ABSTRACT
Background: Although much is known about the association between dietary glycemic load (GL) and type 2 diabetes (T2D), prospective cohort studies have not consistently shown a positive dose-response relation.
Objective: We performed a comprehensive examination of evidence on the dose response that links GL to T2D and sources of heterogeneity among all prospective cohort studies on healthy adults available in the literature.
Design: We conducted a systematic review of all prospective cohort studies and meta-analyses to quantify the GL-T2D relation both without and with adjustment for covariates.
Results: Among 24 prospective cohort studies identified by August 2012, the GL ranged from ∼60 to ∼280 g per daily intake of 2000 kcal (8.4 MJ). In a fully adjusted meta-analysis model, the GL was positively associated with RR of T2D of 1.45 (95% CI: 1.31, 1.61) for a 100-g increment in GL (P < 0.001; n = 24 studies; 7.5 million person-years of follow-up). Sex (P = 0.03), dietary instrument validity (P < 0.001), and ethnicity (European American compared with other; P = 0.04) together explained 97% of the heterogeneity among studies. After adjustment for heterogeneities, we used both funnel and trim-and-fill analyses to identify a negligible publication bias. Multiple influence, cumulative, and forecast analyses indicated that the GL-T2D relation tended to have reached stability and to have been underestimated. The relation was apparent at all doses of GL investigated, although it was statistically significant only at >95 g GL/2000 kcal.
Conclusion: After we accounted for several sources of heterogeneity, findings from prospective cohort studies that related the GL to T2D appear robust and consistently indicate strong and significantly lower T2D risk in persons who consume lower-GL diets. This review was registered at http://www.crd.york.ac.uk/PROSPERO as CRD42011001810.
INTRODUCTION
Carbohydrate foodstuffs that have a low or lower-than-average glycemic load (GL)4 or glycemic index (GI) may reduce risk of several chronic diseases, including type 2 diabetes (T2D) (1). Both FAO (2) and WHO (3), along with many diabetes associations, have issues, support, or make recommendations concerning these nutritional concepts. The GL describes the product of the quality of carbohydrate food (GI) and quantity (weight) of carbohydrate ingested. However, it remains uncertain whether there is a positive dose-response relation that links the GL to T2D, and if so, over what range of GL such a relation exists (1, 4). Because the 2 earliest cohort studies on incident T2D reported a positive relation with GL (5, 6), several studies made less convincing or contradictory observations (7–13), whereas other studies reported strong positive findings (14–17). Reasons for inconsistency in the dose response among these studies have not been examined formally (1, 4) but are of particular interest because an understanding of the reasons could be key to make a decision about the utility of recommending GL (or GI) reduction for lowering T2D risk in apparently healthy individuals.
In the first 2 published studies that prospectively related GL to T2D risk, the positive relation displayed a significant sex difference with a greater magnitude shown in women than in men (5, 6). A subsequent meta-analysis of studies (1) suggested the heterogeneity of findings may have been due in part to the quality of dietary instruments used (food-frequency or diet history questionnaires); those studies that used an instrument reported to have a correlation with food records <0.5 were possibly invalid. Later still, the duration of follow-up was hypothesized as a potential determinant of the strength of a relation between GL and T2D with support from similar observations for coronary heart disease (18). An additional publication indicated that ethnicity was a possible factor that contributes to the strength of the relation between GL and T2D risk (17).
The primary objective of the current study was to determine whether there is a positive dose response that relates GL to incident T2D in apparently healthy adults and, if so, to examine the range of GL dosages that significantly relate to T2D risk. The secondary objective was to examine the 4 predefined hypotheses as sources of heterogeneity, which we defined as the proportion of male study participants (SEX), energy-adjusted and deattenuated dietary instrument correlation for carbohydrate (CORR), number of follow-up years (FUY), and the proportion of participants of European American ethnicity (ETH). These secondary measures were of changes in RR with changes in values of covariates that represented predefined hypotheses. To meet these objectives, we undertook, in a comprehensive and systematic manner, a dose-response meta-analysis that used data from all available prospective cohort studies in the literature on populations of healthy adults.
METHODS
A comprehensive search and systematic assessment of studies and data extraction were conducted in a stepwise process in accordance with our specific objectives following an a priori defined protocol (http://www.crd.york.ac.uk/PROSPERO; CRD42011001810 at). In brief, our protocol made use of several well-established guidelines, such as the Newcastle-Ottawa Scale (NOS) for observational studies (19, 20), the proposed method for reporting of Meta-analyses Of Observational Studies in Epidemiology (21), the proposed method for Strengthening the Reporting of Observational Studies in Epidemiology (22), the proposed Measurement Tool to Assess Systematic Reviews (23), and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (24).
Search strategies
An electronic search for original publication records was conducted on MEDLINE and EMBASE by using PROQUEST (http://search.proquest.com) via the Royal Society of Medicine, London, United Kingdom (http://www.rsm.ac.uk). Additional searchers were made on the Cochrane Library of Systematic Reviews (http://www.thecochranelibrary.com/view/0/index.html), the Centre for Reviews and Dissemination (http://www.york.ac.uk/inst/crd), the PROSPERO Register of Systematic Reviews http://www.york.ac.uk/inst/crd/projects/register.htm), the CDC (http://www.cdc.gov), the NIH (http://www.nih.gov), and Google Scholar (http://scholar.google.co.uk) (see “Supplemental data” in the online issue for details of search strategies). Manual searches were also made of full publications that entered the review process (see “Supplemental data” for a list of both excluded and included studies) and of previous key reviews encountered (1, 4, 25–29).
Searches were conducted for the period from 1997 to August 2012 (week 3) inclusive by using medical subject headings (MeSH), titles, and abstracts. Briefly, terms included carbohydrate, glycemic index, glycemic load, blood glucose, diabetes or diabetes mellitus, incidence, risk factor, hazard assessment, risk assessment, follow-up, association, cohort studies, and prospective studies. Records in process or without assigned medical subject headings were also captured from January 2010 to August 2012 (week 3) by using only titles and abstracts. No language restrictions were applied. Electronic searches were limited to records after 1996, which was just before the first relevant publication (6) in 1997.
Eligibility and study selection
At least 2 authors evaluated titles and abstracts of electronic records for potentially relevant studies by selecting for prospective cohort studies that had incident diabetes (T2D) as an endpoint related to GL in adults of any age or sex and from any part of the globe in any language. Full study reports were retrieved for additional examination by ≥2 authors to assess the number of studies included in each publication and whether our inclusion criteria had been met. For the primary analysis, studies had to be 1) original, 2) have a prospective-cohort design, 3) study an eligible (healthy) population, 4) have an endpoint of T2D either self-reported or clinically confirmed in adults, and 5) have sufficient dietary and other information to provide a rate of change in RR per unit change in GL for at least one identifiable range of GL. For secondary analysis by using covariates, studies had further 6) to identify the population sample as men or women or both, 7) inform directly or indirectly via citation about the validity of the dietary instrument used, 8) provide FUY, and 9) identify the ethnicity of participants. All information had to be either reported or calculable from information available in the literature or obtained by author correspondence.
Exclusion criteria, in addition to those in original publications, were not assigned other than studies had to exclude T2D at baseline, and inclusion criteria had to be met (eg, cross-sectional or case-control studies were not included).
Data extraction and process of analysis
The flowchart shown in Figure 1 illustrates the principle stages and processes of the review undertaken. See Tables S1–S4 under “Supplemental data” in the online issue for a list of extracted data. Extraction was undertaken independently by 2 authors (GL and either RT or HL), and discrepancies were resolved jointly. Data were preserved in a Stata11.2 database file (StataCorp LP) from which they were drawn for calculations and statistical analyses.
FIGURE 1.
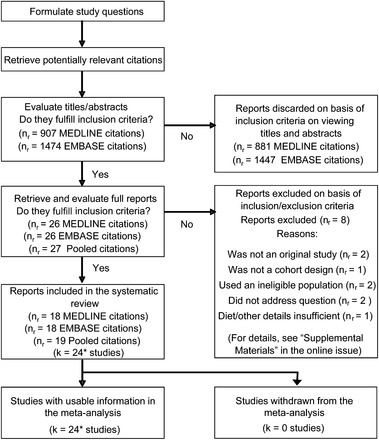
Summary of the study methodology, processes of review, and outcomes of inclusion and exclusion criteria. Reasons for exclusion are detailed online (see “Supplemental data” in the online issue). *Data from 2 reports from the same study at different times (16, 30) were combined before meta-analysis. Literature searches were conducted in week 3 of August 2012. MEDLINE and EMBASE were searched through PROQUEST (http://search.proquest.com) via the Royal Society of Medicine (http://www.rsm.ac.uk; see Search Strategies under “Supplemental data” for details). nr, number of reports; k, number of studies.
