Abstract
Biological nitrogen fixation can fuel CO2 sequestration by forests but can also stimulate soil emissions of nitrous oxide (N2O), a potent greenhouse gas. Here we use a theoretical model to suggest that symbiotic nitrogen-fixing trees could either mitigate (CO2 sequestration outweighs soil N2O emissions) or exacerbate (vice versa) climate change relative to non-fixing trees, depending on their nitrogen fixation strategy (the degree to which they regulate nitrogen fixation to balance nitrogen supply and demand) and on nitrogen deposition. The model posits that nitrogen-fixing trees could exacerbate climate change globally relative to non-fixing trees by the radiative equivalent of 0.77 Pg C yr−1 under nitrogen deposition rates projected for 2030. This value is highly uncertain, but its magnitude suggests that this subject requires further study and that improving the representation of biological nitrogen fixation in climate models could substantially decrease estimates of the extent to which forests will mitigate climate change.
The balance between CO2 sequestration by forests and soil N2O emissions is poorly constrained. Here, the authors use a theoretical model to demonstrate that symbiotic N2-fixing trees can either mitigate climate change or exacerbate it relative to non-fixing trees.
Introduction
Forests currently sequester a quarter of annual anthropogenic CO2 emissions1,2. Nitrogen-fixing tree symbioses, in which bacteria living in root nodules convert atmospheric N2 gas to a plant-available form of nitrogen (N), can provide much of the N needed to drive forest growth3,4. N-fixing trees thus mitigate climate change by sequestering CO2, either directly via their own growth or indirectly via the turnover of their N-rich tissues whose decomposition increases surrounding soil N and plant growth. However, in addition to driving CO2 sequestration, elevated soil N driven by the decomposition of N-rich plant litter can also drive soil emissions of nitrous oxide (N2O)5–10, a potent greenhouse gas11. What is the current balance of the CO2 and N2O effects of N-fixing trees, i.e. the net CO2–N2O effect, and to what degree will it be modified by global change?
Studies on another major N input to forests, atmospheric N deposition, offer insight into the net CO2–N2O effect of N enrichment. N deposition rates are increasing globally due to fossil fuel and fertilizer use12. Although intensifying N deposition is expected to stimulate CO2 sequestration13, it is also expected to stimulate soil N2O emissions14–17 that will offset 18–90% of the negative radiative forcing of this CO2 sequestration15. These studies demonstrate the potential for elevated soil N2O emissions to substantially offset CO2 sequestration driven by N enrichment. However, the balance of the CO2 and N2O effects of biological N fixation, which has fundamentally different dynamics than those of N deposition, is unresolved.
Unlike N deposition, biological N fixation has the capacity to self-regulate, feeding back to ecosystem-scale soil N levels18. A deficiency of N can stimulate N fixation, which can promote plant growth and CO2 sequestration. An excess of N can inhibit N fixation, which is physiologically costly, reducing ecosystem-scale soil N excess and its associated soil N2O emissions. However, the strength of this feedback varies across N-fixing species. Some N-fixing species exhibit a facultative N fixation strategy and feedback to soil N levels3,18–20, downregulating N fixation rates from over 30 to 0 kg N ha−1 yr−1 at the ecosystem scale3. However, other N-fixing species do not regulate their N fixation rate in response to soil N levels, exhibiting an obligate N fixation strategy18,21,22. In this case, N fixation at the ecosystem scale is only downregulated when these species are competitively excluded. However, before competitive exclusion occurs, obligate N-fixing trees can drive substantial soil N2O emissions5. The strong connection between N fixation, soil N enrichment, and soil N2O emissions calls for the explicit consideration of N fixation strategies when estimating the net CO2–N2O effect of forests.
Here we use a theoretical modeling approach to ask two main questions: how do N-fixing trees influence the net CO2–N2O effect of forests, i.e. do N-fixing trees mitigate or exacerbate climate change? How will their influence change under elevated N deposition rates? We use the terms mitigate and exacerbate to highlight that the influence of N-fixing trees is relative to ongoing greenhouse gas effects. In forests, the cooling effect of CO2 sequestration is partially offset by the warming effect of soil N2O emissions2, resulting in a net cooling CO2–N2O effect. We are not suggesting that N-fixing trees can or will change the direction of the net CO2–N2O effect of forests from cooling to warming. The question we address is how N-fixing trees modify CO2 sequestration in comparison to how they modify soil N2O emissions relative to non-fixing trees.
We use a differential equation ecosystem model that captures the fluxes and pools of carbon (C) and N in an ecosystem, and includes competition between N-fixing and non-fixing trees. We validated the model against literature estimates of the relevant fluxes and pools of C and N in tropical, temperate, and boreal forests. The model predicts CO2 sequestration (CO2 effect) and soil N2O emissions (N2O effect) of an ecosystem with a given dominant N fixation strategy. We compute the net CO2–N2O effect of the ecosystem with two complementary methods. The first method compares accumulated CO2 sequestration to accumulated soil N2O emissions after 100 years of ecosystem succession using the global warming potential of N2O. The second method computes the net radiative forcing from continuous CO2 sequestration and soil N2O emissions over 100 years of ecosystem succession. To evaluate the CO2 and N2O effects of N-fixing trees, we compare model ecosystems of non-fixing trees to model ecosystems that contain both N-fixing trees and non-fixing trees. Model ecosystems with N-fixing trees contain one of three empirically supported N fixation strategies18: obligate (fix N at a constant rate per unit biomass), perfectly facultative (downregulate N fixation to perfectly meet their N demand after taking up soil N; hereafter facultative), and incomplete regulator (downregulate N fixation similarly to the facultative strategy but sustain N fixation at a constant minimum rate). The difference in the net CO2–N2O effect between a model ecosystem of non-fixing trees and a model ecosystem with N-fixing trees is the net CO2–N2O effect attributed to the N-fixing trees and is inherently relative to the net CO2–N2O effect of non-fixing trees. To estimate the magnitude of the net CO2–N2O effect of N-fixing trees at the global scale, we parameterized the model for tropical, temperate, and boreal forests, and simulated the model under past (low; pre-Anthropocene23), recent (intermediate; 200124 and 200612), and future N deposition rates (high; 2030 for the SRES A2 scenario12,25). The model suggests that N-fixing trees can either mitigate or exacerbate climate change relative to non-fixing trees, contingent on their N fixation strategy and on N deposition. As N deposition intensifies, N-fixing trees stimulate substantial soil N2O emissions but promote minimal CO2 sequestration, exacerbating climate change relative to non-fixing trees. The goal of this study is not to generate a quantitatively accurate prediction of the net CO2–N2O effect of N-fixing trees. Rather, the objectives are to estimate its potential magnitude, and to generate and explore hypotheses of how N-fixing trees could mitigate or exacerbate climate change. Ultimately, these hypotheses should be analyzed empirically and with Earth System Models.
