SUMMARY
Environmental stress threatens the fidelity of embryonic morphogenesis. Heat, for example, is a teratogen. Yet how heat affects morphogenesis is poorly understood. Here, we identify a heat-inducible actin stress response (ASR) in Drosophila embryos that is mediated by the activation of the actin regulator Cofilin. Similar to ASR in adult mammalian cells, heat stress in fly embryos triggers the assembly of intra-nuclear actin rods. Rods measure up to a few microns in length, and their assembly depends on elevated free nuclear actin concentration and Cofilin. Outside the nucleus, heat stress causes Cofilin-dependent destabilization of filamentous actin (F-actin) in actomyosin networks required for morphogenesis. F-actin destabilization increases the chance of morphogenesis mistakes. Blocking the ASR by reducing Cofilin dosage improves the viability of heat-stressed embryos. However, improved viability correlates with restoring F-actin stability, not rescuing morphogenesis. Thus, ASR endangers embryos, perhaps by shifting actin from cytoplasmic filaments to an elevated nuclear pool.
Graphical Abstract
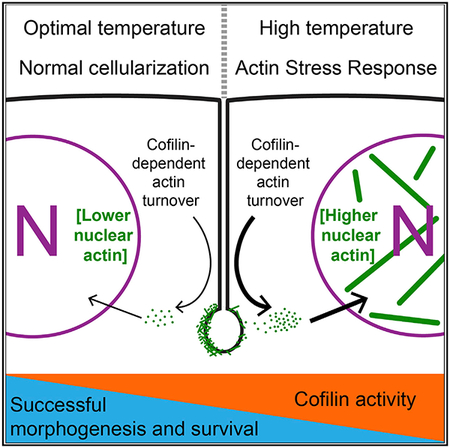
In Brief
Figard et al. show that heat stress induces an actin stress response (ASR) in early Drosophila embryos. This ASR is mediated by a heat-induced increase in Cofilin activity. Increased Cofilin activity destabilizes F-actin structures required for morphogenesis. In addition, the Cofilin-mediated ASR reduces embryo viability.
INTRODUCTION
Environmental stress significantly challenges developing embryos. In humans, prenatal exposure to hypoxia, drugs, pathogens, or high temperature is associated with increased risk of fetal death or physical malformations and/or defects following birth (Edwards, 2006; Dixon et al., 2011; Hamdoun and Epel, 2007). Environmental stress disrupts cellular function in embryos by generating reactive oxygen species, altering gene expression, inducing apoptosis and heterochronicity, and disrupting signaling networks (Parman et al., 1999; Puscheck et al., 2015; Salilew-Wondim et al., 2014; Crews et al., 2016). Whether these stress-induced disruptions impinge on the actin cytoskeleton, the ultimate architectural driver of embryonic morphogenesis, remains unknown.
Recently, the actin cytoskeleton itself has emerged as a mediator of stress response (Amberg et al., 2012; Baird et al., 2014; Bernstein et al., 2006; Chambers et al., 2015; Higuchi et al., 2013). Many cell types, including neurons, myocytes, and epithelial cells, reorganize their F-actin upon exposure to heat or oxidative stress as part of an inducible actin stress response (ASR; reviewed in Bamburg and Bernstein [2016]; Kanellos and Frame [2016]; Munsie and Truant [2012]). This F-actin reorganization is typified by the assembly of actin “rods” in the nucleus or cytoplasm of affected cells (Ashworth et al., 2003; Iida et al., 1986; Minamide et al., 2000; Ono et al., 1993; Sanger et al., 1980; Vandebrouck et al., 2010). In transiently stressed neuronal cells, rod assembly promotes increased cell survival through an unknown mechanism (Bernstein et al., 2006; Munsie et al., 2012). However, the full extent of the ASR’s protective value is still undefined in most cells and is likely to be context dependent.
So far, the ASR has not been described in any embryo. What’s more, like other inducible stress responses in early embryos, it is unclear whether an ASR would be protective or would adversely divert normal developmental programs (Hamdoun and Epel, 2007). Here, we identify a maladaptive ASR in heat-stressed Drosophila embryos. This ASR destabilizes cytoplasmic F-actin structures, compromises cellularization—the first morphogenetic event in fruit fly development—and reduces embryo viability. Intriguingly, reduced viability appears to be more of a consequence of F-actin destabilization than of mild morphogenesis mistakes.
RESULTS
Heat Stress Induces Intra-Nuclear Actin Rod Assembly in Embryos
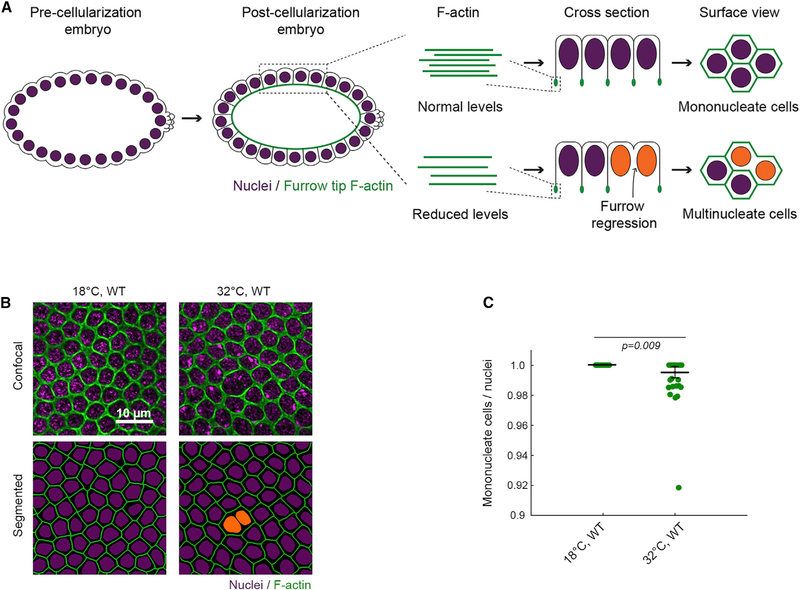
During cellularization, plasma membrane furrows invaginate synchronously to package ~6,000 nuclei of the syncytial embryo into a sheet of mononucleate epithelial cells that immediately goes on to gastrulate and eventually forms the larva. Furrow invagination depends on assembly of F-actin and Myosin-2 structures at furrow tips (Sokac and Wieschaus, 2008; Figure 1A, cross section). Considered in three dimensions, these furrow tip structures make a hexagonal network across the embryo surface, with each hexagon encircling one nucleus (Figure 1A, surface view). Mutations in positive regulators of F-actin (e.g., Diaphanous/Formin) reduce F-actin levels at all furrow tips and result in the regression of a fraction of furrows (Padash Barmchi et al., 2005; Grosshans et al., 2005; Sokac and Wieschaus, 2008; Zheng et al., 2013). Furrow regressions manifest as multinucleate cells. Thus, multinucleation serves as a proxy for compromised F-actin function during cellularization.
Figure 1. Heat Stress Leads to Cellularization Failures.
(A) In embryos with normal levels of F-actin (green), furrows ingress between nuclei (purple) to form mononucleate cells. In embryos with reduced F-actin, some furrows regress, resulting in multinucleate cells (orange nuclei).
(B) Surface views show furrow tip F-actin (phalloidin, green) and nuclei (Hoechst, purple) in wild-type (WT) embryos at indicated temperatures. Multinucleate cells highlighted by orange nuclei in corresponding segmented images.
(C) Severity of multinucleation in embryos at indicated temperatures. Each point represents one embryo (n = 52 embryos per genotype, with 150 nuclei analyzed per embryo; horizontal lines are means ± SE).
