Summary
The peripheral protein quality control (QC) system removes non-native membrane proteins including ΔF508-CFTR, the most common CFTR mutant in cystic fibrosis (CF), from the plasma membrane (PM) for lysosomal degradation by ubiquitination. It remains unclear how unfolded membrane proteins are recognized and targeted for ubiquitination, and how they are removed from the apical plasma membrane. Using comprehensive siRNA screens, we identified RFFL, an E3 ubiquitin (Ub) ligase that directly and selectively recognizes unfolded ΔF508-CFTR through its disordered regions. RFFL retrieves the unfolded CFTR from the PM for lysosomal degradation by chaperone-independent K63-linked poly-ubiquitination. RFFL ablation enhanced the functional expression of cell surface ΔF508-CFTR in the presence of folding corrector molecules, and this effect was further improved by inhibiting the Hsc70-dependent ubiquitination machinery. We propose that multiple peripheral QC mechanisms evolved to dispose of non-native PM proteins and to preserve cellular proteostasis, even at the cost of eliminating partially functional polypeptides.
Introduction
Protein homeostasis (proteostasis) has evolved to preserve the functional integrity of cellular milieu against genetic and environmental stresses by adjusting a complex array of biochemical processes, including the regulation of transcription, translation, protein folding, targeting, posttranslational modifications, and degradation. Multiple mechanisms are operational to ensure the recognition and degradation of non-native polypeptides in various subcellular compartments, including the endoplasmic reticulum (ER), nucleus, mitochondria and cytosol (Brodsky, 2012; Fischer et al., 2012; Gardner et al., 2005; Okiyoneda et al., 2011; Pechmann et al., 2013; Wyatt et al., 2009). Efficient removal of conformationally defective PM proteins is critical to preserve the permeability barrier, transport capacity and signal transduction capacity of the PM in both yeast and higher eukaryotes (Wang et al., 2011; Zhao et al., 2013). Conformational destabilization of PM proteins leads to the chaperone- and/or adaptor-dependent E3 Ub ligase interaction and subsequent poly-ubiquitination (Hein et al., 1995; Li et al., 1999) of ion channels (Apaja et al., 2013; Okiyoneda et al., 2010; Sharma et al., 2004) and receptors (Apaja et al., 2010), which constitutes both an effective endocytic and lysosomal sorting signal. E3 ligases such as chaperone-associated CHIP (Apaja et al., 2010; Okiyoneda et al., 2010) and arrestin-related trafficking adaptor associate Rsp5 are responsible for destabilized PM protein poly-ubiquitination (Lin et al., 2008).
CF transmembrane conductance regulator (CFTR) is a cAMP-dependent anion channel expressed at the apical PM of airways, intestines and pancreatic duct epithelia (Riordan, 2008). Mutations in CFTR cause CF, one of the most prevalent, lethal genetic diseases in Caucasians (Collins, 1992; Riordan, 2008). The most common CF-causing mutation, the deletion of F508 (ΔF508) in nucleotide binding domain (NBD1), imposes a global folding defect on CFTR (Du et al., 2005; Riordan, 2008), which accounts for near complete degradation of the newly synthesized core-glycosylated channel by the ER QC. A small fraction of ΔF508-CFTR that escapes the ER QC and reaches the PM exhibits a gating defect (Dalemans et al., 1991) and an accelerated biochemical turnover (Okiyoneda et al., 2010). The residual amount of ΔF508-CFTR PM expression can be enhanced by low temperature (e.g. 26°C), chemical chaperones (e.g. glycerol) and small molecule correctors that act as pharmacological chaperones (e.g. VX-809) (Denning et al., 1992; Sato et al., 1996; Van Goor et al., 2011). The “rescued” ΔF508-CFTR (rΔF508-CFTR) molecules are partially functional, which can be improved by CFTR gating potentiators (e.g. VX-770) (Van Goor et al., 2011). The combination drug therapy, consisting of VX-809 and VX-770 combination (Orkambi), however results only in modest clinical benefit in ΔF508 homozygous patients (Van Goor et al., 2011; Wainwright et al., 2015). This may be due to the limited conformational correction by VX-809 (Grove et al., 2009) and the VX-770-induced instability at the ER and PM (Cholon et al., 2014; Veit et al., 2014).
Previous studies demonstrate that ubiquitination is pivotal for ΔF508-CFTR ER associated protein degradation (ERAD) by multiple Ub E3 ligases, including Rma1 (Younger et al., 2006), Gp78 (Morito et al., 2008), RNF185 (El Khouri et al., 2013), Ubr1 (Stolz et al., 2013) and the chaperone-associated CHIP (Meacham et al., 2001). Recent ΔF508-CFTR interactome and CFTR correction-related transcriptome analysis identified several E3 ligases (TRIM21, UBR4, RNF215, UBOX5, ASB8, FBXO7, SYVN1 and FBXO2) that could facilitate the ERAD in CF bronchial epithelia (Hegde et al., 2015; Pankow et al., 2015; Ramachandran et al., 2016). rΔF508-CFTR is also ubiquitinated by the peripheral QC (Fu et al., 2015; Okiyoneda et al., 2011; Okiyoneda et al., 2010; Sharma et al., 2004). While MARCH2, C-Cbl and Nedd4–2 are involved in the CFTR ubiquitination regardless of the conformation states (Caohuy et al., 2009; Cheng and Guggino, 2013; Ye et al., 2010), CHIP-UbcH5c ubiquitination machinery selectively recognizes and ubiquitinates the conformationally-defective CFTR through Hsc70/Hsp90 and co-chaperone complexes (Okiyoneda et al., 2010). The CFTR ubiquitination can be counteracted by deubiqutinating enzymes Usp10 in early endosome to prevent the lysosomal degradation (Bomberger et al., 2009). The poly-ubiquitinated ΔF508-CFTR undergoes rapid Dab2 and AP-2 dependent endocytosis (Fu et al., 2015; Fu et al., 2012) and targeting for lysosomal sorting by the ESCRT machinery (Okiyoneda et al., 2010; Sharma et al., 2004). Considering that the ablation of previously identified E3 ligases (CHIP and c-Cbl) only partially inhibited the rapid elimination of rΔF508-CFTR from the PM (Fu et al., 2015; Okiyoneda et al., 2010; Ye et al., 2010), we searched for additional E3 ligases that may be involved in the peripheral QC of ΔF508-CFTR in CF bronchial epithelial cell model. Here, we report the identification of an E3 Ub ligase RFFL as one of the primary E3 enzymes responsible for chaperone-independent ubiquitination and peripheral QC of misfolded CFTR with a unique recognition mechanism.
Results
The cellular basis of rΔF508-CFTR rapid turnover at the apical PM in CFBE
To demonstrate the distinct cellular fate of the rΔF508-CFTR, we determined its turnover at the apical PM in human CF bronchial epithelial cell line, CFBE41o- (CFBE). This experiment is based on the temperature-sensitive conformational defect of ΔF508-CFTR which is partially folded at 26°C, but rapidly unfolded by a shift to 37°C (Okiyoneda et al., 2010). CFBE has been derived from homozygous ΔF508 patient and has no detectable CFTR expression, while retaining characteristics of a native bronchial epithelium to some extent (Ehrhardt et al., 2006). CFBE was engineered to stably express CFTR variants with a 3HA-tag in the 4th extracellular loop (Sharma et al., 2004; Veit et al., 2012). Cell surface ELISA showed that ΔF508-CFTR rescued by low temperature (rΔF508-CFTR) was rapidly eliminated from the PM with a half-life of <1 hour (Figure 1A). Accelerated endocytosis (Figure 1B) and profoundly impaired endocytic recycling (Figure 1C) accounted for the rΔF508-CFTR instability, similar to that reported in non-polarized HeLa cells (Okiyoneda et al., 2010) and consistent with some previous observations in CFBE (Swiatecka-Urban et al., 2005; Varga et al., 2008). As a corollary, the rapid turnover of the complex-glycosylated rΔF508-CFTR was documented by cycloheximide (CHX) chase (Figure 1D), a process likely initiated by the augmented ubiquitination of the mutant in the post-Golgi compartments (Figure 1E). The poly-ubiquitination level of the unfolded rΔF508-CFTR after 37°C exposure was significantly higher than that of the WT and the folded rΔF508-CFTR (Figures 1E–1F), confirming previous observations in non-polarized cells (Okiyoneda et al., 2010). These observations imply that the apical PM QC mechanism can efficiently recognize and ubiquitinate partially unfolded CFTR in CFBE. Ub-chain configuration of CFTR can confer distinct association with various biochemical processes, as exemplified by the preferential proteasomal degradation of substrates harboring K48-linked Ub chains, while endocytosis and lysosomal sorting are the preferential fates of K63-linked Ub chains (Clague and Urbé, 2010). Efficient elimination of CFTR from the apical PM likely requires poly-Ub conjugation, since fusion of WT Ub, but not the mutant variant (Ub-AllRΔG), which is largely resistant to poly-Ub chains formation (Barriere et al., 2006; Okiyoneda et al., 2010) (Figure S1A), profoundly accelerated endocytosis and reduced the half-life of CFTR at the apical PM (Figures 1A–1B). Notably, the functional integrity of the channel was not influenced by Ub fusion to the C-terminal tail (Sharma et al., 2004). To determine the poly-Ub chain configuration of rΔF508-CFTR, we developed an ELISA-based Ub-chain detection assay using K48- and K63-linkage specific anti-Ub antibodies (Newton et al., 2008). To this end, an N-terminal histidine-biotin-histidine (HBH) tag (Tagwerker et al., 2006) was engineered in the ΔF508-CFTR cDNA. The core-glycosylated HBH-tagged rΔF508-CFTR-3HA was eliminated by CHX chase (Figures 1G and S1B) and detergent solubilized complex-glycosylated CFTR was immobilized on neutravidin-coated plates under denaturing conditions and quantified by anti-HA ELISA (Figure S1C). Core-glycosylated ΔF508-CFTR, isolated by culturing without a low temperature rescue, was found to contain K48-, but not K63-linked chains upon proteasome inhibitor MG132 treatment (Figure 1H). In contrast, complex-glycosylate rΔF508-CFTR was modified with both K48- and K63-linked poly-ubiquitination, and thermal unfolding of the rescued channel at 37°C for 1 h increased the abundance of K63-Ub chains by >8-fold but K48-Ub chains by only ~3.4 fold (Figures 1I–1J). The K63-linked poly-ubiquitination was accumulated by bafilomycin A1 that inhibits lysosomal degradation while MG-132 augmented the K48-Ub chains (Figures 1I–1J). These results indicate that both K48- and K63-Ub chains are responsible for the elimination of unfolded ΔF508-CFTR in the post-Golgi compartments, especially for the proteasomal and lysosomal degradation, respectively.
Figure 1. rΔF508-CFTR is ubiquitinated and rapidly eliminated from the apical PM of CFBE at physiologic temperature.
