Abstract
The myocardium consists of numerous cell types embedded in organized layers of extracellular matrix and requires an intricate network of blood and lymphatic vessels and nerves to provide nutrients and electrical coupling to the cells. Although much of the focus has been on cardiomyocytes, these cells make up less than 40% of cells within a healthy adult heart. Therefore, repairing or regenerating cardiac tissue by merely reconstituting cardiomyocytes is a simplistic and ineffective approach. In fact, when injury occurs, cardiac tissue organization is disrupted at the level of the cells, the tissue architecture, and the coordinated interaction among the cells. Thus, reconstitution of a functional tissue must reestablish electrical and mechanical communication between cardiomyocytes and restore their surrounding environment. It is also essential to restore distinctive myocardial features, such as vascular patency and pump function.
In this manuscript, we review the current status, challenges, and future priorities in cardiac regenerative or reparative medicine. In the first part, we provide an overview of our current understanding of heart repair and comment on the main contributors and mechanisms involved in innate regeneration. A brief section is dedicated to the novel concept of “rejuvenation or regeneration”, which we believe may impact future development in the field. The last section describes regenerative therapies, where the most advanced and disruptive strategies used for myocardial repair are discussed. Our recommendations for priority areas in studies of cardiac regeneration or repair are summarized in Tables 1 and 2.
Keywords: myocardial regeneration, regenerative treatments, cardiac rejuvenation, regenerative knowledge gaps
Cardiovascular regenerative and reparative medicine (herein referred to simply as “regenerative”) began about two decades ago with the premise that transplanted cells would proliferate, differentiate into functional cardiomyocytes, and reconstitute the function of damaged heart tissue (1,2). The excitement and promise were high, and cell therapy was viewed as a cure for cardiovascular disease. Within two years, several clinical trials were initiated. However, after two decades of research, cell therapy has not yet been proven to benefit patients with cardiovascular disease. The results have been mixed. Studies of ST-elevation myocardial infarction have been disappointing (3), whereas those of refractory angina consistently indicate beneficial effects (4). Studies of chronic heart failure have been encouraging, although conclusive demonstration of therapeutic efficacy in this setting is still lacking (3,5–7). This has led us to posit that global changes are needed to achieve progress in the field of cardiovascular regenerative medicine. We started creating cooperative multidisciplinary networks, unifying our efforts and knowledge, and coordinating future research (5). Most importantly, a deeper understanding of the fundamental processes that govern the complex phenomenon of regeneration is essential to guide the future development of this field. In this essay, we provide a comprehensive analysis of the complex array of factors involved in cardiac regeneration and repair, and we discuss the main challenges and priority areas for future studies, which are summarized in Tables 1 and 2.
Table 1.
Recommendations for future preclinical studies of cardiac regeneration.
| Component | Questions and Recommendations |
|---|---|
| General | - Does postnatal heart regeneration occur in large mammals (humans)? - What are the causes and mechanism of regenerative silencing in mammals? - To what extent does cardiac rejuvenation affect regenerative mechanisms and cardioprotection? - A deeper understanding of human developmental processes is needed to unravel regenerative biology. Use of organoids, tiny three-dimensional cellular structures resembling the organs, to provide more dynamic and physiological models on a dish, will help to understand development and regeneration. - A clear differentiation of in vitro and in vivo pro-regenerative processes should be established to better define their clinical relevance. - Cardiac molecular imaging should be used for better noninvasive functional characterization of different cell types in health and during heart healing, and as guidance for therapeutic interventions. |
| Cardiomyocytes | - Can human mature cardiomyocytes proliferate and if so, what are the mechanisms that control this process? - Can one identify and track cardiomyocyte subpopulation/s that are capable of division in the adult heart based on ploidy and other characteristics? - Markers of cardiomyocyte proliferation (rather than cell cycle activity) should be identified. - What are the transcriptional pathways underlying cardiomyocyte dedifferentiation, replication, migration, and maturation? - What mechanisms direct cardiomyocytes division as opposed to polyploidization? |
| Inflammatory / Immune cells | - What differentiates pro-regenerative from scar/fibrosis-driving cell signaling? - By which mechanisms do the same signals drive divergent healing processes? - Deeper characterization is needed of the communication among immune cells, fibroblasts, endothelial cells, and cardiomyocytes. - Better understanding of macrophage functions will enable targeting of disease-promoting cellular functions and preservation of macrophage activities or subsets that are essential for defending homeostasis and pro-regenerative healing. |
| Fibroblasts | - Proper definition and characterization of markers of fibroblasts in baseline and activated (myofibroblasts) states are needed. - The molecular circuitry that regulates fibroblasts activation needs to be elucidated to identify possible therapeutic approaches that might limit progressive cardiac fibrosis. - Are fibroblasts the only relevant source of collagen and extracellular matrix production in the heart, either at baseline or with acute and chronic injury? |
| Resident cardiac progenitors / Epicardium | - Efforts should be made to harness the therapeutic effects of the epicardial response and clarify how it is modulated in the setting of regeneration versus scar formation. - The paracrine and progenitor cell components of the epicardial response should be defined and dissected. - Complete molecular and functional characterization of endogenous cardiac progenitors should be pursued. |
| Extracellular matrix (ECM) | - To what extent can components of extracellular matrix induce regeneration in vivo in an otherwise non-regenerative environment? - Can targeting ECM components be a sufficient therapeutic strategy for regeneration? |
Table 2.
Main challenges and recommendations for future preclinical studies of cardiac regenerative therapies.
| Type | Questions and Recommendations |
|---|---|
| General issues regarding preclinical studies | - Animal models: use of comorbid or aged small and large animals might help to increase the translatability of the results. Male and female animals should be similarly represented. The pathophysiology of the underlying cardiac disease and associated comorbidities should be taken into account to decide on the treatment strategy and to interpret the therapeutic effects. - Study design: the rigor guidelines of Circulation Research [8] should be followed in all in vivo studies; in particular, keeping investigators blinded is critical, even in small animal studies. Delivery method should be carefully adjusted to the therapeutic modality and clinical context for maximal efficacy and safety. - Negative results should be reported, as their scientific value is equal to that of positive results. - To promote appropriate translation, it is critical to develop networks of experienced labs that work together to test the safety and efficacy of cell therapy in large animal models with a level of rigor comparable to multicenter clinical trials (CAESAR model), so as to avoid unnecessary or premature clinical studies [9]. |
|
Somatic stem / progenitor cells: -Cardiac: CDCs, c-kit+, CMC -Non-cardiac: BMMNCs, BM-MSCs, AT-MSCs, CD34+cells, UC-MSCs |
- An effort should be made to identify the most effective cell type(s) or cell combinations for cardiac regeneration. Head-to-head comparisons of different cells should be made, preferably comparing dose-response relationships rather than arbitrary doses. It is possible that one specific cell type exhibits superior efficacy or alternatively, combinations of different cell types may be more efficacious in inducing regeneration. - The dose-response relationship should be elucidated for various cell types. - The possible cumulative effects of repeated doses should be assessed for each cell type along with the optimal number, timing, and frequency of repeated doses. - The optimal route of administration (intracoronary, transendocardial, or intravenous) should be determined by direct comparisons of the same cell type given via different routes. - The mechanism by which cell therapy promotes regeneration/repair remains unclear, although there is general agreement that it involves paracrine mechanisms rather than engraftment and differentiation. This is perhaps the single most important unresolved issue in the field. - The immunogenicity of allogeneic cells may be a limitation if repeated dosing is required; this issue should be elucidated. |
| iPSC-derived CMs | - The main challenges related to iPSC-derived cardiomyocytes are: - 1) Heterogeneity: generated cells are frequently a mixture of atrial, ventricular, and conductive cells. Directing the differentiation toward ventricular-type cells may ensure better functional efficacy after transplantation. - 2) Immaturity: generated cardiomyocytes exhibit structural and functional features of neonatal cells with poor integration into the host myocardium and lower long-term stability. To what extent the cells should be matured in vitro before transplantation is still unclear. - Ensuring adequate survival, engraftment, and coupling of transplanted cardiomyocytes with host cells is a priority. - Teratoma formation risk is inherent in the pluripotency of parental cells. Adequate purification of generated cardiomyocytes should be performed before transplantation to prevent oncogenicity. - The evidence that donor age may be associated with increased risk of significant abnormalities in iPSCs should be evaluated. |
| Gene therapy | - Efforts should be made to develop an “ideal” vector, that guarantees adequate gene transfer efficiency with long-term and/or regulated gene expression. Selection of the optimal delivery method is also important. - Use of nonintegrative gene transfer approaches (i.e., plasmid vectors, modRNAs, miRNAs) may reduce the potential risk of tumorigenicity. - With regard to genome editing tools (CRISPR/Cas9, ZFNs, TALENs), several technical aspects should be improved: target-site availability, editing efficiency, and off-target mutagenesis. |
|
Extracellular vesicles - Exosomes - Microvesicles - Vesicle contents |
Because of the heterogeneity of vesicles, standardized methods to characterize their structure and their protein, nucleic acid and lipid content are required. Definition of distinctive vesicular characteristics for different parental cell types would be desirable. Isolation and purification methods should be standardized. Based on their apparently short half-lives following systemic administration, efficient modes of delivery to diseased tissues should be accurately defined. Possible organotropisms of vesicles based on their membrane composition should be carefully evaluated. Careful comparisons of vesicles with parental cells should be done in rigorous in vivo studies More complex vesicle contents (e.g. mitochondria or mtDNA) should begin to be evaluated as next generation options. |
|
Tissue engineering - Hydrogel-based tissue - Stacked cell sheets - Scaffold-free cell aggregates - Vascularized scaffolds |
- In vitro large-scale generation of cardiomyocytes from iPSCs is one of the limiting factors. Related priority questions are outlined above in the corresponding section. - Adequate in vitro vascularization and perfusion of engineered constructs is necessary for generation of sufficiently large (several millimeters) tissue construct to replace a scar. - Generation of well-characterized banks of allogeneic hiPSC lines that offer human leukocyte antigen-matched products for most patients might help to alleviate immunologic problems. - Appropriate models to test safety and efficacy of human heart engineered tissue should be selected and/or developed. |
AT-MSCs: adipose tissue-derived mesenchymal stem cells; BM-MSCs: bone marrow-derived mesenchymal stem cells; BMMNCs: bone marrow mononuclear cells; CDCs: cardiosphere-derived cells; CM: cardiomyocytes; CMCs: cardiac mesenchymal cells; ESCs: embryonic stem cells; iPSCs: induced pluripotent stem cells; UC-MScs: umbilical cord MSCs.
