Abstract
The development, maintenance of healthy bone and regeneration of injured tissue in the human body comprise a set of intricate and finely coordinated processes. However, an analysis of current bone regeneration strategies shows that only a small fraction of well-reported bone biology aspects has been used as inspiration and transposed into the development of therapeutic products. Specific topics that include inter-scale bone structural organization, developmental aspects of bone morphogenesis, bone repair mechanisms, role of specific cells and heterotypic cell contact in the bone niche (including vascularization networks and immune system cells), cell-cell direct and soluble-mediated contact, extracellular matrix composition (with particular focus on the non-soluble fraction of proteins), as well as mechanical aspects of native bone will be the main reviewed topics. In this Review we suggest a systematic parallelization of (i) fundamental well-established biology of bone, (ii) updated and recent advances on the understanding of biological phenomena occurring in native and injured tissue, and (iii) critical discussion of how those individual aspects have been translated into tissue regeneration strategies using biomaterials and other tissue engineering approaches. We aim at presenting a perspective on unexplored aspects of bone physiology and how they could be translated into innovative regeneration-driven concepts.
Keywords: bone physiology, bone microenvironment, biomaterials, biomimetics
1. Introduction
Bone physiology involves the coordinated regulation of a myriad of biological processes that lead tissue development, homeostasis and repair upon trauma [1]. Bone regenerative processes can thus be highly complicated to emulate, partially due to the numerous and multifactorial cues contributing for the regulation of its niche. Those include the tissue’s complex composition and intricate associated pathways involving aspects like bone’s soluble microenvironment, extracellular matrix (ECM) insoluble proteins and glycoprotein composition and renewal, cell-cell and cell-ECM interactions mechanical stimulation, or the role of microRNAs. The understanding of the individual and coordinated role of each intervenient on bone tissue physiology, as well as their interconnected actions, has inspired the design of a plethora of biomimetic and/or bioinspired bone regeneration approaches. In fact, there is an urgent need for the development of new, effective and compatible treatments for bone-related injuries. Age-related fractures are expected to increase in the United States from 2.1 million in 2005 to over 3 million fractures in 2025, solely considering the elderly/aging population at risk [2]. In Europe, the annual number of fractures is estimated to rise 28% from 2010 to 2025, with an absolute increase from 3.5 million to 4.5 million injuries, respectively [3]. The application of concepts learnt from nature for the emulation of the structure and physiology of healthy bone, however, requires insightful and careful approaches. Although mimicking bone’s structure and function based on its physiology is an attractive idea, the implementation of effective reproducible therapies is most often dependent on achieving a balance between sufficient complexity to warrant function, along with ease/speed of manufacturing and regulatory compliance.
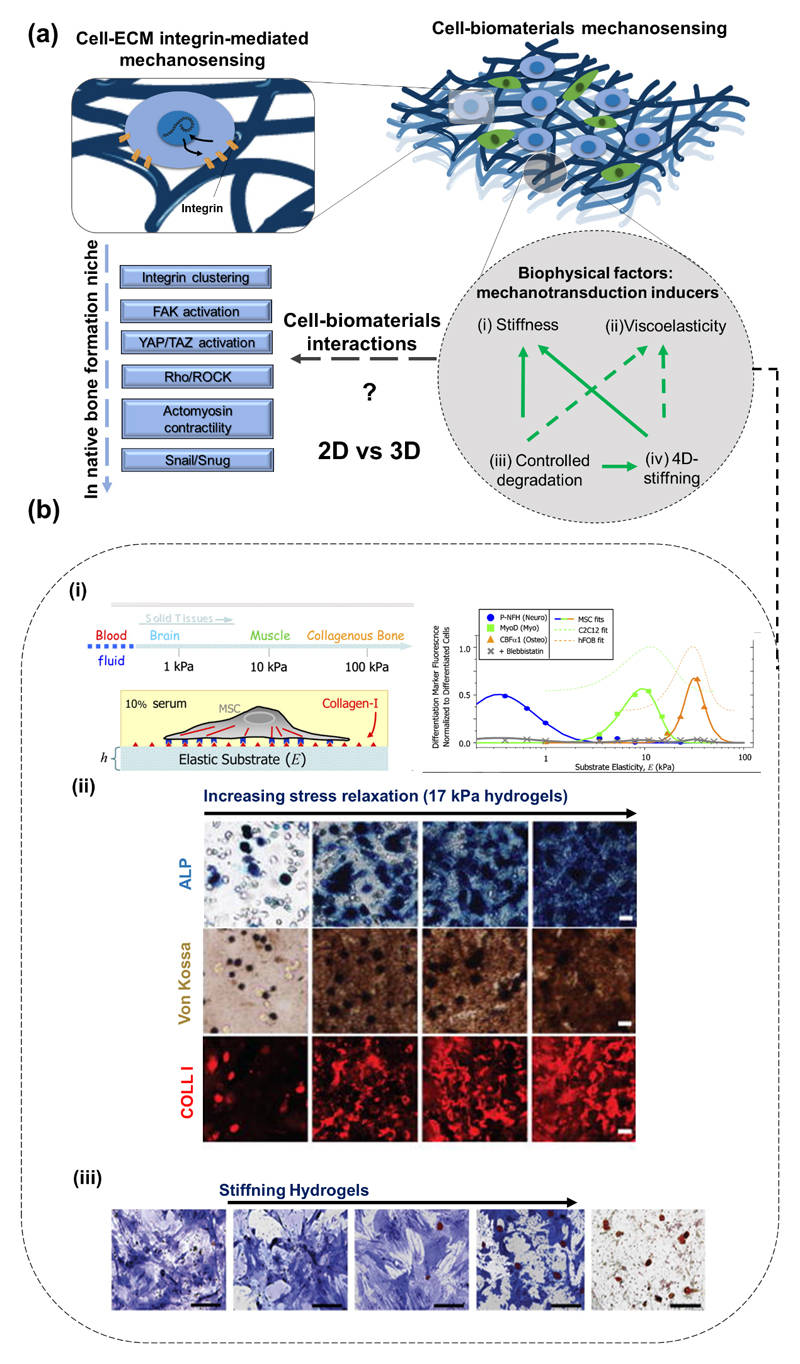
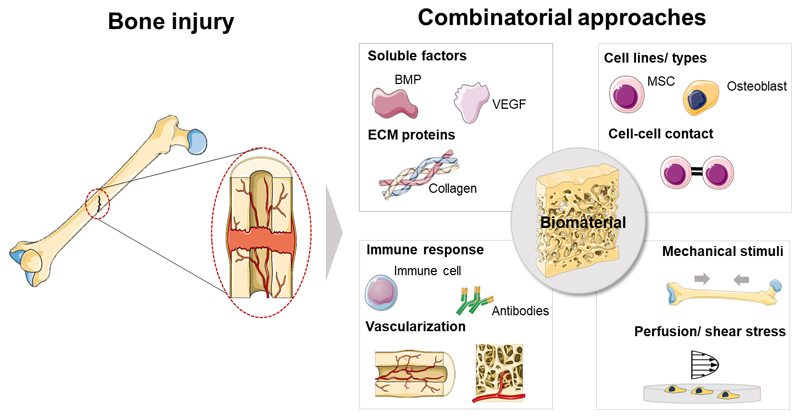
The study of individual factors in biologically non-representative environments in most available literature concerning bone regeneration may be hindering the disclosure of unprecedented valuable results. The effects of (bio)chemical (e.g. presence of soluble cytokines, as bone morphogenic proteins – BMPs), structural and chemical properties (e.g. different biomaterials chemistries and architectures), or externally applied mechanical stimuli (e.g. compressive stress or flow perfusion) on bone regeneration strategies have been explored in a unifactorial fashion, focusing on single stimuli for the design of biomaterials and other regenerative approaches. Interestingly, though, a growing body of knowledge comprising both fundamental and applied studies focusing on bone’s response to engineered cues have undoubtedly proven that bone is a complex and dynamic system, in which different biological processes and structural characteristics play complementary roles towards the successful regeneration and maintenance of bone’s healthy behavior [1]. The advent of stem cells biology, as well as the progressive know-how on the structural, biophysical and biochemical role of the ECM components and the scrutiny of immune cells crosstalk supported recent advances in the design of biomaterials targeting bone regeneration [4]. The knowledge of such complexity may be an effective manner to pave the way for the design of multifactorial strategies targeting bone regeneration and disease treatment [5]. Some high-throughput studies have approached multifactorial aspects of bone regeneration as crosstalk between different cell types (including mineral-forming cells, reabsorption cells, immune cells and vascular cells) [6, 7], the combinatorial role of ECM proteins [8, 9], the effect of physical factors as biomaterials bioactivity, stiffness and viscoelasticity [10, 11], as well as the role of extrinsic mechanical forces actuating in the native bone (e.g., compression and shear stress) [12]. Although some key aspects of bone physiology are well studied and have led the development of therapies, several aspects still remain poorly understood and explored as potential added-value assets to enhance regeneration. Some examples include the crosstalk of bone resident cells with immune cells, the role of hematopoietic stem cells (HSC) on regenerative phenomena, or the tight control of time- and space-coordinated biochemical and biophysical signaling [13].
This Review sets the objective of establishing an unprecedented correlation between (i) well-known and recently reported bone physiology phenomena and (ii) the use of this know-how for the development of well-established or proof-of-concept bone-healing therapeutic approaches. A thorough analysis is performed to identify aspects of bone biology lacking exploitation for regenerative therapies, which may represent a source of innovative ideas for novel and impactful future developments on the field.
1.1. The need for regenerative medicine therapies – analysis of critical aspects to achieve bone regeneration
While bone tissue trauma normally heals by itself, the so-called “critical” defects - with average diameters of 2 cm or higher in humans - do not show this spontaneous ability [14]. Such defects with poor healing ability often derive from tumor ablation, serious injury and orthopedic diseases [15]. Specifically considering physically-caused injuries, including a range of fractures from standard injury to open tibial defects, bone healing repair failure percentage can go from 10 to 50% [16]. Failure on bone healing will ultimately culminate in the suppression of blood supply to the tissue, which will result in the non-union of the bone due to ischemia, osteonecrosis and bone loss [17].
Efforts to repair bone defects excluding the ones that specifically target bone regeneration can be divided in two main segments: (i) implantation of bone grafts (of autologous or allogenic origin) or (ii) development of synthetic permanent bone substitution grafts [18]. Limitations have been reported for both therapeutic approaches. Although commonly applied in clinics and known to foster bone repair, autologous bone grafts, inflict morbidity of the donor’s extraction site [18, 19]. Allogeneic bone grafts may potentially be rejected due to host-to-graft immune response. Moreover, the implantation of allografts requires a complex implantation technique that involves the achievement of constant vascular supply to the site, as well as a maintenance of an adequate mechanical environment to promote vessels formation [20]. Permanent substitution grafts, manufactured from non-degradable materials, have been associated with unwanted side effects including bone resorption, poor osseointegration and triggering of adverse (e.g. allergenic) reactions on the host [18]. Current strategies based on synthetic grafts for bone healing are out of the scope of this Review. Thorough reviews of this important topic related to bone repair therapies can be found in References [21, 22].
Well-reported problems associated with the current bone repair approaches show that there is a dire need for the development and marketplace implementation of new and more efficient strategies. In opposition to previously mentioned techniques focused on tissue repair, tissue engineering seeks the complete regeneration of damaged tissues through the in vitro and/or in vivo-synthesis of novel biological matrices with equivalent properties to those of the original healthy tissue. Four main pillars may be used separately or in combined design strategies to promote bone regeneration: (i) biomaterials, (ii) biomolecules, (iii) cells and (iv) externally-applied stimuli. The following sections of this Review will exploit bioinspired rationales behind the use of different tissue engineering players through a parallelization strategy with native bone’s physiological phenomena.
2. An Inter-Scale View of Bone Structure
Bone is the anatomic structure responsible for the movement, protection, maintenance of mineral homeostasis and structural support of the human body. A fully-grown adult’s skeleton is composed of 206 individual bones. Human bones are divided in five major categories, which include long (aimed at supporting body weight; e.g. clavicles, raddi, metacarpals, tibiae, phalanges, femurs, humeri, metatarsals, fibulae and ulnae), short (providing movement and stability; e.g. tarsal and carpal), flat (targeted at internal organs protection; e.g. skull, sternum, mandible, ribs and scapulae), irregular (e.g. vertebrae, coccyx, sacrum and hyoid) and sesamoid bones (embedded at tendons; e.g. patella) [23].
During tissue formation, two bone types can be identified: (i) the woven/primary bone, which appears during the embryonic development and fracture repair, and consists of osteoid (unmineralized ECM) with collagen fibers that show little or no determined orientation in the three-dimensional (3D) space, along with a random distribution of cells [24, 25]. This is a transient structure, which is later replaced by mature lamellar bone; and (ii) the lamellar/secondary bone, which constitutes the adult skeleton and consists of highly organized sheets of mineralized osteon [26]. This structure is stronger and more rigid, and less elastic than the woven bone [24].
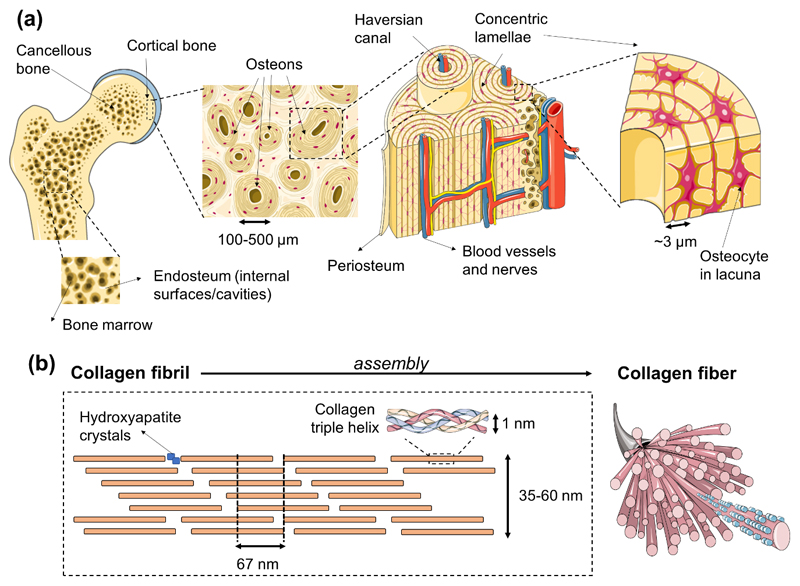
Lamellar bone is constituted by both cortical/compact and trabecular/cancellous bone. The first one is dense, solid and located in the most outer part of the tissue; on its turn, cancellous bone contains a sponge-like structure with interconnecting cavities and is located at the internal section of bone. Both cortical and cancellous bones are composed of osteons. The ratio of both bone types varies according to the anatomical site (e.g. femoral head with a 50:50 cortical to trabecular ratio; vertebra with 25:75; radial diaphysis with 95:5; and overall, the human skeleton with a 80:20 ratio) [1]. On a structural perspective, in cortical bone the lamellae with about 3 μm thickness, is organized into concentric circles, surrounding a vertical Haversian canal containing blood vessels and nerves. This entire structure is designated the osteon or Haversian system, and is the functional unit of bone. The system is formed when osteoclasts remodel existing bone, leaving cylindrical cavities that are subsequently filled with osteoblasts that produce lamellae around the Haversian canal [24, 27]. Osteocytes are located between lamellae, within small cavities known as lacuna, interconnected by a series of tunnels called canaliculi [1, 24]. The trabecular bone is composed of large spaces, with a honeycomb-like network of trabecular plates. The matrix consists of a 3D network of fine columns that crosslink to form the trabeculae [1, 24]. This results in a light and porous bone that is strong against multidirectional forces, and crucial to enable body movement. The spaces between trabeculae are filled with bone marrow. A fibrous connective tissue layer, called periosteum, surrounds the external surface of cortical bone, while in the inner section, a membranous structure - the endosteum - covers the surface of cortical and trabecular bone. The latter is also in contact with the bone marrow space, blood vessels canals and is composed by blood vessels, osteoblasts and osteoclasts [1]. Both periosteum and endosteum have inspired the development of two-dimensional (2D) membranes to mimic these anatomical structures and assist tissue regeneration processes [28].
On a nanoscale perspective, bone is constituted by a plethora of structural proteins and polysaccharides, which main composition is collagen fibrils with diameters between 35 and 60 nm, and up to 1 μm length, organized with a 67 nm periodicity and 40 nm gaps [29–31]. These fibrils are mineralized by the anisotropic and extremely stiff inorganic component - hydroxyapatite crystals [32] - that lay in the collagen gaps [31]. Interestingly, despite the variations on bone structural shapes depending on bone types and throughout different species, the mineralized collagen fibrils observed in humans are highly conserved across species and bone types [33]. Bone organic and inorganic phases are thought to have an interplay that allows load transfer. Gupta et al. [34] showed that both components deform elastically during initial loading, although to different degrees. This specific deformation pattern is hypothesized to culminate in fibril-matrix decoupling targeting the protection of the brittle mineral phase, improving the effective redistribution of strain energy in the tissue. Nanostructured scaffolds based on nanomaterials capable of closely resembling the native ECM structure have been designed. According with some authors, such constructs demonstrate a beneficial effect concerning the formation of functional tissue due to an improvement of the interactions at the cellular and protein level [29, 30]. Although the macro- and microstructure of bone closely replicated using porous scaffolds, the emulation of the osteon organization is dependent on a nanometric control of materials distribution. In fact, a precise combination of micro- and nanometric aspects of the bone structure is fundamental for the development of truly mimetic structured biomaterials. Nonetheless, the effective need for highly organized osteon/adult bone interscale mimetic biomaterials is not unanimous. Effective biomaterials capable of triggering osteogenic differentiation and bone repair have been often based on biophysical and biochemical cues inspired on bone developmental processes, instead of adult bone structural features. These strategies are composed of much simpler units than the adult completely formed bone itself. The role of nanostructured biomaterials as cue providers targeting bone regeneration must not be overlooked. Platforms exhibiting, for example, topographic, pro-differentiation and pro-mineralization cues through techniques as nanopatterning, electrospinning, and development of nanocomposites [30] has led to some of the most interesting outputs in the field. Complete reviews correlating (i) nanomaterials and their similarity with the native bone niche and (ii) their bone regeneration outputs can be found in References [30, 35–38].
3. Bone Development Mechanisms
During mammals’ fetal development and natural bone repair upon injury, bone formation is achieved through two processes: intramembranous and endochondral ossification [1]. The primary structure for these two mechanisms is the woven (or immature hollow) bone, that is readily replaced by the lamellar/secondary bone (parallel fibrils deposited in opposite directions) [25]. The formation of lamellar bone occurs at a much slower rate than that of woven bone [39]. This structure does not appear only in the fetal life, but every time a bone suffers a non-critical injury [25].
3.1. Intramembranous bone formation
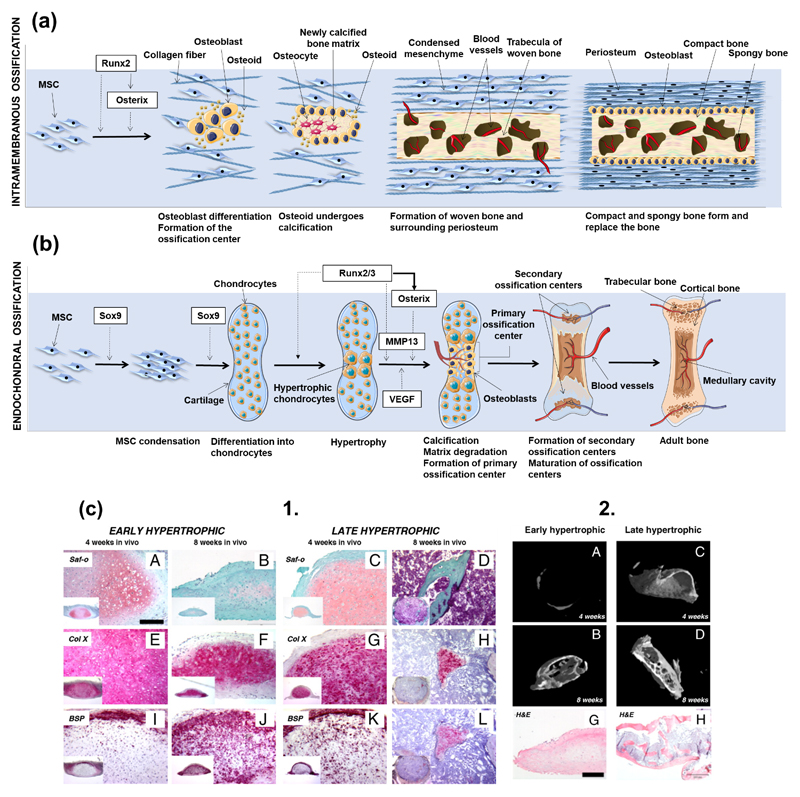
Intramembranous ossification is the most primitive form of ossification, with the first proof of its existence dating to 500 million years BP, in opposition to endochondral ossification, with first reported case with 100 million years BP [40]. In intramembranous ossification, mesenchymal stem cells (MSCs) present in mesenchyme or in the medullary cavity - caused by a bone injury - differentiate into osteoblasts. In fetal development, this process is mainly responsible for the formation of the flat skull bones and some parts of the clavicles [25, 41]. Unlike in the endochondral ossification process, in intramembranous ossification the bone is formed without a cartilaginous intermediate. The formation of a nidus – a cluster of undifferentiated MSCs – is the starting point for the intramembranous ossification process. Cells in these clusters stop their proliferation and develop into the osteoprogenitor phenotype, and then eventually differentiate into osteoblasts, through an intermediate pre-osteoblast lineage [41, 42]. Runx2 is one of the most important early transcription factors responsible for osteoblastic differentiation [43, 44]. The expression of Runx2 is dependent on the Wnt signaling, which leads to high levels of β-catenin in MSCs. In turn, Runx2 induces the later expression of the transcription factor gene Osterix, also involved in the differentiation of MSCs to osteoblasts [45]. After full differentiation, osteoblasts produce a non-mineralized type I collagen-rich fibrillar ECM: the osteoid. While entrapped in this matrix, osteoblasts differentiate into mature osteocytes, and the matrix is further mineralized. The described mechanism is also considered by most authors as the backbone for the formation of subperiosteal bone and, thus, the process behind the woven and lamellar bone type formation in this region [46]. However, this concept has been challenged, and the mechanism behind periosteum surface ossification was suggested as developmentally distinct [45]. A schematic representation of the intramembranous ossification process can be found in Figure 1a.
Figure 1.
Interscale representation of bone. (a) A macroscopic-to-microscopic view of cancellous and cortical bone. Bone marrow lies in the cavities of cancellous bone, which are lined by the endosteum structure. Tightly packed osteons integrate cortical tissue, which is covered by the periosteum membrane. Osteons are formed by Harvasian canals, which contain blood vessels and nerve tissue, surrounded by concentric lamellae that show thicknesses of circa 3 μm. Osteocytes reside in the osteon inside lacuna structures. (b) Bone tissue is constituted at the nanometric scale by collagen fibers that comprise assembled collagen triple helix structures that give rise to the collagen fibril, with a characteristic periodic spacing of 67 nm, and gaps of 40 nm where the mineral component of bone is located.
3.2. Endochondral ossification
During endochondral ossification chondrocytes from surrounding cartilage tissues initially form a matrix template, the growth plate, and then differentiate into bone structures [43]. This ossification process drives the embryonic formation of long bones. When chondrocytes’ morphology is round, these cells synthesize type II collagen [47] and subsequently form a columnar layer, becoming pre-hypertrophic. They eventually differentiate into post-mitotic hypertrophic cells, which release type X collagen, mineralizing the surrounding matrix, leading the formation of the bone structure [47]. During bone formation various cycles of death of hypertrophic chondrocytes occur, which is accompanied by the invasion of blood vessels, leading to the replacement of the initially collagenous matrix by trabecular bone, also known as primary spongiosa [48]. As the process continues, trabecular bone is resorbed, and its center is split into different plates. While chondrocytes are present in the plates the previous process continues [48]. The adequate differentiation of chondrocytes into the hypertrophic phenotype is of extreme importance for the genesis and proliferation of bone tissue [48]. A schematic representation of the endochondral ossification process can be found in Figure 1b.
For endochondral ossification-driven bone formation, some biochemical factors must be present in specific moments of chondrocyte-to-osteoblast differentiation. Those include:
The Sox Trio: Sox9/5/6. These molecules are responsible for the differentiation of MSCs into the chondrogenic phenotype, as well as for the regulation of the expression of critical genes for the formation of cartilaginous matrix [48–50].
Expression of the fibroblast growth factor (FGF) receptor 3 and a membrane-spanning tyrosine kinase receptor by chondrocytes. These cells contain a domain that binds to extracellular ligands, including FGFs, initiating the receptor’s autophosphorylation, as well as the stimulation of the tyrosine kinase activity, leading to the inhibition of proliferation and growth of chondrocytes [51, 52];
Presence of BMPs, which are responsible for the formation of mesenchymal condensations and of joints in initial stages of endochondral ossification. After condensation, when long bones are already formed, BMP-2, -3, -4, -5 and -7 are released in the perichondrium. BMP-2 and -6 are produced by hypertrophic chondrocytes, and BMP-7 by proliferative chondrocytes. BMPs positively regulate chondrocyte proliferation and negatively modulate chondrocyte terminal differentiation [51, 52];
High level expression of parathyroid hormone-related peptide (PTHrP) by hypertrophic chondrocytes. This peptide binds and activates the receptor parathyroid hormone (PTH)/PTHrP, also activated by PTH (main regulator of calcium/phosphate metabolism and remodulation of the bone). In fact, the PTH/PTHrP complex is the main regulator of bone development and mineral ion homeostasis. The PTH peptide acts by maturing the immature chondrocytes to a hypertrophic phenotype. When the chondrocytes express PTHrP or an activated form of the receptor, a decrease on the cartilage maturation and increase in bone formation is observed [53, 54]. For a successful bone formation, hypertrophic chondrocytes must express high levels of alkaline phosphatase (ALP), osteonectin, osteopontin, bone sialoprotein and osteocalcin [55–60].
Indian hedgehog homolog (Ihh). This protein, present in the embryogenic patterning, controls the endochondral bone formation by inhibiting the differentiation of hypertrophic chondrocytes, therefore delaying the mineralization of the matrix. The control of growth plate elongation is not a chondrocyte property, but a property of the growth plate module arising from the interaction with chondrocytes involved in the negative feedback loop of Ihh/PTHrP. Ihh also acts as a chondrocyte proliferation stimulator, through a PTHrP-independent pathway [52, 61].
Runx2, Runx3 and core-binding factor beta subunit (CBFβ). These three transcription factors have been described in the literature as promoters of chondrocytic hypertrophy, complementing each other in the process [62, 63];
The proteins hypoxia-inducible factor-1 (HIF-1α) and vascular endothelial growth factor (VEGF). These two factors are essential for bone vascularization, in which HIF-1α acts by mediating hypoxic responses, allowing the survival of chondrocytes, and targets VEGF. VEGF is responsible for the stimulation of angiogenesis and vasculogenesis, as well as restoration of the oxygen supply in hypoxic conditions. It is hypothesized that these two proteins act together in a pathway that regulates chondrocytes survival [64, 65].
3.3. Development-mimetic strategies to engineer bone tissue
A close look at the embryonic development pathways of bone has served as inspiration for the design of finely tuned regenerative approaches, in strategies addressed as “developmental engineering” [66]. Although endochondral ossification is the pathway that gives rise to most of human bones [24], approaches to differentiate stem cells into functional bone cells (namely, osteoblasts) are mostly based on external stimuli provided to undifferentiated cells that include mineralized/mineralizable platforms, which resembles the intramembranous ossification process [67]. This is a much simpler process when compared to endochondral ossification, thus easier to be carried out in vitro and easier to trace overtime; however, it often results in poor vascularization and limited-area bone regeneration. Consequently, endochondral ossification has been hypothesized as advantageous over intramembranous process for tissue engineering due to its inherent ability to form vascularized bone due to the release of VEGF and MMPs by hypertrophic chondrocytes, which allow overcoming associated hypoxia in the tissue [68]. Despite the successful generation of bone tissue reported for endochondral ossification-mimetic strategies, the implantation of tailored mineralized biomaterial matrices has also enabled high quality bone regeneration, in which the final tissue recapitulates key characteristics of the native precursor, including vascular networks. Examples of tissue engineering strategies focused on both intramembranous and endochondral developmental pathways will be reviewed in the following Sections 3.3.1 and 3.3.2.
3.3.1. Regenerative strategies based on intramembranous ossification: the role of mineralized biomaterial matrices
Mineralized biomaterials have been reported as effective promoters of intramembranous ossification-analogous pathways [69–71]. Although in initial approaches their utility was mostly reported exclusively for the treatment of small scale injuries due to their inability to autonomously induce MSCs differentiation, seminal work by Yuan et al.,in 2010, introduced physicochemical and structural tailoring of calcium phosphate-based ceramics as a way to achieve osteoinductive biomaterials capable of promoting the full regeneration of large-scale defects in sheep and dog ectopic and orthotopic models [72]. Calcium phosphates with different chemical compositions – hydroxyapatite, tricalcium phosphate (TCP), and mixtures of both (biphasic calcium phosphates - BCP) were exposed to different post-synthesis sintering temperatures, so different microstructural features could be obtained (smaller grains for 1150°C, and larger ones for 1300°C). Those materials were tested for their ability to induce in vitro MSCs osteogenic differentiation, as well as in vivo bone formation. TCP showed the highest osteoinductive effect on in vitro cultured MSCs and the strongest ability to induce bone formation, with outputs similar to the implantation of autografts or treatment with recombinant human BMP-2. Moreover, the implantation of TCP avoided the formation of fibrous tissue when compared to the autograft strategy, and promoted a more defect-localized bone formation when compared to BMP-2 administration. An overall analysis of the study by Yuan et al. suggested that elevated specific area achieved through a reduction on grain size accompanied by resorbability features may be the key to process efficient bioceramics targeting bone regeneration [72].
Although bioceramics were proven to promote the regeneration of mineralized tissue on bone defects, the analysis of the de novo formed tissue is often restricted to bone-specific genes and proteins. However, the formation of a vascular network in bone is of utmost importance to achieve highly functional regenerated tissues. Recently, Díaz et al. (2018) evaluated a series of mineralized biomaterials and their ability to induce bone healing in a major cranial defect in the complete absence of growth factors and endogenous cells [73]. Moreover, the invasion of the implanted biomaterials by endothelial cells and the formation of blood vessels was also assessed. Macroporous poly(ethylene glycol)-diacrylate-co-N-acryloyl 6-aminocaproic acid (PEGDA-co-A6ACA), poly(ethylene glycol)-diacrylate (PEGDA) and acryloyl 6 aminocaproic acid (A6ACA) hydrogels were mineralized in vitro through immersion in a Ca2+/PO43- solution and in simulated body fluid (m-SBF). The in vivo performance of the hydrogels was tested before and after the mineralization step. Although endogenous cell proliferation and infiltration and blood vessels formation could be observed in both mineralized and non-mineralized porous biomaterials, the presence of bone forming cells, osteoclast precursors and hard tissue formation was only observed in mineralized biomaterials, suggesting the indispensable role of mineral environments for the promotion of osteogenic differentiation using cell-free and growth factor-free biomaterials [73].
Despite the significant advances concerning the application of calcium phosphates as osteoinducers, their interaction with stem cells and the bone defect moiety is still not completely unravelled [69]. The hypothesis that microarchitectural features act as key drivers for osteogenesis led by calcium phosphates gained momentum during the last decade [74, 75]. Moreover, free ions – specifically calcium - possibly released from these materials to the surrounding environment also showed the ability to induce osteogenesis on MSCs through the stimulation of BMP-2 expression [76]. The full elucidation of the pathways driving bone cells invasion of synthetic mineralized biomaterials, mechanisms leading MSCs osteogenic differentiation and the stimulation of neoangiogenesis in bone defects treated with these materials is in great need to promote the design of rationally tailored mineralized/mineralizable bone regenerative matrices.