PM stability (A), endocytosis rate (B) and recycling rate (C) of CFTR-3HA variants at 37°C in CFBE or CFBE-tet cells were measured by ELISA. ΔF508-CFTR was rescued by 26°C for 2 days followed by 37°C incubation for 1.5 h to induce unfolding. (D) Complex-glycosylated CFTR-3HA stability measured by CHX chase. Ubiquitination level of HBH-CFTR variants was measured by immunoprecipitation at denaturing condition (dIP) and Western blotting (E) and normalized for CFTR in precipitates (F). Non-rescued ΔF508-CFTR was used as a positive control. (G) Isolated HBH-ΔF508-CFTR was visualized by Western blotting. K48 and K63-linked poly-ubiquitination of immature (H) or mature HBH-rΔF508-CFTR (I, J) were quantified by ELISA using the Ub linkage specific antibodies. 10 μM MG-132 or 1 μM BafA1 were treated for 1 h at 37°C. The ubiquitination level was normalized by the CFTR amount quantitated by ELISA using anti-HA antibody (Figure S1C). The number of data from at least two independent experiments is indicated in the parentheses. Data represent mean±SE. See also Figure S1.
Identification of RFFL as E3 ligase of membrane proteins in post-Golgi compartments
To identify E3 ligases that can recognize misfolded rΔF508-CFTR, we performed a loss-of function phenotypic siRNA screen of 636 E3 Ub ligases in CFBE stably expressing the horseradish peroxidase-tagged ΔF508-CFTR (ΔF508-CFTR-HRP). ΔF508-CFTR-HRP was selected as a reporter molecule to confer increased sensitivity and reproducibility to PM detection as compared to the detection of 3HA-tagged ΔF508-CFTR by ELISA (Phuan et al., 2014). Following the silencing of E3 enzymes by pooled siRNAs, the luminescence signal generated by the rΔF508-CFTR-HRP remaining at the PM after rescuing (26°C, 2 days) and unfolding (37°C, 2 h) was measured. As a comparison, the PM density of the C-terminally truncated CD4 (CD4T), a stable PM model protein (Barriere et al., 2006), was also measured by ELISA to eliminate the E3 ligases that non-selectively regulate the PM expression of membrane proteins. The primary screen isolated 37 E3 ligases whose knockdown (KD) more strongly increased the PM level of rΔF508-CFTR-HRP than Hsc70 KD, but marginally affected the CD4T (Figure 2A). These E3 ligases were re-screened to confirm the primary screen, and revealed that KD of 7 E3 ligases had a stronger effect than Hsc70 ablation (Figure S2A). In subsequent studies, we focused on RFFL (also known as CARP2) since its KD increased the PM level of rΔF508-CFTR, while minimally influencing the lysosomal targeting or endosomal recycling other model proteins with distinct sorting signals and mechanisms (Figure 2B). CD4TccUb(AllRΔG) and CD4-Lamp1 are sorted from the PM to lysosomes by recognition of a tetra-mono-Ub and a Tyr-based sorting motif, respectively (Barriere et al., 2006; Rohrer et al., 1996). The CD4Tl-λL57C chimera, which contains the conditionally unfolded bacteriophage λ cytosolic domain, is eliminated by CHIP-mediated ubiquitination from the PM (Apaja et al., 2010) while the transferrin receptor (TfR) (Mukherjee et al., 1997) and the WT CFTR represent endocytic recycling cargos. RFFL KD modestly increased the PM level of T70-CFTR which is a CFTR class VI mutant accelerated the PM turnover (Figure 2B) (Haardt et al., 1999; Sharma et al., 2004), and the PM level of P67L-CFTR, a class II mutant (Sabusap et al., 2016) (Figure S2B). RFFL individual siRNAs also increased rΔF508-CFTR-HRP PM density, implying the on-target effect (Figure 2C). Profound ablation of RFFL by pooled siRNA (siRFFL) increased the complex-glycosylated rΔF508-CFTR expression in CFBE without inducing the ER accumulation of the core-glycosylated form (Figure 2D). Similar effects were observed upon stable RFFL KD by shRNA (Figures 2E and S2C). Using a halide-sensitive YFP quenching assay, it was found that siRFFL significantly enhanced the cAMP stimulated halide conductance conferred by rΔF508-CFTR at the PM in CFBE (Figures 2F–2G). The enhanced functional PM expression is likely due to the increased PM stability of rΔF508-CFTR upon RFFL KD (Figures 2H and S2D). siRFFL also stabilized T70-CFTR and P67L-CFTR at the PM, but not the WT (Figure 2I). The RFFL KD effect was still observed as the increased rΔF508-CFTR-HRP PM level in CFBE-teton cells where the exogenous CFTR-HRP mRNA level was adjusted to lower level of the endogenous CFTR mRNA in Calu-3 cells (Figures S2E–S2F), indicating a possibility that RFFL limits ΔF508-CFTR PM expression at physiological condition.
Figure 2. E3-ligase siRNA screen identified RFFL as a candidate gene that limits the PM density and stability of rΔF508-CFTR in CFBE.
(A) E3 siRNA (636 genes) screening in CFBE transfected with 50 nM siRNA. PM level of rΔF508-CFTR-HRP and CD4T were measured. (B) PM level of CFTR-HRP variants, CD4T chimeras and TfR in CFBE transfected with 50 nM siRNA. (C) rΔF508-CFTR-HRP PM level in CFBE individual RFFL siRNA transfected. (D-E) Immunoblotting of ΔF508-CFTR-3HA in CFBE transfected with siRNA (D) or shRNA stably (E). Na+/K+ ATPase was used as loading control. (F-G) Representative traces (F) and quantification of the initial YFP quenching rate (G) of rΔF508-CFTR function in siRNA transfected CFBE. (H-I) PM stability of CFTR-3HA variants in CFBE measured by ELISA. The number of data from at least two independent experiments is indicated in the parentheses. Data represent mean±SE, n.s., not significant, **p<0.01. See also Figure S2.
RFFL activity promotes rΔF508-CFTR lysosomal targeting
RFFL contains three functional domains; the N-terminal FYVE-like domain that may ensure its association with endosomes (Coumailleau et al., 2004), the catalytic RING domain and the caspase interacting domain (CID), as well as predicted intrinsically disordered regions (DR). The endo-lysosomal localization of RFFL-GFP was confirmed by co-localization with Rab5 (early endosome marker), Rab7 and Rab9 (late endosome markers), as well as lysosome marker Lamp1 (Figures 3A and S3A–S3B). Additionally, RFFL was partially localized at the PM based on its co-localization with wheat germ agglutinin (WGA) in non-permeabilized cells (Figure S3B). RFFL was associated with the cytosolic surface of lysosomes and other acidic endocytic organelles, as indicated by co-staining with LysoTracker (Figure S3C). Deletion of the N-terminal region (ΔNT), containing the FYVE-like domain, changed the RFFL localization to a diffuse cytosolic distribution pattern (Figure 3A).
Figure 3. RFFL ablation attenuates the endocytosis and lysosomal sorting of ΔF508-CFTR.
(A) Fluorescence micrographs of HeLa cells expressing RFFL-GFP or RFFLΔNT-GFP with mRFP-Rab5 or Lamp1-RFP. (B) Endocytosis rate of rΔF508-CFTR, TfR and CD4TccUbAllRΔG, (C) endocytic recycling of rΔF508-CFTR in CFBE upon 50 nM siRNA transfection. CHX chase experiment measuring stability of mature rΔF508-CFTR (D) and WT, T70 and P67L-CFTR-3HA (E). (F) Indirect immunostaining of internalized rΔF508-CFTR-3HA in CFBE immediately after 1 hour 37°C labeling (0 h) and 2 h chase. EEA1 was used as an early endosome marker. (G) Indirect immunostaining of internalized rΔF508-CFTR-3HA in CFBE after 4 h chase with 10 μg/ml leupeptin. Lysosome was labeled with Lysotracker Red. Co-localization of internalized rΔF508-CFTR-3HA with Lysotracker in shNT or shRFFL stably transfecting CFBE was quantified by Pearson’s correlation coefficient (PCC). (H) The mean vesicular pH of endocytic vesicles (>200 vesicles) containing rΔF508-CFTR or CD4Tl-λL57C in siRNA-transfected CFBE (>50 cells) measured by FRIA. The number of data from at least two independent experiments is indicated in the parentheses. Data represent mean±SE, n.s., not significant, *p<0.05, **p<0.01. Bars, 10 μm. See also Figure S3.
Consistent with the pleiotropic subcellular localization of RFFL, siRFFL slowed down endocytosis of rΔF508-CFTR by ~24% of the siNT, but minimally accelerated its endocytic recycling (Figures 3B–3C). siRFFL failed to inhibit the endocytosis of TfR and CD4TccUb(AllRΔG), which are mediated by the AP-2 and Ub-binding adaptors, respectively (Barriere et al., 2006) (Figure 3B). Accordingly, CHX chase experiments showed that siRFFL stabilized the mature but not the immature form of rΔF508-CFTR (Figures 3D and S3D). Similarly, siRFFL stabilized the mature T70-CFTR and P67L-CFTR, but not WT CFTR (Figures 3E and S3E). The RFFL effect is not specific in airway cells because siRFFL increased the mature rΔF508-CFTR in human embryonic kidney 293MSR cells and pancreatic CFPAC-1 cells (Figures S3F–S3H). Immunocytochemical analysis showed that rΔF508-CFTR accumulated at the PM and in EEA1 positive endosomes upon RFFL KD, while most of the channel was eliminated after 2 h chase in control cells (Figure 3F). RFFL KD reduced the accumulation of internalized rΔF508-CFTR in lysosomes after blocking lysosomal proteases (Figure 3G). Furthermore, siRFFL suppressed the rapid endo-lysosomal transfer kinetics of internalized rΔF508-CFTR measured by fluorescence ratio image analysis (FRIA) that monitors the vesicular pH of internalized FITC-labeled anti-HA-CFTR complexes (Barriere and Lukacs, 2008). In siNT treated cells, internalized rΔF508-CFTR was delivered to the lysosomal compartment (pH<5.5) in 30 min, but siRFFL increased the residence time of internalized rΔF508-CFTR in early endosomes (pH ~6.1), but not in recycling endosomes (pH ~6.4), where WT CFTR is typically localized (Figures 3H and S3I). In line with the selective substrate recognition, RFFL KD failed to affect the rapid lysosomal targeting of CD4Tl-λL57C (Figure 3H).
Membrane targeting and disordered regions are required for RFFL interaction with unfolded rΔF508-CFTR
We next investigated the molecular determinants of RFFL in the peripheral QC of rΔF508-CFTR. Pull-down experiments revealed that RFFL fused with V5-tag (RFFL-V5) preferentially interacts with mature HBH-rΔF508-CFTR unfolded at 37°C for 4 h, but not with its WT counterpart, indicating the conformational dependency (Figure 4A). RFFL-His-Biotin (RFFL-HB) physically interacted with the mature rΔF508-CFTR and this interaction was increased by the catalytically inactive RING domain mutations (C316A+C391A, designated as 2CA) (Figures 4B and S4A). The proper localization of RFFL determined by the palmitoylation, but not by the FYVE-like domain, is necessary for its interaction with the CFTR because perturbing the N-terminal region containing predicted palmitoylation sites (Araki et al., 2003) (C5A+C6A+C10A designated as C5610A, Δ2–10 or ΔNT) perturbed the CFTR interaction (Figures 4B–4C and S4A–S4B). However, the proper RFFL localization is not sufficient to interact with the unfolded CFTR. RFFL also requires the disordered regions to interact with CFTR because deleting one of the disordered regions (ΔDR1 and ΔDR2) predicted by PONDR® reduced the CFTR interaction without affecting the localization (Figures 4B and S4B). Pull-down experiments using truncation RFFL mutants supported that both DR1 and DR2 are crucial for efficient CFTR interaction because the RFFL fragment containing both DR1 and DR2 (1–148) more efficiently interacted with the mature rΔF508-CFTR than the RFFL fragment containing only DR1 (e.g. 1–38, 1–88, 1–120) (Figure 4C). These results strongly suggest that DR1 and DR2 are the substrate recognition sites directly interacting with the unfolded CFTR.
