Abstract
Blue cheeses are susceptible to yeast and bacterial growth on their surface, which causes spoilage during ripening process and the formation of slime. The dairy industry frequently control the proliferation of undesirable microorganisms with natamycin and high salt concentration. The green propolis is a complex of substances that presents antimicrobial properties with great potential as preservative in the food industry. The aims of the present study were to identify the mesophilic aerobic microorganisms present on the surface of Gorgonzola-type cheese, evaluate the antifungal and antibacterial effects of the ethanol extract of green propolis (EEP) on the development of those microorganisms and verify the effects of EEP on the sensory quality of cheese. Ten yeast species belonging to genera Yarrowia, Candida, Debaryomyces and Saccharomyces were identified, as well as seven species of bacteria belonging to genera Staphylococcus, Bacillus, Enterococcus, Corynebacterium and Proteus. The EEP showed minimum biocide concentration (MBC), between 0.3% (weight/weight) and 5% for Bacillus cereus and Proteus vulgaris, respectively. Saccharomyces cerevisiae was the most sensitive species (MBC of 0.63%) and Candida parapsilosis the most resistant one (MBC of 5%). In the sensory analysis, the cheeses involved with EEP at 5% concentration did not differ from the control, while at 10%, there was a slight decrease in acceptance. The EEP has potential and feasibility to be used in Gorgonzola-type cheese, inhibiting the main bacteria and yeasts without affecting largely the sensory characteristics of the product.
Keywords: Green propolis, Gorgonzola-type cheese, Antimicrobial activity, Natamycin, Sensory analysis
Introduction
Gorgonzola cheese is an Italian blue cheese made from cow’s milk. The name blue cheese is due to the coloration of the colonies resulted from the development of the fungus Penicillium roqueforti, which grows inside the bulk (Cocolin et al. 2009). Blue cheeses are subject to formation of slime on surface, which begins with the decomposition of nutrients by enzymes produced by microorganisms and residues of lysed cells. In an aqueous medium, these compounds begin to reorganize into polymeric structures that form a polysaccharide matrix (Flemming et al. 2016). Industries control the proliferation of undesirable surface microorganisms, mainly yeasts and aerobic bacteria, with natamycin and high concentration of salt. Natamycin is produced by Streptomyces natalensis and is commonly employed in dairy-based food products to prevent yeasts and mold contamination (El-Diasty et al. 2008). Natamycin is active against yeasts and mold, but not against bacteria, viruses and protozoa (Resa et al. 2014).
Among the alternatives that could complement or replace the use of natamycin, the green propolis stands out for its antimicrobial effect in several studies (Aga et al. 1994; Farnesi et al. 2009; Abubakar et al. 2014). In addition, propolis has already been tested as a food preservative because of the recognized bactericidal and bacteriostatic properties, and the propolis constituents are recognized as safe (GRAS), (Tosi et al. 2007). Costa et al. (2014) studying propolis in biobased packing of cheese and butter, observed excellent results in the preservation with the use of that substance. Propolis is a resinous substance collected by bees of the species Apis mellifera. It is used in the beehive as building material and defensive agent. Propolis has many biological properties such as antibacterial, antifungal, antioxidant, and antiviral activities in its complex and heterogeneous chemical composition (Wagh 2013). There is a growing interest in introducing healthy additives in food and propolis may be an economically viable alternative as preservative in food technology. However, currently there are no studies on the sensory and microbiological effect of propolis on Gorgonzola-type cheese.
Given the above, the objective of this study was to investigate the use of ethanol extract of green propolis (EEP) as preservative of blue cheeses, since the green propolis has antimicrobial properties and provides benefits for health. Therefore, the current study aimed to identify the mesophilic aerobic microorganisms present on the surface of Gorgonzola cheeses, evaluate the antifungal and antibacterial effects of the EEP on the development of the identified microorganisms, analyze the inhibitory effect of EEP and natamycin on the cheese surface and verify the effect of the application of EEP on sensorial quality of the cheeses.
Materials and methods
Collection and obtainment of the ethanol extract of green propolis
The crude green propolis was collected in the region of Campo das Vertentes (Latitude 21°14′S. Longitude 44°59′) in the state of Minas Gerais, Brazil, from beehives of the species Apis mellifera, in the period between August and October of 2015. The extraction of green propolis in ethanol was done according to Park and Ikegaki (1998), with modifications to optimize the extraction. Firstly, the frozen crude propolis was crushed in an industrial blender and then weighed and conditioned in hermetically sealed Erlenmeyers, mixed in 99% ethanol, at a ratio of 40 g of crude propolis/80 g of ethanol. The Erlenmeyer flasks were left to stir (Orbital Stirrer Table SL180/A Solab®) for 20 days at room temperature (25 °C). After this procedure, they were filtered on paper filter (Whatman No. 40) inside a freezer at − 20 °C into an amber vessel, resulting in the prime extract of propolis, with concentration of 33.3% (weight of propolis/weight of propolis + weight of ethanol). The filtered extract was also stored in a freezer at − 20 °C, protected from light and air. The different concentrations of EEP in percentage (weight/weight) that we used in the analyses were obtained by adding ethanol to the prime extract of propolis.
The chemical characterization of the green propolis utilized in this research was exhaustively studied and exposed in Righi et al. (2013).
Identification of microorganisms of Gorgonzola-type cheese
Sampling
The samples were collected from seven brands, with factories located in different regions of Minas Gerais State (Brazil), designated by letters: A (Centro), B (Alto Paranaíba), C (Zona da Mata), D (Noroeste), E (Triângulo Mineiro), F and G (Campo das Vertentes). From each brand, cheeses from three different lots were collected, resulting in 21 samples. Considering the date of production, the ripening period was 3–4 months for all cheeses. Gorgonzola-type cheeses (the term, type, is necessary, because the cheeses were manufactured in Brazil) in their original packaging were identified and duly conditioned under refrigeration and taken to the laboratory where they were stored until the moment of the analyses.