3.3.2. Regenerative strategies based on endochondral ossification
In 1998, Bianco et al. [77] discussed bone formation through the endochondral ossification pathway, by modulating the terminal differentiation of what the authors called the “borderline chondrocyte”. The authors asked whether there was a future for hypertrophic chondrocytes as primary modules for bone regeneration, as these cells induce the differentiation of neighbor MSCs in vivo [77]. It has been later hypothesized that the regeneration of bones natively formed by endochondral ossification would benefit from undergoing the same pathway for their regeneration. With the rise of stem cells as important players on regenerative medicine strategies, the discussion about the selection of the most beneficial way to differentiate cells into functional osteoblasts, and even to fully functional tissues, has gained momentum. Ten years after Bianco and co-workers inquired about the pertinence of using hypertrophic prone-to-mineralization chondrocytes as precursors for bone formation, Jukes et al. [78] introduced the endochondral ossification into the stem cells world for bone tissue engineering by inducing the formation of ectopic bone on animal models using murine embryonic stem cells (ESCs) previously stimulated through the endochondral pathway in vitro. Despite this breakthrough, the validation of the endochondral route as an effective way to promote bone formation was still restricted to ESCs, which are prone to ethical concerns and considered of poor clinical relevance for that reason [78–80]. Only in 2010, Scotti et al. [81] reported the use of clinically relevant and widely available bone marrow-derived MSCs (BMMSCs) to undergo osteogenic differentiation through the remodeling of MSC-originated hypertrophic cartilage templates and generate ectopic bone tissue when implanted in nude mice (Figure 1c). Hypertrophic cartilage in more advanced maturation stages accelerated bone formation, although the implantation of precursor hypertrophic tissues in all stages of maturation rendered bone formation in vivo. Interestingly, gene, protein and structural analysis of the developed tissues showed that morphogenesis occurred with a high level of parallelism with the well-known developmental processes observed for endochondral bone formation in embryos, which included the early activation of Ihh signaling and the in vivo subsequent development of a bony collar, its vascularization and osteoclastic remodeling of cartilaginous precursors.
The main advantage associated with the recapitulation of endochondral ossification is the possibility of engineering a fully functional bone organ, containing a mature vascularized mineralized matrix, as well as a hematopoietic bone marrow component. Indeed, the implantation of different progenitor and stem cell types has led the in vivo recreation of functional hematopoietic niches. Ectopically implanted CD146+ human skeletal progenitor cells were able to induce the formation of a hematopoietic compartment in mice [75], and the formation of a mature HSC niche after embryonic MSCs implantation was reported to be dependent on the endochondral ossification process [83]. The suppression of directly involved factors on the endochondral ossification process, including VEGF and Osterix, inhibited the generation of such hematopoietic niche [83]. The application of tissue engineered constructs as templates for endochondral ossification capable of promoting not only mineralized tissue formation, but also the development of bone-like ossicles featuring vascularization and functional HSCs niche compartments was hypothesized by Scotti et al. [84] in 2013. Human BMMSCs seeded onto type I collagen porous scaffolds, cultured in vitro for 3 weeks in serum-free chondrogenic medium, and for additional 2 weeks in pro-hypertrophic medium, which contained IL-β1 aimed at the acceleration of cartilage mass remodeling [85]. Indeed, pre-treatment with this cytokine results in higher accumulation of matrix metalloproteinase 13 (MMP-13) and DIPEN (an aggrecan epitope exposed after its degradation) after 5 weeks of implantation. After pre-conditioning, the constructs were implanted into nude mice and, extensive remodeling was indeed observed after 12 weeks. At that stage, the formed tissues were similar to native bone’s structure, with an outer layer resembling cortical bone and inner parts with cancellous bone features. Regions identified as hypertrophic cartilage in the first weeks of culture developed into bone marrow and densely mineralized bone tissue. Impressively, mouse sequential bleeding after 1, 2 and 3.5 months after transplantation confirmed the functionality of the ossicle-derived HSCs, capable of multilineage reconstitution.
The achievement of ossicle structures using other sources of adult stem cells, including human adipose-derived stem cells (ASCs), in endochondral ossification-mimetic strategies has been challenging. However, the application of stem cells from abundant and easily retrievable sources is potentially highly valuable. This hypothesis is reinforced by the failure of primary chondrocyte lineages - including fully mature nasal chondrocytes induced in vitro for an hyperthrophic phenotype – on being capable of leading in vivo endochondral ossification; in opposition, implanted tissues prepared from hyperthrophic nasal chondrocytes reverted their phenotype into a hyaline status [86]. In 2016, ASCs assembled as 3D cellular micrometric pellets or adhered onto collagen scaffolds were cultured in chondrogenic cell culture media supplemented with early and, optionally, with late hyperthropic supplements administered on later times of in vitro culture [87]. Those constructs were implanted in female nude mice, and both early and late endochondral ossification templates underwent cartilaginous remodeling and developed functional bone marrow-specific features incorporated in the newly formed ossicles. Reprogrammed cells may also represent a breakthrough in the future obtaining of scalable cell sources capable of undergoing endochondral ossification. Specifically, dermal fibroblasts directly reprogrammed through into the chondrogenic lineage doxycycline-inducible human Sox9 were capable of promoting endochondral ossification in vivo [88, 89].
Despite the clear promise represented by stem or precursor cells as in vitro-modulated templates for in vivo bone formation mostly in immunologically suppressed animal models, the achievement of effective protocols to directly drive endochondral differentiation in immunocompetent models, avoiding the existing in vitro long-term pre-conditioning protocols, would benefit the translational steps needed for the implementation of these techniques into widely available regenerative therapies. Co-culture strategies targeting the understanding of different cell types on hypertrophic cartilage formation and endochondral ossification processes have been a recent matter of interest. Todorov et al. [90] addressed the effect of the presence of monocytes committed to osteoclastogenesis as possible enhancers of tissues’ remodeling through chemotaxis of skeletal and vascular cells. However, the presence of such monocytes did not lead to any improvement of cellular chemotaxis in vivo. Future studies may elucidate the role of different cells types on the successful induction of endochondral ossification as a bone regeneration targeting system.
4. Adult bone physiology
4.1. General aspects
Bone undergoes longitudinal and radial growth, modeling and remodeling during the whole life of adult (i.e. non-embryonic) individuals [1]. Longitudinal and radial growth occur during childhood and adolescence period. On its turn, bone modeling, an anabolic process involving new bone deposition in response to physiological or mechanical factors is less frequent in adults than remodeling. Contrarily to bone remodeling, where osteoclasts and osteoblasts work sequentially in the same bone remodeling unit, in bone modeling bones are shaped or reshaped by the independent action of osteoblasts and osteoclasts, i. e. the activity of both cells may not be coupled anatomically or temporally [91]. This is achieved by the action of bone osteoprogenitor-derived cells: osteoblasts and osteoclasts [92]. Bone morphogenesis regulated by exposure to mechanical challenges is reviewed in more detail in Section 6. Also, the role of different bone resident or migrating cell types, as well as their crosstalk during bone healthy state maintenance and during injury/repair processes are reviewed in Section 5 and further ahead on this section.
Remodeling occurs continuously in human adults to form and maintain the complex and functional skeleton structure [1]. This process helps counter the effects of increasing bone fragility throughout life, allowing for the maintenance of the structural stability of the human body [23, 93]. Bone remodeling is increased during adults’ middle age and happens in four stages: activation (activation and recruitment of osteoprogenitor cells), resorption (resorption of the osteoprogenitor cells by osteoclasts), reversal (transitional phase from bone resorption to bone formation) and formation (matrix synthesis by osteoblasts) [1, 94]. A detailed explanation of the previously mentioned phases is provided by Clarke [1]. In brief, during bone resorption osteoclasts act by removing the “old bone” packets; afterwards, new synthesized matrix is created, along with mineralized tissue. Bone formation and degradation are tightly kept in equilibrium throughout humans’ life by the bone homeostasis and remodeling [1]. Osteoclasts (the promotors of bone resorption) and osteoblasts (involved in bone formation) are the main mediators of this process [1]. The bone unit responsible to maintain this equilibrium is the basic multicellular unit (BMU), composed by osteoclasts, osteoblasts, connective tissue, nerves and blood vessels [6].
4.2. Bone Healing: tissue response upon injury
The maintenance of a fully functional bone system is indispensable for body structural maintenance and organ protection. For that, it is important to have a system that guaranties its integrity [23] through the activation of mechanisms of healing upon fracture avoiding the formation of scar tissues [93]. Fractures are the most common large-organ traumatic injuries in humans. As discussed previously, the repair of bone fractures is a postnatal regenerative process that recapitulates many of the ontological events of embryonic skeletal development [95]. Although fracture repair processes usually restore the damaged skeletal organ to its pre-injury cellular composition, structure and biomechanical function, critical fractures will not heal normally [95]. A complete schematic representation of the process occurring after trauma – bone healing – can be found in Reference [95]. This phenomenon endows several stadia that occur in a sequential manner: (i) inflammation, (ii) soft and hard callus formation and (iii) bone remodeling, which are reviewed in the following topics:
The (i) inflammatory phase, which is characterized by the proliferation and migration of mesenchymal progenitor and blood cells to the healing fracture site [96]. Blood cells that reside in the defect area form a hematoma [97]. Several pro-inflammatory cytokines and growth factors (including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-6, IL-11 and IL-18) are targeted to the defect site in a temporally and spatially controlled manner [95, 98]. These signals recruit inflammatory cells and promote angiogenesis. At this stage, platelets are recruited and activated in the defect site and produce transforming growth factor-β1 (TGF-β1) and platelet derived growth factor (PDGF) [98]. Simultaneously, recruited osteoprogenitor cells produce BMPs which, in coordination with other factors, promote the local recruitment and osteogenic differentiation of MSCs [96, 98].
The (ii) soft callus formation. After the formation of the blood hematoma, blood cells, fibroblasts and immune cells are recruited to the injury site, forming the granulation tissue [99]. Bone is formed in the peripheric regions of the fractures sites via intramembranous ossification after 7 to 10 days after injury, generating the periosteum [96, 99] The inner parts of the fracture (mechanically less stable [100] and contain the granulation tissue) are subsequently replaced by fibrous tissue (mainly composed of fibroblast cells), fibrocartilage and later cartilage of the soft callus. This structure provides a cartilaginous scaffold within the bone fracture site, that acts as both a fixation and stabilization structure and a template for subsequent mineralization. Within soft callus construct, the differentiation of mesenchymal cells into chondrocytes takes place [99]. These cells then proliferate until complete differentiation into a mature hypertrophic phenotype [99]. At this stage, TGF-β2 and -β3, as well as BMPs, mediate cell differentiation and proliferation at the injury site [99–101]. By the process of endochondral ossification, the soft callus is transformed in the hard callus, with a mineralized matrix produced by osteoblasts. At this stage, the formation of the woven bone is initiated [99, 100].
The formed primary bone is gradually replaced by secondary (lamellar) bone, in the (iii) bone remodeling process and osteocytes undergo apoptosis in a reestablishment of the normal bone physiology [99, 100], reaching a physiological status indistinguishable from the pre-fractured condition.
Understanding the fundamental components that make up the ECM and cell components of native bone tissue is vital for the creation of engineered regenerative strategies able to recapitulate the stages of intramembranous and endochondral ossification. Also, unraveling regulatory factors that drive soft callus formation, a key intermediate stage in endochondral ossification, is important when considering strategies to mimic its ECM or in priming its progenitor cells.
A plethora of immune system cells take part of the bone healing process, and their role has been thoroughly reviewed [102, 103]. Macrophages are highly influential of this process, and several studies point to their presence in the healing cascade [98, 99, 104–106]. Their absence in the healing place have been associated to a complete depletion on the regeneration injured tissues [107, 108]. In bone healing, an optimal balance between macrophages with proinflammatory phenotypes - usually addressed as M1 - and pro-healing phenotypes - usually addressed as M2 – is required for an adequate regeneration process. M1 macrophages initiate the inflammatory response [108] and secrete pro-inflammatory cytokines [105], and M2 macrophages are responsible for tissue remodeling, with a phenotype induced by IL-4 and -13, and secreting IL-10 [109]. These two macrophage types work together to start and finish the immune response in an interlocked chain of events. M1 macrophages, not only initiate the inflammatory response but also secrete factors that stimulate the beginning of the angiogenic process [109, 110]. These are gradually substituted by pro-healing M2 macrophages, which promote ECM synthesis, cell proliferation and vessel maturation on the healing site [109, 110]. An unbalance corresponding to a long M1 macrophages permanence at the defect site may lead to an excessive inflammation, that may compromise fracture healing [101]. Spiller and coworkers developed a bone regeneration system based on the controlled release of interferon-gamma (IFN-γ) and IL-4 through a decellularized bone scaffold to reproduce the in vivo transition of M1 to M2 macrophages that ultimately could improve the vascularization of the construct [112]. The rapid release of IFN-γ caused early M1 polarization of macrophages, while the sustained release of IL-4 caused M2 polarization, in vitro [112]. This temporal modulation of macrophage phenotype could be advantageous to improve the vascularization of the scaffolds in vivo. In fact, 3D printed silicate-β-tricalcium phosphate scaffolds loaded with IFN-γ were able to drive the sequential activation of M1 and M2 macrophage polarization states in a temporally-controlled manner [113]. Through the combined action of released silicate and IFN-γ, timely induction of M1 phenotypes in early time points (one day after implantation) and pro-healing polarization (seven days after implantation) triggered enhanced vascularization of the implanted scaffolds [113].
Other cell types are also present in the fracture location, which include monocytes, neutrophils and natural killer (NK) cells [114]. These cells produce cytokines that are responsible for the recruitment and activation of other cells with differentiation and proliferation potential to regenerate the tissue (e.g. osteoprogenitor MSCs) [114]. When osteoprogenitor cells are recruited to the fracture place, their osteogenic differentiation is partially induced by immune cells present at the injury site [111]. T-lymphocytes are also part of the regenerative process: they act by inhibiting the healing process through the action of cytokines IFN-γ and TNF-α [115–118]. Conversely, MSCs have been reported to affect the immune response in a plethora of ways, through suppression or inhibition mechanisms. This response is coordinated by the cellular microenvironment and the MSCs-to-T-lymphocytes ratio, with a high ratio inhibiting the immune response, and a low ratio inducing it [114, 119, 120]. The full elucidation of MSCs/T-cells crosstalk is still dependent on further studies. Interactions between immune cells and bone cells are reviewed in Section 5.3.5, and their applications in biomaterial-based tissue regeneration strategies is summarized in Section 5.4.1 and in Table 1.
Table 1. Cell-cell interactions applied in tissue engineering strategies.
| Type of cell interaction | Tissue engineering approach | Main achievements | References |
|---|---|---|---|
| MSCs-(pre)osteoblasts |
|
|
|
|
|
|
|
| MSCs-vascular cells |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Osteoblasts-osteocytes |
|
|
|
| Osteoblasts-osteoclasts |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Osteocytes-osteoclasts |
|
|
|
|
|
|
|
| Vascular-bone cells |
|
|
|
|
|
|
|
|
|
|
|
| Immune-MSCs/bone cells |
|
|
|
|
|
|
|
| Multi-cultures |
|
|
|
5. The adult bone niche
5.1. Bone primary stem cell niche
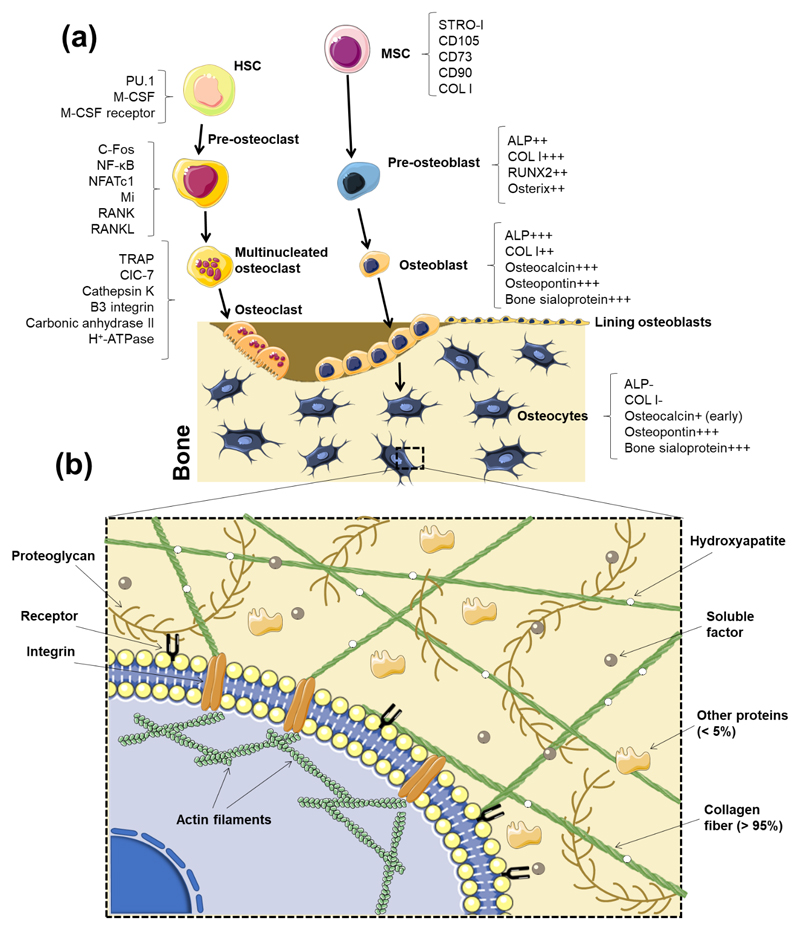
The bone tissue comprises two primary niches: the osteoblastic and the vascular niche [13]. Two stem cells types - HSCs and MSCs – reside in the bone cavity, which is filled with bone marrow and blood vessels [13]. HSCs, which are surrounded by stromal cells in the bone marrow, are responsible for the formation of the immune and blood system, as well as osteoclasts [13, 121]. MSCs also reside in the bone marrow and intervene in the formation of the mesenchymal lineage cells, which include osteoblasts, adipocytes, chondrocytes, fibroblast and other stromal cells. Together, both stem cell types maintain the normal bone homeostasis and cellular generation [13]. Unlike what was thought until recent years, HSCs are not located on the inner surface of the bone. Instead, HSCs were recently described to be on the perivascular niche where they are regulated by growth factors, chemokines and cytokines (e.g.: stem cell factor, chemokine stromal cell-derived factor 1 (CXCL-12) and angiopoientin-I), secreted through CXCL-12-abundant reticular cells, endothelial cells and MSCs [122].
MSCs, characterized by the expression of PDGFRα, CD51, nestin, CD139, interferon-induced GTP-binding protein MX1 (Mx1), leptin receptor (Lepr) and periaxin (Prx), give rise to osteoprogenitor cells that form the osteoblastic niche. Thereafter, the factors referred previously are released to promote a correct HSC maintenance (reviewed by Yin and Li [13]). The maintenance of a functional microenvironment in the bone niche is dependent on the precise level of the hierarchical lineages of the HSCs and MSCs, so osteoblastogenesis and hematopoiesis can maintain a correct balance of osteoblast and osteoclast production. Importantly, N-cadherin positive osteoblasts interact with HSCs and help the anchoring of these cells to the osteoblastic niche [123].
Cell signaling inside the bone niche, for instance between osteoblasts and B lymphocyte precursors, is well known to determine features of the immune system [114]. Immunogenesis will not be discussed in this Review, and bone niche interactions will be described in the scope of the correct function of bone tissue itself. Nonetheless, the importance of a proper function of bone niches for successful bone development must be emphasized due to its crucial contribution for the continuous exportation of immune cells and tissue progenitor cells to the peripheral immune system, thus sustaining tissue repair and regeneration (Figure 2) [124].
Figure 2.
(a) Schematic representation of intramembranous ossification. At an initial stage, MSCs cluster and differentiate into osteoblasts, forming the ossification center. Runx2 is deeply involved in the regulation of osteogenic differentiation, either directly or by inducing the late expression of Osterix. Osteoblasts start to produce the osteoid, which calcifies in few days. Osteoblasts trapped into the calcified matrix differentiate into osteocytes. Vascularized mesenchyme condenses on the external area of the woven bone, generating the periosteum. The woven bone is produced, with vascularized internal spaces that will form the marrow cavity. The surface of trabeculae is filled with matrix forming the compact bone. Spongy bone persists at the inner part. (b) Schematic representation of endochondral ossification. After condensation, MSCs starts to differentiate into chondrocytes, generating a cartilage template. Chondrocytes in the middle of the cartilage become hypertrophic. Sox9 and Runx2/3 are indispensable transcription factors for the initiation of chondrogenesis and the hypertrophy of chondrocytes, respectively. Hypertrophic chondrocytes induce vascular invasion. At this stage, Osterix functions as both a downstream and transcriptional partner of Runx2/3 during calcification and matrix degradation in cartilage, and cooperate with Runx2/3 to induce MMP13 expression. Osteoblasts differentiate from cells brought into the cartilage template with blood vessels invasion, starting to produce bone at a primary ossification center. Bone formation then spreads along the shaft forming secondary ossification centers. Finally, the adult bone, containing both trabecular and cortical bones and the medullary cavity is formed. (c) 1. Scotti et al. [74] induced endochondral bone formation in vitro using human MSCs. Hypertrophic tissue structures were implanted into nude mice to assess their ability to form trabecula bone. Both early (A-J) and later (C-L) hypertrophic samples went towards differentiation after in vivo implantation, although the latter specimen presented a more intense remodeling after 4 weeks (K), with the cartilaginous template almost resorbed after 8 weeks (L). (c) 2. Quantitative microtomography (μCT) of explants demonstrated that the deposition of mineralized matrix at the early hypertrophic samples (A-B) was reduced when compared to the late hypertrophic implanted structures (C-D). In fact, late hypertrophic samples displayed an interconnected network of trabeculae throughout the core after 8 weeks of implantation (D) [81]. Histological analysis by hematoxylin/eosin staining (G-H) revealed the presence of trabecular-like structures in the outer collar and inner core of the late, but not of the early, hypertrophic samples [81]. Figure 2(b) was adapted from Reference [81].
5.2. Bone resident cells
5.2.1. Osteoblasts
The main function of osteoblasts is to synthetize new bone matrix [1]. Different sub-populations of osteoblasts have shown to respond differently to several signals (mechanical, hormonal and from cytokines) [1]. As discussed on Section 3.1, under physiological conditions, MSCs undergo the Wnt/β-catenin pathway to differentiate into the osteoblastic phenotype. Osteoblasts have a cuboidal morphology while proliferating on the bone matrix surfaces, unlike their precursors cells (preosteoblasts), which show a spindle shape [1]. Mature osteoblasts secrete bone ECM proteins, such as type I collagen. Typical gene indicators of osteoblast differentiation are Runx2, distal-less homeobox 5 (Dlx5), Osterix, alias core-binding factor alpha1 (Cbfa1), osteoblast specific factor 2 (Osf2) and ColIA1 [125, 126].
Osteoblasts residing in bone tissue can be divided in two types: mesenchymal (MOBL) or surface osteoblasts (SOBL) [25]. In the bone matrix, the undifferentiated MSCs start to differentiate into MOBL, which secrete collagen throughout the matrix, forming a woven structure. After the creation of sufficient woven bone to form a platform-like structure, SOBL secrete collagen fibrils in a parallel way onto the previously made bone structure, creating the highly oriented lamellar bone. Once this process finishes, osteoblasts are matured into osteocytes surrounded by collagen matrix [25] (Figure 2a).
5.2.1.1. Stem cells differentiation into osteoblasts: exploiting physiological sources
Stem cells osteogenic differentiation, which is commonly targeted in tissue engineering strategies, is divided in three main stages: (i) peak in cell number; (ii) cellular differentiation, initiated with the expression and transcription of ALP; (iii) and a terminal step: production of osteocalcin and osteopontin [92]. In the human body, BMMSCs reside in a specific niche composed of a large variety of support cells that include hematopoietic progenitors; osteoclasts, immune cells and blood cells. The osteogenic differentiation of MSCs is known to be influenced by factors secreted by osteoblasts and osteocytes [6]. This phenomenon occurs through a communication network amongst osteoblasts and osteocytes that enhance a response in the MSCs, when these two bone cell types are in contact [6]. In vitro, the co-culture of MSCs with osteocytes showed greater osteogenic differentiation than the ones with osteoblasts, indicating that osteocytes induce MSCs’ osteogenesis more effectively than osteoblasts. On the other hand, osteoblasts helped the proliferation of the MSCs [6]. The differentiation of MSCs recruited to injured sites is not solely driven by contact with residing cells. Aspects of the fracture environment known to regulate MSCs fate include the control over the mineralized environment, respective release of ionic cues and the fine temporal variation of mechanical properties of the generated ECM during regeneration. Examples of tissue engineered approaches based on the use of in vivo-like mineralized synthetic biomaterials are described in Section 3.3.1. Other aspects such as control over biophysical cues generated throughout the bone repair process, also mimicked through the application of biomaterials with adequate and dynamic mechanical properties, are reviewed in Section 6.2.
Induced osteogenesis of MSCs may provide an important tool for the development of tissue engineering strategies focusing the treatment of large bone defects, which is currently challenging. Stem cells can derive from adult or embryonic sources, or from reprogramed adult cells (human induced pluripotent stem cells; hiPSCs) [127, 128]. Adult human sources of MSCs proven to enable osteogenic differentiation include bone marrow and adipose tissue [129, 130]. Stem cells from postnatal sources can be obtained from placenta, umbilical cord, cord blood and amnion [127, 128]. BMMSCs have been the most commonly used cell type for bone engineering. However, due to the complex and invasive isolation process, the limited cell number and the reduction of differentiation potential with donors age, researchers have been trying to surpass these disadvantages by using other cell sources [131]. Nonetheless, efforts to promote their efficient expansion using non-conventional 3D microcarriers under bioreactor configurations must be addressed. The in vitro expansion of MSCs and their use in combined strategies using microcarriers as cell growth supports and implantable scaffolding materials was recently reviewed in detail by Bunpetch et al. [132]. Human umbilical cord and adipose tissue are routinely discarded as clinical waste and, in the case of adipose tissue, may be used as noncontroversial MSCs sources [133].
hiPSCs represent a promising tool for bone regeneration [134]. These pluripotent cells closely resemble human embryonic stem cells; however, they are obtained through the reprograming of human somatic cells [135]. Using hiPSCs instead of stem cells derived from other sources, can be advantageous since they can be obtained directly from the patient (patient-specific) and overcome any ethical and immunological issues [134]. Their ability to differentiate into the three germ layers enable them to be reprogrammed into different bone cells, namely osteoblasts and osteoclasts, which highlights their potential to be used in cell-based therapies of bone defects and injuries [136, 137]. Methods commonly employed to differentiate ESCs into the osteogenic lineage have been adapted for iPSCs differentiation [138]. Wu et al. recently provided an updated review on the methods used for iPSCs differentiation targeting bone repair [139], which are classified according to (i) their dependence on the production of intermediate embryonic body structures, (ii) the direct generation of iPSCs-derived mesenchymal precursors, and (iii) the direct differentiation of iPSCs into osteoblasts without intermediate steps. The classical protocol for the osteogenic differentiation of iPSCs, based on a protocol established for embryonic stem cells [140], involves the initial formation of embryonic bodies followed by the harvest of iPSC-derived MSCs present in those structures and, finally, their differentiation into osteoblasts using osteogenic media. This protocol, however, was proven as low-yield, and approaches based on the direct seeding of dissociated embryonic bodies onto osteoinductive biomaterials have risen as effective ways of achieving regenerative systems for bone defects using iPSCs [136, 137, 141–143]. Alternatively to the formation of embryonic bodies, the adaptation of a protocol for embryonic cells differentiation [144] rendered iPSCs differentiation into MSC-like cells (often named iPSC-MSCs), which may be potentially differentiated into any mesenchymal lineage – including the osteogenic one. iPSC-MSCs were proven as valuable for the tissue regeneration field [145–147]. It is known that iPSC-MSCs are less responsive to the traditional chemically induced osteogenic differentiation protocol applied to MSCs (comprising ascorbic acid, β-glycerophosphate and dexamethasone), which has led researchers to find alternative differentiation methods [148]. In 2013, de Peppo et al. targeted the use of iPSC-MSCs as components of a tissue engineered system comprising decellularized bone as 3D scaffolds and a perfusion bioreactor. Perfusion conditions led to increasing expression of osteogenic markers, which were kept stable after the subcutaneous implantation of the iPSC in nude mice for 12 weeks [136]. Most of the studies reporting biomaterial-driven osteogenic differentiation of iPSC-MSCs rely on the use of mineralized matrices, either using decellularized bone or synthetic calcium phosphates. In 2018, a strategy to promote localized iPSC-MSCs in situ differentiation through localized BMP-2 recruiting was suggested by the co-injection of cells and a BMP-2 antibody in alginate beads [149]. This strategy avoided the formation of ectopic bone, commonly reported for BMP-2 releasing systems, and has the potential to surpass drawbacks associated to growth factors that include short half-life
The use of iPSCs has not been restricted to their differentiation into osteoblasts. A recent study using iPSC-MSCs also brought to light the potential of iPSCs to be differentiated into cell types other than osteoblasts with extreme relevance for the formation of functional bone, such as osteoclasts. Jeon et al. [7] used iPSC-MSCs differentiated into osteoblasts and osteoclasts differentiated from iPSCs-derived macrophages. iPSC-MSCs and iPSCs-derived macrophages were co-cultured and differentiated in PLGA/PLLA/hydroxyapatite porous scaffolds, rendering de novo-formed bone tissue upon implantation in nude mice.
Although iPSCs osteogenic differentiation relying on mesenchymal-like precursors has received a great deal of attention, the direct differentiation of iPSCs into osteoblasts has also been reported. In a precursor study, Levi et al. used biomaterials with the ability to concomitantly release osteogenic cytokines – BMP-2 - and provide biomineralization cues as boosting agents for in vivo bone formation through iPSCs differentiation [150]. Subsequent studied used methods based on the use of small molecules [151,152], osteogenic scaffolds [153–154], and gene modification to promote the fast, safe and high-yield differentiation of iPSCs and their application in bone regeneration strategies.
In general, protocols targeting the direct differentiation of iPSCs into osteoblasts depend on the use of cytokines, multi-step approaches using different supplements overtime, or in situ cell differentiation seeded onto specific biomaterials. In 2016, Kang et al. reported a breakthrough one-step protocol based on the use of adenosine, a natural occurring nucleoside, as a cell culture medium supplement to directly converse hiPSCs into functional osteoblasts [152]. Adenosine-treated cells seeded onto 3D microporous matrices rendered the successful repair of a critical bone defect, which included the formation of vascularized neobone capable of undergoing resorption.
Despite the clear promise represented by iPSCs as easily obtained cells for tissue regeneration, the field is still not free of challenges that include the delayed or low osteogenic differentiation of some cells that are exposed to differentiation protocols developed so far [148, 155], which may culminate in the long-term formation of dangerous teratoma tissues. Comparison between differentiation protocols, namely a robust parallelization between MSC precursor-based methods and direct differentiation protocols, are still in need.