In particular, we first addressed the primary objective by determining rates of change in RR with rise in GL for each study and combined these rates by using a meta-analysis without covariates. Second, we addressed the secondary objective by meta-analysis with covariates (metaregression) to quantify the hypothesized heterogeneities. Third, we applied various techniques (see Study quality and related risk of bias; see Statistics) to assess how sound the outcomes shown appeared to be. Fourth, we return to our primary concern to ask over what doses of GL an association with T2D may exist after adjustment for covariates.
Calculations
The primary measure was RR (by combining studies that reported risk ratios and ORs). Values of GL (see Table S1 under “Supplemental data” in the online issue) that were based on a white-bread standard were converted to values that were based on the glucose standard (31), which, thus, became smaller. All studies had expressed GL values for given energy intakes, where the energy intakes differed among studies. GL was reexpressed on a common metric with units of grams of GL per 2000 kcal (8.4 MJ). Reported values related to dietary instruments were checked against cited validation studies. This check was essential to ensure that energy-adjusted and deattenuated values of the instrument’s correlation for carbohydrate were captured for CORR. When this was not possible (3 studies; see Table S3), approximate adjustments were made on the basis of average differences between raw and energy-adjusted values and between energy adjusted and deattenuated values imputed from other studies (see Table S3). Values of CORR were for validations of single applications of a dietary instrument. When more than one instrument was used in a study, the correlations from each instrument were averaged so that they were comparable with the application of a single instrument (see Table S3). For a case-by-case description of required data not reported directly in an original publication but otherwise knowable from related information by calculation, see the footnotes to Table S1; such calculations were performed by ≥2 authors independently, with inequalities resolved by discussion. Whenever calculations were considered approximate, the sensitivity of meta-analysis outcomes to approximations was assessed. Authors of original publications were consulted for otherwise unpublished or incalculable information.
Study quality and related risk of bias
Individual studies were assessed independently by 2 authors by using NOS for quality assessment of nonrandomized studies (19, 32) (see “Supplemental data” in the online issue or http://www.crd.york.ac.uk/PROSPEROFILES/1810_PROTOCOL_20111105.pdf for details).
The bias that was due to individual studies retrieved was assessed by the influence of study deletion, and the bias that was due to potential nonretrieved studies was assessed by using funnel plots and trim-and-fill analysis (see Statistics). The bias across studies was examined by an analysis of the influence of individual NOS components of selection, outcome, and comparability of studies and other study characteristics related to diet, population, and study progress (see Statistics).
Statistics
Interrater agreements on extracted data were determined by using the κ coefficient for dichotomous data (agreed compared with disagreed) and weighted κ for study quality in which ≥2 outcomes were possible (33, 34). Pairwise Pearson’s correlations (pwcorr version 3.0.13; Stata release 11.2 SE, 2009; StataCorp LP) between variables before meta-analysis with covariates were explored to alert to potential confounders and tested for potential significance by using the 2-tailed t test for product-moment correlation (35, 36). In meta-analyses, combined means and trends were obtained by using inverse variance with weighting for both random and fixed effects (37). Random effects were chosen when the variance among studies as a percentage of the total variance (I2) was >0% (38, 39). The significance of heterogeneity (I2 >0%) was assessed by using the Q test (38). Associations were assessed for significance by using the asymptotic z test.
In the main analysis, a 2-stage procedure was adopted when findings from cohort studies were assessed. In the first stage, trends for the change of lnRR with the change in energy-adjusted GL (dose-response slope) were obtained for each study separately. Trends were provided or readily calculable for 4 studies in 3 reports (8, 14, 40). All other studies provided several dose-specific RR values relative to a lowest GL referent. For these studies we obtained trends by using the generalized least-squares method for trend estimation of summarized dose-response data as designed for use in meta-analyses of prospective cohort studies (glst version 7.0.0; StataCorp LP) (38, 39, 41). In the second stage, trends with GL (dose-response slopes) from each study were combined by using a meta-analysis to obtain the combined mean of trends, both when no covariate was involved (metan version 3.03; StataCorp LP) (42–44) and when covariates were fitted by using the method of moments (metareg version 2.6.1; StataCorp LP) (45, 46). Covariates were centered and continuous (eg, the validity of a dietary instrument) or centered and dichotomous (eg, European American compared with other ethnicities). The validity of this 2-step approach with these data was assessed by comparison with a one-step glst metaregression (38, 39) with virtually identical outcomes (see Table S6 under “Supplemental data” in the online issue).
Residuals from meta-analyses with and without covariates were assessed visually for asymmetry by using funnel plots (47) and objectively for size and significance of any asymmetry (interpretable as publication bias) by using the trim-and-fill analysis of Duval and Tweedie (metatrim version 1.0.3; StataCorp LP) (48, 49). The last procedure also provided a nonparametric estimate of the number and location of observations required to both fill a funnel and achieve symmetry.
The sensitivity of a model β coefficient to the dropping of individual studies was assessed graphically by using an influence analysis, in which the change in each jth hypothesized covariate β-coefficient caused by the deletion of each ith study (ie, Δβij) was expressed as a proportion of the SE of the coefficient (seij) obtained after the deletion; this yielded a score comparable with the z score for the β coefficient before deletion. This approach was used also to indicate whether the metaregression model was overly complex or not, signified by too large an influence for any study compared with the majority (50). We also expressed the results numerically as the percentage change in the β coefficient that was due to dropping a study from the analysis.
The exploration of the sensitivity of results to a study characteristic or quality item (other than those hypothesized) was by a modification of the influence-analysis procedure. This procedure asked how the β coefficient for each jth hypothesized covariate changed by addition to the metaregression model of a single (vth) explored centered covariable that represented the examined study characteristic or quality item. The change in β (ie, Δβjv) was expressed as a percentage of the β coefficient obtained before addition of the vth variable to the model. An inspection of the influence of individual-study characteristics and quality items was undertaken in preference to assessing the influence of the overall study quality alone (20, 51).
The sensitivity of the overall outcomes to weakness in data was assessed by making alternative assumptions and calculations (see Table S5 under “Supplemental data” in the online issue).
The dose range over which GL remained a positive modifier for incident T2D was assessed by using a graphical display of the trend for the dose response compared with dose. In practice, this was ΔlnRR per g GL compared with the average dose (0.5ΔGL) over which lnRR could change from each Q1 to Qn, where Q1 referred to the lowest numbered quantile and Qn to quantiles that had n > 1 within each study, pooled across all studies. The trend across the difference (Δ lnRR) compared with the average (0.5Δ GL) plot was obtained by using a metaregression with adjustments for the hypothesized covariates. The possibility of a nonlinear trend with GL was allowed by using a cubic spline (mksplin version 1.2.5 with 3 equally dispersed knots; Stata release 11.2 SE, 2009; StataCorp LP).
All analyses were conducted with Stata software (release 11.2 SE, 2009; StataCorp LP) by applying options under kappa, pwcorr, metan, metatrim, glst, metareg, and mksplin commands (40).
RESULTS
Retrieval and selection of literature
Both MEDLINE and EMBASE returned a similar number of potentially relevant publications (26 each) (Figure 1). On examination of full reports, 18 articles each from MEDLINE and EMBASE were relevant, which together totaled 19 original reports after pooling. Data from 2 reports of the same study, which ostensibly differed only in high numbers of follow-up years (20 and 26 y), were combined prior to meta-analysis (16, 30), and some reports included multiple studies on sexes and ethnicities separately (8, 17). This process left 24 original studies for meta-analysis. Reasons for exclusion or not meeting inclusion criteria are summarized in Figure 1 (see online materials for details). Two earlier meta-analyses that mistakenly identified studies to include are also detailed in the supplementary materials. All studies that met inclusion and exclusion criteria were retained in meta-analyses, and none of the studies were withdrawn during synthesis of the evidence.
Study quality and other characteristics
Study qualities according to the NOS scale were good to near optimal and ranged from 6 to 8 (mean: 7.6; n = 24) for possible scores from 0 to 9 (summarized in Table 1; see Table S4 for individual study scores). The preconsultation interrater agreement on NOS between assessors was 97%, which was significantly greater than expected by chance (68%) according to the weighted kappa (κ = 0.87, P < 0.0001 for 10 possible quality scores and n = 24 studies). Authors from 16 studies indicated no conflicting interest. Other authors mostly had earlier publication dates and we found no explicit statements (see Table S4). Author affiliations and sources of funding also gave no concern for potential bias.