Figure 4. RFFL interacts with mature rΔF508-CFTR through disordered regions.
(A) Interaction of complex-glycosylated rΔF508-CFTR, but not the WT counterpart with RFFL-V5 was shown by NA pulldown. (B-C) Interaction of RFFL-HB variants with mature rΔF508-CFTR was shown by NA pulldown in BHK cells treated with 50 μg/ml CHX for 16 h at 26°C and 4 h at 37°C following 26°C rescue to minimize the immature form. Schematic model of RFFL mutants and predicted disordered regions are shown. (D) BiFC signal generation in COS-7 cells co-transfected with ΔF508-CFTR-VN and RFFL-VC with (rΔF) or without 26°C rescue (ΔF). (E) Co-localization of the BiFC signal generated by interaction of rΔF508-CFTR-VN and RFFL-VC with mRFP-Rab5 in COS-7 cells. Bars, 10 μm. See also Figure S4.
To define the subcellular location of the rΔF508-CFTR and RFFL interaction, we performed a bimolecular fluorescence complementation (BiFC) experiment by measuring the complementation of a split Venus fluorescent protein (Kodama and Hu, 2010). To this end, ΔF508-CFTR and RFFL were fused to the N-terminal (VN155; ΔF508-CFTR-VN) and C-terminal fragment of Venus (VC155; RFFL-VC), respectively. Venus fluorescence could only be detected upon co-expression of ΔF508-CFTR-VN with RFFL-VC at the permissive 26°C followed by 37°C unfolding for 2.5 h, but not at 37°C due to the ER retention of the misfolded CFTR (Figure 4D). Venus derived fluorescence was minimal in the presence of ΔF508-CFTR-VN and RFFLΔNT-VC, consistent with the results of the pull-down experiment. The Venus signal was observed at the PM (Figure S4C) and vesicular compartments co-localized with Rab5 (Figure 4E), indicating that RFFL interacts with rΔF508-CFTR at the PM and early endosomes.
To determine whether RFFL recruitment to non-native rΔF508-CFTR is chaperone-dependent as it is the case for CHIP E3 ligase (Okiyoneda et al., 2010; Sharma et al., 2004), we checked the Hsc70 KD effect on the RFFL-rΔF508-CFTR interaction. Hsc70 KD failed to inhibit the RFFL-2CA-HB interaction with mature rΔF508-CFTR, but not with the immature rΔF508-CFTR, indicating that Hsc70 is not required for the RFFL recognition of mature rΔF508-CFTR (Figure S4D). Additionally, RFFL overexpression reduced rΔF508-CFTR even in Hsc70 KD cells, supporting the Hsc70-independent RFFL interaction with rΔF508-CFTR (Figure S4E).
RFFL promotes poly-ubiquitination of rΔF508-CFTR in post-Golgi compartments and in vitro.
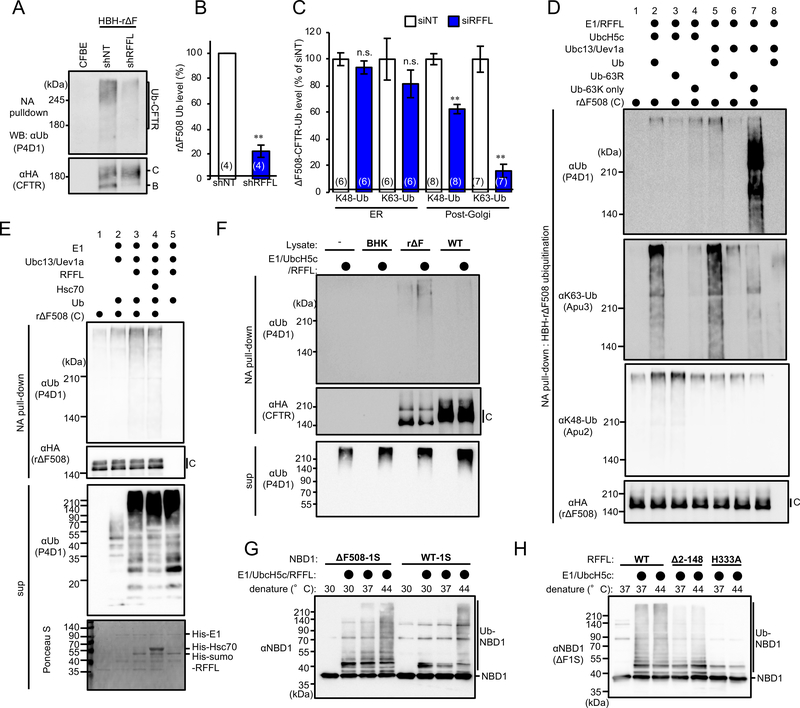
Next, we determined the mature rΔF508-CFTR ubiquitination level in CFBE upon RFFL ablation. As expected, the ubiquitination was reduced by stable RFFL KD or transient siRFFL transfection (Figures 5A–5B and S5A). siRFFL reduced the K48- and K63-linked poly-ubiquitination of complex-glycosylated rΔF508-CFTR by ~40% and ~80%, respectively, while it did not affect ubiquitination of the core-glycosylated form (Figure 5C).
Figure 5. RFFL directly facilitates poly-ubiquitination of unfolded CFTR.
Ubiquitination level of HBH-rΔF508-CFTR variants in CFBE stably transfected with shNT or shRFFL was measured by NA pulldown (A) and normalized for CFTR in precipitates (B). (C) K48- and K63-linked poly-ubiquitination of the complex-glycosylated (post-Golgi) or core-glycosylated (ER) HBH-ΔF508-CFTR in CFBE was quantified by ELISA using the Ub linkage specific antibody. (D) Reconstitution of RFFL-mediated ubiquitination of complex-glycosylated rΔF508-CFTR in vitro. (E) Addition of purified Hsc70 failed to stimulate the RFFL-mediated in vitro CFTR ubiquitination. (F) Distinct susceptibility of the RFFL-mediated ubiquitination between WT and rΔF508-CFTR. (G) Unfolding dependent NBD1 in vitro ubiquitination by RFFL. (H) Removing the N-terminal region reduces the RFFL-mediated NBD1 ubiquitination. The number of data from at least two independent experiments is indicated in the parentheses. Data represent mean±SE, n.s., not significant, **p<0.01. See also Figure S5.
To assess whether RFFL can directly ubiquitinate rΔF508-CFTR, we reconstituted the ubiquitination using purified His6-E1, His6-sumo-UbcH5c and His6-sumo-RFFL from E. coli, and complex-glycosylated HBH-rΔF508-CFTR from BHK cells (Figures S5B–S5C). The activity of the recombinant Ub machinery was confirmed by measuring auto-ubiquitination of RFFL (Figure S5D). Immunoblot analysis showed K63-linked poly-Ub adduct formation on HBH-rΔF508-CFTR in the presence of E1, UbcH5c, RFFL and Ub after 2 h incubation at 37°C, while omitting any of the components or using RFFL-ΔRING, prevented the ubiquitination (Figure S5E). The ubiquitination was also observed after replacing UbcH5c with Ubc13/Uev1a that preferentially catalyzes K63-linked poly-ubiquitination (Hofmann and Pickart, 1999) (Figures 5D and S5F). K48-linked poly-ubiquitination of HBH-rΔF508-CFTR was observed in the presence of UbcH5c, but not Ubc13/Uev1a (Figures 5D and S5F). The RFFL-induced rΔF508-CFTR ubiquitination was reconstituted with a mutant Ub containing only a single Lys63 (Ub-63K), but not with Ub-63R mutated to Lys63 to Arg63 (Figure 5D). These results strongly suggest that RFFL directly stimulates mature rΔF508-CFTR ubiquitination, especially K63-linked poly-ubiquitination. Interestingly, Ub chains containing Ub-63K were strongly detected by the anti-Ub antibody, while detected by the anti-K63-Ub antibody to a lesser extent (Figures 5D and S5F). This observation is probably due to the reduced recognition of Ub-63K by the anti-K63-Ub antibody. In contrast to the chaperone-mediated ubiquitination by CHIP (Okiyoneda et al., 2010; Sharma et al., 2004), the addition of purified Hsc70 failed to facilitate the RFFL-mediated in vitro rΔF508-CFTR ubiquitination (Figure 5E). The RFFL-mediated CFTR ubiquitination seems to be conformation dependent because RFFL minimally stimulated the WT CFTR ubiquitination (Figure 5F). The direct RFFL recognition was also supported by the result that RFFL ubiquitinated purified NBD1 containing ΔF508 and 1S mutations (ΔF1S-NBD1) (Rabeh et al., 2012) (Figure S5G). RFFL preferentially ubiquitinated ΔF1S-NBD1 compared to WT1S-NBD1, and the thermal denaturation stimulated the NBD1 ubiquitination (Figure 5G). The NBD1 ubiquitination was not stimulated by the catalytic inactive H333A mutant, and was largely abolished by removing the N-terminal region of RFFL (Δ2–148), supporting the direct CFTR recognition of RFFL by DR1 and DR2 (Figure 5H).
RFFL inhibition improves the pharmacological rescue of ΔF508-CFTR
The most promising CFTR corrector, VX-809, only modestly improves the PM expression and function of ΔF508-CFTR. This modest effect could in part be attributed to the efficient peripheral degradation of rΔF508-CFTR. siRFFL enhanced the pharmacological rescue efficiency of VX-809 by ~1.6 fold in CFBE (Figures 6A–6C). Similar improvement was observed upon siRFFL treatment combined with distinct classes of correctors (VX-809, C4) and the chemical chaperone, glycerol (designated as Trio) (Okiyoneda et al., 2010; Okiyoneda et al., 2013; Sharma et al., 2004) (Figures 6A–6C). siRFFL also improved the corrector combination effect in NCI-H441 human lung epithelial cells by increasing the PM level of rΔF508-CFTR cells to ~43% of the WT (Figure S6A). Concordantly, siRFFL enhanced the accumulation of complex-glycosylated ΔF508-CFTR in the presence of correctors (Figure 6D). Finally, siRFFL could partially counteract the destabilizing effect of chronic VX-770 treatment on PM stability, density, and function of rΔF508-CFTR (Figures 6E–6H and S6B).
Figure 6. RFFL KD improves the corrector-rescued ΔF508-CFTR function.
(A) PM density of ΔF508-CFTR-HRP in CFBE transfected with 50 nM siRNA and treated with 3 μM VX-809 alone or with 10 μM C4 and 5% glycerol (Trio) for 24 h at 37°C. Representative traces (B) and quantification of the initial YFP quenching rate (C) of ΔF508-CFTR function in CFBE siRNA transfected and pre-treated with DMSO, VX-809 alone or Trio for 24 h at 37°C. (D) ΔF508-CFTR-3HA expression in siRNA transfected CFBE treated with correctors as mentioned above was analyzed by immunoblot. PM stability of ΔF508-CFTR-3HA (E) and density of ΔF508-CFTR-HRP (F) in siRNA transfected CFBE. VX-809 (3 μM) and VX-770 (100 nM) were pre-treated for 24 h at 37°C. (G-H) Representative traces (G) and quantification of the initial YFP quenching rate (H) of ΔF508-CFTR function in CFBE siRNA transfected and pre-treated with DMSO, VX-809 and VX-770 for 24 h at 37°C. The number of data from at least two independent experiments is indicated in the parentheses. Data represent mean±SE, n.s., not significant, *p<0.05, **p<0.01. See also Figure S6.
