Abstract
Background and Purpose
The aim of this study was to determine the effect of food intake on the heart rate (HR) in postural orthostatic tachycardia syndrome (POTS).
Methods
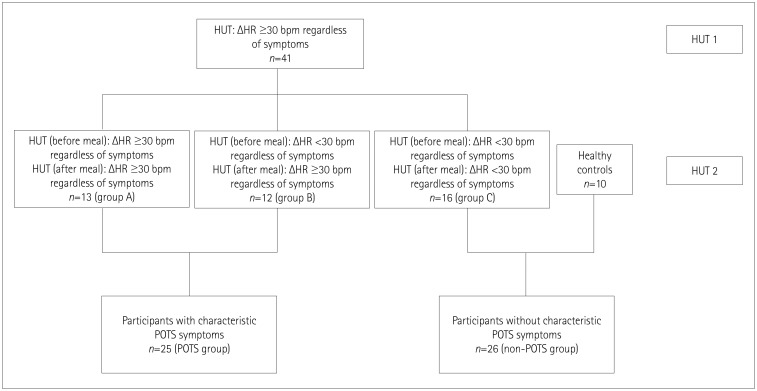
The following five-phase protocol was applied to 41 subjects who had a sustained HR increment of ≥30 beats/min within 10 min of standing in an initial tilt-table test: 1) 10-min supine phase, 2) 10-min 70°-tilted phase, 3) ingestion of 400 mL of Nutridrink Multi Fibre®, 4) 45-min supine phase, and 5) 10-min 70°-tilted phase. Subjects were divided into four groups: A) difference in HR for standing vs. supine (ΔHR) before the meal of ≥30 beats/min (n=13), B) ΔHR <30 beats/min before the meal but ≥30 beats/min after the meal (n=12), and C) ΔHR <30 beats/min both before and after the meal (n=16). Group D consisted of 10 healthy subjects.
Results
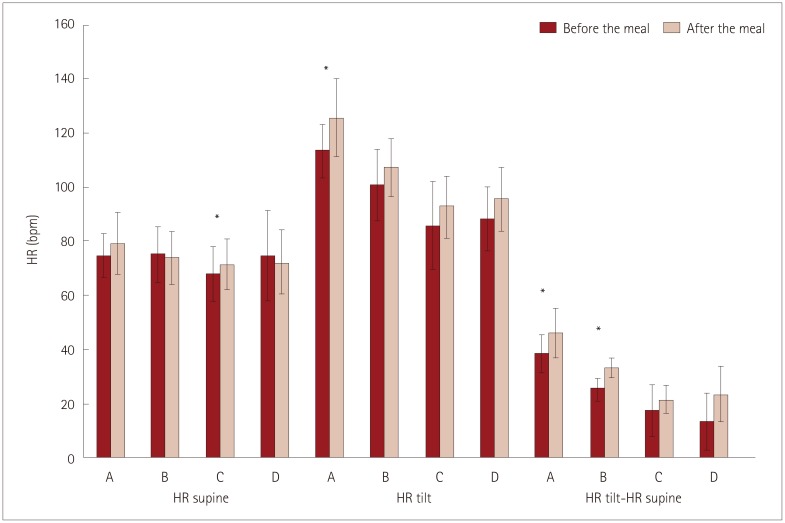
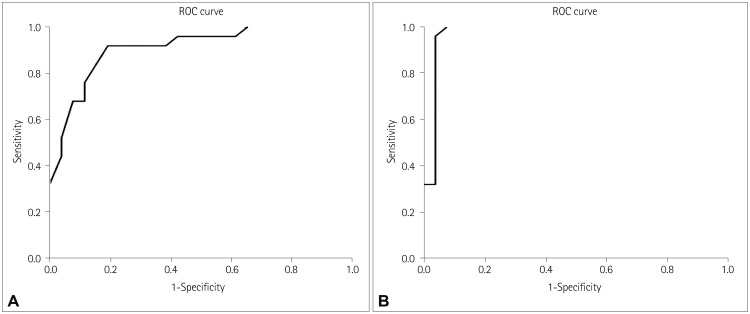
Before the meal, ΔHR was significantly higher in group A than in all of the other groups, and in group B than in group D (p<0.001). After the meal, ΔHR was significantly higher in groups A and B (p<0.001 and p<0.0001, respectively). When we pooled patients (according to their symptoms) from group A and B into a POTS group and from group C and D into a non-POTS group, an increase in HR of 25 beats/min before the meal had a sensitivity of 92.0% and a specificity of 80.8%. After the meal, an increase in HR of 30 beats/min had a sensitivity of 96.0% and a specificity of 96.2%.