Enumeration and isolation of yeast and bacteria
Twenty-five grams of the scraped surface of Gorgonzola-type (1–2 mm thickness) cheese were transferred to plastic bags containing 225 mL of 0.1% peptone water and homogenized in Stomacher® (Mayo Homogenius HG 400, São Paulo, Brazil) for 5 min. Serial dilutions were performed for plating and further isolation. Yeast was cultivated on Dichloran Rose Bengal Chloramphenicol Agar (DRBC, Difco, Becton–Dickinson, Sparks, MD, USA) and incubated at 28 °C/48–72 h. For bacteria, we used Nutrient Agar, incubated at 30 °C/24–48 h. After determining the number of colonies, they were differentiated according to their morphotype. Colonies were selected based on shape characteristics, edge structure, color, texture, appearance, elevation, brightness (Dias and Schwan 2010). After this procedure, each morphotype was sampled by calculating the number of representative isolates, according to the methodology described by Senguna et al. (2009). All isolates were purified and stored in specific medium (agar nutrient for bacteria and yeast extract glucose peptone agar for yeast) containing 40% glycerol, stored at − 80 °C.
Matrix assisted laser desorption/ionization–time of flight mass spectrometer (MALDI-TOF) analysis
The isolates were subjected to Matrix Assisted Laser Desorption/Ionization–Time of Flight Mass Spectrometer (Bruker Daltonics®, Germany) analysis for the identification according to the methodology described by Amorim et al. (2016). All extractions were performed in triplicate and each repetition was spotted three times on the MALDI-TOF stainless steel target. The identification was performed using Biotype library. According to the manufacturer, score values should be 1.99 for reliable identification to the species level. For cluster analysis, the raw spectra were converted into text files using FlexAnalysis software version 3.4 (Bruker Daltonics®, Germany) containing the list of peaks (m/z) and their intensities. Spectra were then attenuated, the base line was subtracted and the signal intensities were normalized using the mMass software version 5.5 (Niedermeyer and Strohalm 2012).
For yeasts, approximately 1 μg (weighted with Mettler Toledo® Ultra Micro balance) biomass from the culture plate was added to a 500 μL tube containing 6 μL of 25% formic acid in water (v·v−1). For bacteria, approximately 1 μg (weighted with Mettler Toledo® Ultra Micro balance) biomass from the culture plate was added to a 500 μL tube containing 6 μL of an organic solution (containing 50% water, 47.5% acetonitrile and 2.5% trifluoroacetic acid [v·v−1]; Fluka, Buchs, Switzerland). For both bacteria and yeast, samples were vortexed for 60 s and then centrifuged at 4000×g for 60 s at room temperature (25 °C). Supernatant of each sample (1 μL) was placed onto the MALDI-TOF stainless plate (MSP 96 target polished steel; Bruker Daltonics®, Germany). When the sample was almost dried on the MALDI-TOF stainless plate, 1 μL of the matrix solution α-cyano-4-hydroxycinnamic acid (CHCA, Fluka; Buchs, Switzerland) saturated in an organic solution was added and gently mixed. Subsequently, air-dried samples were analyzed by MALDI-TOF. Overall, each MALDI-TOF sample was spotted in triplicate to test reproducibility (Amorim et al. 2016).
Prior to the analyses, calibration was performed with a standard bacterial test (Bruker Daltonics®, Germany) containing a previously prepared extract of Escherichia coli K12. The identified Isolates were deposited at the Agricultural Microbiology Cultures Collection (CCMA) of the Department of Biology in Federal University of Lavras (Lavras, M.G., Brazil).
Antimicrobial activity test of ethanol extract of propolis on microorganisms isolated from Gorgonzola-type cheese
For the analysis of the inhibitory effect of EEP, we used the previously isolated bacteria and yeasts, identified and belonging to the public collection of microorganism cultures (CCMA or Agricultural Microbiology Cultures Collection of the Department of Biology in Federal University of Lavras and ATCC or American Type Culture Collection): Staphylococcus saprophyticus (CMMA1318), Staphylococcus equorum (CMMA 1319), Staphylococcus lugdugensis (CMMA 1321), Enterococcus faecalis (ATCC® 376), Proteus vulgaris (CMMA 1322), Corynebacterium flavescens (CMMA 1323) and Bacillus cereus (CMMA 1320) and yeasts Yarrowia lipolytica (CMMA 1324), Debaryomyces hansenii (CMMA 1325), Candida parapsilosis (CMMA 1326), Candida zeylanoides (CMMA 1330), Candida guilliermondii (CMMA 1329), Candida intermedia (CMMA 1331), Candida norvegensis (CMMA 1327), Candida sphaerica (ATCC® 8549), Candida kefir (ATCC® 4922) and Saccharomyces cerevisiae (CMMA 1328).
After bacteria and yeast activation, the inoculums were prepared in saline solution 0.85% (m/v) and the cell suspensions were standardized using the McFarland scale adjusted to 0.5 and spectrophotometer measurements (at wavelength 600 nm), obtaining the mean optical density of 0.09, corresponding to 1.5 × 108 CFU mL−1 (Rehman et al. 2010). The minimum inhibitory concentration (MIC) was determined by using TSB (tryptic soy broth) microdilution technique for bacteria and YEPG (yeast extract glucose peptone agar) broth for yeast according methodology described by Santurio et al. (2011). We used 96-well polystyrene microplates. In each well, 150 μL of pure culture medium were added. In the first row, 150 μL of solution of EEP (with the adequate dilution of medium) were added. The serial dilutions were done using a multichannel pipette (Transferpette®) starting in the first row. The final concentration of inhibitory agent in the culture media in each row was: 10.0%; 5.0%; 2.5%; 1.25%; 0.63%; 0.31% and 0.16%. The inoculation of the microorganism at each well was done through 10 μL of standardized suspension of microorganism using a micropipette (MiniOne®). Two control tests were performed: one without the inoculation of the microorganisms at the well and the other containing the ethanol (instead EEP) in medium at the same concentration of the propolis (10.0%; 5.0%; 2.5%; 1.25%; 0.63%; 0.31% and 0.16%). Analyses with natamycin (Natamax® [natamycin: min. 50%+ lactose max. 50%]), using 96-well polystyrene microplates were performed too, to compare the effect of EEP and natamycin on yeast. The proceeding was the same that we used to determine the MIC with EEP, except for the final concentration of inhibitory agent: 0.04; 0.02; 0.01; 0.005; 0.0025; 0.0013; 0.0006; 0.0003; 0.00,015 and 0.000075% of natamycin in each row (being 0.04% the first and 0.00075% the last row).