Hsc70 ablation improves the ΔF508-CFTR rescue upon RFFL knockdown
To examine if RFFL plays a parallel role with the chaperone and CHIP dependent peripheral QC mechanism (Apaja et al., 2010; Okiyoneda et al., 2010), we measured the rΔF508-CFTR PM stability upon double KD of RFFL and CHIP or Hsc70, members of the chaperone-dependent ubiquitination machinery (Figure 7A). While ablation of RFFL, CHIP or Hsc70 alone modestly increased the rΔF508-CFTR PM stability and density, combining KD of RFFL and Hsc70, but not CHIP, additively enhanced rΔF508-CFTR PM stability, density and function in CFBE (Figures 7B–7D). This could be explained by the additive reduction of K48- and K63-linked poly-ubiquitination of rΔF508-CFTR (Figures 7E–7F). Simultaneous KD of RFFL and Hsc70 failed to enhance the TMEM16A function, a Ca2+-activated Cl− channel, indicating the selectivity of these parallel peripheral QC mechanisms (Figure 7D). Similarly, simultaneous KD of RFFL and Hsc70, but not Hsc70 KD alone, robustly improved the VX-809 correction on the ΔF508-CFTR PM density and function (Figures 7G–7I). Jointly, these results suggest that both the chaperone-dependent and RFFL-mediated peripheral QC mechanisms limit the functional PM expression of ΔF508-CFTR.
Figure 7. Hsc70 and RFFL KD additively mediated ΔF508-CFTR correction.
(A) KD of RFFL, CHIP or Hsc70 in siRNA transfected CFBE were confirmed by Western blotting. PM stability (B) and density (C) of rΔF508-CFTR-HRP in CFBE transfected with siNT, siRFFL, siCHIP or siHsc70 (25 nM each). (D) Function of rΔF508-CFTR or TMEM16A in siRNA transfected CFBE determined by halide sensitive YFP quenching assay. (E) Isolated mature HBH-rΔF508-CFTR in CFBE transfected with siRNA used for Ub ELISA. (F) Ubiquitination of HBH-rΔF508-CFTR in siRNA-transfected CFBE was quantified by ELISA. (G) PM density of ΔF508-CFTR-HRP in siRNA transfected CFBE treated with VX-809 for 24 h at 37°C. Representative traces (H) and quantification of the initial quenching rate (I) of ΔF508-CFTR function in siRNA transfected CFBE treated with VX-809 for 24 h at 37°C. The number of data from at least two independent experiments is indicated in the parentheses. Data represent mean±SE, n.s., not significant, *p<0.05, **p<0.01. (J) A schematic model of CFTR peripheral QC by paralleled RFFL- and chaperone-mediated ubiquitination mechanism.
Discussion
Here, we propose that the Ub ligase RFFL limits the functional expression of rΔF508-CFTR by rapidly eliminating the mutant from the apical PM. The conformationally defective rΔF508-CFTR is susceptible to poly-ubiquitination, rather than mono-ubiquitination by RFFL, facilitating the accelerated endocytosis and impeding its lysosomal degradation. While the former process can be attributed to the Ub-ligase activity of RFFL confined to the PM, the endocytic RFFL pool likely accounts for ubiquitination or re-ubiquitination of internalized ΔF508-CFTR, which could be counteracted by the deubiquitinating activity of USP10 (Bomberger et al., 2009). These opposing mechanisms, depending on the severity of the conformational defect of a single channel, ensure a structural defect-dependent sorting mechanism either for recycling or lysosomal degradation. Because RFFL KD was not sufficient to restore the endocytic recycling of rΔF508-CFTR despite reducing the ubiquitination, the PM stabilization effect seems to be mostly due to the inhibited endocytosis. Molecular determinants for the CFTR recycling efficiency may not only be the ubiquitination states, but also other conformational states. We also found that P67L-CFTR, a class II mutant (Sabusap et al., 2016), exhibits the accelerated turnover in the post-Golgi compartments and PM, a hallmark of class VI mutations including T70-CFTR and rΔF508-CFTR (Veit et al., 2016). Because RFFL KD prevented the peripheral degradation of T70-CFTR and P67L-CFTR, RFFL-mediated ubiquitination could be responsible for the PM instability of CFTR class VI mutants.
Our in vitro assays provide evidence that RFFL directly ubiquitinates complex-glycosylated ΔF508-CFTR in conjunction with the E2 enzymes UbcH5c or Ube13/Uev1a. RFFL predominantly mediates the K63-linked poly-ubiquitination, an efficient signal for endocytosis (Kamsteeg et al., 2006) and lysosomal delivery (Barriere et al., 2007; Huang et al., 2013; Lauwers et al., 2009). RFFL also modestly stimulates the K48-linked poly-ubiquitination involved in the lysosomal degradation (Zhang et al., 2013). Together these culminate in accelerated internalization and efficient lysosomal delivery mediated by clathrin-adaptors (e.g. AP-2, Dab2 (Fu et al., 2015)) and endosomal ESCRT machineries (e.g. Hrs, TSG101 (Okiyoneda et al., 2010; Sharma et al., 2004), respectively. We propose that RFFL recognizes the unfolded regions of CFTR including NBD1 at the PM and endosomes through its disordered regions (DR1 and DR2), similar to San1, a nuclear QC E3 ligase that can directly recognize unfolded substrates by its disordered domains (Rosenbaum et al., 2011). The direct interaction of RFFL with its substrates may account for the substrate selectivity as RFFL KD failed to affect the lysosomal delivery of CD4Tl-λL57C that is ubiquitinated by a chaperone-dependent mechanism (Apaja et al., 2010). Previous studies showed that RFFL overexpression inhibits the endocytic recycling of TfR although this could not be due to the ubiquitination activity because of the RING domain independency (Coumailleau et al., 2004). N-terminal palmitoylation sites observed in SAKURA, the rat ortholog of RFFL (Araki et al., 2003), but not the FYVE-like domain which is distinct from a classical FYVE domain (McDonald and El-Deiry, 2004; Tibbetts et al., 2004), is a determinant for the RFFL membrane localization and a prerequisite for CFTR recognition in vivo.
This study demonstrates that RFFL plays a crucial role in the chaperone-independent peripheral QC mechanism eliminating the conformationally-defective CFTR in the endocytic pathway (Figure 7J). Upon overexpression, RFFL interacts with immature ΔF508-CFTR at the cytoplasmic face of ER, probably through Hsc70, and reduces the protein level. At physiological level, however, RFFL predominantly interacts with mature ΔF508-CFTR at the PM and endosomes, and stimulates its ubiquitination, resulting in rapid endocytosis and lysosomal degradation.
The FDA approved combinatorial pharmacotherapy of ΔF508-CFTR homozygous patients with the folding corrector VX-809 and the gating potentiator VX-770 (Orkambi) can restore the ΔF508-CFTR channel function to ~25% of the WT level in vitro if the potentiator was added acutely (Van Goor et al., 2011). Orkambi ensured modest clinical benefit for patients in a phase III clinical trial (Wainwright et al., 2015); (Jones and Barry, 2015). Recent studies showed that prolonged exposure to VX-770 reduced the PM expression partly by destabilizing the ΔF508-CFTR at the cell surface, involving the peripheral QC mechanism (Cholon et al., 2014; Veit et al., 2014). We show that RFFL ablation improved the VX-809 induced functional ΔF508-CFTR expression by ~2-fold, and reversed the destabilizing effect of chronic VX-770 exposure. The efficacy of ΔF508-CFTR correction was further improved by simultaneous ablation of RFFL and Hsc70, which together robustly counteract the peripheral ubiquitination and PM removal. In contrast to the chaperone-associated ubiquitination mechanism, which potentially affects a broad range of substrate proteins, RFFL-mediated ubiquitination exhibited some degree of substrate specificity. In fact, RFFL knockout mice exhibit no abnormal phenotype (Ahmed et al., 2009) although RFFL is ubiquitously expressed (Coumailleau et al., 2004). Intriguingly, RFFL transcription is stimulated by RAF/MEK/ERK signaling pathway (Gan et al., 2013; Gan et al., 2012) that negatively regulates the ΔF508-CFTR functional expression by unknown mechanisms (Trzcinska-Daneluti et al., 2012; Trzcińska-Daneluti et al., 2015). Despite the fact that RAF/MEK/ERK signaling pathway could also modulate chaperone-mediated ubiquitination (Trzcińska-Daneluti et al., 2015), it may limit the ΔF508-CFTR functional expression by up-regulating RFFL activity.
Taken together, the present results demonstrate that E3 ligase RFFL limits functional PM expression of unfolded ΔF508-CFTR. RFFL gene expression is not up-regulated by ΔF508-CFTR expression or in primary human bronchial epithelial (HBE) cells from CF patients carrying ΔF508 mutation (Figures S7A–S7B). However, counteracting the RFFL activity may provide a preferable therapeutic approach as a CFTR stabilizer that is a class of drugs that extends the PM resident time of CFTR class VI mutants. Despite future studies are needed to validate the impact on the ΔF508-CFTR in CF-HBE cells, developing agents selectively inhibiting the RFFL-mediated CFTR ubiquitination may help improve efficacy of the pharmacological therapy in CF.
STAR METHODS
Contact for Reagent and resource sharing
Further information and requests for reagents may be directed to and will be fulfilled by Tsukasa Okiyoneda (t-okiyoneda@kwansei.ac.jp).
Experimental model and subject details
Cell models
CFBE41o- stably expressing CFTR-3HA variant were generated by lentivirus transduction (Okiyoneda et al., 2013; Phuan et al., 2014; Veit et al., 2014) under puromycin (3 μg/ml), and were grown in minimal essential medium (MEM, Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine, 10 mM HEPES, and 3 μg/ml puromycin. CFBE41o- Tet-on cells stably expressing CFTR-3HA, CFTR-HRP, His6-Biotin-His6 (HBH-tagged CFTR variants, CD4T, CD4TccUb(AllRΔG) or CD4TL-λL57C under a tetracycline-responsive promoter were generated (Okiyoneda et al., 2013; Phuan et al., 2014; Veit et al., 2014) by lentivirus transduction using the Lenti-X TetON Advanced Inducible Expression System (Clontech, Mountain View, CA) under puromycin (3 μg/ml) and G418 selection (0.2 mg/ml), and were grown in MEM (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine, 10 mM HEPES, 3 μg/ml puromycin and 0.2 mg/ml G418. NCI-H441 Tet-on cells stably expressing CFTR-3HA cells (Veit et al., 2012) were grown in RPMI-1640 Medium (ATCC) supplemented with 10% FBS, 3 μg/ml puromycin and 0.2 mg/ml G418. For propagation, the CFBE cells were cultured in plastic dishes coated with an extracellular matrix (ECM mix) consisting of 10 μg/ml human fibronectin (EMD), 30 μg/ml PureCol collagen preparation (Advanced Biomatrix) and 100 μg/ml bovine serum albumin (Sigma-Aldrich). The CFTR-3HA, -HRP or -HBH expression was induced by 0.5 or 1 μg/ml doxycycline treatment for 4 days. Stable RFFL KD CFBE-tet-ΔF508-CFTR-3HA cells were generated by lentivirus expressing shRFFL in pLKO.1-blast vector under blasticidin S (10 μg/ml) selection. CFBE41o- Tet-on cells stably expressing ΔF508-CFTR-3HA and constitutive YFP-H148Q/I152L/F46 (Veit et al., 2014), and HeLa cells expressing ΔF508-CFTR-3HA (Okiyoneda et al., 2010) were grown as previously. BHK cells stably expressing HBH-CFTR-3HA variants were generated as (Sharma et al., 2004) and grown in DMEM/F12 medium (Wako) supplemented with 5% FBS and 500 μM methotrexate. COS7 and 293MSR cells were grown in DMEM medium (Wako) supplemented with 10% FBS and DMEM medium (Wako) supplemented with 10% FBS and 600 μg/ml G418, respectively. CFPAC-1 Tet-on cells stably expressing ΔF508-CFTR-3HA cells generated by lentivirus transduction as described above were grown in Iscove’s Modified Dulbecco’s Medium (IMDM) medium (Wako) supplemented with 10% FBS and 1 μg/ml puromycin.