Conclusions
Food intake can significantly alter the results of the tilt-table test and so should be taken into account when diagnosing POTS.
Keywords: postural orthostatic tachycardia syndrome, food intake, tilt-table test
INTRODUCTION
Postural orthostatic tachycardia syndrome (POTS) is a form of orthostatic intolerance characterized by a sustained excessive increase in the heart rate (HR) after adopting an upright posture. The HR increase in POTS is accompanied by characteristic symptoms such as lightheadedness, weakness, and nausea, which are relieved by adopting a recumbent position.1 This abnormal response to orthostasis could be pathophysiologically explained by insufficient compensatory vascular constriction, excessive central sympathetic tone, hypovolemia with a defect in the renin-angiotensin-aldosterone system, deconditioning, and autoimmune causes, but the exact underlying mechanism remains unknown.1,2 POTS is more common among females and either can appear in isolation or can overlap and be secondary to other conditions such as chronic fatigue syndrome, multiple sclerosis, inappropriate sinus tachycardia, and Ehlers-Danlos syndrome.1,3,4 The exact prevalence of POTS is unknown, mostly because many patients who suffer from this condition have unspecific symptoms, resulting in them being underdiagnosed or misdiagnosed and hence treated for some other condition instead.5 A definitive diagnosis of POTS requires fulfillment of the following criteria reported in 2011 by Freeman et al.,6 a sustained HR increment of ≥30 beats/min within 10 min of standing or during head-up tilting in the absence of orthostatic hypotension and accompanied by characteristic symptoms that are relieved by recumbency. For individuals aged 12–19 years, the required minimum increment in HR is 40 beats/min.6 The tilt-table test is a common diagnostic tool for diagnosing POTS because it enables the positions of the patient to be readily altered between standing and supine while measuring the blood pressure (BP) and HR changes, which are major criteria for POTS.
Meal intake significantly influences the autonomic nervous system, and it is estimated that maintaining the postprandial BP requires a 200% increase in sympathetic nervous activity.7,8 This effect of the sympathetic nervous response seems to be even greater in young adults, which is the population mostly affected by POTS.9 Since several neurological illnesses can influence the postprandial response, it is no wonder that gastroenterologic symptoms are common in the POTS, and postprandial worsening of orthostatic symptoms often occurs.10,11 One study has shown how food ingestion alters the HR and BP upon head-up tilting in POTS patients.11 However, current POTS criteria do not define whether food intake should be taken into account while interpreting the results of tilt-table tests. Based on the hypothesis that food intake prior to tilt-table testing can influence the criteria used to diagnose POTS, the aim of this study was to determine the effect of food intake on the current criteria applied for the diagnosis of POTS.
METHODS
This was a prospective study that was conducted from April 2016 to November 2017 in the Referral Center for Autonomic Nervous System Disorders at University Hospital Center Zagreb.
Eligible participants were consecutive patients referred to the center for tilt-table testing and who had a sustained HR increment of ≥30 beats/min within 10 min of standing or during head-up tilting in the absence of orthostatic hypotension with or without characteristic symptoms for POTS in the baseline examination.6 Exclusion criteria were the presence of any underlying condition that might influence the results in the tilt-table test such as hypertension, diabetes or polyneuropathy. Furthermore, age- and sex-matched healthy controls who had no previous history of events that could be associated with any kind of orthostatic intolerance were included. All participants signed informed-consent forms approved by the Ethical Committee of University Hospital Center Zagreb (approval no. 02/21 AG).
Forty-one participants met the inclusion criteria (29 females; age 29.74±10.61 years, mean±SD), and the control group consisted of 10 healthy individuals (6 females, age 24.25±2.25 years). The study flow chart is presented in Fig. 1. After fulfilling the inclusion criteria, participants were asked to come to the laboratory within 28 days, when they underwent the second tilt-table test comprising the following five phases: 1) 10-min supine phase, 2) 10-min 70°-tilted phase, 3) meal ingestion (5–7 min), 4) 45-min supine phase, 5) 10-min 70°-tilted phase.
Fig. 1. Study flow chart. HR: heart rate, ΔHR: difference in heart rate for tilted vs. supine, HUT: head-up tilt-table test, POTS: postural orthostatic tachycardia syndrome.
