Abstract
Purpose
Precise evaluation of serum testosterone levels is important in making an accurate diagnosis of androgen deficiency. Recent practice guidelines on male androgen deficiency recommend that testosterone be measured in the morning while fasting. Although there is ample evidence regarding morning measurement of testosterone, studies that evaluated the effect of glucose load or meals were limited by inclusion of hypogonadal or diabetic men, and measurement of testosterone was not performed using mass spectrometry.
Methods
Sixty men (23–97 years) without pre-diabetes or diabetes who had normal total testosterone (TT) levels underwent either an oral glucose tolerance test (OGTT) or a mixed meal tolerance test (MMTT) after an overnight fast. Serum samples were collected before and at regular intervals for 2 h (OGTT cohort) or 3 h (MMTT cohort). TT was measured by LC-MS/MS. LH and prolactin were also measured.
Results
TT decreased after a glucose load (mean drop at nadir = 100 ng/dL) and after a mixed meal (drop at nadir = 123 ng/dL). Approximately 11% of men undergoing OGTT and 56% undergoing MMTT experienced a transient decrease in TT below 300 ng/dL, the lower normal limit. Testosterone started declining 20 min into the tests, with average maximum decline at 60 min. Most men still had TT lower than baseline at 120 min. This effect was independent of changes in LH or prolactin.
Conclusion
A glucose load or a mixed meal transiently, but significantly, lowers TT levels in healthy, non-diabetic eugonadal men. These findings support the recommendations that measurement of serum testosterone to diagnose androgen deficiency should be performed while fasting.
Keywords: Sex hormones, Testosterone, LH, Oral glucose tolerance test, Mixed meal tolerance test, Hypogonadism
Introduction
Male hypogonadism is a clinical syndrome resulting from low testosterone levels due to dysfunction of the hypothalamic-pituitary-testicular axis. The diagnosis of hypogonadism is based on the laboratory finding of consistently low serum testosterone levels in the presence of specific symptoms of androgen deficiency [1]. Hence, precise measurement of testosterone is critical in making an accurate diagnosis. As various clinical practice guidelines suggest specific, cutoffs for testosterone levels, care must be taken to avoid factors that transiently lower serum testosterone levels as otherwise these patients might be misclassified as hypogonadal. Use of accurate assays in the measurement of sex steroids is important and has received much emphasis recently [1, 2]. In addition to the assays, several other considerations are also important during the evaluation of androgen deficiency. One important consideration is measurement of serum testosterone in the morning. Indeed, a large body of data confirms that serum testosterone secretion has a diurnal rhythm and its concentration varies widely during the day, with peak levels occurring during the morning hours [3, 4]. Based on these observations, clinical practice guidelines for male androgen deficiency have recommended that serum testosterone levels, in a patient presenting for evaluation of hypogonadism, should be assessed in the morning [1].
Another important consideration is whether measurement of testosterone in the evaluation of androgen deficiency be performed in a fasting state. Although the most recent Endocrine Society Clinical Practice Guidelines recommend that testosterone should be drawn in a fasting state [1], these recommendations are based on studies that either had a small sample size, enrolled a heterogenous population of men or did not use reliable methods for testosterone measurement [5–13]. For instance, some studies enrolled men with impaired glucose tolerance and diabetes [10, 12], conditions known to be associated with low testosterone [14, 15], while other studies enrolled men who were either hypogonadal or had other comorbidities [10–12]. Therefore, inclusion of men with heterogenous characteristics in previous studies makes it difficult to ascribe the decline in serum testosterone concentrations solely to glucose load or meals. Furthermore, studies that did enroll healthy men either had a very small sample size or did not measure serum testosterone with liquid-chromatography-tandem mass spectrometry (LC-MS/MS), the gold-standard method [16]. Not surprisingly, the findings from these studies were not unanimous with some reports showing a transient decrease in total testosterone levels [independent of changes in sex hormone binding globulin (SHBG)] [6–13], while others did not observe any significant change [5]. Furthermore, the response in luteinizing hormone (LH) secretion was also variable and was not helpful in elucidating the mechanisms by which feeding might influence testosterone secretion. Lastly, these studies did not measure serum prolactin levels, a hormone that is known to increase in post-prandial state and suppresses testosterone production by inhibiting gonadotropin releasing hormone (GnRH) synthesis.
