Abstract
Background
This is an updated version of the original Cochrane review published in Issue 12, 2012. That review considered both fibromyalgia and neuropathic pain, but the effects of amitriptyline for fibromyalgia are now dealt with in a separate review.
Amitriptyline is a tricyclic antidepressant that is widely used to treat chronic neuropathic pain (pain due to nerve damage). It is recommended as a first line treatment in many guidelines. Neuropathic pain can be treated with antidepressant drugs in doses below those at which the drugs act as antidepressants.
Objectives
To assess the analgesic efficacy of amitriptyline for relief of chronic neuropathic pain, and the adverse events associated with its use in clinical trials.
Search methods
We searched CENTRAL, MEDLINE, and EMBASE to March 2015, together with two clinical trial registries, and the reference lists of retrieved papers, previous systematic reviews, and other reviews; we also used our own hand searched database for older studies.
Selection criteria
We included randomised, double‐blind studies of at least four weeks' duration comparing amitriptyline with placebo or another active treatment in chronic neuropathic pain conditions.
Data collection and analysis
We performed analysis using three tiers of evidence. First tier evidence derived from data meeting current best standards and subject to minimal risk of bias (outcome equivalent to substantial pain intensity reduction, intention‐to‐treat analysis without imputation for dropouts; at least 200 participants in the comparison, 8 to 12 weeks' duration, parallel design), second tier from data that failed to meet one or more of these criteria and were considered at some risk of bias but with adequate numbers in the comparison, and third tier from data involving small numbers of participants that were considered very likely to be biased or used outcomes of limited clinical utility, or both.
Main results
We included 15 studies from the earlier review and two new studies (17 studies, 1342 participants) in seven neuropathic pain conditions. Eight cross‐over studies with 302 participants had a median of 36 participants, and nine parallel group studies with 1040 participants had a median of 84 participants. Study quality was modest, though most studies were at high risk of bias due to small size.
There was no first‐tier or second‐tier evidence for amitriptyline in treating any neuropathic pain condition. Only third‐tier evidence was available. For only two of seven studies reporting useful efficacy data was amitriptyline significantly better than placebo (very low quality evidence).
More participants experienced at least one adverse event; 55% of participants taking amitriptyline and 36% taking placebo. The risk ratio (RR) was 1.5 (95% confidence interval (CI) 1.3 to 1.8) and the number needed to treat for an additional harmful outcome was 5.2 (3.6 to 9.1) (low quality evidence). Serious adverse events were rare. Adverse event and all‐cause withdrawals were not different, but were rarely reported (very low quality evidence).
Authors' conclusions
Amitriptyline has been a first‐line treatment for neuropathic pain for many years. The fact that there is no supportive unbiased evidence for a beneficial effect is disappointing, but has to be balanced against decades of successful treatment in many people with neuropathic pain. There is no good evidence of a lack of effect; rather our concern should be of overestimation of treatment effect. Amitriptyline should continue to be used as part of the treatment of neuropathic pain, but only a minority of people will achieve satisfactory pain relief. Limited information suggests that failure with one antidepressant does not mean failure with all.
Plain language summary
Amitriptyline for neuropathic pain in adults
Neuropathic pain is pain coming from damaged nerves, and can have a variety of different names. Some of the more common are painful diabetic neuropathy, postherpetic neuralgia, or post‐stroke pain. It is different from pain messages that are carried along healthy nerves from damaged tissue (for example, a fall, or cut, or arthritic knee). Neuropathic pain is treated by different medicines to those used for pain from damaged tissue. Medicines such as paracetamol or ibuprofen are not usually effective in neuropathic pain, while medicines that are sometimes used to treat depression or epilepsy can be very effective in some people with neuropathic pain.
Amitriptyline is an antidepressant, and antidepressants are widely recommended for treating neuropathic pain. Amitriptyline is commonly used to treat neuropathic pain conditions, but an earlier review found no good quality evidence to support its use. Most studies were small, relatively old, and used methods or reported results that we now recognise as making benefits seem better than they are.
In March 2015 we performed searches to look for new studies in adults with neuropathic pain of at least moderate intensity. We found only two additional small studies that did not provide any good quality evidence for either benefit or harm. This is disappointing, but we can still make useful comments about the drug.
Amitriptyline probably does not work in neuropathic pain associated with human immunodeficiency virus (HIV) or treatments for cancer. Amitriptyline probably does work in other types of neuropathic pain, though we cannot be certain of this. Our best guess is that amitriptyline provides pain relief in about 1 in 4 (25%) more people than does placebo, and about 1 in 4 (25%) more people than placebo report having at least one adverse event, which may be troublesome, but probably not serious. We cannot trust either figure based on the information available.
The most important message is that amitriptyline probably does give really good pain relief to some people with neuropathic pain, but only a minority of them; amitriptyline will not work for most people.
Background
This is an update of an earlier review of amitriptyline for neuropathic pain and fibromyalgia originally published in The Cochrane Library in 2012 (Moore 2012a). The effects of amitriptyline for fibromyalgia are now dealt with in a separate review (Moore 2015).
In the update we have used a template for reviews of drugs used to relieve neuropathic pain. The aim is for all reviews to use the same methods, based on current criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Appendix 1).
Description of the condition
The 2011 International Association for the Study of Pain definition of neuropathic pain is "pain caused by a lesion or disease of the somatosensory system" (Jensen 2011), and based on a definition agreed at an earlier consensus meeting (Treede 2008). Neuropathic pain is cause by injury to the nervous tissue, either peripheral or central and it can be followed by plastic changes in the central nervous system (Moisset 2007). The origin of neuropathic pain is complex (Baron 2010; Baron 2012; Tracey 2011; von Hehn 2012), and neuropathic pain features can be found in people with joint pain (Soni 2013).
Many people with neuropathic pain conditions are significantly disabled with moderate or severe pain for many years. Chronic pain conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, employment, and increased healthcare costs (Moore 2014a).
Neuropathic pain is usually divided according to the cause of nerve injury. There may be many causes, but some common causes of neuropathic pain include diabetes (painful diabetic neuropathy, PDN), shingles (postherpetic neuralgia, PHN), amputation (stump and phantom limb pain), neuropathic pain after surgery or trauma, stroke or spinal cord injury, trigeminal neuralgia (TGN), and human immunodeficiency virus (HIV) infection. Sometimes the cause is not known.
In systematic reviews, the overall prevalence of neuropathic pain in the general population is reported to be between 7% and 10% (van Hecke 2014), and about 7% in a systematic review of studies published since 2000 (Moore 2014a). In individual countries, prevalence rates have been reported as 3.3% in Austria (Gustorff 2008), 6.9% in France (Bouhassira 2008), and up to 8% in the UK (Torrance 2006). Some forms of neuropathic pain, such as PDN and post‐surgical chronic pain (which is often neuropathic in origin), are increasing (Hall 2008). The incidence of PHN may decrease where vaccination programmes are introduced; vaccination for herpes zoster is ongoing in the UK, for example.
Estimates of incidence vary between individual studies for particular origins of neuropathic pain, often because of small numbers of cases. In primary care in the UK, between 2002 and 2005, the incidences (per 100,000 person‐years' observation) were 28 (95% confidence interval (CI) 27 to 30) for PHN, 27 (26 to 29) for TGN, 0.8 (0.6 to 1.1) for phantom limb pain, and 21 (20 to 22) for PDN (Hall 2008). Others have estimated an incidence of 4 in 100,000 per year for trigeminal neuralgia (Katusic 1991; Rappaport 1994), and 12.6 per 100,000 person‐years for TGN and 3.9 per 100,000 person‐years for PHN in a study of facial pain in the Netherlands (Koopman 2009). One systematic review of chronic pain demonstrated that some neuropathic pain conditions, such as PDN, can be more common than other neuropathic pain conditions, with prevalence rates up to 400 per 100,000 person years (McQuay 2007).
Neuropathic pain is difficult to treat effectively, with only a minority of people experiencing a clinically relevant benefit from any one intervention. A multidisciplinary approach is now advocated, combining pharmacological interventions with physical or cognitive (or both) interventions. Conventional analgesics like paracetamol and nonsteroidal anti‐inflammatory drugs are not thought to be effective, but are frequently used (Hall 2013; Vo 2009). Some people may derive some benefit from a topical lidocaine patch or low‐concentration topical capsaicin, though evidence about benefits is uncertain (Derry 2012; Derry 2014). High‐concentration topical capsaicin may benefit some people with PHN (Derry 2013). Treatment is often by so‐called 'unconventional analgesics', such as antidepressants such as amitriptyline or duloxetine (Lunn 2014; Sultan 2008), or antiepileptics (gabapentin or pregabalin; Moore 2009; Moore 2014b; Wiffen 2013a).
The proportion of people who achieve worthwhile pain relief (typically at least 50% pain intensity reduction; Moore 2013a) is small, generally only 10% to 25% more than with placebo, with numbers needed to treat for an additional beneficial outcome (NNT) usually between 4 and 10 (Kalso 2013; Moore 2013b). Neuropathic pain is not particularly different from other chronic pain conditions in that only a small proportion of trial participants have a good response to treatment (Moore 2013b).
One overview of treatment guidelines pointed out some general similarities between recommendations, but guidelines are not always consistent with one another (O'Connor 2009), nor followed (Hall 2013). The current National Institute for Health and Care Excellence (NICE) guidance in the UK suggests offering a choice of amitriptyline, duloxetine, gabapentin or pregabalin as initial treatment for neuropathic pain (with the exception of trigeminal neuralgia), with switching if first, second, or third drugs tried are not effective or not tolerated (NICE 2013). Antidepressant drugs are also suggested as first line agents in the latest Canadian guidelines (Moulin 2014), and in updated guidance from the Neuropathic pain Special Interest Group of the International Association for the Study of Pain (Finnerup 2015).
Description of the intervention
Amitriptyline is a tricyclic antidepressant. It is not licensed in the UK for treating neuropathic pain, but is commonly used for various neuropathic pain conditions around the world, irrespective of licensed indications. The drug is available as tablets (10, 25, and 50 mg) and oral solutions. It is usually given at night time in an attempt to reduce any sedative effects during the day. There were over 11 million prescriptions for amitriptyline in England in 2013, mainly for 10 mg and 25 mg tablets (PCA 2014); some of these prescriptions would be for relief of depression. The main adverse effects are due to its anticholinergic activity, and include dry mouth, weight gain, and drowsiness.
How the intervention might work
The mechanism of action of amitriptyline in the treatment of neuropathic pain remains uncertain, although it is known to inhibit both serotonin and noradrenaline reuptake. The mechanism is likely to differ from that in depression since analgesia with antidepressants is often achieved at lower dosage than the onset of any antidepressant effect; adverse events associated with amitriptyline often wane after two or three weeks, when the benefits of the drug become apparent. In addition, there is no correlation between the effect of antidepressants on mood and pain, and antidepressants produce analgesia in people with and without depression (Onghena 1992).
Why it is important to do this review
Amitriptyline is an established pharmacological intervention for chronic neuropathic pain. The earlier review found some evidence of pain relief with amitriptyline compared with placebo for PDN, mixed neuropathic pain, and fibromyalgia, at the expense of increased adverse events, but this was based on small numbers of participants in studies that were susceptible to bias.
It was decided to split reviews combining neuropathic pain conditions with fibromyalgia into separate reviews, so an update was performed at the same time, to capture any new studies.
Like the earlier Cochrane review, this update assessed evidence in ways that make both statistical and clinical sense, and used developing criteria for what constitutes reliable evidence in chronic pain (Appendix 1; Moore 2010a). It followed standards set out in the PaPaS Author and Referee Guidance for pain studies of the Cochrane Pain, Palliative and Supportive Care Group (PaPaS 2012).
Objectives
To assess the analgesic efficacy of amitriptyline for relief of chronic neuropathic pain, and the adverse events associated with its use in clinical trials.
Methods
Criteria for considering studies for this review
Types of studies
We included studies if they were randomised controlled trials (RCTs) with double‐blind assessment of treatment, and outcomes reported ideally after eight weeks of treatment or longer for the highest level of evidence, but accepted studies lasting four to eight weeks as a lower level. Full journal publication was required, with the exception of extended abstracts of otherwise unpublished clinical trials. We did not include short abstracts (usually meeting reports), studies that were non‐randomised, studies of experimental pain, case reports, or clinical observations. We did not include studies with fewer than 10 participants in any treatment arm, or studies of topical administration.
Types of participants
We included adults aged 18 years and above with initial pain of at least moderate intensity. Participants could have one or more of a wide range of chronic neuropathic pain conditions including (but not limited to):
painful diabetic neuropathy;
postherpetic neuralgia;
trigeminal neuralgia;
phantom limb pain;
postoperative or traumatic neuropathic pain;
complex regional pain syndrome;
cancer‐related neuropathy;
Guillain Barré;
HIV neuropathy;
spinal cord injury.
We included studies of participants with more than one type of neuropathic pain; in such cases, we analysed results according to the primary condition.
Types of interventions
Amitriptyline in any dose, by any route other than topical, administered for the relief of neuropathic pain, and compared to placebo or an active comparator. We did not include studies using amitriptyline to treat pain resulting from the use of other drugs.
Types of outcome measures
Studies needed to report pain assessment as either a primary or secondary outcome.
We anticipated that studies would use a variety of outcome measures, with most using standard subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as:
at least 30% pain relief over baseline (moderate);
at least 50% pain relief over baseline (substantial);
much or very much improved on Patient Global Impression of Change (PGIC) (moderate);
very much improved on PGIC (substantial).
These outcomes were used in the earlier version of this review, but are different from many other earlier reviews, concentrating on dichotomous outcomes where pain responses are not normally distributed.
Primary outcomes
Patient reported pain relief of 30% or greater.
Patient reported pain relief of 50% or greater.
Patient Global Impression of Change (PGIC) much or very much improved.
Patient Global Impression of Change (PGIC) very much improved.
Secondary outcomes
Any pain‐related outcome indicating some improvement.
Withdrawals due to lack of efficacy.
Participants experiencing any adverse event.
Participants experiencing any serious adverse event.
Withdrawals due to adverse events.
Specific adverse events, particularly somnolence and dizziness.
Search methods for identification of studies
Electronic searches
We searched the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) (via The Cochrane Library to Issue 9, 2012 for the original review, and via the Cochrane Register of Studies Online (CRSO) from 2012 to 10 March 2015);
MEDLINE (via Ovid) (from inception to September 2012 for the original review, and from 2012 to 10 March 2015);
EMBASE (via Ovid) (from inception to September 2012 for the original review, and from 2012 to 10 March 2015);
Oxford Pain Relief database (Jadad 1996a) for the original review. This database is no longer being updated.
See Appendix 2 for the CENTRAL search strategy, Appendix 3 for the MEDLINE search strategy, and Appendix 4 for the EMBASE search strategy.
There was no language restriction.
Searching other resources
We reviewed the bibliographies of all identified RCTs and review articles, and searched clinical trial databases (ClinicalTrials.gov (ClinicalTrials.gov) and World Health Organization (WHO) ICTRP (apps.who.int/trialsearch/) to identify additional published or unpublished data. We did not contact investigators or study sponsors.
Data collection and analysis
The intention was to perform separate analyses according to particular neuropathic pain conditions. We performed analyses combining different neuropathic pain conditions for exploratory purposes only.