Method details
Transfection
Transient expression of plasmids expressing CFTR and RFFL variants was accomplished using polyethylenimine Max (Polysciences Inc, Warrington, PA). siRNA transfection (50 nM) in CFBE and NCI-H441 cells was accomplished using Lipofectamine RNAiMax transfection reagent (Invitrogen). siRNA transfected cells were used for the experiments 5 days post-transfection. The target sequences of shRNA and siRNA are listed in table S1.
Measurement of CFTR cell-surface density, endocytosis, stability and recycling.
Cell surface density, stability, endocytosis and recycling of CFTR-3HA were measured by ELISA using anti-HA as previously (Okiyoneda et al., 2010; Okiyoneda et al., 2013). Cell-surface density and stability of CFTR-HRP were measured as previously (Phuan et al., 2014; Veit et al., 2014). Cell surface expression of ΔF508-CFTR was induced by 26°C incubation for 36–48 h, followed by 1 h incubation at 37°C to induce the unfolding. Cell surface density and endocytosis of CD4 chimeras and TfR was measured by cell-surface ELISA using anti-CD4 (OKT4, eBioscience Inc, San Diego) and Transferrin Biotin-XX conjugate (ThermoFisher), respectively, as described (Okiyoneda et al., 2010).
Fluorescence ratio image analysis (FRIA)
The methodology for FRIA of endocytic vesicles containing CFTR as a cargo was described in detail (Barriere and Lukacs, 2008) and in polarized cells (Veit et al., 2014). CFBE41o- Tet-on cells expressing ΔF508–3HA or CFTR-wt-3HA were electroporated with indicated siRNAs using Neon® system (Life Technologies). Filter-grown cells were allowed to polarize for 5 days and temperature-rescued for 48 hours at 30°C after the siRNA treatment (25 nM). CFTR-wt-3HA served as a reference point. Before labeling the CFTR, the cells were shifted to 37°C for 1.5 h, after which the CFTR-3HA was labeled with anti-HA antibody and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary Fab (Jackson ImmunoResearch, USA) on ice. Synchronized uptake was performed for indicated times at 37°C. Approximately 300 vesicles were analyzed in each independent experiment, and the graphs show the mean ± SEM of vesicular pH from three independent experiments. FRIA was performed on a Zeiss Observer Z1 inverted fluorescence microscope (Carl Zeiss MicroImaging) equipped with a X-Cite 120Q fluorescence illumination system (Lumen Dynamics Group Inc., Canada) and Evolve 512 EMCCD camera (Photometrics Technology, USA). The acquisition was carried out at 495 ± 5 and 440 ± 10 nm excitation wavelengths using a 535 ± 25-nm emission filter and analyzed with the MetaFluor software (Molecular Devices, Canada).
Pull-down assay and Western blotting
Cells were solubilized in mild lysis buffer (150 mM NaCl, 20 mM Tris, 0.1% NP-40, pH 8.0, supplemented with 1 mM PMSF, 5 μg/ml leupeptin and pepstatin) and the cell lysates were incubated with Neutravidin agarose (ThermoFisher) for 2 hours at 4°C. After being washed 4 times with mild lysis buffer, the complex was eluted in urea elution buffer (8 M urea, 2% SDS, 3 mM biotin) at room temperature for 30 min, and analyzed by Western blotting. Western blotting experiments were performed as previously described (Okiyoneda et al., 2010).
CFTR ubiquitination measurement in vivo
CFTR ubiquitination level in CFBE cells were performed as previously (Okiyoneda et al., 2010). To isolate complex-glycosylated HBH-ΔF508-CFTR in post-Golgi compartments, CFBE cells were rescued at 26°C for 2 days followed by 100 μg/ml cycloheximide (CHX) treatment for 9–12 h at 26°C to minimize the amount of core-glycosylated form in the ER because the core-glycosylated form was mostly eliminated after 7 hours CHX chase at 26°C (Figure.S1B). Cells were then incubated at 37°C for 1 h in the culture medium supplemented with 10 μM MG-132, 1 μM bafilomycin A1, and 10 μM PR-619 to allow the ubiquitinated CFTR to accumulate. Cells were lysed in RIPA buffer and complex-glycosylated HBH-ΔF508-CFTR was purified using Neutravidin agarose under denaturing condition, and analyzed by Western blotting with anti-Ub (P4D1) and anti-HA antibodies. CFTR ubiquitination level (>200 kDa) was measured by densitometry and normalized for the CFTR level in the precipitate.
To quantitate the K48- and K63-linked poly-ubiquitination of HBH-CFTR, cell lysates, as prepared above, were used for the ELISA assay. The core-glycosylated HBH-ΔF508-CFTR in the ER was isolated from cell lysate of CFBE cells cultured only at 37°C by immobilization on Neutravidin coated 96 well white plate (ThermoFisher). The complex-glycosylated HBH-ΔF508-CFTR in post-Golgi compartments was isolated by immobilization on Neutravidin coated 96 well white plate from cell lysate of CFBE cells cultured at 26°C for 2 days, followed by 100 μg/ml CHX chase at 26°C for 9–12 hours to minimize the core-glycosylated form, and 37°C unfolding for 1 hour with 10 μM MG-132 and 10 μM PR-619. Isolated immobilized HBH-ΔF508-CFTR on Neutravidin coated 96 well white plate was denatured in 8 M urea at room temperature for 5 min. After 0.1% BSA blocking following 3 washes with 0.1% NP-40-PBS, the CFTR ubiquitination was detected by anti-K48 Ub (Apu2) and anti-K63 Ub (Apu3) antibodies, and quantified with HRP-conjugated secondary antibody. Linkage specific CFTR ubiquitination level was normalized for the CFTR level quantified by anti-HA antibody.
siRNA screen
Human E3 siRNA library was generated by selecting 636 genes from Human Druggable Genome siRNA Set V4.1 (Qiagen) and Human Refseq NM siRNA Set V4.0 (Qiagen). The E3 siRNA library (636 genes) is listed in Table S2. Pooled siRNA against each E3 ligase was composed of four different siRNA. CFBE-tet-ΔF508-CFTR-HRP were transfected by forward transfection with 50 nM pooled siRNA using lipofectamine RNA Max (Invitrogen, Carlsbad, CA) cells in 96 well white plate, and the PM level of ΔF508-CFTR-HRP was measured 5 days after siRNA transfection. The CFTR expression was induced by 500 ng/ml doxycycline for 4 days. ΔF508-CFTR-HRP were rescued at 26°C for 2 days, followed by 1 hour incubation at 37°C before the experiment. Cells were washed 6 times with PBS, and the HRP activity was assayed by adding 100 μl/well SuperSignal Pico (Pierce). After incubation for 5 minutes, chemiluminescence was measured using a Tecan Infinite M1000 plate reader (Tecan Groups Ltd, Mannedorf, Switzerland) or Valioskan microplate reader (ThermoFisher Scientific, Waltham, MA).
Immunocytochemistry and BiFC assay
Cells transiently expressing RFFL-GFP variants were immunostained as previously (Glozman et al., 2009). For immunostaining, the following primary antibodies were used: anti-Rab5 (C8B1, Cell Signaling Technology), -Rab7 (D95F2, Cell Signaling Technology), Rab9 (D52G8, Cell Signaling Technology), and -EEA1 (3C10, MBL). To label the PM, cells were incubated with 5 μg/ml Wheat Germ Agglutinin (WGA)-Alex594 (ThermoFisher) for 10 min without permeabilization. For lysosome labeling, CFBE cells were pre-treated with 10 μg/ml leupeptin for 24 h, and incubated with 100 nM LysoTracker Red DND-99 (Life Technologies) for 1 h at 37°C. After wash, cells were incubated with or without 10 μg/ml leupeptin for 4 h at 37°C.
Organelle markers mRFP-Rab5 (addgene #14437), mRFP-Rab7 (addgene #14436) or Lamp1-RFP (addgene #1817) were transiently transfected using polyethylenimine Max (Polysciences Inc, Warrington, PA). To label the rΔF508-CFTR-3HA internalized from the PM, cells were incubated with anti-HA antibody (Covance, MMS-101R) at 37°C for 1 hour, and chased for the periods indicated after removing excess antibody by washing. Cells were fixed, permeabilized with 0.1% Triton-X100, and stained with Alexa Fluor® 594-conjugated anti-mouse IgG (ThermoFisher). For BiFC experiments, COS-7 cells were transiently transfected with ΔF508-CFTR-3HA-VN155 and RFFL variants fused to VC155. ΔF508-CFTR-3HA-VN155 was rescued at 26°C for 36 hours, followed by 37°C incubation for 1 hour. Co-expression of ΔF508-CFTR-3HA-VN155 and RFFL-VC155 was confirmed by indirect immunostaining using anti-HA and anti-RFFL antibodies. To examine the cellular localization of BiFC signal, cells were transiently transfected with mRFP-Rab5. Single optical sections were collected on an inverted laser confocal fluorescence microscope (SP8, Leica) equipped with a HC PL APO 63X/NA 1.40 objective. Images were processed with Photoshop CS6 (Adobe). Co-localization of internalized CFTR-3HA with lysosome marker was analyzed by Pearson’s correlation coefficient for CFTR-3HA co-localization with Lysotracker using Volocity 5 (PerkinElmer).
Halide-sensitive YFP quenching assay
ΔF508-CFTR function assay by halide-sensitive YFP fluorescence quenching was performed as described (Veit et al., 2014). CFBE cells expressing both inducible ΔF508-CFTR-3HA and halide sensor YFP-F46L/H148Q/I152L were seeded onto white 96-well microplates and transfected with 50 nM siRNA. Expression of ΔF508-CFTR was induced for 4 days at 37ºC with 500 ng/ml doxycycline, and VX-809 (3 μM) was added during the last 24 hours. For low temperature-rescued ΔF508-CFTR, expression of ΔF508-CFTR was induced for 2 days at 37ºC and followed by rescue for an additional 48 hours at 26ºC. At the time of assay, cells were washed 4 times with 400 μL of phosphate-buffered saline (PBS)-chloride (140 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 1.1 mM MgCl2, 0.7 mM CaCl2, and 5 mM glucose) and incubated with PBS-chloride (50 μL per well), followed by well wise-injection of activator solution (50 μL per well) [20 μM forskolin, 0.5 mM 3-isobutyl-1-mthyl-xanthine (IBMX), 0.5 mM 8-(4-chlorophenylthio)-adenosine-3’,5’-cyclic monophosphate (cpt-cAMP), and 0.1 mM genistein] and incubated for 57 s. The fluorescence was recorded continuously (200 ms per point) for 3 s (baseline) and for 32 s after rapid addition of 100 μL PBS-iodide, in which NaCl was replaced with NaI. After normalization to YFP signals before PBS-iodide injection, the I− influx rate was calculated by linear fitting of the initial slope. Fluorescence was measured using a Varioskan Flash (Thermo Fisher Scientific) with a dual syringe pump (excitation/emission 500/535 nm).