Considering that the influence of a glucose load or meals on morning serum testosterone levels in metabolically healthy eugonadal men has not been evaluated using state-of-the-art assays, we evaluated two cohorts of eugonadal men to understand whether acute feeding affects circulating testosterone concentrations: (1) The first cohort included healthy, non-obese, eugonadal men from the Baltimore Longitudinal Study on Aging (BLSA) without diabetes and a normal oral glucose tolerance test (OGTT) that was performed using frequent blood sampling (OGTT Cohort); (2) The second cohort comprised of healthy, eugonadal, non-obese, non-diabetic men who were part of a protocol to evaluate metabolic response to a mixed meal; these men underwent a mixed meal tolerance test (MMTT) and underwent frequent sampling during the test (MMTT Cohort). Our objective was to determine whether 75 g of glucose load (concentration of glucose in some beverages) or a mixed meal results in any changes in serum testosterone level; to determine whether these changes occur at any specific time points during these tests, and whether oral glucose load or mixed meal has any differential effects on serum testosterone concentrations.
Methods
The OGTT cohort
Design and participants
The Baltimore Longitudinal Study of Aging (BLSA) has been investigating human aging since its establishment by the National Institute on Aging Intramural Research Program in 1958 [17]. The BLSA continuously enrolls healthy community-dwelling volunteers 20 years of age or older, followed at intervals of 1 to 4 years, with older subjects having more frequent follow-up visits.
Men participating in the BLSA who did not have any chronic illness, pre-diabetes, or diabetes, had a normal fasting morning serum testosterone, were 40–100 years old and had available data from their last OGTT formed the analytic sample (51 subjects) for the evaluation of the influence of an OGTT on serum testosterone levels. Participants were classified (normal, pre-diabetes, or diabetes) according to the American Diabetes Association criteria using fasting plasma glucose and/or 2-h post-OGTT glucose levels [18]. Subjects underwent a complete physical examination and measurement of their body mass index (BMI). All men had normal serum total testosterone concentrations (>300 ng/dL) at baseline.
Oral glucose tolerance test
After a 10-hour overnight fast, blood samples were collected immediately before and every 20 min for 120 min after ingestion of 75 g of glucose. Subjects underwent OGTT between 8 and 8:30 a.m.
The MMTT cohort
Design and participants
Ten healthy non-obese men (not part of the BLSA cohort) 21–55 years old were recruited. Prior to enrollment, volunteers were screened for glucose intolerance with a 75 g OGTT and only men without pre-diabetes or diabetes were invited to participate. One participant with a baseline total testosterone below 300 ng/dL was excluded from the study, resulting in an analytical sample of nine subjects.
Mixed meal tolerance test
After fasting for at least 12 h, subjects consumed a standardized meal-replacement (Ensure Plus) solution in the early morning. The solution consisted of 1.5 Cal/mL (15% protein, 58% carbohydrate, 28% fat). The amount of solution given was based on the subject’s screening weight (5 mL of solution/kg of body weight). Blood samples were collected before and every 20 min after the beginning of the meal for the first 180 min.
Sample collection
All samples were collected into EDTA-coated tubes. Immediately after collection, samples were centrifuged at 4 °C, divided into aliquots and immediately frozen on dry ice and stored at −80 °C until analysis.
Measurement of glucose and insulin
Plasma glucose levels were measured with a glucose oxidase analyzer (YSI Incorporated, Yellow Springs, OH, USA), and insulin was measured by enzyme-linked immunosorbent assay (ELISA) (Mercodia Inc., Winston-Salem, NC, USA) with intra-assay variation of 2.8 to 4.0% and inter-assay variation of 2.6 to 3.6%.
Measurement of testosterone, LH, and prolactin
Blood samples collected immediately before and after the glucose load and administration of solution were assessed for testosterone, LH, and prolactin levels. Total testosterone was measured using a LC-MS/MS assay performed in a CDC-certified laboratory with a sensitivity of 2 ng/dL [19]. Luteinizing hormone was measured using a immunofluorometric assay (PerkinElmer, Waltham, MA), with limit of detection of 0.05 U/L. Prolactin was measured using an immunoassay with limit of detection of 1 ng/mL (Quest Diagnostics, Cambridge, MA).