Student’s t test used to calculate p value in (C).
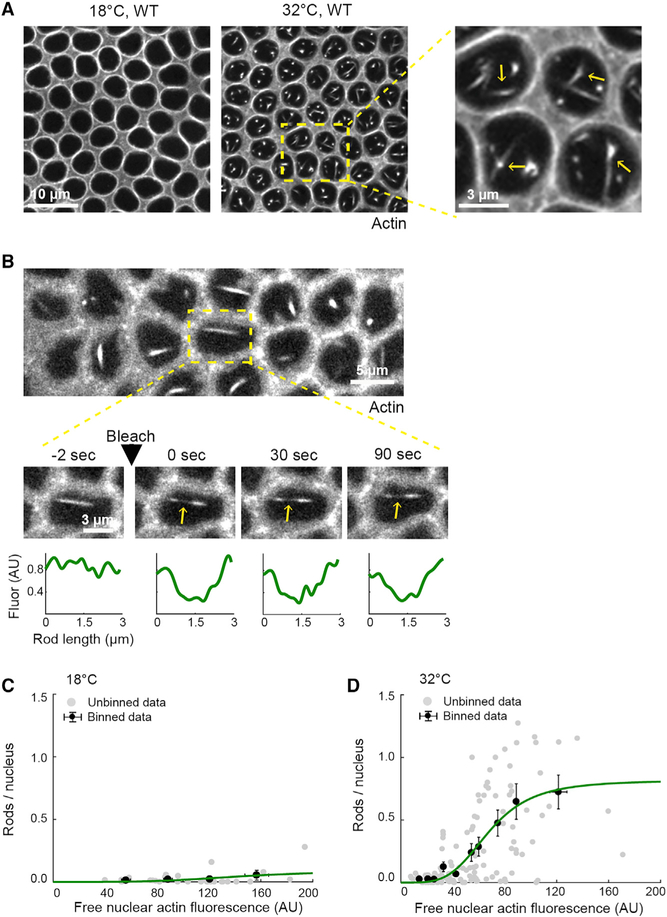
We previously found that wild-type embryos are prone to multi-nucleation when reared under heat stress (Figures 1B and 1C; Zheng et al., 2013), leading us to hypothesize that F-actin is somehow disrupted by high temperature. To test this hypothesis, we first visualized the actin cytoskeleton in live wild-type embryos injected with rhodamine labeled G-actin (G-actinRed). We chose G-actinRed as a probe, because it readily incorporates into furrow tips during cellularization with no known adverse effects on the process (Cao et al., 2008; Xue and Sokac, 2016). Embryos were injected with G-actinRed at 18°C and then imaged at either 18°C or under heat stress at 32°C (Table S1). This is a mild heat stress, given that standard Culturing conditions are 18°C–29°C, and heat shock experiments in Drosophila typically use ≥ 37°C (e.g., Bergh and Arking, 1984; Crews et al., 2016). In injected embryos, single plane, confocal surface views captured furrow tip F-actin, as well as cross sections through nuclei. Strikingly, numerous actin rods were seen inside the nuclei of embryos at 32°C (Figures 2A and S1A). These rods resembled actin rods previously associated with the ASR in heat and oxidatively stressed adult cell types (Kanellos and Frame, 2016).
Figure 2. Intra-Nuclear Actin Rods Assemble in Heat-Stressed Embryos.
(A) Surface views show G-actinRed in furrow tips in live wild-type (WT) embryos at indicated temperatures. Intra-nuclear rods assemble at 32°C (yellow arrows, higher-magnification inset).
(B) Surface views show FRAP of a rod (G-actinRed) in a WT embryo at 32°C. Higher-magnification time-lapse images show boxed ROI, and yellow arrows indicate bleached mid-section of a rod. FRAP kinetics for that rod plotted below.
(C and D) Rod abundance (rods/nucleus) versus free nuclear actin fluorescence in live WT embryos at 18°C (C) and 32°C (D) (n ≥ 31 embryos, with rod abundance counted in ≥ 60 nuclei per embryo; free actin fluorescence averaged from three nuclei per embryo). Each gray point represents one embryo, and black points represent binned data (mean ± SE). The green line represents the binned data fitted to a Hill function with Hill coefficient = 4. Related data are shown in Figure S1.
Actin rods in stressed neurons are “stable,” meaning there is no actin subunit turnover along their length (Bernstein et al., 2006; Minamide et al., 2010). To assess subunit turnover in rods in embryos, we measured fluorescence recovery after photo-bleaching (FRAP) for actin in the central region of rods. Nuclear rods showed no recovery (Figure 2B), whereas F-actin in cytoplasmic furrow tips recovers in tens of seconds (Xue and Sokac, 2016). So, analogous to the ASR in neurons, rods are stable in heat-stressed embryos. Despite this stability, rod assembly is reversible if embryos are shifted back to lower temperature, as previously described for ASR in transiently stressed neurons (Figure S1B; Bamburg and Bernstein, 2016).
We wondered whether intra-nuclear rod assembly depends on free nuclear actin concentration. To probe this dependence, we injected wild-type embryos with a range of G-actinRed concentrations and scored for rod assembly (Figures 2C and 2D). For embryos at 32°C, rod numbers increased with free nuclear actin concentration (Figures 2D). For embryos at 18°C, a few rods formed, but only at ~3-fold higher free nuclear actin level compared to 32°C (Figures 2C and 2D). Thus, rod assembly is concentration dependent at both temperatures, but rods form at lower free actin levels during heat stress. The difference in rod assembly at 18°C versus 32°C was not due to a change in the ratio of free nuclear to free cytoplasmic actin (Figure S1C). Free nuclear and cytoplasmic actin concentrations show the same linear relationship to each other at 18°C and 32°C (Figure S1D), perhaps because embryos are undergoing rapid rounds of mitoses with partial nuclear envelope breakdown. So, regardless of temperature, actin is incorporated into newly reformed nuclei at levels reflecting its free cytoplasmic concentration. Consistent with this, the dependencies between rod assembly and either free nuclear or free cytoplasmic actin concentration were similar (Figures 2C, 2D, S1E, and S1F). Finally, we found a maximum level of free actin in the nucleus that is not exceeded at 32°C, but instead at which additional actin rods are made (Figure S1G). Together, these results support a model where the proportion of free nuclear to free cytoplasmic actin does not change with temperature; however, during heat stress, some additional activity lowers the threshold concentration for actin sequestration in rods.
