Abstract
Liver disease is a significant health problem worldwide with mortality reaching around 2 million deaths a year. Non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD) are the major causes of chronic liver disease. Pathologically, NAFLD and ALD share similar patterns of hepatic disorders ranging from simple steatosis to steatohepatitis, fibrosis and cirrhosis. It is becoming increasingly important to identify new pharmacological targets, given that there is no FDA-approved therapy yet for either NAFLD or ALD. Since the evolution of liver diseases is a multifactorial process, several mechanisms involving parenchymal and non-parenchymal hepatic cells contribute to the initiation and progression of liver pathologies. Moreover, certain protective molecular pathways become repressed during liver injury including signaling pathways such as the cyclic adenosine monophosphate (cAMP) pathway. cAMP, a key second messenger molecule, regulates various cellular functions including lipid metabolism, inflammation, cell differentiation and injury by affecting gene/protein expression and function. This review addresses the current understanding of the role of cAMP metabolism and consequent cAMP signaling pathway(s) in the context of liver health and disease. The cAMP pathway is extremely sophisticated and complex with specific cellular functions dictated by numerous factors such abundance, localization and degradation by phosphodiesterases (PDEs). Furthermore, because of the distinct yet divergent roles of both of its effector molecules, the cAMP pathway is extensively targeted in liver injury to modify its role from physiological to therapeutic, depending on the hepatic condition. This review also examines the behavior of the cAMP-dependent pathway in NAFLD, ALD and in other liver diseases and focuses on PDE inhibition as an excellent therapeutic target in these conditions.
Keywords: Liver, cAMP, PDE, ALD, NAFLD, Rolipram
1. Introduction
The liver is vital for regulating key metabolic processes that result in maintenance of overall energy homeostasis in the body. It has a myriad of functions ranging from xenobiotic detoxification and endobiotic metabolism to synthesis of crucial proteins such as blood clotting factors. Histologically, the liver is predominantly comprised of parenchymal cells (hepatocytes) that constitute approximately 80% of the liver volume. Cells of non-parenchymal origin constitute the rest of the liver, including sinusoidal endothelial cells, resident hepatic macrophages (Kupffer cells) and hepatic stellate cells (HSCs) [1]. Hepatocytes perform basic metabolic functions such as metabolism of lipids and amino acids, biochemical oxidation reactions and detoxification. Kupffer cells play a protective role against gut-derived toxins in the liver and in the regulation of hepatic inflammation by secreting cytokines. HSCs are crucial for vitamin A storage and wound healing, while hepatic sinusoidal cells secrete adhesion molecules and play a role in endocytosis. Hepatic disorders that result in a compromised or dysfunctional liver are brought upon by a multitude of pathogenic mechanisms driven by factors such as hyper-caloric diets, drugs, viral infections, genetic predisposition, alcohol consumption and chemical exposure, to name a few. Since the evolution of liver diseases is a multifactorial process, several mechanisms involving parenchymal and non-parenchymal hepatic cells contribute to the initiation and progression of liver pathologies.
Cyclic nucleotides including cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are key intracellular second messengers affecting multiple cellular processes [2]. This review article addresses the relevance of the cAMP-dependent pathways in regulating the physiologic and pathologic aspects that underlie the development of different types of liver disorders. Additionally, the review evaluates the potential of targeting cAMP metabolism for therapeutic purposes in the treatment and management of various stages of multiple liver diseases.
1.1. Liver disease
There are many types of liver diseases, and they are generally categorized based on the cause or etiology. This review focuses on the most common types of liver diseases, namely non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD). NAFLD is prevalent in approximately 20–30% and 5–18% of the populations in western countries and Asia, respectively, and it affects approximately 30% of the US adult population, most of whom are already predisposed to other metabolic disorders [3, 4]. ALD is prevalent in heavy alcoholic drinkers and it is the eighth most common cause of mortality in the US [5, 6].
Both NAFLD and ALD are complex diseases that are progressive in nature, and encompass a spectrum of disorders that are initially manifested as hepatic fat accumulation or simple steatosis [7, 8]. Steatosis is often accompanied by inflammation, also known as non-alcoholic steatohepatitis (NASH) or alcoholic steatohepatitis (ASH), which is characterized by infiltration and activation of immune cells in the liver. Persistent activation of immune cells could stimulate pro-fibrogenic signaling in hepatic stellate cells leading to fibrosis, and potentially cirrhosis and chronic liver disease leading to liver failure.
Pathologically, ALD is similar to NAFLD, with stages ranging from simple steatosis to alcoholic steatohepatitis (ASH), to fibrosis and cirrhosis. NAFLD and ALD exhibit almost identical clinical and histological features that are often indistinguishable from each other. Although NASH is considered to be milder than ASH [8], both can lead to cirrhosis and, potentially, even liver transplant. ALD is caused by chronic alcohol consumption as opposed to NAFLD that can be associated with other metabolic-related disorders such as obesity, diabetes and insulin resistance, and the metabolic syndrome [11, 12]. Heavy drinkers tend to develop simple steatosis, but only 10–35% of heavy drinkers develop alcoholic hepatitis, and between 15 and 20% of heavy drinkers develop more severe forms of ALD such as fibrosis and cirrhosis [9, 10]. Although NAFLD and ALD bear similar histological and clinical features, the pathophysiological mechanisms underlying disease progression can be quite distinct.
Although causes of steatosis are different in NAFLD and ALD (excess calories vs ethanol metabolism), the mechanisms are very similar. Specifically, dysregulation of lipid metabolism with increased de novo lipogenesis and impaired fatty acid oxidation are the main causes of lipid accumulation within hepatocytes in both diseases [8]. Additionally, changes in enzyme activities critical for lipid synthesis (Acetyl-CoA carboxylase) and degradation (carnitine palmitoyl-transferase) have been reported to play a critical role in the development of steatosis in NAFLD and ALD. Importantly, fatty acid accumulation can lead to lipotoxicity [8]. Specifically, we and others have reported that cytotoxic free fatty acids cause mitochondrial dysfunction in hepatocytes [11–13]. Moreover, damaged hepatocytes produce chemoattractant cytokines leading to neutrophil infiltration into the liver and inflammatory liver injury [11, 13]. Additionally, lipid accumulation triggers organelle dysfunction such as endoplasmic reticulum (ER) stress and oxidative stress, thereby triggering signaling cascades leading to apoptosis [14]. Likewise, free fatty acids can also interfere with insulin signaling, as well as activate death receptors leading to hepatocyte death [15].
On the other hand, in ALD, metabolites of ethanol metabolism can act directly on hepatocytes to induce ER and oxidative stress, eventually triggering pro-apoptotic signaling pathways as well as stimulating the innate immune response [16, 17]. Hepatocyte death also occurs through multiple mechanisms including apoptosis and necroptosis [8]. Ethanol consumption can also increase gut permeability resulting in lipopolysaccharide (LPS) leakage into the circulation, which then activates toll-like receptor 4 (TLR4) on hepatocytes and macrophages to incite inflammatory responses [18]. In addition, hepatic cells can also release extracellular vesicles in ALD, as well as damage-associated molecular patterns (DAMPs), that recruit macrophages thereby leading to HSC activation and migration [19]. HSC activation and phenotypic change into proliferative, contractile, and chemotactic myofibroblasts is the key cellular process of hepatic fibrosis [20–23]. Myofibroblasts migrate and accumulate at the site of injury and produce increasing amounts of extracellular matrix (ECM) components such as collagens and fibronectin [24]. Excessive scar deposition in the liver results in a significant deterioration of liver function, altered blood flow and eventually liver failure [25].
1.2. General cAMP signaling: effectors and regulation
Cyclic AMP, a second messenger molecule discovered over half a century ago by Earl Sutherland and colleagues, is pivotal for many physiological processes [26, 27]. It is synthesized from adenosine triphosphate (ATP) by the enzymes, adenylyl cyclases (ACs). There are ten mammalian AC genes encoding ten isoforms (AC1 to AC10) [28]. AC1 to AC9 are transmembrane ACs (tmACs) which respond to G proteincoupled receptor (GPCR) activation to extracellular hormones and neurotransmitters. Soluble AC is encoded by AC10 gene and responds to Ca2+ and bicarbonate [29–31]. cAMP is the initiating component of the intracellular signal transduction pathway known as cAMP-dependent pathway or adenylyl cyclase pathway. The cAMP-dependent pathway is a G-protein coupled receptor (GPCR)-triggered signaling cascade, wherein GPCRs are a family of integral membrane proteins that respond to a number of extracellular stimuli [32]. When GPCRs are activated by a specific ligand, they undergo a conformational change and activate the stimulatory alpha subunit of the G-protein complex (Gs), which exchanges a guanosine diphosphate for a guanosine triphosphate and is then released from the complex. The activated Gs alpha subunit then binds and activates the molecular signal integrator, adenylyl cyclase, which subsequently catalyzes the conversion of ATP into cAMP. The changes in cAMP levels inside the cell vary in terms of time frame, with transient increases occurring in milliseconds to more stable increases that last from hours to days, depending on the location and activators [33]. Increases in cAMP levels leads to activation of a variety of effector molecules, with the protein kinase A (PKA) family of proteins and the exchange proteins activated by cAMP (EPACs) being the classical and most recognized downstream targets [34]. Increase in cAMP levels can also lead to activation of cyclic nucleotide-gated (CNG) ion channels that regulate signal transduction in the retina (vision) and olfactory receptor neurons (olfaction) as well as the recently discovered popeye domain containing proteins (Popdc) that mediate epithelial cell function and physiology of cardiac and skeletal muscle [35, 36].
The PKA protein was first discovered in 1968 by Edmond H. Fischer and Edwin G. Krebs and further characterized by Susan S. Taylor and colleagues [37–40]. As its name suggests, PKA activation leads to phosphorylation of a number of downstream protein targets that regulate varying cell functions depending on their location. The classical PKA holoenzyme exists as a tetramer with two regulatory subunits that have cAMP binding domains and two catalytic subunits. A rise in cAMP levels leads to binding of two cAMP molecules to each regulatory subunit, which induces a conformational change resulting in detachment of the two regulatory subunits and release of the two catalytic subunits that are now activated. Once freed, the catalytic subunits catalyze the transfer of ATP terminal phosphates to protein substrates at serine or threonine residues (phosphorylation) which causes a change in the substrate’s activity. PKA has been designated as PKA(I) and PKA(II) by differences in the regulatory subunits that interact with an identical catalytic subunit [41]. Different regulatory subunit isoforms are differentially expressed in different tissues and have distinct roles. PKA substrates perform numerous cell functions including regulation of lipid and glucose metabolism, kinases for smooth muscle contraction, dopamine signaling in the brain and renin secretion in the kidney. Apart from directly phosphorylating protein substrates, PKA also regulates gene transcription and protein synthesis by activating the cAMP response element-binding protein (CREB), a transcription factor that binds to DNA sequences called cAMP response elements (CRE) [42].
Historically, it was thought that major effects of cAMP were solely mediated by PKA; however, in 1998, EPAC (exchange protein directly activated by cAMP) was identified as a new family of cAMP sensor proteins [43, 44]. There are two EPAC isoforms, EPAC1 and EPAC2, and both proteins have a cAMP binding domain that is homologous to that of PKA. While EPAC1 is expressed ubiquitously, EPAC2 is mainly expressed in the liver, brain, pancreas and adrenal gland. Upon binding to cAMP, EPAC activates the Ras superfamily small GTPases, Rap1 and Rap2. Cellular functions of EPAC range from regulation of cell adhesion and formation of cell-cell junctions through the EPAC/Rap1 signaling pathway, to mediating adipocyte differentiation and cardiomyocyte hypertrophy, dictated largely by their distinct tissue distribution.
Degradation of cyclic nucleotides is carried out by a group of enzymes known as cyclic nucleotide phosphodiesterases (PDEs). PDEs regulate cAMP and cGMP levels and signaling by their hydrolysis thus affecting cAMP and cGMP-dependent processes [45]. Of the cAMPspecific PDES, the PDE4 family is widely expressed accounting for the majority of cAMP hydrolysis activity in cells, and therefore it has been used as a therapeutic target for various inflammatory diseases as well as for depression and cognitive deficits.
