Abstract
Purpose:
irRECIST were designed to capture atypical responses seen with immunotherapy. We hypothesized that, in patients with metastatic clear cell Renal Cell Carcinoma (mccRCC), candidate biomarkers for nivolumab response would show improved association with clinical endpoints capturing atypical responders (irRECIST) compared to standard clinical endpoints (RECISTv1.1).
Experimental Design:
Endpoints based on RECISTv1·1 (ORR/PFS) or irRECIST (irORR/irPFS) were compared in patients enrolled in the CheckMate-010 trial. Pretreatment tumors were analyzed by PD-L1 and PD-L2 immunohistochemistry, and by multiplex-immunofluorescence for CD8, PD-1, TIM-3 and LAG-3. T-cell activation signatures were assessed by RNAseq.
Results:
Median irPFS was significantly longer than median PFS. irORR was not significantly different from ORR but irPD rate was significantly lower than PD rate. Tumor cell (TC) PD-L1 expression was not associated with PFS or ORR but patients with TC PD-L1 ≥ 1% had longer median irPFS and higher irORR. High percentage of CD8+ tumor infiltrating cells (TIC) that are PD-1+TIM-3−LAG-3− (% CD8+PD-1+TIM-3−LAG-3− TIC) correlated with high levels of T-cell activation and was associated with longer median irPFS and higher irORR. Notably, combination of TC PD-L1 expression with % CD8+PD-1+TIM-3−LAG-3− TIC identified 3 groups of patients for which irPFS and irORR were significantly different.
Conclusions:
Atypical responders to nivolumab were identified in the CheckMate-010 trial. We observed improved association of candidate biomarkers for nivolumab response with endpoints defined by irRECIST compared to RECISTv1.1. TC PD-L1 expression in combination with PD-1 expression on CD8+ TIC may predict outcome on nivolumab in mccRCC.
Introduction
Cancer immunotherapy targeting programmed cell death protein 1 (PD-1) signaling improves overall survival in several tumor types with manageable toxicity and durable responses in a subset of patients (1). In patients with previously-treated metastatic clear cell renal cell carcinoma (mccRCC), nivolumab, a fully human monoclonal antibody against PD-1, demonstrated superior overall survival (OS) and fewer serious adverse events than everolimus in the CheckMate-025 trial, leading to its Food and Drug Administration approval (2). While nivolumab’s favorable therapeutic index makes it an appealing consideration for earlier disease settings, the lack of predictive biomarkers for selecting patients likely to achieve durable benefit limits the ability to establish the value of anti-PD-1s monotherapy in treatment naïve mccRCC patients.
World health organization (WHO) tumor response criteria and the most recent Response Evaluation Criteria in Solid Tumors version-1.1 (RECISTv1.1) are surrogates of survival routinely used by oncologists for clinical decision making (3, 4). Compared to targeted agents and conventional chemotherapy, immune-checkpoint inhibitors can display an atypical pattern of response, where new lesions develop or established lesions grow before an objective response or stable disease is observed (5–10). Immune-related Response Criteria (irRC, adapted from WHO criteria) and subsequently irRECIST, immune-based therapeutics RECIST (iRECIST), and immune-modified RECIST (imRECIST; all adapted from RECISTv1.1) were therefore developed to prevent misclassification of atypical responders as early progressors by the conventional WHO and RECISTv1.1 criteria (11–14). Recent analyses demonstrated that compared to RECISTv1.1, immune-related response criteria may more accurately predict long-term survival outcomes in patients with melanoma and lung cancer treated by PD-1 blockade (15, 16). Although it is increasingly accepted that response per immune-related criteria can more accurately assess benefit from immunotherapy, efforts to identify predictive biomarkers for anti-PD-1 agents have exclusively utilized endpoints based on RECISTv1.1, potentially impairing biomarker discovery.
The present manuscript is based on the analysis of the CheckMate-010 trial, a dose finding study where patients with mccRCC were randomly assigned to three different doses of nivolumab. It should be noted that in the initial publication of the trial, although irRECIST-based endpoints were reported, they were solely used as exploratory efficacy endpoints to demonstrate that, similar to RECIST v1.1-based endpoints, nivolumab efficacy was dose-independent (17). In this study, we first evaluated whether atypical responses to nivolumab, defined by irRECIST, impacted clinical outcome of patients with mccRCC enrolled the trial. We further tested the hypothesis that candidate biomarkers for nivolumab response show improved association with clinical endpoints capturing atypical responders (i.e. irRECIST) compared to standard clinical endpoints (i.e. RECISTv1.1).
Materials and Patients
Patients and tissue specimen
We studied mccRCC patients from the CheckMate-010 trial (BMS-936558, ClinicalTrials.gov_NCT01354431) (17). This trial is a multicenter phase II dose-finding study of nivolumab in patients with mccRCC who received previous regimen of agent targeting vascular endothelial growth factor pathway. Formalin-fixed and paraffin-embedded (FFPE) tumor sections were collected by the sponsor at the time of the trial. Institutional Review Board approval and individual written informed consents were obtained before tissue acquisition, tissue staining, and analysis of clinical information in accord with an assurance filed with and approved by the Department of Health and Human Services.
Clinical endpoints
Progression free survival (PFS) was defined as the time from randomization to documented disease progression per RECIST v1.1 or death. Patients alive and progression-free were censored at the date of last tumor assessment. Objective response rate (ORR) was defined as the proportion of randomized subjects whose best tumor response is either partial response (PR) or complete response (CR) per RECIST v1.1 (3). The definition of irRECIST has been previously published and these criteria have been utilized in previous studies (12, 16–19). Briefly, irRECIST include the following major modifications from RECISTv1.1: 1) requirement to confirm progression ≥ 4 weeks after initial radiological progression and 2) not scoring new small nontarget lesions as progression but using the net tumor burden to define progression.
irRECIST definitions for PFS (irPFS) and ORR (irORR) are similar to PFS and ORR and were derived from modified RECIST (12, 17).
Immunohistochemistry analysis
Programmed cell death 1 ligand 1 (PD-L1) and programmed cell death 1 ligand 2 (PD-L2) immunohistochemistry were performed using a verified assay developed by Dako (clone 28–8) and an in-house validated assay (clone D7U8C, Supplementary Materials and Methods and Supplementary Fig. 1) respectively. Membranous PD-L1 expression and membranous and/or cytoplasmic PD-L2 expression were independently scored as percentage of positive tumor cells by S.S. and J-C.P. that were blinded to patient outcomes. Interscorer discrepancies were resolved by consensus review. Overall tumor PD-L1 expression was quantified using ImageScope Membranous v9-algorithm (Leica).
Multiplex immunofluorescence analysis
Performance of previously validated antibodies for CD8, PD-1, T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) and lymphocyte activation protein-3 (LAG-3) was assessed by staining non-neoplastic tonsil tissue and obtaining a staining pattern overlapping with published data (20–24). CD8, PD-1, TIM-3 and LAG-3 multiplex immunofluorescence was performed by serial staining using the Opal tyramide signal system from Perkin Elmer (Waltham, MA, USA) as described in the Supplementary Materials and Methods and Supplementary Table 1.