Selection of studies
We determined eligibility by reading the abstract of each study identified by the search. We eliminated studies that clearly did not satisfy inclusion criteria and obtained full copies of the remaining studies. Two review authors read these studies independently and reached agreement by discussion. We did not anonymise the studies before assessment.
Data extraction and management
Two review authors independently extracted data using a standard form and checked for agreement before entry into Review Manager 5 (RevMan 2014), or any other analysis method. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design (parallel‐group or cross‐over, placebo or active control, titration schedule), study duration and follow‐up, analgesic outcome measures and results, withdrawals and adverse events (participants experiencing any adverse event, or serious adverse event).
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum (Jadad 1996b).
Two review authors independently assessed the risk of bias for each study, using the criteria outlined in the 'Risk of bias' tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group. We resolved any disagreements by discussion. We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process such as random number table or computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (less than 10% of participants did not complete the study or used 'baseline observation carried forward' (BOCF) analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); high risk of bias (used 'completer' analysis).
Size (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably because the conduct of small studies is more likely to be less rigorous, allowing critical criteria to be compromised (Dechartres 2013; Kjaergard 2001; Nüesch 2010). Studies were considered to be at low risk of bias if they had 200 participants or more, at unclear risk if they had 50 to 200 participants, and at high risk if they had fewer than 50 participants.
Measures of treatment effect
We pooled dichotomous data to calculate risk ratio (RR) with 95% confidence intervals (CI) using a fixed‐effect model unless we found significant statistical heterogeneity (see Assessment of heterogeneity), and calculated NNTs as the reciprocal of the absolute risk reduction (ARR) (McQuay 1998). For unwanted effects, the NNT becomes the number needed to treat to harm (NNH) and is calculated in the same manner. We did not use continuous data in analyses.
Unit of analysis issues
The unit of analysis was the individual participant. For cross‐over studies we planned to use the first period data only, or any useable results if first period data were not available.
Dealing with missing data
We used intention‐to‐treat (ITT) analysis where the ITT population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. We assigned missing participants zero improvement wherever possible.
Assessment of heterogeneity
We dealt with clinical heterogeneity by combining studies that examined similar conditions. We assessed statistical heterogeneity visually (L'Abbé 1987), and with the use of the I2 statistic. When the I2 statistic was greater than 50%, we considered the reasons for this.
Assessment of reporting biases
The aim of this review is to use dichotomous data of known utility (Moore 2010b; Moore 2013a). The review did not depend on what authors of the original studies chose to report or not, though clearly difficulties arose with studies failing to report any dichotomous results. We extracted and used continuous data, which probably poorly reflect efficacy and utility, if useful for illustrative purposes only.
We undertook no assessment of publication bias due to the quality of the data identified, although we had planned to use a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNT of 10 or higher) (Moore 2008).
Data synthesis
We undertook meta‐analysis using a fixed‐effect model. A random‐effects model for meta‐analysis would have been used if there was significant clinical heterogeneity and it was considered appropriate to combine studies.
We assessed data for each painful condition in three tiers, according to outcome and freedom from known sources of bias.
The first tier used data meeting current best standards, where studies reported the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of LOCF or other imputation method other than BOCF for dropouts, reported an ITT analysis, lasted eight or more weeks, had a parallel‐group design, and had at least 200 participants (preferably at least 400) in the comparison (Moore 2010a; Moore 2012b). We planned to report these first‐tier results first.
The second tier used data from at least 200 participants, but where one or more of the above conditions was not met (reporting at least 30% pain intensity reduction, using LOCF or a completer analysis, or lasting four to eight weeks).
The third tier of evidence used data from fewer than 200 participants, or where there were expected to be significant problems because, for example, of very short duration studies of less than four weeks, where there was major heterogeneity between studies, or where there were shortcomings in allocation concealment, attrition, or incomplete outcome data. For this third tier of evidence, no data synthesis is reasonable, and may be misleading, but an indication of beneficial effects might be possible.
Subgroup analysis and investigation of heterogeneity
We planned all analyses to be according to individual painful conditions, because placebo response rates with the same outcome can vary between conditions, as can the drug‐specific effects (Moore 2009). We did not plan subgroup analyses since experience of previous reviews indicated that there would be too few data for any meaningful subgroup analysis.
Sensitivity analysis
We planned no sensitivity analyses because the evidence base was known to be too small to allow reliable analysis. We did examine details of dose escalation schedules in the unlikely situation that this could provide some basis for a sensitivity analysis.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, and Characteristics of studies awaiting classification.
Results of the search
New searches from January 2012 to 10 March 2015 identified 32 potentially relevant studies in CENTRAL, 100 in MEDLINE, and 261 in EMBASE. Of these, four were obtained and read in full to determine inclusion status.
One study still awaits classification because of translation requirements. Keskinbora 2006 is a Turkish report comparing gabapentin and amitriptyline in 46 participants with peripheral neuropathic pain.
Included studies
In this update we included two new studies (203 participants; Boyle 2012; Mishra 2012) and 15 studies from the previous review that fulfilled the inclusion criteria (Figure 1). Studies reporting on efficacy or safety of amitriptyline were carried out in painful diabetic neuropathy (five studies, 654 participants; Anon 2000; Biesbroeck 1995; Boyle 2012; Jose 2007; Max 1992), postherpetic neuralgia (five studies, 227 participants; Graff‐Radford 2000; Max 1988; Rowbotham 2005; Watson 1992; Watson 1998); spinal cord injury (two studies, 122 participants; Cardenas 2002; Rintala 2007), cancer‐related pain (two studies, 162 participants; Kautio 2008; Mishra 2012), and one study each in mixed neuropathic pain (Vrethem 1997), HIV neuropathy (Shlay 1998), and post‐stroke pain (Leijon 1989), with 177 participants in these three studies.
1.
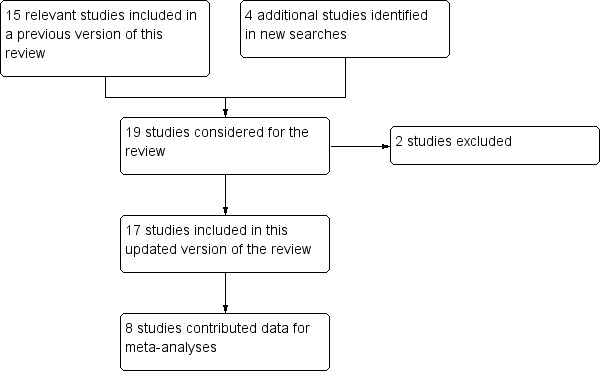
Flow diagram.
The total number of participants in these studies was 1342. Eight studies had a cross‐over design (Jose 2007; Leijon 1989; Max 1992; Max 1988; Rintala 2007; Vrethem 1997; Watson 1992; Watson 1998) and included 302 participants (mean 38, median 36). In these studies we estimated that 262 participants were exposed to amitriptyline, 74 to placebo, and 259 to an active comparator of some description. These studies were not always clear about the number of participants completing each cross‐over and providing data. Nine parallel‐groups studies included 1040 participants (mean 116, median 84). In these studies 425 participants were exposed to amitriptyline, 313 to placebo, and 310 to an active comparator. Overall, the estimates of exposure were 687 to amitriptyline, 387 to placebo, and 560 to active treatments.
The included studies individually involved between 15 and 254 participants; the median study size was 50 participants. Only four studies involved over 100 participants (Anon 2000; Biesbroeck 1995; Mishra 2012; Shlay 1998), and only one more than 100 participants in each treatment arm (Biesbroeck 1995). The median study duration was six weeks; six studies had a shorter duration (Boyle 2012; Leijon 1989; Mishra 2012; Vrethem 1997; Watson 1992; Watson 1998), while one study had a duration of 14 weeks (Shlay 1998).
Excluded studies
We excluded two new studies for this update, making a total of 27 excluded studies (Achar 2010; Banerjee 2013; Bansal 2009; Bowsher 1997; Carasso 1979; Hampf 1989; Kalso 1996; Kaur 2011; Kautio 2009; Kieburtz 1998; Lampl 2002; Max 1987; McQuay 1992; McQuay 1993; Mendel 1986; Mercadante 2002; Morello 1999; Pilowsky 1982; Pilowsky 1990; Robinson 2004; Sharav 1987; Turkington 1980; Vanelderen 2015; Ventafridda 1987; Watson 1982; Wilder‐Smith 2005; Zitman 1990). Reasons for exclusion of studies were: not being convincingly double‐blind, not demonstrating that participants had initial pain of at least moderate intensity, lasting less than four weeks, having fewer than 10 participants in a treatment arm, not having a clear diagnosis of the painful condition, preventative treatments, having a high dropout rate, or not reporting any pain data. Details are in the Characteristics of excluded studies table.
Risk of bias in included studies
Risk of bias is shown in Figure 2 as a summary and in Figure 3 for each included study.
2.
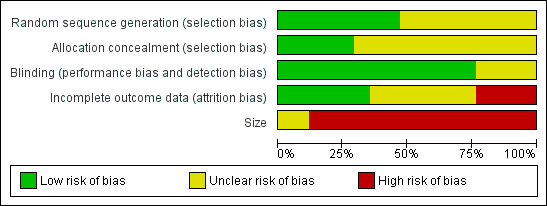
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
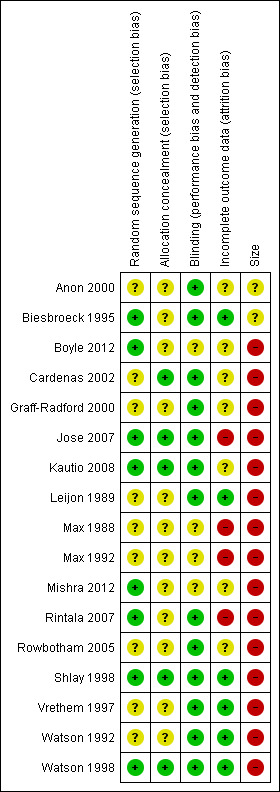
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Quality scores were good using the Oxford Quality Score; four studies scored 3/5 points, 10 scored 4/5, and three scored 5/5.
Allocation
All studies were randomised, but only eight adequately described the method used to generate the random sequence, and only five adequately described how the allocation of the sequence was concealed.
Blinding
All studies were double‐blind, and 13 adequately described the method used to maintain the blinding.
Incomplete outcome data
Eight studies had a cross‐over design. Four cross‐over studies posed difficulties because data on all randomised participants were not available (Jose 2007; Max 1988; Max 1992; Rintala 2007). They tended to report on completers of all cross‐over phases. In only six studies was reporting of a high standard.
Selective reporting
The outcomes specified in the methods of most of these studies were not those sought for the review, so selective reporting bias was not an issue.
Other potential sources of bias
None of the studies included over 200 participants per treatment arm, and only two included 50 to 200 participants (Anon 2000; Biesbroeck 1995). We judged the remaining studies, all with fewer than 50 participants per treatment arm, to be at high risk of bias for this item.
Effects of interventions
Results from individual studies are in Appendix 5 (efficacy) and Appendix 6 (adverse events and withdrawals).
Efficacy
No study in any neuropathic pain condition met the criteria for first‐ or second‐tier evidence.
Painful diabetic neuropathy
Five studies evaluated amitriptyline in PDN (Anon 2000; Boyle 2012; Biesbroeck 1995; Jose 2007; Max 1992). Two were of six weeks' duration and were small cross‐over studies (Jose 2007; Max 1992), while the duration of treatment in the remaining studies was four weeks (Boyle 2012), eight weeks (Biesbroeck 1995), and nine weeks (Anon 2000). All five were active controlled studies comparing amitriptyline (10 to 150 mg daily) with pregabalin (Anon 2000), topical capsaicin (Biesbroeck 1995), duloxetine or pregabalin (Boyle 2012), lamotrigine (Jose 2007), or desipramine or fluoxetine (Max 1992); the Max 1992 study also used a placebo control in its design. The estimate of exposure to interventions was 314 for amitriptyline, 110 for placebo, and 334 for other interventions.
Two studies provided dichotomous efficacy outcomes (Anon 2000; Max 1992).
Third‐tier evidence
None of these studies found any difference between amitriptyline and other active interventions, based mainly on group mean data. Only one small completer analysis from a multiple cross‐over design offers some support for oral amitriptyline being any better than placebo (Figure 4).
4.
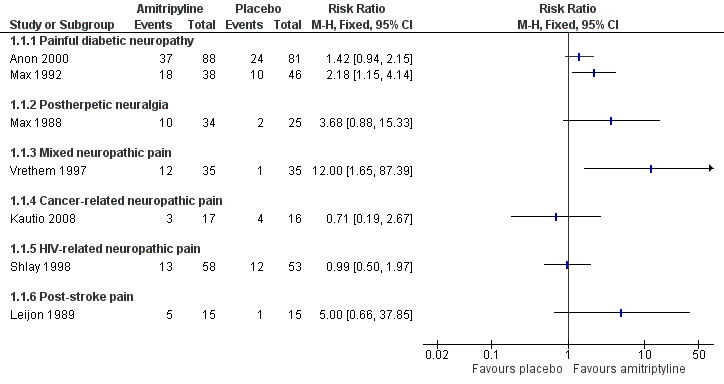
Forest plot of comparison: 1 Amitriptyline versus placebo, outcome: 1.1 Third‐tier efficacy.
Postherpetic neuralgia
Five studies evaluated amitriptyline in PHN; none involved more than 62 participants. Two were of five weeks' duration (Watson 1992; Watson 1998), two of six weeks' duration (Max 1988; Rowbotham 2005), and one of eight weeks' duration (Graff‐Radford 2000). Three were cross‐over studies (Max 1988; Watson 1992; Watson 1998). All studies were active controlled, comparing amitriptyline (25 to 200 mg daily) with fluphenazine and amitriptyline plus fluphenazine (Graff‐Radford 2000), lorazepam (Max 1988), desipramine and fluoxetine (Rowbotham 2005), maprotiline (Watson 1992), and nortriptyline (Watson 1998). Two studies also included a placebo treatment arm (Graff‐Radford 2000; Max 1988). The estimate of exposure to interventions was 227 for amitriptyline, 53 to placebo, and 148 to other interventions.
One study reported no dichotomous outcomes (Graff‐Radford 2000).
Third‐tier evidence
There was no convincing evidence that amitriptyline at various daily doses was better than nortriptyline, maprotiline, desipramine, or fluoxetine. Two studies pointed to amitriptyline being better than placebo (Graff‐Radford 2000; Max 1988), but based on only 84 participants in the comparison. Amitriptyline was possibly better than lorazepam (Max 1988), but not desipramine (Rowbotham 2005), maprotiline (Watson 1992), or nortriptyline (Watson 1998).
Spinal cord injury
Two studies evaluated amitriptyline in spinal cord injury (Cardenas 2002; Rintala 2007); neither involved more than 84 participants. One was of six weeks' duration (Cardenas 2002), and the other had a cross‐over design with nine‐week treatment periods (Rintala 2007). Both were placebo comparisons and one also involved gabapentin as an active comparator (Rintala 2007). The estimate of exposure to interventions was 72 for amitriptyline (10 to 150 mg daily), 65 to placebo, and 26 to other interventions.
Third‐tier evidence
The larger parallel‐group study showed no difference between amitriptyline and placebo in a statistical analysis (Cardenas 2002), but there was some suggestion that amitriptyline may have been somewhat better than placebo in a probable completer analysis in the other study (Rintala 2007).
