Abstract
Background
Non-invasive lung adenocarcinoma could benefit from limited resection, nonetheless, there is a lack of method to determine preoperative tumour invasiveness. We aimed to investigate whether folate receptor-positive circulating tumour cells (FR+-CTCs) in combination with maximum tumour diameter (MTD) determines, before surgery, the invasiveness of small-sized, indeterminate solitary pulmonary nodules (SPNs).
Methods
A total of 382 patients with suspicious lung adenocarcinoma on computed tomography who were expected to undergo lung resection were enrolled in this study at three participating institutes and randomly assigned into training and validation cohorts. Before surgery, 3 mL peripheral blood was collected from all participants. FR+-CTCs were analyzed using immunomagnetic leukocyte depletion and quantitated by ligand-targeted PCR method. After surgery, the resected tissues were diagnosed by pathologists according to IASLC/ATS/ERS classification.
Findings
FR+-CTC levels in the peripheral blood can differentiate benign from malignant nodules with a sensitivity of 78·6%–82·7% and a specificity of 68·8%–78·4%. Both FR+-CTC and MTD are independent predictive indices of invasive tumours for lung adenocarcinoma ≤2 cm based on multivariate analyses. Further, FR+-CTC count in combination with MTD can differentiate non-invasive cancers from invasive cancers with a sensitivity of 63·6%–81·8% and a specificity of 71·4%–89·7%.
Interpretation
Detection of FR+-CTC is a reliable method to differentiate malignancy of indeterminate SPNs. Combining of FR+-CTC count and MTD could possibly enhance preoperative determination of the invasiveness of lung nodules and guide surgeons to select limited lung resection and avoid overtreatment for patients with non-invasive lesions.
Fund
None.
Keywords: Circulating tumour cells, Folate receptor, Non-small cell lung cancer, Tumour invasiveness, Limited lung resection
Research in context.
Evidence before this study
We searched MEDLINE and conference abstracts (American Society of Clinical Oncology annual meeting, World Conference on Lung Cancer) with the terms “circulating tumour cells”, “folate receptor”, “limited lung resection”, “non-small cell lung cancer”, and “tumour invasiveness” to establish the feasibility of this study. Previous clinical evidence has demonstrated that FR+-CTC can be used for differential diagnosis between benign and malignant pulmonary nodules. However, FR+-CTC for the diagnosis of SPNs has not been fully examined in a multi-centre, large-scale, prospective study. More importantly, the utility of FR+-CTC level and its combination with MTD to determine tumour invasiveness was not reported.
Added value of this study
Our study shows that FR+-CTC can effectively discriminate benign and malignant pulmonary tumours and FR+-CTC is an independent predictive factor to differentiate non-invasive adenocarcinoma from invasive adenocarcinoma in small-sized early stage lung adenocarcinoma.
Implications of all the available evidence
To avoid overtreatment, FR+-CTC detection should be performed in patients with indeterminate SPNs. When pathological assessment is not available, FR+-CTC could benefit NSCLC patients who are likely to have non-invasive lesions such that a limited lung resection may be performed.
Alt-text: Unlabelled Box
1. Introduction
Growing use of chest computed tomography (CT) has resulted in the identification of over millions of solitary pulmonary nodules (SPNs) each year [1]. Although only approximately 30% of such SPNs are malignant, decisions to undergo surgical resection or pursue CT surveillance hinge on the probability of malignancy for the identified SPNs [2]. Tissue biopsy, radiographic imaging modalities, and serum biomarkers can help determine the malignancy. However, tissue biopsy is invasive and not practical in a significant portion of patients with small SPNs [3], while other methods suffer from low sensitivity and specificity [[4], [5], [6]]. Thus, a non-invasive and reliable diagnostic assessment of SPNs is needed.
Since the report from the North American Lung Cancer Study Group of a three-fold increase in local recurrence rate and a decrease in overall survival following limited resection, lobectomy has been the standard of care for early-stage non-small cell lung cancer (NSCLC) in the past two decades [7]. Nevertheless, recent studies have shown that sub-lobar resection could be acceptable for patients with non-invasive adenocarcinomas [8]. For patients with stage IA NSCLC, segmentectomy has showed a comparable survival and a better preservation of lung function [9,10]. Indeed, the NCCN guideline suggests that sub-lobar resection is appropriate in patients with peripheral nodule ≤2 cm, and pure adenocarcinoma in situ (AIS) histology [11]. Therefore, development of a non-invasive and sensitive strategy for predicting the invasiveness of lung nodules, particularly in NSCLC, before selecting eligible patients for limited lung resection will significantly benefit patients with non-invasive lung carcinoma, particularly early-stage, undefined small-sized SPNs, by avoiding major lung resections. In the long run, a reliable differentiating strategy for SPNs will significantly contribute to the reduction of lung cancer-associated mortality by guiding adequate and timely treatment and avoiding overtreatment of non-invasive SPNs.
Circulating tumour cells (CTCs), are the cells shed from the tumours and subsequently entered the circulation. With recent technological advances, CTC has emerged as one of the key markers in “liquid biopsy” for cancer management [12]. The CellSearch system, a CTC assay that captures CTCs using magnetic beads linked with monoclonal antibody against epithelial cell adhesion molecule (EpCAM), was approved for prognosis of several metastatic cancers by US FDA [13]. Because lung cancer cells were found to have substantial loss of EpCAM expression due to epithelial-to-mesenchymal transition [14], the CellSearch system has limited sensitivity (23% versus 80% by size-based filtration method) for detecting CTCs in lung cancer.