Multiplex IF stained tissue sections were visualized with the Mantra Quantitative Pathology Workstation (Perkin Elmer). Multispectral images were acquired with the Mantra microscope using a 20x objective. Inform 2.2 software was then used in order to deconvolute the multispectral images, and to segment and phenotype cells (Supplementary Fig. 2). Briefly, for each fluorochrome a spectral library was created on single stained tissue sections; this library was used to deconvolute the multispectral images in spectral images corresponding to a specific fluorochrome (Supplementary Fig. 2A). Cell segmentation based on nuclear DAPI signal, cell size, and CD8, PD-1, TIM3, LAG-3 membranous staining signal was then applied (Supplementary Fig. 2B). The phenotyping step, based on machine learning recognition algorithm, was performed by developing 3 algorithms recognizing: 1) cells mono-stained for CD8 or co-stained for CD8 and PD-1, 2) cells mono-stained for CD8 or co-stained for CD8 and TIM-3, 3) cells mono-stained for CD8 or co-stained for CD8 and LAG-3 (Supplementary Fig. 2C). For each case, positive cells were manually identified until the learning recognition algorithm was concordant with visual count (concordance >90%). Of note, a cell was called CD8+ only if it was recognized as CD8+ by all three different algorithms. For each segmented cell, we therefore obtained information about the presence or the absence of CD8, PD-1, TIM3 or LAG3 staining. Unique phenotyping was performed for each tumor to account for inter sample variability of signal intensities as previously described (25). The percentage and the density of CD8+ cells expressing PD-1, TIM-3, LAG-3 either alone or in different combinations was calculated on a minimum of 5 different 0.36 mm2 images acquired from tumor area containing the highest density of tumor infiltrating CD8+ cells (26). Additional images were taken for tumors with low CD8+ cells to reach a minimum of 100 CD8+ cells counted. Tumor specimens with tumor area below 3.6 mm2 were not scored to minimize tissue selection bias.
Gene expression analysis
RNAs were extracted using the AllPrep DNA/RNA FFPE Mini Kit (Qiagen, Hilden, Germany) from a 5 cm2 tumor-enriched area macrodissected from 4 μm thick FFPE tissue sections prepared form a single tumor block. The tumor-enriched area contained an estimated percentage of tumor cells that ranged from 30% to 70%. Transcriptome capture analysis was performed as described in the Supplementary Materials and Methods. The cytolytic activity (CYT) signature; the effector-T cell (Teff) signature; 18 interferon-gamma (IFN-γ) responsive genes signature were defined based on previously published associations with the respective biology, and/or with clinical outcome (27–29). For each gene signature, an expression score for each patient sample was calculated as the geometric mean normalized count of all component genes in the signature. The geometric mean of the signatures was then log2 transformed to obtain the final signature expression score.
Statistical analysis
Prentice-Wilcoxon and McNemar’s tests were used to assess difference between correlated pairs of irPFS and PFS, and irORR and ORR endpoints respectively. Chi-Square test of equal proportion was used to estimate the difference between the rate of responses using RECISTv1.1 or irRECIST.
Association of tumor cell (TC) PD-L1 and TC PD-L2 positivity with Fuhrman nuclear grade (FNG) was assessed using Chi-square test. Correlation of TC PD-L1 and TC PD-L2 positivity was estimated by calculating Spearman’s rank correlation coefficient. PD-L1 and PD-L2 expression scores were categorized with frequently used PD-L1 cut-off points in immuno-oncology trial and correlated with clinical endpoints. Fisher’s exact test was used for binary endpoints of irORR and ORR and the log-rank test for irPFS and PFS. Superiority of immune-related endpoints (irPFS and irORR) over non-immune-related endpoints (PFS and ORR) was assessed graphically and also numerically via the appropriate test statistic. For example, log-rank test statistic comparing irPFS by PD-L1/PD-L2 was compared with log-rank test statistic comparing PFS by PD-L1/PD-L2.
Multiplex IF assay was performed in a total of 5 batches – Kruskal-Wallis one-way Analysis of variance (ANOVA) test was used to assess differences in median expression score across batches. Correlations of the different fractions or cell densities of CD8+ cells expressing PD-1, TIM-3, or LAG-3 either alone or in different combination were estimated by calculating Pearson’s rank correlation coefficient. Differences between the median percentages or densities of CD8+ cells expressing PD-1, TIM-3, or LAG-3 either alone or in different combination were estimated using Sign test.
Expression scores of the proportion and density of CD8+ cells expressing PD-1, TIM-3, and LAG-3 either alone or in different combinations were correlated with PFS/irPFS and ORR/irORR. The observed expression scores were positively skewed and therefore a cube root transformation of expression scores was taken to reduce the influence of extreme biomarker expression scores on model parameter estimates. There was a total of 33 different combinations of proportion and density of CD8+ PD-1, TIM-3, and/or LAG-3 expression scores. Transformed expression scores was correlated with PFS and irPFS using univariable Cox proportional hazards (PH) model. This approach to correlate continuous expression scores with clinical outcomes better utilizes the information contained in the continuous measurements and allows for assessment of a linear relationship between expression scores and clinical endpoints. In addition, it reduces the chances of false-positive results as a consequence of using various approaches to classify expression scores. The Holm-Bonferroni approach was also used to control the family wise error rate (FWER) (Type I error) at 5% for the hypotheses tests conducted (30). The Holm-Bonferroni approach is uniformly more powerful than the rather conservative classic Bonferroni approach. For interpretation and visualization purposes, each of the biomarkers that meet the FWER control screening threshold (i.e. declared statistically significant) specified above were also classified into binary variables by selecting an optimal threshold that maximizes specificity and sensitivity with respect to irORR (31). At optimized cutoffs, Fisher’s exact test was used for binary endpoints of irORR and the log-rank test for irPFS.
The difference in gene signature expression score distribution between groups were evaluated using a Wilcoxon rank sum test. Signature expression score were correlated with irPFS and irORR using univariable Cox proportional hazards (PH) and binary logistic regression analysis respectively.
One-sided p-values ≤0.05 were considered statistically significant for all analysis.
Results
Patients
From May 2011 to January 2012, 167 previously treated mccRCC patients were randomly assigned to nivolumab 0.3, 2, or 10 mg/kg intravenously once every 3 weeks as previously described (17). Median clinical-follow up of the present study was 38.4 months (min-max: 0.6 – 43.3 months). Baseline demographics, treatment arms and clinical characteristics are presented in Table 1. Since no significant dose-response relationship of nivolumab with primary endpoint (PFS) and secondary endpoints (ORR, irPFS, OS) were previously found, patient cohorts were pooled together in the present study (17). PD-L1, PD-L2 expression and positivity of the immune checkpoint inhibitors PD-1, TIM-3 and LAG-3 on CD8+ tumor-infiltrating cells (TIC) were assessed in 140, 127 and 98 patients, respectively (Supplementary Fig. 3).
Table 1.