Mixed neuropathic pain
One four‐week cross‐over study involving 35 participants compared amitriptyline (75 mg daily) with maprotiline and placebo in mixed neuropathic pain (Vrethem 1997).
Third‐tier evidence
There was no convincing evidence that amitriptyline was better than placebo or maprotiline. This small study indicated that with amitriptyline about a third of participants were pain‐free or much improved, and more than with placebo.
Cancer‐related neuropathic pain
Two studies evaluated amitriptyline (10 mg to 100 mg daily) in cancer‐related neuropathic pain. One was of eight weeks' duration and placebo‐controlled (33 participants; Kautio 2008), and the other of four weeks' duration, comparing amitriptyline with gabapentin, pregabalin, and placebo (120 participants; Mishra 2012).
One study reported no dichotomous outcomes (Mishra 2012).
Third‐tier evidence
There was no convincing evidence that amitriptyline at 10 to 50 mg daily was better than placebo. The small study showed no difference between amitriptyline and placebo. Amitriptyline, gabapentin, and pregabalin all appeared to show a morphine‐sparing effect in the larger study, where mean pain intensity scores decreased in all treatment groups over the duration of the study.
Painful HIV‐related neuropathy
One 14‐week study reporting on 136 participants compared amitriptyline with placebo in painful HIV‐related neuropathy (Shlay 1998).
Third‐tier evidence
There was no convincing evidence that amitriptyline at 25 to 75 mg daily was better than placebo. This study showed no difference between amitriptyline and placebo.
Post‐stroke pain
One four‐week cross‐over study involving 15 participants compared amitriptyline with carbamazepine and placebo in post‐stroke pain (Leijon 1989).
Third‐tier evidence
There was no convincing evidence that amitriptyline at 25 to 75 mg daily was better than placebo. This small study indicated that with amitriptyline about a third of participants were pain‐free or much improved, and more than with placebo.
Adverse events
Participants experiencing at least one adverse event
This outcome was reported by six studies with placebo treatment arms, with 519 participants in the comparison (Anon 2000; Cardenas 2002; Kautio 2008; Leijon 1989; Shlay 1998; Vrethem 1997). At least one adverse event was experienced by 148/269 (55%) of participants taking amitriptyline, and 89/250 (36%) taking placebo. The RR was 1.5 (1.3 to 1.8) (Analysis 1.2), and the NNH was 5.2 (3.6 to 9.1).
1.2. Analysis.
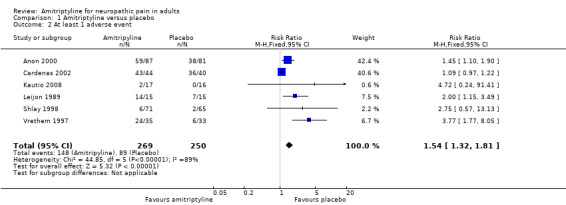
Comparison 1 Amitriptyline versus placebo, Outcome 2 At least 1 adverse event.
Serious adverse events
Three studies reported serious adverse events (Anon 2000; Boyle 2012; Vrethem 1997). Six serious adverse events (including one death) occurred in 83 participants treated with amitriptyline, duloxetine, or pregabalin in Boyle 2012, but the results for individual treatment arms were not reported. In the remaining studies there were 8/122 (6.6%) events with amitriptyline and 2/114 (1.8%) with placebo.
Withdrawals
Two studies reported all‐cause withdrawals (Anon 2000; Cardenas 2002); 31/131 (24%) withdrew for any cause with amitriptyline and 22/121 (18%) with placebo. The RR was 1.3 (0.8 to 2.1); the NNH was not calculated (Analysis 1.3).
1.3. Analysis.
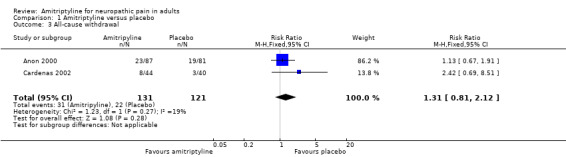
Comparison 1 Amitriptyline versus placebo, Outcome 3 All‐cause withdrawal.
Adverse event withdrawals were reported by three studies with placebo treatment arms (Anon 2000; Max 1988; Rintala 2007). Overall, 25/159 (16%) withdrew because of adverse events with amitriptyline and 10/144 (7%) with placebo. The RR was 2.2 (1.1 to 4.5); the NNH was 11 (6.3 to 57) (Analysis 1.4).
1.4. Analysis.
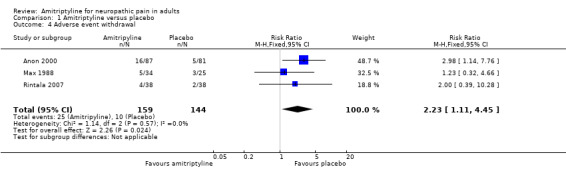
Comparison 1 Amitriptyline versus placebo, Outcome 4 Adverse event withdrawal.
In one active‐controlled study 1/28 participants withdrew due to adverse events with amitriptyline, 3/28 with duloxetine, and 6/27 with pregabalin (Boyle 2012).
One study reported lack of efficacy withdrawals (Anon 2000); 3/87 withdrew because of lack of efficacy with amitriptyline and 9/81 with placebo.
Discussion
Because amitriptyline is a crucially important drug in treating neuropathic pain, and because experience from previous reviews was that most studies would be older, small, and have methodological deficiencies according to present standards of evidence, we felt it appropriate to accept lower standards than those currently demanded for part of our analyses. It is important to recognise that the lower‐level evidence is likely to be subject to various positive biases, and that these lower levels of evidence cannot be used to make cross‐drug comparisons of efficacy with other drugs.
The most important finding of this review was that there were no studies that met current standards of evidence for chronic pain that minimise all known biases (Moore 2010a; Moore 2012b). All the studies accepted for third‐tier evidence contained features of design, conduct, or reporting that are known to be associated with bias in favour of the active treatment. Particular problems were reporting of outcomes of less than 50% pain intensity reduction, or undefined 'improvement', having relatively short duration (although we excluded studies lasting less than four weeks), and studies being small, in circumstances where small studies in chronic pain are known to be associated with over‐estimation of treatment effect (Dechartres 2013; Nüesch 2010), beyond the large random variation that occurs with small pain studies (Moore 1998). That means that the third‐tier efficacy results reported here offer only the best judgement possible on evidence that is not wholly trustworthy.
Summary of main results
There is limited evidence based on small numbers of small studies that amitriptyline may have some benefit in neuropathic pain, with the exception of cancer‐related and HIV‐related neuropathic pain. These latter two conditions are notoriously difficult to treat, with growing evidence that most drugs fail in these conditions. Combining the classic neuropathic pain conditions of painful diabetic neuropathy (PDN), postherpetic neuralgia (PHN), and mixed neuropathic pain for third‐tier evidence gave, in four studies and 382 participants, a statistically significant benefit for amitriptyline compared with placebo (RR 2.0 (1.5 to 2.8)), with an NNT of 5.1 (3.5 to 9.3). Given the caveats above, this is probably an overestimation of treatment effect, but the magnitude and consistency of effect within these studies does provide some confidence that amitriptyline benefits are real, at least for some people.
There are, however, problems with an assumption than amitriptyline is effective. For example, several studies could not differentiate between the efficacy found with amitriptyline and some other drugs, two of which, lamotrigine (Wiffen 2013b) and low dose topical capsaicin (Derry 2012), have evidence of little benefit in neuropathic pain.
Overall completeness and applicability of evidence
The included studies had deficiencies because the design or reporting included features known to be associated with potential bias towards the active treatment over placebo. For example, almost half the studies had a cross‐over design, most were small, some had a relatively short duration, and few had both a placebo group and reported outcomes based on individual participants obtaining a high degree of pain relief. For most specific painful conditions there was only a single small study.
This limits considerably the applicability of the evidence. Although amitriptyline is widely used as the mainstay of treatment of neuropathic pain, there is no unbiased evidence on which to base clinical practice beyond extensive clinical experience, and no evidence for comparison with other potential treatments of neuropathic pain.
There are also significant limits in what the review can say about appropriate doses of amitriptyline. Most studies used dose titration and the range of doses was 10 mg to 150 mg daily.
Quality of the evidence
All studies had to be randomised and double‐blind to be included, and all had to have participants with at least moderate pain relief to ensure that studies were sensitive. No single study fulfilled all the qualities of reliability now used in chronic pain.
Potential biases in the review process
We used an extensive search strategy to identify both published and unpublished studies, based on previous Cochrane reviews and on other reviews with different strategies, and fundamental to all of these was a comprehensive manual journal search for early studies (Jadad 1996a). It is unlikely that relevant high‐quality large studies of amitriptyline in neuropathic pain have been overlooked, especially because amitriptyline is the mainstay of treatment. One unpublished study was consistent with published data (Anon 2000).
Agreements and disagreements with other studies or reviews
Most previous systematic reviews have tended to examine all antidepressants or tricyclic antidepressants as a class of drugs (Attal 2010; Collins 2000; Finnerup 2005; Hempenstall 2005; McQuay 1996; Moulin 2007; Saarto 2007; Wong 2007), mainly because there are few studies with any single antidepressant drug in any single neuropathic pain condition before the advent of duloxetine (Lunn 2014). None of these reviews has considered the additional sources of potential bias revealed in the recent past, and have occasionally concluded that the evidence for antidepressants or tricyclic antidepressant drugs is of high quality, including European guidelines (Attal 2010). It is notable how many authors have been prepared to produce firm guidelines based on tiny amounts of trial data with known evidence problems (Wong 2007). Other reviews have downgraded the quality of evidence regarding amitriptyline (Bril 2011). A more recent review considered all tricyclic antidepressant drugs together, in a pooled analysis of all neuropathic pain conditions (Finnerup 2015). For amitriptyline there was very wide variation in reported NNTs in each trial, ranging between about 2 to 50.
Our earlier review, and this update, are considerably more critical of the quality and quantity of useful data for amitriptyline for treating neuropathic pain, and are part of a series of reviews examining individual drugs rather than combining all together. This is appropriate because there is no good evidence that failure with one molecule will preclude success with another. For example a comparison of amitriptyline with nortriptyline in a cross‐over study in postherpetic neuralgia found that out of 31 participants five had mild or no pain with amitriptyline but moderate to severe pain with nortriptyline, while four had good pain relief with nortriptyline but none with amitriptyline (Watson 1998). This small sample suggests that up to 30% of patients may react differently even to closely related drugs.
The third‐tier estimates of efficacy for amitriptyline in neuropathic pain are of the same order as found for duloxetine in painful diabetic neuropathy (Lunn 2014). Duloxetine studies had many more participants that were parallel‐group, lasting about three months, and better controlled. While the published studies used LOCF imputation, additional analyses explored the use of clinically more relevant BOCF, with outcomes like at least 50% pain relief; these analyses resulted in a small though generally not statistically significant increase (worsening) of NNT (Moore 2014c).
Authors' conclusions
Implications for practice.
For people with chronic neuropathic pain
Amitriptyline has been a first‐line treatment for neuropathic pain for many years. The fact is that there is no supportive unbiased evidence for substantial pain relief has to be balanced against decades of successful treatment in many tens of thousands of people with neuropathic pain. There is no reliable evidence of a lack of effect: rather our concern should be of overestimation of treatment effect.
For clinicians
Amitriptyline should continue to be used as part of the treatment of neuropathic pain, but we should be cognisant of the fact that only a small number of people will achieve satisfactory pain relief.
For policy makers
Amitriptyline should continue to be used as part of the treatment of neuropathic pain, but a range of drugs will be needed to provide good pain relief for a population of people with neuropathic pain.
For funders
Amitriptyline should continue to be used as part of the treatment of neuropathic pain, but a range of drugs will be needed to provide good pain relief for a population of people with neuropathic pain.
Implications for research.
General
There is no convincing evidence about effectiveness of the most commonly used first line therapy for neuropathic pain.
It is unlikely that any large randomised trials of amitriptyline will be conducted in specific neuropathic pain conditions to prove efficacy. Such trials are expensive. The bigger implication is for research in clinical practice, to determine whether there is a sequence of using drugs that will provide overall better clinical effectiveness (Moore 2010c). Another area for research, though extremely difficult, is to identify characteristics that predict which patients are likely to benefit from amitriptyline.
Design
This review highlights the design weaknesses of trials in neuropathic pain. It is notable that probably the only treatment in neuropathic pain that reaches first tier level of evidence is duloxetine in painful diabetic neuropathy, and then because of a post‐hoc individual patient level analysis to change last observation carried forward (LOCF) to baseline observation carried forward (BOCF), and use a common defined outcome (Moore 2014c).
Measurement (endpoints)
There are no lessons here about endpoints. We know that individuals with high levels of pain relief obtain benefit in a range of other areas, like sleep, depression, quality of life, and function.
Comparison between active treatments
A comparison between active treatments is not possible given the present state of knowledge, with generally inadequate trials and reporting.
What's new
| Date | Event | Description |
|---|---|---|
| 28 May 2019 | Amended | Contact details updated. |
| 11 October 2017 | Review declared as stable | No new studies likely to change the conclusions are expected. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 12, 2012
| Date | Event | Description |
|---|---|---|
| 4 April 2019 | Amended | Minor typo corrected in Summary of main results. |
| 3 April 2019 | Amended | Minor typo corrected in Summary of main results. |
| 7 July 2015 | Review declared as stable | This review will be assessed for updating in 2018. |
| 20 March 2015 | New citation required but conclusions have not changed | Previous review split into two new reviews, dealing separately with neuropathic pain and fibromyalgia. Title changed from Amitriptyline for neuropathic pain and fibromyalgia in adults to Amitriptyline for neuropathic pain in adults New studies did not provide data that changed conclusions |
| 10 March 2015 | New search has been performed | New searches run and two new studies (Boyle 2012; Mishra 2012, 203 participants) identified. One small unpublished study awaiting translation and classification |
| 24 September 2010 | Amended | Contact details updated. |
Acknowledgements
Support for this review came from the Oxford Pain Relief Trust.
The protocol for this review was written with funding support from the National Health Service (NHS) Cochrane Collaboration Programme Grant Scheme (UK) and European Union Biomed 2 Grant no. BMH4 CT95 0172 (UK). We are grateful to the peer reviewers for some very useful comments relating to that protocol, and the earlier review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several recent changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. To summarise some of the recent insights that must be considered in this new review:
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011a; Moore 2011b), back pain (Moore 2010c), and arthritis (Moore 2010b), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no‐one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
As a consequence, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials of less than 12 weeks duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010b); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010b; Moore 2013b; Moore 2014b; Straube 2010; Sultan 2008). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so.
Individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a significant way (Hoffman 2010; Moore 2010d; Moore 2014a).
Imputation methods such as last observation carried forward (LOCF), used when participants withdraw from clinical trials, can overstate drug efficacy especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012bMoore 2012b).