The beat-to-beat HR and BP were continuously monitored throughout the test using a Task Force Monitor (CNSystems Medizintechnik, Graz, Austria). The meal consumed by the participants comprised 400 mL of the liquid meal replacement Nutridrink Multi Fibre® at room temperature. One 200-mL pack has an energy content of 300 kcal (100 mL contains 5.8 g of fat, 6 g of protein, and 18 g of carbohydrate). All of the participants had fasted for at least 3 hours prior to the testing. None of the participants received any form of treatment between testing that could affect their POTS symptoms.
In order to investigate the effect of food intake on hemodynamic parameters during the tilt-table test, we divided subjects into four groups: A) difference in HR for standing vs. supine (ΔHR) before the meal of ≥30 beats/min (n=13), B) ΔHR <30 beats/min before the meal but ≥30 beats/min after the meal (n=12), C) ΔHR <30 beats/min both before and after the meal (n=16), and D) healthy subjects (n=10) (Fig. 2).
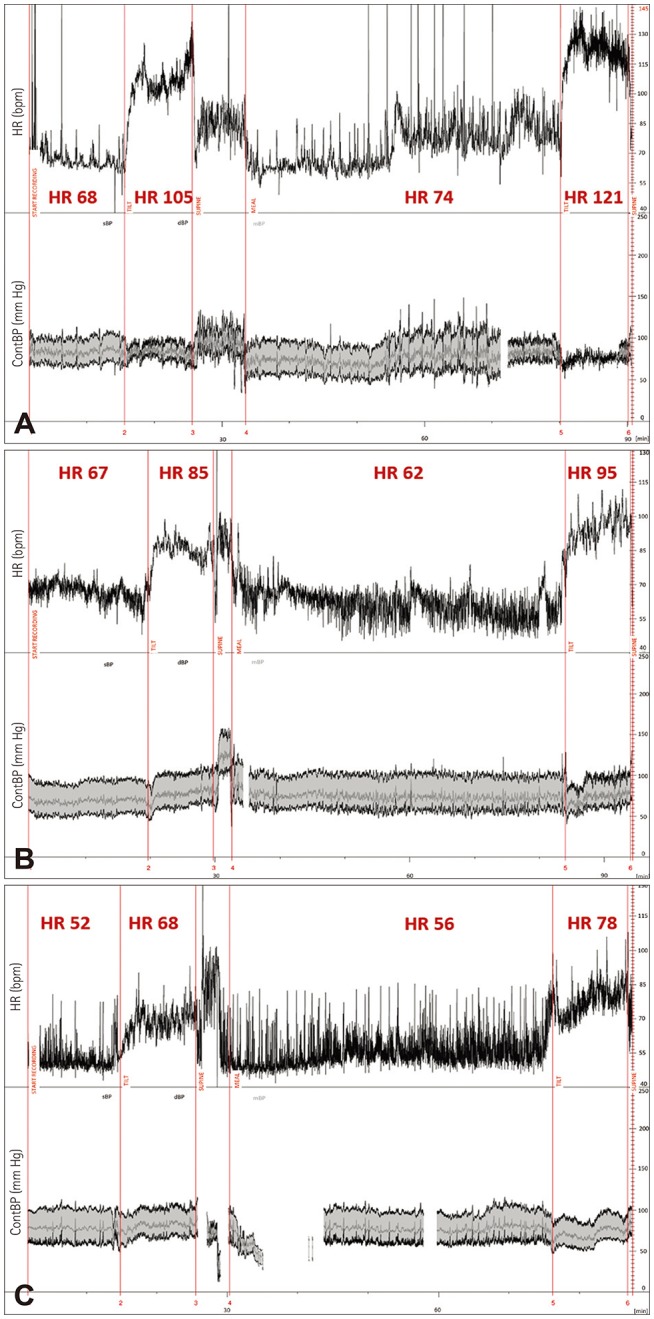
Fig. 2. Example HR and BP tracings from representative patients in groups A (upper part, A), B (middle part, B), and C (lower part, C). ContBP: continuous blood pressure, HR: heart rate.
