Abstract
The findings of studies on the association between physical activity and adiposity are not consistent, and most are cross-sectional and used only self-reported measures. The aims of this study were to evaluate: 1) independent and combined cross-sectional associations of objectively-measured physical activity and sedentary time with body composition outcomes at 30 years, and 2) prospective associations of changes in self-reported physical activity from 23 to 30 years with the same outcomes in participants from the 1982 Pelotas (Brazil) Birth Cohort. Body mass index, waist circumference, visceral abdominal fat, fat mass index, and android/gynoid fat ratio were the outcomes. 3,206 participants were analysed. In cross-sectional analyses, higher objectively-measured moderate-to-vigorous physical activity was associated with lower body mass index (β = 0.017, 95%CI: −0.026; −0.009), waist circumference (β = −0.043, 95%CI: −0.061; −0.025), visceral abdominal fat (β = −0.006, 95%CI: −0.009; −0.003), and fat mass index (β = −0.015, 95%CI: −0.021; −0.009), independent of sedentary time. Sedentary time was independently associated only with higher fat mass index (β = 0.003, 95%CI: 0.001; 0.005). In longitudinal analyses, using self-reported measure, adiposity was lower among those who were consistently active or who became active. Adiposity was similar among the “became inactive” and “consistently inactive” subjects. Our findings suggest metabolic benefits from engagement in physical activity throughout young adulthood, with stronger associations on concurrent levels.
Introduction
The global prevalence of overweight and obesity increased by almost 30% between 1980 and 2013 in adults1. Similarly, in Brazil more than half of adults are overweight and the prevalence of obesity is 11.7% for men and 20.6% for women1. In Pelotas, a southern Brazilian city, there is also evidence of the rapid increase in the prevalence of obesity from adolescence to adulthood2.
The rise in prevalence of overweight/obesity is partly explained by changes in dietary and physical activity patterns3. Physical activity/exercise interventions seem to be effective to reduce anthropometric outcomes such as body mass index (BMI)4. A meta-analysis of exercise interventions studies showed a mean difference of 1.0 kg/m2 and 0.4 kg/m2 on BMI between treatment and control participants in supervised and motivational interventions, respectively4. However, evidence of a preventive association between daily physical activity and body composition is still controversial5–7. Regarding sedentary time, a systematic review of reviews reported that high amount of this behaviour may not exert an independent harmful effect on body composition when moderate-to-vigorous physical activity (MVPA) is taken into account in youth8. However, studies in adult populations on the independent association of sedentary behaviour and adiposity are not consistent9. Some studies using self-reported measurements found an independent positive association between sedentary behaviour and waist circumference10,11, while in a study with objectively measured sedentary time the positive associations with waist circumference and BMI were only significant among participants with insufficient physical activity12.
The reported inconsistency on the associations between physical activity and body composition maybe explained by methodological heterogeneity5–8,13. Studies with adults commonly evaluated participants of several ages, but physical activity and body composition and its relationship may be different from young to older adults. Physical activity and sedentary behaviour are usually assessed by both questionnaires and objective methods, and the kind of measure used may affect the results. The directionality of the association is another methodological issue that should be taken into consideration. Because physical activity may change as a consequence of overweight/obesity, cross-sectional analyses are susceptible to reverse causality bias, which tends to underestimate the association of physical activity with adiposity6. Also, levels of daily physical activity generally decrease with age14, thus it is important to better understand the relationship between changes in physical activity and adiposity in young adulthood. Another important point is that most studies only evaluated adiposity through BMI12,15–17 and waist circumference12,15. Studies using more precise body composition measurements that differentiate fat mass from lean mass are needed to help understand the relationship of physical activity and sedentary time with body composition.
This study aimed at examining the independent and combined cross-sectional associations of objectively measured physical activity and sedentary time with anthropometric and body composition outcomes at 30 years of age. We also evaluated the association of changes in self-reported physical activity from 23 to 30 years of age with anthropometric and body composition outcomes at 30 years of age among individuals who have been prospectively followed since birth, in the city of Pelotas, Brazil. Our hypothesis was that physical activity would be negatively associated with body composition and positively with sedentary time. Moreover, in the combined analyses, we expected that participants with high levels of physical activity still have benefits in body composition regardless of the time spent in sedentary time.
Results
At 23 years of age, 4,297 individuals were examined, which represented a follow-up rate of 77.4% of the original birth cohort (including 282 identified deaths) (Fig. 1). At 30 years of age, 3,701 individuals (68.1% including 325 identified deaths) were examined. Information on self-reported physical activity at both age 23 and 30 years and at least one of the body composition outcomes was available for 3,206 individuals. Table 1 shows that 50.4% of the participants included in the analyses were women and 74.8% had white skin colour. Most individuals belonged to families whose income at birth was lower or equal to three minimum wages (70%), one third of the mothers had ≤4 years of schooling at birth and 6.7% of individuals had a low birth weight. The prevalence of smoking at 23 years of age was 24.3%. At both time points about 50% reported less than 150 minutes per week of leisure-time physical activity. The median of time spent in MVPA at 30 years of age was 16.1 minutes per day (mean: 25.6; SD: 31.2) and in sedentary time was 684.8 minutes per day (mean: 679.7; SD: 88.6). The participants included in the present analyses were slightly more likely to be females and with family income ≤6 minimum wages compared to all participants followed-up at 23 years (Supplementary Table S1).
Figure 1.