Appendix 2. CENTRAL search strategy (via CRSO)
MESH DESCRIPTOR amitriptyline EXPLODE ALL TREES (1002)
(am?tr?pt?lin* or amitriptyliini):TI,AB,KY (2074)
1 OR 2 (2074)
MESH DESCRIPTOR Pain explode all trees (30033)
MESH DESCRIPTOR Peripheral Nervous System Diseases explode all trees (2565)
MESH DESCRIPTOR Somatosensory Disorders explode all trees (703)
MESH DESCRIPTOR Neuralgia EXPLODE ALL TREES (605)
((pain* or discomfort*) and (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)):TI,AB,KY (9635)
((neur* or nerv*) and (compress* or damag*)):TI,AB,KY (1930)
4 OR 5 OR 6 OR 7 OR 8 OR 9 (38890)
3 AND 10 (207)
2012 TO 2015:YR (115373)
11 AND 12 (32)
Appendix 3. MEDLINE (via Ovid) search strategy
Amitriptyline/ (6028)
(am?tr?pt?lin* or amitriptyliini).mp. (8111)
1 or 2 (8111)
exp PAIN/ (314208)
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (118087)
exp SOMATOSENSORY DISORDERS/ (16640)
exp NEURALGIA/ (13991)
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (39812)
((neur* or nerv*) adj6 (compress* or damag*)).mp. (49057)
4 or 5 or 6 or 7 or 8 or 9 (461007)
randomized controlled trial.pt. (386549)
controlled clinical trial.pt. (88799)
randomized.ab. (284481)
placebo.ab. (149366)
drug therapy.fs. (1745898)
randomly.ab. (201462)
trial.ab. (293536)
groups.ab. (1288153)
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (3290048)
3 and 10 and 19 (739)
limit 20 to yr="2012 ‐Current" (100)
Appendix 4. EMBASE (via Ovid) search strategy
Amitriptyline/ (34109)
(am?tr?pt?lin* or amitriptyliini).mp. (34901)
1 or 2 (34901)
exp PAIN/ (876555)
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (52348)
exp SOMATOSENSORY DISORDERS/ (67274)
exp NEURALGIA/ (76377)
(pain* or discomfort*) adj10 (central or complex or nerv* or neuralg* or neuropath*)).mp. (84841)
((neur* or nerv*) adj6 (compress* or damag*)).mp. (71386)
4 or 5 or 6 or 7 or 8 or 9 (1012171)
crossover‐procedure/ (41667)
double‐blind procedure/ (120544)
randomized controlled trial/ randomized controlled trial/ (363694)
(random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or (doubl* adj blind*) or assign* or allocat*).tw. (1288420)
11 or 12 or 13 or 14 (1367733)
3 and 10 and 15 (1576)
limit 16 to yr="2012 ‐Current" (261)
Appendix 5. Summary of outcomes in individual studies: efficacy
| Study |
Treatment (taken at night, unless stated) |
Pain outcome | Other efficacy outcome |
| Anon 2000 | Amitriptyline 75 mg/d = 87 Pregabalin 600 mg/d = 86 Placebo = 81 Treatment taken in divided doses, 3 times daily Titration over first 2 weeks |
Participants with ≥ 50% reduction of pain from baseline Amitriptyline = 40/87 Pregabalin = 34/86 Placebo = 24/81 |
|
| Biesbroeck 1995 | Amitriptyline 25 to 125 mg/d = 117
Capsaicin cream 0.075% = 118 Placebos contained mimicking agents Titration of A over first 4 weeks |
Both treatments produced substantial pain relief ‐ statistically significant from baseline, but no difference between groups Only physician global reported |
Both treatments improved interference with daily activities due to pain, with no difference between groups |
| Boyle 2012 | Amitriptyline 25 mg twice daily, to 25 mg am and 50 mg night = 28 Duloxetine 60 mg am to 60 mg twice daily = 28 Pregabalin 150 mg twice daily to 300 mg twice daily = 27 | No difference between groups in mean pain intensity | |
| Cardenas 2002 | Amitriptyline 10 to 125 mg/d = 44
Placebo = 40
Placebo contained 0.5 mg/d benztropine to mimic dry mouth Titration Week 1 ‐ 10 mg/d Week 2 ‐ 25 mg/d Increased by 25 mg/d each week to max 125 mg/d determined by complete pain relief or max tolerated dose Median max dose = 50 mg/d |
Mean data only No significant difference between groups for any measures except satisfaction with life (favours placebo) | |
| Graff‐Radford 2000 | Amitriptyline 12.5 to 200 mg/d = 12
Fluphenazine 1 to 3 mg/d = 12
Amitriptyline + Fluphenazine = 13
Placebo = 13
Placebo contained glycopyrrolate to mimic dry mouth and constipation
Titration
Amitriptyline by 25 mg each week to max tolerated dose or 200 mg/d
Fluphenazine by 1 mg each week to max 3 mg/d Cross‐over |
Significant decrease in mean pain (using VAS) for amitriptyline and amitriptyline + fluphenazine, but not fluphenazine alone or placebo Amitriptyline + fluphenazine not better than amitriptyline alone | |
| Jose 2007 | Amitriptyline 10 to 50 mg/d = 53
Lamotrigine 50 to 200 mg/d (divided dose) = 46
Titration after 2 weeks if response and tolerated
Amitriptyline ‐ 10, 25, 50 mg
Lamotrigine ‐ 50, 100, 200 mg Cross‐over |
PGIC 50% improvement (efficacy and safety, 100 mm VAS) Amitriptyline = 13/46 Lamotrigine = 19/46 PGIC improvement 25% to 50% Amitriptyline = 5/46 Lamotrigine = 6/46 Majority of patients remained above 30 mm at end (IQR amitriptyline = 40 to 70, lamotrigine = 30 to 70) | No significant difference between groups using median Likert pain and McGill pain Improvements seen from 2nd week onwards |
| Kautio 2008 | Amitriptyline 10 to 50 mg/d = 20 Placebo = 22 Titration by 10 mg/d every week to target dose if tolerated | In patients who remained in study ≥ 4 weeks Patient global assessment at 14 weeks (5‐point scale) 'Complete relief' and 'major relief' Amitriptyline = 3/17 Placebo = 4/16 ≥ 'some relief' Amitriptyline = 8/17 Placebo = 5/16 |
Patient global using numeric scale showed NSD trend for amitriptyline better than placebo NSD between groups for sensory neuropathy (which was generally mild) No significant changes in depression |
| Leijon 1989 | Amitriptyline 25 to 75 mg/d = 15
Carbamazepine 200 to 800 mg/d = 15
Placebo = 15
All medications given in divided doses, am and evening
Forced titration to day 6 for amitriptyline and day 18 for carbamazepine. Reduction allowed for moderate AEs Cross‐over |
Patient global assessment of PR at end of period (5‐point scale) Much improved and pain free (top 2) Amitriptyline = 5/15 Carbamazepine = 2/15 Placebo = 1/15 ≥ Improved (top 3) Amitriptyline = 10/15 Carbamazepine = 5/15 Placebo = 1/15 | Mean PI reduced compared with placebo from 2nd week for amitriptyline, only at 3rd for carbamazepine Depression scores (means) not reduced compared with placebo |
| Max 1988 | Amitriptyline 12.5 to 150 mg/d = 34 Lorazepam 0.5 to 6 mg/d = 40 Placebo = 25 Titration over first 3 weeks to max tolerated dose (rate dependent on age and weight) Medications taken as divided dose, unless patients complained of daytime sedation | From graph
Patient global evaluation ‐ 6‐point scale: 'complete' or 'a lot'
Amitriptyline = 10/34 Lorazepam = 2/40 Placebo = 2/25 'complete", 'a lot' or 'moderate' Amitriptyline = 13/34 Lorazepam = 4/40 Placebo = 6/25 |
At baseline 43 patiernts not depressed, 15 depressed (mostly mild). NSD between depressed and non‐depressed for pain relief |
| Max 1992 | Study 1
Amitriptyline 12.5 to 150 mg/d = 29 + 5 + 20
Desipramine 12.5 to 150 mg/d = 29 + 5 + 20
Study 2
Fluoxetine 20 to 40 mg/d = 28 + 9
Placebo = 28 + 9
Placebo contained 0.125 to 1.5 mg benztropine to mimic dry mouth
Doses titrated up to max tolerated during weeks 1 to 4 Cross‐over. Patients could enter other study after completion of first: 38 completed amitriptyline versus desipramine, and 46 completed fluoxetine versus placebo |
Global rating of pain relief (6‐point scale) at end of treatment period for completers 'complete' or 'a lot: Amitriptyline = 18/38 Desipramine = 15/38 Fluoxetine = 15/46 Placebo = 10/46 |
NSD between amitriptyline and desipramine for mean weekly pain scores |
| Mishra 2012 | Amitriptyline 50 to 100 mg/d = 30 Gabapentin 900 to 1800 mg/d = 30 Pregabalin 150 to 600 mg/d = 30 Placebo = 30 | Mean pain intensity decreased in all groups over duration of study | Apparent morphine‐sparing effect and improvement in functional capacity. Morphine‐sparing and functional capacity were significantly better with pregabalin than the other treatments. |
| Rintala 2007 | Amitriptyline 25 to 150 mg/d = 28 (as 3 doses daily)
Gabapentin 300 to 1200 mg/d = 26 (as 3 doses daily)
Placebo = 25
Placebo contained diphenhydramine 25 to 150 mg/d as 3 doses daily, to mimic side effects of amitriptyline and gabapentin Cross‐over |
≥ 30% PR Patients with low depression score Amitriptyline = 50% Gabapentin = 42.9% Placebo = 35.7% Patients with high depression score Amitriptyline = 62.5% Gabapentin = 12.5% Placebo = 25% Denominators unknown: unclear whether %ages are for patients completing all three phases (do not back calculate to whole numbers) or for all patients taking medication (do not know distribution of depression within groups) | Change in average pain from baseline to week 8: NSD between treatments for patients with low depression scores (n = 2 5) Amitriptyline significantly greater than placebo, and NS greater than gabapentin for patients with high depression scores (n = 13) |
| Rowbotham 2005 | Amitriptyline 25 to 150 mg/d = 17 Desipramine 25 to 150 mg/d = 15 Fluoxetine 10 to 60 mg/d = 15 Titration Doses increased every 2 to 7 days over first 21 days, then kept stable if tolerated Mean dose Amitriptyline = 77 mg/d, desipramine = 93 mg/d, fluoxetine = 44 mg/d | PR at end of treatment (6 weeks) of 'moderate' or better (= ≥ 50% PR) Amitriptyline = 9/17 Desipramine = 12/15 Fluoxetine = 5/15 | NSD between treatments for %age change in daily diary VAS from baseline to start of taper NSD between groups for mean final pain category 2.1 to 3.2 (scale 0 to 5) Minimal changes seen in all groups for symptom checklist scores |
| Shlay 1998 | Amitriptyline 25 to 75 mg/d = 71
Placebo = 65
Titration
A increased every 2 to 3 days to max (Also included acupuncture treatment arms) |
Complete or a lot of relief 6 weeks Amitriptyline = 9/61 Placebo = 13/60 14 weeks Amitriptyline = 13/58 Placebo = 12/53 | Mean changes in PI at weeks 6 and 14, NSD between groups ‐ both improved NSD in QoL or neurologic summary scores |
| Vrethem 1997 | Amitriptyline 25 to 75 mg/d = 36
Maprotiline 25 to 75 mg/d = 36
Placebo = 36
Titration
25 mg on days 1 to 3
50 mg on days 4 to 6
75 mg from day 7 Cross‐over |
Patient global at end of each treatment period (5‐point scale) 'Pain free' and 'much improved' (top 2) Amitriptyline = 12/35 Maprotiline = 4/35 Placebo = 1/35 ≥ 'improved' (top 3) Amitriptyline = 22/35 Maprotiline = 14/35 Placebo = 8/35 | Responder' = PR 20% from baseline Amitriptyline = 20/35 Maprotiline = 15/35 Placebo = 7/35 No difference between responses of diabetics and non‐diabetics |
| Watson 1992 | Amitriptyline = 35
Maprotiline = 35
Titration over first 3 weeks to max tolerated dose
12.5 mg/d increased by 12.5 mg to 25 mg/d mg every 3 to 5 d Cross‐over |
PI at final or 5th week (none, mild, moderate, no changes) None or mild: Amitriptyline = 15/35 Maprotiline = 12/35 'Effectiveness' (excellent, good, improved but unsatisfactory, no change) Excellent or good: Amitriptyline = 14/35 Maprotiline = 6/35 |
NSD between groups for patient estimate of %age improvement in pain
NSD between treatments for depression scores Equal sedative scores for groups |
| Watson 1998 | Amitriptyline = 33
Nortriptyline = 33
Titration over first 3 weeks to max tolerated dose
10 or 20 mg/d increased by 10 mg/d every 3 to 5 d Cross‐over |
Satisfaction with pain relief and tolerable of side effects Amitriptyline = 17/33 Nortriptyline = 15/33 | NSD between groups for pain VAS NSD between groups for pt estimate of %age improvement in pain |
AE: adverse effect; d: day; NS: non‐significant; NSD: non‐significant difference; PGIC: Patient Global Impression of Change; PI: pain intensity; QoL: quality of life; VAS: visual analogue scale
Appendix 6. Summary of outcomes in individual studies: adverse events and withdrawals
| Study |
Treatment (taken at night, unless stated) |
Adverse events | Withdrawals |
| Anon 2000 | Amitriptyline 75 mg/d = 87 Pregabalin 600 mg/d = 86 Placebo = 81 Treatment taken in divided doses, 3 times daily Titration over first 2 weeks |
Patients with ≥ 1 AE: Amitriptyline = 59/87 Pregabalin = 57/86 Placebo = 38/81 Most mild or moderate, 26 severe Patients with SAE: Amitriptyline = 5/87 Pregabalin = 5/86 (1 death, unrelated) Placebo = 2/81 |
All‐cause: Amitriptyline = 23/87, Pregabalin = 24/86, Placebo = 19/81 AE: Amitriptyline = 16/87, Pregabalin = 11/86, Placebo = 5/81 LoE: Amitriptyline = 3/87, Pregabalin = 7/86, Placebo = 9/81 |
| Biesbroeck 1995 | Amitriptyline 25 to 125 mg/d = 117
Capsaicin cream 0.075% = 118 Placebos contained mimicking agents Titration of A over first 4 weeks |
Amitriptyline ‐ GI, anticholinergic, CNS/neuromuscular, cardiovascular, sedative, skin, other Capsaicin ‐ skin, transient cough/sneeze | Not reported |
| Boyle 2012 | Amitriptyline 25 mg twice daily, to 25 mg am and 50 mg night = 28 Duloxetine 60 mg am to 60 mg twice daily = 28 Pregabalin 150 mg twice daily to 300 mg twice daily = 27 | Pregabalin had highest rate of AEs SAE: 6 (1 death, 5 non‐fatal) Did not state which groups |
AE: Amitriptyline 1/28 Duloxetine 3/28 Pregabalin 6/27 |
| Cardenas 2002 | Amitriptyline 10 to 125 mg/d = 44
Placebo = 40
Placebo contained 0.5 mg/d benztropine to mimic dry mouth Titration Week 1 ‐ 10 mg/d Week 2 ‐ 25 mg/d Increased by 25 mg/d each week to max 125 mg/d determined by complete pain relief or max tolerated dose Median max dose = 50 mg/d |
Patients with ≥1 AE:
Amitriptyline = 43/44
Placebo = 36/40
Both drugs: mainly dry mouth, drowsiness, constipation
Increased spasticity amitriptyline > placebo (details for individual events available) |
All‐cause:
Amitriptyline = 8/44, Placebo = 3/40 AE: Amitriptyline = 8/44 (urinary retention ± autonomic dysreflexia (3), constipation (1), other systemic complaints (3)) Placebo = 3/40 (constipation (1), urinary retention/constipation (1), unrelated hospital admission (1)) |
| Graff‐Radford 2000 | Amitriptyline 12.5 to 200 mg/d = 12
Fluphenazine 1 to 3 mg/d = 12
Amitriptyline + Fluphenazine = 13
Placebo = 13
Placebo contained glycopyrrolate to mimic dry mouth and constipation
Titration
Amitriptyline by 25 mg each week to max tolerated dose or 200 mg/d
Fluphenazine by 1 mg each week to max 3 mg/d Cross‐over |