Results
Net CO2–N2O effect of N-fixing trees at the ecosystem scale
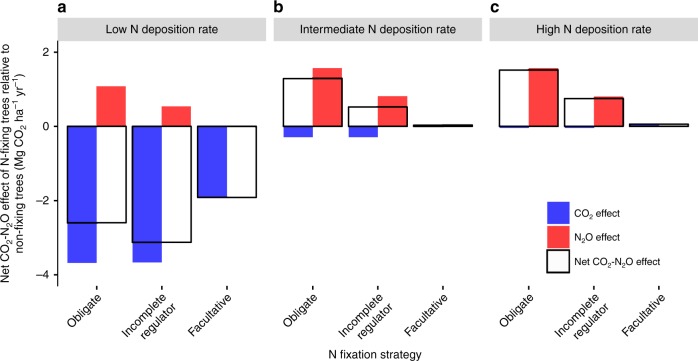
Our model suggests that N-fixing trees can either mitigate climate change relative to non-fixing trees (a negative net CO2–N2O effect of N-fixing trees relative to non-fixing trees) or exacerbate climate change relative to non-fixing trees (a positive net CO2–N2O effect of N-fixing trees relative to non-fixing trees). The main controls that determine this balance are N fixation strategy and N deposition rate (Fig. 1 displays results for tropical forests and Supplementary Figures 1 and 2 display results for temperate and boreal forests respectively; because patterns are analogous between tropical, temperate, and boreal forests we hereafter focus on tropical forests). For N-fixing trees that exacerbate climate change relative to non-fixing trees, soil N2O emissions do not necessarily offset the absolute level of CO2 sequestration (see Supplementary Figure 3 for the absolute net CO2–N2O effects of ecosystems with and without N-fixing trees). Rather, the offset of CO2 sequestration by soil N2O emissions for ecosystems with N-fixing trees is greater than the offset of CO2 sequestration by soil N2O emissions for ecosystems without N-fixing trees. Similarly, for N-fixing trees that mitigate climate change relative to non-fixing trees, the offset of CO2 sequestration by soil N2O emissions for ecosystems with N-fixing trees is lower than the offset of CO2 sequestration by soil N2O emissions for ecosystems without N-fixing trees. Generally, under low N deposition rates, N-fixing trees promote CO2 sequestration but only minimal soil N2O emissions relative to non-fixing trees (Fig. 1a), whereas under high N deposition rates, N-fixing trees stimulate soil N2O emissions but only minimal CO2 sequestration relative to non-fixing trees (Fig. 1c).
Fig. 1.
Modeled CO2 and N2O effects of nitrogen-fixing trees relative to non-fixing trees. The CO2 and N2O effects of N-fixing trees relative to non-fixing trees are shown under a low N deposition rates23, b intermediate N deposition rates12, and c high N deposition rates12. Units are CO2 radiative equivalents, which balance the greenhouse effects of CO2 and N2O using the global warming potential of N2O. A positive net CO2–N2O effect of N-fixing trees relative to non-fixing trees indicates that N-fixing trees have a warming effect relative to non-fixing trees (i.e. N-fixing trees warm more than non-fixing trees but do not necessarily warm overall). A negative net CO2–N2O effect of N-fixing trees relative to non-fixing trees indicates that N-fixing trees have a cooling effect relative to non-fixing trees (i.e. N-fixing trees cool more than non-fixing trees but do not necessarily cool overall). The model is parameterized for a tropical forest
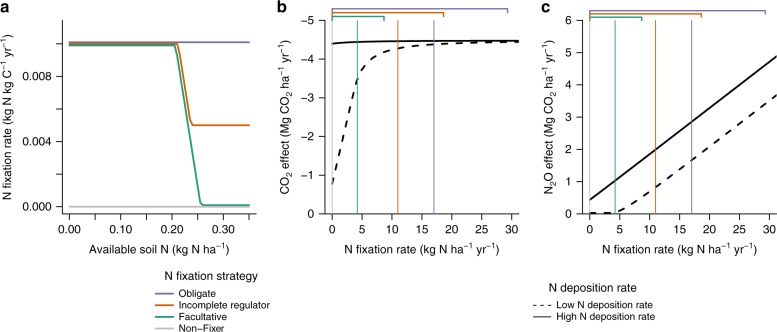
Obligate and incomplete regulator N-fixers sustain N fixation after satisfying their N demand, whereas facultative N-fixers shut off N fixation after satisfying their N demand (Fig. 2a). Over succession, obligate and incomplete regulator N-fixers promote greater N supply to the ecosystem via sustained N fixation than facultative N-fixers (indicated by the vertical lines in Fig. 2b, c). Under low N deposition, N supplied via N fixation by obligate and incomplete regulator N-fixing trees facilitates non-fixing trees in meeting their N demand, amplifying ecosystem-scale CO2 sequestration to a greater extent than that by facultative N-fixing trees (Fig. 2b). However, this N supplied via N fixation also stimulates soil N2O emissions (Fig. 2c). This is especially pronounced for obligate N-fixers, which sustain N fixation at a higher rate than incomplete regulator N-fixers. As such, under low N deposition rates, incomplete regulator N-fixing trees exhibit the greatest net CO2–N2O cooling effect because of their high CO2 effect (Fig. 1a). They are followed by obligate N-fixing trees, which have a similarly high CO2 effect but a higher N2O effect (Fig. 1a). Facultative N-fixing trees, which have a substantially lower CO2 effect, have the lowest net CO2–N2O cooling effect (Fig. 1a).
Fig. 2.