5.2.2. Osteocytes
Osteocytes are fully matured and differentiated osteoblasts which descend from the mesenchymal lineage (Figure 2a). They comprise 90% to 95% of the whole bone cells in adult bone and may live up to decades in their mineralized environment exhibiting a dendritic configuration. Their function is to support the skeleton and bone metabolism. As osteoblasts differentiate into osteocytes, ALP production decreases, while osteocalcin raises [1] (Figure 2a). Other expressed markers, including phosphate-regulating protein with homologies to endopeptidases expressed by genes of the X chromosome (PHEX), matrix extracellular phosphoglycoprotein (MEPE), dentin matrix protein 1 (DMP-1), FGF-23, sclerostin, and oxygen regulated protein (ORP143), are thought to protect osteocytes against hypoxia [156]. These cells were considered as “passive placeholders in bone” in the past. However, they were proven to have numerous functions including bone remodeling - through the activation of both osteoclasts and osteoblasts -, as well as in endocrine cell functioning [156]. The interactions between osteocytes and osteoblasts/osteoclasts are reviewed in Section 5.4. Osteocytes are also responsible for the excretion of proteins such as CD44, galectin 3 and osteocalcin. These proteins have the function to promote cell adhesion and the regulation of mineral exchange in the bone. Osteocytes also produce Runx2 and Osterix, which are required for osteoblast differentiation, and are followed by ALP and collagen, necessary for the formation of the osteoid. Several soluble molecules produced by osteocytes positively interfere with biomineralization (e.g. PHEX, MEPE and DMP-1) [156]. The communication between osteocytes, which occurs mainly by gaps composed of connexin 43 [157, 158], are required for their survival, maturation and correct activity. The diverse roles of osteocytes also encompass their phagocytic activity, during osteolysis, since they have lysosomes in their constitution. One of the most remarkable functions of osteocytes is mechanosensing by translating stress factors into biologic signals [156]. Vazquez et al. [159] developed a 3D in vitro co-culture systems to assess the effect of mechanical loading on the interaction between osteocytes and osteoblasts. They demonstrated that osteocytes subjected to mechanical appropriate mechanical cues act upon osteoblasts towards bone formation [159].
5.2.3. Osteoclasts
Osteoclasts, generated in the bone marrow from mononuclear monocyte-macrophage precursors derived from the hematopoietic lineage [160], are the only cells capable of resorbing bone and, consequently, play an essential role in bone remodeling. The receptor activator of nuclear factor kappa-β ligand (RANKL) and macrophage colony stimulating factor (M-CSF) are reported to drive osteoclasts’ proliferation, differentiation and survival [160, 161]. Bone resorption occurs in the presence of factors secreted by osteoclasts: hydrogen ions that lead the acidification of the resorption compartment dissolving the mineral bone matrix, and cathepsin K, an enzyme responsible for the digestion of the insoluble fraction of the matrix (mainly type I collagen). These cells are bound by the bone matrix through integrins (β1 for collagen, laminin and fibronectin, and αvβ3 for osteopontin and bone sialoprotein) [162]. Such binding polarizes osteoclasts, creating an actin ring that seals the periphery of the ligation of the osteoclasts to the matrix, and a ruffled border in the resorbing surface, which leads to the secretion of H+ ions, followed by the exocytosis of enzymes from the acidified vesicles [162].
5.3. Heterotypic cell-cell interactions in bone
It is well established that bone cells interact in adult bone to regulate homeostasis process, supporting the balance between bone resorption and formation that allows the maintenance of the tissue’s integrity [162]. We review the interactions between the main reported cells that constitute healthy bone - osteoblasts, osteoclasts and osteocytes -, and their crosstalk with the vascular system that irrigates and also constitutes functional bone. Due to their relevance in bone healing, interactions between immune cells and bone resident cells are also reviewed.
5.3.1. Osteocytes-osteoblasts
Bone formation is regulated by several signaling mechanisms, with particular importance for the Wnt/β-catenin pathway [1]. The activation of canonical Wnt signaling in early osteoblasts promotes osteoblast differentiation and bone formation [163], with opposing effects observed when Wnt signaling is disrupted [164]. Osteocytes secrete Wnt antagonists, which include sclerostin and the LRP5/6 inhibitor Dickkopf-related protein 1 (DKK1). Both molecules inhibit osteoblast differentiation and bone formation [165, 166]. Moreover, the in vivo loss of secreted frizzled-related protein 1 (SFRP1), which is a competitive antagonist of Wnt ligand, resulted in increased bone mass and mineral density, as well as in in vitro enhancement of osteoblast proliferation and differentiation into osteocytes [167]. With their capacity to interfere in canonical Wnt signaling, therefore affecting osteoblasts differentiation, osteocytes play a regulatory role in bone formation.
5.3.2. Osteoblasts-osteoclasts
Signaling between osteoblasts and osteoclasts is crucial for osteoclast maturation [1]. It is known that osteoblasts and stromal cells produce RANKL, M-CSF, and osteoprotegerin (OPG), while early osteoclast precursors produce c-Fms (M-CSF receptor) and receptor activator of nuclear factor κ B (RANK) - a receptor for RANKL. RANKL and M-CSF stimulate osteoclast differentiation, while OPG is an inhibitor of RANKL, and competes with RANKL for RANK [1]. Low levels of OPG lead to accelerated osteoclast development, which culminates in osteoporosis: a disease characterized by the disruption of bone resorption/formation balance, and in which bone resorption exceeds bone growth [160]. Although osteoblasts were thought to be the main providers of RANKL to osteoclasts, osteocytes have proven to have an extremely relevant role in this mediation [168].
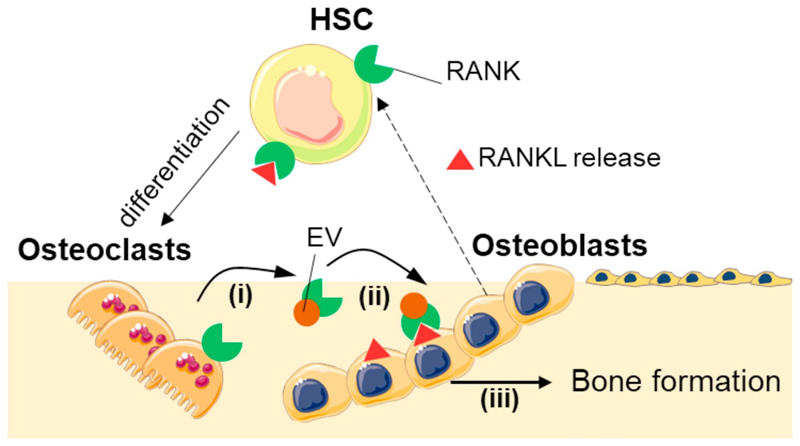
Besides the role of osteoblasts and osteocytes on osteoclastogenesis, the reverse role of osteocytes on osteoblasts’ function has also been a matter of interest. In 2011, osteoclasts were reported as being uncapable of stimulating RANKL reverse signalling in osteoblasts through direct cell interactions [169]. However, in 2018, Ikebuchi et al. presented a breakthrough mechanism driven by extracellular vesicles, in which osteoclasts modulate osteoblasts function and, therefore, bone formation [170]. In the suggested setting, RANKL receptors in osteoblasts are responsible for the reverse RANK-RANKL modulation. Osteocytes release extracellular vesicles containing RANK on their surface, which bind to RANKL on the surface of osteoblasts (and hypothetically osteocytes), triggering intracellular signalling. The authors proved that mTOR pathway was activated, triggering the production of Runx2, and leading to bone formation.
5.3.3. Osteocytes-osteoclasts
Osteocytes – both healthy and apoptotic at microdamage sites – have been reported to recruit osteoclasts to the bone remodeling sites and were shown to send bone resorption cues to those cells [171]. The expression of the RANKL during the dendritic process associated with osteocytes maturation was associated with the osteocyte-led bone resorption [171]. Upon injury, right after damage, pro-apoptotic molecules are released by osteocytes; contrarily, anti-apoptotic molecules are produced at 1-2 mm distance from the cracks [171]. The promotion of a defective performance of osteocytes in mice through β-catenin depletion led to increased osteoclasts activity [171]. This result demonstrated the dependency of the correct regulation of osteoclasts activity on osteocytes, proving osteocytes’ relevant role on the bone remodeling process [171]. Another indication of the close osteocyte/osteoclast interaction was the induced formation of osteocytes, both in vitro and in vivo, by osteoclasts apoptotic bodies; a similar contact with osteoblast-derived apoptotic bodies, however, did show this ability [171, 172]. Mechanistically, the induction of osteocytes formation by exposure to osteoclasts’ apoptotic bodies was not driven by RANKL; instead, it was proved to be a TNF-α-dependent process.
Recent studies have focused on a deeper elucidation of osteocytes-osteoclasts interactions, namely in the understanding of osteoclastogenesis. IL-6 has been reported as a mediator and modulator of this cellular interaction, although the mechanism behind this phenomenon has not been completely unraveled. In 2017, Wu et al. performed the characterization of inflammatory factors present in the serum of ten patients who underwent orthognathic surgery [173]. The authors found that both IL-6 and RANKL were stimulated in serum 3 to 7 days after surgery. The characterization of inflammatory cytokines from patients’ blood, along with an in vitro study in which an osteocyte cells line (MLO-Y4) was stimulated with IL-6 and IL-6 receptor, allowed correlating increased osteocyte-mediated osteoclastogenesis with the presence of IL-6, unraveling its role in the enhanced expression of RANKL.
5.3.4. Vascular cells interactions with bone cells
Bone-associated blood flow controls oxygen and nutrient delivery/exchange in the tissue, and bone formation and resorption are coupled with bone hemodynamics. During endochondral ossification, the vascularization of hypertrophic cartilage is one of the determinant steps for bone elongation. Moreover, in fracture healing, the generation of an efficient new tissue is also dependent on a successful vascularization. A tight connection between the growth of blood vessels in bone and osteogenesis has been reported [174]. Endothelial and osteoblastic cells have a molecular crosstalk in which angiogenesis and osteogenesis are synergistically promoted. Osteoblasts are known to secrete angiogenic factors, including VEGF [175] and erythropoietin [176], which mediate their crosstalk with endothelial cells. Nonetheless, the mechanisms and molecules involved in this process have not yet been fully unraveled.
Bone vasculature has recently been presented as a unique network with substantial differences from other body vascular systems. Interestingly, vascular growth in bone was proven to be obtained by a tissue specific angiogenesis, in which the Notch pathway is responsible for endothelial cell proliferation and blood vessel growth in post-natal long bone. In a study conducted by Ramasamy et al. [177] the authors verified a deficiency on the bone vessel growth and morphology by knocking out the gene responsible for the Notch signaling. In turn, this led to reduced osteogenesis, resulting in the irregularity of bone structure in mice. Huang et al. [178] identified chemokine (C-X-C motif) ligand 9 (Cxcl9) as an angiostatic factor secreted by osteoblasts in the bone marrow environment. Mice with constitutive mTORC1 (an Cxcl9 activator) in osteoblasts demonstrated enhanced VEGF secretion; however, this was accompanied by an unexpected decrease in the phosphorylation of its receptor (VEGFR2), as well as downstream signaling in endothelial cells, and reduced vasculature formation in bone.
The structure of bone vasculature was proved as a unique construction by Kusumbe et al. [179] who identified a new capillary subtype in the murine skeletal system, presenting distinct molecular, morphological and functional properties. These vessels were shown to be crucial for the correct bone development and maintenance, since they generate a distinct molecular and metabolic microenvironment, linking angiogenesis and osteogenesis, and lastly maintaining perivascular osteoprogenitor cells. The study of human vasculature and the identification of capillary subtypes specific to bone may represent a step ahead on the understanding and development of vascularizing strategies. Also, the scrutinization of the individual interactions between human bone vascular cells and other resident bone cell types besides osteoblasts (including osteoblasts and osteoclasts), as well as the comparison of these results with more complex co-culture systems, is still in high demand for a full mastering of biochemical/biophysical signaling dictating the achievement of fully functional bone tissue.
5.3.5. Immune cells interactions with bone cells
Despite not having been reported to reside permanently in healthy mineralized bone tissue, except for osteal macrophages - OsteoMacs, immune cells residing in the bone marrow are in a close anatomic location with bone. The crosstalk between bone and immune system cells has often been overlooked, and usually focuses on the role of such cells in disease [180]. We here report some of the studied cell crosstalk facts involving bone cells and bone-related/bone-constituent (OsteoMacs) immune cells, connected to the regulation of bone’s normal physiology.
OsteoMacs are probably the most studied immune system cells in bone tissue. They reside on the endosteal and periosteal surfaces and compose 10 to 15% of most tissues [94]. In vivo, OsteoMacs form a shell over mature matrix-producing osteoblasts at sites of bone modeling [181]. Depletion of macrophages in vivo results in complete loss of endosteal OsteoMacs and their associated osteoblasts, suggesting that this population is needed to maintain mature osteoblasts in the bone structure [94, 182]. Furthermore, OsteoMacs can also function as osteoclast precursors [156]. Raggatt et al. [183] confirmed that primary OsteoMacs isolated from endosteal bone tissue can differentiate towards the osteoclast lineage in vitro, in the presence of both RANKL and CSF-1 factors.
Immune cells, namely macrophages and monocytes are the first cells to interact and react with foreign pathogens or implanted devices [184]. Despite the advances concerning the elucidation of the role of OsteoMacs in bone biology, little is known about their differentiation behavior in the presence of biomaterials used in bone regenerative strategies, as well as about the factors involved in the process. Most strategies employed in bone biology field are adapted from studies focusing on biomaterials integration by soft tissues and many of them play with properties of the surface biomaterials that ultimately enable the modulation of macrophages behavior [184]. Considering the crucial role of OsteoMacs in bone formation and remodeling, further steps should be taken towards the incorporation of this population into 3D in vitro bone remodeling models as well as bone regenerative approaches. The independent analysis of OsteoMacs on biological tissues has been associated with some cellular detection limitations, and this must be considered while studying the role of these cells, mainly in in vivo settings. Studies targeting in vivo depletion of OsteoMacs often cause collateral decrease in osteoclasts [185]. Indeed, these two cell types show major similarity, which are assigned to their shared progenitor myeloid lineage, produced growth factors and other molecules. While osteoclasts are reported to be easily distinguished from resident macrophages with targeting well-established biomolecular techniques [160], the reverse cell depletion process is not free of risks, leading to the possible misinterpretation of the role of these cells on bone biology. Recently, the identification of the CD169 marker as specific for OsteoMacs allowed an unbiased analysis of their role on bone development [185]. Using this osteoclast-bias-free depletion method, the role of bone resident macrophages as pro-anabolic supporters of osteoblasts function during bone homeostasis and repair was elucidated, both on intramembranous and endochondral pathways [185].
Besides macrophages, other immune cells have deserved attention for their role on healthy bone maintenance. The reduction of bone-related B- and T- lymphocytes in mice have led to osteoporotic scenario [186]. Moreover, it is known that mature B-cells produce more than half of bone marrow-derived OPG, which contributes to osteoclastogenesis restriction [186] and T-lymphocytes are also thought to interact with B-cells to enhance OPG production [94]. HSCs-derived megakaryocytes, known to produce platelets, were shown to enhance the in vitro osteoblast proliferation and differentiation through the expression of RANKL, OPG and some unknown anti-osteoclastic factors [187]. Despite the evident role of a plethora of inflammatory cells in the maintenance of healthy bone status, most studies concerning their role still focus exclusively on post-injury/healing stadia. Nonetheless, currently available information shows the relevance and active role of these cells on basal bone maintenance, and that their depletion often culminates in disease scenario. Therefore, an investment on the elucidation of the role of inflammatory cells on the maintenance of healthy bone may drive the discovery of new diseases, and may also benefit the existing know-how targeting pro-regenerative techniques through the exploitation of novel in vitro-designed co-culture setups.
5.4. Co-cultures for bone regeneration: modulation of stem cell fate and improvement of tissue integration
Cells are often included as part of regenerative therapies due to their ability to naturally synthesize ECM proteins responsible for tissue reshaping and release biomolecules that dictate the success of bone development and correct function. Crosstalk between bone resident cells, immune cells, endothelial cells and MSCs are key players of a coordinate chain of events that occur during bone healing. Since different cell types cohabit in the in vivo environment, modulating each other’s response by direct contact or release of molecules, it is essential to study and develop bone engineering strategies that somehow could recreate and take advantage of specific features of this niche. Table 1 is dedicated to an analysis of already reported cell-cell combinations applied in the context of bone tissue regeneration.
We identify four main axis of co-culture setups applied in bone tissue engineering: (i) co-culture of different primary bone cells, (ii) stem cells and resident bone cells, (iii) bone/stem cells and endothelial cells (targeting vascularization) and (iv) bone/stem cells and immune cells (targeting immunomodulation).
Bone resident cells, i.e. osteoblasts, osteoclasts and osteocytes are the pillars of bone. As reviewed in Section 5.3, dedicated to the description of heterotypic cell interactions, these cells are in constant communication with each other and adjacent cells, modulating their response through a complex network of signaling that provides the simultaneous degradation, formation and maintenance of the tissue [188]. Some of these interactions were transposed to tissue engineering, (i) to developed strategies that aim the recreation of the 3D bone environment and processes and (ii) to improve the quality and performance of bone regeneration approaches. Most studies aimed at the development of in vitro 3D models that recreate some of the in vivo bone events by establishing the interactions between osteoblasts-osteoclasts or osteoblasts-osteocytes into a scaffold/platform or by self-assembling [159, 189–191] (Table 1). Studies focusing on the crosstalk between osteocytes and osteoclasts in 3D biomaterial-based in vitro environments are still absent. In fact, although many fundamental studies have directly addressed these interactions, the transition to a tissue engineering 3D perspective needs to be further explored.
MSCs, addressed in Section 5.2.1.1, are well accepted as relevant cells for implantation in regenerative medicine platforms, owing to their clinical potential and easy retrieval. hiPSCs are another attractive stem cell source due to their ability to be reprogrammed from easily accessible tissues [134]. Although the incorporation of stem cells into bone regenerative strategies was an exceptional achievement, the combination of co-cultures comprising stem cells and adult bone resident cells within 3D cultures is seen a highway to achieve higher complexity models, resulting in regenerated tissues of superior quality [192]. Birmingham et al. [6] investigated whether soluble factors produced by osteoblasts and osteocytes could influence bone formation. By establishing an indirect co-culture that allowed the direct contact between those two bone cells, while restricting it to MSCs, the authors proved the need of the biochemical crosstalk between both bone cells and MSCs to direct them towards the osteoblast lineage, in the absence of osteogenic media [6]. Besides better recreating the bone niche, co-cultures can also function as an alternative to conditioned media used in tissue engineering methods. So far, the characterization of these co-cultures in a 3D context has been essentially made through hydrogel-based strategies [193, 194] (Table 1).
The achievement of constructs rich in functional vascular networks is one of the well-known gold standards of bone regeneration targeting effective osseointegration and defect restoration [195, 196]. Approaches based on the presentation of vascularization-inducing biochemical cues through biomaterials are common to induce vascular cells migration and invasion of scaffolding materials. Endothelial cells aimed at promoting tissue vascularization, however, are used in technologies that either compete or complement the delivery or localized presentation of biochemical cues [197]. While endothelial cells cultured alone showed limited potential for vascular growth, studies have shown that their combination with osteoblasts or BMMSCs as well as with biomolecules involved in osteogenesis enhanced their vascularization capacity [197–203]. The exploitation of co-culture systems is a growing trend to establish biomimetic cell-based approaches targeting functional angiogenesis on regenerative constructs and/or full vascularization [204].
Different cell sources have been suggested as effective promoters of scaffold vascularization agents [205]. The use of mature endothelial cells and EPCs on co-culture strategies targeting bone regeneration using tissue engineered constructs was systematically reviewed by Liu et al. [204] concerning aspects as type of co-cultured cells (adult primary bone cells, stem cells), application of static and dynamic flow/tension regimes (reported to modulate bone formation and ageing processes [206]), type of used ECM-support, as well as critical aspects such as cell culture media used for in vitro experiments, cell seeding methodologies, establishment of direct and indirect co-culture setups, type of animal models, and how these factors affected bone formation and vasculature quality.
Pluripotent stem cells, including ESCs and iPSC are nowadays rising as important sources of endothelial cells [205,207]. Other stem sources, including BMMSCs, have also been suggested as naïve platforms to be differentiated into endothelial/vascular cells [208]. The use of stem cell-derived endothelial cells is nowadays a breakthrough in the generation of high yield in vitro tissues. However, their application in bone regeneration is poorly explored, even though the use of autologous sources of stem cells and further endothelial and tissue-specific differentiation may represent a step forward in the design of low-invasive (e.g. using ASCs) technologies. The characterization of the effect of different co-culture setups is another aspect that is still in need of further in-depth study; efforts to perform relevant characterization have relied on micropatterning strategies (mostly targeting 2D culture systems), use of transwell systems, and microfluidics to generate multicompartment structures [209]. Future optimization of such setups will allow further elucidation of the role of distinct culture setups on the synergic role of vasculature formation and bone tissue development on 3D/4D relevant structures, which will be organized in ECM-like structural organization and temporarily-controlled remodeling.
Controlling immune-mediation as a driving force for bone regeneration has also been explored for tissue-engineered systems. The conjugation of immune system cells with either adult bone cells or stem cells is currently a major trend. Primordial advances on immunoregulation for tissue engineering targeted the direct role of biomaterials upon implantation (or upon cytokine stimulation in vitro) [210–214], or their use as cytokine-release mediators to induce bone formation and successful vascularization [112]. Macrophages are the most widely studied immune cells in co-cultures for bone regeneration [215–217], and most of these studies target the osteogenic differentiation of adult MSCs under 2D direct and paracrine co-culture setups. Indeed, secreted factors from immune cells are described to affect MSCs [218, 219], and synergic effects are reported and suggested as, for example, means to obtain anti-inflammatory macrophages as therapeutic agents [220]. The effect of macrophages’ polarization state has also been correlated with MSCs osteogenic fate promotion [221, 222]. Interestingly, the co-culture of MSCs with M1 macrophage pheonotypes was reported to promote enhanced osteogenic differentiation of BMMSC [221]. Other studies, though, correlate M2 anti-inflammatory phenotypes with higher ALP and mineralization of both bone marrow and adipose tissue stem cells [222, 223]. The discussion over the timely presentation of macrophages with distinctive roles on injury sites on physiological bone healing may hide the clues to design regulated biomaterial and co-culture systems to promote the presence of the most adjuvant immunoregulatory cells on implantation sites.
In a recent approach, the mechanomodulation of macrophages using magnetized superparamagnetic scaffolds allowed driving their phenotype to a M2-like status; media conditioned by these macrophages was later proved to enhance two important bone regeneration features: osteoblasts osteogenesis and endothelial cells angiogenic potential [224]. Indeed, biophysical stimuli (including topographic cues) seem to provide crucial cues to modulate macrophage activity and its subsequent interaction with stem cells [225]. There seems to be a broad room for exploitation of immune cells as bone regeneration adjuvants. While monocytes/macrophages start to see their roles unraveled and potentiated in the field of tissue regeneration, other poorly explored cells with proved potential (e.g. NK cells [226]) may lead to relevant scientific discoveries and technological breakthroughs. The role of lymphocytes as supportive cells for tissue engineering has also been scrutinized [227], mainly taking in consideration their key role in autoimmune diseases as rheumatoid arthritis [228]. Few studies report the characterization of heterotypic cells interactions while cultured on biologically relevant biomaterials, which can be used as in vitro tissue models and/or as implantable devices. Moreover, the results obtained from these studies are difficult to correlate as different biophysical and chemical cues are provided by different materials.
Sophisticated biomaterial designs will be necessary to enable co-culture setups with different and relevant cell types driving effective osteogenic differentiation, osseointegration and beneficial immune response upon implantation. Such co-cultures may be performed in direct or indirect setups with highly complex cellular communication occurring, which may be facilitated by multicompartmental and time-regulated biomaterials. Another interesting route to achieve the precise location of homotypic and heterotypic cell-cell contacts in either implantable or in vivo-formed microtissues may rely on the localized presentation of cell-recognizable domains in distinct fraction of biomaterials. Surface patterning using specific cell-targeting antibodies is one of the most common approaches to control cell positioning. These systems have been suggested in the 1990’s as “immunospots” for disease diagnosis [229, 230], and antibody-coated cell-specific microbeads (so caller “immunobeads”) are routinely used in cell isolation from blood and tissues. This concept was extended for the spatially controlled cellular patterning of in 2D biomaterials [231], as well as in 3D beads targeting the in situ formation of robust microtissues through the selective adhesion of specific cells, namely MSCs and HUVECs, to polymeric injectable biodegradable particles [232, 233]. Cell patterning and specific cell-cell contact may be directed not only by biomaterial-based approaches, but also by cell-driven strategies. Cellular surface engineering, i.e. the direct chemical modification of cellular membrane, may be used to spatially control cell-cell assembly of cells during the formation of highly organized tissues [234–238]. The application of these concepts to bone tissue regeneration may lead the way for the achievement of scaffold-free implantable multicellular microtissues with, for example, evenly distributed vascular networks.
5.5. Protein-mediated cell-cell contact in bone
5.5.1. The role of cadherins, connexins and pannexins
Cells can communicate by two processes, involving indirect and direct contact. Most interactions that occur during bone formation, development and remodeling have been shown to be driven by direct cell contact. Cadherins are the main proteins responsible for cell-cell attachment [252]. These proteins are glycoproteins located at the cell membrane, which promote cell-cell adhesion by a calcium-mediated mechanism. Cadherins (molecular weight around 120 kDa) are constituted by two domains: the extracellular and the transmembrane domain. The calcium-binding site (five repeats; responsible for the ability of cells to bind the same cadherin) is located in the extracellular domain. Cadherins can be classified in the following way: type I and type II. In these two types, cadherins can be divided even more: type I: N-, E, M- and R-; type II: 5 to 12 [252]. The cytoplasmic C-terminal tail of cadherins is responsible for the stabilization of the adhesion. This structure is organized by the binding of cadherin to β-catenin and plakoglobin, which connect cadherins to the actin cytoskeleton, via N-catenin, actinin, ZO-1 and vinculin, in a dynamic process. Adherent junctions, i.e. the junctional structures between two adjoining cells, allow the communication and adhesion between cells [252].
In bone, there are three major cadherins: E-cadherin, N-cadherin and cadherin-11 [253–255]. Cell-cell adhesion mediated by cadherins is essential for the function of bone-forming cells during osteogenesis. The absence of those cadherins was shown to inhibit osteoblasts differentiation [256]. During osteoblast differentiation, cadherin-2 is downregulated over the process, and cadherin-11 becomes the main cadherin for osteoblast functions. For successful osteogenesis, cell-cell contact amongst cells of the osteoblastic lineage and the osteoclasts precursors is necessary. This interaction is mediated by RANK (receptor) and RANKL. RANK is present in osteoclasts precursors, while RANKL is present in the membrane of osteoblastic cells [1, 257, 258].
Connexins are proteins involved in cell-cell contact, allowing the rapid dissemination of molecules (smaller than 1 kDa) and ions by diffusion among cells. They link cells through gap junction channels, that facilitate electrical and chemical coupling [259]. The most widely reported connexin in bone - produced by osteocytes, osteoblasts and osteoclasts - is Cx43 [260]. In addition, both cells also produce Cx37 [261], and osteoblasts produce Cx45 and Cx46 [158]. When osteochondroprogenitors, as well as committed osteoblast progenitors, lack Cx43 protein, there is a decrease in bone mass and density [260]. Interestingly, the deletion of the gene that codes Cx43 from mature osteoblasts and osteocytes did not lead to any effect on bone mineral density or bone length. This suggests that Cx43 is essential for osteochondral progenitors, but not in committed osteoblasts [260]. Cx37 has recently been proved to regulate bone mass [261]. The lack of this connexin led to increase in bone mass. However, this effect was shown to be gender dependent, with males being more affected than females [261]. The higher bone mass observed in individuals with depleted Cx37 is related with a decrease in osteoclast differentiation, driving impaired bone resorption [261].
Pannexins are proteins with a very similar structural topology to connexins. However, their sequence is not homologous with connexins, and they only function as an unpaired channel [262, 263]. One of the genes encoding pannexins - Panx1 - is present in murine osteoblastic cells [264], whereas Panx3 is expressed in various osteoblastic cell lines, primary calvaria cells and in hypertrophic chondrocytes [265–267]. Although some studies have explored the role of pannexins in osteoblast differentiation in vitro, in vivo studies are still needed [262].
5.5.2. The role of protein-mediated cell-cell contact on bone regeneration: a still unexplored concept in tissue regeneration strategies
The modulation of pro-regenerative niches based on the modification of biomaterials with cell-cell contact-mimetic domains has been suggested as a strategy to locally target the differentiation of stem cells, including pluripotent stem cells and MSCs. Toh et al. [268] developed 2D surfaces modified with Matrigel® and E-cadherin micropatterns, to mimic cell-matrix and cell-cell adhesion motifs, respectively. The culture of pluripotent ESCs on the patterns showed that integrin and E-cadherin adhesions were capable of locally promoting distinct cell fates, which culminated in the generation of spatially heterogeneous cell colonies. The role of selectively modified biomaterials on the modulation of clinically relevant iPSCs has also been a matter of debate [269]. Recently, nanoporous and microporous with pore sizes ~5 nm and ~120 μm, respectively, were applied as platforms for the study of the role of cell-cell interactions on the paracrine function of MSCs [270]. The authors hypothesized that cell-cell contact through N-cadherins, as well as cell-matrix interactions, would be increased in biomaterials with higher pore size. Alginate-based hydrogels and porous scaffolds featuring similar mechanical and chemical profiles were synthetized to show different pore size ranges [270]. The secretory profile of MSCs cultured on both biomaterials showed significant differences, and the retrieved conditioned medium from both conditions differently influenced the function of C2C12 myogenic precursors, proving the potential of cadherin-mediating biomaterials as platforms for the modulation of potentially therapeutic cytokines produced by MSCs.
Biomaterials with the ability to present cell-cell contact motifs to cells are still in their early infancy on the bone regeneration field. A study by Cosgrove et al. [9] described the use of a biomaterial-based approach to elucidate the role of cell-ECM and cell-cell contact of MSCs in osteogenesis driven by mechanical transduction phenomenon. The authors modified a hyaluronic acid hydrogel with a HAVDI adhesive motif from N-cadherin (to emulate the cell-cell ligation) and a RGD adhesive motif from fibronectin (to emulate the cell-ECM ligation), for the co-presentation of these motifs. HAVDI ligation decreased the contractible state of the cells (and the nuclear YAP/TAZ location), which led cells to wrongly interpret the ECM stiffness, causing a change in the downstream cell osteogenic differentiation and proliferation. On the same year, Zhu et al. [271] also presented a methacrylated hyaluronic acid-modified hydrogel with N-cadherin and the integrin-binding domains and hypothesized that such moieties would allow presenting an “orthotopic” environment to MSCs. Interestingly, N-cadherin-containing hydrogels improved the osteogenic differentiation of encapsulated cells, both in vitro and in vivo, which seems contradictory with the results presented by Cosgrove et al. [9] in the same year.
The low number of studies reported on the effect of cell-cell domains-presenting biomaterials on the regenerative medicine field, in particular for bone tissue engineering strategies, still hamper a profound discussion about the role of these domains on stem cells differentiation and biological tailoring to achieve improved regeneration outcomes. The opportunity for the development of systematically modified materials that combine ECM and cell-cell interaction cues is a current opportunity for the design of more efficient biomaterials capable of contributing to the elucidation of fundamental aspects of bone niche interactions.
5.6. Soluble biomolecules present in bone environment
5.6.1. The primary role of biomolecules in bone formation and regeneration
Biomolecules play a crucial role in both formation and repair of bone tissue, and are constantly present through all bone formation and repair phases. They are responsible for the recruitment, proliferation, differentiation and migration of the osteoprogenitor cells [2]. As mentioned on Section 4, upon bone injury, immune cells are recruited to the defect site and coordinate actions triggered by the release of cytokines and growth factors, that culminate in the recruitment of MSCs, remodeling and vascularization of the tissue [2]. Bone physiology during development, under healthy conditions and upon regeneration involves the participation of an elevated number of biomolecules. In this Review, we will briefly describe the roles of widely reported BMPs and pro-angiogenic VEGF, as these are the most commonly used cytokines and growth factors on tissue regeneration strategies as externally-provided factors. The delivery of off-the-shelf cellular factors (in cell-free strategies) to regulate bone healing has been recently reviewed [272]. An interesting table that correlates the most important growth factors in bone tissue repair, their function and working period can be found in a recent Review by Wang et al. [273].
