Abstract
Repair mechanisms after acetaminophen (APAP) hepatotoxicity are poorly understood. We recently discovered that phosphatidic acid (PA) increases in mice and humans after APAP overdose, and is critical for liver regeneration. Here, we hypothesized that PA inhibits glycogen synthase kinase-3β (GSK3β), a component of canonical Wnt/β-catenin signaling, after APAP overdose. To test that, we treated mice with 300 mg/kg APAP at 0h followed by vehicle or 20 mg/kg of the glycerol 3-phosphate acyltransferase inhibitor FSG67 at 3, 24 and 48h. Some mice also received the GSK3 inhibitor L803-mts. Blood and liver were collected at multiple time points. Consistent with our earlier results, FSG67 did not affect toxicity (ALT, histology), APAP bioactivation (total glutathione), or oxidative stress (oxidized glutathione), but did reduce expression of proliferating cell nuclear antigen (PCNA) at 52h. We then measured GSK3β phosphorylation and found it was dramatically decreased by FSG67 at 24h, before PCNA dropped. Expression of cyclin D1, downstream of Wnt/β-catenin, was also reduced. To determine if the effect of FSG67 on GSK3β is important, we treated mice with FSG67 and L803-mts after APAP. Importantly, L803-mts rescued hepatocyte proliferation and survival. Our data indicate PA and lysoPA may support recovery after APAP overdose by inhibiting GSK3β.
Keywords: Drug-induced liver injury, lipids, lipid metabolism, lipid signaling, cell growth, biomarkers
1. INTRODUCTION
Acetaminophen (APAP) is a widely used drug (Kaufman et al., 2002), but APAP overdose can cause severe liver injury. It is the primary cause of acute liver failure (ALF) in the US and several other countries (Lee, 2012). The standard treatment for APAP overdose is N-acetylcysteine (NAC). However, NAC is only effective when given <16 h after overdose (Rumack et al., 1981), and nearly half of all patients present at 24 h or later (Craig et al., 2012). A promising approach to the treatment of late-presenting patients could be enhancement of liver regeneration. Therefore, a better understanding of liver regeneration after APAP hepatotoxicity is needed.
There is strong evidence that liver regeneration is critical for survival of ALF patients. Expression of the cell proliferation marker proliferating cell nuclear antigen (PCNA) is higher in tissue from ALF survivors compared to non-survivors (Kayano et al., 1992). Similarly, several serum biomarkers of regeneration are elevated in ALF survivors, including α-fetoprotein (Schmidt and Dalhoff, 2005), vascular endothelial growth factor (VEGF) (Takayama et al., 2011), leukocyte cell-derived chemotaxin 2 (LECT2) (Sato et al., 2004) and the amino acid α-NH2-butyric acid (Rudnick et al., 2009). Finally, two recent meta-analyses of previous studies revealed that experimental use of granulocyte colony stimulating factor (G-CSF), which is thought to enhance liver regeneration through mobilization of stem cells from bone marrow, improves outcome in liver failure patients (Chavez-Topia et al., 2015; Yang et al., 2016).
In this study, we sought to test the effect of phosphatidic acid (PA) on the Wnt/GSK3β/β-catenin signaling axis during liver regeneration after APAP overdose in mice. PA is a universal intermediate in the synthesis of membrane glycerophospholipids and triacylglycerols (Harris and Finck, 2011). In fact, conversion of PA to diacylglycerol by lipin enzymes is the penultimate step in creation of new cell membranes (Harris and Finck, 2011). We recently demonstrated that PA increases in liver tissue and plasma after APAP overdose in both mice and humans due to suppression of hepatic lipins (Lutkewitte et al., 2018). We were surprised by that result, as we had assumed that synthesis of new membrane lipids would be necessary for hepatocyte proliferation. Using the drug FSG67, an inhibitor of glycerol 3-phosphate acyltransferase that is critical for the primary pathway of lysoPA and PA synthesis, we discovered that PA itself can support or enhance liver regeneration (Lutkewitte et al., 2018). However, the mechanisms by which it does so are unknown. Bhushan et al. (2017) recently demonstrated that an inhibitor of glycogen synthase kinase-3 (GSK3) improves liver regeneration after APAP overdose in mice, likely through Wnt/β-catenin signaling. PA is thought to interact with Dishevelled (Dvl) (Simons et al., 2009; Capelluto et al., 2014), a protein involved in the Wnt/β-catenin pathway that is inhibited by GSK3β. It is also known to interact with a number of other kinases and phosphatases in a way that alters their activity (Stace and Ktiskakis, 2006). Based on those data, we hypothesized that PA affects GSK3β signaling. We tested that hypothesis by treating mice with a toxic dose of APAP followed by either vehicle, FSG67, or a combination of FSG67 and the GSK3 inhibitor L803-mts and measuring liver injury, hepatocyte proliferation markers, and Wnt/GSK3β/β-catenin signaling markers at multiple time points. Importantly, previous data from our laboratory already confirmed that FSG67 reduces PA content in the liver (Lutkewitte et al., 2018).
2. MATERIALS AND METHODS
2.1. Animals.
Male C57Bl/6 mice between ages 8 and 12 weeks were purchased from Jackson Laboratories (Bar Harbor, ME) and kept in a temperature-controlled 12h light/dark cycle room with free access to water and food. Mice were fasted overnight prior to a single 300 mg/kg dose of APAP (Sigma, St. Louis, MO) dissolved in warm phosphate-buffered saline (PBS) at 0 h, followed by a 20 or 30 mg/kg dose of FSG67 (Focus Biomolecules, Plymouth Meeting, PA) dissolved in DMSO or an equivalent volume of DMSO vehicle at 3, 24, and 48 h. In two experiments, mice were also treated with 600 nmol of the GSK3 inhibitor, L803-mts/N-myristol-GKEAPPAPPQS(p)P (Genemed Synthesis, Inc., San Francisco, CA), dissolved in warm PBS, in addition to APAP and FSG67. All treatments were administered i.p. Mice were euthanized at 6, 24, and 52 h, and blood and liver tissues were harvested. For each animal, one liver section was fixed in 10% phosphate-buffered formalin for histology. Other liver pieces were flash frozen in liquid nitrogen for later biochemical analyses. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences.
2.2. Clinical chemistry.
Alanine aminotransferase (ALT) was measured in serum using a kit from Point Scientific Inc. (Canton, MI) according to the manufacturer’s instructions.
2.3. qPCR.
