Abstract
Condylar cartilage is a joint cartilage essential for smooth jaw movement. The importance of ciliary proteins in condylar cartilage development has been reported. However, little is known about how ciliary proteins control the homeostasis of condylar cartilage. Here we show that intraflagellar transport 20 (IFT20), a ciliary protein, is required for the maintenance of cartilaginous matrix in condylar cartilage. Utilizing NG2-CreER mice expressed in condylar cartilage, we deleted Ift20 by tamoxifen treatment at juvenile-to-adult stages. In wild-type condylar cartilage, IFT20 was robustly produced in the cis-Golgi, but deletion of Ift20 by tamoxifen induction of NG2-CreER (Ift20:NG2-CreER) resulted in reduced cell proliferation and decreased Golgi size in condylar cartilage. Importantly, while the primary cilia were present in cartilage cells in the condylar layers of wild-type mice, no primary cilia were present in the Ift20:NG2-CreER condylar layers. Consistent with this finding, ciliary-mediated Hedgehog signaling was severely attenuated in Ift20 mutant chondrocytes, and thus the production levels of type X collagen were significantly reduced in Ift20:NG2-CreER mice. These results suggest that IFT20 is required for Golgi size and Hedgehog signaling to maintain cartilaginous matrix in condylar cartilage. Our study highlights the unique function of IFT20 in the homeostasis of condylar cartilage.
Keywords: Condyle, Chondrocyte, Golgi, Intraflagellar transport, Mice
Introduction
Condylar cartilage is required for smooth jaw movement when chewing and speaking. A series of studies have highlighted the importance of the developmental aspects of condylar cartilage (1). Particularly, growth factor signals, including Hedgehog, TGF-β/BMP and Wnt, are essential for the regulation of chondrocyte proliferation and differentiation in condylar cartilage (2) (3) (4). However, little is known about how exactly these growth factor signals are transduced to maintain the homeostasis of condylar cartilage.
Primary cilia are antenna-like organelles that coordinate multiple signaling pathways, and mutations in genes encoding ciliary proteins lead to a diverse set of clinical conditions called “ciliopathies” (5). Importantly, numerous studies have indicated the molecular link between ciliopathies and craniofacial defects (6), and these findings suggest that mutations in ciliary protein genes may negatively impact the morphology and/or function of craniofacial joints. In fact, deletion of Kif3a in chondrocytes results in abnormal condylar cartilage formation and growth (7). However, it remains unclear how ciliary proteins control the maintenance of cartilaginous matrix in condylar cartilage.
Intraflagellar transport (IFT) is the bidirectional transport of multi-subunit protein complexes required for ciliary assembly, and IFT20 is the smallest IFT protein in the IFT-B complex (8). In addition to the traditional role of IFT20 in cilium assembly, several studies have shown that IFT20 has a unique function, e.g. in the control of intracellular membrane trafficking, Cbl-mediated ubiquitination, and nucleation of Golgi-derived microtubules (9-11). In support of these findings, we recently found that both the canonical and non-canonical role of IFT20 in neural crest cells are essential for skull formation (12). However, it remains elusive how IFT20 functions in other craniofacial tissues, including condylar cartilage.
The aim of this study is to investigate the role of IFT20 in condylar cartilage, to identify regulatory mechanisms for the maintenance of cartilaginous matrix.
Materials and methods
Animals
The Animal Welfare Committee and the Institutional Animal Care and Use Committee of The University of Texas Medical School at Houston approved the experimental protocol. Col2a1-CreERT2 mice were generated and maintained as previously described (13). Ift20-floxed mice (14), NG2-CreER mice (15), Aggrecan-CreERT2 mice (16), and Rosa26 reporter mice (17) were obtained from the Jackson Laboratory. Tamoxifen (Sigma; T5648) was administrated intraperitoneally (75 mg/kg) twice a week starting from 5 weeks. We compared Ift20 conditional knockout (Ift20flox/flox: NG2-CreER+) mice with wild-type controls (Ift20flox/flox: NG2-CreER−). These mice were maintained on a mix genetic background.
Histological analysis and immunostaining
Safranin O and X-gal staining was carried out using standard methods. Anti-IFT20 (Proteintech; 13615-1-AP, 1:100), anti-acetylated tubulin (Sigma; T6793, 1:500), anti-gamma tubulin (Sigma; T5326, 1:500), anti-GM130 (BD; 610822, 1:200), anti-Collagen X (Abcam; ab58632, 1:100), and Smoothened (Abcam; ab38686, 1:400) antibodies were used for immunostaining. Cell proliferation was analyzed using a proliferating cell nuclear antigen staining kit (Life Technologies; 931143). Images were captured with an Olympus FluoView 1000 confocal microscope. More than 100 cells in three independent experiments were randomly analyzed and quantified.