Folate receptor (FR) has physiological and pharmacological functions mediating cellular and trans-cellular folate/antifolate transport [15]. FRα, the most widely expressed FR isoform, is aberrantly over-expressed in 77% of lung adenocarcinoma tissues but with low expression level in normal tissues [16,17]. In the circulation, no cells except a rare subgroup of activated macrophages express FR [18], therefore it could serve as a potential biomarker for identifying CTCs in the peripheral blood of lung cancer patients. In this study, we conducted a prospective, multi-center trial to determine the value of folate receptor-positive CTCs (FR+-CTCs) as a predictive index in diagnosing early NSCLC patients with SPNs.
2. Materials and methods
2.1. Study design
This study is a prospective, multi-centre trial conducted at three centres: Shanghai Chest Hospital affiliated to Shanghai Jiao Tong University, Renmin Hospital of Wuhan University, and Shanghai Pulmonary Hospital affiliated to Tongji University. From October 2013 to September 2016, 382 patients with single, suspicious CT-detected pulmonary nodules were enrolled. Inclusion criteria included: 1) patients with single CT-detected SPN of ≤3 cm; 2) suspicious NSCLC patients who are expected to undergo surgery; and 3) sufficient amount of peripheral blood sample collected before surgery. Exclusion criteria included: 1) history of cancer; 2) recent pulmonary infection; 3) previously treated with anti-cancer agents, corticosteroid, or other non-steroid anti-inflammatory drugs; and 4) multiple lung nodules. All resected nodules were examined by H&E pathological staining after surgery. The enrolled subjects were randomly assigned at a 2:1 ratio into training and validation sets, among which 222 subjects were randomly assigned to receive serum biomarker testing for comparison. Lung cancer staging was based on the 7th IASLC TNM Staging System. Benign lung diseases included harmatoma, pneumonia, tuberculosis, intrapulmonary lymph node, bronchiectasis, and granuloma. According to IASLC/ATS/ERS classification of lung adenocarcinoma, AIS is defined as non-invasive adenocarcinoma showing no stromal, vascular, or pleural invasion, while minimally invasive adenocarcinoma (MIA) and invasive pulmonary adenocarcinoma (IPA) are defined as invasive adenocarcinoma containing an invasive component [19]. The Institutional Ethics Committees of the three participating centres have approved this study. An informed consent was obtained from each participant.
2.2. FR+-CTC analysis
FR+-CTC analysis was performed using the CytoploRare® Detection Kit provided by GenoBiotech (China) Co. Ltd. in a blinded manner (Supplementary Fig. 1). Three millilitres of peripheral blood were withdrawn into an EDTA-containing anti-coagulant tube from each subject before surgical operation. The enrichment of CTC was initially achieved by lysis of erythrocytes followed by immuno-magnetic depletion of leukocytes from the whole blood. Then, FR+-CTCs in each sample were quantified by ligand-targeted polymerase chain reaction (LT-PCR) as published before [20]. In brief, a tumour-specific ligand folic acid with a high labelling efficiency to FRα is used to label CTCs and an oligonucleotide that is conjugated to the tumour-specific ligand is used to determine the number of CTCs through conventional PCR. The primer sequences were listed as follows: detection probe (an oligonucleotide that is conjugated to the tumour-specific ligand folic acid), 5′–CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGTTCTAA–3′; forward primer, 5′–TATGATTATGAGGCATGA–3′; reverse primer, 5′–GGTGTCGTGGAGTCG–3′; TaqMan probe, 5′–FAM–CAGTTGAGGGTTC–MGB–3′. The LT-PCR reaction was performed on ABI StepOne instrument (Thermo Fisher Scientific, MA, USA) as follows: denaturation at 95 °C for 2 min, annealing at 40 °C for 30 s, extension at 72 °C for 30 s, and then cooling at 8 °C for 5 min; 40 cycles of denaturation at 95 °C for 10 s, annealing at 35 °C for 30 s, and extension at 72 °C for 10 s. A self-referenced CTC unit derived from standard curve was used to indicate the abundance of FR+-CTCs in 3 mL peripheral blood. For example, 10 CTC units represent 10 FR+-CTCs in 3 mL whole blood. A serial of standards containing oligonucleotides (10−14 to 10−9 M, corresponding to 2 to 2 × 105 CTC units/3 mL blood) are used for FR+-CTC quantification.
2.3. Tumour marker analysis
For the randomly selected portion of patients assigned to receive serum biomarker tests, 3 mL of peripheral blood was withdrawn into a blood coagulation tube. After centrifuging at 3500 rpm for 10 min, the serum was collected for analysis of tumour markers. Carcino-embryonic antigen (CEA), cytokeratin 19 fragments (CYFRA21-1), and neuron-specific enolase (NSE) were analyzed by flow fluorescence assay (Tellgen, Shanghai, China) and squamous cell carcinoma antigen (SCC) was detected by chemiluminescence immunoassay (Abbott Laboratories, IL, USA).
2.4. Immunohistochemistry
Lung adenocarcioma and benign lung disease tissue samples were obtained from selected patients. Serous ovarian cancer tissue samples purchased from OriGene Technologies, Inc. (MD, USA) served as a positive control. FFPE slides (5 μm each) were first deparaffinized and immersed in citrate buffer solution (pH = 6.0) for antigen retrieval. Next, the tissue sections were incubated with anti-FRα monoclonal antibody (R&D Cat# MAB5646, RRID: AB_2278620; diluted at 1:100) overnight at 2 °C, followed by incubation with secondary antibody (TL-125-QPH, Thermo Fisher Scientific, MA, USA) for 15 min at room temperature. After repeated washing, the slides were stained with DAB and counterstained with haematoxylin for visualization. At last, slides were mounted with neutral balsam and observed under a light microscope (Carl Zeiss, Germany).