Considering the different effectors and their unique functions, the cAMP pathway is extremely sophisticated and complex with specific cellular functions being dictated by numerous factors such as cAMP levels and abundance, localization, distribution and behavior of the cAMP receptors when activated, as well as the presence of PDEs.
1.3. cAMP in liver: physiological role, expression, activation
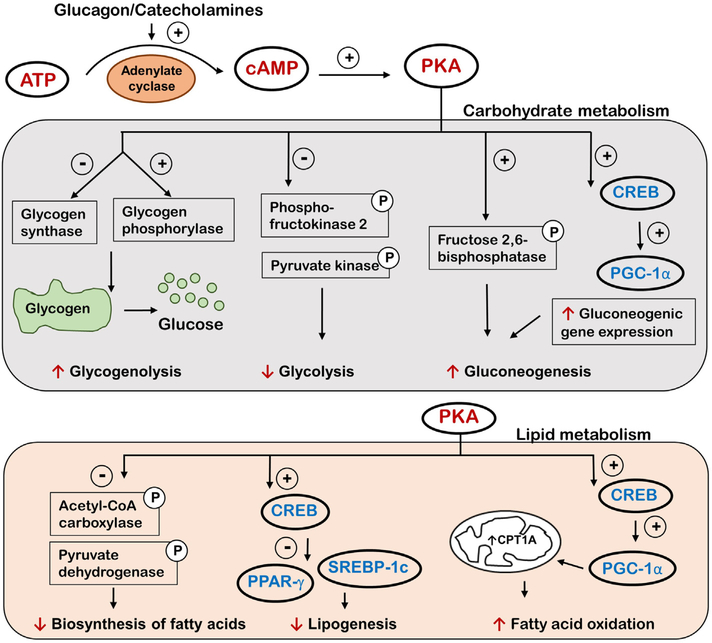
The role of the cAMP/PKA signaling pathway in the liver is well documented with numerous studies demonstrating involvement of the pathway in various metabolic functions through regulation of gene transcription and kinase activity, and key effects include facilitation of carbohydrate and lipid metabolism [46]. Glucagon and catecholamines, such as adrenaline in the liver, activate adenylyl cyclase which synthesizes cAMP, thus giving rise to increasing cAMP levels in the liver [47]. Increased cAMP levels lead to increased glucose production through stimulation of glucose-producing pathways by increasing the transcription of gluconeogenic enzymes, glucose 6-phosphatase (G6Pase), phosphoenolpyruvate carboxykinase (PEPCK) and pyruvate carboxylase (PC) [48]. Additionally, PKA phosphorylates fructose 2,6bisphosphatase, which activates the enzyme, and this leads to stimulation of gluconeogenesis [49]. On the other hand, PKA phosphorylates key enzymes involved in glycolysis (phosphofructokinase-2 and pyruvate kinase) and inactivates them. Furthermore, PKA inhibits glycogen synthase and activates glycogen phosphorylase through phosphorylation, leading to suppressed glycogenesis and activated glycogenolysis, respectively.
Rising hepatic cAMP levels also lead to inhibition of lipogenesis through PKA-mediated phosphorylation of key players in fatty acid synthesis, such as acetyl-CoA carboxylase and pyruvate dehydrogenase, thereby inhibiting their activity [50]. Insulin, the hormone with opposing effects to that of glucagon, can reverse the phosphorylation levels of these enzymes and stimulate lipogenesis while also decreasing glucose production. Interestingly, an important extra-hepatic cAMP effect is to promote the release of insulin from pancreatic beta cells [51]. Insulin then migrates to the liver and adipose tissue and suppresses the accumulation of cAMP, therefore exhibiting a tight co-ordination on both glucose and lipid metabolism in the liver. cAMP also regulates lipid metabolism through cAMP-responsive transcription factors such as CREB proteins that are activated by PKA [52]. Multiple studies have shown that CREB acts as a checkpoint in both glucose and lipid metabolism through its target gene battery. The cAMP/CREB pathway represses hepatic expression of genes involved in lipid synthesis such as the nuclear hormone receptor, peroxisome-proliferator activator receptor gamma (PPAR-γ), a key regulator of lipogenic genes [53]. Activated CREB also stimulates expression of the nuclear hormone receptor coactivator known as peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC-1α), which upregulates gluconeogenic genes as well as genes involved in mitochondrial fatty acid oxidation [54]. Additionally, a recent study reported that CREB can also regulate the activity of sterol regulatory element-binding protein-1c (SREBP-1c), a transcription factor for lipogenic genes involved in de novo hepatic lipogenesis, such as fatty acid synthase, through the insulin induced gene-2 (Insig-2) [55]. The outcomes of PKA activation in the liver are summarized in Fig. 1.
Fig. 1.
A schematic diagram showing the well-characterized outcomes concerning carbohydrate and lipid metabolism as a result of increased intracellular cAMP and PKA activation in the liver. PKA activation results in phosphorylation of different effector molecules that either leads to activation (+) or inactivation (−) with the net effect of increased glucose production and decreased lipid accumulation in the liver. ATP - adenosine triphosphate, cAMP cyclic adenosine monophosphate, PKA - protein kinase A, CREB - cAMP response element-binding protein, PGC-1α - peroxisome proliferator-activated receptor gamma coactivator 1-alpha, PPARγ - peroxisome-proliferator activator receptor gamma, SREBP-1c - sterol regulatory-element binding protein-1c
Elucidating the hepatic role of the other cAMP sensor, EPAC, only began recently as compared to PKA. The cAMP/EPAC pathway is also heavily involved in the maintenance of metabolic homeostasis in the body, such as by inducing leptin resistance, as well as by increasing insulin secretion and sensitivity [56]. Recent papers also demonstrate the role of EPAC as a regulator of fibrosis through multiple pathways including the inhibition of epithelial cell transformation, ECM formation and hepatic stellate cell proliferation and migration [57, 58].
Overall, cAMP plays an important role in regulating hepatic energy metabolism. Moreover, because of the distinct and divergent roles of both PKA and EPAC, it appears that the cAMP pathway is extensively targeted in liver injury. The role of this pathway can also range from physiological to therapeutic, depending on the state of the liver. The next section examines the behavior of the cAMP-dependent pathway in NAFLD, ALD and in other liver diseases, and it will focus on the hepatic functions of the pathway during such diseased conditions.
2. The role of cAMP in NAFLD
Due to its unique hepatic effects that appear beneficial, the role of the cAMP signaling pathway has been investigated in NAFLD. As mentioned previously, NAFLD initially manifests itself as simple steatosis that may progress to steatohepatitis. Therefore, lowering lipid production and accumulation while simultaneously increasing lipid breakdown, should be helpful in attenuating NAFLD. For example, administration of glucagon-like peptide (GLP-1) in obese mice (ob/ob mutant mice) lowered serum alanine aminotransferase (ALT, a marker of liver injury), hepatic oxidative stress and lipid accumulation in the liver. GLP-1 increased hepatocyte cAMP levels and induced genes involved in fatty acid oxidation, while suppressing genes involved in de novo lipogenesis [59]. Additionally, in a mouse model of high fat diet-induced NASH, GLP-1 increased PKA and AMP-activated protein kinase (AMPK) activity, resulting in enhanced expression of PPARα-dependent genes involved in fatty acid β-oxidation [60].
Moreover, a study investigating the therapeutic effects of resveratrol, a naturally occurring polyphenol, in NAFLD demonstrated that its protective effects were mediated, in part, by the cAMP pathway [61]. Specifically, resveratrol improved hepatic steatosis in a high fat diet mouse model of NAFLD via improved fatty acid β-oxidation by inducing autophagy. The authors also showed that resveratrol increased SIRT-1dependent autophagy in hepatocytes via cAMP/PKA pathway. Several other studies have also shown the beneficial role of resveratrol in preventing hepatic steatosis and injury in various NAFLD models [62–66]. Importantly, resveratrol has been demonstrated to increase cAMP by inhibiting PDE4 [67]. A recent study also reported that dietary supplementation of a reduced form of coenzyme Q10 (CoQ10H2) suppressed hepatic PDE4 expression, increased cAMP levels and fatty acid β-oxidation via the SIRT-1/PGC-1α/PPARα pathway, and inhibited the development and progression of obesity and type 2 diabetes in a mouse model [68]. The authors also reported the inhibitory effect of CoQ10H2 on the genes involved in de novo lipogenesis in the liver such as SREBP1c. Thus, the body of evidence available to date supports a role for cAMP as a positive mediator in the attenuation of steatosis and obesity in NAFLD and NASH.
2.1. The role of cAMP in ALD
A plethora of studies have been conducted to elucidate the role of cAMP in ALD. The first report on lower cAMP levels in resting peripheral blood mononuclear cells of alcoholic hepatitis (AH) patients was published in 1983 and suggested that these patients had an immune dysfunction [69]. In 1987, another study confirmed that lymphocytes from alcoholic patients had much lower basal and adenosine receptor stimulated cAMP levels [70]. Later, effects of cAMP in the development of liver injury was examined in a rat model of ALD. Rats were fed alcohol via a permanent intra-gastric cannula for 2 months and a group of animals was given cAMP by intraperitoneal administration. The study showed that cAMP prevented the increase in liver fat accumulation caused by alcohol feeding [71]. The authors suggested that the cAMP effect on alcohol-induced hepatic steatosis was partially mediated by the cAMP effect on the alcohol-metabolizing enzyme, CYP2E1 [71]. In a chronic binge ethanol model, rats demonstrated dysregulated hepatic lipid and glucose metabolism, which were attributed to ethanol-induced defects in nuclear translocation and phosphorylation of CREB [72]. This defect in CREB activation was accompanied by increased lipid accumulation in the liver and suppression of carnitine palmitoyltransferase 1A (CPT1A), the rate limiting enzyme for mitochondrial fatty acid oxidation [73]. Importantly, a recent study from our group using a mouse ALD model demonstrated that chronic alcohol feeding reduced hepatic cAMP levels leading to decreased phospho-CREB levels [74]. We further demonstrated that this decrease in cAMP levels was mediated by increased hepatic PDE4 expression/ activity in both the whole liver as well as isolated hepatocytes. Induction of PDE4 and resultant compromised cAMP signaling contributed to dysregulated fatty acid β-oxidation by decreasing CPT1A expression. Moreover, a PKA specific agonist increased CPT1A levels in primary hepatocytes [74]. In addition to fatty acid oxidation, inhibition of lipid droplet lipolysis via cytosolic lipases in alcohol-exposed hepatocytes has been recently demonstrated [75]. This inhibition was mediated by the inability of alcohol-exposed hepatocytes to activate PKA in response to β-adrenergic stimulation and recruitment of lipases to lipid droplets.
ALD patients often display endotoxemia where gut bacteria and microbial products escape into the systemic circulation due to a leaky gut [76–79]. Endotoxemia leads to dysregulated cytokine metabolism with increased pro-inflammatory (e.g. TNFα, IL-1β) and decreased antiinflammatory cytokine (e.g. IL-10) production. Earlier work by our group demonstrated that chronic ethanol-mediated decreases in cAMP in monocytes and macrophages correlated with enhanced inflammation and LPS-induced TNFα production [80]. Additionally, numerous studies have documented suppression of LPS-induced TNFα production by cAMP in various cell types [81–84]. Importantly, increased inflammatory cytokine expression in Kupffer cells, particularly TNFα, plays a key role in the pathogenesis of alcoholic hepatitis and ALD [76, 85–88]. In fact, a study employing the administration of anti-TNFα antibody to alcohol-fed rats showed beneficial effects against liver injury, while another study demonstrated that mice lacking the TNFα-type I receptor failed to develop alcoholic liver injury [89, 90].
