Abstract
Although adolescents are developmentally distinct from adults, they often receive addiction treatment based on adult models. This is problematic because adolescents face significantly different conditions in addiction treatment, including distinct basic biological and neurodevelopmental stages, unique sociodevelopmental concerns, distinctive addiction trajectories, and in turn, disparate treatment goals and outcomes. In sum, it can be difficult for even savvy clinicians to know how to approach addiction treatment with this important age group.
In an effort to help clinicians and researchers consider substance use via a neurodevelopmental lens, we approached this review with four goals: (1) characterize the prevalence, and related health and safety implications, of substance use within this age group; (2) identify the nature of the adolescent brain, including characteristic features of this phase of neurodevelopment relevant for adolescent substance use treatment; (3) provide an overview of current adolescent addiction interventions and avenues to improve clinical treatment and clinical research efforts for adolescents; and (4) examine the intersection between the nature of the developing brain and adolescent substance use, and utilize that information to inform alternative routes and directions for substance use treatment in this critical age group.
This review concludes by offering a novel neurodevelopmental model and framework to examine substance use interventions, along with a series of recommendations to optimize adolescent substance use treatment and clinical research.
Keywords: adolescent, MRI, treatment, development, addiction
I. Introduction
Adolescence has been argued to be the only developmental period bookended by highly disparate events (Giedd, 2018). It commences with biology, defined as the onset of puberty, and concludes via social construct, typified by the achievement of “independent functioning” such as obtaining a job, completing training, and beginning a family (Giedd, 2018). While the ages for this window vary widely throughout the globe, most agree that the central work of adolescence largely encapsulates ages 13–18 (Giedd, 2018).
Across cultures and societies, it is during this precise developmental period that substance use is most often initiated (Vega et al., 2002), with peak age of first misuse, and related problems following soon thereafter (Wagner, 2002). Given that adolescents have goals, cognitions, and social contexts distinct from adults, it is critical that clinicians and researchers consider adolescent substance use through a neurodevelopmental lens. In the present review, we engage a developmental neuroscience framework to characterize the prevalence, and related health and safety implications of substance use within this age group; identify the nature of the adolescent brain, including characteristic features of this phase of neurodevelopment relevant for adolescent substance use treatment; and provide an overview of current adolescent addiction interventions and avenues to improve clinical treatment and clinical research efforts for adolescents. We then conclude by examining the intersection between the nature of the developing brain and adolescent substance use, and utilize that information to inform alternative routes and highlight promising future directions for substance use treatment in this critical age group.
Due to the inherently polysubstance-using nature of this age group (Karoly et al., 2015, Clark, 2004), we focus this review primarily on the three most frequently used substances by adolescents: alcohol, cannabis and tobacco (Johnston et al., 2018, CDC, 2016). We have not integrated examination of prescription pain/opioid misuse here due to its relatively recent history within adolescent addiction clinical research and treatment settings, and the related dearth of empirical adolescent opioid treatment research in this area (Dash et al., 2018).
Prevalence of adolescent substance use
The rates and patterns of adolescent substance use have maintained historical consistency throughout the past several decades. Alcohol continues to be the top substance used by adolescents across the globe (Cousijn et al., 2018, Feldstein Ewing et al., 2014). Noted in top United States (U.S.) surveys (Johnston et al., 2018, CDC, 2016), despite the legal age being 21 years, alcohol is highly accessible for American youth, with most accessing alcohol through peers or other individuals (CDC, 2016). In turn, it is no surprise that half of American 14-year-olds have consumed alcohol. This rises to 75% by age 18, with half drinking to intoxication (Johnston et al., 2018, CDC, 2016). Relevant to potential neurotoxicity and health impact (Feldstein Ewing et al., 2014, Lisdahl et al., 2013), 20% of adolescents start drinking by age 13 (prior to initiating high school) (CDC, 2016). Interestingly, U.S. surveys reflect a current 10-year low in youth drinking. Yet, rates of youth alcohol use and related problems still remain consequential, in terms of safety and health impact (Johnston et al., 2018).
One currently debated reason for the decline in adolescent alcohol use in the U.S. revolves around recent changes in cannabis legislation (Choo et al., 2016, Feldstein Ewing et al., 2017). For example, treatment providers in states where cannabis is medically and/or recreationally legal are observing some degree of youths’ increasing preference for cannabis over alcohol (Feldstein Ewing et al., 2017). Presently, one quarter of U.S. youth report cannabis use by age 14, with 8% starting by age 13. Of relevance, rates of cannabis use have recently begun to approximate adolescent alcohol use patterns, with half of U.S. teens now using cannabis by age 18 (Johnston et al., 2018, CDC, 2016). This reflects a 22% rise in adolescent cannabis use during the past decade (Johnston et al., 2018).
These startling trends raise the question of whether increased recreational and medical legalization of cannabis are contributing to observed increases in adolescent use (Feldstein Ewing et al., 2017). Unfortunately, requisite data needed to identify causal relationships between changes in cannabis legislation and adolescent use are not yet available, and thus far, evidence has been mixed regarding effects on both adolescent consumption and perceived harmfulness. Further, effects may vary substantially across states/contexts (Ingraham, 2017, Schmidt et al., 2016, Cerda et al., 2018, Cerda et al., 2017, Hasin et al., 2015). Active efforts by scientists and practitioners in recreationally and medically legal areas are needed to disaggregate directional impacts between public policy legislation and adolescent use (National.Academy.of.Sciences, 2017, Feldstein Ewing et al., 2017). For example, in 2017, national survey databases indicated adolescents reported significant drops in their estimations of potential harm and disapproval of cannabis use (Johnston et al., 2018); how this aligns with patterns of adolescent cannabis use and intersects with alcohol use is a critical, and increasingly pressing, empirical public health question that has direct implications for addiction treatment providers working with adolescents in this field (Feldstein Ewing et al., 2017).
In terms of tobacco, one quarter of American youth have tried tobacco, with 7% initiating use before age 13; this rises to a third of youth by age 18, with 10% smoking 10+ cigarettes/day (Johnston et al., 2018, CDC, 2016). National data reflect that American adolescents are moving away from tobacco use, as represented by a 71% drop in past 11 year cigarette use (Johnston et al., 2018). Part of this may be due to teens’ transition to electronic vapor products (“vaping”), a recent newcomer to the world of adolescent substance use. Adolescents report vaping nicotine (25%) and cannabis (11%), preferring flavor-based cartridges (31%), contentiously marketed toward children (Johnston et al., 2018, Lodrup Carlsen et al., 2018). Surveys reflect 20% of U.S. youth have vaped an e-liquid containing nicotine and/or cannabis by age 14, rising to one third by age 18 (Johnston et al., 2018, CDC, 2016).
The ubiquity of adolescent substance use does not equate with its safety
For many clinicians, the most pressing public health concern around adolescent substance use is not that it represents an entry point into a life-long course of protracted addiction. Rather, the more imminent concern for parents and providers is that adolescents will make a risky choice while trying to obtain, use, and/or dispose of substances that result in consequences that cannot be undone (e.g., getting a head injury in an accident; being a victim of sexual assault; making a foolish mistake that costs them life or limb), and that render their life trajectory much more difficult (e.g., posting illicit pictures of themselves on social media; getting arrested; losing a scholarship) (Feldstein Ewing et al., 2016a).
One of the central challenges in this area is that experimentation with substance use is so typical among adolescents that for decades it has been interpreted as largely “normative”, and to some degree to-be-anticipated, and some argue, even developmentally-appropriate (Shedler and Block, 1990). While the initial part of this trajectory has been interpreted as largely “harmless” in terms of behavioral impact, conferring a degree of social advantage, for some, during high school (Feldstein Ewing et al., 2016a), the nature of substance use shifts for many during this period.
Specifically, more than half of adolescents have tried at least one substance by age 18, with many using at least one substance in a “harmful way”, such as binge (heavy) use. Due to the inherently illicit behaviors that must be engaged in to access and utilize most substances (with the exception of prescription opioids, see (Dash et al., 2018)), many youth use substances in ways that increase the likelihood of encountering other forms of harm (e.g., using in an unsupervised setting; using with peers or older adolescents that the youth does not know well; having to navigate getting home from a friend’s house while intoxicated; trying to quickly dispose of substances in areas that could cause damage to the youth and/or their surroundings) (Johnston et al., 2018, CDC, 2016).
In terms of health relevance, large-scale data reflect that health sequelae for substance use for adolescents have become much more severe throughout the past three decades. Not only does morbidity and mortality increase 200–300% during this developmental period (Giedd, 2018), but a recent JAMA review indicates that alcohol use increased morbidity from 5,800 deaths in 1980 to 9,400 deaths in 2014, with years of life lost doubling (Dwyer-Lindgren et al., 2018). Further, substance-related consequences have skyrocketed from 3,300 deaths in 1980 to 33,100 deaths in 2014, with years of life lost up by a factor of nine (Dwyer-Lindgren et al., 2018).
The relevance of these values is that adolescent initiation of substance use plays a key role in this equation. Viner and colleagues (2017) utilize a metric that quantifies impact of adolescent exposure, referred to as adolescent attributable fraction (AAF). This represents the relative impact on mortality that would have occurred in the absence of exposure to the risk (e.g., if no substances had been used during adolescence). In the example of tobacco, this team found that 80% of individuals commence tobacco use during adolescence, contributing to 72% of smoking during the adult years, with a pronounced link particularly for those who initiated smoking before age 16 (Viner et al., 2017). Additionally, a recent Lancet review highlighted that the ages of 15–19 are particularly precarious for adolescents throughout the globe, with accidents and injuries (including road injuries, interpersonal violence, self-harm, drowning, which of relevance, are often intertwined with substance use) as the top cause of death in this age group, and substance use itself a leading cause of disability (Mokdad et al., 2016).
Despite several decades of efforts by experts to identify the best avenues to prevent and reduce adolescent substance use, progress continues to be limited. Given that adolescents have goals, cognitions, and social contexts that are distinct from adults, one avenue that may improve treatment outcomes for this age group is considering substance use via a neurodevelopmental lens (Dwyer-Lindgren et al., 2018).
II. The nature of the adolescent brain: characteristic features relevant for the adolescent addiction treatment context
Despite the rise of scientific interest in the neurodevelopmental period of adolescence (Giedd, 2018), exemplified by recent large-scale research initiatives (e.g., Australian iCATS; European IMAGEN; US NCANDA and ABCD) (Whelan et al., 2014, Jernigan et al., 2018, Simmons et al., 2014, Brown et al., 2015), throughout history contributions of this salient developmental period on health and neural drivers of behavior have largely been overlooked (Bundy et al., 2018). This is relevant because adolescence is, at this moment, being recognized as a highly unique neurodevelopmental period that can impact lifelong health and wellbeing - the chrysalis before adulthood.