1 patient in amitriptyline due to AE (excessive sedation) | Amitriptyline worst for dry mouth Fluphenazine worst for sleepiness |
| Jose 2007 | Amitriptyline 10 to 50 mg/d = 53
Lamotrigine 50 to 200 mg/d (divided dose) = 46
Titration after 2 weeks if response and tolerated
Amitriptyline ‐ 10, 25, 50 mg
Lamotrigine ‐ 50, 100, 200 mg Cross‐over |
Total number of events:
Amitriptyline = 33 (mainly sedative, CNS) Lamotrigine = 11 (mainly skin, creatinine) |
Lost to follow‐up:
Amitriptyline = 7/53, Lamotrigine = 0/46 AE: Amitriptyline = 19/53 (dizziness (4), postural hypertension (2), difficulty urination (1), constipation (1), dry mouth (1), increased sleep (10)) Lamotrigine = 8/46 (rash (3), itching (1), increased creatinine (4)) LoE (titration stopped because no benefit with 2 doses): Amitriptyline = 16/53, Lamotrigine = 22/46 |
| Kautio 2008 | Amitriptyline 10 to 50 mg/d = 20 Placebo = 22 Titration by 10 mg/d every week to target dose if tolerated | Requiring dose reduction ‐ in patients who remained in trial ≥ 4 weeks: Amitriptyline = 2/17 (tiredness, tachycardia) Placebo = 0/16 | Exclusion/withdrawal within first 4 weeks: Amitriptyline = 3 (2 chemo stopped, 1 non compliance) Placebo = 6 (3 AE, 2 chemo stopped, 1 non compliance) |
| Leijon 1989 | Amitriptyline 25 to 75 mg/d = 15
Carbamazepine 200 to 800 mg/d = 15
Placebo = 15
All medications given in divided doses, am and evening
Forced titration to day 6 for Amitriptyline and day 18 for Carbamazepine. Reduction allowed for moderate AEs Cross‐over |
Patients with ≥ 1 AE
Amitriptyline = 14/15
Carbamazepine = 14/15
Placebo = 7/15
Mostly mild Most common Amitriptyline ‐ tiredness, dry mouth Carbamazepine ‐ vertigo, dizziness, gait problems No dose reduction due to AE for amitriptyline 4 dose reductions due to AE for carbamazepine |
1 participant with carbamazepine had treatment stopped at day 25 due to interaction with warfarin |
| Max 1988 | Amitriptyline 12.5 to 150 mg/d = 34 Lorazepam 0.5 to 6 mg/d = 40 Placebo = 25 Titration over first 3 weeks to max tolerated dose (rate dependent on age and weight) Medications taken as divided dose, unless patients complained of daytime sedation | Patients with ≥ 1 AE: Amitriptyline = 88% Lorazepam = 98% Placebo = 72% Most common: Amitriptyline ‐ dry mouth, sedation, dizziness, difficulty urinating Lorazepam ‐ sedation, dizziness, dry mouth, mood change Placebo ‐ dry mouth, sedation, dizziness | AE:
Amitriptyline = 5/34 (urinary retention, sedation, dizziness, palpitations, rash)
Lorazepam = 6/40 (acute depression (4), ataxia, nightmares)
Placebo = 3/25 (dizziness, disorientation, rash) LoE: 3 (group not given) Mediation error: 1 (group not given) Other unrelated: 4 (group not given) |
| Max 1992 | Study 1
Amitriptyline 12.5 to 150 mg/d = 29 + 5 + 20
Desipramine 12.5 to 150 mg/d = 29 + 5 + 20
Study 2
Fluoxetine 20 to 40 mg/d = 28 + 9
Placebo = 28 + 9
Placebo contained 0.125 to 1.5 mg benztropine to mimic dry mouth
Doses titrated up to max tolerated during weeks 1 to 4 Cross‐over. Patients could enter other study after completion of first: 38 completed amitriptyline versus desipramine, and 46 completed fluoxetine versus placebo |
In patients taking both drugs Patients with ≥ 1 AE: Amitriptyline = 31/38 Desipramine = 29/38 Majority were dose limiting Most common (≥ 5%): Amitriptyline = dry mouth, tiredness headache, palpitations, increased sweating, constipation, lightheadedness, orthostatic symptoms Desipramine = dry mouth, tiredness, constipation, insomnia, increased sweating, headache, lightheadedness |
AE: Amitriptyline = 7/54 (confusion 2, ortho hypertension, fatigue, malaise, hypomania, rash) Desipramine = 7/54 (rash 3, ortho hypertension, bundle‐branch block, tremor, fever) A total of 16 participants did not complete Amitriptyline‐Desipramine study due to adverse events or 'voluntary withdrawal' |
| Mishra 2012 | Amitriptyline 50 to 100 mg/d = 30 Gabapentin 900 to 1800 mg/d = 30 Pregabalin 150 to 600 mg/d = 30 Placebo = 30 | Most common were somnolence, dizziness, and dryness of mouth, nausea, and constipation | No data |
| Rintala 2007 | Amitriptyline 25 to 150 mg/d = 28 (as 3 doses daily)
Gabapentin 300 to 1200 mg/d = 26 (as 3 doses daily)
Placebo = 25
Placebo contained diphenhydramine 25 to 150 mg/d as 3 doses daily, to mimic side effects of amitriptyline and gabapentin Cross‐over |
Most commonly reported: Amitriptyline ‐ dry mouth, drowsiness, fatigue, constipation, increased spasticity, dizziness, nausea Gabapentin ‐ dry mouth, drowsiness, fatigue, constipation, dizziness Placebo ‐ dry mouth, drowsiness, fatigue, constipation, increased spasticity | AE:
Amitriptyline = 4/38, Gabapentin = 5/38, Placebo = 2/38 Medical problem: Amitriptyline = 2/38, Gabapentin = 1/38, Placebo = 1/38 Other: Amitriptyline = 1/38, Gabapentin = 0/38, Placebo = 3/38 |
| Rowbotham 2005 | Amitriptyline 25 to 150 mg/d = 17
Desipramine 25 to 150 mg/d = 15
Fluoxetine 10 to 60 mg/d = 15
Titration
Doses increased every 2 to 7 days over first 21 days, then kept stable if tolerated
Mean dose
Amitriptyline = 77 mg/d, Desipramine = 93 mg/d, Fluoxetine = 44 mg/d |
No usable data | All‐cause
Amitriptyline = 2/17, Desipramine = 2/15, Fluoxetine = 5/15 (4 were on opioids) AE: Amitriptyline and desipramine = 3/32 (sedation/cognitive impairment, orthostasis) Fluoxetine = 2/15 (recurrence of atrial fibrillation, hospitalisation for nausea/weakness with hyponatraemia) |
| Shlay 1998 | Amitriptyline 25 to 75 mg/d = 71
Placebo = 65
Titration
A increased every 2 to 3 days to max (Also included acupuncture treatment arms) |
Grade 4 AE (serious) Amitriptyline = 6/71 Placebo = 2/65 | By 14 weeks 35% of patients in either group had discontinued treatment |
| Vrethem 1997 | Amitriptyline 25 to 75 mg/d = 36
Maprotiline 25 to 75 mg/d = 36
Placebo = 36
Titration
25 mg on days 1 to 3
50 mg on days 4 to 6
75 mg from day 7 Cross‐over |
Patients with ≥ 1 AE: Amitriptyline = 24/35 Maprotiline = 23/34 Placebo = 6/33 Most common dry mouth, sedation, vertigo Patients with SAE: Amitriptyline = 3/35 Maproptiline = 2/34 Placebo = 0/33 | 2 patients did not provide any data for any treatment AE: Amitriptyline = 3/35 (hyperglycaemia, severe thirst, urinary retention) Maprotiline = 2/35 (sedation, vertigo and urticaria) |
| Watson 1992 | Amitriptyline = 35
Maprotiline = 35
Titration over first 3 weeks to max tolerated dose
12.5 mg/d increased by 12.5 mg to 25 mg/d mg every 3 to 5 d Cross‐over |
Patients with ≥ 1 AE Amitriptyline = 20/32 Maprotiline = 28/32 (details in table V of study report) | Excl (added back for efficacy):
Amitriptyline = 2 (mouth ulcer, pain remission during washout between treatments)
Maprotiline = 1 (pain remission during washout between treatments) AE: Amitriptyline = 5/35 (dry mouth, constipation, sedation, dizziness, lethargy, mouth ulcers, nausea) Maprotiline = 4/35 (dry mouth, nausea, vomiting, restless legs) |
| Watson 1998 | Amitriptyline = 33
Nortriptyline = 33
Titration over first 3 weeks to max tolerated dose
10 or 20 mg/d increased by 10 mg/d every 3 to 5 d Cross‐over |
Patients with ≥ 1 AE Amitriptyline = 31/33 Nortriptyline = 31/33 (details in table 1 of study report) | Patients "left the study"
Amitriptyline = 1/33 (slurred speech, urinary retention)
Nortriptyline = 1/33 (increased pain, fever, epigastric pain, bad dreams, perspiration) Patients with "intolerable AE ‐ treatment stopped" Amitriptyline = 10/33 Nortriptyline = 5/33 |
AE: adverse effect; CNS: central nervous system; GI: gastrointestinal; LoE: lack of efficacy; SAE: serious adverse effect
Data and analyses
Comparison 1. Amitriptyline versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Third‐tier efficacy | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Painful diabetic neuropathy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Postherpetic neuralgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Mixed neuropathic pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Cancer‐related neuropathic pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 HIV‐related neuropathic pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Post‐stroke pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 At least 1 adverse event | 6 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.32, 1.81] |
| 3 All‐cause withdrawal | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.81, 2.12] |
| 4 Adverse event withdrawal | 3 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.11, 4.45] |
1.1. Analysis.
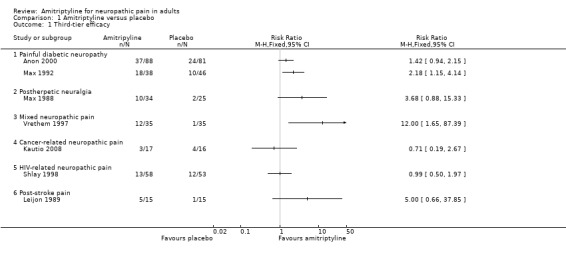
Comparison 1 Amitriptyline versus placebo, Outcome 1 Third‐tier efficacy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anon 2000.
| Methods | R, DB, PC and AC, parallel groups, duration 9 weeks Amitriptyline 75 mg/day (25 mg x 3 daily), pregabalin 600 mg/day (200 mg x 3 daily), placebo Pain assessed periodically up to 9 weeks |
|
| Participants | Adults with painful diabetes neuropathy and pain ≥ 4/10 for at least 1 week N = 254 Mean age 60 years, 37% female Mean baseline score 6.3 to 6.9 |
|
| Interventions | Amitriptyline, n = 87 Pregabalin, n = 86 Placebo, n = 81 |
|
| Outcomes | Pain score Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matched" capsules and placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation not mentioned |
| Size | Unclear risk | 50 to 200 participants/treatment arm |
Biesbroeck 1995.
| Methods | R, DB (DD), PC and AC, parallel groups, duration 8 weeks Amitriptyline taken as single dose, but split (morning and bedtime) if warranted. Initial daily dose of amitriptyline 25 mg, increased to maximum of 125 mg during first 4 weeks. Cream applied to painful area x 4 daily Pain assessed at baseline and every 2 weeks |
|
| Participants | Inclusion: diabetic neuropathy involving feet, ≥ moderate daily pain interfering with activities or sleep N = 235, mean age 60 years (range 21 to 85), M 132/F 103 Mean duration of symptoms > 4 years, mean baseline pain > 60/100 |
|
| Interventions | Amitriptyline capsule (titrated from x 1 to x 5 25 mg/day) + placebo cream, n = 117 Topical capsaicin 0.075% cream + placebo capsule(s), n = 118 Topical capsaicin 0.075% cream + oral amitriptyline capsule(s) ‐ not analysed For first 2 weeks, placebo cream contained methyl nicotinate, a rubefacient that can produce a stinging/burning sensation and erythema (to mimic capsaicin). Placebo capsules contained 0.25 mg benztropine to mimic dry mouth of amitriptyline, and also for first 2 weeks 2 mg diazepam to mimic CNS effects such as sedation 7 day washout for all topical medication and tricyclic antidepressants. Other long‐term oral therapy permitted with no changes to dose or frequency |
|
| Outcomes | Pain intensity Pain relief Interference with activities of life Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W0. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomisation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy method described. Attempt to control for unmasking by adverse effects |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF analysis for efficacy, but no data suitable for analysis. ITT analysis for adverse events |
| Size | Unclear risk | 50 to 200 participants/treatment arm |
Boyle 2012.
| Methods | R, DB, PC, parallel‐group, treatment duration 4 weeks with 8‐day single‐blind placebo run‐in Medication taken in divided doses (morning and evening) Pain intensity assessed at baseline and end of low and high dose periods |
|
| Participants | Inclusion: Adults with type 1 or 2 diabetes, PDN for ≥ 1 year N = 83 (65 completers), mean age 65 years (SD ± 9) M 57, F 26 Baseline PI = 3.1 to 3.5/10 (SD 0.4) in treatment arms |
|
| Interventions | Amitriptyline 25 mg twice daily, titrated to 25 mg am and 50 mg night
Duloxetine 60 mg am titrated to 60 mg twice daily
Pregabalin 150 mg twice daily titrated to 300 mg twice daily States that participants were requested to stop current pain medication ≥ 5 half‐lives before start of study, but that for ethical reasons, opioids and NSAIDs could be continued during study, and paracetamol to maximum 4 g daily allowed as rescue medication |
|
| Outcomes | Pain intensity Quality of life Adverse events |
|
| Notes | Oxford Quality Score: R2, DB1, W0. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization provided by an independent statistician to ensure groups were matched ....." Judged likely to have been computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described ‐ stated to be "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation not reported. Completer analysis for efficacy, but only mean data reported; ITT for safety |
| Size | High risk | < 50 participants per treatment arm |
Cardenas 2002.
| Methods | R, DB, PC, parallel‐group, treatment duration 6 weeks Medication taken as single dose 1 to 2 hours before bedtime. Initial daily dose of amitriptyline 10 mg, increased to 25 mg after 1 week, then by 25 mg each week to maximum of 125 mg if tolerated Pain intensity assessed at baseline and then weekly (average of 3 assessments used in weeks 1 and 6) |
|
| Participants | Inclusion: spinal cord injury > 6 months previously, age 18 to 65 years pain ≥ 3 months with average pain in last month ≥ 3/10 Exclude: history cardiovascular disease, abnormal ECG, seizures, major depressive episode or requiring antidepressant medication, consuming > 2 alcoholic drinks/day N = 84, mean age 42 years, M 67/F 17 Baseline pain intensity > 5/10 |
|
| Interventions | Amitriptyline 25 to 125 mg/day, n = 44 Placebo, n = 40 Placebo contained 0.5 mg benztropine to mimic dry mouth |
|
| Outcomes | Mean pain intensity Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described other than stated as done by Center Pharmacy Investigational Drug Services |
| Allocation concealment (selection bias) | Low risk | Remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical gelatin capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not reported |
| Size | High risk | Fewer than 50 participants/treatment arm |
Graff‐Radford 2000.