Our group further confirmed that cAMP elevating agents have beneficial effects on cytokine production by decreasing the pro-inflammatory cytokine, TNFα, and increasing the anti-inflammatory cytokine, IL-10, in response to LPS [80, 91, 92]. Specifically, we examined the efficacy of Misoprostol (prostaglandin analog) to modulate LPS inducible cytokine responses employing whole blood (ex-vivo) analysis before and after Misoprostol administration to healthy control subjects [91]. Our results showed that Misoprostol reduced LPS inducible TNF while increasing the production of the anti-inflammatory cytokine, IL10, in human subjects. In vitro studies assessing the underlying mechanisms of Misoprostol effect identified increased cAMP/PKA signaling and consequent changes in CRE and NF-κB activity [91]. Additionally, using chromatin immunoprecipitation studies we demonstrated that Misoprostol treatment altered transcription factor and RNA Polymerase II promoter binding, resulting in changes in TNFα and IL-10 mRNA levels. These studies suggested a potential rationale for Misoprostol use in ALD, NAFLD and other liver diseases where inflammation plays an etiologic role.
Furthermore, ALD is often associated with oxidative stress, which can lead to hepatocellular damage and hepatocyte death. A study evaluating ethanol-induced hepatotoxicity and apoptosis in Sprague Dawley rats reported that treatment with 14-deoxyandrographolide, an adenylate cyclase activator, offered protection through upregulation of constitutive nitric oxide synthase (cNOS), eventually improving the redox state [93]. The authors reported that the adenylate cyclase activation leading to upregulated cAMP levels modulated the expression of caveolin-1 and calmodulin leading to inhibition of cNOS-caveolin-1 interaction and upregulation of cNOS, resulting in improved oxidative stress.
Another beneficial mechanistic property that the cAMP/PKA pathway offers in ethanol-induced liver injury is its ability to upregulate alcohol dehydrogenase (ADH) and decrease CYP2E1 activity, thereby interfering with ethanol metabolism [94, 95]. There are three enzymes involved in oxidative metabolism of alcohol: alcohol dehydrogenase (ADH), cytochrome P450 2E1 (CYP2E1) and catalase. The main enzyme involved in alcohol break down is ADH, which converts alcohol into acetaldehyde (which is short-lived), and acetaldehyde is further converted to acetate by aldehyde dehydrogenase. CYP2E1 is active only with large amounts of alcohol consumption, while catalase metabolizes only a small fraction of alcohol in the body. Alcohol metabolism by CYP2E1 often generates large amounts of highly reactive, oxygen-containing molecules or reactive oxygen species (ROS), leading to exacerbated oxidative stress [96]. A study employing a polyphenol treatment as a means to suppress ethanol-induced hepatocyte death also demonstrated that cAMP modulated CYP2E1 and ADH activities, consequently leading to reduced ROS production and cell death [97].
2.2. The role of cAMP in liver fibrosis
Ongoing hepatocyte injury and inflammation result in uncontrolled activation and proliferation of hepatic stellate cells (HSCs) and the development of fibrosis and cirrhosis [98–100]. Liver fibrosis occurs in multiple types of chronic liver injury, and unfortunately, there is no FDA-approved specific therapy for fibrosis. The key cellular process of hepatic fibrosis is activation and phenotypic change of hepatic stellate cells (HSCs) into proliferative, contractile, and chemotactic myofibroblasts [20–23]. Myofibroblasts migrate and accumulate at the site of injury and produce increasing amounts of extracellular matrix (ECM) components such as collagens and fibronectin [24]. Excessive scar deposition results in a significant loss of liver function, altered blood flow and eventually liver failure [25]. Because trans-differentiation of HSCs plays a key role in the development of liver fibrosis, targeting HSC activation has become a focal point in treating liver fibrosis [101, 102].
The cAMP signaling pathway plays a critical role in HSC activation by antagonizing pro-fibrogenic pathways in HSCs [103]. Prior studies showed that quiescent HSCs have high levels of phospho-CREB, which decreases upon HSC activation; whereas activation of PKA or CAMK-II restores phospho-CREB levels and inhibits proliferation of activated HSCs [104, 105].
The protective effects of the cAMP pathway in liver disease presented thus far have been predominantly associated with PKA activation. However, the other arm of the cAMP pathway, via EPAC activation, also plays a major role in dictating the progression and severity of liver injury, especially in relation to HSCs. Because one of the properties of EPAC is to regulate epithelial cell function and ECM formation, it has become a potential therapeutic target for tissue fibrosis [57]. A study evaluating acetaldehyde-induced hepatic stellate cell activation showed that cAMP/PKA and cAMP/EPAC pathways were both involved. They found that acetaldehyde suppresses EPAC1, but increases EPAC2 expression. cAMP analog, Me-cAMP, which activates the EPAC/Rap1 pathway significantly decreased the proliferation of acetaldehyde-stimulated hepatic stellate cells and collagen synthesis, while PKA specific agonist had no significant effect. Moreover, PKA activation led to increased expression HSC activation marker, αSMA and collagen. However, only EPAC2 depletion by siRNA prevented HSC activation demonstrated by decreased alpha smooth muscle actin (αSMA) and collagen expression [106]. Another study using a mouse model of carbon tetrachloride-induced liver fibrosis reported that EPAC-1 expression was reduced in fibrotic livers compared to normal livers [58]. Importantly, they also found that EPAC-1 levels are decreased in human fibrotic livers. Administering the cAMP activator, prostaglandin E2, restored EPAC-1 levels and enhanced its activity, resulting in attenuation of platelet-derived growth factor (PDGF)-induced proliferation and migration of stellate cells [58]. Notably, PDGF and transforming growth factor beta (TGFβ1) suppressed EPAC1 mRNA expression levels in isolated HSCs, with no effect on PKA. However, both PKA and EPAC agonist could attenuate PDGF-induced migration of HSCs In vitro [58]. One study suggested that PKA did not reduce α-SMA levels; however, it still has the capacity to mediate phosphorylation of proteins that regulate the epithelial to mesenchymal transformation (EMT) [107]. The EMT is a mechanistic event leading to fibrosis, where cells of epithelial phenotype transition to a mesenchymal phenotype through increases in α-SMA and decreases in E-cadherin expression. Additionally, both PKA and EPAC have been shown to attenuate TGF-β-mediated inhibition of E-cadherin expression (57). Overall, the cAMP pathway appears to play a promising therapeutic role in liver fibrosis as well.
2.3. The role of cAMP in other liver diseases
Apart from NAFLD and ALD, the role of cAMP in other liver diseases has also been investigated. For example, in drug-induced liver injury, one study showed that increased cAMP levels appeared to provide protection against an acetaminophen-induced liver injury model [108]. In addition, in a study on LPS-induced inflammatory liver injury, cAMPmediated induction of IL-10 (anti-inflammatory) in liver cells using the cAMP analog, dibutyryl cAMP, was also beneficial [109]. Another study of cAMP derivatives in a carbon tetrachloride-induced rat liver injury model showed that the cAMP derivatives not only attenuated inflammation and reduced serum liver enzymes (transaminases), but also inhibited liver transaminase activity and ameliorated cytoplasmic vacuolation induced by carbon tetrachloride [110].
The effect of cAMP signaling on bile acid induced toxicity in hepatocytes has been reported [111–115]. Specifically, it has been demonstrated that increased cAMP, and specifically EPAC signaling, protects hepatocytes from bile acid induced apoptosis via PI3/Akt pathway. Moreover, this protection involves glycogen synthase kinase 3 (GSK3)-mediated inhibition of pro-apoptotic kinase, c-Jun NH2-terminal kinase (JNK) and ER stress [114]. EPAC activation also protects hepatocytes from bile acid induced mitochondrial apoptosis [113, 114]. Another pathway of cAMP-mediated protection involves PKA-mediated phosphorylation of CD95 (FasR) and preventing formation of deathinducing signal complex (DISC), activation of caspases and apoptosis [115, 116].
In a cholestatic liver injury rat model (common bile duct ligation), increased cAMP levels inactivated hepatic stellate cells and attenuated fibrosis [117]. Importantly, the beneficial role of cAMP extends to liver cancer as well, based on a study showing that the cAMP/PKA pathway was protective against hepatocellular carcinoma [118]. The study demonstrated that activated PKA phosphorylated the epidermal growth factor receptor (EGFR) at serine residues to suppress its kinase activity. This, in turn, led to de-phosphorylation of the signal transducer and activator of transcription 3 (STAT3) resulting in repressed STAT3 target genes and inhibition of hepatocarcinogenesis. Additionally, another study utilizing PDE4 inhibitors to increase intracellular cAMP levels reported that cAMP interfered with cell cycle progression (decreased availability of cyclin A and increased expression of p21) and inhibited cell proliferation in a hepatocarcinoma-derived cell line (HepG2 cells) [119].
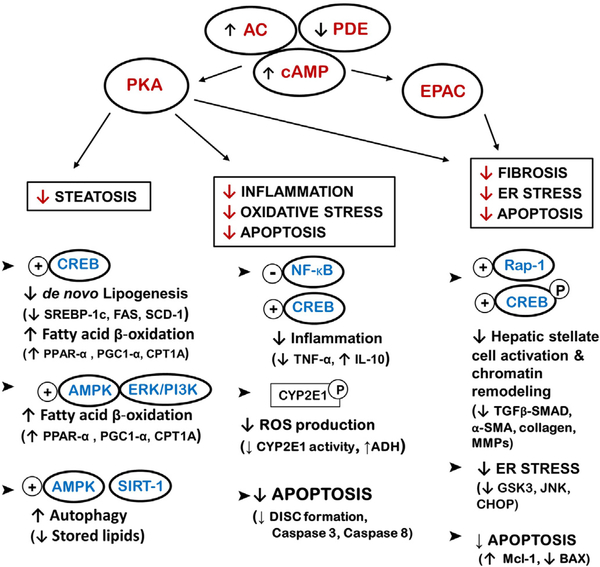
It is important to note that the cAMP pathway, through its two effector molecules, plays a physiological role in maintaining hepatic function and homeostasis while also exhibiting a therapeutic role in the different stages of various liver diseases, irrespective of the etiology (Fig. 2 and Table 1). The reported evidence currently available strongly supports increased intracellular cAMP levels as a potential therapy in multiple forms of liver disease.
Fig. 2.
A schematic diagram cataloguing cAMP/PKA/EPAC-mediators and targets in NAFLD, ALD and liver fibrosis. The diagram also demonstrates pathways modulated by cAMP/PKA/EPAC activation and the overall effects in the liver such as lipid metabolism, inflammation, oxidative and endoplasmic reticulum (ER) stress, apoptosis and fibogenesis. Some of the mediators that have been shown to be key for the beneficial effects of increasing/restoring cAMP in liver disease are mentioned in brackets. AC-adenylate cyclase, PDE-phosphodiesterase, cAMP - cyclic adenosine monophosphate, PKA - protein kinase A, EPAC- exchange protein activated by cAMP, CREB - cAMP response element-binding protein, AMPK - AMP-activated protein kinase, ERK/PI3K extracellular signal-regulated kinases/PI 3-kinases, SREBP-1c - sterol regulatory element-binding protein −1c, FAS - fatty acid synthase, SCD-1 - stearoyl-CoA desaturase, PGC-1α - peroxisome proliferator-activated receptor gamma coactivator 1-alpha, PPAR-α - peroxisome-proliferator activator receptor alpha, CPT1A - carnitine palmitoyl transferase 1A, NF-κB - nuclear factor-kappa B, TNFα - tumor necrosis factor alpha, IL-10 - interleukin 10, ROS - reactive oxygen species, ADH - alcohol dehydrogenase, DISC - death-inducing signaling complex, ER - endoplasmic reticulum, GSK3 - glycogen synthase kinase 3, JNK c-Jun N-terminal kinases, CHOP - C/EBP homologous protein, TGF-β - transforming growth factor beta, α-SMA - alpha smooth muscle actin, MMPs - matrix metalloproteinases.