Baseline Demographics, Treatment Arm, and Clinical Characteristics per Tumor Cell PD-L1 Expression Levels and Percentage Levels of CD8+ Tumor Infiltrating Cell That Are PD-1+TIM-3−LAG-3−
| Characteristic | Total (n=167) | TC PD-L1 | % of CD8+PD-1+TIM-3−LAG-3− TIC | ||
|---|---|---|---|---|---|
| ≥1% n=19 | <1%n=121 | High (≥36%) n=74 | Low (<36%) n=24 | ||
| Age | |||||
| Median (Min, Max) | 61.0(37.0, 81.0) | 61 44, 76 | 60 37, 81 | 59.5 38.0, 81.0 | 60.037.0, 78.0 |
| Gender, (%) | |||||
| Female | 46 (27.5) | 7 (36.8) | 30 (24.8) | 22 (29.7) | 4 (16.7) |
| Male | 121 (72.5) | 12 (63.2) | 91 (75.2) | 52 (70.3) | 20 (83.3) |
| Race | |||||
| ASIAN | 7 (4.2) | 1 (5.3) | 4 (3.3) | 2 (2.7) | - |
| BLACK | 3 (1.8) | 1 (5.3) | 1 (0.8) | 1 (1.4) | 1 (4.2) |
| OTHER | 1 (0.6) | - | 1 (0.8) | - | - |
| WHITE | 156 (93.4) | 17 (89.5) | 115 (95.0) | 71 (95.9) | 23 (95.8) |
| Ethnicity | |||||
| Non-Hispanic | 159 (95.2) | 18 (94.7) | 116 (95.9) | 71 (95.9) | 23 (95.8) |
| Not Reported | 8 (4.8) | 1 (5.3) | 5 (4.1) | 3 (4.1) | 1 (4.2) |
| Treatment Arm, niv. dose | |||||
| 0.3 mg/kg | 59 (35.3) | 6 (31.6) | 44 (36.4) | 24 (32.4) | 10 (41.7) |
| 2 mg/kg | 54 (32.3) | 8 (42.1) | 35 (28.9) | 26 (35.1) | 6 (25.0) |
| 10 mg/kg | 54 (32.3) | 5 (26.3) | 42 (34.7) | 24 (32.4) | 8 (33.3) |
| MSKCC Risk Category | |||||
| Favorable | 54 (32.7) | 5 (27.8) | 41 (34.2) | 29 (39.2) | 4 (16.7) |
| Intermediate | 68 (41.2) | 6 (33.3) | 51 (42.5) | 25 (33.8) | 12 (50.0) |
| Poor | 43 (26.1) | 7 (38.9) | 28 (23.3) | 20 (27.0) | 8 (33.3) |
| Missing | 2 | 1 | 1 | - | - |
| ECOG PS | |||||
| 0 | 38 (22.9) | 5 (27.8) | 26 (21.5) | 16 (21.9) | 3 (12.5) |
| 1 | 108 (65.1) | 11 (61.1) | 81 (66.9) | 50 (68.5) | 15 (62.5) |
| 2 | 20 (12.1) | 2 (11.11) | 14 (11.6) | 7 (9.6) | 6 (25.0) |
| Missing | 1 | 1 | - | 1 | - |
| Number of Prior Therapy Adv/Met Setting | |||||
| 1 | 50 (29.9) | 5 (26.3) | 34 (28.1) | 24 (32.4) | 9 (37.5) |
| >1 | 117 (70.1) | 14 (73.7) | 87 (71.9) | 50 (67.6) | 15 (62.5) |
| Number of Prior Anti-Angiogenic Therapy | |||||
| 1 | 104 (62.3) | 13 (68.4) | 72 (59.5) | 50 (67.6) | 15 (62.5) |
| >1 | 63 (37.7) | 6 (31.6) | 49 (40.5) | 24 (32.4) | 9 (37.5) |
Abbreviations: CD8, cluster of differentiation 8; ECOG PS: eastern cooperative oncology group performance status; LAG-3, lymphocyte activation protein-3; MSKCC: Memorial Sloan Kettering Cancer Center; niv., nivolumab; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1; TC, tumor cell; TIC, tumor infiltrating cell; TIM-3, T-cell immunoglobulin and mucin-domain containing-3.
Comparison of clinical outcomes using RECISTv1.1 and irRECIST
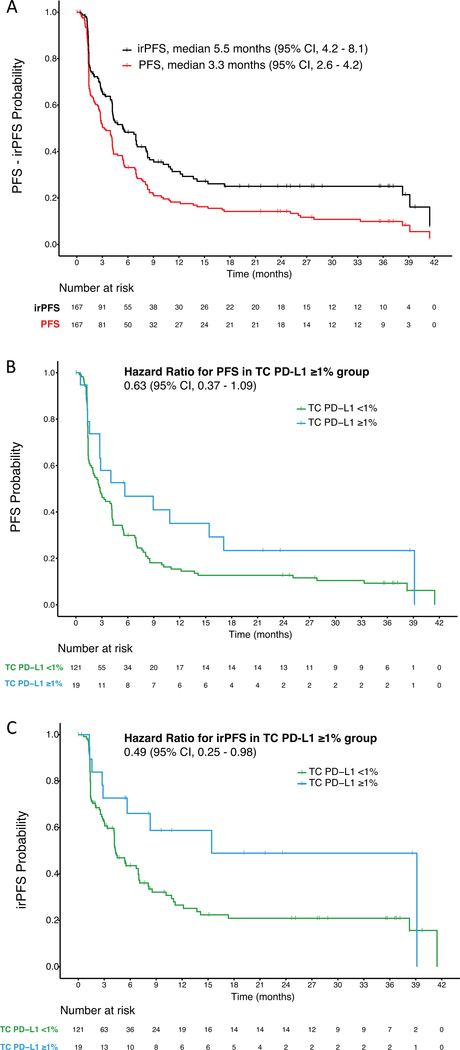
We compared responses evaluated using RECISTv1.1 to those evaluated using irRECIST in the whole cohort (n = 167). RECISTv1.1 were used to calculate PFS and ORR and irRECIST were used to calculate irPFS and irORR. irORR (22.8%) was not significantly different from ORR (21%). However, the percentage of patients with irPD (24.6%) was significantly lower than the percentage of patients with PD (35.3%, p = 0.03) and the percentage of patients with irSD (50.3%) tended to be higher than the percentage of patients with SD (41.3%) (Supplementary Table 2). Median irPFS (5.5 months) was significantly longer than median PFS (3.3 months; p <0.001) (Fig. 1A).
Figure 1:
(A) Kaplan-Meier curves for progression free survival (PFS) and immune-related PFS (irPFS). (B) Kaplan-Meier Curves for progression free survival (PFS) and (C) immune-related PFS (irPFS) per tumor cell (TC) PD-L1 expression levels.
Expression of PD-L1 and PD-L2 and their association with clinical outcomes
In the initial study of the Checkmate-010, TC PD-L1 expression was evaluated in 107 patients using a prototype immunohistochemistry assay based on the 28–8 clone (17). Here, PD-L1 was independently assessed in larger number of patients (n = 140) (Supplementary Fig. 3) using a verified assay developed by Dako using the 28–8 clone. Tissue analyzed included 95 (67.9%) primary tumors, 36 (25.7%) metastases and 9 (6.4%) lesions from unknown sites. TC PD-L1 expression ≥1% was observed in 19 (13.6 %) patients (Table 2, Supplementary Fig. 4) and was associated with high FNG (p = 0.003; Supplementary Table 3).