BMPs are a family of multifunctional growth factors containing over 20 members, generally classified into four categories: BMP-2/4, BMP-5/6/7/8a/8b, BMP-9/10 and BMP-12/13/14 [274]. From those 20 types of identified BMPs, at least 7 have shown osteoinductive potential [275, 276]. Those include protein forms with 30 to 38 kDa molecular weight, composed of two disulfide-linked polypeptide subunits. These proteins are involved in the regulation of cell proliferation, differentiation and matrix biosynthesis during reconstruction process of human bone, in coordination with other molecules. Moreover, BMPs are unique proteins with the ability to induce bone formation individually. While several homodimer forms of BMPs were reported to be osteoinductive, the heterodimer forms of these molecules including BMP-2/-6, BMP-2/-7 and BMP-4/-7 have shown more potency both in vitro and in vivo as compared to the respective homodimer mixtures, with 20-fold higher osteoinductive activity for BMP-2/-7 heterodimer in particular [277]. The higher activity of BMP heterodimers has been associated with higher affinity for receptors than homodimer counterparts [278–280].
The detailed role and structure of each BMP family member is reviewed in Reference [260]. These molecules stimulate the differentiation of MSCs into osteoblastic lineage and promote the proliferation of osteoblasts and chondrocytes, being an active member of endochondral ossification and bone healing process (see Section 3.2 and 4.1) [281, 282]. BMPs bind to receptor complexes consisting of type I and type II transmembrane serine/threonine kinases. The role and associated potency of different BMPs receptors can be found in Reference [276]. BMPs target cells by activating mothers against decapentaplegic (Smads) and mitogen activated protein kinase (MAPK) pathways [281] that converge at transcription factors as Runx2 to promote osteoblast and chondrocyte differentiation from MSCs [44]. In tissue regeneration therapies, bone formation induced by growth factors administration results from a combined action of the administered molecules and endogenous produced factors [276]. Along with BMPs, TGF-β - including TGF-β1, TGF-β2, and TGF-β3 - is a family of proteins also involved in skeletal embryonic development and postnatal bone homeostasis through the same pathways [44].
VEGF, a crucial intervenient of vascular growth, is also deeply involved in the correct bone development and regeneration, linking both osteogenesis and angiogenesis [283, 284]. This growth factor is involved in both intramembranous ossification and endochondral bone formation. In the later, VEGF stimulates vessel invasion and the recruitment of chondrocytes into hypertrophic cartilage while in the former it is released by osteoblasts upon hypoxia exposure and induces endothelial migration and proliferation and vessel permeability [283, 284]. In turn, osteogenic factors such as BMP-2, are produced by endothelial cells, leading to osteoblast differentiation and mineralization [284].
Besides the previous mentioned signaling molecules, others with similar relevance for bone tissue development and regeneration include cytokines, PDGF, FGF, among others. A detailed description of the source, function and target of those proteins can be found at Dimitriou et al. [100].
5.6.2. Biomolecules in tissue engineering strategies
Biomolecules are essential in the bone regeneration process due to their key role on the recruitment of MSCs, tissue remodeling and vascularization [2] upon bone injury. The understanding of the individual tasks, combined actions or even potentiating effects of growth factors have led the development of biomaterial-based structures targeting bone regeneration. Strategies to modulate cell response based on bioactive agents may rely on the controlled release of these molecules through finely controlled drug delivery systems [285]. Systems designed to enable the pulsatile or controlled sequential release of different bioactive agents have been applied in tissue regeneration strategies [286, 287].
BMPs are the mostly studied growth factors for bone tissue engineering. BMP-2 and BMP-7 are the most used ones, with application in clinical medicine. However, they have showed limited success, owing to the reported formation of ectopic bone [288]. Besides promoting osteogenesis, these two BMPs, as well as PDGF and VEGF play a critical role in promoting neovascularization of bone tissue [197, 199, 283]. Since bone is a highly vascularized tissue, the performance of a scaffold can be dependent of its ability to induce new vessel formation in the transplantation site [21]. As these growth factors are often costly, Yu et al. [201] reported a new peptide, designated bone forming peptide-1 (BFP-1), easy to synthetize at lower cost [289], that derives from the immature region of BMP-7. This biomolecule was shown to enhance vascularization through the up-regulation of VEGF receptor gene in endothelial cells [201]. The incorporation of BFP-1 into beta-tricalcium phosphate (β-TCP) scaffolds containing endothelial cells successfully promoted angiogenic functions of endothelial cells of the construct in vitro and enhanced vascularization and bone regeneration in vivo [198].
The release of bioactive molecules in a controlled and site-specific manner is also crucial for the success of tissue engineering strategies. Complete reviews addressing the controlled release of bioactive agents for bone regeneration can be found in References [286, 290–292]. The complex design of biomaterials as a route to mimic naturally occurring or much needed therapeutic phenomena, or as a way to allow effective administration of drugs with paramount importance for bone regeneration, has been explored through the establishment of finely controlled degradation profiles of adequate implantable biomaterials. For instance, a biodegradable drug delivery system designed to enable the pulsatile release of PTH – a FDA-approved drug for the treatment of osteoporosis – for 21 days, presenting a promising method to circumvent the daily injectable administrations of this drug [293]. An advanced 3D scaffold design consisting of finely tuned biodegradability profiles allowed the application of a nanofibrous material loaded with PTH in the high-quality regeneration of a mouse critical size (2.3 mm) calvarial defect, as compared to regular injections of PTH or continuous release of the hormone through a releasing biomaterial [294].
As previously mentioned, growth factors have important effects on the regulation of bone formation, remodeling and regeneration. Their clinical application, however, is often hampered by the administration of supraphysiological doses of soluble compounds reported to be rapidly cleared from the body, and that show limited therapeutic effect, elevated costs, and are associated with serious side effects such as the formation of ectopic bone (e.g. for BMP-2 and BMP-7) [295]. The presentation of growth factors immobilized in biomaterials with controlled spatiotemporal release or localized presentation have been suggested to enhance tissue regeneration outputs obtained from the delivery of these molecules to defect sites. Through the entrapment of BMP-2 in alginate/chitosan self-standing membranes produced by the layer-by-layer electrostatic assembly technique, Caridade et al. [296] achieved a slow-releasing system, in which only a ~15% fraction of the growth factor mass was released from the biomaterial structure after 30 days of immersion in a physiologic-mimetic solution. Slow BMP-2 releasing materials supported the formation of localized ectopic bone fragments in a subcutaneous mouse model, while membranes chemically tailored to promote faster release did not achieve a pro-regenerative performance. Other membrane materials based on electrostatic interactions between natural polysaccharides – chitosan and chondroitin sulfate – also showed the ability to uptake and retain high amounts of growth factors, namely TGF-β3 [297]. These materials released only 1% of the total loaded TGF-β3 mass after 15 days of immersion in an aqueous phosphate buffer, which suggests their possible application as growth factor-presenting materials, with extremely low drug release profiles. The majority of growth factors related with bone regeneration and used as therapeutic agents interact with cells through cell membrane-based mechanisms. In the particular case of BMP-2, the initiation of signaling pathways occurs through the binding of plasma membrane receptors, followed by the phosphorylation of the protein, culminating in the Smad signaling activation [298]. The use of biomaterials to present growth factors to cells, independently of release mechanisms, is then considered an advantageous approach to circumvent excessive cargo release and provide highly localized biochemical signals in defect sites. Cell-interacting biomaterials containing covalently immobilized proteins and growth factors have been shown to modulate stem cells response and drive effective bone repair [299]. Indeed, the modification of biomaterials with covalently attached growth factors was suggested as a promising manner of maintaining physiological levels and prolong their life time [300, 301]. For example, methoxy poly(ethylene glycol) injectable hydrogels covalently modified with BMP-2 through a click reaction showed enhanced synergic osteogenic differentiation of human periodontal ligament cells as compared to soluble BMP-2 formulations [302].
The high cost of recombinant growth factors has driven the design of biomaterials with ECM-mimetic properties, in which these molecules are selectively attracted to specific scaffold locations, which then serve as growth factors reservoirs [303]. One of the most well reported examples of ECM proteins-growth factors physiological interactions is the formation of fibronectin-BMP2 complexes [304]. In biomaterials, however, the effectiveness of the interactions between growth factors and ECM proteins is strictly dependent on protein rearrangement and conformational aspects. Salmerón-Sanchez and co-workers [305] developed an approach to control fibronectin conformation and exploit growth factor (BMP-2) recruitment and presentation to cells. The use of poly(ethyl acrylate) materials allowed adsorbing fibronectin in a fibrillar fashion, in opposition to the globular conformations obtained on control polymeric surfaces. The fibrillar arrangement of fibronectin showed a synergistic presentation of integrin-binding sites and bound BMP-2, which drove MSCs osteogenesis and the full regeneration of a nonhealing bone defect. Other bioinspired studies based on the use of heparin-based domains or charged polyelectrolytes for partially selective growth factors sequestering from blood and plasma components have been incorporated into tissue regenerative approaches [306, 307].
The delivery of extracellular vesicles (EVs) as regeneration modulators is an increasingly relevant trend in the field of tissue engineering [308, 309]. These nanosized structures, which include exosomes and shedding vesicles, produced by cells were once seen as “garbage bags” used to excrete waste [309]. Nowadays, they are considered as powerful tools to induce cell response because they are known to carry biologically relevant cargoes that include proteins, lipids, as well as coding and non-coding RNAs [310]. Reports on the pro-regenerative and immunomodulatory roles of these structures can be found in References [308, 309, 311, 312]. The use of EVs generated in vitro after the differentiation of stem cells into the osteogenic lineage has proven to be effective as inducers of the osteogenic differentiation of naïve stem cells. Moreover, stem cell-conditioned medium and isolated EVs were capable of regulating osteoblasts activity and promote the regeneration of bone defects in vivo, and osteogenic differentiation in vitro [313, 314]. These effects are thought to be mainly derived from the EV-mediated delivery of microRNAs with positive impact on bone formation [315–317]. Human MSCs-derived exosomes retrieved from different stages of stem cells osteogenic differentiation were capable of committing homotypic cells into the same fate; nonetheless, only exosomes from late osteogenic differentiation timepoints were capable of inducing ECM mineralization [318]. microRNA profiling of exosomes from different stages of MSCs osteogenic differentiation showed different patterns, which was partially correlated with the observed findings.
The design of bioinstructive materials capable of surpassing regulatory issues and allow cost-effective and safe drug administration, or even the localized recruitment of endogenous pro-regenerative factors is currently one of the most widely spread trends for bone regeneration therapies development [319]. For example, biomaterial-based synthetic approaches capable of mimicking the microRNA delivery function of EVs are gaining momentum [320, 321]. The integration of the know-how of ECM properties capable of withstanding highly effective growth factor sequestration from the implantation medium with effective mechanisms for delivery of in vitro-generated cargo-loaded vesicles may represent an elegant way to mimic biological functions of the regenerating bone tissue.
5.7. Cells-ECM interactions in bone
5.7.1. Cell-ECM natural interaction in native bone
The ECM is a complex network comprising proteins (soluble and insoluble), growth factors and polysaccharides. It provides physical structure and a biochemical context to the cellular microenvironment [322]. In body tissues, the communication amongst cells and the surrounding ECM is mainly made through three types of proteins: integrins, selectins and immunoglobulin [323]. This adhesion contributes to cell biological processes as immune response, metastases, inflammatory process, division and death of cells, tumor progression and cell polarity. An extensive 3D imaging map of the localization of bone cells, ECM proteins and other molecules as well as potential interactions between them in whole mouse femurs is available at Coutu et al. [324]. Figure 2b shows a schematic of the various interactions that occur in the ECM. The bone matrix is mainly composed of collagen (85-90%) and other types of proteins. The ECM has two mechanisms by which it affects cellular behavior: (i) by the direct interaction with cells, and (ii) by harboring growth factors for cell proliferation and differentiation; biomimetic biomaterials designed to mimic such ECM features are addressed in Sections 5.5. (approach (i)) and 5.6.2 (approach (ii)).
The connection between cells and ECM - case (i) - is made through proteins existing on cell surface, integrins, which regulate not only the cell-to-physical matrix adhesion but are also responsible for some intracellular signals [325]. These proteins recognize specific peptide sequences and bind to specific peptide domains by the presence of two distinct subunits: α and β. The binding of the ligand to this intramembranous protein is dependent on the association of these two subunits, making it possible for only one integrin to recognize and connect to specific types of ECM proteins [326]. A Review by Shekaran et al. [327] focused on the role of different full proteins and peptide domains, as well as cellular interacting integrins, in bone tissue and repair. Recently, a study reported that in an initial stage, fibroblasts α5β1 integrins connected to fibronectin are able to sense mechanical load and activate adhesion-related pathways in less than a second to reinforce adhesion [328]. It is still unknown whether this phenomenon also occurs for bone cells or even stem cells. The elucidation of this effect on a wider range of cells may give further insight on mechanosensing aspects, which may drive cell fate modulation through rapid-acting cell-biomaterials interactions.
The understanding of the role of ECM on bone regeneration has been addressed through combinatorial studies, in which different features of ECM (e.g. biophysical and biochemical aspects) are varied and studied in different proportions. For example, Huang et al. [329] studied the combined effect of mechanical factors - i.e. ECM stiffness - and the presence of ECM insoluble proteins on the osteogenic differentiation of MSCs, cultured as 2D monolayers, in basal medium. The tested ECM cell-binding proteins - type I collagen, fibronectin, vitronectin and laminin - induced the in vitro osteogenic differentiation of MSCs, implying that the right ECM composition is enough to trigger the process. This study also showed that, although type I collagen is the main protein in bone ECM, no difference was observed in its capacity to induce more osteogenic differentiation than the others tested proteins. In fact, fibronectin showed more ability to drive osteogenic differentiation, followed by laminin, type I collagen, and vitronectin. The authors also verified that mechanical stretching of the cells improved differentiation [329]. Another in vitro study by Mathews et al. [330] showed that type I collagen and laminin were the most successful ECM proteins in inducing the proliferation and adhesion of MSCs, and a high percentage of MSCs differentiation occurred by contact with fibronectin, vitronectin and type I collagen. Combinations of adhesive ECM proteins – fibronectin, laminin, osteocalcin – mixed with methacrylate gelatin hydrogels were suggested by Dolatshahi-Pirouz et al. [331] as a way to study the combinatorial role of ECM proteins and soluble factors in BMMSCs, using a high-throughput strategy; mixtures of proteins in the presence of bone-inducing cytokines, resembling the close-to-native complexity of ECM, led to higher osteogenic differentiation of MSCs. Gothard et al. [332] studied the in vivo effect of adding growth factors and osteoinductive soluble molecules to an alginate/demineralized bone ECM hydrogel. All formulations, even the ones excluding growth factors or soluble factors, induced bone formation in rats. The authors hypothesized that this behavior may be related to reminiscent amounts of cytokines in the demineralized bone ECM used to synthetize the hydrogels. The denaturation and fragmentation of the cytokines through UV-irradiation of hydrogels suggested that, indeed, that could be the explanation for the observed general bone formation. Despite the efforts to correlate different biomaterials processed from proteins of the bone or enamel (as it is the other mineralized tissue in the human body) [333], type I collagen and fibronectin remain the two most commonly reported proteins in tissue engineering and osseointegration approaches. Many other proteins integrate bone structure in smaller fractions than type I collagen, but still present important roles in bone physiology. Table 2 focuses on the description of the role of several bone proteins, namely the ones presented in smaller percentages, and whether they have been used in tissue regeneration therapies.
Table 2. Examples of ECM proteins involved in bone and other mineralized tissues (e.g. enamel) formation and regeneration, and their use in tissue engineering systems. Locations in mineralized organs - bone and teeth - are indicated in bold.
| Protein | Physiological localization | Biological function | Ligation site | Bone tissue engineering systems and osseointegration |
|---|---|---|---|---|
| Ameloblastin |
|
|||
| Fibronectin |
|
|||
| Laminin |
|
|
||
| Vitronectin |
|
|
||
| Type I collagen |
|
|
||
| Type IV collagen |
|
|
||
| Type X collagen |
|
|
||
| Osteonectin (SPARC) | ||||
| Osteocalcin |
|
|||
| Biglycan | ||||
| Bone sialoprotein (BSP) |
|
|
||
| Osteopontin (Secreted phosphoprotein 1; OPN) |
|
|||
| Decorin | ||||
| Thrombospondin (type I and type II) |
|
|
|
|
| Tenascin C |
|
|||
| Periostin |
|
|||
| Dentin matrix acidic phosphoprotein 1 (DMP1) |
|
|
||
| Type III collagen | ||||
| Versican |
|
|
||
| TGF-β receptor interacting protein-1 (TRIP-1) |
|
|
|
Despite the undebatable importance of controlling protein composition for the synthesis of bioinspired biomaterials aimed at regulating cell adhesion and function, the immobilization of full proteins onto biomaterials is not strictly necessary to promote integrin-binding events. Recombinant protein fragments and short polypeptides with protein-specific cell binding domains are valid alternatives to achieve cell membrane exposure to those molecules, while enhancing bioactivity and facilitating their incorporation into biomaterials. Aspects that may hamper protein function and efficient binding, including correct protein rearrangement and subsequent exposure of bioactive domains to reach cell membrane, may be surpassed with the use of fragmented proteins peptide domains. The conformation of full-length proteins and their presentation to cells is dictated by factors as biomaterial substrate chemistry. While it is interesting to verify proteins proneness to re-arrange into different configurations in response to substrate chemistry, and although it can be used as a versatile modulator of cell response, it is challenging to predict the behavior of ECM proteins adsorbed into novel or unstudied biomaterials. Keselowsky et al. [334] showed that human fibronectin, at a density of 40 ng/cm2 adsorbed to alkanethiols self-assembled monolayers with highly controlled chemical features, including CH3, OH, COOH and NH2 fixed densities, led to distinct cellular recognition of immature osteoblast-like cells (MC3T3-E1 cell line) through integrin binding mechanisms. The binding of soluble integrins to each substrate after fibronectin adsorption was characterized, and major differences were observed: OH and NH2 substrates led to high amounts of bound α5v1 integrin; COOH surfaces enhanced α5v1 and αvβ3 integrins binding; and CH3 did not promote the binding of any of the integrins. These phenomena led to distinctive modulation of focal adhesions composition and respective signaling provided by cells from each substrate, resulting in distinctive osteogenic differentiation potencies [335]. Substrates with OH and NH2 groups led immature osteoblasts to increased gene expression of osteogenic markers including ALP, bone sialoprotein and osteocalcin, and promoted the deposition of higher amounts of mineralized matrix [335]. The use of anti-fibronectin antibodies to impair integrin/cell binding to fibronectin showed that cell response -in particular mineralization-, was indeed tailored by protein configuration in each chemically distinct substrate.
Although it is well accepted that the incorporation of either full proteins or short peptide sequences into biomaterials as integrin binders are promising strategies to modulate cell response aspects that include osteogenesis [336], a tight control over the spatial distribution of integrin-recognizing ECM domains on biomaterials surfaces has proven to be pivotal to tailor a plethora of cellular phenomena like adhesion, proliferation, migration and multilineage differentiation [337]. Tethering/spacing of ECM proteins on biomaterials surfaces was reported to modulate specific cellular aspects with major influence on bone repair, including control of stem cells fate [338] and endothelial cell spreading and proliferation [339].
Integrin clustering is the key aspect in the determination of cellular adhesion strength to materials and modulation of integrin interaction with plasma membranes proteins, which include talin and vinculin, reported to dictate actin contractility and focal adhesion stabilization [340, 341]. These are key aspects in cellular mechanosensing and response modulation, which are reviewed in Section 6. A recent and complete review addressing the importance of integrin clustering on biomaterials design and cell response modulation can be found in Reference [337]. Interestingly, dramatic differences are known to occur in integrin clustering on 2D substrates and in ECM-resembling 3D materials [342]. In 2D substrates, integrins organize and gather in > 1 μm clusters identified as focal adhesions [343]; in 3D matrices, these structures are often much smaller and show shorter lifetimes [344]. Despite such disparity, 2D biomaterial models have been the most widely used source to understand fundamental aspects of protein/peptides spacing and their effects on cell response. Nonetheless, and despite the difficulty on controlling bioactive domains precise positioning in 3D matrices, a remarkable insight using 2D vs 3D modelling was provided by Lepzelter et al. [342] based on Monte Carlo membrane fluctuation simulations [345].
In tissue regeneration and biomaterial design strategies, ECM composition is often discussed based on direct cell-protein interactions phenomena, or on the ability of ECM structure to bind/recruit organic agents able to interact directly with cells (e.g. growth factors). The role of ECM proteins and specific peptide domains in the formation of inorganic deposits, which are crucial for bone formation, is a field of study that has not received as much attention as direct cells-organic structures contact. Organic components of the ECM are recognized as essential for in vivo recruitment and deposition of high quality minerals, as well as for their stabilization, orientation and growth, in processes independent from the direct action of cells [439–441]. A recent study reports the ability of decellularized native ECMs, such as the one from the periosteum, on the promotion of the nucleation of calcium phosphates and bone-like crystals [442]. Despite the raising evidence that decellularized ECM can be used to promote biomineralization upon implantation, few recent studies address this aspect. Moreover, systematic studies focusing on the design of highly controlled protein (or other ECM components)-based compositions and their effect on bone-mimetic apatite deposition are still in need. An extension of arrays/biomaterial libraries developed in a wide range of studies focused on cellular response control could be an interesting approach to perform a rapid assessment of the biomineralization-induction potential of different biomaterial compositions. A detailed description of the formation of mineralized components of bone is out of the scope of this Review. A systematic revision of bioinspired mineralization occurring in organic ECM frameworks can be found in a complete Review publication by Benesch et al. [439] from 2008, in which the role of individual ECM proteins and peptide domains was systematically described.
6. Bone mechanobiology: the role of externally applied forces and ECM matrix mechanical/viscoelastic properties
6.1. The native mechanical environment of bone
Mechanical and physical signals that occur through walking, running and other types of movements have a crucial role in the induction of osteogenesis, as well as in the maintenance of healthy bone [443]. Interesting observations have been reported during the last years, which correlate the “Mechanostat Theory” with biochemical signaling occurring during bone homeostasis. The “Mechanostat Theory”, suggested by Harold Frost in the 1890’s [443], correlates bone growth and loss with local elastic deformation (in the form of compression and elongation), which occurs in a life-long regime, due to peak forces exerted by surrounding muscles [444]. Tyrovola and Odont [445] reviewed several studies, in which compressive/tensile deformations were applied to bone and periodontal ligament tissues, and in which a correlation between the observed behavior and the OPG/RANKL/RANK system was established. An example that shows the correlation of the “Mechanostat Theory” and the OPG/RANKL/RANK bone remodeling system is the one occurring in the tooth/periodontal ligament interface. The compression of tooth, during orthodontic movement, led to the increase of RANKL concentration [446, 447], promoting osteoclast formation. The tensile stretching applied to the periodontal ligament promoted the increase of osteoblasts OPG concentration in a magnitude-dependent manner [447], while inducing a simultaneous RANKL concentration decrease it. The relative concentrations of OPG and RANKL on both tensioned and compressed sides of tooth regulate local bone modeling, remodeling and root resorption.
Other physical and mechanical factors that influence bone health are drag force and shear stress. Shear stress occurs in bone on the unmineralized matrix around the osteocytes that forms canals by which the interstitial fluid passes, creating a force along the surface, on a parallel fashion [448]. A consequence of bone deformation is the generation of interstitial flow on osteocytes, creating a drag phenomenon on the fibers that connect cells [449–452]. Healthy bone remodels in response to mechanical stresses: in the absence of loading, bone resorption is increased, while in the presence of flow perfusion through the movement of extracellular fluid radially toward the bone cortex [453] bone is known to remodel. Subsequent studies focusing on the role of perfusion on primary bone cells and stem cells behavior have shown increased mineralized matrix deposition in a dose-dependent manner [448, 453]. Weinbaum et al. [450] suggested a mathematical model to explain how bone cells detect mechanical loading, and how flow behaves through the pericellular matrix surrounding an osteocyte process in its canaliculus. Despite the small deformations predicted by the model, and the small dimensions of the pericellular annulus (typically 0.1 μm), the flow shear stress on the membranes of the osteocyte processes was roughly the same as for the vascular endothelium in capillaries. Still little is known about the role of perfusion shear stress and interstitial flow in bone biology [451, 454]. However, it is known that osteocytes are the main mechanosensing cells in bone and that, upon exposure to fluid flow, they stimulate osteoblasts, thus producing more bone tissue and prostaglandins, which are responsible for the activity of osteoblasts and osteoclasts [171]. Early studies focused on unravelling the effect of hydrostatic pressure and substrate stretching on osteocytes behavior [455]. However, flow-induced shear stress has shown to affect osteocytes in a more relevant manner, as compared to osteoblasts [156]. An extensive list of biological phenomena, that has been increasing in the last years, has shown that osteocytes respond to shear stress by releasing nitric oxide (NO), adenosine triphosphate (ATP) and prostaglandins. Moreover, gap junctions and hemichannels are open, and several signaling pathways (e.g. Wnt/β-catenin, protein kinase A (PKA)) are initiated after shear stress induction. The mechanisms for load sensing in osteocytes are thought to depend on the dendritic process, or bending of cilia [171]. Glycocalyces on the surfaces of dendritic processes have been shown to be related with osteocytes mechanosensing; however, on the cell body, different mechanosensing mechanism are known to be active [171]. The TGF-β superfamily - which includes BMPs, activins, and growth differentiation factors (GDFs) - has been suggested as one of the most important mediators of cellular response to physical cues via a feedback loop mechanism, reviewed by Wu et al. [44].
The role of paralogous transcriptional factors Yes-associated protein (YAP) and PDZ-binding motif (TAZ) in osteogenesis are described since 2004 and 2005, respectively. Overtime, contradictory roles of each factor were reported regarding osteogenic differentiation of stem cells. Recently, their role as combinatorial promoters of bone development was reported [456–458]. Upon deletion of YAP/TAZ from skeletal lineage cells, osteogenesis-imperfecta like-phenotypes were generated, and bone properties were reduced through lower collagen content and organization. Homozygous TAZ deletion led to spontaneous fractures on mice, while dual (YAP/TAZ) deletion caused neonatal lethality. Dual deletion led reduced osteoblast activity and increased osteoclast activity, negatively affecting bone synthesis and remodeling.
YAP/TAZ is known to play a crucial role in mechanotransduction phenomena, working as sensors for mechanical cues, with relevant application on growth factors-free approaches for mesenchymal stem cells differentiation, mainly driven through the modulation of ECM (or biomaterials) rigidity. Their role as nuclear relays of mechanical signaling exerted by ECM stiffness and induced cell shape has been associated with Rho GTPase acitivity and tension of the actomyosin of the cytoskeleton [459]. Besides the direct effect of matrix stiffness on cellular mechanosensing via YAP/TAZ, MSCs were shown to be mechanically regulated by shear stress applied to tissues through shear flow; the application of shear stress to MSCs showed improved osteogenic differentiation in a Rho-ROCK dependent manner. [460–462] Important aspects as bone loss related to microgravity conditions experienced during space flights has also been correlated with poor TAZ nuclear accumulation, leading to low osteogenesis [463].
In 2016, two zinc finger repressors – Snail and Slug -, best known for their participation in epithelial-to-mesenchymal transition mechanisms, were described to perform binding interactions with YAP/TAZ, forming complexes, activating YAP/TAZ/TEAS and Runx2 downstream targets that control stem cell osteogenesis [464]. Knockout mouse models targeting Snail, Slug or both combined allowed unraveling a biological mechanism in which both transcription factors cooperatively control stem cell self-renewal, osteogenic differentiation and bone formation [465].
6.2. Modulation of regenerative systems: engineering mechanosensing targeting osteogenic differentiation and bone growth
Biomaterials with different mechanical properties have triggered the differentiation of MSCs into different lineages (Figure 3). The directing effect of microenvironments’ mechanical features on stem cell differentiation were first reported by Engler et al. [466]. Collagen-coated 2D polyacrylamide gels impacted on the fate determination of BMMSCs precisely accordingly to their stiffness. Gels with elastic modulus similar to native adult or developmental tissues directed cells into the neurogenic, myogenic or osteogenic lineages, in a nonmuscle myosin II-dependent pathway. Type I collagen and collagen-coated 2D gels were also applied to study the adipogenic, chondrogenic and smooth cell differentiation of BMMSCs [467], as well as osteogenic differentiation [468]. MSCs retrieved from alternative sources including adipogenic, cardiac and mammary tissue responded to stiffness cues of flat substrates, altering their phenotype to adipocytes, endothelial cells, and epithelial cells, respectively [469–471]. ESCs have also been differentiated into the pancreatic, mesodermal and osteogenic lineages using hyaluronic acid and type I collagen substrates through single mechanotransduction mechanisms [472–474]. Aspects beyond substrates’ modulus, including their presentation to cells and spatial organization were addressed by Yang et al. [475], who designed biomaterials with stiff and soft regions, presented in ordered or random designs. BMMSCs showed higher adhesion and spreading on substrates with higher percentages of stiff regions, in a dose-dependent manner. However, when stiff regions were distributed on the surface on a randomized manner, cells showed lower levels of YAP activation, and their phenotype changed to smaller and rounded. The apparent actin disruption caused by random patterns led to lower ALP expression and higher CD105 (stemness marker) expression.
Figure 3.
(a) Schematic representation of the bone cell differentiation process, generating from mesenchymal and hematopoietic stem cells. Osteoblasts descend from MSCs, which firstly differentiate into pre-osteoblasts. Osteoblasts proliferate and align in the surface of the bone while others undergo maturation into the osteocyte phenotype. HSCs differentiate into pre-osteoclasts, which become multinucleated, and finally originate mature osteoclasts responsible for bone resorption. Factors produced or expressed by different cells present in the bone niche are presented aside each cell type schematic representation. (b) A variety of factors constitute the bone extracellular environment. Biological, physical and topographical features compose a specific microenvironment capable of guiding cells into predetermined phenotypes and function. Cells interact with ECM through receptors and other proteins localized at the surface.
An extremely limited number of physiological phenomena occur in strictly 2D processes, which include, for example, the deposition of osteoblasts on osteoid matrix. However, the emulation of regeneration processes in most human tissues requires the replication of a 3D environment to induce cues presented by native ECM. The establishment of reliable correlations between distinctive mechanical properties of hydrogels and consequent differentiation lineages of cells encapsulated in the 3D environment is a demanding task. Stiffness variation in hydrogels are frequently obtained by varying the extension of crosslinking mechanisms, which often alters physical and chemical aspects of hydrogels that may include (i) exposure to chemically (un)reacted groups; (ii) availability of cell adhesion motifs to surface membrane integrins; (iii) porosity; (iv) pore size; (v) water content; and (vi) viscoelasticity [457–461]. While surface chemistry of hydrogels studied as 2D substrates for stem cell culture could be easily homogenized using, most commonly, type I collagen coatings on the cell-exposed part of the hydrogel [481, 482], the achievement of fully comparable 3D systems that unequivocally allow to isolate the “stiffness” variable are challenging to prepare. Wen et al. [480] partially addressed this question by modulating several aspects of hydrogel design hypothesized to function as a source of bias to 2D collagen-coated polyacrylamide systems. Without any alteration of stiffness values, hydrogels with varying porosity did not alter protein tethering on the biomaterials’ surface, and both ASCs and BMMSCs fate onto the adipogenic and osteogenic lineages, which remained exclusively dependent on the substrates’ elastic modulus. A comprehensive list of 2D and 3D approaches used to study stem cells morphological re-organization and differentiations induced by mechanical properties can be found in Reference [483].