Liver tissues were homogenized using a bead homogenizer and RNA was extracted using RNA-Bee reagent (Tel-Test Inc., Friendswood, TX). Chloroform was then added and the samples were shaken, then allowed to incubate at 4°C for 5 min. After centrifugation (12,000xg, 4°C for 5 min), the aqueous phase was transferred to a new tube and mixed with isopropanol (0.5 mL). The samples were then allowed to sit at room temperature for 10 min, and then RNA was pelleted by centrifugation (12,000xg, 4°C for 5 min). The RNA pellets were then washed with 75% ethanol and re-suspended in RNase-free H2O. RNA concentration and purity was measured using a Nanodrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, USA). Next, 2 μg of RNA was transcribed into cDNA using a High Capacity cDNA Reverse Transcription Kit, (Applied BioSystems, Thermo Fisher Scientific, USA) and an Applied Biosystems Veriti thermocycler. For reverse transcription real-time qPCR, cDNA was mixed with PowerUp SYBR Green Master mix (Applied Biosystems, Thermo Fisher Scientific, USA) along with forward and reverse primers (Table 1) from Integrated DNA Technologies and run on an Applied Biosystems ViiA7 real-time qPCR instrument.
Table 1.
Primer sequences for qPCR analysis.
| Gene symbol | Forward | Reverse |
|---|---|---|
| Wnt4 | AGACGTGCGAGAAACTCAAAG | GGAACTGGTATTGGCACTCCT |
| Wnt5a | CAACTGGCAGGACTTTCTCAA | CATCTCCGATGCCGGAACT |
| PCNA | TTTGAGGCACGCCTGATCC | GGAGACGTGAGACGAGTCCAT |
| Cyclin-D1 | GCGTACCCTGACACCAATCTC | CTCCTCTTCGCACTTCTGCTC |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
2.4. Western blotting.
Liver tissues were homogenized in 25 mM HEPES buffer with 5 mM EDTA (pH 7.4), 0.1% CHAPS, and protease inhibitors, using a bead homogenizer (Thermo Fisher Scientific, Waltham, MA). Protein concentration in the homogenates was measured using the bicinchoninic acid (BCA) assay, and equal amounts of protein were loaded in each lane of a 4-20% Tris-glycine gel. After electrophoresis, proteins were transferred to PVDF membranes with 0.45 μm pores and blocked with 5% milk in Tris-buffered saline with 0.1% Tween 80. After incubation with the appropriate antibodies, protein bands were visualized using the Odyssey Imaging System (LiCor Biosciences, Lincoln, NE). All primary antibodies were used at a 1:1,000 dilution. Primary antibodies were purchased from Cell Signaling Technology (Danvers, MA): phosphorylated GSK3β (Cat. No. 9323), GSK3β (Cat. No. 9315), PCNA (Cat. No. 2586), and β-actin (Cat. No. 4967). Secondary antibodies were purchased from Li-Cor (Lincoln, NE): IRDye 680 goat anti-mouse IgG (Cat. No. 926-68070) and IRDye 800CW goat anti-rabbit IgG (Cat. No. 926-32211) were used at a 1:10,000 dilution.
2.5. Histology and immunohistochemistry.
Formalin-fixed tissue was embedded in paraffin wax and 5 μm sections were mounted on glass slides for hematoxylin and eosin (H&E) staining and immunohistochemistry. H&E staining was performed using a standard protocol. For PCNA staining, the tissue sections were blocked for 10 min each using both peroxidase and protein blocking reagents from Dako (Agilent, Santa Clara, CA) prepared in standard TBS-T buffer. Primary PCNA antibody from Cell Signaling Technology (Danvers, MA) was then added to each section at a 1:250 dilution in Dako antibody diluent and allowed to incubate for 2 h at room temperature. After rinsing with TBS-T, biotinylated goat anti-mouse secondary antibody was added at a dilution of 1:400 in TBS-T for 30 min. Finally, staining was developed using the VECTASTAIN Elite ABC HRP Kit (Vector Laboratories, Burlingame, CA) with Dako DAB+ chromogen substrate (Agilent, Santa Clara, CA). Richard-Allan Scientific hematoxylin (Thermo Fisher Scientific, Waltham, MA) was used to counterstain. For quantification of PCNA by histology, 10-12 serial high power images (400x) were collected from a single liver section from each animal. In each field, all hepatocyte nuclei and PCNA-positive nuclei were separately counted using the Multi-point tool in ImageJ software. The proportion of PCNA-positive nuclei was expressed as a percentage for each animal.
2.6. Glutathione measurement.
Total and oxidized glutathione were measured using a modified Tietze assay, as we previously described in detail (McGill and Jaeschke, 2015).
2.7. Statistics.
Normality was tested using the Shapiro-Wilk test. For normally distributed data, statistical significance was assessed using either Student’s t-test or one-way analysis of variance (ANOVA) with post-hoc Dunn’s test or Student-Newman-Keul’s to compare with the control group. For non-normally distributed data, the Mann-Whitney U-test or the Kruskal-Wallis test were used. All statistical analyses were performed using SigmaPlot 12.5 software (Systat, San Jose, CA). In all cases, p<0.05 was considered significant.