TABLE 1.
Study characteristics in relation to hypothesized sources of heterogeneities, SEX, CORR, FUY, and ETH (n = 24 prospective cohort studies)1
| Pearson’s correlations | ||||||||
| Characteristics | Mean | Min | Max | κ × 102 | Compared with SEX | Compared with CORR | Compared with FUY | Compared with ETH |
| Dietary factors | ||||||||
| Range of glycemic load across all studies (g/2000 kcal)2 | — | 62 | 279 | — | — | — | — | — |
| Study mean glycemic load (g/2000 kcal)2 | 139 | 93 | 231 | 100 | 0.04 | 0.19 | 0.05 | −0.40* |
| Study mean energy intake (kcal/d) | 1917 | 1494 | 2629 | 953 | 0.66* | 0.27 | 0.22 | −0.26 |
| Glucose (= 1) or white bread (= 0) reference | 0.67 | 0 | 1 | 100 | 0.20 | 0.27 | 0.40 | −0.55* |
| Diet assessments (n) | 1.35 | 1 | 7 | 100 | −0.31 | 0.03 | 0.52* | 0.33 |
| Foods in dietary instrument (n) | 125 | 66 | 276 | 100 | 0.31 | 0.50* | 0.17 | −0.07 |
| Dietary instrument (CORR) (fractional) | 0.62 | 0.43 | 0.80 | 100 | 0.08 | — | 0.10 | 0.07 |
| Applicability within population (yes = 1; doubtful = 0) | 0.79 | 0 | 1 | 100 | 0.09 | 0.38 | 0.10 | −0.31 |
| Used energy-adjusted intakes (yes = 1; no = 0) | 0.91 | 0 | 1 | 100 | −0.26 | 0.05 | 0.14 | 0.23 |
| Fiber excluded as confounder (yes = 1; no = 0) | 0.96 | 0 | 1 | 100 | 0.21 | −0.18 | 0.26 | 0.16 |
| Population factors | ||||||||
| T2D excluded at baseline (yes = 1; no = 0) | 1.00 | 1 | 1 | 100 | 0.00 | 0.00 | 0.00 | 0.00 |
| Population sample analyzed (n) | 31,583 | 1898 | 124,907 | 904 | −0.33 | 0.07 | −0.05 | 0.23 |
| ETH (European American = 1; others = 0) | 0.38 | 0 | 1 | 100 | −0.25 | 0.07 | 0.06 | — |
| SEX (M = 1; F = 0) | 0.40 | 0 | 1 | 955 | — | −0.08 | 0.03 | −0.25 |
| Study average BMI (kg/m2) | 26 | 23 | 29 | 100 | 0.04 | −0.21 | −0.05 | −0.18 |
| Age at baseline (y) | 54 | 32 | 75 | 826 | 0.30 | 0.20 | −0.02 | −0.26 |
| Progress factors | ||||||||
| Person-years | 303,071 | 7592 | 2,127,502 | 907 | −0.28 | 0.04 | 0.46* | 0.27 |
| Total no. of cases in a study | 1234 | 99 | 6950 | 100 | −0.25 | −0.14 | 0.62* | 0.11 |
| FUY | 10.1 | 4 | 26 | 100 | 0.03 | 0.10 | — | 0.06 |
| Clinical (= 1) or self-reported (= 0) outcomes | 0.79 | 0 | 1 | 100 | 0.27 | 0.38 | 0.36 | −0.03 |
| Study quality | ||||||||
| Newcastle-Ottawa Scale (0–9) | 7.6 | 6 | 8 | 878 | — | — | — | — |
CORR was energy adjusted and deattenuated in each case, either as reported in the original studies or the cited validation study or as adjusted and deattenuated subsequently (see Table S3 under “Supplemental data” in the online issue). None of the values reported arose from repeated-measures analysis of consecutively used dietary instruments. *Potential to be significant (monovariate P < 0.05) according to a 2-tailed t test applicable to the Pearson’s product-moment correlation. CORR, energy-adjusted and deattenuated dietary instrument correlation for carbohydrate; ETH, proportion of participants of European American ethnicity; FUY, number of follow-up years; Max, maximum; Min, minimum; SEX, proportion of male study participants; T2D, type 2 diabetes.
Adjusted for energy intake.
κ <100% because of inadvertent selection of a value for men only instead of for men and women combined in one study (40).
κ <100% because of inadvertent selection of the total population investigated (n = 7310) instead of the subsample analyzed (n = 5598) in one study (9) and because of an inadvertent typo in an extracted value from another study (15).
κ <100% because of unequal calculated values between authors after accurate extraction of input data from one study (8).
κ <100% because of unequal calculated values between authors after accurate extraction of input data from one study (52); rounding of 37.8 to 37 instead of to 38 after calculations for one study (53), and extraction error for 1 in 5 numbers used in calculations for study of native Hawaiian men (17).
This value was calculated as the product of κ ×102 shown for the population sample analyzed and FUY extracted.
κ in this row is weighted for 10 possible scores (0–9).
Correlations among hypothesized factors (SEX, CORR, FUY, and ETH) and various other study characteristics and overall quality were generally low and nonsignificant (monovariate P > 0.05) (Table 1). Some potentially significant correlations did arise. For example, the study average energy intake correlated with the proportion of participants who were men, with intakes in men that were higher than in women (P = 0.00003 unadjusted; P = 0.0006 after Bonferroni adjustment). Care was taken to assess how much this and other potential correlations (highlighted with asterisks in Table 1) affected the conclusions reached about the hypothesized covariates (see influence analysis in Statistics).
Original articles had RR or trends for RR with GL that had been adjusted for confounding variables. Most commonly, these were (n studies among the 24 studies analyzed); BMI (in kg/m2) (n = 21), physical activity (n = 21), age (n = 15), smoking (n = 15), level of education (n = 14), dietary or cereal fiber intake (n = 13), alcohol intake (n = 11), and family history of diabetes (n = 10). As factors (adjusted = 1; not adjusted = 0), none of these variables correlated significantly with the hypothesized covariates (SEX, CORR, FUY, and ETH). Thus all correlations were <0.25 except for age, which approached a potentially significant correlation with follow-up years (correlation of 0.35; monovariate P = 0.08). The variance in age among studies was explored subsequently as a potential confounder in the current meta-analysis with hypothesized covariates.
Glycemic load
Reported GL values reexpressed in a common metric ranged widely, the lowest of which was 62 g/2000 kcal and the highest of which was 279 g/2000 kcal among all study quantiles. Thus, the global range for GL intake spanned at least 217 g/2000 kcal. Within studies, the range for GL was less and spanned on average 81 g/2000 kcal, which was almost one-third of that for the global range of 217 g/2000 kcal. The range of GL intake within a study (region) varied, the lowest of which was 39 g/2000 kcal and the highest of which was 143 g/2000 kcal. Overall, the mean of study GL intakes was 139 g/2000 kcal (Table 1).
Combined studies: meta-analysis without covariates
Observations made in this and the next section were of central importance. Differences were found among studies for the rise in RR for T2D per 100-g rise in GL (Figure 2). Thus, the inconsistency among all studies (heterogeneity) was high (I2 = 48%) and significant (P = 0.005). Each sex subgroup (males, females, and mixed sexes) contributed heterogeneity (I2 > 0%). By using this approach to analysis, there was only a clearly significant association of T2D with GL in women (P < 0.001 for females, P = 0.060 for mixed sexes, and P = 0.132 for males). Nevertheless, the random-effects meta-analysis provided strong evidence that RR for T2D was significant (P < 0.001) over all studies at 1.27 (95% CI: 1.15, 1.40) for a 100-g rise in GL.
FIGURE 2.
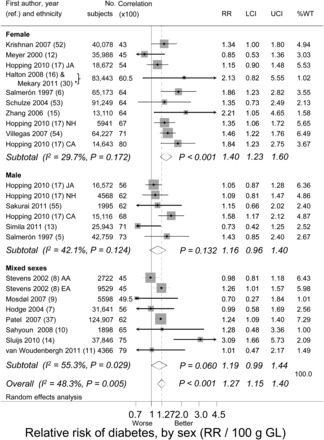
Meta-analysis without covariates that relates RR of type 2 diabetes to GL by sex group. Study means are denoted by gray boxes (larger points have greater weight). Horizontal lines denote 95% CIs. Arrowheads indicate truncations. Diamonds show combined-study means and corresponding 95% CIs. The scale is logarithmic with RR and 95% CIs shown untransformed. Individual studies are identified by first author, date, and citation. P values were calculated by using the z test for RR and the Q test for I2. *Data from the same study reported at different follow-up durations (20 and 26 y) (16, 30) were combined before meta-analysis. AA, African American; CA, Caucasian American; EA, European American; GL, glycemic load; I2, variance among studies as a percentage of the total variance; JA, Japanese American; LCI, lower CI; NH, Native Hawaiian; ref., reference; UCI, upper CI; %WT, weight based on random effects.
