Abstract
Background
Global consumption of protein per capita is rising, while rates of infertility are increasing. However, a clear relationship between protein intake and reproductive health has not been demonstrated. The activation of the quiescent primordial follicles is the first step of folliculogenesis, and their activation must be tightly controlled to prevent premature exhaustion of the ovarian follicular reserve.
Methods
The primordial follicle reserve of wild-type or liver-specific ablation of fibroblast growth factor 21 (FGF21) in mice, subjected to limited or excessive protein diets or oral gavage test, were detected in vivo. Mouse ovary organ cultures were used to examine the direct role of metabolites or metabolic hormones on primordial follicle activation.
Findings
Mouse primordial follicle activation, was reduced by restricted protein intake and was accelerated by excessive protein intake, in an ovarian mTORC1 signaling-dependent manner. Furthermore, restricted or excessive protein intake resulted in an augmentation or decline of oocyte number and fertility at older age, respectively. Liver-specific ablation of FGF21, which resulted in a reduction of 87% in circulating FGF21, abrogated the preserving effect of low-protein intake on primordial follicle pool. Interestingly, FGF21 had no direct effect on the activation of primordial follicles, but instead required an adipokine adiponectin. Moreover, AdipoRon, an oral adiponectin receptor agonist, prevented the over-activation effect of excessive protein intake on primordial follicle activation.
Interpretation
Dietary protein consumption controlled ovarian primordial follicle reserve and fertility, which required coordination between FGF21 and adiponectin.
Fund
Natural Science Foundation of China (Grant 31772616).
Keywords: Adiponectin, FGF21, Primordial follicle, Protein intake
Research in context.
Evidence before this study
Dietary protein intake is closely related to reproduction, but its clear role on follicle development is uncertain. Fibroblast growth factor 21 (FGF21) was shown to mediate the beneficial effects of dietary protein-restriction on energy homeostasis, yet the role of FGF21 on fertility remained controversial.
Added value of this study
Dietary protein consumption correlated closely to the ovarian primordial follicle reserve across different species including pigs and mice. The beneficial effects of low-protein intake on the primordial follicle reserve required FGF21. FGF21 exerted no direct effects on primordial follicle activation, but instead exerted its effects on primordial follicle activation indirectly via adiponectin.
Implications of all the available evidence
More women are having their first babies at delayed age in many developed countries. Meanwhile, the global consumption of protein per capita is rising. Our results suggest that the increased consumption of protein could have harmful effects on their ovarian primordial follicle reserve and fertility when they are pregnant at an older age.
Alt-text: Unlabelled Box
1. Introduction
The primordial follicle, consisting of an oocyte enclosed in a single layer of pre-granulosa cells, is the fundamental storage unit of the female germline. The pool of primordial follicles, which is established around the time of birth and is unable to regenerate in postnatal life [[1], [2], [3], [4]], is crucial for reproductive capacity. Once established the primordial follicle reserve provides all of the oocytes for postpubertal ovulations and is the approximate determinant of reproductive lifespan [5]. The primordial follicles are maintained in a quiescent state, remaining dormant until being activated and recruited into the growing follicular pool [6]. Following activation primordial follicles transit through growth stages and subsequently provide the mature oocytes through the process of folliculogenesis [7]. The dormancy of primordial follicle must be strictly regulated to prevent premature exhaustion of the primordial follicle reserve, which results in termination of the reproductive process and reproductive aging [7].
The mammalian target of the rapamycin complex 1 (mTORC1) pathway has been suggested as the key signaling mechanism for the activation of primordial follicles [7] and mTORC1 signaling both in pre-granulosa cells and oocytes is required [8,9]. The activation of dormant oocytes requires an mTORC1-KITL cascade in primordial follicle granulosa cells and KIT-PI3K signaling in oocytes [9]. Based on those findings, short-term treatment with rapamycin, an inhibitor of mTORC1 signaling, exhibits persistent effects on prolonging the ovarian lifespan irrespective of the age at treatment [10]. Moreover, blocking of ovarian mTORC1 signaling, using the clinically approved drug everolimus (RDA001) or experimental drug INK128, protects ovarian primordial follicles from over-activation and preserves fertility during genotoxic chemotherapy [11]. Therefore, these results highlight the central role of ovarian mTORC1 signaling in control of the ovarian primordial follicle reserve as well as female reproductive lifespan.
Nutrition has been shown to profoundly influence fertility. In recent decades, the average consumption of protein per capita has increased [12], while the accelerated decline of fertility is becoming a serious health problem in high-income countries [13]. Studies conducted on Drosophila melanogaster have revealed that lifespan and fecundity were differentially influenced by the intake of protein-to-carbohydrate ratio, but not energy intake, and the lifespan and lifetime fertility were maximized at low protein-to-carbohydrate ratios [14]. Recently, it was observed that dietary proteins, unlike carbohydrates and lipids, could rescue the nutrient restriction-induced blockade of the reproductive cycle as well as ovarian development in mice [15]. Interestingly, the intake of dietary protein is closely related to mTORC1 signaling [16], possibly attributed to the clear association between amino acids and the activity of mTORC1 signaling [17]. These results suggest a causal role of protein-to-carbohydrate ratio on ovarian primordial follicle activation and maturation but remains to be defined.
In mammals, the liver plays a crucial role in the control of amino acid metabolism, and hepatic fibroblast growth factor 21 (FGF21) is considered as the most important endocrine signal required for the control of energy metabolism and lipid homeostasis by protein restriction [[18], [19], [20], [21], [22]]. Though FGF21 has beneficial effects on metabolic fitness [[23], [24], [25]], the effects of FGF21 on reproduction remained controversial [[26], [27], [28]]. Furthermore, the direct effect of FGF21 on ovarian development is largely unknown, despite receptors for FGF family members being widely expressed in ovaries [29]. Therefore, in the present study the effects of dietary protein level on ovarian primordial follicle activation and subsequent reproductive performance in mice were investigated, as well as the role of hepatic FGF21 in this process.
2. Methods
2.1. Study approval
All animal procedures in this study were handled in accordance with Guide for the Care and Use of Laboratory Animals (National Research Council, Bethesda, MD, USA), and the Institutional Animal Care and Research Committee of Sichuan Agricultural University (SICAU-2015-034).
2.2. Animals and dietary interventions
Four-week old female C57BL/6J mice were obtained from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China) and were housed in groups of three or four per ventilated cage. Mice were kept in temperature controlled (22 ± 1 °C) facilities with a 12-hour light/dark cycle (12-hour light period starting at 06:00). The FGF21 liver-specific knockout mice were generated as previously described [30], in brief, FGF21Liver+/−,Alb-Cre mice were generated by mating FGF21loxp/loxp mice (022361; The Jackson Laboratory, Bar Harbor, ME, USA) with Alb-Cre mice (J003574; Model Animal Research Center, Nanjing University, Nanjing, China) transgenic mice. FGF21Liver−/−,Alb-Cre mice were generated by crossing FGF21Liver+/−,Alb-Cre mice with FGF21loxp/loxp mice. Littermates of FGF21loxp/loxp mice were used as controls. The efficiency of FGF21 knockout was shown previously [30].
Mice were provided ad libitum one of the 3 diets varying in protein and carbohydrate content (Table S1). All diets were custom designed and manufactured in dry, pelleted form by Dossy Experimental Animals Co. LTD (Chengdu, China), including a normal-protein (NP, Cat.# D2014028-N) diet deriving energy 59.26% from carbohydrate, 17.97% from lipid and 22.49% from protein, a low-protein (LP, Cat.# D2014028-L) diet deriving energy 73.15% from carbohydrate, 17.87% from lipid and 9.07% from protein, whereas a high-protein (HP, Cat.# D2014028-H) diet deriving energy 25.44% from carbohydrate, 18.05% from lipid and 56.12% from protein. The food intake was determined every two weeks using each cage as an experimental unit, and the bodyweight of each mouse were measured every two weeks. At 4, 12, 24 and 48 weeks on diets, mice at the stage of diestrus (n = 8–10/group) were euthanized using carbon dioxide followed by cervical dislocation. FGF21LKO mice, mice (n = 6–8) were euthanized for sampling at 12 weeks on diets. Mice (n = 6) were sacrificed for collecting samples 4 weeks after oral AdipoRon administration. Paired ovaries were dissected in ice-cooled PBS to remove excess tissues under stereoscopic microscopes (SZX16, Olympusm, Japan). The left ovary, liver tissues, and inguinal white adipose tissue were snap-frozen in liquid nitrogen followed by storage at −80 °C, and the right ovary were fixed in 4% (weight/volume) paraformaldehyde dehydrated, and embedded in paraffin.