3. RESULTS
3.1. FSG67 reduces liver regeneration after APAP overdose.
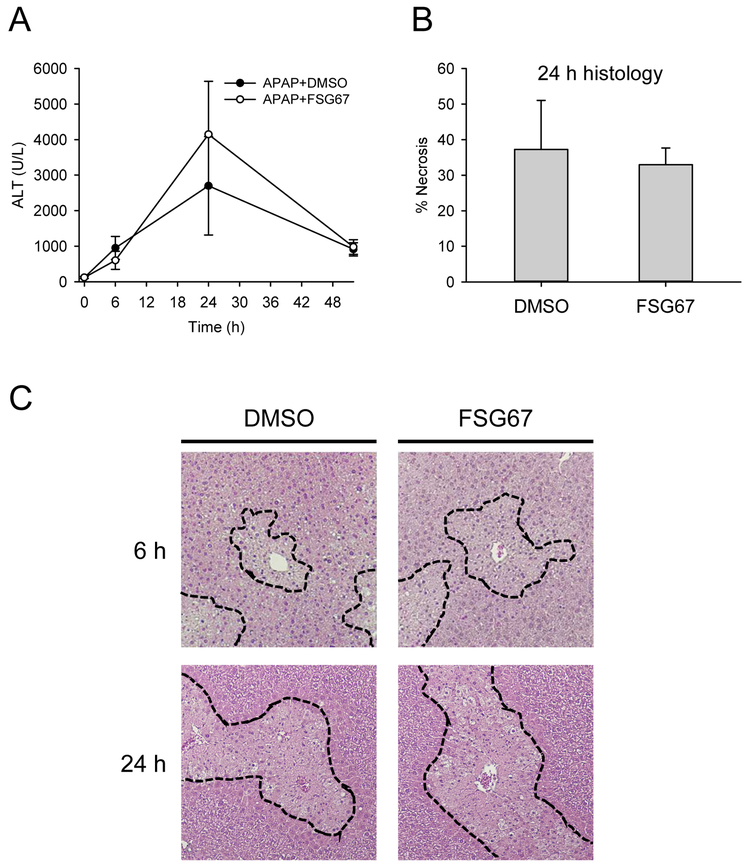
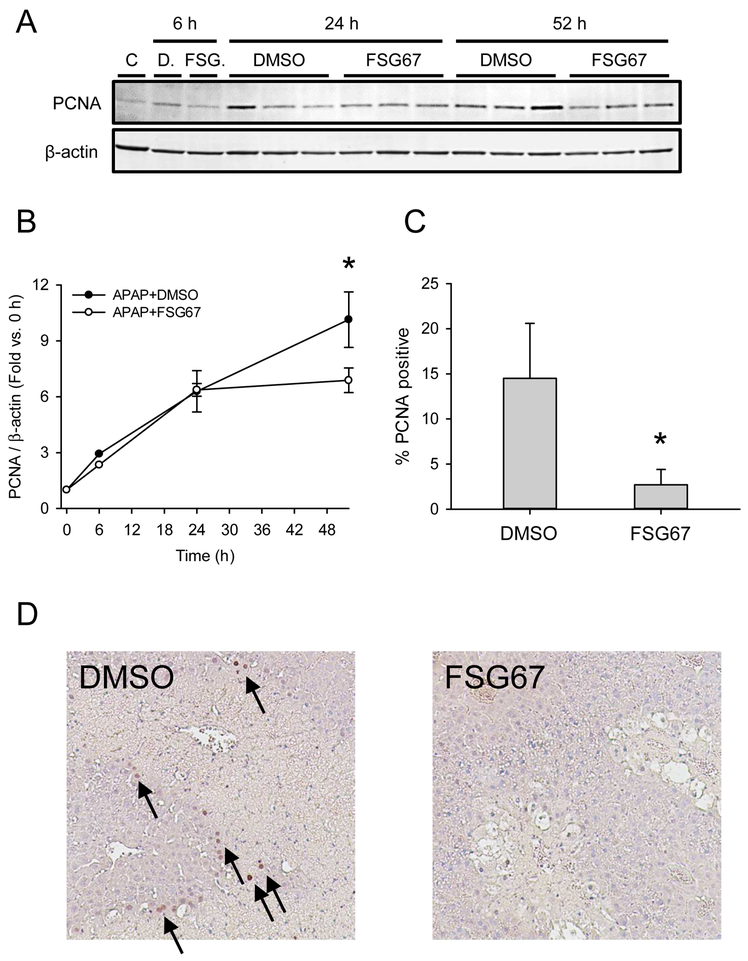
We treated mice with 300 mg/kg APAP followed by either DMSO vehicle or 20 mg/kg FSG67 at 3, 24, and 48 h post-APAP and measured liver injury and regeneration at multiple time points. Although there was a modest trend toward increased plasma alanine aminotransferase (ALT) at 24 h (Fig. 1A), that trend was not observed in histology (Fig. 1B, C). Overall, FSG67 had no significant effect on liver injury (Fig. 1). However, it did reduce expression of proliferating cell nuclear antigen (PCNA) at 52 h by 32±7% based on immunoblotting, and reduced the percentage of hepatocytes positive for PCNA by immunohistochemistry by 81±9% (Fig. 2). These data are consistent with our earlier work that demonstrated that FSG67 treatment blunts liver regeneration at late time points after APAP overdose (Lutkewitte et al., 2018).
Figure 1. FSG67 does not affect liver injury after APAP overdose.
Mice were treated with 300 mg/kg APAP at 0 h followed by either DMSO vehicle or 20 mg/kg FSG67 at 2, 24, and 48 h. Blood and liver tissue were collected at 6, 24 and 52 h. (A) Plasma alanine aminotransferase (ALT) activity over time. (B) Percentage of tissue that was necrotic at 24 h. (C) H&E-stained liver sections at 6 and 24 h. Dashed lines emphasize areas of necrosis. Necrosis is characterized by loss of basophilia, increased eosinophilia, and nuclear shrinkage (pyknosis) at 6 h, and by eosinophilia, cell swelling, and loss of nuclei (karryorhexis) at 24 h. Data expressed as mean±SE for n = 3-6 mice.
Figure 2. FSG67 reduces liver regeneration after APAP overdose.
Mice were treated with 300 mg/kg APAP at 0 h followed by either DMSO vehicle or 20 mg/kg FSG67 at 2, 24, and 48 h. Blood and liver tissue were collected at 6, 24 and 52 h. (A) Immunoblot for proliferating cell nuclear antigen (PCNA) and β-actin. Samples (n = 3-5) were pooled for each of the control (C) and 6 h lanes. The full western blot for the 6 h samples is available in Suppl. Fig. 1. (B) Densitometry analysis of PCNA. (C) Percentage of cells that stained positive for PCNA at 52 h. (D) Representative images of liver sections stained for PCNA. Arrows point to PCNA-positive cells. Data expressed as mean±SE for n = 3-6 mice. *p<0.05 vs. 0 h or DMSO vehicle.
3.2. FSG67 does not affect APAP bioactivation.
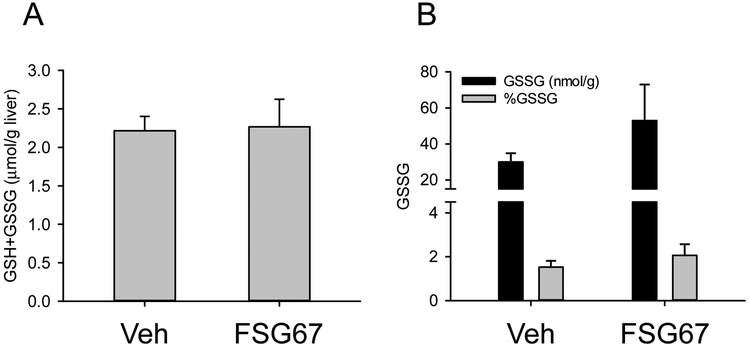
APAP hepatotoxicity depends upon conversion to a reactive metabolite, commonly believed to be N-acetyl-p-benzoquinone imine (NAPQI) (McGill and Jaeschke, 2013). The reactive metabolite binds to and depletes glutathione stores in the liver and causes oxidative stress. As a result, any treatment that prevents or otherwise alters NAPQI formation could affect liver injury or repair after APAP overdose. To verify that treatment with FSG67 did not affect APAP bioactivation or oxidative stress, we measured total (GSH+GSSG) and oxidized (GSSG) glutathione at 6 h post-APAP in mice that also received either vehicle or FSG67. Importantly, FSG67 treatment had no effect on GSH+GSSG or GSSG (Fig. 3). Together with the fact that FSG67 treatment did not affect liver injury, these data demonstrate that FSG67 does not alter APAP bioactivation or subsequent oxidative stress.