2.5. Immunostaining
CTCs were enriched in 3 mL of peripheral blood samples obtained from two selected lung cancer patients using the aforementioned protocol. The enriched CTC samples were fixed by 4% paraformaldehyde for 20 min at 4 °C. Then the cells were labelled with a CD45 monoclonal antibody conjugated to Alexa 488 (Abcam Cat# ab197730; diluted at 1:100) and a fluorescent conjugates of folic acid (10 nM, Wuxi AppTec, Wuxi, China) for 1 h. After that, sample was washed and mounted with DAPI-containing mounting media (Thermo Fisher Scientific, MA, USA), and subsequently subjected to image analysis using a fluorescence microscope (Olympus, Japan).
2.6. Statistical analysis
The sample size was determined based on a previous study by Chen X, et al. [21] The difference of FR+-CTC or biomarker expression levels was compared using the Mann-Whitney U test or Kruskal-Wallis test. The categorical variables were compared using chi-square tests or Fisher's exact tests. Receiver operating characteristic (ROC) analysis was performed and the area under curve (AUC) was calculated to assess the performance of FR+-CTC and other clinical biomarkers in lung cancer diagnosis. Youden index (sensitivity + specificity - 1) was used to identify the optimal cut-off values in the Training set and the diagnostic efficiency was validated in the Validation set. Logistic-regression was performed to examine the efficiency of maximum tumour diameter (MTD), FR+-CTC, and other clinical biomarkers in predicting the pathological invasiveness in lung adenocarcinomas. A logistic-regression model was established based on the training set data. The ability of FR+-CTC in combination with MTD to determine tumour invasiveness was then analyzed by ROC analysis and confirmed using the validation set data. A value of P < ·05 was considered to indicate a statistically significant difference. Statistical tests were performed using SPSS 18·0 software (SPSS, RRID: SCR_002865) and Prism 5·0 (GraphPad Prism, RRID: SCR_002798).
3. Results
3.1. FRα expression in lung cancer tissues
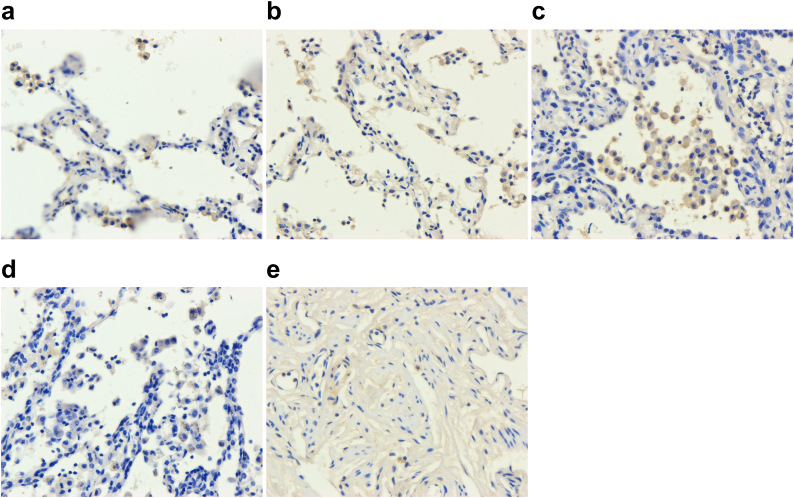
Immunohistochemistry was performed in FFPE samples collected from lung adenocarcinoma patients with different degree of tumour invasiveness and benign lung disease patients. FRα was highly expressed in lung cancer tissues, predominantly localized to the cell membrane (Fig. 1). The expression level of FRα was similar between lung adenocarcinoma patients with different extent of tumour invasiveness, while patients with benign lung disease showed a relatively lower FRα expression level (Supplementary Table 1).
Fig. 1.
Expression level of FRα in lung cancer tissues. Representative images of FFPE tissue of (a) AIS, (b) MIA, (c) IPA, (d) benign lung disease, and (e) serous ovarian cancer (positive control) by immunohistochemistry (400×) for FRα.
3.2. Detection of FRα+-CTC using immunofluorescent staining
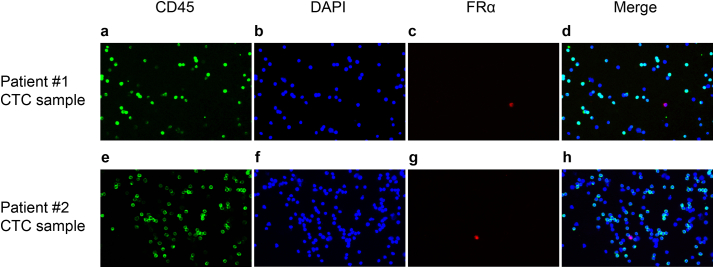
Immunostaining of CD45, FRα, and DAPI was performed in CTC samples enriched from the peripheral blood of two lung cancer patients using negative depletion method. CTCs which were specifically labelled by fluorescent folate conjugates but not CD45 antibody were observed (Fig. 2). In contrast, remaining leukocytes were labelled by CD45 antibody but not fluorescent folate conjugates. There were a few cells present in the two tested patients that were negative for both CD45 and FRα. We suggest that those are white blood cells that lack CD45 expression, such as subtypes of macrophages and monocytes [22]. It is also possible that those are CTCs that lack FRα expression or other circulating endothelial cells [23]. Though these hypotheses require further evaluation.
