Abstract
Objective
Adrenocortical carcinoma (ACC) is a rare malignancy with poor prognosis. ACC was reported in 3.2% patients with Lynch syndrome (LS), however no particular case-detection strategies have been recommended.
Participants
We report a case of a 65-year-old woman who was incidentally discovered with a large adrenal mass during work-up of postmenopausal uterine bleeding. She was recently diagnosed with MSH6 germline mutation after her sister presented with uterine carcinoma in the setting of LS.
Results
Whereas the patient was asymptomatic for overt hormonal excess, biochemical work-up confirmed glucocorticoid autonomy and androgen and estrogen excess. Urine steroid profiling was suggestive of ACC. Adrenalectomy confirmed an oncocytic ACC with focal extracapsular extension into the periadrenal adipose tissue with a Ki-67 of 15% and a peak mitotic count of 40/50 high-power fields.
Conclusion
ACC can be the only manifestation of LS. A best case-detection approach for ACC in the asymptomatic patient with LS is unclear, however urine steroid profiling could be considered.
Keywords: Lynch syndrome, adrenocortical carcinoma, adrenocorticotropic hormone, steroid profiling, diagnosis
Adrenal cortical carcinoma (ACC) is a rare malignancy with an incidence of one to two per one million individuals per year but represents ∼13% of adrenal tumors >4 cm in referral endocrine centers [1]. ACC occurs most frequently in the fifth to sixth decade of life and demonstrates a female-to-male predominance of 2.5 to 1. Although most ACCs are sporadic, these can also occur as part of an hereditary syndrome, such as Li-Fraumeni syndrome, multiple endocrine neoplasia type 1, Beckwith–Wiedemann syndrome, familial adenopolymatosis, neurofibromatosis type 1, Carney complex, and Lynch syndrome (LS) [2]. Despite the large tumor size on presentation, approximately one-half of patients with ACC is discovered incidentally (42%), with a smaller proportion of patients presenting with hormonal excess (31%) or with symptoms of mass effect (20%) or discovered during cancer-staging imaging for another malignancy (6%) and during evaluation of B symptoms (1%) [3]. Early discovery is key to assure a better prognosis.
LS-hereditary nonpolyposis colorectal cancer is an autosomal-dominant hereditary cancer predisposition syndrome caused by germline pathogenic variants in any of DNA mismatch repair genes [4], including MLH1, MSH2, MSH6, and PMS2 [5]. Patients with LS demonstrate a substantial lifetime risk of developing colorectal (80%) and endometrial cancer (60%) [4, 6]. Germline pathogenic variants in the MSH6 gene account for ∼18% of LS cases [7]. Association of ACC with LS has been reported only in several case reports and one small prospective study, totaling 12 patients (Table 1) [5, 8–14].
Table 1.