Figure 3. FSG67 does not affect APAP bioactivation or oxidative stress.
Mice were treated with 300 mg/kg APAP at 0 h followed by either DMSO vehicle or 20 mg/kg FSG67 at 2, 24, and 48 h. Blood and liver tissue were collected at 6 h. (A) Total glutathione (GSH+GSSG) content in the liver. (B) Oxidized glutathione (GSSG) content in the liver. Data expressed as mean±SE for n = 3 mice.
3.3. DMSO vehicle does not affect liver regeneration after APAP overdose.
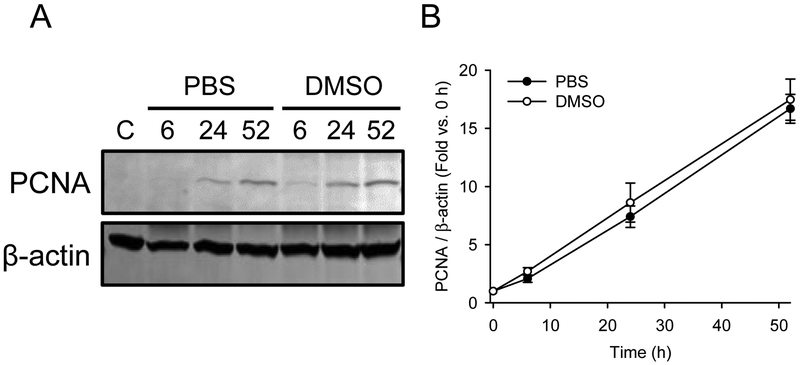
DMSO is known to have numerous biological effects, and it is possible that the DMSO vehicle in our experiments alters liver regeneration signaling or kinetics and affects the results. To test that possibility, we measured PCNA expression over time in mice treated with 300 mg/kg APAP and either saline or 2 mL/kg DMSO vehicle at 3, 24 and 48 h post-APAP. Importantly, we did not observe any differences in the time course of liver regeneration due to DMSO (Fig. 4). Based on that, it seems unlikely that DMSO has an effect on our results with FSG67.
Figure 4. DMSO does not affect liver regeneration after APAP overdose.
Mice were treated with 300 mg/kg APAP at 0 h followed by 2 mL/kg DMSO at 3, 24, and 48 h. Blood and liver tissue were collected at 6, 24 and 52 h. (A) Immunoblots of pooled samples for PCNA and β-actin. (B) Densitometry of immunoblots that were performed with individual samples. Data expressed as mean±SE for n = 4 mice.
3.4. FSG67 reduces GSK3βphosphorylation and signaling after APAP overdose.
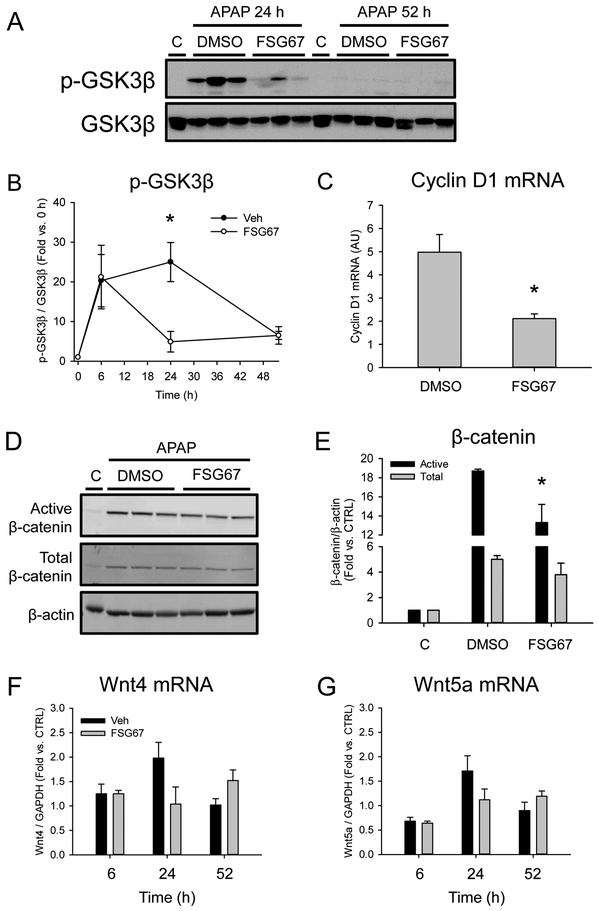
We previously demonstrated that PA likely promotes liver regeneration after APAP overdose in mice. However, the mechanisms by which it does that are not yet known. Bhushan et al. (2017b) recently discovered that inhibition of GSK3β accelerates liver regeneration in APAP hepatotoxicity by increasing β-catenin activity and downstream expression of cyclin D1 and other pro-proliferative genes. We hypothesized that PA enhances liver regeneration by acting on GSK3β. To test that, we measured phosphorylation of GSK3β by immunoblotting and expression of cyclin D1 by qPCR in APAP-treated mice followed by either DMSO vehicle or FSG67. Consistent with our hypothesis, we found that FSG67 dramatically reduced GSK3β phosphorylation (Fig. 5A, B) and cyclin D1 mRNA (Fig. 5C) at 24 h post-APAP. We also observed a comparatively modest (29±10%) but significant decrease in active (non-phosphorylated) β-catenin (Fig. 5D,E), consistent with disruption of Wnt/β-catenin signaling. Although the mean value for total β-catenin was also lower after FSG67 treatment, the difference was not statistically significant. To determine if those effects may be due to a direct interaction between PA and GSK3β or if they are merely downstream of another effect, we also measured expression of the two major isoforms of Wnt that have been implicated in regeneration after APAP hepatotoxicity (Bhushan et al., 2014). Binding of Wnt ligands to the Frizzled receptor immediately precedes GSK3β phosphorylation, so any further upstream effects would likely result in reduced Wnt expression. There was no difference in expression of either Wnt isoform (Fig. 5F,G), which is consistent with the hypothesis that PA directly interacts with GSK3β. However, additional studies are needed to confirm that interaction.
Figure 5. FSG67 reduces GSK3β phosphorylation after APAP overdose.