With visual inspection (Figure 2), the inconsistency among studies within each sex subgroup appeared possibly related to the validity of the dietary instrument used (CORR; tested below, see Strength, stability, and significance of covariates). This possibility confounded attempts to use a subgroup analysis without covariates to assess differences between sexes. Similar observations were made when the rate of change in lnRR per 100 g GL (ie, dose-response) was replaced in each study with the absolute rise in lnRR from the lowest to highest quantile of GL in each study (see Figure S1 under “Supplemental data” in the online issue).
Combined studies: meta-analysis with covariates
Without covariate adjustments, the all-studies combined-mean incremented RR per 100-g GL was 1.27 (95% CI: 1.15, 1.40) as summarized in Table 2 (model 1) (P < 0.001). This relation became progressively stronger while remaining significant after adjustment for SEX, CORR, and ETH but not FUY and reached 1.45 (95% CI: 1.31, 1.61) in the fully adjusted model applied to all studies (Table 2, model 5).
TABLE 2.
RR of T2D when energy-adjusted glycemic load was 100 g higher than the point of reference (RR = 1) for 24 prospective cohort studies1
| Model | Covariates used in the model | RR (95% CI) | P | R2 | I2 | P for I2 |
| % | % | |||||
| 1 | None | 1.27 (1.15, 1.40) | <0.001 | 0 | 48 | 0.005 |
| 2 | SEX | 1.24 (1.12, 1.36) | <0.001 | 18 | 42 | 0.016 |
| 3 | SEX, CORR | 1.39 (1.24, 1.54) | <0.001 | 72 | 20 | 0.20 |
| 4 | SEX, CORR, FUY | 1.39 (1.24, 1.56) | <0.001 | 64 | 23 | 0.16 |
| 5 | SEX, CORR, FUY, ETH | 1.45 (1.31, 1.61) | <0.001 | 97 | 2 | 0.43 |
Values of RR were untransformed after analysis of the ln form (lnRR). Model 1 was a meta-analysis without covariates, Model 2 was adjusted for sex (to 50% male and 50% female). Model 3 was adjusted as for model 2 and for dietary instrument validity (CORR centered on 0.7, which was the approximate middle value for generally acceptable correlations (correlations >0.5). Model 4 was adjusted as for model 3 and for FUY (centered on 10 y, which was the approximate mean for these studies). Model 5 was adjusted as for model 4 and for ETH (centered on 50% European Americans and 50% other ethnicities). Model 5 was also called the fully adjusted model. P values were calculated by using the z test for RR and the Q test for I2. CORR, energy-adjusted and deattenuated dietary instrument correlation for carbohydrate; ETH, proportion of participants of European American ethnicity; FUY, number of follow-up years; I2, heterogeneity that remained after adjustment for covariates; R2, heterogeneity accounted for by adjustments for covariates; SEX, proportion of male study participants; T2D, type 2 diabetes.
The progressive inclusion of covariates in the model, in which, eg, model 2 included the same covariates as in model 1 plus the covariate for SEX, and model 3 included the same covariates as in model 2 plus the covariate for CORR (Table 2), progressively accounted for heterogeneity (inconsistency among studies) with exception for FUY. This effect was indicated by the progressive fall in heterogeneity (I2) from 48% to 2% (models 1–5).
R 2 (Table 2) is a measure of the variance among studies that was fully explained by the covariates. Model 1 was without covariates as explanatory variables, and therefore, had R2 = 0% (the model explained none of the between-study variance). R2 increased progressively to 97% (model 5) because of the inclusion of hypothesized covariates that were due to associations with SEX, CORR, and ETH (but not FUY) so that 3 of the 4 hypothesized covariates explained virtually all variability among studies.
Altogether, cohorts of healthy adults who consumed diets with a high GL appeared to be at 45% greater risk of developing T2D than were cohorts of adults who consumed a diet lower in GL by 100 g (Table 2, model 5).
Sensitivity of RR to retention of FUY in the model
A model that excluded the nonsignificant FUY as covariate exhibited relations for RR that were essentially no different to those in model 5, which included FUY, and thus, RR remained at 1.45 (95% CI: 1.31, 1.61) for a 100-g increment in GL. Meanwhile the among-studies variance remained essentially completely explained by SEX, CORR, and ETH (I2 = 1%, R2 = 99%).
Sensitivity of RR to study deletions
In the fully adjusted model (model 5), the combined-study mean incremental RR for T2D stabilized at ∼0.45 (95% CI: 0.31, 0.61) for a 100-g increase in GL. The deletion of any individual study from the analysis affected this increment by <8%; the increments ranged from 0.42 (95% CI: 0.26, 0.60; P < 0.001, I2 = 4%) after deletion of a study from Hopping et al (17) to 0.48 (95% CI: 0.34, 0.65; P < 0.001, I2 = 0%) after deletion of the study from Krishnan et al (52).
Sensitivity of RR to study quality items
Adjustments to RR, additional to those in model 5, by individual NOS study quality items were negligible and affected the combined study incremental RR by <4% (range: −4% to +4%) (see Table S6). The quality items fell under 3 headings of participant-selection criteria, outcome criteria, and comparability criteria. Sensitivity to these and to individual quality items were assessed. Individual quality items were: whether the sample populations were truly or somewhat truly representative of the average adult population or sex subgroup in the communities; whether subcohorts (by quantile) were selected from the same community; whether exposure to GL was ascertained securely; whether the outcome of interest was absent at the start of the study; whether few subjects were lost to follow-up or the losses were adequately explained; whether the analysis of the study had adjusted for nonnutrient risk factors (smoking, physical activity, and BMI); whether there had been adequate adjustments for nutritional factors (energy, fiber, fat, and alcohol intakes); whether the outcome was assessed adequately; and whether the follow-up was sufficiently long for the outcome to occur (≥4y) (see Table S7 under “Supplemental data” in the online issue for results on individual items).
Sensitivity of RR to other study characteristics
Adjustments to RR, additional to those in model 5, were explored for other study characteristics but were also negligible, each of which affected the combined study incremental RR by ≤10% (range: –4% to +10%). These variables included the study mean GL, the study mean energy intake, whether GL had been expressed on a glucose or a bread standard in the original study, the number of dietary assessments made during the follow-up period, the number of food items in the dietary instrument, whether or not the dietary instrument had been validated within the population studied, whether or not the validation was for energy adjusted intakes, or not dietary (or cereal) fiber had been excluded as a confounder or covariate, the size of the study population sample, the study average BMI, the study average age of participants at baseline, the number of person-years captured in the study, the total number of incident T2D cases captured in the study, and the case ascertainment (ie, self-report compared with a clinical report) (see Table S7 for results on individual items).
Sensitivity of RR to data weaknesses
Weaknesses in data used in the meta-analysis were explored by making a range of alternative assumptions and simulations. Details are provide online (see Table S5) and concerned approximations that were assumed to exist or were introduced by calculations when information was not reported (or not provided in correspondence) directly or indirectly for GL, energy intake, cases by quantile, noncases by quantile, person-years by quantile, and CORR. CORR was the only hypothesized covariable that had been derived in previous experimental work by using estimation procedures. When the assumptions for CORR were varied, RR for a 100-g increment in GL stayed within the range from 1.45 (95% CI: 1.31, 1.61) to 1.49 (95% CI: 1.33, 1.67) compared with 1.45 (95% CI: 1.31, 1.61) as reported in Table 2 (model 5). A lower value was found only when an additional random error for GL was simulated, at which point model 5 yielded, on average for 10 simulations, a consistently lower RR (P < 0.001; t test) of 1.42 (95% CI: 1.28, 1.58) for an increment in GL of 100 ± SD of 10 g. Altogether, the sensitivity analyses (see Table S5) provided confirmation that the assumptions we made introduced either a negligible error or resulted in conservative estimates for the RR-T2D relation with GL.
Publication bias
Asymmetry in a funnel plot (see Figure S2 under “Supplemental data” in the online issue) according to trim-and-fill analysis indicated a 0.00 (95% CI: –0.06, +0.06; P > 0.99) bias in the incremental RR over 100 g GL in the fully adjusted model (model 5). The analysis also provided the location and precision of hypothetical points needed to achieve symmetry and eliminate bias and that represented studies potentially missed by the literature search, but the analysis returned no studies as potentially missing.