Fig. 2.
Immunostaining of CTCs. Immunofluorescence microscopy (20×) demonstrating CD45-negative and FRα-positive CTCs enriched from (a-d) patient #1 and (e-h) patient #2 using negative depletion method, in which CD45, nucleus, and FRα were stained in green, blue, and red, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Patients' characteristics
SPSS was used to randomly assign the recruited patients into training (n = 260) and validation (n = 122) sets. The clinicopathological characteristics of the patients in each study group were shown in Table 1. In brief, MTD showed no significant difference between patients with lung cancer and benign lung disease (P > ·05), while lung cancer patients showed a significantly higher expression of CEA, CYFRA21-1, and NSE compared to benign lung disease patients (P < ·05).
Table 1.
Clinicopathological characteristics of enrolled patients.
| Training set |
Validation set |
|||||
|---|---|---|---|---|---|---|
| Malignant (n = 197) | Benign (n = 63) | P value | Malignant (n = 93) | Benign (n = 29) | P value | |
| Age, median (range), y | 60 (21–81) | 57 (21–72) | 002 | 57 (27–79) | 54 (26–76) | 71 |
| Sex | 03 | 67 | ||||
| Male | 80 (41) | 36 (57) | 40 (43) | 14 (48) | ||
| Female | 117 (59) | 27 (43) | 53 (57) | 15 (52) | ||
| MTD, mean (SD), cm | 1·52 (0·709) | 1·40 (0·656) | 35 | 1·51 (0·74) | 1·51 (0·76) | 98 |
| Serum biomarkers, mean (SD)a | ||||||
| CEA, ng/mL | 2·75 (5·33) | 1.25 (0·53) | 01 | 2.72 (5·47) | 1.44 (0·59) | 37 |
| CYFRA21-1, ng/mL | 2·00 (1·36) | 1·26 (0·75) | <001 | 1·89 (1·38) | 1·53 (0·73) | 67 |
| NSE, ng/mL | 13·93 (7·13) | 11·07 (5·04) | 007 | 12·50 (4·12) | 11·45 (5·63) | 17 |
| SCC, ng/mL | 1·01 (0·76) | 0·89 (0·33) | 26 | 1·07 (0·91) | 1·13 (1·01) | 92 |
| Pathological subtype | ||||||
| Adenocarcinoma | 171 (87) | 85 (91) | ||||
| Squamous cell carcinoma | 10 (5) | 3 (3) | ||||
| Small cell lung cancer | 3 (2) | 0 | ||||
| Others | 13 (7) | 5 (5) | ||||
| Clinical stage | ||||||
| 0 (Tis) | 30 (15) | 21 (23) | ||||
| I | 149 (76) | 66 (71) | ||||
| II | 5 (3) | 3 (3) | ||||
| III | 6 (3) | 2 (2) | ||||
| Uncertain | 7 (4) | 1 (1) | ||||
In the training set, 117 lung cancer patients and 37 benign lung disease patients were randomly selected to receive serum biomarker tests. In the validation set, 52 lung cancer patients and 16 benign lung disease patients were randomly selected to receive serum biomarker tests.
3.4. Value of FR+-CTC in predicting malignancy
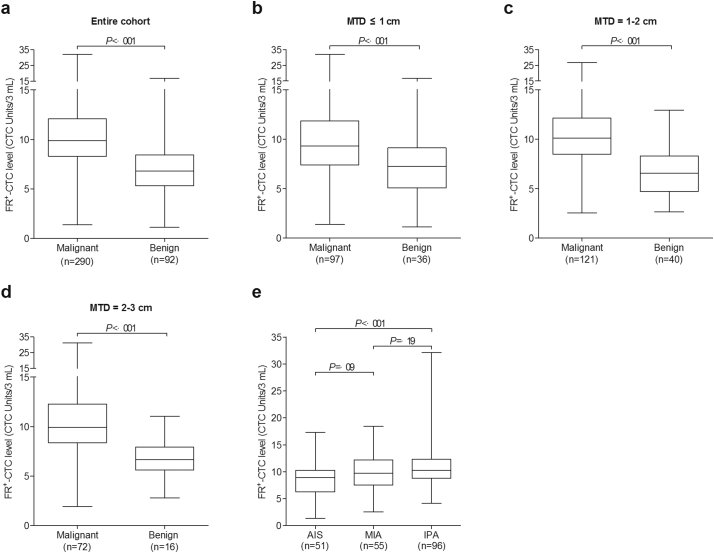
In the entire cohort, the median FR+-CTC level in patients with malignant and benign lung diseases were 9·9 and 6·8 CTC Units/3 mL (P < ·001), respectively (Fig. 3a). The average FR+-CTC level in the two groups were 10·38 and 7·00 CTC Units/3 mL, respectively. Patients were classified into three subgroups with MTD of ≤1 cm, 1–2 cm, or 2–3 cm. In different subgroups, the FR+-CTC levels were all significantly higher in patients with lung cancer than those with benign lung diseases (P < ·001; Fig. 3b–d).
Fig. 3.
Comparison of FR+-CTC level between subgroups. The Box-and-Whiskers plots showing the FR+-CTC level of the malignant group and the benign group in (a) the entire cohort; (b) subgroup of patients with MTD of ≤1 cm, (c) 1–2 cm, and (d) 2–3 cm; and (e) subgroup of patients with small-sized lung adenocarcinoma patients with different tumour invasiveness (AIS, MIA, and IPA). Lines indicate the median and interquartile range of FR+-CTC level. The difference of FR+-CTC levels was compared using the Mann-Whitney U test.