2.3. Serum measurements
Serum levels of amino acids in mice on diets for 12 weeks in a fed state were determined by high-performance liquid chromatography. Commercial enzyme-linked immunosorbent assay kits were used to detect the serum concentrations of FGF21 (MF2100, R&D Systems), IGF1 (MG100, R&D Systems), adiponectin (EZMADP-60K, Millipore) and leptin (EZML-82K, Millipore).
2.4. Culture of mouse ovaries in vitro
Mouse ovary organ culture was conducted as previously described [31]. Briefly, ovaries from newborn (24–36 h) female mice were collected and dissected in DMEM/Ham's F12 (Invitrogen) containing 3 mg/ml BSA (sigma), 1 mM sodium pyruvate (Invitrogen), 50 U/ml penicillin (Invitrogen) and 50 μg/ml streptomycin (Invitrogen). Then the ovaries were cultured in DMEM/Ham's F12 (Invitrogen) containing 3 mg/ml BSA (sigma), 1 mM sodium pyruvate (Invitrogen), 2 × Insulin-Transferrin-Selenium-Ethanolamine (Invitrogen), 50 U/ml penicillin (Invitrogen) and 50 μg/ml streptomycin (Invitrogen), 1 ng/ml EGF (Prospec) on hydrophilic polycarbonate membrane (Millipore) floating in 12-well plates (Nunc) with 800 ml of culture medium (10 ovaries per well). Eight ovaries per well were used to detect protein expression after treatment for 6 h [32]. Then, the remaining ovaries were cultured for 4 days at 37 °C in a humidified atmosphere of 5% CO2 followed by histological analysis. The medium was changed every second day.
Ovary organ culture in vitro was performed to evaluate the effects of amino acid levels in medium on ovarian primordial follicle activation. The ovaries were dissected in amino acid-free DMEM/Ham's F12 containing 1 mM sodium pyruvate (Invitrogen), 50 U/ml penicillin (Invitrogen) and 50 μg/ml streptomycin (Invitrogen), and cultured in amino acid-free DMEM/Ham's F12 containing 1 mM sodium pyruvate (Invitrogen), 2 × Insulin-Transferrin-Selenium-Ethanolamine (Invitrogen), 50 U/ml penicillin (Invitrogen) and 50 μg/ml streptomycin (Invitrogen). Ovaries were cultured with graded level of amino acids according to the serum amino acid composition of mice on diets for 12 weeks when the ovarian primordial follicle counts were significantly affected by diets. For ovaries treated with FGF21, adiponectin and combined supplementation of adiponectin and compound C (20 μM), the treatments were performed 30 min prior to the placement of the ovaries.
2.5. Oral AdipoRon administration
Four-week old mice fed with the LP, NP and HP diets were treated via oral gavage of DMSO (as control) or AdipoRon according to a 2 × 3 factorial design. AdipoRon (Selleck Chemicals, Houston, TX, USA) dissolved in DMSO (Sigma) was suspended in 0.5% carboxymethylcellulose and was then administered orally to each group of mice (n = 6) at a dose of 50 mg/kg per day. This dosage was observed to have beneficial effects on metabolism, lifespan, and anti-apoptosis [[33], [34], [35]]. Mice were sacrificed for sampling 28 days after treatment.
2.6. Histology and follicle counts
The quantification of ovarian follicles was performed as previously described with small modifications [36,37]. In brief, paraffin-embedded ovaries were serially sectioned at 5-μm thickness and stained with H&E for morphological observation. Every fifth section was counted for in vitro cultured ovaries, ovaries of mice on diets for 4 weeks and 12 weeks, and every tenth section on diets for 24 weeks and 48 weeks. Different classes of ovarian follicles were defined as previously described [36]. Those with oocytes surrounded by one layer of flattened five to eight somatic cells were defined as a primordial follicle. Primary follicles consisted of an oocyte surrounded with one layer containing one enlarged cell or a whole layer of cuboidal pre-granulosa cells. Secondary follicles contained more than one layer of granulosa cells but without an antrum. Once an antrum formed in the granulosa cell layers, the follicle was defined as an antral follicle. Oocytes with visible nuclei from primordial, primary, secondary and antral follicles were quantitated. Atretic follicles were defined as previously described [38], briefly, atretic follicles were usually evident at the stage of late secondary or antral follicles, and those follicles had an aberrant oocyte and multiple layers of pycnotic granulosa cells. The total follicle counts at each developmental stage were multiplied by a correction factor of 5 (in vitro cultured, 4 weeks and 12 weeks on diets) or 10 (24 weeks and 48 weeks on diets) to represent the estimated number of total follicles in an ovary.
2.7. RNA extraction and detection of gene expressions
RNA was isolated with Trizol reagent (15596018; Thermo Fisher Scientific) and RNeasy Mini Kit (RR037A; Takara Bio, Kusatsu, Japan), according to the manufacturer's instruction. The cDNA was reversed from RNA (500 ng) with reagents from Takara Bio. Amplification and relative Ct values were determined using an ABI PRISM 7900HT Fast Real-Time PCR system (Thermo Fisher Scientific) using SYB Green Real-Time PCR regent (RR820A; Takara Bio). The cycle threshold (2−ΔΔCt) method was used to calculate the relative gene expression. Gene expression levels were normalized to β-actin expression levels. The primer sequence were Fgf21, forward 5′-CTGGGGGTCTACCAAGCATA-3′ and reverse 5′-CACCCAGGATTTGAATGACC-3′, β-actin forward 5′-GGCTGTATTCCCCTCCATCG-3′ and reverse 5′-CCAGTTGGTAACAATGCCATGT-3′, Igf1 forward 5′-CACATCATGTCGTCTTCACACC-3′ and reverse 5′-GGAAGCAACACTCATCCACAATG-3′, AdipoQ forward 5′-TGTTCCTCTTAATCCTGCCCA-3′ and reverse 5′-CCAACCTGCACAAGTTCCCTT-3′, Pten forward 5′-CGGCAGCATCAAATGTTTCAG-3′and reverse 5′-AACTGGCAGGTAGAAGGCAAC, Lhx8 forward 5′-TCCAGGTTATGCAAGCACAG-3′ and reverse 5′-GGAGTTGTTGGTGAGCATCC-3′, Foxo3a forward 5′-GCAAGCCGTGTACTGTGGA-3′ and reverse 5′-CGGGAGCGCGATGTTATCC-3′.
2.8. Western blotting
Detections of protein expressions were performed for the whole mouse ovaries as previously described in our lab [30]. In brief, each ovary sample was homogenized in 150 μL of lysis buffer (catalog P0013B, Beyotime Biotechnology, Jiangsu, China) supplemented with protease inhibitor cocktail (catalog 04693132001, Roche, USA). The supernatant, after centrifuging at 12,000g for 30 min at 4 °C, was collected to measure the protein content using BCA protein assay kit (catalog 23227, Thermo Scientific, IL, USA) on a plate reader. Proteins were separated on 10% SDS-PAGE gel after boiling at 95 °C for 10 min and was then transferred to an apolyvinylidene fluoride membrane (catalog 1620177, BioRad, CA, USA). The membrane was washed in Tris-buffered saline containing tween (TBST) and blocked with 1% bovine serum albumin in TBST at room temperature for at least 1 h with gentle shaking, followed by incubation overnight at 4 °C with the respective primary antibodies. Antibodies against β-actin (catalog 4970S), p-mTOR (catalog 2971S), mTOR (catalog 2972S), p-S6 (catalog 4858T), S6 (catalog 2217S), p-ERK1/2 (catalog 9201S), ERK1/2 (catalog 9202S), p-AMPK (catalog 2535S) and AMPK (catalog 2793S) were obtained from Cell Signaling Technology (Danvers, MA, USA). After three washes with TBST the following morning, membranes were incubated with the respective secondary antibodies (7074 and 7076, CST) at room temperature for 1 h, after 3 washes. Protein signals were detected by ECL western blotting detection reagent (catalog 1705060, BioRad) on a Molecular Imager ChemiDoc XRS+ System (BioRad). Blots were quantified with Image J software (National Institutes of Health, Bethesda, MD, USA).
2.9. Statistics
One-way analysis of variance (ANOVA) was applied to analyze the difference between three or more groups using Graphpad Prism 6 (GraphPad Software Incorporated, La Jolla, CA, USA), and unpaired t-test was applied to analyze the difference between two groups. For the experiments involving oral AdipoRon treatment, or FGF21 liver-specific knockout mice, data was analyzed using two-way ANOVA test or with un-paired t-test for multiple comparisons in order to determine differences between each group where appropriate. For the mating experiment of mice on protein restricted or excess diets for 48 weeks, the percentage of pregnant mice were analyzed by Chi-square test. Data was presented as mean ± SEM. Statistical significance was set at P < .05.