Mechanisms that drive the CO2 and N2O effects of nitrogen-fixing trees. a N fixation rate as a function of available soil N for the three N fixation strategies examined in the model (the horizontal gray line represents a zero N fixation rate for non-fixing trees). b CO2 effect. CO2 sequestration increases with increasing N fixation rate when N is limiting. When N supply to the ecosystem is sufficient to alleviate N limitation, CO2 sequestration plateaus with increasing N fixation rate. This plateau occurs at a lower N fixation rate under high N deposition than under low N deposition. c N2O effect (displayed in units of CO2 radiative equivalents). Increasing N fixation rate does not stimulate soil N2O emissions when N is limiting. When N supply to the ecosystem is sufficient to alleviate N limitation, soil N2O emissions increase with increasing N fixation rate. This increase occurs at a higher N fixation rate under low N deposition than under high N deposition. The black curves in b and c represent the CO2 and N2O effects respectively of an ecosystem with a tropical forest parameterization, a single biomass C pool, and a prescribed constant N fixation rate per unit biomass C. The vertical purple, orange, and green lines in b and c represent average N fixation rates over 100 years for the three N fixation strategies examined in the model (the vertical gray line represents a zero N fixation rate over 100 years for non-fixing trees). The corresponding brackets indicate the range of N fixation rates over 100 years for the three N fixation strategies examined in the model. The low N deposition rate is from Galloway et al.23 and the high N deposition rate is derived from Dentener et al.12. Overall, a–c show that N fixation drives cooling when N is limiting (low N fixation and/or N deposition) and warming when N is not limiting (high N fixation and/or N deposition)
Increased N supply to the ecosystem via elevated N deposition induces N-fixing trees to downregulate N fixation to the greatest extent possible (Fig. 2a): facultative N-fixers completely downregulate N fixation and incomplete regulator N-fixers partially downregulate N fixation, whereas obligate N-fixers do not downregulate N fixation. Because facultative N-fixing trees completely downregulate N fixation (Fig. 2a), they have a negligible net CO2–N2O effect relative to non-fixing trees under high N deposition rates (Fig. 1c). Under high N deposition rates, N demand is satisfied by N deposition. As such, N fixed by obligate and incomplete regulator N-fixing trees due to sustained N fixation does not contribute to CO2 sequestration (Fig. 2b). Rather, it contributes to soil N2O emissions, which increase indefinitely with increasing N fixation (Fig. 2c). Thus, obligate and incomplete regulator N-fixing trees exhibit a considerable N2O effect, yielding a net CO2–N2O warming effect relative to non-fixing trees (Fig. 1c).
Initial soil N pool sizes do not strongly influence the net CO2–N2O effect of N-fixing trees relative to non-fixing trees (differ by <1 Mg CO2 ha−1 yr−1 between low and high initial soil N pool sizes; Supplementary Figure 4).
Net CO2–N2O effect of N-fixing trees at the global scale
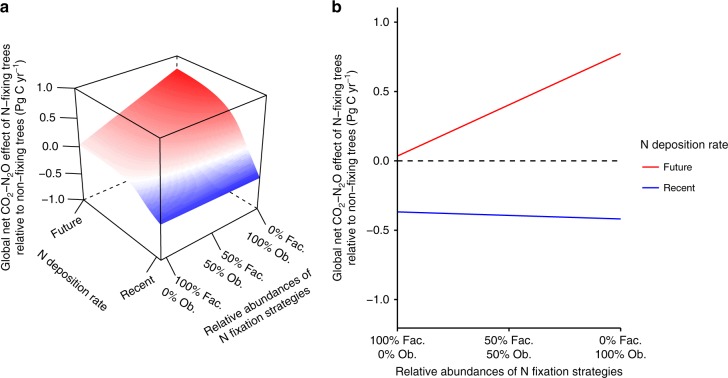
To ascertain how important the climate impacts of N-fixing trees could be, we estimated the net CO2–N2O effect of N-fixing trees at the global scale. Although N-fixing trees play a crucial role in forests, the global distribution of N fixation strategies is not well established26. Accordingly, we made estimates of the global net CO2–N2O effect of N-fixing trees first by examining three basic scenarios: all N-fixing trees are obligate, all N-fixing trees are facultative, and all N-fixing trees are incomplete regulators. Because forests around the globe include an assemblage of these three N fixation strategies18,27, the maximum and minimum of these three basic scenarios provide bounds to the global net CO2–N2O effect of N-fixing trees. We ran each basic scenario under future N deposition rates (for the SRES A2 scenario). Our model suggests that if all N-fixing trees are facultative, they will have an insignificant influence on estimates of the net CO2–N2O effect of global forests (Table 1). At the opposite extreme, if all N-fixing trees are obligate, N-fixing trees will decrease estimates of the net CO2–N2O effect of global forests by the radiative equivalent of 0.77 Pg C yr−1 (Table 1).
Table 1.
Modeled global net CO2–N2O effect of forests and of N-fixing trees relative to non-fixing trees under future N deposition rates (2030 for the SRES A2 scenario)
| Global forest composition | Global net CO2–N2O effect of forests (Pg C yr−1) | Global net CO2–N2O effect of N-fixing trees relative to non-fixing trees (Pg C yr−1) |
|---|---|---|
| Obligate N-fixer and non-fixer | −2.98 | +0.77 |
| Facultative N-fixer and non-fixer | −3.72 | +0.03 |
| Incomplete regulator N-fixer and non-fixer | −3.40 | +0.36 |
| Non-fixer | −3.76 | Not applicable |
Scenarios displayed are: all N-fixing trees are obligate, all N-fixing trees are facultative, and all N-fixing trees are incomplete regulators. Units are C radiative equivalents, which balance the greenhouse effects of CO2 and N2O using the global warming potential of N2O. Negative values in the centre column indicate a net cooling CO2–N2O effect of forests. Positive values in the right-hand column, which are the differences from the non-fixer row in the centre column, indicate that N-fixing trees have a net warming CO2–N2O effect relative to non-fixing trees
NA not applicable
In a further analysis, we determined the global net CO2–N2O effects of N-fixing trees relative to non-fixing trees for a range of relative abundances of ecosystems containing obligate N-fixing trees and ecosystems containing facultative N-fixing trees under a range of N deposition rates (Fig. 3a). Under recent N deposition rates, our assumptions of the relative abundances of ecosystems containing obligate and facultative N-fixing trees have a negligible influence on the global net CO2–N2O effect of N-fixing trees relative to non-fixing trees (Fig. 3b, Supplementary Table 1), whereas under future N deposition rates these assumptions can change this global scale estimate by up to 0.77 Pg C yr−1 (Fig. 3b, Table 1).
Fig. 3.