Previous Reports of Patients With LS and ACC
| Studies | Age | Sex | MSH Type | Microsatellite Stability | Mode of Discovery | Tumor Size, mm | Adrenal Hormone Excess | Treatment | Mitotic Count | Outcome | Criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [8] | 44 | M | NR | NR | NR | NR | NR | NR | NR | Died of disease | NR |
| [9] | 65 | F | MSH2 | MSS | Cushingoid features | NR | ACTH-independent Cushing | S | 130/50 HPF | Died of disease | Didn’t meet Amsterdam criteria |
| [10] | 34 | M | MSH2 | MSS | Symptoms of hypertension and hypokalemia (possible primary hyperaldosteronism) leading to imaging) | 40 | NR (possible primary hyperaldosteronism) | S | NR | Died of disease | Met Amsterdam criteria II |
| [11] | 60 | F | MSH2 | MSS | Follow-up MRI for breast cancer | 51 | NR | S | NR | Alive | Met Amsterdam criteria |
| [5] | 29 | M | MSH2 | MSS | Flank pain | NR | NR | S | 20/50 HPF | Alive | NR |
| [12] | 52 | M | MSH2 | MSS | Genetic evaluation | NR | NR | NR | NR | Alive | Met Amsterdam criteria I |
| 47 | M | MLH1 | MSS | Genetic evaluation | NR | NR | NR | NR | Alive | Met Amsterdam criteria I | |
| 39 | M | MSH6 | MSS | Genetic evaluation | NR | NR | NR | NR | Alive | NR | |
| 42 | F | MSH2 | MSS | Genetic evaluation | NR | NR | NR | NR | Alive | NR | |
| 23 | F | MSH2 | NR | Genetic evaluation | NR | NR | NR | NR | Alive | NR | |
| [13] | 54 | F | MSH2 | NR | Lion pain, weight loss, and lethargy | 140 | None | S | 1/50 HPF | Alive | Met Amsterdam criteria II |
| [14] | 68 | M | MSH2 | NR | Abdominal pain | 41(Extra adrenal) | S | 2/10 HPF | Alive | Met Amsterdam criteria II | |
| This study | 65 | F | MSH6 | NR | Incidental discovery | 92 | Androgen, estrogen excess | S | 40/50 HPF | Alive | NR |
Abbreviations: ACTH, adrenocorticotropic hormone; F, female; HPF, high-power field; M, male; MSS, microsatellite stable; NR, not reported; S, surgical.
1. Case Report
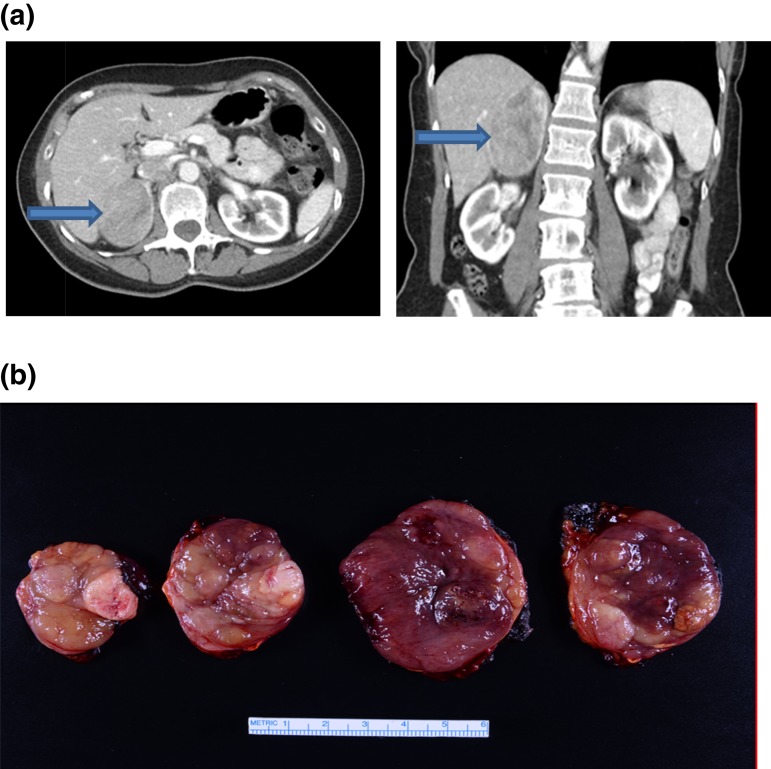
A 65-year-old woman with a past medical history of hypertension presented for evaluation of a recently developed vaginal bleeding. Transvaginal ultrasound demonstrated endometrial thickening and two uterine fibroids. The patient was treated with dilation and curettage of endometrial hyperplasia, and pathology was benign. Incidentally, on the same initial ultrasound, a large heterogeneous mass within the right upper quadrant of the abdomen was also noted. To investigate this finding further, an abdominal CT scan was performed and revealed a heterogeneous right adrenal mass, 6.0 × 5.1 × 7.8 cm (Fig. 1A). The patient was referred to the Mayo Clinic for further evaluation of the right adrenal mass.