3. Results
3.1. Consumption of diets differing in protein content resulted in differential ovarian primordial follicle activation rates
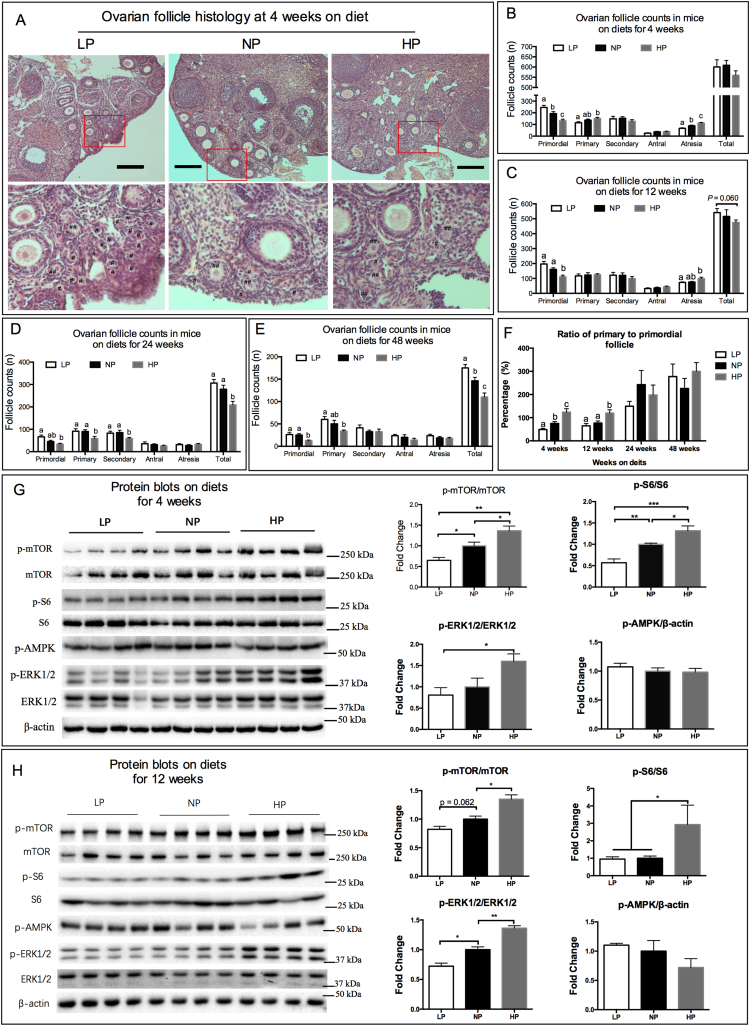
C57BL/6 female mice were fed ad libitum with three types of isocaloric diets varying in the content of protein and carbohydrate (Table S1). Consistent with a recent report [39] food intake was greater in mice fed a LP diet and was lower in mice fed a HP diet, when compared with mice fed a NP diet (Fig. S1A). This resulted in substantial differences in protein intake among the three groups (Fig. S1B). There were no differences in body weight among the three groups within the first 16 weeks of treatment, but body weight was lower in the HP group following 16 weeks on diets when compared to the other two groups (Fig. S1C). The analysis of follicular dynamics showed that the number of primordial follicles per ovary increased in mice fed the LP diet and was decreased in mice fed the HP diet, when compared to mice fed the NP diet for 4 weeks. Meanwhile, the number of primary follicles (Fig. 1A) decreased in mice fed the LP diet, and was increased in mice fed the HP diet, when compared to ovaries in the NP group (Fig. 1B). When mice were fed the HP diet for 12, 24 and 48 weeks (Fig. 1C–E) the number of ovarian primordial follicles were significantly lower compared with mice fed the NP or LP diets. The number of atretic follicles was greater in the mice fed the HP diet compared to mice fed the NP or LP diets (Fig. 1B and C). Moreover, the percentage of ovarian follicles at each developmental stage were calculated (Fig. S1D–G) and showed that the mice fed the LP diet had a greater percentage of primordial follicles and lower percentage of primary follicles, and the mice fed the HP diet had a lower percentage of primordial follicles and a higher percentage of primary follicles (Fig. S1D and E). The ratio of primary to primordial follicles, evidence of follicle activation, was lower in mice fed the LP diet, but was increased in mice fed the HP diet, when compared to mice fed the NP diet for 4 and 12 weeks (Fig. 1F).
Fig. 1.
Consumption of diets differing in protein content resulted in substantial changes of ovarian primordial follicle activation. Ovaries from mice on diets with low protein (LP), normal protein (NP) or excess protein (HP) for 4 weeks, 12 weeks, 24 weeks and 48 weeks were analyzed for follicle counts at different developmental stages (n = 8–10 per group). (A) Representative ovarian histology of mice on diets for 4 weeks. #, primordial follicle. ##, primary follicle. Scale bars: 200 μm. Original magnification: ×100. Follicle counts at each developmental stage were determined at 4 weeks (B), 12 weeks (C), 24 weeks (D) and 48 weeks (E) on diets. (F) Ratio of primary to primordial follicles, based on primordial and primary follicle counts from B-E. Ovarian protein expressions of phosphorylated mTOR, S6, ERK1/2 and AMPK in mice on diets for 4 weeks (G) and 12 weeks (H) as detected by western blotting (n = 8 per group for quantification). Columns with different letter a,b,c denotes P < .05 by un-paired t-test. *P < .05, **P < .01, ***P < .001 by un-paired t-test.
Primordial follicle dormancy is generally maintained by inhibitory factors in oocytes including those encoded by the genes for Pten [37], Lhx8 [40], and Foxo3a [41]. Oocytes from the three groups were isolated and the expression of Pten, Lhx8 and Foxo3a in the oocytes were greater in the LP fed mice when compared to the HP fed mice at week 4 (Fig. S1H–J). The phosphorylation levels of mTOR, and its downstream target S6, in the ovaries were significantly lower in mice fed the LP diet, but were greater in mice fed the HP diet, when compared with the mice fed the NP diet at weeks 4 and 12 (Fig. 1G and H). Similar effects were observed for the phosphorylations of ERK1/2, but not for the phosphorylation of AMPK in the ovaries (Fig. 1G and H). Pig ovaries share similarities with human ovaries including follicle numbers and size [42]. Thus, young female pigs were fed a diet containing adequate protein (AP) for maximum growth as recommended by the NRC nutrient requirement of swine (2012), or a protein-restricted diet (PR) which contained only 50% digestible amino acids of the AP diet for 4 weeks. Ovarian histology revealed that the intake of a protein restricted diet increased the percentage of ovarian primordial follicle in the pigs (68.74% vs 80.39%, P = .012, Fig. S1K), while decreasing the percentage of primary follicles (20.87% vs 15.47%, P = .062, Fig. S1K), secondary follicles (9.46% vs 3.31%, P = .040, Fig. S1K) and the ratio of primary to primordial follicles (31.41% vs 20.49%, P = .027, Fig. S1L), when compared with the AP diet. The number of antral follicles with a diameter between 1–3 mm on the surface of the ovaries was not affected by dietary treatment (Fig. S1M). Western blot analysis revealed that mTORC1 signaling (featured by p-mTOR/mTOR, p-S6/S6) and ERK1/2 signaling in the pig ovaries were down-regulated in the ovaries of pigs fed the LP diet when compared with those fed the AP diet (Fig. S1N). These results demonstrate that the consumption of diets differing in protein content resulted in substantial changes in ovarian primordial follicle activation.