While much of the literature tends to focus on adolescents’ newly piqued penchant for risk behaviors, fewer clinical and research efforts have been dedicated to the highly adaptive aspects of the adolescent period (Giedd, 2015). Data are now showing that the adolescent brain is, in many ways, pre-programmed for resilience (Cousijn et al., 2018, Blakemore, 2018, Giedd, 2018), with the adolescent brain purposefully transitioning from an overproliferation of neurons, characteristic of childhood, to greater specificity and focus of brain networks, demonstrated by increases in strength and purpose (Nelson et al., 2016). In lay terms, the adolescent brain can be seen as the development of a system of functional highways established within the brain during this developmental stretch. Ultimately, across theoretical perspectives, biology and environment dynamically interact throughout this time to generate an enhanced set of developmental tasks during this window; the outgrowth of which sets the foundation for later adaptive network connectivity, structure/function, and resultant behavior and cognition during adulthood. Central facets likely to drive the primary tasks of this neurodevelopmental period are:
(1). Substantive pubertal changes.
Defined as the process by which an individual reaches sexual maturity, puberty is initiated by a series of internal changes that precede external ones; girls often commence ~2 years before boys (Herman-Giddens et al., 2001). The earliest phase includes adrenarche (~age 8). Adrenarche is initiated by the hypothalamic-pituitary-adrenal (HPA) axis, which triggers relevant hormones including dehydro-epiandrosterone (DHEA), dehydroepiandrosterone-sulfate (DHEAS), and testosterone (Barendse et al., 2018). DHEA contributes to axonal and white matter development by stimulating neurogenesis via opposition of the neurotoxic impact of glucorticoids (Barendse et al., 2018, Nguyen et al., 2017). DHEA plays a central role in cortical plasticity in prefrontal (dorsolateral prefrontal cortex, dlPFC; anterior cingulate, ACC), parietal (temporal parietal junction, TPJ), and subcortical structures (amygdala, hippocampus) involved in memory and attention (Nguyen et al., 2013, Nguyen et al., 2017). Subsequently, relevant for the adolescent treatment context, DHEA has cognition-promoting functions during adolescent development, particularly in emotionally “hot” situations (Nguyen et al., 2017, Brumback et al., 2016).
Gonadarche, the maturation of the gonads, follows adrenarche and is characterized by a surge of hormones and rapid physical growth, contributing to sexual dimorphism in the body, face and voice (Crone and Dahl, 2012). Gonardarche begins in the brain, with hypothalamus release of gonadotrophin-releasing hormone (GnRH), in turn activating the resting hypothalamic-pituitary-gonadal (HPG) axis, and pituitary to produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (Crone and Dahl, 2012). FSH and LH generate estrogen (via ovaries) and testosterone (via testes), instrumental in movement toward sexual dimorphism in the body and brain (Draganski et al., 2014, Blakemore et al., 2010).
One particularly robust finding, relevant to the adolescent treatment context, is that pubertal maturation, particularly increased testosterone, promotes structural and functional development of the striatum (Braams et al., 2015, Op de Macks et al., 2011, Herting et al., 2014, Goddings et al., 2014). This is salient, as hormonally-mediated striatal development has been implicated as predictive of risk-taking and substance use during adolescence (Marceau et al., 2018, Braams et al., 2016), above and beyond chronological age. This suggests that variability in gonadal hormones may play a crucial role in organizing striatal development and consequential reward-seeking behavior during adolescence; factors relevant in the adolescent addiction treatment context. While such striatal changes have been examined in the context of negative risk-taking, there is reason to believe that striatal changes also serve a vital role in positive risk-taking, including pursuit of goals and movement into more mature social and cognitive challenges (Davidow et al., 2018) (Crone and Dahl, 2012).
While the hormonal cascade has been established, how each element contributes to the nature of cognitive development and its intersection with adolescent mental health, including substance use and its treatment, has been under-examined (Byrne et al., 2017). Here we highlight the central and interactive roles of DHEA and gonadal hormones in neurocognitive development, and indicate their potential role and impact within the adolescent addiction treatment context.
(2). Surge of cognitive skills.
Another hallmark of adolescence is the steep improvement in cognitive abilities that allow individuals to set and accomplish high-level goals (Davidow et al., 2018). In addition to the widely recognized role of cognitive control, adolescents show an elegant network of development across an array of cognitive skills during this period. Examples of emergent cognitive skills highly relevant to the treatment context include the inception of abstract reasoning, higher-order reasoning, working memory, self-monitoring, and cognitive flexibility (Luna et al., 2004, De Luca et al., 2003). Of note, this array of cognitive skills matures across a range of timelines throughout adolescence. For example, cognitive flexibility is firmly online by early adolescence, whereas working memory shows strongest effects by late adolescence (Luna et al., 2004, De Luca et al., 2003, Anderson et al., 2018). Developmental neuroscientists propose that the heterogeneous patterns of behavior observed throughout adolescence are subserved by the comparably heterogeneous patterns of brain development; this has direct implications for clinical treatment with adolescents.
A rich collection of functional neuroimaging studies in healthy adults compared with lesion patients reveal the centrality of prefrontal and parietal cortices in cognitive control and related cognitive capacities (Miller, 2000, Miller and Cohen, 2001, Knight, 1990). Structural prefrontal and parietal cortical development is typified by cortical thinning (i.e., pruning) in gray matter, along with linear increases in white matter. Marked regional variability is observed; medial and ventral prefrontal cortex (PFC) develop notably sooner than lateral and dorsal PFC (Giedd et al., 1999, Gogtay et al., 2004, Shaw et al., 2008, Tamnes et al., 2017).
Further, these patterns do not linearly associate with emerging cognitive skills. For example, cross-sectional studies of working memory and inhibitory control have, somewhat counterintuitively, shown both increases (Rubia et al., 2006, Rubia et al., 2013, Jolles et al., 2011) and decreases in activation (Velanova et al., 2008, Velanova et al., 2009) of prefrontal and parietal cortex with age, depending on task demands and the precise adolescent age tested. This mirrors the adolescent substance use neuroimaging literature, which has shown the same pattern of counterintuitive increases and decreases in activation (Feldstein Ewing et al., 2014).
One possibility is that age predicts stronger activation in a smaller, more specialized subset of prefrontal and parietal regions, and less reliance upon more diffuse, and less task-relevant regions (Durston et al., 2006, Guassi Moreira et al., 2018). Indeed, a growing number of longitudinal studies support “task-positive” activation increases, and in tandem, less task-irrelevant activation, throughout adolescence (Durston et al., 2006, Simmonds et al., 2017).
Of relevance to the treatment context, these disparate patterns of developmental timing may help explain why developing adolescent cognitive skills like working memory, which is supported by dlPFC (Nee et al., 2013, Moser et al., 2017, Wager and Smith, 2003), along with abstract thinking and reasoning, which rely upon the most rostral portions of dlPFC (Dumontheil, 2014), emerge a bit later in the cognitive skill cascade.
A challenge that stymies succinct characterization of adolescent cognitive development – and likely adolescent substance use treatment as well – is that the same adolescents often show markedly different cognitive skills when placed in different socioemotional contexts. Relative to adults, adolescent performance is more strongly impacted by affectively salient stimuli and by socioemotional contexts (see Section 4 below) (Gardner and Steinberg, 2005, Hare et al., 2008, Somerville et al., 2011, Cohen et al., 2016). This sensitivity to socioemotional contexts is mirrored by the relatively greater responsivity of subcortical structures (ventral striatum, amygdala) as compared with lateral prefrontal regions during this age period; relevant to the treatment context, these networks serve a crucial role in identifying and behaviorally responding to salient cues (Hare et al., 2008, Somerville et al., 2011, Chein et al., 2011, Cohen et al., 2016). Importantly, turning up the socioemotional dial also impacts functional coupling (i.e., connectivity) in nascent prefrontal-parietal (Cohen et al., 2016) and prefrontal-subcortical networks (Somerville et al., 2011). These adolescent-specific differences in circuit-level communication may be crucial for understanding how and why adolescents identify (and communicate about) emotions, particularly when emotions run hot (Casey, 2015).
Similarly, adolescents differ significantly from adults in their emerging skills in engaging cognitive strategies, including reappraisal and emotional distancing, to regulate emotions (Guassi Moreira and Silvers, 2018). A long history of behavioral research has shown that very young children can utilize cognitive regulatory strategies if provided the appropriate scaffolding, but not necessarily when left to their own devices (Moore et al., 1976, Mischel et al., 1989, Mischel and Mischel, 1983, Garnefski and Kraaij, 2006, Williams and McGillicuddy-De Lisi, 1999). Relevant to the treatment context, one’s ability to effectively engage cognitive strategies in the area of self-regulation improves linearly from childhood to adolescence, with performance plateauing at around age 17 (Silvers et al., 2012). The precise age at which adolescents achieve adult-like performance differs for social and non-social stimuli (Silvers et al., 2012), as well as for appetitive and aversive stimuli (Silvers et al., 2017, Silvers et al., 2014).
Adolescents show protracted development, particularly in the context of salient, negative emotions (particularly negative social emotions; see Section 4), and in turn, adolescence is typified by a period of greater experienced negative affect and elevated amygdala response relative to adults (Silvers et al., 2017, Silvers et al., 2012, Silvers et al., 2015). Compared to adolescents, adults show reduced concurrent and sustained amygdala responses to aversive stimuli, and these age-related reductions in amygdala response are mediated by the enhanced role of ventrolateral prefrontal cortex (vlPFC) (Silvers et al., 2017, Silvers et al., 2015). However, the interaction between vlPFC and amygdala responses requires relatively mature prefrontal-amygdala coupling, suggesting that neurobiological development serves as a relevant potential rate-limiting step in adolescents’ ability to navigate certain types of strong emotions, and engage planful behavior in the context of deep negative feelings (Silvers et al., 2017).
Another central hallmark of adolescent cognitive development is the emergence of abstract reasoning (e.g., “formal operations”). Of relevance to the treatment context, abstract reasoning enables an adolescent to generate and systematically evaluate hypotheses (e.g., Erickson et al., 2005) and engage in relational reasoning (Inhelder and Piaget, 1958), which involves the simultaneous consideration of interrelated dimensions and situations necessary to arrive at a conclusion (Osherson and Markman, 1975). Children can solve 0- and 1-relational level problems easily, but only adolescents with sufficient frontal lobe maturation have the capacity to solve 2-relational level problems, wherein two dimensions of variation are simultaneously considered (e.g., Christoff et al., 2001). As behavioral treatment often requires this type of simultaneous processing, the development of this cognitive skill may impact adolescents’ capacity to engage and successfully participate in treatment.
Together, the findings above suggest that adolescence is characterized by an array of novel cognitive skills highly relevant to the treatment context, including the emergent capacity to reason abstractly, to experience and react to negative emotions, and in turn, to develop and engage in purposeful and planful behavior.
(3). Sculpting out of self.