Table 2.
Summary of Efficacy Results per Tumor Cell PD-L1 Expression Levels, Tumor Cell PD-L2 Expression Levels or Percentage Levels of CD8+Tumor Infiltrating Cell That Are PD-1+TIM-3−LAG-3−
| TC PD-L1 Expressiona |
|||||
|---|---|---|---|---|---|
| < 1% (n = 121b) | ≥ 1% (n = 19) | ||||
| Endpoints | n | % | n | % | p-valuec |
| ORR | 22 | 18.8 | 7 | 36.8 | 0.07 |
| 95% CI, % | 12.2 – 27.1 | 16.3 – 61.6 | |||
| irORR | 23 | 19.3 | 9 | 47.4 | 0.01 |
| 95% CI, % | 12.7 – 27.6 | 24.4 – 71.1 | |||
| Median PFS, months | 2.8 | 5.7 | 0.09 | ||
| 95% CI | 1.8 – 4.1 | 1.6 – 17.1 | |||
| Median irPFS, months | 4.3 | 15.4 | 0.04 | ||
| 95% CI | 3.4 – 6.9 | 2.9 – 39.1 | |||
| TC PD-L2 Expressiond |
|||||
|---|---|---|---|---|---|
| < 1% (n = 81b) | ≥ 1% (n = 46) | ||||
| Endpoints | n | % | n | % | p-valuec |
| ORR | 19 | 24.7 | 9 | 19.6 | 0.81 |
| 95% CI, % | 15.6 – 35.8 | 9.4 – 33.9 | |||
| irORR | 19 | 24.1 | 11 | 23.9 | 0.59 |
| 95% CI, % | 15.1 – 35.0 | 12.6 – 38.8 | |||
| Median PFS, months | 3.9 | 2.6 | 0.88 | ||
| 95% CI | 1.9 – 5.4 | 1.4 – 4.2 | |||
| Median irPFS, months | 5.7 | 4.4 | 0.73 | ||
| 95% CI | 4.1 – 8.5 | 2.4 – 8.3 | |||
| % of CD8+PD-1+TIM-3−LAG-3− TICe |
|||||
|---|---|---|---|---|---|
| low (n = 24f) | high (n = 74g) | ||||
| Endpoints | n | % | n | % | p-valuec |
| irORR | 0 | 0 | 21 | 28.8 | 0.001 |
| 95% CI, % | 0.0 – 14.8 | 18.8 – 40.6 | |||
| Median irPFS, months | 2.6 | 6.9 | 0.005 | ||
| 95% CI | 1.4 – 6.7 | 4.2 – 12.2 | |||
For 27 patients either tissues were not available, or staining was not assessable for analysis.
ORR data for 4 patients and irORR data for 2 patients were missing.
Fisher's exact test was used to test the association with irORR and log-rank test was used to test the association with irPFS.
For 40 patients either tissues were not available, or staining was not assessable for analysis.
For 69 patients either tissues were not available, or staining was not assessable for analysis.
irORR data for 1 patient was missing.
irORR data for 1 patient was missing.
Abbreviations: CD8, cluster of differentiation 8; CI, confidence interval; LAG-3, lymphocyte activation protein-3; ir, immune-related; ORR, objective response rate; PD-1, programmed cell death 1; PD-L1/2, programmed cell death 1 ligand 1/2; PFS, progression-free survival; TC, tumor cell; TIC, tumor infiltrating cell; TIM-3, T-cell immunoglobulin and mucin-domain containing-3.
TC PD-L1 expression was not significantly associated with improved PFS (p = 0.09; Table 2 and Fig. 1B). In contrast, median irPFS was significantly longer in the TC PD-L1 expression ≥1% group (15.4 months) compared to the TC PD-L1 <1% group (4.3 months; p = 0.04; Table 2 and Fig. 1C). Although ORR tended to be higher in the TC PD-L1 expression ≥1% group (36.8%) compared to the TC PD-L1 expression <1% group (18.8%), this difference did not reach statistical significance (p = 0.07). On the other hand, irORR was significantly higher in the TC PD-L1 positive ≥1% group (47.4%) compared to the TC PD-L1 <1% group (19.3%; p = 0.01; Table 2). While the patients randomly assigned to nivolumab 0.3, 2, or 10 mg/kg achieved similar clinical efficacy, we also aimed to demonstrate that the association between TC PD-L1 expression and clinical outcome was independent of drug dosing. Indeed, no significant difference in TC PD-L1 positivity score among the different arms of nivolumab-treated patients was observed. Of note baseline demographics and clinical characteristics were also similar between TC PD-L1 expression groups (Table 1). When higher cutoffs were used to define TC PD-L1 positivity (5% and 10%), the proportion of patients with positive tumors became progressively smaller (8% and 5%, respectively), preventing meaningful correlations with clinical endpoints.
PD-L1 expressed on tumor immune cells (IC) can also bind to PD-1 and inhibit the anti-tumor immune response (1, 32). Therefore, we further evaluated overall PD-L1 expression by measuring membranous PD-L1 staining on both TC and IC in 131 patients (Supplementary Fig. 3). Using an arbitrary cutoff of ≥1% PD-L1 positive cells, overall (TC and IC) PD-L1 expression was observed in 29% of patients. No significant association between overall PD-L1 expression and clinical endpoints was found at any tested cutoff (Supplementary Table 4).
To test if TC expression of the second PD-1 ligand, PD-L2, could improve the identification of tumors that respond to anti-PD-1 therapy, TC PD-L2 expression was assessed by immunohistochemistry in 127 patients (Supplementary Fig. 3 and 4) (33).TC PD-L2 expression ≥1% was observed in 46 (36.5%) patients (Table 2) and was not associated with FNG (Supplementary Table 3). No significant association between overall PD-L2 expression and clinical outcomes was found at 1% positivity cutoff (Table 2) or any other tested cutoff (Supplementary Table 5). TC PD-L1 expression did not correlate with TC PD-L2 expression (Supplementary Fig. 5) and only 8 of 126 (6.34%) patients had tumors expressing both PD-L1 (≥1%) and PD-L2 (≥1%). Therefore, we evaluated whether combined TC PD-L1 and/or TC PD-L2 expression could better identify patients that respond to nivolumab. When TC positivity was defined as expression of either or both PD-L1 (≥1% cutoff) and PD-L2 (≥1% cutoff), 55 of 126 patients (43.65%) were categorized as positive. No significant association between PD-L1/PD-L2-positivity and clinical endpoints was found (Supplementary Table 6).
Expression of PD-1, TIM-3, and LAG-3 on CD8+ TIC and their association with clinical outcomes
Human tumors have been shown to contain severely exhausted T- cells expressing multiple immune-checkpoints and it has been proposed that these cells mediate resistance to PD-1 blockade (34, 35). For this reason, we evaluated CD8+ TIC for the expression of the immune-checkpoints PD-1, TIM-3 and LAG-3 in 98 patients (Supplementary Fig. 3). For each tumor, we measured both the absolute number (cell density per mm2) and the relative amount (percentage) of CD8+ TIC expressing the 3 immune-checkpoints either alone or in various combinations by multiplex immunofluorescence.
