoxia (11), increases sympathetic activity, increases vasoconstriction, and increases BP (24, 36). Our past findings support peripheral chemoreflex sensitization by showing enhanced hypoxic ventilatory response in patients with POTS studied without regard for complaints of hyperventilation or dyspnea (46). The hyperpneic response in patients with POTS consisting of raised TV during HUT may be explained by a unique pattern of respiratory muscle recruitment.
In vivo and in situ studies of young animals exposed to chronic intermittent hypoxia demonstrate a breathing pattern of active expiration at rest (55), suggesting that the neural mechanisms responsible for active expiration (normally a passive phenomenon) are hyperactive. The significance of this active expiration (typically employed during exercise) is a reduction in end-expiratory lung volume which lengthens the diaphragm, augmenting subsequent TV beyond that obtained simply by increased inspiratory diaphragmatic excursion. Such increased intraabdominal pressure would further compromise venous return from below the diaphragm that impairs such individuals’ ability to maintain CO during HUT.
It may be useful to classify patients with POTS-HV as having a dysfunctional breathing phenotype (3), as there are a growing number of reviews on the use of breathing exercises to mitigate dysfunctional breathing or reduce dyspnea in pulmonary diseases, such as asthma (49) or chronic obstructive pulmonary disease (18). A relevant example of breathing modifications that may be applicable to our patients with POTS-HV is a study that showed improvement in autonomic function in patients with essential hypertension (33). BP fell over a 3-mo period with both slow and fast breathing training, but Valsalva ratio, HR variation with respiration, and BP response in the hand grip and cold-pressor test showed significant improvement only in patients using slow breathing. Cortical influences can override reflex breathing patterns, and it is conceivable that such learned breathing patterns may at least counteract expiration described above, with consequent improvement in venous return to the heart among other putative benefits.
Hyperventilation in panic disorder.
Epinephrine spillover from the heart is present in patients with panic disorder, even at rest. During panic attacks, there are large increases in circulating epinephrine as well as sympathetic activity in skeletal muscle (5, 12, 23, 54). Beta adrenergic receptors are therefore activated in panic disorder and mediate hyperventilation (16). In addition, epinephrine is known to induce panic attacks with hyperventilation (51). The observed hemodynamic effects of increased epinephrine shown here are as expected.
Voluntary hyperpneic hyperventilation in healthy volunteers.
Voluntary poikilocapnic hyperventilation in normal man produces a small decrease in MAP, a decrease in SVR because of vasodilation, and an increase in HR and CO (4, 38). Under healthy conditions, hypocapnia vasodilates the systemic vasculature because of central and peripheral effects of alkalosis on sympathetic vasoconstriction (4, 21, 29, 38). Venous return is also augmented by actions of the respiratory muscle pump (32). These mechanisms may also occur in hyperventilation during panic disorder, although overshadowed by sympathetic activation. In contrast, voluntary hyperpnea produces directionally opposite changes in CO and SVR in POTS-HV, shown in Table 2, because of increased vasoconstriction and often reduced blood volume or its redistribution in upright patients with POTS, effectively reducing preload (44), as described above.
Upright hyperventilation causes tachycardia, but is it really POTS?
All subjects who hyperventilated experienced excess upright tachycardia and symptoms attributable to hypocapnia (e.g., lightheadedness because of decreased CBF). It would be specious reasoning to include voluntary hyperventilation or panic disorder as forms of POTS. Similarly, perhaps upright spontaneous POTS-HV is best regarded as postural hyperpnea rather than POTS.
Limitations.
TCD only measures blood flow through specific cerebral blood vessels. The MCA is the main vessel supplying the area of the brain activated during executive memory testing. Perfusion during orthostatic stress may vary with brain location, but such variations are often small (8). We did not measure TCD in both hemispheres; previous work showed that MCA CBF was not different between the hemispheres during orthostatic stress (35).
The Modelflow algorithm used by the Finometer yields relative measures of CO. While we standardized supine CO against inert gas rebreathing CO, the inert gas technique requires breathing alterations that greatly affected results.
We did not measure absolute total blood volume or central blood volume. However, we have shown in previous experiments that these quantities are absolutely reduced for patients with POTS with reduced resting CO (44).
Respitrace inductance respiratory plethysmography was used to estimate changes in TV and V̇e. These were calibrated against inductance plethysmography.