Mice were treated with 300 mg/kg APAP at 0 followed by either DMSO vehicle or 20 mg/kg FSG67 at 2, 24, and 48 h. Blood and liver tissue were collected at 6, 24 and 52 h. (A) Immunoblots for phosphorylated GSK3β (p-GSK3β) and total GSK3β. (B) Densitometry of GSK3β immunoblots. (C) Cyclin D1 mRNA. (D) Immnoblots for active β-catenin, total β-catenin, and β-actin at 24 h. (E) Densitometry for β-catenin blots. (F) Wnt4 mRNA. (G) Wnt5 mRNA. All control (C) lanes are pools of the same vehicle-only control animals. The same pool was used for every C lane. Data expressed as mean±SE for n = 3-5 mice. *p<0.05 for DMSO Veh vs. FSG67.
3.5. Inhibition of GSK3 rescues liver regeneration in the presence of FSG67 after APAP overdose.
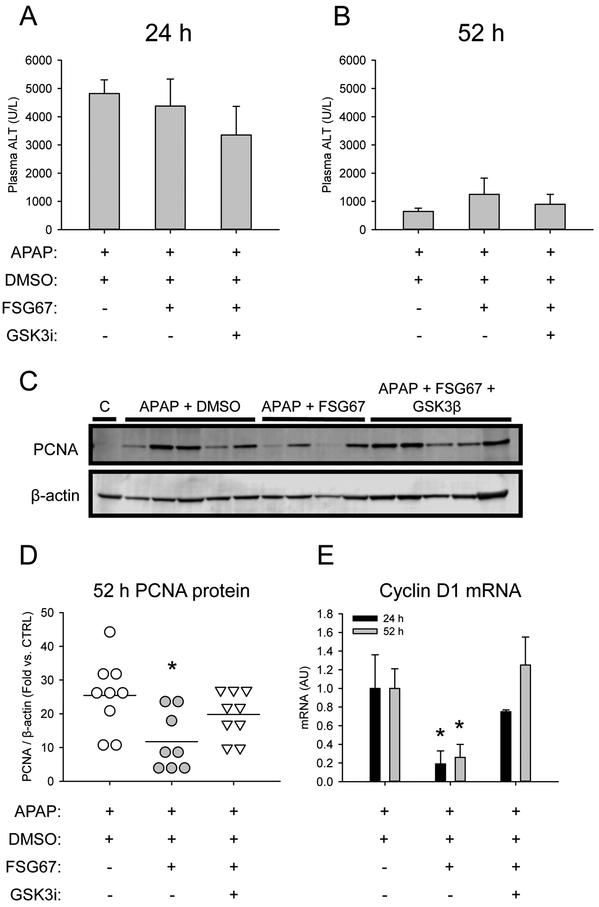
To confirm that FSG67 acts on or upstream of GSK3β and to determine if that effect is important for liver regeneration, we performed a rescue experiment using L803-mts, a highly specific peptide inhibitor of GSK3β. Ten mice each were treated with 300 mg/kg APAP followed by either 2 mL/kg DMSO vehicle, 20 mg/kg FSG67, or 20 mg/kg FSG67 and 600 nmol L803-mts. Mortality in the 52 h mice was 10%, 30% and 10%, respectively, which is consistent with the detrimental effect of FSG67 on liver regeneration and with our hypothesis that L803-mts rescues regeneration. Liver injury and hepatocyte proliferation markers were measured in the surviving mice. Consistent with our earlier results, neither FSG67 alone nor the combination of FSG67 and L803-mts had an effect on liver injury as indicated by plasma ALT (Fig. 6A, B), but both PCNA protein and cyclin D1 mRNA were reduced by FSG67 (Fig. 6C-E). Importantly, L803-mts restored cyclin D1 and PCNA expression to control levels (Fig. 6C-E). To further explore the importance of GSK3β for the effect of FSG67 on liver regeneration, we performed a small 48 h survival study using the same treatment regimen, but with a modestly higher dose of FSG67 (30 mg/kg). The higher dose of FSG67 resulted in 100% mortality in the group treated with APAP and FSG67, which was reduced to 50% by the addition of L803-mts (Table 2). Taken together, these data are consistent with our hypothesis that PA acts on GSK3β and that the effect is critical for liver regeneration and recovery after APAP overdose in mice.
Figure 6. L803-mts rescues liver regeneration in the presence of FSG67 after APAP overdose.
Mice were treated with 300 mg/kg APAP at 0 followed by DMSO vehicle, 20 mg/kg FSG67, or 20 mg/kg FSG67 and 600 nmol L803-mts at 3, 24, and 48 h. Blood and liver tissue were collected at 24 and 52 h. (A) Plasma ALT at 24 h. (B) Plasma ALT at 52 h. (C) Representative immunoblot for PCNA and β-actin at 52 h. (D) Densitometry for immunoblots for PCNA and β-actin at 52 h. (E) Cyclin D1 mRNA at 24 and 52 h. Data expressed as mean±SE for n = 8-9 mice. *p<0.05 for DMSO vs. FSG67..
Table 2.
Survival at 48 h post-APAP.
| Group* | Number | % |
|---|---|---|
| APAP + Vehicle | 4/4 | 100 |
| APAP + FSG67 | 0/4 | 0 |
| APAP + FSG67 + L803-mts | 2/4 | 50 |
300 mg/kg APAP; 2 mL/kg DMSO vehicle; 30 mg/kg FSG67; 600 nmol L803-mts.
4. DISCUSSION
A better understanding of liver regeneration after acute liver injury is needed. Most studies of liver regeneration to date have relied on the partial hepatectomy (PHx) model, in which 2/3 of the liver is removed and the remaining tissue grows to restore the original functional mass. While that is an excellent model to investigate the mechanisms of liver growth after hepatic resection or some cases of liver transplantation, acute liver injury differs from PHx in several major ways. First, acute injury involves widespread cell death within the tissue, which generally does not occur in the PHx model. Second, the cell death in acute intrahepatic injury results in release of damage-associated molecular patterns (DAMPs) that cause systemic sterile inflammation (McGill et al., 2014; Woolbright and Jaeschke, 2015). Third, the sudden removal of a large portion of the liver in the PHx model has dramatic hemodynamic effects (Michalopoulos, 2007; Abshagen et al., 2012; Xie et al., 2014). Acute intrahepatic injury can also disrupt circulation by damaging the sinusoidal endothelium, but the general architecture of the circulatory system is maintained. Fourth, hepatocyte proliferation after PHx is diffuse and panlobular (Fabrikant, 1968), while only hepatocytes around the margins of necrosis proliferate after acute intrahepatic injury. Finally, there is emerging evidence that some hepatocyte proliferation signaling mechanisms differ between acute injury and PHx (Bhushan et al., 2014).