Myocardial regeneration – existing knowledge and gaps
Successfully regenerating human heart microscopically or macroscopically requires reconstitution of multiple cell types in appropriate ratios, forming a cardiac architecture and coordinating cell-cell connections similar to those observed in native cardiac tissue (Figure 1). Orchestrated division, differentiation and de-differentiation, migration, integration, infiltration, and maturation of cardiomyocytes and non-cardiomyocytes within a scaffold are likely required to form a newly functional tissue. Moreover, although regeneration/repair shares many common pathways with organogenesis during embryonic development (10), it involves additional complexities such as injury-related inflammation and the need to remove damaged/necrotic cells.
Figure 1.
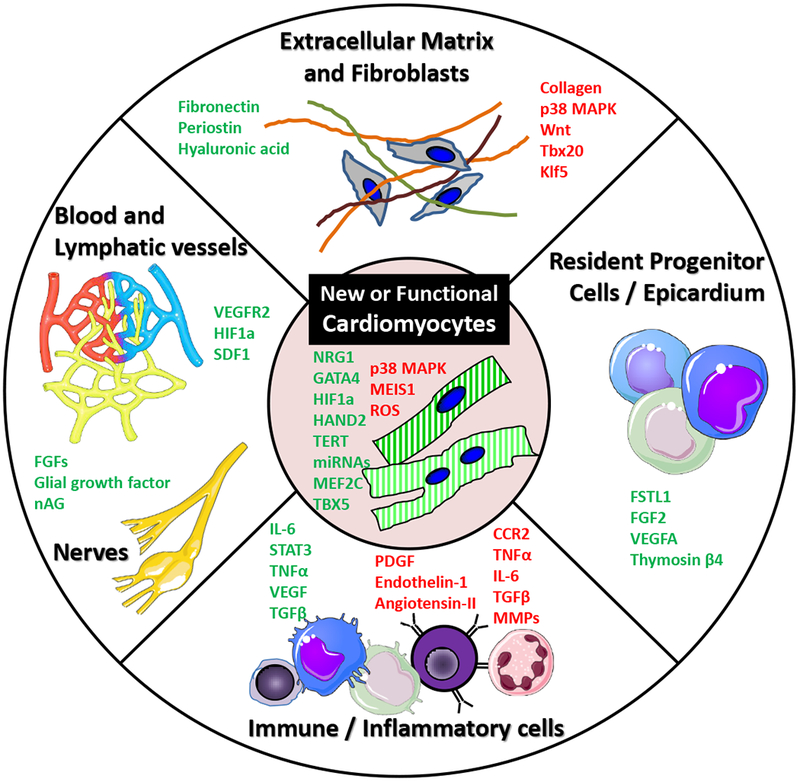
Different targets of the cardiac regenerative process. Although quantitative and qualitative restoration of cardiomyocytes has an important role in cardiac regeneration, other constitutive cardiac components are required to reach an optimal recovery of cardiac architecture and functionality after an injury. Pro-(green) and anti-regenerative (red) factors influencing different heart components are shown. CCR2 indicates C-C chemokine receptor type 2; HIF-1α, hypoxia-inducible factor 1α; IL, interleukin; MAPK, mitogen-activated protein kinase; nAG, newt anterior gradient protein; SDF1, C-X-C motif chemokine 12; STAT3, signal transducer and activator of transcription 3; Tbx, T-box protein; and VEGF, vascular endothelial growth factor.
Despite its complexity, there is evidence that cardiac regeneration occurs in some species. Most of the knowledge on spontaneous cardiac regeneration comes from lower vertebrates, such as teleost fish (zebrafish) and urodeles (newts and salamanders), since they preserve this regenerative capability through their entire life cycle (11–13). More evolved organisms lose the ability to self-regenerate, although the reasons still uncertain. In mammals, a robust regenerative capacity exists in the neonatal period in mice, including after ischemic injury, ventricular resection, and cryo-injury (14,15). Telomere dysfunction and telomerase inactivation soon after birth are some of the hypotheses proposed to explain the loss of regenerative ability after the neonatal period in mice (16). In humans, the existence of cardiac regeneration is still debated. Cases of complete functional recovery and apparent regeneration have been reported in a newborn child with myocardial infarction and in infants after cardiac surgery (17,18). MRI evidence of decreased delayed gadolinium enhancement and increased viable tissue, which is suggestive but not conclusive evidence of myocardial regeneration (19), has been provided by several cell therapy clinical trials (6). These findings suggest the possibility of reactivating or upregulating regeneration-related pathways that are silenced or downregulated at some stage after birth in the human heart. Verifying the existence of cardiac regeneration in humans and unraveling its underlying pathways and control mechanisms are a priority for regenerative medicine research.
Components of myocardial regeneration
As mentioned above, regeneration involves reconstitution of multiple cell types and structures within the heart after an injury. The main challenge is to restore the pool of lost cardiomyocytes, although reconstitution of other cells and scaffold architecture is likewise important.
Sources of new cardiomyocytes
Several potential sources of nascent cardiomyocytes exist. They can either be provided by exogenous cells or, theoretically, derived internally from stem or progenitor cells. Exogenous or extrinsic replacement generally refers to implantation of cardiomyocytes differentiated in vitro from embryonic stem cells or induced pluripotent stem cells (iPSCs). Generation of cardiomyocytes from exogenous cells has been extensively discussed in previous reviews (20,21) and is beyond the scope of this article.
Endogenous repair occurs when cardiomyocytes are generated directly within the heart and is one of the innate mechanisms of regeneration. New cardiomyocytes can potentially arise from cardiomyocytes or putative cardiac stem cells, among others (22–26). Daughter cardiomyocytes, originating by simple division (mitosis) of a parental cell, can also potentially dedifferentiate into a more primitive cell type, proliferate, and then differentiate back into new cardiomyocytes. Finally, differentiation of heart resident stem/progenitor cells into new cardiomyocytes has been postulated to be a mechanism of regeneration, although infrequent. Fate mapping studies in zebrafish and mice have demonstrated that, both after injury and during normal heart homeostasis, almost all new cardiomyocytes arise from the pre-existing pool of cardiomyocytes (25,27,28). This suggests that resident cardiomyocytes are capable of re-entering the cell cycle and dividing under certain circumstances. In most species, cardiomyocytes exit the cell cycle after birth, but in humans, approximately 25% of them undergo a further cycle of nuclear division without cytokinesis resulting in binucleation (29). Increasing evidence, however, suggests that cardiomyocytes do renew even during adulthood, although their proliferation occurs at a very low rate and decreases progressively from 1% per year in a 25-years old adult to 0.45% at age 75 (30). Why the cell cycle is downregulated and more importantly, how to induce cardiomyocytes to re-enter the cell cycle are essential questions that need to be answered. A recent study, using fate mapping of hypoxic cells, identified a rare population of hypoxia-inducible factor 1α (HIF1α) positive cardiomyocytes that importantly contributed to new cardiomyocyte formation in the adult heart (31). Other factors associated with cell cycle regulation in cardiomyocytes are p38 mitogen-activated protein kinase (MAPK) and MEIS1 (32,33), both related to the inhibition of cell proliferation. Neuregulin 1 (NRG1) is an emerging ligand, agonist of ERBB2 and ERBB4 receptor tyrosine kinases of the epidermal growth factor receptor family, that induces proliferation of adult cardiomyocytes in vitro and in vivo (34). Interestingly, NRG1 appears to stimulate proliferation independent of the ploidy of the cell (polyploidization, or presence of more than 2 sets of chromosomes within the cell, is thought to suppress further cell cycle entry).