A core feature of adolescence is a heightened focus on the self, as adolescents increasingly consider who they are and what others might think of them (Pfeifer and Peake, 2012, Sebastian et al., 2008); this shift of focus contributes to the development of cognitive and socio-emotional processes crucial during adulthood, including self-awareness and its impact on increasingly complex decision-making (Blakemore and Robbins, 2012, Pfeifer and Peake, 2012). Increased self-awareness is integral to the formation of self-identity (Pfeifer and Berkman, 2018), including on the level of traits (e.g., academic versus social) (Pfeifer et al., 2013) and other dimensions of culture and identity (Telzer et al., 2013b).
Many developing cognitive processes hinge upon the capacity for meta-cognition, including the capacity to self-reflect sufficiently in order to effectively self-monitor. This may explain why social and cognitive processes appear to co-develop behaviorally and neurally (Blakemore and Choudhury, 2006, Sebastian et al., 2008, Pfeifer and Peake, 2012). Relative to children, in some of these dimensions, adolescents are more akin to adults in arenas of self-processing (Jankowski et al., 2014, Pfeifer et al., 2009, Debbané et al., 2017, Pfeifer et al., 2007, Pfeifer et al., 2013), subserved by the ventromedial prefrontal cortex (vmPFC) and the ventral striatum (VS) (Denny et al., 2012, Pfeifer and Berkman, 2018, Roy et al., 2012). However, highly relevant to the adolescent treatment context, the vmPFC is uniquely sensitive to evaluating the self-relevance of social information during adolescence (Pfeifer et al., 2013, Dégeilh et al., 2015), while the VS is central to “what other people think about you”, a characteristic cognitive feature heightened during adolescence (Jankowski et al., 2014).
While a heightened sense of self is normative during adolescence, the novel experience of self-reflection and self-monitoring has some unpleasant implications. Adolescents’ growing ability to consider what others think about them can be accompanied by natural elevations in attendant negative affect, including fears about social assessment and self-consciousness, both of which peak during adolescence (Rankin et al., 2004, Michiel Westenberg et al., 2004). The enhanced feelings of others’ scrutiny can be activated even during minimally evaluative circumstances – such as simply telling a study participant that a peer is watching them– and has been linked to adolescent-specific increases in medial prefrontal cortex (mPFC) recruitment and mPFC-VS connectivity (Somerville et al., 2013). Greater self-focus also has a darker side. It can lead to rumination, negative affect, and depression (Moberly and Watkins, 2008, Nolen-Hoeksema, 2000), and can contribute to adolescents’ sense that they need to engage in behaviors to improve their social standing and affect, including substance use (Cousijn et al., 2018, Caouette and Feldstein Ewing, 2017).
Together, relevant for the adolescent addiction treatment context, these data suggest that adolescent-emergence of self-focus is supported by functional changes in regions involved in self-cognition, and to a lesser extent – regions involved in cognitive and affective processing – and that these normative changes likely contribute to the characteristic pronounced self-focus experienced by most adolescents.
(4). Adolescents’ changing social landscape.
Developmental psychologists have long observed that adolescents’ growing sense of self-awareness occurs concurrently with substantive shifts in their social landscapes. Only recently, however, have developmental neuroscientists begun to probe the neural underpinnings that correspond to these social behavioral changes (Foulkes and Blakemore, 2018).
Adolescents’ growing sense of self-awareness shifts concurrently with their changing social landscapes. Indeed, adolescents’ desire to establish individuality is almost by necessity coupled with their transition away from their caregivers (Crone and Dahl, 2012). At the same time, adolescents’ increased self-consciousness reflects not only greater self-awareness, but also concern over how their peers perceive them. Social networks expand dramatically as adolescents spend an increasing time with friends and less with family (Wrzus et al., 2013, Larson and Richards, 1991). Beliefs about what constitutes normative behavior among peers (Knoll et al., 2015, Knoll et al., 2017), and being in the presence of peers (Gardner and Steinberg, 2005), impact adolescent decision-making around perceived risk during this period.
It is during this timeframe that most youth begin to make decisions about whether and when to engage in substance use, and these situations often arise in peer-based contexts (Shedler and Block, 1990, Winters, 1999). Despite the panoply of factors involved in this decision, the proportion of alcohol-using friends continues to be the best predictor of adolescents’ decision to engage in substance use (e.g., accounting for 50% of the variance; Chassin et al., 2004). Further, adolescents’ perception of peer substance use behavior has been directly related to both their current substance use, as well as their substance use progressions (e.g., substance use in the following year; D’Amico and McCarthy, 2006, Kilmer et al., 2006)
While neural correlates of peer acceptance and rejection have been evaluated (for a review, see Burnett et al., 2011), few studies have employed ecologically-valid paradigms to evaluate the influence of adolescents’ actual peers on their risk-taking behavior. Chein and colleagues found that adolescents (ages 14–18) (Chein et al., 2011), as contrasted with emerging adults and adults, made more risky driving decisions when they were aware that their friends were in the fMRI control room than when they conducted the task on their own (peer vs. alone). The authors also found greater BOLD response in VS and orbitofrontal cortex (OFC) in the peer versus the alone condition.
Overlapping with other neurodevelopmental theories (e.g., Casey et al., 2008, Ernst et al., 2005), In his 2008 model, Steinberg (e.g., Steinberg, 2008) posits the predominance of the social-emotional network (SEN) during adolescence. Critical nodes within the SEN include the dopaminergic (DA)-pathways of the OFC, nucleus accumbens (NAc), VS, and mPFC. These areas are particularly important for adolescents’ processing of social (peer) information (e.g., Steinberg, 2008, Guyer et al., 2009, Masten et al., 2009). The greater activation of these regions during the neurodevelopmental period of adolescence may be the result of substantive neurodevelopmental changes (including a redistribution of DA receptor density in the PFC, striatum, and NAc). These changes peak during adolescence, resulting in a relatively greater release of DA during this timeframe (e.g., Paus et al., 2008a). Practically, this means that risk taking behaviors, which are inherently exciting, frightening, and fun, may indeed feel much more rewarding during middle adolescence (e.g., Dahl, 2011, Galvan et al., 2007)
While it is clear that peers take on newfound significance during adolescence and that this shift has the potential to increase risk behaviors, an important body of work is beginning to reveal important caveats to this thesis. First, peer influence is also a powerful motivator for prosocial behavior during adolescence (van Hoorn et al., 2016, Foulkes et al., 2018). Relatedly, while neural reward circuits are linked to a variety of risk behaviors during adolescence, VS and vmPFC reactivity to social cues also portend positive, prosocial development (Telzer et al., 2013a) (Pfeifer et al., 2011). Second, while peers become increasingly important during adolescence, this does not render parents as unimportant. Despite spending less time with parents, connectedness with parents can attenuate the impact of the enhanced reward circuit responses typical during adolescence, serving as a protective force insulating adolescents against risk behavior, stress and even depression (Guassi Moreira and Telzer, 2018, Telzer et al., 2015, Guassi Moreira and Telzer, 2015, Doom et al., 2016). In fact, when compared with peers, parents show a significantly greater impact on adolescent decision-making (Welborn et al., 2016, Guassi Moreira et al., in press).
Understanding how adolescents navigate not only risk, but also prosocial peer interactions is one of the ultimate challenges for adolescent addiction treatment developers. We believe that this challenge is not insurmountable (Pfeifer et al., 2011, Paus et al., 2008b). Our task is to determine how best to channel youths’ drive and developmentally-unique cognitive systems to help them make more healthy choices.
Summary.
These four developmental domains interact dynamically throughout adolescence (Crone and Dahl, 2012), and are highly relevant to the adolescent addiction treatment context. For example, an adolescent’s environment can impact the nature and timing of puberty (e.g., family stress and parent conflict can accelerate onset) and vice versa; adolescents who look older may be treated differently than same-age adolescents who appear younger (Ellis and Garber, 2003). These pubertal changes can alter the social spheres that adolescents are introduced to and experience (e.g., more mature girls may find themselves in the company of young men, who are already more advanced in terms of substance use). Moreover, social experiences, in turn, shape adolescents’ cognitive opportunities and related development early (e.g., youth who initiate substances – such as alcohol, cannabis, vaping may be altering the capacity and nature of their brain growth) (Lisdahl et al., 2013). Considering the interplay of these factors is crucial for understanding adolescent development as well as cultivating impactful programs to prevent and treat adolescent substance use.
III. How well do existing treatments for adolescent substance use work?
Throughout the past 3 decades, adolescent addiction treatment has shown some degree of capacity to catalyze and sustain behavior change in adolescents, but overall, results have been underwhelming (Feldstein Ewing et al., 2016a). More specifically, despite several decades of efforts by experts to identify the best avenues to prevent and reduce adolescent substance use, few youth receive treatment (<6%) (SAMHSA, 2014). Of those who do, even when the treatment is grounded in evidence-based approaches and works well for adults, many youth do not show significant long-term changes in their substance use (Jensen et al., 2011, Tripodi et al., 2010), with 86% returning to use within a year of treatment (Winters et al., 2000).
As reviewed in Feldstein Ewing (2016a), this contrasts with the adult addiction literature, wherein a number of psychosocial interventions have much stronger impact in terms of instantiating and sustaining meaningful behavior change (Anton et al., 2006, Project Match Research Group, 1997). For example, meta-analyses examining the efficacy of motivational interviewing (MI) indicate that in the context of addiction treatment, MI’s effect sizes are notably less robust for adolescents (mean d = 0.17) (Jensen et al., 2011) as compared with their impact with adults (mean d = 0.77) (Hettema et al., 2005).
At issue is that most of the interventions clinicians use with adolescents are “borrowed” from adult clinical addiction research (Feldstein Ewing et al., 2016c). Yet the samples and populations utilized in large scale adult addiction studies, such as Projects COMBINE and MATCH (Anton et al., 2006, Project Match Research Group, 1997), included inherently different populations, such as adults who largely self-referred to treatment. As a result, there is a notable gap between the nature of adults from whom these treatments were derived, and the nature of adolescents that we are trying to implement the same interventions with (for more see (Feldstein Ewing et al., 2016a). Ultimately, better targeting with adolescent neurodevelopment in mind is likely to improve adolescent addiction treatment outcomes.
The poor generalizability of “adult” treatment to adolescents revolves around the significantly different conditions that make interactions within adolescent addiction treatment highly disparate from adults; within treatment sessions, adolescents face inherently different neurodevelopmental issues (Giedd, 2015), disparate sociodevelopmental concerns (Blakemore, 2018), are on a different addiction trajectory (Cousijn et al., 2018), and in turn, have different treatment outcome goals than adults (Feldstein Ewing et al., 2016a).
Here, we include a brief overview of the challenges facing adolescent addiction treatment and its reporting, and our recommendations for avenues to improve best practices for clinicians and clinical research in this critical area of adolescent addiction treatment development.
How can adolescent addiction treatment be improved?
(1). Absence of uniformly-agreed upon outcome in the adolescent addiction treatment literature.