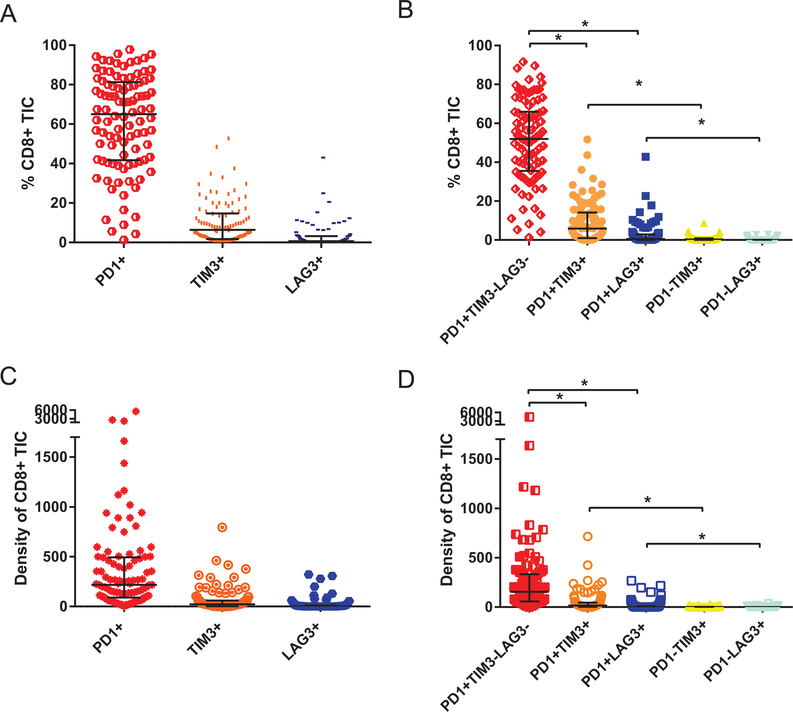
PD-1 was the immune-checkpoint most frequently expressed on CD8+ TIC followed by TIM-3 and LAG-3 (Fig. 2A and 2C). In line with these findings, the median percentage (and median density) of CD8+ TIC expressing PD-1 alone was significantly higher than the median percentage (and median density) of CD8+ TIC expressing PD-1 and TIM-3 or PD-1 and LAG-3. Of note, TIM-3 and LAG-3 were detected more frequently on CD8+PD-1+ TIC compared to CD8+PD-1− TIC (Fig. 2B and 2D).
Figure 2:
(A, B) Vertical scatter plot comparing the median percentage and (C, D) the median density (cell per mm2); of CD8+ tumor infiltrating cell (TIC) Expressing PD-1, TIM-3, LAG-3 either alone (A, C) or in different combinations (B, D). Error bars represent interquartile range. Stars indicate p-value <0.001.
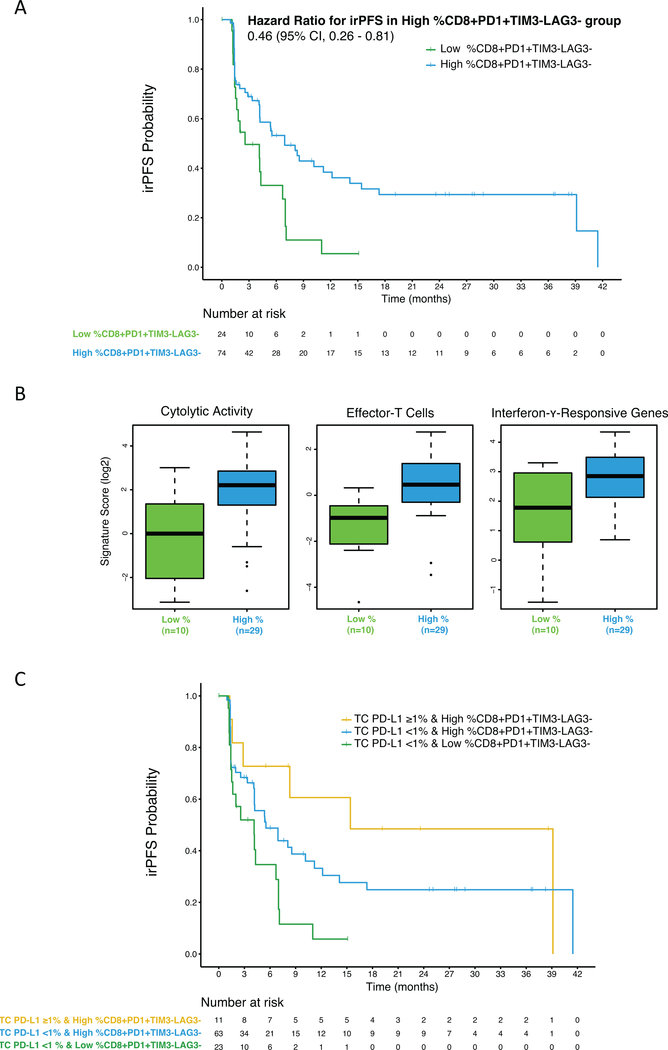
We next investigated whether the expression of PD-1, TIM-3, and LAG-3 (in various combinations) on CD8+ TIC (Supplementary Table 7) was associated with outcome on nivolumab therapy. Biomarker analysis included both the mean percentage and mean density measurements as they were found to be weakly correlated with each other and therefore provide different information on the composition of the tumor microenvironment (Supplementary Fig. 6). To correlate these biomarkers with clinical endpoints, we utilized a stringent approach in which the candidate biomarkers were screened for the presence of a linear relationship between biomarker levels and PFS or irPFS. Chances of false positive results were controlled using the Holm-Bonferroni criteria. Whereas no biomarker was found to be significantly correlated with PFS, three biomarkers (percentage of CD8+ TIC that are PD-1+TIM-3−LAG-3−; density of CD8+ TIC that are PD-1+TIM-3−LAG-3−; percentage of CD8+ TIC that are PD-1+) significantly correlated with irPFS (Supplementary Table 8). Using a cutoff that maximizes sensitivity for irORR, the percentage of CD8+ TIC that are PD-1+TIM-3−LAG-3− (hereafter called CD8+PD-1+TIM-3−LAG-3− TIC) exhibited the best sensitivity and specificity (Table 2 and Supplementary Table 9) and was further explored. At the optimized cutoff of 36%, high percentage of CD8+PD-1+TIM-3−LAG-3− TIC was observed in 74 of 98 (75.5 %) patients. The median irPFS was significantly longer in the group with high percentage of CD8+PD-1+TIM-3−LAG-3− TIC (6.9 months) compared to the group with low percentage of CD8+PD-1+TIM-3−LAG-3− TIC (2.6 months; p = 0.005; Table 2; Fig. 3A). irORR was significantly higher in the group with high percentage of CD8+PD-1+TIM-3−LAG-3− TIC (28.8%) compared to the group with low percentage of CD8+PD-1+TIM-3−LAG-3− TIC (0%; p = 0.001; Table 2). Of note, no significant difference in biomarker expression scores among the three-dose arm of nivolumab-treated patients was observed. Baseline demographics and clinical characteristics were also similar between groups of patients having a high or a low percentage of CD8+PD-1+TIM-3−LAG-3− TIC (Table 1).