Table 1.
cAMP mediators and targets for various pathways in the liver.
| Mediator | Target | Pathway |
|---|---|---|
| PKA | CREB, PPAR-α, CPT1A, PGC1α, SIRT-1, AMPK | Increased fatty acid β-oxidation |
| PKA | AMPK, SREBP1c, ACC, FAS, SCD-1 | Decreased de novo lipogenesis |
| PKA | Cytosolic lipases, ATGL, HSL | Increased β-Adrenergic induced lipolysis |
| PKA | CREB, NF-κB | Decreased inflammation, decreased TNFα and increased anti-inflammatory IL-10 |
| PKA | CD95, FADD, DISC | Decreased bile acid- and TNF- induced apoptosis |
| EPAC | Rap-1, GSKβ, JNK, CHOP | Decreased bile-acid induced ER stress |
| EPAC1 | Mitochondrial Mcl-1, BAX | Decreased bile-acid induced death |
| PKA | αSMA, Collagen | Increased acetaldehyde-induced activation and decreased PDGF-induced migration of HSCs |
| EPAC2 | Rap-1, αSMA, Collagen | Decreased acetaldehyde-induced activation of HSCs |
| EPAC1 | SMAD2/3, αSMA, Collagen | Attenuation of CCl4-induced hepatic fibrosis and TGFβ1- and PDGF-induced proliferation and migration of HSCs |
| PDE4B | cAMP/PKA, NF-κB | Inflammation, TNFα |
| PDE4B | CREB, PGC1α, SIRT-1, CPT1A | Fatty acid β-oxidation |
| PDE [4] | SMAD3, TGFβ1, αSMA, Collagen | Fibrogenesis, HSC activation/proliferation |
3. Therapeutic interventions via cAMP signaling
As clearly described, the cAMP signaling pathway appears to be an excellent pharmacological target for hepatic conditions, including NAFLD and ALD, through its anti-inflammatory, anti-lipogenic, and anti-fibrogenic effects. A number of cAMP agonists and derivatives, as well as adenylate cyclase activators and analogs of PKA and EPAC, have been investigated in multiple disorders. For instance, the naturally occurring adenylate cyclase activator, forskolin, has been proposed as a dietary supplement for obesity as well as a potential drug candidate for cancer therapy [120, 121]. Forskolin has also undergone clinical trials for asthma, has been administered to patients for the treatment of glaucoma, and has been studied for its therapeutic potential role in cardiac and liver fibrosis [122–125]. A forskolin derivative selective for adenylate cyclase 5, colforsin daropate hydrochloride (NKH447), has been approved for the treatment of advanced congestive heart failure [126]. The cAMP analog, bucladesine (dibutyryl cAMP), a compound which mimics the action of endogenous cAMP and is a predominant PKA activator that can resist PDE cleavage, was introduced into clinical trials to treat congestive heart failure, wounds and inflammation [127–129]. Other drugs that target the cAMP system include β2-adrenoceptor agonists, such as salmeterol and formoterol, are used for treating asthma due to their bronchodilatory properties [130]. Longacting β2-adrenergic receptor agonists bind to β 2-adrenergic receptors and induce intracellular cAMP that can also antagonize airway smooth muscles [131]. Analogs of cAMP that selectively activate EPAC have also been developed, including 8-(4-Chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate that is widely used as an EPAC1 and EPAC2 activator in various studies [132]. Intriguingly, sulfonylureas, drugs used in patients with type 2 diabetes to promote insulin release from pancreatic beta cells, have been reported to bind and activate EPAC2, although this hypothesis has been challenged [133, 134]. Nonetheless, EPAC2 activation contributes to amplification in insulin secretion, thus suggesting a role for the pharmaceutical development of cAMP activators in diabetes [135]. Although these are all promising possibilities in terms of therapeutic development, it appears that compounds targeting adenylate cyclases or other effectors of the pathway are not widely recognized and still understudied.
3.1. Role of PDEs and PDE inhibitors
There is another class of cAMP targets, namely phosphodiesterase (PDE) inhibitors, that has been studied extensively and has shown remarkable potential in terms of drug development and proved efficient in liver diseases. PDEs are ubiquitously present in different tissues and cells. Their function is to hydrolyze cAMP and cGMP and to maintain their homeostasis for normal physiological processes. There are 11 different members of the mammalian class PDE superfamily (PDE1 through PDE11) with multiple genes and splicing variants [136, 137]. Given their varying roles, there are at least a 100 different PDE proteins characterized in eukaryotes [45]. Moreover, they are localized in different cellular compartments to regulate the duration and amplitude of cyclic nucleotide signaling within subcellular domains such as the cytosol and plasma membrane. Notably, although PDEs of the same family show divergence in their amino acid sequences, they are functionally related and can share similar substrates. Furthermore, PDEs can be either cAMP-specific or cGMP-specific, or they can hydrolyze both cyclic nucleotides; for example, PDE2 allows for cross-regulation of the cAMP and cGMP pathways. The different PDEs and their substrate specificity and tissue distribution are presented in Table 2. PDEs are thought to be excellent targets for manipulating levels of cyclic nucleotides because they are essentially viewed as end-stage regulators of signal transduction mediated by these important second messenger molecules. Additionally, due to their unique tissue distribution, structural properties, and functional properties, these enzymes have been hypothesized to be as potential targets for pharmacological inhibition since the 1970s [138, 139].
Table 2.
A list of the different phosphodiesterases (PDEs) identified in mammals along with their substrate specificity and tissue distribution.
| PDE | Tissue distribution | Specificity |
|---|---|---|
| PDE 1 | Brain, heart, skeletal muscle and testis | cGMP, cAMP |
| PDE 2 | Adrenal medulla, heart, brown adipose tissue, liver and brain, endothelial cells, macrophages, pulmonary artery | cGMP, cAMP |
| PDE 3 | Heart, platelet, vascular smooth muscle, oocyte, adipocytes, hepatocytes, spermatocytes | cGMP, cAMP |
| PDE 4 | Brain, inflammatory cells, cardiovascular tissues, smooth muscles | cAMP |
| PDE 5 | Lung, heart, cerebellum, less in the rain and kidney | cGMP |
| PDE 6 | Retina | cGMP |
| PDE 7 | Pro-inflammatory and immune cells, endothelial cells, brain, heart, skeletal muscle, pancreas | cAMP |
| PDE 8 | Testis, eye, liver, skeletal muscle, heart, kidney, ovary, brain | cAMP |
| PDE 9 | Kidney, liver, lung, brain, spleen, small intestine | cGMP |
| PDE 10 | Brain, thyroid, testis | cGMP, cAMP |
| PDE 11 | Skeletal muscle, prostate, kidney, liver, pituitary and salivary glands, testis | cGMP, cAMP |
The very first known PDE inhibitor is coffee, due to the presence of the methyl xanthine, caffeine. Coffee is a weak non-selective PDE inhibitor, which was first mentioned by Henry Hyde Salter in his monograph published in 1860 entitled, “On asthma: Its pathology and treatment”. Coffee was recommended as a remedy that “in many cases is more efficacious than any other” [140]. Relevant to liver disease, coffee consumption has been shown to be associated with lower liver injury markers, especially in patients with pre-existing liver disease. Coffee intake was also associated with lower incidences of progressive liver conditions such as fibrosis, cirrhosis and hepatocellular carcinoma [141–144].
PDE inhibitors can prolong the action or effect of cAMP and cGMP signaling by inhibiting cAMP and cGMP degradation by PDEs. Selective PDE inhibitors have been utilized as a novel therapeutic approach in many disorders such as asthma, chronic obstructive pulmonary disease, coronary heart disease, hypertension, infective diseases (such as malaria), as well as in mental conditions such as depression and schizophrenia. Sildenafil, a cGMP-specific PDE5 inhibitor, may be considered a hallmark drug belonging to this class of therapeutic agents. Sildenafil enhances the vasodilatory effects of cGMP and has been available for about two decades for treating erectile dysfunction [145]. Other notable PDE inhibitors that are in clinical use include: cilostazol, a PDE3selective inhibitor used to alleviate intermittent claudication in patients with peripheral vascular disease; pentoxifylline, a competitive nonselective PDE inhibitor and an antagonist of the adenosine 2 receptors, used for managing muscle pain in patients with peripheral artery disease; and theophylline, another nonselective PDE inhibitor, commonly used as a vasodilator [146–148].
Among the cAMP-selective PDE inhibitors, PDE4 inhibitors have been well-explored for therapeutic purposes, since PDE4 is present predominantly in immune cells and cells of the central nervous system. The outcomes of PDE4 inhibition are well-documented and correlated with procognition, wakefulness, neuroprotection and anti-inflammation. Thus, the PDE4 family is a popular therapeutic target for diverse conditions, such as asthma and central nervous system-related disorders [149–151]. Indeed, rolipram, the prototypical PDE4 inhibitor, was initially modeled and designed as an antidepressant drug in the 1990s, but was later discontinued due to its narrow therapeutic window [152]. However, selective PDE4 and dual PDE3/4 inhibitors, including roflumilast, are still considered favorable therapeutic agents for treating various chronic inflammatory diseases pertaining to the respiratory system by virtue of their anti-inflammatory and bronchodilatory properties [153]. Because these agents can cross the blood brain barrier, the oral administration of selective PDE4 inhibitors often produces side effects of the central nervous system and gastrointestinal tract such as nausea, vomiting, diarrhea and dyspepsia. Hence, there is a need for novel and targeted drug delivery systems [154]. In spite of being discontinued, rolipram is still widely used in research to characterize the beneficial effects of PDE4 inhibition, with multiple studies demonstrating its protective effects in auto-immune diseases, spinal cord injury, Alzheimer’s’ disease and acute lung injury, to name a few [155–158]. However, PDE inhibitors have not undergone rigorous clinical trials, nor have they been approved for liver diseases. The following section will discuss current, available studies of cAMP-specific PDE inhibitors in the liver and assess their potential in hepatic disorders.
3.2. PDE inhibition in NAFLD, ALD, fibrosis and other liver diseases
As discussed earlier, endotoxin (LPS)-driven inflammation plays a major role in the pathogenesis of ALD. Marco Conti’s group has demonstrated the essential role of PDE4, specifically PDE4B, in LPS-inducible TNFα production [159, 160]. By using Pde4a, b and d knockout mice, it was established that among the PDE4 sub-family, PDE4B is critical for LPS signaling through TLR4 in macrophages and Pde4b knockout mice are protected from LPS-induced shock [160]. Based on these observations, our group later identified the alcohol-mediated increase of PDE4B as a cause of diminished cAMP levels and an underlying mechanism of alcohol-mediated “priming”; PDE4 inhibition abrogates alcohol-mediated “priming” of monocytes/macrophages and production of high levels of TNFα [92]. Protective effect of PDE4 inhibitors, Rolipram and roflumilast, has also been demonstrated in various murine models of inflammation-driven liver injury [161–165]. Importantly, our recent study demonstrated that chronic alcohol feeding reduced hepatic cAMP leading to decreased phospho-CREB levels in a mouse ALD model [74]. We further showed that this decrease in cAMP levels was mediated by increased hepatic PDE4 expression/ activity in both the liver as well as isolated hepatocytes. Induction of PDE4 and a resultant compromised cAMP signaling contributed to dysregulated fatty acid β-oxidation by decreasing CPT1A expression. Moreover, a PKA specific agonist increased CPT1A levels in primary hepatocytes [74]. Using both pharmacological and gene knockout approaches, we showed that inhibition of PDE4, specifically PDE4B, significantly attenuated alcohol induced hepatic steatosis by preventing the alcohol-mediated decrease in hepatic Cpt1a expression via the Pparα/Sirt1/Pgc1α pathway in vivo [74]. Additionally, Mollmann et al. reported that PDE4 inhibition, using roflumilast, diminished steatohepatitis and improved glucose tolerance in mice fed a high-fat Western-type diet. The authors attributed this action mechanistically to cAMP activation of PKA and CREB leading to increased mitochondrial biogenesis induced by PCG-1α [166]. Mice administered roflumilast also exhibited increased energy expenditure and lower weight gain. Several human studies have also reported the reduction of fat mass and improvement in insulin resistance after treatment with roflumilast [167–169]. Plant flavonoids that are reportedly competitive inhibitors of PDE3 and PDE4 have also been reported to suppress lipogenic pathways and are being considered as potential therapeutic agents for obesity and hepatic steatosis [170]. The well-characterized PDE inhibitor, pentoxifylline, was also found to be protective against NASH induced by the methionine-choline deficient diet through suppressed TNFα production and alleviation of ER stress [171, 172]. Likewise, a meta-analysis of randomized, double-blinded, placebo-controlled trials for the clinical use of pentoxifylline in NAFLD reported that pentoxifylline improved NAFLD parameters such as elevated liver enzyme levels in patients [173].