Statistical analysis
Baseline characteristics were reported using means and standard deviations, while graphical data and changes from baseline were displayed as mean ± standard error of mean. A paired Student’s t-test was performed to evaluate the effects of feeding on baseline values of glucose, insulin, total testosterone, LH, and prolactin at each follow-up timepoint for both cohorts. Bonferroni-Holm correction method was applied to adjust p-values for multiple testing [20]. To explore the association between nadir levels of testosterone and changes in glucose simple linear regression was used. All hypotheses were tested using two-sided type I error α= 0.05. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC), GraphPad Prism version 6 (GraphPad Software, La Jolla, CA), and R software version 3.2.4.
Results
OGTT cohort
Characteristics of the participants
Fifty-one participants formed the analytical sample (Table 1). Their median (range) age was 69 years (range 40 to 97 years), mean BMI was 26 kg/m2 (21.5 to 43.7 kg/m2), and mean serum testosterone level at baseline was 552 ng/dL (316 to 924 ng/dL).
Table 1.
Baseline characteristics of selected participants in the OGTT and MMTT studies
| OGTT (N = 51) | MMTT (N = 9) | |
|---|---|---|
| Demographics | ||
| Age (years) | 68.2 (16.4) | 31.4 (8.9) |
| Weight (kg) | 79.5 (14.2) | 84.9 (11.0) |
| Height (cm) | 174.9 (8.1) | 183.8 (7.6) |
| BMI (kg/m2) | 25.9 (3.8) | 25.1 (2.2) |
| Glycemic parameters | ||
| HbA1C (%) | 5.6 (0.4) | 5.3 (0.3) |
| Fasting glucose (mg/dL) | 89.7 (5.7) | 90.3 (8.5) |
| Fasting insulin (mU/L) | 4.5 (2.7, 8.0) | 5.8 (3.7) |
| Hormones | ||
| Total testosterone (ng/dL) | 552 (142) | 465 (110) |
| Luteinizing hormone (IU/L) | 3.7 (2.7,4.5) | 6.7 (1.5) |
| Prolacin (ng/mL) | 7.1 (2.7) | 15.5 (5.2) |
Data displayed as mean (SD) or median (IQR)
OGTT oral glucose tolerance test, MMTT mixed meal tolerance test
Effect of glucose load on glucose and insulin
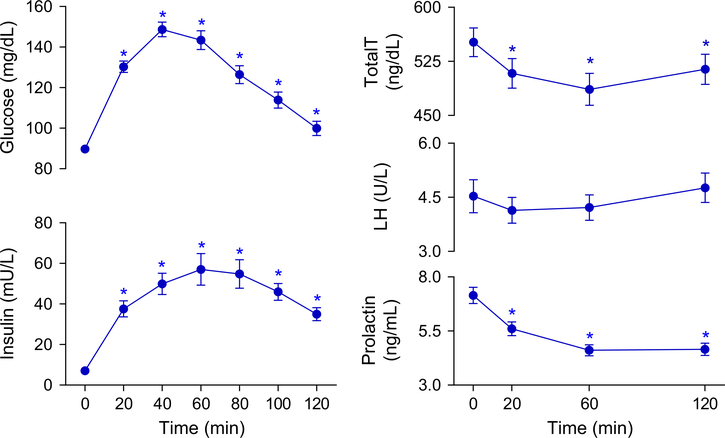
As expected, glucose and insulin levels significantly increased after ingestion of the glucose load, and remained higher than baseline throughout the 120 min of the test (Fig. 1).
Fig. 1.
Mean serum levels of glucose, insulin, prolactin, LH, and total testosterone in metabolically healthy eugonadal men before and 2 h following an oral glucose load. Data are means and error bars are SEM. p-values calculated by paired t-test with Bonferroni-Holm correction comparing baseline to follow-up measurements. *p < 0.05. LH luteinizing hormone, total T total testosterone
Effect of glucose load on testosterone levels
The OGTT significantly lowered plasma total testosterone levels, with a mean reduction of 6.8% at 20 min, 11.5% at 60 min and 6.4% at 120 min (Fig. 1). At the end of the test (120 min), 73% of the subjects had total testosterone levels that continued to be lower compared to their baseline concentrations. Five of the 47 men that had testosterone measured at all time points had at least one testosterone level below 300 ng/dL after consuming the glucose solution: one subject at 20 min, five subjects at 60 min, and one subject at 120 min. Of these 47 men, 41 (87%) had at least one measurement below baseline level, with 10, 22, and 9 subjects achieving a nadir at 20, 60, and 120 min, respectively. Mean total testosterone level at nadir was 452 ± 22 ng/dL (18% reduction). Mean total testosterone level at nadir was 452 ± 22 ng/dL (18% reduction). The increase in glucose concentration at 20-min time-point was positively associated with the percent decrease in total testosterone compared to baseline (p = 0.047; r2= 0.098).