The microplates were incubated in 35–37 °C/24–48 h and 28 °C/48–72 h for bacteria and yeast, respectively. After incubation, the microplates were evaluated and the MICs were defined as the lowest concentrations of inhibitory agent with no turbidity of the culture medium in the row.
The minimal biocide concentration (MBC) was obtained by plating in petri dishes with culture medium, 10 μL aliquots of the well that did not show growth in row. MBC was defined as the lowest concentration of antimicrobial with no growth in petri dishes (Santurio et al. 2011). The experiment was run in triplicate and three replicates.
Evaluation of the antimicrobial activity of the ethanol extract of green propolis and natamycin on the surface of Gorgonzola-type cheese
To evaluate the antimicrobial activity of EEP and natamycin on the surface of Gorgonzola-type cheeses (in sito test), these cheeses without the addition of natamycin were obtained from a local industry, which were used as a culture medium. The cheeses were opened in laminar flow chamber and 5 mm thick slices were removed and transferred to petri dishes. The slices were exposed for 60 min to UV light to promote the inactivation of microorganisms (Chun et al. 2009). In pre-tests, we observed that the UV light exposition for 60 min, does not affect the chemical and physical–chemical characteristics of the cheeses.
The application of the EEP onto the surface of the cheese slices simulated the application pattern of the diluted solution of natamycin in immersion for 5 s, excess drainage and drying, according to the manufacturer (Danisco 2015). Four concentrations of EEP were used: 1.25% (EEP1.25%); 2.50% (EEP2.5%); 5.0% (EEP5%); 10.0% (EEP10%) and a control (C), without addition of inhibitory agent. For natamycin, the treatments in % were: control (C); 0.001; 0.005; 0.025 and 0.125.
Isolates of S. saprophyticus (CMMA 1318), S. equorum (CMMA 1319), Y. lipolytica (CMMA 1324) and D. hansenii (CMMA 1325) were used.
On the surface of each cheese slice, three 10 μL-aliquots of the standardized microbial suspension (cell suspensions were standardized using the McFarland scale adjusted to 0.5 and spectrophotometer measurements (at wavelength 600 nm), obtaining the mean optical density of 0.09, corresponding to 1.5 × 108 CFU mL−1 Rehman et al. 2010) were inoculated. The slices of cheese were incubated at 37 °C for 24–48 h and 25 °C for 48–72 h for bacteria and yeast, respectively, and the presence or absence of microbial growth on the cheese was then observed (this test was similar to the MBC test, but the cheese was the culture medium instead the TSB and YEPG).
Sensory analysis
For the sensory analysis, the project that originated this research was approved by the Research Ethics Committee (Comitê de Ética em Pesquisa com Seres Humanos—COEP) of the Federal University of Lavras under the number 57513116.1.0000.5148. All steps of manipulation and generation of samples to perform the sensory analysis followed the principles of Good Manufacturing Practices.
The main purpose of the sensory analysis was to investigate any possible difference caused with the application of EEP in the sensory quality of cheeses in relation to a cheese with no EEP, since its application was not intended to create a new flavor of cheese.
Cheese preparation
The application of EEP onto the cheese surfaces followed the application patterns of the diluted solution of natamycin (Natamax®), according to the manufacturer (Danisco 2015). The EEP concentrations for application onto cheese surfaces were based on the maximum value of MBC (5%) and 10% concentration. The different treatments of the sensory analyses were designed as follows:
Treatment 1 (T1): Control treatment (no application of EEP)
Treatment 2 (T2): Cheese applied with EEP5%
Treatment 3 (T3): Cheese applied with EEP10%
The triangular-shaped samples used in the sensory analysis had 10 g and encompassed the outermost part and the center of the cheeses, considering that the cheeses have cylindrical format. The external area of the samples, about of 30% with the EEP, was calculated observing the uniformity of the sample, and considering that the cheese will be consumed as a whole (Correa et al. 2018).
Multiple comparison test
In order to verify whether there was a significant difference in the preference among samples with EEP addition as opposed to the samples without EEP, a multiple comparison test (MCT) was performed according to Instituto Adolfo Lutz (2008). The MCT was based on the comparison of a reference sample (R) with the treatments T1, T2 and T3. The R, just like T1, was not added with an inhibitory agent. Comparing R with T1, T2 and T3 is necessary to have a score of reference for the control treatment (T1).
Forty untrained consumers, who consume blue cheese on a regular basis, were invited. They used a scale of seven points (0 = no difference of the R, 1 = very slight difference, 2 = slight/moderate difference, 3 = moderate difference, 4 = moderate/large difference, 5 = large difference, 6 = very large difference) to evaluate taste and odour. The 10 g portions were presented in disposable plastic cups. Each tray with four coded samples was randomly numbered with three digits.
Acceptance test
The cheeses were submitted to the acceptance test (AT) using a hedonic scale of 1–9 according to Instituto Adolfo Lutz (2008), being: 1 = disliked extremely; 2 = disliked very much; 3 = disliked moderately; 4 = disliked slightly; 5 = neither liked nor disliked; 6 = liked slightly; 7 = liked moderately; 8 = liked very much; 9 = liked extremely. The AT was based on the comparison of the T1, T2 and T3.
Ninety-two untrained tasters with knowledge in Food Science and over 18 years were recruited. The tasters were selected because they consume blue cheeses and due to their interest in participating in the test. The tests were performed in individual cabins under red light. Samples coded with three digits were presented simultaneously, in white disposable cups, cut into triangular pieces. The samples were presented at 12 °C.
Statistical analysis of the experimental results
The experiment for the sensory analysis was structured in randomized blocks. Analysis of variance (ANOVA) and test of means (Duncan and Scott-Knott for the MCT and AT respectively) were carried out for significant data at level of significance of 5% using software SISVAR® (Ferreira 2011) and MATLAB® 8.0 (The Math Works Inc., Natick, MA, USA).