Besides hepatic steatosis, PDE inhibition has been strongly implicated in the management of liver fibrosis, which occurs in multiple types of chronic liver injury. Unfortunately, there is no FDA-approved specific therapy for liver fibrosis, and thus, developing novel targets for anti-fibrotic therapy is an important undertaking. The effect of PDE inhibitor, pentoxifylline, on HSC activation and trans-differentiation and the development of fibrosis was also demonstrated in In vitro and in vivo studies, supporting the role of PDE inhibition as a protective mechanism in liver fibrosis [174–178]. More recently, a study demonstrated that pentoxifylline suppressed TGF-β1 expression in activated LX-2 hepatic stellate cells by inhibiting the pro-fibrogenic hedgehog signaling pathway [179]. In addition, pentoxifylline has also been reported to improve complications such as bacterial infection and renal dysfunction in patients with advanced cirrhosis [180]. Using bile-duct ligation as a cholestatic liver injury model, our group demonstrated that induction of hepatic PDE4A, B and D plays a causal role in the development of liver injury and fibrosis [161]. Specifically, we showed that targeting this induction of PDE4 activity by rolipram prevented hepatic inflammation, fibrosis and injury. More importantly, PDE4 induction in hepatic stellate cells preceded the expression of the HSC activation marker, α-SMA, suggesting that PDE4 induction is required for HSC activation process. Indeed, treatment with rolipram markedly attenuated the trans-differentiation of quiescent HSCs into myofibroblasts. PDE4 inhibitors have also been shown to reduce the production and activity of matrix metalloproteinases (MMPs) in human lung fibroblasts, and this could be another potential mechanism of protection in liver fibrosis [181]. MMPs are involved in proteolytic degradation and remodeling of the ECM as well as in the inflammatory process [182]. These findings implicated PDE4 as a driver of fibrogenic gene expression, and hence as a pathogenic mediator of liver fibrosis.
Given the evidence that altered cAMP levels through PDE4 induction can modulate the degree of steatosis, inflammation and fibrosis, PDE4 inhibitors are now being considered for the treatment of metabolic disorders, with some PDE4 inhibitors currently undergoing clinical trials [183]. In 2014, a PDE4 inhibitor (ASP9831) was introduced into phase 1 and phase 2 clinical trials for the treatment of NASH; however, it was not effective in reducing NASH parameters such as elevated ALT/AST, despite a defined mechanism of action [184]. Such failures emphasize the challenges of NASH and ALD treatment, given that the diseases are multi-factorial. There is a critical need for more efficient/reproducible preclinical tests, appropriate drug dosing, and potentially, combinatorial drug therapy.
4. Concluding remarks
Liver diseases such as ALD and NAFLD continue to plague both developed and developing countries. To date, there is no available Food and Drug Administration-approved therapy for either ALD or NAFLD. Therefore, there is an urgent need to identify pathogenic targets for drug therapy. Moreover, because ALD and NAFLD share pathophysiological features, such as steatosis, inflammation, and fibrosis, the identification, development and evaluation of a drug target for one can also be potentially utilized for the other. This review focused on the relevance of cAMP signaling in physiologic and pathologic aspects of liver function. Additionally, it addressed the current understanding of cAMP metabolism and signaling in parenchymal and non-parenchymal hepatic cells, that impact the development of liver pathologies (Fig. 2, Table 1). Because the fate of the cAMP pathway is controlled by PDEs, targeting these enzymes using PDE inhibitors is becoming relevant in the treatment and management of liver diseases. Particularly, based on current preclinical and clinical findings, PDE4specific inhibitors demonstrate therapeutic potential in the management of liver diseases such as NAFLD and ALD. However, more preclinical studies and larger, placebo-controlled clinical trials are warranted to establish their efficacy during different stages of liver disease.
Acknowledgements
We thank Marion McClain and Dr. Swati Joshi-Barve for editing the manuscript. This work was supported by grants from National Institute on Alcohol Abuse and Alcoholism (NIAAA)R44AA021331 (LG), U01AA021901 (CM), P50AA024337 (CM) and National Institute of General Medical Sciences (NIGMS)P20GM113226 (CM).
References
- [1].Kmiec Z, Cooperation of liver cells in health and disease, Adv. Anat. Embryol. Cell Biol 161 (III-XIII) (2001) 1–151. [DOI] [PubMed] [Google Scholar]
- [2].Beavo JA, Brunton LL, Cyclic nucleotide research — still expanding after half a century, Nat. Rev. Mol. Cell Biol 3 (2002) 710–718. [DOI] [PubMed] [Google Scholar]
- [3].Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M, Non alcoholic fatty liver: epidemiology and natural history, Rev. Recent Clin. Trials 9 (2014) 126–133. [DOI] [PubMed] [Google Scholar]
- [4].Vernon G, Baranova A, Younossi ZM, Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults, Aliment. Pharmacol. Ther 34 (2011) 274–285. [DOI] [PubMed] [Google Scholar]
- [5].Singal AK, Anand BS, Recent trends in the epidemiology of alcoholic liver disease, Clin. Liver Dis 2 (2013) 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Im GY, Lucey MR, Practical concerns and controversies in the Management of Alcoholic Hepatitis, Gastroenterol. Hepatol. (N Y) 12 (2016) 478–489. [PMC free article] [PubMed] [Google Scholar]
- [7].Than NN, Newsome PN, A concise review of non-alcoholic fatty liver disease, Atherosclerosis 239 (2015) 192–202. [DOI] [PubMed] [Google Scholar]
- [8].Greuter T, Malhi H, Gores GJ, Shah VH, Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: exploiting similarities and differences in pathogenesis, JCI Insight 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gao B, Bataller R, Alcoholic liver disease: pathogenesis and new therapeutic targets, Gastroenterology 141 (2011) 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Menon KV, Gores GJ, Shah VH, Pathogenesis, diagnosis, and treatment of alcoholic liver disease, Mayo Clin. Proc 76 (2001) 1021–1029. [DOI] [PubMed] [Google Scholar]
- [11].Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M, Hote P, et al. , Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes, Hepatology 46 (2007) 823–830. [DOI] [PubMed] [Google Scholar]
- [12].Shen C, Ma W, Ding L, Li S, Dou X, Song Z, The TLR4-IRE1alpha pathway activation contributes to palmitate-elicited lipotoxicity in hepatocytes, J. Cell. Mol. Med (2018) 1–10, 10.1111/jcmm.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schuster S, Cabrera D, Arrese M, Feldstein AE, Triggering and resolution of inflammation in NASH, Nat. Rev. Gastroenterol. Hepatol 15 (6) (2018) 349–364. [DOI] [PubMed] [Google Scholar]
- [14].Ashraf NU, Sheikh TA, Endoplasmic reticulum stress and oxidative stress in the pathogenesis of non-alcoholic fatty liver disease, Free Radic. Res 49 (2015) 1405–1418. [DOI] [PubMed] [Google Scholar]
- [15].Sommerfeld A, Reinehr R, Haussinger D, Free fatty acids shift insulin-induced hepatocyte proliferation towards CD95-dependent apoptosis, J. Biol. Chem 290 (2015) 4398–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, KurtJones EA, Fitzgerald KA, et al. , STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 16544–16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barnes MA, Roychowdhury S, Nagy LE, Innate immunity and cell death in alcoholic liver disease: role of cytochrome P4502E1, Redox Biol 2 (2014) 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ceccarelli S, Nobili V, Alisi A, Toll-like receptor-mediated signaling cascade as a regulator of the inflammation network during alcoholic liver disease, World J. Gastroenterol 20 (2014) 16443–16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fujii H, Kawada N, Fibrogenesis in alcoholic liver disease, World J. Gastroenterol. 20 (2014) 8048–8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Friedman SL, Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury, J. Biol. Chem. 275 (2000) 2247–2250. [DOI] [PubMed] [Google Scholar]
- [21].Kisseleva T, Brenner DA, Mechanisms of fibrogenesis, Exp. Biol. Med. (Maywood) 233 (2008) 109–122. [DOI] [PubMed] [Google Scholar]
- [22].Aoyama T, Paik YH, Seki E, Toll-like receptor signaling and liver fibrosis, Gastroenterol. Res. Pract 2010. (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kisseleva T, Brenner DA, Anti-fibrogenic strategies and the regression of fibrosis, Best Pract. Res. Clin. Gastroenterol 25 (2011) 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Friedman SL, Mechanisms of hepatic fibrogenesis, Gastroenterology 134 (2008) 1655–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mallat A, Lotersztajn S, Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis, Am. J. Phys. Cell Physiol 305 (2013) C789–C799. [DOI] [PubMed] [Google Scholar]
- [26].Sutherland EW, Rall TW, Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles, J. Biol. Chem 232 (1958) 1077–1091. [PubMed] [Google Scholar]
- [27].Robison GA, Butcher RW, Sutherland EW, Cyclic AMP, Annu. Rev. Biochem 37 (1968) 149–174. [DOI] [PubMed] [Google Scholar]
- [28].Steegborn C, Structure, mechanism, and regulation of soluble adenylyl cyclases similarities and differences to transmembrane adenylyl cyclases, Biochim. Biophys. Acta 2014 (1842) 2535–2547. [DOI] [PubMed] [Google Scholar]
- [29].Rahman N, Buck J, Levin LR, pH sensing via bicarbonate-regulated “soluble” adenylyl cyclase (sAC), Front. Physiol 4 (2013) 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kleinboelting S, Diaz A, Moniot S, van den Heuvel J, Weyand M, Levin LR, Buck J, et al. , Crystal structures of human soluble adenylyl cyclase reveal mechanisms of catalysis and of its activation through bicarbonate, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 3727–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zippin JH, Chen Y, Straub SG, Hess KC, Diaz A, Lee D, Tso P, et al. , CO2/ HCO3(−)- and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor, J. Biol. Chem 288 (2013) 33283–33291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pierce KL, Premont RT, Lefkowitz RJ, Seven-transmembrane receptors, Nat. Rev. Mol. Cell Biol 3 (2002) 639–650. [DOI] [PubMed] [Google Scholar]
- [33].Antoni FA, New paradigms in cAMP signalling, Mol. Cell. Endocrinol 353 (2012) 3–9. [DOI] [PubMed] [Google Scholar]
- [34].Cheng X, Ji Z, Tsalkova T, Mei F, Epac and PKA: a tale of two intracellular cAMP receptors, Acta Biochim. Biophys. Sin. Shanghai 40 (2008) 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kaupp UB, Seifert R, Cyclic nucleotide-gated ion channels, Physiol. Rev 82 (2002) 769–824. [DOI] [PubMed] [Google Scholar]
- [36].Simrick S, Schindler RF, Poon KL, Brand T, Popeye domain-containing proteins and stress-mediated modulation of cardiac pacemaking, Trends Cardiovasc. Med 23 (2013) 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Walsh DA, Perkins JP, Krebs EG, An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle, J. Biol. Chem 243 (1968) 3763–3765. [PubMed] [Google Scholar]