Frequently the efficacy of regenerative approaches is measured by their ability to induce cardiomyocytes proliferation. Therefore, correct evaluation of proliferation is essential and should be differentiated from cell cycle activity, which is the one reflected by the typical readouts of BrdU, Ki67 expression, and Aurora B (35). In fact, the distinction between true cardiomyocyte renewal and polyploidization is one of the central questions discussed in the consensus statement on cardiomyocyte regeneration (36). Finally, whether regenerative therapy-induced cardiomyocyte proliferation is just an epiphenomenon or is responsible for the observed improvement of cardiac function still has to be clarified. In part, this will depend on the number of new cardiomyocytes formed, which must be carefully quantified and reported in experimental studies; simply showing examples of new myocytes is not adequate. In addition, many more questions need to be addressed (Table 1).
Crucial role of inflammatory and immune cells in heart regeneration
Contrary to classical belief, inflammation is not necessarily an impediment to tissue regeneration. In fact, monocytes and macrophages are required for cardiac regeneration (37), and injury-induced cardiomyocyte proliferation is inhibited by immunosuppression (38). Similarly, blockade of interleukin 6 (IL-6) and its downstream effector, signal transducer and activator of transcription 3 (STAT 3), suppresses cardiac regeneration after apical resection in neonatal hearts (38). A recent study analyzed the transcriptional signature after apical resection in three model organisms (zebrafish, axolotl, and neonatal mice) and concluded that activation of C5aR1 (activated by complement components of the innate immune system) promotes cardiomyocyte proliferation. Activation of complement pathway components may therefore mediate successful cardiac regeneration (39).
Technological advances have permitted a better characterization of inflammatory/immune cells and macrophages/monocytes including during cardiac repair. As a result, we know that resident macrophages in the heart are deployed there during embryonic development and they self-renew independently of blood monocytes. Surface expression of C-C chemokine receptor type 2 (CCR2), resulting in CCR2+ and CCR2− cells, varies regionally within the heart and participates in organ development (40). CCR2− macrophages reside preferentially in the myocardial wall, are essential for neonatal cardiac regeneration, and actively participate in normal heart development, specifically in the formation of the coronary vasculature. In contrast, CCR2+ cells are in the endocardium and are dispensable for cardiogenesis. Moreover, they secrete chemoattractants and induce transendothelial leukocyte migration into an injured area during myocardial ischemia. CCR2− embryonic macrophages are replaced by CCR2+ blood monocyte-derived macrophages in adult hearts after injury. Evidence suggests that limiting CCR2+ influx may preserve embryonic CCR2− subsets and improve myocardial repair (41).
Macrophages have a central role in the crosstalk among cardiomyocytes, fibroblasts, endothelial cells, and extracellular matrix (ECM) components (42). They may play a dual role after injury by secreting inflammatory cytokines, activating fibroblasts, remodeling the ECM, and ultimately driving the progression of heart failure (43). On the other hand, they can be pro-regenerative by clearing necrotic debris and stimulating angiogenesis and cardiomyocyte proliferation (44). Harnessing their pro-reparative mechanism(s) to promote heart regeneration vs. their pro-inflammatory effect that exacerbates disease will require better understanding and regulation of different hematopoietic/immune cells involved in homeostasis and injury (3).
The enigmatic fibroblasts
Fibroblasts are the cells that remodel injured myocardium providing structural integrity when other cells are lost. They are known for their primordial role in heart remodeling and fibrosis after injury and their intimate structural and functional interconnection with cardiomyocytes. In a healthy human heart, it is estimated that roughly 50% of the cells are mesenchymal cells, which include fibroblasts and pericytes (45). The majority of cardiac fibroblasts arise during embryonic development – 80% of them are generated from the epicardium through epithelial-to-mesenchymal transition (46). The concept of extra-cardiac cells (i.e., hematopoietic cells) differentiating into cardiac fibroblasts has been ruled out (47). These findings suggest that fibroblasts are formed in an organ-specific manner and may even be tailored to the function of the organ in which they are embedded.
In homeostatic conditions, fibroblasts are found in the adventitia and myocardial interstitium and are critical in maintaining the structural and mechanical properties of the heart. Recent studies indicate that tissues-resident fibroblasts differentiate into and constitute most of disease-activated fibroblasts and myofibroblasts after cardiac injury and in tissue fibrosis and remodeling (46–48).
Many facets of fibroblast biology remain a topic of ongoing debate, likely due to a lack of clear definition of cardiac fibroblasts (49,50). We now know that many functional characteristics attributed to cardiac fibroblasts in health and disease may be equivocal, as they relied on markers that were nonspecific or partially identified the pool of cardiac fibroblasts. Using newly-generated and validated genetic lineage-tracing mouse models, researchers can now more reliably address fundamental concepts in fibroblast biology; some of which are of extreme relevance for regenerative medicine (Table 1). As we unravel increasing evidence that adult fibroblasts share many cardiogenic transcription factors with endogenous cardiac progenitor cells (CPCs; 50), we can begin to address the critical question of whether CPCs and cardiac fibroblasts are the same cell type or arise from the same cells.
Extracellular matrix: the active scenario where everything happens
Although the ECM was initially considered an inert scaffold, we now understand that it is an organized and dynamic mesh of proteins, influencing cell proliferation, migration, intercellular signaling, and growth factor modulation (51). The composition as well as the structure of the ECM influence the regenerative capability of the myocardium. Changes in physical conditions such as pressure overload may influence the architectural properties of the protein mesh. In fact, progressive stiffening and maturation of the ECM in a growing mouse was correlated with cardiomyocyte cell cycle arrest (52). Similarly, in the failing human heart unloaded with a ventricular assist device, cell cycle re-entry has been observed (53).
The ECM is a multi-compartment structure that provides both micro- and macroscopic cues for cell behavior. We propose that the balance between different ECM components differentiates “regenerative ECM”, where cells incorporate and function, from “scarring ECM”, where collagen fibers predominate. Fibronectin, heparin-binding EGF-like growth factor, hyaluronic acid, tenascin C, periostin, and collagen are matricellular proteins. Many of the matricellular proteins stimulate cardiomyocyte proliferation in a paracrine fashion (54–56), whereas collagen type I is thought to directly inhibit regeneration (57). However, scar formation should not be considered necessarily a barrier to regeneration. Although not observed in mammals, in the zebrafish heart after cryoinjury, an extensive initial scar forms, which is replaced by cardiomyocytes over time (58).
The role of other non-protein components of the ECM (extracellular vesicles, non-coding RNAs) in regulation of the structure and function of the matrix is rapidly emerging (59–61). Therapeutic targeting of these molecules may constitute an attractive approach to modulate heart remodeling and eventually, promote regeneration.
Many questions regarding ECM are still unanswered, as listed in Table 1.
Neovascularization and lymphangiogenesis
One of the most important prognostic factors in acute myocardial infarction is whether blood flow is restored by reopening the obstructed coronary artery. A continuous supply of nutrients, as well as routes for eliminating metabolic products, is essential for tissue health. Therefore, reestablishing blood perfusion is required to repair damaged myocardium. Furthermore, the vasculature is a critical component of any newly-built myocardial tissue. In the absence of neovascularization following injury, the heart fails to repair and instead forms extensive fibrotic scar (62). In fact, angiogenesis and arteriogenesis were recently identified as one of the hallmarks and phenomenon that precede cardiomyogenesis (63).
Although the precise mechanisms of neovascularization are not well defined, the epicardium has been proposed as a key player. After injury, the epicardium is reactivated and secretes several classical angiogenic factors, including vascular endothelial growth factor (VEGF), C-X-C motif chemokine 12 (CXCL12 or SDF1), fibroblast growth factor, and retinoic acid (64). These factors are thought to promote epithelial-to-mesenchymal transition of epicardium-derived cells that invade the underlying myocardium, giving rise to pericytes, smooth muscle cells and fibroblasts. However, the origin of new endothelial cells is still unclear.
Our knowledge of the role of the lymphatic system in regeneration is limited. The lymphatic system actively participates in maintaining tissue fluid homeostasis and trafficking of immune cells. As mentioned earlier, inflammation can trigger activation of regenerative pathways (37–39). It is also associated with profound lymphangiogenesis and lymphatic vessel remodeling. Removing the soaring numbers of inflammatory cells, excess cytokines, and cellular debris, and resolving the edema resulting from increased blood vascular permeability are of pivotal importance to facilitate tissue repair (65). In rodents, induction of lymphangiogenesis with VEGF-C, the principal macrophage-derived cytokine mediator of lymphatic formation, improves healing, reduces fibrosis, and preserves myocardial function after myocardial infarction (66).
Nervous system
Since innervation is required to maintain tissue trophy, denervated organs or body parts exhibit progressive atrophy and dysfunction. In recent decades, the role of nerves in regeneration has been demonstrated across different species and several nerve-derived cytokines (fibroblast growth factor, glial growth factor or glial-derived neurotrophic factor, nAG) implicated in the process have been described (67–69). In recent studies, mechanical or chemical denervation of parasympathetic or sympathetic systems impaired cardiac regeneration in mice upon injury (70,71). Conversely, spinal cord stimulation-induced sympathetic innervation of the infarcted heart was associated with improved heart remodeling and function (72). It seems that a critical nerve density is a determining factor in cardiac regeneration and is an evolutionarily conserved pathway among different species (13,67).