| Methods | R, DB (DD), PC, and AC, parallel groups, treatment period 8 weeks Medication taken as single dose at bedtime. Initial daily dose of amitriptyline was 12.5 mg, increased by 25 mg each week to maximum of 200 mg or maximum tolerated dose. Initial daily dose of fluphenazine was 1 mg, titrated to maximum of 3 mg, depending on response. Pain intensity and side effects assessed each week |
|
| Participants | Postherpetic neuralgia with pain for ≥ 6 months ‐ no further details about inclusion/exclusion criteria N = 50 (49 completed), mean age 73 years, M 27/F 22 Mean duration of pain symptoms 33 months, baseline pain 55/100 |
|
| Interventions | Amitriptyline 12.5 mg to 200 mg/day, n = 11 Fluphenazine 1 to 3 mg/day, n = 13 Amitriptyline + fluphenazine 25 to 300/1 to 3 mg/day, n = 12 Placebo, n = 13 No details of washout or permitted medication |
|
| Outcomes | Mean pain intensity Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ states "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy method. "Active" placebo (glycopyrrolate) to mimic anticholinergic side effects |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not described |
| Size | High risk | Fewer than 50 participants/treatment arm |
Jose 2007.
| Methods | R, DB, AC, cross‐over study. 2 x 6‐week treatment periods separated by 2‐week washout Amitriptyline taken as single dose at bedtime, lamotrigine as divided dose, morning and night. Initial daily dose of amitriptyline 10 mg, increasing to 25 mg, and 50 mg after 2 weeks at each dose. Initial daily dose of lamotrigine 25 mg, increasing to 50 mg and 100 mg after 2 weeks at each dose |
|
| Participants | Inclusion: painful diabetic neuropathy, type 2 diabetes, stable glucose‐lowering medication, pain ≥ 5/10 for ≥ 1 month Exclusion: renal or liver disease, epilepsy, psychiatric or cardiac disease, uncontrolled hypertension, peripheral vascular disease, other cause of neuropathy or painful conditions N = 53 (46 completed both periods), mean age 56 years, M 16/F 30 Mean duration of pain symptoms 12 months; mean baseline pain ≥ 70/100 |
|
| Interventions | Amitriptyline 10 to 50 mg/day, n = 53 Lamotrigine 50 to 200 mg/day, n = 46 Antidepressants, anticonvulsants, local anaesthetics and opioids discontinued ≥ 1 month, other PDN medication ≤ 1 week before start of study. Paracetamol ≤ 3 g/day permitted during run‐in and washout periods, except before assessments |
|
| Outcomes | Patient global impression of change (≥ 50% and ≥ 30% improvement) Pain intensity Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "generated using random number tables by block randomisation" |
| Allocation concealment (selection bias) | Low risk | ''Drugs were blinded, packed and numbered serially, and allocated remotely'' ''Drug codes maintained under lock and key'' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy method, "matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis of completers |
| Size | High risk | Fewer than 50 participants/treatment arm |
Kautio 2008.
| Methods | R, DB, PC, parallel groups, 8‐week treatment period Initial daily dose 10 mg, increased by 10 mg/week to maximum dose 50 mg if tolerated Pain symptoms assessed twice weekly, and global improvement of symptoms at end of study |
|
| Participants | Inclusion: cancer patient with chemotherapy‐induced neuropathy, age 18 to 65 years, baseline pain ≥ 3/10, expected survival time ≥ 3 months and neurotoxic chemotherapy of ≥ 2 months Exclusion: other neurological disease, other possible causes of neuropathy, contraindications to amitriptyline therapy N = 42 (33 completed), mean age 54 years, M 12/F 32 |
|
| Interventions | Amitriptyline 10 to 150 mg/day, n = 21 (17 in analysis) Placebo, n = 21 (16 in analysis) Concomitant medication for neuropathic symptoms that inhibits norepinephrine uptake prohibited |
|
| Outcomes | Responder (complete or major relief of neuropathic symptoms) | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Remote allocation (hospital pharmacy) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF on completer analysis as reported. Relevant participants added back for responder analysis |
| Size | High risk | Fewer than 50 participants/treatment arm |
Leijon 1989.
| Methods | R, DB (DD), AC, and PC, cross‐over study. 3 x 4 weeks separated by 1‐week washout. Medication taken as divided doses, morning and night Initial daily dose of amitriptyline 25 mg, increased to 50 mg on day 2, and 75 mg on day 6. Initial daily dose of carbamazepine 200 mg, increased to 400 mg on day 2, 600 mg on day 6, 700 mg on day 15, and 800 mg on day 18. Dose reduction allowed for moderate adverse events Pain assessed twice daily, and global evaluation of effect on pain at end of each period |
|
| Participants | Inclusion: unequivocal stroke episode, constant or intermittent pain which started after stroke and requires treatment, and is not nociceptive, peripheral neuropathic or psychogenic in origin Exclusion: contraindication to study drug, condition would make evaluation difficult N = 15, mean age 66 years, M 12/F 3 Duration of pain 54 months (range 11 to 154), mean baseline pain intensity ˜5/10 |
|
| Interventions | Amitriptyline 25 to 75 mg/day, n = 15 Carbamazepine 200 to 800 mg, n = 14 Placebo, n = 15 |
|
| Outcomes | Patient global evaluation (much improved + pain‐free, and ≥ improved) Mean pain intensity Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ described only as randomised |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double dummy technique", "identical capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in patient global evaluation, no withdrawals |
| Size | High risk | Fewer than 50 participants/treatment arm |
Max 1988.
| Methods | R, DB, PC, and AC, cross‐over study. 2 x 6‐week treatment periods (placebo to active, or active to active) separated by 1‐week washout Medication taken as divided dose morning and evening (unless intolerable daytime sedation). Initial daily dose of amitriptyline 12.5 mg, titrated over first 3 weeks to 150 mg or maximum tolerated dose. Initial daily dose of lorazepam 0.5 mg, titrated over first 3 weeks to 6 mg or maximum tolerated dose. Pain intensity assessed 5 x daily and pain relief at end of each treatment period |
|
| Participants | Postherpetic neuralgia Inclusion: daily pain persisting ≥ 3 months after eruption Exclusion: presence of another type of pain as severe as PHN, depression requiring treatment N = 62 (41 completed both periods, 58 completed at least part of at least one period), median age 72 years, M 31/F 27 Median duration of pain 19 months, baseline pain (in completers) moderate |
|
| Interventions | Amitriptyline 12.5 to 150 mg/day, n = 34 Lorazepam 0.5 to 6 mg/day, n = 40 Placebo, n = 25 2‐week drug‐free washout period before start of study |
|
| Outcomes | Patient global evaluation of treatment at 6 weeks (all periods) Mean pain intensity (first period only) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported ‐ states patients were "randomised into one of four treatment pairs" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported ‐ stated "under double blind conditions" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis |
| Size | High risk | Fewer than 50 participants/treatment arm |
Max 1992.
| Methods | Two R, DB, AC, cross‐over studies. 2 x 6‐week treatment periods separated by 2‐week washout, then option to enter the other study Medication taken as single dose at 9 pm. Dose titrated to maximum tolerated over first 4 weeks of study Pain assessed daily (Gracely scale), and global evaluation of treatment made at end of each treatment phase |
|
| Participants | Inclusion: painful diabetic neuropathy, stable control of diabetes mellitus, ≥ 3 months of daily pain ≥ moderate intensity, not attributable to another cause Exclusion: other pain more severe than neuropathic pain, severe depression, symptomatic coronary artery or peripheral vascular disease, postural hypotension, nephropathy Study 1: N = 29 initially, but unclear how many included in analyses Study 2: N = 28 initially, but unclear how many included in analyses Median age ˜58 years, M:F 3:2 Median duration of pain ˜3 years, mean baseline pain intensity moderate to severe |
|
| Interventions | Study 1 Amitriptyline 12.5 to 150 mg/day Desipramine 12.5 to 150 mg/day Study 2 Fluoxetine 20 to 40 mg/day Placebo Placebo contained 0.125 to 1.5 mg benztropine/day to mimic dry mouth Antidepressant medication stopped ≥ 3 weeks before start of baseline observations. Other analgesic medication stopped if possible, or limited to 1 dose/day for severe pain |
|
| Outcomes | Patient global evaluation of treatment at end of each treatment period. No usable data Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ stated to be "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not clearly described ‐ stated to be "double blind randomisation" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described ‐ stated to be "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis |
| Size | High risk | Fewer than 50 participants/treatment arm |
Mishra 2012.
| Methods | Randomised, double blind, active and placebo controlled, parallel group. Not enriched. No imputation method mentioned Three active treatments, with low starting dose and increases at start of weeks 2 and 3. Total duration 4 weeks Gabapentin 900 mg/d (divided x2) increasing to 1800 mg/d (divided x3) Pregabalin 150 mg/d (divided x2) increasing to 600 mg (divided x2) Amitriptyline 50 mg/d increasing to 100 mg/d at bedtime |
|
| Participants | Cancer with neuropathic pain. N = 120, age and sex distribution not reported. Baseline pain 7.6/10 |
|
| Interventions | Gabapentin 1800 mg daily, n = 30 Pregabalin 600 mg daily, n = 30 Amitriptyline 100 mg daily, n = 30 Placebo, n = 30 |
|
| Outcomes | Mean changes for pain functional capacity and opioid sparing | |
| Notes | Oxford Quality Score: R = 2, DB = 1, W = 0, Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | All drugs encapsulated, but no mention of equal numbers and regimen or double dummy method |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation not mentioned |
| Size | High risk | < 50 participants per treatment arm |
Rintala 2007.
| Methods | R, DB, PC, and AC, cross‐over study. 3 x 9‐week treatment periods separated by 1‐week washout Medication taken as 3 daily doses. Daily dose of amitriptyline 25 mg (days 1 to 3), increased to 50 mg (4 to 5), 75 mg (6 to 7), 100 mg (8 to 14), 125 mg (15 to 21), and 150 mg (22 to 56), then reduced during 9th week of treatment. Daily dose of gabapentin 300 mg (days 1 to 3), increased to 600 mg (4 to 5), 900 mg (6 to 7), 1800 mg (8 to 14), 2400 mg (15 to 21), 3600 mg (22 to 56), then reduced during 9th week of treatment Pain intensity assessed at baseline and end of each treatment period |
|
| Participants | Inclusion: spinal cord injury ≥ 12 months previously, ≥ 1 pain component characteristic of neuropathic pain, present for > 6 months, pain intensity ≥ 5/10, age 18 to 70 years Exclusion: significant cardiac conduction disturbance, history of seizures, liver dysfunction, renal insufficiency, serious psychological disturbance, abuse problem, use of contraindicated medication N = 38 (22 completed all 3 phases), mean age ˜40 years, M 36/F 2 Median duration of pain 5 years, median pain at baseline 6/10 |
|
| Interventions | Amitriptyline 25 to 150 mg/day, n = 28 Gabapentin 300 to 1200 mg/day, n = 26 All pain medication stopped > 1 week before start |
|
| Outcomes | Responder (≥ 30% reduction in pain), by depressive symptoms Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "based on table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical capsules" prepared by commercial compounding company. Each single capsule contained the required dose for the schedule |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis reported |
| Size | High risk | Fewer than 50 participants/treatment arm |
Rowbotham 2005.
| Methods | R, DB (DD), AC, parallel groups, 6‐week treatment period then 2‐week taper Medication taken as divided dose, twice daily. Initial doses were amitriptyline 25 mg/day, desipramine 25 mg/day, fluoxetine 20 mg every other day. Dose increased every 2 to 7 days over first 3 weeks to maximum tolerated or daily doses of amitriptyline 150 mg, desipramine 150 mg, and fluoxetine 60 mg |
|
| Participants | Inclusion: postherpetic neuralgia, age > 40 years, pain ≥ 3 months after healing of rash Exclusion: previous adequate trial of antidepressant for postherpetic neuralgia, previous neurosurgical or neurolytic therapy, separate pain problem of ≥ severity N = 47, mean age 72 years, M 20/F 27 Mean duration of symptoms 42 months, mean baseline pain 54/100 |
|
| Interventions | Amitriptyline 25 to 150 mg/day, n = 17 Desipramine 25 to 150 mg/day, n = 15 Fluoxetine 10 to 60 mg/day, n = 15 |
|
| Outcomes | Responder (≥ moderate pain relief) Mean pain intensity Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ states "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique ‐ states "under double blind conditions .... all subjects took 2 capsules twice a day" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF implied for mean data and categorical data. Comparison shows differences between completers and non‐completers |
| Size | High risk | Fewer than 50 participants/treatment arm |
Shlay 1998.
| Methods | Multicentre, R, DB (part DD), PC, parallel groups, study duration 14 weeks Initial daily dose of amitriptyline 25 mg, increased by 25 mg every 2 to 3 days to maximum 75 mg/day. Medication taken 1 to 2 hours before bedtime. Acupuncture at standard and control points carried out twice weekly, with needles inserted to different depths. Initially participants randomised to 2 x 2 factorial study, where participants received amitriptyline + control acupuncture, standard acupuncture + placebo amitriptyline, amitriptyline + standard acupuncture, or placebo amitriptyline + control acupuncture. Subsequently, participants randomised to amitriptyline versus placebo amitriptyline, or standard acupuncture versus control acupuncture Pain assessed daily using Gracely Scale, and at end of titration and maintenance periods by Patient Global Pain Relief |
|
| Participants | Inclusion: documented history of HIV and symptoms of HIV‐related lower extremity neuropathy, age ≥ 13 years Exclusion: treatment for opportunistic infection or malignancy (except Kaposi sarcoma) Antiretroviral medication allowed throughout study. Analgesic medication could be maintained or reduced, but new treatments discouraged. Tricyclic antidepressants and Monoamine oxidase inhibitors discontinued ≥ 2 weeks before start. N = 125, mean age 41 years, M 124/F 12 |
|
| Interventions | Amitriptyline 25 to 75 mg/day + control acupuncture, n = 33 Acupuncture (standard technique) x 2/week + placebo amitriptyline, n = 31 Amitriptyline + standard acupuncture, n = 32 Placebo amitriptyline + control acupuncture, n = 29 Amitriptyline alone, n = 6 Placebo amitriptyline alone, n = 5 Standard acupuncture alone, n = 58 Control acupuncture alone, n = 56 Antiretroviral therapy permitted, dosages of analgesic medication or herbal therapies maintained or reduced. Initiation of new treatment discouraged. |
|
| Outcomes | Global pain relief at 6 and 14 weeks Mean pain intensity Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedules prepared ''using random blocks stratified by unit'' by university statistical centre |
| Allocation concealment (selection bias) | Low risk | Remote allocation ''by study units by telephoning the statistical center" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo capsules were of "identical" appearance to amitriptyline. Acupuncture control used "control points". Unit pharmacists were only people not blinded to drug assignment, and acupuncturists were only people not blinded to acupuncture assignment. Application of drug treatment effectively blinded, application of acupuncture potentially compromised. Diaries and pain assessments collected by staff blinded to assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two methods used. LOCF ‐ if pain score for week 6 or 14 not available, closest score (4 to 10 week or 11 to 16 week) used. BOCF ‐ assumes no change from pain at baseline. Did not change results |
| Size | High risk | Fewer than 50 participants/treatment arm |
Vrethem 1997.