Within a randomly selected portion of patients, we also compared the diagnostic efficiencies of FR+-CTC with established tumour biomarkers. In a ROC analysis (Table 2), the AUC of FR+-CTC was the highest (Training set: AUC 0·781 [95% confidence interval (CI) 0·698–0·864]; Validation set: AUC 0·792 [95% CI 0·668–0·917]; Supplementary Fig. 2a and b). With 8·3 CTC Units/3 mL as the cut-off value, the sensitivity and specificity for differentiating malignant from benign nodules were 78·6%–82·7% and 68·8%–78·4%, respectively. Notably, when FR+-CTC was combined with CEA, CYFRA21-1, and NSE, the performance improved dramatically (Training set: AUC 0·864 [95% CI 0·807–0·921]; Validation set: AUC 0·802 [95% CI 0·673–0·930]; Supplementary Fig. 2c and d).
Table 2.
Results of ROC analysis (Malignant versus Benign).
| AUC (95% CI) | P value | Cut-off value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Training set | |||||
| MTD | 0·601 (0·506–0·696) | 07 | 1·9 | 33·3 | 91·9 |
| FR+-CTC | 0·781 (0·698–0·864) | <001 | 8·3 | 78·6 | 78·4 |
| CEA | 0·637 (0·543–0·731) | 01 | 1·81 | 38·5 | 91·9 |
| CYFRA21-1 | 0·694 (0·598–0·791) | <001 | 1·15 | 76·1 | 62·2 |
| NSE | 0·648 (0·543–0·752) | 007 | 10·84 | 73·5 | 56·8 |
| SCC | 0·562 (0·457–0·667) | 0·26 | 0·91 | 50·4 | 70·3 |
| CEA + CYFRA21-1 + NSE | 0·742 (0·657–0·828) | <001 | 0·700 | 70·9 | 70·3 |
| FR+-CTC + CEA + CYFRA21-1 + NSE | 0·864 (0·807–0·921) | <001 | 0·825 | 65·8 | 94·6 |
| Validation set | |||||
| MTD | 0·552 (0·386–0·719) | 53 | 1·9 | 73·1 | 43·8 |
| FR+-CTC | 0·792 (0·668–0·917) | <001 | 8·3 | 82·7 | 68·8 |
| CEA | 0·576 (0·425–0·726) | 36 | 1·81 | 40·4 | 62·5 |
| CYFRA21-1 | 0·536 (0·387–0·686) | 67 | 1·15 | 67·3 | 37·5 |
| NSE | 0·614 (0·444–0·784) | 17 | 10·84 | 73·1 | 43·8 |
| SCC | 0·509 (0·338–0·680) | 91 | 0·91 | 46·2 | 50·0 |
| CEA + CYFRA21-1 + NSE | 0·618 (0·463–0·773) | 16 | 0·700 | 69·2 | 37·5 |
| FR+-CTC + CEA + CYFRA21-1 + NSE | 0·802 (0·673–0·930) | <001 | 0·825 | 55·8 | 81·3 |
Only patients who were randomly selected to receive serum biomarker tests (117 lung cancer and 37 benign lung disease patients in the training set, 52 lung cancer and 16 benign lung disease patients in the validation set) were included in this ROC analysis. The logistic equations and cut-off values established from the training set data were confirmed using the validation set data. The equations are as follow: Probability of malignancy = eα/(1 + eα); For “CEA + CYFRA21-1 + NSE”, α = 0·602*CEA + 0·573*CYFRA21-1 + 0·041*NSE – 1·168; For “FR+-CTC + CEA + CYFRA21-1 + NSE”, α = 0·388*FR+-CTC + 0·736*CEA + 0·633*CYFRA21-1 + 0·004*NSE – 4·346.
3.5. Value of FR+-CTC in predicting tumour invasiveness
A total of 202 out of 256 patients with adenocarcinoma had a tumour size of ≤2 cm, which according to established guideline could be eligible for limited resection [11]. Within this subgroup, 51, 55, and 96 patients had pathological classification of AIS, MIA, and IPA, respectively. The baseline characteristics showed no significant difference between groups (P > ·05), while age and MTD were significantly correlated with increasing in tumour invasiveness (P < ·05; Supplementary Table 2).
For FR+-CTC analysis, the median FR+-CTC levels in patients with AIS, MIA, and IPA were 8·9, 9·7, and 10·3 CTC Units/3 mL, respectively (Fig. 3e). The average FR+-CTC level in the three groups were 8·74, 10·03 and 11·14 CTC Units/3 mL, respectively. The FR+-CTC levels between patients in the training and validation sets were similar (Supplementary Table 3). A significant difference in FR+-CTC count was observed between AIS versus IPA (P < ·001), AIS versus MIA + IPA (P = ·002), and AIS + MIA versus IPA (P = ·004).
Logistic regression analysis was performed on the training set. Forward selection (likelihood ratio) algorithm was used to select variables to include in the logistic-regression model (Table 3). Multivariate analysis indicated that FR+-CTC and MTD remained statistically independent predictive factors in classifying non-invasive and invasive cancers.
Table 3.
Logistic analyses of clinical predictors of tumour invasiveness (Training set).