- [38].Knighton DR, Zheng JH, Ten Eyck LF, Xuong NH, Taylor SS, Sowadski JM, Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase, Science 253 (1991) 414–420. [DOI] [PubMed] [Google Scholar]
- [39].Lefkowitz RJ, Pierce KL, Luttrell LM, Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity, Mol. Pharmacol 62 (2002) 971–974. [DOI] [PubMed] [Google Scholar]
- [40].Taylor SS, Yang J, Wu J, Haste NM, Radzio-Andzelm E, Anand G, PKA: a portrait of protein kinase dynamics, Biochim. Biophys. Acta 2004. (1697) 259–269. [DOI] [PubMed] [Google Scholar]
- [41].Taylor SS, Buechler JA, Yonemoto W, cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes, Annu. Rev. Biochem 59 (1990) 971–1005. [DOI] [PubMed] [Google Scholar]
- [42].Shaywitz AJ, Greenberg ME, CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals, Annu. Rev. Biochem 68 (1999) 821–861. [DOI] [PubMed] [Google Scholar]
- [43].de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL, Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP, Nature 396 (1998) 474–477. [DOI] [PubMed] [Google Scholar]
- [44].Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, et al. , A family of cAMP-binding proteins that directly activate Rap1, Science 282 (1998) 2275–2279. [DOI] [PubMed] [Google Scholar]
- [45].Conti M, Beavo J, Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling, Annu. Rev. Biochem 76 (2007) 481–511. [DOI] [PubMed] [Google Scholar]
- [46].Ravnskjaer K, Madiraju A, Montminy M, Role of the cAMP pathway in glucose and lipid metabolism, Handb. Exp. Pharmacol 233 (2016) 29–49. [DOI] [PubMed] [Google Scholar]
- [47].Sutherland EW, Robison GA, The role of cyclic AMP in the control of carbohydrate metabolism, Diabetes 18 (1969) 797–819. [DOI] [PubMed] [Google Scholar]
- [48].Jitrapakdee S, Transcription factors and coactivators controlling nutrient and hormonal regulation of hepatic gluconeogenesis, Int. J. Biochem. Cell Biol 44 (2012) 33–45. [DOI] [PubMed] [Google Scholar]
- [49].Pilkis SJ, Claus TH, el-Maghrabi MR, The role of cyclic AMP in rapid and longterm regulation of gluconeogenesis and glycolysis, Adv. Second Messenger Phosphoprotein Res 22 (1988) 175–191. [PubMed] [Google Scholar]
- [50].Lent BA, Kim KH, Phosphorylation and activation of acetyl-coenzyme A carboxylase kinase by the catalytic subunit of cyclic AMP-dependent protein kinase, Arch. Biochem. Biophys 225 (1983) 972–978. [DOI] [PubMed] [Google Scholar]
- [51].Yajima H, Komatsu M, Schermerhorn T, Aizawa T, Kaneko T, Nagai M, Sharp GW, et al. , cAMP enhances insulin secretion by an action on the ATPsensitive K+ channel-independent pathway of glucose signaling in rat pancreatic islets, Diabetes 48 (1999) 1006–1012. [DOI] [PubMed] [Google Scholar]
- [52].Mayr B, Montminy M, Transcriptional regulation by the phosphorylation-dependent factor CREB, Nat. Rev. Mol. Cell Biol 2 (2001) 599–609. [DOI] [PubMed] [Google Scholar]
- [53].Herzig S, Hedrick S, Morantte I, Koo SH, Galimi F, Montminy M, CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma, Nature 426 (2003) 190–193. [DOI] [PubMed] [Google Scholar]
- [54].Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, et al. , CREB regulates hepatic gluconeogenesis through the coactivator PGC-1, Nature 413 (2001) 179–183. [DOI] [PubMed] [Google Scholar]
- [55].Wang H, Zhao M, Sud N, Christian P, Shen J, Song Y, Pashaj A, et al. , Glucagon regulates hepatic lipid metabolism via cAMP and Insig-2 signaling: implication for the pathogenesis of hypertriglyceridemia and hepatic steatosis, Sci. Rep 6 (2016) 32246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Almahariq M, Mei FC, Cheng X, Cyclic AMP sensor EPAC proteins and energy homeostasis, Trends Endocrinol. Metab 25 (2014) 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Insel PA, Murray F, Yokoyama U, Romano S, Yun H, Brown L, Snead A, et al. , cAMP and Epac in the regulation of tissue fibrosis, Br. J. Pharmacol 166 (2012) 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schippers M, Beljaars L, Post E, Lotersztajn S, Reker-Smit C, Han B, MunozLlancao P, et al. , Upregulation of Epac-1 in hepatic stellate cells by prostaglandin E2 in liver fibrosis is associated with reduced fibrogenesis, J. Pharmacol. Exp. Ther 363 (2017) 126–135. [DOI] [PubMed] [Google Scholar]
- [59].Ding X, Saxena NK, Lin S, Gupta NA, Anania FA, Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice, Hepatology 43 (2006) 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, et al. , Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis, Liver Int. 31 (2011) 1285–1297. [DOI] [PubMed] [Google Scholar]
- [61].Zhang Y, Chen ML, Zhou Y, Yi L, Gao YX, Ran L, Chen SH, et al. , Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway, Mol. Nutr. Food Res 59 (2015) 1443–1457. [DOI] [PubMed] [Google Scholar]
- [62].Alberdi G, Rodriguez VM, Macarulla MT, Miranda J, Churruca I, Portillo MP, Hepatic lipid metabolic pathways modified by resveratrol in rats fed an obesogenic diet, Nutrition 29 (2013) 562–567. [DOI] [PubMed] [Google Scholar]
- [63].Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, et al. , Resveratrol improves health and survival of mice on a high-calorie diet, Nature 444 (2006) 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rivera L, Moron R, Zarzuelo A, Galisteo M, Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats, Biochem. Pharmacol 77 (2009) 1053–1063. [DOI] [PubMed] [Google Scholar]
- [65].Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H, Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase, Acta Pharmacol. Sin 29 (2008) 698–706. [DOI] [PubMed] [Google Scholar]
- [66].Tian Y, Ma J, Wang W, Zhang L, Xu J, Wang K, Li D, Resveratrol supplement inhibited the NF-kappaB inflammation pathway through activating AMPKalphaSIRT1 pathway in mice with fatty liver, Mol. Cell. Biochem 422 (2016) 75–84. [DOI] [PubMed] [Google Scholar]
- [67].Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, et al. , Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases, Cell 148 (2012) 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Xu Z, Huo J, Ding X, Yang M, Li L, Dai J, Hosoe K, et al. , Coenzyme Q10 improves lipid metabolism and ameliorates obesity by regulating CaMKII-mediated PDE4 inhibition, Sci. Rep 7 (2017) 8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Barlas N, Mutchnick MG, Grant GJ, Trainin N, The effect of thymic humoral factor on intracellular lymphocyte cyclic AMP in alcoholic liver disease, Thymus 5 (1983) 433–437. [PubMed] [Google Scholar]
- [70].Diamond I, Wrubel B, Estrin W, Gordon A, Basal and adenosine receptor-stimulated levels of cAMP are reduced in lymphocytes from alcoholic patients, Proc. Natl. Acad. Sci. U. S. A 84 (1987) 1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gouillon ZQ, Miyamoto K, Donohue TM, Wan YJ, French BA, Nagao Y, Fu P, et al. , Role of CYP2E1 in the pathogenesis of alcoholic liver disease: modifications by cAMP and ubiquitin-proteasome pathway, Front. Biosci 4 (1999) A16–A25. [DOI] [PubMed] [Google Scholar]
- [72].Aroor AR, Jackson DE, Shukla SD, Dysregulated phosphorylation and nuclear translocation of cyclic AMP response element binding protein (CREB) in rat liver after chronic ethanol binge, Eur. J. Pharmacol 679 (2012) 101–108. [DOI] [PubMed] [Google Scholar]
- [73].Louet JF, Hayhurst G, Gonzalez FJ, Girard J, Decaux JF, The coactivator PGC1 is involved in the regulation of the liver carnitine palmitoyltransferase I gene expression by cAMP in combination with HNF4 alpha and cAMP-response element-binding protein (CREB), J. Biol. Chem 277 (2002) 37991–38000. [DOI] [PubMed] [Google Scholar]
- [74].Avila DV, Barker DF, Zhang J, Mcclain CJ, Barve S, Gobejishvili L, Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis, J. Pathol 240 (2016) 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schott MB, Rasineni K, Weller SG, Schulze RJ, Sletten AC, Casey CA, Mcniven MA, beta-adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure, J. Biol. Chem 292 (2017) 11815–11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bode C, Bode JC, Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol. Clin. Exp. Res 29 (2005) 166S–171S. [DOI] [PubMed] [Google Scholar]
- [77].Fukui H, Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure, Alcohol. Clin. Exp. Res 29 (2005) 172S–179S. [DOI] [PubMed] [Google Scholar]
- [78].Betrapally NS, Gillevet PM, Bajaj JS, Gut microbiome and liver disease, Transl. Res 179 (2017) 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, et al. , Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease, Gut 65 (2016) 830–839. [DOI] [PubMed] [Google Scholar]
- [80].Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, Mcclain C, Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease, Am. J. Physiol. Gastrointest. Liver Physiol 291 (2006) G681–G688. [DOI] [PubMed] [Google Scholar]
- [81].Shames BD, Mcintyre RC Jr., Bensard DD, Pulido EJ, Selzman CH, Reznikov LL, Harken AH, et al. , Suppression of tumor necrosis factor alpha production by cAMP in human monocytes: dissociation with mRNA level and independent of interleukin-10, J. Surg. Res 99 (2001) 187–193. [DOI] [PubMed] [Google Scholar]
- [82].Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N, Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells, J. Biol. Chem 271 (1996) 20828–20835. [DOI] [PubMed] [Google Scholar]
- [83].Newman WH, Zhang LM, Lee DH, Dalton ML, Warejcka DJ, Castresana MR, Leeper-Woodford SK, Release of tumor necrosis factor-alpha from coronary smooth muscle: activation of NF-kappaB and inhibition by elevated cyclic AMP, J. Surg. Res 80 (1998) 129–135. [DOI] [PubMed] [Google Scholar]
- [84].O’Donnell PM, Taffet SM, The proximal promoter region is essential for lipopolysaccharide induction and cyclic AMP inhibition of mouse tumor necrosis factor-alpha, J. Interf. Cytokine Res 22 (2002) 539–548. [DOI] [PubMed] [Google Scholar]
- [85].Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS, Increased plasma tumor necrosis factor in severe alcoholic hepatitis, Ann. Intern. Med 112 (1990) 917–920. [DOI] [PubMed] [Google Scholar]
- [86].Felver ME, Mezey E, Mcguire M, Mitchell MC, Herlong HF, Veech GA, Veech RL, Plasma tumor necrosis factor alpha predicts decreased long-term survival in severe alcoholic hepatitis, Alcohol. Clin. Exp. Res 14 (1990) 255–259. [DOI] [PubMed] [Google Scholar]
- [87].Khoruts A, Stahnke L, Mcclain CJ, Logan G, Allen JI, Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients, Hepatology 13 (1991) 267–276. [PubMed] [Google Scholar]
- [88].Mcclain C, Barve S, Joshi-Barve S, Song Z, Deaciuc I, Chen T, Hill D, Dysregulated cytokine metabolism, altered hepatic methionine metabolism and proteasome dysfunction in alcoholic liver disease, Alcohol. Clin. Exp. Res 29 (2005) 180S–188S. [DOI] [PubMed] [Google Scholar]
- [89].Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG, Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat, Hepatology 26 (1997) 1530–1537. [DOI] [PubMed] [Google Scholar]
- [90].Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG, Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice, Gastroenterology 117 (1999) 942–952. [DOI] [PubMed] [Google Scholar]