| Methods | R, DB (DD), PC and AC, cross‐over study. 3 x 4‐week treatment periods separated by 1‐week washouts Medication taken at night |
|
| Participants | Inclusion: polyneuropathic pain (diabetic and non‐diabetic) for ≥ 6 months, with ≥ 2 of distal sensory impairment, distal muscle weakness or atrophy, bilateral decrease, loss of tendon reflexes N = 37, age 35 to 83, M 17/F 19 (no data for one participant) Duration of pain 6 to 168 months |
|
| Interventions | Amitriptyline 25 to 75 mg/day, n = 35 Maprotiline 25 to 75 mg/day, n = 35 Placebo, n = 35 2 participants took ≤ 1 dose and provided no data |
|
| Outcomes | Patient global evaluation ("much improved or pain‐free" and "≥ improved") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ stated to be 'randomised' |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double dummy technique", "identical capsules". Adverse events reported to research assistant, then to two independent neurologists if dose changes required; investigators blinded to adverse events |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | True responder data available for all participants for global analysis |
| Size | High risk | Fewer than 50 participants/treatment arm |
Watson 1992.
| Methods | R, DB (DD), AC, cross‐over study. 2 x 5‐week treatment periods separated by 2‐week washout Medication probably taken as single dose. Initial dose of amitriptyline or maprotiline 25 mg (12.5 mg if age > 65 years), increased by 12.5 mg every 3 to 5 days to maximum tolerated dose within 3 weeks Pain intensity assessed at baseline and weekly intervals |
|
| Participants | Inclusion: postherpetic neuralgia, pain symptoms ≥ 3 months and ≥ moderate for half of the day Exclusion: cardiac disease, seizure disorder, other significant pain problem, previous brain damage through injury, stroke etc, alcoholism N = 35, mean age 71 years, M 18/F 17 Median duration of pain 14 months |
|
| Interventions | Amitriptyline ≥ 12.5 mg/day, n = 35 Maprotiline ≥ 12.5 mg/day, n = 35 All antidepressant or neuroleptic medications withdrawn over 3 weeks before start of study. Stable analgesics continued as needed |
|
| Outcomes | Responder (mild or no pain at end of study) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ stated to be "randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Imputation method not reported. Completer analysis reported, but all participants included in responder outcome |
| Size | High risk | Fewer than 50 participants/treatment arm |
Watson 1998.
| Methods | R, DB, AC, cross‐over study. 2 x 5‐week treatment periods separated by 2‐week washout Initial daily dose 20 mg (10 mg if age > 65 years), increased by 10 mg every 3 to 5 days to maximum tolerated within 3 weeks Pain intensity and pain relief assessed at baseline and weekly intervals |
|
| Participants | Inclusion: postherpetic neuralgia, pain symptoms ≥ 3 months and ≥ moderate for half of the day Exclusion: cardiac disease, seizure disorder, other significant pain problem, severe depression, previous brain damage through injury, stroke etc, alcoholism N = 33, mean age 71 years, M 18/F 17 Median duration of pain 14 months |
|
| Interventions | Amitriptyline ≥ 10 mg/day, n = 33 Nortriptyline ≥ 10 mg/day, n = 33 All antidepressant or neuroleptic medications withdrawn over 3 weeks before start of study. Stable analgesics continued as needed |
|
| Outcomes | Responder (satisfaction with pain relief and tolerable side effects) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated "by computer" |
| Allocation concealment (selection bias) | Low risk | Remote allocation and "sequence concealed in sequential, numbered, sealed envelopes" ''Code kept in central pharmacy'' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical blue gelatin capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in responder analysis |
| Size | High risk | Fewer than 50 participants/treatment arm |
AC: active control; ACR: American College of Rheumatology; BOCF: baseline observation carried forward; CNS: central nervous system; DB: double‐blinding; DD: double dummy; ECG: electrocardiogram; F: female; HRS‐D: Hamilton Rating Scale ‐ Depression; ITT: intention‐to‐treat; LOCF: last observation carried forward; M: male; N: number of participants in study; n: number of participants in treatment arm; NSAIDs: non‐steroidal anti‐inflammatory drugs; PC: placebo controlled; PDN: painful diabetic neuropathy; PHN: postherpetic neuralgia; R: randomisation; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Achar 2010 | Not double‐blind |
| Banerjee 2013 | Open label |
| Bansal 2009 | Fewer than half of participants completed 4 weeks of treatment |
| Bowsher 1997 | Pre‐emptive study |
| Carasso 1979 | Single‐blind study |
| Hampf 1989 | Fewer than 10 participants in amitriptyline treatment arm |
| Kalso 1996 | Duration of study < 4 weeks |
| Kaur 2011 | Study described as double‐blind, but tablets supplied by two different pharmaceutical companies as free samples. All authors considered that they were extremely unlikely to be indistinguishable, so study not convincingly double‐blind. |
| Kautio 2009 | Prophylactic treatment, no initial pain requirement |
| Kieburtz 1998 | Inadequate levels of pain at baseline (using Gracely Scale and use of pain medication at baseline) |
| Lampl 2002 | Prophylactic treatment |
| Max 1987 | Some participants had inadequate levels of pain at baseline (using Gracely Scale) |
| McQuay 1992 | Duration of study < 4 weeks |
| McQuay 1993 | Duration of study < 4 weeks |
| Mendel 1986 | Fewer than 10 participants per treatment arm |
| Mercadante 2002 | Duration of study < 4 weeks |
| Morello 1999 | Some participants had inadequate levels of pain at baseline (using Gracely Scale) |
| Pilowsky 1982 | Unclear diagnosis of pain condition ("a wide range of intractable pain problems ..... without readily treatable somatic pathology") |
| Pilowsky 1990 | Study not double‐blind |
| Robinson 2004 | Some participants had inadequate levels of pain at baseline (using categorical scale) |
| Sharav 1987 | Mixed pain conditions. "Most patients had evidence of musculoskeletal pain" |
| Turkington 1980 | No initial pain requirement for inclusion, no baseline pain reported, no pain measurement reported |
| Vanelderen 2015 | Duration of study < 4 weeks |
| Ventafridda 1987 | Duration of study < 4 weeks |
| Watson 1982 | Duration of study < 4 weeks |
| Wilder‐Smith 2005 | Amitriptyline comparison was not blinded |
| Zitman 1990 | Unclear diagnosis of pain condition ("somatoform pain disorder"). Included some participants with < moderate baseline pain intensity |
Characteristics of studies awaiting assessment [ordered by study ID]
Keskinbora 2006.
| Methods | Randomised controlled trial, double‐blind |
| Participants | Peripheral neuropathic pain ‐ burning, stabbing, shooting N = 46 |
| Interventions | Amitriptyline Gabapentin |
| Outcomes | Improvement in pain intensity Patient satisfaction Adverse events |
| Notes | Turkish (with English abstract) ‐ awaiting translation, but probably no evaluable data |
N: number of participants in study
Differences between protocol and review
This update considers neuropathic pain conditions only. Fibromyalgia is the subject of a separate review (Moore 2015).
We have used three‐tiers of evidence, not two, to better distinguish the strength of evidence and in line with other reviews of interventions for neuropathic pain. We assessed the data according to different neuropathic pain conditions, and planned no further subgroup analysis because the amount of data was expected to be small.
Contributions of authors
For the earlier review PW, RAM, and SD wrote the protocol, RAM and SD carried out searches, assessed studies for inclusion, and extracted data. RAM acted as arbitrator. All authors were involved in writing the review.
For this update RAM and SD carried out searches, assessed studies for inclusion, and extracted data. All authors were involved in writing the review.
RAM will be responsible for updating.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
SD has no conflicts relating to this review or any similar product.
PW has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
DA has no conflicts relating to this review or any similar product.
PC has received research support from industry sources at various times but none related to this review.
For transparency SD, PW, and RAM have received research support from charities, government, and industry sources at various times, but none relate to this review. We are funded by the NIHR for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Anon 2000 {published data only}
- Anon. A placebo‐controlled trial of pregabalin and amitriptyline for treatment of painful diabetic peripheral neuropathy. Docstoc.com (accessed 1 September 2012) (date of publication unknown). [http://www.docstoc.com/docs/106010487/PFIZER‐INC‐These‐results‐are‐supplied‐for‐informational‐purposes]
Biesbroeck 1995 {published data only}
- Biesbroeck R, Bril V, Hollander P, Kabadi U, Schwartz S, Singh SP, et al. A double‐blind comparison of topical capsaicin and oral amitriptyline in painful diabetic neuropathy. Advances in Therapy 1995;12(2):111‐20. [PubMed] [Google Scholar]
Boyle 2012 {published data only}
- Boyle J, Eriksson ME, Gribble L, Gouni R, Johnsen S, Coppini DV, et al. Randomized, placebo‐controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care 2012;35(12):2451‐8. [DOI: 10.2337/dc12-0656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardenas 2002 {published data only}
- Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain 2002;96(3):365‐73. [DOI] [PubMed] [Google Scholar]
- Turner JA, Jensen MP, Warms CA, Cardenas DD. Blinding effectiveness and association of pretreatment expectations with pain improvement in a double‐blind randomized controlled trial. Pain 2002;99(1‐2):91‐9. [DOI: 10.1016/S0304-3959(02)00060-X] [DOI] [PubMed] [Google Scholar]
Graff‐Radford 2000 {published data only}
- Graff‐Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clinical Journal of Pain 2000;16(3):188‐92. [DOI] [PubMed] [Google Scholar]
Jose 2007 {published data only}
- Jose VM, Bhansali A, Hota D, Pandhi P. Randomized double‐blind study comparing the efficacy and safety of lamotrigine and amitriptyline in painful diabetic neuropathy. Diabetic Medicine 2007;24(4):377‐83. [DOI: 10.1111/j.1464-5491.2007.02093.x] [DOI] [PubMed] [Google Scholar]
Kautio 2008 {published data only}
- Kautio AL, Haanpää M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy‐induced neuropathic symptoms. Journal of Pain and Symptom Management 2008;35(1):31‐9. [DOI: 10.1016/j.jpainsymman.2007.02.043] [DOI] [PubMed] [Google Scholar]
Leijon 1989 {published data only}
- Leijon G, Boivie J. Central post‐stroke pain ‐ a controlled trial of amitriptyline and carbamazepine. Pain 1989;36(1):27‐36. [DOI] [PubMed] [Google Scholar]
Max 1988 {published data only}
- Max MB, Schafer SC, Culnane M, Smoller B, Dubner R, Gracely RH. Amitriptyline, but not lorazepam, relieves postherpetic neuralgia. Neurology 1988;38(9):1427‐32. [DOI] [PubMed] [Google Scholar]
Max 1992 {published data only}
- Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. New England Journal of Medicine 1992;326(19):1250‐6. [DOI] [PubMed] [Google Scholar]
Mishra 2012 {published data only}
- Mishra S, Bhatnagar S, Goyal GN, Rana SP, Upadhya SP. A comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double‐blind placebo‐controlled study. American Journal of Hospice and Palliative Care 2012;29(3):177‐82. [DOI: 10.1177/1049909111412539] [DOI] [PubMed] [Google Scholar]
Rintala 2007 {published data only}
- Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Archives of Physical Medicine and Rehabilitation 2007;88(12):1547‐60. [DOI: 10.1016/j.apmr.2007.07.038] [DOI] [PubMed] [Google Scholar]
Rowbotham 2005 {published data only}
- Rowbotham MC, Reisner LA, Davies PS, Fields HL. Treatment response in antidepressant‐naïve postherpetic neuralgia patients: double‐blind, randomized trial. Journal of Pain 2005;6(11):741‐6. [DOI] [PubMed] [Google Scholar]
Shlay 1998 {published data only}
- Shlay JC, Chaloner K, Max MB, Flaws B, Reichelderfer P, Wentworth D, et al. Acupuncture and amitriptyline for pain due to HIV‐related peripheral neuropathy: a randomized controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. JAMA 1998;280(18):1590‐5. [DOI] [PubMed] [Google Scholar]
Vrethem 1997 {published data only}
- Vrethem M, Boivie J, Arnqvist H, Holmgren H, Lindström T, Thorell LH. A comparison a amitriptyline and maprotiline in the treatment of painful polyneuropathy in diabetics and nondiabetics. Clinical Journal of Pain 1997;13(4):313‐23. [DOI] [PubMed] [Google Scholar]
Watson 1992 {published data only}
- Watson CP, Chipman M, Reed K, Evans RJ, Birkett N. Amitriptyline versus maprotiline in postherpetic neuralgia: a randomized, double‐blind, crossover trial. Pain 1992;48(1):29‐36. [DOI] [PubMed] [Google Scholar]
Watson 1998 {published data only}
- Watson CP, Vernich L, Chipman M, Reed K. Nortriptyline versus amitriptyline in postherpetic neuralgia: a randomized trial. Neurology 1998;51(4):1166‐71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Achar 2010 {published data only}
- Achar A, Chatterjee G, Ray TG, Naskar B. Comparative study of clinical efficacy with amitriptyline, pregabalin, and amitriptyline plus pregabalin combination in postherpetic neuralgia. Indian Journal of Dermatology, Venereology and Leprology 2010;76(1):63‐5. [DOI] [PubMed] [Google Scholar]
Banerjee 2013 {published data only}
- Banerjee M, Pal S, Bhattacharya B, Ghosh B, Mondal S, Basu J. A comparative study of efficacy and safety of gabapentin versus amitriptyline as coanalgesics in patients receiving opioid analgesics for neuropathic pain in malignancy. Indian Journal of Pharmacology 2013;45(4):334‐8. [DOI: 10.4103/0253-7613.115000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bansal 2009 {published data only}
- Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P. Amitriptyline vs. pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabetic Medicine 2009;26(10):1019‐26. [DOI: 10.1111/j.1464-5491.2009.02806.x] [DOI] [PubMed] [Google Scholar]
Bowsher 1997 {published data only}
- Bowsher D. The effects of pre‐emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double‐blind, placebo‐controlled trial. Journal of Pain and Symptom Management 1997;13(6):327‐31. [DOI] [PubMed] [Google Scholar]
Carasso 1979 {published data only}
- Carasso RL, Yehuda S, Streifler M. Clomipramine and amitriptyline in the treatment of severe pain. International Journal of Neuroscience 1979;9(3):191‐4. [DOI] [PubMed] [Google Scholar]
Hampf 1989 {published data only}
- Hampf G, Bowsher D, Nurmikko T. Distigmine and amitriptyline in the treatment of chronic pain. Anesthesia Progress 1989;36(2):58‐62. [PMC free article] [PubMed] [Google Scholar]
Kalso 1996 {published data only}
- Kalso E, Tasmuth T, Neuvonen PJ. Amitriptyline effectively relieves neuropathic pain following treatment of breast cancer. Pain 1996;64(2):293‐302. [DOI: 10.1016/0304-3959(95)00138-7] [DOI] [PubMed] [Google Scholar]
Kaur 2011 {published data only}
- Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, Chakrabarti A. A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double‐blind, cross‐over clinical trial. Diabetes Care 2011;34(4):818‐22. [DOI: 10.2337/dc10-1793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kautio 2009 {published data only}