Effect on LH and prolactin levels
No significant change in LH levels was observed throughout the 2 h of the test (Fig. 1). Interestingly, serum prolactin levels decreased after glucose ingestion and remained lower than baseline for the remainder of the test (mean reduction of 20.2%, 33.3%, and 32.4% at 20, 60, and 120 min, respectively; Fig. 1).
MMTT cohort
Characteristics of the participants
Nine participants formed the analytical sample (Table 1). The median (range) age was 31 years (23 to 45 years), mean BMI was 25kg/m2 (21.2 to 28.6kg/m2), and mean serum testosterone level at baseline was 465 ng/dL (343 to 625ng/dL).
Effect of mixed meal on glucose and insulin
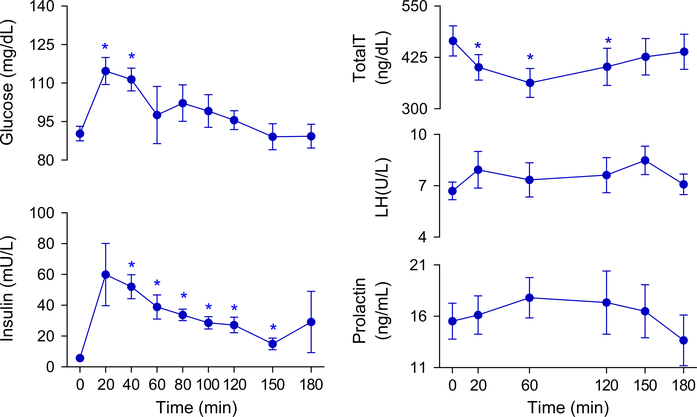
Glucose and insulin concentrations significantly increased after ingestion of the mixed meal, with glucose levels returning towards baseline at 60 min, while insulin levels remained higher than baseline until 150 min after meal ingestion (Fig. 2).
Fig. 2.
Mean serum levels of glucose, insulin, prolactin, LH, and total testosterone in metabolically healthy eugonadal men before and in the 3 h following a mixed meal. Data are means and error bars are SEM. p-values calculated by paired t-test with Bonferroni-Holm correction comparing baseline to follow-up measurements. *p < 0.05. LH luteinizing hormone, total T total testosterone
Effect of mixed meal on testosterone levels
Total testosterone levels decreased significantly after the ingestion of a mixed meal, with a mean reduction of 13.6% at 20 min, 21.4% at 60 min, and 14.9% at 120 min (Fig. 2). Eight men (89%) continued to have persistently lower total testosterone levels compared to baseline at 120 min. Five out of the nine participants (55%) achieved at least one testosterone value below 300 ng/dL after ingesting the mixed meal: one subject at 20 min, four subjects at 60 min, and three subjects at 120 min. At the 150-min time-point, testosterone values in all men had returned to normal. In contrast with the OGTT cohort, all 9 men in the MMTT cohort achieved a lower testosterone value than baseline at some point after ingestion of a mixed meal. Three men achieved a nadir value at 20 min, 2 at 60 min and 4 at 120 min. Mean nadir total testosterone concentration was 342 ± 34 ng/dL (26% reduction).
Effect of mixed meal on LH and prolactin levels
No significant change in LH or prolactin levels were observed throughout the 3 h of the test (Fig. 2).