In addition, the use of nasal probes can underestimate ETCO2 if the probe moves from the nose or if the subject breaths through mouth.
Conclusion.
Hyperventilation is common in postural tachycardia syndrome and has distinctive cardiovascular characteristics when compared with hyperventilation in panic disorder or to voluntary hyperventilation. Hyperventilation in POTS is hyperpnea only, distinct from panic in which tachypnea also occurs. CO is decreased in POTS, whereas peripheral resistance and BP are increased. This is distinct from voluntary hyperventilation where CO is increased and resistance and BP are decreased and from panic where they are all increased.
GRANTS
Funding for this project was provided by Grants RO1-HL-134674 and RO1-HL-112736 from the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.S. and M.S.M. conceived and designed research; M.A.S., C.T., and M.S. performed experiments; J.M.S., P.P., P.V., and M.S.M. analyzed data; J.M.S., P.P., M.A.S., P.V., and M.S.M. interpreted results of experiments; J.M.S. and M.S.M. prepared figures; J.M.S., P.P., and P.V. drafted manuscript; P.P. and M.S.M. edited and revised manuscript; J.M.S., P.P., M.A.S., C.T., M.S., P.V., and M.S.M. approved final version of manuscript.
REFERENCES
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (5th ed.). Washington, D.C.: American Psychiatric Association, 2013. [Google Scholar]
- 2.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc 87: 1214–1225, 2012. doi: 10.1016/j.mayocp.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulding R, Stacey R, Niven R, Fowler SJ. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev 25: 287–294, 2016. doi: 10.1183/16000617.0088-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnum JF, Hickam JB, McINTOSH HD. The effect of hypocapnia on arterial blood pressure. Circulation 9: 89–95, 1954. doi: 10.1161/01.CIR.9.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Coupland NJ, Wilson SJ, Potokar JP, Bell C, Nutt DJ. Increased sympathetic response to standing in panic disorder. Psychiatry Res 118: 69–79, 2003. doi: 10.1016/S0165-1781(03)00045-3. [DOI] [PubMed] [Google Scholar]
- 6.Cowley DS, Roy-Byrne PP. Hyperventilation and panic disorder. Am J Med 83: 929–937, 1987. doi: 10.1016/0002-9343(87)90654-1. [DOI] [PubMed] [Google Scholar]
- 7.de Burgh Daly M, Korner PI, Angell-James JE, Oliver JA. Cardiovascular and respiratory effects of carotid body stimulation in the monkey. Clin Exp Pharmacol Physiol 5: 511–524, 1978. doi: 10.1111/j.1440-1681.1978.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 8.Deegan BM, Cooke JP, Lyons D, Olaighin G, Serrador JM. Cerebral autoregulation in the vertebral and middle cerebral arteries during combine head upright tilt and lower body negative pressure in healthy humans. Conf Proc IEEE Eng Med Biol Soc 2010: 2505–2508, 2010. doi: 10.1109/IEMBS.2010.5626647. [DOI] [PubMed] [Google Scholar]
- 9.Del Pozzi AT, Schwartz CE, Tewari D, Medow MS, Stewart JM. Reduced cerebral blood flow with orthostasis precedes hypocapnic hyperpnea, sympathetic activation, and postural tachycardia syndrome. Hypertension 63: 1302–1308, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dempsey JA, Smith CA. Pathophysiology of human ventilatory control. Eur Respir J 44: 495–512, 2014. doi: 10.1183/09031936.00048514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding Y, Li YL, Schultz HD. Role of blood flow in carotid body chemoreflex function in heart failure. J Physiol 589: 245–258, 2011. doi: 10.1113/jphysiol.2010.200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esler M, Alvarenga M, Lambert G, Kaye D, Hastings J, Jennings G, Morris M, Schwarz R, Richards J. Cardiac sympathetic nerve biology and brain monoamine turnover in panic disorder. Ann N Y Acad Sci 1018: 505–514, 2004. doi: 10.1196/annals.1296.062. [DOI] [PubMed] [Google Scholar]
- 13.Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, Bhella PS, Levine BD. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 55: 2858–2868, 2010. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner WN. The pathophysiology of hyperventilation disorders. Chest 109: 516–534, 1996. doi: 10.1378/chest.109.2.516. [DOI] [PubMed] [Google Scholar]