Cell culture
ATDC5 cells were cultured using alpha-MEM medium (Sigma-Aldrich; M8042) containing 10% fetal bovine serum (FBS) (Sigma-Aldrich; F4135), 2% L-glutamine (Sigma-Aldrich; G7513), and 1% Penicillin-Streptomycin (Sigma-Aldrich; P4333). After reaching 70% confluency, scramble or IFT20 siRNA (Thermo Fisher Scientific; 120nM) was transfected into ATDC5 cells using Lipofectamine RNAiMAX (Thermo Fisher Scientific; 13778075). To induce ciliogenesis, cells were starved with alpha-MEM medium containing 0.1% FBS for 24 hours. To stimulate Hedgehog signaling, ATDC5 cells were treated with Smoothened agonist (Calbiochem; 566661, 100nM) or Purmorphamine (Calbiochem; 540223, 1,000nM) for 24 hours.
Quantitative real-time RT-PCR
Total RNA was extracted using TRIzol Reagent (Thermo Fisher Scientific; 15596-026). Quantitative RT-PCR was carried out using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad; 1725274). The conditions for qRT-PCR were 95°C for 2 min, 95°C for 5 sec, and 60°C for 30 sec, for 40 cycles. Primer sequences for Gli1 were 5’-AGCCCGCTTCTTTGTTAATTTGA-3’ and 5’-CCAAGCCAACTTTATGTCAGGG-3’. Primer sequences for Ptch1 were 5’-CTTCTCCTATCTTCTGACGGGT-3’ and 5’-AAAGAACTGCGGCAAGTTTTTG-3’. Primer sequences for Gapdh were 5’-CGTCCCGTAGACAAAATGGT-3’ and 5 ’-TCAATGAAGGGGTCGTTGAT-3’. Data were normalized to Gapdh levels and quantified using the 2−ΔΔCT method.
Western blotting analysis
Cell lysates were separated by SDS-PAGE (Bio-Rad; 4561036). Anti-IFT20 (Proteintech; 13615-AP, 1:1,000), anti-GAPDH (Cell Signaling technology; 14C10, 1:10,000), and Goat anti-rabbit IgG HRP-conjugate (Millipore sigma; 12-348, 1:5,000) antibodies were used for western blotting. The Clarity Max ECL Substrate (Bio-Rad; 1705061) was used for chemiluminescent detection, and the signals were quantified with the image-J software.
Statistical analysis
A two-tailed Student’s t test was used for the two groups. A p value of less than 0.05 was considered statistically significant.
Results and Discussion
Disruption of Ift20 results in abnormal Golgi size in condylar cartilage
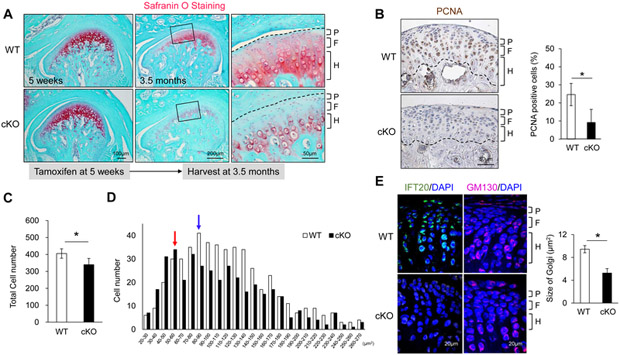
Temporomandibular joint disorder (TMD) is widely recognized as an idiopathic juvenile form of arthritis (18). To identify a Cre line to address the etiology of TMD from juvenile stages in condylar cartilage in mice, we compared the Cre activity among established chondrocyte-specific Cre mouse lines Col2a1-CreERT2, Aggrecan-CreERT2, and Neuron glia antigen-2 (NG2)-CreER. NG2 (Cspg4) encodes a chondroitin sulfate proteoglycan (19). NG2-CreER mice have been used to label glia cells, but this mouse line has not been used for studying condylar cartilage. In our Cre induction schedule starting from 5 weeks, both Col2a1-CreERT2 and Aggrecan-CreERT2 directed mosaic Cre-mediated recombination in condylar cartilage (Fig. 1). On the other hand, NG2-CreER effectively labeled chondrocytes in all polymorphic, flattened, and hypertrophic layers (Fig. 1). These data suggest that NG2-CreER serves as an additional and homogeneous Cre-inducible mouse line to study condylar cartilage. To characterize the function of ciliary proteins in condylar cartilage, we utilized NG2-CreER to delete Ift20 in mice (cKO hereafter). To address the role of IFT20 from juvenile to adult stages, tamoxifen was initiated from 5 weeks of age. Histological analysis showed that the area of the Safranin O-stained layer in cKO mice was smaller to control mice (Fig. 2A). To examine whether the reduction in size of the Safranin O-positive layer was due to decreased numbers of condylar chondrocytes, cell proliferation activity was examined. In cKO mice, a significant reduction in the number of PCNA-positive cells was observed in polymorphic, flattened, and hypertrophic layers (Fig. 2B). Along with the reduction in cell numbers in condylar cartilage (Fig. 2C), the size of condylar chondrocytes in cKO mice was smaller (cell size peak: 50-60μm, red arrow) than in controls (cell size peak: 80-90μm, blue arrow) mice (Fig. 2D). It is known that the Golgi apparatus expands in chondrocytes during the secretion of cartilaginous matrix (20). Therefore, we hypothesized that IFT20 controls the maintenance of Golgi size in condylar cartilage. Interestingly, in