Modeled global CO2 and N2O effects of nitrogen-fixing trees relative to non-fixing trees. a Global net CO2–N2O effect of N-fixing trees relative to non-fixing trees for a range of relative abundances of ecosystems containing obligate N-fixing trees and ecosystems containing facultative N-fixing trees under a range of N deposition rates. Global forest composition ranges from the scenario in which all N-fixing trees are facultative to the scenario in which all N-fixing trees are obligate, i.e. the relative abundances of ecosystems containing obligate N-fixing trees and ecosystems containing facultative N-fixing trees range from 0 to 100% and 100 to 0% respectively. Red represents a warming effect and blue represents a cooling effect relative to non-fixing trees. Global N deposition rate ranges from the minimum recent N deposition rate derived from Vet et al.24 or Dentener et al.12, to the future N deposition rate derived from Dentener et al.12. b Global net CO2–N2O effect of N-fixing trees relative to non-fixing trees under recent and future N deposition rates. The curves in b are cross sections of the extremes of the surface displayed in a. The dotted line is at zero, representing the transition between a cooling effect and a warming effect relative to non-fixing trees. Fac. represents ecosystems containing facultative N-fixing trees and Ob. represents ecosystems containing obligate N-fixing trees
Discussion
Our model identifies N fixation strategy and N deposition rate as the main controls of the net CO2–N2O effect of N-fixing trees at both the ecosystem and global scales (Figs. 1 and 3). In particular, under elevated N deposition rates, our model suggests that N fixation strategy is the key determinant of the net CO2–N2O effect of forests: obligate N-fixing trees exacerbate climate change relative to non-fixing trees, whereas facultative N-fixing trees influence climate change in the same manner as non-fixing trees. The net CO2–N2O effect of N-fixing trees at the global scale under future N deposition rates—up to 0.77 Pg C yr−1 according to our model—is highly uncertain, given the numerous caveats associated with scaling a simple model up to the globe. However, the magnitude of this estimate suggests that N-fixing trees could have a critical influence on the extent to which forests will mitigate climate change. Below, we discuss our current understanding of N fixation strategies and the CO2 and N2O effects of N-fixing trees, how other global change factors could influence the net CO2–N2O effect of N-fixing trees, and extensions of our results to forest management and Earth System Models.
According to our model, N fixation strategies are a key determinant of how N-fixing trees will influence climate change, but the global distribution of N fixation strategies is not well established. There is observational evidence that actinorhizal N-fixing trees in temperate forests are obligate21,22 but that rhizobial N-fixing trees in tropical forests downregulate N fixation (either with a facultative or an incomplete regulator N fixation strategy)3,19,20. Theoretical evidence suggests that a transition from facultative N fixation strategies at lower latitudes to obligate N fixation strategies at higher latitudes could explain the order of magnitude drop in N-fixing tree abundance27 and the differences in successional patterns of N-fixing tree abundance between tropical and temperate forests28,29. Theory also suggests why an obligate N fixation strategy could be more adaptive at higher latitudes: low decomposition rates at low temperatures could lead to sustained N limitation, favoring obligate N fixation30. However, there is limited empirical evidence to support these theories because N fixation strategies are difficult to establish experimentally18. Our study emphasizes the need for a more accurate and extensive description of the distributions of different N fixation strategies given their significant influence on predictions of the net CO2–N2O effect of global forests.
The CO2 sequestration component of our model relies on the theory that N-fixing trees drive forest growth by meeting its N demand, which has some3,4 but not universal31–33 support. For example, Batterman et al.3 found that in a 300-year forest chronosequence in Panama, N-fixing trees provided over 50% of the N demand of early successional forest growth. However, another study from the same region of Panama showed a negligible influence of N-fixing trees on forest growth32. Furthermore, recent studies in Alaska31 and Costa Rica33 have shown that N-fixing trees can even inhibit the growth of surrounding trees and thus inhibit forest growth. These results could be due to non-N limitation and strong competitive effects of N-fixing trees on surrounding trees, although these mechanisms remain speculative. Further research is necessary to determine the predominance and controls of non-facilitative effects of N-fixing trees on forest growth. Additional studies on how N-fixing trees drive soil N2O emissions are also necessary. It is well established that soil N drives soil N2O emissions34,35. However, studies of the extent to which N-fixing trees enrich soil N and stimulate soil N2O emissions are rare, although they demonstrate that N-fixing trees can substantially increase soil N2O emissions5–10 (soil N2O emissions can be up to 12-fold greater in stands of N-fixing trees than in stands of non-fixing trees5). The magnitude of our estimate of the net CO2–N2O effect of N-fixing trees at the global scale highlights the need for further research on the impact of N-fixing trees on soil N2O emissions.
Our analysis focused on a single global change factor—intensifying N deposition—due to its clear link to N supply. However, global change factors beyond N deposition such as increasing temperature, changing precipitation, and CO2 fertilization could also influence the net CO2–N2O effect of N-fixing trees. N-fixing trees are projected to increase in abundance due to increasing temperatures36, which would amplify their net CO2–N2O effect. Additionally, increasing temperatures will increase soil N2O emission rates37,38. N-fixing trees generally have a greater water use efficiency than non-fixing trees39, and are more abundant in arid conditions28,36,40, suggesting that changing precipitation could either increase or decrease N-fixing tree abundance and their net CO2–N2O effect (although forecasted changes in precipitation in the United States and Mexico are projected to have only a minor influence on N-fixing tree abundance36). Additionally, soil moisture strongly controls soil N2O emission rates37,38. CO2 fertilization has been suggested to promote N limitation via increased forest growth41, although empirical evidence is mixed42,43. Intensifying N limitation could promote increasing N fixation rates44,45 and a net CO2–N2O cooling effect of N-fixing trees relative to non-fixing trees, although this response could be limited by other nutrients44,45. Our study only addresses intensifying N deposition as it has a direct influence on N limitation, but other global change factors should also be considered for a comprehensive analysis of how N-fixing trees will mitigate or exacerbate climate change.
Forest management studies have recommended planting N-fixing trees during reforestation to alleviate regenerating forests from N limitation46,47. However, our study suggests that planting obligate and incomplete regulator N-fixing trees may actually exacerbate climate change relative to non-fixing trees under elevated N deposition rates. This finding complements recent empirical evidence that N-fixing trees might not promote forest growth31–33. However, we emphasize that in our study, the net CO2–N2O effect of all forest ecosystems is a cooling effect (Supplementary Figure 3), and we are addressing the relative merit of N-fixing trees (with different N fixation strategies) vs. non-fixing trees. Furthermore, our analysis does not consider the merits of biodiversity or other site-specific factors that could influence the net CO2–N2O effect of N-fixing trees.
Biological N fixation is a significant source of uncertainty in the climate projections of Earth System Models48,49. Our results suggest that including the regulation of biological N fixation in Earth System Models and explicitly considering soil N2O emissions, rather than CO2 sequestration alone, could considerably decrease estimates of the extent to which global forests will mitigate climate change. Global forests currently sequester 2.4 Pg C yr−1 (ref. 1), representing a negative radiative forcing. Our analysis suggests that a single functional group, N-fixing trees, could decrease the magnitude of this negative radiative forcing of forests by up to 32% as N deposition intensifies. The theoretical modeling approach we employ here is only a basic framework for generating hypotheses and exploring their potential limits. We do not claim to have made accurate predictions for the net CO2–N2O effect of N-fixing trees, but rather seek to stimulate discussion on their climate role and suggest further research. In particular, empirical work is necessary to quantify the net CO2–N2O effect of N-fixing trees and improve its representation in Earth System Models, allowing the development of an accurate estimate of the extent to which N-fixing trees and global forests will mitigate or exacerbate climate change.