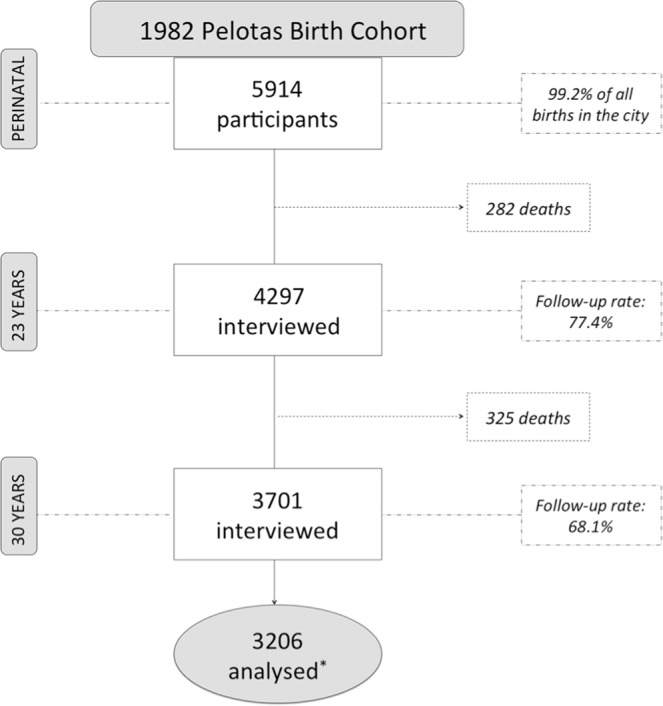
Flow diagram of study participants. *Number of participants who had available information on self-reported physical activity at 23 and 30 years of age and on at least one of the body composition outcomes.
Table 1.
Description of the sample included in the analyses. The 1982 Pelotas (Brazil) Birth Cohort.
| Variable | N | % |
|---|---|---|
| Gender | ||
| Male | 1,590 | 49.6 |
| Female | 1,616 | 50.4 |
| Family income at birth (minimum wages) | ||
| ≤1 | 626 | 19,6 |
| 1.1–3 | 1,607 | 50,4 |
| 3.1–6 | 618 | 19,4 |
| 6.1–10 | 178 | 5,6 |
| >10 | 161 | 5,0 |
| Maternal schooling at birth (years) | ||
| 0–4 | 1,050 | 32.8 |
| 5–8 | 1,382 | 43.2 |
| 9–11 | 345 | 10.8 |
| ≥12 | 424 | 13.2 |
| Skin colour | ||
| White | 2,398 | 74.8 |
| Black | 516 | 16.1 |
| Brown | 181 | 5.6 |
| Yellow | 60 | 1.9 |
| Indigenous | 51 | 1.6 |
| Low birth weight | 216 | 6.7 |
| Socioeconomic status at 23 years | ||
| A (richest) | 181 | 7.3 |
| B | 645 | 26.1 |
| C | 1,016 | 41.1 |
| D | 574 | 23.2 |
| E (poorest) | 58 | 2.3 |
| Schooling at 23 years | ||
| 0–4 | 213 | 6.6 |
| 5–8 | 880 | 27.5 |
| 9–11 | 1,616 | 50.4 |
| ≥12 | 497 | 15.5 |
| Smoking at 23 years | 780 | 24.3 |
| Daily energy intake at 23 years (kcal) [mean-SD] | 3726.8 | 2050 |
| Changes in physical activity from 23 to 30 years – self-reported | ||
| Inactive–Inactive | 1,666 | 52.0 |
| Inactive–Active | 427 | 13.3 |
| Active–Inactive | 610 | 19.0 |
| Active–Active | 503 | 15.7 |
| MVPA at 30 years – accelerometer (min/day) | ||
| 1st tertile (0–8.4) | 908 | 33.5 |
| 2nd tertile (8.5–26.8) | 905 | 33.4 |
| 3rd tertile (26.9–379.6) | 899 | 33.1 |
| SED at 30 years – accelerometer (min/day) | ||
| 1st tertile (113.9–648.0) | 915 | 33.3 |
| 2nd tertile (648.1–718.9) | 920 | 33.4 |
| 3rd tertile (719.0–952.1) | 915 | 33.3 |
MVPA: Moderate-to-vigorous physical activity; SED: sedentary time.
Cross-sectional analyses
Table 2 shows the independent cross-sectional associations between objectively measured MVPA and sedentary time and anthropometric and body composition outcomes at 30 years. In the adjusted analyses, higher MVPA was associated with lower BMI, waist circumference, visceral abdominal fat, fat mass index and android/gynoid fat ratio. These associations persisted even after further adjustment for sedentary time. Conversely, sedentary time was associated only with higher fat mass index after controlling for confounding factors including MVPA. The magnitude of association with fat mass index was five times greater for time spent in MVPA compared with sedentary time.
Table 2.
Crude and adjusted analyses of objectively measured moderate-to-vigorous physical activity and sedentary time (mean of minutes per day) and anthropometric and body composition outcomes at 30 years of age.
| Variables | Crude | Adjusted* | Adjusted** |
|---|---|---|---|
| β (95%CI) | β (95%CI) | β (95%CI) | |
| BMI at 30 years (kg/m 2 ) | |||
| MVPA | −0.018 (−0.025;−0.011) | −0.020 (−0.027;−0.012) | −0.017 (−0.026;−0.009) |
| SED | 0.003 (0.001;0.006) | 0.005 (0.002;0.008) | 0.002 (−0.001;0.005) |
| Waist circumference at 30 years (cm) | |||
| MVPA | −0.017 (−0.032;−0.002) | −0.049 (−0.066;−0.033) | −0.043 (−0.061;−0.025) |
| SED | 0.005 (−0.000;0.010) | 0.013 (0.007;0.019) | 0.005 (−0.001;0.012) |
| Visceral abdominal fat at 30 years (cm) | |||
| MVPA | 0.003 (0.000;0.005) | −0.006 (−0.009;−0.003) | −0.006 (−0.009;−0.003) |
| SED | −0.001 (−0.002;0.000) | 0.001 (0.000;0.002) | 0.000 (−0.001;0.001) |
| Fat mass index at 30 years (kg/m 2 ) | |||
| MVPA | −0.033 (−0.038;−0.028) | −0.018 (−0.023;−0.012) | −0.015 (−0.021;−0.009) |
| SED | 0.006 (0.004;0.008) | 0.005 (0.003;0.007) | 0.003 (0.001;0.005) |
| Android/Gynoid fat ratio at 30 years (g) | |||
| MVPA | 0.001 (−0.000;0.001) | −0.001 (−0.001;−0.000) | −0.001 (−0.001;−0.000) |
| SED | 0.000 (−0.000;0.000) | 0.000 (0.000;0.000) | 0.000 (−0.000;0.000) |
MVPA: Moderate-to-vigorous physical activity; SED: sedentary time.