The current status of the field renders it quite difficult, if not impossible, to compare adolescent treatment outcomes across different treatment approaches (e.g., Black and Chung, 2014). This is in contrast to the adult literature, wherein there are common, widely-agreed upon outcome metrics, such as percent days abstinent (PDA) or drinks per drinking day (DDD) (Anton et al., 2006, Project Match Research Group, 1997). In this brief examination, numerous different categories of outcome variables were reported. The most common included number of substance use days, substance-related consequences, and quantity of substance use.
This range of outcomes is likely to reflect a number of issues; one, as observed throughout the adolescent addiction treatment literature, there may simply be different targets for adolescent treatment response. More likely, this reflects that adolescents often show behavior change within one dimension of substance use (e.g., decrease in alcohol use days), while still retaining high scores on another (e.g., continued alcohol-related problem scores due to ongoing processing for an alcohol-related arrest that occurred prior to change in drinking) (McCambridge and Strang, 2004, Feldstein Ewing et al., 2013). Of greater concern to adolescent addiction practitioners, variance on outcomes may reflect reporting bias that favors treatment outcomes that withstood the test of statistical significance.
One avenue to improve the field may be to report on commonly-agreed upon adolescent treatment outcome measures (see Table 1) and do so regardless of statistical significance. We believe that this recommendation, to move toward a core outcome set in the field of adolescent addiction treatment, is highly important, and follows recent relevant initiatives, including the Scottish National Health System’s core outcome work (The.Scottish.Government, 2015), and the Core Outcome Measure in Effectiveness Trials (COMET) Initiative (Gargon et al., 2017, Williamson et al., 2017). Notably, while these examples serve as excellent models, they have thus far been largely implemented with adult, rather than adolescent, clinical research studies. This fact again highlights the need for identifying and rolling out jointly-agreed upon core outcome metrics for adolescents in addiction treatment.
Table 1:
Recommended methods for examining adolescent treatment response and related neural relevance
| Recommended methods for examining adolescent treatment response | Related neural relevance |
|---|---|
| (1) Work toward consensus for common adolescent treatment outcomes, such as: past month days of substance use | Allows a continuous measure of potential neurotoxic impact in the developing brain |
| (2) Examine pre-post change in interference in functioning, as assessed with empirically-validated “problems” measures | Adolescents may have non-symptomatic substance use; this is one way to disaggregate experimentation from more problem levels of use |
| (3) Conduct comparable follow-up windows of adolescent treatment outcomes such as: 3, 6, 9 and 12 months | Consistent and comparable outcome assessments on a neural level due to rapid neurodevelopment |
| (4) Evaluate and report outcomes across all types of adolescent substance use | Different substances may have different levels of impact and interaction on the developing brain |
| (5) Evaluate parent/family factors in adolescent substance use outcomes | We do not yet have a strong sense of how family interactions affect addiction processing in the developing brain; this is an important direction for future work |
| (6) Evaluate social (peer) relationships in adolescent substance use outcomes | Social (peer) factors are strongly implicated in the developing brain; this is an important direction for adolescent treatment response |
(2). Difficult to determine the degree of clinical relevance and impact in adolescent treatment response.
Additionally, even when effect sizes are significant, it is not clear the degree to which reported outcomes are clinically meaningful with adolescent addiction patients. For example, one less drinking day per month may achieve statistically significance, but not a meaningful clinical change in terms of adolescents’ overall health, social, cognitive, and academic outcomes. In the adult literature, clinical impact has been defined as a statistically significant reduction in initial rates or problem scores, or a halving of initial symptoms (Miller and Manuel, 2008).
As with many other forms of adolescent health risk behaviors (e.g., HIV risk behavior), a central measurement challenge is that adolescents engage in substance use sporadically and inconsistently (Clark, 2004). This makes treatment outcome measurement quite different from adults, whose use is often characterized by heavy, consistent patterns. For example, an adolescent may use alcohol very heavily (binge drink 3x/month over the summer), but then not drink at all during the initial months of the school year (Del Boca et al., 2004).
Another avenue to improve the field is to examine pre-to-post changes in interference in functioning for youth; this represents changes in the degree to which alcohol or other substance use disrupts interactions with peers, with family, with school/other academic, and/or other relevant work/extracurricular obligations. To this end, examining reductions in interference in functioning is likely a more meaningful metric (see Table 1). Examples of measures that can effectively access and assess this factor include the Rutgers Alcohol Problems Index (White and Labouvie, 1989) and the Marijuana Problems Index (Lisdahl et al., 2018).
(3). Wide variance on examined timing.
Adolescent addiction studies continue to reflect the importance, but notable absence, of longitudinal adolescent addiction treatment outcome studies (Larimer and Cronce, 2007). Further, there is substantial variance in the timing of reported outcomes; some studies include end-of-treatment only, while others report data in a variety of windows (e.g., 1 month, 6 weeks, 8 weeks, 3 months).
A third recommendation to improve the field includes examining behavior change, systematically (such as every 3 months; see Table 1) for at least the first year following treatment, to begin to generate comparable windows to examine youth change.
(4). Substance substitution?
While it is clear that adolescents tend to gravitate toward polysubstance, rather than mono-substance, use (Clark, 2004), many adolescent treatment studies do not report treatment outcomes for non-target substances of abuse. For example, many measured treatment outcomes in alcohol (only), cannabis (only), tobacco (only); some examine 2 substance categories, but include inconsistent pairings across each study (e.g., alcohol+cannabis; alcohol+tobacco). This is relevant, as many contemporary adolescent addiction treatment teams are trying to disaggregate whether or not youth are “swapping” out one substance for another, particularly in the changing cannabis and opioid landscapes (Choo et al., 2016, Feldstein Ewing et al., 2017, Dash et al., 2018).
Our fourth recommendation to the field is our encouragement to explicitly examine and report outcomes across all types of substance use, to ensure that we can disaggregate the differential impacts and interactions that each substance (and their intersection) might be having with the developing brain (e.g., Whelan et al., 2014, Karoly et al., 2015). This is likely to become an increasingly important issue in the field of addiction treatment as researchers move towards a precision medicine lens for understanding the genetic, lifestyle, psychological, social, and other bio-behavioral markers associated with treatment responsiveness (Volkow, 2018). Of course, it should be noted that this point applies equally to adult studies, and to psychosocial interventions for most kinds of behavior disorders. Sadly, adolescent treatment has continued to lag behind advances made in other age groups in the journey towards precision medicine (Feldstein Ewing et al., 2016c, Feldstein Ewing et al., 2017, Feldstein Ewing et al., 2016a, Bundy et al., 2018).
Summary.
Many adolescent addiction treatments appear to have clinically meaningful outcomes, but cross-treatment comparison and interpretation is not truly possible in the literature’s current state. At this time, inconsistent targets and timing obscure careful detection of comparative clinically-meaningful treatment gains (Black and Chung, 2014). In turn, it is currently quite difficult to access the driving mechanisms and their intersection with potential developmental cognitive factors, and true treatment success in this age group. In turn, we make these recommendations for the assessment of adolescent treatment outcomes, with examples of how each recommendation maps onto relevant neural targets (Table 1). It will be fascinating to continue to see if and how these developmental neuroscience findings translate to the clinic.
IV. Promising future directions for substance use treatment in this age group.
While the previous section indicates that existing treatments available to adolescents have had difficulty examining behavior change, it is our position that actively considering the nature of the developing adolescent brain can inform the revision and approach of interventions with this age group. In other words, the developing brain gives us an invaluable perspective regarding what might “work” better in this age group in terms of prevention/ intervention.
As summarized in Figure 1, we propose four key neurodevelopmental features of adolescence, and encourage approaching addiction interventions from this foundation as a promising first step in articulating prevention and intervention to the adolescent age group. Compellingly, in largely overlapping neural networks, those four features include: (1) Puberty; (2) Surge of cognitive skills (3) Sculpting out of self; and (4) Changing social landscape. Collectively, consideration of these factors, and their interplay, highlights several important themes to consider in developing novel clinical addiction approaches with this age group.
Figure 1:
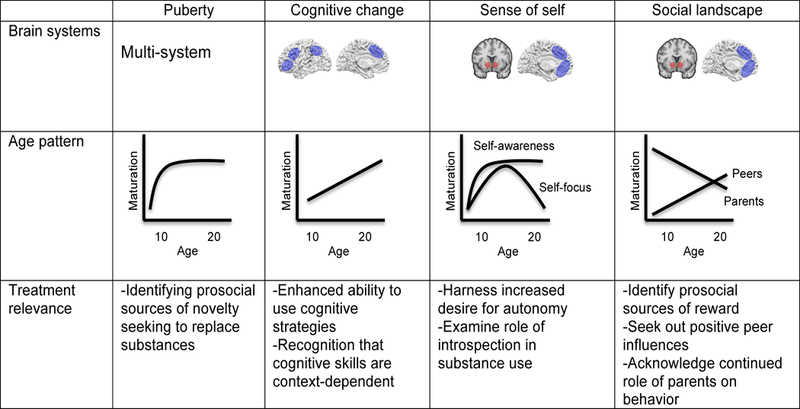
Summary of the tasks of adolescence. Brain systems: Blue designates cortical regions, red subcortical. Common social cognition networks are involved in changes in self-processing and navigating the novel social landscape. Age patterns: Adolescent-emergent, linear and adolescent-specific age patterns are depicted. Treatment relevance: Relationship between tasks and treatments are summarized.
Benefit of a prosocial perspective on prevention and intervention.
First, consistent with G. Stanley Hall’s “storm and stress” (Hall, 1904) perspective of adolescence, many existing adolescent-focused prevention and intervention approaches hinge on “problem-focused” perspectives in substance use and its resolution. However, this does not play to the nature of the adolescent brain, which is increasingly being recognized as evolving and adaptive (Giedd, 2015). As cited by Ellis (Ellis et al., 2017), integrating considerations of adolescent neurodevelopment would likely generate more positive treatment outcomes if we took a positive, adaptive-focused perspective that plays to and enhances adolescents’ existing strengths in resilience, natural penchant to cognitive flexibility, and socially-adaptive and prosocial growth. This is an arena that is gaining increasing traction in adolescent addiction contexts (Cousijn et al., 2018, Feldstein Ewing et al., 2018). Non-traditional, but potentially highly impactful examples here could include clinical approaches that engage adolescents in helping younger peers, pairing problem users up with more successful youth in the same age group, and engaging adolescents in avenues for more successful positive change in their peer and greater social communities (Yeager et al., 2017).
A relevant point in this examination is that while a handful of emerging studies are beginning to include prosocial, resilience-focused models of adolescent behavior (Yeager et al., 2017, Ellis et al., 2017, Cousijn et al., 2018, Feldstein Ewing et al., 2018, Foulkes et al., 2018), a careful synthesis of these models has not yet been created. This is a critical avenue for future work, and will likely require not only examination of quantitative, but also mixed-method, and qualitative research, as much of this emerging research is still in its inception and early stages of implementation.