In order to test the hypothesis, we pooled patients from group A and B into a POTS group and patients from group C and D into a non-POTS group according to their symptoms.12
Statistical analysis was conducted using the SPSS software (version 20; IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test was used to check whether the data conformed to a normal distribution. Differences in the distribution of qualitative variables were determined using the χ2 test, while differences in quantitative variables were determined using the ANOVA with the Bonferroni post-hoc multiple-comparison correction, and using the paired t-test. For the paired t-test, according to Bonferroni multiple-comparison correction, a p value of <0.001 was considered statistically significant. For other comparisons, a p value <0.05 was considered statistically significant. Receiver operating characteristics (ROC) curves were used to interpret the sensitivity and specificity of different cutoff values in differentiating between POTS and non-POTS patients.
RESULTS
Descriptive data and the distributions of HR and BP parameters before and after the meal are presented in Table 1. All of the participants in groups A and B had a 3-month history of symptoms characteristic of POTS. Participants from group C were referred to the laboratory due to at least one episode of vasovagal syncope and they had no characteristic POTS symptoms. There were no intergroup differences in age and sex (p=0.112 and p=0.203, respectively).
Table 1. Descriptive data and distribution of HR and BP parameters before and after the ingestion of a meal.
| Group A (n=13) | Group B (n=12) | Group C (n=16) | Group D (n=10) | p | |
|---|---|---|---|---|---|
| Age, years | 31.46±10.53 | 25.67±7.89 | 32.00±12.06 | 24.25±2.25 | 0.112 |
| Sex, female | 6 | 9 | 13 | 6 | 0.203 |
| Before meal | |||||
| sBP, mm Hg | |||||
| Supine | 113.62±8.47 | 109.50±10.42 | 106.00±13.35 | 112.60±12.17 | 0.291 |
| Tilted | 109.00±11.23 | 109.08±13.86 | 110.00±11.89 | 109.50±15.76 | 0.997 |
| dBP, mm Hg | |||||
| Supine | 72.08±9.34 | 63.25±20.44 | 66.00±8.44 | 68.70±6.53 | 0.324 |
| Tilted | 76.77±11.66 | 73.00±9.40 | 74.19±9.11 | 72.90±9.33 | 0.751 |
| HR, beats/min | |||||
| Supine | 74.85±8.05 | 75.00±10.30 | 68±10.40 | 74.50±16.58 | 0.311 |
| Tilted | 113.31±10.01 | 100.58±12.99 | 85.69±16.33 | 88.10±11.87 | <0.01* |
| After meal | |||||
| sBP, mm Hg | |||||
| Supine | 114.23±8.17 | 109.08±8.80 | 105.19±11.33 | 110.10±13.60 | 0.165 |
| Tilted | 105.23±11.88 | 104.08±14.10 | 107.88±13.45 | 109.20±12.28 | 0.766 |
| dBP, mm Hg | |||||
| Supine | 70.69±8.47 | 66.33±7.33 | 64.88±8.69 | 65.20±9.71 | 0.286 |
| Tilted | 72.77±8.01 | 68.75±9.30 | 72.00±10.55 | 69.50±5.76 | 0.623 |
| HR, beats/min | |||||
| Supine | 79.31±11.54 | 73.83±9.86 | 71.25±9.50 | 72.20±11.87 | 0.217 |
| Tilted | 125.38±14.64 | 107.08±10.78 | 92.75±11.56 | 95.60±11.97 | <0.01† |
Data are n or mean±SD values.
*p<0.01 for group A–C and group A–D, p=0.031 for group B–C, †p=0.003 for group A–B, p<0.01 for group A–C and group A–D, p=0.023 for group B–C.
BP: blood pressure, dBP: diastolic blood pressure, HR: heart rate, sBP: systolic blood pressure.
There were no differences in the supine HR and the supine or tilted systolic BP and diastolic BP before or after the meal between the study groups (all p>0.05). There was a significant difference in the tilted HR before the meal between group A and groups D and C (p<0.00001). Before the meal, ΔHR was significantly higher in group A than in all of the other groups, and in group B than in group D (p<0.001).
Differences in supine HR, tilted HR, and the increase in HR after tilting depending on the ingestion of a meal are presented in Fig. 3.
Fig. 3. Differences in supine HR, tilted HR, and the increase in HR after the tilt depending on the meal. Bar indicate the values before and after the meal, respectively. Note that ΔHR (difference in HR for standing vs. supine) was significantly higher after the meal only in groups A and B (p<0.001 and p<0.0001, respectively). *Indicates significant difference. HR: heart rate.