Range of GL associated with T2D risk
To assess the range over which GL associated with T2D, one-step pooled metaregression analysis was performed on subcohorts (n = 24 studies; including 24 referent and 79 nonreferent cohorts) (Figure 3). The analysis made adjustments for SEX, CORR, FUY, and ETH as in model 5 but allowed the increment in RR from referent to nonreferent cohort to vary with the dose for GL. The dose for GL was the average GL between the referent Q1 and the higher nonreferent Qn, and curvature was permitted by fitting the dose as a cubic spline. The fitted dose-response (trend) was imperfectly linear (horizontal) with a loss of this imperfection when the study of Meyer et al (12) was dropped from the analysis. Nevertheless, when all studies were retained, the GL was significantly related to risk of T2D at all doses >95 g/2000 kcal.
FIGURE 3.
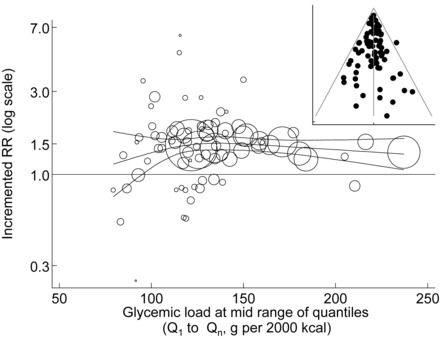
Range over which GL and RR of type 2 diabetes are related. Data points are bubbles and show RRs per 100 g GL for study quantiles from the lowest quantile (Q1) to each higher quantile (Qn) after adjustment for the 4 hypothesized covariates. Bubbles increase in size with increase in weight of the data point. Curvilinearity of the trend and 95% CIs for the adjusted RR per 100 g GL was permitted by fitting a one-step pooled cubic-spline metaregression with 3 equally dispersed knots to the lnRR. Larger bubbles have greater precision and weight. The inset shows the corresponding funnel plot for model residuals. GL, glycemic load.
Model forecasts
We compared our model forecasts with combined estimates of RR related to GL obtained as other authors have done before us (1, 4, 26) by using only lowest and highest quantiles in the meta-analysis without covariates. In this last scenario, the current data set yielded RR of 1.20 (95% CI: 1.11, 1.30) and corresponded to an average range for GL across regions of 81 g from the lowest (Q1) to the highest (Qmax) quantile (see Figure S1 under “Supplemental data” in the online issue).
In model 5 (Table 2), RR after adjustment for SEX, ETH, and CORR equal to 0.7 indicated the higher value for RR of 1.45 (95% CI: 1.31, 1.61) for a 100-g rise in GL (Table 2). The same adjustments with CORR equal to 1.0 (ie, the unattainable ideal) forecasted RR of 2.05 (95% CI: 1.55, 2.65), which was 5 times stronger than the RR of 1.20. With consideration that, globally, a significant association was indicated for GL between ∼100 and 279 g (Figure 3), the same model forecasted RR of 3.56 (95% CI: 2.06, 5.75) over this range for GL, which spanned 179 g GL. This RR (3.56) was 12 times stronger than that obtained by using only the lowest and highest quantiles in the meta-analysis without covariates. Although such forecasts should be considered cautiously and have wide 95% CIs, they provide evidence that a meta-analysis that is without consideration of dose and covariates can substantially underestimate the importance of the GL in affecting T2D incidence both regionally and, more so, globally.
Strength, stability, and significance of covariates
In Model forecasts, the only critical uncertainty about the covariates was the strength (and significance) of the relation between RR and CORR. Without study deletions, RR increased to 2.05 (95% CI: 0.6, 4.7) per unit of CORR (1.0) with P = 0.0005 (Table 3). Any single study deletion from the analysis left CORR significant at P ≤ 0.007. Largest deviations occurred after deletion of observations from Meyer et al (12) and Krishnan et al (52), which left the RR-CORR association between 11% lower and 19% higher, respectively. However, after deletion of both studies, the RR-CORR association was 2.3 (95% CI: 0.7, 5.4), which remained both significant (P = 0.001) and no less strong than when these studies were retained in the analysis.
TABLE 3.
Association of hypothesized determinants with the RR of T2D (n = 24 studies)1
| Increment in RR2 | P-trend | |||||
| Increment in RR2 | Unadjusted | Adjusted3 | R 2 | I 2 | P for I2 | |
| SEX (F > M) | ||||||
| Single covariate model | 0.24 (−0.01, 0.57) | 0.065 | — | 17 | 43 | 0.02 |
| Bi-covariate model (model 3)4 | 0.23 (0.01, 0.47) | 0.036 | 0.036 | 72 | 14 | 0.20 |
| Fully adjusted (model 5)5 | 0.22 (0.02, 0.46) | 0.031 | 0.031 | 97 | 2 | 0.43 |
| CORR (over the maximum range, 0.0–1.0) | ||||||
| Single-covariate model | 2.19 (0.5, 6.0) | 0.004 | — | 53 | 30 | 0.09 |
| Bi-covariate model (model 3)4 | 2.15 (0.5, 5.5) | 0.002 | 0.004 | 72 | 14 | 0.20 |
| Fully adjusted model (model 5)5 | 2.05 (0.6, 4.7) | 0.0005 | 0.001 | 97 | 2 | 0.43 |
| FUY (/10 y) | ||||||
| Single-covariate model | −0.01 (−0.28, 0.26) | 0.92 | — | −9 | 49 | 0.01 |
| Fully adjusted (model 5)5 | 0.00 (−0.19, 0.20) | 0.96 | >0.99 | 97 | 2 | 0.43 |
| ETH (European American > other ethnicities combined) | ||||||
| Single-covariate model | 0.24 (0.01, 0.52) | 0.041 | — | 18 | 43 | 0.016 |
| Fully adjusted model (model 5)5 | 0.22 (0.05, 0.41) | 0.011 | 0.044 | 97 | 2 | 0.43 |
RR of T2D was for an energy-adjusted glycemic load 100 g higher than in the referent quantile. P values were calculated by using the z test for RR and the Q test for I2. CORR, energy-adjusted and deattenuated dietary instrument correlation for carbohydrate; ETH, proportion of participants of European American ethnicity; FUY, number of follow-up years; I2, heterogeneity that remained after adjustment for covariates; R2, heterogeneity accounted for by adjustments for covariates; SEX, proportion of male study participants; T2D, type 2 diabetes.
All values are trends; 95% CIs in parentheses. Trends and 95% CIs are shown untransformed. RR was analyzed as the ln.
Adjusted for 2 or more covariances according to Bonferroni (see footnote 1 of Table 2). The following are listed in order of priority: 1 × P for the first hypothesized determinant (SEX), 2 × P for the second hypothesized determinant (CORR), 3 × P for the third hypothesized determinant (FUY), and 4 × P for the fourth hypothesized determinant (ETH).
Inclusive of the first 2 hypothesized covariates (ie, SEX and CORR).
Inclusive of all 4 hypothesized covariates (ie, SEX, CORR, FUY, and ETH).
The RR-CORR association also remained stable to possible adjustments for any other study characteristic or study quality items explored as centered covariates additional to those hypothesized (see Table S7). Each item had only a minor influence (–10 to +19%) on the size of the RR-CORR association, but this association always remained significant (P < 0.03).
Relations for RR-SEX, RR-ETH, and RR-FUY together with RR-CORR are also shown graphically online (see Figure S3 under “Supplemental data” in the online issue) and are summarized in Table 3. Even after consideration of multiple covariances (and the hierarchy of hypotheses), the relation for RR-SEX was significant (P < 0.031) and that for RR-ETH was significant (P < 0.011) or borderline significant after multiple covariances were accounted for (P = 0.044) (Table 3). Sensitivity analysis shown graphically (see Figure S4 under “Supplemental data” in the online issue) indicated that no one study compared with any other had an unduly large influence on the size of β coefficients for covariates, which was also an indication that model 5 was not unduly complex. Also, neither the RR-SEX nor the RR-ETH association was modified by greater than –23% to +19% when adjusted for an additional (vth) covariate that represented any study quality items and any other study characteristics, none of which were themselves significant (z score <2; P > 0.05) (see Table S7).