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| AIS versus IPA | ||||
| MTD | 41·81 (5·78–302·49) | <001 | 55·54 (5·65–545·99) | ·001 |
| FR+-CTC | 1·33 (1·06–1·66) | 01 | 1·36 (1·01–1·83) | ·04 |
| CEA | 2·10 (0·92–4·81) | 08 | ||
| CYFRA21-1 | 1·18 (0·75–1·84) | 48 | ||
| NSE | 0·98 (0·90–1·06) | 55 | ||
| SCC | 1·19 (0·11–12·71) | 88 | ||
| AIS versus MIA + IPA | ||||
| MTD | 10·71 (2·24–51·11) | 003 | 9·85 (1·92–50·57) | ·006 |
| FR+-CTC | 1·26 (1·05–1·52) | 01 | 1·23 (1·01–1·50) | ·04 |
| CEA | 1·99 (0·90–4·42) | 09 | ||
| CYFRA21-1 | 1·04 (0·71–1·53) | 83 | ||
| NSE | 0·98 (0·91–1·06) | 59 | ||
| SCC | 1·46 (0·37–5·78) | 59 | ||
| AIS + MIA versus IPA | ||||
| MTD | 45·45 (9·12–226·57) | <001 | 53·24 (9·49–298·77) | <001 |
| FR+-CTC | 1·14 (1·01–1·29) | 04 | 1·14 (0·99–1·32) | 07 |
| CEA | 1·31 (0·91–1·90) | 15 | ||
| CYFRA21-1 | 1·31 (0·92–1·87) | 14 | ||
| NSE | 0·98 (0·92–1·05) | 60 | ||
| SCC | 0·64 (0·24–1·69) | 36 | ||
Only patients in the training set, who were randomly selected to receive serum biomarker tests (17 AIS, 22 MIA, and 44 IPA patients), were included in the logistic analyses. The logistic equations established from the training set data were confirmed using the validation set data in ROC analysis (Fig. 3A–F). For combining FR+-CTC and MTD, the equations are as follow: Probability of invasive cancer = eα/(1 + eα); For “AIS versus IPA”, α = 0·308*FR + -CTC + 4·017*MTD - 6·790; For “AIS versus MIA + IPA”, α = 0·210*FR+-CTC + 2·288*MTD - 3·137; For “AIS + MIA versus IPA”, α = 0·133*FR+-CTC + 3·975*MTD - 5·972.
The performance of FR+-CTC in differentiating different pathological subgroups was examined by a ROC analysis. Probability of invasive cancer was calculated using the multivariate logistic-regression model established based on the training set data. The model was then confirmed using the validation set data. FR+-CTC can effectively differentiate between non-invasive and invasive cancers (Supplementary Fig. 3). When combined with MTD, the performance of FR+-CTC further improved (Table 4).
Table 4.
Results of ROC analysis (AIS versus MIA versus IPA).
| Training set |
Validation set |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | P | Cut-off | Sen. (%) | Spec. (%) | AUC (95% CI) | P | Cut-off | Sen. (%) | Spec. (%) | |
| AIS vs IPA | ||||||||||
| MTD | 0·855 (0·736–0·974) | <001 | 1·3 | 72·7 | 88·2 | 0·831 (0·650–1·012) | 009 | 1·3 | 63·6 | 71·4 |
| FR+-CTC | 0·757 (0·627–0·887) | 002 | 9·2 | 75·0 | 70·6 | 0·727 (0·492–0·963) | 08 | 9·2 | 68·2 | 57·1 |
| Combined | 0·893 (0·795–0·991) | <001 | 0·767 | 81·8 | 88·2 | 0·864 (0·676–1·052) | 004 | 0·767 | 72·7 | 85·7 |
| AIS vs MIA + IPA | ||||||||||
| MTD | 0·763 (0·631–0·896) | <001 | 1·1 | 66·7 | 76·5 | 0·758 (0·562–0·955) | 03 | 1·1 | 67·7 | 71·4 |
| FR+-CTC | 0·716 (0·593–0·840) | 006 | 10·3 | 53·0 | 88·2 | 0·685 (0·457–0·913) | 13 | 10·3 | 55·9 | 85·7 |
| Combined | 0·804 (0·687–0·921) | <001 | 0·805 | 69·7 | 82·4 | 0·824 (0·604–1·043) | 008 | 0·805 | 67·7 | 71·4 |
| AIS + MIA vs IPA | ||||||||||
| MTD | 0·863 (0·783–0·942) | <001 | 1·4 | 68·2 | 92·3 | 0·787 (0·646–0·928) | 002 | 1·4 | 63·6 | 79·0 |
| FR+-CTC | 0·650 (0·529–0·771) | 02 | 8·8 | 84·1 | 46·2 | 0.673 (0·508–0·839) | 06 | 8·8 | 77·3 | 42·1 |
| Combined | 0·878 (0·803–0·954) | <001 | 0·577 | 75·0 | 89·7 | 0·830 (0·707–0·953) | <001 | 0·577 | 63·6 | 73·7 |
Only patients who were randomly selected to receive serum biomarker tests (17 AIS, 22 MIA, and 44 IPA patients in the training set; 7 AIS, 12 MIA, and 22 IPA patients in the validation set) were included in this ROC analysis.