The results of the ROC analysis performed for ΔHR before and after the meal for the POTS and non-POTS groups are presented in Fig. 4. According to the ROC curve of the ΔHR before the meal (Fig. 4A), 91% of the total area was under the curve. To differentiate between subjects with POTS, the optimal cutoff for ΔHR was found to be 25 beats/min, which gave a sensitivity of 92.0% and a specificity of 80.8%. A cutoff of 30 beats/min for ΔHR before the meal had a sensitivity of 52.0% and a specificity of 96.2%.
Fig. 4. Results of the ROC analysis performed for Δheart rate before (A) and after (B) the meal. ROC: receiver operating characteristics.
According to the ROC curve of ΔHR after the meal (Fig. 4B), 97% of the total area was under curve. To differentiate between subjects with POTS, the optimal cutoff for ΔHR after the meal was found to be 30 beats/min, which gave a sensitivity of 96.0% and a specificity of 96.2%.
DISCUSSION
The main finding of this study was that the ΔHR cutoff for diagnosing POTS depends on the timing of performing the tilt-table test relative to the food intake.
Several previous studies have shown how food ingestion can influence ΔHR in patients with POTS, but those studies had different aims. A study performed by Tani et al.11 found that there was a significant difference in the tilted HR before and after the meal for both healthy controls and POTS patients (77.0±1.9 vs. 89.1±2.7 beats/min and 120.8±4.6 vs.133.0±5.4 beats/min, respectively). Although differences in ΔHR before and after the meal were not reported for another study, the results showed that the supine HR did not change after the meal in POTS patients; however, during the postprandial orthostatic challenge, POTS patients had a significantly greater ΔHR compared to healthy subjects.13
A particularly interesting observation was that no such influence of food intake on HR was observed in patients with neurally mediated syncope, although a different methodology was used.14 Moreover, several studies have suggested that food intake does not significantly change hemodynamic parameters in most healthy people.15,16
There are several possible explanations for why such differences are observed in patients with POTS. The splanchnic circulation serves as a large reservoir for blood and is thought to be important for BP regulation.17 While normal subjects compensate for this redistribution of the blood volume that happens after the food ingestion.18 patients with chronic generalized autonomic failure do not have recourse to reflex regulation and also develop hypotension and worsening orthostatic hypotension.10,19 If POTS is considered to be a mild form of dysautonomia, the increase in HR after food ingestion may be considered a milder consequence of food intake, comparable to the drop in BP seen in patients with autonomic failure. In line with this, postprandial worsening of orthostatic symptoms commonly develops in both conditions.11,20
One of the main problems in past research into the effects of food intake on hemodynamic responses in neurological conditions was revealed in a recently performed meta-analysis showing that the nutrients ingested, measurement frequency, duration, and position of the subject are not properly defined for the younger neurologic populations that typical suffer from POTS.10 It is surprising that almost all such studies use supine HR and BP measurements after meal ingestion, while most of the symptoms and problems of orthostatic intolerance occur in an upright position. Furthermore, the importance of meal ingestion lies in different hemodynamic responses depending on the meal. In patients with neurally mediated syncope, ingesting either carbohydrate or water results in opposite effects on BP during postural maneuvers, whereas this does not occur in control subjects.14 Even the osmolality of the fluid may affect the hemodynamic responses in patients with orthostatic intolerance.21
One of the limitations of the present study was the possibility of selection bias. Although we enrolled consecutive patients, which patients were referred to the laboratory was dependent on other sources. Furthermore, there is a possibility that some of the patients in group C recovered by the time they were tested for the second time. Finally, we did not classify patients according to POTS subtypes (e.g., neuropathic or hyperadrenergic).