3.2. Fertility was altered by dietary protein restriction or excessive protein intake
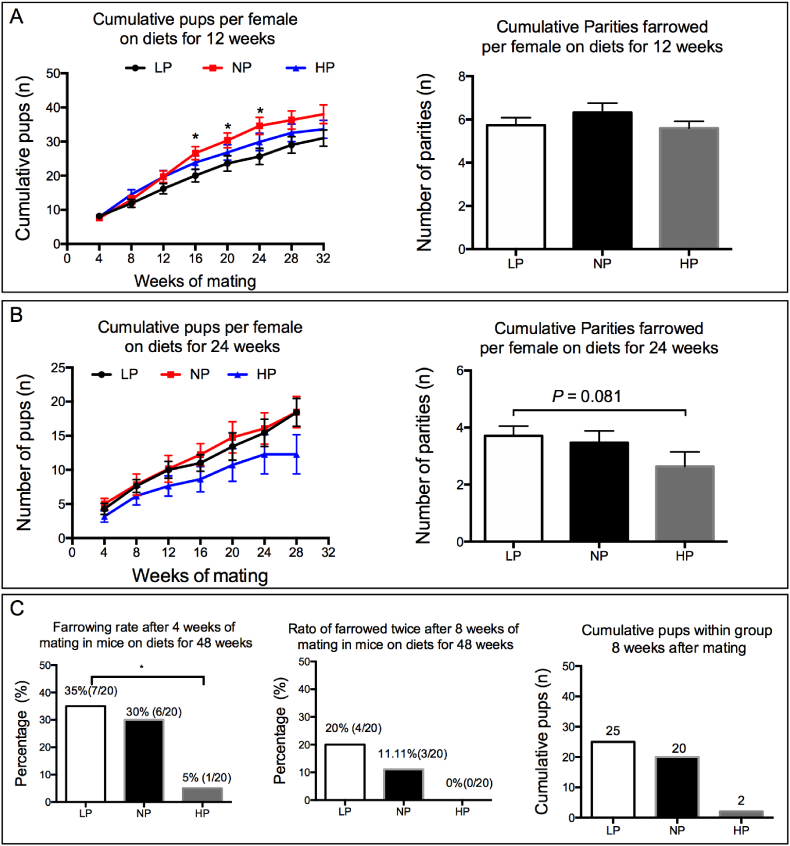
Since the ovarian primordial follicle reserve was profoundly changed by dietary protein intake, we hypothesized that those changes would cause alternations in fertility. Female mice were primed with PMSG and hCG to induce ovulation, as the number of ovulated oocytes not only reflected the ovarian reserve, but also the quality of antral follicles [43]. Results revealed that the number of ovulated oocytes was not affected by dietary treatment for 4, 12 or 24 weeks (Fig. S2A–C), with the exception of a lower ovulation rate in the HP fed mice than in the LP fed mice on diets for 48 weeks (Fig. S2D). Moreover, female mice on diets for 12, 24 and 48 weeks were mated (1:1), with fertile male mice aged 4–8 months, repeatedly for 32, 28 and 8 weeks, respectively. For mice on diets for 12 weeks, followed by a 32-week mating period, the cumulative number of pups per mouse were lower for the LP fed mice than in the NP fed mice at week 16, 20 and 24 of mating, while no difference of cumulative number of pups per mouse was found at weeks 28 and 32 of mating (Fig. 2A). The cumulative parities farrowed during this 32-week mating period was not affected by dietary treatment (Fig. 2A). For mice on diets for 24 weeks followed by a 28-week mating period, the cumulative number of pups per female did not differ among the three groups except for a tendency of a lower cumulative number of pups in the HP fed mice compared to the other two groups (P = .067 by one-way ANOVA test, Fig. 2B). Mice received dietary treatment for 48 weeks were mated to check their fertility. Four weeks after the beginning of mating, the percentage of farrowed mice was greater in the LP group compared to the HP group (35.0% vs 5.0%, un-paired t-test P < .05, Fig. 2C), which was also observed after eight weeks of mating (Fig. 2C). The cumulative number of pups over the 8-week mating period was lower in the HP fed group compared to the other groups (Fig. 2C). Collectively, these results demonstrated that the subsequent fertility was changed due to dietary protein restriction or excess, and long-term overconsumption of protein caused negative effects on fertility.
Fig. 2.
Subsequent fertility was differentially altered after dietary treatment. Female mice on diets for 12, 24 and 48 weeks from each group (n = 20) were mated with fertile male mice at the ratio of 1:1. (A) Cumulative pups and cumulative parities of mice on diets for 12 weeks in the subsequent 32 weeks of mating, * NP vs LP, P < .05 by un-paired t-test. (B) Cumulative pups and cumulative parities of mice on diets for 24 weeks in the subsequent 28 weeks of mating. (C) Percentage of farrowed mice on diets for 48 weeks in the subsequent 8 weeks of mating, * P < .05 by Chi-square test.
3.3. Mediatory role of hepatic FGF21 in the activation of primordial follicles as regulated by different dietary protein intake
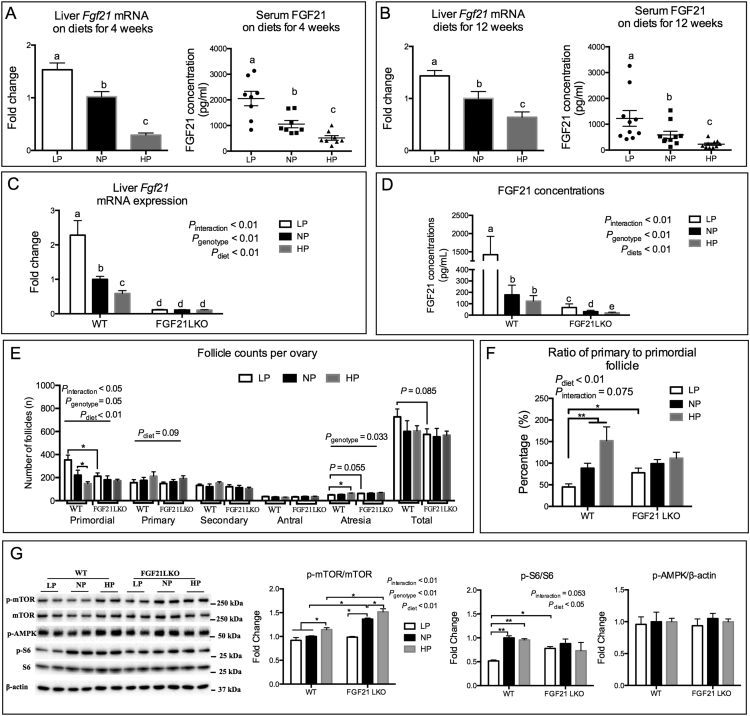
Concentrations of metabolic signals, such as amino acids, IGF1 [15,44] and FGF21 [18,45], are closely correlated to dietary protein intake levels, and are directly or indirectly associated with reproductive function. In this study, the circulating concentrations of amino acids decreased when protein was restricted and were increased when protein intake was in excess (Fig. S3A). Compared to mice fed the NP diet, mice fed the LP diet had greater hepatic Fgf21 expression and elevated serum concentrations of FGF21, which were lower in the mice fed the HP diet (Fig. 3A and B). Furthermore, the changes in FGF21 levels in the liver appear to result from the alteration of serum amino acid levels. There was a dose-dependent decrease in FGF21 expression and secretion during the amino acid treatment in vitro of mouse primary hepatocytes (Fig. S3B and C). Conversely, in vivo, the profile of liver IGF1 expression and secretion (Fig. S3D and E) was not linearly altered as the intake of dietary protein levels increased. These results supported liver-derived FGF21 as a representative endocrine metabolic signal of dietary protein restriction or excess.
Fig. 3.
Mediatory role of hepatic FGF21 in the activation of primordial follicle regulated by dietary protein intake. Liver Fgf21 expression and circulating FGF21 concentration of conventional mice on diets for 4 (A) and 12 weeks (B) as influenced by diets (n = 8–10). Liver Fgf21 expression (C) and circulating FGF21 concentration (D) of FGF21LKO mice on diet for 12 weeks as influenced by dietary protein restriction or excess (n = 6–8). The count of follicle at different developmental stages in each ovary (E), and ratio of primary to primordial follicle (F) of mice were determined. (G) The ovarian protein expressions of phosphorylated mTOR, S6 and AMPK were determined by western blotting (n = 4). Columns with different letter a,b,c denotes P < .05 by un-paired t-test. *P < .05, **P < .01 by un-paired t-test.
Liver FGF21 is the main source of circulating FGF21 [46,47] and metabolic changes seen during protein restriction required hepatic secretion of FGF21 [[18], [19], [20], [21], [22]]. Thus, genetically modified (FGF21LKO) mice, with FGF21 specifically knocked out (KO) in liver cells, were established. The knockout of FGF21 in the liver was confirmed in our previous research [30], as well as by the lack of Fgf21 expression on KO mice used in this study (Fig. 3C). Loss of hepatic FGF21 resulted in an average decrease of 87% (LP, 96%; NP, 85%; HP, 81%) in circulating FGF21 (Fig. 3D). It is worth noting that the circulating FGF21 levels were different in FGF21LKO mice fed different dietary protein levels (Fig. 3D). This indicated that other tissues may also be responsive to dietary protein levels and respond with altered FGF21 secretion. Loss of FGF21 in the livers of the FGF21LKO mice did not affect their daily food intake or bodyweight (Fig. S3F and G).
Similar with what was seen in the wildtype mice (Fig. 1A and B), the LP group had a greater number and percentage of ovarian primordial follicles and lower percentage of primary follicles compared with the HP groups (Fig. 3E and Fig. S3H). Conversely, the HP diet fed mice had a greater number of primary follicles and a lower number of primordial follicles compared with the other two groups (Fig. 3E). The ratio of primary to primordial follicles was lower in the LP fed mice when compared to the other two groups in the WT mice (Fig. 3F). In the FGF21LKO mice, no difference in the number of primary and primordial follicles and their percentages were observed among the different dietary groups (Fig. 3E and Fig. S3H). Furthermore, the phosphorylation levels of mTOR in the ovary were positively correlated to the dietary protein levels in both WT and FGF21LKO mice (P < .01, Fig. 3G). However, FGF21 deficiency in the liver increased the phosphorylation level of ovarian mTOR in the NP and the HP fed mice compared to WT mice fed the same diet (Fig. 3G). The phosphorylation level of ovarian S6 was significantly lower in the LP group when compared to the other groups in WT mice, whereas no difference in S6 phosphorylation was observed between the three dietary groups in the FGF21LKO mice (Fig. 3G). These results demonstrate that hepatic FGF21 is involved in the control of ovarian primordial follicle activation involving dietary protein levels.