The exclusion of the hypothesized but nonsignificant variable FUY from model 5 (Tables 2 and 3) also had a negligible influence on the size of the remaining but significant hypothesized covariables. Increments in RR that were due to a 100-g increment in GL after exclusion of FUY were: for SEX, 0.21 (95% CI: 0.02, 0.42) higher in women than in men, which compared with 0.22 (95% CI: 0.03, 0.46) when FUY was included; for ETH, the value was 0.21 (95% CI: 0.04, 040) higher in European Americans than in other ethnicities combined compared with 0.22 (95% CI: 0.05, 0.41) when FUY was included; and for CORR, the values was higher by 2.01 (95% CI: 0.64, 4.6) per unit increase in CORR compared with 2.05 (95% CI: 0.6, 0.47) when FUY was included. Meanwhile, the variance among studies was still essentially fully explained (I2 = 1%, R2 = 99%).
We concluded that the T2D-GL relation had significant covariates (SEX, CORR, and ETH) that were neither highly sensitive to observations for individual studies nor attributable to an inadequate account of other study characteristics or study quality items or data weaknesses explored.
Cumulative meta-analysis with covariates
Additional evidence on the stability of variable estimates comes from the cumulative meta-analysis (see Figure S5 under “Supplemental data” in the online issue). All cumulative RR values obtained remained positive (RR >1) and stabilized at 1.45. Covariates SEX, CORR, and ETH each approached stability, although with less precision than for RR. By contrast, the cumulative information on FUY was unstable. A positive and seemingly significant association between RR and FUY emerged with the observations of Halton et al (16), who reported results of a 20-y follow-up study (16). However, any relation with FUY was lost progressively when subsequent studies were included in the analysis and was not evident by the time of the study of Mekary et al (30) that reported on 26-y follow-up.
DISCUSSION
Our meta-analysis of 24-prospective cohort studies had 7.5 million person-years of follow-up and revealed a significant and strongly positive relation between GL and T2D risk across a wide range of GL values. Also shown was that the relation was significantly affected by several main sources of heterogeneity. These sources included sex, ethnicity, and the quality of the dietary assessment, although not the duration of follow-up, and explained essentially all of the variance in RR among studies. After adjustment for heterogeneities, the RR stabilized at 1.45 (95% CI: 1.31, 1.61) for a rise in GL of 100 g/2000 kcal. That is, over this dose range, the risk of T2D increased by 45%.
The associations we found with covariates were generally supportive of 3 of the 4 hypotheses addressed and that originated elsewhere (1, 5, 6, 17). The positive dose-dependent GL-T2D relation was stronger in women than in men (SEX), stronger when the dietary instrument had greater validity (CORR), and stronger in European Americans than in the other ethnicities examined as a single group. The duration of follow-up (FUY), which may reflect the duration of exposure to GL or time to develop clinical disease had an unstable relation with RR for T2D, and over all studies, our analysis was unable to confirm that this factor had importance. The positive GL-T2D relation in the current meta-analysis was coherent with meta-analyses of human interventions that showed, for the first time to our knowledge, that both GL (and GI) and fasting blood glucose (and glycated protein) interrelate independently of dietary fiber intake while dependent on the severity of dysglycemia (56, 57).
Our meta-analysis with covariates had several strengths. It combined many studies, small to large cohorts, and few to many cases and, thus, allowed reasonably precise variable estimations, power to detect significant relations, and precise assessment of asymmetric biases. The publication bias was both far from significant and negligible, whereas the sensitivity of each main outcome to each individual study was relatively small. In addition, the main outcomes proved relatively insensitive to surrogacy or confounding by many other study characteristics or quality items assessed. Notably too, we provided an account of essentially all heterogeneity among studies. A major strength was that our 4 hypothesized covariates had been suggested previously, although at a time when only very limited evidence was available. In the current study, 3 of the 4 hypotheses were far from refuted, although only CORR was highly significant over all studies, and SEX and ETH were weakly significant (P < 0.05 after multiple covariates were accounted for). Additional studies are needed to better define how the GL-T2D relation changes with the duration of follow-up because studies with 10–20 y of follow-up are low in number.
A good balance of men, women, and mixed-sex studies have provided us with a reasonable representation of each sex and a postori power to detect a significant effect. Our observation of a sex difference was strengthened by avoiding errors made (1, 26) when data from Patel et al (40) were extracted during a previous meta-analysis of fewer studies. Although the sex difference may be genetic, it could also reflect a sex-related behavior (eg, greater alcohol consumption in men than in women) recently reported as a determinate in women (30, 58). Moreover, the sex difference shown in the current study had an evidence level that was no better than the narrative grade of the original studies (ie, grade B compared with grade A for interventional studies) (59, 60).
Dietary instruments in the reviewed studies had a broad range of validities for the assessment of carbohydrate intake (CORR), which facilitated a precision and power to detect a significant influence. A possible weakness is that CORR is an instrument variable rather than an exposure variable and, thus, had a potential to hide an unidentified exposure. However, in the absence of such an identification, a bias because of inadvertent adjustment for an instrument variable is not thought to present a major threat (61). An additional weakness was that few studies used dietary instruments that had been specifically validated for GL. However, instrumental estimates of carbohydrate and GL intakes appeared highly correlated (30, 62) or yielded similar values for CORR (63). An implication of CORR for the prediction of the size of the GL-T2D relation is that all combined means from a meta-analysis without appropriate covariates will underestimate the importance of GL in the contribution of risk of T2D. Moreover, whenever GL is shown to have a negligible positive effect in observational studies, a low validity for the dietary instrument used needs to be excluded as a possible artifact. Whether an inconsistent association between RR for other chronic diseases and carbohydrate intake (12, 14, 16, 54, 64) would be resolved by examining evidence along lines used here for GL remains to be examined when sufficient observations accumulate.
It has been understood that GI compares favorably with GL in terms of predicting chronic disease risk generally (1), which is a view that has been refuted (4) and affirmed (65) for T2D. However, such comparisons depend on an understanding of the causes of heterogeneity in results for both GI and GL. Furthermore, that the GL-T2D relation depends on CORR raises the interesting question of whether similar results might arise for other chronic diseases, including cancers associated with T2D (66, 67).
The current study is limited in its ability to describe the effect of ethnicity on the relation between GL and T2D. Many ethnic groups have not yet been researched, and other ethnic groups have limited representation. European Americans formed the largest group, and the simplest assessment possible was for a 2-group comparison with a similar number of studies that represented all other ethnicities. Clearly, a distinction between these groups will eventually depend on representation among all other ethnicities. For the present, the data simply support rather than refute differences in RR in defined groups of people. That the strength of the GL-T2D relation was found to depend on ethnicity does not automatically mean GL has less importance in some ethnic groups; this is because, in some instances, it might be due to an overall higher ethnic susceptibility to the incidence of T2D unrelated to GL.
In several important aspects, our meta-analysis went beyond previous work (1, 4, 25–29). First, we applied a dose-response meta-analysis and considered the range of GL that showed significant association. Second, our analyses were much more systematic and comprehensive with an in-depth quantification of heterogeneities. Third, cumulative analyses were conducted with adjustments for several significant covariates. A model that permits adjustment for 4 possible covariates may seem overly complex with ≤24 prospective studies. However, the significant covariates had higher levels of significance when applied simultaneously than singly. Moreover, our cumulative analysis reached toward stable means and trends for RR and significant covariates before the last 10 studies were includes in the analysis. Furthermore, a low sensitivity to the deletion of individual studies was a direct indication that the fully adjusted model (model 5) was not unduly complex.
We showed that the GL-T2D relation operated over a wide range of GL values and was especially significant when the GL was >95 g/2000 kcal. A target of 100 g GL would, in theory, be reached by consuming 100 g carbohydrates with a GI of 100, by 130 g carbohydrates [the US Recommended Dietary Allowance (RDA) for adults (68)] with a GI of 77, or by 200 g carbohydrates with GI of 50, or by 250 g carbohydrates with a GI of 40. The 200–250-g carbohydrate intake approximates the median for Western diets in adults (69). Numerous examples of highly palatable foods have a GI ≤40 (70), which allows for achievement of all these possibilities. Thus, up to and immediately above the carbohydrate RDA for adults, the replacement of high-GI foods with low-GI foods would allow the target to be met. However, when carbohydrate intake is >250 g, high-GI foods would need to be consumed at a lower frequency or by increasing elimination from the diet. At 300 g carbohydrates (60% of energy), the GI would need to be 33 and would be achievable by combining foods with a GI <50.