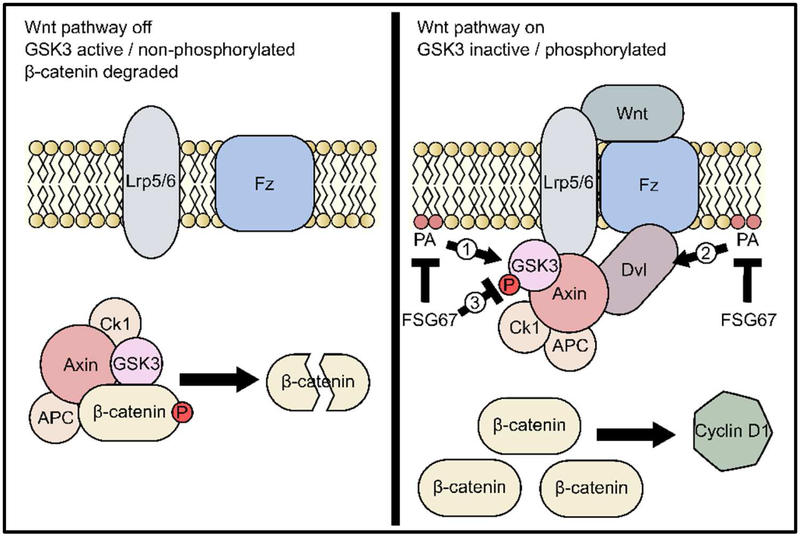
In the present study, we demonstrated that FSG67 reduces hepatocyte proliferation after APAP hepatotoxicity through a mechanism involving GSK3β. Those data indicate that some PA species (including lysoPA) may be important for Wnt/β-catenin signaling during liver regeneration. GSK3β is part of the canonical Wnt/β-catenin signaling pathway, which is known to be important for liver regeneration after both PHx and APAP overdose (Thompson and Monga, 2007; Apte et al., 2009; Nejak-Bowen and Monga, 2011; Bhushan et al., 2014; 2017b). However, the exact mechanism by which PA and GSK3β could interact remains unknown. Bhushan et al. (2014) found that Wnt4 and Wnt5a are likely to be the major Wnt isoforms important for regeneration after APAP overdose. They measured all 11 Wnt isoforms in the liver in the same model of APAP overdose that we used and discovered that only those two were elevated. Furthermore, both were lower at a 2-fold higher dose of APAP at which regeneration was inhibited, demonstrating that they are important (Bhushan et al., 2014). The fact that expression of those isoforms was unchanged by FSG67 in our experiments and that Wnt signaling through the Frizzled (Fzd) and low density lipoprotein receptor-related protein (Lrp) 5/6 co-receptors is the step immediately upstream of GSK3β dephosphorylation/activation (Thompson and Monga, 2007) could mean that PA acts directly on either the receptors or on GSK3β itself, although we cannot rule out that other Wnt isoforms were involved in our specific experiments. Interestingly, the protein disheveled (Dvl) is thought to interact with PA at the plasma membrane (Simons et al., 2009; Capelluto et al., 2014). Dvl is an important inhibitor of the β-catenin destruction complex involving GSK3β. Normally, active non-phosphorylated GSK3β forms the complex with Axin and Apc, and the complex phosphorylates β-catenin to target it for ubiquitination and proteolysis (Thompson and Monga, 2007) (Fig. 7). However, when Wnt proteins bind to Fzd and Lrp5/6, Dvl is recruited to those receptors at the membrane and promotes binding of Lrp5/6 to axin in the destruction complex. Sequestration of the complex at the membrane allows β-catenin to accumulate and promote transcription of cyclin D1 and other pro-proliferative genes in the nucleus (Thompson and Monga, 2007) (Fig. 7). Thus, PA may be important for GSK3β inhibition through its interaction with Dvl. On the other hand, PA is known to interact with many other proteins, especially many kinases and phosphatases (Stace and Ktistakis, 2006), so a direct interaction with GSK3β that affects its phosphorylation and activation status is also possible (Fig. 7). We also cannot rule out the possibility that FSG67 has some other effect on the pathway at this point (Fig. 7). Additional studies using more specific approaches to manipulate PA levels are needed to confirm the effect on GSK3β and to test those additional hypotheses.
Figure 7. Mechanisms by which FSG67 may affect Wnt/β-catenin signaling.
When Wnt/β-catenin signaling is off, the β-catenin destruction complex composed of GSK3β, APC, CK1 and Axin targets β-catenin for destruction. When Wnt binds to Frizzled (Fz) and the Lrp co-receptors, the destruction complex is held inactive at the plasma membrane and β-catenin accumulates and upregulates transcription of pro-proliferation genes like cyclin D1. FSG67 may act by inhibiting synthesis of phosphatidic acid (PA) and lysoPA, which may normally inhibit GSK3β either directly (1) or through an effect mediated by disheveled (Dvl) (2). Alternatively, FSG67 may itself have an effect on GSK3β (3).
In addition to the experiments reported here, we tested the effect of treatment with exogenous PA on liver regeneration after APAP overdose, but the results were inconsistent. Although we observed acceleration of liver regeneration in our initial experiments with PA treatment (data not shown), we were unable to reproduce those results at a later time. The lack of reproducibility may have been due to two factors. First, the formulation of PA may be important. We treated our mice with PA liposomes suspended in saline, and there can be considerable variation in liposome quality between preparations. Also, liposomes are known to be primarily absorbed into the lymphatic system, bypassing the liver. Interestingly, another group dissolved 18:1 lysoPA in DMSO and found that post-treatment improved survival over several days after APAP overdose (Bae et al., 2017; Bae, personal communication). Unfortunately, they did not report ALT and did not quantify necrosis in those particular experiments (Bae et al., 2017), so we do not know if the lysoPA treatment affected the early injury phase. Interestingly, they also reported an effect of pre-treatment with 18:1 lysoPA on GSK3β (Bae et al., 2017), but it was the opposite of our results for PA. While we cannot yet rule out that the effect we have observed with FSG67 is partially due to reduced lysoPA levels in addition to reduced PA, the latter fact argues against that possibility. Nevertheless, the findings of Bae et al. are consistent with ours and indicate that phosphatidic acid species are critical for recovery after APAP overdose. Second, exogenous PA is thought to be rapidly metabolized by lipases in extracellular fluid, forming lysoPA and other products. Indeed, most studies avoid treatment with exogenous PA for that reason.
The TNFα/interleukin-6/STAT3 axis is also believed to be important for liver regeneration in APAP hepatotoxicity (James et al., 2003; 2005), as is epidermal growth factor receptor (EGFR) signaling (Bhushan et al., 2017a). A weakness of our study is that we did not test the effect of FSG67 on those pathways. Furthermore, Wnt/β-catenin signaling is complex, and crosstalk with many other signaling pathways has been described. For example, it was recently demonstrated that insulin-like growth factor-1 (IGF-1) receptor (IGFR1) directly enhances expression of b-catenin target genes in proliferating human hepatocytes (Jamwal et al., 2018), and data from similar studies in other tissues supports that hypothesis (Hu et al., 2012).