Maximizing their drastically developing cognitive skills.
Adolescents are in the midst of experiencing a surge of new cognitive skills; at the most fundamental level, adolescents’ brains are organized toward and ready for adaptation (Giedd, 2015). Leagues ahead of the natural adaptive skills of adults who are often clunky at social modification, adolescents are primed to quickly, and often seamlessly, integrate novel information and behavioral adjustment that facilitates their capacity to rapidly adapt and adjust in numerous (different) novel and shifting social and community contexts (Giedd, 2015, Giedd, 2018). To this end, adolescents are arguably better prepared to modify and shift their behavior successfully than adults (Davidow et al., 2016), who are much less facile in adapting their behavior to be congruent in novel social contexts. Here, we believe that potentially novel clinical approaches, and particularly those that incorporate the role of social and family context in youth substance use behavior, are likely to show promise for catalyzing reductions in adolescent substance use. Examples of this include adolescents’ capacities to drastically reduce use when they are outside of risk environments (e.g., when adolescents with substance use disorders easily transition to zero use during time in experiential education programs). The greatest challenge here may be to identify how best to work with adolescents’ developing cognitive skills to translate potential reductions in use in one context (e.g., while on experiential education programs; while in treatment), to other social contexts (e.g., when they return from programs into social environments that may be populated by substance-using peers, or parents).
Harnessing drive for autonomy.
One aspect of addiction treatment that is largely overlooked in existing treatments is adolescents’ inherent drive for autonomy. The social psychology literature echoes adolescents’ strong desire for sculpting out their own place of standing (Yeager et al., 2017). Here, we believe that non-traditional approaches that allow adolescents to engage and utilize their voice for change, may give them the opportunity to channel this inherent drive toward positive contributions within their peer and sociocultural communities. Non-traditional approaches of promise include encouraging adolescents to engage as advocates for change in positive arenas of interest (e.g., social justice; political domains) (Yeager et al., 2017). Additionally, it is worthwhile to consider that while adolescents are developing their “voice”, they are developing meta-cognition, including how to self-reflect on how their choices and behaviors may impact others, from more immediate levels (friends, family) to broader spheres of communication (e.g., school, social media). Clinical efforts that work with adolescents to identify the lines of connection (e.g., between their developing self → the ideas that their developing self has → the behaviors in which that developing self engages → how those ideas/behaviors can instantiate change in the broader sociopolitical community) may help adolescents play an active role in their evolving sense of self.
Parents are not out of the picture yet.
The last consideration that we believe may be underdiscussed in current addiction approaches, that largely happens on a one-on-one treatment level in adult addiction treatment, is the role of the family in addiction treatment outcomes. While adolescents “look like” adults, by and large, many continue to live in family contexts throughout the adolescent years. Further, data continue to reflect that the developing brain is modified by parent factors (Telzer et al., 2013a) (Guassi Moreira and Telzer, 2018). Similarly, parent and family-based approaches still continue to show some of the largest successes in adolescent addiction treatment outcomes (Feldstein and Miller, 2006). Relevant to the shifting social contexts of adolescents, parents and families can represent one beneficial constant in a world of ever-evolving social landscapes. Thus, here, we encourage clinicians to incorporate parents and families whenever practical and possible to maximize positive development and change.
Summary and Future Directions.
This reviews offers one step toward understanding the nature of the developing adolescent brain, and how those neurodevelopmental data can inform next-step modifications or innovations to adolescent addiction treatment. This represents one critical foundational element in a much-larger cascade of health care approaches for this age group. In other words, in order to have adolescent addiction interventions generate maximal impact, they have to be effective, but they also have to be accessible to and specific for this age group. We suggest four key neurodevelopmental factors that we believe will enhance addiction treatment approaches (e.g., puberty, developing cognitive skills, sense of self, social landscape) and directly-linked clinical approaches that we believe will enhance treatment outcomes in this age group (e.g., prosocial perspectives, developing cognitive skills, drive for autonomy, and inclusion of parents/families). And, we encourage methods for examining adolescent treatment response, and highlight their related neural relevance, including: working towards consensus for common adolescent treatment outcomes (e.g., past month days of substance use); examining pre-post changes in interference in functioning, as assessed with empirically-validated “problems” measures; conducting comparable follow-up windows of adolescent treatment outcomes (e.g., 3, 6, 9, and 12 months); evaluating and reporting outcomes across all types of adolescent substance use; and evaluating parent/family factors in adolescent substance use outcomes. Already, at present, the field is moving very rapidly in the domain of methodological, statistical, and technological innovations, including rapid advances in predictive algorithms and machine learning (Casey et al., 2018). Our team represents one lab explicitly linking the integration of neuroimaging technology into the adolescent treatment context (Feldstein Ewing et al., 2016b), and similar advances in identifying and understanding variability are emerging in other adolescent treatment fields (e.g., eating disorders) (McAdams, 2017). We look forward to seeing innovations that stem from the integration of these neurodevelopmental perspectives in adolescent addiction treatment approaches and outcomes.
Acknowledgements:
This work was supported by NIH/NIAAA (1R01AA023658-01 and K24AA026876-01 PI: Feldstein Ewing; K23 AA025399, PI: Squeglia).
References
- ANDERSON VA, ANDERSON P, NORTHAM E, JACOBS R & CATROPPA C 2018. Development of Executive Functions Through Late Childhood and Adolescence in an Australian Sample. Developmental Neuropsychology, 20, 385–406. [DOI] [PubMed] [Google Scholar]
- ANTON RF, O’MALLEY SS, CIRAULO DA, CISLER RA, COUPER D, DONOVAN DM, GASTFRIEND DR, HOSKING JD, JOHNSON BA, LOCASTRO JS, LONGABAUGH R, MASON BJ, MATTSON ME, MILLER WR, PETTINATI HM, RANDALL CL, SWIFT R, WEISS RD, WILLIAMS LD, ZWEBEN A & COMBINE STUDY RESEARCH GROUP 2006. Combined pharmacotherapies and behavioral interventions for alcohol dependence, the COMBINE Study: A randomized control trial. JAMA: Journal of the American Medical Association, 295, 2003–2017. [DOI] [PubMed] [Google Scholar]
- BARENDSE MEA, SIMMONS JG, BYRNE ML, SEAL ML, PATTON GC, MUNDY L, WOOD SJ, OLSSON CA, ALLEN NB & WHITTLE S 2018. Brain structural connectivity during adrenarche: Associations between hormone levels and white matter microstructure. Psychoneuroendocrinology, 88, 70–77. [DOI] [PubMed] [Google Scholar]
- BLACK JJ & CHUNG T 2014. Mechanisms of change in adolescent substance use treatment: How does treatment work? Substance Abuse, 35, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAKEMORE S-J, BURNETT S & DAHL RE 2010. The role of puberty in the developing adolescent brain. Human Brain Mapping, 31, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAKEMORE SJ 2018. Avoiding social risk in adolescence. Current Directions in Psychological Science, 27, 116–122. [Google Scholar]
- BLAKEMORE SJ & CHOUDHURY S 2006. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry, 47, 296–312. [DOI] [PubMed] [Google Scholar]
- BLAKEMORE SJ & ROBBINS TW 2012. Decision-making in the adolescent brain. Nat Neurosci, 15, 1184–91. [DOI] [PubMed] [Google Scholar]
- BRAAMS BR, PEPER JS, VAN DER HEIDE D, PETERS S & CRONE EA 2016. Nucleus accumbens response to rewards and testosterone levels are related to alcohol use in adolescents and young adults. Developmental Cognitive Neuroscience, 17, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAAMS BR, VAN DUIJVENVOORDE ACK, PEPER JS & CRONE EA 2015. Longitudinal Changes in Adolescent Risk-Taking: A Comprehensive Study of Neural Responses to Rewards, Pubertal Development, and Risk-Taking Behavior. Journal of Neuroscience, 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN SA, BRUMBACK T, TOMLINSON K, CUMMINS K, THOMPSON WK, NAGEL BJ & AL., E. 2015. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs, 76, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUMBACK T, WORLEY M, NGUYEN-LOUIE TT, SQUEGLIA LM, JACOBUS J & TAPERT SF 2016. Neural predictors of alcohol use and psychopathology symptoms in adolescents. Dev Psychopathol, 28, 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUNDY DAP, DE SILVA N, HORTON S, PATTON GC, SCHULZ L, JAMINSON DT, DISEASE.CONTROL.PRIORITIES-3.CHILD.AND.ADOLESCENT.HEALTH & DEVELOPMENT.AUTHORS.GROUP 2018. Investment in child and adolescent health and development: key messages from Disease Control Priorities, 3rd Edition Lancet, 391, 687–699. [DOI] [PubMed] [Google Scholar]
- BURNETT S, SEBASTIAN C, COHEN KADOSH K & BLAKEMORE S-J 2011. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews, 35, 1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYRNE ML, WHITTLE S, VIJAYAKUMAR N, DENNISON M, SIMMONS JG & ALLEN NB 2017. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev Cogn Neurosci, 25, 12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAOUETTE J & FELDSTEIN EWING SW 2017. Four mechanistic models of peer influence on adolescent cannabis use. Current Addiction Reports [DOI] [PMC free article] [PubMed]
- CASEY BJ 2015. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol, 66, 295–319. [DOI] [PubMed] [Google Scholar]
- CASEY BJ, CANNONIER T, CONELY MI, COHEN AO, BARCH DM, HEITZIG MM, SOULES ME, TESLOVICH T, DELLARCO DV, GARAVAN H, ORR CA, WAGER TD, BANICH MT, SPEER NK, SUTHERLAND MT, RIEDEL MC, DICK AA, BJORK JM, THOMAS KM, CHAARANI B, MEJIA MH, HAGLER DJJ, M., D. C., SICAT CS, HARMS MP, DOSENBACH NUF, ROSENBERG M, EARL E, BARTSCH H, WATTS R, POLEMENI JR, KUPERMAN JM, FAIR DA, DALE AM & ABCD.IMAGING.ACQUISITION.WORKGROUP 2018. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci [DOI] [PMC free article] [PubMed]
- CASEY BJ, GETZ S & GALVAN A 2008. The Adolescent Brain. Developmental Review, 28, 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC 2016. Youth Risk Behavior Surveillance System (YRBSS) In: HEALTH, A. A. S. (ed.). Centers for Disease Control and Prevention. [Google Scholar]
- CERDA M, SARVET A, WALL M, FENG T, KEYES KM, GALEA S & HASIN DS 2018. Medical marijuana laws and adolescent use of marijuana and other substances: Alcohol, cigarettes, prescription drugs, adn other illicit drugs. Drug Alcohol Depend, 183, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERDA M, WALL M, FENG T, KEYES KM, SARVET A, SCHULENBERG JE, O’MALLEY PM, PACULA RL, GALEA S & HASIN DS 2017. Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatrics, 171, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHASSIN L, HUSSONG A, BARRERA M, MOLINA B, TRIM R & RITTER J 2004. Adolescent substance use. In: LERNER R & STEINBERG L (eds.) Handbook of adolescent psychology 2nd ed. New York: Wiley. [Google Scholar]