*Adjusted for sex, skin colour, family income at birth, maternal schooling at birth, birth weight, socioeconomic status at 30 years, schooling at 30 years, smoking at 30 years, and daily energy intake at 30 years.
**Adjusted also for moderate-to-vigorous physical activity/sedentary time at 30 years.
Socioeconomic status at 30 years is the covariate with more missing data.
N of the analyses with MVPA from the top to the bottom of the table: crude - 2,699; 2,711; 2,670; 2,616; 2,631; adjusted - 2,145; 2,153; 2,121; 2,072; 2,083.
N of the analyses with SED from the top to the bottom of the table: crude - 2,737; 2,749; 2,078; 2,649; 2,664; adjusted - 2,145; 2,153; 2,121; 2,072; 2,083.
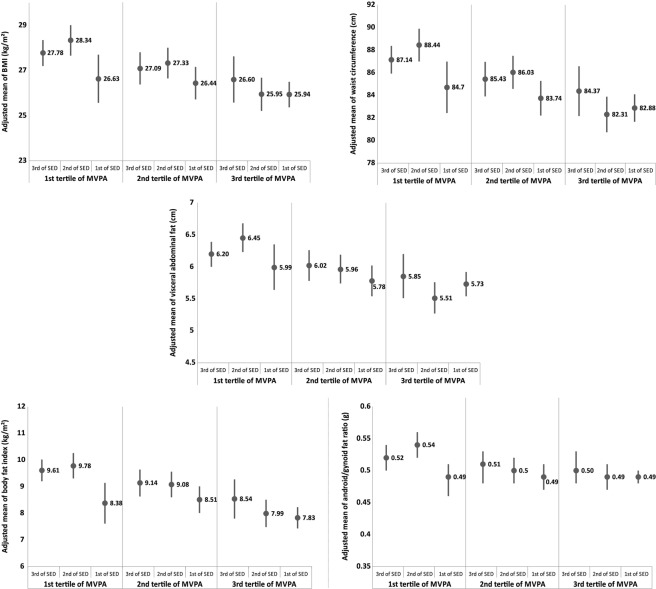
In combined association analyses, individuals with higher amount of MVPA and less amount of sedentary time (e.g. third tertile of MVPA and first and second tertiles of sedentary time) had lower values for all adiposity outcomes than those individuals with less amount of MVPA and higher amount of sedentary time. Nevertheless, among those in the highest tertile of MVPA, high amounts of sedentary time attenuated the benefits of MVPA on anthropometric and body composition outcomes (Fig. 2). The regression coefficients of the combined association analyses are presented in the Supplementary Table S2.
Figure 2.
Adjusted means (95%CI) of anthropometric and body composition outcomes by combined categories of moderate-to-vigorous physical activity and sedentary time at 30 years of age. Adjusted for sex, skin colour, family income at birth, maternal schooling at birth, birth weight, socioeconomic status at 30 years, schooling at 30 years, smoking at 30 years, and daily energy intake at 30 years. MVPA: moderate-to-vigorous physical activity; SED: sedentary time. Socioeconomic status at 30 years is the covariate with more missing data. N of the analyses from the top to the bottom of the figure: 2,145; 2,153; 2,121; 2,072; 2,083.
Longitudinal analyses
Table 3 shows the results for the prospective associations between self-reported changes in physical activity and the outcomes. After controlling for confounding factors, individuals who became active at 30 years and those who were active at both 23 and 30 years had lower BMI [β = −0.66 (95%CI: −1.04; −0.27); β = −0.60 (95%CI: −0.97; −0.23)], smaller waist circumference [β = −1.85 (95%CI: −2.87; −0.84); β = −1.37 (95%CI: −2.35; −0.39)], less visceral abdominal fat [β = −0.46 (95%CI: −0.66; −0.25); β = −0.43 (CI95%: −0.62; −0.23)], lower fat mass index [β = −0.59 (95%CI: −0.98; −0.20); β = −0.53 (95%CI: −0.90; −0.15)] and android/gynoid fat ratio [β = −0.01 (95%CI: −0.03; −0.00); β = −0.04 (95%CI: −0.05; −0.02)] at age 30 years compared to those individuals who were inactive at both ages. Moreover, the regression coefficients for those who were active at 23 years but inactive at 30 years were similar to those who were inactive at both age 23 and 30 years for most outcomes, except for waist circumference. Individuals who became inactive at 30 years had higher waist circumference [β = 1.05 (95%CI: 0.16; 1.94)] than those who were inactive at both time points.
Table 3.
Crude and adjusted analyses of changes in self-reported physical activity from 23 to 30 years of age and anthropometric and body composition outcomes at 30 years of age.