4. Discussion
With the overdiagnosis and overtreatment in lung cancer screening, an efficacious biomarker for assisting LDCT to determine the malignancy and tumour invasiveness of lung nodules is urgently warranted [24]. In this study, we found that FRα was highly expressed in lung cancer tissues and FR+-CTCs can be enriched from peripheral blood of lung cancer patients using the aforementioned negative depletion method and visualized by immunostatining with anti-FRα fluorescent folate conjugates, suggesting that FRα could be an effective “tag” for labelling lung cancer CTCs. We also showed that FR+-CTC detection can effectively differentiate malignant lesions from benign tumours, even in patients with early-stage lung cancers, yielding a high sensitivity and specificity. Consistent with our previous study, the performance of FR+-CTC in lung cancer diagnosis, in terms of AUC, was better than any other established serum biomarkers [20]. When combined with clinical tumour biomarkers, diagnosis can be incrementally improved. Not surprisingly, the sensitivity and specificity were slightly lower in this study than previous ones, because the FR+-CTC level has proven to associate with clinical stage and the majority of the recruited patients were in very early-stage (91·7% cases were stage 0–I) [20,21,25,26]. In addition, FR+-CTC is an independent diagnostic factor regardless of MTD.
Although lobectomy is the standard surgical procedure for lung cancer, a limited resection is suggested for some carefully selected patients, in particular patients with small-sized lesions. Previous report suggested that non-invasive adenocarcinomas are potential candidates for limited resection and have excellent prognosis [8]. Evaluating tumour invasiveness of lung adenocarcinomas preoperatively can be crucial in determining the most adequate surgical procedures. In practice, it is sometimes difficult to eliminate tumour invasion through intra-operative morphological diagnosis based on frozen sections [27]. Lee et al. reported that lesion border and lesion margin on CT imaging that were routinely used in clinical settings showed only moderate inter-observer agreement [28], thereby calling for a more objective and robust parameter other than CT imaging to differentiate non-invasive adenocarcinomas from invasive ones. In this study, our results demonstrated that in small-sized lung adenocarcinoma, FR+-CTC level was significantly lower in non-invasive adenocarcinomas than that in invasive adenocarcinomas. Multivariate analysis revealed that smaller tumour size and lower FR+-CTC level are significant independent differentiators of non-invasive cancer from invasive cancer. It is generally accepted that CTCs can enter the circulation through epithelial-to-mesenchymal transition and the transition to M-type confers the tumour cells with the ability to pass through the wall of blood vessel [29]. Therefore it is probable that the more aggressive tumour cells would be released as CTCs at a very early-stage of cancer and led to a greater increase in the level of CTCs in circulation. This could also explain why tumour invasiveness could lead to a change in FR+-CTC level while the FRα expression levels in tissue were similar. Although the shedding of tumour cells into bloodstream has traditionally been regarded as a late-stage event during the progression of tumour [30], recent studies have demonstrated that CTCs could be detected even at a pre-malignant stage [31], which is in agreement with our hypothesis. In addition, we found that the diagnostic efficiency of FR+-CTC in combination with MTD was better compared to considering FR+-CTC or MTD alone, in classifying non-invasive and invasive cancers. The inclusion of the MIA subgroup leads to a decrease in the diagnostic efficiency of both FR+-CTC and MTD, because the clinical features and aggressiveness of MIA fall between those of AIS and IPA.
The potential limitation of this study is the selection bias. As only patients who were suspicious for lung cancer and underwent surgery resection subsequently were enrolled in the current study, and only patients who received serum biomarker tests were included in the analysis determining tumour invasiveness (leading to the small sample size of the subgroups in the validation set), our results required further validation in a more general population in future. In addition, Teixeira et al. has recently reported several molecular alterations such as methylation changes associated with the progression of pre-invasive lung cancer lesions [32]. Unfortunately, since the majority of the patients were not followed-up after surgical treatment and the mutation statuses were not assessed, the correlation between the FR+-CTC level and those molecular alterations cannot be evaluated.
In conclusion, LT-PCR based FR+-CTC detection can be used as a reliable tool for early diagnosis of NSCLC in patients with undefined SPNs. More importantly, FR+-CTC could be an independent indicator for evaluating tumour invasiveness of small-sized lung adenocarcinoma, and thus opening the opportunity for using CTC to guide clinical decision for limited resection surgery.
Data sharing statement
Data are available upon request from the corresponding author.
Funding sources
None.
Declaration of interests
All authors have no conflicts of interest to declare.
Author contributions
QL was the principal investigator. QZ and QL were responsible for the initial concept of this study. QG, CC, and QL designed the study protocol and oversaw all daily activities of the study. YL, LW, JH, ML, ZD, SY, HZ, and CP were all responsible for the reporting of individual patient data. QZ, LW, JH, and QS did the primary statistical analyses. QZ, JL, SL, CC, and QL led the interpretation of the data and writing of the manuscript. All authors additionally assisted in data interpretation and preparation of the final article.
Acknowledgments
We thank Geno Biotech China Co. Ltd. for providing CytoploRare® Detection Kit and technical support in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.02.028.
Contributor Information
Jiatao Lou, Email: loujiatao@126.com.
Shun Lu, Email: shun_lu@hotmail.com.
Chang Chen, Email: chenthoracic@163.com.