Cofilin Promotes Actin Rod Assembly in Heat-Stressed Embryos
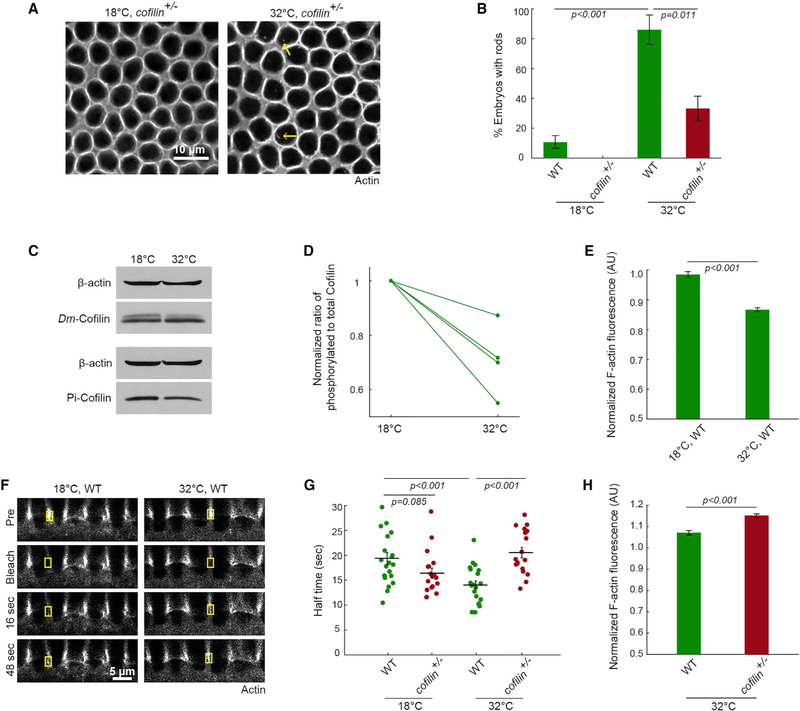
Next, we asked whether Cofilin provides the activity that promotes rod assembly in heat-stressed embryos. In mammalian cell types, ASR is mediated by stress-induced hyper-activation of Cofilin, an actin binding protein with diverse functions that influence both cytoplasmic and nuclear actin (Ashworth et al., 2003; Huang et al., 2008; Kim et al., 2009; Minamide et al., 2000; Ohta et al., 1989). Under normal conditions, Cofilin in the cytoplasm binds and severs F-actin to accelerate filament turnover (Andrianantoandro and Pollard, 2006). Cofilin also binds G-actin and works with importins to shuttle free actin into the nucleus (Dopie et al., 2012, 2015). Finally, Cofilin at high concentrations may stabilize F-actin regardless of cellular location (Andrianantoandro and Pollard, 2006; McCullough et al., 2008). During ASR, Cofilin has been shown to promote rod assembly via nuclear import of free actin and/or stabilization of actin rods (Bamburg et al., 2010; Munsie et al., 2012). To then assay Cofilin’s role in rod assembly in embryos, we injected G-actinRed into embryos expressing a reduced dose of Cofilin (cofilin+/−). These embryos express 60.5% ± 3.8% the wild-type level of Cofilin (mean ± SE; n = 4 experiments). Compared to wild-type, cofilin+/− embryos were less likely to form rods at 32°C (Figures 3A and 3B), supporting that Cofilin is required for rod assembly in heat-stressed embryos. We did not detect Cofilin in rods, despite trying several fixation protocols and Cofilin antibodies. Instead, given the actin concentration dependence of rod assembly, Cofilin may promote rods by somehow keeping free actin levels high in the cytoplasm and/or nucleus.
Figure 3. Cofilin Mediates an ASR that Changes Actin Organization in Nuclei and Cytoplasm.
(A) Surface views show G-actinRed in furrow tips in cofilin+/− embryos at the indicated temperatures. Intra-nuclear rods (yellow arrows) are reduced in cofilin+/− embryos at 32°C.
(B) Percentage of embryos with rods in wild-type (WT) and cofilin+/− embryos at the indicated temperatures (n ≥ 14 embryos per condition, with ~500 nuclei analyzed per embryo; mean ± SE).
(C) Representative western blots for Dm-Cofilin, Pi-cofilin from WT embryos at the indicated temperatures. β-actin is used as a loading control. Antibody validation in Figure S2.
(D) Ratio phosphorylated to total Cofilin in WT embryos at the indicated temperatures (n = 4 independent experiments; mean ± SE).
(E) F-actin levels in furrow tips in WT embryos at the indicated temperatures (n ≥ 29 embryos per temperature, with 15 furrows analyzed per embryo; mean ± SE). Normalization to Histone-GFP embryos at 25°C, according to Figure S3.
(F) Cross sections show FRAP of furrow tip F-actin (G-actinRed) in WT embryos at the indicated temperatures. Yellow boxes show bleached furrow tips. Pre, immediate pre-bleach time point; bleach, immediate post-bleach time point; sec, seconds after bleach.
(G) FRAP kinetics for furrow tip F-actin in WT and cofilin+/− embryos at indicated temperatures. Each point represents one embryo (n ≥ 14 embryos per temperature, with 1–3 furrows analyzed per embryo; horizontal lines represent means ± SE).
(H) F-actin levels in furrow tips in WT and cofilin+/− embryos at 32°C (n R 48 embryos per condition, with 15 furrows analyzed per embryo; mean ± SE). Normalization to Histone-GFP embryos at 32°C.
(E) and (H) correspond to scatterplots in Figures S4C and S4D.
Student’s t test used to calculate p values in (B), (E), (G), and (H).
Cofilin Upregulation Destabilizes Cytoplasmic F-Actin in Heat-Stressed Embryos
We examined Cofilin’s global activity state in whole embryo lysates following heat stress. When Cofilin is dephosphorylated at serine 3 (S3), it can bind G- or F-actin and so is considered active (Bravo-Cordero et al., 2013). Alternatively, when Cofilin is phosphorylated at S3, it cannot bind actin at all and is inactive. We made and validated a polyclonal antibody against Drosophila melanogaster Cofilin (Dm-Cofilin) that detects both phosphorylated and dephosphorylated Cofilin (top and bottom band, respectively; Figures 3C, S2A, and S2B). Using this antibody, the ratio of phosphorylated to total Cofilin was consistently reduced at 32°C (Figures 3C and 3D), indicating a shift of Cofilin to a higher activity state in heat-stressed embryos. Similarly, we saw a reproducible decrease in levels of phosphorylated Cofilin using a commercially available phospho-specific antibody (Pi-Cofilin; Figure 3C).
Apart from rod assembly, Cofilin’s role in F-actin severing predicts that its stress-induced activation would also destabilize normal cytoplasmic F-actin structures (e.g., Ashworth and Molitoris, 1999; Schwartz et al., 1999), and consequently alter the ratio of free to polymer-associated G-actin. In fact, destabilization could generate the free monomer pool that supports rod assembly. However, it is not known whether destabilization of cytoplasmic F-actin is a general feature of ASR. To address this issue, we imaged F-actin levels and dynamics at furrow tips, where Cofilin normally localizes during cellularization (Xue and Sokac, 2016). To quantify F-actin levels, wild-type embryos reared at either 18°C or 32°C were fixed and phalloidin stained in the same tube with internal control embryos reared at 25°C (Histone-GFP; Figure S3). This method avoided tube-to-tube variation in phalloidin staining and allowed normalization to the internal control embryos. We found that heat-stressed embryos have significantly less F-actin at furrow tips (Figures 3E, S3, and S4C). To assay F-actin stability, we performed FRAP using G-actinRed (i.e., faster turnover indicates reduced stability; Mukhina et al., 2007). The half time to recovery for F-actin in furrow tips in wild-type embryos was 19.33 ± 1.15 s at 18°C but sped up to 14.02 ± 0.81 s in embryos at 32°C (Figures 3F and 3G; mean ± SE). No change was seen for the mobile fraction (Figure S4A). These results suggest that the dynamic F-actin in furrow tips is less stable in heat-stressed embryos.
Next, we asked whether Cofilin is driving F-actin destabilization in heat-stressed embryos. In cofilin+/− embryos at 32°C, F-actin levels in furrow tips were fully restored to wild-type levels (normalization to Histone-GFP at 32°C; Figures 3H and S4D). Recovery by FRAP slowed to 20.54 ± 1.04 s (mean ± SE), equivalent to the turnover rate in unstressed wild-type embryos (Figure 3G). No change was seen for the mobile fraction (Figure S4B). Notably, F-actin was stabilized in cofilin+/− embryos only at 32°C, but not at 18°C (Figure 3G), consistent with Cofilin having potent, heat stress-specific, F-actin-destabilizing activity in embryos. Together, our data support a model whereby heat stress induces an ASR in embryos during which increased Cofilin activity alters the actin cytoskeleton in both the cytoplasm (furrow tip F-actin is destabilized) and the nucleus (actin rods assemble).