control condylar cartilage, IFT20 was robustly expressed in the cis-Golgi (Supplemental Fig. 1). In agreement with the pattern of NG2-CreER recombination (Fig. 1), IFT20 was disrupted in all layers of condylar cartilage in cKO mice (Fig. 2E, left panel). Interestingly, the Golgi labeled by GM130, a marker of cis-Golgi, was smaller in cKO compared with control mice (control: 9.42±0.62μm2; cKO: 5.26±0.77μm2; P<0.05) (Fig. 2E, right panel). Because IFT20 can control the nucleation of Golgi-derived microtubules in tumor cells (11), we examined whether IFT20 is capable of regulating Golgi size in chondrocytes. Consistent with our findings in vivo (Fig. 2E), the knockdown of IFT20 by siRNA in ATDC5 cells led to a smaller Golgi shape compared with that seen in control cells (Supplemental Fig. 2). These results indicate that IFT20 is required for maintaining Golgi size when chondrocytes secrete cartilaginous matrix in condylar cartilage.
Fig. 1.
Evaluation of Cre activity in condylar cartilage.
After crossing NG2-CreER, Col2a1-CreERT2 and Aggrecan-CreERT2 mice with Rosa26 (R26) reporter mice, tamoxifen was injected intraperitoneally starting from 5 weeks of age. Condylar cartilage was harvested at 6 weeks and examined. P=Polymorphic layer; F=Flattened layer; H=Hypertrophic layer.
Fig. 2.
IFT20 is important for condylar cartilage maintenance in mice.
(A) The mandibular condyle was stained with Safranin O at 5 weeks and 3.5 months. (B) PCNA immunohistochemistry and quantifications of PCNA-positive cells at 3.5 months. (C) Total cell number was quantified in the condylar cartilage area at 3.5 months. (D) Cell size distribution in condylar cartilage. (E) Localization of IFT20 and cis-Golgi marker GM130 in condylar cartilage. The size of the Golgi apparatus was quantified. P=Polymorphic layer; F=Flattened layer; H=Hypertrophic layer. Data in (B), (C) and (E) are represented as mean ± SD; n=3 in each group. *P<0.05.
IFT20 is required for ciliary-dependent Hedgehog signaling in condylar cartilage
During craniofacial formation, primary cilia transduce numerous developmental signals such as Hedgehog signaling (6). Importantly, mice lacking Indian Hedgehog (Ihh) in chondrocytes display abnormal condylar cartilages (2) (21). This led us to hypothesize that ciliary-dependent Hedgehog signaling may be responsible for the reduction of cartilaginous matrix in cKO mice. To determine whether the loss of primary cilia leads to the attenuation of Hedgehog signaling, the ATDC5 chondrogenic cell line was used to examine the role of IFT20. Compared with control cells, the numbers of primary cilia-positive cells were reduced in si-Ift20 cells (Supplemental Fig. 3). To stimulate hedgehog signaling, both control and si-Ift20 cells were treated with Hedgehog agonists (either SAG or Puromorphine), with control cells showing robust induction of Smoothened (Smo) on the surface of primary cilia (Fig. 3A, upper panel). Whereas around 30-40% of si-Ift20 cells still formed primary cilia (Supplemental Fig. 3), no Smo accumulation was observed in si-Ift20 cells (Fig. 3A, middle and lower panel). To examine whether IFT20-assembled primary cilia transduce Hedgehog signaling, cells were stimulated with Hedgehog agonists, and the expression levels of Gli1 and Ptch1 were quantified. Consistent with absence of Smo induction in primary cilia (Fig. 3A), the expression of both Gli1 and Ptch1 was significantly downregulated in si-Ift20 cells (Fig. 3B). Importantly, while primary cilia were abundantly present in condylar cartilage in control mice, no primary cilia were present in the polymorphic, flattened, and hypertrophic layers of Ift20 cKO mice (Fig. 3C). These results suggest that IFT20 regulates ciliary-dependent Hedgehog signaling in condylar cartilage. Importantly, Hedgehog signaling regulates collagen type X (Col X), a critical cartilaginous matrix component essential for the maturation of chondrocytes (22). Supporting this concept, the production of Col X was significantly reduced in condylar cartilage in cKO mice (Fig. 3D). Taken together, these results suggest that IFT20-assembled primary cilia transduce Hedgehog signaling, which is required for the production of Col X to maintain the maturation of condylar cartilage.