The encapsulation of BMMSCs in alginate hydrogels modified with the integrin-binding peptide RGD showed that hydrogels with elastic modulus ranging from 2.5 to 5 kPa led cells preferentially into the adipogenic lineage, while stiffer hydrogels (11 – 30 kPa) induced osteogenesis [477]. ASCs encapsulated in a bacterial origin polymer – gellan gum (modified with methacrylic groups) – also underwent soluble factors-free osteogenic induction by simple encapsulation in ~50 kPa hydrogels [484]. Interestingly, in both studies the observation of the osteogenic phenotype was not dependent on cell spreading onto the hydrogels [477, 484]. In contrast to primordial studies in 2D substrates, that reported a correlation between cell fate and morphology, in 3D structures cells are able to go through osteogenesis through integrin binding and nanometric rearrangement of adhesion ligands [477]. Other studies targeting stem cells osteogenesis on 3D biomaterials focused on the maintenance of microstructure with variation of mechanical properties through the incorporation of mineralized structures on the surface of 3D porous scaffolds [485], and on the supplementation of fibrin gels with sodium chloride to enhance hydrogels’ mechanical properties and drive osteogenic response [486]. The osteogenic response on poly(ethylene glycol) hydrogels modified with the RGD peptide domain was proved to be integrin dependent [487]. Interestingly, and in contrast with data reported for 2D biomaterials-driven osteogenesis via mechanotransduction pathways, MSCs differentiation did not involve actin filaments and microtubules associated with myosin contractility, neither ROCK activity [487], suggesting that in specific 3D biomaterials formulations osteogenesis mediation may be regulated by different pathways than the ones observed in 2D cultures.
Mechanical transduction targeting bone regeneration has not been limited to materials stiffness. Indeed, the use of 2D-to-3D biomaterials with nanopattern cues has proven to be efficient on driving osteogenesis of stem cells and osteoprogenitor cells in the absence of any soluble cues or substrate chemistry [488–497]. This response is often associated to cytoskeleton organization and generation of large focal adhesions, which has been correlated with possible direct mechanotransduction pathways [498]. A thorough Systematic Review focusing on the osteogenic potential of several nanotopographies, with focus on aspects as size, shape, anisotropy/level of organization of the patterns [499] allowed retrieving some interesting information about osteogenesis-inductive topographic cues: nanopillar features with heights lower than 20 nm and disordered nanopits seem to systematically increase osteogenic differentiation of stem cells. Nonetheless, a careful analysis and studies correlating results obtained with the same materials is necessary to harness the field.
Most studies on mechanotransduction targeting stem cells differentiation focus on the single property of hydrogels’ elastic modulus. Often, purely elastic materials are used as substrate, or the viscoelasticity of the matrixes is not considered or discussed. However, an important aspect of physiological ECMs is their viscoelasticity [500], which leads to stress relaxation that stimulate ECM remodeling through cellular forces. In 2011, Cameron et al. [501] addressed the effect of biomaterials’ creep behavior on MSCs morphology, proliferation and differentiation fate. 2D polyacrylamide materials with constant ~4.7 kPa storage modulus (elastic component) and increasing loss modulus (viscous component) led increased MSCs spread area and proliferation but drove a decrease on focal adhesions size and maturity, which was hypothesized to be related to a creep-mediated loss in cell cytoskeletal tension. ALP activity on MSCs seeded onto higher loss modulus gels was increased, suggesting that the modulation of this factor can trigger osteogenesis, even in soft hydrogels. Chaudhuri et al. [502] investigated the role of stress relaxation on cell spreading on substrates with equal elastic modulus. Fibroblast and osteosarcoma cell lines responded to substrates’ relaxation properties by spreading onto soft materials with high stress relaxation, on a similar manner to cells cultured on stiff materials. Later on, the same team investigated the role of these properties on MSCs differentiation on 3D hydrogels [503]. Alginate hydrogels modified with RGD domains were tuned to show equal elastic modulus, adhesive peptide modification (in two different levels) and degradability, while altering their relaxation times from 70 to 3300 seconds. MSCs adipogenic and osteogenic differentiation were modulated on combined effects of matrix elastic modulus and stress relaxation properties, with 17 kPa hydrogels with fast relaxation properties providing the most advantageous condition for osteogenesis.
The possibility of modulating ECM-cells interactions and consequent cell response through the local modulation of mechanical cues administered to cells is a powerful tool to design highly effective biomaterials independent of recombinant costly growth factors or drugs with possible side effects. While the effect of native ECM and ECM-mimetic biomaterials is well established in the literature, other overlooked aspects of mechanics are now reaching the realms of “biomechanics” and “biophysics”. Stress relaxation is currently under deep scrutinization as a modulator of stem cells differentiation, and aspects as 4D/spatiotemporal modulation of biomaterials properties are now addressed as enablers of ECM remodeling mechanisms [504, 505]. The design of materials with on-demand changing compositions is poorly described. However, their potential as sequential modulators of cell behavior open the possibility of establishing extremely complex cell response patterns and co-cultures with potential high impact in the achievement of biocompatible, bioinstructive and nature-mimetic regenerative systems.
7. Conclusion and future directions
The field of bone regeneration has benefited from the progressive elucidation of anatomical and physiological aspects of native human tissue. Natural occurring bone precursors and also adult tissues’ morphology and composition have inspired therapies targeting bone damage. Some of the most interesting and promising approaches have allowed emulating bone development phenomena by the control of biochemical signaling through the mimicking of endochondral ossification pathway, or by giving biophysical cues to stem cells that resemble the mechanical properties of the unmineralized precursor of bone (the osteoid).
Holistic approaches combining multiscale features and properties have demonstrated high potential for the design of new biomaterials and scaffolds based on bone’s anatomy [506]. Top-down and bottom-up strategies have been integrated, enabling an increase in the complexity of designed materials, and allowing a full-scale control of the scaffolds’ properties. Independently of the strategies used to manufacture implantable biomaterials, well-accepted crucial features must be considered, namely the adequacy of their mechanical properties to the site of implantation, adequate triggering of immune response, and the type and spatial-temporal distribution of constructs’ properties. A strict control over these aspects is a powerful tool to modulate cell fate, guiding cellular behavior and response in vitro and in vivo (e.g. cell adhesion, proliferation, viability, differentiation, matrix production).
Recently, attention has been directed to other aspects of bone biology and their understanding as a source of valid know-how to design novel regenerative approaches. In particular, control over the insoluble fraction of bone - comprising ECM proteins and glycoproteins, as well as cellular structures, and even immobilized growth factors aimed at interacting with cellular membranes – has been identified as a strategic way to direct stem cell fate and promote bone regeneration. In particular, and despite their ubiquitous presence in human ECMs, studies targeting the use of glycans in biomaterials as tools to modulate cell behavior are still rare [507].
Although several co-cultures between resident bone cells and stem cells have played an important role on fundamental studies, their application on biomaterials models as in situ cell delivery systems to promote bone repair is still scarce. Tailoring of inflammation and regeneration-associated aspects are also under rapid exploitation, mainly using macrophages polarization techniques. Nonetheless, there is a need for the elucidation of such co-cultures and for the design of simple yet effective ways to apply these concepts into the clinics. The role of ECM proteins and their interaction with soluble factors present in the bone injury environment are another branch of biomaterials design that we here identify as still underdeveloped. The identification of the principal components affecting bone regeneration may allow processing cost-effective biomaterials with ECM-mimetic features using advanced biotechnological approaches, independent on the use of costly recombinant technologies.
Certainly, the wider understanding, characterization and application of soluble factors (e.g. growth factors) and nanostructures structures containing soluble cues (e.g. EVs) produced by cells themselves are strong trends that show a high impact as versatile tools to direct cell differentiation. In particular for the case of EVs, their application as off-the-shelf naturally produced nanoparticles integrated into biomaterial matrices may represent a valuable tool in future regeneration techniques.
Externally applied stimulation happens in bone in individuals’ everyday life and are known to be crucial for bone remodeling. Recently, normal fetal movement has been highlighted as crucial for the development of high quality newborns’ skeletal growth [508], and mechanical forces in utero were suggested as possibly impactful on the long-term. While some works have focused on the use of bioreactors to mimic compression/elongation and interstitial flow that occur in native bone, with improved outcomes concerning MSCs differentiation into the osteogenic lineage [509, 510] (Figure 4), there is still a long way to pave in the emulation of all relevant mechanical physiological stimulation. A recent study focused on the combinatorial assessment of 3D biomaterial matrices stimulation along with different compression pressures [511]. More studies enabling the unraveling of ECM protein composition, cellular co-cultures setups and physiologically-occurring mechanical stimulation are needed to lift this field from a mostly unifactorial trend to a multifactorial platform (Figure 5). High-throughput screening technologies adapted specifically to tissue regeneration needs, along with the creation of directed and effective high-content analysis methods are promising routes to unravel compositional, interactional and external factors-associated with bone biology. A complete emulation of bone structure and function will be a costly, time consuming and difficult road. On its turn, the identification of key factors leading desirable cell response and implant integration in the wounded tissues would allow the “just-enough” complex design of effective tissue regeneration strategies, enabling the easier selection of components to fit the marketplace using safe, easily regulated and cost-effective strategies.
Figure 4.
Representation of reciprocal interactions between osteoblasts and osteocytes. Osteoblasts (and osteocytes) release RANKL, which binds to hematopoietic stem cells, giving rise to their differentiation into osteoclasts. Ikebuchi et al. [170] proved that osteoclasts are capable of modulating osteoblasts’ ability to form new bone through the release of extracellular vesicles (EVs) that contain RANK on their surface (i). The vesicles migrate to osteoblasts’ surface (ii) leading to the binding of vesicular RANK to RANKL present at osteoblasts’ surface, and directing osteoblast to form new bone (iii). The image was adapted from an original scheme by Zaidi et al. [515].
Figure 5.
(a) Bone mechanical microenvironment is mediated by integrin-mediated binding of bone cells to the ECM. Several pathways are described in the bone healing and homeostasis process, which include integrin clustering in the presence of stiff substrates, leading the activation of focal adhesion kinases (FAKs), which later drives the activation of the YAP/TAZ pathway. Focal adhesions also activate the RHO GTPases, which favor F-actin polymerization through the activation of RHOassociated protein kinase (ROCK) [458]. The Snail/Slug pathway is also known to occur during bone formation [464]. On biomaterials, most of these pathways (with exception to Snail/Slug) have been reported to occur in, for example, MSCs culture on 2D substrates. However, stem cells osteogenic differentiation on specific 3D setups were independent from these well-known mechanisms [487]. So far, the mechanisms driving osteogenic differentiation in 3D matrices in vitro require further exploitation towards full understanding. Interestingly, not only stiffness has been addressed as a modulator of cell response towards the osteogenic commitment. Other properties including viscoelasticity (e.g. stress relaxation) and 4D spatiotemporal degradation or stiffening have been suggested as modulators of stem cells commitment [512]. The variation of physical aspects may impact the measured properties of biomaterials overtime. In the grey circle, continuous green arrow indicates the direct impact of the variation of one factor on other overtime; discontinuous green arrows show properties that will probably influence others. (b) In 2006, the ability of 2D hydrogels’ stiffness to solely direct BMMSCs multilineage differentiation was proven for the first time [466] - example (i); stress relaxation on biomaterials with constant elastic modulus is another factor capable of directing higher production of osteogenesis-related markers by MSCs, including ALP, type I collagen and phosphate deposition (stained by von Kossa) [503] - example (ii); hydrogels with varying properties overtime showed the ability to increase the production of ALP by MSCs - example (iii) [513]. Figure 4(b) was adapted from References [466, 503, 513].
Figure 6.
(a) Engineered devices, combining biomaterials and external stimulus allow mimicking the in vivo-occurring stimuli. Different types of bioreactors allow stimulating cells in distinct manners by mimicking the fluid shear stress through a perfusion flow method and the strain caused by compression. Figure 5(a) was produced using Servier Medical Art. (b) Perfusion flow was successfully applied for the re-cellularization of 3D scaffolds targeting facial bone reconstruction in a porcine model [514]. Indeed, such perfusion flow has proven to be effective on the homogeneous (re)population of large biomaterial and/or decellularized ECMs structures with cells of interest. The acquisition of bone defect morphology and dimensions was performed by microcomputerized tomography (μCT). Decellularized bovine bone was machined to present the exact shape of the defect, and later filled with autologous porcine ASCs, which were cultured in the 3D scaffold under perfusion flow, and later implanted in the bone defect, rendering full wound regeneration. Figure 5(b) is adapted from Reference [514].
Figure 7.
The treatment of bone injuries may benefit from the deconstruction of the native tissue niche and on the application of concepts learnt from basic physiology to the design of efficient regenerative therapies. Although simplistic approaches based on the variation of single factors may be easier to regulate and produce with high fidelity as industrialized systems, bone’s intricate multicellular healing and homeostasis processes – characterized by fine immunological spatiotemporal coordination and unique vascular and mechanical environment – suggest that the combination of specific transversal aspects of bone physiology may hide the cue for more effective, rapid and high-quality bone formation.
Acknowledgements
This work was supported by the European Research Council grant agreement ERC-2014-ADG-669858 (project ATLAS), and developed within the scope of the project CICECO-Aveiro Institute of Materials, POCI-01-0145-FEDER-007679 (FCT Ref. UID /CTM /50011/2013), financed by national funds through the FCT/MEC and when appropriate co-financed by FEDER under the PT2020 Partnership Agreement. C. Martins-Cruz acknowledges the grant BI/U189/7570/2016. M. B. Oliveira acknowledges the support from Portuguese Foundation for Science and Technology − FCT (grant SFRH/BPD/111354/2015). Figure 2 and Figure 5 were produced using templates from Servier Medical Art.
References
- [1].Clarke B. Normal bone anatomy and physiology. Clinical journal of the American Society of Nephrology : CJASN. 2008;3(Suppl 3):S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Quarto R, Giannoni P. Bone Tissue Engineering: Past-Present-Future. Methods in molecular biology (Clifton, NJ) 2016;1416:21–33. doi: 10.1007/978-1-4939-3584-0_2. [DOI] [PubMed] [Google Scholar]
- [3].Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden: A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Archives of Osteoporosis. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu X, Tang X, Gohil SV, Laurencin CT. Biomaterials for Bone Regenerative Engineering. Advanced healthcare materials. 2015;4:1268–85. doi: 10.1002/adhm.201400760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oliveira MB, Mano JF. High-throughput screening for integrative biomaterials design: exploring advances and new trends. Trends in biotechnology. 2014;32:627–36. doi: 10.1016/j.tibtech.2014.09.009. [DOI] [PubMed] [Google Scholar]
- [6].Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, McNamara LM. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. European cells & materials. 2012;23:13–27. doi: 10.22203/ecm.v023a02. [DOI] [PubMed] [Google Scholar]
- [7].Jeon OH, Panicker LM, Lu Q, Chae JJ, Feldman RA, Elisseeff JH. Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Scientific Reports. 2016;6:26761. doi: 10.1038/srep26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hou L, Coller J, Natu V, Hastie TJ, Huang NF. Combinatorial extracellular matrix microenvironments promote survival and phenotype of human induced pluripotent stem cell-derived endothelial cells in hypoxia. Acta Biomater. 2016;44:188–99. doi: 10.1016/j.actbio.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cosgrove BD, Mui KL, Driscoll TP, Caliari SR, Mehta KD, Assoian RK, et al. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat Mater. 2016;15:1297–306. doi: 10.1038/nmat4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen G, Dong C, Yang L, Lv Y. 3D Scaffolds with Different Stiffness but the Same Microstructure for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2015;7:15790–802. doi: 10.1021/acsami.5b02662. [DOI] [PubMed] [Google Scholar]
- [11].Leite ÁJ, Oliveira MB, Caridade SG, Mano JF. Screening of Nanocomposite Scaffolds Arrays Using Superhydrophobic-Wettable Micropatterns. Advanced Functional Materials. 2017;27:1701219–n/a. [Google Scholar]
- [12].Castro E, Mano JF. Magnetic Force-Based Tissue Engineering and Regenerative Medicine. Journal of Biomedical Nanotechnology. 2013;9:1129–36. doi: 10.1166/jbn.2013.1635. [DOI] [PubMed] [Google Scholar]
- [13].Yin T, Li L. The stem cell niches in bone. Journal of Clinical Investigation. 2006;116:1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. The Journal of craniofacial surgery. 1990;1:60–8. doi: 10.1097/00001665-199001000-00011. [DOI] [PubMed] [Google Scholar]
- [15].Pelled G, Ben-Arav A, Hock C, Reynolds DG, Yazici C, Zilberman Y, et al. Direct Gene Therapy for Bone Regeneration: Gene Delivery, Animal Models, and Outcome Measures. Tissue engineering Part B, Reviews. 2010;16:13–20. doi: 10.1089/ten.teb.2009.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kimelman N, Pelled G, Helm GA, Huard J, Schwarz EM, Gazit D. Review: gene- and stem cell-based therapeutics for bone regeneration and repair. Tissue engineering. 2007;13:1135–50. doi: 10.1089/ten.2007.0096. [DOI] [PubMed] [Google Scholar]
- [17].Buckwalter JA. Effects of early motion on healing of musculoskeletal tissues. Hand clinics. 1996;12:13–24. [PubMed] [Google Scholar]
- [18].Bueno EM, Glowacki J. Cell-free and cell-based approaches for bone regeneration. Nature reviews Rheumatology. 2009;5:685–97. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- [19].Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon's point of view. Journal of cellular and molecular medicine. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O’Doherty E, et al. Engineered vascularized bone grafts. Proceedings of the National Academy of Sciences. 2010;107:3311. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends in biotechnology. 2012;30:546–54. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Szpalski C, Barr J, Wetterau M, Saadeh PB, Warren SM. Cranial bone defects: current and future strategies. Neurosurgical focus. 2010;29:E8. doi: 10.3171/2010.9.FOCUS10201. [DOI] [PubMed] [Google Scholar]
- [23].Standring S. Gray's anatomy : the anatomical basis of clinical practice. USA: New York: Elsevier Limited; 2016. [Google Scholar]
- [24].Tzelepi V, Tsamandas AC, Zolota V, Scopa CD. Bone Anatomy, Physiology and Function. In: Kardamakis D, Vassiliou V, Chow E, editors. Bone Metastases: A translational and clinical approach. Dordrecht: Springer Netherlands; 2009. pp. 3–30. [Google Scholar]
- [25].Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. European cells & materials. 2008;15:53–76. doi: 10.22203/ecm.v015a05. [DOI] [PubMed] [Google Scholar]
- [26].Reznikov N, Shahar R, Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014;10:3815–26. doi: 10.1016/j.actbio.2014.05.024. [DOI] [PubMed] [Google Scholar]
- [27].Currey JD. The structure and mechanics of bone. Journal of Materials Science. 2012;47:41–54. [Google Scholar]
- [28].Caridade SG, Mano JF. (*) Engineering Membranes for Bone Regeneration. Tissue Eng Part A. 2017;23:1502–33. doi: 10.1089/ten.tea.2017.0094. [DOI] [PubMed] [Google Scholar]
- [29].Li X, Wang L, Fan Y, Feng Q, Cui FZ, Watari F. Nanostructured scaffolds for bone tissue engineering. Journal of biomedical materials research Part A. 2013;101:2424–35. doi: 10.1002/jbm.a.34539. [DOI] [PubMed] [Google Scholar]
- [30].Gong T, Xie J, Liao J, Zhang T, Lin S, Lin Y. Nanomaterials and bone regeneration. Bone Research. 2015;3:15029. doi: 10.1038/boneres.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Faingold A, Cohen SR, Reznikov N, Wagner HD. Osteonal lamellae elementary units: Lamellar microstructure, curvature and mechanical properties. Acta Biomaterialia. 2013;9:5956–62. doi: 10.1016/j.actbio.2012.11.032. [DOI] [PubMed] [Google Scholar]
- [32].Nair AK, Gautieri A, Chang S-W, Buehler MJ. Molecular mechanics of mineralized collagen fibrils in bone. Nature communications. 2013;4 doi: 10.1038/ncomms2720. 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fratzl P, Weinkamer R. Nature’s hierarchical materials. Progress in Materials Science. 2007;52:1263–334. [Google Scholar]
- [34].Gupta HS, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17741–6. doi: 10.1073/pnas.0604237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Leite AJ, Mano JF. Biomedical applications of natural-based polymers combined with bioactive glass nanoparticles. Journal of Materials Chemistry B. 2017;5:4555–68. doi: 10.1039/c7tb00404d. [DOI] [PubMed] [Google Scholar]
- [36].Li Y, Liu C. Nanomaterial-based bone regeneration. Nanoscale. 2017;9:4862–74. doi: 10.1039/c7nr00835j. [DOI] [PubMed] [Google Scholar]
- [37].Luz GM, Mano JF. Mineralized structures in nature: Examples and inspirations for the design of new composite materials and biomaterials. Composites Science and Technology. 2010;70:1777–88. [Google Scholar]
- [38].Yi H, Ur Rehman F, Zhao C, Liu B, He N. Recent advances in nano scaffolds for bone repair. Bone Research. 2016;4:16050. doi: 10.1038/boneres.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kini U, Nandeesh BN. Physiology of Bone Formation, Remodeling, Metabolism. In: Fogelman I, Gnanasegaran G, van der Wall H, editors. Radionuclide and Hybrid Bone Imaging. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. pp. 29–57. [Google Scholar]
- [40].Cunningham C, Scheuer L, Black S. Developmental Juvenile Osteology (Second Edition) San Diego: Academic Press; 2016. Chapter 3 - Bone Development; pp. 19–35. [Google Scholar]
- [41].Hartwig WC. The anatomy and biology of the human skeleton by D. Gentry Steele and Claud A. Bramblett. Texas A & M University Press, College Station, 1988. Clinical Anatomy. 1990;3:151–3. [Google Scholar]
- [42].Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Annals of the New York Academy of Sciences. 2010;1192:437–43. doi: 10.1111/j.1749-6632.2009.05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Karsenty G. The complexities of skeletal biology. Nature. 2003;423:316–8. doi: 10.1038/nature01654. [DOI] [PubMed] [Google Scholar]
- [44].Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Research. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Karaplis AC. Chapter 3 - Embryonic Development of Bone and Regulation of Intramembranous and Endochondral Bone Formation A2 - Bilezikian, John P. In: Raisz LG, Martin TJ, editors. Principles of Bone Biology (Third Edition) San Diego: Academic Press; 2008. pp. 53–84. [Google Scholar]
- [46].Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nature Reviews Molecular Cell Biology. 2011;13:27. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- [47].Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- [48].Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochemical and Biophysical Research Communications. 2005;328:658–65. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- [49].Nishimura R, Wakabayashi M, Hata K, Matsubara T, Honma S, Wakisaka S, et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. The Journal of biological chemistry. 2012;287:33179–90. doi: 10.1074/jbc.M111.337063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes & development. 2002;16:2813–28. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes & development. 2002;16:1446–65. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- [52].Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP Signaling Integrates Chondrocyte Proliferation and Hypertrophic Differentiation. Developmental Cell. 2002;3:439–49. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- [53].Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, et al. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development (Cambridge, England) 2001;128:4523–34. doi: 10.1242/dev.128.22.4523. [DOI] [PubMed] [Google Scholar]
- [54].Schipani E, Provot S. PTHrP, PTH, and the PTH/PTHrP receptor in endochondral bone development. Birth defects research Part C, Embryo today : reviews. 2003;69:352–62. doi: 10.1002/bdrc.10028. [DOI] [PubMed] [Google Scholar]
- [55].Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression of bone sialoprotein (BSP) in developing human tissues. Calcified Tissue International. 1991;49:421–6. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- [56].Bianco P, Riminucci M, Silvestrini G, Bonucci E, Termine JD, Fisher LW, et al. Localization of bone sialoprotein (BSP) to Golgi and post-Golgi secretory structures in osteoblasts and to discrete sites in early bone matrix. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1993;41:193–203. doi: 10.1177/41.2.8419459. [DOI] [PubMed] [Google Scholar]
- [57].de Bernard B, Bianco P, Bonucci E, Costantini M, Lunazzi GC, Martinuzzi P, et al. Biochemical and immunohistochemical evidence that in cartilage an alkaline phosphatase is a Ca2+-binding glycoprotein. J Cell Biol. 1986;103:1615–23. doi: 10.1083/jcb.103.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Galotto M, Campanile G, Robino G, Cancedda FD, Bianco P, Cancedda R. Hypertrophic chondrocytes undergo further differentiation to osteoblast-like cells and participate in the initial bone formation in developing chick embryo. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1994;9:1239–49. doi: 10.1002/jbmr.5650090814. [DOI] [PubMed] [Google Scholar]
- [59].Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science (New York, NY) 1996;273:663–6. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- [60].Woodard JC, Donovan GA, Fisher LW. Pathogenesis of vitamin (A and D)-induced premature growth-plate closure in calves. Bone. 1997;21:171–82. doi: 10.1016/s8756-3282(97)00099-9. [DOI] [PubMed] [Google Scholar]
- [61].Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science (New York, NY) 1996;273:613–22. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- [62].Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mechanisms of development. 1999;80:159–70. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- [63].Yoshida CA, Furuichi T, Fujita T, Fukuyama R, Kanatani N, Kobayashi S, et al. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nature genetics. 2002;32:633–8. doi: 10.1038/ng1015. [DOI] [PubMed] [Google Scholar]
- [64].Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Current opinion in cell biology. 2001;13:167–71. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- [65].Maes C, Araldi E, Haigh K, Khatri R, Van Looveren R, Giaccia AJ, et al. HIF-1a and VEGF promote chondrocyte survival via complementary mechanisms regulating the oxygen level in the avascular developing growth cartilage. Bone. 48:S68. [Google Scholar]
- [66].Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue engineering Part B, Reviews. 2009;15:395–422. doi: 10.1089/ten.TEB.2009.0461. [DOI] [PubMed] [Google Scholar]
- [67].Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. The New England journal of medicine. 2001;344:385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- [68].Mikael PE, Xin X, Urso M, Jiang X, Wang L, Barnes B, et al. A potential translational approach for bone tissue engineering through endochondral ossification. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference; 2014. pp. 3925–8. [DOI] [PubMed] [Google Scholar]
- [69].Barradas AM, Yuan H, van Blitterswijk CA, Habibovic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. European cells & materials. 2011;21:407–29. doi: 10.22203/ecm.v021a31. discussion 29. [DOI] [PubMed] [Google Scholar]
- [70].Samavedi S, Whittington AR, Goldstein AS. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater. 2013;9:8037–45. doi: 10.1016/j.actbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- [71].Bouler JM, Pilet P, Gauthier O, Verron E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017;53:1–12. doi: 10.1016/j.actbio.2017.01.076. [DOI] [PubMed] [Google Scholar]
- [72].Yuan H, Fernandes H, Habibovic P, de Boer J, Barradas AMC, de Ruiter A, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proceedings of the National Academy of Sciences. 2010 doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].GD EC, S Y-RV, Manando N, Mengqian L, Shyni V. Mineralized Biomaterials Mediated Repair of Bone Defects Through Endogenous Cells. Tissue Engineering Part A. 2018;24:1148–56. doi: 10.1089/ten.tea.2017.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Davison NL, Su J, Yuan H, van den Beucken JJ, de Bruijn JD, Barrere-de Groot F. Influence of surface microstructure and chemistry on osteoinduction and osteoclastogenesis by biphasic calcium phosphate discs. European cells & materials. 2015;29:314–29. doi: 10.22203/ecm.v029a24. [DOI] [PubMed] [Google Scholar]
- [75].Barba A, Diez-Escudero A, Maazouz Y, Rappe K, Espanol M, Montufar EB, et al. Osteoinduction by Foamed and 3D-Printed Calcium Phosphate Scaffolds: Effect of Nanostructure and Pore Architecture. 2017;9:41722–36. doi: 10.1021/acsami.7b14175. [DOI] [PubMed] [Google Scholar]
- [76].Barradas AM, Fernandes HA, Groen N, Chai YC, Schrooten J, van de Peppel J, et al. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials. 2012;33:3205–15. doi: 10.1016/j.biomaterials.2012.01.020. [DOI] [PubMed] [Google Scholar]
- [77].Bianco P, Cancedda FD, Riminucci M, Cancedda R. Bone formation via cartilage models: the "borderline" chondrocyte. Matrix biology : journal of the International Society for Matrix Biology. 1998;17:185–92. doi: 10.1016/s0945-053x(98)90057-9. [DOI] [PubMed] [Google Scholar]
- [78].Jukes JM, Both SK, Leusink A, Sterk LMT, van Blitterswijk CA, de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proceedings of the National Academy of Sciences. 2008;105:6840. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hurlbut WB. Framing the future: embryonic stem cells, ethics and the emerging era of developmental biology. Pediatric research. 2006;59:4r–12r. doi: 10.1203/01.pdr.0000205377.04359.3e. [DOI] [PubMed] [Google Scholar]