F-Actin Destabilization Puts Morphogenesis at Risk in Heat-Stressed Embryos
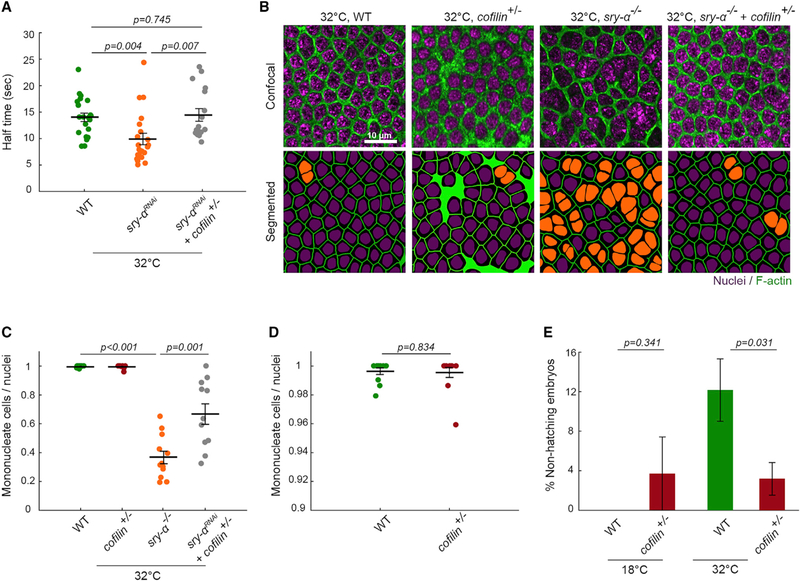
Although stress responses are typically considered protective, we suspected that F-actin destabilization at furrow tips during embryonic ASR is deleterious and could explain why cellularization shows increased multinucleation at 32°C (Figures 1B and 1C). To examine a causal link between F-actin stability and multi-nucleation, we genetically modulated F-actin stability using Cofilin and another F-actin binding protein Sry-α. Sry-α is a positive regulator of F-actin levels in furrow tips and was previously shown to promote successful cellularization at 32°C (Zheng et al., 2013). We knocked down Sry-α function in embryos by RNAi (sry-αRNAi) and found that F-actin at furrow tips turns over very fast at 32°C, with a half time to recovery of 9.93 ± 1.07 s (mean ± SE; Figure 4A), indicating severe destabilization. Again, no change was seen for the mobile fraction (Figure S4E). F-actin destabilization correlated with frequent multinucleation in sry-αRNAi embryos, and sry-α null mutants (sry-α−/−; Figures 4B and 4C). F-actin stability could be restored in sry-αRNA embryos by simultaneously reducing the dosage of Cofilin (sry-αRNAi + cofilin+/−; Figure 4A). This restoration of F-actin stability mitigated the multinucleation phenotype (sry-α−/−+cofilin+/−; Figures 4B and 4C), showing that a minimum level of F-actin stability is required in furrow tips to ensure successful furrow ingression. Thus, the ASR makes cellularization more error-prone by reducing F-actin stability.
Figure 4. ASR Is Maladaptive for Cellularization and Embryo Development.
(A) FRAP kinetics for furrow tip F-actin in embryos of indicated genotypes at 32°C. Each point represents one embryo (n ≥ 14 embryos per genotype, with 1–3 furrows analyzed per embryo).
(B) Surface views show furrow tip F-actin (phalloidin, green) and nuclei (Hoechst, purple) in embryos of indicated genotypes at 32°C. Multinucleate cells highlighted by orange nuclei in corresponding segmented images.
(C) Severity of multinucleation in embryos of indicated genotypes at 32°C. Each point represents one embryo (n ≥ 11 embryos per genotype, with ~150 nuclei analyzed per embryo).
(D) Data from (4C) on shortened y axis to show no difference between wild-type (WT) and cofilin+/− embryos.
(E) Larval hatching rates for indicated conditions (n ≥ 5 independent experiments, with ≥ 9 embryos per experiment; mean ± SE). Corresponding scatterplot in Figure S4F.
Student’s t test used to calculate p values in (A), (C), (D), and (E).
Horizontal lines represent means ± SE in (A), (C), and (D).
Viability, in Addition to Morphogenesis, Is Compromised in Heat-Stressed Embryos
Even if Cofilin-mediated ASR puts cellularization at risk, it could benefit the organism overall (i.e., there might be a trade-off). To address this possibility, we quantified larval hatching rates for wild-type and cofilin+/− embryos. Embryos were reared at 18°C or 32°C, collected in late cellularization, and shifted back to room temperature until larval hatching was scored (Table S1). For the 32°C embryo group, more cofilin+/− embryos survived and hatched than did wild-type (Figures 4E and S4F), suggesting that the ASR is not only disruptive for cellularization but also can be harmful to embryos overall.
We wondered whether the reduced viability of heat-stressed embryos was a result of cellularization mistakes. However, inspection of cofilin+/− embryos showed a similar likelihood of furrow regressions as seen in wild-type embryos (Figures 4C and 4D). We do not know why overstabilization of F-actin at 32°C in cofilin+/− embryos causes multinucleation, though our combined data suggest that some “precise” level of stability is required for best cellularization outcomes. Nonetheless, cofilin+/− embryos had improved viability compared to wild-type embryos, even though they showed the same extent of multinucleation as wild-type. Thus, embryos must be robust to a small number of furrow regressions. Instead, the reduced viability accompanying ASR seems more closely associated with F-actin destabilization in the cytoplasm, perhaps due to more free actin entering the nucleus.
DISCUSSION
Here, we identify an embryonic ASR. While previously ASR was defined in terms of actin rod assembly (e.g., Kanellos and Frame, 2016), our work argues that the actin cytoskeleton is more extensively modified throughout cells. We suggest a model whereby increased Cofilin activity during ASR in fly embryos leads to increased F-actin severing in the cytoplasm and destabilization of normal F-actin structures. This F-actin destabilization has two consequences: first, it puts morphogenesis at risk. Specifically, during cellularization, F-actin destabilization at all furrow tips leads to the stochastic regression of a small number of furrows. Second, cytoplasmic F-actin destabilization increases the free actin monomer pool in the cytoplasm and, consequently, the nucleus, to a level that can promote concentration-dependent actin rod assembly in the nucleus. Rod assembly buffers free nuclear actin levels, perhaps in an attempt to sequester subunits from some adverse function in either the nucleus or cytoplasm. While many details of this model remain to be tested, we believe that the ASR should now be viewed in terms of the entire actin cytoskeleton and all its functions, both nuclear and cytoplasmic.