Discussion
Accurate diagnosis of male androgen deficiency is important so that only those men who are likely to benefit are started on testosterone therapy. Over the years, various clinical practice guidelines on male androgen deficiency syndromes recommend that a diagnosis of hypogonadism be made only in patients with specific signs and symptoms and consistently (and unequivocally) low morning testosterone concentrations [21–24]. The most recent Endocrine Society guidelines also recommended that serum testosterone measurement should be performed while the patient is fasting [1]. These latest recommendations are based on a limited number of previous studies [6–13] that were limited by inclusion of either hypogonadal, prediabetic, or diabetic men. Furthermore, these studies did not utilize gold-standard methods for measurement of testosterone nor measured serum prolactin levels. The present study was designed to overcome these limitations. Herein, we report that ingestion of either a glucose load or a mixed meal results in a significant decrease in serum total testosterone levels (as assessed by LC-MS/MS) in healthy, non-diabetic, eugonadal men. Participants achieved reductions in serum testosterone levels by as much as 39% with the glucose load and 56% after a mixed meal; this drop resulted in serum testosterone levels dropping below the normal range in a significant number of participants. Thus, our observations support the recent recommendations by the Endocrine Society guidelines that measurement of testosterone in the evaluation of androgen deficiency should be performed after an overnight fast [1]. As this reduction in serum testosterone concentrations was non-trivial even in healthy non-diabetic men, it is conceivable that this decrement might be of a greater magnitude in men who are obese or have metabolic syndrome.
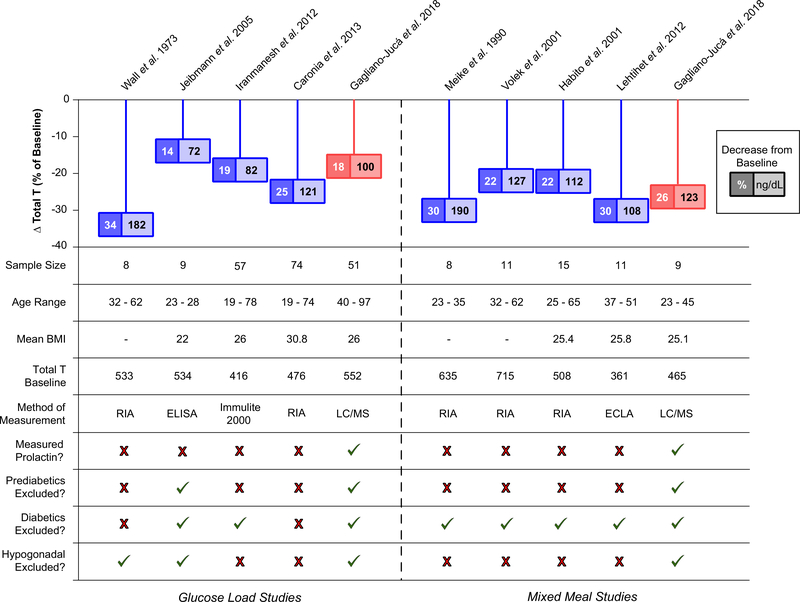
Previous studies had reported mean reduction in serum total testosterone concentrations between 10–35% with either OGTT or MMTT (Fig. 3) [6–13]. Although the exact mechanism by which this reduction occurs remains unclear, none of the previous studies observed any significant change in SHBG concentrations either following a glucose load [10, 11] or administration of meals [5, 8, 9], suggesting that the fall in serum testosterone is real and not simply a reflection of reduction in serum SHBG levels. What remains unclear is whether this decline in testosterone is due to a central process or secondary to reduced testicular secretion as some previous studies reported an increase in LH levels while others showed no change [6, 8, 10]. In the current study, we did not observe any significant change in serum LH levels following either a glucose load or mixed meal despite measuring serum LH levels at multiple time points in both cohorts. The reason for these contrasting findings might be due to the fact that LH is secreted in a pulsatile manner and its measurement at limited time points may not be sufficient to draw meaningful inferences. A previous study that performed serial LH sampling at 10-min intervals for 6 h reported that glucose ingestion dampened LH pulsatility but did not affect basal or total LH secretion [12]. Since the decrease in serum testosterone concentrations observed in these studies (including the current study) was not associated with a parallel decline in serum LH levels, it does suggest that this decline is not solely mediated by a central mechanism; however, the absence of a concurrent rise in LH levels in response to the fall in testosterone does suggest some degree of central involvement. Indeed, exposure of immortalized GnRH cell line to high concentrations of glucose results in impairment in GnRH synthesis and secretion in vitro and leads to their apoptosis. Though speculative, it is conceivable that the sudden increase in serum glucose concentrations might transiently impair GnRH synthesis and/or release [25].
Fig. 3.