There is some evidence that other lipid second messengers are also involved in liver regeneration after acute injury. For example, sphingolipids may promote hepatocyte proliferation after ischemia-reperfusion induced liver injury (Nojima et al., 2015; 2016). It is possible that there is a balance or interaction between synthesis of PA, lysoPA, and other lipids. However, very little is known about that at this point.
DMSO had no effect on liver regeneration in our experiments. DMSO is known to be biologically active. For example, it is a potent inhibitor of cytochrome P450-mediated drug metabolism. In fact, pre- or co-treatment with DMSO is well known to protect against APAP hepatotoxicity by inhibiting NAPQI formation and protein binding (Seigers, 1978; Younes and Siegers, 1980; Jaeschke et al., 2006; Kelava et al., 2010; Du et al., 2013) although we avoided that in our studies by using a 3 h post-treatment with DMSO or FSG67; reactive metabolite formation is complete by 1.5 h post-APAP (McGill et al., 2013), so 3 h post-treatment does not affect the early injury phase. It has also been reported that DMSO inhibits hepatocyte proliferation in vitro (Kost and Michalopoulos, 1991) and enhances activation of natural killer cells during APAP hepatotoxicity in vivo (Masson et al., 2008). Hepatocyte proliferation is obviously critical for liver regeneration. Furthermore, there is evidence that inflammatory cells enhance repair and regeneration after APAP overdose (Ju et al., 2002; Antoniades et al., 2012; Williams et al., 2014; Woolbright and Jaeschke, 2017), and the discovery that NKT-deficient mice have worse injury and outcomes (Martin-Murphy et al., 2013) is consistent with those data. Thus, it was possible that using DMSO as a vehicle for FSG67 altered liver regeneration kinetics or signaling after APAP hepatotoxicity in our experiments. However, we found no differences in regeneration between mice treated with either saline or 2 mL/kg DMSO after APAP overdose.
Our results with DMSO may also be important for treatment of liver diseases, especially ALF, by cell transplantation. Stem cells and other cell types intended for clinical use, including hepatocytes, are often cryopreserved in solutions containing DMSO (Terry et al., 2010; Iansante et al., 2018). However, the effect of DMSO on cell transplantation success has never been systematically tested, despite the fact that it inhibits hepatocyte proliferation in vitro (Kost and Michalopoulos, 1991). Our data are consistent with the null hypothesis that DMSO has no effect on proliferation in vivo, and support the continued use of DMSO in those solutions.
5. CONCLUSIONS
Overall, our data demonstrate that FSG67 blunts liver regeneration after APAP overdose through a mechanism involving GSK3β. Those data are consistent with our hypothesis that PA is critical for liver regeneration by supporting inhibition of GSK3β and promoting Wnt/β-catenin signaling. This discovery has implications for many other liver diseases that involve hepatocyte proliferation, including cirrhosis and hepatocellular carcinoma. Future studies will confirm our results using more specific genetic approaches to manipulate PA, and will elucidate the mechanisms by which PA and GSK3β interact.
Supplementary Material
HIGHLIGHTS.
FSG67 reduces hepatocyte proliferation after APAP hepatotoxicity
FSG67 reduces GSK3β phosphorylation after APAP overdose, increasing its activity
GSK3 inhibition restores normal liver regeneration in the presence of FSG67
PA is likely critical for liver regeneration after APAP hepatotoxicity as a novel regulator of Wnt/β-catenin signaling
ACKNOWLEDGEMENTS
The authors would like to thank the Experimental Pathology (ExPath) Core Laboratory at the University of Arkansas for Medical Sciences, especially Jennifer D. James, HT(ASCP), HTL, QIHC for her development of a modified PCNA staining protocol. This work was supported in part by a Pinnacle Research Award from the American Association for the Study of Liver Diseases (AASLD) Foundation (to MRM) and National Institutes of Health (NIH) grants R01 HL119225 (to BNF), R01 DK117657 (to BNF), R01 DK104735 (to BNF), R01 DK098414 (to UA), R56 DK112768 (to UA), U54 TR001629 (to LPJ) and R42 DK079387 (to LPJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abshagen K, Eipel C, Vollmar B. 2012. A critical appraisal of the hemodynamic signal driving liver regeneration. Langenbecks Arch Surg. 397, 579–90. [DOI] [PubMed] [Google Scholar]
- Antoniades CG1, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C, Wagner B, Barnardo A, Pomplun S, Auzinger G, Bernal W, Heaton N, Vergani D, Thursz MR, Wendon J. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 56, 735–46. [DOI] [PubMed] [Google Scholar]
- Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. 2009. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 175, 1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GH, Lee SK, Kim HS, Lee M, Lee HY, Bae YS. 2017. Lysophosphatidic acid protects against acetaminophen-induced acute liver injury. Exp Mol Med. 49, e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Chavan H, Borude P, Xie Y, Du K, McGill MR, Lebofsky M, Jaeschke H, Krishnamurthy P, Apte U. 2017a. Dual Role of Epidermal Growth Factor Receptor in Liver Injury and Regeneration after Acetaminophen Overdose in Mice. Toxicol Sci. 155, 363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Poudel S, Manley MW Jr, Roy N, Apte U. 2017b. Inhibition of Glycogen Synthase Kinase 3 Accelerated Liver Regeneration after Acetaminophen-Induced Hepatotoxicity in Mice. Am J Pathol. 187, 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U. 2014. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol. 184, 3013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelluto DG, Zhao X, Lucas A, Lemkul JA, Xiao S, Fu X, Sun F, Bevan DR, Finkielstein CV. 2014. Biophysical and molecular-dynamics studies of phosphatidic acid binding by the Dvl-2 DEP domain. Biophys J. 106, 1101–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ, Noreña-Herrera C, Vidaña-Perez D, Delgado-Sanchez G, Uribe M, Barrientos-Gutierrez T. 2015. Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Ann Hepatol. 14, 631–41. [PubMed] [Google Scholar]
- Craig DG, Bates CM, Davidson JS, Martin KG, Hayes PC, Simpson KJ. 2012. Staggered overdose pattern and delay to hospital presentation are associated with adverse outcomes following paracetamol-induced hepatotoxicity. Br J Clin Pharmacol. 73, 285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Williams CD, McGill MR, Xie Y, Farhood A, Vinken M, Jaeschke H. 2013. The gap junction inhibitor 2-aminoethoxy-diphenyl-borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes and c-jun N-terminal kinase activation. Toxicol Appl Pharmacol. 273, 484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrikant JI 1968. The kinetics of cellular proliferation in regenerating liver. J Cell Biol. 36, 551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TE, Finck BN. 2011. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol Metab. 22, 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Lee SY, O'Kusky JR, Ye P. 2012. Signalling through the type 1 insulin-like growth factor receptor (IGF1R) interacts with canonical Wnt signalling to promote neural proliferation in developing brain. ASN Neuro. 4, pii: e00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iansante V, Mitry RR, Filippi C, Fitzpatrick E, Dhawan A. 2018. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. 83, 232–240. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 78, 1670–6. [DOI] [PubMed] [Google Scholar]