Methods
Model description
Our model is an extension of a simple differential equation ecosystem model18,50. It includes a N-fixer biomass C pool (BF, kg C ha−1), a non-fixer biomass C pool (B0, kg C ha−1), a plant-unavailable soil N pool (D, kg N ha−1; detritus), and a plant-available soil N pool (A, kg N ha−1; nitrate, ammonium, and forms of organic N that are accessible to plants). The rates of change of these pools satisfy the following ordinary differential equations (represented by the box diagram in Supplementary Figure 5):
| 1 |
| 2 |
| 3 |
| 4 |
The per capita growth rates of BF and B0 are represented by the functions gF and g0, respectively:
| 5 |
| 6 |
The growth rate of Bi (i = F represents N-fixers, i = 0 represents non-fixers) is determined by Liebig’s law of the minimum51. When Bi is N-limited, gi is a function of the nutrient use efficiency of N (ωi), N uptake rate (νi), and, for BF, N fixation rate per unit biomass C (F). When Bi is not N-limited, gi is limited by some unspecified resource (such as phosphorus, light, or space), represented by a density-dependent function that decreases with increasing total biomass (BF + B0). For non-N-limited growth, βi is the maximum growth rate and γi is the coefficient that determines the extent to which gi is decreased by total biomass. The parameter µi represents the turnover rate, m represents the mineralization rate, φ represents the loss rate of plant-unavailable soil N, I represents the abiotic N input flux, k represents the loss rate of plant-available soil N other than gaseous losses of N2O (leaching of all forms of plant-available soil N and gaseous losses of nitric oxide (NO), ammonium (NH3), and nitrogen gas (N2)), and η represents the gaseous loss rate of plant-available soil N as N2O. We assume that the N2O gaseous loss rate is a linear function of A, following 2006 IPCC Guidelines for National Greenhouse Gas Inventories52. Thus, the atmospheric N2O pool (E; in kg N2O-N ha−1) satisfies the following equation:
| 7 |
The parameter ψ represents the atmospheric N2O removal rate (through photolysis and oxidation reactions11) and is the inverse of the lifetime of N2O in the atmosphere.
Different N fixation strategies (obligate, facultative, and incomplete regulator) are represented by the following equation, which gives N fixation rate per unit biomass C:
| 8 |
The parameter Fmin represents the sustained minimum N fixation rate, and thus describes the gradient of N fixation strategies from obligate N-fixers (Fmin = Fmax, i.e. F is constant), to incomplete regulator N-fixers (0 < Fmin < Fmax), to facultative N-fixers (Fmin = 0). The parameter Fmax is the maximum N fixation rate per unit biomass C.
Model simulations
Simulations of the model were conducted in R using the package deSolve. We parameterized our model for tropical, temperate, and boreal forests (Supplementary Table 2), and conducted the following simulations for each parameterization. We simulated four versions of the model (ecosystems containing only non-fixers, ecosystems containing non-fixers and obligate N-fixers, ecosystems containing non-fixers and facultative N-fixers, and ecosystems containing non-fixers and incomplete regulator N-fixers) for 100 years. We simulated each of the four versions of the model under three N deposition rates: past (low; pre-Anthropocene; from Galloway et al.23), recent (intermediate; 2001 and 2006; from Vet et al.24 and Dentener et al.12 respectively), and future N deposition rates (high; 2030 for the SRES A2 scenario25; from Dentener et al.12) (Supplementary Table 3). N deposition rates for tropical, temperate, and boreal forests were estimated using weighted averages with tropical, temperate, and boreal forest areas (from the 2015 Global Forest Resources Assessment53). The range of N deposition rates can also be representative of varying degrees of N enrichment from other sources (rock weathering N input, turnover, mineralization, etc.). Additionally, we simulated each of the four versions of the model under low, intermediate, and high initial soil N pool sizes (Supplementary Table 4).
CO2 effect, N2O effect, and net CO2–N2O effect
We calculated the CO2 and N2O effects of the ecosystem with two complementary methods. The first method quantifies total change in the sizes of the biomass C pools and the atmospheric N2O pool, converting N2O to CO2 radiative equivalents using global warming potentials. The second method quantifies net radiative forcing from continuous changes in the sizes of the biomass C pools and the atmospheric N2O pool. Both methods calculate the CO2 and N2O effects of the ecosystem over 100 years, similar to the IPCC’s SRES and Representative Concentration Pathways. The first method is easier to compare to studies of standing biomass C pools, whereas the second method gives a more accurate accounting of net radiative forcing. Results given in the main text are from the first method, but both methods give similar results.
For the first method, the CO2 and N2O effects of the ecosystem were calculated as follows:
| 9 |
| 10 |
The global warming potential of N2O over a 100 year time horizon11 (298 kg CO2 per kg N2O) was used to find the CO2 radiative equivalent of soil N2O emissions. The CO2 effect and N2O effect are both given in units of kg CO2 ha−1 yr−1.
For the second method, we adapted an equation for the radiative forcing of a continuous emission pulse from Alvarez et al.54:
| 11 |
| 12 |
is the sequestration of CO2 at time tE. ai and are constants and lifetimes respectively that represent the timescales of different CO2 removal processes55. Removal of CO2 by the terrestrial sink is already included in these CO2 removal processes, and, as such, Eq. (11) is not an ideal representation of the CO2 effect but is effective at demonstrating its general trend. A(tE) is the available soil N pool at time tE. REGHG is the radiative efficiency of the greenhouse gas and was calculated using the following formula from Myhre et al.11 that converts radiative efficiency from units of W m−2 ppbv−1 (standard) to units of W m−2 kg−1:
| 13 |
REGHG,ppbv is the radiative efficiency in units of W m−2 ppbv−1, MA is the mean molar mass of air, MGHG is the molar mass of the greenhouse gas, and TM is the total mass of the atmosphere. Parameter values and descriptions are available in Supplementary Table 5. Results and figures corresponding to those available in the main text are displayed in Supplementary Table 6 and Supplementary Figure 6.