Figure 1.
(a, left) Axial CT image and (right) coronal CT image showing a 6.0 × 5.1 × 7.8-cm right adrenal mass (arrows). (b) Gross pathology serial cut sections of a 9.2-cm right ACC.
Notably, as a result of a recent diagnosis of LS in the patient’s sister, our patient was tested positive for familial pathogenic variant in MSH6. A recent colonoscopy was normal.
A. Investigations
During evaluation in the adrenal clinic, the patient was mostly asymptomatic, although she did complain of some fatigue, loss of appetite, and a 3-pound weight loss over the prior 2 weeks (which she thought was a result of anxiety related to the recent diagnosis of the adrenal mass). On physical examination, she did not have Cushingoid features, acne, or hirsutism. Her blood pressure was 135/83 mmHg, and no clinical features suggestive of primary hyperaldosteronism, such edema or hypokalemia, were present. Biochemical work-up was negative for pheochromocytoma but demonstrated evidence of androgen excess, elevated serum steroid precursors, and estrogen excess, which could have explained the patient’s recent uterine bleeding (Table 2). In addition, the patient demonstrated evidence of adrenocorticotropic hormone (ACTH) independent cortisol excess based on abnormal cortisol concentrations after 1 mg overnight dexamethasone administration, along with low ACTH and elevated 24-hour, urine-free cortisol (Table 2). Urine multisteroid profiling was performed and was highly suspicious for ACC (Table 2). Based on the clinical, biochemical, and imaging presentation, ACC was suspected, and adrenalectomy was recommended.
Table 2.
Results of Biochemical Testing Demonstrate Androgen-, Estrogen-, and Corticotrophin-Independent Cortisol Excess
| Laboratory Test | Before Surgery | 1 Mo After Surgery | Reference Range |
|---|---|---|---|
| 24-h Urine | |||
| Urine-free cortisol, μg/24 h | 68 | N/A | 3.5–45 |
| Serum | |||
| ACTH, pg/mL | <5 | N/A | 7.2–63 |
| 8 am Serum cortisol following 1 mg overnight dexamethasone suppression test, μg/dL |
13 | N/A | <1.8 |
| Aldosterone, ng/dL | 20 | N/A | ≤21 |
| Renin plasma activity, ng/mL/h | 2 | N/A | 0.6–3.0 |
| Androstenedione, ng/dL | 151 | N/A | 30–200 |
| DHEA sulfate, μg/dL | 403 | <15 | <15–157 |
| 17-Hydroxyprogesterone, ng/dL | 167 | <40 | <51 |
| 17-Hydroxypregnenolone, ng/dL | 888 | <16 | 31–455 |
| Total testosterone, ng/dL | 30 | <7 | 8–60 |
| Estradiol, pg/mL | 113 | <10 | <10 (Postmenopausal) |
Abbreviations: DHEA, dehydroepiandrosterone; N/A, not available.
B. Treatment
Patient was treated with an open right adrenalectomy. Final pathology demonstrated a 9.2 × 5.9 × 4.8-cm adrenal oncocytic ACC (Fig. 1B) with focal extracapsular extension into periadrenal adipose tissue, a Ki-67 index of 15%, and a peak mitotic count of 40 mitoses in 50 high-powered fields. Surgical margins were negative for tumor. Postoperatively, the patient was treated with glucocorticoid-replacement therapy, and treatment with mitotane was started 6 weeks after surgery.
C. Outcome and Follow-Up
During the subsequent 26-months of follow-up, the patient remains in remission: imaging demonstrates no evidence for local recurrence or metastatic disease. In addition, patient’s serum and urinary steroid biomarkers are within normal ranges.
2. Discussion
We present a rare case of a patient with LS whose only presentation was incidentally discovered ACC. Outside from ACC, she had no other manifestations of LS at the time of this case report.