- [80].Lo B, Parham L. Ethical Issues in Stem Cell Research. Endocrine Reviews. 2009;30:204–13. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proceedings of the National Academy of Sciences. 2010;107:7251. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- [83].Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–4. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Scotti C, Piccinini E, Takizawa H, Todorov A, Bourgine P, Papadimitropoulos A, et al. Engineering of a functional bone organ through endochondral ossification. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mumme M, Scotti C, Papadimitropoulos A, Todorov A, Hoffmann W, Bocelli-Tyndall C, et al. Interleukin-1beta modulates endochondral ossification by human adult bone marrow stromal cells. European cells & materials. 2012;24:224–36. doi: 10.22203/ecm.v024a16. [DOI] [PubMed] [Google Scholar]
- [86].Pippenger BE, Ventura M, Pelttari K, Feliciano S, Jaquiery C, Scherberich A, et al. Bone-forming capacity of adult human nasal chondrocytes. Journal of cellular and molecular medicine. 2015;19:1390–9. doi: 10.1111/jcmm.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Osinga R, Di Maggio N, Todorov A, Allafi N, Barbero A, Laurent F, et al. Generation of a Bone Organ by Human Adipose-Derived Stromal Cells Through Endochondral Ossification. Stem cells translational medicine. 2016;5:1090–7. doi: 10.5966/sctm.2015-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tam WL, O DF, Hiramatsu K, Tsumaki N, Luyten FP, Roberts SJ. Sox9 Reprogrammed Dermal Fibroblasts Undergo Hypertrophic Differentiation In Vitro and Trigger Endochondral Ossification In Vivo. Cellular Reprogramming. 2014;16:29–39. doi: 10.1089/cell.2013.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wang Y, Wu MH, Cheung MPL, Sham MH, Akiyama H, Chan D, et al. Reprogramming of Dermal Fibroblasts into Osteo-Chondrogenic Cells with Elevated Osteogenic Potency by Defined Transcription Factors. Stem Cell Reports. 2017;8:1587–99. doi: 10.1016/j.stemcr.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Todorov A, Scotti C, Barbero A, Scherberich A, Papadimitropoulos A, Martin I. Monocytes Seeded on Engineered Hypertrophic Cartilage Do Not Enhance Endochondral Ossification Capacity. Tissue engineering Part A. 2017;23:708–15. doi: 10.1089/ten.tea.2016.0553. [DOI] [PubMed] [Google Scholar]
- [91].Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Therapeutic advances in musculoskeletal disease. 2016;8:225–35. doi: 10.1177/1759720X16670154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Huang Z, Nelson ER, Smith RL, Goodman SB. The sequential expression profiles of growth factors from osteoprogenitors [correction of osteroprogenitors] to osteoblasts in vitro. Tissue engineering. 2007;13:2311–20. doi: 10.1089/ten.2006.0423. [DOI] [PubMed] [Google Scholar]
- [93].Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–9. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- [94].Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. The Journal of biological chemistry. 2010;285:25103–8. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nature reviews Rheumatology. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Knight MN, Hankenson KD. Mesenchymal Stem Cells in Bone Regeneration. Advances in Wound Care. 2013;2:306–16. doi: 10.1089/wound.2012.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Schlundt C, Schell H, Goodman SB, Vunjak-Novakovic G, Duda GN, Schmidt-Bleek K. Immune modulation as a therapeutic strategy in bone regeneration. Journal of Experimental Orthopaedics. 2015;2:1. doi: 10.1186/s40634-014-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mountziaris PM, Mikos AG. Modulation of the Inflammatory Response for Enhanced Bone Tissue Regeneration. Tissue engineering Part B, Reviews. 2008;14:179–86. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cameron JA, Milner DJ, Lee JS, Cheng J, Fang NX, Jasiuk IM. Employing the biology of successful fracture repair to heal critical size bone defects. Current topics in microbiology and immunology. 2013;367:113–32. doi: 10.1007/82_2012_291. [DOI] [PubMed] [Google Scholar]
- [100].Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- [101].Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. Journal of cellular biochemistry. 2003;88:873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- [102].El-Jawhari JJ, Jones E, Giannoudis PV. The roles of immune cells in bone healing; what we know, do not know and future perspectives. Injury. 2016;47:2399–406. doi: 10.1016/j.injury.2016.10.008. [DOI] [PubMed] [Google Scholar]
- [103].Baht GS, Vi L, Alman BA. The Role of the Immune Cells in Fracture Healing. Current osteoporosis reports. 2018;16:138–45. doi: 10.1007/s11914-018-0423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:1517–32. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- [105].Park JE, Barbul A. Understanding the role of immune regulation in wound healing. The American Journal of Surgery. 2004;187:S11–S6. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- [106].Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone. 2018;106:78–89. doi: 10.1016/j.bone.2015.10.019. [DOI] [PubMed] [Google Scholar]
- [107].van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJA. Macrophage Depletion Impairs Wound Healing and Increases Left Ventricular Remodeling after Myocardial Injury in Mice. The American Journal of Pathology. 2007;170:818–29. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 2014;184:3192–204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- [109].Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, et al. Macrophage Phenotype as a Predictor of Constructive Remodeling following the Implantation of Biologically Derived Surgical Mesh Materials. Acta biomaterialia. 2012;8:978–87. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–88. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nature reviews Rheumatology. 2012;8:133–43. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- [112].Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Li T, Peng M, Yang Z, Zhou X, Deng Y, Jiang C, et al. 3D-printed IFN-γ-loading calcium silicate-β-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone. Acta Biomaterialia. 2018 doi: 10.1016/j.actbio.2018.03.012. [DOI] [PubMed] [Google Scholar]
- [114].Kovach TK, Dighe AS, Lobo PI, Cui Q. Interactions between MSCs and Immune Cells: Implications for Bone Healing. Journal of Immunology Research. 2015;2015:17. doi: 10.1155/2015/752510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Dighe AS, Yang S, Madhu V, Balian G, Cui Q. Interferon gamma and T cells inhibit osteogenesis induced by allogeneic mesenchymal stromal cells. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31:227–34. doi: 10.1002/jor.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Toben D, Schroeder I, El Khassawna T, Mehta M, Hoffmann JE, Frisch JT, et al. Fracture healing is accelerated in the absence of the adaptive immune system. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:113–24. doi: 10.1002/jbmr.185. [DOI] [PubMed] [Google Scholar]
- [117].Reinke S, Geissler S, Taylor WR, Schmidt-Bleek K, Juelke K, Schwachmeyer V, et al. Terminally differentiated CD8(+) T cells negatively affect bone regeneration in humans. Sci Transl Med. 2013;5:177ra36. doi: 10.1126/scitranslmed.3004754. [DOI] [PubMed] [Google Scholar]
- [118].Colburn NT, Zaal KJ, Wang F, Tuan RS. A role for gamma/delta T cells in a mouse model of fracture healing. Arthritis and rheumatism. 2009;60:1694–703. doi: 10.1002/art.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Najar M, Raicevic G, Boufker HI, Fayyad-Kazan H, De Bruyn C, Meuleman N, et al. Adipose-tissue-derived and Wharton's jelly-derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor. Tissue engineering Part A. 2010;16:3537–46. doi: 10.1089/ten.TEA.2010.0159. [DOI] [PubMed] [Google Scholar]
- [120].Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Pistoia V, et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem cells (Dayton, Ohio) 2007;25:1753–60. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- [121].Gori JL, Butler JM, Chan YY, Chandrasekaran D, Poulos MG, Ginsberg M, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. The Journal of clinical investigation. 2015;125:1243–54. doi: 10.1172/JCI79328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zhang J, Niu C, Ye L, Huang H, He X, Tong W-G, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- [124].Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annual review of immunology. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- [125].Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: Games without frontiers. Archives of Biochemistry and Biophysics. 2014;561:3–12. doi: 10.1016/j.abb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- [126].Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- [127].Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jeon YJ, Kim J, Cho JH, Chung HM, Chae JI. Comparative Analysis of Human Mesenchymal Stem Cells Derived From Bone Marrow, Placenta, and Adipose Tissue as Sources of Cell Therapy. Journal of cellular biochemistry. 2016;117:1112–25. doi: 10.1002/jcb.25395. [DOI] [PubMed] [Google Scholar]
- [129].Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental hematology. 2005;33:1402–16. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [130].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [131].Wen Y, Jiang B, Cui J, Li G, Yu M, Wang F, et al. Superior osteogenic capacity of different mesenchymal stem cells for bone tissue engineering. Oral surgery, oral medicine, oral pathology and oral radiology. 2013;116:e324–32. doi: 10.1016/j.oooo.2012.02.024. [DOI] [PubMed] [Google Scholar]
- [132].Bunpetch V, Zhang Z-Y, Zhang X, Han S, Zongyou P, Wu H, et al. Strategies for MSC expansion and MSC-based microtissue for bone regeneration. Biomaterials. 2017 doi: 10.1016/j.biomaterials.2017.11.023. [DOI] [PubMed] [Google Scholar]
- [133].Zajdel A, Kalucka M, Kokoszka-Mikolaj E, Wilczok A. Osteogenic differentiation of human mesenchymal stem cells from adipose tissue and Wharton's jelly of the umbilical cord. Acta biochimica Polonica. 2017;64:365–9. doi: 10.18388/abp.2016_1488. [DOI] [PubMed] [Google Scholar]
- [134].Csobonyeiova M, Polak S, Zamborsky R, Danisovic L. iPS cell technologies and their prospect for bone regeneration and disease modeling: A mini review. Journal of Advanced Research. 2017;8:321–7. doi: 10.1016/j.jare.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [136].de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, et al. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110:8680–5. doi: 10.1073/pnas.1301190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Xie J, Peng C, Zhao Q, Wang X, Yuan H, Yang L, et al. Osteogenic differentiation and bone regeneration of iPSC-MSCs supported by a biomimetic nanofibrous scaffold. Acta Biomater. 2016;29:365–79. doi: 10.1016/j.actbio.2015.10.007. [DOI] [PubMed] [Google Scholar]
- [138].Lou X. Induced Pluripotent Stem Cells as a new Strategy for Osteogenesis and Bone Regeneration. Stem Cell Reviews and Reports. 2015;11:645–51. doi: 10.1007/s12015-015-9594-8. [DOI] [PubMed] [Google Scholar]
- [139].Wu Q, Yang B, Hu K, Cao C, Man Y, Wang P. Deriving Osteogenic Cells from Induced Pluripotent Stem Cells for Bone Tissue Engineering. Tissue engineering Part B, Reviews. 2017;23:1–8. doi: 10.1089/ten.TEB.2015.0559. [DOI] [PubMed] [Google Scholar]
- [140].Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20641–6. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ardeshirylajimi A, Dinarvand P, Seyedjafari E, Langroudi L, Adegani FJ, Soleimani M. Enhanced reconstruction of rat calvarial defects achieved by plasma-treated electrospun scaffolds and induced pluripotent stem cells. Cell Tissue Res. 2013;354:849–60. doi: 10.1007/s00441-013-1693-8. [DOI] [PubMed] [Google Scholar]
- [142].Ardeshirylajimi A, Hosseinkhani S, Parivar K, Yaghmaie P, Soleimani M. Nanofiber-based polyethersulfone scaffold and efficient differentiation of human induced pluripotent stem cells into osteoblastic lineage. Molecular biology reports. 2013;40:4287–94. doi: 10.1007/s11033-013-2515-5. [DOI] [PubMed] [Google Scholar]
- [143].Duan X, Tu Q, Zhang J, Ye J, Sommer C, Mostoslavsky G, et al. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. Journal of cellular physiology. 2011;226:150–7. doi: 10.1002/jcp.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Karp JM, Ferreira LS, Khademhosseini A, Kwon AH, Yeh J, Langer RS. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem cells (Dayton, Ohio) 2006;24:835–43. doi: 10.1634/stemcells.2005-0383. [DOI] [PubMed] [Google Scholar]
- [145].Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem cells translational medicine. 2012;1:83–95. doi: 10.5966/sctm.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Hynes K, Menicanin D, Mrozik K, Gronthos S, Bartold PM. Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem Cells Dev. 2014;23:1084–96. doi: 10.1089/scd.2013.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zou L, Luo Y, Chen M, Wang G, Ding M, Petersen CC, et al. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Scientific Reports. 2013;3 doi: 10.1038/srep02243. 2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wang P, Liu X, Zhao L, Weir MD, Sun J, Chen W, et al. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015;18:236–48. doi: 10.1016/j.actbio.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wu Q, Yang B, Cao C, Hu K, Wang P, Man Y. Therapeutic antibody directed osteogenic differentiation of induced pluripotent stem cell derived MSCs. Acta Biomaterialia. 2018;74:222–35. doi: 10.1016/j.actbio.2018.05.028. [DOI] [PubMed] [Google Scholar]
- [150].Levi B, Hyun JS, Montoro DT, Lo DD, Chan CK, Hu S, et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20379–84. doi: 10.1073/pnas.1218052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kanke K, Masaki H, Saito T, Komiyama Y, Hojo H, Nakauchi H, et al. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum-free and feeder-free conditions. Stem Cell Reports. 2014;2:751–60. doi: 10.1016/j.stemcr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kang H, Shih YR, Nakasaki M, Kabra H, Varghese S. Small molecule-driven direct conversion of human pluripotent stem cells into functional osteoblasts. Sci Adv. 2016;2:e1600691. doi: 10.1126/sciadv.1600691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kang H, Shih YV, Hwang Y, Wen C, Rao V, Seo T, et al. Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of human induced pluripotent stem cells. Acta Biomater. 2014;10:4961–70. doi: 10.1016/j.actbio.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Jin GZ, Kim TH, Kim JH, Won JE, Yoo SY, Choi SJ, et al. Bone tissue engineering of induced pluripotent stem cells cultured with macrochanneled polymer scaffold. Journal of biomedical materials research Part A. 2013;101:1283–91. doi: 10.1002/jbm.a.34425. [DOI] [PubMed] [Google Scholar]
- [155].Ko JY, Park S, Im GI. Osteogenesis from human induced pluripotent stem cells: an in vitro and in vivo comparison with mesenchymal stem cells. Stem Cells Dev. 2014;23:1788–97. doi: 10.1089/scd.2014.0043. [DOI] [PubMed] [Google Scholar]
- [156].Bonewald LF. The amazing osteocyte. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52:157–66. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Plotkin LI. Connexin 43 hemichannels and intracellular signaling in bone cells. Frontiers in Physiology. 2014;5:131. doi: 10.3389/fphys.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Vazquez M, Evans BA, Riccardi D, Evans SL, Ralphs JR, Dillingham CM, et al. A new method to investigate how mechanical loading of osteocytes controls osteoblasts. Frontiers in endocrinology. 2014;5:208. doi: 10.3389/fendo.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- [161].Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nature reviews Genetics. 2003;4:638–49. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- [162].Matsuo K. Cross-talk among bone cells. Current opinion in nephrology and hypertension. 2009;18:292–7. doi: 10.1097/MNH.0b013e32832b75f1. [DOI] [PubMed] [Google Scholar]
- [163].Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- [164].Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–66. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- [166].Heiland GR, Zwerina K, Baum W, Kireva T, Distler JH, Grisanti M, et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Annals of the rheumatic diseases. 2010;69:2152–9. doi: 10.1136/ard.2010.132852. [DOI] [PubMed] [Google Scholar]
- [167].Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Molecular endocrinology (Baltimore, Md) 2004;18:1222–37. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- [168].Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- [169].Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nature Medicine. 2011;17 doi: 10.1038/nm.2489. 1473. [DOI] [PubMed] [Google Scholar]
- [170].Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561:195–200. doi: 10.1038/s41586-018-0482-7. [DOI] [PubMed] [Google Scholar]
- [171].Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34:658–90. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:915–27. doi: 10.1359/jbmr.080207. [DOI] [PubMed] [Google Scholar]
- [173].Wu Q, Zhou X, Huang D, Ji Y, Kang F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;41:1360–9. doi: 10.1159/000465455. [DOI] [PubMed] [Google Scholar]
- [174].Percival CJ, Richtsmeier JT. Angiogenesis and intramembranous osteogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2013;242:909–22. doi: 10.1002/dvdy.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Beamer B, Hettrich C, Lane J. Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing. HSS journal : the musculoskeletal journal of Hospital for Special Surgery. 2010;6:85–94. doi: 10.1007/s11420-009-9129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Wu C, Giaccia AJ, Rankin EB. Osteoblasts: a novel source of erythropoietin. Current osteoporosis reports. 2014;12:428–32. doi: 10.1007/s11914-014-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–80. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Huang B, Wang W, Li Q, Wang Z, Yan B, Zhang Z, et al. Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone. Nature communications. 2016;7 doi: 10.1038/ncomms13885. 13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Weitzmann MN, Pacifici R. T cells: unexpected players in the bone loss induced by estrogen deficiency and in basal bone homeostasis. Annals of the New York Academy of Sciences. 2007;1116:360–75. doi: 10.1196/annals.1402.068. [DOI] [PubMed] [Google Scholar]
- [181].Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2018 doi: 10.1016/j.biomaterials.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. Journal of immunology (Baltimore, Md : 1950) 2008;181:1232–44. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- [183].Raggatt LJ, Chang MK, Alexander KA, Maylin ER, Walsh NC, Gravallese EM, et al. Osteomacs: Osteoclast precursors during inflammatory bone disease but regulators of physiologic bone remodeling. Bone. 2009;44:S136–S7. [Google Scholar]
- [184].Miron RJ, Bosshardt DD. OsteoMacs: Key players around bone biomaterials. Biomaterials. 2016;82:1–19. doi: 10.1016/j.biomaterials.2015.12.017. [DOI] [PubMed] [Google Scholar]
- [185].Batoon L, Millard SM, Wullschleger ME, Preda C, Wu AC, Kaur S, et al. CD169(+) macrophages are critical for osteoblast maintenance and promote intramembranous and endochondral ossification during bone repair. Biomaterials. 2017 doi: 10.1016/j.biomaterials.2017.10.033. [DOI] [PubMed] [Google Scholar]
- [186].Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–48. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29:403–40. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Gabriela C, Donatella G, Nicola B. The Combined Use of Mesenchymal Stromal Cells and Scaffolds for Bone Repair. Current pharmaceutical design. 2012;18:1796–820. doi: 10.2174/138161212799859648. [DOI] [PubMed] [Google Scholar]
- [189].Clarke MS, Sundaresan A, Vanderburg CR, Banigan MG, Pellis NR. A three-dimensional tissue culture model of bone formation utilizing rotational co-culture of human adult osteoblasts and osteoclasts. Acta Biomater. 2013;9:7908–16. doi: 10.1016/j.actbio.2013.04.051. [DOI] [PubMed] [Google Scholar]
- [190].Hayden RS, Quinn KP, Alonzo CA, Georgakoudi I, Kaplan DL. Quantitative characterization of mineralized silk film remodeling during long-term osteoblast-osteoclast co-culture. Biomaterials. 2014;35:3794–802. doi: 10.1016/j.biomaterials.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Lotz EM, Berger MB, Schwartz Z, Boyan BD. Regulation of osteoclasts by osteoblast lineage cells depends on titanium implant surface properties. Acta Biomater. 2017 doi: 10.1016/j.actbio.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Pirraco RP, Marques AP, Reis RL. Cell interactions in bone tissue engineering. Journal of cellular and molecular medicine. 2010;14:93–102. doi: 10.1111/j.1582-4934.2009.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Bongio M, Lopa S, Gilardi M, Bersini S, Moretti M. A 3D vascularized bone remodeling model combining osteoblasts and osteoclasts in a CaP nanoparticle-enriched matrix. Nanomedicine (London, England) 2016;11:1073–91. doi: 10.2217/nnm-2015-0021. [DOI] [PubMed] [Google Scholar]
- [194].Hammoudi TM, Rivet CA, Kemp ML, Lu H, Temenoff JS. Three-dimensional in vitro tri-culture platform to investigate effects of crosstalk between mesenchymal stem cells, osteoblasts, and adipocytes. Tissue Eng Part A. 2012;18:1686–97. doi: 10.1089/ten.tea.2011.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Almubarak S, Nethercott H, Freeberg M, Beaudon C, Jha A, Jackson W, et al. Tissue engineering strategies for promoting vascularized bone regeneration. Bone. 2016;83:197–209. doi: 10.1016/j.bone.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Mercado-Pagan AE, Stahl AM, Shanjani Y, Yang Y. Vascularization in bone tissue engineering constructs. Annals of biomedical engineering. 2015;43:718–29. doi: 10.1007/s10439-015-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].He X, Dziak R, Yuan X, Mao K, Genco R, Swihart M, et al. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PLoS One. 2013;8:e60473. doi: 10.1371/journal.pone.0060473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Wang H, Cheng H, Tang X, Chen J, Zhang J, Wang W, et al. The synergistic effect of bone forming peptide-1 and endothelial progenitor cells to promote vascularization of tissue engineered bone. Journal of biomedical materials research Part A. 2017 doi: 10.1002/jbm.a.36287. [DOI] [PubMed] [Google Scholar]
- [199].Akar B, Jiang B, Somo SI, Appel AA, Larson JC, Tichauer KM, et al. Biomaterials with persistent growth factor gradients in vivo accelerate vascularized tissue formation. Biomaterials. 2015;72:61–73. doi: 10.1016/j.biomaterials.2015.08.049. [DOI] [PubMed] [Google Scholar]
- [200].Fu WL, Xiang Z, Huang FG, Gu ZP, Yu XX, Cen SQ, et al. Coculture of peripheral blood-derived mesenchymal stem cells and endothelial progenitor cells on strontium-doped calcium polyphosphate scaffolds to generate vascularized engineered bone. Tissue engineering Part A. 2015;21:948–59. doi: 10.1089/ten.TEA.2014.0267. [DOI] [PubMed] [Google Scholar]
- [201].Yu T, Liu Q, Jiang T, Wang X, Yang Y, Kang Y. Channeled β-TCP Scaffolds Promoted Vascularization and Bone Augmentation in Mandible of Beagle Dogs. Advanced Functional Materials. 2016;26:6719–27. [Google Scholar]
- [202].Lee JH, Hah YS, Cho HY, Kim JH, Oh SH, Park BW, et al. Human umbilical cord blood-derived CD34-positive endothelial progenitor cells stimulate osteoblastic differentiation of cultured human periosteal-derived osteoblasts. Tissue engineering Part A. 2014;20:940–53. doi: 10.1089/ten.TEA.2013.0329. [DOI] [PubMed] [Google Scholar]
- [203].Correia CR, Pirraco RP, Cerqueira MT, Marques AP, Reis RL, Mano JF. Semipermeable Capsules Wrapping a Multifunctional and Self-regulated Co-culture Microenvironment for Osteogenic Differentiation. Scientific Reports. 2016;6 doi: 10.1038/srep21883. 21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Liu Y, Chan JK, Teoh SH. Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J Tissue Eng Regen Med. 2015;9:85–105. doi: 10.1002/term.1617. [DOI] [PubMed] [Google Scholar]
- [205].Bajpai VK, Andreadis ST. Stem Cell Sources for Vascular Tissue Engineering and Regeneration. Tissue engineering Part B, Reviews. 2012;18:405–25. doi: 10.1089/ten.teb.2011.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Ramasamy SK, Kusumbe AP, Schiller M, Zeuschner D, Bixel MG, Milia C, et al. Blood flow controls bone vascular function and osteogenesis. Nature communications. 2016;7 doi: 10.1038/ncomms13601. 13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Du C, Narayanan K, Leong MF, Wan AC. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials. 2014;35:6006–14. doi: 10.1016/j.biomaterials.2014.04.011. [DOI] [PubMed] [Google Scholar]
- [208].Cho S-W, Lim SH, Kim I-K, Hong YS, Kim S-S, Yoo KJ, et al. Small-Diameter Blood Vessels Engineered With Bone Marrow–Derived Cells. Annals of Surgery. 2005;241:506–15. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Park H, Lim D-J, Sung M, Lee S-H, Na D, Park H. Microengineered platforms for co-cultured mesenchymal stem cells towards vascularized bone tissue engineering. Tissue Engineering and Regenerative Medicine. 2016;13:465–74. doi: 10.1007/s13770-016-9080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Hu Z, Ma C, Rong X, Zou S, Liu X. Immunomodulatory ECM-like Microspheres for Accelerated Bone Regeneration in Diabetes Mellitus. 2018;10:2377–90. doi: 10.1021/acsami.7b18458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Caires HR, Esteves T, Quelhas P, Barbosa MA, Navarro M, Almeida CR. Macrophage interactions with polylactic acid and chitosan scaffolds lead to improved recruitment of human mesenchymal stem/stromal cells: a comprehensive study with different immune cells. Journal of The Royal Society Interface. 2016;13 doi: 10.1098/rsif.2016.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Hachim D, LoPresti ST, Yates CC, Brown BN. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials. 2017;112:95–107. doi: 10.1016/j.biomaterials.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Graney PL, Roohani-Esfahani SI, Zreiqat H, Spiller KL. In vitro response of macrophages to ceramic scaffolds used for bone regeneration. 2016;13 doi: 10.1098/rsif.2016.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Correia CR, Gaifem J, Oliveira MB, Silvestre R, Mano JF. The influence of surface modified poly(l-lactic acid) films on the differentiation of human monocytes into macrophages. Biomaterials Science. 2017;5:551–60. doi: 10.1039/c6bm00920d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J, et al. Induction of Osteogenesis in Mesenchymal Stem Cells by Activated Monocytes/Macrophages Depends on Oncostatin M Signaling. Stem cells (Dayton, Ohio) 2012;30:762–72. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- [216].Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Scientific Reports. 2016;6 doi: 10.1038/srep38308. 38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Saldaña L, Vallés G, Bensiamar F, Mancebo FJ, García-Rey E, Vilaboa N. Paracrine interactions between mesenchymal stem cells and macrophages are regulated by 1,25-dihydroxyvitamin D3. Scientific Reports. 2017;7 doi: 10.1038/s41598-017-15217-8. 14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Le Blanc K, Davies LC. Mesenchymal stromal cells and the innate immune response. Immunology Letters. 2015;168:140–6. doi: 10.1016/j.imlet.2015.05.004. [DOI] [PubMed] [Google Scholar]
- [219].Lin L, Du L. The role of secreted factors in stem cells-mediated immune regulation. Cellular Immunology. 2017 doi: 10.1016/j.cellimm.2017.07.010. [DOI] [PubMed] [Google Scholar]
- [220].Takizawa N, Okubo N, Kamo M, Chosa N, Mikami T, Suzuki K, et al. Bone marrow-derived mesenchymal stem cells propagate immunosuppressive/anti-inflammatory macrophages in cell-to-cell contact-independent and -dependent manners under hypoxic culture. Experimental Cell Research. 2017;358:411–20. doi: 10.1016/j.yexcr.2017.07.014. [DOI] [PubMed] [Google Scholar]
- [221].Lu LY, Loi F, Nathan K, Lin TH, Pajarinen J, Gibon E, et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2017;35:2378–85. doi: 10.1002/jor.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Zhang Y, Böse T, Unger RE, Jansen JA, Kirkpatrick CJ, van den Beucken JJJP. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell and Tissue Research. 2017;369:273–86. doi: 10.1007/s00441-017-2598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Gong L, Zhao Y, Zhang Y, Ruan Z. The Macrophage Polarization Regulates MSC Osteoblast Differentiation in vitro. Annals of clinical and laboratory science. 2016;46:65–71. [PubMed] [Google Scholar]
- [224].Hao S, Meng J, Zhang Y, Liu J, Nie X, Wu F, et al. Macrophage phenotypic mechanomodulation of enhancing bone regeneration by superparamagnetic scaffold upon magnetization. Biomaterials. 2017;140:16–25. doi: 10.1016/j.biomaterials.2017.06.013. [DOI] [PubMed] [Google Scholar]
- [225].Vallés G, Bensiamar F, Crespo L, Arruebo M, Vilaboa N, Saldaña L. Topographical cues regulate the crosstalk between MSCs and macrophages. Biomaterials. 2015;37:124–33. doi: 10.1016/j.biomaterials.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Almeida Catarina R, Caires Hugo R, Vasconcelos Daniela P, Barbosa Mário A. NAP-2 Secreted by Human NK Cells Can Stimulate Mesenchymal Stem/Stromal Cell Recruitment. Stem Cell Reports. 2016;6:466–73. doi: 10.1016/j.stemcr.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Wang L, Zhao Y, Shi S. Interplay between Mesenchymal Stem Cells and Lymphocytes: Implications for Immunotherapy and Tissue Regeneration. Journal of Dental Research. 2012;91:1003–10. doi: 10.1177/0022034512460404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Osta B, Lavocat F, Eljaafari A, Miossec P. Effects of Interleukin-17A on Osteogenic Differentiation of Isolated Human Mesenchymal Stem Cells. Frontiers in Immunology. 2014;5 doi: 10.3389/fimmu.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Pritchard DJ, Morgan H, Cooper JM. Patterning and Regeneration of Surfaces with Antibodies. Analytical Chemistry. 1995;67:3605–7. doi: 10.1021/ac00115a034. [DOI] [PubMed] [Google Scholar]
- [230].Mooney JF, Hunt AJ, McIntosh JR, Liberko CA, Walba DM, Rogers CT. Patterning of functional antibodies and other proteins by photolithography of silane monolayers. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12287–91. doi: 10.1073/pnas.93.22.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Custódio CA, San Miguel-Arranz V, Gropeanu RA, Gropeanu M, Wirkner M, Reis RL, et al. Photopatterned Antibodies for Selective Cell Attachment. Langmuir. 2014;30:10066–71. doi: 10.1021/la502688h. [DOI] [PubMed] [Google Scholar]
- [232].Custódio CA, Santo VE, Oliveira MB, Gomes ME, Reis RL, Mano JF. Functionalized Microparticles Producing Scaffolds in Combination with Cells. Advanced Functional Materials. 2014;24:1391–400. [Google Scholar]
- [233].Custodio CA, Cerqueira MT, Marques AP, Reis RL, Mano JF. Cell selective chitosan microparticles as injectable cell carriers for tissue regeneration. Biomaterials. 2015;43:23–31. doi: 10.1016/j.biomaterials.2014.11.047. [DOI] [PubMed] [Google Scholar]
- [234].Abbina S, Siren EMJ, Moon H, Kizhakkedathu JN. Surface Engineering for Cell-Based Therapies: Techniques for Manipulating Mammalian Cell Surfaces. ACS Biomater Sci Eng. 2017 doi: 10.1021/acsbiomaterials.7b00514. [DOI] [PubMed] [Google Scholar]
- [235].Rogozhnikov D, O’Brien PJ, Elahipanah S, Yousaf MN. Scaffold Free Bio-orthogonal Assembly of 3-Dimensional Cardiac Tissue via Cell Surface Engineering. Scientific Reports. 2016;6 doi: 10.1038/srep39806. 39806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].O’Brien PJ, Luo W, Rogozhnikov D, Chen J, Yousaf MN. Spheroid and Tissue Assembly via Click Chemistry in Microfluidic Flow. Bioconjugate Chemistry. 2015;26:1939–49. doi: 10.1021/acs.bioconjchem.5b00376. [DOI] [PubMed] [Google Scholar]
- [237].Custódio CA, Mano JF. Cell Surface Engineering to Control Cellular Interactions. ChemNanoMat. 2016;2:376–84. doi: 10.1002/cnma.201600047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Oliveira MB, Hatami J, Mano JF. Coating Strategies Using Layer-by-layer Deposition for Cell Encapsulation. Chemistry, an Asian journal. 2016;11:1753–64. doi: 10.1002/asia.201600145. [DOI] [PubMed] [Google Scholar]
- [239].Occhetta P, Glass N, Otte E, Rasponi M, Cooper-White JJ. Stoichiometric control of live cell mixing to enable fluidically-encoded co-culture models in perfused microbioreactor arrays. Integrative biology : quantitative biosciences from nano to macro. 2016;8:194–204. doi: 10.1039/c5ib00311c. [DOI] [PubMed] [Google Scholar]