Summary of studies evaluating the effect of a glucose load (left) and a mixed meal (right) on serum testosterone levels (both absolute and % changes). BMI body mass index, ECLA electrochemiluminescence immunoassay, ELISA enzyme-linked immunosorbent assay, LC/MS liquid-chromatography tandem mass spectrometry, RIA radioimmunoassay
Hyperprolactinemia, of any etiology, suppresses hypothalamic GnRH synthesis and/or secretion and is an established cause of central hypogonadism [26, 27]. It has also been known that serum prolactin concentrations increase post-prandially, particularly after high-protein meals [28–30]. We had hypothesized that a surge in prolactin levels might be contributory to this transient decrease in serum testosterone levels, however, serum prolactin concentrations did not increase significantly in either cohort, in fact its levels decreased after glucose load. This suggests that postprandial decline in serum testosterone levels is not a consequence of hyperprolactinemia. Another mechanism that might be responsible for post-prandial reduction in testosterone level is increased secretion of inflammatory cytokine tumor necrosis factor-α (TNF-α). This cytokine is known to increase in healthy men in response to glucose administration [31]; it blunts LH response to GnRH administration [32] and directly suppresses testosterone secretion by Leydig cells [33, 34]. The role of cytokines in this context should be explored in future studies.
The observations of the current study have important practical ramifications. Coming for a blood draw after an overnight fast is not convenient for many patients, in particular for older men and those who take medications in the morning. Consequently, many patients ask that instead of a full breakfast they could have orange juice, coffee, or other breakfast beverages before coming to the laboratory. Considering that a cup of some special coffee beverages contains up to 60 g of sugar and that a glass of orange juice contains 30 g of sugar (Supplementary Table), it is conceivable that consumption of these beverages might have an influence on serum testosterone concentrations. Therefore, it is prudent that the practitioners advise their patients to present for testosterone measurement after a complete overnight fast. In select patients suspected of having metabolic syndrome, this fasting visit can also serve as an avenue for measuring certain metabolic parameters such as fasting glucose and lipid profile.
The present study has several strengths: (i) careful exclusion of hypogonadal, prediabetic, and diabetic men to avoid any confounding effect of their condition on changes in testosterone levels; (ii) evaluation of healthy men without chronic illness; (iii) measurement of serum testosterone using LC-MS/MS, the gold-standard method; (iv) measurement of serum prolactin levels in parallel with testosterone measurement; and, (v) measurement of hormonal parameters at multiple time points. Additionally, since intake of pure glucose load or mixed meal may result in variable changes in testosterone levels, we evaluated responses to both interventions. Lastly, the composition of the mixed meal was carefully standardized. The study also has a few limitations. The sample size in the MMTT study was small, though comparable to some previous studies. We did not measure SHBG levels; however, this was intentional as none of the previous studies observed any effect of a glucose load or meals on serum SHBG levels [5, 8–11]. Lastly, we did not measure inflammatory cytokines, which might have shed some additional light on the mechanism behind post-prandial reduction in testosterone.
Conclusion
We demonstrate a significant reduction in concentrations of serum total testosterone in healthy men in response to an oral glucose load (mean reduction of 18% from baseline) and administration of a mixed meal (mean reduction of 26% from baseline. Therefore, measurement of serum testosterone in men presenting for evaluation of hypogonadism should be performed after an overnight fast.
Supplementary Material
Acknowledgments
Funding National Institute on Aging Intramural research grants 03AG-N035 and 15-AG-N074.
Footnotes
Compliance with ethical standards
Conflict of interest Dr. Basaria has no conflict of interest related to the current work. He has previously received grant support from Abbott Pharmaceuticals for investigator-initiated studies unrelated to this study and has previously consulted for AbbVie, Eli Lilly, Inc and Regeneron Pharmaceuticals. The other authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The BLSA study protocol was reviewed by the National Institute of Environmental Health Sciences Institutional Review Board and the study with participants not from the BLSA cohort was approved by the Intramural Research Program of the National Institute on Aging and the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland.
Informed consent Informed consent was obtained from all individual participants included in both studies.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12020-018-1741-y) contains supplementary material, which is available to authorized users.