Only 13 cases (including our case) have been reported so far in the literature (Table 1). The median age of presentation was 47 years (range: 23 to 68), and 46% were women. The first association of ACC with LS was described in the “N family” of two large Midwestern kindreds by H. T. Lynch in 1966 [8]. Proband from the N family died at age 44 from ACC, whereas his siblings presented with multiple primary colon carcinomas, endometrium carcinomas, and other cancers [8]. Later, three other case reports demonstrated an association of ACC with LS in patients with the MSH2 germline mutation [5, 10, 11]. In 2013, a prospective study of 114 patients with ACC demonstrated a 3.2% prevalence of LS, which is higher than in the general population (0.2%) and comparable to the prevalence of LS in patients with colorectal cancer and endometrial cancer (1% to 5%) [12]. In this study, five of 114 patients with ACC and LS had MSH2 (in three patients), MSH6 (one patient), and MLH1 (one patient) germline mutations, with four patients demonstrating microsatellite stability. More recently, two more case reports published in 2016 and 2018 [13, 14] again showed association between LS and ACC with MSH2 germline mutation.
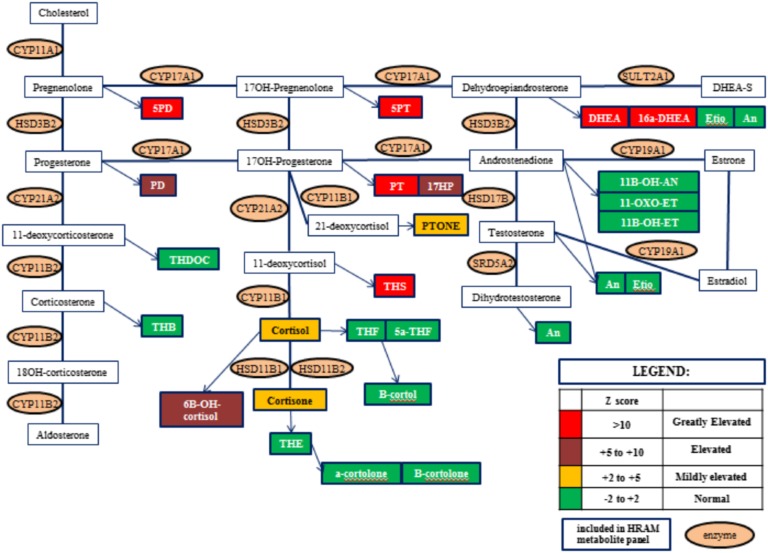
It is challenging to diagnose ACC early in an asymptomatic phase, before substantial growth and metastases occur. The diagnosis of ACC is based on clinical presentation and imaging characteristics of adrenal mass (Hounsfield units >10, size >4 cm, and heterogeneous). In patients with a genetic predisposition of ACC (such as Li-Fraumeni syndrome, Beckwith–Wiedemann syndrome, multiple endocrine neoplasia type 1, familial adenomatous polyposis, neurofibromatosis 1, and LS), the incidence of ACC, although much higher than the general population, is still low to warrant serial imaging. Steroid profiling (Fig. 2) is an attractive alternative that could help diagnose ACC much earlier in the natural history of the disease [15]. In our patient, steroid profiling confirmed our suspicion of ACC after discovery of adrenal mass [16]. Whereas in this case, steroid profiling did not change our management, after appropriate validation, this test could be offered as a case-detection, noninvasive, and radiation-free test to patients at high risk for ACC. Surgical resection is the mainstay of treatment of ACC with a goal to achieve a microscopic tumor clearance (R0) resection. Further management depends on the stage of ACC and prognostic markers derived from pathology examination.
Figure 2.
Urine steroid profiling. HRAM, high resolution, accurate mass.