3.4. FGF21 exerted no direct effects on primordial follicle activation in vitro
To test whether FGF21 directly regulates primordial follicle activation, newborn mouse ovaries were cultured in vitro for 4 days with FGF21 at 0, 50, 200, or 1000 ng/ml. Results showed that FGF21 did not affect the number of ovarian primordial or primary follicles (Fig. 4A), or the ratio of primary to primordial follicles (Fig. S4A). The level of FGF21 also had no effect on ovarian signaling pathways, involved in primordial follicle activation, including mTOR, AMPK, or S6 (Fig. 4B). Also, the obligatory co-receptor of FGF21 beta-Klotho, was undetected by western blot in the ovaries of mice fed with different diets for 4 weeks (Fig. S4B). These results support that FGF21 does not directly activate primordial follicles in the ovary.
Fig. 4.
FGF21 exerted no direct effects on primordial follicle activation in vitro. (A) Newborn mouse ovaries were cultured with different level of FGF21 for 4 days and were stained with H&E to detect follicle counts at different developmental stages. (B) The ovaries cultured for 6 h were subjected to western blotting to detect ovarian protein expression (n = 3 replicates of 8 ovaries). (C) The newborn mouse ovaries were cultured with different levels of amino acids for 4 days to determine follicle counts at different developmental stages. (D) Ovarian protein expression was determined 6 h after in vitro culture (n = 3 replicates of 8 ovaries). Follicle counts are average value of four replicates. Scale bars: 100 μm. Original magnification: ×200. Columns with different letter a,b denotes P < .05 by un-paired t-test. # denotes primary follicle, and ## denotes secondary follicles.
Amino acids are the primary metabolites resulting from dietary protein metabolism. Therefore, four differing levels of amino acids were tested during the in vitro culturing of mouse ovaries. Medium was prepared containing no amino acids (0-AA), low amino acids (LP-AA: 3.93 mM), normal amino acids (NP-AA: 5.14 mM), and high amino acids (HP-AA: 7.74 mM). Levels of amino acids were chosen based on the profiles of circulating amino acids found in the mice fed with the LP, NP and HP diets for 12 weeks (Fig. S3A). The number of primordial and primary follicles (Fig. 4C), ratio of primary to primordial follicles (Fig. S4C), as well as the phosphorylation levels of mTOR and its downstream kinase S6 (Fig. 4D) were significantly inhibited in the no amino acid group compared to the other groups. However, no difference in the number of primordial follicles, primary follicles or the phosphorylation levels of signaling kinase involved in primordial follicle activation were observed in the LP-AA, NP-AA and HP-AA groups (Fig. 4C and D). This indicates that the circulating amino acids derived from the LP diet were sufficient to activate similar numbers of primordial follicles.
3.5. Adiponectin, an FGF21 regulated adipokine, inhibited ovarian primordial follicle activation
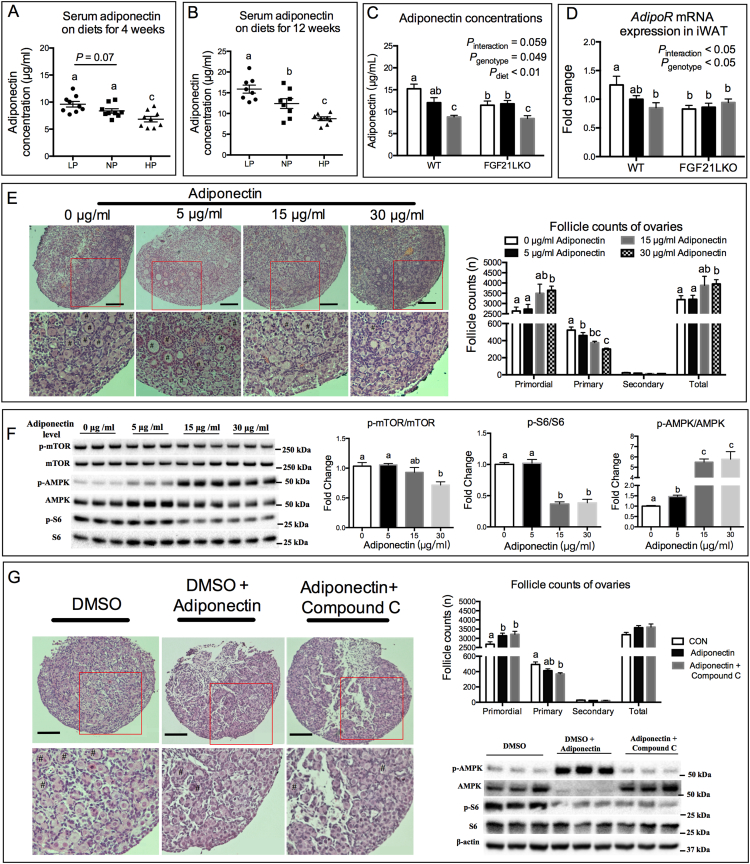
Adipose tissue is a direct target of liver produced FGF21 [30,48,49], and the adipokine adiponectin can be regulated by FGF21 [50,51]. We observed that the serum concentration of adiponectin increased when mice were fed the LP diet and decreased when diets containing excess protein were fed as compared to the NP group (Fig. 5A and B). Conversely, circulating concentrations of leptin, another adipokine, were not affected by dietary protein levels (Fig. S5A and B). Interestingly, FGF21 exposure levels and not amino acid levels resulted in increased expression of AdipoQ, which encodes adiponectin protein, in cultured 3T3L1 adipocytes (Fig. S5C and D). Furthermore, the AdipoQ and serum adiponectin levels were affected by loss of hepatic FGF21 (P = .049) and diets (P < .01) (Fig. 5C and D). The hepatic ablation of FGF21 reduced both the serum adiponectin levels and inguinal white adipose tissue (iWAT) expression levels of AdipoQ in mice fed the LP diet (Fig. 5C and D). On the contrary, the circulating levels of leptin were not affected by dietary protein levels or the ablation of hepatic FGF21 (Fig. S5E).
Fig. 5.
Adiponectin is an FGF21 regulated adipokine that inhibits ovarian primordial follicle activation. Serum adiponectin concentration in mice on diets for 4 (A) and 12 (B) weeks (n = 8–10). Concentration of adiponectin (C) and mRNA expression of AdipoQ (D) in iWAT in WT and FGF21LKO mice on diets for 12 weeks. Follicle counts (E) and ovarian protein expression (F) in newborn mouse ovaries cultured with graded levels of recombinant adiponectin protein. (G) Newborn mouse ovaries were cultured with adiponectin or co-cultured with adiponectin and AMPK inhibitor compound C, and the follicle counts, and ovarian protein expressions were determined. Scale bars: 100 μm. Original magnification: ×200. Follicle counts are average value of four replicates. Columns with different letter a,b,c denotes P < .05 by un-paired t-test. # denotes primary follicle, and ## denotes secondary follicles.
Next, we treated in vitro cultured mouse ovaries with different concentrations of adiponectin (5, 15, 30 μg/ml) and found that adiponectin at 15 and 30 μg/ml significantly decreased the number of activated primordial follicles (Fig. 5E) and the ratio of primary to primordial follicles (Fig. S5F), as compared to untreated controls. Additionally, adiponectin increased the phosphorylation level of AMPK, and down-regulated the phosphorylation levels of mTOR and S6 (Fig. 5F). Compound C, an inhibitor of AMPK signaling, was then used to test whether AMPK mediated the effect of adiponectin on primordial follicle activation. Results revealed that compound C did not change the inhibitory effects of adiponectin on the primordial follicle activation (Fig. 5G and Fig. S5G). Meanwhile, compound C significantly mitigated the phosphorylation of AMPK induced by adiponectin but did not reduce the phosphorylation of S6 (Fig. 5G). These results demonstrate that adipose adiponectin is regulated by hepatic FGF21, which could lead to inhibition of primordial follicle activation in an AMPK-independent manner.