Fig. 3.
IFT20 is essential for ciliary-dependent Hedgehog signaling in condylar cartilage.
(A) ATDC5 cells were transfected with scramble and si-Ift20, then stimulated with Hedgehog agonist, either Purmorphamine (Puro) or Smoothened agonist (SAG). Immunocytochemistry was performed using anti-acetylated tubulin and anti-Smo antibodies and counterstaining with DAPI. (B) qRT-PCR analysis for Gli1 and Ptch1 in control and si-Ift20 treated ATDC5 cells. (C) Primary cilia were examined by immunohistochemistry of condylar cartilage using anti-acetylated tubulin and antigamma-tubulin antibodies at 3.5 months. White arrow shows primary cilia in WT. Asterisks indicate the cells shown in the high-magnification image inserted in (C). P=Polymorphic layer; F=Flattened layer; H=Hypertrophic layer. (D) Col X production was examined by immunohistochemistry. P=Polymorphic layer; F=Flattened layer; H=Hypertrophic layer. (E) A mechanism by which IFT20 controls the maintenance of condylar cartilage. IFT20 is critical for governing the Golgi size and regulating Hedgehog signaling to maintain cartilaginous matrix in condylar cartilage. Data in (B) represented as mean ± SD; n=3 in each group. *P<0.05.
In summary, utilizing the NG2-CreER mouse line, we showed that IFT20 is required for (1) maintenance of the Golgi size, and (2) Hedgehog signaling to preserve cartilaginous matrix in condylar cartilage (Fig. 3E). Our study introduces a unique perspective on the importance of ciliary proteins for condylar cartilage homeostasis, and may contribute to the understanding of the etiology of temporomandibular joint disorders in humans.
Supplementary Material
Supplemental Fig. 1. IFT20 is expressed in the cis-Golgi in condylar cartilage. Cellular localization of IFT20 in WT condylar cartilage at 3.5 months. Anti-GM130 was used to label the cis-Golgi.
Supplemental Fig. 2. IFT20 is critical for maintaining Golgi size in ATDC5 cells. (A) ATDC5 cells were transfected with scramble and two different si-Ift20 RNA (#1 and #2). Reduction of IFT20 levels was confirmed by western blotting. GAPDH was used as a loading control. (B) Quantification analysis of western blot shown in (A). (C) Golgi size was examined by immunocytochemistry using an anti-GM130 antibody in control and si-Ift20 treated ATDC5 cells. (D) The size of the Golgi apparatus was measured and quantified. Data in (B) and (D) represented as mean ± SD; n=3 in each group. *P<0.05.
Supplemental Fig. 3. IFT20 is critical for ciliogenesis in ATDC5 cells. (A) The presence of primary cilia was examined by immunocytochemistry using anti-acetylated tubulin and anti-IFT20 antibodies in control and si-Ift20 treated ATDC5 cells. (B)The number of primary cilia was measured and quantified. Data in (B) represented as mean ± SD; n=3 in each group. *P<0.05.
Highlights:
NG2-CreER mouse serves as an additional and powerful Cre-driver to study condylar cartilage.
Intraflagellar transport 20 (IFT20) is required for maintaining Golgi size in condylar cartilage.
IFT20 is required for ciliary-dependent Hedgehog signaling to produce cartilaginous matrix in condylar cartilage.