- CHEIN J, ALBERT D, O’BRIEN L, UCKERT K & STEINBERG L 2011. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev Sci, 14, F1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOO E, FELDSTEIN EWING SW & LOVEJOY T 2016. Opiates out, cannabis in: Negotiating the unknowns in patient care for chronic pain. JAMA, 316, 1763–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTOFF K, PRABHAKARAN V, DORFMAN J, ZHAO Z, KROGER JK, HOLYOAK KJ & GABRIELI JDE 2001. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage, 14, 1136–1149. [DOI] [PubMed] [Google Scholar]
- CLARK DB 2004. The natural history of adolescent alcohol use disorders. Addiction, 99, 5–22. [DOI] [PubMed] [Google Scholar]
- COHEN AO, BREINER K, STEINBERG L, BONNIE RJ, SCOTT ES, TAYLOR-THOMPSON K, RUDOLPH MD, CHEIN J, RICHESON JA, HELLER AS, SILVERMAN MR, DELLARCO DV, FAIR DA, GALVÁN A & CASEY BJ 2016. When Is an Adolescent an Adult? Assessing Cognitive Control in Emotional and Nonemotional Contexts. Psychological Science, 27, 549–562. [DOI] [PubMed] [Google Scholar]
- COUSIJN J, LUIJTEN M & FELDSTEIN EWING SW 2018. Adolescent resilience to addiction: a social plasticity hypothesis. The Lancet Child and Adolescent Health, 2, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONE EA & DAHL RE 2012. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Review Neuroscience, 13, 636–650. [DOI] [PubMed] [Google Scholar]
- D’AMICO EJ & MCCARTHY DM 2006. Escalation and initiation of younger adolescents’ substance use: The impact of perceived peer use. Journal of Adolescent Health, 39, 481–487. [DOI] [PubMed] [Google Scholar]
- DAHL R 2011. Understanding the Risky Business of Adolescence. Neuron, 69. [DOI] [PubMed] [Google Scholar]
- DASH GF, WILSON AC, MORASCO BJ & FELDSTEIN EWING SW 2018. A model of the intersection of pain and opioid misuse in children and adolescence. Clinical Psychological Science [DOI] [PMC free article] [PubMed]
- DAVIDOW JY, FOERDE K, GALVAN A & SHOHAMY D 2016. An Upside to Reward Sensitivity: The Hippocampus Supports Enhanced Reinforcement Learning in Adolescence. Neuron, 92, 93–99. [DOI] [PubMed] [Google Scholar]
- DAVIDOW JY, INSEL C & SOMERVILLE LH 2018. Adolescent Development of Value-Guided Goal Pursuit. Trends in Cognitive Sciences [DOI] [PubMed]
- DE LUCA CR, WOOD SJ, ANDERSON V, BUCHANAN JA, PROFFITT TM, MAHONY K & PANTELIS C 2003. Normative data from the CANTAB. I: development of executive function over the lifespan. J Clin Exp Neuropsychol, 25, 242–54. [DOI] [PubMed] [Google Scholar]
- DEBBANÉ M, BADOUD D, SANDER D, ELIEZ S, LUYTEN P & VRTIČKA P 2017. Brain activity underlying negative self- and other-perception in adolescents: The role of attachment-derived self-representations. Cognitive, Affective, & Behavioral Neuroscience, 17, 554–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DÉGEILH F, GUILLERY-GIRARD B, DAYAN J, GAUBERT M, CHÉTELAT G, EGLER P-J, BALEYTE J-M, EUSTACHE F & VIARD A 2015. Neural Correlates of Self and Its Interaction With Memory in Healthy Adolescents. Child Development, 86, 1966–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL BOCA FK, DARKES J, GREENBAUM PE & GOLDMAN MS 2004. Up close and personal: Temporal variability in the drinking of individual college students during their first year. Journal of Consulting and Clinical Psychology, 72, 155–164. [DOI] [PubMed] [Google Scholar]
- DENNY BT, KOBER H, WAGER TD & OCHSNER KN 2012. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci, 24, 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOOM JR, DOYLE CM & GUNNAR MR 2016. Social stress buffering by friends in childhood and adolescence: Effects on HPA and oxytocin activity. Social Neuroscience, 12, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAGANSKI B, KOOLSCHIJN PCMP, PEPER JS & CRONE EA 2014. The Influence of Sex Steroids on Structural Brain Maturation in Adolescence. PLoS ONE, 9, e83929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONTHEIL I 2014. Development of abstract thinking during childhood and adolescence: The role of rostrolateral prefrontal cortex. Dev Cogn Neurosci, 10, 57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURSTON S, DAVIDSON MC, TOTTENHAM N, GALVAN A, SPICER J, FOSSELLA JA & CASEY BJ 2006. A shift from diffuse to focal cortical activity with development. Dev Sci, 9, 1–8. [DOI] [PubMed] [Google Scholar]
- DWYER-LINDGREN L, BERTOZZI-VILLA A, STUBBS RW, MOROZOFF C, SHIRUDE S, UNUTZER J, NAGHAVI M, MOKDAD AH & MURRAY CJL 2018. Trends and Patterns of Geographic Variation in Mortality From Substance Use Disorders and Intentional Injuries Among US Counties, 1980–2014. JAMA, 319, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS BJ, BIANCHI J-M, GRISKEVICIUS V & FRANKENHUIS WE 2017. Beyond risk and protective factors: An adaptation-based approach to resilience. Perspectives on Psychological Science, 12, 561–587. [DOI] [PubMed] [Google Scholar]
- ELLIS BJ & GARBER J 2003. Psychosocial Antecedents of Variation in Girls’ Pubertal Timing: Maternal Depression, Stepfather Presence, and Marital and Family Stress. Child Dev, 71, 485–501. [DOI] [PubMed] [Google Scholar]
- ERICKSON SJ, GERSTLE M & FELDSTEIN SW 2005. Brief interventions and motivational interviewing with children, adolescents, and their parents in pediatric health care settings: A review Archives of Pediatrics and Adolescent Medicine, 159, 1173–1180. [DOI] [PubMed] [Google Scholar]
- ERNST M, PINE DS & HARDIN M 2005. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 35, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDSTEIN EWING SW, BLAKEMORE S-J & SAKHARDANDE A 2014. The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. Neuroimage: Clinical [DOI] [PMC free article] [PubMed]
- FELDSTEIN EWING SW, BRYAN AD, ALICANTE T, KORTHUIS PT, HUDSON KA & LOVEJOY T 2018. Three integrated elements of empowerment: HIV prevention with sub-Saharan African adolescents involved in transactional sex. Clinical Practice in Pediatric Psychology
- FELDSTEIN EWING SW, GAUME J & APODACA TR 2016a. Ambivalence: Prerequisite for success in motivational interviewing with adolescents? Addiction [DOI] [PMC free article] [PubMed]
- FELDSTEIN EWING SW, HOUCK JM, YEZHUVATH U, SHOKRI-KOJORI E, TRUITT D & FILBEY FM 2016b. The impact of therapists’ words on the adolescent brain: In the context of addiction treatment. Behav Brain Res, 15, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDSTEIN EWING SW, LOVEJOY T & CHOO E 2017. How has legal recreational cannabis impacted adolescents in your state? A window of opportunity. Am J Public Health, 107, 246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDSTEIN EWING SW, MCEACHERN AD, YEZHUVATH U, BRYAN AD, HUTCHISON KE & FILBEY FM 2013. Integrating brain and behavior: Evaluating adolescents’ response to a cannabis intervention. Psychology of Addictive Behaviors, 27, 510–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDSTEIN EWING SW, TAPERT SF & MOLINA BS 2016c. Uniting adolescent neuroimaging and treatment research: Recommendations in pursuit of improved integration. Neurosci Biobehav Rev, 62, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDSTEIN SW & MILLER WR 2006. Substance abuse and risk-taking among adolescents. Journal of Mental Health, 15, 1–11. [Google Scholar]
- FOULKES L & BLAKEMORE SJ 2018. Studying individual differences in human adolescent brain development. Nat Neurosci, 21, 315–323. [DOI] [PubMed] [Google Scholar]
- FOULKES L, LEUNG JT, FUHRMANN D, KNOLL LJ & BLAKEMORE SJ 2018. Age differences in the prosocial influence effect. Dev Sci, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALVAN A, HARE T, VOSS H, GLOVER G & CASEY BJ 2007. Risk-Taking and the Adolescent Brain: Who is at Risk? Developmental Science, 10, F8–F14. [DOI] [PubMed] [Google Scholar]
- GARDNER M & STEINBERG L 2005. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Dev Psychol, 41, 625–35. [DOI] [PubMed] [Google Scholar]
- GARGON E, WILLIAMSON PR, ALTMAN DG, BLAZEBY JM, TUNIS S & CLARKE M 2017. The COMET Initiative database: progress and activities update (2015). Trials, 18, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARNEFSKI N & KRAAIJ V 2006. Relationships between cognitive emotion regulation strategies and depressive symptoms: A comparative study of five specific samples. Personality and Individual Differences, 40, 1659–1669. [Google Scholar]
- GIEDD JN 2015. The amazing teen brain. Sci Am, 312, 32–37. [DOI] [PubMed] [Google Scholar]
- GIEDD JN 2018. A ripe time for adolescent research. Journal of Research on Adolescence 28, 157–159. [DOI] [PubMed] [Google Scholar]
- GIEDD JN, BLUMENTHAL J, JEFFRIES NO, CASTELLANOS FX, LIU H, ZIJDENBOS A, PAUS T, EVANS AC & RAPOPORT JL 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci, 2, 861–3. [DOI] [PubMed] [Google Scholar]
- GODDINGS A-L, MILLS KL, CLASEN LS, GIEDD JN, VINER RM & BLAKEMORE S-J 2014. The influence of puberty on subcortical brain development. NeuroImage, 88, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOGTAY N, GIEDD JN, LUSK L, HAYASHI KM, GREENSTEIN D, VAITUZIS AC, NUGENT TF 3RD, HERMAN DH, CLASEN LS, TOGA AW, RAPOPORT JL & THOMPSON PM 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A, 101, 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUASSI MOREIRA JF, MCLAUGHLIN KA & SILVERS JA 2018. GUASSI MOREIRA JF & SILVERS JA 2018 In due time: Neurodevelopmental considerations in the study of emotion regulation. In: COLE PM & HOLLENSTEIN T (eds.) Emotion Regulation: Development Across the Life Span Burlington, MA: Taylor & Francis. [Google Scholar]
- GUASSI MOREIRA JF, TASHJIAN SM, GALVAN A & SILVERS JA in press. Parents versus peers: Assessing the impact of social agents on decision making in young adults. Psychological Science [DOI] [PubMed]
- GUASSI MOREIRA JF & TELZER EH 2015. Changes in family cohesion and links to depression during the college transition. Journal of Adolescence, 43, 72–82. [DOI] [PubMed] [Google Scholar]
- GUASSI MOREIRA JF & TELZER EH 2018. Mother still knows best: Maternal influence uniquely modulates adolescent reward sensitivity during risk taking. Developmental Science, 21, e12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUYER AE, MCCLURE-TONE EB, SHIFFRIN ND, PINE DS & NELSON EE 2009. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development, 80, 1000–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL GS 1904. Adolescence: Its psychology and relations to physiology, anthropology, sociology, sex, crime, religion, and education (Vols I & II), New York, D. Appleton & Co. [Google Scholar]
- HARE TA, TOTTENHAM N, GALVAN A, VOSS HU, GLOVER GH & CASEY BJ 2008. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry, 63, 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASIN DS, WALL M, KEYES KM, CERDA M, SCHULENBERG JE, O’MALLEY PM, GALEA S, PACULA RL & FENG T 2015. Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: Results from annual, repeated cross-sectional surveys. The Lancet Psychiatry, 2, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMAN-GIDDENS ME, WANG L & KOCH G 2001. Secondary sexual characteristics in boys: estimates from the national health and nutrition examination survey III, 1988–1994. Arch Pediatr Adolesc Med, 155, 1022–8. [DOI] [PubMed] [Google Scholar]
- HERTING MM, GAUTAM P, SPIELBERG JM, KAN E, DAHL RE & SOWELL ER 2014. The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Human Brain Mapping, 35, 5633–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HETTEMA J, STEELE J & MILLER WR 2005. Motivational Interviewing. Annual Review of Clinical Psychology, 1, 91–111. [DOI] [PubMed] [Google Scholar]
- INGRAHAM C. Following marijuana legalization, teen drug use is down in Colorado. The Washington Post. 2017.