Rejuvenation for regeneration
A decline in regenerative capacity, with subsequent loss of tissue homeostasis and function, is one of the features of aging organs. For instance, the lifelong regenerative capacity of muscle is gradually lost and is minimal in advanced age (73). Emerging evidence indicates that the functional and numerical decline of satellite cells - the skeletal muscle adult stem cells - is a progressive process occurring throughout the lifetime of the organism (74). At advanced old age, these cells become non-functional due to senescence and apoptosis. Tissues and organs with low baseline regenerative capacity, such as the heart, are the most vulnerable to the effect of chronological aging. In fact, aging is one of the most relevant risk factors for cardiovascular disease and poor prognosis (75).
At the cellular level, aged tissues are characterized by the presence of senescent cells which are thought to contribute to the progressive age-related organ dysfunction (76). Senescent cells present extensive transcriptional changes, chronically stimulated DNA-damage response, specific senescence-associated secretome (SASP), and cell-cycle arrest (77). In the heart, virtually any cell type can turn senescent and manifest in a cell-specific manner. Since regeneration is an orchestrated process that involves different cell types, senescence may affect the global process of cardiac regeneration and repair in multiple ways. First, when affecting the cardiomyocytes, the progressively decreased age-related endogenous cardiomyogenesis caused by deactivation of pro-proliferative and activation of pro-senescence pathways (as pointed out above) might be linked to the reduced cardiac regenerative ability after injury in aging individuals (30). Moreover, senescent cardiomyocytes characterized by telomere shortening, DNA damage, and alterations in ploidy (78,79), present structural changes and functional decline, such as cell hypertrophy, abnormal contractility, and abnormal relaxation resulting in age-related diastolic dysfunction. In case of resident CPCs, senescent cells lose the capacity to self-renew and their reparative (proliferation and differentiation) responses are blunted. Progenitor cell-dependent regeneration can be perturbed in a cell-autonomous manner (senescence affects directly the stem and progenitor cells) or by paracrine mechanisms (stem cell niche dysfunction caused by the SASP) (80). Finally, senescent interstitial cells, especially dysfunctional SASP-secreting fibroblasts (81), are essential in spreading the phenomenon of senescence through the whole heart with a direct effect on cells implicated in regeneration (i.e., cardiomyocytes, stem and progenitor cells). Moreover, SASP comprising pro-inflammatory cytokines, chemokines, and proteases (82,83) contributes to ECM remodeling, progressive fibrosis, and increased myocardial stiffness in elderly individuals, while high collagen fibers deposition directly inhibits regeneration (57).
To counteract the deleterious effects of persistent cellular senescence, the anti-aging or rejuvenating therapies target the promotion of cellular youthfulness. A range of interventions, from dietary to epigenetic modifiers, gene editing, and cell-based therapies, have been successfully tested in preclinical studies (75). As confirmation of a close relationship between cellular rejuvenation and regeneration (84), a variety of rejuvenating interventions have been shown to improve the regenerative ability and function of different organs, including the heart (85–87), although the debate is still open (88). So, while the concept remains to be confirmed, rejuvenation by genetic engineering, hypoxic preconditioning, drugs, growth factors, or exosomes may be plausible methods to enhance cardiac regeneration and repair by busting cardiomyocyte, CPCs and other non-parenchymal cell’s viability and function. In conclusion, the notion that cellular rejuvenation, conceived as partial or total reversion of the features of senescence, bringing the cardiac phenotype to a younger state or, at least delaying its further age-related decline, is a new concept that will open new horizons in regenerative medicine research.
Regenerative therapies
Multiple strategies have been used to induce cardiac regeneration in the past two decades. Classification of this broad spectrum of possible approaches in rigid groups is difficult because of their frequently overlapping mechanisms of action. From a conceptual standpoint, these therapies can be categorized as cell-based (i.e., involving transplantation of stem/progenitor cells or cardiomyocytes cultured in vitro into the injured heart) and cell-free (i.e., stimulators of endogenous repair, modified mRNAs, small-molecule chemical compounds, and recombinant proteins delivered with viral or non-viral DNA vectors or extracellular nanovesicles) (Figure 2). Moreover, there is evidence that a multipronged approach, using a combination of different strategies in association with tissue engineering, may be required to achieve complete regeneration of the heart (89). The concept of personalized or precision regenerative medicine is a consideration to be kept in mind when deciding on the treatment strategy. The specific disease phenotype, associated comorbidities and treatments, as well as the genetic background of each individual may be important to select which cell or cell-free product should be used to achieve the desired effect. In this section, we discuss some of the field’s most prominent advances in recent years and those that might change the future of regenerative therapies. Comprehensive reviews on this topic have been published elsewhere (90–92).
Figure 2.
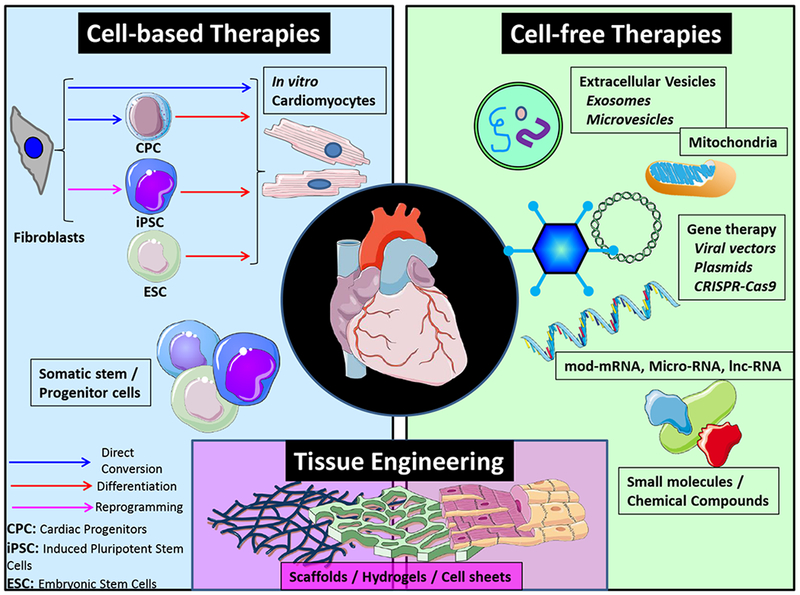
Regenerative therapies. Classification of different type of therapies in cell-based, cell-free or tissue engineering is done mostly from an academic perspective. In reality many of them converge and the combined use of different strategies may lead to better results. CPC indicates cardiac progenitor cell; CRISPR-Cas9, clustered regularly interspaced short palindromic repeats-associated 9; ECS, embryonic stem cell; and iPSC, induced pluripotent stem cell.
Gene therapy and CRISPR/Cas9 genome editing
Gene therapy has been applied to many aspects of cardiovascular research (93); however, from a regenerative perspective applications of special interest include gene therapy to promote vasculogenesis in coronary artery disease, gene therapy targeting adverse myocardial remodeling, and endogenous cardiomyocyte proliferation (94) in heart failure. Regarding the first one, a novel VEGF-DdNdC factor is being tested clinically in refractory angina by using percutaneous delivery of adenoviral vectors (95). The novelty of VEGF-DdNdC is its lymphangiogenic potential, its angiogenic effects, and its stimulation of resident stem/progenitor cells in the treated muscles (96). Regarding adverse remodeling, a recent study has demonstrated that gene therapy targeting telomerase activation in the mouse heart protects from dilation and progressive dysfunction following myocardial infarction, with a concomitant reduction of the scar and increase in survival (97). Elongated telomeres, proliferating cardiomyocytes, and a gene expression switch towards a regeneration signature of neonatal mice in adult animals were thought to underlie the observed functional improvements. This proof-of-concept study indicated that the telomere-telomerase axis can be a conceptually novel, attractive, and promising target for cardiac regeneration and should be further examined in larger studies.
Over the years, promising preclinical gene therapy results have failed to find clinical translation. A low gene transfer and transduction efficiency of only 10-20% in the human heart might have contributed to these negative results (93). Factors such as intrinsic characteristics of the vectors, delivery method, volume of injection, and matrix binding properties of the transgenes affect the final distribution and concentration of the therapeutic product. The use of adeno-associated virus (AAV) with proven long-term efficacy in preclinical models is also limited in the clinical setting by the high prevalence of pre-existing antibodies against many serotypes.
Transfer of nucleic acids with slowly degrading nanoparticles and exosomes has emerged as a potential therapeutic approach, and vectors with better cardiac tropism are being developed. Moreover, fast, efficient, and precise modern genome-editing technology has revolutionized the field of gene therapy in recent years. Among the currently used genome-editing tools, the clustered regularly interspaced short palindromic repeats (CRISPR)-associated 9 (Cas9) system offers several advantages: it is more user-friendly, more economical, faster, and has a higher editing efficiency (98). CRISPR/Cas9 has significantly broadened the application of stem cells in human regenerative medicine in general. Although there is no clear evidence that CRISPR/Cas9 is being used in cardiac regeneration, the potential of this genome-editing tool can be inferred for both in vitro and in vivo therapeutic applications, as well as for increasing our fundamental knowledge on the pathophysiology of the reparative mechanisms. Although tremendously promising, important questions remain about the safety and efficacy of potential gene-editing therapies (Table 2).