Protein purification
His6-E1 (UBE1, addgene #34965), His6-sumo-UbcH5c, His6-sumo-RFFL variants, His6-sumo-NBD1-ΔF1S, His6-sumo-NBD1-WT1S (Rabeh et al., 2012) and GST-RFFL variants were expressed in BL21 rosetta2 E. coli strain (Merck Millipore). Cells were lysed by incubation with 1 mg/ml lysozyme for 30 minutes on ice, followed by sonication.The His-tagged proteins and GST-tagged proteins were purified using Ni-affinity and Glutathione-affinity chromatography, respectively, as described (Rabeh et al., 2012).
In vitro CFTR ubiquitination
Complex-glycosylated HBH-rΔF508-CFTR-3HA was purified from BHK after rescue at 26°C for 36 h, followed by 100 μg/ml CHX treatment for 9 h at 26°C to eliminate the core-glycosylated form. After solubilization in Tris-NP-40 solubilization buffer (20 mM Tris, 150 mM NaCl, 2 mM MgCl2, 10% glycerol, 5 mM imidazole, and 0.1% NP-40 at pH 7.4) supplemented with 5 mM TCEP, 1 mM PMSF, 5 μg/ml leupeptin, 5 μg/ml pepstatin A and 2.5 mM ATP, cell lysate was incubated with Neutravidin agarose (ThermoFisher) for 2 h at 4°C. After being washed 4 times in wash buffer (20 mM Tris, 500 mM NaCl, 2 mM MgCl2, 10% glycerol, 0.1% NP-40 at pH 7.4) supplemented with 5 mM TCEP, 1 mM PMSF, 5 μg/ml leupeptin, 5 μg/ml pepstatin A and 2.5 mM ATP, purified complex-glycosylated HBH-rΔF508-CFTR-3HA bound to the agarose beads was incubated with components of the putative ubiquitination machinery (0.2 μM His6-E1 (BostonBiochem), 6 μM His6-sumo-UbcH5c or 3 μM His6-Ubc13/Uev1a (BostonBiochem), 5 μM His6-sumo-RFFL, 5 μM Hsc70 (StressMarq Biosciences), 20 μM Ub, Ub-63R or Ub-63K (BostonBiochem)) in reaction buffer (20 mM HEPES pH 7.5, 50 mM NaCl, 5 mM MgCl2, 2.5 mM ATP, 5 mM TCEP, 0.1% NP-40, 10 μM MG-132) for 2 h at 37°C. For the comparison of the susceptibility of RFFL-mediated in vitro ubiquitination between rΔF508 and WT-CFTR (Figure 5F), purified HBH-rΔF508- or WT-CFTR bound to neutravidin agarose were incubated with 0.2 μM His6-E1, 6 μM His6-sumo-UbcH5c, 3 μM GST-RFFL, and 20 μM Ub in the reaction buffer for 2 h at 30°C. After the reaction, the supernatant containing E1, E2 and RFFL was collected for Western blotting to confirm the auto-ubiquitination of RFFL. The beads were washed five times in RIPA buffer supplemented with 2 M urea, and HBH-CFTR-3HA was recovered by elution in urea elution buffer (8 M urea, 2% SDS, 3 mM biotin). Eluted HBH-CFTR-3HA was analyzed by Western blotting with antibodies indicated in Figures.
In vitro NBD1 ubiquitination
250 ng of purified His6-sumo-NBD1-ΔF1S or His6-sumo-NBD1-WT1S was mixed with 0.2 μM His6-E1, 4 μM His6-sumo-UbcH5c, 4 μM His6-sumo-RFFL and 20 μM Ub in 15 μl of reaction buffer (20 mM HEPES pH 7.5, 50 mM NaCl, 5 mM MgCl2, 2.5 mM ATP, 2 mM DTT, and 20 μM MG-132) for 2 h at 37°C. To examine the unfolding-dependent ubiquitination, His6-sumo-NBD1 was denatured at 30°C, 37°C or 44°C for 5 min, followed by ubiquitination at 30°C for 2 hours as described above except using 1 μM GST-RFFL variants instead of 4 μM His6-sumo-RFFL. The ubiquitination reaction was stopped by adding SDS sample buffer and boiling for 5 min. The ubiquitination was analyzed with Western blotting using anti-CFTR (L12B4) and anti-Ub (P4D1) antibodies.
mRNA isolation and q-PCR
CFBE were grown on filter supports for 5 days in medium supplemented with the indicated doxycycline concentrations. Human bronchial epithelial cells (HBE) were a kind gift of Walter E. Finkbeiner (UCSF) or were purchased from the Cystic Fibrosis Translational Research center (CFTRc), McGill University. HBE cells were expanded using conditional reprogramming (Avramescu et al., 2017) followed by differentiation on filter supports for ≥ 4 weeks following established protocols (Neuberger et al., 2011). The mRNA isolation and q-PCR was performed as described previously (Veit et al., 2012). The primers are listed in Key Resources Table.
Key Resources Table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| HA (16B12) | BioLegend | Cat#901515 |
| CD4 (OKT4) | eBioscience | Cat#14-0048-82 |
| Ub (P4D1) | Santa Cruz Biotechnology | Cat#sc-8017 |
| K48-Ub (Apu2) | Merck Millipore | Cat#05–1307 |
| K63-Ub (Apu3) | Merck Millipore | Cat#05–1308 |
| Na/K ATPase (H-3) | Santa Cruz Biotechnology | Cat#sc-48345 |
| Rab5 (C8B1) | Cell Signaling Technology | Cat#3547S |
| Rab7 (D95F2) | Cell Signaling Technology | Cat#9367P |
| Rab9 (D52G8) | Cell Signaling Technology | Cat#5118S |
| EEA1 (3C10) | MBL | Cat#PM062 |
| Lamp1 (D2D11) | Cell Signaling Technology | Cat#9091P |
| CFTR (L12B4) | Merck Millipore | Cat#MAB3484 |
| CFTR (M3A7) | Merck Millipore | Cat#MAB3480 |
| RFFL | Sigma | Cat#HPA019492 |
| RFFL | GeneTex | Cat#GTX117832 |
| V5 (6F5) | Wako | Cat#1123591 |
| Hsc70 (B-6) | Santa Cruz Biotechnology | Cat#sc-7298 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| WGA-Alexa Fluor 594 | ThermoFisher | Cat#W11262 |
| DAPI | Wako | Cat#340–07971 |
| LysoTracker Red DND-99 | ThermoFisher | Cat#L7528 |
| VX-809 | AdooQ BioScience | Cat#A10986 |
| VX-770 | AdooQ BioScience | Cat#A10985 |
| corrector 4a (C4) | Merck Millipore | Cat#219673 |
| glycerol | Sigma | Cat#G6279 |
| cycloheximide | Wako | Cat#3720991 |
| MG-132 | Cayman | Cat#10012628 |
| Bafilomycin A1 | Adipogen Life Sciences | Cat#BVT-0252-C100 |
| PR-619 | Merck Millipore | Cat#662141 |
| leupeptin | Wako | Cat#122–03751 |
| Biotin | Wako | Cat#023–08716 |
| Doxycycline | Wako | Cat#049–31121 |
| NEM | Wako | Cat#054–02063 |
| Forskolin | Wako | Cat#067–02191 |
| cpt-cAMP | Santa Cruz Biotechnology | Cat#sc-201569A |
| IBMX | Wako | Cat#095–03413 |
| Transferrin Biotin-XX conjugate | ThermoFisher | Cat#T23363 |
| Lipofectamine RNAiMax | ThermoFisher | Cat#13778150 |
| Neutravidin agarose | ThermoFisher | Cat#29200 |
| Neutravidin coated 96 well white plate | ThermoFisher | Cat#15116 |
| SuperSignal West Pico Chemiluminescent Substrate | ThermoFisher | Cat#34080 |
| Amplex Red | ThermoFisher | Cat#A12222 |
| HRP-neutravidin | ThermoFisher | Cat#31001 |
| Alexa Fluor 488-Streptavidin | ThermoFisher | Cat#S11223 |
| His6-Ubc13/Uev1a complex | Boston Biochem | Cat#E2–664 |
| His6-UBE1 | Boston Biochem | Cat#E-304 |
| His6-sumo-UbcH5c | Boston Biochem | Cat#E2–627 |
| Ub | Boston Biochem | Cat#U-100H |
| Ub-63R | Boston Biochem | Cat#UM-K63R |
| Ub-63K | Boston Biochem | Cat#UM-K630 |
| Hsc70 | StressMarq Biosciences | Cat#SPR-106B |
| Experimental Models: Cell Lines | ||
| CFBE-CFTR-3HA | This paper | N/A |
| CFBE-CFTR-Ub-3HA | This paper | N/A |
| CFBE-CFTR-Ub(AllRΔG)-3HA | This paper | N/A |
| CFBE-ΔF508 CFTR-3HA | (Veit et al., 2012) | N/A |
| CFBE-teton-ΔF508 CFTR-3HA | (Veit et al., 2012) | N/A |
| CFBE-teton-P67L CFTR-3HA | (Veit et al., 2014) | N/A |
| CFBE-teton-ΔF508 CFTR-HRP | (Veit et al., 2012) | N/A |
| CFBE-teton-WT CFTR-HRP | (Veit et al., 2012) | N/A |
| CFBE-teton-T70 CFTR-HRP | This paper | N/A |
| CFBE-teton-HBH-ΔF508 CFTR | This paper | N/A |
| CFBE-teton-HBH-T70 CFTR | This paper | N/A |
| CFBE-teton-HBH-WT CFTR | This paper | N/A |
| CFBE-teton-ΔF508 CFTR-3HA shNT | This paper | N/A |
| CFBE-teton-ΔF508 CFTR-3HA shRFFL-1 | This paper | N/A |
| CFBE-teton-CD4T | This paper | N/A |
| CFBE-teton-CD4TL-lamda | This paper | N/A |
| CFBE-teton-CD4TccUbAllRΔG | This paper | N/A |
| CFBE-teton-CD4Tl-Lamp1 | This paper | N/A |
| CFBE-teton-TMEM16A | (Veit et al., 2012) | N/A |
| NCI-H441-teton-ΔF508-CFTR-3HA | (Veit et al., 2012) | N/A |
| CFPAC-1 | ATCC | Cat#CRL-1918 |
| CFPAC-1-teton-ΔF508-CFTR-3HA | This paper | N/A |
| HeLa-ΔF508 CFTR-3HA | (Okiyoneda et al., 2010) | N/A |
| 293MSR | ThermoFisher | Cat#R79507 |
| 293MSR-ΔF508 CFTR-3HA | This paper | N/A |
| COS7 | JCRB cell bank | Cat#JCRB9127 |
| BHK | (Glozman et al., 2009) | N/A |
| BHK-HBH-ΔF508-CFTR-3HA | This paper | N/A |
| BHK-HBH-CFTR-3HA | This paper | N/A |
| CFBE-teton-ΔF508-CFTR-3HA, YFP-H148Q/I152L/F46L | (Veit et al., 2014) | N/A |
| Calu-3 | ATCC | Cat#HTB-55 |
| Primary HBE WT/WT (age 50, male) | CFTRc | BD150412 |
| Primary HBE WT/WT (unknown age and gender) | CFTRc | BD201111 |
| Primary HBE WT/WT (unknown age and gender) | WE. Finkbeiner (UCSF) | HTBE 13–21 |
| Primary HBE WT/WT (unknown age and gender) | WE. Finkbeiner (UCSF) | HBE 13–39 |
| Primary HBE WT/WT (unknown age and gender) | WE. Finkbeiner (UCSF) | HBE 13–41 |
| Primary CF-HBE ΔF508/ΔF508 (age 30, female) | CFTRc | BCF130409 |
| Primary CF-HBE ΔF508/ΔF508 (age 41, male) | CFTRc | BCF1201209 |
| Primary CF-HBE ΔF508/ΔF508 (age 32, male) | CFTRc | BCF43 |
| Primary CF-HBE ΔF508/ΔF508 (unknown age and gender) | CFTRc | BCF060314 |
| Primary CF-HBE ΔF508/ΔF508 (unknown age and gender) | WE. Finkbeiner (UCSF) | CFBE 13–35 |
| Oligonucleotides | ||
| CFTR-FW (for q-PCR) | This paper | AGTGGAGGAAAGCCTTTGGAGT |
| CFTR-RV (for q-PCR) | This paper | ACAGATCTGAGCCCAACCTCA |
| RFFL-FW (for q-PCR) | This paper | CCCTCGCCTCCTCTCCTA |
| RFFL-RV (for q-PCR) | This paper | GTGGGACCTCCTCAGGCT |
| GAPDH-FW (for q-PCR) | This paper | CATGAGAAGTATGACAACAGCCT |
| GAPDH-RV (for q-PCR) | This paper | AGTCCTTCCACGATACCAAAGT |
| Recombinant DNA | ||
| pLKO-blast-scramble (shNT) | (Bryant et al., 2010) | addgene #26701 |
| shRFFL-1 pLKO-blast | This paper | N/A |
| pDest-eGFP-N1 | (Hong et al., 2010) | addgene #31796 |
| RFFL-GFP | This paper | N/A |
| RFFLΔNT-GFP | This paper | N/A |
| RFFLΔFYVE-GFP | This paper | N/A |
| RFFLΔDR3-GFP | This paper | N/A |
| RFFLΔCID-GFP | This paper | N/A |
| RFFLΔRING-GFP | This paper | N/A |
| mRFP-Rab5 | (Vonderheit and Helenius, 2005) | addgene #14437 |
| Lamp1-RFP | (Sherer et al., 2003) | addgene #1817 |
| mRFP-Rab7 | (Vonderheit and Helenius, 2005) | addgene #14436 |
| RFFL-HB | This paper | N/A |
| RFFLΔNT-HB | This paper | N/A |
| RFFLΔFYVE-HB | This paper | N/A |
| RFFLΔDR3-HB | This paper | N/A |
| RFFLΔCID-HB | This paper | N/A |
| RFFLΔRING-HB | This paper | N/A |
| RFFL-2CA-HB | This paper | N/A |
| RFFLΔDR1-HB | This paper | N/A |
| RFFLΔDR2-HB | This paper | N/A |
| RFFL-C5610A-HB | This paper | N/A |
| RFFLΔ2–10-HB | This paper | N/A |
| RFFL1–38-HB | This paper | N/A |
| RFFL1–88-HB | This paper | N/A |