Qingquan Luo, Email: luoqingquan@hotmail.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Gould M.K., Donington J., Lynch W.R. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.She Y., Zhao L., Dai C. Development and validation of a nomogram to estimate the pretest probability of cancer in Chinese patients with solid solitary pulmonary nodules: a multi-institutional study. J Surg Oncol. 2017;116:756–762. doi: 10.1002/jso.24704. [DOI] [PubMed] [Google Scholar]
- 3.Gould M.K., Fletcher J., Iannettoni M.D. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl) doi: 10.1378/chest.07-1353. (108S–30S) [DOI] [PubMed] [Google Scholar]
- 4.Chun E.J., Lee H.J., Kang W.J. Differentiation between malignancy and inflammation in pulmonary ground-glass nodules: the feasibility of integrated (18)F-FDG PET/CT. Lung Cancer. 2009;65:180–186. doi: 10.1016/j.lungcan.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Hu Q., Xiao P., Li J., Yu P. A retrospective analysis of serum tumor markers found in non-small cell lung cancer. J Cancer Res Ther. 2016;12:117–120. doi: 10.4103/0973-1482.151424. [DOI] [PubMed] [Google Scholar]
- 6.Gelbman B.D., Cham M.D., Kim W. Radiographic and clinical characterization of false negative results from CT-guided needle biopsies of lung nodules. J Thorac Oncol. 2012;7:815–820. doi: 10.1097/JTO.0b013e31824abd9c. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg R.J., Rubinstein L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 8.Yoshizawa A., Motoi N., Riely G.J. Impact of proposed IASLC/ATS/ERS classification of lung adencarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 9.Altorki N.K., Yip R., Hanaoka T. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg. 2014;147:754–762. doi: 10.1016/j.jtcvs.2013.09.065. [DOI] [PubMed] [Google Scholar]
- 10.Harada H., Okada M., Sakamoto T., Matsuoka H., Tsubota N. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg. 2005;80:2041–2045. doi: 10.1016/j.athoracsur.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 11.NCCN NCCN Clinical Practice Guidelines in Oncology, Non-Small Cell Lung Cancer (version 3.2018) https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Available at:)
- 12.Mateo J., Gerlinger M., Rodrigues D.N., de Bono J.S. The promise of circulating tumor cell analysis in cancer management. Genome Biol. 2014;15:448. doi: 10.1186/s13059-014-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristofanilli M., Budd G.T., Ellis M.J. Circulating tumour cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 14.Krebs M.G., Hou J.M., Sloane R. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7:306–315. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 15.Chen C., Ke J., Zhou X.E. Structural basis for molecular recognition of folic acid by folate receptors. Nature. 2013;500:486–489. doi: 10.1038/nature12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nunez M.I., Behrens C., Woods D.M. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J Thorac Oncol. 2012;7:833–840. doi: 10.1097/JTO.0b013e31824de09c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driver B.R., Barrios R., Ge Y., Haque A., Tacha D., Caqle P.T. Folate receptor α expression level correlates with histologic grade in lung adenocarcinoma. Arch Pathol Lab Med. 2016;140:682–685. doi: 10.5858/arpa.2015-0431-OA. [DOI] [PubMed] [Google Scholar]
- 18.He W., Kularatne S.A., Kalli K.R. Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int J Cancer. 2008;123:1968–1973. doi: 10.1002/ijc.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis W.D., Brambilla E., Noquchi M. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011;8:381–385. doi: 10.1513/pats.201107-042ST. [DOI] [PubMed] [Google Scholar]
- 20.Wang L., Wu C., Qiao L. Clinical significance of folate receptor-positive circulating tumor cells detected by ligand-targeted polymerase chain reaction in lung cancer. J Cancer. 2017;8:104–110. doi: 10.7150/jca.16856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X., Zhou F., Li X. Folate receptor-positive circulating tumor cell detected by LT-PCR-based method as a diagnostic biomarker for non-small cell lung cancer. J Thorac Oncol. 2015;10:1163–1171. doi: 10.1097/JTO.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 22.Yu H.K., Lee H.J., Choi H.N. Characterization of CD45-/CD31+/CD105+ circulating cells in the peripheral blood of patients with gynecologic malignancies. Clin Cancer Res. 2013;19:5340–5350. doi: 10.1158/1078-0432.CCR-12-3685. [DOI] [PubMed] [Google Scholar]
- 23.Tropea M.M., Harper B.J., Graninger G.M. Isolation of circulating CD45-, CD34dim cell population and validation of their endothelial phenotype. Thromb Haemost. 2014;112:770–780. doi: 10.1160/TH14-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ten Haaf K., van der Aalst C.M., de Koning H.J. Clinically detected non-aggressive lung cancers: implications for overdiagnosis and overtreatment in lung cancer screening. Thorax. 2018;73:407–408. doi: 10.1136/thoraxjnl-2017-211149. [DOI] [PubMed] [Google Scholar]
- 25.Lou J., Ben S., Yang G. Quantification of rare circulating tumor cells in non-small cell lung cancer by ligand-targeted PCR. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y., Chen Z., Dong J. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol. 2013;6:697–702. doi: 10.1593/tlo.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walts A.E., Marchevsky A.M. Root cause analysis of problems in the frozen section diagnosis of in situ, minimally invasive, and invasive adenocarcinoma of the lung. Arch Pathol Lab Med. 2012;136:1515–1521. doi: 10.5858/arpa.2012-0042-OA. [DOI] [PubMed] [Google Scholar]
- 28.Lee S.M., Park C.M., Goo J.M., Lee H.J., Wi J.Y., Kang C.H. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology. 2013;268:265–273. doi: 10.1148/radiol.13120949. [DOI] [PubMed] [Google Scholar]
- 29.Yu M., Bardia A., Wittner B.S. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebs M.G., Metcalf R.L., Carter L., Brady G., Blackhall F.H., Dive C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11:129–144. doi: 10.1038/nrclinonc.2013.253. [DOI] [PubMed] [Google Scholar]
- 31.Rhim A.D., Mirek E.T., Aiello N.M. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teixeira V.H., Pipinikas C.P., Pennycuick A. Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat Med. 2019 doi: 10.1038/s41591-018-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material