In vivo cell reprogramming: broadening the boundaries of regeneration
In 2006, the field of stem cell biology was revolutionized when mouse embryonic fibroblasts were partially reprogrammed into induced pluripotent stem cells (iPSCs) by the overexpression of four mouse transcription factors, Oct4, Sox2, Klf4, and c-Myc (OSKM), using retroviral vectors. Moreover, this discovery disrupted the classical concept of the unidirectionality of the embryonic development first and aging later, opening a new field of research in regenerative and anti-aging therapies.
Although in the cardiovascular field reprogramming is mostly used to generate cardiomyocytes, there is no reason to believe that this technology should be limited to these cells. By conventional reprogramming methods, cardiomyocytes can be obtained in vitro from terminally differentiated non-cardiac somatic cells (usually fibroblasts), first converted into iPSCs. Many groups are studying the challenges associated with iPSC-differentiated cardiomyocytes such as immaturity and heterogeneity with a consequent risk of arrhythmogenicity once transplanted into the heart (99) (Table 2). The recognition that mature somatic cells can be transdifferentiated into cardiomyocytes, without passing through an intermediate pluripotent state (100) - a phenomenon also referred to as direct conversion or direct reprogramming - has several therapeutic implications. First, by bypassing the pluripotent state, the risk of tumorigenesis may be reduced; second, using patient’s own cells avoids further immunologic issues (similar to iPSCs); and, finally, the ability to directly convert non-cardiomyocytes into cardiomyocytes in vitro offers the enticing possibility to do the same in vivo. Reprogramming by transdifferentiation spontaneously occurs in endogenous regeneration: after injury, transdifferentiation of atrial into ventricular cardiomyocytes or of cardiac fibroblast into endothelial-like cells have been described (91). Exogenously-induced transdifferentiation has been reported in studies that have delivered cardiac developmental transcription factors – GATA4, myocyte-specific enhancer factor 2C (MEF2C), and T-box protein 5 (TBX5) - directly into mouse hearts (101). Interestingly, although the reprogramming efficiency of resident fibroblasts into cardiomyocytes achieved in vivo (10-15%) was similar to that observed by the same group during in vitro conversion experiments, in vivo-generated cardiomyocytes were more fully reprogrammed and more mature (101,102). This suggests that the natural heart environment, absent in a culture dish, may enhance the cell fate switch.
Similarly, a successful transdifferentiation of cardiomyocytes into conduction system cells has been reported in vitro and in vivo by ectopic activation of Tbx18 (103) or the Notch signaling cascade (104). If confirmed in humans, these strategies may revolutionize the treatment of cardiac conduction system disorders.
Finally, in a recent study, partial in vivo reprogramming by cyclic expression of OSKM ameliorated the age-related decline of tissue and organ regenerative capacity, with partial restoration of adult stem cells populations (85). Although that study did not focus on the heart, the lifespan of reprogrammed progeric mice, which otherwise usually die from cardiovascular causes, was prolonged. This study highlights, once more, the connection between pro-regenerative and anti-aging pathways and establishes reprogramming as a possible therapeutic strategy to target them both.
Proof of concept for in vivo reprogramming has been provided in animal models. However, substantial challenges remain (Table 2) that should be addressed before any conclusion concerning the clinical utility of in vivo reprogramming therapies can be made.
Extracellular vesicles and vesicular cargo as future cell-free therapeutics
Many investigators have transplanted somatic stem/progenitor cells into the heart with the hope that these cells would engraft, differentiate, and proliferate to form new myocardium. However, we have learned that most of the favorable effects of these cells are driven by paracrine factors. Therefore, cell-free therapies originated with the idea that delivering these factors alone may be sufficient to activate/potentiate endogenous regeneration by inducing: 1) proliferation of preexisting cardiomyocytes; 2) in vivo reprogramming with generation of a reparative pool of cells (cardiomyocytes, conduction system cells, endothelial cells, etc.); and 3) differentiation of resident progenitor cells.
Exosomes, the smallest of the secreted extracellular vesicles (EVs), are nanoparticles (30-130 nm) that transport thousands of signaling molecules (ribonucleic acids, proteins, lipids). Their potential as biomarkers of disease and as therapeutics is rapidly emerging (105,106). Native exosomes secreted by stem/progenitor cells or manipulated exosomes secreted by engineered stem cells or via treatment of isolated vesicles have been tested therapeutically (107). Exogenously administered exosomes can have short- and long-distance effects by targeting specific cells and inducing molecular modifications in them. They have also been shown to activate immune responses and initiate immunoprotective functions. There has been mounting interest in using acellular therapies with EVs as the next generation of treatments for cardiac repair/regeneration (108,109).
Several preclinical studies suggest that the parental cells’ effects can be recapitulated by secreted EVs. Exosomes secreted by mesenchymal stem cells suppressed vascular remodeling and inflammation in a murine model of pulmonary hypertension (110), modulated angiogenesis, and protected cardiomyocytes from ischemic injury (111). They also exerted pro-regenerative actions in cutaneous and skeletal muscle healing. Cardiosphere-derived cell-secreted exosomes induced cardiomyocyte proliferation and angiogenesis, increased the viable mass, decreased the scar, and preserved cardiac function in acute and chronic myocardial ischemia models (112). There is also evidence that they exert an anti-senescence effect by partly activating the telomere-telomerase axis (87). EVs secreted by iPSC-derived cardiovascular progenitors outperformed both their parent cells and iPSC-cardiomyocytes when injected in peri-infarct myocardial regions in mice by significantly improving cardiac function and stimulating tissue reparative pathways (113).
Many, if not most, of the effects of exosomes are seemingly mediated by their ribonucleic acids (RNA) content, specifically microRNAs (miRs) and other noncoding RNAs. MiRs are small noncoding RNAs belonging to a class of silencing RNAs fundamental in post-transcriptional gene regulation. Their role in cardiac regeneration has been reviewed (114). Different miRs can be used to induce direct conversion of fibroblasts to cardiomyocytes (in vivo reprogramming), to promote cardiomyocyte proliferation, and to drive the differentiation of iPSCs, or cardiac resident progenitors to cardiomyocytes. Importantly, a single miR can influence multiple pathways at once, thus orchestrating and coordinating the whole regenerative process.
Recently, the regenerative properties of some stem cells have been linked to mitochondrial transfer via EVs (115). Moreover, these vesicles can transport the full mitochondrial genome (mtDNA) and transfer it to dysfunctional cells, restoring their activity (116). Since mitochondrial dysfunction and cumulative mtDNA damage are related to cardiac aging and heart failure (117,118), and since the importance of mitochondria in mediating stem cell activity is becoming increasingly evident (119), mitochondria-based therapies may be an attractive option to boost cardiac regeneration in the future. Cardiac transplantation of skeletal muscle-derived mitochondria to ameliorate ischemia/reperfusion injury is being explored in a phase II clinical trial (NCT02851758).
Clinical use of EVs, however, is hindered by formidable logistic and cost-effectiveness issues related to the cost of producing large quantities of EVs for therapeutic administration. Key challenges related to the clinical use of EVs and miRs are summarized in Table 2.
Cardiac tissue engineering. Why we should not give up on bioartificial heart?
The exciting progress from the past decade in tissue engineering and stem cell biology has opened new and promising horizons for the development of 3-dimensional engineered heart constructs (EHC). Development of a perfect EHC or even an entire bioartificial heart is a potentially reachable goal, but doing so requires overcoming a variety of biological and technical challenges. The aim of tissue engineering has traditionally been to provide living, force-producing heart muscle tissue that can be transplanted on an injured heart and restore its normal function. Compared with cell therapy alone, the potential advantages of combining cells with scaffolds or hydrogels are: 1) a higher retention of cells, which can improve their efficacy and safety; 2) selection of cells is performed in vitro, reducing the side effect of massive cell death in situ; and 3) tissues can be better quality-controlled before implantation. It should be noted that all these benefits of EHCs are theoretical, as head-to-head comparative studies with cell therapies have not been performed (120).
Building an engineered tissue requires mimicking native ECM composition and structure to modulate implanted cell behavior. In an engineered tissue, the collagen fiber orientation mediates cell organization and alignment. This cell alignment, in turn, governs contractile forces generated by cardiac cells (121,122). Similarly, ECM components retain and provide necessary cues for cell differentiation (123), which is key for differentiating stem cells towards specific cardiac lineages.
Another interesting concept is the intrinsic bioactivity of the components of the scaffolds/hydrogels. In general, acellular injectable biomaterials have been demonstrated to prevent adverse remodeling after myocardial infarction and some of them to promote angiogenesis in preclinical models (124). However, randomized trials with the natural biomaterial alginate have failed to prove functional benefits (125). In the future, co-delivery of biomaterials with additional cell-free bioactive compounds (EVs, miRs, growth factors, etc.) may be of especial interest. Prolonged release and desired localization of the delivery are some of the advantages that biomaterials can offer to the cell-free therapeutics, increasing their efficacy. Recently, hyaluronic acid hydrogel enriched with miR-302 injections in the mouse heart after myocardial infarction have been shown to increase cardiomyocytes proliferation and improve heart function (126). Research in this field is just beginning.