3.6. Adiponectin receptor agonist AdipoRon inhibits primordial follicle activation
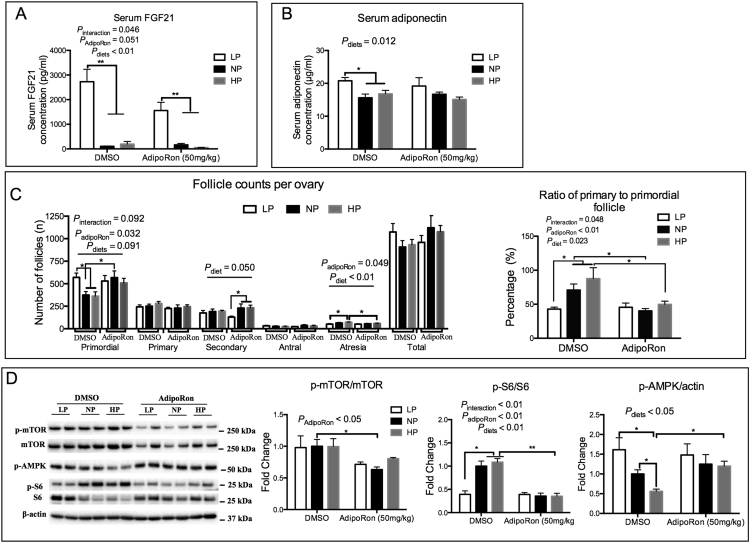
In order to more specifically investigate the potential role of adiponectin in dietary protein related ovarian follicle activation, we utilized the newly developed adiponectin receptor agonist AdipoRon. Firstly, in vitro cultured ovaries were treated with adiponectin or its receptor agonist AdipoRon. We found that the AdipoRon (25 and 50 mM) and adiponectin treatment (15 and 30 μg/ml) had comparable inhibitory effects on the activation of primordial follicles (Fig. S6A). Similar effects were seen in the phosphorylation levels of ovarian AMPK and decreased phosphorylation of S6 when ovaries were treated with adiponectin or AdipoRon (Fig. S6B). Then, AdipoRon or DMSO (as control) was administered orally to mice fed with the LP, NP or HP diets for 28 days. Results showed that body weights were not affected by AdipoRon or dietary protein levels (data not shown). AdipoRon also did not change the secretory pattern of hepatic FGF21 or adipose adiponectin induced by the differing dietary protein levels (Fig. 6A and B). In the DMSO treated mice, the percentage of primordial follicles were greater and the percentage of primary follicle lower in the LP fed mice compared to mice fed the NP or HP diets (Fig. 6C and Fig. S6C). The phosphorylation levels of mTOR and S6 were down-regulated, while the phosphorylation level of AMPK was up-regulated in the LP fed mice (Fig. 6D). However, those changes were absent following AdipoRon treatment (Fig. 6C and D). These results reveal that activation of adiponectin signaling was able to protect the ovarian primordial follicle from HP diet-induced activation.
Fig. 6.
Protection of adiponectin receptor agonist AdipoRon on the primordial follicle activation. Serum FGF21 (A) and adiponectin (B) concentrations in DMSO or AdipoRon treated mice fed different diets. (C) The follicle counts and ratio of primary to primordial follicle in ovaries of mice fed different diets treated with DMSO or AdipoRon. (D) Ovarian protein expression as detected by western blotting. n = 6, *P < .05, **P < .01 by un-paired t-test.
4. Discussion
In this study, we examined the effects of isocaloric diets with different protein levels on ovarian primordial follicle activation and the potential underlying endocrine mechanism(s). Results revealed that the activation of ovarian primordial follicles in mice differed depending on the dietary protein level. In mice subjected to diets low in protein primordial follicle activation was inhibited, whereas primordial follicle activation was accelerated when mice were fed a diet high in protein. The changes in primordial follicle activation in response to the dietary protein levels resulted in substantial differences in both ovarian follicle reserves and female fertility lifespan. We also found that hepatic FGF21 and adipose adiponectin functioned as critical endocrine hormones for the control of primordial activation by dietary protein levels.
In fact, emerging evidence has revealed that the composition of dietary macronutrients including carbohydrates, proteins, and lipids rather than the amount of energy in diets may influence metabolic health, cancer pathogenesis, and lifespan [16,39,52,53]. In particular, a low-protein diet is gradually being accepted as a healthy diet due to its beneficial effects on aging [39,54], energy homeostasis [55], and tumor suppression abilities [16,53]. However, it remains uncertain whether a low protein diet could impact female fertility. There is evidence that sufficient amino acid intake is a requirement for the maintenance of a normal estrous cycle [44]. In the present study, we extended those studies by examining the effects of varying protein intake on the ovarian primordial follicle reserve, an important determinant of reproductive lifespan [5]. The present study revealed that a diet low in protein resulted in the retention of more primordial follicles within the ovaries. Conversely, when protein was provided in excess more primordial follicles were activated to form primary follicles. To date, there are a limited number of studies elucidating the long-term effects of protein intake on reproduction. In a recent study, the estrous cycle and the number of corpora lutea of mice at an older age, which are important signs of ovarian activity and reproductive aging, were maximized with high carbohydrate: low protein ratio diets. However, the number of follicles per ovary were highest with a protein: carbohydrate ratio of 3:1 [56]. This inconsistency might be due to the different criteria in follicle enumeration between our study and theirs [56]. In that study [46], ovaries were serially sectioned at 20 μm thickness and follicles (classified as small preantral, large preantral, small antral, and large antral) were counted on three of the largest ovarian cross-sections. In the present study, ovaries were serially sectioned at 5 μm thickness and every tenth sections were stained for counting. Additionally, the number of follicles at different developmental stages were not presented in that study, it remains unclear whether the number of primordial follicles could be affected by protein intake. Nevertheless, these results demonstrate that consumption of diets varied in protein content exert substantial differences in ovarian follicle development both short-term and long-term.
In our study, mice were subjected to a breeding assay to test the pre-mating nutritional effects on the subsequent lifetime reproductive performance. The mice fed a protein restricted diet for 12 weeks had less cumulative pups from 16 to 24 weeks of the first mating than the normal diet fed mice did (Fig. 2A). This might be due to the lower number of antral follicles in the protein restricted diet fed mice (Fig. 2A). However, the difference of cumulative pups between the LP mice and NP mice disappeared after 28 weeks of mating, indicating a catch-up of fertility. Whether this “catch-up” could be attributed to the restoration of primordial follicle activation requires further study. When the dietary treatments were extended to an older age, the HP diet appeared to impair fertility. This was particularly true when the mice were fed the HP diet for 48 weeks and could be attributed to diminished ovarian reserves. To data, there is a scarcity of evidence elucidating the effects of long-time consumption of a high-protein on fertility in rodent animals, the impairment of fertility in mice with increasing time on a high-protein diet might be attributed to two reasons: firstly, feeding a high-protein diet induced greater number of growing follicles and also follicles undergoing atresia at different times on diets, which diminished the ovarian reserve in particular for those at an older age since the loss of oocytes is irreversible [[1], [2], [3], [4]]; secondly, fewer number of oocytes were ovulated in mice with increasing time on a high-protein diet, which might be not only the results of decreased ovarian reserve, but also due to impairment of mitochondrial function in oocytes. However, whether mitochondrial function in oocytes was impaired by excessive protein intake needs further investigation. The results provide evidence that protein intake levels prior to breeding can affect the reproductive ability of females. However, it is important to note that the duration of dietary intervention appears to influence reproductive outcomes. These results suggest that the increased consumption of protein, coupled with women delaying pregnancy, may exacerbate fertility issues in humans.
The ovarian phosphorylation of mTOR and its downstream target S6 are two critical molecules in mTORC1 signaling and represent the necessary and sufficient signals in order to activate dormant primordial follicles [8,9]. In our study, both the phosphorylation of mTOR and S6 were inhibited in the ovaries of LP fed mice but were elevated in the ovaries of HP fed mice. Recent studies revealed that decreased ovarian mTOR activity was related to a higher primordial follicle reserve [10,11,57], which is consistent with our findings. Thus, changes in ovarian mTORC1 signaling, such as those seen in our study, may represent the mechanism for altering primordial follicle activation. Amino acids, FGF21 and adiponectin were three important metabolic signals that were influenced by dietary protein intake. In the present study, we tested whether the changes in those metabolic signals could exert effects on ovarian mTORC1 signaling and thereby alter primordial follicle activation. The failure of amino acid-free medium to activate dormant primordial follicles could be explained by the inhibition of mTOR phosphorylation, as the stimulation of cellular mTOR phosphorylation by growth factors requires amino acids [58]. It is interesting that the medium amino acid level derived from the serum of LP fed mice was sufficient to activate primordial follicles in cultured ovaries. It surpassed our expectations that an increase of 30% (derived from the serum of the NP fed group) or 97% (derived from the serum of the HP fed group) medium amino acid levels were unable to induce an increase in ovarian mTOR phosphorylation or in the activation of primordial follicles. These data indicated that there might be a threshold level of amino acids required to activate the ovarian mTORC1 signaling pathway and result in primordial follicle activation, above which no further activation is possible.