This work adds to a growing list of examples in which Cofilin emerges as a specific target for, and vulnerability to, environmental stress. Beyond the ASR, Cofilin oxidation and responsiveness to heat shock proteins in environmentally stressed cells affects actin-based behaviors, as well as cell survival via mitochondrial health and apoptotic signaling (Klamt et al., 2009; Klemke et al., 2008; Simard et al., 2011). While Cofilin’s functions will surely differ with context, we suggest it as a key general node in actin-based stress response, acting across diverse cell types and organisms. Our observations in the fly embryo lead us to consider whether stress-induced changes in Cofilin activity could partially explain why high temperature is teratogenic (Auger et al., 2017; Chambers et al., 1998; Dixon et al., 2011; Edwards 2006). Because F-actin remodeling drives morphogenesis (Rodal et al., 2015), we think it possible that Cofilin-mediated stress response could compromise the fidelity of other morphogenetic events, just as it precipitated cellularization failures here. In addition, by changing the levels or organization of nuclear actin, Cofilin-mediated ASR could also alter nuclear events critical to development, including transcription and genome remodeling.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to the Lead Contact, Anna Marie Sokac (sokac@bcm.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Drosophila melanogaster stocks were housed at 22°C on standard molasses food. Most analyses focus on embryos in Bownes Stages 4 through 5 (pre-cellularization through the end of cellularization) (Bownes, 1975), unless noted in the Method Details, such as mixed-stage embryo collections for some Western Blotting experiments.
Fly stocks and genetics
OreR was used as the wild-type stock. For Histone-GFP, embryos were collected from ubi::H2A-GFP (gift of E. Wieschaus). For cofilin+/−, adults from stock tsr1/CyO (Bloomington Stock Center #9107) were crossed with OreR, and F2 embryos collected from F1 tsr1/+ females crossed with either sibling tsr1/+ males (imaging and western blotting experiments) or OreR males (larval hatching assays). For sry-α−/−, embryos were collected from Df(3R)X3F/TM3, Sb (Merrill et al., 1988). For sry-α−/− + cofilin+/−, adults from stock tsr1/CyO were crossed with Df(3R)X3F/TM3, Sb, and F2 embryos collected from F1 tsr1/+; Df(3R)X3F/+ females crossed with sibling tsr1/+; Df(3R)X3F/+ males.
METHOD DETAILS
Embryo collection, fixation, and staining
Embryo collection cups were set up on apple juice plates at 18°C or 32°C according to published protocols (Figard and Sokac, 2011). For F-actin staining, mixed-stage embryos were fixed in 8% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4): n-heptane (1:1) and hand-peeled for staining with 5 U ml−1 Alexa Fluor 488 phalloidin or Alexa Fluor 546 phalloidin (Thermo Scientific, Rockford, IL). For nuclear staining, Hoescht 33342 was used at 1.0 μg ml−1 (Invitrogen, Carlsbad, CA).
Hatching assay
Embryos were reared in collection cups at 18°C or 32°C, following the scheme shown in Table S1. Late cellularization-stage embryos were hand-selected under a dissecting microscope, transferred to fresh apple juice plates, covered in Halocarbon oil 27 (Sigma Aldrich, St. Louis, MO), and incubated at 22°C in humidifying chambers (80×15 mm glass Petri dish lined with moist paper towels). After 48 hours plates were scored for the percent of hatched larvae.
Rhodamine G-actin injections and RNAi
Embryos were collected 30 minutes post-egg laying from collection cups at 18°C. Embryos were mounted in a line on the edge of a glass coverslip with “embryo glue” (a solution made from double-sided tape saturated in n-heptane), dessicated for 7–10 minutes prior to injection, and covered with 1:3 mixture of Halocarbon 700:Halocarbon 27. Lyophilized, rhodamine-labeled, non-muscle G-actin (G-actinRed; Cytoskeleton, Inc., Denver, CO) was resuspended at 5 μg/ml with 1 μL water and 1 μL G-buffer (5 mM Tris-HCl pH 8.0, 0.2 mM ATP, 0.5 mM DTT, and 0.2 mM CaCl2), and ~1 μL of the solution was loaded into a glass capillary needle. Using a custom injection stage and Femtojet Express microinjector (Eppendorf, Hamburg, Germany), ~50 pl of the solution was injected per embryo. Following injection, embryos were incubated at 18°C or 32°C until imaging (Table S1). For sry-αRNAi, embryos were prepared as follows: G-actinRed was resuspended at 5 μg/μl in sry-α double-stranded RNA, previously diluted 1:1 with G-buffer, and ~50 pl was injected per embryo. Double-stranded RNAs for sry-α were prepared as previously described, using primers: sry-α-RNAi-F (5′-TAATACGACTCACTATAGGGTCAGGAGCTAATC-3′) and sry-α-RNAi-R (5′-TAATCAGACTCACTATAGGGCC CAGCATGTCCA-3′) (Zheng et al., 2013). To confirm that neither G-actinRed injection nor FRAP perturbed cellularization, furrow ingression rates were estimated using a custom MATLAB furrow tracking algorithm in wild-type embryos following these manipulations, and were found to be comparable to published rates of furrow ingression for wild-type embryos (Figard et al., 2013).
Image acquisition
Images were collected on a Zeiss LSM 710 confocal microscope with a 40X/1.2 numerical aperture water-immersion objective (Carl Zeiss, Inc., Oberkochen, Germany). For presentation, images were cropped, resized, and adjusted for brightness and contrast in ImageJ/FIJI (Schindelin et al., 2012) or Adobe Photoshop CC (Adobe, San Jose, CA). Channels were adjusted separately in multicolor images.
For FRAP, embryos were imaged at 18 ± 2°C or at 32 ± 2°C in a thermal incubator. G-actinRed was bleached to approximately 50% fluorescence intensity using 100% laser power in cross-section within a 2 μm × 1 μm box for furrow canals or in surface views within a 2 μm × 2 μm box for rods. Fluorescence recovery was recorded at 1 or 2 s intervals for ≥ 80 s, which encompassed the time to full recovery. Bleached furrow canals ingress at the same rate and with the same morphology as their unbleached neighbors, suggesting that bleaching does not induce significant phototoxicity (Figure 3F; Xue and Sokac, 2016).
For imaging actin rods, wild-type or Histone-GFP embryos injected with G-ActinRed were imaged at 18 ± 2°C or at 32 ± 2°C in a thermal incubator, ~10 mm beneath the embryo’s surface, at the mid-section of the nuclei.
Western Blotting
Embryos (200 per condition) in early cellularization were hand-selected under a dissecting microscope and rapidly snap-frozen in liquid nitrogen. Frozen embryos were homogenized on ice in lysis buffer (150 μL 0.05 M Tris pH 8.0, 0.15 M KCl, 0.05M EDTA, 0.5% NP-40, 1X protease inhibitor cocktail (Pierce Protease Inhibitor Mini Tablets, EDTA-free, Thermo Scientific)). After spinning to remove yolk and debris, protein concentrations were determined using a BCA Protein Assay (Thermo Scientific). Equal amounts of protein were loaded per well and separated on hand-cast 12% SDS-PAGE gels. Proteins were transferred to 0.2 μm nitrocellulose (Bio-Rad, Hercules, CA) and probed with 1:50 mouse anti-β-actin (sc-47778, Santa Cruz Biotechnology, Dallas, TX), 1:5000 rabbit anti-Dm-Cofilin, or 1:5000 rabbit anti-Pi-Cofilin (sc-12912-R, Santa Cruz Biotechnology), followed by 1:5000 goat anti-mouse HRP or 1:10,000 goat anti-rabbit HRP secondary antibodies (Jackson Immuno Research, West Grove, PA).
Antibody production and validation
Recombinant protein 6X-His-Dm-Cofilin (Twinstar) was expressed in E. coli strain BL21 and purified in gravity-flow columns (Thermo Scientific) using Nickel-NTA affinity beads (QIAGEN, Hilden, Germany). Antibody was raised in rabbits by Covance (Princeton, NJ), and precipitated from sera using ammonium sulfate (Harlow and Lane, 1988). IgG was further purified using the Melon Gel Purification Kit (Thermo Scientific).