References
- 1.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN,Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA, Testosterone therapy in men with hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab 103(5), 1715–1744 (2018). 10.1210/jc.2018-00229 [DOI] [PubMed] [Google Scholar]
- 2.Wartofsky L, Handelsman DJ, Standardization of hormonal assays for the 21st century. J. Clin. Endocrinol. Metab 95(12), 5141–5143 (2010). 10.1210/jc.2010-2369 [DOI] [PubMed] [Google Scholar]
- 3.Bremner WJ, Vitiello MV, Prinz PN, Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J. Clin. Endocrinol. Metab 56(6), 1278–1281 (1983). 10.1210/jcem-56-6-1278 [DOI] [PubMed] [Google Scholar]
- 4.Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB, Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin. Endocrinol 67(6), 853–862 (2007). 10.1111/j.1365-2265.2007.02976.x [DOI] [PubMed] [Google Scholar]
- 5.Meikle AW, Stringham JD, Woodward MG, McMurry MP, Effects of a fat-containing meal on sex hormones in men. Metab.: Clin. Exp 39(9), 943–946 (1990) [DOI] [PubMed] [Google Scholar]
- 6.Jeibmann A, Zahedi S, Simoni M, Nieschlag E, Byrne MM, Glucagon-like peptide-1 reduces the pulsatile component of testosterone secretion in healthy males. Eur. J. Clin. Investig 35(9), 565–572 (2005). 10.1111/j.1365-2362.2005.01542.x [DOI] [PubMed] [Google Scholar]
- 7.Wall JR, Jarrett RJ, Zimmet PZ, Bailes M, Ramage CM, Fallin plasma-testosterone levels in normal male subjects in response to an oral glucose load. Lancet 1(7810), 967–968 (1973) [DOI] [PubMed] [Google Scholar]
- 8.Lehtihet M, Arver S, Bartuseviciene I, Pousette A, S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 44(6), 405–410 (2012). 10.1111/j.1439-0272.2012.01296.x [DOI] [PubMed] [Google Scholar]
- 9.Habito RC, Ball MJ, Postprandial changes in sex hormones after meals of different composition. Metab.: Clin. Exp 50(5), 505–511 (2001). 10.1053/meta.2001.20973 [DOI] [PubMed] [Google Scholar]
- 10.Caronia LM, Dwyer AA, Hayden D, Amati F, Pitteloud N, Hayes FJ, Abrupt decrease in serum testosterone levels after an oral glucose load in men: implications for screening for hypogonadism. Clin. Endocrinol 78(2), 291–296 (2013). 10.1111/j.1365-2265.2012.04486.x [DOI] [PubMed] [Google Scholar]
- 11.Hjalmarsen A, Aasebo U, Aakvaag A, Jorde R, Sex hormone responses in healthy men and male patients with chronic obstructive pulmonary disease during an oral glucose load. Scand. J. Clin. Lab. Investig 56(7), 635–640 (1996) [DOI] [PubMed] [Google Scholar]
- 12.Iranmanesh A, Lawson D, Veldhuis JD, Glucose ingestionacutely lowers pulsatile LH and basal testosterone secretion in men. Am. J. Physiol. Endocrinol. Metab 302(6), E724–730 (2012). 10.1152/ajpendo.00520.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volek JS, Gomez AL, Love DM, Avery NG, Sharman MJ,Kraemer WJ, Effects of a high-fat diet on postabsorptive and postprandial testosterone responses to a fat-rich meal. Metab.: Clin. Exp 50(11), 1351–1355 (2001) [DOI] [PubMed] [Google Scholar]
- 14.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, Dandona P, Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes care 33(6), 1186–1192 (2010). 10.2337/dc09-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandona P, Dhindsa S, Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J. Clin. Endocrinol. Metab 96(9), 2643–2651 (2011). 10.1210/jc.2010-2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handelsman DJ, Wartofsky L, Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J. Clin. Endocrinol. Metab 98(10), 3971–3973 (2013). 10.1210/jc.2013-3375 [DOI] [PubMed] [Google Scholar]
- 17.Stone JL, Norris AH, Activities and attitudes of participants in the Baltimore longitudinal study. J. Gerontol. 21(4), 575–580 (1966) [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes, A., Standards of medical care in diabetes-2010. Diabetes Care 33 Suppl 1, S11–61 (2010). 10.2337/dc10-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS, Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J. Clin. Endocrinol. Metab 96(8), 2430–2439 (2011). 10.1210/jc.2010-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holm S, A simple sequentially rejective multiple test procedure. Scand. J. Stat 6(2), 65–70 (1979) [Google Scholar]