- [240].Correia CR, Santos TC, Pirraco RP, Cerqueira MT, Marques AP, Reis RL, et al. In vivo osteogenic differentiation of stem cells inside compartmentalized capsules loaded with co-cultured endothelial cells. Acta Biomaterialia. doi: 10.1016/j.actbio.2017.02.007. [DOI] [PubMed] [Google Scholar]
- [241].Kuss MA, Wu S, Wang Y, Untrauer JB, Li W, Lim JY, et al. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. Journal of biomedical materials research Part B, Applied biomaterials. 2017 doi: 10.1002/jbm.b.33994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Liu X, Chen W, Zhang C, Thein-Han W, Hu K, Reynolds MA, et al. Co-Seeding Human Endothelial Cells with Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells on Calcium Phosphate Scaffold Enhances Osteogenesis and Vascularization in Rats. Tissue Eng Part A. 2017;23:546–55. doi: 10.1089/ten.tea.2016.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Huang X, Li C, Zhu B, Wang H, Luo X, Wei L. Co-cultured hBMSCs and HUVECs on human bio-derived bone scaffolds provide support for the long-term ex vivo culture of HSC/HPCs. Journal of biomedical materials research Part A. 2016;104:1221–30. doi: 10.1002/jbm.a.35656. [DOI] [PubMed] [Google Scholar]
- [244].Hwang MP, Subbiah R, Kim IG, Lee KE, Park J, Kim SH, et al. Approximating bone ECM: Crosslinking directs individual and coupled osteoblast/osteoclast behavior. Biomaterials. 2016;103:22–32. doi: 10.1016/j.biomaterials.2016.06.052. [DOI] [PubMed] [Google Scholar]
- [245].Elango J, Sanchez C, de Val J, Henrotin Y, Wang S, Motaung K. Cross-talk between primary osteocytes and bone marrow macrophages for osteoclastogenesis upon collagen treatment. 2018;8 doi: 10.1038/s41598-018-23532-x. 5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Middleton K, Al-Dujaili S, Mei X, Gunther A, You L. Microfluidic co-culture platform for investigating osteocyte-osteoclast signalling during fluid shear stress mechanostimulation. Journal of biomechanics. 2017;59:35–42. doi: 10.1016/j.jbiomech.2017.05.012. [DOI] [PubMed] [Google Scholar]
- [247].Santos MI, Unger RE, Sousa RA, Reis RL, Kirkpatrick CJ. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30:4407–15. doi: 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- [248].Xing Z, Xue Y, Finne-Wistrand A, Yang ZQ, Mustafa K. Copolymer cell/scaffold constructs for bone tissue engineering: co-culture of low ratios of human endothelial and osteoblast-like cells in a dynamic culture system. Journal of biomedical materials research Part A. 2013;101:1113–20. doi: 10.1002/jbm.a.34414. [DOI] [PubMed] [Google Scholar]
- [249].Thein-Han W, Xu HH. Prevascularization of a gas-foaming macroporous calcium phosphate cement scaffold via coculture of human umbilical vein endothelial cells and osteoblasts. Tissue Eng Part A. 2013;19:1675–85. doi: 10.1089/ten.tea.2012.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Tu MG, Chen YW, Shie MY. Macrophage-mediated osteogenesis activation in co-culture with osteoblast on calcium silicate cement. J Mater Sci Mater Med. 2015;26:276. doi: 10.1007/s10856-015-5607-z. [DOI] [PubMed] [Google Scholar]
- [251].Gamblin AL, Renaud A, Charrier C, Hulin P, Louarn G, Heymann D, et al. Osteoblastic and osteoclastic differentiation of human mesenchymal stem cells and monocytes in a miniaturized three-dimensional culture with mineral granules. Acta Biomater. 2014;10:5139–47. doi: 10.1016/j.actbio.2014.08.033. [DOI] [PubMed] [Google Scholar]
- [252].Marie PJ. Role of N-cadherin in bone formation. Journal of cellular physiology. 2002;190:297–305. doi: 10.1002/jcp.10073. [DOI] [PubMed] [Google Scholar]
- [253].Cheng SL, Lecanda F, Davidson MK, Warlow PM, Zhang SF, Zhang L, et al. Human osteoblasts express a repertoire of cadherins, which are critical for BMP-2-induced osteogenic differentiation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998;13:633–44. doi: 10.1359/jbmr.1998.13.4.633. [DOI] [PubMed] [Google Scholar]
- [254].Ferrari SL, Traianedes K, Thorne M, Lafage-Proust MH, Genever P, Cecchini MG, et al. A role for N-cadherin in the development of the differentiated osteoblastic phenotype. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15:198–208. doi: 10.1359/jbmr.2000.15.2.198. [DOI] [PubMed] [Google Scholar]
- [255].Kawaguchi J, Kii I, Sugiyama Y, Takeshita S, Kudo A. The transition of cadherin expression in osteoblast differentiation from mesenchymal cells: consistent expression of cadherin-11 in osteoblast lineage. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16:260–9. doi: 10.1359/jbmr.2001.16.2.260. [DOI] [PubMed] [Google Scholar]
- [256].Marie PJ, Haÿ E. Cadherins and Wnt signalling: a functional link controlling bone formation. BoneKEy Reports. 2013;2:330. doi: 10.1038/bonekey.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Stains JP, Civitelli R. Cell-cell interactions in regulating osteogenesis and osteoblast function. Birth defects research Part C, Embryo today : reviews. 2005;75:72–80. doi: 10.1002/bdrc.20034. [DOI] [PubMed] [Google Scholar]
- [258].Marie PJ, Hay E, Modrowski D, Revollo L, Mbalaviele G, Civitelli R. Cadherin-mediated cell-cell adhesion and signaling in the skeleton. Calcif Tissue Int. 2014;94:46–54. doi: 10.1007/s00223-013-9733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Ribeiro-Rodrigues TM, Martins-Marques T. Role of connexin 43 in different forms of intercellular communication - gap junctions, extracellular vesicles and tunnelling nanotubes. 2017;130:3619–30. doi: 10.1242/jcs.200667. [DOI] [PubMed] [Google Scholar]
- [260].Xu H, Gu S, Riquelme MA, Burra S, Callaway D, Cheng H, et al. Connexin 43 channels are essential for normal bone structure and osteocyte viability. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30:436–48. doi: 10.1002/jbmr.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Pacheco-Costa R, Hassan I, Reginato RD, Davis HM, Bruzzaniti A, Allen MR, et al. High bone mass in mice lacking Cx37 because of defective osteoclast differentiation. The Journal of biological chemistry. 2014;289:8508–20. doi: 10.1074/jbc.M113.529735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Plotkin LI, Stains JP. Connexins and pannexins in the skeleton: gap junctions, hemichannels and more. Cellular and molecular life sciences : CMLS. 2015;72:2853–67. doi: 10.1007/s00018-015-1963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochimica et biophysica acta. 2013;1828:15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- [264].Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, et al. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. Journal of cell science. 2007;120:3772–83. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- [265].Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol. 2011;193:1257–74. doi: 10.1083/jcb.201101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Ishikawa M, Iwamoto T, Fukumoto S, Yamada Y. Pannexin 3 inhibits proliferation of osteoprogenitor cells by regulating Wnt and p21 signaling. The Journal of biological chemistry. 2014;289:2839–51. doi: 10.1074/jbc.M113.523241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, et al. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. The Journal of biological chemistry. 2010;285:18948–58. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Toh YC, Xing J, Yu H. Modulation of integrin and E-cadherin-mediated adhesions to spatially control heterogeneity in human pluripotent stem cell differentiation. Biomaterials. 2015;50:87–97. doi: 10.1016/j.biomaterials.2015.01.019. [DOI] [PubMed] [Google Scholar]
- [269].Tong Z, Solanki A, Hamilos A, Levy O, Wen K, Yin X, et al. Application of biomaterials to advance induced pluripotent stem cell research and therapy. The EMBO journal. 2015;34:987–1008. doi: 10.15252/embj.201490756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Qazi TH, Mooney DJ, Duda GN, Geissler S. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials. 2017;140:103–14. doi: 10.1016/j.biomaterials.2017.06.019. [DOI] [PubMed] [Google Scholar]
- [271].Zhu M, Lin S, Sun Y, Feng Q, Li G, Bian L. Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche. Biomaterials. 2016;77:44–52. doi: 10.1016/j.biomaterials.2015.10.072. [DOI] [PubMed] [Google Scholar]
- [272].Haumer A, Bourgine PE, Occhetta P, Born G, Tasso R, Martin I. Delivery of cellular factors to regulate bone healing. Adv Drug Deliv Rev. 2018 doi: 10.1016/j.addr.2018.01.010. [DOI] [PubMed] [Google Scholar]
- [273].Wang Q, Yan J, Yang J, Li B. Nanomaterials promise better bone repair. Materials Today. 2016;19:451–63. [Google Scholar]
- [274].Miyazono K, Maeda S, Imamura T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine & Growth Factor Reviews. 2005;16:251–63. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- [275].Riley EH, Lane JM, Urist MR, Lyons KM, Lieberman JR. Bone morphogenetic protein-2: biology and applications. Clinical orthopaedics and related research. 1996:39–46. [PubMed] [Google Scholar]
- [276].Kempen DH, Creemers LB, Alblas J, Lu L, Verbout AJ, Yaszemski MJ, et al. Growth factor interactions in bone regeneration. Tissue engineering Part B, Reviews. 2010;16:551–66. doi: 10.1089/ten.teb.2010.0176. [DOI] [PubMed] [Google Scholar]
- [277].Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, et al. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth factors (Chur, Switzerland) 1996;13:291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- [278].Hazama M, Aono A, Ueno N, Fujisawa Y. Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem Biophys Res Commun. 1995;209:859–66. doi: 10.1006/bbrc.1995.1578. [DOI] [PubMed] [Google Scholar]
- [279].Valera E, Isaacs MJ, Kawakami Y, Izpisúa Belmonte JC, Choe S. BMP-2/6 Heterodimer Is More Effective than BMP-2 or BMP-6 Homodimers as Inductor of Differentiation of Human Embryonic Stem Cells. PLOS ONE. 2010;5:e11167. doi: 10.1371/journal.pone.0011167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Neugebauer JM, Kwon S, Kim HS, Donley N, Tilak A, Sopory S, et al. The prodomain of BMP4 is necessary and sufficient to generate stable BMP4/7 heterodimers with enhanced bioactivity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E2307–16. doi: 10.1073/pnas.1501449112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Yang J, Shi P, Tu M, Wang Y, Liu M, Fan F, et al. Bone morphogenetic proteins: Relationship between molecular structure and their osteogenic activity. Food Science and Human Wellness. 2014;3:127–35. [Google Scholar]
- [282].Marsell R, Einhorn TA. The role of endogenous bone morphogenetic proteins in normal skeletal repair. Injury. 2009;40(Suppl 3):S4–7. doi: 10.1016/S0020-1383(09)70003-8. [DOI] [PubMed] [Google Scholar]
- [283].Grosso A, Burger MG, Lunger A, Schaefer DJ, Banfi A, Di Maggio N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Frontiers in Bioengineering and Biotechnology. 2017;5 doi: 10.3389/fbioe.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Hu K, Olsen BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–8. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Santo VE, Gomes ME, Mano JF, Reis RL. From nano- to macro-scale: nanotechnology approaches for spatially controlled delivery of bioactive factors for bone and cartilage engineering. Nanomedicine (London, England) 2012;7:1045–66. doi: 10.2217/nnm.12.78. [DOI] [PubMed] [Google Scholar]
- [286].Lavrador P, Gaspar VM, Mano JF. Stimuli-responsive nanocarriers for delivery of bone therapeutics – Barriers and progresses. Journal of Controlled Release. 2018;273:51–67. doi: 10.1016/j.jconrel.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering--Part II: challenges on the evolution from single to multiple bioactive factor delivery. Tissue engineering Part B, Reviews. 2013;19:327–52. doi: 10.1089/ten.teb.2012.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) The spine journal : official journal of the North American Spine Society. 2008;8:1011–8. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- [289].Lee YJ, Lee JH, Cho HJ, Kim HK, Yoon TR, Shin H. Electrospun fibers immobilized with bone forming peptide-1 derived from BMP7 for guided bone regeneration. Biomaterials. 2013;34:5059–69. doi: 10.1016/j.biomaterials.2013.03.051. [DOI] [PubMed] [Google Scholar]
- [290].Vo TN, Kasper FK, Mikos AG. Strategies for Controlled Delivery of Growth Factors and Cells for Bone Regeneration. Advanced drug delivery reviews. 2012;64:1292–309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Koutsopoulos S, Unsworth LD, Nagai Y, Zhang S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4623–8. doi: 10.1073/pnas.0807506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Oliveira JM, Salgado AJ, Sousa N, Mano JF, Reis RL. Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies—A review. Progress in Polymer Science. 2010;35:1163–94. [Google Scholar]
- [293].Dang M, Koh AJ, Jin X, McCauley LK, Ma PX. Local pulsatile PTH delivery regenerates bone defects via enhanced bone remodeling in a cell-free scaffold. Biomaterials. 2017;114:1–9. doi: 10.1016/j.biomaterials.2016.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Ulery BD. Biomaterials-based biologic burst release builds better bone. Science Translational Medicine. 2016;8:366ec189–366ec189. doi: 10.1126/scitranslmed.aal2797. [DOI] [PubMed] [Google Scholar]
- [295].James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, et al. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue engineering Part B, Reviews. 2016;22:284–97. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Caridade SG, Monge C, Almodovar J, Guillot R, Lavaud J, Josserand V, et al. Myoconductive and osteoinductive free-standing polysaccharide membranes. Acta Biomater. 2015;15:139–49. doi: 10.1016/j.actbio.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Rodrigues MN, Oliveira MB, Costa RR, Mano JF. Chitosan/Chondroitin Sulfate Membranes Produced by Polyelectrolyte Complexation for Cartilage Engineering. Biomacromolecules. 2016;17:2178–88. doi: 10.1021/acs.biomac.6b00399. [DOI] [PubMed] [Google Scholar]
- [298].Bonor J, Adams EL, Bragdon B, Moseychuk O, Czymmek KJ, Nohe A. Initiation of BMP2 Signaling in Domains on the Plasma Membrane. Journal of cellular physiology. 2012;227:2880–8. doi: 10.1002/jcp.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Wang Z, Wang Z, Lu WW, Zhen W, Yang D, Peng S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. Npg Asia Materials. 2017;9:e435. [Google Scholar]
- [300].Kim MJ, Lee B, Yang K, Park J, Jeon S, Um SH, et al. BMP-2 peptide-functionalized nanopatterned substrates for enhanced osteogenic differentiation of human mesenchymal stem cells. Biomaterials. 2013;34:7236–46. doi: 10.1016/j.biomaterials.2013.06.019. [DOI] [PubMed] [Google Scholar]
- [301].Tabisz B, Schmitz W, Schmitz M, Luehmann T, Heusler E, Rybak JC, et al. Site-Directed Immobilization of BMP-2: Two Approaches for the Production of Innovative Osteoinductive Scaffolds. 2017;18:695–708. doi: 10.1021/acs.biomac.6b01407. [DOI] [PubMed] [Google Scholar]
- [302].Park SH, Kwon JS, Lee BS, Park JH, Lee BK, Yun J-H, et al. BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells. Scientific Reports. 2017;7:6603. doi: 10.1038/s41598-017-06911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Hauff K, Zambarda C, Dietrich M, Halbig M, Grab AL, Medda R, et al. Matrix-Immobilized BMP-2 on Microcontact Printed Fibronectin as an in vitro Tool to Study BMP-Mediated Signaling and Cell Migration. Frontiers in Bioengineering and Biotechnology. 2015;3 doi: 10.3389/fbioe.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Fitzpatrick V, Fourel L, Destaing O, Gilde F, Albigès-Rizo C, Picart C, et al. Signal mingle: Micropatterns of BMP-2 and fibronectin on soft biopolymeric films regulate myoblast shape and SMAD signaling. Scientific Reports. 2017;7:41479. doi: 10.1038/srep41479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Llopis-Hernández V, Cantini M, González-García C, Cheng ZA, Yang J, Tsimbouri PM, et al. Material-driven fibronectin assembly for high-efficiency presentation of growth factors. Science Advances. 2016;2:e1600188. doi: 10.1126/sciadv.1600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Oliveira SM, Santo VE, Gomes ME, Reis RL, Mano JF. Layer-by-layer assembled cell instructive nanocoatings containing platelet lysate. Biomaterials. 2015;48:56–65. doi: 10.1016/j.biomaterials.2015.01.020. [DOI] [PubMed] [Google Scholar]
- [307].Hudalla GA, Murphy WL. Biomaterials that regulate growth factor activity via bioinspired interactions. Advanced functional materials. 2011;21:1754–68. doi: 10.1002/adfm.201002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Bjorge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine - a new paradigm for tissue repair. Biomaterials Science. 2018;6:60–78. doi: 10.1039/c7bm00479f. [DOI] [PubMed] [Google Scholar]
- [309].Taverna S, Pucci M, Alessandro R. Extracellular vesicles: small bricks for tissue repair/regeneration. Annals of Translational Medicine. 2017;5:83. doi: 10.21037/atm.2017.01.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Silva AM, Teixeira JH, Almeida MI, Gonçalves RM, Barbosa MA, Santos SG. Extracellular Vesicles: Immunomodulatory messengers in the context of tissue repair/regeneration. European Journal of Pharmaceutical Sciences. 2017;98:86–95. doi: 10.1016/j.ejps.2016.09.017. [DOI] [PubMed] [Google Scholar]
- [312].Chen B, Li Q, Zhao B, Wang Y. Stem Cell-Derived Extracellular Vesicles as a Novel Potential Therapeutic Tool for Tissue Repair. Stem cells translational medicine. 2017;6:1753–8. doi: 10.1002/sctm.16-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Scientific Reports. 2016;6:21961. doi: 10.1038/srep21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Martins M, Ribeiro D, Martins A, Reis Rui L, Neves Nuno M. Extracellular Vesicles Derived from Osteogenically Induced Human Bone Marrow Mesenchymal Stem Cells Can Modulate Lineage Commitment. Stem Cell Reports. 2016;6:284–91. doi: 10.1016/j.stemcr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Xie Y, Chen Y, Zhang L, Ge W, Tang P. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. Journal of cellular and molecular medicine. 2017;21:1033–41. doi: 10.1111/jcmm.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Xu J-F, Yang G-h, Pan X-H, Zhang S-J, Zhao C, Qiu B-S, et al. Altered MicroRNA Expression Profile in Exosomes during Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. PLOS ONE. 2014;9:e114627. doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Baglio SR, Devescovi V, Granchi D, Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527:321–31. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- [318].Wang X, Omar O, Vazirisani F, Thomsen P, Ekstrom K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. 2018;13:e0193059. doi: 10.1371/journal.pone.0193059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Custódio CA, Reis RL, Mano JF. Engineering Biomolecular Microenvironments for Cell Instructive Biomaterials. Advanced healthcare materials. 2014;3:797–810. doi: 10.1002/adhm.201300603. [DOI] [PubMed] [Google Scholar]
- [320].Sriram M, Sainitya R, Kalyanaraman V, Dhivya S, Selvamurugan N. Biomaterials mediated microRNA delivery for bone tissue engineering. International Journal of Biological Macromolecules. 2015;74:404–12. doi: 10.1016/j.ijbiomac.2014.12.034. [DOI] [PubMed] [Google Scholar]
- [321].Zhang X, Li Y, Chen YE, Chen J, Ma PX. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nature communications. 2016;7 doi: 10.1038/ncomms10376. 10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Wade RJ, Burdick JA. Engineering ECM signals into biomaterials. Materials Today. 2012;15:454–9. [Google Scholar]
- [323].Campbell ID, Humphries MJ. Integrin Structure, Activation, and Interactions. Cold Spring Harbor Perspectives in Biology. 2011;3:a004994. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Coutu DL, Kokkaliaris KD, Kunz L, Schroeder T. Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. Nature Biotechnology. 2017;35:1202. doi: 10.1038/nbt.4006. [DOI] [PubMed] [Google Scholar]
- [325].Hidalgo-Bastida LA, Cartmell SH. Mesenchymal stem cells, osteoblasts and extracellular matrix proteins: enhancing cell adhesion and differentiation for bone tissue engineering. Tissue engineering Part B, Reviews. 2010;16:405–12. doi: 10.1089/ten.TEB.2009.0714. [DOI] [PubMed] [Google Scholar]
- [326].Mbalaviele G, Shin CS, Civitelli R. Perspective: Cell–Cell Adhesion and Signaling Through Cadherins: Connecting Bone Cells in Their Microenvironment. Journal of Bone and Mineral Research. 2006;21:1821–7. doi: 10.1359/jbmr.060811. [DOI] [PubMed] [Google Scholar]
- [327].Shekaran A, Garcia AJ. Extracellular matrix-mimetic adhesive biomaterials for bone repair. Journal of biomedical materials research Part A. 2011;96:261–72. doi: 10.1002/jbm.a.32979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Strohmeyer N, Bharadwaj M, Costell M, Fässler R, Müller DJ. Fibronectin-bound α5β1 integrins sense load and signal to reinforce adhesion in less than a second. Nature Materials. 2017;16:1262. doi: 10.1038/nmat5023. [DOI] [PubMed] [Google Scholar]
- [329].Huang CH, Chen MH, Young TH, Jeng JH, Chen YJ. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. Journal of cellular biochemistry. 2009;108:1263–73. doi: 10.1002/jcb.22356. [DOI] [PubMed] [Google Scholar]
- [330].Mathews S, Bhonde R, Gupta PK, Totey S. Extracellular matrix protein mediated regulation of the osteoblast differentiation of bone marrow derived human mesenchymal stem cells. Differentiation; research in biological diversity. 2012;84:185–92. doi: 10.1016/j.diff.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [331].Dolatshahi-Pirouz A, Nikkhah M, Gaharwar AK, Hashmi B, Guermani E, Aliabadi H, et al. A combinatorial cell-laden gel microarray for inducing osteogenic differentiation of human mesenchymal stem cells. Scientific Reports. 2014;4:3896. doi: 10.1038/srep03896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Gothard D, Smith EL, Kanczler JM, Black CR, Wells JA, Roberts CA, et al. In Vivo Assessment of Bone Regeneration in Alginate/Bone ECM Hydrogels with Incorporated Skeletal Stem Cells and Single Growth Factors. PLoS ONE. 2015;10:e0145080. doi: 10.1371/journal.pone.0145080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Ravindran S, George A. Multifunctional ECM proteins in bone and teeth. Experimental Cell Research. 2014;325:148–54. doi: 10.1016/j.yexcr.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25:5947–54. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- [335].Keselowsky BG, Collard DM, García AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proceedings of the National Academy of Sciences. 2005;102:5953–7. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Gandavarapu NR, Alge DL, Anseth KS. Osteogenic differentiation of human mesenchymal stem cells on α5 integrin binding peptide hydrogels is dependent on substrate elasticity. Biomaterials science. 2014;2:352–61. doi: 10.1039/C3BM60149H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Karimi F, O'Connor AJ. Integrin Clustering Matters: A Review of Biomaterials Functionalized with Multivalent Integrin-Binding Ligands to Improve Cell Adhesion, Migration, Differentiation, Angiogenesis, and Biomedical Device Integration. 2018:e1701324. doi: 10.1002/adhm.201701324. [DOI] [PubMed] [Google Scholar]
- [338].Trappmann B, Gautrot JE, Connelly JT, Strange DGT, Li Y, Oyen ML, et al. Extracellular-matrix tethering regulates stem-cell fate. Nature Materials. 2012;11:642. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- [339].Slater JH, Frey W. Nanopatterning of fibronectin and the influence of integrin clustering on endothelial cell spreading and proliferation. Journal of biomedical materials research Part A. 2008;87:176–95. doi: 10.1002/jbm.a.31725. [DOI] [PubMed] [Google Scholar]
- [340].Calderwood DA, Ginsberg MH. Talin forges the links between integrins and actin. Nature cell biology. 2003;5:694. doi: 10.1038/ncb0803-694. [DOI] [PubMed] [Google Scholar]
- [341].Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nature reviews Molecular cell biology. 2010;11:633–43. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Lepzelter D, Bates O, Zaman M. Integrin Clustering in Two and Three Dimensions. Langmuir. 2012;28:5379–86. doi: 10.1021/la203725a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. Journal of cell science. 2001;114:3583–90. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- [344].Kubow KE, Horwitz AR. Reducing background fluorescence reveals adhesions in 3D matrices. Nature cell biology. 2011;13:3–5. doi: 10.1038/ncb0111-3. author reply -7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Lin LC, Brown FL. Brownian dynamics in Fourier space: membrane simulations over long length and time scales. Physical review letters. 2004;93 doi: 10.1103/PhysRevLett.93.256001. 256001. [DOI] [PubMed] [Google Scholar]
- [346].Lu X, Li W, Fukumoto S, Yamada Y, Evans C, Diekwisch TGH, et al. The Ameloblastin extracellular matrix molecule enhances bone fracture resistance and promotes rapid bone fracture healing. Matrix biology : journal of the International Society for Matrix Biology. 2016;52–54:113–26. doi: 10.1016/j.matbio.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Hatakeyama J, Fukumoto S, Nakamura T, Haruyama N, Suzuki S, Hatakeyama Y, et al. Synergistic roles of amelogenin and ameloblastin. Journal of dental research. 2009;88:318–22. doi: 10.1177/0022034509334749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Hirose N, Shimazu A, Watanabe M, Tanimoto K, Koyota S, Sugiyama T, et al. Ameloblastin in Hertwig's epithelial root sheath regulates tooth root formation and development. PLoS One. 2013;8:e54449. doi: 10.1371/journal.pone.0054449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Tamburstuen MV, Reseland JE, Spahr A, Brookes SJ, Kvalheim G, Slaby I, et al. Ameloblastin expression and putative autoregulation in mesenchymal cells suggest a role in early bone formation and repair. Bone. 2011;48:406–13. doi: 10.1016/j.bone.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Spahr A, Lyngstadaas SP, Slaby I, Haller B, Boeckh C, Tsoulfidou F, et al. Expression of amelin and trauma-induced dentin formation. Clinical oral investigations. 2002;6:51–7. doi: 10.1007/s00784-001-0139-y. [DOI] [PubMed] [Google Scholar]
- [351].Zhang X, Diekwisch TGH, Luan X. Structure and Function of Ameloblastin as an Extracellular Matrix Protein: Adhesion, Calcium Binding, and CD63-Interaction in Human and Mouse. European journal of oral sciences. 2011;119:270–9. doi: 10.1111/j.1600-0722.2011.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Iizuka S, Kudo Y, Yoshida M, Tsunematsu T, Yoshiko Y, Uchida T, et al. Ameloblastin Regulates Osteogenic Differentiation by Inhibiting Src Kinase via Cross Talk between Integrin β1 and CD63. Molecular and Cellular Biology. 2011;31:783–92. doi: 10.1128/MCB.00912-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Cowles EA, DeRome ME, Pastizzo G, Brailey LL, Gronowicz GA. Mineralization and the expression of matrix proteins during in vivo bone development. Calcified tissue international. 1998;62:74–82. doi: 10.1007/s002239900397. [DOI] [PubMed] [Google Scholar]
- [354].Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802 doi: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- [355].Pankov R, Yamada KM. Fibronectin at a glance. Journal of cell science. 2002;115:3861. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- [356].Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. Journal of cell science. 1997;110:2187. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- [357].Liao Y-F, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA Segment of Fibronectin Is a Ligand for Integrins α9β1 and α4β1 Providing a Novel Mechanism for Regulating Cell Adhesion by Alternative Splicing. Journal of Biological Chemistry. 2002;277:14467–74. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- [358].Higuchi A, Ling Q-D, Hsu S-T, Umezawa A. Biomimetic Cell Culture Proteins as Extracellular Matrices for Stem Cell Differentiation. Chemical Reviews. 2012;112:4507–40. doi: 10.1021/cr3000169. [DOI] [PubMed] [Google Scholar]
- [359].Agarwal R, Gonzalez-Garcia C, Torstrick B, Guldberg RE, Salmeron-Sanchez M, Garcia AJ. Simple coating with fibronectin fragment enhances stainless steel screw osseointegration in healthy and osteoporotic rats. Biomaterials. 2015;63:137–45. doi: 10.1016/j.biomaterials.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Kim S, Myung W-C, Lee J-S, Cha J-K, Jung U-W, Yang H-C, et al. The effect of fibronectin-coated implant on canine osseointegration. Journal of Periodontal & Implant Science. 2011;41:242–7. doi: 10.5051/jpis.2011.41.5.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Kammerer PW, Lehnert M, Al-Nawas B, Kumar VV, Hagmann S, Alshihri A, et al. Osseoconductivity of a Specific Streptavidin-Biotin-Fibronectin Surface Coating of Biotinylated Titanium Implants - A Rabbit Animal Study. Clinical implant dentistry and related research. 2015;17(Suppl 2):e601–12. doi: 10.1111/cid.12292. [DOI] [PubMed] [Google Scholar]
- [362].Schonmeyr BH, Wong AK, Li S, Gewalli F, Cordiero PG, Mehrara BJ. Treatment of hydroxyapatite scaffolds with fibronectin and fetal calf serum increases osteoblast adhesion and proliferation in vitro. Plastic and reconstructive surgery. 2008;121:751–62. doi: 10.1097/01.prs.0000299312.02227.81. [DOI] [PubMed] [Google Scholar]
- [363].Mohamadyar-Toupkanlou F, Vasheghani-Farahani E, Hanaee-Ahvaz H, Soleimani M, Dodel M, Havasi P, et al. Osteogenic Differentiation of MSCs on Fibronectin-Coated and nHA-Modified Scaffolds. ASAIO journal (American Society for Artificial Internal Organs : 1992) 2017;63:684–91. doi: 10.1097/MAT.0000000000000551. [DOI] [PubMed] [Google Scholar]
- [364].Subbiah R, Du P, Van SY, Suhaeri M, Hwang MP, Lee K, et al. Fibronectin-tethered graphene oxide as an artificial matrix for osteogenesis. Biomedical materials (Bristol, England) 2014;9 doi: 10.1088/1748-6041/9/6/065003. 065003. [DOI] [PubMed] [Google Scholar]
- [365].Zhang Y, Xiang Q, Dong S, Li C, Zhou Y. Fabrication and characterization of a recombinant fibronectin/cadherin bio-inspired ceramic surface and its influence on adhesion and ossification in vitro. Acta Biomater. 2010;6:776–85. doi: 10.1016/j.actbio.2009.08.025. [DOI] [PubMed] [Google Scholar]
- [366].Xing J, Mei T, Luo K, Li Z, Yang A, Li Z, et al. A nano-scaled and multi-layered recombinant fibronectin/cadherin chimera composite selectively concentrates osteogenesis-related cells and factors to aid bone repair. Acta Biomater. 2017;53:470–82. doi: 10.1016/j.actbio.2017.02.016. [DOI] [PubMed] [Google Scholar]
- [367].Gu YC, Kortesmaa J, Tryggvason K, Persson J, Ekblom P, Jacobsen SE, et al. Laminin isoform-specific promotion of adhesion and migration of human bone marrow progenitor cells. Blood. 2003;101:877–85. doi: 10.1182/blood-2002-03-0796. [DOI] [PubMed] [Google Scholar]
- [368].Kang HK, Kim OB, Min SK, Jung SY, Jang DH, Kwon TK, et al. The effect of the DLTIDDSYWYRI motif of the human laminin alpha2 chain on implant osseointegration. Biomaterials. 2013;34:4027–37. doi: 10.1016/j.biomaterials.2013.02.023. [DOI] [PubMed] [Google Scholar]
- [369].Bougas K, Jimbo R, Xue Y, Mustafa K, Wennerberg A. Novel Implant Coating Agent Promotes Gene Expression of Osteogenic Markers in Rats during Early Osseointegration. International Journal of Biomaterials. 2012;2012:9. doi: 10.1155/2012/579274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Bougas K, Jimbo R, Vandeweghe S, Hayashi M, Bryington M, Kozai Y, et al. Bone apposition to laminin-1 coated implants: histologic and 3D evaluation. International Journal of Oral and Maxillofacial Surgery. 2013;42:677–82. doi: 10.1016/j.ijom.2012.11.008. [DOI] [PubMed] [Google Scholar]
- [371].Bougas K, Stenport VF, Currie F, Wennerberg A. Laminin Coating Promotes Calcium Phosphate Precipitation on Titanium Discs in vitro. Journal of oral & maxillofacial research. 2012;2:e5. doi: 10.5037/jomr.2011.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Francisco AT, Hwang PY, Jeong CG, Jing L, Chen J, Setton LA. Photocrosslinkable laminin-functionalized polyethylene glycol hydrogel for intervertebral disc regeneration. Acta Biomater. 2014;10:1102–11. doi: 10.1016/j.actbio.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Francisco AT, Mancino RJ, Bowles RD, Brunger JM, Tainter DM, Chen YT, et al. Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials. 2013;34:7381–8. doi: 10.1016/j.biomaterials.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Kumagai T, Lee I, Ono Y, Maeno M, Takagi M. Ultrastructural localization and biochemical characterization of vitronectin in developing rat bone. The Histochemical journal. 1998;30:111–9. doi: 10.1023/a:1003235100960. [DOI] [PubMed] [Google Scholar]