Results and discussion
Identification of microorganisms by MALDI-TOF and frequency in the cheeses studied
The number of isolates of each species of yeast and bacteria identified from the different brands of Gorgonzola-type cheese, is shown in Table 1.
Table 1.
Number of yeast and bacteria isolates identified in Gorgonzole-type cheeses of the different brands (A, B, C, D, E, F, and G)
| Species | Brands | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | ||
| Y. lipolytica | 14 | 15 | 2 | 6 | 13 | – | 10 | 60 |
| C. sphaerica | 7 | – | – | – | – | – | 7 | 14 |
| D. hansenii | 10 | – | 23 | 7 | – | 3 | 9 | 52 |
| C. parapsolosis | 6 | – | 6 | – | – | – | 3 | 15 |
| C. intermedia | 6 | – | – | – | 9 | – | – | 15 |
| C. norvegensis | 3 | – | – | 6 | – | – | – | 9 |
| S. cerevisiae | 9 | – | – | – | – | – | – | 9 |
| C. guilliermondii | – | – | – | – | – | 8 | – | 8 |
| C. zeylanoides | – | – | – | – | – | 9 | – | 9 |
| C. kefir | 3 | – | – | – | – | – | – | 3 |
| S. saprophyticus | 19 | 27 | 12 | – | 11 | – | – | 69 |
| S. equorum | 24 | 29 | 4 | 16 | 21 | 32 | 34 | 160 |
| B. cereus | 2 | – | – | – | – | – | – | 2 |
| E. faecalis | 14 | – | – | – | – | 5 | 7 | 26 |
| C. flavescens | 23 | – | – | – | – | – | – | 23 |
| S. lugdugensis | – | – | – | 8 | – | – | – | 8 |
| P. vulgaris | – | – | – | – | – | 11 | – | 11 |
– Indicates absence of isolates for the species
Among the species of yeast, Y. lipolytica and D. hansenii stand out as the greatest number of isolates from the cheeses. The predominant bacteria species in all regions were S. equorum, with a total of 160 isolates, and S. saprophyticus, with 19 and 27 isolates for brands A and B, respectively, totalizing 69 isolates in all brands.
The prevalence of the microorganism species identified and their importance are covered in other scientific studies. According to Ozturk and Sagdic (2014) and Mendoza et al. (2014), D. hansenii has high lipolytic activity and low proteolytic activity, while Y. lipolitica presents high lipolytic and proteolytic activities. The high amount of nutrients and enzyme production by these microorganisms contribute to accelerate the deterioration of blue cheeses and explain the high frequency at which they appear. The formation of slime occurs in a symbiotic process of microorganisms in which several components are degraded and synthesized according to the metabolic characteristics of each species. The slime in the surface of the cheeses is formed by the growth of microorganisms and the more intense the enzymatic activity, the greater the tendency to form slime. Water presence and proteolytic, lipolytic and exopolyssaccharide decomposition are fundamental for the generation of molecules for the formation of the polymer that constitutes slime (Flemming et al. 2016).
As observed in current study, Irlinger and Bergere (1999) and Banjara et al. (2015) highlight the importance of genus Staphylococcus in cheeses. According to these authors, the genus Staphylococcus is the main one found at the beginning of maturation (within 4 days) being replaced by coryneform bacteria in the third week of maturation. On the other hand, the species C. flavescens was found only in brand A in our research.
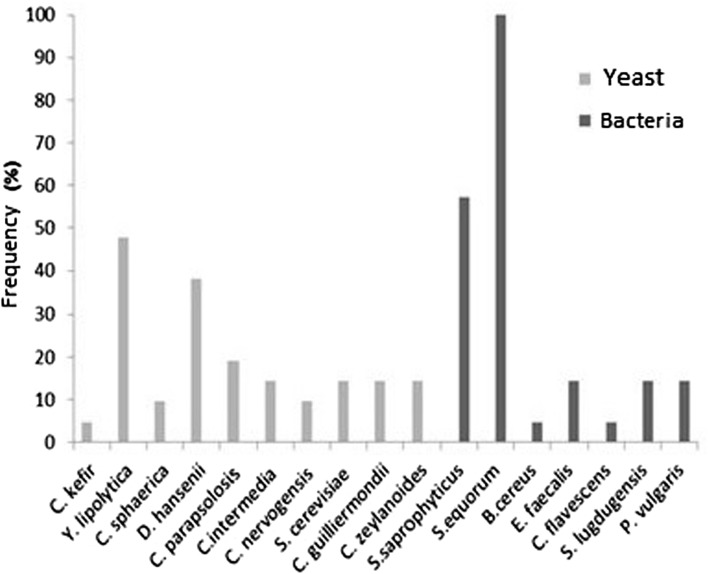
The frequency of occurrence (in %) of each species of yeast and bacteria in the Gorgonzola-type cheeses, in relation of the total amount of cheeses studied in this work is described in Fig. 1.
Fig. 1.
Frequency of yeast and bacteria species given in percentage, on the total amount of Gorgonzola-type cheese
The species Y. lipolytica was found in 47.62% of the cheeses analyzed, followed by D. hansenii, which was identified in 38.10% of the cheeses. As well as Y. lipolytica, D. hansenii is a common species in several environments (Lima et al. 2009). S. equorum and S. saprophyticus were the most prevalent bacteria, with frequencies of 100.00 and 57.14%, respectively. Alibi et al. (2016) point out that in the bacterial community of cheeses, halotolerant coryneforms are among the most prevalent ones, although the most prevalent bacteria in this study belonged to genus Staphylococcus.
The formation of slime on the surface of the cheese is increased by the lipolytic activity of yeasts, mainly Y. lipolytica (Flemming et al. 2016). The yeasts more associated with blue cheese, according to Mounier (2008) and Almeida et al. (2014) are Candida famata, Candida catenulata, Candida lipolytica, Trichosporon cutaneum and Zygosaccharomyces spp. genera. In Roquefort cheeses, the species Dabaryomyces hansenii, Candida sphaerica and other species of genus Candida are the most frequent ones. These statements were corroborated by the results observed in this study. Eliskases-Lechner and Ginzinger (1995) defended that the most frequent species on the surface of blue cheeses are Debaryomyces hansenii, Kluyveromyces marxianus and Yarrowia lipolytica.