- [91].Gobejishvili L, Ghare S, Khan R, Cambon A, Barker DF, Barve S, Mcclain C, et al. , Misoprostol modulates cytokine expression through a cAMP pathway: potential therapeutic implication for liver disease, Clin. Immunol 161 (2015) 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gobejishvili L, Barve S, Joshi-Barve S, Mcclain C, Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: relevance to alcoholic liver disease, Am. J. Physiol. Gastrointest. Liver Physiol 295 (2008) G718–G724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mandal S, Nelson VK, Mukhopadhyay S, Bandhopadhyay S, Maganti L, Ghoshal N, Sen G, et al. , 14-Deoxyandrographolide targets adenylate cyclase and prevents ethanol-induced liver injury through constitutive NOS dependent reduced redox signaling in rats, Food Chem. Toxicol 59 (2013) 236–248. [DOI] [PubMed] [Google Scholar]
- [94].Potter JJ, Macdougald OA, Mezey E, Regulation of rat alcohol dehydrogenase by cyclic AMP in primary hepatocyte culture, Arch. Biochem. Biophys 321 (1995) 329–335. [DOI] [PubMed] [Google Scholar]
- [95].Oesch-Bartlomowicz B, Padma PR, Becker R, Richter B, Hengstler JG, Freeman JE, Wolf CR, et al. , Differential modulation of CYP2E1 activity by cAMP-dependent protein kinase upon Ser129 replacement, Exp. Cell Res 242 (1998) 294–302. [DOI] [PubMed] [Google Scholar]
- [96].Cederbaum AI, Wu D, Mari M, Bai J, CYP2E1-dependent toxicity and oxidative stress in HepG2 cells, Free Radic. Biol. Med 31 (2001) 1539–1543. [DOI] [PubMed] [Google Scholar]
- [97].Yamashita H, Goto M, Matsui-Yuasa I, Kojima-Yuasa A, Ecklonia cava polyphenol has a protective effect against ethanol-induced liver injury in a cyclic AMPdependent manner, Mar. Drugs 13 (2015) 3877–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Higashi T, Friedman SL, Hoshida Y, Hepatic stellate cells as key target in liver fibrosis, Adv. Drug Deliv. Rev 121 (2017) 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Iredale JP, Pellicoro A, Fallowfield JA, Liver fibrosis: understanding the dynamics of bidirectional wound repair to inform the Design of Markers and Therapies, Dig. Dis 35 (2017) 310–313. [DOI] [PubMed] [Google Scholar]
- [100].Koyama Y, Brenner DA, Liver inflammation and fibrosis, J. Clin. Invest 127 (2017) 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Li JT, Liao ZX, Ping J, Xu D, Wang H, Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies, J. Gastroenterol 43 (2008) 419–428. [DOI] [PubMed] [Google Scholar]
- [102].Tsuchida T, Friedman SL, Mechanisms of hepatic stellate cell activation, Nat. Rev. Gastroenterol. Hepatol 14 (2017) 397–411. [DOI] [PubMed] [Google Scholar]
- [103].Lopez-Sanchez I, Dunkel Y, Roh YS, Mittal Y, De Minicis S, Muranyi A, Singh S, et al. , GIV/Girdin is a central hub for profibrogenic signalling networks during liver fibrosis, Nat. Commun 5 (2014) 4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Houglum K, Lee KS, Chojkier M, Proliferation of hepatic stellate cells is inhibited by phosphorylation of CREB on serine 133, J. Clin. Invest 99 (1997) 1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kawada N, Kuroki T, Kobayashi K, Inoue M, Kaneda K, Inhibition of myofibroblastic transformation of cultured rat hepatic stellate cells by methylxanthines and dibutyryl cAMP, Dig. Dis. Sci 41 (1996) 1022–1029. [DOI] [PubMed] [Google Scholar]
- [106].Yang Y, Yang F, Wu X, Lv X, Li J, EPAC activation inhibits acetaldehyde-induced activation and proliferation of hepatic stellate cell via Rap1, Can. J. Physiol. Pharmacol 94 (2016) 498–507. [DOI] [PubMed] [Google Scholar]
- [107].Macpherson MR, Molina P, Souchelnytskyi S, Wernstedt C, Martin-Perez J, Portillo F, Cano A, Phosphorylation of serine 11 and serine 92 as new positive regulators of human Snail1 function: potential involvement of casein kinase-2 and the cAMP-activated kinase protein kinase A, Mol. Biol. Cell 21 (2010) 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kelava T, Cavar I, Vukojevic K, Saraga-Babic M, Culo F, The effect of glucagon and cyclic adenosine monophosphate on acute liver damage induced by acetaminophen, Histol. Histopathol 28 (2013) 245–255. [DOI] [PubMed] [Google Scholar]
- [109].Arai T, Hiromatsu K, Kobayashi N, Takano M, Ishida H, Nimura Y, Yoshikai Y, IL-10 is involved in the protective effect of dibutyryl cyclic adenosine monophosphate on endotoxin-induced inflammatory liver injury, J. Immunol 155 (1995) 5743–5749. [PubMed] [Google Scholar]
- [110].Saito M, Nasu A, Kataoka S, Yamaji N, Ichikawa A, Effect of adenosine 3′,5′cyclic monophosphate derivatives on acute liver injury induced by carbon tetrachloride, Aust. J. Pharm 15 (1992) 449–454. [DOI] [PubMed] [Google Scholar]
- [111].Cullen KA, Mccool J, Anwer MS, Webster CR, Activation of cAMP-guanine exchange factor confers PKA-independent protection from hepatocyte apoptosis, Am. J. Physiol. Gastrointest. Liver Physiol 287 (2004) G334–G343. [DOI] [PubMed] [Google Scholar]
- [112].Webster CR, Boria P, Usechak P, Anwer MS, S-adenosylmethionine and cAMP confer differential cytoprotection against bile acid-induced apoptosis in canine renal tubular cells and primary rat hepatocytes, Vet. Ther 3 (2002) 474–484. [PubMed] [Google Scholar]
- [113].Webster CR, Usechak P, Anwer MS, cAMP inhibits bile acid-induced apoptosis by blocking caspase activation and cytochrome c release, Am. J. Physiol. Gastrointest. Liver Physiol 283 (2002) G727–G738. [DOI] [PubMed] [Google Scholar]
- [114].Johnston A, Ponzetti K, Anwer MS, Webster CR, cAMP-guanine exchange factor protection from bile acid-induced hepatocyte apoptosis involves glycogen synthase kinase regulation of c-Jun NH2-terminal kinase, Am. J. Physiol. Gastrointest. Liver Physiol 301 (2011) G385–G400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Reinehr R, Haussinger D, Inhibition of bile salt-induced apoptosis by cyclic AMP involves serine/threonine phosphorylation of CD95, Gastroenterology 126 (2004) 249–262. [DOI] [PubMed] [Google Scholar]
- [116].Bhattacharjee R, Xiang W, Wang Y, Zhang X, Billiar TR, cAMP prevents TNFinduced apoptosis through inhibiting DISC complex formation in rat hepatocytes, Biochem. Biophys. Res. Commun 423 (2012) 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Abdel Kawy HS, Cilostazol attenuates cholestatic liver injury and its complications in common bile duct ligated rats, Eur. J. Pharmacol 752 (2015) 8–17. [DOI] [PubMed] [Google Scholar]
- [118].Zhou M, Mok MT, Sun H, Chan AW, Huang Y, Cheng AS, Xu G, The antidiabetic drug exenatide, a glucagon-like peptide-1 receptor agonist, counteracts hepatocarcinogenesis through cAMP-PKA-EGFR-STAT3 axis, Oncogene 36 (2017) 4135–4149. [DOI] [PubMed] [Google Scholar]
- [119].Massimi M, Cardarelli S, Galli F, Giardi MF, Ragusa F, Panera N, Cinque B, et al. , Increase of intracellular cyclic AMP by PDE4 inhibitors affects HepG2 cell cycle progression and survival, J. Cell. Biochem 118 (2017) 1401–1411. [DOI] [PubMed] [Google Scholar]
- [120].Rios-Hoyo A, Gutierrez-Salmean G, New dietary supplements for obesity: what we currently know, Curr. Obes. Rep 5 (2016) 262–270. [DOI] [PubMed] [Google Scholar]
- [121].Sapio L, Gallo M, Illiano M, Chiosi E, Naviglio D, Spina A, Naviglio S, The natural cAMP elevating compound Forskolin in Cancer therapy: is it time? J. Cell. Physiol 232 (2017) 922–927. [DOI] [PubMed] [Google Scholar]
- [122].Huerta M, Urzua Z, Trujillo X, Gonzalez-Sanchez R, Trujillo-Hernandez B, Forskolin compared with beclomethasone for prevention of asthma attacks: a single-blind clinical trial, J. Int. Med. Res 38 (2010) 661–668. [DOI] [PubMed] [Google Scholar]
- [123].Vetrugno M, Uva MG, Russo V, Iester M, Ciancaglini M, Brusini P, Centofanti M, et al. , Oral administration of forskolin and rutin contributes to intraocular pressure control in primary open angle glaucoma patients under maximum tolerated medical therapy, J. Ocul. Pharmacol. Ther. 28 (2012) 536–541. [DOI] [PubMed] [Google Scholar]
- [124].Lu D, Aroonsakool N, Yokoyama U, Patel HH, Insel PA, Increase in cellular cyclic AMP concentrations reverses the profibrogenic phenotype of cardiac myofibroblasts: a novel therapeutic approach for cardiac fibrosis, Mol. Pharmacol 84 (2013) 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].El-Agroudy NN, El-Naga RN, El-Razeq RA, El-Demerdash E, Forskolin, a hedgehog signalling inhibitor, attenuates carbon tetrachloride-induced liver fibrosis in rats, Br. J. Pharmacol 173 (2016) 3248–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hosono M, Cardiovascular effects of colforsin daropate hydrochloride, a novel drug for the treatment of acute heart failure, Nihon Yakurigaku Zasshi 114 (1999) 83–88. [DOI] [PubMed] [Google Scholar]
- [127].Miyagi Y, Sasayama S, Nakajima H, Fujita M, Asanoi H, Comparative hemodynamic effects of intravenous dobutamine and dibutyryl cyclic AMP, a new inotropic agent, in severe congestive heart failure, J. Cardiovasc. Pharmacol 15 (1990) 138–143. [DOI] [PubMed] [Google Scholar]
- [128].Toba K, Sudoh N, Nagano K, Eto M, Mizuno Y, Nakagawa H, Kawabata Y, et al. , Randomized prospective trial of gentian violet with dibutyryl cAMP and povidone-iodine with sugar as treatment for pressure sores infected with methicillin-resistant Staphylococcus aureus in elderly patients, Nihon Ronen Igakkai Zasshi 34 (1997) 577–582. [DOI] [PubMed] [Google Scholar]
- [129].Rundfeldt C, Steckel H, Sorensen T, Wlaz P, The stable cyclic adenosine monophosphate analogue, dibutyryl cyclo-adenosine monophosphate (bucladesine), is active in a model of acute skin inflammation, Arch. Dermatol. Res 304 (2012) 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Giembycz MA, Kaur M, Leigh R, Newton R, A holy grail of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids, Br. J. Pharmacol 153 (2008) 1090–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Billington CK, Ojo OO, Penn RB, Ito S, cAMP regulation of airway smooth muscle function, Pulm. Pharmacol. Ther 26 (2013) 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, et al. , A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK, Nat. Cell Biol 4 (2002) 901–906. [DOI] [PubMed] [Google Scholar]
- [133].Takahashi T, Shibasaki T, Takahashi H, Sugawara K, Ono A, Inoue N, Furuya T, et al. , Antidiabetic sulfonylureas and cAMP cooperatively activate Epac2A, Sci. Signal 6 (298) (2013) ra94. [DOI] [PubMed] [Google Scholar]
- [134].Rehmann H, Epac2: a sulfonylurea receptor? Biochem. Soc. Trans 40 (2012) 6–10. [DOI] [PubMed] [Google Scholar]
- [135].Henquin JC, Nenquin M, Activators of PKA and Epac distinctly influence insulin secretion and cytosolic Ca2+ in female mouse islets stimulated by glucose and tolbutamide, Endocrinology 155 (2014) 3274–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Conti M, Phosphodiesterases and cyclic nucleotide signaling in endocrine cells, Mol. Endocrinol 14 (2000) 1317–1327. [DOI] [PubMed] [Google Scholar]
- [137].Houslay MD, Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown, Trends Biochem. Sci 35 (2010) 91–100. [DOI] [PubMed] [Google Scholar]
- [138].Weiss B, Hait WN, Selective cyclic nucleotide phosphodiesterase inhibitors as potential therapeutic agents, Annu. Rev. Pharmacol. Toxicol 17 (1977) 441–477. [DOI] [PubMed] [Google Scholar]
- [139].Jeon YH, Heo YS, Kim CM, Hyun YL, Lee TG, Ro S, Cho JM, Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development, Cell. Mol. Life Sci 62 (2005) 1198–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Salter HH, On Asthma: Its Pathology and Treatment: Churchill, (1868).