Phosphatase assay
Collections of mixed-stage embryos were snap-frozen in microcentrifuge tubes in liquid nitrogen. Frozen embryos were homogenized on ice in EDTA-free lysis buffer (200 μL 0.05 M Tris pH 8.0, 0.15 M KCl, 0.5% NP-40, 1X protease inhibitor cocktail (Pierce Protease Inhibitor Mini Tablets, EDTA-free, Thermo Scientific)). For lysates with phosphatase inhibitor, final concentration of 0.04 M sodium orthovanadate (Santa Cruz Biotechnology) and 0.20 M sodium fluoride (Sigma Aldrich) were included in the lysis buffer. For phosphatase reactions, 80 μg embryo protein (calculated from BCA assay, Thermo Scientific) were incubated with 4000 units of λ phosphatase in λ phosphatase reaction buffer supplemented with MnCl2 per the manufacturer’s instructions (Santa Cruz Biotechnology) for 20 minutes at 30°C. Equal concentrations of protein from each reaction were separated on hand-cast 12% SDS-PAGE gels, followed by Western Blotting.
QUANTIFICATION AND STATISTICAL ANALYSIS
Student’s t tests were performed using GraphPad QuickCalcs (GraphPad, San Diego, CA). Comparisons with p values ≤ 0.05 were considered to be significant. Specific information regarding p values and n values can be found in the figure legends. All graphs were generated in MATLAB (MathWorks, Natick, MA) and edited in Adobe Illustrator CC (Adobe, San Jose, CA). Figures were assembled in Adobe Photoshop CC.
Multinucleation quantification
The percent of embryos displaying multinucleation was counted manually using raw, single plane, surface view images collected at the furrow canals, where an entire embryo side was visible (≥1000 nuclei assayed per embryo). The ratio of mononucleate cells to nuclei was determined by manually counting in two quadrants from a raw, single plane, surface view image collected at the furrow canals (quadrant size = 2500 μm2); and the mean was calculated per embryo. For presentation of the multinucleation phenotype, images were segmented using our previously described custom MATLAB code (Zheng et al., 2013).
F-actin levels quantification
We quantified F-actin levels in furrow tips of embryos with 3–6 μm furrow lengths using custom MATLAB code, as previously described (Zheng et al., 2013), with the following modification: To control for tube-to-tube variation in immunostaining, we used His-tone-GFP embryos collected at 25°C (for temperature comparisons) or 32°C (for genotype comparisons) as internal controls that were mixed into each tube of experimental wild-type or cofilin+/− embryos collected at either 18°C or 32°C, following the scheme shown in Table S1. GFP signal distinguished the experimental embryos from Histone-GFP controls. We confirmed that differences between wild-type and Histone-GFP embryos at 25°C were insignificant, with a ratio of 1.006 ± 0.035 (n = 3 experiments from 37 embryos for each genotype; mean ± SE). Thus, we normalized F-actin levels as follows: The F-actin fluorescence value from each embryo in an experimental group was normalized to the F-actin fluorescence value from each Histone-GFP embryo in the same tube, generating a pairwise series of normalized F-actin levels. To compare between experimental conditions, pairwise normalized F-actin levels were pooled from each experimental condition (temperature or genotype) and used to calculate average and standard error of the mean. An alternative method, in which F-actin levels from each embryo in an experimental group were normalized to the average of the corresponding Histone-GFP embryos, was also performed and gave similar results.
FRAP quantification
Fluorescence intensity was measured using FIJI/ImageJ. Intensity of the bleached furrow canal (IFRAP) was normalized (INORM) by the intensity of an unbleached furrow canal (IREF) and the data fitted using two methods (Hardy, 2012; Phair et al., 2004). In both methods, IREF-PRE and IFRAP-PRE are the pre-bleach fluorescence intensity in the respective region, and I0 is the normalized intensity after bleach. In method 1: INORM(t) = (IFRAP(t)*IREF-PRE)/(IFRAP-PRE*IREF(t)). Using MATLAB, recovery was fit to a single exponential function: INORM(t) = Imax−(Imax−I0)*e(−k*t). In method 2: INORM(t) = IFRAP(t)/IFRAP-PRE. Using MATLAB, recovery was fit to a modified exponential function: INORM(t) = (Imax−I0)*e(-k*t) −Ka*t, where Ka is the acquisition bleaching rate calculated by the slope of the reference fluorescence intensity. For both methods, the mobile fraction and half-time to recovery were calculated by the following equations: Mobile fraction = (Imax−I0) − (1−I0) and Half time = −ln(0.5)/k. The mobile fraction and half-time values calculated using method 1 are plotted throughout. The same trends were confirmed using method 2.
Actin rod abundance quantification
For rod quantifications, we chose G-actinRed imaging in live embryos to avoid confounding results that were likely to be introduced by poor retention of rods in chemically fixed embryos. (Note that while rods could be minimally preserved by fixation and stained with phalloidin in some embryos, to the eye, rod numbers and size were drastically reduced compared to live embryos). To compare rod formation between temperatures and genotypes, we quantified the percent embryos containing rods within each experiment. However, the same trends were confirmed using two additional measurements of rod abundance: rods per nucleus, as well as percent nuclei containing rods.
For rod abundance quantifications, the total number of nuclei and rods were manually counted and expressed as a ratio (rods / nucleus). Each image was assigned a code so that the experimenter performing the quantifications was blind to the conditions. Free nuclear actin fluorescence and free cytoplasmic actin fluorescence were quantified by averaging fluorescence measurements from 2 μm × 2 μm boxes in three nuclei or three cytoplasmic (non-furrow) regions per embryo. To generate the plots in Figure 2C, 2D, S1E, S1F, and S1G, MATLAB was used to bin data along the x axis with equal numbers of data points in each bin. Binned data were fit to a Hill Function.
Western blot quantification
Films were scanned in black and white at 600 dpi and quantified after applying the “Subtract Background” algorithm in Fiji/ImageJ and then inverting. Per blot (bit depth = 8), integrated intensities for each band were normalized against the value of the 18°C band for that antibody or Cofilin species (i.e., phosphorylated or de-phosphorylated) so that results from different experiments could be related. The average and standard deviation for the normalized values for eight or four experiments for actin or Cofilin, respectively, were calculated. Dm-Cofilin blots were used to calculate the ratio of phosphorylated to total Cofilin. Consistent with the decrease in this ratio, the Pi-Cofilin values consistently decreased in four out of four experiments.