Building a bioartificial heart remains a laudable and achievable end-goal of cardiovascular bioengineering (127). With the creation of decellularized, biocompatible whole-heart scaffolds, many of the obstacles to engineering a complex vascularized cardiac tissue seem to have been obviated. The current challenge is to improve the processes required for recellularization - including those for cells, bioreactors, and physiologic conditioning. Generation of sufficient numbers of cardiac cells, repopulation of the scaffold, and the maturation of a functional recellularized human-sized heart are some of the hurdles to be overcome. Maintaining sterility and quantifying readiness of the nascent organs are also critical for success (128). At present, the largest recellularized heart has been the decellularized porcine heart reseeded with neonatal murine cardiac cells and human umbilical cord endothelial cells under simulated physiological conditions. The coronary arteries were re-endothelialized, and measurable intrinsic myocardial electrical activity was observed in the newly generated organs after 10 days (129). These promising results suggest that we may be able to build a bioartificial heart that can be transplanted in the near future, if we overcome the issue of cost - the largest barrier to success in the field.
Conclusions
Despite the complexity of the biological processes that underlie regeneration and the suboptimal results obtained in many clinical studies to date, we have gained enormous knowledge in this field in recent years. We need to proceed with caution, responsibility, and scientific rigor. From a fundamental perspective, the classical concept of regeneration whereby a scar is replaced by large numbers of cardiomyocytes (as many as a billion), which were lost after a myocardial infarction, may not be plausible. Instead, we should consider regeneration as a global and balanced process, by involving the entirety of cardiac structures and cells types and by incorporating cellular rejuvenation as a new biological target. By replacing the classical concept with this new paradigm, we will approach the goal with a more realistic and achievable first step. Tables 1 and 2 summarize current challenges and priority areas for future studies, which we believe should be addressed to move the field forward. Future research in regenerative medicine should focus on these questions; this will help attain the ultimate goal of building a functional heart.
Acknowledgments
Sources of Funding
The authors would like to acknowledge the following sources: NIH Grants HL113530 and HL-78825; the Hungarian National Research, Development, and Innovation Office (OTKA KH_17 125570, NVKP 16-1-2016-0017 National Heart Program, and VEKOP-2.3.2-16-2016-00002) and the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the Therapeutic Development thematic programme of the Semmelweis University; G. Harold and Leila Y. Mathers Charitable Foundation and Universidad Catolica San Antonio de Murcia (UCAM); the ISCIII and FEDER (TerCel RD16/0011/0005) and ERANET II (Nanoreheart); the Ministerio de Economía, Industria y Competitividad (SAF2017-84324-C2-R), Fundació La MARATÓ de TV3 (201502, 201516), CIBER Cardiovascular (CB16/11/00403), and AdvanceCat 2014.
Nonstandard Abbreviations and Acronyms:
- AAV
adeno-associated virus
- AT-MSCs
adipose tissue-derived mesenchymal stem cells
- BM-MSCs
bone marrow-derived mesenchymal stem cells
- BMMNCs
bone marrow mononuclear cells
- CAESAR
Consortium for Preclinical Assessment of Cardioprotective Therapies
- CCR2
C-C chemokine receptor type 2
- CDCs
cardiosphere-derived cells
- CM
cardiomyocytes
- CMCs
cardiac mesenchymal cells
- CPC
cardiac progenitor cells
- CRISPR
clustered regularly interspaced short palindromic repeats
- CXCL12 or SDF1
C-X-C motif chemokine 12
- ECM
Extracellular matrix
- EGF
epidermal growth factor
- EHC
engineered heart constructs
- ESCs
embryonic stem cells
- EVs
extracellular vesicles
- GATA4
cardiac developmental transcription factors
- HIF1α
hypoxia-inducible factor 1α
- IL-6
interleukin 6
- iPSCs
induced pluripotent stem cells
- MAPK
mitogen-activated protein kinase
- MEF2C
myocyte-specific enhancer factor 2C
- mtDNA
mitochondrial genome
- NRG1
neuregulin 1
- OSKM
transcription factors Oct4, Sox2, Klf4, and c-Myc
- STAT 3
signal transducer and activator of transcription 3
- SASP
senescence-associated secretome
- TBX5
T-box protein 5
- miRs
microRNAs
- UC-MScs
umbilical cord MSCs
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
DT holds a financial interest in Miromatrix, Inc. and is entitled to sales royalty through the University of Minnesota for products related to the research described in this paper. This relationship has been reviewed and managed by the University of Minnesota and the Texas Heart Institute in accordance with its conflict of interest policies. This does not alter the authors’ adherence to the Circulation Research’s policies on sharing data and materials. PF is the founder and CEO of Pharmahungary, a group of R&D companies.
References
- 1.Hagège AA, Vilquin JT, Bruneval P, Menasché P. Regeneration of the myocardium: a new role in the treatment of ischemic heart disease? Hypertension 2001;38:1413–5. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med 1998;4:929–33. [DOI] [PubMed] [Google Scholar]
- 3.Wysoczynski M, Khan A, Bolli R. New paradigms in cell therapy: Repeated dosing, intravenous delivery,immunomodulatory actions, and new cell types. Circ Res 123:138–158, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AR, Farid TA, Pathan A, Tripathi A, Ghafghazi S, Wysoczynski M, Bolli R. Impact of Cell Therapy on Myocardial Perfusion and Cardiovascular Outcomes in Patients With Angina Refractory to Medical Therapy: A Systematic Review and Meta-Analysis. Circ Res 2016;118:984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Avilés F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R, Charron D, Fuster V, Janssens S, Kastrup J, Kim HS, Lüscher TF, Martin JF, Menasché P, Simari RD, Stone GW, Terzic A, Willerson JT, Wu JC; TACTICS (Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes). Global position paper on cardiovascular regenerative medicine. Eur Heart J 2017;38:2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee MN, Bolli R, Hare JM. Clinical Studies of Cell Therapy in Cardiovascular Medicine: Recent Developments and Future Directions. Circ Res 2018;123:266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madonna R, Van Laake LW, Davidson SM, Engel FB, Hausenloy DJ, Lecour S, Leor J, Perrino C, Schulz R, Ytrehus K, Landmesser U, Mummery CL, Janssens S, Willerson J, Eschenhagen T, Ferdinandy P, Sluijter JP. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J 2016;37:1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolli R New initiatives to improve the rigor and reproducibility of articles published in Circulation Research. Circ Res 2017; 121:472–479. [DOI] [PubMed] [Google Scholar]
- 9.Chamuleau SAJ, van der Naald M, Climent A, Kraaijveld AO, Wever K, Dunker D, Aviles F, Bolli R; on behalf of the Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes (TACTICS) group. Translational research in cardiovascular repair: A call for a paradigm shift. Circ Res 2018; 122:310–318. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Izpisua Belmonte JC. Stem Cells: A Renaissance in Human Biology Research. Cell 2016;165:1572–1585. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol. 2012;28:719–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witman N, Murtuza B, Davis B, Arner A, Morrison JI. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev Biol 2011;354:67–76. [DOI] [PubMed] [Google Scholar]
- 13.Uygur A, Lee RT. Mechanisms of Cardiac Regeneration. Dev Cell 2016;36:362–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science 2011;331:1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant DM, O’Meara CC, Ho NN, Gannon J, Cai L, Lee RT. A systematic analysis of neonatal mouse heart regeneration after apical resection. J Mol Cell Cardiol 2015;79:315–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aix E, Gutiérrez-Gutiérrez Ó, Sánchez-Ferrer C, Aguado T, Flores I. Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation. J Cell Biol 2016;213:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsang V, Yacoub M, Sridharan S, Burch M, Radley-Smith R, Khaghani A, Savoldo B, Amrolia PJ Late donor cardiectomy after paediatric heterotopic cardiac transplantation. Lancet 2009;374:387–92. [DOI] [PubMed] [Google Scholar]
- 18.Haubner BJ, Schneider J, Schweigmann U, Schuetz T, Dichtl W, Velik-Salchner C, Stein JI, Penninger JM. Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ Res 2016;118:216–21. [DOI] [PubMed] [Google Scholar]
- 19.Naumova AV, Modo M, Moore A, Murry CE, Frank JA. Clinical imaging in regenerative medicine. Nat Biotechnol 2014;32:804–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida Y, Yamanaka S. Induced Pluripotent Stem Cells 10 Years Later: For Cardiac Applications. Circ Res 2017;120:1958–1968. [DOI] [PubMed] [Google Scholar]
- 21.Duelen R, Sampaolesi M. Stem Cell Technology in Cardiac Regeneration: A Pluripotent Stem Cell Promise. EBioMedicine 2017;16:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sdek P, Zhao P, Wang Y, Huang CJ, Ko CY, Butler PC, Weiss JN, Maclellan WR. Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. J Cell Biol. 2011;194:407–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–962. [DOI] [PubMed] [Google Scholar]
- 24.Ausma J, Litjens N, Lenders MH, Duimel H, Mast F, Wouters L, Ramaekers F, Allessie M, Borgers M. Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat. J Mol Cell Cardiol. 2001;33:2083–2083. [DOI] [PubMed] [Google Scholar]