Figure 3:
(A) Kaplan-Meier curves for immune-related progression free survival (irPFS) per percentage levels of CD8+ Tumor Infiltrating Cell that Are PD-1+ TIM-3− LAG-3− (CD8+ PD-1+ TIM-3− LAG-3− TIC). (B) Association between the percentage levels of CD8+ PD-1+ TIM-3− LAG-3− TIC and T-cell activation signature scores. (C) Kaplan-Meier curves for irPFS per TC PD-L1 expression levels combined with the percentage levels of CD8+ PD-1+ TIM-3− LAG-3− TIC.
The high percentage of CD8+PD-1+TIM-3−LAG-3− TIC associated to nivolumab response might identify patients with an active anti-tumor immune response in their pre-treatment tumors. To test this hypothesis, we performed RNAseq on a subset of tumors with available tissue (Supplementary Fig. 3) and interrogated three gene expression signatures associated with T cell activation: the CYT signature, the Teff signature, and the IFN-γ responsive gene signature (27–29). Thirty-nine of 47 patients with gene expression signature data had also IF data. In line with our hypothesis, high percentage of CD8+PD-1+TIM-3−LAG-3− TIC (≥36%) was found to be associated with high expression levels of the CYT, Teff and IFN-γ signatures (p = 0.004, 0.001 and 0.007 respectively, Fig. 3B). None of the three signatures were significantly associated with clinical outcomes.
Predictive value of TC PD-L1 expression combined with the percentage of CD8+PD-1+TIM-3−LAG-3− TIC
We next tested whether the combination of TC PD-L1 expression with the percentage of CD8+PD-1+TIM-3−LAG-3− TIC could improve the ability to identify patients that respond to nivolumab. Ninety-seven patients had data for both biomarkers. Among these, 11 patients had both high TC PD-L1 expression (≥1%) and high percentage of CD8+PD-1+TIM-3−LAG-3− TIC (≥36%), and 63 patients had low TC PD-L1 expression (<1%) and high percentage of CD8+PD-1+TIM-3−LAG-3− TIC. All 23 patients with low percentage of CD8+PD-1+TIM-3−LAG-3− TIC (<36%) were found to have low TC PD-L1 expression.
Both median irPFS and irORR were significantly different among three patient groups (p = 0.013 and p = 0.001, respectively; Fig. 3C, Table 3). Specifically, median irPFS was longest in the group with both high TC PD-L1 expression and high percentage of CD8+PD-1+TIM-3 LAG-3− TIC (15.4 months), intermediate in the group with low TC PD-L1 expression and high percentage of CD8+PD-1+TIM-3−LAG-3− TIC (5.5 months) and shortest in the group with both low TC PD-L1 expression and low percentage of CD8+PD-1+TIM-3−LAG-3− TIC (4.1 months). Similarly, irORR was highest in the group with both high TC PD-L1 expression and high percentage of CD8+PD-1+TIM-3−LAG-3− TIC (54.5%), intermediate in the group with low TC PD-L1 expression and high percentage of CD8+PD-1+TIM-3−LAG-3− TIC (24.2%) and lowest in the group with both low TC PD-L1 expression and low percentage of CD8+PD-1+TIM-3−LAG-3− TIC (0%).
Table 3.
Summary of Efficacy Results Per Tumor Cell PD-L1 Expression Levels Combined with the Percentage Levels of CD8+ Tumor Infiltrating Cell that Are PD-1+TIM-3−LAG-3− a
| TC PD-L1 expression ≥1% and high % of CD8+ PD-1+TIM-3−LAG-3− TIC (n = 11) | TC PD-L1 expression <1% and high % of CD8+PD-1+TIM-3−LAG-3− TIC (n = 63b) | TC PD-L1 expression <1% and low % of CD8+PD-1+TIM-3−LAG-3− TIC (n = 23) | |||||
|---|---|---|---|---|---|---|---|
| Endpoints | n | % | n | % | n | % | p-valuec |
| irORR | 6 | 54.5 | 15 | 24.2 | 0 | 0.0 | 0.001 |
| 95% CI, % | 23.4 – 83.3 | 14.2 – 36.7 | 0.0 – 15.4 | ||||
| Median irPFS, months | 15.4 | 5.5 | 4.1 | 0.013 | |||
| 95% CI | 1.6 – 39.1 | 4.1 – 10.2 | 1.4 – 6.7 | ||||
Tissue or staining for PD-L1 and multiplex IF were not available for 70 patients.
irORR data for 2 patients were missing.
Fisher's exact test was used to test the association with irORR and log-rank test was used to test the association with irPFS.
Abbreviations: CD8, cluster of differentiation 8; CI, confidence interval; LAG-3, lymphocyte activation protein-3; ir, immune-related; ORR, objective response rate; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1; PFS, progression-free survival; TC, tumor cell; TIC, tumor infiltrating cell; TIM-3, T-cell immunoglobulin and mucin-domain containing-3.
Discussion
Our analyses of the CheckMate-010 cohort show that irRECIST enable the capture of patients with mccRCC who displayed atypical responses to nivolumab therapy, which had a significant impact on median PFS. Furthermore, we showed that candidate biomarkers for nivolumab response had an improved association with clinical endpoints defined by irRECIST relative to RECISTv1.1. By utilizing irRECIST, we developed a novel model combining TC PD-L1 expression and percentage of CD8+ TIC that express PD-1 (but not the other immune-checkpoints TIM-3 or LAG-3). This proposed model allowed stratification of nivolumab-treated patients in three groups with significantly different irPFS and irORR.
Clinical experience increasingly indicates that traditional response criteria cannot adequately assess benefit from immunotherapy. In line with results obtained in melanoma and lung cancer, our study showed that within the CheckMate-010 trial, a subset of patients with mccRCC treated with nivolumab experienced objective response or stable disease by irRECIST, but PD by RECISTv1.1 (14–16). Reclassification of the CheckMate-010 trial patient outcomes by irRECIST resulted in significantly longer irPFS compared to PFS. These data have important implications for clinical management of patients as well as for correlative biomarker studies. Our results indicate that utilization of RECISTv1.1 underestimates the number of mccRCC patients that benefit from nivolumab therapy. Our findings also support the concept that misclassification of atypical responders by RECISTv1.1 has the potential to confound biomarker analyses for immune-checkpoint inhibitors and thus undermine the development of robust patient selection criteria for these agents. Indeed, in line with previously published data, we found that TC PD-L1 expression was not correlated with clinical outcome by RECISTv1.1 in the CheckMate-010 trial patients (17). However, TC PD-L1 expression was found to be significantly associated with both higher irORR and longer irPFS. These associations are notable given that tumor PD-L1 expression has been associated with poor prognosis in patients with RCC treated with either prior ineffective therapies or vascular endothelial growth factor receptor (VEGFR) blockers (21, 36–38). Of note, overall PD-L1 expression (including expression in both TC and IC) was not associated with clinical endpoints suggesting that in ccRCC, PD-L1 expression on TC (but not IC) is a major driver of immune evasion that is reversed by PD-1 blockade. However, further studies are needed to specifically address the role of IC PD-L1 expression in predicting response to nivolumab. In spite of the association of TC PD-L1 expression with clinical outcomes, our data show that this marker alone fails to reliably identify all patients likely to benefit from therapy.