DATA AND SOFTWARE AVAILABILITY
All raw data, image files, and custom MATLAB algorithms are available upon request to the Lead Contact.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-β-actin, mouse | SCBT | Cat#sc-47778; RRID: AB_626632 |
| Anti-Drosophila melanogaster Cofilin (Dm-Cofilin), rabbit | This study | N/A |
| Anti-PhosphoCofilin (Pi-Cofilin), rabbit | SCBT | Cat#sc-12912-R; RRID:AB_673572 |
| Anti-Mouse HRP | Jackson Immuno Research | Cat#115–035–003; RRID:AB_10015289 |
| Anti-Rabbit HRP | Jackson Immuno Research | Cat#111–035–144; RRID:AB_2307391 |
| Bacterial and Virus Strains | ||
| BL21 (DE3) | Thermo Scientific | Cat#C600003 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Actin protein (rhodamine), human platelet (G-actinred) | Cytoskeleton, Inc. | Cat#APHL99-E |
| 6X-His-Dm-Cofilin | This study | N/A |
| Alexa Fluor 488 phalloidin | Invitrogen/Thermo Scientific | Cat#A12379 |
| Alexa Fluor 546 phalloidin | Invitrogen/Thermo Scientific | Cat#A22283 |
| Hoechst 33342 | Invitrogen/Fisher Scientific | Cat#H3570 |
| Halocarbon 27 oil | Sigma-Aldrich | Cat#H8773–100ML |
| Halocarbon 700 oil | Sigma-Aldrich | Cat#H8898–100ML |
| Nickel-NTA Agarose | QIAGEN | Cat#30210 |
| Pierce Protease Inhibitor Mini Tablets, EDTA-free | Thermo Scientific | Cat#A32955 |
| Critical Commercial Assays | ||
| Melon Gel IgG Spin Purification Kit | Thermo Scientific | Cat#45206 |
| Lambda phosphatase | SCBT | Cat#sc-200312A |
| BCA Protein Assay | Thermo Scientific | Cat#23225 |
| Pierce Disposable Columns (10 mL) | Thermo Scientific | Cat#29924 |
| Amicon-Ultra 15 mL centrifugal filters, MWCO 30 kDa | Millipore-Sigma | Cat#UFC903008 |
| Experimental Models: Organisms/Strains | ||
| Oregon R (OreR) | DGGR | Cat#109612; RRID:DGGR_109612 |
| ubi::H2A-GFP (Histone-GFP) | Gift of E. Wieschaus | N/A |
| Tsr1/CyO (1/2 cofilin) | BDSC | Cat#9107; RRID:BDSC_9107 |
| Df(3R)X3F/TM3, Sb (sry-α −/−) | Merrill et al., 1988 | N/A |
| Oligonucleotides | ||
| Primer: Forward: sry-α-RNAi-F (5’-TAATACGACTCACT ATAGGGTCAGGAGCTAATC-3’); Reverse: sry-α-RNAi-R (5’-TAATCAGACTCACTATAGGGCCCAGCATGTCCA-3’) | Zheng etal., 2013 | N/A |
| Recombinant DNA | ||
| pET45b-tsr(B/X)(6X-His-Dm-Cofilin) | This study | N/A |
| Software and Algorithms | ||
| ImageJ/FIJI | NIH | https://fiji.sc |
| Adobe Photoshop CC | Adobe | https://www.adobe.com/creativecloud.html |
| Adobe Illustrator CC | Adobe | https://www.adobe.com/creativecloud.html |
| MATLAB | MathWorks | https://www.mathworks.com/products/matlab.html |
| Segmentation Algorithm (custom MATLAB code) | Zheng etal., 2013 | N/A |
| Furrow tracking algorithm (custom MATLAB code) | Zheng etal., 2013 | N/A |
| Furrow canal intensity algorithm (custom MATLAB code) | Zheng etal., 2013 | N/A |
Highlights.
Drosophila embryos mount an actin stress response (ASR) against heat stress
Cofilin mediates the ASR, affecting actin in both the nucleus and the cytoplasm
In nuclei, actin rods assemble; in cytoplasm, F-actin structures are destabilized
F-actin destabilization disrupts morphogenesis, and embryo ASR is overall maladaptive
ACKNOWLEDGMENTS
We gratefully acknowledge the computing resources provided by the CIBR Center of Baylor College of Medicine. This work was supported by grants from the NIH (R01 GM115111 to L.F., L.Z., Z.X., H.S., and A.M.S.; T32 GM008231 to N.B.). S.C. and I.G. are supported by a grant from the NIH (R01 GM082837), grants from the NSF (PHY-1147498, PHY-1430124, and PHY-1427654), a John S. Dunn Collaborative Research Award, and a Welch Foundation grant (Q-1759).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found with this article online at https://doi.org/10.1016/j.celrep.2019.02.092.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Amberg D, Leadsham JE, Kotiadis V, and Gourlay CW (2012). Cellular ageing and the actin cytoskeleton. Subcell. Biochem 57, 331–352. [DOI] [PubMed] [Google Scholar]
- Andrianantoandro E, and Pollard TD (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24, 13–23. [DOI] [PubMed] [Google Scholar]
- Ashworth SL, and Molitoris BA (1999). Pathophysiology and functional significance of apical membrane disruption during ischemia. Curr. Opin. Nephrol. Hypertens 8, 449–458. [DOI] [PubMed] [Google Scholar]
- Ashworth SL, Southgate EL, Sandoval RM, Meberg PJ, Bamburg JR, and Molitoris BA (2003). ADF/cofilin mediates actin cytoskeletal alterations in LLC-PK cells during ATP depletion. Am. J. Physiol. Renal Physiol 284, F852–F862. [DOI] [PubMed] [Google Scholar]
- Auger N, Fraser WD, Sauve R, Bilodeau-Bertrand M, and Kosatsky T (2017). Risk of congenital heart defects after ambient heat exposure early in pregnancy. Environ. Health Perspect 125, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NA, Douglas PM, Simic MS, Grant AR, Moresco JJ, Wolff SC, Yates JR 3rd, Manning G, and Dillin A (2014). HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science 346, 360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, and Bernstein BW (2016). Actin dynamics and cofilin-actin rods in alzheimer disease. Cytoskeleton (Hoboken) 73, 477–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, Bernstein BW, Davis RC, Flynn KC, Goldsbury C, Jensen JR, Maloney MT, Marsden IT, Minamide LS, Pak CW, et al. (2010). ADF/cofilin-actin rods in neurodegenerative diseases. Curr. Alzheimer Res 7, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh S, and Arking R (1984). Development profile of the heat shock response in early embryos of Drosophila. J. Exp. Zool 231, 379–391. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Chen H, Boyle JA, and Bamburg JR (2006). Formation of actin-ADF/cofilin rods transiently retards decline of mitochondrial potential and ATP in stressed neurons. Am. J. Physiol. Cell Physiol 291, C828–C839. [DOI] [PubMed] [Google Scholar]
- Bownes M (1975). A photographic study of development in the living embryo of Drosophila melanogaster. J. Embryol. Exp. Morphol 33, 789–801. [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Magalhaes MAO, Eddy RJ, Hodgson L, and Condeelis J (2013). Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell Biol 14, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Albertson R, Riggs B, Field CM, and Sullivan W (2008). Nuf, a Rab11 effector, maintains cytokinetic furrow integrity by promoting local actin polymerization. J. Cell Biol 182, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Johnson KA, Dick LM, Felix RJ, and Jones KL (1998). Maternal fever and birth outcome: a prospective study. Teratology 58, 251–257. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Dalton LE, Clarke HJ, Malzer E, Dominicus CS, Patel V, Moorhead G, Ron D, and Marciniak SJ (2015). Actin dynamics tune the integrated stress response by regulating eukaryotic initiation factor 2a dephosphorylation. eLife 4, e04872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews SM, McCleery WT, and Hutson MS (2016). Pathway to a pheno-copy: heat stress effects in early embryogenesis. Dev. Dyn 245, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MJ, Marazita ML, Beaty TH, and Murray JC (2011). Cleft lip and palate: understanding genetic and environmental influences. Nat. Rev. Genet 12, 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopie J, Skarp KP, Rajakylä EK, Tanhuanpää K, and Vartiainen MK (2012). Active maintenance of nuclear actin by importin 9 supports transcription. Proc. Natl. Acad. Sci. USA 109, E544–E552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopie J, Rajakylä EK, Joensuu MS, Huet G, Ferrantelli E, Xie T, Jää-linoja H, Jokitalo E, and Vartiainen MK (2015). Genome-wide RNAi screen for nuclear actin reveals a network of cofilin regulators. J. Cell Sci 128, 2388–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ (2006). Review: hyperthermia and fever during pregnancy. Birth Defects Res. A Clin. Mol. Teratol 76, 507–516. [DOI] [PubMed] [Google Scholar]