Acknowledgments
We thank Dr. Akiko Nishiyama for the NG2-CreER mice, Dr. Benoit de Crombrugghe for the Aggrecan-CreERT2 mice, Dr. Philippe Soriano for the Rosa26 reporter mice, and Dr. Gregory J Pazour for the Ift20-floxed mice. We thank Dr. Richard Behringer for helpful comments on the manuscript. We also thank Patricia Fonseca for editorial assistance. This study was supported by a research grant NIDCR/NIH R01DE025897 (Y.K.).
Footnotes
Disclosures: All authors state that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hinton RJ, Jing J, Feng JQ. Genetic Influences on Temporomandibular Joint Development and Growth. Current topics in developmental biology. 2015;115:85–109. [DOI] [PubMed] [Google Scholar]
- 2.Shibukawa Y, Young B, Wu C, et al. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236(2):426–34. [DOI] [PubMed] [Google Scholar]
- 3.Jing J, Hinton RJ, Mishina Y, Liu Y, Zhou X, Feng JQ. Critical role of Bmpr1a in mandibular condyle growth. Connective tissue research. 2014;55 Suppl 1:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Wang T, Hamilton JL, Chen D. Wnt/beta-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis. Current rheumatology reports. 2017;19(9):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Current biology : CB. 2009;19(13):R526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brugmann SA, Cordero DR, Helms JA. Craniofacial ciliopathies: A new classification for craniofacial disorders. American journal of medical genetics Part A. 2010;152a(12):2995–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinumatsu T, Shibukawa Y, Yasuda T, et al. TMJ development and growth require primary cilia function. Journal of dental research. 2011;90(8):988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan X, Yang S. Primary Cilia and Intraflagellar Transport Proteins in Bone and Cartilage. Journal of dental research. 2016;95(12):1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmid FM, Schou KB, Vilhelm MJ. IFT20 modulates ciliary PDGFRalpha signaling by regulating the stability of Cbl E3 ubiquitin ligases. 2018;217(1):151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finetti F, Paccani SR, Riparbelli MG, et al. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nature cell biology. 2009;11(11): 1332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishita M, Park SY, Nishio T, et al. Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. 2017;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noda K, Kitami M, Kitami K, Kaku M, Komatsu Y. Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(19):E2589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M, Lichtler AC, Sheu TJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis (New York, NY : 2000). 2007;45(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonassen JA, San Agustin J, Follit JA, Pazour GJ. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. The Journal of cell biology. 2008;183(3):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development (Cambridge, England). 2011;138(4):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis (New York, NY : 2000). 2009;47(12):805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soriano P Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics. 1999;21(1):70–1. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira CL, Silva MA, Felicio CM. Signs and symptoms of temporomandibular disorders in women and men. CoDAS. 2016;28(1):17–21. [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, Stallcup WB. The primary structure of NG2, a novel membrane-spanning proteoglycan. The Journal of cell biology. 1991;114(2):359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron DA. The Golgi apparatus in bone and cartilage cells. Clinical orthopaedics and related research. 1968;58:191–211. [PubMed] [Google Scholar]
- 21.Kurio N, Saunders C, Bechtold TE, et al. Roles of Ihh signaling in chondroprogenitor function in postnatal condylar cartilage. Matrix biology : journal of the International Society for Matrix Biology. 2018;67:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amano K, Densmore M, Nishimura R, Lanske B. Indian hedgehog signaling regulates transcription and expression of collagen type X via Runx2/Smads interactions. The Journal of biological chemistry. 2014;289(36):24898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. IFT20 is expressed in the cis-Golgi in condylar cartilage. Cellular localization of IFT20 in WT condylar cartilage at 3.5 months. Anti-GM130 was used to label the cis-Golgi.
Supplemental Fig. 2. IFT20 is critical for maintaining Golgi size in ATDC5 cells. (A) ATDC5 cells were transfected with scramble and two different si-Ift20 RNA (#1 and #2). Reduction of IFT20 levels was confirmed by western blotting. GAPDH was used as a loading control. (B) Quantification analysis of western blot shown in (A). (C) Golgi size was examined by immunocytochemistry using an anti-GM130 antibody in control and si-Ift20 treated ATDC5 cells. (D) The size of the Golgi apparatus was measured and quantified. Data in (B) and (D) represented as mean ± SD; n=3 in each group. *P<0.05.
Supplemental Fig. 3. IFT20 is critical for ciliogenesis in ATDC5 cells. (A) The presence of primary cilia was examined by immunocytochemistry using anti-acetylated tubulin and anti-IFT20 antibodies in control and si-Ift20 treated ATDC5 cells. (B)The number of primary cilia was measured and quantified. Data in (B) represented as mean ± SD; n=3 in each group. *P<0.05.