| RFFL1–120-HB | This paper | N/A |
| RFFL1–148-HB | This paper | N/A |
| RFFL1–200-HB | This paper | N/A |
| RFFL1–220-HB | This paper | N/A |
| RFFLΔNYAP-HB | This paper | N/A |
| pLX304 | (Yang et al., 2011) | addgene #25890 |
| GFP-V5 | This paper | N/A |
| RFFL1-V5 | This paper | N/A |
| ΔF508 CFTR-VN | This paper | N/A |
| RFFL-VC | This paper | N/A |
| RFFLΔNT-VC | This paper | N/A |
| pET26b-NBD1 ΔF-1S | (Rabeh et al., 2012) | N/A |
| pET26b-NBD1 WT-1S | (Rabeh et al., 2012) | N/A |
| pCold1-His-SUMO RFFL | This paper | N/A |
| pCold1-His-SUMO RFFLΔRING | This paper | N/A |
| pCold1-His- SUMO UbcH5c | This paper (Y. Sato, Univ. of Tokyo) | N/A |
| pDest-GST-RFFL | This paper | N/A |
| pDest-GST-RFFLΔ2–148 | This paper | N/A |
| pDest-GST-RFFL-H333A | This paper | N/A |
| Software and Algorithms | ||
| PONDR® | (Romero et al., 2001) | http://www.pondr.com |
Statistical analysis
For quantification, replicate experiments were repeated at least two times, and data were expressed at means ± SEM. Statistical significance was assessed by two-tailed paired Student’s t test using Excel software (Microsoft).
Supplementary Material
Acknowledgements
We thank S. Suzuki, M. Morikawa-Okiyoneda for technical supports, Y. Sato, C. Wolberger, R. Shaw, A. Helenius, W. Mothes, C. Hu, K. Mostov, D. Root, R. Shaw, CD. Hu for plasmids and WE. Finkbeiner for primary cells. We also thank G. Lesage for preparation of custom E3 siRNA library and T. Harris for carefully reading the manuscript. This work was supported by JSPS KAKENHI Grant Numbers JP25893275, 15H05643, 15H01192, grants from The Mitsubishi Foundation, Uehara Memorial Foundation, The Sumitomo Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Astellas Foundation for Research on Metabolic Disorders to TO, Work in GLL lab was supported by NIH-NIDDK, CIHR and Cystic Fibrosis Canada. GL Lukacs is a recipient of a Canada Research Chair.
Footnotes
Declaration of Interests
The authors declare no competing financial interests. T.O. has a patent pending in Japan for methodology to identify inhibitors of RFFL-mediated CFTR ubiquitination (2017–047626).
References
- Ahmed AU, Moulin M, Coumailleau F, Wong WW, Miasari M, Carter H, Silke J, Cohen-Tannoudji M, Vince JE, and Vaux DL (2009). CARP2 deficiency does not alter induction of NF-kappaB by TNFalpha. Curr Biol 19, R15–17; [DOI] [PubMed] [Google Scholar]
- Apaja PM, Foo B, Okiyoneda T, Valinsky WC, Barriere H, Atanasiu R, Ficker E, Lukacs GL, and Shrier A (2013). Ubiquitination-dependent quality control of hERG K+ channel with acquired and inherited conformational defect at the plasma membrane. Mol Biol Cell 24, 3787–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apaja PM, Xu H, and Lukacs GL (2010). Quality control for unfolded proteins at the plasma membrane. J Cell Biol 191, 553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Kawamura M, Suzuki T, Matsuda N, Kanbe D, Ishii K, Ichikawa T, Kumanishi T, Chiba T, Tanaka K, et al. (2003). A palmitoylated RING finger ubiquitin ligase and its homologue in the brain membranes. J Neurochem 86, 749–762. [DOI] [PubMed] [Google Scholar]
- Avramescu RG, Kai Y, Xu H, Bidaud-Meynard A, Schnúr A, Frenkiel S, Matouk E, Veit G, and Lukacs GL (2017). Mutation-specific downregulation of CFTR2 variants by gating potentiators. Hum Mol Genet 26, 4873–4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriere H, and Lukacs GL (2008). Analysis of endocytic trafficking by single-cell fluorescence ratio imaging. Curr Protoc Cell Biol [DOI] [PubMed] [Google Scholar]
- Barriere H, Nemes C, Du K, and Lukacs GL (2007). Plasticity of polyubiquitin recognition as lysosomal targeting signals by the endosomal sorting machinery. Mol Biol Cell 18, 3952–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriere H, Nemes C, Lechardeur D, Khan-Mohammad M, Fruh K, and Lukacs GL (2006). Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in Mammalian cells. Traffic 7, 282–297. [DOI] [PubMed] [Google Scholar]
- Bomberger JM, Barnaby RL, and Stanton BA (2009). The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem 284, 18778–18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL (2012). Cleaning up: ER-associated degradation to the rescue. Cell 151, 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, and Mostov KE (2010). A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12, 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caohuy H, Jozwik C, and Pollard HB (2009). Rescue of DeltaF508-CFTR by the SGK1/Nedd4–2 signaling pathway. J Biol Chem 284, 25241–25253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, and Guggino W (2013). Ubiquitination and degradation of CFTR by the E3 ubiquitin ligase MARCH2 through its association with adaptor proteins CAL and STX6. PLoS One 8, e68001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholon DM, Quinney NL, Fulcher ML, Esther CR, Das J, Dokholyan NV, Randell SH, Boucher RC, and Gentzsch M (2014). Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med 6, 246ra296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague MJ, and Urbé S (2010). Ubiquitin: same molecule, different degradation pathways. Cell 143, 682–685. [DOI] [PubMed] [Google Scholar]
- Collins FS (1992). Cystic fibrosis: molecular biology and therapeutic implications. Science 256, 774–779. [DOI] [PubMed] [Google Scholar]
- Coumailleau F, Das V, Alcover A, Raposo G, Vandormael-Pournin S, Le Bras S, Baldacci P, Dautry-Varsat A, Babinet C, and Cohen-Tannoudji M (2004). Over-expression of Rififylin, a new RING finger and FYVE-like domain-containing protein, inhibits recycling from the endocytic recycling compartment. Mol Biol Cell 15, 4444–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq JP, and Lazdunski M (1991). Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 354, 526–528. [DOI] [PubMed] [Google Scholar]
- Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, and Welsh MJ (1992). Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358, 761–764. [DOI] [PubMed] [Google Scholar]
- Du K, Sharma M, and Lukacs GL (2005). The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol 12, 17–25. [DOI] [PubMed] [Google Scholar]
- Ehrhardt C, Collnot EM, Baldes C, Becker U, Laue M, Kim KJ, and Lehr CM (2006). Towards an in vitro model of cystic fibrosis small airway epithelium: characterisation of the human bronchial epithelial cell line CFBE41o-. Cell Tissue Res 323, 405–415. [DOI] [PubMed] [Google Scholar]
- El Khouri E, Le Pavec G, Toledano MB, and Delaunay-Moisan A (2013). RNF185 is a novel E3 ligase of endoplasmic reticulum-associated degradation (ERAD) that targets cystic fibrosis transmembrane conductance regulator (CFTR). J Biol Chem 288, 31177–31191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer F, Hamann A, and Osiewacz HD (2012). Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci 37, 284–292. [DOI] [PubMed] [Google Scholar]
- Fu L, Rab A, Tang L, Bebok Z, Rowe SM, Bartoszewski R, and Collawn JF (2015). ΔF508 CFTR surface stability is regulated by DAB2 and CHIP-mediated ubiquitination in post-endocytic compartments. PLoS One 10, e0123131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Rab A, Tang LP, Rowe SM, Bebok Z, and Collawn JF (2012). Dab2 is a key regulator of endocytosis and post-endocytic trafficking of the cystic fibrosis transmembrane conductance regulator. Biochem J 441, 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Wang C, Patel M, Kreutz B, Zhou M, Kozasa T, and Wu D (2013). Different Raf protein kinases mediate different signaling pathways to stimulate E3 ligase RFFL gene expression in cell migration regulation. J Biol Chem 288, 33978–33984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Wang J, Wang C, Sommer E, Kozasa T, Srinivasula S, Alessi D, Offermanns S, Simon MI, and Wu D (2012). PRR5L degradation promotes mTORC2-mediated PKC-δ phosphorylation and cell migration downstream of Gα12. Nat Cell Biol 14, 686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RG, Nelson ZW, and Gottschling DE (2005). Degradation-mediated protein quality control in the nucleus. Cell 120, 803–815. [DOI] [PubMed] [Google Scholar]
- Glozman R, Okiyoneda T, Mulvihill CM, Rini JM, Barriere H, and Lukacs GL (2009). N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J Cell Biol 184, 847–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove DE, Rosser MF, Ren HY, Naren AP, and Cyr DM (2009). Mechanisms for rescue of correctable folding defects in CFTRDelta F508. Mol Biol Cell 20, 4059–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haardt M, Benharouga M, Lechardeur D, Kartner N, and Lukacs GL (1999). C-terminal truncations destabilize the cystic fibrosis transmembrane conductance regulator without impairing its biogenesis. A novel class of mutation. J Biol Chem 274, 21873–21877. [DOI] [PubMed] [Google Scholar]
- Hegde RN, Parashuraman S, Iorio F, Ciciriello F, Capuani F, Carissimo A, Carrella D, Belcastro V, Subramanian A, Bounti L, et al. (2015). Unravelling druggable signalling networks that control F508del-CFTR proteostasis. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein C, Springael JY, Volland C, Haguenauer-Tsapis R, and André B (1995). NPl1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin-protein ligase. Mol Microbiol 18, 77–87. [DOI] [PubMed] [Google Scholar]
- Hofmann RM, and Pickart CM (1999). Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96, 645–653. [DOI] [PubMed] [Google Scholar]
- Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, and Shaw RM (2010). BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol 8, e1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Zeng X, Kim W, Balasubramani M, Fortian A, Gygi SP, Yates NA, and Sorkin A (2013). Lysine 63-linked polyubiquitination is required for EGF receptor degradation. Proc Natl Acad Sci U S A 110, 15722–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, and Barry PJ (2015). Lumacaftor/ivacaftor for patients homozygous for Phe508del-CFTR: should we curb our enthusiasm? Thorax 70, 615–616. [DOI] [PubMed] [Google Scholar]
- Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, and Deen PM (2006). Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci U S A 103, 18344–18349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y, and Hu CD (2010). An improved bimolecular fluorescence complementation assay with a high signal-to-noise ratio. Biotechniques 49, 793–805. [DOI] [PubMed] [Google Scholar]
- Lauwers E, Jacob C, and André B (2009). K63-linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J Cell Biol 185, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kane T, Tipper C, Spatrick P, and Jenness DD (1999). Yeast mutants affecting possible quality control of plasma membrane proteins. Mol Cell Biol 19, 3588–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, MacGurn JA, Chu T, Stefan CJ, and Emr SD (2008). Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 135, 714–725. [DOI] [PubMed] [Google Scholar]
- McDonald ER, and El-Deiry WS (2004). Suppression of caspase-8- and −10-associated RING proteins results in sensitization to death ligands and inhibition of tumor cell growth. Proc Natl Acad Sci U S A 101, 6170–6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham GC, Patterson C, Zhang W, Younger JM, and Cyr DM (2001). The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol 3, 100–105. [DOI] [PubMed] [Google Scholar]
- Morito D, Hirao K, Oda Y, Hosokawa N, Tokunaga F, Cyr DM, Tanaka K, Iwai K, and Nagata K (2008). Gp78 cooperates with RMA1 in endoplasmic reticulum-associated degradation of CFTRDeltaF508. Mol Biol Cell 19, 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, and Maxfield FR (1997). Endocytosis. Physiol Rev 77, 759–803. [DOI] [PubMed] [Google Scholar]
- Neuberger T, Burton B, Clark H, and Van Goor F (2011). Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the pre-clinical testing of CFTR modulators. Methods Mol Biol 741, 39–54. [DOI] [PubMed] [Google Scholar]
- Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, et al. (2008). Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134, 668–678. [DOI] [PubMed] [Google Scholar]
- Okiyoneda T, Apaja PM, and Lukacs GL (2011). Protein quality control at the plasma membrane. Curr Opin Cell Biol 23, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiyoneda T, Barrière H, Bagdány M, Rabeh WM, Du K, Höhfeld J, Young JC, and Lukacs GL (2010). Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329, 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiyoneda T, Veit G, Dekkers JF, Bagdany M, Soya N, Xu H, Roldan A, Verkman AS, Kurth M, Simon A, et al. (2013). Mechanism-based corrector combination restores ÄF508-CFTR folding and function. Nat Chem Biol 9, 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow S, Bamberger C, Calzolari D, Martínez-Bartolomé S, Lavallée-Adam M, Balch WE, and Yates JR (2015). ΔF508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature 528, 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechmann S, Willmund F, and Frydman J (2013). The ribosome as a hub for protein quality control. Mol Cell 49, 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuan PW, Veit G, Tan J, Roldan A, Finkbeiner WE, Lukacs GL, and Verkman AS (2014). Synergy-based small-molecule screen using a human lung epithelial cell line yields brosis. Natureienceators.t augment VX-809 maximal efficacy. Mol Pharmacol 86, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabeh WM, Bossard F, Xu H, Okiyoneda T, Bagdany M, Mulvihill CM, Du K, di Bernardo S, Liu Y, Konermann L, et al. (2012). Correction of Both NBD1 Energetics and Domain Interface Is Required to Restore Delta F508 CFTR Folding and Function. Cell 148, 150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Osterhaus SR, Parekh KR, Jacobi AM, Behlke MA, and McCray PB (2016). SYVN1, NEDD8, and FBXO2 Proteins Regulate a human lung epithelial celmembrane Conductance Regulator (CFTR) Ubiquitin-mediated Proteasomal Degradation. J Biol Chem 291, 25489–25504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR (2008). CFTR function and prospects for therapy. Annu Rev Biochem 77, 701–726. [DOI] [PubMed] [Google Scholar]
- Rohrer J, Schweizer A, Russell D, and Kornfeld S (1996). The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J Cell Biol 132, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, and Dunker AK (2001). Sequence complexity of disordered protein. Proteins 42, 38–48. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JC, Fredrickson EK, Oeser ML, Garrett-Engele CM, Locke MN, Richardson LA, Nelson ZW, Hetrick ED, Milac TI, Gottschling DE, et al. (2011). Disorder targets misorder in nuclear quality control degradation: a disordered ubiquitin ligase directly recognizes its misfolded substrates. Mol Cell 41, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabusap CM, Wang W, McNicholas CM, Chung WJ, Fu L, Wen H, Mazur M, Kirk KL, Collawn JF, Hong JS, et al. (2016). Analysis of cystic fibrosis-associated P67L CFTR illustrates barriers to personalized therapeutics for orphan diseases. JCI Insight 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Ward CL, Krouse ME, Wine JJ, and Kopito RR (1996). Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J Biol Chem 271, 635–638. [DOI] [PubMed] [Google Scholar]
- Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, et al. (2004). Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol 164, 923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Ingmundson A, Horner SM, Cicchetti G, Allen PG, Pypaert M, Cunningham JM, and Mothes W (2003). Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic 4, 785–801. [DOI] [PubMed] [Google Scholar]
- Stolz A, Besser S, Hottmann H, and Wolf DH (2013). Previously unknown role for the ubiquitin ligase Ubr1 in endoplasmic reticulum-associated protein degradation. Proc Natl Acad Sci U S A 110, 15271–15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatecka-Urban A, Brown A, Moreau-Marquis S, Renuka J, Coutermarsh B, Barnaby R, Karlson KH, Flotte TR, Fukuda M, Langford GM, et al. (2005). The short apical membrane half-life of rescued {Delta}F508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of {Delta}F508-CFTR in polarized human airway epithelial cells. J Biol Chem 280, 36762–36772. [DOI] [PubMed] [Google Scholar]
- Tagwerker C, Flick K, Cui M, Guerrero C, Dou Y, Auer B, Baldi P, Huang L, and Kaiser P (2006). A tandem affinity tag for two-step purification under fully denaturing conditions: application in ubiquitin profiling and protein complex identification combined with in vivocross-linking. Mol Cell Proteomics 5, 737–748. [DOI] [PubMed] [Google Scholar]
- Tibbetts MD, Shiozaki EN, Gu L, McDonald ER, El-Deiry WS, and Shi Y (2004). Crystal structure of a FYVE-type zinc finger domain from the caspase regulator CARP2. Structure 12, 2257–2263. [DOI] [PubMed] [Google Scholar]
- Trzcinska-Daneluti AM, Nguyen L, Jiang C, Fladd C, Uehling D, Prakesch M, Al-awar R, and Rotin D (2012). Use of kinase inhibitors to correct se regulator CARP2. Structureditions: ap 11, 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzcińska-Daneluti AM, Chen A, Nguyen L, Murchie R, Jiang C, Moffat J, Pelletier L, and Rotin D (2015). RNA Interference Screen to Identify Kinases That Suppress Rescue of ΔF508-CFTR. Mol Cell Proteomics 14, 1569–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, et al. (2011). Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 108, 18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Goldstein RF, Jurkuvenaite A, Chen L, Matalon S, Sorscher EJ, Bebok Z, and Collawn JF (2008). Enhanced cell-surface stability of rescued DeltaF508 cystic fibrosis transmembrane conductance regulator (CFTR) by pharmacological chaperones. Biochem J 410, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE, et al. (2016). From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 27, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, et al. (2014). Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med 6, 246ra297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G, Bossard F, Goepp J, Verkman AS, Galietta LJ, Hanrahan JW, and Lukacs GL (2012). Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell 23, 4188–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheit A, and Helenius A (2005). Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol 3, e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, et al. (2015). Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med 373, 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Thibault G, and Ng DT (2011). Routing misfolded proteins through the multivesicular body (MVB) pathway protects against proteotoxicity. J Biol Chem 286, 29376–29387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt A, Yerbury J, Poon S, Dabbs R, and Wilson M (2009). Chapter 6: The chaperone action of Clusterin and its putative role in quality control of extracellular protein folding. Adv Cancer Res 104, 89–114. [DOI] [PubMed] [Google Scholar]
- Yang X, Boehm JS, Salehi-Ashtiani K, Hao T, Shen Y, Lubonja R, Thomas SR, Alkan O, Bhimdi T, Green TM, et al. (2011). A public genome-scale lentiviral expression library of human ORFs. Nat Methods 8, 659–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Cihil K, Stolz DB, Pilewski JM, Stanton BA, and Swiatecka-Urban A (2010). c-Cbl facilitates endocytosis and lysosomal degradation of cystic fibrosis transmembrane conductance regulator in human airway epithelial cells. J Biol Chem 285, 27008–27018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger JM, Chen L, Ren HY, Rosser MF, Turnbull EL, Fan CY, Patterson C, and Cyr DM (2006). Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell 126, 571–582. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xu M, Scotti E, Chen ZJ, and Tontonoz P (2013). Both K63 and K48 ubiquitin linkages signal lysosomal degradation of the LDL receptor. J Lipid Res 54, 1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Macgurn JA, Liu M, and Emr S (2013). The ART-Rsp5 ubiquitin ligase network comprises a plasma membrane quality control system that protects yeast cells from proteotoxic stress. Elife 2, e00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.