The occurrence of species Debaryomyces hansenii does not show specificity of environments. This statement was emphasized by Banjara et al. (2015), who, when investigating the species of fungi in cheeses of various types acquired in the commerce of Lincoln—Nebraska (USA)—a region of temperate climate, very different from the state of Minas Gerais—observed that the species mentioned was the most abundant one, found in 79% of all the cheeses in their study. Ceugniez et al. (2015) when studying the microorganisms present in Tomme d’orchies cheese (Nomain, France) observed by means of biochemical analyses and genetic sequencing that most of the yeasts isolated belonged to the species Yarrowia lipolytica, Debaryomyces hansenii, Kluyveromyces lactis and Kluyveromyces marxianus.
Evaluation of inhibitory effect of the propolis extract and natamycin in vitro
The ranges of MIC, MBC of EEP and ethanol expressed as percentage (w/w) over yeast and bacterial species were shown in Table 2.
Table 2.
Minimum inhibitory concentrations (MIC) and minimum biocide concentrations (MBC) of EEP, ethanol control and natamycin over isolated bacteria and yeast species of Gorgonzola-type cheese
| Inhibitory agent (%) | ||||
|---|---|---|---|---|
| Ethanol control | EEP | |||
| MIC | MBC | MIC | MBC | |
| Yeasts | ||||
| Candida parapsilosis | 10.0 | > 10.00 | 1.25 | 5.00 |
| Yarrowia lipolytica | > 10.0 | > 10.00 | 1.25 | 2.50 |
| Debaryomyces hansenii | > 10.0 | > 10.00 | 1.25 | 2.50 |
| Candida zeylanoides | 2.50 | 10.00 | 0.63 | 2.50 |
| Candida guilliermondii | 5.00 | 10.00 | 1.25 | 2.50 |
| Candida intermedia | 5.00 | 10.00 | 0.63 | 1.25 |
| Candida norvegensis | 5.00 | 5.00 | 1.25 | 1.25 |
| Candida sphaerica | 5.00 | 10.00 | 0.63 | 1.25 |
| Candida kefir | 2.50 | 5.00 | 1.25 | 2.50 |
| Saccharomyces cerevisiae | 10.00 | > 10.00 | 0.63 | 0.63 |
| Bacteria | ||||
| Staphylococcus lugdugensis | 5.00 | > 10.00 | 0.63 | 1.25 |
| Enterococcus faecalis | 5.00 | 10.00 | 0.63 | 1.25 |
| Staphylococcus saprophyticus | 5.00 | 10.00 | 0.31 | 0.63 |
| Staphylococcus equorum | 5.00 | 10.00 | 0.31 | 0.63 |
| Proteus vulgaris | 10.00 | > 10.00 | 2.50 | 5.00 |
| Corynebacterium flavescens | 1.25 | 5.00 | 0.16 | 0.63 |
| Bacillus cereus | 5.00 | 10.00 | 0.16 | 0.31 |
| Yeasts | Natamycin (%) | |
|---|---|---|
| MIC | MBC | |
| Yarrowia lipolytica | 0.00015 | 0.00130 |
| Candida parapsolosis | 0.00060 | 0.00130 |
| Candida norvegensis | 0.00030 | 0.00060 |
| Saccharomyces cerevisiae | 0.00015 | 0.00030 |
| Candida guilliermondii | 0.00015 | 0.00030 |
| Candida zeylanoides | 0.00015 | 0.00030 |
| Candida sphaerica | 0.00015 | 0.00030 |
| Debaryomyces hansenii | 0.00015 | 0.00015 |
| Candida intermedia | 0.00015 | 0.00015 |
| Candida kefir | 0.00015 | 0.00030 |
Among yeast and bacteria species, the concentrations of alcohol required to promote the inactivation of microorganisms were always greater than the EEP, indicating that the effect of EEP comes mainly from the chemical constitution of propolis, with ethanol being only a solubilizing agent of their compounds. Among the yeast species, all required concentrations greater than or equal to 10% ethanol for inactivation except for C. norvegensis (5.0% ethanol). Regarding the concentrations of EEP, C. parapsilosis required 5.0% for inactivation, being the most resistant one. S. cerevisiae was the most sensitive yeast species to EEP. The other species of yeast had MBC of EEP between 1.25 and 2.50%. Among the species of bacteria, S. saprophyiticus, S. equorum (most frequent) and C. flavescens required 0.63% of EEP to reach MBC, whereas B. cereus was the most sensitive, being inactivated with 0.31%. The most resistant bacteria to EEP and ethanol was P. vulgaris, the only gram-negative species identified. Propolis has higher antimicrobial activity against gram-positive bacteria compared to gram-negative bacteria. One of the explanatory hypotheses is that gram-negative bacteria have a more complex cell wall and higher phospholipid content than gram-positive ones (Silici and Kutluca 2005).
The efficiency of green propolis when compared to ethanol is explained by the presence of several inhibitory compounds such as flavonoids (García-Lafuente et al. 2009). Propolis components have effects on the permeability of the cytoplasmic membrane to the ions, causing the dissipation of the membrane potential, which characterizes an ionophore substance. The effect may further decrease the resistance of cells to other antibacterial compounds explaining the greater sensitivity to gram-positive rather than gram-negative microorganisms (Abubakar et al. 2014). When the antimicrobial activities of natamycin and EEP were compared, natamycin was superior to the EEP. MBC values of natamycin were 0.00015–0.0013%, whereas for the EEP were 0.63–5.00%. Despite the superiority of natamycin in relation to propolis, natamycin is an antibiotic (Dalhoff and Levy 2015).
Another extremely relevant factor regarding propolis is that it causes bacterial inhibition. However, the antifungal activity of natamycin depends on its binding to sterols on the cell membrane, especially ergosterol, which is the main sterol of fungal membranes. These compounds are present in the membranes of fungus, but not in bacteria (Thomas et al. 2005).