- James LP, Kurten RC, Lamps LW, McCullough S, Hinson JA. 2005. Tumour necrosis factor receptor 1 and hepatocyte regeneration in acetaminophen toxicity: a kinetic study of proliferating cell nuclear antigen and cytokine expression. Basic Clin Pharmacol Toxicol. 97, 8–14. [DOI] [PubMed] [Google Scholar]
- James LP, Lamps LW, McCullough S, Hinson JA. 2003. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun. 309, 857–863. [DOI] [PubMed] [Google Scholar]
- Jamwal G, Singh G, Dar MS, Singh P, Bano N, Syed SH, Sandhu P, Akhter Y, Monga SP, Dar MJ. 2018. Identification of a unique loss-of-function mutation in IGF1R and a crosstalk between IGF1R and Wnt/β-catenin signaling pathways. Biochim Biophys Acta Mol Cell Res. 1865, 920–931. [DOI] [PubMed] [Google Scholar]
- Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. 2002. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 15, 1504–13. [DOI] [PubMed] [Google Scholar]
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. 2002. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 287, 337–44. [DOI] [PubMed] [Google Scholar]
- Kayano K, Yasunaga M, Kubota M, Takenaka K, Mori K, Yamashita A, Kubo Y, Sakaida I, Okita K, Sanuki K. 1992. Detection of proliferating hepatocytes by immunohistochemical staining for proliferating cell nuclear antigen (PCNA) in patients with acute hepatic failure. Liver. 12, 132–6. [DOI] [PubMed] [Google Scholar]
- Kelava T, Cavar I, Culo F. 2010. Influence of small doses of various drug vehicles on acetaminophen-induced liver injury. Can J Physiol Pharmacol. 88, 960–7. [DOI] [PubMed] [Google Scholar]
- Kost DP, Michalopoulos GK. 1991. Effect of 2% dimethyl sulfoxide on the mitogenic properties of epidermal growth factor and hepatocyte growth factor in primary hepatocyte culture. J Cell Physiol. 147, 274–80. [DOI] [PubMed] [Google Scholar]
- Lee WM. 2012. Acute liver failure. Semin Respir Crit Care Med. 33, 36–45. [DOI] [PubMed] [Google Scholar]
- Lutkewitte AJ, Schweitzer GG, Kennon-McGill S, Clemens MM, James LP, Jaeschke H, Finck BN, McGill MR. Lipin deactivation after acetaminophen overdose causes phosphatidic acid accumulation in liver and plasma in mice and humans and enhances liver regeneration. Food Chem Toxicol. 115, 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Murphy BV, Kominsky DJ, Orlicky DJ, Donohue TM Jr, Ju C. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology. 57, 1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson MJ, Carpenter LD, Graf ML, Pohl LR. 2008. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology. 48, 889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. 2013. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 30, 2174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. 2015. A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol Mech Methods. 25, 589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. 2013. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 269, 240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Staggs VS, Sharpe MR, Lee WM, Jaeschke H; Acute Liver Failure Study Group. 2014. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology. 60, 1336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK. 2007. Liver regeneration. J Cell Physiol. 213, 286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejak-Bowen KN, Monga SP. 2011. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 21, 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Freeman CM, Gulbins E, Lentsch AB. 2015. Sphingolipids in liver injury, repair and regeneration. Biol Chem. 396, 633–43 [DOI] [PubMed] [Google Scholar]
- Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. 2016. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 64, 60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick DA, Dietzen DJ, Turmelle YP, Shepherd R, Zhang S, Belle SH, Squires R; Pediatric Acute Liver Failure Study Group. 2009. Serum alpha-NH-butyric acid may predict spontaneous survival in pediatric acute liver failure. Pediatr Transplant. 13, 223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumack BH, Peterson RC, Koch GG, Amara IA. 1981. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 141, 380–5. [DOI] [PubMed] [Google Scholar]
- Sato Y, Watanabe H, Kameyama H, Kobayashi T, Yamamoto S, Takeishi T, Hirano K, Oya H, Nakatsuka H, Watanabe T, Kokai H, Yamagoe S, Suzuki K, Oya K, Kojima K, Hatakeyama [Google Scholar]
- Schmidt LE, Dalhoff K. 2005. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology. 41, 26–31. [DOI] [PubMed] [Google Scholar]
- Siegers CP. 1978. Antidotal effects of dimethyl sulphoxide against paracetamol-, bromobenzene-, and thioacetamide-induced hepatotoxicity. J Pharm Pharmacol. 30, 375–7. [DOI] [PubMed] [Google Scholar]
- Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee HJ, Wu AL, Fang Y, Satlin LM, Dow JT, Chen J, Zheng J, Boutros M, Mlodzik M. 2009. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol. 11, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stace CL, Ktistakis NT. 2006. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim Biophys Acta. 1761, 913–26. [DOI] [PubMed] [Google Scholar]
- Takayama H, Miyake Y, Nouso K, Ikeda F, Shiraha H, Takaki A, Kobashi H, Yamamoto K. 2011. Serum levels of platelet-derived growth factor-BB and vascular endothelial growth factor as prognostic factors for patients with fulminant hepatic failure. J Gastroenterol Hepatol. 26, 116–21. [DOI] [PubMed] [Google Scholar]
- Terry C, Dhawan A, Mitry RR, Lehec SC, Hughes RD. 2010. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transpl. 16, 229–37. [DOI] [PubMed] [Google Scholar]
- Thompson MD, Monga SP. 2007. WNT/beta-catenin signaling in liver health and disease. Hepatology. 45, 1298–305. [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. 2014. Toxicol Appl Pharmacol. 275, 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. 2015. Sterile inflammation in acute liver injury: myth or mystery? Expert Rev Gastroenterol Hepatol. 9, 1027–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J Hepatol. 66, 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Yang Y, Shi Y, Lv F, He J, Chen Z. 2016. Effects of Granulocyte Colony-Stimulating Factor on Patients with Liver Failure: a Meta-Analysis. J Clin Transl Hepatol. 4, 90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M, Siegers CP. Inhibition of the hepatotoxicity of paracetamol and its irreversible binding to rat liver microsomal protein. Arch Toxicol. 45, 61–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.