- 21.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM, Task Force ES, Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab 95(6), 2536–2559 (2010). 10.1210/jc.20092354 [DOI] [PubMed] [Google Scholar]
- 22.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM, Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab 91 (6), 1995–2010 (2006). 10.1210/jc.2005-2847 [DOI] [PubMed] [Google Scholar]
- 23.Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, Lightner DJ, Miner MM, Murad MH, Nelson CJ, Platz EA, Ramanathan LV, Lewis RW, Evaluation and management of testosterone deficiency: AUA guideline. J. Urol (2018). 10.1016/j.juro.2018.03.115 [DOI] [PubMed] [Google Scholar]
- 24.Dean JD, McMahon CG, Guay AT, Morgentaler A, Althof SE, Becher EF, Bivalacqua TJ, Burnett AL, Buvat J, El Meliegy A, Hellstrom WJ, Jannini EA, Maggi M, McCullough A, Torres LO, Zitzmann M, The International Society for Sexual Medicine’s Process of Care for the assessment and management of testosterone deficiency in adult men. J. Sex. Med 12 (8), 1660–1686 (2015). 10.1111/jsm.12952 [DOI] [PubMed] [Google Scholar]
- 25.Pal L, Chu HP, Shu J, Topalli I, Santoro N, Karkanias G, In vitro evidence of glucose-induced toxicity in GnRH secreting neurons: high glucose concentrations influence GnRH secretion, impair cell viability, and induce apoptosis in the GT1–1 neuronal cell line. Fertil. Steril 88(4 Suppl), 1143–1149 (2007). 10.1016/j.fertnstert.2007.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung CY, Prolactin suppresses luteinizing hormone secretion and pituitary responsiveness to luteinizing hormone-releasing hormone by a direct action at the anterior pituitary. Endocrinology 113(2), 632–638 (1983). 10.1210/endo-113-2-632 [DOI] [PubMed] [Google Scholar]
- 27.Thorner MO, Ryan SM, Wass JA, Jones A, Bouloux P, Williams S, Besser GM, Effect of the dopamine agonist, lergotrile mesylate, on circulating anterior pituitary hormones in man. J. Clin. Endocrinol. Metab 47(2), 372–378 (1978). 10.1210/jcem-47-2-372 [DOI] [PubMed] [Google Scholar]
- 28.Ishizuka B, Quigley ME, Yen SS, Pituitary hormone release in response to food ingestion: evidence for neuroendocrine signals from gut to brain. J. Clin. Endocrinol. Metab 57(6), 1111–1116 (1983). 10.1210/jcem-57-6-1111 [DOI] [PubMed] [Google Scholar]
- 29.Carlson HE, Wasser HL, Levin SR, Wilkins JN, Prolactin stimulation by meals is related to protein content. J. Clin. Endocrinol. Metab 57(2), 334–338 (1983). 10.1210/jcem-57-2-334 [DOI] [PubMed] [Google Scholar]
- 30.Carlson HE, Prolactin stimulation by protein is mediated by amino acids in humans. J. Clin. Endocrinol. Metab 69(1), 7–14 (1989). 10.1210/jcem-69-1-7 [DOI] [PubMed] [Google Scholar]
- 31.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D, Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106(16), 2067–2072 (2002) [DOI] [PubMed] [Google Scholar]
- 32.Gaillard RC, Turnill D, Sappino P, Muller AF, Tumor necrosis factor alpha inhibits the hormonal response of the pituitary gland to hypothalamic releasing factors. Endocrinology 127(1), 101–106 (1990). 10.1210/endo-127-1-101 [DOI] [PubMed] [Google Scholar]
- 33.Mauduit C, Gasnier F, Rey C, Chauvin MA, Stocco DM, Louisot P, Benahmed M, Tumor necrosis factor-alpha inhibits leydig cell steroidogenesis through a decrease in steroidogenic acute regulatory protein expression. Endocrinology 139(6), 2863–2868 (1998). 10.1210/endo.139.6.6077 [DOI] [PubMed] [Google Scholar]
- 34.Morales V, Santana P, Diaz R, Tabraue C, Gallardo G, Lopez Blanco F, Hernandez I, Fanjul LF, Ruiz de Galarreta CM, Intratesticular delivery of tumor necrosis factor-alpha and ceramide directly abrogates steroidogenic acute regulatory protein expression and Leydig cell steroidogenesis in adult rats. Endocrinology 144(11), 4763–4772 (2003). 10.1210/en.2003-0569 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.