Antimicrobial activity of ethanol extract of green propolis and natamycin on Gorgonzola-type cheese surface
The result of the antimicrobial activity of four concentrations of EEP and natamycin on the four species of the most frequent microorganisms in Gorgonzola-type cheese is described in Table 3.
Table 3.
Growth of microorganisms inoculated onto surface of Gorgonzola-type cheese previously applied from EEP and natamycin
| Species | EEP (%) | |||
|---|---|---|---|---|
| 1.25 | 2.50 | 5.00 | 10.00 | |
| Yarrowia lipolytica | − | − | − | − |
| Debaromyces hansenii | + | − | − | − |
| Staphylococcus saprophyticus | − | − | − | − |
| Staphylococcus equorum | − | − | − | − |
| Species | Natamycin (%) | |||
|---|---|---|---|---|
| 0.001 | 0.005 | 0.025 | 0.125 | |
| Yarrowia lipolytica | + | + | + | − |
| Debariomyces hansenii | + | + | − | − |
+ growth
− no growth
The concentrations of natamycin required to inhibit yeast growth on the cheese surface were lower than EEP concentrations. As observed in Table 3, the concentration of EEP for total inhibition of Y. lipolytica and D. hansenii occurred from 1.25% for Y. lipolytica and 2.50% for D. hansenii, whereas bacteria (S. saprophyticus and S. equorum) were inhibited from the concentration of 1.25%. Y. lipolytica was more sensitive to propolis and more resistant to natamycin when compared to D. hansenii. Natamycin completely inhibited yeasts tested at concentration above 0.025% for D. hansenii and 0.125% for Y. lipolytica. A better performance of propolis on the inhibition of Y. lipolytica relative to the cheese test (2.5% versus 1.25% of EEP) is observed. In relation to natamycin, there was reduction of inhibition of both D. hansenii and Y. lipolytica (0.025% and 0.125% of D. hansenii and Y. lipolytica respectively against MBC of 0.00015% and 0.0013% of D. hansenii and Y. lipolytica, respectively). Probably this effect was due to the fact that EEP (which was diluted in ethanol and is rich in resin) had formed a protective film on the cheese surface at drying, which made it difficult for the microorganisms to access the cheese nutrients. Resa et al. (2014) inferred in their study (with natamycin pulverized and solubilized in starch film) that effect of pulverized natamycin on cheeses is not prolonged. The authors observed that the growth of S. cerevisiae, Z. rouxii and Y. lipolytica occurred after 24 h on the pulverized cheeses, while on the cheeses covered by the starch film, yeast remained inhibited.
Costa et al. (2014), in their study concerning red propolis in biobased packing of cheese and butter, concluded that the propolis have a simultaneous antimicrobial and antioxidant bi-functional effect and the extracts of red propolis can be a competitive alternative in reducing the use of synthetic packaging films in the storage of some foods.
For the concentrations of EEP and natamycin (2.5% and 0.125% respectively) necessary to inhibit the main yeasts in the in situ test (see Table 3), the average cost to prepare a EEP2.5% was approximately US$1.75/L, considering the cost of 1 kg of crude green propolis in Minas Gerais State, approximately US$70.00. There is no official quotation of crude propolis in Brazil. For the natamycin, the average cost to prepare a solution of 0.125% of concentration was approximately US$0.18/L, considering the average cost of 1 kg of pure natamycin in the markets of Minas Gerais State, approximately US$150.00. Besides that, propolis has inhibitory effect in bacteria and is considered a health product (this may aggregate value to cheeses). In spite of the fact that natamycin is recognized as cheap, it is an antibiotic. Dalhoff and Levy (2015) observed that yeast (Candida spp.) that colonized the intestine, became resistant to treatment in patients submitted to natamycin treatment due to fungal bowel infection.
Sensory analysis: multiple comparison and acceptance test
The mean scores of the MCT and the AT of Gorgonzola-type cheese applied with EEP5% (T2) and EEP10% (T3) onto its surface, comparing to the control treatment (T1), are described in Table 4.
Table 4.
Mean scores of the multiple comparison test and the acceptance test of the treatments (T1, T2 and T3) for the Gorgonzola-type cheese applied from EEP
| Treatment** | Multiple comparison test* | |
|---|---|---|
| Taste | Odour | |
| T1 | 1.50a | 1.23a |
| T2 | 2.36b | 2.18b |
| T3 | 2.76b | 2.94c |
| Treatment** | Acceptance test | ||
|---|---|---|---|
| Taste | Odour | Overall impression | |
| T1 | 6.89a | 6.50a | 6.64a |
| T2 | 6.53a | 6.79a | 6.80a |
| T3 | 5.82b | 5.86b | 5.98b |
* The treatments C, EEP5% and EEP10% were compared with reference sample
**Equal letters in the columns indicate absence of significant differences in Duncan Test (multiple comparison tests) and Scott-Knottt (Acceptance test) at the level of 5% of significance
T1: Control treatment
T2: Application of EEP5%
T3: Application of EEP10%
In the MCT, there was significant difference among the treatments. Regarding taste attribute, there was a significant difference (p < 0.05) comparing T1with T2 and T3, in which the addition of more extract resulted in a slight perception of the presence of EEP on the cheese. On the other hand, when comparing T2 and T3, no significant difference was observed for the taste. Regarding odour, there was a significant difference (p < 0.05) among treatments, in which a higher addition of the extract resulted in a slight perception. These results indicated that the cheeses with EEP on the surface differ in relation to the T1. The tasters noticed difference between the R and the T1 (averages of 1.50 and 1.23 for taste and odour respectively), a common fact when it comes to sensory evaluation with untrained people. The greatest difference perceived was in odour, considering T1 and T3 (2.94 scale).