| Variables | Mean (95%CI) | Crude β (95%CI) | Adjusted* β (95%CI) |
|---|---|---|---|
| BMI at 30 years (kg/m 2 ) | p = 0.042 | p < 0.001a | |
| Inactive–Inactive | 26.88 (26.60;27.16) | Ref | Ref |
| Inactive–Active | 26.53 (26.03;27.02) | −0.36 (−0.95;0.24) | −0.66 (−1.04;−0.27) |
| Active–Inactive | 27.41 (26.97;27.86) | 0.53 (0.01;1.05) | 0.05 (−0.29;0.40) |
| Active–Active | 26.63 (26.22;27.04) | −0.25 (−0.81;0.31) | −0.60 (−0.97;−0.23) |
| Waist circumference at 30 years (cm) | p < 0.001 | p < 0.001b | |
| Inactive–Inactive | 84.02 (83.40;84.65) | Ref | Ref |
| Inactive–Active | 83.76 (82.64;84.89) | −0.26 (−1.60;1.09) | −1.85 (−2.87;−0.84) |
| Active–Inactive | 87.64 (86.62;88.67) | 3.62 (2.45;4.79) | 1.05 (0.16;1.94) |
| Active–Active | 86.07 (85.08;87.06) | 2.05 (0.79;3.30) | −1.37 (−2.35;−0.39) |
| Visceral abdominal fat at 30 years (cm) | p < 0.001 | p < 0.001b | |
| Inactive–Inactive | 5.76 (5.66;5.87) | Ref | Ref |
| Inactive–Active | 5.68 (5.48;5.88) | −0.08 (−0.31;0.14) | −0.46 (−0.66;−0.25) |
| Active–Inactive | 6.43 (6.25;6.61) | 0.67 (0.47;0.86) | −0.02 (−0.20;0.15) |
| Active–Active | 6.15 (5.99;6.32) | 0.39 (0.18;0.60) | −0.43 (−0.62;−0.23) |
| Fat mass index at 30 years (kg/m 2 ) | p < 0.001 | p < 0.001b | |
| Inactive–Inactive | 9.46 (9.24;9.67) | Ref | Ref |
| Inactive–Active | 8.32 (7.92;8.72) | −1.14 (−1.60;−0.67) | −0.59 (−0.98;−0.20) |
| Active–Inactive | 8.39 (8.05;8.74) | −1.06 (−1.47;−0.66) | 0.26 (−0.08;0.61) |
| Active–Active | 7.36 (7.01;7.71) | −2.09 (−2.53;−1.66) | −0.53 (−0.90;−0.15) |
| Android/Gynoid fat ratio at 30 years (g) | p < 0.001 | p < 0.001b | |
| Inactive–Inactive | 0.49 (0.48;0.50) | Ref | Ref |
| Inactive–Active | 0.50 (0.48;0.51) | 0.01 (−0.01;0.03) | −0.01 (−0.03;−0.00) |
| Active–Inactive | 0.53 (0.52;0.55) | 0.05 (0.03;0.06) | 0.00 (−0.01;0.02) |
| Active–Active | 0.51 (0.49;0.52) | 0.02 (0.01;0.03) | −0.04 (−0.05;−0.02) |
BMI: body mass index.
*Adjusted for sex, skin colour, family income at birth, maternal schooling at birth, birth weight, socioeconomic status at 23 years, schooling at 23 years, smoking at 23 years, and daily energy intake at 23 years.
aAdjusted also for BMI at 23 years.
bAdjusted also for waist circumference at 23 years.
Socioeconomic status at 23 years is the covariate with more missing data.
N of the analyses from the top to the bottom of the table: crude - 3,184; 3,201; 3,151; 3,081; 3,099; adjusted - 2,355; 2,457; 2,421; 2,375; 2,387.
Discussion
In the present study, we aimed to investigate the independent and combined cross-sectional associations of objectively measured physical activity and sedentary time, as well as longitudinal association of changes in self-reported physical activity from 23 to 30 years of age with anthropometric and body composition outcomes at 30 years of age. Confirming our hypothesis, we observed that physical activity levels are inversely associated with outcomes related to adiposity in cross-sectional and longitudinal analyses.
Reviews and meta-analyses summarizing cross-sectional and longitudinal studies report limited evidence that physical activity prevents weight gain/obesity4–7,18. However, some of these reviews evaluated only weight gain as the outcome5,18, others included only experimental studies4,18 and one evaluated only studies with obese individuals7. The inconsistent results found in these reviews may be explained by the heterogeneity among studies in relation to of age of the samples, the different types of measurement methods for physical activity and different study designs.
In our cross-sectional analyses, objectively measured physical activity at 30 years was associated with lower adiposity. Similar to our observations, most cross-sectional studies in adults have found an inverse association between physical activity and body composition, although their findings are exclusively based on self-reported data19–21. By using an objective measure of physical activity, consistent associations were found between MVPA and all anthropometric and body composition outcomes in the present study. An increase in 30 minutes per day of MVPA was associated with a 0.5 kg/m2 lower BMI and fat mass index and a 1.3 cm lower waist circumference. These ‘effects’ are meaningful, especially if we consider that it was adjusted for several covariates including sedentary time and daily energy intake. Also, given that few young adults achieve high levels of MVPA, a small ‘effect’ in a large population can be significant in a public health perspective.
Objectively measured sedentary time was only associated with higher fat mass index independent of MVPA, refuting our hypothesis of a consistent association. Some studies with adults investigating the independent association of sedentary behaviour have found a significant association with higher waist circumference10,11, but these studies only used self-reported data. On the other hand, another study with adults that also used objective measure did not find an independent association with waist circumference and BMI12. In the combined association analyses, the findings confirmed our hypothesis. Belonging to the top tertile of MVPA was associated with lower adiposity regardless of the tertile of sedentary time. For those in the lower and intermediate tertiles of MVPA, lower adiposity was found among those individuals with lower amount of sedentary time. These results suggest that spending high amount of time in physical activity appears enough to obtain an impact on anthropometric and body composition measurements, although prolonged sedentary time will attenuate the magnitude of the association. These findings corroborate with the results of previous studies12,22. Wanner et al. found that the odds of overweight/obesity was lower for individuals with high level of leisure-time physical activity independently of the amount of sitting time (both self-reported data), although the odds ratio was attenuated among those with high levels of leisure-time physical activity and high sitting time compared to those in with high physical activity and low sitting time for most body composition outcomes22. Barone Gibbs et al. evaluated the association of sedentary behaviour with body composition outcomes in two categories of physical activity in middle-aged adults using objective measures and observed that cross-sectional and longitudinal positive associations between sedentary behaviour and adiposity were only evident among individuals with insufficient levels of physical activity12.