- [141].Wadhawan M, Anand AC, Coffee and liver disease, J. Clin. Exp. Hepatol 6 (2016) 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C, Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and metaanalysis of prospective studies, Eur. J. Cancer Prev 26 (2017) 368–377. [DOI] [PubMed] [Google Scholar]
- [143].Kennedy OJ, Roderick P, Buchanan R, Fallowfield JA, Hayes PC, Parkes J, Systematic review with meta-analysis: coffee consumption and the risk of cirrhosis, Aliment. Pharmacol. Ther 43 (2016) 562–574. [DOI] [PubMed] [Google Scholar]
- [144].Hodge A, Lim S, Goh E, Wong O, Marsh P, Knight V, Sievert W, et al. , Coffee intake is associated with a lower liver stiffness in patients with non-alcoholic fatty liver disease, hepatitis C, and hepatitis B, Nutrients 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, et al. , Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction, Int. J. Impot. Res 8 (1996) 47–52. [PubMed] [Google Scholar]
- [146].Bedenis R, Stewart M, Cleanthis M, Robless P, Mikhailidis DP, Stansby G, Cilostazol for intermittent claudication, Cochrane Database Syst. Rev (10) (2014) CD003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Olin JW, Sealove BA, Peripheral artery disease: current insight into the disease and its diagnosis and management, Mayo Clin. Proc 85 (2010) 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Mokry J, Urbanova A, Kertys M, Mokra D, Inhibitors of phosphodiesterases in the treatment of cough, Respir. Physiol. Neurobiol (2018), 10.1016/j.resp.2018.01.008 (in press). [DOI] [PubMed] [Google Scholar]
- [149].Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E, Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of longlasting long-term potentiation and improves memory, Proc. Natl. Acad. Sci. U. S. A 95 (1998) 15020–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Dyke HJ, Montana JG, Update on the therapeutic potential of PDE4 inhibitors, Expert Opin. Investig. Drugs 11 (2002) 1–13. [DOI] [PubMed] [Google Scholar]
- [151].Chen RW, Williams AJ, Liao Z, Yao C, Tortella FC, Dave JR, Broad spectrum neuroprotection profile of phosphodiesterase inhibitors as related to modulation of cell-cycle elements and caspase-3 activation, Neurosci. Lett 418 (2007) 165–169. [DOI] [PubMed] [Google Scholar]
- [152].Zhu J, Mix E, Winblad B, The antidepressant and antiinflammatory effects of rolipram in the central nervous system, CNS Drug Rev 7 (2001) 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Abbott-Banner KH, Page CP, Dual PDE3/4 and PDE4 inhibitors: novel treatments for COPD and other inflammatory airway diseases, Basic Clin. Pharmacol. Toxicol 114 (2014) 365–376. [DOI] [PubMed] [Google Scholar]
- [154].Press NJ, Banner KH, PDE4 inhibitors - a review of the current field, Prog. Med. Chem 47 (2009) 37–74. [DOI] [PubMed] [Google Scholar]
- [155].Kumar N, Goldminz AM, Kim N, Gottlieb AB, Phosphodiesterase 4-targeted treatments for autoimmune diseases, BMC Med. 11 (2013) 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Garcia-Osta A, Cuadrado-Tejedor M, Garcia-Barroso C, Oyarzabal J, Franco R, Phosphodiesterases as therapeutic targets for Alzheimer’s disease, ACS Chem. Neurosci 3 (2012) 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Hannila SS, Filbin MT, The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury, Exp. Neurol 209 (2008) 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Fang CL, Wen CJ, Aljuffali IA, Sung CT, Huang CL, Fang JY, Passive targeting of phosphatiosomes increases rolipram delivery to the lungs for treatment of acute lung injury: an animal study, J. Control. Release 213 (2015) 69–78. [DOI] [PubMed] [Google Scholar]
- [159].Jin SL, Conti M, Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses, Proc. Natl. Acad. Sci. U. S. A 99 (2002) 7628–7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Jin SL, Lan L, Zoudilova M, Conti M, Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages, J. Immunol 175 (2005) 1523–1531. [DOI] [PubMed] [Google Scholar]
- [161].Gobejishvili L, Barve S, Breitkopf-Heinlein K, Li Y, Zhang J, Avila DV, Dooley S, et al. , Rolipram attenuates bile duct ligation-induced liver injury in rats: a potential pathogenic role of PDE4, J. Pharmacol. Exp. Ther 347 (2013) 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Taguchi I, Oka K, Kitamura K, Sugiura M, Oku A, Matsumoto M, Protection by a cyclic AMP-specific phosphodiesterase inhibitor, rolipram, and dibutyryl cyclic AMP against Propionibacterium acnes and lipopolysaccharide-induced mouse hepatitis, Inflamm. Res 48 (1999) 380–385. [DOI] [PubMed] [Google Scholar]
- [163].Matsuhashi T, Otaka M, Odashima M, Jin M, Komatsu K, Konishi N, Wada I, et al. , Specific type IV phosphodiesterase inhibitor ameliorates thioacetamide-induced liver injury in rats, J. Gastroenterol. Hepatol 20 (2005) 135–140. [DOI] [PubMed] [Google Scholar]
- [164].Fischer W, Schudt C, Wendel A, Protection by phosphodiesterase inhibitors against endotoxin-induced liver injury in galactosamine-sensitized mice, Biochem. Pharmacol 45 (1993) 2399–2404. [DOI] [PubMed] [Google Scholar]
- [165].Feng H, Chen J, Wang H, Cheng Y, Zou Z, Zhong Q, Xu J, Roflumilast reverses polymicrobial sepsis-induced liver damage by inhibiting inflammation in mice, Lab. Investig 97 (2017) 1008–1019. [DOI] [PubMed] [Google Scholar]
- [166].Mollmann J, Kahles F, Lebherz C, Kappel B, Baeck C, Tacke F, Werner C, et al. , The PDE4 inhibitor roflumilast reduces weight gain by increasing energy expenditure and leads to improved glucose metabolism, Diabetes Obes. Metab 19 (2017) 496–508. [DOI] [PubMed] [Google Scholar]
- [167].Wouters EF, Bredenbroker D, Teichmann P, Brose M, Rabe KF, Fabbri LM, Goke B, Effect of the phosphodiesterase 4 inhibitor roflumilast on glucose metabolism in patients with treatment-naive, newly diagnosed type 2 diabetes mellitus, J. Clin. Endocrinol. Metab 97 (2012) E1720–E1725. [DOI] [PubMed] [Google Scholar]
- [168].Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJM, et al. , Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials, Lancet 374 (2009) 685–694. [DOI] [PubMed] [Google Scholar]
- [169].Jensterle M, Kocjan T, Janez A, Phosphodiesterase 4 inhibition as a potential new therapeutic target in obese women with polycystic ovary syndrome, J. Clin. Endocrinol. Metab 99 (2014) E1476–E1481. [DOI] [PubMed] [Google Scholar]
- [170].Peluso MR, Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase, and modulate lipid homeostasis in adipose tissue and liver, Exp. Biol. Med. (Maywood) 231 (2006) 1287–1299. [DOI] [PubMed] [Google Scholar]
- [171].Chae MK, Park SG, Song SO, Kang ES, Cha BS, Lee HC, Lee BW, Pentoxifylline attenuates methionine- and choline-deficient-diet-induced steatohepatitis by suppressing TNF-alpha expression and endoplasmic reticulum stress, Exp. Diabetes Res 2012 (2012) 762565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Yalcin M, Akarsu M, Celik A, Sagol O, Tunali S, Ertener O, Bengi G, et al. , A comparison of the effects of infliximab, adalimumab, and pentoxifylline on rats with non-alcoholic steatohepatitis, Turk J Gastroenterol 25 (Suppl. 1) (2014) 167–175. [DOI] [PubMed] [Google Scholar]
- [173].Zeng T, Zhang CL, Zhao XL, Xie KQ, Pentoxifylline for the treatment of nonalcoholic fatty liver disease: a meta-analysis of randomized double-blind, placebo-controlled studies, Eur. J. Gastroenterol. Hepatol 26 (2014) 646–653. [DOI] [PubMed] [Google Scholar]
- [174].Desmouliere A, Xu G, Costa AM, Yousef IM, Gabbiani G, Tuchweber B, Effect of pentoxifylline on early proliferation and phenotypic modulation of fibrogenic cells in two rat models of liver fibrosis and on cultured hepatic stellate cells, J. Hepatol. 30 (1999) 621–631. [DOI] [PubMed] [Google Scholar]
- [175].Movassaghi S, Nadia Sharifi Z, Mohammadzadeh F, Soleimani M, Pentoxifylline protects the rat liver against fibrosis and apoptosis induced by acute administration of 3,4-Methylenedioxymethamphetamine (MDMA or ecstasy), Iran J. Basic Med. Sci 16 (2013) 922–927. [PMC free article] [PubMed] [Google Scholar]
- [176].Zhang D, Jiang H, Wang Y, Ma J, Pentoxifylline inhibits hepatic stellate cells proliferation via the Raf/ERK pathway, APMIS 120 (2012) 572–581. [DOI] [PubMed] [Google Scholar]
- [177].Toda K, Kumagai N, Kaneko F, Tsunematsu S, Tsuchimoto K, Saito H, Hibi T, Pentoxifylline prevents pig serum-induced rat liver fibrosis by inhibiting interleukin-6 production, J. Gastroenterol. Hepatol. 24 (2009) 860–865. [DOI] [PubMed] [Google Scholar]
- [178].Hernandez E, Bucio L, Souza V, Escobar MC, Gomez-Quiroz LE, Farfan B, Kershenobich D, et al. , Pentoxifylline downregulates alpha (I) collagen expression by the inhibition of Ikappabalpha degradation in liver stellate cells, Cell Biol. Toxicol 24 (2008) 303–314. [DOI] [PubMed] [Google Scholar]
- [179].Li H, Hua J, Guo CX, Wang WX, Wang BJ, Yang DL, Wei P, et al. , Pentoxifylline inhibits liver fibrosis via hedgehog signaling pathway, J. Huazhong Univ. Sci. Technol. Med. Sci 36 (2016) 372–376. [DOI] [PubMed] [Google Scholar]
- [180].Lebrec D, Thabut D, Oberti F, Perarnau JM, Condat B, Barraud H, Saliba F, et al. , Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis, Gastroenterology 138 (2010) 1755–1762. [DOI] [PubMed] [Google Scholar]
- [181].Brown WM, Treating COPD with PDE 4 inhibitors, Int. J. Chron. Obstruct Pulmonol. Dis 2 (2007) 517–533. [PMC free article] [PubMed] [Google Scholar]
- [182].Duarte S, Baber J, Fujii T, Coito AJ, Matrix metalloproteinases in liver injury, repair and fibrosis, Matrix Biol. 44–46 (2015) 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Wu C, Rajagopalan S, Phosphodiesterase-4 inhibition as a therapeutic strategy for metabolic disorders, Obes. Rev 17 (2016) 429–441. [DOI] [PubMed] [Google Scholar]
- [184].Ratziu V, Bedossa P, Francque SM, Larrey D, Aithal GP, Serfaty L, Voiculescu M, et al. , Lack of efficacy of an inhibitor of PDE4 in phase 1 and 2 trials of patients with nonalcoholic steatohepatitis, Clin. Gastroenterol. Hepatol 12 (2014) 1724–1730 (e1725). [DOI] [PubMed] [Google Scholar]