In the AT, the T2 and the T1 did not differ significantly in the Scott-Knott’s test at level of 5% significance, for the odour, taste and overall impression attributes. Regarding the T3, there was a decrease in the mean scores of acceptance for the three attributes analyzed, which indicated that at this concentration, EEP begins to interfere negatively with the product. However, this interference is discrete, because the scale is close to “liked slightly” while for the T1 and T2, the average scores were close to “liked moderately”. Such small difference is feasible when it comes to Gorgonzola cheese, because it has a strong taste and odour due to the development of the fungus Penicillium roqueforti and to production of volatile compounds from the metabolism of fat and proteins. This helps to mask the strong taste and odour of EEP. According to Figueiredo et al. (2015), green propolis presents balsamic, bitter, spicy and astringent flavors. Its odour can be floral, strong and camphorous. In the current study, the EEP5% was able to inhibit the most resistant microorganisms in the MBC test and the in situ test; at 2.5% concentration, EEP inhibited all the microorganisms identified with higher frequency on the cheeses surface. These facts make it safe to use EEP5% without impairing the sensory quality of the cheeses.
In relation of the effect of the EEP to the beneficial microorganisms (e.g., P. roqueforti and lactic bacteria), pre-tests indicated that the EEP do not diffuse itself to interior of the cheeses. As the undesirable microorganisms grow on the surface, the application of EEP to the bulk or to the milk causes serious adversities to cheese taste and odour (due to volume of EEP necessary to protect the surface too), even to Gorgonzola cheese, which have a naturally pronounced taste. Thus, since P. roqueforti grows mainly in the interior of the cheeses, only the surface should be protected.
Conclusion
The current study identified the mesophilic aerobic microorganisms present on the surface of Gorgonzola-type cheeses produced in Minas Gerais State (Brazil), evaluated the antimicrobial effects of the EEP on the development of these microorganisms and carried out a sensory evaluation of Gorgonzola-type cheeses with application of EEP on their surface. Y. lipolytica and D. hansenii stand out as the greatest number of isolates from the cheeses (60 and 52 isolates in total respectively). For the bacteria species, S. equorum and S. saprophyticus are the most prevalent, with a total of 160 and 69 isolates respectively. The concentration of EEP on the MBC analysis necessary to inactivation of C. parapsilosis was 5.0%, being the most resistant one. S. cerevisiae was the most sensitive one (EEP at 0.63% concentration). Among the species of bacteria, S. saprophyiticus, S. equorum and C. flavescens required 0.63% of EEP to reach MBC. With respect to the inhibition test on the surface of cheeses (in situ test), the concentration of EEP necessary for inhibition of Y. lipolytica and D. hansenii were 1.25% for Y. lipolytica and 2.50% for D. hansenii whereas natamycin completely inhibited that yeasts at concentration above 0.025% for D. hansenii and 0.125% for Y. lipolytica. Concerning sensory attributes, the EEP can be safely applied at concentration of 5.0% onto the Gorgonzola-type cheese surface, because at this concentration, there is no interference in taste and odour, and the microorganisms are completely inhibited.
This study confirms the potential of the EEP against spoilage bacteria and yeasts of the Gongonzola-type cheese, and its use as preservative, thus reducing the levels of antibiotics ingested by the consumer.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abubakar MB, Abdullah WZ, Sulaiman SA, Ang BS. Polyphenols as key players for the antileukaemic effects of propolis. Evid Based Complement Alternat Med. 2014;1:1–11. doi: 10.1155/2014/371730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aga H, Shibuya T, Sugimoto T, Kurimoto M, Nakajima SH. Isolation and identification of antimicrobial compounds in Brazilian propolis. Biosci Biotechnol Biochem. 1994;58:945–946. doi: 10.1271/bbb.58.945. [DOI] [Google Scholar]
- Alibi S, Ferjani A, Boukadida F. Implication of Corynebacterium species in food’s contamination. J Coast Life Med. 2016;9:123–156. [Google Scholar]
- Almeida M, et al. Construction of a dairy microbial genome catalog opens new perspectives for the metagenomic analysis of dairy fermented products. BMC Genomics. 2014;15:1101–1120. doi: 10.1186/1471-2164-15-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim JC, Schwan RF, Duarte WF. Sugar cane spirit (cachaça): effects of mixed inoculum of yeasts on the sensory and chemical characteristics. Food Res Int. 2016;85:76–83. doi: 10.1016/j.foodres.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Banjara N, Suhr MJ, Hallen-Adams HE. Diversity of yeast and mold species from a variety of cheese types. Curr Microbiol. 2015;70(6):792–800. doi: 10.1007/s00284-015-0790-1. [DOI] [PubMed] [Google Scholar]
- Ceugniez A, Drider D, Jacques P, Coucheney F. Yeast diversity in a traditional French cheese “Tomme d’Orchies” reveals infrequent and frequent species with associated benefits. Food Microbiol. 2015;53:177–184. doi: 10.1016/j.fm.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Chun H, Kim J, Chung K, Won M, Song KB. Inactivation kinetics of Listeria monocytogenes, Salmonella enterica serovar Typhimurium, and Campylobacter jejuni in ready-to-eat sliced ham using UV-C irradiation. Meat Sci. 2009;83:599–603. doi: 10.1016/j.meatsci.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Cocolin L, Numera D, Alessandria V, Rantsiou K, Dolci P, Grassi MA, Lomanaco S, Civera T. Microbial ecology of Gorgonzola rinds and occurrence of different biotypes of Listeria monocytogenes. Int J Food Microbiol. 2009;31:200–205. doi: 10.1016/j.ijfoodmicro.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Correa FT, van Mullem JJ, Correa PT, Abreu LR, Carneiro JDS, Pinto SM. Development of cheese wrapped in barks of reforestation trees. J Culin Sci Technol. 2018 [Google Scholar]