In our longitudinal analyses, we found that changes in self-reported leisure-time physical activity from 23 to 30 years of age were associated with anthropometric and body composition outcomes at 30 years of age. Being consistently active and becoming active between 23 and 30 years of age were associated with lower levels of adiposity compared with those consistently inactive. Regarding longitudinal observational studies in adults, studies with self-reported physical activity have shown negative associations with adiposity12,15,16,22. A cohort of middle-aged and older women from United States found that levels of vigorous-intensity and total physical activity were inversely associated with risk of becoming overweight or obese16. A prospective study with middle-aged men from France and Ireland showed that higher levels of vigorous-intensity physical activity at baseline were associated with lower BMI, waist circumference and BMI change in 5 years15. Corroborating with our findings, a cohort from Switzerland observed that remaining inactive or becoming inactive was positively associated with obesity and anthropometric measurements in middle-aged adults22. We found that individuals who were active at 23 years but became inactive at 30 years were similar in body composition to individuals who were inactive at both ages. This suggests that there may not be lasting association of physical activity on anthropometric and body composition outcomes in the assessed period and the current status of physical activity is more important than the previous one. Therefore, it is necessary to keep active throughout adulthood in order to maintaining the benefits on body composition.
Controversially, longitudinal studies in adults with objective measures have shown inconsistent results on the subject12,17,23. A cohort study from United States did not find association between objectively measured MVPA at baseline and 5-year change in BMI and waist circumference in middle-aged adults12. A study in Norway also did not find an association between objectively measured MVPA at baseline and body weight change in 6 years, although body weight at baseline was associated with a decrease in MVPA17. Another study using data from the UK Biobank found a longitudinal association between higher BMI and waist circumference at baseline with lower activity levels at follow-up23.
The negative association between physical activity and body composition may be due to the increase in the total energy expenditure24. Besides this, other adaptations caused by physical activity practice could occur in the body and explain our findings. Physical activity, especially MVPA, may stimulate greater fat oxidation and oxygen consumption, increasing resting metabolic rate24–26. Moreover, sedentary activities may impair energy balance and contribute to obesity and weight gain12,27, since sedentary behaviour has lower energy costs than light-intensity physical activity28. In our combined analyses, we only evaluated MVPA and sedentary time and found that the association of MVPA with body composition appears attenuated by high sedentary time. The absence of assessment regarding the effect of light-intensity physical activity on body composition is due to the potential dependence between this behaviour and sedentary time. Despite the recognised independence of MPVA and sedentary time, when individuals might present high periods in both conditions, large changes in sedentary time are expected to provide large increments in light-intensity physical activity, which would show the same effect on body composition, but in the opposite direction.
Our study has several strengths. It was carried out in a large population-based cohort with high rates of follow-up, which minimize the likelihood of selection bias. Also, our longitudinal component allows the assessment of temporality between changes in physical activity and later body composition. All measurements of body composition were performed with high standard instruments and physical activity and sedentary time were objectively measured at 30 years of age, ensuring a good quality of exposures and outcomes measures. Finally, we found associations of physical activity with all anthropometric and body composition outcomes, reinforcing the consistency in our findings.
The major limitation of our study was the lack of some body composition measures before 30 years of age. As visceral abdominal fat, fat mass index and android/gynoid fat ratio were evaluated only at one time point, we cannot specifically evaluate whether these associations are bi-directional. However, we adjusted the analyses of these outcomes for waist circumference at age 23 as a proxy of previous adiposity. Also, we evaluated the possibility of bi-directional association with our longitudinal data and we did not find significant association between BMI and waist circumference at 23 years as exposure and self-reported leisure-time physical activity at 30 years as outcome (Supplementary Table S3). However, we cannot refute bi-directional association for our cross-sectional analyses using accelerometer data. The direction of the association is another methodological issue that should be taken into consideration. Because physical activity may be reduced as a consequence of overweight/obesity, cross-sectional analyses are susceptible to bias due to reverse causality. Indeed, a bi-directional association between adiposity and physical activity has been suggested in studies examining causality using Mendelian randomization29. Another limitation of the present study is the self-reported data for changes in physical activity. We performed cross-sectional analyses using objective measures but longitudinal analyses using self-reported data. Comparing the results of different measures is complicated. Besides the temporal differences in this case, we believe we are evaluating different constructs of physical activity. While the self-reported data in our study included only leisure-time and did not consider the intensity of physical activity, the objectively measured MVPA was not specific to the leisure-time domain and assess a wider set of daily activities. Also, self-reported measurements tend to overestimate levels of physical activity compared to accelerometer data30,31. Thus, self-reported data may lead to misclassification, reducing the chance of identifying associations. We can speculate that if we had objectively measures for our longitudinal analyses, the strength of the association could be even greater.
In conclusion, our findings suggest a negative cross-sectional association between MVPA and adiposity in young adults and spending excessive time sedentary only partly attenuated the magnitude of this association. Becoming active at 30 years or being consistently active between 23 and 30 years was associated with lower adiposity at 30 years, while becoming inactive at 30 years have similar impact on adiposity than being consistently inactive at both time points. These results suggest that being consistently active during young adulthood may reduce the risk of developing unhealthy levels of adiposity. Longitudinal independent and combined associations of objectively measured physical activity and sedentary time with body composition, with both exposures and outcomes assessed at least in two moments, should be investigated in further studies.
Methods
Study design
In 1982, all maternity hospitals in Pelotas, Brazil, were visited daily and all live births were identified. All mothers who lived in the urban areas of the city were invited to participate in the Pelotas 1892 birth cohort study together with their children (participation rate: 99.2%). All individuals have been followed-up several times throughout childhood and adolescence. The study was approved by the School of Medicine Ethics Committee of the Federal University of Pelotas and all participants signed the informed consent form. All methods were performed in accordance with relevant guidelines and regulations. Further details on the study methodology can be found elsewhere32,33.