In conclusion, food intake can significantly alter the results of the tilt-table test and should be taken into account when diagnosing POTS. Performing the tilt-table test after consuming a controlled meal might increase the sensitivity and specificity of the test; however, this hypothesis should be tested in a larger sample of patients and healthy subjects.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep. 2015;15:60. doi: 10.1007/s11910-015-0583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raj SR. Postural tachycardia syndrome (POTS) Circulation. 2013;127:2336–2342. doi: 10.1161/CIRCULATIONAHA.112.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Adamec I, Lovrić M, Žaper D, Barušić AK, Bach I, Junaković A, et al. Postural orthostatic tachycardia syndrome associated with multiple sclerosis. Auton Neurosci. 2013;173:65–68. doi: 10.1016/j.autneu.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal AK, Garg R, Ritch A, Sarkar P. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478–480. doi: 10.1136/pgmj.2006.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 7.Trahair LG, Horowitz M, Jones KL. Postprandial hypotension: a systematic review. J Am Med Dir Assoc. 2014;15:394–409. doi: 10.1016/j.jamda.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Masuda Y, Kawamura A. Role of the autonomic nervous system in postprandial hypotension in elderly persons. J Cardiovasc Pharmacol. 2003;42(Suppl 1):S23–S26. doi: 10.1097/00005344-200312001-00007. [DOI] [PubMed] [Google Scholar]
- 9.Fagius J, Ellerfelt K, Lithell H, Berne C. Increase in muscle nerve sympathetic activity after glucose intake is blunted in the elderly. Clin Auton Res. 1996;6:195–203. doi: 10.1007/BF02291134. [DOI] [PubMed] [Google Scholar]
- 10.Pavelić A, Krbot Skorić M, Crnošija L, Habek M. Postprandial hypotension in neurological disorders: systematic review and meta-analysis. Clin Auton Res. 2017;27:263–271. doi: 10.1007/s10286-017-0440-8. [DOI] [PubMed] [Google Scholar]
- 11.Tani H, Singer W, McPhee BR, Opfer-Gehrking TL, Haruma K, Kajiyama G, et al. Splanchnic-mesenteric capacitance bed in the postural tachycardia syndrome (POTS) Auton Neurosci. 2000;86:107–113. doi: 10.1016/S1566-0702(00)00205-8. [DOI] [PubMed] [Google Scholar]
- 12.Crnošija L, Krbot Skorić M, Adamec I, Lovrić M, Junaković A, Mišmaš A, et al. Hemodynamic profile and heart rate variability in hyperadrenergic versus non-hyperadrenergic postural orthostatic tachycardia syndrome. Clin Neurophysiol. 2016;127:1639–1644. doi: 10.1016/j.clinph.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Seligman WH, Low DA, Asahina M, Mathias CJ. Abnormal gastric myoelectrical activity in postural tachycardia syndrome. Clin Auton Res. 2013;23:73–80. doi: 10.1007/s10286-012-0185-3. [DOI] [PubMed] [Google Scholar]
- 14.Pitt MS, Hainsworth R. Contrasting effects of carbohydrate and water on blood pressure responses to postural maneuvers in patients with posturally related (vasovagal) syncope. Clin Auton Res. 2004;14:249–254. doi: 10.1007/s10286-004-0198-7. [DOI] [PubMed] [Google Scholar]
- 15.Imai C, Muratani H, Kimura Y, Kanzato N, Takishita S, Fukiyama K. Effects of meal ingestion and active standing on blood pressure in patients ≥60 years of age. Am J Cardiol. 1998;81:1310–1314. doi: 10.1016/s0002-9149(98)00163-5. [DOI] [PubMed] [Google Scholar]
- 16.Puisieux F, Boumbar Y, Bulckaen H, Bonnin E, Houssin F, Dewailly P. Intraindividual variability in orthostatic blood pressure changes among older adults: the influence of meals. J Am Geriatr Soc. 1999;47:1332–1336. doi: 10.1111/j.1532-5415.1999.tb07434.x. [DOI] [PubMed] [Google Scholar]
- 17.Rowell LB. Regulation of splanchnic blood flow in man. Physiologist. 1973;16:127–142. [PubMed] [Google Scholar]
- 18.Fujimura J, Camilleri M, Low PA, Novak V, Novak P, Opfer-Gehrking TL. Effect of perturbations and a meal on superior mesenteric artery flow in patients with orthostatic hypotension. J Auton Nerv Syst. 1997;67:15–23. doi: 10.1016/s0165-1838(97)00087-8. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhuri KR, Thomaides T, Hernandez P, Alam M, Mathias CJ. Noninvasive quantification of superior mesenteric artery blood flow during sympathoneural activation in normal subjects. Clin Auton Res. 1991;1:37–42. doi: 10.1007/BF01826056. [DOI] [PubMed] [Google Scholar]
- 20.Mathias CJ, Mallipeddi R, Bleasdale-Barr K. Symptoms associated with orthostatic hypotension in pure autonomic failure and multiple system atrophy. J Neurol. 1999;246:893–898. doi: 10.1007/s004150050479. [DOI] [PubMed] [Google Scholar]
- 21.Z'Graggen WJ, Hess CW, Humm AM. Acute fluid ingestion in the treatment of orthostatic intolerance - important implications for daily practice. Eur J Neurol. 2010;17:1370–1376. doi: 10.1111/j.1468-1331.2010.03030.x. [DOI] [PubMed] [Google Scholar]