- Costa SS, Druzian JI, Machado BAS, Souza SO, Guimarães AG. Bi-functional biobased packing of the cassava starch, glycerol, licuri nanocellulose and red propolis. PLoS ONE. 2014;9:e112554. doi: 10.1371/journal.pone.0112554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalhoff AAH, Levy SB. Does use of the polyene natamycin as a food preservative jeopardise the clinical efficacy of amphotericin B? A word of concern. Int J Antimicrob Agents. 2015;45:564–567. doi: 10.1016/j.ijantimicag.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Danisco (2015) The cheese corner. http://www.danisco.com/food-beverages/dairy/insights-for-cultured-dairy-newsletter/september-2015/the-cheese-corner/
- Dias DR, Schwan RF (2010) Isolamento e identificação de leveduras. In: Moreira FMS, Huising EJ, Bignell DE (eds) Manual de Biologia dos Solos Tropicais - Amostragem e Caracterização da Biodiversidade, vol 1, pp 227–277
- El-Diasty E, El-Kaseh R, Salem R. The effect of natamycin on keeping quality and organoleptic characters of yoghurt. Arab J Biotechnol. 2008;12(1):41–48. [Google Scholar]
- Eliskases-Lechner F, Ginzinger W. The yeast flora of surface-ripened cheeses. Milchwissenschaft. 1995;50:458–462. [Google Scholar]
- Farnesi AP, Aquino-Ferreira R, Jong D, Bastos JK, Soares AEE. Effects of stingless bee and honey bee propolis on four species of bacteria. Genet Mol Res. 2009;8:635–640. doi: 10.4238/vol8-2kerr023. [DOI] [PubMed] [Google Scholar]
- Ferreira DF. Sisvar: a computer statistical analysis system. Ciênc e Agrotec. 2011;35:1039–1042. doi: 10.1590/S1413-70542011000600001. [DOI] [Google Scholar]
- Figueiredo FJB, et al. Physicochemical characterization and flavonoid contents of artisanal Brazilian green propolis. Int J Pharm Pharm Sci. 2015;7:64–68. [Google Scholar]
- Flemming HC, Neu T, Wingender J. The perfect slime: microbial extracellular polymeric substances (EPS) London: IWA Publishing; 2016. p. 336p. [Google Scholar]
- García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- Instituto Adolfo Lutz (2008) Métodos físico-químicos para análise de alimentos/coordenadores Odair Zenebon, Neus Sadocco Pascuet e Paulo Tiglea. São Paulo, Brazil 1:100–620
- Irlinger F, Bergere JL. Use of conventional biochemical tests and analyses of ribotype patterns for classification of micrococci isolated from dairy products. J Dairy Res. 1999;66:91–103. doi: 10.1017/S0022029998003203. [DOI] [Google Scholar]
- Lima CDLC, Lima LA, Cerqueira MMOP, Ferreira EG, Rosa CA. Lactic acid bacteria and yeasts associated with the artisanal Minas cheese produced in the region of Serra do Salitre, Minas Gerais. Arq Bras de Med Vet e Zootec. 2009;61:266–272. doi: 10.1590/S0102-09352009000100037. [DOI] [Google Scholar]
- Mendoza LM, Padilla B, Belloch C, Vignolo G. Diversity and enzymatic profile of yeast isolated from traditional llama meat sausages from north-western Andean region of Argentina. Food Res Int. 2014;62:572–579. doi: 10.1016/j.foodres.2014.04.008. [DOI] [Google Scholar]
- Mounier J, Monnet C, Jacques N, Vallaeys T, Arditi R, Sarthou AS, Hélias A, Irlinger F. Microbial interactions within a cheese microbial community. ASM. 2008;74:172–181. doi: 10.1128/AEM.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer THJ, Strohalm M. Mass as a software tool for the annotation of cyclic peptide tandem mass spectra. PLoS ONE. 2012;7(9):e44913. doi: 10.1371/journal.pone.0044913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk I, Sagdic O. Biodiversity of yeast mycobiota in “sucuk,” a traditional Turkish fermented dry sausage: phenotypic and genotypic identification, functional and technological properties. J Food Sci. 2014;79:2315–2322. doi: 10.1111/1750-3841.12662. [DOI] [PubMed] [Google Scholar]
- Park KY, Ikegaki M. Praparation of water and ethanolic extracts of propolis and evaluations of the preparations. Biosci Biotechnol Biochem. 1998;62(11):2230–2232. doi: 10.1271/bbb.62.2230. [DOI] [PubMed] [Google Scholar]
- Rehman S, Reehana K, Khursheed AB, Alsaba FR, Abdul SS, Mohd SA. Isolation, characterisation and antibacterial activity studies of coumarins from Rhododendron lepidotum Wall. ex G. Don, Ericaceae. Braz J Pharm. 2010;20(6):886–890. doi: 10.1590/S0102-695X2010005000037. [DOI] [Google Scholar]
- Resa CPO, Jagus RJ, Gerscheson LN. Natamycin efficiency for controlling yeast growth in models systems and on cheese surfaces. Food Control. 2014;35:101–108. doi: 10.1016/j.foodcont.2013.06.049. [DOI] [Google Scholar]
- Righi AA, Negri G, Salatino A. Comparative chemistry of propolis from eight Brazilian localities. Evid Based Complement Alternat Med. 2013;15:12. doi: 10.1155/2013/267878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santurio DF, Costa MM, Maboni G, Cavalheiro CP, Sá MF, Pozzo M, Alves SH, Fries LLM. Atividade antimicrobiana de óleos essenciais de condimentos frente a amostras de Escherichia coli isoladas de aves e bovinos. Ciênc Rur. 2011;41(6):1051–1056. doi: 10.1590/S0103-84782011005000067. [DOI] [Google Scholar]
- Senguna IY, Nielsen DS, Karapinar M, Jakobsen M. Identification of lactic acid bacteria isolated from Tarhana, a traditional Turkish fermented food. Int J Food Microbiol. 2009;135:105–111. doi: 10.1016/j.ijfoodmicro.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Silici S, Kutluca S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J Ethnopharmacol. 2005;99:69–73. doi: 10.1016/j.jep.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Thomas LV, Ingram- RE, Bevis HE, Brightwell P, Wilson N, Delves-Broughton J. Natamycin control of yeast spoilage of wine. Food Prot Trends. 2005;25:510–517. [Google Scholar]
- Tosi EA, Ré E, Ortega ME, Cazzoli AF. Food preservative based on propolis: bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli. Food Chem. 2007;104:1025–1209. doi: 10.1016/j.foodchem.2007.01.011. [DOI] [Google Scholar]
- Wagh VD. Propolis: a wonder bees product and its pharmacological potentials. Adv Pharm Sci. 2013;5:145–172. doi: 10.1155/2013/308249. [DOI] [PMC free article] [PubMed] [Google Scholar]