- 15.Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97: 291–301, 1999. doi: 10.1042/cs0970291. [DOI] [PubMed] [Google Scholar]
- 16.Heistad DD, Wheeler RC, Mark AL, Schmid PG, Abboud FM. Effects of adrenergic stimulation on ventilation in man. J Clin Invest 51: 1469–1475, 1972. doi: 10.1172/JCI106943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibbert G, Pilsbury D. Hyperventilation in panic attacks. Ambulant monitoring of transcutaneous carbon dioxide. Br J Psychiatry 153: 76–80, 1988. doi: 10.1192/bjp.153.1.76. [DOI] [PubMed] [Google Scholar]
- 18.Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 10: CD008250, 2012. doi: 10.1002/14651858.CD008250.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iturriaga R, Andrade DC, Del Rio R. Enhanced carotid body chemosensory activity and the cardiovascular alterations induced by intermittent hypoxia. Front Physiol 5: 468, 2014. doi: 10.3389/fphys.2014.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 96: 575–580, 1997. doi: 10.1161/01.CIR.96.2.575. [DOI] [PubMed] [Google Scholar]
- 21.Kontos HA, Richardson DW, Raper AJ, Zubair-ul-Hassan, Patterson JL Jr. Mechanisms of action of hypocapnic alkalosis on limb blood vessels in man and dog. Am J Physiol 223: 1296–1307, 1972. doi: 10.1152/ajplegacy.1972.223.6.1296. [DOI] [PubMed] [Google Scholar]
- 22.Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med 347: 43–53, 2002. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- 23.Lambert E, Hotchkin E, Alvarenga M, Pier C, Richards J, Barton D, Dawood T, Esler M, Lambert G. Single-unit analysis of sympathetic nervous discharges in patients with panic disorder. J Physiol 570: 637–643, 2006. doi: 10.1113/jphysiol.2005.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leuenberger UA, Brubaker D, Quraishi SA, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci 121: 87–93, 2005. [Erratum in Auton Neurosci 183:120, 2014]. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Han Z, Chen S, Liao Y, Wang Y, Liu P, Chen Y, Tang C, Lin J, Du J, Jin H. Total peripheral vascular resistance, cardiac output, and plasma C-type natriuretic Peptide level in children with postural tachycardia syndrome. J Pediatr 166: 1385–1389, 2015. doi: 10.1016/j.jpeds.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS). Neurology 45, Suppl 5: S19–S25, 1995. [PubMed] [Google Scholar]
- 27.Low PA, Opfer-Gehrking TL, Textor SC, Schondorf R, Suarez GA, Fealey RD, Camilleri M. Comparison of the postural tachycardia syndrome (POTS) with orthostatic hypotension due to autonomic failure. J Auton Nerv Syst 50: 181–188, 1994. doi: 10.1016/0165-1838(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 28.Malmberg LP, Tamminen K, Sovijärvi AR. Orthostatic increase of respiratory gas exchange in hyperventilation syndrome. Thorax 55: 295–301, 2000. doi: 10.1136/thorax.55.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74: 543–594, 1994. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- 30.Masaoka Y, Homma I. Anxiety and respiratory patterns: their relationship during mental stress and physical load. Int J Psychophysiol 27: 153–159, 1997. doi: 10.1016/S0167-8760(97)00052-4. [DOI] [PubMed] [Google Scholar]
- 31.Medow MS, Stewart JM. The postural tachycardia syndrome. Cardiol Rev 15: 67–75, 2007. doi: 10.1097/01.crd.0000233768.68421.40. [DOI] [PubMed] [Google Scholar]
- 32.Miller JD, Pegelow DF, Jacques AJ, Dempsey JA. Skeletal muscle pump versus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J Physiol 563: 925–943, 2005. doi: 10.1113/jphysiol.2004.076422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mourya M, Mahajan AS, Singh NP, Jain AK. Effect of slow- and fast-breathing exercises on autonomic functions in patients with essential hypertension. J Altern Complement Med 15: 711–717, 2009. doi: 10.1089/acm.2008.0609. [DOI] [PubMed] [Google Scholar]
- 34.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 29: 1876–1881, 1998. doi: 10.1161/01.STR.29.9.1876. [DOI] [PubMed] [Google Scholar]