In the present study, the Fgf21 expression was affected by amino acid level in a dose-dependent manner in primary culture of mouse hepatocytes, and these results are in agreement with previous research which found that amino acids (particularly the non-essential amino acids) could directly controlled FGF21 expression and secretion in hepatocytes [20]. However, it is worth noting that diet low in protein content also had greater percentage of carbohydrates, and the dietary treatment is in fact the consumption of diets with different ratios of protein to carbohydrate. Indeed, the carbohydrates could induce FGF21 expression and secretion [59,60]. Using the geometric framework, it was observed that both carbohydrate and protein regulated the expression and secretion of FGF21, and it was maximally induced under low protein, high carbohydrate intakes [45]. Therefore, FGF21 secretion could be a potential metabolic checkpoint of consuming diets with different protein-to-carbohydrate ratio. In line with this notion, recent evidence revealed that liver FGF21 mediated the effects of protein restriction on liver lipid metabolism and subcutaneous white adipose tissue metabolism [[20], [21], [22]]. However, the other liver-derived metabolic hormone IGF1 were affected to a lesser extent by dietary protein levels, which was consistent with the recent study which reported that FGF21, rather than IGF1, could be a representative hormone of protein intake level [61]. Recent studies also provide evidence that FGF21 is involved in reproduction [[26], [27], [28]]. The fact that a liver specific FGF21 deficiency abolishes the changes in primordial follicle activation seen, suggests that hepatic FGF21 may serve as a mediator in the process. Reproduction is an energy-expending process which requires sufficient ATP provision for the reproductive tract, results in the present study confirmed that FGF21 regulated female fertility, further indicating that energy metabolism and reproduction are coupled. However, our in vitro study revealed that there was no direct effect of FGF21 on ovarian mTORC1 signaling and the subsequent primordial follicle activation, despite the presence of receptors for FGFs in ovaries [29]. Usually, the action of FGF21 on energy metabolism requires beta-klotho (KLB) [62,63], which was not observed in ovaries by WB or in situ hybridization (data not shown).
It was observed that FGF21 was not a direct physiological regulator of fertility in mice [27], which was consistent with our results. Consumption of a high-fat diet was able to reverse the infertility of FGF21-Tg mice, suggesting that FGF21 might cooperated with adipokines to preserve fertility [27]. Moreover, it was observed that the control of energy metabolism by FGF21 required adiponectin [50,51]. In the present study, the AdipoQ mRNA expression was upregulated in adipocytes by FGF21 (Fig. S5D), and the bodyweight of LP mice similar compared with the NP and HP mice despite of greater food intake, which might be possibly the result of increased adipose energy expenditure induced by FGF21, as previously described [20,21]. Next, we hypothesized that FGF21 might mediate the effect of dietary protein level on ovarian primordial follicle activation via adiponectin. Circulating adiponectin concentrations, rather another adipokine leptin, were affected by diets, which agreed with the recent research [61]. Liver specific FGF21 deficiency decreased adiponectin concentrations when mice were fed with protein restricted diets, indicating that only high circulating levels of FGF21 regulate the secretion of adiponectin. Further, the direct effect of adiponectin on primordial follicle activation was confirmed in in vitro cultured ovaries in the present study. It was shown that adiponectin at physiological levels significantly reduced primordial follicle activation, likely via the inhibition of mTORC1 signaling [64]. Notably, AMPK signaling is accepted as an important target of adiponectin [33], and we also observed an up-regulation of AMPK signaling by adiponectin in vitro. However, the inhibition of ovarian primordial follicle activation by adiponectin might be independent of AMPK signaling, as blockage of AMPK activity failed to abrogate the effects of adiponectin on primordial follicle activation. AdipoRon is a recently developed oral adiponectin receptor agonist [33] which was observed to exert beneficial effects on energy metabolism [33,34], myocardial function [66], heart function [67], and diabetic nephropathy [68]. Our study showed that AdipoRon treatment could prevent the excessive activation of primordial follicles in the high protein diet fed mice. However, recent evidence revealed that FGF21 could regulates energy metabolism via both adipose-dependent and -independent mechanisms [65], it remains unclear whether other adipose-independent pathway played a role in the control of ovarian primordial follicle activation by protein restriction.
In conclusion, we have demonstrated that excess protein intake accelerates the activation of primordial follicles but was delayed by low protein intake. The effects of protein intake level on primordial follicle activation may be mediated by hepatic FGF21 and adipose secreted adiponectin. Our study provides nutritional solutions to preserve ovarian primordial follicles for women with advanced reproductive age and potentially protect those at increased risk of primordial follicle loss, such as those undergoing genotoxic chemotherapy therapy [69,70].
5. Limitation of study
The results of the present study provide significant insights into nutritional regulation of fertility via effects on primordial follicle activation. However, there are several insufficiencies in this study. Firstly, despite the effects of ovarian primordial follicle activation by liver FGF21, the breeding assay was not conducted in FGF21LKO mice due to a limited number of female FGF21LKO mice, and thus it remains unclear whether the alternations of follicle development would cause difference in the subsequent fertility of those mice. Secondly, the loss of liver FGF21 abrogated the effects of protein intake on primordial follicle activation, and the adipose adiponectin was responsible, at least partly. However, loss of liver FGF21 failed to affect the adiponectin concentrations in mice fed the NP and HP diets. FGF21 in adipose tissue might also play a role in adiponectin secretion, thus the whole-body loss of FGF21 should be further used to address the role of the FGF21-adiponectin axis in the control of primordial follicle activation in mice fed different levels of protein. Thirdly, double knockout of liver FGF21 and adipose adiponectin could be a better animal model to test the hypothesis of this study and requires further investigation. Finally, despite the beneficial effects of a low-protein diet on primordial follicle reserve, an extremely low-protein diet might induce protein malnutrition and reproductive failure, thus it remains unclear the optimal intake of protein-to-carbohydrate ratio on follicle reserve without inducing protein undernutrition.
Declaration of interests
None of the authors have a conflict of interests to declare.
Author contributions
YZ, LH, BF, DW conceived, designed and conducted the experiment. JL, DJ, XH, YZ, ZL, LY, CJ, XJ conducted the experiment. ZY, LC, ZF, YL, SX, JL conducted and analyzed the data. YZ, BF, XJ and DW wrote the paper.
Declarations of interest
None.
Acknowledgments
This study was funded in part by National Natural Science Foundation of China, PR China (Grant 31772616). The funding source had no role in study design, collection, analysis, and interpretation of data, writing of the report, or in the decision to submit the paper for publication. The authors also wish to thank the laboratory staff for their ongoing assistance and Prof. Paul Dyce for editing the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.02.020.