For both methods, the net CO2–N2O effect reflects the balance of CO2 sequestration and soil N2O emissions and is thus calculated as the sum of the CO2 effect and N2O effect. A negative net CO2–N2O effect indicates a cooling effect (CO2 sequestration exceeds soil N2O emissions) and a positive net CO2–N2O effect indicates a warming effect (soil N2O emissions exceed CO2 sequestration).
Model validity
The model accurately estimates CO2 sequestration and soil N2O emissions under recent N deposition rates. For tropical forests, the total biomass C equilibrium of the model is 124 Mg C ha−1 (see Supplementary Note 1 for equilibria analysis), which is similar to Batterman et al.3, which reported 128 Mg C ha−1 in old growth tropical forests. For temperate forests, the total biomass C equilibrium of the model is 145 Mg C ha−1, which is similar to Pregitzer et al.56, which reported 171 Mg C ha−1 in old growth temperate forests. For boreal forests, the total biomass C equilibrium of the model is 75 Mg C ha−1, which is similar to Pregitzer et al.56, which reported 81 Mg C ha−1 in old growth boreal forests.
For tropical forests, the soil N2O emission rate of the model ranges between 0 and 6.97 kg N2O-N ha−1 yr−1. This is less than the default value used by the IPCC for tropical forests52 (16 kg N2O-N ha−1 yr−1) but is similar to values from Stehfest and Bouwman57 (1.37 kg N2O-N ha−1 yr−1). For temperate forests, the soil N2O emission rate of the model ranges between 0 and 0.29 kg N2O-N ha−1 yr−1. This is again less than the default value used by the IPCC for temperate forests52 (8 kg N2O-N ha−1 yr−1) but is similar to values from Stehfest and Bouwman57 (0.64 kg N2O-N ha−1 yr−1). For boreal forests, the soil N2O emission rate of the model ranges between 0 and 0.13 kg N2O-N ha−1 yr−1. This is similar to values from Pihlatie et al.58 (−0.67 to 0.39 kg N2O-N ha−1 yr−1).
For tropical forests, the N fixation rate of the model ranges between 0 and 29 kg N ha−1 yr−1, which is similar to values from Batterman et al.3 (0–29 kg N ha−1 yr−1), Sullivan et al.20 (1.2–14.4 kg N ha−1 yr−1), and Winbourne et al.59 (0.3–22.75 kg N ha−1 yr−1). For temperate forests, the N fixation rate of the model ranges between 0 and 10 kg N ha−1 yr−1, which is similar to values from Menge and Hedin22 (0–11 kg N ha−1 yr−1). For boreal forests, the N fixation rate of the model ranges between 0 and 6 kg N ha−1 yr−1, which is similar to values from Blundon and Dale60 (0.3 kg N ha−1 yr−1). Other reported N fixation rates for temperate forests21,61 (33–150 kg N ha−1 yr−1) and boreal forests62,63 (38–107 kg N ha−1 yr−1) are substantially higher, but N-fixing trees are often rare or absent in temperate and boreal forests27. As such, the average N fixation rates across temperate and boreal forests are likely within the range of the N fixation rates of our model.
Global scale estimate
We applied the net CO2–N2O effect calculated with tropical, temperate, and boreal forest parameterizations to tropical, temperate, and boreal forest areas (from the 2015 Global Forest Resources Assessment53) respectively. Many forests are recovering from a past disturbance, imparting a variegated age distribution on global forests64. Because the net CO2–N2O effect (Eqs. (9) and (10)) is averaged over the first 100 years of ecosystem succession, it roughly encompasses the age distribution of global forests.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank P. Akana, R. Arkebauer, T. Bytnerowicz, A. Huddell, A. Petach, A. Quebbeman, and B. Taylor for discussions and comments on the manuscript. This study was supported by the National Oceanic and Atmospheric Administration’s Climate Program Office's Atmospheric Chemistry, Carbon Cycle, and Climate program, award NA15OAR4310065, and by the National Science Foundation, award DEB-1457650. We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada. Cette recherche a été financée par le Conseil de recherches en sciences naturelles et en génie du Canada.
Author contributions
S.K.-G. and D.M. designed the study. S.K.-G. performed the analysis and wrote the initial draft. Both authors contributed to the writing.
Code availability
Code used for analyses and figures has been archived in a GitHub repository (http://github.com/siankg/Nfixation_CO2N2O, 10.5281/zenodo.2576173).
Data availability
Data sharing is not applicable to this study as no data were generated or analyzed, aside from the simulated data created by the model and code.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Ute Skiba and the other anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09424-2.
References
- 1.Pan Y, et al. A large and persistent carbon sink in the world’s forests. Science. 2011;333:988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 2.Ciais, P. et al. in Climate Change 2013: The Physical Science Basis (eds Stocker, T. et al.) 465–570 (IPCC, Cambridge Univ. Press, Cambridge, United Kingdom and New York, NY, USA. 2013).
- 3.Batterman SA, et al. Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature. 2013;502:224–227. doi: 10.1038/nature12525. [DOI] [PubMed] [Google Scholar]
- 4.Gehring C, Vlek PLG, De Souza LAG, Denich M. Biological nitrogen fixation in secondary regrowth and mature rainforest of central Amazonia. Agric. Ecosyst. Environ. 2005;111:237–252. doi: 10.1016/j.agee.2005.06.009. [DOI] [Google Scholar]
- 5.Bühlmann T, Caprez R, Hiltbrunner E, Körner C, Niklaus PA. Nitrogen fixation by Alnus species boosts soil nitrous oxide emissions. Eur. J. Soil Sci. 2017;68:740–748. doi: 10.1111/ejss.12457. [DOI] [Google Scholar]
- 6.Erickson H, Keller M, Davidson EA. Nitrogen oxide fluxes and nitrogen cycling during postagricultural succession and forest fertilization in the humid tropics. Ecosystems. 2001;4:67–84. doi: 10.1007/s100210000060. [DOI] [Google Scholar]
- 7.Erickson H, Davidson EA, Keller M. Former land-use and tree species affect nitrogen oxide emissions from a tropical dry forest. Oecologia. 2002;130:297–308. doi: 10.1007/s004420100801. [DOI] [PubMed] [Google Scholar]
- 8.Erickson HE, Perakis SS. Soil fluxes of methane, nitrous oxide, and nitric oxide from aggrading forests in coastal Oregon. Soil Biol. Biochem. 2014;76:268–277. doi: 10.1016/j.soilbio.2014.05.024. [DOI] [Google Scholar]
- 9.Hall SJ, Asner GP. Biological invasion alters regional nitrogen-oxide emissions from tropical rainforests. Glob. Chang. Biol. 2007;13:2143–2160. doi: 10.1111/j.1365-2486.2007.01410.x. [DOI] [Google Scholar]
- 10.Soper FM, et al. Remotely sensed canopy nitrogen correlates with nitrous oxide emissions in a lowland tropical rainforest. Ecology. 2018;99:2080–2089. doi: 10.1002/ecy.2434. [DOI] [PubMed] [Google Scholar]
- 11.Myhre, G. et al. in Climate Change 2013: The Physical Science Basis (eds Stocker, T. et al.) 659–740 (IPCC, Cambridge Univ. Press, Cambridge, United Kingdom and New York, NY, USA 2013).