- Kautio AL, Haanpää M, Leminen A, Kalso E, Kautiainen H, Saarto T. Amitriptyline in the prevention of chemotherapy‐induced neuropathic symptoms. Anticancer Research 2009;29(7):2601‐6. [PubMed] [Google Scholar]
Kieburtz 1998 {published data only}
- Kieburtz K, Simpson D, Yiannoutsos C, Max MB, Hall CD, Ellis RJ, et al. A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection. AIDS Clinical Trial Group 242 Protocol Team. Neurology 1998;51(6):1682‐8. [DOI] [PubMed] [Google Scholar]
Lampl 2002 {published data only}
- Lampl C, Huber G, Adl J, Luthringshausen G, Franz G, Marecek S, et al. Amitriptyline in the prophylaxis of central poststroke pain. Preliminary results of 39 patients in a placebo‐controlled, long‐term study. Stroke 2002;33(12):3030‐2. [DOI] [PubMed] [Google Scholar]
Max 1987 {published data only}
- Max MB, Culnane M, Schafer SC, Gracely RH, Walther DJ, Smoller B, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987;37(4):589‐96. [DOI] [PubMed] [Google Scholar]
McQuay 1992 {published data only}
- McQuay HJ, Carroll D, Glynn CJ. Low dose amitriptyline in the treatment of chronic pain. Anaesthesia 1992;47(8):646‐52. [DOI] [PubMed] [Google Scholar]
McQuay 1993 {published data only}
- McQuay HJ, Carroll D, Glynn CJ. Dose‐response for analgesic effect of amitriptyline in chronic pain. Anaesthesia 1993;48(3):281‐5. [DOI] [PubMed] [Google Scholar]
Mendel 1986 {published data only}
- Mendel CM, Klein RF, Chappell DA, Dere WH, Gertz BJ, Karam JH, et al. A trial of amitriptyline and fluphenazine in the treatment of painful diabetic neuropathy. JAMA 1986;255(5):637‐9. [PubMed] [Google Scholar]
Mercadante 2002 {published data only}
- Mercadante S, Arcuri E, Tirelli W, Villari P, Casuccio A. Amitriptyline in neuropathic cancer pain in patients on morphine therapy: a randomized placebo‐controlled, double‐blind crossover study. Tumori 2002;88(3):239‐42. [DOI] [PubMed] [Google Scholar]
Morello 1999 {published data only}
- Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double‐blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Archives of Internal Medicine 1999;159(16):1931‐7. [DOI] [PubMed] [Google Scholar]
Pilowsky 1982 {published data only}
- Pilowsky I, Hallett EC, Bassett DL, Thomas PG, Penhall RK. A controlled study of amitriptyline in the treatment of chronic pain. Pain 1982;14(2):169‐79. [DOI] [PubMed] [Google Scholar]
Pilowsky 1990 {published data only}
- Pilowsky I, Barrow CG. A controlled study of psychotherapy and amitriptyline used individually and in combination in the treatment of chronic intractable, 'psychogenic' pain. Pain 1990;40(1):3‐19. [DOI] [PubMed] [Google Scholar]
Robinson 2004 {published data only}
- Robinson LR, Czerniecki JM, Ehde DM, Edwards WT, Judish DA, Goldberg ML, et al. Trial of amitriptyline for relief of pain in amputees: results of a randomized controlled study. Archives of Physical Medicine and Rehabilitation 2004;85(1):1‐6. [DOI: 10.1016/S0003-9993(03)00476-3] [DOI] [PubMed] [Google Scholar]
Sharav 1987 {published data only}
- Sharav Y, Singer E, Schmidt E, Dionne RA, Dubner R. The analgesic effect of amitriptyline on chronic facial pain. Pain 1987;31(2):199‐209. [DOI] [PubMed] [Google Scholar]
Turkington 1980 {published data only}
- Turkington RW. Depression masquerading as diabetic neuropathy. JAMA 1980;243(11):1147‐50. [PubMed] [Google Scholar]
Vanelderen 2015 {published data only}
- Vanelderen P, Zundert J, Kozicz T, Puylaert M, Vooght P, Mestrum R, et al. Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo‐controlled, double‐blind clinical trial with amitriptyline as a comparator. Anesthesiology 2015;122(2):399‐406. [DOI: 10.1097/ALN.0000000000000508] [DOI] [PubMed] [Google Scholar]
Ventafridda 1987 {published data only}
- Ventafridda V, Bonezzi C, Caraceni A, Conno F, Guarise G, Ramella G, et al. Antidepressants for cancer pain and other painful syndromes with deafferentation component: comparison of amitriptyline and trazodone. Italian Journal of Neurological Sciences 1987;8(6):579‐87. [DOI] [PubMed] [Google Scholar]
- Ventafridda V, Caraceni A, Saita L, Bonezzi C, Conno F, Guarise G. Trazodone for deafferentation pain. Comparison with amitriptyline. Psychopharmacology (Berl) 1988;95 Suppl:S44‐9. [DOI] [PubMed] [Google Scholar]
Watson 1982 {published data only}
- Watson CP, Evans RJ, Reed K, Merskey H, Goldsmith L, Warsh J. Amitriptyline versus placebo in postherpetic neuralgia. Neurology 1982;32(6):671‐3. [DOI] [PubMed] [Google Scholar]
Wilder‐Smith 2005 {published data only}
- Wilder‐Smith CH, Hill LT, Laurent S. Postamputation pain and sensory changes in treatment‐naive patients: characteristics and responses to treatment with tramadol, amitriptyline, and placebo. Anesthesiology 2005;103(3):619‐28. [DOI] [PubMed] [Google Scholar]
Zitman 1990 {published data only}
- Zitman FG, Linssen AC, Edelbroek PM, Stijnen T. Low dose amitriptyline in chronic pain: the gain is modest. Pain 1990;42(1):35‐42. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Keskinbora 2006 {published data only}
- Keskinbora K, Pekel AF, Aydinli I. Comparison of efficacy of gabapentin and amitriptyline in the management of peripheral neuropathic pain. Agri (Algoloji) Dernegi'nin Yayin organidir 2006;18(2):34‐40. [PubMed] [Google Scholar]
Additional references
Attal 2010
- Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. European Journal of Neurology 2010;17(9):1113‐e88. [DOI: 10.1111/j.1468-1331.2010.02999.x] [DOI] [PubMed] [Google Scholar]
Baron 2010
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurology 2010;9(8):807‐19. [DOI: 10.1016/S1474-4422(10)70143-5] [DOI] [PubMed] [Google Scholar]
Baron 2012
- Baron R, Wasner G, Binder A. Chronic pain: genes, plasticity, and phenotypes. Lancet Neurology 2012;11(1):19‐21. [DOI: 10.1016/S1474-4422(11)70281-] [DOI] [PubMed] [Google Scholar]
Bouhassira 2008
- Bouhassira D, Lantéri‐Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136(3):380‐7. [DOI: 10.1016/j.pain.2007.08.013] [DOI] [PubMed] [Google Scholar]
Bril 2011
- Bril V, England J, Franklin GM, Backonja M, Cohen J, Toro D, et al. Evidence‐based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM&R 2011;3(4):345‐52.e21. [DOI: 10.1016/j.pmrj.2011.03.008] [DOI] [PubMed] [Google Scholar]
Collins 2000
- Collins SL, Moore RA, McQuay HJ, Wiffen P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. Journal of Pain and Symptom Management 2000;20(6):449‐58. [DOI] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012
- Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010111] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Sven‐Rice A, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD007393.pub3] [DOI] [PubMed] [Google Scholar]
Derry 2014
- Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD010958.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Finnerup 2005
- Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005;5(3):289‐305. [DOI: 10.1016/j.pain.2005.08.013] [DOI] [PubMed] [Google Scholar]
Finnerup 2015
- Finnerup NB, Attal, N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta‐analysis. Lancet Neurology 2015;14(2):162‐73. [DOI: 10.1016/S1474-4422(14)70251-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gustorff 2008
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self‐reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiologica Scandinavica 2008;52(1):132‐6. [DOI] [PubMed] [Google Scholar]
Hall 2008
- Hall GC, Carroll D, McQuay HJ. Primary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002‐2005. BMC Family Practice 2008;9:26. [DOI: 10.1186/1471-2296-9-26] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2013
- Hall GC, Morant SV, Carroll D, Gabriel ZL, McQuay HJ. An observational descriptive study of the epidemiology and treatment of neuropathic pain in a UK general population. BMC Family Practice 2013;14:28. [DOI: 10.1186/1471-2296-14-28] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hempenstall 2005
- Hempenstall K, Nurmikko TJ, Johnson RW, A'Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Medicine 2005;2(7):e164. [DOI: 10.1371/journal.pmed.0020164] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 2010
- Hoffman DL, Sadosky A, Dukes EM, Alvir J. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy?. Pain 2010;149(2):194‐201. [DOI: 10.1016/j.pain.2009.09.017] [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore RA, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: ] [DOI] [PubMed] [Google Scholar]
Jensen 2011
- Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, et al. A new definition of neuropathic pain. Pain 2011;152(10):2204‐5. [DOI: 10.1016/j.pain.2011.06.017] [DOI] [PubMed] [Google Scholar]
Kalso 2013
- Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ 2013;347:f7339. [DOI: 10.1136/bmj.f7339] [DOI] [PubMed] [Google Scholar]
Katusic 1991
- Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945‐1984. Neuroepidemiology 1991;10:276‐81. [DOI: 10.1159/000110284] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Koopman 2009
- Koopman JS, Dieleman JP, Huygen FJ, Mos M, Martin CG, Sturkenboom MC. Incidence of facial pain in the general population. Pain 2009;147(1‐3):122‐7. [DOI: 10.1016/j.pain.2009.08.023] [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
Lunn 2014
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐263048‐2] [Google Scholar]
McQuay 2007
- McQuay HJ, Smith LA, Moore RA. Chronic pain. In: Stevens A, Raferty J, Mant J, Simpson S editor(s). Health Care Needs Assessment. Oxford: Radcliffe Publishing Ltd, 2007:519‐600. [ISBN: 978‐1‐84619‐063‐6] [Google Scholar]
Moisset 2007
- Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimaging 2007;37(Suppl 1):S80‐8. [DOI: 10.1016/j.neuroimage.2007.03.054] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978–0–931092–69–5] [Google Scholar]
Moore 2009
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007076] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010a
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010c
- Moore RA, Derry S, McQuay HJ, Straube S, Aldington D, Wiffen P, et al. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain 2010;149(2):173‐6. [DOI: 10.1016/j.pain.2009.08.007] [DOI] [PubMed] [Google Scholar]
Moore 2010d
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. [DOI: 10.1016/j.pain.2010.02.039] [DOI] [PubMed] [Google Scholar]
Moore 2011a
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Moore 2011b
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. [DOI: 10.1097/EJA.0b013e328343c569] [DOI] [PubMed] [Google Scholar]
Moore 2012b
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265‐8. [DOI: 10.1016/j.pain.2011.10.00] [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. [DOI: 10.1111/papr.12050] [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD007938.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014c
- Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions ‐ individual patient data responder analysis. European Journal of Pain 2014;18(1):67‐75. [DOI: 10.1002/j.1532-2149.2013.00341.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for fibromyalgia in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD011824] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moulin 2007
- Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain ‐ consensus statement and guidelines from the Canadian Pain Society. Pain Research and Management 2007;12(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moulin 2014
- Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, et al. Pharmacological management of chronic neuropathic pain: Revised consensus statement from the Canadian Pain Society. Pain Research and Management 2014;19(6):328‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence (NICE). Neuropathic pain ‐ pharmacological management: the pharmacological management of neuropathic pain in adults in non‐specialist settings, 2013. www.nice.org.uk/guidance/cg173 (accessed 19 October 2014).
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2009
- O'Connor, AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. American Journal of Medicine 2009;122(10 Suppl):S22‐32. [DOI: 10.1016/j.amjmed.2009.04.007] [DOI] [PubMed] [Google Scholar]
Onghena 1992
- Onghena P, Houdenhove B. Antidepressant‐induced analgesia in chronic non‐malignant pain: a meta‐analysis of 39 placebo‐controlled studies. Pain 1992;49:205‐20. [DOI] [PubMed] [Google Scholar]
PaPaS 2012
- PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 9 March 2015).
PCA 2014
- Anonymous. Prescription cost analysis, England 2013. Available at: hscic.gov.uk/catalogue/PUB13887/pres‐cost‐anal‐eng‐2013‐rep.pdf (accessed 9 March 2015). Health and Social Care Information Centre, 2014:100‐1. [ISBN: 978‐1‐78386‐089‐0]
Rappaport 1994
- Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self‐sustaining discharge in the trigeminal ganglion. Pain 1994;56:127‐38. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Soni 2013
- Soni A, Batra R, Gwilym S, Spector T, Hart D, Arden N, et al. Neuropathic features of joint pain: a community‐based study. Arthritis & Rheumatism 2013;65(7):1942‐9. [DOI: 10.1002/art.37962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2010
- Straube S, Derry S, Moore RA, Paine J, McQuay HJ. Pregabalin in fibromyalgia ‐ responder analysis from individual patient data. BMC Musculoskeletal Disorders 2010;11:150. [DOI: 10.1186/1471-2474-11-150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sultan 2008
- Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology 2008;8:29. [DOI: 10.1186/1471-2377-8-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torrance 2006
- Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. Journal of Pain 2006;7(4):281‐9. [DOI: 10.1016/j.jpain.2005.11.008] [DOI] [PubMed] [Google Scholar]
Tracey 2011
- Tracey I. Can neuroimaging studies identify pain endophenotypes in humans?. Nature Reviews Neurology 2011;7(3):173‐81. [DOI: 10.1038/nrneurol.2011.4] [DOI] [PubMed] [Google Scholar]
Treede 2008
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70(18):1630‐5. [DOI: 10.1212/01.wnl.0000282763.29778.59] [DOI] [PubMed] [Google Scholar]
van Hecke 2014
- Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155(4):654‐62. [DOI: 10.1016/j.pain.2013.11.013] [DOI] [PubMed] [Google Scholar]
Vo 2009
- Vo T, Rice AS, Dworkin RH. Non‐steroidal anti‐inflammatory drugs for neuropathic pain: how do we explain continued widespread use?. Pain 2009;143(3):169‐71. [DOI: 10.1016/j.pain.2009.03.013] [DOI] [PubMed] [Google Scholar]
von Hehn 2012
- Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012;73(4):638‐52. [DOI: 10.1016/j.neuron.2012.02.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2013a
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice ASC, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2013b
- Wiffen PJ, Derry S, Moore RA. Lamotrigine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD006044.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2007
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007;335:87. [DOI: 10.1136/bmj.39213.565972.AE] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
McQuay 1996
- McQuay HJ, Tramer M, Nye BA, Carroll D, Wiffen PJ, Moore RA. A systematic review of antidepressants in neuropathic pain. Pain 1996;68:217‐27. [DOI] [PubMed] [Google Scholar]
Moore 2012a
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008242.pub2] [DOI] [PubMed] [Google Scholar]
Saarto 2007
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005454.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