Appendix A. Supplementary data
Supplementary material
References
- 1.Lei L., Spradling A.C. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci U S A. 2013;110(21):8585–8590. doi: 10.1073/pnas.1306189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang H., Zheng W., Shen Y., Adhikari D., Ueno H., Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci U S A. 2012;109(31):12580–12585. doi: 10.1073/pnas.1206600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H., Liu L., Li X. Life-long in vivo cell-lineage tracing shows that no oogenesis originates from putative germline stem cells in adult mice. Proc Natl Acad Sci U S A. 2014;111(50):17983–17988. doi: 10.1073/pnas.1421047111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H., Panula S., Petropoulos S. Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med. 2015;21(10):1116–1118. doi: 10.1038/nm.3775. [DOI] [PubMed] [Google Scholar]
- 5.McGee E.A., Hsueh A.J. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 6.Faddy M.J., Gosden R.G., Gougeon A., Richardson S.J., Nelson J.F. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update. 2015;21(6):779–786. doi: 10.1093/humupd/dmv037. [DOI] [PubMed] [Google Scholar]
- 8.Adhikari D., Zheng W., Shen Y. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2009;19(3):397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H., Risal S., Gorre N. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24(21):2501–2508. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Dou X., Sun Y., Li J. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell. 2017;16(4):825–836. doi: 10.1111/acel.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman K.N., Chenette D., Arju R. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc Natl Acad Sci U S A. 2017;114(12):3186–3191. doi: 10.1073/pnas.1617233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfray H.C.J., Aveyard P., Garnett T. Meat consumption, health, and the environment. Science. 2018;361(6399) doi: 10.1126/science.aam5324. (eaam5324–10) [DOI] [PubMed] [Google Scholar]
- 13.Fritz R., Jindal S. Reproductive aging and elective fertility preservation. J Ovarian Res. 2018;11(1):66. doi: 10.1186/s13048-018-0438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K.P., Simpson S.J., Clissold F.J. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008;105(7):2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torre Della S., Rando G., Meda C. Amino acid-dependent activation of liver estrogen receptor alpha integrates metabolic and reproductive functions via IGF-1. Cell Metab. 2011;13(2):205–214. doi: 10.1016/j.cmet.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Levine M.E., Suarez J.A., Brandhorst S. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efeyan A., Zoncu R., Sabatini D.M. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18(9):524–533. doi: 10.1016/j.molmed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laeger T., Henagan T.M., Albarado D.C. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laeger T., Albarado D.C., Burke S.J. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 2016;16(3):707–716. doi: 10.1016/j.celrep.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maida A., Zota A., Sjøberg K.A. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J Clin Invest. 2016;126(9):3263–3278. doi: 10.1172/JCI85946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-Martí A., Garcia-Guasch M., Tresserra-Rimbau A. A low-protein diet induces body weight loss and browning of subcutaneous white adipose tissue through enhanced expression of hepatic fibroblast growth factor 21 (FGF21) Mol Nutr Food Res. 2017:61(8). doi: 10.1002/mnfr.201600725. [DOI] [PubMed] [Google Scholar]
- 22.Maida A., Zota A., Vegiopoulos A. Dietary protein dilution limits dyslipidemia in obesity through FGF21-driven fatty acid clearance. J Nutr Biochem. 2018;57:189–196. doi: 10.1016/j.jnutbio.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78(1):223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 24.Kharitonenkov A., DiMarchi R. FGF21 revolutions: recent advances illuminating FGF21 biology and medicinal properties. Trends Endocrinol Metab. 2015;26(11):608–617. doi: 10.1016/j.tem.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Staiger H., Keuper M., Berti L., Hrabě de Angelis M., Häring H.-U. Fibroblast growth factor 21—metabolic role in mice and men. Endocr Rev. 2017;38(5):468–488. doi: 10.1210/er.2017-00016. [DOI] [PubMed] [Google Scholar]
- 26.Owen B.M., Bookout A.L., Ding X. FGF21 contributes to neuroendocrine control of female reproduction. Nat Med. 2013;19(9):1153–1156. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singhal G., Douris N., Fish A.J. Fibroblast growth factor 21 has no direct role in regulating fertility in female mice. Mol Metab. 2016;5(8):690–698. doi: 10.1016/j.molmet.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C., Messina A., Somm E. KLB, encoding β-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol Med. 2017;9(10):1379–1397. doi: 10.15252/emmm.201607376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price C.A. Mechanisms of fibroblast growth factor signaling in the ovarian follicle. J Endocrinol. 2015;228(2):R31–R43. doi: 10.1530/JOE-15-0414. [DOI] [PubMed] [Google Scholar]
- 30.Hua L., Zhuo Y., Jiang D. Identification of hepatic fibroblast growth factor 21 as a mediator in 17β-estradiol–induced white adipose tissue browning. FASEB J. 2018;32(10):5602–5611. doi: 10.1096/fj.201800240R. [DOI] [PubMed] [Google Scholar]
- 31.Morgan S., Campbell L., Allison V., Murray A., Spears N. Culture and co-culture of mouse ovaries and ovarian follicles. J Vis Exp. 2015;97:1–10. doi: 10.3791/52458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y., Zhang Y., Li J. MAPK3/1 participates in the activation of primordial follicles through mTORC1-KITL signaling. J Cell Physiol. 2017;233(1):226–237. doi: 10.1002/jcp.25868. [DOI] [PubMed] [Google Scholar]
- 33.Okada-Iwabu M., Yamauchi T., Iwabu M. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503(7477):493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Wan Y., Ye G. Hepatoprotective effects of AdipoRon against d-galactosamine-induced liver injury in mice. Eur J Pharm Sci. 2016;93(C):123–131. doi: 10.1016/j.ejps.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Fairaq A., Shawky N.M., Osman I., Pichavaram P., Segar L. AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: implications toward suppression of neointimal hyperplasia. Pharmacol Res. 2017;119:289–302. doi: 10.1016/j.phrs.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers M., Britt K.L., Wreford N.G.M., Ebling F.J.P., Kerr J.B. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- 37.Reddy P., Liu L., Adhikari D. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle Pool. Science. 2008;319(5863):611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 38.Matikainen T., Perez G.I., Jurisicova A. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28(4):355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 39.Solon-Biet S.M., McMahon A.C., Ballard J.W. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren Y., Suzuki H., Jagarlamudi K. Lhx8 regulates primordial follicle activation and postnatal folliculogenesis. BMC Biol. 2015;13:39. doi: 10.1186/s12915-015-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omari S., Michel M., Ding J. Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nat Commun. 2013;4:1843–1847. doi: 10.1038/ncomms2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin J., Emery B.R., Huang I., Peterson C.M., Carrell D.T. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human) J Exp Clin Assist Reprod. 2006;3(1):2. doi: 10.1186/1743-1050-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Meir A., Burstein E., Borrego-Alvarez A. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14(5):887–895. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narita K., Nagao K., Bannai M. Dietary deficiency of essential amino acids rapidly induces cessation of the rat estrous cycle. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0028136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solon-Biet S.M., Cogger V.C., Pulpitel T. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 2016;24(4):555–565. doi: 10.1016/j.cmet.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Markan K.R., Naber M.C., Ameka M.K. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63(12):4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holstein-Rathlou Von S., BonDurant L.D., Peltekian L. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 2016;23(2):335–343. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher F.M., Kleiner S., Douris N. FGF21 regulates PGC-1 and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minard A.Y., Tan S.X., Yang P. mTORC1 is a major regulatory node in the FGF21 signaling network in adipocytes. Cell Rep. 2016;17(1):29–36. doi: 10.1016/j.celrep.2016.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Z., Tian H., Lam K.S. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17(5):779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Holland W.L., Adams A.C., Brozinick J.T. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17(5):790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solon-Biet S.M., Mitchell S.J., Coogan S.C. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep. 2015;11(10):1529–1534. doi: 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubio-Patiño C., Bossowski J.P., De Donatis G.M. Low-protein diet induces IRE1a-dependent anticancer immunosurveillance. Cell Metab. 2018;27(4):828–842. doi: 10.1016/j.cmet.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Hine C., Harputlugil E., Zhang Y. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160(1–2):132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Treviño-Villarreal J.H., Reynolds J.S., Bartelt A. Dietary protein restriction reduces circulating VLDL triglyceride levels via CREBH-APOA5-dependent and -independent mechanisms. JCI Insight. 2018;3(21) doi: 10.1172/jci.insight.99470. (pii: 99470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solon-Biet S.M., Walters K.A., Simanainen U.K. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc Natl Acad Sci U S A. 2015;112(11):3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou L., Xie Y., Li S. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR Signaling pathway in vivo. J Ovarian Res. 2017;10(1):56. doi: 10.1186/s13048-017-0350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sancak Y., Peterson T.R., Shaul Y.D. The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iizuka K., Takeda J., Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009;583(17):2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 60.Søberg S., Sandholt C.H., Jespersen N.Z. FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 2017;25(5):1045–1053. doi: 10.1016/j.cmet.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Wahl D., Solon-Biet S.M., Wang Q.P. Comparing the effects of low-protein and high-carbohydrate diets and caloric restriction on brain aging in mice. Cell Rep. 2018;25(8):2234–2243. doi: 10.1016/j.celrep.2018.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding X., Boney-Montoya J., Owen B.M. Beta-Klotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16(3):387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Owen B.M., Ding X., Morgan D.A. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20(4):670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang Z.Z., Hu M.W., Ma X.S. LKB1 acts as a critical gatekeeper of ovarian primordial follicle pool. Oncotarget. 2015;7(5):5738–5753. doi: 10.18632/oncotarget.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.BonDurant L.D., Ameka M., Naber M.C. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 2017;25(4):935–944. doi: 10.1016/j.cmet.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Liang B., Lau W.B. Restoring diabetes-induced autophagic flux arrest in ischemic/reperfused heart by ADIPOR (adiponectin receptor) activation involves both AMPK-dependent and AMPK-independent signaling. Autophagy. 2017;13(11):1855–1869. doi: 10.1080/15548627.2017.1358848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y., Zhao J., Li R. AdipoRon, the first orally active adiponectin receptor activator, attenuates postischemic myocardial apoptosis through both AMPK-mediated and AMPK-independent signalings. Am J Physiol Endocrinol Metab. 2015;309(3):E275–E282. doi: 10.1152/ajpendo.00577.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim Y., Lim J.H., Kim M.Y. The Adiponectin receptor agonist AdipoRon ameliorates diabetic nephropathy in a model of type 2 diabetes. J Am Soc Nephrol. 2018;29(4):1108–1127. doi: 10.1681/ASN.2017060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jang H., Lee O.H., Lee Y. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal Res. 2016;60(3):336–347. doi: 10.1111/jpi.12316. [DOI] [PubMed] [Google Scholar]
- 70.Jang H., Na Y., Hong K. Synergistic effect of melatonin and ghrelin in preventing cisplatin-induced ovarian damage via regulation of FOXO3a phosphorylation and binding to the p27Kip1 promoter in primordial follicles. J Pineal Res. 2017;63(3) doi: 10.1111/jpi.12432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material