- INHELDER B & PIAGET J 1958. The growth of logical thinking from childhood to adolescence, New York, NY, Basic Books. [Google Scholar]
- JANKOWSKI KF, MOORE WE, MERCHANT JS, KAHN LE & PFEIFER JH 2014. But do you think I’m cool? Developmental Cognitive Neuroscience, 8, 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN CD, CUSHING CC, AYLWARD BS, CRAIG JT, SORELL DM & STEELE RG 2011. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: A meta-analytic review. Journal of Consulting and Clinical Psychology, 79, 433–440. [DOI] [PubMed] [Google Scholar]
- JERNIGAN TL, BROWN SA & ABCD.CONSORTIUM.COORDINATORS 2018. Introduction. Dev Cogn Neurosci [DOI] [PMC free article] [PubMed]
- JOHNSTON LD, MIECH RA, O’MALLEY PM, BACHMAN JG, SCHULENBERG JE & PATRICK ME 2018. Monitoring the Future national survey results on drug use: 1975–2017: Overview, key findings on adolescent drug use, Ann Arbor, Michigan, University of Michigan. [Google Scholar]
- JOLLES DD, KLEIBEUKER SW, ROMBOUTS SARB & CRONE EA 2011. Developmental differences in prefrontal activation during working memory maintenance and manipulation for different memory loads. Developmental Science, 14, 713–724. [DOI] [PubMed] [Google Scholar]
- KAROLY H, BRYAN AD, WEILAND BJ, MAYER AR, DODD AB & FELDSTEIN EWING SW 2015. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci [DOI] [PMC free article] [PubMed]
- KILMER JR, WALKER DD, LEE CM, PALMER RS, MALLETT KA, FABIANO P & LARIMER ME 2006. Misperceptions of college student marijuana use: Implications for prevention. Journal of Studies on Alcohol, 67, 277–281. [DOI] [PubMed] [Google Scholar]
- KNIGHT RT 1990. Neural mechanisms of event related potentials: evidence from human lesion studies. In: ROHRBAUGH JW, PARASURAMAN R & JOHNSON R (eds.) Event-related brain potentials: basic issues and applications New York: Oxford University Press. [Google Scholar]
- KNOLL LJ, LEUNG JT, FOULKES L & BLAKEMORE S-J 2017. Age-related differences in social influence on risk perception depend on the direction of influence. Journal of Adolescence, 60, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOLL LJ, MAGIS-WEINBERG L, SPEEKENBRINK M & BLAKEMORE S-J 2015. Social Influence on Risk Perception During Adolescence. Psychological Science, 26, 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARIMER ME & CRONCE JM 2007. Identification, prevention, and treatment revisited: Individual-focused college drinking prevention strategies 1999–2006. Addictive Behaviors, 32, 2439–2468. [DOI] [PubMed] [Google Scholar]
- LARSON R & RICHARDS MH 1991. Daily companionship in late childhood and early adolescence: changing developmental contexts. Child Dev, 62, 284–300. [DOI] [PubMed] [Google Scholar]
- LISDAHL KM, GILBART ER, WRIGHT NE & SHOLLENBARGER S 2013. Dare to Delay? The Impacts of Adolescent Alcohol and Marijuana Use Onset on Cognition, Brain Structure, and Function. Front Psychiatry, 4, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISDAHL KM, SHER KJ, CONWAY KP, GONZALEZ R, FELDSTEIN EWING SW, NIXON SJ, TAPERT SF, BARTSCH H, GOLDSTEIN RZ & HEITZIG MM 2018. Adolescent brain cognitive development (ABCD) study: Overview of substance use assessment methods. Dev Cogn Neurosci [DOI] [PMC free article] [PubMed]
- LODRUP CARLSEN KC, SKJERVEN HO & CARLSEN KH 2018. The toxicity of E-cigarettes and children’s respiratory health. Paediatric Respiratory Reviews [DOI] [PubMed]
- LUNA B, GARVER KE, URBAN TA, LAZAR NA & SWEENEY JA 2004. Maturation of Cognitive Processes From Late Childhood to Adulthood. Child Development, 75, 1357–1372. [DOI] [PubMed] [Google Scholar]
- MARCEAU K, KIRISCI L & TARTER RE 2018. Correspondence of Pubertal Neuroendocrine and Tanner Stage Changes in Boys and Associations With Substance Use. Child Development [DOI] [PMC free article] [PubMed]
- MASTEN CL, EISENBERG NI, BOROFSKY LA, PFEIFER JH, MCNEALY K, MAZZIOTTA JC & DAPRETTO M 2009. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Soc Cogn Affect Neurosci, 4, 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCADAMS CJ 2017. Changing the brain to treat anorexia nervosa. The Lancet Psychiatry, 4, 262–263. [DOI] [PubMed] [Google Scholar]
- MCCAMBRIDGE J & STRANG J 2004. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: Results from a multi-site cluster randomized trial. Addiction, 99, 39–52. [DOI] [PubMed] [Google Scholar]
- MICHIEL WESTENBERG P, DREWES MJ, GOEDHART AW, SIEBELINK BM & TREFFERS PDA 2004. A developmental analysis of self-reported fears in late childhood through mid-adolescence: social-evaluative fears on the rise? Journal of Child Psychology and Psychiatry, 45, 481–495. [DOI] [PubMed] [Google Scholar]
- MILLER EK 2000. The prefrontal cortex and cognitive control. Nat Rev Neurosci, 1, 59–65. [DOI] [PubMed] [Google Scholar]
- MILLER EK & COHEN JD 2001. An integrative theory of prefrontal cortex function. Annu Review Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- MILLER WR & MANUEL JK 2008. How large must a treatment effect be before it matters to practitioners? An estimation method and demonstration. Drug and Alcohol Review, 27, 524–528. [DOI] [PubMed] [Google Scholar]
- MISCHEL HN & MISCHEL W 1983. The development of children’s knowledge of self-control strategies. Child Dev, 54, 603–619. [Google Scholar]
- MISCHEL W, SHODA Y & RODRIGUEZ MI 1989. Delay of gratification in children. Science, 244, 933–8. [DOI] [PubMed] [Google Scholar]
- MOBERLY NJ & WATKINS ER 2008. Ruminative self-focus and negative affect: An experience sampling study. Journal of Abnormal Psychology, 117, 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOKDAD AH, FOROUZANFAR MH, DAOUD F, MOKDAD AA, EL BCHERAOUI C, MORADI-LAKEH M & AL., E. 2016. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet, 387, 2383–2401. [DOI] [PubMed] [Google Scholar]
- MOORE B, MISCHEL W & ZEISS A 1976. Comparative effects of the reward stimulus and its cognitive representation in voluntary delay. Journal of Personality and Social Psychology, 34, 419–424. [Google Scholar]
- MOSER DA, DOUCET GE, ING A, DIMA D, SCHUMANN G, BILDER RM & FRANGOU S 2017. An integrated brain–behavior model for working memory. Molecular Psychiatry [DOI] [PMC free article] [PubMed]
- NATIONAL.ACADEMY.OF.SCIENCES 2017. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research National Academies Press. [PubMed] [Google Scholar]
- NEE DE, BROWN JW, ASKREN MK, BERMAN MG, DEMIRALP E, KRAWITZ A & JONIDES J 2013. A Meta-analysis of Executive Components of Working Memory. Cerebral Cortex, 23, 264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON EE, JARCHO JM & GUYER AE 2016. Social re-orientation and brain development: an expanded and updated review. Dev Cogn Neurosci, 17, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN TV, MCCRACKEN JT, DUCHARME S, CROPP BF, BOTTERON KN, EVANS AC & KARAMA S 2013. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J Neurosci, 33, 10840–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN TV, WU M, LEW J, ALBAUGH MD, BOTTERON KN, HUDZIAK JJ, FONOV VS, COLLINS DL, CAMPBELL BC, BOOIJ L, HERBA C, MONNIER P, DUCHARME S & MCCRACKEN JT 2017. Dehydroepiandrosterone impacts working memory by shaping cortico-hippocampal structural covariance during development. Psychoneuroendocrinology, 86, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLEN-HOEKSEMA S 2000. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol, 109, 504–11. [PubMed] [Google Scholar]
- OP DE MACKS ZA, MOOR BG, OVERGAAUW S, GÜROĞLU B, DAHL RE & CRONE EA 2011. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Developmental Cognitive Neuroscience, 1, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHERSON D & MARKMAN E 1975. Language and the ability to evaluate contradictions and tautologies. Cognition, 3, 213–226. [Google Scholar]
- PAUS T, KESHAVAN M & GIEDD JN 2008a. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience, 9, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUS T, TORO R, LEONARD G, LERNER JV, LERNER RM, PERRON M, PIKE BG, RICHER L & STEINBERG L 2008b. Morphological properties of the action-observation cortical network in adolescents with low and high resistance to peer influence. Social Neuroscience, 3, 303–316. [DOI] [PubMed] [Google Scholar]
- PFEIFER JH & BERKMAN ET 2018. The Development of Self and Identity in Adolescence: Neural Evidence and Implications for a Value-Based Choice Perspective on Motivated Behavior. Child Development Perspectives [DOI] [PMC free article] [PubMed]
- PFEIFER JH, KAHN LE, MERCHANT JS, PEAKE SJ, VEROUDE K, MASTEN CL, LIEBERMAN MD, MAZZIOTTA JC & DAPRETTO M 2013. Longitudinal Change in the Neural Bases of Adolescent Social Self-Evaluations: Effects of Age and Pubertal Development. Journal of Neuroscience, 33, 7415–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEIFER JH, LIEBERMAN MD & DAPRETTO M 2007. “I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. J Cogn Neurosci, 19, 1323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEIFER JH, MASTEN CL, BOROFSKY LA, DAPRETTO M, FULIGNI AJ & LIEBERMAN MD 2009. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev, 80, 1016–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEIFER JH, MASTEN CL, MOORE WE III, OSWALD TM, MAZZIOTTA JC, LACOBONI M & DAPRETTO M 2011. Entering Adolescence: Resistance to Peer Influence, Risky Behavior, and Neural Changes in Emotion Reactivity. Neuron, 69, 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEIFER JH & PEAKE SJ 2012. Self-development: Integrating cognitive, socioemotional, and neuroimaging perspectives. Developmental Cognitive Neuroscience, 2, 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROJECT MATCH RESEARCH GROUP 1997. Matching alcoholism treatments to client heterogeneity: Project MATCH Posttreatment drinking outcomes. Journal of Studies on Alcohol, 58, 7–29. [PubMed] [Google Scholar]