- 35.Ocon AJ, Messer ZR, Medow MS, Stewart JM. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci (Lond) 122: 227–238, 2012. doi: 10.1042/CS20110241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol 174: 156–161, 2010. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J 6: 84–99, 2006. [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson DW, Kontos HA, Raper AJ, Patterson JL Jr. Systemic circulatory responses to hypocapnia in man. Am J Physiol 223: 1308–1312, 1972. doi: 10.1152/ajplegacy.1972.223.6.1308. [DOI] [PubMed] [Google Scholar]
- 39.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci 317: 75–77, 1999. doi: 10.1016/S0002-9629(15)40480-X. [DOI] [PubMed] [Google Scholar]
- 40.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 43: 132–137, 1993. doi: 10.1212/WNL.43.1_Part_1.132. [DOI] [PubMed] [Google Scholar]
- 41.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 160: 222–226, 2012. doi: 10.1016/j.jpeds.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart JM. Common syndromes of orthostatic intolerance. Pediatrics 131: 968–980, 2013. doi: 10.1542/peds.2012-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol 291: H904–H913, 2006. doi: 10.1152/ajpheart.01359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 290: H665–H673, 2006. doi: 10.1152/ajpheart.00784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart JM, Taneja I, Medow MS. Reduced body mass index is associated with increased angiotensin II in young women with postural tachycardia syndrome. Clin Sci (Lond) 113: 449–457, 2007. doi: 10.1042/CS20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taneja I, Medow MS, Clarke DA, Ocon AJ, Stewart JM. Baroreceptor unloading in postural tachycardia syndrome augments peripheral chemoreceptor sensitivity and decreases central chemoreceptor sensitivity. Am J Physiol Heart Circ Physiol 301: H173–H179, 2011. doi: 10.1152/ajpheart.01211.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tani H, Singer W, McPhee BR, Opfer-Gehrking TL, Haruma K, Kajiyama G, Low PA. Splanchnic-mesenteric capacitance bed in the postural tachycardia syndrome (POTS). Auton Neurosci 86: 107–113, 2000. doi: 10.1016/S1566-0702(00)00205-8. [DOI] [PubMed] [Google Scholar]
- 48.Thijs RD, van den Aardweg JG, Reijntjes RH, van Dijk JG, van Lieshout JJ. Contrasting effects of isocapnic and hypocapnic hyperventilation on orthostatic circulatory control. J Appl Physiol (1985) 105: 1069–1075, 2008. doi: 10.1152/japplphysiol.00003.2008. [DOI] [PubMed] [Google Scholar]
- 49.Thomas M, McKinley RK, Mellor S, Watkin G, Holloway E, Scullion J, Shaw DE, Wardlaw A, Price D, Pavord I. Breathing exercises for asthma: a randomised controlled trial. Thorax 64: 55–61, 2009. doi: 10.1136/thx.2008.100867. [DOI] [PubMed] [Google Scholar]
- 50.Tipton MJ, Harper A, Paton JFR, Costello JT. The human ventilatory response to stress: rate or depth? J Physiol 595: 5729–5752, 2017. doi: 10.1113/JP274596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Zijderveld GA, Veltman DJ, van Dyck R, van Doornen LJ. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res 33: 73–78, 1999. doi: 10.1016/S0022-3956(98)00051-X. [DOI] [PubMed] [Google Scholar]
- 52.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 112: 157–165, 2007. doi: 10.1042/CS20060091. [DOI] [PubMed] [Google Scholar]
- 53.Wilhelm FH, Gevirtz R, Roth WT. Respiratory dysregulation in anxiety, functional cardiac, and pain disorders. Assessment, phenomenology, and treatment. Behav Modif 25: 513–545, 2001. doi: 10.1177/0145445501254003. [DOI] [PubMed] [Google Scholar]
- 54.Wilkinson DJ, Thompson JM, Lambert GW, Jennings GL, Schwarz RG, Jefferys D, Turner AG, Esler MD. Sympathetic activity in patients with panic disorder at rest, under laboratory mental stress, and during panic attacks. Arch Gen Psychiatry 55: 511–520, 1998. doi: 10.1001/archpsyc.55.6.511. [DOI] [PubMed] [Google Scholar]
- 55.Zoccal DB. Peripheral chemoreceptors and cardiorespiratory coupling: a link to sympatho-excitation. Exp Physiol 100: 143–148, 2015. doi: 10.1113/expphysiol.2014.079558. [DOI] [PMC free article] [PubMed] [Google Scholar]