From October 2004 to August 2005 (mean age: 22.8 years), a census was carried out in the urban area of the city in order to identify all individuals belonging to the cohort. Individuals were interviewed and examined during home visits. Between June 2012 to February 2013 (mean age: 30.2 years), cohort members were invited to attend the research clinic for clinical examinations and interviews.
Physical activity and sedentary time
For the cross-sectional analyses, objective measures collected when participants were 30 years old were used. In 2012–13, physical activity was objectively measured using the GENEActiv accelerometer (ActivInsights, Kimbolton, UK). This is a wrist-worn triaxial device, which provides raw data acceleration expressed in milli-g units. Validity parameters of GENEActiv to estimate physical activity energy expenditure and the comparability between this device and other traditional accelerometer brand are available elsewhere34–36. Participants were invited to wear the accelerometer, and those who agreed were instructed to wear the device continuously for a period between four to seven days, including at least one weekend day. Disabled participants, those who were living in other cities or unable to wear the accelerometers during work-time were excluded from the measurements. Furthermore, women who were pregnant during the visit were contacted after delivery and invited to wear the accelerometer. Complete accelerometer data collection protocol is available elsewhere37. The R-package GGIR [http:/cran.r-project.org]34 was used to analyse binary files from the GENEActiv accelerometers, which includes, in summary, a post-calibration process using local gravity as a reference35, non-wear detection, and calculation of the vector magnitude of activity-related acceleration using the Euclidian Norm minus 1 g (ENMO = )34. Records with post-calibration error lower than 20 mg, with at least one complete 24-h cycle with data collected for every 15-min period and at least two days of wear duration were included in the present analysis. Two summary variables were derived, time spent in MVPA and sedentary time. MVPA was estimated in 10-min bouts in which at least 80% of each time-window spent with acceleration equal to or higher than 100 mg38,39. The assessment of sedentary time was restricted for an expected awake period arbitrarily defined from 7 am to 11 pm, and sedentary time was defined by non-bouted time with intensity < 50 mg.
For the longitudinal analyses, self-reported physical activity data when participants were 23 and 30 years old were used. In the 2004–5 visits, physical activity was measured using the long version of the International Physical Activity Questionnaire (IPAQ). This questionnaire assesses walking, moderate and vigorous physical activity according to frequency and duration in the following domains: occupational; household; leisure-time and commuting40. In 2012–13 visits, physical activity was measured through a questionnaire regarding the duration and weekly frequency of leisure-time physical activity. The questionnaire included a list of activities constructed based on results from a pilot study identifying the most frequent activities practiced by young adults. A cut-off point of 150 minutes per week for leisure-time physical activity was used to classify individuals as active or inactive41. Leisure-time physical activity at ages 23 and 30 was divided in four categories: “consistently inactive” (inactive at both time points), “increased activity” (moving from inactive to active), “decreased activity” (moving from active to inactive), and “consistently active” (being active at both time points).
Anthropometry and body composition
In the present study, we also evaluated anthropometric and body composition outcomes. The outcomes for cross-sectional and longitudinal analyses were the same. BMI was estimated from weight and height measurements in the 2004–5 and 2012–13 visits. Weight was measured using the Bod POD® scale and height with a portable stadiometer (aluminium and wood) with precision of 0.1 cm. Waist circumference was measured also in the 2004–5 and 2012–13 visits. The measurement was made twice, after gentle expiration, using a flexible tape (Cescorf®, Porto Alegre, Brazil) with precision of 0.1 cm at the narrowest part of the trunk, identified as the midpoint between the lowest rib margin and the iliac crest. If the difference between the measurements was greater than 1 cm, two additional measures were taken and the mean of the two closest measurements was used. Visceral abdominal fat thickness was estimated in the 2012–13 visits using a Toshiba Xario (Toshiba Medical Systems Corp., Tokyo, Japan) ultrasound with a 3.5-MHz convex probe, according to validated protocols42,43. Visceral fat thickness was estimated by measuring the distance between the peritoneum and lumbar spine at the intersection between the xyphoid line and the waist circumference. Measurements were taken from static images at the end of a quiet expiration. Body composition was estimated with dual-energy x-ray absorptiometry (DXA Lunar Prodigy) in the 2012–13 visits, and fat mass index (Fat mass (kg)/height (m2) was calculated. Android/gynoid fat ratio was estimated dividing the fat mass in the android region by the gynoid region. The fat regions were automatically identified by the equipment software. Pregnant women were excluded from the analyses.
Covariates
The following variables measured at birth were considered as possible confounders for all analyses: sex, family income (in minimum wages), maternal schooling (in complete years), self-reported maternal skin colour, and birth weight (recorded by the hospital staff using calibrated paediatric scales). Other covariates measured at 23 and 30 years were: socioeconomic status which was developed by the Brazilian Association of Survey Companies (A, B, C, D, and E)44 and achieved schooling of the cohort member (in complete years), tobacco smoking (those subjects who reported smoking in the last week were considered as smokers), and daily energy intake. The variables family income and maternal schooling did not present a high correlation in the present sample (r = 0.57), so were both included in the adjusted analyses model. Daily energy intake (kcal) was assessed using a self-administered food frequency questionnaire that gathered information on the intake of 88 food items. Based on references available to estimate macronutrients45–47, the amount (grams) of carbohydrates, proteins and fats of each food item was obtained and the daily energy intake was estimated by multiplying the amount of carbohydrates and proteins by 4 kcal/g and the amount of fats by 9 kcal/g. The covariates included in the adjusted analyses were based on conceptual model according to the literature.