- Figard L, and Sokac AM (2011). Imaging cell shape change in living Drosophila embryos. J. Vis. Exp 49, 2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figard L, Xu H, Garcia HG, Golding I, and Sokac AM (2013). The plasma membrane flattens out to fuel cell-surface growth during Drosophila cellularization. Dev. Cell 27, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans J, Wenzl C, Herz HM, Bartoszewski S, Schnorrer F, Vogt N, Schwarz H, and Müller HA (2005). RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development 132, 1009–1020. [DOI] [PubMed] [Google Scholar]
- Hamdoun A, and Epel D (2007). Embryo stability and vulnerability in an always changing world. Proc. Natl. Acad. Sci. USA 104, 1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy LR (2012). Fluorescence recovery after photobleaching (FRAP) with a focus on F-actin. Curr. Protoc. Neurosci Chapter 2, Unit 2.17 10.1002/0471142301.ns0217s61. [DOI] [PubMed] [Google Scholar]
- Harlow E, and Lane D (1988). Antibodies: A Laboratory Manual (Cold Spring Harbor Laboratory Press; ). [Google Scholar]
- Higuchi R, Vevea JD, Swayne TC, Chojnowski R, Hill V, Boldogh IR, and Pon LA (2013). Actin dynamics affect mitochondrial quality control and aging in budding yeast. Curr. Biol 23, 2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TY, Minamide LS, Bamburg JR, and Bokoch GM (2008). Chronophin mediates an ATP-sensing mechanism for cofilin dephosphorylation and neuronal cofilin-actin rod formation. Dev. Cell 15, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K, Iida H, and Yahara I (1986). Heat shock induction of intranuclear actin rods in cultured mammalian cells. Exp. Cell Res 165, 207–215. [DOI] [PubMed] [Google Scholar]
- Kanellos G, and Frame MC (2016). Cellular functions of the ADF/cofilin family at a glance. J. Cell Sci 129, 3211–3218. [DOI] [PubMed] [Google Scholar]
- Kim JS, Huang TY, and Bokoch GM (2009). Reactive oxygen species regulate a slingshot-cofilin activation pathway. Mol. Biol. Cell 20, 2650–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamt F, Zdanov S, Levine RL, Pariser A, Zhang Y, Zhang B, Yu LR, Veenstra TD, and Shacter E (2009). Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nat. Cell Biol 11, 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke M, Wabnitz GH, Funke F, Funk B, Kirchgessner H, and Sam-stag Y (2008). Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity 29, 404–413. [DOI] [PubMed] [Google Scholar]
- McCullough BR, Blanchoin L, Martiel JL, and De la Cruz EM (2008). Cofilin increases the bending flexibility of actin filaments: implications for severing and cell mechanics. J. Mol. Biol 381, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill PT, Sweeton D, and Wieschaus E (1988). Requirements for auto-somal gene activity during precellular stages of Drosophila melanogaster. Development 104, 495–509. [DOI] [PubMed] [Google Scholar]
- Minamide LS, Striegl AM, Boyle JA, Meberg PJ, and Bamburg JR (2000). Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat. Cell Biol 2, 628–636. [DOI] [PubMed] [Google Scholar]
- Minamide LS, Maiti S, Boyle JA, Davis RC, Coppinger JA, Bao Y, Huang TY, Yates J, Bokoch GM, and Bamburg JR (2010). Isolation and characterization of cytoplasmic cofilin-actin rods. J. Biol. Chem 285, 5450–5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhina S, Wang YL, and Murata-Hori M (2007). Alpha-actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev. Cell 13, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsie LN, and Truant R (2012). The role of the cofilin-actin rod stress response in neurodegenerative diseases uncovers potential new drug targets. Bioarchitecture 2, 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsie LN, Desmond CR, and Truant R (2012). Cofilin nuclear-cytoplasmic shuttling affects cofilin-actin rod formation during stress. J. Cell Sci 125, 3977–3988. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Nishida E, Sakai H, and Miyamoto E (1989). Dephosphorylation of cofilin accompanies heat shock-induced nuclear accumulation of cofilin. J. Biol. Chem 264, 16143–16148. [PubMed] [Google Scholar]
- Ono S, Abe H, Nagaoka R, and Obinata T (1993). Colocalization of ADF and cofilin in intranuclear actin rods of cultured muscle cells. J. Muscle Res. Cell Motil 14, 195–204. [DOI] [PubMed] [Google Scholar]
- Padash Barmchi M, Rogers S, and Häcker U (2005). DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J. Cell Biol 168, 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parman T, Wiley MJ, and Wells PG (1999). Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat. Med 5, 582–585. [DOI] [PubMed] [Google Scholar]
- Phair RD, Gorski SA, and Misteli T (2004). Measurement of dynamic protein binding to chromatin in vivo, using photobleaching microscopy. Methods Enzymol 375, 393–414. [DOI] [PubMed] [Google Scholar]
- Puscheck EE, Awonuga AO, Yang Y, Jiang Z, and Rappolee DA (2015). Molecular biology of the stress response in the early embryo and its stem cells. Adv. Exp. Med. Biol 843, 77–128. [DOI] [PubMed] [Google Scholar]
- Rodal AA, Del Signore SJ, and Martin AC (2015). Drosophila comes of age as a model system for understanding the function of cytoskeletal proteins in cells, tissues, and organisms. Cytoskeleton (Hoboken) 72, 207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salilew-Wondim D, Tesfaye D, Hoelker M, and Schellander K (2014). Embryo transcriptome response to environmental factors: implication for its survival under suboptimal conditions. Anim. Reprod. Sci 149, 30–38. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Gwinn J, and Sanger JM (1980). Dissolution of cytoplasmic actin bundles and the induction of nuclear actin bundles by dimethyl sulfoxide. J. Exp. Zool 213, 227–230. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Hosford M, Sandoval RM, Wagner MC, Atkinson SJ, Bamburg J, and Molitoris BA (1999). Ischemia activates actin depolymerizing factor: role in proximal tubule microvillar actin alterations. Am. J. Physiol 276, F544–F551. [DOI] [PubMed] [Google Scholar]
- Simard JP, Reynolds DN, Kraguljac AP, Smith GS, and Mosser DD (2011). Overexpression of HSP70 inhibits cofilin phosphorylation and promotes lymphocyte migration in heat-stressed cells. J. Cell Sci 124, 2367–2374. [DOI] [PubMed] [Google Scholar]
- Sokac AM, and Wieschaus E (2008). Zygotically controlled F-actin establishes cortical compartments to stabilize furrows during Drosophila cellularization. J. Cell Sci 121, 1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck A, Domazetovska A, Mokbel N, Cooper ST, Ilkovski B, and North KN (2010). In vitro analysis of rod composition and actin dynamics in inherited myopathies. J. Neuropathol. Exp. Neurol 69, 429–441. [DOI] [PubMed] [Google Scholar]
- Xue Z, and Sokac AM (2016). -Back-to-back mechanisms drive actomyosin ring closure during Drosophila embryo cleavage. J. Cell Biol 215, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Sepúlveda LA, Lua RC, Lichtarge O, Golding I, and Sokac AM (2013). The maternal-to-zygotic transition targets actin to promote robustness during morphogenesis. PLoS Genet 9, e1003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data, image files, and custom MATLAB algorithms are available upon request to the Lead Contact.