- 25.Jopling C, Sleep E, Raya M, Martí M, Raya A, Izpisúa Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010;464:606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senyo Samuel E., Steinhauser Matthew L., Pizzimenti Christie L., Yang Vicky K., Cai Lei, Wang Mei, Wu Ting-Di, Jean-Luc Guerquin-Kern Claude P. Lechene, Lee Richard T.. Mammalian Heart Renewal by Preexisting Cardiomyocytes. Nature 2013; 493: 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 2010;464:601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahuja P, Sdek P, MacLellan WR. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev 2007;87:521–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 2015;523:226–30. [DOI] [PubMed] [Google Scholar]
- 32.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev 2005;19:1175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 2013;497:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gemberling M, Karra R, Dickson AL, Poss KD. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife. 2015. April 1;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zebrowski DC, Becker R, Engel FB. Am J Physiol Heart.2016;310:H1045–54 [DOI] [PubMed] [Google Scholar]
- 36.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, Giacca M, Hare JM, Houser S, Lee RT, Marbán E, Martin JF, Molkentin JD, Murry CE, Riley PR, Ruiz-Lozano P, Sadek HA, Sussman MA, Hill JA. Cardiomyocyte Regeneration: A Consensus Statement. Circulation 2017;136:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest 2014;124:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han C, Nie Y, Lian H, Liu R, He F, Huang H, Hu S. Acute inflammation stimulates a regenerative response in the neonatal mouse heart. Cell Res 2015;25:1137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Natarajan N, Abbas Y, Bryant DM, Gonzalez-Rosa JM, Sharpe M, Uygur A, Cocco-Delgado LH, Ho NN, Gerard NP, Gerard CJ, Macrae CA, Burns CE, Burns CG, Whited JL, Lee RT. Complement Receptor C5aR1 Plays an Evolutionarily Conserved Role in Successful Cardiac Regeneration. Circulation 2018. doi: 10.1161/CIRCULATIONAHA.117.030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive Embryonic Macrophages are Required for Coronary Development and Maturation. Circ Res 2016;118:1498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K, Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 2013. 21;127:2038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honold L, Nahrendorf M. Resident and Monocyte-Derived Macrophages in Cardiovascular Disease. Circ Res 2018;122:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leask A Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res 2015;116:1269–1276. [DOI] [PubMed] [Google Scholar]
- 44.Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, Adachi H, Yashiro H, Suzuki K. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest 2016;126:2151–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisén J. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015;161:1566–75. [DOI] [PubMed] [Google Scholar]
- 46.Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 2014;124:2921–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Müller AM, Volz KS, Tang Z, Red-Horse K, Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res 2014;115:625–35. [DOI] [PubMed] [Google Scholar]
- 48.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, J Lin SC, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 2016;7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tallquist MD, Molkentin JD. Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol 2017;14:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furtado MB, Nim HT, Boyd SE, Rosenthal NA. View from the heart: cardiac fibroblasts in development, scarring and regeneration. Development 2016;143:387–97. [DOI] [PubMed] [Google Scholar]
- 51.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. Elife 2015;4. doi: 10.7554/eLife.07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canseco DC, Kimura W, Garg S, Mukherjee S, Bhattacharya S, Abdisalaam S, Das S, Asaithamby A, Mammen PP, Sadek HA. Human ventricular unloading induces cardiomyocyte proliferation. J Am Coll Cardiol 2015;65:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 2007;13:962–9. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Karra R, Dickson AL, Poss KD. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev Biol 2013;382:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Missinato MA, Tobita K, Romano N, Carroll JA, Tsang M. Extracellular component hyaluronic acid and its receptor Hmmr are required for epicardial EMT during heart regeneration. Cardiovasc Res 2015;107:487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satoh A, Hirata A, Makanae A. Collagen reconstitution is inversely correlated with induction of limb regeneration in Ambystoma mexicanum. Zoolog Sci 2012;29:191–7. [DOI] [PubMed] [Google Scholar]
- 58.González-Rosa JM, Martín V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 2011;138:1663–74. [DOI] [PubMed] [Google Scholar]
- 59.Huang ZP, Ding Y, Chen J, et al. Long non-coding RNAs link extracellular matrix gene expression to ischemic cardiomyopathy. Cardiovasc Res 2016;112:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Micheletti R, et al. Sci Transl Med 2017;9:395; Micheletti R, Plaisance I, Abraham BJ, Sarre A, Ting CC, Alexanian M, Maric D, Maison D, Nemir M, Young RA, Schroen B, González A, Ounzain S, Pedrazzini T. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci Transl Med 2017;9:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.An M, Kwon K, Park J, Ryu DR, Shin JA, Lee Kang J, Choi JH, Park EM, Lee KE, Woo M, Kim M. Extracellular matrix-derived extracellular vesicles promote cardiomyocyte growth and electrical activity in engineered cardiac atria. Biomaterials 2017;146:49–59. [DOI] [PubMed] [Google Scholar]
- 62.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 2006;127:607–19. [DOI] [PubMed] [Google Scholar]
- 63.Ingason AB, Goldstone AB, Paulsen MJ, Thakore AD, Truong VN, Edwards BB, Eskandari A, Bollig T, Steele AN, Woo YJ. Angiogenesis precedes cardiomyocyte migration in regenerating mammalian hearts. J Thorac Cardiovasc Surg 2018;155:1118–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, Gise Av, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, Pu WT. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest 2011;121:1894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Tanaka M, Shimokawa R, Kimura-Matsumoto M, Morita H, Sato S, Kamata I, Ishii T. Lymphangiogenesis in myocardial remodelling after infarction. Histopathology 2007;51:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henri O, Pouehe C, Houssari M, Galas L, Nicol L, Edwards-Lévy F, Henry JP, Dumesnil A, Boukhalfa I, Banquet S, Schapman D, Thuillez C, Richard V, Mulder P, Brakenhielm E. Selective Stimulation of Cardiac Lymphangiogenesis Reduces Myocardial Edema and Fibrosis Leading to Improved Cardiac Function Following Myocardial Infarction. Circulation 2016;133:1484–97. [DOI] [PubMed] [Google Scholar]
- 67.Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 2007;318:772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lucas D, Scheiermann C, Chow A, Kunisaki Y, Bruns I, Barrick C, Tessarollo L, Frenette PS. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med 2013;19:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rojas-Muñoz A, Rajadhyksha S, Gilmour D, van Bebber F, Antos C, Rodríguez Esteban C, Nüsslein-Volhard C, Izpisúa Belmonte JC. ErbB2 and ErbB3 regulate amputation-induced proliferation and migration during vertebrate regeneration. Dev Biol 2009;327:177–90. [DOI] [PubMed] [Google Scholar]
- 70.Mahmoud AI, O’Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, Gannon JB, Cai L, Choi WY, Egnaczyk GF, Burns CE, Burns CG, MacRae CA, Poss KD, Lee RT. Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration. Dev Cell 2015;34:387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White IA, Gordon J, Balkan W, Hare JM. Sympathetic Reinnervation Is Required for Mammalian Cardiac Regeneration. Circ Res 2015;117:990–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao SY, Liu Y, Zuo M, Zhang Y, Yue W, Au KW, Lai WH, Wu Y, Shuto C, Chen P, Siu CW, Schwartz PJ, Tse HF. Remodelling of cardiac sympathetic re-innervation with thoracic spinal cord stimulation improves left ventricular function in a porcine model of heart failure. Europace 2015;17:1875–83. [DOI] [PubMed] [Google Scholar]
- 73.García-Prat L, Sousa-Victor P, Muñoz-Cánoves P. Functional dysregulation of stem cells during aging: a focus on skeletal muscle stem cells. FEBS J 2013;280:4051–4062. [DOI] [PubMed] [Google Scholar]
- 74.Bengal E, Perdiguero E, Serrano AL, Muñoz-Cánoves P. Rejuvenating stem cells to restore muscle regeneration in aging. F1000Res. 2017. January 25;6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gude NA, Broughton KM, Firouzi F, Sussman MA. Cardiac ageing: extrinsic and intrinsic factors in cellular renewal and senescence. Nat Rev Cardiol 2018;15:523–542. [DOI] [PubMed] [Google Scholar]
- 76.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol 2005;40:634–642. [DOI] [PubMed] [Google Scholar]
- 77.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 2015;21:1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papp Z, Czuriga D, Balogh L, Balogh A, Borbely A. How cardiomyocytes make the heart old. Curr Pharm Biotechnol 2012;13:2515–2521. [PubMed] [Google Scholar]