- [375].Seiffert D. Detection of vitronectin in mineralized bone matrix. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1996;44:275–80. doi: 10.1177/44.3.8648088. [DOI] [PubMed] [Google Scholar]
- [376].Preissner KT. Structure and Biological Role of Vitronectin. Annual Review of Cell Biology. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- [377].Schleicher I, Parker A, Leavesley D, Crawford R, Upton Z, Xiao Y. Surface modification by complexes of vitronectin and growth factors for serum-free culture of human osteoblasts. Tissue engineering. 2005;11:1688–98. doi: 10.1089/ten.2005.11.1688. [DOI] [PubMed] [Google Scholar]
- [378].Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Advanced Drug Delivery Reviews. 2003;55:1531–46. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- [379].Ferreira AM, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta Biomaterialia. 2012;8:3191–200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- [380].Sartori M, Giavaresi G, Parrilli A, Ferrari A, Aldini NN, Morra M, et al. Collagen type I coating stimulates bone regeneration and osteointegration of titanium implants in the osteopenic rat. International orthopaedics. 2015;39:2041–52. doi: 10.1007/s00264-015-2926-0. [DOI] [PubMed] [Google Scholar]
- [381].Chamieh F, Collignon A-M, Coyac BR, Lesieur J, Ribes S, Sadoine J, et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Scientific Reports. 2016;6 doi: 10.1038/srep38814. 38814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Bi F, Shi Z, Liu A, Guo P, Yan S. Anterior cruciate ligament reconstruction in a rabbit model using silk-collagen scaffold and comparison with autograft. PLoS One. 2015;10:e0125900. doi: 10.1371/journal.pone.0125900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Wang X, Wang G, Liu L, Zhang D. The mechanism of a chitosan-collagen composite film used as biomaterial support for MC3T3-E1 cell differentiation. Scientific Reports. 2016;6 doi: 10.1038/srep39322. 39322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Guillaume O, Naqvi SM, Lennon K, Buckley CT. Enhancing cell migration in shape-memory alginate-collagen composite scaffolds: In vitro and ex vivo assessment for intervertebral disc repair. Journal of biomaterials applications. 2015;29:1230–46. doi: 10.1177/0885328214557905. [DOI] [PubMed] [Google Scholar]
- [385].Eweida A, Schulte M, Frisch O, Kneser U, Harhaus L. The impact of various scaffold components on vascularized bone constructs. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2017;45:881–90. doi: 10.1016/j.jcms.2017.02.016. [DOI] [PubMed] [Google Scholar]
- [386].Foidart J-M, Reddi AH. Immunofluorescent localization of type IV collagen and laminin during endochondral bone differentiation and regulation by pituitary growth hormone. Developmental Biology. 1980;75:130–6. doi: 10.1016/0012-1606(80)90149-9. [DOI] [PubMed] [Google Scholar]
- [387].Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor β type 1 binds to collagen IV of basement membrane matrix: Implications for development. Developmental Biology. 1991;143:303–8. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- [388].Paralkar VM, Weeks BS, Yu YM, Kleinman HK, Reddi AH. Recombinant human bone morphogenetic protein 2B stimulates PC12 cell differentiation: potentiation and binding to type IV collagen. The Journal of cell biology. 1992;119:1721. doi: 10.1083/jcb.119.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Gao TJ, Lindholm TS, Kommonen B, Ragni P, Paronzini A, Lindholm TC, et al. Enhanced healing of segmental tibial defects in sheep by a composite bone substitute composed of tricalcium phosphate cylinder, bone morphogenetic protein, and type IV collagen. Journal of biomedical materials research. 1996;32:505–12. doi: 10.1002/(SICI)1097-4636(199612)32:4<505::AID-JBM2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- [390].Rosati R, Horan GS, Pinero GJ, Garofalo S, Keene DR, Horton WA, et al. Normal long bone growth and development in type X collagen-null mice. Nature genetics. 1994;8:129–35. doi: 10.1038/ng1094-129. [DOI] [PubMed] [Google Scholar]
- [391].Kirsch T, von der Mark K. Ca2+ binding properties of type X collagen. FEBS Lett. 1991;294:149–52. doi: 10.1016/0014-5793(91)81363-d. [DOI] [PubMed] [Google Scholar]
- [392].Young MF, Kerr JM, Ibaraki K, Heegaard AM, Robey PG. Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clinical orthopaedics and related research. 1992:275–94. [PubMed] [Google Scholar]
- [393].Alford AI, Hankenson KD. Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–57. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- [394].Delany AM, Kalajzic I, Bradshaw AD, Sage EH, Canalis E. Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology. 2003;144:2588–96. doi: 10.1210/en.2002-221044. [DOI] [PubMed] [Google Scholar]
- [395].Bradshaw AD. The role of SPARC in extracellular matrix assembly. Journal of Cell Communication and Signaling. 2009;3:239–46. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [396].Alford AI, Kozloff KM, Hankenson KD. Extracellular matrix networks in bone remodeling. The International Journal of Biochemistry & Cell Biology. 2015;65:20–31. doi: 10.1016/j.biocel.2015.05.008. [DOI] [PubMed] [Google Scholar]
- [397].Liao S, Ngiam M, Chan CK, Ramakrishna S. Fabrication of nano-hydroxyapatite/collagen/osteonectin composites for bone graft applications. Biomedical materials (Bristol, England) 2009;4 doi: 10.1088/1748-6041/4/2/025019. 025019. [DOI] [PubMed] [Google Scholar]
- [398].Pataquiva-Mateus AY, Wu HC, Lucchesi C, Ferraz MP, Monteiro FJ, Spector M. Supplementation of collagen scaffolds with SPARC to facilitate mineralization. Journal of biomedical materials research Part B, Applied biomaterials. 2012;100:862–70. doi: 10.1002/jbm.b.32650. [DOI] [PubMed] [Google Scholar]
- [399].Zoch ML, Clemens TL, Riddle RC. New Insights into the Biology of Osteocalcin. Bone. 2016;82:42–9. doi: 10.1016/j.bone.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Knepper-Nicolai B, Reinstorf A, Hofinger I, Flade K, Wenz R, Pompe W. Influence of osteocalcin and collagen I on the mechanical and biological properties of Biocement D. Biomolecular engineering. 2002;19:227–31. doi: 10.1016/s1389-0344(02)00036-9. [DOI] [PubMed] [Google Scholar]
- [401].Parisuthiman D, Mochida Y, Duarte WR, Yamauchi M. Biglycan Modulates Osteoblast Differentiation and Matrix Mineralization. Journal of Bone and Mineral Research. 2005;20:1878–86. doi: 10.1359/JBMR.050612. [DOI] [PubMed] [Google Scholar]
- [402].Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular assembly. The FEBS journal. 2013;280:2120–37. doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Chen XD, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:948–58. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- [404].Douglas T, Heinemann S, Hempel U, Mietrach C, Knieb C, Bierbaum S, et al. Characterization of collagen II fibrils containing biglycan and their effect as a coating on osteoblast adhesion and proliferation. J Mater Sci Mater Med. 2008;19:1653–60. doi: 10.1007/s10856-007-3250-z. [DOI] [PubMed] [Google Scholar]
- [405].Douglas T, Hempel U, Mietrach C, Viola M, Vigetti D, Heinemann S, et al. Influence of collagen-fibril-based coatings containing decorin and biglycan on osteoblast behavior. Journal of biomedical materials research Part A. 2008;84:805–16. doi: 10.1002/jbm.a.31501. [DOI] [PubMed] [Google Scholar]
- [406].Malaval L, Wade-Guéye NM, Boudiffa M, Fei J, Zirngibl R, Chen F, et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. The Journal of Experimental Medicine. 2008;205:1145–53. doi: 10.1084/jem.20071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Bellahcene A, Bonjean K, Fohr B, Fedarko NS, Robey FA, Young MF, et al. Bone sialoprotein mediates human endothelial cell attachment and migration and promotes angiogenesis. Circulation research. 2000;86:885–91. doi: 10.1161/01.res.86.8.885. [DOI] [PubMed] [Google Scholar]
- [408].Ganss B, Kim RH, Sodek J. Bone sialoprotein. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- [409].Im BJ, Lee SC, Lee MH, Leesungbok R, Ahn SJ, Kang YG, et al. Promotion of osteoblastic differentiation and osteogenic transcription factor expression on a microgroove titanium surface with immobilized fibronectin or bone sialoprotein II. Biomedical materials (Bristol, England) 2016;11 doi: 10.1088/1748-6041/11/3/035020. 035020. [DOI] [PubMed] [Google Scholar]
- [410].Baranowski A, Klein A, Ritz U, Ackermann A, Anthonissen J, Kaufmann KB, et al. Surface Functionalization of Orthopedic Titanium Implants with Bone Sialoprotein. PLoS One. 2016;11:e0153978. doi: 10.1371/journal.pone.0153978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Klein A, Baranowski A. Effect of bone sialoprotein coated three-dimensional printed calcium phosphate scaffolds on primary human osteoblasts. 2018 doi: 10.1002/jbm.b.34073. [DOI] [PubMed] [Google Scholar]
- [412].Chan WD, Goldberg HA, Hunter GK, Dixon SJ, Rizkalla AS. Modification of polymer networks with bone sialoprotein promotes cell attachment and spreading. Journal of Biomedical Materials Research Part A. 2010;94A:945–52. doi: 10.1002/jbm.a.32715. [DOI] [PubMed] [Google Scholar]
- [413].Ishijima M, Tsuji K, Rittling SR, Yamashita T, Kurosawa H, Denhardt DT, et al. Resistance to unloading-induced three-dimensional bone loss in osteopontin-deficient mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17:661–7. doi: 10.1359/jbmr.2002.17.4.661. [DOI] [PubMed] [Google Scholar]
- [414].Hunter GK. Role of osteopontin in modulation of hydroxyapatite formation. Calcified tissue international. 2013;93:348–54. doi: 10.1007/s00223-013-9698-6. [DOI] [PubMed] [Google Scholar]
- [415].Jensen T, Baas J, Dolathshahi-Pirouz A, Jacobsen T, Singh G, Nygaard JV, et al. Osteopontin functionalization of hydroxyapatite nanoparticles in a PDLLA matrix promotes bone formation. Journal of biomedical materials research Part A. 2011;99:94–101. doi: 10.1002/jbm.a.33166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [416].Fiorellini JP, Glindmann S, Salcedo J, Weber HP, Park CJ, Sarmiento HL. The Effect of Osteopontin and an Osteopontin-Derived Synthetic Peptide Coating on Osseointegration of Implants in a Canine Model. The International journal of periodontics & restorative dentistry. 2016;36:e88–e94. doi: 10.11607/prd.2830. [DOI] [PubMed] [Google Scholar]
- [417].He X, Yang X, Jabbari E. Combined effect of osteopontin and BMP-2 derived peptides grafted to an adhesive hydrogel on osteogenic and vasculogenic differentiation of marrow stromal cells. Langmuir : the ACS journal of surfaces and colloids. 2012;28:5387–97. doi: 10.1021/la205005h. [DOI] [PubMed] [Google Scholar]
- [418].Jensen T, Dolatshahi-Pirouz A, Foss M, Baas J, Lovmand J, Duch M, et al. Interaction of human mesenchymal stem cells with osteopontin coated hydroxyapatite surfaces. Colloids and surfaces B, Biointerfaces. 2010;75:186–93. doi: 10.1016/j.colsurfb.2009.08.029. [DOI] [PubMed] [Google Scholar]
- [419].Bierbaum S, Douglas T, Hanke T, Scharnweber D, Tippelt S, Monsees TK, et al. Collageneous matrix coatings on titanium implants modified with decorin and chondroitin sulfate: characterization and influence on osteoblastic cells. Journal of biomedical materials research Part A. 2006;77:551–62. doi: 10.1002/jbm.a.30572. [DOI] [PubMed] [Google Scholar]
- [420].Kannus P, Jozsa L, Järvinen TAH, Järvinen TLN, Kvist M, Natri A, et al. Location and Distribution of Non-collagenous Matrix Proteins in Musculoskeletal Tissues of Rat. The Histochemical journal. 1998;30:799–810. doi: 10.1023/a:1003448106673. [DOI] [PubMed] [Google Scholar]
- [421].Bornstein P, Armstrong LC, Hankenson KD, Kyriakides TR, Yang Z. Thrombospondin 2, a matricellular protein with diverse functions. Matrix biology : journal of the International Society for Matrix Biology. 2000;19:557–68. doi: 10.1016/s0945-053x(00)00104-9. [DOI] [PubMed] [Google Scholar]
- [422].Carron JA, Wagstaff SC, Gallagher JA, Bowler WB. A CD36-binding peptide from thrombospondin-1 can stimulate resorption by osteoclasts in vitro. Biochem Biophys Res Commun. 2000;270:1124–7. doi: 10.1006/bbrc.2000.2574. [DOI] [PubMed] [Google Scholar]
- [423].Hankenson KD, Bain SD, Kyriakides TR, Smith EA, Goldstein SA, Bornstein P. Increased marrow-derived osteoprogenitor cells and endosteal bone formation in mice lacking thrombospondin 2. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15:851–62. doi: 10.1359/jbmr.2000.15.5.851. [DOI] [PubMed] [Google Scholar]
- [424].Midwood KS, Chiquet M, Tucker RP, Orend G. Tenascin-C at a glance. Journal of cell science. 2016;129:4321–7. doi: 10.1242/jcs.190546. [DOI] [PubMed] [Google Scholar]
- [425].Giblin SP, Midwood KS. Tenascin-C: Form versus function. Cell Adhesion & Migration. 2015;9:48–82. doi: 10.4161/19336918.2014.987587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [426].He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2003;2:552–8. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- [427].Narayanan K, Gajjeraman S, Ramachandran A, Hao J, George A. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. The Journal of biological chemistry. 2006;281:19064–71. doi: 10.1074/jbc.M600714200. [DOI] [PubMed] [Google Scholar]
- [428].Volk SW, Shah SR, Cohen AJ, Wang Y, Brisson BK, Vogel LK, et al. Type III collagen regulates osteoblastogenesis and the quantity of trabecular bone. Calcified tissue international. 2014;94:621–31. doi: 10.1007/s00223-014-9843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [429].Keene DR, Sakai LY, Burgeson RE. Human bone contains type III collagen, type VI collagen, and fibrillin: type III collagen is present on specific fibers that may mediate attachment of tendons, ligaments, and periosteum to calcified bone cortex. Journal of Histochemistry & Cytochemistry. 1991;39:59–69. doi: 10.1177/39.1.1983874. [DOI] [PubMed] [Google Scholar]
- [430].Kim JK, Xu Y, Xu X, Keene DR, Gurusiddappa S, Liang X, et al. A Novel Binding Site in Collagen Type III for Integrins α1β1 and α2β1. Journal of Biological Chemistry. 2005;280:32512–20. doi: 10.1074/jbc.M502431200. [DOI] [PubMed] [Google Scholar]
- [431].van der Plas RM, Gomes L, Marquart JA, Vink T, Meijers JC, de Groot PG, et al. Binding of von Willebrand factor to collagen type III: role of specific amino acids in the collagen binding domain of vWF and effects of neighboring domains. Thrombosis and haemostasis. 2000;84:1005–11. [PubMed] [Google Scholar]
- [432].Fujita K, Nozaki K, Horiuchi N, Yamashita K, Miura H, Nagai A. Regulation of periodontal ligament-derived cells by type III collagen-coated hydroxyapatite. Bio-medical materials and engineering. 2018;29:15–27. doi: 10.3233/BME-171709. [DOI] [PubMed] [Google Scholar]
- [433].Ghanaati S. Non-cross-linked porcine-based collagen I-III membranes do not require high vascularization rates for their integration within the implantation bed: a paradigm shift. Acta Biomater. 2012;8:3061–72. doi: 10.1016/j.actbio.2012.04.041. [DOI] [PubMed] [Google Scholar]
- [434].Nakamura M, Sone S, Takahashi I, Mizoguchi I, Echigo S, Sasano Y. Expression of Versican and ADAMTS1, 4, and 5 During Bone Development in the Rat Mandible and Hind Limb. Journal of Histochemistry and Cytochemistry. 2005;53:1553–62. doi: 10.1369/jhc.5A6669.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [435].Sotoodehnejadnematalahi F, Burke B. Structure, function and regulation of versican: the most abundant type of proteoglycan in the extracellular matrix. Acta medica Iranica. 2013;51:740–50. [PubMed] [Google Scholar]
- [436].Maurice SB, Bell T, Daniels T, Fetterly CR, Nelson DR, Winwood PJ, et al. Tibial bone versican content decreases with zoledronate treatment in adult mice. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25:1975–81. doi: 10.1007/s00198-014-2700-y. [DOI] [PubMed] [Google Scholar]
- [437].Metz-Estrella D, Jonason JH, Sheu TJ, Mroczek-Johnston RM, Puzas JE. TRIP-1: a regulator of osteoblast function. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:1576–84. doi: 10.1002/jbmr.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [438].Choy L, Derynck R. The Type II Transforming Growth Factor (TGF)-β Receptor-interacting Protein TRIP-1 Acts as a Modulator of the TGF-β Response. Journal of Biological Chemistry. 1998;273:31455–62. doi: 10.1074/jbc.273.47.31455. [DOI] [PubMed] [Google Scholar]
- [439].Benesch J, Mano JF, Reis RL. Proteins and Their Peptide Motifs in Acellular Apatite Mineralization of Scaffolds for Tissue Engineering. Tissue Engineering Part B: Reviews. 2008;14:433–45. doi: 10.1089/ten.teb.2008.0121. [DOI] [PubMed] [Google Scholar]
- [440].Boskey AL. Matrix proteins and mineralization: an overview. Connective tissue research. 1996;35:357–63. doi: 10.3109/03008209609029212. [DOI] [PubMed] [Google Scholar]
- [441].Roach HI. Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell biology international. 1994;18:617–28. doi: 10.1006/cbir.1994.1088. [DOI] [PubMed] [Google Scholar]
- [442].Lin X, Zhao C, Zhu P, Chen J, Yu H, Cai Y, et al. Periosteum Extracellular-Matrix-Mediated Acellular Mineralization during Bone Formation. Advanced healthcare materials. 2018;7 doi: 10.1002/adhm.201700660. 1700660. [DOI] [PubMed] [Google Scholar]
- [443].Frost HM. From Wolff's law to the Utah paradigm: insights about bone physiology and its clinical applications. The Anatomical record. 2001;262:398–419. doi: 10.1002/ar.1049. [DOI] [PubMed] [Google Scholar]
- [444].Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30:781–6. doi: 10.1016/s8756-3282(02)00707-x. [DOI] [PubMed] [Google Scholar]
- [445].Tyrovola JB. The 'Mechanostat Theory' of Frost and the OPG/RANKL/RANK System. Journal of cellular biochemistry. 2015;116:2724–9. doi: 10.1002/jcb.25265. [DOI] [PubMed] [Google Scholar]
- [446].Shiotani A, Shibasaki Y, Sasaki T. Localization of receptor activator of NFκB ligand, RANKL, in periodontal tissues during experimental movement of rat molars. Journal of Electron Microscopy. 2001;50:365–9. doi: 10.1093/jmicro/50.4.365. [DOI] [PubMed] [Google Scholar]
- [447].Oshiro T, Shiotani A, Shibasaki Y, Sasaki T. Osteoclast induction in periodontal tissue during experimental movement of incisors in osteoprotegerin-deficient mice. The Anatomical record. 2002;266:218–25. doi: 10.1002/ar.10061. [DOI] [PubMed] [Google Scholar]
- [448].Wittkowske C, Reilly GC, Lacroix D, Perrault CM. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Frontiers in Bioengineering and Biotechnology. 2016;4:87. doi: 10.3389/fbioe.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [449].Cowin SC, Moss-Salentijn L, Moss ML. Candidates for the mechanosensory system in bone. Journal of biomechanical engineering. 1991;113:191–7. doi: 10.1115/1.2891234. [DOI] [PubMed] [Google Scholar]
- [450].Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. Journal of biomechanics. 1994;27:339–60. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- [451].Fritton SP, Weinbaum S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annual review of fluid mechanics. 2009;41:347–74. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [452].Lanza Robert. Front Matter A2. In: Langer R, Vacanti J, editors. Principles of Tissue Engineering (Third Edition) Burlington: Academic Press; 2007. p. iii. [Google Scholar]
- [453].Roberts WE, Hartsfield JK. Bone development and function: genetic and environmental mechanisms. Seminars in Orthodontics. 2004;10:100–22. [Google Scholar]
- [454].Xiao Z, Quarles LD. Physiological mechanisms and therapeutic potential of bone mechanosensing. Reviews in endocrine & metabolic disorders. 2015;16:115–29. doi: 10.1007/s11154-015-9313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [455].Huang C, Ogawa R. Mechanotransduction in bone repair and regeneration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:3625–32. doi: 10.1096/fj.10-157370. [DOI] [PubMed] [Google Scholar]
- [456].Kegelman CD, Mason DE, Dawahare JH, Horan DJ, Vigil GD, Howard SS, et al. Skeletal cell YAP and TAZ combinatorially promote bone development. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2018 doi: 10.1096/fj.201700872R. fj201700872R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [457].Kegelman CD, Mason DE, Dawahare JH, Vigil GD, Howard SS, Bellido TM, et al. Skeletal Cell YAP And TAZ Redundantly Promote Bone Development By Regulation Of Collagen I Expression And Organization. bioRxiv. 2017 [Google Scholar]
- [458].Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nature Reviews Molecular Cell Biology. 2017;18:758. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [459].Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL. Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells. Biophysical journal. 2015;108:2783–93. doi: 10.1016/j.bpj.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [460].Zhong W, Tian K, Zheng X, Li L, Zhang W, Wang S, et al. Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by Yes-associated protein. Stem Cells Dev. 2013;22:2083–93. doi: 10.1089/scd.2012.0685. [DOI] [PubMed] [Google Scholar]
- [461].Zhong W, Zhang W, Wang S, Qin J. Regulation of fibrochondrogenesis of mesenchymal stem cells in an integrated microfluidic platform embedded with biomimetic nanofibrous scaffolds. PLoS One. 2013;8:e61283. doi: 10.1371/journal.pone.0061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [462].Kim KM, Choi YJ, Hwang JH, Kim AR, Cho HJ, Hwang ES, et al. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PLoS One. 2014;9:e92427. doi: 10.1371/journal.pone.0092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [463].Chen Z, Luo Q, Lin C, Kuang D, Song G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Sci Rep. 2016;6 doi: 10.1038/srep30322. 30322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [464].Tang Y, Feinberg T, Keller ET, Li XY, Weiss SJ. Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nature cell biology. 2016;18:917–29. doi: 10.1038/ncb3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [465].Tang Y, Weiss SJ. Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation. Cell cycle (Georgetown, Tex) 2017;16:399–405. doi: 10.1080/15384101.2017.1280643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [466].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [467].Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32:3921–30. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [468].Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:730–8. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- [469].Kshitiz, Hubbi ME, Ahn EH, Downey J, Afzal J, Kim DH, et al. Matrix rigidity controls endothelial differentiation and morphogenesis of cardiac precursors. Science signaling. 2012;5 doi: 10.1126/scisignal.2003002. ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [470].Lui C, Lee K, Nelson CM. Matrix compliance and RhoA direct the differentiation of mammary progenitor cells. Biomechanics and modeling in mechanobiology. 2012;11:1241–9. doi: 10.1007/s10237-011-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [471].Young DA, Choi YS, Engler AJ, Christman KL. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials. 2013;34:8581–8. doi: 10.1016/j.biomaterials.2013.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [472].Evans ND, Gentleman E, Chen X, Roberts CJ, Polak JM, Stevens MM. Extracellular matrix-mediated osteogenic differentiation of murine embryonic stem cells. Biomaterials. 2010;31:3244–52. doi: 10.1016/j.biomaterials.2010.01.039. [DOI] [PubMed] [Google Scholar]
- [473].Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. European cells & materials. 2009;18:1–13. doi: 10.22203/ecm.v018a01. discussion -4. [DOI] [PubMed] [Google Scholar]
- [474].Narayanan K, Lim VY, Shen J, Tan ZW, Rajendran D, Luo SC, et al. Extracellular matrix-mediated differentiation of human embryonic stem cells: differentiation to insulin-secreting beta cells. Tissue engineering Part A. 2014;20:424–33. doi: 10.1089/ten.TEA.2013.0257. [DOI] [PubMed] [Google Scholar]
- [475].Yang C, DelRio FW, Ma H, Killaars AR, Basta LP, Kyburz KA, et al. Spatially patterned matrix elasticity directs stem cell fate. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E4439–45. doi: 10.1073/pnas.1609731113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [476].Buwalda SJ, Vermonden T, Hennink WE. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules. 2017;18:316–30. doi: 10.1021/acs.biomac.6b01604. [DOI] [PubMed] [Google Scholar]
- [477].Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Materials. 2010;9:518. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [478].Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881–93. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [479].Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nature Materials. 2013;12:458. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [480].Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nature Materials. 2014;13:979. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [481].Leach JK, Whitehead J. Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration. ACS Biomater Sci Eng. 2017 doi: 10.1021/acsbiomaterials.6b00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [482].McMurray RJ, Dalby MJ, Tsimbouri PM. Using biomaterials to study stem cell mechanotransduction, growth and differentiation. J Tissue Eng Regen Med. 2015;9:528–39. doi: 10.1002/term.1957. [DOI] [PubMed] [Google Scholar]
- [483].Lv H, Li L, Sun M, Zhang Y, Chen L, Rong Y, et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem cell research & therapy. 2015;6:103. doi: 10.1186/s13287-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [484].Oliveira MB, Custódio CA, Gasperini L, Reis RL, Mano JF. Autonomous osteogenic differentiation of hASCs encapsulated in methacrylated gellan-gum hydrogels. Acta Biomaterialia. 2016;41:119–32. doi: 10.1016/j.actbio.2016.05.033. [DOI] [PubMed] [Google Scholar]
- [485].Chen G, Dong C, Yang L, Lv Y. 3D Scaffolds with Different Stiffness but the Same Microstructure for Bone Tissue Engineering. ACS applied materials & interfaces. 2015;7:15790–802. doi: 10.1021/acsami.5b02662. [DOI] [PubMed] [Google Scholar]
- [486].Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences. 2013;110:3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [487].Parekh SH, Chatterjee K, Lin-Gibson S, Moore NM, Cicerone MT, Young MF, et al. Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeletal tension. Biomaterials. 2011;32:2256–64. doi: 10.1016/j.biomaterials.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [488].Kaur G, Valarmathi MT, Potts JD, Jabbari E, Sabo-Attwood T, Wang Q. Regulation of osteogenic differentiation of rat bone marrow stromal cells on 2D nanorod substrates. Biomaterials. 2010;31:1732–41. doi: 10.1016/j.biomaterials.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [489].de Peppo GM, Agheli H, Karlsson C, Ekstrom K, Brisby H, Lenneras M, et al. Osteogenic response of human mesenchymal stem cells to well-defined nanoscale topography in vitro. International journal of nanomedicine. 2014;9:2499–515. doi: 10.2147/IJN.S58805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [490].You M-H, Kwak MK, Kim D-H, Kim K, Levchenko A, Kim D-Y, et al. Synergistically Enhanced Osteogenic Differentiation of Human Mesenchymal Stem Cells by Culture on Nanostructured Surfaces with Induction Media. Biomacromolecules. 2010;11:1856–62. doi: 10.1021/bm100374n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [491].Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Materials. 2007;6:997. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- [492].Kantawong F, Burgess KEV, Jayawardena K, Hart A, Burchmore RJ, Gadegaard N, et al. Whole proteome analysis of osteoprogenitor differentiation induced by disordered nanotopography and mediated by ERK signalling. Biomaterials. 2009;30:4723–31. doi: 10.1016/j.biomaterials.2009.05.040. [DOI] [PubMed] [Google Scholar]
- [493].Lavenus S, Berreur M, Trichet V, Pilet P, Louarn G, Layrolle P. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. European cells & materials. 2011;22:84–96. doi: 10.22203/ecm.v022a07. discussion. [DOI] [PubMed] [Google Scholar]
- [494].McNamara LE, Sjostrom T, Burgess KE, Kim JJ, Liu E, Gordonov S, et al. Skeletal stem cell physiology on functionally distinct titania nanotopographies. Biomaterials. 2011;32:7403–10. doi: 10.1016/j.biomaterials.2011.06.063. [DOI] [PubMed] [Google Scholar]
- [495].Sjostrom T, McNamara LE, Meek RM, Dalby MJ, Su B. 2D and 3D nanopatterning of titanium for enhancing osteoinduction of stem cells at implant surfaces. Advanced healthcare materials. 2013;2:1285–93. doi: 10.1002/adhm.201200353. [DOI] [PubMed] [Google Scholar]
- [496].Silverwood RK, Fairhurst PG, Sjostrom T, Welsh F, Sun Y, Li G, et al. Analysis of Osteoclastogenesis/Osteoblastogenesis on Nanotopographical Titania Surfaces. Advanced healthcare materials. 2016;5:947–55. doi: 10.1002/adhm.201500664. [DOI] [PubMed] [Google Scholar]
- [497].McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nature Materials. 2011;10:637. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- [498].Teo BK, Wong ST, Lim CK, Kung TY, Yap CH, Ramagopal Y, et al. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS nano. 2013;7:4785–98. doi: 10.1021/nn304966z. [DOI] [PubMed] [Google Scholar]
- [499].Dobbenga S, Fratila-Apachitei LE, Zadpoor AA. Nanopattern-induced osteogenic differentiation of stem cells - A systematic review. Acta Biomater. 2016;46:3–14. doi: 10.1016/j.actbio.2016.09.031. [DOI] [PubMed] [Google Scholar]
- [500].McKinnon DD, Domaille DW, Cha JN, Anseth KS. Biophysically Defined and Cytocompatible Covalently Adaptable Networks as Viscoelastic 3D Cell Culture Systems. Advanced Materials. 2014;26:865–72. doi: 10.1002/adma.201303680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [501].Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32:5979–93. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- [502].Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, et al. Substrate stress relaxation regulates cell spreading. Nature communications. 2015;6 doi: 10.1038/ncomms7365. 6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [503].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15:326–34. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [504].Hilderbrand AM, Ovadia EM, Rehmann MS, Kharkar PM, Guo C, Kloxin AM. Biomaterials for 4D stem cell culture. Current opinion in solid state & materials science. 2016;20:212–24. doi: 10.1016/j.cossms.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [505].Brown TE, Anseth KS. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chemical Society reviews. 2017;46:6532–52. doi: 10.1039/c7cs00445a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [506].Oliveira SM, Reis RL, Mano JF. Towards the design of 3D multiscale instructive tissue engineering constructs: Current approaches and trends. Biotechnology Advances. 2015;33:842–55. doi: 10.1016/j.biotechadv.2015.05.007. [DOI] [PubMed] [Google Scholar]
- [507].Sgambato A, Russo L, Montesi M, Panseri S, Marcacci M, Carava E, et al. Different Sialoside Epitopes on Collagen Film Surfaces Direct Mesenchymal Stem Cell Fate. ACS applied materials & interfaces. 2016;8:14952–7. doi: 10.1021/acsami.5b08270. [DOI] [PubMed] [Google Scholar]
- [508].Verbruggen SW, Kainz B, Shelmerdine SC, Hajnal JV, Rutherford MA, Arthurs OJ, et al. Stresses and strains on the human fetal skeleton during development. Journal of The Royal Society Interface. 2018;15 doi: 10.1098/rsif.2017.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [509].Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proceedings of the National Academy of Sciences. 2002;99:12600–5. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [510].Hoffmann W, Feliciano S, Martin I, de Wild M, Wendt D. Novel Perfused Compression Bioreactor System as an in vitro Model to Investigate Fracture Healing. Frontiers in Bioengineering and Biotechnology. 2015;3:10. doi: 10.3389/fbioe.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [511].Seo J, Shin JY, Leijten J, Jeon O, Bal Ozturk A, Rouwkema J, et al. Interconnectable Dynamic Compression Bioreactors for Combinatorial Screening of Cell Mechanobiology in Three-dimensions. ACS applied materials & interfaces. 2018 doi: 10.1021/acsami.7b17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [512].Peng Y, Liu Q, He T, Ye K, Yao X, Ding J. Degradation rate affords a dynamic cue to regulate stem cells beyond varied matrix stiffness. Biomaterials. 2018 doi: 10.1016/j.biomaterials.2018.04.021. [DOI] [PubMed] [Google Scholar]
- [513].Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nature communications. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- [514].Bhumiratana S, Bernhard JC, Alfi DM, Yeager K, Eton RE, Bova J, et al. Tissue-engineered autologous grafts for facial bone reconstruction. Science Translational Medicine. 2016;8:343ra83–ra83. doi: 10.1126/scitranslmed.aad5904. [DOI] [PMC free article] [PubMed] [Google Scholar]