Statistical analyses
Crude and adjusted linear regression models were performed to examine the associations between exposures and outcomes. We graphically tested the normality and homoscedasticity (homogeneity of variance) of residuals. First, we assessed the cross-sectional associations between objectively measured MVPA or sedentary time, both in continuous forms, with anthropometric and body composition outcomes at 30 years of age. Multivariate models were adjusted for sex, family income at birth, maternal schooling at birth, maternal skin colour, birth weight, socioeconomic status and achieved schooling of the member cohort at 30 years, smoking at 30 years and daily energy intake at 30 years. In an additional model MVPA and sedentary time at 30 years were mutually adjusted to identify their independent contributions. Linear regression models were performed also to assess the cross-sectional association of combined categories of tertiles of MVPA and sedentary time with anthropometric and body composition outcomes at 30 years. In the adjusted analyses, the same model was used except the adjustment for MVPA or sedentary time. Finally, we examine the prospective association between categories of changes in physical activity from 23 to 30 years of age and anthropometric and body composition outcomes at 30 years. Multivariate models of these analyses were adjusted for sex, family income at birth, maternal schooling at birth, maternal skin colour, birth weight, socioeconomic status and achieved schooling of the member cohort at 23 years, smoking at 23 years, and daily energy intake at 23 years. Also, we adjusted the model for a baseline anthropometric measure, BMI at 23 years only when the outcome was BMI at 30 years and waist circumference at 23 years for the other outcomes. Exploratory analyses showed that the results were similar between males and females; therefore, the analyses were not stratified by sex. Effect sizes are β-coefficients and 95% CI. All statistical analyses were performed using Stata version 12 (StataCorp, College Station, TX, USA).
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Supplementary information
Acknowledgements
This article is based on data from the study “1982 Pelotas Birth Cohort” conducted by Postgraduate Program in Epidemiology at Federal University of Pelotas with the collaboration of the Brazilian Public Health Association (ABRASCO). From 2004 to 2013, the Wellcome Trust supported the 1982 birth cohort study. The Brazilian National Research Council (CNPq) and Rio Grande do Sul State Research Support Foundation (FAPERGS) also supported this phase of the study. The International Development Research Center, World Health Organization, Overseas Development Administration, European Union, National Support Program for Centers of Excellence (PRONEX) and the Brazilian Ministry of Health supported previous phases of the study. SB, KKO and EDLR are supported by the Medical Research Council (Unit Programme numbers: MC_UU_12015/2 and MC_UU_12015/4).
Author Contributions
B.G.C.S., I.C.M.S., U.E. and B.L.H. designed the present study. B.G.C.S. and B.L.H. led the analysis of this paper. I.C.M.S., U.E., S.B., K.K.O. contributed to results interpretation. B.G.C.S. drafted the manuscript. E.D.L.R., N.P.L., S.G.S. and G.V.A.F. collaborated with the critical revision of the manuscript. All authors read, revised and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41935-2.
References
- 1.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima NP, et al. Evolução do excesso de peso e obesidade até a idade adulta, Pelotas, Rio Grande do Sul, Brasil, 1982–2012. Cad. Saúde Pública. 2015;31:2017–2025. doi: 10.1590/0102-311X00173814. [DOI] [PubMed] [Google Scholar]
- 3.Schrauwen P, Westerterp KR. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr. 2000;84:417–427. doi: 10.1017/S0007114500001720. [DOI] [PubMed] [Google Scholar]
- 4.Conn VS, Hafdahl A, Phillips LJ, Ruppar TM, Chase JA. Impact of physical activity interventions on anthropometric outcomes: systematic review and meta-analysis. J Prim Prev. 2014;35:203–215. doi: 10.1007/s10935-014-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain–a systematic review. Obes Rev. 2000;1:95–111. doi: 10.1046/j.1467-789x.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilks DC, Besson H, Lindroos AK, Ekelund U. Objectively measured physical activity and obesity prevention in children, adolescents and adults: a systematic review of prospective studies. Obes Rev. 2011;12:e119–129. doi: 10.1111/j.1467-789X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- 7.Baillot A, et al. Impact of physical activity and fitness in class II and III obese individuals: a systematic review. Obes Rev. 2014;15:721–739. doi: 10.1111/obr.12171. [DOI] [PubMed] [Google Scholar]
- 8.Biddle SJ, Garcia Bengoechea E, Wiesner G. Sedentary behaviour and adiposity in youth: a systematic review of reviews and analysis of causality. Int J Behav Nutr Phys Act. 2017;14:43. doi: 10.1186/s12966-017-0497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biddle SJH, et al. Screen Time, Other Sedentary Behaviours, and Obesity Risk in Adults: A Review of Reviews. Curr Obes Rep. 2017;6:134–147. doi: 10.1007/s13679-017-0256-9. [DOI] [PubMed] [Google Scholar]
- 10.Shibata AI, et al. Physical Activity, Television Viewing Time, and 12-Year Changes in Waist Circumference. Med Sci Sports Exerc. 2016;48:633–640. doi: 10.1249/MSS.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knaeps S, et al. Ten-year change in sedentary behaviour, moderate-to-vigorous physical activity, cardiorespiratory fitness and cardiometabolic risk: independent associations and mediation analysis. Br J Sports Med. 2018;52:1063–1068. doi: 10.1136/bjsports-2016-096083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barone Gibbs B, et al. Sedentary Time, Physical Activity, and Adiposity: Cross-sectional and Longitudinal Associations in CARDIA. Am J Prev Med. 2017;53:764–771. doi: 10.1016/j.amepre.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince SA, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corder, K. et al. Change in physical activity from adolescence to early adulthood: a systematic review and meta-analysis of longitudinal cohort studies. Br J Sports Med (2017). [DOI] [PMC free article] [PubMed]