For this reason, we tested other candidate biomarkers to develop a multi-marker model that demonstrates a greater predictive value than PD-L1 expression alone. Persistent exposure to antigen during chronic infection or cancer induces T cells exhaustion, a cell state characterized by co-expression of multiple immune-checkpoints, and that has been shown to limit cell proliferation, and reduced cytotoxic activities (34). Rejuvenation of exhausted T cells can be achieved by PD-1 blockade, and combined inhibition of PD-1 with TIM-3 or LAG-3 induces synergistic reduction of tumor growth (35, 39, 40). Here, we found that both high percentages of CD8+ TIC expressing PD-1 and high percentages of CD8+ TIC expressing PD-1 but not TIM-3 and LAG-3 are positively associated with longer irPFS on nivolumab. The association with higher irORR was strongest for the subset of CD8+ TIC expressing PD-1, but not TIM-3 or LAG-3, suggesting that the higher predictive value of these cells might be related to their less exhausted phenotype and their ability to be more efficiently reactivate with PD-1 blockade. In line with these findings, we showed that a high percentage of CD8+ TIC expressing PD-1 but not TIM-3 and LAG-3 was associated with high levels of T-cell activation, as assessed by gene expression analysis. The rejuvenation of highly exhausted may require combination therapy including agents targeting PD-1, TIM-3 and LAG-3.
Remarkably, in our cohort, all patients with low percentage of CD8+ TIC expressing PD-1 (but not TIM-3 or LAG-3) were also negative for TC PD-L1 expression and did not respond to nivolumab. On the other hand, among patients with a high percentage of CD8+ TIC expressing PD-1 (but not TIM-3 or LAG-3), the subset of patients with positive TC PD-L1 expression displayed highest irORR and longest irPFS. Overall, our results suggest that (a) patients with high expression of the therapy target PD-1, on TIC, and of its ligand PD-L1, on TC are the most likely to benefit from nivolumab; (b) patients with high expression of PD-1 on TIC in absence of TC PD-L1 expression have an intermediate chance to respond to nivolumab; (c) patients with neither high PD-1 expression on TIC nor TC PD-L1 expression are resistant to therapy. Validation of this model could help identify patients who would benefit sufficiently from nivolumab monotherapy in the treatment naive or adjuvant settings.
The analyses presented here have several potential limitations. First, in our cohort, patients were randomly assigned to receive three different doses of nivolumab. However, it was previously reported that all the tested clinical endpoints (ORR, PFS, irPFS) were not significantly different in the three randomized arms (17). Moreover, we demonstrated that our candidate biomarkers expression scores were similar between the three nivolumab treatment groups. Taken together, these data strongly suggest that the randomized drug dosing design of the trial has very limited impact on our results.
Another limitation of the study is the analysis of a single tumor sample for each patient. As intratumor heterogeneity is well-recognized in RCC, biomarker measurement in a single tumor region (mostly from the primary tumor) might not reflect its expression in the patient’s metastatic lesions that are the target of systemic therapy (41). The design of clinical trials that include collection of both pretreatment primary tumors and metastatic lesions for correlative studies would be very helpful to address the impact of tumor heterogeneity in biomarker discovery and validation.
Further limitations of our work include the prospective-retrospective nature of the study and the use of samples from a non-controlled clinical trial where post-nivolumab therapy confounded an OS analysis. For this reason, we chose to focus on clinical endpoints more directly impacted by the initial therapy (e.g. ORR/irORR and PFS/irPFS). In the CheckMate-025 trial, there was no association between PD-L1 expression and improved OS on nivolumab after a minimum follow-up of 14 months (2). It remains to be tested if the multi-factorial model we developed by studying irRECIST endpoints in a mature CheckMate-010 cohort can identify durable responders more accurately than PD-L1 expression alone when the CheckMate-025 trial reaches longer follow-up. Ultimately, our model needs to be validated in prospective biomarker-driven trials such as Hoosier Clinical Research Network GU-260 (NCT03117309).
Supplementary Material
Statement of translational relevance.
The use of radiological response criteria capturing atypical responses to immunotherapy has been recently shown to be a better surrogate marker of antitumor effect with anti-PD-1/PD-L1 agents in melanoma and lung cancer. In line with these findings, our study demonstrates that atypical responses from nivolumab captured by irRECIST translate into longer clinical benefit in patient with mccRCC. Our results also indicate that the use of conventional RECIST is likely to negatively impact the association of candidate predictive biomarkers with outcome by failing to accurately identify patients benefiting from nivolumab therapy. Our findings support the more extensive use of immune-related response criteria in the assessment of response to immunotherapy in mccRCC setting and in the execution of correlative studies for predictive biomarker discovery and validation to immune-checkpoint blockade.
Acknowledgments
S.S., P.J.C., G.J.F., A.H.S., T.K.C., M.B.A., and D.F.M. have received support from NIH/NCI DF/HCC Kidney Cancer SPORE P50-CA101942. S.A.K. has received support from NIH/NCI R50CA211482. T.K.C. has received support from The Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at Dana-Farber Cancer Institute.