- 12.Dentener F, et al. Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Glob. Biogeochem. Cycles. 2006;20:1–21. doi: 10.1029/2005GB002672. [DOI] [Google Scholar]
- 13.Magnani F, et al. The human footprint in the carbon cycle of temperate and boreal forests. Nature. 2007;447:849–851. doi: 10.1038/nature05847. [DOI] [PubMed] [Google Scholar]
- 14.Zaehle S, Ciais P, Friend AD, Prieur V. Carbon benefits of anthropogenic reactive nitrogen offset by nitrous oxide emissions. Nat. Geosci. 2011;4:601–605. doi: 10.1038/ngeo1207. [DOI] [Google Scholar]
- 15.Reay DS, Dentener FJ, Smith P, Grace J, Feely RA. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008;1:430–437. doi: 10.1038/ngeo230. [DOI] [Google Scholar]
- 16.Tian H, et al. North American terrestrial CO2 uptake largely offset by CH4 and N2O emissions: toward a full accounting of the greenhouse gas budget. Clim. Change. 2015;129:413–426. doi: 10.1007/s10584-014-1072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Greaver TL. A review of nitrogen enrichment effects on three biogenic GHGs: The CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol. Lett. 2009;12:1103–1117. doi: 10.1111/j.1461-0248.2009.01351.x. [DOI] [PubMed] [Google Scholar]
- 18.Menge, D. N. L., Wolf, A. A. & Funk, J. L. Diversity of nitrogen fixation strategies in Mediterranean legumes. Nat. Plants 1,15064 (2015). [DOI] [PubMed]
- 19.Barron AR, Purves DW, Hedin LO. Facultative nitrogen fixation by canopy legumes in a lowland tropical forest. Oecologia. 2011;165:511–520. doi: 10.1007/s00442-010-1838-3. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan BW, et al. Spatially robust estimates of biological nitrogen (N) fixation imply substantial human alteration of the tropical N cycle. Proc. Natl Acad. Sci. USA. 2014;111:8101–8106. doi: 10.1073/pnas.1320646111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binkley, D., Cromack Jr., K. & Baker, D. D. in The Biology and Management of Red Alder (eds Hibbs, D. E. et al.) 57–72 (Oregon State Univ. Press, Corvallis, 1994).
- 22.Menge DNL, Hedin LO. Nitrogen fixation in different biogeochemical niches along a 120 000-year chronosequence in New Zealand. Ecology. 2009;90:2190–2201. doi: 10.1890/08-0877.1. [DOI] [PubMed] [Google Scholar]
- 23.Galloway JN, et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science. 2008;320:889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 24.Vet R, et al. A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 2014;93:3–100. doi: 10.1016/j.atmosenv.2013.10.060. [DOI] [Google Scholar]
- 25.Nakicenovic, N. and Swart, R. (eds) Emissions Scenarios (IPCC, Cambridge Univ. Press, Cambridge, 2000).
- 26.Vitousek, P. M., Menge, D. N., Reed, S. C. & Cleveland, C. C. Biological nitrogen fixation: rates, patterns and ecological controls in terrestrial ecosystems. Phil. Trans. R. Soc. B. 368, 20130119 (2013). [DOI] [PMC free article] [PubMed]
- 27.Menge DNL, Lichstein JW, Ángeles-Pérez G. Nitrogen fixation strategies can explain the latitudinal shift in nitrogen-fixing tree abundance. Ecology. 2014;95:2236–2245. doi: 10.1890/13-2124.1. [DOI] [PubMed] [Google Scholar]
- 28.Gei M, et al. Legume abundance along successional and rainfall gradients in Neotropical forests. Nat. Ecol. Evol. 2018;2:1104–1111. doi: 10.1038/s41559-018-0559-6. [DOI] [PubMed] [Google Scholar]
- 29.Menge, D. N. L., Denoyer, J. L. & Lichstein, J. W. Phylogenetic constraints do not explain the rarity of nitrogen-fixing trees in late-successional temperate forests. PLoS ONE5, e12056 (2010). [DOI] [PMC free article] [PubMed]
- 30.Sheffer, E., Batterman, S. A., Levin, S. A. & Hedin, L. O. Biome-scale nitrogen fixation strategies selected by climatic constraints on nitrogen cycle. Nat. Plants 1, 15182 (2015). [DOI] [PubMed]
- 31.Chapin FS, III, Conway AJ, Johnstone JF, Hollingsworth TN, Hollingsworth J. Absence of net long-term successional facilitation by alder in a boreal Alaska floodplain. Ecology. 2016;97:2986–2997. doi: 10.1002/ecy.1529. [DOI] [PubMed] [Google Scholar]
- 32.Lai HR, Hall JS, Batterman SA, Turner BL, van Breugel M. Nitrogen fixer abundance has no effect on biomass recovery during tropical secondary forest succession. J. Ecol. 2018;106:1415–1427. doi: 10.1111/1365-2745.12979. [DOI] [Google Scholar]
- 33.Taylor BN, Chazdon RL, Bachelot B, Menge DNL. Nitrogen-fixing trees inhibit growth of regenerating Costa Rican rainforests. Proc. Natl Acad. Sci. USA. 2017;114:8817–8822. doi: 10.1073/pnas.1707094114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aronson EL, Allison SD. Meta-analysis of environmental impacts on nitrous oxide release in response to N amendment. Front. Microbiol. 2012;3:1–6. doi: 10.3389/fmicb.2012.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall SJ, Matson PA. Nitrogen oxide emissions after nitrogen additions in tropical forests. Nature. 1999;400:152–155. doi: 10.1038/22094. [DOI] [Google Scholar]
- 36.Liao W, Menge DNL, Lichstein JW, Ángeles-Pérez G. Global climate change will increase the abundance of symbiotic nitrogen-fixing trees in much of North America. Glob. Chang. Biol. 2017;23:4777–4787. doi: 10.1111/gcb.13716. [DOI] [PubMed] [Google Scholar]
- 37.Griffis TJ, et al. Nitrous oxide emissions are enhanced in a warmer and wetter world. Proc. Natl Acad. Sci. USA. 2017;114:12081–12085. doi: 10.1073/pnas.1704552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith KA, et al. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003;54:779–791. doi: 10.1046/j.1351-0754.2003.0567.x. [DOI] [Google Scholar]