- 79.Sheydina A, Riordon DR, Boheler KR. Molecular mechanisms of cardiomyocyte aging. Clin Sci 2011;121: 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Deursen JM. The role of senescent cells in ageing. Nature 2014;509:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev 2008;129, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beyret E, Martinez Redondo P, Platero Luengo A, Izpisua Belmonte JC. Elixir of Life: Thwarting Aging With Regenerative Reprogramming. Circ Res 2018;122:128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JL, Xu J, Rodriguez Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua Belmonte JC. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell 2016;167:1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madonna R, Taylor DA, Geng YJ, De Caterina R, Shelat H, Perin EC, Willerson JT. Transplantation of mesenchymal cells rejuvenated by the overexpression of telomerase and myocardin promotes revascularization and tissue repair in a murine model of hindlimb ischemia. Circ Res 2013;113:902–14. [DOI] [PubMed] [Google Scholar]
- 87.Grigorian-Shamagian L, Liu W, Fereydooni S, Middleton RC, Valle J, Cho JH, Marbán E. Cardiac and systemic rejuvenation after cardiosphere-derived cell therapy in senescent rats. Eur Heart J 2017;38:2957–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao ZA, Han X, Lei W, Li J, Yang Z, Wu J, Yao M, Lu XA, He L, Chen Y, Zhou B, Hu S. Lack of Cardiac Improvement After Cardiosphere-Derived Cell Transplantation in Aging Mouse Hearts. Circ Res 2018;123:e21–e31. [DOI] [PubMed] [Google Scholar]
- 89.Perea-Gil I, Gálvez-Montón C, Prat-Vidal C, Jorba I, Segú-Vergés C, Roura S, Soler-Botija C, Iborra-Egea O, Revuelta-López E, Fernández MA, Farré R, Navajas D, Bayes-Genis A. Head-to-head comparison of two engineered cardiac grafts for myocardial repair: From scaffold characterization to pre-clinical testing. Sci Rep 2018;8:6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Mol Med 2011;3:701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sahara M, Santoro F, Chien KR. Programming and reprogramming a human heart cell. EMBO J 2015;34:710–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madonna R, Ferdinandy P, De Caterina R, Willerson JT, Marian AJ. Recent developments in cardiovascular stem cells. Circ Res 2014;115:e71–8. [DOI] [PubMed] [Google Scholar]
- 93.Ylä-Herttuala S, Baker AH. Cardiovascular Gene Therapy: Past, Present, and Future. Mol Ther 2017;25:1095–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gabisonia K, Recchia FA. Gene Therapy for Heart Failure: New Perspectives. Curr Heart Fail Rep 2018;15:340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hartikainen J, Hassinen I, Hedman A, Kivelä A, Saraste A, Knuuti J, Husso M, Mussalo H, Hedman M, Rissanen TT, Toivanen P, Heikura T, Witztum JL, Tsimikas S, Ylä-Herttuala S. Adenoviral intramyocardial VEGF-DΔNΔC gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. Eur Heart J 2017;38:2547–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hassinen I, Hartikainen J, Hedman A, Kivel€a A, Saraste A, Knuuti J, Husso M, Mussalo H, Hedman M, Toivonen P, Heikura T, Yl€a-Herttuala S, Adenoviral intramyocardial VEGF-D transfer increases myocardial perfusion in refractory angina patients. Circulation 2015;132:A11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bär C, Bernardes de Jesus B, Serrano R, Tejera A, Ayuso E, Jimenez V, Formentini I, Bobadilla M, Mizrahi J, de Martino A, Gomez G, Pisano D, Mulero F, Wollert KC, Bosch F, Blasco MA. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat Commun 2014;5:5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strong A, Musunuru K. Genome editing in cardiovascular diseases. Nat Rev Cardiol 2017;14:11–20. [DOI] [PubMed] [Google Scholar]
- 99.Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016;538:388–391. [DOI] [PubMed] [Google Scholar]
- 100.Sancho-Martinez I, Baek SH, Izpisua Belmonte JC. Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol 2012;14:892–9. [DOI] [PubMed] [Google Scholar]
- 101.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010;142:375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 2012;485:593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kapoor N, Liang W, Marbán E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol 2013;31:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rentschler S, Yen AH, Lu J, Petrenko NB, Lu MM, Manderfield LJ, Patel VV, Fishman GI, Epstein JA. Myocardial Notch signaling reprograms cardiomyocytes to a conduction-like phenotype. Circulation 2012;126:1058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jansen F, Li Q, Pfeifer A, Werner N. Endothelial- and Immune Cell-Derived Extracellular Vesicles in the Regulation of Cardiovascular Health and Disease. JACC Basic Transl Sci 2017; DOI: 10.1016/j.jacbts.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Monguió-Tortajada M, Roura S, Gálvez-Montón C, Pujal JM, Aran G, Sanjurjo L, Franquesa M, Sarrias MR, Bayes-Genis A, Borràs FE. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: implications for nanomedicine. Theranostics 2017;7:270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Conlan RS, Pisano S, Oliveira MI, Ferrari M, Mendes Pinto I. Exosomes as Reconfigurable Therapeutic Systems. Trends Mol Med 2017;23:636–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garikipati VNS, Shoja-Taheri F, Davis ME, Kishore R. Extracellular Vesicles and the Application of System Biology and Computational Modeling in Cardiac Repair. Circ Res 2018;123:188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sluijter JP, Davidson S, Boulanger C, Buzás E, de Kleijn DP, Engel F, Giricz Z, Hausenloy D, Kishore R, Lecour S, Leor J, Madonna R, Perrino C, Prunier F, Sahoo S, Schiffelers R, Schulz R, Van Laake L, Ytrehus K, Ferdinandy P. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2018;114:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012;126:2601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang K, Ma R, Cai W, Huang W, Paul C, Liang J, Wang Y, Zhao T, Kim HW, Xu M, Millard RW, Wen Z, Wang Y. Exosomes Secreted from CXCR4 Overexpressing Mesenchymal Stem Cells Promote Cardioprotection via Akt Signaling Pathway following Myocardial Infarction. Stem Cells Int. 2015;2015:659890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2014;2:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.El Harane N, Kervadec A, Bellamy V, Pidial L, Neametalla HJ, Perier MC, Lima Correa B, Thiébault L, Cagnard N, Duché A, Brunaud C, Lemitre M, Gauthier J, Bourdillon AT, Renault MP, Hovhannisyan Y, Paiva S, Colas AR, Agbulut O, Hagège A, Silvestre JS, Menasché P, Renault NKE. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur Heart J 2018;39:1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hodgkinson CP, Kang MH, Dal-Pra S, Mirotsou M, Dzau VJ. MicroRNAs and Cardiac Regeneration. Circ Res 2015;116:1700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci 2018;25:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, Galkin A, Thakur BK, Soplop N, Uryu K, Hoshino A, Norton L, Bonafé M, Cricca M, Gasparre G, Lyden D, Bromberg J. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci 2017;114:E9066–E9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JG, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ, Gheorghiade M. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol 2017;14:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moslehi J, DePinho RA, Sahin E. Telomeres and mitochondria in the aging heart. Circ Res 2012;110:1226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang H, Menzies KJ, Auwerx J. The role of mitochondria in stem cell fate and aging. Development 2018;145. doi: 10.1242/dev.143420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: state of the art. Circ Res 2014;114:354–67. [DOI] [PubMed] [Google Scholar]
- 121.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation 1992;85:1743–5. [DOI] [PubMed] [Google Scholar]
- 122.Schwan J, Kwaczala AT, Ryan TJ, Bartulos O, Ren Y, Sewanan LR, Morris AH, Jacoby DL, Qyang Y, Campbell SG. Anisotropic engineered heart tissue made from laser-cut decellularized myocardium. Sci Rep 2016;6:32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakayama KH, Hou L, Huang NF. Role of Extracellular Matrix Signaling Cues in Modulating Cell Fate Commitment for Cardiovascular Tissue Engineering. Advanced healthcare materials. 2014;3:628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A, Mahmoudi M, Malkovskiy AV, Rajadas J, Butte MJ, Bernstein D, Ruiz-Lozano P. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 2013;34:9048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rao SV, Zeymer U, Douglas PS, Al-Khalidi H, White JA, Liu J, Levy H, Guetta V, Gibson CM, Tanguay JF, Vermeersch P, Roncalli J, Kasprzak JD, Henry TD, Frey N, Kracoff O, Traverse JH, Chew DP, Lopez-Sendon J, Heyrman R, Krucoff MW. Bioabsorbable Intracoronary Matrix for Prevention of Ventricular Remodeling After Myocardial Infarction. J Am Coll Cardiol 2016;68:715–23. [DOI] [PubMed] [Google Scholar]
- 126.Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, Cavanaugh CA, Zhou S, Kanade R, Atluri P, Morrisey EE, Burdick JA. Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng 2017;1:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taylor DA, Parikh RB, Sampaio LC. Bioengineering Hearts: Simple yet Complex. Curr Stem Cell Rep 2017;3:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taylor DA, Elgalad A, Sampaio LC. What will it take before a bioengineered heart will be implanted in patients? Curr Opin Organ Transplant 2018. doi: 10.1097/MOT.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 129.Weymann A, Patil NP, Sabashnikov A, Jungebluth P, Korkmaz S, Li S, Veres G, Soos P, Ishtok R, Chaimow N, Pätzold I, Czerny N, Schies C, Schmack B, Popov AF, Simon AR, Karck M, Szabo G. Bioartificial heart: a human-sized porcine model-the way ahead. PLoS One 2014;9:e111591. [DOI] [PMC free article] [PubMed] [Google Scholar]