Nutritionists are likely to be interested in the limitations that the current evidence placed on the contribution to GL and carbohydrate intake that can be made by sugars. With an assumption of a GI of 68 for sucrose (31), 147 g alone would contribute a GL of 100 g in a 2000-kcal/d diet, and at 4 kcal/g sucrose, this would be 29% of energy. These amounts already exceed both the RDA of 130 g carbohydrate/d and the upper intake for sugars of 25% of energy intake in the United States (68) [and 10% elsewhere (70)]. Therefore the target of a 100-g GL in a 2000 kcal/d diet would not pose a more stringent limitation on sucrose intake than would either the RDA for carbohydrate or upper intake for sugars. Similar considerations hold for pure fructose and high-fructose corn syrup. Therefore, the current evidence is consistent with a view that, for carbohydrate nutrition in general, with regard to GL, sugars can be treated as just another carbohydrate together with starches. For both types of carbohydrates, the measure of grams of GL per 2000 kcal becomes a factor (aside from energy intake) that explains the risk of T2D. However, an intake of 100 g pure fructose/d (equivalent to 20% of 2000 kcal) has been recommended by the American Heart Association as an upper limit for normal to borderline hypertriglyceridemic individuals (71).
Weaknesses exist in these approaches to target a 100-g GL in 2000 kcal and in the underpinning meta-analyses and were inherent to the reviewed studies. These weaknesses are that the methodology for the assessment of the GI of foods is imprecise (72, 73) and varies with ripeness, processing, and chewing, and in observational studies foods are reported rather than provided. However, with 7.5 million years of follow-up, the use of energy-adjusted nutrient values, deattenuated correlations, multiple dietary assessments in studies of longer follow-up, and precision for GL expressed and recorded per grams of fresh-weight food, such weaknesses appeared not to be as great as often envisaged (58, 74–77).
In conclusion, the prospective association between GL and T2D risk is moderate to strong, depends on the prevailing circumstance, and appears to have greater importance globally than regionally. The relation appears stronger in studies that use dietary instruments of greater validity (as defined in the current study), women, and European Americans. Persons who consume diets >100 g GL/2000 kcal appear at progressively greater risk of T2D with greater GL intake. Altogether, our meta-analysis supports that GL is an important and underestimated dietary characteristic that, among others, contributes significantly to the incidence of T2D.
Supplementary Material
Acknowledgments
We are indebted to the many study participants and authors of the original studies. We especially thank the following published authors for their responses to requests for additional or confirmatory information: AM Hodge, DR Jacobs Jr (Meyer et al), G Maskarinec (Hopping et al), and RA Mekary and cocommentators WC Willett, FB Hu, A Patel, NR Sahyoun, J Stevens, GJ van Woudenbergh, and R Villegas. We also thank N Orsini (Karolinska Instituten, Stockholm, Sweden) for help with the glst metaregression program. The review was initiated by Independent Nutrition Logic Ltd and funded unconditionally by Beneo-Palatinit GmbH, which is a producer of low-glycemic carbohydrates. Acknowledged authors of reviewed publications provided additional data.
The authors’ responsibilities were as follows—GL: was the guarantor and was responsible for the protocol, literature search, extraction, analysis, synthesis, and initial production of the manuscript; RT and HL: shared responsibility for independent searches and extraction of data from the literature and comments on the manuscript; SL: collaborated to provide advice on the study design, analysis, and interpretation of findings and participated in the writing of the manuscript. GL and HL hold shares in Independent Nutrition Logic Ltd, and at the time of this study, RT was partly employed by Independent Nutrition Logic Ltd. Independent Nutrition Logic Ltd is an independent consultancy that takes commissions from many organizations, a full list of which is shown at www.inlogic.co.uk. SL reported no conflicts of interest. Neither the funding body nor any of its employees, affiliations, institutions, associations or competitors had any role in any part of the initiation or conduct of this review.
ABBREVIATIONS
- CORR
energy-adjusted and deattenuated dietary instrument correlation for carbohydrate
- ETH
proportion of participants of European American ethnicity
- FUY
number of follow-up years
- GI
glycemic index
- GL
glycemic load
- NOS
Newcastle-Ottawa Scale
- Q1
Qmax, and Qn, the lowest, highest, and intermediate-and-highest numbered quantiles in a study
- RDA
Recommended Dietary Allowance
- SEX
proportion of male study participants
- T2D
type 2 diabetes
FOOTNOTES
Supported by Beneo-Palatinit GmbH, Mannheim, Germany.
REFERENCES
- 1. Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC.. Glycemic index, glycemic load, and chronic disease risk–a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–37. [DOI] [PubMed] [Google Scholar]
- 2. WHO/FAO. Carbohydrates in human nutrition. FAO Food and Nutrition Paper No 66. Rome, Italy: Food and Agriculture Organisation, 1998. [Google Scholar]
- 3. Mann J.. Dietary carbohydrate: relationship to cardiovascular disease and disorders of carbohydrate metabolism. Eur J Clin Nutr 2007;61(suppl 1):S100–11. [DOI] [PubMed] [Google Scholar]
- 4. Dong JY, Zhang L, Zhang YH, Qin LQ.. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br J Nutr 2011;106:1649–54. [DOI] [PubMed] [Google Scholar]
- 5. Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC.. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20:545–50. [DOI] [PubMed] [Google Scholar]
- 6. Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC.. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7. [DOI] [PubMed] [Google Scholar]
- 7. Hodge AM, English DR, O’Dea K, Giles GG.. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004;27:2701–6. [DOI] [PubMed] [Google Scholar]
- 8. Stevens J, Ahn K.. Juhaeri, Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care 2002;25:1715–21. [DOI] [PubMed] [Google Scholar]
- 9. Mosdøl A, Witte DR, Frost G, Marmot MG, Brunner EJ.. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Am J Clin Nutr 2007;86:988–94. [DOI] [PubMed] [Google Scholar]
- 10. Sahyoun NR, Anderson AL, Tylavsky FA, Lee JS, Sellmeyer DE, Harris TB;. Health, Aging, and Body Composition Study. Dietary glycemic index and glycemic load and the risk of type 2 diabetes in older adults. Am J Clin Nutr 2008;87:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Woudenbergh GJ, Kuijsten A, Sijbrands EJ, Hofman A, Witteman JC, Feskens EJ.. Glycemic index and glycemic load and their association with C-reactive protein and incident type 2 diabetes. J Nutr Metab 2011;2011:623076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyer KA, Kushi LH, Jacobs DR Jr, Slavin J, Sellers TA, Folsom AR.. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. [DOI] [PubMed] [Google Scholar]
- 13. Similä ME, Valsta LM, Kontto JP, Albanes D, Virtamo J.. Low-, medium- and high-glycaemic index carbohydrates and risk of type 2 diabetes in men. Br J Nutr 2011;105:1258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sluijs I, van der Schouw YT, van der AD, Spijkerman AM, Hu FB, Grobbee DE, Beulens JW.. Carbohydrate quantity and quality and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition-Netherlands (EPIC-NL) study. Am J Clin Nutr 2010;92:905–11. [DOI] [PubMed] [Google Scholar]
- 15. Zhang C, Liu S, Solomon CG, Hu FB.. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006;29:2223–30. [DOI] [PubMed] [Google Scholar]
- 16. Halton TL, Liu S, Manson JE, Hu FB.. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am J Clin Nutr 2008;87:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hopping BN, Erber E, Grandinetti A, Verheus M, Kolonel LN, Maskarinec G.. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J Nutr 2010;140:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB.. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med 2006;355:1991–2002. [DOI] [PubMed] [Google Scholar]
- 19. Wells G, Shea S, O’Connell D, Robertson J, Peterson P, Welch V, Losos M, Tugwell P.. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. URL: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Buce.pdf. Accessed 28th August 2009.