- RANKIN JL, LANE DJ, GIBBONS FX & GERRARD M 2004. Adolescent Self-Consciousness: Longitudinal Age Changes and Gender Differences in Two Cohorts. Journal of Research on Adolescence, 14, 1–21. [Google Scholar]
- ROY M, SHOHAMY D & WAGER TD 2012. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci, 16, 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIA K, LIM L, ECKER C, HALARI R, GIAMPIETRO V, SIMMONS A, BRAMMER M & SMITH A 2013. Effects of age and gender on neural networks of motor response inhibition: From adolescence to mid-adulthood. NeuroImage, 83, 690–703. [DOI] [PubMed] [Google Scholar]
- RUBIA K, SMITH AB, WOOLLEY J, NOSARTI C, HEYMAN I, TAYLOR E & BRAMMER M 2006. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp, 27, 973–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings NSDUH Series H-48 Rockville, MD. [Google Scholar]
- SCHMIDT LA, JACOBS LM & SPETZ J 2016. Young People’s More Permissive Views About Marijuana: Local Impact of State Laws or National Trend? Am J Public Health, 106, 1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEBASTIAN C, BURNETT S & BLAKEMORE SJ 2008. Development of the self-concept during adolescence. Trends Cogn Sci, 12, 441–6. [DOI] [PubMed] [Google Scholar]
- SHAW P, KABANI NJ, LERCH JP, ECKSTRAND K, LENROOT R, GOGTAY N, GREENSTEIN D, CLASEN L, EVANS A, RAPOPORT JL, GIEDD JN & WISE SP 2008. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci, 28, 3586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEDLER J & BLOCK J 1990. Adolescent drug use and psychological health: A longitudinal inquiry. American Psychologist, 45, 612–630. [DOI] [PubMed] [Google Scholar]
- SILVERS JA, INSEL C, POWERS A, FRANZ P, HELION C, MARTIN RE, WEBER J, MISCHEL W, CASEY BJ & OCHSNER KN 2017. vlPFC–vmPFC–Amygdala Interactions Underlie Age-Related Differences in Cognitive Regulation of Emotion. Cerebral Cortex, 27, 3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERS JA, INSEL C, POWERS A, FRANZ P, WEBER J, MISCHEL W, CASEY BJ & OCHSNER KN 2014. Curbing craving: Behavioral and brain evidence that children regulate craving when instructed to do so but have higher baseline craving than adults. Psychological Science, 25, 1932–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERS JA, MCRAE K, GABRIELI JD, GROSS JJ, REMY KA & OCHSNER KN 2012. Age-Related Differences in Emotional Reactivity, Regulation, and Rejection Sensitivity in Adolescence. Emotion [DOI] [PMC free article] [PubMed]
- SILVERS JA, SHU J, HUBBARD AD, WEBER J & OCHSNER KN 2015. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Developmental Science, 18, 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMMONDS DJ, HALLQUIST MN & LUNA B 2017. Protracted development of executive and mnemonic brain systems underlying working memory in adolescence: A longitudinal fMRI study. NeuroImage, 157, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMMONS JG, WHITTLE S, PATTON GC, DUDGEON P, OLSSON CA, BYRNE ML, MUNDY LK, SEAL ML & ALLEN NB 2014. Study protocol: imaging brain development in the Childhood to Adolescence Transition Study (iCATS). BMC Pediatr, 14, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMERVILLE LH, HARE T & CASEY BJ 2011. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci, 23, 2123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMERVILLE LH, JONES RM, RUBERRY EJ, DYKE JP, GLOVER G & CASEY BJ 2013. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol Sci, 24, 1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG L 2008. A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28, 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMNES CK, HERTING MM, GODDINGS A-L, MEUWESE R, BLAKEMORE S-J, DAHL RE, GÜROĞLU B, RAZNAHAN A, SOWELL ER, CRONE EA & MILLS KL 2017. Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. The Journal of Neuroscience, 37, 3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TELZER EH, FULIGNI AJ, LIEBERMAN MD & GALVÁN A 2013a. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Developmental Cognitive Neuroscience, 3, 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TELZER EH, HUMPHREYS KL, SHAPIRO M & TOTTENHAM N 2013b. Amygdala Sensitivity to Race Is Not Present in Childhood but Emerges over Adolescence. Journal of Cognitive Neuroscience, 25, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TELZER EH, ICHIEN NT & QU Y 2015. Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social Cognitive and Affective Neuroscience, 10, 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THE.SCOTTISH.GOVERNMENT 2015. National Health and Wellbeing Outcomes: A framework for improving the planning and delivery of integrated health and social care services In: HEALTH AND SOCIAL CARE INTEGRATION, P. B. J. W. S. A. (ed.). [Google Scholar]
- TRIPODI SJ, BENDER K, LITSCHGE C & VAUGHN MG 2010. Interventions for reducing adolescent alcohol abuse: A meta-analytic review. Archives of Pediatrics and Adolescent Medicine, 164, 85–91. [DOI] [PubMed] [Google Scholar]
- VAN HOORN J, VAN DIJK E, MEUWESE R, RIEFFE C & CRONE EA 2016. Peer Influence on Prosocial Behavior in Adolescence. Journal of Research on Adolescence, 26, 90–100. [Google Scholar]
- VEGA WA, AGUILAR-GAXIOLA S, ANDRADE L, BIJL R, BORGES G, CARAVEO-ANDUAGA JJ, DEWIT DJ, HEERINGA SG, KESSLER RC, KOLODY B, MERIKANGAS KR, MOLNAR BE, WALTERS EE, WARNER LA & WITTCHEN H-U 2002. Prevalence and age of onset for drug use in seven international sites: results from the international consortium of psychiatric epidemiology. Drug and Alcohol Dependence, 68, 285–297. [DOI] [PubMed] [Google Scholar]
- VELANOVA K, WHEELER ME & LUNA B 2008. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex, 18, 2505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELANOVA K, WHEELER ME & LUNA B 2009. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J Neurosci, 29, 12558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINER RM, HARGREAVES DS, MOTTA JVDS, HORTA B, MOKDAD AH & PATTON GC 2017. Adolescence and Later Life Disease Burden: Quantifying the Contribution of Adolescent Tobacco Initiation From Longitudinal Cohorts. J Adolesc Health, 61, 171–178. [DOI] [PubMed] [Google Scholar]
- VOLKOW ND 2018. Toward precision medicine in addiction treatment. Am J Addict, 27, 35–36. [DOI] [PubMed] [Google Scholar]
- WAGER TD & SMITH EE 2003. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci, 3, 255–74. [DOI] [PubMed] [Google Scholar]
- WAGNER F 2002. From First Drug Use to Drug Dependence Developmental Periods of Risk for Dependence upon Marijuana, Cocaine, and Alcohol. Neuropsychopharmacology, 26, 479–488. [DOI] [PubMed] [Google Scholar]
- WELBORN BL, LIEBERMAN MD, GOLDENBERG D, FULIGNI AJ, GALVÁN A & TELZER EH 2016. Neural mechanisms of social influence in adolescence. Social Cognitive and Affective Neuroscience, 11, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHELAN R, WATTS R, ORR C & ALTHOFF RR AE, BANASCHEWSKI T, BARKER GJ, BOKDE AL, BÜCHEL C, CARVALHO FM, CONROD PJ, FLOR H, FAUTH-BÜHLER M, FROUIN V, GALLINAT J, GAN G, GOWLAND P, HEINZ A, ITTERMANN B, LAWRENCE C, MANN K, MARTINOT JL, NEES F, ORTIZ N, PAILLÈRE-MARTINOT ML, PAUS T, PAUSOVA Z, RIETSCHEL M, ROBBINS TW, SMOLKA MN, STRÖHLE A, SCHUMANN G, GARAVAN H; IMAGEN CONSORTIUM. 2014. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature, 512, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE HR & LABOUVIE EW 1989. Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol, 50, 30–37. [DOI] [PubMed] [Google Scholar]
- WILLIAMS K & MCGILLICUDDY-DE LISI A 1999. Coping strategies in adolescents. Journal of Applied Developmental Psychology, 20, 537–549. [Google Scholar]
- WILLIAMSON PR, ALTMAN DG, BAGLEY H, BARNES KL, BLAZEBY JM, BROOKES ST, CLARKE M, GARGON E, GORST S, HARMAN N, KIRKHAM JJ, MCNAIR A, PRINSEN CAC, SCHMITT J, TERWEE CB & YOUNG B 2017. The COMET Handbook: Version 1.0. Trials, 18, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTERS KC 1999. Treating adolescents with substance use disorders: An overview of practice issues and treatment outcomes. Substance Abuse, 20, 203–225. [DOI] [PubMed] [Google Scholar]
- WINTERS KC, STINCHFIELD RD, OPLAND E, WELLER C & LATIMER WW 2000. The effectiveness of the Minnesota Model approach in the treatment of adolescent drug abusers. Addiction, 95, 601–12. [DOI] [PubMed] [Google Scholar]
- WRZUS C, HÄNEL M, WAGNER J & NEYER FJ 2013. Social network changes and life events across the life span: A meta-analysis. Psychological Bulletin, 139, 53–80. [DOI] [PubMed] [Google Scholar]
- YEAGER DS, DAHL RE & DWECK CS 2017. Why interventions to influence adolescent behavior often fail but could succeed. Perspectives on Psychological Science, 13, 101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]