- 15.Wagner A, et al. Leisure-time physical activity and regular walking or cycling to work are associated with adiposity and 5 y weight gain in middle-aged men: the PRIME Study. Int J Obes Relat Metab Disord. 2001;25:940–948. doi: 10.1038/sj.ijo.0801635. [DOI] [PubMed] [Google Scholar]
- 16.Britton KA, et al. Physical activity and the risk of becoming overweight or obese in middle-aged and older women. Obesity (Silver Spring. Md.) 2012;20:1096–1103. doi: 10.1038/oby.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekelund U, et al. Objectively measured sedentary time and physical activity and associations with body weight gain: does body weight determine a decline in moderate and vigorous intensity physical activity? Int J Obes (Lond) 2017;41:1769–1774. doi: 10.1038/ijo.2017.186. [DOI] [PubMed] [Google Scholar]
- 18.Hebden L, Chey T, Allman-Farinelli M. Lifestyle intervention for preventing weight gain in young adults: a systematic review and meta-analysis of RCTs. Obes Rev. 2012;13:692–710. doi: 10.1111/j.1467-789X.2012.00990.x. [DOI] [PubMed] [Google Scholar]
- 19.Besson H, et al. A cross-sectional analysis of physical activity and obesity indicators in European participants of the EPIC-PANACEA study. Int J Obes (Lond) 2009;33:497–506. doi: 10.1038/ijo.2009.25. [DOI] [PubMed] [Google Scholar]
- 20.Bradbury, K. E., Guo, W., Cairns, B. J., Armstrong, M. E. G. & Key, T. J. Association between physical activity and body fat percentage, with adjustment for BMI: a large cross-sectional analysis of UK Biobank. BMJ Open7 (2017). [DOI] [PMC free article] [PubMed]
- 21.Du H, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr. 2013;97:487–496. doi: 10.3945/ajcn.112.046854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanner M, et al. Associations between domains of physical activity, sitting time, and different measures of overweight and obesity. Prev Med Rep. 2016;3:177–184. doi: 10.1016/j.pmedr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, et al. Adiposity and grip strength as long-term predictors of objectively measured physical activity in 93 015 adults: the UK Biobank study. Int J Obes (Lond) 2017;41:1361–1368. doi: 10.1038/ijo.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshioka M, et al. Impact of high-intensity exercise on energy expenditure, lipid oxidation and body fatness. Int J Obes Relat Metab Disord. 2001;25:332–339. doi: 10.1038/sj.ijo.0801554. [DOI] [PubMed] [Google Scholar]
- 25.Spriet LL. Regulation of skeletal muscle fat oxidation during exercise in humans. Med Sci Sports Exerc. 2002;34:1477–1484. doi: 10.1097/00005768-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Knab AM, et al. A 45-minute vigorous exercise bout increases metabolic rate for 14 hours. Med Sci Sports Exerc. 2011;43:1643–1648. doi: 10.1249/MSS.0b013e3182118891. [DOI] [PubMed] [Google Scholar]
- 27.Bann D, et al. Light Intensity physical activity and sedentary behavior in relation to body mass index and grip strength in older adults: cross-sectional findings from the Lifestyle Interventions and Independence for Elders (LIFE) study. PloS one. 2015;10:e0116058. doi: 10.1371/journal.pone.0116058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ainsworth BE, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 29.Richmond RC, et al. Assessing causality in the association between child adiposity and physical activity levels: a Mendelian randomization analysis. Plos Med. 2014;11:e1001618. doi: 10.1371/journal.pmed.1001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc. 2014;46:99–106. doi: 10.1249/MSS.0b013e3182a0595f. [DOI] [PubMed] [Google Scholar]
- 31.Palta P, et al. Self-reported and accelerometer-measured physical activity by body mass index in US Hispanic/Latino adults: HCHS/SOL. Prev Med Rep. 2015;2:824–828. doi: 10.1016/j.pmedr.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Victora CG, Barros FC. Cohort profile: the 1982 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2006;35:237–242. doi: 10.1093/ije/dyi290. [DOI] [PubMed] [Google Scholar]
- 33.Horta BL, et al. Cohort Profile Update: The 1982 Pelotas (Brazil) Birth Cohort Study. Int J Epidemiol. 2015;44(441):441a–441e. doi: 10.1093/ije/dyv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Hees VT, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PloS one. 2013;8:e61691. doi: 10.1371/journal.pone.0061691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Hees VT, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Applied Physiol (Bethesda. Md.: 1985) 2014;117:738–744. doi: 10.1152/japplphysiol.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowlands AV, et al. Comparability of measured acceleration from accelerometry-based activity monitors. Med Sci Sports Exerc. 2015;47:201–210. doi: 10.1249/MSS.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 37.Knuth AG, et al. Methodological description of accelerometry for measuring physical activity in the 1993 and 2004 Pelotas (Brazil) birth cohorts. Cad Saude Publica. 2013;29:557–565. doi: 10.1590/S0102-311X2013000300013. [DOI] [PubMed] [Google Scholar]
- 38.Hildebrand M, VT VANH, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46:1816–1824. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 39.da Silva IC, et al. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol. 2014;43:1959–1968. doi: 10.1093/ije/dyu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craig CL, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 41.WHO. Global Recommendations on Physical Activity for Health, (Geneva: World Health Organization, 2010). [PubMed]
- 42.Stolk RP, et al. Validity and reproducibility of ultrasonography for the measurement of intra-abdominal adipose tissue. J Obes Relat Metab Disord. 2001;25:1346–1351. doi: 10.1038/sj.ijo.0801734. [DOI] [PubMed] [Google Scholar]
- 43.Rolfe Ede L, et al. Association between birth weight and visceral fat in adults. Am J Clin Nutr. 2010;92:347–352. doi: 10.3945/ajcn.2010.29247. [DOI] [PubMed] [Google Scholar]
- 44.ABEP, B.A.o.S.C. Brazilian Economic Classification Criteria. http://www.abep.org/criterio-brasil (2012).
- 45.Franco, G. Tabela de composição química dos alimentos, (Atheneu: Rio de Janeiro, 1999).
- 46.NEPA, N.d.E.e.P.e.A. Tabela brasileira de composição de alimentos - TACO, (UNICAMP: Campinas, 2006).
- 47.Agriculture, U.S.D.o. National Nutrient Database for Standard Reference, (National Agricultural Library 2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.