- 20. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.0.0 Cambridge, United Kingdom: The Cochrane Collaboration, 2008. Available from: http://www.cochrane.org/training/cochrane-handbook#previous and from http://www.mrc-bsu.cam.ac.uk/cochrane/handbook500/ (cited 15 February 2008). [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB.. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 22. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, Henry DA, Boers M.. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013–20. [DOI] [PubMed] [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D.. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 25. Chiu CJ, Liu S, Willett WC, Wolever TM, Brand-Miller JC, Barclay AW, Taylor A.. Informing food choices and health outcomes by use of the dietary glycemic index. Nutr Rev 2011;69:231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu S, Chou EL.. Dietary glycemic load and type 2 diabetes: modeling the glucose-raising potential of carbohydrates for prevention. Am J Clin Nutr 2010;92:675–7. [DOI] [PubMed] [Google Scholar]
- 27. Murakami K, Okubo H, Sasaki S.. Effect of dietary factors on incidence of type 2 diabetes: a systematic review of cohort studies. J Nutr Sci Vitaminol (Tokyo) 2005;51:292–310. [DOI] [PubMed] [Google Scholar]
- 28. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB.. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–40. [DOI] [PubMed] [Google Scholar]
- 29. Hare-Bruun H, Nielsen BM, Grau K, Oxlund AL, Heitmann BL.. Should glycemic index and glycemic load be considered in dietary recommendations? Nutr Rev 2008;66:569–90. [DOI] [PubMed] [Google Scholar]
- 30. Mekary RA, Rimm EB, Giovannucci E, Stampfer MJ, Willett WC, Ludwig DS, Hu FB.. Joint association of glycemic load and alcohol intake with type 2 diabetes incidence in women. Am J Clin Nutr 2011;94:1525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Foster-Powell K, Holt SH, Brand-Miller JC.. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56. [DOI] [PubMed] [Google Scholar]
- 32. Al-Harbi K, Farrokhyar F, Mulla S, Fitzgerald P.. Classification and appraisal of the level of clinical evidence of publications from the Canadian Association of Pediatric Surgeons for the past 10 years. J Pediatr Surg 2009;44:1013–7. [DOI] [PubMed] [Google Scholar]
- 33. Landis JR, Koch GG.. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 34. StataCorp. κ-Interrater agreement In: Stata base reference manuals. College Station, TX: StataCorp LP, 2009:802–15. [Google Scholar]
- 35. Altman GD.. Practical statistics for medical research. London, United Kingdom: Chapman & Hall, 1991. [Google Scholar]
- 36. StataCorp. Correlate—correlations (covariances) of variables or coefficients. In: Stata base reference manuals. College Station, TX: StataCorp LP, 2009:324–31. [Google Scholar]
- 37. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 38. Orsini N, Bellocco R, Greenland S.. Generalized least squares for trend estimation of summarized dose-response data. In: Sterne J, ed. Meta-analysis in Stata: an updated collection from the Stata Journal. College Station, TX: Stata Press, 2009:200–17. [Google Scholar]
- 39. Orsini N, Bellocco R, Greenland S.. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal 2006;6:40–57. [Google Scholar]
- 40. Patel AV, McCullough ML, Pavluck AL, Jacobs EJ, Thun MJ, Calle EE.. Glycemic load, glycemic index, and carbohydrate intake in relation to pancreatic cancer risk in a large US cohort. Cancer Causes Control 2007;18:287–94. [DOI] [PubMed] [Google Scholar]
- 41. Greenland S, Longnecker MP.. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 42. Bradburn MJ, Deeks JJ, Altman D.. Metan—a command for meta-analysis in Stata. Stata Tech Bull 1998;44:4–15. [Google Scholar]
- 43. Bradburn MJ, Deeks JJ, Altman D.. Metan—a command for meta-analysis in Stata In: Sterne JAC, ed. Meta-analysis in Stata: an updated collection from the Stata Journal. College Station, TX: Stata Press, 2009:3–28. [Google Scholar]
- 44. Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman D.. Metan: fixed- and random-effects meta-analysis In: Sterne JAC, ed. Meta-analysis in Stata: an updated collection from the Stata Journal. College Station, TX: Stata Press, 2009:29–54. [Google Scholar]
- 45. Sharp S.. Meta-analysis Regression. Stata Tech Bull 1998;42:16–22. [Google Scholar]
- 46. Sharp S.. Meta-analysis regression In: JAC S, ed. Meta-analysis in Stata: an updated collection from the Stata Journal. College Station, TX: Stata Press, 2009:97–106. [Google Scholar]
- 47. Egger M, Davey Smith G, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duval S, Tweedie R.. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000;95:89–98. [Google Scholar]
- 49. Steichen TJ.. Nonparametric trim and fill analysis of publication bias in meta-analysis In: Sterne JAC, ed. Meta-analysis in Stata: an updated collection from the Stata Journal. College Station, TX: Stata Press, 2009:165–77. [Google Scholar]
- 50. Dupont WD.. Statistical modelling in biometical researchers: a simple introduction to the analysis of complex data. Cambridge, United Kingdom: Cambridge University Press, 2002. [Google Scholar]
- 51. Jüni P, Witschi A, Bloch R, Egger M.. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054–60. [DOI] [PubMed] [Google Scholar]
- 52. Krishnan S, Rosenberg L, Singer M, Hu FB, Djousse L, Cupples LA, Palmer JR.. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch Intern Med 2007;167:2304–9. [DOI] [PubMed] [Google Scholar]
- 53. Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB.. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 2004;80:348–56. [DOI] [PubMed] [Google Scholar]
- 54. Villegas R, Liu S, Gao YT, Yang G, Li H, Zheng W, Shu XO.. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med 2007;167:2310–6. [DOI] [PubMed] [Google Scholar]
- 55. Sakurai M, Nakamura K, Miura K, Takamura T, Yoshita K, Morikawa Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y et al. Dietary glycemic index and risk of type 2 diabetes mellitus in middle-aged Japanese men. Metabolism 2012;61:47–55. [DOI] [PubMed] [Google Scholar]
- 56. Livesey G, Taylor R, Hulshof T, Howlett J.. Glycemic response and health a systematic review and meta-analysis: the database, study characteristics, and macronutrient intakes. Am J Clin Nutr 2008;87:223S–36S. [DOI] [PubMed] [Google Scholar]
- 57. Livesey G, Taylor R, Hulshof T, Howlett J.. Glycemic response and health a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr 2008;87:258S–68S. [DOI] [PubMed] [Google Scholar]
- 58. Livesey G.. Joint association of glycemic load and alcohol intake with type 2 diabetes incidence in women. Am J Clin Nutr 2012;95:983. [DOI] [PubMed] [Google Scholar]
- 59. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weightman A, Ellis S, Cullum A, Sander Turley R.. Grading evidence and recommendations for public health interventions: developing and piloting a framework. 2005. Available from: http://www.nice.org.uk/niceMedia/docs/grading_evidence.pdf (cited 11 September 2001).
- 61. Myers JA, Rassen JA, Gagne JJ, Huybrechts KF, Schneeweiss S, Rothman KJ, Joffe MM, Glynn RJ.. Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol 2011;174:1213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Beulens JW, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, Grobbee DE, van der Schouw YT.. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol 2007;50:14–21. [DOI] [PubMed] [Google Scholar]
- 63. Levitan EB, Mittleman MA, Hakansson N, Wolk A.. Dietary glycemic index, dietary glycemic load, and cardiovascular disease in middle-aged and older Swedish men. Am J Clin Nutr 2007;85:1521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Salmerón J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC.. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019–26. [DOI] [PubMed] [Google Scholar]
- 65. Schulz M, Liese AD, Fang F, Gilliard TS, Karter AJ.. Is the association between dietary glycemic index and type 2 diabetes modified by waist circumference? Diabetes Care 2006;29:1102–4. [DOI] [PubMed] [Google Scholar]
- 66. Noto H, Osame K, Sasazuki T, Noda M.. Substantially increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis of epidemiologic evidence in Japan. J Diabetes Complications 2010;24:345–53. [DOI] [PubMed] [Google Scholar]
- 67. Sun G, Kashyap SR.. Cancer risk in type 2 diabetes mellitus: metabolic links and therapeutic considerations. J Nutr Metab 2011;2011:708183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. IOM. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Macronutrients and healthful diets. Washington, DC: National Academies Press, 2003. [Google Scholar]
- 69. FAO/WHO. Carbohydrates in human nutrition. FAO Food and Nutrition Paper No 66. Rome, Italy: Food and Agriculture Organisation, 1998. Available from: http://www.fao.org/docrep/w8079e/w8079e00.htm (cited 2003). [Google Scholar]
- 70. Atkinson FS, Foster-Powell K, Brand-Miller JC.. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011;123:2292–333. [DOI] [PubMed] [Google Scholar]
- 72. Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever THM.. Glycaemic index methodology. Nutr Res Rev 2005;18:145–71. [DOI] [PubMed] [Google Scholar]
- 73. Wolever TM, Vorster HH, Bjorck I, Brand-Miller J, Brighenti F, Mann JI, Ramdath DD, Granfeldt Y, Holt S, Perry TL et al. Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr 2003;57:475–82. [DOI] [PubMed] [Google Scholar]
- 74. Hu FB.. Obesity epidemiology. Oxford, United Kingdom: Oxford University Press, 2008. [Google Scholar]
- 75. Willett W.. Nutritional Epidemiology. New York, NY: Oxford University Press, 1998. [Google Scholar]
- 76. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC.. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 77. Livesey G.. Glycemic response and toleration. In: O’Donnell K, Kearsley MW, eds. Sweeteners and wugar alternatives in food technology, 2nd ed. Oxford, United Kingdom: Wiley-Blackwell, 2012:1–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