- 39.Adams MA, Turnbull TL, Sprent JI, Buchmann N. Legumes are different: leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl Acad. Sci. USA. 2016;113:4098–4103. doi: 10.1073/pnas.1523936113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellegrini AFA, Staver AC, Hedin LO, Charles-Dominique T, Tourgee A. Aridity, not fire, favors nitrogen-fixing plants across tropical savanna and forest biomes. Ecology. 2016;97:2177–2183. doi: 10.1002/ecy.1504. [DOI] [PubMed] [Google Scholar]
- 41.Wieder WR, Cleveland CC, Smith WK, Todd-Brown K. Future productivity and carbon storage limited by terrestrial nutrient availability. Nat. Geosci. 2015;8:441–444. doi: 10.1038/ngeo2413. [DOI] [Google Scholar]
- 42.Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl Acad. Sci. USA. 2010;107:19368–19373. doi: 10.1073/pnas.1006463107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy HR, et al. Re-assessment of plant carbon dynamics at the Duke free-air CO2 enrichment site: interactions of atmospheric [CO2] with nitrogen and water availability over stand development. New Phytol. 2010;185:514–528. doi: 10.1111/j.1469-8137.2009.03078.x. [DOI] [PubMed] [Google Scholar]
- 44.Hungate BA. CO2 elicits long-term decline in nitrogen fixation. Science. 2004;304:1291. doi: 10.1126/science.1095549. [DOI] [PubMed] [Google Scholar]
- 45.van Groenigen KJ, et al. Element interactions limit soil carbon storage. Proc. Natl Acad. Sci. USA. 2006;103:6571–6574. doi: 10.1073/pnas.0509038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunningham SC, et al. Balancing the environmental benefits of reforestation in agricultural regions. Perspect. Plant Ecol. Evol. Syst. 2015;17:301–317. doi: 10.1016/j.ppees.2015.06.001. [DOI] [Google Scholar]
- 47.Macedo MO, et al. Changes in soil C and N stocks and nutrient dynamics 13 years after recovery of degraded land using leguminous nitrogen-fixing trees. For. Ecol. Manag. 2008;255:1516–1524. doi: 10.1016/j.foreco.2007.11.007. [DOI] [Google Scholar]
- 48.Stocker BD, et al. Terrestrial nitrogen cycling in Earth system models revisited. New Phytol. 2016;210:1165–1168. doi: 10.1111/nph.13997. [DOI] [PubMed] [Google Scholar]
- 49.Wieder, W. R., Cleveland, C. C., Lawrence, D. M. & Bonan, G. B. Effects of model structural uncertainty on carbon cycle projections: biological nitrogen fixation as a case study. Environ. Res. Lett. 10, 044016 (2015).
- 50.Vitousek, P. M., Hedin, L. O., Matson, P. A., Fownes, J. H. & Neff, J. in Successes, Limitations and Frontiers in Ecosystem Science (eds Pace, M. L. & Groffman, P. M.) 432–451 (Springer, New York, 1998).
- 51.Liebig, J. Organic Chemistry in its Application to Agriculture and Physiology (Taylor and Walton, London, 1840).
- 52.De Klein, C. et al. in IPCC Guidelines for National Greenhouse Gas Inventories Vol. 4 (eds Eggleston, H. S. et al.) 11.1–11.54 (IPCC, IGES, Hayama, Japan 2006).
- 53.MacDicken K. Global Forest Resources Assessment 2015: what, why and how? For. Ecol. Manag. 2015;325:3–8. doi: 10.1016/j.foreco.2015.02.006. [DOI] [Google Scholar]
- 54.Alvarez RA, Pacala SW, Winebrake JJ, Chameides WL, Hamburg SP. Greater focus needed on methane leakage from natural gas infrastructure. Proc. Natl Acad. Sci. USA. 2012;109:6435–6440. doi: 10.1073/pnas.1202407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joos F, et al. Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: a multi-model analysis. Atmos. Chem. Phys. 2013;13:2793–2825. doi: 10.5194/acp-13-2793-2013. [DOI] [Google Scholar]
- 56.Pregitzer KS, Euskirchen ES. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004;10:2052–2077. doi: 10.1111/j.1365-2486.2004.00866.x. [DOI] [Google Scholar]
- 57.Stehfest E, Bouwman L. N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosystems. 2006;74:207–228. doi: 10.1007/s10705-006-9000-7. [DOI] [Google Scholar]
- 58.Pihlatie M, et al. Gas concentration driven fluxes of nitrous oxide and carbon dioxide in boreal forest soils. Tellus B: Chem. Phys. Meteorol. 2007;59:458–469. doi: 10.1111/j.1600-0889.2007.00278.x. [DOI] [Google Scholar]
- 59.Winbourne JB, et al. Nitrogen cycling during secondary succession in Atlantic Forest of Bahia, Brazil. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-19403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blundon DJ, Dale MRT. Dinitrogen fixation (acetylene reduction) in primary succession near Mount Robson, British Columbia, Canada. Arct. Alp. Res. 1990;22:255–263. doi: 10.2307/1551588. [DOI] [Google Scholar]
- 61.Boring LR, Swank WT. The role of black locust (Robinia pseudoacacia) in forest succession. J. Ecol. 1984;72:749–766. doi: 10.2307/2259529. [DOI] [Google Scholar]
- 62.Ruess RW, McFarland JM, Trummer LM, Rohrs-Richey JK. Disease-mediated declines in N-fixation inputs by Alnus tenuifolia to early-successional floodplains in interior and south-central Alaska. Ecosystems. 2009;12:489–502. doi: 10.1007/s10021-009-9237-5. [DOI] [Google Scholar]
- 63.Uliassi DD, Ruess RW. Limitations to symbiotic nitrogen fixation in primary succession on the Tanana River floodplain. Ecology. 2002;83:88–103. doi: 10.1890/0012-9658(2002)083[0088:LTSNFI]2.0.CO;2. [DOI] [Google Scholar]
- 64.Pan Y, et al. Age structure and disturbance legacy of North American forests. Biogeosciences. 2011;8:715–732. doi: 10.5194/bg-8-715-2011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code used for analyses and figures has been archived in a GitHub repository (http://github.com/siankg/Nfixation_CO2N2O, 10.5281/zenodo.2576173).
Data sharing is not applicable to this study as no data were generated or analyzed, aside from the simulated data created by the model and code.