3. Conclusion
In summary, we present a patient with LS whose only manifestation was ACC, adding to the available literature of only 12 cases. We also demonstrated that steroid profiling could serve as a noninvasive diagnostic and potentially as a case-detection tool for patients at risk for ACC.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACC
adrenocortical carcinoma
- ACTH
adrenocorticotropic hormone
- LS
Lynch syndrome
References and Notes
- 1. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 2. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, Hartman RP, Dean DS, Thomas MA, Shah MZ, Herndon J, McKenzie TJ, Arlt W, Young WF Jr, Bancos I. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919–932. [DOI] [PubMed] [Google Scholar]
- 5. Karamurzin Y, Zeng Z, Stadler ZK, Zhang L, Ouansafi I, Al-Ahmadie HA, Sempoux C, Saltz LB, Soslow RA, O’Reilly EM, Paty PB, Coit DG, Shia J, Klimstra DS. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum Pathol. 2012;43(10):1677–1687. [DOI] [PubMed] [Google Scholar]
- 6. Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, Wang F, Bandipalliam P, Syngal S, Gruber SB. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137(5):1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Houlleberghs H, Goverde A, Lusseveld J, Dekker M, Bruno MJ, Menko FH, Mensenkamp AR, Spaander MCW, Wagner A, Hofstra RMW, Te Riele H. Suspected Lynch syndrome associated MSH6 variants: a functional assay to determine their pathogenicity. PLoS Genet. 2017;13(5):e1006765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lynch HT, Shaw MW, Magnuson CW, Larsen AL, Krush AJ. Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med. 1966;117(2):206–212. [PubMed] [Google Scholar]
- 9. Berends MJ, Cats A, Hollema H, Karrenbeld A, Beentjes JA, Sijmons RH, Mensink RG, Hofstra RM, Verschueren RC, Kleibeuker JH. Adrenocortical adenocarcinoma in an MSH2 carrier: coincidence or causal relation? Hum Pathol. 2000;31(12):1522–1527. [DOI] [PubMed] [Google Scholar]
- 10. Broaddus RR, Lynch PM, Lu KH, Luthra R, Michelson SJ. Unusual tumors associated with the hereditary nonpolyposis colorectal cancer syndrome. Mod Pathol. 2004;17(8):981–989. [DOI] [PubMed] [Google Scholar]
- 11. Medina-Arana V, Delgado L, González L, Bravo A, Díaz H, Salido E, Riverol D, González-Aguilera JJ, Fernández-Peralta AM. Adrenocortical carcinoma, an unusual extracolonic tumor associated with Lynch II syndrome. Fam Cancer. 2011;10(2):265–271. [DOI] [PubMed] [Google Scholar]
- 12. Raymond VM, Everett JN, Furtado LV, Gustafson SL, Jungbluth CR, Gruber SB, Hammer GD, Stoffel EM, Greenson JK, Giordano TJ, Else T. Adrenocortical carcinoma is a Lynch syndrome-associated cancer. J Clin Oncol. 2013;31(24):3012–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Challis BG, Kandasamy N, Powlson AS, Koulouri O, Annamalai AK, Happerfield L, Marker AJ, Arends MJ, Nik-Zainal S, Gurnell M. Familial adrenocortical carcinoma in association with Lynch syndrome. J Clin Endocrinol Metab. 2016;101(6):2269–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wright JP, Montgomery KW, Tierney J, Gilbert J, Solórzano CC, Idrees K. Ectopic, retroperitoneal adrenocortical carcinoma in the setting of Lynch syndrome. Fam Cancer. 2018;17(3):381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bancos I, Arlt W. Diagnosis of a malignant adrenal mass: the role of urinary steroid metabolite profiling. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):200–207. [DOI] [PubMed] [Google Scholar]
- 16. Hines JM, Bancos I, Bancos C, Singh RD, Avula AV, Young WF, Grebe SK, Singh RJ. High-resolution, accurate-mass (HRAM) mass spectrometry urine steroid profiling in the diagnosis of adrenal disorders. Clin Chem. 2017;63(12):1824–1835. [DOI] [PubMed] [Google Scholar]