Abbreviations list:
- ANOVA
analysis of variance
- CD8
cluster of differentiation 8
- CI
confidence interval
- CR
complete response
- CYT
cytolytic activity
- DAPI
4′,6-diamidino-2-phenylindole
- ECOG PS
eastern cooperative oncology group performance status
- FFPE
formalin-fixed and paraffin-embedded
- FNG
Fuhrman nuclear grade
- FWER
family wise error rate
- IC
immune cell
- IFN-γ
interferon-gamma
- imRECIST
immune-modified RECIST
- ir
immune-related
- irRC
immune-related response criteria
- iRECIST
immune-based therapeutics RECIST
- LAG-3
lymphocyte activation protein-3
- mccRCC
metastatic clear cell renal cell carcinoma
- MSKCC
Memorial Sloan Kettering cancer center
- ORR
objective response rate
- niv
nivolumab
- OS
overall survival
- PD-1
programmed cell death 1
- PD-L1
programmed cell death 1 ligand 1
- PD-L2
programmed cell death 1 ligand 2
- PFS
progression free survival
- PH
proportional hazard
- PR
partial response
- RECISTv1.1
response evaluation criteria in solid tumor version-1.1
- RNAseq
RNA sequencing
- SD
stable disease
- TC
tumor cell
- TIC
tumor infiltrating cell
- TIM-3
T-cell immunoglobulin and mucin-domain containing-3
- Teff
effector T-cell
- VEGFR
vascular endothelial growth factor receptor
- WHO
world health organization
Footnotes
Conflict of interest disclosure statement:
C.E. Horak, M. Wind-Rotolo and Y. Ishii are employee of Bristol-Myers Squibb. C.E. Horak and M. Wind-Rotolo have stock or other ownership interests from Bristol-Myers Squibb. P.J. Catalano has consulting or advisory role for Lilly. G.J. Freeman has consulting or advisory role for Novartis, Lilly, Roche/Genentech, Bristol-Myers Squibb, Bethyl Laboratories, Xios Therapeutics, Quiet Therapeutics, Seattle Genetics; patents, royalties, or other intellectual property from Novartis, Roche/Genentech, Bristol-Myers Squibb/Medarex, Amplimmune/Astrazeneca, Merck, EMD Serono, Boehringer Ingelheim and research funding from Novartis, Roche/Genentech. A.H. Sharpe has consulting or advisory role for Surface Oncology, SQ2 Biotech, Adaptimmune, Bethyl Laboratories, Xios Therapeutics, Quiet Therapeutics, Seattle Genetics; patents, royalties, other intellectual property from Novartis, Boehringer Ingelheim, Astrazeneca, Dako/Agilent Technologies, Bristol-Myers Squibb, Roche, Merck, EMD Serono and research funding from Novartis, Roche/Genentech, VCB, Ipsen. F.S. Hodi has consulting or advisory role for Novartis, Genentech/Roche, Merck Sharp & Dohme, Amgen, EMD Serono and research funding from Novartis, Roche/Genentech, Bristol-Myers Squibb, Merck Sharp & Dohme. R.J. Motzer has consulting or advisory role for Novartis, Pfizer, Exelixis, Eisai, Merck and research funding from Pfizer, GlaxoSmithKline, Bristol-Myers Squibb, Eisai, Novartis, Roche/Genentech. T.K. Choueiri has consulting or advisory role for Novartis, Pfizer, Exelixis, Prometheus, Agilent, Bayer, GlaxoSmithKline, Merck, Bristol-Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Peleton Therapeutics and research funding from Pfizer, Novartis, Merck, Exelixis, Tracon Pharma, GlaxoSmithKline, Bristol-Myers Squibb, AstraZeneca, Peleton Therapeutics, Roche/Genentech, Celldex, Agensys. C.J. Wu has consulting or advisory role for Neon Therapeutics; patents, royalties, other intellectual property from Neon Therapeutics; and stock or other ownership interests from Neon therapeutics. M.B. Atkins has consulting or advisory role for Roche/Genentech, Pfizer, Novartis, X4 pharma, Genoptix, Bristol-Myers Squibb, Nektar, Merck, Exelixis, Acceleron Pharma, Peloton, Eisai, Celldex, Alexion Pharmaceuticals, AstraZeneca/MedImmune, Gactone Pharma, Agenus, Idera, Argos Therapeutics, Array Biopharma. D.F. McDermott has consulting or advisory role for Roche/Genentech, Pfizer, Novartis, X4 pharma, Bristol-Myers Squibb, Eisai, Array Biopharma, Alexion Pharmaceuticals and research funding from Prometheus. S. Signoretti has consulting or advisory role for AstraZeneca/MedImmune, Merck, AACR, NCI; patents, royalties, other intellectual property from Biogenex Laboratories; and research funding from AstraZeneca, Exelixis, Bristol-Myers Squibb. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother. 2017. May;66(5):551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015. November 05;373(19):1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. January;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 4.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. January 01;47(1):207–14. [DOI] [PubMed] [Google Scholar]
- 5.Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015. November 01;33(31):3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal GM, Theoret MR, Pazdur R. Treatment Beyond Progression With Immune Checkpoint Inhibitors-Known Unknowns. JAMA Oncol. 2017. November 01;3(11):1473–4. [DOI] [PubMed] [Google Scholar]
- 7.Hales RK, Banchereau J, Ribas A, Tarhini AA, Weber JS, Fox BA, et al. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010. October;21(10):1944–51. [DOI] [PubMed] [Google Scholar]
- 8.Elias R, Kapur P, Pedrosa I, Brugarolas J. Renal Cell Carcinoma Pseudoprogression with Clinical Deterioration: To Hospice and Back. Clin Genitourin Cancer. 2018. July 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong AS, Thian YL, Kapur J, Leong CN, Kee P, Lee CT, et al. Pushing the limits of immune-related response: a case of “extreme pseudoprogression”. Cancer Immunol Immunother. 2018. July;67(7):1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Velasco G, Krajewski KM, Albiges L, Awad MM, Bellmunt J, Hodi FS, et al. Radiologic Heterogeneity in Responses to Anti-PD-1/PD-L1 Therapy in Metastatic Renal Cell Carcinoma. Cancer Immunol Res. 2016. January;4(1):12–7. [DOI] [PubMed] [Google Scholar]
- 11.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009. December 01;15(23):7412–20. [DOI] [PubMed] [Google Scholar]
- 12.Bohnsack O, Hoos A, Ludajic K. Adaptation and modification of the immune related response criteria (IRRC): IrRECIST. Journal of Clinical Oncology. 2014. 2014/05/20;32(15_suppl):e22121–e. [Google Scholar]
- 13.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017. March;18(3):e143–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol. 2018. March 20;36(9):850–8. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016. May 01;34(13):1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018. January;88:38–47. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015. May 01;33(13):1430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishino M, Gargano M, Suda M, Ramaiya NH, Hodi FS. Optimizing immune-related tumor response assessment: does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer. 2014;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Janne PA, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015. January 22;372(4):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giraldo NA, Becht E, Pages F, Skliris G, Verkarre V, Vano Y, et al. Orchestration and Prognostic Significance of Immune Checkpoints in the Microenvironment of Primary and Metastatic Renal Cell Cancer. Clin Cancer Res. 2015. July 01;21(13):3031–40. [DOI] [PubMed] [Google Scholar]
- 22.Granier C, Dariane C, Combe P, Verkarre V, Urien S, Badoual C, et al. Tim-3 Expression on Tumor-Infiltrating PD-1+CD8+ T Cells Correlates with Poor Clinical Outcome in Renal Cell Carcinoma. Cancer Res. 2017. March 01;77(5):1075–82. [DOI] [PubMed] [Google Scholar]
- 23.Mason DY, Cordell JL, Gaulard P, Tse AG, Brown MH. Immunohistological detection of human cytotoxic/suppressor T cells using antibodies to a CD8 peptide sequence. J Clin Pathol. 1992. December;45(12):1084–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan J, Zhang Y, Zhang JP, Liang J, Li L, Zheng L. Tim-3 expression defines regulatory T cells in human tumors. PLoS One. 2013;8(3):e58006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carey CD, Gusenleitner D, Lipschitz M, Roemer MGM, Stack EC, Gjini E, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood. 2017. November 30;130(22):2420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001. July 1;61(13):5132–6. [PubMed] [Google Scholar]
- 27.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017. August 1;127(8):2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018. June;24(6):749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015. January 15;160(1–2):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holm S A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- 31.Lopez-Raton M, Rodriguez-Alvarez MX, Cadarso-Suarez C, Gude-Sampedro F. OptimalCutpoints: An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. 2014. 2014 2014-11-13;61(8):36. [Google Scholar]
- 32.Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med. 2016. November 03;375(18):1767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012:656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015. August;15(8):486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008. September 30;105(39):15016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res. 2015. March 01;21(5):1071–7. [DOI] [PubMed] [Google Scholar]
- 37.Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008. August 15;14(16):5150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006. April 01;66(7):3381–5. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nat Rev Immunol. 2015. January;15(1):45–56. [DOI] [PubMed] [Google Scholar]
- 40.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016. May 17;44(5):989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, et al. Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol Res. 2015. October;3(10):1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.